CHAPTER 32 Clinical Risk Assessment
Definitions
Risk assessment is defined by numerous components.2,29 Risk is the probability that an individual will develop a specific disease in a given period. The risk of developing the disease will vary from individual to individual.
Risk factors may be environmental, behavioral, or biologic factors that, when present, increase the likelihood that an individual will develop the disease. Risk factors are identified through longitudinal studies of patients with the disease of interest. Exposure to a risk factor or factors may occur at a single point in time; over multiple, separate points in time; or continuously. However, to be identified as a risk factor, the exposure must occur before disease onset. Interventions often can be identified and when implemented, can help modify risk factors.
The term risk determinant/background characteristic, which is sometimes substituted for the term risk factor, should be reserved for those risk factors that cannot be modified.
Risk indicators are probable or putative risk factors that have been identified in cross-sectional studies but not confirmed through longitudinal studies.
Risk predictors/markers, although associated with increased risk for disease, do not cause the disease.
These factors also are identified in cross-sectional and longitudinal studies.
Box 32-1 lists elements of these categories of risk for periodontal disease.
Risk Factors for Periodontal Disease
Tobacco Smoking
Tobacco smoking is a well-established risk factor for periodontitis.2,32 A direct relationship exists between smoking and the prevalence of periodontal disease (see Chapter 26). This association is independent of other factors such as oral hygiene or age.18 Studies comparing the response to periodontal therapy in smokers, previous smokers, and nonsmokers have shown that smoking has a negative impact on the response to therapy. However, former smokers respond similarly to nonsmokers.3 These studies demonstrate the therapeutic impact of intervention strategies on patients who smoke (see Chapter 26).
Diabetes
Diabetes is a clear risk factor for periodontitis.2 Epidemiologic data demonstrate that the prevalence and severity of periodontitis are significantly higher in patients with type 1 or type 2 diabetes mellitus than in those without diabetes, and that the level of diabetic control is an important variable in this relationship (see Chapter 5).
Pathogenic Bacteria and Microbial Tooth Deposits
It is well documented that accumulation of bacterial plaque at the gingival margin results in the development of gingivitis and that the gingivitis can be reversed with the implementation of oral hygiene measures.25 These studies demonstrate a causal relationship between accumulation of bacterial plaque and gingival inflammation. However, a causal relationship between plaque accumulation and periodontitis has been more difficult to establish. Often, patients with severe loss of attachment have minimal levels of bacterial plaque on the affected teeth, indicating that the quantity of plaque is not of major importance in the disease process. However, although quantity may not indicate risk, there is evidence that the composition, or quality, of the complex plaque biofilm is of importance.
In terms of quality of plaque, three specific bacteria have been identified as etiologic agents for periodontitis: Aggregatibacter actinomycetemcomitans (formerly Actinobacillus actinomycetemcomitans), Porphyromonas gingivalis, and Tannerella forsythia (formerly Bacteroides forsythus).11 P. gingivalis and T. forsythia are often found in chronic periodontitis, whereas A. actinomycetemcomitans is often associated with aggressive periodontitis. Cross-sectional and longitudinal studies support the delineation of these three bacteria as risk factors for periodontal disease. Additional evidence that these organisms are causal agents includes the following16:
Although not completely supported by these criteria for causation, moderate evidence also suggests that Campylobacter rectus, Eubacterium nodatum, Fusobacterium nucleatum, Prevotella intermedia/nigrescens, Peptostreptococcus micros, Streptococcus intermedius, and Treponema denticola are etiologic factors in periodontitis.11
Therefore the quantity of plaque present may not be as important as the quality of the plaque in determining risk for periodontitis.
Anatomic factors, such as furcations, root concavities, developmental grooves, cervical enamel projections, enamel pearls, and bifurcation ridges, may predispose the periodontium to disease as a result of their potential to harbor bacterial plaque and present a challenge to the clinician during instrumentation. Similarly, the presence of subgingival and overhanging margins can result in increased plaque accumulation, increased inflammation, and increased bone loss. Although not clearly defined as risk factors for periodontitis, anatomic factors and restorative factors that influence plaque accumulation may play a role in disease susceptibility for specific teeth.7
The presence of calculus, which serves as a reservoir for bacterial plaque, has been suggested as a risk factor for periodontitis. Although the presence of some calculus in healthy individuals receiving routine dental care does not result in significant loss of attachment, the presence of calculus in other groups of patients, such as those not receiving regular care and patients with poorly controlled diabetes, can have a negative impact on periodontal health.29
Risk Determinants/Background Characteristics for Periodontal Disease
Genetic Factors
Evidence indicates that genetic differences between individuals may explain why some patients develop periodontal disease and others do not. Studies conducted in twins have shown that genetic factors influence clinical measures of gingivitis, probing pocket depth, attachment loss, and interproximal bone height.26-28 The familial aggregation seen in localized and generalized aggressive periodontitis also is indicative of genetic involvement in these diseases (see Chapter 18).
Kornman et al21 demonstrated that alterations in specific genes encoding the inflammatory cytokines interleukin-1α (IL-1α) and interleukin-1β (IL-1β) were associated with severe chronic periodontitis in nonsmoking subjects.21 However, results of other studies have shown limited association between these altered genes and the presence of periodontitis. Overall, it appears that changes in the IL-1 genes may be only one of several genetic changes involved in the risk for chronic periodontitis. Therefore, although the alteration in the IL-1 genes may be a valid marker for periodontitis in defined populations, its usefulness as a genetic marker in the general population may be limited.20
Immunologic alterations, such as neutrophil abnormalities,17 monocytic hyperresponsiveness to lipopolysaccharide stimulation in patients with localized aggressive periodontitis,37 and alterations in the monocyte/macrophage receptors for the Fc portion of antibody,20,47 also appear to be under genetic control. In addition, genetics play a role in regulating the titer of the protective immunoglobulin G2 (IgG2) antibody response to A. actinomycetemcomitans in patients with aggressive periodontitis15 (seeChapter 24).
Age
Both the prevalence and severity of periodontal disease increase with age.8,31,32 It is possible that degenerative changes related to aging may increase susceptibility to periodontitis. However, it also is possible that the attachment loss and bone loss seen in older individuals are the result of prolonged exposure to other risk factors over a person’s life, creating a cumulative effect over time. In support of this, studies have shown minimal loss of attachment in aging subjects enrolled in preventive programs throughout their lives.33,34 Therefore it is suggested that periodontal disease is not an inevitable consequence of the aging process and that aging alone does not increase disease susceptibility. However, it remains to be determined whether changes related to the aging process, such as intake of medications, decreased immune function, and altered nutritional status, interact with other well-defined risk factors to increase susceptibility to periodontitis.
Evidence of loss of attachment may have more consequences in younger patients. The younger the patient, the longer the patient has for exposure to causative factors. In addition, aggressive periodontitis in young individuals often is associated with an unmodifiable risk factor such as a genetic predisposition to disease.32 Therefore young individuals with periodontal disease may be at greater risk for continued disease as they age.
Gender
Gender plays a role in periodontal disease.2 Surveys conducted in the United States since 1960 demonstrate that men have more loss of attachment than women.42,44,45 In addition, men have poorer oral hygiene than women, as evidenced by higher levels of plaque and calculus.1,43,45 Therefore gender differences in prevalence and severity of periodontitis appear to be related to preventive practices rather than any genetic factor.
![]() Science Transfer
Science Transfer
The presence of dental plaque over time is the major risk factor for developing periodontitis. The qualitative bacterial makeup of the biofilm also can affect the risk of having periodontal disease. Smoking not only increases the risk of a patient having periodontal bone loss but also negatively affects treatment outcomes. Smoking cessation can overcome the bad effects on treatment prognosis. Smoking patients should be involved in smoking cessation programs so that their periodontal prognosis is optimized. Several genetic factors, including alteration of the genes responsible for interleukin-1 (IL-1) levels, do affect the risk of periodontal disease but apparently do not predict treatment outcomes.
Diabetes and acquired immunodeficiency syndrome (AIDS) are also systemic factors that increase the risk of periodontal destruction. The role of osteoporosis is not clearly documented, and a patient’s age plays a role mainly by the increased time the plaque bacteria has in contact with periodontal tissues. Many older patients involved in a lifelong preventive periodontal program show minimal loss of attachment.
Socioeconomic Status
Gingivitis and poor oral hygiene can be related to lower socioeconomic status (SES).2,42,44 This can most likely be attributed to decreased dental awareness and decreased frequency of dental visits compared with more educated individuals with higher SES. After adjusting for other risk factors, such as smoking and poor oral hygiene, lower SES alone does not result in increased risk for periodontitis (see Chapter 5).
Stress
The incidence of necrotizing ulcerative gingivitis increases during periods of emotional and physiologic stress, suggesting a link between the two.10,38 Emotional stress may interfere with normal immune function6,40 and may result in increased levels of circulating hormones, which can affect the periodontium.36 Stressful life events, such as bereavement and divorce, appear to lead to a greater prevalence of periodontal disease,13 and an apparent association exists between psychosocial factors and risk behaviors such as smoking, poor oral hygiene, and chronic periodontitis.9 Adult patients with periodontitis who are resistant to therapy are more stressed than those who respond to therapy.5 Individuals with financial strain, distress, depression, or inadequate coping mechanisms have more severe loss of attachment.12 Although epidemiologic data on the relationship between stress and periodontal disease are limited, stress may be a putative risk factor for periodontitis.32
Risk Indicators for Periodontal Disease
Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome
It has been hypothesized that the immune dysfunction associated with human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) increases susceptibility to periodontal disease (see Chapter 19). Early reports on the periodontal status of patients with AIDS or individuals who are HIV seropositive revealed that these patients often had severe periodontal destruction characteristic of necrotizing ulcerative periodontitis.48 More recent reports, however, have failed to demonstrate significant differences in the periodontal status of individuals with HIV infection and healthy controls.24,41 The apparent discrepancy in these reports may have been caused by the inclusion of patients with AIDS (versus patients who were exclusively HIV seropositive) in some studies.32
Conflicting results also exist in studies examining the level of immunosuppression and severity of periodontal destruction. Some studies support that as the degree of immunosuppression increases in adults with AIDS, periodontal pocket formation and loss of clinical attachment also increase. Results of other studies have found no relationship between periodontal diseases and HIV/AIDS status. Evidence also suggests that AIDS-affected individuals who practice good preventive oral health measures, including effective home care and seeking appropriate professional therapy, can maintain periodontal health.39 Therefore, although it seems reasonable to hypothesize that HIV infection and immunosuppression are risk factors for periodontal disease, the evidence is not conclusive.2,32
Osteoporosis
Osteoporosis has been suggested as another risk factor for periodontitis. Although studies in animal models indicate that osteoporosis does not initiate periodontitis, evidence indicates that the reduced bone mass seen in osteoporosis may aggravate periodontal disease progression.4,23 However, reports in humans are conflicting. In a study of 12 women with osteoporosis and 14 healthy women, von Wowern et al46 reported that the women with osteoporosis had greater loss of attachment than the control subjects. In contrast, Kribbs22 examined pocket depth, bleeding on probing, and gingival recession in women with and without osteoporosis. Although the two groups had significant differences in bone mass, no differences in periodontal status were noted. However, it appears that a link may exist between osteoporosis and periodontitis, and additional studies may need to be conducted to determine if osteoporosis is a true risk factor for periodontal disease.14,19
Infrequent Dental Visits
Identifying failure to visit the dentist regularly as a risk factor for periodontitis is controversial.29 One study demonstrated an increased risk for severe periodontitis in patients who had not visited the dentist for 3 or more years, whereas another demonstrated that there was no more loss of attachment or bone loss in individuals who did not seek dental care compared with those who did over a 6-year period. However, differences in the ages of the subjects in these two studies may explain the different results. Additional longitudinal and intervention studies are necessary to determine if infrequency of dental visits is a risk factor for periodontal disease.
Risk Markers/Predictors for Periodontal Disease
Previous History of Periodontal Disease
A history of previous periodontal disease is a good clinical predictor of risk for future disease.29 Patients with the most severe existing loss of attachment are at the greatest risk for future loss of attachment. Conversely, patients currently free of periodontitis have a decreased risk for developing loss of attachment compared with those who currently have periodontitis (seeChapter 5).
Bleeding on probing is the best clinical indicator of gingival inflammation.29 Although this indicator alone does not serve as a predictor for loss of attachment, bleeding on probing coupled with increasing pocket depth may serve as an excellent predictor for future loss of attachment. Lack of bleeding on probing does appear to serve as an excellent indicator of periodontal health.
Clinical Risk Assessment for Periodontal Disease
Information concerning individual risk for developing periodontal disease is obtained through careful evaluation of the patient’s demographic data, medical history, dental history, and clinical examination (Box 32-2). The elements that contribute to increased risk can be identified through the collection of demographic data, including the patient’s age, gender, and SES. The medical history may reveal elements such as a history of diabetes, smoking, HIV/AIDS, or osteoporosis, as well as the perceived level of stress. The dental history can reveal a family history of early tooth loss (suggestive of a genetic predisposition for aggressive periodontitis), a previous history of periodontal disease, and information concerning the frequency of oral health care in the past. Important elements identified during the clinical examination can include the location and extent of bacterial plaque accumulation, presence of plaque-retentive factors (e.g., overhanging restorations, subgingival margins), presence of anatomic plaque-retentive areas (e.g., grooves, furcation involvements), presence of calculus, extent of attachment loss, and presence or absence of bleeding on probing.
BOX 32-2 Clinical Risk Assessment for Periodontal Disease
Once the social, demographic, medical history, dental history, and clinical presentation data are collected, they must be analyzed to identify patients at risk for developing periodontal disease. This analysis can be accomplished by the health care provider and/or through the use of a computer-based risk assessment tool. One example of this type of tool is the Periodontal Assessment Tool (PAT), which is one component of the PreViser Oral Health Information Suite (OHIS). The PAT utilizes input from twenty-three items that are part of a routine periodontal examination to generate both a quantification of current disease and a risk score for future disease. The score, which is based on an individual patient’s set of risk factors and history, ranges from 1 (lowest risk) to 5 (highest risk). It has been proposed that utilizing this tool will provide a more objective, quantitative way to assess risk for periodontitis than clinical opinion.30,35.Ultimately, a thorough risk assessment should improve clinical decision making, as well as patient outcomes.
Once an at-risk patient is identified and a diagnosis is made, the treatment plan may be modified accordingly (Figure 32-1). For example, patients with a history of cigarette smoking should be informed of the relationship between smoking and periodontitis. They also should be informed of the impact of smoking on their prognosis and the likelihood of success of their periodontal therapy if they continue to smoke. Part of their recommended treatment plan for initial therapy may include referral to a smoking cessation program or implementation of self-administered smoking cessation aids. As another example, a patient diagnosed with severe, chronic periodontitis may be encouraged to be tested for the IL-1–positive genotype. If positive, the patient’s treatment may involve the administration of systemic antimicrobial agents and host modifiers that would not be used in a patient without this genetic marker. If alterations in the host response are identified, the prognosis and treatment plan may be modified. Previously identified risk elements also may need to be reassessed at the reevaluation stage of treatment. This is especially important in patients who do not respond favorably to periodontal therapy (see Figure 32-1).
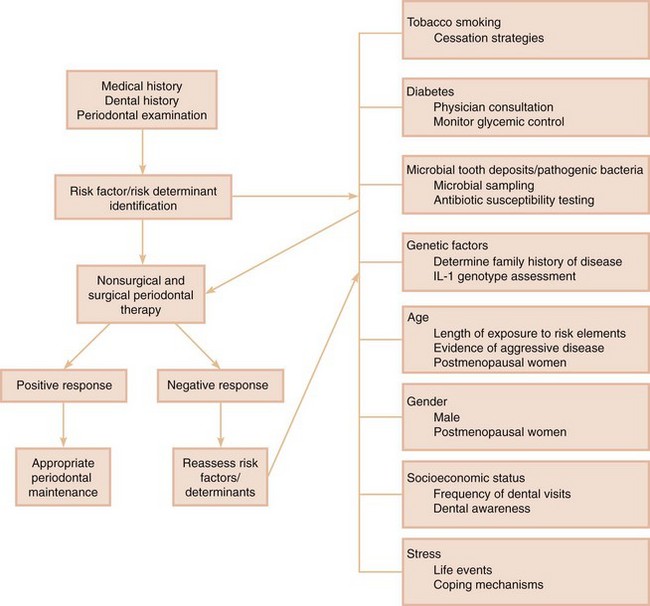
Figure 32-1 Risk assessment in periodontal therapy. The major risk elements to be considered in the diagnosis and treatment of periodontal disease are indicated. The importance of risk assessment before the initiation of therapy is highlighted, as well as the need for reassessment after a negative response to therapy.
Conclusion
Risk assessment involves identifying elements that either may predispose a patient to developing periodontal disease or may influence the progression of disease that already exists. In either case, these patients may require modification of their prognosis and treatment plan. In addition to an evaluation of the factors contributing to their risk, these patients should be educated concerning their risk, and when appropriate, suitable intervention strategies should be implemented.
1 Abdellatif HM, Burt BA. An epidemiological investigation into the relative importance of age and oral hygiene status as determinants of periodontitis. J Dent Res. 1987;66:13.
2 American Academy of Periodontology. Position paper: Epidemiology of periodontal diseases. J Periodontol. 1996;67:935.
3 American Academy of Periodontology. Position paper: Tobacco use and the periodontal patient. J Periodontol. 1999;70:1419.
4 Aufdemorte TB, Boyan BD, Fox WC, et al. Diagnostic tools and biologic markers: animal models in the study of osteoporosis and oral bone loss. J Bone Miner Res. 1993;8(suppl 1):S29.
5 Axtelius B, Söderfeldt B, Nilsson A, et al. Therapy-resistant periodontitis: psychosocial characteristics. J Clin Periodontol. 1998;25:482.
6 Ballieux RE. Impact of mental stress on the immune response. J Clin Periodontol. 1991;18:427.
7 Blieden TM. Tooth-related issues. Ann Periodontol. 1999;4:91.
8 Burt BA. Periodontitis and aging: reviewing recent evidence. J Am Dent Assoc. 1994;125:273.
9 Croucher R, Marcenes WS, Torres MC, et al. The relationship between life-events and periodontitis: a case-control study. J Clin Periodontol. 1997;24:39.
10 Enwonwu CO. Epidemiological and biochemical studies of necrotizing ulcerative gingivitis and noma (cancrum oris) in Nigerian children. Arch Oral Biol. 1972;17:1357.
11 Genco R, Kornman K, Williams R, et al. Consensus report: Periodontal diseases: pathogenesis and microbial factors. Ann Periodontol. 1996;1:926.
12 Genco RJ, Ho AW, Grossi SG, et al. Relationship of stress, distress, and inadequate coping behaviors to periodontal disease. J Periodontol. 1999;70:711.
13 Green LW, Tryon WW, Marks B, et al. Periodontal disease as a function of life events stress. J Stress. 1986;12:32.
14 Guers NC, Lewis CE, Jeffcoat MJ. Osteoporosis and periodontal disease progression. Periodontol 2000. 2003;32:105.
15 Gunsolley JC, Tew JG, Gooss CM, et al. Effects of race, smoking and immunoglobulin allotypes on IgG subclass concentrations. J Periodontal Res. 1997;32:381.
16 Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78.
17 Hart TC, Shapira L, Van Dyke TE. Neutrophil defects as risk factors for periodontal diseases. J Periodontol. 1994;65:521.
18 Ismail AI, Burt BA, Eklund SA. Epidemiologic patterns of smoking and periodontal disease in the United States. J Am Dent Assoc. 1983;106:617.
19 Kinane DF. Periodontitis modified by systemic factors. Ann Periodontol. 1999;4:55.
20 Kinane DF, Hart TC. Genes and gene polymorphisms associated with periodontal disease. Crit Rev Oral Biol Med. 2003;14:430.
21 Kornman KS, Crane S, Wang HY, et al. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997;24:72.
22 Kribbs PJ. Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent. 1990;63:218.
23 Krook L, Whalen JP, Lesser GV, et al. Experimental studies on osteoporosis. Methods Achiev Exp Pathol. 1975;7:72.
24 Lamster IB, Begg MD, Mitchell L, et al. Oral manifestations of HIV infection in homosexual men and intravenous drug users: study design and relationship of epidemiologic, clinical, and immunologic parameter to oral lesions. Oral Surg Oral Med Oral Pathol. 1994;78:163.
25 Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177.
26 Michalowicz BS, Aeppli DP, Kuba RK, et al. A twin study of genetic variation in proportional radiographic alveolar bone height. J Dent Res. 1991;70:1431.
27 Michalowicz BS, Aeppli DP, Virag JG, et al. Risk findings in adult twins. J Periodontol. 1991;62:293.
28 Michalowicz BS, Diehl SR, Gunsolley JC, et al. Evidence for a substantial genetic basis for risk of adult periodontitis. J Periodontol. 2000;71:1699.
29 Page RC, Beck JD. Risk assessment for periodontal diseases. Int Dent J. 1997;47:61.
30 Page RC, Martin JA, Loeb CF. The oral health information suite (OHIS): its use in the management of periodontal disease. J Dent Edu. 2005;69:509.
31 Papapanou PN. Epidemiology and natural history of periodontal disease. In: Lang NP, Karring T, editors. Proceedings of the First European Workshop on Periodontology. London: Quintessence, 1994.
32 Papapanou PN. Risk assessments in the diagnosis and treatment of periodontal diseases. J Den Educ. 1998;62:822.
33 Papapanou PN, Lindhe J. Preservation of probing attachment and alveolar bone levels in two random population samples. J Clin Periodontol. 1992;19:583.
34 Papapanou PN, Lindhe J, Sterrett JD, et al. Considerations on the contribution of aging to loss of periodontal tissue support. J Clin Periodontol. 1991;18:611.
35 Persson GR, Mancl LS, Martin J, Page RC. Assessing periodontal disease risk. A comparison of clinicians’ assessment versus a computerized tool. JADA. 2003;134:575.
36 Rose RM. Endocrine responses to stressful psychological events. Psychiatr Clin North Am. 1980;3:251.
37 Shapira L, Soskolone WA, Van Dyke TE, et al. Prostaglandin E2 secretion, cell maturation, and CD14 expression by monocyte-derived macrophages from localized juvenile periodontitis patients. J Periodontol. 1996;67:224.
38 Shields WD. Acute necrotizing ulcerative gingivitis: a study of some of the contributing factors and their validity in an Army population. J Periodontol. 1977;48:346.
39 Stanford TW, Rees TD. Acquired immune suppression and other risk factors/indicators for periodontal disease progression. Periodontol 2000. 2003;32:118.
40 Sternberg EM, Chrousos GP, Wilder RL, et al. The stress response and the regulation of inflammatory disease. Ann Intern Med. 1992;117:854.
41 Swango PA, Kleinman DV, Konzelman JL. HIV and periodontal health: a study of military personnel with HIV. J Am Dent Assoc. 1991;122:49.
42 US Public Health Service, National Center for Health Statistics, Periodontal disease in adults, United States, 1960–1962, PHS Pub No 1000, Series 11, No 12, 1965, US Government Printing Office, Washington, DC
43 US Public Health Service, National Center for Health Statistics, Oral hygiene in adults, United States, 1960–1962, PHS Pub No 1000, Series 11, No 16, 1966, US Government Printing Office, Washington, DC
44 US Public Health Service, National Center for Health Statistics, Basic data on dental examination findings of persons 1–74 years, United States, 1971–1974, DHEW PHS Pub No 79-1662, Series 11, No 214, 1979, US Government Printing Office, Washington, DC
45 US Public Health Service, National Institute of Dental Research (NIDR), Oral health of United States adults: national findings, NIH Pub No 87-2868, 1987, NIDR, Bethesda, Md
46 von Wowern J, Klausen B, Kollerup G. Oseoporosis : a risk factor in periodontal disease. J Periodontol. 1994;65:134.
47 Wilson ME, Kalmar JR. FcγIIa (CD32): a potential marker defining susceptibility to localized juvenile periodontitis. J Periodontol. 1996;76:323.
48 Winkler JR, Herrera C, Westenhouse J, et al. Periodontal disease in HIV-infected and uninfected homosexual and bisexual men. AIDS. 1992;6:1041. (letter)