CHAPTER 56 Gingival Surgical Techniques
Periodontal pocket reduction surgery limited to the gingival tissues only and not involving the underlying osseous structures, without the use of flap surgery, can be classified as gingival curettage and gingivectomy. Current understanding of disease etiology and therapy limits the use of both techniques, but their place in surgical therapy is essential.
Gingival Curettage
The word curettage is used in periodontics to mean the scraping of the gingival wall of a periodontal pocket to remove diseased soft tissue. Scaling refers to the removal of deposits from the root surface, whereas planing means smoothing the root to remove infected and necrotic tooth substance. Scaling and root planing may inadvertently include various degrees of curettage. However, they are different procedures with different rationales and indications. Both should be considered separate parts of periodontal treatment.
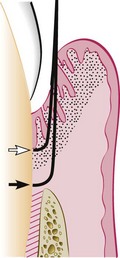
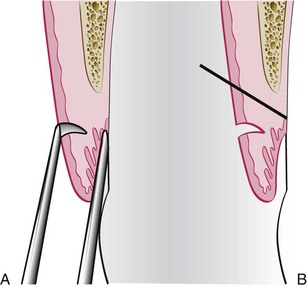
Curettage in periodontics has been defined as gingival and subgingival curettage (Figure 56-1). Gingival curettage consists of the removal of the inflamed soft tissue lateral to the pocket wall and the junctional epithelium. Subgingival curettage refers to the procedure that is performed apical to the junctional epithelium and severing the connective tissue attachment down to the osseous crest.
It should also be understood that some degree of curettage is accomplished unintentionally during scaling and root planing and is referred to inadvertent curettage. This chapter refers to the intentional curettage performed during the same visit as scaling and root planing, or as a separate procedure to reduce pocket depth by enhancing gingival shrinkage, new connective tissue attachment, or both.
Rationale
Curettage accomplishes the removal of the chronically inflamed granulation tissue that forms in the lateral wall of the periodontal pocket. This tissue, in addition to the usual components of granulation tissues (fibroblastic and angioblastic proliferation), contains areas of chronic inflammation and may also have pieces of dislodged calculus and bacterial colonies. The latter may perpetuate the pathologic features of the tissue and hinder healing.
This inflamed granulation tissue is lined by epithelium, and deep strands of epithelium penetrate into the tissue. The presence of this epithelium is construed as a barrier to the attachment of new fibers in the area.
When the root is thoroughly planed, the major source of bacteria disappears, and the pathologic changes in the tissues adjacent to the pocket resolve with no need to eliminate the inflamed granulation tissue by curettage. The existing granulation tissue is slowly resorbed and the bacteria present in the tissue without replenishment of their numbers from the plaque in the pocket are destroyed by the defense mechanisms of the host. Therefore the need for curettage to eliminate the inflamed granulation tissue appears questionable.* It has been shown that scaling and root planing with additional curettage do not improve the condition of the periodontal tissues beyond the improvement resulting from scaling and root planing alone.
Curettage may also eliminate all or most of the epithelium that lines the pocket wall and the underlying junctional epithelium. Curettage for this purpose is still valid, particularly when an attempt is made for new attachment, as occurs in intrabony pockets. However, opinions differ regarding whether scaling and curettage consistently remove the pocket lining and the junctional epithelium. Some investigators report that scaling and root planing tear the epithelial lining of the pocket without removing either it or the junctional epithelium.33 Others claim that both epithelial structures6,7,32 and sometimes the underlying inflamed connective tissue34 are removed by curettage. Some investigators have reported that the removal of the pocket lining and junctional epithelium by curettage is not complete.51,54,57
Curettage and Esthetics
The awareness of esthetics in periodontal therapy has become an integral part of care in the modern practice of periodontics. In the past, pocket elimination was the primary goal of therapy and little regard was given to the esthetic result. Rapid shrinkage of the gingival tissue was the goal to eliminate the pocket. Currently, esthetics is a major consideration of therapy, especially in the maxillary anterior area (teeth #6 to 11), and every effort is made to minimize gingival tissue shrinkage and the preservation of the interdental papilla.
A compromise therapy is feasible in the anterior maxilla; this therapy consists of thorough subgingival root planing while attempting to not detach the connective tissue attachment beneath the junctional epithelium. Gingival curettage should be avoided. The granulation tissue in the lateral wall of the pocket, in an environment free of plaque and calculus, becomes connective tissue, thereby minimizing gingival shrinkage. Thus, although complete pocket elimination is not accomplished, the inflammatory changes are reduced or eliminated and the interdental papilla and the esthetic appearance of the area are preserved.
There are many instances in which a surgical flap is necessary for access to the root surface for scaling and root planing. A surgical technique specially designed to minimize gingival recession and preserve the interdental papilla is the papilla preservation technique (see Chapter 57).
Another important precaution is to avoid root planing apical to the base of the pocket to the osseous crest The removal of the junctional epithelium and disruption of the connective tissue attachment exposes the nondiseased portion of the cementum. Root planing and the removal of the nondiseased cementum may result in excessive shrinkage of the gingiva, which results in increased gingival recession.
Indications
Indications for curettage are very limited. It can be used after scaling and root planing for the following purposes:
Procedure
Basic Technique
Curettage does not eliminate the causes of inflammation (i.e., bacterial plaque and deposits). Therefore curettage should always be preceded by scaling and root planing, which is the basic periodontal therapy procedure (see Chapter 45). The use of local infiltrative anesthesia for scaling and root planing is optional. However, gingival curettage will always require some type of local anesthesia.
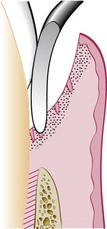
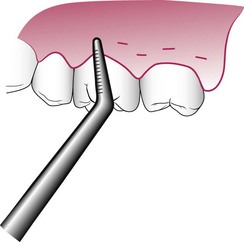
The curette is selected so that the cutting edge is against the tissue (e.g., Gracey #13-14 for mesial surfaces, Gracey #11-12 for distal surfaces). Curettage can also be performed with a 4R-4L Columbia Universal curette. The instrument is inserted to engage the inner lining of the pocket wall and is carried along the soft tissue, usually in a horizontal stroke (Figure 56-2). The pocket wall may be supported by gentle finger pressure on the external surface. The curette is then placed under the cut edge of the junctional epithelium to undermine it.
In subgingival curettage, the tissues attached between the bottom of the pocket and the alveolar crest are removed with a scooping motion of the curette to the tooth surface (Figure 56-3). The area is flushed to remove debris, and the tissue is partly adapted to the tooth by gentle finger pressure. In some cases, suturing of separated papillae and application of a periodontal pack may be indicated.
Other Techniques
Other techniques for gingival curettage include the excisional new attachment procedure, ultrasonic curettage, and the use of caustic drugs:
Excisional New Attachment Procedure
The excisional new attachment procedure (ENAP) has been developed and used by the United States (US) Naval Dental Corps.40,62,63 It is a definitive subgingival curettage procedure performed with a knife. The ENAP technique is as follows:
Ultrasonic Curettage
The use of ultrasonic devices has been recommended for gingival curettage.35 When applied to the gingiva of experimental animals, ultrasonic vibrations disrupt tissue continuity, lift off the epithelium, dismember collagen bundles, and alter the morphologic features of fibroblast nuclei.20 Ultrasound is effective for debriding the epithelial lining of periodontal pockets. This results in a narrow band of necrotic tissue (microcauterization), which strips off the inner lining of the pocket.
The Morse scaler-shaped and rod-shaped ultrasonic instruments are used for this purpose. Some investigators found ultrasonic instruments to be as effective as manual instruments for curettage35,50,64 but resulted in less inflammation and less removal of underlying connective tissue. The gingiva can be made more rigid for ultrasonic curettage by injecting anesthetic solution directly into it.10
Caustic Drugs
Since early in the development of periodontal procedures,53,61 the use of caustic drugs has been recommended to induce a chemical curettage of the lateral wall of the pocket or even the selective elimination of the epithelium. Drugs, such as sodium sulfide, alkaline sodium hypochlorite solution (Antiformin),8,24,26 and phenol,4,9 have been proposed and then discarded after studies indicated their ineffectiveness.5,18,26 The extent of tissue destruction with these drugs cannot be controlled, and they may increase rather than reduce the amount of tissue to be removed by enzymes and phagocytes.
Healing after Scaling and Curettage
Immediately after curettage, a blood clot fills the pocket area, which is totally or partially devoid of epithelial lining. Hemorrhage is also present in the tissues with dilated capillaries and abundant polymorphonuclear leukocytes (PMNs), which appear on the wound surface. This is followed by a rapid proliferation of granulation tissue with a decrease in the number of small blood vessels as the tissue matures.
Restoration and epithelialization of the sulcus generally require 2 to 7 days,27,34,37,57 and restoration of the junctional epithelium occurs in animals as early as 5 days after treatment. Immature collagen fibers appear within 21 days. Healthy gingival fibers inadvertently severed from the tooth and tears in the epithelium33,46 are repaired in the healing process. Several investigators have reported that in monkeys11,62 and humans58 treated by scaling and curettage, healing results in the formation of a long, thin junctional epithelium with no new connective tissue attachment. In some cases, this long epithelium is interrupted by “windows” of connective tissue attachment.11
Clinical Appearance after Scaling and Curettage
Immediately after scaling and curettage, the gingiva appears hemorrhagic and bright red. After 1 week, the gingiva appears reduced in height because of an apical shift in the position of the gingival margin. The gingiva is also darker red than normal but much less so than on previous days. After 2 weeks and with proper oral hygiene, the normal color, consistency, surface texture, and contour of the gingiva are attained and the gingival margin is well adapted to the tooth.
Gingivectomy
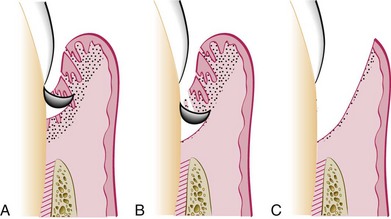
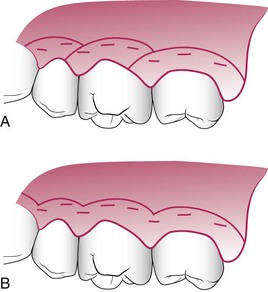
Gingivectomy means excision of the gingiva. By removing the pocket wall, gingivectomy provides visibility and accessibility for complete calculus removal and thorough smoothing of the roots (Figure 56-5). This creates a favorable environment for gingival healing and restoration of a physiologic gingival contour.
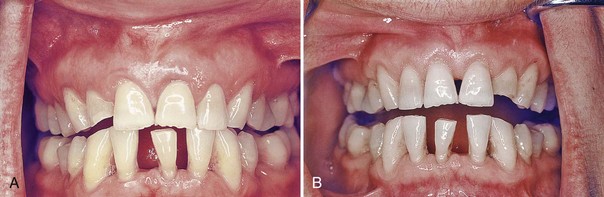
Figure 56-5 Results obtained by treating a suprabony pocket with gingivectomy. A, Before treatment. B, After treatment.
The gingivectomy technique was widely performed in the past. Improved understanding of healing mechanisms and the development of more sophisticated flap methods have relegated the gingivectomy to a lesser role in the current repertoire of available techniques. However, it remains an effective form of treatment when indicated (see Figure 56-5).
Indications and Contraindications
The gingivectomy technique may be performed for the following indications16:
Contraindications to gingivectomy include the following:
The gingivectomy technique may be performed by means of scalpels, electrodes, lasers, or chemicals. All these techniques are reviewed here, although the surgical method is the only technique recommended.
Surgical Gingivectomy
Step 1
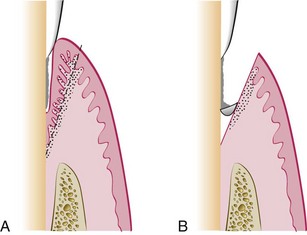
The pockets on each surface are explored with a periodontal probe and marked with a pocket marker (Figures 56-6 and 56-7). Each pocket is marked in several areas to outline its course on each surface.
Step 2
Periodontal knives (e.g., Kirkland knives) are used for incisions on the facial and lingual surfaces and those distal to the terminal tooth in the arch. Orban periodontal knives are used for interdental incisions. Bard-Parker blades #12 and #15, as well as scissors, are used as auxiliary instruments.
The incision is started apical to the points marking the course of the pockets48,56 and is directed coronally to a point between the base of the pocket and the crest of the bone. It should be as close as possible to the bone without exposing it, to remove the soft tissue coronal to the bone. Exposure of bone is undesirable. If it occurs, healing usually presents minimal complications if the area is adequately covered by the periodontal pack.
Either interrupted or continuous incisions may be used (Figure 56-8). The incision should be beveled at approximately 45 degrees to the tooth surface and recreate the normal festooned pattern of the gingiva. Failure to bevel the incision will leave a broad, fibrous plateau, which will take a longer time to develop a physiologic contour. In the interim, plaque and calculus may lead to the recurrence of pockets.
Step 3
Remove the excised pocket wall, clean the area, and closely examine the root surface. The most apical zone consists of a light, bandlike zone where the tissues were attached. Coronally, calculus remnants, root caries, or resorption may be found. Granulation tissue may be seen on the excised soft tissue (Figure 56-9).
Gingivoplasty
Gingivoplasty is similar to gingivectomy, but its objective is different. Gingivectomy is performed to eliminate periodontal pockets and includes reshaping as part of the technique. Gingivoplasty is a reshaping of the gingiva to create physiologic gingival contours with the sole purpose of recontouring the gingiva in the absence of pockets.19
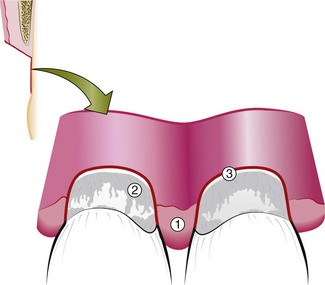
Gingival and periodontal disease often produces deformities in the gingiva that is conducive for plaque accumulation and food debris, which prolongs and aggravates the disease process. Such deformities include (1) gingival clefts and craters, (2) craterlike interdental papillae caused by acute necrotizing ulcerative gingivitis, and (3) gingival enlargements.
Gingivoplasty may be accomplished with a periodontal knife, a scalpel, rotary coarse diamond stones,44 or electrodes.13 The technique resembles that of festooning of a artificial denture, which consists of tapering the gingival margin, creating a scalloped marginal outline, thinning the attached gingiva, creating vertical interdental grooves, and shaping the interdental papillae.
Healing after Surgical Gingivectomy
The initial response after gingivectomy is the formation of a protective surface blood clot. The underlying tissue becomes acutely inflamed with necrosis. The clot is then replaced by granulation tissue. In 24 hours, there is an increase in new connective tissue cells, which are mainly angioblasts beneath the surface layer of inflammation and necrotic tissue. By the third day, numerous young fibroblasts are located in the area.47 The highly vascular granulation tissue grows coronally, creating a new free gingival margin and sulcus.41 Capillaries derived from blood vessels of the periodontal ligament migrate into the granulation tissue and within 2 weeks, they connect with the gingival vessels.59
After 12 to 24 hours, epithelial cells at the margins of the wound begin to migrate over the granulation tissue, separating it from the contaminated surface layer of the clot. Epithelial activity at the margins reaches a peak in 24 to 36 hours.13
The new epithelial cells arise from the basal and deeper spinous layers of the epithelial wound edge and migrate over the wound over a fibrin layer that is later resorbed and replaced by a connective tissue bed.25 The epithelial cells advance by a tumbling action with the cells becoming fixed to the substrate by hemidesmosomes and a new basement lamina.23,28
After 5 to 14 days, surface epithelialization is generally complete. During the first 4 weeks after gingivectomy, keratinization is less than it was before surgery. Complete epithelial repair takes about 1 month.52 Vasodilation and vascularity begin to decrease after the fourth day of healing and appear to be almost normal by the sixteenth day.36 Complete repair of the connective tissue takes about 7 weeks.52
The flow of gingival fluid in humans is initially increased after gingivectomy and diminishes as healing progresses.1,49 Maximal flow is reached after 1 week, coinciding with the time of maximal inflammation.
Although the tissue changes that occur in postgingivectomy healing are the same in all individuals, the time required for complete healing varies considerably, depending on the area of the incised surface and interference from local irritation and infection. In patients with physiologic gingival melanosis, the pigmentation is diminished in the healed gingiva.
![]() Science Transfer
Science Transfer
Gingival curettage procedures involve root planing plus a deliberate curettage of the soft tissues lining the pocket carried out under local anesthesia. These procedures result in improvement of periodontal health but generally are not as effective as periodontal surgical approaches. Therefore these treatments are used in patients in which surgical procedures are contraindicated by the patient’s systemic status or patients who will not accept surgical therapy. In the anterior segments, gingival curettage gives a better esthetic outcome than periodontal surgery and may be used even though the pocket depth reduction is compromised. The excisional new attachment procedure (ENAP) removes the soft tissue pocket lining with a scalpel and then sutures the tissue at its original level. Recently, ENAP has been done with a neodymium:yttrium-aluminum-garnet (Nd-YAG) laser, and preliminary reports show evidence of periodontal regeneration.
Gingivectomy is rarely used to treat periodontitis and even patients with extensive gingival hyperplasia are best treated with flap surgery so that the underlying bone defects can be visualized, treated, and then covered with soft tissue. A few patients needing crown lengthening can be treated with gingivectomy when there is no need for osseous surgery to establish an adequate biologic width.
Laser gingivectomy with carbon dioxide (CO2) laser or Nd-YAG laser gives a more precise tissue contouring than tissue removal with surgical blades and may be another treatment option. Electrosurgical gingivectomies may cause delayed healing, and the surgeon must be careful not to place the electrodes close to the bone margins because sequestration of bone can occur.
Gingivectomy by Electrosurgery
Advantages
Electrosurgery permits an adequate contouring of the tissue and controls hemorrhage.15,17,39
Disadvantages
Electrosurgery cannot be used in patients who have incompatible or poorly shielded cardiac pacemakers. The treatment causes an unpleasant odor. If the electrosurgery point touches the bone, irreparable damage can be inflicted.2,17,22,44 Furthermore, the heat generated by injudicious use can cause tissue damage and loss of periodontal support when the electrode is used close to bone. When the electrode touches the root, areas of cementum can be burned.60 Therefore the use of electrosurgery should be limited to superficial procedures such as removal of gingival enlargements, gingivoplasty, relocation of frenum and muscle attachments, and incision of periodontal abscesses and pericoronal flaps. Extreme care should be used to avoid contacting the tooth surface. Electrosurgery should not be used for procedures that involve proximity to the bone, such as flap operations, or for mucogingival surgery.
Technique
The removal of gingival enlargements and gingivoplasty39 is performed with the needle electrode, supplemented by the small ovoid loop or the diamond-shaped electrodes for festooning. A blended cutting and coagulating (fully rectified) current is used. In all reshaping procedures, the electrode is activated and moved in a concise “shaving” motion.
In the treatment of acute periodontal abscesses, the incision to establish drainage can be made with the needle electrode without exerting pressure. The incision remains open because the edges are sealed by the current. After the acute symptoms subside, the regular procedure for the treatment of the periodontal abscess is followed (see Chapter 42).
For hemostasis, the ball electrode is used. Hemorrhage must be controlled by direct pressure (using air, compress, or hemostat) first; then the surface is lightly touched with a coagulating current. Electrosurgery is helpful for the control of isolated bleeding points. Bleeding areas located interproximally are reached with a thin, bar-shaped electrode.
Frenum and muscle attachments can be relocated to facilitate pocket elimination using a loop electrode. For this purpose, the frenum or muscle is stretched and sectioned with the loop electrode and a coagulating current. For cases of acute pericoronitis, drainage may be obtained by incising the flap with a bent-needle electrode. A loop electrode is used to remove the flap after the acute symptoms subside.
Healing after Electrosurgery
Some investigators report no significant differences in gingival healing after resection by electrosurgery and resection with periodontal knives.14,31 Other researchers find delayed healing, greater reduction in gingival height, and more bone injury after electrosurgery.44 There appears to be little difference in the results obtained after shallow gingival resection with electrosurgery and that with periodontal knives.12 However, when used for deep resections close to bone, electrosurgery can produce gingival recession, bone necrosis and sequestration, loss of bone height, furcation exposure, and tooth mobility. These problems do not occur with the use of periodontal knives.2,17
Laser Gingivectomy
The lasers most often used in dentistry are the carbon dioxide (CO2) and the neodymium:yttrium-aluminum-garnet (Nd:YAG), which have wavelengths of 10,600 nm and 1064 nm, respectively, both in the infrared range; they must be combined with other types of visible lasers for the beam to be seen and aimed.
The CO2 laser has been used for the excision of gingival growths,3,42 although healing is delayed compared with healing after conventional scalpel gingivectomy.21,30,43 The use of a laser for oral surgery requires precautionary measures to avoid reflecting the beam on instrument surfaces, which could result in injury to neighboring tissues and the eyes of the operator. (Chapter 64 includes further information on laser therapy.)
Gingivectomy by Chemosurgery
Techniques to remove the gingiva using chemicals, such as 5% paraformaldehyde38 or potassium hydroxide,29 have been described in the past but are not currently used. They are presented here to provide a historical perspective.
The chemical gingivectomy has the following disadvantages:
Summary
The gingivectomy surgical technique has a long history of use in periodontal surgery. This technique has been attempted using scalpels, electrosurgery, laser, and chemical cautery. Even though this technique may have some use for the minimal reduction of redundant gingival tissue, many limiting factors must be considered. Current periodontal surgery must consider the (1) conservation of keratinized gingiva, (2) minimal gingival tissue loss to maintain esthetics, (3) adequate access to the osseous defects for definitive defect correction, and (4) minimal postsurgical discomfort and bleeding by attempting surgical procedures that will allow primary closure. The gingivectomy surgical technique has limited use in current surgical therapy because it does not satisfy these considerations in periodontal therapy. The clinician must carefully evaluate each case as to the proper application of this surgical procedure.
1 Arnold R, Lunstad G, Bissada N, et al. Alterations in crevicular fluid flow during healing following gingival surgery. J Periodontal Res. 1966;1:303.
2 Azzi R, Kenney EB, Tsao TF, et al. The effect of electrosurgery upon alveolar bone. J Periodontol. 1983;54:96.
3 Barak S, Kaplan H. The CO2 laser in the surgical excision of gingival hyperplasia caused by nifedipine. J Clin Periodontol. 1988;15:633.
4 Barkann A. A conservative technique for the eradication of a pyorrhea product. J Am Dent Assoc. 1939;26:61.
5 Beube FE. An experimental study of the use of sodium sulphide solution in treatment of periodontal pockets. J Periodontol. 1939;10:49.
6 Beube FE. Treatment methods for marginal gingivitis and periodontitis. Texas Dent J. 1953;71:427.
7 Blass JL, Lite T. Gingival healing following surgical curettage: a histopathologic study. NY Dent J. 1959;25:127.
8 Box KF. Periodontal disease and treatment. J Ontario Dent Assoc. 1952;29:194.
9 Bunting RW. The control and treatment of pyorrhea by subgingival surgery. J Am Dent Assoc. 1928;15:119.
10 Burman LR, Alderman LE, Ewen SJ. Clinical application of ultrasonic vibrations for supragingival calculus and stain removal. J Dent Med. 1958;13:156.
11 Caton JC, Zander HA. The attachment between tooth and gingival tissues after periodic root planing and soft tissue curettage. J Periodontol. 1979;50:462.
12 Eisenmann D, Malone WF, Kusek J. Electron microscopic evaluation of electrosurgery. Oral Surg. 1970;29:660.
13 Engler WO, Ramfjord S, Hiniker JJ. Healing following simple gingivectomy: a tritiated thymidine radioautographic study. I. Epithelialization. J Periodontol. 1966;37:289.
14 Fisher SE, Frame JW, Browne RM, et al. A comparative histological study of wound healing following CO2 laser.and conventional surgical excision of the buccal mucosa. Arch Oral Biol. 1983;28:287.
15 Flocken JE. Electrosurgical management of soft tissues and restoration dentistry. Dent Clin North Am. 1980;24:247.
16 Glickman I. The results obtained with the unembellished gingivectomy technique in a clinical study in humans. J Periodontol. 1956;27:247.
17 Glickman I, Imber LR. Comparison of gingival resection with electrosurgery and periodontal knives: biometric and histologic study. J Periodontol. 1970;41:242.
18 Glickman J, Patur B. Histologic study of the effect of Antiformin on the soft tissue wall of periodontal pockets in humans. J Am Dent Assoc. 1955;51:420.
19 Goldman HM. The development of physiologic gingival contours by gingivoplasty. Oral Surg. 1950;3:879.
20 Goldman HM. Histologic assay of healing following ultrasonic curettage versus hand instrument curettage. Oral Med Oral Pathol. 1961;14:925.
21 Gottsegen R, Ammons WFJr. Position paper: Research in lasers in periodontics. Chicago: American Academy of Periodontology; 1992.
22 Henning F. Healing of gingivectomy wounds in the rat: reestablishment of the epithelial seal. J Periodontol. 1968;39:265.
23 Henning F. Epithelial mitotic activity after gingivectomy: relationship to reattachment. J Periodontal Res. 1969;4:319.
24 Hunter HA. A study of tissues treated with Antiformin citric acid. J Can Dent Assoc. 1955;21:344.
25 Innes PB. An electron microscopic study of the regeneration of gingival epithelium following gingivectomy in the dog. J Periodontal Res. 1970;5:196.
26 Johnson RW, Waerhaug J. Effect of Antiformin on gingival tissue. J Periodontol. 1956;27:24.
27 Kon S, Novaes AB, Ruben MP, et al. Visualization of microvascularization of the healing periodontal wound. II. Curettage. J Periodontol. 1969;40:96.
28 Krawczyk WS. A pattern of epithelial cell migration during wound healing. J Cell Biol. 1971;49:247.
29 Löe H. Chemical gingivectomy: effect of potassium hydroxide on periodontal tissues. Acta Odontol Scand. 1961;19:517.
30 Loumanen M. A comparative study of healing of laser and scalpel incision wounds in rat oral mucosa. Scand J Dent Res. 1987;95:65.
31 Malone WF, Eisenmann D, Kusck J. Interceptive periodontics with electrosurgery. J Prosthet Dent. 1969;22:555.
32 Morris ML. The removal of the pocket and attachment epithelium in humans: a histological study. J Periodontol. 1954;25:7.
33 Moskow BS. The response of the gingival sulcus to instrumentation: a histologic investigation. I. The scaling procedure. J Periodontol. 1962;33:282.
34 Moskow BS. The response of the gingival sulcus to instrumentation: a histologic investigation. II. Gingival curettage. J Periodontol. 1964;35:112.
35 Nadler H. Removal of crevicular epithelium by ultrasonic curettes. J Periodontol. 1962;33:220.
36 Novaes AB, Kon S, Ruben MP, et al. Visualization of the microvascularization of the healing periodontal wound. III. Gingivectomy. J Periodontol. 1969;40:359.
37 O’Bannon JY. The gingival tissues before and after scaling the teeth. J Periodontol. 1964;35:69.
38 Orban B. New methods in periodontal treatment. Bur. 1942;42:116.
39 Oringer MJ. Electrosurgery for definitive conservative modern periodontal therapy. Dent Clin North Am. 1969;13:53.
40 Periodontics syllabus: NAVED P5110, 1975, US Naval Dental Corps, p 113.
41 Persson PA. The healing process in the marginal periodontium after gingivectomy with special regard to the regeneration of epithelium (an experimental study on dogs). Odontol T. 1959;67:593.
42 Pick RM, Pecaro BC, Silberman CJ. The laser gingivectomy: the use of CO2 laser for the removal of phenytoin hyperplasia. J Periodontol. 1985;56:492.
43 Pogrel MA, Yen CK, Hanser LS. A comparison of carbon dioxide laser, liquid nitrogen cryosurgery and scalpel wound in healing. Oral Surg Med Pathol. 1990;69:269.
44 Pope JW, Gargiulo AW, Staffileno H, et al. Effects of electrosurgery on wound healing in dogs. Periodontics. 1968;6:30.
45 Ramfjord SP, Ash MMJr. Periodontology and periodontics. Philadelphia: Saunders; 1979.
46 Ramfjord SP, Kiester G. The gingival sulcus and the periodontal pocket immediately following scaling of the teeth. J Periodontol. 1954;25:167.
47 Ramfjord SP, Engler WD, Hiniker JJ. A radiographic study of healing following simple gingivectomy. II. The connective tissue. J Periodontol. 1966;37:179.
48 Ritchey B, Orban B. The periodontal pocket. J Periodontol. 1952;23:199.
49 Sandalli P, Wade AB. Alterations in crevicular fluid flow during healing following gingivectomy and flap procedures. J Periodontal Res. 1969;4:314.
50 Sanderson AD. Gingival curettage by hand and ultrasonic instruments: a histologic comparison. J Periodontol. 1966;37:279.
51 Sato M. Histopathological study of the healing process after surgical treatment for alveolar pyorrhea. Bull Tokyo Dent College. 1960;1:71.
52 Stanton G, Levy M, Stahl SS. Collagen restoration in healing human gingiva. J Dent Res. 1969;48:27.
53 Stewart H. Partial removal of cementum and decalcification of tooth in the treatment of pyorrhea alveolaris. Dent Cosmos. 1899;41:617.
54 Stone S, Ramfjord SP, Waldron J. Scaling and gingival curettage: a radioautographic study. J Periodontol. 1966;37:415.
55 Tonna E, Stahl SS. A polarized light microscopic study of rat periodontal ligament following surgical and chemical gingival trauma. Helv Odontol Acta. 1967;11:90.
56 Waerhaug J. Depth of incision in gingivectomy. Oral Surg. 1955;8:707.
57 Waerhaug J. Microscopic demonstration of tissue reaction incident to removal of subgingival calculus. J Periodontol. 1955;26:26.
58 Waerhaug J. Healing of the dentoepithelial junction following subgingival plaque control. I. As observed in human biopsy material. J Periodontol. 1978;49:1.
59 Watanabe Y, Suzuki S. An experimental study in capillary vascularization in the periodontal tissue following gingivectomy or flap operation. J Dent Res. 1963;42:758.
60 Wilhelmsen NR, Ramfjord SP, Blankenship JR. Effects of electrosurgery on the gingival attachment in rhesus monkeys. J Periodontol. 1976;47:160.
61 Younger WJ. Some of the latest phases in implantations and other procedures. Dent Cosmos. 1893;35:102.
62 Yukna RA. A clinical and histological study of healing following the excisional new attachment procedure in rhesus monkeys. J Periodontol. 1976;47:701.
63 Yukna RA, Bowers GM, Lawrence JJ, et al. A clinical study of healing in humans following the excisional new attachment procedure. J Periodontol. 1976;47:696.
64 Zach L, Cohen G. The histologic response to ultrasonic curettage. J Dent Res. 1961;40:751.