CHAPTER 72 Localized Bone Augmentation and Implant Site Development
One of the most critical aspects of creating an esthetic implant restoration is surgical placement of the implant in a “prosthetically-driven” position to restore the natural tooth position and to emulate the natural emergence of a tooth from the soft tissues. Implants placed without regard for prosthetic position often result in dental restorations that are functionally and esthetically compromised, and patients are left with a less-than-optimal reconstruction. Placement of dental implant(s) in an esthetically and functionally optimal position requires reconstruction of deficient alveolar ridges (or preservation of ridges when teeth are to be extracted).
Periodontal bone loss, gingival recession, tooth loss, and long-term use of removable appliances typically result in alveolar defects that prevent the placement of implants in an optimal prosthetic position. It also leads to soft tissue deficiencies that are unacceptable. Fortunately, continuous innovations in surgical techniques and advances in the biologic understanding of bone-regenerative techniques have led to advanced implant procedures and an increased predictability in the reconstruction of alveolar ridge defects.27,38
Standard implant placement surgery, as described in Chapter 71, is based on adequate bone volume and quality in the desired implant location. The time-tested standard protocol allows for adequate remodeling and maturation of bone, with healing periods of 3 to 6 months. Recent implant procedures often challenge these original conventions by placing implants in areas with inadequate bone volume, simultaneously augmenting bone, and restoring or loading implants after shorter healing periods. This chapter presents an overview of surgical bone augmentation procedures used to correct or to prevent alveolar ridge deficiencies for the optimal placement of dental implants.
Guided Bone Regeneration
Much of what can be achieved with implant surgery and specifically with bone augmentation procedures is directly related to the achievements and understanding of guided bone regeneration (GBR). Historically, augmentation or “regeneration” of alveolar bone lost following tooth removal, alveolar bone resorption, or traumatic injury presented a significant challenge for clinicians. Allowed to heal without the intervention of regenerative procedures, extraction site defects (especially those lacking a self-supporting bone structure) heal with fibrous connective tissue or scar formation and often did not fill with bone. The surrounding soft tissues collapsed into the bone defect, leaving an alveolar ridge deficiency with respect to the natural tooth position and jaw shape. If a removable prosthetic appliance was used, the alveolar ridge continued to resorb over time.
Periodontal studies during the last several decades have led to new techniques and a new treatment approach referred to as guided tissue regeneration (GTR). Briefly, this concept is based on the principle that specific cells contribute to the formation of specific tissues. Exclusion of the faster-growing epithelium and connective tissue from a periodontal wound for a minimum of 6 to 8 weeks allows the slower-growing tissues to occupy the space adjacent to the tooth. Osteoblasts, cementoblasts, and periodontal ligament cells are then afforded the opportunity to regenerate a new periodontal attachment (defined by new bone and new connective tissue fibers inserted into newly formed cementum) on the previously diseased root surface. Chapter 61 includes a discussion on the concepts of GTR as related to periodontal regeneration.
The same basic principle of GTR has been applied to alveolar bone defects to regenerate new bone.13 Using a canine model, Schenk et al47 demonstrated with histology that bone regeneration in membrane-protected defects healed in a sequence of steps that simulated bone formation after tooth extraction. They found that after blood clot formation, bone regeneration was initiated by the formation of woven bone initially along new blood vasculature at the periphery of the defect. The new vascular supply emanated from the existing bone bed (recipient site). Cortical perforations surgically created in the recipient site are thought to enhance the blood supply and cellular access to the bone grafted area. The woven bone, which is formed quickly with a disorganized, immature structure, was subsequently replaced by lamellar bone with an organized, mature structure. Over time, bone remodeling continued with new, secondary osteons being formed.
This concept employed the same principles of specific tissue exclusion but was not associated with teeth. Rather, it was bone that was being isolated from the surrounding soft tissues. Thus the term applied to this technique was guided bone regeneration (GBR). Because the objective of GBR is to regenerate a single tissue, namely bone, it is theoretically easier to accomplish than GTR, which strives to regenerate multiple tissues simultaneously in a complex relationship.
Interestingly, long before the current concepts of GBR were introduced, Murray and co-workers40 demonstrated that when a cavity with a source of osteoblasts and a blood supply was isolated from adjacent soft tissues, it could fill with bone, whereas if the space were not protected, it would fill with fibrous connective tissue. In addition to this observation, they suggested that a bone graft placed in the space might interfere with bone formation because the graft would need to be resorbed before bone could occupy the space.
Bone is a unique tissue that has the capacity to regenerate itself completely. Because of its rigid calcified structure, however, bone has specific requirements that must be respected to achieve regeneration. Because the calcified structure of bone is not conducive to perfusion, new bone formation is critically dependent on establishing an adequate blood supply through the ingrowth of new vasculature while maintaining rigid fixation or stabilization for bone formation. Any movement of the segments of bone relative to one another (even micromotion) during healing results in disruption of the blood supply and a change in the type of tissue formed in the site from mineralized bone to fibrous connective tissue. Table 72-1 lists the biologic requirements for bone regeneration along with the associated component of GBR surgical procedures needed to accomplish bone regeneration.
TABLE 72-1 Biologic Requirements for Bone Regeneration
| Requirement | Surgical Procedure |
|---|---|
| Blood supply | Cortical perforations |
| Stabilization | Fixation screws, membrane tacks |
| Osteoblasts | Autogenous bone (graft or recipient site) |
| Confined space | Barrier membrane |
| Space maintenance | Tenting screws, bone graft materials |
| Wound coverage | Flap management, tension-free suturing |
Barrier Membranes
Barrier membranes are biologically inert materials that serve to protect the blood clot and prevent soft tissue cells (epithelium and connective tissue) from migrating into the bone defect, allowing osteogenic cells to be established. Membranes have been manufactured from biocompatible materials that are either nonresorbable and resorbable. The ideal properties of a barrier membrane are (1) biocompatibility, (2) space maintenance, (3) cell occlusiveness, (4) good handling properties, and (5) resorbability or ease of removal (nonresorbable). Advantages and disadvantages of the resorbable versus nonresorbable membranes are outlined next.
Nonresorbable Barrier Membranes
Various nonresorbable materials have been used as barrier membranes, including latex and Teflon. Teflon, an expanded polytetrafluoroethylene membrane (ePTFE, Gore-Tex Periodontal and Bone Regenerative Membranes, Gore and Associates, Flagstaff, AZ), has been used extensively as a barrier membrane in both GTR and GBR procedures. A variety of shapes and sizes have been designed to be custom-fit around teeth and osseous defects. These barrier membranes are nonresorbable and thus require a subsequent surgical procedure to remove them. The advantage of a nonresorbable barrier membrane is its ability to maintain separation of tissues over an extended time. Unless the barrier is exposed, it can remain in place for several months to years. Typically, GBR membranes are removed after 6 to 12 months.
The disadvantage of a nonresorbable barrier membrane is that if it becomes exposed, it will not heal (i.e., wound will remain open) spontaneously and may become progressively more exposed. Exposed membranes become contaminated with oral bacteria, which may lead to infection of the site and result in bone loss. Therefore exposed membranes must be removed. Contamination or early removal may also result in less bone regeneration.
Space can be maintained under a barrier membrane with bone graft material or tenting screws, thereby facilitating the regeneration of increased bone volume. Stiffer or titanium-reinforced (TR) membranes (Gore and Associates, Flagstaff, AZ) with space-maintaining capabilities can regenerate bone without the need for bone grafts or tenting apparatus.36,47 Stiffer membranes are able to promote significant amounts of new bone and maintain sufficient space without the addition of supportive devices. Ridge augmentation can be enhanced with a TR membrane in conjunction with implant placement in localized bone defects.24
Resorbable Barrier Membranes
The use of resorbable membranes continues to attract widespread interest primarily because it eliminates the need for an additional surgery to be removed. Copolymers of polylactide and polyglycolide (PLA/PGA) have been used to construct biodegradable membranes. Today, collagen-based resorbable barrier membranes are used extensively. The primary advantage of a resorbable membrane is the elimination of surgical reentry for membrane removal. In the case of a subsequent implant placement procedure (or exposure surgery), this may not be a significant advantage. The other advantage is that resorbable membranes are less likely to become exposed and are less problematic if they do become exposed.
A possible disadvantage is that many resorbable membranes degrade before bone formation is completed, and the degradation process may produce varying degrees of inflammation.61 Recent developments include cross-linking of collagen to increase resistance to biodegradation and thus increase longevity of the barrier function.17 Fortunately, the mild inflammatory reaction caused by bioresorbable membranes does not seem to interfere with osteogenesis. Another disadvantage is that resorbable barrier membranes are quite pliable. The lack of stiffness often results in collapse of the membrane into the defect area.46 Thus resorbable membranes are best suited for situations that allow the graft material or hardware (tenting screws, plates) or the adjacent alveolar bone to maintain the desired dimensions.
Human histology has demonstrated that resorbable barrier membranes support the growth of new bone when used in GBR procedures for horizontal, vertical, sinus, and extraction socket defects.18,20,37 They have also been shown to reduce bone resorption when used over a monocortical bone block to augment horizontally deficient ridges.58 Geurs et al20 demonstrated that a bioabsorbable barrier membrane, used in a GBR procedure along with an allograft, was able to facilitate new bone formation. At present, it can be stated that biodegradable membranes have the potential to support bone formation if they are supported by bone graft material to resist collapse and if they are long-lasting enough to maintain their barrier function for extended periods in small to moderate bone defects.28,29
Bone Graft Materials
Unlike other tissues, bone has the unique capacity to completely regenerate itself. The major limiting factors are maintenance of space and structure for bone formation. Bone graft materials have been used to facilitate bone formation within a given space by occupying that space and allowing the subsequent bone growth (and graft replacement) to take place on the structure. The biologic mechanisms that support the use of bone graft materials are osteoconduction, osteoinduction, and osteogenesis.
Osteoconduction is the formation of bone by osteoblasts from the margins of the defect on the bone graft material. Materials that are osteoconductive serve as a scaffold for bone growth. They neither inhibit nor induce bone formation. They simply allow the normal formation of bone by osteoblasts into the grafted defect along the surface of the graft material. Osteoconductive bone graft materials facilitate bone formation by bridging the gap between the existing bone and a distant location that otherwise would not be occupied by bone.
Osteoinduction involves new bone formation through stimulation of osteoprogenitors from the defect (or from the vasculature) to differentiate into osteoblasts and begin forming new bone. This induction of the bone-forming process by cells that would otherwise remain inactive occurs through cell mediators that “turn on” these bone-forming cells. The most widely studied of these mediators is the family of bone morphogenic proteins (BMPs). See Chapter 73 for a review of BMP use in bone augmentation.
Osteogenesis occurs when living osteoblasts are part of the bone graft, as in autogenous bone transplantation. Given an adequate blood supply and cellular viability, these transplanted osteoblasts form new centers of ossification within the graft. Thus, in addition to the bone formation from osteoblasts that already exist in the defect, osteoblasts added as part of the bone graft also form ossification centers and contribute to the total capacity for bone formation.
Numerous bone graft materials have been used to aid in the reconstruction of bone defects. These range from allografts (derived from the same species) to xenografts (derived from a different species) and alloplast or synthetic graft materials. At a minimum, bone graft materials should be osteoconductive. Bone graft materials that are also osteoinductive are believed to be more advantageous than those that are only osteoconductive. Table 72-2 lists the properties of different classes of bone graft materials.
Demineralized freeze-dried bone allograft (DFDBA) is thought to have osteoinductive effects because viable BMPs within the donor tissue matrix are exposed by the decalcification process.57 In contrast to this view, more recent reports have suggested that bone augmentation with DFDBA is not osteoinductive because it does not contain the BMPs necessary to induce bone formation.4,8 Schwartz et al48 reported that variations in the amount of bone formation induced by BMPs in DFDBA may be related to the source (i.e., donor tissue) of the bone and the techniques used to process it. In addition to processing variations, it has been demonstrated that young donor bone results in significantly greater quantities of BMPs retained in the bone allograft matrix compared with older donor bone.49 Therefore the source of donor bone can greatly influence its osteoinductive capacity.
Bone graft materials help maintain space under a barrier membrane to facilitate the formation of bone within a confined space. Perhaps more importantly, bone graft materials should facilitate neovascularization and the migration of osteoprogenitors. Because the size of the bone graft particles determines the resultant space available (between particles) for osseous formation, particle size has been carefully selected according to this concept. The typical size of bone graft particles ranges from 100 to 1000 mm, which is conducive to neovascularization and the ingrowth of bone. Bone forms in cones called osteons with a central blood supply. The dimension of these cones (100-µm radius) is determined by the distance that the central vasculature supply can provide nutrients to cells at the edges of the osteon.
Autogenous Bone
Compared with other bone graft materials, autogenous bone is thought to be the best bone graft material because, in addition to being osteoconductive, it is osteoinductive and osteogenic. Furthermore, barring contamination, there is no risk of rejection or adverse reaction to the autogenous graft material. Intraoral sources of autogenous bone include edentulous spaces, maxillary tuberosity, mandibular ramus, mandibular symphysis, and extraction sites. Bone harvested from a recent extraction site (e.g., about 6 to 12 weeks healing) may have the advantage of increased osteogenic activity compared with other sites, which are more static and undergoing little or no osteogenesis. The maxillary tuberosity provides a more cellular source of autogenous bone compared with other sites. However, the trabecular nature of this site provides a lesser quantity of mineralized matrix, and the resultant total volume of bone available for grafting is often inadequate. For greater amounts of bone, it is more desirable to harvest bone from the mandibular ramus or symphysis. This bone, which is typically more cortical, can be harvested and used as a monocortical block graft or it can be ground or shaved into small fragments and used as a particulate graft.
Although the mandibular ramus and symphysis offer good sources of bone for grafting, clinicians are sometimes reluctant to harvest bone from these sites because of an increased risk of morbidity from the surgical procedure. Risks of surgery in the mandibular symphysis region include postoperative bleeding, bruising, wound dehiscence, damage to lower incisors, disfigurement, and injury to nerves. Nerve injury may be the most significant concern because it can be a long-term (possibly permanent), annoying alteration in sensation of the lower lip, chin, anterior teeth, and gingiva for the patient. A more serious risk is the alteration of facial appearance, particularly when the facial muscles are completely elevated from the bone beyond the inferior border of the mandible. A condition referred to as “witch’s chin” can occur when the facial muscles and overlying skin of the chin fall, causing a disfiguring sag of facial tissues after surgery.
A retrospective analysis of 48 chin graft–harvesting procedures, suggests that maintaining a 5-mm margin of safety between graft harvest sites and vital structures (e.g., lower incisors, the inferior border of the chin, and the mental foramen) will minimize postoperative complications.21 In the 48 procedures, postoperative sequelae included bruising of the lower face (48/48), bruising of the upper neck (6/48), and paresthesia of the lower lip and incisors (6/48). No patients experienced facial disfigurement or muscle prolapse (chin droop). Three of the six patients with paresthesia experienced transient symptoms and recovered completely within 2 months, whereas symptoms persisted longer than 6 months in the other three patients. Not surprisingly, the larger harvest defects (trephined six-ring sites) resulted in a higher incidence of paresthesia, which was longer lasting than that of the smaller defects (trephined four-ring sites). Harvesting bone in a custom-shaped “block” did not result in paresthesia, presumably because these harvest sites were smaller than the four-ring and six-ring trephine-harvested sites.
Observation of the following basic principles can minimize the risk of postoperative morbidity:
When harvesting autogenous bone, regardless of site or method used, it is important to use techniques that prevent overheating and maintain viability of the bone cells. Exceeding 47° C (116.6° F) is known to cause bone necrosis.16 Thus the use of drills, trephines, or saws to cut bone should always be done with profuse irrigation to keep instruments and bone cooled. Precision of osseous cuts can be facilitated with new technologies such as piezoelectric bone surgery (see Chapter 75).
Patients often present for implant planning after tooth loss and alveolar ridge resorption. In these cases, the clinician is obligated to perform augmentation procedures to reconstruct lost bone and place implants in a prosthetically-driven position.
Surgical reconstructive procedures for the preparation and placement of dental implants have become more numerous and complex. Depending on the size and morphology of the defect, various augmentation procedures can be used. These procedures have been categorized according to the deficient dimension: horizontal or vertical. Methods used to augment horizontal, as well as vertical, bone deficiencies include particulate bone grafts and monocortical block grafts. Barrier membranes can be used with bone grafts to reconstruct all types of alveolar bone defects. See Chapter 73 for a review of procedures used to achieve vertical augmentation. All the proven principles of GBR and flap management must be followed to achieve good results. These include generating a blood supply; maintaining a stable, protected space for bone growth; and achieving tension-free flap wound closure.
Flap Management
Soft tissue management is a critical aspect of bone augmentation procedures. Incisions, reflection, and manipulation should be designed to optimize blood supply and wound closure. The design and management of mucoperiosteal flaps must consider the increased dimensions of the ridge after augmentation as well as esthetics and approximation of the wound margins. The surgical procedure must be executed with the utmost of care in order to preserve the vascularity of the flap and to minimize tissue injury.1
Several flap techniques maintain a “submerged” position of bone grafts and barrier membranes during the entire healing process, including a remote or displaced incision.9,25 The advantage of a remote incision is that the wound opening is positioned away from the graft. A conventional crestal incision can be used, even in large supracrestal defects, as long as a periosteal releasing incision and coronal advancement of the flap achieve a tension-free closure.30 Most reports suggest removing sutures approximately 10 to 14 days after surgery. It is also suggested that no prosthesis be inserted for 2 to 3 weeks after surgery to avoid pressure over the wound during the early healing phase.
General concepts for flap management associated with ridge augmentation include the following:
Horizontal Bone Augmentation
A deficiency in the horizontal dimension of bone may be minimal, such as a dehiscence or fenestration of an implant surface, or it may be more significant, such that the implant would have more than one axial surface exposed while having some bone along the entire vertical length. Dehiscence defects can usually be managed during implant placement because most of the implant is covered and stabilized by native bone. If the horizontal deficiency is large and the implant placement would result in significant exposure (i.e., implant body is significantly outside the alveolar bone), it may be better to reconstruct the bone first, in a staged approach, with a subsequent surgery for implant placement.
Although reconstruction of deficient ridges with bone grafts alone (i.e., without barrier membrane) has proved to be effective, variable resorption of the grafted bone has been reported. Preliminary results in a 1- to 3-year study using autografts harvested from the maxillary tuberosity showed an increased ridge width, but resorption of 50% of the graft volume was also noted.55 Buser et al9 investigated the lateral ridge augmentation procedure using an autograft from the retromolar or symphysis area covered by a membrane in 40 consecutively treated patients and noted no clinical signs of resorption of the block graft. The researchers emphasized a remote incision technique, perforation of the cortex, stable placement of corticocancellous autografts, precise adaptation and stabilization (with miniscrews) of the ePTFE membranes, and a tension-free primary soft tissue closure. After 7 to 13 months, the sites were reopened for membrane removal and implant placement. Of the 40 patients, 38 exhibited excellent ridge augmentation, whereas 2 sites showed some soft tissue encapsulation of the grafted bone.
Nevins and Mellonig42 and Doblin et al14 reported an increased amount of new bone using freeze-dried bone allografts (FDBAs) with membranes, even in the presence of membrane exposure. The biopsies showed viable bone cells and visible osteocytes in lacunae, and a 9-month specimen showed no remaining allograft material.
On the other hand, there are some contradictory results using DFDBA and membrane combinations.2,7,8 In a human study, seven paired extraction sockets were grafted with either DFDBA or autologous bone. Sites were reentered and biopsied after 3 to 13 months to evaluate bone formation. Histologic specimens revealed dead particles of DFDBA with no evidence of bone formation on the surface and no evidence of osteoclastic resorption. Conversely, the autogenous sites revealed vascular channels with woven and lamellar bone. Some nonvital, cortical bone chips were observed with osteoclastic resorption.
Figure 72-1 (online) shows an example of the lack of bone formation around DFDBA particles used in a ridge augmentation procedure after more than 20 months of healing under a nonresorbable ePTFE barrier membrane.
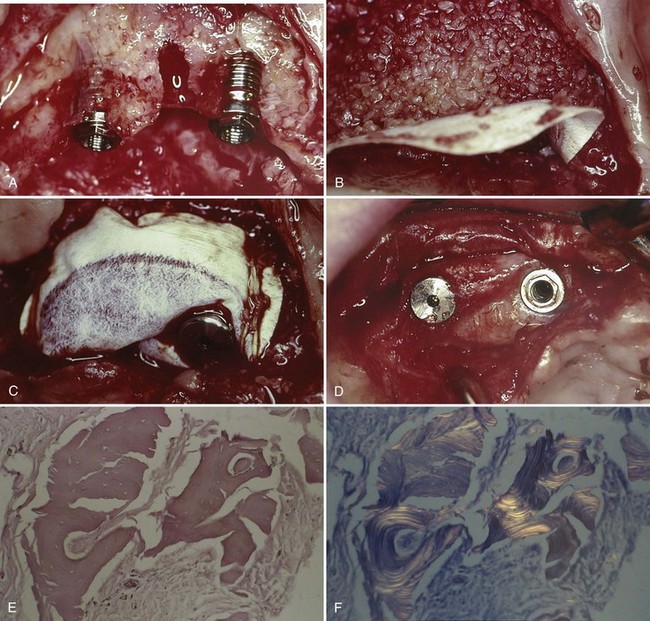
Figure 72-1 A, Clinical view of maxillary alveolar ridge after removal of a failed implant (center defect) and placement of two implants in the bone adjacent to this sites. B, The narrow ridge was grafted with decalcified freeze-dried bone allograft (DFDBA) particulate bone graft material. The graft material was completely covered by a nonresorbable, expanded polytetrafluoroethylene (ePTFE) barrier membrane. C, The ePTFE barrier membrane was secured with the cover screw of one implant and sutures that were secured to the periosteum. D, Clinical view of DFDBA-augmented ridge after more than 20 months healing under a nonresorbable, ePTFE barrier membrane. The membrane remained submerged throughout the entire healing period. E, H & E light microscopic view of specimen harvested from the DFDBA-augmented ridge. Note there are no bone cells (osteoblasts, osteoclasts, or osteocytes), no bone formation, and no osteoclastic resorption of DFDBA particles. There is no evidence of inflammation. Fibroblasts and connective tissue surround the “dead” DFDBA particles. F, Polarized light microscopic view of the same specimen (E) harvested from the DFDBA-augmented ridge. Note the appearance of lamellar layers demonstrating the presence of the DFDBA particles.
Particulate Bone Graft
Advantages of particulate bone grafts (or bone chips) are that the smaller pieces of bone demonstrate more rapid ingrowth of blood vessels (revascularization), larger osteoconduction surface, more exposure of osteoinductive growth factors, and easier biologic remodeling compared with a bone block for reconstruction of large defects. However, particulate grafts often lack a rigid, supportive structure and are much more easily displaced than block grafts.
Autologous particulated bone grafts can be harvested from any edentulous jaw site, either in smaller “particle” or in a larger “block” of bone. If the bone has been harvested in a block, a bone mill is necessary to “particulate” the bone and prepare it for transplantation into the bone defect (Figure 72-2, online).
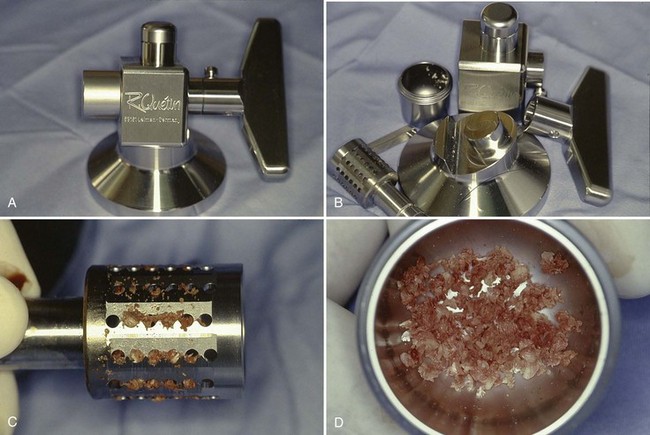
Figure 72-2 A, R. Quetin bone mill (assembled). B, R. Quetin bone mill (disassembled). C, Ground bone observed in blades of bone mill. D, Autogenous block bone ground into evenly sized particulate bone with bone mill suitable for bone grafting.
Particulate grafts are indicated (1) in defects with multiple osseous walls that will contain the graft or (2) in dehiscence or fenestration defects when implants are placed during the bone augmentation procedure. If a bone defect does not have sufficient osseous walls to contain the graft, the barrier membrane (placed in contact with the native bone) should be secured along the periphery with tacks, screws, or sutures. This bone graft and secured barrier membrane combination becomes an environment that is stable and supports new bone formation. Figure 72-3 depicts the use of a barrier membrane in combination with a particulate bone graft to treat a mandibular horizontal defect.
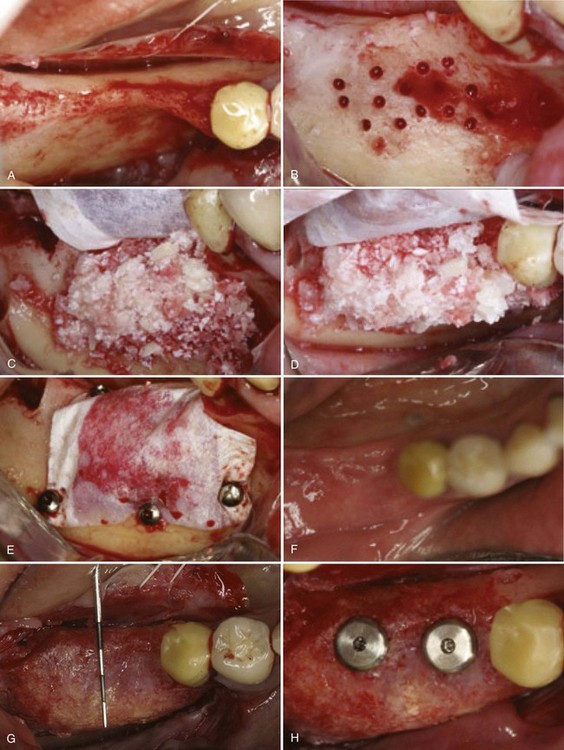
Figure 72-3 Horizontal bone augmentation with expanded polytetrafluoroethylene (ePTFE) barrier membrane. Bone graft consists of a mixture of autogenous particles harvested from the alveolar ridge and Bio-OssOsteohealth Company, A Division of Luipold Pharmaceuticals, Inc., Shirley, NY. A, Partially edentulous posterior mandible with narrow buccolingual dimensions. B, Cortical perforations made in buccal bone with small round bur to enhance blood supply to grafted area. C, Placement of ePTFE barrier membrane and mixture of autogenous and Bio-Oss particulate bone graft. D, View of C from occlusal perspective to visualize horizontal augmentation. E, Barrier membrane secured to native bone with fixation screws. F, Clinical photograph of healed site prior to membrane removal. Soft tissue closure maintained throughout healing without membrane exposure. G, Clinical photograph of healed ridge after membrane removal. Dimensions of alveolar ridge are significantly wider as demonstrated with periodontal probe. H, Two standard-diameter implants placed in “prosthetically-driven” ideal position. Notice there are no implant exposures.
(Images courtesy Dr. Istvan A. Urban, Budapest, Hungary.)
Monocortical Block Graft
Horizontal alveolar deficiencies that might be challenging to reconstruct with particulate grafts can easily be reconstructed with a monocortical block bone graft. The technique uses a cortical block of bone harvested from a remote site and used to increase the width of bone. The block graft taken from an intraoral (e.g., mandibular symphysis or ramus) or extraoral (e.g., iliac crest or tibia) site is fixated to the prepared recipient site with screws. The overlying soft tissues can be separated from the bone graft with a barrier membrane or simply covered with the mucoperiosteal flap. Fixation hardware (screws and plates) should be removed after an adequate period of healing (approximately 6 months). The disadvantage of this technique is the biologic limitation of revascularizing large bone blocks. It therefore is crucial to have sufficient osteogenic cells in the residual surface of the surrounding bone and to limit this technique to horizontal augmentation and only minimal vertical defects.
Figure 72-4 shows the use of a monocortical block graft to reconstruct a horizontal deficiency in the posterior right mandible. The patient presented with a loss of the buccal cortical plate of bone after a traumatic extraction of endodontically treated tooth #29. The surgical extraction also resulted in a nonrestorable cut into the mesial root of tooth #30. Recommended treatment included extraction of tooth #30, monocortical block graft reconstruction of the buccal defect at site #29, and bone augmentation of the extraction socket #30. The procedure for this case follows.
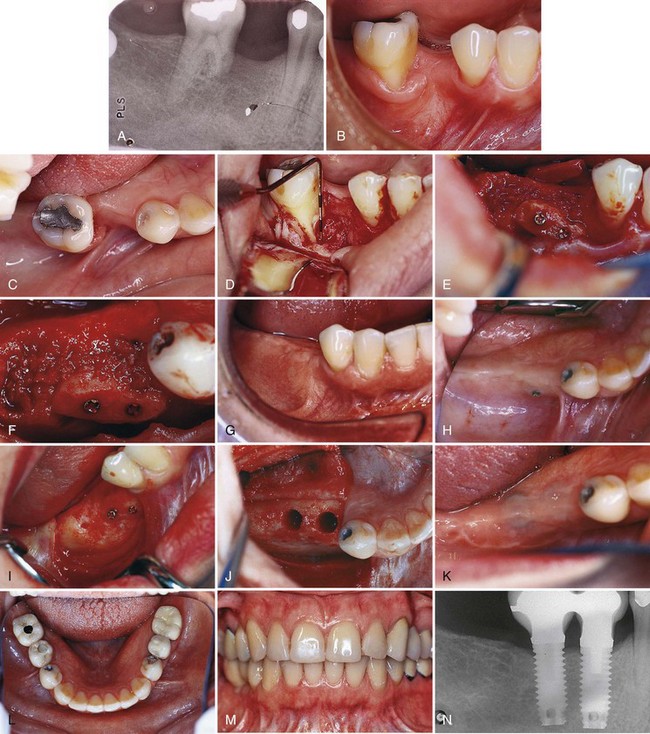
Figure 72-4 Use of a monocortical block graft to reconstruct a horizontal deficiency in the posterior right mandible. A, Periapical radiograph shows missing tooth #29 and damaged (i.e., cut) mesial root #30. B and C, Labial and occlusal views, respectively, of site reveal deficient alveolar ridge on buccal side of #29. D, Full-thickness flap reflection reveals the extent of missing bone in the buccal aspect of site #29, as well as the periodontal defect and damaged mesial root #30. E and F, Autogenous monocortical bone block graft secured to native alveolar bone with fixation screws. G, Good tissue healing after block graft, with evidence of a widened alveolar ridge. H, After 6 months of healing, posterior fixation screw is observed protruding through the mucosa. I, Full-thickness flap reveals that bone resorption has resulted in exposure of part of the fixation screw. J, Osteotomy prepared for wide-diameter implants, taking care to avoid making the labial bone “graft” too thin. K, Complete closure and good healing of wound after implant placement. L and M, Clinical photographs of completed restorations. N, Final radiograph shows good restoration contours on wide-diameter implants.
Procedure
After local anesthesia, an incision was made in keratinized tissue along the crest and around the molar tooth (#30) with a vertical releasing incision mesial to the first bicuspid (#28). A full-thickness flap was elevated to expose the alveolar bone (Figure 72-4, D). All soft tissues were thoroughly removed from the recipient site before bone grafting. After simple forceps delivery of tooth #30, the defect to be grafted was measured to determine the size of block graft to harvest from the mandibular symphysis. Several bleeding points were created using a small round bur.
The autogenous monocortical block graft was harvested from the mandibular symphysis. It was cut to an appropriate size and mortised to intimately fit the recipient defect site. Once properly positioned, the graft was fixated with two fixation screws (Leibinger, Kalamazoo, MI) that passed through the graft and into the existing native alveolar bone. A periosteal releasing incision was used to sever the periosteum from anterior to posterior and facilitate coronal advancement of the mucogingival flap.
After 6 months of healing, a full-thickness mucoperiosteal flap was elevated to expose the alveolar bone sites #29 and 30. Mild resorption of the monocortical block graft is evident. Notice that the position of the head of the fixation screws (especially the posterior screw) is more protruded than the grafted bone as a consequence of bone remodeling and resorption (see Figure 72-4, H and I).
The fixation screws are removed, and the sites are prepared in the usual manner for the placement of two screw-type, wide-diameter implants (Implant Innovations, Palm Beach Gardens, FL). Care is taken to avoid preparing the grafted site too wide or too far labially because the grafted bone may be vulnerable to fracture or additional resorption (Figure 72-4, J).
Simultaneous Implant Placement
Large alveolar bone defects need to be augmented before implant placement and require a healing period of 6 months or longer. In selected cases, it is possible to perform a bone augmentation procedure simultaneously with the implant placement. It is essential to achieve good implant stability in the existing native bone so that endosseous integration can occur.
A very predictable osseous defect to manage with simultaneous implant placement is the implant dehiscence or fenestration defect. Fenestration defects are exposures of the implant’s axial surface that do not include the coronal aspect of the implant (Figure 72-5). Dehiscence defects expose a part of the axial surface, including the coronal aspect of the implant, while maintaining sufficient bone volume around all remaining implant surfaces (Figure 72-6). In a dehiscence defect the implant remains within the confines of the existing bone.
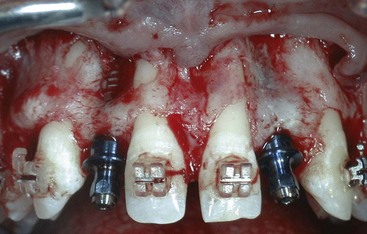
Figure 72-5 Fenestration defect observed in very thin maxillary anterior sites. Implants placed in the maxillary lateral incisor positions demonstrating fenestration defects due to the concavities in the apical area. Notice that the natural teeth (centrals and cuspids) also demonstrate fenestrations. Bone will grow across this type of defect in a very predictable manner because the implants are stable; the defect is small and surrounded by bone.
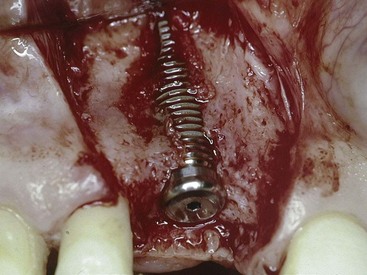
Figure 72-6 Dehiscence defect observed during placement of an implant in the maxillary central incisor position. Note that the implant is completely surrounded by existing native bone except for the facial surface, which is exposed. Bone will grow across this type of defect in a very predictable manner because the implant is stable and the exposed surface is relatively flat with bone on all surfaces.
Fenestration and dehiscence defects have been managed with barrier membranes or simply with flap closure. Bone grafts have also been used. The only controlled comparison studies between membrane treatment and periosteal flap coverage of exposed implant surfaces in humans demonstrated that the membrane treatment was far superior with regard to bone fill.11 Another controlled study in humans showed better results in the membrane groups; four of six sites (66%) treated with a membrane resulted in 95% to 100% elimination of the dehiscence and total coverage of the threads. In the control sites, only 2 of 6 sites (33%) showed moderate-to-complete bone fill.43 All other clinical studies are in the form of case reports.38 Figure 72-7, M, demonstrates coverage of an implant dehiscence using a barrier membrane. Admittedly, without a biopsy, it cannot be determined whether the tissue covering the implant is bone or firm connective tissue.22,26
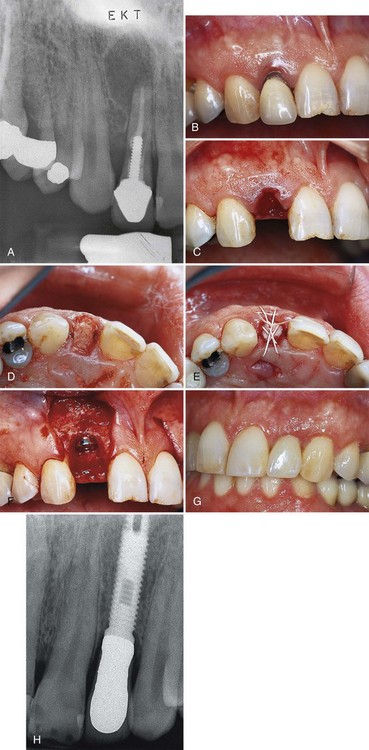
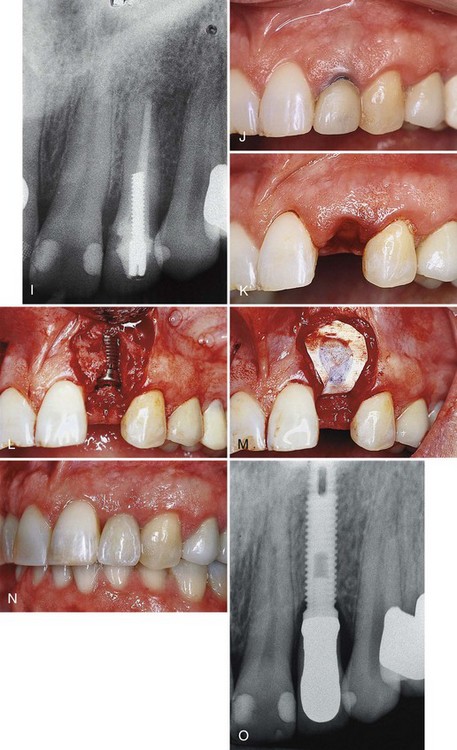
Figure 72-7 Use of staged (A to H) and delayed (I to O) implant placement after extraction of two maxillary lateral incisors in one individual. A, Periapical radiograph of tooth #7 with a large radiolucent lesion around the apex and periodontal bone loss along the distal interproximal area. B, Preoperative photograph of tooth #7 with gingival recession and marginal inflammation. C, Atraumatic extraction or tooth #7 without tissue incision or tissue elevation. Palpation reveals no facial bone present at the time of extraction. D, Decalcified freeze-dried bone allograft (DFDBA) condensed into extraction site. E, Expanded polytetrafluoroethylene (ePTFE) barrier membrane positioned over graft and held in place with sutures. F, Six months after the extraction/graft, the implant is placed. Notice the implant is completely covered with bone. G, Final restoration. H, Final radiograph of delayed implant placement. I, Periapical radiograph of tooth #10. J, Preoperative photograph of tooth #10 with exposed gingival margin. K, Atraumatic extraction of tooth #10 without tissue incision or tissue elevation. Palpation reveals no facial bone present, and dehiscence is expected. L, Two months after extraction, the implant is placed with dehiscence defect. M, Guided bone regeneration (GBR) accomplished with ePTFE barrier membrane positioned over the dehiscence. N, Final restoration. O, Final radiograph.
(Figures A, B, C, F, G, J, M, N, and O from Klokkevold PR et al: Pract Periodont Aesthet Dent 11:603, 1999.)
A 1-year multicenter study evaluating 55 Brånemark implants (i.e., machined-surface, external hex) with bone dehiscence in 45 patients, treated by ePTFE membrane alone, demonstrated an average bone fill of 82%.12 The average initial defect height was 4.7 mm. The 1-year follow-up of these implants demonstrated a favorable response to loading. Of the 55 implants, a total of 6 failed, corresponding to a cumulative survival rate of 84.7% in the maxilla and 95.0% in the mandible, which is similar to previously published results for this implant design.
A clinical report on the use of TR membranes demonstrated the biologic potential to fill a large protected space in four patients.24 Bone dehiscence at implant sites ranged from 5 to 12 mm (mean, 8.2 mm). They were covered with a TR membrane alone (no graft). Reentry after 7 to 8 months of submerged healing found complete bone coverage over all implants. Radiographic evaluation demonstrated the implants functioning with normal crestal bone support after 1 year.
No clinical comparisons are available in the literature evaluating the placement of bone grafts with or without barrier membranes on implant dehiscence defects. Most evidence supports the use of graft materials in conjunction with membrane treatment, especially the use of FDBA in conjunction with GBR. In a study with 40 patients, 110 implants were placed in conjunction with barrier membranes and FDBA; a success rate of 96.8% was achieved with complete bone fill (defined as >90% fill of dehiscence).45 This study reported a membrane exposure rate of 29% but noted little adverse effect on the bone regeneration.
With respect to ridge preservation, Becker et al6 recently reported the effect of barrier membranes and autogenous bone grafts on the preservation of ridge width around implants. They evaluated the ridge width around 76 implants in 61 patients from a case series database. Three groups were compared, including 34 implants treated with GBR (barrier membranes), 27 implants treated with autogenous bone grafts, and 15 implants placed without the need for ridge preservation/augmentation procedures (control group). The results revealed that implant sites treated with barrier membranes and autogenous bone grafts lost an average of 0.1 mm and 0.8 mm, respectively, more than the no augmentation group.
Another study evaluated the possibility of regenerating bone around implants placed in extraction sockets.30 Bone augmentation was achieved with human DFDBA particles mixed with tetracycline packed around the exposed implant surfaces. Implants and graft material were covered with e-PTFE barrier membranes with complete flap closure for 4 to 6 months. The results demonstrated complete bone regeneration in all cases except when barrier membranes were prematurely exposed and removed. One-year posttreatment histologic evaluation of regenerated bone revealed remnants of the DFDBA graft particles in direct contact with vital bone tissue (woven and lamellar bone). Osteoblasts were observed and appeared to be actively engaged in bone formation adjacent to graft particles. Figure 72-7, L and M, demonstrates a simultaneous GBR and implant placement in a dehiscence defect.
Complications
Bone augmentation procedures used to increase the bone volume in deficient alveolar ridges have been successful and have enabled the placement of implants into prosthetically-driven positions.51 Unfortunately, these augmentation procedures carry an increased risk of morbidity and can require secondary surgeries to correct problems resulting from the procedure.60 The subsequent corrective surgeries required to correct problems add surgical time and complexity to the implant therapy.
Surgical complications are reported for a variety of bone reconstructive techniques.15 A review assessed the number and types of complications associated with bone reconstructive procedures for endosseous implants.56 The review of literature (1976–1994) included 2315 implants in 733 autogenous block, particulate, and various other bone graft materials. Complications reported included bleeding, postoperative infection, bone fracture, nerve dysfunction, perforation of the mucosa, loss of a portion of the bone graft, pain, decubital ulcers, sinusitis, and wound dehiscence. Wound dehiscence seemed to have the most deleterious effect on implant survival. This finding emphasizes the importance of flap management.
Typical findings include less bone fill with early exposure and membrane removal versus retaining the membrane without exposure for 6 to 8 months.25,53 Buccolingual ridge deficiencies were treated in a prospective study involving 19 patients using ePTFE membranes and miniscrews as fixation and tenting devices.32 The group of defects, which healed uneventfully, yielded on reentry a 90% to 100% bone regeneration compared with the maximal volume of the space defined by the membrane placement. In the exposed-membrane group, the percentage of regenerated bone ranged from 0% to 62%. When a late membrane removal was performed (3 to 5 months postsurgically), the regeneration varied between 42% and 62%. The authors concluded that the length of membrane healing and size of the defect played a significant role in the amount of new bone formation.32
Other authors have reported successful bone fill in situations in which the membranes had to be removed because of an early exposure.42,52 A significantly greater fill of the osseous defects at the grafted sites was noted. The authors concluded that the regeneration of bone around the implants appeared most dependent on the anatomy of the bony defect at the time of implant placement.
Although the effect or amount of regenerated bone with regard to membrane exposure is somewhat contradictory, the goal should be to keep the membranes covered during the healing period so that the risk of infection and soft tissue and esthetic problems can be minimized or eliminated. Again, the importance of flap management for ridge augmentation procedures should be stressed. See Chapter 77 for more information and details regarding surgical complications and failures.
Alveolar Ridge Preservation/Management of Extractions
Because tooth extraction (or tooth loss) often results in alveolar ridge resorption or collapse, preservation of bone volume at the time of extraction is a desirable goal. Most bone loss after extraction occurs in the first 6 to 24 months.10 Therefore, when clinicians are afforded the opportunity to intervene at the time of extraction, preservation of alveolar bone should be initiated. A conservative approach to the management of extraction sites can eliminate or significantly reduce the necessity of advanced bone augmentation procedures.
When extracting a tooth and preparing for implant placement, alveolar bone resorption should be prevented. Experimental animal studies have shown that the use of a barrier membrane enhances the predictability of bone fill in the extraction site and therefore maintains original bone volume when compared with mucoperiosteal flap coverage alone.7 Clinical studies have also demonstrated the benefits of a regenerative approach to tooth extraction.34,35,41 These authors found that a nonresorbable barrier membrane resulted in minimal resorption of alveolar ridge size and shape.
Although earlier studies have proposed the concept of treating extraction sites without flap closure (i.e., an exposed membrane used to cover the graft), more recent studies concluded that complete wound closure over the physical barrier might be associated with greater bone fill.5,53 The decision about whether to advance a flap to achieve wound closure must be weighed against the soft tissue changes that will be created and may need to be corrected (i.e., mucogingival junction discrepancies and esthetic problems).
The timing of implant placement relative to the time of extraction has been widely debated. Depending on the quantity, quality, and support of existing bone, as well as the preferences of the clinician and patient, the placement of implants after tooth extraction can be immediate, delayed, or staged. By definition, immediate implant placement occurs at the time of extraction. Delayed implant placement is performed approximately 2 months after extraction to allow for soft tissue healing. Staged implant placement allows for substantial bone healing within the extraction site, which typically requires 4 to 6 months or longer.
Tooth extraction is managed with an atraumatic surgical technique that uses a narrow, flat instrument (e.g., Periotome, Hu-Friedy, Chicago) directed apically into the sulcus to sever the periodontal ligament and slightly expand the adjacent periodontal tissues. The tooth is elevated and removed with forceps using a gentle, rotational movement. New techniques for atraumatic extraction are described in Chapter 75. Buccolingual forces are avoided to prevent damaging the integrity of the labial bone. No incisions are made, and care is taken to avoid soft tissue reflection. In this manner, soft tissues maintain their structural anatomy, and the periosteum (blood supply to the bone) remains intact. If the tooth has multiple roots, curved roots, or other anatomic features that make removal difficult, it may be necessary to cut the tooth using a high-speed rotary drill or other cutting device and remove it in smaller pieces. It is important to cut only tooth structure and avoid cutting (overheating) bone when using high-speed rotary drills. The bone within the extraction site is completely debrided of soft tissue with surgical curettes. After debridement, the extraction site is thoroughly irrigated with sterile saline. Finally, the clinician can evaluate the bone level and socket anatomy to determine whether to bone-graft the site and when to place the implant (immediate, delayed, or staged placement).
Delayed Implant Placement
Delayed implant placement shares some advantages of immediate implant placement, including extraction site preservation, and offers additional advantages. Unlike immediate implant placement, which is deficient of soft tissue for coverage, the delayed–implant placement technique allows time for soft tissue healing to close the wound.23 The delayed-placement technique still reduces the length of treatment by several months because it is not necessary to wait for complete bone healing. Furthermore, because bone formation is active within the first few months after tooth extraction, the delayed technique may facilitate more osteogenesis adjacent to the implant.
The primary advantage of delayed implant placement is that by allowing for soft tissue healing and closure of the extraction site, mucogingival flap advancement is not necessary. This alleviates the need for additional surgeries to correct mucogingival discrepancies. Delayed implant placement also allows time for resolution of infections that may have been present within the extraction site.
As with immediate implant placement, similar limitations of bone support and implant stability exist for delayed placement. The normal osseous healing that occurs within the first 2 months does not significantly affect the anatomy of the alveolar bone. Therefore limitations in bone support after 2 months of healing are similar to those that exist at the time of extraction.
Staged Implant Placement
Staged implant placement allows adequate time for osseous healing. This may be complete osseous healing of an extraction site without a bone graft (if circumferential bone support is good) or with a bone graft. Staged implant placement, by definition, allows for complete hard and soft tissue healing and permits the placement of implants into healed bone sites with adequate coverage by hard and soft tissues.51 This eliminates the necessity of mucogingival flap advancement, allows for the resolution of preexisting infections, and prevents soft tissue invasion. Furthermore, by using an extended healing period, the grafted bone also has the opportunity to become vascularized. Bone grafts performed simultaneously with implant placement do not share this advantage.
The primary disadvantage of staged implant placement is the length of time required for bone healing.
Delayed Versus Staged Technique
Delayed and staged techniques for implant placement are demonstrated here in one individual using two extraction sites with similar bone morphologies in the anterior maxilla (see Figure 72-7). Both techniques facilitate the esthetic placement of implants into prosthetically-driven positions. Delayed and staged approaches maintain alveolar bone volume, reduce the need for advanced bone augmentation, and eliminate the need for subsequent mucogingival surgery. The timing and management of delayed versus staged implant placement is described in the next section.
To decide which implant placement method to use, the quantity and location of bone surrounding the tooth should be assessed. Once the patient has been anesthetized, a periodontal probe can be used to “sound” for the level of bone support through the soft tissue. Using this method, the bone levels surrounding the tooth can be mapped. Bone support that surrounds the extraction site can also be evaluated and confirmed after tooth removal by palpation, probing, and direct (internal) visualization.
If the tooth to be extracted has sufficient bone support on all surfaces, the extraction site can be expected to fill with bone without additional augmentation procedures, except when the labial bone is very thin. A simple extraction followed by a healing period of 4 to 6 months could be sufficient for complete osseous healing. Subsequently, an implant could be placed in the usual manner without the need for bone augmentation. Conversely, if little or no bone exists on the labial surface, it should be anticipated that the site would require bone augmentation to facilitate placement of the implant. In this case, bone grafting at the time of extraction can be used to maintain the alveolar ridge dimensions occupied by the tooth.
Immediate Implant Placement
The primary advantage of immediate implant placement is the reduction of the healing time, which translates to an earlier restorative time (i.e., shorter time to completion for the patient).33,39,50,59 Because the implant is placed at the time of extraction, the bone-to-implant healing begins immediately with extraction site healing. Another advantage is that the normal bone healing, which generally occurs within the extraction site, takes effect around the implant. This bone-forming activity may enhance the bone-to-implant contact compared with an implant placed in a less osteogenically active site.
Possible disadvantages of immediate implant placement include the need for subsequent mucogingival surgeries to correct tissues moved by repositioned flaps and the need for bone grafting to fill extraction site defects around the implant. If inadequate bone exists to stabilize the implant, immediate implant placement is not recommended.
When a two-stage implant is placed at the time of tooth extraction, the mucogingival flap is advanced, with releasing incisions, to cover the implant completely (exception: one-stage implants). It may also be necessary to graft bone into the extraction site in areas that do not contact the implant to avoid soft tissue invasion around the implant.50 A 1-year study of 49 immediate extraction site implants treated by a membrane alone demonstrated a 93.6% bone fill. After 1 year (postloading), the implant success rate was 93.9%.5
Although some have advocated submerging implants placed in extraction sockets with flap advancement,23 others have demonstrated success with a nonsubmerged approach. Implants may be placed in extraction sockets along with bone augmentation without flap advancement using a one-stage implant placement approach. Clinical studies evaluating the outcome of bone augmentation around implants placed in extraction sockets reveal good bone fill.31 The placement of 21 transmucosal implants in immediate extraction sites treated with a barrier membrane were tested for the implant success rate and the percentage of bone fill. Of 21 transmucosal implants, 20 yielded complete bone fill and coverage of the entire plasma-coated implant surface.
A clinical report on the use of resorbable collagen membranes around extraction site implants demonstrated a variable degree of bone fill in nine patients.44 More clinical review of the use of resorbable membranes for GBR is required because evidence is insufficient to evaluate the predictability.
In a study of 30 patients, the use of autografts alone in 54 extraction sites was highly effective for simultaneously placed implants completely within the envelope of bone.3 The study showed that extraction sites, including those with a buccal dehiscence, could be treated with autografts alone. Because ungrafted sites were not evaluated, the absolute need to graft small defects adjacent to implants was not ascertained by this study. In another study, implants placed in extraction sockets were tested for their potential to regenerate bone with allograft alone, a membrane alone, and a combination treatment.19 Reentry confirmed 100% thread coverage in all but one implant in the “no-wall” group treated with DFDBA alone. A clinical study of five patients evaluated different treatment modalities for extraction site implants together with bone graft combinations.54 This small study supported the concept that “non–space-making defects” are best treated with a combination of barrier membrane and an autograft or allograft as compared to treatment with a nonreinforced membrane alone (without a graft).
Immediate placement of an implant into the extraction socket in a one-stage approach along with immediate provisionalization is perhaps the best way to manage the hard and soft tissues following extraction (Figure 72-8, A to J). The immediate placement of a provisional restoration is the best way to support the soft tissues (papilla and marginal gingiva) following tooth extraction.
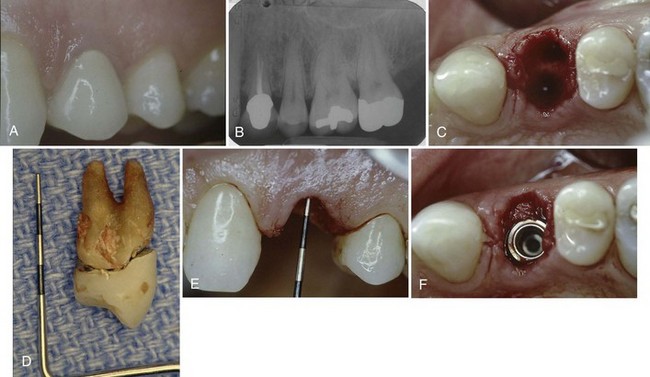
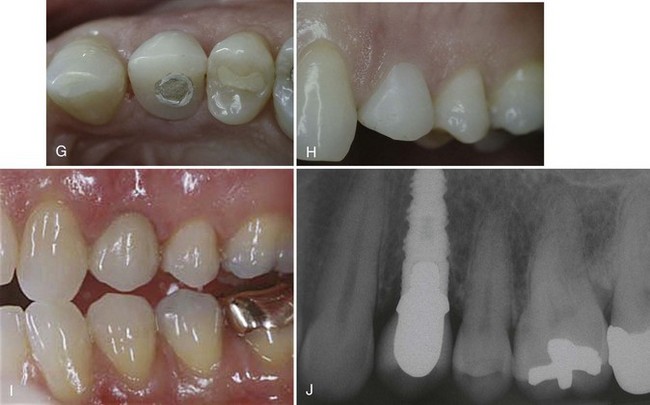
Figure 72-8 Immediate implant placement after extraction of a maxillary first premolar. A, Preoperative clinical photograph of tooth #12, which fractured and was deemed nonrestorable. B, Periapical radiograph of tooth #12. It is root canal–treated and has a full-coverage porcelain-fused-to-metal (PFM) crown. C, Atraumatic extraction of tooth #12 without incisions or tissue elevation. D, Extracted tooth. E, Palpation and examination with probe reveals intact bony walls around the extraction socket. The facial bone wall is observed to be about 2 mm below the facial gingival margin. F, The palatal aspect of the extraction socket is prepared, and a tapered implant is placed to emerge through the central fossa. Autogenous bone chips (harvested from the maxillary tuberosity) are condensed into the labial aspect of the extraction socket to support the facial bone and soft tissue contours. G, An immediate provisional restoration is fabricated (indirectly in the laboratory) and delivered at the time of implant placement. The palatal cusp is not replaced in this provisional to avoid function and the occlusion is checked for light centric contacts only. H, Facial view of provisional restoration at the time of delivery. Notice how the interdental papilla and facial gingival margin is well supported by the contours of the provisional restoration. I, Final restoration at time of delivery (approximately 4 months after implant placement). J, Final radiograph of definitive restoration.
Conclusion
Localized bone augmentation procedures allow clinicians to reconstruct horizontal alveolar ridge deficiencies and replace missing teeth with dental implants in a prosthetically-driven position with natural appearance and function. In many cases, implants can be placed simultaneously with the augmentation procedure. In cases of advanced bone resorption, ridge augmentation before implant placement may be a better choice. Bone augmentation procedures can also be used to preserve alveolar dimensions following tooth extraction. If adequate bone exists to stabilize an implant, these procedures may be combined. The predictable outcome of these procedures depends on several biologic principles that must be followed. Diagnosis, treatment planning, careful execution of the surgical treatment, postoperative follow-up, and appropriate implant loading are all important factors in achieving success.
![]() Science Transfer
Science Transfer
Implants may be placed immediately into extraction sites when there is sufficient bone apically and horizontally to stabilize and cover the implant surfaces. This gives the simplest method to replace an extracted tooth and to maintain soft tissue contours. In cases where there is insufficient bone for this procedure, a variety of bone graft materials can be placed in the socket, covered with a resorbable collagen membrane and sutures placed to hold the soft tissues without the need for complete flap closure. Implants can then be placed in the augmented site in 4 to 6 months.
Bone dehiscences or fenestrations present during implant placement are the easiest defects to resolve. These are successfully treated with a variety of bone graft materials used in conjunction with resorbable barrier membranes.
Localized ridge augmentation in a horizontal direction is generally carried out with autogenous veneer bone grafts harvested from the mandibular ramus or with bone graft materials covered with a titanium reinforced Teflon membrane with water tight flap closure being essential. These procedures may result in some postoperative morbidities including pain, swelling, and bruising.
Vertical augmentation of deficient alveolar ridges using bone grafts and membranes is more difficult and less predictable than techniques to improve the width of the alveolus. For this reason, bone distraction techniques are becoming more often applied to these difficult cases. Bioactive molecules such as BMP II are currently being evaluated in clinical trials and will add additional bone forming capacity to current surgeries.
1 Bahat O, Handelsman M. Periodontal reconstructive flaps–classification and surgical considerations. Int J Periodontics Restorative Dent. 1991;11:480-487.
2 Becker W, Becker BE, Caffesse R. A comparison of demineralized freeze-dried bone and autologous bone to induce bone formation in human extraction sockets. J Periodontol. 1994;65:1128-1133.
3 Becker W, Becker BE, Polizzi G. Autogenous bone grafting of bone defects adjacent to implants placed into immediate extraction sockets in patients: a prospective study. Int J Oral Maxillofac Implants. 1994;9:398.
4 Becker W, Clokie C, Sennerby L, et al. Histologic findings after implantation and evaluation of different grafting materials and titanium micro screws into extraction sockets: case reports. J Periodontol. 1998;69:414-421.
5 Becker W, Dahlin C, Becker BE, et al. The use of e-PTFE barrier membranes for bone promotion around titanium implants placed into extraction sockets: a prospective multicenter study. Int J Oral Maxillofac Implants. 1994;9:31-40.
6 Becker W, Hujoel P, Becker BE. Effect of barrier membranes and autologous bone grafts on ridge width preservation around implants. Clin Implant Dent Relat Res. 2002;4:143-149.
7 Becker W, Schenk R, Higuchi K, et al. Variations in bone regeneration adjacent to implants augmented with barrier membranes alone or with demineralized freeze-dried bone or autologous grafts: a study in dogs. Int J Oral Maxillofac Implants. 1995;10:143-154.
8 Becker W, Urist MR, Tucker LM, et al. Human demineralized freeze-dried bone: inadequate induced bone formation in athymic mice. A preliminary report. J Periodontol. 1995;66:822-828.
9 Buser D, Dula K, Hirt HP, Schenk RK. Lateral ridge augmentation using autografts and barrier membranes: a clinical study with 40 partially edentulous patients. J Oral Maxillofac Surg. 1996;54:420-432. discussion 432-423
10 Carlsson GE, Persson G. Morphologic changes of the mandible after extraction and wearing of dentures. A longitudinal, clinical, and x-ray cephalometric study covering 5 years. Odontol Revy. 1967;18:27-54.
11 Dahlin C, Andersson L, Linde A. Bone augmentation at fenestrated implants by an osteopromotive membrane technique. A controlled clinical study. Clin Oral Implants Res. 1991;2:159-165.
12 Dahlin C, Lekholm U, Becker W, et al. Treatment of fenestration and dehiscence bone defects around oral implants using the guided tissue regeneration technique: a prospective multicenter study. Int J Oral Maxillofac Implants. 1995;10:312-318.
13 Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81:672-676.
14 Doblin JM, Salkin LM, Mellado JR, et al. A histologic evaluation of localized ridge augmentation utilizing DFDBA in combination with e-PTFE membranes and stainless steel bone pins in humans. Int J Periodontics Restorative Dent. 1996;16:120-129.
15 Donovan MG, Dickerson NC, Hanson LJ, Gustafson RB. Maxillary and mandibular reconstruction using calvarial bone grafts and Branemark implants: a preliminary report. J Oral Maxillofac Surg. 1994;52:588-594.
16 Eriksson AR, Albrektsson T. Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent. 1983;50:101-107.
17 Friedmann A, Strietzel FP, Maretzki B, et al. Histological assessment of augmented jaw bone utilizing a new collagen barrier membrane compared to a standard barrier membrane to protect a granular bone substitute material. Clin Oral Implants Res. 2002;13:587-594.
18 Fugazzotto PA. GBR using bovine bone matrix and resorbable and nonresorbable membranes. Part 1: histologic results. Int J Periodontics Restorative Dent. 2003;23:361-369.
19 Gelb DA. Immediate implant surgery: three-year retrospective evaluation of 50 consecutive cases. Int J Oral Maxillofac Implants. 1993;8:388-399.
20 Geurs NC, Korostoff JM, Vassilopoulos PJ, et al. Clinical and histologic assessment of lateral alveolar ridge augmentation using a synthetic long-term bioabsorbable membrane and an allograft. J Periodontol. 2008;79:1133-1140.
21 Hunt D, Jovanovic S. Autogenous bone harvesting: a clinical graft technique for particulate and amniocortical bone blocks. Int J Periodontics Restorative Dent. 1999;19:165.
22 Jensen OT, Greer ROJr, Johnson L, Kassebaum D. Vertical guided bone-graft augmentation in a new canine mandibular model. Int J Oral Maxillofac Implants. 1995;10:335-344.
23 Jovanovic SA, Buser D: Guided bone regeneration in dehiscence defects and delayed extraction sockets In: Quintessence Chicago 1994.
24 Jovanovic SA, Nevins M. Bone formation utilizing titanium-reinforced barrier membranes. Int J Periodontics Restorative Dent. 1995;15:56.
25 Jovanovic SA, Spiekermann H, Richter EJ. Bone regeneration around titanium dental implants in dehisced defect sites: a clinical study. Int J Oral Maxillofac Implants. 1992;7:233-245.
26 Klokkevold PR, Han TJ, Camargo PM. Aesthetic management of extractions for implant site development: delayed versus staged implant placement. Pract Periodontics Aesthet Dent. 1999;11:603-610. quiz 612
27 Klokkevold PR, Newman MG. Current status of dental implants: a periodontal perspective. Int J Oral Maxillofac Implants. 2000;15:56-65.
28 Kostopoulos L, Karring T. Augmentation of the rat mandible using guided tissue regeneration. Clin Oral Implants Res. 1994;5:75-82.
29 Kostopoulos L, Karring T. Guided bone regeneration in mandibular defects in rats using a bioresorbable polymer. Clin Oral Implants Res. 1994;5:66-74.
30 Landsberg CJ, Grosskopf A, Weinreb M. Clinical and biological observations of demineralized freeze-dried bone allografts in augmentation procedures around dental implants. Int J Oral Maxillofac Implants. 1994;9:586.
31 Lang NP, Bragger U, Hammerle CH, Sutter F. Immediate transmucosal implants using the principle of guided tissue regeneration. I. Rationale, clinical procedures and 30-month results. Clin Oral Implants Res. 1994;5:154-163.
32 Lang NP, Hammerle CH, Bragger U, et al. Guided tissue regeneration in jawbone defects prior to implant placement. Clin Oral Implants Res. 1994;5:92-97.
33 Lazzara RJ. Immediate implant placement into extraction sites: surgical and restorative advantages. Int J Periodontics Restorative Dent. 1989;9:332-343.
34 Lekovic V, Camargo PM, Klokkevold PR, et al. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J Periodontol. 1998;69:1044-1049.
35 Lekovic V, Kenney EB, Weinlaender M, et al. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontol. 1997;68:563-570.
36 Linde A, Thoren C, Dahlin C, Sandberg E. Creation of new bone by an osteopromotive membrane technique: an experimental study in rats. J Oral Maxillofac Surg. 1993;51:892-897.
37 Llambes F, Silvestre FJ, Caffesse R. Vertical guided bone regeneration with bioabsorbable barriers. J Periodontol. 2007;78:2036-2042.
38 Mellonig JT, Nevins M. Guided bone regeneration of bone defects associated with implants: an evidence-based outcome assessment. Int J Periodontics Restorative Dent. 1995;15:168-185.
39 Missika P, Abbou M, Rahal B. Osseous regeneration in immediate postextraction implant placement: a literature review and clinical evaluation. Pract Periodontics Aesthet Dent. 1997;9:165-175. quiz 176
40 Murray G, Holden R, Roschlau W. Experimental and clinical study of new growth of bone in a cavity. Am J Surg. 1957;93:385.
41 Nemcovsky CE, Serfaty V. Alveolar ridge preservation following extraction of maxillary anterior teeth. Report on 23 consecutive cases. J Periodontol. 1996;67:390-395.
42 Nevins M, Mellonig JT. The advantages of localized ridge augmentation prior to implant placement: a staged event. Int J Periodontics Restorative Dent. 1994;14:96-111.
43 Palmer RM, Floyd PD, Palmer PJ, et al. Healing of implant dehiscence defects with and without expanded polytetrafluoroethylene membranes: a controlled clinical and histological study. Clin Oral Implants Res. 1994;5:98-104.
44 Parodi R, Santarelli G, Carusi G. Application of slow-resorbing collagen membrane to periodontal and peri-implant guided tissue regeneration. Int J Periodontics Restorative Dent. 1996;16:174-185.
45 Rominger JW, Triplett RG. The use of guided tissue regeneration to improve implant osseointegration. J Oral Maxillofac Surg. 1994;52:106-112. discussion 112-103
46 Sandberg E, Dahlin C, Linde A. Bone regeneration by the osteopromotion technique using bioabsorbable membranes: an experimental study in rats. J Oral Maxillofac Surg. 1993;51:1106-1114.
47 Schenk RK, Buser D, Hardwick WR, Dahlin C. Healing pattern of bone regeneration in membrane-protected defects: a histologic study in the canine mandible. Int J Oral Maxillofac Implants. 1994;9:13-29.
48 Schwartz Z, Mellonig JT, Carnes DLJr, et al. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation. J Periodontol. 1996;67:918-926.
49 Schwartz Z, Somers A, Mellonig JT, et al. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation is dependent on donor age but not gender. J Periodontol. 1998;69:470-478.
50 Schwartz-Arad D, Chaushu G. Placement of implants into fresh extraction sites: 4 to 7 years retrospective evaluation of 95 immediate implants. J Periodontol. 1997;68:1110-1116.
51 Shanaman RH. The use of guided tissue regeneration to facilitate ideal prosthetic placement of implants. Int J Periodontics Restorative Dent. 1992;12:256-265.
52 Shanaman RH. A retrospective study of 237 sites treated consecutively with guided tissue regeneration. Int J Periodontics Restorative Dent. 1994;14:292-301.
53 Simion M, Baldoni M, Rossi P, Zaffe D. A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period. Int J Periodontics Restorative Dent. 1994;14:166-180.
54 Simion M, Dahlin C, Trisi P, Piattelli A. Qualitative and quantitative comparative study on different filling materials used in bone tissue regeneration: a controlled clinical study. Int J Periodontics Restorative Dent. 1994;14:198-215.
55 ten Bruggenkate CM, Kraaijenhagen HA, van der Kwast WA, et al. Autogenous maxillary bone grafts in conjunction with placement of I.T.I. endosseous implants. A preliminary report. Int J Oral Maxillofac Surg. 1992;21:81-84.
56 Tolman DE. Reconstructive procedures with endosseous implants in grafted bone: a review of the literature. Int J Oral Maxillofac Implants. 1995;10:275-294.
57 Urist MR. Bone: formation by autoinduction. Science. 1965;150:893-899.
58 von Arx T, Buser D. Horizontal ridge augmentation using autogenous block grafts and the guided bone regeneration technique with collagen membranes: a clinical study with 42 patients. Clin Oral Implants Res. 2006;17:359-366.
59 Wilson TGJr, Schenk R, Buser D, Cochran D. Implants placed in immediate extraction sites: a report of histologic and histometric analyses of human biopsies. Int J Oral Maxillofac Implants. 1998;13:333-341.
60 Yildirim M, Hanisch O, Spiekermann H. Simultaneous hard and soft tissue augmentation for implant-supported single-tooth restorations. Pract Periodontics Aesthet Dent. 1997;9:1023-1031. quiz 1032
61 Zellin G, Gritli-Linde A, Linde A. Healing of mandibular defects with different biodegradable and non-biodegradable membranes: an experimental study in rats. Biomaterials. 1995;16:601-609.
