Nutrition
• Explain the importance of a balance between energy intake and energy requirements.
• List the end products of carbohydrate, protein, and fat metabolism.
• Explain the significance of saturated, unsaturated, and polyunsaturated fats.
• Describe the food guide pyramid and discuss its value in planning meals for good nutrition.
• List the current dietary guidelines for the general population.
• Explain the variance in nutritional requirements throughout growth and development.
• Discuss the major methods of nutritional assessment.
• Identify three major nutritional problems and describe patients at risk.
• Establish a plan of care to meet the nutritional needs of a patient.
• Describe the procedure for initiating and maintaining enteral feedings.
• Describe the methods to avoid complications of enteral feedings.
• Describe the methods for avoiding complications of parenteral nutrition.
• Discuss medical nutrition therapy in relation to three medical conditions.
• Discuss diet counseling and patient teaching in relation to patient expectations.
http://evolve.elsevier.com/Potter/fundamentals/
Nutrition is a basic component of health and is essential for normal growth and development, tissue maintenance and repair, cellular metabolism, and organ function. The human body needs an adequate supply of nutrients for essential functions of cells. Food security is critical for all members of a household. This means that all household members have access to sufficient, safe, and nutritious food to maintain a healthy lifestyle; sufficient food is available on a consistent basis; and the household has resources to obtain appropriate food for a nutritious diet. Food also holds symbolic meaning. Giving or taking food is part of ceremonies, social gatherings, holiday traditions, religious events, the celebration of birth, and the mourning of death. The difficulty of the decision to withdraw food in a terminal illness, even in the form of intravenous (IV) nutrients, is a testament to the symbolic power of food and feeding.
Florence Nightingale understood the importance of nutrition, stressing a nurse’s role in the science and art of feeding during the mid-1800s (Dossey, 1999). Since then the nurse’s role in nutrition and diet therapy has changed. Medical nutrition therapy (MNT) uses nutrition therapy and counseling to manage diseases (American Dietetic Association, 2010b). In some illnesses such as type 1 diabetes mellitus (DM) or mild hypertension, diet therapy is often the major treatment for disease control (ADA, 2008; American Heart Association, 2010). Other conditions such as severe inflammatory bowel disease require specialized nutrition support such as enteral nutrition (EN) or parenteral nutrition (PN). Current standards of care promote optimal nutrition in all patients (American Heart Association, 2010; ACS, 2011).
The U.S. Department of Health and Human Services (USDHHS) and the Public Health Service established nutritional goals and objectives for Healthy People 2020 (USDHHS, 2010). Healthy People 2020 is the United States’ contribution to the “Health for All” strategy of the World Health Organization (WHO, 2010). Healthy People 2020 (Box 44-1) continues the objectives initiated in Healthy People 2000 and Healthy People 2010, with overall goals of promoting health and reducing chronic disease. All nutrition-related objectives include baseline data from which progress is measured. The challenge remains to motivate consumers to put these dietary recommendations into practice.
Scientific Knowledge Base
Nutrients: The Biochemical Units of Nutrition
The body requires fuel to provide energy for cellular metabolism and repair, organ function, growth, and body movement. The basal metabolic rate (BMR) is the energy needed to maintain life-sustaining activities (breathing, circulation, heart rate, and temperature) for a specific period of time at rest. Factors such as age, body mass, gender, fever, starvation, menstruation, illness, injury, infection, activity level, or thyroid function affect energy requirements. The resting energy expenditure (REE), or resting metabolic rate, is the amount of energy that an individual needs to consume over a 24-hour period for the body to maintain all of its internal working activities while at rest. Factors that affect metabolism include illness, pregnancy, lactation, and activity level.
In general, when energy requirements are completely met by kilocalorie (kcal) intake in food, weight does not change. When the kilocalories ingested exceed a person’s energy demands, the individual gains weight. If the kilocalories ingested fail to meet a person’s energy requirements, the individual loses weight.
Nutrients are the elements necessary for the normal function of numerous body processes. Energy needs are met from a variety of nutrients: carbohydrates, proteins, fats, water, vitamins, and minerals. Food is sometimes described according to its nutrient density (i.e., the proportion of essential nutrients to the number of kilocalories). High–nutrient dense foods such as fruits and vegetables provide a large number of nutrients in relationship to kilocalories. Low–nutrient dense foods such as alcohol or sugar are high in kilocalories but nutrient poor.
Carbohydrates
Carbohydrates, composed of carbon, hydrogen, and oxygen, are the main source of energy in the diet. Each gram of carbohydrate produces 4 kcal/g and serves as the main source of fuel (glucose) for the brain, skeletal muscles during exercise, erythrocyte and leukocyte production, and cell function of the renal medulla. People obtain carbohydrates primarily from plant foods, except for lactose (milk sugar). They are classified according to their carbohydrate units, or saccharides.
Monosaccharides such as glucose (dextrose) or fructose cannot be broken down into a more basic carbohydrate unit. Disaccharides such as sucrose, lactose, and maltose are composed of two monosaccharides and water. Both monosaccharides and disaccharides are classified as simple carbohydrates and are found primarily in sugars. Polysaccharides such as glycogen are made up of many carbohydrate units (i.e., complex carbohydrates). They are insoluble in water and digested to varying degrees. Starches are polysaccharides.
The body is unable to digest some polysaccharides because humans do not have enzymes capable of breaking them down. Fiber is a polysaccharide that is the structural part of plants that is not broken down by the human digestive enzymes. Because fiber is not broken down, it does not contribute calories to the diet. Insoluble fibers are not digestible and include cellulose, hemicellulose, and lignin. Soluble fibers dissolve in water and include barley, cereal grains, cornmeal, and oats.
Proteins
Proteins provide a source of energy (4 kcal/g), and they are essential for synthesis (building) of body tissue in growth, maintenance, and repair. Collagen, hormones, enzymes, immune cells, deoxyribonucleic acid (DNA), and ribonucleic acid (RNA) are all made of protein. In addition, blood clotting, fluid regulation, and acid-base balance require proteins. These proteins transport nutrients and many drugs in the blood. Ingestion of proteins maintains nitrogen balance.
The simplest form of protein is the amino acid, which is made up of hydrogen, oxygen, carbon, and nitrogen. The body does not synthesize indispensable amino acids; thus these need to be provided in the diet. Examples of indispensable amino acids are histidine, lysine, and phenylalanine. The body synthesizes dispensable amino acids. Examples of amino acids synthesized in the body are alanine, asparagine, and glutamic acid. Amino acids can link together. Albumin and insulin are simple proteins because they contain only amino acids or their derivatives. The combination of a simple protein with a nonprotein substance produces a complex protein such as lipoprotein, formed by a combination of a lipid and a simple protein.
A complete protein, also called a high-quality protein, contains all essential amino acids in sufficient quantity to support growth and maintain nitrogen balance. Examples of foods that contain complete proteins are fish, chicken, soybeans, turkey, and cheese. Incomplete proteins are missing one or more of the nine indispensable amino acids and include cereals, legumes (beans, peas), and vegetables. Complementary proteins are pairs of incomplete proteins that, when combined, supply the total amount of protein provided by complete protein sources.
Nitrogen balance is achieved when the intake and output of nitrogen are equal. When the intake of nitrogen is greater than the output, the body is in positive nitrogen balance. Positive nitrogen balance is required for growth, normal pregnancy, maintenance of lean muscle mass and vital organs, and wound healing. The body uses nitrogen to build, repair, and replace body tissues. Negative nitrogen balance occurs when the body loses more nitrogen than it gains (e.g., with infection, burns, fever, starvation, head injury, and trauma). The increased nitrogen loss is the result of body tissue destruction or loss of nitrogen-containing body fluids. Nutrition during this period needs to provide nutrients to put patients into positive balance for healing.
Protein provides energy; however, because of the essential role of protein in growth, maintenance, and repair, a diet needs to provide adequate kilocalories from nonprotein sources. When there is sufficient carbohydrate in the diet to meet the energy needs of the body, protein is spared as an energy source.
Fats
Fats (lipids) are the most calorie-dense nutrient, providing 9 kcal/g. Fats are composed of triglycerides and fatty acids. Triglycerides circulate in the blood and are composed of three fatty acids attached to a glycerol. Fatty acids are composed of chains of carbon and hydrogen atoms with an acid group on one end of the chain and a methyl group at the other. Fatty acids can be saturated, in which each carbon in the chain has two attached hydrogen atoms; or unsaturated, in which an unequal number of hydrogen atoms are attached and the carbon atoms attach to each other with a double bond. Monounsaturated fatty acids have one double bond, whereas polyunsaturated fatty acids have two or more double carbon bonds. The various types of fatty acids have significance for health and the incidence of disease and are referred to in dietary guidelines.
Fatty acids are also classified as essential or nonessential. Linoleic acid, an unsaturated fatty acid, is the only essential fatty acid in humans. Linolenic acid and arachidonic acid (also unsaturated fatty acids) are important for metabolic processes but are manufactured by the body when linoleic acid is available. Deficiency occurs when fat intake falls below 10% of daily nutrition. Most animal fats have high proportions of saturated fatty acids, whereas vegetable fats have higher amounts of unsaturated and polyunsaturated fatty acids.
Water
Water is critical because cell function depends on a fluid environment. Water makes up 60% to 70% of total body weight. The percent of total body water is greater for lean people than obese people because muscle contains more water than any other tissue except blood. Infants have the greatest percentage of total body water, and older people have the least. When deprived of water, a person cannot survive for more than a few days.
An individual meets fluid needs by drinking liquids and eating solid foods high in water content such as fresh fruits and vegetables. Water is also produced during digestion when food is oxidized. In a healthy individual fluid intake from all sources equals fluid output through elimination, respiration, and sweating (see Chapters 41 and 45). An ill person has an increased need for fluid (e.g., with fever or gastrointestinal [GI] losses). By contrast, he or she also has a decreased ability to excrete fluid (e.g., with cardiopulmonary or renal disease), which often leads to the need for fluid restriction.
Vitamins
Vitamins are organic substances present in small amounts in foods that are essential to normal metabolism. They are chemicals that act as catalysts in biochemical reactions. When there is enough of any specific vitamin to meet the body’s catalytic demands, the rest of the vitamin supply acts as a free chemical and is often toxic to the body. Certain vitamins are currently of interest in their role as antioxidants. These vitamins neutralize substances called free radicals, which produce oxidative damage to body cells and tissues. Researchers think that oxidative damage increases a person’s risk for various cancers. These vitamins include betacarotene and vitamins A, C, and E (Nix, 2009).
The body is unable to synthesize vitamins in the required amounts and depends on dietary intake. Vitamin content is usually highest in fresh foods that are used quickly after minimal exposure to heat, air, or water. Vitamins are classified as fat soluble and water soluble.
Fat-Soluble Vitamins: The fat-soluble vitamins (A, D, E, and K) are stored in the fatty compartments of the body. With the exception of vitamin D, people acquire vitamins through dietary intake. Hypervitaminosis of fat-soluble vitamins results from megadoses (intentional or unintentional) of supplemental vitamins, excessive amounts in fortified food, and large intake of fish oils.
Water-Soluble Vitamins: The water-soluble vitamins are vitamin C and the B complex (which is eight vitamins). The body does not store water-soluble vitamins; thus they need to be provided in daily food intake. Water-soluble vitamins absorb easily from the GI tract. Although they are not stored, toxicity can still occur.
Minerals
Minerals are inorganic elements essential to the body as catalysts in biochemical reactions. They are classified as macrominerals when the daily requirement is 100 mg or more and microminerals or trace elements when less than 100 mg is needed daily. Macrominerals help to balance the pH of the body, and specific amounts are necessary in the blood and cells to promote acid-base balance. Interactions occur among trace minerals. For example, excess of one trace mineral sometimes causes deficiency of another. Selenium is a trace element that also has antioxidant properties. Silicon, vanadium, nickel, tin, cadmium, arsenic, aluminum, and boron play an unidentified role in nutrition. Arsenic, aluminum, and cadmium have toxic effects.
Anatomy and Physiology of the Digestive System
Digestion of food is the mechanical breakdown that results from chewing, churning, and mixing with fluid and chemical reactions in which food is reduced to its simplest form. Each part of the GI system has an important digestive or absorptive function (Fig. 44-1). Enzymes are the proteinlike substances that act as catalysts to speed up chemical reactions. They are an essential part of the chemistry of digestion.
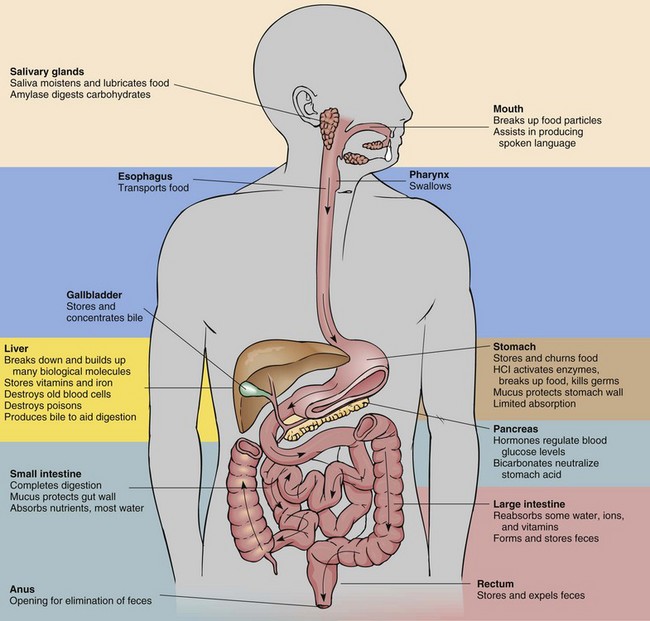
FIG. 44-1 Summary of digestive system anatomy/organ function. HCl, Hydrochloric acid. (From Rolin Graphics.)
Most enzymes have one specific function. Each enzyme works best at a specific pH. For example, the enzyme amylase in the saliva breaks down starches into sugars. The secretions of the GI tract have very different pH levels. For example, saliva is relatively neutral, gastric juice is highly acidic, and the secretions of the small intestine are alkaline.
The mechanical, chemical, and hormonal activities of digestion are interdependent. Enzyme activity depends on the mechanical breakdown of food to increase its surface area for chemical action. Hormones regulate the flow of digestive secretions needed for enzyme supply. Physical, chemical, and hormonal factors regulate the secretion of digestive juices and the motility of the GI tract. Nerve stimulation from the parasympathetic nervous system (e.g., the vagus nerve) increases GI tract action.
Digestion begins in the mouth, where chewing mechanically breaks down food. The food mixes with saliva, which contains ptyalin (salivary amylase), an enzyme that acts on cooked starch to begin its conversion to maltose. The longer an individual chews food, the more starch digestion occurs in the mouth. Proteins and fats are broken down physically but remain unchanged chemically because enzymes in the mouth do not react with these nutrients. Chewing reduces food particles to a size suitable for swallowing, and saliva provides lubrication to further ease swallowing of the food. The epiglottis is a flap of skin that closes over the trachea as a person swallows to prevent aspiration. Swallowed food enters the esophagus, and wavelike muscular contractions (peristalsis) move the food to the base of the esophagus, above the cardiac sphincter. Pressure from a bolus of food at the cardiac sphincter causes it to relax, allowing the food to enter the fundus, or uppermost portion, of the stomach.
The chief cells in the stomach secrete pepsinogen; and the pyloric glands secrete gastrin, a hormone that triggers parietal cells to secrete hydrochloric acid (HCl). The parietal cells also secrete HCl and intrinsic factor (IF), which is necessary for absorption of vitamin B12 in the ileum. HCl turns pepsinogen into pepsin, a protein-splitting enzyme. The body produces gastric lipase and amylase to begin fat and starch digestion, respectively. A thick layer of mucus protects the lining of the stomach from autodigestion. Alcohol and aspirin are two substances directly absorbed through the lining of the stomach. The stomach acts as a reservoir where food remains for approximately 3 hours, with a range of 1 to 7 hours.
Food leaves the antrum, or distal stomach, through the pyloric sphincter and enters the duodenum. Food is now an acidic, liquefied mass called chyme. Chyme flows into the duodenum and quickly mixes with bile, intestinal juices, and pancreatic secretions. The small intestine secretes the hormones secretin and cholecystokinin (CCK). Secretin activates release of bicarbonate from the pancreas, raising the pH of chyme. CCK inhibits further gastrin secretion and initiates release of additional digestive enzymes from the pancreas and gallbladder.
Bile is manufactured in the liver and concentrated and stored in the gallbladder. It acts as a detergent because it emulsifies fat to permit enzyme action while suspending fatty acids in solution. Pancreatic secretions contain six enzymes: amylase to digest starch; lipase to break down emulsified fats; and trypsin, elastase, chymotrypsin, and carboxypeptidase to break down proteins.
Peristalsis continues in the small intestine, mixing the secretions with chyme. The mixture becomes increasingly alkaline, inhibiting the action of the gastric enzymes and promoting the action of the duodenal secretions. Epithelial cells in the small intestinal villi secrete enzymes (e.g., sucrase, lactase, maltase, lipase, and peptidase) to facilitate digestion. The major portion of digestion occurs in the small intestine, producing glucose, fructose, and galactose from carbohydrates; amino acids and dipeptides from proteins; and fatty acids, glycerides, and glycerol from lipids. Peristalsis usually takes approximately 5 hours to pass food through the small intestine.
Absorption
The small intestine is the primary absorption site for nutrients. It is lined with fingerlike projections called villi. Villi increase the surface area available for absorption. The body absorbs nutrients by means of passive diffusion, osmosis, active transport, and pinocytosis (Table 44-1).
TABLE 44-1
Mechanisms for Intestinal Absorption of Nutrients
| MECHANISM | DEFINITION |
| Active transport | An energy-dependent process whereby particles move from an area of greater concentration to an area of lesser concentration. A special “carrier” moves the particle across the cell membrane. |
| Passive diffusion | The force by which particles move outward from an area of greater concentration to lesser concentration. The particles do not need a special “carrier” to move outward in all directions. |
| Osmosis | Movement of water through a membrane that separates solutions of different concentrations. Water moves to equalize the concentration pressures on both sides of the membrane. |
| Pinocytosis | Engulfing of large molecules of nutrients by the absorbing cell when the molecule attaches to the absorbing cell membrane. |
Data from Nix S: Williams’ basic nutrition and diet therapy, ed 13, St Louis, 2009, Mosby.
Carbohydrates, protein, minerals, and water-soluble vitamins are absorbed by the small intestine, processed in the liver, and released into the portal vein circulation. Fatty acids are absorbed in the lymphatic circulatory systems through lacteal ducts at the center of each microvilli in the small intestine.
Approximately 85% to 90% of water is absorbed in the small intestine (Huether et al., 2008). Approximately 8.5 L of GI secretions and 1.5 L of oral intake are managed daily within the GI tract. The small intestine resorbs 9.5 L, and the colon absorbs approximately 0.4 L. The remaining 0.1 L is eliminated in feces. In addition, electrolytes and minerals are absorbed in the colon, and bacteria synthesize vitamin K and some B-complex vitamins. Finally, feces are formed for elimination.
Metabolism and Storage of Nutrients
Metabolism refers to all of the biochemical reactions within the cells of the body. Metabolic processes are anabolic (building) or catabolic (breaking down). Anabolism is the building of more complex biochemical substances by synthesis of nutrients. Anabolism occurs when an individual adds lean muscle through diet and exercise. Amino acids are anabolized into tissues, hormones, and enzymes. Normal metabolism and anabolism are physiologically possible when the body is in positive nitrogen balance. Catabolism is the breakdown of biochemical substances into simpler substances and occurs during physiological states of negative nitrogen balance. Starvation is an example of catabolism when wasting of body tissues occurs.
Nutrients absorbed in the intestines, including water, are transported through the circulatory system to the body tissues. Through the chemical changes of metabolism, the body converts nutrients into a number of required substances. Carbohydrates, protein, and fat are metabolized to produce chemical energy and maintain a balance between anabolism and catabolism. To carry out the work of the body, the chemical energy produced by metabolism converts to other types of energy by different tissues. Muscle contraction involves mechanical energy, nervous system function involves electrical energy, and the mechanisms of heat production involve thermal energy.
Some of the nutrients required by the body are stored in tissues. The major form of body reserve energy is fat, stored as adipose tissue. Protein is stored in muscle mass. When the energy requirements of the body exceed the energy supplied by ingested nutrients, stored energy is used. Monoglycerides from the digested portion of fats are converted to glucose by gluconeogenesis. Amino acids are also converted to fat and stored or catabolized into energy through gluconeogenesis. All body cells except red blood cells and neurons oxidize fatty acids into ketones for energy when dietary carbohydrates (glucose) are not adequate. Glycogen, synthesized from glucose, provides energy during brief periods of fasting (e.g., during sleep). It is stored in small reserves in liver and muscle tissue. Nutrient metabolism consists of three main processes:
Elimination
Chyme moves by peristaltic action through the ileocecal valve into the large intestine, where it becomes feces (see Chapter 46). Water absorbs in the mucosa as feces move toward the rectum. The longer the material stays in the large intestine, the more water is absorbed, causing the feces to become firmer. Exercise and fiber stimulate peristalsis, and water maintains consistency. Feces contain cellulose and similar indigestible substances, sloughed epithelial cells from the GI tract, digestive secretions, water, and microbes.
Dietary Guidelines
Dietary reference intakes (DRIs) present evidence-based criteria for an acceptable range of amounts of vitamins and nutrients for each gender and age-group (Institute of Medicine, 2006). There are four components to the DRIs. The estimated average requirement (EAR) is the recommended amount of a nutrient that appears sufficient to maintain a specific body function for 50% of the population based on age and gender. The recommended dietary allowance (RDA) is the average needs of 98% of the population, not the exact needs of the individual. The adequate intake (AI) is the suggested intake for individuals based on observed or experimentally determined estimates of nutrient intakes and is used when there is not enough evidence to set the RDA. The tolerable upper intake level (UL) is the highest level that likely poses no risk of adverse health events. It is not a recommended level of intake (Tolerable upper level intake, 2010).
Food Guidelines
The U.S. Department of Agriculture (USDA) and the U.S. Department of Health and Human Services (USDHHS) published the Dietary Guidelines for Americans 2010 and provide average daily consumption guidelines for the five food groups: grains, vegetables, fruits, dairy products, and meats (Box 44-2). These guidelines are for Americans over the age of 2 years. As a nurse, consider the food preferences of patients from different racial and ethnic groups, vegetarians, and others when planning diets. The ChooseMyPlate program was developed by the U.S. Department of Agriculture to replace the My Food Pyramid program. ChooseMyPlate provides a basic guide for making food choices for a healthy lifestyle (Fig. 44-2). The ChooseMyPlate program includes guidelines for balancing calories; decreasing portion size; increasing healthy foods; increasing water consumption; and decreasing fats, sodium, and sugars (USDA, 2011a).

FIG. 44-2 ChooseMyPlate. (From US Department of Agriculture: ChooseMyPlate, 2011, http://www.choosemyplate.gov).
Daily Values
The Food and Drug Administration (FDA) created daily values for food labels in response to the 1990 Nutrition Labeling and Education Act (NLEA). The FDA first established two sets of reference values. The referenced daily intakes (RDIs) are the first set, comprising protein, vitamins, and minerals based on the RDA. The daily reference values (DRVs) make up the second set and consist of nutrients such as total fat, saturated fat, cholesterol, carbohydrates, fiber, sodium, and potassium. Combined, both sets make up the daily values used on food labels (USFDA, 2008). Daily values did not replace RDAs but provided a separate, more understandable format for the public. Daily values are based on percentages of a diet consisting of 2000 kcal/day for adults and children 4 years or older.
Nursing Knowledge Base
Sociological, cultural, psychological, and emotional factors are associated with eating and drinking in all societies. Holidays and events are celebrated with food, food is brought to those who are grieving, and food is used for medicinal purposes. It is incorporated into family traditions and rituals and is often associated with eating behaviors. You need to understand patients’ values, beliefs, and attitudes about food and how these values affect food purchase, preparation, and intake to affect eating patterns.
Nutritional requirements depend on many factors. Individual caloric and nutrient requirements vary by stage of development, body composition, activity levels, pregnancy and lactation, and the presence of disease. Registered dietitians (RDs) use predictive equations that take into account some of these factors to estimate patients’ nutritional requirements.
Factors Influencing Nutrition
Environmental factors beyond the control of individuals contribute to the development of obesity. Obesity is an epidemic in the United States. The prevalence of obesity in adults has doubled since 1980, with 33% of adults in the United States overweight, 34% obese, and 6 % extremely obese (body mass index [BMI] ≥40) (Khan et al., 2009). Proposed contributing factors are sedentary lifestyle, work schedules, and poor meal choices often related to the increasing frequency of eating away from home and eating fast food (Kruskall, 2006). The likelihood of healthy eating and participation in exercise or other activities of healthy living is limited by environmental factors. Lack of access to full-service grocery stores, high cost of healthy food, widespread availability of less healthy foods in fast-food restaurants, widespread advertising of less healthy food, and lack of access to safe places to play and exercise are environmental factors that contribute to obesity (Khan et al., 2009).
Developmental Needs
Infants Through School-Age: Rapid growth and high protein, vitamin, mineral, and energy requirements mark the developmental stage of infancy. The average birth weight of an American baby is 3.2 to 3.4 kg (7 to 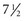 pounds). An infant usually doubles birth weight at 4 to 5 months and triples it at 1 year. Infants need an energy intake of approximately 90 to 110 kcal/kg of body weight, with premature infants needing 105 to 130 kcal/kg per day (Nix, 2009). Commercial formulas and human breast milk both provide approximately 20 kcal/oz. A full-term newborn is able to digest and absorb simple carbohydrates, proteins, and a moderate amount of emulsified fat. Infants need about 100 to 120 mL/kg/day of fluid because a large portion of total body weight is water.
pounds). An infant usually doubles birth weight at 4 to 5 months and triples it at 1 year. Infants need an energy intake of approximately 90 to 110 kcal/kg of body weight, with premature infants needing 105 to 130 kcal/kg per day (Nix, 2009). Commercial formulas and human breast milk both provide approximately 20 kcal/oz. A full-term newborn is able to digest and absorb simple carbohydrates, proteins, and a moderate amount of emulsified fat. Infants need about 100 to 120 mL/kg/day of fluid because a large portion of total body weight is water.
Breastfeeding: The American Dietetic Association strongly supports exclusive breastfeeding for the first 6 months of life and breastfeeding with complementary foods from 6 to 12 months (American Dietetic Association, 2009). Breastfeeding has multiple benefits for both infant and mother, including fewer food allergies and intolerances; fewer infant infections; easier digestion; convenience, availability, and freshness; temperature always correct; economical because it is less expensive than formula; and increased time for mother and infant interaction.
Formula: Infant formulas contain the approximate nutrient composition of human milk. Protein in the formula is typically whey, soy, cow’s milk base, casein hydrolysate, or elemental amino acids. The American Academy of Pediatrics sets standards for the level of nutrients in infant formulas. Soy protein–based formulas are used for infants allergic or intolerant to cow’s milk (Nix, 2009).
Infants should not have regular cow’s milk during the first year of life. It is too concentrated for an infant’s kidneys to manage, increases the risk of milk product allergies, and is a poor source of iron and vitamins C and E (Nix, 2009). Honey and corn syrup are potential sources of botulism toxin and should not be used in an infant’s diet. This toxin is potentially fatal in children under 1 year of age (Nix, 2009).
Introduction to Solid Food: Breast milk or formula provides sufficient nutrition for the first 4 to 6 months of life. The development of fine-motor skills of the hand and fingers parallels an infant’s interest in food and self-feeding. Iron-fortified cereals are typically the first semisolid food to be introduced. For infants 4 to 11 months, cereals are the most important nonmilk source of protein (Fox et al., 2006).
The addition of foods to an infant’s diet is governed by an infant’s nutrient needs, physical readiness to handle different forms of foods, and the need to detect and control allergic reactions. Foods such as wheat, egg white, nuts, citrus juice, and chocolate have a high incidence of allergies and should be added late (Nix, 2009). Caregivers should introduce new foods one at a time, approximately 4 to 7 days apart to identify allergies. It is best to introduce new foods before milk or other foods to avoid satiety (Hockenberry and Wilson, 2011).
The growth rate slows during toddler years (1 to 3 years). A toddler needs fewer kilocalories but an increased amount of protein in relation to body weight; consequently appetite often decreases at 18 months of age. Toddlers exhibit strong food preferences and become picky eaters. Small frequent meals consisting of breakfast, lunch, and dinner with three interspersed high nutrient–dense snacks help improve nutritional intake (Hockenberry and Wilson, 2011). Calcium and phosphorus are important for healthy bone growth.
Toddlers who consume more than 24 ounces of milk daily in place of other foods sometimes develop milk anemia because milk is a poor source of iron. Toddlers need to drink whole milk until the age of 2 years to make sure that there is adequate intake of fatty acids necessary for brain and neurological development. Certain foods such as hot dogs, candy, nuts, grapes, raw vegetables, and popcorn have been implicated in choking deaths and need to be avoided. Dietary requirements for preschoolers (3 to 5 years) are similar to those for toddlers. They consume slightly more than toddlers, and nutrient density is more important than quantity.
School-age children, 6 to 12 years old, grow at a slower and steadier rate, with a gradual decline in energy requirements per unit of body weight. Despite better appetites and more varied food intake, you need to assess school-age children’s diets carefully for adequate protein and vitamins A and C. They often fail to eat a proper breakfast and have unsupervised intake at school. High fat, sugar, and salt result from too-liberal intake of snack foods. Physical activity level decreases consistently, and high-calorie, readily available food increases in consumption, leading to an increase in childhood obesity (Budd and Hayman, 2008).
In the last 20 years the prevalence of overweight children has risen. The percent of obesity in children ages 6 to 11 years has doubled to 17%, and the percent of overweight adolescents has more than tripled to 17.6% (Li and Hooker, 2010). A combination of factors contributes to the problem, including a diet rich in high-calorie foods, food advertising targeting children, inactivity, genetic predisposition, use of food as a coping mechanism for stress or boredom or as a reward or celebration, and family and social factors (Budd and Hayman, 2008). Childhood obesity contributes to medical problems related to the cardiovascular system, endocrine system, and mental health (Budd and Hayman, 2008). As a result of the obesity, the incidence of type II diabetes in children is also increasing. Prevention of childhood obesity is critical because of the long-term effects. Family education is an important component in decreasing the prevalence of this problem. Promote healthy food choices and eating in moderation along with increased physical activity.
Adolescents: During adolescence physiological age is a better guide to nutritional needs than chronological age. Energy needs increase to meet greater metabolic demands of growth. Daily requirement of protein also increases. Calcium is essential for the rapid bone growth of adolescence, and girls need a continuous source of iron to replace menstrual losses. Boys also need adequate iron for muscle development. Iodine supports increased thyroid activity, and use of iodized table salt ensures availability. B-complex vitamins are necessary to support heightened metabolic activity.
Many factors other than nutritional needs influence the adolescent’s diet, including concern about body image and appearance, desire for independence, eating at fast-food restaurants, peer pressure, and fad diets. Nutritional deficiencies often occur in adolescent girls as a result of dieting and use of oral contraceptives. An adolescent boy’s diet is often inadequate in total kilocalories, protein, iron, folic acid, B vitamins, and iodine. Snacks provide approximately 25% of a teenager’s total dietary intake. Fast food, particularly value-size or super-size meals, is common and adds extra salt, fat, and kilocalories (Budd and Hayman, 2008). Skipping meals or eating meals with unhealthy choices of snacks contributes to nutrient deficiency and obesity (Hockenberry and Wilson, 2011).
Fortified foods (nutrients added) are important sources of vitamins and minerals. Snack food from the dairy and fruit and vegetable groups are good choices. To counter obesity, increasing physical activity is often more important than curbing intake. The onset of eating disorders such as anorexia nervosa or bulimia nervosa often occurs during adolescence. Recognition of eating disorders is essential for early intervention (Box 44-3).
Sports and regular moderate-to-intense exercise necessitate dietary modification to meet increased energy needs for adolescents. Carbohydrates, both simple and complex, are the main source of energy, providing 55% to 60% of total daily kilocalories. Protein needs increase to 1 to 1.5 g/kg/day. Fat needs do not increase. Adequate hydration is very important. Adolescents need to ingest water before and after exercise to prevent dehydration, especially in hot, humid environments. Vitamin and mineral supplements are not required, but intake of iron-rich foods is required to prevent anemia.
Parents have more influence on adolescents’ diets than they believe. Effective strategies include limiting the amount of unhealthy food choices kept at home, encouraging smart snacks such as fruit vegetables or string cheese, and enhancing the appearance and taste of healthy foods (Mayo Clinic Staff, 2009). Making healthy food choices more convenient at home and at fast-food restaurants and discouraging adolescents from eating while watching television are ways to promote healthy eating (Befort et al., 2006).
Pregnancy occurring within 4 years of menarche places a mother and fetus at risk because of anatomical and physiological immaturity. Malnutrition at the time of conception increases risk to the adolescent and her fetus. Most teenage girls do not want to gain weight. Counseling related to nutritional needs of pregnancy is often difficult, and teens tolerate suggestions better than rigid directions. The diet of pregnant adolescents is often deficient in calcium, iron, and vitamins A and C. Prenatal vitamin and mineral supplements are recommended.
Young and Middle Adults: There is a reduction in nutrient demands as the growth period ends. Mature adults need nutrients for energy, maintenance, and repair. Energy needs usually decline over the years. Obesity becomes a problem because of decreased physical exercise, dining out more often, and increased ability to afford more luxury foods. Adult women who use oral contraceptives often need extra vitamins. Iron and calcium intake continues to be important.
Pregnancy: Poor nutrition during pregnancy causes low birth weight in infants and decreases chances of survival. Generally the needs of a fetus are met at the expense of the mother. However, if nutrient sources are not available, both suffer. The nutritional status of the mother at the time of conception is important. Significant aspects of fetal growth and development often occur before the mother suspects the pregnancy. The energy requirements of pregnancy are related to the mother’s body weight and activity. The quality of nutrition during pregnancy is important, and food intake in the first trimester includes balanced portions of essential nutrients with emphasis on quality. Protein intake throughout pregnancy needs to increase to 60 g daily. Calcium intake is especially critical in the third trimester, when fetal bones are mineralized. Iron needs to be supplemented to provide for increased maternal blood volume, fetal blood storage, and blood loss during delivery.
Folic acid intake is particularly important for deoxyribonucleic acid (DNA) synthesis and the growth of red blood cells. Inadequate intake can lead to fetal neural tube defects, anencephaly, or maternal megaloblastic anemia (Nix, 2009). Women of childbearing age need to consume 400 mcg of folic acid daily, increasing to 600 mcg daily during pregnancy. Prenatal care usually includes vitamin and mineral supplementation to ensure daily intakes; however, pregnant women should not take additional supplements beyond prescribed amounts.
Lactation: The lactating woman needs 500 kcal/day above the usual allowance because the production of milk increases energy requirements. Protein requirements during lactation are greater than those required during pregnancy. The need for calcium remains the same as during pregnancy. There is an increased need for vitamins A and C. Daily intake of water-soluble vitamins (B and C) is necessary to ensure adequate levels in breast milk. Fluid intake needs to be adequate but not excessive. Caffeine, alcohol, and drugs are excreted in breast milk and should be avoided.
Older Adults: Adults 65 years and older have a decreased need for energy because their metabolic rate slows with age. However, vitamin and mineral requirements remain unchanged from middle adulthood. Numerous factors influence the nutritional status of the older adult (Box 44-4). Age-related changes in appetite, taste, smell, and the digestive system affect nutrition (Touhy and Jett, 2010). For example, older adults often experience a decrease in taste cells that alters food flavor and may decrease intake. Multiple factors contribute to the risk of food insecurity in the older adult. Income is significant because living on a fixed income often reduces the amount of money available to buy food. Health is another important influence that affects a person’s desire and ability to eat. Lack of transportation or ability to get to the grocery store because of mobility problems contributes to inability to purchase adequate and nutritious food. Often availability of nutritionally adequate and safe foods is limited or uncertain.
Maintaining good oral health is significant throughout adulthood, particularly as an individual ages. Difficulty chewing, missing teeth, having teeth in poor condition, and oral pain result from poor oral health. These often contribute to malnutrition and dehydration in older adults (Touhy and Jett, 2010). Poor oral hygiene and periodontal disease are potential risk factors for systemic diseases such as joint infections, ischemic stroke, cardiovascular disease, DM, and aspiration pneumonia (O’Connor, 2008).
The older adult is often on a therapeutic diet or has difficulty eating because of physical symptoms, lack of teeth, or dentures or is at risk for drug-nutrient interactions (Table 44-2). Caution older adults to avoid grapefruit and grapefruit juice because they alter absorption of many drugs. Thirst sensation diminishes, leading to inadequate fluid intake or dehydration (see Chapter 41). Symptoms of dehydration in older adults include confusion; weakness; hot, dry skin; furrowed tongue; rapid pulse; and high urinary sodium. Some older adults avoid meats because of cost or because they are difficult to chew. Cream soups and meat-based vegetable soups are nutrient-dense sources of protein. Cheese, eggs, and peanut butter are also useful high-protein alternatives. Milk continues to be an important food for older women and men who need adequate calcium to protect against osteoporosis (a decrease of bone mass density). Screening and treatment are necessary for both older men and women. Vitamin D supplements are important for improving strength and balance, strengthening bone health, and preventing bone fractures and falls (Park et al., 2008). The diet of older adults needs to contain choices from all food groups and often requires a vitamin and mineral supplement. MyPlate for Older Adults addresses the specific nutritional needs for older adults and encourages physical activity (Tufts University, 2011).
TABLE 44-2
Sample of Drug-Nutrient Interactions*
| DRUG | EFFECT |
| Analgesic | |
| Acetaminophen | Decreased drug absorption with food; overdose associated with liver failure |
| Aspirin | Absorbed directly through stomach; decreased drug absorption with food; decreased folic acid, vitamins C and K, and iron absorption |
| Antacid | |
| Aluminum hydroxide | Decreased phosphate absorption |
| Sodium bicarbonate | Decreased folic acid absorption |
| Antiarrhythmic | |
| Amiodarone (Codarone) | Taste alteration |
| Digitalis | Anorexia, decreased renal clearance in older people |
| Antibiotic | |
| Penicillin | Decreased drug absorption with food, taste alteration |
| Cephalosporin | Decreased vitamin K |
| Rifampin (Rifadin) | Decreased vitamin B6, niacin, vitamin D |
| Tetracycline | Decreased drug absorption with milk and antacids; decreased nutrient absorption of calcium, riboflavin, vitamin C caused by binding |
| Trimethoprim/sulfamethoxazole | Decreased folic acid |
| Anticoagulant | |
| Warfarin (Coumadin) | Acts as antagonist to vitamin K |
| Anticonvulsant | |
| Carbamazepine (Tegretol) | Increased drug absorption with food |
| Phenytoin (Dilantin) | Decreased calcium absorption; decreased vitamins D and K and folic acid; taste alteration; decreased drug absorption with food |
| Antidepressant | |
| Amitriptyline | Appetite stimulant |
| Clomipramine (Anafranil) | Taste alteration, appetite stimulant |
| Fluoxetine (Prozac) (selective serotonin reuptake inhibitors [SSRIs]) | Taste alteration, anorexia |
| Antihypertensive | |
| Captopril (Capoten) | Taste alteration, anorexia |
| Hydralazine | Enhanced drug absorption with food, decreased vitamin B6 |
| Labetalol (Normodyne) | Taste alteration (weight gain for all beta-blockers) |
| Methyldopa | Decreased vitamin B12, folic acid, iron |
| Antiinflammatory | |
| All steroids | Increased appetite and weight, increased folic acid, decreased calcium (osteoporosis with long-term use), promotes gluconeogenesis of protein |
| Antiparkinson | |
| Levodopa (Dopar) | Taste alteration, decreased vitamin B6 and drug absorption with food |
| Antipsychotic | |
| Chlorpromazine | Increased appetite |
| Thiothixene | Decreased riboflavin, increased need |
| Bronchodilator | |
| Albuterol sulfate | Appetite stimulant |
| Theophylline | Anorexia |
| Cholesterol Lowering | |
| Cholestyramine (Prevalite) | Decreased fat-soluble vitamins (A, D, E, K); vitamin B12; iron |
| Diuretic | |
| Furosemide (Lasix) | Decreased drug absorption with food |
| Spironolactone (Aldactone) | Increased drug absorption with food |
| Thiazides | Decreased magnesium, zinc, and potassium |
| Laxative | |
| Mineral oil | Decreased absorption of fat-soluble vitamins (A, D, E, K), carotene |
| Platelet Aggregate Inhibitor | |
| Dipyridamole (Persantine) | Decreased drug absorption with food |
| Potassium Replacement | |
| Potassium chloride | Decreased vitamin B12 |
| Tranquilizer | |
| Benzodiazepines | Increased appetite |
*Not intended to be an exhaustive or all-inclusive list. Always check pharmacology references before administering medications.
Data from Hermann J: Nutrient and drug interactions, http://pods.dasnr.okstate.edu/docushare/dsweb/Get/Document-2458/T-3120web.pdf; accessed October 31, 2010; Lehne RA: Pharmacology for nursing care, ed 7, St Louis, 2010, Saunders.
The USDHHS Administration on Aging (AOA) requires states to provide nutrition screening services to older adults who benefit from home-delivered or congregate meal services. This program requires meals to provide at least one third of the DRI for an older adult and meet the Dietary Guidelines for Americans (American Dietetic Association, 2010a). Homebound older adults with chronic illnesses have additional nutritional risks. They frequently live alone with little or no social or financial resources to assist in obtaining or preparing nutritionally sound meals, contributing to the risk for food insecurity. Approximately 19% of older adults experience some degree of food insecurity as a result of low income or poverty (American Dietetic Association, 2010a). Increased nutrition screening by the nurse results in early recognition and treatment of nutritional deficiencies. Undernourishment of older adults often results in health problems that lead to admission to acute care hospitals or long-term care facilities.
Alternative Food Patterns
Long before the FDA issued recommended allowances and guidelines, many people followed special patterns of food intake based on religion (Table 44-3), cultural background (Box 44-5), ethics, health beliefs, personal preference, or concern for the efficient use of land to produce food. Such special diets are not necessarily more or less nutritious than diets based on the MyPlate or other nutritional guidelines because good nutrition depends on a balanced intake of all required nutrients.
Vegetarian Diet
A common alternative dietary pattern is the vegetarian diet. Vegetarianism is the consumption of a diet consisting predominantly of plant foods. Some vegetarians are ovolactovegetarian (avoid meat, fish, and poultry but eat eggs and milk), lactovegetarians (drink milk but avoid eggs), or vegans (consume only plant foods). Through careful selection of foods, individuals following a vegetarian diet can meet recommendations for proteins and essential nutrients (Nix, 2009). Zen macrobiotic (primarily brown rice, other grains, and herb teas) and fruitarian (only fruit, nuts, honey, and olive oil) diets are nutrient poor and frequently result in malnutrition. Knowledge related to complementary use of high and low biological value proteins is necessary. Children who follow a vegetarian diet are especially at risk for protein and vitamin deficiencies such as vitamin B12. Careful planning helps to ensure a balanced, healthy diet.
Critical Thinking
Effective critical thinking requires a synthesis of knowledge, experience, information collected from patients, critical thinking attitudes, and intellectual and professional standards. Clinical judgments require you to anticipate the required information, analyze the data, and make decisions regarding patient care. Critical thinking is a dynamic process. During assessment (Fig. 44-3) consider all elements that build toward making appropriate nursing diagnoses.
Integrate knowledge from nursing and other disciplines, previous experiences, and information gathered from patients and families regarding customary food preferences and recent diet history. Use of professional standards such as the DRIs, the USDA MyPlate dietary guidelines, and Healthy People 2020 objectives provide guidelines to assess and maintain patients’ nutritional status. Other professional standards by the American Heart Association (AHA, 2010), the American Diabetes Association (ADA, 2008), The American Cancer Society (ACS, 2011), and the American Society for Parenteral and Enteral Nutrition (ASPEN) (Bankhead et al., 2009) are available. These standards are evidence based and regularly updated for optimal patient care.
Nursing Process
Apply the nursing process and use a critical thinking approach in your care of patients. The nursing process provides a clinical decision-making approach for you to develop and implement an individualized plan of care.
Assessment
During the assessment process, thoroughly assess each patient and critically analyze findings to ensure that you make patient-centered clinical decisions required for safe nursing care. Early recognition of malnourished or at-risk patients has a strong positive influence on both short- and long-term health outcomes. Studies indicate that 40% to 55% of adult hospitalized patients are either malnourished or at risk for malnutrition (Mason, 2006). Patients who are malnourished on admission are at greater risk of life-threatening complications such as arrhythmia, sepsis, or hemorrhage during hospitalization.
Through the Patient’s Eyes
Assess patients’ nutritional status by using the nursing history to gather information about factors that usually influence nutrition. As the nurse you are in an excellent position to recognize signs of poor nutrition and take steps to initiate change. Close contact with patients and their families enables you to make observations about physical status, food intake, food preferences, weight changes, and response to therapy. Always ask patients about their food preferences, their values regarding nutrition, and what they expect from nutritional therapy. In attempting to affect eating patterns, you need to understand patient’s values, beliefs, and attitudes about food. Also assess family traditions and rituals related to food, cultural values and beliefs, and nutritional needs. Determine how these factors affect food purchase, preparation, and intake.
Screening
Nutrition screening is an essential part of an initial assessment. Screening a patient is a quick method of identifying malnutrition or risk of malnutrition using sample tools (Charney, 2008). Nutrition screening tools need to gather data on the current condition, stability of the condition, assessment of whether it will worsen, and if the disease process accelerates. These tools typically include objective measures such as height, weight, weight change, primary diagnosis, and the presence of other co-morbidities (Charney, 2008). Combine multiple objective measures with subjective measures related to nutrition to adequately screen for nutritional problems. Identification of risk factors such as unintentional weight loss, presence of a modified diet, or the presence of altered nutritional symptoms (i.e., nausea, vomiting, diarrhea, and constipation) requires nutritional consultation.
Several standardized nutrition screening tools are available for use in the outpatient setting. The Subjective Global Assessment (SGA) uses the patient history, weight, and physical assessment data to evaluate nutritional status (Charney, 2008). SGA is a simple, inexpensive technique that is able to predict nutrition-related complications. The Mini Nutritional Assessment (MNA) (Fig. 44-4) was developed to use for screening older adults in home care programs, nursing homes, and hospitals. The tool has 18 items that are divided into screening and assessment. If a patient scores 11 or less on the screening portion, the health care provider completes the assessment portion (Kondrup et al., 2003). A total score of less than 17 indicates protein-energy malnutrition (Guigoz et al., 1996; Guigoz and Vellas, 1999). The Malnutrition Screening Tool (MST) is an effective measure of nutritional problems for patients in a variety of health care settings (Charney, 2008).

FIG. 44-4 Mini Nutritional Assessment (MNA). (Copyright © Nestlé, 1994, Revision 2009. N67200 12/99 10M.)
Assess patients for malnutrition when they have conditions that interfere with their ability to ingest, digest, or absorb adequate nutrients. Use standardized tools to assess nutrition risks when possible. Congenital anomalies and surgical revisions of the GI tract interfere with normal function. Patients fed only by IV infusion of 5% or 10% dextrose are at risk for nutritional deficiencies. Chronic diseases or increased metabolic requirements are risk factors for development of nutritional problems. Infants and older adults are at greatest risk.
Anthropometry
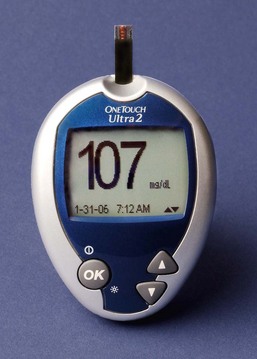
Anthropometry is a measurement system of the size and makeup of the body. Nurses obtain height and weight for each patient on hospital admission or entry into any health care setting. If you are not able to measure height with the patient standing, position him or her lying flat in bed as straight as possible with arms folded on the chest and measure him or her lengthwise. Serial measures of weight over time provide more useful information than one measurement. The patient needs to be weighed at the same time each day, on the same scale, and with the same clothing or linen. Document his or her weight and compare height and weight to standards for height-weight relationships. An ideal body weight (IBW) provides an estimate of what a person should weigh. Rapid weight gain or loss is important to note because it usually reflects fluid shifts. One pint or 500 mL of fluid equals 1 lb (0.45 kg). For example, for a patient with renal failure, a weight increase of 2 lbs (0.90 kg) in 24 hours is significant because it usually indicates that the patient has retained a liter (1000 mL) of fluid.
Other anthropometric measurements often obtained by dietitians help identify nutritional problems. These include the ratio of height-to-wrist circumference, midupper arm circumference (MAC), triceps skinfold (TSF), and midupper arm muscle circumference (MAMC). A dietitian will compare values for MAC, TSF, and MAMC to standards and calculate them as a percentage of the standard. Changes in values for an individual over time are of greater significance than isolated measurements (Nix, 2009).
Body mass index (BMI) measures weight corrected for height and serves as an alternative to traditional height-weight relationships. Calculate BMI by dividing the patient’s weight in kilograms by height in meters squared: Weight (kg) divided by height2 (m2). For example, a patient who weighs 165 lbs (75 kg) and is 1.8 m (5 feet 9 inches) tall has a BMI of 23.15 (75 ÷ 1.82 = 23.15). The website for the National Heart Lung and Blood Institute (http://www.nhlbisupport.com/bmi/) provides an easy way to calculate BMI. A patient is overweight if his or her BMI is 25 to 30. A BMI of greater than 30 is defined as obesity and places a patient at higher medical risk of coronary heart disease, some cancers, DM, and hypertension.
Laboratory and Biochemical Tests
No single laboratory or biochemical test is diagnostic for malnutrition. Factors that frequently alter test results include fluid balance, liver function, kidney function, and the presence of disease. Common laboratory tests used to study nutritional status include measures of plasma proteins such as albumin, transferrin, prealbumin, retinol binding protein, total iron-binding capacity, and hemoglobin. After feeding, the response time for changes in these proteins ranges from hours to weeks. The metabolic half-life of albumin is 21 days, transferrin is 8 days, prealbumin is 2 days, and retinol binding protein is 12 hours. Use this information to determine the most effective measure of plasma proteins for your patients. Factors that affect serum albumin levels include hydration; hemorrhage; renal or hepatic disease; large amounts of drainage from wounds, drains, burns, or the GI tract; steroid administration; exogenous albumin infusions; age; and trauma, burns, stress, or surgery. Albumin level is a better indicator for chronic illnesses, whereas prealbumin level is preferred for acute conditions (Pagana and Pagana, 2009).
Nitrogen balance is important to determining serum protein status (see discussion of protein in this chapter). Calculate nitrogen balance by dividing 6.25 into the total grams of protein ingested in a day (24 hours). Use laboratory analysis of a 24-hour urinary urea nitrogen (UUN) to determine nitrogen output. For patients with diarrhea or fistula drainage, estimate a further addition of 2 to 4 g of nitrogen output. Calculate nitrogen balance by subtracting the nitrogen output from the nitrogen intake. A positive 2- to 3-g nitrogen balance is necessary for anabolism. By contrast, negative nitrogen balance is present when catabolic states exist.
Diet History and Health History
In addition to the general nursing history, use data from a more specific diet history to assess a patient’s actual or potential needs. Box 44-6 lists some specific assessment questions to ask in the diet history. The diet history focuses on a patient’s habitual intake of foods and liquids and includes information about preferences, allergies, and other relevant areas such as the patient’s ability to obtain food. Gather information about the patient’s illness/activity level to determine energy needs and compare food intake. Your nursing assessment of nutrition includes health status; age; cultural background (see Box 44-5); religious food patterns (see Table 44-3); socioeconomic status; personal food preferences; psychological factors; use of alcohol or illegal drugs; use of vitamin, mineral, or herbal supplements; prescription or over-the-counter (OTC) drugs (see Table 44-2); and the patient’s general nutrition knowledge.
In outpatient settings the patient keeps a 3- to 7-day food diary. This allows you to calculate nutritional intake and to compare it with DRI to see if the patient’s dietary habits are adequate. Use food questionnaires to establish patterns over time (Bankhead et al., 2009). In health care settings nurses collaborate with RDs to complete calorie counts for patients.
Physical Examination
The physical examination is one of the most important aspects of a nutritional assessment. Because improper nutrition affects all body systems, observe for malnutrition during physical assessment (see Chapter 30). Complete the general physical assessment of body systems and recheck relevant areas to evaluate a patient’s nutritional status. The clinical signs of nutritional status (Table 44-4) serve as guidelines for observation during physical assessment.
TABLE 44-4
Physical Signs of Nutritional Status
| BODY AREA | SIGNS OF GOOD NUTRITION | SIGNS OF POOR NUTRITION |
| General appearance | Alert: responsive | Listless, apathetic, cachectic |
| Weight | Weight normal for height, age, body build | Obesity (usually 10% above ideal body weight [IBW]) or underweight (special concern for underweight) |
| Posture | Erect posture; straight arms and legs | Sagging shoulders; sunken chest; humped back |
| Muscles | Well-developed, firm; good tone; some fat under skin | Flaccid, poor tone, underdeveloped tone; “wasted” appearance; impaired ability to walk properly |
| Nervous system control | Good attention span; not irritable or restless; normal reflexes; psychological stability | Inattention; irritability; confusion; burning and tingling of hands and feet (paresthesia); loss of position and vibratory sense; weakness and tenderness of muscles (may result in inability to walk); decrease or loss of ankle and knee reflexes; absent vibratory sense |
| Gastrointestinal function | Good appetite and digestion; normal regular elimination; no palpable organs or masses | Anorexia; indigestion; constipation or diarrhea; liver or spleen enlargement |
| Cardiovascular function | Normal heart rate and rhythm; lack of murmurs; normal blood pressure for age | Rapid heart rate (above 100 beats/min), enlarged heart; abnormal rhythm; elevated blood pressure |
| General vitality | Endurance; energy; sleeps well; vigorous | Easily fatigued; no energy; falls asleep easily; tired and apathetic |
| Hair | Shiny, lustrous; firm; not easily plucked; healthy scalp | Stringy, dull, brittle, dry, thin, and sparse, depigmented; easily plucked |
| Skin (general) | Smooth and slightly moist skin with good color | Rough, dry, scaly, pale, pigmented, irritated; bruises; petechiae; subcutaneous fat loss |
| Face and neck | Uniform color; smooth, pink, healthy appearance; not swollen | Greasy, discolored, scaly, swollen; dark skin over cheeks and under eyes; lumpiness or flakiness of skin around nose and mouth |
| Lips | Smooth; good color; moist; not chapped or swollen | Dry, scaly, swollen; redness and swelling (cheilosis); angular lesions at corners of mouth; fissures or scars (stomatitis) |
| Mouth, oral membranes | Reddish-pink mucous membranes in oral cavity | Swollen, boggy oral mucous membranes |
| Gums | Good pink color; healthy and red; no swelling or bleeding | Spongy gums that bleed easily; marginal redness, inflammation; receding |
| Tongue | Good pink or deep reddish color; no swelling; smooth, presence of surface papillae; lack of lesions | Swelling, scarlet and raw; magenta, beefiness (glossitis); hyperemic and hypertrophic papillae; atrophic papillae |
| Teeth | No cavities; no pain; bright, straight; no crowding; well-shaped jaw; clean with no discoloration | Unfilled caries; missing teeth; worn surfaces; mottled (fluorosis), malpositioned |
| Eyes | Bright, clear, shiny; no sores at corner of eyelids; moist and healthy pink conjunctivae; prominent blood vessels; no fatigue circles beneath eyes | Eye membranes pale (pale conjunctivas); redness of membrane (conjunctival injection); dryness; signs of infection; Bitot’s spots; redness and fissuring of eyelid corners (angular palpebritis); dryness of eye membrane (conjunctival xerosis); dull appearance of cornea (corneal xerosis); soft cornea (keratomalacia) |
| Neck (glands) | No enlargement | Thyroid or lymph node enlargement |
| Nails | Firm, pink | Spoon shape (koilonychia); brittleness; ridges |
| Legs, feet | No tenderness, weakness, or swelling; good color | Edema; tender calf; tingling; weakness |
| Skeleton | No malformations | Bowlegs; knock-knees; chest deformity at diaphragm; prominent scapulae and ribs |
From Nix S: Williams’ basic nutrition and diet therapy, ed 13, St Louis, 2009, Mosby.
Dysphagia
Dysphagia refers to difficulty swallowing. The causes (Box 44-7) and complications of dysphagia vary. Complications include aspiration pneumonia, dehydration, decreased nutritional status, and weight loss. Dysphagia leads to disability or decreased functional status, increased length of stay and cost of care, increased likelihood of discharge to institutionalized care, and increased mortality (Ashley et al., 2006).
Be aware of warning signs for dysphagia. They include cough during eating; change in voice tone or quality after swallowing; abnormal movements of the mouth, tongue, or lips; and slow, weak, imprecise, or uncoordinated speech. Abnormal gag, delayed swallowing, incomplete oral clearance or pocketing, regurgitation, pharyngeal pooling, delayed or absent trigger of swallow, and inability to speak consistently are other signs of dysphagia. Patients with dysphagia often do not show overt signs such as coughing when food enters the airway. Silent aspiration is aspiration that occurs in patients with neurological problems that lead to decreased sensation. It often occurs without a cough, and symptoms usually do not appear for 24 hours (Palmer and Metheny, 2008). Silent aspiration accounts for most of the 40% to 70% of aspiration in patients with dysphagia following stroke (Kwon et al., 2006).
Dysphagia often leads to an inadequate amount of food intake, which often results in malnutrition. Frequently patients with dysphagia become frustrated with eating and show changes in skinfold thickness and albumin. Adjustment to new dietary restrictions during the rehabilitation period affects intake for long periods of time. Malnutrition significantly slows swallowing recovery and may increase mortality (Robbins et al., 2007).
Dysphagia screening quickly identifies problems with swallowing and helps nurses initiate referrals for more in-depth assessment by a speech pathologist (Skill 44-1 on pp. 1026-1027). Early and ongoing assessment of patients with swallowing difficulties and use of a valid dysphagia screening tool increase quality of care and decrease incidence of aspiration pneumonia (AY Cichero et al., 2009). Dysphagia screening includes medical record review; observation of a patient at a meal for change in voice quality, posture, and head control; percentage of meal consumed; eating time; drooling or leakage of liquids and solids; cough during/after a swallow; facial or tongue weakness; palatal movement; difficulty with secretions; pocketing; choking; and presence of voluntary and dry cough. A number of validated screening tools are available, such as the Bedside Swallowing Assessment, Burke Dysphagia Screening Test, Acute Stroke Dysphagia Screen, and Standardized Swallowing Assessment (Edmiaston et al., 2010). The Acute Stroke Dysphagia screen is an easily administered and reliable tool for health care professionals who are not speech-language pathologists. Screening for and treatment of dysphagia requires a multidisciplinary team approach of nurses, RDs, health care providers, and speech language pathologists (SLPs) (Robbins et al., 2007).
Nursing Diagnosis
Cluster all assessment data to identify actual or at-risk nursing diagnoses (Box 44-8). A nutritional problem often occurs when overall intake is significantly decreased or increased or when one or more nutrients are not ingested, completely digested, or completely absorbed. Nursing diagnoses are related to either the actual nutrition problems (e.g., inadequate intake) or problems that place the patient at risk for nutritional deficiencies such as oral trauma, severe burns, or infections.
Select a nursing diagnostic statement based on defining characteristics in the assessment database. Make sure the nursing diagnosis is as precise as possible. The following are examples of nursing diagnoses that apply to nutritional problems:
• Imbalanced nutrition: less than body requirements
• Imbalanced nutrition: more than body requirements
• Risk for imbalanced nutrition: more than body requirements
Be sure to select the appropriate related factor for a nursing diagnosis. Related factors need to be accurate so you select the appropriate interventions. In addition, there are also clinical situations in which patients have multiple related problems. The concept map in Fig. 44-5 shows the relationship of nursing diagnoses for Mrs. Cooper.
Planning
Planning to maintain patients’ optimal nutritional status requires a higher level of care than simply correcting nutritional problems. Often there is a need for patients to make long-term changes for nutrition to improve. Synthesis of patient information from multiple sources is necessary to create an individualized approach of care that is relevant to a patient’s needs and situation (Fig. 44-6). Apply critical thinking to ensure that you consider all data sources in developing a patient’s plan of care. The accurate identification of nursing diagnoses related to patients’ nutritional problems results in a care plan that is relevant and appropriate (see the Nursing Care Plan). Referring to professional standards for nutrition is especially important during this step, because published standards are based on scientific findings.
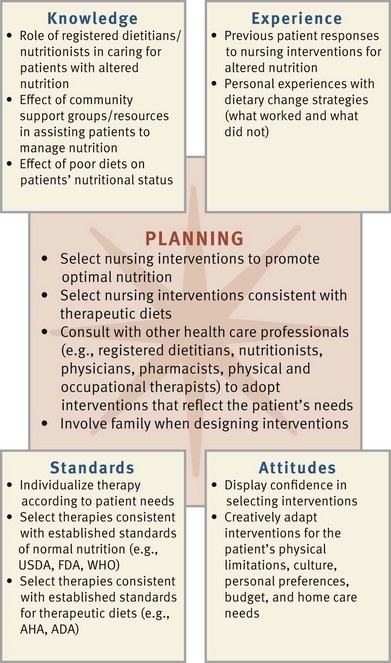
FIG. 44-6 Critical thinking model for nutrition planning. ADA, American Diabetes Association; AHA, American Heart Association; FDA, Food and Drug Administration; USDA, U.S. Department of Agriculture; WHO, World Health Organization.
Goals and Outcomes
Goals and outcomes of care reflect a patient’s physiological, therapeutic, and individualized needs. Nutrition education and counseling are important to prevent disease and promote health. Patients on therapeutic diets need to understand the implications of their diets and how prescribed diets help to control their illnesses. When planning care, be aware of all factors that influence a patient’s food intake. In one study patients with heart failure identified that decreased hunger, diet restrictions, fatigue, shortness of breath, anxiety, and sadness influenced their food intake (Lennie et al., 2006).
Individualized planning is essential. Explore patients’ feelings about their weight and diet and help them set realistic and achievable goals (Daniels, 2006). Mutually planned goals negotiated among the patient, RD, and nurse ensure success. For the patient with heart failure described previously, an overall goal is “Patient will achieve appropriate BMI height-weight range or be within 10% of IBW.” The following outcomes assist in achievement of this goal:
• Patient’s daily nutritional intake meets the minimal DRIs.
• Patient’s daily nutritional fat intake is less than 30%.
• Patient removes sugared beverages from diet.
• Patient refrains from eating unhealthy foods between meals and after dinner.
Meeting nutritional goals requires input from the patient and the multidisciplinary team. Knowledge of the role of each discipline in providing nutrition support is necessary to maximize nutritional outcomes. For example, collaboration with an RD helps develop appropriate nutrition treatment plans. Calorie counts are frequently ordered, and assistance is necessary in obtaining accurate data. An effective plan of care requires accurate exchange of information among disciplines.
Setting Priorities
After identifying patients’ nursing diagnoses, you determine priorities in order to plan timely and successful interventions. For example, managing a patient’s oral pain will be a priority over the diagnosis of imbalanced nutrition: less than body requirements if the patient is unable to swallow and maintain adequate food intake. Deficient knowledge regarding diet therapy will be a priority if it is necessary to promote long-term and effective weight loss.
During acute illness or surgery the intake of food is often altered in the perioperative period. The priority of care is to provide optimal preoperative nutrition support in patients with malnutrition. The priority for the resumption of food intake after surgery depends on the return of bowel function, the extent of the surgical procedure, and the presence of any complications (see Chapter 50). For example, when patients have oral and throat surgery, they chew and swallow food in the presence of excision sites, sutures, or tissue manipulated during surgery. The priority of care is to first provide comfort and pain control. Then address nutritional priorities and plan care to maintain nutrition that does not cause pain or injury to the healing tissues.
The patient and family must collaborate with the nurse in planning care and setting priorities. This is important because food preferences, food purchases, and preparation involve the entire family. The plan of care cannot succeed without their commitment to, involvement in, and understanding of the nutritional priorities.
Teamwork and Collaboration
The care of the patient often extends beyond the acute hospital setting, requiring continued collaboration among members of the health care team. It is important that discharge planning include nutritional interventions as patients return to their homes or extended care facilities. Communicate patient goals and planned interventions to all team members to achieve expected patient outcomes. Consult with an SLP, RD, pharmacist, and/or occupational therapist when working with patients with dysphagia or who need ongoing nutritional assessment and interventions to meet their nutritional needs.
Enteral tube feedings are often administered into the stomach or intestines via a tube inserted through the nose or a percutaneous access (Skills 44-2 and 44-3 on pp. 1028-1035). These enteral feedings supplement a patient’s oral nutritional intake in the home, acute care, extended care, or rehabilitation setting when they cannot meet their nutritional needs by mouth. Regardless of the setting, the RD assesses and monitors the patient’s nutritional status and intake and makes recommendations for changes. RDs are expert in the choice of enteral formulas and dietary modifications required for specific disease states. Long-term management of nutritional problems is a challenge that requires collaboration among the patient, family, and health care team members.
Patients who cannot tolerate nutrition through the GI tract receive parenteral nutrition, a solution consisting of glucose, amino acids, lipids, minerals, electrolytes, trace elements, and vitamins, through an indwelling peripheral or central venous catheter (CVC) (see Chapter 41). The pharmacist is an expert who reviews medications to identify drug-nutrient interactions. Pharmacists are also experts in preparing mixtures of total parenteral nutrition (TPN).
When patients have difficulty feeding themselves, occupational therapists work with them and their families to identify assistive devices. Devices such as utensils with large handles and plates with elevated sides help a patient with self-feeding. An SLP helps a patient with swallowing exercises and techniques to reduce the risk of aspiration. Occupational therapists also help patients maintain function in the home setting by rearranging food preparation areas in an effort to maximize a patient’s functional capacity.
Implementation
Diet therapies are numerous and are chosen on the basis of a patient’s overall health status, ability to eat and digest normally, and long-term nutritional needs. The focus of health promotion is to educate patients and family caregivers about balanced nutrition and to assist them in obtaining resources to eat high-quality meals. In acute care, your role as a nurse is to manage acute conditions that alter patients’ nutritional status and assist in ways to promote their appetite and ability to take in nutrients. Patients who are ill or debilitated often have poor appetites (anorexia). Anorexia has many causes (e.g., pain, fatigue, and the effects of medications). Help patients understand the factors that cause anorexia and use creative approaches to stimulate appetite. In the restorative care setting, you will assist patients in learning how to follow the therapeutic diets necessary for recovery and treatment of chronic health conditions.
Health Promotion
As a nurse you are in a key position to educate patients about healthy diet choices and good nutrition. Incorporating knowledge of nutrition into patients’ lifestyles serves to prevent the development of many diseases. Outpatient and community-based settings are optimal locations for nursing assessment of nutritional practices and status. Early identification of potential or actual problems is the best way to avoid more serious problems. Similarly, in other health care settings patients with nutritional problems such as obesity often require assistance in menu planning and compliance strategies. Your role as educator includes educating families and providing information about community resources. Telephone numbers of an RD or nurse for follow-up questions are always a part of counseling.
Meal planning takes into account the family’s budget and different preferences of family members. Choose specific foods on the basis of the dietary prescription and recommended food groups. For families on limited budgets use substitutes. For example, bean or cheese dishes often replace meat in a meal, and evaporated milk or dry skim milk is used for cooking. Have patients modify the method of preparation when it is necessary to minimize certain substances. Baking rather than frying reduces fat intake, and patients can use lemon juice or spices to add flavor to low-sodium diets.
Planning menus a week in advance has several benefits. It helps ensure good nutrition or compliance with a specific diet and helps a family stay within their allotted budget. Nurses or RDs need to check menus for content. Often a simple tip is helpful in meal planning such as avoiding grocery shopping when hungry, which can lead to spur-of-the-moment purchases of more expensive or less nutritious foods that are not included in meal plans. The U.S. Department of Agriculture (USDA, 2011b) provides sample weekly meal-planning services for a range of budgets on its website.
Support individuals who are interested in losing weight. A high percentage of those who attempt to lose weight are unsuccessful, regaining lost weight over time. Diet and exercise compliance affects success with weight loss. Information on weight loss is available from multiple sources. Help patients develop a successful weight-loss plan that considers their preferences and resources and includes awareness of portion sizes and knowledge of energy content of food (Kruskall, 2006).
Food safety is an important public health issue. Foodborne bacteria can occur from improper food cleaning, preparation, or poor hygiene practices of food workers. Health care professionals not only need to be aware of the factors related to food safety but also should provide patient education to reduce the risks for foodborne illnesses (Table 44-5; Box 44-9).
TABLE 44-5
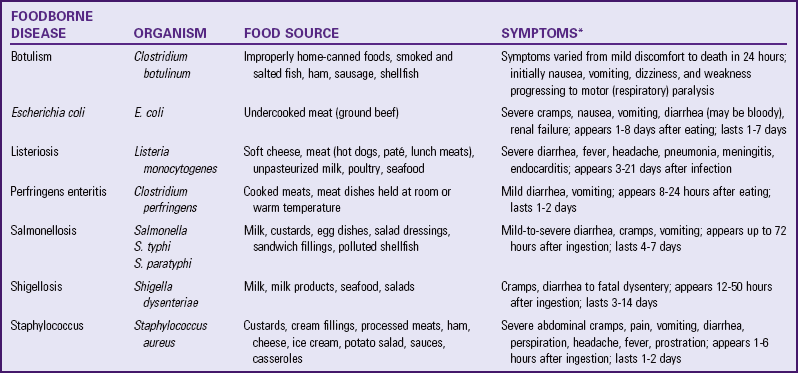
*Symptoms are generally most severe for youngest and oldest age-groups.
From Nix S: Williams’ basic nutrition and diet therapy, ed 13, St Louis, 2009, Mosby.
Acute Care
The nutritional care of acutely ill patients requires a nurse to consider a variety of factors that influence nutritional intake. Diagnostic testing and procedures in the acute care setting disrupt food intake. Often as preparation for or immediately following a diagnostic procedure, a patient is to receive nothing by mouth (NPO). Mealtimes in a health care setting are frequently interrupted, or patients have poor appetites. Patients often are too fatigued or uncomfortable to eat. It is important to continuously assess a patient’s nutritional status and adopt interventions that promote normal intake, digestion, and metabolism of nutrients. Patients who are NPO and receive only standard IV fluids for more than 4 to 7 days are at nutritional risk.
Advancing Diets: Acute and chronic conditions affect a patient’s immune system and nutritional status. Patients with decreased immune function (e.g., from cancer, chemotherapy, human immunodeficiency virus/acquired immunodeficiency syndrome [HIV/AIDS], or organ transplants) require special diets that decrease their exposure to microorganisms and are higher in selected nutrients. Table 44-6 gives an overview of the immune system, the malnutrition impact, and which nutrients are beneficial. In addition, patients who are ill, who have had surgical procedures, or who were NPO for a period of time have specialized dietary needs. Health care providers order a gradual progression of dietary intake or therapeutic diet to manage patients’ illness (Box 44-10).
TABLE 44-6
Nutrition and the Immune System
| IMMUNE/PHYSIOLOGICAL COMPONENT | MALNUTRITION EFFECT | VITAL NUTRIENT |
| Antibodies | Decreased amount | Protein, vitamins A, B6, B12, C, folic acid, thiamin, biotin, riboflavin, niacin |
| GI tract | Systemic movement of bacteria | Arginine, glutamine, omega-3 fatty acids |
| Granulocytes and macrocytes | Longer time for phagocytosis kill time and lymphocyte activation | Protein, vitamins A, B6, B12, C, folic acid, thiamin, riboflavin, niacin, zinc, iron |
| Mucus | Flat microvilli in GI tract, decreased antibody secretion | Vitamins B12, B6, C, biotin |
| Skin | Integrity compromised, density reduced, wound healing slowed | Protein, vitamins A, B12, C, niacin, copper, zinc |
| T-lymphocytes | Depressed T-cell distribution | Protein, arginine, iron, zinc, omega-3 fatty acids, vitamins A, B6, B12, folic acid, thiamin, riboflavin, niacin, pantothenic acid |
Modified from Grodner M, Long S, DeYoung S: Foundations and clinical applications of nutrition: a nursing approach, ed 5, St Louis, 2012, Mosby.
Promoting Appetite: Providing an environment that promotes nutritional intake includes keeping a patient’s environment free of odors, providing oral hygiene as needed to remove unpleasant tastes, and maintaining patient comfort. Offering smaller, more frequent meals often helps. In addition, certain medications affect dietary intake and nutrient use. For example, medications such as insulin, glucocorticoids, and thyroid hormones affect metabolism. Other medications such as antifungal agents frequently affect taste. Some of the psychotropic medications affect appetite, cause nausea, and also alter taste. The nurse and RD help patients to select foods that reduce the altered taste sensations or nausea. Consult with an RD regarding seasonings that may be used to improve food taste. In other situations medications need to be changed. Assessing patients for the need for pharmacological agents to stimulate appetite such as cyproheptadine (Periactin), megestrol (Megace), or dronabinol (Marinol) or to manage symptoms that interfere with nutrition requires health care provider consultation.
Mealtime is usually a social activity. If appropriate, encourage visitors to eat with the patient. When patients experience anorexia, encourage other nurses or care providers to converse and engage them in conversation. Mealtime is also an excellent opportunity for patient education. Instruct a patient about any therapeutic diets, medications, energy conservation measures, or adaptive devices to assist with independent feeding.
Assisting Patients with Oral Feeding: When a patient needs help with eating, it is important to protect his or her safety, independence, and dignity. Clear the table or over-bed tray of clutter. Assess his or her risk of aspiration (see Skill 44-1). Patients at high risk for aspiration have decreased level of alertness, decreased gag and/or cough reflexes, and difficulty managing saliva (see Assessment section of this chapter).
Patients with dysphagia are at risk for aspiration and need more assistance with feeding and swallowing. An SLP identifies patients at risk and provides recommendations for therapy (Nowlin, 2006). Provide a 30-minute rest period before eating (Palmer and Metheny, 2008). Position the patient in an upright, seated position in a chair or raise the head of the bed to 90 degrees. Have the patient flex the head slightly to a chin-down position to help prevent aspiration. If the patient has unilateral weakness, teach him or her and the caregiver to place food in the stronger side of the mouth. With the help of an SLP, determine the viscosity of foods that the patient tolerates best through the use of trials of different consistencies of foods and fluids. Thicker fluids are generally easier to swallow. The American Dietetic Association published the National Dysphagia Diet Task Force National Dysphagia Diet in 2002 to provide uniformity of diets provided to patients with dysphagia (NDDTF, 2002). There are four levels of diet: dysphagia puree, dysphagia mechanically altered, dysphagia advanced, and regular. The four levels of liquid include thin liquids (low viscosity), nectar-like liquids (medium viscosity), honey-like liquids (viscosity of honey), and spoon-thick liquids (viscosity of pudding) (NDDTF, 2002).
Feed a patient with dysphagia slowly, providing smaller-size bites. Allow him or her to chew thoroughly and swallow the bite before taking another. More frequent chewing and swallowing assessments throughout the meal are necessary. Allow the patient time to empty the mouth after each spoonful, matching the speed of feeding to the patient’s readiness (see Skill 44-1). If the patient begins to cough or choke, remove the food immediately (Nowlin, 2006). Sometimes it is necessary to have oral suction equipment available at the patient’s bedside.
Provide opportunities for patients to direct the order in which they want to eat the food items and how fast they wish to eat. Determine the patient’s food preferences; and, unless contraindicated, try to have these items included on his or her dietary tray. Ask the patient if the food is the right temperature. These seem like small acts, but they go a long way in maintaining the patient’s sense of independence.
Patients with visual deficits also need special assistance. Patients with decreased vision are able to independently feed themselves when they are given adequate information. Identify the food location on a meal plate as if it were a clock (e.g., meat at 9 o’clock and vegetable at 3 o’clock). Tell the patient where the beverages are located in relation to the plate. Be sure that other care providers set the meal tray and plate in the same manner. Patients with impaired vision and those with decreased motor skills are more independent during mealtimes with the use of large-handled adaptive utensils (Fig. 44-7). These are easier to grip and manipulate.
Enteral Tube Feeding: Enteral nutrition (EN) provides nutrients into the GI tract. It is the preferred method of meeting nutritional needs if a patient is unable to swallow or take in nutrients orally yet has a functioning GI tract. Enteral nutrition provides physiological, safe, and economical nutritional support. Patients with enteral feedings receive formula via nasogastric, jejunal, or gastric tubes. Patients with a low risk of gastric reflux receive gastric feedings; however, if there is a risk of gastric reflux, which leads to aspiration, jejunal feeding is preferred. Box 44-11 lists indications for tube feeding. Enteral tube feedings can easily be given in the home setting by either the nurse or a family caregiver. After an enteral tube is inserted, verification of tube placement by x-ray film examination needs to occur before the patient receives the first enteral feeding (see Skill 44-2).
An enteral formula is usually one of four types. Polymeric (1 to 2 kcal/mL) includes milk-based blenderized foods prepared by hospital dietary staff or in a patient’s home. The polymeric classification also includes commercially prepared whole-nutrient formulas. For this type of formula to be effective, the patient’s GI tract needs to be able to absorb whole nutrients. The second type, modular formulas (3.8 to 4 kcal/mL), are single macronutrient (e.g., protein, glucose, polymers, or lipids) preparations and are not nutritionally complete. This type of formula is added to other foods to meet a patient’s individual nutritional needs. The third type, elemental formulas (1 to 3 kcal/mL), contain predigested nutrients that are easier for a partially dysfunctional GI tract to absorb. Finally, specialty formulas (1 to 2 kcal/mL) are designed to meet specific nutritional needs in certain illness (e.g., liver failure, pulmonary disease, or HIV infection).
Tube feedings are typically started at full strength at slow rates (see Skill 44-3 and Box 44-12). Increase the hourly rate every 8 to 12 hours per health care provider’s order if no signs of intolerance appear (high gastric residuals, nausea, cramping, vomiting, and diarrhea). Studies have demonstrated a beneficial effect of enteral feedings compared with PN. Feeding by the enteral route reduces sepsis, minimizes the hypermetabolic response to trauma, decreases hospital mortality, and maintains intestinal structure and function (Khalid et al., 2010). Enteral nutrition is successful within 24 to 48 hours after surgery or trauma to provide fluids, electrolytes, and nutritional support. Gastric ileus prevents nasogastric feedings from being given. Nasointestinal or jejunal tubes allow successful postpyloric feeding because formula is placed directly into the small intestine or jejunum or beyond the pyloric sphincter of the stomach (Bankhead et al., 2009).
A serious complication associated with enteral feedings is aspiration of formula into the tracheobronchial tree. Aspiration of enteral formula into the lungs irritates the bronchial mucosa, resulting in decreased blood supply to affected pulmonary tissue (McCance et al., 2010). This leads to necrotizing infection, pneumonia, and potential abscess formation. The high glucose content of a feeding serves as a bacterial medium for growth, promoting infection. Acute respiratory distress syndrome (ARDS) is also an outcome frequently associated with pulmonary aspiration. Some of the common conditions that increase the risk of aspiration include coughing, gastroesophageal reflux disease (GERD), nasotracheal suctioning, an artificial airway, decreased level of consciousness, and lying flat. Prokinetic medications such as metoclopramide, erythromycin, or cisapride promote gastric emptying and decrease the risk of aspiration (Bourgault et al., 2007; Metheny, 2006). Keep the head of the bed elevated a minimum of 30 degrees, preferably 45 degrees, unless medically contraindicated (Bankhead et al., 2009). Measure gastric residual volumes (GRVs) every 4 to 6 hours in patients receiving continuous feedings and immediately before the feeding in patients receiving intermittent feedings (Metheny et al., 2006). Delayed gastric emptying is a concern if 250 mL or more remains in a patient’s stomach on two consecutive assessments (1 hour apart) or if a single GRV measurement exceeds 500 mL (Bankhead et al., 2009). There is a lack of consensus for recommendations for stopping feedings; decisions to stop feedings must include assessment of patient’s condition (Metheny et al., 2008). The North American Summit on Aspiration in the Critically Ill Patient recommends the following: (1) stop feedings immediately if aspiration occurs; (2) withhold feedings and reassess patient tolerance to feedings if GRV is over 500 mL; (3) routinely evaluate the patient for aspiration; and (4) use nursing measures to reduce the risk of aspiration if GRV is between 250 and 500 mL (Bankhead et al., 2009).
Enteral Access Tubes: When patients are unable to ingest food but are still able to digest and absorb nutrients, enteral tube feeding is indicated. Feeding tubes are inserted through the nose (nasogastric or nasointestinal), surgically (gastrostomy or jejunostomy), or endoscopically (percutaneous endoscopic gastrostomy or jejunostomy [PEG or PEJ]). If EN therapy is for less than 4 weeks, total, nasogastric, or nasojejunal feeding tubes may be used. Surgical or endoscopically placed tubes are preferred for long-term feeding (more than 4 weeks) to reduce the discomfort of a nasal tube and provide a more secure, reliable access (Rolandelli et al., 2005). Some patients such as those with gastroparesis (decreased or absent innervation to the stomach that results in delayed gastric emptying) or esophageal reflux or with a history of aspiration pneumonia require placement of tubes beyond the stomach into the intestine (Cirgin Ellett, 2006; Metheny et al., 2007).
Most health care settings use small-bore feeding tubes because they create less discomfort for a patient (Fig. 44-8). For the adult most of these tubes are 8- to 12-Fr and 36 to 44 inches (90 to 110 cm) long. A stylet is often used during insertion of a small-bore tube to stiffen it. The stylet is removed when the correct position of the feeding tube is confirmed. Skill 44-3 describes the procedure for initiating beginning nasogastric, gastrostomy, and jejunostomy enteral feedings.
Historically nurses verified feeding tube placement by injecting air through the tube while auscultating the stomach for a gurgling or bubbling sound or asking the patient to speak. Auscultation has repeatedly been shown to be ineffective in detecting tubes accidentally placed in the lung (Bourgault et al., 2007). Some patients are able to speak despite placement of feeding tubes in the lung (Rolandelli et al., 2005). Furthermore, auscultation is not effective in distinguishing between gastric and intestinal placement for feeding tubes (Rauen et al., 2008). The measurement of pH of secretions withdrawn from the feeding tube helps to differentiate the location of the tube (Box 44-13). At present the most reliable method for verification of placement of small-bore feeding tubes is x-ray film examination (Box 44-14).
The addition of blue food coloring to enteral formula to assist with the detection of formula aspirated into the lung, presumably by staining the tracheobronchial secretions, is no longer used. The FDA issued a public health advisory reporting an association between use of Blue No. 1 food coloring and patient deaths (McClave et al., 2009).
Table 44-7 outlines major complications of EN. Of special note, patients who are severely malnourished are at risk for electrolyte disturbances from re-feeding syndrome during EN or PN therapy. In re-feeding syndrome potassium, magnesium, and phosphate move intracellularly, resulting in low serum (extracellular) levels and edema. These changes cause cardiac dysrhythmias, heart failure, respiratory distress, convulsions, coma, or death.
Parenteral Nutrition: Parenteral nutrition (PN) is a form of specialized nutrition support in which nutrients are provided intravenously. A basic PN formula is a combination of crystalline amino acids, hypertonic dextrose, electrolytes, vitamins, and trace elements. TPN, administered through a central line, is a 2-in-1 formula in which fat emulsions are administered separately from the protein and dextrose solution (Krzywda and Meyer, 2010). Safe administration depends on appropriate assessment of nutrition needs, meticulous management of the central venous catheter (CVC), and careful monitoring to prevent or treat metabolic complications. PN is administered in a variety of settings, including a patient’s home. Regardless of the setting, adhere to principles of asepsis and infusion management to ensure safe nutrition support.
Patients who are unable to digest or absorb EN benefit from PN. Patients in highly stressed physiological states such as sepsis, head injury, or burns are candidates for PN therapy (see Box 44-11).
Clinical and laboratory monitoring by a multidisciplinary team is required throughout PN therapy. The need for continued PN is consistently reevaluated. The goal to move toward use of the GI tract is constant (McClave et al., 2009). Disuse of the GI tract has been associated with villus atrophy and generalized cell shrinkage. As a result of disuse of the GI tract, bacteria may move from the unused gut into the bloodstream, resulting in gram-negative septicemia.
Intravenous fat emulsions are sometimes added to PN to provide supplemental kilocalories, prevent essential fatty acid deficiencies, and help control hyperglycemia during periods of stress (Phillips, 2010). Administer these emulsions through a separate peripheral line, through the central line by using Y-connector tubing (see Chapter 41), or as an admixture to the PN solution. The addition of fat emulsion to a PN solution is called a 3-in-1 admixture or total nutrient admixture. The patient receives it over a 24-hour period. Do not use the admixture if you observe oil droplets or an oily or creamy layer on the surface of the admixture. This observation indicates that the emulsion has broken into large lipid droplets that cause fat emboli if administered. IV fat emulsions are white and opaque. Take care to avoid confusing enteral formula with parenteral lipids.
Initiating Parenteral Nutrition: Patients with short-term nutritional needs often receive IV solutions of less than 10% dextrose via a peripheral vein in combination with amino acids and lipids. Peripheral solutions are not as calorically dense as TPN solutions and therefore are usually temporary. PN with greater than 10% dextrose requires a CVC that a health care provider places into a high-flow central vein such as the superior vena cava under sterile conditions (see Chapter 41). If you are using a CVC that has multiple lumens, use a port that is exclusively dedicated for the TPN. Label the port for TPN and do not infuse other solutions or medications through it (Krzywda and Meyer, 2010). Nurses with special training insert peripherally inserted central catheters (PICCs) that are started in a vein of the arm and threaded into the subclavian or superior vena cava vein.
After catheter placement, wait to flush and use the catheter until the position is radiographically confirmed. The health care provider secures the CVC with a securement device and covers the site with a sterile dressing. A PICC is usually stabilized with sterile strips of tape and a sterile dressing. A chest x-ray film examination verifies catheter tip placement for a CVC or PICC before starting a PN infusion (DeChicco et al., 2007).
Before beginning any PN infusion, verify the health care provider’s order and inspect the solution for particulate matter or a break in the fat emulsion. Always use an infusion pump to deliver a constant rate. The initial rate delivers no more than 50% of estimated needs for the first 24 to 48 hours. The rate is gradually increased until a patient’s complete nutrition needs are supplied (National Guideline Clearinghouse, 2008). Patients receiving PN at home frequently administer the entire daily solution over 12 hours at night. This allows the patient to disconnect from the infusion each morning, flush the central line, and have independent mobility during the day. Home PN therapy often interferes with patients’ normal activities, causing a poorer quality of life (Siepler, 2007).
Preventing Complications: Complications of PN include catheter-related problems and metabolic alterations (Table 44-8). Pneumothorax results from a puncture insult to the pulmonary system and involves the accumulation of air in the pleural cavity with subsequent collapse of the lung and impaired breathing. Pneumothorax is usually accompanied by symptoms of sudden sharp chest pain, dyspnea, and coughing. In relation to PN, pneumothorax most often occurs during CVC placement. Monitor a patient with a CVC for the first 24 hours for signs and symptoms of pulmonary distress.
TABLE 44-8
Metabolic Complications of Parenteral Nutrition
| PROBLEM | SIGNS/SYMPTOMS | INTERVENTION |
| Electrolyte imbalance | See Chapter 41 for signs of deficiency/toxicity | Check TPN for supplemental electrolyte levels. Notify health care provider of imbalances. Maintain steady rate of infusion. Monitor intake and output. |
| Hypercapnia | Increased oxygen consumption, CO2, respiratory quotient (>1), and minute ventilation | Ventilator-dependent patients are at risk; provide 30% to 60% of energy requirements per health care provider’s order. |
| Hypoglycemia | Diaphoresis, shakiness, confusion, loss of consciousness | To prevent hypoglycemia, do not abruptly discontinue TPN but taper rate down to within 10% of infusion rate 1 to 2 hours before stopping. If you suspect hypoglycemia, test blood glucose and administer IV bolus of 50% dextrose or glucagon per order or protocol if necessary. |
| Hyperglycemia | Thirst, headache, lethargy, increased urination | Monitor blood glucose level every 6 hours. Initiate TPN slowly and taper up to maximal infusion rate to prevent hyperglycemia. Additional insulin may be required during therapy if problem persists or patient has diabetes mellitus. |
| Hyperglycemic hyperosmolar nonketotic coma (HHNKC) or hyperosmolar hyperglycemic nonketotic syndrome (HHNS) | Hyperglycemia (>500 mg/dl), glycosuria, serum osmolarity >350 mOsm/L, confusion, azotemia, headache, severe signs of dehydration (see Chapter 41), hypernatremia, metabolic acidosis, convulsions, coma | Monitor blood glucose, BUN, serum osmolarity, glucose in urine, and fluid losses; administer insulin as ordered; replace fluids as ordered; maintain constant infusion rate; and provide 30% of daily energy needs as fat. Patients at risk are those receiving steroids; older adults diagnosed with diabetes, who have impaired renal or pancreatic function or increased metabolism, or who are septic. |
BUN, Blood urea nitrogen; IV, intravenous; TPN, total parenteral nutrition.
An air embolus possibly occurs during insertion of the catheter or when changing the tubing or cap. Turn the patient into a left lateral decubitus position, and have the patient perform a Valsalva maneuver (holding the breath and “bearing down”) during catheter insertion to help prevent air embolus. The increased venous pressure created by the maneuver prevents air from entering the bloodstream. Maintaining integrity of the closed IV system also helps prevent air embolus.
Catheter occlusion is present when there is sluggish or no flow through the catheter. Temporarily stop the infusion and flush with saline or heparin per protocol or orders. If this is unsuccessful, attempt to aspirate a clot. If still unsuccessful, follow institution protocol for use of a thrombolytic agent (e.g., urokinase).
Suspect catheter sepsis if a patient develops fever, chills, or glucose intolerance and has a positive blood culture. To prevent infection, change the TPN infusion tubing every 24 hours. Do not hang a single container of PN for more than 24 hours or lipids more than 12 hours. Change the administration system every 72 hours when infusing a 2-in-1 solution and every 24 hours for a 3-in-1 solution (Phillips, 2010). During CVC dressing changes, always use a sterile mask and gloves and assess insertion sites for signs and symptoms of infection (see Chapter 41). Change the CVC dressing per institution policy and anytime it becomes wet or contaminated. Use either alcohol or an alcoholic solution of chlorhexidine gluconate to clean the injection port or catheter hub 15 seconds before and after each time it is used (National Guideline Clearinghouse, 2008). Use a 1.2-micron filter for 3-in-1 formulas and an inline 0.22-micron filter for PN solutions that do not include IV fat emulsions (Task Force for the Revision of Safe Practices for Parenteral Nutrition, 2004).
PN solutions contain most of the major electrolytes, vitamins, and minerals. Patients also need supplemental vitamin K as ordered throughout therapy. Vitamin K is synthesized by microflora found in the jejunum and ileum with normal use of the GI tract; however, because PN circumvents GI use, patients need to receive exogenous vitamin K.
Electrolyte and mineral imbalances often occur. Administration of concentrated glucose is accompanied by increases in endogenous insulin production, which causes cations (potassium, magnesium, and phosphorus) to move intracellularly. Monitor blood glucose levels every 6 hours to assess for hyperglycemia and administer supplemental insulin as needed (Phillips, 2010) (Skill 44-4 on pp. 1035-1039).
Too-rapid administration of hypertonic dextrose can result in an osmotic diuresis and dehydration (see Chapter 41). If an infusion falls behind schedule, do not increase the rate in an attempt to catch up. Sudden discontinuation of a solution can cause hypoglycemia. Usually 10% dextrose is infused when PN solution is suddenly discontinued. Patients with diabetes are more at risk.
The goal is to move patients from PN to EN and/or oral feeding. Once patients are meeting one third to one half of their kilocalorie needs per day, PN is usually decreased to half the original volume. EN feedings are then increased to meet needs. Patients who make the transition from PN to oral feedings typically have early satiety and decreased appetite. PN is gradually decreased in response to increased oral intake. If oral intake is inadequate, small frequent meals are helpful. Calorie/protein counts are recommended when patients begin taking soft foods. When 75% of needs are being met by enteral feedings or reliable dietary intake, PN therapy is usually discontinued. PN may also be discontinued if complications occur or the health care provider determines that it is not benefiting the patient (Krzywda and Meyer, 2010).
Restorative and Continuing Care
Patients discharged from a hospital with diet prescriptions often need dietary education to plan meals that meet specific therapeutic requirements. Restorative care includes both immediate postsurgical care and routine medical care and therefore includes patients in the hospital and at home. The following sections address nutritional interventions for some common disease states.
Medical Nutrition Therapy: Optimal nutrition is important in health and illness, but the specific dietary intake pattern that results in optimal nutrition is modified for patients with particular diseases. Medical nutrition therapy (MNT) is the use of specific nutritional therapies to treat an illness, injury, or condition. MNT is necessary to help the body metabolize certain nutrients, correct nutritional deficiencies related to the disease, and eliminate foods that may exacerbate disease symptoms. It is most effective using a team approach that promotes collaboration between the health care team and an RD (American Dietetic Association, 2010b).
Gastrointestinal Diseases: Peptic ulcers are controlled with regular meals and medications such as histamine receptor antagonists that block secretion of HCl or proton pump inhibitors. Marshall and Warren first identified Helicobacter pylori in 1984. H. pylori is a bacterium that causes up to 85% of peptic ulcers and is confirmed by laboratory tests or a biopsy during endoscopy (Nix, 2009). It is treated with antibiotics that control the bacterial infection. Stress and overproduction of gastric HCl also irritate a pre-existing ulcer. Encourage patients to avoid foods that increase stomach acidity and pain such as caffeine, decaffeinated coffee, frequent milk intake, citric acid juices, and certain seasonings (hot chili peppers, chili powder, black pepper). Discourage smoking, alcohol, aspirin, and nonsteroidal antiinflammatory drugs (NSAIDs). Teach patients to eat a well-balanced, healthy diet; avoid eating large meals; and eat three regular meals (or several small meals) without snacks, especially at bedtime (Nix, 2009). Family members of the patient with a H. pylori infection also need to be tested and treated if indicated.
Inflammatory bowel disease includes Crohn’s disease and idiopathic ulcerative colitis. Treatment of acute inflammatory bowel disease includes elemental diets (formula with the nutrients in their simplest form ready for absorption) or PN when symptoms such as diarrhea and weight loss are prevalent. In the chronic stage of the disease a regular highly-nourishing diet is appropriate. Vitamins and iron supplements are often required to correct or prevent anemia. Patients manage irritable bowel syndrome by increasing fiber, reducing fat, avoiding large meals, and avoiding lactose or sorbitol-containing foods for susceptible individuals.
The treatment of malabsorption syndromes such as celiac disease includes a gluten-free diet. Gluten is present in wheat, rye, barley, and oats. Short-bowel syndrome results from extensive resection of bowel, after which patients suffer from malabsorption caused by lack of intestinal surface area. These patients require lifetime feeding with either elemental enteral formulas or PN.
Diverticulitis is a condition that results from an inflammation of diverticula, which are abnormal but common pouchlike herniations that occur in the bowel lining. This condition is nutritionally treated with a moderate- or low-residue diet until the infection subsides. Afterward a high-fiber diet is generally prescribed for chronic diverticula problems.
Diabetes Mellitus: Type 1 diabetes mellitus (DM) requires both insulin and dietary restrictions for optimal control, with treatment beginning at diagnosis (ADA, 2008). By contrast, patients often control type 2 DM initially with exercise and diet therapy. If these measures prove ineffective, it is common to add oral medications. Insulin injections often follow if type 2 diabetes worsens or fails to respond to these initial interventions.
Individualize the diet according to a patient’s age, build, weight, and activity level. Maintaining a prescribed carbohydrate intake is the key in diabetes management. A diet that includes carbohydrates from fruits, vegetables, whole grains, legumes, and low-fat milk is recommended (American Dietetic Association, 2010b). Monitoring carbohydrate consumption is a key strategy in achieving glycemic control (ADA, 2008). Limit saturated fat to less than 7% of the total calories and cholesterol intake to less than 200 mg/day. Also recommended are a variety of foods containing fiber. Patients are able to substitute sucrose-containing foods for carbohydrates but need to make sure to avoid excess energy intake. Sugar alcohols and non-nutritive sweeteners are able to be eaten as long as the recommended daily intake levels are followed (ADA, 2008). Patients with diabetes and normal renal function should continue to consume usual amounts of protein (15% to 20% of energy) (ADA, 2008).
The goal of MNT treatment is to have glycemic levels that are normal or as close to normal as safely possible; lipid and lipoprotein profiles that decrease the risk of microvascular (e.g., renal and eye disease), cardiovascular, neurological, and peripheral vascular complications; and blood pressure in the normal or near-normal range (ADA, 2008). Be aware of signs and symptoms of hypoglycemia and hyperglycemia.
Cardiovascular Diseases: The American Heart Association (AHA) dietary guidelines (AHA, 2010) are intended to reduce risk factors for the development of hypertension and coronary artery disease. Diet therapy for reducing the risk of cardiovascular disease includes balancing calorie intake with exercise to maintain a healthy body weight; eating a diet high in fruits, vegetables, and whole-grain high-fiber foods; eating fish at least 2 times per week; and limiting food and beverages that are high in added sugar and salt. The AHA guidelines also recommend limiting saturated fat to less than 7%, trans-fat to less than 1%, and cholesterol to less than 300 mg/day. To accomplish this goal, patients choose lean meats and vegetables, use fat-free dairy products, and limit intake of fats and sodium (Nix, 2009).
Cancer and Cancer Treatment: Malignant cells compete with normal cells for nutrients, increasing a patient’s metabolic needs. Most cancer treatments cause nutritional problems. Patients with cancer often experience anorexia, nausea, vomiting, and taste distortions. The goal of nutrition therapy is to meet the increased metabolic needs of a patient (Nix, 2009). Malnutrition in cancer is associated with increased morbidity and mortality. Enhanced nutritional status often improves a patient’s quality of life.
Radiation therapy destroys rapidly dividing malignant cells; however, other normal rapidly dividing cells such as the epithelial lining of the GI tract are often affected. Radiation therapy causes anorexia, stomatitis, severe diarrhea, strictures of the intestine, and pain. Radiation treatment of the head and neck region causes taste and smell disturbances, decreased salivation, and dysphagia. Nutrition management of a patient with cancer focuses on maximizing intake of nutrients and fluids. Individualize diet choices to a patient’s needs, symptoms, and situation (Nix, 2009). Use creative approaches to manage alterations in taste and smell. For example, patients with altered taste often prefer chilled foods or foods that are spicy. Encourage patients to eat small frequent meals and snacks that are nutritious and easy to digest.
Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome: Patients with HIV/AIDS typically experience body wasting and severe weight loss. The wasting is related to anorexia, stomatitis, oral thrush infection, nausea, or recurrent vomiting, all resulting in inadequate intake. Factors associated with weight loss and malnutrition include severe diarrhea, GI malabsorption, and altered metabolism of nutrients. Systemic infection results in hypermetabolism from cytokine elevation. Often the medications taken to treat HIV infection cause side effects that alter nutritional status.
Restorative care of malnutrition resulting from AIDS focuses on maximizing kilocalories and nutrients. Diagnose and address each cause of nutritional depletion in the care plan. Individually tailored nutrition support progresses in stages from oral, to enteral, and finally to parenteral. Good hand hygiene and food safety are essential because of a patient’s reduced resistance to infection. For example, minimization of exposure to Cryptosporidium in drinking water, lakes, or swimming pools is important. Small, frequent, nutrient-dense meals that limit fatty and overly sweet foods are easier to tolerate. Patients benefit from eating cold foods and drier or saltier foods with fluid in between (Nix, 2009).
Evaluation
Patients expect competent and accurate care. If ongoing nutrition therapies do not result in successful outcomes, patients expect nurses to recognize this and alter the plan of care accordingly. Expectations and health care values held by nurses frequently differ from those held by patients. Successful interventions and outcomes require nurses to know what patients expect in addition to nursing knowledge and skill. Work closely with patients to define their expectations, and talk with them about their concerns if their expectations are not realistic. Consider the limits of their conditions and treatment, their dietary preferences, and their cultural beliefs when evaluating outcomes.
Patient Outcomes
Care plans need to reflect achievable goals and outcomes. Evaluate the actual outcomes of nursing actions and compare with expected outcomes to determine if the goals are met (Fig. 44-9). Multidisciplinary collaboration remains essential in providing nutritional support. Nutrition therapy does not always produce rapid results. Ongoing comparisons need to be made with baseline measures of weight, serum albumin or prealbumin, and protein and kilocalorie intake. If you do not observe gradual weight gain or if weight loss continues, evaluate the dietary EN prescription and determine if the patient is experiencing any adverse effects from medications that are affecting his or her nutritional status. Changes in condition also indicate a need to change the nutritional plan of care. Consult multidisciplinary members of the health care team in an effort to better individualize this plan. The patient is an active participant whenever possible. In the end, a patient’s ability to incorporate dietary changes into his or her lifestyle with the least amount of stress or disruption facilitates attainment of outcome measures. When expected outcomes are not met, revise the nursing interventions or expected outcomes based on the patient’s needs or preferences. When outcomes are not met, ask questions such as “How has your appetite been?” “Have you noticed a change in your weight?” “How much would you like to weigh?” or “Have you changed your exercise pattern?”
Safety Guidelines for Nursing Skills
Ensuring patient safety is an essential role of the professional nurse. To ensure patient safety, communicate clearly with members of the health care team, assess and incorporate the patient’s priorities of care and preferences, and use the best evidence when making decisions about your patient’s care. When performing the skills in this chapter, remember the following points to ensure safe, individualized patient care.
• Use aseptic technique when preparing and delivering enteral feedings. Check agency policy for wearing gloves when handling feedings (Bankhead et al., 2009).
• Label enteral equipment with patient name, room number, formula name, rate, date and time of initiation, and nurse initials (Bankhead et al., 2009).
• Practice “right patient, right formula, right tube” by matching formula and rate to feeding order and verifying that an enteral tubing set connects formula to a feeding tube (Bankhead et al., 2009).
• Have a patient sit upright or elevate the head of the bed a minimum of 30 (preferably 45) degrees unless medically contraindicated for patients receiving enteral feedings (Bankhead et al., 2009).
• Trace all lines and tubing back to the patient to ensure that you have only enteral-to-enteral connections (Bankhead et al., 2009).
• Do not add food coloring or dye to EN. Use of dye has been linked to hypotension, metabolic acidosis, and death (Metheny et al., 2007).
• Refer to manufacturer guidelines to determine hang time for enteral feedings. Maximum hang time for formula is 8 hours in an open system and 24 to 48 hours in a closed, ready-to-hang system (if it remains closed). There is increased risk of bacterial growth in feedings that exceed the recommended hang time.
• Auscultation is not a reliable method for verification of nasogastric or nasointestinal tube placement because a tube inadvertently placed in the lungs, pharynx, or esophagus also transmits a sound similar to that of air entering the stomach (Metheny et al., 2007; Serna and McCarthy, 2006).
• Continuous enteral feedings and PN are always administered using an infusion pump.
Skill 44-1 Aspiration Precautions
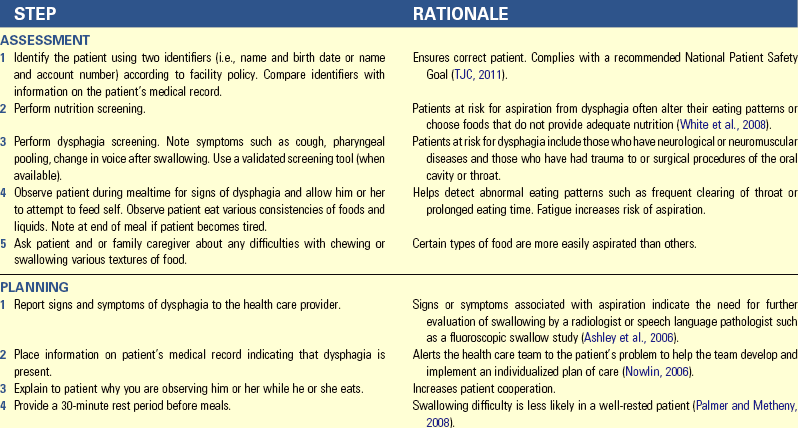

Unexpected Outcomes and Related Interventions:
1. Patient coughs, gags, complains of food “stuck in throat,” or has pockets of food in mouth.
• Patient may require a swallowing evaluation.
• Initiate consultation with a speech-language pathologist (SLP) for swallowing exercises and techniques to improve swallowing and reduce risk of aspiration.
• Notify health care provider and SLP of any symptoms that occurred during meal and which foods caused the symptoms.
• Document the following in the patient’s medical record: patient’s tolerance of various food textures, amount of assistance required, position during meal, absence or presence of any symptoms of dysphagia, and amount eaten.
• Report any coughing, gagging, choking, or swallowing difficulties to nurse in charge or health care provider.
Skill 44-2 Inserting a Small-bore Nasoenteric Tube for Enteral Feedings
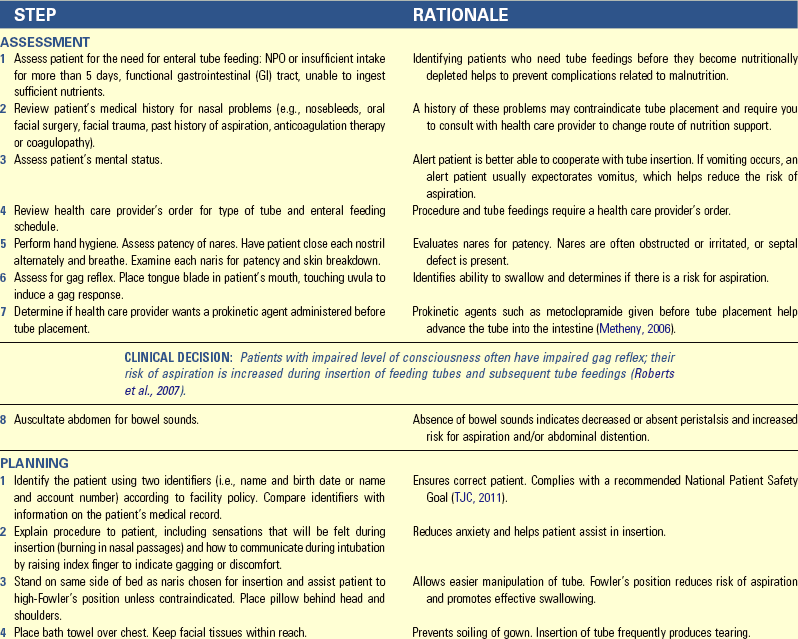
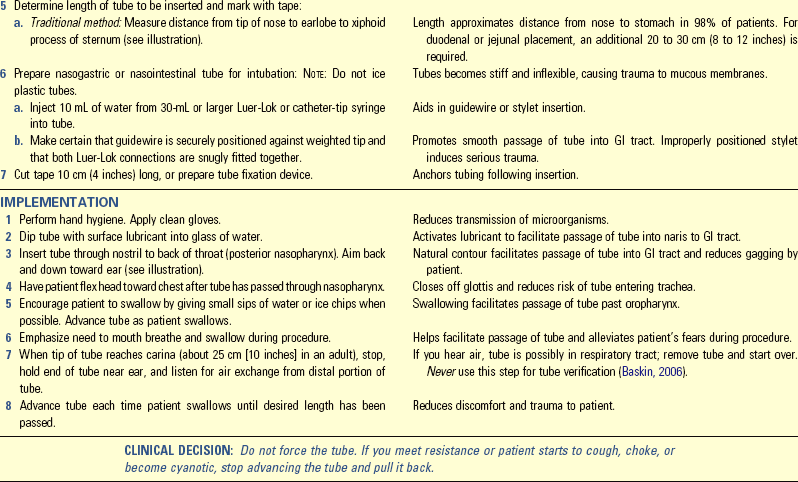
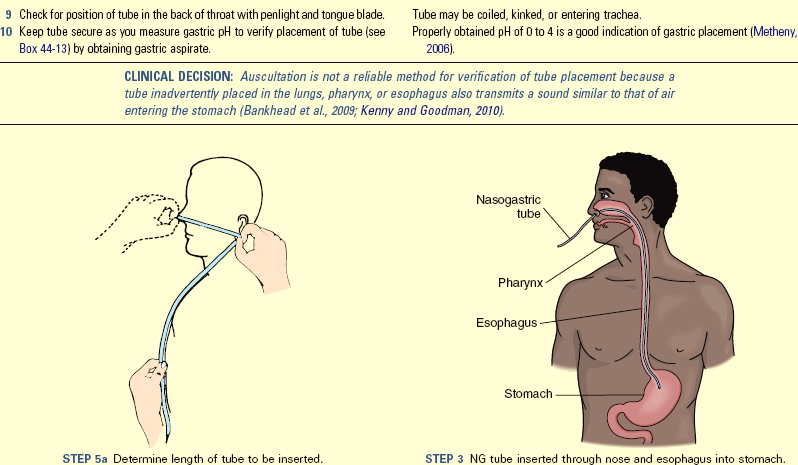
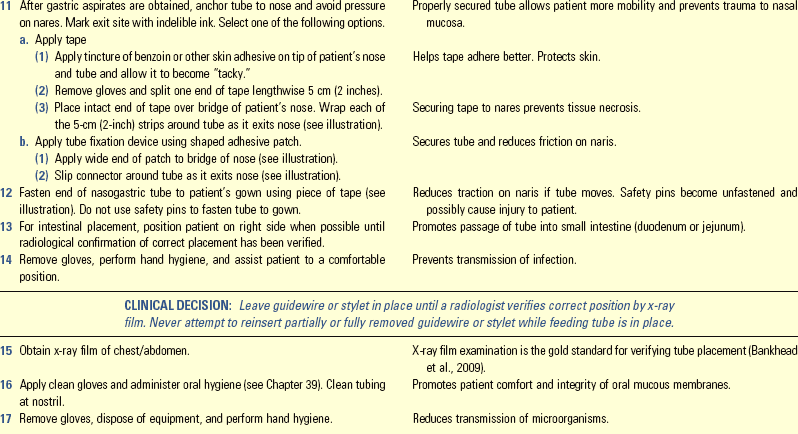
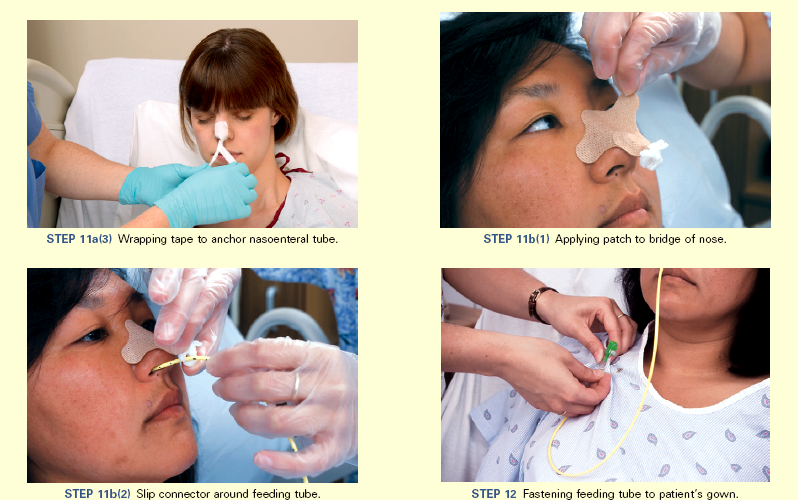

Unexpected Outcomes and Related Interventions:
1. Aspiration of stomach contents into the respiratory tract
• Suction nasotracheally and orotracheally.
• Consult health care provider immediately to order chest x-ray film examination.
2. Displacement of feeding tube to another site (e.g., from duodenum to stomach, mark at exit site if tube is moved); possibly occurs when patient coughs or vomits
Skill 44-3 Administering Enteral Feedings via Nasoenteric, Gastrostomy, or Jejunostomy Tubes
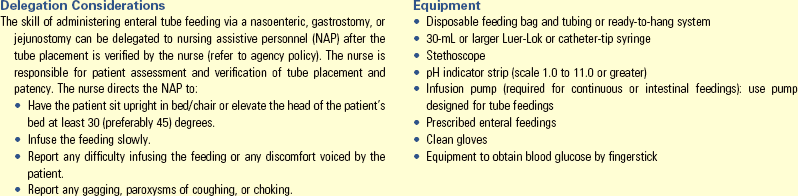
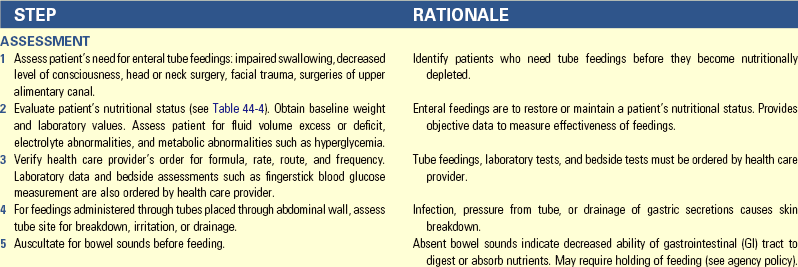
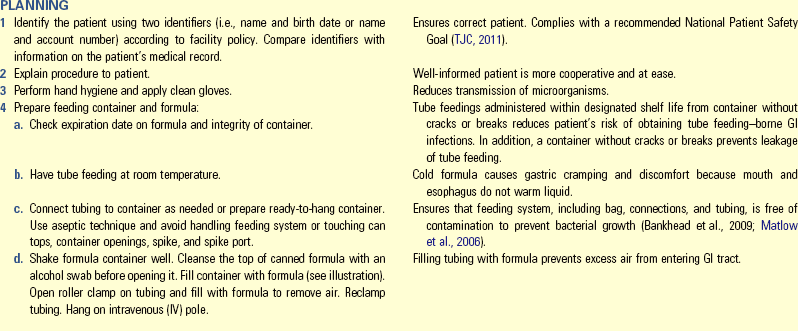


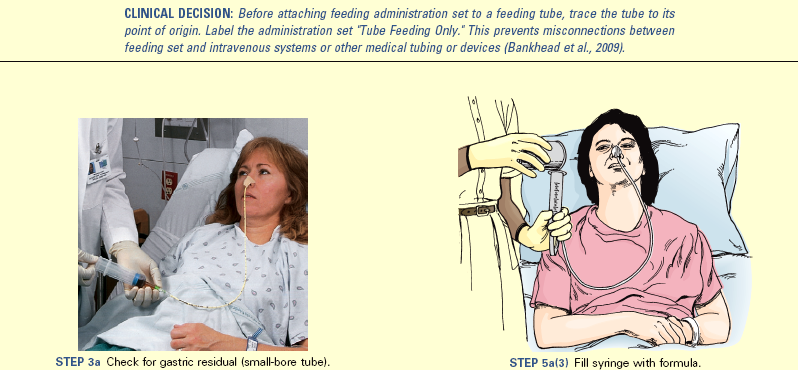
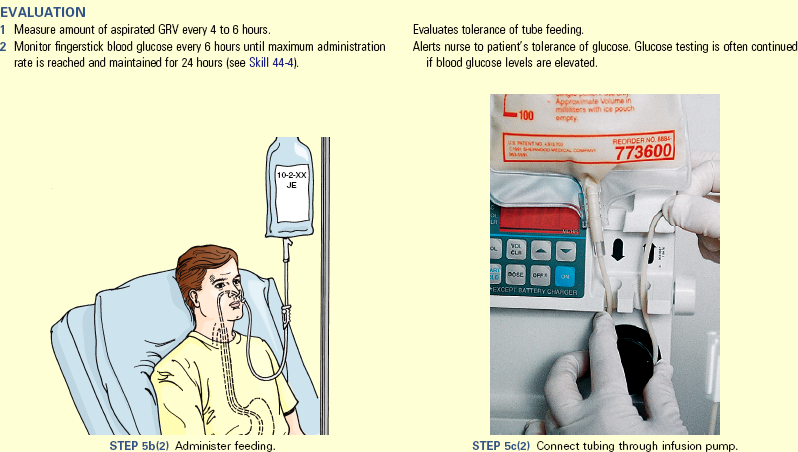

Unexpected Outcomes and Related Interventions (in addition to those in Skill 44-1):
1. GRV exceeds 250 mL for each of two consecutive assessments (see agency policy).
• Hold feeding and notify health care provider..
• Maintain patient in upright position in chair/bed or elevate HOB at least 30 (preferably 45) degrees.
2. Patient develops diarrhea 3 times or more in 24 hours.
• Notify health care provider.
• Institute skin care measures.
• Consider change in antibiotics, only for patients receiving antibiotics.
3. Patient develops nausea, vomits, and aspirates formula when gastric emptying is delayed or formula is administered too rapidly and produces vomiting.
• Teach patient or family caregiver how to determine correct placement of feeding tube.
• Inform patient or family caregiver of signs associated with pulmonary aspiration, delayed gastric emptying.
• Reinforce signs and symptoms associated with feeding tube complications and when to call health care provider.
• Explain and demonstrate how to do skin care around gastrostomy or jejunostomy tube and explain signs and symptoms of infection at the insertion site.
Skill 44-4 Blood Glucose Monitoring
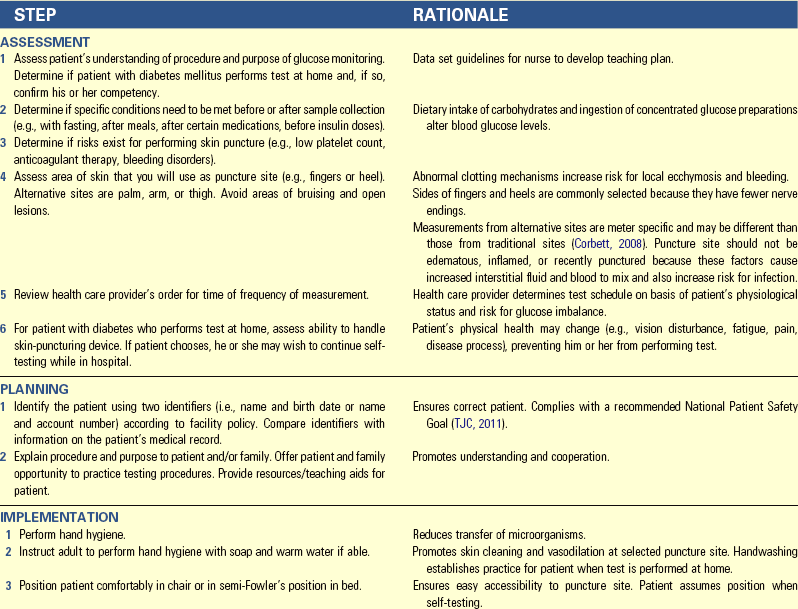

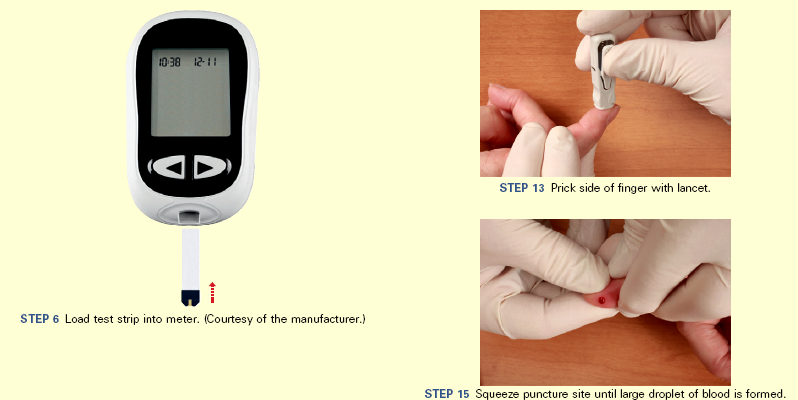


Unexpected Outcomes and Related Interventions:
1. Puncture site continues to bleed or is bruised.
2. Glucose meter malfunctions.
3. Blood glucose level is above or below target range.
• Continue to monitor patient.
• Follow agency protocol for laboratory confirmation testing of very high or very low results. Laboratory testing is generally considered more accurate.
• Check medical record to see if there is a medication order for deviations in glucose level; if not, notify health care provider.
• Administer insulin or carbohydrate source as ordered (depending on glucose level).
• Record glucose results on appropriate flow sheet and describe response, including presence or absence of pain or excessive oozing of blood at puncture site.
• Describe explanations or teaching provided in medical record.
• Report blood glucose levels out of target range and take appropriate action for hypoglycemia or hyperglycemia.
Key Points
• Ingestion of a diet balanced with carbohydrates, fats, proteins, vitamin, and minerals provides the essential nutrients to carry out the normal physiological functioning of the body throughout the life span.
• Through digestion food is broken down into its simplest form for absorption. Digestion and absorption occur mainly in the small intestine.
• Guidelines for dietary change recommend reduced fat, saturated fat, sodium, refined sugar, and cholesterol and increased intake of complex carbohydrates and fiber.
• Because improper nutrition affects all body systems, nutritional assessment includes a review of total physical assessment.
• Enteral feedings are for patients who are unable to ingest food but are able to digest and absorb food in the gastrointestinal tract.
• EN protects intestinal structure and function and enhances immunity.
• TPN supplies essential nutrients in appropriate amounts to support life through the administration of a concentrated nutrient solution into the superior vena cava near the right atrium of the heart.
• MNT is a recognized treatment modality for both acute and chronic disease states.
• One of the most important responsibilities of a nurse administering enteral feedings is to take precautions to prevent patients from aspirating feeding formula.
• Special diets alter the composition, texture, digestibility, and residue of foods to suit the patient’s particular needs.
Clinical Application Questions
Preparing for Clinical Practice
1. As part of your next visit to the senior citizens’ center where Mrs. Cooper lives, you plan to present a program to the residents to help decrease their risk of cardiovascular disease. Using your knowledge of medical nutrition therapy (MNT), summarize five points that you will include in the program for the residents.
2. Six months later, Mrs. Cooper is admitted to the hospital for a viral infection. She had a recent weight loss of 6 pounds in the week before admission and lost an additional 4 pounds during the week of hospitalization. Her appetite is poor; she has frequent nausea and vomiting. Her abdomen is soft and nontender, and bowel sounds are present. The health care provider orders enteral feedings to be started.
a. What type of tube should be selected?
b. How is the tube placement verified?
3. Two years after her husband died, Mrs. Cooper suffered a stroke and developed dysphagia. Develop a plan of care for assisting Mrs. Cooper with meals to reduce the risk of aspiration.
![]() Answers to Clinical Application Questions can be found on the Evolve website.
Answers to Clinical Application Questions can be found on the Evolve website.
Are You Ready to Test Your Nursing Knowledge?
1. Which statement made by an adult patient demonstrates understanding of healthy nutrition teaching?
1. I need to stop eating red meat.
2. I will increase the servings of fruit juice to four a day.
3. I will make sure that I eat a balanced diet and exercise regularly.
4. I will not eat so many dark green vegetables and eat more yellow vegetables.
2. The nurse teaches a patient who has had surgery to increase which nutrient to help with tissue repair?
3. The nurse is caring for a patient experiencing dysphagia. Which interventions help decrease the risk of aspiration during feeding? (Select all that apply.)
1. Sit the patient upright in a chair.
2. Give liquids at the end of the meal.
3. Place food in the strong side of the mouth.
4. Provide thin foods to make it easier to swallow.
5. Feed the patient slowly, allowing time to chew and swallow.
6. Encourage patient to lie down to rest for 30 minutes after eating.
4. The nurse suspects that the patient receiving parenteral nutrition (PN) through a central venous catheter (CVC) has an air embolus. What action does the nurse need to take first?
1. Raise head of bed to 90 degrees
2. Turn patient to left lateral decubitus position
5. Which action is initially taken by the nurse to verify correct position of a newly placed small-bore feeding tube?
1. Placing an order for x-ray film examination to check position
2. Confirming the distal mark on the feeding tube after taping
3. Testing the pH of the gastric contents and observing the color
4. Auscultating over the gastric area as air is injected into the tube
6. The catheter of the patient receiving parenteral nutrition (PN) becomes occluded. Place the steps for caring for the occluded catheter in the order in which the nurse would perform them.
1. Attempt to aspirate a clot.
2. Temporarily stop the infusion.
7. Based on knowledge of peptic ulcer disease (PUD), the nurse anticipates the presence of which bacteria when reviewing the laboratory data for a patient suspected of having PUD?
8. The nurse is assessing a patient receiving enteral feedings via a small-bore nasogastric tube. Which assessment findings need further intervention?
1. Gastric pH of 4.0 during placement check
2. Weight gain of 1 pound over the course of a week
3. Active bowel sounds in the four abdominal quadrants
4. Gastric residual aspirate of 350 mL for the second consecutive time
9. The home care nurse is seeing the following patients. Which patient is at greatest risk for experiencing inadequate nutrition?
1. A 55-year-old obese man recently diagnosed with diabetes mellitus
2. A recently widowed 76-year-old woman recovering from a mild stroke
3. A 22-year-old mother with a 3-year-old toddler who had tonsillectomy surgery
4. A 46-year-old man recovering at home following coronary artery bypass surgery
10. The nurse is checking feeding tube placement. Place the steps in the proper sequence.
1. Draw 5 to 10 mL gastric aspirate into syringe.
3. Mix aspirate in syringe and place in medicine cup.
4. Observe color of gastric aspirate.
5. Perform hand hygiene and put on clean gloves.
11. Which statement made by a patient of a 2-month-old infant requires further education?
1. I’ll continue to use formula for the baby until he is a least a year old.
2. I’ll make sure that I purchase iron-fortified formula.
3. I’ll start feeding the baby cereal at 4 months.
4. I’m going to alternate formula with whole milk starting next month.
12. The nurse is teaching a program on healthy nutrition at the senior community center. Which points should be included in the program for older adults? (Select all that apply.)
1. Avoid grapefruit and grapefruit juice, which impair drug absorption.
2. Increase the amount of carbohydrates for energy.
3. Take a multivitamin that includes vitamin D for bone health.
13. The nurse sees the nursing assistive personnel (NAP) perform the following for a patient receiving continuous enteral feedings. What intervention does the nurse need to address immediately with the NAP? The NAP:
1. Fastens the tube to the gown with tape.
2. Places the patient supine while giving a bath.
14. The patient receiving total parenteral nutrition (TPN) asks the nurse why his blood glucose is being checked since he does not have diabetes. What is the best response by the nurse?
1. TPN can cause hyperglycemia, and it is important to keep your blood glucose level in an acceptable range.
2. The high concentration of dextrose in the TPN can give you diabetes; thus you need to be monitored closely.
3. Monitoring your blood glucose level helps to determine the dose of insulin that you need to absorb the TPN.
4. Checking your blood glucose level regularly helps to determine if the TPN is effective as a nutrition intervention.
15. The nurse is performing blood glucose monitoring for a patient receiving parenteral nutrition. Place the steps of the procedure in the correct sequence.
Answers: 1. 3; 2. 2; 3. 1, 3, 5; 4. 2; 5. 1; 6. 2, 3, 1, 4; 7. 4; 8. 4; 9. 2; 10. 5, 2, 1, 4, 3, 6, 7; 11. 4; 12. 1, 3, 4; 13. 2; 14. 1; 15. 6, 2, 3, 1, 5, 4, 7.
References
American Cancer Society (ACS), American Cancer Society guidelines on nutrition and physical activity for cancer prevention 2011. http://www.cancer.org/acs/groups/cid/documents/webcontent/002577-pdf.pdf [Accessed October 17, 2011].
American Diabetes Association (ADA). Position statement: nutrition recommendations and interventions for diabetes. Diabetes Care. 2008;31(suppl 1):561.
American Dietetic Association. Position of the American Dietetic Association: Promoting and supporting breastfeeding. J Am Diet Assoc. 2009;109:1926.
American Dietetic Association. Position of the American Dietetic Association, American Society for Nutrition, and Society for Nutrition Education: Food and nutrition programs for community-residing older adults. J Am Diet Assoc. 2010;110(3):463.
American Dietetic Association. Position of the American Dietetic Association: Integration of medical nutrition therapy and pharmacotherapy. J Am Diet Assoc. 2010;110(6):950.
American Heart Association, Diet and lifestyle recommendations revision 2010. http://www.heart.org/HEARTORG/GettingHealthy/Diet-and-Lifestyle-Recommendations_UCM_305855_Article.jsp [Accessed October 17, 2011].
Andrews, MM, Boyle, JS. Transcultural concepts in nursing care, ed 5. Philadelphia: Lippincott Williams & Wilkins; 2008.
Ashley, J, et al. Speech, language, and swallowing disorders in the older adult. Clin Geriatr Med. 2006;22:291.
Baskin, WN. Acute complications associated with bedside placement of feeding tubes. Nutr Clin Pract. 2006;21:40.
Budd, GM, Hayman, LL. Addressing the childhood obesity crisis: a call to action. Matern Child Nurs. 2008;33(2):111.
Charney, P. Nutrition screening vs nutrition assessment: how do they differ? Nutr Clin Pract. 2008;23(4):366.
Corbett, JV. Laboratory tests and diagnostic procedures with nursing diagnoses, ed 7. Upper Saddle River, NJ: Prentice-Hall; 2008.
Daniels, J. Obesity: America’s epidemic. Am J Nurs. 2006;106(1):40.
Delegge, DH. Managing gastric residual volumes in the critically ill patient: An update. Curr Opin Nutr Metab Care. 2011;14:193.
DiMaria-Ghalili, RA, Nutrition in the elderly: nursing standard of practice protocol: nutrition in aging 2008. http://consultgerirn.org/topics/nutrition_in_the_elderly/want_to_know_more [Accessed October 17, 2011].
Dossey, B. Florence Nightingale: mystic, visionary, and healer. Philadelphia: Springhouse; 1999.
Ebersole, P, et al. Toward healthy aging: human needs and nursing response, ed 7. St Louis: Mosby; 2008.
Giger, JN, Davidhizar, RE. Transcultural nursing: assessment and intervention, ed 5. St Louis: Mosby; 2008.
Hockenberry, MJ, Wilson, D. Wong’s nursing care of infants and children, ed 9. St Louis: Mosby; 2011.
Huether, SE, et al. Understanding pathophysiology, ed 4. St Louis: Mosby; 2008.
Institute of Medicine, Dietary reference intakes: essential nutrient guide 2006. http://iom.edu/Reports/2006/Dietary-Reference-Intakes-Essential-Guide-Nutrient-Requirements.aspx [Accessed October 31, 2010].
Khan, LK, et al. Recommended community strategies and measurements to prevent obesity in the United States. MMWR Morb Mortal Wkly Rep. 2009;58(RR-7):1.
Kondrup, J, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr. 2003;22(4):415.
Krondl, M, et al. Helping older adults meet nutritional challenges. J Nutr Elderly. 2008;27(3/4):2005.
Kruskall, LJ. Portion distortion: sizing up food servings. ACSM Health Fitness J. 2006;10(3):8.
Krzywda, EA, Meyer, D. Parenteral nutrition. In Alexander M, et al, eds.: Infusion nursing society infusion nursing: an evidence-based approach, ed 3, St Louis: Saunders, 2010.
Lehne, RA. Pharmacology for nursing care, ed 7. St Louis: Saunders; 2010.
Li, J, Hooker, NH. Childhood obesity and schools: evidence from the national survey of children’s health. J School Health. 2010;80(2):96.
Mason, P. Undernutrition in hospital: causes and consequences. Hosp Pharm. 2006;13:353.
Mayo Clinic Staff, Teen weight loss: healthy habits count 2009. http://www.mayoclinic.com/health/teen-weight-loss/WT00012 [Accessed October 17, 2011].
McCance, KL, et al. Pathophysiology: the biologic basis for disease in adults and children, ed 6. St Louis: Mosby; 2010.
McClave, SA, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. J Parenter Enter Nutr. 2009;33(3):277.
Meiner, SE. Gerontologic nursing, ed 4. St Louis: Mosby; 2011.
Metheny, NA, et al. Tracheobronchial aspiration of gastric contents in critically ill tube-fed patients: frequency, outcomes, and risk factors. Crit Care Med. 2006;34(4):1007.
Metheny, NA, et al. Gastric residual volume and aspiration in critically ill patients receiving gastric feedings. Am J Crit Care. 2008;17(6):512.
National Dysphagia Diet Task Force (NDDTF). National Dysphagia Diet: standardization for optimal care. Chicago: American Dietetic Association; 2002.
National Guideline Clearinghouse, Strategies to prevent central-line associated bloodstream infections in acute care hospitals 2008. http://www.guideline.gov/content.aspx?id=13395&search=prevention+of+healthcare+associated+infection [Accessed October 17, 2011].
Nix, S. Williams’ basic nutrition and diet therapy, ed 13. St Louis: Mosby; 2009.
Nowlin, A. The dysphagia dilemma: how you can help. RN. 2006;69(6):44.
O’Connor, L. Oral health care. In Capezuti E, et al, eds.: Evidence-based geriatric nursing protocols for best practice, ed 3, New York: Springer, 2008.
Pagana, KD, Pagana, TJ. Mosby’s diagnostic and laboratory test reference, ed 9. St Louis: Mosby; 2009.
Palmer, JL, Metheny, NA. Preventing aspiration in older adults with dysphagia. Am J Nurs. 2008;108(2):40.
Park, S, et al. Vitamin and mineral supplements: barriers and challenges for older adults. J Nutr Elderly. 2008;27(3/4):297.
Phillips, LD. Manual of IV therapeutics: evidence-based practice for infusion therapy,, ed 5. Philadelphia: FA Davis; 2010.
Rauen, CA, et al. Seven evidence-based practice habits: putting some sacred cows to pasture. Crit Care Nurs. 2008;28(2):98.
Robbins, J, et al. Team management of dysphagia in the institutional setting. J Nutr Elderly. 2007;26(3/4):59.
Roberts, S, et al. Devices and techniques for bedside enteral feeding tube placement. Nutr Clin Pract. 2007;22:412.
Rolandelli, RH, et al. Clinical nutrition: enteral feeding and tube feeding. Philadelphia: Saunders; 2005.
Serna, ED, McCarthy, MS. Heads up to prevent aspiration during enteral feeding. Nursing. 2006;36(1):76.
Siepler, J. Principles and strategies for monitoring home parenteral nutrition. Nutr Clin Pract. 2007;22(3):340.
Task Force for the Revision of Safe Practices for Parenteral Nutrition. Safe practices for parenteral nutrition. J Parenter Enter Nutr. 2004;28(6):S39.
The Joint Commission (TJC), 2011 National patient safety goals (NPGs) 2011. http://www.jointcommission.org/standards_information/npsgs.aspx
2010. Tolerable upper level intake. National Institutes of Health, 2010. http://ods.od.nih.gov/pubs/conferences/tolerable_upper_intake.pdf [Accessed October 19, 2010].
Touhy, TA, Jett, KF. Ebersole and Hess’ gerontological nursing healthy aging. St Louis: Mosby; 2010.
Tufts University. MyPlate for Older Adults. http://nutrition.tufts.edu/research/myplate-older-adults, 2011. [Accessed February 7, 2012].
US Department of Agriculture (USDA), Choose MyPlate 2011. http://www.choosemyplate.gov [Accessed October 17, 2011].
US Department of Agriculture (USDA), Shopping, cooking, and meal planning 2011. http://www.nutrition.gov/nal_display/index.php?info_center=11&tax_level=2&tax_subject=391&level3_id=0&level4_id=0&level5_id=0&topic_id=1756&&placement_default=0 [Accessed October 17, 2011].
US Department of Health and Human Services (USDHHS), Healthy people 2020 2010. http://www.healthypeople.gov/hp2020/objectives [Accessed October 17, 2011].
US Food and Drug Administration (USFDA). Appendix F. Calculate the percent daily value for the appropriate nutrients. http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/FoodLabelingNutrition/FoodLabelingGuide/ucm064928.htm, 2008. [Accessed October 17, 2011].
White, G, et al. Dysphagia: cause, assessment, and management. Geriatrics. 2008;3(5):15.
World Health Organization (WHO), Food security 2010. http://www.who.int/trade/glossary/story028/en [Accessed October 17, 2011].
Research References
AY Cichero, J, et al. Triaging dysphagia: nurse screening for dysphagia in an acute hospital. J Clin Nurs. 2009;18:1649.
Bankhead, R, et al. Enteral nutrition practice recommendations. JPEN J Parenter Enteral Nutr. 2009;33:122.
Befort, C, et al. Fruit, vegetable and fat intake among non-Hispanic black and non-Hispanic white adolescents: associations with home availability and food consumption settings. J Am Diet Assoc. 2006;106(3):367.
Bourgault, AN, et al. Development of evidence-based guidelines and critical care nurses’ knowledge of enteral feedings. Crit Care Nurse. 2007;27(4):17.
Chang, C, Roberts, B. Feeding difficulty in older adults with dementia. J Clin Nurs. 2008;17:2266.
Cirgin Ellett, ML. Important facts about intestinal feeding tube placement. Gastroenterol Nurs. 2006;29(2):112.
DeChicco, R, et al. Tip position of long-term central venous access devices used for parenteral nutrition. J Parenter Enter Nutr. 2007;31(5):382.
Edmiaston, J, et al. Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010;19(4):357.
Fox, MK, et al. Sources of energy and nutrients in the diets of infants and toddlers. J Am Diet Assoc. 2006;106(suppl 1):S28e1.
Frey, K, Ramsberger, G. Comparison of outcomes before and after implementation of a water protocol for patients with cerebrovascular accident and dysphagia. J Neurosci Nurs. 2011;43(3):165–170.
Guigoz, Y, Vellas, B. The Mini Nutritional Assessment (MNA) for grading the nutritional state of elderly patients: presentation of the MNA, history and validation. Nestle Nutr Workshop Ser Clin Perform Programme. 1999;1:3.
Guigoz, YB, et al. Assessing the nutritional status of the elderly: the Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev. 1996;54(1 pt 2):S59.
Huang, G, et al. Training in swallowing prevents aspiration pneumonia in stroke patients with dysphagia. J Int Med Res. 2006;34(3):303.
Kenny, DJ, Goodman, P. Care of the patient with enteral tube feeding: an evidence-based practice protocol. Nurs Res. 2010;59(1S):S22.
Khalid, I, et al. early enteral nutrition and outcomes of critically ill patients treated with vasopressors and mechanical ventilation. Am J Crit Care. 2010;19(3):261.
Kwon, HM, et al. The pneumonia score: a simple grading scale for prediction of pneumonia after acute stroke. Am J Infect Control. 2006;34(2):64.
Lennie, TA, et al. Factors influencing food intake in patients with heart failure: a comparison with healthy elders. J Cardiovasc Nurs. 2006;21(2):123.
Matlow, A, et al. Enteral tube hub as a reservoir for the transmissible enteric bacteria. Am J Infect Control. 2006;34(3):131.
Metheny, NA. Preventing respiratory complications of tube feedings: evidence-based practice. Am J Crit Care. 2006;15(4):360.
Metheny, NA, et al. Complications related to feeding tube placement. Curr Opin Gastroenterol. 2007;23:178.
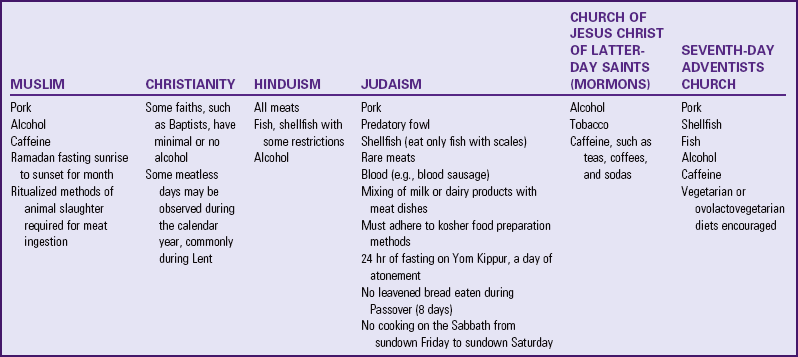
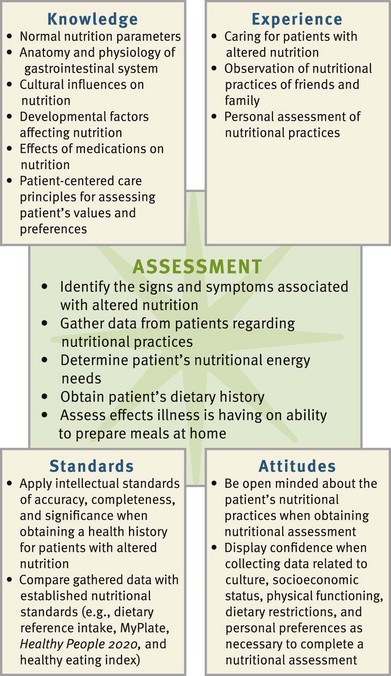
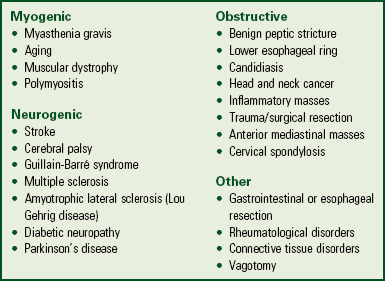
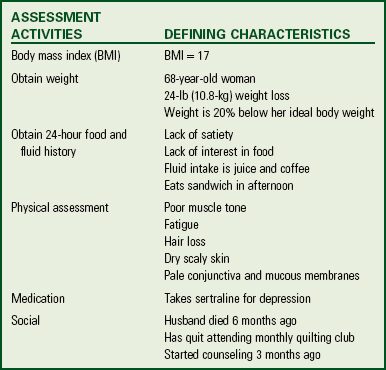




 to 1 lb (0.2 to 0.45 kg) per week.
to 1 lb (0.2 to 0.45 kg) per week.