Fluid, Electrolyte, and Acid-Base Balance
• Describe the processes involved in regulating extracellular fluid volume, body fluid osmolality, and fluid distribution.
• Describe the processes involved in regulating plasma concentrations of potassium, calcium, magnesium, and phosphate ions.
• Describe the processes involved in regulating acid-base balance.
• Describe common fluid, electrolyte, and acid-base imbalances.
• Identify risk factors for fluid, electrolyte, and acid-base imbalances.
• Choose appropriate clinical assessments for specific fluid, electrolyte, and acid-base imbalances.
• Interpret basic fluid, electrolyte, and acid-base laboratory values.
• Apply the nursing process when caring for patients with fluid, electrolyte, and acid-base imbalances.
• Discuss purpose and procedure for initiation and maintenance of intravenous therapy.
• Calculate an intravenous flow rate.
• Describe how to measure and record fluid intake and output.
• Explain how to change intravenous solutions and tubing and discontinue an infusion.
• Describe potential complications of intravenous therapy and what to do if they occur.
• Discuss the procedure for initiating a blood transfusion and interventions to manage a transfusion reaction.
http://evolve.elsevier.com/Potter/fundamentals/
• Skills Performance Checklists
• Interactive Learning Activities
Fluid surrounds all the cells in the body and is also inside cells. Body fluids contain electrolytes such as sodium and potassium; they also have a certain degree of acidity. Fluid, electrolyte, and acid-base balances within the body maintain the health and function of all body systems. The characteristics of body fluids influence body system function because of their effects on cell function. These characteristics include the fluid amount (volume), concentration (osmolality), composition (electrolyte concentration), and degree of acidity (pH). All of these characteristics have regulatory mechanisms, which keeps them in balance for normal function. In this chapter you learn how the body normally maintains fluid, electrolyte, and acid-base balance. You also learn how imbalances develop; how various fluid, electrolyte, and acid-base imbalances affect patients; and ways to help patients maintain or restore balance safely.
Scientific Knowledge Base
This section provides the foundation for your critical thinking regarding patients who have or are at risk of having, fluid, electrolyte, or acid-base imbalances.
Location and Movement of Water and Electrolytes
Water makes up a substantial proportion of body weight. In fact, about 60% of the body weight of an adult man is water. This proportion decreases with age; approximately 50% of an older man’s weight is water. Women typically have less water content than men. Obese people have less water in their bodies than lean people because fat contains less water than muscle. The term fluid means water that contains dissolved or suspended substances such as glucose, mineral salts, and proteins.
Fluid Compartments
Body fluids are located in two distinct compartments: extracellular fluid (ECF) outside the cells, and intracellular fluid (ICF) inside the cells (Fig. 41-1). In adults ICF is approximately two thirds of total body water. ECF is approximately one third of total body water. ECF has two major divisions (intravascular fluid and interstitial fluid) and a minor division (transcellular fluids). Intravascular fluid is the liquid portion of the blood (i.e., the plasma). Interstitial fluid is located between the cells and outside the blood vessels. Transcellular fluids such as cerebrospinal, pleural, peritoneal, and synovial fluids are secreted by epithelial cells (Hall, 2011).
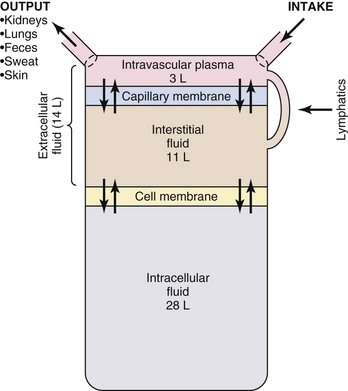
FIG. 41-1 Body fluid compartments. (From Hall JE: Guyton and Hall textbook of medical physiology, ed 12, Philadelphia, 2011, Saunders.)
Fluid in the body compartments contains mineral salts known technically as electrolytes. An electrolyte is a compound that separates into ions (charged particles) when it dissolves in water. Ions that are positively charged are called cations; ions that are negatively charged are called anions. Cations in body fluids are sodium (Na+), potassium (K+), calcium (Ca2+), and magnesium ions (Mg2+). Anions in body fluids are chloride (Cl−) and bicarbonate (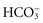 ). Anions and cations combine to make salts. If you put table salt (NaCl) in water, it separates into Na+ and Cl−. Other combinations of anions and cations do the same. Clinical laboratories usually report electrolyte measurements in milliequivalents per liter (mEq/L) or millimoles per liter (mmol/L), two different units of concentration (Table 41-1). Millimoles per liter represent the number of milligrams of the electrolyte divided by its molecular weight that are contained in a liter of the fluid being measured (usually blood plasma or serum). Milliequivalents per liter is the millimoles per liter multiplied by the electrolyte charge (e.g., 1 for Na+, 2 for Ca2+). A milliequivalent of one electrolyte can combine with a milliequivalent of another electrolyte, which is why this measurement unit is used (Rose, 2011).
). Anions and cations combine to make salts. If you put table salt (NaCl) in water, it separates into Na+ and Cl−. Other combinations of anions and cations do the same. Clinical laboratories usually report electrolyte measurements in milliequivalents per liter (mEq/L) or millimoles per liter (mmol/L), two different units of concentration (Table 41-1). Millimoles per liter represent the number of milligrams of the electrolyte divided by its molecular weight that are contained in a liter of the fluid being measured (usually blood plasma or serum). Milliequivalents per liter is the millimoles per liter multiplied by the electrolyte charge (e.g., 1 for Na+, 2 for Ca2+). A milliequivalent of one electrolyte can combine with a milliequivalent of another electrolyte, which is why this measurement unit is used (Rose, 2011).
Fluid that contains a large number of dissolved particles is more concentrated than the same amount of fluid that contains only a few particles. Osmolality of a fluid is a measure of the number of particles per kilogram of water. Some particles (e.g., urea) pass easily through cell membranes; others such as Na+ cannot cross easily. The particles that cannot cross cell membranes easily (nonpermeant particles) determine tonicity of a fluid (Caon, 2008). A fluid with the same concentration of nonpermeant particles as normal blood is called isotonic. A hypotonic solution is more dilute than the blood, and a hypertonic solution is more concentrated than normal blood (Fig. 41-2).
Movement of Water and Electrolytes
Active transport, diffusion, osmosis, and filtration are processes that move water and electrolytes between body compartments. These processes maintain equal osmolality in all compartments while allowing for different electrolyte concentrations.
Active Transport: Fluids in different body compartments have different concentrations of electrolytes that are necessary for normal function. For example, concentrations of Na+, Cl−, and 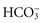 are higher in the ECF than in the ICF, whereas the concentrations of K+, Mg2+, and PO3−4 are higher in the ICF than in the ECF. Cells maintain their high intracellular electrolyte concentration by active transport. Active transport requires energy in the form of adenosine triphosphate (ATP) to move electrolytes across cell membranes against the concentration gradient (from areas of lower concentration to areas of higher concentration). One example of active transport is the sodium-potassium pump, which moves Na+ out of a cell and K+ into it, keeping ICF lower in Na+ and higher in K+ than the ECF.
are higher in the ECF than in the ICF, whereas the concentrations of K+, Mg2+, and PO3−4 are higher in the ICF than in the ECF. Cells maintain their high intracellular electrolyte concentration by active transport. Active transport requires energy in the form of adenosine triphosphate (ATP) to move electrolytes across cell membranes against the concentration gradient (from areas of lower concentration to areas of higher concentration). One example of active transport is the sodium-potassium pump, which moves Na+ out of a cell and K+ into it, keeping ICF lower in Na+ and higher in K+ than the ECF.
Diffusion: Diffusion is passive movement of electrolytes or other particles down the concentration gradient (from areas of higher concentration to areas of lower concentration). Within a body compartment electrolytes diffuse easily by random movements until the concentration is the same in all areas. However, diffusion of electrolytes across cell membranes requires proteins that serve as ion channels. For example, when a sodium channel in a cell membrane is open, Na+ diffuses passively across the cell membrane into the ICF because concentration is lower in the ICF. Opening of ion channels is tightly controlled and plays an important part in muscle and nerve function.
Osmosis: Water moves across cell membranes by osmosis, a process by which water moves through a membrane that separates fluids with different particle concentrations (Fig. 41-3). Cell membranes are semipermeable, which means that water crosses them easily but they are not freely permeable to many types of particles, including electrolytes such as sodium and potassium. These semipermeable cell membranes separate interstitial fluid from ICF. The fluid in each of these compartments exerts osmotic pressure, an inward-pulling force caused by particles in the fluid. The particles already inside the cell exert ICF osmotic pressure, which tends to pull water into the cell. The particles in the interstitial fluid exert interstitial fluid osmotic pressure, which tends to pull water out of the cell. Water moves into the compartment that has a higher osmotic pressure (inward-pulling force) until the particle concentration is equal in the two compartments.
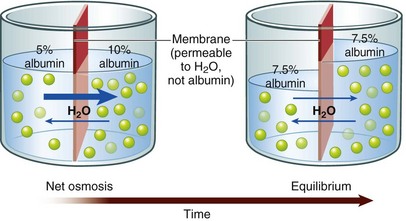
FIG. 41-3 Osmosis moves water through semipermeable membrane. (From Patton KT, Thibodeau GA: Anatomy and physiology, ed 7, St Louis, 2010, Mosby.)
If the particle concentration in the interstitial compartment changes, osmosis occurs rapidly and moves water into or out of cells to equalize the osmotic pressures. For example, when a hypotonic solution (more dilute than normal body fluids) is administered intravenously, it dilutes the interstitial fluid, decreasing its osmotic pressure below intracellular osmotic pressure. Water moves rapidly into cells until the two osmotic pressures are equal again. On the other hand, infusion of a hypertonic intravenous (IV) solution (more concentrated than normal body fluids) causes water to leave cells by osmosis to equalize the osmolality between interstitial and intracellular compartments.
Filtration: Fluid moves into and out of capillaries (between the vascular and interstitial compartments) by the process of filtration (Fig. 41-4). Filtration is the net effect of four forces, two that tend to move fluid out of capillaries and small venules and two that tend to move fluid back into them (Rose, 2011). Hydrostatic pressure is the force of the fluid pressing outward against a surface. Similarly, capillary hydrostatic pressure is a relatively strong outward-pushing force that helps move fluid from capillaries into the interstitial area. Interstitial fluid hydrostatic pressure is a weaker opposing force that tends to push fluid back into capillaries.
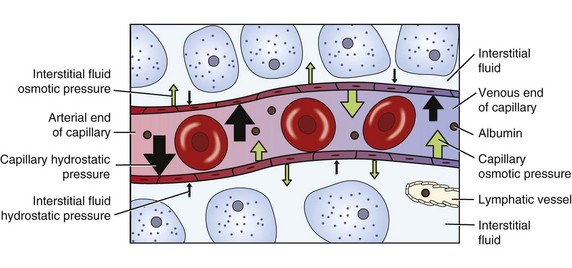
FIG. 41-4 Capillary filtration moves fluid between vascular and interstitial compartments. (From Copstead LC, Banasik JL: Pathophysiology, ed 4, St Louis, 2010, Saunders.)
Blood contains albumin and other proteins known as colloids. These proteins are much larger than electrolytes, glucose, and other molecules that dissolve easily. Most colloids are too large to leave capillaries in the fluid that is filtered, so they remain in the blood. Because they are particles, colloids exert osmotic pressure. Blood colloid osmotic pressure, also called oncotic pressure, is an inward-pulling force caused by blood proteins that helps move fluid from the interstitial area back into capillaries. Interstitial fluid colloid osmotic pressure normally is a very small opposing force.
At the arterial end of a normal capillary, capillary hydrostatic pressure is strongest, and fluid moves from the capillary into the interstitial area, bringing nutrients to cells. At the venous end capillary hydrostatic pressure is weaker, and the colloid osmotic pressure of the blood is stronger. Thus fluid moves into the capillary at the venous end, removing waste products from cellular metabolism. Lymph vessels remove any extra fluid and proteins that have leaked into the interstitial fluid.
Disease processes and other factors that alter these forces may cause accumulation of excess fluid in the interstitial space, known as edema. For example, people with heart failure develop edema. In this situation, venous congestion from a weakened heart, which no longer pumps effectively, increases capillary hydrostatic pressure, causing edema by moving excessive fluid into the interstitial space. Inflammation is another cause of edema. It increases capillary blood flow and allows capillaries to leak colloids into the interstitial space. The resulting increased capillary hydrostatic pressure and increased interstitial colloid osmotic pressure produce localized edema in the inflamed tissues.
Fluid Balance
Fluid homeostasis is the dynamic interplay of three processes: fluid intake and absorption, fluid distribution, and fluid output (Felver, 2010b). Human total daily fluid output consists of hypotonic sodium-containing fluid. People must have intake of an equivalent amount of hypotonic sodium-containing fluid (or water plus foods with some salt) to maintain fluid balance.
Fluid Intake
Fluid intake occurs orally through drinking but also through eating because most foods contain some water. Food metabolism creates additional water. Average fluid intake from these routes for healthy adults is about 2300 mL, although it varies widely (Table 41-2). Other routes of fluid intake include IV, rectal (e.g., enemas), and irrigation of body cavities that can absorb fluid.
TABLE 41-2
Healthy Adult Average Daily Fluid Intake and Output
| FLUID INTAKE | NORMAL | PROLONGED HEAVY EXERCISE |
| Fluids ingested: | ||
| Oral | 1100-1400 mL | ? |
| Foods | 800-1000 mL | ? |
| Metabolism | 300 mL | 200 mL |
| TOTAL | 2200-2700 mL | ? |
| FLUID OUTPUT | ||
| Skin (insensible and sweat) | 500-600 mL | 5350 mL |
| Insensible-lungs | 400 mL | 650 mL |
| GI | 100-200 mL | 100 mL |
| Urine | 1200-1500 mL | 500 mL |
| TOTAL | 2200-2700 mL | 6600 mL |
Data from Heitz UE, Horne MM: Mosby’s pocket guide series: Fluid, electrolyte and acid base balance, ed 5, St Louis, 2005, Mosby; Hall JE: Guyton and Hall textbook of medical physiology, ed 12, Philadelphia, 2011, Saunders.
Although you might think that the major regulator of oral fluid intake is thirst, habit and social reasons actually account for most fluid intake (Johnson, 2007). Thirst, the conscious desire for water, is an important regulator of fluid intake when plasma osmolality increases (osmoreceptor-mediated thirst) or the blood volume decreases (baroreceptor-mediated thirst and angiotensin II– and III–mediated thirst). The thirst-control mechanism is located within the hypothalamus in the brain (Fig. 41-5). Osmoreceptors continually monitor plasma osmolality; when it increases, they cause thirst by stimulating neurons in the hypothalamus. Dry oral mucous membranes also cause thirst. People who are alert can obtain fluid or communicate their thirst to others, and fluid intake restores fluid balance. Infants, patients with neurological or psychological problems, and some older adults who are unable to perceive or communicate their thirst are at risk for dehydration.
Fluid Distribution
The term fluid distribution means the movement of fluid among its various compartments. Fluid distribution between the extracellular and intracellular compartments occurs by osmosis. Fluid distribution between the vascular and interstitial portions of the ECF occurs by filtration.
Fluid Output
Fluid output normally occurs through four organs: the skin, lungs, gastrointestinal (GI) tract, and kidneys. Examples of abnormal fluid output include vomiting, wound drainage, or hemorrhage (Felver, 2010b). Table 41-2 shows average amounts of fluid excretion for healthy adults, although urine output varies greatly, depending on fluid intake. Insensible (not visible) water loss through the skin and lungs is continuous. It increases when a person has a fever or a recent burn to the skin (Metheny, 2010). Sweat, which is visible and contains sodium, occurs intermittently and increases fluid output substantially. The GI tract plays a vital role in fluid balance. Approximately 3 to 6 L of fluid moves into the GI tract daily and then returns again to the ECF. The average adult normally excretes only 100 mL of fluid each day through feces. However, diarrhea causes a large fluid output from the GI tract.
The kidneys are the major regulator of fluid output because they respond to hormones that influence urine production. When healthy adults drink more water, they increase urine production to maintain fluid balance. If they drink less water, sweat a lot, or lose fluid by vomiting, their urine volume decreases to maintain fluid balance. These adjustments primarily are caused by the actions of antidiuretic hormone (ADH), the renin-angiotensin-aldosterone system (RAAS), and atrial natriuretic peptides (ANPs) (Goldstein et al., 2010) (Fig. 41-6).
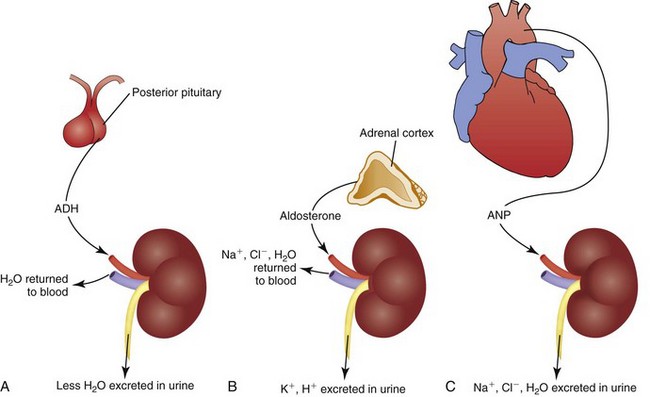
FIG. 41-6 Major hormones that influence renal fluid excretion. A, Antidiuretic hormone (ADH). B, Aldosterone. C, Atrial natriuretic peptide (ANP).
Antidiuretic Hormone: ADH regulates the osmolality of the body fluids by influencing how much water is excreted in urine. It is synthesized by neurons in the hypothalamus that release it from the posterior pituitary gland. ADH circulates in the blood to the kidneys, where it acts on the collecting ducts (Koeppen and Stanton, 2008). Its name—antidiuretic hormone—tells you what it does. It causes renal cells to resorb water, taking water from the renal tubular fluid and putting it back in the blood. This action decreases urine volume, concentrating the urine while diluting the blood by adding water to it (see Fig. 41-6, A).
People normally have some ADH release to maintain fluid balance. More ADH is released if body fluids become more concentrated. Factors that increase ADH levels include severely decreased blood volume (e.g., dehydration, hemorrhage), pain, stressors, and some medications.
ADH levels decrease if body fluids become too dilute. This allows more water to be excreted in urine, creating a larger volume of dilute urine and concentrating the body fluids back to normal osmolality. For example, ethyl alcohol decreases ADH release, which causes people to urinate frequently when they drink alcoholic beverages.
Renin-Angiotensin-Aldosterone System: The renin-angiotensin-aldosterone system (RAAS) regulates ECF volume by influencing how much sodium and water are excreted in urine. It also contributes to regulation of blood pressure. Specialized cells in the kidneys release the enzyme renin, which acts on angiotensinogen, an inactive protein secreted by the liver that circulates in the blood. Renin converts angiotensinogen to angiotensin I, which other enzymes in the lung capillaries convert to angiotensin II (Koeppen and Stanton, 2008). Angiotensin II has several functions, one of which is vasoconstriction in some vascular beds. The important fluid homeostasis functions of angiotensin II include stimulation of aldosterone release from the adrenal cortex.
Aldosterone circulates to the kidneys, where it causes resorption of sodium and water in isotonic proportion in the distal renal tubules. Removing sodium and water from the renal tubules and returning it to the blood increases the volume of the ECF (see Fig. 41-6, B). Aldosterone also contributes to electrolyte and acid-base balance by increasing urinary excretion of potassium and hydrogen ions.
To maintain fluid balance, normally some action of the RAAS occurs. Certain stimuli increase or decrease the activity of this system to restore fluid balance. For example, if hemorrhage or vomiting decreases the extracellular fluid volume (ECV), blood flow decreases through the renal arteries, and more renin is released. This increased RAAS activity causes more sodium and water retention, helping to restore ECV.
Atrial Natriuretic Peptide: Atrial natriuretic peptide (ANP) also regulates ECV by influencing how much sodium and water are excreted in urine. Cells in the atria of the heart release ANP when they are stretched (e.g., by an increased ECV). ANP is a weak hormone that inhibits ADH by increasing the loss of sodium and water in the urine (see Fig. 41-6, C). Thus ANP opposes the effect of aldosterone (Koeppen and Stanton, 2008).
Fluid Imbalances
If disease processes, medications, or other factors disrupt fluid intake or output, imbalances sometimes occur (Felver, 2010b). For example, with diarrhea there is an increase in fluid output, and a fluid imbalance (dehydration) occurs if fluid intake does not increase appropriately. There are two major types of fluid imbalances: volume imbalances and osmolality imbalances (Fig. 41-7). Volume imbalances are disturbances of the amount of fluid in the extracellular compartment. Osmolality imbalances are disturbances of the concentration of body fluids. Volume and osmolality imbalances occur separately or in combination.
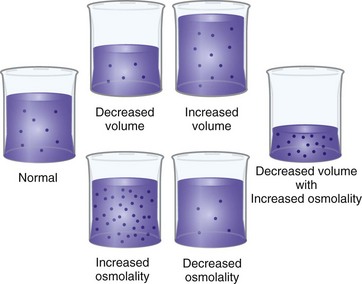
FIG. 41-7 Fluid volume and osmolality imbalances. (From Copstead LC, Banasik JL: Pathophysiology online for pathophysiology, ed 4, St Louis, 2010, Mosby.)
Extracellular Fluid Volume Imbalances
In an ECV imbalance there is either too little (ECV deficit) or too much (ECV excess) isotonic fluid. ECV deficit is present when there is insufficient isotonic fluid in the extracellular compartment. Remember that there is a lot of sodium in normal ECF. With ECV deficit, output of isotonic fluid exceeds intake of sodium-containing fluid. Because ECF is both vascular and interstitial, signs and symptoms arise from lack of volume in both of these compartments. Table 41-3 lists specific causes and signs and symptoms of ECV deficit. The term hypovolemia means decreased vascular volume and often is used when discussing ECV deficit (Metheny, 2010).
TABLE 41-3
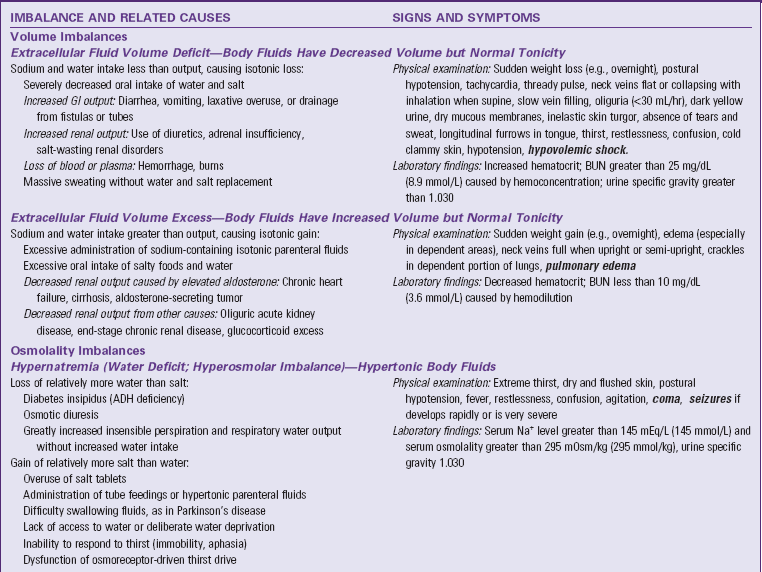
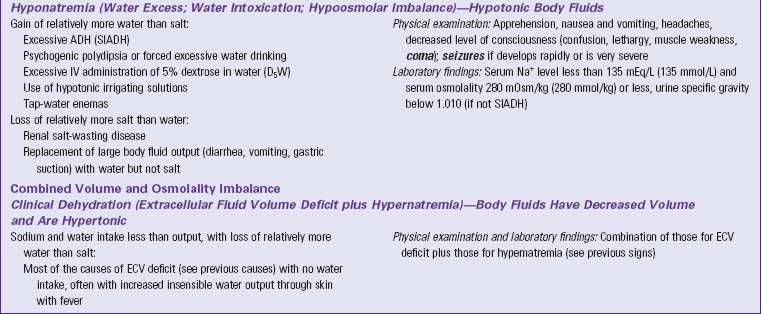
ADH, Antidiuretic hormone; BUN, blood urea nitrogen; ECV, extracellular fluid volume; GI, gastrointestinal; IV, intravenous; SIADH, syndrome of inappropriate secretion of antidiuretic hormone.
Text in bold italics denotes potentially life-threatening manifestations.
ECV excess occurs when there is too much isotonic fluid in the extracellular compartment. Intake of sodium-containing isotonic fluid has exceeded fluid output. For example, when you eat more salty foods than usual and drink water, you may notice that your ankles swell or rings on your fingers feel tight and you gain 2 lbs (1 kg) or more overnight. These are manifestations of mild ECV excess. See Table 41-3 for other specific causes and signs and symptoms.
Osmolality Imbalances
In an osmolality imbalance body fluids become hypertonic or hypotonic, which causes osmotic shifts of water across cell membranes. The osmolality imbalances are called hypernatremia and hyponatremia.
Hypernatremia, also called water deficit, is a hypertonic condition. Two general causes make body fluids too concentrated: loss of relatively more water than salt or gain of relatively more salt than water (Felver, 2010b). Table 41-3 lists specific causes under these categories. When the interstitial fluid becomes hypertonic, water leaves cells by osmosis, and they shrivel. Signs and symptoms of hypernatremia are those of cerebral dysfunction, which arise when brain cells shrivel. Hypernatremia may occur in combination with ECV deficit; this combined disorder is called clinical dehydration.
Hyponatremia, also called water excess or water intoxication, is a hypotonic condition. It arises from gain of relatively more water than salt or loss of relatively more salt than water (Felver, 2010b) (see Table 41-3). The excessively dilute condition of interstitial fluid causes water to enter cells by osmosis, causing the cells to swell. Signs and symptoms of cerebral dysfunction occur when brain cells swell.
Clinical Dehydration
ECV deficit and hypernatremia often occur at the same time; this combination is called clinical dehydration (Bryant, 2007). The ECV is too low, and the body fluids are too concentrated. Clinical dehydration is common with gastroenteritis or other causes of severe vomiting and diarrhea when people are not able to replace their fluid output with enough intake of dilute sodium-containing fluids. Signs and symptoms of clinical dehydration are those of both ECV deficit and hypernatremia (see Table 41-3).
Electrolyte Balance
You can best understand electrolyte balance by considering the three processes involved in electrolyte homeostasis: electrolyte intake and absorption, electrolyte distribution, and electrolyte output (Table 41-4) (Felver, 2010b). Although sodium is an electrolyte, it is not included here because serum sodium imbalances are the osmolality imbalances discussed previously.
TABLE 41-4
Electrolyte Intake and Absorption, Distribution, and Output
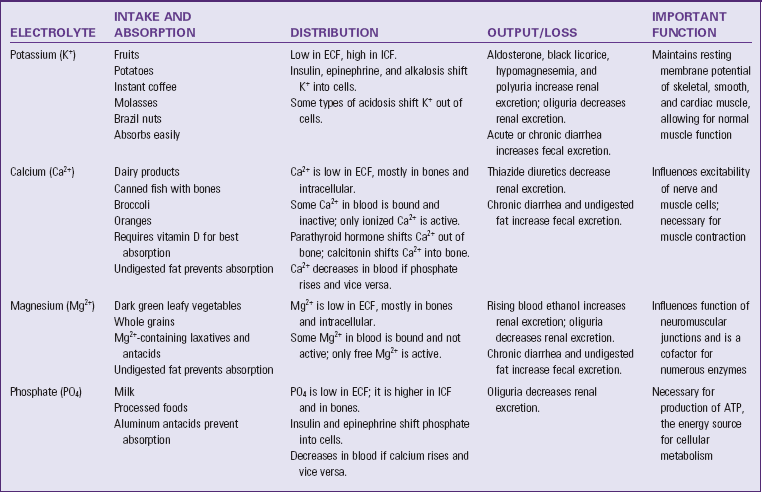
ATP, Adenosine triphosphate; ECF, extracellular fluid; ICF, intracellular fluid.
Electrolyte distribution is an important issue. Plasma concentrations of K+, Ca2+, Mg2+, and phosphate are very low compared with their concentrations in cells and bone (Metheny, 2010). These concentration differences are necessary for normal muscle and nerve function. The electrolyte values that you review from laboratory reports are measured in blood serum and do not measure intracellular levels.
Electrolyte output occurs through normal excretion in urine, feces, and sweat. Output also occurs through vomiting, drainage tubes or fistulas. When electrolyte output increases, electrolyte intake must increase to maintain electrolyte balance. Similarly, if electrolyte output decreases such as with oliguria, electrolyte intake must also decrease to maintain balance (Felver, 2010b).
Electrolyte Imbalances
Factors such as diarrhea, endocrine disorders, and medications that disrupt electrolyte homeostasis cause electrolyte imbalances. Electrolyte intake greater than electrolyte output or a shift of electrolytes from cells or bone into the ECF causes plasma electrolyte excess. Electrolyte intake less than electrolyte output or shift of electrolyte from the ECF into cells or bone causes plasma electrolyte deficit (Felver, 2010b).
Potassium Imbalances
Hypokalemia is abnormally low potassium concentration in the blood. Hypokalemia results from decreased potassium intake and absorption, a shift of potassium from the ECF into cells, and an increased potassium output (Table 41-5). Common causes of hypokalemia from increased potassium output include diarrhea, repeated vomiting, and use of potassium-wasting diuretics. People who have these conditions need to increase their potassium intake to reduce their risk of hypokalemia. Hypokalemia causes muscle weakness, which becomes life threatening if it includes respiratory muscles and potentially life-threatening cardiac dysrhythmias.
TABLE 41-5
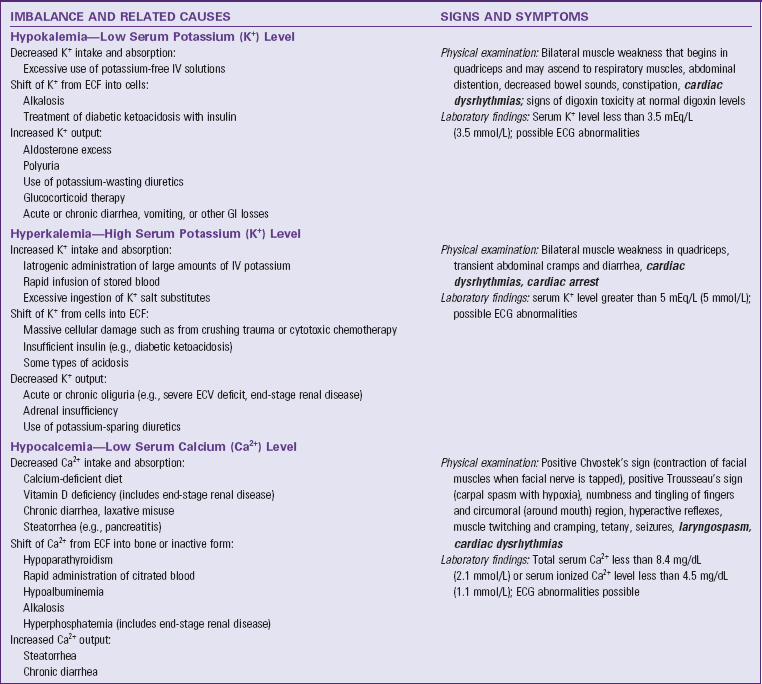
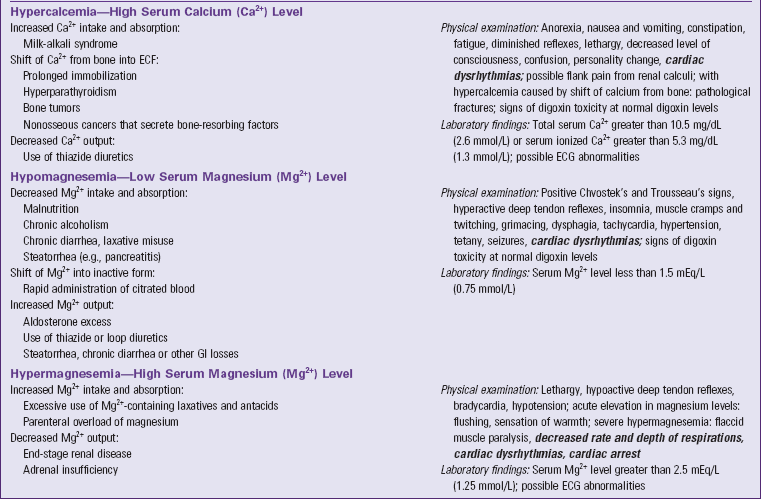
ECF, Extracellular fluid, ECG, electrocardiogram, ECV, extracellular fluid volume, GI, gastrointestinal, IV, intravenous. Text in bold italics denotes potentially life-threatening manifestations.
Data from Felver L: Fluid and electrolyte homeostasis and imbalances. In Copstead LC, Banasik JL: Pathophysiology, ed 4, St Louis, 2010b, Saunders; Goldstein MB et al: Fluid, electrolyte and acid-base physiology: a problem-based approach, ed 4, St Louis, 2010, Saunders; and Rose BD: Clinical physiology of acid-base disorders, ed 6, New York, 2011, McGraw-Hill.
Hyperkalemia is abnormally high potassium ion concentration in the blood. Its general causes are increased potassium intake and absorption, shift of potassium from cells into the ECF, and decreased potassium output (see Table 41-5). People who have oliguria (decreased urine output) are at high risk of hyperkalemia from the resultant decreased potassium output unless their potassium intake also decreases substantially. Understanding this principle helps you remember to check urine output before you administer IV solutions containing potassium. Hyperkalemia can cause muscle weakness, potentially life-threatening cardiac dysrhythmias, and cardiac arrest.
Calcium Imbalances
Hypocalcemia is abnormally low calcium concentration in the blood. The physiologically active form of calcium in the blood is ionized calcium. Total blood calcium also contains inactive forms that are bound to plasma proteins and small anions such as citrate. Factors that cause too much ionized calcium to shift to the bound forms cause symptomatic ionized hypocalcemia. Table 41-5 summarizes general causes. People who have acute pancreatitis frequently develop hypocalcemia because calcium binds to undigested fat in their feces and is excreted. This process decreases absorption of dietary calcium and also increases calcium output by preventing resorption of calcium contained in GI fluids. Hypocalcemia increases neuromuscular excitability, the basis for its signs and symptoms.
Hypercalcemia is abnormally high calcium concentration in the blood. Hypercalcemia results from increased calcium intake and absorption, shift of calcium from bones into the ECF, and decreased calcium output (see Table 41-5). patients with cancer often develop hypercalcemia because some cancer cells secrete chemicals into the blood that are related to parathyroid hormone. When these chemicals reach the bones, they cause shift of calcium from bones into the ECF. This weakens bones, and the person sometimes develops pathological fractures (i.e., bone breakage caused by forces that would not break a healthy bone). Hypercalcemia decreases neuromuscular excitability, the basis for its other signs and symptoms, the most common of which is lethargy.
Magnesium Imbalances
Hypomagnesemia is abnormally low magnesium concentration in the blood. Its general causes are decreased magnesium intake and absorption, shift of plasma magnesium to its inactive bound form, and increased magnesium output (see Table 41-5). Signs and symptoms are similar to those of hypocalcemia because hypomagnesemia also increases neuromuscular excitability.
Hypermagnesemia is abnormally high magnesium concentration in the blood (see Table 41-5). End-stage renal disease causes hypermagnesemia unless the person decreases magnesium intake to match the decreased output. Signs and symptoms are caused by decreased neuromuscular excitability, with lethargy and decreased deep tendon reflexes being most common.
Acid-Base Balance
For optimal cell function the body maintains a balance between acids and bases. Acid-base homeostasis is the dynamic interplay of three processes: acid production, acid buffering, and acid excretion (Felver, 2010a). Normal acid-base balance is maintained with acid excretion equal to acid production. Acids release hydrogen (H+) ions; bases (alkaline substances) take up H+ ions. The more H+ ions that are present, the more acidic is the solution.
The degree of acidity in blood and other body fluids is reported from the clinical laboratory as pH. The pH scale goes from 1.0 (very acid) to 14.0 (very alkaline; basic). A pH of 7.0 is considered neutral. The normal pH range of adult arterial blood is 7.35 to 7.45. Maintaining pH within this normal range is very important for optimal cell function. If the pH goes outside the normal range, enzymes within cells do not function properly; hemoglobin does not manage oxygen properly; and serious physiological problems occur, including death. Laboratory tests of a sample of arterial blood called arterial blood gases (ABGs) are used to monitor a patient’s acid-base balance (Kramer and Raymond, 2009) (Table 41-6).
Acid Production
Cellular metabolism constantly creates two types of acids: carbonic acid and metabolic acids (Fig. 41-8). Cells produce carbon dioxide (CO2), which acts like an acid in the body by converting to carbonic acid (H2CO3):
Metabolic acids are any acids that are not carbonic acid. They include citric acid, lactic acid, and many others.
Acid Buffering
Buffers are pairs of chemicals that work together to maintain normal pH of body fluids. If there are too many free H+ ions, a buffer takes them up so they no longer are free. If there are too few, a buffer can release H+ ions to prevent an acid-base imbalance. Buffers work rapidly, within seconds.
All body fluids contain buffers. The major buffer in the ECF is the bicarbonate (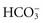 ) buffer system, which buffers metabolic acids. It consists of a lot of bicarbonate and a small amount of carbonic acid (normally a 20 to 1 ratio). Addition of H+ released by a metabolic acid to a bicarbonate ion makes more carbonic acid. Now the H+ is no longer free and will not decrease the blood pH:
) buffer system, which buffers metabolic acids. It consists of a lot of bicarbonate and a small amount of carbonic acid (normally a 20 to 1 ratio). Addition of H+ released by a metabolic acid to a bicarbonate ion makes more carbonic acid. Now the H+ is no longer free and will not decrease the blood pH:
If there are too few H+ ions, the carbonic acid portion of the buffer pair will release some, increasing the bicarbonate, again returning pH to normal.
Other buffers include hemoglobin, protein buffers, and phosphate buffers. Cellular and bone buffers also contribute. Buffers normally keep the blood from becoming too acid when acids that are produced by cells circulate to the lungs and kidneys for excretion.
Acid Excretion
The body has two acid-excretion systems: lungs and kidneys. The lungs excrete carbonic acid; the kidneys excrete metabolic acids (see Fig. 41-8).
Excretion of Carbonic Acid: When you exhale, you excrete carbonic acid in the form of CO2 and water. If the PaCO2 (i.e., level of CO2 in the blood) rises, the chemoreceptors trigger faster and deeper respirations to excrete the excess. If the PaCO2 falls, the chemoreceptors trigger slower and shallower respirations so more of the CO2 produced by cells remains in the blood and makes up the deficit. These alterations in respiratory rate and depth maintain the carbonic acid portion of acid-base balance (Coggon, 2008a). Sometimes people who have lung disease have difficulty with normal excretion of carbonic acid, which causes it to accumulate and make the blood more acid.
Excretion of Metabolic Acids: The kidneys excrete all acids except carbonic acid. They secrete H+ into the renal tubular fluid, putting  back into the blood at the same time. If there are too many H+ ions in the blood, renal cells move more H+ ions into the renal tubules for excretion, retaining more
back into the blood at the same time. If there are too many H+ ions in the blood, renal cells move more H+ ions into the renal tubules for excretion, retaining more 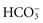 in the process. If there are too few H+ ions in the blood, renal cells secrete fewer H+ ions.
in the process. If there are too few H+ ions in the blood, renal cells secrete fewer H+ ions.
Phosphate buffers in the renal tubular fluid keep the urine from becoming too acidic when the kidneys excrete H+ ions. If the kidneys need to excrete a lot of H+, renal tubular cells secrete ammonia, which combines with the H+ ions in the tubules to make 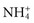 , ammonium ions. Buffering by phosphate and the creation of NH4+ turn free H+ ions into other molecules in the renal tubular fluid (Rose, 2011). This process enables metabolic acid excretion in urine without making urine too acidic. People who have kidney disease often have difficulty with normal excretion of metabolic acids.
, ammonium ions. Buffering by phosphate and the creation of NH4+ turn free H+ ions into other molecules in the renal tubular fluid (Rose, 2011). This process enables metabolic acid excretion in urine without making urine too acidic. People who have kidney disease often have difficulty with normal excretion of metabolic acids.
Acid-Base Imbalances
People develop acid-base imbalances when their normal homeostatic mechanisms are dysfunctional or overwhelmed. The term acidosis describes a condition that tends to make the blood relatively too acidic. Because our cells produce two types of acid, there are two different types of acidosis: respiratory acidosis and metabolic acidosis. The term alkalosis describes a condition that tends to make the blood relatively too basic (alkaline). There are two types of alkalosis: respiratory alkalosis and metabolic alkalosis.
The body has compensatory mechanisms that limit the extent of pH change with acid-base imbalances (Coggon, 2008b; Rose, 2011). It is important to remember that the kidney or lung cannot compensate for itself. Therefore the kidneys compensate for respiratory acid-base imbalances; the respiratory system compensates for metabolic acid-base imbalances. These compensatory mechanisms do not correct the problem, but they assist the body to adapt. However, if the underlying condition is not corrected, these compensatory mechanisms will fail.
Respiratory Acidosis
Respiratory acidosis arises from alveolar hypoventilation; the lungs are unable to excrete enough CO2. The PaCO2 rises, creating an excess of carbonic acid in the blood, which decreases pH (Table 41-7). The kidneys compensate by increasing excretion of metabolic acids in the urine, which increases blood bicarbonate. This compensatory process is slow, often taking 24 hours to show clinical effect and 3 to 5 days to reach steady state. Decreased cerebrospinal fluid (CSF) pH and intracellular pH of brain cells cause decreased level of consciousness.
Respiratory Alkalosis
Respiratory alkalosis arises from alveolar hyperventilation; the lungs excrete too much carbonic acid (CO2 and water). The PaCO2 falls, creating a deficit of carbonic acid in the blood, which increases pH (see Table 41-7). Respiratory alkalosis usually is short lived; thus the kidneys do not have time to compensate. When the pH of blood, CSF, and ICF increases acutely, cell membrane excitability also increases, giving rise to neurological symptoms such as excitement, confusion, and paresthesias. If the pH rises high enough, central nervous system (CNS) depression can occur.
Metabolic Acidosis
Metabolic acidosis occurs from an increase of metabolic acid or a decrease of base (bicarbonate). The kidneys are unable to excrete enough metabolic acids, which accumulate in the blood, or bicarbonate is removed from the body directly as with diarrhea (see Table 41-7). In either case the blood 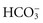 decreases, and the pH falls. With an increase of metabolic acids, blood
decreases, and the pH falls. With an increase of metabolic acids, blood 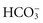 decreases because it is used to buffer metabolic acids. Similarly, when patients have conditions that cause the removal of
decreases because it is used to buffer metabolic acids. Similarly, when patients have conditions that cause the removal of 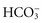 , the amount of
, the amount of 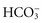 in the blood decreases. To help identify the specific cause, health care providers and the laboratory calculate the anion gap, a reflection of unmeasured anions in plasma. You calculate anion gap by subtracting the sum of plasma concentrations of the anions Cl− and
in the blood decreases. To help identify the specific cause, health care providers and the laboratory calculate the anion gap, a reflection of unmeasured anions in plasma. You calculate anion gap by subtracting the sum of plasma concentrations of the anions Cl− and 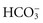 from the plasma concentration of the cation Na+ (Rose, 2011). When reviewing laboratory reports, check the reference values from the laboratory that measured the electrolyte concentrations (Table 41-8).
from the plasma concentration of the cation Na+ (Rose, 2011). When reviewing laboratory reports, check the reference values from the laboratory that measured the electrolyte concentrations (Table 41-8).
TABLE 41-8
Anion Gap in Metabolic Acidosis
| ANION GAP TYPE | VALUES (WITHOUT K+) | CAUSES |
| Normal anion gap | 5-11 mEq/L (5-11 mmol/L) Varies, depending on laboratory |
Excess output of bicarbonate: Diarrhea, pancreatic fistula, intestinal decompression, renal tubular acidosis Increase of chloride-containing acid: Parenteral HCl therapy |
| High anion gap | Greater than 11 mEq/L (11 mmol/L) Varies, depending on laboratory | Increase of any acid except HCl: Ketoacids (DKA, starvation, alcoholism), lactic acid (circulatory shock, extreme exercise), excessive normal metabolic acids (oliguric acute kidney injury, end-stage renal disease, severe hyperthyroidism, burns, severe infection), unusual organic acids (salicylate overdose, acids metabolized from methanol, ethylene glycol, paraldehyde) |
Data from Rose BD: Clinical physiology of acid-base disorders, ed 6, New York, 2011, McGraw-Hill.
The abnormally low pH in metabolic acidosis stimulates the chemoreceptors so the respiratory system compensates for the acidosis by hyperventilation. Compensatory hyperventilation begins in a few minutes and removes carbonic acid from the body. This process does not correct the problem, but it helps limit the pH decrease. Metabolic acidosis decreases one’s level of consciousness (see Table 41-7).
Metabolic Alkalosis
Metabolic alkalosis occurs from a direct increase of base (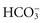 ) or a decrease of metabolic acid, which increases blood
) or a decrease of metabolic acid, which increases blood 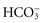 by releasing it from its buffering function. Common causes include vomiting and gastric suction (see Table 41-7). The respiratory compensation for metabolic alkalosis is hypoventilation. The decreased rate and depth of respiration allow carbonic acid to increase in the blood, as seen by an increased PaCO2. The need for oxygen may limit the degree of respiratory compensation for metabolic alkalosis. Because
by releasing it from its buffering function. Common causes include vomiting and gastric suction (see Table 41-7). The respiratory compensation for metabolic alkalosis is hypoventilation. The decreased rate and depth of respiration allow carbonic acid to increase in the blood, as seen by an increased PaCO2. The need for oxygen may limit the degree of respiratory compensation for metabolic alkalosis. Because 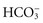 crosses the blood-brain barrier with difficulty, neurological signs and symptoms are less severe or even absent with metabolic alkalosis (Rose, 2011).
crosses the blood-brain barrier with difficulty, neurological signs and symptoms are less severe or even absent with metabolic alkalosis (Rose, 2011).
Nursing Knowledge Base
You will apply knowledge about fluid, electrolyte, and acid-base imbalance in many clinical settings. Use the scientific knowledge base in clinical decision making to provide safe, optimal fluid therapy. For example, apply knowledge of risk factors for fluid imbalances and physiology of normal aging when assessing older adults, knowing that this age-group has high risk of fluid imbalances (Bryant, 2007). Nursing knowledge includes questions to ask to elicit risk factors for fluid, electrolyte, and acid-base imbalance; specific clinical assessments for signs and symptoms of these imbalances; and nursing and collaborative interventions to maintain or restore fluid and electrolyte balance (Metheny, 2010). Skills and techniques for safe IV therapy, are a vital area of the nursing knowledge base and the focus of much nursing research to support evidence-based practice.
Critical Thinking
Successful critical thinking requires a synthesis of knowledge, experience, information gathered from patients, critical thinking attitudes, and intellectual and professional standards. Clinical judgments require you to anticipate the information necessary to analyze the data and make decisions regarding patient care. Patients’ conditions are always changing. During assessment, consider all critical thinking elements and data about the specific patient to develop appropriate nursing diagnoses.
In the case of fluid, electrolyte, and acid-base balance, you integrate knowledge of physiology, pathophysiology, and pharmacology and previous experiences and information gathered from patients. Critical analysis of data enables an understanding of how fluid, electrolyte, and acid-base imbalances affect a specific patient and his or her family. In addition, critical thinking attitudes such as accountability, discipline, and integrity assist you in identifying appropriate nursing diagnoses and planning successful interventions. Professional standards such as the Infusion Nurses Society (INS) standards of practice (INS, 2011) provide valuable guidance for appropriate assessment.
Nursing Process
Apply the nursing process and use a critical thinking approach in your care of patients. The nursing process provides a clinical decision-making approach for you to develop and implement an individualized plan of care. For patients at high risk for fluid, electrolyte, and/or acid-base imbalances or those who already have these imbalances, an individualized approach is the foundation for safe and effective patient-centered nursing care.
Assessment
During the assessment process, thoroughly assess each patient and critically analyze findings to ensure you make patient-centered clinical decisions required for safe nursing care. Using a systematic approach in assessment enables you to help patients maintain or restore fluid, electrolyte, and acid-base balances safely (Fig. 41-9).
Through the Patient’s Eyes
A patient’s fluid, electrolyte, or acid-base imbalance is sometimes so severe that it prevents initial discussion of his or her expressed needs, values, and preferences. However, when a patient is alert enough to discuss care, you need to elicit this information. Focus on the patient’s experience with fluid, electrolyte, or acid-base alterations and his or her perceptions of the illness. For example, for a patient who is hospitalized for clinical dehydration from diarrhea, ask if he or she has experienced dehydration previously, and assess his or her interpretation of the signs and symptoms experienced and possible causes. For example, ask how the person manages diarrhea at home to assess the patient’s understanding of how to prevent the imbalances from occurring in the future. Assess potential barriers to rehydration, such as concerns regarding IV therapy or lack of availability of favorite fluids at the preferred fluid temperature. Ask about the patient’s greatest concerns regarding fluid status to build the basis for active partnership in planning, implementing, and evaluating patient-centered care.
Nursing History
Clinical assessment begins with a patient history designed to reveal risk factors that cause or contribute to fluid, electrolyte, and acid-base imbalances (Table 41-9). Ask specific, focused questions to identify factors that contribute to a patient’s potential imbalances (Box 41-1).
TABLE 41-9
Risk Factors for Fluid, Electrolyte, and Acid-Base Imbalances
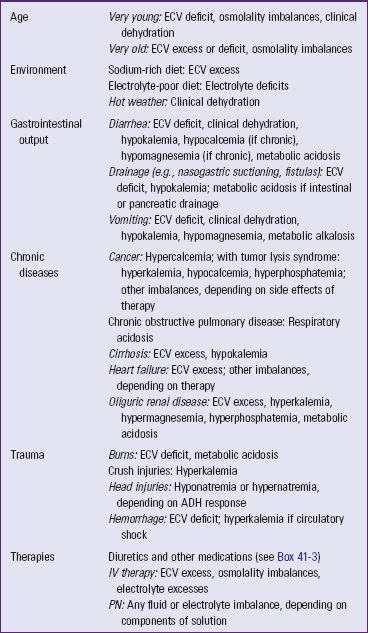
ADH, Antidiuretic hormone; ECV, extracellular fluid volume; IV, intravenous; PN, parenteral nutrition.
Age
First assess a patient’s age. An infant’s proportion of total body water (70% to 80% total body weight) is greater than that of children or adults. Infants and young children have greater water needs and immature kidneys (Hockenberry and Wilson, 2011). They are at greater risk for ECV deficit and hypernatremia because body water loss is proportionately greater per kilogram of weight.
Children who are between the ages of 2 and 12 frequently respond to illnesses with fevers of higher temperatures and longer duration than those of adults (Hockenberry and Wilson, 2011). At any age fever increases the rate of insensible water loss. Adolescents have increased metabolism and increased water production because of their rapid growth changes. Fluctuations in fluid balance are greater in adolescent girls because of hormonal changes associated with the menstrual cycle.
Older adults experience a number of age-related changes that potentially affect fluid, electrolyte, and acid-base balances (Box 41-2). They often have more difficulty recovering from imbalances resulting from the combined effect of normal aging, various disease conditions, and multiple medications.
Environment
Hot environments increase fluid output through sweating. Sweat is a hypotonic sodium-containing fluid. Excessive sweating without adequate replacement of salt and water can lead to ECV deficit, hypernatremia, or clinical dehydration. Ask patients about their normal level of physical work and whether they engage in vigorous exercise in hot environments. Do the patients have fluid replacements containing salt available during exercise and activity?
Dietary Intake
Assess dietary intake of fluids; salt; and foods rich in potassium, calcium, and magnesium (see Table 41-4). Ask patients if they follow weight-loss diets. Starvation diets or those with high fat and no carbohydrate content often lead to metabolic acidosis (see Table 41-7). In addition, assess the patient’s ability to chew and swallow, which, if altered, interferes with adequate intake of electrolyte-rich foods and fluids.
Lifestyle
Take an alcohol intake history. Chronic alcohol abuse commonly causes hypomagnesemia, in part because it increases renal magnesium excretion.
Medications
Obtain a complete list of your patient’s current medications, including over-the-counter (OTC) and herbal preparations, to assess the risk for fluid, electrolyte, and acid-base imbalances (Box 41-3). Use a drug reference book or reputable online database to check the potential effects of other medications. Ask specifically about the use of baking soda as an antacid, which can cause ECV excess because of its high sodium content that holds water in the extracellular compartments. For an individual who uses laxatives, ask about the consistency and frequency of stools. Multiple loose stools remove fluid and electrolytes from the body, thus causing numerous imbalances.
Medical History
Surgery causes a physiological stress response, which increases with extensive surgery and blood loss. In the second to fifth postoperative day, increased secretion of aldosterone, glucocorticoids, and ADH cause increased ECV, decreased osmolality, and increased potassium excretion (Monahan et al., 2007). In otherwise healthy patients these imbalances resolve without difficulty, but patients who have preexisting illnesses or additional risk factors often need treatment during this time period.
Gastrointestinal Output
Increased output of fluid through the GI tract is a common and important cause of fluid, electrolyte, and acid-base imbalances that requires careful assessment. Vomiting and diarrhea, either acute or chronic, can cause ECV deficit, hypernatremia, clinical dehydration, and hypokalemia by increasing the output of fluid, Na+, and K+. In addition, chronic diarrhea can cause hypocalcemia and hypomagnesemia by decreasing electrolyte absorption. Removal of gastric acid from the body through vomiting or nasogastric suction can cause metabolic alkalosis. In contrast, removal of the bicarbonate-rich intestinal or pancreatic fluids through diarrhea, intestinal suction, or fistula can cause metabolic acidosis.
Acute Illness or Trauma
Acute conditions that place patients at high risk for fluid, electrolyte, and acid-base alterations include respiratory diseases, burns, trauma, GI alterations, and acute oliguric renal disease.
Respiratory Disorders: Many acute respiratory disorders predispose patients to respiratory acidosis. For example, bacterial pneumonia causes alveoli to fill with exudate that impairs gas exchange, causing the patient to retain carbon dioxide, which leads to increased PaCO2 and respiratory acidosis.
Burns: Burns place patients at high risk for ECV deficit from numerous mechanisms, including plasma-to-interstitial fluid shift and increased evaporative and exudate output. The greater the body surface burned, the greater is the fluid loss (Copstead and Banasik, 2010). Patients with burns often develop metabolic acidosis because of greatly increased cellular metabolism, which produces more metabolic acids than their kidneys are able to excrete.
Trauma: Hemorrhage from any type of trauma causes ECV deficit from blood loss. Some types of trauma create additional risks. For example, crush injuries cause hyperkalemia. The trauma from the crush injury destroys the cellular structure, resulting in a massive release of intracellular K+ into the blood.
Head injury typically alters ADH secretion. It may cause diabetes insipidus (secretion of too little ADH), in which patients excrete large volumes of very dilute urine and develop hypernatremia. In contrast, head injury may cause the syndrome of inappropriate antidiuretic hormone (SIADH), in which excess secretion of ADH causes hyponatremia by retaining too much water and concentrating the urine (Copstead and Banasik, 2010).
Chronic Illness
Many chronic diseases create ongoing risk of fluid, electrolyte, and acid-base imbalances. In addition, the treatment regimens for chronic disease often cause imbalances. Assess patients for the presence of these conditions.
Cancer: The types of fluid and electrolyte imbalances that occur with cancer depend on the type and progression of the cancer and treatment regimen. Many patients with cancer develop hypercalcemia when their cancer cells secrete chemicals that circulate into bones and cause calcium to enter the blood. Other fluid and electrolyte imbalances occur in cancer because some types of tumors cause metabolic and endocrine abnormalities. In addition, patients with cancer are at risk for fluid and electrolyte imbalances as a result of the side effects (e.g., anorexia, diarrhea) of chemotherapy, biological response modifiers, or radiation (Copstead and Banasik, 2010).
Heart Failure: Patients who have chronic heart failure have diminished cardiac output, which reduces kidney perfusion and activates the RAAS. The action of aldosterone on the kidneys causes ECV excess and risk of hypokalemia. Most diuretics used to treat heart failure increase the risk of hypokalemia while reducing the ECV excess. Dietary sodium restriction is important with heart failure because Na+ holds water in the ECF, making the ECV excess worse. In severe heart failure restriction of both fluid and sodium is prescribed to decrease the workload of the heart by reducing excess circulating fluid volume (Copstead and Banasik, 2010).
Oliguric Renal Disease: Oliguria occurs when the kidneys have a reduced capacity to make urine. Some conditions, such as acute rephritis, cause a sudden onset of oliguria, whereas other problems, such as chronic kidney disease, lead to chronic oliguria. Oliguric renal disease prevents normal excretion of fluid, electrolytes, and metabolic acids, resulting in ECV excess, hyperkalemia, hypermagnesemia, hyperphosphatemia, and metabolic acidosis. The severity of these imbalances is proportional to the degree of renal failure. Although chronic kidney disease is progressive, successful management of imbalances is possible with dietary restriction of sodium and other electrolytes, fluid restriction in severe cases, and eventually dialysis or renal transplant (Copstead and Banasik, 2010).
Physical Assessment
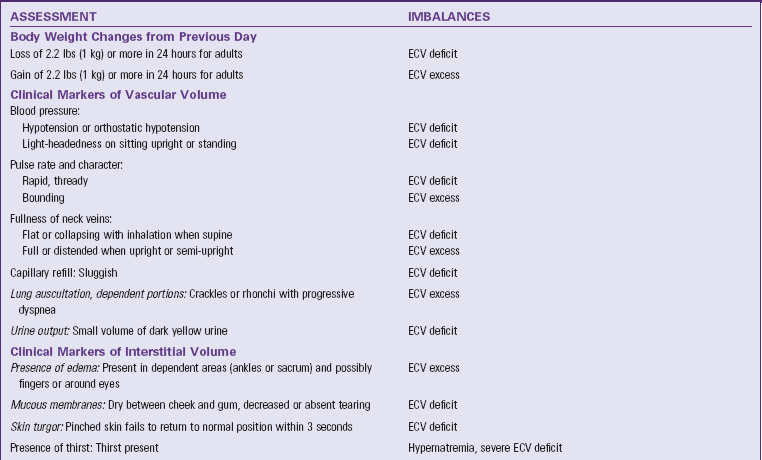
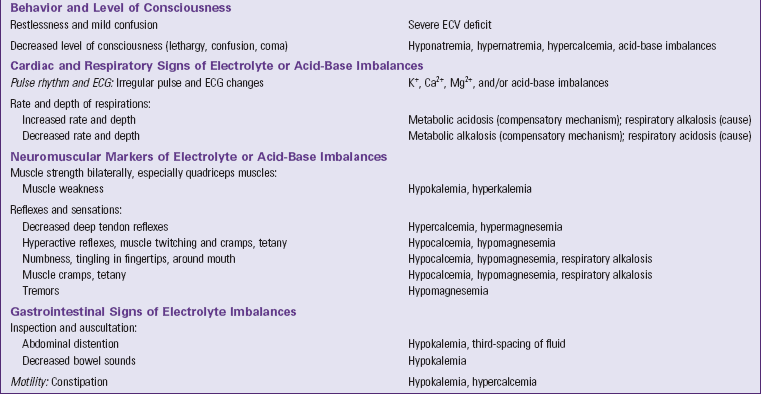
Data gathered through a focused physical assessment validates and extends the information collected in the patient history. Table 41-10 summarizes focused assessments for patients with fluid, electrolyte, and acid-base imbalances. Focus your assessment on the areas pertinent to each patient situation. For example, for patients at risk of fluid imbalances, focus your assessment on body weight changes, clinical markers of vascular and interstitial volume, thirst, behavior changes, and level of consciousness. Additional focused assessments for patients at high risk of electrolyte and acid-base imbalances include specific cardiac, respiratory, neuromuscular, and GI markers. Grouping your assessments under these categories helps you know which assessments to prioritize and enables you to assess effectively.
TABLE 41-10
Focused Nursing Assessments for Patients with Fluid, Electrolyte, and Acid-Base Imbalances
Daily Weights and Fluid Intake and Output Measurement
Daily weights are an important indicator of fluid status (Metheny, 2010). Each kilogram (2.2 lbs) of weight gained or lost overnight is equal to 1 L of fluid retained or lost. These fluid gains or losses indicate changes in the amount of total body fluid, usually ECF, but do not indicate shift between body compartments. Weigh patients with heart failure and those who are at high risk for or actually have ECV excess daily. Daily weights are also useful for patients with clinical dehydration or other causes or risks for ECV deficit. Weigh the patient at the same time each day with the same scale after a patient voids. Calibrate the scale each day or routinely. The patient needs to wear the same clothes or clothes that weigh the same; if using a bed scale, use the same number of sheets on the scale with each weighing. Compare the weight of each day with that of the previous day to determine fluid gains or losses. Look at the weights over several days to recognize trends. Interpretation of daily weights guides medical therapy and nursing care. Teach patients with heart failure to take and record their daily weights at home and to contact their health care provider if their weight increases suddenly by a set amount (obtain parameters from their health care providers). Recognizing trends in daily weights taken at home is important. Research shows that patients who are hospitalized for decompensated heart failure often experience steady increases in daily weights during the week before hospitalization. A weight gain of more than 2.2 lbs (1 kg) was associated with increased risk of hospitalization because of heart failure (Chaudry et al., 2007).
Measuring and recording all liquid intake and output (I&O) during a 24-hour period is an important aspect of fluid balance assessment. Compare a patient’s 24-hour intake with his or her 24-hour output. The two measures should be approximately equal if the person has normal fluid balance. To interpret situations in which I&O are substantially different, consider the individual patient. For example, if intake is substantially greater than output, there are two possibilities: the patient may be gaining excessive fluid or may be returning to normal fluid status by replacing fluid lost previously from the body. Similarly, if intake is substantially smaller than output, there are also two possibilities: The patient may be losing needed fluid from the body and developing ECV deficit and/or hypernatremia or may be returning to normal fluid status by excreting excessive fluid gained previously.
In most health care settings I&O measurement is a nursing assessment. Some agencies require a health care provider’s order for I&O. If you want to measure I&O for a patient with compromised fluid status, check your agency policies to determine whether you can institute it or if you need a health care provider’s order.
Fluid intake includes all liquids that a person eats (e.g., gelatin, ice cream, soup), drinks, (e.g., water, coffee, juice), or receives through nasogastric or jejunostomy feeding tubes (see Chapter 44). IV fluids (continuous infusions and intermittent IV piggybacks) and blood components are also sources of intake. Water swallowed while taking pills and liquid medications also counts as intake. A patient receiving tube feedings often receives numerous liquid medications, and water is used to flush the tube before and/or after medications. Over a 24-hour period these liquids amount to significant intake and always are recorded on the I&O record. Ask patients who are alert and oriented to assist with measuring their oral intake and explain to families why they should not drink or eat from the patient’s meal trays or water pitcher.
Fluid output includes urine, diarrhea, vomitus, gastric suction, and drainage from postsurgical wounds or other tubes (see Chapter 50). Record a patient’s urinary output after each voiding. Instruct patients who are alert, oriented, and ambulatory to save their urine in a calibrated insert, which attaches to the rim of the toilet bowl (Fig. 41-10). Teach patients and families the purpose of I&O measurements. Teach them to notify the nurse or nursing assistive personnel (NAP) to empty any container with voided fluid or how to measure and empty the container themselves and report the result appropriately. Patients need to have good vision and motor skills to perform these measurements. Active involvement of patient and family is an aspect of patient-centered care that is essential to maintaining accurate I&O measurements. When a patient has an indwelling urinary catheter, drainage tube, or suction, record output (e.g., at the end of each nursing shift or every hour) as the patient’s condition requires.
You can delegate portions of I&O measurement and recording to NAP with competent skills in measurement. Research shows that visual estimates of fluid volumes often are unreliable; actual measurement is preferable (McConnell et al., 2007). In many institutions NAP record oral intake but not intake through feeding or IV tubes, which are nursing responsibilities. Similarly NAP often record urine, diarrhea, and vomitus output but not drainage through tubes. The responsible registered nurse (RN) or licensed practical nurse/licensed vocational nurse (LPN/LVN) and the NAP work as a team to record measurements in the designated location in the electronic health record (EHR), often on a flow sheet with other information. The EHR program usually calculates the 24-hour totals. If an EHR is not used, record I&O on paper forms attached to the bedside chart or room door. You or the NAP calculate the 24-hour totals (see agency policy). Accurate I&O facilitates ongoing evaluation of a patient’s hydration status.
Laboratory Values
Review the patient’s laboratory test results and compare them with the normal ranges to obtain further objective data about fluid, electrolyte, and acid-base balances. Normal and abnormal test results are summarized in Tables 41-1, 41-3, 41-5, 41-6, and 41-7. The frequency of electrolyte level measurements depends on the severity of the patient’s illness. Analysis of laboratory results requires a good medical clinician, especially if a person develops an acute imbalance while also having a chronic disease. Serum electrolyte tests usually are performed routinely on any patient entering a hospital to screen for imbalances and serve as a baseline for future comparisons.
Nursing Diagnosis
When caring for patients with suspected fluid, electrolyte, and acid-base imbalances, it is particularly important to use critical thinking to formulate nursing diagnoses. The assessment data that establish the risk for or the actual presence of a nursing diagnosis in these areas are often subtle, and patterns and trends emerge only when there has been astute assessment. Multiple body systems are often involved; careful clustering of defining characteristics leads to selection of the appropriate diagnoses (Box 41-4).
In addition to the accurate clustering of assessment data, an important part of formulating nursing diagnoses is identifying the relevant causative or related factor. You choose interventions that treat or modify the related factor for the diagnosis to be resolved. For example, deficient fluid volume related to loss of GI fluids from vomiting requires therapies that manage the patients’ emesis and restore fluid volume with IV therapy. In contrast, the diagnosis of deficient fluid volume related to elevated body temperature requires therapies to lower the patient’s body temperature and replace lost body fluids through oral fluid replacement or possibly IV therapy. Possible nursing diagnoses for patients with fluid, electrolyte, and acid-base alterations include the following:
Planning
During the planning process use critical thinking to synthesize information from multiple resources (Fig. 41-11). Ensure that the patient’s plan of care integrates both scientific and nursing knowledge and all of the information that you collected about the individual patient.
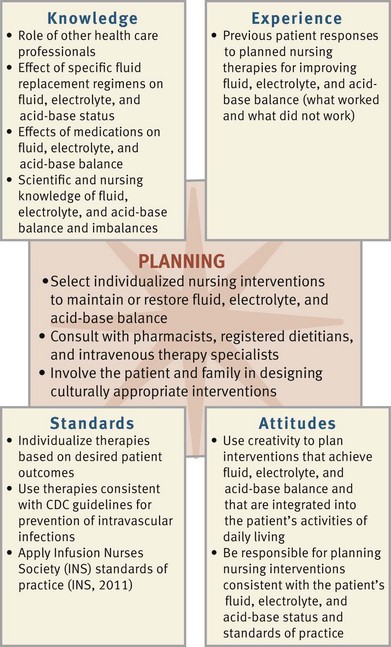
FIG. 41-11 Critical thinking model for fluid, electrolyte, and acid-base balances planning. CDC, Centers for Disease Control and Prevention.
Goals and Outcomes
Establish an individual patient plan of care for each nursing diagnosis (see the Nursing Care Plan) that includes mutually established patient goals for each diagnosis. Goals need to be individualized and realistic with measurable outcomes. For example, with a nursing diagnosis of deficient fluid volume, the following related outcomes may be established for the goal, “The patient will achieve normal hydration status at discharge”:
Setting Priorities
The patient’s clinical condition determines which of the nursing diagnoses takes the greatest priority. Many nursing diagnoses in the area of fluid, electrolyte, and acid-base balances are of highest priority because the consequences for the patient can be serious or even life threatening. For example, in the concept map (Fig. 41-12 on p. 903) for Mrs. Beck, the occurrence of vomiting and diarrhea created a high-priority nursing diagnosis of fluid volume deficit. In this situation intervention is necessary to help resolve her vomiting and diarrhea and replace her deficient fluid volume. If these priorities are unmet, Mrs. Beck’s fluid imbalance likely will worsen.
Teamwork and Collaboration
Consultation with a patient’s health care provider helps to set realistic time frames for the goals of care, particularly when the patient’s physiological status is unstable. Ongoing communication and consultation are important because the patient’s condition can change quickly. Collaboration with the patient and family and other members of the interdisciplinary health care team such as IV therapy and pharmacy assists in achieving patient outcomes. Patient and family are very helpful in identifying approaches for successful therapies, such as ways to increase fluid intake. Incorporate patient preferences and resources into the plan of care. Do not delegate administration of IV fluid and hemodynamic assessment to NAP. When the patient is stable, you can delegate daily weights, I&O, and direct physical care to NAP.
Begin discharge planning early for patients with acute or chronic fluid and electrolyte disturbances by anticipating the needs of the patient and family as they transition to another setting. In the hospital, collaboration with other members of the health care team ensures that care will continue in the home or long-term care setting with few disruptions. You ensure that therapeutic regimens established in one setting continue through completion at the next setting. For example, for a patient who is discharged on IV therapy, you assess the knowledge and skills of the family member or friend who is to assume caregiving responsibilities and initiate a referral to home IV therapy as soon as possible. Close collaboration with members of the health care team such as the patient’s health care provider, dietitian, and pharmacist is essential to ensure positive patient outcomes. A dietitian is a valuable resource in recommending food sources to increase or reduce intake of specific electrolytes (see Chapter 44). A pharmacist helps identify medications or combinations of medications likely to cause electrolyte or acid-base disturbances and offer information regarding patient education about side effects to anticipate for prescribed drugs. The patient’s health care provider directs the treatment of fluid, electrolyte, or acid-base imbalances.
Implementation
Health promotion activities focus primarily on patient education. Teach patients and caregivers to recognize risk factors for developing imbalances and implement appropriate preventive measures. For example, parents of infants need to understand that GI losses lead quickly to serious imbalances; therefore, when vomiting or diarrhea occurs in an infant, they need to promptly rehydrate with sodium-containing fluid or seek health care to restore normal balance. People of any age need to learn to replace body fluid losses with sodium-containing fluid and water.
Patients with chronic health alterations often are at risk for developing fluid, electrolyte, and acid-base imbalances. They need to understand their own risk factors and the measures to be taken to avoid imbalances. For example, patients with end-stage renal disease often need to restrict intake of fluid, sodium, potassium, magnesium, and phosphate. Through diet education these patients learn the types of foods to avoid and the suitable volume of fluid that they are permitted daily (see Chapter 44). Teach patients with chronic diseases and their family caregivers the early signs and symptoms of the fluid, electrolyte, and acid-base imbalances for which they are at risk and what to do if these occur.
Acute Care
Although fluid, electrolyte, and/or acid-base imbalances occur in all settings, they are common in acute care. Acute care nurses administer medications and oral and IV fluids to replace fluid and electrolyte deficits or maintain normal homeostasis; they also assist with restricting intake as part of therapy for excesses.
Enteral Replacement of Fluids: Oral replacement of fluids and electrolytes is appropriate as long as the patient is not so physiologically unstable that oral fluids cannot be replaced rapidly. Oral replacement of fluids is contraindicated when the patient has a mechanical obstruction of the GI tract, is at high risk for aspiration, or has impaired swallowing. Some patients unable to tolerate solid foods are still able to ingest fluids. Strategies to encourage fluid intake include offering small sips of fluid frequently, popsicles, and ice chips. Record one half the volume of the ice chips in I&O measurement. For example, if a patient ingests 240 mL of ice chips, you record 120 mL of intake. Encourage patients to keep their own record of intake to involve them actively. Family members who are properly instructed can also assist. Pay attention to each patient’s preferred temperature of oral fluids. Cultural beliefs regarding appropriate fluid temperature may interfere with fluid intake unless the fluid with the preferred temperature is available (Box 41-5).
When replacing fluids by mouth in a patient with ECV deficit, choose fluids that contain sodium (e.g., Pedialyte and Gastrolyte). Liquids containing lactose, caffeine, or low-sodium content are not appropriate when a patient has diarrhea.
A feeding tube is appropriate when the patient’s GI tract is healthy but the patient cannot ingest fluids (e.g., after oral surgery or with impaired swallowing). Options for administering fluids include gastrostomy or jejunostomy instillations or infusions through small-bore nasogastric feeding tubes (see Chapter 44).
Restriction of Fluids: Patients who have hyponatremia usually require restricted water intake. Patients who have very severe ECV excess sometimes have both sodium and fluid restrictions. Fluid restriction often is difficult for patients, particularly if they take medications that dry the oral mucous membranes or if they are mouth breathers. Explain the reason that fluids are restricted and ensure that the patient and family visitors know the amount of fluid permitted orally and understand that ice chips, gelatin, and ice cream are fluids. Help the patient decide the amount of fluid to drink with each meal, between meals, before bed, and with medications. It is important to allow patients to choose preferred fluids unless contraindicated. Frequently patients on fluid restriction can swallow a number of pills with as little as 1 oz (30 mL) of liquid.
In acute care settings fluid restrictions usually allot half the total oral fluids between 7 am and 3 pm, the period when patients are more active, receive two meals, and take most of their oral medications. Offer the remainder of the fluids during the evening and night shifts. Patients on fluid restriction need frequent mouth care to moisten mucous membranes, decrease the chance of mucosal drying and cracking, and maintain comfort (see Chapter 39).
Parenteral Replacement of Fluids and Electrolytes: Fluid and electrolytes may be replaced through infusion of fluids directly into veins (intravenously) rather than via the digestive system. Parenteral replacement includes parenteral nutrition (PN), IV fluid and electrolyte therapy (crystalloids), and blood and blood component (colloids) administration. IV devices are called peripheral IVs when the catheter tip lies in a vein in one of the extremities; they are called central venous IVs when the catheter tip lies in the central circulatory system (e.g., in the vena cava close to the right atrium of the heart) (Fig. 41-13).
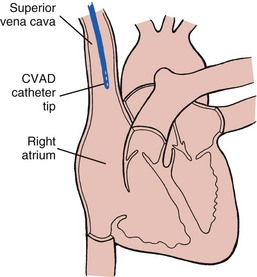
FIG. 41-13 Central venous lines deliver intravenous fluid into superior vena cava near heart. CVAD, Central venous access device.
Practice standard body fluid precautions when administering parenteral fluids (see Chapter 28) to minimize your own risk for exposure to bloodborne pathogens. Read and understand the policy and procedures for parenteral infusions at the institution for which you work.
Parenteral Nutrition: PN, also called total parenteral nutrition (TPN), is IV administration of a complex, highly concentrated solution containing nutrients and electrolytes that is formulated to meet a patient’s needs. Depending on their osmolality, PN solutions are administered through a central IV catheter (high osmolality) or peripherally (lower osmolality). Chapter 44 reviews principles and guidelines for PN administration, which is used when patients are unable to receive enough nutrition orally or through enteral feeding.
Intravenous Therapy (Crystalloids): The goal of IV fluid administration is to correct or prevent fluid and electrolyte disturbances. It allows for direct access to the vascular system, permitting the continuous infusion of fluids over a period of time. You regulate IV fluid therapy continuously because of ongoing changes in a patient’s fluid and electrolyte balance. To provide safe and appropriate therapy to patients who require IV fluids, you need knowledge of the correct ordered solution, the reason the solution was ordered, the equipment needed, the procedures required to initiate an infusion, how to regulate the infusion rate and maintain the system, how to identify and correct problems, and how to discontinue the infusion.
Types of Solutions: Many prepared IV solutions are available for use (Table 41-11). An IV solution is isotonic, hypotonic, or hypertonic. Isotonic solutions have the same effective osmolality as body fluids. Sodium-containing isotonic solutions such as normal saline are indicated for ECV replacement to prevent or treat ECV deficit. Hypotonic solutions have an effective osmolality less than body fluids, thus decreasing osmolality by diluting body fluids and moving water into cells. Hypertonic solutions have an effective osmolality greater than body fluids. If they are hypertonic sodium-containing solutions, they increase osmolality rapidly and pull water out of cells, causing them to shrivel (David, 2007). The decision to use a hypotonic or hypertonic solution is based on the patient’s specific fluid and electrolyte imbalance. For example, a patient with hypernatremia that cannot be treated with oral water generally receives a hypotonic IV solution to dilute the ECF and rehydrate cells. Too rapid or excessive infusion of any IV fluid has the potential to cause serious patient problems.
Additives such as potassium chloride (KCl) are common in IV solutions. A health care provider’s order is necessary if an IV is to have additives added (e.g., 1000 mL D5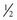 NS with 20 mEq KCl at 125 mL/hr). Administer KCl carefully because hyperkalemia can cause fatal cardiac dysrhythmias. Under no circumstances should it be administered by IV push (directly through a port in IV tubing). Verify that a patient has adequate kidney function and urine output before administering an IV solution containing potassium. Patients with normal renal function who are receiving nothing by mouth should have potassium added to IV solutions. The body cannot conserve potassium, and the kidneys continue to excrete potassium even when the plasma level falls. Without potassium intake, hypokalemia develops quickly.
NS with 20 mEq KCl at 125 mL/hr). Administer KCl carefully because hyperkalemia can cause fatal cardiac dysrhythmias. Under no circumstances should it be administered by IV push (directly through a port in IV tubing). Verify that a patient has adequate kidney function and urine output before administering an IV solution containing potassium. Patients with normal renal function who are receiving nothing by mouth should have potassium added to IV solutions. The body cannot conserve potassium, and the kidneys continue to excrete potassium even when the plasma level falls. Without potassium intake, hypokalemia develops quickly.
Vascular Access Devices: Vascular access devices (VADs) are catheters or infusion ports designed for repeated access to the vascular system. Peripheral catheters are for short-term use (e.g., fluid restoration after surgery and short-term antibiotic administration). Devices for long-term use include central catheters and implanted ports, which empty into a central vein. Remember that the term central applies to the location of the catheter tip, not to the insertion site. Peripherally inserted central catheters (PICC lines) enter a peripheral arm vein and extend through the venous system to the superior vena cava where they terminate. Other central lines enter a central vein such as the subclavian or jugular vein or are tunneled through subcutaneous tissue before entering a central vein. Central lines are more effective than peripheral catheters for administering large volumes of fluid, PN, and medications or fluids that irritate veins. Proper care of central line insertion sites is critical for the prevention of catheter-related bloodstream infections (CRBSI). The National Quality Forum (NQF) (2010) identified CRBSIs as one of their endorsed patient safety measures that health care institutions are encouraged to report. Beginning in October of 2008, the Centers for Medicare and Medicaid Services (CMS) no longer reimburses over and above the typical inpatient prospective payment system rate for care required to manage and correct a CRBSI. This means that a hospital is not paid for the added costs and hospital days needed to treat it. Nurses require specialized education regarding care of central venous catheters and implanted infusion ports. Nursing responsibilities for central lines include careful monitoring, flushing to keep the line patent, and site care and dressing changes to prevent CRBSIs.
Equipment: Correct selection and preparation of IV equipment assists in safe and quick placement of an IV line. Because fluids infuse directly into the bloodstream, sterile technique is necessary. Organize all equipment at the bedside for an efficient insertion. IV equipment includes VADs, tourniquet, clean gloves, dressings, IV fluid containers, various types of tubing, and electronic infusion devices (EIDs), also called infusion pumps. VADs that are short, peripheral IV catheters are available in a variety of gauges such as the commonly used 20 and 22 gauges. A larger gauge indicates a smaller-diameter catheter. A peripheral VAD is called an over-the-needle catheter; it consists of a small plastic tube or catheter threaded over a sharp stylet (needle). Once you insert the stylet and advance the catheter into the vein, you withdraw the stylet, leaving the catheter in place. These devices have a safety mechanism that covers the sharp stylet when withdrawing it to reduce the risk of needlestick injury (Fig. 41-14). Needleless systems allow you to make connections without using needles, which reduces needlestick injuries (Hadaway and Richardson, 2010).
The main IV fluid used in a continuous infusion flows through tubing called the primary line. The primary line connects to the IV catheter. Injectable medications such as antibiotics are usually added to a small IV solution bag and “piggybacked” as a secondary set into the primary line or as a primary intermittent infusion to be administered over a 30- to 60-minute period (see Chapter 31). The type and amount of solution are prescribed by the patient’s health care provider and depend on the medication added and the patient’s physiological status. If an IV infusion is connected to an EID, use the tubing designated for that EID. For gravity-flow IVs (not using an EID), select tubing as described in the equipment list of Skill 41-1 on pp. 916-925. Add IV extension tubing to increase the length of the primary line, which reduces pulling of the tubing and increases a patient’s mobility in changing position.
Initiating the Intravenous Line: After you collect the equipment at the patient’s bedside, prepare to insert the IV line by assessing the patient for a venipuncture site (see Skill 41-1). The most common IV sites are on the inner arm (Fig. 41-15). Do not use hand veins on older adults or ambulatory patients. IV insertion in a foot vein is common with children, but avoid these sites in adults because of the increased risk of thrombophlebitis (INS, 2011).
As you assess a patient for potential venipuncture sites, consider conditions that exclude certain sites. Venipuncture is contraindicated in a site that has signs of infection, infiltration, or thrombosis. An infected site is red, tender, swollen, and possibly warm to the touch. Exudate may be present. Do not use an infected site because of the danger of introducing bacteria from the skin surface into the bloodstream. Avoid using an extremity with a vascular (dialysis) graft/fistula or on the same side as a mastectomy. Avoid areas of flexion if possible (INS, 2011). Choose the most distal appropriate site (INS, 2011). Using a distal site first allows for the use of proximal sites later if the patient needs a venipuncture site change.
Venipuncture is a technique in which a vein is punctured through the skin by a sharp rigid stylet (e.g., metal needle). The stylet is partially covered either with a plastic catheter or a needle attached to a syringe. General purposes of venipuncture are to collect a blood specimen, start an IV infusion, provide vascular access for later use, instill a medication, or inject a radiopaque or other tracer for special diagnostic examinations. Skill 41-1 describes venipuncture for peripheral IV fluid infusion, incorporating INS (2011) standards of practice. It takes practice to become proficient in venipuncture. Only experienced practitioners perform it for patients whose veins are fragile or collapse easily, such as older adults. Box 41-6 describes principles to follow for venipuncture in older adults.
Nurses require specialized knowledge and education to place PICCs. Some central lines and implanted ports require insertion by physicians or advanced practice nurses. Both types of central catheters require close monitoring and maintenance. This chapter focuses on peripheral catheters.
Regulating the Infusion Flow Rate: After initiating a peripheral IV infusion and checking it for patency, regulate the rate of infusion according to the health care provider’s orders (Skill 41-2 on pp. 925-929). For patient safety avoid uncontrolled flow of IV fluid into a patient. You are responsible for calculating the flow rate per hour that delivers the IV fluid in the prescribed time frame. The correct IV infusion rate ensures patient safety by preventing too-slow or too-rapid administration of IV fluids. An infusion rate that is too slow often leads to further physiological compromise in a patient who is dehydrated, in circulatory shock, or critically ill. An infusion rate that is too rapid overloads the patient with IV fluid, causing fluid and electrolyte imbalances and cardiac complications in vulnerable patients (e.g., older adults or patients with preexisting heart disease).
Electronic infusion devices (EIDs), also called IV pumps or infusion pumps, deliver an accurate hourly IV infusion rate. EIDs use positive pressure to deliver a measured amount of fluid during a specified unit of time (e.g., 125 mL/hr). Familiarize yourself with the brand of EID in use at your agency so you are able to accurately set the flow rate. Many EIDs have capabilities that allow for single- and multiple-solution infusions at different rates. A variety of electronic detectors and alarms respond to air in IV lines, occlusion, completion of infusion, high and low pressure, and low battery power.
When you open a roller clamp or other type of clamp on an infusion tubing that is not yet properly inserted in an EID or on a gravity-flow IV system, the IV fluid infuses very rapidly. Nonelectronic volume control devices are used occasionally with an IV solution infused by gravity to prevent accidental infusion of a large fluid volume. These devices hang between the IV bag and the patient and hold only a small volume of fluid that can infuse into the patient. Regardless of the device in use, monitor the patient regularly to verify correct infusion of IV fluids. Patency of an IV catheter means that IV fluid flows easily through it. For patency there must be no clots at the tip of the catheter, and the catheter tip must not be against the vein wall. A blocked catheter slows or stops the rate of infusion of the IV fluids. IV flow rate also can be slowed by infiltration, vasospasm, a knot or kink in the tubing, external pressure on the tubing, and position changes of the patient’s extremity. If the flow decreases or stops and the EID is working correctly, inspect the tubing. Sometimes the patient is lying or sitting on it. Also inspect the area around the insertion site for anything that obstructs the flow of IV fluids. For gravity flow, the height of the container influences flow rate. Raising the container usually increases the rate because of increased driving pressure.
Flexion of an extremity, particularly at the wrist or elbow, can decrease IV flow rate by compressing the vein. Although VAD placement in areas of flexion is discouraged, occasionally it becomes necessary. In that case INS standards specify use of an arm board or other joint stabilization device to protect the IV site by keeping the joint extended (INS, 2011). Use padding with arm boards because they may cause skin or nerve damage from pressure. Starting an infusion in a new location rather than relying on a site that causes problems may be more comfortable for a patient. Before discontinuing the current infusion, choose another site and start the infusion to verify that the patient has other accessible veins.
Maintaining the System: After placing an IV line and regulating the flow rate, maintain the IV system. Line maintenance involves (1) keeping the system sterile and intact; (2) changing IV fluid containers, tubing, and contaminated site dressings; (3) assisting a patient with self-care activities so as not to disrupt the system; and (4) monitoring for complications of IV therapy. The frequency and options for maintaining the system are identified in agency policies.
An important component of patient care is maintaining the integrity of an IV line to prevent infection. Potential sites for contamination of a VAD are shown in Fig. 41-16. Inserting an IV line under appropriate aseptic technique reduces the chances of contamination from the patient’s skin microflora. After insertion the conscientious use of infection control principles, including thorough hand hygiene before and after handling any part of the IV system and maintaining sterility of the system during tubing and fluid container changes, prevents infection.
Always maintain the integrity of an IV system. Never disconnect tubing because it becomes tangled or it might seem more convenient for positioning or moving a patient or applying a gown. If a patient needs more room to maneuver, use aseptic technique to add extension tubing to an IV line. However, keep the use of extension tubing to a minimum, because each connection of tubing provides opportunity for contamination. Never let IV tubing touch the floor. You do not use stopcocks for connecting more than one solution to a single IV site because they are sources of contamination (CDC, 2002; INS, 2011). IV tubing contains needleless injection ports through which syringes or other adaptors can be inserted for medication administration. Clean an injection port thoroughly with 2% chlorhexidine (preferred), 70% alcohol, or povidone-iodine solution and let it dry before accessing the system (INS, 2011).
Protective devices designed to prevent movement or accidental dislodgment of a VAD are called catheter stabilization devices (Fig. 41-17). These devices are available in many hospitals, and nurses decide whether or not to use them when starting an IV line (Box 41-7). This is a patient safety issue. INS standards indicate that use of these devices is preferable over taping when feasible (INS, 2011).
Changing Intravenous Fluid Containers, Tubing, and Dressings: Patients receiving IV therapy over several days require periodic changes of IV fluid containers (Skill 41-3 on pp. 929-934). It is important to organize tasks so you can change containers rapidly before a thrombus forms in the catheter.
Recommended frequency of IV tubing change depends on whether it is used for continuous or intermittent infusion. INS (2011) standards specify that continuous infusion tubing changes occur no more frequently than every 96 hours unless the tubing has been compromised or has become contaminated, which requires immediate tubing change. In contrast, change tubing for intermittent infusion every 24 hours because of the increased risk of contamination from opening the IV system (INS, 2011). Blood, blood components, and lipids are likely to promote bacterial growth in tubing. INS standards (2011) specify tubing changes every 4 hours for blood and blood components and every 24 hours for continuous IV lipids. For lipids use tubing that is free of diethylhexyl-phthalate (DEHP), a toxin that leaches into lipid solutions (INS, 2011). Whenever possible, schedule tubing changes when it is time to hang a new IV container to decrease risk of infection (INS, 2011). To prevent entry of bacteria into the bloodstream, maintain sterility during tubing and IV fluid container changes (INS, 2011).
A sterile dressing over an IV site reduces the entrance of bacteria into the insertion site. Transparent dressings, the most common type, help secure the VAD, allow continuous visual inspection of the IV site, and become less easily soiled or moistened than gauze dressings. You leave transparent dressings in place until the IV tubing is replaced (INS, 2011). If a gauze dressing is used, change it every 48 hours (INS, 2011). Both types of dressings must be changed when the IV device is removed or replaced or when the dressing becomes damp, loosened, or soiled (INS, 2011) (Skill 41-4 on pp. 934-936).
Assisting Patients to Protect Intravenous Integrity: To prevent the accidental disruption of an IV system, a patient often needs assistance with hygiene, comfort measures, meals, and ambulation. Changing gowns is difficult for a patient with an IV in the arm. Teach nursing assistive personnel (NAP) and patients that they must not break the integrity of an IV line to change a gown because it leads to contamination. It helps to use a gown with snaps along the top sleeve seam to facilitate changing the gown without disturbing the venipuncture site. Change regular gowns by following these steps for maximum speed and arm mobility:
1. To remove a gown, remove the sleeve of the gown from the arm without the IV line, maintaining the patient’s privacy.
2. Remove the sleeve of the gown from the arm with the IV line.
3. Remove the IV solution container from its stand and pass it and the tubing through the sleeve. (If this involves removing the tubing from an EID, use the roller clamp to slow the infusion to prevent the accidental infusion of a large volume of solution or medication).
4. To apply a gown, place the IV solution container and tubing through the sleeve of the clean gown and hang it on its stand. (If the IV line is controlled by an EID, reassemble, turn on the pump, and open the roller clamp.)
5. Place the arm with the IV line through the gown sleeve.
6. Place the arm without the IV line through the gown sleeve.
A patient with an arm or a hand infusion is able to walk unless contraindicated. Offer a rolling IV pole on wheels. Help the patient to get out of bed and place the IV pole next to the involved arm. Teach the patient to hold on to the pole with the involved hand and to push it while walking. Check that the IV container is at the proper height, there is no tension on the tubing, and the flow rate is correct. Instruct the patient to report any blood in the tubing, a stoppage in the flow, or increased discomfort.
Complications of Intravenous Therapy: Table 41-12 presents complications of IV therapy, with assessments and nursing interventions. A potentially dangerous complication of IV therapy is circulatory overload with IV solution, which occurs when a patient receives too-rapid administration or an excessive amount of fluids. Assessment findings depend on the type of IV solution that infuses in excess (see Table 41-12). The signs and symptoms often arise rapidly, which highlights the importance of frequent assessment of patients receiving IV therapy.
TABLE 41-12
Complications of Intravenous Therapy with Nursing Interventions
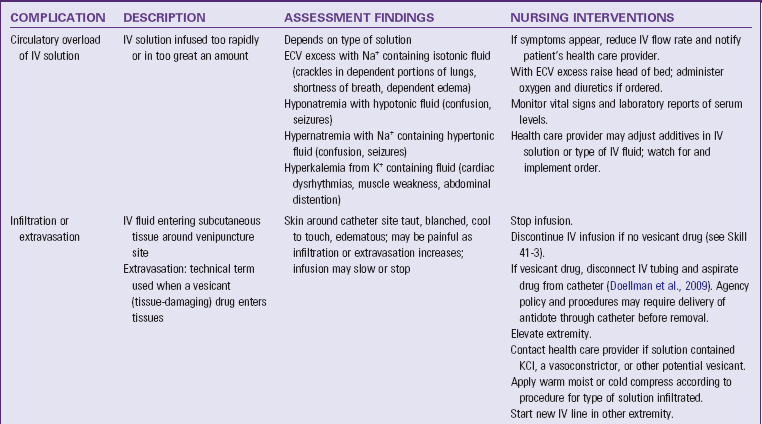
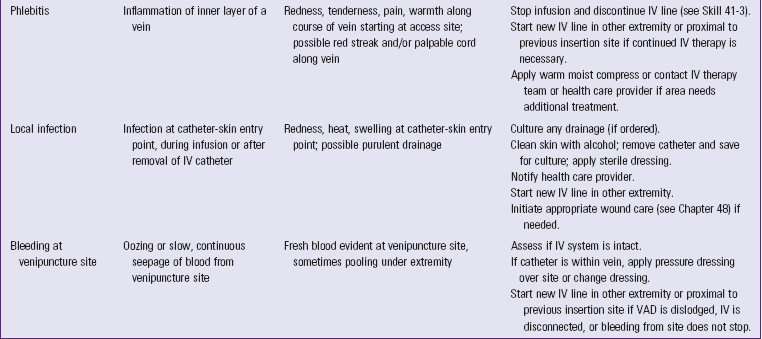
ECV, Extracellular volume; IV, intravenous; VAD, vascular access device.
Infiltration occurs when an IV catheter becomes dislodged or a vein ruptures and IV fluids inadvertently enter subcutaneous tissue around the venipuncture site. When the IV fluid contains additives that damage tissue, extravasation occurs (Hadaway, 2007). Infiltration or extravasation causes coolness, paleness, and swelling of the area. When infiltration occurs, immediately assess for any additives in the infiltrated fluid to determine what type of action is necessary to prevent local tissue damage and sloughing. Vasoconstrictors, high-dose potassium, and other IV additives in subcutaneous tissue need different treatments from those needed for an infiltrated additive-free IV (Doellman et al., 2009) (see Table 41-12). Although the INS removed their previous infiltration scale from their 2011 standards because of insufficient research validation, the society does recommend use of an infiltration scale to provide objectivity in infiltration measurement (Table 41-13).
TABLE 41-13
From Groll D et al: Evaluation of the psychometric properties of the phlebitis and infiltration scales for the assessment of complications of peripheral vascular access devices, J Infus Nurs 33(6):385, 2010.
Phlebitis (i.e., inflammation of a vein) results from chemical, mechanical, or bacterial causes. Risk factors for phlebitis include acidic or hypertonic IV solutions; rapid IV rate; IV drugs such as KCl, vancomycin, and penicillin; VAD inserted in area of flexion, poorly secured catheter; poor hand hygiene; and lack of aseptic technique (Roszell and Jones, 2010). The typical signs of inflammation (i.e., heat, erythema [redness], tenderness) occur along the course of the vein (Table 41-14). Phlebitis can be dangerous because blood clots (thrombophlebitis) form along the vein and in some cases cause emboli. This may cause permanent damage to veins. Although some agencies require routine removal of VADs and site rotation to help prevent phlebitis and other complications, the INS Standards of Practice (2011) recommend replacement of a peripheral IV catheter only if clinically indicated in adults (Webster et al., 2010). Avoid routine replacement of peripheral IV catheters in infants and children (INS, 2011).
TABLE 41-14
| GRADE | CLINICAL CRITERIA |
| 0 | No symptoms |
| 1 | Erythema at access site with or without pain |
| 2 | Pain at access site with erythema and/or edema |
| 3 | Pain at access site with erythema and/or edema; streak formation; palpable venous cord |
| 4 | Pain at access site with erythema and/or edema; streak formation; palpable venous cord >2.54 cm (1 inch) in length; purulent drainage |
From Infusion Nurses Society: Infusion nursing standards of practice, J Intraven Nurs 29(15), 2006.
In the absence of phlebitis, local infection at the venipuncture site is usually caused by poor aseptic technique during catheter insertion, daily monitoring, or catheter removal. Early recognition of local infection and treatment are important to prevent bacteria from entering the bloodstream (see Table 41-12).
Bleeding can occur around the venipuncture site during the infusion or through the catheter or tubing if these become disconnected inadvertently (see Table 41-12). Bleeding is more common in patients who receive heparin or other anticoagulants or who have a bleeding disorder (e.g., hemophilia or thrombocytopenia).
Discontinuing Peripheral Intravenous Access: Discontinue IV access after infusion of the prescribed amount of fluid; when infiltration, phlebitis, or local infection occurs; or if the IV catheter develops a thrombus at its tip. Skill 41-3 presents the steps for discontinuing peripheral IV access. You help patients and families understand that moving from IV infusion to oral fluid intake is a sign of progress toward recovery.
Blood Transfusion: Blood transfusion, or blood component therapy, is the IV administration of whole blood or a blood component such as packed red blood cells (RBCs), platelets, or plasma. Objectives for administering blood transfusions include (1) increasing circulating blood volume after surgery, trauma, or hemorrhage; (2) increasing the number of RBCs and maintaining hemoglobin levels in patients with severe anemia; and (3) providing selected cellular components as replacement therapy (e.g., clotting factors, platelets, albumin).
Blood Groups and Types: Blood transfusions must be matched to each patient to avoid incompatibility. RBCs have antigens in their membranes; the plasma contains antibodies against specific RBC antigens. If incompatible blood is transfused (i.e., a patient’s RBC antigens differ from those transfused), the patient’s antibodies trigger RBC destruction in a potentially dangerous transfusion reaction (i.e., an immune response to the transfused blood components).
The most important grouping for transfusion purposes is the ABO system, which identifies A, B, O, and AB blood types. Determination of blood type is based on the presence or absence of A and B red blood cell (RBC) antigens. Individuals with type A blood have A antigens on their RBCs and anti-B antibodies in their plasma. Individuals with type B blood have B antigens on their RBCs and anti-A antibodies in their plasma. A person who has type AB blood has both A and B antigens on the RBCs and no antibodies against either antigen in the plasma. A type O individual has neither A nor B antigens on RBCs but has both anti-A and anti-B antibodies in the plasma (Trick, 2010). Table 41-15 shows the compatibilities between blood types of donors and recipients. People with type O blood are considered universal blood donors because they can donate packed RBCs and platelets to people with any ABO blood type. People with type AB blood are called universal blood recipients because they can receive packed RBCs and platelets of any ABO type.
TABLE 41-15
ABO Compatibilities for Transfusion Therapy
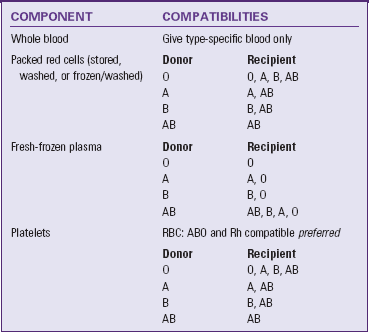
ABO, Blood group consisting of groups A, AB, B, and O.
From Alexander M et al: Infusion nursing: an evidence-based approach, ed 3, St Louis, 2010, Saunders.
Another consideration when matching blood components for transfusions is the Rh factor, which refers to another antigen in RBC membranes. Most people have this antigen and are Rh positive; a person without it is Rh negative. People who are Rh negative receive only Rh-negative blood components.
Autologous Transfusion: Autologous transfusion (autotransfusion) is the collection and reinfusion of a patient’s own blood. Blood for an autologous transfusion most commonly is obtained by preoperative donation up to 6 weeks before a scheduled surgery (e.g., heart, orthopedic, plastic, or gynecological). A patient can donate several units of blood, depending on the type of surgery and his or her ability to maintain an acceptable hematocrit. Blood for autologous transfusion is also obtained at the time of surgery by normovolemic hemodilution or through blood salvage (e.g., during surgery for liver transplantation, trauma, or vascular and orthopedic conditions). After surgery blood is salvaged from drainage from chest tubes or joint cavities. Autologous transfusions are safer for patients because they decrease the risk of mismatched blood and exposure to bloodborne infectious agents (Trick, 2010).
Transfusing Blood: Transfusion of blood or blood components is a nursing procedure that requires an order from a health care provider. A blood transfusion reaction is one of the National Quality Forum’s patient safety measures that should be included in a health care institution’s public reporting of safety events (NQF, 2010). Patient safety is a nursing priority, and patient assessment, verification of health care provider’s order, and verification of correct blood products for the correct patient are imperative.
Perform a thorough patient assessment before initiating a transfusion and monitor carefully during and after the transfusion. Assessment is critical because of the risk of transfusion reactions. Pretransfusion assessment includes establishing whether the patient knows the reason for the blood transfusion and whether he or she has ever had a previous transfusion or transfusion reaction. A patient who has had a transfusion reaction is usually at no greater risk for a reaction with a subsequent transfusion. However, he or she may be anxious about the transfusion, requiring nursing intervention. Before beginning a transfusion, explain the procedure and instruct the patient to report any side effects (e.g., chills, dizziness, or fever) once the transfusion begins. Ensure that he or she has signed an informed consent. Patients with certain cultural backgrounds may refuse blood transfusions (see Box 41-5).
Because of the danger of transfusion reactions, your pretransfusion assessment always includes the patient’s baseline vital signs. These data allow you to identify when vital sign changes occur as a result of a transfusion reaction.
For patient safety always verify three things: that blood components delivered are the ones that were ordered; that blood delivered to the patient is compatible with the blood type listed in the medical record; and that the right patient receives the blood. Together two RNs or one RN and an LPN (check agency policy and procedures) must check the label on the blood product against the medical record and against the patient’s identification number, blood group, and complete name. If even a minor discrepancy exists, do not give the blood; notify the blood bank immediately to prevent infusion errors.
When administering a transfusion you need an appropriate-size IV catheter and blood administration tubing that has a special in-line filter (Fig. 41-18). Adults require a large catheter (e.g., 18- or 20-gauge) because blood is more viscous than crystalloid IV fluids. Children with small veins use a smaller catheter. Prime the tubing with 0.9% sodium chloride (normal saline) to prevent hemolysis or breakdown of RBCs. Initiate a transfusion slowly to allow for the early detection of a transfusion reaction. Maintain the ordered infusion rate, monitor for side effects, assess vital signs, and promptly record all findings. It is important to stay with the patient during the first 15 minutes, the time when a reaction is most likely to occur. After the initial time period, continue to monitor the patient and obtain vital signs periodically during the transfusion as directed by agency policy. If a transfusion reaction is anticipated or suspected, obtain vital signs more frequently (Table 41-16).
TABLE 41-16
Acute Adverse Effects of Transfusions
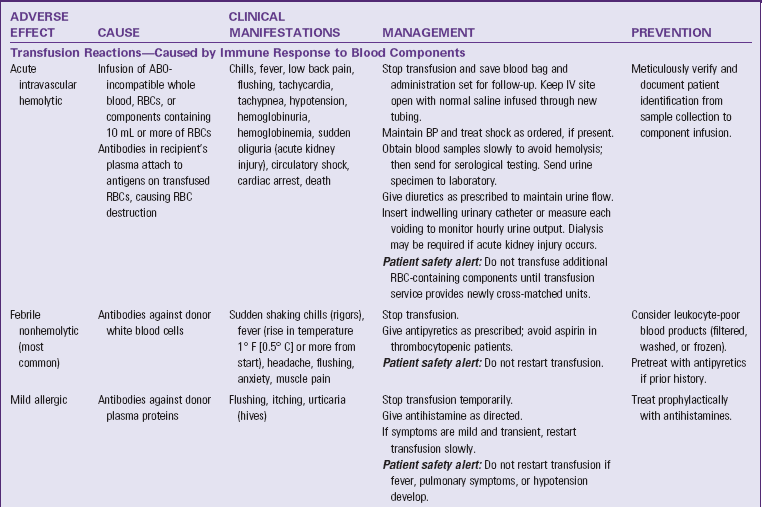
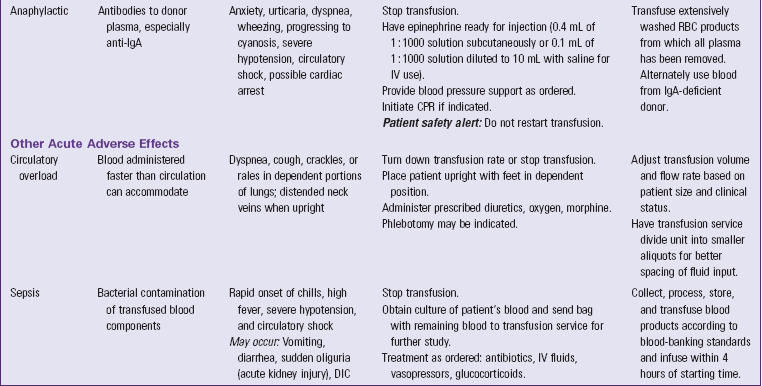
ABO, Blood group consisting of groups A, AB, B, and O; BP, blood pressure; CPR, cardiopulmonary resuscitation; DIC, disseminated intravascular coagulation; IgA, immunoglobulin A; IV, intravenous; RBC, red blood cell.
Data from Roback J et al., editors: Technical manual, ed 16, Bethesda, Md, 2008, American Association of Blood Banks; and Trick NL: Blood component therapy. In Alexander M et al: Infusion nursing: an evidence-based approach, ed 3, St Louis, 2010, Saunders.
The transfusion rate usually is specified in the health care provider’s orders. Ideally a unit of whole blood or packed RBCs is transfused in 2 hours. This time can be lengthened to 4 hours if the patient is at risk for ECV excess. Beyond 4 hours there is a risk for bacterial contamination of the blood.
When patients have a severe blood loss such as with hemorrhage, they often receive rapid transfusions through a central venous catheter. A blood-warming device often is necessary because the tip of the central venous catheter lies in the superior vena cava, above the right atrium. Rapid administration of cold blood can cause cardiac dysrhythmias. Patients who receive large-volume transfusion of citrated blood have high risk of hyperkalemia, hypocalcemia, hypomagnesemia, and metabolic alkalosis.
Transfusion Reactions and Other Adverse Effects: A transfusion reaction is an immune system reaction to the transfusion that ranges from a mild response to severe anaphylactic shock or acute intravascular hemolysis, both of which are life threatening. Table 41-16 presents the causes, manifestations, management, and prevention of transfusion reactions. Prompt intervention when a transfusion reaction occurs maintains or restores the patient’s physiological stability. When you suspect acute intravascular hemolysis, do the following (Trick, 2010):
• Stop the transfusion immediately.
• Keep the IV line open by replacing the IV tubing down to the catheter hub with new tubing and running 0.9% sodium chloride (normal saline).
• Do not turn off the blood and simply turn on the 0.9% sodium chloride (normal saline) that is connected to the Y-tubing infusion set. This would cause blood remaining in the IV tubing to infuse into the patient. Even a small amount of mismatched blood can cause a major reaction.
• Immediately notify the health care provider or emergency response team.
• Remain with the patient, observing signs and symptoms and monitoring vital signs as often as every 5 minutes.
• Prepare to administer emergency drugs such as antihistamines, vasopressors, fluids, and corticosteroids per health care provider order or protocol.
• Prepare to perform cardiopulmonary resuscitation.
• Save the blood container, tubing, attached labels, and transfusion record for return to the blood bank.
• Obtain blood and urine specimens per health care provider order or protocol.
Acute adverse effects that do not involve an immune response to the blood components also can occur during a transfusion (see Table 41-16). Circulatory overload is a risk when a patient receives massive whole blood or packed RBC transfusions for massive hemorrhagic shock or when a patient with normal blood volume receives blood. Patients particularly at risk for circulatory overload are older adults and those with cardiopulmonary diseases. Transfusion of blood components that are contaminated with bacteria, especially gram-negative bacteria, can cause sepsis.
Another category of adverse transfusion effects is diseases transmitted by blood from infected donors who are asymptomatic. Symptoms of these conditions may arise long after the transfusion. Diseases transmitted through transfusions include hepatitis B and C, human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS), and cytomegalovirus infection (CDC, 2010). In the United States all units of blood for blood banks undergo screening for HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), and syphilis, which reduces the risk of acquiring these bloodborne infections.
Interventions for Electrolyte Imbalances: In addition to the administration of prescribed medical therapies, there are nursing interventions for preserving or restoring electrolyte imbalance. For example, people who have hypokalemia or hypercalcemia often need bowel management for constipation. Patient safety interventions to prevent falls (see Chapter 27) are vital for patients who become lethargic from hypercalcemia and those with muscle weakness. Patients who have hypercalcemia need an increased fluid intake to prevent renal damage; nurses can help them meet the oral fluid intake goals. Teach patients the reasons for their therapies and the importance of balancing electrolyte I&O to prevent imbalances in the future.
Interventions for Acid-Base Imbalances: Nursing interventions to promote acid-base balance support prescribed medical therapies and aim at reversing the existing acid-base imbalance while providing for patient safety. when imbalances are life threatening, they require rapid treatment. Maintain a functional IV line and check the health care provider’s orders frequently for new medications or fluids. Give fluid and electrolyte replacement and prescribed drugs such as insulin promptly. In addition, monitor patients closely for changes in their status. Use protective measures such as side rails for patients with decreased level of consciousness. Support compensatory hyperventilation for patients with metabolic acidosis by keeping their oral mucous membranes moist and positioning them to facilitate chest expansion. Chapter 40 reviews appropriate therapies for patients with respiratory acidosis. Patients with acid-base imbalances often require repeated ABG analysis.
Arterial Blood Gases: Determination of a patient’s acid-base status requires obtaining a sample of arterial blood for laboratory testing. An ABG reveals acid-base status and the adequacy of ventilation and oxygenation. A qualified RN or other health care provider draws arterial blood from a peripheral artery (usually the radial) or from an existing arterial line (see agency policy and procedures). Before an arterial blood draw ensure that the patient has an ulnar pulse to prevent loss of blood flow to the hand if the radial artery is damaged. After the ABG puncture apply pressure to the puncture site for at least 5 minutes to reduce the risk of hematoma formation. A longer time is necessary if the patient takes anticoagulant medications. Reassess the radial pulse after removing the pressure. After obtaining the specimen, take care to prevent air from entering the syringe because this alters the blood gas values. To reduce oxygen usage by blood cells, submerge the syringe in crushed ice and transport it immediately to the laboratory.
Restorative Care
After experiencing acute alterations in fluid, electrolyte, or acid-base balance, patients often require ongoing maintenance to prevent a recurrence of health alterations. Older adults require special considerations to prevent complications from developing (see Box 41-2).
Home Intravenous Therapy: IV therapy often continues in the home setting for patients requiring long-term hydration, PN, or long-term medication administration. A home IV therapy nurse works closely with the patient to ensure that a sterile IV system is maintained and complications can be avoided or recognized promptly. Box 41-8 summarizes patient education guidelines for home IV therapy.
Nutritional Support: Most patients who have had electrolyte disorders or metabolic acid-base imbalances require ongoing nutritional support. Depending on the type of disorder, fluid or food intake may be encouraged or restricted (see Chapter 44). Patients or family members who are responsible for meal preparation need to learn to understand nutritional content of foods and read the labels of commercially prepared foods.
Medication Safety: Numerous medications, OTC drugs, and herbal preparations contain components or create potential side effects that can alter fluid and electrolyte balance. Patients with chronic disease who are receiving multiple medications and those with renal disorders are at significant risk for alterations. Once patients return to a restorative care setting, whether in the home, long-term care, or other setting, drug safety is very important. Patient and family education regarding potential side effects and drug interactions that can alter fluid, electrolyte, or acid-base balance is essential. Review all medications with patients, and encourage them to consult with their local pharmacist, especially if they wish to try a new OTC drug or herbal preparation.
Evaluation
Review with patients how well their major concerns regarding fluid, electrolyte, or acid-base situations were alleviated or addressed. For example, ask a person admitted with dehydration who was concerned about falling due to light-headedness, “How confident are you in your ability to stand without getting light-headed now?” If the patient’s concern was feeling uncomfortable with very dry mouth, ask, “How does your mouth feel now?” If the patient’s concerns involved having a better understanding of a chronic problem, focus the evaluation on the patient’s view of the patient education provided. A patient’s perspectives regarding care often depend in part on involvement of family and friends. If patients have concerns about returning home or to a different care setting, it is important to evaluate how well prepared they feel for the transition from acute care.
Patient Outcomes
Evaluate the effectiveness of interventions using the goals and outcomes established for the patient’s nursing diagnoses. Evaluation of a patient’s clinical status is especially important if acute fluid, electrolyte, and/or acid-base imbalances exist. A patient’s condition can change very quickly, and it is important to recognize impending problems by integrating information about his or her presenting risk factors, clinical status, effects of the present treatment regimen, and potential causative agent. Knowledge of how various pathophysiological conditions affect fluid, electrolyte, and acid-base balance; the effects of medications and fluids; and the patient’s presenting clinical status aid in evaluation (Fig. 41-19).
Compare your current assessment findings with the previous patient assessment. For example, a patient’s hypokalemia demonstrates improvement when the serum potassium is increasing toward normal and the physical signs and symptoms of hypokalemia begin to disappear or lessen in intensity. Specifically the patient’s heart rhythm becomes more regular, and normal bowel function returns.
For patients with less acute alterations, evaluation likely occurs over a longer period of time. In this situation evaluation may be more focused on behavioral changes (e.g., the patient’s adherence to dietary restrictions and medication schedules). Another important element of evaluation is the family’s ability to anticipate alterations and prevent problems from recurring.
The patient’s level of progress determines whether the plan of care needs to continue or be revised. If goals are not met, you may need to consult a health care provider to discuss additional methods such as increasing the frequency of an intervention (e.g., providing more fluids to a dehydrated patient), introducing a new therapy (e.g., initiating insertion of an IV line), or discontinuing a particular therapy. Once outcomes are met, the nursing diagnosis is resolved, and you are able to focus on other priorities, including maintaining normal fluid, electrolyte, and acid-base balance. If established outcomes are not achieved, explore factors that contributed to why the planned outcomes were not met. Modification of the care plan occurs after this evaluation. Questions asked if outcomes are not achieved may include the following:
• “What difficulties are you having with measuring your I&O daily and keeping a record?”
• “What barriers are you experiencing to obtaining the potassium-rich foods you need?”
• “Are you continuing to have frequent loose stools or diarrhea?”
• “Have you purchased an antacid, or are you still using baking soda as an antacid?”
Safety Guidelines for Nursing Skills
Ensuring patient safety is an essential role of the professional nurse. To ensure patient safety, communicate clearly with members of the health care team, assess and incorporate the patient’s priorities of care and preferences, and use the best evidence when making decisions about your patient’s care. When performing the skills in this chapter, remember the following points to ensure safe, individualized patient care:
• Check that you have the necessary information, a health care provider’s order if required, and equipment available for the procedure before beginning.
• Before initiation of therapy, check patient identification using two patient identifiers, and assess the appropriate route and rate of infusion and potential incompatabilities between infusing fluids and medications (INS, 2011).
• Determine if the patient has a latex allergy and use nonlatex items if allergy is present (INS, 2011).
• Use special designated tubing for the brand of EID and for blood transfusions and some medications.
• Review the steps of the procedure mentally before entering a patient’s room (i.e., consider modifications that you may need to make for this specific patient and verify that the type of IV solution is appropriate for this patient).
• Maintain strict aseptic and sterile techniques when required and sterility and integrity of the IV system to prevent bloodstream infections (INS, 2011).
• If you contaminate a sterile object during the procedure, do not use it. Use a new sterile one.
• Use standard body fluid precautions during procedures and place all disposable blood-contaminated items and sharp items in designated puncture-resistant biohazard containers (INS, 2011).
Skill 41-1 Initiating Intravenous Therapy 
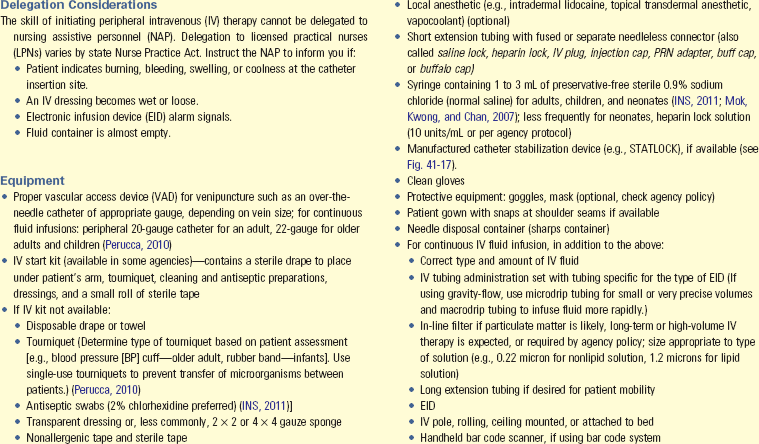
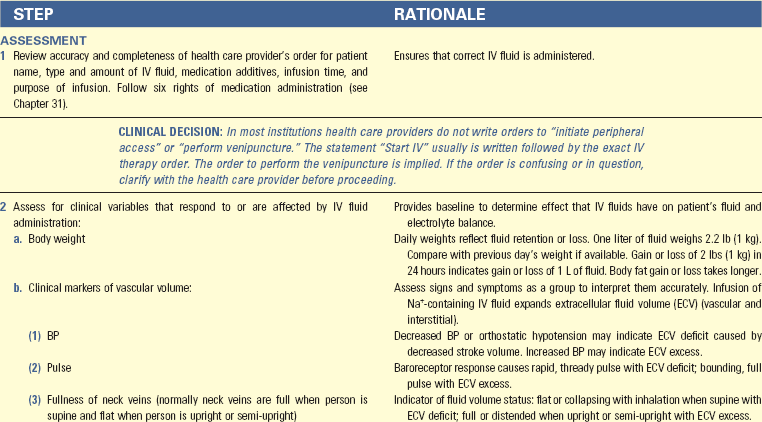


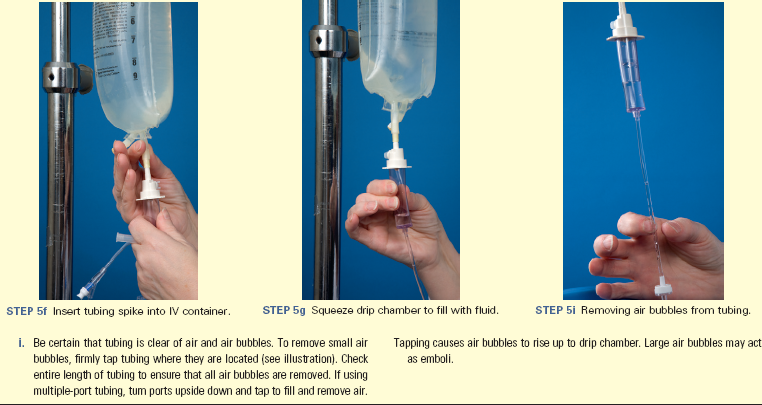
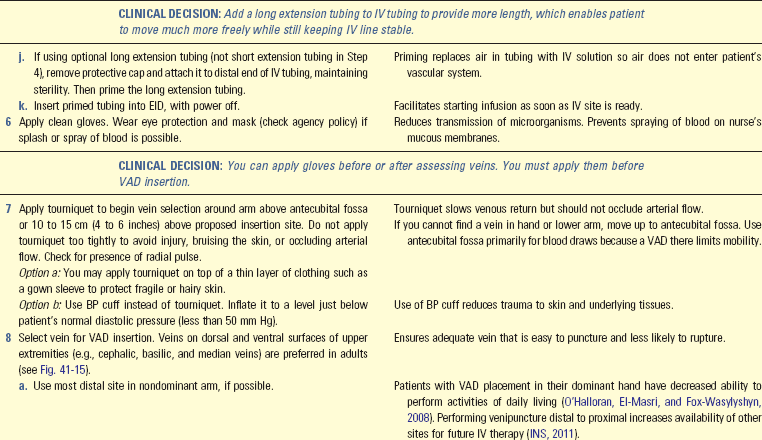
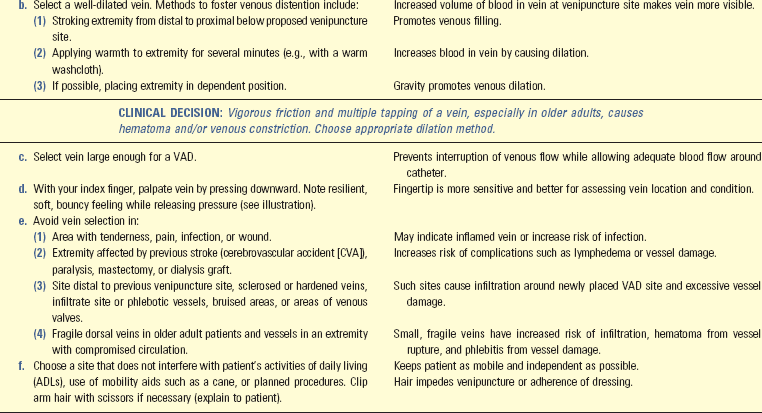
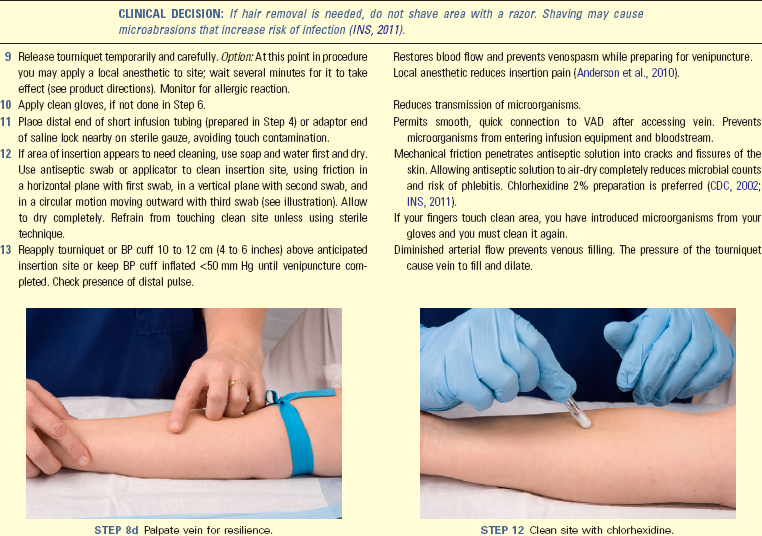

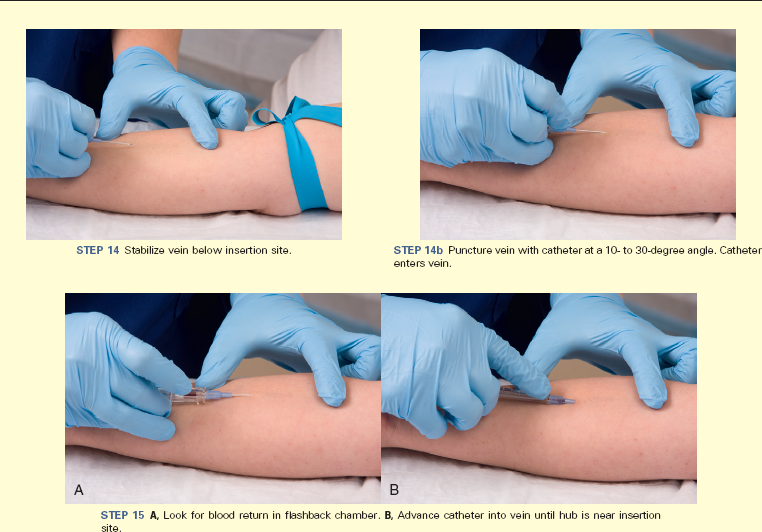

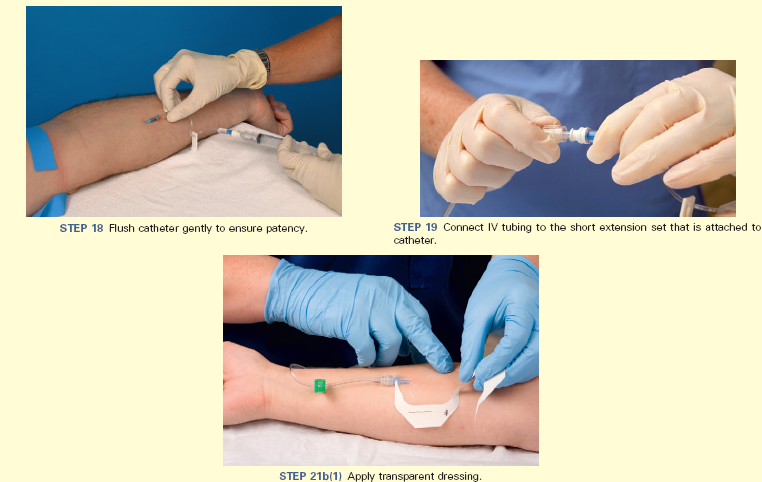
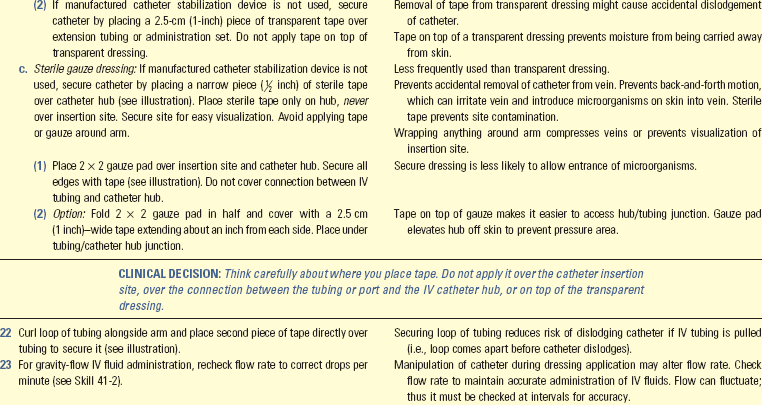
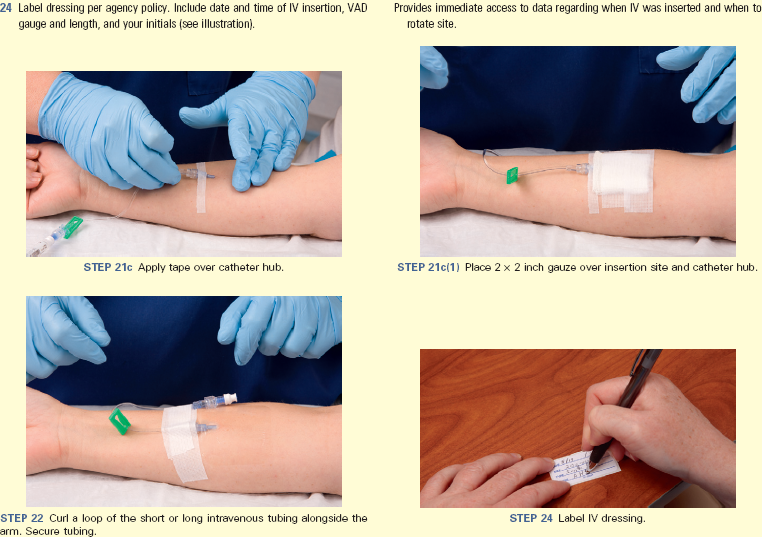
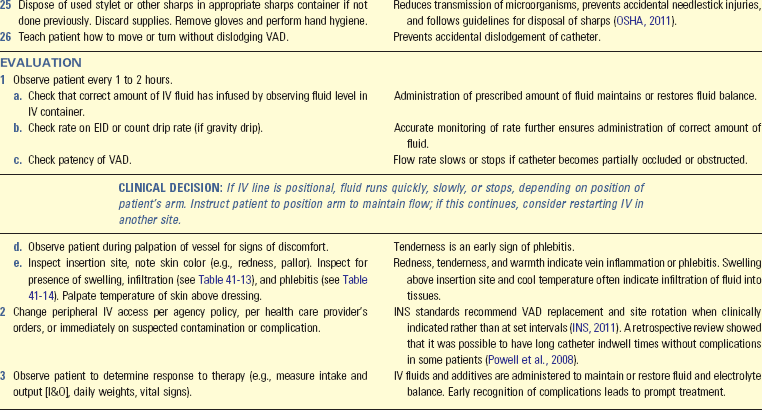
Unexpected Outcomes and Related Interventions:
1. Overload of IV solution, infiltration, phlebitis, local infection, and bleeding at venipuncture site
• See Table 41-12.
• Document in nurses’ notes or other designated location in an electronic health record (EHR) date and time of insertion; number and sites of attempts, precise description of insertion site (e.g., cephalic vein on dorsal surface of right lower arm, 2.5 cm above wrist); catheter gauge, type, length, and brand; type of dressing and catheter stabilization; flow rate; type of infusion, EID use, and your identity (INS, 2011). If using bar-code system, fluid type and time record automatically.
• Record patient’s status, IV fluid, amount infused, and integrity and patency of system according to agency policy.
• Report to oncoming nursing staff: type of fluid, flow rate, status of VAD, amount of fluid remaining in present container, expected time to hang subsequent IV container, and patient condition.
• Ensure that patient is able and willing to self-administer IV therapy or that a reliable family caregiver will provide IV therapy at home.
• Teach patient and caregiver information needed to administer IV therapy safely (see Box 41-8).
Skill 41-2 Regulating Intravenous Flow Rate 

Unexpected Outcomes and Related Interventions:
1. Circulatory overload of IV solution occurs.
• See Table 41-12.
2. IV fluid container empties with subsequent loss of VAD patency.
• Record rate of infusion in milliliters per hour (or drops per minute with gravity flow) in designated location in the patient’s medical record.
• Document use of EID or volume-control device.
• Immediately record any new IV fluid rate.
• At change of shift or when leaving on break, report rate and volume left of infusion to nurse in charge or next nurse assigned to care for patient.
• Ensure that patient is able and willing to operate infusion pump and administer IV therapy. If he or she is unable to provide self-care, be sure that a reliable family caregiver is available in the home.
• Ensure that EID functions properly before use and that patient’s electrical outlets are properly grounded.
• Teach patient and caregiver what EID alarms mean, methods to troubleshoot them, and how to disconnect the tubing from the EID pump in case of pump failure. Provide a 24-hour access telephone number for patient to call for assistance.
• If using gravity administration, teach patient and family caregiver how to time drops per minute using watch with second hand.
Skill 41-3 Maintenance of Intravenous System 

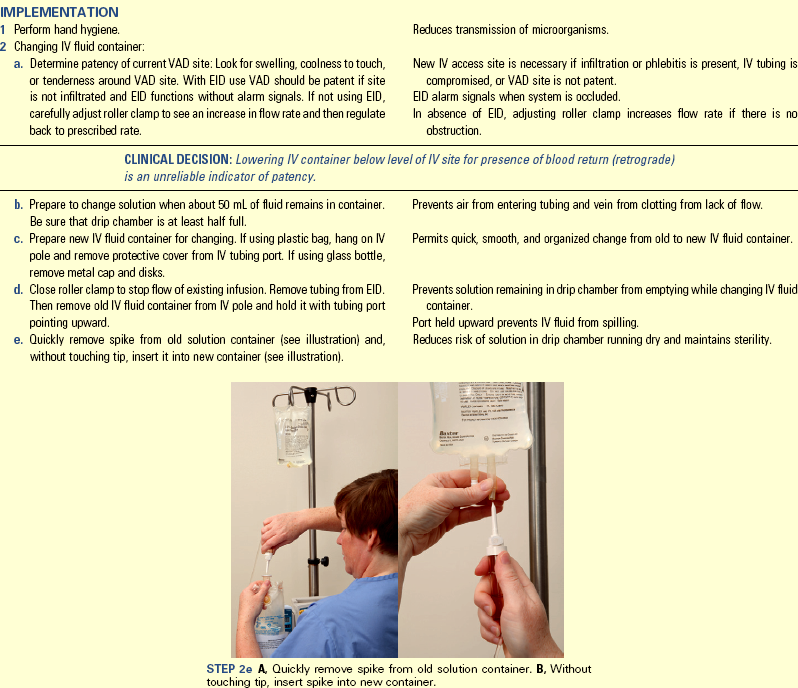

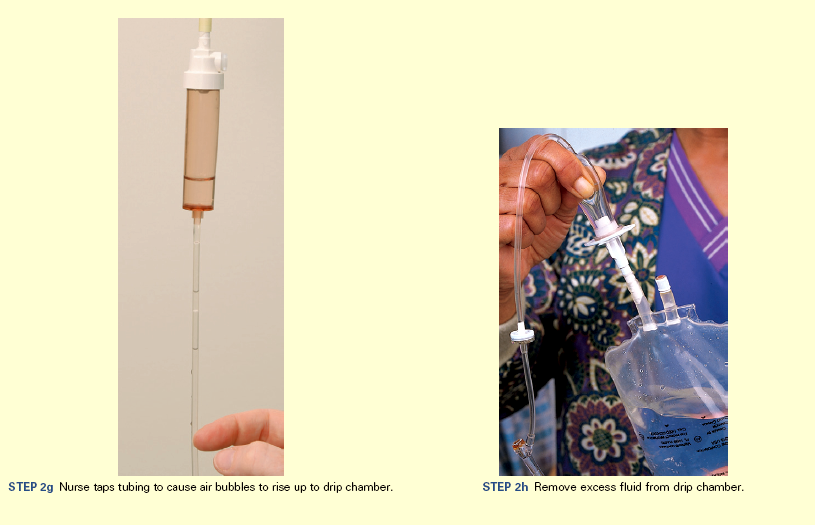
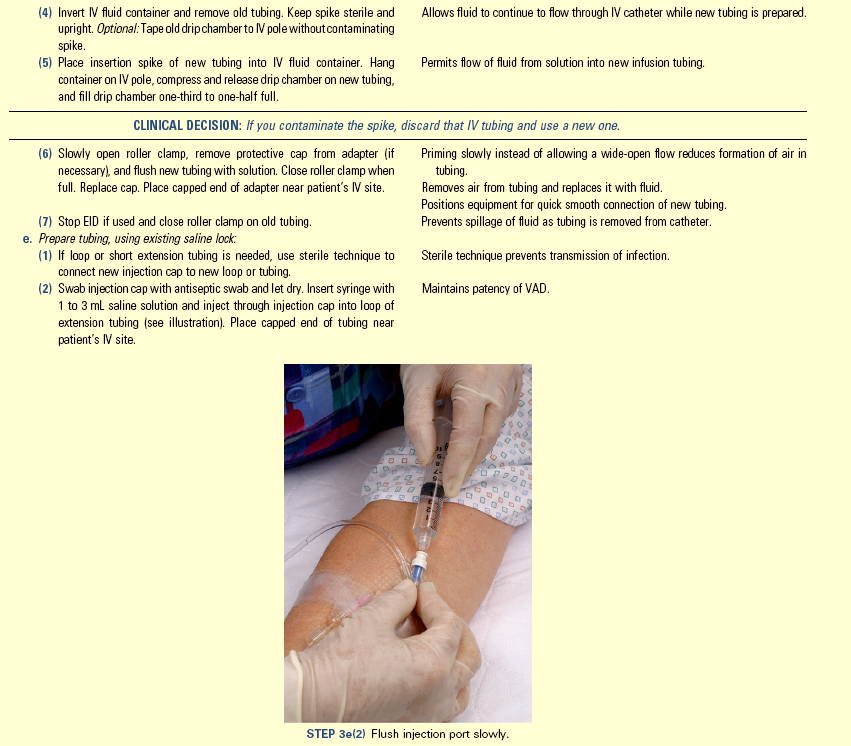

Unexpected Outcomes and Related Interventions:
1. Flow rate is incorrect; patient receives too little or too much fluid.
• Readjust infusion rate to ordered rate.
• Evaluate patient for adverse effects; notify health care provider if apparent.
• Determine and correct cause of incorrect flow rate (e.g., positional change that might affect rate, EID malfunction (or poor height of IV container with gravity flow), kinking or obstruction of tubing, infiltration or other complications of VAD site).
• Notify health care provider if patient’s anticipated infusion is 100 to 200 mL less than or greater than expected (check agency policy).
2. Catheter tip is missing after withdrawal.
3. See Table 41-12 for infiltration, phlebitis, and local infection.
• Record amount and type of fluid infused, amount and type of fluid started, and tubing change on patient’s medical record according to agency policy. If using bar-code system, fluid type and time record automatically in an EHR.
• Record time that peripheral IV access was discontinued on patient’s record according to agency policy. Include site assessment information and status of catheter, including gauge, length, and catheter tip integrity.
• Ensure that patient is able and willing to self-manage IV therapy (including changing IV containers) or that reliable family caregiver is at home to provide IV care.
• Instruct patient or caregiver in procedure for performing an IV solution and tubing change.
• Instruct patient or caregiver to notify health care provider if bleeding or drainage is noted at insertion site or if pain or tenderness occurs up to 4 days after catheter removal.
Skill 41-4 Changing a Peripheral Intravenous Dressing 


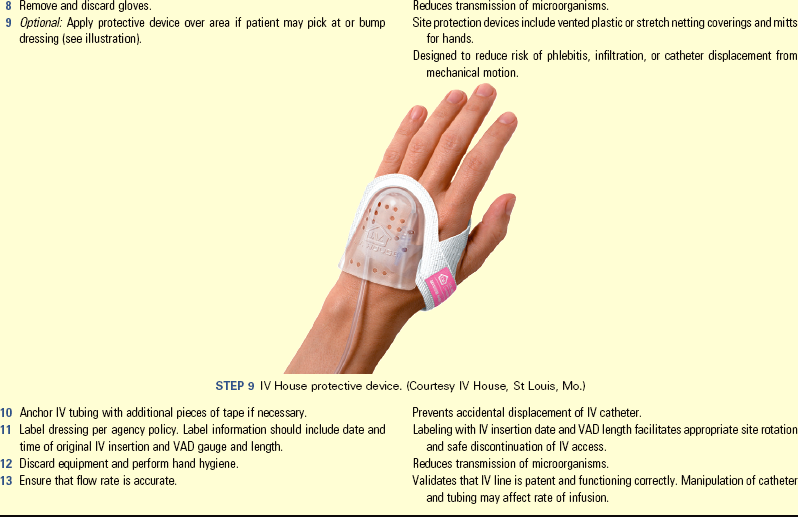
Unexpected Outcomes and Related Interventions:
1. VAD is accidentally dislodged or removed.
• Start new IV line in other extremity or proximal to previous insertion site if continued therapy is necessary.
2. IV site develops infiltration, phlebitis, and local infection.
• See Table 41-12.
• Record time that peripheral dressing was changed, reason for change, type of dressing material used, patency of system, and description of venipuncture site.
• Report to charge nurse or oncoming nursing shift that dressing was changed and any significant information about integrity of system.
• Report any complications to health care provider and document them.
Key Points
• Body fluids containing water, Na+, and other electrolytes are distributed between ECF and ICF compartments.
• A dynamic interplay of fluid intake and absorption, fluid distribution, and fluid output determines fluid balance.
• Fluid moves between blood vessels and interstitial fluid by filtration; water moves between ECF and ICF by osmosis.
• ECV deficit and excess are abnormal volumes of isotonic fluid, manifested as sudden changes in body weight and changes in markers of vascular and interstitial volume.
• Osmolality imbalances are abnormal concentrations of body fluids, manifested as altered serum Na+ levels and decreased level of consciousness.
• Interplay of electrolyte intake and absorption, electrolyte distribution, and electrolyte output determines balance of K+, Ca2+, Mg2+, and phosphate.
• Interplay of acid production, acid buffering, and acid excretion determines acid-base balance.
• Acid-base imbalances are caused by excesses or deficits of carbonic or metabolic acids, manifested as changes in level of consciousness and abnormalities of PaCO2, 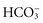 , and pH.
, and pH.
• Patients who are very young or very old, whose I&O of fluid and/or electrolytes are not equal, or who have various chronic diseases or trauma are at high risk for fluid, electrolyte, and acid-base imbalances.
• Treatment for ECV excess is Na+ restriction and fluid restriction if severe; treatment for hyponatremia usually is water restriction.
• Prevention and treatment of ECV deficit, hypernatremia, and electrolyte deficits are accomplished with enteral or parenteral administration of appropriate fluid.
• Initiation and maintenance of IV therapy require clinical decision making, skill, and organized procedures to maintain sterility and patency of the system.
• Good IV practice requires periodic updating of procedures based on current research evidence.
• Nurses monitor vigilantly for complications of IV therapy, which include fluid overload, infiltration, phlebitis, local infection, and bleeding at the infusion site.
• Administration of blood or blood products requires a specific procedure for correctly identifying patient and blood product and responding to transfusion reactions quickly.
• Patient and family teaching is important for preventing fluid, electrolyte, and acid-base imbalances and effective restorative care.
Clinical Application Questions
Preparing for Clinical Practice
Mrs. Hilda Beck is a 72-year-old seen by her health care provider this morning after falling at home because she became light-headed after vomiting and having diarrhea that has lasted over 24 hours. She was admitted for oral and intravenous (IV) fluid therapy.
1. Why is Mrs. Beck likely becoming light-headed? When should you expect this to resolve?
2. Mrs. Beck’s IV fluid order is 1000 mL 0.9% sodium chloride to run over 8 hours. Calculate the milliliters per hour that you should program into her infusion pump.
3. you auscultate crackles in Mrs. Beck’s lung bases while her IV is infusing. What is your next action?
![]() Answers to Clinical Application Questions can be found on the Evolve website.
Answers to Clinical Application Questions can be found on the Evolve website.
Are You Ready to Test Your Nursing Knowledge?
1. A patient who is comatose is admitted to the hospital with an unknown history. Respirations are deep and rapid. Arterial blood gas levels on admission are pH, 7.20; PaCO2, 21 mm Hg; PaO2, 92 mm Hg; and 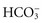 , 8. You interpret these laboratory values to indicate:
, 8. You interpret these laboratory values to indicate:
2. A patient with a cardiac history is taking the diuretic furosemide (Lasix) and is seen in the emergency department for muscle weakness. which laboratory value do you assess first?
3. Which of these patients do you expect will need teaching regarding dietary sodium restriction?
1. An 88-year-old with a fractured femur scheduled for surgery
2. A 65-year-old recently diagnosed with heart failure
3. A 50-year-old recently diagnosed with asthma and diabetes
4. A 20-year-old with vomiting and diarrhea from gastroenteritis
4. You teach patients to replace sweat, vomiting, or diarrhea fluid losses with which type of fluid?
5. You assess four patients. Which patient is at greatest risk for the development of hypocalcemia?
1. 56-year-old with acute kidney renal failure
2. 40-year-old with appendicitis
6. Which of the following activities can you delegate to nursing assistive personnel (NAP)? (Select all that apply.)
1. Measuring oral intake and urine output
2. Preparing intravenous (IV) tubing for routine change
7. Place the following steps for intravenous (IV) catheter insertion in the correct order:
2. Open and prepare infusion set.
3. Select appropriate vein and insert catheter.
4. Use two identifiers to ensure correct patient.
5. Assess for risk factors such as age or platelet count.
6. Carefully check the health care provider’s order for the IV therapy.
8. Assessment findings consistent with intravenous (IV) fluid infiltration include: (Select all that apply.)
9. Which of the following defining characteristics is consistent with fluid volume deficit?
1. A 1-lb (0.5 kg) weight loss, pale yellow urine
2. Engorged neck veins when upright, bradycardia
10. Which of the following assessments do you perform routinely when an older adult patient is receiving intravenous 0.9% NaCl?
11. While receiving a blood transfusion, your patient develops chills, tachycardia, and flushing. What is your priority action?
12. The health care provider’s order is 1000 mL 0.9% NaCl with 20 mEq K+ intravenously over 8 hours. Which assessment finding causes you to clarify the order with the health care provider before hanging this fluid?
13. Your patient who has diabetic ketoacidosis is breathing rapidly and deeply. Intravenous (IV) fluids and other treatments have just been started. What should you do about this patient’s breathing?
1. Notify her health care provider that she is hyperventilating
2. Provide frequent oral care to keep her mucous membranes moist
3. Ask her to breathe slower and help her to calm down and relax
14. Your patient had 200 mL of ice chips and 900 mL intravenous (IV) fluid during your shift. Which total intake should you record?
15. The health care provider’s order is 1000 mL 0.9% NaCl IV over 6 hours. Which rate do you program into the infusion pump?
Answers: 1. 1; 2. 4; 3. 2; 4. 2; 5. 3; 6. 1, 3; 7. 6, 5, 4, 1, 2, 3; 8. 1, 4; 9. 3; 10. 1; 11. 4; 12. 4; 13. 2; 14. 3; 15. 2.
References
Bryant, H. Dehydration in older people: assessment and management. Emerg Nurs. 2007;15(4):22.
Caon, M. Osmoles, osmolality and osmotic pressure: clarifying the puzzle of solution concentration. Contemp Nurs. 2008;29(1):92.
Centers for Disease Control and Prevention (CDC). Guidelines for the prevention of intravascular catheter-related infections. MMWR Morb Mortal Wkly Rep. 51(RR-10), 2002.
Centers for Disease Control and Prevention (CDC). HIV transmission through transfusion—Missouri and Colorado, 2008. MMWR Morb Mortal Wkly Rep. 59(41), 2010.
Coggon, JM. Arterial blood gas analysis 1: understanding ABG reports. Nurs Times. 2008;104(18):18.
Coggon, JM. Arterial blood gas analysis 2: compensatory mechanisms. Nurs Times. 2008;104(19):24.
Copstead, LC, Banasik, JL. Pathophysiology online for pathophysiology, ed 4. St Louis: Mosby; 2010.
Cronenwett, L, et al. Quality and safety education for nurses. Nurs Outlook. 2007;55(3):122.
David, K. IV fluids: do you know what’s hanging and why? RN. 2007;70(10):35.
Doellman, D, et al. Infiltration and extravasation: update on prevention and management. J Infus Nurs. 2009;32(4):203.
Fabian, B. Infusion therapy in the older adult. In Alexander M, et al, eds.: Infusion nursing an evidence-based approach, ed 3, St Louis: Saunders, 2010.
Felver, L. Acid-base homeostasis and imbalances. In Copstead LC, Banasik JL, eds.: Pathophysiology, ed 4, St. Louis: Saunders, 2010.
Felver, L. Fluid and electrolyte homeostasis and imbalances. In Copstead LC, Banasik JL, eds.: Pathophysiology, ed 4, St Louis: Saunders, 2010.
Giger, JN, Davidhizar, RE. Transcultural nursing: assessment and intervention, ed 4. St Louis: Mosby; 2008.
Goldstein, MB, et al. Fluid, electrolyte and acid-base physiology: a problem-based approach, ed 4. St Louis: Saunders; 2010.
Hadaway, L. Infiltration and extravasation: preventing a complication of IV catheterization. Am J Nurs. 2007;107(8):64.
Hadaway, L, Richardson, D. Needleless connectors: a primer on terminology. J Infus Nurs. 2010;33(1):22.
Hall, JE. Guyton and Hall textbook of medical physiology, ed 12. Philadelphia: Saunders; 2011.
Hockenberry, MJ, Wilson, D. Wong’s nursing care of infants and children, ed 9. St Louis: Mosby; 2011.
Infusion Nurses Society (INS). Infusion nursing standards of practice. J Infus Nurs. 2011;34(1S):S1.
Johnson, A. Psychobiology of thirst and salt appetite. Med Sci Sports Exercise. 2007;39(8):1388.
Koeppen, BM, Stanton, BA. Berne & Levy physiology, ed 6. St Louis: Mosby; 2008.
Kramer, BJ, Raymond, MK. Arterial blood gases. RN. 2009;72(4):22.
Lehne, RA. Pharmacology for nursing care, ed 7. Philadelphia: Saunders; 2010.
Meiner, SE. Gerontologic nursing, ed 4. St Louis: Mosby; 2011.
Metheny, NM. Fluids and electrolytes balance: nursing applications, ed 5. Sudbury, Mass: Jones & Bartlett Learning; 2010.
Monahan, F, et al. Phipps’ medical-surgical nursing: health and illness perspectives, ed 8. St Louis: Mosby; 2007.
National Quality Forum (NQF). National Voluntary Consensus Standards for Public Reporting of Patient Safety Event Information: a consensus report. Washington, DC: NQF; 2010.
Occupational Safety and Health Administration [OSHA]. Bloodborne pathogen and needlestick prevention. United States Department of Labor, last updated July 11, 2011 http://www.osha.gov/SLTC/bloodbornepathogens/index.html. [Accessed October 9, 2011].
Perucca, R. Peripheral venous access devices. In Alexander M, et al, eds.: Infusion nursing an evidence-based approach, ed 3, St Louis: Saunders, 2010.
Rose, BD. Clinical physiology of acid-base disorders, ed 6. New York: McGraw-Hill; 2011.
Scales, K, Pilsworth, J. The importance of fluid balance in clinical practice. Nurs Standard. 2008;22(4):50.
The Joint Commission (TJC), 2011 National patient safety goals (NPGs). TJC; 2011. http://jointcommission.org/standards_information/npsgs.aspx [Last accessed October 10, 2011].
Trick, NL. Blood component therapy. In Alexander M, et al, eds.: Infusion nursing an evidence-based approach, ed 3, St Louis: Saunders, 2010.
Wotton, K, Crannitch, K, Munt, R. Prevalence, risk factors and strategies to prevent dehydration in older adults. Contemp Nurs. 2008;31(1):44.
Research References
Anderson, S, et al. Administration of local anesthetic agents to decrease pain associated with peripheral vascular access. J Infus Nurs. 2010;33(6):353.
Chaudry, SI, et al. Patterns of weight change preceding hospitalization for heart failure. Circulation. 2007;116(14):1549.
Harnage, SA. Achieving zero catheter related bloodstream infections: 15 months success in a community based medical center. J Assoc Vasc Access. 2007;12(4):218.
Hertzel, C, Sousa, VD. The use of smart pumps for preventing medication errors. J Infus Nurs. 2009;32(5):257.
McConnell, JS, et al. “About a cupful”—a prospective study into accuracy of volume estimation by medical and nursing staff. Accident Emerg Nurs. 2007;15(2):101.
Mok, E, Kwong, TK, Chan, MF. A randomized controlled trial for maintaining peripheral intravenous lock in children. Int J Nurs Pract. 2007;13(1):33.
O’Halloran, L, El-Masri, MM, Fox-Wasylyshyn, SM. Home intravenous therapy and the ability to perform self-care activities of daily living. J Infus Nurs. 2008;31(6):367.
Poon, EG, et al. Effect of bar-code technology on the safety of medication administration. New Engl J Med. 2010;362(18):1698.
Powell, J, Tarnow, KG, Perucca, R. The relationship between peripheral intravenous catheter indwell time and the incidence of phlebitis. J Infus Nurs. 2008;31(1):39.
Roszell, S, Jones, C. Intravenous administration issues: a comparison of intravenous insertions and complications in vancomycin versus other antibiotics. J Infus Nurs. 2010;33(2):112.
Schears, GJ. Summary of product trials for 10,164 patients: comparing an intravenous stabilizing device to tape. J Infus Nurs. 2006;29(4):225.
Smith, B. Peripheral intravenous catheter dwell times: a comparison of three securement methods for implementation of a 96-hour scheduled change protocol. J Infus Nurs. 2006;29(1):17.
Webster, J, et al. Clinically indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. (3):2010. [CD007798].
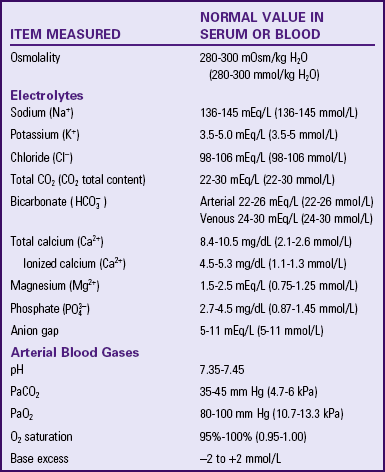
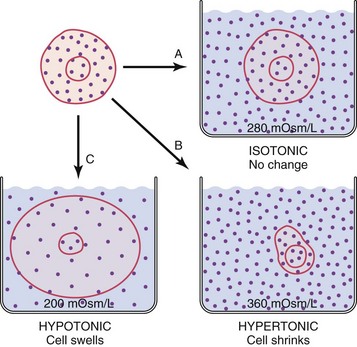
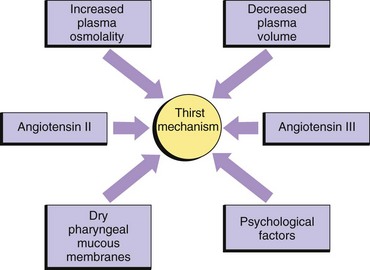
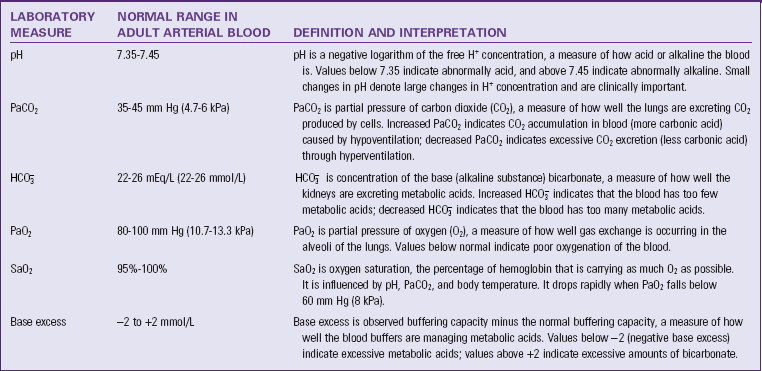
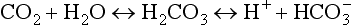
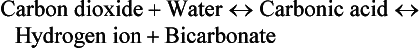
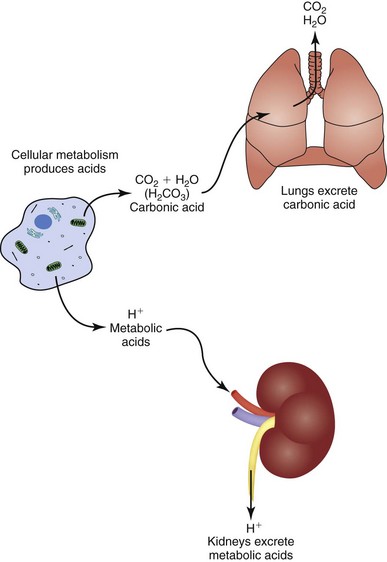

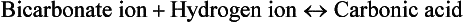
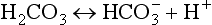
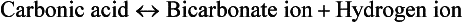
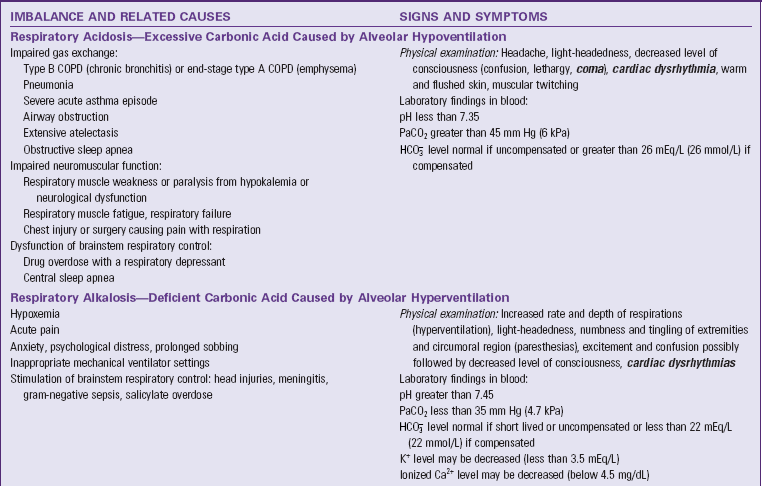
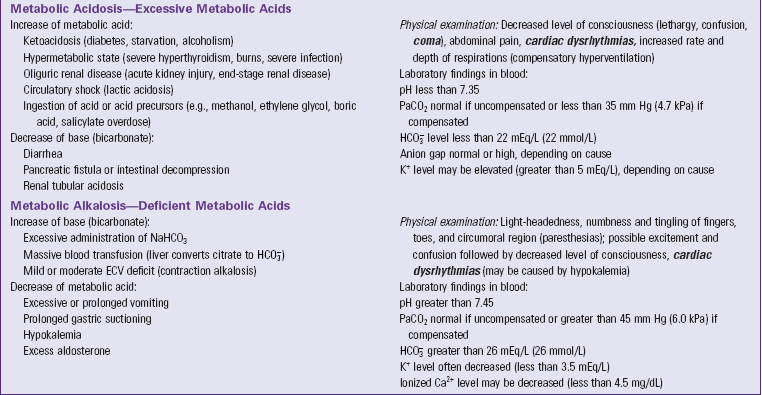
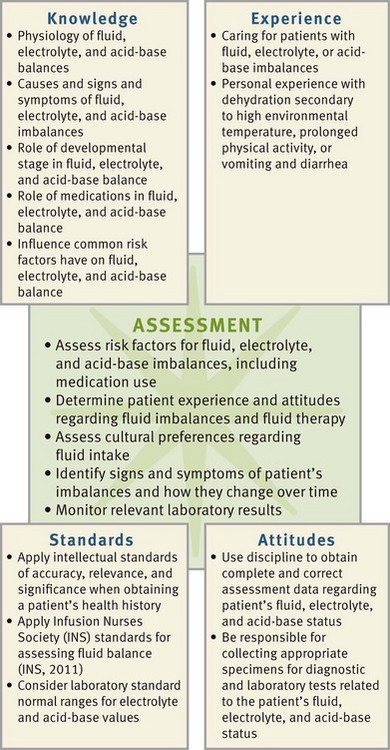
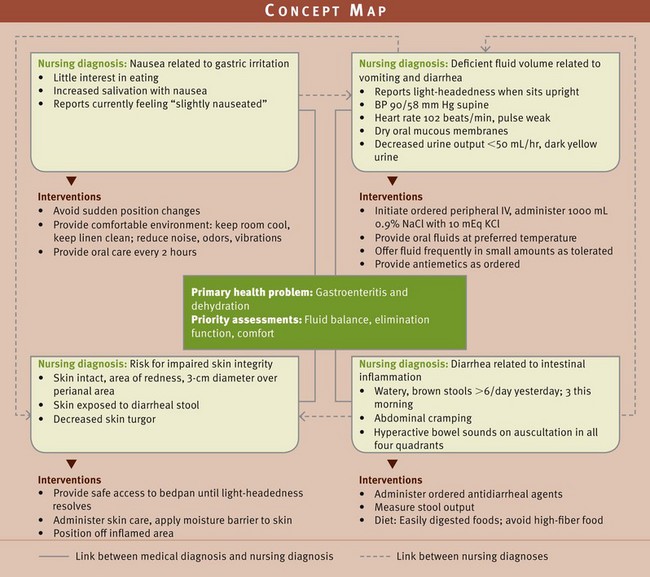

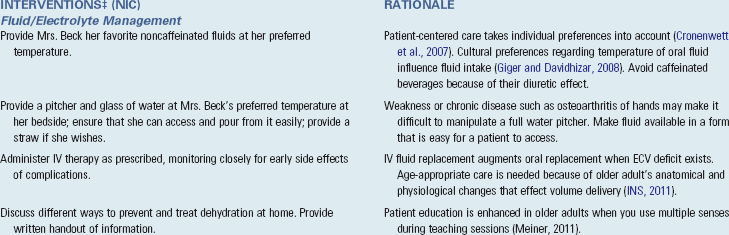

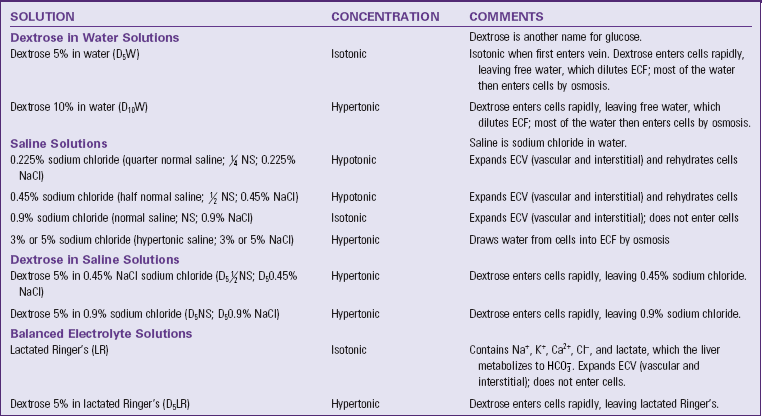
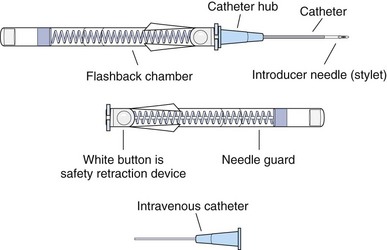
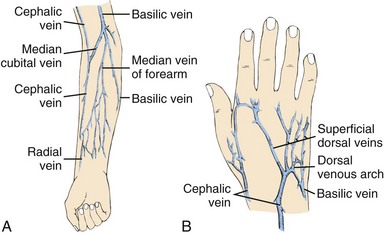
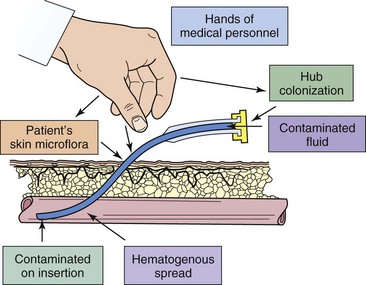
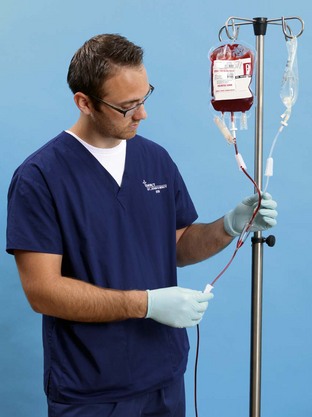
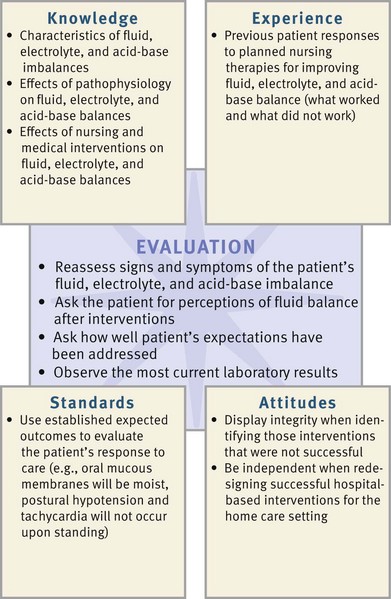
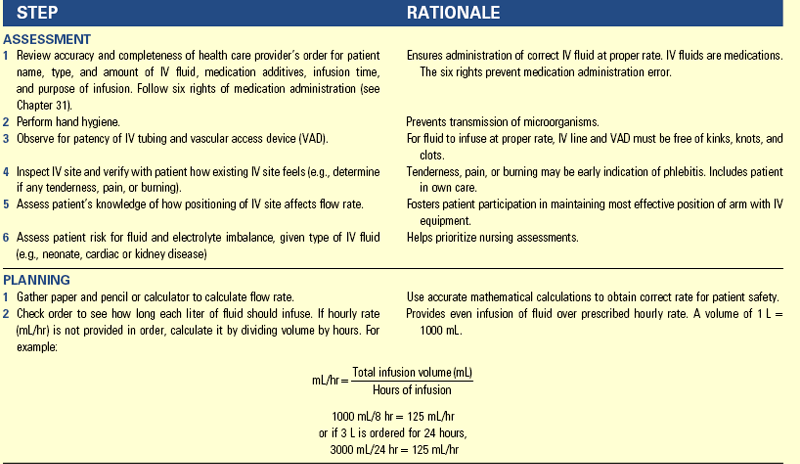


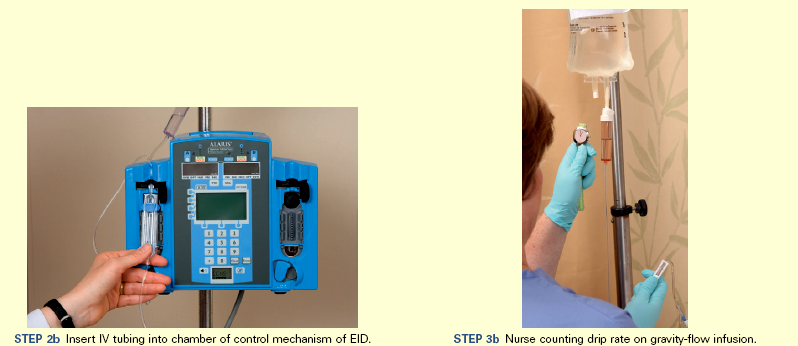


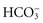 in it
in it