Chapter 33 Midwifery and obstetric emergencies
The immediate management of the emergencies discussed in this chapter are dependent on the prompt action of the midwife. Recognition of the problem and the instigation of emergency measures may determine the outcome for the mother or the fetus. The midwife should keep calm and try to explain as much as possible to the mother and her partner to ensure her consent and cooperation for procedures that may be needed. All midwifery and medical staff must be updated on the signs and symptoms of critical illness from both obstetric and non-obstetric causes and regular drills should be held to maintain and improve those skills. They should be trained in basic life support to a nationally recognized level and emergency drills for maternal resuscitation should be regularly practised in clinical areas in all maternity units.
Vasa praevia
The term vasa praevia is used when a fetal blood vessel lies over the os, in front of the presenting part. This occurs when fetal vessels from a velamentous insertion of the cord or to a succenturiate lobe (Ch. 11) cross the area of the internal os to the placenta. The fetus is in jeopardy, owing to the risk of rupture of the vessels, which could lead to exsanguination. Good outcome depends on antenatal diagnosis and delivery by caesarean section before the membranes rupture (Oyelese & Smulian 2006).
Vasa praevia may be diagnosed antenatally using ultrasound. Sometimes, vasa praevia will be palpated on vaginal examination when the membranes are still intact. If it is suspected a speculum examination should be made.
Ruptured vasa praevia
When the membranes rupture in a case of vasa praevia, a fetal vessel may also rupture. This leads to exsanguination of the fetus unless birth occurs within minutes.
Diagnosis
Fresh vaginal bleeding, particularly if it commences at the same time as rupture of the membranes, may be due to ruptured vasa praevia. Fetal distress disproportionate to blood loss may be suggestive of vasa praevia.
Management
The midwife should call for urgent medical assistance. The fetal heart rate should be monitored. If the mother is in the first stage of labour and the fetus is still alive, an emergency caesarean section is carried out. If in the second stage of labour, delivery should be expedited and a vaginal birth may be achieved. Caesarean section may be carried out but mode of birth will be dependent on parity and fetal condition.
There is a high fetal mortality associated with this emergency and a paediatrician should therefore be present for the birth. If the baby is born alive, resuscitation, haemoglobin estimation and a blood transfusion will be necessary.
Presentation and prolapse of the umbilical cord (see Box 33.1)
Predisposing factors
These are the same for both presentation and prolapse of the cord. Any situation where the presenting part is neither well applied to the lower uterine segment nor well down in the pelvis may make it possible for a loop of cord to slip down in front of the presenting part. Such situations include:
Cord presentation
This occurs when the umbilical cord lies in front of the presenting part, with the fetal membranes still intact.
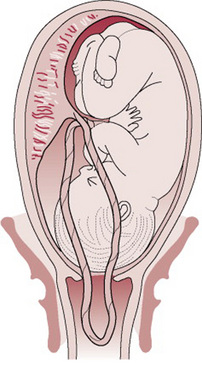
Cord prolapse
The cord lies in front of the presenting part and the fetal membranes are ruptured (see Fig. 33.1).
High head
If the membranes rupture spontaneously when the fetal head is high, a loop of cord may be able to pass between the uterine wall and the fetus resulting in its lying in front of the presenting part. As the presenting part descends the cord becomes trapped and occluded.
Multiparity
The presenting part may not be engaged when the membranes rupture and malpresentation is more common.
Prematurity
The size of the fetus in relation to the pelvis and the uterus allows the cord to prolapse. Babies of very low birth weight (<1500 g) are particularly vulnerable (Mesleh et al 1993, Lin 2006).
Malpresentation
Cord prolapse is associated with breech presentation, especially complete or footling breech. This relates to the ill-fitting nature of the presenting parts and also the proximity of the umbilicus to the buttocks. In this situation, the degree of compression may be less than with a cephalic presentation, but there is still a danger of asphyxia.
Shoulder and compound presentation and transverse lie carry a high risk of prolapse of the cord, occurring with spontaneous rupture of the membranes.
Face and brow presentations are less common causes of cord prolapse (see Ch. 31).
Multiple pregnancy
Malpresentation, particularly of the second twin, is more common in multiple pregnancy.
Polyhydramnios
The cord is liable to be swept down in a gush of liquor if the membranes rupture spontaneously. Controlled release of liquor during artificial rupture of the membranes is sometimes performed to try to prevent this.
Murphy & MacKenzie (1995) found in 55 cases out of a total 132 that none of the above factors could be attributed as the reason for cord prolapse.
Cord presentation
This is diagnosed on vaginal examination when the cord is felt behind intact membranes. It is, however, rarely detected but may be associated with aberrations in fetal heart monitoring such as decelerations, which occur if the cord becomes compressed.
Management
Under no circumstances should the membranes be ruptured. The midwife should discontinue the vaginal examination, in order to reduce the risk of rupturing the membranes. Medical aid should be summoned. To assess fetal well-being continuous electronic fetal monitoring should be commenced or the fetal heart should be auscultated as continuously as possible. The mother should be helped into a position that will reduce the likelihood of cord compression. Caesarean section is the most likely outcome.
Cord prolapse
Diagnosis
Whenever there are factors present that predispose to cord prolapse (Fig. 33.1), a vaginal examination should be performed immediately on spontaneous rupture of membranes.
Bradycardia, and variable or prolonged decelerations of the fetal heart are associated with cord compression, which may be caused by cord prolapse. The diagnosis of cord prolapse is made when the cord is felt below or beside the presenting part on vaginal examination. The cord may be felt in the vagina or in the cervical os or a loop of cord may be visible at the vulva (Lin 2006).
Immediate action
Where the diagnosis of cord prolapse is made, the time should be noted and the midwife should call for urgent assistance. The midwife should explain her findings and emergency measures that may be needed to the mother and her birth partner.
If an oxytocin infusion is in progress this should be stopped. If the cord lies outside the vagina, then it should be gently replaced to prevent spasm, to maintain temperature and prevent drying. Administering oxygen to the mother by face mask at 4 L/min may improve fetal oxygenation.
Relieving pressure on the cord
The risks to the fetus are hypoxia and death as a result of cord compression. The risks are greatest with prematurity and low birth weight (Murphy & MacKenzie 1995). The midwife may need to keep her fingers in the vagina and hold the presenting part off the umbilical cord, especially during a contraction. The mother can be helped to change position to further reduce pressure on the cord. Raising her pelvis and buttocks or adopting the knee–chest position will cause the fetus to gravitate towards the diaphragm (Fig. 33.2). The foot of the bed may also be raised (Trendelenburg position) to relieve compression on the cord. Alternatively, the mother can be helped to lie on her left side, with a wedge or pillow elevating her hips (exaggerated Sims’ position) (Fig. 33.3). There is some evidence to suggest that bladder filling may also be an effective technique for managing cord prolapse (Houghton 2006, Katz et al 1988). A self-retaining 16G Foley catheter is used to instil approximately 500–700 mL of sterile saline into the bladder. The full bladder can relieve compression of the cord by elevating the presenting part about 2 cm above the ischial spines until delivery by caesarean section. The bladder would be drained in theatre immediately before delivery.
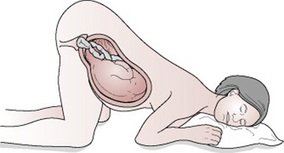
Figure 33.2 Knee–chest position. Pressure on the umbilical cord is relieved as the fetus gravitates towards the fundus.
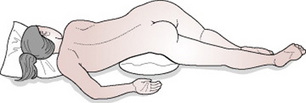
Figure 33.3 Exaggerated Sims’ position. Pillows or wedges are used to elevate the woman’s buttocks to relieve pressure on the umbilical cord.
Birth must be expedited with the greatest possible speed to reduce the mortality and morbidity associated with this condition. Caesarean section is the treatment of choice in those instances where the fetus is still alive and vaginal birth is not imminent.
If cord prolapse is diagnosed in the second stage of labour, with a multiparous mother, the midwife may perform an episiotomy to expedite the birth. Where the presentation is cephalic, assisted birth may be achieved through ventouse or forceps.
If cord prolapse occurs in the community, emergency transfer to hospital is essential. The midwife should carry out the same procedures to relieve the compression on the cord. Consultant unit staff should be informed and be prepared to perform an emergency caesarean section.
Shoulder dystocia
Definition
The term ‘shoulder dystocia’ is used in this chapter to describe failure of the shoulders to traverse the pelvis spontaneously after birth of the head (Smeltzer 1986). However, a universally accepted definition of shoulder dystocia has yet to be produced (Roberts 1994).
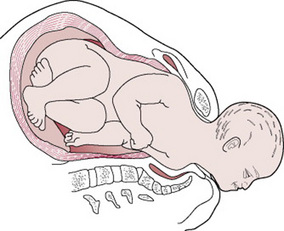
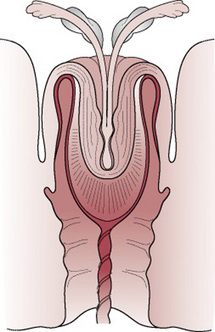
The anterior shoulder becomes trapped behind or on the symphysis pubis, while the posterior shoulder may be in the hollow of the sacrum or high above the sacral promontory (Fig. 33.4). This is, therefore, a bony dystocia, and traction at this point will further impact the anterior shoulder, impeding attempts at delivery.
Incidence
Shoulder dystocia is not a common emergency: the incidence is reported as varying between 0.37% and 1.1% (Bahar 1996).
Risk factors
Although it would be useful to identify those women at risk from a birth complicated by shoulder dystocia, most risk factors can give only a high index of suspicion (Al-Najashi et al 1989). Antenatally, these risk factors include post-term pregnancy, high parity, maternal age over 35 and maternal obesity (weight over 90 kg).
Fetal macrosomia (birth weight over 4000 g) has been associated with an increased risk of shoulder dystocia, the incidence increasing as birth weight increases (Delpapa & Mueller-Heubach 1991, Hall 1996). However, ultrasound scanning for prediction of macrosomia to prevent shoulder dystocia has a poor record of success (Combs et al 1993, Hall 1996). If a large baby is suspected then this fact must be communicated clearly to the team caring for the woman in labour (CESDI 1999, p 47).
Maternal diabetes and gestational diabetes have been identified as important risk factors (Athukorala 2007). In diabetic women, a previous birth complicated by shoulder dystocia increases the risk of recurrence to 9.8%; this compares with a risk of recurrence of 0.58% in the general population (Smith et al 1994).
In labour, risk factors that have been consistently linked with shoulder dystocia include oxytocin augmentation, prolonged labour, prolonged second stage of labour and operative deliveries (Al-Najashi et al 1989, Bahar 1996, Benedetti & Gabbe 1978, Keller et al 1991). For a clinically suspected large baby the delivery team must be alert for the possibility of shoulder dystocia (CESDI 1999).
Warning signs and diagnosis
The birth may have been uncomplicated initially, but the head may have advanced slowly and the chin may have had difficulty in sweeping over the perineum. Once the head is born, it may look as if it is trying to return into the vagina, which is caused by reverse traction.
Shoulder dystocia is diagnosed when manoeuvres normally used by the midwife fail to assist the birth (Resnik 1980).
Management (see Box 33.2 for the HELPERR mnemonic)
Upon diagnosing shoulder dystocia, the midwife must summon help immediately. An obstetrician, an anaesthetist and a person proficient in neonatal resuscitation should be called.
Shoulder dystocia is a frightening experience for the mother, for her partner and for the midwife. The midwife should keep calm and try to explain as much as possible to the mother to ensure her full cooperation for the manoeuvres that may be needed to complete the birth.
The purpose of all these manoeuvres is to disimpact the shoulders. The principle of using the most simple manoeuvres first should be applied. The midwife will need to make an accurate and detailed record of the type of manoeuvre(s) used and the time taken, the amount of force used and the outcome of each manoeuvre attempted.
Non-invasive procedures
Change in maternal position
Any change in the maternal position may be useful to help release the fetal shoulders as shoulder dystocia is a mechanical obstruction. However, certain manoeuvres have proved useful and are described below. It is anticipated that following the use of one or more of these manoeuvres the midwife should be able to proceed with the birth.
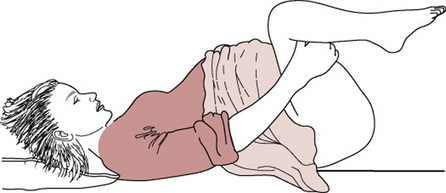
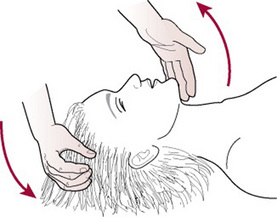
The McRoberts manoeuvre
This manoeuvre involves helping the woman to lie flat and to bring her knees up to her chest as far as possible (Fig. 33.5).
This manoeuvre will rotate the angle of the symphysis pubis superiorly and use the weight of the mother’s legs to create gentle pressure on her abdomen, releasing the impaction of the anterior shoulder (Gonik et al 1983, 1989). It is the manoeuvre associated with the lowest level of morbidity and requires the least force to assist the birth (Bahar 1996, Gross et al 1987, Nocon et al 1993).
Suprapubic pressure
Pressure should be exerted on the side of the fetal back and towards the fetal chest. This manoeuvre may help to adduct the shoulders and push the anterior shoulder away from the symphysis pubis into the larger oblique or transverse diameter (Fig. 33.6).
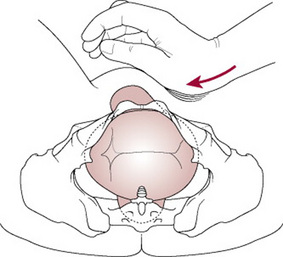
Figure 33.6 Correct application of suprapubic pressure for shoulder dystocia.
(After Pauerstein C 1987, with permission.)
All fours position
The all-fours position (or Gaskin manoeuvre) is achieved by assisting the mother onto her hands and knees; the act of turning the mother may be the most useful aspect of this manoeuvre (Bruner et al 1998). In shoulder dystocia, the impaction is at the pelvic inlet and the force of gravity will keep the fetus against the anterior aspect of the mother’s uterus and pelvis. However, this manoeuvre may be especially helpful if the posterior shoulder is impacted behind the sacral promontory as this position optimizes space available in the sacral curve and may allow the posterior shoulder to be delivered first. In a retrospective study of 82 consecutive cases of shoulder dystocia and the all fours manoeuvre, 83% of women were delivered with no other manoeuvres required (Bruner et al 1998). Manipulative manoeuvres can be performed while the woman is on all fours but clear verbal communication is needed as eye contact is difficult.
Manipulative procedures
Where non-invasive procedures have not been successful, direct manipulation of the fetus must now be attempted.
Positioning of the mother
The McRoberts position as detailed above can be used, or the mother could be placed in the lithotomy position with her buttocks well over the end of the bed so that there is no restriction on the sacrum. If neither the McRoberts nor lithotomy positions are appropriate, then the all-fours position may prove useful. Any of the following manoeuvres can be undertaken with the mother in one of these positions.
Episiotomy
The problem facing the midwife is an obstruction at the pelvic inlet which is a bony dystocia, not an obstruction caused by soft tissue. Although episiotomy will not help to release the shoulders per se, the midwife should consider the need to perform one (see Ch. 28) to gain access to the fetus without tearing the perineum and vaginal walls.
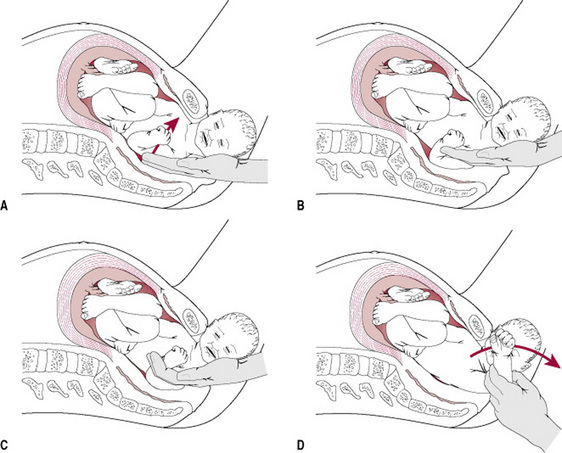
Rubin’s manoeuvre
This manoeuvre (Rubin 1964) requires the midwife to identify the posterior shoulder on vaginal examination, then to push the posterior shoulder in the direction of the fetal chest, thus rotating the anterior shoulder away from the symphysis pubis. By adducting the shoulders this manoeuvre reduces the 12 cm bisacromial diameter (Fig. 33.7).
Woods’ manoeuvre
Woods’ (1943) manoeuvre requires the midwife to insert her hand into the vagina and identify the fetal chest. Then, by exerting pressure on to the posterior fetal shoulder, rotation is achieved. Although this manoeuvre does abduct the shoulders, it will rotate the shoulders into a more favourable diameter and enable the midwife to complete the birth (Fig. 33.8).
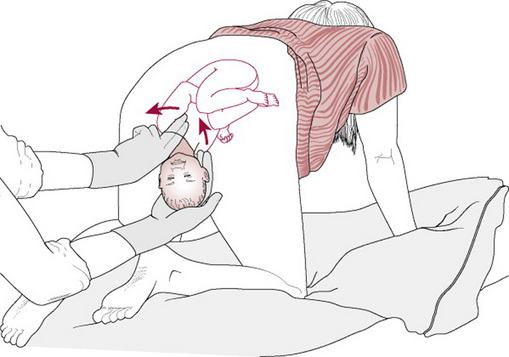
Delivery of the posterior arm
The midwife has to insert her hand into the vagina making use of the space created by the hollow of the sacrum (Fig. 33.9A, B). Then two fingers splint the humerus of the posterior arm (Fig. 33.9C), flex the elbow and sweep the forearm over the chest to deliver the hand (Fig. 33.9D) (O’Leary 1992). If the rest of the birth is not then accomplished, the second arm can be delivered following rotation of the shoulder using either Woods’ or Rubin’s manoeuvre or by reversing the Løvset manoeuvre (see Ch. 31).
Zavanelli manoeuvre
If the manoeuvres described above have been unsuccessful, the obstetrician may consider the Zavanelli manoeuvre (Sandberg 1985) as a last hope for birth of a live baby.
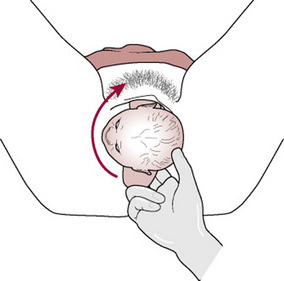
The Zavanelli manoeuvre requires the reversal of the mechanisms of birth so far and reinsertion of the fetal head into the vagina. Delivery is then completed by caesarean section.
Method. The head is returned to its pre-restitution position (Fig. 33.10A). Pressure is then exerted on-to the occiput and the head is returned to the vagina (Fig. 33.10B). Prompt delivery by caesarean section is then required.
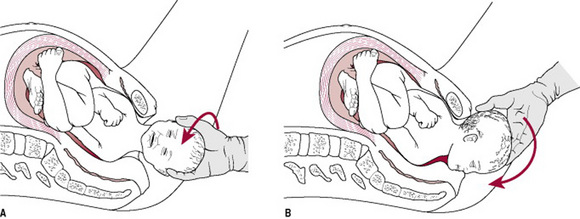
Figure 33.10 The Zavanelli manoeuvre: (A) Head being returned to direct anteroposterior (pre-restitution) position. (B) Head being returned to the vagina.
(After Sandberg 1985, with permission.)
Symphysiotomy
Symphysiotomy is the surgical separation of the symphysis pubis and is used to enlarge the pelvis to enable the birth. It is usually performed in cases of cephalopelvic disproportion and is used more routinely in the developing world. There are a few recorded cases where symphysiotomy has been used successfully to relieve shoulder dystocia (Reid & Osuagwu 1999), but the procedure has usually been associated with a high level of maternal morbidity. The rarity of reported cases makes it difficult to assess the technique for the relief of shoulder dystocia.
Outcomes following shoulder dystocia
Maternal
Approximately two-thirds will have a blood loss of >1000 mL from injury associated with the birth (Benedetti & Gabbe 1978). Maternal death from uterine rupture has been reported following the use of fundal pressure (Seigworth 1966) and from haemorrhage during and following the birth (O’Leary 1992).
Fetal
Neonatal asphyxia may occur following shoulder dystocia in 5.7–9.7% of cases and the attending paediatrician must be experienced in neonatal resuscitation (CESDI 1999, Naef & Morrison 1994).
Brachial plexus injury with damage to cervical nerve roots 5 and 6 may result in an Erb’s palsy (see also Ch. 45). This is commonly associated with shoulder dystocia when the head and neck have been twisted and pulled (Ubachs et al 1995).
Neonatal morbidity may be as high as 42% following shoulder dystocia. Fetal damage may occur even with excellent management using appropriate obstetric manoeuvres (Naef & Morrison 1994). Shoulder dystocia remains a cause of intrapartum fetal death (CESDI 1999).
The midwife must ensure that she takes opportunities to practise the practical skills needed to complete a birth complicated by shoulder dystocia. Mannequins may help in simulation training and practice drills (Crofts et al 2006).
Rupture of the uterus
Rupture of the uterus is one of the most serious complications in midwifery and obstetrics. It is often fatal for the fetus and may also be responsible for the death of the mother. It remains a significant problem worldwide. With effective antenatal and intrapartum care some cases may be avoided.
Rupture of the uterus is defined as being complete or incomplete:
Dehiscence of an existing uterine scar may also occur. This involves rupture of the uterine wall but the fetal membranes remain intact. The fetus is retained within the uterus and not expelled into the peritoneal cavity (Cunningham et al 1997).
Causes
Cases of spontaneous rupture of an unscarred uterus in primigravid mothers are reported in the literature (Guirgis & Kettle 1989, Roberts & Trew 1991), but are rare.
Rupture of the uterus can be precipitated in the following circumstances:
Signs of intrapartum rupture of the uterus
Complete rupture of a previously non-scarred uterus may be accompanied by sudden collapse of the mother, who complains of severe abdominal pain. The maternal pulse rate increases; simultaneously, alterations of the fetal heart may occur, including the presence of variable decelerations (Flannelly et al 1993, Phelan 1990). Three intrapartum fetal deaths associated with ruptured uterus were reported (Acolet 2007). There may be evidence of fresh vaginal bleeding. The uterine contractions may stop and the contour of the abdomen alters. The fetus becomes palpable in the abdomen as the presenting part regresses. The degree and speed of the mother’s collapse and shock depend on the extent of the rupture and the blood loss (Box 33.3).
Incomplete rupture of the uterus
Incomplete rupture may have an insidious onset found only after birth or during a caesarean section. This type is more commonly associated with previous caesarean section. Blood loss associated with dehiscence, or incomplete rupture, can be scanty, as the rupture occurs along the fibrous scar tissue which is avascular (O’Connor & Gaughan 1993).
Whenever shock during the third stage of labour is more severe than the type of birth or blood loss warrants, or the mother fails to respond to treatment given, the possibility of incomplete rupture should be considered. Incomplete rupture may also be manifest as a cause of abdominal pain and or postpartum haemorrhage following vaginal birth.
Management
All units should have a protocol for dealing with uterine rupture. An immediate caesarean section is performed in the hope of delivering a live baby. Following the birth of the baby and placenta, the extent of the rupture can be assessed. Choice between the options to perform a hysterectomy or to repair the rupture depends on the extent of the trauma and the mother’s condition. Further clinical assessment will include evaluation of the need for blood replacement and management of any shock.
The mother will be unprepared for the events that have occurred and therefore may be totally opposed to hysterectomy. Reports of successful pregnancy following repair of uterine rupture are available (O’Connor & Gaughan 1993).
Rupture of the uterus following previous caesarean section
The risk of uterine rupture is increased for those women who have a uterine scar. Studies cite figures of between 0.3 and 0.7% of labours following a previous caesarean section (Miller et al 1994, Vause & Macintosh 1999). Rates of rupture are lowest following a lower segment caesarean section.
Amniotic fluid embolism
Amniotic fluid embolism (AFE) is rare, unpredictable and unpreventable (Lewis 2007). AFE occurs when amniotic fluid enters the maternal circulation via the uterus or placental site; maternal collapse can be rapidly progressive.
The body responds to AFE in two phases. The initial phase is one of pulmonary vasospasm causing hypoxia, hypotension, pulmonary oedema and cardiovascular collapse. The second phase sees the development of left ventricular failure, with haemorrhage and coagulation disorder and further uncontrollable haemorrhage. Mortality and morbidity are high (Lewis 2007) though early diagnosis may lead to better outcome (Tuffnell 2002) and early transfer to an intensive care unit is associated with decreased morbidity.
Emergency drills for maternal resuscitation should be regularly practised in clinical areas in all maternity units (Lewis 2007).
Predisposing factors
Amniotic fluid embolism can occur at any gestation. It is mostly associated with labour and its immediate aftermath, but cases in early pregnancy and postpartum have been documented (Clark et al 1995).
Chance entry of amniotic fluid into the circulation under pressure may occur through the uterine sinuses of the placental bed (Clark et al 1995).
The barrier between the maternal circulation and the amniotic sac may be breached during periods of raised intra-amniotic pressure, such as termination of pregnancy or during placental abruption. Procedures, such as ARM and insertion of an intrauterine catheter, have been associated with AFE. Amniotic fluid embolism can also occur during an intrauterine manipulation, such as internal podalic version or during a caesarean section.
It is a condition that is difficult to predict and equally difficult to prevent. Amniotic fluid embolism is associated with a high maternal mortality rate. A total of 17 women died in the years 2003–2005, diagnosis having been confirmed clinically and by post mortem (Lewis 2007).
Clinical signs and symptoms (Box 33.4)
Premonitory signs and symptoms (restlessness, abnormal behaviour, respiratory distress and cyanosis) may occur before collapse (Lewis 2007). There is maternal hypotension and uterine hypertonus. The latter will induce fetal compromise and is in response to uterine hypoxia. Cardiopulmonary arrest follows quickly. Only minutes may elapse before arrest. Blood coagulopathy develops following the initial collapse, if the mother survives.
Emergency action
Any one of the above symptoms is indicative of an acute emergency. As the mother is likely to be in a state of collapse, resuscitation needs to be commenced at once. An emergency team should be called, since the midwife responsible for the care of the mother requires immediate help. If collapse occurs in a community setting, basic life support should be commenced prior to the arrival of emergency services.
Despite improvements in intensive care the outcome of this condition is poor. Specific management for the condition is life support, and high levels of oxygen are required. Mothers who survive are likely to have suffered a degree of neurological impairment (Clark et al 1995).
Complications of amniotic fluid embolism
Disseminated intravascular coagulation (DIC) is likely to occur within 30 min of the initial collapse. In some cases the mother bleeds heavily prior to developing amniotic fluid embolism, which contributes to the severity of her condition. It has also been reported that the amniotic fluid has the ability to suppress the myometrium, resulting in uterine atony.
Acute renal failure is a complication of the excessive blood loss and the prolonged hypovolaemic hypotension. The mother will require continuous assessment of urinary output, using an indwelling catheter. Accurate records of fluid intake and urinary output and urinalysis should be maintained by the midwife. A urinary output of <30 mL/hr should be reported, as should the presence of proteinuria. Prompt transfer to an intensive therapy unit for specialized care improves the outcome in AFE (Lewis 2007). Midwifery support and advice should be continued for the family. The mother should be given the opportunity to talk about emergency treatment when she has recovered sufficiently (Mapp 2005, Mapp & Hudson 2005).
Effect of amniotic fluid embolism on the fetus
Perinatal mortality and morbidity are high where amniotic fluid embolism occurs before the birth of the baby. Delay in the time from initial maternal collapse to delivery needs to be minimal if fetal compromise or death is to be avoided. However, maternal resuscitation may, at that time, be a priority. Box 33.5 is a summary of the key points relating to amniotic fluid embolism.
All cases of suspected or proven amniotic fluid embolism, whether fatal or not, should be reported to the National Amniotic Fluid Embolism Register: UKOSS, National Perinatal Epidemiology Unit, University of Oxford, Old Road Campus, Old Road, Headington, Oxford OX3 7LF.
Acute inversion of the uterus
This is a rare but potentially life-threatening complication of the third stage of labour. It occurs in approximately 1 in 20 000 births (Calder 2000). A midwife’s awareness of the precipitating factors enables her to take preventive measures to avoid this emergency.
Causes
Causes of acute inversion are associated with uterine atony and cervical dilatation, and include:
Careful management of the third stage of labour is needed to prevent inversion. In active management of the third stage, palpation of the fundus is essential to confirm that contraction has taken place, prior to controlled cord traction.
Warning signs and diagnosis
The major sign of acute inversion is profound shock and usually haemorrhage. The blood loss is within a range of 800–1800 mL (Platt & Druzin 1981, Watson et al 1980).
Inversion of the uterus will cause the woman severe abdominal pain. On palpation of the uterus, the midwife may feel an indentation of the fundus. Where there is a major degree of inversion the uterus may not be palpable abdominally but may be felt upon vaginal examination or in a severe case the uterus may be seen at the vulva.
The pain is thought to be caused by the stretching of the peritoneal nerves and the ovaries being pulled as the fundus inverts. Bleeding may or may not be present, depending on the degree of placental adherence to the uterine wall. The cause of the symptoms may not be readily apparent and diagnosis may be missed if inversion is incomplete.
Management
Immediate action
A swift response is needed to reduce the risks to the mother.
Throughout the events the mother and her partner should be kept informed of what is happening. Assessment of vital signs, including level of consciousness, is of utmost importance.
Medical management
The hydrostatic method of replacement involves the instillation of several litres of warm saline infused through a giving set into the vagina. The pressure of the fluid builds up in the vagina and restores the uterus to the normal position, while the operator seals off the introitus by hand or using a soft ventouse cup.
If the inversion cannot be replaced manually a cervical constriction ring may have developed. Drugs can be given to relax the constriction and facilitate the return of the uterus to its normal position. Surgical correction via a laparotomy may be needed to correct inversion. Full support and explanation of the emergency should be offered to the mother in the postnatal period (Mapp 2005, Mapp & Hudson 2005).
Basic life-support measures
Cardiac arrests are rare in maternity units but they can and do happen and their management is sometimes suboptimal. All medical and midwifery staff should be trained to a nationally recognized level: Basic Life Support, Immediate Life Support or Advanced Life support (BLS, ILS and ALS), as appropriate (Lewis 2007).
Emergency drills for maternal resuscitation should be regularly practised in clinical areas in all maternity units. These drills should include the identification of the equipment required and appropriate methods for ensuring that cardiac arrest teams know the location of the maternity unit and theatres in order to arrive promptly. Specialized courses such as Advanced Life Support in Obstetrics (ALSO) and Managing Obstetric Emergencies and Trauma (MOET) provide additional training for obstetric, midwifery and other staff.
Standards of basic life support have been agreed internationally for health professionals and lay people (Resuscitation Council UK 2005). Basic life support refers to the maintenance of an airway and support for breathing, without any specialist equipment other than possibly a pharyngeal airway. The basic principles are:
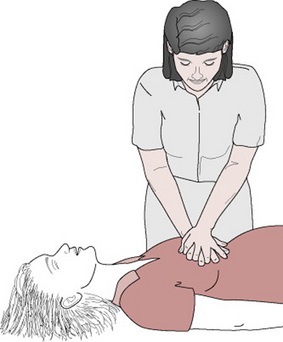
Figure 33.13 Chest compression. The midwife leans well over the patient, with arms straight. Hands are one on top of the other with fingers interlinked. The heel of the hand is used to compress the chest.
Chest compression and rescue breathing should be continued until help arrives and until those experienced in resuscitation are able to take over. A ratio of 30 chest compression to two breaths should be maintained (Resuscitation Council UK 2005).
The exact sequences of resuscitation will depend on the training of staff and their experience in assessment of breathing and circulation.
These measures are summarized in Box 33.6.
Shock
Shock is a complex syndrome involving a reduction in blood flow to the tissues that may result in irreversible organ damage and progressive collapse of the circulatory system. If left untreated it will result in death. Shock can be acute but prompt treatment results in recovery, with little detrimental effect on the mother. However, inadequate treatment or failure to initiate effective treatment can result in a chronic condition ending in multisystem organ failure, which may be fatal.
Shock can be classified as follows:
This section deals with the principles of hypovolaemic shock and septic shock, either of which may develop as a consequence of childbirth.
Hypovolaemic shock
This is caused by any loss of circulating fluid volume, as in haemorrhage, but may also occur when there is severe vomiting. The body reacts to the loss of circulating fluid in stages as follows:
Initial stage
The reduction in fluid or blood decreases the venous return to the heart. The ventricles of the heart are inadequately filled, causing a reduction in stroke volume and cardiac output. As cardiac output and venous return fall, the blood pressure is reduced. The drop in blood pressure decreases the supply of oxygen to the tissues and cell function is affected.
Compensatory stage
The drop in cardiac output produces a response from the sympathetic nervous system through the activation of receptors in the aorta and carotid arteries. Blood is redistributed to the vital organs. Vessels in the gastrointestinal tract, kidneys, skin and lungs constrict. This response is seen by the skin becoming pale and cool. Peristalsis slows, urinary output is reduced and exchange of gas in the lungs is impaired as blood flow diminishes. The heart rate increases in an attempt to improve cardiac output and blood pressure. The pupils of the eyes dilate. The sweat glands are stimulated and the skin becomes moist and clammy. Adrenaline (epinephrine) is released from the adrenal medulla and aldosterone from the adrenal cortex. Antidiuretic hormone (ADH) is secreted from the posterior lobe of the pituitary. Their combined effect is to cause vasoconstriction, increased cardiac output and a decrease in urinary output. Venous return to the heart will increase but, unless the fluid loss is replaced, will not be sustained.
Progressive stage
This stage leads to multisystem failure. Compensatory mechanisms begin to fail, with vital organs lacking adequate perfusion. Volume depletion causes a further fall in blood pressure and cardiac output. The coronary arteries suffer lack of supply. Peripheral circulation is poor, with weak or absent pulses.
Effect of shock on organs and systems
The human body is able to compensate for loss of up to 10% of blood volume, principally by vasoconstriction. When that loss reaches 20–25%, however, the compensatory mechanisms begin to decline and fail. In pregnancy the plasma volume increases, as does the red cell mass. The increase is not proportionate, but allows a healthy pregnant woman to sustain significant blood loss at birth as the plasma volume is reduced with little disturbance to normal haemodynamics. In a woman who has not had a healthy increase in plasma volume, or has sustained an antepartum haemorrhage, a much lower blood loss is required to have a pathological effect on the body and its systems. Individual organs are affected as below.
Brain
The level of consciousness deteriorates as cerebral blood flow is compromised. The mother will become increasingly unresponsive. She may not respond to verbal stimuli and there is a gradual reduction in the response elicited from painful stimulation.
Lungs
Gas exchange is impaired as the physiological dead space increases within the lungs. Levels of carbon dioxide rise and arterial oxygen levels fall. Ischaemia within the lungs alters the production of surfactant and, as a result of this, the alveoli collapse. Oedema in the lungs, due to increased permeability, exacerbates the existing problem of diffusion of oxygen. Atelectasis, oedema and reduced compliance impair ventilation and gaseous exchange, leading ultimately to respiratory failure. This is known as adult respiratory distress syndrome (ARDS).
Kidneys
The renal tubules become ischaemic, owing to the reduction in blood supply. As the kidneys fail, urine output falls to less than 20 mL/hr. The body does not excrete waste products such as urea and creatinine, so levels of these in the blood rise.
Gastrointestinal tract
The gut becomes ischaemic and its ability to function as a barrier against infection wanes. Gram-negative bacteria are able to enter the circulation.
Liver
Drug and hormone metabolism ceases, as does the conjugation of bilirubin. Unconjugated bilirubin builds up and jaundice develops. Protection from infection is further reduced as the liver fails to act as a filter. Metabolism of waste products does not occur, so there is a build-up of lactic acid and ammonia in the blood. Death of hepatic cells releases liver enzymes into the circulation.
Management
Urgent resuscitation is needed to prevent the mother’s condition deteriorating and causing irreversible damage. Women who decline blood products must have their wishes respected and a treatment plan in case of haemorrhage should be discussed with them before labour (Lewis 2007).
Assessment of clinical condition
An interprofessional team approach to management should be adopted to ensure that the correct level of expertise is available. A clear protocol for the management of shock should be used, with the midwife fully aware of key personnel required. Once the mother’s immediate condition is stable, the midwife should continue to assess and record the woman’s condition or liaise with staff on the intensive care unit if the woman is transferred.
Hypovolaemic shock in pregnancy will reduce placental perfusion and oxygenation to the fetus. This will result in fetal distress and possibly death. Where maternal shock is caused by antepartum factors, the midwife should determine whether the fetal heart is present, but as swift and aggressive treatment may be required to save the mother’s life this should be the first priority.
Detailed observation charts including fluid balance should be accurately maintained. The extent of the mother’s illness may require her transfer to a critical care unit.
Observations and clinical signs of deterioration in hypovolaemic shock
Box 33.7 is a summary of key points relating to hypovolaemic shock.
Central venous pressure
Central venous pressure (CVP) is the pressure in the right atrium or superior vena cava. It is an indicator of the volume of blood returning to the heart and reflects the competence of the heart as a pump and the peripheral vascular resistance. In the presence of acute peripheral circulatory failure, which accompanies severe shock, the monitoring of CVP aids assessment of blood loss and indicates the fluid replacement required. In such a situation it is extremely dangerous to base an intravenous regimen on guesswork. Hyper- or hypovolaemia, cardiac and renal failure may result.
The normal pressure varies between 5 and 10 cm H2O. In shock the pressure will be persistently low (i.e. below 5 cm) and may even register a negative reading, indicating hypovolaemia. The correct volume of replacement fluids may then be assessed with greater accuracy.
Method of measuring CVP
A catheter is inserted by a doctor (usually an anaesthetist) into a major vein such as the subclavian or external jugular vein and advanced into the right atrium. The catheter is then connected to a manometer and an intravenous infusion using a three-way tap.
To take a manometer reading, the mother should be lying flat and the base of the manometer should be calibrated to measure 0 cm of water when aligned with the level of the right atrium (Fig. 33.14). This point is level with a mid-axillary line for most people. The three-way tap is opened and filled with intravenous fluid. The fluid will fall and rise with respiratory effort and should be allowed to stabilize before a reading is taken. The highest level the fluid reaches is used for the CVP measurement. Once the reading is completed the tap is returned to the infusion position.
A baseline observation is taken when the CVP catheter is inserted and the position in which the mother was lying is noted. Minor changes in position should be noted, as they may alter the CVP reading.
Principles of care of CVP lines
Additional complications include pneumothorax, hydrothorax, trauma to lung or veins and cardiac arrhythmias during and due to insertion.
Septic shock
This is a distributive form of shock, where an overwhelming infection develops. Certain organisms produce toxins that cause fluid to be lost from the circulation into the tissues. The commonest form of sepsis causing death in childbearing in the UK is reported to be that caused by beta haemolytic Streptococcus pyrogenes (Lancefield Group A) (Lewis 2007). This is a Gram-positive organism, responding to intravenous antibiotics, specifically those that are penicillin based. In the general population, infections from Gram-negative organisms such as Escherichia coli, Proteus or Pseudomonas pyocyaneus are predominant, which are common pathogens in the female genital tract.
The placental site is the main point of entry for an infection associated with pregnancy and childbirth. This may occur following prolonged rupture of fetal membranes, obstetric trauma, septic abortion or in the presence of retained placental tissue. A total of 22 women died over 3 years as a result of genital tract sepsis in the last Confidential Enquiry (Lewis 2007). Of these, 18 were counted as Direct deaths, one as early pregnancy and three late deaths, i.e. >6 weeks postpartum.
Clinical signs
The mother may present with a sudden onset of tachycardia, pyrexia, rigors and tachypnoea. The mother may also exhibit a change in her mental state and gastrointestinal symptoms are common in pelvic sepsis. Signs of shock, including hypotension, develop in septic shock as the condition takes hold.
Haemorrhage may be present. This could be a direct result of events due to childbearing, but it occurs in septic shock because of DIC (Ch. 20).
Management
This is based on preventing further deterioration by restoring circulatory volume and then eradication of the infection. Replacement of fluid volume will restore perfusion of the vital organs. Satisfactory oxygenation is also needed.
Measures are needed to identify the source of infection and to protect against reinfection by maintaining high standards of care in clinical procedures. A full infection screening should be carried out including a high vaginal swab, midstream specimen of urine and blood cultures. Infusion sites and indwelling catheters should be checked for signs of contamination and changed as appropriate. Rigorous treatment with intravenous antibiotics, after blood cultures have been taken, is essential to halt the illness.
Retained products of conception can be detected on ultrasound, and these can then be removed.
In all situations where the mother requires to be transferred for critical or intensive care, relatives should be kept informed of progress. The midwife may be the person with whom the relatives have formed a relationship and therefore is relied on to give information.
Conclusion
The emergency situations included in this chapter are rare, but the actions of the midwife are fundamental to the well-being of mother, baby and also the partner. Awareness of local emergency procedures and knowledge of correct use of any supportive equipment are essential. Midwives in all practice settings should maintain skills that enable them to act in an emergency. The use of multiprofessional workshops to rehearse simulated situations can ensure that all members of the care team know exactly what is required when needed. Midwives should also engage in reviews of practice to ensure that policies and protocols are regularly reviewed to incorporate best practice and current evidence.
Acolet D. CEMACH Confidential Enquiries into Maternal and Child Health. Perinatal Mortality 2005 England and Wales and Northern Ireland, CEMACH, London, 2007. Online. Available: www.cemach.org.uk.
Alderson P, Schierhout G, Roberts I, et al. Colloids versus crystalloids for fluid resuscitation in critically ill patients (Cochrane review). The Cochrane Library. (Issue 3):2001. Update Software Oxford
Al-Najashi S, Al-Suleiman SA, El-Yahia A, et al. Shoulder dystocia a clinical study of 56 cases. Australian and New Zealand Journal of Obstetrics and Gynaecology. 1989;29:129-131.
Athukorala C, Crowther C, Wilson K, et al. Women with gestational diabetes mellitus in the ACHOIS trial: Risk factors for shoulder dystocia. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2007;47:37-41.
Awwad JT, Azar GB, Aswad NK, et al. Uterine rupture in pregnancy caused by blast injury with fetal survival. Journal of Obstetrics and Gynaecology. 1993;13(6):448.
Bahar AM. Risk factors and fetal outcome in cases of shoulder dystocia compared with normal deliveries of a similar birthweight. British Journal of Obstetrics and Gynaecology. 1996;103:868-872.
Benedetti TJ, Gabbe SG. Shoulder dystocia: a complication of fetal macrosomia and prolonged second stage of labour with mid pelvic delivery. Obstetrics and Gynecology. 1978;52(5):526-529.
Brar HS, Greenspoon JS, Platt LD, et al. Acute puerperal uterine inversion. New approaches to management. Journal of Reproductive Medicine. 1989;34(2):173-177.
Bruner JP, Drummond SB, Meenan AL, et al. Journal of Reproductive Medicine. 1998;43(5):439-443.
Calder AA. Emergencies in operative obstetrics. Baillière’s Clinical Obstetrics and Gynaecology. 2000;14(1):43-55.
CESDI (Confidential enquires into stillbirths and deaths in infancy). Sixth annual report. London: Maternal and Child Health Research Consortium, 1999.
Clark SL, Hankins GD V, Dudley DA, et al. Amniotic fluid embolism: an analysis of the national registry. American Journal of Obstetrics and Gynecology. 1995;172:1158-1169.
Combs CA, Singh NB, Khoury JC. Elective induction versus spontaneous labour after sonographic diagnosis of fetal macrosomia. Obstetrics and Gynecology. 1993;81(4):492-496.
Crofts JF, Bartlett C, Ellis D, et al. Training for shoulder dystocia. A trial of simulation using low-fidelity and high fidelity mannequins. Obstetrics and Gynecology. 2006;108(6):1477-1485.
Cunningham FG, MacDonald PC, Gant NF, et al. Williams obstetrics, 20th edn. Prentice Hall, London, 1997;773.
Delpapa E, Mueller-Heubach E. Pregnancy outcome following ultrasound diagnosis of macrosomia. Obstetrics and Gynecology. 1991;78(1):340-343.
DoH (Department of Health), Welsh Office, Scottish Home and Health Department, Department of Health and Social Services, Northern Ireland. Report on confidential enquiries into maternal deaths in the United Kingdom 1991–1993. London: HMSO, 1996.
Flannelly GM, Turner MJ, Rassmussen MJ, et al. Rupture of the uterus in Dublin: an update. Journal of Obstetrics and Gynaecology. 1993;13:440-443.
Gonik B, Allen Stringer C, Held B. An alternate maneuver for management of shoulder dystocia. American Journal of Obstetrics and Gynecology. 1983;145:882-883.
Gonik B, Allen R, Sorab J. Objective evaluation of the shoulder dystocia phenomenon: effect of maternal pelvic orientation on force reduction. Obstetrics and Gynecology. 1989;74(1):44-48.
Gross SJ, Shime J, Forrine D. Shoulder dystocia: predictors and outcome. A five year review. American Journal of Obstetrics and Gynecology. 1987;56(2):334-336.
Guirgis RR, Kettle MJ. Uterine rupture in a primigravid patient. Journal of Obstetrics and Gynaecology. 1989;9(3):214-215.
Hall M. Guessing the weight of the baby. British Journal of Obstetrics and Gynaecology. 1996;103:734-736.
Houghton G. Bladder filling: an effective technique for managing cord prolapse British. Journal of Midwifery. 2006;14(2):88-89.
Howe RS. Third trimester uterine rupture following hysteroscopic uterine perforation. Obstetrics and Gynecology. 1993;81:827-829.
Katz Z, Shoham Z, Lancet M, et al. Management of labour with umbilical cord prolapse: a five year study. Obstetrics and Gynaecology. 1988;72(2):278-281.
Keller JD, Lopez JA, Dooley SL, et al. Shoulder dystocia and birth trauma in gestational diabetes: a five year experience. American Journal of Obstetrics and Gynecology. 1991;165:928-930.
Lewis G. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving mothers’ lives: reviewing maternal deaths to make motherhood safer 2003–2005. The seventh report on Confidential Enquiries into Maternal Deaths in the United Kingdom. CEMACH, London, 2007.
Lin MG. Umbilical cord prolapse. Obstetrical and Gynecological Survey. 2006;61(4):269-277.
Lydon-Rochelle M, Holt VL, Easterling TR, et al. Risk of uterine rupture among women with a prior cesarean delivery. New England Journal of Medicine. 2001;345(1):3-8.
Mallett J, Dougherty L, editors. The Royal Marsden NHS Trust manual of clinical nursing procedures, 5th edn, Oxford: Blackwell Science, 2000.
Mapp T. Feelings and fears during obstetric emergencies 2. British Journal of Midwifery. 2005;13(1):36-40.
Mapp T, Hudson K. Feelings and fears during obstetric emergencies 1. British Journal of Midwifery. 2005;13(1):30-35.
Mesleh R, Sultan M, Sabagh T, et al. Umbilical cord prolapse. Journal of Obstetrics and Gynaecology. 1993;13(1):24-28.
Michiels I, De Valck C, De Loor J, et al. Spontaneous uterine rupture during pregnancy, related to a horse fall 8 weeks earlier. Acta Obstetrica et Gynecologica Scandinavica. 2007;86(3):380-381.
Miller DA, Diaz FG, Paul RH. Vaginal birth after cesarean: a ten year experience. Obstetrics and Gynecology. 1994;84:255-258.
Murphy DJ, MacKenzie IZ. The mortality and morbidity associated with umbilical cord prolapse. British Journal of Obstetrics and Gynaecology. 1995;102:826-830.
Naef RW, Morrison JC. Guidelines for management of shoulder dystocia. Journal of Perinatology. 1994;15(6):435-441.
Nocon JJ, McKenzie DK, Thomas LJ, et al. Shoulder dystocia: an analysis of risk and obstetric maneuvers. American Journal of Obstetrics and Gynecology. 1993;168(6):1732-1739.
O’Connor RA, Gaughan B. Rupture of the gravid uterus and its management. Journal of Obstetrics and Gynaecology. 1993;13:29-33.
O’Leary JA. Shoulder dystocia and birth injury: prevention and treatment. New York: McGraw-Hill, 1992.
Oyelese Y, Smulian JC. Placenta previa, placenta accreta, and vasa previa Obstetrics and Gynecology. 2006;107(4):927-941.
Pauerstein C, editor. Clinical obstetrics. New York: Churchill Livingstone, 1987.
Phelan JP. Uterine rupture. Clinical Obstetrics and Gynecology. 1990;33(3):432-437.
Platt LD, Druzin ML. Acute puerperal inversion of the uterus. American Journal of Obstetrics and Gynecology. 1981;141(2):187-190.
Reid PC, Osuagwu FI. Symphysiotomy in shoulder dystocia. Journal of Obstetrics and Gynaecology. 1999;19(6):664-666.
Resnik R. Management of shoulder girdle dystocia. Clinical Obstetrics and Gynecology. 1980;23(2):559-564.
Resuscitation Council UK Resuscitation guidelines. Resuscitation Council (UK) Trading, London, 2005.
Roberts L. Shoulder dystocia. Studd J, editor. Progress in obstetrics and gynaecology, Vol 11. Churchill Livingstone, Edinburgh, 1994;201-216.
Roberts L, Trew G. Uterine rupture in a primigravida. Journal of Obstetrics and Gynaecology. 1991;11(4):261-262.
Rubin A. Management of shoulder dystocia. Journal of the American Medical Association. 1964;189:835.
Sandberg EC. The Zavanelli maneuver: a potentially revolutionary method for the resolution of shoulder dystocia. American Journal of Obstetrics and Gynecology. 1985;152:479-487.
Seigworth GR. Shoulder dystocia: review of five years experience. Obstetrics and Gynecology. 1966;25(6):764-767.
Smeltzer JS. Prevention and management of shoulder dystocia. Clinical Obstetrics and Gynecology. 1986;29(2):299-308.
Smith RB, Lane C, Pearson JF. Shoulder dystocia: what happens at the next delivery? British Journal of Obstetrics and Gynaecology. 1994;101:713-715.
Sweet BR, Tiran D. Mayes’ midwifery. London: Baillière Tindall, 1996.
Tuffnell DJ. Amniotic fluid embolism Maternal morbidity and mortality. London: RCOG, 2002.
Ubachs JMH, Slooff ACJ, Peeters LLH. Obstetric antecedents of surgically treated obstetric brachial plexus injuries. British Journal of Obstetrics and Gynaecology. 1995;102:813-817.
Usta IM, Hamdi MA, Abu Musa AA, et al. Pregnancy outcome in patients with previous uterine rupture. Acta Obstetrica et Gynecologica Scandinavica. 2007;86(2):172-176.
Vause S, Macintosh M. Use of prostaglandins to induce labour in women with a caesarean scar. British Medical Journal. 1999;318:1056-1058.
Watson P, Besch N, Bowes WA. Management of acute and subacute puerperal inversion of the uterus. Obstetrics and Gynecology. 1980;55(1):12-16.
Woods CE. A principle of physics as applied to shoulder delivery. American Journal of Obstetrics and Gynecology. 1943;45:796-805.
Allott H. A grief shared. British Medical Journal. 1994;6308:602.
A chilling contemporary account of a delivery complicated by shoulder dystocia.
Brook LA, Weindling AM. The paediatric. Clinical focus: shoulder dystocia. Clinical Risk. 1995;1(2):55-60.
Shoulder dystocia from the paediatrician’s perspective; useful descriptions of possible sequelae.
Grady K, Prasad BGR, Howell C. Cardiopulmonary resuscitation in the non-pregnant and pregnant patient. Managing Obstetric Emergencies and Trauma. RCOG Press, London, 2003.
An in depth view of obstetric management.
Hofmeyr GJ, Mohlala BKF. Hypovolaemic shock – best practice in research. Clinical Obstetrics and Gynaecology. 2001;15(4):645-662.
This article presents best practice for the management of hypovolaemic shock in childbearing. It revisits the pathophysiology of the condition and examines some of the evidence that informs current practice.
Jevon P, Raby M. Resuscitation in pregnancy. A practical approach. Books for Midwives, Hale, 2001.
The authors of this book are a resuscitation officer and midwife respectively and they have provided a concise guide that addresses the key issues of resuscitation. The topics covered include those required for effective practice, as well as ethical and professional issues.
Johnstone FD, Myerscough PR. Shoulder dystocia. British Journal of Obstetrics and Gynaecology. 1998;105:811-815.
Gives clinically useful overview of shoulder dystocia from an obstetric perspective.
RCM (Royal College of Midwives). Clinical risk management. Shoulder dystocia. RCM Midwives Journal. 2000;3(11):348-351.
Looks at shoulder dystocia from a risk management perspective with the authority of the RCM.
Resuscitation Council UK, Resuscitation guidelines. London: Resuscitation Council (UK) Trading, 2005.
This publication provides internationally agreed information and guidance on resuscitation and emergency life support. The website www.resus.org.uk contains a range of information and posters that can be downloaded and publications that can be ordered.
Knight M, Kurinczuk JJ, Tuffnell D. The UK Obstetric Surveillance System for rare disorders of pregnancy. British Journal of Obstetrics and Gynaecology. 2005;112(3):263-265.
UKOSS. A system set up to study rare disorders of pregnancy. It is has become part of routine midwifery practice to provide information for the UKOSS Surveillance System, which are used to study uncommon disorders of pregnancy.www.npeu.ox.ac.uk/UKOSS