Chapter 45 Trauma during birth, haemorrhage and convulsions
This chapter focuses on complications occurring in specifically vulnerable babies; the midwife’s awareness of this vulnerability may prevent such complications. However, if a complication does occur, the midwife must report it to the baby’s doctor and may work with that doctor and/or a wider multiprofessional team to diagnose it and implement effective treatment. Parents may be distressed when their baby suffers a complication and the midwife helps them to understand the complication, facilitating their discussions with the multiprofessional team members, and assisting them to care for their baby.
The chapter presents information on
Trauma during birth
Despite skilled midwifery and obstetric care in developed, Western societies and a reduction in the incidence, birth trauma still occurs. Efforts continue to reduce the incidence even further.
Trauma to skin and superficial tissues
Skin
Skin damage is often iatrogenic, resulting from forceps blades (Plate 28), vacuum extractor cups, scalp electrodes and scalpels. Poorly applied forceps blades or vacuum extractor cup may result in scalp abrasion (Plate 29), although fewer problems occur with softer vacuum extractor cups. Forceps blades may cause bruising or superficial fat necrosis. Scalp electrodes cause puncture wounds, as do fetal blood sampling techniques. Occasionally, during uterine incision at caesarean section, laceration of the baby’s skin may occur.
Superficial fat necrosis is very rare and usually presents between days 1–28 with well-defined areas of induration where pressure was applied (Dudink et al 2003). All other skin injuries should be detected during the midwife’s detailed examination of the baby immediately after birth. All trauma should be made known to the parents and reported to the baby’s doctor.
Abrasions and lacerations should be kept clean and dry. If there are signs of infection, further medical consultation should be sought by the midwife or parents. Antibiotics may be required. Deeper lacerations may require closure with butterfly strips or sutures. Healing is usually rapid with no residual scarring (Sorantin et al 2006). There is no specific management for fat necrosis that should spontaneously resolve (Dudink et al 2003).
Superficial tissues
Soft tissue trauma involves oedematous swellings and/or bruising. During labour the fetal part overlying the cervical os may be subjected to pressure, a ‘girdle of contact’, with reduced venous return and resultant congestion and oedema.
Caput succedaneum
With cephalic presentation, there may be a diffuse oedematous swelling under the scalp and above the periosteum, called a caput succedaneum (Fig. 45.1). With an occipitoanterior position, one caput succedaneum may be present. With an occipitoposterior position, a caput succedaneum may form, but if the occiput rotates anteriorly a second caput succedaneum can develop. A second caput succedaneum may also form if, during the second stage of labour, the birth of the head is delayed and the perineum acts as another ‘girdle of contact’. A ‘false’ caput succedaneum can also occur if a vacuum extractor cup is used; because of its distinctive shape, the swelling is known as a ‘chignon’ (Plate 29).
A caput succedaneum is present at birth, does not usually enlarge, can ‘pit’ on pressure, can cross a suture line and the oedema may move to the dependent area of the scalp (Furdon & Clark 2001). The baby will usually experience some discomfort and, although care continues as normal, gentle handling is appropriate. Abrasion of a chignon is possible.
The swelling is usually self-limiting, resolving by 36 hrs of life, with no longer-term consequences (Sorantin et al 2006). An abraded chignon usually heals rapidly if the area is kept clean, dry and is not irritated.
Other injuries
The cervical os may restrict venous return when the fetal presentation is not cephalic. When the face presents, it becomes congested and bruised and the eyes and lips become oedematous. In a breech presentation the fetus will develop bruised and oedematous genitalia and buttocks. This type of trauma is apparent at birth. The baby experiences discomfort and pain therefore gentle handling is essential and mild analgesia may be required.
For babies with bruised or oedematous buttocks, maintaining nappy area hygiene is important and needs to be accomplished without inflicting further trauma to the skin. Barrier ointment or cream applications may be required if disposable nappies designed to limit the contact of urine and faecal fluid with the skin are not available. If skin excoriation does occur, the infection risk increases and consultation with a wound care specialist nurse may be required to ensure best skin care practice.
Uncomplicated oedema and bruising usually resolve within days. However, if the baby suffers significant trauma during a vaginal breech birth, resulting serious complications require specific treatment, and take longer to resolve. These complications include excessive haemolysis resulting in hyperbilirubinaemia; excessive blood loss resulting in hypovolaemia, shock, anaemia and disseminated intravascular coagulation (DIC); and damage to muscles resulting in difficulties with micturition and defecation.
Muscle trauma
Injuries to muscle result from tearing or when the blood supply is disrupted.
Torticollis
The most commonly damaged muscle is the sternomastoid muscle. The right and left sternomastoid muscles run from the respective side of the top of the sternum, along the right or left side of the neck and are inserted into the mastoid process of the right or left temporal bone (Tortora & Derrickson 2006). When contracted simultaneously, these muscles allow the head to flex. When contracted separately, each turns the head to the opposite side.
Excessive traction or twisting causing tearing to one of these muscles can occur during the birth of the anterior shoulder of a fetus with a cephalic presentation, or during rotation of the shoulders when the fetus is being born by vaginal breech or caesarean section. A 1–3 cm, apparently painless, hard lump of blood and fibrous tissue is felt on the affected sternomastoid muscle. The muscle length is shortened, therefore the neck is twisted to the affected side: a torticollis or wry neck. If the techniques for assisting at these stages of birth are correctly applied, torticollis is preventable (Butler 2003).
Torticollis management involves carers and parents performing passive muscle-stretching exercises initially under the guidance of a physiotherapist, and actively encouraging the baby to move the neck. The swelling will usually resolve over several weeks to months with minimal sequelae. Occasionally surgical intervention is required if there is no resolution by 1 year. Follow-up to ensure achievement of normal movement is recommended (Butler 2003).
Nerve trauma
The nerves most commonly traumatized are the facial and brachial plexus nerves. Spinal cord injury is very rare and is not discussed here; an excellent explanation is given in Brand (2006).
Facial nerve
The facial nerve runs close to the skin surface and is vulnerable to compression resulting in unilateral facial palsy. Compression may occur in the uterus but is more likely during birth by the maternal sacral promontory or by a mis-applied forceps blade. On the affected side, the baby appears to have no nasolabial fold, the eyelid remains open and the mouth is drawn over to the unaffected side (Plate 30). The baby will drool excessively, may be unable to form an effective seal on the breast or teat, resulting in initial feeding difficulties, and may also have difficulty swallowing (Parker 2006).
There is no specific treatment. If the eyelid remains open, regular instillation of methyl cellulose eye drops lubricate the eyeball. Feeding difficulties are usually overcome by the baby’s own adaptation, although alternative feeding positions may help. Spontaneous resolution is usually within 7–10 days; this may extend to months or years if the damage is severe. Cosmetic surgical interventions for the most severely affected babies may be required (Parker 2006).
Brachial plexus
Nerve roots exiting from the spine at the fifth to eighth cervical and the first thoracic vertebrae form a matrix of nerves in the neck and shoulder; the brachial plexus (Tortora & Derrickson 2006). Evans-Jones et al (2003) report a stable incidence of 0.42/1000 live births over 40 years for congenital (obstetric) brachial plexus palsy in the United Kingdom (UK) and the Republic of Ireland. Brachial plexus trauma usually results from excessive lateral flexion, rotation or traction of the head and neck during vaginal breech birth or when shoulder dystocia occurs. This force may or may not be excessive or inappropriate (Evans-Jones et al 2003). It is suggested that elective caesarean section for all babies who present breech will prevent such trauma (Dyachenko et al 2006, Evans-Jones et al 2003).
The possible damage ranges from oedema to haemorrhage to tearing of the nerves. There are three main injuries: Erb’s palsy, Klumpke’s palsy and total brachial plexus palsy. These injuries can be unilateral or bilateral.
Erb’s palsy
There is paralysis of the shoulder and arm (not the hand) due to damage to the upper brachial plexus involving the fifth and sixth cervical nerve roots (Parker 2006). The baby’s affected arm is inwardly rotated, the elbow extended, the wrist pronated and flexed and the hand partially closed; the ‘waiter’s tip position’. The arm is limp, although some movement of the fingers and arm is possible (Plate 31).
Klumpke’s palsy
The shoulder and upper arm are unaffected but the lower arm, wrist and hand are paralysed resulting in wrist drop and no grasp reflex. This is due to damage to the lower brachial plexus involving the seventh and eighth cervical and the first thoracic nerve roots.
Total brachial plexus palsy
There is complete paralysis of the shoulder, arm and hand, lack of sensation, and circulatory problems due to damage to all brachial plexus nerve roots. If there is bilateral paralysis, spinal injury should be suspected.
All types of brachial plexus trauma will require further investigations such as X-ray and ultrasound scanning (USS) of the clavicle, arm, chest and cervical spine, and assessment of the joints. Passive movements of the joints and limb can be initiated under the direction of a physiotherapist. At 1 month of age, magnetic resonance imaging can offer specific data on nerve damage.
Complete recovery within 6 months is expected for 52% of babies, 2% will have no recovery and 46% will have incomplete recovery (Evans-Jones et al 2003). Erb’s palsy tends to resolve more quickly than the other forms. Regular functional follow-up assessments are recommended. Babies with no functional recovery by 6 months may require microsurgical nerve repair (DiTaranto et al 2004, Evans-Jones et al 2003).
Fractures
Fractures are rare but the most commonly affected bones are the clavicle, humerus, femur and those of the skull. With all such fractures, a ‘crack’ may be heard during the birth.
Clavicle
Clavicular fractures are the second most common type of birth trauma after skin and superficial tissue injury (Parker 2006). Fractures can occur with shoulder dystocia or a vaginal breech birth, or if the baby is macrosomic. The affected clavicle is usually the one that was nearest the maternal symphysis pubis. Brachial plexus and phrenic nerve injuries should be excluded in the affected baby.
Humerus
Midshaft fractures can occur if with shoulder dystocia or during a vaginal breech birth the extended arm is forced down and born.
Femur
Midshaft fractures can occur during vaginal breech birth if the extended legs are forced down and born.
Management of fractures
With most fractures, distortion, deformity, swelling or bruising are usually evident on examination; crepitus may be felt; the baby feels pain and is reluctant to move the affected area. An X-ray examination can usually confirm the diagnosis.
The baby requires careful handling to avoid further pain, and mild analgesia, such as paracetamol may be required (British National Formulary for Children 2005).
Fractures of the clavicle require no specific treatment. To immobilize a fractured humerus, place a pad in the axilla and firmly splint the arm with the elbow bent across the chest with a bandage, ensuring respirations are not embarrassed. Immobilize a fractured femur using a splint and bandage. Traction and plaster casting may be required (Parker 2006). Stable union of a fractured clavicle usually occurs in 7–10 days, while the humerus and femur take 2–3 weeks.
Skull
Although rare, these fractures, linear or depressed, may occur during prolonged or difficult instrumental births. There may be no signs but an overlying cephalhaematoma, or signs of associated complications such as intracranial haemorrhage or neurological disturbances, may suggest a fracture’s presence.
X-ray examination can confirm the fracture. An USS may help diagnose associated haemorrhage. Linear fractures usually require no treatment. A depressed skull fracture is a concavity of the bone with no break. External repair using vacuum apparatus is a more recent alternative to the traditional surgical intervention (Sorantin et al 2006). Leakage of CSF through the ear or nose requires antibiotic therapy. Treatment of associated complications is necessary.
Linear skull fractures usually heal quickly with no sequelae. Depressed fractures have a similarly optimistic outcome except if complications occur, when permanent neurological damage is likely (Paige & Carney 2002).
Haemorrhage
Blood volume in the term baby is approximately 80–100 ml/kg and in the pre-term baby 90–105 mL/kg; therefore even a small haemorrhage can be potentially fatal. In this section, haemorrhages are discussed according to their principal cause, or in relation to other factors.
Haemorrhage due to trauma
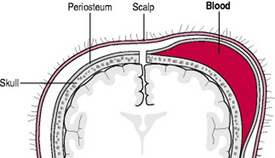
Cephalhaematoma
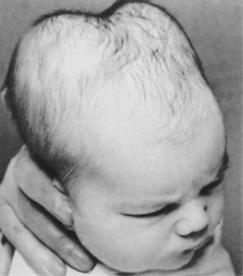
A cephalhaematoma is an effusion of blood under the periosteum that covers the skull bones (Fig. 45.2). During a vaginal birth, with friction between the fetal skull and maternal pelvic bones, such as in cephalopelvic disproportion or precipitate labour, the periosteum is torn from the bone, causing bleeding underneath. Cephalhaematomas may also occur during vacuum-assisted births. Because the fetal or newborn skull bones are not fused, and as the periosteum is adherent to the edges of the skull bones, a cephalhaematoma is confined to one bone. However, more than one bone may be affected; therefore multiple cephalhaematomas may develop. A double cephalhaematoma is usually bilateral (Fig. 45.3).
A cephalhaematoma is not present at birth; the swelling appears after 12 hrs, grows larger over subsequent days and can persist for weeks. The swelling is firm, does not pit on pressure, does not cross a suture and is fixed (Furdon & Clark 2001).
No treatment is necessary and the swelling subsides when the blood is reabsorbed. Haemolysis of the extravasated blood may result in hyperbilirubinaemia. A ridge of bone, felt round the periphery of the swelling, is due to the accumulation of osteoblasts.
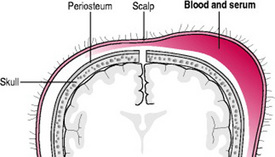
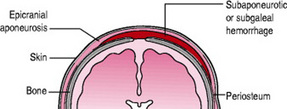
Subaponeurotic haemorrhage
A subaponeurotic (or subgaleal) haemorrhage is rare. Under the scalp, the epicranial aponeurosis, a sheet of fibrous tissue that covers the cranial vault allowing for muscles to attach to the bone, provides a potential space above the periosteum through which veins travel. Excessive traction on these veins results in haemorrhage, the epicranial aponeurosis is pulled away from the periosteum of the skull bones and swelling is evident (Fig. 45.4). Subaponeurotic haemorrhage may occur with any type of birth but is more often associated with vacuum-assisted births, primiparous women, severe dystocia, occipitolateral or posterior head positions, pre-term babies, precipitate births, macrosomia, coagulopathies and male babies (Sansoucie & Cavaliere 2003).
The swelling is present at birth, increases in size and is a firm, fluctuant mass. The scalp is movable rather than fixed. The swelling can cross sutures and extend into the subcutaneous tissue of neck and eyelids. The baby experiences pain with head movement or handling of the swelling.
Some subaponeurotic haemorrhages are small but there is a risk of excessive haemorrhage and severe shock. This emergency situation requires immediate medical assistance; stabilization and full supportive care, including blood transfusion. Subaponeurotic haemorrhage is associated with a mortality rate of 25% (Sorantin et al 2006).
With a smaller haemorrhage and in the babies who survive a larger haemorrhage, the blood is reabsorbed and the swelling and bruising resolve over 2–3 weeks. Hyperbilirubinaemia complicates recovery.
Subdural haemorrhage
A sickle-shaped, double fold of dura mater, the falx cerebri, dips into the fissure between the cerebral hemispheres. Attached at right angles to the falx cerebri, between the cerebrum and the cerebellum, is a horseshoe-shaped fold of dura mater; the tentorium cerebelli. In these folds of dura run large venous sinuses draining blood from the brain.
Normally moulding of the skull bones and stretching of the underlying structures during birth are well tolerated. Trauma to the fetal head, such as excessive compression or abnormal stretching, may tear the dura, particularly the tentorium cerebelli, rupturing venous sinuses and resulting in a subdural haemorrhage. Predisposing factors include rapid, abnormal or excessive moulding, such as in precipitate labour or rapid birth, malpositions, malpresentations, cephalopelvic disproportion, or undue compression during forceps manoeuvres (Smith 2006). Subdural haemorrhage may be fatal.
A baby with a small haemorrhage may demonstrate no signs and resolution is spontaneous. Alternatively the haemorrhage may initially be small but if blood continues to leak, the signs develop over several days. As blood accumulates, there is cerebral irritation, cerebral oedema and raised intracranial pressure. The baby is likely to vomit, be non-responsive, and have a bulging anterior fontanelle, abnormal eye movements, apnoea, bradycardia and convulsions. If the subdural haemorrhage is initially large, the baby is likely to be severely asphyxiated and difficult to resuscitate (Smith 2006).
Diagnosis is confirmed by cranial USS. Supportive treatment focuses on replacing blood volume and controlling the consequences of asphyxia and raised intracranial pressure. Subdural taps may be required to drain large collections of blood.
Haemorrhage due to disruptions in blood flow
Subarachnoid haemorrhage
A primary subarachnoid haemorrhage involves bleeding directly into the subarachnoid space. Pre-term babies who suffer hypoxia at birth resulting in disruption of cerebral blood flow, and term babies who suffer traumatic births are vulnerable (Levene et al 2000, Smith 2006). A secondary haemorrhage involves leakage of blood into the subarachnoid space from an intraventricular haemorrhage (Smith 2006).
The affected baby may have generalized convulsions from the second day of life, pre-term babies may have apnoeic episodes, but otherwise the baby appears normal and some babies exhibit no signs. Subarachnoid haemorrhage is difficult to see on USS, although computerized tomography scanning can demonstrate the haemorrhage. If a lumbar puncture is performed, the CSF will be uniformly bloodstained. Management includes replacement of blood volume, and control of the consequences of asphyxia and convulsions. The condition is usually self-limiting.
Post-haemorrhagic hydrocephalus may occur but drainage is not usually required and recovery is usually favourable (Levene et al 2000).
Germinal matrix/intraventricular haemorrhage and intraparenchymal lesions
Germinal matrix/intraventricular haemorrhage (GMH/IVH) and intraparenchymal lesions (IPL) primarily affect babies of <32 weeks’ gestation and those weighing <1500 g, although term babies can be affected (Rennie & Roberton 2002). The incidence and severity of these haemorrhages/lesions are inversely correlated with gestational age (Smith 2006).
In 1978, Papile et al proposed the following grading system. A grade 1 haemorrhage into the germinal matrix, is a periventricular or subependymal haemorrhage. Extension of the haemorrhage into the lateral ventricle(s), results in an intraventricular haemorrhage (IVH) or grade 2 haemorrhage. The choroid plexus of the lateral ventricles normally produces CSF. If a grade 2 haemorrhage is complicated by blockage to the outflow of CSF, post-haemorrhagic hydrocephalus develops and the ventricles dilate; a grade 3 IVH. A grade 3 haemorrhage may extend into the cerebral tissue, giving rise to a periventricular/parenchymal haemorrhage, known as a grade 4 haemorrhage.
Volpe (1997) proposed that the intraventricular clot in the grade 3 haemorrhage disrupts venous drainage, causing stasis and infarction. Reperfusion of the area caused haemorrhage into the infarcted area and necrotic damage of the white matter. Volpe (1997) reclassified a grade 4 haemorrhage as a complication of a grade 3 IVH, referring to it as a periventricular haemorrhagic infarction (PHI).
More recently, the term ‘periventricular’ and Papile et al’s (1978) grading system have been reconsidered. Improved imaging techniques show that periventricular/parenchymal damage may not always be due to haemorrhage and the lesions vary in size, nature and location. There is still debate about the classification, but Rennie & Roberton (2002) suggest descriptions and the terms GMH/IVH/IPL are now preferred as they indicate the location of the haemorrhage/lesion. An IVH can be with or without ventricular dilatation.
The stage of brain development is a crucial factor in the aetiology of GMH/IVH/IPL. The two lateral ventricles are lined with ependymal tissue. Tissue lying immediately next to the ependyma is the germinal matrix, also known as the subependymal layer. From 8 to 28 weeks’ gestation, neuroblasts are produced in the germinal matrix and migrate to the cerebral cortex. During this period, a rich blood supply is provided to the germinal matrix through fragile immature capillaries that lack supporting muscle or collagen fibres. These vessels are particularly vulnerable to increases in cerebral blood flow, rupturing easily causing haemorrhage. After 32 weeks’ gestation the germinal matrix becomes less active and by term has almost completely involuted. At the same time the capillaries become more stable; therefore GMH/IVH/IPL in more mature babies are less common than in those <32 weeks’ gestation (Kirby 2002, Rennie & Roberton 2002).
In the baby of <32 weeks’ gestation, the arterial supply to the area around the lateral ventricles is limited, with arteries forming an end zone or watershed that is relatively poorly supplied with blood. With cerebral blood flow reductions, this area is vulnerable to ischaemic damage, demonstrated as an IPL. The venous drainage from white matter and the deep areas of the brain, including the lateral ventricles, involves a peculiar U-turn route in the area of the germinal matrix. Disruptions to venous flow lead to congestion, with a risk of venous infarctions and ischaemia, demonstrated as an IPL. With reperfusion of these ischaemic areas, there may be haemorrhage, porencephalic cysts may develop and are demonstrated as IPLs (Cullens 2000, Kirby 2002, Rennie & Roberton 2002).
Throughout the perinatal period multiple factors may affect cerebral haemodynamics resulting in GMH/IVH/IPL. Early factors include obstetric haemorrhage, lack of antenatal steroids, birth outside a regional unit, chorioamnionitis, low one minute Apgar score, bruising at birth and low umbilical artery pH. Later risk factors include acidosis, hypotension, hypertension, respiratory distress syndrome (RDS) requiring mechanical ventilation, ‘fighting the ventilator’, apnoea, rapid volume expansion, rapid administration of sodium bicarbonate or other hyperosmolar solutions, pneumothorax and patent ductus arteriosus (Paige & Carney 2002). Also implicated are excessive handling, exposure to light and noise, lateral flexion of the baby’s head and crying.
Approximately 50% of GMHs are small, have a ‘silent’ onset, and are detectable only on USS. If the haemorrhage is larger or extends, the clinical features may gradually appear and worsen, including apnoeic episodes that become more frequent and severe, bradycardia, pallor, falling packed cell volume, tense anterior fontanelle, metabolic acidosis and convulsions. The baby may be limp or unresponsive. If the haemorrhage is large and sudden in onset, apnoea and circulatory collapse may present (Cullens 2000, Rennie & Roberton 2002).
At-risk babies are screened by 7 days of life for GMH/IVH/IPL using cranial USS. Serial scanning of a lesion may determine any increase, extension or complication.
Care of at-risk babies is focused on prevention (Blackburn 2003). The birth should be in a regional obstetric unit with intensive neonatal facilities. Prenatal maternal steroid administration and postnatal surfactant replacement therapy reduce the incidence of GMH/IVH/IPL. Postnatally, haemodynamic stability is essential, as is prevention of complications. The baby’s needs related to respiration and acid–base balance, prevention of hypoxic events, circulation and blood pressure, temperature control, nutrition and elimination, and pain control and comfort must be carefully assessed and meticulously met, with continuing evaluation and appropriate adjustments to care. Mechanical ventilation may be required and endotracheal tube suction should be performed only when necessary. Sophisticated monitoring equipment and the judicious use of analgesic, sedative and inotropic drugs may assist achieving and maintaining stability. If complications develop, such as pneumothorax or patent ductus arteriosus, these should be quickly detected and effectively treated. The baby’s developmental needs should be met, particularly in relation to supportive flexed positioning, reduction in bright lighting, a quiet, undisturbed environment and appropriate interaction with parents and others (Blackburn 2003, Paige & Carney 2002, Rennie & Roberton 2002).
Despite preventative measures, babies do develop GMH/IVH/IPL. The outcome depends on the nature of the lesion. The neurological prognosis for babies with a GMH or a small IVH is usually good. An IVH associated with ventricular dilatation may resolve spontaneously with no long-term consequences. However, with a large IVH and ventricular dilatation, the accumulating CSF may require temporary drainage using ventricular taps or external ventricular drainage. Some babies may require permanent CSF drainage via a ‘shunt’: i.e. a drainage tube surgically inserted into the ventricular system is connected to a one-way valve placed subcutaneously behind the ear. The valve’s outflow tube is attached to a catheter allowing drainage of the CSF into a large vein in the neck, or into the peritoneum, where it is reabsorbed and eliminated (Blackburn 2003). The prognosis for these babies is less good. Approximately 50% of babies with a massive IVH die, usually within 48 hrs of the onset and 90% of those who survive develop physical and/or neurological and/or intellectual impairment/s. The prognosis for babies with IPL is variable. If the IPL develops into a porencephalic cyst, hemiplegic cerebral palsy is likely, as is developmental delay. Long-term follow-up is essential and parents need much support (Blackburn 2003, Paige & Carney 2002, Rennie & Roberton 2002).
Periventricular leucomalacia
Although not strictly a haemorrhage, periventricular leucomalacia (PVL) is included because of its association with GMH/IVH/IPL. Between 27 and 30 weeks’ gestation, the area of white matter around the lateral ventricles and within the watershed area of the deep cerebral arteries is undergoing considerable development. It is sensitive to any insult that results in reduced cerebral perfusion, such as those associated with GMH/IVH/IPL (Levene et al 2000), and intrauterine infection with or without ruptured membranes (Rennie & Roberton 2002). Reduced perfusion results in areas of ischaemia and degeneration of the nerve fibre tracts, disrupting nerve pathways between areas of the brain and between the brain and spinal cord. This softening and necrosis of tissue is PVL, seen on USS but more clearly with magnetic resonance imaging.
Similar pathogenesis is seen in the older pre-term and term baby, but the lesion occurs in the subcortical region rather than the periventricular region. This is because the watershed moves away from the ventricles to the cortex once the germinal matrix involutes. These lesions are known as subcortical leucomalacia (Levene et al 2000).
Care instituted to reduce the incidence of GMH/IVH/IPL may reduce the incidence of PVL or the severity of the related ischaemic damage. The prognosis is variable. The areas may become cystic, porencephalic cysts, that may regress with little resulting impairment. However bilateral occipital cysts usually result in spastic diplegic cerebral palsy (Rennie & Roberton 2002).
Vitamin K deficiency bleeding
Vitamin K deficiency bleeding (VKDB) may occur up to 12 months of age, although it more commonly occurs between birth and 8 weeks of life. It was previously known as haemorrhagic disease of the newborn (HDN). Several proteins, factor II (prothrombin), factor VII (proconvertin), factor IX (plasma thromboplastin component), factor X (thrombokinase) and proteins C and S, require vitamin K for their conversion to active clotting factors. A deficiency of vitamin K, as in VKDB, leads to a deficiency of these clotting factors and resultant bleeding.
Vitamin K1 (phytomenadione) is poorly transferred across the placenta; therefore fetal stores are low. Any stores are quickly depleted after birth and so, for normal clotting to occur, the baby must receive dietary vitamin K1, the absorption of which requires fat and bile salts. Vitamin K2 (menaquinone) is synthesized by bowel flora and may assist in the conversion of proteins to active clotting factors. Because the neonate’s bowel is sterile, vitamin K2 production is restricted until colonization has occurred. Therefore all newborns are deficient in vitamin K and vulnerable to VKDB.
There are three forms of VKDB that were first described by Lane and Hathaway (1985):
Early VKDB is rare, principally affecting babies born to women who, during pregnancy, have taken warfarin, barbiturates, phenytoin, carbamazepine, cephalosporins, or tuberculostatics for treatment of their medical conditions. As these drugs interfere with vitamin K metabolism, avoidance during pregnancy reduces the risk of early VKDB. Taking vitamin K1 supplements during pregnancy may prevent early VKDB but study results are variable (Moskowitz & Karpatkin 2005).
The babies most susceptible to developing classic VKDB are those with birth trauma, asphyxia, postnatal hypoxia and those who are pre-term, or of low birthweight. They are more likely to spontaneously bleed or have invasive interventions resulting in bleeding that cannot be controlled. Disruptions to the colonization of the bowel due to antibiotic therapy, or lack of or poor enteral feeding, may also result in classic VKDB. Breastfed babies produce bifidobacteria in their bowel that inhibit menaquinone production. Serum menaquinone is naturally low in the newborn breastfed baby, resulting in increased susceptibility to VKDB, although there are doubts as to the role of the menaquinones produced in the bowel, emphasizing the importance of dietary vitamin K1 (Gasking 1998).
The amount of vitamin K1 in breastmilk is naturally low, although colostrum and hind milk do contain higher levels than foremilk. The vitamin K1 in breastmilk is considered insufficient for the exclusively breastfed newborn. Cow’s milk has higher concentrations of vitamin K1 than breastmilk, although the levels are still low. Artificial infant formulae are fortified with vitamin K1, offering some prophylaxis against VKDB (Manco-Johnson et al 2002).
Late VKDB occurs almost exclusively in breastfed babies. However, babies who have liver disease or a condition that disrupts vitamin K1’s absorption from the bowel, for example cystic fibrosis, may develop late VKDB (Manco-Johnson et al 2002).
The baby may have bruising; or bleeding from the umbilicus, puncture sites, the nose or the scalp; or severe jaundice for >1 week and/or persistent jaundice for >2 weeks. Gastrointestinal bleeding manifests as melaena and haematemesis. In early and late VKDB, there may be extracranial and intracranial bleeding. With severe haemorrhage, circulatory collapse occurs. Late VKDB is associated with higher mortality and morbidity. Blood tests reveal prolonged prothrombin time (PT) and partial thromboplastin time (PTT), with a normal platelet count (Rennie & Roberton 2002).
Babies diagnosed with VKDB require investigation and monitoring to assess their need for treatment. With all forms of VKDB, the baby will require administration of vitamin K1, 1–2 mg intramuscularly. In severe cases, when coagulation is grossly abnormal and there is severe bleeding, replacement of deficient clotting factors is essential. If circulatory collapse and severe anaemia occur, blood transfusion or exchange transfusion may be required. Affected babies usually require other supportive therapy to assist in their recovery.
As VKDB is a potentially fatal condition, prophylactic administration of vitamin K became the norm. Various types, routes and doses were used, but vitamin K1 1 mg given intramuscularly within the first hour after birth became the most effective practice, despite some disquiet over such invasive prophylaxis (Soin & Katesmark 1993).
When two case-control studies reported an association between intramuscular vitamin K1 administration and childhood cancers (Golding et al 1990, 1992), controversy increased. This finding was not confirmed in subsequent case-control studies (Ansell et al 1996, Ekelund et al 1993, Klebanoff et al 1993, McKinney et al 1998, von Kries et al 1996). However, one large retrospective case-control study reported a significant association between the administration of vitamin K1 intramuscularly and the development of acute lymphoblastic leukaemia in 1–6 year olds, although not with other cancers (Parker et al 1998).
These studies were limited by their design, with unreliable record keeping of the route and dose of vitamin K1. The statistical association reported by Golding et al (1990, 1992) and Parker et al (1998) indicates that the children with childhood cancers were more likely to have been given vitamin K1 intramuscularly at birth, but not that the vitamin K1 caused the cancer. The more recent UK Childhood Cancer Study (Fear et al 2003) showed no causal link with vitamin K1.
There was a reassessment of vitamin K1 prophylaxis in the 1990s and a variety of products and regimens emerged. The most recent recommendations offer two prophylactic regimes using vitamin K1 as Konakion MM Paediatric 2 mg/0.2 mL (Roche 2005). For all pre-term babies, all ‘at risk’ term babies, and healthy term babies whose parents choose, an intramuscular injection of vitamin K1 should be administered within one hour of birth to give a transient peak serum concentration, reducing the risk of early VKDB (Puckett & Offringa 2000). Some vitamin K1 remains within the muscle and acts as a slow release depot, providing prophylaxis for classic and probably also for late VKDB (Hey 2003, Wariyar et al 2000). The dose of vitamin K1 varies according to the baby’s weight and local guidelines should be consulted.
For healthy term babies whose parents decline the single intramuscular injection of vitamin K1 (phytomenadione), the following oral prophylaxis regimen should be instituted (Roche 2005). Oral vitamin K1 2.0 mg is given on the first and seventh days of life. If the baby is breastfed, a further dose is required at one month and monthly doses thereafter until solid food and/or milk formula feeding is introduced. This regimen should reduce the risk of all forms of VKDB; however this is dependent on the involvement, motivation and compliance of healthcare professionals and parents. Medical advice should be sought if the baby vomits within 1 hrs of oral administration.
Greer et al (1997) and Bolisetty et al (1998) demonstrated that administration of vitamin K1 to lactating women increased the levels of vitamin K1 in the maternal serum and breastmilk. Their babies had higher serum levels of vitamin K1, giving some VKDB prophylaxis. However, some of these babies were also given intramuscular vitamin K1, affecting their serum levels of vitamin K1. Wariyar et al (2000) concluded that while maternal prophylaxis during breastfeeding was a possible alternative to prophylaxis for the baby, it was a more complicated strategy.
All parents should be given the opportunity to discuss vitamin K1 prophylaxis during pregnancy, understand the specific management of pre-term and ‘at risk’ babies, and agree on their choice of prophylaxis. They should also understand the signs and treatment of VKDB, especially if their baby has one or more of the risk factors.
Thrombocytopenia
Thrombocytopenia is defined as a platelet count of <150 000/μL (alternatively described as 150 × 109/L), and severe thrombocytopenia is a platelet count of <50 000/μL (Roberts & Murray 2003). It results from a decreased rate of formation of platelets or an increased rate of consumption. In the general neonatal population, thrombocytopenia is rare (Castro et al 2007), however, it develops in up to 35% of pre-term and sick babies and in 50% of babies who require intensive care (Chakravorty et al 2005, Roberts & Murray 2003). Babies at risk of developing thrombocytopenia include those who have:
Fetal thrombocytopenia, due to congenital infection or an inherited condition, may be monitored during pregnancy to determine the need for maternal immunoglobulin administration and/or prenatal intrauterine platelet transfusions (Roberts & Murray 2003).
Postnatally, early onset thrombocytopenia is mild and due to a lack of platelet production associated with placental insufficiency (Chakravorty et al 2005). Immune thrombocytopenias, asphyxia, disseminated intravascular coagulation and congenital infections also result in early onset cases. Later onset thrombocytopenia is caused by bacterial sepsis and/or necrotizing enterocolitis in 90% of cases and is usually severe (Roberts & Murray 2006). For many babies, multiple causative factors are involved.
A petechial rash appears soon after birth, presenting in a mild case with a few localized petechiae. In a severe case there is widespread and serious haemorrhage from multiple sites. Intracranial haemorrhage may be fatal. Diagnosis is based on history, clinical examination and a reduced platelet count. It is differentiated from other haemorrhagic disorders because coagulation times, fibrin degradation products and red blood cell morphology are normal. Mild early onset cases are usually self-limiting, and require no treatment (Chakravorty et al 2005). In immune-mediated thrombocytopenia, intravenous immunoglobulin administration may help (Levene et al 2000). In severe later onset cases, transfusions of platelet concentrate are required, although the optimum regime is yet to be determined (Chakravorty et al 2005).
Disseminated intravascular coagulation (consumptive coagulopathy)
Disseminated intravascular coagulation (DIC) is an acquired coagulation disorder associated with the release of thromboplastin from damaged tissue, stimulating abnormal coagulation and fibrinolysis with widespread deposition of fibrin in the microcirculation and excessive consumption of clotting factors and platelets. DIC is secondary to primary conditions. Maternal causes include pre-eclampsia, eclampsia and placental abruption. Fetal causes include severe fetal distress, the presence of a dead twin in the uterus and traumatic birth. Neonatal causes include conditions resulting in hypoxia and acidosis, severe infections, hypothermia, hypotension and thrombocytopenia (Baldwin 2001/2002, Chalmers 2004).
As clotting factors and platelets are depleted and fibrinolysis is stimulated, the baby will develop a generalized purpuric rash and bleed from multiple sites. With stimulation of the clotting cascade, multiple microthrombi appear in the circulation. These can occlude vessels, with organ and tissue ischaemia and damage, particularly affecting the kidneys, resulting in haematuria and reduced urine output (Chalmers 2004). The baby becomes anaemic due to haemorrhage and fragmentation of red cells by the fibrin deposits in blood vessels (Baldwin 2001/2002).
The diagnosis is made from clinical signs and laboratory findings that show a low platelet count, low fibrinogen level, distorted and fragmented red blood cells, low haemoglobin and raised fibrin degradation products (FDPs) with a prolonged PT and PTT (Chalmers 2004).
Treatment includes correction of the underlying cause if possible and full supportive care. Control of DIC requires transfusions of fresh frozen plasma, concentrated clotting factors and platelets. Cryoprecipitate is an excellent source of fibrinogen. If there is anaemia, transfusions of whole blood or red cell concentrate are required. Occasionally an exchange transfusion of fresh heparinized blood may be performed, to remove FDPs while replacing the clotting factors. If treatment of the primary disorder and/or replacement of clotting factors is ineffective, the administration of heparin may reduce fibrin deposition (Baldwin 2001/2002).
The prognosis depends on the severity of the primary condition, as well as of the DIC, and the baby’s response to treatment.
Haemorrhage related to other causes
Umbilical haemorrhage
This usually occurs as a result of a poorly applied cord ligature. The use of plastic cord clamps has almost eliminated this type of haemorrhage, although it is essential to avoid catching or pulling the clamp. Tampering with partially separated cords before they are ready to separate is discouraged. Umbilical haemorrhage is a potential cause of death. A purse-string suture should be inserted if bleeding continues after 15 or 20 min of manual pressure.
Vaginal bleeding
A small temporary vaginal discharge of bloodstained mucus occurring in the first days of life, pseudomenstruation, is due to the withdrawal of maternal oestrogen. This is a normal expectation but is included here for completeness. Parents need to know that this is a possibility and is self-limiting. Continued or excessive vaginal bleeding warrants further investigation to exclude pathological causes.
Haematemesis and melaena
These signs may present when the baby has swallowed maternal blood during birth, or from cracked nipples during breastfeeding. The diagnosis must be differentiated from VKDB, from other causes of haematemesis that include oesophageal, gastric or duodenal ulceration, and from other causes of melaena, that include necrotizing enterocolitis and anal fissures. These causes need specific and usually urgent treatment.
If the cause is swallowed blood, the condition is self-limiting and requires no specific treatment. If the cause is cracked nipples, appropriate treatment for the mother must be implemented.
Haematuria
Haematuria may be associated with coagulopathies, urinary tract infections and structural abnormalities of the urinary tract. Birth trauma may cause renal contusion and haematuria. Occasionally, after suprapubic aspiration of urine, transient mild haematuria may be observed. Treatment of the primary cause should resolve the haematuria.
Bleeding associated with intravascular access
Some sick or pre-term babies require the insertion of catheters, lines or cannulae into central or peripheral arteries or veins, or both, to provide routes for blood sampling and the infusion of fluids and drugs. However, there is a risk of severe external haemorrhage if there is dislodgement of these from the vessel or accidental disconnection from the sampling or infusion equipment, and of severe haemorrhage if a central vessel is punctured internally.
Skilled technique, close observation and careful handling of babies with intravascular access are imperative to prevent these potentially fatal haemorrhages. If an external haemorrhage does occur, continuous pressure should be applied to the site until natural haemostasis occurs or until haemostatic sutures are inserted. If there is external bleeding from an umbilical vessel, the cord stump should be squeezed between the fingers until haemostasis occurs. A replacement transfusion of whole blood or packed red cells may be required. Internal haemorrhage may require surgical intervention.
Convulsions
A convulsion (seizure/fit) is a sign of neurological disturbance, not a disease, and the occurrence of a convulsion is a medical emergency (Granelli & McGrath 2004). Because the newborn brain is still developing, its function is immature and there is an imbalance between stimulation and inhibition of neural networks, convulsions present quite differently in the neonate and may be more difficult to recognize than those of later infancy, childhood or adulthood. The incidence of convulsions is suggested as 5–8/1000 live births (Levene et al 2000).
Convulsive movement can be differentiated from jitteriness or tremors in that, with the latter two, the movements are rapid, rhythmic, equal, are often stimulated or made worse by disturbance and can be stopped by touching or flexing the affected limb. They are normal in an active, hungry baby and are of no consequence, although their occurrence should be documented. Convulsive movements tend to be slower, less equal, are not necessarily stimulated by disturbance, cannot be stopped by restraint and are always pathological (Granelli & McGrath 2004).
Abnormal, sudden or repetitive movements of any part of the body that are not controlled by repositioning or containment holds require investigation. Levene et al (2000) suggest that the type of movement can help classify the convulsion as subtle, tonic, multifocal clonic, focal clonic or myoclonic. Granelli & McGrath (2004) describe the specific appearance of convulsions as follows.
During a convulsion the baby may have tachycardia, hypertension, raised cerebral blood flow and raised intracranial pressure, that predispose to serious complications.
Many conditions cause newborn convulsions and they are classified into central nervous system, metabolic, other and idiopathic causes (Table 45.1).
Table 45.1 Selected causes of neonatal convulsions
| Category | Selected causes |
|---|---|
| Central nervous system | Intracranial haemorrhage |
| Intracerebral haemorrhage | |
| Hypoxic-ischaemic encephalopathy | |
| Kernicterus | |
| Congenital abnormalities | |
| Metabolic | Acquired disorders of metabolism |
| Hypo- and hyperglycaemia | |
| Hypo- and hypercalcaemia | |
| Hypo- and hypernatraemia | |
| Inborn errors of metabolism | |
| Other | Hypoxia |
| Congenital infections | |
| Severe postnatally acquired infections | |
| Neonatal abstinence syndrome | |
| Hyperthermia | |
| Idiopathic | Unknown |
If a convulsion is suspected, a complete history and physical and laboratory investigations related to the possible cause would be undertaken. An EEG may help detect abnormal electrical brain activity and guide treatment (Levene et al 2000). Rennie & Boylan (2007) contend that detection of convulsions and response to anticonvulsants using continuous EEG recordings requires further large-scale study.
Immediate treatment necessitates obtaining assistance from a doctor while ensuring that the baby has a clear airway and adequate ventilation, either spontaneously or mechanically. The baby can be turned to the semiprone position, with the head in a neutral position. Gentle oral and nasal suction may be required to remove any milk or mucus. If the baby is breathing spontaneously but is cyanosed, facial oxygen is given. Active resuscitation may be required. The need for intravenous access should be assessed. Any necessary handling must be gentle and the baby is usually nursed in an incubator to allow for observation and temperature regulation (Granelli & McGrath 2004).
It is important that the nature of the convulsion is documented, noting the type of movement, the areas affected, its length, colour change, change in heart rate, respiratory rate or blood pressure and immediate sequelae.
The aims of care are to treat the primary cause (details of which are not discussed in this chapter), and the pharmacologic control of the convulsions. The latter is controversial due to the potential for damage from the drugs versus the potential damage from the convulsion on the developing brain (Rennie & Boylan 2007). Cochrane reviews suggest that while there is no robust research evidence for the use of any anticonvulsants in neonates, there is consensus for the use of such drugs particularly when the baby experiences prolonged or frequent convulsions (Booth & Evans 2004; Evans & Levene 2001).
If pharmacological treatment is prescribed, the drugs most commonly used are phenobarbital and phenytoin. Newer anticonvulsants such as topiramate and levetiracetam are still being evaluated but may have fewer side-effects (Rennie & Boylan 2007). Anticonvulsant therapy may be discontinued when convulsions cease, preferably before the baby is discharged home (Levene et al 2000). The need for well-conducted randomized controlled trials to determine the most effective treatment remains. The outcome for babies who have convulsions is also controversial and statistics vary. Granelli & McGrath (2004) suggest that the prognosis depends on the cause and type of convulsion, whether it was demonstrated on EEG and whether the tracing became normal following treatment, what type of treatment was used and how long it was before any treatment was successful. Pre-term babies and those babies with congenital malformations of the brain, hypoxic-ischaemic encephalopathy, IVH and IPL, or types of bacterial meningitis tend to have a higher mortality or a poor neurological outcome. Babies with late hypocalcaemia, hyponatraemia, benign familial neonatal seizures or primary subarachnoid haemorrhage are more likely to survive neurologically intact (Paige & Carney 2002). Babies with subtle, generalized tonic and some myoclonic convulsions have a poorer neurological outcome than babies experiencing other types of convulsions. Babies who have short-lived convulsions that respond well to treatment usually have a better outcome than babies who have prolonged and difficult to treat convulsions (Granelli & McGrath 2004).
Parents
The care of parents is more comprehensively discussed elsewhere, and in this section only specific aspects will be summarized. Trauma during birth, haemorrhage and convulsions are unexpected complications and parents may be shocked and anxious, and perhaps find themselves in a crisis situation (Fowlie & McHaffie 2004, McGrath 2003, Seigel et al 2002). However, not all parents experience such feelings and some can adapt quickly to their baby’s condition (Carter et al 2005, Greig 2002).
The extent of the midwife’s and other professionals’ contact with parents will depend on circumstances but the experiences parents have at this time have longer-term implications for them, their response to the situation, their relationships with the multiprofessional teams involved in their care as well as their interaction with and care of their baby. One of the most important aspects of caring for the parents is in relation to communication. All parents are entitled to be given information about their baby’s condition, treatment and care in ways that are considered best practice. The ‘Right From The Start template’ provides an excellent guide (Arnold 2006), and the principles related to the baby, parents and family are summarized as follows:
Parental involvement in their baby’s care is essential and the family-centered care/partnership with parents approach should now pervade all midwifery and neonatal settings. Midwives and neonatal nurses have an important role in promoting adaptive coping mechanisms and guiding parents to appropriate resources and support services (Fowlie & McHaffie 2004, McGrath 2003). The premature baby charity BLISS (2005) offers a helpful information guide for parents and their website (www.bliss.org.uk) includes a parent message board. Additional support and information is available from specialized outside agencies and Wilson (2006) suggests that the charity Contact a Family is also a useful resource (www.cafamily.org.uk) in the longer term.
Ansell P, Bull D, Roman E. Childhood leukaemia and intramuscular vitamin K: findings from a case control study. British Medical Journal. 1996;313:204-205.
Arnold L. Working with families affected by a disability or health condition from pregnancy to pre-school. A support pack for health professionals, 15 April Contact a Family, London, 2007. Online. Available: http://www.cafamily.org.uk/HealthSupportPack.pdf, 2006.
Baldwin KB. Neonatal Disseminated Intravascular Coagulation. Central Lines. 17(6), 2001/2. 1,2,4-6,10,11
Blackburn ST. Assessment and management of the neurologic system. In: Kenner C, Wright-Lott J, editors. Comprehensive neonatal nursing. A physiologic perspective. 3rd edn. Philadelphia: Saunders; 2003:624-660.
BLISS. Parent Information Guide, 3rd edn. London: BLISS, 2005.
Bolisetty S, Gupta JM, Graham GG, et al. Vitamin K in pre-term breast milk with maternal supplementation. Acta Paediatrica. 1998;87(9):960-962.
Booth D, Evans DJ. Anticonvulsants for neonates with seizures. Cochrane Database of Systematic Reviews, 3. 2004; CD004218
Brand MC. Part 1: Recognizing neonatal spinal cord injury. Advances in Neonatal Care. 2006;6(1):15-24.
British National Formulary for Children. BMJ Publishing, London, 2005.
Butler JM. Assessment and management of the musculoskeletal system. In: Kenner C, Wright-Lott J, editors. Comprehensive neonatal nursing. A physiologic perspective. 3rd edn. Philadelphia: Saunders; 2003:661-672.
Carter JD, Mulder RT, Bartram AF, et al. Infants in a neonatal intensive care unit: parental response. Archives of Disease in Childhood Fetal and Neonatal. 2005;90(2):F109-F113.
Castro V, Kroll H, Origa AF, et al. A prospective study on the prevalence and risk factors for neonatal thrombocytopenia and platelet alloimmunization among 9332 unselected Brazilian newborns. Transfusion. 2007;47(1):59-66.
Chakravorty S, Murray NA, Roberts IA. Neonatal thrombocytopenia. Early Human Development. 2005;81(1):35-41.
Chalmers EA. Neonatal coagulation problems. Archives of Disease in Childhood Fetal and Neonatal. 2004;89(6):F475-F478.
Cullens V. Brain injury in the premature infant. In: Boxwell G, editor. Neonatal intensive care nursing. London: Routledge; 2000:152-163.
DiTaranto P, Campagna L, Price AE, et al. Outcomes following nonoperative treatment of brachial plexus birth injuries. Journal of Child Neurology. 2004;19(2):87-90.
Dudink J, Walther FJ, Beekman RP. Subcutaneous fat necrosis of the newborn: hypercalcaemia with hepatic and atrial myocardial calcification. Archives of Disease in Childhood Fetal and Neonatal. 2003;88(4):F343-F345.
Dyachenko A, Ciampi A, Fahey J, et al. Prediction of risk for shoulder dystocia with neonatal injury. American Journal of Obstetrics and Gynaecology. 2006;195(6):1544-1549.
Ekelund H, Finnström O, Gunnarskog J, et al. Administration of vitamin K to newborn infants and childhood cancer. British Medical Journal. 1993;307:89-91.
Evans DJ, Levene MI. Anticonvulsants for preventing mortality and morbidity in full term newborns with perinatal asphyxia. Cochrane Database of Systematic Reviews. 2, 2001. CD001240
Evans-Jones G, Kay SPJ, Weindling AM, et al. Congenital brachial palsy: incidence, causes, and outcome in the United Kingdom and Republic of Ireland. Archives of Disease in Childhood Fetal and Neonatal. 2003;88(3):F185-F189.
Fear NT, Roman E, Ansell P, et al. Vitamin K and childhood cancer: a report from the United Kingdom Childhood Cancer Study. British Journal of Cancer. 2003;89(7):1228-1231.
Fowlie PW, McHaffie H. Supporting parents in the neonatal unit. British Medical Journal. 2004;329:1336-1338.
Furdon S, Clark D. Differentiating scalp swelling in the newborn. Advances in Neonatal Care. 2001;1(1):22-27.
Gasking D. Vitamin K: implications for prophylaxis. Journal of Neonatal Nursing. 1998;4(1):29-33.
Golding J, Patterson M, Kinlen LJ. Factors associated with childhood cancer in a national cohort study. British Journal of Cancer. 1990;62:304-308.
Golding J, Greenwood R, Birmingham K, et al. Childhood cancer, intramuscular vitamin K and pethidine given during labour. British Medical Journal. 1992;305:341-346.
Granelli SLP, McGrath JM. Neonatal Seizures. Diagnosis, pharmacologic interventions, and outcomes. Journal of Perinatal and Neonatal Nursing. 2004;18(3):275-287.
Greer FR, Marshall SP, Foley AL, et al. Improving the vitamin K status of breastfeeding infants with maternal vitamin K supplements. Pediatrics. 1997;99(1):88-92.
Greig C. Prenatal preparation of parents for neonatal care: A comparative descriptive study. University of Edinburgh, 2002. PhD thesis
Hey E. Vitamin K – what, why and when. Archives of Disease in Childhood Fetal and Neonatal. 2003;88(2):F80-F83.
Kirby CL. Posthemorrhagic hydrocephalus: A complication of intraventricular hemorrhage. Neonatal Network. 2002;21(1):59-68.
Klebanoff MA, Read JS, Mills JL, et al. The risk of childhood cancer after neonatal exposure to vitamin K. New England Journal of Medicine. 1993;329(13):905-908.
Lane PA, Hathaway WE. Vitamin K in infancy. Journal of Pediatrics. 1985;106:351-359.
Levene MI, Tudehope D, Thearle MJ. Essentials of neonatal medicine, 3rd edn. Oxford: Blackwell Science, 2000.
Manco-Johnson M, Rodden DJ, Collins S. Newborn hematology. In: Merenstein GB, Gardner SL, editors. Handbook of neonatal intensive care. 5th edn. Mosby: St Louis; 2002:419-442.
McGrath JM. Family-centered care. In: Kenner C, Wright-Lott J, editors. Comprehensive neonatal nursing. A physiologic perspective. 3rd edn. Philadelphia: Saunders; 2003:89-107.
McKinney PA, Jaszczak E, Findlay E, et al. Case-control study of childhood leukaemia and cancer in Scotland: findings for neonatal intramuscular vitamin K. British Medical Journal. 1998;316:173-179.
Moskowitz NP, Karpatkin M. Coagulation problems in the newborn. Current Paediatrics. 2005;15(1):50-56.
Paige PL, Carney PR. Neurologic disorders. In: Merenstein GB, Gardner SL, editors. Handbook of neonatal intensive care. 5th edn. St Louis: Mosby; 2002:644-678.
Papile LA, Burnstein J, Burnstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 gm. Journal of Pediatrics. 1978;92:529-534.
Parker LA. Part 2: Birth trauma: Injuries to the intraabdominal organs, peripheral nerves, and skeletal system. Advances in Neonatal Care. 2006;6(1):7-11.
Parker L, Cole M, Craft AW, et al. Neonatal vitamin K administration and childhood cancer in the north of England: retrospective case-control study. British Medical Journal. 1998;305:341-346.
Puckett RM, Offringa M. Prophylactic vitamin K for vitamin K deficiency bleeding in neonates. Cochrane Database of Systematic Reviews. 4, 2000. CD002776
Rennie JM, Boylan G. Treatment of neonatal seizures. Archives of Disease in Childhood Fetal and Neonatal. 2007;92:F148-F150.
Rennie JM, Roberton NRC, editors. A manual of neonatal intensive care, 4th edn, London: Arnold, 2002.
Roberts IA, Murray NA. Neonatal thrombocytopenia. Archives of Disease in Childhood Fetal and Neonatal. 2003;88(5):F359-F364.
Roberts IA, Murray NA. Neonatal thrombocytopenia. Current Haematological Reports. 2006;5(1):55-63.
Roche. How to use Konakion MM Paediatric 2mg/0.2ml (phytomenadione) for the prophylaxis of vitamin K deficiency bleeding (VKDB). Hertfordshire: Roche, 2005.
Sansoucie DA, Cavaliere TA. Newborn and infant assessment. In: Kenner C, Wright-Lott J, editors. Comprehensive neonatal nursing. A physiologic perspective. 3rd edn. Philadelphia: Saunders; 2003:308-347.
Seigel R, Gardner SL, Merenstein GB. Families in crisis: theoretical and practical considerations. In: Merenstein GB, Gardner SL, editors. Handbook of neonatal intensive care. 5th edn. St Louis: Mosby; 2002:725-753.
Smith C. Intracranial haemorrhage in infants. Current Diagnostic Pathology. 2006;12(3):184-190.
Soin H, Katesmark M. By muscle or mouth. Nursing Times. 1993;89(42):32-33.
Sorantin E, Brader P, Thimary F. Neonatal trauma. European Journal of Radiology. 2006;60(2):199-207.
Thomas R, Harvey D. Colour guide: neonatology, 2nd edn. Edinburgh: Churchill Livingstone, 1997.
Tortora GJ, Derrickson B. Principles of anatomy and physiology, 11th edn. Hoboken: John Wiley, 2006.
Volpe JJ. Brain injury in the premature infant. Clinics in Perinatology. 1997;24(3):567-587.
von Kries R, Gobel U, Hachmeister A, et al. Vitamin K and childhood cancer: a population-based case-control study in Lower Saxony, Germany. British Medical Journal. 1996;313:199-203.
Wariyar U, Hilton S, Pagan J, et al. Six years’ experience of prophylactic oral vitamin K. Archives of Disease in Childhood Fetal and Neonatal. 2000;82:F64-F68.
Wilson AK. Contact a family – not just a red book!. Infant. 2006;2(4):142-145.
Boxwell G, editor. Neonatal intensive care nursing. London: Routledge, 2000.
This book is primarily written for neonatal nurses and teachers. Student midwives and midwives would benefit from the additional more detailed information about many of the conditions addressed in this chapter. Chapter 2, 7, 8, 9, 17 and 19 are recommended.
Hankins GDV, Clark SM, Munn MB. Cesarean section on request at 39 week: impact on shoulder dystocia, fetal trauma, neonatal encephalopathy, and intrauterine fetal demise. Seminars in Perinatology. 2006;30(5):276-287.
Given the controversies about increasing rates, these American authors offer convincing arguments in support of elective caesarean section to prevent complications.
Rennie JM. Roberton’s textbook of neonatology, 4th edn. London: Elsevier, 2005.
A classic British textbook that gives excellent explanations of physiology and discusses the management of neonatal complications, albeit from a mainly medical perspective.