The Child with Renal Dysfunction
http://evolve.elsevier.com/wong/ncic
Administration of Medication, Ch. 27
Collection of Specimens, Ch. 27
Defects of the Genitourinary Tract, Ch. 11
Family-Centered Care of the Child with Chronic Illness or Disability, Ch. 22
Family-Centered Home Care, Ch. 25
Physical Examination: Genitalia, Ch. 6
Renal Structure and Function
The kidney’s primary responsibility is to maintain the composition and volume of the body fluids in equilibrium. To maintain this constant internal environment, the kidney must respond appropriately to alterations in the internal environment caused by variations in dietary intake and extrarenal losses of water and solutes. This is accomplished by the formation of urine (the product of glomerular filtration), tubular reabsorption, and tubular secretion. Reabsorption is the transport of a substance from the tubular lumen to the blood in surrounding vessels. Secretion is transport in the opposite direction (i.e., from the blood to the lumen). These processes are either active or passive. Excretion is the elimination of a substance from the body, in this case urine.
A secondary function of the kidney is the production of certain humoral substances. One such substance is an enzyme, erythropoietin-stimulating factor (or erythrogenin), which acts on a plasma globulin to form erythropoietin, which in turn stimulates erythropoiesis in the bone marrow. Its production increases in the presence of hypoxia and androgens. Few red blood cells form in the absence of erythropoietin, which accounts somewhat for the anemia associated with advanced kidney disease. The kidney also secretes another enzyme, renin, in response to reduced blood volume, decreased blood pressure, or increased secretion of catecholamines. Renin stimulates the production of the angiotensins, which produce arteriolar constriction and an elevation in blood pressure and stimulate the production of aldosterone by the adrenal cortex.
Renal Physiology
The structural and functional unit of the kidney is the nephron, which contains a complex system of tubules, arterioles, venules, and capillaries (Fig. 30-1, A). The nephron consists of the Bowman capsule, which encloses a tuft of capillaries and is joined successively to the proximal convoluted tubule, the loop of Henle, the distal convoluted tubule, and the straight or collecting duct (Fig. 30-1, B). Collecting tubules join larger ducts, and all the larger collecting ducts of one renal pyramid join to form a single duct that opens into a minor calyx. A number of calyces empty into one of several major calyces that converge into the renal pelvis. The renal pelvis narrows after it leaves the kidney and forms what then becomes a ureter, through which urine drains into the urinary bladder.
Pathophysiology Review
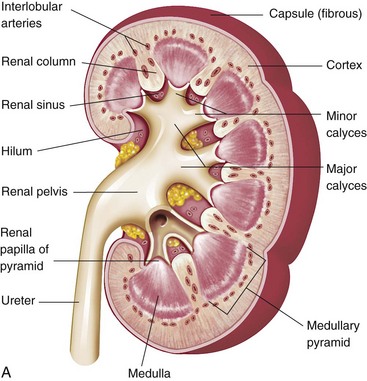
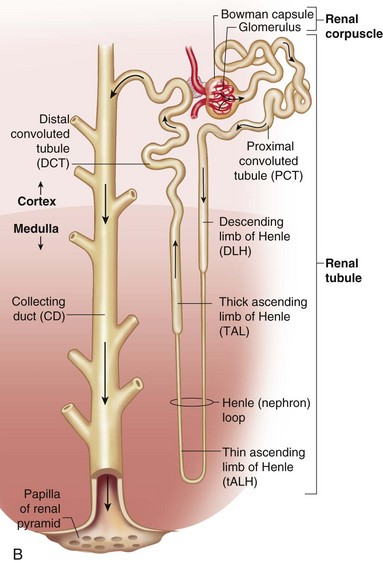
Fig. 30-1 A, Kidney structure. B, Components of the nephron. (From Patton KT, Thibodeau GA: Anatomy and physiology, ed 7, St Louis, 2010, Mosby.)
The blood supply to the kidneys constitutes approximately one fifth of the total cardiac output; therefore profuse bleeding can accompany renal trauma. Because interstitial tissue is sparse, individual nephrons with their blood vessel component are closely packed together. A sizable afferent arteriole, which separates into capillary loops that constitute the glomerular tuft, supplies each nephron. Blood leaves by a smaller efferent arteriole. From there the efferent arterioles branch into a peritubular capillary network and hairpin loops called the vasa recta, which parallel the loops of Henle and collecting ducts. The total surface area of the renal capillaries is approximately equal to the total surface area of the tubules.
The Bowman capsule is composed of two cellular layers that separate the blood from the glomerular filtrate: the capillary endothelium and a layer of tubular epithelial lining cells. Situated between these layers is the basal lamina, or basement membrane. This glomerular membrane is permeable because the capillary endothelium is fenestrated with pores, or fenestrae. Also, the outer surface of the glomerular epithelium consists of fingerlike projections (pseudopodia, or podocytes), which cover the entire surface to form slits called slit pores. The basement membrane has no visible openings but behaves as though it contains pores or channels. Consequently, the glomerular filtrate (which has essentially the same composition as plasma except for the large protein molecules and cellular elements) passes through these three layers at a rapid rate. The structure of these layers becomes altered in kidney disease.
Glomerular Filtration
Filtration through the glomerular capillaries is governed by the same mechanism as filtration across other capillaries in the body (i.e., the size of the capillary bed, the permeability of the capillaries, and the hydrostatic and osmotic pressure gradients across the capillaries). The filtration capacity of the glomerulus is the product of permeability of the glomerular capillaries and three pressure forces: glomerular hydrostatic pressure, colloidal osmotic (oncotic) pressure (COP), and intracapsular pressure.
Blood enters the nephron at a substantial pressure. This hydrostatic pressure forces plasma fluid and solutes through the capillary membrane and into the unit’s collecting apparatus. As this filtrate travels through the renal tubules, water and solutes are selectively reabsorbed back into the vascular compartment. That which is not reabsorbed is excreted as urine. Filtration takes place as long as hydrostatic pressure within the glomerular capillaries exceeds the opposing COP of the plasma proteins. If the pressure becomes equal through decreased hydrostatic pressure or decreased COP, no further filtration takes place. In a state of dehydration, more water is reabsorbed; when water intake is increased, more is excreted as urine. In conditions that produce osmotic diuresis (i.e., when large solutes, such as glucose, are filtered through the capillaries in such excessive amounts that they cannot be reabsorbed), the osmotic attraction of the solute causes less water to be reabsorbed, resulting in water being excreted in the urine with the solute.
Tubular Function
The function of the renal tubules is to modify the glomerular filtrate. Tubular cells may add more of a substance to the filtrate (tubular secretion), remove some or all of a substance from the filtrate (tubular reabsorption), or both. The reabsorption is selective and discriminating for substances essential to body processes and equilibrium, whereas nonessential substances are eliminated as waste. The substances are secreted or reabsorbed in the tubules by osmosis, passive movement down a chemical or electric gradient, or are actively transported against these gradients. These processes operate throughout the length of the tubules, but there are variations in the types, amounts, and mechanisms by which substances are secreted or reabsorbed in the different tubular segments. The cellular characteristics of each segment are largely responsible for these variations (see Fig. 30-1).
Active transport mechanisms move vital substances both inward and outward from the tubular filtrate. For example, the proximal tubule reabsorbs essential substances such as glucose, amino acids, and sodium ions and returns them directly to the blood. Active transport mechanisms here, as elsewhere, have a limited capacity, or threshold, for moving the solute. When the maximum of the transport mechanism is reached, no more substance is reabsorbed, and the remainder is excreted in the urine. For example, when blood glucose concentrations exceed their transport capacity, the surplus remains in the filtrate to be excreted in the urine (glycosuria). When two substances share a common transport mechanism, the first substance may be blocked by the addition of a second substance (selective inhibition). The effect of many therapeutic agents (e.g., diuretics) depends on this process.
Electrolytes are moved by both active transport and diffusion; the transport of certain electrolytes, particularly sodium, has important effects on other substances. For example, sodium is actively transported from all parts of the nephron. The movement of sodium ions produces both an electric and an osmotic gradient, which causes chloride ions and water to diffuse from the tubules in an effort to establish equilibrium. This is the obligatory water reabsorption in the kidneys. There is a limit to the concentration gradient against which sodium can be transported out; therefore, when larger than normal amounts of sodium ions remain in the tubules, water is obliged to remain with the sodium.
Under normal conditions the kidneys are able to adjust the urine and solute excretion in response to the requirements for body water and electrolyte balance. They are able to excrete or conserve both water and most electrolytes in addition to excreting the end products of protein metabolism, principally urea. The volume of urine excreted by the kidneys in a given period depends on the water balance (including intravascular filtration pressure), the quantity of solutes presented to the kidneys, and the capacity of the kidneys to dilute or concentrate the filtrate.
Renal Development and Function in Early Infancy
![]() Development of the kidney begins within the first weeks of embryonic life but is not completed until about the end of the first year after birth. The nephrons increase in number throughout gestation and reach their full complement by birth. However, at this point they are immature and less efficient than at later ages. Many of the tubular sections are not fully formed, and the glomeruli enlarge considerably after birth.
Development of the kidney begins within the first weeks of embryonic life but is not completed until about the end of the first year after birth. The nephrons increase in number throughout gestation and reach their full complement by birth. However, at this point they are immature and less efficient than at later ages. Many of the tubular sections are not fully formed, and the glomeruli enlarge considerably after birth.
Glomerular filtration and absorption are relatively low in the infant and do not reach adult values until between 1 and 2 years of age. This appears to be related to a barrier imposed by more cuboidal-shaped glomerular epithelial cells and higher afferent arteriole resistance. Consequently, the newborn is unable to dispose of excess water and solutes rapidly or efficiently.
The tubular length of nephrons is highly variable. Glomerular size is less variable. The juxtaglomerular nephrons show more advanced development than the cortical nephrons. The loop of Henle (the site of the urine-concentrating mechanism) is short in the newborn, which reduces the ability to reabsorb sodium and water and therefore produces very dilute urine; however, the newborn pituitary gland secretes adequate amounts of antidiuretic hormone. The length of tubules gradually increases until concentrating ability reaches adult levels by approximately the third month of life. Urea synthesis and excretion are slower during this time, and the newborn retains large quantities of nitrogen and essential electrolytes to meet the needs for growth in the first weeks of life. Consequently, the excretory burden is minimized. The lower concentration of urea, the principal end product of nitrogen metabolism, also reduces concentrating capacity because it contributes to the concentration mechanism.
Other characteristics of the newborn’s kidneys result in renal function that differs from that of older children and adults. Because of some as yet undetermined cause, newborn infants are unable to excrete a water load at rates similar to those of older persons. Hydrogen ion excretion is reduced, acid secretion is lower for the first year of life, and plasma bicarbonate levels are low. Because of these inadequacies of the kidney and because of less efficient blood buffers, the newborn is more liable to develop severe metabolic acidosis. Sodium excretion is reduced in the immediate newborn period, and the kidneys are less able to adapt to sodium deficiencies and excesses. For example, an isotonic saline infusion may produce edema because of impaired ability to eliminate excess. Conversely, inadequate reabsorption of sodium from the tubules may increase sodium losses in disorders such as vomiting or diarrhea. Moreover, infants have a diminished capacity to reabsorb glucose and, during the first few days, to produce ammonium ions.
The kidney functions during fetal life and produces urine that contributes to the amniotic fluid volume. The 24-hour urine volume is low at birth, rapidly increases in the neonatal period, and steadily increases with normal growth. (See Appendix C.) The kidneys continue to grow in size until body growth is complete in adolescence.
Renal Pelvis and Ureters: Structure and Function*
The renal pelvis is a funnel-shaped structure that originates at the major calyces and terminates in the funnel-shaped ureteropelvic junction. The ureter is a thin mucomuscular tube that extends from the ureteropelvic to the ureterovesical junction in the base of the bladder. Three areas—the ureteropelvic junction, the ureterovesical junction, and the segment nearest the sacroiliac junction—are particularly narrow and prone to obstruction when a solid body (such as a urinary calculus [“stone”]) passes.
The principal function of the renal pelvis and ureter is the transport of urine from the kidney to the bladder. Urine is moved via a process called peristalsis, whereby muscular movements originating in the renal pelvis propel a bolus of urine toward the urinary bladder for storage and eventual evacuation when the child urinates. The renal pelvis stores only a relatively small volume of urine (approximately 15 ml in adults) before a contraction is triggered that pushes the urine toward the bladder. The forward movement of urine from the kidney to the bladder is called efflux, whereas abnormal (or backward) urine movement is termed reflux. Aside from mechanical stretching, neurogenic and hormonal factors modulate ureteral peristalsis.
The ureterovesical junction joins the ureters and bladder. It is made up of three principal components: the lowest segment of the ureter, the trigone muscle, and the adjacent bladder wall. The ureters allow the passage of urine from the upper urinary tracts while preventing regurgitation of urine from the bladder to the ureters. During bladder filling, intravesical pressure remains relatively low and the detrusor muscle remains in a relaxed state. A peristaltic contraction of the ureter propels urine into the bladder. During micturition, the intravesical pressure rises as the detrusor muscle contracts; this raises the potential for harmful reflux into the upper urinary tracts. Several mechanisms in the normal ureterovesical junction act together to prevent reflux. The terminal (intravesical) ureteral segment tunnels through the bladder wall at an oblique angle. During bladder contraction, tension in the detrusor muscle squeezes the intravesical ureter closed. The trigone muscle that surrounds the ureteral orifice of the terminal ureter enhances this process. In addition, the longitudinally arranged muscle of the intravesical ureter contracts, providing further resistance to reflux. Anatomic defects of the ureterovesical junction, such as lateral displacement of the ureter or reduced length of the intravesical ureter, predispose the child to primary reflux. In children with normal anatomy, voiding dysfunction associated with infections and high bladder pressures predisposes them to secondary reflux (Sillen, 2008).
Urethrovesical Unit: Structure and Function*
The urethrovesical unit consists of the bladder, urethra, and pelvic muscles; it is also called the lower urinary tract. The urinary bladder is a muscle-lined sac that stores and empties itself of urine. In the infant the bladder lies entirely in the abdomen. The bladder assumes its place in the true pelvis shortly before puberty. This change in position is due to the maturation of the pelvic bone rather than migration of the bladder and urethra.
![]() The bladder has two inlets (the ureteral orifices) and a single outlet (the urethral orifice). The base of the bladder is a relatively fixed, triangular area consisting of the bladder neck and trigone. In contrast, the body of the bladder is distensible, changing from a tetrahedron (four-sided shape) when relatively empty to a nearly spherical shape as the bladder fills.
The bladder has two inlets (the ureteral orifices) and a single outlet (the urethral orifice). The base of the bladder is a relatively fixed, triangular area consisting of the bladder neck and trigone. In contrast, the body of the bladder is distensible, changing from a tetrahedron (four-sided shape) when relatively empty to a nearly spherical shape as the bladder fills.
One of the four layers of the bladder wall consists of smooth muscle bundles that promote bladder evacuation via micturition. Collectively this muscular tunic is called the detrusor. The muscular tunic of the bladder wall also contains collagen, a tough, nonelastic substance that maintains the integrity of the bladder wall while also preventing overdistention. Certain pathologic factors, including denervation of the bladder and obstruction of the outlet, may cause an overabundance of collagen in the detrusor muscle. This causes a loss of bladder compliance (distensibility), abnormally high filling pressures, and trabeculation of the bladder wall.
The urethra is a mucomuscular tube that connects the external meatus and the bladder. The male urethra originates at the bladder neck, piercing the prostate and pelvic floor before tunneling through the posterior portion of the penis and terminating at the glans penis. The proximal portion of the urethra comprises the sphincter mechanism, whereas the distal portion serves as a conduit for the passage of urine or semen. The urethral meatus is a vertical slit located at the summit of the glans penis.
The female urethra follows a relatively short, straight course compared with the male. It originates at the bladder base and terminates at an external meatus located immediately superior to (in front of) the vaginal orifice. The distal two thirds of the female urethra are fused with the vaginal wall.
The primary responsibilities of the bladder are to store urine manufactured by the kidneys and to evacuate this urine at regular intervals via the process of micturition. During infancy the bladder is expected to empty spontaneously; by the fourth year of life (or earlier) the child is expected to gain control of detrusor and urethral sphincter function. Control of the urethrovesical unit is referred to as urinary continence. Continent individuals are expected to hold their urine for at least 2 hours while awake. During sleeping hours they may arise once to urinate, although many children and young adults sleep for 8 hours or more without interruption. Three factors—anatomic integrity of the lower urinary tract, detrusor control, and competence of the urethral sphincter mechanism—must function normally for an individual to achieve and maintain continence.
Detrusor control requires successful integration of neurologic structures in the brain, spinal cord, and peripheral nervous systems. The brain influences bladder function via its inhibitory role on detrusor contractions. The stable detrusor contracts only when its owner gives permission. Several areas of the brain act together to control detrusor stability. A pathologic condition of one of these areas is known to produce detrusor instability, or the loss of control over detrusor contractions.
The spinal cord influences lower urinary tract function because it transmits messages between the brain and the target organ. Two areas in the spinal cord are particularly significant. The thoracolumbar cord (spinal levels T10-L2) influences bladder and urethral sphincter function. Sympathetic impulses from the brain travel to the bladder body and smooth muscle of the urethra, causing relaxation of the detrusor muscle and contraction of urethral smooth muscle. This combination of actions promotes bladder filling and storage of urine. The sacral spinal cord (spinal segments S2-S4) influences the bladder muscle, promoting micturition. Parasympathetic impulses travel from these nuclei, causing contraction of the detrusor muscle and indirectly promoting relaxation of smooth muscle in the urethra.
Two peripheral nerve plexuses directly influence control of the detrusor muscle. The pelvic plexus provides parasympathetic innervation to the bladder and urethra, and the inferior hypogastric plexus provides sympathetic innervation (Sugarman, 2000).
The final mechanism responsible for the attainment and maintenance of continence is the urethral sphincter mechanism. Traditionally two sphincters are described. The internal sphincter consists of the smooth muscle of the bladder and proximal urethra, and the external sphincter consists of the periurethral striated muscle. However, it is better to describe a single mechanism consisting of elements of compression and elements of tension.
Elements of compression are necessary for the urethra to form a watertight seal between episodes of urination. The softness (collapsibility) of the urethral wall is important for continence, particularly when a catheter alters urethral integrity. The mucus produced by the epithelium further enhances the watertight seal of the urethra. The mucus reduces surface tension, promoting collapse of the walls and sealing the microscopic fissures against urinary leakage.
The vascular cushion also acts as an element of compression (in addition to producing tension), contributing to urethral closure during physical stress. The vascular cushion, or network of the arterioles, venules, and arteriovenous communications in the urethra, promotes urethral compression by transmitting pressure from the muscles surrounding the urethra and those intrinsic to its walls. The vascular cushion contributes to urethral closure pressure because it is filled with an incompressible fluid that has its own intrinsic pressure.
The elements of tension in the urethral sphincter mechanism consist of the vascular cushion, intrinsic smooth and skeletal muscles, and periurethral striated muscle. These muscles are specially innervated to maintain the tension needed for urethral closure between episodes of micturition and to provide an extra measure of urethral tension, which is needed when significant physical exertion stresses sphincter closure. The pelvic muscles receive somatic innervation, which allows voluntary interruption of the urinary stream and provides added protection against precipitous rises in abdominal pressure.
Clinical Manifestations
As in most disorders of childhood, the incidence and type of kidney or urinary tract dysfunction change with the child’s age and maturation. In addition, the presenting complaints and their significance vary with maturation. For example, a complaint of enuresis has greater significance at age 8 years than at age 4. In the newborn, urinary tract disorders are associated with a number of obvious malformations of other body systems, including the curious and unexplained but frequent association between malformed or low-set ears and urinary tract anomalies. Important signs and symptoms that suggest possible renal or genitourinary tract disease in children at different ages are outlined in Box 30-1.
Many clinical manifestations are common to a variety of childhood disorders, but their presence is an indication to obtain further information from the patient history, family history, and laboratory studies as part of a complete physical examination. Radiographic studies and renal biopsy can be used to further evaluate suspected kidney disease.
Laboratory Tests
Both urine and blood studies contribute vital information for the detection of renal problems. The single most important test is probably routine urinalysis. Specific urine and blood tests provide additional information.
Glomerular filtration rate is a measure of the amount of plasma from which a given substance is totally cleared in 1 minute. Clearance is calculated from the ratio of substance excreted to the concentration of that substance in the plasma. A number of substances can be used, but the most useful clinical estimation of glomerular filtration is the clearance of creatinine, an end product of protein metabolism in muscle and a substance that is freely filtered by the glomerulus and secreted by renal tubular cells. The production and secretion of creatinine remain relatively constant from day to day, and its appearance in the urine is determined by the serum level. When the collection is complete and accurately timed, the results are fairly reliable and compare favorably with clearance of other substances (e.g., insulin) that require special equipment and long immobilization of the child to evaluate.
Any significant degree of renal disease can diminish the glomerular filtration rate, but renal vascular disease and diseases of the glomerulus have the most immediate effect. The nurse’s responsibility in this test is collection of urine, usually a 12- or 24-hour specimen.
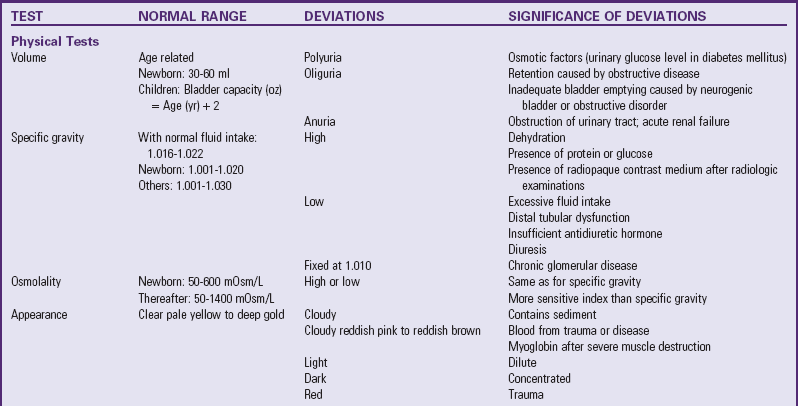
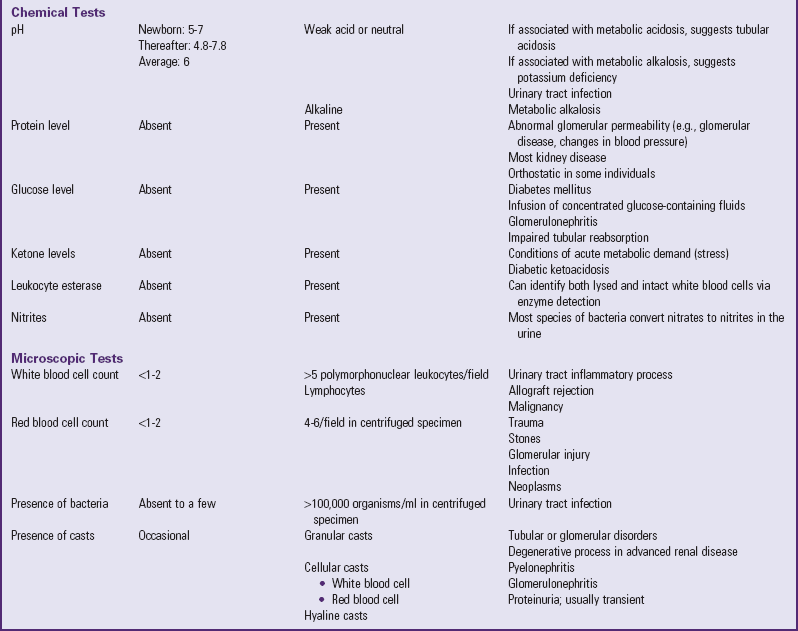
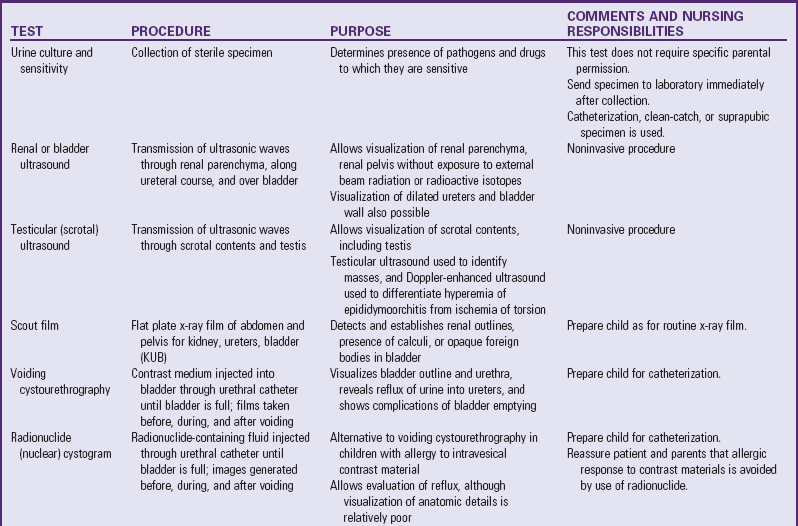
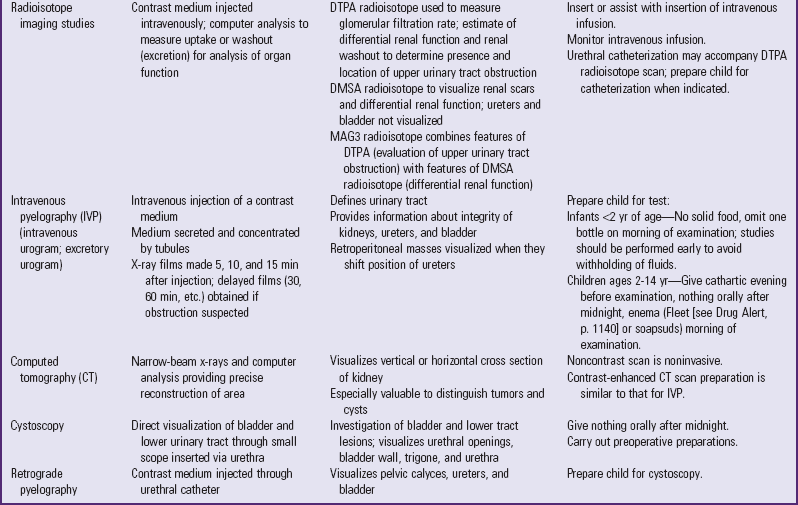
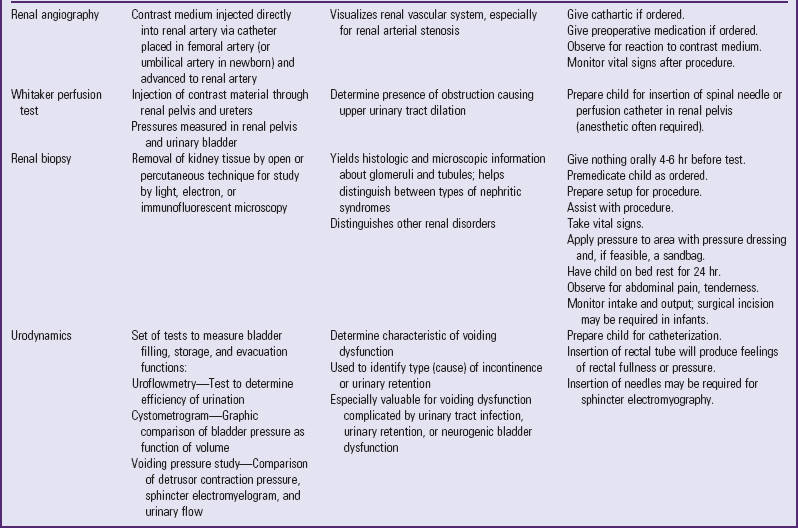
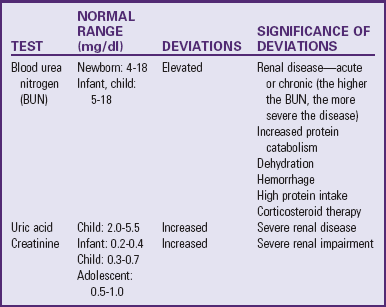
Table 30-1 outlines the major urine and blood tests. Radiologic and other tests of urinary system function are described in Table 30-2. Blood tests of renal function are outlined in Table 30-3.
Nursing Care Management
Nursing responsibilities in the assessment of renal disorders and diseases begins with observation of the child for any manifestations that might indicate dysfunction. The most significant ongoing assessments in children with renal conditions are accurate measurement and recording of weight, intake and output, and blood pressure. (See Chapter 6.) These assessments are necessary not only for children with known renal dysfunction but also for those children at risk for developing renal complications (e.g., children in shock, postoperative patients).
In addition to the general manifestations of renal conditions (see Box 30-1), many conditions have specific characteristics that distinguish them from other disorders. These are discussed as appropriate throughout the chapter.
The nurse is generally responsible for preparing infants, children, and parents for tests and collection of urine and (sometimes) blood specimens. (See Preparation for Diagnostic and Therapeutic Procedures, and Collection of Specimens, Chapter 27.) Nurses observe the characteristics of the urine collected, often perform any of a number of tests on urine specimens (e.g., urine specific gravity, protein, blood, glucose, ketones), and assist with more complex diagnostic tests (e.g., radiography, cystoscopy). Nurses must be familiar with significant laboratory tests, their implications, and preprocedural care.
Genitourinary Tract Disorders
![]() Urinary tract infection (UTI) is a clinical condition that may involve the urethra and bladder (lower urinary tract) and the ureters, renal pelvis, calyces, and renal parenchyma (upper urinary tract). Because it is often impossible to localize the infection, the broad designation UTI is applied to the presence of significant numbers of microorganisms anywhere within the urinary tract (except the distal third of the urethra, which is usually colonized with bacteria).
Urinary tract infection (UTI) is a clinical condition that may involve the urethra and bladder (lower urinary tract) and the ureters, renal pelvis, calyces, and renal parenchyma (upper urinary tract). Because it is often impossible to localize the infection, the broad designation UTI is applied to the presence of significant numbers of microorganisms anywhere within the urinary tract (except the distal third of the urethra, which is usually colonized with bacteria).
![]() Critical Thinking Case Studies—Urinary Tract Infection
Critical Thinking Case Studies—Urinary Tract Infection
Infection of the urinary tract may be present with or without clinical symptoms. As a result, the site of infection is often difficult to pinpoint with accuracy. The various terms used to describe urinary tract disorders are defined in Box 30-2.
The peak incidence of UTI not caused by structural anomalies occurs between 2 and 6 years of age. Except for the neonatal period, females have a 10 to 30 times greater risk for developing UTI than males. It is estimated that 5% to 6% of girls will have had at least one episode of bacteriuria between the time they enter first grade and graduate from high school. The likelihood of recurrence is 50% or greater in girls; the recurrence rate is lower in boys (Mingin, Hinds, Nguyen, et al, 2004).
Approximately, 20% to 25% of children with a negative radiologic evaluation have recurrent UTI (Mingin, Hinds, Nguyen, et al, 2004; Bratslavsky, Feustel, Aslan, et al, 2004). UTI in newborns differs in some respects from infections occurring in older children. In the neonatal age-group, boys with UTIs outnumber girls. At all ages asymptomatic bacteriuria is more common than symptomatic disease. An increased incidence of UTI is observed in adolescents, especially those with evidence of sexual activity.
Etiology
A variety of organisms can be responsible for UTI. Escherichia coli (80% of cases) and other gram-negative enteric organisms are most commonly implicated; all are common to the anal, perineal, and perianal region. Other organisms associated with UTI include Proteus, Pseudomonas, Klebsiella, Staphylococcus aureus, Haemophilus, and coagulase-negative staphylococci. A number of factors contribute to the development of UTI, including anatomic, physical, and chemical conditions or properties of the host’s urinary tract.
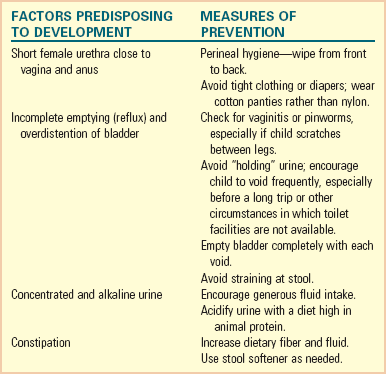
Anatomic and Physical Factors: The structure of the lower urinary tract is believed to account for the increased incidence of bacteriuria in females. The short urethra, which measures approximately 2 cm (0.75 inch) in young girls and 4 cm (1.5 inches) in mature women, provides a ready pathway for invasion of organisms. In addition, the closure of the urethra at the end of micturition may return contaminated bacteria to the bladder.
The longer male urethra (as long as 20 cm [8 inches] in an adult) and the antibacterial properties of prostatic secretions inhibit the entry and growth of pathogens. The presence or absence of the foreskin contributes to the differences in UTI rates in infants. Infant girls had a 5% incidence of developing a UTI, whereas UTI rates were 21% in uncircumcised infant boys and only 2% in circumcised infant boys (Zorc, Levine, Platt, et al, 2005; Shaikh, Morone, Bost, et al, 2008). The presence of a foreskin is associated with a preputial colonization of uropathic bacteria that can ascend the urethra easily (Balat, Karakok, Guler, et al, 2008). The incidence of renal scarring is greatest in patients whose first infection occurs during infancy.
The single most important host factor influencing the occurrence of UTI is urinary stasis. Ordinarily urine is sterile, but at 37° C (98.6° F) it provides an excellent culture medium. Under normal conditions the act of completely and repeatedly emptying the bladder flushes away any organisms before they have an opportunity to multiply and invade surrounding tissue. However, urine that remains in the bladder allows bacteria from the urethra to rapidly become established in the rich medium.
Incomplete bladder emptying (stasis) may result from reflux (see p. 1138 for a discussion of reflux), anatomic abnormalities (especially those involving the ureters), or extrinsic ureteral or bladder compression. The pressure of overdistention within the bladder may increase the risk of infection by decreasing host resistance, probably as a result of lessened blood flow to the mucosa. This often occurs in a neurogenic bladder or as a consequence of voluntarily holding back urine despite the urge to void.
Urinary stasis may also occur because of dysfunctional voiding. Dysfunctional voiding refers to an abnormality in either the storage or emptying phase of micturition and is associated with urgency, frequency, incontinence, UTI, and secondary vesicoureteral reflux (VUR) (Berry, 2005). Children may also exhibit bowel elimination symptoms such as constipation (Plachter, Schulman, and Canning, 1999) (Box 30-3).
Extrinsic factors that may be responsible for functional bladder neck obstruction are pregnancy and chronic and intermittent constipation. In both conditions the full uterus or rectum displaces the bladder and posterior urethra in the fixed and limited space of the bony pelvis, causing obstruction, incomplete micturition, and urinary stasis. Treating constipation and administering antibiotic therapy for UTI reduces the recurrence of infection. Failure to relieve the fecal retention in spite of adequate treatment of the UTI may result in recurrence.
Other extrinsic factors that can contribute to UTI include catheters, especially short-term indwelling catheters, and administration of antimicrobial agents. Antimicrobials alter the host’s normal perineal flora, allowing easier colonization of uropathogens. Tight clothing or diapers, poor hygiene, and local inflammation, such as from vaginitis, masturbation, or pinworm infestation, may also increase the risk of ascending infection. The essential oils in bubble baths and shampoos can irritate the urethra of both boys and girls, causing painful and frequent urination. Therefore bubble baths are discouraged. There is no evidence that plain tub baths increase the risk of UTI, but infections have been related to the use of hot tub or whirlpool baths. Sexual intercourse may produce transient bacteriuria in females and is associated with an increased risk of UTI.
Altered Urine and Bladder Chemistry: Several mechanical and chemical characteristics of the urinary tract promote urine sterility. Adequate fluid intake promotes urinary transport and lowers the concentration of pathogens (and nutrients) in the urine. Diuresis also enhances the antibacterial properties of the renal medulla, probably as a result of increased blood flow, which hastens leukocytosis; diuresis promotes the mechanical removal of pathogens (see Research Focus box).
Pathophysiology
After invasion by bacteria, the first line of defense in the lower urinary tract is complete evacuation by voiding. Inflammation in the bladder and urethral walls is apparent within 30 minutes of invasion by a bacterial pathogen. Polymorphonuclear leukocytes rapidly migrate to the bladder wall, which becomes completely injected within 2 hours. Complete evacuation of the bladder is particularly important for the eradication of bacteria from the urine. Urination not only removes bacteria and associated toxins contained in the urine, it also allows more efficient destruction of the bacteria remaining on the thin film of urine that is closely adherent to the vesical wall.
Recurrent infection of the urinary bladder predisposes the individual to transient episodes of VUR. After resolution of the infection the reflux is not detectable on voiding cystourethrography (VCUG). Although it is known that certain adherent bacteria promote urinary system dilation, the relationship between bladder wall inflammation and ureterovesical junction competence remains unclear (Wein, Kavoussi, Norvick, et al, 2006).
Clinical Manifestations
The clinical manifestations of UTIs depend on the child’s age. In newborn infants and children less than 2 years of age the signs are characteristically nonspecific. They more nearly resemble gastrointestinal tract disorders: failure to thrive, feeding problems, vomiting, diarrhea, abdominal distention, and jaundice. Newborns may have fever, hypothermia, or sepsis. Other evidence includes frequent or infrequent voiding, constant squirming and irritability, strong-smelling urine, and an abnormal stream. A persistent diaper rash is also a helpful clue.
The classic symptoms of UTI are often observed in children more than 2 years of age. These include enuresis or daytime incontinence in the child who has been toilet trained, fever, strong- or foul-smelling urine, increased frequency of urination, dysuria, or urgency. The children may also complain of abdominal pain or costovertebral angle tenderness (flank pain). Some patients are seen with hematuria. Some preschoolers may vomit. Infants and young boys frequently develop obstructive uropathy, which is characterized by dribbling of urine, straining with urination, or a decrease in the force and size of the urinary stream. High fever and chills accompanied by flank pain, severe abdominal pain, and leukocytosis suggest pyelonephritis. However, flank pain and tenderness may be the only indication of pyelonephritis on physical examination.
Manifestations in adolescents are more specific. Symptoms of lower tract infections include frequency and painful urination of a small amount of turbulent urine that may be grossly bloody. Fever is usually absent. Upper tract infection is characterized by fever; chills; flank pain; and lower tract symptoms, which may appear 1 or 2 days after the upper tract symptoms.
Many UTIs in children are asymptomatic or atypical in clinical presentation, and many complaints may be unrelated to the urinary tract. Many are treated as respiratory or gastrointestinal tract infections. It is important to identify these children so treatment can be initiated. Significant scarring can occur, especially in infants and young children.
Diagnostic Evaluation
The diagnosis of UTI depends on a high degree of suspicion, evaluation of the history and physical examination, and urinalysis and culture. Urine with a possible infection appears cloudy, hazy, or thick with noticeable strands of mucus and pus; it also smells fishy and unpleasant, even when fresh. A presumptive UTI diagnosis can be made on the basis of microscopic examination of the urine, which often reveals pyuria (5 to 8 white blood cells/ml of uncentrifuged urine) and the presence of at least one bacterium in a Gram stain. However, a normal urinalysis may also be present in conditions of asymptomatic bacteriuria.
Detection of bacteria in a urine culture confirms the diagnosis of UTI, but urine collection is often difficult, especially in infants and small children. (See Collection of Specimens, Chapter 27.) Several factors may alter a urine specimen. Contamination of a specimen by organisms from sources other than the urine is the most common cause of false-positive results. Bag urine specimens are commonly contaminated by perineal and perianal flora and are usually considered inadequate for a definitive diagnosis. The American Academy of Pediatrics recommends collecting urine by the bag to determine whether it is necessary to obtain a catheterized urine specimen for culture (Wald, 2005).
Unless the specimen is a first morning sample, a recent high fluid intake may indicate a falsely low organism count. Therefore do not encourage children to drink large volumes of water in an attempt to obtain a specimen quickly.
The most accurate tests of bacterial content are suprapubic aspiration (for children <2 years of age) and properly performed bladder catheterization (as long as the first few milliliters are excluded from collection). Care of a urine specimen obtained for culture is an important nursing responsibility related to diagnosis. The specimen should be taken to the laboratory for culture immediately. If culture is delayed, place the sample in a refrigerator for up to 24 hours, but storage can result in a loss of formed elements, such as blood cells and casts (Froom, Bieganiec, Ehrenrich, et al, 2000) (see Evidence-Based Practice box).
Tests to detect bacteriuria are being used with increased frequency in UTI screening. The plastic dipstick and agar-coated slide tests are quick and inexpensive methods for detecting infection before obtaining final culture results. The presence of nitrites on dipstick analysis of urine has been shown to have a predictive value of as much as 100% (identifies infected urine) (Raymond and Sauvestre, 1998). The absence of nitrites and leukocyte esterase in combination has been shown to have 92% negative predictive value (identifies uninfected urine) (Raymond and Sauvestre, 1998) (see Table 30-1). The agar-coated slides have a positive predictive value (identify infected urine) of 96% and negative predictive value (identify uninfected urine) of 99.8% (Colodner and Keness, 2000). These test results are used to initiate treatment of UTI while culture results are pending. It is important to remember that some organisms that cause UTI are non–nitrite producing (e.g., Pseudomonas organisms).
Localization of the infection site may involve more specific tests, including ureteral catheterization, bladder washout procedures, and radioisotope renography. Other tests, such as ultrasonography, VCUG, intravenous pyelography, and dimercaptosuccinic acid (DMSA) scan, may be performed after the infection subsides to identify anatomic abnormalities contributing to the development of infection and existing kidney changes from a recurrent infection.
Therapeutic Management
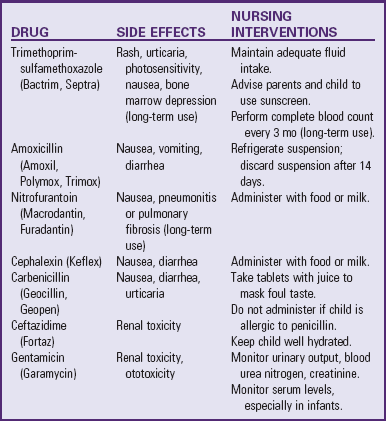
The objectives of treatment of children with UTI are to (1) eliminate the current infection, (2) identify contributing factors to reduce the risk of recurrence, (3) prevent urosepsis, and (4) preserve renal function (American Academy of Pediatrics, 1999). Antibiotic therapy depends on laboratory culture and sensitivity tests. Nonetheless, empiric therapy on the basis of the child’s history and presenting symptoms may be necessary when fever or systemic illness complicates UTI. Common antiinfective agents used for UTI include the penicillins, sulfonamide (including trimethoprim-sulfamethoxazole), the cephalosporins, nitrofurantoin, and the tetracyclines. All antibiotics may cause side effects or prove ineffective because of bacterial resistance (Table 30-4).
Children with suspected pyelonephritis and fever are admitted to the hospital and given appropriate antibiotics intravenously for a minimum of 48 hours. Blood and urine cultures are obtained on admission and after therapy. Urine cultures are usually repeated at monthly intervals for 3 months and at 3-month intervals for another 6 months.
Renal scarring can develop during the initial infection, especially in younger children. Therefore some practitioners believe that the first UTI in childhood necessitates radiologic evaluation, regardless of the patient’s age and sex. The standard assessment of UTI with renal ultrasound and VCUG is now being reconsidered. Instead the “top-down” approach of early DMSA renal scanning to identify acute pyelonephritis and renal injury is often recommended (Tseng, Lin, Lo, et al, 2007; Hardy and Austin, 2008). Children with a negative DMSA scan during their first UTI rarely have VUR. Negative DMSA results could reduce the need for children to undergo the often traumatic VCUG (Tseng, Lin, Lo, et al, 2007; Hardy and Austin, 2008).
Anatomic defects such as primary reflux or bladder neck obstruction may require surgical correction to prevent recurrent infection or may indicate the need for prophylactic antibiotics and careful follow-up monitoring. Follow-up study is an important component of medical management, since the relapse rate is high and recurrent infection tends to occur 1 to 2 months after termination of treatment. The aim of therapy and careful follow-up in such cases is to prevent morbidity and reduce the chance of renal scarring.
Prognosis: With prompt and adequate treatment at the time of diagnosis, the long-term prognosis for UTIs is usually excellent. The hazard of progressive renal injury is greatest when infection occurs in young children (especially <2 years of age) and is associated with congenital renal malformations and reflux. Therefore early diagnosis of children at risk is particularly important during infancy and toddlerhood.
Nursing Care Management
Objectives of nursing care include identification of children with UTI and education of parents and children regarding prevention and treatment of infection. Aside from the influence of renal abnormalities, girls between the ages of 2 and 6 years are in general a high-risk group. Because they are not a captive population, mass screening is difficult. However, the annual health examination should include a routine urinalysis. In addition, nurses should instruct parents to observe regularly for clues that suggest UTI. Unfortunately, the signs of UTI are not as evident as those of upper respiratory tract infection. Therefore many cases go undetected because no one thought to investigate this common problem.
Because infants and young children are unable to express their feelings and sensations verbally, it is difficult to detect any discomfort they may be experiencing from dysuria. A careful history regarding voiding habits, stooling pattern, and episodes of unexplained irritability may assist in detecting less obvious cases of UTI. Encourage parents to observe for specific clues of UTI in suspected cases (see Critical Thinking Exercise).
Collecting an appropriate specimen is essential when the nurse suspects infection. It is the nurse’s responsibility to take every precaution to obtain acceptable, clean-voided specimens in order to avoid using other collecting procedures except when absolutely indicated.
Other tests are often performed to detect anatomic defects. Prepare children for these tests as appropriate for their age. Children who are old enough to understand need an explanation of the procedure, its purpose, and what they will experience. (See Preparation for Diagnostic and Therapeutic Procedures, Chapter 27.) Sometimes a simple description of the urinary system is helpful. For children under 3 to 4 years of age, the nurse can explain the procedure using a doll. For those who are older, a simple drawing of the bladder, urethra, ureters, and kidneys makes the explanation more understandable. Especially with preschool children, the nurse must clarify that the urinary tract is separate from any sexual function and that the test is for a problem that they did not cause. It is not uncommon for children to associate blame for perceived wrongdoing (e.g., masturbation) or unacceptable thoughts with the reason for the illness or tests.
Children may be treated as outpatients to avoid overnight separation from home. In such cases be careful not to overlook the need for adequate preparation. If surgery is subsequently indicated, the children will have an understanding of these procedures, which helps decrease fear and anxiety regarding more extensive medical-surgical intervention.
Because antiinfective drugs are indicated in the treatment of UTI, the nurse teaches the patient and parents the appropriate dosage and scheduling and provides suggestions for administration of the agent. Certain drugs are available in liquid form; others are available only in capsule or pill form. In general, capsules are separated and pills are crushed, with their contents mixed into a small volume of food or chilled liquid to mask a disagreeable taste. A simple suggestion is to introduce a medicine in divided doses and mixed with a flavored gelatin in an ice cube tray. Children tolerate other medications best with a small portion of partially frozen grape or apple juice.
Encourage adequate fluid intake for the prevention and treatment of UTI, since bacterial eradication from the urinary tract is partially dependent on urine flow and voiding frequency (Dacher and Savoye-Collet, 2004; Wilde and Brasch, 2008). It is recommended that a person drink 100 ml/kg, or approximately 50 ml/lb of body weight, daily. The patient should primarily drink clear liquids. Have children avoid caffeinated or carbonated beverages because of their potentially irritative effect on the bladder mucosa. The child who is febrile and unable to drink liquids is given intravenous (IV) hydration until the fever resolves and oral liquids are tolerated.
Prevention: Prevention is the most important goal in both primary and recurrent infection. Most preventive measures are simple, ordinary hygienic habits that should be a routine part of daily care. Investigate any signs of intestinal parasites (e.g., scratching between the legs and around the anal area) and treat them appropriately. Advise sexually active adolescent girls to urinate as soon as possible after intercourse to flush out bacteria introduced during sex play. Also teach parents and older children health practices that prevent UTI (see Nursing Care Guidelines box).
Children who experience recurrent febrile UTIs or recurrent infections complicated by VUR may be given a suppressive or prophylactic antibiotic for a period of months or several years. The medication is commonly administered once a day; the patient and parents are advised to give the antibiotic before sleep because this represents the longest period without voiding. A sulfonamide-trimethoprim, nitrofurantoin, or cephalosporin is often used for antibiotic prophylaxis.
Vesicoureteral Reflux
VUR refers to the retrograde flow of bladder urine into the ureters. Reflux increases the chance for and perpetuates febrile UTI because with each void urine is swept up the ureters and then allowed to empty after voiding. Therefore the residual urine from the ureters remains in the bladder until the next void. The International Classification System describes the degree of reflux from the bladder into upper genitourinary tract structures (Fig. 30-2).
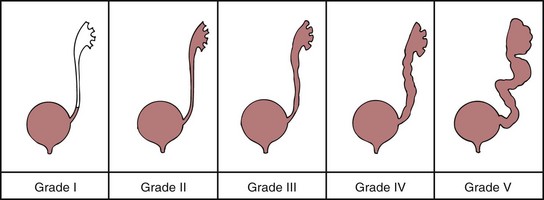
Fig. 30-2 Grades of reflux. (From Retik AB, Cukier J, editors: Pediatric urology, Baltimore, 1987, Williams & Wilkins.)
Primary reflux results from a congenital anomaly that affects the ureterovesical junction. Ectopic or orthotopic implantation of the ureter, abnormal tunneling of the intramural ureteral segment, and defects in the configuration of the ureter orifice are associated with primary reflux. Primary reflux has a significant familial pattern; the incidence of reflux in siblings of affected children has been reported as 36%. Moreover, screening should occur early, since siblings evaluated after 2 years of age had twice the risk of already having renal damage as detected by renal scan (Houle, Cheikhelard, Barrieras, et al, 2004). Although reflux occurs more often in females, siblings of both genders should be screened. One study found affected brothers of male children with reflux had a higher grade of reflux and were more likely to have renal scarring than female siblings (Pirker, Mohanan, Colhoun, et al, 2006) Screening for reflux in siblings through 72 months of age is recommended to prevent renal damage, which can occur in the absence of symptomatic UTI.
Secondary reflux occurs as a result of an acquired condition such as UTI or obstruction. UTI can produce transient reflux. Neuropathic bladder dysfunction, particularly when poor bladder compliance coexists with bladder outlet obstruction, may produce secondary reflux as urine seeks to escape the high pressures of the lower urinary tract. Obstruction may also be due to renal stones, strictures, or tumors affecting the urinary tract. Children who routinely “hold in” urine (dysfunctional voiding) may ultimately develop secondary reflux (Dacher and Savoye-Collet, 2004; Berry, 2005).
Reflux with infection is the most common cause of pyelonephritis in children. Refluxed urine ascending into the collecting tubules of the nephrons allows the microorganisms to gain access to the renal parenchyma, initiating renal scarring. The shape of renal papillae and the angle of entry of the collecting ducts change with advancing age, making intrarenal reflux difficult. Therefore most renal scars associated with reflux occur at a young age and are present at the time of diagnosis; few develop after 5 to 6 years of age. Between 30% and 60% of children with VUR have evidence of renal scarring, and scarring is almost always found in association with reflux. Careful routine follow-up care is a critical part of management of children with UTIs; children with reflux as documented by VCUG are assessed repeatedly during ensuing years.
Therapeutic Management
In most cases of VUR, conservative, nonoperative therapy is effective in controlling infection. There is a high incidence of spontaneous resolution over time—approximately 20% to 30% for each 2-year period throughout childhood. An 80% probability of remission may occur in grades I and II reflux when managed medically (Koff, 1997). Therapy consists of continuous low-dose antibacterial therapy with frequent urine cultures, which can usually be performed at home by the dip slide or dipstick methods. This long-term therapy requires medical supervision and reliable, cooperative parents. Surgical correction of reflux may be required for grades IV and V reflux. Grade III is managed conservatively unless there are complications.
The major indications for surgical intervention include significant anatomic abnormality at the ureterovesical junction, recurrent UTIs, high grades of VUR, noncompliance with medical therapy, intolerance to antibiotics, and VUR after puberty in females. Antireflux surgery consists of reimplantation of the ureters.
Renal ultrasonography is performed 1 month postoperatively to check for ureteral obstruction. If there is no obstruction, antibiotic therapy is discontinued. A renal ultrasound and VCUG are recommended 6 months after surgery. Because of the anxiety experienced by some children undergoing catheterization for cystography and the high success rate of antireflux surgical procedures, some practitioners may omit VCUG at this time. Two years after surgery, the child is seen for a final renal ultrasound to assess renal growth.
For some children with less severe reflux, a minimally invasive endoscopic option (subtrigonal injection, or STING) for the treatment of VUR is an attractive alternative to years of daily antibiotics or surgical intervention. During cystoscopy, the surgeon locates the affected ureter and injects a gel-like bulking agent—dextranomer–hyaluronic acid copolymer (Deflux)—into the mucous membrane where the ureter enters the bladder. This injected material creates a bulge, which results in elongation of the tunnel and passive closure of the ureteral orifice, making the retrograde flow of bladder urine more difficult. In addition to being an outpatient procedure requiring only a brief anesthetic, the procedure requires no incision, so it decreases the child’s discomfort (Lavelle, Conlin, and Skoog, 2005). A follow-up VCUG occurs 3 months after injection to assess for correction of reflux. In higher grades of reflux, more than one injection may be required to achieve resolution. Overall cure rates relate to degree of reflux and range from 77% after the first injection to 96% after the second injection (Wadie, Tirabassi, Courtney, et al, 2007; Cerwinka, Scherz, and Kirsch, 2008).
Nursing Care Management
The primary nursing goal in children receiving medical therapy is encouraging compliance. Emphasize the importance of maintaining the medical regimen to parents and older children. The medications prescribed are usually well tolerated by children, but parents may need help encouraging children to take the medication. The methods described in Chapter 27 provide some guidelines for administration and encouraging compliance. The importance of hygiene and a frequent voiding schedule are also discussed.
Because siblings are at risk for VUR, nurses should encourage parents to have their other children screened using renal ultrasonography and cystography. All children require age-appropriate preparation for the tests. Atraumatic care includes using lidocaine jelly before catheterization. (See Collection of Specimens, Chapter 27.)
Glomerular Disease
Acute glomerulonephritis (AGN) as a classification includes a number of distinct entities. It may be a primary event or a manifestation of a systemic disorder (Table 30-5), and the disease can range from minimum to severe. The common features include oliguria, edema, hypertension and circulatory congestion, hematuria, and proteinuria. Most cases are postinfectious and have been associated with pneumococcal, streptococcal, and viral infections. All postinfectious diseases are presumed to result from immune complex formation and glomerular deposition, and the clinical presentations may be indistinguishable. Postinfectious glomerulonephritis exhibits a better clinical course than other acute proliferative glomerulonephritis (Ozaltin, Besbas, Bakkaloglu, et al, 2005).
Acute poststreptococcal glomerulonephritis (APSGN) is the most common of the noninfectious renal diseases in childhood and the one for which a cause can be established in the majority of cases. APSGN can occur at any age but primarily affects early school-age children, with a peak age of onset of 6 to 7 years (Hahn, Knox, and Forman, 2005). It is uncommon in children younger than 2 years of age.
Etiology
It is now generally accepted that APSGN is an immune-complex disease (i.e., a reaction that occurs as a by-product of an antecedent streptococcal infection with certain strains of the group A β-hemolytic streptococci). Most streptococcal infections do not cause APSGN. A latent period of 10 to 14 days occurs between the streptococcal infection of the throat or skin and the onset of clinical manifestations. The peak incidence of disease corresponds to the incidence of streptococcal infections. Disease secondary to streptococcal pharyngitis is more common in the winter or spring. However, when associated with pyoderma (principally impetigo), it may be more prevalent in later summer or early fall, especially in warmer climates. Multiple cases tend to occur in families. Second attacks are rare.
Pathophysiology
The mechanism by which the reaction takes place is still speculative. The most popular proposal to explain the pathologic process is that the streptococcal infection is followed by the release of a membranelike material from the specific organism into the circulation. Because it is antigenic, antibodies are formed and an immune-complex reaction occurs after the appropriate period. These immune complexes become trapped in the glomerular capillary loop.
The kidney itself appears normal or moderately enlarged, but microscopic examination reveals a diffuse proliferative and exudative process. Glomerular capillary loops are almost obliterated by swelling, and infiltration with polymorphonuclear leukocytes adds to the appearance of increased cellularity. Consequently, the glomeruli appear dense and lobulated. Examination with the electron microscope reveals discrete nodules or “humps” in the basement membrane, which are identified as deposits of immune complexes. These deposits are not evident after approximately 6 weeks.
Endothelial cell proliferation and edema occlude the capillary lumen of affected glomeruli, and the afferent arteriole is probably constricted by vasospasm, both of which significantly reduce the glomerular filtration rate. This occurs without a proportional decrease in renal blood flow and results in a reduced capacity to form filtrate from the glomerular plasma flow. Vascular and tubular changes are mild and nonspecific; therefore tubular function is less severely impaired.
The decreased filtration of plasma results in an excessive accumulation of water and retention of sodium. These cause expanded plasma and interstitial fluid volumes that lead to circulatory congestion and edema. It is unclear whether a decreased glomerular filtration rate, increased capillary permeability, or vascular spasm is responsible for these various manifestations. The cause of the hypertension associated with AGN is also unexplained. Plasma renin activity is low during the acute phase, but hypervolemia may be a factor.
Clinical Manifestations
Typically, affected children are in good health until they experience the antecedent infection. In some instances there is no history of an infection, or it is described as only a mild cold. The onset of nephritis appears after an average latent period of approximately 10 days. Because the child appears well during this time, parents may not recognize the association.
Initial signs of nephrotic reaction include puffiness of the face, especially around the eyes (periorbital edema); anorexia; and the passage of cola-colored urine. The edema is more prominent in the face in the morning but spreads during the day to involve the extremities and abdomen. The edema is only moderate and may not be appreciated by someone unfamiliar with the child’s normal appearance. The urine is cloudy, smoky brown, or what parents describe as resembling tea or cola, and it is severely reduced in volume.
The child is pale, irritable, and lethargic and appears unwell but seldom expresses specific complaints. Older children may complain of headaches, abdominal discomfort, and dysuria. On examination there is usually a mild to moderate elevation in blood pressure compared with normal values for age. Occasionally a child will have an onset with severe symptoms such as seizures from hypertensive encephalopathy, pulmonary and circulatory congestion, or hematuria in the absence of hypertension and edema. Table 30-6 compares APSGN and minimal change nephrotic syndrome (MCNS).
TABLE 30-6
COMPARISON OF POSTSTREPTOCOCCAL GLOMERULONEPHRITIS AND NEPHROTIC SYNDROME
| MANIFESTATIONS | ACUTE POSTSTREPTOCOCCAL GLOMERULONEPHRITIS | MINIMAL CHANGE NEPHROTIC SYNDROME |
| Streptococcal antibody titers | Elevated | Normal |
| Blood pressure | Elevated | Normal or decreased |
| Edema | Primarily periorbital and peripheral | Generalized, severe |
| Circulatory congestion | Common | Absent |
| Proteinuria | Mild to moderate | Massive |
| Hematuria | Gross or microscopic | Microscopic or none |
| Red blood cell casts | Present | Absent |
| Azotemia | Present | Absent |
| Serum potassium levels | Normal or increased | Normal |
| Serum protein levels | Minimum reduction | Markedly decreased |
| Serum lipid levels | Normal | Elevated |
| Peak age at onset (yr) | 5-7 | 2-3 |
Clinical Course: The acute edematous phase of glomerulonephritis usually persists from 4 to 10 days but may persist for 2 or 3 weeks, during which time the child remains listless, anorexic, and apathetic. The weight fluctuates, the urine remains smoky brown, and the blood pressure may suddenly reach dangerously high levels at any time during this phase.
The first sign of improvement is a small increase in urinary output with a corresponding decrease in body weight. With diuresis the child begins to feel better, the appetite improves, and the blood pressure decreases to normal with the reduction of edema. Gross hematuria diminishes, in part because of dilution of the red blood cells in the more dilute urine, but microscopic hematuria may persist for weeks or months. Blood urea nitrogen (BUN) and creatinine levels decrease during diuresis and usually return to normal. A slight to moderate proteinuria may persist for several weeks.
Prognosis: Almost all children correctly diagnosed as having APSGN recover completely, and specific immunity is conferred so that subsequent recurrences are uncommon (Kasahara, Hayakawa, Okubo, et al, 2001). Deaths from complications still occur but fortunately are rare. A few of these children may develop chronic disease, but many of these cases are believed to be (probably) different glomerular diseases misdiagnosed as poststreptococcal disease.
Complications: The major complications that may develop during the acute phase of glomerulonephritis are hypertensive encephalopathy, acute cardiac decompensation, and acute renal failure (ARF). Normally, cerebral blood flow responds to acute arterial hypertension by vasoconstriction. However, acute and severe hypertension may cause this protective autoregulation of cerebral blood flow to fail, leading to hyperperfusion of the brain and cerebral edema. The premonitory signs of encephalopathy are headache, dizziness, abdominal discomfort, and vomiting. If the condition progresses, there may be transient loss of vision or hemiparesis, disorientation, and generalized tonic-clonic seizures.
Hypervolemia, not cardiac failure, causes cardiac decompensation during the acute edematous phase of nephritis. However, signs of circulatory congestion are evident. The heart is enlarged, and increased pulmonary vascular markings are evident on x-ray examination. Increased pulmonary capillary permeability is also believed to be an important factor in the development of pulmonary edema.
ARF with persistent oliguria or anuria is an uncommon complication but one that requires an appropriate treatment regimen.
Diagnostic Evaluation
Urinalysis during the acute phase characteristically shows hematuria, proteinuria, and increased specific gravity. The specific gravity is moderately elevated and seldom exceeds 1.020. Proteinuria generally parallels the hematuria but is not the massive proteinuria seen in nephrotic syndrome. Gross discoloration of urine reflects its red blood cell and hemoglobin content. Microscopic examination of the sediment shows many red blood cells, leukocytes, epithelial cells, and granular and red blood cell casts. Bacteria are not seen, and urine cultures are negative.
Unless the disease has progressed to renal failure, the blood examination reveals normal electrolyte (sodium, potassium, and chloride ions) and carbon dioxide levels. Azotemia resulting from impaired glomerular filtration is reflected in elevated BUN and creatinine levels in at least 50% of cases. When proteinuria is heavy, there may be changes associated with nephrotic syndrome (i.e., transient hypoproteinemia and hyperlipidemia).
Cultures of the pharynx are positive for streptococci in only a few cases, and the numbers are not significantly greater than the normal carrier incidence in many communities. Positive cultures help establish a diagnosis. Cultures should be obtained from other household members, and persons positive for group A streptococci should receive a course of antistreptococcal therapy.
Serologic tests are needed for diagnosis. Antibody responses to the extracellular products of the streptococci provide indirect evidence of previous streptococcal infection. These include antistreptolysin O (ASO), antistreptokinase (ASKase), antihyaluronidase (AHase), antideoxyribonuclease B (ADNase-B), and antinicotyladenine dinucleotidase (ANADase). The ASO titer is the most familiar and readily available test for streptococcal antibodies. ASO appears in the serum approximately 10 days after the initial infection; however, there is no correlation between the degree of elevation and the severity or prognosis of the glomerulonephritis. It is a useful diagnostic tool when nephritis follows a pharyngeal infection but is of less value after pyoderma. An ASO titer of 250 Todd units or higher is of diagnostic significance, as is a rising titer in two samples taken 1 week apart. More consistent and reliable antibody tests following streptococcal skin infections are elevated AHase and ADNase-B titers.
Of more importance for clinical serologic diagnosis is measurement of the serum complement level (C3). Serum C3 level is decreased initially but returns to normal 8 to 10 weeks after onset of the glomerulonephritis. Other studies include a chest x-ray examination, which shows characteristic generalized cardiac enlargement, pulmonary congestion, and pleural effusion during the edematous phase of acute disease. Renal biopsy for diagnostic purposes is seldom required but may be useful in the diagnosis of atypical cases.
Therapeutic Management
No specific treatment is available for AGN, but recovery is spontaneous and uneventful in most cases. Management consists of general supportive measures and early recognition and treatment of complications. Children who have normal blood pressure and a satisfactory urinary output can generally be treated at home. Those with substantial edema, hypertension, gross hematuria, or significant oliguria are often hospitalized because of the unpredictability of complications. Short hospitalization is the rule in uncomplicated cases; prolonged hospitalization is required only for children with severely impaired renal function.
General Measures: Bed rest is no longer recommended during the acute phase because ambulation does not seem to have an adverse effect on the course of the disease once the gross hematuria, edema, hypertension, and azotemia have ceased. Because they are generally listless and experience fatigue and malaise, most children voluntarily restrict their activities during the most active phase of the disease.
Fluid Balance: Regular measurement of vital signs, body weight, and intake and output is essential to monitor the disease’s progress and detect complications that may appear at any time during the course of the disease. A record of daily weight is the most useful means to assess fluid balance and should be kept for children treated at home and for those who are hospitalized. Sodium and water restriction is useful when the output is significantly reduced (<2 to 3 dl/24 hr). In these children the water allowed is equivalent to the calculated insensible loss plus the volume of urine excreted.
Diuretics are of limited value when severe renal failure is present, since little sodium reaches the distal tubules as a result of the reduced filtration rate. However, when renal failure is not severe, diuretic therapy (usually furosemide [Lasix]) is helpful if significant edema and fluid overload are present. Rarely, children with AGN develop ARF with oliguria that significantly alters the fluid and electrolyte balance. These children require careful management that may include peritoneal dialysis (PD) or hemodialysis.
Loss of glomerular filtration in children with severe forms of APSGN may produce electrolyte imbalances, especially hyperkalemia, acidosis, hypocalcemia, and hyperphosphatemia. Management of these electrolyte disturbances is described under Acute Renal Failure.
Hypertension: Acute hypertension must be anticipated and identified early. Blood pressure measurements are taken every 4 to 6 hours. Significant but not severe hypertension is controlled with loop diuretics. Other antihypertensive drugs, such as calcium channel blockers, beta blockers, or angiotensin-converting enzyme inhibitors, may be needed in severe cases. Seizure activity associated with hypertensive encephalopathy requires anticonvulsant therapy and antihypertensive agents (see Renal Failure, p. 1162, for management of severe hypertension).
Nutrition: Dietary restrictions depend on the stage and severity of the disease, especially the extent of edema. A regular diet is permitted in uncomplicated cases, but sodium intake is usually limited (no salt is added to foods). Moderate sodium restriction is usually instituted for children with hypertension or edema. Foods with substantial amounts of potassium are generally restricted during the period of oliguria. Protein restriction is reserved only for children with severe azotemia resulting from prolonged oliguria. The loss of appetite associated with the disease usually limits the protein intake sufficiently.
Antibiotics: Antibiotic therapy is indicated only for those children with evidence of persistent streptococcal infections. Antibiotics do not alter the course of the disease but are often recommended to prevent transmission of nephritogenic streptococci to other family members. Authorities are divided on the use of prophylactic antimicrobials for other family members.
Nursing Care Management
Nursing care of the child with glomerulonephritis involves careful assessment of the disease status, with regular monitoring of vital signs (including frequent measurement of blood pressure), fluid balance, and behavior. Vital signs provide clues to the severity of the disease and early signs of complications. The nurse carefully measures them and records and reports any abnormalities. The nurse notes the volume and character of urine and weighs the child daily. Assessment of the child’s appearance for signs of cerebral complications is an important nursing function because the severity of the acute phase is variable and unpredictable. The child with edema, hypertension, and gross hematuria may be subject to complications, and anticipatory preparations are included in the Nursing Care Plan on p. 1171.
For most children a regular diet is allowed but should contain no added salt. Foods high in sodium and salted treats are eliminated, and the nurse should advise parents and friends against bringing items such as potato chips or pretzels. However, the total amount of salt ingested is usually less than prescribed because of poor appetite. Fluid restriction, if prescribed, is more difficult; the amount permitted should be evenly divided throughout the waking hours and served in small cups to give the illusion of larger servings. Meal preparation and service require special attention because the child has a poor appetite and is indifferent to meals during the acute phase. Collaboration with parents and the dietitian and special consideration for food preferences will facilitate meal planning.
During the acute phase children are generally content to lie in bed. As they begin to feel better and their symptoms subside, they will want to be up and about. Activities should be planned to allow for frequent rest periods and avoidance of fatigue.
Children with mild edema and no hypertension, as well as convalescent children being treated at home, need follow-up care. Parents are instructed regarding general measures, including activity, diet, and prevention of infection. Strenuous activity is usually restricted until there is no evidence of proteinuria or macroscopic hematuria. Health supervision is continued with weekly, followed by monthly, visits for evaluation and urinalysis. Parent education and support in preparation for discharge and home care include education in home management and the need for follow-up care and health supervision.
Chronic or Progressive Glomerulonephritis
The majority of cases of renal glomerular disease are AGN, MCNS, and glomerulonephritis associated with systemic diseases. These pose relatively few problems of diagnosis, and their natural course is fairly predictable. A few cases present a prolonged course and a poor ultimate prognosis. They are a rather heterogeneous group and are defined by correlating the clinical manifestations, pathologic conditions, and natural course of the individual diseases.
Chronic glomerulonephritis (CGN) describes a variety of different disease processes that may be distinguished from each other by renal biopsy. These include membranoproliferative glomerulonephritis, membranous glomerulonephritis, focal segmental glomerulosclerosis, and immunoglobulin A nephropathy. In CGN tissue damage and progression to fibrosis are related to the immune response that brings about inflammation, failure to activate glomerular repair, and excessive fibrogenic activity (Coppo and Amore, 2004). Rapidly progressive glomerulonephritis is the term used to describe an acute illness with severe, acute onset that causes rapidly progressive deterioration of renal function in weeks to months. Renal biopsy of these patients shows a variety of diseases with the common feature of greater than 50% glomerular crescents found in the biopsy section.
Pathophysiology
In most cases of CGN, immunologic mechanisms can be implicated either through direct attack on the kidney or secondary to the accumulation of immune complexes in the glomerular filter or fibrin deposition from previously damaged glomeruli. Either can contribute to further glomerular damage and can initiate chronic changes in the glomerular structure (Coppo and Amore, 2004). In many cases there is no history of an acute glomerular disease. In other cases CGN may represent one of a succession of exacerbations of a preexisting disease. CGN that is not associated with other diseases may go undetected for years and be relatively asymptomatic until kidney destruction produces marked reduction in renal function. Consequently, the disease is more common in adolescents than in younger children. Renal insufficiency with all its manifestations occurs as the ultimate event.
Clinical Manifestations
The clinical manifestations and laboratory findings reflect deteriorating renal function. Nephrotic syndrome often develops. Hypertension, edema, proteinuria, cardiac failure, dyspnea, osteodystrophy, and anemia are common manifestations of progressive disease.
Diagnostic Evaluation
Laboratory findings may include proteinuria, with casts and red and white blood cells. Elevated BUN, creatinine, and uric acid levels are evidence of failing renal function. Electrolyte alterations include metabolic acidosis, elevated potassium, elevated phosphorus, and decreased calcium levels. The renal insufficiency may extend from 5 to 15 years and even longer, or rapid deterioration may progress to end-stage renal disease (ESRD).
Therapeutic Management
Early in the course of the disease, treatment is appropriate to the underlying disease and is largely symptomatic in most cases. Directs nursing efforts toward providing optimum conditions for the child’s physical, psychologic, and social development. As few restrictions as feasible are imposed, and the child is allowed to live as normal a life as possible for as long as possible. Some forms of CGN are treated with corticosteroids or cytotoxic agents. Marked hypertension is controlled with antihypertensive agents, and anemia may require recombinant erythropoietin and iron supplements. Ultimately, dialysis and transplantation may be needed to restore relatively good health; however, these alternatives are reserved until renal failure is far advanced. (See Chronic Renal Failure, p. 1167, for more detailed management of specific problems.) Children with rapidly progressive glomerulonephritis are usually referred to a center specializing in renal disease.
Nephrotic Syndrome
Nephrotic syndrome is the most common presentation of glomerular injury in children. It is defined as massive proteinuria, hypoalbuminemia, hyperlipidemia, and edema, but the disorder is a clinical manifestation of a large number of distinct glomerular disorders in which increased glomerular permeability to plasma protein results in massive urinary protein loss (Bagga and Mantan, 2005). After a description of the three major forms of nephrotic syndrome, the remainder of the discussion is devoted to minimal change disease.
Types of Nephrotic Syndrome
Nephrotic syndrome can be classified as primary, when the syndrome is restricted to glomerular injury, or secondary, when it develops as part of a systemic illness. Although it may have several different histologic variations, the most common form of the primary disease is MCNS. A congenital form is also recognized.
Minimal Change Nephrotic Syndrome: Approximately 80% of cases of nephrotic syndrome in children result from MCNS. MCNS can be seen at any age but is predominantly a disease of the preschool child. The disease is rare in children younger than 6 months of age, uncommon in infants younger than 1 year of age, and unusual after the age of 8 years. The incidence of the disease in North America is approximately 2 to 7 per 100,000 children per year, and males outnumber females 2 : 1. In adolescence the ratio is 1 : 1.
The cause of MCNS (also known as idiopathic nephrosis, minimal lesion nephrosis, nil disease, childhood nephrosis, lipoid nephrosis, or uncomplicated nephrosis) remains obscure. A nonspecific illness, usually a viral upper respiratory tract infection, often precedes the manifestations by 4 to 8 days but is considered to be a precipitating factor rather than a cause.
Secondary Nephrotic Syndrome: Nephrotic syndrome may occur after or in association with glomerular damage of known or presumed cause. Prominent among causes of glomerular damage is AGN or CGN. Less commonly, secondary nephrotic syndrome occurs during the course of collagen vascular diseases (such as disseminated lupus erythematosus and anaphylactoid purpura) or as a result of toxicity to drugs (such as trimethadione and heavy metals), stings, or venom. Nephrotic syndrome is the major presenting symptom of renal disease in pediatric patients with acquired immunodeficiency syndrome. Diverse, rare causes are sickle cell disease, hepatitis, malaria, cyanotic heart disease, tuberculosis, infected ventriculojugular shunts, renal vein thrombosis, or malignancies.
Congenital Nephrotic Syndrome: The hereditary form of nephrotic syndrome is caused by a recessive gene on an autosome. Infants who have nephrotic syndrome are small for gestational age, and proteinuria and edema manifest early. The disease does not respond to the usual therapy, and death in the first year or 2 of life is the rule if the infant does not receive dialysis or a successful kidney transplant.
Pathophysiology
The pathogenesis of MCNS is not completely understood. A metabolic, biochemical, or physiochemical disturbance in the basement membrane of the glomeruli may lead to increased permeability to protein, but the causes and mechanisms are only speculative.
The glomerular membrane, which is normally impermeable to albumin and other large proteins, becomes permeable to proteins, especially albumin, which leak through the membrane and are lost in urine (hyperalbuminuria). This reduces the serum albumin level (hypoalbuminemia), which decreases the COP in the capillaries (Bagga and Mantan, 2005). As a result, the hydrostatic pressure exceeds the pull of the COP, and fluid accumulates in the interstitial spaces and body cavities, particularly the abdominal cavity (ascites). The shift of fluid from the plasma to the interstitial spaces reduces the vascular fluid volume (hypovolemia), which in turn stimulates the renin-angiotensin system and the secretion of antidiuretic hormone and aldosterone. Tubular reabsorption of sodium and water increases in an attempt to increase intravascular volume. The elevation of serum cholesterol, phospholipids, and triglycerides is not fully understood. The sequence of events in nephrotic syndrome is diagrammed in Fig. 30-3.
Clinical Manifestations
A previously well child begins to gain weight, which progresses insidiously over a period of days or weeks. Puffiness of the face, especially around the eyes, is apparent on arising in the morning but subsides during the day, when swelling of the abdomen and lower extremities is more prominent. The generalized edema may develop so slowly that parents consider it a sign of healthy growth. Although an acute infection may precipitate severe generalized edema (anasarca), the usual course is one of progressive weight gain until either a rapid or a gradual increase in edema prompts the family to seek medical evaluation. Usually present are periorbital edema, abdominal swelling from ascites, and labial or scrotal swelling. Edema of the intestinal mucosa may cause diarrhea, loss of appetite, and poor intestinal absorption. The volume of urine is decreased, and it appears darkly opalescent and frothy.
The child often has extreme skin pallor and may experience skin breakdown during periods of severe edema. The child is irritable and may be more easily fatigued or lethargic but does not appear seriously ill. Weight loss from poor appetite and loss of protein is not uncommon, although it is often obscured by edema. Changes in the nails appear as white (Muehrcke) lines parallel to the lunula, which are caused by prolonged hypoalbuminemia. The blood pressure is usually normal or slightly decreased. The child is more susceptible to infection, especially cellulitis, pneumonia, peritonitis, or sepsis.
In rare cases children with MCNS have significant or persistent hypertension, gross or persistent hematuria, or significant or persistent azotemia (increased nitrogenous products in the blood).