Ruminant and Swine Anesthesia
After completion of this chapter, the reader will be able to:
• Describe the main physiologic and anatomic differences that influence anesthetic management of ruminants and swine.
• Explain how to prepare a ruminant or porcine patient for anesthesia.
• Select an anesthetic protocol for an American Society of Anesthesiologists (ASA) PS1 or PS2 adult cow, small ruminant, and pig.
• Explain how to intubate an adult cow, a small ruminant or calf, and a pig.
• Explain the importance of positioning of ruminants under general anesthesia.
• Explain how to position a ruminant for recovery.
• Explain the anesthetic concerns and challenges regarding anesthetizing pigs.
RUMINANTS
The basic principles of anesthesia discussed in Chapter 8 apply to ruminant anesthesia. Ruminants do not pose quite the same challenge to the anesthetist as horses; however, an understanding of their unique digestive physiology is important because it affects the well-being of the patient under general anesthesia. In addition, ruminants are presented for general anesthesia less frequently than small animals or horses are, so it takes longer to gain anesthetic experience. There are several reasons for this. Because of their relatively calm nature, ruminants require general anesthesia for relatively few procedures. Many surgeries can be conducted using local or regional anesthetic techniques (see Chapter 6) in conjunction with physical restraint. Consideration must also be given to drug withdrawal times when dealing with animals that produce milk or meat for human consumption. Finally, administration of general anesthesia to production animals is often uneconomical.
Ruminant patients range in size from a few kilograms (lambs and kids) to over 1000 kg (adult bull). Thus, as with horses, specialized tilt tables, head gates, hoists, and transporters may be required to allow the veterinarian to perform surgery or procedures such as hoof-trimming safely without injury to the animal. Equipment suitable for anesthetizing small animal patients is commonly used for smaller patients (sheep, goats, calves). However if young cattle >150 kg and adult cattle are to undergo general anesthesia, the anesthetist must have access to a large animal anesthesia machine and be familiar with its operation as well as that of related equipment and monitors.
The main anesthetic concerns in ruminants result from their unique digestive anatomy and physiology. Ruminants constantly produce large volumes of saliva compared with other species, and this is generally not inhibited by general anesthesia. They are thus prone to aspiration if the airway is not protected. In addition, regurgitation of ruminal contents (known as regurgitus) can occur at any stage of general anesthesia and occur most commonly in light and deep planes. Fermentation in the rumen is only slightly decreased by general anesthesia; thus ruminants are predisposed to bloat, as they cannot eructate when they are unconscious.
As with small animal anesthesia, neuromuscular blockers are rarely used in general practice but are sometimes used to provide muscle relaxation for ocular and orthopedic procedures in veterinary schools and referral practices.
PATIENT PREPARATION
The reader is referred to Chapters 2 and 8 for a detailed discussion of the essentials of patient preparation before anesthesia. See Procedure 10-1, p. 294, for additional tasks pertinent to preparation of the ruminant patient undergoing general anesthesia. In addition, the anesthetist should be familiar with operation of additional equipment such as surgical tables, transporters, head gates, and hoists that will be used during the anesthetic episode.
It is key to ensure that ruminants have been adequately fasted before anesthesia. Fasting reduces the size of the rumen and also decreases microbial activity. This in turn decreases gas production during anesthesia. Normally ruminants eructate to expel the gas from the rumen; however, under anesthesia this does not happen, which can lead to bloating. A bloated rumen can put pressure on the diaphragm and large blood vessels (aorta, caudal vena cava) in the abdomen, resulting in respiratory as well as circulatory compromise. Once an anesthetized ruminant has developed severe bloat, it can be very difficult to treat and may lead to the death of the patient if it goes unnoticed or untreated. Bloat often goes unrecognized when a patient is small and covered by surgical drapes. Signs include development of a distended, tight abdomen, decreased blood pressure, increased heart rate, and decreased ventilation.
SELECTING A PROTOCOL
A suitable protocol takes into account the minimum patient database, the patient’s physical status class, and the type and duration of procedure to be performed. Regardless of the protocol, the correct drugs and amounts must be prepared. Ill, geriatric, pediatric, or otherwise compromised patients (physical status class P3 to P5) require use of modified protocols based on the patient’s primary condition. Management of these cases can be quite challenging and requires customization of the anesthetic protocol by the veterinarian-in-charge (VIC).
SUMMARY OF A GENERAL ANESTHETIC PROCEDURE
The dynamics for the commonly used protocols in ruminant anesthesia are very similar to those for small animals (see Chapter 8, p. 236), with the exception that induction with an intramuscular (IM) agent is not commonly done in clinical practice.
Equipment Preparation
During a typical anesthetic induction, many events occur in rapid succession. Anesthetic agents are administered, the patient becomes unconscious and recumbent, and the endotracheal tube is placed, secured, and cuffed. The patient is then lifted or hoisted onto the surgery table, the endotracheal tube is connected to the anesthetic machine, and the anesthetic gas level is adjusted, all within the first few minutes. Because these events follow one another so rapidly, the technician does not have the luxury of leaving the patient to locate equipment (Figure 10-1). For this reason, all equipment must be carefully gathered, checked, and organized before commencement of the procedure.
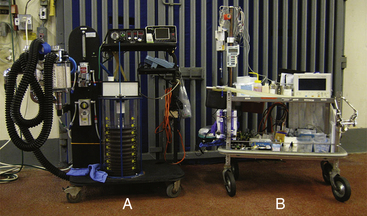
FIGURE 10-1 Anesthetic equipment for anesthetizing a large ruminant. A, Large animal anesthetic machine and ventilator. B, An anesthetic cart with drugs, syringes, endotracheal tubes, a mouth gag for adult cattle, and anesthetic monitor ready for anesthetizing a large ruminant.
Patients weighing more than 150 kg are usually placed on a large animal anesthetic machine (see Figure 9-1). Most large animal anesthetic machines incorporate a ventilator (see Figure 9-1, D). Both the circle system and the ventilator of the machine should be checked before use. Small ruminants and calves weighing less than 150 kg can be placed on a small animal anesthetic machine. Hypothermia is uncommon in anesthetized adult cattle; however, devices such as warm air blankets or warm water circulating blankets can be used to maintain body temperature in small ruminants and calves as for small animal patients.
Any specialized equipment required for restraining or positioning anesthetized ruminants, such as head gates, transporters, and tilt tables, should be checked. In addition to the items from the standard checklist, it is extremely helpful to have suction available for small ruminants to allow feed material, regurgitus, or saliva to be removed from the pharynx during intubation.
A crash cart containing emergency equipment and drugs should also always be available.
Premedication or Sedation
Many ruminants are calm and tractable enough to allow intravenous (IV) catheterization and induction of anesthesia with minimal or no premedication and mild restraint. Adult cattle are typically restrained using the head gate of a transporter or chute, or against a tilt-table. Premedication is often reserved for patients that are aggressive, excited, or stressed. Despite the fact that many ruminants do not require sedation before anesthesia, premedication will still provide benefits such as decreased dose of induction and maintenance drugs, muscle relaxation, and analgesia.
Anticholinergic drugs are not used to premedicate ruminants because they do not reduce salivation but instead cause the saliva to become thick and ropy. These thicker strands of saliva are more easily aspirated. This class of drugs is therefore reserved for treatment of arrhythmias and for cardiopulmonary resuscitation (CPR) in this species. See Protocol 10-1 for sedative and premedication drugs and doses in ruminants.
Anesthetic Induction
Anesthetic induction is the process by which an animal loses consciousness and enters surgical anesthesia. Of the goals set out for induction in Chapter 8, it is particularly important to gain control of and protect the airway as quickly as possible in ruminants.
What follows is a description of specific techniques used to induce anesthesia. See Protocol 10-2 for IV induction protocols in ruminants, and Procedure 10-2, p. 290, for the sequence of events for induction with an IV agent and maintenance with an inhalant agent in a ruminant.
Intravenous Induction
Induction in large cattle may occur in a special induction stall that has padded walls and often a padded floor, in a transporter, or on a tilt table. Smaller ruminants can generally undergo induction next to the surgery table or, if small or severely compromised, while lying on the surgery table. Although ruminants do not typically become excited during induction of anesthesia, the goal with adult cattle is similar to that in horses: to rapidly produce unconsciousness and minimize injury of the patient or personnel. Drugs are thus given to the larger ruminants as a bolus, with the exception of “double drip” (see Protocol 10-2 and Figure 10-2), which is administered rapidly intravenously to effect. Smaller ruminants, particularly those that are compromised, can be given induction drugs to effect as for small animal patients.

FIGURE 10-2 “Double drip.” A 500-mL bag of 5% dextrose in water containing 5% guaifenesin, to which 5 mL of ketamine has been added. Final concentrations are therefore 1 mg of ketamine per milliliter and 50 mg of guaifenesin per milliliter.
Once the patient is unconscious, it should be kept in sternal recumbency for intubation whenever possible. It is important to be vigilant for regurgitation, which can occur at any point of the anesthetic procedure but occurs most frequently when anesthesia is light or too deep. If regurgitation occurs the head should immediately be positioned so that it is lower than the body to prevent aspiration.
Once anesthesia has been induced, the vital signs should be briefly checked before intubation. See Procedure 10-3, p. 295, for IV induction techniques in adult cattle.
Endotracheal Intubation
After induction of general anesthesia, an endotracheal tube is usually placed in the patient’s airway. The advantages of intubation are discussed in Chapter 4 on p. 98 and Chapter 8 on p. 245.
Equipment for Endotracheal Intubation
The following equipment is required for endotracheal intubation:
• Appropriately sized endotracheal tubes (at least two of slightly different diameters)
• Stylette (small ruminants and calves only)
• A mouth gag to hold the jaws apart (adult cattle only)
• Laryngoscope (small ruminants and calves)
• Gauze sponge to grasp the tongue (if preferred)
• A syringe to inflate the cuff (10 mL for small ruminants and calves or 60 mL for adult cattle)
Selecting an Endotracheal Tube
The general principles for selecting an endotracheal tube are discussed in Chapter 8. Adult cattle typically require a 22-mm–, 26-mm–, or 30-mm–diameter cuffed tube. Typically two tube sizes are selected for intubation—the anticipated size and one size smaller. Small ruminants and calves require smaller tubes.
Endotracheal tubes are further discussed in Chapter 4.
Preparing the Tube
Before an endotracheal tube is used, it must be checked for integrity. It should be clean, sanitized, and free of blockages, holes, deterioration, or other damage. The connector must be securely attached, and the cuff must inflate and hold pressure after detaching the syringe from the valve. The tube may be lubricated with a small amount of sterile water-soluble lubricant, although this is not essential in adult cattle.
Intubation Procedure
The procedure for intubation of small ruminants and calves differs from that for adult cattle.
Small Ruminants and Calves: Intubation in small ruminants and calves is accomplished as for small animal patients. The oral opening is small in these patients compared with the distance between the mouth and larynx, so visualization of the airway can be challenging. In addition, the caudal half of the tongue is thickened, which further obstructs the anesthetist’s view. Attempting to pass the endotracheal tube alone typically completely obstructs the view, making successful placement extremely challenging, and more a matter of luck than skill. Thus using a stylette that protrudes beyond the end of the tube allows better visualization of the larynx.
With the head extended by an assistant, the anesthetist places a laryngoscope to visualize the larynx. It often helps to grasp the tongue with a gauze sponge and gently pull it forward. The anesthetist then passes the stylette into the airway, taking care not to cause injury to the larynx or trachea. The endotracheal tube can then be passed over the stylette and into the larynx (Figure 10-3).
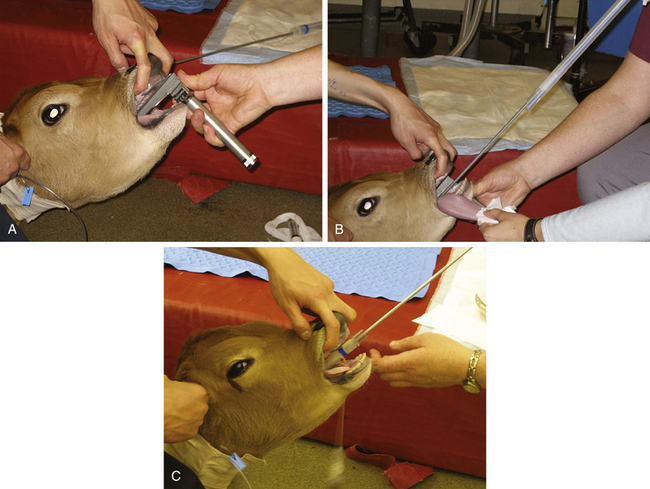
FIGURE 10-3 Intubation of a calf. A, After induction, an assistant holds the patient’s head in an extended position. The anesthetist places a laryngoscope and directly visualizes the larynx. The tongue of ruminants, particularly sheep and goats, has a raised area in the caudal portion that makes correct positioning of the laryngoscope more difficult than in small animals. B, A stylette, which is pre-placed within and extends beyond the endotracheal tube, is advanced until it is positioned 2 to 5 cm within the larynx. C, The tube is then advanced over the stylette into the trachea. The stylette can then be removed. This technique is also used for intubating sheep and goats.
The cuff is inflated as for small animal intubation (see Chapter 8).
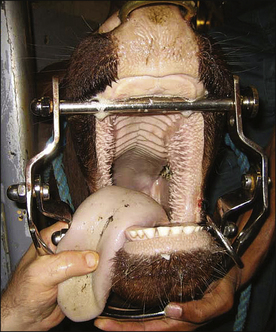
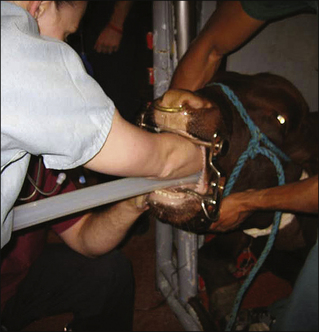
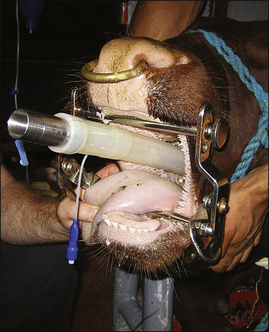
Adult Cattle: Adult cattle are intubated manually using a blind technique (Procedure 10-4, p. 295). A speculum or mouth gag is placed, which prevents the cow from closing its mouth. This protects the anesthetist’s arm and hand from being damaged if the cow should become lightly anesthetized enough to chew. The anesthetist then inserts the nondominant hand into the mouth up to the larynx, holding (and protecting) the endotracheal tube in this hand. The dominant hand holds the connector end of the tube and is used to advance the tube. Once the nondominant hand is at the level of the larynx, the anesthetist palpates the epiglottis, reflects it forward if necessary, and directs the end of the endotracheal tube into the trachea, advancing it into the larynx with the dominant hand. Extending the head and neck of the cow, although sometimes challenging because of the weight, is often helpful while the tube is being passed. On successful placement, the endotracheal cuff is inflated. The tube is then tied in place, either to the halter or around the muzzle as in a dog.
Maintenance of Anesthesia
After anesthetic induction and endotracheal intubation, patients that are in a light plane of anesthesia must be brought into surgical anesthesia. When the patient reaches
the desired anesthetic depth, general anesthesia is most commonly maintained with inhalant anesthetics but can also be maintained with total intravenous anesthesia (TIVA). See Protocol 10-3 for maintenance protocols in ruminants.
Maintenance of Anesthesia with Inhalant
Maintenance of anesthesia in small ruminants and calves is similar to that in small animals (see Procedure 8-7). Maintenance of anesthesia in adult cattle is similar to that in horses (see Procedure 9-6). Healthy ruminants typically have relatively few problems during the maintenance phase of anesthesia. Blood pressure is usually well maintained and is often much higher than that seen in small animal and equine patients. Ruminants do, however, tend to hypoventilate and are often observed to breathe rapidly and shallowly, somewhat like a panting dog. This type of breathing pattern may lead to hypercarbia, hypoxemia, and difficulty keeping the patient anesthetized because of inadequate delivery of inhalant anesthetic to the lungs. Patients that demonstrate this breathing pattern should be placed on a ventilator (see Chapter 6).
Most ruminants have accessible arteries in their ears, and these are often catheterized so that blood pressure can be monitored directly and blood samples can be taken for blood gas analysis, particularly for long surgeries or in compromised patients.
Patient Positioning, Comfort, and Safety
During anesthetic induction and maintenance, a number of considerations must be observed to ensure that the patient is not harmed. Many of the same principles of small animal anesthesia apply to ruminant anesthesia (see Chapter 8, p. 252). Additional concerns include the following:
• All ruminants should be positioned for surgery with the mouth lower than the pharynx to allow drainage of saliva and any regurgitated material from the mouth, preventing buildup in the pharynx, which could lead to aspiration in recovery.
• Ruminants, even large cattle, are not predisposed to developing myopathy or neuropathy as horses are; however, appropriate physical support and padding during anesthesia is prudent (Figure 10-4).
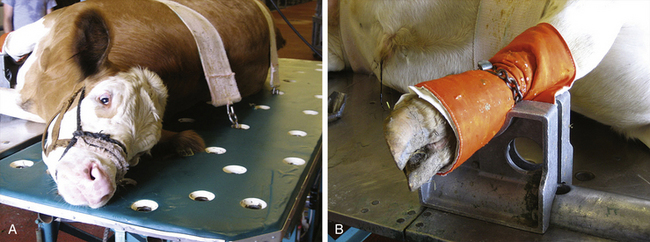
FIGURE 10-4 Restraint of a bull on a tilt table. A, A bull appropriately restrained with two support bands on a tilt table that has a padded surface. The bull’s halter is secured by passing a rope through one of the holes and tying it underneath the table. B, Appropriate padding of the bull’s distal right forelimb, which is placed in a metal foot support.
Anesthetic Recovery
Unlike horses, ruminants are generally content to lie in sternal recumbency after anesthesia. The development of complications from anesthetic recovery is generally limited to the residual effects of bloat. Ruminants rarely develop nasal edema during anesthesia and usually do not require nasal intubation. See Procedure 10-5, p. 296, for information regarding anesthetic recovery.
Preparation for Recovery
On completion of the procedure, turn off the inhalant and transfer the patient to a padded recovery stall where it can be extubated and monitored (large cattle) or to a quiet, clean area on the floor (small ruminant). Support or prop the patient in sternal recumbency with the mouth lower than the pharynx. This allows eructation as the patient regains consciousness, as well as drainage of saliva and/or regurgitus that may have accumulated during anesthesia (Figure 10-5).
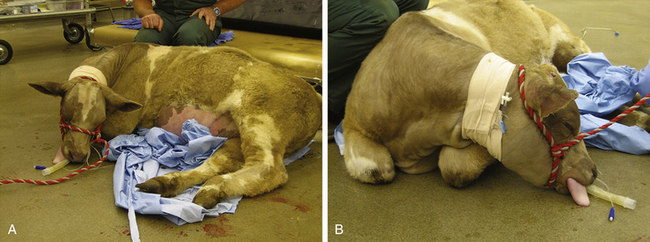
FIGURE 10-5 Appropriate positioning of a ruminant for anesthetic recovery. A, This heifer recovering from anesthesia for umbilical hernia repair is placed in sternal recumbency and maintained in that position, in this case by an assistant, to allow for eructation. The head is positioned so that the pharynx is higher than the mouth, thus allowing for drainage of saliva and regurgitus. B, Folding the front limbs underneath the heifer makes it easier to keep her in sternal recumbency.
Extubation
In contrast to other patients, the endotracheal tube cuff should be either kept inflated or only partially deflated in order to prevent aspiration of any material that may have become lodged in the pharynx during anesthesia. The anesthetist should wait for strong swallowing movements or coughing before extubation. Both before and after removal, keep the neck in a natural but extended position to protect the airway. Remove the endotracheal tube gently using a slow, steady motion. If there is difficulty removing the tube, remove some more air from the cuff and try again.
Postanesthetic Period
Once a ruminant is lying in sternal recumbency without support and is no longer in danger of bloating, it can be left unattended. Many ruminants will lie quietly after anesthesia, standing only some time after the anesthetic period, unless they are stimulated to rise. It is not necessary to withhold food or water from ruminants postoperatively unless specifically instructed to do so.
SWINE
Pigs are challenging patients to restrain, sedate, and anesthetize because of unique characteristics in this species that make physical examination, sedation, IV catheterization, and intubation much more difficult than in the species discussed so far.
PHYSICAL EXAMINATION
In most swine, examination is impossible beyond general observation of the animal, assessment of respiratory rate and character, and observation of obvious problems such as nasal discharge. Restraint of conscious pigs typically results in them squealing in protest, making procedures such as thoracic auscultation impossible. The anesthetist must often rely on patient history to determine health status. Pigs also do not have readily accessible peripheral veins or arteries, making further investigation of cardiovascular status and obtaining blood samples very difficult to impossible without causing extreme stress to the animal and to the handler.
SUMMARY OF A GENERAL ANESTHETIC PROCEDURE
Sedative drugs are most commonly administered by IM injection in pigs owing to the lack of easily accessible peripheral veins and the presence of a thick layer of subcutaneous fat, which makes IM administration of drugs difficult without the use of needles that are at least 11⁄2 inches long. The most accessible site for IM injection is in the muscles of the neck caudal to the ear and at least 3 to 5 cm lateral to the dorsal midline (Figure 10-6).

FIGURE 10-6 Pig sedated with Telazol-ketamine-xylazine (TKX). This pig became laterally recumbent 15 minutes after IM injection of TKX (the blood on the side of the neck marks the site of IM injection).
Swine are generally considered to be the most resistant to sedative drugs of the domestic species, and many protocols for IM sedation, premedication, or total injectable anesthesia include a tranquilizer or sedative, an opioid, and a dissociative. Various combinations of drugs have been used to sedate swine (Protocol 10-4). Generally, drug combinations that include a dissociative produce more predictable and heavier sedation that, in some pigs, may produce anesthesia for short surgical and nonsurgical procedures. A combination that is widely used to produce heavy sedation to anesthesia is Telazol, ketamine, and xylazine, or TKX (Procedure 10-6, p. 296).
Anesthetic Induction
Using a combination of drugs such as TKX will often induce anesthesia in pigs. The eyes of pigs are very small and sunken in and do not provide reliable information about depth of anesthesia. Readiness for intubation is often best assessed by seeing if the mouth can be opened without resistance. If after administration of TKX the patient is not quite deeply anesthetized enough to intubate, the anesthetist has two options. He or she can place an IV catheter in an aural vein (Figure 10-7) and administer small increments of an IV induction drug such as ketamine (i.e., 0.5- to 1.0-mg/kg boluses), or deepen anesthesia by administering an inhalant anesthetic via face mask (Figure 10-8).
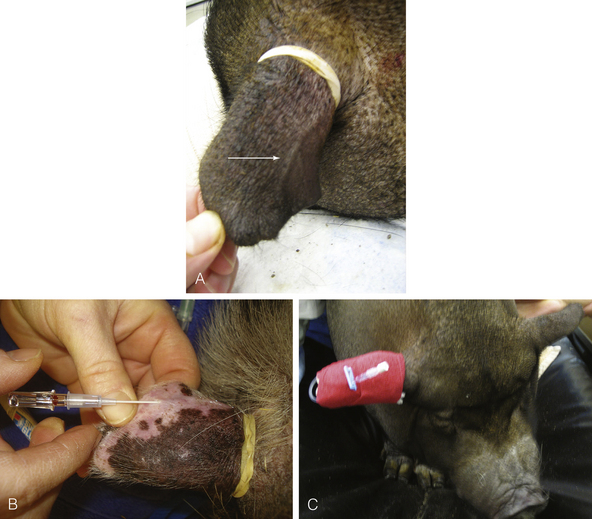
FIGURE 10-7 Placing a catheter in an aural vein of a pig. A, A rubber band placed around the base of the ear acts as a tourniquet, causing the marginal ear vein to distend (white arrow). B, Placement of an IV catheter in the vein. Note the blood flashback in the hub of the catheter. Because of the small size of ear veins in pigs, blood does not commonly flow out of the catheter after the stylette has been removed. After the catheter has been placed, the rubber band is removed. It is usually easier to cut the rubber band off, taking care not to cut the ear, than risk dislodging the catheter while moving the rubber band over the catheter and off the ear. C, If required, the catheter can be secured and wrapped to allow for administration of postoperative intravenous medication.
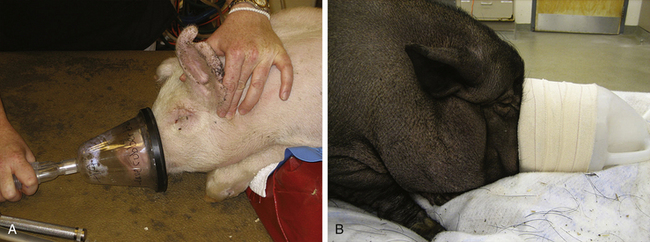
FIGURE 10-8 Administering an inhalant anesthetic via a face mask in a pig. A, Use of a small animal clear face mask in a small pig. B, Use of a homemade face mask in a large pig. In both cases it is important that the mask be tight fitting to avoid pollution of the work area and to ensure that the inhalant anesthetic is not diluted by room air, which will delay induction.
Intubation
Intubation of swine is particularly challenging because of poor visualization resulting from the limited opening of the mouth, the relatively narrow dental arcade, and the
anatomy of the larynx and proximal trachea. A ventral laryngeal diverticulum is present into which the tube can easily be misdirected, and the laryngotracheal junction is at an angle rather than being straight as is seen in other domestic species. Finally, the larynx in pigs is sensitive and may spasm when stimulated, making intubation even harder. The novice anesthetist should seek assistance from an experienced person when intubating a pig, as it is easy to damage the larynx by forcing the tube. There are several methods of intubating pigs. The pig may be placed in either sternal or dorsal recumbency. Similarly to small ruminants, a straight stylette is placed within the tube and extends beyond it. With a laryngoscope used to visualize the airway, the stylette is passed into the larynx, bypassing the diverticulum. The tube can then be gently threaded over the stylette into the trachea. Care must be taken with the stylette to avoid damage to the larynx and trachea (Figure 10-9). Alternatively, a stylette with a 20- to 30-degree curve in it is placed in the tube, ensuring that it does not extend beyond the end of the tube. The tube is inserted into the larynx with the convex surface against the palate. Once the tube is placed in the larynx, the tube and stylette are rotated 180 degrees and advanced into the trachea. Patience should be exercised, and if anesthesia becomes light during intubation, further attempts at intubation should be halted until IV or inhalant drugs are administered and an appropriate depth of anesthesia for intubation is achieved.
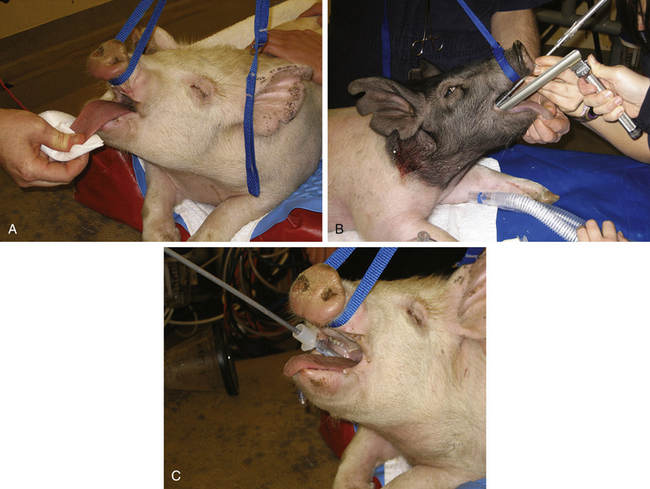
FIGURE 10-9 Intubation of a pig. A, After anesthetic induction, the tongue is gently pulled forward and down while the upper jaw is pulled upward using a lead. B, With a laryngoscope used to aid visualization, a stylette placed through and beyond the end of an endotracheal tube is advanced into the larynx. C, The tube is advanced over the stylette into the larynx.
Maintenance of Anesthesia
Anesthesia in most pigs can be maintained with inhalant anesthetic delivered using a small animal anesthetic machine and circle system. Maintenance of anesthesia is similar to that in small animal patients (see Chapter 8). In the case of very large pigs that can be intubated with a size 16-mm endotracheal tube, a large animal machine can be used.
Monitoring
Pigs can be challenging to monitor effectively, because they have few palpable peripheral arteries and their cone-shaped legs make the use of blood pressure cuffs, which are designed for the more cylindric arms of people, more difficult. In most pigs pulses can be palpated in the ear and on the medical aspect of the carpus. In smaller pigs the brachial artery may be palpable, a Doppler signal may be obtained from it, and oscillometric cuffs will often give pressure readings (Figure 10-10). A Doppler signal is also relatively easy to elicit from the tail artery, which runs in the ventral midline of the tail. Pulse oximeter transmission probes will usually work on the tongue but can also be placed on other areas such as the snout and ears of pink pigs (Figure 10-11).
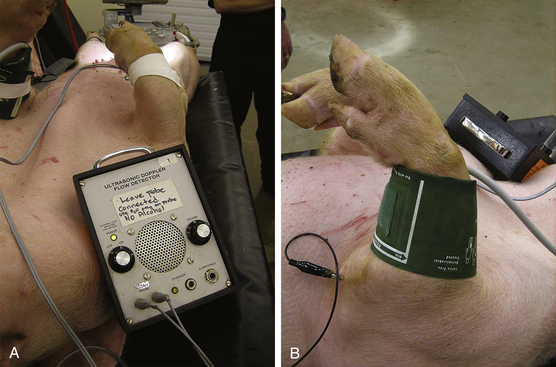
FIGURE 10-10 Placement of Doppler probe and oscillometric blood pressure cuff on the forelimb of a small pig. A, Placement of the Doppler probe on the medial aspect of the carpus. B, Placement of an oscillometric blood pressure cuff on the forelimb.
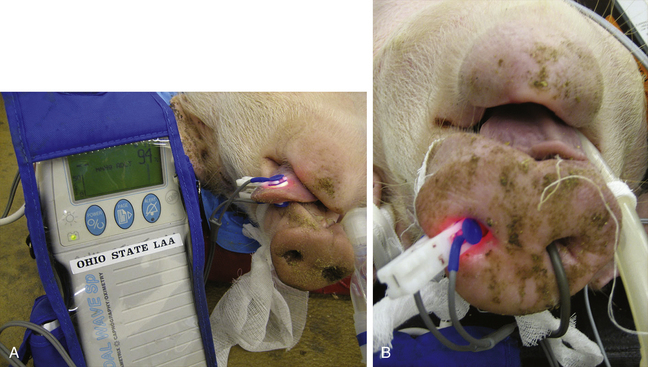
FIGURE 10-11 Pulse oximetry probe placement on a pig. A, Placement of a pulse oximetry transmission probe on a pig’s tongue. B, Placement of a pulse oximetry transmission probe on a pig’s snout. Note the temperature probe entering the pig’s right nostril.
The respiratory system can be monitored by observing the breathing bag and with capnometry as for other species.
Porcine Stress Syndrome
Porcine stress syndrome, also known as malignant hyperthermia, has been associated with anesthesia, particularly inhalant anesthetics. This metabolic condition occurs in affected animals because of a mutation in one of the genes that controls calcium metabolism in the muscle. Signs include muscle rigidity, rapid rise in temperature, hypercapnia, hyperkalemia, and death. Treatment includes immediate termination of all anesthetic drugs, delivery of oxygen at high flow rates, and treatment with dantrolene.
Recovery
The general principles applicable to extubation and recovery for small animals apply to pigs, including detection and treatment of hypothermia. Typically the IV catheter is removed before full awakening, although if a pig is to be hospitalized it may be prudent to secure the IV catheter for administration of IV medications (see Figure 10-7, C).
KEY POINTS
1. The main anesthetic concerns in ruminants arise from their unique digestive anatomy and physiology.
2. Ruminants are susceptible to bloat, regurgitation, and hypoventilation under general anesthesia.
3. Ruminants are generally very tractable, and surgery, particularly flank laparotomy, is often accomplished using local anesthesia with or without sedation.
4. Intubation is performed under direct visualization with a laryngoscope in small ruminants and calves, often with the use of a stylette.
5. Intubation of adult cattle is performed manually using a blind technique that involves direct palpation of the larynx.
6. During recovery from anesthesia ruminants should be placed in sternal recumbency to allow for eructation. The endotracheal tube should be left in place with the cuff partially inflated to minimize the risk of aspirating rumen contents or saliva.
7. Preoperative physical examination of swine is limited to observation.
8. The main anesthetic concerns in swine are inability to perform blood work, resistance to sedation, paucity of peripheral veins and arteries, and difficulty of intubation.
9. Pigs are resistant to sedation compared with other species. The combination of Telazol, ketamine, and xylazine (TKX) is commonly used to provide heavy sedation or total intravenous anesthesia (TIVA) in pigs.
10. After sedation or induction, the ear vein is usually accessible for catheterization in pigs.
11. Intubation of swine is challenging because of poor visualization of the larynx, and the potential for laryngospasm.
12. There are few palpable arteries in pigs, making monitoring difficult. Oscillometric blood pressure cuffs may not work well on the cone-shaped limbs of pigs.
REVIEW QUESTIONS
1. Comparing the sensitivity of cattle, horses, and swine to xylazine, which of the following is true?
a. Cattle are more sensitive than horses, which are more sensitive than swine.
b. Cattle are more sensitive than swine, which are more sensitive than horses.
c. Horses are more sensitive than cattle, which are more sensitive than swine.
d. Swine are more sensitive than cattle, which are more sensitive than horses.
2. An anticholinergic is an essential component of premedication in ruminants.
3. “Double drip” contains which two drugs?
4. You plan to anesthetize a 1000-kg bull and maintain anesthesia using an inhalant technique. Which of the following statements regarding intubation is correct?
a. The inhalant can be safely delivered via a face mask.
b. You will need a laryngoscope to visualize the larynx.
5. It is common for anesthetized ruminants to hypoventilate.
6. Positioning the head of an anesthetized ruminant with the pharynx higher than the mouth helps to prevent:
7. Ruminants should be placed in sternal recumbency during recovery to allow them to:
8. Intubation is made easier in pigs by:
9. Which of the following statements regarding porcine anesthesia is true?
a. Oscillometric blood pressure monitors work well in pigs.
b. All pigs should have complete blood work before anesthesia.
c. Pigs are very sensitive to alpha2-agonists.
d. Intravenous sedation is virtually impossible in healthy pigs.
10. Which of the following is not a sign of porcine stress syndrome?