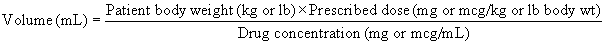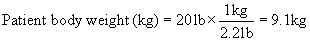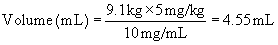Canine and Feline Anesthesia
Summary of a General Anesthetic Procedure
Anesthetic Induction with an Intramuscular Agent or Combination
Anesthetic Induction with an Intravenous Injection of an Ultra–Short-Acting Agent to Effect
Total Intravenous Anesthesia by Intravenous Boluses of an Ultra–Short-Acting Agent
Total Intravenous Anesthesia by Constant Rate Infusion
Induction and Maintenance with an Inhalant Agent
Intravenous Induction and Maintenance with an Inhalant Agent
Maintenance with an Inhalant Agent
Maintenance with Repeat Boluses of Propofol or Another Ultra–Short-Acting Agent
Maintenance with a Constant Rate Infusion
After completion of this chapter, the reader will be able to:
• Describe anesthetic techniques commonly used in small animal practices.
• List strategies used to minimize adverse effects when selecting an anesthetic protocol.
• Describe how different methods of anesthetic induction and maintenance influence the dynamics of an anesthetic event.
• Prepare a small animal patient, anesthetic equipment, and anesthetic agents and adjuncts for general anesthesia.
• Describe induction of general anesthesia by intravenous (IV) injection of an ultra–short-acting agent, by mask or chamber induction, or by intramuscular (IM) injection.
• Explain cautions and risks associated with each method of anesthetic induction, and strategies to maximize patient safety.
• List reasons for, advantages of, and potential complications of endotracheal intubation.
• Explain how to do each of the following: (1) select and prepare an endotracheal tube (ETT) for placement; (2) place an ETT in a dog or cat; (3) check for proper placement; (4) inflate the cuff; (5) minimize laryngospasm; and (6) extubate a patient during anesthetic recovery.
• Describe maintenance of general anesthesia by administration of an inhalant agent, injection of repeat IV boluses of an ultra–short-acting agent, or constant rate infusion (CRI).
• List principles of providing for patient positioning, comfort, and safety during anesthetic maintenance.
• List factors that affect patient recovery from anesthesia, the signs of recovery, appropriate monitoring during recovery, and oxygen therapy during recovery.
• Describe general nursing care during the postanesthetic period.
Small animal∗ patients are restrained and anesthetized by means of a variety of techniques including general anesthesia, sedation, neuroleptanalgesia, and local and regional anesthesia. Of these options, general anesthesia is most commonly used for several reasons. The immobilization and unconsciousness associated with general anesthesia enable most procedures to be performed more quickly and safely than is possible with alternative techniques. General anesthetics also produce analgesia during the period of unconsciousness and, if used along with an analgesic as a part of a balanced protocol, during the preoperative and postoperative periods as well. In addition, general anesthetic procedures can be performed with readily available resources and at a reasonable cost. In dogs and cats general anesthesia is induced and maintained using balanced protocols, exclusive use of inhalants, intramuscular (IM) protocols, and total intravenous (IV) techniques.
Mild to heavy sedation and neuroleptanalgesia are also frequently used to facilitate diagnostic and therapeutic procedures in small animals that are frightened, aggressive, or in pain. Box 8-1 lists some of the procedures commonly performed with patients under sedation or neuroleptanalgesia. Box 8-2 shows American College of Veterinary Anesthesiologists (ACVA) monitoring guidelines for sedated patients.
Although less frequently employed than general anesthesia, sedation, or neuroleptanalgesia, local and regional techniques are used along with general anesthesia in small animals to provide additional analgesia for dental, abdominal, orthopedic, and occasionally other procedures. Neuromuscular blockers are rarely used in general practice but are sometimes used to provide muscle relaxation for ocular and orthopedic procedures in veterinary schools and referral practices.
PATIENT PREPARATION
The veterinary technician in any busy small animal practice routinely has a constant stream of demands placed on him or her from the time of arrival to the end of the day. In order to function effectively in this environment, the technician must manage time efficiently so that the most pressing patient needs are met first. After essential tasks have been managed, often little time is left for less critical needs such as patient preparation. The technician must resist the temptation to limit or eliminate this important step, however, because incomplete patient preparation often results in complications ranging from mild to life-threatening, especially in anesthetized animals that are not young and healthy. Any time saved by abbreviating patient preparation is often offset managing problems that could have been prevented had it been given due attention. The reader is referred to Chapter 2 for a detailed discussion of the essentials of patient preparation. Procedure 8-1, p. 258, shows a summary of the steps required to prepare a small animal patient for general anesthesia.
SELECTING A PROTOCOL
An anesthetic protocol is a list of the anesthetics and adjuncts prescribed for a particular patient, including dosages, routes, and order of administration. The veterinarian-in-charge (VIC) commonly selects anesthetic protocols, although he or she may authorize the experienced technician to suggest a protocol, which then must be approved before administration. A suitable protocol takes into account the minimum patient database, the patient’s physical status class, and the procedure to be performed. Specific drug choices are also influenced by training, clinical experience, and personal preference and therefore vary widely from doctor to doctor.
When the protocol is known, drug doses, oxygen flow rates, and fluid administration rates must be calculated and checked carefully by the veterinary technician to ensure that the correct drugs and amounts are prepared. Box 8-3 shows the steps required to calculate the doses of most injectable drugs. Ill, geriatric, pediatric, or otherwise compromised patients (physical status class P3 to P5) require use of modified protocols based on the patient’s primary condition. (See Chapter 12 for recommendations regarding class P3 to P5 small animal patients.) Management of these cases can be quite challenging and requires customization of the anesthetic protocol by the VIC.
As discussed in the chapter on monitoring, anesthesia affects central nervous system (CNS) vital centers. Every anesthetized patient is at risk for potentially serious adverse effects such as hypotension, hypoventilation, hypoxemia, and hypothermia because of the actions of anesthetics and adjuncts on these centers. When choosing the protocol and preparing the agents, the anesthetist can use a number of strategies to minimize these adverse effects, as follows:
• Unless the procedure must be performed immediately for the patient’s well-being, correct significant physiologic abnormalities such as dehydration, hypotension, and anemia before anesthesia.
• Base the anesthetic protocol on results of the minimum database. Do not use a single, standard protocol for all patients.
• Use a balanced protocol consisting of multiple agents. This approach reduces the required dose of any one agent, thus minimizing the adverse effects of all agents.
• Double-check all injectable drug doses before administration, and ensure that the concentration of an agent drawn into a syringe is the same as that used for the drug calculations.
• Label all syringes containing injectable anesthetic agents with the name of the patient, the name of the drug, and the drug concentration if more than one concentration is available.
• Administer no more than the minimum dose of drug needed to achieve the desired level of anesthesia.
• Unless told otherwise, administer all IV agents “to effect.”
SUMMARY OF A GENERAL ANESTHETIC PROCEDURE
In order to give anesthetic agents safely, it is important to have a clear understanding of the dynamics of an anesthetic procedure. The word dynamics refers to the changes in the patient’s level of consciousness over time, including when, how extensively, and how quickly these changes occur. Armed with this understanding, the anesthetist is able to administer agents effectively, rapidly detect adverse reactions, and quickly recognize and respond to patient needs. An anesthetist without this knowledge is unable to make sound decisions, differentiate normal from abnormal responses, or react rapidly enough to protect the patient.
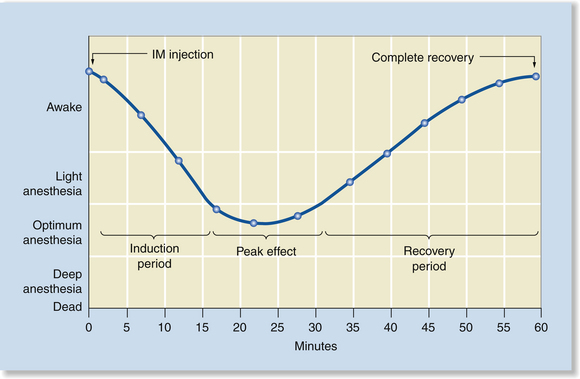
The protocol is the primary determinant of these dynamics. The agent used and the route of administration affect how quickly the patient becomes unconscious and awakens and the amount of control the anesthetist has over anesthetic depth. For instance, when given intramuscularly, most drugs begin to act, reach peak effect, and “wear off” relatively slowly and they afford the anesthetist very little control over anesthetic depth. In contrast, the commonly used inhalant agents act, reach peak effect, and are eliminated relatively quickly and they give the anesthetist excellent control. The dynamics for each of the commonly used protocols are summarized in the following sections.
Anesthetic Induction with an Intramuscular Agent or Combination
When anesthetics are administered by IM injection, anesthetic depth gradually increases after injection (typically over 5 to 20 minutes) and, after peak effect, gradually decreases as the agent is metabolized (typically over 30 to 60 minutes or longer). Once the injection has been given, the anesthetist has little control over changes in depth or the peak effect. If the depth is inadequate, the anesthetist can give additional anesthetic, but if depth is excessive, the anesthetist can only monitor and support the patient until the agent is metabolized and the patient recovers naturally. The only exception to this is when using opioids, benzodiazepines, and alpha2-agonists. In these cases the anesthetist can decrease anesthetic depth rapidly by administering the corresponding antagonist (reversal agent) (Figure 8-1).
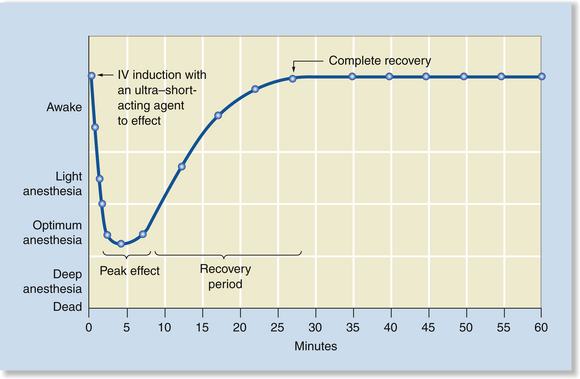
Anesthetic Induction with an Intravenous Injection of an Ultra–Short-Acting Agent to Effect
Anesthetic induction with an intravenous injection of an ultra–short-acting agent to effect is a technique used for short procedures that require less than 10 minutes of anesthesia, such as removal of a Steinman bone pin, examination of the pharynx and larynx, or changing of a bandage. Propofol, methohexital, thiopental sodium, or etomidate may be used this way. When anesthesia is induced by IV injection, anesthetic depth typically increases rapidly over 15 seconds to a few minutes after the initial injection, then decreases gradually over 10 to 20 minutes. When using this technique, the anesthetist controls the peak effect and can increase anesthetic depth rapidly by giving additional boluses of the drug. However, as with IM anesthesia, there is little control over duration, and anesthetic depth cannot be decreased (Figure 8-2).
Total Intravenous Anesthesia by Intravenous Boluses of an Ultra–Short-Acting Agent
With total intravenous anesthesia (TIVA) by IV boluses of an ultra–short-acting agent, anesthesia is induced as previously described and then is,maintained by giving additional boluses every 3 to 5 minutes as needed to maintain surgical anesthesia. This technique is acceptable for noninvasive procedures of short to moderate length but is somewhat cumbersome for major surgeries because it is somewhat challenging to keep the patient at an optimum anesthetic depth even with constant monitoring. Propofol is the agent most commonly used to provide TIVA, although methohexital and etomidate are alternatives. With this technique the anesthetist can increase the depth rapidly by giving incremental boluses, but—as with IM administration—if the patient is deeply anesthetized, the anesthetist can only support the patient and wait until the agent is metabolized and the anesthetic depth naturally decreases (Figure 8-3).
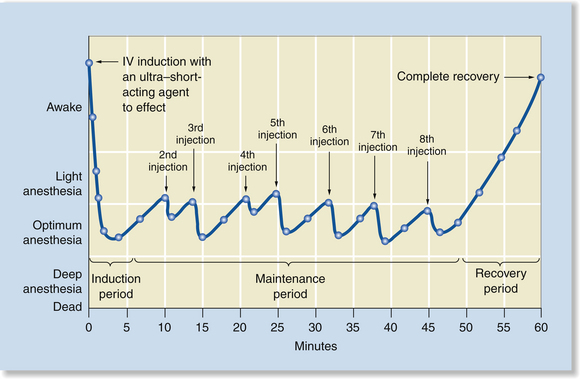
FIGURE 8-3 Total intravenous anesthesia (TIVA) by IV boluses of an ultra–short-acting agent. Anesthetic induction is very rapid, peak effect is short, and surgical anesthesia is maintained with administration of repeat boluses every 3 to 5 minutes to effect. As with a single injection, recovery is gradual after the final bolus but relatively rapid as the agent is metabolized or redistributed.
Total Intravenous Anesthesia by Constant Rate Infusion
TIVA by constant rate infusion (CRI) is similar to TIVA by bolus injections, except that anesthesia is maintained by infusing small amounts of anesthetic constantly with a syringe pump. Although similar in effect to maintenance with bolus injections, this technique moderates and slows down changes in depth by avoiding sudden infusion of a large amount of drug. Instead, only the amount needed to maintain anesthesia is infused on a continuous basis for the duration of the procedure (Figure 8-4).
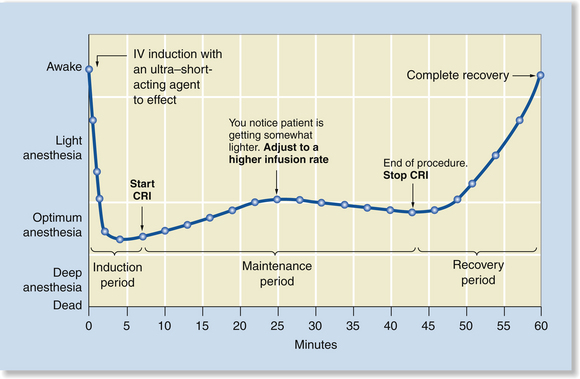
FIGURE 8-4 Total intravenous anesthesia (TIVA) by constant rate infusion (CRI). Anesthetic induction is very rapid, peak effect is short, and surgical anesthesia is maintained with a CRI. The rate of infusion is adjusted based on assessment of the anesthetic depth. After discontinuation of the CRI, recovery is gradual, but relatively rapid as the agent is metabolized or redistributed.
Induction and Maintenance with an Inhalant Agent
The use of an inhalation agent for induction and maintenance differs from injection techniques in several respects. Anesthetic induction with the commonly used inhalant agents is usually faster than IM induction but slower than IV induction (about 5 to 8 minutes with isoflurane or sevoflurane). Also, the anesthetist has excellent control over depth and can either increase or decrease depth relatively rapidly by changing the vaporizer dial setting. With inhalant agents, however, there is a delay between the time the dial setting is changed and the time it takes for patient anesthetic depth to change, because it takes at least several minutes for the new concentration to fill the breathing circuit, reach the patient’s lungs, and equilibrate with the blood and CNS.
The time required for anesthetic depth to change is influenced by a number of factors including the patient’s respiratory drive, the agent used, the carrier gas flow rate, the type of breathing circuit, and the volume of the breathing circuit. For this reason, vaporizer setting adjustments must be anticipated as much as possible through close monitoring. In general, if a patient’s anesthetic depth is significantly light or deep, larger dial changes are warranted, whereas if the patient is slightly too lightly or deeply anesthetized, more subtle changes are needed. The anesthetist will develop a “feel” for the exact amount to change the dial setting in any given circumstance through experience (Figure 8-5).
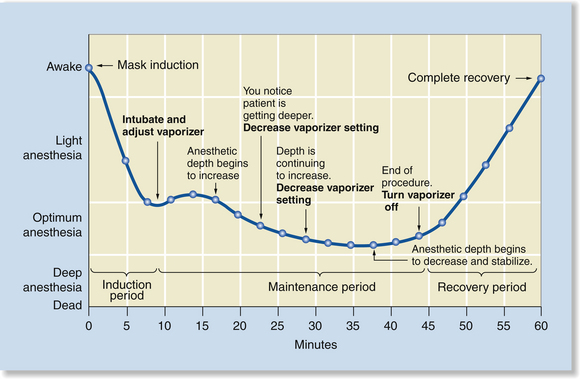
FIGURE 8-5 Induction and maintenance with an inhalant agent. Induction is relatively rapid, but gradual. Anesthesia is maintained by continued administration of the inhalant agent. The percent administered is adjusted based on assessment of the anesthetic depth. After discontinuation of the agent, recovery is gradual, but relatively rapid as the agent is exhaled. Note that there is a delay after any change in the vaporizer setting. This is typical of inhalant anesthesia because of the time required for the concentration of the agent in the breathing circuit to reach the dialed concentration.
Intravenous Induction and Maintenance with an Inhalant Agent
Intravenous induction and maintenance with an inhalant agent is the most commonly used method of inducing and maintaining general anesthesia in small animal patients. It has dynamic elements of both IV and inhalant administration, as illustrated in Figure 8-6, including rapid induction, good control over both increases and decreases in anesthetic depth, and a relatively rapid recovery. The sequence of events associated with this technique is summarized in Procedure 8-2, p. 258.
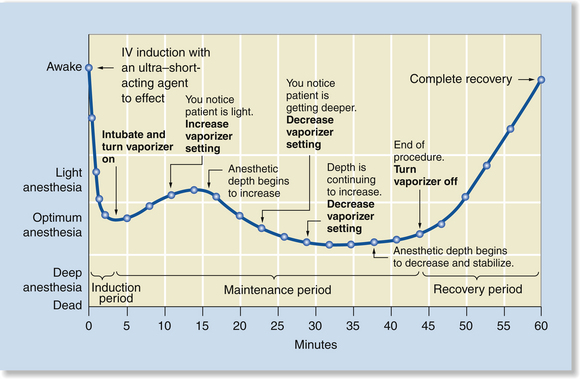
FIGURE 8-6 Intravenous (IV) induction and maintenance with an inhalant agent. This method has features of induction with an IV injection of an ultra–short-acting agent to effect and induction and maintenance with an inhalant agent. Anesthetic induction is very rapid, and the dynamics of maintenance are identical to those shown in Figure 8-5.
EQUIPMENT PREPARATION
During a typical anesthetic induction, many events occur in rapid succession. Anesthetic agents are administered; the patient becomes unconscious and recumbent; the endotracheal tube is placed, secured, cuffed, and attached to the machine; the anesthetic vaporizer is turned on; the patient is positioned and monitored; and adjustments are made as needed, all within the first few minutes. Because these events follow one another so rapidly, the technician does not have the luxury of leaving the patient to locate equipment. For this reason, all equipment must be carefully gathered, checked, and organized before commencement of the procedure.
Unless IM or total IV techniques are used, most small animal patients are anesthetized by means of a small animal anesthesia machine configured as a semiclosed rebreathing system. Patients weighing less than 2.5 to 3 kg require a non-rebreathing circuit. Equipment required to intubate the patient, give injections, and administer fluids is also required—as is, especially if surgery will be performed, equipment designed to prevent hypothermia. A crash cart containing emergency equipment and drugs should also always be available.
PREMEDICATION OR SEDATION
Premedication refers to the administration of anesthetic agents and adjuncts to calm and prepare the patient for anesthetic induction. Preanesthetic medications are chosen specifically to produce a set of desired effects such as sedation, cholinergic blockade, analgesia, and muscle relaxation. Tranquilizers, alpha2-agonists, opioids, dissociatives, and anticholinergics are commonly used as preanesthetic medications and are most often given intramuscularly. See Protocols 8-1 and 8-2 for preanesthetic and sedative protocols in dogs and cats.
After IM injection of preanesthetic medications, the patient should be placed in a location that is quiet, but also one that permits close observation until the agent takes effect (for many drugs given intramuscularly, about 15 to 20 minutes). If the patient is excited or stimulated during this time, the beneficial effects of the drugs may be diminished. Especially if the patient is heavily sedated, observation every few minutes is paramount to permit prompt intervention in the event that the patient experiences complications. Once the patient is adequately sedated, anesthetic induction should immediately follow, or, as an alternative, these protocols can be used alone to provide sedation for diagnostic and therapeutic procedures.
ANESTHETIC INDUCTION
Anesthetic induction is the process by which an animal loses consciousness and enters surgical anesthesia. The goal of anesthetic induction is to take the patient from consciousness to stage III anesthesia smoothly and rapidly, so that an endotracheal tube can be placed. During any induction the patient passes through the excitement stage and therefore may show signs of uncoordination or struggling, followed by progressive relaxation and unconsciousness. Excitement and struggling during induction hamper restraint, increase the risk of unadvertent perivascular drug injection, and predispose the patient to traumatic injury, vomiting, cardiac arrhythmias, and other
adverse effects, and so should be minimized through administration of preanesthetic medications.During induction, the patient should be sufficiently anesthetized to permit intubation, but anesthesia should generally be kept light until the tube is placed. At this point the anesthetic depth can be adjusted as needed. Induction is most commonly accomplished by IV administration of injectable agents or by administration of inhalant agents via a mask or chamber. IM administration of drugs is a third method, which, although less common in general practice, is frequently used in animal shelters to induce and maintain anesthesia in patients undergoing routine procedures such as spays and castrations. Of these techniques, IV induction has the advantage of producing unconsciousness within seconds to a few minutes at most. Therefore most animals that undergo induction by this method pass through the excitement stage quickly, allowing rapid control of the airway. In contrast, mask or chamber induction typically
takes at least 5 to 10 minutes, increasing the likelihood of undesirable effects. Induction after IM injection typically takes about 10 to 20 minutes but results in smooth, gradual CNS depression with little apparent excitement. What follows is a description of specific techniques used to induce anesthesia.
Intravenous Induction
Agents commonly used to induce general anesthesia in dogs and cats by IV injection include (1) a mixture of equal volumes of ketamine and diazepam or midazolam, (2) propofol, (3) neuroleptanalgesics, (4) thiopental sodium, (5) etomidate, and (6) various other combinations containing dissociatives, tranquilizers, and opioids. Protocol 8-3 shows IV induction protocols in dogs and cats.
To induce general anesthesia by the IV route, a volume of the agent to be administered is calculated based on a prescribed dose and drawn into a syringe. The agent is then injected directly into the vein, or into a winged-infusion set or indwelling catheter to effect until the patient can be intubated or until the patient is at an adequate plane of anesthesia for completion of the planned procedure.
The term “to effect” means that only the amount of injectable anesthetic necessary to produce unconsciousness is given, rather than administering the entire dose calculated on a milligram per kilogram basis. This technique is necessary because the amount of drug needed to induce or maintain anesthesia cannot be accurately predicted for a given patient, and most anesthetic agents have narrow therapeutic indices.
For example, the amount of thiopental sodium required to induce anesthesia in a quiet, older dog may be one quarter to one half of the dose required for an active, young dog of equivalent body weight. Similarly, a cat with a urinary obstruction may be deeply anesthetized after receiving a very small dose of ketamine, whereas a healthy cat may require several times more to reach an equivalent depth. The drugs used for premedication also affect the dose of general anesthetic required. For example, a patient that has not received any premedications may require two or three times as much as a patient that has been premedicated with a neuroleptanalgesic combination to reach a comparable plane of anesthesia.
For these reasons, IV drugs are given as a series of bolus injections and discontinued when the desired depth is reached—a process known as titration. A competent anesthetist monitors the patient closely and alters the amount of anesthetic given to suit the patient’s requirements, rather than relying solely on a calculated dose. (See Procedure 8-3, p. 259, for IV induction techniques in dogs and cats.)
After induction with the commonly used IV injectable agents, the duration of anesthesia varies but is usually no more than 10 to 20 minutes. If more than 20 minutes is required, anesthesia is maintained with inhalation anesthetics or administration of propofol, methohexital, or etomidate by repeat boluses or CRI. This is not recommended with other injectable agents such as thiopental sodium, because if such drugs are given this way, large amounts of anesthetic will accumulate in the body and prolong recovery.
Inhalation Agents
Inhalation agents commonly used to induce general anesthesia in dogs and cats include isoflurane and sevoflurane. These agents are administered by means of a facemask or anesthetic chamber. Protocol 8-4 shows inhalant induction protocols in dogs and cats.
Mask Induction
Mask induction involves administration of an inhalant anesthetic such as isoflurane or sevoflurane via a facemask. This method of induction is feasible only with use of inhalation anesthetics with a low blood-gas solubility coefficient, such as isoflurane or sevoflurane, because the rapid induction time associated with these agents results in passage through stage II anesthesia quickly enough to minimize excitement. In contrast, mask induction is difficult and, in some cases, not possible with agents with a higher solubility coefficient because of the prolonged length of time required to reach stage III anesthesia. For some patients in critical condition, induction by mask may be safer than induction with injectable agents because the anesthetist can decrease anesthetic depth or discontinue the agent by adjusting the vaporizer setting if problems arise.
Mask induction is challenging for several reasons. Many patients struggle, necessitating skillful restraint (enough to prevent operator and patient injury, but not so much as to restrict chest excursions or the airway). The fear associated with passage through stage I and the excitement associated with passage through stage II cause release of epinephrine and other catecholamines, which can predispose the patient to cardiac arrhythmias, hypotension, and other adverse effects. Also, mucous membrane color and refill time as well as ocular indicators of anesthetic depth are not as easily observed because the mask partially obscures the eyes and muzzle, although monitoring requirements are no different with this method of induction.
The mask should be carefully fitted before commencement of mask induction. There should be a reasonably tight seal between the muzzle and the rubber gasket without constriction of the tissues or discomfort. The mask should be long enough to accommodate the full length of the muzzle (so that the nares do not press against the end of the mask when the muzzle is fully inserted), but not too long. Be aware that relatively higher oxygen flow rates are required than when an endotracheal tube is used. The technique for mask induction is described and illustrated in Procedure 8-4, p. 259.
There are several cautions associated with mask induction with which the anesthetist must be aware:
• Mask induction may result in significant exposure of personnel to waste anesthetic gas, because no matter how well the mask is fitted, some leakage is inevitable. Therefore adequate room ventilation is necessary to prevent excess inhalation of waste gas (see Chapter 13).
• If the animal resists mask induction, struggling may cause the release of epinephrine, which predisposes the patient to potentially fatal cardiac arrhythmias and hypotension. To avoid this, induce anesthesia in only calm or sedated patients by mask.
• Because of the longer induction time compared with IV administration, mask induction is not appropriate for patients with poor respiratory function (e.g., upper airway disease or obstruction, difficult breathing because of brachycephalic conformation, diaphragmatic hernia, pleural effusion, or pulmonary edema), unfasted patients, or patients at risk for vomiting during induction. These patients must be intubated immediately to prevent serious adverse effects and permit rapid control of the airway and ventilatory support.
• The anesthetist must ensure that the airway is kept open at all times during mask induction. The mask must not occlude the patient’s nostrils, as might happen with a cat or brachycephalic patient if the mask is too small or tight. The anesthetist must not compress the airway or chest during restraint.
Although a mask can be used to maintain anesthesia, the anesthetist is not able to protect the airway, prevent aspiration, provide ventilatory support, or observe respirations as readily as when a tube is present. For these reasons, most anesthetists intubate the patient immediately after induction.
Chamber Induction
Chamber induction involves placing the patient in a closed chamber infused with anesthetic gas. This technique is feasible only for patients small enough to fit comfortably into a chamber (typically weighing less than 5 to 7 kg) and is therefore primarily used for small patients that are aggressive or difficult to handle.
Most chambers are small clear boxes that resemble a 5-gallon aquarium (see Figure 4-6). The chamber should be examined before induction. A tight-fitting lid and two ports (one for entry of fresh gas and another for exit of excess gas) are required. Although there is more than one way to supply oxygen and anesthetic gases to a chamber, a common way is to remove the Y-piece from the corrugated breathing tubes of a semiclosed rebreathing system and attach the inspiratory tube to one chamber port and the expiratory tube to the other port. Place the patient in the chamber, close the lid, and proceed, following the instructions summarized in Procedure 8-5, p. 260, and illustrated in Figure 4-6.
Anesthetic chambers allow the induction of anesthesia in even the most uncooperative animal but are associated with complications from stress, trauma, vomiting, airway blockage, and other issues:
• Because it is impossible to accurately assess most monitoring parameters while the patient is inside a chamber, the anesthetist must be vigilant and prepared to act quickly if the patient shows signs of compromise.
• As with mask induction, there is some risk of regurgitation or vomiting, especially in the nonfasted patient. Because the airway is unprotected, aspiration of stomach contents may occur.
• There is considerable risk of exposure of hospital personnel to waste anesthetic gas, particularly when removing the patient from the chamber. The chamber must be equipped with a scavenger, and ideally the anesthetic gas should be evacuated before the chamber is opened so that waste gas exposure can be avoided. This is often not possible in a clinical setting, however, because of the need to remove the patient quickly.
• As with induction by mask, epinephrine release will predispose the patient to cardiac arrhythmias and hypotension.
• As with mask induction, chamber induction is not appropriate for patients in which rapid control of the airway is required.
Intramuscular Induction
Agents commonly used to induce general anesthesia in dogs and cats by IM injection include neuroleptanalgesic combinations and a variety of combinations of tranquilizers, dissociatives, and opioids. IM induction is useful for animals in which IV injections are difficult, such as ferrets and very young puppies and kittens. IM induction using restraint equipment such as a squeeze cage or rabies pole is necessary to induce anesthesia in extremely aggressive domestic animals. IM induction is also standard in animals that are difficult to approach or impossible to handle or in which IV or mask induction not feasible (e.g., wild animals and captive animals in zoos). In these animals, dissociative-tranquilizer mixtures, neuroleptanalgesics, or opioids (particularly etorphine and carfentanil) are usually administered by means of a blowpipe or tranquilizing gun.
Induction by IM injection differs from IV induction in several important respects.
• IM injections cannot be titrated or given “to effect.” Usually the entire calculated dose is given at once.
• In general, the dose for IM injection is about twice the corresponding IV dose.
• Drugs administered by the IM route require more time to reach a high enough concentration in the brain to induce anesthesia. IM induction is therefore characterized by a relatively slow onset of anesthesia compared with IV induction (typically 10 to 20 minutes). Occasionally, if drugs are deposited in a fascial plane between muscles, or in subcutaneous (SC) tissue, slow or incomplete absorption may result in an even longer induction or a blunted effect.
• After peak effect, if the patient’s depth of anesthesia is still inadequate, additional drug must be given or an inhalant agent must be administered with a mask until the patient can be intubated. (Remember that some drugs such as propofol, thiopental sodium, and etomidate must not be given intramuscularly.)
• IM induction is characterized by a lengthy recovery period because the animal requires considerable time to metabolize the relatively large dose of drug given by this route.
The differences between IM and IV administration are illustrated by the use of ketamine in cats. When ketamine is given intravenously at a dose of 5 mg/kg, induction of anesthesia occurs in less than 1 minute. Alternatively, the drug can be given intramuscularly at a dose of 15 mg/kg, inducing anesthesia in 5 to 10 minutes. Recovery from IV ketamine administration usually begins in 10 to 15 minutes, and healthy animals often appear fully recovered within 1 to 2 hours. In contrast, complete recovery from IM ketamine administration may require several hours.
Oral Administration
Oral administration of certain anesthetics (most notably ketamine) is occasionally used in circumstances in which injection is dangerous or difficult, such as induction of anesthesia in feral cats. With an open-ended tom-cat urethral catheter and syringe, the drug is forcefully squirted into the animal’s mouth from a distance when the patient opens its mouth to hiss or vocalize. When this route is used, care must be taken to avoid pulmonary aspiration or contact with the eyes. Alternatively, the agent can be mixed with a small amount of palatable food. Because ketamine is not labeled for oral use, administration by this route constitutes extra-label use.
Other routes of administration for injectable agents include the SC, rectal, and intraperitoneal routes. For dogs and cats, these routes are too slow or impractical for routine use.
ENDOTRACHEAL INTUBATION
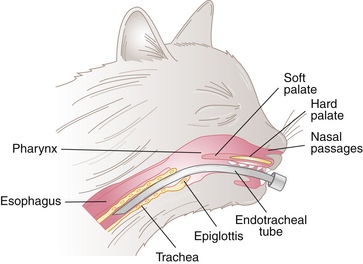
After induction of general anesthesia, an endotracheal tube is usually placed in the patient’s airway. This tube conducts air or anesthetic gases directly from the oral cavity to the trachea, bypassing the nasal passages and pharynx (Figure 8-7). Placement of an endotracheal tube is especially beneficial for maintaining anesthesia with inhalant anesthetics, but also offers several key advantages even when anesthesia is maintained with injectable protocols.
• When properly maintained, an endotracheal tube helps to maintain an open airway, decreasing the likelihood of airway obstruction caused by patient position, collapse of pharyngeal tissues, foreign material, or any other cause. Because of the importance of maintaining a patent airway, it is customary to leave the tube in place throughout anesthesia and into the recovery period, until the animal regains the swallowing reflex.
• Intubation allows more efficient delivery of anesthetic gas to the animal than does a mask. Because gas flow rates can be lowered, intubation results in reduced exposure of hospital personnel to waste anesthetic gas and increased economy.
• Use of an endotracheal tube with an inflated cuff reduces the risk of aspiration of vomitus, blood, saliva, or other material that may be present in the oral cavity or breathing passages. This material may accumulate during any procedure; however, the risk of aspiration is particularly high during oral surgery or dentistry and in patients that have not been fasted.
• An endotracheal tube of the correct diameter and length will improve efficiency of gas exchange by reducing the amount of anatomic dead space. Anatomic dead space is composed of the portions of the breathing passages that contain air but in which no gas exchange can occur (i.e., the mouth, nasal passages, pharynx, trachea, and bronchi). With the anatomic dead space minimized, the endotracheal tube ensures that a larger proportion of the gas delivered to the patient reaches the exchange surface in the alveoli.
• The anesthetist can support ventilation in intubated patients by manual or mechanical means. Manual ventilation refers to forced delivery of oxygen and anesthetic gases by squeezing the reservoir bag of the anesthetic machine. Mechanical ventilation refers to use of a mechanical ventilator to achieve the same result. Manual and mechanical ventilation are discussed in detail in Chapter 6. Because of the respiratory depression associated with anesthesia, periodic manual or mechanical ventilation is necessary in most anesthetized patients to ensure adequate gas exchange. Intermittent mandatory manual or mechanical ventilation is required in patients that have been given neuromuscular blocking agents or in which the thoracic cavity is open.
• Endotracheal intubation followed by manual or mechanical ventilation is also essential for patients in respiratory or cardiac arrest. For this reason it is advisable to have a laryngoscope and an endotracheal tube of correct size readily available for all anesthetized patients, even if endotracheal intubation is not planned.
Equipment for Endotracheal Intubation
The following equipment is required to perform endotracheal intubation (Figure 8-8):
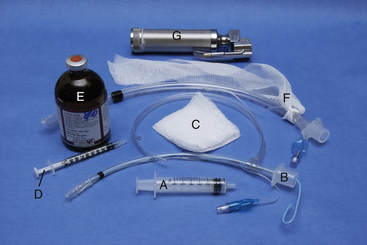
FIGURE 8-8 Intubation equipment. A, Cuffing syringe; B, 4-mm internal diameter (ID), polyvinyl chloride (PVC) endotracheal tube (ETT) with stylette and intravenous (IV) tubing tie; C, 3 × 3 gauze sponge; D, 1-mL syringe containing 0.1 mL of injectable lidocaine; E, 2% lidocaine injectable solution; F, 8-mm ID PVC ETT with gauze tie; G, laryngoscope (optional).
• Appropriately sized endotracheal tubes (at least three of slightly different diameters)
• A 2-foot length of IV tubing or rolled gauze to secure the tube
• A gauze sponge to grasp the tongue
• A 12-mL syringe to inflate the cuff
• A stylette if using a narrow-diameter tube (this is most important for cats) or any other tube that requires additional support
• Lidocaine injectable solution or lidocaine gel to control laryngospasm (in cats only)
• A laryngoscope with an appropriately sized blade if desired
Selecting an Endotracheal Tube
Endotracheal tubes are available in a wide variety of diameters and lengths. The tube must be of a diameter that is small enough to allow placement without causing trauma to the trachea, but large enough to produce a seal when the cuff is inflated. It must be of sufficient length to reach the thoracic inlet when fully inserted, but must not be so long as to reach the main stem bronchi or to extend beyond the end of the muzzle when inserted the correct distance.
At least three tubes of slightly different diameters should be selected so that you are prepared if your first choice does not fit. The size required by any given patient is influenced by patient species, conformation, and breed. For instance, cats require smaller-diameter tubes than dogs of comparable body weight. Most brachycephalic breeds require a tube several sizes smaller than mesocephalic or dolichocephalic breeds. An obese patient will require a smaller tube, and an emaciated patient will require a larger tube than a patient of normal conformation that weighs the same.
In view of these variances, the following guidelines can be used to estimate the proper size based on patient body weight. Most cats require a 3- to 4.5-mm tube. A dog weighing 20 kg will require a 9.5- to 10-mm tube. Increase or decrease the size approximately 1 mm for each 5 kg of body weight under or over 20 kg. For example, prepare a 7.5- to 8-mm tube for a 10-kg patient, and a 10.5- to 11-mm tube for a 25-kg patient. This guideline applies to canine patients weighing about 10 to 40 kg. (See Table 8-1 for size recommendations.)
TABLE 8-1
Recommended Endotracheal Tube Sizes
| Species and Body Weight (kg) | Tube Size (Internal Diameter [mm]) |
| Cat | |
| 1 | 2.5-3 |
| 2-4 | 3.5-4 |
| 5 or greater | 4.5-5 |
| Dog | |
| 2 | 5 |
| 4 | 5.5-6 |
| 7 | 6.5-7 |
| 10 | 7.5-8 |
| 15 | 8.5-9 |
| 20 | 9.5-10 |
| 25 | 10.5-11 |
| 30 | 11.5-12 |
| 40 | 13-14 |
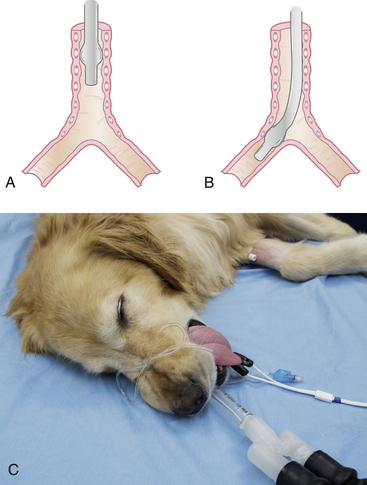
Next determine if the tube is the appropriate length. The endotracheal tube should ideally extend from the tip of the nose to the thoracic inlet. If it is too short, it may not be long enough to reach the trachea at all. If the tube is too long, one of two problems may occur. If inserted too far, the beveled end may advance into only one main stem bronchus, thus supplying only one lung with oxygen and anesthetic. If inserted only to the thoracic inlet, the portion of the tube extending from the mouth will increase mechanical dead space (Figure 8-9). Either situation predisposes the patient to hypoventilation and hypoxemia. If a tube of the appropriate length is unavailable, the machine end of the tube can be trimmed, with care being taken to avoid cutting into the cuff inflation apparatus. Endotracheal tubes are further discussed in Chapter 4.
Preparing the Tube
Before an endotracheal tube is used, it must be checked for integrity. It should be clean, sanitized, and free of blockages, holes, deterioration, or other damage. The connector must be securely attached, and the cuff must inflate and hold pressure after the syringe is detached from the valve. A soft or narrow tube may require use of a stylette, which does not extend beyond the end of the tube, to stiffen it during placement. The tube should be lubricated with a small amount of sterile water-soluble lubricant or with the patient’s saliva immediately before placement.
Intubation Procedure
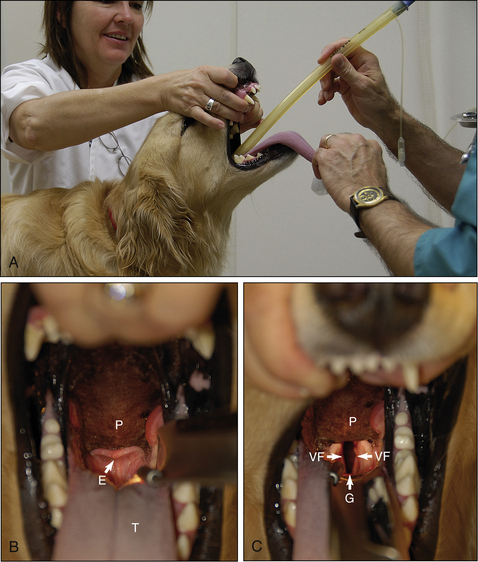
Successful endotracheal tube placement requires knowledge of the anatomy of the pharynx and larynx including the glottis, epiglottis, vocal folds, and soft palate. (See Figure 8-10 for a review of the anatomy.) Careful restraint, positioning, and lighting are necessary to maximize visibility of the larynx. Very subtle differences in these factors can make the difference between success and failure. This is especially true in cats. If you cannot easily see the larynx, insist that your assistant alter the patient position, the way the head is held, or the lighting until you can. A failure to do this frequently results in delay, frustration, and failure to successfully intubate the patient. A little time spent optimizing these factors is well worth the effort.
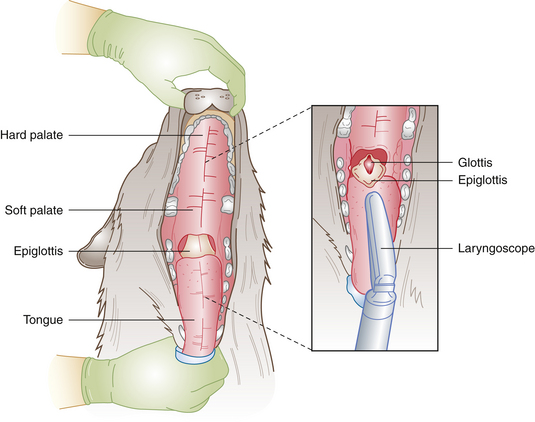
FIGURE 8-10 Anatomy of the pharynx. When the epiglottis is depressed, the glottis is exposed. The endotracheal tube is then advanced through the glottis.
Endotracheal intubation is performed essentially the same way in both dogs and cats, although it is typically more challenging in cats owing to the smaller size of the larynx, decreased visibility, and the predisposition to laryngospasm typical of feline patients.
First prepare the necessary equipment, and prepare the patient for anesthetic induction. Induce anesthesia by IV injection, mask, or chamber until the patient is in a state of readiness for intubation. This requires administration of anesthetic, combined with careful monitoring, until the patient passes through stage II. Readiness for intubation is characterized by unconsciousness, a lack of voluntary movement, an absent pedal reflex, sufficient muscle relaxation to allow the mouth to be held open, and no swallowing when the tongue is grasped. As soon as the patient reaches this point, proceed with intubation as described in Procedure 8-6, p. 260.
In most cases intubation must be performed rapidly and efficiently, because the window for successful intubation is short (typically 1 to 2 minutes with injectable protocols and often less than a minute with inhalation protocols). Immediately after intubation, place the patient in lateral recumbency, secure and cuff the tube, turn on the oxygen, attach the breathing circuit, turn on the anesthetic vaporizer, and immediately start monitoring.
With typical protocols (assuming the patient has not been overdosed), many patients are in a light plane of stage III anesthesia after intubation, although the stage and plane vary case to case. If the patient is in a light plane (as characterized by one or two forceful exhalations immediately after placement, strong jaw tone, present palpebral reflex, and in some cases spontaneous movement, swallowing, or chewing), use high oxygen flow, and keep the inhalant anesthetic agent on induction level (3% to 5% isoflurane or 4% to 6% sevoflurane), until the patient begins to enter a deeper plane. Then immediately decrease the vaporizer setting to a safe level while continuing to monitor. Adjustments are then made in response to monitoring parameters. As soon as the patient’s condition is stable, attach any mechanical monitoring devices and prepare the patient for the procedure. Details of the intubation procedure are given and illustrated in Procedure 8-6, p.
Checking for Proper Placement
The entrance to the esophagus lies just dorsal to the entrance to the trachea, and although difficult to see, easily accommodates the endotracheal tube. Inadvertent misplacement in the esophagus is common and sometimes not detected, because air may appear to move in and out of the tube and reservoir bag even if the tube is not in the trachea. This will result in an inability to keep the patient anesthetized, and possible airway blockage and hypoxemia. The following techniques may be used to confirm proper placement in the trachea:
• Revisualize the larynx, and confirm that the tube is in the correct location.
• Watch for expansion and contraction of the reservoir bag as the animal breathes.
• Feel for air movement from the tube connector as the patient exhales or when light, quick pressure is applied to the chest wall.
• Watch for fogging of the tube with condensation during exhalation.
• Check that the motion of the unidirectional valves coincides with breathing. The inhalation valve should open as the patient inhales, and the exhalation valve should open when the patient exhales.
• Palpate the neck. The trachea is the only naturally firm structure in the neck. If the tube is inside the trachea, only the trachea will be palpable. If the tube is in the esophagus, both the tube and trachea may be palpable. It is not always easy to feel the tube, however, so mastery of this technique requires practice.
• The ability of the patient to vocalize (growl, whine, or cry) indicates a misplaced tube. This is because vocalization requires the vocal cords to vibrate together, which is impossible if the tube is properly placed.
• Many patients, especially if in a light plane of anesthesia, will cough or exhale forcefully during intubation. This is indicative of proper placement, although not all patients exhibit this sign, especially if anesthesia is deep.
• Connecting a capnograph to the endotracheal tube will reveal an appropriate waveform and level of end-tidal CO2 if the animal is correctly intubated.
Securing the Tube
The tube is secured in place with a 2-foot-long piece of rolled gauze or IV tubing. Tie the gauze around the tube near the connector using a surgeon’s knot, or IV tubing around the tube using a Lark’s head knot. Do not place the tie around the small tube supplying the pilot balloon. Be sure that the tie is secure enough that it does not slide up or down the tube, but not so tight that it compresses the tube. Then tie the loose ends over the nose for dolichocephalic dogs or behind the head for cats and brachycephalic dogs. When properly secured, the tube should not move in or out when manipulated (Figure 8-11).
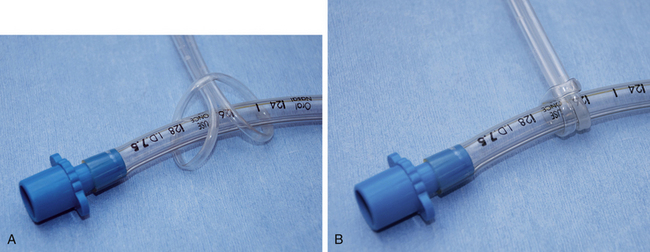
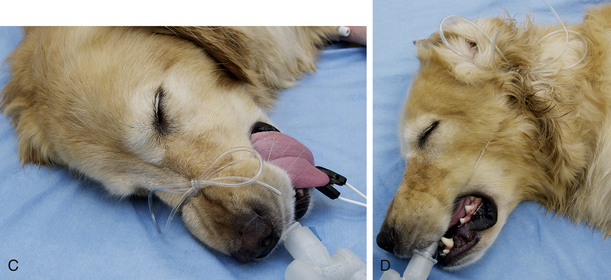
FIGURE 8-11 A, Endotracheal tube (ETT) with intravenous (IV) tubing tie showing lark’s head knot. B, ETT with lark’s head knot pulled tight to prevent the tube from slipping. C, ETT IV tubing tie firmly secured around the nose of a dolichocephalic animal to prevent movement. D, ETT IV tubing tie secured around the back of the head. Used for cats, brachycephalic dogs, and possibly for patients undergoing dental cleanings.
Cuff Inflation
Immediately after successful intubation, the cuff of the endotracheal tube must be gently inflated until a seal is formed between the trachea and the cuff. “Cuffing” prevents leakage of anesthetic gases and inhalation of room air, which will result in a variety of complications including contamination of the surgery suite with waste gases and difficulty keeping the patient anesthetized. To inflate the cuff, first extend the patient’s head to straighten the airway. Attach an air-filled 12-mL syringe to the valve port. Have an assistant close the pop-off valve and gently compress the reservoir bag, watching the pressure manometer. Listen for gas leakage around the tube, which may sound like a soft hiss or gurgling. Slowly inflate the cuff until the leaking just ceases at a pressure of 20 cm H2O but resumes at higher pressures. Avoid overinflation of the cuff, which can result in a variety of mild to serious complications. Inflation should be checked again after 15 or 30 minutes of anesthesia, because tracheal diameter may increase as a result of muscle relaxation, or a slow, undetected leak in the cuff or pilot line may cause the cuff to deflate.
Sometimes the anesthetist may see or hear recommendations that suggest use of a specific volume of air to inflate the cuff. These recommendations are not valid and in fact are potentially dangerous, because the volume necessary to seal the cuff depends on the relationship among the external diameter of the tube, the internal diameter of the trachea, and the size and type of the cuff and is thus somewhat different for every patient and anesthetic procedure.
Laryngospasm
Laryngospasm is a reflex closure of the glottis in response to contact with any object or substance. This reflex will cause the glottis to forcibly close during intubation. This complication is most commonly encountered in cats, swine, and small ruminants, especially when in a light plane of anesthesia. It is extremely difficult to place a tube in a patient experiencing laryngospasm because the glottis closes as soon as it is touched with the tube and cannot be forced open without damaging the larynx.
Laryngospasm is frustrating, makes successful intubation difficult, and in extreme cases causes hypoxemia and cyanosis. If the patient becomes cyanotic, immediately release the tongue and administer oxygen by mask. In most cases the cyanosis will quickly resolve. Laryngospasm can be prevented by using one or more of the following strategies.
• Apply no more than 0.1 mL of 2% injectable lidocaine directly to the glottis before placement. Aerosolize it with a 25- to 26-gauge needle, or apply 2 to 4 drops on the arytenoid cartilages with a tom-cat catheter attached to a 1-mL syringe. Wait 30 to 60 seconds for the lidocaine to take effect before attempting intubation. As an alternative, a small amount of lidocaine gel can be gently applied with a sterile cotton swab.
• Be sure the patient is adequately anesthetized before attempting to intubate the patient, because increased anesthetic depth decreases the incidence and severity of laryngospasm.
• Prepare carefully, wait for the glottis to open before attempting placement, and try to get the tube in the first time. Repeat attempts worsen laryngospasm.
• Do not force the tube! This can lead to severe and potentially life-threatening complications including tracheal rupture, pneumothorax, and pneumomediastinum.
Complications of Intubation
A number of hazards are associated with endotracheal intubation (Box 8-4). Most are associated with tracheal irritation, trauma, or failure to protect the airway. Although the larynx and trachea of mammals are relatively resilient structures, excessive force will result in damage, perforation, rupture, or irritation of the delicate mucosa. An endotracheal tube must therefore be chosen, maintained, placed, and monitored with care.
• Intubation may stimulate the vagus nerve and cause an increase in parasympathetic tone, particularly in brachycephalic dogs. This in turn may cause bradycardia, hypotension, and cardiac arrhythmias. Occasionally, cardiac arrest may occur, particularly in an animal with preexisting cardiovascular disease. Anticholinergics given in the preanesthetic period help to prevent parasympathetic stimulation.
• Some animals are difficult to intubate. Brachycephalic dogs, for example, have a large amount of redundant tissue within the oral cavity that falls over the back of the pharynx when the animal’s mouth is opened, obscuring the glottis. To compound this challenge, these animals must be intubated quickly after induction to prevent airway collapse, so the anesthetist must use all means to facilitate intubation. A laryngoscope is helpful in these patients to increase visibility of the oropharynx, to gently retract the redundant tissue away from the glottis, and to avoid airway trauma during intubation.
• Overzealous efforts to intubate may damage the larynx, pharynx, or soft palate. Particular care should be used when intubating cats, which have a narrow glottis that is easily traumatized. Irritation of the larynx during intubation causes laryngospasm, which can occlude the airway if severe.
• Overinflation of the cuff may cause pressure necrosis of the tracheal mucosa. Cats are particularly sensitive to pressure necrosis from endotracheal tubes, and it is sometimes recommended that uncuffed tubes or tubes with low-pressure cuffs be used with this species. Some anesthetists suggest that if tubes with high-pressure cuffs are used, the cuff should be inflated for no longer than 30 minutes before being deflated and moved slightly to a new location in the trachea. Others suggest that more risk to the patient, through accidental extubation, inadvertent esophageal intubation, or damage to the tracheal mucosa, is caused by moving the tube.
• Endotracheal tubes may become obstructed by saliva, mucus, blood, or foreign material such as gauze. This material may also occlude the patient end of the tube after use, making this a hazard for the next patient if the tube is not cleaned properly. Obstruction may also occur if the tube is kinked or twisted or if the end is occluded against the wall of the trachea. If the endotracheal tube is obstructed for any of these reasons, the patient will not receive oxygen, resulting in hypoxemia.
• Intubated animals require careful monitoring during recovery to ensure that the tube is removed when the animal begins to swallow. If the patient regains consciousness with the tube in place, the patient may chew the tube. In fact, patients can chew the tube in half and aspirate the distal portion into the airway. The presence of such a tracheal foreign body is dangerous, requires bronchoscopy to remove, and is difficult to explain to the owner.
• Although endotracheal tubes used in human patients are routinely discarded after a single use, tubes used in veterinary patients are commonly reused. Tubes must therefore be thoroughly disinfected between patients to prevent the spread of infectious diseases such as infectious tracheobronchitis (“kennel cough”). Keep in mind that red rubber tubes soaked in a disinfectant solution for too long or in a solution that is too concentrated will become impregnated with the disinfectant. When next used, the disinfectant may irritate the tracheal mucosa. This can result in coughing after anesthesia or may even cause the tracheal mucosa to slough.
• Despite all precautions, some intubated patients will develop minor irritation of the trachea and larynx. Animal owners should be warned that it is common for animals to cough for 1 to 2 days after anesthesia if an endotracheal tube is used.
Because of the problems associated with the use of endotracheal tubes and for reasons of convenience, not all animals undergo endotracheal intubation during anesthesia. If an endotracheal tube is not used, the inhalation agent is delivered by mask throughout the procedure. Animals lightly anesthetized with IM or IV agents for the performance of short procedures may not require the use of an endotracheal tube if the animal maintains the ability to swallow throughout the anesthesia and can breathe adequately. There should be no sounds indicating airway obstruction (such as fluid sounds in the airway), and the chest wall should move normally. With these exceptions, intubation is recommended for safety reasons if inhalation anesthetics are used or if a lengthy procedure is performed with the patient under injectable anesthesia.
MAINTENANCE OF ANESTHESIA
After anesthetic induction and endotracheal intubation, patients that are in a light plane of anesthesia must be brought into surgical anesthesia. When the patient reaches the desired anesthetic depth, general anesthesia is maintained with injectable anesthetics, inhalant anesthetics, or a combination thereof. General anesthesia is most commonly maintained with inhalant agents delivered via an anesthetic machine. Less frequently, anesthesia is maintained with IV boluses every 3 to 5 minutes or a CRI of an ultra–short-acting agent such as propofol, or with IM protocols. See Protocol 8-5 for maintenance protocols in dogs and cats, and Procedure 8-7, p. 262, for details regarding anesthetic maintenance.
Maintenance with an Inhalant Agent
To maintain general anesthesia with an inhalant agent, periodic changes in the vaporizer dial setting must be made based on information derived from physical and machine generated monitoring parameters. Frequent and effective monitoring is paramount, so that subtle changes in anesthetic depth are detected in time to make adjustments before the anesthetic depth becomes seriously too light or deep, thus risking that the patient will move or become aware or will be endangered.
In general, if the anesthetic depth is slightly too light or deep, small dial changes are necessary (about 0.5% to 1% increments with isoflurane and sevoflurane). In contrast, if anesthetic depth is significantly light or deep, large dial changes are necessary. For instance, if the patient’s anesthetic depth is significantly light (patient exhibits spontaneous movement, swallowing, active reflexes, strong muscle tone), settings of 3% to 5% isoflurane or 4% to 6% sevoflurane and a high oxygen flow rate are generally necessary to bring the patient back into surgical anesthesia. If the patient’s anesthetic depth is significantly too deep (e.g., absent reflexes; flaccid jaw tone; central, dilated pupils), then the vaporizer should be turned off, the oxygen flow increased, and the patient monitored
carefully until signs of decreased depth are evident. Be aware that these are only general guidelines, and each case must be handled differently as the circumstances warrant.
Maintenance with Repeat Boluses of Propofol or Another Ultra–Short-Acting Agent
As when maintaining anesthesia with an inhalant agent, frequent and effective monitoring is necessary so that the anesthetist has an accurate assessment of the patient’s condition and anesthetic depth. Every 3 to 5 minutes, additional boluses are administered to effect, often in volumes of approximately 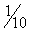 to ¼ of that required for induction, as needed to maintain depth at an optimal plane. If the anesthetist is distracted during this process, there is a danger that the patient will unexpectedly awake in the midst of a procedure, endangering itself and hospital staff.
to ¼ of that required for induction, as needed to maintain depth at an optimal plane. If the anesthetist is distracted during this process, there is a danger that the patient will unexpectedly awake in the midst of a procedure, endangering itself and hospital staff.
Maintenance with a Constant Rate Infusion
Before induction and intubation, a calculated volume of anesthetic is drawn into a syringe large enough to accommodate the anticipated amount needed to maintain surgical anesthesia for the duration of the procedure. The syringe is placed in a syringe pump that has been programmed with an infusion rate based on a calculated dose, and attached to a winged infusion set, which in turn is placed in the injection port of an IV administration set. After induction, the pump is started, causing the agent to be delivered at a constant rate. Based on results of monitoring parameters, subtle changes are made in the infusion rate as needed to maintain surgical anesthesia. This method of administration is most suited to propofol, which does not accumulate in body fat stores, because it is rapidly metabolized. Although etomidate or methohexital can also be used to maintain anesthesia, these agents are rarely used for this purpose in small animal practice.
Maintenance with Injectable and Inhalant Agents
As an alternative, inhalant and injectable agents can be used in combination to maintain anesthesia. For example, if a patient in which anesthesia is maintained with an inhalant agent shows a response to a painful surgical stimulus or wakes prematurely, small doses of an opioid or other appropriate injectable agent can be given intravenously to rapidly deepen the anesthetic plane.
Maintenance with an Intramuscular Injection
Finally, anesthesia can be induced and maintained with a single IM injection. IM protocols generally produce general anesthesia of relatively short duration (only long enough to perform routine procedures such as spays and castrations). This technique is most commonly used in shelter medicine for elective surgeries in cats. Only tranquilizers, alpha2-agonists, opioids, dissociatives, and anticholinergics can be given intramuscularly. In contrast, propofol, etomidate, and barbiturates should be given only by the IV route. Protocol 8-6 describes IM anesthetic protocols in cats.
PATIENT POSITIONING, COMFORT, AND SAFETY
During anesthetic induction and maintenance, a number of considerations must be observed to ensure that the patient is not harmed.
• During induction the animal should be supported as it loses consciousness. Particular care should be taken to be sure that the animal does not strike its head on the table during induction or transfer to surgery.
• After IV induction, as soon as the patient is intubated, remove the needle and syringe to avoid accidental overdose in the event that the syringe plunger is accidentally pushed while the patient is being moved.
• Immediately after intubation, lay the patient in lateral recumbency, then secure and cuff the tube.
• Before preparing the patient for surgery, the anesthetist must ensure that the patient’s endotracheal tube is correctly placed (i.e., in the trachea) and that the cuff is functional and inflated. Once the surgical preparation begins, it is difficult to position the animal to allow reintubation without compromising aseptic technique.
• Check the endotracheal tube for kinks or bends. An open airway must be maintained at all times (Figure 8-12).
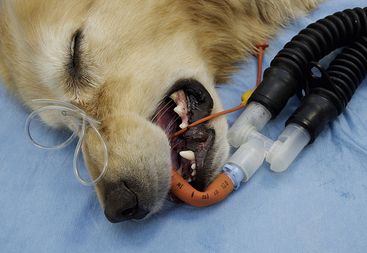
FIGURE 8-12 In this patient the endotracheal tube is kinked, and the airway is blocked because of the position of the breathing tubes in relation to the position of the patient.
• Any time an intubated patient is turned over, the endotracheal tube should be temporarily disconnected from the anesthetic circuit. Rolling or twisting the animal while it is still connected to the circuit may cause the endotracheal tube to twist and collapse, resulting in an airway obstruction, or may cause the distal end of the tube to traumatize or lacerate the trachea.
• The hoses of the anesthetic machine should be supported so that there is no drag on the endotracheal tube. This could result in tracheal trauma or displacement of the tube.
• The position of the hoses and endotracheal tube should be checked during patient transfer and after repositioning. Hyperflexion of the neck should be avoided because it may lead to endotracheal tube obstruction.
• The reservoir bag should be placed so that it is clearly visible at all times.
• When positioning the animal on the surgery table, the anesthetist should ensure that the animal assumes as normal a posture as possible. In particular, hyperextension or hyperflexion of the limbs should be avoided because either may result in permanent neurologic injury.
• Heavy drapes or instruments must not compress the chest of small patients, because this may interfere with respiration.
• Do not overtighten leg restraint ropes, or circulation may be compromised.
• Place the patient on a heat-retaining surface such as a warm-water circulating blanket. Do not use an electric heating pad, which can burn the patient!
• If one lung is diseased, place the normal side up whenever possible, to maximize oxygen exchange.
• Tilting the surgery table so that the patient’s head is down gives the surgeon easier access to some abdominal organs, particularly the uterus and ovaries. However, the anesthetist should be aware that more than a 15-degree elevation of the caudal aspect of the body may cause the abdominal organs to compress the diaphragm, which may compromise heart and lung function. Head-down tilted positions should be avoided entirely in animals with breathing difficulties, especially those with diaphragmatic hernia.
• Artificial tear solution or other corneal lubricant should be instilled into the eyes of an anesthetized patient every hour, unless the patient is undergoing ocular surgery. This is particularly important if an anticholinergic is used. General anesthesia decreases tear secretion for a period of up to 24 hours after anesthesia, and some dogs may need periodic application of a corneal lubricant for up to 36 hours after anesthesia. Cats maintain some tear production throughout anesthesia; however, lubrication is advisable if an anticholinergic or ketamine is given.
ANESTHETIC RECOVERY
The anesthetic recovery period may be defined as the period between the time the anesthetic is discontinued and the time the animal is able to stand and walk without assistance. The length of the recovery period depends on many factors, including the following:
• The length of the anesthetic period. As a general rule, the longer the patient is under anesthesia, the longer the expected recovery.
• The condition of the patient. Lengthy recoveries are seen in animals that have almost any debilitating disease (particularly liver and kidney disease).
• The type of anesthetic given and the route of administration. Lengthy recoveries are more common if an injectable agent is given intramuscularly rather than intravenously.
• The patient’s temperature. Hypothermic patients are slow to metabolize and excrete anesthetic drugs.
• The breed of the patient. Certain canine breeds (e.g., greyhounds, salukis, Afghan hounds, whippets, and Russian wolfhounds) are slow to recover from certain anesthetic agents, especially barbiturates.
Anesthetist’s Role in the Recovery Period
The anesthetist’s duty toward the patient does not end until the patient is awake and sternal. On completion of the procedure, the patient should be transferred to a recovery area where it can be monitored. A crash cart,
monitoring equipment, and oxygen should be readily available. The anesthetist must remain vigilant during recovery because this is often one of the most dangerous periods even for animals that have had no problems during induction or maintenance.
During anesthetic recovery, the anesthetist must fulfill each of the following responsibilities:
• Discontinue administration of all anesthetic agents.
• Monitor the patient on a continual basis.
• Administer oxygen as necessary.
• Administer reversal agents (see Protocol 8-7 for reversal agent protocols in dogs and Protocol 8-8 for reversal agent protocols in cats).
• Maintain a patent airway, and extubate the patient at the appropriate time.
• Provide general nursing care (reassure the patient, provide patient hygiene, warm the patient, and prevent self-injury).
• Provide adequate analgesia, and administer other medications as requested by the veterinarian.
Procedure 8-8, p. 262, shows the sequence of events during the recovery period.
Signs of Recovery
An animal recovering from general anesthesia gradually progresses back through the anesthetic stages and planes. As the animal moves from deep to moderate to light anesthesia, vital signs and reflexes change in predictable ways. The heart rate, respiratory rate, and respiratory volume increase. After assuming a ventromedial position, the eyeballs move back to a central position. Reflex responses return, and muscle tone strengthens. The animal may shiver, swallow, chew, or attempt to lick. Shortly after swallowing reflexes return, the animal will normally show signs of consciousness, including voluntary movement of the head or limbs and possibly vocalization.
While passing through stage II, some patients may exhibit a variety of alarming signs including head-bobbing, delirium, hyperventilation, head-thrashing, and rapid limb paddling, especially if not premedicated. Occasionally a patient may attempt to stand and fall or may appear blind and bump into the sides of the cage. Some patients (particularly those recovering from ketamine anesthesia) may chew at their paws or claw their faces. Animals showing these signs of a “rough” or “stormy” recovery usually return to normal within a short time, but steps must be taken to prevent self-trauma or disruption of the surgical wound. Administration of preanesthetic medications before the procedure often prevents or moderates these signs, but additional tranquilization or administration of analgesics during the postoperative period may be necessary in these patients.
Monitoring
During recovery, the patient must be watched continuously at close range because a recovering animal may develop hypoxemia, cardiac arrhythmias, or other complications yet show no signs that are evident to the casual observer. Evaluation from across the room is not acceptable because problems such as vomiting, chewing the tube, and airway occlusion occur with some regularity and must be managed without delay to prevent serious consequences. These problems are discussed in detail in Chapter 12.
To minimize these risks, the patient should be positioned in the cage so that mucous membranes and respirations can be observed. Vital signs should be evaluated at least every 5 minutes. Abnormal vital signs or a delayed return to consciousness may indicate a variety of serious conditions that must be treated promptly by the veterinarian, such as shock, hemorrhage, hypoglycemia, or hypothermia. See Box 8-5 for ACVA monitoring guidelines for the recovery period.
Oxygen Therapy
As soon as the anesthetic depth decreases sufficiently, most recovering patients begin to shiver. Shivering is a protective response to hypothermia that raises the body temperature. During shivering, contracting muscle tissue converts oxygen and chemical fuel into heat. Consequently, muscle contractions associated with shivering increase oxygen consumption. Oxygen administration during recovery is necessary to meet these needs and to compensate for residual respiratory depression until anesthetic depth decreases.
Oxygen should be administered via the endotracheal tube at a rate of 50 to 100 mL/kg/min (0.5 to 1 L/10 kg body weight up to a maximum of 5 L/min) for 5 minutes after discontinuation of the anesthetic or until the animal swallows. This route of administration allows waste anesthetic gases to be scavenged and gives the anesthetist the ability to bag the patient during recovery to help reinflate collapsed alveoli.
If the patient is at a light depth and must be extubated, oxygen can be administered by mask. Some patients do not tolerate a mask without becoming agitated, however, and may require an alternative means of oxygen delivery. The following options are alternatives to the use of a mask during the postoperative period.
• An oxygen source such as the Y-piece or the fresh gas inlet of a non-rebreathing system can be placed near the nasal openings.
• An Elizabethan collar can be placed around the patient’s neck, with an oxygen line secured to the inside of the collar. The front of the collar is covered with cellophane, with a small ventilation hole. A flow rate of 1 L/min provides 30% to 40% oxygen.
• Oxygen can be delivered via a nasal catheter. A lubricated soft red rubber catheter (5 to 10 French depending on the size of the patient) is introduced into the ventral nasal meatus. Intranasal proparacaine or 2% lidocaine can be used to desensitize the nasal tissues to allow insertion. The catheter is advanced to the level of the carnassial teeth and attached to the dorsum of the nose with tissue glue (cyanoacrylate). A flow rate of 100 to 150 mL/kg provides 30% to 50% oxygen. Alternatively, nasal prongs used in human hospitals may be used. They can be secured to the patient with tissue glue or staples.
Note that a patient that is intubated and breathing oxygen from an anesthetic machine receives close to 100% oxygen. This is beneficial for the relatively short duration of most procedures, but prolonged inhalation of high levels of oxygen (e.g., greater than 50% oxygen for more than 24 hours) can be toxic.
Extubation
To prepare the patient for extubation, deflate the cuff by drawing out all the air until the pilot balloon is empty. Untie the tube so that it can be rapidly removed. At all times during recovery, keep the neck in a natural but extended position to protect the airway. Some anesthetists prefer to deflate the cuff and untie the gauze before signs of arousal are seen so that the tube can be quickly removed when swallowing occurs.
As soon as the patient shows signs of imminent arousal, the endotracheal tube must be removed using a slow, steady motion. In dogs, return of the swallowing reflex is the most appropriate time to remove the tube because this reflex will help protect the animal from pulmonary aspiration if vomiting occurs during recovery. Animals that show voluntary limb, head, or chewing movements are close to consciousness, however, and should be extubated even if swallowing has not been observed. These patients typically swallow on removal of the endotracheal tube.
A notable exception to this general rule is brachycephalic dogs. In these patients, many anesthetists prefer to delay extubation until the patient is able to lift its head unassisted, as early extubation may lead to respiratory distress from upper airway obstruction. In these patients it is wise to prepare by having a laryngoscope, an endotracheal tube (the same size as or one size smaller than used during the procedure), and the appropriate dose of an ultra–short-acting IV induction agent such as propofol nearby in case reintubation is necessary.
If a recovering patient shows signs of respiratory distress after extubation, the anesthetist must determine whether this is because of pulmonary disease (in which case oxygen therapy may be needed) or upper airway obstruction. One helpful clue is that upper airway obstruction is often associated with stridor (noisy respirations), especially on inspiration. If obstruction is present, the anesthetist should reposition the patient and gently pull the tongue forward. If the obstruction persists, it may be necessary to reinduce and reintubate the patient.
In cats the endotracheal tube may be removed when signs of impending arousal are observed. These include swallowing, an active palpebral reflex, and voluntary limb, tail, or head movements. Delaying extubation is not advisable in cats because it may predispose the patient to laryngospasm.
After dental cleaning, oral surgery, or any other procedure in which blood or other fluids are present in the oral cavity, the cuff should be left partially inflated during removal to sweep out the fluid, and prevent it from entering the airways. After extubation, all animals should be placed in lateral or sternal recumbency with the neck extended. This position helps maintain a patent airway. Occasionally, fluid or mucus may accumulate in the pharynx or trachea and must be removed by suction. The anesthetist should do this before the patient is fully awake to avoid being bitten.
POSTANESTHETIC PERIOD
In the postanesthetic period, patients require general nursing care to ease recovery, ensure their safety, and prepare them for return to the hospital ward. The anesthetist should be sensitive to the fact that the animal has no means of understanding the events that have produced the disorientation, fear, and discomfort that often accompanies the return to consciousness. Quiet, calm handling, reassurance, and attention to the patient’s comfort level are therefore essential.
The anesthetist can take several steps to minimize patient discomfort during recovery. All ties restraining the animal to the surgery table should be removed before the animal regains consciousness. The anesthetist should ensure that all accessory procedures, such as bandaging, chest tube placement, and urinary catheterization, have been completed and that all monitoring equipment probes, cuffs, and electrocardiographic electrodes have been removed. The anesthetist should also be gentle when moving a recovering patient, so as not to increase discomfort and pain.
The IV catheter should be left in place until the endotracheal tube has been removed and it is clear that the patient is recovering normally. This provides venous access in the event that the patient’s current condition or an unexpected complication necessitates administration of IV drugs or fluids.
Patient recovery may be hastened by gentle stimulation in the form of talking softly to the patient, patting or rubbing the chest, turning the patient, or gently moving the endotracheal tube. These actions stimulate breathing and increase the flow of information to the reticular activating system (RAS) of the brain—the area responsible for maintaining consciousness in the awake animal. A lack of stimulation of the RAS may cause drowsiness in the conscious animal. It is therefore speculated that stimulation of this area may help the animal to awaken.
A recovering patient should also be turned every 10 to 15 minutes to prevent pooling of blood in the dependent lung and tissues—a condition called hypostatic congestion. When the intubated patient is turned from one side to another, the breathing circuit must be briefly detached from the endotracheal tube, and the head and neck should be turned as a single unit to minimize the risk of tracheal damage by the tube. Although not proven, there may be a risk of causing the stomach to twist if a patient is turned by moving the limbs over the body from one side to the other. For this reason, some professionals recommend turning a patient by sliding the limbs under the body instead, especially if the patient is a member of a breed prone to gastric dilatation–volvulus.
Never leave a recovering patient in an open cage or on a table unattended, because once spontaneous movement returns, a recovering patient may fall and be injured. Food and water should not be left in the cage during recovery because it is possible for a recovering animal to drown in a water bowl or suffocate in a food bowl. Some patients may be able to drink soon after standing; however, most have little appetite for food for several hours after recovery anyway. Vomiting during the recovery period is not uncommon, but provided the patient is conscious, this is seldom dangerous.
Nursing care of hypothermic patients should include the provision of heat. This can be accomplished by a variety of means including warm air or water blankets, heat lamps, warm water bottles, and incubators. Never use electric heating pads, which have a significant potential to burn a recovering patient. Box 8-6 lists recommended heat sources. Recovering patients are unable to voluntarily move away from a heat source if excessive; therefore the anesthetist must prevent burns by ensuring that the heat source never exceeds 42° C (approximately 107.6° F). Gradual rewarming is preferred to rapid rewarming because the latter may cause dilation of cutaneous vessels, leading to hypotension.
Analgesics should be administered as requested by the veterinarian, preferably before the onset of pain (see Chapter 7). If analgesia is adequate, the patient should be able to sleep comfortably and should demonstrate minimal signs of discomfort. A change in the dose or frequency of administration or use of a different or additional agent may be necessary if postoperative pain is not well controlled.
After recovery the anesthetist must provide ongoing care and prepare the patient for release or continued hospitalization. Most small animal patients should be given nothing by mouth for the first hour or two and no food for at least several hours. On discharge, the client should be instructed to reintroduce water gradually after arriving home and to feed a small meal after several hours. Exceptions to these guidelines include very small and neonatal patients, which require reintroduction of water and food more rapidly.
KEY POINTS
1. Small animal patients are restrained and anesthetized with a variety of techniques, but general anesthesia is most commonly selected for many procedures. In many practices intravenous (IV) or mask induction followed by maintenance with an inhalant agent is most commonly employed.
2. When a protocol is being selected, great care must be used in calculating drug dosages, oxygen flow rates, and fluid administration rates, and in drawing up and administering anesthetic agents and adjuncts.
3. To safely and effectively anesthetize a patient, the anesthetist must have a clear understanding of the expected sequence of events and dynamics associated with each anesthetic technique.
4. Equipment must be carefully prepared before anesthetic induction, because once induction commences, the anesthetist has little to no time to gather missing equipment.
5. The goal of anesthetic induction is to take the patient from consciousness to stage III anesthesia smoothly and rapidly. This requires finesse, watchfulness, and an ability to make decisions rapidly and act quickly based on patient monitoring parameters.
6. IV induction agents are most often given “to effect” by using a process called titration. When inducing anesthesia by IV injection, in general the entire calculated dose should not be administered at once.
7. Mask and chamber inductions involve risks of which the anesthetist must be aware to prevent serious complications. Vigilant patient monitoring is required when these methods are used.
8. There are many advantages to endotracheal intubation, which justify this technique even in patients anesthetized only with injectable drugs.
9. Successful and safe endotracheal intubation requires careful preparation, excellent technique, and watchfulness for potential complications.
10. The anesthetic period can be divided into preanesthesia, induction, maintenance, and recovery. Traditionally, anesthetic depth has been described in terms of stages and planes of anesthesia, with stage III, plane 2, being suitable for most surgical procedures.
11. Patient positioning, comfort, and safety must be considered during anesthetic maintenance to avoid problems such as traumatic injury, accidental overdose, airway blockage, hypoxemia, circulatory compromise, burns, hypostatic congestion, and corneal drying.
12. The length of the recovery period depends on many factors, including the anesthetic protocol and the patient’s condition. Return to consciousness is accompanied by increasing heart and respiratory rates, increased reflex responses, and voluntary movement.
13. During the recovery period, the anesthetist must continue to monitor the patient’s vital signs, particularly temperature, as anesthetic complications commonly occur during this period. It is often helpful to administer oxygen for several minutes after the anesthetic has been discontinued.
14. In dogs extubation should occur when the swallowing reflex returns. In cats extubation should occur when the patient shows signs of impending arousal, such as voluntary movements, swallowing, or active reflexes.
15. Other duties during recovery include stimulation of the patient, administration of oxygen, postoperative analgesia, and general nursing care.
16. Anesthetic safety is improved through the use of preanesthetic agents, use of the minimum effective dosages, selection of a protocol well suited to the patient, and close monitoring of the patient.
REVIEW QUESTIONS
1. Consider the following scenario: You are performing a procedure on a canine patient that you feel is at an appropriate anesthetic depth. Which of the following settings would be most appropriate if using sevoflurane in this patient?
2. Which of the following statements about how to induce anesthesia in a young healthy patient using an IV agent such as thiopental or propofol is least accurate?
a. If, after giving the initial amount, the patient is not adequately anesthetized to allow intubation, give the rest of the calculated dose.
b. Draw up the calculated dose, give about ¼ to ½ first, and give the rest to effect.
3. Which of the following statements regarding IV induction agents is least accurate?
a. Ketamine-diazepam takes slightly longer to act and lasts somewhat longer than propofol.
b. When using propofol, give about 25% to 50% of the calculated dose every 30 seconds to effect.
c. Significantly lower doses of thiopental sodium must be used in sick, old, or debilitated patients.
d. Thiopental sodium is generally given intravenously but can be given intramuscularly in uncooperative patients.
4. Which of the following barbiturates can be used to maintain anesthesia without prolonging recovery?
5. Which of the following statements about thiopental sodium is least accurate?
a. It is likely to cause tissue necrosis if injected outside of the vein.
b. Due to the patient’s age and condition, you may have to give less of the drug.
c. It commonly induces seizures during recovery.
d. It is more likely than propofol to depress respirations in neonates.
e. It is more likely than propofol to cause cardiac arrhythmias such as bigeminy and PVCs.
6. Which of the following statements concerning endotracheal intubation is false?
a. Choosing a tube that is too short may cause increased mechanical dead space.
b. Choosing a tube that is too small may result in increased resistance to breathing.
c. Choosing a tube that is too long may result in hypoxemia.
d. Failure to cuff the tube may result in aspiration of foreign material.
7. Which of the following is an indicator that the endotracheal tube has been placed in the esophagus instead of the trachea?
a. The patient coughs when the tube is placed.
b. The unidirectional valves don’t move.
c. The pressure manometer indicates 0 to 2 cm H2O while the patient is spontaneously breathing.
8. The term atelectasis refers to:
a. Increased fluid in the alveoli
b. Hyperinflation of the alveoli
9. After an anesthetic procedure, when is it best to extubate a dog?
a. Right after you turn off the vaporizer
b. About 10 minutes after turning off the vaporizer
10. Hypostatic congestion may be present at the end of the anesthetic protocol. This term refers to the:
a. Accumulation of mucus in the trachea
b. Pooling of blood in the dependent lung
c. Leakage of fluid into the chest
d. Pooling of ingesta in one area of the gastrointestinal tract
11. Which of the following protocols would result in the fastest induction time and the best control over anesthetic depth?
a. IM induction and maintenance
b. IV induction with an ultra–short-acting agent, and maintenance with bolus injections of the same
c. Inhalant induction and maintenance
d. IV induction with an ultra–short-acting agent, and maintenance with an inhalant
12. Mask inductions in small animal patients are:
a. Easier for the anesthetist than IV inductions
b. Less likely to cause cardiac arrhythmias and hypotension than IV inductions
c. An excellent technique for brachycephalic breeds
d. Possible only with use of inhalant agents with a low solubility coefficient
13. Which of the following techniques are used most often in most general small animal practices?
a. General anesthesia and sedation
b. Sedatives and local techniques
14. An 18-kg adult dog of normal conformation would likely require a __________ endotracheal tube.
The following questions may have more than one correct answer. Select all that apply.
15. An endotracheal tube is used to:
16. Problems associated with endotracheal intubation include:
17. During anesthetic maintenance:
a. The patient should be supported as it loses consciousness
b. The patient should be placed on an electric heating pad to prevent hypothermia
c. If a patient has one diseased lung, it should be positioned with the diseased side down
d. The anesthetist should avoid any more than a 15-degree elevation of the rear quarters
18. The length of the anesthetic recovery period may be influenced by:
19. Which of the following is/are not a normal sign(s) of recovery?
20. Which of the following actions is appropriate during anesthetic recovery?