Chapter 3 Therapeutic modalities
Introduction
Therapeutics in its broadest sense covers all types of intervention aimed at alleviating the effects of disease. The term ‘therapeutics’ generally relates to procedures based on accepted principles of medical science, that is, on ‘conventional’ rather than ‘alternative’ medical practice.
The account of drug discovery presented in this book relates exclusively to conventional medicine – and for this we make no apology – but it needs to be realized that the therapeutic landscape is actually much broader, and includes many non-pharmacological procedures in the domain of conventional medicine, as well as quasi-pharmacological practices in the ‘alternative’ domain.
As discussed in Chapter 2, the desired effect of any therapeutic intervention is to improve symptoms or prognosis or both. From a pathological point of view, therapeutic interventions may be directed at disease prevention, alleviation of the effects of existing disease, or permanent cure (i.e. restoration to a state of function and prognosis equivalent to those of a healthy individual of the same age, without the need for continuing therapeutic intervention). In practice, there are relatively few truly curative interventions, and they are mainly confined to certain surgical procedures (e.g. removal of circumscribed tumours, fixing of broken bones) and chemotherapy of some infectious and malignant disorders. Most therapeutic interventions aim to alleviate symptoms and/or improve prognosis, and there is increasing emphasis on disease prevention as an objective.
It is important to realize that many types of intervention are carried out with therapeutic intent whose efficacy has not been rigorously tested. This includes not only the myriad alternative medical practices, but also many accepted conventional therapies for which a sound scientific basis may exist but which have not been subjected to rigorous clinical trials.
Therapeutic interventions that lie within the field of conventional medicine can be divided into the following broad categories:
• Advice and counselling (e.g. genetic counselling)
• Psychological treatments (e.g. cognitive therapies for anxiety disorders, depression, etc.)
• Dietary and nutritional treatments (e.g. gluten-free diets for coeliac disease, diabetic diets, etc.)
• Physical treatments, including surgery, radiotherapy
• Pharmacological treatments – encompassing the whole of conventional drug therapy
• Biological and biopharmaceutical treatments, a broad category including vaccination, transplantation, blood transfusion, biopharmaceuticals (see Chapters 12 and 13), in vitro fertilization, etc.
On the fringe of conventional medicine are preparations that fall into the category of ‘nutriceuticals’ or ‘cosmeceuticals’. Nutriceuticals include a range of dietary preparations, such as slimming diets, and diets supplemented with vitamins, minerals, antioxidants, unsaturated fatty acids, fibre, etc. These preparations generally have some scientific rationale, although their efficacy has not, in most cases, been established by controlled trials. They are not subject to formal regulatory approval, so long as they do not contain artificial additives other than those that have been approved for use in foods. Cosmeceuticals is a fancy name for cosmetic products similarly supplemented with substances claimed to reduce skin wrinkles, promote hair growth, etc. These products achieve very large sales, and some pharmaceutical companies have expanded their business in this direction. We do not discuss these fringe ‘ceuticals’ in this book.
Within each of the medical categories listed above lies a range of procedures: at one end of the spectrum are procedures that have been fully tried and tested and are recognized by medical authorities; at the other is outright quackery of all kinds. Somewhere between lie widely used ‘complementary’ procedures, practised in some cases under the auspices of officially recognized bodies, which have no firm scientific foundation. Here we find, among psychological treatments, hypnotherapy and analytical psychotherapy; among nutritional treatments, ‘health foods’, added vitamins, and diets claimed to avoid ill-defined food allergies; among physical treatments, acupuncture and osteopathy; among chemical treatments, homeopathy, herbalism and aromatherapy. Biological procedures lying in this grey area between scientific medicine and quackery are uncommon (and we should probably be grateful for this) – unless one counts colonic irrigation and swimming with dolphins.
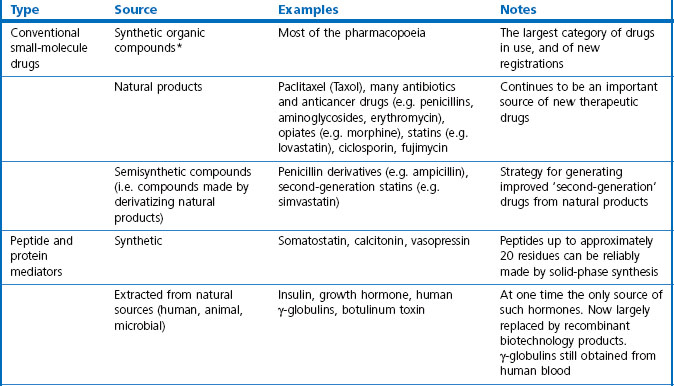
In this book we are concerned with the last two treatment categories on the list, summarized in Table 3.1, and in this chapter we consider the current status and future prospects of the three main fields; namely, ‘conventional’ therapeutic drugs, biopharmaceuticals and various biological therapies.
Conventional therapeutic drugs
Small-molecule drugs, either synthetic compounds or natural products, have for a long time been the mainstay of therapeutics and are likely to remain so, despite the rapid growth of biopharmaceuticals in recent years. For their advantages and disadvantages see Box 3.1.
Box 3.1
Advantages and disadvantages of small-molecule drugs
• ‘Chemical space’ is so vast that synthetic chemicals, according to many experts, have the potential to bind specifically to any chosen biological target: the right molecule exists; it is just a matter of finding it.
• Doctors and patients are thoroughly familiar with conventional drugs as medicines, and the many different routes of administration that are available. Clinical pharmacology in its broadest sense has become part of the knowledge base of every practising doctor, and indeed, part of everyday culture. Although sections of the public may remain suspicious of drugs, there are few who will refuse to use them when the need arises.
• Oral administration is often possible, as well as other routes where appropriate.
• From the industry perspective, small-molecule drugs make up more than three-quarters of new products registered over the past decade. Pharmaceutical companies have long experience in developing, registering, producing, packaging and marketing such products.
• Therapeutic peptides are generally straightforward to design (as Nature has done the job), and are usually non-toxic.
• As emphasized elsewhere in this book, the flow of new small-molecule drugs seems to be diminishing, despite increasing R&D expenditure.
• Side effects and toxicity remain a serious and unpredictable problem, causing failures in late development, or even after registration. One reason for this is that the selectivity of drug molecules with respect to biological targets is by no means perfect, and is in general less good than with biopharmaceuticals.
• Humans and other animals have highly developed mechanisms for eliminating foreign molecules, so drug design often has to contend with pharmacokinetic problems.
• Oral absorption is poor for many compounds. Peptides cannot be given orally.
Although the pre-eminent role of conventional small-molecule drugs may decline as biopharmaceutical products grow in importance, few doubt that they will continue to play a major role in medical treatment. New technologies described in Section 2, particularly automated chemistry, high-throughput screening and genomic approaches to target identification, have already brought about an acceleration of drug discovery, the fruits of which are only just beginning to appear. There are also high expectations that more sophisticated drug delivery systems (see Chapter 16) will allow drugs to act much more selectively where they are needed, and thus reduce the burden of side effects.
Biopharmaceuticals
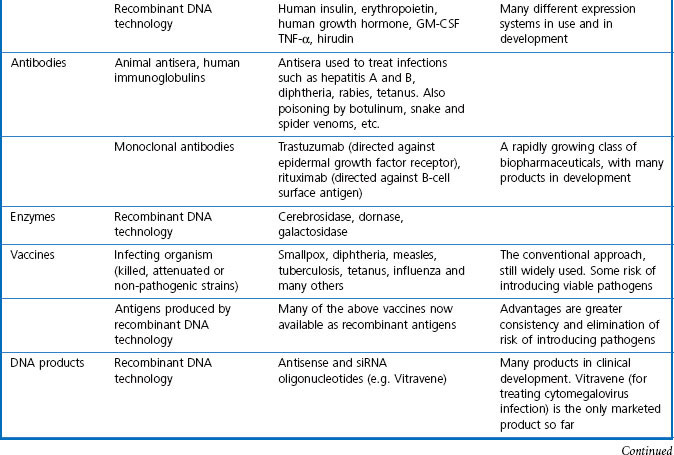
For the purposes of this book, biopharmaceuticals are therapeutic protein or nucleic acid preparations made by techniques involving recombinant DNA technology (Walsh, 2003), although smaller nucleotide assemblies are now being made using a chemical approach. Although proteins such as insulin and growth hormone, extracted from human or animal tissues, have long been used therapeutically, the era of biopharmaceuticals began in 1982 with the development by Eli Lilly of recombinant human insulin (Humulin), made by genetically engineered Escherichia coli. Recombinant human growth hormone (also produced in E. coli), erythropoietin (Epogen) and tissue plasminogen activator (tPA) made by engineered mammalian cells followed during the 1980s. This was the birth of the biopharmaceutical industry, and since then new bioengineered proteins have contributed an increasing proportion of new medicines to be registered (see Table 3.1 for some examples, and Chapters 12 and 22 for more details). The scope of protein biopharmaceuticals includes copies of endogenous mediators, blood clotting factors, enzyme preparations and monoclonal antibodies, as well as vaccines. See Box 3.2 for their advantages and disadvantages. This field has now matured to the point that we are now facing the prospect of biosimilars consequent on the expiry of the first set of important patents in 2004 and notwithstanding the difficulty of defining ‘difference’ in the biologicals space (Covic and Kuhlmann 2007).
Box 3.2
Advantages and disadvantages of biopharmaceuticals
• The main benefit offered by biopharmaceutical products is that they open up the scope of protein therapeutics, which was previously limited to proteins that could be extracted from animal or human sources.
• The discovery process for new biopharmaceuticals is often quicker and more straightforward than with synthetic compounds, as screening and lead optimization are not required.
• Unexpected toxicity is less common than with synthetic molecules.
• The risk of immune responses to non-human proteins – a problem with porcine or bovine insulins – is avoided by expressing the human sequence.
• The risk of transmitting virus or prion infections is avoided.
• Producing biopharmaceuticals on a commercial scale is expensive, requiring complex purification and quality control procedures.
• The products are not orally active and often have short plasma half-lives, so special delivery systems may be required, adding further to costs. Like other proteins, biopharmaceutical products do not cross the blood–brain barrier.
• For the above reasons, development generally costs more and takes longer, than it does for synthetic drugs.
• Many biopharmaceuticals are species specific in their effects, making tests of efficacy in animal models difficult or impossible.
Immunization against infectious diseases dates from 1796, when Jenner first immunized patients against smallpox by infecting them with the relatively harmless cowpox. Many other immunization procedures were developed in the 19th century, and from the 20th century onwards pharmaceutical companies began producing standardized versions of the antigens, often the attenuated or modified organisms themselves, as well as antisera which would give immediate passive protection against disease organisms. Vaccines and immune approaches to controlling disease are still a major concern, and increasingly biotechnology-derived vaccines are being developed to improve the efficacy of, and reduce the risks associated with, preparations made from infectious material.
Overall, biopharmaceuticals offer great promise for the future, and rapid progress is being made in the technologies used to produce them (Scrip Report, 2001; Covic and Kuhlmann 2007). Currently, nearly all approved biopharmaceuticals are proteins, the majority being copies of endogenous mediators, monoclonal antibodies or vaccines. Many of the clinically useful hormones and mediators that we currently know about have already been produced as biopharmaceuticals, but future advances are rapidly being made as basic research discovers new protein signalling mechanisms. Monoclonal antibodies and antibody mimicking scaffold proteins (see Chapter 13) offer much broader possibilities, and progress is being facilitated by identifying the genes for important functional proteins, such as key enzymes, transporters, etc. Once the DNA sequence of a putative target is known, its amino acid sequence can be inferred and an antibody or antibody-mimetic protein can be produced, even if the target protein is of such low abundance that it cannot be isolated biochemically.
Following the wave of successes by the biotechnology industry in producing biopharmaceuticals such as human insulin, erythropoietin and growth hormone during the 1980s and 1990s, medical biotechnology expanded into many other fields, including the development of therapeutic modalities beyond therapeutic proteins and antibodies. Next we briefly discuss two important developments still in the experimental phase, namely gene-based and cell-based therapies, which are under very active investigation.
Gene therapy
Recombinant DNA technology offers the promise of altering the genetic material of cells and thereby correcting the results of genetic defects, whether inherited or acquired. The techniques for manipulating cellular DNA that underpin much of modern molecular biology have great versatility, and can in principle be applied to therapeutic as well as experimental endeavours. Even where the genetic basis of the disease is not well understood, it should be possible to counteract its effects by genetic, as distinct from pharmacological, means. Further technical information about gene therapy is given in Chapter 12, and in reference works such as Meager (1999), Templeton and Lasic (2000), Kresina (2001), Brooks (2002) and Sheridan (2011). Gene therapy has been actively investigated for more than two decades, and many clinical trials have been performed. So far, however, the results have proved disappointing, and there are currently (at the time of publishing) no gene therapy products approved for clinical use, although many clinical trials are still in progress (Sheridan 2011).
The most widely investigated approach involves introducing new genes to replace missing or dysfunctional ones; this is most commonly done by engineering the new gene into a modified virus (the vector), which has the ability to enter the host cell, causing expression of the artificially introduced gene until the cell dies or expels the foreign DNA. Such non-integrated DNA is usually eliminated quite quickly and is not passed on to the cell’s progeny, and so this type of transfection is generally only appropriate in situations where transient expression is all that is required. Retroviral vectors are able to incorporate the new DNA into the host cell’s chromosomes, where it will remain and be expressed during the lifetime of the cell and will be passed on to any progeny of that cell. More elaborate gene therapy protocols for treating single-gene disorders are designed actually to correct the disease-producing sequence mutation in the host genome, or to alter gene expression so as to silence dysfunctional genes.
At one time gene therapy directed at germline cells was considered a possibility, the advantage being that an inherited gene defect could be prevented from affecting progeny, and effectively eliminated for good. The serious risks and ethical objections to such human genetic engineering, however, have led to a worldwide ban on germ-cell gene-therapy experiments, and efforts are restricted to somatic cell treatments.
How much impact has gene therapy had so far as a therapeutic approach, and what can be expected of it in the future? The first successful trial of gene therapy to be reported was by Anderson and colleagues, who used it in 1990 to replace the dysfunctional gene for the enzyme adenosine deaminase (ADA). ADA deficiency causes severe combined immunodeficiency syndrome (SCID), a rare condition which prevents the normal immune response to pathogens, and means that the child can only survive in a germ-free environment. This first gene-therapy trial was successful in partly restoring ADA function, but by no means curative. Hundreds of clinical trials were performed during the 1990s, mainly in three clinical areas, namely cancer, AIDS and single-gene inherited disorders such as cystic fibrosis, haemophilia and SCID. Most of these used viral vectors to deliver the DNA, though some used liposome-packaged DNA or other non-viral vectors for this purpose. The genetic material was delivered systemically in some cases, by intravenous or subcutaneous injection; in other cases it was injected directly into solid tumours. An alternative strategy was to harvest bone marrow cells from the patient, transfect these with the necessary DNA construct ex vivo, and return them to the patient so that the genetically modified cells would recolonize the bone marrow and provide the required protein. These techniques had been extensively worked out in laboratory animals, but the clinical results were uniformly disappointing, mainly because transfection rates were too low and expression was too transient. Repeat administration of viral vectors often elicited an immune response which inactivated the vector. So the very high expectation in the early 1990s that gene therapy would revolutionize treatment in many areas of medicine, from arthritis to mental illness, quickly gave way to much more guarded optimism, and in some cases pessimistic dismissal of the whole concept. There were, however, a few cases in which SCID in children was successfully – and apparently permanently – cured by gene therapy, and there were other trials in haemophilia and certain cancers where results looked promising. Alarm bells sounded, first in 1999 when a teenager, Jesse Gelsinger, who was participating in a gene therapy trial in Philadelphia, developed an intense immunological reaction and suddenly died 4 days after treatment. Official scrutiny uncovered many other cases of adverse reactions that had not been reported as they should have been. Many ongoing trials were halted, and much tighter controls were imposed. Subsequently, in 2000, immune function was successfully restored in 18 SCID children, 17 of whom were alive 5 years later (the first therapeutic success for human gene therapy), but two later developed leukaemia, thought to be because integration of the retroviral transgene occurred in a way that activated a cancer-promoting gene, raising even more serious concerns about the long-term side effects of gene therapy.
In the much more cautious atmosphere now prevailing, some carefully controlled trials are beginning to give positive results, mainly in the treatment of haemophilia, but overall, the general view is that gene therapy, while showing great theoretical potential, has so far proved disappointing in its clinical efficacy, amid concerns about its long-term safety and ongoing problems in designing effective delivery systems (see commentaries by Cavazzana-Calvo et al., 2004; Relph et al., 2004; Sheridan, 2011). Pessimists refer to a decade of failure and note that hundreds of trials have failed so far to produce a single approved therapy. A quick survey of the literature, however, shows a profusion of laboratory studies aimed at improving the technology, and exploring many new ideas for using gene therapy in numerous conditions, ranging from transplant rejection to psychiatric disorders.
The main problems to be overcome are (a) to find delivery vectors that are efficient and selective enough to transfect most or all of the target cells without affecting other cells; (b) to produce long-lasting expression of the therapeutic gene; and (c) to avoid serious adverse effects. Additionally, a method for reversing the effect by turning the foreign gene off if things go wrong would be highly desirable, but has not so far been addressed in trials.
Antisense DNA and small interfering RNA (siRNA) have been investigated as an alternative to the DNA strategies outlined above. Antisense DNA consists of an oligonucleotide sequence complementary to part of a known mRNA sequence. The antisense DNA binds to the mRNA and, by mechanisms that are not fully understood, blocks expression very selectively, though only for as long as the antisense DNA remains in the cell. The practical problems of developing therapeutic antisense reagents are considerable, as unmodified oligonucleotides are quickly degraded in plasma and do not enter cells readily, so either chemical modification or special delivery systems such as liposomal packaging are required (see Kole et al., 2012). So far only one antisense preparation has been approved for clinical use, an oligonucleotide used to treat an ocular virus infection in AIDS patients. Ribozymes, specific mRNA sequences that inactivate genes by catalysing DNA cleavage, are being investigated as an alternative to antisense DNA, but so far none has been approved for clinical use. The recent past has seen an explosion of the evaluation of siRNAs following the award of the Nobel Prize for its discovery in 2006 and the demonstration that it can be used to silence important genes in non-human primates (Zimmerman et al., 2006). Most major pharmaceutical companies made large investments in the technology in the hope that it would have broad utility and lend itself to systemic medication against a variety of targets but it now seems that the problems of delivery of the synthetic nucleotide 23mers is a roadblock that is very hard to overcome and most of the early clinical trials currently in progress are using direct injection to the target tissues (Ledford, 2010).
In addition to their chequered clinical trials history, gene therapy products share with other biopharmaceuticals many features that cause major pharmaceutical companies to shy away from investing heavily in such products. The reagents are large molecules, or viruses, that have to be delivered to the appropriate sites in tissues, often to particular cells and with high efficiency. Supplying gene therapy reagents via the bloodstream is only effective for luminal vascular targets, and topical administration is usually needed. Viral vectors do not spread far from the site of injection, nor do they infect all cell types. The vectors have their separate toxicology issues. Commercial production, quality control, formulation and delivery often present problems.
In summary, the theoretical potential of gene therapy is enormous, and the ingenuity being applied to making it work is very impressive. Still, after 30 years of intense research effort no product has been developed, and many of the fundamental problems in delivering genes effectively and controllably still seem far from solution. Most likely, a few effective products for a few specific diseases will be developed and marketed in the next few years, and this trickle will probably grow until gene therapy makes a significant contribution to mainstream therapeutics. Whether it will grow eventually to a flood that supplants much of conventional therapeutics, or whether it will remain hampered by technical problems, nobody can say at this stage. In the foreseeable future, gene therapy is likely to gain acceptance as a useful adjunct to conventional chemotherapy for cancer and viral infections, particularly AIDS. The Holy Grail of a cure for inherited diseases such as cystic fibrosis still seems some way off.
Cell-based therapies
Cell-replacement therapies offer the possibility of effective treatment for various kinds of degenerative disease, and much hope currently rests on the potential uses of stem cells, which are undifferentiated progenitor cells that can be maintained in tissue culture and, by the application of appropriate growth factors, be induced to differentiate into functional cells of various kinds. Their ability to divide in culture means that the stock of cells can be expanded as required (see Atala et al., 2011; Lanza et al., 2007).
Autologous cell grafts (i.e. returning treated cells to the same individual) are quite widely used for treating leukaemias and similar malignancies of bone marrow cells. A sample of the patient’s bone marrow is taken, cleansed of malignant cells, expanded, and returned to colonize the bone marrow after the patient has been treated with high-dose chemotherapy or radiotherapy to eradicate all resident bone marrow cells. Bone marrow is particularly suitable for this kind of therapy because it is rich in stem cells, and can be recolonized with ‘clean’ cells injected into the bloodstream.
Apart from this established procedure for treating bone marrow malignancies, only two cell-based therapeutic products have so far gained FDA approval: preparations of autologous chondrocytes used to repair cartilage defects, and autologous keratinocytes, used for treating burns. Other potential applications which have been the focus of much experimental work are reviewed by Fodor (2003). They include:
• Neuronal cells injected into the brain (Isaacson, 2003) to treat neurodegenerative diseases such as Parkinson’s disease (loss of dopaminergic neurons), amyotrophic lateral sclerosis (loss of cholinergic neurons) and Huntington’s disease (loss of GABA neurons)
• Insulin-secreting cells to treat insulin-dependent diabetes mellitus
• Cardiac muscle cells to restore function after myocardial infarction.
The major obstacle to further development of such cell-based therapies is that the use of embryonic tissues – the preferred source of stem cells – is severely restricted for ethical reasons. Although stem cells can be harvested from adult tissues and organs, they are less satisfactory. Like gene therapy, cell-based therapeutics could in principle have many important applications, and the technical problems that currently stand in the way are the subject of intensive research efforts. Biotechnology companies are active in developing the necessary tools and reagents that are likely to be needed to select and prepare cells for transplantation.
Tissue and organ transplantation
Transplantation of human organs, such as heart, liver, kidneys and corneas, is of course a well established procedure, many of the problems of rejection having been largely solved by the use of immunosuppressant drugs such as ciclosporin and fujimycin. Better techniques for preventing rejection, including procedures based on gene therapy, are likely to be developed, but the main obstacle remains the limited supply of healthy human organs, and there is little reason to think that this will change in the foreseeable future. The possibility of xenotransplantation – the use of non-human organs, usually from pigs – has received much attention. Cross-species transplants are normally rejected within minutes by a process known as hyperacute rejection. Transgenic pigs whose organs are rendered resistant to hyperacute rejection have been produced, but trials in humans have so far been ruled out because of the risk of introducing pig retroviruses into humans. Despite much discussion and arguments on both sides, there is no sign of this embargo being lifted. Organ transplantation requires such a high degree of organization to get the correct matched organs to the right patients at the right time, as well as advanced surgical and follow-up resources, that it will remain an option only for the privileged minority.
Building two- (e.g. skin) and three-dimensional (e.g. a heart valve) structures that are intended to function mechanically, either from host cells or from banked, certified primordial or stem cell populations, is at the cutting edge of tissue engineering efforts. The aim is to fashion these tissues and organ parts around artificial scaffold materials, and to do this in culture under the control of appropriate growth and differentiation factors. The development of biocompatible scaffolding materials, and achieving the right growth conditions, are problems where much remains to be done. Artificial skin preparations recently became available and others will probably follow.
Also in an early, albeit encouraging, state of development are bionic devices – the integration of mechanical and electronic prostheses with the human body – which will go beyond what has already been accomplished with devices such as cochlear implants and neurally controlled limb prostheses. The role of the major pharmaceutical companies in this highly technological area is likely to be small. The economic realities of the relatively small patient populations will most likely be the limiting factor governing the full implementation of integrated bionics.
Summary
Small organic molecule drugs of molecular weight <500 Da are the preferred therapeutic modality of the major pharmaceutical companies for most disease applications but the landscape is now changing and it has been predicted that by 2014 the most important therapies for treating human diseases will be biologicals (Reuters Factbox, 2010). The development over the years of large, chemically diverse small-molecule libraries, many already with ‘drug-like’ properties (see Chapters 8 and 9) built into their structure, reinforces the commitment of the industry to this approach. However, protein and peptide therapeutics also have their place in the pharmaceutical armamentarium, especially with respect to the immune system and hormonal dysregulation. Many pharmaceutical companies began with immune antisera and vaccines, but the first specialized biotechnology companies took advantage of recombinant DNA methods to produce therapeutic proteins. Although the major pharmaceutical companies have almost all adopted protein therapeutics in addition to their traditional small molecule approach, many of the advances in the field have been made by specialist biotechnology companies.
Protein- and DNA- and RNA-based biopharmaceuticals often face difficult pharmacokinetic problems, in particular poor absorption, rapid degradation, and inability to enter cells or cross the blood–brain barrier. Their successful development therefore often depends on developing suitable delivery systems that help to overcome these problems. For this reason (and also to improve the performance of conventional therapeutic drugs) drug delivery technology (see Chapter 16) is currently receiving a great deal of attention, with many new polymer- and liposome-based formulations being invented and tested. The right delivery system is as necessary as the right drug, and for biopharmaceuticals the two will generally need to be developed in tandem, rather than first developing a compound and then optimizing the delivery system (which is the development strategy usually adopted for small-molecule drugs).
Somatic (non-germline) gene therapy initially was thought to have great promise for curing inborn errors that lead to disease. More than 30 years later, although the technology and our understanding have greatly improved, clinical success has proved elusive. Optimizing vectors and delivery systems so as to produce long-lasting gene expression in the tissues where it is needed has proved much more difficult than expected. Nevertheless, there is reason for optimism in the long term. Currently, gene therapy development is being directed mainly at life-threatening disorders such as cancer, AIDs and haemophilia, where the need is greatest and the risks are balanced by the severity of the diseases. It is likely to be another decade or two before gene therapy begins to make a broader clinical impact.
The involvement of pharmaceutical companies in the transplantation field is largely confined to improving the immunosuppressant drugs that are needed to protect transplants from immune rejection. The use of transplants is severely restricted by the availability of human organs, and hopes for improving the situation by the use of xenografts are unlikely to be realized in the foreseeable future. Stem-cell technologies are likely to be used successfully for certain kinds of tissue repair and cell replacement; biotechnology companies, rather than pharmaceutical companies, are likely to make the running in these new fields although some large firms are establishing departments of regenerative medicine. Currently, techniques such as bone marrow transplants are being developed and used successfully by clinical teams without any necessary input from commercial research. Probably their use will become more routine, but it seems unlikely that the market size for commercial products in this area will be enough for a large pharmaceutical company.
Atala A, Lanza R, Thomson JA, Nerem R. Principles of regenerative medicine, 2nd ed, New York: Academic Press, 2011.
Brooks G, ed. Gene therapy: the use of DNA as a drug. New York: John Wiley and Sons, 2002.
Cavazzana-Calvo M, Thrasher A, Mavilio F. The future of gene therapy. Nature. 2004;427:779–781.
Covic A, Kuhlmann MK. Biosimilars: recent developments. International Urology and Nephrology. 2007;39:261–266.
Fodor WL. Tissue engineering and cell based therapies, from the bench to the clinic: the potential to replace, repair and regenerate. Reproductive Biology and Endocrinology. 2003;1:102–107.
Isaacson O. The production and use of cells as therapeutic agents in neurodegenerative diseases. Lancet Neurology. 2003;2:417–424.
Kole R, Krainer AR, Altman S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nature Rev Drug Discov. 2012;11:125–140.
Kresina TF, ed. An introduction to molecular medicine and gene therapy. New York: John Wiley and Sons, 2001.
Lanza R, Langer R, Vacanti JP. Principles of tissue engineering. New York: Academic Press, 2007.
Ledford H. Drug giants turn their backs on RNA interference Nature. 2010;468:487.
Meager A. Gene therapy technologies: applications and regulations from laboratory to clinic. Chichester: John Wiley and Sons; 1999.
Relph K, Harrington K, Pandha H. Recent developments and current status of gene therapy using viral vectors in the United Kingdom. British Medical Journal. 2004;329:839–842.
Reuters Factbox. Apr 13th 2010 Worlds top 2014 vs 2010. www.reuters.com/article/2010/04/13.
Scrip Report. (2001) Biopharmaceuticals: a new era of discovery in the biotechnology revolution.
Sheridan C. Gene therapy finds its niche. Nature Biotechnology. 2011;29:121–128.
Templeton NS, Lasic DD. Gene therapy: therapeutic mechanisms and strategies. New York: Marcel Dekker; 2000.
Walsh G. Biopharmaceuticals, 2nd ed. Chichester: John Wiley and Sons Ltd; 2003.
Zimmerman TS, Lee ACH, Akinc A, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114.