Chapter 83Skeletal Muscle and Lameness
Diagnosis of Specific Muscle Disorders in the Horse
Diagnosis of a particular muscle disorder is best accomplished with a thorough neuromuscular examination. The key components of the examination include the following.
History
A history of stiffness, muscle cramping, pain, muscle fasciculations, exercise intolerance, undiagnosed lameness, weakness, or muscle atrophy may all indicate a muscle disorder. Further characterization requires a detailed account of the horse’s performance level, exercise schedule, previous lameness, diet, vaccination history, signs of respiratory disease, duration, severity and frequency of muscle problem, any factors that initiate the muscle problem, and all medications with which the horse is being treated.
Physical Examination
A detailed evaluation of the muscular system includes inspection of the horse for symmetry of muscle mass while standing with forelimbs and hindlimbs exactly square. Any evidence of fine tremors or fasciculations should be noted before palpating the horse. Horses originating in the southwestern United States that have muscle pain and fasciculations should have their ears examined with an otoscope for ear ticks (Otobius megnini).1 The entire muscle mass of the horse should be palpated for heat, pain, swelling, or atrophy comparing contralateral muscle groups. Firm, deep palpation of the lumbar, gluteal, and semimembranosus and semitendinosus muscles may reveal pain, cramps, or fibrosis. The triceps, pectoral, gluteal, and semitendinosus muscles should be tapped with a fist or percussion hammer and observed for a prolonged contracture suggestive of myotonia. Running a blunt instrument such as artery forceps, a needle cap, or a pen over the lumbar and gluteal muscles should illicit extension (swayback), followed by flexion (hogback) in healthy horses. Guarding against movement may reflect abnormalities in the pelvic or thoracolumbar muscles, or pain associated with the thoracolumbar spine (see Chapter 52) or sacroiliac joints (see Chapter 51). The horse should be observed at the walk and the trot for any gait abnormalities, and some horses should be ridden.
Ancillary Diagnostic Tests
Muscle Enzymes
Skeletal muscle necrosis may be identified by determining the activity in blood of serum enzymes or proteins that are normally present in high concentration within intact muscle cells but leak out into the bloodstream following cell damage. Three enzymes are used routinely to assess muscle necrosis: creatine kinase (CK), aspartate transaminase (AST), and lactate dehydrogenase (LDH). Serum myoglobin has also been used as a marker of acute muscle necrosis.2,3 The permeability of the muscle cell membrane, rate of enzyme production, alternate tissue sources of the enzyme, and rate of enzyme excretion/degradation may also influence serum enzyme activities.
Serum Creatine Kinase
Isoforms of CK are found in skeletal muscle (MM), cardiac muscle (MB), and nervous tissue (BB). CK is a relatively low-molecular-weight protein (80,000 Da) that is intimately involved in energy production within the cell cytoplasm. It is liberated within hours of muscle damage, or increased cell membrane permeability, into the extracellular fluid and usually peaks at 4 to 6 hours after muscle injury (half-life [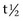 ] is 108 min).4 A threefold to fivefold increase in serum CK from normal values is believed to represent necrosis of approximately 20 g of muscle tissue.5 Rhabdomyolysis results in a proportionately greater increase in the MM isoform than the MB isoform, although some investigators disagree with the tissue specificity of serum CK isoforms in the horse.6 Limited elevations in CK (<1000 U/L; high range of normal value = 380 U/L ) may accompany training or transport.7 Extreme fatiguing exercise (e.g., endurance rides or the cross-country phase of a Three Day Event) may result in CK activities being increased to more than 1000 U/L, but usually less than 5000 U/L. Under these circumstances, serum CK activities rapidly return to baseline (i.e., <350 U/L in 24 to 48 hours). Recumbent horses also may have slightly elevated CK activities that are usually less than 3000 U/L. In contrast, more substantial elevations (from several thousand to hundreds of thousands of units per liter) in the activity of this enzyme may occur with rhabdomyolysis.3
] is 108 min).4 A threefold to fivefold increase in serum CK from normal values is believed to represent necrosis of approximately 20 g of muscle tissue.5 Rhabdomyolysis results in a proportionately greater increase in the MM isoform than the MB isoform, although some investigators disagree with the tissue specificity of serum CK isoforms in the horse.6 Limited elevations in CK (<1000 U/L; high range of normal value = 380 U/L ) may accompany training or transport.7 Extreme fatiguing exercise (e.g., endurance rides or the cross-country phase of a Three Day Event) may result in CK activities being increased to more than 1000 U/L, but usually less than 5000 U/L. Under these circumstances, serum CK activities rapidly return to baseline (i.e., <350 U/L in 24 to 48 hours). Recumbent horses also may have slightly elevated CK activities that are usually less than 3000 U/L. In contrast, more substantial elevations (from several thousand to hundreds of thousands of units per liter) in the activity of this enzyme may occur with rhabdomyolysis.3
Serum Aspartate Transaminase
Serum AST, previously known as serum glutamic-oxaloacetic acid and aspartate aminotransferase, is a larger-molecular-weight protein that has high activity in skeletal and cardiac muscle and also in liver, red blood cells, and other tissues. Elevations in AST are not specific for myonecrosis, and increases could be the result of hemolysis, muscle, liver, or other organ damage. AST activity increases more slowly in response to myonecrosis than does CK, often peaking between 12 and 24 hours after the insult. In addition, AST is cleared slowly by the reticuloendothelial system and may persist for 2 to 3 weeks after rhabdomyolysis (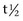 is 7 to 10 days).4,8
is 7 to 10 days).4,8
By comparing serial activities of CK and AST, information concerning the progression of myonecrosis or muscle cell membrane permeability may be derived. Elevations in CK and AST reflect relatively recent or active myonecrosis or muscle cell stress; persistently elevated serum CK indicates that myonecrosis or muscle stress is likely ongoing. Elevated AST activity accompanied by decreasing or normal CK activity indicates that myonecrosis has ceased. The degree of elevation of CK and AST does not necessarily reflect the severity of clinical signs.
Serum Lactate Dehydrogenase
LDH is a tetramer made up of combinations of the M and H subunits, with five isoenzyme forms found in various organs within the body. Electrophoretic separation suggests that the M4 (LDH5) and M3H (LDH4) isoforms are found predominantly in skeletal muscle. Elevations in LDH may be detected in horses with rhabdomyolysis, myocardial necrosis, and/or hepatic necrosis.7 Therefore concurrent measurement of serum CK is necessary to establish that rhabdomyolysis is present.
Myoglobin
Elevation in plasma/serum myoglobin concentrations indicates acute muscle damage. Myoglobin is a low-molecular-weight protein (16,500 Da) that leaks into plasma immediately after muscle damage and is rapidly cleared in the urine by the kidney. Approximately 200 g or more of muscle must be damaged before it is detectable in the urine in people.9 Normal serum concentrations in resting horses have been determined by nephelometry (range, 0 to 9 mcg/L), with measured concentrations with rhabdomyolysis ranging from 10,000 to 800,000 mcg/L.2,3
Exercise Response Test
Diagnosing chronic exertional rhabdomyolysis (ER) may be problematic in horses that do not have acute clinical signs and have normal serum AST and CK at rest. In such horses, an exercise challenge can be helpful to detect subclinical ER. In addition, quantifying the extent of rhabdomyolysis during mild exercise is helpful in deciding how rapidly to put a horse back into training. Blood samples should be taken before exercise and 4 to 6 hours after exercise to evaluate peak changes in CK. Serum CK activity measured immediately postexercise will not reflect the amount of damage occurring during the exercise test. Small fluctuations in serum CK activity may occur with exercise from enhanced muscle membrane permeability, particularly if exercise is prolonged or strenuous and the horse is untrained.10 A submaximal exercise test is often valuable for detecting rhabdomyolysis because it provides more consistent evidence of subclinical rhabdomyolysis than maximal exercise tests.3,11 Fifteen minutes of trotting is often sufficient to produce subclinical muscle damage in horses prone to chronic exertional myopathies.12 If signs of stiffness develop before this, exercise should be concluded. A normal response would be less than a threefold to fourfold increase from basal CK.
Thermography
Thermography may be useful for identification of superficial abnormal temperature changes from muscle damage but has little value in horses with deeper injuries. However, there are many potentially confusing issues such as recent removal of a rug or tack. Careful comparisons of the left and right sides should be made. Muscle inflammation is seen as an area of increased temperature in the skin directly overlying the affected muscle. The most common sites of muscle strain identified thermographically include the longissimus dorsi, the origin or body of the middle gluteal, the insertion of the gluteals on the greater and third trochanters of the femur, biceps femoris, semitendinosus, semimembranosus, and adductor muscles.13
Nuclear Scintigraphy
Nuclear scintigraphy is useful for identification of some forms of muscle damage and may alert the clinician to an area of deep muscle damage that had not been suspected based on clinical examination. In human athletes, technetium-99m stannous pyrophosphate has been used to assess the degree of skeletal muscle damage and to delineate areas of damage.14,15 It is thought that abnormal uptake of the radiopharmaceutical reflects an early stage of muscle damage from episodic ischemia, which is reversible in some fibers but may lead to muscle necrosis in others.14
Technetium-99m–methylene diphosphonate (MDP) is taken up in some damaged muscle in the horse and is best seen in the bone (delayed) phase images, that is, 3 hours after injection. Scintigraphy has been used most commonly in horses with a history of poor performance, with or without stiffness after exercise, to confirm a diagnosis of equine rhabdomyolysis.16 The mechanism of MDP binding is unknown, but the release of large amounts of calcium from damaged muscle or the exposure of calcium binding sites on protein macromolecules in the damaged muscle may be responsible. Diffuse linear areas of increased radiopharmaceutical uptake (IRU) are commonly seen in the caudal epaxial muscles and the muscles of the hindquarters and thigh in some but not all horses with ER (Figure 83-5). Less commonly there is IRU in the triceps and latissimus dorsi muscles.
The use of scintigraphy for the diagnosis of other muscle injuries has not been documented in the horse, but in one author’s experience (SJD) it can be helpful in some horses with either proximal forelimb or hindlimb muscle injuries. Uptake of the radiopharmaceutical tends to be much more focal and much less intense than in horses with rhabdomyolysis. In some, but not all, horses the region of IRU has correlated with a region of increased echogenicity identified ultrasonographically.
Ultrasonography
Diagnostic ultrasonography is potentially useful for identification of muscle trauma and fibrosis, provided that there is physical disruption of the muscle and assuming that one knows where to look. Muscles have a rather typical striated echogenic pattern,17,18 but this varies according to the muscle group, and careful comparisons must be made between similar sites in contralateral limbs, in both transverse and longitudinal images. The appearance of muscle is also sensitive to the way the horse is standing and whether the muscle is under tension; therefore it is important that the horse is standing squarely and bearing weight evenly. Muscle fascia appears as well-defined relatively echogenic bands. Care must be taken in identifying large vessels and artifacts created by them.
In an acute injury, muscle fiber disruption is seen as relatively hypoechoic areas within muscle, with loss of the normal muscle fiber striation. The jagged edge of the margin of the torn muscle may be increased in echogenicity. Tears in the muscle fascia may be identified. The muscle defect may be filled by a loculated hematoma that is slowly replaced by hypoechogenic granulation tissue. With muscle fiber repair there is a progressive increase in echogenicity. Relatively hyperechogenic regions may develop as a result of fibrous scarring, which may result in long-term gait abnormalities. Hyperechogenic regions causing shadowing artifacts reflect mineralization.
Muscle Biopsy
The routine examination of muscle biopsies has resulted in the identification of a number of specific equine myopathies. To fully characterize a neuromuscular disorder and its rate of progression, muscle fiber sizes, shapes, and fiber type distribution, mitochondrial distribution, polysaccharide staining pattern, neuromuscular junctions, nerve branches, connective tissue, and blood vessels should be examined in frozen sections using a battery of tinctorial and histochemical stains.19
A number of basic pathological responses of muscle can be identified in formalin-fixed, paraffin-embedded sections. These include inflammation, muscle fiber necrosis, muscle fiber regeneration, variations in muscle fiber sizes and shapes, alterations in the number of cell nuclei, vacuolar change, and proliferation of connective tissue. However, there are many pathological alterations that cannot be detected in formalin-fixed tissue but can readily be seen in histochemical stains of fresh-frozen biopsy samples.20 Histochemical stains of frozen tissue allow muscle fiber types to be distinguished, differentiation between neurogenic and myogenic atrophy, characterization of vacuolar storage material, characterization of inclusion bodies, and assessment of mitochondrial density. In addition, frozen samples may be used for biochemical analysis of substrate concentrations and enzyme activities, as well as DNA isolation.
When considering collection of muscle biopsies, some general guidelines are applicable. Preferably, samples should be collected from what is considered abnormal/diseased muscle. A 6-mm outer diameter (Jorgen KRUUSE A/S, Langeskov, Denmark) percutaneous needle biopsy technique can be used to obtain small muscle samples through a 1.5-cm skin incision using a local anesthetic solution subcutaneously. If this technique is used, enough muscle should be obtained to form a 1.5-cm2 sample at a minimum. However, these samples do not tolerate well shipment to an outside laboratory. The optimum biopsy for shipment of histopathological tissues to a laboratory is collected using surgical or open techniques and performed under local analgesia. Care must be exercised to infiltrate only the subcutaneous tissues, not the muscle, with the local anesthetic solution. The objective is to obtain approximately 2-cm3 of tissue; hence a suitably long skin incision is required. Subsequently two parallel incisions 2 cm apart should be made longitudinal to the muscle fibers with a scalpel. The muscle should only be handled in one corner using forceps, and care should be taken not to crush the tissue. The muscle sample is then excised by transverse incisions 2 cm apart, and the tissue is fixed appropriately.
Samples submitted for routine histopathology can be placed in formalin. Samples for histochemical analysis require fixation in isopentane (methylbutane) chilled in liquid nitrogen to ensure rapid freezing and minimization of freeze artifact. In the field, where freezing is not possible, fresh samples wrapped in gauze slightly moistened with saline can be shipped in a water-tight hard container on icepacks to specialized laboratories. Samples that potentially may be used for biochemical analysis should be immediately frozen in liquid nitrogen. Samples for electron microscopy (EM) require appropriate fixation in glutaraldehyde preparations. Ideally, thin sections of muscle for EM should be clamped in vivo to maintain fibers at a resting length before they are excised. However, if pathology other than the alignment of thick and thin myofilaments is to be investigated, small muscle pieces can be excised and placed directly in appropriate EM fixative.
Responses of strips of fresh muscle to stimuli such as caffeine, halothane, and a variety of other agents can be performed on site by specialized laboratories, but these tests are largely research tools.21,22
Electromyography
A specific diagnosis of the cause of muscle atrophy, muscle fasciculations, or myotonic dimpling after tapping the muscle can be aided by performing electromyography (EMG). EMG of normal skeletal muscle shows a brief burst of electrical activity when the needle is inserted in muscle and then quiescence, unless motor units are recruited (motor unit action potentials), or the needle is very close to a motor endplate (miniature endplate potentials). Normal muscle shows little spontaneous electrical activity unless the muscle contracts or the horse moves. Motor unit action potentials can be evaluated to assess amplitude, duration, phase, and number of phases. Myopathic changes include a decrease in duration and amplitude of motor unit action potentials.23,24 Horses with abnormalities in the electrical conduction system of muscle, or denervation of motor units, show abnormal spontaneous electrical activity in the form of fibrillation potentials, positive sharp waves, myotonic discharges, or complex repetitive discharges.
Based on the information obtained on neuromuscular examination and muscle biopsy, a diagnosis can usually be obtained. The following classification system may be helpful to narrow down rule-outs for muscle disease in horses:
Muscular Pain, Strain, and Tears
The role of muscle pain and injury in lameness and poor performance in the horse is rather poorly recognized. In human athletes, muscle fatigue, muscle stiffness, and muscle soreness are well-recognized entities, although the pathological processes in the absence of detectable structural abnormalities are not completely understood. Increased intramuscular pressure may be associated with muscle pain after prolonged vigorous exercise in human athletes.14
Delayed-onset muscular stiffness or soreness (DOMS) is recognized in people as pain that develops 24 to 48 hours after unaccustomed use of certain muscles and usually resolves spontaneously, assuming the muscles are not overworked again.25 Continued overstress may result in structural damage to myofilaments. However, specific training involving the activity that provoked the original DOMS decreases the amount of soreness associated with that condition over time.
Muscle soreness in the pectoral region after repeated jumping efforts is commonly recognized, especially in event horses several hours after completing the cross-country phase of a Three Day Event.26 It seems to improve with massage.
Muscle fiber tearing and hemorrhage can result in acute muscular pain in human athletes. A palpable defect or swelling can be detected in superficial muscles. For deeper muscles, ultrasonography is required for accurate diagnosis.
Muscle fibrosis and mineralization have been well documented in the horse following tearing of the semimembranosus and semitendinosus muscles (see Fibrotic Myopathy section, page 558), but acute lesions here and elsewhere in the limbs have been poorly documented.27-29 The use of diagnostic ultrasonography17,18 has helped in the diagnosis of both acute and more chronic muscle lesions, but diagnosis often remains a challenge because of the deep location of some affected muscles and the lack of localizing clinical signs.
In one author’s (SJD) experience, the most commonly recognized muscle injury sites in the forelimb include biceps brachii, brachiocephalicus, the pectorals, and the musculotendonous junction of the superficial digital flexor (Figures 83-1 to 83-4). In the hindlimb, semimembranosus and semitendinosus, adductor, gracilis, gluteal, and gastrocnemius muscle injuries have been recognized most frequently. Acute muscle tearing and hemorrhage can result in severe pain and lameness and other clinical signs mimicking colic. Swelling around the damaged muscle, assuming it is superficial, may not appear until 24 to 48 hours later.
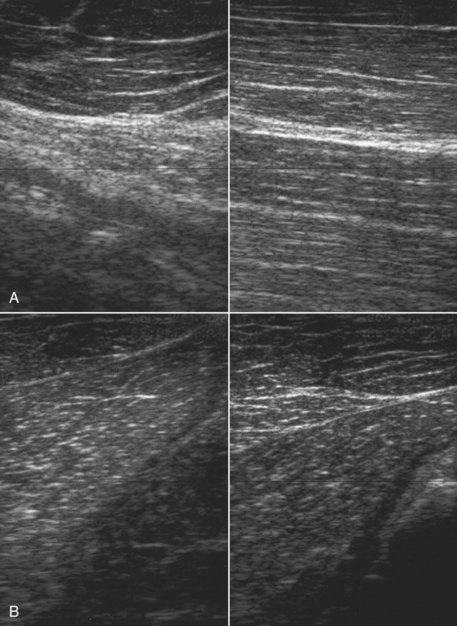
Fig. 83-1 A and B, Longitudinal ultrasonographic images of the muscles at the base of the neck on the left and right sides of an advanced-level event horse with restricted forelimb gait (left images indicate left side; right images indicate right side). The horse had severe pain and tension in the strap muscles at the base of the neck on the left side and slight muscle atrophy. Note the increased echogenicity of the deeper muscle on the left side compared with the right. The horse was treated by H-wave stimulation that resulted in progressive relief of the muscle spasm and clinically significant improvement in gait and ability to jump.
Fig. 83-2 Transverse ultrasonographic images of the right (left) and left (right) gracilis muscles of a 12-year-old Thoroughbred cross event horse with acute-onset right hindlimb lameness of 6 days’ duration, with slight swelling of the muscle, pain on palpation, and diffuse edematous swelling in the crus. At the time of lameness onset, the horse showed signs attributed to colic. A focal region of increased echogenicity in the right gracilis muscle is caused by muscle fiber tearing and hemorrhage.
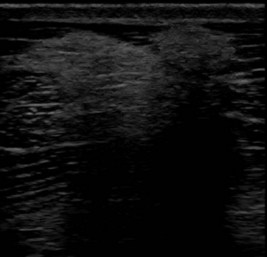
Fig. 83-3 Transverse ultrasonographic image of the left brachiocephalicus muscle of a Grand Prix dressage horse that showed left forelimb lameness only when performing lateral movements, such as half pass. The lameness was not altered by any local analgesic technique. There is a focal area of increased echogenicity, caused by muscle fibrosis, resulting in acoustic shadowing.
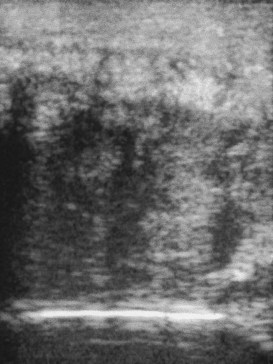
Fig. 83-4 Longitudinal ultrasonographic image of the cranial aspect of the antebrachium of a 7-year-old hunter. The horse had developed acute-onset lameness 3 months previously, associated with substantial soft tissue swelling on the cranial aspect of the antebrachium. The extensor carpi radialis muscle is enlarged greatly and has hypoechoic and hyperechoic regions, with little normal muscle architecture.
Muscle tension and spasm in the thoracolumbar region are well-documented sources of pain contributing to poor performance in association with primary hindlimb lameness, but primary muscle pain in this region has often tended to be overlooked by many veterinarians, although recognized by physiotherapists. Localized muscle soreness and the interpretation of abnormal sensitivity of acupuncture points are potentially confusing. Protective muscle spasm may also develop secondary to a primary lesion of either the thoracolumbar spine or the sacroiliac region (see Chapters 49 to 52).
Jeffcott et al30 demonstrated that injection of lactic acid into the left longissimus dorsi muscles could significantly diminish performance of Standardbred (STB) trotters worked at speed on a treadmill, although changes in gait were subtle.
One author (SJD) has seen a number of horses that had suddenly lost performance during competition or training, either following a particularly extravagant jump or after an awkward jump. The horses had subsequently become reluctant either to jump or to gallop downhill. Clinical examination revealed intense muscle spasm in the caudal thoracic and lumbar regions, with associated pain. Manipulation to release muscle spasm resulted in relief of pain and rapid restoration of normal performance. Acute back muscle pain may also be induced by a fall.
Localized back muscle soreness is readily induced by a poorly fitting saddle. It may also be caused by a rider who either sits crookedly or is unable to ride completely in balance with the horse. This may be because of the ineptitude of the rider or the shape of the saddle and the way in which it sits on a particular horse and thus positions the rider. Such muscle pain is usually localized to the saddle area and may be associated with slight soft tissue swelling. Thermographic examination may be helpful to demonstrate to an owner the associated localized inflammation. Pressure measurements can also be used (see Figure 117-6), although there is some variability in the accuracy of different commercially available systems.
Diagnosis
History and Clinical Examination
It is important to establish whether there was a history of a fall or other traumatic event, the duration of clinical signs, whether swelling was noted, and whether the horse had exhibited lameness or had performed poorly.
The detection of muscle swelling from recent trauma or loss of muscle bulk as a result of fibrosis, chronic injury, or atrophy requires the horse to stand completely squarely, bearing weight evenly on all limbs and looking straight ahead. The horse should be appraised visually and by careful, systematic palpation, looking for defects in the muscle, muscle swelling, areas of abnormal muscle firmness from fibrosis, muscle tension, or spasm, and areas of pain.
In an acute injury resulting in muscle tearing or rupture, it may be possible to palpate a defect in the very early stages, but this will become filled with hemorrhage, inflammatory exudate, and edema. Careful palpation should enable detection of most acute superficial muscle injuries, but localization of deeper muscle injury may be more difficult. Identification of chronic muscle strain is more challenging because clinical signs are more subtle. The horse must be as relaxed as possible to assess properly the response to firm and deep palpation. If the muscle is sore, the horse may react by increasing tension in anticipation of pain or by pulling away. There may be “knots” within the area of damaged muscle.
The neck, limbs, and thoracolumbar region should be moved passively to detect any limitations in movement or pain induced by movement. The horse should be observed moving at both the walk and the trot to identify any characteristic gait abnormalities, such as an abnormal hind foot placement from fibrotic myopathy (see Chapter 48) or sinking of a front fetlock as a result of rupture at the musculotendonous junction of the superficial digital flexor (see Chapter 13)![]() . However, it must be borne in mind that muscle soreness resulting in compromise of performance may not result in overt lameness because pain may only be induced when the muscle contracts strongly or is stretched maximally. Pain associated with a brachiocephalicus muscle in a dressage horse may only be evident in particular movements such as half pass (see Figure 83-3). A show jumper with sore gluteal muscles may not push off as strongly with the affected limb, resulting in the hindquarters drifting toward the ipsilateral side as the horse jumps.
. However, it must be borne in mind that muscle soreness resulting in compromise of performance may not result in overt lameness because pain may only be induced when the muscle contracts strongly or is stretched maximally. Pain associated with a brachiocephalicus muscle in a dressage horse may only be evident in particular movements such as half pass (see Figure 83-3). A show jumper with sore gluteal muscles may not push off as strongly with the affected limb, resulting in the hindquarters drifting toward the ipsilateral side as the horse jumps.
Muscle Stimulation
Muscle-stimulating machines can be helpful in the identification of superficial muscle injuries. Intermittent electrical stimulation of focal areas within a muscle results in muscle contraction and relaxation. The strength of stimulus can be varied. Horses vary in sensitivity and tolerance of the procedure, so careful comparisons must be made between the left and right sides. Damaged muscle tends to respond greater to lower stimulus strength, and contraction and relaxation are less smooth and may induce pain.
Thermography, Nuclear Scintigraphy, and Ultrasonography
Thermography, nuclear scintigraphy, and ultrasonography are discussed on pages 819 and 820.
Muscle Enzymes
Determination of serum muscle enzyme concentrations is rarely of value in identification of muscle soreness or trauma but is useful for detecting horses with rhabdomyolysis (see page 813).
Treatment
The aims of treatment include repair of damaged muscle, relief of both muscle spasm and pain, restoration of normal circulation, minimization of fibrous scar formation, and remobilization of muscles. The precise mode of treatment will depend on the type of muscle injury and the stage of injury and repair. Treatment modalities include laser,31,32 therapeutic ultrasound,31 H wave, transcutaneous electrical stimulation, electromagnetic therapy,33 massage,26 passive stretching combined with box rest, and a graduated, controlled exercise program. Relief of acute muscle spasm may require chiropractic manipulation. During return to work, the exercise program must be carefully moderated according to the site of the injury and to avoid overstress in the early stages of repair while encouraging a progressive increase in strength. These subjects are discussed more fully elsewhere (see Chapters 92 through 96).
Prevention
Because equine muscle injuries are poorly recognized there has been little work on prevention. However, there is evidence to document the beneficial effects of warm-up before strenuous exercise.34-36 Warm-up enhances blood flow to active muscle and increases muscle temperature. This results in better oxygen delivery to exercising muscle, improved enzyme function, and increased range of motion. The best warm-up program for each type of exercise remains poorly defined; however, warm-up should aim to prepare the physiological systems, without contributing to excessive heat generation or fatigue.
Exertional Rhabdomyolysis
ER has numerous etiologies and is a common complex cause of poor performance. About 3% of exercising horses had an episode of ER in the past 12 months.37 It occurs in a variety of breeds, including draft breeds, Warmbloods, Thoroughbreds (TB), STBs, Arabians, Morgans, Quarter Horses, Appaloosa, American Paint horses, and many more.37-41 In draft breeds, ER can be particularly debilitating, and terms such as Monday morning disease, azoturia, or paralytic myoglobinuria are used.42 A milder syndrome occurs in lighter breeds, and the terms tying-up, set fast, myositis, and chronic intermittent rhabdomyolysis are used to describe muscle necrosis or muscle cell stress following any form of exercise in the lighter horse breeds.43,44 Several specific causes have been identified for ER. Otherwise successful athletic horses may have a sporadic episode of ER from extrinsic causes such as dietary imbalances, concurrent respiratory infections, and inappropriate training regimens. In horses chronically afflicted with ER there may be an intrinsic dysfunction of muscle metabolism or muscle contraction.
Sporadic Exertional Rhabdomyolysis
The most common extrinsic cause of sporadic ER is exercise that exceeds the horse’s underlying state of training.44 Horses that are advanced too quickly in training, horses that are only ridden sporadically while being continually fed full rations, and horses performing strenuous exercise such as racing or endurance riding without sufficient conditioning commonly develop rhabdomyolysis. In addition, rhabdomyolysis may be more common in horses exercising during an outbreak of respiratory disease. Both equine herpesvirus–1 and equine influenza virus have been implicated as causative agents.38,45
Clinical Signs
Classically, horses lose impulsion and develop a stiff, stilted gait, particularly involving the hindquarters during exercise. There is excessive sweating and a high respiratory rate from pain. The horse may be unable to walk forward after resting because of firm painful muscle contractures involving the back and hindquarters. Signs are most commonly seen after 15 to 30 minutes of light exercise.40,46 A horse with severe rhabdomyolysis shows signs of colic, becomes recumbent, and develops occult myoglobinuria. The urine may be discolored and has an abnormal smell. Attempts to move more severely affected animals may result in extreme pain, obvious anxiety, and exacerbation of the condition.
ER is often symmetrical, involving gluteal, biceps femoris, semitendinosus, and semimembranosus muscles. Forelimbs are less commonly overtly affected. ER may accompany the exhaustion syndrome in endurance horses with concurrent evidence of a rapid heart rate, dehydration, hyperthermia, synchronous diaphragmatic flutter, and collapse.47 Muscle contractures are not always consistent in either endurance horses or event horses with ER.
Diagnosis is usually obvious based on the clinical signs, but measurement of serum muscle enzyme activities provides an assessment of the severity of muscle damage and confirms the diagnosis. The degree of elevation of muscle enzyme activity does not necessarily reflect the severity of clinical signs.
Treatment of Acute Rhabdomyolysis
If an attack has occurred during exercise some distance from where the horse is normally stabled, the horse should not be made to walk home. It should be transported back home or left in a nearby stable. If an attack has occurred at a competition, the horse should be treated there and should not be transported home over a long distance until at least 24 to 48 hours later.
The objectives of treatment are to relieve anxiety and muscle pain, as well as to correct fluid and acid-base deficits. Acetylpromazine (0.04 to 0.07 mg/kg), an α-adrenergic antagonist, is helpful in relieving anxiety and may increase muscle blood flow. Its use is contraindicated in dehydrated horses. Alternatively, xylazine (0.4 to 0.8 mg/kg) may provide short-term relief from anxiety. In horses with extreme pain, detomidine (0.02 to 0.04 mcg/kg) combined with butorphanol (0.01 to 0.04 mg/kg) provides excellent sedation and analgesia. Nonsteroidal antiinflammatory drugs (NSAIDs) such as ketoprofen (2.2 mg/kg), phenylbutazone (2.2 to 4.4 mg/kg), or flunixin meglumine (1.1 mg/kg) provide additional pain relief. Analgesic treatment is continued to effect, but most horses are relatively pain free within 18 to 24 hours.
Intravenous or intragastric dimethyl sulfoxide (as a <20% solution) can be used as an antioxidant, antiinflammatory, and osmotic diuretic in severely affected horses. Corticosteroid administration is advocated by some veterinarians in the acute stage. If the horse is recumbent, methyl prednisolone succinate (2 to 4 mg/kg intravenously [IV]) should be given once. Muscle relaxants such as methocarbamol (5 to 22 mg/kg, IV slowly) seem to produce variable results, possibly depending on the dosage used. The administration of dantrium sodium (2 to 4 mg/kg orally [PO]) in severely affected horses may decrease muscle contractures and possibly prevent further activation of muscle necrosis. This can be repeated in 4 to 6 hours.
Severe ER can lead to renal compromise from ischemia and the combined nephrotoxic effects of myoglobinuria, dehydration, and NSAIDs. The first priority in horses with hemoconcentration, or myoglobinuria, is to reestablish fluid balance and induce diuresis. In horses with mild rhabdomyolysis, administration of fluids via a nasogastric tube may be adequate, but generally fluids are better given intravenously. Balanced polyionic electrolyte solutions are best. If severe ER is present, then isotonic saline or 2.5% dextrose in 0.45% saline may be necessary because horses often have hyponatremia, hypochloremia, and hyperkalemia. If hypocalcemia is present, then supplementing intravenous fluids with 100 to 200 mL of 24% calcium borogluconate is recommended, but serum calcium should not exceed a low normal range. Affected horses are usually alkalotic, making bicarbonate therapy inappropriate.48
Ten liters of fluids may be given rapidly. The total fluid replacement is based on an estimation of the degree of dehydration and the clinical response: if the horse is mildly dehydrated (5%), give 10 L fast and then 15 L over the next 4 to 6 hours; if dehydration is severe (20%), give 10 L fast and then 50 L at 4 L/h. If the horse is recumbent, consider using at least two intravenous giving sets and infusing into both the jugular and the cephalic veins. Suture the catheters in place.
Ideally, reassessment of the packed cell volume and concentrations of total plasma protein and serum electrolytes after the initial period of therapy should provide a successful guide for the therapeutic regimen. However, in the practical situation, the clinical response to therapy is usually an adequate indicator. In severely affected horses, regular monitoring of blood urea nitrogen and/or serum creatinine is advised to assess the extent of renal damage. Diuretics are usually contraindicated unless the horse is in oliguric renal failure.
Horses should be stall rested on a hay diet for a few days. Small paddock turnout in a quiet area for a few hours twice a day is then helpful. Horses may be handwalked at this time but not for more than 5 to 10 minutes at a time. For horses with extrinsic (sporadic) ER, rest with regular access to a paddock should continue until serum muscle enzyme activities are normal. Training should be resumed gradually, and a regular exercise schedule that matches the degree of exertion to the horse’s underlying state of training should be established. Avoid lunging exercise until the horse is back in normal work. If the horse has a day or several days off, the dietary energy concentrations should be reduced accordingly.
Chronic Exertional Rhabdomyolysis
Horses that have repeated episodes of ER from a young age, or from the time of purchase, or when they are put back into training after a long period of rest may have a chronic dietary imbalance or underlying intrinsic abnormality of muscle function. Chronic forms of ER are seen in many breeds of horses, including draft horses, Warmbloods, Quarter Horses, American Paint horses, Appaloosas, TBs, Arabians, STBs, Morgans, and crossbreds.39,44,49-55 Many of the horses with intrinsic muscle defects will have repeated episodes of rhabdomyolysis with minimal exercise, even when the dietary and training recommendations for sporadic ER are followed. Three specific intrinsic causes of ER have been identified to date: recurrent exertional rhabdomyolysis (RER),56 type 1 polysaccharide storage myopathy (PSSM),57,58 and type 2 PSSM.59 It is likely that there are other specific causes that have yet to be identified (idiopathic chronic ER). In all these intrinsic forms of chronic ER, it appears that there are specific environmental stimuli that are necessary to trigger muscle necrosis in genetically susceptible animals.46,56 Horses cannot be cured of a susceptibility to this condition, but if the specific disease is identified, changes in management can be implemented to minimize episodes of rhabdomyolysis.
Chronic Dietary Imbalance
Horses consuming a high-grain diet appear to be more likely to develop ER than horses fed a low-grain or fat-supplemented diet.46,56,60 The grain itself may not be responsible for rhabdomyolysis; however, high starch intake may trigger rhabdomyolysis in horses with particular myopathies such as RER and PSSM.
Electrolyte depletion in horses can occur from dietary deficiency and losses in sweat with strenuous exercise. Sodium, potassium, magnesium, and calcium play key roles in muscle fiber contractility. With severe acute electrolyte depletion, such as that found after endurance exercise, serum electrolytes may be below normal ranges.47 With chronic dietary depletion, however, serum concentrations may not reflect total body electrolyte imbalances.61,62 Work by Harris et al61,62 established renal fractional excretions as a technique to evaluate electrolyte concentrations in horses with chronic ER. Blood and urine samples are obtained concurrently, and creatinine and electrolyte concentrations are measured in both. [Serum creatinine]/[urine creatinine] multiplied by the reciprocal for urine and serum electrolyte concentrations × 100 provides the fractional excretion of a particular electrolyte. It can be difficult to obtain consistently representative renal fractional excretions of electrolytes in horses from catheterized samples.63 In the United Kingdom, a number of horses with chronic ER had low fractional excretions of sodium, and daily dietary supplementation of 60 g (2 oz) of NaCl resulted in abatement of clinical signs. Other horses had high phosphorus excretion, suggesting a dietary calcium/phosphorus imbalance, and decreasing bran while providing a daily calcium supplement (60 g of CaCO3) was helpful in reducing clinical signs of ER.62 Hypokalemia was suggested to play a role in chronic ER, although it is not a common finding in horses consuming adequate quantities of forage.64,65 Supplementation with good quality forage or 30 g of KCl/day (“lite” salt) is recommended for horses with low renal fraction excretion of potassium.
Another postulated cause of ER is the increased generation of free radicals from oxidative metabolism associated with exercise. Selenium, acting via the enzyme glutathione peroxidase, and vitamin E, acting within the lipid component of cell membranes, scavenge free radicals and prevent lipid peroxidation of cell membranes. Primary selenium deficiency is common in young horses living in areas with selenium-deficient soil; however, it has rarely been demonstrated as a cause of ER.40 In fact, many racehorses with chronic ER have higher concentrations of selenium and vitamin E from zealous dietary supplementation by the owner.66 Riding horses, however, that are not on green pasture for 2 or more years may develop a deficiency of vitamin E, which could contribute to muscle soreness. It is not known whether horses that experience repeated episodes of ER may generate more free radicals than normal horses. A higher generation of free radicals in horses with chronic ER may explain the perceived benefit of repeated administration of selenium and vitamin E in TB horses with chronic ER.67 Adequate values are more than 0.07 mcg/mL for blood selenium and more than 2.0 mcg/mL for serum vitamin E.
Recurrent Exertional Rhabdomyolysis
About 5% to 10% of TB racehorses develop ER during a racing season, and 75% of these horses have more than four episodes in 4 months.55 Approximately 6% of National Hunt horses68 and 13% of polo horses develop ER.69 Horses with a nervous disposition, especially fillies, are highly predisposed.56 Research studies suggest that a subset of TB horses with chronic ER have RER.11,12,22 RER appears to be an inherited, intermittent, stress-induced defect in the regulation of muscle contraction.12,22 A breeding trial using TB horses with RER confirmed that the characteristic abnormality in muscle contracture is inherited in an autosomal dominant fashion.70 Recurrent episodes of rhabdomyolysis in STBs and Arabian horses may be from a similar abnormality, but this has not been confirmed. A heritable basis for RER in STBs was supported by equine lymphocyte antigen profiles in affected horses.50
Factors that trigger RER in susceptible horses include gender, temperament, excitement, stress, dietary starch, exercise duration/intensity, season, and lameness.40,41,56,71 Females are most commonly afflicted with RER (67% female, 33% male), particularly those that are 2 years of age and in race training.56,71 Nervous horses are five times more likely to develop RER, and horses with lameness are four times more likely to develop RER. Susceptible horses receiving more than 5 kg of concentrate feed (oats, corn, molasses mix) are more likely to develop RER than those receiving 2.5 kg of concentrate feed/day.56 Dietary effects of high carbohydrate in horses with RER may in part be related to the psychogenic effects of grain on excitability. In horses with RER, glycogen storage does not increase substantially.60,72 Inclement weather has been cited as a trigger of RER, and the condition is reported more commonly in the autumn and winter in the United Kingdom.40
A contribution of reproductive hormones to triggering RER was postulated because the incidence appears to be highest in mares.41,56,71 Many owners report that episodes of RER occur most commonly during estrus, but in one study of racehorses, no direct correlation was shown between progesterone fluctuations and serum CK activity.73 It is likely that the estrus cycle is one of many factors that combine to trigger RER in susceptible horses. Many racetrack practitioners report that the incidence of RER declines when susceptible mares are treated with testosterone. Hypothyroidism and lactic acidosis were suggested as a cause of RER but have never been substantiated.3,60,74-76
Clinical Signs
Horses with RER have intermittent elevations in serum CK activity.3,11,67,68 Episodes of muscle stiffness, sweating, and firm muscle contractures often occur in horses once they become fit and are frequently associated with excitement at the time of exercise. In TBs, RER occurs most frequently during training when horses are held to a slow gallop.56 In STBs, RER often occurs 15 to 30 minutes into jogging.3 Obvious clinical signs are rare after racing. A history of poor performance and elevated serum AST and CK may be the only presenting complaints in some horses. Older TBs used as riding horses may have very intermittent episodes of RER associated with layup of fit horses. Event horses often develop clinical signs after the steeplechase phase or in the “10 minute box” of a long-format Three Day Event, or less commonly after warm-up for the cross-country phase of a short-format Three Day Event or during the cross-country phase. Muscle stiffness, reluctance to collect, and lack of power may be present on a continual basis between episodes in some of these older horses. Arabian horses often develop clinical signs with little exertion, frequently in association with excitement.
In some riding horses, the clinical signs of a mild attack may be very subtle and are easily missed by the rider. The horse is maintained in work and may actually experience daily episodes resulting in cumulative muscle damage (Figure 83-5).
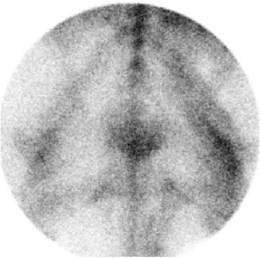
Fig. 83-5 Dorsal scintigraphic image of the pelvic region of an 8-year-old show jumper mare with a history of poor performance and reluctance to work. Cranial is at the top. There are linear areas of moderately increased radiopharmaceutical uptake in the muscles, associated with chronic, recurrent exertional rhabdomyolysis.
Diagnosis
A submaximal exercise test (page 819) is useful for identification of RER in a horse with a history of poor performance unrelated to signs typical of tying-up. Nuclear scintigraphy (page 819) may demonstrate IRU in the affected muscles of some, but not all, affected horses.
Muscle biopsies from horses with RER that are in training have a characteristic histological appearance (Figure 83-6). These horses have increased numbers of centrally located nuclei in mature muscle fibers. They may have evidence of varying stages of muscle degeneration and regeneration, and they have normal to slightly increased muscle glycogen staining.11 Histopathological changes are often lacking in horses that have been laid up for a period before biopsy. In addition, RER is characterized by abnormal sensitivity of intact muscle bundles to contractures induced by the addition of caffeine or halothane to a muscle bath.21,22 Elevated myoplasmic calcium concentration was reported in muscle of horses with acute ER77 and in myoblasts derived from TBs with RER.12 There are physiological similarities between the contracture results of RER and contracture tests for malignant hyperthermia (MH). Biochemical studies of isolated muscle cell membranes78 and linkage analysis79 do not support an identical biochemical basis for MH and RER. The increased halothane sensitivity of the muscles in RER horses may explain why many TBs with RER develop rhabdomyolysis under halothane anesthesia.80
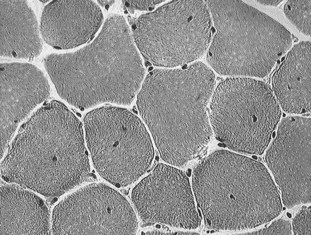
Fig. 83-6 Cryostat-cut sections of a biopsy of the semitendinosus muscle of a Thoroughbred with recurrent exertional rhabdomyolysis (RER) stained with hematoxylin and eosin. Note the presence of centrally located nuclei in mature muscle fibers. This is a common feature of biopsies of horses with RER.
Management
In the past, horses have been box stall rested for several weeks following an episode of RER. It is our opinion that this is counterproductive and increases the likelihood that the horses will develop rhabdomyolysis when put back into training. The initial muscle pain usually subsides within 24 hours of acute RER, and daily turnout in a small paddock can be provided at this time. Subsequently, a gradual return to performance is recommended once serum CK is close to normal range.
Prevention
Prevention of further episodes of RER in susceptible horses includes standardized daily routines and providing an environment that minimizes stress. This should include desensitizing horses to stressful situations, moving to a quiet area of the barn, regular turnout, and positioning near compatible horses. Daily exercise is essential, whether in the form of turnout, walking on a horse walker, lunging, or riding. There should be a long, slow, warm-up period.
The diet should be adjusted to include a balanced vitamin and mineral supplement, high-quality hay (but not alfalfa), and a minimum of soluble carbohydrates (<3 kg of high-starch concentrate). Postexercise serum CK activity in horses with RER is influenced by the amount of energy horses are fed daily, as well as the composition of the diet. When 500-kg TBs with RER were fed 88 MJ (21 Mcal) of energy and exercised for 30 minutes a day, serum CK activity after exercise was within the normal range. There was no measurable effect of feeding a starch-based (2.5 kg of an oat, corn, molasses mix) or a fat-based (2.3 kg of rice bran; 20% digestible energy [DE] from fat) supplement with hay on muscle necrosis.60 However, when the energy level was increased to a level that was closer to what is normally fed to racehorses (117 MJ or 28 Mcal), the source of energy had a significant effect on postexercise CK activity. Horses fed a high proportion of energy in the form of starch (5 kg of oat, corn, molasses mix) had significantly higher CK levels after exercise than horses fed 25% of total DE as fat (Re-Leve, Hallway Feeds, Lexington, Kentucky, United States).72 Therefore it would appear that TBs susceptible to RER that are in moderate to high intensity training should be fed concentrate feeds that are relatively low in starch (<20% DE) and high in fat (20% DE or a feed that is 10% to 12.5% fat by weight) to minimize postexercise muscle damage. Vegetable oils can be used to supplement low-starch feeds (a maximum of 600 mL/day, with 800 U of vitamin E). However, it is difficult to achieve adequate caloric intake for TBs in race training by adding vegetable oil to low-starch feeds such as hay cubes. For hard keepers, a complete low-starch, high-fat pelleted feed containing vegetable oils and/or rice bran works well and provides a balanced ration. One such feed, Re-Leve, has been shown to lower serum CK activity in exercising TBs with RER. No more than half of the total of forage should be fed as alfalfa: lower-starch hays such as grass or meadow, timothy, brome, or oat hay are preferable. Corn and barley should be avoided because they are particularly high in starch.
The use of low doses of acetylpromazine tranquilizers (0.005 to 0.01 mg/kg) 30 minutes before exercise is believed to help some nervous or excitable horses. Long-acting tranquilizers such as fluphenazine have been used successfully by some practitioners. Dantrolene (2 to 4 mg/kg PO) given 1 hour before exercise has proven to be effective in preventing RER in some horses.81,82 Dantrolene is used to prevent MH in people and swine by decreasing the release of calcium from the calcium release channel. Phenytoin has also been advocated as a treatment for horses with RER.83 Dosages are adjusted in horses to maintain serum levels of 8 to 10 mcg/mL. Initial doses begin at 6 to 8 mg/kg PO for 3 to 5 days. Doses can be increased by 1-mg/kg increments every 3 days until RER is prevented but should be cut back if horses appear drowsy. If possible, serum phenytoin concentrations should be assessed regularly at the initiation of treatment. Phenytoin acts on a number of ion channels within muscle and nerves, including sodium and calcium channels. Unfortunately, long-term treatment with dantrolene or phenytoin is expensive, and efficacy has not been established.
In some mares in which episodes of RER coincide with estrus, suppression of estrus using progesterone implants or injections may be helpful. This should be done in conjunction with dietary and training alterations. Anecdotal information from racehorse trainers suggests injection with testosterone cypionate decreases RER in TB mares; however, this may be a banned substance in many racing districts.
The above management is effective in reducing RER in many horses, but some remain intractable. Nervous young racing fillies and older riding horses, especially event horses, present the greatest challenge in management.
Polysaccharide Storage Myopathy
A subset of horses with chronic ER have a storage disorder characterized by the accumulation of glycogen and an abnormal polysaccharide in skeletal muscle.58 Abnormal polysaccharide accumulation occurs in a variety of European and North American breeds of riding and driving horses that have a history of muscle stiffness and increased serum CK activity after exercise.51,53,84,85
Recently an autosomal dominant gain-of-function mutation was identified in the glycogen synthase gene (GYS1) in Quarter Horses with PSSM.57 This mutation is found in at least 20 different breeds of horses in Europe and North America. Affected breeds include Quarter Horses; American Paint horses; Appaloosas; draft horses, including American Cream, Belgian, Percheron, Cob Normande, Trekpard, Haflinger, Morgan, Mustang, Rocky Mountain Horse, and Tennessee Walking Horse breeds; mixed breed horse; Cobs; Hanoverians; Rheinlander; and Warmbloods of unspecified type.53,59 Because some horses with elevated muscle glycogen content do not possess the GYS1 mutation, PSSM is now subdivided into type 1 and type 2.59 Type 1 PSSM refers to horses with the GYS1 mutation. The prevalence of the PSSM from the GYS1 mutation ranges from 8% in Quarter Horses and Paints52 to 36% in Belgian draft horses,54 with no reported cases in the few TBs, STBs, and Arabians tested.53,59 Type 2 refers to horses with excessive muscle glycogen that do not have the GYS1 mutation. Type 2 PSSM appears to account for about 25% of PSSM seen in Quarter Horse–related breeds and 80% of PSSM seen in Warmblood breeds.59 The acronyms EPSM and EPSSM have also been used for polysaccharide storage myopathy, although they do not indicate a specific genotype.86
Clinical Signs
Horses with both forms of PSSM often have a calm and sedate demeanor. Clinical signs of muscle stiffness, reluctance to exercise, exercise intolerance, or overt muscle contractures and reluctance to move usually are apparent at the commencement of training.46,51 Most horses have numerous episodes of ER or a consistent history of poor performance; however, some mildly affected horses have only one or two episodes of ER per year.46 In Quarter Horses, type 1 PSSM has the highest frequency in halter horses, followed by pleasure horses and working cow horses. It is uncommon in racing Quarter Horses. Serum CK activities are often elevated in untreated Quarter Horses, even when horses are rested, and while clinical signs are active, CK will usually increase by 1000 U/L or more 4 hours after 15 minutes of exercise at a trot.87 Muscle atrophy, renal failure, and severe colic-like pain are less common presenting complaints. Both type 1 and type 2 PSSM occur in Quarter Horse and Paint horse foals and weanlings without rhabdomyolysis necessarily being induced by exercise (see page 829).
Draft horses with type 1 PSSM show signs of a generalized decrease in muscle mass, overt muscle atrophy, weakness in the hindlimbs with difficulty rising, reluctance to backup, and gait abnormalities.54,55,88-90 ER can be a debilitating feature of type 1 PSSM in draft breeds. Although shivers was suggested to be a sign of PSSM, recent studies show there is no causal relationship (see Chapter 48).54 Draft horses with PSSM often have only a mild elevation in serum CK and AST. The prevalence of type 1 PSSM is so high in many draft breeds that many homozygous affected horses can be identified.91 These homozygotes may have more severe clinical signs.
Warmbloods derived from draft crosses may have type 1 PSSM. Many European Warmblood breeds are more frequently affected by type 2 PSSM.59 One of the most common presenting complaints in Warmbloods with PSSM is a gait abnormality characterized by lack of impulsion and shifting undiagnosed lameness.51 Warmbloods with PSSM also present with sore muscles and ER.
Diagnosis
A diagnosis of type 1 PSSM can be made by testing whole blood or hair root samples for the GYS1 mutation (http://www.vdl.umn.edu/vdl/ourservices/neuromuscular.html). Type 2 PSSM at present must still be diagnosed by muscle biopsy. The distinctive features of type 1 PSSM in muscle biopsy samples are numerous subsarcolemmal vacuoles and dense, crystalline periodic acid–Schiff (PAS)-positive, amylase-resistant inclusions in fast twitch fibers.58 A false-negative diagnosis of type 1 PSSM by muscle biopsy may occur if biopsy samples are small or if horses are less than 1 year of age. Muscle biopsies from horses with type 2 PSSM have increased PAS staining for glycogen and aggregates of granular PAS-positive polysaccharide in the cytoplasm or under the sarcolemma.91 The PAS-positive polysaccharide in type 2 PSSM is frequently, but not always, amylase sensitive. False-positive diagnosis is possible for type 2 PSSM in highly trained horses that normally have higher muscle glycogen concentrations or in formalin-fixed sections that show a greater deposition of subsarcolemmal glycogen in healthy horses. Other features that may be present with both forms of PSSM include muscle necrosis, macrophage infiltration of myofibers, regenerative fibers, and atrophied type 2 fibers.
Etiology
Muscle glycogen concentrations in horses with type 1 PSSM are 1.5 to 4 times normal, and glucose-6-phosphate concentrations are up to 10 times normal.92 Increased muscle glycogen concentrations in type 1 PSSM appear to be from increased and unregulated glycogen synthase activity as a result of a gain-of-function mutation in the GYS1 gene.57 Horses with PSSM show increased glycogen synthase activity both in the basal state and when stimulated by glucose-6-phosphate. Elevated insulin in the bloodstream can further enhance glycogen synthase activity. Abnormal amylase-resistant polysaccharide appears to result from production of a polysaccharide with a higher ratio of straight glucose chains, which are created by glycogen synthase, relative to branched chains, which are created by the less active glycogen branching enzyme. Rhabdomyolysis in type 1 PSSM appears to result from a deficiency of energy within individual contracting muscle fibers.93 The genetic defect combined with a high-starch diet appears to produce substrate-limited muscle oxidative metabolism by both impairing production of acetyl coenzyme A from glycogen and by preventing lipolysis and delivery of free fatty acids to skeletal muscle mediated by insulin release. Clinical signs of muscle pain in horses with the GYS1 mutation may be exacerbated by enhanced individual insulin sensitivity,92 as well as by meals that produce elevated blood glucose and insulin levels.94 The etiology of type 2 PSSM is not known at present.
Management
Horses with acute rhabdomyolysis can be managed in a fashion similar to that described for sporadic rhabdomyolysis (see page 824).87 Episodes of rhabdomyolysis can be lowered by 75% or more by feeding a diet that is low in starch, supplemented with fat and high in fiber.46,94 The primary factor to consider in PSSM horses is their body weight and energy requirements. Overweight horses should begin with a weight-reduction program consisting of grass hay, a ration balancer, a grazing muzzle, and daily exercise following an overnight fast, which elevates plasma free fatty acid levels through lipolysis. Horses with PSSM are much less tolerant of dietary starch and sugar than horses with RER. Horses with a normal body weight should consume diets consisting of good quality grass hay and no high-starch concentrate feed. Instead, a low-starch fat-supplemented feed, such as rice bran–based feeds, complete feeds designed for PSSM, or 250 to 500 mL (1 to 2 cups) of vegetable oil soaked into a low-starch pellet should be provided.94,95 Although 1 lb of fat per day has been recommended on some Internet websites, recent research has shown that half as much fat usually works well for many horses with type 1 PSSM.94 The amount of digestible energy obtained through starch and sugar should be less than 10% and fat more than 13% per day. Daily exercise is absolutely essential to return PSSM horses to athletic endeavors. As much daily turnout as possible combined with a very gradual training program has a significant impact on decreasing serum CK activity within 30 days.94 Box stall rest for more than 12 h/day appears to increase the incidence of rhabdomyolysis in these horses.
Malignant Hyperthermia
Etiology
An autosomal dominant mutation in the skeletal muscle ryanodine receptor (RYR1), which is altered in pigs and people with MH, was originally identified in Quarter Horses that developed severe reactions to general anesthesia.96 The prevalence of the RYR1 mutation is in general low; however, within a family of Quarter Horses with type 1 PSSM the prevalence is high.
Clinical Signs
Horses with both the MH mutation and the GYS1 mutation for PSSM show intermittent but severe signs of muscle pain, stiffness, cramping, and sudden death associated with mild exertion.97 Quarter Horses with MH that were masked with halothane and then intubated and maintained on halothane showed a marked increase in body temperature, metabolic acidosis, rigor, and death.98
Diagnosis
Genetic testing is recommended in Quarter Horse and Paint horses with a family history of postanesthetic complications or with difficult-to-manage forms of PSSM. Testing is available through the Veterinary Diagnostic Laboratory at the University of Minnesota (http://www.vdl.umn.edu/vdl/ourservices/neuromuscular.html).
Treatment
The most successful outcome for a horse with suspected MH would be pretreatment with oral dantrolene (4 mg/kg) 30 to 60 minutes before induction of general anesthesia. There is no cost-effective means to deliver dantrolene to horses intravenously once an episode has begun. Unfortunately, once a fulminant episode is underway it is difficult to prevent cardiac arrest. Horses with both PSSM and MH respond to a low-starch, high-fat diet and regular daily exercise.97
Nonexertional Rhabdomyolysis
Although exertion is the most common trigger for rhabdomyolysis in horses, many horses can suffer severe rhabdomyolysis without any preceding exertion. Nonexertional rhabdomyolysis may have metabolic, immune-mediated, infectious, nutritional, or toxic causes.
Metabolic Myopathies
Polysaccharide Storage Myopathy
Clinical Signs
The most common form of PSSM causes the development of muscle necrosis, pain, and firm muscles in adult horses with the onset of training (see section on Polysaccharide Storage Myopathy, page 828). A subset of horses with both type 1 and type 2 PSSM and a concurrent illness may develop rhabdomyolysis without any preceding exercise. Rhabdomyolysis may be so severe that horses become recumbent and unable to rise. Initial muscle swelling and firmness may give way to substantial atrophy after a few days. This form of PSSM was described in weanling and yearling Quarter Horses with concurrent bacterial pneumonia or diarrhea.99 In addition to serum CK greater than 200,000 U/L, electrolyte abnormalities such as hyponatremia, hypochloremia, hypocalcemia, hyperphosphatemia, and hyperkalemia are common as a result of the loss of partitioning between extracellular and the large intramuscular fluid compartment and, in some horses, renal compromise.100 Foals with type 2 PSSM may have a history of a stiff gait and difficulty producing the power required to stand successfully from a recumbent position without assistance. Serum CK activity in these foals may only be moderately elevated, and the primary clinical signs are weakness and pain. Horses with both type 1 PSSM and the mutation for MH (RYR1) can develop severe nonexertional rhabdomyolysis and sudden death.
Diagnosis
A diagnosis of type 1 PSSM and MH can be made by genetic testing. A diagnosis of type 2 PSSM is based on identification of abnormal polysaccharide in PAS stains of muscle biopsies (Figure 83-7). A diagnosis may be complicated by the fact that rhabdomyolysis in weanlings with PSSM may precede the later stage of accumulation of abnormal polysaccharide.101 The diagnosis of PSSM in a weanling may be supported by the identification of PSSM in a muscle biopsy from the dam or sire.99
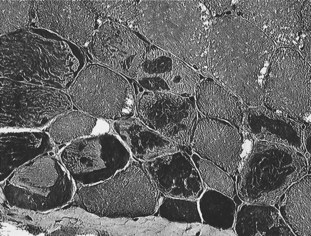
Fig. 83-7 Cryostat-cut sections of a semitendinosus biopsy of a horse with polysaccharide storage myopathy stained with periodic acid–Schiff (PAS) and counterstained with hematoxylin. Note the presence of white subsarcolemmal vacuoles in some fibers and the dark PAS-positive granular inclusions representing abnormal polysaccharide in numerous other muscle fibers.
There are many other differential diagnoses for acute rhabdomyolysis in foals. Neonatal foals may have modest increases in CK at birth in conjunction with elevated creatinine and a history of placentitis or dystocia.102 Septicemia could potentially cause an inflammatory reaction in skeletal muscle of neonatal foals. Most commonly, myodegeneration is caused by vitamin E and selenium deficiency with subsequent peroxidation of cell membranes by oxygen free radicals (see page 834).103 Young Quarter Horses have developed acute rhabdomyolysis on infection with Streptococcus equi (see page 832).104 Mild elevations in CK also occur in Quarter Horses with glycogen branching enzyme deficiency (GBED).
Treatment
Acute treatment of rhabdomyolysis is similar to that recommended in the Sporadic Rhabdomyolysis section (pages 824 and 825). Special considerations for foals include stall confinement in those showing pronounced stiffness or weakness and assistance to rise and suckle every hour. Nutritional support in the form of mare’s milk or foal pellets should be supplied if they do not suckle adequately. Either the mare should be switched to a low-starch high-fat supplement that the foal can share or the mare’s grain should be kept inaccessible to the foal. In recumbent foals, in addition to intravenous fluids and nutritional support, a constant rate infusion of detomidine, lidocaine, butorphanol, or ketamine may be useful to control severe pain and struggling. Dantrolene sodium (4 mg/kg, every 4 to 6 hours, PO) may decrease muscle cell necrosis. Assistance to stand every few hours either manually or using a sling is necessary to improve muscle function and circulation. Cautious handwalking for no more than a few minutes at a time is recommended once the foal is stable and strong enough to ambulate. Whenever possible, stall confinement should be limited to less than 48 hours after the episode of rhabdomyolysis and stiffness subside because prolonged stall confinement may result in an increased incidence of rhabdomyolysis episodes as a result of PSSM. Small paddock turnout with limited ability to move around is recommended once stiffness subsides.
Dietary management of weanling foals is similar to that of adults except that foals have a higher protein requirement than adults, especially with regard to lysine. Feeding alfalfa hay rather than grass hay, combined with balanced commercial low-starch high-fat ration, is recommended to meet nutritional needs of weanlings. In some foals, this may provide excessive sugar, and grass hay may be necessary. As much turnout as possible with other horses to encourage daily exercise is recommended for long-term management of foals with PSSM.
The prognosis for weanlings with nonexertional rhabdomyolysis is guarded because of the high likelihood of recurrence.
Glycogen Branching Enzyme Deficiency (GBED)
Clinical Signs
GBED is a fatal autosomal recessive glycogen storage disorder distinct from PSSM in Quarter Horse and Paint foals.105,106 A mutation in the glycogen branching enzyme gene (GBE1) causes a deficiency in the glycogen branching enzyme (GBE) responsible for producing a normally configured glycogen molecule in numerous tissues.107 Approximately 8% of Quarter Horses and Paint horses are carriers of the mutation, and 3% of second and third trimester aborted fetuses submitted to two diagnostic laboratories were found to be homozygous for GBED.108 Pleasure horses and working cow horses have the highest incidence of GBED carriers.109 Many foals with GBED are undiagnosed because of the similarity of clinical signs with many neonatal diseases and the current lack of awareness of available genetic testing for stillborn foals and aborted fetuses.
Clinical signs appear to be caused by a lack of intracellular glucose stores for normal tissue metabolism. Foals may be aborted, stillborn, weak after birth, or live to up to 18 weeks of age. Death may be sudden when foals are exercised on pasture, associated with weak respiratory muscles, or the result of euthanasia from persistent recumbency. Treatable flexural deformities of all limbs and recurrent hypoglycemia occur in some affected foals. Persistent leukopenia, intermittent hypoglycemia, and moderate elevations in serum CK (1000 to 15,000 U/L), AST, and γ-glutamyl transferase activities are common laboratory findings.
Diagnosis
Routine post mortem examination usually reveals few abnormalities apart from pulmonary edema in some foals and basophilic inclusions in skeletal muscle and cardiac tissues in foals more than 1 month of age. PAS staining of muscle, heart, and sometimes liver shows notable lack of normal PAS staining for glycogen and a variable amount of abnormal PAS-positive globular or crystalline intracellular inclusions. EM and iodine spectra absorption indicated that the polysaccharide is filamentous, with a very minimally branched structure. The most accurate diagnosis of GBED can be obtained through genetic testing of the foal for homozygous status or the dam/sire for heterozygous status. Many stallion owners offer a free repeat breeding to owners that lose foals, and if a diagnosis is not established, the owner will have a 25% chance of having another GBED-affected offspring on repeat breeding. The Veterinary Genetics Laboratory at the University of California, Davis (www.vgl.ucdavis.edu) and Vet Gen in Michigan (www.vetgen.com) are licensed by the University of Minnesota to test for GBED. Mane or tail hairs with roots intact can be submitted. Diagnostic laboratories should be encouraged to screen aborted fetuses for GBED either through PAS staining of cardiac samples or via genetic testing. Testing also should be strongly advised for prepurchase evaluation of broodmares or stallions.
Inflammatory Myopathies
Immune-Mediated Myopathies
Infarctive Hemorrhagic Purpura
Mild elevations in serum CK activity have been observed in conjunction with purpura hemorrhagica in horses. Rarely, some horses vaccinated for, or exposed to, S. equi within the past month develop variable edema, extremely high serum CK activity, acute colic, firm swellings within muscle and under the skin, and unilateral lameness.110 Infarctions of skeletal muscle, subcutaneous tissue, and focal areas of the gastrointestinal tract and lungs resulting from a severe vasculopathy are found in these horses. Infarctive purpura is characterized by leukocytosis, hyperfibrinogenemia, and hypoalbuminemia. A diagnosis is often established based on clinical signs of marked pain, inflammatory leukogram, low serum albumin concentration, leukocytoclastic vasculitis in skin and affected tissues, and very high S. equi M protein titer. Successful treatment requires early detection, penicillin for 14 days, and prolonged high doses of dexamethasone (0.12 to 0.2 mg/kg) for at least 10 days, followed by tapering doses of prednisolone at an initial dose of 2 mg/kg. Aggressive steroid treatment is required because horses treated with lower doses progress to intestinal infarction and death.
Immune-Mediated Myositis
Immune-mediated polymyositis (IMM) often occurs in Quarter Horses, although other breeds may be affected. Horses are usually either 8 or less or 16 or more years of age.111,112 In approximately one third of horses with IMM, a triggering factor appears to have been exposure to S. equi or a respiratory disease.111
Clinical Signs
The most prominent clinical sign of IMM in Quarter Horses is rapid onset of muscle atrophy, particularly affecting the back and croup muscles, accompanied by stiffness and malaise. Atrophy may progress to involve 50% of the horses’ muscle mass within 1 week and may lead to generalized weakness. Focal symmetrical atrophy of cervical muscles has been reported in a pony with IMM.112
Diagnosis
Hematological abnormalities are usually restricted to mild to moderate elevations in serum CK and AST activity. However, in some horses with chronic myositis, serum muscle enzyme activities are normal. Muscle biopsy of epaxial and gluteal muscles shows lymphocytic vasculitis, anguloid atrophy, myofiber infiltration with lymphocytes, fiber necrosis with macrophage infiltration, and regeneration in acute stages (Figure 83-8). Biopsies of semitendinosus or semimembranosus muscles may show some evidence of atrophy and vasculitis, but substantial inflammatory infiltrates may be absent in these muscles. The extent of the inflammatory infiltrates in epaxial muscles is such that a diagnosis can often be established from several formalin-fixed samples obtained using an 18-gauge disposable biopsy needle.
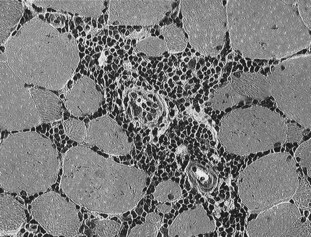
Fig. 83-8 Immune-mediated myositis: Cryostat-cut sections of a biopsy of the longissimus dorsi from a horse with rapid muscle atrophy stained with hematoxylin and eosin. Note the prominent cuffing of the blood vessels with mononuclear cell infiltrates and the lymphocytes surrounding a number of muscle fibers that is characteristic of immune-mediated myopathy in Quarter Horses.
The lymphocytic infiltrates seen in muscle samples from horses with IMM contain a high CD4/CD8 ratio with no evidence of IgG binding to myofibers.111 The reason why specific muscle groups are affected in horses with IMM is unclear.
Treatment
Horses with concurrent evidence of streptococcal infection should be treated with antibiotics. It is prudent to avoid intramuscular injections. Administration of corticosteroids appears to immediately improve signs of malaise and inappetence and prevents further progression of muscle atrophy. Recommended dosages are dexamethasone (0.05 mg/kg) for 3 days, followed by prednisolone (1 mg/kg for 7 to 10 days) tapered by 100 mg/week over 1 month. Serum CK activity often normalizes after 7 to 10 days of treatment. Muscle mass will usually gradually recover over 2 to 3 months even without corticosteroid treatment. Recurrence of atrophy in susceptible horses is common and may require reintroduction of corticosteroid therapy.
Infectious Myopathies
Virus-Associated Myositis
Necrosis of skeletal and cardiac muscle may occur in association with viral diseases such as equine influenza and equine infectious anemia. In most situations, viral-induced muscle damage represents a component of systemic multiple organ system involvement. Equine influenza A2 has been found to cause severe rhabdomyolysis, and equine herpesvirus–1 has been reported to induce primary muscle stiffness and clinical signs resembling ER.38,45
Sarcocystosis Myositis
Cysts of the sporozoan parasite Sarcocystis are commonly seen in routine histological sections of the heart, esophageal, and skeletal muscle. More than 90% of horses more than 8 years of age have sarcocysts in esophageal muscles, and 6% of healthy horses have one or more nonreactive sarcocysts in gluteal muscle biopsies.52 Cysts usually pose no problem, but multisystemic dysfunction occurs with heavy infestations. Horses with heavy infestation show signs of fever, anorexia, stiffness, weight loss, muscle fasciculations, atrophy, and weakness.113 Diagnosis of sarcocystosis requires history, clinical signs, laboratory evaluation, and the demonstration of an inflammatory reaction to immature cysts in muscle biopsies. Infection occurs from contamination of feed by infected canine feces. Successful treatment of one horse with sarcocystosis using phenylbutazone, trimethoprim sulfa, and pyrimethamine was reported.113
Streptococcus equi Rhabdomyolysis
Severe rhabdomyolysis was reported in young Quarter Horses in association with concurrent Streptococcus equi equi submandibular lymphadenopathy and/or guttural pouch empyema.104 Myonecrosis could be from toxic shock resulting from profound nonspecific T-cell stimulation by streptococcal superantigens and the release of high levels of inflammatory cytokines. An alternative explanation may be bacteremia with local multiplication and production of exotoxins or proteases within skeletal muscle. S. equi bacteria were identified in affected muscle using immunofluorescent stains for both Lancefield group C carbohydrate and S. equi M protein. There is currently no evidence that the S. equi involved is an atypical genetic strain of S. equi.
Clinical Signs
Horses develop a stiff gait that progresses rapidly to severely painful, firm, swollen, epaxial, and gluteal muscles. Horses often become recumbent, unable to rise, and unrelenting pain may necessitate euthanasia within 24 to 48 hours.
Diagnosis
Hematological abnormalities include mature neutrophilia, hyperfibrinogenemia, and marked elevations in CK (115,000 to 587,000 U/L) and AST (600 to 14,500 U/L) concentrations. Titers to the M protein of S. equi are low in affected horses, unless horses are recently vaccinated for strangles. Titers to another protein called S. equi myosin binding protein were high in a small number of horses that were tested.104 At post mortem, large, pale areas of necrotic muscle are evident in hindlimb and lumbar muscles. The histopathological lesions are characterized by severe acute myonecrosis, with a degree of macrophage infiltration. Sublumbar muscles often show the most severe and chronic necrosis as indicated by greater macrophage infiltration of myofibers.
Treatment
A high mortality rate has been reported. Appropriate therapy includes intravenous penicillin therapy combined with an antimicrobial that inhibits protein synthesis, such as rifampin. In addition, flushing infected guttural pouches and draining abscessed lymph nodes will diminish the bacterial load. NSAIDs and possibly high doses of short-acting corticosteroids may assist in diminishing the inflammatory response. Control of unrelenting pain is a major challenge in horses with severe rhabdomyolysis. Constant-rate infusion of lidocaine, detomidine, or ketamine may provide better anxiety and pain relief than periodic injections of tranquilizers. Horses should be placed in a deeply bedded stall and moved from side to side every 4 hours if they are unable to rise. Some horses may benefit from a sling if they will bear weight on their hindlimbs when assisted to stand.
Anaplasma-Associated Rhabdomyolysis
Clinical signs of acute rhabdomyolysis were reported in a horse in conjunction with Anaplasma phagocytophilia infection (formerly Ehrlichia equi).114 Typical clinical signs of fever, malaise, and limb edema are accompanied by severe muscle stiffness. Hematological findings include anemia, thrombocytopenia, neutropenia, morula visible in granulocytes, and marked elevations in serum CK and AST concentrations. A diagnosis is confirmed by polymerase chain reaction testing of blood for A. phagocytophilia. A direct toxic effect of A. phagocytophilia on muscle cells is postulated. The horse in this report was successfully treated with supportive care and oxytetracycline.114
Clostridial Myositis
Clostridium septicum, Clostridium chauvoei, Clostridium sporogenes, and mixed infections cause acute myonecrosis in horses with a high fatality rate. Clostridium perfringens type A is another cause of myonecrosis; however, with early and aggressive treatment mortality rates for infection with this bacterium are about 20%.115 There is usually only one primary site of infection that is associated with an injection or deep wound.116,117 Clostridial spores may lie dormant in skeletal muscle,118 or direct clostridial spore deposition into the tissue may occur in association with a penetrating injury. If suitable necrotic conditions exist, the spores convert to the vegetative, toxin-producing form of the organism. Powerful exotoxins responsible for the local necrotizing myositis and systemic toxemia are released by proliferating clostridia. Myocardial damage occurs in some horses.
Clinical Signs
Horses often have a history of colic, vaccination, exertional myopathy, penetrating wound, and/or intramuscular injections in the preceding 48 hours. Affected horses usually rapidly develop depression, swelling, and crepitus at the injection site, as well as a fever, toxemia, and tachypnea.116 Crepitus is not always present.115 Tremors, ataxia, dyspnea, recumbency, coma, and death may occur within the next 12 to 24 hours. Hematology and serum biochemical analyses usually reflect a generalized state of debilitation and toxemia (e.g., hemoconcentration and a stress/toxic leukogram may be present). Moderate elevations in the activities of serum CK and serum AST usually occur; however, they often do not reflect the toxicity of clostridial myonecrosis.
Diagnosis
Ultrasonographic evaluation of swollen areas may reveal fluid and characteristic hyperechogenic gas accumulation. A specific diagnosis is best made from aspirates of affected tissues examined via direct smears, fluorescent antibody staining, and anaerobic bacterial culture. Cut tissue from the affected area may reveal abundant serosanguineous fluid with an odor of rancid butter. At post mortem, swelling, crepitus, and autolysis are rapid, and blood-stained fluid is often observed discharging from body orifices.
Treatment
Aggressive antibiotic therapy, wound fenestration, aggressive surgical debridement over the entire affected area, and supportive care are the hallmarks of successful treatment.115 High doses of intravenous potassium penicillin are recommended every 2 to 4 hours until the horse is stable (1 to 5 days), combined or followed by oral metronidazole. Supportive fluid therapy and antiinflammatory agents for control of pain and swelling are recommended. Short-acting corticosteroids such as prednisolone or hydrocortisone may be used for initial therapy of systemic/toxic shock, but continued use is contraindicated in the face of overwhelming infection. Extensive skin sloughing over the affected area is common in surviving horses.
Postinjection Muscle Soreness and Abscessation
Intramuscular injection of a variety of drugs and vaccines may be followed by severe localized muscle soreness, with or without swelling. Injections in the neck may result in neck stiffness and a bilaterally shortened forelimb stride. Injections in the gluteal muscle mass or thigh region can cause unilateral hindlimb stiffness and lameness. These reactions are usually transitory, but occasionally muscle abscessation is a sequel.
A muscle abscess results in localized swelling, heat, and pain. The extent of the abscess, its depth, and the thickness of the walls may be determined ultrasonographically. Surgical drainage or complete abscess excision is required.
Other Muscle Abscesses
Suppurative myositis may arise through penetrating injuries, hematogenous spread of infection, or via local spread of infection. Initially there is an ill-defined cellulitis, which may heal or progress to a well-defined abscess (Figure 83-9). An abscess may heal, expand, or fistulate, usually to the skin surface. Once fistulated, the abscess may collapse and heal or result in a chronic granuloma with intermittent discharge. Staphylococcus aureus, S. equi, and Corynebacterium pseudotuberculosis are commonly isolated from skeletal muscle abscessation.

Fig. 83-9 Transverse ultrasonographic image of the lateral aspect of the antebrachium of a pony with cellulitis previously unresponsive to antimicrobial treatment. There was extensive soft tissue swelling throughout the antebrachium and carpal regions, with effusion in the carpal sheath and the tendon sheaths of extensor carpi radialis and the long and lateral digital extensor tendons. The pony was severely lame. There is a loculated, partly fluid-filled developing abscess (arrow).
The effect of an abscess on the horse’s gait depends on its location and can vary from mild stiffness to severe lameness.
Diagnosis is confirmed by ultrasonography or by culture of aspirated fluid. Abscesses lying deep within muscles can be difficult to diagnose. There may be an elevated fibrinogen level and nucleated white blood cell count. The synergistic hemolysin inhibition test, detecting antibodies to C. pseudotuberculosis, can be helpful for detection of internal abscesses.99
Treatment consists of poulticing, lancing, flushing, and draining. Occasionally surgical excision may be required for complete removal. The use of antimicrobial drugs is controversial but may be recommended for corynebacterial abscesses. Commonly recommended drugs include procaine penicillin (20,000 U/kg IM twice daily) and crystalline penicillin (20,000 to 40,000 U/kg IV four times daily) alone or in combination with rifampin (5 mg/kg PO twice daily). If antimicrobial therapy is used, it should be continued for several weeks.
Prognosis is usually good for horses with superficial abscesses. Horses with deep abscesses are more difficult to manage successfully. Prolonged resolution or recrudescence often occurs with corynebacterial abscesses.
Traumatic Myopathies
Postanesthetic Myopathy
Postanesthetic myopathy occurs as a unilateral problem, such as triceps myopathy, a bilateral problem, such as hindlimb adductor myopathies, or as a generalized condition.102 In horses anesthetized in lateral recumbency, the dependent triceps muscle is most commonly affected, whereas the longissimus dorsi and gluteal muscles are usually affected in horses positioned in dorsal recumbency.
Hypoperfusion of muscle groups is the most important causative factor, often the result of increased intracompartmental pressure. Positioning of the horse on the operating table is critical. Compressing muscles against a hard, unyielding surface, venous drainage obstruction, and hypotension may all be contributory factors. Generalized myopathies may occur independent of positioning, and hypotension may play a greater role in these horses. The longer the duration of general anesthesia, the higher the risk.
Clinical Signs
Clinical signs are usually evident as soon as the horse tries to stand but may be delayed for up to 2 hours. Horses with severe myopathy may be unable to stand. Triceps myopathy is often characterized by a dropped elbow stance similar to that seen in horses with radial nerve paralysis. Involvement of the gluteal muscles may result in unwillingness to bear weight on the hindlimbs. Horses may appear distressed, with profuse sweating, tachycardia, and tachypnea. The degree of distress depends on the severity of the muscle damage. Affected muscles may feel very firm to near hard. There may be localized swelling.
Diagnosis
Diagnosis is confirmed by measurement of serum CK concentration. The concentration is often increased after general anesthesia, but levels greater than 2000 U/L indicate the presence of myositis. The CK concentration may be normal immediately after the horse stands, but as blood flow returns to the affected muscles, the concentration will increase and peak 4 hours after initiation of the event.
Treatment and Prognosis
Treatment aims to relieve distress and pain, to encourage perfusion of muscles, and to keep the horse standing if at all possible. Pain relief is provided by NSAIDs, such as phenylbutazone (4.4 mg/kg) or flunixin meglumine (1 mg/kg), combined with opiate analgesics (e.g., butorphanol, 0.1 mg/kg) if necessary. Sedation with detomidine in horses with severe pain or acepromazine (0.05 mg/kg) in those with mild pain may be required. Constant-rate infusions of detomidine or butorphanol may be beneficial for pain control. Fluid therapy is important to maintain renal perfusion and urine output and to ensure adequate muscle perfusion. A balanced polyionic electrolyte solution should be infused at up to 20 mL/kg/h for the first several hours.
The prognosis for horses with unilateral myopathy is usually good, although with severe damage there may be residual muscle fibrosis, which may compromise function. With persistent clinical signs, ultrasonographic examination may be useful to determine the extent and severity of muscle damage.119 The prognosis for generalized myopathy is more guarded.
Prevention
Prevention requires careful preoperative planning to minimize the time under general anesthesia, careful positioning of the horse on the operating table, and maintenance of arterial blood pressure greater than 60 mm Hg using fluid therapy and inotropic agents such as dobutamine (1 to 5 mcg/kg/min). The position of the horse on the operating table is in part dictated by the surgical procedure. With horses in lateral recumbency, the dependent forelimb should be pulled forward, and the upper limb should be supported at least parallel to the table. The hindlimbs should be supported parallel to, or above parallel, and adequately separated to permit venous drainage. With horses in dorsal recumbency, support of the forelimbs or hindlimbs in a semiflexed position using an overhead hoist or side bars can be helpful.
Gastrocnemius Muscle Injury
Gastrocnemius muscle injury in foals and adult horses is discussed in Chapter 80.
Compartment-like Syndrome in the Antebrachium
Compartment syndrome is a condition in which high pressure within a closed fascial space reduces capillary blood perfusion below a level necessary for tissue viability. The syndrome develops in skeletal muscle enclosed by relatively noncompliant osseofascial boundaries.122 Elevated intracompartmental pressure may result from increased intracompartmental fluid or from decreased compartment size. Increased pressure from fluid accumulation may be the result of muscle trauma and hemorrhage or a sequel to prolonged ischemia and subsequent reperfusion and edema. Prolonged elevated pressure greater than 30 mm Hg resulted in myonecrosis in the dog.123
A compartment-like syndrome was described in the horse involving the caudolateral muscle compartment of the antebrachium.124 This encloses the lateral digital extensor, ulnaris lateralis, superficial and deep digital flexor and flexor carpi ulnaris muscles, and also the median artery, vein, and nerve.
Lameness is a sequel to trauma and is acute and severe in onset, with reluctance to bear weight on the limb associated with firm swelling on the caudolateral aspect of the antebrachium. Digital pulse amplitudes in the more distal part of the limb may be reduced if the limb is flexed or extended. With time, the affected muscle mass may become cold.
Treatment is by fasciotomy. Incising the superficial fascia over ulnaris lateralis resulted in immediate separation of the fascia by 3 to 4 cm and rapid relief of clinical signs.124
 Nutritional Myopathies
Nutritional Myopathies
John Maas and Stephanie J. Valberg
Nutritional Myodegeneration
Etiology
Nutritional myodegeneration (NMD) (white muscle disease, nutritional muscular dystrophy) is an acute degenerative disease of cardiac and skeletal muscle caused by a dietary deficiency of selenium and/or vitamin E.103,125 Marginally to severely selenium-deficient areas occur in regions of numerous countries throughout the world.125,126 This syndrome occurs in young, rapidly growing foals born to dams that consumed selenium-deficient diets during gestation. The disease has also been implicated in masseter muscle myopathy and occasionally nonexertional rhabdomyolysis in adult horses. Selenium and vitamin E appear to be synergistic in preventing NMD. However, based on prophylaxis and response to treatment, selenium deficiency appears to be the most important contributor.
Clinical Signs
Foals with primary necrosis of myocardium and respiratory muscles have dyspnea, a rapid irregular heartbeat, profound weakness, recumbency, and sudden death.100,103 The skeletal muscle form of NMD frequently has a slower onset of muscular weakness, trembling, stiffness, or inability to stand. Most affected foals are able to stand only for short periods. Supporting muscle groups may appear swollen and may be hard and painful on palpation. Commonly affected muscle groups include the gastrocnemius, semitendinosus, semimembranosus, and biceps femoris and muscles of the lumbar, gluteal, and neck regions. If the diaphragm and intercostal muscles are affected, the foal may show respiratory distress and evidence of increased abdominal effort when breathing. Myocardial damage and signs consistent with cardiac dysfunction may be apparent. Dysphagia from necrosis in the tongue may be the only sign in some affected foals and is frequently accompanied by aspiration pneumonia. Foals with primary muscular NMD often show improvement after a few days of rest and treatment with selenium and can often stand and walk within 3 to 5 days. In the western United States, selenium-responsive NMD is seen in adult horses that were extremely deficient in selenium during the winter (snow on the ground), and the only clinical signs have been myoglobinuria (red snow) and mild stiffness in the morning.
Clinical Pathology
Substantially elevated serum CK concentration in the thousands to hundreds of thousands of units per liter along with high AST and LDH activities occur during myodegeneration. Progressively decreasing CK activity can be used as a prognostic indicator of cessation of myodegeneration. Hyperkalemia, hyperphosphatemia, hyponatremia, hypochloremia, and hypocalcemia can occur with severe rhabdomyolysis when the normal distinction between extracellular and intracellular compartments is destroyed by massive tissue necrosis.100 Myoglobinuria is common. Elevated serum protein concentrations and hemoconcentration commonly reflect dehydration in foals unable to nurse or drink water, as well as fluid shifts into damaged tissues. Neutrophilia is common because of the high incidence of aspiration pneumonia.
Diagnosis
Whole blood selenium and plasma vitamin E samples can be used to assess intermediate to long-term nutritional status. Whole blood selenium analysis is preferred over plasma and serum.127 Whole blood selenium concentrations ranging from 0.07 to more than 0.1 ppm are considered normal. Short-term oral or intramuscular supplementation of selenium can confuse interpretation of circulating selenium concentration. Selenium-dependent glutathione peroxidase (GSH-Px) formed in the red cells during erythropoiesis also provides an index of body selenium status. Adequate GSH-Px activities are greater than 20 to 50 U/mg of hemoglobin/min in horses. However, GSH-Px reference values are only specific to the laboratory where the analysis is performed and must be validated by comparison with blood selenium concentration. The activity of GSH-Px in red blood cells of domestic species remains constant for 4 to 6 days when maintained at 39° F (4° C); after this time, significant decreases occur. The critical concentration of vitamin E (α-tocopherol) in plasma is 1.1 to 2 ppm. Vitamin E deteriorates rapidly in plasma samples. Therefore plasma samples for α-tocopherol analysis need to be put on ice immediately, protected from the light by wrapping in tin foil, and stored frozen (−21° F, −70° C) if analysis is to be delayed.
Pathology
Bilaterally symmetrical myodegeneration is a consistent finding in NMD.103 Skeletal muscle degeneration is characterized by pale discoloration and a dry appearance of affected muscle, white streaks in muscle bundles, calcification, and intramuscular edema. The white streaks seen in muscle bundles represent bands of coagulation necrosis or, in horses with chronic disease where insults may have occurred weeks before, may represent fibrosis and calcification. Affected muscle bundles are often adjacent to apparently normal or minimally affected muscle. Histologically, affected muscle fibers may be hypercontracted and fragmented, with some mineralization of muscle fibers and macrophage infiltration.128 Tissue biopsies and tissue specimens obtained at necropsy can be assayed for selenium content. Normal liver concentrations of selenium are 1.05 to 3.5 ppm on a dry matter (DM) basis. Fresh liver is approximately 30% DM in adult horses.
Pathophysiology
During normal cellular metabolism, highly reactive forms of oxygen (free radicals) are produced. These include hydrogen peroxide, hydroperoxides, lipoperoxides, superoxide, various hydroxy radicals, and singlet oxygen. Vitamin E is active within the cell membrane as a lipid-soluble antioxidant that scavenges free radicals that otherwise might react with unsaturated fatty acids to form lipid hydroperoxides. In contrast, GSH-Px destroys hydrogen peroxide and lipoperoxides that have already been formed and converts them to water or relatively harmless alcohols. Other enzymes such as catalase and superoxide dismutase are also involved in this protective process. It is believed that a deficiency of selenium results in rhabdomyolysis through oxidant damage to muscle cell membranes. The precise interrelationships between selenium, vitamin E, other metabolic factors, and triggering mechanisms in NMD are not fully understood because many horses deficient in selenium and/or vitamin E have no evidence of muscle disease. In certain situations, deficiencies of both selenium and vitamin E are necessary for disease to occur. In other horses, NMD can occur when a deficiency of only one of the nutrients is present and the other is normal in blood and tissues.
Treatment and Prognosis
Myocardial damage is often extensive and incompatible with life with the cardiac form of NMD. Only rarely is treatment successful. In contrast, the skeletal form of NMD is more generally amenable to treatment, although the prognosis for clinical recovery from the skeletal form of NMD is guarded and depends often on whether secondary complications such as respiratory disease develop. In all horses with NMD, therapy should involve specific supplementation with selenium and vitamin E and general supportive care.
Alleviation of selenium-responsive NMD requires the use of injectable selenium products. These are available with selenium concentrations varying from 1 mg/mL of selenium to 5 mg/mL, with all products containing 50 mg/mL (68 U) of vitamin E as dl-α-tocopherol acetate. The label dose for selenium is 0.055 to 0.067 mg/kg (2.5 to 3 mg/45 kg) body weight given intramuscularly or subcutaneously. Dosage of these injectable products should not be greatly increased above the label dose to prevent an inadvertent selenium toxicosis. Absorption and distribution of injectable selenium occur rapidly and may account for the rapid improvement in clinical signs seen in reversible cases.
The amount of vitamin E in vitamin E/selenium combinations is insufficient for vitamin E supplementation. Injectable vitamin E products are now available that contain 300 U vitamin E/mL as d-α-tocopherol (Vital E, Schering-Plough Animal Health, Boxmeer, The Netherlands). Administration of these products increases the tissue and/or plasma level of vitamin E activity for approximately 3 weeks. The bioavailability of vitamin E from injectable products depends on the form of vitamin E (the alcohol form, d-α-tocopherol, being the most active) and the amount and quality of the solution emulsifier used. Oral vitamin E supplementation is a good approach to supplement dietary levels with natural vitamin E providing the highest bioavailability. Daily recommended levels of supplementation for horses range from 600 to 1800 mg of dl-α-tocopheryl acetate.129 Oral α-tocopherol is now available for all species and contains 500 U vitamin E/mL (Emcelle, Stuart Products, Inc., Bedford, Texas, United States). The recommended dosage of this product is 0.5 to 1.5 U/kg body weight.
Supportive therapy may include administration of antibiotics to help combat secondary pneumonia and infected decubital lesions that are common in recumbent horses. If dysphagic, horses should be fed via nasogastric tube, and provision of adequate energy intake and attention to the fluid and electrolyte balance are of critical importance if recovery is to be successful. Hyperkalemia may be life threatening in affected foals. Mineralocorticoids, alkalinizing fluids, and dextrose and insulin may be used to reduce circulating potassium concentrations.100
Prevention and Control
NMD is prevented and controlled through supplementation of selenium and vitamin E. Oral supplementation for horses at 1 mg/day of selenium increases blood selenium concentrations above levels known to be associated with NMD.130,131 Supplementation of pregnant mares is advised in areas known to be selenium deficient; however, only limited selenium may cross the placenta.107 Supplementation during lactation increases the levels of selenium in milk and thus provides a potential means of selenium supplementation in foals; however, evidence in cattle indicates that this increased level of selenium in milk may not meet the foal’s nutrient requirements.132 Supplementation of selenium by use of injectable selenium products alone would require treatment every 30 to 45 days and would provide only partial amounts of the recommended level of selenium.133
Regardless of the method of supplementation, periodic blood (or tissue) sampling of horses at risk is necessary to ensure maintenance of desired levels of selenium. In high-risk areas, samples should be taken every 60 to 90 days to determine selenium status in susceptible horses and every 6 to 12 months to monitor supplementation. Based on these assessments, adjustments to the rate or extent of selenium supplementation may be made. Feeding horses properly prepared and stored hay and grain or allowing them access to high-quality green forage should ensure adequate vitamin E intake.
Toxic Myopathies
Ingestion of a number of toxic substances in feed or forage may cause rhabdomyolysis in horses.
Ionophores
Ionophores are commonly added to ruminant feeds to promote growth and to control coccidia. However, horses are 10 times more sensitive to the toxic effects of ionophores in feed than cattle. When equine feeds are inadvertently contaminated with ionophores or horses eat cattle feed, cardiomyopathy is the most common chronic sequela, although some horses may die acutely with colic-like signs, myoglobinuria, hypokalemia, cardiac arrhythmia, and tachypnea.134
Pasture Myopathies
Tremetone
Horses ingesting 0.5% to 2% body weight of tremetone-containing plants are likely to die from skeletal muscle and cardiac muscle necrosis. Horses show marked depression, weakness, low head posture, and increased cardiac and respiratory rate. Serum AST and CK concentrations are often markedly elevated, and serum electrolyte abnormalities such as hypocalcemia, hyponatremia, hypochloremia, hyperkalemia, and hyperphosphatemia may be present. Treatment is generally supportive as described in the section on acute ER. Tremetone was identified in white snakeroot (Eupatorium rugosum) and rayless goldenrod (Isocoma wrightii). White snakeroot grows in shaded areas of the eastern and central United States.135-137 Rayless goldenrod is common in the southwestern United States on open pastures. Tremetone remains active in the hay and in the stalks of the dead plants on pasture, so both the fresh and the dried form of the plants should be kept from horses.136
Cassia occidentalis
Muscle necrosis may also occur in horses ingesting Cassia occidentalis seeds prevalent in the southeastern United States.138 Horses develop incoordination, recumbency, and death. Gross skeletal muscle lesions are not present, but histopathological lesions include segmental myonecrosis.
Atypical Myoglobinuria
Atypical myoglobinuria occurs sporadically in horses kept at pasture, usually with no supplemental feeding.140,141 It has been recognized most commonly in the United Kingdom and Europe, but a similar syndrome has been seen in the midwestern United States.142 The cause is unknown but appears to involve disruption in lipid metabolism from exposure to a toxin in well-grazed, cool wet pastures.143,144 It occurs most often in spring and autumn and is often associated with a sudden deterioration in weather conditions.141,145 The clinical signs are sudden in onset and rapidly progressive, frequently resulting in death. Several horses in a group may be affected, although some may remain without symptoms. Affected horses are reluctant to move, have muscle weakness, fasciculations, and may become recumbent. Choke may be present, and gut sounds may be reduced, with a reduction in fecal production, although appetite may be unaffected. Heart rates may be markedly elevated, and pulmonary edema may be present. The horses do not show signs of pain, despite post mortem evidence of widespread myopathy. Metabolic and respiratory acidosis, elevated cardiac troponin I concentration, substantial increases in serum CK and AST levels, and myoglobinuria are common.
Post mortem examination reveals widespread myodegeneration in skeletal muscles and the myocardium. Special stains for lipid reveal excessive lipid storage in the heart, diaphragm, and other oxidative postural muscles.142,143 Multiple chain lengths of acylcarnitines are elevated in urine or plasma.144 Supportive therapy including antioxidants such as vitamin C, vitamin E, riboflavin, and intravenous fluids containing dextrose is recommended. Mortality is high in affected horses.
Disorders Associated with Muscle Fasciculations or Myotonia
A variety of muscle disorders exist in the horse that can cause muscle fasciculations, muscle cramping, and, in some, recumbency. These include metabolic alkalosis combined with hypocalcemia, hypomagnesemia, infestation with ear ticks, myotonia congenita and dystrophic myotonia, and hyperkalemic periodic paralysis. With the exception of ear ticks, these disorders are not associated with a marked elevation in serum CK activity.
Hypocalcemia in Horses
Hypocalcemia (lactation tetany, transport tetany, idiopathic hypocalcemia, and eclampsia) is a relatively rare disorder in horses. Clinical signs of increased muscle tone may resemble tetanus. Horses may show a stiff, stilted gait, rear limb ataxia, muscle fasciculations (especially temporal, masseter, and triceps muscles), trismus, dysphagia, salivation, anxiety, profuse sweating, tachycardia, elevated body temperature, cardiac dysrhythmias, synchronous diaphragmatic flutter, convulsions, coma, and death.146 Clinical signs of excitability are usually seen when serum calcium values are less than normal but more than 8 mg/dL. Values of 5 to 8 mg/dL usually produce tetanic spasms and incoordination. Concentrations less than 5 mg/dL usually result in recumbency and stupor. This disorder often progresses and may cause death within 48 hours, particularly in lactating mares. A metabolic alkalosis, hypomagnesemia/hypermagnesemia, and hyperphosphatemia/hypophosphatemia have all been found in association with hypocalcemia in horses.147,148
Treatment involves the intravenous administration of calcium solutions such as 20% calcium borogluconate or those recommended for the treatment of parturient paresis in cattle. Administration of these solutions at the rate of 250 to 500 mL/500 kg diluted 1 : 4 with saline or dextrose will often result in full recovery, although in some horses it may take several days.146 Relapses do occur. If no response to an initial infusion occurs, a second dose may be given 15 to 30 minutes later. Cardiac rate and rhythm should be closely monitored when calcium-containing solutions are infused intravenously.
Ear Tick–Associated Muscle Cramping
Intermittent painful muscle cramps not associated with exercise were described in horses with severe Otobius. megnini infestations.1 These horses show intermittent signs of severe muscle cramping of pectoral, triceps, abdominal, or semitendinosus/semimembranosus muscles lasting from minutes to a few hours, with severe pain that often resembles colic. Horses may fall over when stimulated. Between muscle cramps, horses appear to be normal. Percussion of triceps, pectoral, or semitendinosus muscles results in a typical myotonic cramp. Horses have serum CK activities ranging from 4000 to 170,000 U/L. Numerous ear ticks, O. megnini, can be identified in the external ear canal of affected horses. Without treatment for ear ticks, the spasms continue; however, local treatment of the ear ticks using pyrethrins and piperonyl butoxide results in recovery within 12 to 36 hours later. Acepromazine is helpful to relieve painful cramping.
Myotonia
Myotonic muscle disorders represent a heterogeneous group of clinically similar diseases that share the feature of delayed relaxation of muscle after mechanical stimulation or voluntary contraction. Skeletal muscle ion channel dysfunction producing abnormal muscle membrane excitability or abnormalities in mRNA processing149,150 appear to be the shared abnormality among myotonias in many species.
Clinical Signs
Foals with myotonia congenita usually have conspicuously well-developed musculature and mild hindlimb stiffness.151-153 Gait abnormalities are usually most pronounced when exercise begins and frequently diminish as exercise continues. Bilateral bulging (dimpling) of the thigh and rump muscles is often obvious and exacerbated by stimulation of affected muscles by percussion. Affected muscles may remain contracted for up to 1 minute or more with subsequent slow relaxation. A variety of breeds of horses have been described with this disorder, but an inherited basis for myotonia has not been established.
A more severe progressive form of myotonia that eventually results in debilitating muscle atrophy, fibrosis, and stiffness has been observed in Quarter Horse foals.154-157 At birth, foals with myotonia dystrophica appear well-muscled, and slight stiffness disappears with exercise. As the condition progresses, muscle hypertrophy is less obvious, atrophy may occur, and exercise may produce debilitating muscle contractures. Foals may become so stiff they are unable to stand. This condition resembles myotonia dystrophica in people in that numerous organ systems may be involved. Retinal dysplasia, lenticular opacities, and gonadal hypoplasia have been reported in one such Quarter Horse foal.155
Diagnosis
A diagnosis of both forms of myotonia is best made by EMG. High-frequency crescendo-decrescendo action potentials with a classic “dive-bomber” pattern are characteristic for myotonia. Muscle biopsies may be normal or show fiber hypertrophy and an increased proportion of type 1 fibers with myotonia congenita. Muscle biopsies from horses with myotonia dystrophica show fiber-type grouping, increased numbers of cells with centrally located nuclei, sarcoplasmic bodies, ring fibers, and fibrosis.154,156
Treatment
Foals with myotonia congenita do not usually demonstrate progression of clinical signs beyond 6 to 12 months of age. Phenytoin may diminish clinical signs, but no long-term treatment is available.83 The prognosis for foals with myotonia dystrophica is extremely poor, and most foals are euthanized by 1 year of age.
Stiff-Horse Syndrome
A condition called stiff-horse syndrome has been described in Belgium and is characterized by stiffness and muscle spasms, associated with an abnormally straddled posture.158 The muscle spasms were typically triggered by voluntary movements, such as going to eat from the manger or being led from the stable. EMG showed persistent motor unit activity in the axial and gluteal muscles. The clinical signs were responsive to oral prednisolone therapy but recurred after treatment stopped. Several similar cases have been seen in the United Kingdom.159,160 The etiology of the condition is currently unknown. Similar conditions have been recognized in people, some of which are thought to be autoimmune.
Muscle Fasciculation of Unknown Cause
One horse had a history of sudden onset severe hyperesthesia and intense muscle fasciculations of the longissimus dorsi muscles in the thoracolumbar region, stimulated by light touch.159 No underlying cause was identified. It was speculated that pain may be neuropathic in origin, and the horse responded to treatment with gabapentin (4 mg/kg PO BID), with complete resolution of clinical signs.
Muscle Fasciculation Associated with Osteoarthritis of the Caudal Cervical Synovial Articulations
Several horses have shown an episodic transient forelimb lameness, unresponsive to local analgesic techniques in association with ipsilateral or bilateral fasciculation of the pectoral and shoulder muscles after exercise, and neck stiffness.159 All have had massive enlargement of the synovial articulations between the sixth and seventh cervical vertebrae, with ventral buttressing. Episodic nerve root impingement is thought to be the underlying cause of the muscle fasciculation and lameness. Intraarticular medication with corticosteroids has resulted in short term (months) resolution of clinical signs.
 Hyperkalemic Periodic Paralysis
Hyperkalemic Periodic Paralysis
Hyperkalemic periodic paralysis (HyPP) is an autosomal dominant trait affecting Quarter Horses, American Paint horses, Appaloosas, and Quarter Horse crossbreds worldwide.161-163 The genetic disease has been linked to a prolific Quarter Horse sire named Impressive. Current estimates indicate that 4% of the Quarter Horse breed may be affected.162,164,165 Affected horses may have been preferentially selected as breeding stock for pronounced muscle development, and there is evidence of selection of HyPP-affected horses as superior halter horses by show judges.164
Clinical Signs
Clinical signs among horses carrying the same mutation range from asymptomatic to daily muscle fasciculations and weakness. In the majority of horses, intermittent clinical signs begin by 2 to 3 years of age, with no apparent abnormalities between episodes. Ingestion of diets high in potassium (>1.1%), such as those containing alfalfa hay, molasses, electrolyte supplements, and kelp-based supplements, or sudden dietary changes commonly trigger episodes.161,162,166-168 Fasting, general anesthesia or heavy sedation, trailer rides, and stress may also precipitate clinical signs; however, the onset of signs is often unpredictable without a definable cause. Other possible precipitating factors that have been noted in people and horses are exposure to cold, fasting, pregnancy, and concurrent disease and rest following exercise. Exercise per se does not appear to stimulate clinical signs, and serum CK shows no change to very modest increases during episodic fasciculations and weakness.
In most horses, clinical episodes begin with a brief period of myotonia, with some horses showing prolapse of the third eyelid. Sweating and muscular fasciculations are observed commonly in the flanks, neck, and shoulders. The muscle fasciculations become more generalized as additional muscle groups are involved. Stimulation and attempts to move may exacerbate muscular fasciculations. Some horses may develop severe muscle cramping. Muscular weakness during episodes is a common characteristic of HyPP. Horses remain standing during mild attacks. In more severe attacks, clinical signs may progress to apparent weakness with swaying, staggering, dog sitting, or recumbency within a few minutes. Heart and respiratory rates may be elevated, and horses may show manifestations of stress yet remain relatively bright and alert during episodes. Affected horses usually respond adversely to noise and painful stimuli during attacks. Episodes last for variable periods, usually from 15 to 60 minutes. Several horses have died during acute episodes. Respiratory distress occurs in some horses as a result of paralysis of upper respiratory muscles, and a tracheostomy may be necessary. In addition, young horses that are homozygous for the HyPP trait have been observed to manifest a respiratory stridor and periodically may develop obstruction of the upper respiratory tract.169,170 Horses homozygous for HyPP may have dysphagia or respiratory distress, and endoscopic findings include pharyngeal collapse and edema, laryngopalatal dislocation, and laryngeal paralysis. Once the episode subsides, horses appear normal with no apparent or minimal gait abnormalities. Although HyPP horses appear normal between attacks, examination using EMG reveals abnormal fibrillation potentials, complex repetitive discharges with occasional myotonic potentials, and trains of doublets between episodes.162,171
Etiology
HyPP is caused by a point mutation that results in a phenylalanine/leucine substitution in a key part of the voltage-dependent skeletal muscle sodium channel α-subunit.163 In horses with HyPP, the resting membrane potential is closer to firing than in normal horses. Sodium channels are normally briefly activated during the initial phase of the muscle action potential. The HyPP mutation results in a failure of a subpopulation of sodium channels to inactivate when serum potassium concentrations are increased. As a result, an excessive inward flux of sodium and outward flux of potassium ensues, resulting in persistent depolarization of muscle cells and temporary weakness.
Diagnosis
In most affected horses, hyperkalemia (6 to 9 mEq/L), hemoconcentration, and hyponatremia occur during episodes, but acid-base balance is normal.161,162 Serum potassium concentrations return to normal following cessation of clinical signs. Some affected horses may have normal serum potassium concentrations during minor episodes of muscle fasciculations. Differential diagnoses for hyperkalemia include delay before serum centrifugation, hemolysis, acidosis, renal failure, severe rhabdomyolysis, and high-intensity exercise.
Because veterinarians may not be present during acute episodes, the definitive test for identifying HyPP is the demonstration of the base-pair sequence substitution in the abnormal segment of the DNA encoding for the α-subunit of the sodium channel. Submission of mane or tail hair should be made to appropriate genetic laboratories.
Descent from the stallion Impressive on the sire’s or dam’s side in a horse with episodic muscle tremors is strongly suggestive of HyPP. To increase public awareness of this genetic defect, mandatory testing for HyPP with results designated on the Registration Certificate began for foals descending from Impressive born after January 1, 1998. In response to requests from the membership, in 2004 the American Quarter Horse Association Stud Book and Registration Committee ruled that foals born in 2007 and later testing homozygously affected for HyPP (H/H) will not be eligible for registration.
Treatment
Low-grade exercise can sometimes abort a mild episode, or if horses are just beginning to exhibit clinical signs. Feeding grain or corn syrup to stimulate insulin-mediated movement of potassium across cell membranes may also be helpful. Other treatment options that may abort an episode include intramuscular administration of epinephrine (3 mL of 1 : 1000/500 kg) and administration of acetazolamide (3 mg/kg PO every 8 to 12 hours). Many horses experience spontaneous recovery from episodes of paralysis and appear normal by the time a veterinarian arrives.
During severe episodes, administration of calcium gluconate (0.2 to 0.4 mL/kg of a 23% solution diluted in 1 L of 5% dextrose) will often provide immediate improvement. An increase in extracellular calcium concentration raises the muscle membrane threshold potential, which decreases membrane hyperexcitability. To reduce the serum potassium, intravenous dextrose (6 mL/kg of a 5% solution) alone or combined with sodium bicarbonate (1 to 2 mEq/kg) can be used to enhance intracellular movement of potassium. With severe respiratory obstruction, a tracheostomy may be necessary.
Control
Decreasing dietary potassium and increasing renal losses of potassium are the primary steps taken to prevent HyPP episodes.167,168 Avoid feeding high-potassium feeds such as many electrolyte and kelp supplements, alfalfa hay, canary grass hay, orchard grass hay, brome hay, soybean meal, and molasses. Optimally, later cuts of timothy or Bermuda grass hay; grains such as oats, corn, wheat, and barley; and beet pulp should be fed in small meals several times a day. Regular exercise and/or frequent access to a large paddock or yard are also beneficial. Pasture works well for horses with HyPP because the high water content of pasture grass makes it unlikely that horses will consume large amounts of potassium in a short period. Ideally, horses with recurrent episodes of HyPP should be fed a diet containing between 0.6% and 1.1% total potassium concentration. Because there is a wide variation in potassium concentration of forages depending on maturity and soils, it is advisable to have feeds analyzed for potassium concentrations and other nutrient requirements. Commercially available complete feeds with a guaranteed K+ content may be more convenient for some HyPP horses, especially for owners with few horses.
For horses with recurrent episodes of muscle fasciculations even with dietary alterations, acetazolamide (2 to 4 mg/kg PO every 8 to 12 hours) or hydrochlorothiazide (0.5 to 1 mg/kg PO every 12 hours) may be helpful. These agents work through different mechanisms; however, both cause increased renal potassium ATPase activity. In addition, acetazolamide stabilizes blood glucose and potassium by stimulating insulin secretion. Breed registries and other associations may have restrictions on the use of these drugs during competitions because diuretics mask prohibited substances.
Prognosis
In most horses, HyPP is a manageable disorder, although recurrent episodes may occur and severe episodes may be fatal. Owners of affected horses should be strongly discouraged from breeding these horses for the long-term health of the Quarter Horse and other breeds. Breeding of an affected horse to a normal horse has a 50% chance of producing a foal with HyPP. Owners of affected horses should advise veterinarians of HyPP status before general anesthesia or procedures requiring heavy sedation.