Chapter 68Pathophysiology of Tendon Injury
Functional Anatomy of Equine Tendons and Ligaments
Tendons passively transfer force generated by muscle to bony attachments on the opposite side of a joint, or joints, to provide movement. In contrast the function of a ligament is to resist distraction of its two bony attachments (e.g., collateral ligaments and suspensory ligament [SL]). Although this function is true for most tendons and ligaments, the horse has evolved its digital flexor tendons and SL to exhibit additional functions. These tendons and ligaments, situated on the palmar distal aspect of the equine limb (Figure 68-1), receive large weight-bearing loads because of the hyperextended metacarpophalangeal and metatarsophalangeal joints. As a result the tendons and ligaments on the palmar aspect of the distal limb act to support the metacarpophalangeal and metatarsophalangeal joint during normal weight bearing.
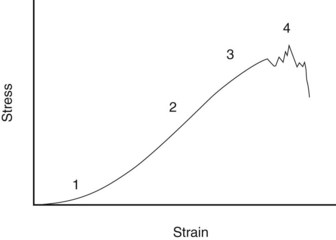
Fig. 68-1 Simplified stress-strain curve for tendon. 1, Toe region; 2, linear deformation; 3, yield, 4, rupture.
(From Goodship AE, Birch HL, Wilson AM: The pathobiology and repair of tendon and ligament injury, Vet Clin North Am Equine Pract 10:323, 1994.)
In addition, the equine digital flexor tendons exhibit considerable elasticity that is used to store energy for energy-efficient locomotion.1 In the case of the superficial digital flexor tendon (SDFT), its muscle is highly pennate (the muscle fibers are arranged at an oblique angle to the line of pull of the muscle, which maximizes power and minimizes contraction distance) and is unable to contract by more than a few millimeters.2 Therefore the action of the muscle, together with its accessory ligament, is largely passive to fix the origin of the SDFT in space. Although the muscle contracts only a short distance, its action, together with the tendon elasticity, also provides shock absorption.2 The gait of a horse at speed can be compared with a weight (the horse’s body) bouncing up and down on elastic springs (the digital flexor tendons and SL) in a similar fashion to a pogo stick’s bouncing.3 This arrangement allows horses to reach and maintain high speeds while minimizing energy expenditure (see Chapter 26).
Functional Characteristics
Biomechanical Properties
When a tendon is loaded, it stretches. The relationship between the force and the elongation defines the structural properties of the specific tendon or ligament. Because these properties depend on the size of the structure, comparison between tendons and ligaments is better made from the material properties of the tissue, which are determined by plotting the force per unit area (stress) against the percentage elongation (strain). A stylized example of such a stress-strain curve for tendon is shown in Figure 68-1. The curve has the following four regions:
Biomechanical Parameters
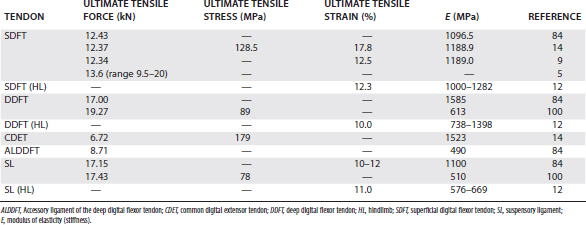
A number of simple biomechanical parameters, which are derived from their structural and material properties, can be ascribed to tendons. Some of the values of these parameters for the palmar supporting structures of the distal limb are shown in Table 68-1. A parameter not frequently calculated, but probably more relevant to the in vivo situation, is the force or stress at the yield point, after which irreversible damage is occurring.
Structural Properties
The ultimate tensile strength is the load at which the tendon breaks. The SDFT receives in excess of 1 metric ton at maximum weight bearing in vivo and breaks at 1.2 to 2 metric tons when tested ex vivo in a materials testing apparatus. Within any population of horses, large variation occurs in the ultimate tensile force, with up to a twofold difference between the weakest and strongest tendons.5
Stiffness is the load required to extend a tendon by a unit length and is the parameter that has to be optimized to the weight of the horse for the tendon to act efficiently as a spring.
Material Properties
One property is the ultimate tensile stress. As the SDFT is only about 1 cm2 in cross-sectional area, the ultimate tensile stress (force at failure per unit area) in the horse is close to 100 MPa, which is at the upper limit of previously documented figures for other species (45 to 125 MPa).6,7 The large variation seen for the structural strength also exists for ultimate tensile stress, indicating that the variation does not result merely from differences in cross-sectional area. It is hypothesized that the horses with weaker tendons are more prone to tendon injury. Another property is the modulus of elasticity or stiffness (E), which is a constant determined from the ratio of stress to strain for the linear part of the stress-strain curve. The modulus of elasticity for the SDFT is about 1000 MPa. Frequently it is correlated with ultimate tensile stress, so that the stronger the tendon, the stiffer it is.
The property, ultimate tensile strain, is the percentage extension of the tendon at its breaking point. In vitro testing of equine digital flexor tendons indicates that they usually extend by 10% to 12% of the original length before they rupture, although values of up to 20% have been reported.8 However, the ultimate tensile strain reflects only the final strain before rupture and includes that yield portion of the stress-strain curve that represents irreversible damage to the tendon tissue (see Figure 68-1). In addition, the ultimate tensile strain is not constant along the length of the SDFT in vitro9; the highest ultimate tensile strain occurs in the metacarpal region (the region most frequently injured).
In vivo, the normal strains in the digital flexor tendons (in ponies) are about 2% to 4% at the walk and 4% to 6% at the trot.10 At the gallop in Thoroughbreds (TBs), maximum strains in the metacarpal region of the SDFT can reach 16%.11 Such strains are far greater than usually expected in tendons from most species and reflect the highly specialized nature of the equine digital flexor tendon. If these high strains are truly representative of the strains within the tendon, they indicate that equine tendon is operating at or close to its ultimate tensile strain. This suggests little tolerance in the system, which explains the high incidence of injury in this structure. However, some caution in the interpretation of in vitro measurements is necessary, because studies have shown different results obtained between in vivo and in vitro tests.10
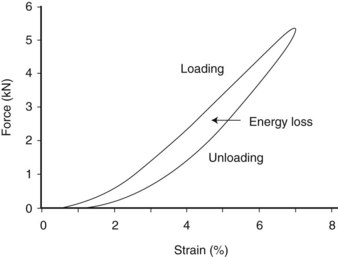
Hysteresis
Hysteresis refers to the energy loss between the loading and unloading cycles of tendon (Figure 68-2), determined from the area between these two curves. Hysteresis is usually about 5% in equine tendons.12 Some of this energy is responsible for the rise in temperature within the tendon core associated with repeated loading (as in an exercising horse), which has been suggested as a causative factor in equine superficial digital flexor tendonitis13 (see the following discussion).
Classification of Tendons and the Relationship to Function
Research has demonstrated that tendons possess different properties depending on function.8,14 The tendons in the horse, like those in people, can be divided into two broad categories: those with the primary function of withstanding the weight of the horse (weight-bearing tendons) and those with the primary function of flexing, extending, or rotating joints (positional tendons). Weight-bearing tendons, such as the equine digital flexor tendons, are more elastic than positional tendons (e.g., the equine digital extensor tendons), which reflects the function of the digital flexor tendons as elastic energy stores. Positional tendons require stiffness for accurate positioning of the limb or digit. Human finger tendons are stiff for such a purpose, and although equine digital extensor tendons are not required for accurate placement of the digit, they nevertheless resemble this category of positional tendons. 14 These differences in biomechanical properties are reflected in the anatomical features of the tendons.
Anatomical Structure
Morphology of Tendons
Tendon is composed of a hierarchical structure of subunits. To the naked eye, in cross-section the tendon substance is divided into a number of fascicles, which are in turn composed of ever decreasingly sized subunits: fibers and then fibrils.
The fascicles are held together by the loose connective tissue, the endotendon, which is confluent with the outside of the tendon, the epitendon. The endotendon contains vascular and neural elements. In regions where the tendons are not surrounded by a tendon sheath, a thick fibrous layer, the paratendon, further surrounds the tendon.
Crimp
In longitudinal section, under a light microscope, the collagen fibers in tendon have a wavy appearance known as crimp. This pattern is responsible in part for the elasticity of the tendon, and it is eliminated in the toe region of the stress-strain curve when the mechanical behavior of the tendon is nonlinear. A generalized reduction in the crimp angle occurs with aging, with a differentially greater reduction in the central fibers.15,16 As a tendon stretches, the central fibers straighten first and therefore receive differentially greater load than the peripheral fibers, which may explain the site of pathological damage in those horses with centrally positioned core lesions. The reason for lesions situated peripherally in a tendon is less clear, unless these are also focal regions of the tendons that have developed atypically straightened fibers. Lesions involving the entire cross-section of the tendon represent a more generalized disruption of the tendon matrix.
Collagen Fibril Diameter
The collagen fibrils are composed of many triple helical collagen molecules arranged in a quarter stagger, which gives a characteristic banding pattern on electron microscopy. These collagen molecules are synthesized by the tenocytes within the tendon, where early events of collagen fibril formation are associated with cellular projections (fibripositors),17,18 which are thought to be responsible for laying down the template of linearly arranged collagen fibrils during development. Subsequent enlargement of the collagen fibrils occurs extracellularly, by covalent intermolecular cross-linking (see the following discussion). Fusion of adjacent fibrils is responsible for the increasing size of collagen fibrils with age.19,20 Soon after birth, foals develop the characteristic of a bimodal or trimodal pattern of fibril diameters in the SDFT, in which the fibrils can be grouped into two or three populations (small [40 nm], medium [120 nm], and large [>200 nm]), whereas positional tendons tend to have a more consistent unimodal distribution of larger diameter fibrils in adults.21
Associated Structures
Blood Supply
Tendons obtain nutrients from two primary processes: perfusion and diffusion. Diffusion of nutrients from compartments other than blood occurs predominantly where the tendon is enclosed in a sheath, the synovial fluid playing an important role in tendon nutrition.
The principal blood supply in tendon arises from three sources: proximally, the musculotendonous junction; distally, the osseous insertion; and between these two, the tendon is supplied by intratendonous and extratendonous vessels. The extratendonous supply arises from the paratendon in extrasynovial tendon and from mesotendon attachments within synovial tendon sheaths (such as the vinculum between the fetlock annular ligament and the SDFT). The predominance of either source in the midtendon region depends on the species and the tendon. In the equine SDFT, two major parallel vessels run longitudinally in the lateral and medial borders of the midmetacarpal tendon, accompanied by an extensive anastomosing network of vessels.22 These vessels anastomose with paratendon blood vessels, although removal of the paratendon blood supply in the horse failed to produce gross pathological damage. However, ligation of the intratendonous supply in the midmetacarpal region produced ischemic pathological damage, demonstrating the importance of the intratendonous supply. The deep digital flexor tendon (DDFT) also has an anastomosing vascular network, except for its dorsal aspect as it passes over the metacarpophalangeal joint, where it has a more fibrocartilaginous phenotype to resist the compressive forces in this region.23
Tendon has been shown to have a good blood supply based on a number of techniques, usually involving clearance measurements of various radionuclides injected intratendonously (most commonly 133Xe and 24Na). The SDFT appears to have good blood supply similar to that of resting skeletal muscle, although findings have been inconsistent among studies and among animals on successive measurements. The large variation in the blood flow under different circumstances may indicate that external factors, as yet undefined, influence blood flow on a day-to-day basis.
Differences in blood flow between the SDFT and DDFT are affected by age, exercise, and injury (Figure 68-3).24 The SDFT has a slightly higher blood flow than the DDFT, which reflects its good vascular anatomy (see the previous discussion). However, studies showed similar functional blood flow throughout the metacarpal region of the SDFT, although histologically and microangiographically the middle and distal regions were less well vascularized.25 However, not surprisingly, the DDFT in the metacarpophalangeal joint region has a significantly lower blood flow, associated with its fibrocartilaginous phenotype, with few blood vessels because of the high compressive forces in this region, which would limit any blood flow.
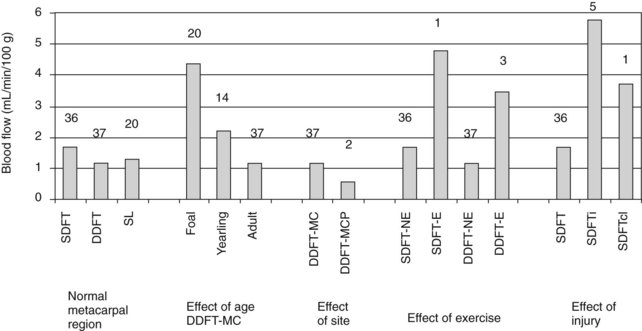
Fig. 68-3 Absolute blood flow in equine digital flexor tendons derived from 133Xe clearance half-times. Numbers above the columns indicate the numbers of tendons evaluated. DDFT, Deep digital flexor tendon; E, exercised; MC, metacarpal region; MCP, metacarpophalangeal region; NE, not exercised; SDFT, superficial digital flexor tendon; SDFTcl, contralateral “normal” SDFT; SDFTi, superficial digital flexor tendonitis.
(From Jones AJ: Normal and diseased equine digital flexor tendon: blood flow, biochemical and serological studies, PhD thesis, 1993, University of London.)
The blood flow appears to be considerably higher in foals than in adult horses, with a gradual decline in blood flow to the adult level by 3 years of age.
Exercise induces an increase in blood flow (about 200%), although this increase is delayed in animals not previously trained. The tendon blood supply therefore appears to exhibit a fitness memory.
Injury provokes a considerable increase in blood flow (>300%), which occurs in both clinically affected and clinically unaffected limbs, consistent with the bilateral nature of tendonitis in the horse, even though one limb is more severely affected than the other. Other measurements carried out in injured tendons have yielded variable results, which have been interpreted as representing the coexistence of fibrous tissue with low blood flow and hyperemic areas of acutely inflamed tendon.
Cellular Components
Although the biomechanical characteristics of tendon are determined by the composition and organization of the extracellular matrix, tenocytes are essential for the formation and maintenance of tendon tissue. At least three different populations of tenocytes are identifiable within the fascicles of normal equine tendon and ligament8,26 (Figure 68-4):

Fig. 68-4 Histological features of equine tendon and ligament. A, Foal superficial digital flexor tendon (SDFT) showing obvious crimp and predominantly type II cells (arrow). B, Young SDFT showing reduction in crimp and increased number of type I cells (arrow). Note also the endotendon septa (star). C, Aged deep digital flexor tendon from the metacarpophalangeal region showing acellular regions and type III cells (arrow), resembling the chondrocyte phenotype associated with compressive loading in this region. D, Chondroid metaplasia (arrow) in an aged SDFT. The acellular areas are visible between the regions of chondroid metaplasia. E, Suspensory ligament branch showing the lines of type II cells (arrows) characteristic of ligament.
The proportion of these cells varies between tendons and ligaments, with tendon site, and with age.27 Young tendon has considerably larger numbers of type II cells arranged between collagen bundles. With aging, type I cells predominate, whereas in the areas subjected to compressive forces, type III cells can be identified.
The activity of these different cell types is unknown. The different cell types identifiable histologically may represent different cell lines or different states of extracellular matrix production. A reasonable assumption is to suppose that type II and III cells are metabolically more active and are responsible for maintaining the tendon extracellular matrix, although the metabolic activity of the type I cells cannot be discounted. There appear to be phenotypic differences between cells recovered from different tendons maintained in vitro, which reflect the matrix of the tendon from which they were derived. Tenocytes recovered from flexor tendons exhibit high cartilage oligomeric matrix protein (COMP) synthesis (see later), whereas those recovered from extensor tendons produce much less COMP but are metabolically more active.28
Ligament has a much higher cell population, with a predominance of type II cells arranged in columns. In the SDFT of the horse, which has greater numbers of cells during growth, total tendon cell numbers remain relatively constant after skeletal maturity. However, acellular areas develop, especially in the center of the SDFT in the metacarpal region, although the degree of acellularity is not particularly related to age.29 More active, type II cells can be found surrounding the fibrils in these regions, as well as chondroid metaplasia. Other areas, typified by the DDFT in the metacarpophalangeal region, also have acellular regions associated with a fibrocartilaginous phenotype (type III cells and cartilage-like matrix) as a result of concurrent compressive forces as the tendon wraps around the metacarpophalangeal joint. Other cells are associated with the tendon, namely the paratendon, epitendon, and endotendon fibroblasts and the synovial-like cells of the epitendon within the tendon sheaths. These cell populations may also play important roles in maintaining tendon tissue, especially because the endotendon harbors certain growth factors, such as transforming growth factor-β (TGF-β) (Figure 68-5).30 Furthermore, as the endotendon contains the vasculature, it is also likely to contain a source of pluripotential cells, which may be responsible for intrinsic healing mechanisms,31,32 although pluripotentiality in cells derived from tendon has showed inferior capabilities compared with cells recovered from a more conventional source, the bone marrow.33
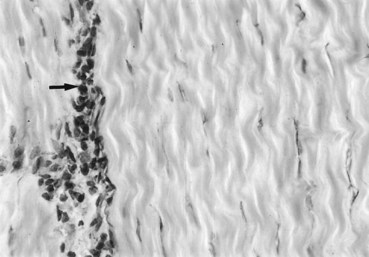
Fig. 68-5 Immunohistochemical staining for transforming growth factor–β3 in equine superficial digital flexor tendon. Note the concentration of stain (arrow) in the endotendon septa.
(Courtesy Eddy Cauvin, Lyon, France.)
The regulation of tenocyte metabolism still is not understood fully but probably relies on a combination of mechanical and cytokine stimuli. Tenocytes have been shown to sense and react rapidly to mechanical stimuli in vitro.34 However, equine tenocytes in culture require the addition of a suitable growth factor to initiate a synthetic response to load.28 The use of confocal microscopy has provided an insight into the relationship among tenocytes. Staining with a membrane dye revealed extensive cytoplasmic extensions from tenocytes, which form a complex meshwork around the collagen bundles. Gap junctions exist between cytoplasmic extensions, which would provide an ideal arrangement for the coordinated biosynthetic reactions to mechanical stimuli.35
Of the multitude of growth factors having effects on connective tissues, TGF-β and insulin-like growth factor 1 have been investigated the most in equine tendon.30,36,37 The synthesis and distribution of TGF-β isoforms in equine digital flexor tendon vary with age. The highest levels are observed in young equine digital flexor tendon, especially within the endotendon.30 Levels decline after skeletal maturity, especially in the tendon fascicles themselves, and this may result in a relative lack of tenocyte synthetic activity after skeletal maturity. However, it is not yet clear which are the most fundamental growth factors in equine tendons and how the growth factor milieu acts to cause tendon matrix synthesis and repair.
Molecular Composition of Tendon Matrix
Tendons are composed predominantly of extracellular matrix, within which is a wide array of proteins, organized and interacting to produce the mechanical properties of tendon. The tendon extracellular matrix is composed predominantly of water (about 65% wet weight), collagen (about 30% wet weight), and noncollagenous glycoproteins (about 5% wet weight).
Collagen
About 80% of the dry weight of the tendon is collagen, of which the predominant collagen type is type I (>95%).8 Type III collagen is present in the endotendon and increases as the horse ages. Type II (the collagen of articular cartilage) is likely to occur at the same sites in the horse as described in other species—namely, tendon insertions and where tendons develop fibrocartilage-like tissue associated with a change in the direction of pull around bony prominences (e.g., at the metacarpophalangeal joint).
Collagen fibrils are strong, but the bonds formed between these fibrils and the higher order subunits are more likely to determine the strength of the tissue. The major covalent cross-link of type I collagen in tendon is between hydroxylysine and lysine residues.38 Lysine and hydroxylysine are converted to the respective aldehydes by the action of the enzyme lysyl oxidase, which is inhibited by β-aminopropionitrile fumarate, a chemical that has been used in the treatment of equine tendon injuries. These lysine and hydroxylysine aldehydes then can form a number of different types of cross-links: reducible (e.g., dihydroxylysinonorleucine and hydroxylysinonorleucine) or nonreducible (hydroxylysylpyridinoline). The reducible cross-links become reduced with age so that at maturity their level is less than 10% of the level in the foal.39
Noncovalent cross-links (electrostatic in nature) are provided by the proteoglycans and other glycoproteins, especially the small proteoglycan decorin, which coats the collagen fibril. Although individually these cross-links are less strong than the covalent cross-links, the high number and involvement in the higher order organization of the collagen network make them potentially major determinants of tendon mechanical properties.
Noncollagenous Glycoproteins
Cartilage Oligomeric Matrix Protein
COMP is a large molecule consisting of five subunits, bound via disulfide bonds at their N-termini to form a five-armed protein, with a bouquet of arms with globular C-terminal domains that can interact with other matrix components (Figure 68-6).40,41 Although initially thought to be restricted in distribution to cartilage, COMP subsequently was found largely in tissues whose function primarily is to resist load. Thus COMP is found in significant amounts in tendon, ligament, cartilage, intervertebral disk, and meniscus. In equine digital flexor tendons COMP shows large variation with site and age.42 Levels are low at birth and in the digital extensor tendon at all ages but accumulate within the digital flexors rapidly with weight bearing. Levels peak in the metacarpal region of the SDFT (at about 3% dry weight of tendon) at skeletal maturity and subsequently decline (Figure 68-7). Levels peak at a lower level in the metacarpophalangeal regions and in the DDFT but are maintained in the former.
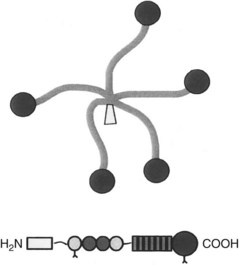
Fig. 68-6 Illustration of the cartilage oligomeric matrix protein molecule.
(Courtesy K. Rosenberg, Lund, Sweden.)

Fig. 68-7 The variation of cartilage oligomeric matrix protein levels with age in the metacarpal region of the superficial digital flexor tendon.
(Modified from Smith RK, Zunino L, Webbon PM, et al: The distribution of cartilage oligomeric matrix protein [COMP] in tendon and its variation with tendon site, age and load, Matrix Biol 16:255, 1997.)
The function of COMP has not yet been elucidated completely, but COMP is known to bind fibrillar collagens (I, II, and IX),43,44 and a mutation in the human COMP gene is responsible for pseudoachondroplasia, characterized by lax tendons and ligaments, short stature, and early-onset osteoarthritis.45,46 Although COMP may have a structural role, present research data suggest that it also may act to bring collagen molecules together to form fibrils, and it may assist in the organization of the collagen network.47 This role may explain the decline in COMP levels after skeletal maturity in the metacarpal region, because the collagen matrix has been formed and limited remodeling occurs in an adult. In the equine SDFT, a significant correlation has been shown between ultimate tensile stress and COMP levels at skeletal maturity.48 Thus high levels of COMP during development potentially enable the formation of a high-quality tendon matrix.
Proteoglycans
Proteoglycans are a group of molecules that possess a protein core and a side chain of sugars (glycosaminoglycans, or GAGs). The sugar side chains are highly variable in type and length so that great diversity exists even within a given tissue. Work on a variety of soft tissues, especially articular cartilage, has demonstrated a large number of different proteoglycans that are vital to maintaining the structural integrity of the tissue by playing structural roles or by regulating the metabolism of the tissue.
Proteoglycans are largely divided into two broad categories, the large and small proteoglycans. The large proteoglycans are exemplified by the major proteoglycan of cartilage, aggrecan, and the fibroblast-derived large proteoglycan, versican. These molecules possess a large number of GAG side chains, and some can form aggregates with hyaluronic acid. With the repulsion of the negatively charged GAG chains, this molecule has a bottle-brush shape and can trap large quantities of water. The swelling potential for this molecule, when restrained by the collagen network of cartilage, produces a structural matrix ideally suited to resisting compression. In tendon, areas subjected to compressive forces develop a matrix rich in these large proteoglycans, such as in the DDFT and SDFT in the metacarpophalangeal region.
The small proteoglycans such as decorin, biglycan, fibromodulin, and lumican usually have only one or two GAG side chains. Many of these proteoglycans have wide tissue distribution and both structural and regulatory roles. A number of these proteoglycans bind to other members of the extracellular matrix. Thus decorin, the most common proteoglycan in tensional tendon (e.g., metacarpal region of the digital flexor tendons), has been shown to bind to fibrils of type I collagen.49 Decorin is thought to be responsible for regulating collagen fibril diameter and, together with the other small proteoglycans, may be responsible for providing electrostatic cross-links between fibrils, thus being also an important determinant of tendon strength. Targeted disruption of the genes for some of these small proteoglycans has confirmed a suggested role in maintaining tissue structural integrity. Deleting or knocking out the decorin gene results in variably sized collagen fibrils and poor mechanical strength in skin,50 whereas targeted disruption of the fibromodulin gene causes altered collagen fibril morphology and reduced mechanical strength in tendon.51
Other Noncollagenous Glycoproteins
A number of other noncollagenous glycoproteins have been described in tendon, including elastin (not thought to be important for tendon elasticity in the horse),39 fibronectin (which is up-regulated after injury), thrombospondin-4, PRELP (proline arginine-rich end leucine-rich repeat protein), and tenascin-C. However, the functions of these, and others that have yet to be characterized, have not been determined fully.
Types of Tendon Injury
Tendons can withstand intrinsic (overstrain) or extrinsic (percutaneous) injury or displacement. The most common injury in the horse is the intrinsic injury of the SDFT in the metacarpal region. Epidemiological data have indicated a frequency of 24% in National Hunt horses in training, rising to as high as 40% in some yards.52,53 Much of our understanding of tendon physiology (as described previously) and pathogenesis relates to the SDFT. Insertional injuries, although common in the human athlete, are rarer in the horse and more commonly are seen associated with the SL rather than the SDFT.
Clinical superficial digital flexor tendonopathy* varies in severity from individual fibril or fiber slippage to individual fibril or fiber rupture and ultimately to complete rupture of tendon with progressive involvement of more groups of fibers and fascicles. Although some overstrain injuries can be caused by simple overload of the tendon, clinical tendonopathy in many mature horses is believed to be preceded by subclinical degeneration of the tendon matrix. This is based on a number of observations. First, postmortem examination of tendons of horses euthanized for reasons other than tendonopathy revealed low-grade pathological damage ranging from acellular areas, chondroid metaplasia, and cyst formation.29,54 Second, tendonopathy is frequently a bilateral disease, although one limb is more severely affected than the other. Although bilateral changes sometimes can be difficult to identify clinically, ultrasonography often confirms some degree of bilateral involvement.52 Third, research has identified a number of changes that occur within tendons, associated with aging and exercise, that are believed to be the major drivers of tendon degeneration that precedes clinical injury.
Mechanisms of Tendon Injury: Effect of Aging and Exercise
Various controlled exercise studies in adult and young horses (Table 68-2) have provided considerable information on the effect of exercise on normal equine tendons. In none of these studies was there any indication of clinical tendonopathy induced by the exercise protocols.
Regional differences in collagen fibril diameter have been seen in long-term exercised older horses, but not in short-term exercised or younger horses.55 Within the central region of the SDFT there was a higher proportion of smaller fibrils in comparison with controls (Figure 68-8). The higher proportion of small fibrils did not correlate with new collagen formation and thus may result from disassembly of the larger-diameter fibrils, rather than the formation of new collagen, which would indicate an adaptive response. Furthermore, the reduction in crimp pattern seen with aging was accelerated by the exercise protocols in adult horses.56
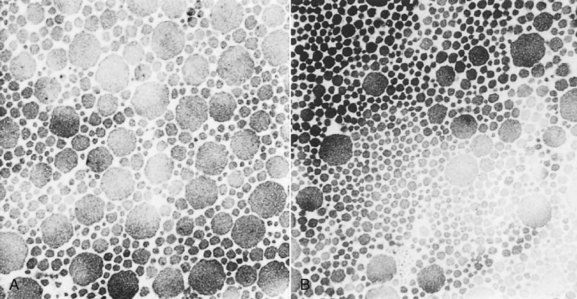
Fig. 68-8 Difference in collagen fibril populations in control and treadmill-exercised yearlings. A, Control. B, Treadmill exercised. Note the increased proportion of small-diameter fibrils compared with the nonexercised cohort (A).
(From Patterson-Kane JC, Firth EC, Parry DAD, et al: Comparison of collagen fibril populations in the superficial digital flexor tendons of exercised and nonexercised Thoroughbreds, Equine Vet J 29:121, 1997.)
Changes in molecular composition also occurred in long-term exercise studies, with a reduction in GAG content and an accelerated loss of COMP in the center of the tendon.57-59 In contrast, molecular analysis of tendons recovered post mortem with central discoloration but no previous diagnosis of superficial digital flexor tendonitis demonstrated an increase in type III collagen and GAG.60 Because these tendons were enlarged substantially and had central hypoechoic lesions when examined by ultrasonography in vitro (Figure 68-9), these molecular changes probably reflect a reparative response rather than a degenerative change associated with aging and exercise.

Fig. 68-9 A, Transverse section of the superficial digital flexor tendon from the midmetacarpal region, showing asymptomatic central discoloration observed in some tendons at postmortem examination. B and C, Note the abnormalities demonstrated when the tendon is examined by ultrasonography in a water bath. The central hypoechoic region and increase in the tendon cross-sectional area in the transverse scans (B) and the central disruption of the fiber alignment pattern in the longitudinal view (C) resemble the changes observed with ultrasonography in clinical tendonitis.
(From Goodship AE, Birch HL: Exercise effects on the skeletal tissues. In Back W, Clayton H, eds: Equine locomotion, London, 2001, Saunders.)
In young growing horses, removal of load from tendon results in a lack of the normal COMP accumulation in tendon with growth, whereas removal of load after COMP has accumulated does not alter its levels in the tendon. Data have shown an association between COMP levels and tendon strength at skeletal maturity,48 so too little exercise may inhibit the ability of the tendon to develop quality tendon matrix. However, exercise studies during skeletal development indicated that tendons are more easily damaged if the exercise level is too high.61
These controlled exercise studies suggest that exercise accelerates a degenerative change that occurs inevitably with aging. Thus the research data suggest that after skeletal maturity a tendon has limited ability to adapt. Instead, cumulative fatigue damage weakens the tendon matrix and allows the initiation of clinical tendonitis when loading overcomes the resistive strength of the tendon, and the tissue tears. In support of this hypothesis, epidemiological studies documented a strong association of age and exercise with the incidence of tendon injury in both horses and people.53,62,63
Further confirmation of cellular activity during growth, but not after skeletal maturity, has been provided by studies of matrix turnover and gene expression. The turnover of collagen was determined for experimental animals, but not for the horse. In experimental animals, collagen turnover was high in neonates and growing animals, but it declined to low levels in adults.64,65 In bovine digital flexor tendons, matrix gene expression, as determined by in situ hybridization, was easily detectable in young, growing calves, but no gene activity was present in the metacarpal region in adults.65 Interestingly, gene activity persisted in the metacarpophalangeal region, which may explain the relative resistance to injury of this region, because of its capability to remodel microdamage. In support of this hypothesis, COMP levels in this region of the equine SDFT and DDFT do not decline after skeletal maturity. The absence of synthetic activity in the metacarpal region appears to arise from a number of mechanisms. Research has shown that flexor tenocytes recovered from older tendons have reduced innate responsiveness28 as well as having reduced levels of anabolic growth factors in the matrix, which act synergistically to elaborate an adaptive response. In studies investigating TGF-β in equine tendons, young equine tendon had high levels, but amounts declined after skeletal maturity.30 Furthermore, there are reduced cell numbers in old tendon, and cells show no ability to increase with exercise,27 reducing the cellular capacity for a response. Gap junction communication, evident in young tendon, through which a synthetic response can be coordinated, is dramatically reduced in older tendon.66
In contrast, young growing tendon does appear to be sensitive to the effects of loading and exercise. The adaptive response of a growing animal may not be constant, and research data suggest that any response may be most pronounced early in life and may decline with growth. The level and amount of work necessary to induce this response are unknown, although, by analogy with bone remodeling in response to load, high strain rates may be the most effective. In support of this hypothesis, epidemiological studies indicated that horses are less prone to metacarpal fracture when they begin training earlier.67 However, as with other skeletal tissue development, such as cartilage, it is hypothesized that a window of opportunity may be exploited to optimize conditioning of tendons for athletic performance (Figure 68-10). The large variation seen in the mechanical properties within a population could be accounted for by variations in tendon development or in rate of accumulation of microdamage, both caused by environmental factors, or it could be genetically determined. Although genetic factors have been linked to Achilles tendonitis in people,68-70 no such genetic determinants have yet been identified in horses, although one study gave a heritability coefficient for SDFT tendonopathy of 0.17.71 Such genetic factors could act by provoking the development of a less competent matrix or giving rise to a poor conformation that may predispose to tendonitis.72 However, the relative contributions of genetics and environment are so closely entwined that it is difficult to apportion causation specifically between the two.
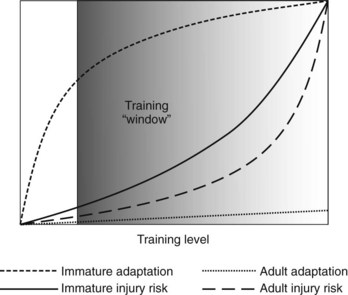
Fig. 68-10 Hypothesized schematic representation of the adaptive response and injury risk for growing (immature) and adult (>2 years of age) equine digital flexor tendons.
(Modified from Goodship AE, Birch HL: Exercise effects on the skeletal tissues. In Back W, Clayton H, eds: Equine locomotion, London, 2001, Saunders.)
Hypothesized Mechanisms of Tendon Degeneration
Mechanical Influences
Sudden overextension of the metacarpophalangeal joint may cause mechanical disruption of the digital flexor tendons. Although this may be the mechanism of certain tendon injuries, such as deep digital flexor tendonitis, direct low-grade mechanical forces, such as experienced under maximal loading, could be responsible for the cumulative fatigue microdamage of the tendon matrix. Subsequent clinical tendonitis is initiated by similar, or sudden supramaximal, loading after the accumulation of microdamage.
Physical Influences: Exercise-Induced Hyperthermia
Because of the hysteresis loop, when a tendon is loaded and unloaded, a loss of stored energy as heat results in temperature increases within the equine digital flexor tendons. Thermocouples have been placed inside the SDFT, and these have recorded temperatures of up to 45° C during periods of galloping.13 Such temperatures are used to kill neoplastic cells therapeutically, so it was hypothesized that these temperatures would interfere with tenocyte metabolism and possibly destroy the cells. However, in vitro experiments have shown that tenocytes are much more resistant to these temperature increases in comparison with other fibroblast-like cells.73 However, these experiments were performed on tenocytes in suspension culture, and more recent experiments in two-dimensional culture have suggested that such temperature rises can adversely influence gap junction communication between cells.74 It is certainly possible that although the cells remain viable with such temperature increases, hyperemia may still adversely influence tendon matrix quality. An alteration in the normal balance between synthesis and resorption activity of tenocytes or a direct denaturing effect on tendon extracellular matrix may occur.
Vascular Theories
Blood flow through tendon is a complex issue, and its relevance to clinical injury is still unsubstantiated. Under maximal loading, blood flow is limited or abolished within the tendon because of the compressive forces generated by the lengthening of the tendon, and this may give rise to relative hypoxia. Indeed, color flow Doppler ultrasonography of equine tendons shows no detectable flow when a horse is standing still, and even flow in the blood vessels of the distal aspect of the limb is sluggish until the horse moves. With injury, an increase in blood flow can be detected using Doppler ultrasonography, but only when the weight-bearing load is removed from the limb when it is raised off the ground. Some areas of equine digital flexor tendons are relatively poorly perfused (e.g., the dorsal portion of the DDFT in the metacarpophalangeal joint region), and this level (but not just the dorsal surface) is a site predisposed to deep digital flexor tendonitis. However, this area also shows histological adaptation to the relatively ischemic environment, with fewer cells and increased amounts of the compression-resisting extracellular matrix components. Furthermore, the tendon may receive some of its nutrition at this site by diffusion from the digital flexor tendon sheath synovial fluid. Similar alterations in the extracellular matrix composition are seen at the corresponding positions in the SDFT, and one could assume that the forces on this tendon would be similar to those of the DDFT at the same site. Therefore a reduced blood flow would also be expected at this level, and yet clinically this region is invariably spared injury in all but the most severe tendonitis.
Equine tendon cells do rely at least in part on oxidative metabolism, although blocking aerobic metabolism does not prevent normal cell proliferation.75 Based on studies in other species, tenocytes may be more resistant to hypoxia than other, similar fibroblast-like cells.
Laser Doppler flowmetry has suggested that a change in blood supply is not the initiating cause in human Achilles tendonitis,76 in contrast to the previously proposed hypoxic cause for tendonitis based on electron microscopic investigations of normal and diseased Achilles tendons.77
Another result of poor perfusion under loading is the generation of toxic free radicals when perfusion is restored. Such reperfusion injury also was proposed as a causative factor for tendonitis through the destruction of tenocytes or tendon matrix by the free radicals, although this at present remains a speculative mechanism.
Proteolytic Enzymes
Various stimuli, including those mentioned previously, could result in the synthesis, release, or activation of proteolytic enzymes. Relatively little information is available on the constitutive or induced expression of proteases in tendon, although activity of procollagenase and aggrecanase was described in human and bovine tendon explants in vitro.78,79 An imbalance between the matrix synthesis and degradation of various extracellular matrix proteins is a possible mechanism whereby the tendon can be weakened and predisposed to clinical tendonitis, and in vitro studies have supported this as a mechanism for the weakening of tendon by cyclical mechanical load.80 Interestingly, the influence of cyclical loading in inducing proteolytic enzyme activity appears to be exaggerated with age, possibly explaining in part the age-related increase in injury risk.80
Factors Affecting the Loading of the Superficial Digital Flexor Tendon and Initiation of Clinical Tendonitis
Peak SDFT forces are responsible for initiating clinical tendonitis. When a tendon has been weakened sufficiently by a preceding degenerative change, factors that increase the peak loading of the SDFT therefore also act to increase the risk of clinical tendonitis.
The SDFT is loaded preferentially at the early stage of the stride,8,10,81 which represents the time of highest injury risk. External factors, such as the rider’s weight or hard ground, increase these peak forces, although possibly only in certain tendons. Data have suggested that landing from a jump increases the peak forces in the SDFT but not the SL.82 The greater height and number of fences jumped at Grand Prix–level show jumping would explain the higher incidence of injury in these horses compared with those competing at a lower level (see Chapters 69 and 115).
The influence of conformation on the risk of superficial digital flexor tendonitis is not clear. Most studies have failed to find an association between conformation and an increased risk of tendonitis.83 However, one study did identify upright metacarpophalangeal joint conformation as being associated with increased risk.72 Foot conformation may also have an influence on the loading of the SDFT (and SL). The lowering of the toe with respect to the heel, or the raising of the heel with respect to the toe, results in reduced loading of the secondary supporter of the metacarpophalangeal joint, the DDFT, thereby increasing the loading of the primary supporter of the joint, the SL (and possibly the SDFT), often only detectable at a trot.11,84,85 As a corollary to this, the long-toe, low-heel conformation characteristic of TBs may actually protect horses from superficial digital flexor tendonitis. However, such alterations in tendon loading may be only short-lived.
The ground surface also potentially influences the loading of the SDFT. Soft ground may predispose to increased strains in the SDFT by allowing the toe to sink. However, using sand has been shown largely to have little effect on strains of the SDFT and DDFT.10 The effect of ground surface on the incidence of tendonitis is probably more related to determining the speed of the horse. Speed is correlated with strains of the SDFT and correlated with the incidence of SDFT tendonitis in racehorses (see Chapter 69Chapter 106Chapter 107Chapter 108Chapter 112). Thus ground surfaces that slow the horse tend to be protective of SDFT tendonitis, whereas the driest and hardest racecourses are associated with the highest incidence of tendonitis.
Many horses develop tendonitis toward the end of a race or event when they are fatigued. Fatigue will cause greater incoordination, which can result in increased peak loads on the SDFT, thereby increasing the risk of injury.
Because tendon degeneration appears to be related to the number of loading cycles, the greater the exercise history and age, the more at risk the horse becomes. This certainly explains the strong association between age and tendonitis and may explain why older, sedentary horses still can develop tendonitis. Because the subclinical phase of tendon degeneration affects both limbs similarly, clinical superficial digital flexor tendonitis is frequently bilateral. Changes are frequently observed with ultrasonography on both limbs,52 although one limb is usually more severely affected than the other.
Strategies for Preventing Tendon Disease
Based on the previously described influences on tendon metabolism and function, a number of preventative strategies can be proposed. First, maximizing the quality of tendon before skeletal maturity with the early introduction of controlled exercise may be possible, thereby reducing the incidence of tendon injury in subsequent racing and competition (Figure 68-11). To test this hypothesis for tendons, two studies have investigated the ability of early exercise regimens to induce a beneficial adaptive response in equine digital flexor tendons. However, neither treadmill exercise nor exercise over ground induced any measurable effects on the digital flexor tendons,86,87 apart from an accelerated rate of growth in the size of the tendon.88 This may have been because the functional ability of the tendon to withstand training and racing was not evaluated or because the additional exercise given to the exercised group of foals was insufficient. Alternatively, the natural exercise performed by foals at pasture included a large amount of jumping activity at play, which is perfectly suited to imparting high strain rates onto the digital flexor tendons, and this potentially induces maximum adaptation in both groups.

Fig. 68-11 Strategy for the prevention of tendonitis in the horse. The dotted line refers to a horse that develops strong tendons, whereas the dashed line represents a horse with poor-quality tendons at skeletal maturity (which appears to be at about 2 years of age). The latter experiences tendonitis during its racing and competitive career because of inevitable and cumulative fatigue damage to the tendon, whereas the former, although having the same degeneration, starts from a stronger point and therefore does not develop tendonitis. The early introduction of exercise during development potentially improves tendon quality (arrow), thereby subsequently reducing the incidence of tendonitis.
A second approach could be to identify genetic influences on the risk of tendonopathy. Certainly, the occurrence of tendonopathy in young flat racehorses may be expected to be more related to genetic factors because the injury is occurring very early in the horses’s exercise history, before degeneration could have accumulated. Work is currently underway to identify any such genetic risks that could potentially be reduced by selective breeding.
Risk factors for clinical injury have yet to be fully identified, but research has indicated that certain racing surfaces (e.g., hard ground89) and shoe types (e.g., toe grabs in Standardbreds, which increase the risk of suspensory disease90) are associated with tendon or ligament disease, and hence avoiding them can reduce the frequency of injury substantially. Epidemiological research into identifying these risk factors systematically is hence warranted and currently underway.91
Addressing the biological basis of tendon degeneration provides a more widespread approach to preventing tendon disease in all athletic horses. The reactivation of the tenocyte synthetic machinery may be more difficult to accomplish because of the factors mentioned earlier. Therefore slowing or stopping the degenerative mechanisms is the strategy most likely to be successful.
Finally, the early detection of subtle clinical disease, or ideally in the subclinical phase of tendon degeneration, may allow an alteration of training to minimize the risk of progression to more severe disease. Although the subclinical phase is currently difficult to identify by palpation or ultrasonographic examination because it causes minimal, if any, inflammatory reaction, in the future, serological assays92 to detect matrix proteins released from the tendon may prove useful for detecting and monitoring this phase. Results to date are not promising.
Pathological Conditions and Phases of Tendon Healing
Once load has overcome the structural strength of the tendon, the tendon tears. The extent of this damage can vary from subtle individual fiber tears, through central defects, to generalized involvement and ultimately rupture. However, even in the last situation, the paratendon usually remains intact. After this tissue failure, repair takes place by three separate but overlapping clinical phases: the acute inflammatory phase, the subacute reparative phase, and the chronic remodeling phase.
The acute inflammatory phase begins with the onset of the clinical injury and lasts usually only 1 to 2 weeks, although this is in part determined by the severity of the injury and the antiinflammatory therapy initiated. This phase is characterized by substantial inflammation, with intratendonous hemorrhage, increased blood supply and edema, and the infiltration of leukocytes, initially neutrophils, but followed by macrophages and monocytes. The pronounced inflammation, if unchecked, results in the release of proteolytic enzymes, which, although directed at removing necrotic collagen, also digest relatively intact tendon collagen, which may cause the expansion of the lesion in the few days after the onset of the clinical tendonitis.
The subacute reparative phase begins within a few days of the injury, overlapping with the acute phase, and peaks after about 3 weeks.93,94 This phase is characterized by a strong angiogenic response and the accumulation of fibroblasts within the damaged tissue. These fibroblasts probably can be derived from a number of sources including the resident tenocytes, endotendon, and paratendon cells, and monocytes of vascular origin, although studies using transgenic rats have indicated that, at least in this species, the majority of the new cells that remain in the tendon after repair are derived from local tissues.95 Increased levels of inflammation appear to be related to increased cellular infiltration and the amount of fibrosis occurring subsequently. The invading cells are responsible for synthesizing scar tissue (tendon tissue is not regenerated), characterized by small collagen fibrils, with an increased proportion of type III collagen,96,97 organized into haphazardly arranged fascicles. The scar tissue formed is initially weaker than tendon tissue, and hence healing tendon is predisposed to reinjury at the injury site. Such episodes of reinjury perpetuate the first two phases and increase the amount of damaged tendon and hence the severity of the injury.
The absence of a paratendon and an externally derived blood supply within a tendon sheath may explain the relative poor response in healing in these areas. The formation of adhesions within tendon sheaths, although responsible for limiting the movement of the tendons subsequently, provides a method of allowing angiogenesis and the infiltration of cells into damaged tendon tissue within a tendon sheath. Hence, although adhesions have deleterious effects on the function of tendons, they are a normal response to encourage tendon healing in this region.
During the chronic remodeling phase, which begins several months after the injury, the scar tissue slowly remodels over a number of months. This remodeling process is associated with an increase in the proportion of type I collagen,98 the major component of normal tendon. However, in spite of this conversion the tissue never becomes normal tendon tissue, although it is probably more functional. Controlled loading (exercise) during this phase may help promote this conversion and, even more important, may align the collagen fibrils in the direction of force, which further improves the mechanical properties of the scar tissue. This aspect of the remodeling process is followed by assessing the fiber alignment score on ultrasonographic images (see Chapter 69).
Reinjury is unfortunately common, even after healing is complete, in the same tendon, the contralateral tendon, or other supporting structures of the metacarpophalangeal joint. As the injured tendon remodels, it becomes stronger, so that fully healed tendon (15 to 18 months after injury) is frequently stronger than normal tendon.99 However, remodeled tendon has poor elasticity, resulting in increased strain in adjacent, relatively undamaged regions of the tendon. Therefore if reinjury occurs in the same tendon, it frequently occurs at adjacent or remote sites to the original injury. Subsequent injury to the contralateral tendon is also effectively a reinjury because of the bilateral nature of the preceding degeneration and clinical tendonitis.100 Subsequent injury to the SL may be the consequence of some loss of support of the metacarpophalangeal joint, caused by substantial lengthening of the SDFT, which can occur with severe superficial digital flexor tendonitis. The SL may also suffer cumulative microdamage, which would increase further its susceptibility to injury.