Chapter 2 Perception
Chapter contents
Horses have been described as being among the most perceptive of animals.1 By studying the sensory perception of horses, we gain valuable insights into their behavior, but we should also be reminded of the care we must take to be consistent in the subtleties of our body language when handling and training them. The differences between human and equine perceptions of the external environment can be explained, in part at least, by the differences in their sensory structures. The horse’s adept perception has allowed it to be constantly aware of changes occurring in its surroundings and this has played a pivotal role in the success of the species. An appreciation and understanding of the horse’s well-developed sensory system are valuable tools, particularly when attempting to understand distinctive aspects of equine behavior.
Vision
The equine eye is among the largest, and held by some to be the largest, in terms of absolute dimensions, of any terrestrial mammal.2,3 Leaving aside the aesthetic appeal of this, it suggests that the horse relies heavily on visual information about its environment. With large retinae and a relative image magnification that is 50% greater than that of humans,4 the horse’s eyes allow it to visualize a wide panorama of the horizon and also the area ahead where feet will be placed and fodder will be selected. As a herbivorous flight animal, the horse has good distance vision, allowing it to scan widely for danger and, despite being relatively poor at accommodation, with a vertical field of 178°,4 it is able to visualize the ground immediately ahead while grazing.
Horse eyes occupy a lateral position on the head, affording a panoramic view in front and on both sides, with only a narrow blind area to the rear (Fig. 2.1). The narrow blind zone at the back of the horse of approximately 20° for each eye,5 can be unveiled by a slight turn of the head. For example, when kicking with its hindlegs, a horse may turn its head to ensure that its target is no longer in the blind area. The width of the blind zone is determined by the level at which the head is carried. As most practitioners appreciate, the blind zone can be effectively increased by cupping one hand around the lateral canthus, an intervention which pacifies many horses that have learned to anticipate aversive stimuli as part of veterinary intervention (see Ch. 14).5
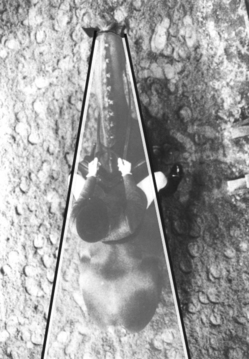
Figure 2.1 Aerial view of a horse showing the blind spot to its rear. The width of the blind spot is influenced by the horse’s head carriage.
(Adapted, with permission, from photograph 6.1A in Equestrian Technique by Tris Roberts, London: JA Allen; 1992.)
The price horses pay for having laterally placed eyes is that the muzzle gets in the way of forward vision. Depending on the carriage of the head, the particular breed and the setting of the eyes, there is a blind zone extending almost 2 m directly in front of the horse. When the head is down the horse’s binocular field is located down the nose in the direction of grass. Therefore horses can see where they eat especially well. This is why, if they do want to see directly in front rather than down the nose, horses have to lift up the nose and point it at the object of interest.
The exposition of more sclera is often noted in anxious animals (Fig. 2.2) because the eyes are opened wider to take in more visual information that may help them resolve the situation, while a fixed eye may be associated with reasonably chronic distress. It is widely believed that the extent of oscillation of eye movements and the amount of sclera shown can be helpful in assessing the disposition of horses,6 but, if the horse has a relatively small iris, that may detract from the reliability of this effect.

Figure 2.2 Rearing horse showing the white of its eye.
(Reproduced with permission of the Captive Animals Protection Society.)
Acuity
There are a number of aspects of vision that can be measured. Visual acuity describes the ability to distinguish the fine details of an object. Clearly, the nearer an object is to the eye, the finer the detail that can be distinguished. Horses’ eyes are geared to be focused largely on more distant features and, like those of many mammal species, appear to have limited accommodation, i.e. the ability to focus on very close objects less than about 1 m away. Animals that have been trained to discriminate between panels painted gray and those bearing black and white stripes can expose their species’ acuity. As panels with ever finer stripes are presented to the observer, the discrimination task becomes more difficult (Fig. 2.3). The point at which the lines blur together and appear to be gray (the limit of acuity) is marked by a failure to discriminate. Most birds that have been tested excel at this task. They can distinguish extremely narrow stripes that occupy as little as 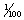 th of a degree of their visual field. Horses can perceive stripes that fill about
th of a degree of their visual field. Horses can perceive stripes that fill about 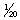 th of a degree.
th of a degree.

Figure 2.3 A system of vertical lines can be presented progressively closer to one another to measure visual acuity.
(Reproduced with permission of Alison Harman.)
An interesting way of expressing the horse’s acuity is in comparison with normal human vision, as 20/33. This indicates that a horse can only discern at 20 m what a human can at 33 m,7 and this compares favorably with the dog (20/50) and the cat (20/100).7 Horses’ eyes are extremely sensitive to movement in all areas of their visual field, but human peripheral vision is considered a good approximation of the visual detail horses can appreciate.8
The visual field is affected by the corpora nigra, which are found on the upper margin of the pupillary aperture, possibly as an anti-glare device.9 The corpora nigra contain an intricate network of blood vessels, which suggests that they might also be used to oxygenate the anterior chamber of the eye (Alison Harman, personal communication 2002). For horses to see through their narrow pupils, they adjust their head position up and down or side to side. Best frontal vision of the ground in front is achieved when the horse flexes slightly at the poll. Horses commonly hold their heads in this position when they are moving in slower gaits. This was thought to improve focus and enhance images of the ground ahead.6 However, when over-flexed so that the nose is behind the vertical, the horse cannot see the space in front of it and so, when being ridden, may occasionally collide with objects, people and other horses if not directed (Fig. 2.4).

Figure 2.4 (A) The visual field in front of a horse when allowed to carry its head naturally. (B) The blind area in front of a horse when over-bent.
((B) Reproduced with permission of the Captive Animals Protection Society.)
A functionally important blind spot is created when a horse is ridden with its nasal planum behind the vertical. The blind spot is formed to the front of the horse, and is believed to be as wide as the body. Thus, when a horse is being ridden in such a fashion it cannot see directly in front of itself. Hyperflexed horses are said to be showing signs of submission and ‘listening to their riders’, but it is possible that compromising a horse in this way makes it more reliant on the rider to avoid obstacles and such that it seems more biddable. Before using physical constraints, such as tie-downs and standing martingales to keep the head down or overchecks to keep it up, one should consider the effect of these restraints on the horse’s ability to convey itself safely over rough terrain and most especially over jumps. The effect on vision is subject to current debate since Bartos et al10 drew attention to the ability of the horse to rotate its eyeball to maintain the pupil in a horizontal position. These authors claimed that the horse maintains the ‘horizontal eyeball position regardless of head position’, suggesting that this rotation overcomes any deficit in vision that might otherwise arise from the horse having its nose pointed caudally by the rider. However, they did not report the head positions they examined, and their photos seemed to imply that the nose was always somewhat in front of the vertical. They were not concerned with forward binocular vision and did not use any technique such as ophthalmoscopy to determine visual field11 or alignment of the pupil relative to the corpora nigra, so it is difficult to comment on their findings. Some recent data counter the generalization that pupil rotation can overcome all changes in head position, indicating that in extreme head positions the pupil is not parallel to the ground12, but further work is needed to confirm this.
The horse has mostly monocular vision, i.e. each eye sees a completely different field of view. However, horses have a small binocular field at the front of the monocular fields. Therefore, the horse can adjust its view to overlap the visual fields of both eyes and achieve a binocular view (Fig. 2.5). This binocular field of view allows the horse to observe the ground in front with both eyes.

Figure 2.5 The visual field of one eye of a horse. Horse and human – (A) rear view, (B) front view – are standing looking out over Perth from a viewpoint in Kings Park. So in front of them they see Perth and to the back of them is a man taking a photo of Perth. The human view (C) is of Perth itself and not much more, since our vision is so frontal. Also we see just the very middle in high acuity. The horse (D) by contrast, sees Perth and everything else simultaneously right back to the man taking the photograph.
(Reproduced with permission of Alison Harman.)
To see objects at a greater distance, the horse rotates its nose upwards because its binocular overlap is oriented down the nose (Fig. 2.6). It is believed that when focusing on objects to the fore, horses may momentarily lose the ability to observe from the rear and to the sides.7 That said, when taking off for a jump, horses sometimes tilt their head sideways, using their lateral vision to get a better look at jumps as they get up close. Perhaps this is why blinkers have found little favor in show-jumping circles, the traditional source of a multiplicity of gadgets. Blinkers are most effective in preventing shying and have been favored by carriage drivers because they make horses less likely to attempt to turn in the shafts or bolt. It is also suggested that blinkers can render a horse more responsive to voice commands used to increase speed because they prevent it from seeing when the driver is neither carrying nor about to use a whip (Les Holmes, personal communication 2002). Blinkers on racing animals bring rather different benefits – especially, it seems, when used for the first time. It has been suggested that, if it is a generally low-ranking animal, a racehorse that sees another horse approaching from behind is more likely to defer to the challenger if not wearing blinkers. This assumes that more high-ranking horses are more motivated to take the lead in a galloping herd, a hypothesis that has yet to be tested.


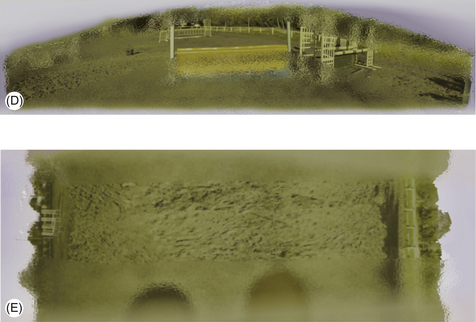
Figure 2.6 (A, B) Show jumper approaching a jump with the horse’s head either (A) up or (B) down. Visual field is indicated. (C–E) A view of what a horse sees when approaching a jump with head (D) up or (E) down. (D) shows that the horse sees the jump and also a lot of other things out to the side. (E) shows that if you hold the head down it sees its foot and knee and a bit of the world out to the side. Perhaps this is why a horse may be more compliant when its head is held down, since it is unlikely to make much sense of the visual world. (C) Shows what a human sees over the jump (remember we only really see the middle of our visual field in high acuity).
(Reproduced with permission of Alison Harman.)
It has been shown that the horse’s eye has a visual streak (Fig. 2.7). This linear retinal region contains high concentrations of ganglion cells, while low concentrations appear in the peripheral regions. Concentrations in the visual streak reach 6100 cells/mm2, with the peripheral regions ranging between 150 and 200 cells/mm2.11 It is worth noting that the distribution of ganglion cells within the visual streak of horses depends on skull shape. Long-skulled (dolichocephalic) breeds, such as the Standardbred, have less centralization of their ganglion cells than the short-skulled (brachycephalic breeds), such as the Arabian, and are therefore thought to have better peripheral vision13 (Fig. 2.8).
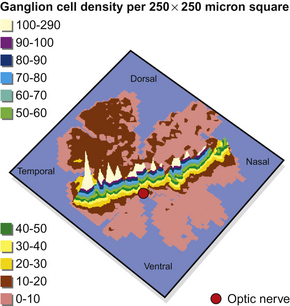
Figure 2.7 The distribution of ganglion cells on a flat-mount of a horse retina. High concentrations are represented by the peaks.
(Reproduced with permission of Alison Harman.)
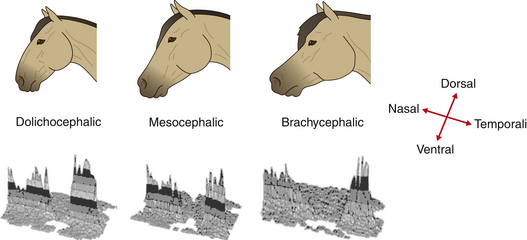
Figure 2.8 Retinal ganglion cell density maps from horses of three breeds showing the dorsal part of the retina is in the background, ventral is in the foreground, nasal is to the left, and temporal is to the right. Each shaded band represents 400 cells/mm2. Studies of the retinae of horses with different skull shapes have shown that (at least some of) the neural tissue of morphologically diverse breeds differs.
Depth perception
It was long believed that animals with laterally placed eyes and extensive monocular visual fields did not have stereopsis – the ability to see in stereo and perceive depth. However, studies14 have demonstrated that the horse’s binocular field is an arc of approximately 60° in front of the head, affording good stereopsis and thresholds of depth detection comparable to those of cats and pigeons. These findings indicate that larger interocular distances, as found in the horse, may provide useful depth judgment. This may have arisen because the horse has evolved to make judgments over a range of several meters, whereas ground-feeding birds, such as pigeons, with an extremely small interocular distance, have to focus at a distance of only a few centimeters. Horses also use monocular depth cues to judge distance.4 This makes sense because they spend so much of their day with their heads to the ground, a position that makes stereopsis redundant.
Harman et al11 suggest that when a horse lifts its head the binocular area of vision is directed at the horizon, enabling scanning and depth perception. In this position monocular lateral vision is compromised. However, when the head is lowered and the binocular vision is directed at the area directly in front of the head, the lateral monocular fields afford good lateral horizon vision.
Effective use of the binocular field is required when a horse attempts to discern an object that is close and low. The horse is best able to use its binocular field of view by arching the neck and rotating the head. It can focus on the object by simultaneous rotation of the eye downward to optimize orientation of the visual streak (see p. 37).
Stimulus visibility
Factors that affect the visibility of a stimulus for a horse include size of the object, contrast and environmental illumination. When a moving horse spots something underfoot, it not only looks at the stimulus, but is also likely to change speed.15 The level of arousal plays a part in the recognition of stimuli, an outcome that may be influenced by the horse’s age and training because recognition of distant stimuli on the ground is facilitated by carrying the head at a lower angle. Saslow15 found that younger animals tend to carry their heads higher and therefore may not notice stimuli as readily as older horses, especially those that have been trained not to carry their neck straight and head high.
Saslow15 also found that horses were able to discern stimuli better in overcast rather than bright sunny conditions. This suggests that the equine rod-dominated eye may not find bright conditions as favorable as dull conditions. That said, naïve horses are often reluctant to make the transition from a well-illuminated area into relative darkness. This is part of the challenge that trailers (floats in the USA) represent. The high proportion of rods to cones (generally 20:1)16 gives the horse excellent night vision but insufficient to make them innately fearless of areas that are poorly lit. As we will see in Chapter 4, horses will work to keep a stable illuminated, and this further explains the aversiveness of small dark spaces, including trailers (Fig. 2.9). A reflective layer of cells behind the retina, the tapetum, enhances vision in poor light. Acting like a mirror, the tapetum reflects light back on to the retina, enabling further light to be gathered. The downside of this arrangement is that image quality is somewhat compromised. Because several receptors may be stimulated by incoming light, acuity can be reduced and the image becomes fuzzy. This effect has been likened to the pixelation of a low-resolution digital image.4
Accommodation
Horses have a small degree of ciliary accommodation,17 which relies on the contraction of ciliary muscles and contractile fibers that extend into the corpora nigra. However, despite weak lens muscles, horses’ optics allow them to see, on the whole, between about 1 m away and infinity, with little need to vary the lens. Accommodation is required if there is a need to see even closer than that with high acuity. Horses rarely need to do that; indeed because the eye’s proximity to objects is generally limited by the length of the nose,18 things very close are felt via the skin and vibrissae of the muzzle, so highly focused vision is not essential.
According to what was originally referred to as the ‘ramp retina’ theory, it was believed that the distance from the nodal point of the eye to the retina varied so that the dorsal retina was farther away than the more central and ventral regions. This theory was supported by the observation that horses are likely to exhibit characteristic head-moving behavior when looking at things. For example, the head may be raised unusually high and the nose pointed forward when observing an object of interest to the fore. The horse may arch its neck sideways (cock its head) to look at an object of unusual interest beside it (Fig. 2.10).

Figure 2.10 Horse raising and tilting its head to look at a pony foal.
(Reproduced with permission of Animal Science Dept, Iowa State University.)
First refuted by Sivak & Allen,19 the ramp retina theory has fallen out of favor. By demonstrating that, except for the far dorsal and far ventral retina, the distance between the retina and lens was the same at all points on the retina, Harman et al11 confirmed the absence of any ramp. Therefore, because movement of the head would not alter the focus of the image on the retina, they inferred that the horse has dynamic accommodation ability.
The arrangement of its visual streak gives the horse a very narrow, but panoramic view. The reason the horse cocks its head sideways is to ‘look’ at an object with the visual streak. Movement of the head may bring into focus images that originally fell onto the regions of low acuity, in the same way that we may see movement in our peripheral visual field and turn towards it to see, with our high-acuity retinal region, what is the cause of the movement. The horse sees a movement in the peripheral visual field and reacts defensively. This may explain why a horse will suddenly raise its head and shy away from an object that has suddenly entered its field of view.
Accommodation in the horse appears to be no more than one diopter (light-bending power) in either direction.11 In optical terms, horses are emmetropic with limited accommodation, which means they can see everything well but cannot focus close up. The normal horse’s eye appears to be correctly focused with a tendency to long-sightedness (hyperopia) when older. It has been suggested, though, that domestication, inbreeding and constant stabling may lead to horses becoming myopic.11 Though yet to be tested, the hypothesis (derived from work in human infants exposed to night lights while asleep) is that if a young horse has limited possibilities to focus into the distance and instead looks only at close objects (because the stable is often a visually limited environment with dim light), then it may have a tendency to be shorter sighted (Alison Harman, personal communication 2001).
Color vision
Leblanc & Bouissou20 showed that mares, when presented with their own and an alien foal, used visual recognition from a distance to identify their offspring. However, when the mare was presented with foals of similar coat color, other sensory responses were required for identification. Notwithstanding this interesting exception, horses have relatively little need for color vision. The equine retina does, however, provide both morphological and electrophysiological evidence for color vision. Although rods dominate, both cones and rods are present in the retina, and there is clear functional duality of responses indicative of cones and rods.21
It has been suggested that, like all mammals (with the exception of primates, some of which are trichromats), horses are dichromats22 and that they struggle to discriminate between green and grays of similar brightness. Smith & Goldman21 suggested that the color discrimination of the horse has no neutral point at which color can be distinguished from gray. Their horses responded to blue, green, red or yellow versus gray at any brightness.
Different colors, from the short-wavelength purples and blues, through the spectrum to the long wavelengths can be tested using color boards arranged in pairs. If the horse distinguishes between colors correctly it finds that it can push the board to reveal a food reward (see the case study at the end of this chapter). However, the problem is to ensure that one adequately controls for brightness or luminance.23 Early studies on color vision in horses trained horses to choose between a colored stimulus and a gray one.21,22 In an attempt to eliminate brightness cues, several gray stimuli were used for comparison with each color. Conflicting results from these studies suggest problems with methodology and raised the possibility that horses may be better than humans at discriminating between gray panels of different luminance.
Investigations of equine responses to color discrimination tests can be further thwarted by lack of motivation in the horses. Horses have been trained to use the color of a central panel to signal a correct (left or right) lever-pressing response.24 However, discrimination performance was better when the combinations were differentially reinforced by two types of food than when by a single reinforcer. Interestingly, the stimulus color of the preceding trial interfered with discrimination performance on a given trial.
The most recent behavioral studies seem25,26 to confirm that horses have only dichromatic vision, similar to red-green color deficiencies found in some humans27 in that they fail to discriminate the color red as easily as they discriminate the other colors from grey. The continued exploration of equine vision, and perhaps even the painting of obstacles for horses in competitions, including show-jumping, should take account of these findings. Retrospective studies of the competition performance of 72 show-jumpers attempting to jump a total of 343 obstacles showed that the number of faults at a particular obstacle depended on obstacle-type, height and arrangement but also color. For example, obstacles of two contrasting colors were jumped without fault more often than those of one (light or dark) color.28
Given that cones are maximally sensitive to particular wavelengths of light as determined by their opsin content, analysis of pigment provides the clearest evidence for dichromatic vision in horses. Microscopic studies of the retina support evidence from recent behavior studies29 by showing that there are two peaks in the spectral sensitivity of equine cones at 428 nm and 539 nm.30 This translates into two basic hues: pastel blue and yellow. It is important to remember that, for dichromats, there are no intermediate hues as there are in the visual world of trichromats, such as humans. Instead, when colors from the two ends of the dichromatic spectrum are mixed, the result is a desaturated version of one of the basic hues or an achromatic region (white or gray). These differences are represented most clearly by the color wheels in Figure 2.11.
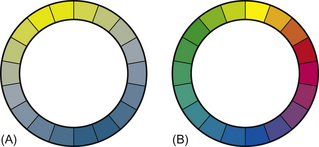
Figure 2.11 The differences between (A) the dichromatic color vision of the horse and (B) that of trichromats, such as humans, are most notable in the number of different colors seen.24
(Reproduced with permission of Joseph Carroll.)
Foal vision
Consistent visual stimulation during neonatal life is required for proper development of the visual pathways.4 When a foal is born the eyes are open and it is assumed that there is some degree of visual function. Important functions of the visual system develop after eye opening. In humans or cats, for example, limited or no input to one eye renders it amblyopic or functionally blind, since no allocation is made for its input in the visual cortex. Similarly, the development of a binocular field, i.e. when both eyes are in register, takes place some time after birth (up to several weeks in cats, 5 years in humans). This is the ‘critical period’ during which the input to the visual cortex becomes fixed. We do not know what this period is in equids, but by extrapolation from cats and humans, it probably occupies the first few months (Alison Harman, personal communication 2002).
It has been suggested that neonatal foals are short-sighted because the muscles used in accommodation are relatively weak.6 This may account for their apparent reliance upon tactile and gustatory exploration, and their occasional collisions with objects, including fences. Exploring ways in which the eye of the neonatal foal is functionally different to that of the mature horse, Enzerink31 found that foals developed a menace response (avoidance of a hand raised up suddenly to the level of the horse’s eye – a crude indication of ophthalmic health) several days post-partum. It is suggested that with open eyelids and the lack of a menace response, foals could be predisposed to eye trauma. However, the absence of a menace response does not provide sufficient reason to stable foals in the first weeks post-partum for fear of globe trauma. Newborn foals are generally well protected by the mare, and globe trauma is not a common finding in healthy foals. The pupillary light response is evident from birth; however, a functional visual cortex is not required to initiate the response and, therefore, a positive response does not exclude a visual deficit.31
Problems with vision
Horses with partial blindness are potentially more dangerous than those that are fully blind, because they may suddenly see objects and react with surprise. Horses with impaired vision often flick their ears rapidly during locomotion and show excessive sensitivity to sounds. Extraorbital causes of impaired vision may include lesions in the brainstem, cerebral cortex, optic nerve or superior colliculi as well as electric shock, serum hepatitis, or poisoning (for example with hypericum, lead and selenium). On the other hand extreme sensitivity to light is noted in recurrent uveitis, equine viral arteritis and riboflavin deficiency.
Chemoreception
In the horse, smell and taste are linked neurologically as they are in many other species.
Smell
Horses familiarize themselves with foreign objects by smelling them. There is evidence that horses investigate urine odor for information that may help them to discriminate between conspecifics on the basis of broad categories, such as sex or reproductive status.32 Social exchange by sniffing one another’s breath usually, but not always, with a closed mouth, represents an important part of greeting rituals between horses. Forced exhalations help to drive air from the nasal cavity in advance of deep inhalations that allow the horse to sample odor molecules. Rarely do humans allow sufficient time for horses to gather much of this sort of information. At the same time, we should remember that because of the combined effect of bathing, using soaps, changing clothes and manipulating all sorts of materials with our hands, our odors are likely to change over time in a way that thwarts their reliability for horses. Horses use odors to recognize familiar foods and those for which they have a particular need.33 The strategic use of agents, such as peppermint essence, that mask the flavor of food and water can overcome capriciousness when horses are presented with novel resources, for instance, as a result of transit, competition or sale.
Olfactory receptors that generate the sense of smell are found in the upper part of the nasal cavity within the mucous membrane. Odorous molecules bind with these receptors and initiate the neural signals that may be processed into strong associations, some social and others sexual. Having a long nose, the horse has a predictably large area of olfactory mucosa (see Ch. 3).
In addition to the conventional olfactory system, the horse contains an accessory olfactory system in the form of the vomeronasal organ (VNO, also known as the Organ of Jacobson). This paired tubular organ is also present in many other animals. It is found inside the horse’s nose within the hard palate and is used to detect pheromones in urine and other moderately volatile odors. The horse uses its VNO during the flehmen response in which it raises its head and rolls back its upper lip (often anthropomorphically labeled a laugh), forcing smell-laden air through slits in the nasal cavity into the VNO (Fig. 2.12). The response is often seen in horses conducting a thorough investigation of other horses’ urine and feces but may also occur when they encounter novel flavors and nasal irritants. Although gravity assists in this sampling procedure, it has been shown that the lumen of the tubular portion of the VNO alternatively expands and contracts to pump its content in the direction of the accessory olfactory bulb.34 In contrast to many other species, the VNO of the horse does not open into the oral cavity.35 Rather than being restricted to exhibition of flehmen only after direct contact of the lips or tongue with urine, horses are unique in that they can flehmen in response to volatile substances borne in the air.35

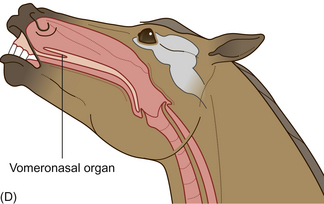
Figure 2.12 Flehmen response seen in adult and juvenile horses of both sexes but most commonly observed in mature stallions, especially in response to estrous mares. The typical response includes (A) elevation of the head, rolling back of the eyes, rotation of the ears to the side and (B) eversion of the upper lip. It may also involve (C) some flicking of the tongue and lateral rolling of the head. (D) Section of horse’s head showing position of vomeronasal organ during flehmen.
(Reproduced with permission of: (B) Jo-Anne Rooker; (C) Francis Burton. (D) Redrawn from Waring 198340 with permission.)
Colts show more flehmen than fillies but intriguingly foals of both sexes show the response more often than do their mothers.35 This suggests that the response has a role in the development of both sexual surveillance and pheromone processing. That said, foals do not appear able to discriminate between estrous and non-estrous urine.36 As yet, the importance of pheromones in triggering maturational change in adolescent horses can only be inferred from studies in other species. The flehmen response offers a powerful signal to observing horses and seems to have an important role in courtship. Stallions can discriminate between estrous and non-estrous mares, but their ability to do so seems to depend on supportive visual and auditory cues rather than on olfactory stimuli alone.37,38
The recent emergence of a commercial equine appeasing pheromone (EAP), a synthetic analogue of the esters found in the skin of a lactating mare’s udder being marketed as a calmative, offers a fascinating insight into the importance to horses of various volatile molecules that humans cannot detect. EAP may facilitate habituation to acute stressors or to stressful situations. For example, it has been reported to reduce restless behaviors in foals at the time of weaning and in reactive horses presented with stimuli associated with clipping.39 The treatment effects of EAP were said to persist after six weeks.
Taste
Taste, like smell, is a result of interactions of chemical stimuli with receptors on a mucous membrane. These receptors are papillae found on the tongue (Fig. 2.13). The taste sensations perceived by the horse are presumed to be gradations of salt, sour, sweet and bitter.40 Umami has not been tested.

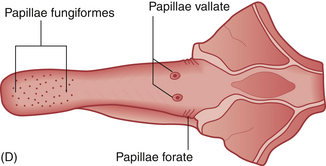
Figure 2.13 (A–C) Horses often attempt to get rid of foul-tasting materials (including many oral medications) from their mouths. (D) Distribution on the equine tongue of the papillae that house taste.
(Reproduced with permission of: (A, B) Jo-Anne Rooker; (C) Tanya Grassi. (D) Redrawn from Waring 198340 with permission.)
Just as it is pivotal in the early bonding of a mare with her foal, taste may be used when two horses groom one another.41 Taste may also help horses to determine the caloric content of foods, as well as allowing animals to discriminate among different foods and exercise their preferences.42 Studies have shown that horses will learn to avoid a food associated with illness.43 Gustation may also provide nutritional information about food. For example, if the horse’s diet is deficient in salt, it may preferentially select feedstuff higher in salt content over another not so high.44
While the sense of taste may also provide information about the toxicity of food, this faculty is far from foolproof.41 It appears that horses differ individually in their ability to avoid bitter additives and Senecio species, including ragwort.45 This may have practical implications in deciding which horses may safely graze on pastures infested with toxic plants.
Taste also regulates digestive processes that initiate further processes such as enzymatic secretions. A normal appetite in the horse is determined primarily on the basis of pre-gastric stimuli such as taste, texture and smell.46 The regulation of feed intake is also greatly influenced by taste, and this is of importance in maintaining the body’s normal chemical balance. Habituation by gradual exposure to increasingly concentrated solutions of innately aversive chemicals has been reported in horses as an adjunct means of modulating water intake in performance horses.47
Hearing
The equine sense of hearing is very well developed. The horse’s funnel-shaped ears can move in unison or independently of each other. Using 10 muscles, the ears can be moved around a lateral arc of 180°, enabling accurate location of the source of the sound.48 Horses with impaired hearing may show more drooping of the ears. The direction in which the ears point helps to indicate in which direction a horse’s attention is focused (Fig. 2.14). This seems particularly useful when horses, as group animals, may have their vision obscured by the bodies of their companions. As such, ear direction may contribute to social referencing in horses. When outdoors, horses seem to be able to use body positioning in sound detection, e.g. it is suggested that, if they position themselves appropriately, horses can amplify sounds by bouncing them off their shoulders.6 Horses are able to locate the source of a sound within an arc of approximately 25°. This compares poorly with hunting species such as dogs and humans that are accurate within a degree. However, it seems that horses are well-equipped to hear faint noises. For example, they can respond to sounds from up to 4400 m away.49

Figure 2.14 The direction of the ears of this horse in a roundpen indicates that it has its attention on the handler.
Horses are better than humans at discriminating between noises of similar loudness. Horses can also protect their ears from very loud noises by laying them flat. The aversive effects of a combatant’s squealing during a fight may be tempered by this response. In comparison with the human, the horse is able to hear higher-pitched sounds. A human’s range of hearing is between 20 Hz and 20 kHz while a horse’s is 55 Hz to 33.5 kHz,50 being most sensitive to sounds in the range 1–16 kHz, a broader range than most mammals. Horses can therefore hear high-pitched sounds that we cannot, but not some of the lower frequency sounds that we can hear. This is thought to arise from the shorter interaural distance horses have compared with humans.51 Equine sensitivity to ultrasound helps in determining the source of noises. It may be that we should become more sophisticated in our exploitation of this difference, for example in the development of training aids and secondary reinforcers. There seems to be an interaction between visual and auditory perception, with an especially interesting correlation across a number of mammalian species between sound localization ability and the width of the field of best vision.52 Species with small foveae or areae centralis have good localization thresholds while those with large fovea have poor localization thresholds. With its characteristic visual streak the horse falls into the latter category, having a long narrow field of good vision that most probably allows it to pinpoint the likely source of a sound without needing accurate identification of the auditory locus.29
There is some suggestion that horses can respond (with nervousness and vocalization) to sounds of very low frequencies, such as geographical vibrations preceding earthquakes.53 It is thought that they do not ‘hear’ as such, but can detect the vibrations through the hoof.
Studies have shown that there is no difference in hearing ability between adult mares and geldings.54 However, there is a significant difference between ‘adult’ horses and ‘old’ horses, suggesting that the ability to hear sound of a higher frequency decreases with age.54
We do well to talk to horses with whom we seek to form a bond. Unlike the visual and olfactory properties we provide, our voice is constant and is therefore a more reliable cue for recognition.55
Touch
The sense of touch is variable over different areas of the horse’s body. The withers, mouth, flank and elbow regions are very sensitive areas. Some horses dislike their ears, eyes, groin and bulbs of the heels being touched. As herd animals it is important that they are sensitive to the presence of others at their sides. This may help them to move as a cohesive social group in times of danger and to initiate bouts of mutual grooming (see Fig. 2.15 and Chs 5 and 10). When riders use their legs to move horses beneath them they are capitalizing on this innate sensitivity and do well to preserve sensitivity to pressure signals from their legs. But, by the same token, from early foundation training we usually expect horses to habituate quickly to relentless pressure from the girth in virtually the same region.56
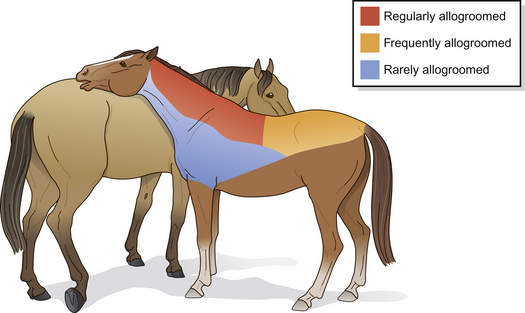
Figure 2.15 Mutual grooming map.
(Redrawn after Feh & de Mazieres 199359, with permission from Elsevier Science.)
The vibrissae around the eyes and muzzle have a rich afferent nerve supply.57,58 The apparently disorganized beard of vibrissae in the neonatal foal is thought to facilitate location of the teat.6 Vibrissae inform the horse of its distance from a given surface and may even be able to detect vibrational energy (sound). Together with the lips, they gather tactile information during grazing and head-rubbing. Horses are said to test electric fences with these whiskers before touching them. It has been suggested that the inability to detect fixed objects is a contributory factor to facial trauma in horses subjected to road transport subsequent to whisker trimming (Amy Coffman, personal communication 2002). Because vibrissae can be identified as anatomically different from normal hair coat, the trimming of whiskers has been outlawed in Germany (Andreas Briese, personal communication 2002). In the mouse, it has been shown that each vibrissa has its own small region of sensory cortex, a so-called whisker barrel, one per whisker, each of which can be clearly seen in brain sections (Alison Harman, personal communication 2002). This dedication of a portion of the cortex to each vibrissa indicates that they must be extremely important sensory instruments which should not be removed for cosmetic purposes.
Pain results in the release of pain mediators that act on the specific nociceptors. The nociceptors generate electrical potential in response to traumatic stimulation such as tissue-damaging pressure, intense heat, irritating chemical substances and skin abrasion.60 As the severity of the stimulus intensifies, there will be an increased frequency of action potential generation. The sensory cortex in the brain (see Ch. 3) creates the perception of pain and makes the horse aware of the strength and position of the pain stimulus. In response to pain there may also be activation of the sympathetic nervous system, causing a range of physiological responses. A painful stimulus also gives rise to a behavioral response and an emotional component that may include fear and anxiety.61 It seems naïve to imagine that whipping horses as is traditional in racing does not cause pain. Padded whips are now required in both Australia and the UK and there are clear rules about how these can and cannot be used. However, for these devices to be effective they must activate mechanoreceptors in the horse’s skin and, despite the padding, deformation of tissues remains a consequence (Fig. 2.16). The justification for whipping tired horses is now being questioned in the scientific literature.61

Figure 2.16 Unpadded (A) and padded whips (B) are likely to cause comparable pain if they cause similar indentations in the skin and if unpadded sections of padded whips impact upon the skin.
(Reproduced with permission of Liss Ralston.)
Sensitivity of the skin varies according to the thickness of the horse’s coat, thickness of its skin and receptor density in different areas. There are distinct receptors in the skin that respond to heat and cold (thermoreceptors), touch, pressure and vibration (mechanoreceptors) and pain (nociceptors). It is worth noting that a common feature of all skin nociceptors is that they often become less responsive if the stimulus is repeated at frequent intervals60 (see Habituation in Ch. 4).
The distribution of different types of sensory end organs changes in different parts of the body. When practitioners twitch their patients’ upper lips (see Ch. 14) they are capitalizing on the fact that this area is rich in three types of nerve endings that detect touch, pressure and pain. The mechanism by which this helps to pacify fractious animals is discussed further in Chapters 3 and 15. It is worth remembering that the buccal mucosa is as sensitive as the skin to tactile stimuli. The discriminative ability horses show when they empty their mouths of fine inedible material taken in during grazing, accounts for the rarity of intestinal foreign bodies in horses compared with, say, cattle. We have exploited this sensitivity by using oral discomfort to control horses. We should respect this sensitivity and avoid the heavy-handed rein-pulling that ultimately destroys it. The growing debate about ethical equitation62 will undoubtedly force us to examine the merits of severe bits (Fig. 2.17) and the use of constricting nosebands that reduce the behavioral manifestations of oral pain in competition horses.63,64 Given the importance of tactile stimulation for communication both within human–horse dyads and among horses, it is surprising that this topic has not been more thoroughly explored by equine scientists.

Figure 2.17 Severe bits frequently cause oral conflict behaviors, if the horse is not stopped from opening its mouth by a jaw-clamping noseband.
(Reproduced with permission of Julie Taylor.)
Summary of Key Points
• a caudal blind spot that accounts for a proportion of startle responses
• dichromatic color vision (i.e. like a color-blind person)
• a sense of taste that discriminates between safe and toxic plants with variable accuracy
• highly developed accessory olfaction
• the ability to hear within and beyond the range of human hearing
Case study
Rascal, a 9-year-old cob, is one of a group of horses at Brackenhurst College, UK, being taught to select certain colors in chromatic pairs as part of an investigation of perceptual ability. He can differentiate between white and yellow.
The animals in this study chose between two boxes that were identical, except for the color of a card displayed on the front of each. In Rascal’s case a yellow card was on the ‘correct’ box, which opened to allow the horse to gain the reward, and a white card was on the ‘incorrect’ box, which was locked. Once successful, a horse can quickly learn some other pairs of colors, unless it cannot distinguish between them, or if a color choice has been used in a previous pairing during training. Horses have excellent memories, so they do not easily learn discrimination reversals, even after long breaks.
The horse was first led into the testing arena and helped by the trainer to open the boxes so that the boxes were associated with hidden food rewards (Fig. 2.18). Next day, the horse was allowed to investigate the boxes itself with the trainer walking alongside. Next, the horse learned to approach the devices without being led by a human. It was released from a start line about 6 m from the boxes and allowed to explore alone. Five training sessions, each lasting for about 15 minutes, were given over a period of 3 weeks. Eventually, using only the visual cues, 70% of trained horses confidently selected the ‘correct’ box every time.
In shaping the final behavior, the trainer randomly assigned the ‘correct’ choice to a left or right position (with a maximum of three consecutive trials in the same position). This was to make sure that the horse was using the colored card to make a choice and not the position of the box, since spatial cues seem stronger than visual ones for equids.
At first, the horse was encouraged to investigate both boxes to discover that only one of the pair of boxes would open and contained the reward. Later, after obtaining the reward, the horse was not allowed to investigate the other (locked) box, because this negatively punished incorrect selection. If the horse made the ‘wrong’ choice, it was then allowed to change its choice and go to the other ‘correct’ box. After four consecutive ‘wrong’ choices, the horse was guided to the ‘correct’ box.
As training progressed, the horse was no longer allowed to swap, but was led back to the starting line to try again after an incorrect choice. This increased the ‘cost’ of failure, making it more important for the horse to get it right first time, and so improved learning.
As opposed to those occasional (often nervous) animals that appear to have no primary motivation to investigate the devices, relaxed horses are most suitable for this type of training. Interestingly, some horses primarily trained as riding animals seem to have preconceptions about being led by humans and do not readily take the opportunity to make choices in the presence of humans. These individuals were reluctant to take the lead and had to be removed from the experimental group.
Clearly, we need more of this type of research because, among other outcomes, it helps to remind us that horses are more than simply draft or riding animals. Many novice observers and even some with equestrian experience are surprised to see horses doing something other than being ridden.
References
1. Blake H. Thinking with horses. London: Souvenir Press, 1977.
2. Soemmerring DW. A comment on the horizontal sections of eyes in man and animals. Anderson SR, Munk O, eds. Schepelern HD, transl. Copenhagen: Bogtrykkeriet Forum; 1971.
3. Knill LM, Eagleton RD, Harver E. Physical optics of the equine eye. Am J Vet Res. 1977;38(6):735–737.
4. Roberts SM. Equine vision and optics. Vet Clin North Am Equine Pract. 1992;8(3):451–457.
5. Beaver BV. Equine vision. Vet Med Small Animal Clinician. 1982:175–178.
6. Fraser AF. The behaviour of the horse. Wallingford, UK: CAB International, 1992.
7. Budiansky S. The nature of horses — exploring equine evolution, intelligence and behavior. New York: Free Press, 1997.
8. Saslow CA. Understanding the perceptual world of horses. Appl Anim Behav Sci. 2002;78(2–4):209–224.
9. Crispin SM. Vision in domestic animals. In: Phillipson AT, Hall LW, Pritchard WR. Scientific foundations of veterinary medicine. London: Heinemann Medical, 1980.
10. Bartos L, Bartosová J, Starostová L. Position of the head is not associated with changes in horse vision. Equine Vet J. 2008;40(6):599–601.
11. Harman AM, Moore S, Hoskins R, Keller P. Horse vision and the explanation of visual behaviour originally explained by the ‘ramp retina’. Equine Vet J. 1999;31(5):384–390.
12. McGreevy PD, Harman A, McLean AN, Hawson A. Over-flexing the horse’s neck: a modern equestrian obsession? J Vet Behav. 2010;5:180–186.
13. Evans KE, McGreevy PD. The distribution of ganglion cells in the equine retina and its relationship to skull morphology. Anat Histol Embryol. 2006;35:1–6.
14. Timney B, Keil K. Local and global stereopsis in the horse. Vision Res. 1999;39:1861–1867.
15. Saslow CA. Factors affecting stimulus visibility for horses. Appl Anim Behav Sci. 1999;61:273–284.
16. Wouters L, De Moor A. Ultrastructure of the pigment epithelium and the photoreceptors in the retina of the horse. Am J Vet Res. 1979;40:1066–1071.
17. Prince JH, Diesem CD, Eglitis I, Ruskell GL. Anatomy and histology of the eye and orbit in domestic animals. Springfield, IL: CC Thomas, 1960.
18. Farrall H, Handscombe M. Equine vision. Equine Vet J. 1999;31(5):354–355.
19. Sivak JG, Allen DB. An evaluation of the ramp retina on the horse eye. Vision Res. 1975;15:1353–1356.
20. Leblanc MA, Bouissou MF. Development of a test to study maternal recognition of young in horses. Biol Behav. 1981;6(4):283–290.
21. Smith S, Goldman L. Colour discrimination in horses. Appl Anim Behav Sci. 1999;62:13–25.
22. Pick DF, Lovell G, Brown S, Dail D. Equine colour perception revisited. Appl Anim Behav Sci. 1994;42:61–65.
23. Macuda T, Timney B. Luminance and chromatic discrimination in the horse (Equus caballus). Behav Process. 1999;44(3):301–307.
24. Miyashita Y, Nakajima S, Imada H. Differential outcome effect in the horse. J Exp Anal Behav. 2000;74(2):245–254.
25. Hall CA, Cassaday HJ. An investigation into the effect of floor colour on the behaviour of the horse. Appl Anim Behav Sci. 2006;99:301–314.
26. Hall CA, Cassaday HJ, Vincent CJ, Derrington AM. Cone excitation ratios correlate with color discrimination performance in the horse (Equus caballus). J Comp Psychol. 2006;120(4):438–448.
27. Murphy J, Hall C, Arkins S. What Horses and Humans See: A Comparative Review. Int J Zool. 2009. ID 721798
28. Stachurska A, Pieta M, Nesteruk E. Which obstacles are most problematic for jumping horses? Appl Anim Behav Sci. 2002;77(3):197–207.
29. Timney B, Macuda T. Vision and hearing in horses. J Am Vet Med Assoc. 2001;218(10):1567–1574.
30. Carroll J, Murphy CJ, Neitz M, et al. Photopigment basis for dichromatic color vision in the horse. J Vision. 2001;1:80–87.
31. Enzerink E. The menace response and the pupillary light reflex in neonatal foals. Equine Vet J. 1998;30(6):546–548.
32. Hothersall B, Harris P, Sörtoft L, Nicol CJ. Discrimination between conspecific odour samples in the horse (Equus caballus). Appl Anim Behav Sci. 2010;126:37–44.
33. McGreevy PD, Hawson LA, Habermann TC, Cattle SR. Geophagia in horses: a short note on 13 cases. Appl Anim Behav Sci. 2001;71:119–215.
34. Whitten WK. Vomeronasal organ and the accessory olfactory system. Appl Anim Behav Sci. 1985;14:105–109.
35. Crowell-Davis SL. Developmental behavior. Vet Clin North Am Equine Pract: Behav. 1986:573–590.
36. Weeks JW, Crowell-Davis SL, Heusner G. Development of a differential flehmen response in Equus caballus. Appl Anim Behav Sci. 2002;78(2–4):329–335.
37. Anderson TM, Pickett BW, Heird JC, Squires EL. Effect of blocking vision and olfaction on sexual responses of haltered or loose stallions. J Equine Vet Sci. 1996;16:254–261.
38. Houpt KA, Guida L. Flehmen. Equine Pract. 1984;6(3):32–35.
39. van Sommeren A, van Dierendonck M. The use of equine appeasing pheromone to reduce ethological and physiological stress symptoms in horses. J Vet Behav. 2010;5:213–214.
40. Waring GH. Horse behavior: behavioral traits and adaptations of domestic and wild horses, including ponies. Park Ridge, NJ: Noyes, 1983.
41. Kiley-Worthington M. The behaviour of horses in relation to management and training. London: JA Allen, 1987.
42. Houpt KA, Wolski T. Domestic animal behavior for veterinarians and animal scientists. Ames: Iowa State University Press, 1982.
43. Houpt KA, Zahorik DM, Swartzman-Andert JA. Taste aversion learning in horses. J Anim Sci. 1990;68:2340–2344.
44. Salter RE, Pluth DJ. Determinants of mineral lick utilization by feral horses. Northwest Sci. 1980;54:109–118.
45. Marinier SL, Alexander AJ. Selective grazing behaviour in horses: development of methodology and preliminary use of tests to measure individual grazing ability. Appl Anim Behav Sci. 1991;30(3–4):203–221.
46. Ralston SL. Controls of feeding in horses. J Anim Sci. 1984;59(5):1354–1359.
47. Murphy K, Wishart S, Mills D. The acceptability of various flavoured solutions by Thoroughbred horses. The role of the horse in Europe. Equine Vet J Suppl. 1999;28:67.
48. Vallencien B. Comparative anatomy and physiology of the auditory organ in vertebrates. In: Busnel RG, ed. Acoustic behavior of animals. Amsterdam: Elsevier; 1963:522–556.
49. Busnel RG. On certain aspects of animal acoustic signals. In: Busnel RG, ed. Acoustic behaviour of animals. Amsterdam: Elsevier; 1963:69–111.
50. Heffner RS, Heffner HE. Hearing in large mammals: horses (Equus caballus) and cattle (Bos taurus). Behav Neurosci. 1983;97:299–309.
51. Heffner HE, Heffner RS. Sound localization in large mammals: localization of complex sounds by horses. Behav Neurosci. 1984;98:541–555.
52. Heffner RS, Heffner HE. Visual factors in sound localization in mammals. J Comp Neurol. 1992;317:219–232.
53. Penick J, Jr. The New Madrid earthquakes of 1811–1812. Columbia: University of Missouri Press, 1976.
54. Ödberg FO. A study of the hearing ability of horses. Equine Vet J. 1978;10(2):82–83.
55. McGreevy PD, Oddie C, Burton FL, McLean AN. The horse–human dyad: Can we align horse training and handling activities with the equid social ethogram? Vet J. 2009;181(1):12–18.
56. McGreevy PD, McLean AN. Equitation science. Oxford: Wiley-Blackwell, 2010.
57. Talukdar AH, Calhoun ML, Stinson AW. Microscopic anatomy of the skin of the horse. Am J Vet Res. 1972;31:1751–1754.
58. Van Niekerk HP. Ethological studies within the man–horse relationship. J S Afr Vet Assoc. 1980;51(4):237–238.
59. Feh C, de Mazieres J. Grooming at a preferred site reduces heart rates in horses. Anim Behav. 1993;46:1191–1194.
60. Swenson MJ, Reece WO. Dukes’ physiology of domestic animals, 11th edn., Ithaca, NY: Cornell University Press, 1993.
61. Evans DL, McGreevy PD. An investigation of racing performance and whip use by jockeys in Thoroughbred races. PLoS. 2010;6(1):e15622.
62. Jones B, McGreevy PD. Ethical equitation: applying a cost-benefit approach. J Vet Behav. 2010;5:196–202.
63. McLean AN, McGreevy PD. Ethical equitation: capping the price horses pay for human glory. J Vet Behav. 2010;5:203–209.
64. McLean AN, McGreevy PD. Horse-training techniques that may defy the principles of learning theory. J Veterinary Behav. 2010;5:187–195.

