Chapter 4 Learning
Chapter contents
Learning theory
Equids can learn remarkable behavioral responses (Fig. 4.1), but if we are to consider principles in horse-training from a rigorous scientific perspective, there is an abiding need to demystify traditional horse-training jargon and couch the following discussion in the language of learning theory. This unifying language explains what happens at a mechanistic level in all effective training systems.1 Using this approach veterinarians and equine scientists can be agents of change by counseling owners and trainers on effective and humane techniques in training and retraining. A glossary of equestrian terms appears at the end of this book, so this chapter avoids any such esoteric labels. The original rules of what we call learning theory were first set down by psychologists and behaviorists who used clinically controlled, some would say sterile, stimuli. These days the study of animal learning is increasingly the pursuit of cognitive ethologists. These are the behavioral scientists who, when considering the way in which a member of a species processes information, emphasize the importance of the environment for which that species evolved and determine how the biology of a species can influence its behavior.2
Figure 4.1 Denver, the swinging mule, changing his head and neck position to shift his center of gravity and swing a platform.
The definition of learning
Broadly speaking, a stimulus is any detectable change in an animal’s environment. A response is any behavior or physiological event. The usual technical definition of learning or conditioning, as it is often called, is any relatively permanent change in the probability of a response occurring as a result of experience. Importantly, this refers to a response and not a cognitive outcome, such as knowledge.
Not all changes in behavior are consequences of learning. The reference to a ‘relatively permanent change’ is added to exclude modifications of behavior by motivational factors, physiological variables or fatigue. A thirsty horse that drinks despite having refused water some hours earlier has changed its behavior but is not considered to have learnt anything in the interim. Instead, its motivation to drink has changed as a result of shifts in variables such as blood volume and the concentration of sodium in body fluids. Similarly, fatigue can change behavior, transforming a rearing colt into a weary slug, but its effects could not be described as relatively permanent.
Sufficient reflexive responses cannot possibly be built into each foal to meet every challenge throughout life, so learning enables young horses to use their experience to update their behaviors according to their developing circumstances. Although only cursorily studied in any empirical sense, it is likely that horses are particularly attentive when learning to do what they fill most of their days with – grazing. Foals sample their mothers’ feces when learning to select safe vegetation types, while adult horses retain the ability to review preferences in diet according to the proximate consequences of consumption.3
Intelligence
The intelligence of animals is difficult to assess. Intelligence may be shown when the animal learns to ignore irrelevant stimuli, just as it also learns to react to significant stimuli. This permits the behavioral developments that make discrimination possible. The horse’s integration with its domestic environment is facilitated by this ability to compare and contrast. Most horses can accurately discriminate between stimuli and evaluate them, e.g. they can differentiate sounds, visual features of special significance, the identities of people, and so on.4
Veterinarians are often asked to comment on the intelligence of horses. For example, some owners are intrigued to know whether they should credit their horses with the cognitive talents of dogs. Attempts to compare brain to bodyweight to arrive at a quantitative comparison between species are rarely well received by horse lovers since, using this measure, the horse approximately equates with the turtle. Furthermore the same ratio credits the shrew with more brain power than the human. One of the core problems here seems to lie in the definition of intelligence, because it is a nebulous construct. It may be preferable to speak of the ability to learn, but even that approach may be flawed when experimental designs fail to select naïve subjects. We should bear in mind that conclusions about equine reasoning abilities (or lack thereof) may be biased because they have been based on observations that appear to have been made almost exclusively with horses that have been trained by negative reinforcement (Amy Coffman, personal communication 2002). It is possible that this may have affected their performance, e.g. by reducing their willingness to risk making mistakes.
Several attempts have been made to compare learning abilities in horses with those of other species and to compare the ability of different breeds of horses.5,6 All of the tests between species are confounded by the differences in physical ability and sensory acuity between species, as these may account for the differences in perceived ‘intelligence’. Add to this the species’ differences in motivation and the variation in salience of a single type of apparatus and one begins to see how such comparisons are inherently flawed. One can see how difficult it is to devise a test that two species can undertake with equity. Experimenters have yet to make the necessary adjustments to stimuli so that members of two species can detect and respond to them with equal ease, speed and motivation. The same criticism can be leveled at experiments that compare the problem-solving ability of breeds within a species or, for that matter, bloodlines within a breed.7 Selection of breed traits can impair or enhance performance in various ways, including emotionality.
Imprinting and socialization
Some neonate animals show evidence of imprinting in a special category of learning that occurs during a highly sensitive period in their development, generally at the time of their first stimulation from the outside world. Spalding8 was the first to make systematic observations of this phenomenon, reporting that shortly after hatching, young chicks followed any moving object. Later, Lorenz9 made the topic popular among behavioral scientists. He described imprinting as a unique process in precocial birds occurring exclusively in a sensitive period during which 2–3-day-old hatchlings begin to studiously follow a ‘mother figure’. Typically, this figure is the hatchlings’ real mother, but Lorenz recognized that, during their growth period, they would follow almost any moving object. Lorenz posited that imprinting was irreversible and, given that birds hatched by another species often court and attempt to mate with the foster species, that it determined future mating partners.
For equine practitioners, the questions are: does imprinting occur in horses; and, if so, is it the same as in precocial birds; and is it of any use in training and management? Veterinarian Robert Miller10 introduced the term imprinting to describe the behavior of young foals and suggested that the sensitive period for foals, when the ‘following response’ is learned, is within the first 48 hours of life. At this time the foal’s mother is usually the nearest large moving creature in its world and it is to her that the foal bonds.
Imprinting is mediated by the sense of sight, but sound and olfaction are also involved. Having described imprinting as a process in foals, Miller also introduced the prospect of imprint training as a viable methodology for modifying the behavior of newborn foals in a bid to reduce the prevalence of aversive reactions and defensive aggression while at the same time sensitizing the foal for pressure release.10,11 Miller’s observations about the phenomenon of imprinting are noteworthy, because like gallinaceous birds, horses are precocial and are mobile soon after birth. If Miller’s claims are correct, we should spend time imprint training foals rather than schooling larger, stronger and more dangerous youngsters.
Imprint training as recommended by Miller is an exhaustive process, requiring habituation of the foal to common stimuli as well as sensitization to other salient stimuli. It involves between 30–50 interactions, which Miller calls ‘stimulations’, of each body region of the foal. Imprint training begins with drying the foal and cutting its umbilical cord. On day one, habituation begins with the foal being held securely before it stands. The holding persists until the foal relaxes and ceases to resist. Then it is rubbed all over its body until it again shows relaxation. Beginning with the ears (including ear canals), face, upper lip, mouth, tongue and nostrils, this rubbing is repeated. Rubbing the eyes, neck, thorax, back, legs, feet, rump, tail, perineum and external genitalia follows.
The next steps are rather controversial because they seem to amount to flooding with novel aversive stimuli, including artificial devices such as clippers and a rectal thermometer, as well as sham rectal examinations. Further habituation to unusual stimuli follows, with the handler rubbing a piece of crackling plastic over the foal’s entire body until ‘panic subsides’. Gunfire, hissing sprayers, whistles, loud music, flapping flags and swinging ropes are also included in the imprinting program. Habituation to pressure around the girth region follows with the handler’s arms surrounding the foal and compressing rhythmically until any resistance abates.
After habituation, sensitization to pressures for leading via the headcollar is undertaken. This is not exclusively part of so-called imprint training, but is really the first step in negative reinforcement training. Training the foal to lead at an early age, as long as it does not compromise foal safety or the mare–foal bond, can be justified because it enhances foal and handler safety during any veterinary intervention before foundation training.
The increase in popularity of imprint training over recent years has triggered interest in the process from scientists, not least because of the possible implication of flooding and learned helplessness in techniques revolving around restraint and aversive stimuli. The ensuing research suggests significant limitations to Miller’s schema. Concerns have been raised for the wellbeing of foals that undergo significant human interventions shortly after birth. Henry et al12 noted that foals separated from their dams for just one hour went on to show patterns of strong dependence on their mothers, little play, social withdrawal and aggressiveness. The perceived need for persistence in application of restraint as part of the Miller regime remains a contentious issue. Restraint may increase latency to stand, but such delays are not accompanied by any accompanying increase in the latency to nurse.13 However, because restraint of struggling foals may increase their risk of injury, veterinarians should consider this outcome carefully before advocating the Miller system. Similarly, as we shall see, they should be mindful that there is insufficient evidence to demonstrate that handling at later ages could not have similar results.14
The suggestion that foals be stimulated until they appear sleepy rather than reactive is central here. We cannot assume that sleepiness indicates emotional acquiescence. There is a need for surveillance of foals’ physiological response to this intervention, which may allow us to assess any deleterious effects of elements of the training program and refine the entire approach.
The sensitive period during which foals can acquire any special learning remains elusive. Mal & McCall15 concluded that handling throughout the first 42 days of life improved performance in a halter-training task compared with handling from days 43 to 84. Concurring with Miller about the likely existence of a critical period, they suggested that this period lies within the first 42 days of life.
Heird et al16 reported that when moderately handled from weaning until 18 months of age, foals showed enhanced problem-solving abilities. Extensively handled horses were slower navigating through a maze than minimally handled horses, but faster than unhandled horses. That said, we should consider the possibility that the aversiveness of confinement within the maze was greatest in the unhabituated, unhandled foals and that problem-solving in and therefore progress through the maze may have been hampered simply by fear. On the other hand, the extensively handled horses, having been trained to wait for cues, may have been more dependent on human signals to advance through the maze. Heird et al16 also reported that horses which had been extensively handled were less emotional, showed a higher maze-learning performance and were more trainable for riding than those that had received less early handling. This evidence supports handling protocols that reduce flight response through habituation, approaches that do not necessitate imprint training per se.
Most of the recent literature suggests that imprint training of foals does not correspond to any natural analogue in the equid ethogram and that, during imprint training, the foals frequently resist strongly and are distressed.17 Whether this early stress can be justified in terms of later benefits is still subject to debate.18 Williams et al19,20 showed that handling foals at birth and/or 12, 24 and 48 hours after birth, had no beneficial effect on their later behavior when tested at 1, 2 and 3 months of age19 or at 6 months of age.19,20
The distinctions among habituation, any equine analogue of filial imprinting (as described in birds) and the more radical elements recommended by Miller are far from simple. This explains why some studies have shown some positive effects of imprint training. For example, foals handled early tend to approach familiar humans12 and accept foot handling21 more readily than unhandled ones. However, aversive stimuli were not rendered more tolerable by imprint training. For example, at 3 or 4 months of age, fitting halters or clipping was as disturbing for handled foals as it was for unhandled foals.13,19,21 Significantly, foals that had been imprinted and then went without regular later handling were as difficult to approach as controls22 (Table 4.1).
Handling after the putative sensitive period seems to have only transient benefits.24 While Mal et al25 found no beneficial effects when foals were handled (stroking, haltering or picking up feet) during the first 7 days, subsequent evidence has emerged to suggest that, when subjected to similar handling at 14 days of age, foals were more tractable at 3 months than non-handled foals. However, differences between the handled and unhandled foals were less apparent when tested at 6 months of age and had disappeared completely by one year of age.22
Higher trainability16 and easier handling,26 has been reported in foals handled regularly from weaning. Similarly, Hausberger et al18 proposed that any benefits of early handling rely on regular repetitions of handling. Compared with unhandled foals, handling foals 5 days per week (including catching, leading, picking up feet, grooming and being approached by an unfamiliar human) until they are 2 years of age improved manageability at 12, 18 and 24 months.23 The optimal duration of the handling period is still unclear, but the optimal time for handling seems to be at weaning since, in contrast to imprint training, this would reduce the chance of interfering with the mare–foal bond. It should be followed up with regular handling.
Hausberger et al18 point out the absence of evidence in foals for the existence of a sensitive period in development that assists in the formation of a foal–human bond. They emphasize that the affiliative qualities of human–horse interactions may be more important than when the interactions occur. Certainly, prior to weaning, foals seem to find being scratched highly reinforcing and humans may be better at scratching some body parts than other horses are.2 In a similar vein, Søndergaard & Halekoh27 proposed that unhandled 2-year-olds become as familiar as handled animals, probably because humans bring food.
Given that foals learn from their dams, it is worth noting that the reactions of the mare during handling have an impact on the foal’s behavior. Sigurjonsdottir & Gunnarsson22 found that the mare’s nervousness correlated positively with the imprinted foal’s resistance to capture, haltering and leading at the age of 4 months. In essence, they demonstrated that the calmer the mare, the easier the foal was to handle. This finding could simply reflect social learning or the inheritance of flightiness. However, more direct activities, such as brushing and hand-feeding of the mare in the presence of the foal had a lasting, positive effect.28 One year later, without any handling in the interim, foals handled in this way were more easily approached in the paddock and stroked by a familiar or unfamiliar human, whereas controls showed greater resistance to capture and to the presence of humans. Hausberger et al18 concluded that establishing a positive human–dam relationship may therefore be critical in creating a durable improvement to the manageability of foals. At the same time as offering such lessons with the dam, handlers should interact gently and consistently with the foals themselves since, as long as they cause no flight response and they do not disrupt the mare–foal bond, regular training activities that fully align with learning theory and, in particular, pressure release contingencies, can be learned at an early age (Fig. 4.2).
The research on imprint training seems to underline the difficulty in dissecting any equine analogue of filial imprinting from habituation and socialization. Socialization involves giving foals opportunities to become acquainted with stimuli they will spend time with in later life. This principle is the core of the lessons foals can learn from watching their dams calmly interacting with humans. They also learn from their peers and require a rich social environment to develop normal social skills, low adult–young ratios being associated with subsequent aggression in foals.29 Intriguingly, it has been reported that foals of high-ranking dams remain closer to their mothers during socialization.30 Although foals reared without peers are bolder with humans,31 it is possible for them to learn to become too familiar with handlers. Grzimek16 separated a neonatal foal from its own species for 64 days and found that it developed a fear of conspecifics and a social preference for humans over equids. Similarly, Williams32 noted that artificially reared foals failed to respond to the social signals of conspecifics and sought human company rather than that of other horses. The socialization benefit is an important reason that fostering orphan foals on to nurse mares is preferable to hand-rearing (see Ch. 12).33 It is not clear how labile the unwelcome consequences of mal-imprinting are in later life. Some owners speak of foals that have mal-imprinted as having a lack of ‘respect’, e.g. ‘because they have no fear, they climb on top of you’. As with any training, the key to finding the best course is to be consistent. If you do not want a horse that takes dangerous liberties in later life, then do not allow it to practice such responses as a foal and certainly never reward it, however inadvertently, for doing so. Familiarity does not necessarily breed contempt. However, just as a mare disciplines her foal before and during weaning by punishing behavior she finds unacceptable, so the human handler might be justified in doing the same to avoid the youngster growing up with a litany of inappropriate responses and no ‘respect’ for humans. The problems with punishment are discussed later in this chapter but in brief it is safe only when accurately timed and as subtle as possible. For these reasons, it demands experienced horsemanship. Novices rearing orphan foals rarely strike the right balance between adequate physical contact and consistent use of pressure-release training. Sensitization in early handling programs is important as a precursor to negative reinforcement. It trains the foal to move away from exogenous tactile stimuli as it must to become a safe and responsive working animal in later life.
In a novel object test, the cardiac responses of Dutch Warmblood youngsters with regular training were compared with youngsters that had received additional training from the age of 5 months onwards. The animals were tested at 9, 10, 21 and 22 months of age.34 The regular youngsters showed greater increases in mean heart rate that could not be entirely explained by the physical activity and decreases in HRV measures. Because these horses showed individual consistency of these heart rate variables at all ages, the authors suggested that non-motor cardiac responses might be useful in quantifying certain aspects of a horse’s temperament.34 Data of this sort strongly imply that individualized attention to the behavioral conditioning of young stock pays dividends throughout their lives.
In conclusion, while noting the helpful efforts of researchers in this domain, we should accept that a great deal of confusion prevails about best practice in the formative years of a horse’s life. This highlights the need for experimental designs to provide data that are more readily applicable to the needs of horse producers and equestrians.
Non-associative learning
Imprinting aside, there are two major categories of learning: non-associative and associative. In non-associative learning the animal is exposed to a single stimulus to which it can become habituated or sensitized, while in associative learning, a relationship is established between at least two stimuli. There are two subdivisions under the umbrella of associative learning. These are classical conditioning and operant conditioning. The latter, as we will see, is important for animals to be able to solve novel problems in their environment. The central tenets of learning theory merit detailed consideration by those embarking on behavior modification in practice because the ontogeny and resolution of inappropriate behavior patterns can be explained with reference to learning theory. If we can appreciate how a horse finds benefits from aggressive responses to humans, we can understand why meeting such responses with violence rarely, if ever, helps. If we can understand how to train a horse to bow, we can apply similar techniques to modify the behavior of a horse that has learned to avoid being loaded onto trailers.
The most basic form of learning involves cumulative experience of stimuli by themselves. This gives the horse data about the relevance of stimuli in its environment.
Habituation
Habituation is said to have occurred when repeated presentations of a stimulus by itself cause a decrease in the response (Fig. 4.3). It is really the simplest form of learning. Consider the training of a police horse (see case study below) that must be gradually exposed to more and more of the potentially frightening stimuli that it will later encounter when out on patrol. The people delivering these stimuli in training are familiar to the horse and started their disturbances at a considerable distance from it. Only when the horse ignores the rumpus at a certain noise level and a certain distance are these variables made more threatening and proximate. Similar approaches are used to train stunt horses for film and exhibition work (Fig. 4.4).

Figure 4.4 Stunt pony, Mr Pie, galloping through fire.
(Photograph courtesy of Amanda Saville-Weeks.)
The likelihood of habituation and its rate are dependent on the nature of the stimulus, the rate of stimulus presentation and the regularity with which it is presented. When habituating a horse to a stimulus, it is essential that the stimulus be repeated well past the point of habituation. To stop prematurely can teach the animal exactly the opposite of what is desired. Habituated responses show spontaneous recovery when stimulation is withheld. This means that exposure to the relevant stimuli must continue at intervals to prevent the original response (e.g. a flight response) from recurring.
Habituation is central to horse taming and pivotal in early handling regimens. Recent work by Jezierski et al23 using Konik horses, confirms that habituation to handling can reduce reactivity. Konik horses are a primitive breed unlikely to have been selected to be easily managed by humans.35 Horses exposed to daily handling (10 minutes per day over 5 days per week) from 2 weeks of age displayed a reduced heart rate and enhanced manageability compared with horses born and reared up to weaning age in a forest environment free from human contact.23 The early handling intervention made horses much easier to manage than non-handled foals in a variety of tests given at 6, 12, 18 and 25 months. Again, it pays to consider the extent of any moderating influence from these foals’ dams, that were exposed to humans as part of the experimental procedure.
Desensitization and counter-conditioning
In behavior modification, the step-by-step process of weakening an unwelcome fear response to a given stimulus or set of stimuli to the point of extinction is often labeled systematic desensitization. If the horse is relaxed when exposed to barely perceptible measures of the causative factors one at a time, it can learn to behave passively rather than fearfully. The emphasis in this approach has to be on the gradual introduction of increasing aliquots of the problematic stimuli. Too fast an advance is marked by a return to the fearful response or an alternative evasion. If this arises, exposure to the stimuli must be returned to the level to which the horse had successfully habituated.
If, rather than simply habituating horses to aversive stimuli, we expose them to pleasant coincidental consequences, counter-conditioning may occur. This helps to facilitate the emergence of an alternative, more appropriate response to the aversive stimulus. Despite encouraging data to support the use of this approach,36 this is a sadly under-exploited strategy in the management of horse behavior. Any veterinarian who appreciates the motivation most equids have to feed should be able to harness this drive to train appropriate behaviors. For example, if one is required to administer eye drops to a horse one can either struggle with it as it wrestles its head away or condition an appropriate head posture using food. The principles of learning theory that apply when horses learn to tilt their heads to nuzzle in the pockets of owners and find titbits can be used for veterinary purposes. For sustained performance, the rewards must outweigh the costs and, because horses learn to avoid aversive stimuli more than some other domestic species such as the dog,37 this means that the daily value of the reinforcements must eclipse the aversiveness of the total daily dose of eye drops. Unfortunately, for some veterinarians and owners, simple habituation to periorbital palpation is too time consuming and therefore counter-conditioning holds even less appeal. Because they are reliable sources of ocular discomfort, these are the people whom equine ophthalmic patients learn to avoid.
It is important to recognize that simply offering food in the presence of a fear-eliciting stimulus is an inadequate approach since the horse may be chewing a mouthful of food and therefore gaining gratification from it while demonstrating a flight response. Rather than allowing the fearful horse to stuff his head into a bucket of grain during exposure, a simple gratifying distraction, effective trainers use valuable rewards that are small enough to be consumed quickly. These rewards are used when the horse that tends to run away with an elevated head posture offers approximations of a more appropriate response such as turning towards the stimulus and lowering its head.38 By waiting for the horse to offer the appropriate response rather than forcing it to ‘behave’ with aversive stimuli, such as buccal discomfort where rein pressure is used, the trainer allows the horse to learn the consequences of its own actions in bringing about a reward. This elegant approach, which allows the horse to have some control over the situation, requires all the features of good training practice, including sensitive shaping technique (see p. 96) and, above all, consistency.39
A less sophisticated approach to fearfulness in horses that may, at first glance, seem expeditious, is flooding, which involves the over-exposure to the causative stimuli until the response disappears. A familiar example of flooding is seen in traditional horse ‘breaking’ when a saddle is strapped to a naïve horse that is then allowed to buck in an attempt to rid itself of the saddle until it gives up. Driven by the belief that the horse is not responding because it is no longer fearful, many misguided handlers reach for this approach to all manner of fear-elicting stimuli. Unfortunately the horse has no control over the situation and can learn that it is simply helpless to respond. Where this learning is context specific (see below), the lessons are only partially learned and the horse may emerge with extreme fear responses in slightly different circumstances. Trainers of police horses generally do not use flooding because the horses must offer learned responses in a very wide variety of contexts (see case study at the end of the chapter). Flooding in combination with physical restraint and muscle relaxants such as succinylcholine chloride has been described, with warnings about the dangers of the subjects damaging themselves as they struggle or collapse to the ground.40 Flooding that leads to apathy may be justified in certain aggressive horses, as an alternative to euthanasia, but for some it remains questionable on welfare grounds.
Sensitization
Sensitization is the opposite of habituation in that there is an increase in a response after repeated presentations of the stimulus by itself. The stimulus has to be intrinsically unpleasant or aversive. Sensitization usually happens when an individual cannot escape or make an avoidance response to prevent repeated exposure to a stimulus that is intrinsically unpleasant or aversive. This is particularly likely if the subject is highly aroused. If one recalls the magnified unpleasantness of a dripping tap when searching for sleep, the effect of sensitization becomes clear. Stimuli over which the horse learns it has minimal control (e.g. bot flies) are those to which it can become most rapidly sensitized and sensitization can override habituation. For example, if while completing his training, the police horse described above is involved in a road traffic accident every day for a month, he would reliably become sensitized to motor vehicles and perhaps even become phobic so that just the sound or sight of them might be sufficient to send him into a flight response.
Associative learning
When horses make links between stimuli and responses or, to use training terms, cues and outcomes, they are undergoing associative learning.
Classical conditioning
Classical conditioning is the acquisition of a response to a new stimulus by association with an old stimulus. It involves coupling a stimulus with an innate behavior or physiological response. It is also labeled Pavlovian conditioning because it has its origins in primarily unrelated saliva collection experiments conducted by Ivan Pavlov.
Pavlov had spotted his experimental dogs salivating when they heard his technician tinkling a bell as he approached the kennels to feed them. To determine how accurately a dog could develop such associations, Pavlov decided to replace the sound of the bell with more easily varied sounds made by a buzzer (and later a metronome). He surgically implanted a tube to collect saliva from each dog to measure its rate of production (Fig. 4.5). Pavlov used this apparatus to demonstrate the strength of the links between a novel stimulus (the buzzer), to a physiological stimulus (food in the mouth) and a response (salivation). The dogs quickly began to salivate in response to the buzzer, a new stimulus that had previously been irrelevant or neutral. The labels Pavlov created for the elements in this process are still used today. Before the learning experience, only meat powder, the unconditioned stimulus (US), produced salivation as an unconditioned response (UR) (Fig. 4.6). After learning, the buzzer became a conditioned stimulus (CS) and the salivation response to the conditioned stimulus became the conditioned response (CR, also known as the learned response).
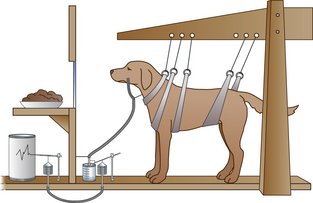
Figure 4.5 Illustration of one of Pavlov’s dogs in its experimental apparatus used for measuring the rate of salivation as an indicator of the strength of associations between conditioned stimuli and food.
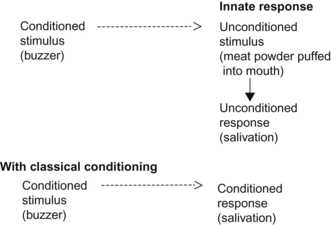
Figure 4.6 Conditioning rather than leadership qualities provides a more plausible explanation of leading behaviors. A horse will lead forward from pressure even from a well-trained dog.
Crucially, in classical conditioning, the sound of a buzzer was followed by the delivery of food to the mouth, regardless of what the dog might have done when it heard the buzzer. Classical conditioning enables the animal to associate events over which it has no control. This increases the predictability of an environment. The more frequently and consistently the neutral stimulus is paired with the unconditioned stimulus, the more rapidly the association will be made. In some cases, usually involving the most fundamental pain or pleasure, the association is formed with a single experience.41 We know that fear-related responses are particularly resistant to extinction42 and that single-trial learning of flight responses can persist for years.43
A good example comes from horse-breeding units. Learning about sex seems especially likely as a consequence of classical conditioning. Some stallions get aroused when they hear the sound of the bridle used to control them in the service pen. Their excitement increases when they are led to the service barn – hence, through classical conditioning another reliable environmental cue becomes associated with copulation. Racetrack grooms use classical conditioning when they whistle each time they see their charges urinating. Once the association between the whistling and urination is made, the horses urinate on cue for post-race urine tests (see Ch. 9). Riders use classical conditioning when they replace pressure cues from the bit and the rider’s legs with previously neutral signals such as changes in their position (seat).
Pavlov recorded an interesting footnote to his studies: he noted that his dogs would race ahead of their handlers to get to the experimental area. They would not wait for a stimulus that made their mouths water, they would actively try to put themselves into situations and perform activities that led to rewards. This was a result of trial-and-error learning and brings us neatly to the other important category: operant conditioning.
Operant conditioning
An operant response is a voluntary activity that brings about a reward. In operant conditioning, the buzzer might still be presented but the dog must make a particular response before food is consumed. In other words, there is a special link (what learning theorists call a contingency) between a particular behavioral response and a food reward. Operant conditioning underpins most of the techniques that work well in equestrian contexts (Fig. 4.7).

Figure 4.7 Conditioning rather than leadership qualities provides a more plausible explanation of leading behaviors. A horse will lead forward from pressure even from a well-trained dog.
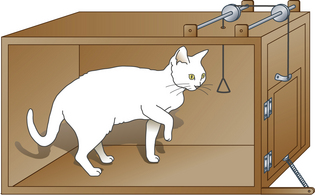
While Pavlov was concentrating on the physiological responses of dogs in harnesses, Thorndike44 was studying the behavioral responses of cats in puzzle boxes. Instead of delivering food independently of behavior whenever a signal had been presented, Thorndike delivered it once his animals had responded. In a body of work intended to discredit the notion that animals are capable of reason, Thorndike44 described the behavior of a naïve cat in a specially designed box (Fig. 4.8).
Without any food or other home comforts, life was rather dull and unsustainable in the puzzle box. The cat could get out – but only by pulling a trigger. Motivated to access food outside the box, Thorndike’s cats would eventually learn to escape by operating the trigger that released the door latch. Once out of the box, they would get their food. Thorndike called this trial-and-error learning, but this label has largely been replaced by the terms instrumental learning and operant conditioning. The animal sees a cue (the trigger), performs a response (pulling) and gets a reward (liberty and food). In any horse-training or retraining situation, trainers should challenge themselves to be clear about the horse’s perception of the same three critical elements: cue, response and reward.45 The effect of the reward is to strengthen the correct response. This is known as reinforcement. The term reinforcement refers to the process in which a reinforcer follows a particular behavior so that the frequency (or probability) of that behavior increases.
On successive occasions in his free-operant studies (see below), Thorndike noted that the cat’s latency to escape fell but only very gradually. The absence of a sudden drop in the latency to learn was sufficient evidence for Thorndike to assert that his cats were not using thought or reason to solve the problem. Thorndike’s work was followed by that of Skinner,46 who maintained that almost any response could be learned by an animal that is given opportunities to operate within its environment to obtain a desired outcome.
Rocky, the 8-year-old Irish Draft horse in Chapter 1 (see Fig. 1.12), learned within 4 weeks of being at a new home that unlocking the bolt on his stable door allowed him to enrich his environment. He learned that the behavior had a pleasant result. However, he does not attempt to escape if a chain is slung across the threshold of his doorway and loosely tied with a single strand of twine. It appears that the open door affords him a better view and, for the time being at least, satisfies his motivation to observe the activity on the yard.
Operant or instrumental conditioning consists of presenting or omitting some reward or punishment when the animal makes a specific response.47 The likelihood of an association arising depends on the relationship between the first event and the second via stimulus–response–reinforcement chains. Operant conditioning enables an animal to associate events over which it has control. This increases the controllability of the environment, which represents the crucial difference between classical (Pavlovian) and operant conditioning. Operant conditioning can have potential benefits for horse welfare by improving choice. In classical conditioning, rewards become associated with stimuli, while in operant conditioning they become associated with responses.
Operant conditioning has been studied experimentally in two types of situations: ‘discrete trial’ situations and ‘free operant’ situations. In a ‘discrete trial’ situation, a subject is exposed to the learning task, required to make a response, and then withdrawn from the situation. For example, a horse may have to choose one of two directions, left or right, in a simple T-maze. In a ‘free operant’ situation, on the other hand, the subject is allowed to operate freely on its environment. For example, a horse placed in a paddock and free to do whatever it likes, can be conditioned by reinforcement under a given set of contingencies. Horses such as those that open their stable doors or dunk their own hay are good examples of free-operant conditioning. Like Thorndike’s cats and the rats that pressed levers in Skinner’s laboratory, they work within their environment to effect a reward. In equine contexts, there are many examples of individuals acquiring a response to avoid an aversive outcome. Several studies suggest that lack of control over aversive events can bring about major behavioral and physiological changes. For example, after being exposed to uncontrollable electric shocks, rats have increased gastric ulceration, increased defecation rates and are more susceptible to certain cancers when compared with individuals that retain control over comparable shocks.
Operant devices can be used to determine the preference animals have for certain environmental parameters. Horses have been trained to break photoelectric beams to turn on lighting in their accommodation and, using this device, have demonstrated a preference for an illuminated environment.48 Others have been trained to nuzzle buttons to access resources (Fig. 4.9).

Figure 4.9 Horse performing an operant task: pressing a button with its muzzle.
(Photograph courtesy of Katherine Houpt.)
Conditioned horses have been used to study the effects of psychotropic pharmaceuticals. In one example, horses were trained to interrupt a light beam for a reward of oats.49 They developed their own individual response rates, which remained stable at between 5 and 35 responses per minute for each horse over a period of months. Reserpine (5 mg/ horse, i.v.) depressed the response rate in all horses tested. This depression was maximal between 3 and 5 days after treatment and lasted for up to 10 days. In part, the lasting nature of the drug accounts for its appeal to those seeking to modulate equine flight responses, especially during traditional breaking (2.5–10 mg s.i.d. or b.i.d. orally for up to 30 days50) (see also Ch. 3).
Training and behavior modification
Learning allows animals to use information about the world to tailor their responses to environmental change. By avoiding pain and discomfort, animals can make their life more pleasant. Even invertebrates such as flies, slugs and ants show impressive forms of learning when avoiding unpleasant stimuli. When animals cannot evade pain or aversive stimuli, they become distressed; but if this is chronic in nature, they develop learned helplessness and fail to make responses that were once appropriate.
We manipulate animals’ experience to train them. Training generally means drawing out desirable behaviors and suppressing undesirable innate behaviors to institute novel responses.
In training horses we have exploited their need as prey animals to avoid discomfort and, for that matter, threats of discomfort.37 Crudely speaking, we apply pressure to attain the desired response and remove it when we get the desired response. The aim of training is to install signals (cues) that result in predictable behavior patterns. When the association between pressure, response and timely release becomes highly predictable, a habit emerges. If a habit does not develop, it could be that the animal has learned that there is no response that can reliably cause the pressure to be released. This may reflect inconsistency on the part of trainers. Unfortunately, it is possible to identify many horses in all spheres of ridden and draft work that have developed such learned helplessness and are often labeled ‘stubborn’ (see Ch. 13).51
The ability of horses to learn is one reason humans find them useful and valuable.47 The changing role of the horse demands a greater emphasis on humane treatment and the development of more sophisticated research into horse education.45 In this context, veterinarians and equine scientists can play an important role as innovators countering traditional horse-lore that resists technological advances.52
Training naïve animals is generally preferable to training animals with inappropriate experience.53 If an animal is pre-exposed to a conditioned stimulus for a number of trials before structured conditioning commences, i.e. before reinforcement, the acquisition of a conditioned response to that stimulus will be retarded. The animal has simply learned to ignore the stimulus because it has no important consequences.
With a sound grip on learning theory, all practitioners can apply therapeutic behavior modification programs that identify the motivation, remove the rewarding aspects of the unwelcome behavior and reinforce a more appropriate alternative. If the strategy does not work, one can be sure that training has been insufficient to establish the new associations or that the reinforcement for the new response is insufficient to overcome gratification from the existing behavior. Due consideration of equine ethology usually serves to explain failures of this sort. If the salience of a cue or the value of a reward is insufficient, no amount of training expertise will effect a desired response.
Training horses, whether under-saddle or in-hand, stabled or in the paddock, basic, advanced or remedial, usually involves operant principles. Before giving a reward, the trainer must wait until the animal produces or begins to produce the desired activity. Rewarding the desired behavior relates to the law of effect, which states that whatever behavior immediately precedes reinforcement will be strengthened.
Reinforcers and punishments
Be it positive or negative, reinforcement will always make a response more likely in future. Conversely, positive or negative punishment will generally make a response less likely in future (Table 4.2).
Table 4.2 Punishment versus reinforcement – effect of the treatment
| Response becomes more likely in future | Response becomes less likely in future |
|---|---|
| Positive reinforcement – titbit reinforces begging | Positive punishment – applying tension on the rein increases discomfort in the mouth |
| Negative reinforcement – easing tension on the rein reduces discomfort in the mouth | Negative punishment – complete removal of food extinguishes begging |
Both punishment and negative reinforcement can be applied as consequences of behavior and so are central to operant conditioning. Many trainers claim not to use negative reinforcement but are instead simply confused by a term that may have unpleasant connotations.54 In this context, negative is used in the mathematical sense and refers to removing (subtracting) something from the animal’s world. Similarly, positive refers to adding. So when trainers reinforce a behavior by removing something unpleasant, they make the behavior more likely in future. For example, if a trainer stops tapping a horse with a whip (see case study in Ch. 13) when it moves in the desired direction, he makes the horse more likely to move away from the whip when the tapping resumes, i.e. the ‘move away’ response has been negatively reinforced. ‘Positive’ and ‘negative’ when applied to reinforcement are not value judgements, as in ‘good’ or ‘bad’, but arithmetical descriptions of whether the behaviour is reinforced by having something added or something taken away, e.g. pressure (Fig. 4.10). For example, when the horse responds to a turn signal and the rein pressure is immediately released, negative reinforcement has been applied.
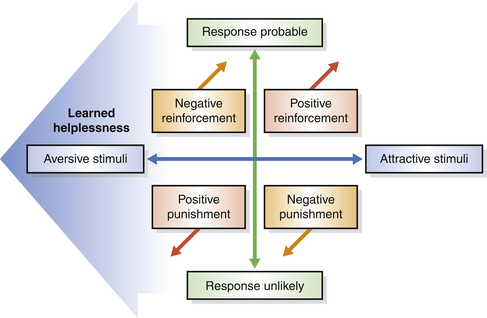
Figure 4.10 The likelihood of a horse in training offering a particular response is a result of the level of aversiveness or attractiveness of the stimulus. Within this structure lie all forms of reinforcement.
Negative punishment or omission forms an important part of our attempts to improve or modify responses. Most readers will agree that a horse being encouraged to perform a new behavior in the manège will first attempt to use an established response. The absence of reinforcement at that point makes repetition of an unwanted established response less likely. If reinforcement has been omitted, the horse has been negatively punished. This forces any horse labeled as having either any talent or even a temper to try new solutions to its problem. An educated horse has learned that it can use a variety of responses to solve the problems presented by hand and leg signals. It is consistency in both the presentation of such problems and the provision of consequences that distinguishes good trainers.
In the interests of clarity, I encourage all trainers and animal educators to consider carefully their use of these terms. Punishment is not in itself a dirty word. Nor is negative. Both negative punishment and positive punishment can be extremely mild. That said, it is easier to resolve problem behaviors that reflect training errors in positive reinforcement than those that emerge from punishment.55 The degree to which one relies on either reinforcers or punishers and the consistency with which one applies them are what matter to the animal.
Using reinforcers
A reinforcer is anything that increases the frequency of the particular behavior that it follows. A response will increase in strength when followed by a reward. The merit of a reinforcer can be measured only in terms of the degree to which it makes the behavior more likely in future. If a trainer’s saying ‘good boy’ in response to a horse’s leg-yielding has no effect on the horse’s future behavior then, according to this definition, reinforcement has not occurred. The trainer’s words have had a neutral or even confusing effect. The definition does not describe how or why some events act as reinforcers. Whether some event is called a reinforcer is purely a matter of the effect it had.
Consideration of the type of reinforcer is important. Discrimination performance in horses improves if there is a differential outcome in reward.56 In a discrimination task where the color of the center panel signaled that either a left or a right lever response was correct, horses performed best when different reinforcers (food pellets for one lever; chopped carrot for the other) were linked with each lever, than when reinforcers were randomly assigned or identical.56 Therefore many different contextual features associated with both stimuli and reinforcers can be integrated to maximize differentiation and thus enhance performance.35 However, in ridden horse contexts, only a limited variety of reinforcement is available. This generally involves the removal or release of pressure.
Palatable foods are generally more reinforcing if they are not normally part of the horse’s diet. Horses that have been exposed to these foods may perform with a higher level of consistency for this type of reinforcer. Because they can recognize these types of food as highly palatable and receive them infrequently, they seem more motivated to work for them. However, this is less effective in naïve horses because of neophobia (literally, fear of the new).
Significantly, horses work to avoid negative reinforcement, even when this interferes with their access to positive reinforcement.35 In practical terms, this has meant that although such rewards appear frequently in experimental protocols, little attention has been paid to the merits of reinforcing desirable behavior under-saddle with positive reinforcers. The problem is that it is difficult to deliver food immediately after the horse offers a desirable response. Therefore devices that instantly deliver rewards to the mouth allow the time between performance of the desired behavior and its reinforcement to be minimized, effectively enhancing the speed of learning. Preliminary research into such devices has produced encouraging results.57 That said, since the horse has evolved to stop moving when it finds pleasing food, such devices may not necessarily align well with ridden work since this relies more heavily on chains of locomotory rather than postural responses.57
Food is not the only reward that can be used. The other obvious one is water, when given to subjects that have been kept thirsty. For example, although questionable on humane grounds, this approach is acknowledged as one of many effective means of motivating a horse to enter a trailer.
Reinforcers can be either primary or secondary. Primary reinforcers are any resources that animals have evolved to seek. If the animal’s motivation is correctly predicted, food, water, physical contact, sex, play, liberty, sanctuary and companionship can all be used as primary reinforcers. Secondary reinforcers are stimuli that are not intrinsically rewarding but that have become associated with the kind of primary resources listed above. These associations make great sense in evolutionary terms since an auditory, olfactory or visual cue that has become reliably linked with a primary reinforcer will hold an animal’s interest much longer than a neutral stimulus.
Consider the way horses are often praised with tactile stimuli: they can either be scratched at the withers or patted on the neck. Horses have evolved to find grooming one another rewarding. Indeed, when horses indulge in the familiar ‘I’ll scratch your back if you scratch mine’ occupation, reduced heart rates suggest that they may be gaining pleasure or stress reduction from the stimulation. So, a scratch on the correct part of the withers can represent a primary reinforcer. By comparison, the far more common practice of patting horses on the neck is reinforcing only if the owner has coupled the pat with something pleasant.4 Because horses have not evolved to be motivated to behave in certain ways for pats on their necks, the intervention has to be conditioned as a secondary reinforcer (e.g. by patting immediately before feeding).
Reinforcement schedules
Until a behavior is firmly fixed as a conditioned behavioral response, the trainer must be consistent in applying signals and in granting rewards. If consistent reinforcement is not provided for correct responses, the horse’s behavior becomes unpredictable. As we see in Chapter 13, this is especially important when using pressure/release pairings. Horses cannot be expected to learn well if exposed to delayed reinforcement, as they do not relate the reinforcement to the behavior. Immediate comfort or immediate relief from discomfort is what works for horses.
Once a behavior is consistently elicited as a conditioned response, it can be enhanced by means of a variable reward schedule. The desired behavior can then be rewarded unpredictably. It is also necessary to obtain the same results in multiple locations in order for the behavior to become generalized and for context specificity (see later) to be overcome. As will be discussed in Chapter 13 on Training, this step is sometimes labeled ‘proof’ (i.e. that the learning is no longer context specific).58
Myers and Mesker59 showed that a horse could respond to different fixed-ratio positive reinforcement schedules (in which partial reinforcement is delivered on the basis of the number of correct responses made) and fixed-interval positive reinforcement schedules (in which reinforcement becomes available again only after some specified time has elapsed). They reported that few reinforcements were required under each new presentation schedule to get predictable response rates from the horse. Their results ranked horses’ responses to the different reinforcement schedules as similar to the responses of tropical aquarium fish, guinea pigs and octopi.
Shaping behavior
Although Skinner’s findings with his lever-pressing rats may seem to represent common sense, his school of thought has produced some intriguing principles. Perhaps one of the simplest yet most powerful of these is shaping. This is the concept of reinforcing successive approximations to the final (desired) response. The technique allows a trainer to move from a situation where it is impossible to reinforce a desired response (because that response never occurs) to one where the targeted response is occurring, being reinforced, and increasing in reliability. If trainers wish to reinforce particular responses, they can either wait for the behavior to occur spontaneously, which can be readily reinforced if the behavior occurs frequently, or they can shape the behavior pattern. In seeking to train complex behaviors or those that occur uncommonly in an animal, the trainer will usually opt to reinforce successive approximations of the final behavior (Fig. 4.11).
Figure 4.11 Arabian gelding being shaped to bow. Generally, at each successive iteration of the behavior, the reward is withheld until the horse shows an improvement on its previous best.
(Reproduced by permission of the University of Bristol, Department of Clinical Veterinary Science.)
Crucially, shaping relies on sparing and grading the reinforcement so that the animal does not stagnate. So, for example, when training a horse to approach a target (in so-called target training) a reward is given only when the horse travels closer to the paddle or stick (or whatever is being used as a target) or does so with more speed than on previous occasions. A common characteristic among good trainers is their ability to recognize an opportunity to reinforce improved ‘approximations’. While poorer trainers complain that their animals fail to understand what is being asked of them and feel that the animals have peaked in their training, superior trainers have the sense and patience to capitalize on each tiny improvement as the only way of moving toward the final response.
While shaping a new behavior or, for that matter, modifying an existing one, it is important to reward target behaviors as soon as they happen. Any delay in rewarding the improvement will lessen the effect of that reward. This may be because it allows the subject to perform another response during the delay interval and it is then potentially this response that is reinforced. An example might be rewarding a horse for jumping a fence very cleanly by giving him a sugar cube. To administer the sugar while riding, you would have to bend forward and place it in front of the horse’s mouth. Since this could not be achieved safely at speed, you would probably slow down or even halt. Instead of learning to jump ever more cleanly, the horse would predictably learn to slow down and halt, these being the behaviors closest to it receiving the reward.
Clicker training
Perhaps the most popular example of a secondary reinforcer is the sound made by a so-called ‘clicker’, the handy device used by thousands of trainers worldwide.60 Clickers develop an association for the animal that allows the trainer to bridge the gap between the time at which an animal performs a response correctly and the arrival of a primary reinforcer (most commonly a food reward). Essentially the clicker comes to mean ‘Yes, that’s good – expect a reward any second now’. When a clicker is first used, the correct association is established by making the sound just before giving a delicious reward. Through classical conditioning repetition assures the animal of the signal’s reliability.
As discussed in subsequent chapters, clicker training can be extremely helpful in remedial behavior modification. Moreover, after traditional negative reinforcement based conditioning has established desirable responses, clicker training is suitable for refining them (Fig. 4.12). The benefits of positive reinforcement as applied in clicker training should not be underestimated. There are many impressive accounts of successful clicker training, including the use of the device to train a sour dressage horse to work with its ears pricked forward. It would be interesting to examine the effect of such a cosmetically palliative approach on the physiological stress responses in such animals. If nothing else, clicker training may be more humane than traditional negative reinforcement training. With clicker training, ‘what you click is what you get’, therefore if your observations and timing are not perfect you will inadvertently shape some behaviors you do not want. So a trainer’s poor timing can make clicker training ineffective. In contrast, poor timing in traditional negative reinforcement training can amount to abuse (see Ch. 13).
Any secondary reinforcer can be instituted in this way. The only significant feature of a commercial clicker device is that the sound it makes is sharp and distinctive. The brevity and clarity of a clicker facilitates precise reinforcement of brief behaviors, such as the bringing forward of the ears. Being pocket-sized or attachable to keyrings, clickers are convenient, but by no means unique. Indeed, as long as they cannot be confused with words that appear in common parlance, human vocalizations (so-called clicker words) are even more readily available. Some horses are fearful of the click per se and are therefore candidates for alternative secondary reinforcers such as a whistle. The use of clicker training principles for shaping and modifying unwanted behaviors, such as reluctance to load, merits serious consideration. By deconstructing a response into its constituent parts and rewarding the horse for successful completion of each, we can demonstrate to the horse that its fears of undertaking the complex behavior as a whole may have been unjustified.
Secondary reinforcers are most effectively established when presented before or up until the presentation of a primary reinforcer. Simultaneous presentation of a reward and a novel secondary stimulus is less likely to work because the primary reinforcer will block or overshadow the new stimulus. Similarly, presentation of the secondary stimulus after the primary reinforcer is unproductive, because although an association will exist between the two, it does not help the animal to predict the arrival of a reward.
The speed or strength of learning increases with the size and attractiveness of the reinforcer. This is why horses will learn to run faster to the sound of a rattling bucket than they will to the rustling of a haynet. Many horses respond well to carrots as the primary reinforcer in a clicker training protocol but only if carrots are not routinely offered in regular meals. The relationship between motivation and the reinforcing value of any food should be considered here. However, as we have seen, a degree of familiarity is altogether desirable since some horses will not work for novel foods regardless of their apparent suitability. So when selecting primary reinforcers, experienced trainers observe the horse’s responses to determine the reinforcing value of a novel reward.
Horses show a rapid decline in interest in responding for the secondary reinforcer only, and the temporal link between primary and secondary reinforcers is also critical.60 Thus the fundamental rules of learning theory apply, and trainers who build the firmest association between the primary and secondary reinforcers by ensuring that the ‘clicker never lies’ (i.e. it always predicts the arrival of a primary reinforcer) can most effectively shape desirable behaviors. The use of secondary reinforcement seems to increase a horse’s interest in performing novel tasks,60 and this creativity in the horse’s approach to problem solving accounts for the growing appeal of clicker training in behavior modification programs, especially where traditional remedial approaches have failed (see Chs 13 and 15).
When shaping alternative responses, practitioners find that continuous reinforcement schedules rapidly increase the response rate of new behaviors.60 However, once the behavior has been shaped and is under stimulus control (see below), intermittent reinforcement can be used (with a resultant increase in the resistance to extinction). That said, during intermittent reinforcement each click must be ‘honored’ with a reward since failure to do so weakens the reinforcing effect of the click.
Contiguity
The principle of contiguity states that temporally close events will become associated. Giving a sugar lump to a horse two minutes after a pat on the neck will not develop a useful association with the pat. The lump has to arrive within seconds of the pat if the latter is to become reinforcing. The time interval between stimuli is not necessarily the most important criterion in establishing an association. Events far apart in time can still be associated as long as there is a high predictive link between them. The best example of this is food aversion learning that helps animals to avoid food items that have previously made them ill. Novel flavors are more likely to be associated with later sickness and therefore the horses may be alert to this possibility when they consume novel foods.
Operant conditioning has been used to indicate the behavioral effects of drugs. Since the reward is usually food, a decrease in the rate of responding suggests that the drug administered is either a depressant or an anorexic agent. Food aversion learning has also been used by scientists interested in the consequences of proprietary drugs on horse welfare. The aversive effect of drugs can be calibrated by the degree to which food associated with it is subsequently avoided.
Punishment
Horses can learn from both unpleasant and pleasant experiences. Haag et al61 found that there was significant correlation between a pony’s rank in learning ability to navigate through a maze for a food reward and its rank in learning ability to avoid mild electric shocks. When shocks are used to teach a horse how to navigate through a maze, subjects take significantly longer to make their choices than horses who were not punished and simply found their way through by trial-and-error. So punishment can stifle creativity and impede a horse’s innate problem-solving skills.55 One should bear this phenomenon in mind when horses that are familiar with human handlers are asked to solve problems in the presence of humans. It is possible that horses have learned that it is best not to offer creative solutions to problems since this can lead to punishment.
Punishment decreases the likelihood that a behavior will be repeated. Positive punishments are those that are instinctively recognized by the horse as detrimental. For example, a horse that bites at a human’s arm and in doing so impacts on a spiky object concealed in the sleeve, experiences a positive punishment that is clearly linked to the unacceptable action. Negative punishments involve the removal of desired things in response to particular behaviors. In free-ranging adult horses, resources are rarely withdrawn as a result of an individual’s behavior. This may be why negative punishment is not an important feature of equestrianism. The chain of association between events is weaker if the events involve removal of consequences. That said, it is worth noting an interesting example of negative punishment when unbridling a horse after work. Most horses relish a head massage when the bridle is removed. Indeed some learn to rub their head against the nearest human even before the bridle is removed. This is annoying and can be dangerous for personnel. To meet the horse’s needs for cutaneous comfort on one’s own terms it is possible to negatively punish a horse for head rubbing. This simply involves massaging its head only when it is still. If the horse moves its head, the pleasurable massage stops immediately. Most horses rapidly learn to remain perfectly still under such conditions.
Negative reinforcement involves the conditioning of preceding signals that predict the potentially aversive stimulus. Punishment, on the other hand, is more complex in that it represents a form of backward chaining because any signaling of the aversive stimulus is either absent or follows the undesirable behavior. Trainers who use punishment to eliminate undesirable behavior must ensure that the wrong association is not created. The unthinking rider who thrashes a horse for knocking down a fence runs the risk of associating fence jumping, be it clean or sloppy, with being hit. Rather than correctly associating a painful consequence with the undesirable behavior, many animals learn to fear the trainer or the training area (Fig. 4.13).

Figure 4.13 Horses can be taught that the only means of escaping aversive stimuli (the whip) is to rear. Discomfort is apparent on the face of this animal as the tight side reins thwart his attempts to balance on his hindlegs.
(Reproduced with permission of the Captive Animals Protection Society.)
Despite some of the problems that are associated with the use of punishers, they remain popular. Free-ranging equids communicate with their companions using mild aggression and reliable threats of aggression. This may make horses innately tolerant of such negative stimuli but is no excuse for physical abuse by humans.
The punishment procedure makes the onset of an aversive stimulus contingent on a particular response. The punishment procedure may or may not lead to a reduction in the response. The situation is complicated because the punishing stimulus also elicits other responses that may actually increase the performance of the ‘punished response’. Whipping a horse for bolting will usually serve to rocket it forth once more. Bit pressure is usually increased prior to such whippings and with discomfort in both the mouth and on the flank, the horse enters a conflict situation (see Ch. 13).
Presentation of aversive stimuli will usually produce an overall suppression of behavior in general. Therefore, a reduction in performing a response may have nothing to do with the specific link between the response and the ‘punishing’ stimulus. So, in beaten horses, confusion and flight responses are more common than useful learned associations.
The effectiveness of punishments is limited by a number of other factors, including punishment intensity. The more motivated an animal is to perform an action, the greater the intensity of the punishment required to stop it.
Warnings of punishment could be useful, just as clickers are predictors of reinforcement. Therefore it seems likely that both horses and riders could benefit from a device that warns them of imminent rein pressure. There is evidence that horses can learn to take evasive action when they hear noises that warn of aversive stimuli. Early data suggest that reins that stretch and make a noise before reaching the limit of their elasticity and causing discomfort in the mouth are good for horse welfare and indeed rider education.
Contingency
Contingency is the temporal relationship, or correlation, between two repeated events or between repeated responses and reinforcers. It describes the extent to which two events are related, ranging from complete consistency – so that if one event occurs then the other will follow, even if after a considerable time – through completely independent, to a consistent negative relationship where the occurrence of one event indicates the non-occurrence of the other. Contiguity is the closeness of two events in space or time. Contiguity and contingency should not be confused with one another. Most forms of conditioning, except perhaps taste aversion learning (see later), require both contingency (relatedness) and contiguity (closeness in time) between two events (in the case of classical conditioning) or between a response and a reinforcer (in the case of operant conditioning).
As indicated above, it is possible for there to be a very strong contingency between two events that do not occur close together in time. Conversely two events can sometimes occur close together in time but overall there can be a weak or zero contingency between them (independence). In many ways one can think of contingencies as consistent links between events that transcend time. Again, effective training requires that both contiguity and contingency are applied.
The contingency between the response of the horse and its subsequent reinforcement relies on an attentive trainer. To use clicker training as an example: effective linkage of the primary and secondary reinforcers relies on the arrival of a primary reinforcer being contingent on the sound of a click but, as we have seen, contingency varies with the consistency of the training method. Contingency reaches 100% when the ‘clicker never lies’. If a food reward arrives without a click or the clicker is sounded without the subsequent arrival of food then the contingency is weakened.
Generalization and discrimination
Pavlov found that almost any stimulus could act as a conditioned stimulus provided it did not produce too strong a response of its own. In very hungry dogs, even painful stimuli such as electric shocks delivered to the paws, which initially caused flinching and distress, quite soon evoked salivation if paired with food.
A colored board in front of Pavlov’s dogs could be used to present visual images with an infinite variety of colors and shapes. Pavlov carried out exhaustive tests using this apparatus and a variety of tactile, visual or auditory stimuli. He found that if a dog was conditioned to salivate when a pure tone of perhaps 800 Hz was sounded, it would also salivate but to a lesser extent when other tones were given. This is now known as generalization. The dog generalized its responses to include stimuli similar to the conditioned one, and the more similar they were the more the dog salivated.
Generalization
Stimulus generalization occurs when a response reinforced in the presence of one stimulus is also elicited by similar stimuli. For example, a horse might be trained to nudge an orange panel for a reward. If the color of the panel is changed to yellow the horse will still nudge, though possibly at a lower rate. We capitalize on stimulus generalization when we use mature educated horses (so-called schoolmasters) to educate novice riders. The horse generalizes the rider’s cues to others it remembers from previous, preferably better, riders and obligingly executes the required maneuver. Generalization is at play when horses learn to offer the same responses that they have developed in the home schooling manège to, say, a dressage arena at a competition. This demonstrates that the trained maneuvers are not context specific to the stimuli that surrounded the animal during its early learning at home. Barrier trials in racing are useful in a similar fashion since they give young racehorses the opportunity to transfer appropriate responses from starting stalls at the training center to starting stalls in general.
Stimulus generalization works against us when we encounter horses that ‘hate vets’. Such animals have learned to generalize the stimuli from one human wearing veterinary smells and bearing needles to all humans that meet the same criteria. But by recognizing and mimicking the stimuli horses use to identify vets and making appropriate associations with them, owners can train horses to tolerate and even enjoy vets.
Discrimination
The opposite process to generalization is discrimination. Horses naturally discriminate to some extent, otherwise they would respond equally to all stimuli. Discrimination can be accelerated if, as well as rewarding the right stimuli, the horse is slightly punished when it responds to others. This is called conditioned discrimination and has been of enormous benefit in working out the sensory capabilities of animals (see the case study of Rascal performing a visual discrimination task in Ch. 2).
Commands used to cue a behavior can be the product of discrimination. When a horse learns that certain cues set the stage for a specific response, the cues (i.e. the commands, signals or aids) are labeled discriminative stimuli. The horse is said to be under stimulus control when it always responds to the specific cue and does not offer the response until or unless it has been cued. By rewarding horses for responding appropriately to stimuli that are less and less obvious, we can foster the power to discriminate between the stimuli that are rewarded and all other background information that would otherwise prevail. Discrimination is what allows us to train dogs to detect drugs, pigs to locate truffles and chickens to identify images of familiar feathered friends. A similar process is at play when we train horses to respond to smaller and smaller cues in training. To obey a trainer’s leg signal to pirouette, a horse must first be persuaded to rotate on a much larger surface with the use of much less subtle stimuli. All horses can learn to discriminate. Unfortunately, however, only the good trainers learn to deliver cues with sufficient subtlety to capitalize on this ability.
Whether positive or negative, reinforcers are effective only if the recipient is motivated appropriately. Relative to normal or fat horses, those in poor body condition may make horses be less likely to err in discrimination tasks for food rewards.62 Rather than true learning ability differences, this seems to reflect the lack of motivation in replete horses.
Although the only data available are from experiments using stimuli such as cards and panels that are arguably less salient to horses than, say, grazing-related stimuli, the ability of horses to discriminate is impressive (e.g. see Sappington & Goldman63). It appears that, although horses can learn complex discrimination problems based on sameness,64 we have yet to show that they can generalize this learning to situations involving novel stimulus presentation.35
While stimuli that have become generalized may be ignored, those that are enhanced are more likely to elicit a response. Stimulus enhancement describes the process by which an animal’s attention can be drawn toward a given stimulus and increase the animal’s motivation to approach the stimulus.65 For example, the sound of a rattling bucket will prompt experienced horses to approach the bucket and eat.
As with all species, learning in the horse reflects core survival requirements. Scientists generally discuss issues of cognition, learning and memory without using the term intelligence.66 So, leaving intelligence aside, the complexity of learning is captured by a hierarchy of learning abilities from habituation to conceptualisation (Table 4.3).
Table 4.3 Hierarchy of learning abilities
| Level | Learning |
|---|---|
| Learning not to respond to a repeated stimulus that has no consequences | |
| Making responses to a new stimulus that has been repeatedly paired with an established effective stimulus | |
| Learning to repeat a voluntary response for reinforcement or not to repeat a voluntary response to avoid punishment | |
| Learning a sequence of responses to obtain a reinforcement at the end of the sequence | |
| Learning to make an operant response to only one set of stimuli from more than one set of stimuli applied concurrently | |
| Discrimination learning based on some common characteristic shared by a number of stimuli | |
| Learning of concepts that emerge from the relationship between stimuli such as ‘A and B’ (conjunctive), ‘A or B’ (disjunctive), and ‘If A, then B’ (conditional) | |
| Logical reasoning, such as ‘Option A is likely’ if and only if ‘Option B is present’ |
Adapted from Thomas 198667 and Murphy & Arkins 2007.68
As mammals, horses have brain characteristics that suggest their learning mechanisms may be similar to those of humans (see Ch. 3). Like humans, horses are proficient at trial-and-error learning (learning to perform a reaction through reward), classical conditioning (learning associations between events) and habituation (learning to ignore aversive stimuli that do not hurt). Beyond these basic learning mechanisms, horses can also generalize stimuli, and can even categorize objects based on similar physical characteristics.69 That said, cognitive research has yet to produce empirical evidence that horses can develop abstract concepts.70 Indeed, there is no peer-reviewed published evidence that horses possess significant higher mental abilities beyond generalizing and categorizing (level 5 in Table 4.3). Attributing human cognitive powers to horses is a double-edged sword. It may appear to honor horses’ minds, but it inadvertently excuses violence in training since a horse that thinks as we do must surely know what we want it to do and can reason why it is being punished. Without downplaying the unique features of equine intelligence, scientists look for the most parsimonious explanations of phenomena. What horse people may regard as examples of reasoning in their horses usually turn out to be trial-and-error learning. The pony that fiddles with the gate latch and learns to open it is a typical example. It is clever, but it is not an example of reasoning.
Extinction
Extinction occurs if the learned response occurs but is no longer followed by reinforcement or if a conditioned stimulus (such as a clicker) is always presented without the unconditioned stimulus (such as the reward). The effect of these procedures is an eventual reduction in response strength, as measured, for example, in rate of response. If horses do not get their expected rewards, they are less likely to behave in ways that have previously paid off. The behaviors drop out and extinction is said to have taken place. This is what happens when horses that nip and nuzzle humans for food stop doing so because they are never rewarded. Often extinction is accompanied by not simply an absence of the learned responses but a reversion to innate behaviors. Occasionally animals may experiment at this point by adopting sequences of other learned behaviors in an attempt to acquire rewards. Where possible, practitioners should plan ahead to determine which of these should be reinforced.
Because extinction does not occur in a vacuum, context-specific stimuli that were present during learning of the unwelcome response can retard extinction. This explains why behavior-modification programs that advocate removal of perceived rewards work best if imposed at the same time as a fundamental environmental shift such as a change of stable.
Frustration effect
There are some intriguing outcomes associated with extinction. Early in extinction it is usual for a so-called ‘frustration effect’ to occur. This describes the way responses usually get transiently more frequent before they disappear. For example, rather like a human repeatedly pressing the ‘on’ switch of a faulty television set, a horse that has superstitiously learned to kick the stable door to get food will kick it harder if the food delivery is delayed. One theory proposes that the subject responds faster because it is ‘frustrated’. To avoid misinterpreting an extinction-based behavior-modification program as a failure, it is important for therapists to be aware that the frustration effect occurs.
Spontaneous recovery
Presentation of the conditioned stimulus alone causes extinction. Extinction can apply to any behavior that occurs and is no longer reinforced. Both welcome and unwelcome behavioral responses will weaken in the absence of reinforcement. If, after extinction has been completed, the animal is given a break from training and then presented with the conditioned stimulus again, the previously extinguished response may suddenly reappear.41 This transient rebound in response strength after a ‘rest’, following extinction, is called spontaneous recovery. This possibility is sometimes overlooked in behavior therapy designed to eliminate unwelcome behaviors by extinction. If an undesirable behavior makes a return, personnel may forget the strength of the original response when they compare current behavior with previous behavior. So, when the response recurs after a long absence, the owner’s conclusion may be slightly damning with remarks like ‘the removal of rewards hasn’t helped’ or ‘the animal has regressed’. In fact, this is the typical pattern found in extinction. This is particularly important with originally fearful responses that have been modified by counter-conditioning because these responses can show spontaneous recovery if reinforcement is withheld. To prevent the original fearful response recurring, the trainer must continue to expose the animal to the relevant stimuli from time to time.
Memory
Memory is the retention or storage of information and therefore the basis for all higher forms of learning. The laying down of memory traces is considered to occur in stages, but can be thought of as a continuum. Fraser71 rather boldly suggests that the length of time an animal can remember a specific signal of training or command can be taken as some measure of intelligence. However, horses that learn a task most readily are not necessarily those that have the best memories.7
Sensory memory refers to memory traces formed within the sensory areas of the CNS (see Ch. 3) whenever any sensory receptor is activated. It has been estimated that only about 2% of sensory input is ever committed to permanent memory. For input to pass from sensory memory to short-term memory there must be some kind of response to the experience. The trace is more likely to be stored if the experience was bad (the speed with which horses learn to avoid electric fences is pertinent here). Animals under the influence of sedatives, tranquilizers, etc. have their sensory experiences dulled and therefore have great problems remembering new experiences. Veterinarians do well to remember this before they prescribe psychoactive drugs for behavior modification. This explains the poor results obtained when horses that are fearful of, say, clippers are treated with acepromazine or detomidine: they show no attenuation of the fear response when presented with clippers after the effects of the sedative have worn off. Similarly, studies on the effect of reserpine on unhandled yearlings72 suggest that the attenuating effect it has on heart rate response to the presence of handlers is transient. This is state-dependent memory. There is also place-dependent memory, which is considerable in horses. When teaching a young horse a new response it is useful to go back to the same spot in the arena to rehearse it. Because confusion often prevails when an animal is in a highly emotional state, an upset and anxious horse does not learn desired responses as well as a calm subject. Hence classical riders advocate the practice of the systematic elimination of resistance. This approach requires that the horse must be calm before being cued to perform. After stress, conflict or confusion during training, the good trainer waits for the calm to return before resuming work. Even though classical riders often apply very subtle pressure-based stimuli, one can see how the return of calm would be less elusive if one were using positive reinforcement.
We know that primary memory involves continuous neural activity because if anything interrupts the activity (for example, a blow to the head, drugs, an unexpected interruption) then the short-term memory trace is lost. Short-term memory appears to have a temporal sequence, so what goes in first will be replaced by later inputs if the memory is not repeated (learned). This repetition is essential for pushing the memory into the long-term stage. The mechanism for the long-term storage of memory traces is thought to be biochemical. Being physical, they do not depend on sustained neural activity. Specifically, long-term memory depends on alteration in gene expression, which influences the formation of either persistent proteins or nerve growth factor.
There are many impressive accounts, anecdotal and scientific, of the resilience of equine memory traces.3,38,73,74 An established means of testing memory in equids is to offer 20 pairs of patterns (Fig. 4.14). The horse is taught that regardless of its position, one of each pair will always yield a food reward when pressed. Deterioration depends on the extent to which horses are taught new associations and perhaps new apparatus in the interim, but the reported frequency of correct choices when the same apparatus is presented after 6 months is approximately 85%. Horses and ponies compare well with donkeys and elephants using this experimental paradigm.75
Figure 4.14 Twenty pairs of patterns used for equine memory tests, including those by Dixon73 and Voith.74 To obtain food the horse had to select the patterns that appear on the left of each pair in this illustration.
An interesting phenomenon is the effect of a period without conditioning, because the temporal distribution of training trials influences equine learning abilities.76 While this is challenged by data showing that horses learn more quickly and make fewer errors if they are trained daily,77 it accounts for the reported effectiveness and popularity of turn-out or spelling periods during the early education of horses, even though plausible mechanisms have yet to be investigated.
The robust nature of equine memory helps to explain the poor performance of horses in discrimination reversal tests5,47 in which, for example, a turn to the left is reinforced for a given number of trials and then the reverse, a turn to the right, is required.
Additional topics in equine learning
As we begin to appreciate the complexities of studying equine learning scientifically, the role of cognition in adaptive behavior is becoming clearer.
Learning sets
Horses learning to use experimental apparatus, such as in the pairs discrimination tests in Figure 4.14, often take time to grasp the challenge of discriminating between operant panels. Once they have established the learning set, their responses become more rapid and accurate. Such horses are described as having learned to learn. When they build on a basic groundwork and then begin training more high school maneuvers, dressage trainers report a similar phenomenon. That is, having reached a certain understanding about what is being asked of them, educated horses learn to pay attention to yet finer stimuli and climb a steep learning curve that takes them to Grand Prix level.
Insight versus simple learning
Using insight, an animal can arrive at a solution without trial and error. The possibility of such complex learning has to be approached with caution since apparently complex patterns of learning can often be explained in terms of simple associative learning. Take, for example, the story of Clever Hans, a horse that lived in Austria in the 1900s.78 Hailed as an equine arithmetic genius, Hans appeared to be able to count and do basic algebra. When set any puzzle with a number as its answer, Hans would begin to count out the solution with his hoof. He would never tap too many times; always stopping at the right number. This looked like complex learning and was the subject of detailed scientific scrutiny. His owner, Baron von Osten, was justifiably proud of him and their fame spread. They were fêted far and wide until a panel of scientists assembled to determine the status of the horse’s IQ. By placing a screen between the horse and any observers, they found his accuracy was entirely dependent on being able to see his audience. A question was posed and the counting commenced. The answer was reached and the counting continued. On and on it went because the horse had received no cue to stop. Hans had learned that he would be rewarded if his foot movements stopped when he detected subtle behavioral changes in human observers. These cues reliably emerged whenever he was set a problem and, of course, they always coincided with the human’s anticipation of the arrival of the right answer. It is believed that von Osten leaned forward as Hans approached the correct number and adopted a more upright posture when it had been reached. Furthermore it is thought that his brimmed hat (Fig. 4.15) helped to accentuate this visual cue. In addition, maybe the observers smiled, grimaced, braced themselves or changed their breathing patterns. Whatever they did constituted a sufficient cue for the horse to stop counting.

Figure 4.15 (A) Clever Hans and Baron von Osten. (B–E) Clever Hans undertaking a series of tests intended to demonstrate problem solving without trial-and-error.
(Reproduced with permission from Pfungst.78)
So, rather than complex learning, simple associative learning could explain the illusion. Hans was responding to tiny physical changes in the behavior of his trainer and observers as they became more tense and anxious that he was about to count too many times and disappoint them. He was declared a fraud even though he was in fact a brilliant judge of minute detail who had trained himself to spot these cues and earn rewards. This should serve as testament to visual acuity in the horse and remind us of the central importance of consistent body language when handling and training horses. The same principle has subsequently been used many times in entertainment to give the illusion of such unlikely academic brilliance.
Maze tests
Maze tests have been used extensively to measure the learning ability of animals. These typically involve a T-maze, in which an animal is presented with a choice to go left or right; the wrong choice runs into a dead end, the correct choice to an exit to food, water or an area with other animals (Fig. 4.16).79 Mazes have merit because they allow the animal to demonstrate its ability without human presence and thus avoid the Clever Hans effect. The absence of humans helps to eliminate other operator effects in horses, especially those that have been previously handled. It is worth noting, however, that the absence of humans is far from absolute in maze studies since they have to lead the horse to the starting point in the maze and retrieve it at the end point. It is important, therefore, to establish baseline results for each individual in an attempt to control for horses that, for example, may prefer not to be left or those that dislike being caught.
Figure 4.16 A horse about to enter a T-maze.
(© Stephen Budiansky. Redrawn, with permission, from Budiansky.79)
Differences between types of horses in maze-learning ability are intriguing. Foals learned which way to turn in a maze in fewer trials than their dams,80 and adult horses were particularly slow to learn a reversal, i.e. turning right after learning to turn left. Houpt et al80 investigated how a foal’s early experience with its dam could influence its learning ability. Using a single-choice maze, they compared the learning abilities of foals raised with their dams with those of orphan foals. Although the orphan foals spent more time in the maze during their first exposure to it, the learning abilities of the two groups did not differ.
Previously unhandled and extensively handled horses learned less quickly in a maze than moderately handled horses.53 This may have been because the previously unhandled horses were still adjusting to being handled, and the extensively handled horses were dependent on human contact to show them the way. In contrast Marinier & Alexander3 found no correlation between horses’ abilities to learn a maze and the extent to which they had been handled earlier. Maze studies suggest that a social rank does not predict problem-solving ability. A study of artificially reared and naturally reared foals81 that showed the latter group to take longer to solve a maze problem, alluded to the role of dependence on a leader. Perhaps we have yet to recognize the role of bonded pairs in problem-solving situations relevant to free-ranging equids. Other explanations for differences in learning ability in a maze also include the motivation for the reward and inhibition resulting from nervousness.
Many horses begin maze trials with a clear preference for one side or the other. An example comes from Kratzer et al47 who allowed 37 yearling geldings, fed diets with a range of protein contents, to wander through a T-maze on a daily basis. At the beginning of the experiment when the horses were allowed to freely walk through the maze five times with both exits open, 5 of the 37 horses chose the right side every time, and 5 chose the left side every time. For the next 5 days, the right side was open and the left side was blocked. Therefore horses with a left preference had to return to the bifurcation before completing their trip through the maze. Although on the fifth day of the experiment the mean number of wrong turns per trial fell from 0.65 to 0.27, about 20% of the youngsters still turned left as their initial choice on entering the maze.
Social learning
The transmission of new information and behavioral strategies among animals is slowly becoming more clearly understood. When we consider the transfer of information between animals it is important to distinguish between social facilitation, stimulus enhancement and true cultural transmission. In social facilitation, innate behaviors are initiated or increased in rate or frequency by the presence of another animal carrying out those behaviors. Horses, for example, are more likely to drink when they see others drinking. Stimulus enhancement is the ethological term that describes the ability of one animal (the so-called demonstrator) to draw the attention of another animal (the observer) to the location of reinforcement opportunity rather than an activity per se. These hard-wired copying responses commonly found in social animals are distinct from true cultural transmission, which is considered a higher mental ability and describes the observational learning of a novel behavior without trial-and-error experience.
This is taking us into the realms of what is known as observational learning and allelomimetic behavior. In laboratory settings, a variety of species, including chickens, grey parrots, quails, ravens, cats, octopi and chimpanzees, have been shown to pass information about foraging strategies from one individual to another. It makes sense for offspring to learn from their parents and for social animals to learn from one another, for example, about what food is safe to consume (see Ch. 8).
As social animals with excellent vision, one would predict that horses would use observations to improve their biological fitness (Fig. 4.17). It makes sense that animals that conform to a form of social order should be able to learn from pre-trained conspecifics; for example, young farm horses were traditionally harnessed alongside a steady mature plough horse to be taught verbal driving commands (Fig. 4.18). But this is associative and not necessarily observational learning. While horses seem good at hard-wired copying, the empirical evidence of true cultural transmission is rather scarce. Controlled studies in the horse have provided some evidence that stimulus enhancement may result from the behavior of demonstrator horses as they approach and interact with experimental apparatus that delivers food (Fig. 4.19).82 However, they have failed to demonstrate that observational learning enhances an individual’s ability to perform an operant task57 or make a choice between two feeding sites.82 As such they have generally challenged the notion that stereotypies can be acquired by mimicry.

Figure 4.18 Schoolmasters can be hitched with youngsters to assist in the training of draft horses.
(Photograph courtesy of Les Holmes.)

Figure 4.19 Results of tests of observational learning in horses have proved disappointing when compared with those for other domestic species.
(Reproduced by permission of the University of Bristol, Department of Clinical Veterinary Science.)
Studies in other species demonstrate that animals do not observe all conspecifics with the same enthusiasm. It may be that we have failed to ask the right questions of horses that are appropriately related in terms of rank, kinship or affiliation to demonstrators. There are likely to be serious limitations on what can be learned by observation. However, there is some evidence that learning to follow a human can develop through observation and that relative social rank affects the way observer horses may learn this from potential demonstrator horses,83 higher-ranking horses offering a more compelling model than equally familiar but lower-ranking conspecifics (the challenges of defining and measuring relative social rank are explored in the next chapter). Feeding behaviors are more likely to be transmitted than those such as maintenance behaviors with less obvious proximate advantages to the performer. Glibly, one might add that if horses learned to respond to riders by observing other horses being ridden, dressage arenas would most surely have been constructed with gallery-style stabling all around them.
Future studies on social learning should focus on the role of the dam as tutor to her foal because vertical transmission of information seems the most likely form of observational learning in a social species. Houpt et al80 reported no evidence of foals learning a spatial task from their dams. However, foals that were exposed in the first 5 days of life to humans as they groomed and fed their mothers during a short period (total of 1.25 h) approached and initiated physical interactions with humans sooner than those subjected to forced handling that included imprinting and haltering.28 Studies of this sort may help us to explore how we provide cooperative teaching to equine subjects, e.g. by interacting with relevant objects verbally.84 The importance of pursuing this line of enquiry lies chiefly in the notion that any training program or technique instituted by humans could be improved by incorporating appropriate elements of the horse’s highly developed social behavioral and learning repertoire and that, fundamentally, social learning may operate quite differently from mainstream learning theory.85
Obedience trainers report that motivation is enhanced in dogs that are allowed to observe others working with their trainer. This reflects dogs’ motivation to play (part of the so-called ‘will to please’) and the increasing use of rewards, especially toys, in their training. Although the opportunity to observe has little effect on the dogs’ ability to complete a task, it certainly seems to enhance their desire to get involved. The equivalent in horses will continue to elude us as long as we persist in using only negative reinforcement. The laudable use of educated horses in training novices to jump is sadly flawed as an example of effective use of observational learning in horse-training, because it relies on the youngsters’ motivation to follow (other horses) rather than to jump per se.
Error-free learning
In operant-conditioning, the most humane exposure of an animal to correction seems to arise in so-called error-free learning, in which circumstances are modified to reduce the chances of errors being offered. For example, in training horses to jump obstacles, a jumping lane can be used to instill the behavior pattern of negotiating the obstacle without testing the opportunities for evasion and acquiring inappropriate responses. Unfortunately, arranging schooling sessions so that opportunities for errors do not arise is far easier said than done. Given this reality and the likelihood that horses are learning all the time, it pays to bear in mind the following maxim:
Just because you don’t intend to teach a horse something doesn’t mean that you won’t; and just because you are not aware of having taught a horse something doesn’t mean that you didn’t.86
Influences on learning
Breed differences in normal behavior
When the learning ability of different breeds is compared we do well to consider breed differences in perception and normal behavior that may create a bias. While many of the behavioral differences among breeds reflect the uses for which they have been bred87 (see Table 1.2 on p. 8), the origins of others are rather more obscure. For example, Icelandic horses are said to have a particularly good homing instinct, though it is not clear whether they have advanced skills or a heightened motivation to return to their home range.
Bagshaw et al88 reported a breed effect of an oral dose of l-tryptophan on horses’ reactions to 15 minutes of acute isolation stress. Arabians were more active and vocalized more in isolation compared with Standardbreds. This study also showed that the Arabian had lower resting serotonin concentrations than the Standardbreds and that Arabians on a separate farm had lower serotonin than 10 Swedish Warmbloods fed the same feed and housed under the same conditions.88
If we assume that all horses are equally able to deliver signals from within the equine ethogram, some interesting differences seem to emerge in the extent to which they signal. Standardbreds have been noted to be more likely to demonstrate snapping behavior in estrus than Arabians.89 This intriguing finding merits further exploration. It is possible that this is a form of displacement behavior that masks behavioral conflict. When 224 adult riding horses’ responses to humans were observed and scored on the basis of their posture, French Saddlebreds showed friendly behavior more often than Anglo-arabs, whereas Thoroughbreds were more indifferent.90
In addition to the intriguing, intrinsic behavioral differences between breeds, breed differences in learning ability have been reported by several authors. Mader & Price91 found that Quarterhorses learned a visual discrimination task more readily than Thoroughbreds, a finding attributed to the relative distractability of the Thoroughbreds. However, the importance of the experimental apparatus in reducing fearfulness may have been confirmed by Sappington et al,5 who found no difference in the learning ability of Quarterhorses, Thoroughbreds and Arabians. Hot-blooded and warm-blooded horses learned an operant task less quickly than cold-blooded horses.82 As with any comparison of problem-solving exercises for a reward, it pays to consider whether all one really ends up testing is the motivation to access the resource. Thoroughbreds are often fussy feeders. Being generally less motivated to eat they may be disinclined to solve problems for food rewards. Thoroughbreds are also more reactive (see the discussion of emotionality in the subsection on temperament, below) and therefore may take longer to approach novel pieces of apparatus calmly. Also, because they tend to be stress susceptible, learning may be reduced as a result of generalized anxiety.
Six personality components reliably reported in horses are dominance, anxiousness, excitability, protection, sociability and inquisitiveness.92 When eight different breeds were surveyed to explore differences across these six personality components, variability between personality components depended on breed.93 Anxiousness and excitability showed most variability, and the Thoroughbred, Arab, and Welsh ponies and cobs were ranked as the top three breeds on both of these components. In contrast, dominance and protection varied the least, with Shetland pony and Irish Draft horse being ranked as the lowest on this component.
Some intriguing individual differences in temperament, in correlation with the height of facial whorls, have been reported. Horses with a single whorl above or between the eyes are said to be of an ‘uncomplicated’ nature, whereas horses with a single whorl below the eyes are said to be ‘unusually imaginative and intelligent’.94,95 Notwithstanding the problems that arise when one attempts to measure intelligence, these reports merit thorough investigation because similar reports in cattle have been ratified by repeated studies.96–98
Temperament
Temperament is a nebulous construct and therefore is difficult to define, so a range of approaches to definitions exist. In humans, temperament has been defined as a person’s behavioral tendencies that appear early in life and continue throughout life.99 The main premise of temperament is that it manifests at an early age, is elicited in specific situations, and remains relatively stable over time.100 The word ‘personality’ is used interchangeably with temperament101 and is generally accepted as describing stable and enduring behavioral tendencies.102 A number of temperament tests for horses have now been reported,103–106 and it is possible that in combination they may form a suite of tests that may be useful in identifying the best horses for certain sorts of work.107
‘Horsonality’ has emerged in a bid to capture the individual differences that characterize equine temperaments.108 This is an interesting advance but one that may suffer considerably from lay interpretations. ‘Horsonality’ includes features such as laterality, compliance and trainability. Laterality is of growing interest to equitation scientists as the impact of left- and right-hemispheric dominance and consequent motor preferences become more clearly understood.
It is important to note that emotionality (or nervousness) has an influence on behavior. McCann et al109 scored emotionality in 32 Quarterhorse yearlings based upon their response to a series of procedures, including standing in a chute, being identified and being released from the chute. The nervous yearlings tended to have a higher overall activity index than the normal yearlings. This correlated with heart rates. Escape tendencies, reactivity to people, behavior after release and overall emotionality contributed to the horses’ being classified as highly nervous, nervous, normal or quiet. Emotionality of the horses affected their frequency of eating and drinking, defecation, locomotion and contact with the other members of the group.
It seems that the learning ability of an individual horse is profoundly influenced by emotionality. Fortunately it is becoming clear that measurement of emotionality as an outcome of avoidance learning tests is possible and that early detection of this quality in yearlings gives a reliable prediction for life and is more consistent than responses in reward-learning tests.110 It may be that time spent grading young horses in this way could reap economic benefits for the industry in the long term.110 Tailoring training schedules and ultimately jobs to meet the individual emotional profiles of horses rather than assuming that all of them can do a single form of work may save time and money. That said, care must be taken to avoid oversimplifying equine temperament tests, because they do not show significant interrelationships.111 It is suggested that several different qualities of a horse’s personality must be assessed before predictions of its working performance can be made with any confidence. So, tests used to predict a horse’s performance as, say, a show-jumper must include its response to novel objects, handling, avoidance learning, reward learning and technique in free jumping.111 The limitations of these assessments reflect the additional role of physical development, long-term training and the skill of the horse’s future riders.111
Stallions and colts are generally less timid than mares and fillies.112 This, along with the absence of reproductive cycles, which have the potential to interrupt training schedules113 and produce variability in competitive performance, accounts for male horses being favored for show-jumping and eventing. Meanwhile, the dynamic presence of stallions predisposes them for work in elite dressage. Behavioral tests comparing saddle mares with draft mares showed the former to be more fearful, and indicated that pregnant mares demonstrated fewer fear responses than non-pregnant mares.114
There are anecdotal reports that socially high-ranking horses can be difficult to train because they are more likely than passive, subordinate animals to deploy evasions.79 However, social rank appears unrelated to learning ability in visual discrimination,6 simple maze,61,80 and avoidance learning tests,61 suggesting that there is a need for more work in this domain. Indeed the whole concept of dominance and the translation of equine social order to horse–human interactions has been questioned.2 The individual differences in emotionality and trainability demand the collection of data from a battery of temperament tests.23,112,113,115–117 Given the wastage that results from behavior problems and the advent of databases for logging the careers of sport-horses, it may be that the time is right for the FEI to develop an international model of breeding stock selection and agree upon a suite of tests that help select breeding stock on the basis of their being tolerant and trainable.
Other differences among breeds include a predisposition to stereotypic behaviors. Horses may inherit both sensitivity to stressors and an ability to express a stereotypy.118 Certain breeds are more prone to stereotypies than others. Usually these are also the breeds that are managed most intensively. Standardbreds are a possible exception here in that they are regularly stabled but show fewer stereotypies than similarly managed Thoroughbreds.119 Thoroughbreds appear particularly predisposed to cribbing and weaving, while Arabians tend to stall walk more.120 These trends may be of significance to horse-trainers since some retardation in learning (specifically persistence in tasks that are no longer rewarding) has been shown to accompany stereotypies.121,122
Summary of Key Points
• Learning facilitates adaptation to novel environments.
• Horses learn in the same way as other species.
• Horses are remarkably adaptable and tolerant.
• A good lesson provides a novel environment with stimuli to which the horse must learn appropriate responses.
• Habituation is the pivotal technique for overcoming flight responses.
• Most horse-training involves operant conditioning.
• Shaping relies on reserving reinforcement until an improved response appears.
• Horses are generally trained with negative rather than positive stimuli, but innovations that accentuate the positive are being successfully developed.
• Clicker training offers a simple method for shaping appropriate responses and therefore provides a basic framework for retraining many unwelcome responses, especially in the unridden horse.
• Consistency and timing are the hallmarks of good training.
Case study
Star is a 6-year-old Thoroughbred being trained to work in the mounted police force. Mounted police perform a wide variety of duties, ranging from park and street patrols to parades, escorts, demonstrations and crowd control at special functions. Whenever on duty, police horses work in pairs and are required to remain calm and steady regardless of highly unusual circumstances. This requires the horse to be relaxed and unflappable, and to remain stationary unless instructed otherwise.
It is central to this training that the horse learns to ignore stimuli from crowds and respond only to those from its rider. It must stand firm under physical pressure from a noisy, pushy crowd and push a pedestrian when so directed by its rider. In other words, the strongest of equine instincts, the flight response, must be vanquished. When given signals, the horse must learn to follow them precisely, irrespective of jostling people, noises, lights and other distractions. Owners who have encountered unwelcome flight responses, including shying (see Ch. 15), could learn a great deal from this case study.
Horses newly acquired by the police force spend 6 weeks being evaluated by a general trainer. Selection is based on temperament and responsiveness. After 6 weeks of assessment, each horse is paired with a single rider for both training and operational work over a number of years, so that a bond develops between mount and rider that, over time, increases the horse’s capacity to cope with new situations. The benefits of this appear in later schooling, where the horse is required to perform unfamiliar tasks, facilitated through rider reassurance.
Initially, the horses are given basic education, including responses to leg, seat and rein signals. On command, they will then move sideways, forwards or backwards, and it is essential that they will stand for extended periods. They are handled daily during grooming and saddling so that they become accepting of contact on all areas, especially sensitive regions such as groin or head. The basic training program generally continues for 6 weeks before specific behaviors are trained.
There is considerable attrition in the selection and training of police remounts. Of the horses that commence training, 10% go on to perform all desired behaviors adequately while only 5% do extremely well. Flighty or nervous horses are unsuitable for crowd control. Mares and stallions are also avoided, as they are considered rather less predictable than geldings and are regarded as being easily distracted when working with other horses.
To train the horse to ignore pressure cues from bystanders, it is first cued to stand with a pedestrian in front of it touching its neck and chest. Next, the rider makes the horse stand still while the pedestrian applies pressure. Although horses have a tendency to move away from persistent pressure, the rider ensures that the horse responds only to commands from him. The horse is then prompted with leg and rein signals to advance toward the applied pressure, that is, toward the pedestrian.
The next stage is to simulate contact from multiple directions within a crowd by organizing several people to surround the horse (Fig. 4.20). The horse is again cued to respond only to the rider’s signals, and walk forward as directed. Discrimination is required in that the horse must learn to discern between pressure on the flank that comes from the rider and that coming from other humans. The most difficult step is training the horse to continue walking even when pedestrians in front of it do not retreat. This is achieved by continuing to apply leg signals until the horse responds.
Gradually other distractions are added to the horse’s repertoire, including pedestrians holding or waving strange objects, or broadcasting loud noises. When multiple novel stimuli are used together, the greatest risk is that they will frighten the horse. In other words, advancing too far too quickly is a real danger. If the horse behaves fearfully, the training reverts to the last step with which the horse was under stimulus control, before the challenging step is attempted again. Verbal and physical reassurance from the rider is considered particularly important during this stage.
Training sessions are given every day, with the length of an average training session being 45 minutes. As horses are worked every day, it becomes an accepted routine, and no specific attempt is made to change motivation prior to a session.
Reinforcement of crowd-control responses is not part of a police horse’s routine training. Basic training is reinforced whenever horses are ridden, but specific maneuvers, such as quadrilles, are revised only in an intensive lead-up to a particular event, such as a public display. Training is then tailored specifically to that event, using appropriate lighting, sound effects and props.
References
1. Goodwin D, McGreevy P, Waran N, McLean A. How equitation science can elucidate and refine horsemanship techniques. Vet J. 2009;181(1):5–11.
2. McGreevy PD, Oddie C, Burton FL, McLean AN. The horse–human dyad: Can we align horse training and handling activities with the equid social ethogram? Vet J. 2009;181(1):12–18.
3. Marinier SL, Alexander AJ. The use of a maze in testing learning and memory in horses. Appl Anim Behav Sci. 1994;39:177–182.
4. Fraser AC. Restraint in the horse. Vet Rec. 1967;80(2):56–64.
5. Sappington BKF, McCall CA, Coleman DA, et al. A preliminary study of the relationship between discrimination reversal learning and performance tasks in yearling and 2-year-old horses. Appl Anim Behav Sci. 1997;53(3):157–166.
6. Mader DR, Price EO. Discrimination learning in horses: effects of breed, age and social dominance. J Anim Sci. 1982;50:962–965.
7. Wolff A, Hausberger M. Learning and memorisation of two different tasks: the effects of age, sex and sire. Appl Anim Behav Sci. 1995;46(3/4):137–143.
8. Spalding D. Instinct: with observations on young animals. Macmillan’s magazine. 1873;27:283–293. (Reprinted in Br J Anim Behav 1954; 2:2–11.)
9. Lorenz KZ. The companion in the bird’s world. Auk. 1937;54:245–273.
10. Miller RM. Imprint training the newborn foal. Colorado Springs: Western Horseman, 1998.
11. Miller RM. Fallacious studies of foal imprint training. J Equine Vet Sci. 2001;21(3):102–103.
12. Henry S, Richard-Yris M-A, Tordjman S, Hausberger M. Neonatal Handling Affects Durably Bonding and Social Development. PLoS ONE. 2009;4(4):e5216.
13. Simpson BS. Neonatal foal handling. Appl Anim Behav Sci. 2002;78(2–4):309–323.
14. Macleay JM. Smart horse: training your horse with the science of natural horsemanship. Fort Collins, CO: J and J Press, 2000. 101–105
15. Mal ME, McCall CA. The influence of handling during different ages on a halter training test in foals. Appl Anim Behav Sci. 1996;50(2):115–120.
16. Heird JC, Whitaker DD, Bell RW, et al. The effects of handling at different ages on the subsequent learning ability of 2-year-old horses. Appl Anim Behav Sci. 1986;15:15–25.
17. Diehl NK, Egan B, Tozer P. Intensive, early handling of neonatal foals: mare–foal interactions. Havemeyer Workshop on Horse Behavior and Welfare, Holar, Iceland, 2002:40–46.
18. Hausberger M, Roche H, Henry S, Visser EK. A review of the human–horse relationship. Appl Anim Behav Sci. 2007;109:1–24.
19. Williams JL, Friend TH, Toscano MJ, et al. The effects of early training sessions on the reactions of foals at 1, 2, and 3 months of age. Appl Anim Behav Sci. 2002;77(2):105–114.
20. Williams JL, Friend TH, Collins MN, et al. Effects of imprint training procedure at birth on the reactions of foals at age six months. Equine Vet J. 2003;35:127–132.
21. Spier SJ, Pusterla JB, Villarroel A, Pusterla N. Outcome of tactile conditioning of neonates, or ‘imprint training’ on selected handling measures in foals. Vet J. 2004;168:252–258.
22. Sigurjonsdottir H, Gunnarsson V. Controlled study of early handling and training of Icelandic foals. Dorothy Russell Havemeyer Workshop Horse Behavior and Welfare. McDonnell S, Mills D (eds.). Holar, Iceland, 13–16 June; 2002.
23. Jezierski T, Jaworski Z, Gorecka A. Effects of handling on behaviour and heart rate in Konik horses: comparison of stable and forest reared youngstock. Appl Anim Behav Sci. 1999;62:1–11.
24. Lansade L, Bertrand M, Bouissou M-F. Effects of neonatal handling on subsequent manageability, reactivity and learning ability of foals. Appl Anim Behav Sci. 2005;92:143–158.
25. Mal ME, McCall CA, Cummins KA, Newland MC. Influence of pre-weaning handling methods on post-weaning learning ability and manageability of foals. Appl Anim Behav Sci. 1994;40(3/4):187–195.
26. Lansade L, Bertrand M, Boivin X, Bouissou M-F. Effects of handling at weaning on manageability and reactivity of foals. Appl Anim Behav Sci. 2004;87:131–149.
27. Søndergaard E, Halekoh U. Young horses’ reactions to humans in relation to handling and social environment. Appl Anim Behav Sci. 2003;84:265–280.
28. Henry S, Hemery D, Richard M-A, Hausberger M. Human–mare relationships and behaviour of foals toward humans. Appl Anim Behav Sci. 2005;93:341–362.
29. Bourjade M, de Boyer des Roches A, Hausberger M. Adult-Young Ratio, a Major Factor Regulating Social Behaviour of Young: A Horse Study. PLoS ONE. 2009;4(3):e4888.
30. Heitor F, Vicente L. Maternal care and foal social relationships in a herd of Sorraia horses: Influence of maternal rank and experience. Appl Anim Behav Sci. 2008;113:189–205.
31. Goodwin D, Hughes CF. Horse play. Havemeyer Workshop on Horse Behavior and Welfare, Holar, Iceland 2002:102–105.
32. Williams M. Effect of artificial rearing on social behavior of foals. Equine Vet J. 1974;6:17–18.
33. Kelly AJ. Practical tips on fostering. In: Harris PA, Gomarsall GM, Davidson HPB, Green RE, eds. Proceedings of the BEVA specialist days on behaviour and nutrition. Newmarket, Suffolk, UK 1999:39–41.
34. Visser EK, van Reenen CG, van der Werf JTN, et al. Heart rate and heart rate variability during a novel object test and a handling test in young horses. Physiol Behav. 2002;76(2):289–296.
35. Nicol CJ. Equine learning: progress and suggestions for future research. Appl Anim Behav Sci. 2002;78(2–4):193–208.
36. Gough MR. A note on the use of behavioural modification to aid clipping ponies. Appl Anim Behav Sci. 1999;63(2):171–175.
37. Casey RA. Recognising the importance of pain in the diagnosis of equine behavioural problems. In: Harris PA, Gomarsall GM, Davidson HPB, Green RE, eds. Proceedings of the BEVA specialist days on behaviour and nutrition. Newmarket, Suffolk, UK 1999:25–28.
38. McGreevy PD. Why does my horse…? London: Souvenir Press, 1996.
39. McGreevy PD, Boakes RA. Carrots and sticks: the principles of animal training. Cambridge: Cambridge University Press, 2007.
40. Miller RM. Psychological effects of succinylcholine chloride immobilization on the horse. Vet Med/Small Anim Clin. 1966;61:941–944.
41. Lieberman DA. Learning: behavior and cognition. Pacific Grove, CA: Brooks/Cole, 1993.
42. LeDoux JE. Emotion, memory and the brain. Sci Am. 1994;270(6):32–39.
43. McGreevy PD, McLean AN. Equitation science. Oxford: Wiley-Blackwell, 2010.
44. Thorndike EL. Animal Intelligence. New York: Macmillan, 1911.
45. McGreevy PD. The advent of equitation science. Vet J. 2007;174:492–500.
46. Skinner BF. The behavior of organisms. New York: Appleton-Century-Crofts, 1938.
47. Kratzer DD, Netherland WM, Pulse RE, Baker JP. Maze learning in Quarterhorses. J Anim Sci. 1977;45:896–902.
48. Houpt KA, Houpt TR. Social and illumination preferences of mares. Equine Pract. 1992;14(6):11–16.
49. Shults T, Combie J, Dougherty J, Tobin T. Variable-interval responding in the horse: a sensitive method of quantitating effects of centrally acting drugs. Am J Vet Res. 1982;43(7):1143–1146.
50. McDonnell SM. Pharmacological aids to behaviour modification in horses. Equine Vet J Suppl. 1998;27:50–51. (abstr)
51. Hall CA, Goodwin D, Heleski C, et al. Is there evidence of ‘Learned Helplessness’ in horses? Proceedings of the 3rd International Equitation Science Symposium, Michigan. Goodwin D, Heleski C, McGreevy PD, et al (eds.). Michigan, USA: MSU; 2007.
52. Derksen FJ, Clayton HM. Is equitation science important to veterinarians? Vet J. 2007;174:452–453.
53. Heird JC, Lennon AM, Bell RW. Effects of early experience on the learning ability of horses. J Anim Sci. 1981;53:1204–1209.
54. Warren-Smith AK, McGreevy PD. Equestrian coaches’ understanding and application of learning theory in horse training. Anthrozoos. 2008;21(2):153–162.
55. McGreevy PD, McLean AN. Punishment in horse-training and the concept of ethical equitation. J Vet Behav. 2009;4(5):193–197.
56. Miyashita Y, Nakajima S, Imada H. Differential outcome effect in the horse. J Exp Anal Behav. 2000;74(2):245–254.
57. Warren-Smith AK, McGreevy PD. The use of blended positive and negative reinforcement in shaping the halt response of horses (Equus caballus). Anim Welf. 2007;16:481–488.
58. McGreevy PD, McLean AN. The roles of learning theory and ethology in equitation. J Vet Behav. 2007;2:108–118.
59. Myers RD, Mesker DC. Operant responding in a horse under several schedules of reinforcement. J Exp Anal Behav. 1960;3:161–164.
60. McCall CA, Burgin SE. Equine utilization of secondary reinforcement during response extinction and acquisition. Appl Anim Behav Sci. 2002;78(2–4):253–262.
61. Haag EL, Rudman R, Houpt KA. Avoidance, maze learning and social dominance in ponies. J Anim Sci. 1980;50(2):329–335.
62. McCall CA. The effect of body condition of horses on discrimination learning abilities. Appl Anim Behav Sci. 1989;22(3–4):327–334.
63. Sappington BKF, Goldman L. Discrimination learning and concept formation in the Arabian horse. J Anim Sci. 1994;72(12):3080–3087.
64. Flannery B. Relational discrimination learning in horses. Appl Anim Behav Sci. 1997;54(4):267–280.
65. McQuoid LM, Galef BG, Jr. Social influences on feeding site selection by Burmese fowl (Gallus gallus). J Comp Psychol. 1992;106:136–141.
66. Linklater WL. Equine learning in a wider context – opportunities for integrative pluralism. Behav Processes. 2007;76:53–56.
67. Thomas RK. Vertebrate intelligence: a review of the laboratory research. In: Hoage RJ, Goldman L. Animal Intelligence: Insights into the Animal Mind. Goldman. Washington DC: Smithsonian Institution Press; 1986:37–56.
68. Murphy J, Arkins S. Equine learning behaviour. Behav Processes. 2007;76:1–13.
69. Hanggi EB. Categorization learning in horses (Equus caballus). J Comp Psychol. 1999;113:243–252.
70. Nicol CJ. Farm animal cognition. J Anim Sci. 1996;62:375–391.
71. Fraser AF. The behaviour of the horse. London: CAB International, 1992.
72. McCann JS, Heird JC, Bell RW, Lutherer LO. Normal and more highly reactive horses, II: The effect of handling and reserpine on the cardiac response to stimuli. Appl Anim Behav Sci. 1988;19:215–226.
73. Dixon JC. Pattern discrimination, learning set, and memory in a pony. Paper presented at the Midwestern Psychological Association Convention, Chicago, 1966.
74. Voith VL. Pattern discrimination, learning set formation, memory retention, spatial and visual reversal learning by the horse. Thesis, Ohio State University, Columbus, 1975.
75. Waring GH. Horse behavior: the behavioral traits and adaptations of domestic and wild horses including ponies. Park Ridge, NJ: Noyes, 1983.
76. Rubin L, Oppegard C, Hintz HF. The effect of varying the temporal distribution of conditioning trials on equine learning behaviour. J Anim Sci. 1980;50:1184–1187.
77. Kusunose R, Yamanobe A. The effect of training schedule on learned tasks in yearling horses. Appl Anim Behav Sci. 2002;78(2–4):225–233.
78. Pfungst O. Clever Hans: the horse of Mr von Osten. New York: Holt, Rinehard & Winston, 1965.
79. Budiansky S. The nature of horses: exploring equine evolution, intelligence and behavior. New York: Free Press, 1997.
80. Houpt KA, Parsons MS, Hintz HF. The learning ability of orphan foals, of normal foals and of their mothers. J Anim Sci. 1982;55:1027–1032.
81. Houpt KA, Hintz HF. Some effects of maternal deprivation on maintenance behaviour, spatial relationships and responses to environmental novelty in foals. Appl Anim Ethol. 1983;9(3/4):221–230.
82. Clarke J, Nicol CJ, Jones R, McGreevy PD. Effects of observational learning on food selection in horses. Appl Anim Behav Sci. 1996;50:177–184.
83. Krueger K, Heinze J. Horse sense: social status of horses (Equus caballus) affects their likelihood of copying other horses’ behaviour. Anim Cogn. 2008;11:431–439.
84. Hothersall B, Nicol C. Equine learning behaviour: accounting for ecological constraints and relationships with humans in experimental design. Behav Processes. 2007;76:45–48.
85. Krueger K, Flauger B. Social learning in horses from a novel perspective. Behav Processes. 2007;76:37–39.
86. Hagerbaumer J. Exploring the equine mind with learning and memory studies. In The thinking horse. Guelph: Equine Research Centre; 1995.
87. Hausberger M, le Scolan N, Bruderer C, Pierre JS. [Temperament in the horse: factors in play and practical implications.] (French) 24 journée de la Recherche Equine, 1998, Institut du Cheval, Paris, France, 1998; 159–169.
88. Bagshaw CS, Ralston SL, Fisher H. Behavioural and physiological effects of orally administered tryptophan on horses subjected to acute isolation stress. Appl Anim Behav Sci. 1994;40:1–12.
89. Houpt KA, Kusunose R. Genetics of behaviour. In: Bowling AT, Ruvinsky A. The genetics of the horse. Wallingford, Oxon: CAB International, 2000.
90. Hausberger A, Muller C. A brief note on some possible factors involved in the reactions of horses to humans. Appl Anim Behav Sci. 2002;76(4):339–344.
91. Mader DR, Price EO. Discrimination learning in horses: effect of breed, age and social dominance. J Anim Sci. 1980;50:962–965.
92. Lloyd AS, Martin JE, Bornett-Gauci HLI, Wilkinson RG. Evaluation of a novel method of horse personality assessment: rater agreement and links to behaviour. Appl Anim Behav Sci. 2007;105:205–222.
93. Lloyd AS, Martin JE, Bornett-Gauci HLI, Wilkinson RG. Horse personality: Variation between breeds. Appl Anim Behav Sci. 2008;112:369–383.
94. Tellington-Jones L, Bruns V. The Tellington-Jones equine awareness method. Buckingham: Kenilworth Press, 1985.
95. Tellington-Jones L. Getting in touch with horses. Buckingham: Kenilworth Press, 1995.
96. Grandin T, Deesing MJ, Struthers JJ, Swinker AM. Cattle with hair whorl patterns above the eyes are more behaviorally agitated during restraint. Appl Anim Behav Sci. 1995;46:117–123.
97. Randle HD. Facial hair whorl position and temperament in cattle. Appl Anim Behav Sci. 1998;56:139–147.
98. Lanier JL, Grandin T, Green R, et al. A note on hair whorl position and cattle temperament in the auction ring. Appl Anim Behav Sci. 2001;73:93–101.
99. Jones AC, Gosling SD. Temperament and personality in dogs (Canis familiaris): A review and evaluation of past research. Appl Anim Behav Sci. 2005;95:1–53.
100. Diederich C, Giffroy J-M. Behavioural testing in dogs: A review of methodology in search for standardisation. Appl Anim Behav Sci. 2006;97(1):51–72.
101. Svartberg K, Forkman B. Personality traits in the domestic dog (Canis familiaris). Appl Anim Behav Sci. 2002;79(2):133–155.
102. Ley J, Bennett P. Measuring personality in dogs. J Vet Behav. 2008;3(4):182.
103. Mackenzie SA, Thiboutot E. Stimulus reactivity tests for the domestic horse (Equus caballus). Equine Pract. 1997;19(7):21–22.
104. Anderson MK, Friend TH, Evans JW, Bushong DM. Behavioral assessment of horses in therapeutic riding programs. Appl Anim Behav Sci. 1999;63(1):11–24.
105. Le Scolan N, Hausberger M, Wolff A. Stability over situations in temperamental traits of horses as revealed by experimental and scoring approaches. Behav Process. 1997;41:257–266.
106. Seaman SC, Davidson HPB, Waran NK. How reliable is temperament assessment in the domestic horse (Equus caballus)? Appl Anim Behav Sci. 2002;78(2–4):175–191.
107. Houpt KA, Rudman R. Foreword to special issue on equine behaviour. Appl Anim Behav Sci. 2002;78:83–85.
108. Visser EK. Horsonality: a study on the personality of the horse, Unpublished PhD thesis, Wageningen University and Research Center Publications (Netherlands); 2002.
109. McCann JS, Heird JC, Bell RW, Lutherer LO. Normal and more highly reactive horses, I: Heart rate, respiration rate and behavioural observations. Appl Anim Behav Sci. 1988;19:201–214.
110. Visser EK, van Reenen CG, Schilder MBH, et al. Learning performances in young horses using two different learning tests. Appl Anim Behav Sci. 2003;80(4):311–326.
111. Visser EK, van Reenen CG, Engel B, et al. The association between performance in show-jumping and personality traits early in life. Appl Anim Behav Sci. 2003;82(4):279–295.
112. Budzynski M, Slomka Z, Soltys L, et al. [Characteristics of nervous activity in Arab horses.] (Polish). Annales Universitatis Mariae Curie-Sklodowska, EE Zootechnica. 1997;15:165–175.
113. Fiske JC, Potter GD. Discrimination reversal learning in yearling horses. J Anim Sci. 1979;49:583–588.
114. Vierin M, Bouissou MF, Vandeheede M, et al. Development of a method for measuring fear reactions in the horse. 24 Journée de la Récherche Equine, 1998, Institut du Cheval, Paris, France, 1998:171–183.
115. Budzynski M. [Repeatability and heritability of the assessment of nervous type in horses.] (Polish). Annales Universitatis Mariae Curie-Sklodowska, EE Zootechnica. 1983;1:229–232.
116. Budzynski M. [Timidity test to assess the degree of nervousness of horses.] (Polish). Medycyna Weterinaryjna. 1984;40(3):156–158.
117. Budzynski M, Soltys L, Slomka Z. [The nervous excitability of Hutsul horses.] (Polish). Zetzyty Naukowe Akademii Rolniczej im H Kollataja w Krakowie, Sesja, Naukowe. 1991;29:41–45.
118. Luescher UA, McKeown DB, Halip J. Reviewing the causes of obsessive-compulsive disorder in horses. Vet Med. 1991;86:527–530.
119. Luescher UA, McKeown DB, Dean H. A cross-sectional study on compulsive behaviour (stable vices) in horses. Equine Vet J Suppl. 1998;27:14–18.
120. McGreevy PD, French NP, Nicol CJ. The prevalence of abnormal behaviours in dressage, eventing and endurance horses in relation to stabling. Vet Rec. 1995;137:36–37.
121. Garner JP, Mason GJ. Evidence for a relationship between cage stereotypies and behavioural disinhibition in laboratory rodents. Behav Brain Res. 2002;136(1):83–92.
122. Parker M, Redhead ES, Goodwin D, McBride SD. Impaired instrumental choice in crib-biting horses (Equus caballus). Behav Brain Res. 2008;191:137–140.
123. Houpt KA. Imprinting training and conditioned taste aversion. Behav Processes. 2007;76:14–16.