Chapter 3 Behavior and the brain
Chapter contents
Introduction
From the brain, and from the brain only, arise our pleasures, joys, laughter and jests, as well as our sorrows, pains, griefs and tears ….
Only relatively recently has it been accepted that the ‘mental state’ of humans and animals, the emotional, instinctive and cognitive foundations of behavior, are inseparable from their somatic aspects. This artificial schism between psychology and neurology began more than four centuries ago when the philosopher René Descartes was trying to study the human body. The Catholic church expressed its dissatisfaction with this study of God’s handiwork, and in response Descartes struck a deal with the church – human existence was divided into two realms: the physical body, which he would study, and the mental/spiritual realm, which would remain the exclusive domain of the church. This artificial construct dividing the mental and physical has guided much of scientific and medical thought ever since, to the detriment of human and animal patients.1 Increasingly powerful molecular tools are being used by neuroscientists to elucidate the relationship between anatomy, physiology and specific functions in perception, thought and movement. Classic psychiatric diseases are not (yet) clearly defined in horses, but an appreciation of basic neuroanatomy and neurophysiology is fundamental to understanding the influence of neuropathology and psychopharmacology on equine behavior (Fig. 3.1).
Fundamentals of functional and behavioral neuroanatomy
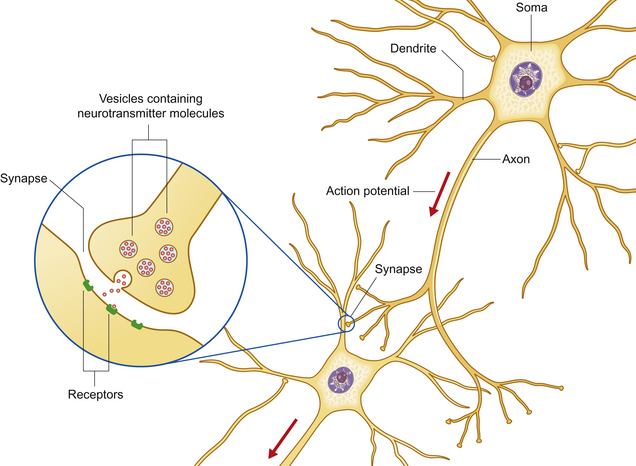
The nervous system processes external stimuli into neuronal impulses resulting in neurotransmitter release, and integrates these with motivations and emotional stimuli to direct the actions of motor units. These enable the animal to react to its environment and influence the behavior of others. The function of the nervous system fundamentally depends on a group of specialized cells called neurons – polarized, elongated cells that are uniquely capable of extremely rapid, intercellular communication. Neurons have a receptor region (the dendritic zone), a cell body (soma) containing the nucleus, and an axon conducting impulses from the dendritic zone to synapse with other neurons (Fig. 3.2). The cell bodies collectively make up the ‘gray’ matter of the central nervous system (CNS), whereas the axons are found in ‘white’ matter. The shape, size and position of the soma as well as the length and branching of the proximal and distal processes differ greatly between neuronal populations. Brainstem axons may have a length of only a few micrometers, whereas the recurrent laryngeal nerve (the nerve that supplies the larynx) is over 3 meters long! Axons often project in groups or bundles that collectively form tracts in the CNS and nerves in the peripheral nervous system. Neurons make up only a small proportion of the total number of cells in the nervous system; the majority are neuroglial cells [Gr. glia, glue] such as astrocytes, cells with important supportive and metabolic functions.
Major components of the central nervous system
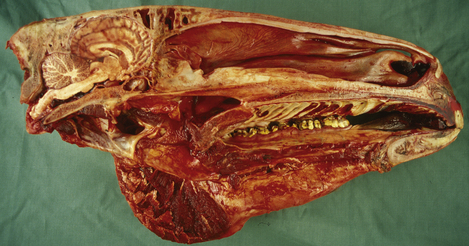
This section serves to introduce the fundamentals of neuroanatomy, a subject that has been comprehensively addressed by de Lahunta2 and reviewed by Kaplan & Sadock3 and Behan.4 The CNS consists of the brain and spinal cord (Fig. 3.3). The brain includes the two cerebral hemispheres, which are roughly mirror images of one another, and the brainstem, a narrow structure through which all the pathways entering and leaving the two hemispheres must pass and which comprises the centers that control breathing, heart rate, eye movement and many other critical functions. Caudal to the cerebral hemispheres is the cerebellum, a structure that helps to control movement and balance. The caudal part of the brainstem flows into the spinal cord, the point of exit for nerves on their way out to innervate muscles and the point of entry for sensory fibers returning from the body’s sensory organs. All the nerves outside the central nervous system are collectively called the peripheral nervous system. The two cerebral hemispheres are built around a connecting system of hollow spaces called the ventricular system. The ventricles are filled with cerebrospinal fluid (CSF). This clear fluid also bathes the surface of the CNS, providing mechanical support to the CNS, and playing an important role in maintaining a constant chemical environment. CSF is produced by modified blood vessels known as the choroid plexus located inside the ventricles.
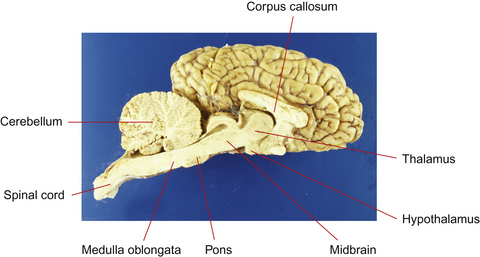
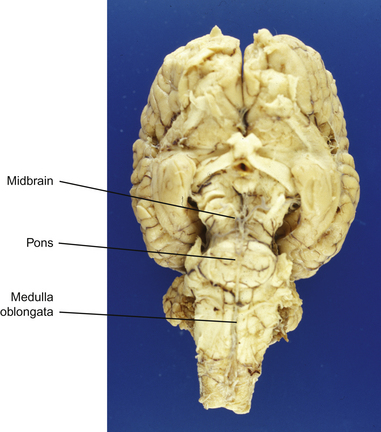
Figure 3.3 Sagittal section of equine brain outlining the major components of the brainstem.
(Photograph courtesy of Keith Ellis.)
There are four major divisions of the CNS:
• forebrain, composed of the cerebral hemispheres, basal nuclei, hypothalamus and thalamus
• brainstem, composed of the midbrain, pons and medulla oblongata
Neurons of similar function are grouped together in ‘laminae’ in the cortex, ‘nuclei’ in the brainstem and ‘columns’ in the spinal cord. Axons from sensory and motor nerves enter and leave the CNS as spinal nerves from the spinal cord and cranial nerves from the brain.
Forebrain
Cerebrum
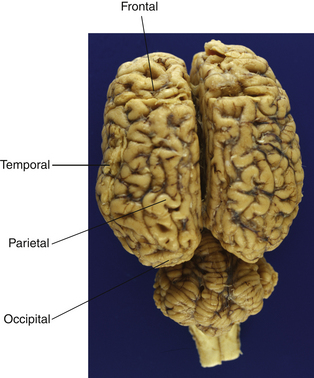
The cerebrum is the most rostral (forward) division of the brain and is divided into two cerebral hemispheres (Fig. 3.4). Cell bodies of the neurons in the cerebrum are located in two general areas: on the outside surface of the cortex (folded into gyri and sulci) and deep to the surface in the basal nuclei (Figs 3.5 and 3.6). Evidence from human studies suggests that the cerebrum is the region of the brain responsible for perception, emotion, voluntary movement and most learning. These functions will be considered in more detail later.
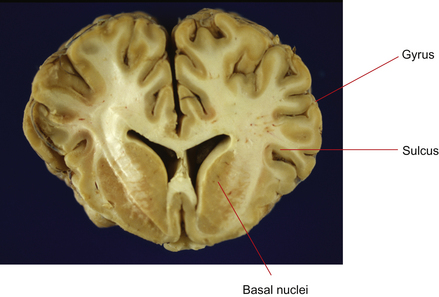
Figure 3.5 Transverse section of cerebrum at the level of the basal nuclei. Neurons in the forebrain are aggregated on the surface of the cortex and in the basal nuclei.
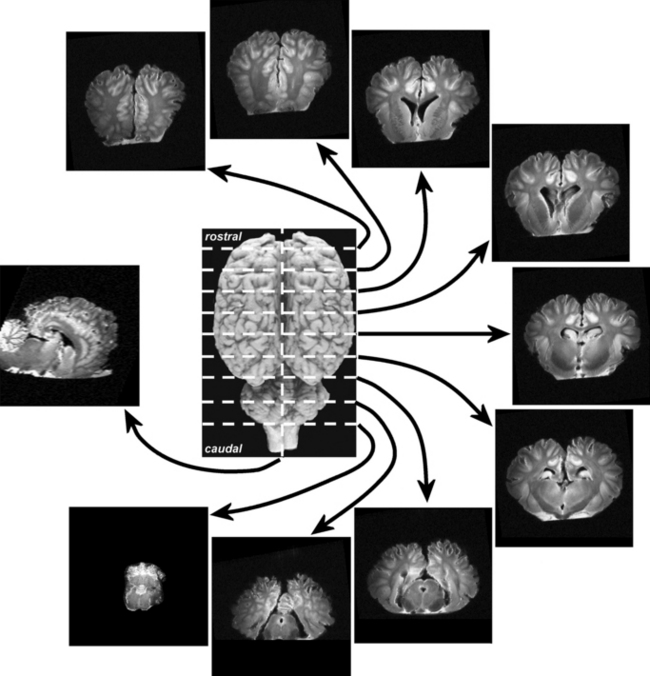
Figure 3.6 One sagittal and nine transverse sections of a T2-weighted proton density magnetic resonance imaging (MRI) scan of an equine brain.
(With permission of Luke Henderson, Kevin Keay and Paul McGreevy.)
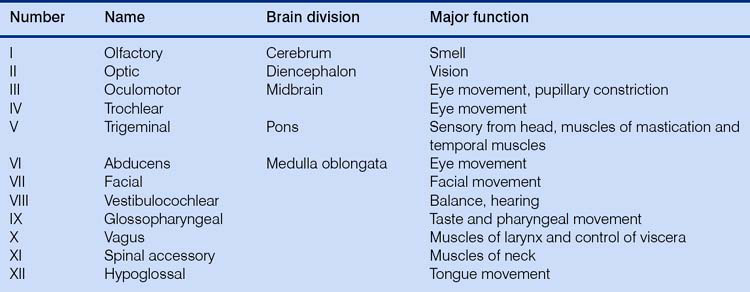
The tremendous number of neurons in the cerebrum have axons arranged in large fiber bundles. Notably the internal capsule serves as the major projection pathway to connect the cerebrum with the brainstem, and the corpus callosum consists of axons connecting the left and right cerebral hemispheres across the midline. Only one cranial nerve (Table 3.1) – the olfactory nerve, which transmits sensations of smell – is associated with the forebrain (this really is not a ‘nerve’ at all; in the horse it is a relatively large structure, and it probably received its name on account of its diminutive nerve-like appearance in the human brain).
Thalamus and hypothalamus (diencephalon)
The most prominent components of the diencephalon are the thalamus and the hypothalamus. The thalamus functions as an integrating system to relay pathways to and from the brainstem and higher centers in the cerebrum. With the exception of impulses from the olfactory nerve, all impulses heading to the cerebrum must synapse in the thalamus; reciprocal fibers there play a critical role in the filtering of sensory input, and in abnormal states they may generate false signals or inappropriately suppress sensation. The thalamus may also serve directly as the site of conscious perception of some sensations5 and plays a pivotal role in maintaining consciousness, focusing attention, and initiating sleep.6
The hypothalamus is the higher center for regulation of autonomic motor activity. It functions without direct voluntary control, but is influenced by the cerebral cortex. There is evidence that stimuli associated with emotionally relevant events may have a significant effect on hypothalamic hormonal regulation. These hormonal consequences seem to influence further behavior and responses in relevant situations, especially aggression and sexual behavior.7 Seasonal polyestrus behavior of mares is initiated by stimulation of the pineal gland by light, either natural or artificial, causing a reduction of melatonin secretion. This in turn allows gonadotropin-releasing hormone (GnRH) to be secreted by the hypothalamus, ultimately resulting in the secretion of estrogen and estrus behavior (the exact connection between decreased melatonin production and increased GnRH concentrations is not yet understood).
Brainstem
Despite its small size, the brainstem is arguably the most important part of the nervous system. It is the link between the cerebral cortex and the spinal cord and it contains the nuclei of most of the cranial nerves and the regulatory centers for cardiac and pulmonary function.
Midbrain
The midbrain is the most rostral portion of the brainstem and contains the pathways connecting the brainstem and cerebrum. It contains the red nucleus, an important nucleus containing ‘upper motor neurons’ that initiate the gait of horses (these are neurons in the brain that innervate and control the lower motor neurons in the brainstem or spinal cord). Two of the three nuclei controlling eye movement – cranial nerves (CNs; see Table 3.1) III (oculomotor) and IV (trochlear) – are found in the midbrain. The dorsal components of the midbrain (rostral and caudal colliculi) are linked with reflex functions such as blinking and, in concert with other structures, reacting (shying) to visual or auditory stimuli.
Pons
The pons lies caudal to the midbrain. The pons is the portion of the brainstem that contains the motor neurons of the trigeminal nerve (CN V), responsible for the muscles of mastication (CN V is also the afferent system for facial sensation, but those neurons are distributed throughout the brainstem).
Medulla oblongata
All of the ascending and descending pathways (directional terms that strictly speaking are only correct for upright primates) between the spinal cord and the brain pass through the medulla oblongata, and the nuclei of the last seven cranial nerves (CNs VI–XII) as well as the sensory nuclei involved in proprioception are located there (Fig. 3.7).
Cerebellum
The cerebellum lies dorsal to the pons. It modulates the tone and relative degrees of contraction of opposing muscles needed for smooth motion, by integrating complex inputs from the brainstem, cerebrum and spinal cord. The reason for the ‘jerky’ movement of foals is because the cerebellum is still developing (but is far, far more developed than the neonatal human brain!). Functional imaging studies in humans have shown the cerebellum to be active during mere imagination of motor acts.8 Foals suffering from a degeneration of the cerebellum, ‘cerebellar abiotrophy’, are strikingly uncoordinated, with strong, jerky movements and prominent tremors, particularly of the head, just before initiating a movement (intention tremors).
Spinal cord
The spinal cord is the most caudal part of the central nervous system. It receives and processes sensory information from the viscera, skin, joints, limbs and trunk, and controls movement of the body, parasympathetic outflow to urogenital structures and sympathetic outflow to the entire body. Neural networks in the spinal cord, referred to as ‘central pattern generators’, produce the rhythmic movements of specific gaits such as trotting, pacing or galloping, even when isolated from the brain and sensory inputs. Supraspinal sensory, and neuromodulatory influences, modulated by training, interact with the central pattern generators to shape the final motor output.9 The gray matter containing the neurons has a roughly H-shaped outline in the center of the spinal cord. Motor neurons are found in the ventral horn, while sensory neurons are present in the dorsal horn. Ascending sensory information passes along fiber tracts (funiculi) to sensory nuclei in the brainstem and cerebellum. Upper motor neurons in the brainstem control movement by innervating lower motor neurons in the ventral horn of the gray matter of the spinal cord, resulting in muscle contraction.
Behavioral neuroanatomy
One organizational principle of the nervous system is the use of parallel processing, in which sensory, motor and cognitive functions can be served by more than one pathway.10 Simplistically, the brainstem and thalamic reticular activating system provide arousal and initiate attention; the caudal part of the forebrain integrates perceptions and, at a conscious level, the frontal cortex initiates voluntary movement and executes plans.
The cerebrum
The cerebral cortex has achieved maximal prominence in humans where, were it spread out flat, it would measure about 45 cm2. In order to fit within the confines of the skull, the cortex of phylogenetically advanced animals such as primates and horses is folded into grooves (sulci) alternating with prominences (gyri). The brains of more ‘primitive’ mammals, such as rodents, have a smooth surface. The neuronal layers of the cortex form the gray matter, while the underlying white matter is composed of axons traveling to and from the gray matter.
The cerebral cortex is divided into two hemispheres, which have a contralateral relationship with the body – for example impulses originating in the left hemisphere affect the right half of the body. Within the cortex, human studies have suggested a hemispheric dichotomy of emotional representation, with the left hemisphere housing the analytical mind and the right hemisphere appearing to be dominant in functions involving emotion.11 This may well apply to horses to some extent.
The hemispheres are somewhat arbitrarily divided into four lobes (see Fig. 3.4) named for the overlying bones: frontal, occipital, parietal and temporal. There is a rough correspondence between these loosely defined anatomical regions and their function, a relationship that is most obvious in humans but appears to hold reasonably well for horses. The parietal lobe contains primary somatosensory and motor areas, while the occipital lobe is almost exclusively committed to visual processing. The temporal lobe includes the primary auditory cortex in addition to structures involved in memory, including the hippocampus and the amygdala. The temporal and frontal lobes influence emotions, and epilepsy of the temporal lobes can manifest solely as behavior alterations, such as unprovoked aggression and extreme irrational fear, hyposexuality and tail chasing in small animals.12,13 Temporal lobe epilepsy has not been shown to occur in the horse, but one could reasonably expect that it does.
The frontal lobe, and particularly the prefrontal cortex, plays a very significant role in the coordination of goal-directed behavior and evaluates the effect of such behavior via reinforcement/punishment outcomes. The frontal lobe also allows the conscious initiation of movement (although once a gait is ‘initiated’ it is controlled largely by the brainstem and spinal cord of the horse). In most behaviors, sensory systems project to association areas where sensory information is interpreted in terms of memories and motivations. Training horses would not be possible without this system.
Evolutionary brain divisions
A further, and rather more functional, classification recognizes three divisions of cerebral cortex: the archicortex, paleocortex and neocortex. These are distinguished on a developmental evolutionary basis and by the number of layers of neurons.
The archicortex and paleocortex are phylogenetically old regions and consist of three layers of cells (laminae) as opposed to the six laminae that characterize the neocortex. The archicortex is composed of medial structures included in the limbic system, such as the hippocampus and amygdala, and is involved mostly with emotion or behavior. The paleocortex is represented by one lobe on both sides of the brain, the piriform lobes, concerned with smell. The neocortex comprises the rest of the cerebral cortex and includes somatosensory and motor areas, visual and auditory cortical areas, and the association cortex. The association cortex is devoted to the collection of information, prioritization of its relative importance and decisions about suitable responses. Horses have representatives of each type of cortex.
In humans, the association cortex appears to be responsible for the ability to think, create, conceptualize and problem solve, and in comparative neuroanatomical studies, the massive size of the frontal lobes is the main feature that distinguishes the human brain from that of other primates. It has been proposed that cognitive abilities of particular species are directly correlated with the challenges of securing food, and that grazing ungulates are to some degree removed from the evolution of higher mental abilities in terms of food procurement. This is reflected in the relatively small size of the equine forebrain compared with that of social carnivores and primates. In terms of solving problems, horses are considerably slower to solve novel problems through rule-learning abilities than species such as primates and higher carnivores.
The limbic system
The components of the limbic system are hard to define, as various authors include different structures and areas in their definitions. It is usually agreed that it consists of those structures forming the border (‘limbic’) of the rostral end of the brainstem. This includes subcortical nuclei such as the hypothalamus and amygdaloid complex as well as the hippocampus, [Gr. hippocampos, sea-horse] an ancient, horseshoe shaped, rolled-up gyrus located within the temporal lobes. In amphibians and reptiles the limbic structures are devoted in large measure to processing olfactory input, a role superseded in mammals by important functions in memory, learning and social and emotional behavior.14
Olfaction remains an important sensory system, and olfaction receptor genes are the largest family of genes currently known to exist. Each receptor protein in the nasal mucosa is highly selective and will bind only a select group of odorants. Horses have a large and well-developed olfactory system which plays a role in appetite and food intake, sexual desire, mating, recognition of friends and foes and the accompanying emotional changes associated with these experiences. An associated sensory apparatus is the vomeronasal organ (VNO), an elongated pouch-like structure ventral to the rostral nasal meatus and lined with olfactory receptors. Scent information, particularly from pheromone molecules, is detected in the VNO (assisted by the ‘flehmen’ response; see Ch. 2). Olfactory signals do not synapse directly in the thalamus but project directly to the frontal lobe and the limbic system. The strong association between odors and memory is rooted in this ancient function of the limbic system.
One nucleus in the limbic system, the amygdala, receives fibers from all sensory areas and appears to assign emotional significance to memories as well as mediating the expression of emotions associated with self-preservation, such as fear.14 Surgical removal of the amygdala results in a loss of fear, changes in social behavior and aggressiveness.
The reciprocal connections between the amygdala and the neocortex could provide the means by which conscious thought and learning can suppress reflex emotional responses such as fears. Certain sensory inputs may trigger a very strong reaction, the flight response, which has evolved as a protection from predation. Horses’ ability to hear a wider range of high-frequency tones, such as the sound of twigs broken by the paws of a stalking predator, are naturally linked to an evasive (bolting) response. This is highly relevant to the training of horses, in which natural fearful responses have to be overcome.
Memory
Learning mechanisms and memory are fundamental to how the brain processes information (see Ch. 4). There is no universally agreed model of how memory works, but it is agreed that a memory is a set of encoded neural connections. Encoding can take place in several parts of the brain, and neural connections are likely to be widespread. Genetically determined positional cues probably steer growing fibers toward the general target with which to synapse, but fine-tuning of the pattern of projections is accomplished by activity-dependent mechanisms. Synaptic relationships are constantly being remodeled through increases or decreases in the size and strength of individual synapses, as well as the formation of new synapses and the elimination of unnecessary ones. This plasticity of cortical representation may not only underlie learning but may allow for recovery from brain lesions.
A widely held hypothesis is that learning occurs through ‘long-term potentiation’, and this has recently been confirmed in rat models.15 Long-term potentiation produces changes in synapses that are necessary to acquire and store new information. Synapses become increasingly sensitive so that a constant level of presynaptic stimulation becomes converted into a larger postsynaptic output. Certain forms of long-term potentiation have been shown to depend on the activation of NMDA receptors (see following sections). Long-term potentiation can be demonstrated in vitro by recording electrical potentials of single hippocampal neurons, and can be prevented by applying NMDA receptor antagonists to the preparations.
The three brain regions that appear to be critical to the formation of memories are the medial temporal lobe and certain thalamic and basal forebrain nuclei. Aside from sensory projection areas, specific areas of cerebral cortex can be damaged with little specific change in learned behavior. Thus these neural substrates are not necessarily the locations in which memory representations are stored but are areas thought to be critical to the normal functioning of the system. It is by modifying the synaptic connections between neurons that processors for most brain functions, including emotion, motivation and motor function, are built.16
Two basic time-scales of learning have been identified: short-term memory and long-term memory (some classifications also include an additional two time scales: immediate and intermediate memory). Neural activity related to short-term memory has been observed in many brain areas, but the mechanism by which this activity can outlast a transient sensory stimulus is still unknown. The transfer of short-term memory (which is easily disrupted) to long-term memory (a more stable anatomical or neurochemical change in the nervous system) depends on the hippocampus and related structures in the medial temporal lobe. Damage to the hippocampus results in an animal’s inability to store recent memories but does not interfere with memories already consolidated before damage occurred.
Cognitive psychological studies have suggested that there are two further distinctions within the domain of long-term memory: the conscious recall of information (explicit memory), and the unconscious use of information about motor skills and procedure (implicit memory). Explicit memory relies on a set of structures in the medial temporal lobe, particularly the hippocampus, diencephalon and gyri in the temporal lobe. The learning of motor skills, allowing movements to be made more quickly and accurately with practice, requires constant feedback from the sensory and association areas for completion but, with practice, these become encoded within a number of cortical areas as well as the basal nuclei and the cerebellum.17 The learning of basic strides by foals as well as the complex responses we expect from horses following sensory cues from the rider, require the formation of long-term implicit memory traces.
Neurophysiology and neurochemistry
Neuroanatomy does not in itself explain how the brain controls behavior. Insight into the effect of neuropathology on behavior, as well as the action of neuropharmacologic drugs, requires an appreciation of the function and underlying physiology of the nervous system.
Electrophysiology
Emotions and behavior are ultimately determined by the release of specific neurotransmitters (reviewed in the following sections), which influence the activity of other neurons. Their release is made possible by the electrophysiological activity of excitable cells in the nervous system. Regardless of cell size and shape, transmitter biochemistry or behavioral function, almost all neurons receive and convey information using electrical signals, the so-called action potentials. Action potentials are rapid, transient all-or-none impulses caused by sodium ions entering the axon and potassium ions rushing out. This changes the difference in electrical potential between the inside and outside of the axon. In most neurons the essence of neuronal processing occurs in the regulation of whether or not an action potential is generated. Action potentials are generated at a specialized trigger region within the origin of the axon, and from there are conducted down the axon in one direction only at rates of 1–100 m/s (360 km/hour!). Near its end, the tubular axon divides into fine branches that form synapses, with other neurons and with effector organs, such as muscle.
The ease with which an action potential can be initiated in an individual neuron depends on the relative difference in concentration of sodium and potassium ions inside and outside the axon. The difference is maintained by the action of ion pumps and ion channels. Variation in the resulting electrical difference results in neurons having various thresholds, excitability properties and firing patterns. In the resting state ion channels are closed, but they open in response to the binding of a number of specific molecules at the synapse, or secondary to changes in the membrane potential. Excitatory neurotransmitters act to open cation channels and increase the likelihood of the generation of an action potential. Inhibitory neurotransmitters on the other hand, open anion (chloride) channels that reduce the electrical difference between the inside and outside of the axon membrane and decrease the likelihood of the generation of an action potential. The modulation of the Na1/K1 pump is believed to be one of the fundamental mechanisms for learning,18 by manipulating long-term potentiation.
At the distal end of the axon, the action potential influences other neurons by affecting the synapse. When an action potential reaches the presynaptic terminals of the appropriate synapses, neurotransmitter-containing synaptic vesicles fuse with the presynaptic membrane and release their content into the synaptic cleft. The neurotransmitters bind with specific molecules in the postsynaptic membrane, the receptors, which cause an alteration in the transmembrane potential to either increase or decrease the likelihood of an action potential being generated by the postsynaptic cell.
A single transmitter can produce several distinct effects by activating different types of receptors, and there is now considerable evidence that synapses can be modified functionally and anatomically during development by experience and learning. Training (‘schooling’) of laboratory animals has been shown to produce measurable changes in synaptic interactions.15
Neurotransmitters
Neurotransmitters are classically defined as substances that are synthesized in a neuron, released into the synaptic cleft and then exert a defined action on the postsynaptic neuron by modulating cellular excitability. They are absolutely cardinal to the normal function of the nervous system, and it is worth spending a little bit of time reviewing their function. Their physiology and pharmacology have been well reviewed by Kaplan & Sadock3 and Dodman et al.12 Abnormal levels of neuropeptides have unequivocally been shown to be involved in human psychiatric disorders, and it is also clear that the relative amount and location of neurochemicals can all influence ‘abnormal’ behavior. Rarely if ever can a solitary and unambiguous shift in one neurochemical cause these conditions. Instead, changes in the action of several neurotransmitters or their interaction with receptors are necessary.
Three classes of neurotransmitters classically transmit information in the nervous system: biogenic amines, amino acids and neuropeptides (Table 3.2). Recent data have led to the identification of several novel classes of neurotransmitters, including nucleotides, prostaglandins and gases such as nitric oxide. Some neurotransmitters have in addition been shown to influence gene expression. The field of neurochemistry has breached the bounds of the mere study of chemical mediation of nerve impulses and has developed into a broad discipline that overlaps neuroanatomy, developmental neurobiology and behavioral genetics.
Table 3.2 Classification of neurotransmittersa
| Neurotransmitter class | Components | Neurotransmitter |
|---|---|---|
| Biogenic amines | Quaternary amines | Acetylcholine, histamine |
| Catecholamines | Adrenaline (epinephrine) | |
| Noradrenaline (norepinephrine) | ||
| Dopamine | ||
| Indolamines | Serotonin, melatonin | |
| Amino acids | Excitatory | Glutamate |
| Inhibitory | GABA | |
| Inhibitory and excitatory | Glycine | |
| Neuropeptides | Calcitonin gene-related peptide | |
| Substance P | ||
| Vasoactive intestinal peptide | ||
| Opioids | Endorphins, enkephalins | |
| Hypocretin | ||
| Many others | ||
| Nucleotides | Adenosine | |
| Prostaglandins | Arachidonic acid | |
| Gases | Nitric oxide | |
| Carbon monoxide |
a There is no clear consensus on the classification of neurotransmitters, and the list of examples is not exhaustive. Additional neurotransmitters are constantly being added.
It is appropriate at this stage to review the classification and function of some of the principal neurotransmitters that are known to have a role in modifying behavior.
Biogenic amines
The biogenic amines are bioactive amines, derivatives of ammonia (NH3) in which one or more hydrogen atoms have been replaced by hydrocarbon groups. This includes dopamine, adrenaline (epinephrine), noradrenaline (norepinephrine), serotonin, acetylcholine and melatonin. A common feature of all biogenic amine neurotransmitters is that they are initially synthesized in the axon terminal by enzymes produced in the cell body and transported down the axon. After release, a significant amount of noradrenaline, serotonin and dopamine in the synaptic cleft is taken up again by a presynaptic transporter protein to be repackaged into vesicles, or degraded by the enzyme monoamine oxidase. A number of psychopharmacologic agents affect amine reuptake, synthesis or degradation.
Adrenaline and noradrenaline
Adrenaline and noradrenaline, along with dopamine, are classified as catecholamines and are synthesized from the precursor tyrosine in a common biosynthetic pathway. Although adrenaline is in higher concentration in serum and is the catecholamine that causes the sweating and increased heart rate when horses are stressed or excited, noradrenaline is more abundant in the brain. Noradrenergic neurons in the medulla project to the hypothalamus and control cardiovascular and endocrine functions, while equivalent neurons in the reticular formation of the pons mainly provide projections to the spinal cord that modulate autonomic reflexes and pain sensations. A further group in the locus ceruleus in the dorsal part of the pons has wide projections to the rest of the CNS. Although the exact function of the locus ceruleus is unknown, increased activity in this region is associated with anxiety, and it is particularly active in animals undergoing stress. Noradrenergic activity in humans is correlated with aggressive behavior, and specific adrenergic receptor blockers have beneficial effects in violent patients.19 Antidepressant drugs such as the tricyclic antidepressants and monoamine oxidase inhibitors increase levels of noradrenaline by blocking its reuptake and catabolism. Their use is indicated in narcoleptic horses (see following sections), but potentially serious adverse effects may be seen with large doses.20
Dopamine
Dopamine-secreting neuronal groups in the substantia nigra in the midbrain send major ascending dopaminergic inputs to the forebrain, including the cerebrum, limbic system and basal nuclei. An important role of dopamine in human medicine concerns the control that dopamine receptors in the basal nuclei have on the initiation of motor responses and the regulation of movement (affected in Parkinson’s disease and Tourette’s syndrome). The significant association between Parkinson’s disease and depression suggests that the dopamine nigrostriatal tract is involved in control of mood as well as motor control. The only example of selective basal nuclei pathology in horses is equine nigropallidal encephalomalacia following ingestion of yellow star thistle (Centaurea solstitialis). Pan-necrosis of the basal nuclei results in devastating dystonia and rigidity of the muscles of mastication in affected horses.
Pathways to the frontal and temporal cortices have been implicated in emotion, thought and memory storage. A strong association has been found between high levels of detachment and a particular subtype of dopamine receptor, D2 receptors.21 Drugs that selectively bind D2 receptors have anti-aggressive effects in human patients.22 Lesion studies indicate that dopaminergic systems innervating the basal nuclei contribute to feeding, drinking and other motivated behaviors in a crucial way, and their pharmacologic blockade impairs feeding behavior. 23,24
The dopaminergic systems may be particularly involved in the brain’s reward system, leading to addiction, not only to abused drugs such as nicotine, amphetamines and cocaine, which directly increase dopamine levels, but also to the biochemical rewards associated with equine stereotypic behaviors. There is evidence that high levels of dopaminergic activity within the basal ganglia play an important role in many stereotypies.25 Dopaminergic activity is modulated by many neurotransmitters, including endogenous opioids and glutamate, and exogenous NMDA receptor blockers have been shown to suppress crib-biting in horses.26
Serotonin
Serotonin is principally produced by neurons in the rostral pons and midbrain. It is synthesized from the amino acid tryptophan, and oral intake of tryptophan supplements may increase serotonin levels in the brain. It has been used to ‘sedate’ horses and may have a very mild effect; however, other work indicates that tryptophan may actually stimulate horses rather than having a sedative effect.27 Serotonin (5-HT) – or its precursor, 5-hydroxytryptophan (5-HTP) – may induce sleep and, importantly, appears to reduce anxiety. The 5-HT system is the mediator of learned and sustained fear responses, and decreased 5-HT levels have been associated with violent psychopathological behavior in humans.28 Potent hallucinogens such as lysergic acid diethylamide (LSD) are structurally similar to 5-HT. It has been argued that serotonin plays a role in the behavioral facilitation system that initiates responses to the environment, and the behavioral inhibition system that arrests ongoing behavior. A decrease in serotonergic transmission leads to an inability to adopt passive or waiting attitudes, or to accept situations that necessitate or create strong inhibitory tendencies. This is particularly relevant to frustrating situations such as encountered by horses kept in what are essentially unnatural environments. Stereotypic behavior patterns in human medicine, often grouped in a classification known as obsessive–compulsive disorders, appear to be partially attributable to decreased 5-HT and abnormal endorphin metabolism. Parallel examples of ritualistic and stereotypic behaviors, including wind-sucking and crib-biting in horses are recognized in veterinary medicine.29 Many antidepressants, discussed further in the following section, such as the tricyclic antidepressants and fluoxetine (Prozac®) block serotonin reuptake transporters, thus increasing the amount of serotonin in the synaptic cleft.
Acetylcholine
This is a very prominent neurotransmitter in both the central and peripheral nervous system, as it is the major neurotransmitter of the parasympathetic branch of the autonomic nervous system as well as the preganglionic synapses of sympathetic neurons. Neurons in nuclei of the thalamus and brainstem project to the cortex and limbic system and affect behavior patterns. Acetylcholine, one of the principal neurotransmitters involved in the propagation of aggressive, predatory behavior, is important in regulating wake and sleep cycles and has been shown to increase the strength of synaptic connections in the hippocampus. Cholinergic enhancement has been shown to improve memory performance in humans.30
Amino acids
Amino acids are molecules containing both an amine and a carboxylic acid group. Amino acid neurotransmitters are the most abundant in the brain. The major excitatory neurotransmitter is glutamate, opposed by the major inhibitory neurotransmitter gamma-aminobutyric acid (GABA). A simplified way to look at brain biochemistry is as a balance between those two neurotransmitters alone, with all other neurotransmitters simply being involved in modulating that balance.
GABA
GABA is the primary neurotransmitter in intrinsic neurons that function as local mediators for inhibitory feedback loops. Major tranquilizing and anticonvulsant agents, including benzodiazepines (e.g. diazepam) and barbiturates act primarily through GABAergic mechanisms. A further inhibitory neurotransmitter, which is predominantly active in the spinal cord, is the amino acid glycine.
Glutamate
It is now generally agreed that glutamate is the major excitatory neurotransmitter in the brain, and it is believed that 70% of the fast excitatory CNS synapses use glutamate as a transmitter. There are five types of glutamate receptors of which N-methyl-d-aspartate (NMDA) is the best understood because it may play a crucial role in learning and memory as well as in aggressive and defensive behavior.
Glutamate has recently gained unparalleled prominence in the field of excitotoxicity (for review see Hahn & Mayhew31). Oxygen metabolism results in the generation of free-radical molecules, which can result in considerable subcellular damage. Neurons appear to be at particular risk of free-radical damage, and excitotoxicity is now thought to be a potential contributor to the pathogenesis of a large number of diseases, including equine motor neuron disease.32
Peptides
An ever-growing list of neuroactive peptides, such as calcitonin gene-related peptide (CGRP), substance P, vasoactive intestinal peptide (VIP) and hypocretin (see subsection on narcolepsy), act as neurotransmitters or, more commonly, modulate pre- or postsynaptic transmission. Nevertheless the actual molar concentration of any given peptide in the brain is maximally two to three orders of magnitude lower than that of the biogenic amines, acetylcholine and amino acids. Unlike classic neurotransmitters that are synthesized at the synapse, neuropeptides are synthesized in the cell body, from where they are transported down the axon. Neuropeptides thus take a comparatively long time to replenish, cannot be recycled, and have a longer duration of action than the amino acids or biogenic amines.33
Opioids
Opioids are a prominent member of the neuropeptide group. The opium poppy was cultivated in lower Mesopotamia in 3400 bc, where it was referred to as the ‘joy plant’, but it was not until the 1970s that this large family of neuropeptides, the endogenous opioids (endorphins), were isolated from brain extracts. Four classes of endogenous opioids are now recognized, and they are principally involved in the regulation of stress, pain and mood, in addition to potentiating effects on adrenergic and glutamatergic neurotransmission. They are known to have a major role in social affiliation and caring behavior in humans, and there is good evidence for opioid peptides mediating defensive and defeat responses during conflict. Opioid receptors are found closely associated, both functionally and anatomically, with dopaminergic and serotonergic neurons, and are distributed in regions such as the limbic system, hypothalamus and basal nuclei. Endorphin systems are probably involved in the effectiveness of the twitch (see Ch. 14), since its action is blocked by naloxone and its application increases plasma concentrations of beta-endorphin.34 A tandem role of endorphins and serotonin has been suggested in stereotypies and has received considerable attention in the study of crib-biting, not least because it may contribute to the phenomenon of emancipation (see Ch. 8).
Equine psychopharmacologic agents
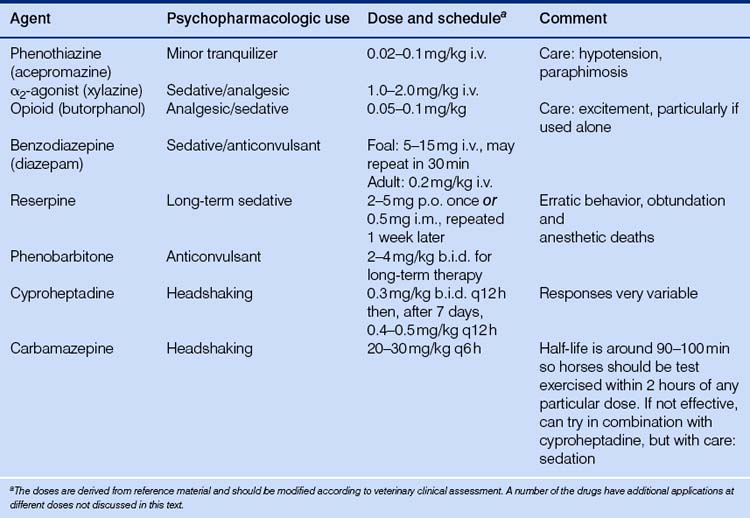
The availability of chemical restraint has revolutionized the number and type of procedures that can be performed on standing horses. The following is a summary of a few of the commonly used behavior-modifying pharmacologic agents used in horses (Table 3.3).
Phenothiazines
Phenothiazine tranquilizers were introduced into clinical veterinary medicine in the 1950s. Phenothiazines such as acepromazine (and older compounds, including chlorpromazine and promazine hydrochloride) interfere principally with the central actions of dopamine but also affect noradrenaline and adrenaline, resulting in mild brainstem depression. This class of drugs is no longer used to sedate animals heavily, but their calming effects have been valuable in calming unfriendly and apprehensive animals. Individual responses are extremely variable, and there is generally a markedly reduced effect on excited horses. Acetyl promazine maleate given at 0.03–0.1 mg/kg i.v. or i.m. has reduced some stereotypies (by reducing all activity?), and the same drug at higher doses eliminated suspected opioid-induced pacing postoperatively.35 They interfere with the central actions of the excitement-producing catecholamines, adrenaline, noradrenaline and dopamine.36 Phenothiazines, however, have no analgesic properties and they decrease gastrointestinal tone and secretions due to CNS depression and anticholinergic actions. Priapism and paraphimosis (penile protrusions) are a recognized risk of using phenothiazines in geldings and particularly stallions. The mechanism is unknown but is attributed to a blockade of adrenergic and dopaminergic receptors centrally and peripherally.
α2-agonists
Agonists of the α2 subgroup of adrenoreceptors were developed as antihypertensive agents for use in humans, and are now far more commonly used for veterinary procedures in horses than are phenothiazines, because they result in more consistent and profound sedation. The behavioral effects of α2-agonists such as xylazine (Rompun™), detomidine and romifidine are dose related and vary from subtle calming to profound sedation accompanied by head drooping and ataxia. Exaggerated reflex kicking responses have been associated with their use, even in profoundly sedated horses, an effect that is reduced when α2-agonists are combined with opiates such as butorphanol. The α2-agonists are commonly used with ketamine, a dissociative anesthetic, to induce anesthesia. Pharmacologically they are classified as analgesics as well as sedatives37 and result in sedation by binding to central and peripheral presynaptic inhibitory α2-receptors. These are inhibitory to the release of noradrenaline and thus act to hyperpolarize the postsynaptic neuron.38
Opioids
Opioids are exogenous substances that bind specific subpopulations of central and peripheral opioid receptors, and at standard doses have analgesic properties without loss of proprioception or consciousness. The classification of opioid drugs has changed as the molecular pharmacology of opioid receptors has been clarified, but it has traditionally been based on their action on the three main classes of receptors: m, k and d. Thus morphine, fentanyl, codeine and heroin are classed as ‘agonists’ while butorphanol (Torbugesic™) is categorized as an agonist-antagonist compound due to its effect on k and m receptors, respectively. The agonist-antagonist agents have fewer undesirable side effects such as excitation.
Opioid agonists and agonist-antagonists are used in equine medicine for their sedative and analgesic action, and common practice is to mix them with an α2-agonist or phenothiazine, particularly to prevent the α2-associated ‘reflex’ kicking. An example of a pure opiate antagonist is naloxone, notably used in veterinary medicine in trials designed to establish whether endogenous opioid blockade will result in diminished stereotypic behavior. Naloxone administered i.v. had a dose-related effect on eliminating crib-biting for up to 2 days, but it also resulted in abdominal distress.35 However, resting behavior was also significantly increased in crib-biting horses, and it may be that the stereotypy reduction was due to a sedative effect of the opiate antagonist.
Benzodiazepines
The most prominent benzodiazepine is diazepam (Valium™), a drug noted for its anxiolytic, sedative, anticonvulsive and muscle-relaxing properties. It is used frequently for sedation in foals and is the drug of choice for the treatment of status epilepticus. Benzodiazepines, along with barbiturates, open axonal chloride channels thus hyperpolarizing membranes and leading to inhibition of CNS function.39 Along with barbiturates they potentiate GABA receptors and increase mean GABA channel opening times.40
Phenobarbitone
Phenobarbitone is a long-acting barbiturate used in the management of seizures and occasionally as a tranquilizer. The primary mechanism of the action of barbiturates is to increase inhibition by acting indirectly as GABA agonists, increasing the neuronal threshold of electrical excitability. It is the initial drug of choice for treating seizure disorders in horses and can initially result in profound sedation.
Reserpine
Reserpine is an alkaloid extracted from the root of Rauwolfia serpentine, a climbing shrub indigenous to India. It was introduced into veterinary medicine in the 1950s and its calming properties were exploited for management and training purposes. It has essentially been dropped from clinical use as a tranquilizer. Reserpine causes depletion of biogenic amine concentrations in the brain, including serotonin, noradrenaline and dopamine. Because noradrenaline is an excitatory neurohormone in the brain, its depletion explains the calming or tranquilizing effect of reserpine in the horse.41 It is still available in oral and injectable preparations as an antihypertensive and antipsychotic drug in human patients, and is on occasion used to calm nervous horses. There is apparently a large variability in the pharmacokinetics of reserpine in horses, and adverse reactions, including erratic behavior and obtundation, and anesthetic deaths due to hypotension, have been recorded. Reserpine should be avoided in stallions and should not be administered prior to surgery.
Fluoxetine
Folk medicine has long used extracts from the flower and leaves of St John’s wort (Hypericum perforatum) to treat mild depressive disorders, but it is only recently that the ability of St John’s wort to inhibit serotonin and dopamine reuptake has been recognized. The antidepressant and anti-obsessional actions of fluoxetine (Prozac®) are similarly thought to be linked to its ability to block presynaptic uptake channels and act as a selective serotonin reuptake inhibitor (SSRI). Fluoxetine has fewer of the anticholinergic, sedative and cardiovascular side effects than the classic tricyclic antidepressant drugs, probably because of comparatively lower opiate, catecholamine and dopamine membrane receptor binding.42 It is the most commonly used specific 5-HT reuptake blocker in companion animal medicine, and is used in an oral preparation to treat stereotypies, aggression and fear, and anxiety.43,44 Fluoxetine has not been thoroughly evaluated in horses, but its use is likely to increase given the prominence of the drug in human and small-animal medicine.
Cyproheptadine
Cyproheptadine is a specific serotonin receptor antagonist with histamine receptor blocking and anticholinergic and sedative effects. It may also stimulate ACTH secretion and has a role in altering pain sensations. It is used in human medicine for treatment of severe anorexia nervosa, and encouraging results have been reported in the use of cyproheptadine for the treatment of horses with hyperadrenocorticism45 and headshaking,46 a condition that may involve altered pain perception.
Carbamazepine
Carbamazepine is a tricyclic anticonvulsant that increases serotonin levels by blocking the neuronal serotonin transporter.47 It can be used in the treatment of most types of human epilepsy, although its anti-seizure mechanism of action is incompletely understood. Carbamazepine in humans is increasingly being used for its anti-aggressive effects and to treat a variety of neuropsychiatric disorders. It has been successfully employed as a treatment of trigeminal neuralgia in humans, an effect that may explain its efficacy in ameliorating the signs of headshaking.48 There are a great many reports in the literature of paradoxical and severe side effects of this drug in human patients,49–51 and care is advised in its use in veterinary medicine.
The neurological examination
The first steps in evaluating behavior cases involve obtaining a detailed history and performing a medical examination to rule out any underlying clinical condition. The behavior changes noted as abnormal by the owner may be associated with pain, metabolic changes, weakness or sleep attacks. A systematic neurological examination is considered fundamental to a psychiatric investigation of human patients presenting with recent-onset psychosis or acute changes in mental status.52 Similarly, it is appropriate to have an appreciation of diseases of the equine nervous system known to alter behavior of the horse. A detailed neurological examination, including assessment of mentation, cranial nerves and sensory and motor systems, should be included in the investigation of any horse presented with a history of a recent change in behavior, or suspected of having an altered state of mentation.
The physical examination is designed to disclose signs of general disease or pain that may have an influence on behavior, while the objective of the neurological examination is to determine if the change in behavior could be explained by an underlying neurological deficit, and to define the anatomical portion of the nervous system responsible for the clinical signs. This is an absolute prerequisite to allow reasonable differential diagnoses to be formulated. Readers are referred to Mayhew53 for a detailed description of how to perform and interpret the neurological examination of the horse.
The neurological examination consists of a careful evaluation of behavior, mental status, cranial nerve function, gait and postural reactions. Because the clinician is unable to ask for voluntary responses, the neurological examination of the horse involves testing simple and complex reflex pathways and interpreting the site of the lesion in light of the anatomy of the reflexes tested. A simple reflex arc consists of a sensory neuron, one or more interneurons and a motor neuron that innervates muscles effecting the response. Reflexes occur without connections from ascending or descending central tracts, although input from higher centers classically inhibits excessive reflex movement. Discrete areas of the nervous system are extremely specialized and, by accurately localizing clinical signs, the anatomical location of a lesion can be determined. This requires an assessment of all the principal components of the nervous system, and abbreviating the neurological examination is discouraged. If it is determined that neurological deficits other than a subtle change in behavior are present, then it is appropriate to proceed with further diagnostics before initiating protocols to treat a primary behavioral problem.
Behavior and mental status
As explained in the introductory chapter, the owner of the horse should be questioned about the onset of the behavioral change, the nature of the signs, and any previous or associated clinical problems. The signalment of the animal has to be taken into consideration, and the presence of possible external triggers, such as auditory or tactile stimuli, should be explored (see Ch. 1). Importantly, the mental status of the animal must be assessed, since neuropathological lesions inducing a change in behavior can also be expected to affect the general function of the cerebral hemispheres as well as neural activity in the brainstem and diencephalon.
The various terms that describe alterations of mental status in horses include obtundation, stupor, coma, hyperactivity and aggression. Further signs of a subtle change in sensorium comprise continual yawning, asymmetric drifting or circling and, in foals, lack of recognition of the mare. More severe forebrain disease may lead to head pressing (Fig. 3.8), head deviation and propulsive walking or circling, aggression and self-mutilation, adopting bizarre postures and leaving food in the mouth. These signs usually reflect disturbances in the diencephalon and forebrain, and often implicate some portion of the limbic system.2 Large, space-occupying lesions in the cerebral hemispheres may result in locomotor or postural disturbances. If the lesion is unilateral the animal may circle toward the side of the lesion (because, for that patient, the spatial field contralateral to the lesion ‘ceases to exist’?). Lesions of localized regions of cortex, for example the occipital lobe, can result in a specific sensory loss such as blindness with normal pupillary light responses (central blindness). Lesions of the frontal lobes may result in behavioral changes such as lack of recognition of familiar people or objects, while damage to the temporal lobe can result in changes in eating behaviors, sexual habits, and temperament.
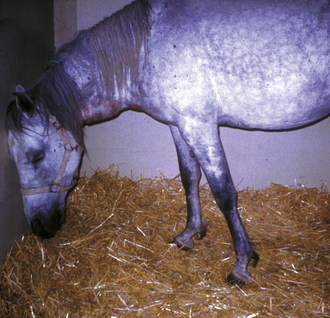
Figure 3.8 Horse showing intermittent somnolence and head pressing as a result of liver failure and hepatoencephalopathy caused by pyrrolizidine alkaloid (ragwort) toxicity.
(Photograph courtesy of Joe Mayhew.)
Seizures are a clinical sign strongly indicating the presence of forebrain disease. They result from multiple, synchronous discharges from a population of neurons producing a variety of undirected, uncontrolled, unorganized movements or changes of mentation. The seizure may be preceded by an aura (actually a part of the seizure but one that does not include involuntary movement) in which the horse is distracted from its environment or is restless. The actual seizure is called the ictus, and in horses is expressed by involuntary changes in muscle tone or movement. The wave of abnormal neurophysiological activity can remain in one region causing, for example, rhythmic movement of muscles of the face, or can spread to the other cortical hemisphere. If there is a generalized excitation, lower motor neurons in brainstem and spinal cord can become involved, resulting in a generalized seizure with rigidity, recumbency and tonic and clonic limb movements (Fig. 3.9). The animal loses consciousness during a generalized seizure, but recovers in a few minutes. This may be followed by a post-ictal phase of obtundation and temporary blindness, which can last for hours or, in the case of foals, days.53
Cranial nerves
Because of the close anatomical relationship between the forebrain and the brainstem, diseases affecting the prosencephalon often also result in dysfunction of one or more cranial nerves. The examination of cranial nerves consists of the evaluation of a series of reflexes and responses involving one or more cranial nerves (see Table 3.1).
The head should be initially examined for asymmetries of posture, facial expression or muscle mass. A head tilt (lateral deviation of the poll), suggests the presence of a vestibular disturbance, and this can be emphasized by applying a blindfold. Ptosis, weak eyelids on palpation, pulling of the muzzle to one side or decreased ear tone are signs of facial nerve (CN VII) paresis. Temporal or masseter muscle atrophy is seen with pathology of the motor branch of the trigeminal nerve (CN V). Atrophy or severe weakness of the tongue is caused by hypoglossal (CN XII) paresis. The function of the sensory branches of CN V is tested by reflex responses to light pricking of the ears, lips or nasal mucosa. Animals with diffuse cerebral dysfunction or focal parietal lobe pathology may show signs of nasal hypalgesia even when CN V is not affected.
The eyes deserve close attention as the ocular muscles are innervated and controlled with great precision. Vision requires intact eyes, optic nerves (CN II) and central visual pathways. Most of the objects viewed with one eye are perceived in the contralateral occipital lobe, but impulses subsequently cross to the opposite hemisphere via the corpus callosum.54
Vision is assessed by making a threatening gesture towards each eye, resulting in blinking (the menace response). Care must be taken not to stimulate the cornea or the palpebrae with air current. While the precise central relay of this reflex is not known, it is thought to include several different brain regions, including the visual pathways, as well as the motor cortex, pons, cerebellum, facial motor nucleus, facial nerve and orbicularis oculi muscle in the eyelid. The menace response is probably mediated by subcortical neural circuits as suggested by work on both rodents55 and human56 and non-human primates.57 Suddenly appearing, ‘looming’ visual stimuli activate midbrain circuits that produce conjugate eye, head and neck movements away from the source of the stimulus.55 Foals up to 2 weeks of age, and animals affected by cerebellar disease, may not respond to this test of vision by blinking. They should nevertheless react to a menacing stimulus by retracting the eyeball, resulting in a brief protrusion of the third eyelid, and/or by pulling away the head.
Eye movement is assessed by moving the head from side to side and looking for normal nystagmus, with the fast phase occurring in the direction of movement. Abnormal, horizontal or vertical nystagmus indicates a lesion in the vestibular system, which influences the cranial nerves innervating the external muscles of the eye: CNs III, IV and VI. Strabismus (abnormal eye position) in horses is rarely due to dysfunction of the latter nerves and is more likely to be caused by mechanical interference with eye movements, but ventral strabismus noted after raising the head is common with ipsilateral vestibular disease.
The pupils should be observed for size and symmetry, and the pupillary light response (CN II and III) assessed by observing pupillary constriction after shining light into the eye. Consensual responses are difficult to evaluate in horses, due to the lateral placement of the globes, and instead the light is swung briskly from eye to eye, observing for a further constriction of the pupil when the light is directed into the pupil previously constricted by the consensual response. Normal pupillary light responses in the presence of blindness suggests pathology of the occipital cortex (central blindness).
The obvious presence of the nictitating membrane is notable, and could indicate the presence of enophthalmos, such as can be caused by sympathetic denervation (Horner’s syndrome) or, classically, tetanus.
Gait and postural reactions
CNS disease resulting in behavioral changes may also cause gait and postural reaction deficits if the brainstem and associated ascending and descending tracts are affected. Ataxia (lack of coordination due to a deficit in proprioception pathways) and weakness (due to upper motor neuron pathology) can underlie changes in gait that cannot be explained by a musculoskeletal lesion. Ataxia is assessed by determining the responses to postural reactions, maneuvers designed to accentuate more subtle deficits. The horse should be examined when walking in a straight line, circling tightly, being led up and down a slope with the head extended. Stepping on the opposite foot, pivoting on a limb, increased or decreased joint movement and excessive circumduction of a limb may be signs of neurological disease. Laterally pulling on the tail while walking is a sensitive test for weakness caused by the interruption of the connection between upper motor neurons in the brain and lower motor neurons in the spinal cord, so called upper motor neuron weakness. Hopping the horse, by flexing one thoracic limb and pushing the animal to the opposite side, can accentuate both ataxia and weakness of subtle thoracic limb signs.
Neurological diseases with behavioral signs
Diseases diffusely affecting the forebrain can be associated with changes in behavior. However, this is highly unlikely to occur in the absence of further clinical signs such as failure to recognize familiar companions, continual yawning, facial twitches (partial seizures) and drifting to one side when blindfolded. More severe disease can, in addition, be expected to result in circling toward the affected side, obtundation, head pressing and generalized seizures. Focal lesions in the midline of the brain affecting the ventromedial hypothalamic nucleus or rostral hypothalamus, or bilateral damage of the hypothalamus or limbic systems, may further cause aggression or disturbances in eating, drinking or sexual behavior.7 It should be remembered that CNS neoplasia is extremely rare in the horse! The following is a brief discussion of diseases of the horse that result in clinical signs which include changes in behavior, but readers are urged to further consult more comprehensive texts.53,58,59
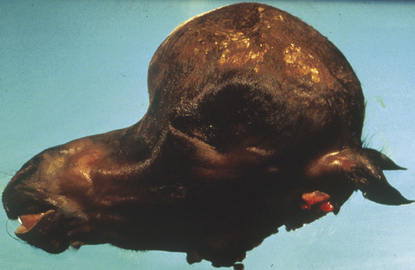
Hydrocephalus
Hydrocephalus is a condition marked by an excessive accumulation of CSF resulting in dilation of the cerebral ventricles and raised intracranial pressure. It may result in enlargement of the cranium (Fig. 3.10) and atrophy of brain parenchyma. This can be secondary to some obstruction of normal CSF circulation or the site of CSF absorption into the venous system. On occasion hydrocephalus, even with a severe loss of cortical tissue, is found as an incidental finding in foals with apparently normal behavior. Hydrocephalus is uncommon in adult horses compared with other domestic animals, as neoplasia is exceedingly rare in the brain of this species. It can be associated with massive cholesterol accumulation in the choroid plexus, cholesterinic granulomas, believed to result from choroid plexus congestion and hemorrhage.60 These can be massive structures and surgical removal has not been reported.
Infectious diseases
Bacterial meningitis
This is rarely seen in adults but is not uncommon in foals, in which it is usually associated with failure of passive transfer of immunoglobulins. Physical and neurological examination findings can include fever, obtundation, ataxia, aimless wandering, abnormal vocalization, collapse and seizures. The prognosis is poor. Attempted therapy consists of long-term antibiotic administration and intensive nursing care.
Diffuse encephalomyelitides
Numerous viruses across the world cause encephalitis or, more commonly, encephalomyelitis (inflammation of the brain and spinal cord), in horses. Most are caused by arboviruses (arthropod-borne viruses), which include viruses from a number of families using an insect or tick vector. Most but not all occur in warm climates, as the vector can survive through the winter. The most prominent of these are the alphaviruses (Eastern, Western and Venezuelan viruses), which are found in the Americas. A variety of other arboviruses such as Semliki Forest, Japanese B, St Louis encephalitis, equine infectious anemia and equine encephalosis viruses, however, also result in cerebral disease in horses.59 Aujeszky’s disease (pseudorabies) has occasionally been reported to cause disease in horses,61 and Borna disease,62 Hendra virus63 and West Nile virus have recently received attention.64 Clinical signs vary in severity depending on the virus, but the majority result in clinical signs attributable to diffuse disease, which may include fever, blindness, ataxia, seizures and hyperexcitability. The prognosis depends on the specific virus involved and is especially poor for Eastern and Venezuelan encephalitis.
A rare virus that deserves additional mention because of its public health significance is rabies, a disease that accounts for thousands of human deaths worldwide each year. The disease can initially cause diverse clinical signs in horses, including lameness, colic, tetanus and peripheral neuropathies.65 In a group of experimentally infected horses the most frequently observed clinical sign was muscle tremors, with pharyngeal spasm or pharyngeal paresis, ataxia and lethargy or somnolence also being commonly observed.66 The virus passes from the tissues at the bite wounds via peripheral nerves to the CNS by retrograde axoplasmic flow. Once in the CNS the virus disseminates widely in the brain and spinal cord, resulting in a variety of clinical signs. In the brainstem (‘dumb’) form, obtundation and dementia with ataxia, drooling and pharyngeal paralysis occur, while the cerebral (‘furious’) form is characterized by vocalization, photo- and hydrophobia, hyperesthesia and seizures, as well as changes in behavior marked by aggressive and destructive behavior. The behavioral changes are believed to be principally due to pathology of the limbic system, and reflect a strategy by the virus that serves to enhance the likelihood of transfer of the agent.67 Viral pathology centered on the spinal cord results in ascending paralysis, and this forms, more often than the cerebral and brainstem forms, seems to result in asymmetric self-mutilation.
Diffuse disease with cerebral signs has also been reported due to bacteria such as the spirochete Borrelia burgdorferi, which causes Lyme disease.68 Protozoal diseases, including equine protozoal myeloencephalitis in the Americas69 and trypanosomiasis in tropical and subtropical countries,70 can on occasion present primarily with forebrain disease.
Brain abscess
Primary brain abscesses in horses are rare but do occur following a history of strangles (Streptococcus equi, subspecies equi) or during an acute septicemic infection in foals (Fig. 3.11). A thorough physical examination may determine whether pyogenic disease or multiple foci are present, and cerebrospinal fluid often has increased inflammatory cells and mildly increased protein concentrations. Displacement and compression of the neural tissue by the abscess, and the resulting disruption of the vasculature and creation of secondary vasogenic edema, leads to brain swelling and increased intracranial pressure. Compulsive circling, obtundation, symmetric or asymmetric central blindness and gait changes often follow. Treatment with antimicrobial therapy is rarely rewarding, and surgical drainage should be considered if the abscess is found to be accessible following a computed tomographic scan.
Parasitic encephalomyelitis
Verminous encephalomyelitis can be due to the aberrant migration of an endoparasite normally found in horses, or due to the presence of a parasite not usually present in horses. Clinical signs usually reflect the random migration of the organism, classically Strongylus vulgaris, in the brain or spinal cord. Some nematodes, such as the filarioid Setaria spp. and the rhabditoid Halicephalobus gingivalis, can result in diffuse forebrain disease with behavioral signs such as blindness, circling, obtundation and head pressing. In addition to the direct tissue destruction caused by the migrating parasite, the presence of the organism (usually Strongylus vulgaris) in the brachiocephalic arteries can result in a thromboembolic shower to the ipsilateral cerebrum. The resulting acute-onset seizures, obtundation and changes in mentation can be severe. The diagnosis is made on the basis of the history and neurological examination findings and may be supported by serum and CSF neutrophilia or eosinophilia. Treatment is based on anti-inflammatory and anti-parasitic therapy, and the prognosis depends on whether clinical signs were caused by the thromboembolic shower or actual parasite migration (worse prognosis).
Metabolic disorders
Hepatic encephalopathy
Liver failure is a prominent cause of cerebral dysfunction in the horse and can be due to primary hepatic pathology or, rarely, shunts bypassing the liver; these connect the portal circulation from the intestines to the systemic circulation (portosystemic shunts) in foals.71 Liver pathology can be acute following Theiler’s disease (serum hepatitis), acute toxicosis with mycotoxins or pyrrolizidine alkaloids (found in plants such as ragwort), liver abscessation, suppurative cholangitis or cholelithiasis. Horses with acute liver failure classically present with obtundation, anorexia, and with CNS derangements such as head pressing and aimless wandering (see Fig. 3.8). Icterus and elevation of liver-specific serum biochemistry values are normally a feature of acute liver failure. In chronic liver failure, hepatic insufficiency occurs because hepatocellular compromise is such that the functional reserve of the organ is exceeded.72 Weight loss is often the most prominent feature of chronic liver failure, and icterus and elevated serum liver enzymes may not be prominent. The most common cause of chronic hepatic failure is exposure to hepatotoxic plants, particularly those containing pyrrolizidine alkaloids (e.g. ragwort). See Savage73 and Barton & Morris74 for detailed discussions of the etiologies of liver pathology in horses.
The clinical manifestations range from minimal changes in behavior and motor activity to overt obtundation, anxiety and coma, consistent with a global depression of CNS function. This appears to arise as a consequence of a net increase in inhibitory neurotransmission due to an imbalance between GABA and glutamate receptor agonists.75 Of the many compounds that accumulate in the circulation principally as a consequence of impaired liver function, ammonia is considered to play an important role in the onset of hepatic encephalopathy.76 Ammonia is excitotoxic, as it is associated with impaired reuptake of glutamate into nerve endings and astrocytes. This increases the concentration of glutamate in the synapse, ultimately leading to a down-regulation of NMDA receptors and decreased activity of this essential excitatory neurotransmitter (see subsection on aminoacid neurotransmitters, above). At the same time, increased inhibitory neurotransmission occurs, due to increased brain levels of benzodiazepines and GABA receptors and the direct interaction of ammonia with one of the GABA receptors. In addition astrocyte metabolism of ammonia to glutamine facilitates plasma-to-brain transport of aromatic amino acids such as tryptophan and tyrosine, direct precursors of the inhibitory neurotransmitters serotonin and dopamine.
Central nervous system energy deprivation
Hypoxia, ischemia and hypoglycemia result in a fall of intracellular energy levels and neuronal necrosis. Neurons in specific anatomical areas in the forebrain and brainstem, including specific populations in the cerebral cortex, hippocampus, amygdala, thalamic nuclei and cerebellum, are especially vulnerable. Excitatory neurotransmitters, principally glutamate, are important final mediators of neuronal death in hypoxia and ischemia.77
Hypoglycemia
Significant hypoglycemia produces transient behavioral signs such as confusion and obtundation. In adults it can be associated with hepatic failure, or hyperinsulinism due to inappropriate administration of insulin in treating hyperlipemia or pancreatic neoplasia. Hypoglycemia is more prevalent in foals and can be found secondary to septicemia, potentially due to depletion of glycogen stores, impaired gluconeogenesis and increased peripheral glucose utilization. It is also found in foals with a decreased milk intake, particularly while in intensive care, and is associated with seizures.
Anesthetic hypoxia/anoxia
Respiratory or cardiac failure during anesthesia can lead to hypoxic brain damage. Central blindness and obtundation can be immediate sequelae. If such animals are euthanized, they are found to have an encephalopathy that can be mapped to those areas of the brain sensitive to energy deprivation of neurons58 such as the superficial cortical laminae.78 Long-term nursing can be rewarding, and aggressive therapy with the free-radical scavenger dimethyl-sulfoxide is appropriate.
Hypoxic ischemic syndrome
Hypoxic ischemic (neonatal maladjustment) syndrome is a well-recognized but poorly understood disorder of neonatal foals (Fig. 3.12). It affects full-term foals with normal maturity, although a very similar syndrome may be present in full-term, dysmature foals.79 Affected individuals typically are normal immediately after birth but within a few hours to days postpartum become disinterested in nursing, may wander aimlessly and rarely make peculiar vocalizations, so-called ‘barking’. Further deterioration is marked by recumbency, seizures and obtundation leading to coma and death. The neuropathological findings vary with the duration of clinical signs but typically include ischemic neuronal necrosis and patchy, focal and diffuse hemorrhages, sometimes accompanied by edema in specific sites of the brain and occasionally spinal cord. The pathogenesis is unknown but the lesions suggest an hypoxic and/or ischemic insult around the time of birth,80 such as premature breakage of the umbilicus when mares rise from the ground in response to human interference. Therapy involves controlling any seizures with diazepam and extended, intensive nursing care.53 Diagnosis is complicated by concurrent septicemia and hypoglycemia.
Leukoencephalomalacia
Long-term ingestion of corn affected by the fungus Fusarium moniliforme can result in single cases or outbreaks of leukoencephalomalacia. Liquefactive necrosis of cerebral white matter classically leads to acute onset of signs of dementia, obtundation, central blindness, incoordination, facial paralysis and death in a few hours to a day. Mildly affected horses that recover may have permanent cognitive deficits. The toxins responsible are secondary metabolites of the fungi, principally fumonisin B1,81,82 causing vascular lesions in the white matter of subcortical regions as well as in the cerebellum, brainstem and spinal cord. Hepatic disease frequently coexists.58 Confirming the source of an outbreak is complicated by the fact that corn samples commonly contain fungal spores.
Epilepsy
Seizures, transient and involuntary changes in behavior or neurological status due to abnormal electrical discharges in forebrain neurons, may be related to the syndromes discussed above. In small animals, seizures can manifest as anything from a subtle alteration in alertness to a generalized tonic–clonic event. Subtle behavioral changes may be manifested during auras, while profound behavioral alterations, including hysteria, rage and ‘fly biting’, can be the primary clinical sign during some forms of seizures (partial complex seizures) depending on the seizure focus.13,83 In humans and dogs partial complex seizures are based in the limbic system, and the amygdala is known to be particularly sensitive to seizure activity. Horses have a relatively high seizure threshold and, unlike in humans and dogs, idiopathic juvenile-onset epilepsy in which no brain lesion can be identified has not been shown to occur. Recurring seizures have been reported in adults, associated with organic lesions, and particularly in foals that may have benign epilepsy that they grow out of.
Seizures have a tendency to occur when the horse is undisturbed, and the first indication of epilepsy in a horse may be unexplained injuries that occur overnight. Any episode that compromises the awareness of an animal as large as a horse must be considered extremely dangerous, and it is strongly recommended that a horse not be ridden unless it has been seizure free without medication for at least 6 months.
Narcolepsy
Narcolepsy is an incurable, non-progressive sleep disorder characterized by striking transitions from wakefulness into rapid eye movement (REM) sleep without passing through slow wave sleep (see Ch. 10). In humans the clinical features include overwhelming episodes of sleep, excessive daytime somnolence, hypnagogic hallucinations, disturbed nocturnal sleep, cataplexy (sudden loss of muscle tone) and sleep paralysis. A syndrome that has the clinical appearance of narcolepsy has been noted in older horses (usually when undisturbed, e.g. in the back of a paddock) and Shetland and miniature horse foals.84 The clinical signs can range from buckling at the knees, usually when the horse is quiet, to total collapse and areflexia with maintenance of some eye and facial responses and normal cardiovascular function.53 The syndrome resolves in some foals. Demonstrating the existence of narcolepsy in horses by classical electroencephalographic techniques may prove to be very difficult, as ongoing work (Colette Williams, personal communication) indicates that horses, unlike humans and dogs, may normally be able to go into REM sleep directly from a period of arousal. This may make eminent sense for a prey species as it would enable the horse to inspect its surroundings before becoming recumbent and losing muscle tone in REM sleep. In humans, some breeds of dogs and miniature horses, an hereditary background has been demonstrated. Canine85 and human86 narcolepsy is associated with dysfunction of a group of neurotransmitters involved in sleep regulation and satiety, the hypocretin system. In narcoleptic foals, narcoleptic episodes are often associated with nursing, and it is intriguing that the hypocretin system is involved in the regulation of sleep as well as appetite. The tricyclic antidepressant imipramine has resolved the signs of narcolepsy in horses for many hours87 but is rarely indicated for long-term management.
Headshaking
Headshaking is a condition in which the horse shakes its head in the absence of obvious external stimuli, and with such frequency and violence that it becomes difficult to ride, or the horse appears to be distressed. Headshaking is most often noted in middle-aged horses during spring or summer and is anecdotally associated with horses asked to use gaits with sustained neck flexion, such as dressage animals. The syndrome appears to be progressive in many cases, with increases in severity and loss of the characteristic seasonality. A great deal of research has been applied to this frustrating condition, but the etiology is far from clear.
Several causes have been suggested, including middle-ear disorders, ear mites, cranial nerve disorders, guttural pouch mycosis, dental periapical osteitis, allergic rhinitis and vasomotor rhinitis; however, only very rarely can it be shown that correction of the abnormality leads to elimination of the headshaking.88 Clinicians in California examined the role of light on headshaking behavior of horses and determined that the condition appeared to be light-stimulated in approximately 60% of the horses. It was suggested that the close association of the optic and trigeminal nerves in the midbrain may allow optic stimulation to cause referred itching, tingling or electric-like sensations in areas innervated by branches of CN V, a condition similar to ‘photic sneezing’ in man.46 The majority of horses with idiopathic headshaking showed some improvement or resolution of signs after oral administration of cyproheptadine.89 This contrasts with studies performed in Great Britain,48 in which a photic etiology could not be established and where cyproheptadine alone was ineffective; 65% of those cases however showed a 90–100% improvement following local anesthesia of the root of the maxillary branch of CN V, and combination therapy of cyproheptadine and carbamazepine resulted in very significant improvement in the majority of cases.
It was concluded that a trigeminal neuritis or neuralgia may be the basis of the underlying etiopathology of idiopathic equine headshaking. The etiology, just as in the human equivalent, is not known. The sodium channel-blocking effects of carbamazepine90 are used to make a diagnosis of trigeminal neuralgia in humans, where similar clinical signs of sporadic or persistent mild to extreme facial pain is associated with the distribution of the trigeminal nerve. In the human condition, ‘trigger factors’ are a consistent feature; this may correspond with the intermittent seasonal nature of the equine disorder. Human trigeminal neuralgia has a much greater prevalence in multiple sclerosis patients,91 and the disease may be associated with demyelination, but so far no direct clinical evidence for this has been established.92 In spite of exhaustive studies on the peripheral portion of the trigeminal nerve, ganglion and sensory trigeminal nucleus, however, no consistent detectable pathology has been identified (Derek Knottenbelt, personal communication 2001).
There are currently no consistently useful therapies, and, although a particular case may respond to single or multiple management or therapeutic measures, the prognosis for cases of idiopathic headshaking is poor. The reported efficacy of the antihistamine hydroxyzine in some headshaking cases93 may be attributed to the fact that rhinitis due to an allergic response may be the trigger factor rather than the specific pathology underlying this disorder. Furthermore, hydroxyzine does possess some sodium channel-blocking effects,94 so it is difficult to establish whether decreased histamine release or sodium blockade is producing the benefit in these isolated individual cases.
There is much research remaining to be done to understand this distressing disorder that regularly results in euthanasia of otherwise healthy horses.
Acknowledgments
The author would like to thank Professor Joe Mayhew for many years of instruction, and Mr Keith Ellis for remarkable tolerance and exceptional photography.
Summary of Key Points
• Major components of the central nervous system are the forebrain (cerebral hemispheres and thalamus), the brainstem (midbrain, pons and medulla oblongata), the cerebellum and the spinal cord.
• The brainstem and thalamic reticular activating system provide arousal and set up attention; the caudal part of the forebrain integrates perceptions, and the frontal cortex initiates voluntary movement and executes plans.
• The parietal lobe comprises primary sensory and motor areas; the occipital lobe is concerned with visual processing; the temporal lobe includes areas devoted to memory and hearing; the hippocampus and the amygdaloid nuclei are involved with memory and emotion; the frontal lobe is concerned with planning and control of movement.
• The limbic system is involved with memory, social and emotional behavior.
• Brain regions that appear to be critical to the formation of memories are the medial temporal lobe and certain thalamic and basal forebrain nuclei.
• Behavior is based on neurons receiving and conveying information using action potentials that trigger the release of neurotransmitters.
• The main neurotransmitter classes are the biogenic amines, amino acids, neuropeptides, nucleotides, prostaglandins and gases.
• Psychopharmacologic agents act by modifying neurotransmitters.
• The neurological examination is carried out to determine whether the change in behavior could be explained by an underlying neurological deficit, and to define the anatomical portion of the nervous system responsible for the clinical signs.
• A number of neurological diseases affecting the forebrain produce changes in behavior.
Case study 1
The author has examined several horses with recent changes in behavior such as becoming ‘hot’, difficult to ride, unwilling to move forward or being prone to frequent and sudden bucking and bolting. A neurological consult was sought, usually because the owner was concerned that the horse may have a ‘brain tumor’. Careful neurological examinations did not reveal changes in mentation or other neurological signs in any of these cases. Several of these horses were euthanized and a postmortem examination further failed to determine morbid neuronal pathology.
Case study 2
An adult Thoroughbred mare known to be ‘difficult’ was brought to a farrier for corrective shoeing. During shoeing she became very belligerent, pushing through handlers, not standing still and acting aggressively. She was dealt with as a ‘difficult’ and ‘badly behaved’ horse by the farriers until, after about one hour of this, the experienced groom realized that the behavior was pathological. She was examined by a veterinary surgeon who found severe icterus. STAT liver enzyme analysis confirmed the diagnosis of acute liver failure and she was euthanized.
(Courtesy of Professor Joe Mayhew.)
References
1. McMillan FD, Rollin BE. The presence of mind: on reunifying the animal mind and body. J Am Vet Med Assoc. 2001;218(11):1723–1727.
2. de Lahunta A. Veterinary neuroanatomy and clinical neurology. Philadelphia: WB Saunders, 1983.
3. Kaplan HI, Sadock BJ. The brain and behavior. Kaplan and Sadock’s synopsis of psychiatry. Baltimore: Williams & Wilkins, 1998.
4. Behan M. Neuroanatomy. Basic science course in veterinary and comparative neurology and neurosurgery, Madison, WI; 1998.
5. Peele TL. The neuroanatomic basis for clinical neurology. New York: McGraw-Hill, 1977.
6. Newman J. Thalamic contributions to attention and consciousness. Conscious Cogn. 1995;4(2):172–193.
7. Van de Poll NE, Van Goozen SH. Hypothalamic involvement in sexuality and hostility: comparative psychological aspects. Prog Brain Res. 1992;93(93):343–361.
8. Luft AR, Skale JM, Stefanou A, et al. Comparing motion- and imagery-related activation in the human cerebellum: a functional MRI study. Hum Brain Mapp. 1998;6(2):105–113.
9. MacKay-Lyons M. Central pattern generation of locomotion: a review of the evidence. Phys Ther. 2002;82(1):69–83.
10. Kandel ER, Schwartz JH, Jessel TM. Principles of neural science. New York: McGraw-Hill, 2000.
11. Walker SF. Lateralization of functions in the vertebrate brain: a review. Br J Psychol. 1980;71(3):329–367.
12. Dodman NH, Miczek KA, Knowles K, et al. Phenobarbital-responsive episodic dyscontrol (rage) in dogs. J Am Vet Med Assoc. 1992;201(10):1580–1583.
13. Feeney DM, Gullotta FP, Gilmore W. Hyposexuality produced by temporal lobe epilepsy in the cat. Epilepsia. 1998;39(2):140–149.
14. Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends Neurosci. 1993;16(8):328–333.
15. Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290(5491):533–536.
16. Rolls ET. Memory systems in the brain. Annu Rev Psychol. 2000;51:599–630.
17. Willingham DB. Systems of memory in the human brain. Neuron. 1997;18:5–8.
18. Brunelli M, Garcia-Gil M, Mozzachiodi R, et al. Neurobiological principles of learning and memory. Archives Italiennes de Biologie. 1997;135(1):15–36.
19. Elliott FA. Propanolol for the control of beligerent behavior following acute brain damage. Ann Neurol. 1977;1:489–491.
20. Peck KE, Hines MT, Mealey KL, Mealey RH. Pharmacokinetics of imipramine in narcoleptic horses. Am J Vet Res. 2001;62(5):783–786.
21. Breier A, Kestler L, Adler C, et al. Dopamine D2 receptor density and personal detachment in healthy subjects. Am J Psychiatry. 1998;155(10):1440–1442.
22. Hector RI. The use of clozapine in the treatment of aggressive schizophrenia. Can J Psychiatry. 1998;43(5):466–472.
23. Galey D, Simon H, Le Moa M. Behavioral effects of lesions in the A10 dopaminergic area of the rat. Brain Res. 1977;124(1):83–97.
24. Johansson AK, Bergvall AH, Hansen S. Behavioral disinhibition following basal forebrain excitotoxin lesions: alcohol consumption, defensive aggression, impulsivity and serotonin levels. Behav Brain Res. 1999;102(1–2):17–29.
25. Dodman NH. Veterinary models of obsessive-compulsive disorder. In: Jenicke MA, Baer L, Minichiello WA. Obsessive-compulsive disorders: practical management. St Louis, MO: Mosby; 1998:318–334.
26. Rendon RA, Shuster L, Dodman NH. The effect of the NMDA receptor blocker, dextromethorphan, on cribbing in horses. Pharmacol Biochem Behav. 2001;68(1):49–51.
27. Bagshaw CS, Ralston SL, Fisher H. Behavioral and physiological effect of orally administered tryptophan on horses subjected to acute isolation stress. Appl Anim Behav Sci. 1994;40(1):1–12.
28. Lee R, Coccaro E. The neuropsychopharmacology of criminality and aggression. Can J Psychiatry. 2001;46(1):35–44.
29. Overall KL. Self-injurious behavior and obsessive-compulsive disorder in domestic animals. In: Dodman NH, Shuster L. Psychopharmacology of animal behavior disorders. Malden, MA: Blackwell Science, 1998.
30. Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290(5500):2315–2319.
31. Hahn CN, Mayhew IG. Equine neurodegenerative diseases – stressed neurons and other radical ideas. Vet J. 1997;154(3):173–174.
32. Divers TJ, Mohammed HO, Cummings JF, et al. Equine motor neuron disease: findings in 28 horses and proposal of a pathophysiological mechanism for the disease. Equine Vet J. 1994;26(5):409–415.
33. Cooper JR, Bloom FE, Rothe RH. Neuroactive peptides. The biochemical basis of neuropharmacology. Oxford: Oxford University Press, 1996. 410–458
34. Lagerweij E, Nelis PC, Wiegant VM, van Ree JM. The twitch in horses: a variant of acupuncture. Science. 1984;225(4667):1172–1174.
35. Marsden MD. Behavioural problems. In: Collahan PT, Mayhew IG, Merritt AM, Moore JN, eds. Equine medicine and surgery, vol 1. St Louis, MO: Mosby; 1999:914–931.
36. Muir WW. Standing chemical restraint in horses. In: Muir WW, Hubbell JAE. Equine anaesthesia: monitoring and emergency therapy. London: Mosby Year Book; 1991:247–266.
37. Booth NH, McDonald LE. Veterinary pharmacology and therapeutics. Ames: The Iowa State University Press, 1982.
38. Hedrick MS, Ryan ML, Bisgard GE. Recurrent laryngeal nerve activation by alpha 2 adrenergic agonists in goats. Respir Physiol. 1995;101:129–137.
39. Hubbell JAE, Muir WW. Standing chemical restraint. In: Reed SM, Bayly WW. Equine internal medicine. Baltimore: WB Saunders; 1998:188–189.
40. Birnir B, Eghbali M, Cox GB, Gage PW. GABA concentration sets the conductance of delayed GABAA channels in outside-out patches from rat hippocampal neurons. J Membr Biol. 2001;181(3):171–183.
41. Tobin T. A review of the pharmacology of reserpine in the horse. J Eq Med Surg. 1978;2(10):433–438.
42. CPS. Compendium of pharmaceuticals and specialties. Canadian Pharmaceutical Association, 1997.
43. Dodman NHD, Shuster R, Mertens L, et al. Use of fluoxetine to treat dominance aggression in dogs. J Am Vet Med Assoc. 1996;209(9):1585–1587.
44. Romatowski J. Two cases of fluoxetine-responsive behavior disorders in cats. Feline Pract. 1998;26(1):14–15.
45. Fey K, Jonigkeit E, Moritz A. Zum equinen Cushing-Syndrom (ECS). Fallbericht, Literaturauswertung zu Diagnostik und Therapie sowie wesentliche Unterschiede zum Cushing-Syndrome des Hundes. Tierarztliche Praxis. Ausgabe G, GrossTiere Nutztiere. 1998;26(1):41–47.
46. Madigan JE, Kortz G, Murphy C, Rodger L. Photic headshaking in the horse: 7 cases. Equine Vet J Suppl. 1995;27(4):306–311.
47. Dailey JW, Reith ME, Steidley KR, et al. Carbamazepine-induced release of serotonin from rat hippocampus in vitro. Epilepsia. 1998;39(10):1054–1063.
48. Newton SA, Knottenbelt DC, Eldridge PR. Headshaking in horses: possible aetiopathogenesis suggested by the results of diagnostic tests and several treatment regimes used in 20 cases. Equine Vet J Suppl. 2000;32(3):208–216.
49. Pellock JM. Carbamazepine side effects in children and adults. Epilepsia. 1987;28(suppl 3):S64–70.
50. Herranz JL, Armijo JA, Arteaga R. Clinical side effects of phenobarbital, primidone, phenytoin, carbamazepine, and valproate during monotherapy in children. Epilepsia. 1988;29(6):794–804.
51. Langbehn DR, Alexander B. Increased risk of side-effects in psychiatric patients treated with clozapine and carbamazepine: a reanalysis. Pharmacopsychiatry. 2000;33(5):196.
52. Sanders RD, Keshavan MS. The neurologic examination in adult psychiatry: from soft signs to hard science. J Neuropsychiatry Clin Neurosci. 1998;10(4):395–404.
53. Mayhew IG. Large animal neurology: a handbook for veterinary clinicians. Philadelphia: Lea & Febiger, 1989.
54. Hanggi EB. Interocular transfer of learning in horses (Equus caballus). J Equine Vet Sci. 1999;19(8):518–524.
55. Westby GW, Keay KA, Redgrave P, et al. Output pathways from the rat superior colliculus mediating approach and avoidance have different sensory properties. Exp Brain Res. 1990;81(3):626–638.
56. King SM, Dykeman C, Redgrave P, Dean P. Use of a distracting task to obtain defensive head movements to looming visual stimuli by human adults in a laboratory setting. Perception. 1992;21(2):245–259.
57. King SM, Cowey A. Defensive responses to looming visual stimuli in monkeys with unilateral striate cortex ablation. Neuropsychologia. 1992;30(11):1017–1024.
58. Summers BA, Cummings JF, de Lahunta A. Veterinary neuropathology. St Louis, MO: Mosby, 1995.
59. Hahn CN, Mayhew IG, MacKay RJ. The nervous system. In: Colahan PT, Mayhew IG, Merritt AM, Moore JN. Equine medicine and surgery. St Louis, MO: Mosby; 1999:863–995.
60. Jackson CA, deLahunta A, Dykes NL, Divers TJ. Neurological manifestation of cholesterinic granulomas in three horses. Vet Rec. 1994;135(10):228–230.
61. Kimman TG, Binkhorst GJ, van den Ingh TS, et al. Aujeszky’s disease in horses fulfils Koch’s postulates. Vet Rec. 1991;128(5):103–106.
62. Richt JA, Grabner A, Herzog S. Borna disease in horses. Vet Clin North Am Equine Pract. 2000;16(3):579–595.
63. Selvey LA, Wells RM, McCormack JG, et al. Infection of humans and horses by a newly described morbillivirus. Med J Austr. 1995;162(12):642–645.
64. Cantile C, Di Guardo G, Eleni C, Arispici M. Clinical and neuropathological features of West Nile virus equine encephalomyelitis in Italy. Equine Vet J. 2000;32(1):31–35.
65. Joyce JR, Russel LH. Clinical signs of rabies in horses. Compend Contin Ed. 1981;3:56–61.
66. Hudson LC, Weinstock D, Jordan T, Bold-Fletcher NO. Clinical presentation of experimentally induced rabies in horses. Zentralbl Veterinarmed. 1996;43(5):277–285.
67. Johnson RT. Selective vulnerability of neural cells to viral infections. Brain. 1980;103(3):447–472.
68. Hahn CN, Mayhew IG, Whitwell KE, et al. A possible case of Lyme borreliosis in a horse in the UK. Equine Vet J. 1996;28(1):84–88.
69. MacKay RJ. Equine protozoal myeloencephalitis. Vet Clin North Am Equine Pract. 1997;13(1):79–96.
70. Sewell MMH, Brocklesby DW. Animal diseases in the tropics. London: Baillière Tindall, 1990.
71. Hillyer MH, Holt PE, Barr FJ, et al. Clinical signs and radiographic diagnosis of a portosystemic shunt in a foal. Vet Rec. 1993;132(18):457–460.
72. Rose RJ, Hodgson DR. Manual of equine practice. Sydney: WB Saunders, 1993.
73. Savage JC. Diseases of the liver. In: Colahan PT, Mayhew IG, Merritt AM, Moore JN. Equine medicine and surgery. St Louis, MO: Mosby; 1999:816–833.
74. Barton MH, Morris DD. Diseases of the liver. In: Reed SM, Bayly WW. Equine internal medicine. Baltimore: WB Saunders; 1998:707–738.
75. Jones EA. Pathogenesis of hepatic encephalopathy. Clin Liver Dis. 2000;4(2):467–485.
76. Albrecht J, Jones EA. Hepatic encephalopathy: molecular mechanisms underlying the clinical syndrome. J Neurol Sci. 1999;170(2):138–146.
77. Matsumoto K, Graf R, Rosner G, et al. Elevation of neuroactive substances in the cortex of cats during prolonged focal ischemia. J Cereb Blood Flow Metab. 1993;13(4):586–594.
78. Auer RN, Siesjo BK. Biological differences between ischemia, hypoglycemia, and epilepsy. Ann Neurol. 1988;24(6):699–707.
79. Rossdale PD. Clinical view of disturbances in equine foetal maturation. Equine Vet J Suppl. 1993;14:3–7.
80. Palmer AC, Rossdale PD. Neuropathological changes associated with the neonatal maladjustment syndrome in the thoroughbred foal. Res Vet Sci. 1976;20(3):267–275.
81. Thiel PG. Levels of fumonisins B1 and B2 in feeds associated with confirmed cases of equine leukoencephalomalacia. J Agric Food Chem. 1991;39:109–111.
82. Wilkins PA. A herd outbreak of equine leukoencephalomalacia. Cornell Veterinarian. 1994;84:53–59.
83. Dodman NH, Knowles KE, Shuster L, et al. Behavioral changes associated with suspected complex partial seizures in bull terriers. J Am Vet Med Assoc. 1996;208(5):688–691.
84. Lunn DP, Cuddon PA, Shaftoe S, Archer RM. Familial occurrence of narcolepsy in miniature horses. Equine Vet J. 1993;25(6):483–487.
85. Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98(3):365–376.
86. Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27(3):469–474.
87. Sweeney CR, Hendricks JC, Beech J, Morrison AR. Narcolepsy in a horse. J Am Vet Med Assoc. 1983;183(1):126–128.
88. Lane JG, Mair TS. Observations on headshaking in the horse. Equine Vet J Suppl. 1987;19(4):331–336.
89. Madigan JE. Evaluation and treatment of headshaking syndrome. International Congress on Equine Clinical Behaviour 1996, Basel, Switzerland.
90. Rizzo MA. Successful treatment of painful traumatic mononeuropathy with carbamazepine: insights into a possible molecular pain mechanism. J Neurol Sci. 1997;152(1):103–106.
91. Katusic S, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Ann Neurol. 1990;27(1):89–95.
92. Newton SA. The functional anatomy of the trigeminal nerve of the horse. PhD Thesis, Liverpool University, 2001.
93. McGorum BC, Dixon PM. Vasomotor rhinitis with headshaking in a pony. Equine Vet J. 1990;22(3):220–222.
94. Martindale: the extra pharmacopoeia. London: Pharmaceutical Press; 1972–1996.