Chapter 10 Body care
Chapter contents
Grooming
In addition to maintaining integument health, grooming behaviors can contribute to affirming social bonds not least by reinforcing affiliations and sharing odors. Evidence that body odors prompt horses to undertake longer investigative sniffing than either urine or feces1 should remind us that horses rely chiefly on scent to recognize familiar conspecifics once they are close.
Mutual grooming
Mutual grooming not only allows horses to reach areas that defy self-grooming strategies, but also facilitates exchange of odors2 and has been shown to reduce heart rate when conducted in certain parts of the mane and withers.3 Grooming of withers by humans has a similar calming effect.4 The effects on cardiac function are particularly striking in foals, with a mean reduction of 14% in heart rate being reported when preferred areas were scratched by humans.3 This phenomenon is regularly harnessed by personnel who want to reward horses of any age without using food.
Mutual grooming can begin in the first week of life5,6 but peaks in the second and third months of life,7 a period during which foals seem to find physical contact intensely gratifying. In the first instance the foal’s mutual grooming partner is its dam, who may dismiss other grooming partners if her foal solicits her attention by attempting to allogroom.5 Although some mature horses never allogroom, most regularly indulge in reciprocated activity of this sort with favored affiliates for periods of about three minutes at a time.8 Broadly speaking, regardless of their age, females spend more time mutually grooming than males. Mutual grooming partners are usually preferred associates that are close in social rank.9 In a natal band, mares and their offspring mutually groom kin rather than unrelated conspecifics.10 The absence of a stallion seems to bring with it some liberation in that grooming partners are preferentially from same sex-age groups.11 The stallions in multi-stallion harems will mutually groom one another.12
Mutual grooming often starts at the cranial neck and proceeds to the withers (Fig. 10.1), the shoulders and then to the tail-head, sometimes changing sides (see Fig. 2.15).13 While youngsters often attempt to begin allogrooming, such overtures are rarely reciprocated by unrelated adults.5 As discussed in Chapter 5, there is equivocal evidence for the role of rank when a bout of mutual grooming begins.5,11 Reports indicate that low-ranking individuals groom more and initiate more groomings and that allogrooming may have a role in appeasement.12 However, most bouts are ended by the departure of the higher-ranking member of the dyad.5 Mutual grooming between foals is often observed before or after a bout of play.14

Figure 10.1 Mutual grooming helps equids to develop and maintain pair bonds.
(Photograph courtesy of Sandra Hannan.)
The frequency of mutual grooming is subject to daily and seasonal variation, with predictable troughs that coincide with recumbency.15 Shedding the winter coat and the seasonal peak in idling behavior account for annual peaks in mutual grooming (i.e. April and July in northern hemisphere studies5). When allowed to interact freely with other horses after 9 months of social deprivation, colts stabled singly showed significantly more social grooming than colts stabled in groups, which may reflect a build-up of motivation (a post-inhibitory rebound effect).16
Self-grooming
Self-grooming brings out a great deal of resourcefulness in horses as they use their hooves, their mouths and objects in their environment to relieve irritation (Fig. 10.2). The frequency of self-grooming peaks at 12.3 times per hour in weeks 5–8 of a foal’s life.17 The relative proportion of body parts and the innate flexibility of foals both help to explain their relatively enhanced ability to self-groom and may account for the decline in frequency of self-grooming to 1.2–2.2 times per hour in adults.18 While their dams focus self-grooming on rolling and rubbing against the forelimbs, conspecifics or inanimate objects, foals are more frequently found scratching anteriorly with their hindlimbs or nibbling posteriorly.17
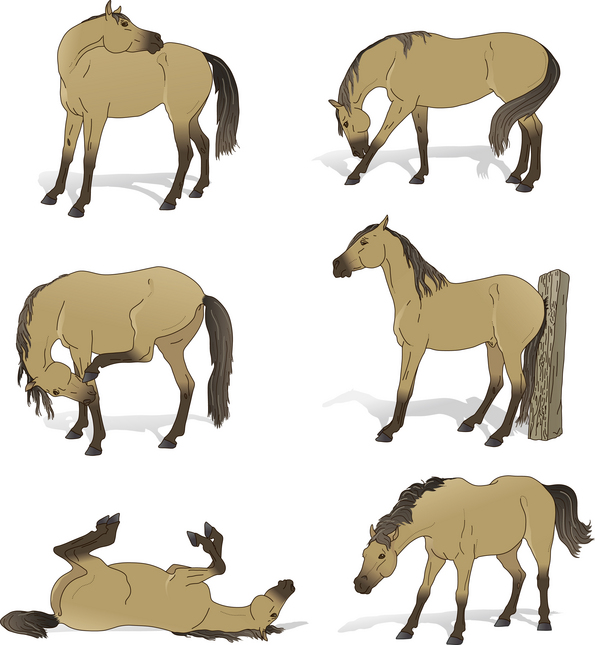
Figure 10.2 Grooming styles used by horses include rolling, shaking, rubbing, scratching and nibbling (of both the back and forelegs).
(Redrawn, with permission, from Waring.13)
Along with appetite and growth, pelage cycles are related to the duration of the photoperiod.18 Therefore the amount of self-grooming peaks with the shedding of the winter coat.
Scratching by humans can act as a highly valuable primary reinforcer, so an appreciation of the pleasure that grooming may bring can help to consolidate the horse–human bond. However, grooming by humans must take account of the variable sensitivity of different individuals and breeds.2 The need to have a clean horse has been overstated by some horse-care manuals. Further, the perceived need to pull manes and tails undoubtedly irritates many horses and possibly compromises the horse–handler bond.
Rolling
Because preferred substrates are sand, fine dry soil and occasionally mud, horses at pasture tend to select bare patches on which to roll, often beside gates or water troughs.19 Some feral horses have been observed to roll preferentially in water.8 With more than 80% of rolling occurring where another horse has rolled,2 its function is believed to include the opportunity to deposit scent over the body. The fact that horses, especially males,20 smell chosen sites before and after rolling appears to support this view.
The head and neck help to propel the horse laterally while rolling (Fig. 10.3).13 Similarly, lateral flexion of the vertebral column and thrashing of hindlegs seems to help the horse to balance itself on the dorsal midline.13 Rolling horses usually return to the same side they started lying on but often repeat the roll before rising.13

Figure 10.3 (A) Sequence showing horse rolling. (B) After a bath horses seem more itchy than normal and enjoy rolling, especially in dusty substrates.
((A) Redrawn, with permission, from Waring.13)
The physical environment can affect horses’ inclination to get down onto the ground. For example, there is evidence that small boxes inhibit sternal recumbency.21 Apart from some foaling boxes, the space on the floor of most stables and many custom-built sand rolls is less than the width of most natural rolling sites. This helps to explain why we should be pleased that more horses do not get cast (stuck against the walls of stables or upside down in their stables). The use of anti-casting rails is a measure believed to help inverted horses to right themselves when stuck against the side of a stable. These rails are set about one meter from the floor, although their height should, strictly speaking, depend on that of the occupant. They work by providing some purchase against which the horse can push in a bid to move itself away from the wall.
Bedding materials such as straw that lend themselves to being banked up against the side of the stable wall are likely to reduce the risk of casting. Whatever bedding is selected must be managed carefully to maintain air hygiene. Beds that drain readily are less likely to be accompanied by high ammonia concentrations or allow humidity to rise to concentrations that increase the viability of fungal spores implicated in lower-airway disease.22 Bedding preference studies suggest that the appeal of straw depends on its being deep and is linked to its alternative use as a source of dietary fiber.23,24
Shaking
Shaking is frequently seen after untacking, rolling and recumbency. It involves coordinated contraction of the superficial musculature that travels caudally from the head leading to vibration and rotation of the skin on the body. The neck is lowered prior to shaking,13 and a wide placement of the forelegs is assumed. Males and non-estrous females often shake after urinating.13
Head and neck involvement is variable. Nodding and shaking the head can send the mane in different directions in a bid to dislodge insects. Before rectal palpation became established as a means of pregnancy diagnosis, folklore maintained that if water was squirted into their ears, mares would not shake their heads if pregnant.
Rubbing
Rubbing can involve fixed objects or the muzzle.13 The latter is used to reach places, such as the barrel and forelegs, that are hard to rub against fixed objects. Foals may rub target areas, such as the head, neck, croup and buttocks, on objects for as long as 15 minutes at a time.13 Less frequently horses are observed using low branches to rub their backs and may sometimes straddle vegetation on which they rub their ventral surface by walking forwards and backwards.13 Rubbing the vulva, tail and buttocks has been recorded in mares,13 with accompanying signs of pleasure, such as lip quivering, suggestive of masturbation.5
Scratching
After turning its head and neck to the rear, a horse can use its hindlimbs to scratch parts of the cranial and anterior cervical region. More common in foals than in adults,17 this response is thought to be retained by more ponies than horses.13 Indeed, some lice-ridden ponies may go on to insert a pastern into the mouth and proceed to mumble on the limb.25
Nibbling and licking
The use of teeth in body care varies from a rhythmic scratching action by the upper incisors, to small bites. While the areas that can be reached by the teeth include the sides and loins (see self-mutilation in Ch. 11) and even the hindlegs (Fig. 10.4), the forelegs seem to receive especially detailed attention that incorporates considerable licking. This is a means of removing bot eggs from the hairs that effectively facilitates their ingestion. Although licking seldom occurs as part of mutual grooming,13 the licking of a neonate by its dam is probably the most important instance of a horse using its tongue during grooming a conspecific. This can last for 30 minutes and, as well as consolidating the mother–infant bond, it provides tactile stimuli that may have a role in stimulating responses such as standing. Licking by a stallion as part of courtship is a means of tasting rather than grooming.
Pest avoidance
Flies bite and spread diseases, such as conjunctival infections, and proteins in the saliva of Culicoides spp. are the stimuli responsible for the often grotesque self-mutilation of sweet itch (Queensland itch, summer itch, summer eczema). Apart from ill-advised excoriation, horses have developed adaptive behavioral responses to combat pests. For example, in foals, the percutaneous invasion of the third-stage larvae of Strongyloides westeri is reported to cause repeated episodes of frenzied behavior lasting about 30 minutes.26 The energy expended in motor responses such as tail-swishing to reduce irritation of insect origin may be considerable, and movement to areas (such as cooler parts of the home range) that provide respite may therefore be seen as forms of energy conservation.27 Tabanid flies drive free-ranging Camargue horses to change their time-budgets and use of their home ranges, apparently in a bid to capitalize on the prevailing winds in key areas.28 Similarly, Assateague ponies have been noted to use inshore water and snow patches as refuges from tabanids.27 For horses, insects are not the only airborne pest. Avoidance measures have also been demonstrated in response to the vocalization of vampire bats.29
With pliable, mobile skin and extensive subcutaneous muscles, horses are among the best skin-twitching animals. The sensitivity of horse skin, for example, to alighting flies as they stimulate the panniculus response, should serve to remind riders how little pressure is needed for signaling and how heavy whip use can compromise welfare (Fig. 10.5). Riding instructors who find themselves telling their pupils to kick their mounts should consider the lesson they are providing for both rider and horse. While stamping, kicking and shaking of the whole body or head are all employed to dislodge insects, the forelock and the tail can act both as screens and swats in combating fly problems. The effectiveness of mutual tail-swishing as a means of reducing the harm caused by flies is reflected by there being demonstrably fewer bites per horse in animals that live in large groups.30 Horses frequently swing their heads in the direction of flies on their flanks in a motor pattern similar to an agonistic head threat that occasionally includes a consummative nip at their own skin.13 Along with pawing, tail-swishing increases with ambient temperature in horses while being prepared for work.31 Since this effect is not a function of fly numbers, it has been suggested that such comfort behaviors may be useful indicators of irritation in horses, especially where ambient temperatures are high.31

Figure 10.5 Heavy whip use can compromise welfare, especially in sensitive parts of the body such as the abdominal wall. Here you can see the whip has disappeared into an indentation it has made in the skin of the flank. Such indentations and flank strikes occur with troubling frequency.
(Reproduced with permission of Liss Ralston.)
The use of other horses’ tails in helping to reduce the frustration of flies on the face is established in foalhood as foals frequently rest within the shelter of their dams’ swatting tails.7 The solitary housing of horses denies them this facility and should oblige owners to provide such animals with some form of screen. The importance of the tail in body care is implied by reports of horses with docked tails occasionally using sticks to scratch their sides.32 Veterinarians who prioritize the welfare of animals in their care recognize the function of the tail as a source of comfort and therefore do not dock horses for cosmetic reasons.
Behavioral thermoregulation
Horses often surprise owners with their reluctance to use field shelters. This rejection of enclosed space relates to their dislike of confinement and reduced surveillance and visual contact with conspecifics. That said, horses are often seen using shaded areas, natural windbreaks, sun-baking and occasionally wading in water to regulate their body temperature. Evaporative heat losses are thought to be increased by the sprinkling effect of tail-swishing while standing in water33 and by the occasional instances of nose-dunking in water (Fig. 10.6). Thermoregulation is often made difficult by rugs, especially in warm weather when rugs are sometimes used to prevent sun-bleaching. Discomfort caused by over-heating, skin disorders and generally badly fitted rugs may prompt some horses to become rug chewers. Other horses, especially youngsters, may adopt this response as a form of object play.
Rest and sleep
Fraser34 identifies four resting states: idling, resting, drowsing and sleeping. He also gives a detailed account of pandiculation (stretching) elsewhere.34
Idling is adopted as a passive waiting style between more animated activities and involves stationary standing with some limb-shifting and positional changes. It can occur as a group activity, in contrast to recumbent sleep, which is rarely undertaken by all the members of a group at one time.35 Seasonal peaks in resting behaviors occur in the summer5 when forage is relatively plentiful and there is pressure to seek shade and avoid insects. Common sites are usually selected for lying, a behavioral mechanism thought to help produce a group odor.2 Elevated sites are often selected since they provide refuge from heat and flies.36 Resting occurs in the standing or recumbent position and in the wakeful periods between sleep and drowsing. Generally, light horses spend less time standing to rest than draft types.37
Drowsing
A drowsing horse stands with its eyelids partly opened and its head hanging at a medium height (Fig. 10.7). The flexion of a single hindlimb is facilitated by the reciprocal apparatus in the hindlimb, which means that movements of the stifle joint drive those of the hock.38 Thus, once the stifle is fixed in position, the hock is obliged to follow suit. In combination with the stay apparatus found in the forelimb as a means of supporting the fetlock, this allows the horse to remain upright with a minimum of muscular effort. The advantages of remaining standing include being able to achieve a quick escape if threatened, but also to avoid cardiorespiratory compromise that comes with recumbency. Interestingly, adopting certain postures for rest has been shown to have familial tendencies.39
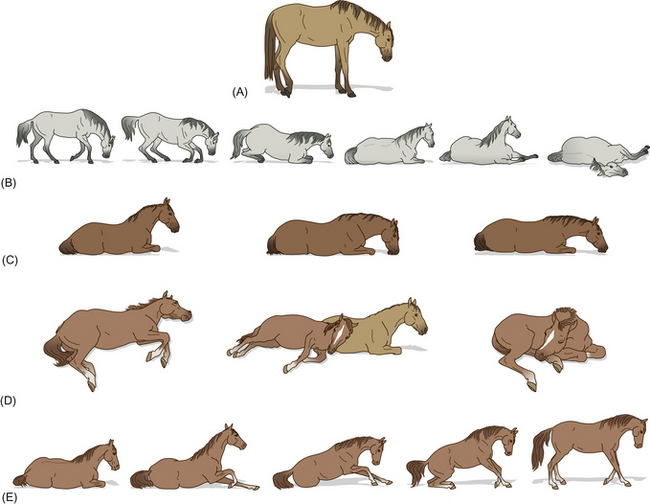
Figure 10.7 A horse’s posture changes in various states of activation: (A) resting with weight distributed among only three legs; (B) lying down; (C and D) recumbent resting attitudes; (E) arising.
(Redrawn, with permission, from Waring.13)
Sleep
The distinction between drowsing and sleep necessitates understanding the different physiological characteristics of various states of wakefulness. In adult horses, sleep occupies 3–5 hours per day, while drowsing consumes a further 2 hours.40 Postural changes tend to accompany certain states – for example, although the eyes remain partly open, the head gradually descends as the horse goes from drowsing to sleep.36 Animals may rest without sleeping and while the distinction between drowsiness and sleep is reasonably subtle, since they may appear very similar externally, electrophysiological studies of animals in various stages of sensitivity to their environment have helped to unpick the differences. Although such measurements require subjects to be restrained, we can assume that the same differences apply in unrestrained horses. Using data extrapolated from restrained subjects, inferences can be made from the posture of horses indulging in various rest behaviors.
The two types of sleep clearly defined in horses are slow-wave sleep (SWS) and rapid-eye-movement (REM) sleep (also known as deep or paradoxical sleep).40 The electroencephalogram (EEG) waves of SWS, as the name suggests, are characterized by a low frequency, a feature that disappears as the horse drifts into REM sleep. Some muscular tone is retained in SWS, even when the head is resting on the ground during sternal recumbency. It is the only form of sleep that occurs in standing horses. Although it may increase in horses that are unable or reluctant to lie down, SWS cannot compensate for lost REM. This is illustrated by post-inhibitory rebound in REM sleep after periods without recumbency.40 Along with the suggestion that horses do not lie down unless they are confident about their surroundings,35,40 this accounts for some of the concerns for the welfare of horses that do not or cannot lie down.41
REM sleep was dubbed paradoxical because, while its EEG is similar to that of wakefulness (Fig. 10.8A), it has the highest arousal threshold. Cardiac and respiratory rates decrease in all forms of sleep.42 REM sleep is peculiar in that it involves the absence of postural tone (and therefore the eyes are shut). It occurs entirely during recumbency and is marked by the greatest degree of variability in cardiac and respiratory frequencies of any state, including wakefulness. As its name implies, REM sleep is also often accompanied by rapid eye movements. In REM sleep during lateral recumbency, limb movements, especially of the upper limbs, may occur.13
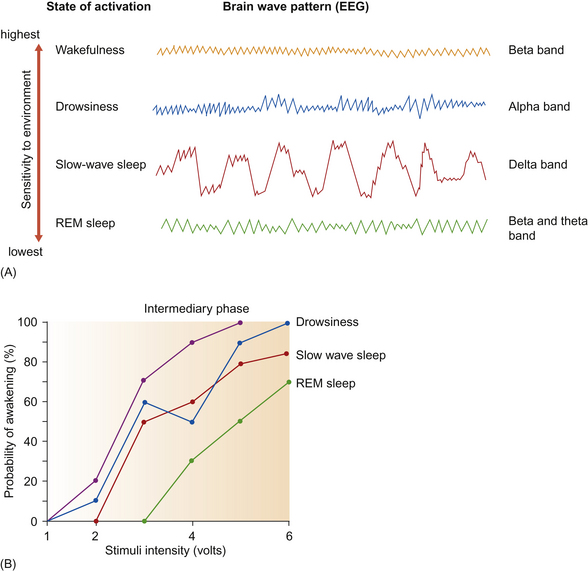
Figure 10.8 (A) In various states of activation in mammals. (B) Variations in arousal threshold from drowsiness to REM sleep.
(After Dallaire.40)
In studies of adult horses, REM sleep has not been observed to occur without a preceding period of SWS, and this gives rise to the term ‘sleep cycle’. Horses, like most non-primates, are polyphasic sleepers in that they sleep in more than one phase throughout the 24-hour period, with one or more sleep cycles occurring in each phase. During the night, horses have about six sleep phases within which the mean duration of sleep cycles is 15 minutes and the mean duration of SWS and REM sleep they contain are 6.4 and 4.2 minutes, respectively.40 The balance comprises drowsing within a sleep cycle and a third type of sleep labeled the intermediary phase.40 Although it has an EEG pattern similar to that of drowsiness, intermediary sleep is characterized by an arousal threshold closer to that of wakefulness than that of drowsing (Fig. 10.8B).40 Found in almost 30% of sleep cycles, the intermediary phase may provide a protective punctuation of sleep that helps to prompt extra surveillance before deep sleep.40
Time spent lying increases in horses when they are exercised,43 and good deep bedding may therefore help to keep performance horses in peak condition by facilitating adequate rest. While the mean time spent in continuous sternal recumbency is 23 minutes,35 twice as much time is generally spent in lateral recumbency. In sternal recumbency horses lie asymmetrically with their hindquarters rotated so that the lateral aspect of the lower limb is on the ground. Rising from this position, once the upper foreleg has been extended, is achieved chiefly by a thrust from the hindquarters (Fig. 10.9).35 In lateral recumbency the importance of the upper limb in rising quickly is demonstrable in that it is almost always anterior to the lower limb, which is usually flexed to some extent.35 As with rolling, it seems probable that horses demonstrate laterality in their preferred side for recumbency.

Figure 10.9 The main muscular effort used in standing after a period of recumbency comes from the hindquarters.
(Photograph courtesy of Francis Burton.)
The timing of sleep is important, since management schedules sometimes interfere with innate rhythms that facilitate sleep. Although most sleep occurs between 2000 and 0500 hours, horses also often sleep in the first 2 hours after midday.40 It is therefore good practice to keep activity in a stable yard to a minimum during the early afternoon.35
The restorative functions of sleep have been compartmentalized by Dallaire,40 who proposes that during SWS sleep the brain is resting, whereas during REM sleep the absence of muscle tone suggests that the muscles are resting. Fraser35 proposes that during REM sleep the brain consolidates long-term memory traces and the forebrain is functionally disconnected so that the brainstem must be responsible for any accompanying limb movements.
The effect of environment
While domestic horses tend to rest in sheltered areas, Przewalski horses when released into extensive enclosures have been noted to rest in the highest parts of the available terrain.44
The floor area of a stable can influence the maintenance behavior of horses within it. The duration of sternal recumbency was significantly longer in large boxes (2.5 × height of the horse squared) than in the small boxes (1.5 × height of the horse squared). Interestingly, horses at pasture show slightly less total sleep and more drowsiness than those kept in separate stalls (Fig. 10.10).40 Drowsing at pasture studied in a small number of ponies occurred only while they were standing. These data may suggest that the external environment may require the deployment of more surveillance than the stable. However, a similar trend toward an increase in the proportion of SWS is reported in sensory-deprivation studies on ponies, which implies that a barren environment may be sufficient to modify sleep patterns, regardless of any concurrent perception of security.45
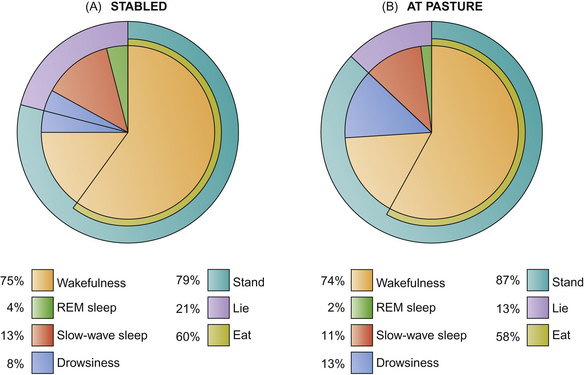
Figure 10.10 The distribution of the four states of activation change according to whether the horse is stabled individually (n = 5) or kept at pasture (n = 2).
The weather may also influence rest behavior. For example, in especially hot weather, perhaps because of the prevalence of flies and the resultant need for mutual tail-swishing, horses may rest only in the standing position.46 The standing position is also favored for rest during the colder months of the year,47 perhaps because it involves less heat loss through convection. Daylength, via the action of serotonin and thus melatonin, can influence sleep patterns, and it is reported that tryptophan, a precursor of serotonin, may be used clinically to help sleep.35
The effect of rank and group size
Resting postures are subject to individual preferences.40 However, the standing position is adopted for rest preferentially by adult mares more than males or youngsters.47 Among adults, the highest-ranking member of a band is often the first to lie down.48 Furthermore, it is believed that the effects of a novel environment in making horses that are unfamiliar with an enclosure reluctant to lie down may be diluted if a resident horse is present to offer an example.40 There are data suggesting that as group size rises within a population, time spent resting in the standing position increases at the expense of time spent in recumbency (Fig. 10.11).47
The effect of age
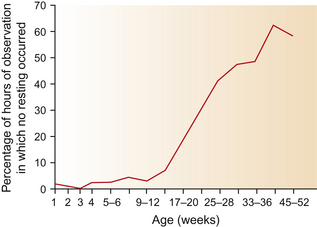
Compared with adults, foals have a greater need for sleep and are less capricious about recumbency, so they often rest in groups.35 Both SWS and REM sleep occur in foals, but their proportions differ.7 With their mothers nearby, newborn foals spend about a third of their time recumbent. When they lie in lateral recumbency, they enter REM sleep and their limbs may move as if running.7 It may be that learning is consolidated during these periods.49 It has been suggested that because it is associated with a reluctance to lie down and therefore may jeopardize sleep patterns and possibly even learning, extended episodes of road transit could be highly undesirable for foals.7
Interesting differences between colts and fillies in the amount and characteristics of recumbent rest in the first week of life are emerging. Fillies have been noted spending 42% of their time in recumbent rest while for colts this is only 20%. Furthermore colts rested upright more and stood more (20% and 28%) than fillies (4% and 23%, respectively) (Machteld van Dierendonck, personal communication 2002). After a peak of the daily time budget spent in recumbency that occurs in the first week of life,50 the need for rest declines as a foal matures, with a striking shift occurring at about 3 months of age (Fig. 10.12).
At this time, foals generally become more social with their conspecifics and especially their peers, if any are present.5 Because of their attachment to their dams, who must graze for extended periods to meet the nutritional demands of lactation, foals do most of their resting in lawn areas.19 They are more likely to rest either upright or recumbent if the dam is resting upright.50 Foals spend only 3.5% of their time resting upright in the first week of life but, as time spent recumbent falls, this steadily increases to a peak of 23% in week 13.50 Meanwhile, nursing mares reach a peak of 32.5% of their time resting upright in week 13 post-partum.50
Play
Activities involving a sense of pleasure and elements of surprise but apparently no immediate function are broadly characterized as play.51 The benefits of play (apparent or otherwise) must outweigh the costs in terms of energy and associated risks.52 One of the immediate rewards of play may well be pleasure, but this is difficult to study. Often initiated by grooming bouts and oral snapping actions, play may establish or strengthen social bonds within a pair or group.53 The value of play is illustrated by the way in which bachelor groups can draw colt foals towards them, with the opportunity to play appearing to be the chief attractant.
Most of the galloping and fast twists and turns used in anti-predator behavior are incorporated into play activities by foals. Sexually dimorphic play-fighting preferences reflect adult agonistic responses, with fillies tending to fight by kicking with their hindlegs as a mare does, while colts prefer to rear, bite and chase each other.12 By practicing these actions before they are required in a serious situation, the foal develops its neuromuscular pathways, the activity becomes learned and so may be executed when needed with ease or flair. Play promotes and regulates developmental rates and therefore contributes to the development of physical strength, endurance and skill.53
Fraser35 argues that the urge to play is derived from a basic need to have optimal blood flow to the muscles. This may provide a mechanism to explain post-inhibitory rebound in locomotory responses after confinement16,40 and why rain can prompt locomotory activity, especially in youngsters. Other than its physiological functions, play may be a form of training that offers problem-solving opportunities and experience that can yield specific information. This is likely to give young animals tactical and behavioral flexibility to fight, find mates and escape predators as adults.52 Play in adult horses, which usually takes the form of object or locomotory play,7 may be a luxury afforded by domestic circumstances and occur as evidence of post-inhibitory rebound or a response to lack of appropriate stimulation from conspecifics at other times.
Play is greatly reduced during periods of extreme ambient temperatures, food scarcity, and other occasions of physiological or psychological stress.13 While animals that have not had their basic food and water requirements met do not play, a low play drive can also indicate ill health or poor nutrition. The range of play behaviors may also be limited by a dearth of playmates.
Prospective studies may demonstrate links between features of play and neuromuscular development, social rank and competitive performance. Rees54 suggests that a horse’s personality as manifested by its mannerisms during play, may be an indication of what to expect when the same horse is under-saddle, especially when presented with an unfamiliar or unsettling situation. Indeed, Fraser35 pursues this idea by advocating that we examine horses’ personalities before assigning their work, or that we even attempt to create play responses in work situations because, he proposes, this will maximize the horse’s willingness to complete the task. For example, a horse that often displays flamboyant paces when playing may experience a play response to the challenges of dressage.35 This is supported by studies of play responses of 698 horses (representing 16 breeds and 13 types) that showed type, rather than breed or sex, was the major influence on free operant responses.55
Playfulness may affect the human–horse bond. For example, Fraser35 endeavored to explain most equestrian pursuits in terms of game theory,56 suggesting that horses learned rules of games when they were being schooled. However, the validity of this approach has been questioned by those who propose that equestrian activities such as jumping easily avoided obstacles or moving repeatedly in circles must appear rather pointless to the equids involved.54,57
Play can be considered under three broad divisions: locomotory play, interactive play and manipulative play.
Locomotory play
Locomotory play begins within a few hours after birth and takes the form of exaggerated or vigorous physical activity, with the foal moving in circles close to the dam, and exhibiting actions such as spinning, and kicking.34 Other labels used to categorize locomotory play include frolic, run, chase, buck, jump, leap and prance.14 Solitary running and bucking are noted more frequently in fillies than in colts.58 The lengths of locomotory play bouts can be related to the increasing confidence of the foal as it gradually moves farther away from the dam. Williams59 notes that locomotory play often seems unfinished, since it is often ended by some form of distraction, such as spotting a shadow. Rather than being unconsummated, bouts of locomotory play may end this way for sound reasons that currently elude us. All age groups of domestic horses may indulge in this form of play without conspecific company.7
Interactive play
If opportunities are available, interactive play occurs among horses of various ages (Fig. 10.13) and its nature seems to depend on the age and sex of the participants. Characterized by repeated physical contact between participants, it often mimics combat between harem stallions but lacks the characteristic vocalizations, persistent ear-pinning and heightened risk of injury.7 It is possible to subdivide interactive play as play fighting and play sexual behavior,14 but in the current text interactive play is considered in the light of the partners involved. Play partners can be adult horses or other foals.

Figure 10.13 Play fighting is recognized among adult companions as they become familiar with one another, especially in bachelor groups.
(Photograph courtesy of Francis Burton.)
Play between foals and adult horses
During the month of dependence7 the focal point of the foal’s life is its dam. In the first week of life the foal will spend most of its time with its mother.5 She allows it to play on or around her, showing considerable tolerance (Fig. 10.14). Types of play behaviors include nibbling and chewing the mane and tail of the mare, biting and bumping her legs and sides, and attempting to climb over or jump the mare when she is lying down.7 At first this play is rough and there is little the mare can do other than tolerate it. But after 2 weeks or so, as the foal becomes more gentle, his dam will have the opportunity to teach him the benefits of mutual grooming.54 Play between the mare and her foal decreases as the foal spends an increasing amount of time with its peers. Although time spent playing does not differ between fillies and colts, the types of games and, therefore, the energy consumption do.58 For example, interactive play bouts of colts are longer than those of fillies.58
Play between foals and an adult other than their mother is rare. Mares will often threaten foals that are not their own, while young and barren mares may show more tolerance. Colts engage in this form of interaction more often than fillies.58 Snapping is usually exhibited before and during these interactions, especially when the adult is a stallion.34 Free-ranging stallions allow foals (again, more commonly colts than fillies5) to investigate or play with them and are generally careful in their treatment of youngsters, often withdrawing from play fights rather than responding agonistically.5 This gentle attitude may continue to be displayed until the foals reach puberty, at which point they often begin to be treated as competition, if male, or with relative indifference, if female. Kiley-Worthington2 notes that the stallion may ultimately eject both colts and fillies from the natal band, but more common causes of natal dispersal are considered in Chapter 5.
Play among foals
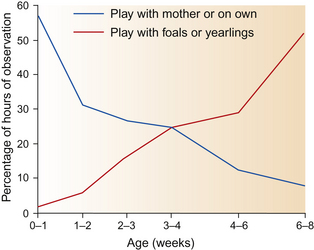
A neonatal foal’s social interactions with other foals gradually increase in frequency so that at 3–4 weeks of age the average number of solitary play bouts matches the average number of bouts of social play (Fig. 10.15).5 Invitations to play often begin with staring, tentative sniffing and head-tossing approaches that initially amount to no more than touching muzzles followed by galloping retreats and flamboyant bucking (Fig. 10.16).34 Colts are more proactive than fillies in using these invitations to initiate play with their peers.37
Possibly because they often get variable responses from adults other than their dams, foals tend to associate more often with other foals.60 Foals tend to affiliate and play with one other foal regularly (Fig. 10.17). The nature of the play within these dyads differs according to the sex of the combination, most notably after the first month of life. Tyler5 indicates that 50% of play involves a colt and a filly, 34% involves two colts and 16% involves two fillies. In the first 8 weeks of life, colts are generally more active and graze less than fillies.61 Colts attempt to mount their peers or, for that matter, their dam about seven times more often than do fillies,5 who are seldom seen mounting after the first month.13 The peak of mounting by all foals coincides with the foal heat. Mounting by foals is tolerated by mares and stallions alike.58

Figure 10.17 As they mature, foals increasingly associate with other foals.
(Photograph courtesy of Michael Jervis-Chaston.)
The pair-bonds that form between fillies may be lifelong.2 While pairs of fillies engage in locomotory play and mutual grooming,54 mock fights are more prevalent and indeed rougher among pairs of colts. Mock fighting includes inhibited biting on the head and neck of the opponent and attempting to unbalance the opponent by pushing against him. Interactive play of this sort may be regularly interrupted by bouts of mutual grooming,13 occasional mounting6 and regular flehmen-like responses.62 It has been suggested that mock fights end with the subordinate player offering a final rear that prompts the higher-ranking player to give chase.10 Mock fighting also occurs in mixed-sex pairs but it is readily curtailed by outright aggression on the part of the filly if it becomes too rough.7 It is likely that play between sexes is the natural forum in which colts learn about tactful courtship.7 Colts that have bonded with colts rather than fillies will nevertheless approach fillies, apparently for mutual grooming.13 Occasionally, the colt will begin to nibble a filly’s hindquarters and sometimes then attempt to mount her. This usually elicits aggression from the filly.
Manipulative play
Manipulative play involves nosing, sniffing, licking, nibbling or biting at objects, from stones and sticks to the manes and tails of other horses, and may progress to pulling, lifting, carrying or apparent attempts at throwing.7 The feet may also be used in manipulation, e.g. when pawing at water or kicking at an object on the ground. Object play seen in foals58 often follows a repetitive pattern of advancing upon and retreating from the object, with the integration of some locomotory activity. So objects, including other horses, may be the focus of locomotory play as foals circle them and run to and from them.14
It has been suggested that, in the absence of conspecifics, providing objects to foals isolated at the time of weaning may improve their welfare.63 Paddocks give weanlings the opportunity to play, and this may help to explain why they are associated with more normal time-budgeting than stables.64 In their relative isolation, stabled horses often learn to occupy themselves by manipulating objects in their restricted environment. Water, haynets, chains and bedding provide serviceable playthings.54 Nipping and nibbling are classic play actions, and horses often chew at wooden rails when confined and on lead ropes when tied-up. Through manipulative play, some horses learn self-reinforcing responses, such as opening stable doors (see Fig. 1.12) and untying themselves.
The social environment of foals influences object play in that foals without peers tend to manipulate objects for longer than socially kept foals.65 Kiley-Worthington1 proposes that horses deprived of visual contact with conspecifics may interact with items in their stable to make noise in a bid to keep in touch with others. When owners provide horses with stable toys, they should bear in mind that there is little or no research to confirm their effectiveness in enriching the environment, that the novelty value of such devices can rapidly dwindle and that horses require several toys that can be alternated.57
Behavioral anomalies in maintenance behavior
Unmanageable play
Owners sometimes complain about behaviors that, while emerging from a motivation to play and perform vigorous exercise, are nonetheless dangerous. While examples in ridden horses include bucking and pulling at the gallop, similar problems on the ground include pulling away at the time of turn-out, and rearing in hand. These are manifestations of the horse being other than under stimulus control. Horses that are fed too much grain are prime candidates for exuberant locomotory displays. This trend is compounded by their being the horses that generally spend most of the day confined. Fortunately, the managers of stable yards in which play gets out of hand are beginning to appreciate the need for relatively more forage in diets and the provision of a recreation area for affiliated inmates.
It is important to remember the role of learning in these responses. Very often they appear in experimental situations and are allowed to be reinforced. For this reason, thoughtful riders never allow horses to begin to find rewards in pulling but instead respond to playful bucking by reducing the pace and returning to the spot where the buck occurred in a bid to show the horse that it is not effective as a means of progress. Horses that are allowed to pull under-saddle rapidly habituate to bit pressure and soon become horses that can bolt. This is one of the reasons some racehorses are difficult to re-educate as safe riding animals for leisure. Therefore, the racing industry should recognize that heavy hands make horses lean on the bit, and are responsible for considerable wastage. Grading-up to a more severe bit is a very short-term solution to the problem, which lies not so much in the horse’s mouth as at the other end of the rein. With consistency at its core, retraining as discussed in Chapter 13 is the best means of preventing battles between horse and rider from escalating.
Interactive play under-saddle can cause horses to use agonistic responses when jostling for position as they begin vigorous exercise – for example, at a place in a familiar route that has become associated with faster gaits. The danger here is that because communication between such horses is compromised by human intervention, both horses and riders may be injured. The safest approach to this contingency is to keep ridden horses at least one body length apart and to keep higher-ranking horses that may be more likely to kick if they are inadvertently challenged, at the back of the group.
Because they are impressionable and can quickly find reinforcement in locomotory play, youngsters should always be handled by experienced personnel. Preventing inappropriate responses not only involves eliminating inappropriate learning opportunities, but also instituting a routine in husbandry techniques. For example, prior to turn-out, it is excellent practice to turn all horses round to face the gateway through which they have just passed. With this routine in place, youngsters learn that they must change direction before being released and this has a tempering effect on the speed with which they pass through the gateway. Older horses, which may kick out simply as a form of post-inhibitory rebound after confinement, are always released facing a direction that is safe for personnel.
Refusal to lie down
While a horse that shows increased resting should be examined for other evidence of illness,35 less recumbency than normal is also a cause for concern. Refusal to lie down has been listed among ‘vices and bad habits’.66 It was unwelcome because it was often associated with a lack of musculoskeletal rest, and a resultant abbreviation of the horse’s working life. The popularity of standing stalls may have contributed to this complaint’s prevalence. When 16 mares were confined in standing stalls for 6 months, 9 were not observed in recumbency (on videotapes that recorded each horse for 24 hours every week), suggesting that horses with no previous experience of such confinement may be reluctant to lie down.41 Thirteen of the mares dropped to their knees at least once during the 6-month observation period, a response attributed to their being in REM sleep while standing.41 (Readers are directed to Chapter 3 for a consideration of narcolepsy.)
Although horses spend 90% of their time standing and when they lie for longer than is typical there may be a health or welfare problem,64 it is important that provision be made for them to lie down whenever they wish.67 While reluctance to rise is associated with musculoskeletal disorders and most commonly lower limb pain (most notably laminitis),13 reluctance to lie down may occur through:
• neophobia, e.g. in a new stable
• confinement and perhaps fear of becoming trapped in a particularly small stable
• inappropriate substrate, e.g. lack of bedding for a stabled horse or lack of dry surfaces for a horse at pasture
• pain, e.g. an arthritic horse may be disinclined to lie down because lying down and, more especially, standing up may be associated with pain.33 Pain is also the reason horses show reluctance to lie down as a feature of peritonitis and purpura haemorrhagica.13
Inappropriate responses to being groomed
While some horses enjoy being groomed to the extent that they attempt to return the favor and in so doing inadvertently hurt, or more frequently alarm, their grooms, others find the intervention ticklish, distressing or even painful.
Hand-reared foals and single foals that attract the status of pets are more likely to regard humans as suitable mutual grooming partners. Key trigger spots are the part of the neck and withers in which foals find grooming most gratifying (for physiological reasons discussed in Ch. 2). The unwelcome consequences of early learning of this sort should be considered before misguided personnel are permitted to indulge a foal’s attempts to reciprocate. Occasionally, owners actively encourage this form of bonding in juvenile and mature horses, despite horses having powerful jaws that can cause inadvertent damage to human skin54 (Fig. 10.18) but the majority find it inappropriate and seek to eliminate the response. Direct punishment by hitting the muzzle is inadvisable, since it frequently makes the recipient head-shy. To shape the behavior more appropriately handlers should apply negative punishment by stopping the reinforcing stimulation of these premium areas when the horse turns its head toward them. The horse quickly learns that to receive more of the preferred attention it must face away from the groomer.

Figure 10.18 Mutual grooming between horse and human. When they have their withers scratched, many horses will attempt to reciprocate as a form of mutual grooming. In most cases this is discouraged because people are fearful of being bitten.
(Photograph courtesy of Sandro Nocentini.)
Breed and individual differences in skin sensitivity through different parts of the body should be accommodated by selecting brushes and modifying brushing technique so that minimal reaction is evoked.2 The alternative to this considerate, adaptive approach is to simply persist in tickling and annoying the horse, which is likely to signal its frustration with tail-swishing. If this signal is ignored, the behavior may mature to kick threats and eventually kicks. This effectively lowers the horse’s threshold to kick and makes it generally more dangerous to be around. Since sensitivity, especially in the groin, is innate, it requires extensive innocuous stimulation before any habituation occurs and, moreover, it is a feature likely to return after a period without stimulation.
Sensitivity in the girth region has been considered part of the so-called cold-back syndrome, an abiding equine enigma that seems related more to somatic pain than wilful disobedience or a desire to avoid work. While it is certainly possible to modify aspects of the evasive strategies learned by horses that seem to suffer such pain, therapy should focus on controlling or eliminating the pain.
Many horses find some husbandry techniques, such as tail and mane pulling, distressing. The repeated use of the twitch to accomplish routine procedures in such horses does nothing to reduce their anticipation of the distress caused by the intervention and, indeed, handling is often made yet more difficult when the horse learns to evade the twitch. Instead of struggling intermittently with a distressed animal and teaching it to struggle more the next time, or waiting for learned helplessness to occur, stable managers aiming for long-term success gradually combine small tolerable exposure to stimuli associated with the procedure before the arrival of meals as the first step in a counter-conditioning program.68
Another management technique that can present problems is trimming ear hair. Some humans find ear hair unacceptable, but its function seems related to preventing entry of foreign bodies so its removal is difficult to justify for purely esthetic reasons. Occasionally a build-up of wax on the aural hair can be removed with judicious use of scissors, but pulling such hair is inhumane and the proximate pain involved has the potential to teach the subject to be head-shy after remarkably few lessons.
Summary of Key Points
• Grooming plays a major role in maintaining health.
• Stabling can compromise a horse’s ability to perform many normal maintenance responses.
• Mutual grooming facilitates social bonds.
• The quantity and quality of sleep can be influenced by age, rank, group size, diet and environmental stimuli.
• Prior to 1 month of age, play is more likely to be solitary or with the mother.
• After 1 month of age, play is more likely to be with peers.
Case study
A 12-year-old Thoroughbred mare had learned to nip her owner when having her girth tightened and to do so very deftly and with little warning. She had learned to get her head out of the way very quickly to avoid punishment but at the same time she was becoming tense and rather head-shy during this part of the tacking-up process.
Before the mare’s behavior could be modified the eliciting causes were investigated. The mare was highly reactive to palpation in the girth region. The unusual sensitivity and muscular guarding was ameliorated only slightly by a short course of non-steroidal anti-inflammatory drugs, and the hyper-reactivity of the girth region was considered to be a chronic condition that could be managed (in conjunction with the use of long-term analgesics) rather than cured. Managing the condition involved purchasing some new saddlery and instituting a novel girthing routine:
• the saddle was re-fitted so that it gave a great deal of clearance over the withers
• the existing girth was replaced with a sheepskin-covered cushioned girth with 10–15 cm of elastic at either end.
To overcome the context-specific elements in the mare’s response, she was moved to a new part of the yard at the start of the new routine. Palpation of the pectoral region and the thoracic region medial to the elbows was conducted in conjunction with counter-conditioning. If the mare kept looking forward and did not swing her head when touched in these areas she was rewarded with scratching of the lower neck and withers or premium food items.
It was important to implement control of the head movements with consistent commands from the handler. Using the pressure-release system detailed in Chapter 13, the head was turned by pressure on the headcollar to teach the mare that it was appropriate to turn on command. She was rewarded for these turns with rubbing on the forehead and then the head was manually turned back, followed by more rewarding. This palpation program was applied twice daily for 1 week before any girthing.
In a bid to break down learning from previous episodes, the girth was fastened from the off-side. The horse was led around the yard three or four times before the forelegs were stretched to smooth the skin under the girth and were held in the stretched position for 30 seconds before being returned to the ground. In addition to mounting from each side, mounting from a block or some other support became obligatory regardless of the weight of the rider. Once mounted, the rider gradually tightened the girth with one hand while scratching the lower neck and withers with the other.
Two months after presentation, the biting had stopped completely. The mare could best be described as tolerant of the girth. The search for a more substantial cure for her primary girth pain continues.
References
1. Hothersall B, Harris P, Sörtoft L, Nicol CJ. Discrimination between conspecific odour samples in the horse (Equus caballus). Appl Anim Behav Sci. 2010;126:37–44.
2. Kiley-Worthington M. The behaviour of horses in relation to management and training. London: JA Allen, 1987.
3. Feh C, de Mazieres J. Grooming at a preferred site reduces heart rates in horses. Anim Behav. 1993;46:1191–1194.
4. Normando S, Haverbeke A, Meers L, et al. Heart rate reduction by grooming in horses (Equus caballus). Havemeyer Workshop on Horse Behaviour and Welfare, Holar, Iceland, 2002: 23–25.
5. Tyler SJ. The behaviour and social organization of the New Forest ponies. Anim Behav Monogr. 1972;5:85–196.
6. Blakeslee JK. Mother–young relationships and related behavior among free-ranging Appaloosa horse. Thesis, Idaho State University, Pocatello, 1974.
7. Crowell-Davis SL. Developmental behavior. Vet Clin North Am Equine Pract: Behav. 1986:573–590.
8. Feist JD, McCullough DR. Behaviour patterns and communication in feral horses. Z Tierpsychol. 1976;41:337–371.
9. Houpt KA. Equine maintenance behavior: feeding, drinking, coat care and behavioral thermoregulation. Havemeyer Workshop on Horse Behaviour and Welfare, Holar, Iceland 2002; 92–95.
10. Wells SM, von Goldschmidt-Rothschild B. Social behaviour and relationships in a herd of Camargue horses. Z Tierpsychol. 1979;49:363–380.
11. Clutton-Brock TH, Greenwood PJ, Powell RP. Rank and relationships in Highland ponies and Highland cows. Z Tierpsychol. 1976;41:202–216.
12. Feh C. Relationships and communication in socially natural horse herds. Havemeyer Workshop on Horse Behaviour and Welfare, Holar, Iceland 2002; 84–91.
13. Waring GH. Horse behavior: the behavioral traits and adaptations of domestic and wild horses including ponies. Park Ridge, NJ: Noyes, 1983.
14. McDonnell SM, Poulin A. Equid play ethogram. Appl Anim Behav Sci. 2001;78(2–4):263–295.
15. Keiper RR, Keenan MA. Nocturnal activity patterns of feral ponies. J Mammal. 1980;61:116–118.
16. Christensen JW, Ladewig J, Sondergaard E, Malmkvist J. Effects of individual versus group stabling on social behaviour in domestic stallions. Appl Anim Behav Sci. 2002;75(3):233–248.
17. Crowell-Davis SL. Self-grooming by mares and foals of the Welsh pony (Equus caballus). Appl Anim Behav Sci. 1987;17(3/4):197–208.
18. Fuller Z, Cox JE, Argo CM. Photoperiodic entrainment of seasonal changes in the appetite, feeding behaviour, growth rate and pelage of pony colts. Anim Sci. 2001;72(1):65–74.
19. Ödberg FO, Francis-Smith K. A study on eliminative and grazing behaviour–the use of the field by captive horses. Equine Vet J. 1976;8(4):147–149.
20. Saslow CA. Understanding the perceptual world of horses. Appl Anim Behav Sci. 2002;78(2–4):209–224.
21. Raabymagle P, Ladewig J. Lying behavior in horses in relation to box size. J Equine Vet Sci. 2006;26:11–17.
22. Clarke AF. Stables. In: Wathes CM, Charles DR. Livestock housing. Wallingford, Oxon: CAB International; 1994:379–403.
23. McGreevy PD, Cripps PJ, French NP, et al. Management factors associated with stereotypic and redirected behaviour in the Thoroughbred horse. Equine Vet J. 1995;27:86–91.
24. Mills DS, Eckley S, Cooper JJ. Thoroughbred bedding preferences, associated behaviour differences and their implications for equine welfare. Anim Sci. 2000;70(1):95–106.
25. McGreevy PD. Why does my horse …? London: Souvenir Press, 1996.
26. Dewes HF. The association between weather, frenzied behaviour, percutaneous invasion by Strongyloides westeri larvae and Rhodococcus equi disease in foals. NZ Vet J. 1989;37(2):69–73.
27. Keiper RR, Berger J. Refuge-seeking and pest avoidance by feral horses in desert and island environments. Appl Animal Ethol. 1982;9(2):111–120.
28. Hughes CF, Goodwin D, Harris PA, Davidson HPB. The effect of social environment on the development of object play in the domestic horse foals. Havemeyer Workshop on Horse Behaviour and Welfare, Holar, Iceland, 2002: 21–22.
29. Delpietro HA. Case reports of defensive behaviour in equine and bovine subjects in response to vocalisation of the common vampire bat (Desmodus rotundus). Appl Anim Behav Sci. 1989;22(3–4):377–380.
30. Duncan P, Vigne N. The effect of group size in horses on the rate of attacks by blood-sucking flies. Biol Behav. 1979;5:55–60.
31. Kasper M, Beck AM. Effect of environmental temperature on the behaviour of Clydesdales during preparation time before athletic performances. Equine Pract. 1997;19:25–28.
32. Lawick-Goodall JV. Tool-using in primates and other vertebrates. In: Lehrman DS, Hinde RA, Shaw E, eds. Adv Study Behav 1970; 3:195–250.
33. Boyd L, Keiper R. Behavioral ecology of feral horses. Havemeyer Workshop on Horse Behaviour and Welfare, Holar, Iceland, 2002:70–73.
34. Fraser AF. Curious idling. Appl Animal Ethol. 1983;10:159–164.
35. Fraser AF. The behaviour of the horse. Wallingford, Oxon: CAB International, 1992.
36. King SRB. Home range and habitat use of free-ranging Przewalski horses at Hustai National Park, Mongolia. Appl Anim Behav Sci. 2002;78(2–4):103–113.
37. Flannigan G, Stookey JM. Day-time time budgets of pregnant mares housed in tie stalls: a comparison of draft versus light mares. Appl Anim Behav Sci. 2002;78(2–4):125–143.
38. van Weeren PR, Jansen MO, ven den Bogert AJ, Barneveld A. A kinematic and strain gauge study of the reciprocal apparatus in the equine hindlimb. J Biomech. 1992;25:1291–1301.
39. Wolff A, Hausberger M. Behaviour of foals before weaning may have some genetic basis. Ethology. 1994;96(1):1–10.
40. Dallaire A. Rest behavior. Vet Clin North Am Equine Pract: Behav. 1986:591–607.
41. Houpt KA, Houpt TR, Johnson JL, et al. The effect of exercise deprivation on the behaviour and physiology of straight-stall confined pregnant mares. Anim Welfare. 2001;10(3):257–267.
42. Parmeggianni PL. Behavioral phenomenology of sleep (somatic and vegetative). Experientia. 1980;36:6–11.
43. Caanitz H, O’Leary L, Houpt K, et al. Effect of exercise on behaviour. Appl Anim Behav Sci. 1991;31(1–2):1–12.
44. van Dierendonck MC, Bandi N, Batdorj D, et al. Behavioural observations of re-introduced Takhi or Przewalski horses in Mongolia. Appl Anim Behav Sci. 1996;50:95–114.
45. Dallaire A, Ruckebusch Y. Sleep patterns in the pony with observations on partial perceptual deprivation. Physiol Behav. 1974;12:789–796.
46. Boy V, Duncan P. Time-budgets of Camargue horses, I: Developmental changes in the time-budgets of foals. Behaviour. 1979;71:187–202.
47. Duncan P. Time-budgets of Camargue horses, II: Time-budgets of adult horses and weaned sub-adults. Behaviour. 1980;72:26–49.
48. Ruckebusch Y. The relevance of drowsiness in the circadian cycle of farm animals. Anim Behav. 1972;20:637–643.
49. Fischbein W, Gutwein BM. Paradoxical sleep and memory storage processes. Behav Biol. 1977;19:425–464.
50. Crowell-Davis SL. Daytime rest behavior of the Welsh pony (Equus caballus). Appl Anim Behav Sci. 1994;40(3/4):197–210.
51. McFarland D. The Oxford companion to animal behaviour. New York: Oxford University Press, 1987.
52. Bekoff M. The essentials of good play. BBC Wildlife. 2000;18(8):46–53.
53. Fagen R. Animal play behaviour. Oxford: Oxford University Press, 1981.
54. Rees L. The horse’s mind. London: Stanley Paul, 1984.
55. Hausberger M, le Scolan N, Bruderer C, Pierre JS. [Temperament in the horse: factors in play and practical implications] (French). 24 Journée de la Recherche Equine, Institut du Cheval, Paris. 1998:159–169.
56. Maynard-Smith J. Evolution and the theory of games. Cambridge: Cambridge University Press, 1982. 263–297
57. Goodwin D, Hughes CF. Horse play. Havemeyer Workshop on Horse Behaviour and Welfare, Holar, Iceland, 2002: 102–105.
58. Crowell-Davis SL, Houpt KA, Kane L. Play development in Welsh pony (Equus caballus). Appl Anim Behav Sci. 1987;18(2):119–131.
59. Williams M. Horse psychology. London: JA Allen, 1976.
60. Crowell-Davis SL, Houpt KA, Carini CM. Mutual grooming and nearest neighbor relationships among foals of Equus caballus. Appl Anim Behav Sci. 1986;15:113–123.
61. Duncan P, Harvey PH, Wells SM. On lactation and associated behaviour in a natural herd of horses. Anim Behav. 1984;32(1):255–263.
62. Schoen AMS, Banks EM, Curtis SE. Behavior of young Shetland and Welsh ponies (Equus caballus). Biol Behav. 1976;1:199–216.
63. Mills DS, Nankervis KJ. Equine behaviour: principles and practice. Oxford: Blackwell Science, 1999.
64. Heleski CR, Shelle AC, Nielsen BD, Zanella AJ. Influence of housing on weanling horse behavior and subsequent welfare. Appl Anim Behav Sci. 2002;78(2–4):297–308.
65. Hughes RD, Duncan P, Dawson J. Interactions between Camargue horses and horseflies. Bull Entomol Res. 1981;71(2):227–242.
66. Hayes MH. Veterinary notes for horse-owners. London: Stanley Paul, 1968.
67. Houpt KA. Equine welfare. In: Houpt KA, ed. Recent advances in companion animal behavior problems. Ithaca, NY: International Veterinary Information Services, 2001. A0810. 1201
68. Gough MR. A note on the use of behavioural modification to aid clipping ponies. Appl Anim Behav Sci. 1999;63(2):171–175.