Chapter 7 Locomotory behavior
Chapter contents
Fetal movements
The movements of the equine fetus have been studied with ultrasonography in a bid to explore the extent of normal locomotion in utero and its roles in optimizing presentation before labor and preparing the musculoskeletal system for work immediately post-partum. In addition, ultrasonography has demonstrated that sucking and swallowing is common in fetal foals, a finding that explains the development and strength of the sucking reflex at birth.1
Simple movements, such as the extension or flexion of a limb or the vertebral column, can be detected from the third month of gestation.1 As the fetus matures, these combine and become repeated to form complex movements that eventually appear coordinated.1 The hooves are tipped with collagenous eponychia, which prevents damage to the amnion and beyond. Most of the coordinated kinetic activity of the foal, including extension of the forelimbs and head toward the maternal pelvis, occurs well in advance of uterine contractions, sometimes as much as 12 days earlier.1 These final adjustments are considered critical in the avoidance of dystocia.2
While simple movements may occur 55 times per hour from the fifth to the ninth month of gestation,1 complex movements as frequent as 84 times per hour have been recorded in the 3 days pre-partum.1 The frequency of the final complex movements that ultimately change the fetal position from supine to prone and occasionally from posterior to anterior1 is illustrated in Figure 7.1.
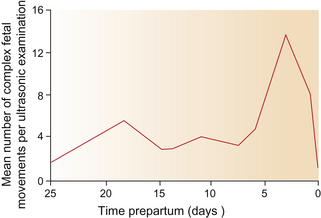
Figure 7.1 Frequency of the final complex movements in the terminal stages of gestation. Complex movements of the equine fetus become more frequent until a position suitable for engagement in the maternal pelvis is achieved.
(After Fraser.1)
Infant growth and movements
An appreciation of the normal development of foals is useful for practitioners, since deviations from the typical developmental pattern can indicate abnormal progression in a neonatal foal.3 Fraser1 suggests that five steps can be identified in the development of the primary mobility required of a foal before it takes its first mouthful of milk and the self-reinforcement of feeding can take place (Table 7.1).
Table 7.1 The steps involved in achieving primary mobility in the equine neonate
| Step | Foal’s responses | Dam’s responses |
|---|---|---|
| Recumbent coordination | ||
| Rising and quadrupedal stability | ||
| Ambulation | ||
| Maternal orientation | ||
| Teat-seeking and sucking |
After Fraser.1
The order in which features of the ethogram emerge is described in Figure 7.2. Foals that accomplish one phase quicker than the mean often show similar precociousness in other domains.1 Perhaps because they have shorter long bones and therefore require less leverage to stand, pony foals are usually stable on their feet within half an hour,1 much earlier than neonatal Thoroughbreds. The time taken to stand depends on the sex of the foal (average for Thoroughbred fillies being 56.3 minutes and for colts 70.6 minutes, n = 127)4 – a difference attributed to the lighter bodyweight of fillies, who therefore require relatively less strength to rise. Unsurprisingly, fillies are the first to feed, generally having their first drink 1.5 hours after birth while colts take an average of 2 hours before feeding.1
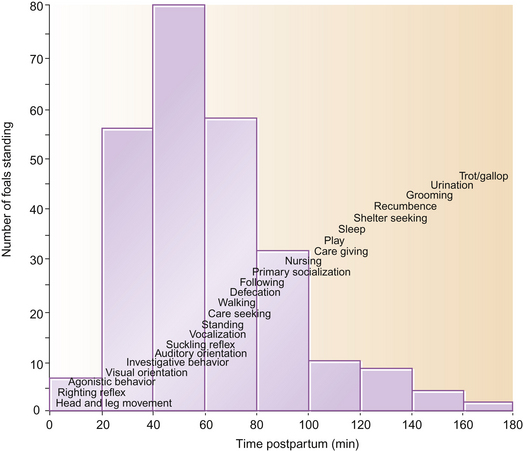
Figure 7.2 The distribution of time taken to stand, and the progressive appearance of novel responses, in the equine neonate.
After the first drink, a melange of rest and sleep occupy most of the foal’s first week but, along with locomotory play, this is punctuated by feeding every 15–30 minutes.1
A daily tally of motor activities in the equine neonate is given in Table 7.2. The prevalence of vigorous stretching (or pandiculation) from the first day of life suggests that it is not only pleasurable as a comfort behavior, but also that it occurs as a result of uterine confinement.1 Stretching may occur when the foal is recumbent or upright (Fig. 7.3). The importance of stretching is dealt with elegantly elsewhere1 but can be summarized in terms of its role in athletic development, growth and joint correctness. By the third day of life, estimates of 80 or so stretches per day have been recorded.1 Some support for the putative orthopedic benefits of stretching comes from the coincidental peak in the frequency of stretching on day 3 of life, the age by which marginally contracted tendons have self-corrected. It would be interesting to investigate the effect of post-foaling thoracic trauma5 on the frequency of holistic stretching.
Table 7.2 Frequency of motor patterns in the equine neonate
| Activity | Daily frequency |
|---|---|
| Defecation | 2–4 |
| Urination | 4–10 |
| Walking | 8–14 |
| Sleeping | 20–25 |
| Sucking | 18–24 |
| Stretching (recumbent and upright) | 40–60 |
After Fraser.1
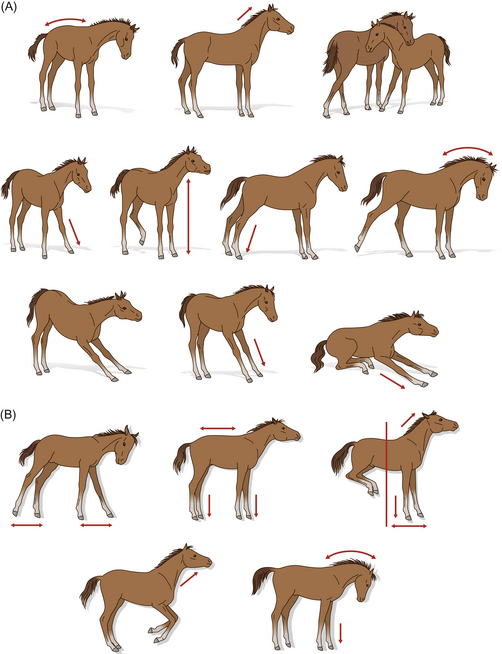
Figure 7.3 Stretching can be observed before and after locomotory activity and may occur when the foal is (A) upright or (B) recumbent.
(Redrawn, with permission, from Fraser.1)
In the first 6 weeks of a foal’s life, most vigorous exercise takes the form of play,6 but the motivation to rest, play and feed changes with age. Crowell-Davis7 identifies three periods of behavioral development in foals, all of which involve different proportions of locomotory activity:
Adult kinetics
Horses have been shown to travel on open ranges (for example, to water) up to 65–80 km daily. For pastured horses, movement during grazing is the main initiator of locomotion, and an estimated daily figure for kinetic behavior in this context is 20 km.1 The distance that grazing horses travel depends on the location of water, the availability of food and the time that has to be spent foraging.
As a means of returning horses to their home range and indeed their social group, homing can occur over distances of more than 15 km.3 The stimuli used to guide this locomotion probably involve olfaction that relies on ground-based stimuli, but they remain the subject of some debate,8,9 especially since homing may even traverse water.3
While incessant walking has been reported as a presenting sign in serum hepatitis,3 route-tracing in the form of box-walking (stall-walking in the USA) is far more common and emerges as a stereotypic behavior in animals that fail to cope with social isolation (see Ch. 5). Conversely, stereotypies are reported less frequently as exercise is increased.10 Locomotion is useful as an indicator of distress, especially when conducted at the expense of feeding; for example, solitary horses walk and trot three times more often than those that can make auditory, visual and physical contacts with other horses.11 Furthermore, locomotion’s role in recreation is considerable, with 75% of the kinetic activity of foals, for example, being in the form of play.12 The need for spontaneous exercise is emphasized by reports of post-inhibitory rebound in locomotion among horses after periods of confinement.13
While locomotion is an integral part of equine anti-predator strategy and ingestive behavior, it is also used in communication and courtship. Exercise tends to affect other behaviors. For example, exercised horses spend more time drinking and lying but urinate less than unexercised controls.14 Tantalizing insights into the effect of training on behavior have been offered by studies showing that horses that lose races tend to be more aroused and require greater control in the parade ring/mounting yard than winners.15 Some of the more important dependent variables from these studies are summarized in Table 7.3. For a more comprehensive list and detailed consideration of likely reasons for these variables being significant to performance, readers are directed to an informative and entertaining book, Watching Racehorses.15
Table 7.3 Some of the features of a racehorse’s behavior and presentation that can be used to predict poor performance (not winning)
| Location | Variable | Extent to which this variable predicts poor performance |
|---|---|---|
| Birdcagea | Weaving and repetitive head movements | ******** |
| Kicking | ***** | |
| Pawing | ** | |
| Parade ring | Crossover (figure-of eight or grackle) noseband | ******** |
| Gaping mouth | **** | |
| Twisting neck | *** | |
| Nose roll (sheepskin noseband) | *** | |
| 3rd metacarpal bandages | *** | |
| Other bandages | ************** | |
| Pacifiers (meshed eye protection) | ** | |
| Mounting yard | Slow gait | ******** |
| Fast gait or circling | ***** | |
| Bucking | ***** | |
| Balking | **** | |
| Courtship behaviors | *** | |
| Ears pointing laterally or aborally | ** | |
| Defecating | ** | |
| Flicking ears | * | |
| Track | Late | ******** |
| Conflict behaviors | **** |
a In Australia, racehorses can be observed in a gallery of open-front standing stalls called the birdcage, prior to being paraded (Fig. 7.4).
Adapted from Watching Racehorses15 with kind permission of Geoffrey Hutson.

Figure 7.4 Horse in cross ties pawing in the standing stalls of a birdcage gallery at a racecourse.
(Reproduced, with permission, from Hutson.15)
Sidedness and symmetry
Although the forelegs alternate in leading during grazing, the time horses spend with the left leg advanced is generally longer than for the right (Fig. 7.5). This manifests as a significant directional bias to graze with the left foreleg in advance of the right.16 This tendency seems breed-dependent with Thoroughbreds showing it more than Standardbreds who in turn show it more than Quarterhorses.17 If advancing a forelimb when grazing reflects greater mobility on that side of midline, then it is possible that the brains of left-preferent animals are right-hemisphere dominant. However, a counter argument is that the non-advanced limb is the more critical for survival because it supports more weight, reflects greater agility on the weight-bearing side of the animal and is arguably better positioned to launch the animal into a flight response and a more dominant left turn (owing to the abduction of the right foreleg in the stance phase). We are yet to study how this affects ridden work, such as canter leads and preferred side for poll-flexion and lateral bend.

Figure 7.5 Even in the absence of lameness, some foals lock in a strong preference for an unbalanced grazing stance before weaning. There is a general population bias to advance the left foreleg. (Figure from www.avma.org)
Even in symmetrical gaits, such as the trot and the pace, asymmetries often become evident at high speeds. In pacers, temporal gait variables, including suspension times, may differ between the left and right couplets, while in trotters high speeds have been used to expose left-right asymmetries that are particularly apparent in the hindlimbs.18 This is not necessarily an effect of training to work in one direction. Slight individual tendencies toward either left or right laterality in general responses, such as in lying down,1 and in foraging, have been reported by others.19,20 Kinematic differences between the left and right limbs have also been reported in 8-month-old Standardbreds that have yet to be trained.21
It is reported that, when jumping, most horses seem to lead with and land with the right leg.1 However, other workers have shown this preference only when horses are jumping on a right-handed turn.22
Lateral flexion
If by design or by default a horse fails to use its hindlimbs in the same path as its forelimbs, it is said to be traveling in two tracks (or two-tracking). Horses can perform a number of lateral movements, most but not all of which require some flexion of the vertebral column.
In the leg yield, the neck and vertebral column are straight except for a slight flexion at the atlanto-occipital joint that flexes the head away from the direction of motion. For the movements in which the vertebral column is flexed, it is easiest to consider the horse moving relative to an imaginary straight line. In some of the movements the neck and forequarters align with the line and the hindquarters deviate to the side (travers, renvers). In other movements the hindlegs follow the line while the forequarters deviate from it (shoulder-in, contra shoulder-in) (Fig. 7.6).
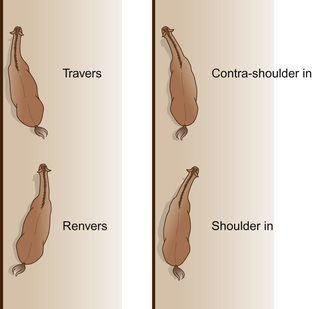
Figure 7.6 The labels for lateral movements depend on the lateral flexion of the horse’s vertebral column and the direction of movement relative to the side of the school.
Naming lateral movements depends on the direction in which the horse’s body is bent relative to the direction of movement around the arena – clockwise (on the right rein) or counter-clockwise (on the left rein). When the convex curve of the body forms the leading edge of the horse as it travels forward, the maneuver is called shoulder-in. For the reverse maneuver, in which the direction of flexion is the direction of locomotion, the terms travers or renvers are used. Half pass (Fig. 7.7) is the same as travers except that it is performed on a diagonal line across the arena.
The extent to which lateral movement (i.e. steering) in racehorses is affected by lateralized use of the whip has been questioned by photographic evidence from New South Wales and Victoria, Australian states in which horses race in opposite directions. Of 200 jockeys racing counter-clockwise, 183 (91.5%) were holding the whip in the right hand and of the 200 jockeys racing clockwise, 107 (53.5%) were holding the whip in the right hand. The primary conclusion is that the data indicate that placement of the whip appears to be primarily determined by handedness of the jockey, not by the direction of the track. Given that over half of NSW jockeys hold the whip in the inside hand, this study challenges the view that the whip is used for steering in NSW.
Simple turns and pirouettes
Turns on the forehand and turns on the quarters are achieved by the rider signaling for more movement by the quarters and forehand respectively. In their elementary forms they are conducted at the rhythm of a collected walk, but when the same principles are applied in advanced horses, elaborate balancing maneuvers, such as canter pirouettes (Fig. 7.8), result. However, despite the theoretical requirement that a good canter pirouette be conducted with the same tempo and rhythm as the collected canter, this is rarely the case. Analysis of individual medal finalists at the Barcelona Olympics showed that pirouettes were performed at about two-thirds of the tempo of a collected canter, had no suspension phase, and were dissociated to the extent that they could be considered to have a four-beat rhythm.23
This chapter will consider the gaits and maneuvers of ridden horses and those at liberty (Fig. 7.9).
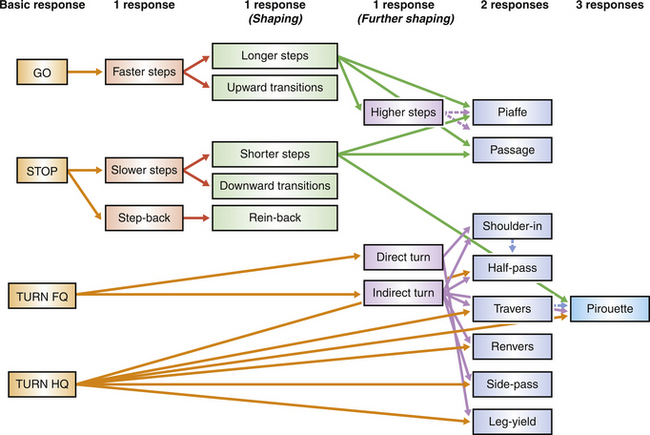
Figure 7.9 All movements required of trained horses are derived from the basic responses of acceleration, deceleration, turning the forequarters (FQ) and turning the hindquarters (HQ). Dotted arrows refer to shaped preparatory movements that usually precede elicitation of the targeted movements.
(Reproduced with permission of Australian Equine Behaviour Centre.)
Gaits
By measuring oxygen consumption in trained ponies that change gait on command, Hoyt & Taylor24 demonstrated that walking is the gait that needs least energy at low speeds, trotting at intermediate speeds, and galloping at high speeds. This accounts for the evolution of gaits as adaptations for economical locomotion within a range of speeds.
It is argued that, in many ways, the physics of equine locomotion have evolved to their limits in that, for example, if the horse’s legs were any longer, its hindlegs would be repeatedly interfering with its forelegs, as happens when the hurried (so-called butcher’s boy) trot leads to forging. Budiansky25 points out that this is the reason species such as the giraffe, with its relatively longer legs, cannot trot.
The care with which horses place their feet at speed is remarkable. With only one toe upon which to land there is little margin for error. Balance is matched by the tremendous care horses take over their hooves. This book would not be a book for hippophiles without the oft-quoted adage ‘no foot – no horse’. This appears here to remind us that horses seem to live by the very same dictum. Their motivation to avoid getting their feet trapped accounts for the reluctance of some horses to accept hoof-care. Considerable neural circuitry is required for any animal to plot a safe course for four limbs, especially as the feet disappear from sight so rapidly. To perform this at the speeds horses reach and through the terrain they can traverse helps to explain why so much of the brain has to be devoted to coordinating limbs and controlling locomotion (see Ch. 3).
Much has been said about the need for good gaits, since they give horses a head-start in training for jumping and dressage. The term kinematics describes scientific measuring of gait. Gifted horse breeders using a visual impression, and indeed scientists using kinematic data, can predict future gait performance of foals by the age of about 4 months.26,27 This is because the kinematic indicators of gait quality (swing duration, the maximal protraction-retraction angle and the maximal flexion of the hock joint)28,29 show a good correlation between foals of this age and adults. A horse’s conformation affects the way it moves, the extent to which its rider is displaced and the work it can successfully perform. For example, although their relative stance durations are similar, ponies make shorter strides than horses, evidenced by a shorter stance and swing duration.29 As a further example, although there are occasional exceptions, Arabian horses are not generally suited to dressage because of their conformation: they frequently have disunited canters, and the hips are rotated, which has the effect of raising the tuber ishii so that their hindlegs ‘trail’ out behind and abduct during lengthened strides. For this reason Arabians often have trouble collecting their hindquarters (Andrew McLean, personal communication 2002).
Gaits are either asymmetrical or symmetrical. If some legs appear to work together and others do not, the horse is doing an asymmetrical gait – either a canter or gallop. As shown in Table 7.4, all of the lateral, diagonal and square gaits are symmetrical, in that the legs on one side of the horse mirror the actions of those on the other. When the legs appear to move independently of one another, not moving forward as ipsilateral or diagonal pairs, the horse is doing a square gait, which may be either a walk, flat walk, running walk or tölt. If the legs on one side appear to move in opposite directions, the horse is doing a diagonal gait which will be either a trot, fox walk or fox trot. If the legs on one side move forward at the same time, the horse is doing a lateral gait which may be a pace, stepping pace, or rack.
Walk
The normal equine walk is a four-beat gait in which the feet move individually and sequentially in diagonals as follows: right fore, left hind, left fore, right hind (Fig. 7.10). At any one time the horse is supported by two feet (about 60% of the time in elite dressage horses30) or three (about 40% of the time in elite dressage horses30).
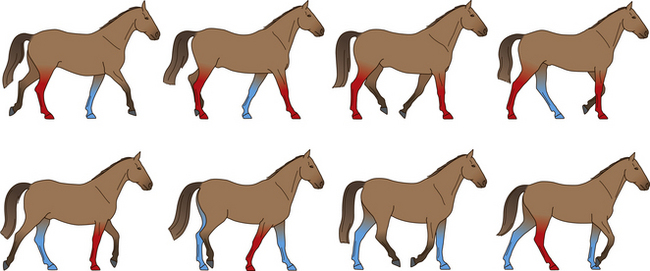
Figure 7.10 The cycle of movements at the walk. The weight-bearing limbs at each stride are indicated.
Four types of walk are recognized by the FEI: collected, medium, extended and free. In the free, performed only in the lower levels of competitive dressage, the horse is allowed to stretch the neck. As can be seen from Table 7.5, differences in the kinematics of the medium and extended walks of dressage horses are not significant. This helps to explain why accelerometers may one day be used to increase objectivity in scoring some aspects of dressage performances (such as the purity of gaits).
Table 7.5 Mean values for stride kinematics of the collected, medium and extended walk in dressage horses
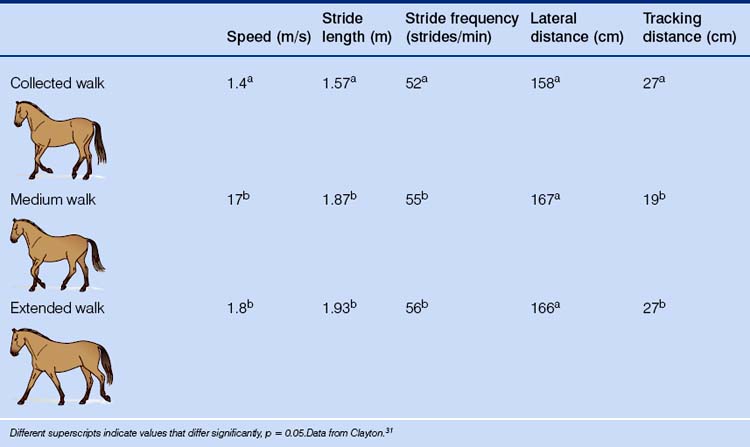
As with all transitions in dressage, great importance is given to maintaining a given rhythm and tempo, so different gaits and variations within gaits are achieved by changes in stride length independent of stride frequency. Despite its being a rule of the FEI that the same stride frequency be maintained at all types of walk, this has proved difficult to demonstrate even in advanced horses.31 Irregularities in rhythm arise because lateral couplets are included.31
Stride-length is of interest to equestrians because the hindfoot should at least reach the impression made by the ipsilateral hoof (this is also known as tracking-up). If this is not the case in walks other than the collected walk, the horse is either being badly ridden or is in poor condition. In horses that have been educated for self-carriage (see Ch. 13), there is a longer stance duration (the time during which weight is borne) in the hindlimbs than the forelimbs at both the collected and extended walk.31,32
In the show ring, the ability of American gaited horses to demonstrate an exaggerated reach with the forelimbs and a substantial over-stride with the hindlimbs (the so-called ‘big lick’) is highly prized.33 While it can be shaped behaviorally, this attribute can also be enhanced by abusive interventions. The practice of ‘soring’ to make horses’ soles, heels or pasterns hypersensitive in a bid to emphasize their ability to lift their feet extravagantly has been subject to considerable scrutiny by the welfare lobby. Indeed it is the focus of legislation and industry self-regulation, but sadly, as fast as the industry agrees on a protocol to identify cases of soring, certain trainers develop new means of avoiding detection.33
Flat and running walks
The flat walk is the same as an ordinary walk, speeded up a little. A good running walk (Fig. 7.11) is the same as a flat walk, with more speed again. In Icelandics it is called a tölt and attracts different additional labels according to the speed with which it is performed (see hreina tölt, below). The fastest of the four-beat show gaits, the running walk, is characteristic of the Tennessee Walking Horse. Maximal stride frequencies are accompanied by tremendous stride lengths rather than the high foreleg action of the rack.3
Diagonal and lateral walks
Horses can perform a diagonal walk in which the footfalls occur as diagonal couplets. The lateral walk (or fox-walk) is characterized by movement of the first foreleg being followed by movement of the ipsilateral hindleg, contralateral foreleg and contralateral hindleg. This gives rise to a lateral rocking action. An extreme example of this is the gait called the fox-trot, in which diagonal pairs of hooves lift off and move forward together, with the front landing noticeably before the hind of the pair.
Trot
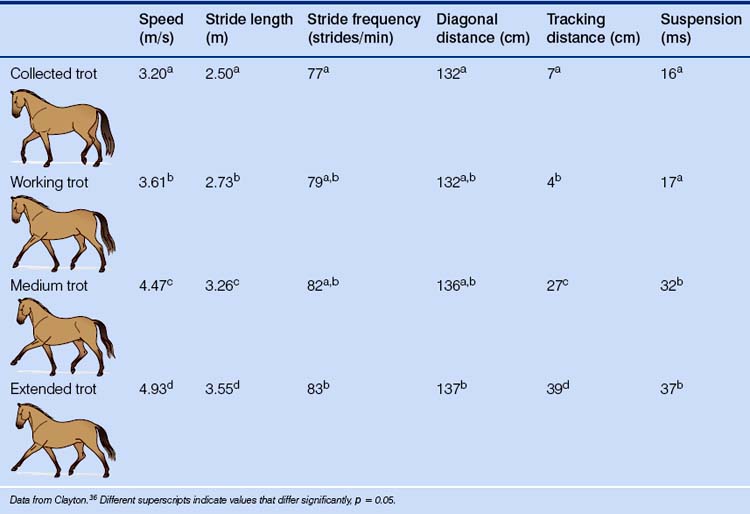
The trot is a two-beat gait in which the diagonal pairs of limbs move in apparent synchrony and the footfalls of the diagonal limb pairs are evenly spaced in time (Fig. 7.12).34 The four recognized types of trot (collected, working, medium and extended) are distinguished by their speed, stride length, stride frequency, lateral distance and tracking distance (Table 7.6).
Figure 7.12 The cycle of movements at the trot. The weight-bearing limbs at each stride are indicated.
Table 7.6 Mean values for stride kinematics of the collected, working, medium and extended trot in FEI-level dressage horses
The interval between the contacts of the fore- and hindlimb with the ground is termed the diagonal advanced displacement. This value is positive if the hindlimb contacts the ground before the forelimb and is negative if vice versa (and zero if both contact simultaneously) (Fig. 7.13). Although it is no longer, strictly speaking a trot (since, according to FEI Rules of Dressage,35 a trot must be a two-beat gait), a trot with a positive diagonal advanced displacement is considered desirable for dressage. The extended trot variable that correlates closest with high scores in elite dressage competitions is stride length (Fig. 7.14).30

Figure 7.13 Negative diagonal advanced placement (DAP) occurs when the foreleg begins its stance phase before its diagonal hindlimb. Positive DAP is when the opposite situation occurs: the hindlimb begins its stance phase before the forelimb.
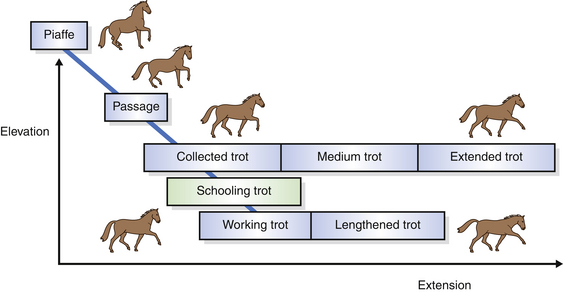
Figure 7.14 This graph shows the increased collection and simultaneous shortening of the stride as the horse becomes more educated toward piaffe.
(Reproduced with permission of Australian Equine Behaviour Centre.)
At the trot, stride length depends on the distance between both diagonal pairs of hoofprints (diagonal distance) and lateral pairs (tracking distance). To increase stride length, the horse must increase the suspension phase of the trot by pushing off the ground with a greater vertical velocity (Fig. 7.15).36 Ponies have a larger range of protraction and retraction than horses, with a more protracted forelimb and a more retracted hindlimb, therefore they demonstrate a more extended trot.29 However, they move with a stiffer trot than the supple trot of horses, which show a larger maximal fetlock extension during the stance phase.29
Pace
The most lateral gait, the pace, is innate for some horses (e.g. Icelandics) but can be acquired by others. The progeny of pacers almost always pace, whereas only some 20% of the offspring of trotters are innate pacers.37 As with the trot, there are two periods of suspension in each pace stride. Ipsilateral couplets are used almost synchronously so that the left fore is swung with the left hind, and the right fore with the right hind. The hindfoot makes contact with the ground before the ipsilateral foreleg (Fig. 7.16).38 Although the pace is often described as a two-beat gait, there is sufficient dissociation between the footfalls at racing speed for it to be considered a four-beat gait.35 A pacing horse tends to swing or ‘wag’ its hindquarters from side to side, while its whole body may appear to rise and fall in a motion similar to the trot.
Stepping pace
In five-gaited show horses, the stepping (or broken) pace is one of the acquired gaits characterized by lateral rather than diagonal couplets, and the return of the hindleg to the ground before the ipsilateral foreleg, which undergoes pronounced elevation (Fig. 7.17). The horse is supported by one, two or three legs at a time.
Rack
In five-gaited show horses, such as occur within the American Saddlebred to name but one of the numerous so-called gaited breeds, the footfall sequences of the lateral walk are applied in a variation called the rack. The beat is an even 1–2–3–4 (Fig. 7.18). With striking animation achieved largely through heightened foreleg action, the rack can be performed with considerable speed, stride frequencies reaching 120 per minute.39 The horse is supported first by two, then by one hoof at a time, jumping forward between transverse pairs of legs (both front, both hind). There is a moment when the horse’s weight is supported by first one hindhoof, then by one front hoof.
When Icelandics travel at the hreina (meaning clear) tölt, there is no ‘jump’ in the movement. The hreina tölt has the footfall sequences of the lateral walk, with an even 1–2–3–4 beat. Only one or two feet support the horse’s weight, with never a moment of suspension or a moment of three-legged weight bearing. In the higher speeds the two-feet-weight-bearing phase becomes shorter while the one-foot-weight-bearing phase becomes longer. Since the horse never takes its weight from the ground, this gait is very comfortable for the rider, and allows the horse to be sure-footed in uneven terrain. Icelandic horses are the only breed in which individuals can innately show both pace and tölt from slow to gallop speed.
Passage and piaffe
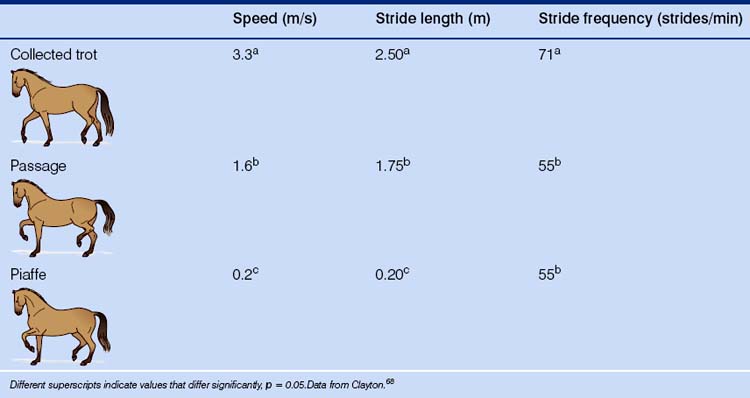
With diagonal pairs of fore- and hindlimbs moving more or less in synchrony, the passage and its almost stationary analogue, the piaffe, are similar in footfall sequencing to the trot but differ from even a collected trot in terms of speed and stride length (Table 7.7). With two suspensions per stride and a characteristically long positive diagonal advanced displacement, the passage involves marked elevation of the limbs, which visibly pause for a moment before descending. It is seen in free-ranging horses as the prancing display characteristic of stallions (see Ch. 6).3 Classically, the toe of the forehoof should be elevated to the middle of the contralateral cannon, while the hindhoof should rise slightly above the contralateral fetlock. Notably, none of the individual medal finalists in the Barcelona Olympics demonstrated this degree of forelimb elevation.40
Table 7.7 Mean values for stride kinematics of the collected trot, passage and piaffe in dressage horses in the individual medal finals of the Barcelona Olympics
Studies of the ground reaction forces during passage demonstrate its similarity to the collected trot, in that the hindlimbs provide forward and upward propulsion while the forelimbs have the higher peak vertical force and elevate the forehand while providing longitudinal retardation.35 Conversely, the ground reaction forces from the piaffe show that the forelimb provides all there is of longitudinal propulsion, while the hindlimb has a retarding influence.35 The piaffe shares a momentary pause at the peak of elevation in the swing phase, but can be readily distinguished from all other variants of the trot by having little, if any, forward movement and no suspension phase – there is always at least one hoof in contact with the ground.
Canter and gallop
The canter, with three beats to the stride (Fig. 7.19), and the gallop (Fig. 7.20), with four, are asymmetrical gaits and are labeled left or right in reference to the leading foreleg and ipsilateral hindleg. The body pivots over the leading leg, which is the last leg to lift off the ground. As the horse leans toward or makes a turn to the right, it usually leads with its right leg, and vice versa. So, a right-lead canter footfall sequence is as follows: left hind, right hind/left fore, right fore. Conversely, a left-lead canter footfall sequence is: right hind, left hind/right fore, left fore.
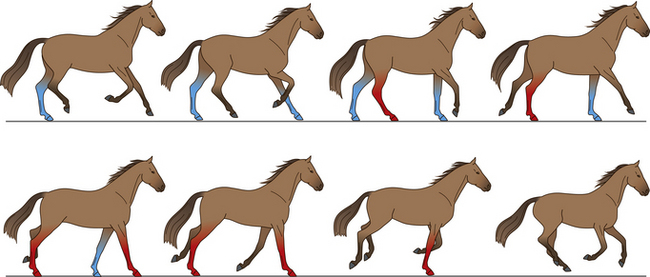
Figure 7.19 The cycle of movements at the canter (right-lead). The weight-bearing limbs at each stride are indicated.
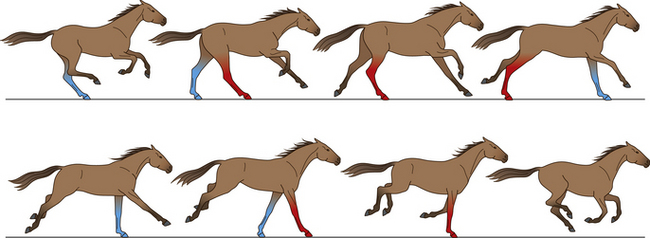
Figure 7.20 The cycle of movements at the gallop (right-lead). The weight-bearing limbs at each stride are indicated.
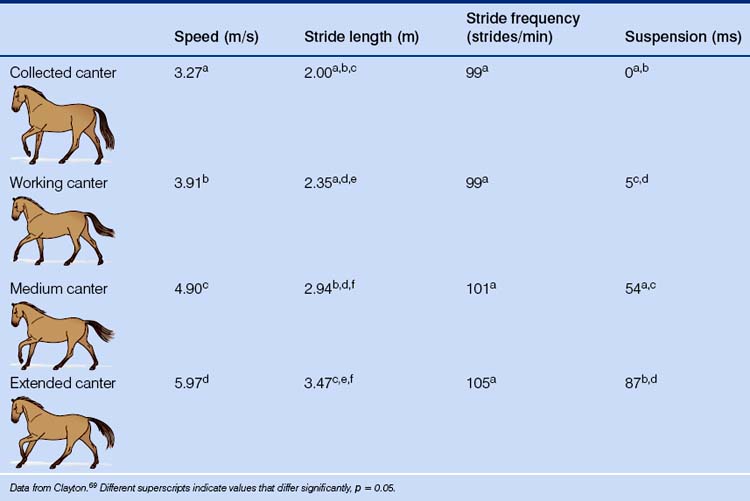
Speed can be used to distinguish the four types of canter recognized by the FEI (Table 7.8), with stride length being the cardinal marker of elite horses performing the extended canter.30 In its slowest form, the canter may adopt a four-beat pattern as the second and third feet meet the ground separately. Paradoxically, as the speed increases, the four beats return with a diagonal or transverse sequencing that distinguishes the equine gallop from the lateral or rotary gallop of the cheetah.
Table 7.8 Mean values for stride kinematics in the collected, working, medium and extended canters in FEI-level dressage horses
Although minimal dorsolateral flexion of the vertebral column is noted during galloping, systematic angular displacement of the neck occurs, presumably to assist balance. The angulation correlates with support periods of the individual legs so, effectively, the head is raised maximally shortly after the lead foreleg leaves the ground.41 The rolling action of the neck gives the impression of the horse’s cervical musculature lifting the body forward as it descends3 (Fig. 7.21). This notion is further supported by evidence that the semispinalis capitis and splenius muscles differ in terms of their fiber type and histochemistry.42
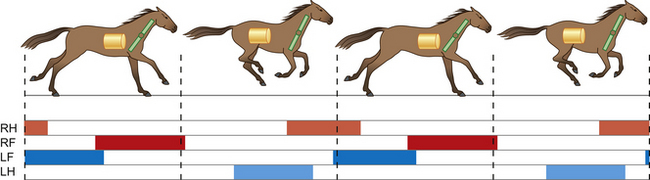
Figure 7.21 The pendular action of the head and neck are coordinated with the footfalls of the gallop stride, so that propulsion from each limb is optimized sequentially. (The barrel represents the centre of gravity.)
(Copyright Stephen Budiansky. Redrawn, with permission, from Budiansky.25)
Neck movement is not a feature of natural equine locomotion that is encouraged in dressage and the show ring, where maintaining head carriage is considered desirable. This is problematic for horses since there may be a modern obsession with imposing neck flexion on ridden horses. The longitudinal neck flexion of the degree desirable by popular opinion in ridden horses is not a common feature of unridden horses moving naturally.43 Regardless of head restraint, the vertebral column, rather like the frame of a bellows, contributes to the forces of ventilation as it undergoes flexion and extension. For this reason each gallop stride is accompanied by a respiratory cycle with the horse exhaling as it lands on its leading foreleg.44
It is fascinating to note that, without prompting by riders, horses need considerable space to canter readily. It has been demonstrated that the tendency for horses to canter is restricted in fields of 1.5 ha or less.45 Therefore, the dimensions of available paddocks should be borne in mind when turnout is advocated as an effective means of normalizing a stabled horse’s behavior.
Airs above the ground
A number of in-place leaps can be achieved with meticulous training (Fig. 7.22). Evocative as they are of the military origins of dressage, they are not performed in FEI competitions but instead form part of demonstrations of haute école training. All of these maneuvers involve the horse beginning by elevating its forehand. With the forelegs tightly flexed and the vertebral column inclined between 30° and 45° above the horizontal, this is called the levade. Maintaining this posture requires balance, strength and the ability to flex the hindquarters profoundly. A series of levades joined together by the horse rocking smoothly forward and punctuated briefly by the forelegs contacting the ground is called mezair.
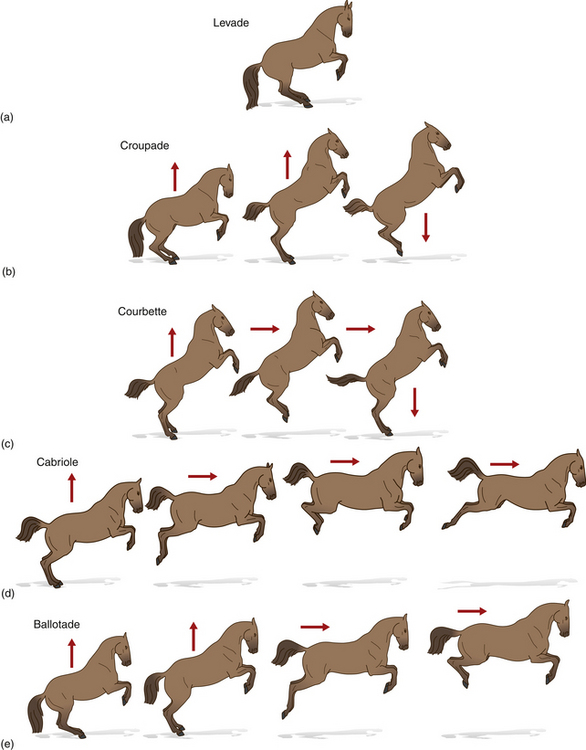
Figure 7.22 The airs above the ground: (A) levade; (B) croupade; (C) courbette; (D) cabriole; (E) ballotade.
(Redrawn, with permission, from Waring.3)
If the horse leaps off the ground from a levade and lands in virtually the same place, it is said to have performed a croupade. This is distinct from a courbette, which integrates deliberate forward movement within the leap. The cabriole is a smooth sequence of movements in which the horse emerges from levade to take off and then kick backwards with the hindlimbs while at the peak of the airborne phase. In the ballotade the horse leaves the ground in much the same fashion, but tucks its hindlegs underneath it.
Other locomotory maneuvers
Jumping
Unless trained to jump, horses generally avoid ditches and horizontal obstacles. Being able to see where to place their feet is critical for their safety. To gallop full-tilt into the unknown is inherently aversive and so must be trained. This explains why foals are not seen spontaneously leaping out of paddocks for the sheer joy of jumping. Of course, some horses are sufficiently trained to tackle fences without their jockeys, but this reflects their conditioning and the herd effect rather than any thrill they may feel from soaring over a man-made obstacle. Horses that bolt toward jumps in show-jumping and cross-country are recognized as a danger to themselves and their riders. Running at hazards should not be confused with choosing to jump.46 Experienced horses approach fences in an active canter.47 This makes sense because, in its simplest form, jumping can be viewed as an animated canter stride, except that at the moment of take-off a half-bound is inserted that aligns the hindlimbs so that both can be used to push off the ground together.34 In 92 of 96 jumps analyzed (over oxers; see Glossary), the leading forelimb was the appendage closest to the fence at take-off.48 In the remaining four jumps in this series, the leading hindleg was the appendage closest to the fence at take-off. The non-leading hindlimb is usually placed between the hoofprints of the two forelimbs at take-off.48
Although limb placements do not differ between a vertical fence and an oxer, more faults occur when horses jump the former,34 suggesting that horses regularly get too close to fences. Forelimb errors, often forced by riders, are involved in the vast majority of jumping faults (e.g. 87% in one study of trunk accelerations49).
A study of 72 horses jumping in 609 rounds of competition produced some fascinating results that demonstrated that the number of faults at a particular obstacle depended on several major variables: the obstacle-type, height, color and arrangement.50 Attempts to jump the third and fourth obstacles in the courses were faulty most often. Furthermore, while walls were the most often avoided by lateral run-outs, uprights and oxers were the most frequently knocked down. The least number of faults was accrued at the second obstacle in a combination compared with the first and third.50
A trajectory of 45° is implied by riding manuals that advocate the ideal take-off point for a simple vertical fence as a distance equal to its height. However, this dictum has been questioned in the light of data that demonstrate Grand Prix show-jumpers using reasonably consistent take-off distances over a variety of upright and spread fences.25 In contrast to a standard show-jumping technique, steeplechasing is associated with placing both hindlimbs closer to the fence than the leading forelimb.47
When a horse lands after a jump, the non-leading forelimb is the first to meet the ground. It provides forward propulsion that carries the horse along, in contrast to the next limb to make contact, the leading forelimb, which has an effective braking action. The return of the trailing hindlimb, which provides more forward thrust, means that the horse has resumed a normal canter stride by the time the fourth foot falls. Good jumpers naturally tuck their forelegs more than average jumpers, and therefore, in untrained horses, this feature and the absence of excessive speed while approaching fences are considered the best predictors of later competitive success.51
Bucking
Arching and humping the vertebral column in combination with retrograde propulsion of the forehand (‘pig-rooting’) occur in play and as a resistance to forward movement in horses under-saddle or on the lunge. Classically, the horse is said to be bucking when the hindlegs leave the ground. If the horse vaults into the air completely, it is described as buck-jumping. Although bucking is often interpreted as a demonstration of joie de vivre, it is worth remembering that in evolutionary terms it may have been a maneuver to dislodge a predator or, possibly, to signal to predators ‘don’t bother with me, I’m in the very best of health’. It is important to eliminate pain, either from the saddle or in the horse’s vertebral column, before assuming that a horse that starts to buck is simply being disobedient (see Ch. 15).
Rearing
Generally an unwelcome response in ridden horses, rearing is a defensive maneuver used to evade aversive stimuli suddenly appearing to the fore, and an intrinsic part of the horse’s agonistic repertoire. It is difficult to resolve because as an evasion it is often the result of conflicting rein pressure and leg pressure. For that reason, there is little left for the rider to do in the case of a rearing horse but to hang on. It is easy to see how a rear can be unintentionally reinforced as the rider removes pressure from the legs in a bid to stay on-board. Since it can be difficult for horses to balance on their hindlegs, the greatest danger is that the horse may fall to one side and crush the rider. For this reason many texts advise the rider to dismount from a rearing horse as soon as possible. However, to eliminate any reinforcing outcomes if the behavior has emerged as an evasion, it is important to drive the horse forward towards the stimulus that evokes the resistance. Therefore, many experienced riders choose to stay on-board to pursue the lesson. They often turn the horse as it descends to the ground so that they have an increased chance of triggering forward movement by avoiding the stimuli that prompted the horse to rear.
Interestingly, in horses, such as circus animals, taught to rear, this maneuver is one of the first responses that proves difficult to achieve as the horse approaches old age. The strength a horse needs to support itself on its hindlegs and balance there is considerable and can prove limiting for these performers, as it does for geriatric stallions when required to mount mares.
Swimming
When swimming, the horse uses its hooves as paddles with a trotting action, but is required to keep the neck extended and the head relatively still. Despite the buoyancy afforded by displacing such a large volume of water, the effort required to move in the water with such small paddles, while inflating the lungs against the pressure of the surrounding water, makes swimming very hard work (Fig. 7.23). This accounts for much of the grunting one hears from horses as they swim. The benefits of swimming in the conditioning of racehorses and as a therapy for orthopedic injuries are well recognized but may be offset by the respiratory effort required from the horse, the coaxing required by personnel and the risk of injury associated with the activity.
Influences on locomotion
The effect of conditioning
Naïve riding animals may trip when ridden, simply because they are unused to supporting the weight of a rider. At the other end of the equine educational spectrum, correctly trained dressage horses move in self-carriage, which implies lightness of the forehand and a greater reliance on the hindquarters for propulsion.34 At the walk and the trot, trained horses have an increased ratio of swing to stride duration. This is achieved by a short stance duration and reduced flexion in the hindlimb, which combine so that maximal protraction occurs earlier in the stride. The action is characterized by increased fetlock extension, which is said to illustrate that more weight is being carried by the hindlimbs. This is what equestrians refer to as ‘engagement of the hindquarters’. Most notable at the trot, the hindlimbs generate what is termed ‘impulsion’ by operating faster and with less limb flexion. Although these variables may seem of importance chiefly to dressage competitors, similar changes as a result of fitness-training are reported in young Standardbreds trained for 5 months. They show increased carpal and fetlock extension in the forelimb and increased tarsal and fetlock extension in the hindlimb.52 The gait of pastured horses is characteristically more relaxed, with the forelimb acting as a passive strut and therefore giving the impression of movement on the forehand.34
The effect of the rider
Kinematics change when a horse carries a rider,53 and the effects tend to be amplified as a function of the rider’s weight. When first ridden under-saddle as part of foundation training, horses show movement that is described as being short in front.34 This reflects the need to increase muscular strength and a new balance in order to support the rider. With training, most young horses find their balance and are therefore able to carry themselves ‘on the bit’ while being ridden (see Ch. 13).34 The extent to which a rider can help a horse balance is the stuff of riding manuals rather than a horse behavior text. However, it is worth noting that, with experience, and as their balance improves, riders adopt a more upright position in the saddle and other characteristics of a good seat as illustrated in Figure 7.24. Although this posture may make humans more effective as riders, because it makes them more stable in the saddle and increases the flexibility of the legs for delivering signals, it is not clear whether it makes the horse’s task easier. With the rider in a more upright position, the centre of gravity shifts to the hindquarters, which, in an educated animal, are required to do proportionally more work than when at pasture.
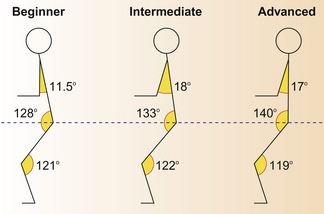
Figure 7.24 At a sitting trot, the angles of the arm, hip joint and knee joint of the beginner, intermediate and advanced riders show significant differences that can have an effect on the way in which the horse can carry itself.
(Redrawn from Schills.)
During the rise phase of a rising trot, riders may set up a left–right asymmetry in ground reaction forces, but competent riders are able to compensate for this by shifting part of the weight to the hindlimbs.54
A great deal of effort on the part of riders goes into pursuit of a desirable outline in their horses, which involves the nasal planum being within 6° of the vertical. To the novice observer, this gives the illusion of the horse being in self carriage and therefore well-trained. Unfortunately, regardless of whether the horse has learned to carry itself that way or whether it has simply lost the battle with its rider to keep its head in a more natural position, the effect of head and neck flexion is variable upper airway obstruction (Fig. 7.25).55
It has been shown that in 87% of cases, faults attracted during jumping can be blamed on forelimb error,49 and they are usually the result of inappropriate stimuli from the rider.56 Interestingly, over-jumping occurs when horses are ridden by less competent riders. The effect of dead weight on a horse’s ability to jump is seen chiefly in the clearance achieved by the leading forelimb and the extension of the fetlock and carpal joints on landing.57 Because these indices are respectively precursors of falls over solid fences and strains of the suspensory ligament and the superficial digital flexor tendon,34 these data led to the abolition of the minimum weight rule for eventers.
Responses to the whip
In a study of Quarterhorses at the gallop, the use of a whip on the shoulder of the leading forelimb, in rhythm with the stride, did not increase speed but reduced stride length and increased stride frequency.58 Analysis of racetrack patrol videos has shown that 38% of breakdown injuries occur after use of the whip.59 (This may reflect the rider’s response to the horse beginning to pull up with an acute lameness.)
When measurements of whip strikes and sectional times during each of the final three 200-m sections of 5 races were analyzed, jockeys in more advanced placings at the final 400-m and 200-m positions in the races were seen to whip their horses more frequently.60 However, horses, on average, achieved highest speeds in the 600-m to 400-m section when there was no whip use, and the increased whip use was most frequent in the final two 200-m sections when horses were fatigued. This increased whip use was not associated with significant variation in velocity as a predictor of superior placing at the finish. Under an ethical framework that considers costs paid by horses against benefits accrued by humans,61 these data make whipping tired horses in the name of sport very difficult to justify.
Responses to confinement and refusal to load
Many horses seem to find pleasure in exercise.9 When mares were confined in standing stalls for 6 months, they showed no significant differences in baseline plasma cortisol concentrations or in cortisol responses to ACTH compared with exercised controls.62 However, in the same way that crib-biters show more of the behavior after a period of deprivation, confined horses show a post-inhibitory rebound in locomotion, indicating a compensatory response to exercise deprivation.13 When, after 6 months of confinement, exercise was permitted, a group of confined mares showed an increase in plasma cortisol concentration, whereas mares given 30 minutes of exercise per day showed a decrease.62
Unfortunately, because the motivation for movement and indeed general activity is greater in horses kept in confined and isolated environments,63 turning-out can be associated with injury. Airs above the ground are not uncommon when confined horses are turned-out (Fig. 7.26). This can have harmful effects on the musculoskeletal system, which is generally in poor condition as a result of confinement.64

Figure 7.26 A 27-year-old horse frolicking enthusiastically after 3 months of box rest.
(Reproduced by permission of the University of Bristol, Department of Clinical Veterinary Science.)
Confinement does not just bring reduced opportunities for exercise, but is often also associated with the absence of companions, a reduced ability to observe surroundings, and relative darkness. The enriching effects of being at pasture may help to account for data showing that training takes less time in pastured horses than stabled horses.65 Horses in a dark stabled environment have demonstrated a preference for a lighted environment by learning to turn on lights by means of an operant device (trial-and-error conditioning).11 Similarly, since stress responses are associated with confinement in stalls,63 one would predict that horses would prefer to remain outside a trailer (a float in the USA and Australia) (see Fig. 2.7). There is generally very little reinforcement to be found in a confined space and, therefore, using the principles of learning theory, one would predict that the likelihood of the horse entering the trailer would naturally decline. This is often what happens when horses that naïvely load once or twice learn that it is a response that yields few rewards. If, when they balk and refuse to load, they are summarily punished, some learn to associate the presentation of the trailer with pain and back away from it.66 The subsequent introduction of ropes to drag the horse in, and various aversive stimuli to send the horse forward, do nothing to dissuade the horse of these associations. The learned fear abates only when the horse is allowed time to enter the confined space at its own pace and find reinforcement therein. Because of the horse’s resistance to behavioral extinction,9 this training should be conducted at home under relaxed conditions.
Owners of horses that load without being reinforced are fortunate to have obliging, tolerant and adaptable animals. Owners of horses that associate no benefits with being loaded do well to approach this problem by reinstalling the leading signals (see Ch. 13) and by instituting a remedial reward system. Given the time such an approach takes to bring results, there is a strong argument for putting rewards in place for all novice horses until loading becomes a habit. The rewards used include premium food items and the opportunity to join a favored affiliate. Offering youngsters daily feeds in a stable (then in a horse-box and then a trailer) is a simple means of creating positive associations with confinement.
The effect of warming up
Routine exercises before competitive work help to warm up the muscles, establish rhythm and reacquaint the horse with the tasks currently required. The effects of such warm-up exercises on stride length are considerable. In endurance horses, stride length at the walk and at the trot, respectively, can increase to 115% and 151% of their pre-race values after 41 km.67
Hyperflexion or rollkur (or long-deep-and-round or low-deep-and-round) are terms used to describe a technique where the horse’s neck is dorsoventrally hyperflexed as a result of bit pressure to the point where the horse’s chin may touch its pectoral region. It is used in training and warming-up horses prior to competition but would attract penalties if seen in dressage competitions themselves, since the horse would be judged to be overbent (the nasal plane being carried behind the vertical). Proponents of this method claim that they shape this response gradually and that the horse is ridden in this ‘frame’ (posture) for only short periods. However, many observers have described longer durations,70,71 and the mechanisms by which this technique might achieve greater flexion of the hock joints (as is claimed) are not yet known. It is plausible that any advantages conferred by this technique in achieving higher steps occur as a result of post-inhibitory rebound where the extension of the nuchal ligament and concomitant alterations in the back muscles may, when released, permit higher steps.
Hyperflexion is believed to decrease stride length and increase elevation of the hindlimbs, while also increasing the dorsoventral oscillation of the lumbar vertebrae.72 Certainly, its current prevalence among elite dressage competitors strongly suggests that it lends some competitive advantage in that its use must help to produce a performance the judges wish to see, even though it is a warm-up practice that is at odds with what is required in the competition itself.
Summary of Key Points
• Movements in utero are of tremendous importance in priming a foal for precocial existence.
• Horses have a behavioral need to stretch and locomote.
• Gaits have evolved for economical energy use at any given speed.
• Kinematic studies are useful for understanding the within-gait differences required in dressage.
• Riders, whips, conditioning and warming-up can influence locomotion in ridden horses.
References
1. Fraser AF. The behavior of the horse. London: CAB International, 1992.
2. Rossdale PD, Ricketts SW. Equine stud farm medicine, 2nd edn. London: Baillière Tindall, 1980.
3. Waring GH. Horse behavior: the behavioral traits and adaptations of domestic and wild horses including ponies. Park Ridge, NJ: Noyes, 1983.
4. Campitelli S, Carenzi C, Verga M. Factors which influence parturition in the mare and development of the foal. Appl Anim Ethol. 1982;9(1):7–14.
5. Jean D, Laverty S, Halley J, et al. Thoracic trauma in newborn foals. Equine Vet J. 1999;31(2):149–152.
6. Fagen RM, George TK. Play behavior and exercise in young ponies (Equus caballus L). Behav Ecol Sociobiol. 1977;2:267–269.
7. Crowell-Davis SL. Developmental behavior. Vet Clin North Am Equine Pract: Behav. 1986:573–590.
8. Williams M. Horse psychology. London: JA Allen, 1976.
9. Kiley-Worthington M. The behavior of horses in relation to management and training. London: JA Allen, 1987.
10. McBride SD, Long L. Management of horses showing stereotypic behavior, owner perception and the implications for welfare. Vet Rec. 2001;148(26):799–802.
11. Houpt KA, Houpt TR. Social and illumination preferences of mares. J Anim Sci. 1989;66:2159–2164.
12. Fraser AF. Ontogeny of behavior in the foal. Appl Anim Ethol. 1980;6(3):303.
13. Freire R, Buckley P, Cooper JJ. Effects of different forms of exercise on post-inhibitory rebound behaviour and unwanted behaviour in stabled horses. Equine Vet J. 2008;41(5):487–492.
14. Caanitz H, O’Leary L, Houpt K, et al. Effect of exercise on behavior. Appl Anim Behav Sci. 1991;31(1–2):1–12.
15. Hutson GD. Watching racehorses: a guide to betting on behavior. Melbourne: Clifton Press, 2002.
16. McGreevy PD, Rogers LJ. Motor and sensory laterality in Thoroughbred horses. Appl Anim Behav Sci. 2005;92(4):337–352.
17. McGreevy PD, Thomson PC. Differences in motor laterality in breeds of performance horse. Appl Anim Behav Sci. 2006;99(1–2):183–190.
18. Drevemo S, Fredricson I, Dalin G, Bjorne K. Equine locomotion, 2: The analysis of coordination between limbs of trotting Standardbreds. Equine Vet J. 1980;12:66–70.
19. Grzimek B. Die Rechts- und Linkshandigkeit bei Pferden, Papageien und Affen. Z Tierpsychol. 1949;6:406–432.
20. McGreevy PD. The functional significance of stereotypies in the stabled horse. PhD thesis, University of Bristol, 1995.
21. Drevemo S, Fredricson I, Hjerten G, McMiken D. Early development of gait asymmetries in trotting Standardbred colts. Equine Vet J. 1987;19:189–191.
22. Leach DH, Ormrod K, Clayton HM. Stride characteristics of horses competing in Grand Prix jumping. Am J Vet Res. 1984;45(5):888–892.
23. Burns TE, Clayton HM. Comparison of the temporal kinematics of the canter pirouette and collected canter. Equine Vet J Suppl. 1997;23:58–61.
24. Hoyt DF, Taylor CR. Gait and the energetics of locomotion in horses. Nature. 1981;292:23–24.
25. Budiansky S. The nature of horses – exploring equine evolution, intelligence and behavior. New York: Free Press, 1997.
26. Clayton HM. Gait analysis as a predictive tool in performance horses. J Equine Vet Sci. 1989;9:335–336.
27. Back W, Schamhardt HC, Hartman W, et al. Predictive value of foal kinematics for adult locomotor performance. Res Vet Sci. 1995;59:64–69.
28. Back W, Barneveld A, Schamhardt HC, et al. Kinematic detection of superior gait quality in young trotting warmbloods. Vet Quart. 1994;16:S91–96.
29. Back W, Schamhardt HC, van Weeren PR, Barneveld A. A comparison between the trot of pony and horse foals to characterise equine locomotion at young age. Equine Vet J Suppl. 1999;30:240–244.
30. Deuel NR, Park J. The gait patterns of Olympic dressage horses. Int J Sport Biomech. 1990;6:198–226.
31. Clayton HM. Comparison of the stride kinematics of the collected, medium and extended walks in horses. Am J Vet Res. 1995;56:849–852.
32. Hodson E, Clayton HM, Lanovaz JL. Temporal analysis of walk movements in the Grand Prix dressage test at the 1996 Olympic Games. Appl Anim Behav Sci. 1999;62:89–97.
33. DeHaven RW. The Horse Protection Act – case study in industry self-regulation. Animal Welfare Forum: Equine Welfare. J Am Vet Med Assoc. 2000;216(8):1250–1253.
34. Clayton HM. Performance in equestrian sports. In: Back W, Clayton HM. Equine locomotion. London: WB Saunders; 2001:193–226.
35. Fédération Equestre Internationale. Rules for dressage events. 22nd edition, Lausanne, Switzerland; 2008.
36. Clayton HM. Comparison of the stride kinematics of the collected, working, medium and extended trot in horses. Equine Vet J. 1994;26:230–234.
37. Cothran EG, MacCluer JW, Weitkamp LR, Bailey E. Genetic differentiation associated with gait within American Standardbred horses. Anim Genetics. 1987;18:285–296.
38. Wilson BD, Neal RJ, Howard A, Groenendyk S. The gait of pacers, 1: Kinematics of the racing stride. Equine Vet J. 1988;20:341–346.
39. Hildebrand M. Symmetrical gaits of horses. Science. 1965;186:1112–1113.
40. Argue CK. The kinematics of piaffe, passage and collected trot of dressage horses. PhD thesis, University of Saskatchewan, Saskatoon, 1994.
41. Rooney JR. The role of the neck on locomotion. Mod Vet Prac. 1978;59:211–213.
42. Gellman KS, Bertram JEA, Hermanson JW. Morphology, histochemistry, and function of epaxial cervical musculature in the horse (Equus caballus). J Morphol. 2002;251(2):182–194.
43. McGreevy PD, Harman A, McLean AN, Hawson LA. Over-flexing the horse’s neck: a modern equestrian obsession? J Vet Behav. 2010;5:180–186.
44. Young IS, RMcN Alexander, Woakes AJ, et al. The synchronisation of ventilation and locomotion in horses (Equus caballus). J Exp Biol. 1992;166:19–31.
45. Kusunose R, Hatakeyama H, Kubo K, et al. [Behavioral studies on yearling horses in field environments, I: Effects of field size on the behavior of horses] [Japanese]. Bull Equine Res Inst. 1985;22:1–7.
46. McLean AN, McGreevy PD. Ethical equitation: capping the price horses pay for human glory. J Vet Behav. 2010;5:203–209.
47. Leach DH, Ormrod K. The technique of jumping a steeplechase fence by competing event-horses. Appl Anim Behav Sci. 1984;12:15–24.
48. Clayton HM, Barlow DA. The effect of fence height and width on the limb placements of show-jumping horses. J Equine Vet Sci. 1989;9:179–185.
49. Barrey E, Galloux P. Analysis of the equine jumping technique by accelerometry. Equine Vet J Suppl. 1997;23:45–49.
50. Stachurska A, Pieta M, Nesteruk E. Which obstacles are most problematic for jumping horses? Appl Anim Behav Sci. 2002;77(3):197–207.
51. Powers PNR, Harrison AJ. A study on the techniques used by untrained horses during loose jumping. J Equine Vet Sci. 2000;20:845.
52. van Weeren PR, van den Bogert AJ, Back W, et al. Kinematics of the Standardbred trotter measured at 6, 7, 8 and 9 months on a treadmill, before and after 5 months of pre-race training. Acta Anat. 1993;146:154–161.
53. Sloet van Oldruitenborgh-Oosterbaan MM, van Schamhardt HC, Barneveld A. Effects of weight and riding on workload and locomotion during treadmill exercise. Equine Exer Physiol. 1995;4:413–417.
54. Schamhardt HC, Merkens HW, van Osch GJVM. Ground reaction force analysis of horse ridden at walk and trot. Equine Exer Physiol. 1991;3:120–127.
55. Petsche VM, Derksen FJ, Berney CE, Robinson NE. Effect of head position on upper airway function in exercising horses. Equine Vet J Suppl. 1995;18:18–22.
56. Lauk HD, Auer J, von Plocki KA. Zum Problem ‘Barren’ Uberblick, biomechanische Berechnungen und Verhaltungsbeobachtungen. Pferdeheilkunde. 1991;7:225–235.
57. Clayton HM. Effect of added weight on landing kinematics of jumping horses. Equine Vet J Suppl. 1997;23:50–53.
58. Deuel NR, Lawrence LM. Effects of urging by the rider on equine gallop stride limb contacts. Proc Equine Nutr Physiol. 1987;10:487–494.
59. Ueda Y, Yoshida K, Oikawa M. Analyses of race accident conditions through use of patrol video. J Equine Vet Sci. 1993;13:707–710.
60. Evans DL, McGreevy PD. An investigation of racing performance and whip use by jockeys in Thoroughbred races. PLoS ONE. 2011;6(1):e15622.
61. Jones B, McGreevy PD. Ethical equitation: applying a cost-benefit approach. J Vet Behav. 2010;5:196–202.
62. Houpt K, Houpt TR, Johnson JL, et al. The effect of exercise deprivation on the behavior and physiology of straight-stall confined pregnant mares. Anim Welfare. 2001;10(3):257–267.
63. Mal ME, Friend TH, Lay DC, et al. Physiological responses of mares to short-term confinement and social isolation. J Equine Vet Sci. 1991;11(2):96–102.
64. Bell RA, Nielsen BD, Waite K, et al. Daily access to pasture turnout prevents loss of mineral in the third metacarpus of Arabian weanlings. J Anim Sci. 2001;79(5):1142–1150.
65. Rivera E, Benjamin S, Nielsen B, et al. Behavioral and physiological responses of horses to initial training: the comparison between pastured versus stalled horses. Appl Anim Behav Sci. 2002;78(2–4):235–252.
66. Cooper JJ. Comparative learning theory and its application in the training of horses. Equine Vet J Suppl. 1998;27:39–43.
67. Lewczuk D, Pfeffer M. Investigation on the horses’ stride length during endurance race competition using video image analysis. In In: Conference on Equine Sports Medicine and Science. The Netherlands: Wageningen Pers; 1998. 249–250
68. Clayton HM. Classification of collected trot, passage and piaffe using stance phase temporal variables. Equine Vet J Suppl. 1997;23:54–57.
69. Clayton HM. Comparison of the collected, working, medium and extended canters. Equine Vet J Suppl. 1994;17:16–19.
70. Jenkins S. Dutch investigation into ‘Rollkur’ lunging. Horse and Hound, 14 October. http://www.horseandhound.co.uk/news/397/149313.html Accessed 26th February, 2008.
71. Thomsen L, Taylor J. Blue Tongue Video FAQ. Epona TV. http://epona.tv/uk/news/show/artikel/blue-tongue-video-faq/?tx_ttnews%5BbackPid%5D=388&cHash=17bfce3a7d. Accessed 2nd December, 2009.
72. van Weeren PR, Meyer H, Johnston C, et al. The effect of different head and neck positions on the thoracolumbar kinematics in the unridden horse. In: Report of the FEI Veterinary and Dressage Committee’s Workshop. The use of over-bending (‘Rollkur) in FEI Competition, 31 January, during the FEI Veterinary Committee meeting at the Olympic Museum, Lausanne. 8; 2006.