Screening for Endocrine and Metabolic Disease
Endocrinology is the study of ductless (endocrine) glands that produce hormones. A hormone acts as a chemical agent that is transported by the bloodstream to target tissues, where it regulates or modifies the activity of the target cell.
The endocrine system cannot be understood fully without consideration of the effects of the nervous system on the endocrine system. The endocrine system works with the nervous system to regulate metabolism, water and salt balance, blood pressure, response to stress, and sexual reproduction.
The endocrine system is slower in response and takes longer to act than the nervous system in transferring biochemical information. The pituitary (hypophysis), thyroid, parathyroids, adrenals, and pineal are glands of the endocrine system whose functions are solely endocrine related and have no other metabolic functions (Fig. 11-1). The hypothalamus controls pituitary function and thus has an important indirect influence on the other glands of the endocrine system. Feedback mechanisms exist to keep hormones at normal levels.
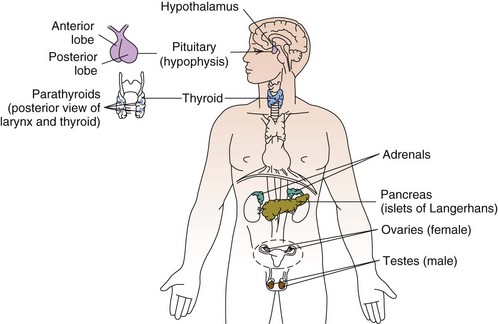
Fig. 11-1 Location of the nine endocrine glands. Not shown: adipose tissue (now classified as the largest endocrine gland in the body).
The endocrine system meets the nervous system in a complex series of interactions that link behavioral-neural-endocrine-immunologic responses. The hypothalamus and the pituitary form an integrated axis that maintains control over much of the endocrine system. The discovery and study of this complex interface axis is called psychoneuroimmunology (PNI) and has provided a new understanding of interactive biologic signaling.
The hypothalamus exerts direct control over both the anterior and posterior portions of the pituitary gland and can synthesize and release hormones from its axon terminals directly into the blood circulation. These neurosecretory cells are so-called because the neurons have a hormone-secreting function. Although neurons can have a hormone-secreting function, the opposite pathway is also present. Hormones that can stimulate the neural mechanism (e.g., acetylcholine) are called neurohormones. Acetylcholine is a neurotransmitter and a neurohormone. It is released at synapses to allow messages to pass along a nerve network, resulting in the release of both hormones and chemicals.
Associated Neuromuscular And Musculoskeletal Signs And Symptoms
The musculoskeletal system is composed of a variety of connective tissue structures in which normal growth and development are influenced strongly and sometimes controlled by various hormones and metabolic processes. Alterations in these control systems can result in structural changes and altered function of various connective tissues, producing systemic and musculoskeletal signs and symptoms (Table 11-1).
TABLE 11-1
Signs and Symptoms of Endocrine Dysfunction
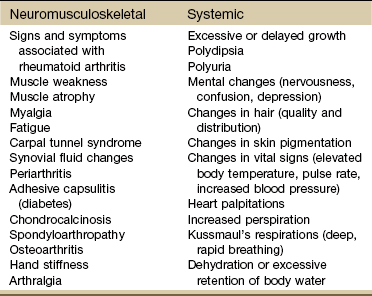
From Goodman CC, Fuller KS: Pathology: implications for the physical therapist, ed 3, Philadelphia, 2009, WB Saunders.
Muscle Weakness, Myalgia, and Fatigue
Muscle weakness, myalgia, and fatigue may be early manifestations of thyroid or parathyroid disease, acromegaly, diabetes, Cushing’s syndrome, and osteomalacia. Proximal muscle weakness associated with endocrine disease is usually painless and unrelated to either the severity or the duration of the underlying disease. The muscular system is sometimes but not always restored with effective treatment of the underlying condition.
Bilateral Carpal Tunnel Syndrome
Bilateral carpal tunnel syndrome (CTS), resulting from median nerve compression at the wrist, is a common finding in a variety of systemic and neuromusculoskeletal conditions1-3 but especially with certain endocrine and metabolic disorders (Table 11-2).4 The fact that the majority of persons with CTS are women at or near menopause suggests that the soft tissues about the wrist could be affected in some way by hormones.5-8
TABLE 11-2
Causes of Carpal Tunnel Syndrome

*The role of repetitive activities and occupational factors (e.g., hand use of any type and keyboard or computer work in particular) has been questioned as a direct cause of carpal tunnel syndrome (CTS) and remains under investigation; sufficient evidence to implicate hand use of any type linked with CTS remains unproven.3,4
Modified from Goodman CC, Fuller K: Pathology: implications for the physical therapist, ed 3, Philadelphia, 2009, Saunders.
Thickening of the transverse carpal ligament in certain systemic disorders (e.g., acromegaly, myxedema) may be sufficient to compress the median nerve. Any condition that increases the volume of the contents of the carpal tunnel (e.g., neoplasm, calcium, and gouty tophi deposits) can compress the median nerve.
The signs and symptoms often associated with CTS include paresthesia, tingling, and numbness and/or pain (or burning pain) with cutaneous distribution of the median nerve to the thumb, index, middle, and radial half of the ring finger. Nocturnal paresthesia is a common complaint, and this discomfort causes sleep disruption. It can be partially relieved by shaking of the hand or changing wrist and hand position. Pain may radiate into the palm and up the forearm and arm.9
It should be noted that bilateral tarsal syndrome affecting the feet also can occur either alone or in conjunction with CTS, although the incidence of tarsal tunnel syndrome is not high (see further discussion of tarsal tunnel syndrome in relation to carpal tunnel syndrome in Chapter 9). Bilateral median nerve neuritis can be characteristic of many systemic diseases, including rheumatoid arthritis, myxedema, localized amyloidosis, sarcoidosis, and infiltrative leukemia.10,11
Whenever a client presents with bilateral symptoms, it represents a red flag. With bilateral CTS the therapist can screen for medical disease by using the Special Questions to Ask: Bilateral Carpal Tunnel Syndrome section (see Appendix B-4).
Periarthritis and Calcific Tendinitis
Periarthritis (inflammation of periarticular structures, including the tendons, ligaments, and joint capsule) and calcific tendinitis occur most often in the shoulders of people who have endocrine disease. Treatment of the underlying endocrine impairment often improves the clinical picture; physical therapy intervention may have a temporary palliative effect.
Chondrocalcinosis
Chondrocalcinosis is the deposition of calcium salts in the cartilage of joints. When accompanied by attacks of gout-like symptoms, it is called pseudogout. Chondrocalcinosis is commonly seen on x-ray films as calcified hyaline or fibrous cartilage. There is an associated underlying endocrine or metabolic disease in approximately 5% to 10% of individuals with chondrocalcinosis (Table 11-3).
Spondyloarthropathy and Osteoarthritis
Spondyloarthropathy (disease of joints of the spine) and osteoarthritis occur in individuals with various metabolic or endocrine diseases, including hemochromatosis (disorder of iron metabolism with excess deposition of iron in the tissues; also known as bronze diabetes and iron storage disease), ochronosis (metabolic disorder resulting in discoloration of body tissues caused by deposits of alkapton bodies), acromegaly, and diabetes mellitus.
Endocrine Pathophysiology
Disorders of the endocrine glands can be classified as primary (dysfunction of the gland itself) or secondary (dysfunction of an outside stimulus to the gland) and are a result of either an excess or an insufficiency of hormonal secretions.
Secondary dysfunction may also occur (iatrogenically) as a result of chemotherapy, surgical removal of the glands, therapy for a nonendocrine disorder (e.g., the use of large doses of corticosteroids resulting in Cushing’s syndrome), or excessive therapy for an endocrine disorder.
Pituitary Gland
Diabetes insipidus (DI) is caused by a lack of secretion or action of vasopressin (antidiuretic hormone [ADH]). This hormone normally stimulates the distal tubules of the kidneys to reabsorb water. Without ADH, water moving through the kidney is not reabsorbed but is lost in the urine, resulting in severe water loss and dehydration through diuresis.
There are two main types of DI: central DI and nephrogenic DI. Central DI, which is the most common type, can be idiopathic (primary) or related to other causes (secondary), such as pituitary trauma, head injury (including neurosurgery), infections such as meningitis or encephalitis, pituitary neoplasm, anorexia, and vascular lesions such as aneurysms. Nephrogenic DI occurs as a result of some medications (e.g., lithium, phenytoin, corticosteroids, anticholinergics), alcohol, electrolyte imbalances such as hypercalcemia and hypokalemia, and diseases affecting the renal system (e.g., sarcoidosis, multiple myeloma, pyelonephritis, systemic lupus erythematosus).
If the person with DI is unconscious or confused and is unable to take in necessary fluids to replace those fluids lost, rapid dehydration, shock, and death can occur. Because sleep is interrupted by the persistent need to void (nocturia), fatigue and irritability result.
Syndrome of Inappropriate Secretion of Antidiuretic Hormone
Syndrome of inappropriate secretion of ADH (SIADH) is an excess or inappropriate secretion of vasopressin that results in marked retention of water in excess of sodium in the body. Urine output decreases dramatically as the body retains large amounts of water. Almost all the excess water is distributed within body cells, causing intracellular water gain and cellular swelling (water intoxication).
Risk Factors: Risk factors for the development of SIADH include pituitary damage caused by infection, trauma, or neoplasm; secretion of vasopressin-like substances from some types of malignant tumors (particularly pulmonary malignancies); and thoracic pressure changes from compression of pulmonary or cardiac pressure receptors, or both.
Clinical Presentation: Symptoms of SIADH are the clinical opposite of symptoms of DI. They are the result of water retention and the subsequent dilution of sodium in the blood serum and body cells. Neurologic and neuromuscular signs and symptoms predominate and are directly related to the swelling of brain tissue and sodium changes within neuromuscular tissues.
Acromegaly
Acromegaly is an abnormal enlargement of the extremities of the skeleton resulting from hypersecretion of growth hormone (GH) from the pituitary gland. This condition is relatively rare and occurs in adults, most often owing to a tumor of the pituitary gland. In children, overproduction of GH stimulates growth of long bones and results in gigantism, in which the child grows to exaggerated heights. With adults, growth of the long bones has already stopped, so the bones most affected are those of the face, jaw, hands, and feet. Other signs and symptoms include amenorrhea (in women), diabetes mellitus, profuse sweating, and hypertension.
Clinical Presentation: Degenerative arthropathy may be seen in the peripheral joints of a client with acromegaly, most frequently attacking the large joints. On x-ray studies, osteophyte formation may be seen, along with widening of the joint space because of increased cartilage thickness. In late-stage disease, joint spaces become narrowed, and occasionally chondrocalcinosis may be present.
Stiffness of the hand, typically of both hands, is associated with a broad enlargement of the fingers from bony overgrowth and with thickening of the soft tissue. Thickening and widening of the phalangeal tufts are typical x-ray findings in soft tissue. In clients with these x-ray findings, much of the pain and stiffness is believed to be due to premature osteoarthritis.
CTS is seen in up to 50% of people with acromegaly. The CTS that occurs with this growth disorder is thought to be caused by compression of the median nerve at the wrist from soft tissue hypertrophy or bony overgrowth or by hypertrophy of the median nerve itself.
Myopathy in people with acromegaly is commonly reported but poorly understood. Changes in muscle size and strength are associated with acromegaly and are probably multifactorial in origin. Screening individuals with acromegaly for muscle weakness and poor exercise tolerance is now recommended.12
About half the individuals with acromegaly have back pain. X-ray studies demonstrate increased intervertebral disk spaces and large osteophytes along the anterior longitudinal ligament (ALL), mimicking diffuse idiopathic skeletal hyperostosis (DISH).
DISH (also known as Forestier’s disease) is characterized by abnormal ossification of the ALL, resulting in an x-ray image of large osteophytes seemingly “flowing” along the anterior border of the spine. DISH is particularly common in the thoracic spine and has been reported to be more prevalent among persons with diabetes than among the nondiabetic population. DISH appears to be an age-related predisposition to ossification of tendon, joint capsule, and ligamentous attachments. Identification of the presence of DISH syndrome prior to surgery is important in the prevention of heterotropic bone formation.13
Adrenal Glands
The adrenals are two small glands located on the upper part of each kidney. Each adrenal gland consists of two relatively discrete parts: an outer cortex and an inner medulla. The outer cortex is responsible for the secretion of mineralocorticoids (steroid hormones that regulate fluid and mineral balance), glucocorticoids (steroid hormones responsible for controlling the metabolism of glucose), and androgens (sex hormones). The centrally located adrenal medulla is derived from neural tissue and secretes epinephrine and norepinephrine. Together, the adrenal cortex and medulla are major factors in the body’s response to stress.
Adrenal Insufficiency
Primary Adrenal Insufficiency: Chronic adrenocortical insufficiency (hyposecretion by the adrenal glands) may be primary or secondary. Primary adrenal insufficiency is also referred to as Addison’s disease (hypofunction), named after the physician who first studied and described the associated symptoms. It can be treated by the administration of exogenous cortisol (one of the adrenocortical hormones).
Primary adrenal insufficiency occurs when a disorder exists within the adrenal gland itself. This adrenal gland disorder results in decreased production of cortisol and aldosterone, two of the primary adrenocortical hormones. The most common cause of primary adrenal insufficiency is an autoimmune process that causes destruction of the adrenal cortex.
The most striking physical finding in the person with primary adrenal insufficiency is the increased pigmentation of the skin and mucous membranes. This discoloration may vary in the white population from a slight tan or a few black freckles to an intense generalized pigmentation, which has resulted in persons being mistakenly considered to be of a darker-skinned race. Members of darker-skinned races may develop a slate-gray color that may be obvious only to family members.
Melanin, the major product of the melanocyte, is largely responsible for the coloring of skin. In primary adrenal insufficiency, the increase in pigmentation is initiated by the excessive secretion of melanocyte-stimulating hormone (MSH) that occurs in association with increased secretion of adrenocorticotropic hormone (ACTH). ACTH is increased in an attempt to stimulate the diseased adrenal glands to produce and release more cortisol.
Most commonly, pigmentation is visible over extensor surfaces such as the backs of the hands; elbows; knees; and creases of the hands, lips, and mouth. Increased pigmentation of scars formed after the onset of the disease is common. However, it is possible for a person with primary adrenal insufficiency to demonstrate no significant increase in pigmentation.
Secondary Adrenal Insufficiency: Secondary adrenal insufficiency refers to a dysfunction of the gland because of insufficient stimulation of the cortex owing to a lack of pituitary ACTH. Causes of secondary disease include tumors of the hypothalamus or pituitary, removal of the pituitary, or rapid withdrawal of corticosteroid drugs. Clinical manifestations of secondary disease do not occur until the adrenals are almost completely nonfunctional and are primarily related to cortisol deficiency only.
Cushing’s Syndrome
Cushing’s syndrome (hyperfunction of the adrenal gland) is a general term for increased secretion of cortisol by the adrenal cortex. When corticosteroids are administered externally, a condition of hypercortisolism called iatrogenic Cushing’s syndrome occurs, producing a group of associated signs and symptoms. Hypercortisolism caused by excess secretion of ACTH (e.g., from pituitary stimulation) is called ACTH-dependent Cushing’s syndrome.14
Therapists often treat people who have developed Cushing’s syndrome after these clients have received large doses of cortisol (also known as hydrocortisone) or cortisol derivatives (e.g., dexamethasone) for a number of inflammatory disorders (Case Example 11-1).
It is important to remember that whenever corticosteroids are administered externally, the increase in serum cortisol levels triggers a negative feedback signal to the anterior pituitary gland to stop adrenal stimulation. Adrenal atrophy occurs during this time, and adrenal insufficiency will result if external corticosteroids are abruptly withdrawn. Corticosteroid medications must be reduced gradually so that normal adrenal function can return.
Because cortisol suppresses the inflammatory response of the body, it can mask early signs of infection. Any unexplained fever without other symptoms should be a warning to the therapist of the need for medical follow-up.
Effects of Cortisol on Connective Tissue: Overproduction of cortisol or closely related glucocorticoids by abnormal adrenocortical tissue leads to a protein catabolic state. This overproduction causes liberation of amino acids from muscle tissue. The resultant weakened protein structures (muscle and elastic tissue) cause a protuberant abdomen, poor wound healing, generalized muscle weakness, and marked osteoporosis (demineralization of bone causing reduced bone mass), which is made worse by an excessive loss of calcium in the urine.
Excessive glucose resulting from this protein catabolic state is transformed mainly into fat and appears in characteristic sites, such as the abdomen, supraclavicular fat pads, and facial cheeks. The change in facial appearance may not be readily apparent to the client or to the therapist, but pictures of the client taken over a period of years may provide a visual record of those changes.
The effect of increased circulating levels of cortisol on the muscles of clients varies from slight to very marked. There may be so much muscle wasting that the condition simulates muscular dystrophy. Marked weakness of the quadriceps muscle often prevents affected clients from rising out of a chair unassisted. Those with Cushing’s syndrome of long duration almost always demonstrate demineralization of bone. In severe cases, this condition may lead to pathologic fractures, but it results more commonly in wedging of the vertebrae, kyphosis, bone pain, and back pain
Obesity, diabetes, polycystic ovarian syndrome, and other metabolic/endocrine problems can resemble Cushing’s syndrome. It is important to recognize critical indicators of this particular disorder, such as excessive hair growth, moonface, mood disorders, and increased muscle weakness, as indicators for further endocrine diagnostic testing.15
The poor wound healing that is characteristic of this syndrome becomes a problem when any surgical procedures are required. Inhibition of collagen formation with corticosteroid therapy is responsible for the frequency of wound breakdown in postsurgical clients.
Thyroid Gland
The thyroid gland is located in the anterior portion of the lower neck below the larynx, on both sides of and anterior to the trachea. The chief hormones produced by the thyroid are thyroxine (T4), triiodothyronine (T3), and calcitonin. Both T3 and T4 regulate the metabolic rate of the body and increase protein synthesis. Calcitonin has a weak physiologic effect on calcium and phosphorus balance in the body.
Genetics plays a role in thyroid disease. A family history of thyroid disease is a risk factor. Age and gender are also factors; most cases occur after age 50. Women are more likely than men to develop thyroid dysfunction.15 Data gathered on the medical history of the orthopedic physical therapy outpatient population indicate a 7% incidence of thyroid disease in the female population.16
Thyroid function is regulated by the hypothalamus and pituitary feedback controls, as well as by an intrinsic regulator mechanism within the gland itself. Basic thyroid disorders of significance to physical therapy practice include goiter, hyperthyroidism, hypothyroidism, and cancer. Alterations in thyroid function produce changes in hair, nails, skin, eyes, gastrointestinal (GI) tract, respiratory tract, heart and blood vessels, nervous tissue, bone, and muscle.
The risk of having thyroid diseases increases with age, but in people older than 60 years of age, it becomes more difficult to detect because it masquerades as other problems such as heart disease, depression, or dementia. Fatigue and weakness may be the first symptoms among older adults, often mistaken or attributed to normal aging. New-onset depression in the older adult population and anxiety syndromes are also symptoms that can indicate thyroid dysfunction.17
On the other hand, thyroid dysfunction can mimic signs and symptoms of aging such as hair loss, fatigue, and depression. The therapist may recognize problems early and make a medical referral, minimizing the client’s symptoms. A simple and inexpensive blood test called a thyroid-stimulating hormone (TSH) test is usually recommended to show whether the thyroid gland is hyperfunctioning or hypofunctioning.
Goiter
Goiter, an enlargement of the thyroid gland, occurs in areas of the world where iodine (necessary for the production of thyroid hormone) is deficient in the diet. It is believed that when factors (e.g., a lack of iodine) inhibit normal thyroid hormone production, hypersecretion of TSH occurs because of a lack of a negative feedback loop. The TSH increase results in an increase in thyroid mass.
Pressure on the trachea and esophagus causes difficulty in breathing, dysphagia, and hoarseness. With the use of iodized salt, this problem has almost been eliminated in the United States. Although the younger population in the United States may be goiter free, older adults may have developed goiter during their childhood or adolescent years and may still have clinical manifestations of this disorder.
Thyroiditis
Thyroiditis is an inflammation of the thyroid gland. Causes can include infection and autoimmune processes. The most common form of this problem is a chronic thyroiditis called Hashimoto’s thyroiditis. This condition affects women more frequently than men and is most often seen in the 30- to 50-year-old age group. Destruction of the thyroid gland from this condition can cause eventual hypothyroidism (Case Example 11-2).
Usually, both sides of the gland are enlarged, although one side may be larger than the other. Other symptoms are related to the functional state of the gland itself. Early involvement may cause mild symptoms of hyperthyroidism, whereas later symptoms cause hypothyroidism.
Hyperthyroidism
Hyperthyroidism (hyperfunction), or thyrotoxicosis, refers to those disorders in which the thyroid gland secretes excessive amounts of thyroid hormone. Graves’ disease is a common type of excessive thyroid activity characterized by a generalized enlargement of the gland (or goiter leading to a swollen neck) and often, protruding eyes caused by retraction of the eyelids and inflammation of the ocular muscles.
Clinical Presentation: Excessive thyroid hormone creates a generalized elevation in body metabolism. The effects of thyrotoxicosis occur gradually and are manifested in almost every system (Fig. 11-3 and Table 11-4).
TABLE 11-4
Systemic Manifestations of Hyperthyroidism
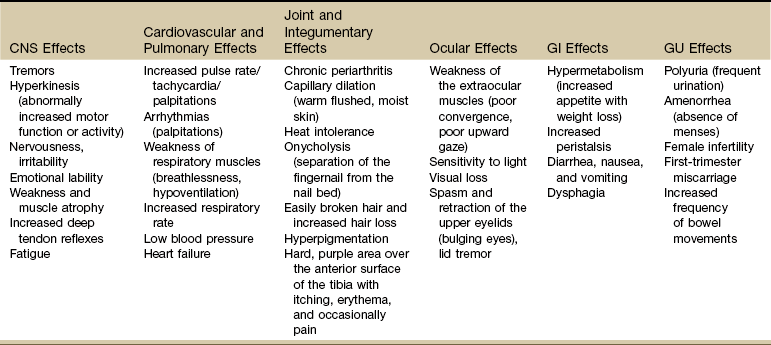
CNS, Central nervous system; GI, gastrointestinal; GU, genitourinary.
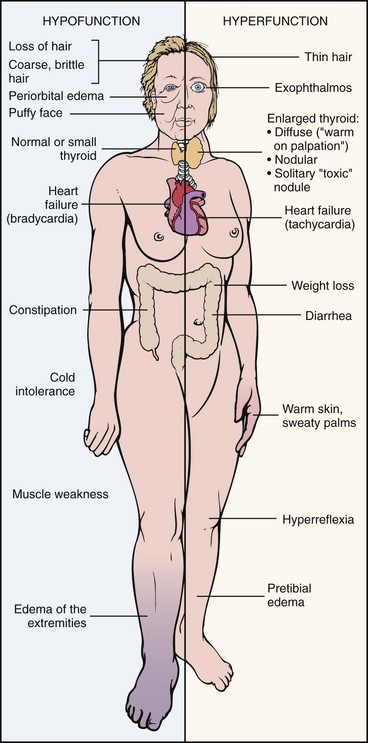
Fig. 11-3 Comparison of hyperthyroidism and hypothyroidism. (From Damjanov I: Pathology for the health-related profession, ed 3, Philadelphia, 2006, WB Saunders. Used with permission.)
In more than 50% of adults older than 70, three common signs are tachycardia, fatigue, and weight loss. In clients younger than 50, clinical signs and symptoms found most often include tachycardia, hyperactive reflexes, increased sweating, heat intolerance, fatigue, tremor, nervousness, polydipsia, weakness, increased appetite, dyspnea, and weight loss.18
Chronic periarthritis is also associated with hyperthyroidism. Inflammation that involves the periarticular structures, including the tendons, ligaments, and joint capsule, is termed periarthritis. The syndrome is associated with pain and reduced range of motion. Calcification, whether periarticular or tendinous, may be seen on x-ray studies. Both periarthritis and calcific tendinitis occur most often in the shoulder, and both are common findings in clients who have endocrine disease (Case Example 11-3).
Painful restriction of shoulder motion associated with periarthritis has been widely described among clients of all ages with hyperthyroidism. The involvement can be unilateral or bilateral and can worsen progressively to become adhesive capsulitis (frozen shoulder). Acute calcific tendinitis of the wrist also has been described in such clients. Although antiinflammatory agents may be needed for the acute symptoms, chronic periarthritis usually responds to treatment of the underlying hyperthyroidism.
Proximal muscle weakness (most marked in the pelvic girdle and thigh muscles), accompanied by muscle atrophy known as myopathy, occurs in up to 70% of people with hyperthyroidism. Muscle strength returns to normal in about 2 months after medical treatment, whereas muscle wasting resolves more slowly. In severe cases normal strength may not be restored for months.
The incidence of myasthenia gravis is increased in clients with hyperthyroidism, which in turn can aggravate muscle weakness. If the hyperthyroidism is corrected, improvement of myasthenia gravis follows in about two-thirds of clients.
Thyroid Storm: Life-threatening complications with hyperthyroidism are rare but still important for the therapist to recognize. Unrecognized disease, untreated disease, or incorrect treatment can result in a condition called thyroid storm. In addition, precipitating factors, such as trauma, infection, or surgery, can turn well-controlled hyperthyroidism into a thyroid storm.
Thyroid storm is characterized by signs and symptoms of hypermetabolism including severe tachycardia with heart failure, shock, and hyperthermia (up to 105.3° F [40.7° C]). Restlessness, agitation, chest pain, abdominal pain, nausea and vomiting, and coma can occur. Immediate medical referral is required to return the client to a normal thyroid state and prevent cardiovascular or hyperthermic collapse. Look for a recent history of the precipitating factors mentioned.
Hypothyroidism
Hypothyroidism (hypofunction) is more common than hyperthyroidism, results from insufficient thyroid hormone, and creates a generalized depression of body metabolism. Hypothyroidism in fetal development and infants is usually a result of absent thyroid tissue and hereditary defects in thyroid hormone synthesis. Untreated congenital hypothyroidism is referred to as cretinism.
The condition may be classified as either primary or secondary. Primary hypothyroidism results from reduced functional thyroid tissue mass or impaired hormonal synthesis or release (e.g., iodine deficiency, loss of thyroid tissue, autoimmune thyroiditis). Secondary hypothyroidism (which accounts for a small percentage of all cases of hypothyroidism) occurs as a result of inadequate stimulation of the gland because of anterior pituitary gland dysfunction.
Risk Factors: Women are 10 times more likely than men to have hypothyroidism. More than 10% of women over age 65 and 15% over age 70 are diagnosed with this disorder. Risk factors include surgical removal of the thyroid gland, external irradiation, and some medications (e.g., lithium, amiodarone).
Clinical Presentation: As with all disorders affecting the thyroid and parathyroid glands, clinical signs and symptoms affect many systems of the body (Table 11-5). Because the thyroid hormones play such an important role in the body’s metabolism, lack of these hormones seriously upsets the balance of body processes.
TABLE 11-5
Systemic Manifestations of Hypothyroidism
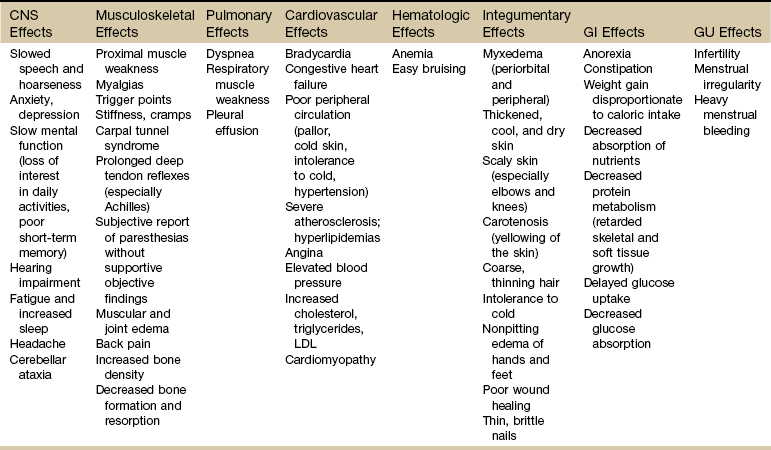
CNS, Central nervous system; LDL, low-density lipoprotein; GI, gastrointestinal; GU, genitourinary.
Among the primary symptoms associated with hypothyroidism are intolerance to cold, excessive fatigue and drowsiness, headaches, and weight gain. In women, menstrual bleeding may become irregular, and premenstrual syndrome (PMS) may worsen. Physical assessment often reveals dryness of the skin and increasing thinness and brittleness of the hair and nails. There may be nodules or other irregularities of the thyroid palpable during anterior neck examination.
Ichthyosis, or dry scaly skin (resembling fish scales; the word ichthyosis is derived from the Latin word ichthus, which means “fish”), may be an inherited dermatologic condition (Fig. 11-4). It may also be the result of a thyroid condition. It must not be assumed that clients who present with this condition are merely in need of better hydration or regular use of skin lotion. A medical referral is needed to rule out underlying pathology.
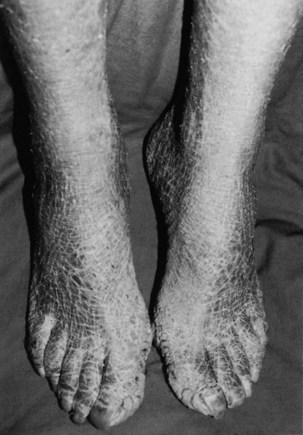
Fig. 11-4 Ichthyosis of the legs in a woman with severe hypothyroidism. (From Callen JP, Jorizzo J, Greer KE, et al: Dermatological signs of internal disease, Philadelphia, 1988, WB Saunders. Used with permission.)
Myxedema: A characteristic sign of hypothyroidism and more rarely associated with hyperthyroidism (Graves’ disease) is myxedema (often used synonymously with hypothyroidism). Myxedema is a result of an alteration in the composition of the dermis and other tissues, causing connective tissues to be separated by increased amounts of mucopolysaccharides and proteins.
This mucopolysaccharide-protein complex binds with water, causing a nonpitting, boggy edema, especially around the eyes, hands, and feet and in the supraclavicular fossae (Case Example 11-4). The binding of this protein-mucopolysaccharide complex causes thickening of the tongue and the laryngeal and pharyngeal mucous membranes. This results in hoarseness and thick, slurred speech, which are also characteristic of untreated hypothyroidism.
Clients who have myxedematous hypothyroidism may demonstrate synovial fluid that is highly distinctive. The fluid’s high viscosity results in a slow fluid wave that creates a sluggish “bulge” sign visible at the knee joint. Often, the fluid contains calcium pyrophosphate dihydrate (CPPD) crystal deposits that may be associated with chondrocalcinosis (deposit of calcium salts in joint cartilage). Thus a finding of a highly viscous, “noninflammatory” joint effusion containing CPPD crystals may suggest to the physician possible underlying hypothyroidism.
When such clients with hypothyroidism have been treated with thyroid replacement, some have experienced attacks of acute pseudogout caused by CPPD crystals remaining in the synovial fluid.
Neuromuscular Symptoms: Neuromuscular symptoms are among the most common manifestations of hypothyroidism. Flexor tenosynovitis with stiffness often accompanies CTS in people with hypothyroidism. CTS can develop before other signs of hypothyroidism become evident. It is thought that this CTS arises from deposition of myxedematous tissue in the carpal tunnel area. Acroparesthesias may occur as a result of median nerve compression at the wrist. The paresthesias are almost always located bilaterally in the hands. Most clients do not require surgical treatment because the symptoms respond to thyroid replacement.
Proximal muscle weakness sometimes accompanied by pain is common in clients who have hypothyroidism. As mentioned earlier, muscle weakness is not always related to either the severity or the duration of hypothyroidism and can be present several months before the diagnosis of hypothyroidism is made. Muscle bulk is usually normal; muscle hypertrophy is rare. Deep tendon reflexes are characterized by slowed muscle contraction and relaxation (prolonged reflex).
Characteristically, the muscular complaints of the client with hypothyroidism are aches and pains and cramps or stiffness. Involved muscles are particularly likely to develop persistent myofascial trigger points (TrPs). Of particular interest to the therapist is the concept that clinically any compromise of the energy metabolism of muscle aggravates and perpetuates TrPs. Treatment of the underlying hypothyroidism is essential in eliminating the TrPs,19 but new research also supports the need for soft tissue treatment to achieve full recovery.20
There appears to be an association between hypothyroidism and fibromyalgia syndrome (FMS). Individuals with FMS and clients with undiagnosed myofascial symptoms may benefit from a medical referral for evaluation of thyroid function.21-24
Neoplasms
Cancer of the thyroid is a relatively uncommon, slow-growing neoplasm that rarely metastasizes. It is often the incidental finding in persons being treated for other disorders (e.g., musculoskeletal disorders involving the head and neck). Primary cancers of other endocrine organs are rare and are not encountered by the clinical therapist very often.
Risk factors for thyroid cancer include female gender, age over 40 years, Caucasian race, iodine deficiency, family history of thyroid cancer, and being exposed to radioactive iodine (I-131), especially as children. In addition, nuclear power plant fallout could expose large numbers of people to I-131 and subsequent thyroid cancer. The use of potassium iodide (KI) can protect the thyroid from the adverse effects of I-131 and is recommended to be made available in areas of the country near nuclear power plants in case of nuclear fallout.25 The initial manifestation in adults and especially in children is a palpable lymph node or nodule in the neck lateral to the sternocleidomastoid muscle in the lower portion of the posterior triangle overlying the scalene muscles26 (Fig. 11-5).
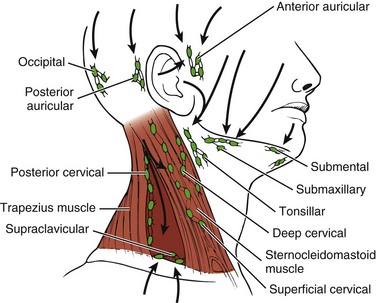
Fig. 11-5 Lymph node regions of the head and neck. Palpable nodal disease associated with thyroid carcinoma is commonly located lateral to the sternocleidomastoid muscle in the lower portion of the posterior triangle overlying the scalene muscles (dark red triangle). (Modified from Swartz MH: Textbook of physical diagnosis, Philadelphia, 1989, WB Saunders.)
A physician must evaluate any client with a palpable nodule because a palpable nodule is often clinically indistinguishable from a mass associated with a benign condition. The presence of new-onset hoarseness, hemoptysis, or elevated blood pressure is a red-flag symptom for systemic disease.
Parathyroid Glands
Two parathyroid glands are located on the posterior surface of each lobe of the thyroid gland. These glands secrete parathyroid hormone (PTH), which regulates calcium and phosphorus metabolism. Parathyroid disorders include hyperparathyroidism and hypoparathyroidism.
The therapist may see clients with parathyroid disorders in acute care settings and postoperatively because these disorders can result from diseases and surgical procedures. If damage or removal of these glands occurs, the resulting hypoparathyroidism (temporary or permanent) causes hypocalcemia, which can result in cardiac arrhythmias and neuromuscular irritability (tetany).
Disorders of the parathyroid glands may produce periarthritis and tendinitis. Both types of inflammation may be crystal induced and can be associated with periarticular or tendinous calcification.
Hyperparathyroidism
Hyperparathyroidism (hyperfunction), or the excessive secretion of PTH, disrupts calcium, phosphate, and bone metabolism. The primary function of PTH is to maintain a normal serum calcium level. Elevated PTH causes release of calcium by the bone and accumulation of calcium in the bloodstream.
Symptoms of hyperparathyroidism are related to this release of bone calcium into the bloodstream. This causes demineralization of bone and subsequent loss of bone strength and density. At the same time, the increase of calcium in the bloodstream can cause many other problems within the body, such as renal stones. The incidence of hyperparathyroidism is highest in postmenopausal women.27
The major cause of primary hyperparathyroidism is a tumor of a parathyroid gland, which results in the autonomous secretion of PTH. Renal failure, another common cause of hyperparathyroidism, causes hypocalcemia and stimulates PTH production. Hyperplasia of the gland occurs as it attempts to raise the blood serum calcium levels. Thiazide diuretics (used for hypertension) and lithium carbonate (used for some psychiatric problems) can exacerbate or even cause hyperparathyroid disorders.28
Clinical Presentation: Many systems of the body are affected by hyperparathyroidism (Table 11-6). Proximal muscle weakness and fatigability are common findings and may be secondary to a peripheral neuropathic process. Myopathy of respiratory muscles with associated respiratory involvement often goes unnoticed. Striking reversal of muscle weakness and atrophy occur with successful treatment of the underlying hyperparathyroidism.
TABLE 11-6
Systemic Manifestations of Hyperparathyroidism
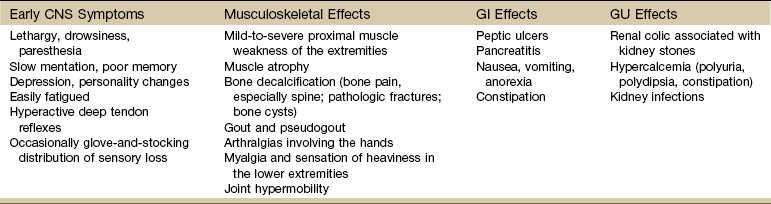
CNS, Central nervous system; GI, gastrointestinal; GU, genitourinary.
Other symptoms associated with hyperparathyroidism are muscle weakness, loss of appetite, weight loss, nausea and vomiting, depression, and increased thirst and urination (Case Example 11-5). Hyperparathyroidism can also cause GI problems, pancreatitis, bone decalcification, and psychotic paranoia (Fig. 11-6).
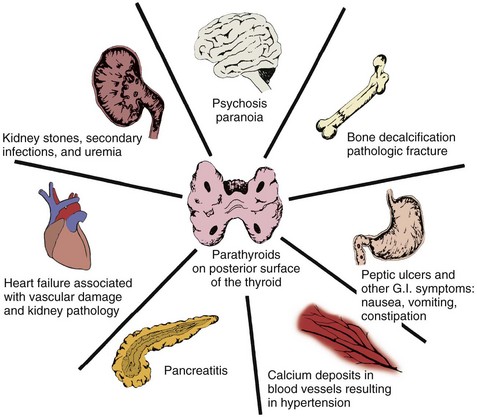
Fig. 11-6 The pathologic processes of body structures as a result of excess parathyroid hormone. (From Muthe NC: Endocrinology: a nursing approach, Boston, 1981, Little, Brown.)
Bone erosion, bone resorption, and subsequent bone destruction from hypercalcemia associated with hyperparathyroidism occurs rarely today. In most cases, hypercalcemia is mild and detected before any significant skeletal disease develops. The classic bone disease osteitis fibrosa cystica affects persons with primary or renal hyperparathyroidism. Bone lesions called Brown tumors appear at the end stages of the cystic osteitis fibrosa. There are increasing reports of this condition in hyperparathyroidism secondary to renal failure because of the increasing survival rates of clients on hemodialysis.
Currently, skeletal manifestations of primary hyperparathyroidism are more likely to include bone pain secondary to osteopenia, especially diffuse osteopenia of the spine with possible vertebral fractures. In addition, a number of articular and periarticular disorders have been recognized in association with primary hyperparathyroidism. The therapist may encounter cases of ruptured tendons caused by bone resorption in clients with hyperparathyroidism.
Inflammatory erosive polyarthritis may be associated with chondrocalcinosis and CPPD deposits in the synovial fluid. This erosion is called osteogenic synovitis. Concurrent illness and surgery (most often parathyroidectomy) are recognized inducers of acute arthritic episodes.
Hypoparathyroidism
Hypoparathyroidism (hypofunction), or insufficient secretion of PTH, most commonly results from accidental removal or injury of the parathyroid gland during thyroid or anterior neck surgery. A less common form of the disease can occur from a genetic autoimmune destruction of the gland. Hypofunction of the parathyroid gland results in insufficient secretion of PTH and subsequent hypocalcemia, hyperphosphatemia, and pronounced neuromuscular and cardiac irritability.
Clinical Presentation: Hypocalcemia occurs when the parathyroids become inactive. The resultant deficiency of calcium in the blood alters the function of many tissues in the body. These altered functions are described by the systemic manifestations of signs and symptoms associated with hypoparathyroidism (Table 11-7).
TABLE 11-7
Systemic Manifestations of Hypoparathyroidism
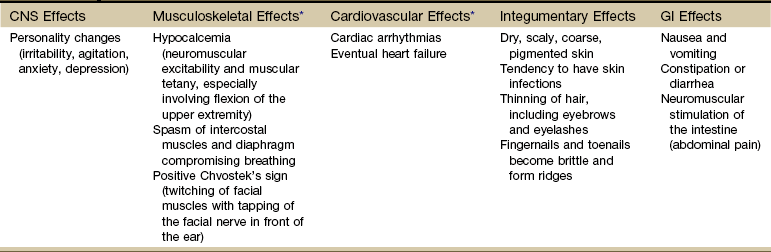
CNS, Central nervous system; GI, gastrointestinal.
*The most common and important effects for the therapist to be aware of are the musculoskeletal and cardiovascular effects.
The most significant clinical consequence of hypocalcemia is neuromuscular irritability. This irritability results in muscle spasms, paresthesias, tetany, and life-threatening cardiac arrhythmias. Muscle weakness and pain have been reported along with hypocalcemia in clients with hypoparathyroidism.
Hypoparathyroidism is primarily treated through pharmacologic management with intravenous calcium gluconate, oral calcium salts, and vitamin D. Acute hypoparathyroidism is a life-threatening emergency and is treated rapidly with calcium replacement, anticonvulsants, and prevention of airway obstruction.
Pancreas
The pancreas is a fish-shaped organ that lies behind the stomach. Its head and neck are located in the curve of the duodenum, and its body extends horizontally across the posterior abdominal wall.
The pancreas has dual functions. It acts as both an endocrine gland, secreting the hormones insulin and glucagon, and an exocrine gland, producing digestive enzymes. Disorders of endocrine function are included in this chapter, whereas disorders of exocrine function affecting digestion are included in Chapter 8.
Diabetes Mellitus
Diabetes mellitus (DM) is a chronic disorder caused by deficient insulin or defective insulin action in the body. It is characterized by hyperglycemia (excess glucose in the blood) and disruption of the metabolism of carbohydrates, fats, and proteins. Over time, it results in serious small vessel and large vessel vascular complications and neuropathies.
Diabetes is the leading cause of end-stage renal disease (ESRD) [kidney failure requiring dialysis or transplantation], nontraumatic lower extremity amputations, and new cases of blindness among adults in the United States, and a major cause of heart disease and stroke.29,30
Type 1 DM is a condition in which little or no insulin is produced. It occurs in about 10% of all cases and usually occurs in children or young adults. Type 2 DM commonly occurs after age 40 and is a condition of defective insulin and/or impaired cell receptor binding of insulin. Table 11-8 depicts the major differences between type 1 and type 2 in presentation and treatment. There has been some discussion as to whether Alzheimer’s disease is type 3 diabetes (“brain diabetes”), unique to the brain or if diabetes is just a risk factor for Alzheimer’s disease.31 A relationship between DM and dementia is undeniable, with numerous studies concluding that DM increases the risk of cognitive decline and dementia, including Alzheimer’s disease.32
Native Americans, Latino Americans, Native Hawaiians, and some Asian Americans and Pacific Islanders have been identified at particularly high risk for type 2 DM and its complications.33 Lack of exercise and obesity are two major risk factors for type 2 DM. As a result of these lifestyle factors (sedentary lifestyle, obesity), the overall number of persons in the United States with diabetes has increased from 10 million in 1977 to 24 million in 2007 and is projected to double or triple by 2050 if current trends in diabetes prevalence continue.34,35
Clinical Presentation: Specific physiologic changes occur when insulin is lacking or ineffective. Normally, the blood glucose level rises after a meal. A large amount of this glucose is taken up by the liver for storage or for use by other tissues such as skeletal muscle and fat. When insulin function is impaired, the glucose in the general circulation is not taken up or removed by these tissues; thus it continues to accumulate in the blood. Because new glucose has not been “deposited” into the liver, the liver synthesizes more glucose and releases it into the general circulation, which increases the already elevated blood glucose level.
Protein synthesis is also impaired because amino acid transport into cells requires insulin. The metabolism of fats and fatty acids is altered, and instead of fat formation, fat breakdown begins in an attempt to liberate more glucose. The oxidation of these fats causes the formation of ketone bodies. Because the formation of these ketones can be rapid, they can build quickly and reach very high levels in the bloodstream. When the renal threshold for ketones is exceeded, the ketones appear in the urine as acetone (ketonuria).
The accumulation of high levels of glucose in the blood creates a hyperosmotic condition in the blood serum. This highly concentrated blood serum then “pulls” fluid from the interstitial areas, and fluid is lost through the kidneys (osmotic diuresis). Because large quantities of urine are excreted (polyuria), serious fluid losses occur, and the conscious individual becomes extremely thirsty and drinks large amounts of water (polydipsia). In addition, the kidney is unable to resorb all the glucose, so glucose begins to be excreted in the urine (glycosuria).
Certain medications can cause or contribute to hyperglycemia. Corticosteroids taken orally have the greatest glucogenic effect. Any person with diabetes taking corticosteroid medications must be monitored for changes in blood glucose levels.
Other hormones produced by the body also affect blood glucose levels and can have a direct influence on the severity of diabetic symptoms. Epinephrine, glucocorticoids, and growth hormone can cause significant elevations in blood glucose levels by mobilizing stored glucose to blood glucose during times of physical or psychologic stress.
When persons with DM are under stress, such as during surgery, trauma, pregnancy, puberty, or infectious states, blood glucose levels can rise and result in the need for increased amounts of insulin. If these insulin needs cannot be met, a hyperglycemic emergency such as diabetic ketoacidosis can result.
It is essential to remember that clients with DM who are under stress will have increased insulin requirements and may become symptomatic even though their disease is usually well controlled in normal circumstances.
Diagnosis: To be diagnosed with diabetes, a person must have fasting plasma glucose (FPG) readings of 126 mg/dL or higher on two different days. The previous cutoff, set in 1979, was 140 mg/dL. This change occurred as a result of research showing that individuals with readings as low as the mid-120s have already started developing tissue damage from diabetes. A value greater than 100 mg/dL is a risk factor for future diabetes and cardiovascular disease. It has been suggested that the term “prediabetes” is no longer used: the person either has diabetes or does not.36
The American Diabetes Association offers consumers a risk test for diabetes (http://www.diabetes.org/risk-test.jsp). All adults should take this risk test; anyone 45 or older should be tested for diabetes every 3 years. Individuals with elevated FPG values as described should be tested every 1 to 2 years. The therapist can offer at-risk clients information on increased activity and exercise as a means of lowering their risk of developing diabetes.37
Physical Complications: At presentation, the client with DM may have a variety of serious physical problems. Infection and atherosclerosis are the two primary long-term complications of this disease and are the usual causes of severe illness and death in the person with diabetes.
Blood vessels and nerves sustain major pathologic changes in the person affected by DM. Atherosclerosis in both large vessels (macrovascular changes) and small vessels (microvascular changes) develops at a much earlier age and progresses much faster in the individual with DM. The blood vessel changes result in decreased blood vessel lumen size, compromised blood flow, and resultant tissue ischemia. The pathologic end-products are cerebrovascular disease (CVD), coronary artery disease (CAD), renal artery stenosis, and peripheral vascular disease (PVD).
Microvascular changes, characterized by the thickening of capillaries and damage to the basement membrane, result in diabetic nephropathy (kidney disease) and diabetic retinopathy (disease of the retina). Diabetes is the leading cause of kidney failure and new cases of blindness in the United States as of 2007.30,38
Poorly controlled DM can lead to various tissue changes that result in impaired wound healing. Decreased circulation to the skin can further delay or diminish healing. Skin eruptions called xanthomas (Fig. 11-7) may appear when high lipid levels (e.g., cholesterol and triglycerides) in the blood cause fat deposits in the skin over extensor surfaces such as the elbows, knees, back of the head and neck, and heels. Yellow patches on the eyelids are another sign of hyperlipidemia. Medical referral is required to normalize lipid levels.
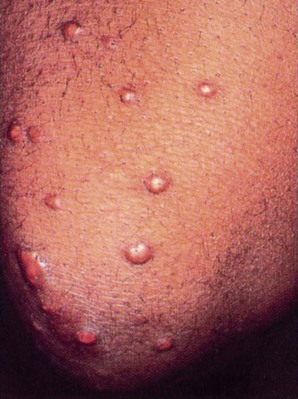
Fig. 11-7 Multiple eruptive xanthomas over the extensor surface of the elbow in a client with poorly controlled diabetes. These lipid-filled nodules characterized by an intracellular accumulation of cholesterol develop in the skin, often around the extensor tendons. Medical referral is required; xanthomas in this population are a sign that the health-care team, including the therapist, must work with the client to provide further education about diabetes, gain better control of glucose levels, and prevent avoidable complications. These skin lesions will go away when the diabetes is under control. Xanthomas can occur in any condition with disturbances of lipoprotein metabolism (not just diabetes). (From Callen JP, Jorizzo J, Greer KE, et al: Dermatological signs of internal disease, Philadelphia, 1988, WB Saunders. Used with permission.)
Physical Complications of Diabetes Mellitus:
Depression: Depression is common in individuals with type 2 diabetes (see Box 3-10) and is linked with increased mortality in this population.39 Adults with diabetes and depression are less likely to follow recommendations for nutrition and exercise. They are less likely to check their blood glucose levels routinely and more likely to take drug “holidays” from their other medications (e.g., for hyperlipidemia or hypertension). Clients with diabetes who are depressed are more likely to miss health care appointments for prevention and intervention.40,41
Diabetic Neuropathy: Neuropathy is the most common chronic complication of long-term DM. Neuropathy in the client with DM is thought to be related to the accumulation in the nerve cells of sorbitol, a by-product of improper glucose metabolism. This accumulation then results in abnormal fluid and electrolyte shifts and nerve cell dysfunction. The combination of this metabolic derangement and the diminished vascular perfusion to nerve tissues contributes to the severe problem of diabetic neuropathy.
Risk factors: Other than glycemic control, there is no curative intervention for diabetic neuropathy. Identifying potentially modifiable risk factors for neuropathy is crucial; the therapist can have a key role in providing risk factor assessment for clients with diabetes.
Risk factors for the development of diabetic neuropathy include the duration and severity of diabetes, elevated triglycerides, higher body mass index (BMI), and a history of smoking or hypertension.42,43
Clinical presentation: Neuropathy may affect the central nervous system, peripheral nervous system, or autonomic nervous system. Peripheral neuropathy usually develops first as a sensory impairment of the extremities. Autonomic involvement is more common with long-standing disease.
Most common among the peripheral neuropathies are chronic sensorimotor distal symmetric polyneuropathy (DPN).44 Polyneuropathy affects peripheral nerves in distal lower extremities, causing burning and numbness in the feet. It can result in muscle weakness, atrophy, and foot drop. Diabetic neuropathy can produce a syndrome of bilateral but asymmetric proximal muscle weakness called diabetic amyotrophy. Although the muscle enzyme levels are usually normal, muscle biopsy reveals atrophy of type II muscle fibers.
CTS (mononeuropathy) is also a common finding in persons with DM; it represents one form of diabetic neuropathy. As many as 5% to 16% of people with CTS have underlying diabetes. The mechanism is thought to be ischemia of the median nerve resulting from diabetes-related microvascular damage. This ischemia then causes increased sensitivity to even minor pressure exerted in the carpal tunnel area.
Autonomic involvement affects the pace of the heart beat, blood pressure, sweating, and bladder function and can cause symptoms such as erectile dysfunction and gastroparesis (delayed stomach emptying).42
Charcot’s joint, or neuropathic arthropathy, is a well-known complication of DM. This condition is due at least in part to the loss of proprioceptive sensation that marks diabetic neuropathy. Severe degenerative arthritis similar to Charcot’s joint has been noted in clients with CPPD crystal deposition disease. Shoulder, hand, and foot disorders are very common, and evaluation of clients with DM should include examination of these areas (Case Example 11-6).45,46
The large- and small-vessel changes that occur with DM contribute to the changes in the feet of individuals with diabetes. Sensory neuropathy, which may lead to painless trauma and ulceration, can progress to infection. Neuropathy can result in drying and cracking of the skin, which creates more openings for bacteria to enter. The combination of all these factors can ultimately lead to gangrene and eventually require amputation. Prevention of these problems by meticulous care of the diabetic foot can reduce the need for amputation by 50% to 75%.
An annual foot screen by a health care provider is currently recommended for anyone with diabetes. This screen includes examination of toenails for length, thickness, and ingrown position. All calluses should be examined because ulceration can occur underneath them. General skin integrity, color, circulation, and structure should also be assessed.47
Whether a poorly controlled blood glucose level is a causative factor in the development of the long-term physical complications of diabetes is still controversial, but it does seem clear that these complications increase with the duration of the disease. Stable glycemic control (between 80 and 110 mg/dL), which prevents the fluctuation of blood glucose levels, has been shown to be helpful in decreasing neuropathic pain (and of course other complications).48
Periarthritis: Musculoskeletal disorders of the hand and shoulder, including periarthritis of the shoulder, is five times as common in this group as it is in individuals who do not have diabetes. The condition most often affects insulin-dependent people, and involvement is typically bilateral.
The mechanism of this association is unclear, but it is believed to be related to fibroblast proliferation in the connective tissue structures around joints or to microangiopathy (disorder involving small blood vessels) involving the tendon sheaths. This periarthritic condition can behave unpredictably: It may regress spontaneously, remain stable, or progress to adhesive capsulitis or frozen shoulder.49
Hand Stiffness: Diabetic stiff hand, LJM syndrome, cheirarthritis (inflammation of the hand and finger joints), and diabetic contractures are common in both types of DM in direct relation to the presence and duration of microvascular complications.
Flexor tenosynovitis, caused by accumulation of excessive dermal collagen in the fingers, results in thickening and induration of the skin around the joints. This condition can lead to sclerodactyly (hardening and shrinking of fingers and toes), which in turn can mimic scleroderma.
Dupuytren’s contracture has a strong association with DM. This syndrome is characterized by nodular thickening of the palmar fascia and flexion contracture of the digits. Clients usually have pain in the palm and digits, with decreased mobility and contracture of the fingers. In clients with diabetes, Dupuytren’s contracture must be differentiated from LJM, which may involve the entire hand and is frequently bilateral, and from flexor tenosynovitis, which is marked by trigger finger.
Individuals with DM may develop CRPS (formerly called reflex sympathetic dystrophy [RSD] syndrome), which is characterized by pain, hyperesthesia, vasomotor and dystrophic skin changes, and tenderness and swelling around the hands and feet.
Intervention: Medical management of the client with diabetes is directed primarily toward maintenance of blood glucose values within the range of 80 to 120 mg/dL. The three primary treatment modalities used in the management of DM are diet, exercise, and medication (insulin and oral hypoglycemic agents; Table 11-9).
TABLE 11-9
Types of Insulin and Insulin Action
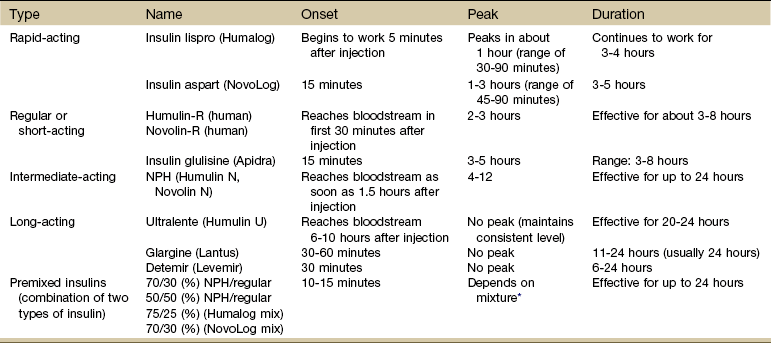
Onset is how long it takes before the insulin reaches the bloodstream and starts to lower glucose levels.
Peak is the time when insulin reaches its maximum strength.
Duration defines how long the insulin continues to lower blood glucose.
*When all mixtures are considered, the range is 1 to 12 hours.
Data from Micromedex Healthcare Series [Internet database]. Greenwood Village, CO, Thomson Reuters Healthcare Inc. Updated periodically. Compiled by Tanner Higginbotham, PharmD. University of Montana Drug Information Service, 2010.
Recommended preventive care services, such as yearly eye and foot examinations, as well as measurements of glycosylated hemoglobin (A1C) two or more times per year are critical in the prevention of diabetic complications such as blindness, amputation, and cardiovascular disease.50 A1C (also known as glycosylated hemoglobin, glycated hemoglobin, or glycohemoglobin) is an accurate, objective measurement of chronic glycemia in diabetes.
Most laboratories list the normal reference range as 4% to 6%. A1C equal or greater than 6.5% on two consecutive occasions is diagnostic of diabetes but must be confirmed by fasting glucose levels.51 The goal is to maintain consistent A1C levels below 7% (American Diabetes Association recommendation),36 which correlates to an average daily blood glucose below 150 to 154 mg/dL. This recommendation (and the plasma glucose levels used to diagnose diabetes) were determined based on the presence of retinopathy at these thresholds.
The guideline for A1C levels applies to the general population; individuals with a history of severe hypoglycemia, limited life expectancy, advanced diabetes-related complications, and extensive comorbid complications may be advised by their medical doctors to follow levels at or above 7%.36 The Association of Endocrinologists recommends A1C levels of 6.5% or lower (average blood sugar reading of 135 mg/dL over a 2- to 3-month period).The A1C measurement gives the client and the therapist an indication of how successful diet, exercise, and medication are in controlling glucose levels over time. It can be used as a baseline from which to evaluate results of intervention. An A1C value greater than 10% warrants immediate medical attention (usually insulin treatment).51
For individuals with type 2 diabetes and the following factors, an A1C goal of less than 8% may be more appropriate than an A1C goal of less than 7%.51
• Known cardiovascular disease or high risk cardiovascular risk
• Inability to recognize and treat hypoglycemia, history of severe hypoglycemia requiring assistance
• Inability to comply with standard goals such as polypharmacy issues
• Limited life expectancy or estimated survival of less than 10 years
• Extensive comorbid conditions such as renal failure, liver failure, and end-stage disease complications
The therapist can conduct a careful screening examination (Box 11-1). All individuals with type 2 diabetes should be screened at the time of diagnosis and annually thereafter for diabetic peripheral neuropathies. Individuals with type 1 diabetes should be screened 5 years after diagnosis and annually thereafter. Screening should include checking knee and ankle reflexes, examining sensory function in the feet, asking about neuropathic symptoms, and examining the distal extremities for ulcers, calluses, and deformities.44
Exercise-Related Complications: Any exercise can improve the body’s ability to use insulin. Exercise causes a decrease in the amount of insulin the pancreas releases because muscle contractions are increasing blood glucose uptake. For the person taking insulin, exercise adds to the effects of the insulin, dropping blood sugars to dangerously low levels. Exercise for the person with DM must be planned and instituted cautiously and monitored carefully because significant complications can result from exercise of higher intensity or longer duration.
Exercise-related complications can be prevented by careful monitoring of the client’s blood glucose level before, during, and after strenuous exercise sessions. (Safe levels are individually determined but usually fall between 100 and 250 mg/dL; between 250 and 300 mg/dL is considered the “caution zone.”) The following recommendations are general guidelines. These are not necessarily “fasting levels” (unless the person has not eaten for the last 12 hours for some reason). Exceptions are common, depending on the type of exercise, training level of the participant, expected glycemic pattern, and whether the individual is using an insulin pump.
If the blood glucose level is between 250 and 300 mg/dL at the start of the exercise, the client may be experiencing a state of insulin deficiency and should test urine for ketones, an indication that the body does not have enough insulin to control the blood sugar and is breaking down fat for energy. Exercise is likely to raise the blood sugars more; the exercise session should be postponed until the blood glucose level is under better control. Blood glucose levels of 300 mg/dL or higher indicate the blood sugar level is too high to exercise safely, putting the client at risk for ketoacidosis. Exercise should be postponed until the blood glucose level drops to a safe pre-exercise range (between 100 to 250 mg/dL, possibly up to 300 mg/dL as described).
If the blood glucose level is less than 100 mg/dL, a 10- to 15-g carbohydrate snack should be given and the glucose retested in 15 minutes to ensure an appropriate level.
Clients with active retinopathy and nephropathy should avoid high-intensity exercise that causes significant increases in blood pressure because such increases can cause further damage to the retinas and kidneys. Any exercise that places the head below the waist causing increased intrathoracic and intracranial pressures can also aggravate retinal problems. Screening for neuropathies by testing deep tendon reflexes and vibratory and position sense are also very important in the prevention of exercise-related complications such as ulcerations or fractures.
It is very important to have the client avoid insulin injection to active extremities within 1 hour of exercise because insulin is absorbed much more quickly in an active extremity. It is important to know the type, dose, and time of the client’s insulin injections so that exercise is not planned for the peak activity times of the insulin.
Clients with type 1 diabetes may need to reduce the insulin dose or increase food intake when initiating an exercise program. During prolonged activities, a 10- to 15-g carbohydrate snack is recommended for each 30 minutes of activity. Activities should be promptly stopped with the development of any symptoms of hypoglycemia, and blood glucose should be tested. In addition, individuals with diabetes should not exercise alone. Partners, teammates, and coaches must be educated regarding the possibility of hypoglycemia and the way to manage it.
Insulin Pump During Exercise: People with type 1 diabetes (and some individuals with insulin-requiring type 2 diabetes) may be using an insulin pump. Continuous subcutaneous insulin infusion (CSII) therapy, known as insulin pump therapy, can bring the hormonal and metabolic responses to exercise close to normal for the individual with diabetes.
Although there are many benefits of pump use for active individuals with diabetes, there are a few drawbacks as well.52 Exercise can speed the development of diabetic ketoacidosis (DKA) when there is an interruption of insulin delivery, which can quickly become a life-threatening condition.
Other considerations include the effect of excessive perspiration or water on the infusion set (needle into the skin at the infusion site gets displaced), ambient temperature (insulin degrades under extreme conditions of heat or cold), and the effect of movement or contact at the infusion site (this causes skin irritation).
Insulin pump users who have pre-exercise blood glucose levels less than 100 mg/dL may not need a carbohydrate snack because they can reduce or suspend base insulin levels during an activity. The insulin reductions and required level of carbohydrate intake needed depends on the intensity and duration of the activity.52
The therapist should become familiar with the features of each pump in use by clients. Knowledge of basic guiding principles for exercise with diabetes and general recommendations for insulin regimen changes is also helpful.
Severe Hyperglycemic States
The two primary life-threatening metabolic conditions that can develop if uncontrolled or untreated DM progresses to a state of severe hyperglycemia (more than 400 mg/dL) are DKA and hyperglycemic, hyperosmolar, nonketotic coma (HHNC; Table 11-10).
DKA occurs with severe insulin deficiency caused by either undiagnosed DM or a situation in which the insulin needs of the person become greater than usual (e.g., infection, trauma, surgery, emotional stress). It is most often seen in the client with type 1 diabetes but can in rare situations occur in the client with type 2 diabetes. Medical treatment is necessary.
HHNC occurs most commonly in the older adult with type 2 diabetes. This complication is extremely serious and, in many cases, fatal. Factors that can precipitate this crisis are infections (e.g., pneumonia); medications that elevate the blood glucose level (e.g., corticosteroids); and procedures such as dialysis, surgery, or total parenteral nutrition (TPN).
There are specific clinical features that identify HHNC. Some of these are similar to those of DKA, such as severe hyperglycemia (1000 to 2000 mg/dL) and dehydration. The major differentiating feature between DKA and HHNC, however, is the absence of ketosis in HHNC.
Because it is likely that the therapist will work with clients who have diabetes, it is imperative that the clinical symptoms of DM and its potentially life-threatening metabolic states are understood. If anyone with diabetes arrives for a clinical appointment in a confused or lethargic state or is exhibiting changes in mental function, fingerstick glucose testing should be performed. Immediate physician referral is necessary.
Hypoglycemia
Hypoglycemia (blood glucose of less than 70 mg/dL) is a major complication of the use of insulin or oral hypoglycemic agents. Hypoglycemia is usually the result of a decrease in food intake or an increase in physical activity in relation to insulin administration. It is a potentially lethal problem. The hypoglycemic state interrupts the oxygen consumption of nervous system tissue. Repeated or prolonged attacks can result in irreversible brain damage and death.
Hypoglycemia Associated With Diabetes Mellitus: Hypoglycemia during or after exercise can be a problem for anyone with diabetes. This condition results as glucose is used by the working muscles, if the circulating level of injected insulin is too high, or both. The degree of hypoglycemia depends on such factors as pre-exercise blood glucose levels, duration and intensity of exercise, and blood insulin concentration.
Clinical Presentation: The severity and number of signs and symptoms depend on the individual client and the rapidity of the drop in blood glucose. It is important to note that clients can exhibit signs and symptoms of hypoglycemia when their elevated blood glucose level drops rapidly to a level that is still elevated (e.g., 400 to 200 mg/dL). The rapidity of the drop is the stimulus for sympathetic activity; even though a blood glucose level appears elevated, clients may still have hypoglycemia.
Clients receiving beta-adrenergic blockers (e.g., propranolol) can be at special risk for hypoglycemia by the actions of this medication. These beta-blockers inhibit the normal physiologic response of the body to the hypoglycemic state or block the appearance of the sympathetic manifestations of hypoglycemia. Clients may also have hypoglycemia during nighttime sleep (most often related to the use of intermediate- and long-acting insulins given more than once a day), with the only symptoms being nightmares, sweating, or headache.
Intervention: Hypoglycemia can be treated in the conscious client by immediate administration of sugar. It is always safer to give the sugar, even when there is doubt concerning the origin of symptoms (DKA and HHNC can also have similar central nervous system symptoms at presentation). Most often, 10 to 15 g of carbohydrate are sufficient to reverse the episode of hypoglycemia. Immediate-acting glucose sources should be kept in every physical therapy department (e.g.,  cup of fruit juice or sugared cola, 8 ounces of milk, two packets of sugar, 2-ounce tube of honey or cake-decorating gel).
cup of fruit juice or sugared cola, 8 ounces of milk, two packets of sugar, 2-ounce tube of honey or cake-decorating gel).
Most people with diabetes carry a rapid-acting source of carbohydrate, such as readily absorbable glucose tablets, so that it is available for use if a hypoglycemic episode occurs. Some individuals use intramuscular glucagon. If the client loses consciousness, emergency personnel must be notified, and glucose will be administered intravenously.
Any episode or suspected episode of hypoglycemia must be treated promptly and must be reported to the client’s physician. It is important to question each client who has diabetes regarding his or her individual response to hypoglycemia. Information regarding individual symptoms, frequency of episodes, and precipitating factors may be invaluable to the therapist in preventing or minimizing a hypoglycemic attack.
Other Hypoglycemic States
Other conditions that can cause hypoglycemic states are usually related to hormonal deficiencies (e.g., cortisol, glucagon, ACTH) or overproduction of insulin or insulin-like material from tumors.
Reactive hypoglycemia, also known as functional hypoglycemia, occurs after the intake of a meal and usually results from stomach or duodenal surgery. This condition involves rapid stomach emptying with rapid rises of glucose levels. Glucose then rapidly falls to below normal levels as an exaggerated response of insulin secretion develops. The cause of reactive hypoglycemia is unknown.
Introduction To Metabolism
As noted earlier, the endocrine system works with the nervous system to regulate and integrate the body’s metabolic activities. The rate of metabolism can be increased by exercise, elevated body temperature (e.g., high fever), hormonal activity (e.g., thyroxine, insulin, epinephrine), and specific dynamic action that occurs after ingestion of a meal. All metabolic functions require proper fluid and acid-base balance. Although acid-base metabolism is not in itself a sign or a symptom, the consequences of an acid-base metabolism disorder can result in many clinical signs and symptoms.
Therapists are unlikely to evaluate someone with a primary musculoskeletal lesion that reflects an underlying metabolic disorder. However, many inpatients in hospitals and some outpatients may be affected by disturbances in acid-base metabolism and other specific metabolic disorders. Only those conditions that are likely to be encountered by a therapist are included in this text.
Fluid Imbalances
Fluid deficit can occur as a result of two primary types of imbalance. There is either a loss of water without loss of solutes or a loss of both water and solutes.
The loss of body water without solutes results in the excess concentration of body solutes within the interstitial and intravascular compartments. To preserve equilibrium, water will then be forced to shift by osmosis from inside cells to these outside compartments.
If this state persists, large amounts of body water will be shifted and excreted (osmotic diuresis), and severe cellular dehydration will result. This type of imbalance can occur as a result of several conditions:
• Decreased water intake (e.g., unavailability, unconsciousness)
• Water loss without proportionate solute loss (e.g., prolonged hyperventilation, diabetes insipidus)
• Increased solute intake without proportionate water intake (tube feeding)
• Excess accumulation of solutes (e.g., high glucose levels such as in DM)
The second type of fluid imbalance results from a loss of both water and solutes. Causes of the loss of both water and solutes include hemorrhage, profuse perspiration (e.g., marathon runners), and loss of gastrointestinal tract secretions (e.g., vomiting, diarrhea, draining fistulas, ileostomy). Postsurgical patients who have had joint replacements, hip fractures, multiple trauma, or neurosurgery often lose blood and become hypovolemic despite efforts to maintain their homeostasis through blood transfusion and fluid replacement.
Severe losses of water or solutes (or both) can lead to dehydration and hypovolemic shock. It is important for the therapist to be aware of possible fluid losses or water shifts in any client who is already compromised by advanced age or by a situation, such as an ileostomy or tracheostomy, that results in a continuous loss of fluid. Because the response to fluid loss is highly individual, it is important to recognize the early clinical symptoms of fluid loss and to carefully monitor vital signs and clinical symptoms in clients who are at risk, especially the elderly, the very young, or the chronically ill.53
Athletes and normal adults may experience orthostatic hypotension when slightly dehydrated, especially when intense exercise increases the core body temperature. The normal vascular system can accommodate this effectively.
Fluid Excess
Fluid excess can occur in two major forms: water intoxication (excess of water without an excess of solutes) or edema (excess of both solutes and water).
Because the etiologic complex, symptoms, and outcomes related to these problems are substantially different, these fluid imbalances are discussed separately.
Water Intoxication: Water intoxication (resulting in hyponatremia) is an excess of extracellular water in relationship to solutes. The extracellular fluid (ECF) becomes diluted, and water must then move into cells to equalize solute concentration on both sides of the cell membrane. High water consumption without solute replacement can result in hyponatremia, a potentially lethal situation.
Water excess can be caused by an accumulation of solute-free fluid. An increase in solute-free fluid usually occurs because of excess ADH (tumors, endocrine disorders) or intake of large amounts of only tap water without balanced solute ingestion. The latter situation occurs most often in older adults who drink additional water after having the flu, with its associated vomiting and diarrhea, or in athletes who have lost large amounts of body fluids during exercise that have been replaced with only water.
Symptoms of water intoxication are largely neurologic because of the shifting of water into brain tissues and resultant dilution of sodium in the vascular space.
Edema: An excess of solutes and water is called isotonic volume excess. The excess fluid is retained in the extracellular compartment and results in fluid accumulation in the interstitial spaces (edema). Edema can be produced by many different situations, most commonly including vein obstruction, decreased cardiac output, endocrine imbalances, and loss of serum proteins (e.g., burns, liver disease, allergic reactions).
Diuretic medications are used frequently to treat volume excess. Various diuretic medications may be used depending on the underlying cause of the problem and the desired effect of the drug. The most commonly used are the thiazide diuretics (e.g., chlorothiazide, hydrochlorothiazide). It is important to assess clients who take diuretic therapy for potential fluid loss and dehydration by observing for clinical symptoms of both.
These medications inhibit sodium and water resorption by the kidneys. Potassium is usually also lost with the sodium and water, so continuous replacement of potassium is a major concern for anyone receiving non–potassium-sparing diuretics. It is essential to monitor clients who take diuretics for signs and symptoms of potassium depletion.
It is also very important to check laboratory data for the potassium level in any client taking diuretics, particularly before exercise. Any value below the normal range (less than 3.5 mEq/L) is potentially dangerous and could result in a lethal cardiac arrhythmia even with moderate cardiovascular exercise.
For clients on diuretics, the therapist must observe for the appearance of symptoms consistent with dehydration or potassium depletion. Any concerns should be discussed with a physician before physical therapy intervention.
Metabolic Disorders
Metabolic syndrome (sometimes referred to as prediabetes type 2 but again, the term “prediabetes” is being questioned) is a group of signs and symptoms that are actually risk factors strongly linked to type 2 diabetes, cardiovascular disease, and stroke. This condition is characterized by insulin resistance and seems to be on the rise in Americans because of lifestyle and metabolic risk factors.
Insulin resistance is a generalized metabolic disorder in which the body cannot use insulin efficiently. Not only do the cells become resistant to insulin, but the cells themselves lose receptor sites (outside and inside the cell membrane). Insulin, which acts like a key to let glucose into the cells, cannot find a keyhole (receptor site) to open the door and let the glucose in.54 This loss of receptor site and decreased receptor site receptivity is why the metabolic syndrome is also called the insulin resistance syndrome.
Some people are genetically predisposed to insulin resistance. Acquired factors, such as excess body fat and physical inactivity, can elicit insulin resistance and the metabolic syndrome in these people. Most people with insulin resistance have abdominal obesity. The biologic mechanisms at the molecular level between insulin resistance and metabolic risk factors are complex and not fully understood.55
Risk Factors and Red Flags: Serious health complications can be reduced by identifying risk factors early through screening. The dominant underlying risk factors for this syndrome appear to be sedentary lifestyle with little to no physical activity or exercise, abdominal obesity, and insulin resistance. Other risk factors include family history of metabolic syndrome, type 2 diabetes, hypertension, elevated fasting glucose (100 mg/dL or more), elevated triglyceride levels (150 mg/dL or more), and low high-density lipoprotein (HDL) (men: less than 40 mg/dL; women: less than 50 mg/dL).56,57
During any person’s examination, the therapist should watch for red flags of metabolic syndrome, including BMI above 30, waist circumference (see Clinical Signs and Symptoms on the next page), elevated blood pressure, and signs of insulin resistance (e.g., acanthosis nigricans [Fig. 11-8], fatigue and decreased energy, depression, drowsiness after meals).
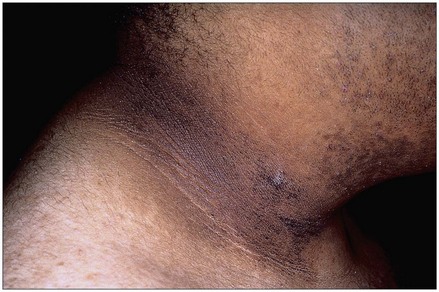
Fig. 11-8 Acanthosis nigricans is a brown to black hyperpigmentation of the skin with a soft to velvet-like feel to it. It is usually found in body folds, such as the posterior and lateral folds of the neck, the axilla, groin, umbilicus, forehead, and other areas. Acanthosis nigricans may be genetically inherited but is also associated with obesity or endocrine disorders such as hypothyroidism or hyperthyroidism, acromegaly, polycystic ovary disease, insulin-resistant diabetes, or Cushing’s disease. (From Bolognia JL, Jorizzo JL, Rapini RP: Dermatology, ed 2, St. Louis, 2007, Mosby.)
Metabolic Alkalosis
Metabolic alkalosis results from metabolic disturbances that cause either an increase in available bases or a loss of nonrespiratory body acids. Blood pH (hydrogen ion concentration in the ECF, a measure of metabolic process function and homeostasis) rises to a level greater than 7.45 (Table 11-11).
TABLE 11-11
Laboratory Values: Metabolic Acidosis and Alkalosis
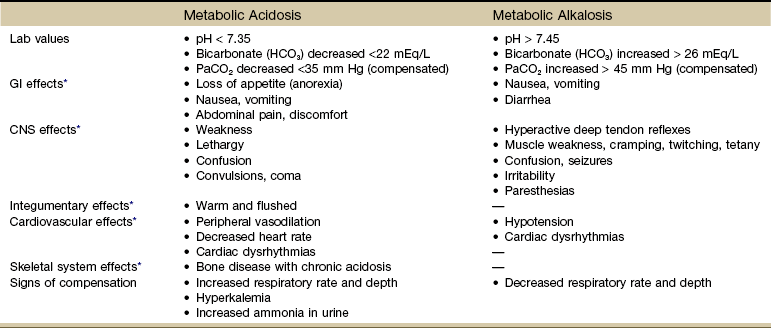
CNS, Central nervous system; GI, gastrointestinal.
Normal values for pH = 7.35-7.45, PaCO2 = 35-45 mm Hg; and HCO3 = 22-36 mEq/L
*Signs and symptoms.
Common causes of metabolic alkalosis include excessive vomiting or upper gastrointestinal suctioning, diuretic therapy, or ingestion of large quantities of base substances such as antacids.
Decreased respirations may occur as the respiratory system attempts to compensate by buffering the basic environment. The lungs attempt to retain carbon dioxide (CO2) and thus hydrogen ions (H).
It is important for the therapist to ask clients about the use of magnesium-containing antacids because symptoms of alkalosis can affect muscular function by causing muscle fasciculation and cramping. Prevention of problems related to alkalosis may be accomplished by education of the client regarding antacid use.
Metabolic Acidosis
Metabolic or nonrespiratory acidosis is an accumulation of fixed (nonvolatile) acids or a deficit of bases. Blood pH decreases to a level below 7.35 (see Table 11-11). Common causes of metabolic acidosis include DKA, lactic acidosis from a wide range of conditions (e.g., smoke inhalation, sepsis, cardiopulmonary failure, adverse reaction to drugs, alcohol abuse, liver disease, cancer), renal failure, severe diarrhea, and drug (including alcohol) or chemical toxicity.
Ketoacidosis occurs because insufficiency of insulin for the proper use of glucose (or increased insulin requirements) results in increased breakdown of fat. This accelerated fat breakdown produces ketones and other acids. These acids accumulate to high levels. While the body attempts to neutralize these increased acids, the plasma bicarbonate (HCO3) is used up.
Chronic kidney disease with renal failure results in acidosis because the failing kidney not only is unable to rid the body of excess acids but also cannot produce necessary bicarbonate.
Lactic acidosis occurs as excess lactic acid is produced during strenuous exercise or when oxygen is insufficient for proper use of carbohydrate (CHO), glucose, and water (H2O).
Intestinal and pancreatic secretions are highly alkaline so that severe diarrhea depletes the body of these necessary bases. Metabolic acidosis can result from ingestion of large quantities of acetylsalicylic acid (salicylates); symptoms of possible metabolic acidosis should be carefully assessed in clients undergoing high-dose aspirin therapy.
Hyperventilation may occur as the respiratory system attempts to rid the body of excess acid by increasing the rate and depth of respiration. The result is an increase in the amount of carbon dioxide and hydrogen excreted through the respiratory system.
Gout
Primary gout is the manifestation of an inherited inborn error of purine metabolism characterized by an elevated serum uric acid (hyperuricemia). Excess uric acid in the blood can result in the formation of tiny uric acid crystals that collect in the joints, triggering a painful inflammatory response.
Gout affects men predominantly, and the usual form of primary gout is uncommon before the third decade, with its peak incidence in the 40s and 50s. The frequency of gout in women approaches that in men after menopause when estrogen, which helps clear uric acid from the kidneys, declines dramatically.59 Gout may occur as a result of another disorder or of its therapy. This is referred to as secondary gout. Secondary gout may be associated with neoplasm, renal disease, or other metabolic disorders such as diabetes and hyperlipidemia (excess serum lipids).60
Risk Factors: Increased serum uric acid levels are associated with middle age, menopause, obesity, white race, stress (including surgery and medical illness), and high dietary intake of purine-rich foods. A variety of medications (e.g., penicillin, insulin, or thiazide diuretics) may increase the serum uric acid level or decrease uric acid excretion, as may a number of acute or chronic disorders other than gout (Table 11-12).
TABLE 11-12
Causes of Secondary Hyperuricemia
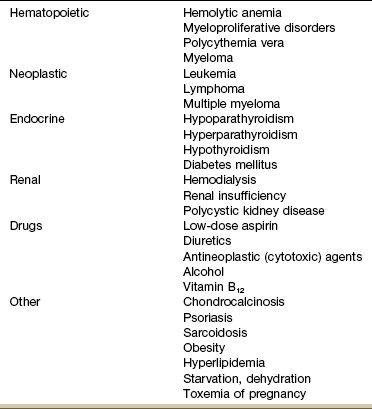
From Wade JP, Liang MH: Avoiding common pitfalls in the diagnosis of gout, J Musculoskel Med 5(8):16–27, 1988.
High intake of meat (beef, pork, lamb) and seafood consumption has been associated with increased risk of gout, whereas high intake of low-fat dairy products and vitamin C supplementation can lower urate levels and reduce the risk of gout. Purine-rich vegetables, protein intake, low-to-moderate alcohol consumption, and BMI are not associated with an increase in gout.61-63
Many diseases have a presentation similar to that of acute gouty arthritis. Gout and septic arthritis occasionally occur together. The diagnosis of gout must be based on the demonstration of monosodium urate crystals by synovial fluid analysis rather than on the clinical presentation alone.
Clinical Presentation: Uric acid is usually dissolved in the blood until it is passed through the kidneys into the urine and then excreted. In individuals with gout, the uric acid changes into crystals (urate) that deposit in joints (causing gouty arthritis) and other tissues such as the kidneys, causing renal disease.
Most renal disease in clients with gout is the result of coexisting conditions such as hypertension or atherosclerosis. Renal dysfunction can occur as a result of urate-related parenchymal damage without the existence of other comorbidities. Urate nephropathy is relatively rare; the therapist is more likely to see a client receiving cyclosporine after a heart or kidney transplant who develops gout during the first year after the transplant.64
The most usual symptom of gout is acute monarticular arthritis. The individual may be awakened from sleep with exquisite pain in the affected joint; any pressure (even the touch of clothes or bed sheets) on the joint is intolerable. Redness and swelling occur within a few hours, sometimes accompanied by low-grade fever and chills. Untreated, the attack lasts from 10 days to 2 weeks. Later, episodes may develop more gradually, affecting more than one joint as the disease progresses.
The peripheral joints of the hands and feet are involved, with 90% of gouty clients having attacks in the metatarsophalangeal joint of the great toe. Other typical sites of initial involvement (in order of frequency) are the instep, ankle, heel, knee, and wrist, although any joint in the body may be involved.
In chronic gouty arthritis, periarticular and subcutaneous deposits of sodium urate (or urate salts) form; these are referred to as tophus (tophi). These deposits produce an acute inflammatory response that leads to acute arthritis and later to chronic arthritis. Enlarged tophi on the joints of the hands and feet may erupt and discharge chalky masses of urate crystals.
The formation of tophi is directly related to the elevation of serum urate; the higher the client’s serum urate concentration, the higher the rate of urate deposition in soft tissue. Before urate-lowering agents became available, 30% to 50% of people with acute gouty arthritis developed tophi. Today, chronic tophaceous gout is rarely seen.
The therapist should refer anyone taking urate-lowering drugs for gout who is having recurrent symptoms. It may be necessary to adjust medication levels. The therapist can reinforce the need for compliance with the management program and provide more education about controlling hypertension and obesity through diet and exercise. Avoidance of alcohol (especially beer), dehydration, and trauma to the extremities are other important components of effective management.64
Pseudogout: Pseudogout is an arthritic condition caused by calcium pyrophosphate dihydrate (CPPD) crystals. It occurs about one-eighth as often as gout and may be hereditary or secondary to other disease processes (hyperparathyroidism is the most common one; Case Example 11-7).
Pseudogout is marked by attacks of gout-like symptoms, usually affecting a single joint (particularly the knee) and associated with chondrocalcinosis (deposition of calcium salts in joint cartilage). In anyone with pseudogout, routine x-ray studies of the knee and wrist frequently demonstrate cartilage calcification, or chondrocalcinosis. Because these changes are found in up to 10% of older adults, diagnosis must be made through aspiration of synovial fluid to identify the CPPD crystals.
Hemochromatosis
Hemochromatosis, also termed hematochromatosis, is an inborn error of iron metabolism. Mutations of the hemochromatosis gene (HFE) have been identified to help in better defining the type of disease present in an individual.65
The cardinal defect in hemochromatosis is the lack of regulation of iron absorption, but the exact mechanism is unknown. The intestinal tract absorbs more iron than is required, thus producing an excess with progressive tissue damage in parenchymal organs from iron retention.
Hemochromatosis is found five to ten times more often in men than in women because women lose blood through menstruation and pregnancy. Men seldom have symptoms until after 50 years of age and are rarely symptomatic before 30 years of age. Because of menstrual blood loss, women display symptoms 10 years later than men (median age: 60 years).
Ascorbic acid (vitamin C) and alcohol seem to accelerate the absorption of dietary iron. The high incidence of alcoholism among clients with hemochromatosis (40%) supports this concept.
Clinical Presentation: For many years, hemochromatosis was identified by a classic clinical triad of enlarged liver, skin hyperpigmentation, and diabetes. The term bronze diabetes was used to describe this presentation. Hyperpigmentation is caused by an increased number of melanocytes and a thinning of the epidermis. However, hemochromatosis may have many different signs and symptoms, confusing early diagnosis (Case Example 11-8).
In its early stages, hemochromatosis produces no symptoms because it takes many years of iron accumulation to produce warning signs or symptoms. Unfortunately, when the disease becomes evident, it is often too late because iron accumulation has caused irreversible tissue or end-organ damage in the heart, liver, endocrine glands, skin, joints, bone, and pancreas. About half the clients with hemochromatosis will develop arthritis.
Hemochromatosis has a well-known association with chondrocalcinosis (deposition of calcium salts in the cartilage of joints). Acute attacks of synovitis can occur, which may resemble a rheumatoid flare. A biopsy of synovial tissue reveals iron deposition in the cells of the synovial lining that is noninflammatory.
Arthritis may be the presenting symptom of hemochromatosis, but it usually occurs after diagnosis and is more severe in adults older than 50. Arthritic manifestations are diverse, and joint damage occurs not from iron but from deposition of CPPD crystals.
The distribution of joint involvement may resemble rheumatoid arthritis, affecting the metacarpophalangeal (MCP) joints, in particular the second and third MCP joints. However, reduced MCP flexion is not accompanied by ulnar deviation. The arthritis can progress, and large joints may become involved, particularly the hips, knees, and shoulders.
Metabolic Bone Disease
Of the numerous metabolic disorders involving connective tissue, only the most commonly occurring diseases that would appear in a physical therapy setting are discussed in this text. These include osteoporosis, osteomalacia, and Paget’s disease.
Osteoporosis: Osteoporosis, meaning “porous bone” and defined as a decreased mass per unit volume of normally mineralized bone compared with age- and sex-matched controls, is the most prevalent bone disease in the world.
Osteoporosis is classified as primary or secondary. Primary osteoporosis is the deterioration of bone mass unassociated with other chronic illnesses or diseases. It is usually related to the aging process, including decreased gonadal function. Idiopathic, postmenopausal, and senile osteoporosis are included in the primary osteoporosis classification. Postmenopausal osteoporosis is associated with accelerated bone loss in the perimenopausal and postmenopausal period, accompanied by high fracture rates, particularly involving the vertebrae. Senile osteoporosis, or age-related osteoporosis, increases with advancing age; it is caused by the bone loss that normally accompanies aging.
Secondary osteoporosis may accompany various endocrine and metabolic disorders (e.g., hyperthyroidism, hyperparathyroidism, hypogonadism, Cushing’s disease, and diabetes mellitus) that can produce associated osteopenia conditions (Table 11-13). Endocrine-mediated bone loss can produce osteoporosis because numerous endocrine hormones affect skeletal remodeling and hence skeletal mass.
Secondary osteoporosis is associated with other disorders that contribute to accelerated bone loss, such as chronic renal failure, rheumatoid arthritis, malabsorption syndromes related to gastrointestinal and hepatic disease, chronic respiratory disease, malignancies, and chronic chemical dependency (e.g., alcoholism).
Transient osteoporosis of the hip (TOH), a rare but temporary presentation of spontaneous osteoporosis of the femoral head (and sometimes femoral neck and acetabulum), is discussed in Chapter 16.
Risk Factors: There is consensus that risk factors associated with osteoporosis and osteoporotic fractures have been identified but which target groups should be screened (e.g., all adults, all postmenopausal women, adults 50 years old and older) varies among groups and organizations.66
United States Preventive Services Task Force (USPSTF) now recommends that all women 65 years of age and older should be screened for osteoporosis. Despite the facts that approximately half of postmenopausal women will sustain an osteoporosis-related fracture and 15% will sustain a hip fracture in their lifetime, 75% of American women between the ages of 45 and 75 years have never discussed osteoporosis with their physician.67,68 Plus, a gap exists in the number of people who present with osteoporotic fractures and those who are treated for the underlying cause of their fracture.69
Comparison of bone mineral density (BMD) to peak bone mass of women who are at peak bone mass is the designated T-score. T-scores are used as a method of describing severity of risk (Table 11-14). Therapists can be involved in primary prevention and education, encouraging and instructing consumers and clients in risk assessment and risk factor reduction (Fig. 11-9 and Box 11-2).
TABLE 11-14
| Status | T-Scores | Interpretation |
| Normal | −1.0 or above | T-score (BMD) is within (or above) 1 SD of the young adult reference mean. |
| Osteopenia (low bone mass) | −1.0 to −2.5 | T-score is 1.0 to 2.5 SDs below young adult mean for age. |
| Osteoporosis | −2.5 or less | T-score is 2.5 or more SDs below mean for age. |
| Severe osteoporosis | −2.5 or less with one or more fragility fractures | BMD is 2.5 or more SDs below mean for age. |
The T-score compares one person’s BMD in standard deviations with the average peak BMD in healthy young persons. Sometimes, Z-scores are used, which compare one individual’s BMD with the mean BMD of persons in the same age group, rather than with the normal values listed for young adults.
The World Health Organization (WHO) has proposed the clinical definition of osteoporosis in the table based on epidemiologic data that link low bone mass with increased fracture risk.
In study populations of Caucasian postmenopausal women, a BMD that was lower than 2.5 SD of normal peak bone mass was associated with a fracture prevalence; 50% of women with bone mass at this level had at least one bone fracture.93
On the basis of these data, the WHO defined osteoporosis as BMD 2.5 or more SD below peak bone mass, osteopenia as bone mass between 1.0 and 2.5 SD below peak, and normal as 1.0 SD below normal peak bone mass or higher.
The WHO criteria apply only to Caucasian, postmenopausal women, and not men, premenopausal women, or women of ethnicity other than Caucasian. T-score conversion based on the NHANES database is available for clinically significant low bone mass in other population groups at http://courses.washington.edu/bonephys/opbmdtz.html.
SD, Standard deviation; BMD, bone mineral density.
Data from World Health Organization: Criteria for defining bone density, WHO, 1994. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis, Geneva, 1994, World Health Organization. Technical Report Series, No. 843.

Fig. 11-9 If you answer “yes” to three or more of these questions, you may be at greater risk for developing osteoporosis, or “brittle bone disease,” and you should contact your physician for further information.
The World Health Organization (WHO) has developed a computerized tool called the Fracture Risk Assessment (FRAX) to calculate the 10-year risk of sustaining a fracture (www.shef.ac.uk/FRAX). It is based on individual patient models that integrate the risks associated with clinical risk factors, as well as BMD at the femoral neck. Medication treatment is recommended for postmenopausal women and men age 50 or older who have a 3% to 10% risk of a hip fracture or 20% risk of major fracture elsewhere in the body (e.g., wrist, spine, upper arm) according to the test.
Screening should begin at 60 years of age in women with key risk factors70 (Box 11-3). Screening begins earlier for anyone who has a fracture after age 50 or a maternal history of fracture after age 50.71 Key risk factors include women who have body weight less than or equal to 125 pounds (57 kg)71 and who do not currently use estrogen.
There is little known evidence to support the use of other individual risk factors, such as smoking, caffeine or alcohol use, low calcium, or low vitamin D intake, because those factors have not been shown to be independent predictors of low bone density.72 Medications used in high doses or for long-term use, such as thyroid supplements, corticosteroids, anticoagulants, lithium, and anticonvulsants, can contribute to the development of secondary osteoporosis.73
Primary prevention and education begins in childhood and adolescence, which are recognized as critical time periods for the development of normal peak bone mass. Diet and bone-building exercises during this critical period are essential in the development of adequate bone mass.
Men can be affected, especially those who smoke, drink alcohol moderately, fail to maintain a calcium-rich diet, have a sedentary lifestyle, or have a family history of fractures or those undergoing dialysis or long-term steroid administration. Some data suggest that men do not receive treatment for osteoporosis or counseling for osteoporosis prevention as aggressively as women.74
Additionally, researchers are beginning to examine the environmental influences associated with industrialized countries such as the United States. For example, although menopause is universal and the resulting estrogen deficiency is presumably similar for all women, differences in the occurrence of osteoporosis among countries cannot be explained only on the basis of estrogen deficiency.75 Countries with the highest incidence of osteoporosis also have a high incidence of heart disease and the highest consumption of carbohydrates, fat, protein, salt, and caffeine.
Clinical Presentation: Osteoporosis is a silent disease with no visible signs or symptoms until bone loss is sufficient to result in fracture. Osteoporosis associated with aging involves fractures of the proximal femur and vertebrae as well as the hip, pelvis, proximal humerus, distal radius, and tibia.
Postmenopausal osteoporosis is associated with accelerated bone loss in the perimenopausal period accompanied by high fracture rates, particularly involving the vertebrae. Vertebral compression fracture will be likely in 25% of women older than 65 and 50% of women 80 years and older (Case Example 11-9).76
Mild-to-severe back pain and loss of height may be the only early signs observed. Changes in bone density do not show up on x-ray films until there is a 30% loss. The cardinal features of established osteoporosis are bone fracture, pain, and deformity.
More than half the women in the United States who are 50 years of age or older are likely to have radiologically detectable evidence of abnormally decreased bone mass (osteopenia) in the spine. More than a third of these women develop major orthopedic problems related to osteoporosis. Most fractures sustained by women older than 50 are secondary to osteoporosis.
Osteomalacia: Osteomalacia is a softening of the bones caused by a vitamin D deficiency in adults and resulting from impaired mineralization in bone matrix. This failure in mineralization results in a reduced rate of bone formation. The deficiency may be due to lack of exposure to ultraviolet rays, inadequate intake of vitamin D in the diet, failure to absorb or use vitamin D, increased catabolism of vitamin D, a renal tubular defect, or a pathologically reduced number of vitamin D receptor sites in tissues.
The disease is characterized by decalcification of the bones, particularly those of the spine, pelvis, and lower extremities. X-ray examination reveals transverse, fracture-like lines in the affected bones and areas of demineralization in the matrix of the bone. These pseudofractures, known as Looser’s transformation zones, are bilateral. The most common sites are the ribs, long bones, lateral scapular margin, upper femur, and pubic rami. As the bones soften, they become bent, flattened, or otherwise deformed. Looser’s zones are believed to result from pressure on the softened bone by the nutrient arteries of its blood supply.
Severe bone pain, skeletal deformities, fractures, and severe muscle weakness and pain are common in people with osteomalacia. Clients typically complain of muscle weakness and pain that sometimes mimics polymyositis or muscular dystrophy.
A similar condition in children, occurring before epiphyseal plate closure, is called rickets. In children with rickets, x-ray findings include the well-known bowing of the long bones, in addition to widening, fraying, and clubbing of the areas of active bone growth. These areas especially include the metaphyseal ends of the long bones and the sternal ends of the ribs, the so-called rachitic rosary.
Paget’s Disease: Paget’s disease (osteitis deformans), named after Sir James Paget from the mid-1880s, is a focal inflammatory condition of the skeleton that produces disordered bone remodeling. Bone is resorbed and formed at an increased rate and in a haphazard fashion. As a result, the new bone is larger, less compact, more vascular, and more susceptible to fracture than normal bone.
Risk Factors: Paget’s disease is the most common skeletal disorder after osteoporosis, affecting men more often than women by a 3 : 2 ratio. Although Paget’s disease affects 2% to 5% of the population older than 40, it is most commonly seen in people older than 70, most of whom are asymptomatic.77 It is more prevalent in Europe and Australia and in people of Anglo-Saxon descent.
Genetic factors are important in the pathogenesis of Paget’s disease; in many families, the disease is inherited in an autosomal-dominant manner. Specific genetic mutations are being identified.78 Although the cause of this condition remains unknown, available evidence points to a slow viral infection in genetically predisposed individuals.79 Evidence for a major genetic component is supported by 40% of affected individuals having affected first-degree relatives.
There are no known ways to prevent Paget’s disease. Eating a healthy diet with sufficient calcium and vitamin D and getting exercise are critical in maintaining skeletal and joint function.79
Clinical Presentation: The severity of involvement and associated clinical characteristics vary greatly. Although some people are asymptomatic, with very limited bone involvement, others manifest a disabling, painful form of Paget’s disease that is characterized by skeletal pain and bones that are extremely deformed and easily fractured. Bones most commonly involved include (in decreasing order) the pelvis, lumbar spine, sacrum, femur, tibia, skull, shoulders, thoracic spine, cervical spine, and ribs.
Bone pain associated with Paget’s disease is described as aching, deep and boring, worse at night, and diminishing but not disappearing with physical activity. Muscular pain may be referred from involved bony structures or as a result of mechanical changes caused by joint deformities.
Other complications include a variety of nerve compression syndromes, secondary osteoarthritis, and vertebral compression and collapse. Rarely, Paget’s disease converts to a malignant neoplasm (osteogenic sarcoma of the femur or humerus) in older adults with extensive Paget’s disease. Metastases are common at the time of diagnosis; survival rates are very poor.80
Physician Referral
Disorders of the endocrine and metabolic systems may appear with recognizable clinical signs and symptoms but almost always require a combination of clinical and laboratory findings for accurate identification.
The therapist is encouraged to complete a thorough Family/Personal History form, augmented by the screening interview, and careful clinical observations, to provide the physician with pertinent screening information when making a referral. When appropriate, the Osteoporosis Screening Evaluation (see Fig. 11-9; see also Appendix C-6) may be helpful. In most cases, the client who has suffered from an endocrine disorder has already been diagnosed and may have been referred for physical therapy for some other musculoskeletal complaint. Such clients may have musculoskeletal problems that can be affected by symptoms associated with hormone imbalances (see Tables 11-4 through 11-7).
Diseases of the endocrine-metabolic system, such as diabetes, obesity, and thyroid abnormalities, account for some of the most common disorders encountered in a physical therapy practice. In recent years, new laboratory techniques have greatly enhanced the physician’s ability to diagnose these diseases.
Nevertheless, in many cases, the disorder remains unrecognized until relatively late in its course; signs and symptoms may be attributed to some other disease process or musculoskeletal disorder (e.g., weakness may be the major complaint in Addison’s disease). Thus any client who has any of the generalized signs and symptoms associated with the endocrine system (see Box 4-19) without an obvious or already known cause should be further evaluated by a physician.
Guidelines for Immediate Medical Attention
• Any person with diabetes who is confused, lethargic, exhibiting changes in mental function, profuse sweating (without exercise), or demonstrating signs of DKA should receive medical attention. (Perform a fingerstick glucose test to help evaluate the situation.)
• Likewise, any episode or suspected episode of hypoglycemia must be treated promptly and reported to the client’s physician.
• Signs of potassium depletion (e.g., muscle weakness or cramping, fatigue, cardiac arrhythmias, abdominal distention, nausea and vomiting) or fluid dehydration in a client who is taking non–potassium-sparing diuretics requires medical attention. Consultation with the physician is advised before exercising the individual.
• Signs of thyroid storm (tachycardia, elevated core body temperature, restlessness, agitation, abdominal pain, nausea, vomiting); observe clients with known history of hyperthyroidism carefully postoperatively or following trauma or infection.
Guidelines for Physician Referral
• Any unexplained fever without other symptoms in a person taking corticosteroids may be an indication of infection and should be evaluated by a physician.
• Palpable nodules or a palpable mass in the supraclavicular area or the scalene triangle, or both (see Fig. 11-5), especially if accompanied by new-onset hoarseness, hemoptysis, or elevated blood pressure, must be evaluated by a physician.
• Any episode (especially a series of episodes) of hypoglycemia in the client with diabetes should be reported to the physician.
• The presence of multiple eruptive xanthomas on the extensor tendons of anyone with diabetes may signal uncontrolled glycemia and requires medical referral to normalize lipid levels; exercise remains a key to the management of this condition.
• Signs of fluid loss or dehydration in anyone taking diuretics should be reported to the physician.
• Recurrent arthritic symptoms in a client with gout who is already taking urate-lowering drugs require medical referral for review of medication.
Clues to Symptoms of Endocrine or Metabolic Origin
• Endocrine or metabolic disease has been previously diagnosed. Bilateral CTS, proximal muscle weakness, and periarthritis of the shoulder(s) are common in persons with certain endocrine and metabolic diseases. Look for other associated signs and symptoms of endocrine or metabolic disease (see Box 4-19).
• Long-term use of corticosteroids can result in classic symptoms referred to as Cushing’s syndrome.
Clinical Presentation
• Identified trigger points are not eliminated or relieved by trigger point therapy. Observe for signs and symptoms of hypothyroidism.
• Palpable lymph node(s) or nodule(s) in the scalene triangle (see Fig. 11-5), especially when accompanied by new-onset hoarseness, hemoptysis, or elevated blood pressure.
• Anyone with muscle weakness and fatigue who is taking diuretics may be experiencing symptoms of potassium depletion. Assess for cardiac arrhythmias, and ask about nausea and vomiting.
• Muscle fasciculation and cramping may be associated with antacid use (metabolic alkalosis).
Associated Signs and Symptoms
• Watch for anyone with arthralgias, hand pain and stiffness, or muscle weakness with an accompanying cluster of signs and symptoms of endocrine or metabolic disorders (see Box 4-19).
Clues to Recognizing Osteoporosis
• Pain is usually severe and localized to the site of fracture (usually mid-thoracic, lower thoracic, and lumbar spine vertebrae).
• Pain may radiate to the abdomen or flanks.
• Aggravating factors: prolonged sitting, standing, bending, or performing Valsalva’s maneuver.
• Alleviating factors: sidelying with hips and knees flexed.
• Sitting up from supine requires rolling to the side first.
• Not usually accompanied by sciatica or chronic pain from nerve root impingement
• Tenderness to palpation over the fracture site
• Rib or spinal deformity, dowager’s hump (cervical kyphosis)
1. What are the most common musculoskeletal symptoms associated with endocrine disorders?
2. What systemic conditions can cause carpal tunnel syndrome?
3. What are the mechanisms by which carpal tunnel syndrome occurs?
4. Disorders of the endocrine glands can be caused by:
5. List three of the most common symptoms of diabetes mellitus.
1. _______________________________________________
6. What is the primary difference between the two hyperglycemic states: diabetic ketoacidosis (DKA) and hyperglycemic, hyperosmolar, nonketotic coma (HHNC)?
7. Is it safe to administer a source of sugar to a lethargic or unconscious person with diabetes?
8. Clients with diabetes insipidus (DI) would most likely come to the therapist with which of the following clinical symptoms?
9. Clients who are taking corticosteroid medications should be monitored for the onset of Cushing’s syndrome. You will need to monitor your client for which of the following problems?
10. Signs and symptoms of Cushing’s syndrome in an adult taking oral steroids may include:
a. Increased thirst, decreased urination, and decreased appetite
b. Low white blood cell count and reduced platelet count
11. Parathyroid hormone (PTH) secretion is particularly important in the metabolism of bone. The client with an oversecreting parathyroid gland would most likely have:
12. Which glycosylated hemoglobin (A1C) value is within the recommended range?
13. A 38-year-old man comes to the clinic for low back pain. He has a new diagnosis of Graves’ disease. When asked if there are any other symptoms of any kind, he replies “increased appetite and excessive sweating.” When you perform a neurologic screening examination, what might be present that would be associated with the Graves’ disease?
a. Hyporeflexia but no change in strength
b. Hyporeflexia with decreased muscle strength
14. All of the following are common signs or symptoms of insulin resistance except:
References
1. Schnetzler, KA. Acute carpal tunnel syndrome. J Am Acad Orthop Surg. 2008;16:276–282.
2. Bickel, KD. Carpal tunnel syndrome. J Hand Surg. 2010;35A:147–152.
3. Palmer, KT. Carpal tunnel syndrome and its relation to occupation: a systematic literature review. Occup Med. 2007;57:57–66.
4. Lozano-Calderon, S. The quality and strength of evidence for etiology: example of carpal tunnel syndrome. J Hand Surg. 2008;33A(4):525–538.
5. Phalen, GS. The carpal tunnel syndrome: seventeen years’ experience in diagnosis and treatment of six hundred and fifty-four hands. J Bone Joint Surg. 1966;48A(2):211–228.
6. Grossman, LA, Kaplan, HJ, Ownby, FD. Carpal tunnel syndrome: initial manifestation of systemic disease. JAMA. 1961;176:259–261.
7. Wluka, AE, Cicuttini, FM, Spector, TD. Menopause, oestrogens, and arthritis. Maturitas. 2000;35(3):183–189.
8. Ferry, S, Hannaford, P, Warskyj, M, et al. Carpal tunnel syndrome: a nested case-control study of risk factors in women. Am J Epidemiol. 2000;151(6):566–574.
9. Michlovitz, S. Conservative interventions for carpal tunnel syndrome. J Orthop Sports Phys Ther. 2004;34(10):591–598.
10. Grokoest, AW, Demartini, FE. Systemic disease and carpal tunnel syndrome. JAMA. 1954;155:635–637.
11. Katz, JN. Clinical practice: carpal tunnel syndrome. N Engl J Med. 2002;346:1807.
12. McNab, T, Khandwala, H, Acromegaly as an endocrine form of myopathy: a case report and review of the literature. Endocr Prac. 2005;11;1:18–22. Available online at www.medscape.com/viewarticle/501408. [Accessed January 21, 2011].
13. Rothschild, B. Hyperostosis associated with hip surgery? J Musculoskel Med. 21(5), 2004.
14. Holcomb, S. Confronting Cushing’s syndrome. Nursing 2005. 2005;35(9):32–36.
15. Holcomb, SS. Detecting thyroid disease. Nursing 2005. 2005;35(10):S4–S9.
16. Boissonnault, WG, Koopmeiners, MB. Medical history profile: orthopaedic physical therapy outpatients. J Orthop Sports Phys Ther. 1994;20(1):2–10.
17. President and Fellows of Harvard College, Harvard Medical School: Thyroid diseases—a special health report. Harvard Health Newsletter. 2004.
18. Trivalle, C, Doucet, J, Chassagne, P, et al. Differences in the signs and symptoms of hypothyroidism in older and younger patients. J Am Geriatr Soc. 1996;1(44):50–53.
19. Lowe, JC, Honeyman-Lowe, G. The metabolic treatment of fibromyalgia. Lafayette, CO: McDowell Health Science Books; 2000.
20. Lowe, JC, Lowe, G. Facilitating the decrease in fibromyalgia pain during metabolic rehabilitation: an essential role for soft tissue therapies. J Bodywork Movement Ther. 1998;2(4):208–217.
21. Lowe, JC, Reichman, AJ, Honeyman, GS, et al. Thyroid status of fibromyalgia patients. Clin Bull Myofascial Ther. 1998;3(1):47–53.
22. Lowe, JC, Reichman, AJ, Yellin, BA. A case-control study of metabolic therapy for fibromyalgia: long term follow-up comparison of treated and untreated patients. Clin Bull Myofascial Ther. 1998;3(1):65–79.
23. Geenen, R, Jacobs, JW, Bijlsma, JW. Evaluation and management of endocrine dysfunction in fibromyalgia. Rheum Dis Clin North Am. 2002;28(2):389–404.
24. Garrison, RL, Breeding, PC. A metabolic basis for fibromyalgia and its related disorders: the possible role of resistance to thyroid hormone. Med Hypotheses. 2003;61(2):182–189.
25. An anti-nuclear shield for your thyroid. Johns Hopkins Med Lett. 2002;14(8):5–7.
26. Gagel, RF, Goepfert, H, Callender, DL. Changing concepts in the pathogenesis and management of thyroid carcinoma. CA Cancer J Clin. 1996;46(5):261–283.
27. Strewler, G. Primary hyperparathyroidism does not progress in most patients. JAMA. 2005;293:1772–1779.
28. Utiger, R. The physician’s perspective. NEJM Health News. 2003;9(1):5.
29. Morbidity and Mortality Weekly Report. November is American Diabetes Month. MMWR. 2010;59(42):1361.
30. Burrows, NR. Incidence of end-stage renal disease attributed to diabetes among persons with diagnosed diabetes in the United States and Puerto Rico. MMWR. 2010;59(42):1361–1366.
31. New York Academy of Sciences. Is Alzheimer’s disease type 3 diabetes? Webinar presented by the Biochemical Pharmacology Discussion Group and the American Chemical Society. http://www.nyas.org/events/WebinarDetail.aspx?cid=25aebb2b-0529-4cc1-92b0-c38c38b3d34f, Oct. 27, 2009. [Available online at Accessed March 3, 2011].
32. Wood, L, Setter, SM, Type 3 Diabetes: brain diabetes? US Pharm. 2010;35;5:36–41. Available online at http://www.uspharmacist.com/content/d/feature/c/20754/. [Accessed March 3, 2011].
33. Centers for Disease Control and Prevention. Diabetes: National Diabetes Fact Sheet for the United States. http://www.cdc.gov/diabetes/statistics/index.htm, 2010. [Available online at Accessed January 21, 2011].
34. Boyle, JP. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29.
35. Centers for Disease Control and Prevention (CDC). Number of Americans with diabetes projected to double or triple by 2050. Older, more diverse populations and longer lifespans contribute to increase. http://www.cdc.gov/media/pressrel/2010/r101022.html, October 22, 2010. [Available online at Accessed March 3, 2011].
36. American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl 1):S11–S61.
37. Norris SL, Zhang X, Avenell A, et al: Long-term non-pharmacological weight loss interventions for adults with prediabetes. Cochrane Database Syst Rev 2:CD005270, April 18, 2005.
38. US Renal Data System. USRDS 2009 annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009.
39. Katon, WJ, Rutter, C, Simon, G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005;28(11):2668–2672.
40. Ciechanowski, P, Russo, J, Katon, W, et al. Where is the patient? The association of psychosocial factors and missed primary care appointments in patients with diabetes. Gen Hosp Psychiatry. 2006;28(1):9–17.
41. Katon, W, Cantrell, CR, Sokol, MC, et al. Impact of antidepressant drug adherence on comorbid medication use and resource utilization. Arch Intern Med. 2005;165(21):2497–2503.
42. Barclay, L, Modifiable risk factors may be linked to risk of developing diabetic neuropathy. N Engl J Med 2005;352:341–350. 408–409 Available online at http://www.medscape.com/viewarticle/498185. [Accessed January 11, 2011].
43. Tesfaye, S, Chaturvedi, N, Eaton, SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–350.
44. Said, G, Diabetic neuropathy—a review. Nat Clin Pract Neurol. 2007;3;6:331–340. Available online at http://www.medscape.com/viewarticle/558574. [Accessed Dec. 13, 2010].
45. Cagliero, E, Apruzzese, W, Perlmutter, GS. Watch for hand, shoulder disorders in patients with diabetes. Am J Med. 2002;112:487–490.
46. Cullen, A, Oflouglu, O, Donthineni, R, Neuropathic arthropathy of the shoulder, Charcot shoulder. Medscape. 2005:7;1. Available online at www.medscape.com/viewarticle/496650). [Accessed on January 21, 2011].
47. Scarborough, P. Diabetes care: tests and measures for the foot and lower extremity. Acute Care Perspectives. 2002;11(4):1–6.
48. Boulton, A. Treatment of symptomatic diabetic neuropathy. Diabetes Metab Res Rev. 2003;19(Suppl 1):S16–S21.
49. Kelly, J. Manipulation for frozen shoulder. J Musculoskel Med. 2003;20(2):58.
50. Mukhtar, Q, Pan, L, Jack, L, et al. Prevalence of receiving multiple preventive care services among adults with diabetes—United States, 2002–2004. MMWR. 2005;54(44):1130.
51. Agency for Healthcare Research and Quality (AHRQ). Diagnosis and management of type 2 diabetes mellitus in adults. Updated by ECRI Institute http://guideline.gov/content.aspx?f=rss&id=24137, 2010. [Available online at Accessed March 2, 2011].
52. Colberg, SR, Walsh, J, Pumping insulin during exercise. Phys Sports Med. April 2002:30;4. Available online at http://www.physsportsmed.com/index.php?article=255. [Accessed January 20, 2011].
53. Auber, G. Taking the heat off: how to manage heat injuries. Nursing 2004. 2004;34(7):50–52.
54. Appel, SJ. Sizing up patients for metabolic syndrome. Nursing 2005. 2005;35(12):20–21.
55. Lorenzo, C. The National Cholesterol Education Program—Adult Treatment Panel III, International Diabetes Federation and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30(1):8–13.
56. Alberti, KG, Eckel, RH, Grundy, SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645.
57. Olatunbosun, ST. Insulin resistance, eMedicine. Updated 5/16/10. Available online at http://emedicine.medscape.com/article/122501-print. [Accessed Sept. 15, 2010].
58. Rosenzweig, JL. Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(10):3671–3689.
59. Chen, L, Schumacher, R. Gout and gout mimickers: 20 clinical pearls. J Musculoskel Med. 2003;20(5):254–258.
60. Kurakula, PC, Keenan, RT. Diagnosis and management of gout: an update. J Musculoskel Med. 2010;27(suppl):S13–S19.
61. Barclay, L, Lie, D, Dietary risk factors for gout clarified. Medscape Medical News March 2004;10. Available online at http://www.medscape.com/viewarticle/471444. [Accessed January 21, 2011].
62. Choi, HK. A prescription for lifestyle change in patients with hyperuricemia and gout. Curr Opin Rheumatol. 2010;22(2):165–172.
63. Choi, HK. Alcohol intake and risk of incident gout in men: a prospective study. Lancet. 2004;363:1277–1281.
64. Wiese, W, Sanders, LS, Wortmann, RL. Gout: effective strategies for acute and long-term control. J Musculoskel Med. 2004;21(10):510–519.
65. Stevens, S, Edwards, C. Recognizing and managing hemochromatosis and hemiarthropathy. J Musculoskel Med. 2004;21(4):212–225.
66. Agency for Healthcare Research & Quality (AHRQ). Screening and risk assessment for osteoporosis in postmenopausal women. Revised 2011. Available online at http://guideline.gov/syntheses/synthesis.aspx?f=rss&id=25304. [Alternate website].
67. Edwards, BJ, Brooks, ER, Langman, CB. Osteoporosis screening of postmenopausal women in the primary care setting; a case-based approach. Gend Med. 2004;1(2):70–85.
68. Feldstein, AC, Nichols, GA, Elmer, JP, et al. Older women with fractures: patients falling through the cracks of guideline-recommended osteoporosis screening and treatment. J Bone Joint Surg. 2003;85A(12):2294–2302.
69. Dipaola, CP. Survey of spine surgeons on attitudes regarding osteoporosis and osteomalacia screening and treatment for fractures, fusion surgery, and pseudoarthrosis. Spine J. 2009;9(7):537–544.
70. Focus on Healthy Aging. Expert panel urges mass screening for osteoporosis, older women. Mt Sinai School Med. 2003;6(1):2.
71. Black, DM, Steinbuch, M, Palermo, L, et al. An assessment tool for predicting fracture risk in postmenopausal women. Osteoporosis Int. 2001;12(7):519–528.
72. Maricic, M, Gluck, O. Osteoporosis: 20 clinical pearls. J Musculoskel Med. 2003;20(11):508–512.
73. Bones are big news. Johns Hopkins Med Lett Health After 50. 2005;17(1):4–5.
74. Kiebzak, G, Beinart, G, Perser, K, et al. Undertreatment of osteoporosis in men with hip fracture. Arch Intern Med. 2002;162(19):2217–2222.
75. Simmons, G. Far Eastern osteoporosis study. Hong Kong: The Gordon Simmons Research Group Ltd; 1996.
76. DePalma, M, Slipman, C. Managing osteoporotic vertebral compression fractures. J Musculoskel Med. 2005;22(9):445–454.
77. Papapoulos, SE. Paget’s disease of bone: clinical, pathogenetic, and therapeutic aspects. Baillieres Clin Endocrinol Metab. 1997;11(1):117–143.
78. Daroszewska, A, Ralston, SH. Genetics of Paget’s disease of bone. Clin Sci (London). 2005;109(3):257–263.
79. Roodman, GD, Windle, JJ. Paget disease of bone. J Clin Invest. 2005;115(2):200–208.
80. American Academy of Orthopedic Surgeons. Paget’s disease of bone. http://www.orthoinfo.aaos.org, Oct. 2005. [Available online at (See tumors). Accessed January 21, 2011].
81. Lydick, E, Cook, K, Turpin, J, et al. Development and validation of a simple questionnaire to facilitate identification of women likely to have low bone density. Am J Man Care. 1998;4:37–48.
82. Elliott, ME, Drinka, PJ, Krause, P, et al. Osteoporosis assessment strategies for male nursing home residents. Maturitas. July 2004;48(3):225–233.
83. National Osteoporosis Foundation (NOF). Standing tall for you. Prevention: who’s at risk? Available online at http://www.nof.org. [Accessed January 10, 2011].
84. Orwoll, ES, Bauer, DC, Vogt, TM, et al. Axial bone mass in older women. Ann Intern Med. 1996;124:187–196.
85. Funk, D, Swank, AM, Adam, KJ, et al. Efficacy of moist heat pack application over static stretching on hamstring flexibility. J Strength Cond Res. 2001;15(1):123–126.
86. Daly, MP, Berman, BM. Rehabilitation of the elderly patient with osteoarthritis. Clin Geriatr Med. 1993;9:783–801.
87. Cottingham, JT, Maitland, J. A three-paradigm treatment model using soft tissue mobilization and guided movement-awareness techniques for a patient with chronic low back pain: a case study. J Orthop Sports Phys Ther. 1997;26(3):155–167.
88. Hayden, JA, Van Tulder, MW, Malmivaara, AV, Koes, BW. Meta-analysis: Exercise therapy for non-specific low back pain. Ann Intern Med. 2005;142(9):765–775.
89. Heggeness, MH. Spinal fracture with neurological deficit in osteoporosis. Osteoporos Int. 1993;3(4):215–221.
90. D’Costa, H, George, G, Parry, M, et al. Pitfalls in the clinical diagnosis of vertebral fractures: a case series in which posterior midline tenderness was absent. Emerg Med J. 2005;22:330–332.
91. Nevitt, MC, Ettinger, B, Black, DM, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med. 1998;128(10):793–800.
92. Fink, HA, Milaetz, DL, Palermo, L, et al. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res. 2005;20(7):1216–1222.
93. The WHO Study Group, Assessment of fracture risk and its application to screening for postmenopausal osteoporosis, WHO Technical Report Series 843. Geneva: World Health Organization, 1994.

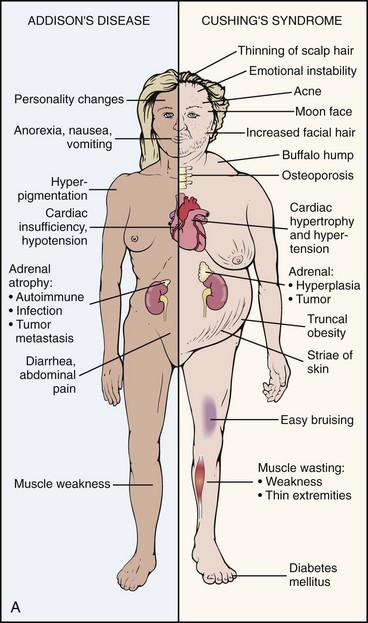
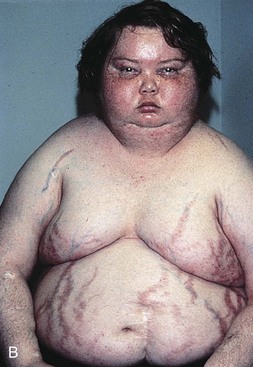
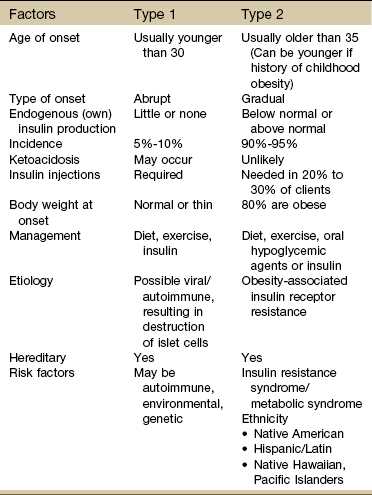
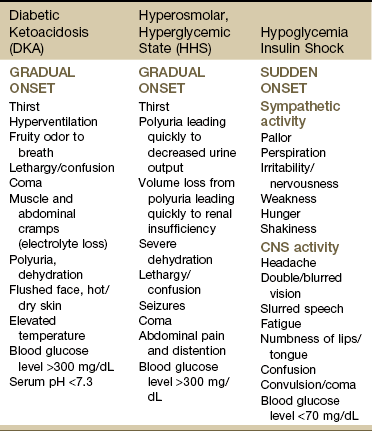
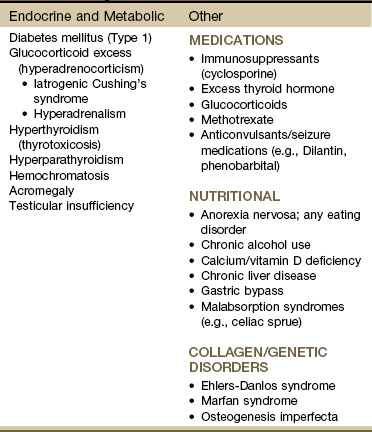
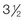 minutes on the treadmill at 1.0 mph (zero grade) because of increasing pain. Treadmill walking was used to measure progress because one of the client’s goals was to be able to walk up to 1 mile.
minutes on the treadmill at 1.0 mph (zero grade) because of increasing pain. Treadmill walking was used to measure progress because one of the client’s goals was to be able to walk up to 1 mile. Key Points to Remember
Key Points to Remember