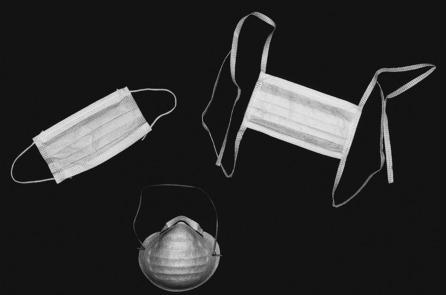
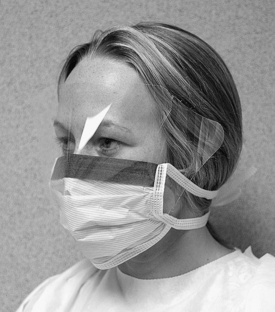
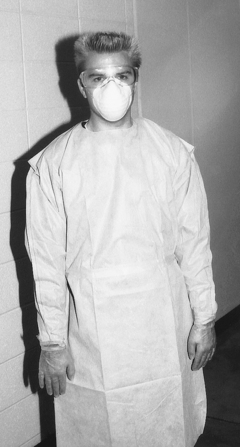
Protective Barriers
After completing this chapter, the student should be able to do the following:
 Describe tasks in the dental office that require the use of personal protective equipment (i.e., gloves, masks, protective eyewear, and protective clothing).
Describe tasks in the dental office that require the use of personal protective equipment (i.e., gloves, masks, protective eyewear, and protective clothing).
 Describe the limitations of personal protective equipment.
Describe the limitations of personal protective equipment.
 Describe when personal protective barriers should be changed.
Describe when personal protective barriers should be changed.
 Compare the different types of gloves and describe the potentially harmful reactions to gloves.
Compare the different types of gloves and describe the potentially harmful reactions to gloves.
 Describe the procedures and types of products available for hand hygiene.
Describe the procedures and types of products available for hand hygiene.
 List the sequence of donning and removing personal protective barriers.
List the sequence of donning and removing personal protective barriers.
Chapter 3 describes the steps in development of an infectious disease, indicating that contamination of the body with microorganisms must occur before disease can develop. Prevention of this exposure or contamination (when possible) is always better than to rely totally on the resistance of the body to fight off disease agents after contamination. Preventing exposure means to avoid contact with the microorganisms, and one accomplishes this in two ways. One way is to prevent the microorganisms from escaping from their source (e.g., in the dental office the main source of microorganisms is the patient’s mouth). Totally preventing microorganisms from escaping the patient’s mouth during care is impossible because microorganisms exit the patient’s mouth on instruments, fingers, and supplies used intraorally, and they escape in aerosols and spatter droplets generated during the use of the air-water syringe, slow- and high-speed handpieces, and ultrasonic scalers. However, use of a rubber dam and preprocedure mouth rinsing can reduce this escape of microorganisms from the patient’s mouth, as described in Chapter 14.
The second way to prevent exposure is to use barriers to prevent contact with microorganisms escaping from their sources. In dentistry this involves the use of protective barriers such as gloves, masks, protective eyewear, and protective clothing. The Occupational Safety and Health Administration blood-borne pathogens standard (see Chapter 8 and Appendix H) indicates that in facilities where exposure to blood-borne pathogens may occur (e.g., dental offices), the employer is responsible for providing, maintaining, cleaning/laundering, disposing of, and ensuring the use of protective barriers, sometimes referred to as personal protective equipment.
GLOVES
Gloves protect dental team members from direct contact with microorganisms present in patients’ mouths and on contaminated surfaces, and they also protect patients from microorganisms on the hands of the dental team.
PROTECTION OF THE DENTAL TEAM
Although intact skin is an excellent barrier to disease agents, a small or even invisible cut appears like the Grand Canyon to microorganisms. Thus small cuts and abrasions can serve as routes of entry of microorganisms into the body, causing a skin infection or other more widespread diseases. One study showed that fourth-year dental students had an average of four areas of trauma on their hands; 12% of the traumas became painful on contact with alcohol, suggesting open skin (cut or abrasion). Some visually intact areas also give a painful response with alcohol, particularly around the fingernails. Thus even a close visual inspection of the hands may not detect all possible portals of entry for microorganisms.
Cuts on unprotected hands are suggested by many to be an important reason for the high occurrence of hepatitis B in dental and medical personnel who do not use gloves routinely. Analyses of the disease herpetic whitlow (herpes simplex virus infection around the fingernails) before the routine gloving era of dentistry also indicate that this condition occurred more frequently in dentists than in others. Another protective value of wearing gloves in the office is protection against contact with chemicals (such as cleaners, disinfectants, sterilants, and x-ray developing solutions) and some dental materials that may irritate the skin. Heat-resistant gloves also protect against burns when heat processing instruments.
PROTECTION OF PATIENTS
Microorganisms are present on just about every surface in the office that has not just been cleaned and disinfected. Microorganisms are there because they settle out of the air or because of contact with other contaminated surfaces. Thus ungloved hands become contaminated with microorganisms on touching just about any environmental surface and from direct contact with fluids or surfaces in a patient’s mouth. If these contaminating microorganisms are not removed by handwashing (as described subsequently) or covered up with gloves, they may be transmitted to a patient.
A dental patient’s blood has been shown to be retained under the fingernails of a dental team member for several days, even with handwashing. This residue could serve as a source of infection for subsequent patients. Thus routine gloving can prevent blood or saliva impaction in those areas (particularly around and under the fingernails) that are difficult to clean.
Microorganisms present in blood may exit the body through small cuts in the skin. This process probably is enhanced if these cuts are moistened, as when a dental team member does not wear gloves when working in a patient’s mouth. This route of disease spread from dental team member to patient may have been important in several of the 10 reported cases of hepatitis B spread from infected dentists to patients (see Chapter 6). These infected dentists did not use gloves routinely for patient care.
One of the most clearly documented cases of disease spread in a dental office resulted from not using gloves routinely for patient care. An ungloved hygienist with dermatitis on her hands and fingers cared for a patient with active herpes labialis (herpes simplex infection on the lips). About a week later, vesicles of herpetic whitlow developed on the hygienist’s hands. Before any sign of her infection had appeared, however, she had unknowingly spread the virus to at least 20 other patients, who developed intraoral herpes lesions. When the vesicles appeared on the hygienist’s hands, she began to routinely wear gloves, which prevented further spread of the virus to any more patients.
This case demonstrates three modes of disease spread in the office (as discussed in Chapter 8): first, from patient to dental team member; second, from dental team member to patient; and third, from patient to patient. In this instance, all three modes of disease spread could have been prevented by routine gloving with every patient. Another important point demonstrated by this case is that dermatitis greatly reduces the effectiveness of handwashing in removing contaminating disease agents. Less vigorous handwashing is performed because of the painful dermatitis, and the dermatitis itself provides additional places on the hands where microorganisms can “hide” from the mechanical action of handwashing.
Uses and Types
Box 10-1 lists the several types of gloves available for various uses in the dental setting. Some have specific uses, and others have multiple uses (Figure 10-1).
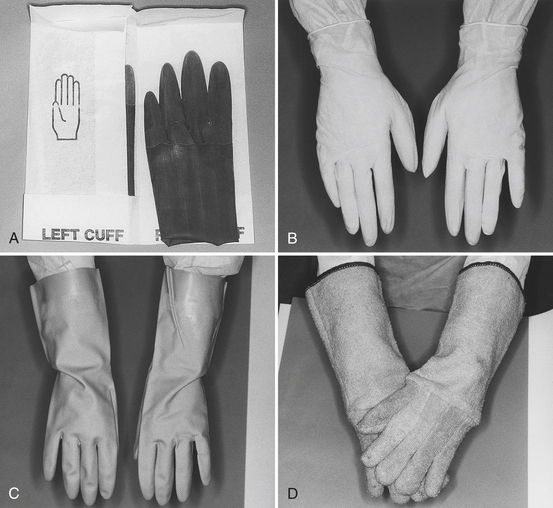
FIGURE 10-1 Gloves used in dentistry. A, Sterile surgeon’s gloves. B, Examination gloves. C, Utility gloves. D, Heat-resistant gloves.
PATIENT CARE ACTIVITIES
Dental professionals should wear disposable gloves during all patient care activities where a potential exists for direct hand contact with saliva, blood, or other oral fluids, mucous membranes, and nonintact skin and when handling items or surfaces contaminated with body fluids or potentially infectious materials.
Gloves used for patient care are not to be reused on a subsequent patient. Dental professionals also should not wash patient care gloves with any detergent or chemical; washing may weaken stabilizers in the glove material or enhance penetration (cause wicking) of material through inherent defects. One may rinse off powder (cornstarch) on fresh gloves with plain water before patient care, if desired.
If the dental professional leaves the chairside during patient care, the best procedure is first to remove the gloves and don a fresh pair on returning to chairside. This change prevents contamination of any surfaces one may touch when away from chairside and prevents contaminating the patient with microorganisms already present on those same surfaces. One also should remember that any surface at chairside that is touched with contaminated gloves and also may be touched during the care of the next patient must have been covered previously to prevent cross-contamination or must be precleaned and disinfected before care of the next patient (see Chapter 12 on surface asepsis). An alternative to changing gloves in these situations is to use inexpensive copolymer or plastic gloves or a sheet of plastic wrap over the patient care gloves (overgloving) to prevent spread of the patient’s microorganisms to surfaces that are touched. One then removes the overgloves before resuming care on the patient.
Another important aspect of glove use during patient care is that one must remove torn or punctured gloves as soon as possible, followed immediately by handwashing and donning of fresh gloves.
Sterile latex or vinyl gloves are used during surgical procedures, but nonsterile gloves are appropriate for most other dental procedures. Surgeon’s gloves are provided in half-sizes ranging from 5 to 9 and usually provide the best fit because the gloves are made for the right hand and left hand. Most examination gloves are ambidextrous in that any glove can be used on the right or left hand. These gloves usually are provided in extra-small, small, medium, and large sizes, but some brands may be sized from 5 to 9. Latex gloves are thought by most to give a better fit than vinyl gloves, but this may not always be true. Use of gloves that fit properly is important to ensure efficient handling of items and to prevent fatigue of the hands.
Use of powderless gloves, reduced-protein latex gloves, nonlatex gloves, and dermal gloves relates to the irritations and allergic reactions some persons have to gloves. See the discussion under Harmful Reactions to Gloves.
OPERATORY CLEANUP AND INSTRUMENT PROCESSING
To provide more protection for the hands during operatory cleanup and handling of instruments than that provided by the thin latex or vinyl patient care gloves or thin copolymer or plastic gloves, one should use utility gloves of nitrile or heavy latex when preparing and using chemicals, precleaning and disinfecting contaminated surfaces, and handling contaminated items during instrument processing. Each person in the office needing these gloves should have his or her own pair or pairs of gloves. The heavy utility gloves are reusable and can be washed with an antimicrobial handwashing agent, rinsed, and dried.
One should use heat-resistant gloves for handling hot items when unloading sterilizers or whenever a risk of burning the hands or forearms exists.
OTHER ACTIVITIES
The dental professional should wear gloves whenever a chance exists for contact with items potentially contaminated with pathogenic microorganisms, such as when handling appliances or equipment in the dental laboratory that have not been decontaminated; contaminated laundry; contaminated waste, tissue, or teeth; and containers of blood, saliva, or other infectious material if a risk of spilling or splashing exists.
Limitations
The manufacturing process for patient care gloves may result in a low level of pinholes; however, the U.S. Food and Drug Administration has placed strict requirements on glove manufacturers to help ensure a high quality of these medical devices. Some manufacturers even test each glove for defects before selling them.
Although gloves provide a high level of protection against direct contact with infectious agents through touching, they offer little protection against injuries with sharp objects such as instruments, needles, and scalpel blades. Thus one still must handle contaminated sharps safely, even while wearing gloves. Sharps injuries also can and do occur through the heavy utility gloves during instrument processing.
One should not use gloves that are torn or have other noticeable defects. One should not reuse heavy utility gloves if they are peeling, cracking, discolored, torn, punctured, or show any other signs of deterioration.
One should not store gloves in direct sunlight or in high dust areas.
Harmful Reactions to Gloves
Some health care workers and patients have harmful reactions when they come into contact with latex gloves or with airborne glove materials. Because these reactions result from contact with latex proteins or other chemicals in the gloves, a brief description of glove manufacturing may help explain the origin of these chemicals.
Latex gloves are manufactured from latex extracted from the rubber tree Hevea brasiliensis, which grows in tropical areas throughout the world. The latex is in the form of a milky fluid to which anticoagulants and preservatives are first added. The latex fluid itself contains the rubber material (cis-1,4 polyisoprene), along with proteins, lipids, and carbohydrates. This latex material then is compounded by adding up to 200 different chemicals, depending on the desired characteristics of the final product. These chemicals are antidegradants, vulcanizing agents that make the latex elastic, accelerators, retarders, promoters, pigments, activators, and mold-releasing agents and also may include fragrances, emulsifiers, stabilizers, biocides, and ultraviolet light absorbers. Hand-shaped porcelain formers coated with more chemicals and with cornstarch powder as a releasing agent are dipped into the compounded latex. The formers then are passed through ovens and a warm-water leaching bath to remove some of the latex proteins and other chemicals. Further treatments include adding more cornstarch or a special chlorination treatment if powder-free gloves are desired. Items manufactured from natural latex are referred to as natural rubber latex (NRL) products.
Although allergies to the NRL in gloves are the major area of concern, contact with other latex-containing products (Box 10-2) besides gloves also may induce a reaction. Currently, about 1% to 6% of the general population and 8% to 12% of regularly exposed health care workers are estimated to be sensitive to latex.
Three types of reactions may occur with gloving. One is a skin reaction to irritants in the gloves called irritant contact dermatitis, and the other two are the immunologic reactions of allergic contact dermatitis and latex allergy.
IRRITANT CONTACT DERMATITIS
Most reactions from wearing gloves result from a nonimmunologic irritation of the skin from nonlatex chemicals in the gloves or applied to the hands. In these instances the skin on the hands becomes dry, reddened, itchy, and sometimes cracked in severe cases.
Conditions that may initiate or aggravate this dermatitis include handwashing with irritating cleaners or antiseptics, failure to rinse completely, failure to dry hands thoroughly after rinsing, excessive perspiration on the hands, and irritation from cornstarch powder.
To reverse and prevent recurrence of irritant contact dermatitis, one should attempt to identify the irritant by first making sure one is performing proper hand hygiene (Figure 10-2). One also should consider changing gloves or handwashing agent brands. When changing glove brands, one should be sure to determine whether a different brand is really a different type of glove. Because the same glove may be sold under many different brands, one should compare samples before purchasing “new” gloves in large volumes.
ALLERGIC CONTACT DERMATITIS
Four types of immunologic hypersensitivities or allergies (I to IV) occur. Two of these types (I and IV) involve reactions to gloves, and both types require a person first to become sensitized to an agent (called an allergen) that on subsequent contact causes a harmful reaction. Allergic contact dermatitis is type IV hypersensitivity, also called delayed hypersensitivity, and is the most frequently occurring immunologic reaction to gloves, accounting for about 80% of the cases. Type I hypersensitivity to glove latex is described subsequently.
Allergic contact dermatitis is almost always limited to the areas of contact and is characterized by initial itching, redness, and vesicles within 24 to 48 hours, followed by dry skin, fissures, and sores. The reaction to poison ivy also is a type IV hypersensitivity. Allergic contact dermatitis caused by contact with gloves results from exposure to one of the many chemicals added during latex harvesting, processing, or glove manufacturing. The most common chemical sensitizers are the accelerators used in vulcanization: thiurams, carbamates, and mercaptobenzothiazoles. Vulcanization is the polymerizing process that makes the latex elastic. In two studies of patients with allergic reactions to gloves, 72% and 83% of patients were patch test positive for thiurams and 25% and 22% were positive for carbamates. The patch test contains small amounts of different chemicals that when applied to the skin of an allergic person will produce a small reaction indicating the specific causative agent.
Eliminating contact with the sensitizing agent is the only way to prevent allergic contact dermatitis. Glove manufacturers attempt to control the addition of chemicals to latex products, but a confusing point is the labeling of gloves as “hypoallergenic” just because they may contain reduced levels of certain chemicals. Such gloves are not free of all potentially sensitizing chemicals and may still be able to induce harmful reactions. The Food and Drug Administration now prohibits such labeling.
LATEX ALLERGY
MECHANISMS: Latex allergy is the third type of reaction to gloves and the second type involving an immune response. Latex allergy is a type I hypersensitivity, also called an immediate hypersensitivity. In this instance, a person is allergic to the naturally occurring proteins present in latex. A person who develops a latex allergy first becomes sensitized by one or more exposures to latex protein allergens before reaction to a subsequent exposure can occur. In sensitization, immunoglobulin E antibodies develop after exposure to the latex protein allergens. These antibodies bind to special cells in the body called mast cells, but a harmful reaction does not occur yet. When more latex protein allergens enter the body (with subsequent exposure), the mast cells are stimulated to produce substances that can cause skin reactions and occasionally more serious systemic reactions that affect blood flow and breathing or cause anaphylaxis.
SYMPTOMS: The symptoms of a latex allergy reaction usually begin within 20 minutes or so after contact with NRL products and may include skin reactions of urticaria (hives), redness, burning, and itching. More severe reactions may involve respiratory symptoms such as runny nose; sneezing; watery, itchy eyes; scratchy throat; and asthma (difficult breathing, coughing spells, and wheezing). Anaphylactic shock rarely occurs as the first sign of latex allergy but could occur with subsequent exposures. Of the 80 cases of anaphylactic shock to NRL products reported in the medical literature up to 1995, 15 persons died.
AIRBORNE LATEX PROTEINS: Of particular concern in latex allergy is the potential for exposure to the NRL protein allergens present in the glove cornstarch powder. These proteins can migrate from the glove into the cornstarch, which causes more protein to be associated with the skin. This cornstarch becomes aerosolized when gloves are removed from boxes and when they are “snapped” during donning or removal. If a latex-sensitive person inhales airborne latex proteins, that person may have a serious respiratory or systemic reaction. Another important aspect of protein-laden cornstarch is that after it is airborne, it can travel extensively throughout the office, clinic, or building and expose many persons to these allergens. Studies of other allergy-causing substances have shown that the higher the overall exposure in a population, the greater the likelihood that more individuals will become sensitized. Unfortunately, the amount of latex exposure needed to produce sensitization or to produce a reaction is not known. However, reductions in exposure are known to decrease sensitization.
PREVENTION OR MANAGEMENT OF LATEX ALLERGIES
ALLERGIES IN THE DENTAL WORKER: As with all other NRL allergies, avoiding contact/exposure with latex protein is necessary to prevent reactions in a sensitive person. In the dental setting this is accomplished by use of nonlatex gloves and other items and establishment of a “latex safe” environment (or at least a reduced presence of latex). If the dental team uses latex gloves, the gloves should be the powder-free, reduced-protein type to eliminate airborne latex protein allergens. The National Institute for Occupational Safety and Health, a division of the Centers for Disease Control and Prevention, has issued recommendations for preventing latex allergies (Box 10-3).
ALLERGIES IN PATIENTS: The first approach to addressing latex allergies in dental patients is to include appropriate questions in the medical history (Box 10-4). Sensitivities to NRL proteins are greater in certain individuals, and this includes persons who have occupational exposure to latex. Persons with spina bifida, urogenital anomalies, and spinal cord injuries are considered at high risk. A history of allergies in the patient or patient’s family is also important to consider. The same is true if a person is allergic to foods, particularly bananas, chestnuts, avocados, or kiwis. Provision of dental care for a latex-allergic patient should be done in an environment with latex as low as reasonably possible (known as ALARP). The following will help the dental team achieve this:
1. Provide treatment in a specially prepared room as first patient of the day.
a. Staff members are not to wear latex while preparing treatment room.
b. They are to handle all items that will contact patient with nonlatex gloves.
c. No one who has worn latex gloves that day should enter the treatment room.
2. Minimize previous contact of patient care items with latex-containing materials.
3. Prevent latex from directly contacting the patient during treatment (use latex alternatives).
4. Eliminate patient exposure to airborne latex protein in glove powder.
5. Have dental team members wear non–latex-containing items that may contact the patient.
HAND HYGIENE
Hands are one of the most important sources of microorganisms in disease spread. Hand hygiene is an important type of personal hygiene for everyone but is a primary disease prevention procedure for health care workers in dentistry and medicine.
Two types of microbial flora are on the hands: resident skin flora and transient skin flora. The resident skin flora consists of microorganisms that colonize the skin and become permanent residents. These microorganisms are always on the skin and can never be removed totally, even with a surgical scrub (likely because the resident flora can exist even several layers under the surface in the stratum corneum), but their numbers can be reduced. Although members of the resident skin flora can cause infection when directly or indirectly spread to others, they are likely less important in disease spread than the second type of skin flora, the transient skin flora. The microorganisms of this flora contaminate the hands during the touching of or other exposure to contaminated surfaces. They usually do not colonize and do not survive on the hands for long periods, thus they are called members of the transient flora (they come and go). The transient flora serves as a source of disease spread because it can contain just about any pathogenic microorganism, depending on how the hands become contaminated. Fortunately, the transient flora can be removed or greatly reduced by routine handwashing, usually because the contaminating microorganisms remain primarily on the outer layers of the skin.
Although handwashing clearly reduces the spread of disease agents, it also reduces the number of microorganisms that may contaminate and subsequently cause a harmful infection of or through the hands. Thus handwashing protects patients and the dental team.
Hand Hygiene Agents
Although plain soap without antimicrobial activity performs well in removing dirt and transient microorganisms from the hands, it has little effect on the resident flora. Health care personnel handwashing products contain low to medium levels of antimicrobial agents. Their frequent use in routine handwashing procedures minimizes the number of transient microorganisms on the hands and aids in reducing the number of resident bacteria by means of their bactericidal chemical activity. Surgical scrub products contain the highest levels of antimicrobial agents and are used in a more vigorous scrubbing procedure when maximum reduction in transient and resident flora is desired, such as before surgical procedures. Nevertheless, the surgical scrub procedure will not sterilize the hands.
When the hands contain no visible soil, alcohol-based hand rubs without water and without rinsing have been shown to be effective in hand antisepsis (Table 10-1). These hand rubs also can be used after surgical scrubbing with plain soap and water.
TABLE 10-1
Antimicrobial Spectrum of Some Hand Hygiene Agents∗
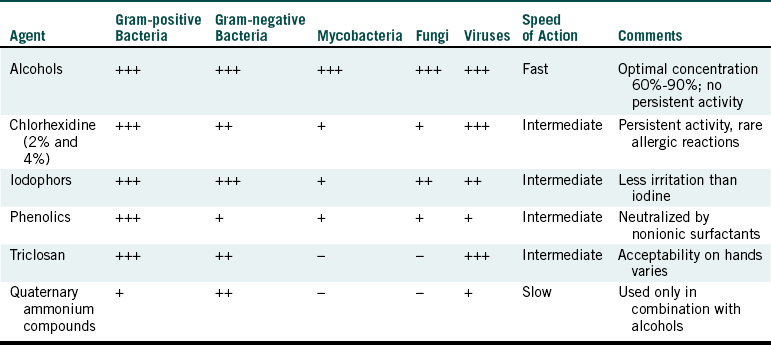
+++, Excellent; ++, good but does not include the entire bacterial spectrum; +, fair; - no activity.
∗Adapted from Centers for Disease Control and Prevention: Guideline for hand hygiene in health-care settings, MMWR 51 (No. RR-16):45, 2002.
Common antimicrobial agents in hand hygiene products include alcohols (ethanol, isopropanol, propanol), chlorhexidine digluconate, iodophor, para-chlorometaxylenol (PCMX or chloroxylenol), triclosan, and quaternary ammonium compounds (see Table 10-1). Chlorhexidine digluconate is well known for its widespread antimicrobial activity and its residual activity (prolonged antimicrobial effect). Chlorhexidine digluconate binds to the skin to give prolonged release of antimicrobial activity. Triclosan also may have a prolonged effect, whereas persistence of para-chlorometaxylenol on skin may be less. Iodophor and quaternary ammonium compounds do not exhibit prolonged activity.
Recently, more emphasis has been placed on the use of alcohol-based hand rubs. In the past these agents were recommended mainly to be most useful when no sinks were available because rinsing the hands after using the hand rubs is not necessary. A person just rubs the hands together until the gel or foam dries. However, several studies have shown that alcohol products can reduce viable bacterial counts on the hands more than plain soap or antimicrobial soap when relatively small amounts of proteinaceous material (e.g., blood or saliva) are present. Thus the hand rubs are given a more prominent role in hand hygiene procedures when no visible soil appears on the hands. Alcohol hand rubs may increase the frequency of hand hygiene because they are quick and easy to use. Alcohols dry the skin, but this effect has been reduced or eliminated by addition of emollients or other skin-conditioning agents such as glycerol in the hand rub preparations.
Hand Hygiene Procedures
The mechanical action of handwashing with a plain or antimicrobial soap is important to suspend dirt and microorganisms from the skin surface so they can be rinsed away with water. When the hands are not visibly soiled, one can use an alcohol-based hand rub for hand antisepsis. Step-by-step procedures for routine hand hygiene and surgical scrubbing are given in Procedure 10-1 and partially illustrated in Figure 10-2.
Aseptic techniques associated with hand hygiene include the use of faucets controlled by foot pedals, elbow handles, “electric eyes,” or ultrasonics. If these devices are not available, then one can use plastic surface barriers to cover the faucet handles. Foot-operated or electric eye soap dispensers also prevent contamination of the hands from multiuse soap containers. Squeeze-bottle soap dispensers facilitate cross-contamination and are not recommended. Bar soaps in soap dishes also tend to accumulate skin and environmental microorganisms and are not recommended for health care facilities. Liquid hand care products should be stored in closed containers in either disposable containers or containers that are washed and dried before refilling. One also should not add soap or lotion to top off a partially empty container. One should wash the container before refilling it.
Box 10-5 lists times when one should perform hand hygiene. The importance of performing hand hygiene before gloving and after removing gloves may not be obvious. When the skin is occluded (tightly covered up) with gloves, members of the resident flora, and to a lesser extent the transient flora, dramatically increase. This increase may be as great as 4000-fold per hour. This increased growth of microorganisms in the warm, moist environment under gloves can cause skin irritation. Washing hands or using an alcohol-based hand rub before gloving reduces the number of microorganisms to begin with, and washing after removing gloves reduces the number of those that have increased, as well as the transient microorganisms that may have contacted the skin through defects in the gloves. Handwashing after removal of gloves also helps remove any powder containing latex protein, other chemicals from the gloves, and sweat. So if you use alcohol hand rubs throughout the day, periodically (e.g., after every 4 to 5 hand rubs) wash and rinse your hands to remove this buildup of debris.
Other Hand Hygiene Considerations
Dental team members can use lotions to prevent skin dryness associated with handwashing. Lotions with a base of petroleum, lanolin, mineral oil, palm oil, or coconut oil have detrimental effects on latex gloves, so these types should be used only at the end of the day. Lotions containing aloe vera, glycerin, vitamin E, or vitamin A are fine to use. One should keep nails short to allow for thorough cleaning and to prevent glove tears. One should not wear artificial nails, which can harbor microbes. One should not wear hand or arm jewelry during surgical procedures or wear hand or nail jewelry during nonsurgical procedures if they make donning gloves more difficult or compromise the appropriate fit and integrity of the gloves.
MASKS
Masks were developed originally to reduce the chances of postoperative infections in patients that were caused by microorganisms in the respiratory tracts of the surgeons. Although some controversy exists as to the ability of masks to accomplish their original purpose, wearing a mask is still standard practice during surgery or at any other time when reducing the spread of potential respiratory disease agents may be important. In recent years a face mask has been viewed also as a means to protect the one who wears the mask from disease agents that might be present in sprays, spatter, or even some aerosol particles of body fluids or other potentially infectious materials. In dentistry, masks mainly protect mucous membranes of the nose and mouth of the dental team from contact with sprays or spatter of oral fluids from the patient or from items contaminated with patient fluids. A much lesser degree of protection to the dental team occurs against inhalation of aerosolized particles of oral fluids that may contain infectious disease agents. Masks worn by the dental team may give some protection to the patients.
Uses and Types
The dental team should wear surgical masks any time a risk exists of spraying or spattering of fluids that may contain potentially infectious disease agents. This may occur during patient care activities involving high-speed or low-speed handpieces, ultrasonic scalers, air/water syringes, or oral irrigators and during grinding or polishing of items that may be contaminated with patient fluids. The mask should be changed with every patient because its outer surface becomes contaminated with droplets from sprays of oral fluids from the previous patient or from touching the mask with saliva-coated fingers. A mask also protects from splashes during instrument processing. Contamination of ultrasonic or other cleaning solutions may occur if the cleaning basket or instruments are accidentally dropped into the solution. Rinsing of instruments under tap water also may cause splashing.
Face masks are composed of synthetic material that serves to filter out at least 95% of small particles that directly contact the mask. In 1995 the National Institute of Occupational Safety (a part of the Public Health Service) indicated that it would certify three classes of filters, N-, R-, and P-series with three levels of filter efficiency—95%, 99%, and 99.97%—in each class. All filter tests are to use particles of aerosol size: 0.3-mm aerodynamic mass median diameter. The filterability of masks is referred to as Bacterial Filtration Efficiency (BFE) or Particle Filtration Efficiency (PFE). The surgical masks commonly used in dentistry are dome shaped or pliable. They may be secured with an elastic band, ear loops, or ties (Figure 10-3). Although these masks protect the mucous membranes of the mouth and nose from direct contact with droplets of oral fluids, they give limited protection against small aerosol particles important in airborne infections such as severe acute respiratory syndrome (SARS). Even though the masks are form fitting over the bridge of the nose to reduce fogging of eyewear, they still “leak” around the edges. Protection against airborne infection requires the use of a respirator (e.g., N-95 respirator) that fits in such a way that forces inhaled air to pass through the filter rather than around the edge of the mask. Respirators are more difficult to breathe through, and their use must be preceded by training and by fit testing to ensure safe use and proper functioning. Because patients with serious airborne disease (e.g., SARS) should be treated in special facilities, respirators are not part of the normal personal protective equipment needed in dental offices.
Limitations
Because surgical masks do not provide a perfect seal around the edges, unfiltered exhaled and inhaled air can pass through these sites. Thus selection of a mask that fits the face well is important to minimize passage of unfiltered air. Also, when a mask becomes wet from moist exhaled air, the resistance to airflow through the mask increases, causing more unfiltered air to pass by the edges of the mask. Thus one should replace wet masks, approximately every 20 minutes, to maintain high filterability.
PROTECTIVE EYEWEAR
A variety of disease agents may cause harmful infection of the eyes or enter the associated mucous membranes and cause systemic infections. An example is the herpes simplex virus, which may be present in sprays, spatter, or aerosols of oral fluids from a patient. Another example is the hepatitis B virus, which may use the eye as a portal of entry into the body and cause hepatitis B.
Besides protecting against infectious disease agents, eyewear also protects against physical damage to the eyes by propelled objects such as tooth fragments or small pieces of a restorative material exiting a patient’s mouth during cavity preparation. Impact damage to the eyes can occur from any polishing, grinding, or buffing procedure, whether performed in a patient’s mouth, at chairside, or in the dental laboratory. Eyewear also can protect against eye damage from ultraviolet irradiation and from splashes of chemicals used at chairside or for cleaning instruments and surfaces, disinfecting surfaces, developing radiographs, or working in the dental laboratory.
The dental team should offer patients eye protection during treatment. Reports of eye damage to patients include impalement of a patient’s eye by an excavator, corneal abrasion from an exploding anesthetic carpule and from a piece of acrylic denture tooth, and subconjunctival hemorrhage after a dentist hit a patient’s eye with his thumb. Instruments and chemicals should not be passed over the head of the patient. If a patient wears prescription eyeglasses, the patient should be allowed to continue to wear them during care; other patients should be provided with eye protection. Disposable eyewear should be provided, or patient eyewear can be decontaminated between uses.
Uses and Types
One should wear protective eyewear whenever contamination of the eyes with aerosols, sprays, or splashes of body fluids or chemicals is possible and whenever projectiles may be generated during any grinding, polishing, or buffing procedure with rotary instruments or equipment. Protective eyewear worn by the dental team should be decontaminated thoroughly before reuse with subsequent patients. Appropriate eye protection also should be used with ultraviolet irradiation. Table 10-2 compares different types of eye protection in relation to the American National Standard for Occupational and Educational Eye and Face Protection, Z87.1-1989. The design, construction, testing, and use of eye and face protection devices should be in accordance with this standard developed by the American National Standards Institute.
TABLE 10-2
Comparison of Eye Protection Devices
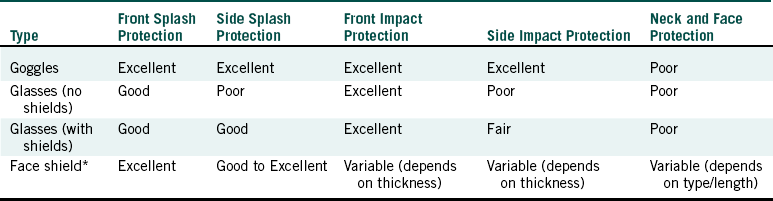
∗Should include side and top protection.
Adapted from American National Standards Institute: Occupational and educational eye and face protection: Z87.1-1989, New York, 1989, American National Standards Institute; and Palenik CJ, Miller CH: Protecting your eyes: it’s the law, Trends Tech Contemp Dent Lab 8:69-74, 1991.
Limitations
Goggles that may be used by themselves or over prescription glasses (Figure 10-4) may not be attractive, but they give the greatest eye protection against front and side splashes and impacts. Although glasses give protection against front splashes and impacts, side protection is poor unless they have shields (Figure 10-5). The degree of impact protection from projectiles depends on the strength of the lenses as determined by the American National Standards Institute standard. Some protective eyeglasses have replaceable lenses (if scratching occurs), have antifogging properties, and are autoclavable. The Occupational Safety and Health Administration blood-borne pathogens standard (see Chapter 8 and Appendix H) indicates that appropriate protective eyewear, in relation to splashes or sprays of body fluids, provides protection to the front and the sides of the eyes. So eyewear should have side shields (Figure 10-6). Masks with attached eye protection are also available (Figure 10-7).
If one uses face shields, the shields should be chin length, provide top protection, and be curved to provide side protection. Some face shields are made of thin plastic and may not offer adequate protection against particles with a high-impact velocity. One also should wear masks with face shields to reduce inhalation of fluid aerosols and dust particles.
PROTECTIVE CLOTHING
Potentially infectious microorganisms may be present in the aerosols, sprays, spatter, and droplets from the oral fluids of patients. These microorganisms not only contaminate unprotected eyes and mucous membranes of the mouth and nose but also contaminate other body sites of the dental team, including the forearms and chest area. Larger droplets also may settle on the lap while one is seated at chairside. Outer protective clothing can protect against this contamination, which otherwise may lead to infection through nonintact skin or at least to spread of the contamination from office to home or elsewhere on unprotected clothing. Changing of obviously contaminated protective clothing before providing care for the next patient is perceived as providing patient protection. Covering up microorganisms present on street clothes with protective clothing also is perceived to provide some degree of patient protection. This action prevents shedding of microorganisms from street clothes into the air near a patient who may have open tissue. Although it seems reasonable to use protective clothing for dental team and patient protection, little evidence is available on the extent to which this prevents disease spread.
Uses and Types
Dental team members should wear protective clothing whenever a chance exists for contamination of skin or other clothing with spray or splashes of saliva, blood, or other potentially infectious materials. Thus the same conditions at chairside that require use of masks and protective eyewear also require use of protective clothing. If this clothing becomes visibly soiled, the person should change clothing before caring for the next patient and should put on fresh protective clothing before surgery. One should remove protective clothing when leaving clinical areas and should not wear such clothing in lunch rooms, restrooms, or outside the office. The Occupational Safety and Health Administration blood-borne pathogens standard also indicates that employees cannot take home contaminated clothing and linens for laundering. Laundering is the responsibility of the employer through laundering in the office or by contracting with a commercial laundering service. Although not required by any law, dental team members also should consider having work shoes to wear only in the office.
Protective clothing is the outer layer of clothing that protects/covers underlying work clothes, street clothes, undergarments, or skin. Examples include uniforms, clinic jackets, lab coats, aprons, and gowns. This clothing should protect against contamination of underlying clothes or skin, and materials with the greatest resistance to fluids provide the greatest protection. Few, if any, chairside dental procedures require fluid-proof clothing. Head covers and shoe covers also are not mandated for use in dentistry, but head covers may be appropriate to give maximum patient protection during surgery.
A convenient approach to office management of protective clothing involves use of disposable gowns with long sleeves and a high neck to cover regular work clothes (Figure 10-8). For routine dental procedures, one may change these clothes at least once a day (e.g., over the lunch hour) or more frequently if they become visibly soiled. Another approach is use of reusable protective clothing such as uniforms, lab coats, or other attire that may be put on at the beginning of the day, but it must be changed for lunch, changed when it becomes visibly soiled, and removed before one leaves the office. Use of protective clothing that is pulled on and removed over the head is not wise because removal may contaminate the face and head with the outside of the clothing.
PLACING AND REMOVING BARRIERS
Chapter 17 presents all of the steps involved in chairside asepsis. However, procedures for putting on and taking off barriers are discussed further in this section. Putting on and taking off barriers should be done in a sequence that limits further spread of microorganisms. This is true when preparing for routine and surgical procedures. For this discussion, it is assumed that the operatory has been cleaned and disinfected and that surface covers have been placed, sterilized instrument packages or cassettes have been placed on the bracket table or cart, the patient has been seated, and the history and any discussions have been completed. The important point to remember about the sequence of putting on protective barriers is to put on gloves last to avoid contaminating the gloves before they are used in the patient’s mouth.
The first step is to put on protective clothing(Box 10-6) and place the patient’s bib. One then unpackages the instruments and supply items without directly touching them and next puts on mask and eyewear. Just before performing treatment, one washes, rinses, and dries the hands and puts on gloves. One should not touch any contaminated items or surfaces with the gloves before they go into the patient’s mouth.
After patient treatment, one considers two key issues when removing the now-contaminated protective barriers:
• The gloves can contaminate anything they touch.
• The hands may become contaminated if one touches certain parts of the protective barriers with ungloved hands.
One approach to removing contaminated barriers is given in Box 10-6 and illustrated in Figure 10-9. If one is going to remove contaminated protective clothing, this step is first. To remove a disposable gown, one pulls it off over gloved hands, turning it inside out and immediately placing it into a waste receptacle. When removing disposable or reusable protective clothing, one should not touch underlying clothes or skin with the contaminated gloves (see Figure 10-9, A). Next, one removes gloves and washes the hands. When removing the gloves, one should not touch the skin but rather pinch the gloves in the wrist area on one hand, stretch the glove out away from the wrist, and pull the glove off (see Figure 10-9, B). One then places the ungloved thumb under the edge of the other glove, stretches the glove out away from the hand, slides it completely off, and places it into the waste receptacle (see Figure 10-9, C). One removes eyeglasses by touching them only on the ear rests (which usually are not contaminated) and placing them in an appropriate area for subsequent decontamination. One removes the mask by touching only the elastic bands around the head or ears or only the ties in back of the head (see Figure 10-9, D). One immediately should discard the mask into the waste receptacle. One then washes, rinses, and dries the hands. These barriers need not be managed as regulated waste unless they are for some unusual reason dripping wet with blood or saliva. One can just place them in the regular waste receptacle.
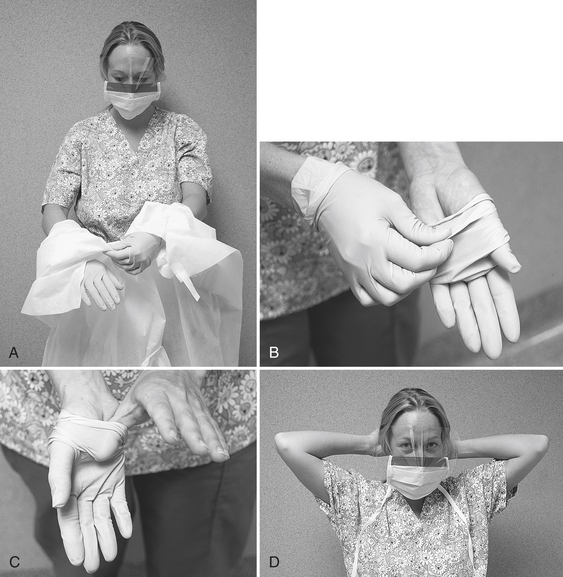
FIGURE 10-9 Removing personal protective equipment (also see Box 10-6). A, Remove gown by reaching back and pulling it off over the gloves, turning it inside out. B, Remove the first glove by pinching it at the wrist and pulling it off toward the fingers. C, Remove the other glove by slipping the thumb under it and pulling it off toward the fingers. D, Remove eyewear by grasping the ear rests, and remove the mask by touching only the elastic bands or ties.
PROPERTIES OF HAND HYGIENE AGENTS AND BARRIERS
Boxes 10-7 and 10-8 give properties to consider when selecting hand hygiene products, gloves, masks, eyeglasses, and gowns.
American Dental Association. Councils on Scientific Affairs and Dental Practice, Instruments and Equipment, Dental Therapeutics, Dental Research and Dental Practice: Infection control recommendations for the dental office and the dental laboratory. J Am Dent Assoc. 1996;127:672–680.
Balazy, A., et al. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Cont. 2006;34:51–57.
Belkin, N.L. Gowns and drapes for the level of exposure anticipated. Bull Am Coll Surg. 2002;87:20–22.
Centers for Disease Control and Prevention. Guideline for hand hygiene in health-care settings. MMWR. 2002;51(No. RR-16):1–45.
Hamann, C.P., DePaola, L.G., Rodgers, P.A. Occupation-related allergies in dentistry. JADA. 2005;136:500510.
Larson, E.L. Effective hand degerming in the presence of blood. J Emerg Med. 1992;10:7–11.
Larson, E., Kumudini, M., Laughton, B.A. Influence of two handwashing frequencies on reduction in colonizing flora with three handwashing products used by health care personnel. Am J Infect Control. 1989;17:83–89.
Manzella, J.P., et al. An outbreak of herpes simplex virus type I gingivostomatitis in a dental hygiene practice. JAMA. 1989;252:2019–2222.
Miller, C.H. Hand hygiene above, under, and beyond the surface. Dent Prods Rpt. 2007;39:134.
Miller, C.H. Select and protect. Dent Prod Rpt. 2007;41:156–160.
Review Questions
______1. Wearing gloves at chairside helps prevent which of the following routes of entry of patient’s microbes into the body?
______2. The first handwashing of the day involves the use of:
______3. When should an alcohol-based hand rub be used?
b. only following handwashing with another antimicrobial agent
______4. Heavy utility gloves should be used:
a. for all intraoral procedures
b. to rebag a dental unit with fresh surface covers
______5. Masks should be used:
______6. A mask should be replaced:
______7. The last of the personal protective barriers to be put on before patient treatment begins are:
______8. Which bacteria on the hands are the most important in spreading disease?
______9. The most common type of skin reaction to gloves is:
______10. When leaving chairside to get a new package of sterile instruments from the sterilizing room, what barrier should be removed and then replaced on returning to chairside?
______11. Which microorganisms are removed by routine handwashing?
______12. Wet masks should be changed because they:
______13. The mechanism of allergic contact dermatitis is the same as: