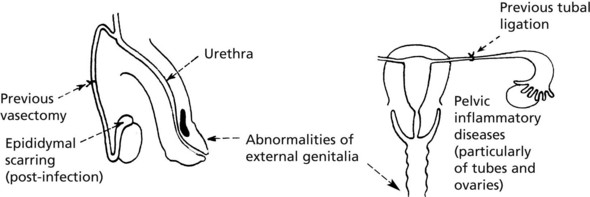
Chapter 12 Genitourinary System
Kidney – Structure and Function
The kidney has several functions:
Many substances will show altered values in renal failure, but the following are always elevated and are commonly used in estimating the progress of the disease.
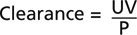 where:
where:
In practice renal function is now commonly assessed by estimated glomerular filtration rate (eGFR), which combines serum creatinine with the age and sex of patient.
Chronic Kidney Disease can be classified as follows:
| CKD | Stage | eGFR/mL/min/1.73m2 |
|---|---|---|
| 1 | with an abnormality* | >90 |
| 2 | with an abnormality* | 60–89 |
| 3 | 30–59 | |
| 4 | 15–29 | |
| 5 | <15 |
The structure and function and diseases will be discussed separately, but diseases of one may well affect others.
Glomerular Structure and Function
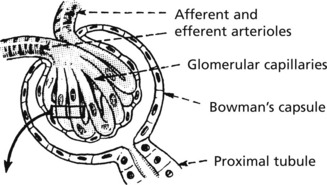
The glomerulus, of which there are over 600000 in the adult, consists of an invagination of a capillary network, derived from the afferent arteriole, into Bowman’s capsule – the beginning of the proximal tubule.
The glomerulus is an efficient filter due to the large surface area of the glomerular capillaries.
Ultrastructure
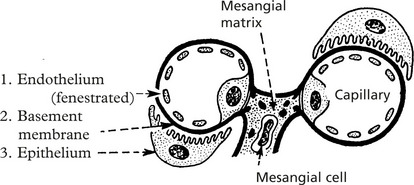
The barrier separating blood from the lumen of the matrix nephron consists of 3 layers:
Mesangial cells have three functions:
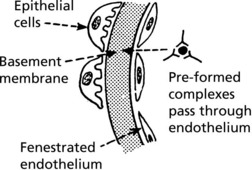
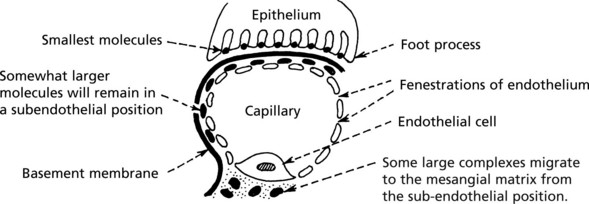
These layers form a complicated sieve controlling glomerular permeability. Wide pores (70–100 nm) in the endothelium allow all components to reach the basement membrane.
The basement membrane produced by the endothelium and epithelium has a strong anionic (negative) charge which repels major plasma proteins (also anionic). It has a central dense layer (lamina densa).
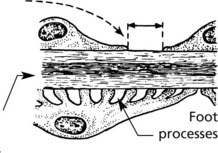
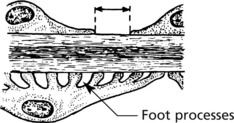
The epithelial cells are attached to the basement membrane by foot processes, separated by ‘slit pores’ 30–60 nm in diameter.
Filtration
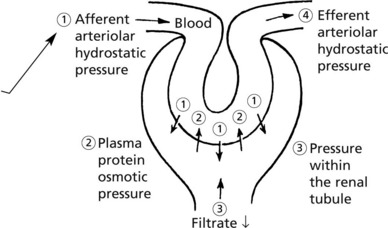
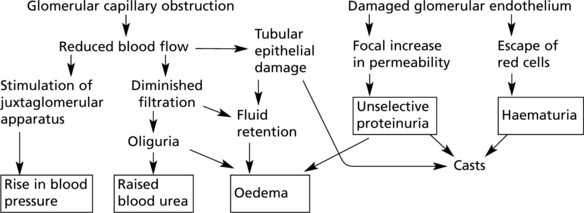
The glomerular filtrate is virtually protein free plasma. Its rate of production is dependent upon:
Variation in any of these factors affects the output of urine. Diminished urinary output results from a reduction in renal blood flow as in shock, from an increase in osmotic pressure as in haemoconcentration, or from obstruction to the outflow of urine.
Glomerular Diseases
The mechanisms underlying these changes are as follows:
These lead to 4 main clinical syndromes:
The main forms of glomerular disease are described in the following pages.
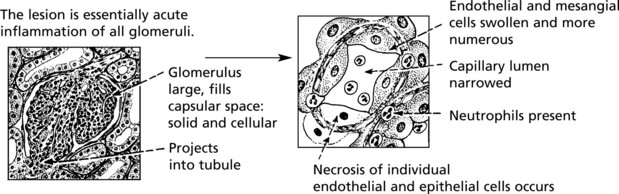
Acute Diffuse Proliferative Glomerulonephritis
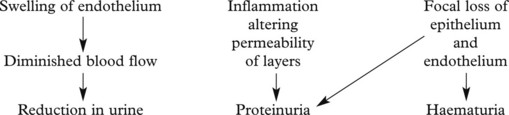
This disease classically follows 2–3 weeks after an infection – usually pharyngitis due to Group A haemolytic streptococci. It is commonest in children and young adults who develop the NEPHRITIC SYNDROME: oliguria, proteinuria, haematuria (urine is smoky and dark), moderate hypertension and facial (periorbital) oedema. This disease is now uncommon in fully developed countries.
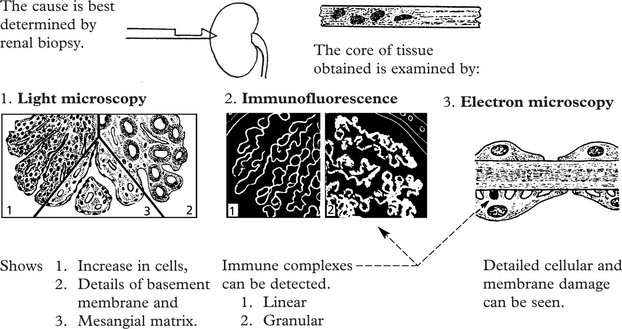
Immune complexes are identified by:
The clinical findings can be correlated with pathology as follows:
Prognosis – The disease usually resolves in 1–2 weeks, particularly in children, but in adults complications are more common.
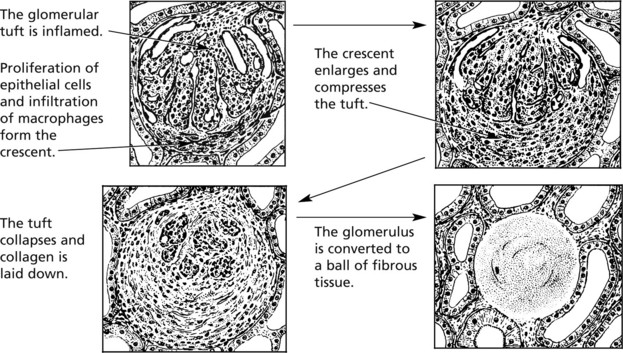
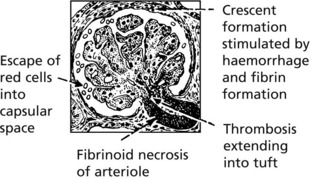
Crescentic (Rapidly Progressive) Glomerulonephritis
Without treatment, this disease progresses to end-stage renal failure in weeks or months. The histological hallmark is crescent formation in >50% of glomeruli.
Many types of glomerulonephritis can progress to crescentic GN. Examples include:
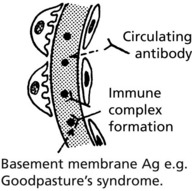
In this serious disorder there is both renal and often pulmonary damage. Immunofluorescence shows the cause – an antibody to type IV collagen in the glomerular basement membrane (anti-GBM) which also damages pulmonary alveolar membranes.
IF shows linear deposits of IgG and C3 in the capillary basement membranes
Prognosis – Without treatment, most patients die within 6 months.
Immunosuppressive drugs (e.g. steroids, cyclophosphamide) and plasma exchange to (remove anti-GBM antibodies) improve the prognosis but many patients require dialysis or transplantation. Hypertension is a further serious complication.
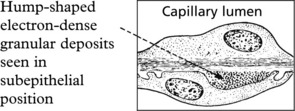
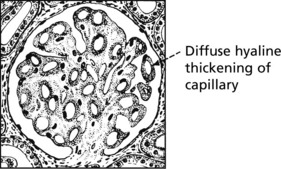
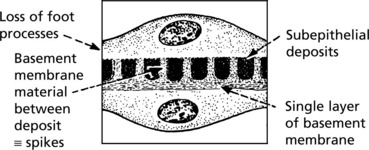
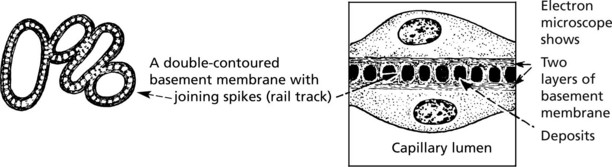
Membranous Glomerulonephritis
This accounts for around 30% of cases of the nephrotic syndrome in adults; some patients present with asymptomatic proteinuria.
Aetiology
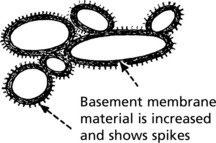
Pathology – There is generalised thickening of capillary basement membrane.
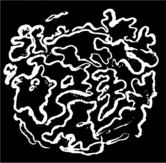
Specific silver staining of the basement membrane shows a typical pattern.
Immunofluorescence reveals deposition of IgG in the capillary walls.
Electron microscope findings explain this appearance.
In the later stages there is a massive increase in basement membrane material.
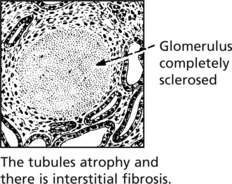
Tubular changes – In the early stages, protein droplets and lipid globules appear in the tubular epithelium. If the disease progresses there is atrophy of the tubules and interstitial fibrosis.
Prognosis – In about 25% of patients the disease remits spontaneously, the remainder continuing to have proteinuria and in 40% chronic renal failure eventually supervenes.
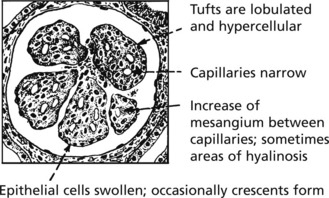
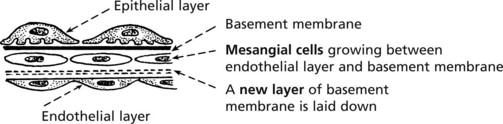
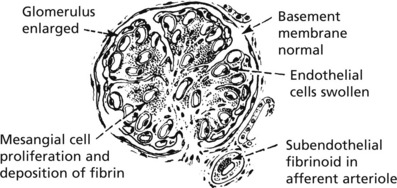
Mesangiocapillary (Membranoproliferative) Glomerulonephritis
In this form of glomerulonephritis there is an increase both in cells and mesangial matrix within glomeruli.
Silver staining of basement membrane
Duplication of basement membrane as in membranous glomerulonephritis, but without spikes.
These changes are due to ‘mesangial interposition’
Using electron microscopy and immunofluorescence, 2 types are identified.
Aetiology
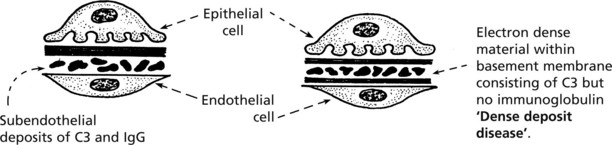
Complement activation is a feature of both forms. In Type 1 this is due to immune complexes by the classical pathway (p.99). In Type 2 there is activation by the alternative pathway by an autoantibody which stabilises C3 converting enzyme. Serum C3 is low in both forms.
Clinical Features
Children and young adults are usually affected. They present with the nephrotic syndrome (50%), the nephritic syndrome or asymptomatic haematuria or proteinuria.
In half of patients there is progression to renal failure (with hypertension) within a decade. The disease often recurs in the subsequently transplanted kidney.
Focal Glomerulonephritis
In contrast to the glomerular diseases discussed already, focal glomerulonephritis affects only a proportion of glomeruli (focal) and only part of those glomeruli (segmental).
In some cases, part of the tuft becomes necrotic and there is related inflammation (focal segmental necrotising glomerulonephritis). Crescents are sometimes seen. This pattern is seen in:
The pattern is associated with haematuria or nephrotic syndrome. Segmental lesions heal by fibrosis.
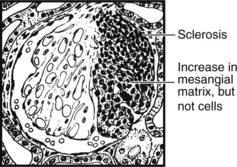
Focal Segmental Glomerulosclerosis (FSGS)
In this pattern of disease, a segment of glomerulus undergoes sclerosis without inflammation, but with an increase in mesangial matrix.
Originally regarded as a variant of minimal change nephropathy, primary FSGS presents as with haematoma or nephrotic syndrome with progression to chronic renal failure. It may recur within a transplanted kidney.
Secondary FSGS may complicate a variety of preexisting conditions including those reflux nephropathy and intravenous drug use.
IgA Nephropathy
This is the commonest form of glomerulonephritis worldwide. It can present with microscopic or macroscopic haematuria or the nephrotic syndrome and may lead to chronic renal failure. It typically affects young males, who often suffer recurrent episodes after upper respiratory infections although all ages can be affected.
The serum IgA level is raised and glomerular damage is due to IgA immune complexes. Sometimes crescentic glomerulonephritis is seen.
Mechanism
IgA immune complexes in blood → Glomeruli → Often focal deposition → Activation of complement (C3) → Often focal damage
Shows deposits of IgA and C3 within the mesangium: the capillary loops are not usually affected.
Note: The focal deposition is dependent on the molecular size of the complex and failure of mesangial clearance.
Henoch–Schönlein purpura has similar renal changes but also skin rash and gastrointestinal symptoms.
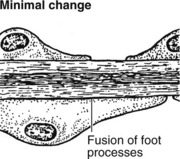
Minimal Change Glomerulonephritis
This disorder affects any age but is the major cause of nephrotic syndrome in children. It typically remits spontaneously and responds to a short course of steroids. Recurrences are quite common.
The glomeruli are histologically normal, but electron microscopy reveals fusion of podocyte foot processes. This is a finding in many causes of proteinuria. No immune complexes are found on immunofluorescence or electron microscopy.
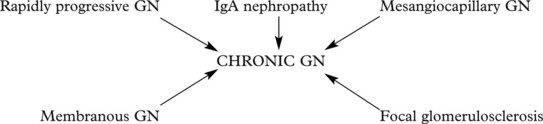
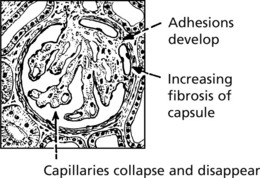
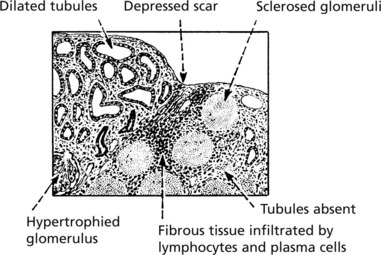
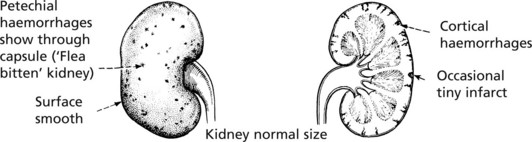
Chronic Glomerulonephritis (GN)
This is the end stage of many forms of glomerulonephritis, but most patients present at this stage without a history of previous renal disease.
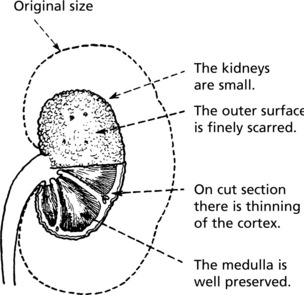
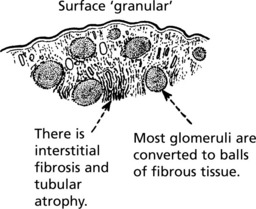
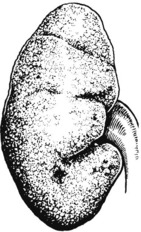
Pathology The kidneys are both small – granular contracted kidney.
In chronic glomerulonephritis a vicious cycle is set up.
Patients may first present with chronic renal failure, or this may develop after years of glomerulonephritis.
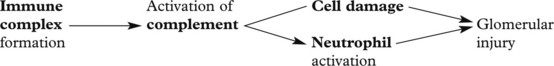
Glomerulonephritis – Disease Mechanisms
The mechanism in most forms of GN is:
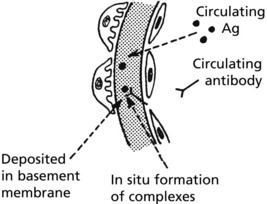
The glomerulus is central to immune complex deposition and formation due to its fenestrated endothelium and high intraluminal pressure.
Immune complexes can occur in glomeruli as follows:
Localisation of Complexes
This depends on the size of the complexes, their shape and electrical charge, and their ability to penetrate the basement membrane.
Other factors modifying the pathological changes are:
Note: In addition to its damaging consequences, complement activation does solubilise complexes allowing their disposal. Thus complement deficiency may predispose to immune complex disease, e.g. in systemic lupus erythematosus (SLE).
Glomerular Disease in Systemic Disorders
Systemic Lupus Erythematosus (SLE)
At least 50% of patients with SLE have clinical evidence of renal involvement, and almost all will have abnormalities on renal biopsy.
The renal changes form a spectrum.
On immunofluorescence, IgG, C3 are almost always present and IgA, IgM, Clq and C4 are often also seen.
Amyloidosis
The general features of amyloidosis have already been described (p.24). Amyloid is deposited around the capillary basement membranes of the glomeruli and in the renal vessels and interstitium.
Gross proteinuria leading to a nephrotic syndrome is common. Interstitial fibrosis results from tubular degeneration and ischaemia due to glomerular and arteriolar lesions. Chronic renal failure results.
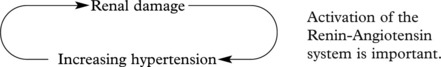
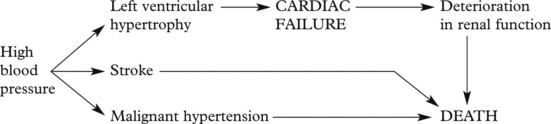
The Kidney and Hypertension
There are two aspects to the relationship of the kidney and high blood pressure.
A vicious circle can be set up:
The renal consequences of benign and malignant hypertension differ, but in each, vascular changes are important.
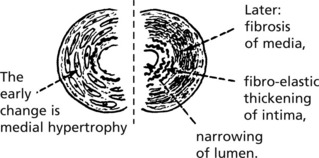
Benign Hypertension
These arteriolar changes lead to glomerular ischaemia.
These changes are patchy in distribution so that renal failure rarely occurs.
It is now thought that small emboli from atheroma of the aorta are responsible for much of the scarring in benign hypertension.
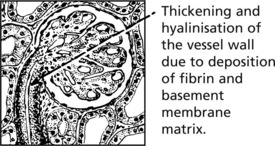
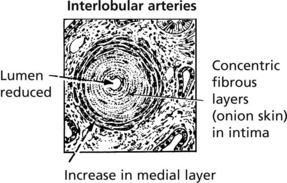
Malignant Hypertension
In this form of hypertension which may arise de novo or on a background of benign hypertension. There is often an underlying cause for the hypertension the blood pressure rises very rapidly and damages renal arteries, arterioles and glomeruli. Renal failure is common unless the disease is treated.
Afferent arterioles: the hallmark is fibrinoid necrosis.
Fibrinoid necrosis heals with the production of an ‘onion-skin’ lesion.
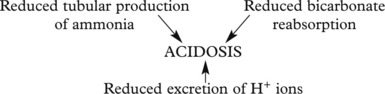
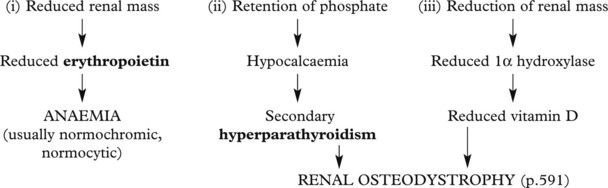
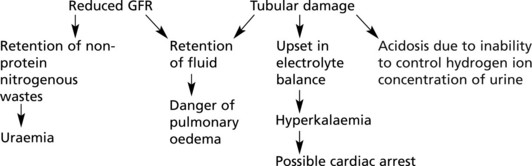
Chronic Renal Failure
Progressive renal damage in many kidney diseases eventually leads to chronic renal failure. The major causes include:
In many cases the underlying cause cannot be determined.
The severity of renal failure is monitored by the serum urea, creatinine and by the glomerular filtration rate (GFR).
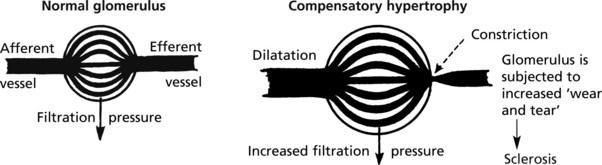
As nephrons are lost, the surviving nephrons show (1) compensatory hypertrophy and are (2) continually active with no ‘down time’. (In the normal kidney, the nephrons do not all function simultaneously.)
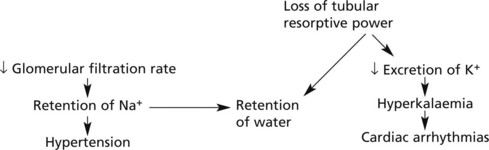
Effects of chronic renal failure:
The kidney also fails to excrete nitrogenous waste products, which accumulate. Although urea concentrations rise and are used to monitor renal function, retention of urea is not in itself harmful.
In uraemia, patients are lethargic. Anorexia, nausea and vomiting are common, with confusion, convulsions and death.
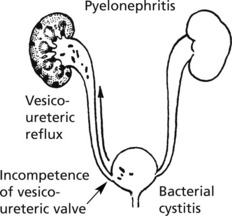
Infections of the Kidney and Urinary Tract
The kidney can become infected through 2 main routes.
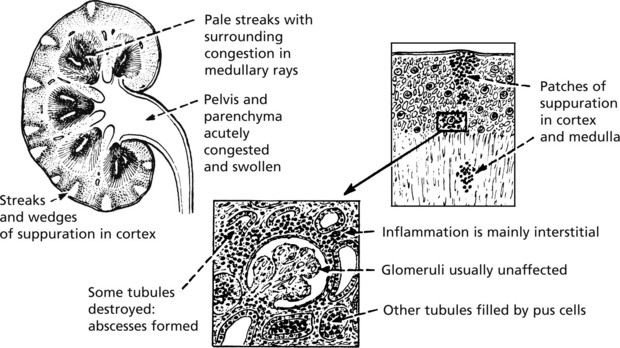
Acute Pyelonephritis
Causes
In women, cystitis is common, particularly in the sexually active. Ascending infection is often provoked by vesico-ureteric reflux during micturition. Pyelonephritis is common in pregnancy.
In men, urinary tract obstruction is usually found, typically due to prostatic enlargement.
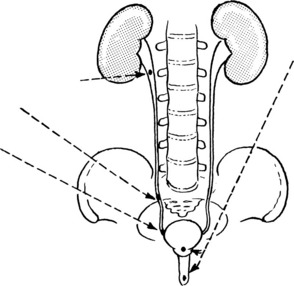
A number of causes of obstruction are seen in both sexes e.g. calculi or tumours in renal pelvis, tumours pressing on ureter, calculi in ureters and tumours of bladder.
Influence of Urinary Tract Obstruction
Obstruction of the urinary tract acts in 3 ways to promote infection:
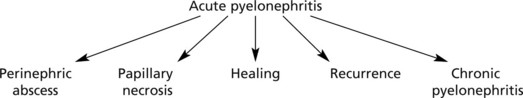
Progress The possibilities are as follows:
Perinephric abscess and papillary necrosis are now rare due to specific antibiotic therapy.
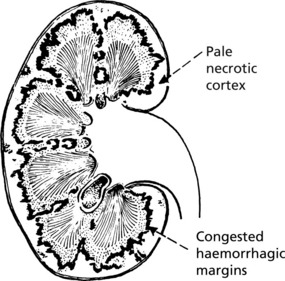
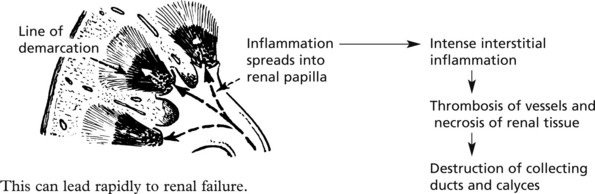
Papillary necrosis (maximal at the kidney poles) is most likely to occur in cases associated with urinary obstruction, diabetes, analgesic nephropathy (e.g. aspirin), sickle cell anaemia and severe hypotension.
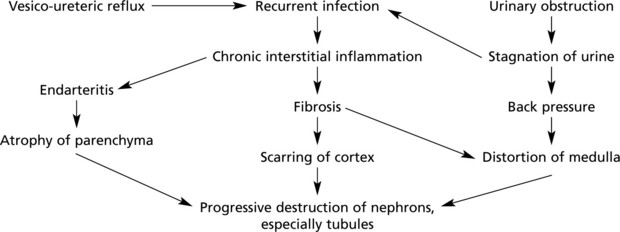
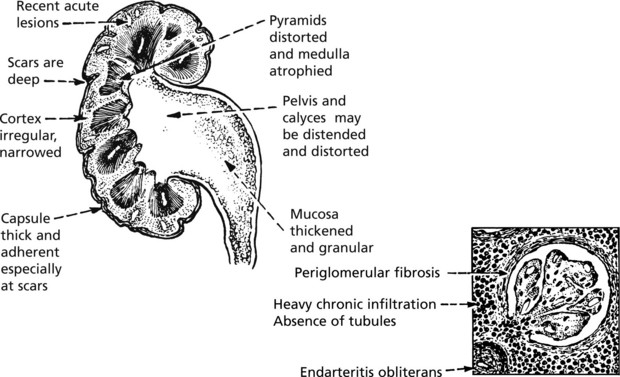
Chronic Pyelonephritis
Chronic pyelonephritis is essentially the result of repeated attacks of inflammation and healing. Vesico-ureteric reflux in early life, often associated with congenital anomalies of the urinary tract, is now regarded as important. The process can be visualised as follows:
Kidney function may be further diminished by the onset of hypertension. In cases with urinary obstruction, the external size of the kidney may remain normal or even be increased. Those with no underlying abnormality, commoner in the female, show progressive contraction of the kidney which becomes greyish white.
Microscopically, there is interstitial fibrosis, inflammation and loss of parenchyma.
Depending on the cause, only one or both kidneys may be affected to variable degrees (c.f. chronic glomerulonephritis).
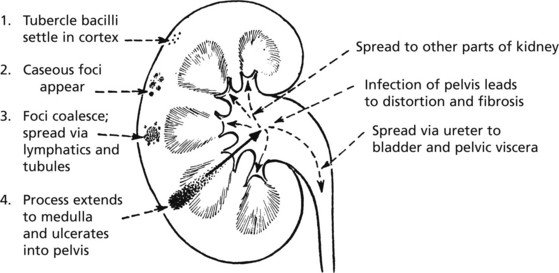
Tuberculosis of the Kidney
Uncommon in many Western countries. It is due to blood spread of infection from another site, e.g. the lungs. Even less commonly, there may be an ascending infection from some other part of the genitourinary system, e.g. epididymis.
As usual, the tuberculous process develops slowly and lesions in the lungs which are the source of infection may have healed and disappeared by the time kidney damage is clinically apparent. The disease is commonly unilateral.
Intercurrent Renal Conditions
Diabetes
Renal complications are common in diabetes. Up to 30% of diabetics develop proteinuria, usually after many years and may go on to chronic renal failure.
The renal lesion is a special form of the ‘small vessel disease’ seen systemically in insulin dependent diabetes, e.g. diabetic retinopathy. Rarely similar changes can be seen in non diabetics.
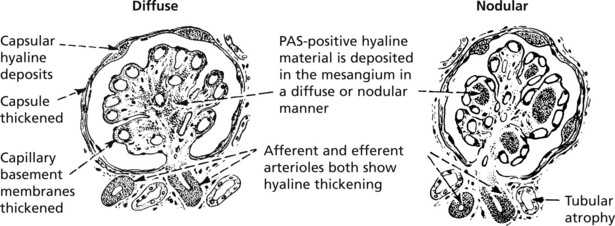
Diabetic glomerulosclerosis. This takes one of two forms:
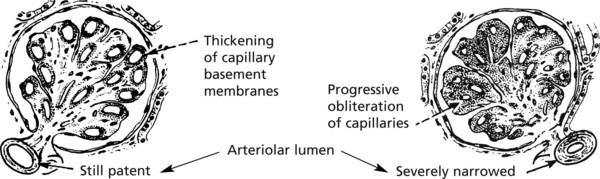
The basement membrane thickening is due to deposition of abnormally glycosylated proteins. The nodular form is often termed the Kimmelstiel–Wilson lesion. Progressive closure of capillaries can occur together with fibrous obliteration of the capsular space and the whole glomerulus. Haemodynamic changes, especially hyperfiltration, lead to sclerosis. Secondary tubular atrophy follows.
Clinical Effects
Screening for microalbuminuria is important to detect early disease. Proteinuria may result in the nephrotic syndrome. Hypertension leads to further renal damage: control of blood pressure, and of diabetes itself, is important to delay the onset of renal failure. Inhibitors of angiotensin activity are particularly effective.
Renal Function and Pregnancy
Four conditions affecting renal function may arise in pregnancy.
EM examination shows mesangial deposits of fibrin, fibrinogen, IgM and complement.
The lesion appears to resolve rapidly after birth.
Acute tubular necrosis, is associated with complications of pregnancy causing shock, e.g. septic abortion, retroplacental haemorrhage and postpartum haemorrhage.
In more severe cases bilateral cortical necrosis may occur especially if disseminated intravascular coagulation (DIC) is superadded.
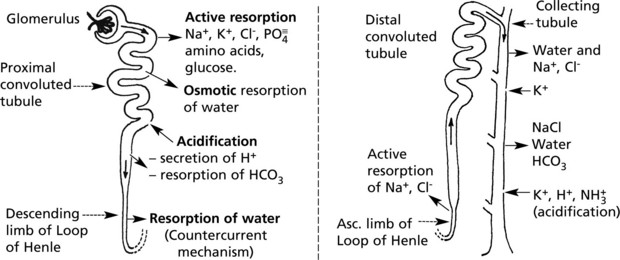
Renal Tubule – Structure and Function
The renal tubules modify the glomerular filtrate. Initially isotonic and neutral, it becomes hypertonic and quite strongly acid. Within 24 hours, 180 litres of filtrate are reduced to 1.5 litres of urine. The main functions of this process are:
Three mechanisms are involved:
Note: Active reabsorption requires energy, and there is a limit to the capacity of the process – maximal tubular capacity (Tm). When this is exceeded, the particular substance involved will appear in the urine. Glucose is an example. In diabetes, the amount of glucose in the filtrate far exceeds the absorptive capacity, and glycosuria results.
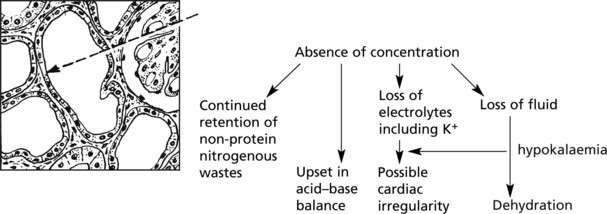
Effects of Tubular Damage
Damage to the tubules results in gross biochemical changes.
Glomerular lesions and pathological changes in the renal pelvis also upset tubular function by interfering with blood supply.
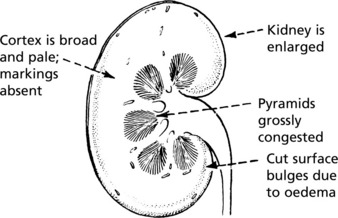
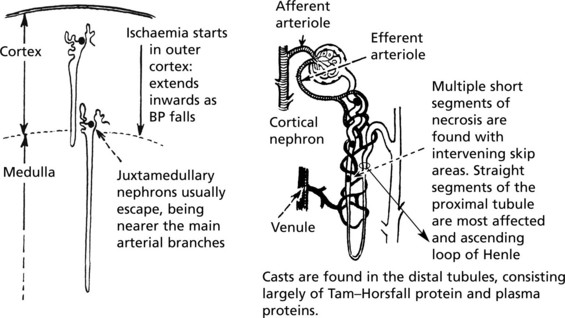
Acute Kidney Injury (AKI) (Acute Tubular Necrosis)
This arises in 2 circumstances:
The gross appearance of the kidneys is the same in both groups.
Acute Kidney Injury (Acute Tubular Necrosis)
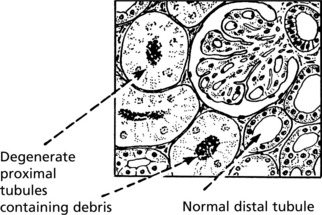
Toxic AKI
The lesions are evenly distributed, affecting all nephrons. They are maximal in the proximal tubules and are the direct result of the toxin, the action of which is intensified by the concentrating activity of the tubule.
Clinical Effects
There are two clinical phases:
The glomerular filtration rate is greatly reduced due to reduced renal blood flow. Unselective reabsorption of the filtrate occurs through the damaged tubule. The effects are:
This occurs following healing of the lesions. The damaged tubular epithelium is replaced by a simple type which has not yet developed selective activities. Large volumes of dilute urine are passed.
The tubular epithelium has a great capacity for regeneration and ultimately regains its selective powers and the prognosis is good.
Tubulo-Interstitial Diseases
In this group of disorders there is damage to the renal tubules and to the interstitial tissues. The main forms are:
Tubulo-Interstitial Nephritis
There are two types – acute and chronic, both typically associated with an immunological reaction to drugs.
ACUTE: Symptoms develop 10–14 days after exposure to drugs e.g. methicillin and NSAIDs e.g. mefenamic acid. Patients are febrile, there is haematuria, proteinuria. Arthralgia is common. Renal impairment varies in severity – may cause ACUTE RENAL FAILURE.
Prognosis
CHRONIC: Long-standing interstitial nephritis leads to interstitial fibrosis, inflammation and continuing tubular damage with tubular loss and atrophy. Many patients present with chronic renal failure. ‘Balkan nephropathy’ found in the river Danube valley is due to ingestion of aristolochic acid, found in herbal remedies.
Analgesic Nephropathy
This is a distinctive disorder caused by long term exposure to analgesics e.g. phenacetin (historically) and NSAIDs. Interstitial inflammation and tubular damage may proceed rapidly to papillary necrosis (p.463).
There is an associated increased risk of transitional cell carcinoma of the kidney and ureter (p.481).
Both minimal change and membranous glomerulonephritis may complicate NSAID therapy.
Metabolic Tubular Lesions
These are due to metabolic defects.
The following are some examples:
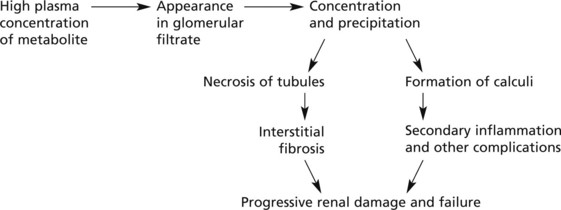
Calcium is deposited in tubular epithelial cells with subsequent fibrosis and calcification – nephrocalcinosis.
The usual causes of hypercalcaemia are malignant tumours, primary hyperparathyroidism, hypervitaminosis D and sarcoidosis.
Increased primary secretion of uric acid leads to uric acid crystal formation in the acid environment of the distal tubule. Renal uric acid stones can occur.
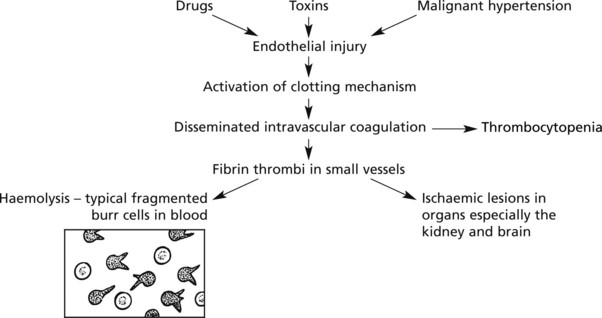
Thrombotic Microangiopathies (Haemolytic Uraemic Syndrome)
This is a rare complication of many conditions.
The renal lesions are similar to those seen in malignant hypertension. There is fibrinoid necrosis of the afferent arterioles and capillaries of the glomeruli, resulting in tubular necrosis. In the most extreme cases, bilateral cortical necrosis of the kidneys may occur. Clinical manifestations include renal failure, haemolytic anaemia, hypertension, and sometimes purpura with thrombocytopenia.
Pathological Complications of Renal Replacement Therapies
The prognosis of end-stage renal failure has been greatly improved by (1) Dialysis and (2) Renal Transplantation. The following possible pathological complications are important:
Amyloidosis largely affecting osteoarticular tissues (e.g. carpal tunnel syndrome: joint stiffness bone cysts) due to raised circulating β2 microglobulin. Visceral involvement is rare and late.
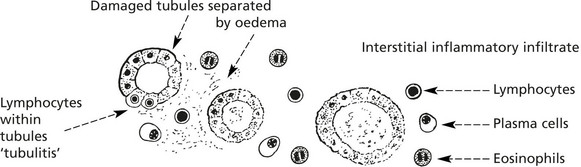
Rejection is the main complication, depending on the extent of HLA matching between donor and recipient. The Banff classification is used to type rejection of which there are four forms:
Pre-existing antibodies against donor HLA or ABO → Binding to endothelial cells → Thrombosis and necrosis
Cell mediated response against donor cells → Interstitial lymphocytic infiltrate and ‘tubulitis’ → Tubular destruction
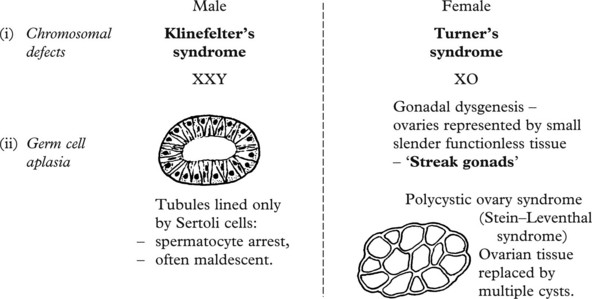
Congenital Disorders
There are many forms of malpositions and malformations of the kidney:
One or both kidneys fail to reach the normal adult position. The condition causes difficulty:
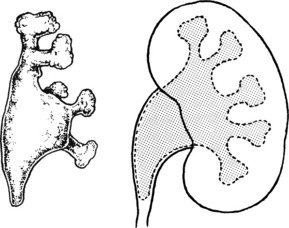
This is usually partial, producing the so-called ‘horse-shoe’ kidney. The ureters may be partially obstructed leading to hydronephrosis, infection and stones.
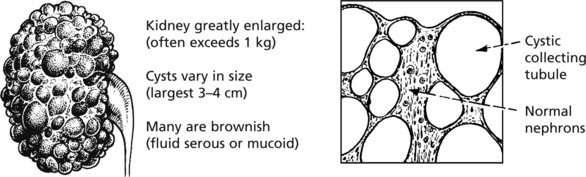
Polycystic Kidney Disease
This occurs in two main forms:
This is relatively common, and accounts for 5–10% of cases of chronic renal failure. The kidneys are converted into a mass of cysts with loss of renal parenchyma.
It is an inherited autosomal dominant trait due to two genes, PKD-1 (85% of cases) which encodes a protein polycystin-1, and PKD-2 (15%) associated with milder disease.
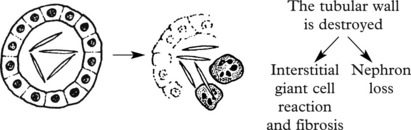
The precise defect is unclear but defects of the cilia of tubular epithelial cells appear responsible. There is cyst formation in some of the collecting tubules, causing a mixture of normal and abnormal nephrons. Cystic change develops after birth and is progressive, resulting in atrophy of normal nephrons by pressure.
Cysts also occur in the liver. Death from cerebral haemorrhage is more frequent than expected, partly due to the hypertension secondary to the kidney disorder and in some cases to Berry aneurysms of cerebral arteries.
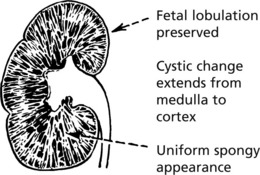
This is a rare condition, which may present in the perinatal period or later in childhood.
The nephrons are said to be normal in number and formation. Cystic dilatation is situated in the terminal branches of the collecting tubules. The disease, if severe, is incompatible with life, and death occurs shortly after birth. It is an autosomal recessive trait due to mutations of the PKHD-1 gene. Congenital hepatic fibrosis often dominates in children who survive infancy.
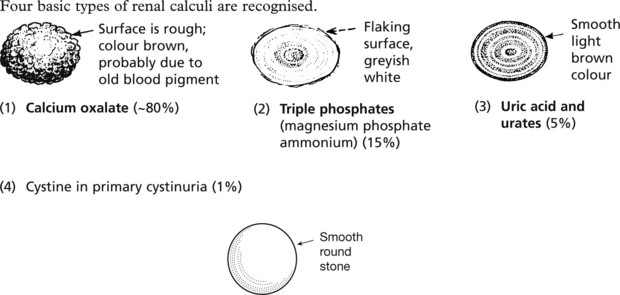
Urinary Calculi
Stones may form in the renal pelvis, ureter or bladder.
Commonly the stones are mixed.
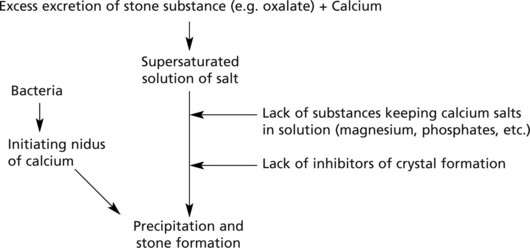
Mode of formation. There are two steps – nucleation followed by aggregation:
Predisposing Factors
Tumours of the Kidney
Malignant Tumours
Three are of importance: renal cell carcinoma, transitional cell carcinoma and nephroblastoma.
Renal Cell Carcinoma
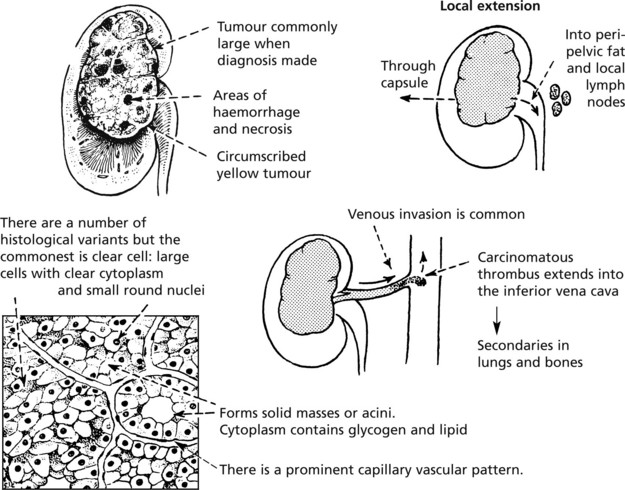
This is the commonest (90%) primary malignant renal tumour and arises from tubular epithelium. It has a typical appearance.
Systemic Effects
Patients with renal carcinoma may have:
Transitional cell carcinoma of the renal pelvis is discussed on p.483.
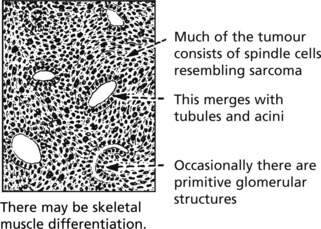
Nephroblastoma (Wilms’ Tumour)
This is one of the commonest malignant tumours of childhood usually presenting between 2 to 5 years. About 10% are bilateral. It is an embryonic type of tumour derived from ‘nephrogenic rests’, and forms a large well-circumscribed growth which rapidly invades blood vessels, giving rise to pulmonary secondaries. With modern therapy 90% long-term survival rates are achieved.
Mutation of the Wilms’ tumour gene (WT-1) on chromosome 11 and a variety of other genes appear to be responsible for the development of this tumour.
Diseases of the Urinary Tract
The urinary tract is a collecting and discharge system. There are 4 main pathological processes affecting its function: (1) obstruction, (2) infection, (3) calculus formation and (4) neoplastic disease.
Obstruction
Acute obstruction, if complete, causes rapid cessation of urine production. If both kidneys are involved, renal failure quickly follows. Chronic obstruction is more common and leads to anatomical changes, with sequels.
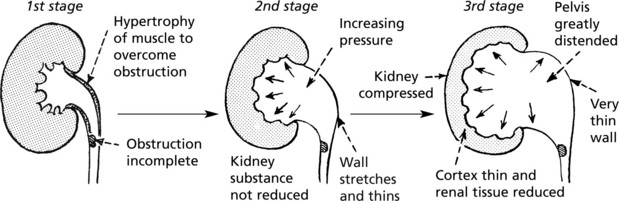
Hydronephrosis
This is a dilatation of the renal pelvis and calyces, due to chronic incomplete or intermittent obstruction.
Microscopically, there is tubular atrophy and glomerular scarring. Superimposed infection is common and aggravates the renal damage.
Aetiology
The condition may affect one or both kidneys. This is related to the site of obstruction.
Unilateral obstruction: above the bladder
Gross hydronephrosis is more commonly unilateral.
Bilateral obstruction: in or around bladder or urethra. The ureters are also affected and become dilated and tortuous.
Urinary Tract Infection
Acute Cystitis
The bladder shows the usual signs of inflammation. Small haemorrhages are common in the oedematous mucosa. The causes have already been discussed.
Chronic Cystitis
This is the result of repeated attacks of acute cystitis, but it is usually associated with obstruction of the urethra causing stasis of urine.
Mixed infection including B. proteus is common and, together with urea-splitting organisms forming ammonia, result in an alkaline urine. Phosphates precipitate and can form crumbling whitish calculi.
Interstitial Cystitis
This disorder usually affects women who complain of intermittent pain, frequency and dysuria without bacterial infection.
Mast cells are commonly seen in the mucosa which may show typical ulcers (Hunner ulcers). The cause is unknown.
Tuberculous Cystitis
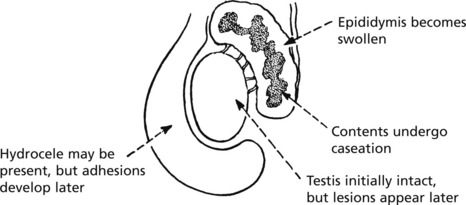
This is always secondary to a tuberculous infection elsewhere, usually in the kidney. Less commonly, the epididymis is the source of infection. Small tubercles form in the submucous layer, usually at the bladder base. These ulcerate and secondary infection is common.
Urethritis
Acute inflammation of the urethra is commonly due to the gonococcus, but another form is now more common in Western countries: so-called non-specific urethritis is often due to chlamydia infection.
In Reiter’s syndrome, in addition to urethritis, there is arthritis and conjunctivitis.
Tumours of the Urothelium
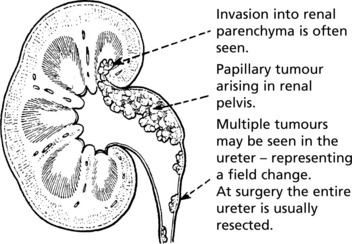
Transitional Cell Carcinoma
This tumour arises from the epithelium of the renal pelvis and is similar to the commoner transitional cell carcinoma of the bladder, with which it shares aetiological factors.
Patients usually present with haematuria.
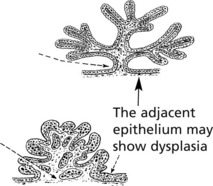
Transitional cell carcinomas occur in the bladder, ureters and renal pelvis. They arise from the stratified transitional epithelium lining the tract and are all considered to be carcinomas but show a wide spectrum of malignant potential. They are graded I to III as follows, according to the World Health Organisation (WHO) classification. They can be papillary or solid in growth pattern.
GRADE I – These are usually Papillary Tumours with uniform, well-differentiated epithelial covering showing no mitotic activity, a slender stalk and delicate branching fronds. Haemorrhage is common.
GRADE II – These also are mainly Papillary. The epithelial cells show mitoses and nuclear pleomorphism.
GRADE III are often solid sessile tumours. They show marked nuclear pleomorphism, mitotic activity and aggressive growth. Ulceration and necrosis are common: invasion into the bladder muscle and lymphatic invasion are usual.
Squamous carcinoma and adenocarcinoma are rare tumours. Rhabdomyosarcoma may occur in the bladder in children.
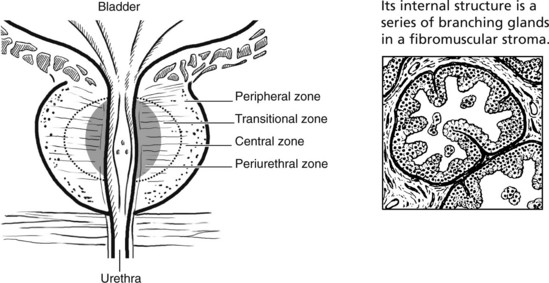
The Prostate
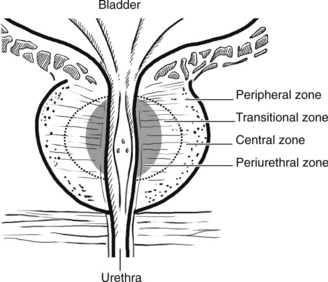
The prostate surrounds the bladder neck and urethra. Although traditionally divided into five lobes, it is now regarded as having four major zones, which tend to be affected by different disorders.
There are three main disorders of the prostate:
Prostatitis
Diseases of the Prostate
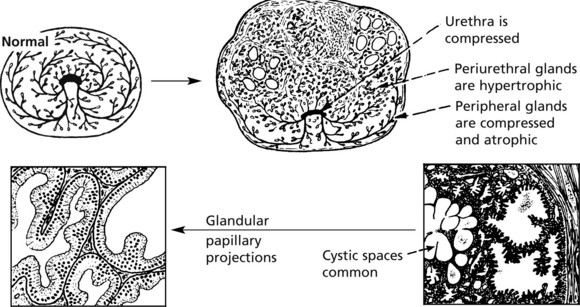
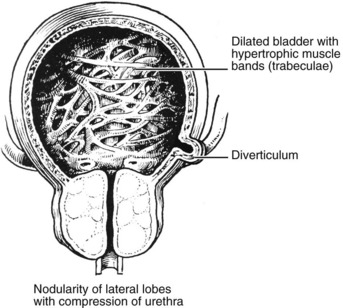
Benign Prostatic Hyperplasia
This is an extremely common finding in over 70% of men over 60 years. The prostate is enlarged often over 100 g (normal prostate 20 g). The transitional and periurethral zones are most affected.
Adenocarcinoma of the Prostate
This is now the commonest cancer in men in the UK and a significant cause of cancer death. It is rare below the age of 40 and rises to very high prevalence in men over 80.
The GLEASON system (Grades 2 through 10) indicates the degree of differentiation from (2) very well differentiated to (10) aggressive anaplastic tumours. Grading correlates well with prognosis.
Spread
Biochemical Tests
Prostatic intraepithelial neoplasia (PIN), analogous to CIN (p.503), has been recognised as a pre-invasive stage of the disease. High grade PIN is strongly associated with development of invasive cancer.
Diseases of the Penis
Tumours of the Penis
Papillomatous proliferations (condylomata acuminata), usually arise in the coronal sulcus or glans. They are due to sexually transmitted infection by Human Papilloma Virus (HPV) Types 6 and 11, and are analogous to similar lesions in the female genitalia
Peyronie’s disease is a fibromatosis related to Dupuytren’s contracture, which causes bending of the penis.
Precancerous Lesions
Epithelial dysplasia and carcinoma-in-situ can affect the penis (Bowen’s disease). This is related to HPV infection (Type 16).
Carcinoma of the Penis
Accounts for less than 1% of all male malignancy and affects middle-aged and elderly men. The site of growth is usually in the preputial area, often in the coronal sulcus, but it extends to involve the glans penis. Two forms of growth occur:
The growth spreads by the lymphatics to the regional (inguinal) nodes. Distant metastases occur late. The prognosis is reasonably good, 5-year survival rate being greater than 50%.
Diseases of the Testis
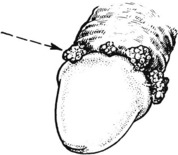
Epididymitis and Orchitis
Acute inflammation due to bacteria is uncommon in these organs. Spread of urinary infection via the vas deferens does occasionally occur and may result in suppuration. Gonorrhoea and chlamydia are seen in young men.
In 20% of adult cases of mumps, the epididymis and testis become acutely inflamed. The condition is usually unilateral, but if bilateral there is a distinct danger of subsequent infertility.
Acute inflammation of the testis may be confused with torsion. Torsion generally occurs within the tunica vaginalis and leads to obstruction of the testicular vessels. Infarction results, with destruction of the tissue. Recurrent minor degrees of torsion can cause atrophy. Since the torsion may be bilateral, prophylactic surgery may reduce the risk of a second event.
Chronic inflammations are important, although rare, in this region.
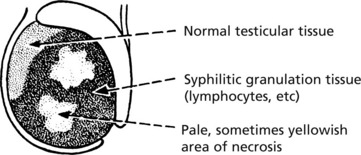
Chronic Granulomatous Orchitis
This is a condition of unknown origin. The testis is infiltrated by macrophages, plasma cells and lymphocytes. Atrophy of the germinal epithelium occurs and the testis becomes fibrotic.
Tumours of the Testis
Germ Cell Tumours of the Testis
These tumours make up 2% of cancers in men and the incidence is rising steeply in Western countries. They are the commonest form of malignancy in young men. Tumours are more common in undescended testes.
The 2 main tumour types are seminoma and teratoma.
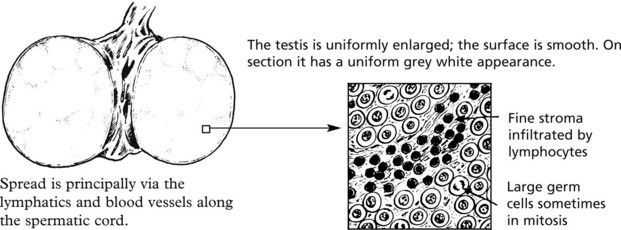
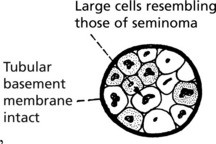
Seminoma This corresponds to the dysgerminoma in the female. It is rare before puberty and has its peak incidence in adults in their 30s. It accounts for 50% of all testicular tumours.
The tumour is extremely radiosensitive and chemosensitive. Orchidectomy and adjuvant therapy give a >95% cure rate. In older men ‘spermatocytic seminoma’ is a rare variant but has an excellent prognosis.
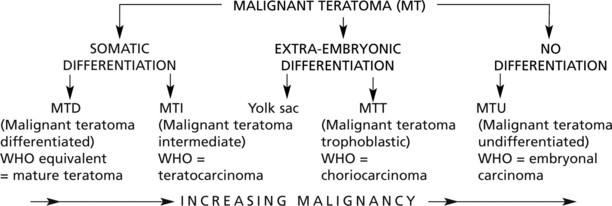
Malignant teratoma (non-seminomatous germ cell tumour)
This type represents 35% of malignant testicular tumours. It takes origin from totipotent germ cells capable of differentiating into derivatives of ectoderm, endoderm and mesoderm. It is customary to classify them according to the degree and type of differentiation exhibited.
The tumours form a spectrum of well differentiated to anaplastic highly malignant growths and are classified in various ways. The following is often used:
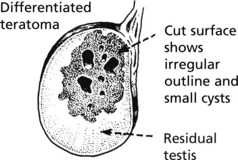
These tumours, unlike seminoma, are usually irregular in shape and show focal haemorrhage and necrosis: in the better differentiated tumours small cysts are common.
Miscellaneous Tumours
The following tumours are rare:
It should be remembered that leukaemia often involves the testes (p.438).
Rarely paratesticular sarcomas occur, e.g. rhabdomyosarcoma in children.











 pages 463, 470, 471
pages 463, 470, 471