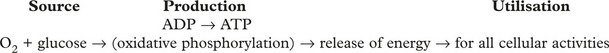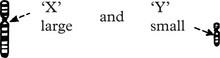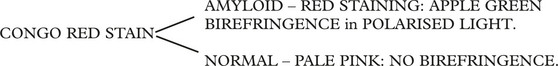Chapter 1 Cell and Tissue Damage
Cellular Physiology And Pathology
Understanding disease requires knowledge of cellular function and dysfunction.
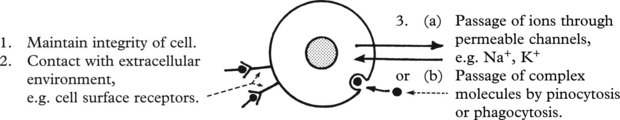
Plasma Membranes
Membrane damage may lead to cellular dysfunction or death.
MITOCHONDRIA These are the main sites of ENERGY production.
Disorder of energy production affects all cellular functions.
Nucleus
The nucleus controls all cellular activities through the action of at least 10 000 genes, each of which encodes a protein with structural, enzymatic or control functions.
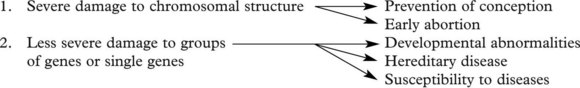
Damage to DNA (e.g. by ionising radiation) is particularly likely in dividing cells. There are effective repair mechanisms but severe damage usually leads to cell death by apoptosis (see p.4).
Germ cell DNA damage (i.e. to spermatogonia or oocytes)
Somatic Cell DNA Damage
This is acquired during life. Damage to stem cells is especially important.
The development of cancer (p.152) through activation of oncogenes or loss of tumour suppressor genes is the most important example.
Control of Cell Number
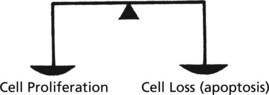
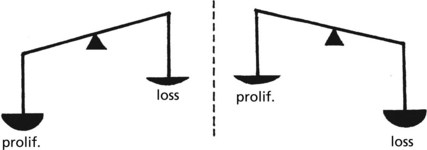
In adult life the CELL NUMBER is fairly constant. Complex control mechanisms evenly balance new cell production with cell loss.
During SOMATIC GROWTH cell proliferation outweighs cell loss.
In the ATROPHY of old age, cell loss outweighs cell proliferation.
In many diseases the balance is lost.
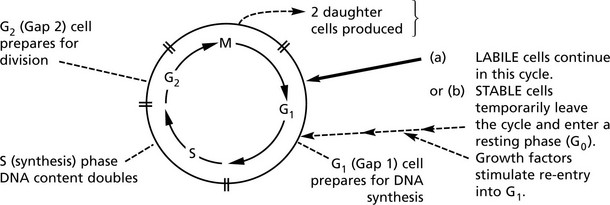
Cell Proliferation – the Cell Cycle
In adult life, cells can be classified into 3 groups according to their proliferation potential.
| Proliferation potential | Examples | |
|---|---|---|
| 1. LABILE CELLS | rapid proliferation and cell turnover | gut-lining epithelial cells |
| 2. STABLE CELLS | slow proliferation and cell turnover | hepatocytes |
| 3. PERMANENT CELLS | NOT able to proliferate | neurones |
In preparation for division, a cell passes through 4 consecutive phases (G1, S, G2, M) ending in Mitosis (M).
A variety of growth promotors, inhibitors and drugs influence the cycle at various stages.
Knowledge of the cycle underlies the understanding of mechanisms involved in HEALING and REPAIR and in CARCINOGENESIS.
Apoptosis
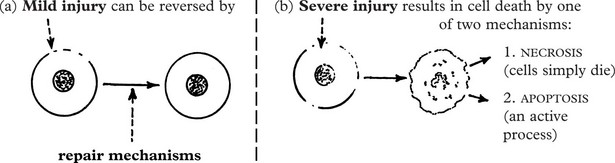
A variety of noxious agents can damage cells. These include:
The severity of the injury determines the outcome:
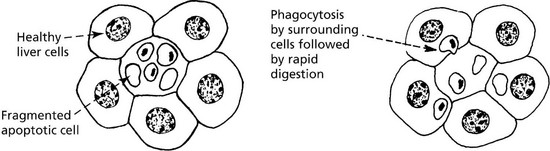
Apoptosis (programmed cell death) (Greek: apoptosis = falling off, like leaves from a tree) is an important process in health and disease by which, unlike necrosis (p. 5), abnormal or unwanted cells are eliminated. It involves activation of a programmed series of events co-ordinated by a dedicated set of gene products. It is an active process. A few examples are:
Cell Damage
Necrosis
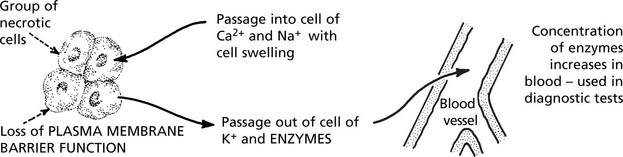
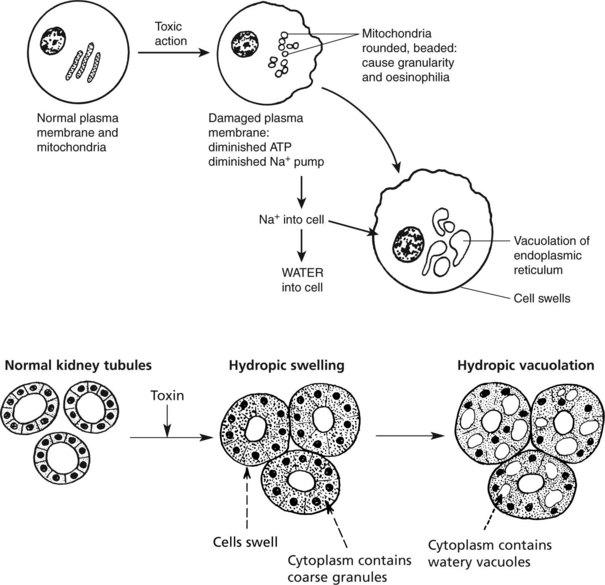
This term describes death of a cell or group of cells, typically following severe hypoxia, physical or chemical injury. Death of the cell is associated with rapid depletion of intracellular energy systems. Initially there are no morphological changes, but within a few hours the cell membrane and intracellular organelles are disrupted. Electron microscopy demonstrates these changes earlier than light microscopy.
What we recognise as necrosis is due to denaturation and coagulation of protein and/or digestion of the cell by released enzymes. There are several naked eye appearances depending on which of these predominates.
Necrosis
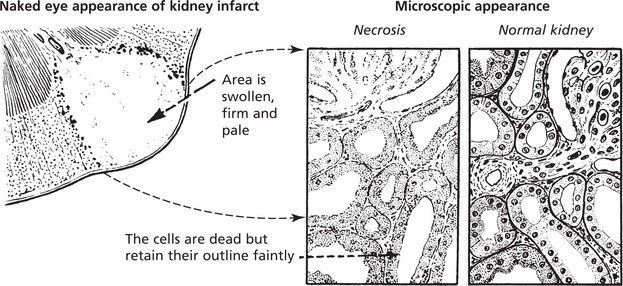
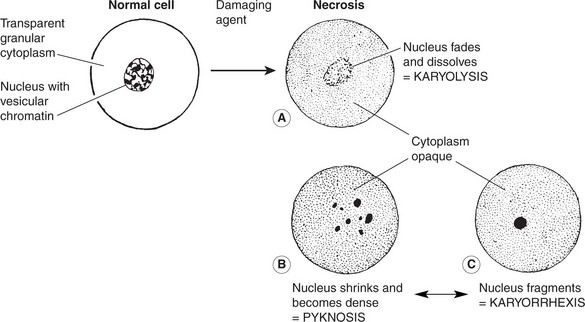
Coagulative Necrosis
This type of necrosis is frequently caused by lack of blood supply, e.g. infarcts of the heart, spleen and kidney.
The following changes are seen using the light microscope:
At electron microscope (E.M.) level, in addition to the above nuclear changes, disorganisation and disintegration of the cytoplasmic organelles and severe damage to the plasma membrane are seen with the formation of surface blebs.
Colliquative Necrosis (Liquefactive Necrosis)
Death of cells in the brain result in liquefactive necrosis in which the dead cells are broken down to form a liquid mass. There is complete loss of structure (see p. 535).
An abscess (see p.39, 40) is another example of colliquative necrosis.
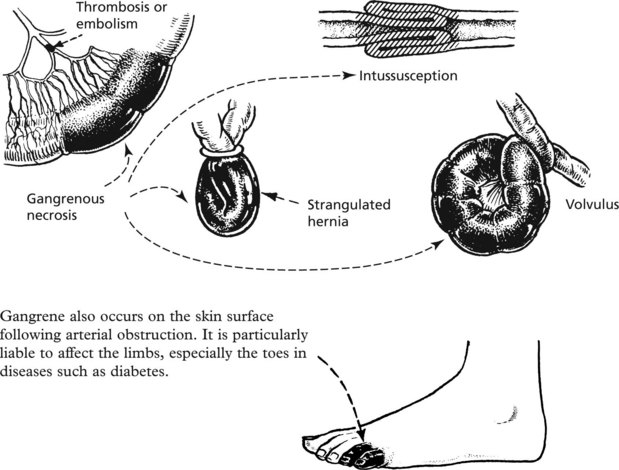
Gangrene
This is a complication of necrosis, which occurs when tissues are invaded by bacteria which release proteolytic enzymes. These enzymes degrade the necrotic tissue releasing foul-smelling gases. The tissue becomes green or black due to breakdown of haemoglobin. Obstruction of the blood supply to the bowel, for example, is almost inevitably followed by gangrene:
A special type of gangrene follows infection with clostridial organisms (gas gangrene; see p.70).
Caseous Necrosis
This is commonly seen in tuberculosis (p. 72). The necrotic tissue has a cream-cheesy appearance.
Necrosis – Autolysis
Cell Damage – Hydropic Swelling
The various agents which can cause cell necrosis may also cause lesser cell damage which is reversible when the injurious agent is removed.
The appearances and effects are considered under these headings: 1. hydropic swelling, 2. fatty change, 3. radiation and 4. atrophy.
Cell Damage – Fatty Change
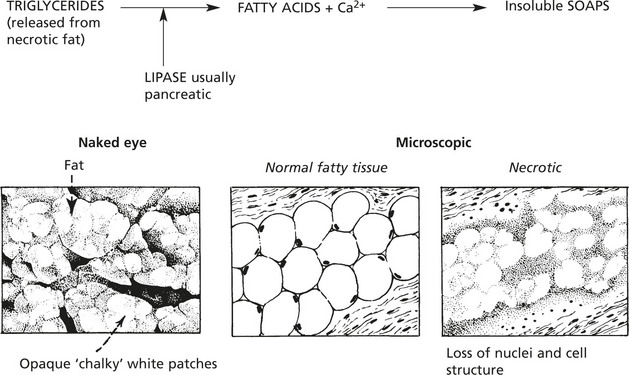
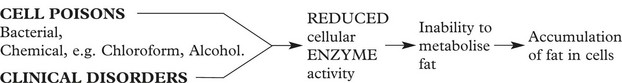
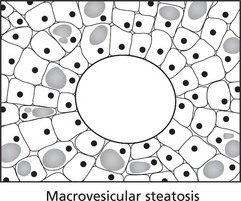
Fatty Change
This is accumulation of fat in non-fatty tissues, e.g. skeletal muscles and the heart.
These tissues cannot metabolise the amount of lipid presented to them, resulting in its accumulation within the cells. Examples include:
In normal non-fatty tissues the intracellular fat is not visible by light microscopy using conventional fat stains.
In fatty change, the accumulated fat is visualised using frozen sections and fat-soluble dyes: e.g. Sudan, in routine paraffin sections the fat has been dissolved and is indicated by clear vacuoles.
For example, in the LIVER, the increase of deposited fat causes enlargement of the organ.
Effects of Fatty Change
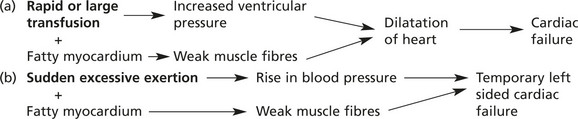
Impairment of cellular function is usually due to the pathological process causing the fatty change (e.g. anoxia in severe anaemia) and not to the physical presence of fat within the cell. In the liver, for example, very large accumulations of fat do not impair basic liver functions.
A possible exception is the myocardium in certain circumstances.
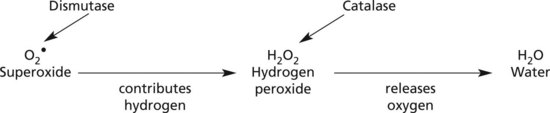
Cell Damage – Free Radicals
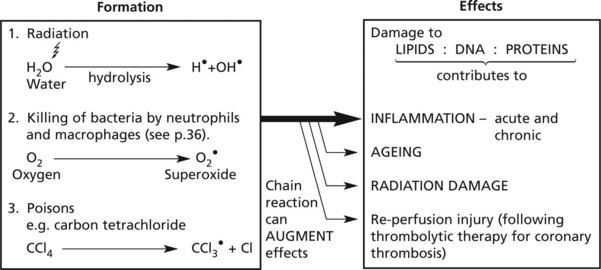
The formation of free radicals is an important contributory factor in many disease processes.
Free radicals are molecules with a single unpaired electron in an outer orbital position: they react with and modify a wide range of molecules, particularly lipids (of cell membranes), DNA and proteins.
A dot above the chemical symbol indicates the presence of a free radical: e.g. OH• = hydroxyl radical, O2• = superoxide radical.
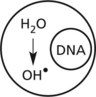
Cell Damage – Radiation
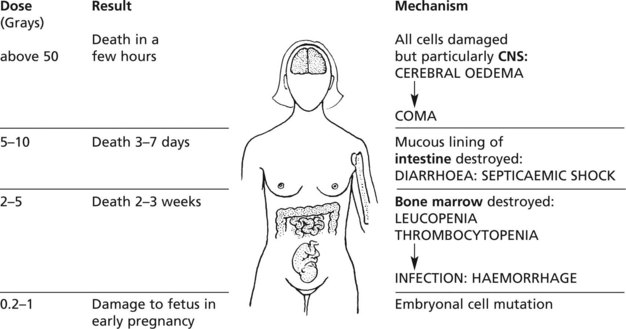
High energy (ionising) radiation, particularly in the form of gamma (γ) or X-rays can cause serious cellular and tissue damage. At cellular level there are 2 mechanisms involving:
A large dose of radiation hydrolyses water producing damaging free hydroxyl radicals: acute cell death follows
These effects will be considered under 3 headings:
Injury to organs which are difficult to shield is seen, for example, in skin, eyes, alimentary tract, bladder, lungs, gonads and spinal cord.
The mechanisms are described on page 149.
Note: Total body irradiation is used in treatment of some leukaemias.
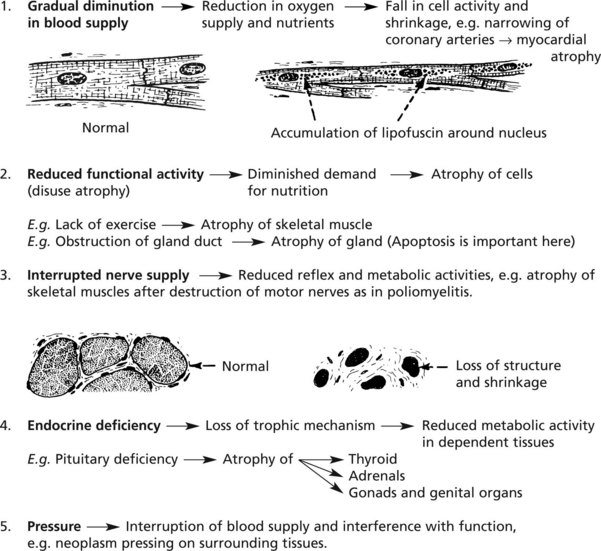
Atrophy
This is a simple decrease in cell size or number, resulting in a shrinkage of affected tissues and organs. The most common example is atrophy of old age (see p.15).
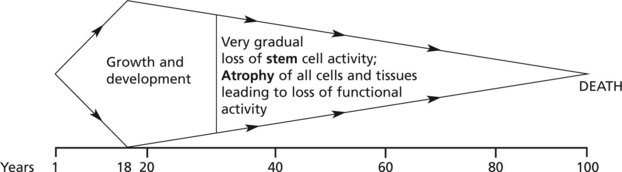
Ageing
The distinction between ‘true’ ageing and ageing complicated by disease processes may be difficult; since therapy can be directed at the latter the distinction is important in clinical practice.
The changes associated with true ageing would be seen in a theoretical ‘ideal’ environment (minimal stress).
The following diagram is illustrative. The main controlling factors are intrinsic, i.e. genetic:
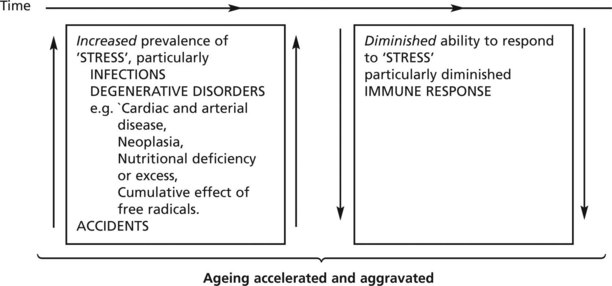
In the real environment this theoretical concept of ageing is accelerated and aggravated by two groups of extrinsic factors:
Note: Death is only very rarely, if ever, due to ‘pure’ ageing. It is the result of disease – either a single process or, with increasing age, more often several causes.
Heredity, Genes and Disease
Cell Nucleus and Chromosomes
The cell NUCLEUS contains chromosomes, which transmit hereditary traits from one generation to the next and also control the synthesis of all the proteins in the body.
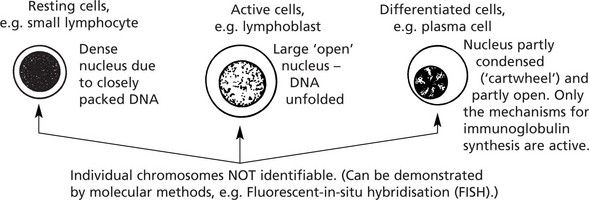
The 46 chromosomes which most human nuclei contain are not identifiable in differentiated cells or cells in the non-proliferating phase of the cell cycle (G0).
The different morphological appearances of nuclei in histological sections indicate the amount of nuclear activity.
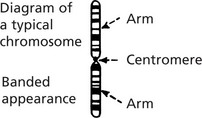
Chromosomes
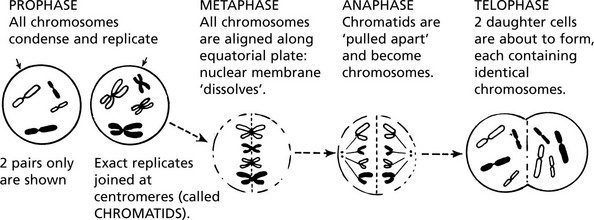
During mitosis the chromosomes condense into specific morphological forms which are identifiable by light microscopy: when colchicine is added to a cell culture, mitosis is arrested at the metaphase: chromosomes are then separable and can be studied.
The following features are specific to each chromosome:
Using these morphological criteria each chromosome is identified and numbered 1 to 22.
The sex chromosomes are labelled
Note: This appearance represents a very condensed and coiled molecular arrangement, i.e. inactive.
A typical normal chromosome map (karyotype) shows 22 pairs of different but identifiable chromosomes……………………………………………… plus 2 sex chromosomes,
Deoxyribonucleic Acid (DNA)
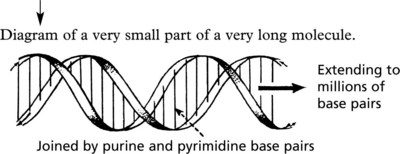
Since Watson and Crick defined the molecular structure of DNA in 1953, there has been a great increase in knowledge of the ‘genetic code’. Each chromosome is a very long single molecule of deoxyribonucleic acid (DNA), condensed during mitosis. It is extended to its characteristic structure when active:
2 long spirals of nucleotides (consisting of a deoxyribose (sugar) + phosphate) around a central axis, complementary but running in opposite directions.
The function is to initiate and control the synthesis of proteins from amino-acids. All types of protein (structural proteins, hormones, receptors, intra-cellular messengers, etc.) are encoded along the molecule.
A GENE is the unit of the chromosome responsible for the synthesis of a single specific protein. Genes vary in length but on average occupy about 20 000 base pairs of the molecule.
There are over 10 000 genes in all the human chromosomes, not all are active: some are repetitive: some form clusters subserving related activities (e.g. MHC (HLA) locus, see p.91).
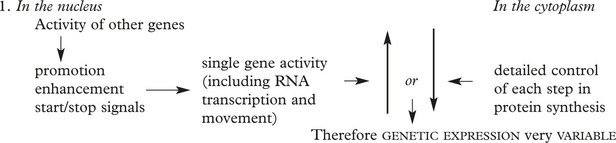
There is a complex regulation of gene activity involving stop and start signals, promotor and enhancer functions all within the DNA structure. The entire human genome has now been sequenced.
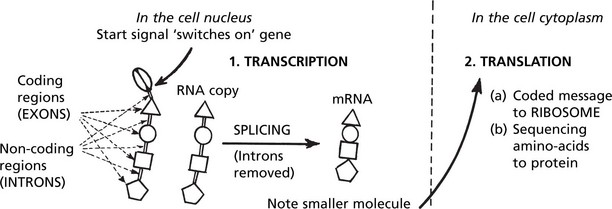
Gene Expression and Protein Synthesis
A gene consists of coding regions (EXONS) interspersed with non-coding regions (INTRONS). In transcription the introns are eliminated and all the exons are transcribed to a ribonucleic acid form (mRNA) which is a copy of the DNA-code sequencing the amino-acids required for the production of a specific protein.
Mitosis and Meiosis
MITOSIS is the process by which somatic cells proliferate ensuring exact replication of the daughter cells. Following the stimulus to proliferate, the chromosomes condense and replicate exactly.
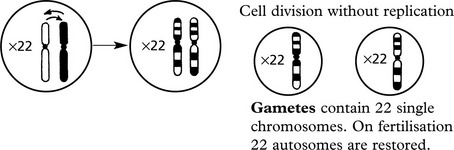
MEIOSIS is a complex process occurring during gametogenesis: it involves the reduction and division of chromosomes in such a way that: 1. a random mixture of both parental genes is present in the gamete and 2. the chances are equal for fertilisation to result in either sex. This simple diagram shows the important results of meiosis.
Primitive germ cell contains 46 chromosomes (i.e. 22 autosomes + XX or XY)
Note: In this simplified diagram a preliminary replication before meiosis and mitotic divisions after meiosis are omitted. The chances of error are greatly increased.
Genetic Abnormalities and Associated Disorders
It is not surprising that errors arise during these complex genetic activities. germ cells and proliferating somatic cells (including stem cells) are susceptible to such errors.
They may occur spontaneously or be the result of external influences.
It is important to distinguish between germ cell and somatic cell abnormalities.
Germ Cell Abnormalities
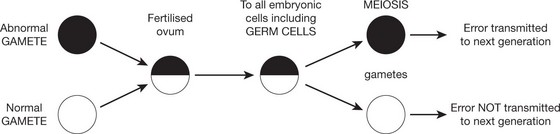
Errors which have arisen during germ cell development are transferred to the fertilised ovum and thence to all cells including the germ cells of the new individual.
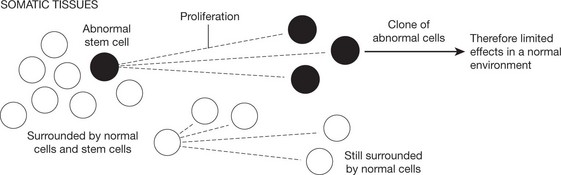
Abnormalities Arising in Somatic Cells
These tend to cause restricted effects: they are not transmitted to the next generation.
Types of Genetic Abnormalities
They range from large, involving whole chromosomes, through parts of chromosomes, to gene clusters and single genes.
Chromosomal Abnormalities
Such gross chromosomal abnormalities occurring during gametogenesis or at fertilisation are usually incompatible with life and are a common cause of spontaneous abortion.
Down’s syndrome is a good example of AUTOSOMAL TRISOMY and is due to an extra chromosome (i.e. Trisomy 21 – karyotype 47XX + 21 or 47XY + 21).
The abnormality occurs in utero after fertilisation of the ovum and is therefore autosomal. Increasing maternal age is a potentiating factor in Down’s syndrome and many other genetic defects.
Despite the existence of efficient repair mechanisms structural errors do arise when the long DNA molecules are accidentally broken during the physical changes occurring at replication. They include, for example, duplication and deletion of gene clusters and single genes: and translocation of fragments of DNA between chromosomes.
Single Gene Disorders
Factors regulating the production of the final specific protein are extremely complex.
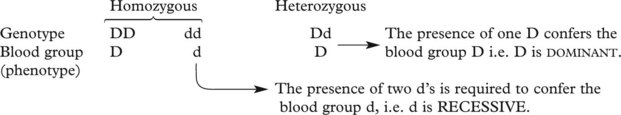
This concept is important in inherited single gene disorders.
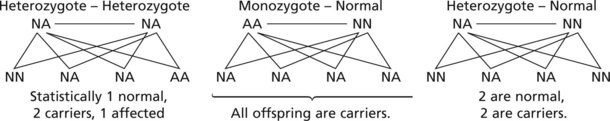
The concept of dominant and recessive traits is useful in genetic counselling. Long lists of dominant and recessive disorders are available: only a few important examples are given.
Metabolic Disorders (Inborn Errors of Metabolism)
These are inherited disorders of single genes which code for the enzymes concerned in many metabolic pathways. The clinical effects show considerable variation in severity.
Examples are: disorders of carbohydrate (including glycogen storage), lipid and amino-acid metabolism: lysosomal storage: and membrane transport (including cystic fibrosis).
Note: Not all single gene abnormalities cause, by themselves, significant pathological effects. As indicated above, the controlling factors are complex and include the important effects of ‘modifying genes’. It seems likely that abnormal recessive genes exist in the normal population but only present as clinical disorders in rare circumstances.
Multifactorial Disorders
Most human diseases have a genetic component but environmental factors usually play a very important part in the pathogenesis.
Examples are: atopic (allergic) disorders: diabetes mellitus, hypertension, rheumatoid arthritis and various infections.
Somatic Cell Genetic Disorders
When mutation occurs after fertilisation of the ovum and at any stage throughout life the effects are limited to the disordered cell(s) and progeny. The clinical effects tend to be localised.
neoplasms and hamartomas are examples of somatic cell genetic disorders (see Carcinogenesis).
Note: In the systematic section of this book significant genetic contribution to the pathogenesis of diseases will be recorded.
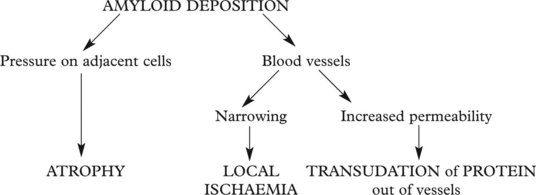
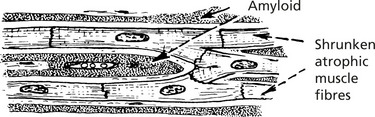
Amyloid Deposition
Amyloid is a waxy substance deposited in the extracellular tissues, particularly around blood vessels and in basement membranes. Various forms of amyloid are seen and they have varying effects. Amyloid is resistant to degradation and its deposition tends to progress relentlessly.
Nature of Amyloid
Chemical: amyloid fibrils are made up of:
Whatever its composition, amyloid fibrils are arranged in a β-pleated configuration: this accounts for its resistance to degradation and its staining properties.
On Light Microscopy
On electronmicroscopy – closely packed interlacing fibrils 70–100 nm in diameter.
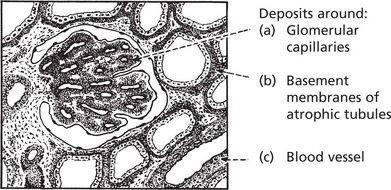
Kidney
In severe cases the kidneys are pale, firm and waxy, and with the iodine test the glomeruli stand out as brown dots to the naked eye.
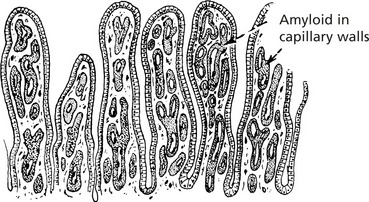
Gastrointestinal Tract
Due to altered permeability of the capillaries, the patient suffers from diarrhoea and protein loss. There may also be malabsorption, nutritional deficiencies and electrolyte imbalance.
Heart
Amyloid deposition occurs around the cardiac muscle fibres and the capillary basement membranes. The heart is enlarged with thick walls. Much of the thickness is due to amyloid deposition.
Effects: Cardiac failure develops mainly due to the mechanical effect of amyloid, making the heart muscle stiff and preventing cardiac filling (restrictive cardiomyopathy). The blood supply to the muscle fibres is also impaired.
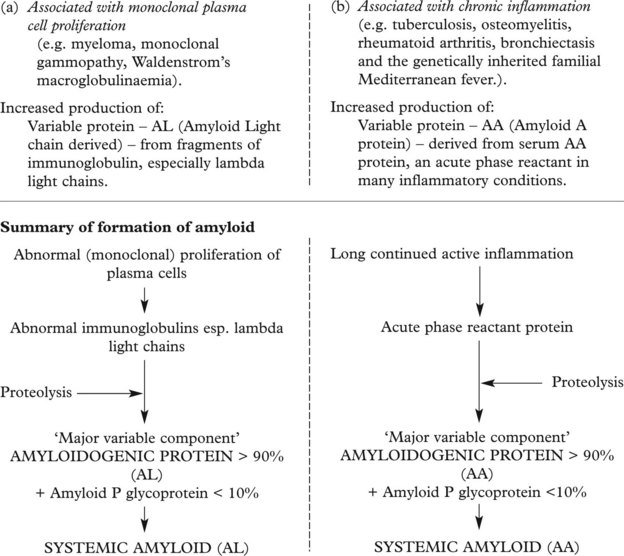
Amyloid Classification
In endocrine tumours – amyloid derived from polypeptide hormones, e.g. medullary carcinoma of thyroid – calcitonin derived amyloid.
In old age – the amyloidogenic protein is related to pre-albumin. Deposits of amyloid occur in the heart, brain and joints. In Alzheimer’s disease (p.544) local amyloid deposition in the brain is important.
Calcification
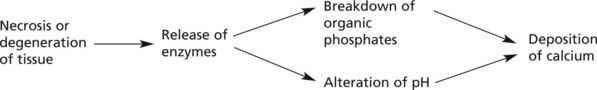
Abnormal deposits of calcium occur in 2 circumstances: dystrophic calcification and metastatic calcification.
Local deposits of calcium may occur in:
The mechanism may be as follows:
Note: Ca2+ is radio-opaque and therefore can be seen on X-rays where it is of diagnostic use, e.g. in old healed disease (e.g. tuberculosis) and also in some tumours, e.g. breast cancer where very small deposits of calcium may be present.
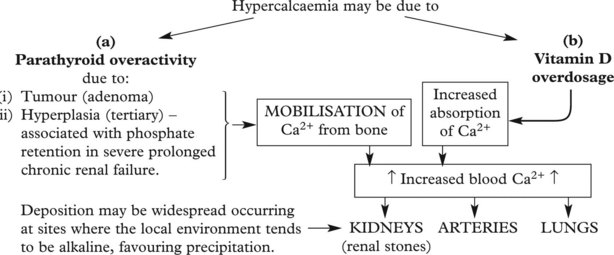
In this case there is an increase in the calcium phosphate product in the blood (usually hypercalcaemia).
Some malignant tumours, e.g. breast and lung, are associated with hypercalcaemia and metastatic calcification.
The mechanisms are: 1. secretion by the tumour of a protein (parathyroid hormone related peptide (PTHrP)) which mimics the action of parathormone and 2. the local release of bone resorbing cytokines by tumour metastases in bones.
Endogenous Pigmentation
Iron-Containing Pigment
Two main pigments are derived from the breakdown of red blood cells:
1. haemosiderin and 2. bilirubin.
The detailed mechanism is described on page 340 in relation to jaundice.
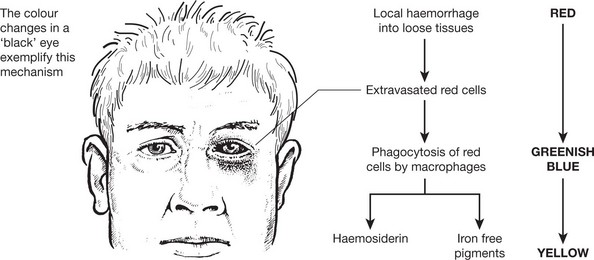
Haemosiderin
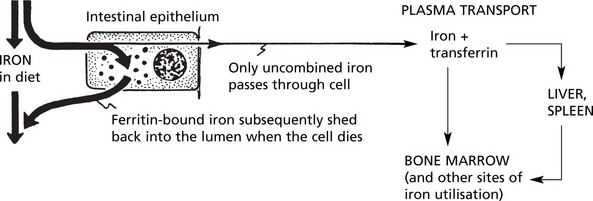
The iron derived from red cell breakdown is held in the spleen, liver and bone marrow, combined with apoferritin. In the plasma it is transported by transferrin. The two mechanisms maintain an equilibrium between the iron contents in these three sites. When the amount of iron within the cells becomes excessive and overloads the ferritin system, it is deposited in a brown granular form – haemosiderin. This occurs in 2 situations:
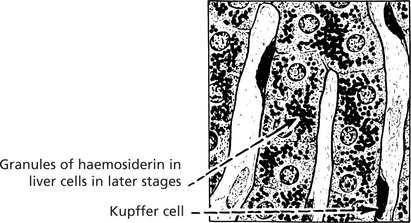
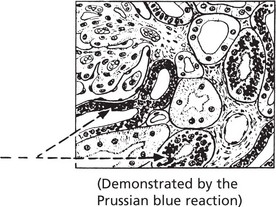
This is seen in the liver, spleen and sometimes the kidneys in cases of haemolytic anaemia, and in patients requiring repeated blood transfusion. The change is most dramatic in the liver, which becomes deep brown. Iron is found in the Kupffer cells first and, later, in liver cells. A Perl’s stain highlights the iron deposits, which are stained blue. Iron deposits per se very rarely cause organ damage.
The ferritin content of the intestinal epithelium, iron saturation of the plasma, stores of iron in the liver and spleen and the demand for iron by the bone marrow form a balancing mechanism preventing overloading of any part of the system.
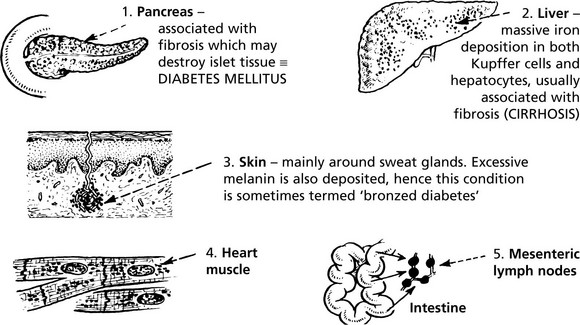
In haemochromatosis there is an inherited defect on chromosome 6 resulting in uncontrolled absorption of iron. The system becomes overloaded and iron is deposited as haemosiderin in many sites, the main ones being:
Haemosiderin may be found in almost any site in the body.
Haemoglobin
Intravascular haemolysis can result in haemoglobin appearing in the urine, giving it a dull red colour. In severe acute haemolysis (e.g. incompatible blood transfusion) acute renal tubular necrosis occurs. In chronic haemolysis (e.g. paroxysmal haemoglobinuria), some of the haemoglobin is reabsorbed and subsequently broken down so that iron, as haemosiderin, appears in the renal tubular epithelium.
Exogenous Pigmentation – Degenerations
Exogenous Pigmentation
Pigments may be introduced by inhalation, ingestion or injection.
Inhalation
The commonest substances inhaled are coal dust (carbon) – black, and stone dust (silica) – grey.
The particles reach the alveoli especially if the bronchial ciliary action is disturbed by chronic bronchitis.
Ingestion
Pigmentation can be caused by chronic ingestion of metals such as silver or lead. In both, the skin has a metallic hue, and in the case of lead a blue line appears on the gums due to interaction between lead and hydrogen sulphide. Excessive intake of carrots can lead to yellowish red skin pigmentation caused by carotene.
Degenerations
This is a descriptive term meaning a glossy, refractile appearance, seen in sections stained with haematoxylin and eosin. It is most commonly encountered in the form of dense collagen, particularly in benign tumours such as leiomyomas where the collagen has replaced muscle fibres, and in blood vessels in arteriosclerosis.
The term ‘Mallory’s hyaline’ refers to an intracellular accumulation of cytoskeletal proteins, e.g. in alcoholic liver disease (p.348).
This is a common change in epithelial tumours which secrete mucin. In these cases the epithelial cells undergo degeneration and appear to dissolve in the mucin.
Connective tissue may also accumulate mucin. Spaces filled with mucopolysaccharides (e.g. hyaluronic acid) appear between the fibres. The change is common in some tumours and the term ‘myxomatous’ may be used.