CHAPTER 116 Sexually Transmitted Infections
CHAPTER 116 Sexually Transmitted Infections
Adolescents have the highest rates of sexually transmitted infections (STIs). Biologic agents, such as Chlamydia trachomatis, may predispose adolescents to certain STIs. Compared with adults, sexually active adolescents are more likely to believe that they will not contract an STI, more likely to come into contact with an infected sexual partner, less likely to receive health care when an STI develops, and less likely to be compliant with treatment for an STI.
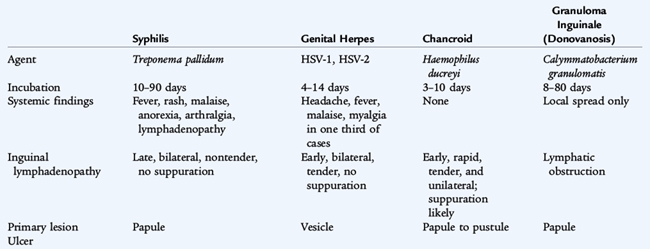
Although numerous organisms cause STIs, the diseases can be grouped by the characteristic clinical presentation. Urethritis and endocervicitis (Table 116-1) are characteristic of Neisseria gonorrhoeae and C. trachomatis and are the most common STIs. More than 70% of genital chlamydial infections in women are asymptomatic. Genital ulcers (Table 116-2) are characteristic of syphilis (Treponema pallidum), genital herpes simplex virus (HSV) infections, chancroid (Haemophilus ducreyi), and granuloma inguinale, also known as donovanosis (Calymmatobacterium granulomatis). Vaginal discharge (Table 116-3) is a symptom of trichomoniasis (Trichomonas vaginalis) and is part of the spectrum of vulvovaginitis (see Chapter 115), which is not always associated with sexual activity. Human papillomavirus (HPV) cause condylomata acuminata, or genital warts (Table 116-4) and are the major risk factor for cervical, vulvar, and vaginal cancers.
TABLE 116-1 Features of Sexually Transmitted Infections Caused by Chlamydia trachomatis and Neisseria gonorrhoeae*
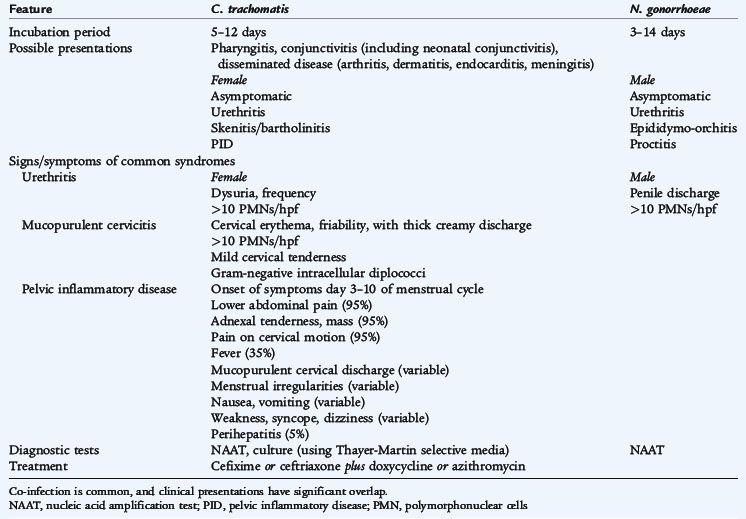
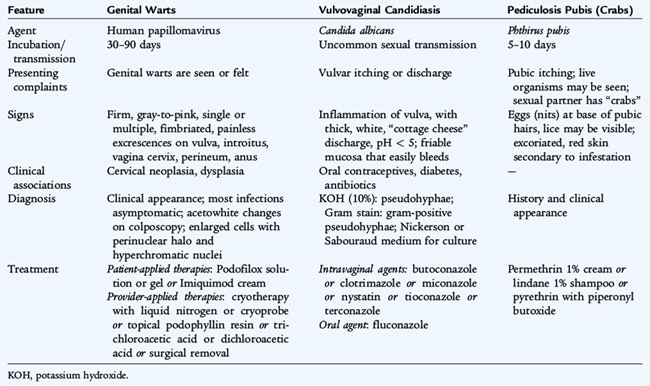
TABLE 116-4 Features of Sexually Transmitted Infections Characterized by Nonulcerative External Genital Symptoms
STIs are associated with significant physiologic and psychological morbidity. Early diagnosis and treatment are important for preventing medical complications and infertility. All STIs are preventable; primary prevention of STIs should be a goal for all health care providers for adolescents. Diagnosis of an STI necessitates evaluation or treatment for concomitant STIs and notification and treatment of sexual partners.
Many infections that are not traditionally considered STIs are sexually transmissible including those caused by human immunodeficiency virus (HIV), human T cell leukemia viruses types I and II, cytomegalovirus (CMV), Epstein-Barr virus (EBV), human herpes virus (HHV-6, HHV-7), hepatitis B virus (HBV), molluscum contagiosum virus, and Sarcoptes scabiei. The presence of any STI suggests behavior that increases risk for HIV (see Chapter 125), and HIV counseling and testing should be provided to all adolescents with STIs. Acquisition of gonorrhea, syphilis, HSV-2, and trichomoniasis in prepubertal children beyond the neonatal period indicates sexual contact and signifies the need to investigate for possible sexual abuse (see Chapter 22). The association of vulvovaginitis and genital HPV infection, which may result from skin or genital HPV types, with sexual abuse is less certain.
PELVIC INFLAMMATORY DISEASE
Direct extension of N. gonorrhoeae, often in combination with C. trachomatis, to the endometrium, fallopian tubes, and peritoneum causes pelvic inflammatory disease. Complications of pelvic inflammatory disease include tubo-ovarian abscess and perihepatitis (Fitz-Hugh–Curtis syndrome), which is inflammation of the capsule of the liver. A major sequela of pelvic inflammatory disease is infertility. The differential diagnosis of pelvic inflammatory disease includes ectopic pregnancy, septic abortion, ovarian cyst torsion or rupture, UTI, appendicitis, mesenteric lymphadenitis, and inflammatory bowel disease. Pelvic ultrasound may detect thickened adnexal structures and is the imaging study of choice for other possible diagnoses. Clinical criteria for diagnosis of pelvic inflammatory disease are lower abdominal tenderness, uterine/adnexal tenderness, and cervical motion tenderness. Additional criteria that support the diagnosis are fever, mucopurulent vaginal discharge, elevated white blood cell (WBC) count (in 45%), elevated erythrocyte sedimentation rate (ESR) (in 65%) or C-reactive protein (CRP), and documented infection with N. gonorrhoeae or C. trachomatis. Adolescents should be hospitalized for treatment if there is uncertainty about the diagnosis; pregnancy; no clinical response to oral therapy with 72 hours; inability to adhere to or tolerate oral therapy; a tubo-ovarian abscess; or severe illness with high fever, nausea, and vomiting. The recommended parenteral treatment is cefotetan or cefoxitin, plus doxycycline orally. The recommended oral treatment of pelvic inflammatory disease for younger persons is ceftriaxone in a single dose, plus doxycycline orally, for 14 days, with or without metronidazole for 14 days. Follow-up examination should be performed within 72 hours, with hospitalization for parenteral therapy if there has not been clinical improvement.
GONORRHEA (Neisseria gonorrhoeae)
N. gonorrhoeae, a gram-negative coccus, is often seen as diplococci. Gonorrhea is a common STI among adolescents. The greatest increase in incidence is in the 15- to 19-year-old age group, especially among girls. The organism causes infection at the site of acquisition, which commonly results in mucopurulent cervicitis and urethritis (see Table 116-1). Disseminated gonococcal infections can occur with hematogenous spread and results in petechial or pustular acral skin lesions, asymmetrical arthralgia, tenosynovitis or septic arthritis, and occasionally endocarditis or meningitis. Perinatal transmission of maternal infection can lead to neonatal sepsis and meningitis (see Chapter 65) and ophthalmia neonatorum (see Chapter 119).
Treatment regimens should be effective against N. gonorrhoeae and C. trachomatis because of the high frequency of concomitant infection. Increasing rates of resistance to fluoroquinolones limit treatment options. A single dose of intramuscular (IM) ceftriaxone (125 mg) or oral cefixime (400 mg) is recommended for uncomplicated gonococcal infections of the cervix, urethra, and rectum. Hospitalization and treatment with ceftriaxone are recommended for disseminated gonococcal infections. For all gonococcal infections, azithromycin or doxycycline also is administered unless chlamydial infection is excluded. Sexual partners should be notified and treated.
CHLAMYDIA INFECTION (Chlamydia trachomatis)
Chlamydiae are obligate intracellular bacteria with a biphasic life cycle, existing as relatively inert elementary bodies in their extracellular form and as reticulate bodies when phagocytosed and replicating within a phagosome. Reticulate bodies divide by binary fission and, after 48 to 72 hours, reorganize into elementary bodies that are released from the cell. Chlamydia infects nonciliated squamocolumnar cells and the transitional epithelial cells that line the mucosa of the urethra, cervix, rectum, and conjunctiva.
C. trachomatis serovars D through K cause urethritis, cervicitis, pelvic inflammatory disease, inclusion conjunctivitis in newborns, and infant pneumonia. C. trachomatis serovars L1–3 cause lymphogranuloma venereum, an infrequent STI characterized by unilateral, painful inguinal lymphadenitis. C. trachomatis serovars A, B, Ba, and C produce trachoma (hyperendemic blinding), which eventually leads to blindness from extensive local scarring.
Chlamydia is the most frequently diagnosed bacterial STI in adolescents and accounts for most cases of nongonococcal urethritis and cervicitis (see Table 116-1). There is a 5:1 female-to-male ratio. Males often have dysuria and a mucopurulent discharge, although approximately 25% may be asymptomatic. Women are more often asymptomatic (approximately 70%) or may have minimal symptoms including dysuria, mild abdominal pain, or vaginal discharge. Prepubertal girls may have vaginitis. At least 30% of persons with gonococcal cervicitis, urethritis, proctitis, or epididymitis have a concomitant C. trachomatis infection.
Chlamydia infection usually is diagnosed by detection of bacterial nucleic acids (PCR tests, using ligase chain reaction and transcription-mediated amplification) of cervical, urethral, and early morning first-voided urine specimens. Amplification tests have supplanted less sensitive culture and ELISA (enzyme-linked immunosorbent assay) tests. Because of false positive results, only culture should be used for medicolegal purposes to confirm C. trachomatis infection in cases of suspected sexual abuse.
Treatment regimens should be effective against C. trachomatis and N. gonorrhoeae because of the high frequency of concomitant infection. A single oral dose of azithromycin (1 g) or doxycycline for 7 days is recommended, which can be combined with a single oral dose of cefixime (400 mg) to treat concomitant gonorrhea infection. Sexual partners should be notified and treated.
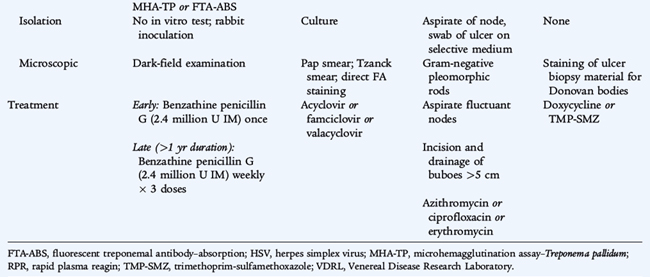
SYPHILIS (Treponema pallidum)
Syphilis is caused by T. pallidum, a long, slender, coiled spirochete. It cannot be cultivated routinely in vitro but can be observed by dark-field microscopy. Untreated infection progresses through several clinical stages (see Table 116-2). Primary syphilis is manifested as a single, painless genital ulcer, or chancre, usually on the genitalia, that appears 3 to 6 weeks after inoculation. Secondary syphilis follows 6 to 8 weeks later and is manifested as fever, generalized lymphadenopathy, and a disseminated maculopapular rash that also is present on the palms and soles. Plaquelike skin lesions, condylomata lata, and mucous membrane lesions occur and are infectious. Tertiary syphilis is a slowly progressive disease that involves the cardiovascular, neurologic, and musculoskeletal systems and is not seen in children. Latent syphilis is asymptomatic infection that is detected by serologic testing. Early latent syphilis indicates acquisition within the preceding year; all other cases of latent syphilis are either late latent syphilis or designated latent syphilis of unknown duration. Infection can be passed from pregnant women and infect their infants resulting in congenital syphilis (see Chapter 66).
The diagnosis of syphilis is based on serologic testing. Nontreponemal antibody tests, the Venereal Disease Research Laboratory (VDRL) test, and the rapid plasma reagin (RPR) test are screening tests and can be quantified as titers increase with increasing duration of infection and decrease in response to therapy. A nonquantitative VDRL test can be performed on cerebrospinal fluid (CSF), but is insensitive. Rheumatic disease and other infectious diseases may cause false positive results. Confirmatory, specific treponemal antibody tests, the microhemagglutination assay–T. pallidum and fluorescent treponemal antibody-absorption, are more specific and are used to confirm the diagnosis of syphilis. These tests usually remain positive for life even if the infection is treated and cured. Dark-field examination of chancres, mucous membranes, or cutaneous lesions may reveal motile organisms.
The treatment of choice for all stages of syphilis is penicillin G. Primary syphilis, secondary syphilis, and early latent syphilis is treated with a single IM dose of benzathine penicillin G. Tertiary, late latent, and latent syphilis of unknown duration is treated with three doses at 1-week intervals. Neurosyphilis is treated with intravenous (IV) aqueous crystalline penicillin G for 10 to 14 days. A systemic, febrile Jarisch-Herxheimer reaction occurs in 15% to 20% of syphilitic patients treated with penicillin.
HERPES SIMPLEX VIRUS INFECTION
HSV-1 and HSV-2 are large, double-stranded DNA viruses of the herpesvirus family that have a linear genome contained within an icosahedral capsid. There is significant DNA homology between types 1 and 2. The virus initially infects mucosal surfaces and enters cutaneous neurons where it migrates along the axons to the sensory ganglia. As viral replication occurs in the ganglia, infectious virus moves along the axon to infect and destroy epithelial cells. Infection may disseminate to other organs in immunocompromised patients. Virus latency is maintained in the ganglia where it undergoes periodic reactivation and replication triggered by undefined events. Although either virus can be found in any site, nongenital type 1 (HSV-1) more commonly occurs above the waist (central nervous system, eyes, mouth), and genital type 2 (HSV-2) more commonly involves genitalia and skin below the waist. Reinfection can occur with exposure to the other type or even a second strain of the same type.
Primary genital herpes is characterized by painful, multiple, grouped vesicles or ulcerative and crusted external genital lesions on an erythematous base (see Table 116-2). In females, the cervix also is involved. Symptoms may include regional lymphadenopathy, discharge, and dysuria. Primary illness lasts 10 to 20 days with recurrences in 50% to 80% of patients. Secondary, recurrent, or reactivation eruptions are not as dramatic and are not associated with systemic symptoms. In primary infection, viral shedding lasts 10 to 14 days, and vesicles and ulcers resolve in 16 to 20 days. In recurrent disease, often with several episodes annually, virus shedding lasts for less than 7 days, and vesicles resolve in 8 to 10 days. Many persons experience five to eight recurrences per year. Some primary and many secondary lesions are asymptomatic.
Viral cultures are the most reliable method of diagnosis and show cytopathic effect in 2 to 5 days. The diagnosis is suggested by a Tzanck smear or Papanicolaou smear showing characteristic multinucleated giant cells with intranuclear inclusions. Latency develops as the virus becomes dormant in the sacral nerve ganglion. Serologic testing is useful only for primary infection, to show seroconversion between acute and convalescent sera. There is much overlap between types 1 and 2 in serologic tests for HSV. Titers are not helpful in guiding management of recurrences.
Oral acyclovir, famciclovir, and valacyclovir are effective treatments in reducing the severity and duration of symptoms in primary cases, and may reduce recurrences. Once-daily suppressive therapy reduces the frequency of genital herpes recurrences by 70% to 80% among patients who have frequent recurrences (>6 recurrences per year). Local hygiene and sitz baths may relieve some discomfort. The use of condoms provides some protection against sexual transmission of HSV.
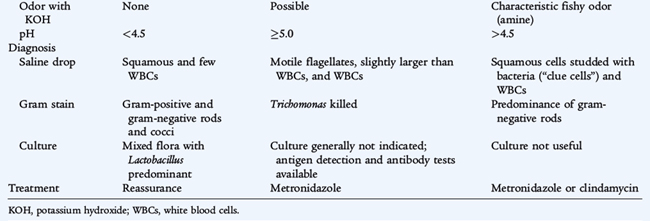
TRICHOMONIASIS (Trichomonas vaginalis)
Trichomoniasis is caused by the protozoan T. vaginalis and often is associated with other STIs such as gonorrhea and Chlamydia infection. Infected males either are asymptomatic or have nongonococcal urethritis. Infected females have vaginitis with thin, malodorous, frothy yellow-green discharge, vulvar irritation, and cervical “strawberry hemorrhages” (see Table 116-3). The diagnosis is based on visualization of motile, flagellated protozoans in the urine or in a saline wet mount, which has a sensitivity of only 60% to 70%. Culture is the most sensitive method of diagnosis. Single-dose treatment of both sexual partners with oral metronidazole (2 g) is recommended.
GENITAL WARTS (HUMAN PAPILLOMAVIRUSES)
HPV infections, the cause of genital warts (condylomata acuminata), may be the most common STI with an estimated prevalence of 20 million active infections and over 6 million new infections each year, mostly in 15- to 24-year-olds. Most HPV infections are asymptomatic or subclinical. HPV types 6 and 11 cause 90% of genital warts and are nononcogenic. HPV types 16 and 18 are associated with 70% of cases of cervical cancer. HPV types 31, 33, and 35 are also ongogenic.
Genital warts can occur on the squamous epithelium or mucous membranes of the genital and perineal structures of females and males (see Table 116-4). Genital warts are usually multiple, firm, gray-to-pink excrescences. Untreated genital warts may remain unchanged, increase in size or number, or resolve spontaneously. They can become tender if macerated or secondarily infected. The diagnosis usually is established by appearance without biopsy. The differential diagnosis of genital warts includes condylomata lata (secondary syphilis) and tumors.
The goal of treatment is removal of symptomatic warts to induce wart-free periods. On cornified skin, patient-applied therapies include podofilox solution or gel or imiquimod cream. Provider-applied therapies include cryotherapy with liquid nitrogen or cryoprobe, topical podophyllin resin, and trichloroacetic acid or dichloroacetic acid, all of which can be applied until the lesions regress. An alternative is surgical removal. Intralesional interferon and laser surgery also have been effective. Factors that may influence selection of treatment include wart number, size, anatomic sites, wart morphology, patient preference, cost of treatment, convenience, adverse effects, and provider experience. Recurrences after treatment are common and are commonly asymptomatic.
PUBIC LICE (Phthirus pubis)
Pubic lice, or pediculosis pubis, are caused by infestation with Phthirus pubis, the pubic crab louse. The louse is predominantly sexually transmitted and lives out its life cycle on pubic hair, where it causes characteristic, intense pruritus (see Table 116-4). Erythematous papules and egg cases (nits) are not seen before puberty. Treatment consists of education regarding personal and environmental hygiene and the application of an appropriate pediculicide, such as permethrin 1% cream, lindane 1% shampoo, or pyrethrins with piperonyl butoxide. Bedding and clothing should be decontaminated (machine washed and machine dried using the heat cycle or dry cleaned) or removed from body contact for at least 72 hours.