Bacterial Diseases
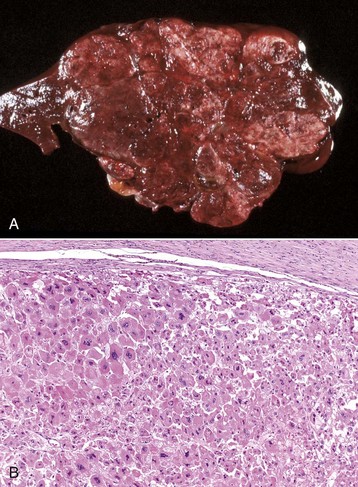
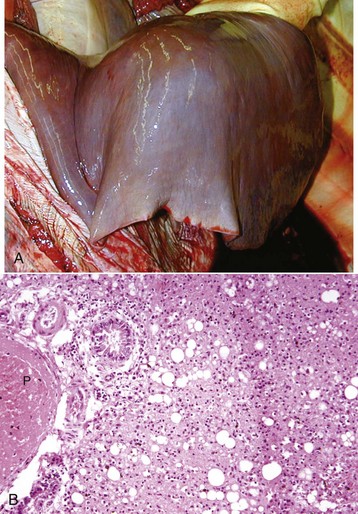
Liver Abscesses and Granulomas: Bacteria can reach the liver via a number of different routes and form abscesses (Figs. 8-41 to 8-43). Routes include the following:
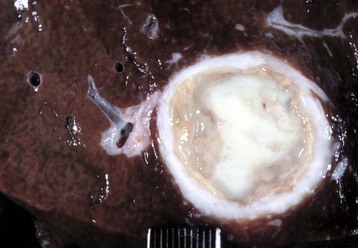
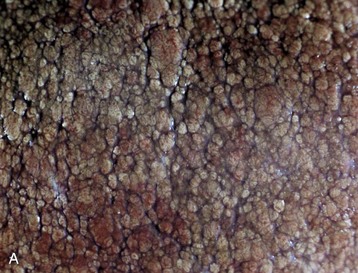
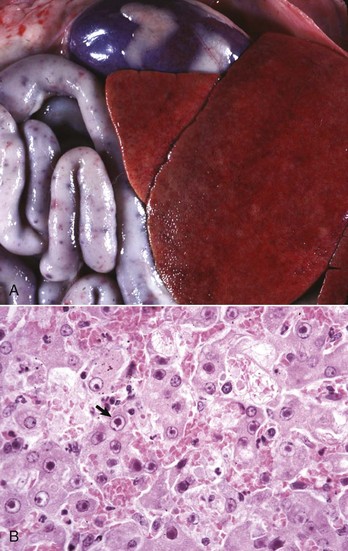
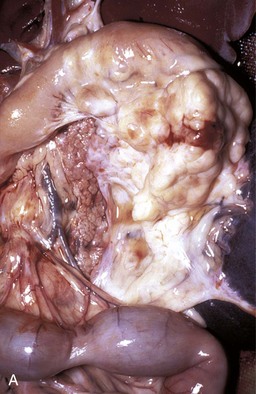
Fig. 8-41 Chronic hepatic abscesses, Corynebacterium pseudotuberculosis, liver, sheep.
Note the thick fibrous capsule and the characteristic pale caseous exudate produced by Corynebacterium pseudotuberculosis in sheep. (Courtesy College of Veterinary Medicine, North Carolina State University.)
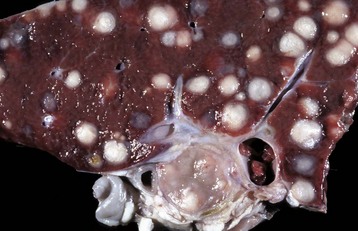
Fig. 8-42 Hepatic abscesses, Rhodococcus equi, liver, goat.
Disseminated hepatic abscesses in a goat caused by Rhodococcus equi. This lesion is more commonly found in foals. (Courtesy Dr. P. Stromberg, College of Veterinary Medicine, The Ohio State University.)
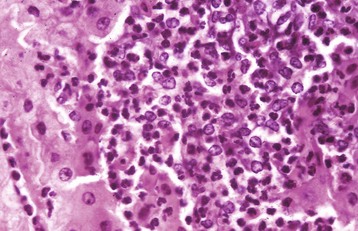
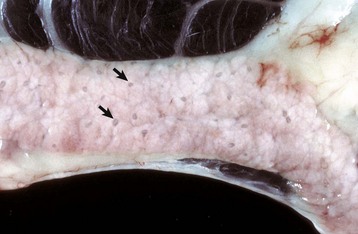
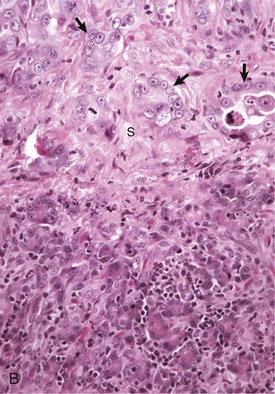
Fig. 8-43 Hepatic abscess, liver, cow.
An abscess in the liver is similar to those in other tissues and consists of an infiltrate of neutrophils, degenerating neutrophils, and necrotic tissue debris. H&E stain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
• The umbilical veins from umbilical infections in newborn animals
• The hepatic artery, as part of a generalized bacteremia
• Ascending infection of the biliary system
• Direct extension of an inflammatory process from tissues immediately adjacent to the liver, such as the reticulum
Both Gram-positive and Gram-negative organisms can cause hepatic abscesses. In adult small animals, hepatic abscesses are often caused by any of a variety of enteric species, as well as Francisella spp., Nocardia asteroides, and Actinomyces spp. Bacterial infections of the liver and subsequent formation of hepatic abscesses or foci of necrosis are especially common in neonatal foals and ruminants, in addition to feedlot cattle. In feedlot cattle, hepatic abscesses usually occur as a sequel to toxic rumenitis because damage to the ruminal mucosa allows ruminal microflora, particularly Fusobacterium necrophorum, to enter the portal circulation. After initially localizing within the liver, bacteria proliferate and produce focal areas of hepatocellular necrosis and hepatitis that can in time develop into hepatic abscesses (Fig. 8-44). Liver abscesses of cattle frequently are incidental lesions, but they can cause weight loss and decreased milk production. Less commonly, a hepatic abscess encroaches on the lumen of either a hepatic vein or the caudal vena cava. This can cause phlebitis that results in mural thrombosis, and because of the obstruction of the outflow to the venous drainage of the liver, passive congestion of the liver and portal hypertension can occur (Fig. 8-45). Detachment of portions of these mural thrombi can produce septic thromboemboli that lodge in the lungs. Rupture of hepatic abscesses directly into the hepatic vein or into the caudal vena cava occurs sporadically in cattle and may result in fatal septic embolization of the lungs. Sometimes death can be sudden from the blockage of large areas of pulmonary capillaries by the exudate. Hepatic abscesses derived from bacteria arriving via the portal vein may not be evenly distributed throughout the liver, possibly because of selective distribution of portal blood into different liver lobes, termed portal streaming. Occasionally, fungi, such as Mucor sp., that proliferate in areas of ruminal ulceration invade the portal circulation and are carried to the liver and there cause extensive areas of necrosis and inflammation (Fig. 8-46).
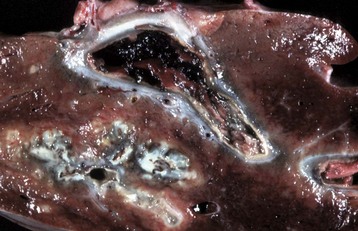
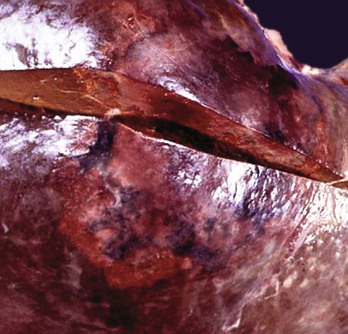
Fig. 8-44 Hepatic abscess, Fusobacterium necrophorum, liver, cow.
Foci of necrosis and abscess formation. Abscesses, such as this one, can erode the wall of a hepatic vein or the caudal vena cava, rupture, and release their contents into the bloodstream. (Courtesy Dr. P. Stromberg, College of Veterinary Medicine, The Ohio State University.)
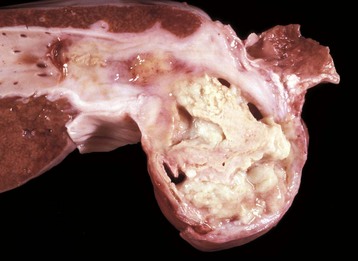
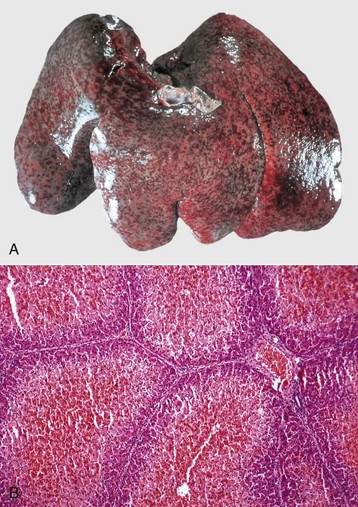
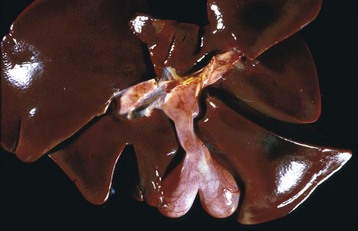
Fig. 8-45 Hepatic abscess, caudal vena cava, cow.
A hepatic abscess has eroded the wall of the vena cava, ruptured, and released its contents into the caudal vena cava. (Courtesy College of Veterinary Medicine, North Carolina State University.)
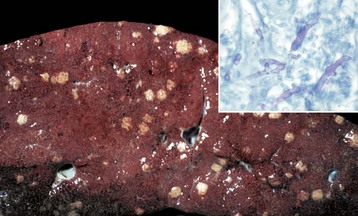
Fig. 8-46 Multiple necrotic foci, disseminated fungal infection (Mucor spp.), liver, cow.
Mucor spp. enter the portal blood after ulcerative rumenitis and cause focal necrosis and inflammation in the liver. Inset, The hyphae of the causative organism (pink) are usually evident within the granuloma. Periodic acid–Schiff (PAS) reaction. (Figure courtesy College of Veterinary Medicine, University of Illinois. Inset courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
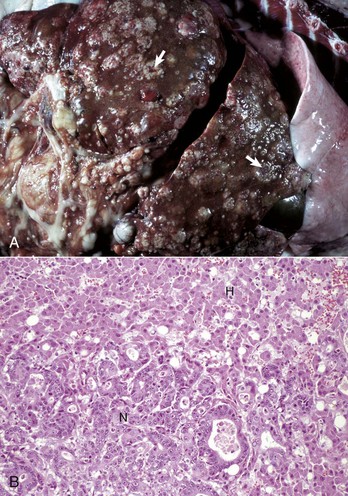
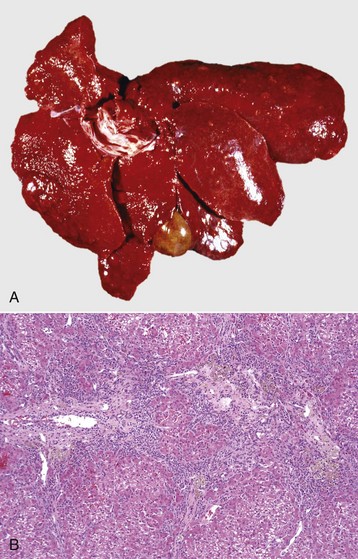
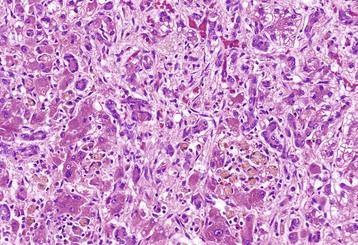
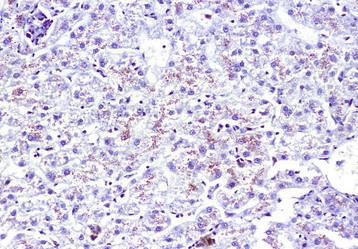
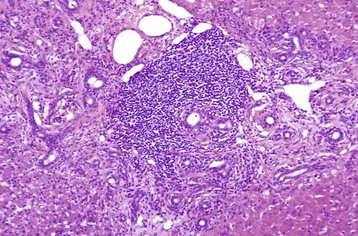
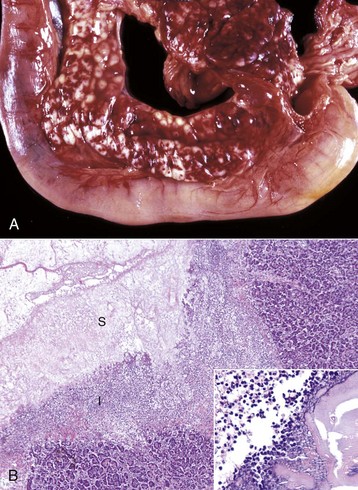
Tuberculosis (Mycobacterium bovis) has been eradicated from almost all of the United States (US), but its occurrence in other countries varies with the effectiveness of control efforts. The primary site of the disease is pulmonary with subsequent dissemination to other organs, including the liver. Other domestic animal species can be infected with Mycobacterium bovis, and it is also a zoonotic microbe. Mycobacterium avium-intracellulare complex can occur in domestic animals, especially dogs, in the southern areas of the US. Granulomas are randomly distributed (i.e., hematogenous spread) in the liver. They have a central core of cell debris, caseation, and granulomatous inflammation surrounded by a fibrous capsule (Fig. 8-47).
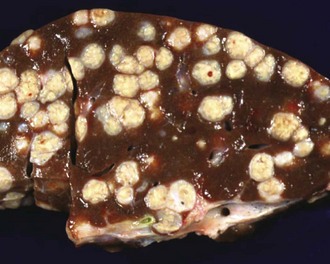
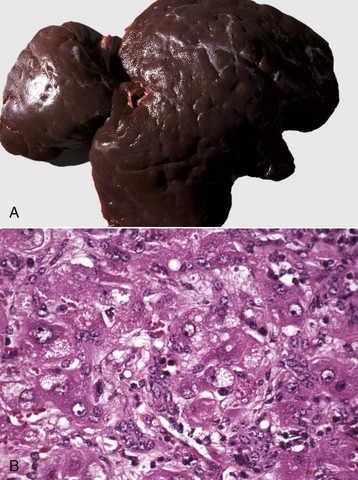
Fig. 8-47 Multiple caseous granulomas, tuberculosis, Mycobacterium bovis, liver, cow.
Hepatic tuberculosis is characterized by random multifocal pale white-to-yellow caseous granulomas on the capsular and cut surfaces. (Courtesy Dr. M. Domingo, Autonomous University of Barcelona; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
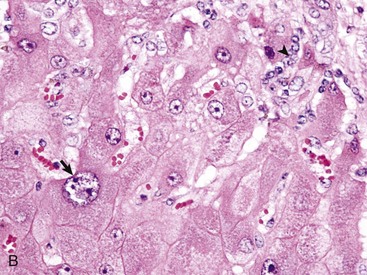
Tyzzer’s Disease: This disease is caused by Clostridium piliforme (formerly Bacillus piliformis), a Gram-negative obligate intracellular parasite. It is well recognized in laboratory animals but occurs only sporadically in domestic animals. Infection is most common in foals but has been described in calves, cats, and dogs and many other species. Typically, only very young or immunocompromised animals are affected. The bacteria are found in the intestinal tract of rodents. Infection is most likely through the oral route. The mechanisms of attachment and entry into host cells are unknown. After colonization of the gastrointestinal tract, organisms penetrate into the portal venous drainage and enter the liver. The disease is characterized by enlarged, edematous, and hemorrhagic abdominal lymph nodes, hepatic enlargement, and the presence of randomly distributed, pale foci of hepatocellular necrosis surrounded by a variably intense inflammatory infiltrate of neutrophils and mononuclear cells (Fig. 8-48, A). Diagnosis requires the demonstration of the characteristic, elongated large bacilli within viable hepatocytes at the margins of necrotic foci (Fig. 8-48, B). Silver stains, such as Warthin-Starry or Gomori’s silver stain, are frequently used for this purpose (Fig. 8-48, C).
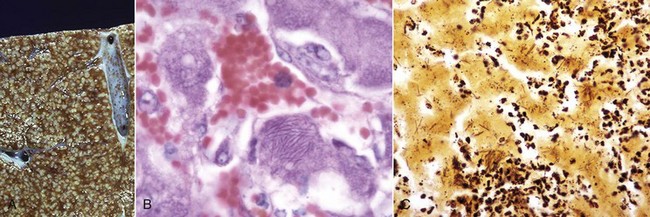
Fig. 8-48 Tyzzer’s disease (Clostridium piliforme).
A, Liver, horse. Disseminated gray-white 1- to 2-mm foci of necrosis surrounded by suppurative inflammation. B, Foal. Clostridium piliforme can be identified by the haphazard distribution of filamentous bacteria in the cytoplasm of hepatocytes. Giemsa stain. C, Foal. Clostridium piliforme can be readily seen with special stains such as Giemsa and Warthin-Starry. Warthin-Starry stain. (A courtesy Dr. R.C. Giles, University of Kentucky; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B and C courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Leptospirosis: Leptospirosis is caused by infection with the Gram-negative, thin, spiral, and motile bacterium of the genus Leptospira. There are two species, of which Leptospira interrogans is capable of causing disease in animals. The taxonomy of these organisms is complicated because there are more than 23 antigenically distinct pathogenic serogroups and 200 serovars. Each serovar can differ with respect to the species affected, organs affected, and severity of disease. Leptospires enter the body through the mucous membranes or through the skin if its barrier functions have been disrupted. Contaminated water, bedding, and soil are common sources of infection because the organism is shed in urine. Fetuses can develop transplacental infection and are often aborted. Infection can involve red blood cells, kidney, liver, and a number of other tissues, depending on the infecting serovar. The liver is often involved in acute, severe leptospirosis of all domestic species because a number of serovars cause intravascular hemolytic anemia leading to ischemic injury to centrilobular areas. Furthermore, organisms can be seen in large numbers in the liver after silver staining methods, although the direct effects of leptospira toxins on hepatocytes are less well established.
Gross lesions include icterus when animals are infected with serovars that produce hemolysis. Hepatic hemorrhage and ascites can occur, depending on the course of infection and the serovar involved. In some cases, acute infection can cause focal necrosis in addition to or instead of centrilobular necrosis. A common but nonspecific change in the liver of infected dogs is dissociation of hepatocytes. Affected cells become rounded and have eosinophilic granular cytoplasm and dark, shrunken hyperbasophilic nuclei. Bile casts in canaliculi are often apparent. Kupffer cells may contain abundant hemosiderin. Infection of dogs with Leptospira grippotyphosa has been reported to produce chronic (chronic-active) hepatitis, but it is unlikely that leptospira are involved in the pathogenesis of the majority of spontaneous cases of chronic hepatitis.
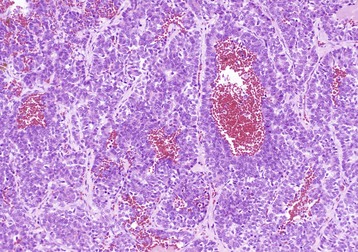
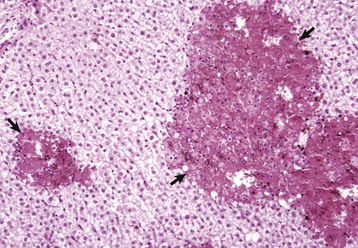
Other Bacterial Infections: These diseases are grouped together because they all arise from a bacteremia that occurs during a systemic infection. A comprehensive list of systemic infections that may produce hepatocellular necrosis and hepatitis is beyond the scope of this chapter, but examples include Yersinia pseudotuberculosis, Salmonella spp. (lesions present within the liver are discrete accumulations of mixed mononuclear inflammatory cells, which often are referred to as paratyphoid nodules) and Brucella spp. infection in many species (Fig. 8-49, A and B). Haemophilus agni and Pasteurella haemolytica can present as infections in sheep. Other infections include Arcanobacter pyogenes (Actinomyces pyogenes) of the bovine fetus and neonate, Campylobacter fetus ssp. fetus in fetal and neonatal lambs (Fig. 8-50), Actinobacillus equuli infection of neonatal foals, and Nocardia asteroides infection of dogs. Yersinia tularensis (Francisella tularensis), the cause of tularemia, can occur in cats and dogs. Bacterial infections such as these may produce lesions within the liver that range from small foci of hepatic necrosis to multiple, large abscesses. Determination of the specific causative agent often depends on bacterial isolation and characterization.
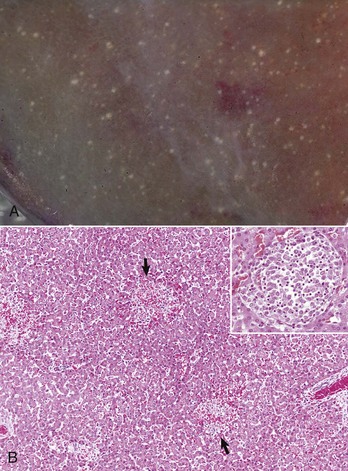
Fig. 8-49 Hepatic salmonellosis, liver.
A, Diaphragmatic surface, cow. Random 1- to 2-mm foci of focal necrosis in a cow with Salmonella septicemia. Multiple pale subcapsular foci of necrosis are evident. B, Pig, Later in the disease process, the necrotic foci are infiltrated by macrophages and form discrete granulomas termed paratyphoid nodules (arrows). H&E stain. Inset, Higher magnification of a paratyphoid nodule. H&E stain. (A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. Inset courtesy Dr. J. Simon, College of Veterinary Medicine, University of Illinois.)
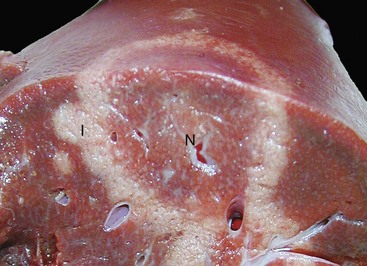
Fig. 8-50 Hepatic campylobacteriosis, multifocal necrotizing hepatitis, liver, capsular and cut surfaces, lamb fetus.
The lesion consists of a necrotic center (coagulation necrosis) (N), which in older lesions is distinctly tan and depressed. This center is surrounded by a white to gray rim of inflammatory cells (I). The cut surface (lower) illustrates the same changes and the extent of the necrosis into the hepatic parenchyma. (Courtesy Drs. C. Lichtensteiger and R. Doty, College of Veterinary Medicine, University of Illinois.)
Bacillary hemoglobinuria and infectious necrotic hepatitis, both due to species of Clostridia, are described in detail in the section on Disorders of Ruminants.
Protozoal Diseases
The liver can be involved in systemic infections with Toxoplasma gondii, Neospora sp., and other less common protozoa (Web Fig. 8-8). Liver lesions are usually characterized by multifocal necrosis and inflammation. Inflammatory cells include neutrophils, macrophages, and smaller numbers of other inflammatory cells. Free tachyzoites or cysts containing bradyzoites can be found within necrotic areas or adjacent to them. Although there are subtle physical differences between the organisms, immunologic testing, such as immunohistochemical staining, is a more reliable means to separate the two organisms.
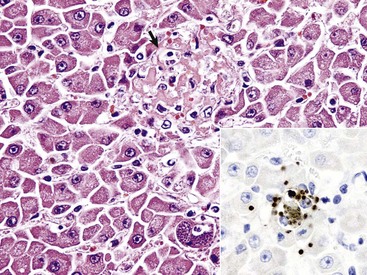
Web Fig. 8-8 Hepatitis with necrosis, toxoplasmosis, liver, cat.
Note the focus of acute hepatic necrosis (arrow). Several cysts filled with bradyzoites are present in a cell, likely a Kupffer cell. H&E stain. Inset, The brown oval structures are bradyzoites that have been labeled with DAB chromogen in an immunohistochemical reaction to confirm toxoplasmosis as the agent. IHC stain. (Courtesy Dr. K. Vashisht, College of Veterinary Medicine, University of Illinois.)
Dimorphic Fungal Diseases
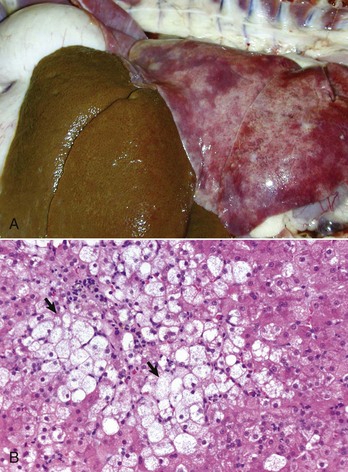
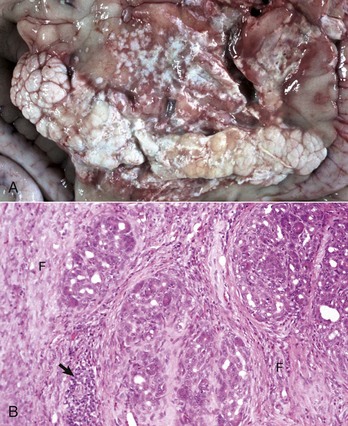
Systemic involvement with dimorphic fungi often includes the liver. There are several genera of fungi that may involve the liver including, Blastomyces, Coccidioides, Aspergillus, and Histoplasma. Histoplasmosis is a fungal disease that is endemic in the US and Canada and can occur occasionally in other areas. It is caused by Histoplasma capsulatum, a soil-dwelling organism. Dogs are affected most often. The route of infection is primarily through inhalation, although ingestion is also a possible route. In some circumstances pulmonary infections become disseminated and affect a variety of visceral organs, including the liver. Lesions in the liver consist of a multifocal distribution of granulomas with intralesional yeast forms of the organism. Numerous yeast forms can be found in the cytoplasm of macrophages and can be readily stained with the periodic acid–Schiff (PAS) reaction (Fig. 8-51, A and B).
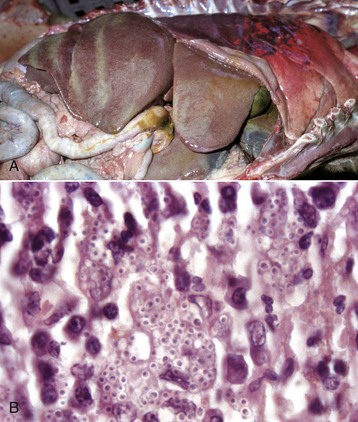
Fig. 8-51 Hepatic histoplasmosis, liver, dog.
A, In disseminated cases, Histoplasma capsulatum can involve the liver. Affected livers tend to be enlarged and pale mahogany from the diffuse hypertrophy and proliferation of Kupffer cells and macrophages. B, Note the yeast form of Histoplasma in the cytoplasm of Kupffer cells and macrophages. H&E stain. (A courtesy College of Veterinary Medicine, University of Illinois. B courtesy Dr. J. Simon, College of Veterinary Medicine, University of Illinois.)
Parasitic Diseases
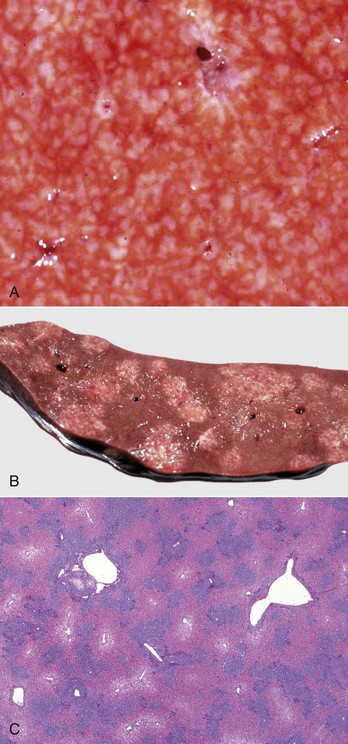
Nematodes: Migration of larvae through the liver is a common component of a nematode’s life cycle in domestic animals. As larvae travel through the liver, they produce local tracts of hepatocellular necrosis that are accompanied by inflammation. These tracts are eventually replaced with connective tissue that matures into fibrous scars and which are especially prominent on the capsular surface (Fig. 8-52). These capsular scars appear as pale areas, and the term milk-spotted liver has been used to describe livers in pigs scarred by migrating larvae of Ascaris suum. Larvae occasionally become entrapped within the liver or its capsule and are walled off within abscesses or granulomas. Examples of chronic hepatitis or hepatic scarring as a consequence of larval migration include migration of ascarids in several species of domestic animals, such as Stephanurus dentatus in pigs, and Strongylus sp. in the horse. Infection of the liver with adult nematodes is considerably less common than larval migration. Calodium hepatica occasionally may be found in the hepatic parenchyma of dogs and cats where the ova provoke granulomatous inflammation.
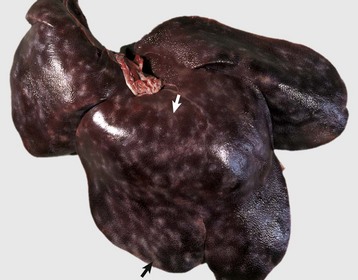
Fig. 8-52 Capsular and portal fibrosis (milk-spotted liver), Ascaris suum larval migration, liver, diaphragmatic surface, pig.
Fibrous tissue (scars) has been deposited in the migration tracks of the ascarid larvae and in adjacent portal areas (arrows). (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Dogs with heartworm infection (Dirofilaria immitis) occasionally develop vena caval syndrome, also known as the postcaval syndrome, which is characterized by DIC, intravascular hemolysis, and acute hepatic failure. The syndrome typically occurs in dogs with large numbers of adult worms in the vena cava and their more usual location within the right side of the heart and pulmonary artery (Fig. 8-53). The liver is engorged with blood as a consequence of severe passive congestion from the partial blockage of the caudal vena cava. It is proposed that mechanical factors produced by the presence of large numbers of worms in the right atrium or caudal vena cava are the cause of intravascular hemolysis, which characterizes vena caval syndrome, although other theories suggest that there may be a hypersensitivity reaction to antigens released by the worms.
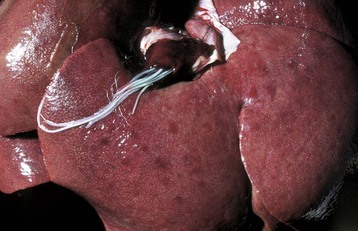
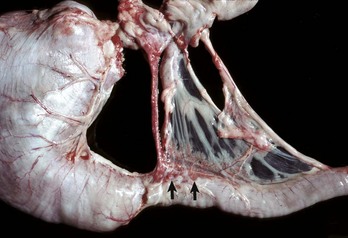
Fig. 8-53 Dirofilariasis, vena caval syndrome, caudal vena cava at the level of the liver, dog.
Large collections of adult Dirofilaria immitis are present in the caudal vena cava. The condition is rapidly fatal unless the nematodes are removed. (Courtesy Dr. C.S. Patton, College of Veterinary Medicine, University of Tennessee.)
Cestodes: A number of cestodes occur within the hepatobiliary system of domestic animals. Those cestode parasites of greatest clinical significance develop encysted forms within the liver of the intermediate hosts. The most important are larval cestodes of the genus Taenia; adults inhabit the gastrointestinal tract of carnivores and usually are innocuous to their definitive host. The ova ingested by an intermediate host develop into embryos, which penetrate the wall of the gut and then are distributed via the blood to virtually any site in the body. Parasitic cysts develop within the tissue of the intermediate host, and the life cycle of the parasite is completed when the cysts are ingested by the definitive host. Although the liver is but one organ in the intermediate host that may be affected, hepatic involvement is common because portal blood, in which embryos migrate, drains into the liver before flowing to the systemic circulation.
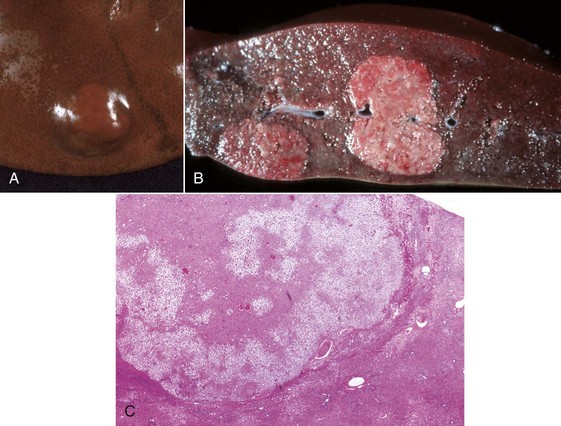
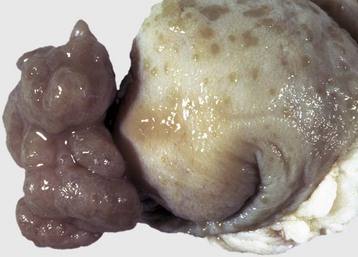
The adult cestode Taenia hydatigena shows up in the small intestine of dogs, whereas its intermediate stage, Cysticercus tenuicollis, appears in the peritoneal cavity of a variety of species, including horses, ruminants, and pigs (Fig. 8-54). Immature cysticerci migrate in the liver and can induce extensive damage if infection is heavy; lesions present are comparable to those induced by migration of immature Fasciola hepatica.
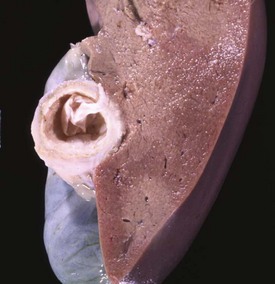
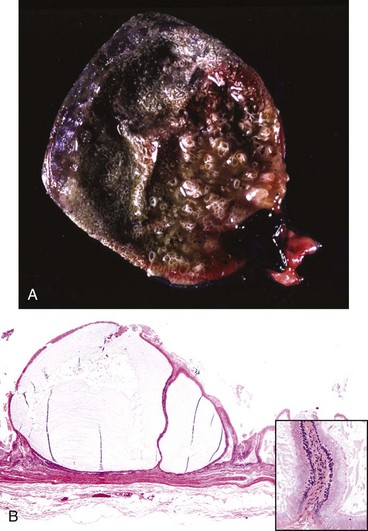
Fig. 8-54 Cysticercosis, liver, cut surface, sheep.
The thick fibrous capsule usually indicates the death of the larva. (Courtesy Dr. K. Read, College of Veterinary Medicine, Texas A&M University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Hydatid liver disease is common in some countries. Echinococcus granulosus is a cestode that parasitizes canids as the definitive host, and hydatid cysts can develop in many different intermediate host animal species, including humans. The dog-sheep cycle is most important in many geographic areas. Pastured cattle are also commonly affected in other geographic locations. Adult worms in the intestines of dogs pass proglottids into the dog’s stool and thereby contaminate pastures. Ova are then ingested by sheep, cattle, or other species. Embryos may develop into hydatid cysts in virtually any organ in the intermediate host, but the liver and lungs are commonly affected. These cysts are usually less than 10 cm in diameter but can attain quite a spectacular size, particularly in humans. Hydatid cysts, even when present in large numbers, rarely cause overt clinical signs of disease in domestic animals.
Cestode adults occurring within the hepatobiliary system include Stilesia hepatica, Stilesia globipunctata, and Thysanosoma actinoides, all of which can inhabit the bile duct of ruminants. Infections with these parasites may result in chronic inflammation of the biliary tract, but they usually do not produce clinical signs of hepatic dysfunction.
Trematodes: The majority of parasitic hepatic injury caused by trematodes is produced by members of three major families. These include the families Fasciolidae, Dicrocoelidae, and Opisthorchidae.
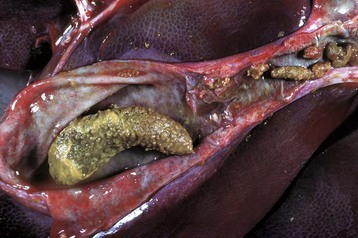
The principal liver fluke disease of sheep and cattle and occasionally other species is caused by Fasciola hepatica. Hepatic fascioliasis occurs throughout the world in areas where climatic conditions, typically in low swampy areas, are suitable for the survival of aquatic snails, which serve as intermediate hosts for the parasites. Adult Fasciola hepatica are leaf-shaped parasites that inhabit the biliary system; their eggs pass via the bile to the intestinal tract and eventually are passed in the feces. Larvae (miracidium) then must develop in the snail intermediate host (genus Lymnaea). Cercariae that leave the snail encyst on herbage where they develop into infectious metacercariae. Metacercariae are ingested by the ruminant host and penetrate the wall of the duodenum to enter the peritoneal cavity and subsequently enter the liver. They migrate within the liver before taking up residence within the bile ducts. Migration of immature flukes through the liver produces hemorrhagic tracts of necrotic liver parenchyma. These tracts are grossly visible and in acute infection are dark red, but with time become paler than the surrounding parenchyma. Repair is often by fibrosis. A variety of untoward sequelae can follow these migrations, including acute peritonitis; hepatic abscesses; death of the host as a consequence of acute, widespread hepatic necrosis produced by a massive infiltration of immature flukes; and the proliferation of spores of Clostridium haemolyticum or Clostridium novyi in necrotic tissue, which causes the subsequent development of bacillary hemoglobinuria or infectious necrotic hepatitis, respectively.
Mature flukes reside in the larger extrahepatic and intrahepatic bile ducts and cause cholangitis. Chronic cholangitis and bile duct obstruction lead to ectasia and stenosis of the ducts and periductular fibrosis that thickens the walls so that the ducts become increasingly prominent. Mineralization may occur producing the classic “pipestem” appearance of diseased bile ducts. The contents of the bile ducts are often dark brown and viscous caused by a combination of abnormal bile, cellular debris, and the iron-porphyrin pigment excreted by the flukes. Obstruction of the ducts leads to cholestasis. Animals with chronic liver fluke disease are often in poor body condition.
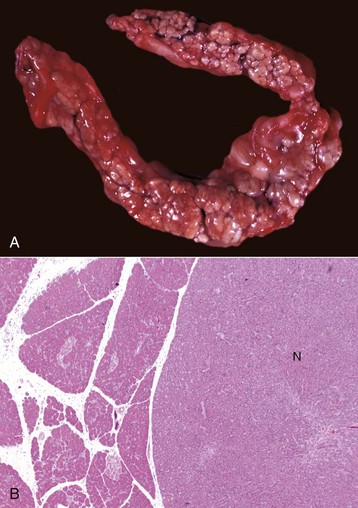
Fasciola gigantica and Fascioloides magna are important causes of liver fluke disease of ruminants in some parts of the world. Fasciola gigantica is most common in areas of Africa and surrounding countries, and Fascioloides magna is found in North America. The adults of Fasciola gigantica and Fasciola hepatica reside in the bile ducts (Fig. 8-55). In contrast, adult Fascioloides magna, whose normal hosts are elk and white-tailed deer, reside in the hepatic parenchyma in aberrant hosts, such as cattle and sheep. In cattle, the immature Fascioloides magna flukes cause extensive tissue damage as they migrate through the liver (Fig. 8-56), but the adults are enclosed by fibrous connective tissue in cysts containing a black fluid. In sheep and goats, the flukes continuously migrate through the liver, causing extensive damage and eventual death.
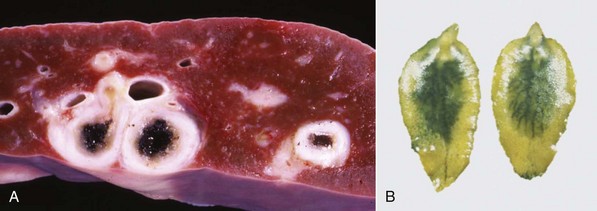
Fig. 8-55 Fasciola hepatica infection.
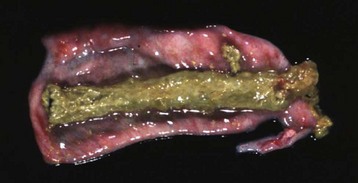
A, Chronic intrahepatic cholangitis (Fasciola hepatica), liver, cow. When Fasciola hepatica metacercariae are ingested, they migrate to the liver and then take up residence within the bile ducts. Mature flukes reside in the larger extrahepatic and intrahepatic bile ducts and cause chronic cholangitis and bile duct obstruction that lead to ectasia and stenosis of the ducts and periductular fibrosis that thickens the walls so that the ducts become increasingly prominent, as shown here. B, Adult Fasciola hepatica are leaf-shaped flukes that inhabit the biliary system; their eggs pass via the bile into the intestinal tract and eventually are passed in the feces. (A courtesy Dr. K. Read, College of Veterinary Medicine, Texas A&M University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B courtesy Dr. T. Boosinger, College of Veterinary Medicine, Auburn University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
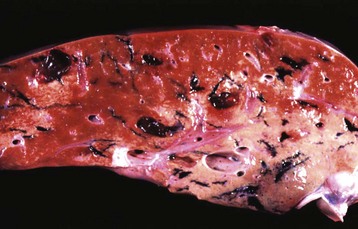
Fig. 8-56 Fluke migration tracts, fascioloidiasis, liver, cow.
Migration of Fascioloides magna through the bovine liver produces extensive parenchymal damage. A black excretory pigment deposited by the fluke discolors the migration tracks black. (Courtesy Dr. J. Wright, College of Veterinary Medicine, North Carolina State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Other trematodes that may inhabit the bile ducts include Dicrocoelium dendriticum in horses, ruminants, pigs, dogs, and cats; Eurytrema pancreaticum and Eurytrema coelomaticum in ruminants; Opisthorchis tenuicollis in pigs, dogs, and cats and Opisthorchis felineus in dogs and cats; Pseudamphistomum truncatum, Metorchis conjunctus, Metorchis albidus, Parametorchis complexus, Concinnum procyonis, and Platynosomum fastosum in dogs and cats. All are capable of inducing changes similar to but usually considerably milder than those caused by Fasciola hepatica. In addition, they occasionally cause obstruction of the biliary ducts.
Cats, and less often dogs, can develop pronounced chronic cholangitis from infections with flukes, most often Opisthorchiidae. Microscopically, larger intrahepatic bile ducts are dramatically thickened by concentric fibrosis and the duct lumen is usually dilated, often with papillary projections of biliary epithelium into the lumen (Fig. 8-57). A mild-to-moderate inflammatory infiltrate of neutrophils and macrophages is often found in and around the ducts, and the portal tracts are infiltrated by neutrophils, lymphocytes, and plasma cells. Eosinophils are generally uncommon. It is often difficult to detect adult flukes or ova in affected animals.
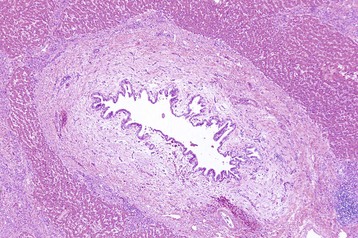
Fig. 8-57 Chronic intrahepatic cholangitis, liver, cat.
Fluke infections of the biliary tree of cats produce a characteristically pronounced periductular fibrosis, dilated bile duct, and papillary projections of biliary epithelium, although flukes may be difficult to find. H&E stain. (Courtesy Dr. J.M. Cullen, College of Veterinary Medicine, North Carolina State University.)
Dogs can be infected with the schistosome Heterobilharzia americana, which is normally a parasite in raccoons. Ova shed into water by infected raccoons release miracidia, which penetrate host snails. Dogs become infected when their skin is penetrated by cercariae, which are released from the intermediate snail hosts. Granulomatous lesions of the liver, pancreas, intestines, and mesentery result when ova released by adult schistosomes lodge in affected tissue and incite an inflammatory reaction.