Endocrine System*
Structure and Function
Endocrine glands are collections of specialized cells that synthesize, store, and directly release their secretions, such as polypeptides, steroids, and amino acid derivatives—including catecholamines and thyroid hormones—into the bloodstream, resulting in physiologic effects on target cells distant from the glands. They are sensing and signaling devices located in the extracellular fluid compartment that are capable of responding to changes in the internal and external environments to coordinate a multiplicity of activities that maintain homeostasis.
Signaling molecules are grouped into three general categories according to the source of the signal and the location of the target on which the signal has an effect (Fig. 12-1). In autocrine signaling, cells respond to signals that they themselves secrete. Molecules produced by one cell that act on a neighboring cell are characteristic of paracrine signaling. The final pattern of signaling, which is the focus of this chapter, is endocrine signaling, whereby hormones produced by cells of endocrine organs are released into the circulation and act on distant target cells.
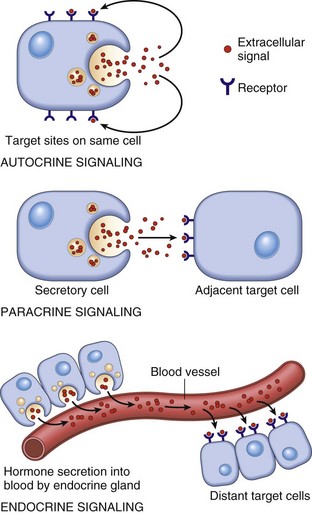
Fig. 12-1 Schematic diagram of the patterns of intercellular signaling (see text). (Modified from Lodish H, Baltimore D, Berk A, et al, editors: Molecular cell biology, ed 3, New York, 1995, WH Freeman.)
Endocrine cells that produce polypeptide hormones have a well-developed, rough endoplasmic reticulum that assembles the hormone, and a prominent perinuclear Golgi apparatus that packages the hormone into granules for intracellular storage and transport. Secretory granules are unique to polypeptide hormone- and catecholamine-secreting endocrine cells and provide a mechanism for intracellular storage of substantial amounts of preformed active hormone. When the cell receives a signal to secrete a hormone, secretory granules are moved to the periphery of the endocrine cell, most likely by the contraction of microfilaments. After release of the peptides into the bloodstream, they bind to receptors on the surface of target cells, activating a series of intracellular events often mediated by second messengers such as cyclic adenosine monophosphate (cAMP), protein kinases, or calcium.
Steroid hormone–secreting cells are characterized by large cytoplasmic lipid bodies that contain cholesterol and other precursor molecules. The lipid bodies are in close proximity to an extensive smooth endoplasmic reticulum and large mitochondria. The latter have hydroxylase and dehydrogenase enzyme systems that attach various side chains to the basic steroid nucleus. Steroid-producing cells lack secretory granules and do not store significant amounts of preformed hormone. They depend on continued biosynthesis to maintain the normal secretory rate of a particular hormone. Steroid hormones enter target cells by diffusion across the plasma membrane and then bind to nuclear or cytosolic receptors.
In general, endocrine organs are composed of islands of secretory epithelial cells delineated by a fine fibrovascular stroma rich in capillaries. With the exception of thyroid follicular epithelial cells, endocrine cells are arranged in cords or packets. Endocrine cells that secrete polypeptide hormones and catecholamines typically contain abundant, lacy to finely granular, pale eosinophilic cytoplasm, which is immunoreactive for chromogranins and synaptophysin that are present in secretory granules and microvesicles, respectively. Steroid hormone–secreting endocrine cells also contain abundant cytoplasm that appears foamy because of the presence of numerous lipid vacuoles.
Pituitary Gland (Hypophysis)
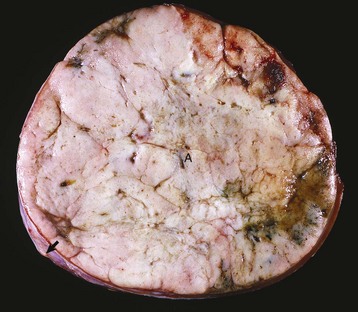
The adenohypophysis consists of three portions: the pars distalis, pars tuberalis, and pars intermedia (Fig. 12-2). In many animal species, the adenohypophysis completely surrounds the pars nervosa of the neurohypophyseal system. The pars distalis is the largest and is composed of several different endocrine cell populations surrounded by abundant capillaries to facilitate secretion of their trophic hormones (Web Fig. 12-1).
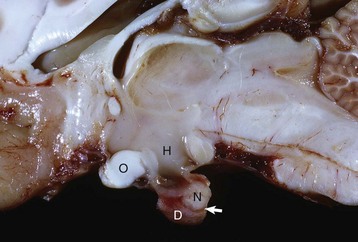
Fig. 12-2 Pituitary gland and brainstem, normal dog.
Longitudinal section of the pituitary region illustrating the close relationship to the optic chiasm (O), hypothalamus (H), and overlying brain. The pars distalis (D) forms a major part of the adenohypophysis and completely surrounds the pars nervosa (N). The residual lumen of Rathke’s pouch (arrow) separates the pars distalis and pars nervosa and is lined by the pars intermedia. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
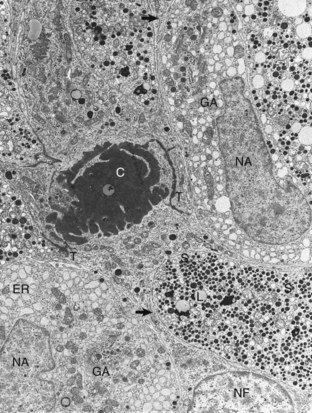
Web Fig. 12-1 Pituitary gland, pars distalis, normal dog.
Follicular cells (NF) in the pars distalis form a framework and extend cytoplasmic processes (arrows) around extracellular accumulations of colloid (C). Adjacent follicular cells are joined by prominent terminal bars (T). Acidophils in the storage phase of the secretory cycle contain numerous large, uniformly electron-dense secretory granules (S), scattered lipofuscin (L) bodies, a small amount of endoplasmic reticulum (ER), and a small Golgi apparatus. Hypertrophied acidophils (NA) have few mature secretory granules but many distended profiles of endoplasmic reticulum and large Golgi apparatuses (GA) associated with prosecretory granules in the process of formation. TEM. Uranyl acetate and lead citrate stain. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
The pars tuberalis is an extension of the adenohypophysis and consists of dorsal projections of parenchymal cells along the infundibular stalk. It primarily functions as a scaffold for the capillary network of the hypophyseal portal system as it courses from the median eminence to the pars distalis. The pars intermedia is located between the pars distalis and pars nervosa; it lines the residual lumen of Rathke’s pouch and has two populations of cells in certain species. In the dog, one of these cell types (B cell) synthesizes and secretes adrenocorticotropic hormone (ACTH).
A specific population of endocrine cells is present in the pars distalis (and also in the pars intermedia of dogs for ACTH secretion) that synthesizes, processes, and secretes each of the pituitary trophic hormones (Fig. 12-3). Secretory cells in the adenohypophysis are classified as acidophils, basophils, and chromophobes based on the reactions of their secretory granules with pH-dependent histochemical stains (Fig. 12-4). Based on contemporary specific immunohistochemical staining, acidophils can be further subclassified functionally into somatotrophs that secrete growth hormone (GH; somatotrophin) and lactotrophs that secrete prolactin. Basophils include gonadotrophs that secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thyrotrophs that secrete thyrotrophic hormone (thyroid-stimulating hormone [TSH]), and ACTH-secreting corticotrophs. Chromophobes are pituitary cells that by light microscopy lack stainable cytoplasmic secretory granules. They include the pituitary cells involved with the synthesis of ACTH and melanocyte-stimulating hormone (MSH) in some species, nonsecretory follicular (stellate) cells, degranulated chromophils (acidophils and basophils) in the actively synthesizing phase of the secretory cycle, and undifferentiated stem cells of the adenohypophysis.
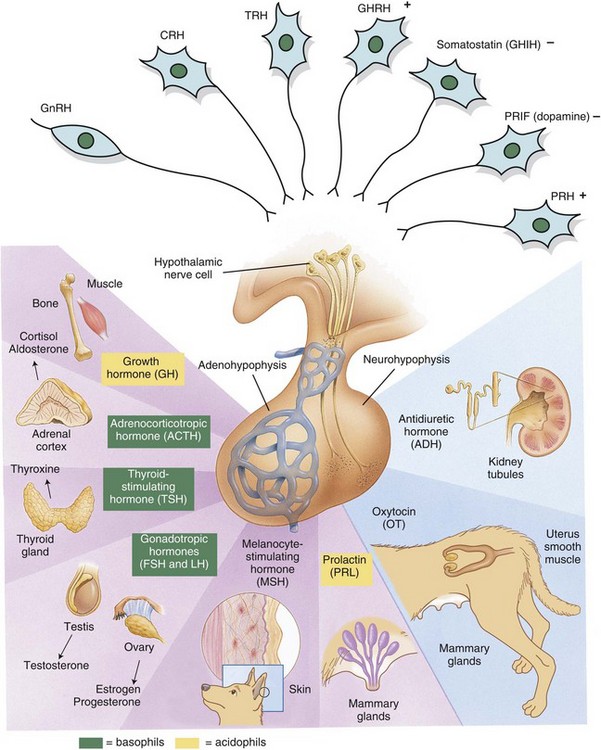
Fig. 12-3 Hypothalamic-pituitary-target gland axis.
Releasing hormones produced by the hypothalamus act on anterior or posterior portions of the pituitary gland to release trophic hormones. Trophic hormones act on specific endocrine glands, stimulating them to produce hormones that exert ultimate actions on downstream tissues. CRH, Corticotropin-releasing hormone; FSH, follicle-stimulating hormone; GHIH, growth hormone–inhibiting hormone; GHRH, growth hormone–releasing hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; PRH, prolactin-releasing hormone; PRIF, prolactin release–inhibiting factor; TRH, thyrotropin-releasing hormone. (Modified from Huether SE, McCance KL: Understanding pathophysiology, ed 2, St Louis, 2000, Mosby; and Squire L, Bloom F, McConnell S: Fundamental neuroscience, ed 2, San Diego, 2003, Academic Press.)
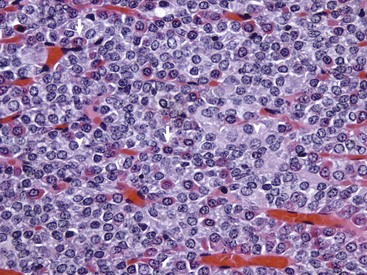
Fig. 12-4 Pars distalis, normal dog.
The pars distalis is composed of acidophils (arrows), basophils (none shown here) and chromophobes (arrowheads). H&E stain. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Each type of endocrine cell in the adenohypophysis is under the control of a specific releasing hormone or factor from the hypothalamus (see Fig. 12-3). These releasing hormones are small peptides synthesized and secreted by neurons of the hypothalamus. They are transported by axonal processes to the median eminence, where they are released into capillaries and conveyed by the hypophyseal portal system to specific endocrine cells in the adenohypophysis. Each hormone stimulates the rapid release of secretory granules containing a specific preformed trophic hormone.
The neurohypophysis is subdivided into three anatomic parts. The pars nervosa (posterior lobe) represents the distal component of the neurohypophyseal system. The infundibular stalk joins the pars nervosa to the overlying hypothalamus and is composed of axonal processes from neurosecretory neurons. It also has numerous capillaries, supported by modified glial cells or pituicytes, which are termination sites for the nonmyelinated axonal processes of neurosecretory neurons in the hypothalamus. The neurohypophyseal hormones, oxytocin and antidiuretic hormone (ADH) or vasopressin, are synthesized in the cell body of hypothalamic neurons, packaged into secretory granules, transported by long axonal processes of the hypothalamohypophyseal tract to axons in the pars nervosa, and released into the capillary bed of the hypothalamohypophyseal portal system.
Neurosecretory neurons in the hypothalamus secrete hormone in response to neural input from higher centers, resulting in hormonal secretion. ADH and oxytocin are nonapeptides synthesized by neurons situated either in the supraoptic or paraventricular nuclei of the hypothalamus. ADH and its corresponding neurophysin appear to be synthesized as part of a common larger biosynthetic precursor molecule, termed propressophysin. The hormones are packaged with a corresponding binding protein (i.e., neurophysin) into membrane-limited neurosecretory granules and transported to the pars nervosa for release into the circulation.
Adrenal Gland
The adrenal glands of mammals consist of two distinct parts that differ not only in morphology and function but also in embryologic origin. Because of their close structural relationships, the outer cortex and inner medulla of the adrenal glands usually have been considered parts of one organ (Fig. 12-5). The adrenal cortex develops from cells of the coelomic epithelium that are of mesodermal origin. The chromaffin tissue and sympathetic ganglion cells of the adrenal medulla are derived from ectoderm of the neural crest.
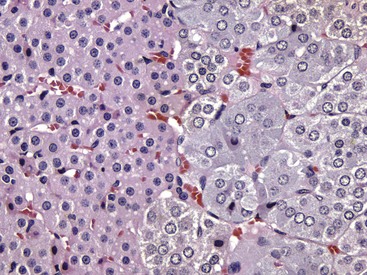
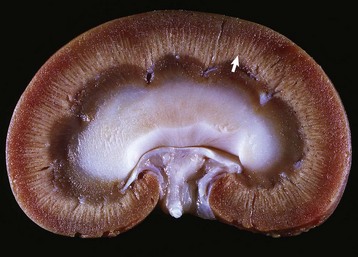
Fig. 12-5 Adrenal gland, normal dog.
Interface between the finely vacuolated (lipid droplets) cells of the adrenocortical zona reticularis (left) and chromaffin cells of the adrenal medulla (right). H&E stain. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
The adrenal cortex of normal dogs is firm, yellow, and nearly uniformly thick. The soft, brown medulla is surrounded by the cortex. In normal dogs, the cortical-to-medullary ratio is approximately 2 : 1. The adrenal glands are richly vascularized, and a sinusoidal network, which demarcates the cell columns of the adrenal cortex, empties into the venous tree at the periphery of the medulla.
The adrenal cortex microscopically and functionally is subdivided into three layers or zones, although the demarcation between zones often is not distinct. The zona glomerulosa or multiformis (outer zone) adjacent to the capsule is composed of columns of cells that have a sigmoid or arclike arrangement. It represents about 15% of the cortical volume and is responsible for the secretion of mineralocorticoid hormones. The secretory cells of the zona fasciculata (middle zone) are arranged in long anastomosing cords separated by numerous small capillaries. This zone constitutes about 80% of the cortical volume, is composed of cells that contain abundant cytoplasmic lipid, and is responsible for the secretion of the glucocorticoid hormones. The zona reticularis (inner zone) accounts for the remaining 5% of the cortical volume. The secretory cells, arranged in small groups surrounded by capillaries, are responsible for the secretion of sex steroids.
Mineralocorticoids are adrenal steroids that principally affect ion transport by epithelial cells and cause excretion of potassium and conservation of sodium. The most potent and important naturally occurring mineralocorticoid is aldosterone. Enzymatically controlled electrolyte pumps in epithelial cells of the renal tubule and sweat glands respond to mineralocorticoids by conserving sodium and chloride and by excreting potassium. In the distal convoluted tubule of the mammalian nephron, a cation-exchange mechanism is responsible for the resorption of sodium from the glomerular filtrate and secretion of potassium into the lumen (Fig. 12-6). These reactions are accelerated by mineralocorticoids but still proceed, although at a much slower rate in their absence. Lack of mineralocorticoid secretion, such as in the Addison’s-like disease of dogs, can result in lethal retention of potassium and loss of sodium.
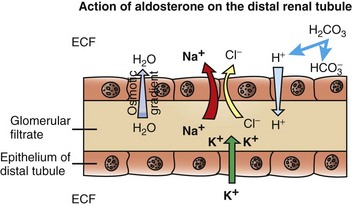
Fig. 12-6 Aldosterone secreted by the zona glomerulosa of the adrenal cortex acts on the distal portions of the nephron to increase tubular excretion of potassium and increase resorption of sodium (and secondarily of chloride).
The resulting osmotic gradient facilitates movement of water from the glomerular filtrate into the extracellular fluid (ECF). (Redrawn with permission from Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Cortisol and lesser amounts of corticosterone are the most important naturally occurring glucocorticoid hormones secreted by the adrenal glands in many animal species. In general, the actions of glucocorticoids on carbohydrate, protein, and lipid metabolism result in sparing of glucose, a tendency to hyperglycemia, and increased glucose production. In addition, glucocorticoids decrease lipogenesis and increase lipolysis in adipose tissue, which results in release of glycerol and free fatty acids.
Glucocorticoids also function to suppress both inflammatory and immunologic responses, thereby reducing the necrosis and fibroplasia that can occur with these responses. However, under the influence of increased concentrations of glucocorticoids, an animal has reduced resistance to bacteria, viruses, and fungi. Glucocorticoids can impair the immunologic response at any stage from the initial interaction and processing of antigens by cells of the monocyte-macrophage system through the induction and proliferation of immunocompetent lymphocytes and subsequent antibody production. Inhibition of a number of functions of lymphoid cells by glucocorticoids forms part of the basis for immunosuppression.
Glucocorticoids exert a profound negative effect on wound healing. Dogs with hypercortisolism can have wound dehiscence (Fig. 12-7). The basic mechanism is inhibition of fibroblast proliferation and collagen synthesis, leading to a decrease in scar tissue formation.
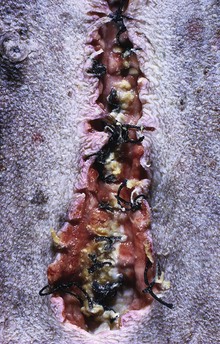
Fig. 12-7 Dehiscence of surgical wound, skin, ventral abdomen, dog.
Wounds heal slowly in dogs with cortisol excess because of an inhibition of fibroblastic proliferation. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Sex hormones (e.g., progesterone, estrogens, and androgens) are synthesized in small amounts by secretory cells of the zona reticularis of the adrenal cortex. Excessive adrenal sex steroids secreted by a neoplasm arising in the zona reticularis can occur infrequently, resulting in clinical manifestations of virilism, precocious sexual development, or feminization (effects depend on which steroid is secreted in excess, the sex of the patient, and the age at onset).
Adrenal Medulla
The adrenal medulla is derived from neuroectoderm of the neural crest and produces catecholamine hormones. The main biosynthetic pathway for catecholamines in mammals starts with tyrosine that is converted first to 1-dihydroxyphenylalanine (dopa) by tyrosine hydroxylase. Dopa is then decarboxylated by 3,4-amino acid decarboxylase to 3,4-dihydroxyphenylethylamine (dopamine), which subsequently undergoes β-hydroxylation by dopamine β-oxidase to form norepinephrine. In mammals the medulla is completely surrounded by the adrenal cortex, and venous blood from the cortex bathes the medullary cells. This blood has the greatest concentration of corticosteroids of any fluid in the body. This close anatomic association between the adrenal cortex and medulla in mammals is not fortuitous because the N-methylating enzyme, phenylethanolamine-N-methyl transferase, which converts norepinephrine to epinephrine, is corticosteroid hormone dependent.
Thyroid Gland
The thyroid gland in most animal species has two lobes, one on each lateral surface of the trachea. In pigs, the main lobe of the thyroid gland is on the midline in the ventral cervical region with dorsolateral projections from each side. In dogs, the right lobe of the thyroid gland is situated slightly cranial to the left lobe and almost touches the caudal aspect of the larynx.
The thyroid gland is the largest of the endocrine organs that function exclusively as endocrine glands. The basic histologic structure of the thyroid gland is unique among endocrine glands and consists of follicles of varying size (20 to 250 µm), which contain colloid produced by the follicular cells. The follicular cells are cuboidal to columnar and are orientated so that their secretory pole is directed toward the lumen of the follicle. An extensive network of interfollicular and intrafollicular capillaries provides the follicular cells with an abundant blood supply. Follicular cells have extensive profiles of rough endoplasmic reticulum for synthesis and a large Golgi apparatus for packaging of substantial amounts of protein, which are then transported into the follicular lumen. The luminal side of follicular cells in contact with the colloid has numerous microvilli (Web Fig. 12-2).
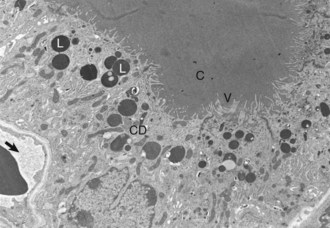
Web Fig. 12-2 Thyroid follicular cells, thyroid gland, normal dog.
Thyroid follicular cells with long microvilli (V) that extend into the colloid (C) within the follicular lumen. Numerous lysosomes (L) and colloid droplets (CD) are present in the apical portion of the follicular cells. An interfollicular capillary (arrow) is present at the base of the follicle. TEM. Uranyl acetate and lead citrate stain. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
The synthesis of thyroid hormones is unique among those of the endocrine glands because the final assembly of hormone occurs extracellularly within the follicular lumen. Follicular cells trap essential raw materials, such as iodide from the blood, by a sodium-iodide symporter in the basolateral plasma membrane and then transport them rapidly against a concentration gradient to the lumen, where the iodide is oxidized by thyroid peroxidase in the microvilli to iodine (I2) (Fig. 12-8). The assembly of thyroid hormones within the follicular lumen is made possible by a unique protein, thyroglobulin. Thyroglobulin is a high molecular weight (600,000 to 750,000 Da) glycoprotein synthesized in successive subunits on the ribosomes of the endoplasmic reticulum in follicular cells. The constituent amino acids (tyrosine and others) and carbohydrates (e.g., mannose, fructose, galactose) are derived from the circulation. Recently synthesized thyroglobulin (17S) leaves the Golgi apparatus and is packaged into apical vesicles that are extruded into the follicular lumen (see Fig. 12-8). The amino acid tyrosine, an essential component of thyroid hormones, is incorporated within the molecular structure of thyroglobulin. Iodine is bound to tyrosyl residues in thyroglobulin at the apical surface of follicular cells to form monoiodotyrosine (MIT) and diiodotyrosine (DIT) (see Fig. 12-8). The resulting MIT and DIT combine to form the two biologically active iodothyronines, T4 and T3, secreted by the thyroid gland.
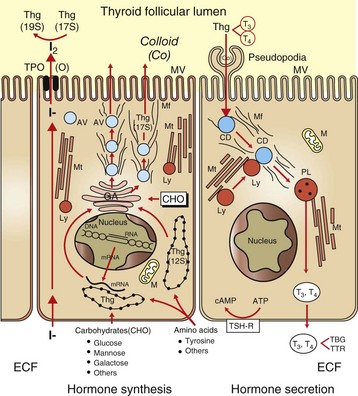
Fig. 12-8 Thyroid follicular cells illustrating two-way traffic of materials from capillaries into the follicular lumen.
Raw materials, such as iodide, are concentrated by follicular cells and rapidly transported into the lumen (left side of drawing). Amino acids (tyrosine and others) and sugars are assembled by follicular cells into thyroglobulin (Thg), packaged into apical vesicles (AV) and released into the lumen. The iodination of tyrosyl residues with the thyroglobulin molecule to form thyroid hormones occurs within the follicular lumen. Elongation of microvilli (MV) and endocytosis of colloid (Co) by follicular cells occur in response to thyroid-stimulating hormone (TSH) stimulation (right side of drawing). The intracellular colloid droplets (CD) fuse with lysosomal bodies (Ly), active thyroid hormone is enzymatically cleaved from thyroglobulin, and free T4 and T3 are released into the circulation. ATP, Adenosine triphosphate; cAMP, cyclic adenosine monophosphate; CHO, carbohydrates; ECF, extracellular fluid; GA, Golgi apparatus; M, mitochondrion; Mf, microfilaments; Mt, microtubules; PL, phagolysosome; TBG, thyroid-binding globulin; TPO, thyroid peroxidase; TSH-R, thyroid-stimulating hormone receptor; TTR, transthyretin. (From Capen CC: Pathophysiology of the thyroid gland. In Dunlop RH, Malbert C-H, editors: Veterinary pathophysiology, Ames, IA, 2004, Blackwell Publishing.)
The secretion of thyroid hormones into the bloodstream from colloid is initiated by elongation of microvilli and formation of pseudopodia on the luminal surface of follicular cells. In response to TSH, these extend into the follicular lumen and indiscriminately phagocytose the adjacent colloid. Colloid droplets within follicular cells fuse with numerous lysosomes. T3 and T4 are released from the thyroglobulin molecule, diffuse across the follicular cell basement membrane, and enter into adjacent capillaries. Negative feedback control of thyroid hormone secretion is accomplished by the coordinated response of the adenohypophysis and certain hypothalamic nuclei to concentrations of T4 and T3 in the blood.
TSH is delivered to thyroid follicular cells where it binds to the basilar aspect of the cell, activates adenyl cyclase, and increases the rate of all biochemical reactions concerned with the biosynthesis and secretion of thyroidal hormones. If the secretion of TSH is sustained (hours or days), thyroid follicular cells become more columnar and follicular lumina become smaller as a result of increased uptake of colloid by endocytosis (Fig. 12-9). Numerous periodic acid–Schiff (PAS)-positive colloid droplets are present in the luminal aspect of the hypertrophied follicular cells. The converse occurs in the thyroid gland in response to increases in circulating T4 and T3, which cause a corresponding decrease in TSH. Thyroid follicles become enlarged and distended with colloid as a result of decreased TSH-mediated endocytosis of colloid. Follicular cells lining the involuted follicles become low cuboidal, with only a few endocytic vacuoles at the interface between the colloid and follicular cells (Fig. 12-10).
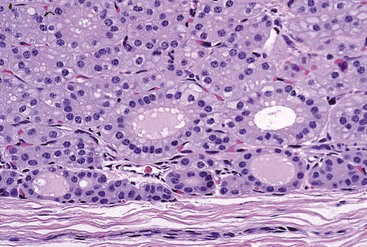
Fig. 12-9 Hyperplasia, thyroid gland, horse.
Follicular epithelial cells following prolonged thyroid-stimulating hormone stimulation are columnar. Note the many collapsed follicles. The lumens of remaining follicles contain pale pink colloid and have numerous endocytic vacuoles at the epithelial cell-follicular lumen interface. H&E stain. (Courtesy Dr. B. Harmon, College of Veterinary Medicine, The University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
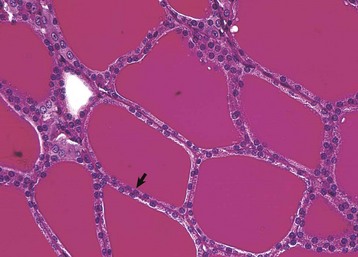
Fig. 12-10 Atrophy, thyroid gland, dog.
Thyroid follicular epithelial cells (arrow) after long-term administration of exogenous thyroxine are cuboidal and follicular lumens are distended with dense colloid. Periodic acid–Schiff reaction. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
T4 and T3, once released into the circulation, act on many different target cells in the body. The overall functions of T4 and T3 are similar, although much of the biologic activity appears to be the result of monodeiodination of T4 to 3,5,3′-triiodothyronine (T3) before they interact with high-affinity nuclear receptors in target cells. In certain conditions, such as protein starvation during the neonatal period, hepatic and renal disease, and fever, T4 is preferentially monodeiodinated to 3,3′,5′-triiodothyronine (reverse T3). Because reverse T3 produced by target cells is biologically inactive, monodeiodination to form reverse T3 provides a mechanism by which the overall metabolic effects of thyroid hormones are attenuated. The subcellular mechanism of action of thyroid hormones resembles that of steroids, in that free hormone enters target cells and binds initially to cytosol-binding proteins and subsequently to high-affinity nuclear receptors. Binding of thyroid hormone to receptors on the inner mitochondrial membrane is responsible for the early activation of energy metabolism and increased oxidative phosphorylation.
Thyroid C (Parafollicular) Cells
Calcitonin is secreted by a second endocrine cell population, C or parafollicular cells, in the mammalian thyroid gland. These cells are situated either in the follicular wall, within the basement membrane between follicular cells, or in small groups adjacent to interfollicular capillaries between follicles (Web Fig. 12-3). C cells do not border the follicular colloid directly, and their secretory pole is oriented toward the interfollicular capillaries. The distinctive feature of C cells is the presence of numerous small, membrane-limited, cytoplasmic secretory granules, which are immunoreactive for calcitonin.
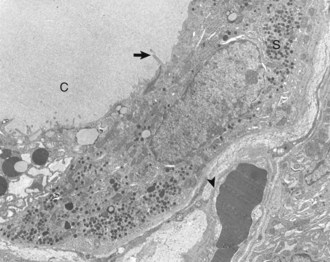
Web Fig. 12-3 Thyroid C cell, thyroid gland, normal dog.
Thyroid C (parafollicular) cell with numerous secretory granules (S) and moderate development of Golgi apparatus and rough endoplasmic reticulum. Microvilli from follicular cells (arrow) extend into the colloid of the follicular lumen (C). The secretory polarity of the C cell is directed toward an interfollicular capillary (arrowhead) with fenestrae. TEM. Uranyl acetate and lead citrate stain. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Calcitonin is a polypeptide hormone secreted according to the calcium ion concentration in plasma and extracellular fluids. The rate of calcitonin secretion is upregulated in response to increased blood calcium concentrations.
C cells store substantial amounts of calcitonin in their cytoplasm, and the hormone is discharged rapidly into interfollicular capillaries in response to hypercalcemia (Fig. 12-11). C cells respond to long-term hypercalcemia by hyperplasia. When the blood calcium concentration is reduced, the stimulus for calcitonin secretion is diminished, and numerous secretory granules accumulate in the cytoplasm of C cells (see Fig. 12-11). Calcitonin exerts its function by interacting with target cells located primarily in bone and kidneys. The actions of parathyroid hormone (PTH) and calcitonin are antagonistic on bone resorption, but synergistic in decreasing the renal tubular reabsorption of phosphorus.
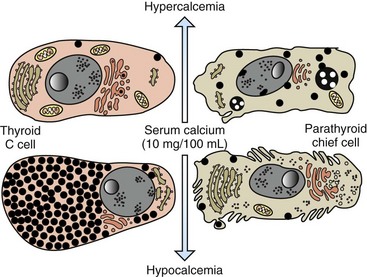
Fig. 12-11 Response of thyroid C cells and parathyroid chief cells to hypercalcemia and hypocalcemia.
C cells accumulate secretory granules in response to hypocalcemia, whereas chief cells are nearly degranulated but have an increased development of synthetic and secretory organelles. In response to hypercalcemia, C cells are degranulated and parathyroid chief cells are predominantly in the inactive stage of the secretory cycle. (Redrawn with permission from Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Parathyroid Glands
Parathyroid glands in most animal species consist of two pairs of glands situated in the cranial cervical region. The dog and cat have both external and internal parathyroid glands located near the thyroid gland. Other animal species, such as the pig, have only a single pair of parathyroid glands cranial to the thyroid gland, embedded either in the thymus in young animals or in adipose connective tissue in adult animals. In cattle and sheep, the larger external parathyroid gland is located a considerable distance cranial to the thyroid gland in the loose connective tissue along the common carotid artery. The smaller internal parathyroid glands are situated on the dorsal and medial surface of the thyroid gland. In horses, the larger (“lower”) parathyroid gland is located a considerable distance from the thyroid gland in the caudal cervical region, near the bifurcation of the bicarotid trunk at the level of the first rib, whereas the smaller (“upper”) parathyroid gland is situated near the thyroid gland.
The parathyroid glands of animals are composed predominantly of chief cells in different stages of secretory activity (Fig. 12-12). Oxyphil cells, often forming nodules, are also present in parathyroid glands of senile horses and cattle. They are larger than chief cells, and their abundant cytoplasm is filled with numerous large, often bizarre-shaped mitochondria and few secretory granules. Although their presence has been associated with hyperparathyroidism in certain species, the extent of their functional capacity remains controversial.
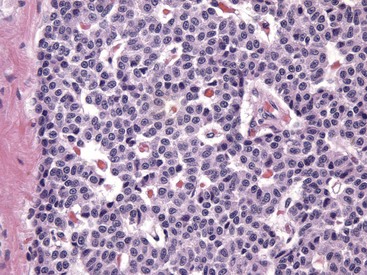
Fig. 12-12 Parathyroid gland, normal dog.
Numerous chief cells are separated and supported by a fine fibrovascular stroma. H&E stain. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Biologically active PTH secreted by chief cells is a straight-chain polypeptide consisting of 84 amino acid residues, with a molecular weight of approximately 9500 Da. Secretory cells in the parathyroid glands of most animals store relatively small amounts of preformed hormone but are capable of responding to minor fluctuations in calcium ion concentration rapidly, by altering the rate of hormonal secretion, and more slowly, by altering the rate of hormonal synthesis (Fig. 12-13). In contrast to most endocrine organs that are under complex control, the parathyroid glands have a unique feedback control system based primarily on the concentration of calcium and, to a lesser extent, of magnesium ions in blood. Calcium ion concentration controls not only the rate of biosynthesis and secretion of PTH but also other metabolic and intracellular degradative processes within chief cells. Increased calcium ion concentration in extracellular fluids rapidly inhibits the uptake of amino acids by chief cells, and consequently synthesis of proPTH, its conversion to PTH, and secretion of stored PTH (see Fig. 12-13).
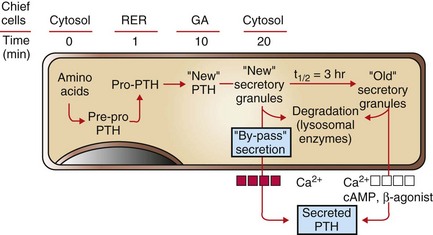
Fig. 12-13 Bypass secretion of parathyroid hormone (PTH) in response to increased demand signaled by decreased blood calcium ion concentration.
Recently synthesized and processed active PTH can be released directly without entering the storage pool of mature (“old”) secretory granules in the cytoplasm of chief cells. PTH from the storage pool can be mobilized by cyclic adenosine monophosphate (cAMP) and β-agonists, such as epinephrine, norepinephrine, and isoproterenol, and by lowered blood calcium ion, whereas secretion from the pool of recently synthesized PTH can be stimulated only by a decreased calcium ion concentration. RER, Rough endoplasmic reticulum; GA, Golgi apparatus. (Redrawn with permission from Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
PTH is the principal hormone involved in the minute-to-minute, fine regulation of blood calcium concentration (total and ionic calcium) in mammals. It does this by directly influencing the function of target cells located primarily in bone and the kidneys, and indirectly acting in the intestine to maintain plasma calcium concentration sufficient to ensure the optimal functioning of a wide variety of body cells. The overall action of PTH on bone is to mobilize calcium into extracellular fluids (Fig. 12-14). Bone responds to PTH by increasing the activity of osteoclasts and osteocytes existing in bone.
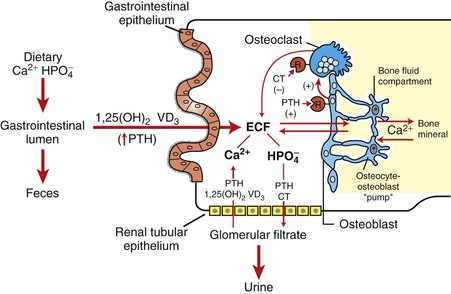
Fig. 12-14 Interrelation of parathyroid hormone (PTH), calcitonin (CT), and 1,25-dihydroxycholecalciferol (1,25-[OH]2 VD3) in hormonal regulation of calcium and phosphorus in extracellular fluids (ECF). (Redrawn with permission from Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
PTH has a rapid (within 5 to 10 minutes) and direct effect on renal tubular function, leading to decreased reabsorption of phosphorus and consequently the development of phosphaturia. The site of action where PTH blocks tubular reabsorption of phosphorus has been determined to be the proximal tubule. The ability of PTH to enhance the renal absorption of calcium also is of considerable importance in the maintenance of calcium homeostasis. This effect of PTH on tubular reabsorption of calcium appears to be a result of a direct action on the distal convoluted tubule. Calcitonin and PTH, acting in concert, provide a dual negative feedback control mechanism to maintain the concentration of calcium in extracellular fluids within narrow limits.
The third major hormone involved in the regulation of calcium metabolism and skeletal remodeling is cholecalciferol or vitamin D3 (see Fig. 12-14). Cholecalciferol is ingested in small amounts in the diet and can be synthesized in the epidermis from precursor molecules (e.g., 7-dehydrocholesterol) through a provitamin D3 intermediate form in response to ultraviolet light. The active metabolites of vitamin D increase the absorption of calcium and phosphorus from the intestine and thereby maintain adequate concentrations of these electrolytes in the extracellular fluids as required for the appropriate mineralization of bone matrix. From a functional point of view, vitamin D brings about the retention of sufficient mineral ions to ensure mineralization of bone matrix, whereas PTH maintains the proper ratio of calcium to phosphorus in extracellular fluids. The major target tissue for 1,25-(OH)2D3 is the mucosa of the small intestine, where it increases the active transcellular transport of calcium (cranial small intestine) and phosphorus (caudal small intestine).
Pancreatic Islets
The endocrine function of the pancreas is performed by small groups of cells, the islets of Langerhans (Fig. 12-15), which are completely surrounded by acinar or exocrine cells that produce digestive enzymes. During embryonic development of the pancreas, a close relationship exists between the endocrine and exocrine portions. Evidence suggests that islet, acinar, and ductal cells arise from a common multipotential precursor cell. In early embryonic development, the endocrine cells are integrated within the exocrine matrix of the pancreatic bud. They subsequently accumulate in nonvascularized clusters and later become separated from the exocrine tissue and then independently vascularized.
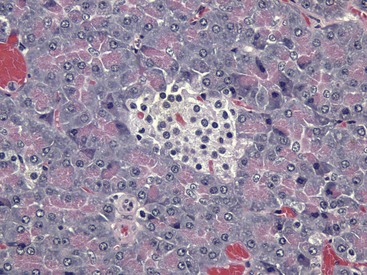
Fig. 12-15 Pancreatic islet, normal dog.
The islet is surrounded by the exocrine pancreas. H&E stain. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
The pancreatic islets of normal animals contain multiple types of cells. The predominant secretory cells are the β cells, which function in the biosynthesis of insulin but co-secrete islet amyloid polypeptide. The glucagon-secreting α cells are less numerous than β cells. δ Cells and F, or PP, cells in the islets secrete somatostatin and pancreatic polypeptide, respectively. The different cell types of the endocrine pancreatic cells can be differentiated by cytochemical and immunohistochemical techniques, and by electron microscopy (Web Fig. 12-4). The α, β, and δ cells have well-developed rough endoplasmic reticulum and Golgi complexes that participate in the biosynthesis of polypeptide hormones, as well as numerous secretory granules in the cytoplasm. Each type of endocrine cell in the pancreatic islets has secretory granules with distinct ultrastructural characteristics, which can be used to identify the cell types; however, immunohistochemical identification of the specific islet hormone is a more accurate method of identifying different cell types in the pancreatic islets.
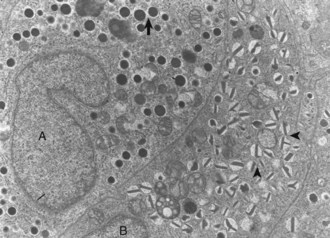
Web Fig. 12-4 Pancreatic islet, normal dog.
Differences in secretion granules between β cells (B) and α cells (A); the internal cores of secretion granules in insulin-secreting β cells (arrowheads) are bar- or Y-shaped, with a prominent space between the limiting membrane and internal core. Secretion granules of the glucagon-secreting α cells have an electron-dense, circular, internal core with a narrow submembranous space (arrow). TEM. Uranyl acetate and lead citrate stain. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
The major physiologic stimulus for the release of insulin from β cells is glucose. An appropriate concentration of calcium ion in extracellular fluids is required for induction of insulin release from β cells. Insulin is a powerful hormone with broad biologic influences that either directly or indirectly affects the structure and function of every organ in the body. Tissues especially responsive to insulin include skeletal and cardiac muscle, adipose tissue, fibroblasts, hepatocytes, leukocytes, mammary glands, cartilage, bone, skin, aorta, pituitary gland, and peripheral nerves. The principal function of insulin is to stimulate anabolic reactions involving carbohydrates, lipids, proteins, and nucleic acids. It catalyzes the formation of macromolecules used in cell structure, energy stores, and regulation of many cell functions. Hepatocytes, adipose cells, and muscle are three principal target sites for insulin. In general, insulin increases the transfer of glucose and certain other monosaccharides, some amino acids and fatty acids, and potassium and magnesium ions across the plasma membrane of target cells. In addition, it enhances glucose oxidation and glycogenesis and stimulates lipogenesis and the formation of adenosine triphosphate (ATP), DNA, and RNA. Insulin also decreases the rate of lipolysis, proteolysis, ketogenesis, and gluconeogenesis.
Glucagon is a hormone that stimulates energy release from target cells and is secreted in response to a reduction in blood glucose concentration. It mobilizes stores of energy-yielding nutrients by increasing glycogenolysis, gluconeogenesis, and lipolysis, thereby increasing the blood concentration of glucose. At physiologic concentrations, glucagon increases both hepatic glycogenolysis and gluconeogenesis. Insulin and glucagon act in concert to maintain the concentration of glucose in extracellular fluids within relatively narrow limits. A glucose sensor in the pancreatic islets controls the relative proportion of these two biologic antagonists. Glucagon controls glucose influx into the extracellular space from the hepatocytes, and insulin controls glucose efflux from the extracellular space into such insulin-sensitive cells as adipocytes, myocytes, and hepatocytes.
Pineal Gland
The pineal gland is a neuroendocrine organ that influences circadian rhythm and is intimately associated with the brain. It is composed of loose neuroglial stroma containing nests of pinealocytes and scattered calcified bodies known as brain sand or corpora arenacea. Pinealocytes secrete the hormone-like compound melatonin during periods of darkness. In addition to its role in circadian rhythms, melatonin is thought to influence seasonal reproductive activity in mammals through inhibition of gonadotropin-releasing hormone (GnRH).
Chemoreceptor Organs
Chemoreceptor tissue is present at several sites in the body, including the carotid body, aortic bodies, nodose ganglion of the vagus nerve, ciliary ganglion in the orbit, pancreas, bodies on the internal jugular vein below the middle ear, and glomus jugular along the recurrent branch of the glossopharyngeal nerve. The chemoreceptor organs are sensitive indicators of changes in the blood carbon dioxide content, pH, and oxygen tension, thereby aiding in the regulation of respiration and circulation. Carotid and aortic bodies can initiate an increase in the depth, minute volume, and rate of respiration via parasympathetic nerves, and an increase in heart rate and elevation of arterial blood pressure via the sympathetic nervous system. The bodies are composed of parenchymal (chemoreceptor) and stellate (sustentacular) cells. Nerve endings with synaptic vesicles, as well as nerve fibers, occur in close association with the chemoreceptor cells.
Portals of Entry
The portals of entry for the inflammatory agents affecting endocrine glands include hematogenous spread and direct extension. Autoimmune and infectious inflammatory diseases affect various endocrine glands. The pathogenesis of autoimmune diseases typically involves autoreactive T lymphocytes and autoantibodies, both of which gain access to the endocrine gland via the blood. Bacterial, viral, and fungal diseases preferentially restricted to individual endocrine glands rarely occur; however, endocrine glands are equally vulnerable to involvement in systemic diseases. Endocrine glands, such as the pituitary and pineal glands and the thyroid and parathyroid glands, can also become secondarily involved through direct extension from the meninges and larynx, respectively.
The endocrine system is unique in the fact that many of the disease processes affecting it involve disturbances of growth such as hyperplasia and neoplasia. The histopathologic differentiation among nodular hyperplasia, adenoma, and carcinoma is often more difficult in endocrine glands than in most other organs of the body. However, criteria used to differentiate these proliferative lesions should be established and applied in a uniform manner in the evaluation of proliferative lesions in endocrine glands. For many endocrine glands, there appears to be a continuous spectrum of proliferative lesions derived from a specific population of secretory cells between focal or nodular hyperplasia and adenomas.
Excessive focal growth of endocrine cells is the consequence of aberrant secretion of growth- and/or function-stimulating hormone(s) and has been referred to as nonneoplastic endocrine hyperplasia. Nodules arising in hyperplastic endocrine glands can be polyclonal and of clonal origin. Hyperfunction and cellular hypertrophy caused by nonneoplastic endocrine hyperplasia are considered to be largely reversible on cessation of the inciting stimulus; however, chronic and severe hyperplasia of endocrine tissues is not always fully reversible.
Endocrine glands appear predisposed to the development of an increased incidence of neoplasms after prolonged stimulation of a population of secretory cells. Long-continued stimulation can lead to the development of clones of cells within the hyperplastic endocrine glands that grow more rapidly and are consequently more susceptible to genetic alterations, resulting in neoplastic transformation on exposure to the appropriate combination of promoting agents.
Responses to Injury
Pathogenic Mechanisms of Endocrine Diseases
Although injury of cells in endocrine glands is often attributable to processes, such as necrosis, inflammation, and autoimmunity, discussed in Chapters 1, 3, and 5, respectively, many diseases of endocrine glands are characterized by dramatic functional disturbances and characteristic clinicopathologic alterations affecting one or more body systems. The affected animal can have changes primarily involving the skin (alopecia caused by hypothyroidism), nervous system (seizures caused by hyperinsulinism), urinary system (polyuria caused by diabetes mellitus, diabetes insipidus, and hyperadrenocorticism), or skeletal system (fractures induced by hyperparathyroidism). There are several mechanisms that can disrupt normal endocrine function, with the majority of processes resulting in insufficient or excessive hormone production.
Primary Hypofunction of an Endocrine Gland
Hormone secretion is subnormal because of extensive destruction of secretory cells by a disease process, the failure of an endocrine gland to develop properly, or the result of a specific biochemical defect in the synthetic pathway of a hormone. In animals, immune-mediated injury characterized by notable infiltration of lymphocytes and plasma cells and deposition of electron-dense immune complexes along basement membranes causes hypofunction via progressive destruction of secretory parenchyma in one or more endocrine glands.
Failure of development also results in primary hypofunction of an endocrine gland. The classic example of this mechanism in animals is the failure of oropharyngeal ectoderm to differentiate completely into trophic hormone–secreting cells of the adenohypophysis in dogs, resulting in pituitary dwarfism (see the section on Disorders of Dogs).
Secondary Hypofunction of an Endocrine Gland
In secondary hypofunction of an endocrine gland, a destructive lesion in one organ, such as the pituitary gland, interferes with the secretion of a trophic hormone. This results in hypofunction of the target endocrine gland. Large, endocrinologically inactive pituitary neoplasms in adult dogs, cats, and other animals can interfere with the secretion of multiple pituitary trophic hormones and result in clinically detectable hypofunction of the adrenal cortex (Fig. 12-16), follicular cells of the thyroid gland, and gonads.
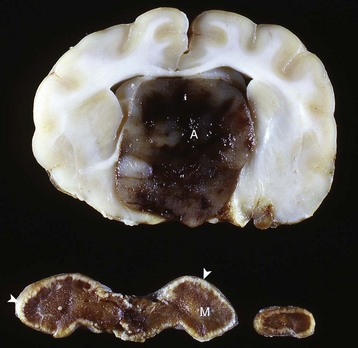
Fig. 12-16 Secondary hypofunction of adrenal glands, brain, pituitary gland and left (longitudinal section) and right (cross section) adrenal glands, dog.
A large nonfunctional chromophobe adenoma (A) has invaded and completely destroyed the adenohypophysis and hypothalamus, and infiltrated into the thalamus. Destruction of the adenohypophysis has resulted in a lack of secretion of thyrotropin, adrenocorticotropin, and other pituitary trophic hormones, resulting in severe bilateral (symmetrical) trophic atrophy of the adrenal cortex (arrowheads), especially the adrenocorticotropic hormone–dependent zona fasciculata and zona reticularis, and consequently, in a relatively more prominent medulla (M). (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Primary Hyperfunction of an Endocrine Gland
Primary hyperfunction of an endocrine gland is one of the most important pathologic mechanisms of endocrine disease in animals. The cells of a lesion, often a neoplasm derived from endocrine cells, autonomously synthesize and secrete a hormone at a rate in excess of the body’s ability to use and degrade it, thereby resulting in a syndrome caused by hormone excess. Examples are summarized in Table 12-1.
TABLE 12-1
Primary Hyperfunction of an Endocrine Gland
| Neoplasia | Hormone | Lesion/Sign |
| Acidophil adenoma (pituitary gland) | Growth hormone | Acromegaly |
| Adrenal cortical adenoma/carcinoma | Estrogen | Feminization |
| Pheochromocytoma (adrenal medulla) | Norepinephrine | Hypertension |
| Thyroid follicular cell adenoma | T4, T3 | ↑Basal metabolic rate |
| C-cell adenoma/carcinoma (thyroid gland) | Calcitonin | Osteosclerosis |
| Parathyroid gland chief cell adenoma | Parathyroid hormone | Fibrous osteodystrophy |
| Pancreatic β-cell adenoma/carcinoma | Insulin | Hypoglycemia |
Secondary Hyperfunction of an Endocrine Gland
In this mechanism of endocrine disease, a lesion in one organ (e.g., adenohypophysis) secretes an excess of a trophic hormone that leads to long-term stimulation and hypersecretion of a hormone by a target organ. The classic example in animals is the ACTH-secreting neoplasm derived from pituitary corticotrophs in dogs and cats (Fig. 12-17). The functional disturbances and lesions are caused by increased blood cortisol concentrations resulting from the ACTH-stimulated hypertrophy and hyperplasia of the cells of the zonae fasciculata and reticularis of the adrenal cortex. In some aging dogs with notable adrenal cortical enlargement and functional disturbances of cortisol excess, no gross or histopathologic evidence of a neoplasm is present in the pituitary gland. These animals can have a change in negative feedback control as the result of an age-related increase in monoamine oxidase-β in the hypothalamus and increased metabolism of dopamine. The outcome is reduced inhibition of ACTH production by the pars intermedia of the pituitary gland leading to severe corticotroph hyperplasia, increased ACTH concentration in the blood, and long-term stimulation of the adrenal cortex, resulting in the syndrome of cortisol excess.
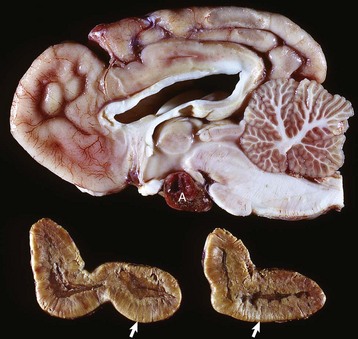
Fig. 12-17 Secondary hyperfunction of adrenal glands, brain, pituitary gland and left and right adrenal glands, dog.
Corticotroph (adrenocorticotropic hormone [ACTH]-secreting) chromophobe adenoma (A) in the pituitary gland and bilateral (symmetrical) enlargement of the adrenal glands. The chronic secretion of ACTH has resulted in bilateral (symmetrical) hypertrophy and hyperplasia of secretory cells of the zona fasciculata and zona reticularis in the adrenal cortex (arrows) and excessive secretion of cortisol. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Hypersecretion of Hormones or Hormonelike Factors by Nonendocrine Neoplasms
Certain neoplasms of nonendocrine tissue in animals secrete either new humoral substances or hormones that share chemical and/or biologic characteristics with the “native” hormones secreted by an endocrine gland. Most of the recently discovered humoral substances secreted by nonendocrine neoplasms are peptides rather than steroids, iodothyronines, or catecholamines. The nonpeptide hormones require more complex biosynthetic pathways and are infrequently produced by cancer cells. Pseudohyperparathyroidism or humoral hypercalcemia of malignancy is a clinical syndrome primarily produced by the autonomous hypersecretion of PTH–related peptide (PTHrP) by cancer cells. PTHrP interacts with the parathyroid hormone receptor in target cells (e.g., bone and kidneys) and results in persistent, often life-threatening hypercalcemia. A well-characterized example of this disease mechanism in animals is the adenocarcinoma of the apocrine glands of the anal sac in dogs (see the section on Disorders of Dogs). These neoplasms produce PTHrP, which mimics the action of PTH and results in an accelerated mobilization of calcium from bone by osteoclasts leading to the development of persistent hypercalcemia. Serum PTH concentrations are lower in dogs with apocrine carcinomas than in controls, and PTH concentrations are undetectable in neoplastic tissue.
Endocrine Dysfunction Caused by Failure of Target Cell Response
Failure of target cells to respond to a hormone can be caused by a lack of adenyl cyclase in the cell membrane or to an alteration in hormone receptors on the cell surface. Hormone is secreted in normal or increased amounts by the cells of the endocrine gland. For example, insulin resistance in obese animals can result from a decrease or downregulation of receptors on the surface of target cells. Receptor downregulation develops in response to a chronic increase in insulin stimulated by the hyperglycemia resulting from excessive food intake. Secretory cells in the corresponding endocrine gland (i.e., pancreatic islets) undergo compensatory hypertrophy and hyperplasia in an attempt to secrete additional hormone.
Endocrine Hyperactivity Caused by Diseases of Other Organs
The best-characterized example of endocrine hyperactivity caused by disease of other organs is the hyperparathyroidism that develops as a result of chronic renal failure or nutritional imbalance. In the renal form, hyperphosphatemia occurs because of a decreased glomerular filtration rate, resulting in a reciprocal decline in serum calcium and parathyroid stimulation. Subsequently the progressive destruction of cells of the proximal convoluted tubules interferes with the metabolic activation of vitamin D by 1α-hydroxylase in the kidneys, leading to decreased intestinal calcium absorption and continued parathyroid stimulation. This rate-limiting step in the metabolism of vitamin D is controlled by multiple factors, including levels of PTH, serum phosphorus, and several other hormones. The intestinal absorption of calcium is impaired and results in the development of progressive hypocalcemia; the hypocalcemia leads to long-term parathyroid gland stimulation and subsequently to the development of generalized demineralization of the skeleton. Nutritional hyperparathyroidism develops in animals fed abnormal diets that are either too low in calcium, too high in phosphorus, or deficient in cholecalciferol.
Endocrine Dysfunction Resulting from Abnormal Hormone Degradation
A decreased rate of hormonal degradation resulting in persistently elevated blood concentrations that simulate a syndrome of hypersecretion is the most common mechanism reported in domestic animal species. The classic example of this mechanism is the syndrome of hyperestrogenism-induced feminization secondary to decreased hepatic degradation of estrogens in patients with cirrhosis. Hypercalcemia, caused in part by a decrease in the ability of proximal convoluted tubular epithelial cells in the diseased kidneys to degrade PTH (along with a decrease in urinary excretion of calcium), is occasionally reported in dogs with chronic renal disease.
Iatrogenic Syndromes of Hormone Excess
The administration of an exogenous hormone can influence the activity of target cells either directly or indirectly and result in important functional disturbances. It is well recognized that the chronic administration of potent preparations of adrenal cortical steroids at inappropriately large daily doses (for the symptomatic treatment of various diseases) can produce most of the functional disturbances that are secondary to an endogenous hypersecretion of cortisol. Increased concentrations of exogenous cortisol result in notable atrophy of the adrenal cortex, particularly the ACTH-dependent zonae fasciculata and reticularis (Fig. 12-18). Similarly the administration of excessively large doses of insulin can result in hypoglycemia, and an excess of T4 or T3 can result in hyperthyroidism, especially in certain species, such as cats, which have limited capacities to conjugate T4 with glucuronic acid and thus enhance its biliary excretion.
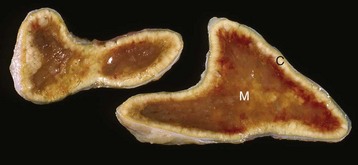
Fig. 12-18 Iatrogenic hyperadrenocorticism, left and right adrenal glands, dog.
Hyperadrenocorticism, caused by long-term administration of exogenous corticosteroids, has resulted in notable trophic atrophy of the adrenocorticotropic hormone–dependent zona fasciculata and zona reticularis of the adrenal cortex (C). The adrenal medulla (M) comprises a relatively greater percentage of the atrophic adrenal gland than of a normal adrenal gland. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
The administration of progestogens to dogs indirectly results in a syndrome of growth hormone excess. For example, the injection of medroxyprogesterone acetate for the prevention of estrus in dogs stimulates the expression of the growth hormone gene in the mammary glands and results in elevated circulating growth hormone concentrations, producing many of the clinical manifestations of acromegaly. The excessive skinfolds (Fig. 12-19), expansion of interdigital spaces, and abdominal enlargement in dogs with iatrogenic acromegaly are related to the protein anabolic effects of growth hormone. Elongation of bones in response to exogenous progestogens requires functional growth plates and osteogenic surfaces.

Fig. 12-19 Iatrogenic acromegaly, beagle (center) compared with unaffected littermates (left and right).
Note the coarseness of the facial features and the markedly thick folds of the skin of the face. These characteristic changes are the result of the protein anabolic effects of somatotropin (produced by hyperplastic mammary ductular epithelial cells), which have been stimulated by the administration of exogenous medroxyprogesterone acetate. (Courtesy Dr. P. Concannon, College of Veterinary Medicine, Cornell University.)
Defense Mechanisms
The primary defense mechanism employed by the endocrine system is the hierarchy of hormonal regulation known as the hypothalamic-pituitary-target gland axis (see Fig. 12-3). The hypothalamus produces a number of releasing and inhibitory hormones in response to sensory input from the central nervous system (CNS). These releasing and inhibitory hormones act on anterior or posterior portions of the pituitary gland to stimulate or prevent the release of trophic hormones. Trophic hormones act on specific endocrine glands, stimulating them to produce hormones that exert ultimate actions on downstream tissues. Under normal circumstances, the action of a hormone is self-limiting because of the existence of negative feedback loops for each hormone series in which secretion of a particular hormone ultimately leads to inhibition of its subsequent secretion. Negative feedback from target endocrine glands can be directed at the hypothalamus, the pituitary gland, or both.
Disorders of Domestic Animals
Disorders known or thought to have a genetic basis and/or be inherited are listed in Web Table 12-1.
WEB TABLE 12-1
Inherited Endocrine Diseases of Animals
| Condition | Species/Breed | Pattern of Inheritance |
| Adenohypophyseal aplasia | Jersey and Guernsey cattle | Unknown |
| Pituitary dwarfism | German shepherds | Autosomal recessive |
| Hypoadrenocorticism | Bearded collies, Nova Scotia duck tolling retriever, Portuguese water dogs, standard poodles | Unknown or autosomal recessive |
| Adrenal hyperplasia-like syndrome | Chow Chows, Pomeranians, poodles, Samoyeds | Unknown |
| Dyshormonogenetic goiter | Abyssinian cats, Afrikaner cattle, rat and toy fox terriers, Saanen dwarf goats, sheep | Autosomal recessive |
Disorders of the Adenohypophysis
Hypopituitarism and Neoplasms of the Adenohypophysis
Aplasia and Prolonged Gestation: See the section on Disorders of Ruminants for a discussion of aplasia and prolonged gestation.
Pituitary Cysts and Pituitary Dwarfism: See the section on Disorders of Dogs for a discussion of pituitary cysts and pituitary dwarfism.
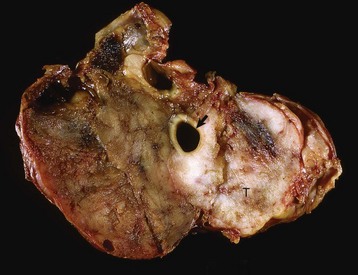
Endocrinologically Inactive Chromophobe Adenomas: Nonfunctional pituitary neoplasms occur in dogs and cats but are uncommon in other species. Although chromophobe adenomas seem endocrinologically inactive, they can cause significant functional disturbances and clinical signs by compressing and causing atrophy of adjacent portions of the pituitary gland, as well as dorsal extension into the overlying brain (Fig. 12-20). The clinical disturbances result either from the lack of secreted pituitary trophic hormones and subsequent diminished target organ function (e.g., adrenal cortex; see Fig. 12-16) or from dysfunction of the CNS. Affected animals often have decreased spontaneous activity, incoordination, and disturbances of balance; are weak; and sometimes collapse after exercise. Chronically affected animals are blind and have dilated and fixed pupils because of compression and disruption of the optic nerves by dorsal extension of the pituitary neoplasms (see Fig. 12-20). Endocrinologically inactive pituitary adenomas often become large before they cause clinical signs or kill the animal (see Figs. 12-16 and 12-20).
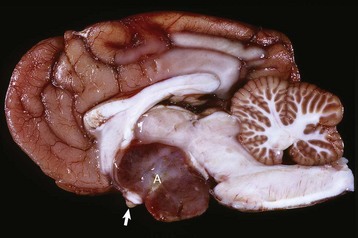
Fig. 12-20 Adenoma, pituitary gland, dog.
A large pituitary adenoma (A) has extended dorsally and compresses the overlying brain. The optic chiasm (arrow) is also severely compressed. The adenohypophysis, neurohypophysis, and hypothalamus have been destroyed by the neoplasm. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Clinical signs reported with nonfunctional pituitary adenomas and hypopituitarism are not specific and could be confused with other disorders of the CNS, such as brain neoplasms and encephalitis, or with chronic renal disease. There is no effect on body stature secondary to compression of the pars distalis and interference with growth hormone secretion because these neoplasms usually arise in adult animals that have already completed their growth. However, atrophy of the skin and loss of muscle mass could be related in part to a lack of the protein anabolic effects of growth hormone. Impaired secretion of pituitary trophic hormones often leads to a reduced basal metabolic rate because of decreased TSH secretion and hypoglycemia secondary to trophic atrophy of the adrenal cortex (see Fig. 12-16).
Pituitary Gland Carcinomas: Pituitary gland carcinomas are uncommon neoplasms compared with adenomas but have been seen in older dogs and cattle. They usually are endocrinologically inactive but can cause significant functional disturbances by destroying the pars distalis and neurohypophyseal system, leading to panhypopituitarism and diabetes insipidus. Carcinomas are large and invade extensively into the overlying brain, along the ventral aspect of the cranial cavity, and into the basisphenoid bone where they cause osteolysis. Metastases occur infrequently to cervical lymph nodes or distant sites such as the spleen or liver. Carcinomas are highly cellular and often have large areas of hemorrhage and necrosis. Giant cells, nuclear pleomorphism, and mitotic figures are encountered more frequently than in adenomas.
Craniopharyngiomas (Intracranial Germ Cell Tumors): Craniopharyngiomas are benign neoplasms derived from epithelial remnants of the oropharyngeal ectoderm of the craniopharyngeal duct (Rathke’s pouch). They often occur in animals younger than those with other types of pituitary neoplasms and are present in either suprasellar or infrasellar locations. Craniopharyngioma is associated with dwarfism in young dogs because it causes subnormal secretion of somatotrophin and other trophic hormones at an early age, before closure of the growth plates.
The reclassification of some pleomorphic neoplasms in the suprasellar region of younger dogs from craniopharyngiomas to germ cell tumors has recently been proposed. The diagnosis of germ cell tumors was based on three criteria: (1) midline suprasellar location, (2) presence within the tumor of several distinct cell types (one population resembles a seminoma or dysgerminoma and others suggest teratomatous differentiation into secretory glandular and squamous elements), and (3) α-fetoprotein immunoreactivity.
Craniopharyngiomas and suprasellar germ cell tumors often are large and grow along the ventral aspect of the brain, where they can surround several cranial nerves. In addition, they extend dorsally into the hypothalamus and thalamus (Fig. 12-21). The resulting clinical signs often occur because of a combination of the following:
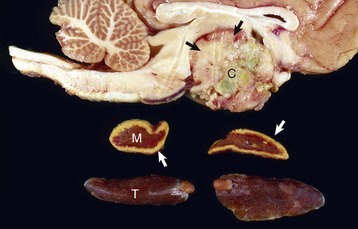
Fig. 12-21 Craniopharyngioma (C), pituitary area, left and right adrenal glands, left and right thyroid glands, dog.
The neoplasm has extended dorsally through the hypothalamus and compressed the thalamus (black arrows). The neoplasm has also destroyed the adenohypophysis and neurohypophysis, resulting in severe bilateral (symmetrical) trophic atrophy of the adrenal cortices (white arrows). The adrenal glands consist predominantly of medulla (M) surrounded by a thin rim of cortex (capsule plus zona glomerulosa). Although the thyroid follicular cells are atrophic, the overall gland (T) size is within normal limits because of colloid involution of the follicles. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
• A lack of secretion of pituitary trophic hormones resulting in trophic atrophy and subnormal function of the adrenal cortex and thyroid gland, atrophy of the gonads, and failure to attain somatic maturation because of a lack of secretion of growth hormone
• Disturbances in water metabolism (polyuria, polydipsia, low urine specific gravity, and osmolality) resulting from an interference in the synthesis and release of ADH by the large neoplasm
• Deficits in cranial nerve function
• CNS dysfunction caused by extension into the overlying brain
Microscopically, craniopharyngiomas have alternating solid and cystic areas. The solid areas are composed of nests of cuboidal, columnar, or squamous epithelial cells with focal areas of mineralization. The cystic spaces are lined by either columnar or squamous cells and contain keratin debris and colloid.
Hyperpituitarism and Neoplasms of the Adenohypophysis
Pars Intermedia Adenomas: Adenomas derived from cells of the pars intermedia are the most common type of pituitary gland neoplasm in horses and the second most common type in dogs, but they are rare in other species. Adenomas develop in older horses, more frequently in females. Nonbrachycephalic breeds of dogs have adenomas of the pars intermedia more often than brachycephalic breeds.
Adenomas of the pars intermedia in dogs are either functionally inactive and result in varying degrees of hypopituitarism and diabetes insipidus, or endocrinologically active and secrete excessive levels of ACTH, leading to bilateral adrenal cortical hyperplasia and a syndrome of cortisol excess. Endocrinologically active (ACTH-secreting) adenomas of the pars intermedia in dogs have prominent groups of corticotrophs that have abundant eosinophilic cytoplasm and more widely scattered follicles.
Two cell populations have been identified in the pars intermedia of normal dogs by immunohistochemistry. The predominant cell type (A cell) is strongly immunoreactive for α-MSH, as in the pars intermedia of other species, whereas the second cell type (B cell) in the canine pars intermedia is strongly immunoreactive for ACTH. This second cell population accounts for the high bioactive ACTH concentration found in the pars intermedia of dogs and most likely gives rise to corticotroph adenomas of the pars intermedia in dogs with the syndrome of cortisol excess.
The clinical syndrome reported with neoplasms of the pars intermedia in horses is characterized by polyuria, polydipsia, polyphagia, muscle weakness, somnolence, intermittent hyperpyrexia, and generalized hyperhidrosis. The affected horses often develop hirsutism because of a failure to seasonally shed hair. The hair over most of the trunk and extremities is long (up to 10 to 12 cm), abnormally thick and wavy, and often matted (Fig. 12-22). Horses with larger neoplasms sometimes have insulin-resistant hyperglycemia and glycosuria, probably resulting from the downregulation of insulin receptors on target cells induced by chronic excessive intake of feed and hyperinsulinemia. The disturbances in carbohydrate metabolism, ravenous appetite, hypertrichosis, and hyperhidrosis reflect deranged hypothalamic function caused by compression of the overlying hypothalamus by the large pituitary neoplasms. The hypothalamus is the primary center for homeostatic regulation of body temperature, appetite, and cyclic shedding of hair.

Fig. 12-22 Hirsutism, skin, horse.
The hirsutism is the result of a failure to shed hair because of hypothalamic compression by an adenoma of the pars intermedia. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
In addition to their space-occupying effects, some adenomas of the pars intermedia are endocrinologically active. Plasma cortisol and immunoreactive adrenocorticotropin (iACTH, molecular weight 4500 Da) concentrations can be modestly elevated in horses with pars intermedia adenomas. The cortisol concentrations often lack normal diurnal rhythm and are not suppressed by either large or small doses of dexamethasone. The modest increases of plasma iACTH appear to be caused by the different processing of proopiomelanocortin (POMC) in neoplasms derived from cells of the pars intermedia. This could explain the normal or slightly increased blood cortisol concentrations and normal or mildly hyperplastic adrenal cortices observed in some horses with adenomas of the pars intermedia. The concentrations of ACTH in the neoplasm have been reported to be six times that of the normal pars intermedia and only approach the concentrations found in the pars distalis of normal horses. The plasma and neoplasm concentrations of pars intermedia–derived peptides (corticotrophin-like intermediate lobe peptide [CLIP], α-MSH, β-MSH, and β-endorphin [β-END]) are disproportionately increased (40 times or more) compared with those of ACTH, apparently as the result of selective posttranslational processing of POMC in a manner similar to that of the normal pars intermedia.
Adenomas of the pars intermedia have immunohistochemical staining similar to that of the nonneoplastic equine pars intermedia. The findings include a strong, diffuse cytoplasmic reaction for POMC, a moderately strong reaction for α-MSH and β-END, a weak reaction for ACTH, and negative immunostaining for prolactin, glial fibrillary acidic protein, and neuron-specific enolase. Two antisera directed against different parts of the N-terminal fragment of human (h) POMC differ in their immunoreactivity. Immunostaining of neoplastic cells was stronger with anti-h1-48 N-POMC antisera than with anti-h1-76 N-POMC antisera. These immunohistochemical findings support the biochemical studies that suggest horses with pituitary adenomas derived from the cells of the pars intermedia develop a unique clinical syndrome that is the result of hypothalamic and neurohypophyseal derangement and an autonomous production of excess amounts of POMC-derived peptides. Although many of the functional disturbances in horses with pituitary adenomas (e.g., diabetes insipidus, polyphagia, hyperpyrexia, hyperhidrosis, and hirsutism) appear to be the result of hypothalamic or neurohypophyseal dysfunction, other behavioral signs (e.g., docility and diminished responsiveness to painful stimuli) could be related to increased plasma and cerebrospinal fluid concentrations of β-END. The clinical syndrome in horses with pituitary neoplasms is distinctly different from that of Cushing’s disease in dogs and cats.
In dogs, adenomas of the pars intermedia result in only a moderate enlargement of the pituitary gland. The pars distalis is readily identifiable and sharply demarcated from the anterior margin of the neoplasm, usually by an incomplete layer of condensed stroma. The neoplasm can extend across the residual hypophyseal lumen and result in compression atrophy, but it usually does not invade the pars distalis. The posterior lobe is incorporated within the neoplasm, but the infundibular stalk is intact. Histologically, canine pars intermedia adenomas are composed of numerous large colloid-filled follicles lined by partially ciliated simple columnar epithelium scattered among nests of variably sized chromophobic cells.
In horses, adenomas of the pars intermedia often are large neoplasms that extend out of the fossa hypophysialis and severely compress the overlying hypothalamus (Fig. 12-23). The adenomas are yellow to white and multinodular and enclose the pars nervosa. When the neoplasm is incised, the pars distalis usually can be identified as a compressed subcapsular rim of tissue on the anterior margin. A sharp line of demarcation remains between the neoplasm and the compressed pars distalis. The neoplastic cells are arranged in cords and nests along the capillaries and connective tissue septae and are large, cylindrical, spindle-shaped, or polyhedral with oval hyperchromatic nuclei (Fig. 12-24). The pattern is often reminiscent of that of the prominent pars intermedia of normal horses. Ribbons of more cuboidal to columnar neoplastic cells occasionally form follicular structures that have dense eosinophilic colloid.
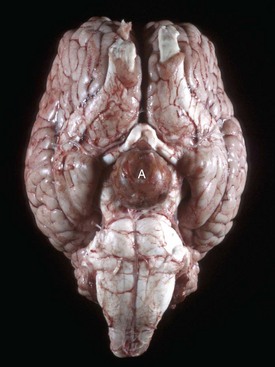
Fig. 12-23 Adenoma, brain, pituitary gland, horse.
The pituitary gland is notably enlarged because of an adenoma (A) of the pars intermedia. (Courtesy College of Veterinary Medicine, University of Illinois.)
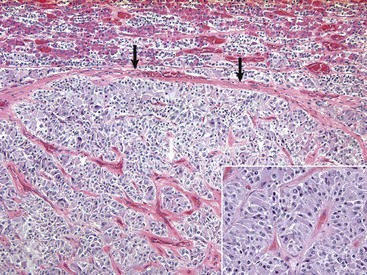
Fig. 12-24 Adenoma, pituitary gland, pars intermedia, horse.
The adenoma is composed of cords and ribbons of well-differentiated, tall cuboidal to columnar cells with ample amounts of granular basophilic cytoplasm. Note the normal pituitary gland (top quarter of figure) that is demarcated from the adenoma by a band of connective tissue (arrows). The expanding volume of the adenoma has compressed the normal gland. Inset, Higher magnification of the cells of the adenoma. H&E stain. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Adrenocorticotropic Hormone-Secreting (Corticotroph) Adenomas: Functional (endocrinologically active) neoplasms arise in the pituitary gland of dogs, particularly boxers, Boston terriers, and dachshunds, and increasingly in cats. They are derived from corticotroph (ACTH-secreting) cells in the pars distalis but can also arise from the pars intermedia. These neoplasms cause a clinical syndrome of cortisol excess (Cushing’s disease), resulting in gluconeogenic, lipolytic, protein catabolic, and antiinflammatory activities.
The pituitary gland is consistently enlarged (see Fig. 12-17); however, neither the occurrence nor the severity of functional disturbances appears to be directly related to the size of the neoplasm. Because the diaphragma sellae is incomplete in these species, the line of least resistance is dorsal expansion of the gradually enlarging pituitary mass. This results in invagination into the infundibular cavity, dilation of the infundibular recess and the third ventricle with eventual compression or replacement of the hypothalamus, and possible extension of the neoplasm into the thalamus (see Fig. 12-16).
Bilateral enlargement of the adrenal glands occurs with functional corticotroph adenomas (see Fig. 12-17). This enlargement is due to cortical hyperplasia, primarily of the zonae fasciculata and reticularis. Nodules of yellow-orange cortical tissue are often found outside the capsule and extending down into and compressing the adrenal medulla.
Pituitary corticotroph adenomas are composed of well-differentiated, large or small, chromophobic cells supported by fine connective tissue septa. The cytoplasm of the neoplastic cells usually is devoid of secretory granules but demonstrates immunoreactivity for ACTH +/− MSH, and hormone-containing secretory granules can be demonstrated by electron microscopy.
Disorders of the Neurohypophysis
Diabetes insipidus results when inadequate ADH is produced (hypophyseal form) or when target cells in the kidneys lack the biochemical pathways necessary to respond to the secretion of normal or increased circulating concentrations of ADH (nephrogenic form). The hypophyseal form of diabetes insipidus results from compression and destruction of the pars nervosa, infundibular stalk, or supraoptic nucleus in the hypothalamus. The disruption of ADH synthesis or secretion in hypophyseal diabetes insipidus can be a result of a large pituitary neoplasm, a dorsally expanding cyst, inflammatory granuloma, or traumatic injury to the skull with hemorrhage and glial proliferation in the neurohypophyseal tissue. Compression or disruption of the posterior lobe, infundibular stalk, and hypothalamus by neoplastic cells interrupts the transport of ADH in nonmyelinated axons from the site of production, primarily in the supraoptic nucleus of the hypothalamus, to the site of release in the capillary plexus of the pars nervosa.
Animals with diabetes insipidus excrete large volumes of hypotonic urine, which in turn necessitates the ingestion of large amounts of water to prevent dehydration and hyperosmolality of body fluids. Urine osmolality is decreased below normal plasma osmolality (approximately 300 mmol/L) in both hypophyseal and nephrogenic forms of diabetes insipidus. In response to water deprivation, urine osmolality still remains below that of the plasma in both forms of diabetes insipidus in contrast to increased osmolality observed in normal animals. Urine osmolality is increased above that of plasma in response to exogenous ADH in the hypophyseal form, but this increase does not occur in nephrogenic diabetes insipidus, a useful feature in differential diagnosis.
Disorders of the Adrenal Cortex
Hypoadrenocorticism (Addison’s Disease)
Adrenal cortical insufficiency was the first recognized endocrine disease and is a common endocrinopathy in dogs; however, it can occur in all animal species. Heritable hypoadrenocorticism has been reported in standard poodles, Portuguese water dogs, bearded collies, and Nova Scotia duck tolling retrievers. Clinical signs are a result of deficient production of any or all classes of corticosteroids (mineralocorticoids, glucocorticoids, and adrenal sex steroids). The synthesis and secretion of mineralocorticoids are reduced, resulting in marked alterations of serum potassium, sodium, and chloride concentrations (see Fig. 12-6). Less potassium is excreted by the kidneys (hypokaluria), resulting in severe hyperkalemia. Less sodium and chloride are reabsorbed from renal tubules, leading to varying degrees of hypernaturia and hyperchloriduria and a corresponding decline in blood concentrations of these ions. The severe hyperkalemia frequently produces notable cardiovascular disturbances. The pronounced bradycardia that develops in some dogs (heart rate of 50 or fewer beats per minute) does not change with exercise but does predispose to weakness and circulatory collapse after minimal exertion.
A decreased production of glucocorticoids results in several characteristic functional disturbances of hypoadrenocorticism. A failure of gluconeogenesis and increased sensitivity to insulin contributes to the development of moderate hypoglycemia. Hyperpigmentation of the skin occurs in some dogs with long-standing adrenocortical insufficiency. This lesion results from a lack of negative feedback to the pituitary gland and the increased release of ACTH (and possibly MSH). The plasma cortisol concentrations in dogs with hypoadrenocorticism are low and range from 0.1 to 1.5 µg/dL. Because of the severe atrophy of the adrenal cortex, little or no increase in blood cortisol concentration results after the administration of ACTH.
Adrenalitis: Bacterial and parasitic agents frequently localize in the adrenal glands and produce varying degrees of inflammation and necrosis. Focal inflammatory processes usually are suppurative, arising in the course of bacterial septicemias. The adrenal capsule provides an effective barrier against direct invasion by inflammatory reactions in adjacent tissue. Granulomatous adrenalitis caused by Histoplasma capsulatum, Coccidioides immitis, or Cryptococcus neoformans occasionally occurs in dogs and cats. Multiple granulomas with central areas of necrosis and calcification can destroy nearly the entire adrenal cortex. Toxoplasma gondii produces necrosis with an infiltration of histiocytes in the adrenal cortex of many species of animals. Experimental evidence suggests that large local concentrations of antiinflammatory steroids in the adrenal cortex (e.g., cortisol and corticosterone) suppress local cell-mediated immunity and permit the progressive growth of certain fungi (e.g., Histoplasma capsulatum, Coccidioides immitis), protozoa (e.g., Babesia darlingi, Babesia jellisoni, or Toxoplasma gondii), and bacteria (e.g., Mycobacterium tuberculosis).
Adrenocortical Hemorrhage (Waterhouse-Friderichsen Syndrome): Massive, diffuse, often bilateral adrenal cortical hemorrhage, a condition known as Waterhouse-Friderichsen syndrome, is an uncommon but fatal consequence of overwhelming sepsis (Fig. 12-25). Although frequently seen in conjunction with endotoxic shock, Waterhouse-Friderichsen syndrome can also result from Gram-positive organisms and noninfectious causes such as anticoagulant therapy and trauma.
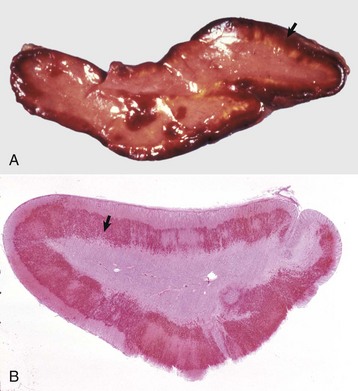
Fig. 12-25 Adrenocortical hemorrhage (Waterhouse-Friderichsen syndrome), adrenal gland, horse.
A, Diffuse hemorrhage (arrow) affecting the adrenal cortex is frequently seen in endotoxic shock. B, Subgross photomicrograph of diffuse hemorrhage (arrow) affecting the adrenal cortex. H&E stain. (Courtesy College of Veterinary Medicine, University of Illinois.)
Idiopathic Adrenocortical Atrophy: See the section on Disorders of Dogs for a discussion of idiopathic adrenocortical atrophy.
Hyperadrenocorticism (Cushing’s Syndrome or Disease)
Cortisol excess, or Cushing’s syndrome, is one of the most common endocrinopathies in adult and aged dogs, increasingly in cats and rarely in other domestic animals. The clinical manifestations and lesions characteristic of the syndrome of hyperadrenocorticism result primarily from chronic overproduction of cortisol by hyperactive cells of the adrenal cortex. Affected animals develop a spectrum of functional disturbances and lesions resulting from the combined glyconeogenetic, lipolytic, protein catabolic, and antiinflammatory effects of the glucocorticoid hormones on many organs. The disease is insidious and slowly progressive. The increase in circulating cortisol concentrations in dogs with hyperadrenocorticism can result from one of several different pathogenic mechanisms. The most common cause of Cushing’s syndrome is Cushing’s disease in which a functional corticotroph (ACTH-secreting) adenoma of the pituitary gland causes bilateral adrenal cortical hypertrophy and hyperplasia (Fig. 12-26). The cortex of each adrenal gland is widened considerably as a result of diffuse and nodular hyperplasia, primarily in the zona fasciculata. Functional adrenal gland neoplasms are an infrequent (10% to 15% of cases) cause of Cushing’s syndrome in the dog. Many of the clinical signs and lesions of naturally occurring hyperadrenocorticism can be induced by the long-term, daily administration of large doses of corticosteroids. To accurately separate the different pathogenetic mechanisms responsible for cortisol excess, plasma cortisol concentrations must be evaluated with the animal in the basal state and then after dexamethasone (large or small dose) suppression and ACTH stimulation.
Fig. 12-26 Adrenal cortices, disturbances of growth, adrenal glands, dogs.
A, Laminar hyperplasia: diffuse thickening of the zona fasciculata and zona reticularis secondary to the effects of a “functional” corticotroph adenoma in the pituitary gland. B, Nodular hyperplasia: multiple discrete hyperplastic nodules extending from the cortex into the medulla. C, Adenoma: note the well-demarcated, large, yellow-tan adenoma compressing the adjacent medulla. D, Adenocarcinoma: large carcinoma with hemorrhage and necrosis. (A courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University. B courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. C courtesy Dr. B. Weeks, College of Veterinary Medicine, Texas A&M University and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. D courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Appetite and food intake often are increased as a direct result of either the hypercortisolism or damage caused by compression of the hypothalamic appetite centers by a large, dorsally expanding pituitary gland neoplasm. The muscles of the extremities and abdomen are weakened and atrophied, resulting in gradual abdominal enlargement (Fig. 12-27), lordosis, muscle trembling, and a straight-legged, skeletal-braced posture assumed to support the body’s weight. Hepatomegaly caused by increased deposits of lipid and glycogen (steroid hepatopathy; see Fig. 8-73) can contribute to the development of the distended, often pendulous, abdomen. The muscular asthenia and wasting are the result of increased catabolism of structural proteins combined with diminished protein synthesis in skeletal myocytes from the influence of long-term cortisol excess. Cutaneous lesions occur frequently with hyperadrenocorticism. The initial changes in the skin often are observed over points of wear (e.g., neck, flanks, or behind the ears) and bony prominences. These initial cutaneous changes spread in a bilaterally symmetric pattern to involve a significant percentage of the body surface (see Fig. 12-27). Cutaneous lesions caused by excessive cortisol include atrophy of the epidermis and pilosebaceous units, combined with loss of collagen and elastin in the dermis and subcutis. Cutaneous calcification or calcinosis cutis is a characteristic lesion and occurs in up to 30% of dogs with hypercortisolism. Numerous calcium crystals are deposited along collagen and elastin fibers in the dermis and can penetrate through the atrophic and thinned epidermis, primarily in dogs and often with normal blood calcium and phosphorus concentrations. This manifestation of hypercortisolism is most likely related to the glyconeogenetic and protein catabolic action of cortisol, which results in the rearrangement of the molecular structure of such proteins as collagen and elastin, and the formation of an organic matrix that attracts and binds calcium. Severe calcification also occurs in other tissues such as lungs, active skeletal muscle, and the stomach.

Fig. 12-27 Cushing’s-like disease, hypercortisolism, dog, poodle.
Hypercortisolism after exogenous corticosteroid administration for the treatment of idiopathic adrenal cortical hyperplasia. Muscle asthenia is the cause of the pendulous abdomen. Note the alopecia of the skin of the abdomen, ventral cervical region, and tail. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Hyperaldosteronism (Conn’s Syndrome)
Hyperaldosteronism is an increasingly recognized entity in cats and rarely in dogs because of unilateral or bilateral aldosterone-secreting adrenocortical neoplasms or adrenal hyperplasia. Unilateral adrenal adenomas are more common in cats. An adrenocortical carcinoma concomitantly secreting aldosterone, corticosterone, and cortisol has been reported. Older cats present with systemic hypertension and polymyopathy associated with hypernatremia and hypokalemia, respectively.
Congenital Adrenal Hyperplasia (Adrenogenital Syndrome): Congenital adrenal hyperplasia is a condition of hypoadrenocorticism and hyperadrenocorticism. Affected individuals are deficient in an enzyme, such as 21-hydroxylase, which is involved in both mineralocorticoid and glucocorticoid synthesis. As a consequence of reduced cortisol levels, there is a compensatory increase in ACTH secretion resulting in adrenal cortical hyperplasia. Subsequent steroid synthesis is diverted to the androgenic pathway, which is independent of 21-hydroxylase. Excessive androgen production leads to virilization and sexual ambiguity in newborns and premature closure of epiphyses. Pomeranians are predisposed to a congenital adrenal hyperplasia–like syndrome and associated dermatosis; however, no mutations in the 21-hydroxylase enzyme have been found in the small group of Pomeranians evaluated.
Hyperplasia and Neoplasia of Adrenal Cortex:
Accessory Adrenal Tissue: Accessory or ectopic adrenal cortical tissue is common in the adrenal glands of adult to aged animals and can be found in the capsule, cortex, and medulla. Many of these nodules arise either as evaginations of the cortex into the capsule and surrounding periadrenal adipose tissue or invaginations of the cortex into the medulla.
Hyperplasia: Nodular hyperplasia is common in the adrenal glands as well-defined spherical nodules in the cortex or attached to the capsule (see Fig. 12-26). Hyperplastic nodules are usually multiple, bilateral and yellow, and involve any of the three zones of the cortex. Histologically, the nodules near the capsule resemble the zona glomerulosa and sometimes the zona fasciculata. The lipid content in these hyperplastic nodules is retained in circumstances that reduce the amount of lipid in the normal adrenal cortex. Hyperplastic cortical nodules are most common in older horses, dogs, and cats. Nodular hyperplasia of the zona reticularis has been seen in animals with functional disturbances suggestive of androgen excess (e.g., greater muscle mass, well-developed crest, clitoral hypertrophy, and mammary gland involution).
Diffuse cortical hyperplasia results in a uniform, usually bilateral, enlargement of the adrenal cortices (see Fig. 12-26). Notable hypertrophy and hyperplasia of cells of the zonae fasciculata and reticularis occur in response to an autonomous hypersecretion of ACTH by a corticotroph adenoma of the pituitary gland (see Fig. 12-17). The cytoplasm of the hyperplastic cells of the zona fasciculata is vacuolated because of the lipid content. Cells of the outer zona glomerulosa can be compressed by the expansion of the inner two zones. Nodular hyperplasia can be present in a diffusely hyperplastic cortex.
Cortical Adenomas and Carcinomas: Adenomas of the adrenal cortex occur most frequently in older dogs and only sporadically in horses, cattle, and sheep. Castrated male goats have a greater incidence of cortical adenomas than intact males. Although these neoplasms are usually incidental findings at necropsy, they are occasionally endocrinologically active. Cortical adenomas are well demarcated and usually are a single, unilateral nodule (see Fig. 12-26). Larger cortical adenomas are yellow to red, distort the contour of the gland, compress the adjacent cortical parenchyma, and are partially or completely encapsulated. Cortical adenomas often develop in an adrenal gland with multiple nodules of hyperplasia and can be difficult to differentiate grossly from hyperplastic nodules. However, these hyperplastic nodules consist of multiple small foci, usually in both adrenal glands, without encapsulation, and often with extracapsular nodules of hyperplastic cortical tissue. Cortical adenomas are composed of well-differentiated cells that resemble secretory cells of the normal zona fasciculata or zona reticularis. The cytoplasm of neoplastic cells is abundant and lightly eosinophilic and filled with lipid droplets. Adenomas are partially or completely surrounded by a fibrous connective tissue capsule of varying thickness and a rim of compressed cortical parenchyma.
Adrenal cortical carcinomas occur less frequently than adenomas and have been reported most often in cattle and older dogs, but they also occur infrequently in other species. Carcinomas develop in adult to older dogs with no apparent breed or sex prevalence. Adrenal carcinomas are larger than adenomas, more likely to be bilateral, and frequently obliterate the affected gland(s). In dogs, they are composed of variegated, yellow-red, friable tissue (see Fig. 12-26) and can invade extensively into surrounding structures, such as the wall of the caudal vena cava, resulting in thrombus formation. Carcinomas attain considerable size in cattle (up to 10 cm or more in diameter) and have multiple areas of calcification or ossification.
Carcinomas are composed of more highly pleomorphic secretory cells than adenomas and are subdivided by a fine fibrovascular stroma. The growth pattern of neoplastic cells varies between neoplasms and within the same carcinoma and can be trabecular, lobular, or focal. Neoplastic cells usually are large and polyhedral, and they have prominent nucleoli and densely eosinophilic or vacuolated cytoplasm.
Carcinomas and adenomas of the adrenal cortex in dogs occasionally are functional and secrete excessive amounts of cortisol. The clinical syndrome produced by excessive secretion by adrenal cortical carcinomas can be complicated by signs produced by compression of adjacent organs when the neoplasm is large; by invasion into the aorta or caudal vena cava, which can lead to intraabdominal hemorrhage; and by metastases to distant sites (e.g., liver, kidneys, mesenteric lymph nodes, and lungs). Functional cortical adenomas and carcinomas are responsible for severe atrophy of the contralateral adrenal cortex because of negative feedback inhibition of pituitary ACTH secretion by the increased blood cortisol concentrations (Fig. 12-28). The atrophic cortex consists primarily of the adrenal capsule and zona glomerulosa. Atrophy is also present in the remnants of the adrenal cortex compressed by functional adenomas. Because of the lack of cortical tissue, the adrenal medulla appears more prominent (see Fig. 12-28).
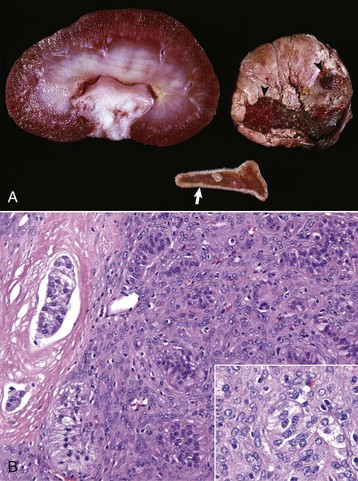
Fig. 12-28 Adrenocortical carcinoma and contralateral cortical atrophy, adrenal glands, dog.
A, The adrenal gland (right) has a large adrenocortical carcinoma that is almost half the size of an adult kidney (left). Multifocal to coalescing areas of hemorrhage and necrosis are apparent (arrowheads) in this tumor. The cortex of the contralateral adrenal gland (lower) is notably thinned (arrow) because of severe trophic atrophy of the zona fasciculata and zona reticularis. Also see Fig. 12-31. B, Note the invasive adrenocortical carcinoma (right) and the vascular invasion characteristic of this tumor in the left margin of the figure. Inset: Cells are haphazardly arranged and there is marked variation of cellular and nuclear size, typical of the cells that form anaplastic neoplasms. H&E stain. (A courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University. B courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Functional Proliferative Lesions in Ferrets: Adrenal gland neoplasms are the second most common neoplasm reported in ferrets and are recognized with increasing frequency as more ferrets are kept as pets and living longer. The adrenal gland enlargements are either bilateral (approximately 45%) as a result of diffuse (most frequent) or nodular hyperplasia, or unilateral (approximately 55%) as a result of adrenal cortical carcinoma or cortical adenoma. Adrenal gland neoplasms develop in adult ferrets (mean age 5 years) with females more frequently affected than males (sex ratio ≥2 : 1), and in animals gonadectomized at an early age (2 to 4 months). The latter finding is attributed to chronic trophic stimulation of the zona reticularis by LH.
A unique histologic feature of adrenocortical carcinomas in ferrets is the presence of a spindle cell component to the neoplastic cortical cells. These spindle cells express smooth muscle actin and arise from capsular or subcapsular smooth muscle cells, or they might represent a morphologically distinct adrenocortical cell. An emerging histologic variant of ferret adrenocortical carcinomas demonstrating increased invasiveness is characterized by myxoid differentiation. In addition, immunoreactivity to the transcription factor GATA-4 has been identified as a marker of anaplasia in ferret adrenocortical tumors.
Clinical signs in ferrets with adrenal cortical neoplasms include vulvar enlargement, bilaterally symmetric alopecia (especially on the ventral abdomen and medial aspects of the rear legs), polyuria, polydipsia, and the presence of a palpable mass at the cranial pole of the kidneys (left side greater frequency than right). Other functional disturbances, including anemia and thrombocytopenia, pyometra, and endometrial hyperplasia in females and squamous metaplasia of prostatic ductular epithelium and cystic prostatic disease in males, are changes consistent with an overproduction of estrogenic steroids by the adrenal gland neoplasms. Some of the functional disturbances resemble those seen in intact females with persistent or prolonged estrus; ferrets are seasonally polyestrous and are induced ovulators. About one-third of ferrets with adrenal cortical neoplasms also have neoplasms derived from the insulin-producing B cells of the pancreatic islets. These neoplasms can produce increased concentrations of serum insulin resulting in hypoglycemia that can lead to seizures, episodic lethargy, ptyalism, ataxia, and hind leg weakness.
The most consistent endocrinologic change in ferrets with adrenal gland neoplasms is increased plasma concentrations of estradiol-17β. It is presumed that the estradiol-17β is produced directly by the neoplastic cells, but alternatively, the adrenal gland neoplasms could secrete androgenic steroids that are aromatized in the skin and possibly elsewhere to estrogenic steroids. No increase in circulating estradiol-17β concentrations is found in response to exogenous ACTH, but plasma concentrations decrease after adrenalectomy. Plasma cortisol and corticosterone concentrations in ferrets with adrenal gland neoplasms are in the low-to-normal range and are not increased greatly in response to exogenous ACTH. Plasma cortisol is not decreased after unilateral adrenalectomy, and the contralateral adrenal cortex is not atrophic as would be expected if the adrenal gland neoplasm was secreting excess cortisol. The clinical signs in ferrets with adrenal cortical neoplasms can be effectively reversed by adrenalectomy (especially of the left side if there is no macroscopic enlargement) but not by chemotherapy with mitotane (o,p′-DDD). Complete regrowth of hair usually occurs by 2 to 3 months after adrenalectomy.
Disorders of the Adrenal Medulla
Adrenal Medullary Hyperplasia: Diffuse or nodular adrenal medullary hyperplasia appears to precede the development of pheochromocytoma in bulls with C-cell neoplasms of the thyroid gland. The proliferated chromaffin cells are nonencapsulated but compress the surrounding adrenal cortex (Fig. 12-29). Hyperplastic cells are round to oval and have pale basophilic cytoplasm. Some bulls with prominent diffuse medullary hyperplasia also often have a few small proliferative nodules. Medullary hyperplasia is diagnosed on the basis of the following criteria: an increased adrenal weight, a decrease in cortical to medullary ratio because of an increase in the size and number of medullary cells, and numerous mitotic figures in the adrenal medulla.
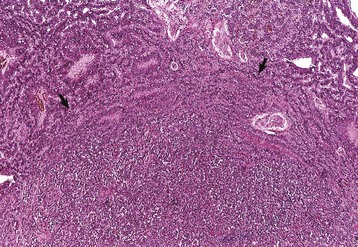
Fig. 12-29 Hyperplasia, adrenal medulla, bull.
Bilateral diffuse hyperplasia of adrenal medulla in a bull with a concomitant C-cell carcinoma of the thyroid gland. The expanded adrenal medulla (bottom) has compressed the adjacent adrenal cortex (arrows). H&E stain. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Neuroblastomas and Ganglioneuromas: Neuroblastomas usually occur in young animals, arise from primitive neuroectodermal cells, and form large intraabdominal masses. The tumors are composed of small neoplastic cells that have hyperchromatic nuclei and scant amounts of cytoplasm and resemble lymphocytes. Neoplastic cells tend to aggregate around blood vessels, forming pseudorosettes. Neurofibrils or unmyelinated nerve fibers can be demonstrated in neuroblastomas.
Ganglioneuromas are benign neoplasms composed of multipolar ganglion cells and neurofibrils and have a prominent fibrous connective tissue stroma. The surrounding adrenal cortex is often severely compressed. Occasionally, neoplastic cells in adrenal medullary neoplasms differentiate into two cell lines, resulting in pheochromocytoma and ganglioneuroma in the same adrenal gland.
Pheochromocytomas: Pheochromocytomas are the most common neoplasms in the adrenal medullas of animals, occurring most often in cattle and dogs and infrequently in other species. Calcitonin-secreting C-cell neoplasms of the thyroid gland occasionally develop concurrently with pheochromocytomas in bulls. Extraadrenal pheochromocytomas, referred to as paragangliomas, also occur infrequently in the abdomen.
Pheochromocytomas often are large (10 cm or more in diameter) and replace most of the affected adrenal gland. Smaller neoplasms are completely surrounded by a thin, compressed rim of adrenal cortex (Fig. 12-30). Large pheochromocytomas are multilobular and variegated light brown to yellow-red as a result of areas of hemorrhage and necrosis. Malignant pheochromocytomas invade through the capsule of an adrenal gland into adjacent structures, such as the caudal vena cava (Fig. 12-31), and metastasize to distant sites, including the liver, regional lumbar aortic and renal lymph nodes, or lungs. Histologically, neoplastic cells vary from small, round to polyhedral cells to large, pleomorphic cells with multiple hyperchromatic nuclei. The cytoplasm is lightly eosinophilic, finely granular, and often indistinct.
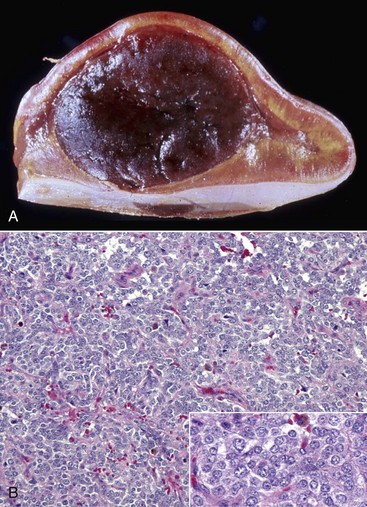
Fig. 12-30 Pheochromocytoma, adrenal gland, horse.
A, Pheochromocytoma compressing the adjacent unaffected adrenal cortex. Note the yellow color of the adrenal cortex attributable to the accumulation of intracellular lipids used to synthesize cortisol. Also see Fig. 12-31. B, Neoplastic chromaffin cells are haphazardly arranged in poorly demarcated lobules of varied sizes. There is moderate variation in cellular and nuclear size and shape. Inset: Higher magnification of the chromaffin cells. H&E stain. (A courtesy Dr. B. Weeks, College of Veterinary Medicine, Texas A&M University and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
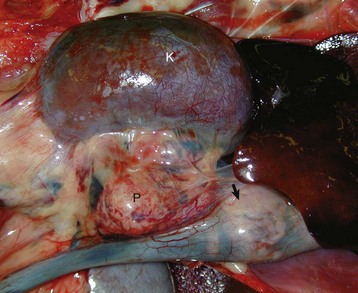
Fig. 12-31 Pheochromocytoma, kidney, adrenal gland, caudal vena cava, dog.
A large pheochromocytoma (P) has obliterated the adrenal gland medial to the kidney (K) and has extensively invaded into the lumen of the caudal vena cava (arrow). (Courtesy Dr. A. Paulman, College of Veterinary Medicine, University of Illinois.)
Functional pheochromocytomas composed of epinephrine- and/or norepinephrine-secreting cells have been reported infrequently in animals. Tachycardia, edema, and cardiac hypertrophy observed in several dogs and horses with pheochromocytomas were attributed to excessive catecholamine secretion. Arteriolar sclerosis and widespread medial hyperplasia of arterioles have been reported in dogs with pheochromocytomas and clinical signs suggestive of paroxysmal hypertension. Hypertension was detected in 43% of dogs with pheochromocytomas; all hypertensive dogs had concurrent diseases that could have contributed to the increase in blood pressure. Infrequently, hyperadrenocorticism occurs concurrently in dogs with pheochromocytoma and could contribute to the development of hypertension, particularly during digital manipulation of the affected adrenal gland during surgery.
Norepinephrine is the predominant catecholamine extracted from pheochromocytomas in dogs. This is similar to the finding in normal pups, in which norepinephrine is the predominant catecholamine; in adult dogs, epinephrine predominates in adrenal medullary tissues. The catecholamine content of pheochromocytomas in bulls with concurrent C-cell neoplasms of the thyroid gland is greater than in the normal adrenal medulla. Urinary excretion of free unconjugated catecholamines and metabolites of catecholamines, such as vanillylmandelic acid, are increased in bulls with pheochromocytomas.
Disorders of the Thyroid Gland
Accessory Thyroid Tissue: The thyroid gland originates embryologically as a thickened plate of epithelium in the ventral aspect of the pharynx. The complex embryogenesis of the thyroid frequently leads to the formation of accessory thyroid tissue. This accessory thyroid tissue is most typically located in the mediastinum but can be located anywhere from the base of the tongue to the diaphragm. About 50% of adult dogs have nodules of accessory thyroid tissue embedded in the adipose around the base of the heart and the origin of the aorta. The follicular structure and function are the same as those of the main thyroid lobes. Attempts to induce hypothyroidism in the dog by surgical thyroidectomy are usually unsuccessful because the accessory thyroid tissue readily responds to the prompt increase in endogenous TSH secretion and can undergo sufficient hyperplasia to sustain adequate hormone production. This accessory thyroid tissue can also undergo neoplastic transformation.
Thyroglossal Duct Cysts: Thyroglossal duct cysts develop most frequently in dogs and pigs and are present occasionally in other animals. They form as a result of persistence of portions of the midline embryologic primordia of the thyroid gland, which migrate caudally from the ventral aspect of the primitive pharynx to form the thyroid lobes postnatally. These cysts are present in the ventral aspect of the cervical region in dogs and are fluctuant masses that can rupture and form a tract to the exterior. Their lining epithelium consists of multiple layers of follicular cells in which colloid-containing follicles are found occasionally. These cells sometimes undergo neoplastic transformation and give rise to papillary carcinomas.
Hypothyroidism
Hypothyroidism is a well-recognized and clinically important disease in dogs but is encountered only occasionally in other animals. Although the disease occurs in many adult purebred and mixed breed dogs, certain breeds, such as the golden retriever, Doberman pinscher, dachshund, Shetland sheep dog, Irish setter, miniature schnauzer, cocker spaniel, and Airedale, are more commonly affected. Hypothyroidism in dogs is usually the result of primary lesions in the thyroid gland, particularly idiopathic follicular collapse and lymphocytic thyroiditis. Less common causes of hypothyroidism include bilateral nonfunctional follicular cell neoplasms and severe iodine-deficient goiter. Hypothyroidism caused by long-standing pituitary gland or hypothalamic lesions, which prevent the release of either TSH or thyrotropin-releasing hormone, is encountered infrequently in the dog. In these cases, the thyroid gland is moderately reduced in size and composed of colloid-distended follicles, lined by flattened follicular cells.
Many functional disturbances reported with hypothyroidism occur because of a reduction in basal metabolic rate. A gain in body weight without an associated change in appetite occurs in some hypothyroid dogs. Thinning of the hair coat is often accompanied by bilaterally symmetric alopecia. Areas affected initially by hair loss are those receiving frictional wear such as the tail and cervical area.
Hyperkeratosis is a consistent finding in hypothyroidism and results clinically in an increased scaliness of the skin. When severe, it occurs as circular scaly patches resembling seborrhea. Microscopically, hyperkeratosis (Fig. 12-32) involves the external root sheath, resulting in follicular keratosis. Hyperpigmentation, especially in such localized areas of alopecia as the dorsal aspect of the nose and distal portion of the tail, occurs in many dogs with hypothyroidism. Myxedema may also develop because of the accumulation of glycosaminoglycans and hyaluronic acid in the dermis and subcutis. These substances bind considerable amounts of water, which results in notable thickening of the skin. Microscopically, mucins appear as granular or fibrillar material in hematoxylin and eosin (H&E)-stained sections.
Fig. 12-32 Hypothyroidism, skin, dog.
Hyperkeratosis (arrows) has resulted in thickening of the epidermis of the skin. H&E stain. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Reproductive abnormalities include lack of libido, reduced sperm count, abnormal or absent estrus cycles, and reduced conception rates. In chronic hypothyroidism, spermatogenic epithelium in the testes is often notably atrophic.
Hypothyroidism in dogs is accompanied by decreased circulating thyroidal hormone concentrations and decreased 131I uptake by the thyroid gland. In dogs with hypothyroidism, the concentration of T4 is usually below 0.8 µg/dL (normal 1.5 to 3.4 µg/dL) and that of T3 is below 50 ng/dL (normal 48 to 150 ng/dL). In the euthyroid dog, T4 concentrations will at least double 8 hours after intravenous or intramuscular injection of TSH. In dogs with hypothyroidism, the T4 concentrations do not change significantly after injection of TSH.
Serum cholesterol concentrations are often greatly increased (300 to 900 mg/dL) in many hypothyroid dogs (normal 40 to 80 mg/dL). The marked hypercholesterolemia in long-standing and severe hypothyroidism results in a variety of secondary lesions, including atherosclerosis, hepatomegaly, and glomerular and corneal lipidosis. Atherosclerosis of coronary (Web Fig. 12-5; also see Fig. 10-38) and cerebral vessels develops in dogs that have severe hypothyroidism and long-standing hyperlipidemia.
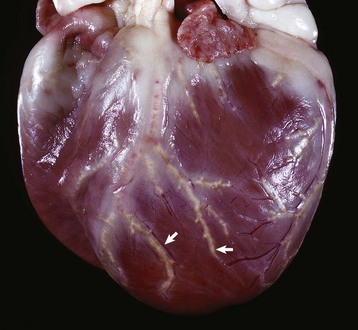
Web Fig. 12-5 Atherosclerosis, hypothyroidism with marked hyperlipidemia, heart, coronary arteries, dog.
Note the atherosclerosis (arrows) of the coronary arteries, which are thickened, firm, yellow-white, and often beaded. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Congenital Dyshormonogenetic Goiter: Congenital dyshormonogenetic goiter in sheep (Corriedale, Dorset Horn, Merino, and Romney breeds), Afrikaner cattle, Saanen dwarf goats, dogs (rat and toy fox terriers), and cats (Abyssinian and Japanese) is inherited as an autosomal recessive trait. Indications that affected young are clinically hypothyroid include subnormal growth rate, abnormal wool or guard hair development, subcutaneous myxedematous swellings, lethargy, and abnormal mentation.
Thyroid lobes are symmetrically enlarged at birth because of an intense diffuse hyperplasia of follicular cells (Fig. 12-33). Thyroid follicles are often collapsed because of lack of colloid resulting from the notable endocytic activity. Follicles are lined by tall columnar follicular cells, which have dilated profiles of rough endoplasmic reticulum, large mitochondria, and dense lysosomal granules associated with the Golgi apparatus, but have few thyroglobulin-containing apical vesicles near the luminal plasma membrane. Numerous long microvilli extend into the follicular lumen.
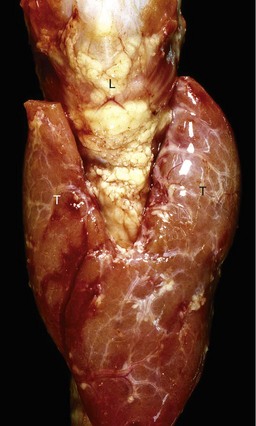
Fig. 12-33 Congenital dyshormonogenetic goiter, thyroid gland, lamb.
The symmetrically enlarged thyroid (T) lobes are fused at the midline ventral to the larynx (L) and trachea. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Although thyroid scintigraphy can be normal, circulating T3 and T4 concentrations after TSH stimulation are consistently low. The lack of a defect in iodide uptake or organification, plus an absence of normal 19S thyroglobulin in goitrous thyroids and only minute amounts of thyroglobulin-related antigens (0.01% of normal) suggest impaired thyroglobulin biosynthesis in sheep, cattle, and goats. Organification defects caused by mutations in thyroid peroxidase have been documented in dogs and cats.
Idiopathic Follicular Atrophy: In follicular atrophy, or “collapse,” the loss of follicular epithelium and disruption of follicles is progressive and the gland is replaced by adipose connective tissue with only a minimal inflammatory response. The gland usually is smaller and lighter in color than normal (Fig. 12-34). The early lesion in dogs with mild clinical signs of hypothyroidism appears to be confined to one part of the thyroid gland. The affected part is composed of small follicles that contain little colloid and are lined by tall columnar follicular cells. A more advanced form of follicular atrophy is present in dogs with clinical hypothyroidism and low blood concentrations of thyroidal hormones. These thyroid glands are notably reduced in size and are composed predominantly of adipose connective tissue with only a few clusters of small follicles containing vacuolated colloid.
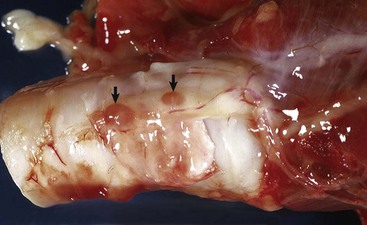
Fig. 12-34 Atrophic thyroid gland, dog.
The thyroid gland on the lateral surface of the trachea is very small and pale tan. As a result, the two parathyroid glands are very prominent (arrows). (Courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Lymphocytic (Immune-Mediated) Thyroiditis: Lymphocytic thyroiditis in dogs and a large European population of horses closely resembles Hashimoto’s disease of humans with evidence suggesting a polygenic mode of inheritance, particularly in affected breeds such as laboratory beagles, giant schnauzers, Doberman pinschers, and Labrador retrievers. The immunologic basis of the development of chronic lymphocytic thyroiditis is through production of autoantibodies, usually directed against thyroglobulin or a microsomal antigen such as thyroperoxidase and infrequently against the TSH receptor, a nuclear antigen, or a second colloid antigen from thyroid follicular cells.
Microscopic lesions consist of multifocal to diffuse infiltrates of lymphocytes which occasionally form nodules, plasma cells, and macrophages. Thyroid follicles are small and lined by columnar epithelial cells; lymphocytes, macrophages, and degenerate follicular cells are often present in the colloid, which is vacuolated (Fig. 12-35; also see Web Fig. 12-6). Thyroid C cells are present as small nests or nodules between follicles and often are more prominent than those in normal dogs. Some remaining follicular cells appear to be transformed into large oxyphilic cells with densely eosinophilic granular cytoplasm.
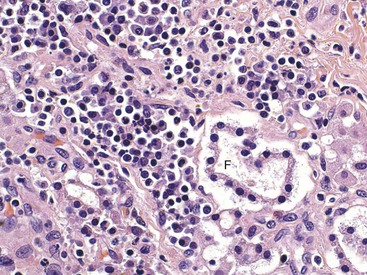
Fig. 12-35 Lymphoplasmacytic thyroiditis, severe hypothyroidism, dog.
Numerous lymphocytes, plasma cells, and a few macrophages have infiltrated the thyroid gland. Only occasional follicles (F) remain. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
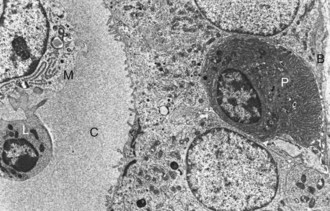
Web Fig. 12-6 Lymphocytic thyroiditis in a dog with severe hypothyroidism.
A lymphocyte (L) and macrophage (M) are present in the colloid (C) of a thyroid follicle. A plasma cell (P) within the follicular basement membrane (B) is infiltrating between thyroid follicular cells. TEM. Uranyl acetate and lead citrate stain. (From Gosselin SJ, Capen CC, Martin SL: Vet Immunol Immunopathol 3:185-201, 1982.)
Hyperthyroidism
Hyperthyroidism, or thyrotoxicosis resulting from elevated circulating levels of T4 and T3, commonly occurs in middle-aged to older cats and rarely in dogs. The condition in cats is associated with a spectrum of functional proliferative lesions, whereas in dogs it results from thyroid neoplasms.
Clinical signs result from an increased rate of basal metabolism. Consequently, animals are polyuric and polydipsic and frequently lose weight despite a voracious appetite. Weakness and fatigue, nervousness or hyperexcitability, and hyperthermia and heat intolerance are also present. In addition, cardiac manifestations of hyperthyroidism, such as tachycardia and dysrhythmias, are well documented in animals; however, secondary hypertrophic cardiomyopathy characterized by concentric hypertrophy is primarily restricted to cats.
In general, the likelihood of animals developing clinical hyperthyroidism secondary to thyroid neoplasms depends on (1) the capability of neoplastic cells to synthesize T4 and T3 (e.g., well-differentiated thyroid neoplasms that form follicles and produce colloid are more likely to synthesize thyroid hormones than poorly differentiated solid neoplasms) and (2) the extent of the increase in circulating concentrations of T4 and T3, which depends on a balance between the rate of secretion of thyroid hormones by the neoplasm, and the rate of degradation of thyroid hormones. Dogs have a much more efficient enterohepatic excretory mechanism for thyroid hormones, which are difficult to overload, than cats; therefore clinical hyperthyroidism in dogs with functional thyroid follicular cell neoplasms is infrequent. Cats are very sensitive to phenol and phenol derivatives and have a poor ability to conjugate phenolic compounds, such as T4, with glucuronic acid and excrete the T4 glucuronide into the bile. The capacity of conjugation of T3 with sulfate is limited and easily overloaded in cats.
Functional Proliferative Lesions in Cats: A spectrum of proliferative lesions that secrete excess thyroidal hormones (e.g., adenomas and multinodular hyperplasia) is common in the thyroid glands of adult and aged cats. Since the late 1970s, there has been a dramatic increase in the incidence of neoplasms and other focal proliferative lesions in the thyroid glands of cats and these have resulted in hyperthyroidism. Hyperthyroidism is one of the two most common endocrine diseases in adult to aged cats (diabetes mellitus is the other). Before 1980, clinical hyperthyroidism was infrequently diagnosed in cats. The reason for the apparently increased incidence is uncertain but appears to be related in part to (1) a larger population of geriatric cats receiving veterinary medical care, (2) improved assays for thyroid hormones, (3) detailed characterization of the clinical syndrome, and (4) increased awareness by veterinary clinicians of its common occurrence in adult to aged cats. In addition, there does appear to be a real increase in the incidence of feline hyperthyroidism over the past 30 years. Potential risk factors that have been reported include a predominantly indoor environment, regular treatment with flea powders, exposures to herbicides and fertilizers, a diet primarily of canned food, and non-Siamese breeds (10 times greater occurrence). It has been suggested that wide variations (excessive to inadequate) in dietary iodine intake over prolonged periods could play a role in the pathogenesis of hyperthyroidism in cats.
The disease in cats is mechanistically different from that of Graves’ disease in human patients in that hyperthyroid cats do not have increased concentrations of circulating thyroid-stimulating immunoglobulins, which are comparable to long-acting thyroid stimulator (LATS) in humans (LATS is an autoantibody that binds to the TSH receptor and activates follicular cells). Purified immunoglobulin G (IgG) prepared from hyperthyroid cats significantly increases 3H-thymidine incorporation into DNA and stimulates thyroid follicular cell proliferation fifteenfold but does not stimulate intracellular cAMP production. The 3H-thymidine incorporation can be completely inhibited by a specific TSH receptor blocking antibody. These data suggest that increased titers of thyroid growth-stimulating immunoglobulins are present in cats with hyperthyroidism and most likely act by the TSH receptor. In cats, the disease most closely resembles toxic nodular goiter in human patients with mutations in genes encoding for either the TSH receptor or the Gsα protein. Mutations in Gsα that correspond to the mutations in humans have been reported in a small group of hyperthyroid cats. Gsα is a G protein that mediates cAMP-dependent TSH signaling. Follicular cell proliferation, differentiation, and secretion of thyroid hormone result from constitutive activation of TSH signaling. In a separate study, all cases of feline follicular hyperplasia and adenomas evaluated by immunohistochemistry demonstrated overexpression of the c-ras oncogene. As was the case for activating Gsα mutations, overexpression of c-ras results in constitutive mitogenesis of thyroid follicular epithelial cells. Hyperplastic and neoplastic thyroid tissue from cats is transplantable into athymic (nude) mice and continues to overproduce T4 and T3 in a subcutaneous location.
Follicular cell adenomas, which often develop in a thyroid gland with multinodular hyperplasia, are more common than thyroid carcinomas. Follicles in the rim of thyroid tissue around a functional adenoma are notably enlarged and distended with colloid (colloid involution). The follicular cells are low cuboidal and atrophic with little evidence of endocytic activity in response to the increased concentrations of circulating thyroid hormones. In cats with solitary adenomas, the opposite thyroid lobe should be examined carefully for evidence of nodular hyperplasia or microadenomas. These small foci of multinodular follicular cell hyperplasia can be the cause of hyperthyroidism recurring several months to a year or more after surgical removal of a functional adenoma.
Hyperthyroidism in cats also occurs in association with bilateral multinodular adenomatous hyperplasia (goiter), which usually causes only slight enlargement of the affected lobe(s) (Fig. 12-36). In contrast to adenomas, areas of nodular hyperplasia are not encapsulated and the adjacent thyroid tissue is not compressed. Microscopically, hyperplastic nodules are composed of irregularly shaped, colloid-filled follicles lined by cuboidal follicular cells, and these nodules are considered a preneoplastic lesion as they can coalesce and form a thyroid follicular cell adenoma.
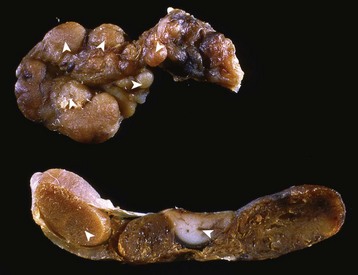
Fig. 12-36 Hyperplasia, hyperthyroidism, left and right thyroid glands, cat.
Multinodular follicular cell hyperplasia (arrowheads) involves both thyroid lobes. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Cats with hyperthyroidism usually have notably increased serum T4 and T3 concentrations, but some euthyroid cats have histologic evidence of nodular disease. The serum T4 concentrations in cats with hyperthyroidism range from 3.4 to 30 µg/dL (normal 1.5 to 5 µg/dL), and serum T3 concentrations range from 179 to 470 ng/dL (normal 60 to 200 ng/dL). Moderately increased serum enzyme activities, including serum alanine aminotransferase (ALT, SGPT), serum aspartate aminotransferase (AST, SGOT), and especially alkaline phosphatase, occur in hyperthyroid cats.
Hyperthyroid cats often have disturbances of calcium homeostasis and a concomitant diffuse chief cell hyperplasia in the parathyroid glands. Blood ionized calcium and plasma creatinine concentrations are significantly decreased, whereas plasma phosphorus and intact PTH concentrations are increased. Hyperparathyroidism occurs in 77% of hyperthyroid cats, with PTH concentrations elevated up to 19 times that of the upper limit of the reference range. Hyperphosphatemia is present in approximately 40% of hyperthyroid cats. The mechanisms for the development of hyperphosphatemia in feline hyperthyroidism are uncertain but could be related in part to polyphagia, resulting in increased intestinal phosphorus absorption, increased catabolism of muscle proteins, release of phosphorus because of the gluconeogenic effects of the increased thyroid hormone concentrations, and increased bone resorption with release of phosphorus into the blood. Hyperparathyroidism and chief cell hyperplasia appear to be related to the reciprocal decline in amounts of circulating ionized calcium in response to the hyperphosphatemia. Increased blood phosphorus concentrations also could decrease renal 1α-hydroxylase activity and decrease the production of the active form of vitamin D, thereby reducing intestinal calcium absorption. However, circulating concentrations of 1,25-(OH)2-dihydroxycholecalciferol were not decreased in a limited number of hyperthyroid cats evaluated.
Hyperplasia of Thyroid Follicular Cells (Goiter): Goiter is a clinical term used to describe a nonneoplastic and noninflammatory enlargement of the thyroid gland (Fig. 12-37). It develops in all domestic mammals as a result of hyperplasia of follicular cells. Certain forms of thyroid hyperplasia, especially nodular, are difficult to differentiate from adenomas. The major pathogenetic mechanisms for the development of thyroid hyperplasia include iodine-deficient diets, goitrogenic compounds that interfere with thyroxinogenesis, excess dietary iodide, and genetically determined defects in the enzymes or thyroglobulin that are essential for the biosynthesis of thyroidal hormones. All of these result in inadequate thyroxine synthesis and decreased blood concentrations of T4 and T3, which are detected by the hypothalamus. This in turn stimulates the pituitary gland to increase the secretion of TSH, resulting in hypertrophy and hyperplasia of follicular cells.
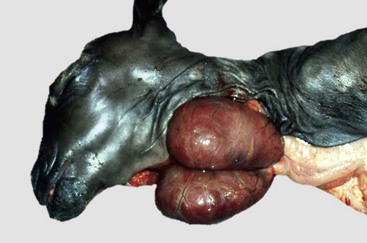
Fig. 12-37 Hyperplasia, thyroid goiter, goat.
Deficiency of maternal dietary iodine during pregnancy has resulted in bilateral hyperplasia (and hypertrophy) of thyroid follicular epithelial cells in this neonatal goat, which has resulted in a symmetric enlargement of the glands (goiter). (Courtesy Dr. O. Hedstrom, College of Veterinary Medicine, Oregon State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Diffuse Hyperplastic and Colloid Goiter: Dietary iodine deficiency that resulted in diffuse hyperplastic goiter was common in many areas of the world before the widespread addition of iodized salt to animal diets. Iodine-deficient goiter still occurs worldwide in domestic animals, but cases are sporadic and few animals are affected. Marginally iodine-deficient diets that contain goitrogenic compounds can cause severe thyroid follicular cell hyperplasia and goiter. Goitrogenic substances include glucocorticoids, phenobarbital, sulfonamides, thiocyanate, perchlorate, and a number of plants of the family Brassicaceae. Offspring of females fed iodine-deficient diets are likely to develop severe thyroidal follicular cell hyperplasia and have clinical signs of hypothyroidism. Both lateral lobes of the thyroid gland are uniformly enlarged in young animals as a result of diffuse hypertrophy and hyperplasia of follicular cells (see Fig. 12-9). The enlargements, when extensive, result in palpable or visible swellings in the cranial ventral cervical area. The affected lobes are firm and dark red because an extensive interfollicular capillary network develops under the influence of long-term TSH stimulation.
Colloid goiter represents the involutionary phase of diffuse hyperplastic goiter in both young and adult animals. It develops either after sufficient amounts of iodide have been added to the diet or after the requirements for thyroid hormones have diminished as the animal ages. The notably hyperplastic follicular cells continue to produce colloid, but endocytosis of colloid from the lumen is decreased. This is a consequence of the diminished TSH concentrations produced in response to the return of blood T4 and T3 concentrations to normal. Both thyroid lobes are diffusely enlarged but are more translucent and lighter in color than in hyperplastic goiter. These differences in macroscopic appearances are the result of less vascularity in colloid goiter and development of macrofollicles distended with colloid. Follicles are progressively distended (see Fig. 12-10) with densely eosinophilic colloid because of diminished TSH-induced endocytosis. As a result, follicular cells lining the macrofollicles are flattened and atrophic. The interface between the colloid and luminal surface of the follicular cells is smooth, and the cells lack the endocytic vacuoles characteristic of actively secreting thyroid follicular cells. Some involuted follicles in colloid goiter have remnants of the papillary projections on follicular cells extending into their lumens.
The changes in diffuse hyperplastic and colloid goiters are uniform throughout the diffusely enlarged thyroid lobes. Follicles are irregular in size and shape in hyperplastic goiter because they contain varying amounts of colloid, which is lightly eosinophilic and vacuolated. Some follicles collapse because of the lack of colloid. The lining epithelial cells are columnar and have deeply eosinophilic cytoplasm and small hyperchromatic nuclei, which are often situated in the basilar portions of the cells. The follicles are lined by single or multiple layers of hyperplastic follicular cells that in some follicles form papillary projections into the lumens (Fig. 12-38). Similar proliferative changes are present in ectopic thyroid tissue in the neck and mediastinum.
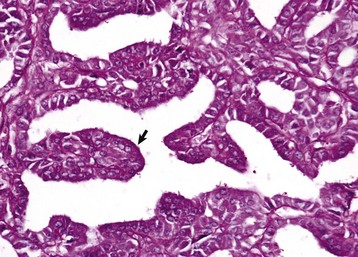
Fig. 12-38 Hyperplastic goiter, thyroid gland, dog.
Hyperplastic follicular epithelium forms a papillary projection (arrow), which extends into the follicular lumen devoid of colloid. Note that the majority of follicular lumens are small and collapsed. Periodic acid–Schiff reaction. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Although seemingly paradoxic, an excess of iodide in the diet also can result in thyroid hyperplasia in animals. Foals of mares fed dry seaweed containing excessive iodide develop thyroid hyperplasia and clinically evident goiter. The thyroid glands of suckling animals are exposed to greater blood iodide concentrations than those of the dam because iodide is concentrated first by the placenta and then by the mammary gland. Increased blood iodide interferes with one or more steps of thyroidal hormone synthesis and secretion, leading to lowered blood T4 and T3 concentrations and resulting in a compensatory increase in pituitary TSH secretion. Excess iodine blocks the release of T3 and T4 by interfering with proteolysis of colloid by lysosomal enzymes in thyroid follicular cells.
Multifocal Nodular Hyperplasia: Multifocal nodular hyperplasia in thyroid glands occurs in old horses, cats, and dogs and appears as multiple, white-to-tan nodules of variable size (see Fig. 12-36), giving affected lobes a moderately enlarged and irregular contour. Multifocal nodular goiter in most animals except cats is endocrinologically inactive and is an incidental lesion at necropsy. However, functional thyroid adenomas often develop in the thyroid glands of aged cats with hyperthyroidism and multinodular hyperplasia of follicular cells. In contrast to thyroid adenomas, the areas of nodular hyperplasia are not encapsulated and cause minimal to no compression of adjacent parenchyma. Thus nodular goiter consists of multiple foci of hyperplastic follicular cells that are sharply demarcated but not encapsulated from the adjacent thyroid tissue.
The microscopic appearance of nodular hyperplasia often varies. Some hyperplastic follicular cells form small follicles with little or no colloid. Other nodules are composed of larger, irregularly shaped follicles lined by one or more layers of columnar cells that form papillary projections into the lumen. Some of the follicles have undergone colloid involution and are filled with densely eosinophilic colloid. These changes appear to be the result of alternating periods of hyperplasia and colloid involution in the thyroid glands of aged animals.
Epithelial Neoplasms of the Thyroid Gland
Follicular Cell Adenomas: Adenomas usually are white-to-tan, small, solid nodules that are well demarcated from the adjacent thyroid parenchyma (Fig. 12-39). The affected thyroid lobe is only moderately enlarged and distorted; usually only a single adenoma is present in a thyroid lobe. A distinct, white, fibrous connective tissue capsule of variable thickness separates the adenoma from the adjacent compressed parenchyma. Some thyroid adenomas are composed of thin-walled cysts filled with a yellow-red fluid. Their external surfaces are smooth and covered by an extensive network of blood vessels. Small masses of neoplastic tissue remain in the wall and form rugose projections into the cyst. Adenomas are histologically classified as follicular and papillary types; the follicular type is more common in the thyroid gland of animals.
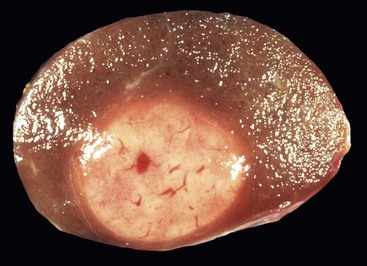
Fig. 12-39 Thyroid adenoma, thyroid gland, horse.
The thyroid gland contains an expansile, encapsulated, round, white-yellow mass that is well demarcated from normal thyroid gland. Note the prominent fibrous connective tissue capsule surrounding the adenoma. (Courtesy College of Veterinary Medicine, University of Illinois.)
Follicular Cell Carcinomas: In dogs, thyroid carcinomas occur more often than adenomas, but in cats adenomas are more common. Boxers develop thyroid carcinomas more frequently than any other breed of dog, but beagles and golden retrievers have a higher risk of developing thyroid carcinomas. Approximately 60% of thyroid carcinomas in dogs are clinically detectable by palpation as a firm mass in the neck and by evidence of respiratory distress caused by tracheal compression. Carcinomas become fixed in position as a result of extensive local invasion of adjacent structures, whereas localized adenomas are freely movable under the skin. Neoplasms of thyroid origin also occur in accessory thyroidal tissue, located anywhere between the base of the tongue and the cranial mediastinum.
Thyroid carcinomas often grow rapidly and invade adjacent structures such as the trachea, esophagus, and larynx (Fig. 12-40). The earliest and most frequent site of metastasis is to lungs because thyroid carcinomas, early in the course of development, invade branches of the thyroid vein. The retropharyngeal and caudal cervical lymph nodes are infrequent sites of metastases.
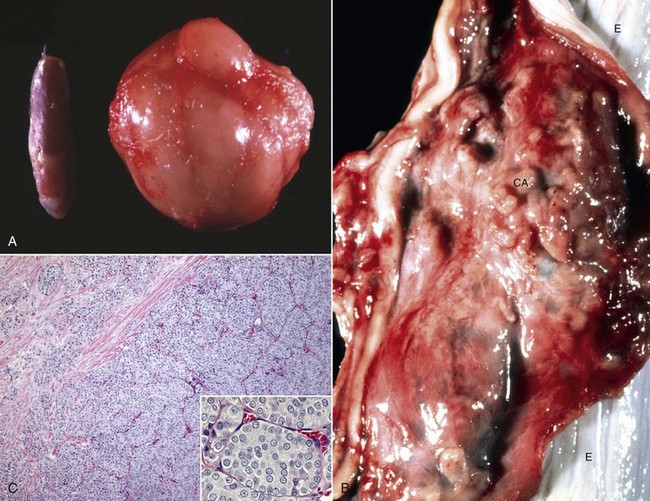
Fig. 12-40 Thyroid carcinoma, thyroid gland, dogs.
A, A large follicular carcinoma (right side) has obliterated the normal structure of the thyroid gland. A normal thyroid gland is shown on the left. B, The poorly demarcated and well-vascularized thyroid carcinoma (CA) is locally invasive and has extended into the wall of the esophagus. E, Esophageal mucosa. C, Neoplastic cells are haphazardly arranged into discrete islands. There is moderate variation in cellular and nuclear size and shape. Note the invasion of the tumor into the capsule of the thyroid gland (upper left). Inset, Higher magnification of the cells in the thyroid carcinoma. H&E stain. (A courtesy Dr. W. Crowell, College of Veterinary Medicine, The University of Georgia and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B courtesy College of Veterinary Medicine, University of Illinois. C courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Thyroid C (Ultimobranchial) Cell Neoplasms: Neoplasms derived from C cells of the thyroid gland occur most frequently in adult to aged bulls, occasionally in horses and dogs, and infrequently in other species. The incidence of C-cell neoplasms increases with advancing age in bulls that also typically have increased vertebral bone density. A high percentage of aged bulls fed calcium-rich diets develop C-cell neoplasms (30%) or hyperplasia of C cells and ultimobranchial derivatives (15% to 20%). The cause of C-cell neoplasms is unknown, but the chronic stimulation of C cells by large concentrations of calcium absorbed from the digestive tract can be responsible for their high incidence. A significant decline in the incidence of C-cell neoplasms occurs when the excessive calcium intake of bulls is reduced. Cows fed similar rations rarely develop proliferative lesions of C cells because of the greater physiologic requirements of pregnancy and lactation for calcium.
C-cell neoplasms in bulls are commonly found in association with other endocrine neoplasms, especially bilateral pheochromocytomas and occasionally pituitary adenomas. There are two distinct forms of the familial cancer syndrome known as multiple endocrine neoplasia (MEN) in humans. MEN1 is due to a disease-causing mutation in the MEN1 gene and is characterized by primary hyperparathyroidism, pancreatic islet cell tumors that are predominantly gastrinomas, and pituitary tumors that are predominantly prolactinomas. Other lesions that can be associated with MEN1 include duodenal gastrinomas, carcinoids, thyroid adenomas, adrenocortical tumors, and lipomas. Mutations in the receptor tyrosine kinase RET result in MEN2. There are two subtypes, MEN2A (also known as Sipple’s syndrome) and MEN2B, both of which are associated with C-cell tumors, primary hyperparathyroidism, and pheochromocytomas. In addition, MEN2B patients frequently have mucosal neuromas and a marfanoid stature. There are increasing reports of MEN in veterinary medicine in cats, dogs, horses, and cattle. However, mutations in MEN1 or RET have never been documented in these cases.
Both C-cell adenomas and carcinomas can contain deposits of amyloid. The source of the localized amyloid deposits in the neoplasms is uncertain, but the amyloid appears to be produced by the neoplastic cells, as amyloid deposits are not present in other organs. Amyloid deposition is consistently documented with medullary thyroid carcinoma in humans, and amyloid has also been reported in certain other endocrine neoplasms. Amyloid in C-cell neoplasms is present between neoplastic cells, around vessels, and in the interstitium. Amyloid has been observed in bulls, horses, and dogs but in amounts varying (minimal to substantial) from case to case. Localized amyloid in C-cell tumors is derived from calcitonin.
Adenomas: C-cell adenomas occur as discrete, single, or multiple gray to tan nodules in one or both thyroid lobes. Adenomas are smaller (approximately 1 to 3 cm in diameter) than carcinomas and are separated from the adjacent thyroid gland parenchyma, which is compressed by a thin, fibrous connective tissue capsule. Larger C-cell adenomas replace most of the thyroid lobe, but a rim of dark, brown-red thyroid gland often is present on one side (Fig. 12-41). Histologically, thyroid C-cell adenomas are discrete, expansive masses composed of cells larger than a colloid-distended follicle. The adenoma is well-circumscribed or partially encapsulated, and adjacent follicles are compressed to varying degrees. The neoplastic C cells are well-differentiated and have abundant to clear, pale eosinophilic cytoplasm.
Carcinomas: Thyroid C-cell carcinomas result in extensive multinodular enlargements of one or both thyroid lobes and can replace an entire thyroid gland. Thyroid C-cell neoplasms in bulls and other animal species are firm, and in some areas, the stroma consists of dense bands of fibrous connective tissue. Multiple metastases occur in the cranial cervical lymph nodes (Fig. 12-42). These nodes are usually large and have areas of necrosis and hemorrhage. Pulmonary metastases appear as discrete tan nodules and occur infrequently. C-cell carcinomas are composed of neoplastic cells that are more pleomorphic than cells of adenomas. The carcinomatous cells are poorly differentiated, polyhedral to spindle-shaped, and have pale eosinophilic, finely granular, indistinct cytoplasm.

Fig. 12-42 C-cell carcinoma and metastases, thyroid and cervical lymph nodes, Holstein bull.
Note the swellings in the neck (arrows) as a result of metastases to the cervical lymph nodes. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Ultimobranchial neoplasms in the thyroid glands of bulls often have a more complex histologic structure than the typical C-cell (medullary) carcinoma in dogs and horses. The neoplasm is composed of differentiated C cells arranged as focal accumulations of neoplastic cells with abundant pale eosinophilic cytoplasm, either within the wall of the thyroid and ultimobranchial follicles or present as larger solid nodules. Ultimobranchial thyroid neoplasms often are accompanied by multifocal hyperplasia of C cells in other parts of the thyroid. The neoplastic C cells often are embedded in an increased amount of hyalinized stroma, and there are deposits of amyloid in some neoplasms. Portions of the thyroid neoplasm in bulls that are derived from less differentiated ultimobranchial remnants consist of follicle-like structures, cysts, and tubules composed of immature small basophilic cells. These neoplasms in bulls and other species closely resemble undifferentiated or stem cells of the normal ultimobranchial body that can differentiate into both C cells and follicular cells. Thyroid follicles and cribriform structures with colloid-like material formed by cells resembling differentiated follicular cells often are present in the neoplasms in close association with these more primitive ultimobranchial-derived structures. Histologically, the structure of ultimobranchial neoplasms in bulls is heterogeneous and resembles a variant of thyroid carcinoma in humans. This neoplasm, designated as an intermediate type of differentiated carcinoma, has structural and immunohistochemical characteristics of both C-cell (medullary) and follicular carcinomas.
Disorders of the Parathyroid Glands
Parathyroid (Kürsteiner’s) Cysts: Small cysts within the parenchyma of the parathyroid glands or in the immediate vicinity of the glands occur frequently in dogs but only occasionally in other animal species (Fig. 12-43). Parathyroid gland cysts are usually multiloculated, lined by a cuboidal to columnar, often ciliated epithelium, and filled with a densely eosinophilic proteinaceous material. Parathyroid gland cysts appear to develop from persistent and dilated remnants of the duct that connects the parathyroid and thymic primordia during embryonic development. Parathyroid gland cysts are distinct from midline cysts derived from remnants of the thyroglossal duct. The latter are lined by multilayered thyroidogenic epithelium that often has colloid-containing follicles and are usually located near the midline, from the base of the tongue to the mediastinum.
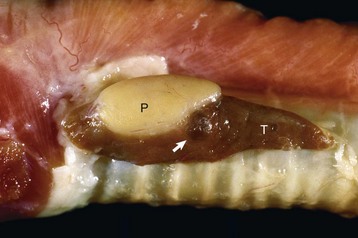
Fig. 12-43 Parathyroid cyst (left external), parathyroid gland, dog.
The parathyroid cyst (arrow) was formed from the persistent and distended embryonic duct that connects parathyroid-thymic primordia in the III and IV pharyngeal pouches (Kürsteiner’s cyst). All parathyroid glands (P) were hyperplastic because of chronic renal failure. T, Thyroid gland. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Hypoparathyroidism
In hypoparathyroidism, either the parathyroid glands secrete subnormal amounts of PTH or the hormone secreted is unable to interact with target cells. Hypoparathyroidism has been recognized in dogs, particularly in smaller breeds such as schnauzers and terriers, and infrequently in other animal species. Idiopathic hypoparathyroidism in adult dogs usually is caused by a diffuse lymphocytic parathyroiditis. Other infrequent causes of hypoparathyroidism include invasion and destruction of parathyroid glands by primary or metastatic neoplasms and trophic atrophy of parathyroid glands resulting from long-term hypercalcemia. In addition, the parathyroid glands are sometimes damaged or inadvertently removed during surgery involving the thyroid glands.
The functional disturbances and clinical manifestations of hypoparathyroidism primarily are the result of increased neuromuscular excitability and tetany. Because of the lack of PTH, bone resorption is decreased and blood calcium concentrations diminish progressively to 4 to 6 mg/dL (Fig. 12-44). Affected animals are restless, nervous, ataxic, and weak and have intermittent tremors of separate muscle groups. Tremors can progress to generalized tetany and convulsive seizures. Concurrently, blood phosphorus concentrations are increased because of increased renal tubular reabsorption.
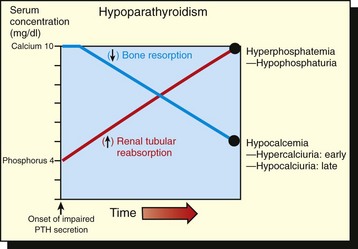
Fig. 12-44 Schematic diagram of the alterations in serum calcium and phosphorus concentrations in response to an inadequate secretion of parathyroid hormone (PTH).
A progressive increase in serum phosphorus concentration and a notable decline in the concentration of serum calcium resulted in increased neuromuscular excitability and tetany. (Redrawn with permission from Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Lymphocytic Parathyroiditis: Lymphocytic parathyroiditis is characterized by extensive degeneration of chief cells and replacement fibrosis. Mild lesions include infiltrates of lymphocytes and plasma cells and nodular hyperplasia of chief cells. Later, the parathyroid gland is completely replaced by lymphocytes, fibroblasts, and neocapillaries with few remaining viable chief cells (Web Fig. 12-7). Lymphocytic parathyroiditis develops by an immune-mediated mechanism, as evidenced by the production of a similar destruction of parathyroid gland parenchyma and lymphocytic infiltration in dogs injected with emulsions of parathyroid gland in adjuvant.
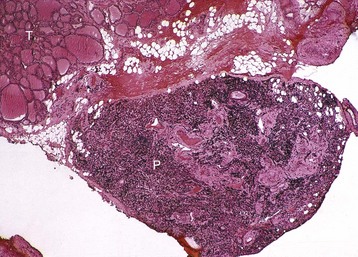
Web Fig. 12-7 Diffuse lymphocytic parathyroiditis, parathyroid gland, dog.
The external parathyroid gland (P) has been completely replaced by lymphocytes, plasma cells, fibroblasts, and neocapillaries. T, Thyroid gland. H&E stain. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Hyperparathyroidism
Primary Hyperparathyroidism: Functional Chief Cell Neoplasms: Adenomas and carcinomas of parathyroid glands often secrete excessive amounts of PTH, resulting in a syndrome of primary hyperparathyroidism. Prolonged increased secretion of PTH accelerates osteolytic and osteoclastic bone resorption. Mineral is removed from the skeleton at an accelerated rate, and bone is replaced by immature fibrous connective tissue. The lesions of fibrous osteodystrophy are generalized throughout the skeleton but are accentuated in certain areas (e.g., maxilla, mandible, and subperiosteal areas of long bones).
Adenomas of parathyroid glands are encountered in older animals, particularly dogs, but parathyroid carcinomas are uncommon. Chief cell adenomas usually cause considerable enlargement of a single parathyroid gland. Parathyroid adenomas are encapsulated and sharply demarcated from the adjacent thyroid gland (Fig. 12-45).
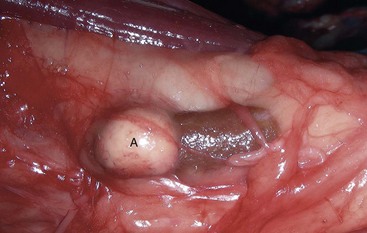
Fig. 12-45 Adenoma, parathyroid gland, dog.
Note the large parathyroid adenoma (A), well demarcated from the thyroid gland. (Courtesy College of Veterinary Medicine, University of Illinois.)
Parathyroid gland adenomas are composed of small, closely packed groups of chief cells delineated by delicate vascular connective tissue septa that have many capillaries (Fig. 12-46). The neoplastic chief cells are round to polyhedral and have pale, expanded eosinophilic cytoplasm in which there are a few electron-dense secretory granules and prominent arrays of endoplasmic reticulum and Golgi complexes (Web Fig. 12-8).
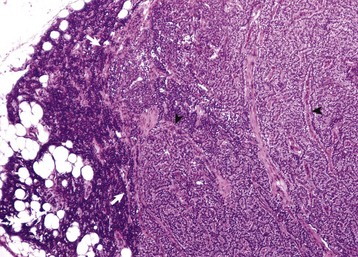
Fig. 12-46 Adenoma, parathyroid gland, dog.
The adenoma consists of closely packed chief cells arranged in small groups separated by fine fibrous septae containing capillaries (arrowheads). It is partially encapsulated and has compressed the adjacent, nonneoplastic parathyroid tissue (arrows), which has undergone trophic atrophy. H&E stain. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
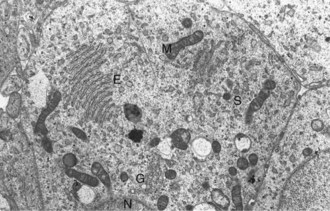
Web Fig. 12-8 Adenoma, parathyroid gland, dog.
Active chief cells have large lamellar arrays of rough endoplasmic reticulum (E), prominent Golgi apparatus (G), and large mitochondria (M) but few secretory granules (S). N, Nucleus of chief cell. TEM. Uranyl acetate and lead citrate stain. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
The functional disturbances observed with endocrinologically active chief cell neoplasms are primarily the result of hypercalcemia and weakening of bones by excessive PTH-stimulated resorption of calcium. Cortical bone is thinned as a result of increased resorption by osteoclasts stimulated by the autonomous secretion of PTH (Fig. 12-47). Lameness can result from fractures of long bones that occur after relatively minor physical traumas. Compression fractures occur in vertebral bodies, resulting in pressure on the spinal cord and nerves that leads to motor and/or sensory dysfunction. Facial hyperostosis, caused by extensive osteoblastic proliferation and deposition of poorly mineralized osteoid and loosening or loss of teeth from alveolar sockets has been observed in dogs with primary hyperparathyroidism. Hypercalcemia results in anorexia, vomiting, constipation, depression, polyuria, polydipsia, and generalized muscular weakness because of decreased neuromuscular excitability.
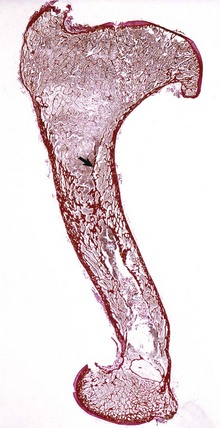
Fig. 12-47 Primary hyperparathyroidism, humerus, dog.
Severe thinning of cortical bone and large resorptive cavities (arrow) have resulted from localized resorption of bone by osteoclasts. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
The most practical laboratory test to aid in the diagnosis of primary hyperparathyroidism is quantification of the concentrations of total blood calcium, phosphorus, and circulating PTH (N-terminal or immunoradiometric [IRMA] assay). Dogs with primary hyperparathyroidism have greatly increased blood calcium concentrations (12 to 20 mg/dL or above). Blood phosphorus concentrations are low to normal (4 mg/dL or less) as a result of inhibition of renal tubular phosphorus reabsorption by the autonomous secretion of PTH. The urinary excretion of calcium and phosphorus is increased, which can predispose to the development of nephrocalcinosis and urolithiasis.
Secondary Hyperparathyroidism:
Nutritional Imbalances: Nutritional hyperparathyroidism occurs commonly in cats, dogs, and horses. The increased secretion of PTH is a compensatory mechanism induced by nutritional imbalances. Such imbalances occur in diets low in calcium, diets with an excess of phosphorus, and diets with normal or low content of calcium and in New World primates housed indoors and fed diets with inadequate amounts of vitamin D3. The significant result is hypocalcemia, which stimulates the parathyroid glands. Blood phosphorus concentrations, when elevated, contribute indirectly to parathyroid stimulation by decreasing blood calcium.
In response to the diet-induced hypocalcemia, chief cells undergo hypertrophy and hyperplasia with increased amounts of lightly eosinophilic or vacuolated cytoplasm. The organelles involved with protein synthesis (endoplasmic reticulum) and packaging of secretory products (Golgi apparatus) are well developed. Many chronically stimulated chief cells with well-developed secretory organelles accumulate glycogen after 5 to 14 weeks.
The most frequent nutritional imbalance causing hyperparathyroidism is the ingestion of excessive amounts of phosphorus. Hyperphosphatemia stimulates the parathyroid gland indirectly by lowering blood calcium. Horses that develop the disease usually have been fed grain diets with below-average-quality roughage. Evidence of excessive phosphorus intake might be difficult to determine, as the excess phosphorus could have been supplied as a bran supplement to the grain ration. The disease in horses is commonly referred to as “big head” because of the fact that the fibrous osteodystrophy is typically hyperostotic and most severe in the mandible and maxilla (see Fig. 16-55). The diet is usually palatable and nutritious, except for its excessive phosphorus and marginal or deficient calcium content. A diet deficient in calcium fails to supply the daily calcium requirement, and hypocalcemia develops, even though a greater proportion of calcium ingested is absorbed. Changes in concentrations of calcium and phosphorus in urine are more consistent and useful in the clinical diagnosis of nutritional secondary hyperparathyroidism in horses than changes in blood concentrations of these minerals. The increased secretion of PTH acts on normal kidneys to notably increase urinary phosphorus excretion and to decrease calcium loss in the urine.
Renal Disease: Hyperparathyroidism as a complication of chronic renal failure is characterized by excessive production of PTH in response to chronic hypocalcemia. When the renal disease is extensive enough to reduce the glomerular filtration rate, phosphorus is retained and hyperphosphatemia develops. The increased blood phosphorus concentration depresses ionized blood calcium concentration, resulting in parathyroid gland stimulation. Chronic renal disease also impairs the production of 1,25-(OH)2D3 by the kidneys, thereby diminishing intestinal calcium transport and increasing mobilization of calcium from the skeleton. All parathyroid glands undergo notable chief cell hyperplasia, and the bones subsequently develop varying degrees of generalized fibrous osteodystrophy. The fibrous osteodystrophy that occurs with chronic renal failure is also most severe in the skull, but it is usually osteoporotic. Affected animals, especially dogs, have supple mandibles and/or maxillas known as “rubber jaws” (see Figs. 16-54 and 16-55).
Pseudohyperparathyroidism: Humoral Hypercalcemia of Malignancy
Hypercalcemia in animals is a common disorder with many causes. The most common form is cancer-associated hypercalcemia or humoral hypercalcemia of malignancy (HHM). Notable increases in serum calcium concentration resulting from an imbalance of calcium released from bones, excreted by the kidneys, or absorbed from the intestinal tract are reported with apocrine gland adenocarcinomas, metastases of solid neoplasms to bone, and hematologic malignancies such as lymphoma (lymphosarcoma).
The clinical signs of hypercalcemia are similar regardless of the underlying cause and depend on the rapidity of onset of increased concentrations of serum ionized calcium. Animals with total serum calcium values in excess of 16 mg/dL (4 mmol/L) generally have the most severe clinical signs. Exceptions to this rule occur, and some animals with severe hypercalcemia have only mild clinical signs. Horses and rabbits have normal total serum calcium concentrations greater than those found in other domestic animals, which should be considered before hypercalcemia is diagnosed in these species. Metabolic acidosis enhances the severity of clinical signs because it results in an increase in the ionized fraction of serum calcium. Increased serum ionized calcium induces clinical signs relating to the gastrointestinal, neuromuscular, cardiovascular, and renal systems.
The parathyroid glands in animals with HHM are small and difficult to locate or undetectable grossly. The parathyroid glands respond to the persistent cancer-associated hypercalcemia by trophic atrophy. Atrophic parathyroid glands in dogs are characterized by narrow cords of inactive chief cells with an abundant fibrous connective tissue stroma and widened perivascular spaces. Thyroid C cells undergo either diffuse or nodular hyperplasia in response to the persistent elevation in blood calcium.
Renal calcification has been detected microscopically in approximately 90% of dogs with HHM caused by anal sac apocrine gland adenocarcinomas, particularly when the calcium × phosphorus product is ≥50. Tubular calcification is most pronounced near the corticomedullary junction but is also present in cortical and inner medullary tubules, Bowman’s capsule, and glomerular tufts. Calcification also occurs in the fundic gastric mucosa and endocardium.
Excessive secretion of biologically active PTHrP plays a central role in the pathogenesis of hypercalcemia in most forms of HHM; however, cytokines, such as interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), transforming growth factor-α (TGF-α) and TGF-β, or 1,25-dihydroxyvitamin D, can have synergistic or cooperative actions with PTHrP. Before PTHrP was identified, it was well understood that nonparathyroid neoplasms and humoral hypercalcemia of malignancy induced a syndrome that mimicked primary hyperparathyroidism because of secretion of a PTH-like factor that was antigenically unrelated to PTH. Purification of the substance with PTH-like activity from the adenocarcinoma derived from apocrine glands of the anal sac in dogs and multiple human neoplasms with documented HHM resulted in the discovery of PTHrP. PTHrP also can be demonstrated by immunohistochemical and biochemical analysis in a number of tissues, in which it appears to function primarily as a paracrine factor.
Apocrine Gland Adenocarcinoma: See the section on Disorders of Dogs for a discussion of apocrine gland adenocarcinoma.
Neoplasms Metastatic to Bone: Solid neoplasms that metastasize widely to bone and grow locally can produce hypercalcemia by inducing local bone resorption. This is not common in animals but is an important cause of HHM in humans, particularly patients with metastatic breast and lung carcinomas.
The pathogenesis of enhanced bone resorption is not well understood, but the two primary mechanisms include (1) secretion of cytokines or factors that stimulate local bone resorption and (2) indirect stimulation of bone resorption by tumor-induced cytokine secretion from local immune or bone cells. Cytokines or factors that are secreted by neoplastic cells and stimulate local bone resorption include PTHrP, TGF-α and TGF-β, and prostaglandins, especially prostaglandin E2.
Lymphoma (Lymphosarcoma): Lymphoma is the most common neoplasm associated with hypercalcemia in dogs and cats. Estimates of the prevalence of hypercalcemia in dogs with lymphoma vary from 20% to 40%. Peripheral lymph node enlargement is present in some cases; cranial mediastinal or visceral nodes usually are involved. The hypercalcemia results from the production of humoral substances by neoplastic cells and/or from physical disruption of trabecular bone by lymphoma in bone marrow. Serum immunoreactive PTH concentrations are subnormal, and plasma prostaglandin E2 concentrations are not different from those of controls.
Most dogs with lymphoma and HHM have significantly increased circulating PTHrP concentrations, but these concentrations are lower (2 to 15 pmol) than in dogs with carcinomas and HHM; there is no correlation with serum calcium concentration. The latter indicates that PTHrP is an important marker in dogs with HHM and lymphoma but is not the sole humoral factor responsible for the stimulation of osteoclasts and development of hypercalcemia. It is likely that cytokines, such as IL-1 or TNF, function synergistically with PTHrP to induce HHM in dogs with lymphoma. Some dogs with lymphoma and hypercalcemia have increased serum 1,25-dihydroxyvitamin D concentrations that could be responsible for or contribute to the induction of hypercalcemia. PTH (N-terminal) concentrations in dogs with lymphoma and hypercalcemia usually are in the normal range; however, a few dogs have concentrations that are increased slightly above the normal range.
Parturient Hypocalcemia
Parturient paresis in dairy cattle is a complex metabolic disease characterized by the development of severe hypocalcemia and hypophosphatemia near the time of parturition and the initiation of lactation. The serum calcium concentration decreases to less than 50% of normal in spite of an increased secretion of PTH by the cow’s parathyroid glands. Bone resorption remains minimal, and few osteoclasts are present on bone surfaces. Biochemical and ultrastructural studies in cows indicate that the parathyroid glands are capable of responding to the acute hypocalcemia by increasing development of the cellular organelles responsible for hormonal synthesis and by increasing secretion of PTH.
The composition of the diet fed to dairy cows is a significant factor in the pathogenesis of parturient hypocalcemia. Diets with excessive calcium have been incriminated as significantly increasing the incidence of the disease. Conversely, diets low in calcium or diets supplemented with therapeutic doses of vitamin D reduce the incidence of parturient hypocalcemia. Calcium homeostasis in pregnant cows fed an excessive calcium diet appears to be maintained principally by intestinal calcium absorption (Fig. 12-48). This greater reliance on intestinal absorption rather than on PTH-stimulated bone resorption probably is a significant factor in the more frequent development of profound hypocalcemia near parturition in cows fed excessive calcium prepartal diets.
Fig. 12-48 Schematic diagram of calcium homeostasis in cows fed a high-calcium prepartal diet.
In this case, calcium homeostasis is primarily dependent on intestinal calcium absorption. The rate of bone resorption is low, and parathyroid glands are inactive. Anorexia and gastrointestinal stasis that often occur near parturition interrupt the major inflow of calcium into the extracellular fluid calcium pool. Outflow of calcium with the onset of lactation exceeds the rate of inflow into the calcium pool, and cows develop a progressive hypocalcemia and paresis. CT, Calcitonin; PTH, parathyroid hormone. (Redrawn with permission from Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
An increased prepartal secretion of calcitonin in cows that develop parturient hypocalcemia or milk fever, especially those fed excessive calcium diets, could be a factor contributing to the inability of increased PTH concentrations to mobilize calcium rapidly from skeletal reserves and to maintain blood calcium concentrations during the critical periparturient period. The thyroidal content of calcitonin is reduced, many C cells are degranulated, and plasma concentrations of calcitonin often are increased in cows before the development of profound hypocalcemia.
Disorders of Pancreatic Islet Cells
Hypofunction of Pancreatic Islet Cells
Diabetes Mellitus: Diabetes mellitus is a metabolic disorder that results from a reduction of insulin available for normal function of many cells in the body. In some cases, increased concentrations of glucagon contribute to development of persistent hyperglycemia. Insulin unavailability could be due to degenerative changes in β cells of the pancreatic islets, reduced effectiveness of the hormone because of the formation of antiinsulin antibodies or inactive complexes, damage to β cells from immune-mediated islet cytotoxicity, or inappropriate secretion of hormones by neoplasms in other endocrine organs.
Diabetes mellitus is a common endocrinopathy in dogs and cats. Most cases of spontaneous diabetes occur in mature dogs and in females approximately twice as often as males. An increased incidence of diabetes mellitus has been observed in certain small breeds of dogs, such as miniature poodles, dachshunds, and terriers, but nearly all breeds of dogs can be affected.
Development of diabetes mellitus in young dogs is the result of idiopathic atrophy of the pancreas, acute pancreatitis with necrosis and hemorrhage, and aplasia of pancreatic islets; however, diabetes mellitus can also occur secondary to other endocrine disorders such as acromegaly, Cushing’s syndrome, hyperaldosteronism, hyperthyroidism and various neoplasms. The pancreas with idiopathic atrophy is reduced to a third or less of its size. Hypoplasia of pancreatic islets in which the islets are absent but the pancreatic acini and ducts are present and functional has been a cause of diabetes mellitus in young dogs (2 to 3 months of age).
In the pathogenesis of diabetes mellitus, several factors are responsible for the decreased availability of insulin. Destruction of islets secondary to severe pancreatitis or the selective degeneration of islet cells are the most common causes in animals. In dogs, the pancreatic islets are often destroyed because of inflammation of the exocrine pancreas. Chronic relapsing pancreatitis with progressive loss of both exocrine and endocrine cells and replacement by fibrous connective tissue is a frequent cause of diabetes mellitus. In these dogs, the pancreas becomes firm, multinodular, and has scattered areas of hemorrhage and necrosis after a recent relapse (Fig. 12-49). Later in the course of the disease, all that remains of the pancreas is a thin fibrous band or nodule near the duodenum and stomach (Fig. 12-50).
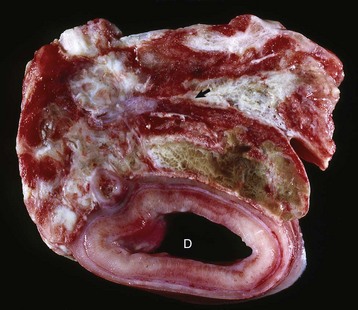
Fig. 12-49 Chronic relapsing pancreatitis, pancreas and duodenum, cross-section, dog.
The pancreas is multinodular and firm with areas of hemorrhage (arrow), fibrosis, and necrosis. D, Duodenum. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
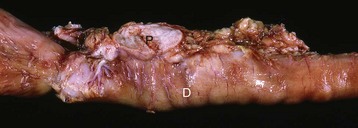
Fig. 12-50 Chronic pancreatitis, pancreas, dog.
The pancreas (P) is markedly atrophied and its parenchyma almost completely replaced by fibrous connective tissue in “end-stage” pancreatitis. D, Duodenum. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Histologically, β cells are reduced in number and remaining cells have vacuolated cytoplasm (Fig. 12-51). The cytoplasm of β cells is distended by massive amounts of glycogen particles (Web Fig. 12-9). Hydropic degeneration with glycogen accumulation, especially in cats, appears to develop in β cells in response to long-term overstimulation or exhaustion because of peripheral insulin resistance. When disease is chronic, the islets are difficult to find. Immune-mediated isletitis characterized by a progressive lymphoplasmacytic infiltration and selective destruction of β cells of the islets is another cause of diabetes mellitus (type 1) occasionally in dogs.
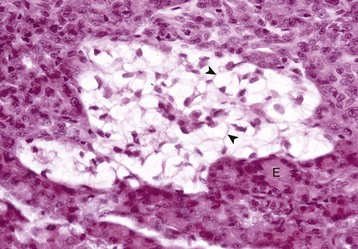
Fig. 12-51 Hydropic (“vacuolar”) degeneration, pancreatic islet, cat.
Discrete vacuoles (arrowheads) are present in the cytoplasm of β cells. E, Exocrine pancreas. H&E stain. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
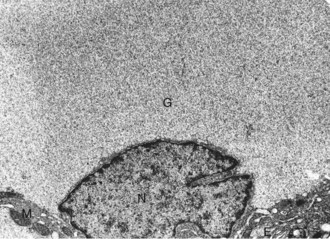
Web Fig. 12-9 Glycogen accumulation in the β cell, pancreatic islet, cat.
The nucleus (N) and secretory organelles are displaced to the periphery of the β cell by the accumulation of glycogen (G) particles. M, Mitochondria; E, endoplasmic reticulum. TEM. Uranyl acetate and lead citrate stain. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Another common pancreatic change in cats with diabetes is the selective deposition of amyloid in islets, resulting in degenerative changes in α cells and β cells (Fig. 12-52). Scattered amyloid deposits in pancreatic islets that increase progressively with age occur in the pancreatic islets of many cats without clinically apparent diabetes mellitus. Islet amyloid polypeptide (IAPP) (37 amino acids) and insulin are both secreted by β cells. The IAPP in cats has a unique amino acid sequence (amino acids 25 to 28: alanine, isoleucine, leucine, and serine), which is predisposed to polymerization and accumulation in pancreatic islets. As the insoluble amyloid fibers progressively accumulate in the islets, they disrupt β cells and surround islet capillaries, leading to the subsequent degeneration of islet cells.
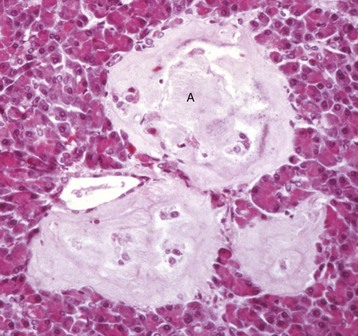
Fig. 12-52 Amyloidosis, pancreatic islets, cat.
Note the deposits of amyloid (A) and degeneration and loss of islet cells. H&E stain. (Courtesy College of Veterinary Medicine, University of Illinois.)
Complete expression of the complex metabolic disturbances in diabetes mellitus appears to be the result of a bihormonal abnormality. Although a relative or absolute deficiency of insulin action in response to a rising extracellular glucose concentration has been long recognized as the sine qua non of diabetes mellitus, the importance of an absolute or relative increase of glucagon secretion in some forms of the disease in certain species has been appreciated only recently. Hyperglucagonemia in diabetic patients can be the result of increased secretion of pancreatic glucagon, enteroglucagon, or both. An increased blood glucagon concentration contributes to the severe endogenous hyperglycemia by mobilizing hepatic stores of glucose and to the development of ketoacidosis by increasing the oxidation of fatty acids by hepatocytes. The major glucoregulatory consequence of insulin deficiency is reduction in the movement of glucose into insulin-sensitive tissues (e.g., liver, adipose tissue, and muscle), with a corresponding increase in hepatic glucose production, resulting in notable endogenous hyperglycemia.
The onset of diabetes mellitus is insidious, and the clinical course is often chronic. Clinically, dogs with diabetes mellitus exhibit polydipsia, polyuria, weight loss in spite of polyphagia, bilateral cataracts, and weakness. The disturbances in water metabolism primarily have an osmotic basis. In dogs with persistent hyperglycemia and glycosuria, the renal tubular epithelium is unable to concentrate the urine effectively against the osmotic attraction of the glucose in the glomerular filtrate.
Diabetic animals have diminished resistance to bacterial and fungal infections, and diseases, such as suppurative cystitis, prostatitis, bronchopneumonia, and dermatitis, often become chronic or recurrent. This increased susceptibility to infection in patients with poorly controlled diabetes can be related in part to impaired chemotactic, phagocytic, and microbicidal functions and decreased adherence of polymorphonuclear leukocytes. The impaired microbicidal function of leukocytes can have a metabolic basis resulting from diminished production of cellular energy from glucose. Radiographic evidence of emphysematous cystitis is strongly suggestive of diabetes mellitus. Infections of the urinary bladder with glucose-fermenting organisms, such as Proteus sp., Aerobacter aerogenes, and Escherichia coli result in gas formation in the wall and lumen. Emphysema also develops in the wall of the gallbladder in some diabetic dogs.
Hepatomegaly occurs as a result of fatty degeneration (see Figs. 1-45 and 8-38). Lipids accumulate in the liver as the result of increased lipid mobilization, and hepatocytes injured by ketonemia have decreased usage of lipids. Individual hepatocytes are greatly enlarged by multiple droplets of lipid (Web Fig. 12-10). If the accumulation of lipids is extensive and long-standing, cirrhosis develops. The liver remains enlarged, and its surface becomes coarsely nodular because of extensive remodeling of hepatic parenchyma (Web Fig. 12-11; also see Fig. 8-21). Individual hepatocytes degenerate and are replaced by regenerative and hyperplastic nodules and interlobular fibrosis. Icterus and bilirubinuria often accompany severe cirrhosis.
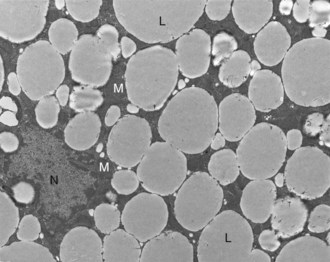
Web Fig. 12-10 Fatty degeneration, liver, dog.
The cytoplasm contains multiple large lipid droplets (L) that have displaced the nucleus (N) peripherally and compressed cytoplasmic organelles such as mitochondria (M). TEM. Uranyl acetate and lead citrate stain. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
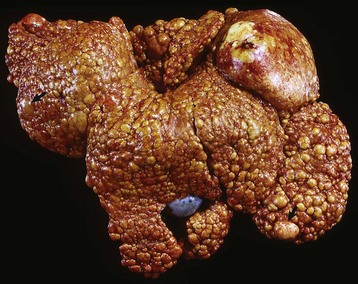
Web Fig. 12-11 Cirrhosis, liver, diaphragmatic surface, dog.
All lobes of the liver are considerably firm and have a coarsely nodular surface. The nodules (arrows) represent areas of regenerative hyperplasia of hepatocytes. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Cataracts often develop in dogs with poorly controlled diabetes mellitus. They are stellate or asteroid in shape (Web Fig. 12-12; also see Fig. 20-95) and initially appear along the suture lines of lenticular fibers. Cataract formation in the diabetic patient is related to the unique sorbitol pathway by which glucose is metabolized in the lens. Glucose is first converted to sorbitol by the enzyme aldose reductase and subsequently to fructose by sorbitol dehydrogenase. These sugar alcohols accumulate within the lens and result in an intracellular accumulation of solute and hypertonicity. The initial structural change in the lens consists of swelling and hydropic degeneration of lenticular fibers. In long-standing cases of diabetes mellitus, swelling of lenticular fibers continues until the majority of lenticular fibers are affected. Later, macromolecular aggregation or precipitation of normally translucent lenticular proteins develops, accompanied by disruption of lenticular fibers and formation of interfibrillar clefts. The result is diffuse, often bilateral lens opacity observed in animals with chronic diabetes mellitus.
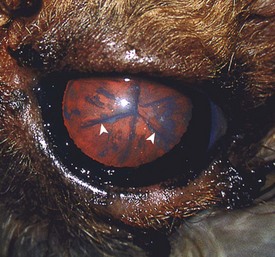
Web Fig. 12-12 Diabetes mellitus, eyeball, lens, dog.
Early stellate lesion (arrowheads) along the sutures of the lens. In dogs (not in cats), cataracts (stellate lesions) can occur in poorly regulated diabetes mellitus because glucose is metabolized in the lens by a sorbitol pathway, which leads to edema of the lens. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Other extrapancreatic lesions of diabetes mellitus, such as chronic renal disease, blindness, and gangrene, are the result of microangiopathy characterized by thickening of the capillary basement membrane. Dogs with long-standing, poorly controlled, spontaneous diabetes mellitus develop nodular or diffuse glomerulosclerosis characterized by PAS-positive fibrillar deposits of glycoprotein, which occasionally form spherical nodules in the glomerular capillary tufts. Other renal lesions include accumulation of glycogen within cells of the loop of Henle (intracytoplasmic) and distal convoluted tubule (intranuclear) (Web Fig. 12-13).
Hyperfunction of Pancreatic Islet Cells
β-Cell (Insulin-Secreting) Neoplasms (Insulinomas): The neoplasms most frequently arising in pancreatic islets are adenomas and carcinomas derived from β cells. These neoplasms are often endocrinologically active and produce dramatic functional disturbances. Other pancreatic neoplasms appear to be derived from multipotential ductal epithelial cells that differentiate into one of several other cell types in the pancreatic islets. β-Cell neoplasms are seen most frequently in dogs 5 to 12 years of age (mean 9 years). These neoplasms also occur in older cattle and can cause periodic convulsions. Carcinomas of the pancreatic islets are more common than adenomas in dogs and are commonly found in the duodenal (right) lobe of the pancreas. Carcinomas of the pancreatic islets can be differentiated grossly from adenomas by their larger size, multilobular appearance, extensive invasion of adjacent parenchyma and lymph vessels, and metastases to extrapancreatic sites, such as regional lymph nodes (e.g., duodenal, mesenteric, hepatic, and splenic), liver, mesentery, and omentum.
The clinical alterations observed with functional β-cell neoplasms are the result of excessive insulin secretion and the development of severe hypoglycemia. The clinical signs are not specific for the hyperinsulinism produced by β-cell neoplasms. Initial signs include weakness, fatigue after vigorous exercise, generalized muscular twitching and weakness, ataxia, mental confusion, and changes in temperament. Dogs are easily agitated and have intermittent periods of excitability and restlessness. Periodic convulsive seizures of the tonic-clonic type occur later in the disease and increase progressively in frequency and intensity.
The predominance of clinical signs relating to the CNS demonstrates the primary dependence of the brain on glucose metabolism for energy. The failure to recognize dogs with functional islet cell neoplasms when they have clinical signs suggestive of primary disease of the nervous system has frequently led to the misdiagnoses of idiopathic epilepsy, brain neoplasm, or other organic neurologic disease. Repeated episodes of prolonged and severe hypoglycemia result in neuronal necrosis and permanent neurologic disability with terminal coma and eventually death.
Adenomas of the β cells of the pancreatic islets typically exist as single, yellow to dark red, small (1 to 3 cm), spherical nodules. The neoplasms are of similar consistency to or slightly firmer than that of the surrounding pancreas. Adenomas occur as a single nodule or less often as multiple nodules in one or both lobes and/or body of the pancreas. Islet cell adenomas are sharply delineated and surrounded by a thin capsule of fibrous connective tissue (Fig. 12-53). Small nests of acinar epithelial cells are sometimes present throughout the neoplasm but are more common at the periphery. Numerous connective tissue septa containing small capillaries radiate from the capsule into the neoplasm and subdivide the neoplasm into small lobules or packets. Well-differentiated neoplastic cells are round to polyhedral with distinct cell borders and pale eosinophilic, finely granular cytoplasm.
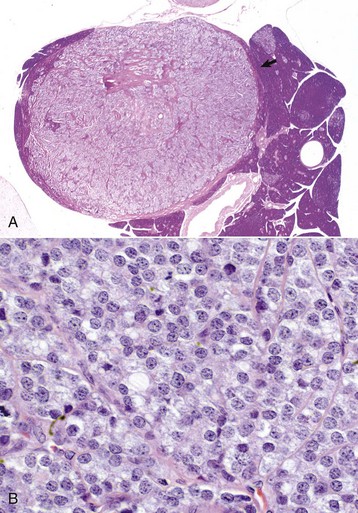
Fig. 12-53 β-Cell adenoma, pancreatic islet, dog.
A, A solid islet adenoma, surrounded by a fibrous capsule of variable thickness, has compressed the adjacent exocrine pancreas (arrow). H&E stain. B, The neoplastic β-cells are arranged into packets and have abundant finely granular cytoplasm characteristic of neuroendocrine tumors. H&E stain. (A courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University. B courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Islet cell carcinomas are consistently larger than adenomas, are multilobular, and invade into and through the fibrous capsule of the pancreas (Fig. 12-54). The dense bands of fibrous tissue that course through the neoplasm give rise to fine connective tissue septa containing capillaries that subdivide the neoplasm into small cords or lobules. Well-differentiated neoplastic cells in islet cell carcinomas are closely packed but are less uniform in size and shape than the cells in adenomas. They are round to polyhedral and have a granular eosinophilic cytoplasm. Mitotic figures are infrequent. Microscopic evidence of distinct local tissue invasion is the principal criterion for the diagnosis of islet cell carcinoma.
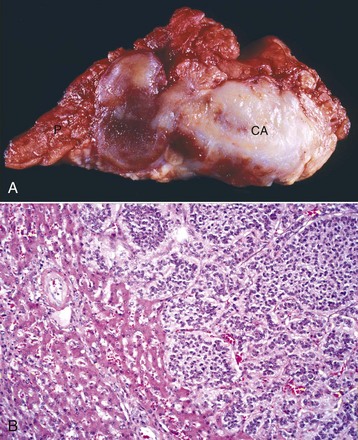
Fig. 12-54 β-cell carcinoma, pancreatic islet, dog.
A, The whitish-red carcinoma (CA) is well demarcated from the lobular exocrine pancreas (P). B, The β-cell carcinoma (right side of figure) has metastasized to the liver and has expanded to compress adjacent hepatic parenchyma and invade sinusoids. H&E stain. (A courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University. B courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Cells composing functional islet cell neoplasms in dogs usually have histochemical and immunohistochemical characteristics of β cells. Ultrastructurally, the neoplastic β cells are electron dense because of numerous cytoplasmic organelles.
Non-β (Gastrin-Secreting) Islet Cell Neoplasms (Gastrinomas): Gastrin-secreting, non-β islet cell neoplasms of the pancreas have been reported in dogs and cats. The hypersecretion of gastrin in humans results in the well-documented Zollinger-Ellison syndrome, consisting of hypersecretion of gastric acid and recurrent peptic ulceration in the gastrointestinal tract. The non-β islet cell neoplasms derived from ectopic amine precursor uptake decarboxylase (APUD) cells in the pancreas produce an excess of gastrin, which normally is secreted by cells of the antral and duodenal mucosa. The incidence of gastrin-secreting pancreatic neoplasms in dogs and cats is uncertain, but it appears to be uncommon compared with that of insulin-secreting β-cell neoplasms. In the comparatively few canine and feline cases studied, the clinical signs have included anorexia, vomiting of blood-tinged material, intermittent diarrhea, progressive weight loss, and dehydration, the result of the multiple ulcerations of the gastrointestinal mucosa because of gastrin-stimulated gastric acid hypersecretion (Zollinger-Ellison–like syndrome).
Animals with Zollinger-Ellison–like syndrome have single or multiple, variably sized neoplasms in the pancreas. These are firm, have increased amounts of fibrous connective tissue, and although partially encapsulated, the neoplasm usually extends into the surrounding pancreas.
Three histologic patterns of gastrin-secreting islet neoplasms in dogs are recognized: (1) the ribbon or trabecular pattern; (2) solid nests of cells with a delicate, highly vascularized stroma; and (3) an acinar pattern with cuboidal neoplastic cells arranged around a central lumen. The stroma is prominent and hyalinized in some gastrin-secreting neoplasms.
Canine gastrin-secreting islet cell neoplasms invade locally into the adjacent pancreas and often metastasize to regional lymph nodes and to the liver. These dogs have either single or multiple ulcerations of the gastric and/or duodenal mucosa and blood in the gut lumen.
Other non-β islet cell neoplasms reported in animals are those of glucagon-secreting α cells. Glucagonomas have been reported rarely in dogs and are associated with superficial necrolytic dermatitis.
Disorders of the Pineal Gland
Pineal Tumors
Pineal tumors are exceedingly rare in animals. The cases in domestic animals are isolated reports in a cow, goat, and horse. Tumors are classified as well-differentiated pinealocytomas, anaplastic pinealoblastomas, or mixed tumors. Immunoreactivity to synaptophysin has been shown to be the most consistent marker for pineal tumors.
Disorders of the Chemoreceptor Organs
Although chemoreceptor tissue appears to be widely distributed in the body, neoplasms develop principally in the aortic and carotid bodies. Aortic body chemodectomas are encountered more frequently in animals than those of the carotid body, but the reverse is true for humans. These neoplasms develop primarily in dogs and infrequently in cats and cattle. Brachycephalic breeds of dogs, such as the boxer and Boston terrier, are highly predisposed to develop neoplasms of the aortic and carotid bodies.
Neoplasms of the Aortic Body: Aortic body neoplasms appear most frequently as a single mass or as multiple nodules near the base of the heart (Fig. 12-55). They vary considerably in size (from 0.5 to 12.5 cm), with carcinomas larger than adenomas. Solitary small adenomas either are attached to the adventitia of the pulmonary artery and ascending aorta or are embedded in the adipose connective tissue between these major vessels. They have a smooth external surface and on cross-section are white and mottled with red-to-brown areas. Larger adenomas are multilobular and can compress and indent the wall of the atria, displace the trachea, and partially surround the major vessels at the base of the heart. Aortic body carcinomas are less frequent than adenomas in dogs. Carcinomas infiltrate the wall of the pulmonary artery and form papillary projections into its lumen or invade through the wall into the lumen of the atria. Although neoplastic cells often invade blood vessels, metastases to the lungs and liver occur infrequently in dogs.
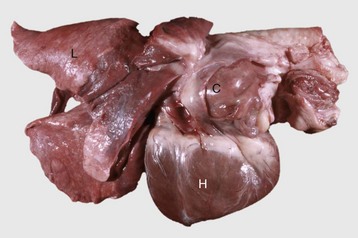
Fig. 12-55 Carcinoma, aortic body, dog.
Note the large mass (C) at the base of the heart (H). Contiguous portions of the right-middle and diaphragmatic lung lobes are atelectatic. L, Lungs. (Courtesy College of Veterinary Medicine, University of Illinois.)
Neoplasms of the aortic bodies in animals are not functional (i.e., they do not secrete excess hormone into the circulation), but as a space-occupying lesion they can produce a variety of functional disturbances. Larger aortic body adenomas and carcinomas put pressure on the atria, vena cava, or both and cause manifestations of cardiac decompensation. A higher percentage of aortic body neoplasms than carotid body neoplasms are benign. Aortic body carcinomas invade locally into the atria, pericardium, and adjacent vessels.
Neoplasms of the Carotid Body: Carotid body neoplasms arise near the bifurcation of the common carotid artery in the cranial cervical area. They are usually unilateral, only rarely bilateral, and are slow growing. Adenomas vary from approximately 1 to 4 cm in diameter, are well encapsulated, and have a smooth external surface. The bifurcation of the common carotid artery is incorporated in the mass, and neoplastic cells are firmly adhered to the tunica adventitia. Adenomas are firm, white with scattered areas of hemorrhage, and are extremely vascular.
Carotid body carcinomas are larger, more coarsely multinodular than adenomas, invade the capsule, and penetrate into the walls of adjacent blood and lymph vessels. The external jugular vein and some cranial nerves can be incorporated into the neoplasm. Metastases of carotid body carcinomas occur in approximately 30% of cases and have been found in the lungs, tracheobronchial and mediastinal lymph nodes, liver, pancreas, and kidneys. Multicentric neoplastic transformation of chemoreceptor tissue (aortic and carotid bodies) occurs frequently in brachycephalic breeds of dogs.
The microscopic features of chemoreceptor neoplasms (“chemodectomas”) are essentially similar, whether they are derived from the carotid or the aortic body. The neoplasm is subdivided into lobules by prominent branching trabeculae of connective tissue, which originate from the fibrous capsule. Neoplasms are further subdivided into smaller nests by fine septa of collagenous and reticulin fibers and small capillaries. Neoplastic cells are commonly aligned along and around small capillaries and are discrete, round to polyhedral, and closely packed, with pale eosinophilic, finely granular, and often vacuolated cytoplasm.
Although the cause of carotid and aortic body neoplasms is unknown, a genetic predisposition aggravated by chronic hypoxia could account for the greater risk of development in certain brachycephalic breeds such as the boxer and Boston terrier. Carotid bodies of several mammalian species, including dogs, have developed hyperplastic foci when the animals were subjected to the chronic hypoxia of high altitude. Humans living at high altitudes have 10 times the frequency of chemodectomas as those residing at sea level.
Heart-Base Neoplasms Derived from Ectopic Thyroid Gland Tissue: Adenomas and carcinomas derived from ectopic thyroid gland account for approximately 5% to 10% of “heart base” neoplasms in dogs. They often compress or invade structures in the cranial mediastinum near the base of the heart (Fig. 12-56). Ectopic thyroid gland neoplasms have a compact cellular (solid) pattern that is difficult to distinguish histologically from that of aortic body neoplasms. Cells of ectopic thyroid gland neoplasms generally are smaller than those of aortic body neoplasms and have more hyperchromatic nuclei and an eosinophilic cytoplasm. Thyroidal neoplasms of follicular cells are not consistently subdivided into small packets by fine strands of connective tissue. Giant cells are infrequent in ectopic thyroid gland neoplasms, and the stroma is not prominent. Usually, primitive follicular structures or colloid-containing follicles can be demonstrated in ectopic thyroid gland neoplasms but not in aortic body neoplasms.
Obesity
Obesity, defined as the accumulation of excessive amounts of adipose tissue in the body, is an increasing problem affecting approximately 35% of dogs and cats. The primary cause is a positive energy balance, but there are many contributory factors, including breed predisposition, advancing age, sex, gonadal status, diet, and lifestyle. In addition, there is an unclear causal sequence between obesity and various endocrinopathies, including hyperadrenocorticism, hypothyroidism, and diabetes mellitus.
Adipose tissue is composed of unilocular white adipocytes and multilocular brown adipocytes; both store energy in the form of lipid, while brown adipocytes are also involved in thermogenesis. Preadipocytes and pluripotent stem cells are responsible for the expansion of adipose tissue in obesity. Adipose tissue is also recognized as an endocrine organ that synthesizes and secretes adipokines such as leptin and adiponectin. In fact, maintaining the balance between these opposing adipokines is essential for the prevention of obesity. Leptin is critical for appetite suppression and energy expenditure via thermogenesis but is also proinflammatory. Obesity ensues with leptin or leptin receptor deficiency, as well as decreased end-organ response to leptin in the hypothalamus. Interestingly, breed, glucocorticosteroid therapy, and neutering have been shown to impact leptin concentrations in dogs and/or cats. Adiponectin is essential for enhanced glucose uptake and metabolism and in contrast to leptin is antiinflammatory. Decreased circulating adiponectin levels have been reported in obese dogs and cats; however, altered proportions of high and low molecular weight adiponectin have not been examined as they have in humans. Monocytes and macrophages, the number and activity of which increase with expansion of adipose tissue, are also critical to the development of obesity. Together with white adipocytes, they produce procoagulant, acute phase reactant, and proinflammatory cytokines, particularly TNF-α and IL-6.
Disorders of Horses
See the section on Disorders of the Adenohypophysis, Disorders of Domestic Animals for a discussion of hyperpituitarism and neoplasms of the adenohypophysis.
Lymphocytic (Immune-Mediated) Thyroiditis
See the section on Disorders of the Thyroid Gland, Disorders of Domestic Animals for a discussion of hypothyroidism.
Nutritional Hyperparathyroidism
See the section on Disorders of the Parathyroid Gland, Disorders of Domestic Animals for a discussion of hyperparathyroidism.
Pinealitis
Pinealitis accompanying uveitis is well established in animal models of experimental autoimmune uveoretinitis after immunization with retinal antigens. Because the pineal gland is rarely, if ever, histologically examined during active uveitis in humans with autoimmune uveitis, there is no direct evidence of concurrent pineal pathology, but it is suspected. However, there are reports documenting lymphocytic and eosinophilic pinealitis in horses with recurrent uveitis.
Disorders of Ruminants (Cattle, Sheep, and Goats)
Aplasia and Prolonged Gestation
Subnormal function of the fetal endocrine system, seen especially in ruminants, can disrupt normal fetal development and result in prolonged gestation. In Guernsey and Jersey cattle, a genetically determined failure of development (aplasia) of the adenohypophysis has been described, but the neurohypophysis develops normally. This aplasia results in a lack of fetal pituitary trophic hormone secretion during the last trimester and hypoplasia of target endocrine organs, namely the adrenal cortex, gonads, and follicular cells of the thyroid gland. Fetal development is normal up to approximately 7 months of gestation, but then fetal growth ceases, irrespective of the length of time the viable fetus is retained in the uterus.
Prolongation of gestation occurs in ewes that ingest the plant Veratrum californicum between days 12 and 14 of gestation. Veratrum californicum contains potent alkaloids that inhibit neural tube development, resulting in cyclopia and extensive malformations of the CNS and hypothalamus in lambs. Arrhinencephalia and lack of development of nasal bones accompany the formation of a proboscis-like structure. Although the adenohypophysis may be present in some cases, it is unable to secrete normal amounts of trophic hormones (ACTH) because it lacks the necessary fine control derived from the hypothalamic-releasing hormones. Pregnancy is maintained until a cesarean section is performed or the lamb dies in utero. Although the lambs retained in utero continue to grow beyond the normal gestation period, target endocrine organs in the fetus are hypoplastic, and the adrenal cortex does not differentiate completely into the three distinctive zones that secrete corticosteroid hormones.
The following concepts have emerged from the study of prolonged gestation in cattle and sheep:
1. Fetal hormones are necessary for final growth and development of the fetus in certain animals.
2. Normal parturition at term in these species requires an intact fetal hypothalamic-pituitary-adrenocortical axis working in concert with trophoblasts of the placenta.
Although the presence or absence of functional adenohypophyseal tissue determines whether the fetus continues to grow in utero, the pathogenesis of prolonged gestation is similar in these two examples. The subnormal development of the fetal adrenal cortex in calves and lambs results in an inadequate secretion of cortisol and a failure to induce 17α-hydroxylase in the placenta, which converts precursor molecules, such as progesterone, to estrogen. Thus the dam’s circulating progesterone is maintained near midgestational concentrations, and the notable increase in estrogens that normally occurs at term resulting in parturition is absent. The estrogen surge, when it occurs, stimulates prostaglandin synthesis in the uterus, which results in smooth muscle contractions and biochemical changes in collagen along the birth canal that normally permit delivery of the fetus.
Congenital Dyshormonogenetic Goiter
See the section on Disorders of the Thyroid Gland, Disorders of Domestic Animals for a discussion of hypothyroidism.
Disorders of Dogs
Pituitary Cysts and Pituitary Dwarfism
Pituitary dwarfism in dogs is usually the result of a failure of the oropharyngeal ectoderm of Rathke’s pouch to differentiate into trophic hormone–secreting cells of the pars distalis. This results in a progressively enlarging, multiloculated cyst and an absence of the adenohypophysis (Fig. 12-57). Other cysts originating in the craniopharyngeal duct or the pharyngeal hypophysis can also become large enough to induce clinical signs.
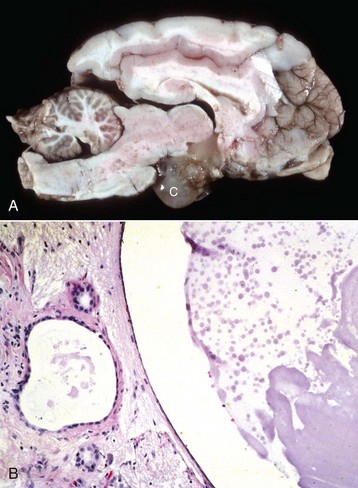
Fig. 12-57 Cystic Rathke’s pouch, brain, sagittal section, dog.
A, A large, multiloculated cyst (C) is present on the ventral aspect of this brain where the adenohypophysis would normally be located. B, High magnification: the cyst is shown in the right half of the figure; the left half shows poorly organized and differentiated adenohypophysis. H&E stain. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Juvenile panhypopituitarism occurs most frequently in German shepherd dogs, but it has been reported in Spitz, toy pinscher, and Karelian bear dogs. The dwarf pups appear normal from birth to about 2 months of age. Subsequently, the slower growth, retention of puppy hair coat, and lack of primary guard hairs gradually become evident (Fig. 12-58). A bilaterally symmetric alopecia develops gradually and often progresses to complete alopecia except for the head and tufts of hair on the legs. There is progressive hyperpigmentation of the skin until the skin is uniformly brownish-black over most of the body. Adult German shepherd dogs with panhypopituitarism vary in size from as small as 2 kg to nearly half normal size, depending on whether the failure of formation of the adenohypophysis is partial or complete.

Fig. 12-58 Panhypopituitarism (“pituitary dwarfism”), 5-month-old German shepherd and littermate.
The unaffected littermate weighed 27.3 kg, whereas the dwarf puppy weighed only 4 kg. The pituitary dwarf has retained its puppy hair coat. (From Alexander JE: Can Vet J 3:83, 1962.)
The mode of inheritance is simple autosomal recessive. The activity of somatomedin, also known as insulin-like growth factor (a non–species-specific, cartilage growth-promoting peptide whose production in the liver and plasma activity is controlled by somatotropin) is low in dwarf dogs. Intermediate somatomedin activity is present in phenotypically normal ancestors suspected to be heterozygous carriers. Assays for somatomedin provide a useful indirect measurement of circulating growth hormone activity in dogs with suspected pituitary dwarfism if canine growth hormone assays are not available.
Craniopharyngioma
See the section on Disorders of the Adenohypophysis, Disorders of Domestic Animals for a discussion of hypopituitarism and neoplasms of the adenohypophysis.
Pars Intermedia Adenomas
See the section on Disorders of the Adenohypophysis, Disorders of Domestic Animals for a discussion of hyperpituitarism and neoplasms of the adenohypophysis.
ACTH-secreting (Corticotroph) Adenomas
See the section on Disorders of the Adenohypophysis, Disorders of Domestic Animals for a discussion of hyperpituitarism and neoplasms of the adenohypophysis.
Pituitary Apoplexy
Pituitary apoplexy is acute infarction or hemorrhage within a pituitary neoplasm or nonneoplastic pituitary gland. It has been reported in 5 dogs ranging in age from 4 to 12 years with no breed predisposition. Corticotroph adenomas of the pars distalis were present in 4/5 dogs. Although the exact pathogenesis of the hemorrhage and necrosis, particularly in nonneoplastic glands, has yet to be elucidated, they are hypothesized to be secondary to alterations in blood supply.
Idiopathic Adrenocortical Atrophy
Bilateral idiopathic adrenal cortical atrophy is an entity in young adult dogs that results in hypoadrenocorticism. The adrenal cortex is reduced to one-tenth or less of its normal thickness because of a marked reduction of all layers of the cortex. It consists primarily of the adrenal capsule (Fig. 12-59). Thus the adrenal medulla becomes relatively more prominent and along with the capsule, makes up the bulk of the remaining adrenal gland. The precise pathogenesis of idiopathic adrenal cortical atrophy is unknown, but the lesion is most likely immune-mediated. Early in the disease, multiple foci of lymphocytes and plasma cells are interspersed between the adrenal sinusoids and adrenal cortical cells. The capsule is thickened by condensation from the collapse of the adrenal cortex and fibroblastic proliferation. Pituitary gland lesions have not been observed in dogs with idiopathic adrenal cortical atrophy involving all three zones of the adrenal cortex, including the zona glomerulosa, which is not under ACTH control. By comparison, atrophy of the adrenal cortex caused by a destructive pituitary gland lesion that decreases ACTH secretion is characterized by severe atrophy only of the two inner cortical zones (zonae fasciculata and reticularis). The zona glomerulosa remains intact (see Fig. 12-16), and thus these animals do not have electrolyte abnormalities because the secretion of aldosterone remains within normal limits.
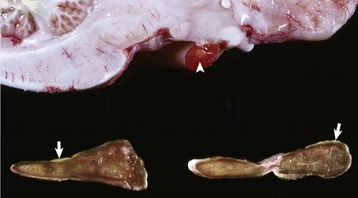
Fig. 12-59 Adrenal cortical atrophy, brainstem and pituitary gland, left and right adrenal glands, dog.
Note the marked bilateral atrophy of the cortices of the adrenal glands (arrows). Atrophy of all three cortical layers is characteristic of hypoadrenocorticism. The pituitary gland (arrowhead) is grossly normal but had microscopic evidence of corticotroph hyperplasia. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
Hypothyroidism
See the section on Disorders of the Thyroid Gland, Disorders of Domestic Animals for a discussion of hypothyroidism.
Renal Hyperparathyroidism
See the section of Disorders of the Parathyroid Gland, Disorders of Domestic Animals for a discussion on hyperparathyroidism.
Apocrine Gland Adenocarcinoma
A syndrome of HHM in aged, primarily female dogs with adenocarcinomas derived from apocrine glands of the anal sac has been characterized. Although a large case series of cats in the United Kingdom with apocrine gland adenocarcinoma was recently published, serum calcium was only measured in 5/64 cats with 1/5 having a marginal elevation and no clinical signs attributed to hypercalcemia. Dogs have persistent hypercalcemia (mean 16.2 mg/dL) and often mild hypophosphatemia, both of which return to normal after surgical excision of the neoplasm. The hypercalcemia persists after removal of the parathyroid glands, suggesting that the humoral factor produced by neoplastic cells does not stimulate an increased secretion of PTH. Increased circulating concentrations of PTHrP have been observed in hypercalcemic dogs with anal sac apocrine gland adenocarcinomas. The neoplasms are malignant, and most have metastasized to regional lumbar aortic lymph nodes at the time of initial presentation. Clinical signs include generalized muscular weakness, anorexia, vomiting, bradycardia, depression, polyuria, and polydipsia. These signs are primarily the result of severe hypercalcemia.
Apocrine gland adenocarcinomas develop as firm, usually unilateral masses, ventrolateral to the anus, and close to the anal sac but not attached to the overlying skin (Fig. 12-60). The neoplasm arises in the wall of the anal sac and projects as a variably-sized mass into the lumen (Fig. 12-61).
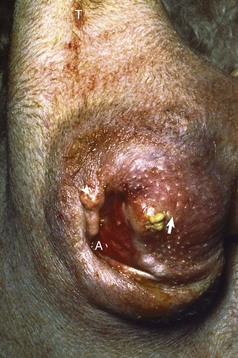
Fig. 12-60 Adenocarcinoma, apocrine glands of right anal sac, anus, dog.
The right perianal region is distended by an adenocarcinoma (arrow), which has compressed the right side of the anus. It also projects as two nodules (arrowhead) on the dorsolateral margin of the anus. T, Tail; A, anus. (Courtesy Dr. C. Capen, College of Veterinary Medicine, The Ohio State University.)
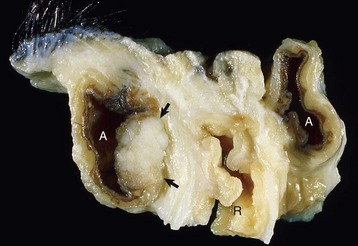
Fig. 12-61 Adenocarcinoma, apocrine glands, anal sac, dorsal plane, formalin-fixed specimen, dog.
A 1-cm diameter nodule (arrows) derived from apocrine glands of the wall of the right anal sac (glands of the perianal sinus) protrudes into the lumen of the right anal sac. Anal sacs (A) are present on both sides of the rectum (R). (From Meuten DJ, Cooper BJ, Capen CC, et al: Vet Pathol 18:454-471, 1981.)
Histologically, apocrine gland adenocarcinomas are distinct from the more common perianal (circumanal) gland tumor, which arises in hepatoid glands. The majority of apocrine gland neoplasms histologically contain glandular and solid areas. Neoplastic cells form acini and papillary tufts that project into the acinar lumens Fig. 12-62; Web Fig. 12-14). The solid pattern is characterized by sheets, microlobules, and packets separated by a thin fibrovascular stroma. Pseudorosettes are common in solid areas adjacent to small blood vessels.
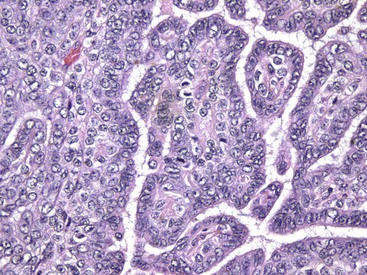
Fig. 12-62 Adenocarcinoma, apocrine glands, anal sac, dog.
Neoplastic cells form poorly arranged glands with papillary projections. There is moderate variation in cellular and nuclear size and shape as well as several mitoses, all characteristic of anaplasia. H&E. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
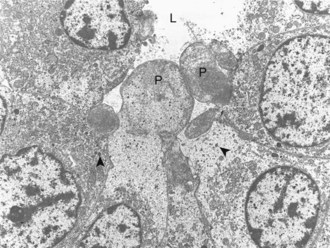
Web Fig. 12-14 Adenocarcinoma, anal sac, dog.
Projections (P) of apical cytoplasm extend into the acinar lumen (L). Small membrane-limited secretory granules (arrowheads) are present in the cytoplasm. TEM. Uranyl acetate and lead citrate stain. (From Meuten DJ, Capen CC, Kociba GJ, et al: Am J Pathol 107:167-175, 1982.)
Disorders of Cats
Adrenocorticotropic Hormone-Secreting (Corticotroph) Adenomas
See the section on Disorders of the Adenohypophysis for a discussion of hyperpituitarism and neoplasms of the adenohypophysis.
Hyperaldosteronism
See the section on Disorders of the Adrenal Cortex, Disorders of Domestic Animals for a discussion of hyperaldosteronism.
Hypothyroidism
See the section on Disorders of the Thyroid Gland, Disorders of Domestic Animals for a discussion of hypothyroidism.
Barber, LG. Thyroid tumors in dogs and cats. Vet Clin Small Anim. 2007;37:755–773.
Bertolini, G, Rossetti, E, Caldin, M. Pituitary apoplexy-like disease in 4 dogs. J Vet Intern Med. 2007;21:1251–1257.
Chiaramonte, D, Greco, DS. Feline adrenal disorders. Clin Tech Small Anim Pract. 2007;22:26–31.
Hughes, AM, Nelson, RW, Famula, TR, et al. Clinical features and heritability of hypoadrenocorticism in Nova Scotia duck tolling retrievers: 25 cases (1994-2006). JAVMA. 2007;231:407–412.
Murray, RD, Horsfield, JE, McCormick, WD, et al. Historical and current perspectives on the treatment, control and pathogenesis of milk fever in dairy cattle. Vet Rec. 2008;163:561–565.
Perillo, A, Passantino, G, Passantino, L, et al. First observation of an Hashimoto thyroiditis-like disease in horses from eastern Europe: histopathological and immunological findings. Immunopharmacol Immunotoxicol. 2005;27:241–253.
Pettigrew, R, Fyfe, JC, Gregory, BL, et al. CNS hypomyelination in rat terrier dogs with congenital goiter and a mutation in the thyroid peroxidase gene. Vet Pathol. 2007;44:50–56.
Wakeling, J, Smith, K, Scase, T, et al. Subclinical hyperthyroidism in cats: a spontaneous model of subclinical toxic nodular goiter in humans? Thyroid. 2007;17:1201–1209.
Wilbe, M, Sundberg, K, Hansen, IR, et al. Increased genetic risk or protection for canine autoimmune lymphocytic thyroiditis in giant schnauzers depends on DLA class II genotype. Tissue Antigens. 2010;75:712–719.
Zoran, DL. Obesity in dogs and cats: a metabolic and endocrine disorder. Vet Clin Small Anim. 2010;40:221–239.
*In memory of Dr. Charles C. Capen, Department of Veterinary Biosciences, The Ohio State University, who made contributions to this chapter in the fourth edition.