Volvulus and Torsion
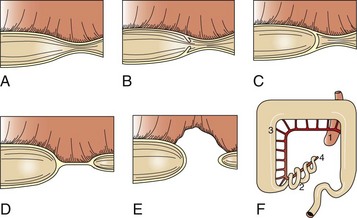
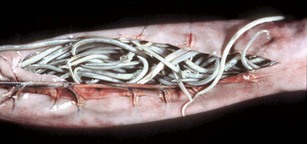
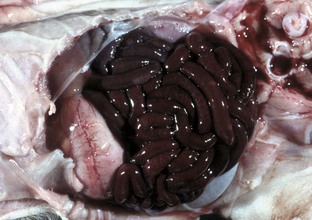
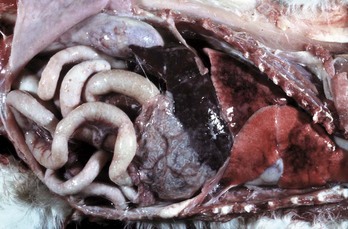
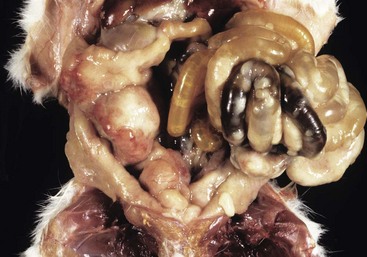
A volvulus is a twisting of the intestine on its mesenteric axis. A torsion is a rotation of a tubular organ along its long axis. The latter is most common in the cecum of cattle and horses and occasionally of the abomasum of calves (see Web Fig. 7-10). Both volvulus and torsion result in compression of the mesenteric veins and arteries resulting in ischemia initially followed by obstruction—veins first and later as the pressure on the mesenteric vessels increases, the arteries. Infarction is a result of occlusion of the thin-walled mesenteric veins. Because the mesenteric arterial supply is anatomically more resistant to occlusion, blood is pumped into the twisted segment but cannot drain. Edema, congestion, hemorrhage, and eventual necrosis result (Figs. 7-107 and 7-108). It is probable that the mechanism of intestinal twisting is secondary to movement of the walls of the abdominal cavity (i.e., the intestine stays still and the horse rolls or otherwise moves around the static intestine).
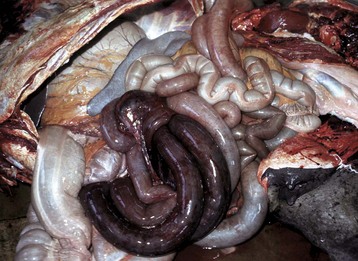
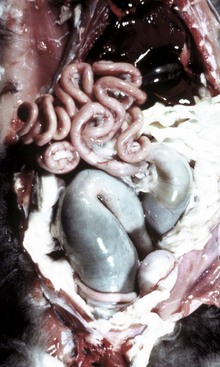
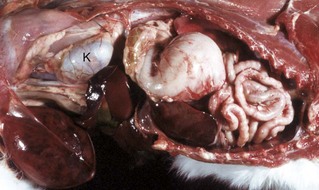
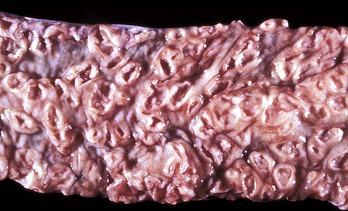
Fig. 7-107 Infarction, small intestine, horse.
Volvulus of the intestine has resulted in vascular compromise and infarction of several loops of bowel. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
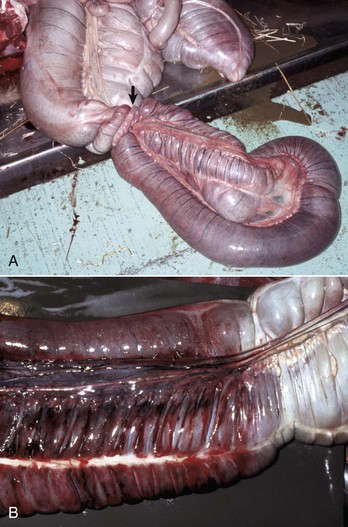
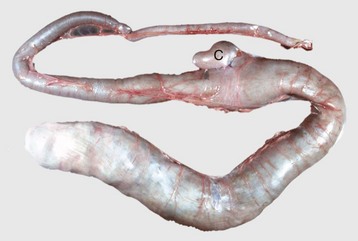
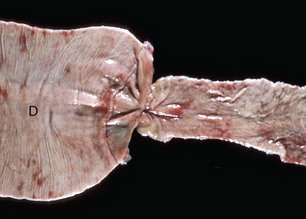
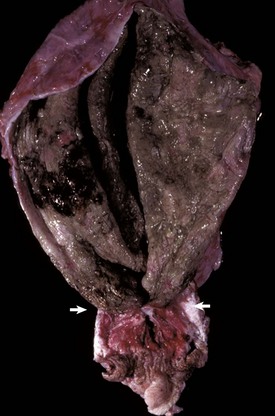
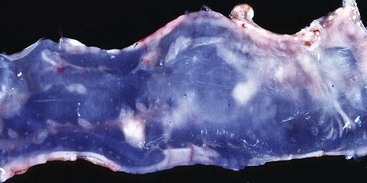
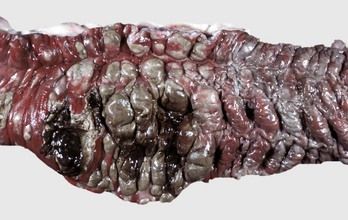
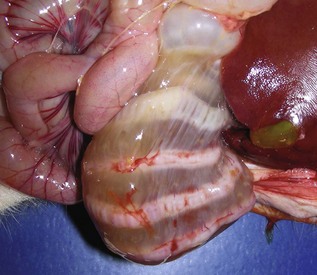
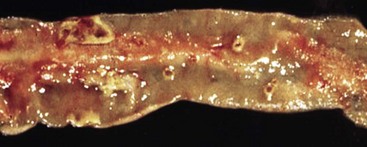
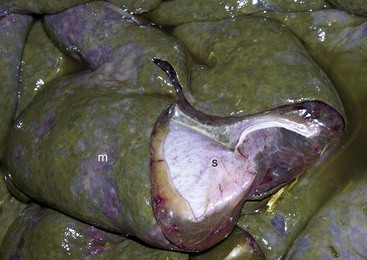
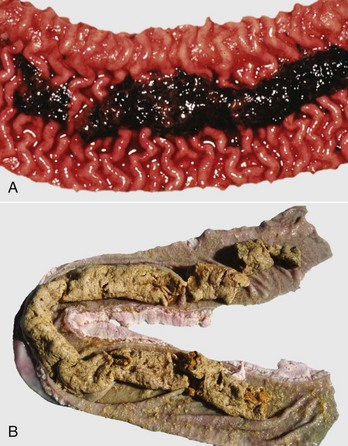
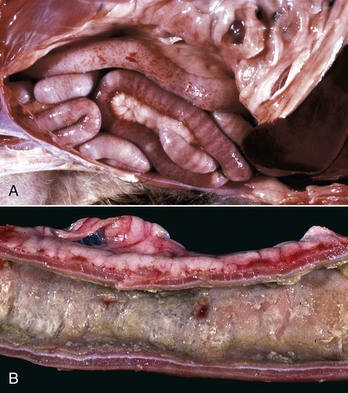
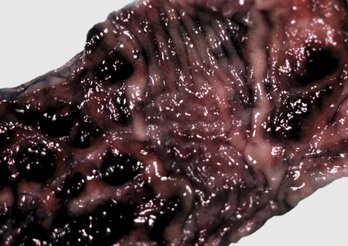
Fig. 7-108 Torsion, large colon, horse.
A, Rotation of the colon on its long axis has resulted in severe colic with strangulation (arrow). Note the red to blue discoloration of the colon distal to the torsion caused by obstruction of venous blood flow. B, Note the sharp line of demarcation (point where the torsion occurred) between viable colon (to the right) and nonviable colon (to the left) caused by obstruction of venous blood flow. In this case, the torsion was not found at the time of necropsy; however, a torsion will commonly untwist itself (reduce itself) during transport of the dead animal to the postmortem room. (A courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University. B courtesy Dr. M.D. McCracken, College of Veterinary Medicine, University of Tennessee.)
At surgery or necropsy, the twisted segment of intestine is distended with gas and fluid and is discolored either dark red or black (see Fig. 2-42). There is usually a sharp line of demarcation between the affected and normal intestine. This line marks the site for surgical resection. A volvulus may result in a rotation of the intestine up to 720 degrees, either clockwise or counterclockwise on its mesenteric axis. Therefore surgical correction of a volvulus may be difficult and complex. It is very important to determine the viability of the bowel after reduction of a volvulus. The affected segment of intestine is often necrotic, congested, and hemorrhagic. Intestinal stasis and toxemia and/or bacteremia may result from bacterial overgrowth and anoxic bowel necrosis. Reperfusion injury may also occur. Toxemia and intestinal rupture may result in death.
Volvulus of the equine large intestine occurs most commonly in the left colon. In equids, the left ventral colon is an extension of the right ventral colon beginning at the sternal flexure. The left ventral colon doubles back on itself in the pelvic inlet to form the left dorsal colon. This pelvic flexure can be palpated rectally. The left dorsal colon becomes the right dorsal colon at the diaphragmatic flexure. The diaphragmatic flexure lies cranial to the sternal flexure and usually contacts the ventral body wall. The left dorsal colon is sacculated with one taenia; the left ventral colon is sacculated with four taeniae. When twisting occurs, it is usually clockwise around the mesocolon and is thus a volvulus. Torsion of the large colon of mares accounts for half of their intestinal displacements in the peripartum period.
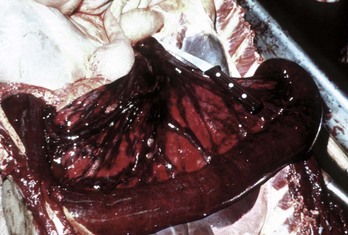
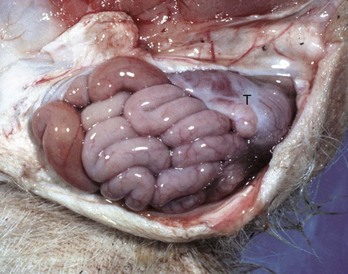
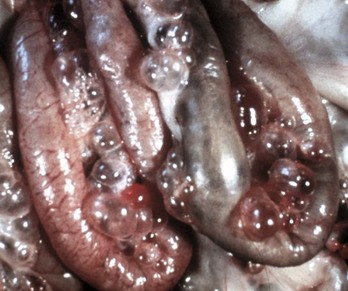
A peculiar type of intestinal strangulation occurs in horses in which lipomas, which are pedunculated, wrap around the intestinal mesentery or the bowel, causing ischemia, colic, and death (Fig. 7-109). Pedunculated lipomas may rotate about their pedicle cutting off their own blood supply. When this occurs, they undergo mineralization and sometimes ossification. The stalk may become necrotic and break, leaving a free-floating lipoma within the abdominal cavity where it apparently does no harm. However, most mesenteric lipomas are of no clinical consequence. Rarely, intestinal strangulation by pedunculated lipomas has been reported in the dog.
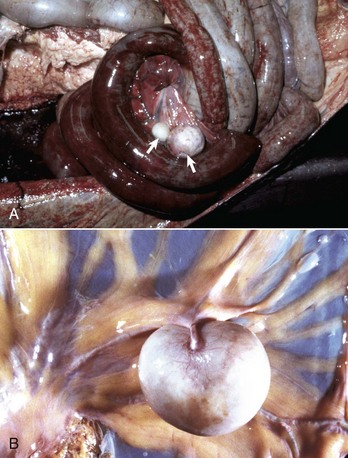
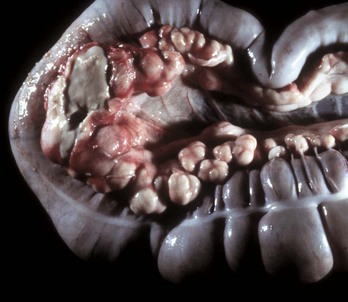
Fig. 7-109 Pedunculated lipomas.
A, Intestinal strangulation by pedunculated lipomas, small intestine, horse. Two lipomas (arrows) have wrapped around the mesentery and strangled the bowel resulting in infarction. B, Mesentery, horse. Closer view of a pedunculated lipoma. (A courtesy College of Veterinary Medicine, Cornell University. B courtesy College of Veterinary Medicine, University of Illinois.)
Miscellaneous Diseases and Conditions
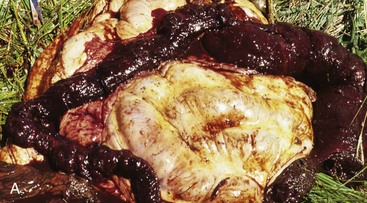
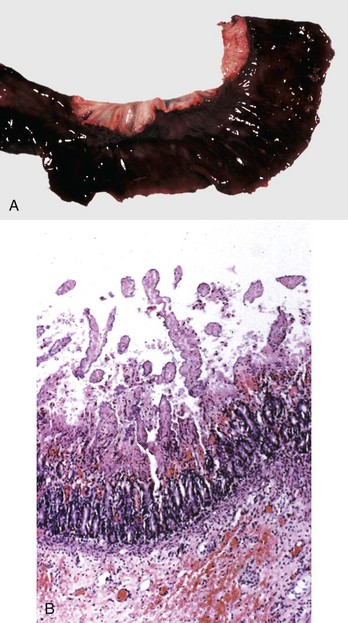
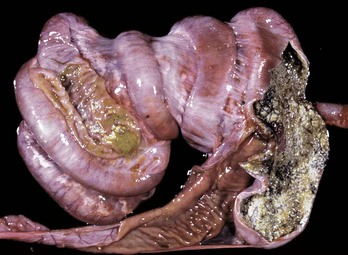
Cecal or large intestinal rupture occurs most commonly in postparturient mares (Fig. 7-110) but can also result from impaction and as a complication of anesthesia. The sites of rupture vary, and the mechanisms are unknown. Iatrogenic rectal tearing may occur secondary to rectal palpation (Fig. 7-111). The presence of blood on a rectal sleeve after palpation is cause for concern because peritonitis may be the result of penetration of the peritoneal cavity, especially if the tear occurs ventrally.
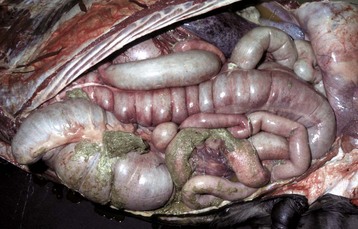
Fig. 7-110 Fibrinous peritonitis, abdomen, horse.
The presence of fibrin and ingesta adherent to serosal surfaces indicates antemortem perforation or rupture of the intestine. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
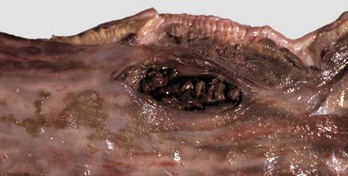
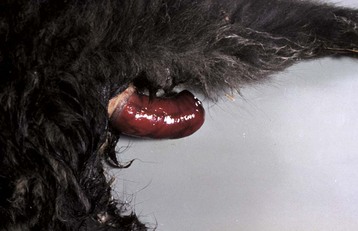
Fig. 7-111 Ulceration, rectum, horse.
Hemorrhage, ulcers, and tears in the rectum are often caused by inexperienced persons or overly vigorous rectal palpation. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
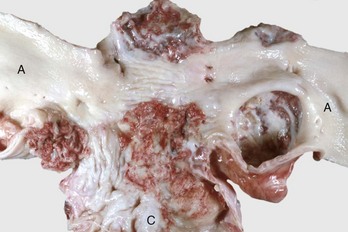
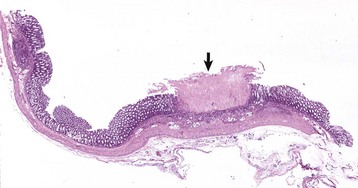
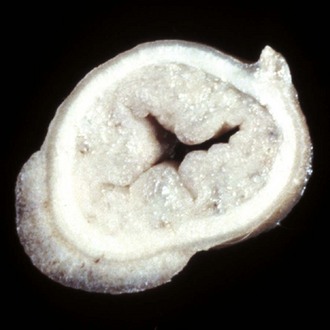
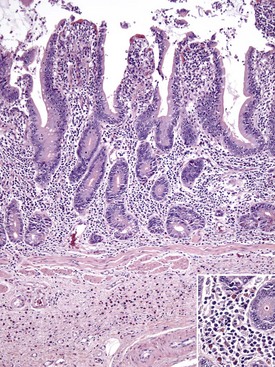
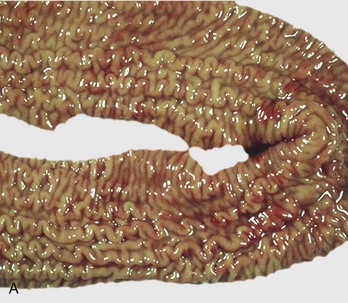
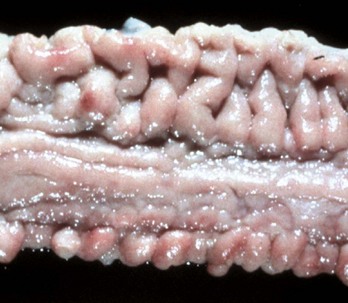
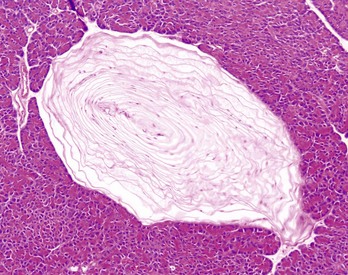
Diverticula (singular, diverticulum) are epithelium-lined cavities that are derived from mucosal epithelium that extend through the muscularis mucosa, submucosa, and muscularis and often reach the serosa, where they sometimes rupture, causing peritonitis (Figs. 7-112 and 7-113; Web Fig. 7-16). This can occur in any part of the tubular gut, including the esophagus and cecum.
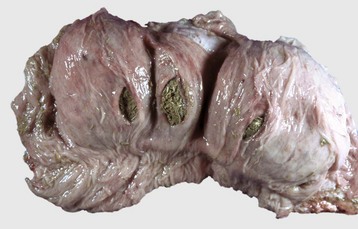
Fig. 7-112 Diverticula, cecum, horse.
Diverticula are mucosal outpouchings into the subjacent smooth muscle layers of the colon. They are filled with ingesta and lined by intact mucosa. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
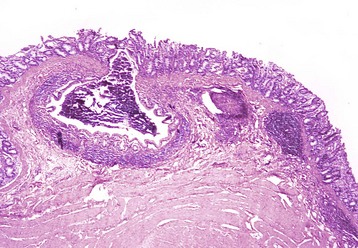
Fig. 7-113 Diverticulum, colon, cow.
A diverticulum lined by superficial mucosa has penetrated through the submucosa to lie next to the muscularis. H&E stain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
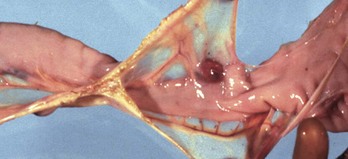
Web Fig. 7-16 Mesodiverticulum, ileum, horse.
An intestinal mucosal outpouch has penetrated the wall of the intestine and extended into the mesentery (red nodule). (Courtesy Dr. J. King, College of Veterinary Medicine, Cornell University.)
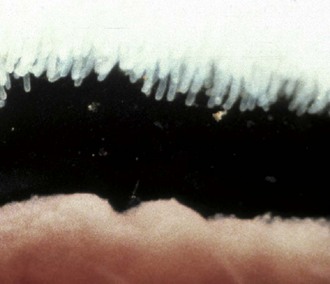
Muscular hypertrophy of the distal ileum is an idiopathic condition of horses and pigs. Although generally an incidental finding, hypertrophy of the tunica muscularis can lead to impaction and rupture of the ileum. The lesion in horses is sometimes segmental, affecting the ileum and variably the jejunum. Often, the lesion is a sequela of muscular hypertrophy caused by a damaged or stenotic ileocecal valve. Muscular hypertrophy of equids may also affect the duodenum and jejunum in association with diverticula in those gut segments. Horses with muscular hypertrophy of the distal ileum may have mild colic, occasional diarrhea, and weight loss. Often, muscular hypertrophy is asymptomatic. Muscular hypertrophy of the ileum in pigs generally occurs as an idiopathic, asymptomatic lesion. Muscular hypertrophy of the tunica muscularis associated with diverticulosis of the ileum has been recorded in young Yorkshire pigs and in Romney Marsh and Hampshire sheep. The lesion is suspected to be secondary to a functional obstruction of the ileocecal valve. Diverticulosis and/or intestinal rupture may result.
Cats can have a severe hypertrophy of the inner, circular layer of the tunica muscularis of the ileum and sometimes the jejunum. In cats with hypereosinophilic syndrome, a disease characterized by intramural eosinophil infiltrates, hypertrophy of the gastric antrum, and small intestinal musculature can occur. Muscular hypertrophy of the intestine and medial hyperplasia of the pulmonary arteries occur in cats given large oral doses of Toxocara cati larvae. These conditions are often accompanied by diarrhea and eosinophilic enteritis. Fibrosis of the lamina propria and hypertrophy of the inner layer of the tunica muscularis may result in a stiff, thickened intestine.
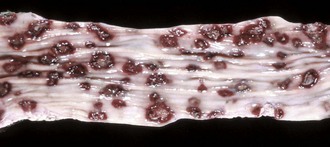
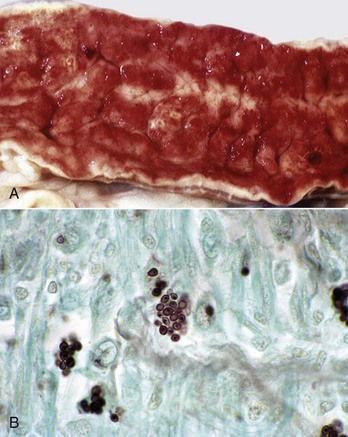
Another unique lesion in the horse is Hemomelasma ilei. These lesions are pink to black plaques that vary in length from several millimeters to many centimeters and can occur anywhere in the intestinal subserosa but are generally limited to the ileum (Fig. 7-114; also see Fig. 3-39). They are attributed to larval migrations of strongyles (usually Strongylus edentatus) and are located on the antimesenteric serosal surface. However, parasites have never been reported in the lesions and therefore the cause of hemomelasma ilei is unknown. They are generally of no clinical consequence but can on occasion lead to intestinal strictures and intermittent colic.
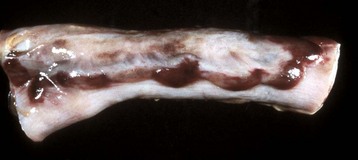
Fig. 7-114 Hemomelasma ilei, ileum, horse.
Hemorrhagic and siderotic fibrovascular plaques on the antimesenteric serosa are attributed to strongyle larval migration (Strongylus edentatus), but this association has never been demonstrated. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Intestinal ceroidosis or leiomyometaplasia is also called brown dog gut. The discolored intestinal smooth muscle may occur in association with chronic enteritis and pancreatitis. Experimentally, leiomyometaplasia can be produced in dogs by vitamin E deficiency, in association with excess dietary lipids. The canine and human intestinal pigmentation is the result of vitamin E deficiency. The dietary requirement for vitamin E is proportional to the concentration of polyunsaturated fatty acids in the diet. Intestinal ceroidosis probably does not cause clinical signs but may be an indicator of a metabolic or nutritional disorder. In this condition, the intestinal serosa varies from tan to dark brown (Fig. 7-115). The stomach and large bowel are variously affected, as is the small intestine. Accumulation of brown, granular, acid-fast–staining lipofuscin in the perinuclear lysosomes of the leiomyocytes is characteristic of this condition.
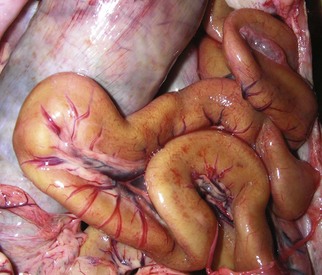
Fig. 7-115 Leiomyometaplasia, intestine, dog.
“Brown dog gut” is a rare condition caused by the accumulation of a brown pigment now known to be ceroid (formerly called lipofuscin) in the lysosomes of smooth muscle cells of the tunica muscularis. It is a dietary condition associated with vitamin E deficiency. (Courtesy Dr. L. Borst, College of Veterinary Medicine, University of Illinois.)
Amyloidosis occasionally is present in the intestinal and vascular walls of the lamina propria and muscularis in association with systemic amyloid AA infiltrations in a variety of animal species of all ages.
Tiger striping is a nonspecific congestion of colonic ridges secondary to diarrhea and/or tenesmus (Web Fig. 7-17). The red and pale longitudinal stripes are formed by the congested tips of the folds alternating with the uncongested mucosa between them.
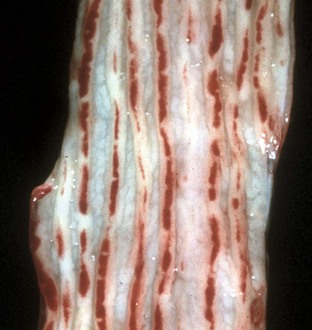
Web Fig. 7-17 Tiger striping, colon, dog.
Nonspecific congestion and hemorrhage of the colonic ridges is due to tenesmus and/or diarrhea. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Small Intestinal Intoxicants
Because most toxins enter the body through ingestion, those that are irritants can cause contact lesions in the oral cavity, esophagus, stomach, and intestine. The lesions that result are generally those of hemorrhage and inflammation. In many cases of intoxication, induction of vomiting is contraindicated because what burns going down will also burn coming up. For some intoxicants, multidrug resistance (MDR1) gene products of enterocytes are part of the detoxification process. In addition, P450 enzymes are present on villus enterocytes, although in much lesser amounts than in the liver. They are in highest concentration in the jejunum and decrease aborally. In humans, ingestion of grapefruit juice interferes with the function of these enzymes, sometimes resulting in enhanced oral drug availability.
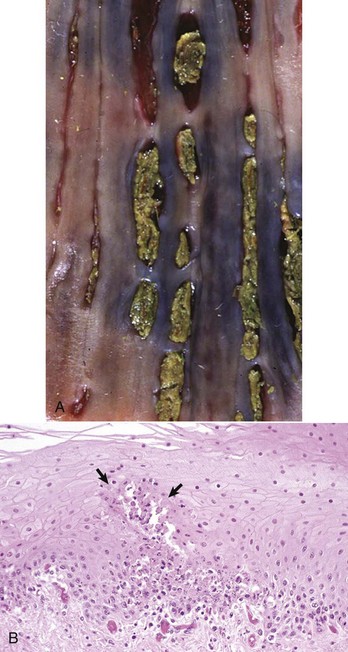
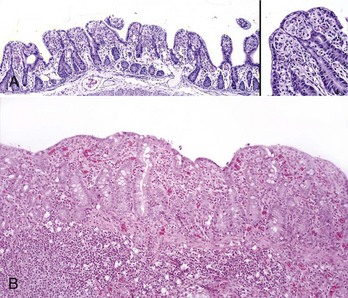
The numbers and types of chemicals and intoxicants animals are exposed to make a listing of them a monumental undertaking. A few examples are phosphorus, arsenic, bracken fern (cattle), mercury, oak, copper, nitrate, thallium, and blister beetles. Blister beetles, a specific toxicity, are sometimes incorporated into crimped hay (Fig. 7-116). They contain a topical irritant called cantharidin. Lesions include sloughing of the epithelium of the stomach and enterocytes of the proximal small intestine (Fig. 7-117). In addition, cantharidin can cause hemorrhagic ulcers of the urinary bladder and myocardial necrosis.

Fig. 7-116 Striped blister beetles.
Numerous species of blister beetles (Epicauta spp.), such as gray, black, and striped, can be found throughout the United States. They contain a vesicant (blister-causing substance) that causes inflammation and blistering of mucosal surfaces when they are ingested. Usually, these beetles are trapped and crushed in crimped hay. (Courtesy Dr. W. Crowell, College of Veterinary Medicine, University of Georgia; and Noah’s Arkive, College of Veterinary Medicine, University of Georgia.)
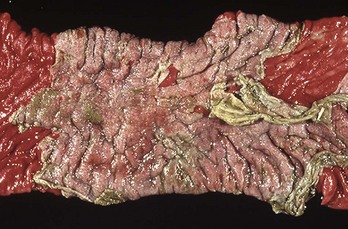
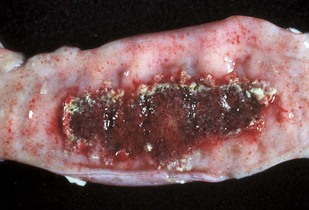
Fig. 7-117 Acute necrohemorrhagic enteritis, small intestine, horse.
The severe necrosis with sloughing of intestinal mucosa is the result of cantharidin, a toxin contained in ingested blister beetles. (Courtesy Dr. R. Panciera, School of Veterinary Medicine, Oklahoma State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Although not generally considered an intoxicant, corticosteroids cause colonic perforation in some treated dogs and can delay GI healing. They do this by decreasing cell turnover, decreasing mucus production, and stimulating gastrin secretion, leading to increased acid production. NSAIDs can cause right dorsal colitis in equids. This colitis is characterized by necrosis, resulting in erosions and ulcers. Epithelial loss may be severe, with only regenerating, rounded islands of normal mucosa remaining. The massive edema of the denuded intestine causes rupture of the submucosa in an elongated diamond-like pattern. The mechanism of injury is direct by topical application (oral administration) and through inhibition of prostaglandin synthesis. Neutrophils play a role by increasing synthesis of tumor necrosis factor-α, leukotriene B4, and upregulation of leukocyte adhesion molecules.
Diseases of the Intestinal Epithelium
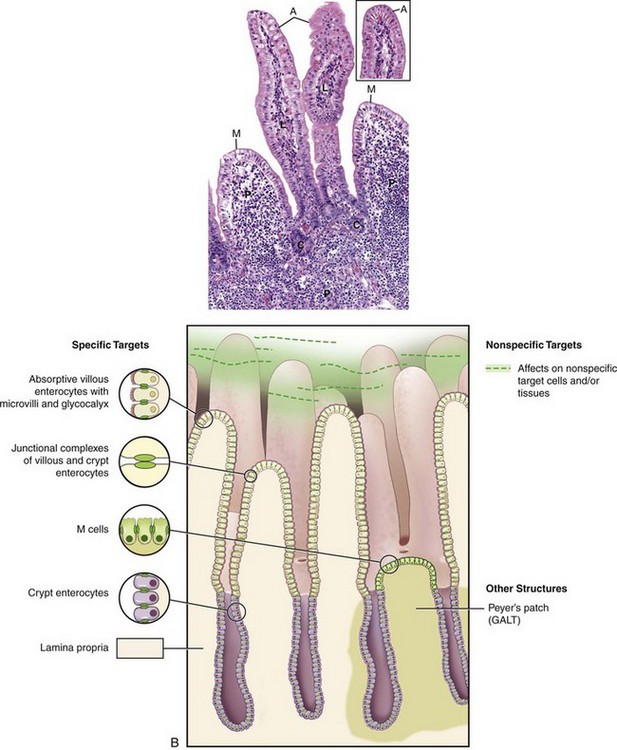
A number of diseases are characterized by colonization or destruction of the epithelial components of the intestinal mucosa. Although the disease-producing effects of pathogens are complex and multifactorial, a simplified understanding of the principal cell under attack is helpful in predicting disease outcome and managing treatment.
Diseases of the Absorptive Enterocytes: A number of agents have a tropism for the absorptive cells lining the intestinal villi. These agents include viruses such as rotavirus, enteric coronavirus, and the coronavirus of transmissible gastroenteritis of pigs. Intracellular bacteria and parasites can likewise invade and multiply in absorptive epithelial cells. Examples include the agents of swine dysentery (Brachyspira hyodysenteriae), coccidia, and cryptosporidium.
Some pathogens with a tropism for absorptive lining cells of the intestine cause destruction of these cells. This results in loss of enterocytes and at least temporary villous atrophy. The loss of the absorptive-digestive villous enterocytes causes maldigestion, and malabsorption results. Furthermore, because ingesta and normal alimentary secretions are unabsorbed, they are degraded further and fermented in the intestine by bacteria, increasing the osmolality of intestinal contents, with a subsequent increase in the fluid content of the bowel.
Because the regenerative crypt cells are not attacked by pathogens with tropism for villous enterocytes, diseases with villous enterocyte damage are not necessarily fatal. The lost cells are replaced by the maturing cells migrating along the basement membrane from the crypt to the villus. The naked basement membrane contracts, causing villous atrophy. This contraction may be a function of the smooth muscle in the lamina propria. The functionally immature migrating crypt cells cover the villi. Often, these immature cells become squamoid in an effort to cover the maximum area of basement membrane. However, if naked basement membranes contact each other, they will adhere, resulting not only in villous blunting but also in villous fusion, preventing the reformation of normal villi.
Diseases of Undifferentiated Crypt Cells: Loss of the undifferentiated epithelial cells in the base of the crypts means loss of the cells capable of rapid mitosis, and thus regeneration of the epithelium is impaired. Therefore the clinical effect of crypt cell loss can be delayed for several days because the villi are initially still covered by enterocytes. This type of loss is more severe and often fatal, compared with villous enterocyte loss. Agents that target and destroy crypt cells are called radiomimetic because they mimic the effects of radiation on the rapidly dividing enterocytes. Examples of these agents include the parvoviruses of carnivores, BVD virus, rinderpest virus, and some mycotoxins such as vomitoxin.
Enterotoxic Escherichia coli infection of neonatal pigs, calves, lambs, and humans causes what is known as a secretory diarrhea. These bacteria are able to colonize the small intestinal enterocytes by way of their surface or pilus antigens, which anchor them to the enterocytes. Different pilus antigens adhere to glycoconjugate receptors on enterocytes in different regions of the small intestine. Thus these bacteria are not washed out by peristalsis. Because the enterocytes are not damaged, no lesions are observed, although microscopically the bacteria can be seen attached to the epithelial surface. The bacteria produce a toxin that causes enterocytes to secrete water and electrolytes. Although cAMP and cyclic guanosine monophosphate (cGMP) mediate this process, the exact mechanism by which this secretion occurs is unknown. Some secretions, especially those of Cl−, occur via the crypt cells. Intestinal secretion exceeds the ability of the colon to absorb the surplus fluid. The net result is diarrhea.
Abnormalities of the Microvilli and Glycocalyx: Because the microvilli and glycocalyx on the villous enterocytes are largely responsible for the immense surface area and the enzymes responsible for nutrient digestion and absorption, it follows that damage to either of these structures can result in intestinal malfunction and resultant diarrhea. A prime example of this is human lactose intolerance. Such persons lack lactase in the glycocalyx. Because of this lack, they are unable to digest lactose from dairy products. The lack of lactase results in failure of uptake of milk sugar, and the lactose is fermented by bacteria in the colon. This results in an osmotic drain of fluid into the gut with resultant diarrhea. Thus the malabsorption in this case is limited to a single substrate. Histologically, the intestine is normal.
Some bacteria, such as attaching and effacing Escherichia coli, damage the microvilli by their attachment. This attachment disrupts enzyme systems housed in the microvilli and glycocalyx and causes diarrhea. The antibiotic neomycin can similarly cause fragmentation of microvilli and destruction of the glycocalyx with resultant diarrhea. Cessation of neomycin therapy results in a return to normal structure and function.
Diseases in which the Epithelial Targets are Unknown or Nonspecific: In a number of enteric diseases, the targeted epithelial cell is unknown or nonspecific. Clostridium perfringens type C is a pathogen of neonatal pigs, lambs, calves, and foals. Unlike the ETEC, which produces a toxin affecting enterocytes, Clostridium perfringens produces a nonspecific cytotoxin. This toxin causes necrosis of villous absorptive cells, which then extends to the lamina propria and blood vessels. The result is massive and acute necrohemorrhagic enteritis.
Separation of Apical Junctional Complexes: Apical junctional complexes, also called tight junctions or zona occludens, join enterocytes to each other. Transmembrane proteins, such as claudin, occludin, tricellulin, junction-associated molecules (JAMs), and the coxsackie virus and adenovirus receptor (CAR), form tight junctions. Normally, these junctions are a barrier to macromolecular transepithelial transport. In certain diseases, such as ostertagiosis, Salmonella Typhimurium in vitro, Clostridium perfringens, alimentary anthrax, and enterohemorrhagic Escherichia coli, these tight junctions are pathologically opened through effects of bacterial toxins and products on transmembrane proteins, allowing transport of macromolecules into the intestinal (abomasal) lumen. This opening of tight junctions is also important in allowing macromolecules, such as immunoglobulin, into the lumen where the pathogen can be attacked.
Parasitic Enteritides
Parasites of the intestinal tract are legion in the various domestic animal species. Refer to a parasitology textbook for specific information regarding the life cycles and identification of the various species. Diagnosis of enteric parasitism is generally performed via fecal flotation or intestinal scrapings.
Amebiasis: Entamoeba spp. are obligate intracellular parasites with a direct life cycle. The portal of entry is oral. Trophozoites are produced that dwell in the intestinal lumen. They may also invade through the intestinal wall and go to many other organs, such as the liver, brain, and lung, especially in humans, in whom microabscesses may form. Cysts are excreted with formed feces and continue their life cycle when ingested by another host. Trophozoites are more likely seen in diarrheic feces. Because cysts are the infective form, diarrheic feces of dogs are not usually considered to be especially dangerous to humans or other animals. The trophozoites vary from 12 to 30 µm in diameter, and the cysts vary from 10 to 20 µm with four nuclei. Contact of ameba and host cells is likely mediated by adhesins. Soluble factors produced by the parasite mediate pathogenicity.
Entamoeba histolytica is zoonotic in humans, other primates, dogs, cats, and other animals. Disease is serious in humans. Lesions include colonic congestion, petechia, and ulceration (ulcerative colitis). This colitis may be acute or chronic, bloody or mucoid. In tissue, the amebas may be as large as 50 µm and often form typical flask-shaped ulcers spanning the mucosa and submucosa of the colon. After penetrating the surface mucus and adhering to the colonic enterocytes, Entamoeba histolytica releases amebophores (channel-forming peptides) that lyse the enterocytes without killing the ameba.
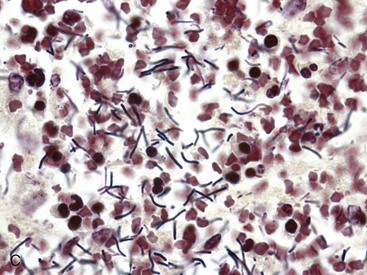
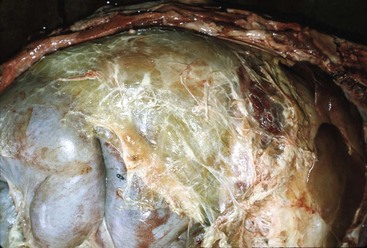
Balantidiasis (Balantidium coli): Balantidium coIi is a normal inhabitant of the cecum and colon of primates, including humans, and swine. It is large (50-60 µm × 25-45 µm) and ciliated. Dogs with whipworm infestation may become infested after contact with infected pigs. In general, Balantidium is an opportunistic pathogen associated with enteric disease (Fig. 7-167).
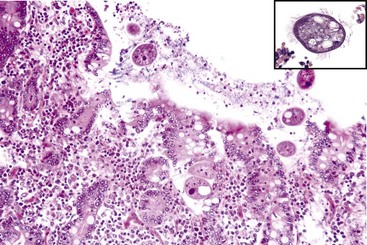
Fig. 7-167 Balantidium coli, colon, pig.
Balantidium coli is an opportunistic flagellated protozoan that is normally present in the pig intestine. This pig had concurrent proliferative (Lawsonia) enteritis. H&E stain. Inset, Higher magnification of Balantidium coli. H&E stain. (Courtesy of Dr. C. Löhr, College of Veterinary Medicine, Oregon State University. Insert, Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
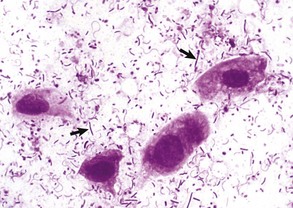
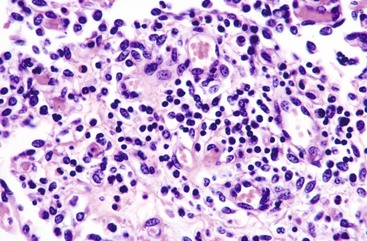
Trichomoniasis: Tritrichomonas foetus is a sexually transmitted pathogen of cattle. Cats, especially those less than a year of age housed in groups, have a tendency toward large bowel diarrhea when infected with this flagellate. Diagnosis is often made by visualization of motile flagellates on fecal wet mounts. Histologic diagnosis is most accurate when at least six biopsy sections of colon containing surface mucus are examined. PCR on paraffin-embedded tissue has also been successful, even in the absence of histologic evidence of the parasite. Infection occurs in the ileum, cecum, and colon. Lesions include mild-to-moderate colitis, with microabscesses and occasional extension of infection into the lamina propria. There may be colonic enterocyte attenuation and/or increased mitotic activity in the crypts. The 5 µm by 7 µm teardrop-shaped parasites can often been seen in surface mucus, within colonic glands, and occasionally within macrophages and lymphatics. Thus the parasite is enteroinvasive under certain circumstances. Flagella are not visible on H&E staining. There is no effective treatment. The diarrheal disease in cats generally resolves within 2 years of onset.
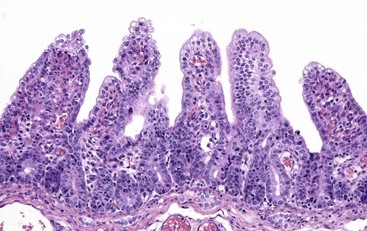
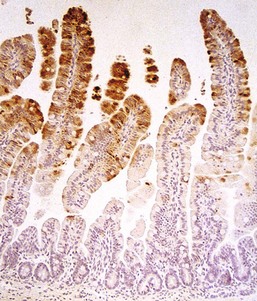
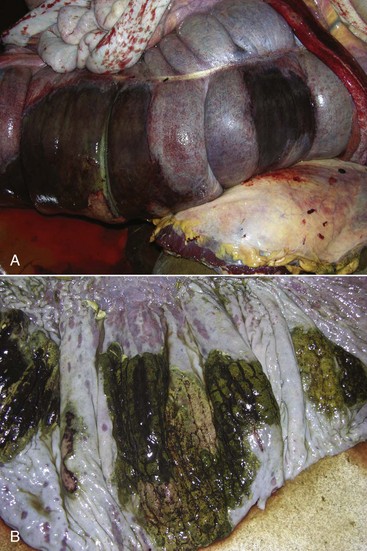
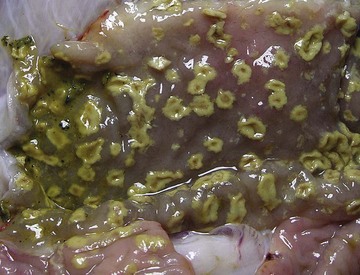
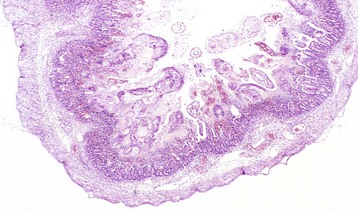
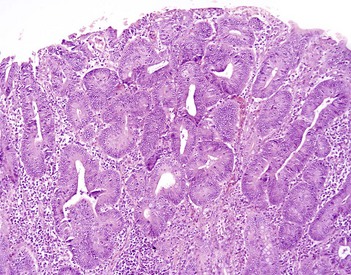
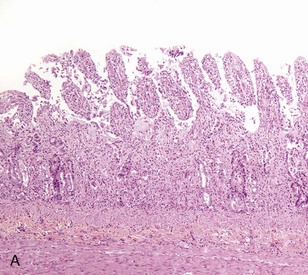
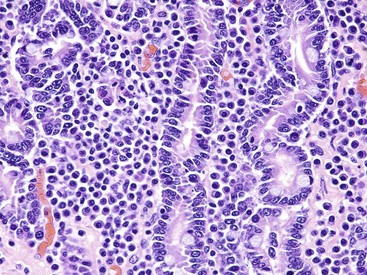
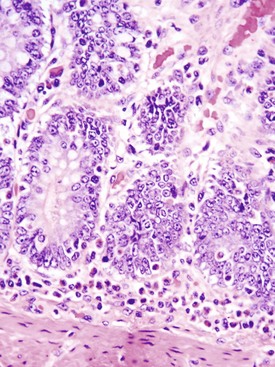
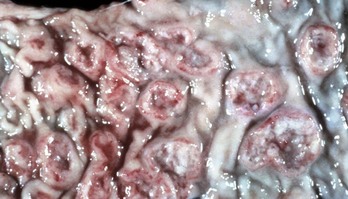
Coccidiosis: Coccidia are exquisitely host- and tissue-specific protozoa. They are obligate intracellular pathogens. Lesions vary from proliferative in sheep and goats (Fig. 7-168) to hemorrhagic in dogs, cats, and cattle (Fig. 7-169). In pigs, a fibrinonecrotic pseudomembrane, without blood, in 5- to 7-day-old animals is characteristic of enteric coccidiosis (Fig. 7-170). Eimeria macusaniensis is a relatively common cause of sickness and death in new world camelids of all ages. Gross lesions, even in heavily infested animals, are minimal to absent. In many cases, fecal examinations are negative.
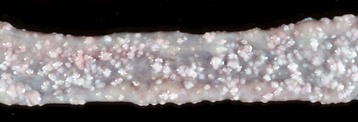
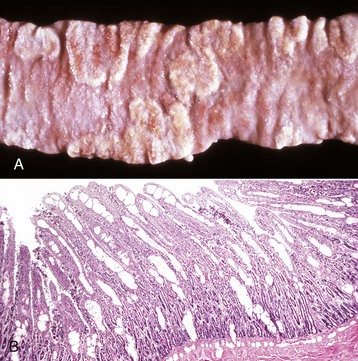
Fig. 7-168 Multifocal proliferative enteritis, small intestine, goat.
Proliferative nodules (also see Fig. 7-172) in the small intestinal mucosa are characteristic of ovine and caprine coccidiosis. Sporozoites and merozoites infect enterocytes and replicate, stimulating hyperplasia of enterocytes. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)

Fig. 7-169 Necrohemorrhagic enteritis, small intestine, calf.
Coccidiosis in cattle, dogs, and cats is characterized by intestinal hemorrhage. Hemorrhagic diarrheic feces may be visible on the perineum and hind legs. In severe cases, there may be anemia, which will be evident as pale external mucous membranes. (Courtesy College of Veterinary Medicine, Cornell University.)

Fig. 7-170 Fibrinonecrotic enteritis, small intestine, pig.
Pseudomembranes are characteristic of porcine coccidiosis. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Most species of Eimeria and Isospora infect villous or crypt epithelial cells, more rarely lacteals, the lamina propria, and regional lymph nodes. The coccidia undergo one or more asexual reproductive cycles within enterocytes. The resulting sporozoites produce schizonts containing merozoites, which infect additional enterocytes.
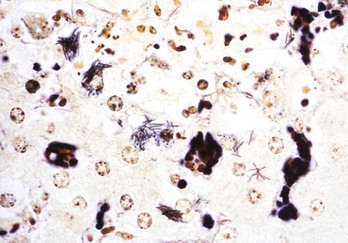
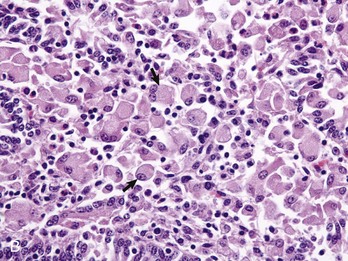
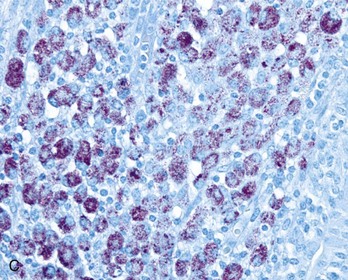
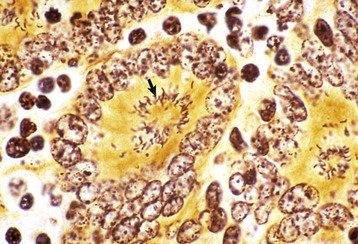
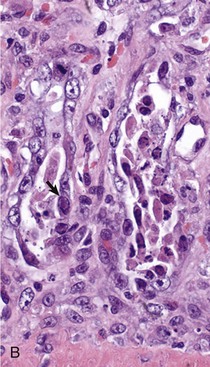
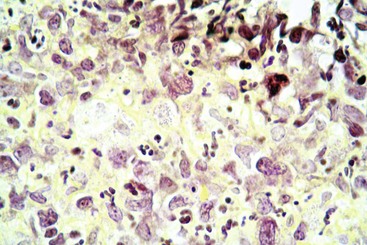
Merozoites produce gamonts that differentiate into microgametes and macrogametes (Fig. 7-171). Microgametes fertilize macrogametes, producing zygotes that develop into oocysts. When a small number of coccidia parasitize the intestine of otherwise healthy young growing animals, little disease results. However, when animals are in crowded conditions associated with poor sanitation, fecal-oral transmission of large numbers of organisms can occur. It is in these circumstances, compounded by malnutrition and intercurrent infections or parasitism, that clinical disease results. Enterocyte rupture occurs in all stages of the parasite’s life cycle. Clinical disease depends on parasitic load and varies by animal species. Because of diminished epithelial turnover in young animals, they are most susceptible to disease.
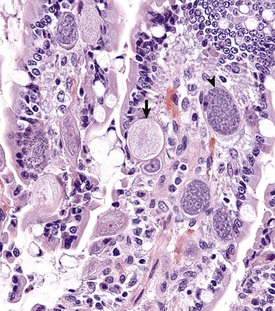
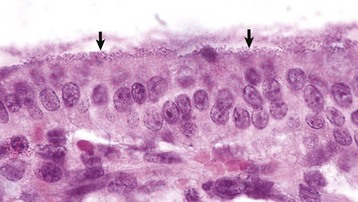
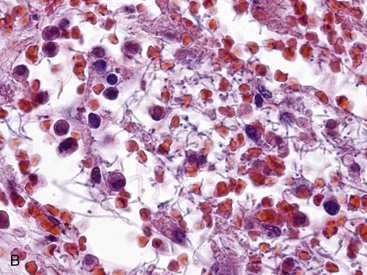
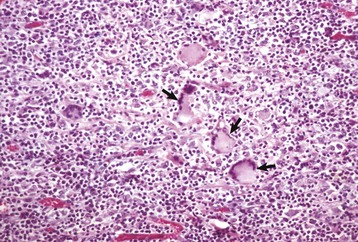
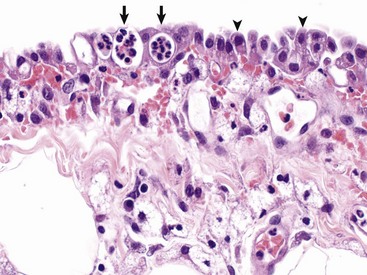
Fig. 7-171 Sexual stages of intestinal coccidiosis. small intestine, cow.
Note that the mucosal epithelial cells are distended with microgametes (arrow) and macrogametes (arrowhead). H&E stain. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
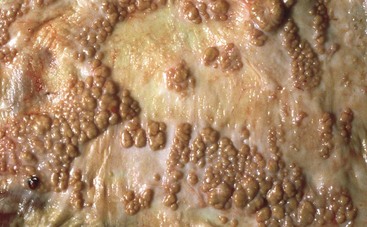
Gross lesions of coccidiosis are variable by host species, parasite species, and intestinal location. Bleeding is variably present both within species and among species. Coccidiosis in sheep and goats is characterized by enterocyte proliferation that is visible grossly as mucosal nodules (Fig. 7-172). The large schizonts of some species are sometimes grossly visible as well. Eimeria leuckarti of equids is asymptomatic. In dogs and cats a slightly different organism, Cystoisospora, is responsible for disease. Intestinal toxoplasmosis of felids is an important zoonotic concern, especially for pregnant women.
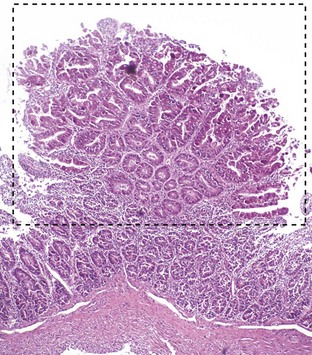
Fig. 7-172 Proliferative enteritis, small intestine, goat.
Coccidia-induced enterocyte hyperplasia results in nodule formation (area identified by dashed-lines) as seen in Fig. 7-168. Note the hyperplastic enterocytes lining crypts within the nodule. H&E stain. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
“Poor doing” associated with diarrhea is characteristic of clinical coccidiosis. Depending on the host species and the region of intestine that is affected, infected fresh blood may be present in the feces. The presence of tenesmus is variable. Oocysts are usually demonstrable in the feces.
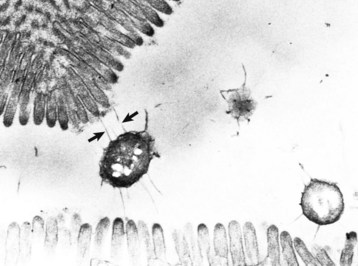
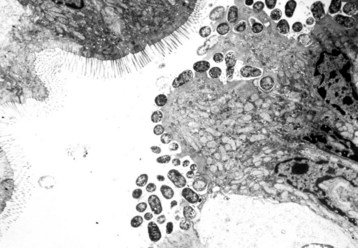
Cryptosporidiosis: Cryptosporidium parvum is a ubiquitous protozoan pathogen of mammals. Often waterborne, it is a significant cause of municipal water contamination. Although it causes a self-limiting infection in immunocompetent animals, the very young or immunocompromised individuals, such as AIDS patients, suffer from intractable diarrhea. When treating calves, veterinarians and veterinary students are at particular risk for infection. Cryptosporidia attach to surface epithelial cells of the stomach, small intestine, or colon. The protozoa displace the microvilli and are enclosed by surface cell membranes. Thus the parasite lives in a unique environment described as intracellular but extracytoplasmic (Web Fig. 7-21). Microgametes, macrogametes, schizonts, trophozoites, meronts, merozoites, and oocysts can be demonstrated in the intestine adjacent to, or attached to, epithelial cells. Oocysts are 4 to 5 µm in diameter and are shed in the feces. Studies have indicated that there are species-specific tropisms or biotypes of cryptosporidia. Previously, fecal contamination of water supplies by ruminants was believed to be the cause of most human outbreaks. Molecular typing of the organism has shown in many disease outbreaks that contamination with human feces and human-specific cryptosporidia causes most human epidemics.
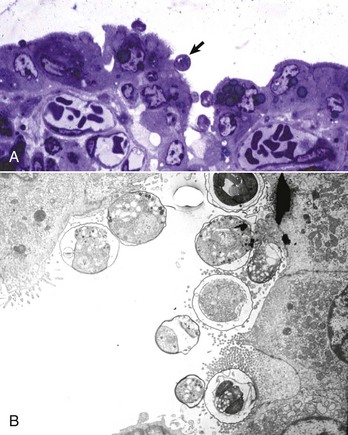
Web Fig. 7-21 Cryptosporidiosis, small intestine.
A, Cow. Cryptosporidia (arrow) are attached to the microvillus border of the enterocyte membrane. Plastic-embedded, toluidine blue–stained section. B, Rabbit. The cryptosporidia form a trilaminated enveloping membrane on fusion with the enterocyte membrane. Their location is thus intracellular but extracytoplasmic. Microvilli are effaced. TEM. Uranyl acetate and lead citrate stain. (A courtesy Dr. A.R. Doster, University of Nebraska; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Oocysts can be identified in feces by Sheather’s sucrose flotation and a modified acid-fast stain. Cryptosporidiosis causes subacute or chronic, sometimes bloody, watery diarrhea. The mechanism of diarrhea involves more than just cell loss. Prostaglandins, perhaps secreted by macrophages, increase anion (Cl−) secretion through cAMP and inhibit sodium absorption and thus water absorption. In addition, Cryptosporidium parvum interferes with interferon-γ (IFN-γ) gene expression of host cells thus contributing to immune evasion by the parasite. There is associated dehydration and electrolyte loss. Although the disease can be fatal, particularly in the presence of other pathogens, it is often self-limiting in immunocompetent individuals. In these cases, the illness resolves spontaneously in about a week.
Affected portions of the GI tract are diffusely reddened and have fluid contents. The organisms appear as tiny blue (hematoxylinophilic) dots attached to the epithelial cells of affected segments. In addition to the dot forms, ring- and banana-shaped organisms are readily seen in Giemsa-stained sections. The lesions of enteritis or colitis consist of decreased mucosal (villous) height, irregular mucosal thickness, crypt necrosis, hyperemia, and an increase in lymphocytes and plasma cells in the lamina propria. Villous atrophy and fusion of the villi of the small intestine are the end result. Because of the intracellular, extracytoplasmic location of the parasite, chemotherapeutic intervention is ineffective. There are few chemicals that can decontaminate the environment. Clorox, for example, is used experimentally to purify the parasites.
Giardiasis: Giardiasis has been reported in many species, including humans, dogs, cats, horses, cattle, rabbits, guinea pigs, hamsters, rats, mice, chinchillas, and parakeets. In clinical veterinary practice, giardiasis is frequently recognized in puppies and kittens and causes concern among owners because of its zoonotic potential. Prevalence of the parasite in humans in the developed world is estimated at 2% to 5%. Giardiasis is caused by a pear-shaped protozoan with posterior flagella, a ventral sucker, and four nuclei, two of which resemble eyes (Fig. 7-173). Giardia lamblia parasitizes the small intestine, particularly the duodenum. Giardia attach to the microvillous border of epithelial cells, producing membrane damage. Although generally asymptomatic, diarrhea may result in very young animals or in animals otherwise immunologically deficient.
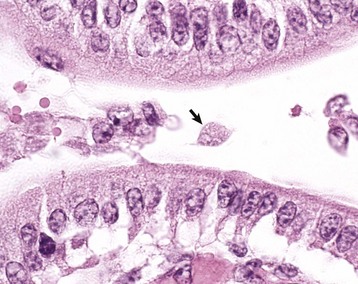
Fig. 7-173 Giardiasis, small intestine, dog.
A single pear-shaped flagellated protozoa is readily visible in the intestinal lumen (arrow). H&E stain. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Giardia spp. have been shown to induce apoptosis of enterocytes thus increasing membrane permeability. In large numbers, the parasites decrease the absorption of simple sugars and disaccharides secondary to microvillous destruction. Ingesta are then fermented by bacterial flora, creating gas and osmotically drawing water into the intestinal lumen. An enterotoxin stimulates intestinal Cl− secretion. Clinical cases of giardiasis have brown, fluid diarrhea, and abdominal discomfort without fever, weight loss, melena, and/or steatorrhea. The diagnosis is made by demonstrating Giardia in preparations of fresh feces or in histologic sections by identifying the organisms either with H&E or Giemsa stains.
Ascariasis: Ascarids are easily recognized as proximal-intestinal, luminal nematodes that are smooth and white. They are round on cross-section, thus giving them the appellation of roundworms together with the other nematodes. They vary greatly in length; the larger the host species, the larger the ascarids. They are 3 to 4 cm long in small animals and attain lengths of 40 to 50 cm in pigs and horses. Ascarids of domestic animals belong to the genera Ascaris (pigs), Parascaris (horses), and Toxocara (dogs, cats, and humans). The young of these species acquire larval ascarids by intrauterine transmission during the last 7 to 10 days of gestation, through the milk of the dam, and later in life through parasite ova contamination of the environment. After ingestion, infective larvae penetrate the intestine and migrate to the liver via the portal circulation. From there the larvae migrate via the caudal vena cava to the lungs. After leaving the circulation and entering the alveoli, the larvae undergo development, and are coughed into the pharynx and swallowed. Development to adults occurs in the intestine. Ova passed in the feces complete the life cycle.
Alternatively, Toxascaris leonina of canids and felids is ingested via an intermediate host. Hepatopulmonary migration does not occur. Lesions produced by ascarid larval migration include canine multifocal eosinophilic gastroenteritis and visceral larval migrans. Animals affected with heavy ascarid burdens lose weight, grow poorly as a result of competition for nutrients between luminal parasites and the host, and often have a pear-shaped abdomen when held vertically. Adult worms may be vomited or passed in the diarrheic feces. A hacking cough termed thumping is a sign of pulmonary larva migrans, especially in pigs. Anthelmintic administration can cause a rapid die off of adult ascarids, resulting in intestinal occlusion (see Web Fig. 7-14). Ascarids continue to migrate after the death of the host and may be found in aberrant locations such as the bile duct, stomach, oral cavity, pancreatic duct, and abdomen (Fig. 7-174).
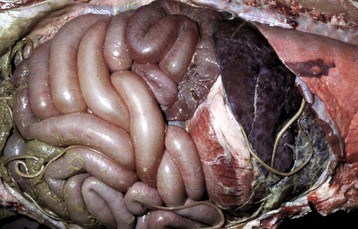
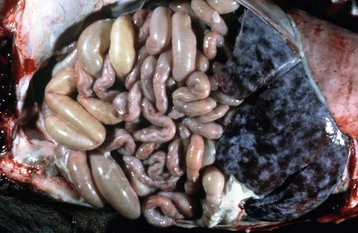
Fig. 7-174 Fibrinous peritonitis, abdomen, pig.
The presence of fibrin along with ascarids in this pig’s abdomen indicates that the intestinal rupture occurred antemortem. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Hookworm Disease: Parasitism by hookworms varies from asymptomatic to fatal based on the challenge dose of parasites, the host’s age, nutritional status, and likely its immunologic state. When death occurs, it is by exsanguination because hookworms are blood eaters (Fig. 7-175). Challenge dosage is often exacerbated by poor nutritional and sanitary conditions, mild climatic conditions, and moisture. Hookworms are generally small nematodes, 1 to 1.5 cm long. Their habitat is usually the proximal small intestine. Genera include Ancylostoma and Uncinaria in dogs, Bunostomum in ruminants, Globocephalus in pigs, and Ancylostoma and Necator in humans. Ancylostoma caninum in dogs has zoonotic potential. Environmental contamination occurs from the large number of eggs produced in the intestine. The first- through third-stage larvae feed on environmental bacteria. Third-stage larvae are infective and enter the host either by ingestion or direct dermal penetration. From either point of entry, they migrate through the pulmonary system, through somatic tissue to the uterus, or through mucosal tissue. Larvae may also be present in colostrum. The final destination is the intestine, where eggs are produced, completing the life cycle.
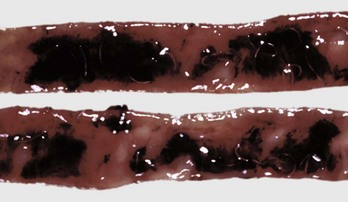
Fig. 7-175 Hookworms, hemorrhagic enteritis, small intestine, dog.
Where hookworms have detached, hemorrhage is present. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Because prenatal infections with hookworms do not become patent for 11 days, fecal examinations may be negative. Otherwise, fecal examination, especially in young animals with anemia, is diagnostic of this disease. Adult hookworms bury into the villous, ingesting tissue, mucus, and blood (Fig. 7-176). When the worm moves to another attachment site, blood may continue to flow from the wound for 30 minutes.
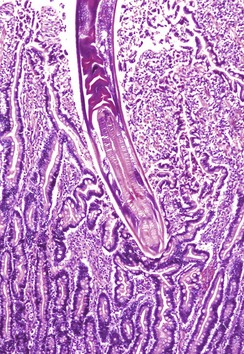
Fig. 7-176 Hookworm enteritis, intestine, dog.
A hookworm has burrowed deep into and attached to the mucosa. H&E stain. (Courtesy College of Veterinary Medicine, Cornell University.)
Trichuriasis: Trichurids, or whipworms, are long and slender at their anterior ends and may be numerous within the cecum and colon. Trichurids have a direct life cycle. The name Trichuris translates to “whip-tail,” which is a misnomer because the parasite actually has a “whip-head” that invades and attaches to the mucosa of the cecum, colon, and rectum. Although the parasite ingests blood, anemia is rarely a clinical symptom. Bloody diarrhea may be present. Different species are parasites of carnivores, ruminants, pigs, and humans. The disease in each species is similar. The horse does not have a whipworm.
Trichuris eggs are elongate, or football-shaped, with an operculum at either end, and are very resistant to environmental conditions. Most infections are asymptomatic and the complete life cycle may take up to 3 months. Therefore repeated dewormings are necessary to eliminate infection, even in the absence of fecal ova. Symptoms may be vague, with only paroxysmal diarrhea. Gross enteric lesions vary from mild to erosive and ulcerative.
Strongyloidosis: Strongyloides spp. are unique in having free-living and parasitic forms. Rhabditiform larvae may develop parthenogenetically. Free-living parasites are both male and female and undergo sexual reproduction. Enteritis can be severe; larvae or larvated eggs are in the feces of infected animals.
Strongyloides stercoralis of dogs is zoonotic. Strongyloides spp. also infect horses, pigs, and cats. Geographic differences in parasite populations account for differences in virulence within host species. Hyperinfection and autoinfection may occur, adding to the parasite burden. Larvae may enter the host by skin penetration, or less often by ingestion. Strongyloides spp. infection may be acquired in utero and through colostrum and milk. Larvae migrate to the bloodstream and lungs. When they gain access to alveoli, they subsequently migrate to airways, where they are carried, via the mucociliary elevator, to the pharyngeal cavity and are swallowed. Small intestinal parasitism is characterized by larvae residing within superficial mucosa (Web Fig. 7-22). Epithelial destruction by the parasites may result in villous atrophy and crypt hyperplasia. The nonspecific clinical signs include diarrhea, hypoproteinemia, weight loss, and dehydration. Rhabditiform dermatitis may also occur.
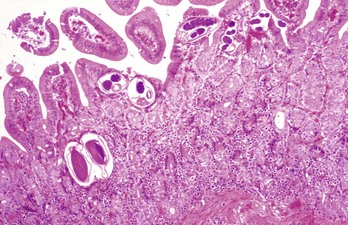
Web Fig. 7-22 Strongyloidosis, small intestine, horse.
Cross-sections of the parasite (Strongyloides westeri) are present in the superficial mucosa. Note the mild chronic inflammatory response with some eosinophils in the lamina propria. H&E stain. (Courtesy Dr. C.S. Patton, College of Veterinary Medicine, University of Tennessee.)
Trichostrongylosis: Trichostrongyles are small nematodes that parasitize the small intestine of ruminants. Mild climates promote clinical disease. These parasites have a direct life cycle. Third-stage larvae are rendered infective in the acid environment of the abomasum. The larvae burrow in between crypt enterocytes, but do not generally penetrate the basement membrane. Paradoxically, crypt hyperplasia is followed by villous atrophy. As with most other parasitisms, crowding, poor sanitation, and inadequate nutrition potentiate disease. Protein leakage into the intestinal lumen together with absorptive enterocyte loss leads to diarrhea, cachexia, and its metabolic consequences, which can be severe and widespread through many organ systems.
Cyathostomiasis: In ponies and horses under 5 years of age in temperate climates, sudden emergence of massive numbers of fourth- and fifth-stage cyathostome larvae from the cecum and colon results in necroulcerative hemorrhagic typhlocolitis. Ova are generally not detected in feces but larvae are often visible.
Nematodirosis: Nematodirus nematodes are parasites of the cranial small intestine of ruminants. The life cycle is direct. Unlike the case with other strongyles, Nematodirus larvae within ova are resistant to cold temperatures. In fact, the ova must overwinter to be infective. This is evolutionally interesting because it allows for a new crop of susceptible hosts, particularly lambs and calves each year. Fourth- and fifth-stage larvae reside in deeper layers of the mucosa than do the trichostrongyles. Villous atrophy of the cranial small intestine is the predominant histologic lesion. Nematodirus spp. do not generally cause disease except in association with other parasites. Signs include green diarrhea, weight loss, and hypoproteinemia secondary to weight loss and inappetence.
Cooperiosis: A small intestinal parasite of ruminants, Cooperia nematodes—unlike other trichostrongyles—do not burrow into the intestine. Rather, they reside between villi, causing pressure necrosis. Their life cycle and clinical signs are similar to that of the other strongyles already described.
Oesophagostomum: The nodular worms of ruminants (Oesophagostomum columbianum, Oesophagostomum radiatum) and pigs (Oesophagostomum dentatum) cause subserosal mineralized nodules that are characteristic of the disease. These nodules generally are of no clinical significance, but they make the intestines unsuitable for use as sausage casings. Occasionally, they are associated with, and can be the cause of, intussusceptions.
Third-stage larvae of Oesophagostomum columbianum of sheep are ingested, penetrate deeply into the small intestinal wall, excyst, and molt to fourth-stage larvae, which mature in the colon. They may encyst in the colonic wall and become mineralized subserosal nodules or may mature to adults. Disease is more severe in nutritionally debilitated animals. Most infestations are asymptomatic. Oesophagostomum radiatum of cattle may produce inappetence, hypoproteinemia from damaged enterocyte tight junctions, and anemia and hemorrhage from consumptive coagulopathy induced by the parasites. Nodules may also form, as in sheep. Oesophagostomiasis in pigs is usually asymptomatic, although ill thrift and malaise secondary to typhlocolitis may occur.
Pinworms: Oxyuris equi is the most common pinworm of domestic animals. The parasites occupy the lumen of the distal intestine of horses and occasionally cause rectal pruritus by laying their eggs on the perineal region. Enterobius vermicularis is the pinworm of primates and great apes. It is not zoonotic and is generally of little clinical consequence.
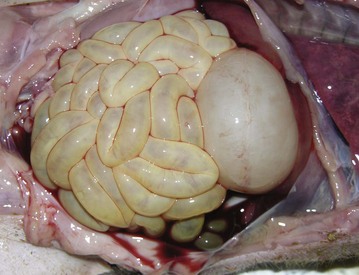
Cestodes: Tapeworms, although frequently found in the alimentary system, are generally of little clinical significance. They require two and sometimes three hosts, often including arthropods and other invertebrates, to complete their life cycles. Tapeworms attach to the gut wall by means of their anterior scolex, which may have hooks in addition to four suckers (Fig. 7-177). Although they can cause some damage at the site of attachment, generally they compete with the host for nutrients. Lacking an alimentary system, they absorb nutrients through their surface. Tapeworms are flat, segmented, and hermaphroditic, reproducing by addition of segments or proglottids. Examples of tapeworms are Anoplocephala spp. in horses, Moniezia spp. in ruminants, and Diphyllobothrium and Dipylidium spp. in dogs and cats. Mesocestoides spp. can infect dogs and cats. In some cases, this parasite can perforate through the intestine and proliferate in the peritoneal cavity (Fig. 7-178).
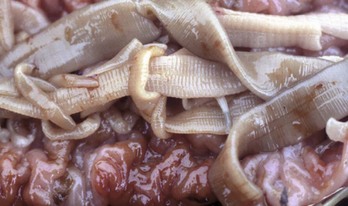
Fig. 7-177 Cestodiasis, small intestine, fur seal.
Segmented tapeworms are present in this otherwise normal intestine. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
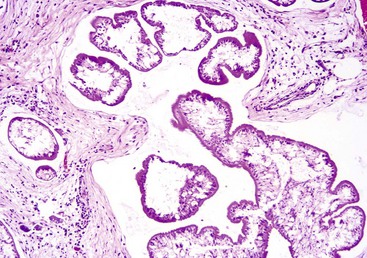
Fig. 7-178 Mesocestoides infection, peritoneum, dog.
Encysted larval cestodes have elicited a granulomatous inflammatory reaction in the peritoneum of this dog. H&E stain. (Courtesy Dr. C. Löhr, College of Veterinary Medicine, Oregon State University.)
Taenia and Echinococcus spp. are the most destructive of the cestodes. Although carnivores are the definitive hosts, the larval forms reside in the viscera and body cavities of the intermediate hosts, usually ruminants, pigs, horses, or rodents (see Fig. 8-54). Humans can also become infected, and sometimes it takes 20 or 30 years for clinical disease to appear. The damage in the intermediate hosts may be quite severe.
Trematodes: Trematodes are uncommon parasites of the alimentary tract. Nanophyetus salmincola uses a snail and a fish as intermediate hosts. It carries the rickettsia responsible for salmon poisoning in the Northwestern US. Lesions of the intestine are hemorrhagic enteritis.
Alaria spp. can attach to the small intestine of dogs and cats, but are generally innocuous. The mesocercariae can cause tissue damage during their migrations through body organs of the host. Paratenic hosts are frogs, snakes, and mice.
Schistosomiasis of ruminants, pigs, horses, and dogs can cause granulomatous intestinal lesions with protein loss secondary to the parasite’s presence in mesenteric veins after migration through the liver. Parasites are acquired by direct penetration of the skin by cercariae.
Acanthocephalans: The thorny-headed worm of pigs, Macracanthorhynchus hirudinaceus, is a small intestinal parasite with a soil-based arthropod intermediate host such as dung beetles. They are thus more common, as are many other parasites in a variety of mammalian species, in “free range” animals. They are occasionally misidentified as tapeworms, which they superficially resemble. However, they are not truly segmented parasites. They occasionally penetrate the bowel wall at the site of parasite attachment, causing peritonitis.
Prosthenorchis spp. are acanthocephalids of primates. Cockroaches are the intermediate hosts.
Intestinal Neoplasia: Neoplasms of various types occur in the GI system of domestic animals. Those of the oral cavity and stomach have already been discussed. Intestinal neoplasms are diagnosed most frequently in dogs and cats, in large part because of their longer lifespans. Additionally, pets live in close harmony with their human companions, and thus it is possible that some of the same environmental factors that cause human cancer may also cause similar problems in animals.
In dogs, benign neoplasms of the intestinal tract are most commonly adenomas or polyps (see Fig. 6-4), and their malignant counterparts adenocarcinomas. Dogs and cats infrequently develop intestinal mast cell tumors and plasmacytomas. Smooth muscle neoplasms termed leiomyomas and leiomyosarcomas arise from existing intestinal muscular layers. An important caveat in diagnosing these spindle cell tumors is that some of them when examined immunohistochemically are composed of undifferentiated cells with an uncertain histogenesis. These neoplasms have been reported in dogs, horses, rats and primates. They are termed GI stromal tumors (GIST). Supposition exists that these neoplasms arise from the interstitial cells of Cajal which normally become the pacemaker cells of the gut. Most are KIT (CD117) positive (proto-oncogene c-kit).
Lymphoma can be solitary, metastatic, or multicentric. In cats, the most common neoplasms include alimentary lymphoma (Fig. 7-179); mastocytomas (Fig. 7-180), which are associated with ulceration; adenomas; adenocarcinomas; and carcinoids. In canids, 5% to 7% of lymphomas are GI. Those of the GI tract are epitheliotropic and primarily T lymphocyte in origin. In humans, most GI lymphomas are B lymphocyte in origin. In sheep, adenocarcinomas of the intestine are fairly common and are virus-induced. In cows, alimentary lymphoma is most common. Horses rarely have intestinal neoplasms develop.
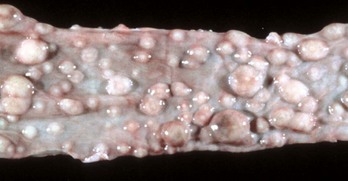
Fig. 7-179 Lymphoma (lymphosarcoma), colon, cat.
Numerous submucosal nodules contain neoplastic lymphocytes. Note that the mucosal epithelium is intact (smooth and shiny) and not ulcerated. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
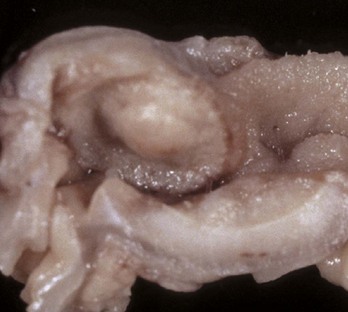
Fig. 7-180 Mast cell tumor, small intestine, cat.
The submucosal nodule contains neoplastic mast cells. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)