Classification of Pneumonias
Few subjects in veterinary pathology have caused so much debate as the classification of pneumonias. Historically, pneumonias in animals have been classified or named based on the following:
1. Presumed cause, with names such as viral pneumonia, Pasteurella pneumonia, distemper pneumonia, verminous pneumonia, chemical pneumonia, and hypersensitivity pneumonitis
2. Type of exudation, with names such as suppurative pneumonia, fibrinous pneumonia, and pyogranulomatous pneumonia
3. Morphologic features, with names such as gangrenous pneumonia, proliferative pneumonia, and embolic pneumonia
4. Distribution of lesions, with names such as focal pneumonia, cranioventral pneumonia, diffuse pneumonia, and lobar pneumonia
5. Epidemiologic attributes, with names such as enzootic pneumonia, contagious bovine pleuropneumonia, and “shipping fever”
6. Geographic regions, with names such as Montana progressive pneumonia
7. Miscellaneous attributes, with names such as atypical pneumonia, cuffing pneumonia, progressive pneumonia, aspiration pneumonia, pneumonitis, farmer’s lung, and extrinsic allergic alveolitis
Until a universal and systematic nomenclature for animal pneumonias are established, veterinarians should be acquainted with this heterogeneous list of names and should be well aware that one disease may be known by different names. In pigs, for instance, enzootic pneumonia, virus pneumonia, and Mycoplasma pneumonia all refer to the same disease caused by Mycoplasma hyopneumoniae (Table 9-5).
TABLE 9-5
Morphologic Types of Pneumonias in Domestic Animals
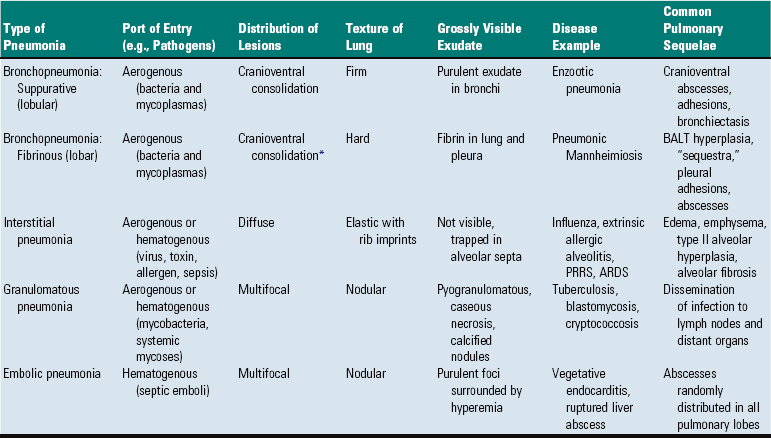
ARDS, Adult respiratory distress syndrome; BALT, bronchial-associated lymphoid tissue; PRRS, porcine reproductive and respiratory syndrome.
*Porcine pleuropneumonia is an exception because it often involves the caudal lobes.
The word pneumonitis has been used by some as a synonym for pneumonia; however, others have restricted this term to chronic proliferative inflammation generally involving the alveolar interstitium and with little or no evidence of exudate. In this chapter, the word pneumonia is used for any inflammatory lesion in the lungs, regardless of whether it is exudative or proliferative, alveolar, or interstitial.
On the basis of texture, distribution, appearance, and exudation, pneumonias can be grossly diagnosed into four morphologically distinct types: bronchopneumonia, interstitial pneumonia, embolic pneumonia, and granulomatous pneumonia. By using this classification, it is possible to predict with some degree of certainty the likely cause (virus, bacteria, fungi, or parasites), routes of entry (aerogenous versus hematogenous), and possible sequelae. These four morphologic types allow the clinician or pathologists to predict the most likely etiology and therefore facilitate the decision as to what samples need to be taken and which tests should be requested to the diagnostic laboratory (i.e., histopathology, bacteriology, virology, or toxicology). However, overlapping of these four types of pneumonias is possible, and sometimes two morphologic types may be present in the same lung.
The criteria used to classify pneumonias grossly into bronchopneumonia, interstitial pneumonia, embolic pneumonia, and granulomatous pneumonia are based on morphologic changes, including distribution, texture, color, and general appearance of the affected lungs (see Table 9-5). Distribution of the inflammatory lesions in the lungs can be (1) cranioventral, as in most bronchopneumonias; (2) multifocal, as in embolic pneumonias; (3) diffuse, as in interstitial pneumonias; or (4) locally extensive, as in granulomatous pneumonias (Fig. 9-55). Texture of pneumonic lungs can be firmer or harder (bronchopneumonias), more elastic (rubbery) than normal lungs (interstitial pneumonias), or have a nodular feeling (granulomatous pneumonias). Describing in words the palpable difference between the texture of a normal lung compared with the firm or hard texture of a consolidated lung can be a difficult undertaking. An analogy illustrating this difference based on touching the parts of the face with the tip of your finger has been advocated by some pathologists. The texture of a normal lung is comparable to the texture of the center of the cheek. Firm consolidation is comparable to the texture of the tip of the nose, and hard consolidation is comparable to the texture of the forehead. The term consolidation is frequently used to describe a firm or hard lung filled with exudate.
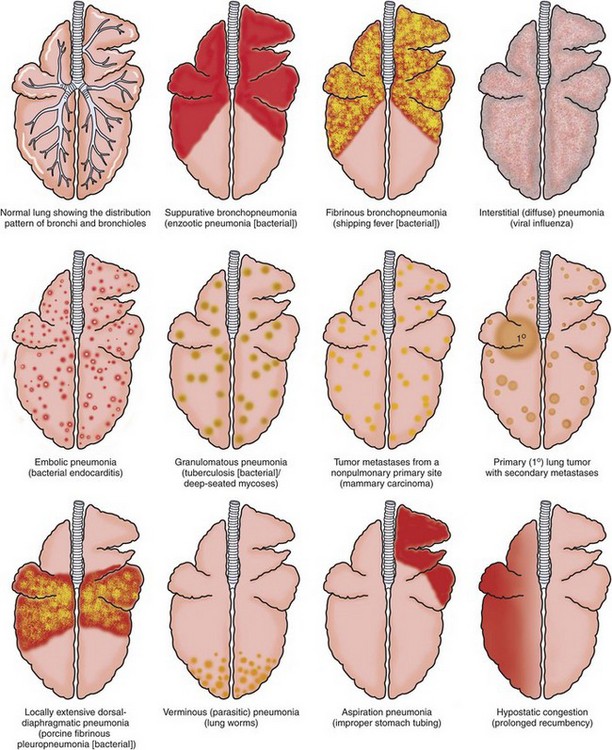
Fig. 9-55 Schematic diagram of the patterns of pneumonia and lung lesions.
A dorsal view of the bovine lung illustrates these patterns. They can readily be extrapolated to the lungs of other domestic animal species. (Courtesy Drs. A. López, Atlantic Veterinary College and J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Changes in the appearance of pneumonic lungs include abnormal color, presence of nodules or exudate, fibrinous or fibrous adhesions, and presence of rib imprints on serosal surfaces (see Fig. 9-55). On cut surfaces, pneumonic lungs may have exudate, hemorrhage, edema, necrosis, abscesses, bronchiectasis, granulomas or pyogranulomas, and fibrosis, depending on the stage.
Palpation and careful observation of the lungs are essential in the diagnosis of pneumonia. (For details, see the section on Examination of the Respiratory Tract.)
Bronchopneumonia: Bronchopneumonia refers to a particular type of pneumonia in which injury and the inflammatory process take place primarily in the bronchial, bronchiolar, and alveolar lumens. Bronchopneumonia is undoubtedly the most common type of pneumonia seen in domestic animals and is with few exceptions characterized by cranioventral consolidation of the lungs (Fig. 9-56). The reason why bronchopneumonias in animals are almost always restricted to the cranioventral portions of the lungs is not well understood. Possible factors contributing to this topographic selectivity within the lungs include (1) gravitational sedimentation of the exudate; (2) greater deposition of infectious organisms; (3) inadequate defense mechanisms; (4) reduced vascular perfusion; (5) shortness and abrupt branching of airways; and (6) regional differences in ventilation.
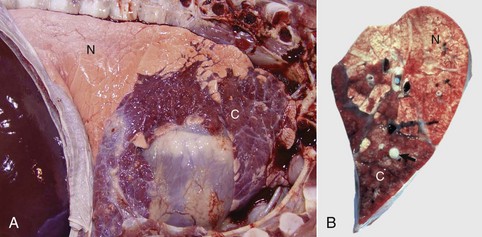
Fig. 9-56 Suppurative bronchopneumonia, enzootic pneumonia, lung, calf.
A, Cranioventral consolidation (C) of the lung involves approximately 40% of pulmonary parenchyma. Most of the caudal lung is normal (N). B, Cut surface. Consolidated lung is dark red to mahogany (C), and a major bronchus contains purulent exudate (arrow). N, Normal. (A courtesy Dr. A. López, Atlantic Veterinary College. B courtesy Ontario Veterinary College.)
The term cranioventral in veterinary anatomy is the equivalent of “anterosuperior” in human anatomy. The latter is defined as “in front (ventral) and above (cranial).” Thus applied to the lung of animals, “cranioventral” means the ventral portion of the cranial lobe. However, by common usage in veterinary pathology, the term cranioventral used to describe the location of lesions in pneumonias has come to mean “cranial and ventral.” Thus it includes pneumonias affecting not only the ventral portion of the cranial lobe (true cranioventral) but also those cases in which the pneumonia has involved the ventral portions of adjacent lung lobes—initially the middle and then caudal on the right and the caudal lobe on the left side.
Bronchopneumonias are generally caused by bacteria and mycoplasmas, by bronchoaspiration of feed or gastric contents, or by improper tubing. As a rule, the pathogens causing bronchopneumonias arrive in the lungs via inspired air (aerogenous), either from infected aerosols or from the nasal flora. Before establishing infection, pathogens must overwhelm or evade the pulmonary defense mechanism. The initial injury in bronchopneumonias is centered on the mucosa of bronchioles; from there, the inflammatory process can spread downward to distal portions of the alveoli and upward to the bronchi. Typically, for bronchopneumonias, the inflammatory exudates collect in the bronchial, bronchiolar, and alveolar lumina leaving the alveolar interstitium unchanged, except for hyperemia. Through the pores of Kohn, the lesions and exudate can spread centripetally to adjacent alveoli until most or all of the alveoli in an individual lobule are involved. If the inflammatory process cannot control the inciting cause of injury, the lesions spread rapidly from lobule to lobule through alveolar pores and destroyed alveolar walls, until an entire lobe or large portion of a lung is involved. The lesion tends to spread centrifugally, with the older lesions in the center, and exudate can be coughed up and then aspirated into other lobules, where the inflammatory process starts again.
At the early stages of bronchopneumonia, the pulmonary vessels are engorged with blood (active hyperemia) and the bronchi, bronchioles, and alveoli contain some fluid (permeability edema). In cases in which pulmonary injury is mild to moderate, cytokines locally released in the lung cause rapid recruitment of neutrophils and alveolar macrophages into bronchioles and alveoli (Fig. 9-57). When pulmonary injury is much more severe, proinflammatory cytokines induce more pronounced vascular changes by further opening endothelial gaps, thus increasing vascular permeability resulting in fibrinous exudates and sometimes hemorrhage in the alveoli. Alterations in permeability can be further exacerbated by structural damage to pulmonary capillaries and vessels directly caused by microbial toxins. The final result of these functional and structural changes is that blood vessels become notably permeable and allow substantial leakage of plasma fluid and proteins (fibrinogen) into the alveoli. Filling of alveoli, bronchioles, and small bronchi with inflammatory exudate progressively obliterates airspaces, and as a consequence of this process, portions of severely affected (consolidated) lungs sink to the bottom of the container when placed in fixative. The replacement of air by exudate also changes the texture of the lungs, and depending on the severity of bronchopneumonia, the texture varies from firmer to harder than normal.
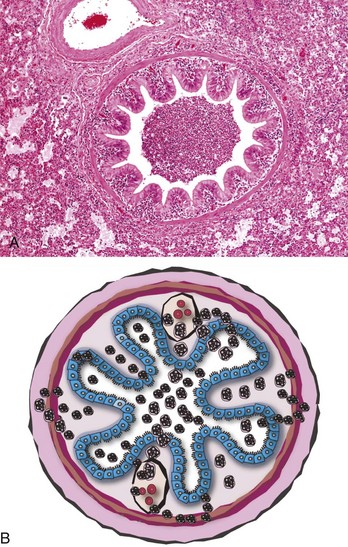
Fig. 9-57 Suppurative bronchopneumonia, lung, pig.
A, Note the bronchiole plugged with purulent exudate. The alveoli are filled with leukocytes and some edematous fluid. H&E stain. B, Schematic diagram of acute bronchiolitis. Note the neutrophils exiting the submucosal capillaries (leukocyte adhesion cascade; see Chapter 3) and moving into the walls of the bronchioles (blue cells = ciliated mucosal epithelium) and then into the bronchiolar lumen. (Courtesy Dr. A. López, Atlantic Veterinary College.)
The term consolidation is used at gross examination when the texture of pneumonic lung becomes firmer or harder than normal as a result of loss of airspaces because of exudation and atelectasis. (For details, see the discussion of lung texture in the section on Classification of Pneumonias). Inflammatory consolidation of lungs has been referred to in the past as hepatization because the affected lung had the appearance and texture of liver. The process was referred to as red hepatization in acute cases in which there was notable active hyperemia with little exudation of neutrophils; conversely, the process was referred to as gray hepatization in those chronic cases in which hyperemia was no longer present, but there was abundant exudation of neutrophils and macrophages. This terminology, although used for and applicable to human pneumonias, is rarely used in veterinary medicine primarily because the evolution of pneumonic processes in animals does not necessarily follow the red-to-gray hepatization pattern.
Bronchopneumonias can be arbitrarily subdivided into suppurative bronchopneumonia if the exudate is predominantly composed of neutrophils, and fibrinous bronchopneumonia if fibrin is the predominant component of the exudate (see Table 9-5). It is important to note that some pathologists use the term fibrinous pneumonia or lobar pneumonia as a synonym for fibrinous bronchopneumonia and bronchopneumonia or lobular pneumonia as a synonym for suppurative bronchopneumonia. Human pneumonias for many years have been classified based on their etiology and morphology, which explains why pneumococcal pneumonia (Streptococcus pneumoniae) has been synonymous with lobar pneumonia. In the old literature, four distinct stages of pneumococcal pneumonia were described as (1) congestion, (2) red hepatization (liver texture), (3) grey hepatization, and (4) resolution. Because of the use of effective antibiotics and prevention, pneumococcal pneumonia and its four classic stages are rarely seen, thus this terminology has been largely abandoned. Currently, the term bronchopneumonia is widely used for both suppurative and fibrinous consolidation of the lungs because both forms of inflammation have essentially the same pathogenesis in which the pathogens reach the lung by the aerogenous route, injury occurs initially in the bronchial and bronchiolar regions, and the inflammatory process extends centrifugally deep into the alveoli. It must be emphasized that it is the severity of pulmonary injury that largely determines whether bronchopneumonia becomes suppurative or fibrinous. In some instances, however, it is difficult to discriminate between suppurative and fibrinous bronchopneumonia because both types can coexist (fibrinosuppurative bronchopneumonia), and one type can progress to the other.
Suppurative Bronchopneumonia: Suppurative bronchopneumonia is characterized by cranioventral consolidation of lungs (see Figs. 9-56 and 9-57), with typically purulent or mucopurulent exudate present in the airways. This exudate can be best demonstrated by expressing intrapulmonary bronchi, thus forcing exudate out of the bronchi (see Fig. 9-56). The inflammatory process in suppurative bronchopneumonia is generally confined to individual lobules, and as a result of this distribution, the lobular pattern of the lung becomes notably emphasized. This pattern is particularly obvious in cattle and pigs because these species have prominent lobulation of the lungs. The gross appearance often resembles an irregular checkerboard because of an admixture of normal and abnormal (consolidated) lobules (see Fig. 9-56). Because of this typical lobular distribution, suppurative bronchopneumonias are also referred to as lobular pneumonias.
Different inflammatory phases occur in suppurative bronchopneumonia where the color and appearance of consolidated lungs varies considerably, depending on the virulence of offending organisms and chronicity of the lesion. The typical phases of suppurative bronchopneumonia could be summarized as follows:
1. During the first 12 hours when bacteria are rapidly multiplying, the lungs become hyperemic and edematous.
2. Soon after, neutrophils start filling the airways and by 48 hours the parenchyma starts to consolidate and becomes firm in texture.
3. Three to 5 days later, hyperemic changes are less obvious, but the bronchial, bronchiolar, and alveolar spaces continue to fill with neutrophils and macrophages, and the affected lung sinks when placed in formalin. At this stage, the affected lung has a gray-pink color, and on cut surface, purulent exudate can be expressed from bronchi.
4. In favorable conditions where the infection is under control of the host defense mechanisms, the inflammatory processes begin to regress, a phase known as resolution. Complete resolution in favorable conditions could take 1 to 2 weeks.
5. In animals in which the lung infection cannot be rapidly contained, inflammatory lesions can progress into a chronic phase. Around 7 to 10 days after infection, the lungs become pale gray and take a “fish flesh” appearance. This appearance is the result of purulent and catarrhal inflammation, obstructive atelectasis, mononuclear cell infiltration, peribronchial and peribronchiolar lymphoid hyperplasia, and early alveolar fibrosis.
Complete resolution is unusual in chronic bronchopneumonia, and lung scars, such as pleural and pulmonary fibrosis; bronchiectasis as a consequence of chronic destructive bronchitis (see bronchiectasis); atelectasis; pleural adhesions; and lung abscesses may remain unresolved for a long time. “Enzootic pneumonias” of ruminants and pigs are typical examples of chronic suppurative bronchopneumonias.
Microscopically, acute suppurative bronchopneumonias are characterized by hyperemia, abundant neutrophils, macrophages, and cellular debris within the lumen of bronchi, bronchioles, and alveoli (see Fig. 9-57). Recruitment of leukocytes is promoted by cytokines, complement, and other chemotactic factors that are released in response to alveolar injury or by the chemotactic effect of bacterial toxins, particularly endotoxin. In most severe cases, purulent or mucopurulent exudates completely obliterate the entire lumen of bronchi, bronchioles, and alveoli.
If suppurative bronchopneumonia is merely the response to a transient pulmonary injury or a mild infection, lesions resolve uneventfully. Within 7 to 10 days, cellular exudate can be removed from the lungs via the mucociliary escalator, and complete resolution may take place within 4 weeks. In other cases, if injury or infection is persistent, suppurative bronchopneumonia can become chronic with goblet cell hyperplasia, an important component of the inflammatory process. Depending on the proportion of pus and mucus, the exudate in chronic suppurative bronchopneumonia varies from mucopurulent to mucoid. A mucoid exudate is found in the more chronic stages when the consolidated lung has a “fish flesh” appearance.
Hyperplasia of BALT is another change commonly seen in chronic suppurative bronchopneumonias; it appears grossly as conspicuous white nodules (cuffs) around bronchial walls (cuffing pneumonia). This hyperplastic change merely indicates a normal reaction of lymphoid tissue to infection. Further sequelae of chronic suppurative bronchopneumonia include bronchiectasis (see Fig. 9-51), pulmonary abscesses, pleural adhesions (from pleuritis), and atelectasis and emphysema from completely or partially obstructed bronchi or bronchioles (e.g., bronchiectasis).
Clinically, suppurative bronchopneumonias can be acute and fulminating but are often chronic, depending on the etiologic agent, stressors affecting the host, and immune status. The most common pathogens causing suppurative bronchopneumonia in domestic animals include Pasteurella multocida, Bordetella bronchiseptica, Arcanobacterium (Actinomyces) pyogenes, Streptococcus spp., Escherichia coli, and several species of mycoplasmas. Most of these organisms are secondary pathogens requiring a preceding impairment of the pulmonary defense mechanisms to allow them to colonize the lungs and establish an infection. Suppurative bronchopneumonia can also result from aspiration of bland material (e.g., milk). Pulmonary gangrene may ensue when the bronchopneumonic lung is invaded by saprophytic bacteria (aspiration pneumonia).
Fibrinous Bronchopneumonia: Fibrinous bronchopneumonia is similar to suppurative bronchopneumonia except that the predominant exudate is fibrinous rather than neutrophilic. With only a few exceptions, fibrinous bronchopneumonias also have a cranioventral distribution (Fig. 9-58; see Fig. 9-55). However, exudation is not restricted to the boundaries of individual pulmonary lobules, as is the case in suppurative bronchopneumonias. Instead the inflammatory process in fibrinous pneumonias involves numerous contiguous lobules and the exudate moves quickly through pulmonary tissue until the entire pulmonary lobe is rapidly affected. Because of the involvement of the entire lobe and pleural surface fibrinous bronchopneumonias are also referred to as lobar pneumonias or pleuropneumonias. In general terms, fibrinous bronchopneumonias are the result of more severe pulmonary injury and thus cause death earlier in the sequence of the inflammatory process than suppurative bronchopneumonias. Even in cases in which fibrinous bronchopneumonia involves 30% or less of the total area, clinical signs and death can occur as a result of severe toxemia and sepsis.
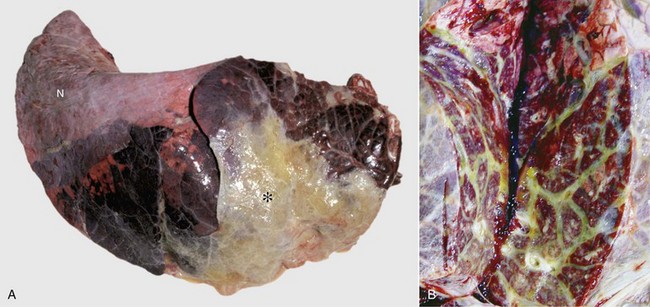
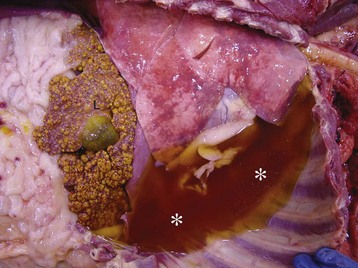
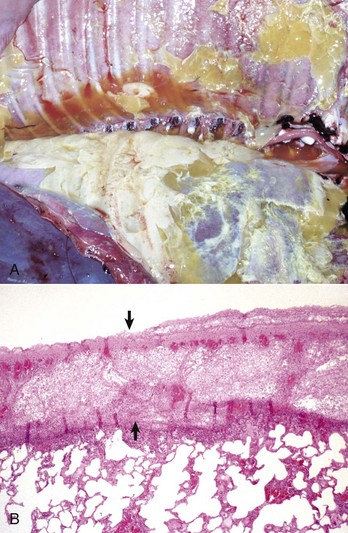
Fig. 9-58 Fibrinous bronchopneumonia (pleuropneumonia), right lung, steer.
A, The pneumonia has a cranioventral distribution that extends into the middle and caudal lobes and affects approximately 80% of the lung parenchyma. The lung is firm, swollen, and covered with yellow fibrin (*). The dorsal portion of the caudal lung is normal (N). B, Cut surface. Affected parenchyma appears dark and hyperemic as compared with more normal lung (top quarter of figure). Interlobular septa are prominent (yellow bands) due to the accumulation of fibrin and edema fluid. This type of lesion is typical of Mannheimia haemolytica infection in cattle (shipping fever). (A courtesy Ontario Veterinary College. B courtesy Dr. A. López, Atlantic Veterinary College.)
The gross appearance of fibrinous bronchopneumonia depends on the age and severity of the lesion and on whether the pleural surface or the cut surface of the lung is viewed. Externally, early stages of fibrinous bronchopneumonias are characterized by severe congestion and hemorrhage, giving the affected lungs a characteristically intense red discoloration. A few hours later, fibrin starts to accumulate on the pleural surface, giving the pleura a ground glass appearance and eventually forming plaques of fibrinous exudate over a red, dark lung (see Fig. 9-58). At this stage, a yellow fluid starts to accumulate in the thoracic cavity. The color of fibrin deposited over the pleural surface is also variable. It can be yellow when the exudate is formed primarily by fibrin, tan when fibrin is mixed with blood, and gray when a large number of leukocytes are part of the fibrinous plaque. Because of the tendency of fibrin to deposit on the pleural surface, some pathologists use the term pleuropneumonia as a synonym for fibrinous bronchopneumonia.
On the cut surface, early stages of fibrinous bronchopneumonia appear as simple red consolidation (see Fig. 9-58). In more advanced cases (24 hours), fibrinous bronchopneumonia is generally accompanied by notable dilation and thrombosis of lymph vessels and edema of interlobular septa. This distention of the interlobular septa gives affected lungs a typical marbled appearance. Distinct focal areas of coagulative necrosis in the pulmonary parenchyma are also common in fibrinous bronchopneumonia such as in shipping fever pneumonia and contagious bovine pleuropneumonia. In animals that survive the early stage of fibrinous bronchopneumonia, pulmonary necrosis often develops into pulmonary “sequestra,” which are isolated pieces of necrotic lung encapsulated by connective tissue. Pulmonary sequestra result from extensive necrosis of lung tissue either from severe ischemia (infarct) caused by thrombosis of a major pulmonary vessel such as in contagious bovine pleuropneumonia, or from the effect of necrotizing toxins released by pathogenic bacteria such as Mannheimia haemolytica. Sequestra in veterinary pathology should not be confused with “bronchopulmonary sequestration,” a term used in human pathology to describe a congenital malformation in which whole lobes or parts of the lung develop without normal connections to the airway or vascular systems.
Microscopically, in the initial stage of fibrinous bronchopneumonia, there is massive exudation of plasma proteins into the bronchioles and alveoli, and as a result most of the airspaces become obliterated by fluid and fibrin. Leakage of fibrin and fluid into alveolar lumina is because of extensive disruption of the integrity and increased permeability of the blood-air barrier. Fibrinous exudates can move from alveolus to alveolus through the pores of Kohn. Because fibrin is chemotactic for neutrophils, these types of leukocytes are always present a few hours after the onset of fibrinous inflammation. As inflammation progresses (3 to 5 days), fluid exudate is gradually replaced by fibrinocellular exudates composed of fibrin, neutrophils, macrophages, and necrotic debris (Fig. 9-59). In chronic cases (after 7 days), there is notable fibrosis of the interlobular septa and pleura.
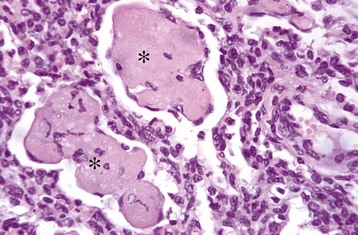
Fig. 9-59 Fibrinous bronchopneumonia, chronic, lung, calf.
Note large aggregates of condensed fibrin (asterisks) surrounded and infiltrated by phagocytic cells. H&E stain. (Courtesy Dr. A. López, Atlantic Veterinary College.)
In contrast to suppurative bronchopneumonia, fibrinous bronchopneumonia rarely resolves completely, thus leaving noticeable scars in the form of pulmonary fibrosis and pleural adhesions. The most common sequelae found in animals surviving an acute episode of fibrinous bronchopneumonia include bronchiolitis obliterans, in which organized exudate becomes attached to the bronchiolar lumen; gangrene, when saprophytic bacteria colonize necrotic lung; pulmonary sequestra; pulmonary fibrosis; abscesses; and chronic pleuritis with pleural adhesions. In some cases, pleuritis can be so extensive that fibrous adhesions extend onto the pericardial sac. Pathogens causing fibrinous bronchopneumonias in domestic animals include Mannheimia (Pasteurella) haemolytica (pneumonic Mannheimiosis), Histophilus somni (formerly Haemophilus somnus), Actinobacillus pleuropneumoniae (porcine pleuropneumonia), Mycoplasma bovis, and Mycoplasma mycoides ssp. mycoides small colony type (contagious bovine pleuropneumonia). Fibrinous bronchopneumonia and pulmonary gangrene can also be the result of bronchoaspiration of irritant materials such as gastric contents.
Fulminating hemorrhagic bronchopneumonia can be caused by highly pathogenic bacteria such as Bacillus anthracis. Although the lesions in anthrax are primarily related to a severe septicemia and sepsis, anthrax should always be suspected in animals with sudden death and exhibiting severe acute fibrinohemorrhagic pneumonia, splenomegaly, and multisystemic hemorrhages. Animals are considered good sentinels for anthrax in cases of bioterrorism.
Interstitial Pneumonia: Interstitial pneumonia refers to that type of pneumonia in which injury and the inflammatory process take place primarily in any of the three layers of the alveolar walls (endothelium, basement membrane, and alveolar epithelium) and the contiguous bronchiolar interstitium. This type of pneumonia is the most difficult to diagnose at necropsy and requires microscopic confirmation as it is easily mistaken in the lung showing congestion, edema, hyperinflation, or emphysema.
The pathogenesis of interstitial pneumonia is complex and can result from aerogenous injury to the alveolar epithelium (type I and II pneumonocytes) or from hematogenous injury to the alveolar capillary endothelium or alveolar basement membrane. Aerogenous inhalation of toxic gases (i.e., ozone, NO2) or toxic fumes (smoke inhalation) and infection with pneumotropic viruses (influenza, IBR, EVR, or canine distemper) can damage the alveolar epithelium. Inhaled antigens, such as fungal spores, combine with circulating antibodies and form deposits of antigen-antibody complexes (type III hypersensitivity) in the alveolar wall, which initiate a cascade of inflammatory responses and injury (allergic alveolitis). Hematogenous injury to the vascular endothelium occurs in septicemias (sepsis), DIC, larva migrans (Ascaris suum), toxins absorbed in the alimentary tract (endotoxin) or toxic metabolites locally generated in the lungs (3 methylindole, paraquat), release of free radicals in alveolar capillaries (ARDS), and viremias and infections with endotheliotropic viruses (CAV and classic swine fever [hog cholera]).
Interstitial pneumonias in domestic animals, like those in humans, are subdivided, based on some morphologic features, into acute and chronic. It should be kept in mind, however, that not all acute interstitial pneumonias are fatal and that they do not necessarily progress to the chronic form.
Acute Interstitial Pneumonias: Acute interstitial pneumonias begin with injury to either type I pneumonocytes or alveolar capillary endothelium, which provokes a disruption of the blood-air barrier and a subsequent exudation of plasma proteins into the alveolar space. This leakage of proteinaceous fluid into the alveolar lumen constitutes the exudative phase of acute interstitial pneumonia. In some cases of diffuse alveolar damage, exuded plasma proteins mix with lipids and other components of pulmonary surfactant and form elongated membranes that become partially attached to the alveolar basement membrane and bronchiolar walls. These membranes are referred to as hyaline membranes because of their hyaline appearance (eosinophilic, homogeneous, and amorphous) microscopically (see Figs. 9-37 and 9-45). In addition to intraalveolar exudation of fluid, inflammatory edema and neutrophils accumulate in the alveolar interstitium and cause thickening of the alveolar walls. This acute exudative phase is generally followed a few days later by the proliferative phase of acute interstitial pneumonias characterized by hyperplasia of type II pneumonocytes to replace the lost type I alveolar cells. Type II pneumonocytes are in fact progenitor cells that differentiate and replace necrotic type I pneumonocytes (see the section on General Aspects of Lung Inflammation). As a consequence, the alveolar walls become increasingly thickened. This process is in part the reason why lungs become rubbery on palpation, what prevents their normal collapse after the thorax is opened, and why the cut surface of the lung has a “meaty” appearance.
Acute interstitial pneumonias are often mild and transient, especially those caused by some respiratory viruses, such as those responsible for equine and porcine influenza. These mild forms of pneumonia are rarely seen in the postmortem room because they are not fatal and do not leave significant sequelae (see the section on Defense Mechanisms of the Exchange System). In severe cases of acute interstitial pneumonias, animals may die of respiratory failure, usually as a result of diffuse alveolar damage, a profuse exudative phase (leakage of proteinaceous fluid) leading to a fatal pulmonary edema. Examples of this type of fatal acute interstitial pneumonia are bovine pulmonary edema and emphysema, and ARDS in all species.
Gross lesions: In contrast to bronchopneumonias, in which distribution of lesions is generally cranioventral, in acute or chronic interstitial pneumonias, lesions are more diffusely distributed and generally involve all pulmonary lobes, or in some cases, they appear to be more pronounced in the dorsocaudal aspects of the lungs. Three important gross features of interstitial pneumonia are (1) the failure of lungs to collapse when the thoracic cavity is opened, (2) the occasional presence of rib impressions on the lung’s pleural surface indicating poor deflation, and (3) the lack of visible exudates in airways unless complicated with secondary bacterial pneumonia (Fig. 9-60; also see Fig. 9-55). The color of affected lungs varies from diffusely red in acute cases to diffusely pale gray to a mottled red, pale appearance in chronic ones. Pale lungs are caused by severe obliteration of alveolar capillaries (reduced blood-tissue ratio), especially evident when there is fibrosis of the alveolar walls. The texture of lungs with uncomplicated interstitial pneumonia is typically elastic or rubbery, but definitive diagnosis based on texture alone is difficult and requires histopathologic examination. On a cut surface, the lungs may appear and feel more “meaty” (having the texture of raw meat) and have no evidence of exudate in the bronchi or pleura (see Fig. 9-60). In acute interstitial pneumonias, particularly in cattle, there is frequently pulmonary edema (exudative phase) and interstitial emphysema secondary to partial obstruction of bronchioles by edema fluid and strenuous air gasping before death. Because edema tends to gravitate into the cranioventral portions of the lungs, and emphysema is often more obvious in the dorsocaudal aspects, acute interstitial pneumonias in cattle occasionally have a gross cranioventral-like pattern that may resemble bronchopneumonia, although the texture is different. Lungs are notably heavy because of the edema and the infiltrative and proliferative changes.
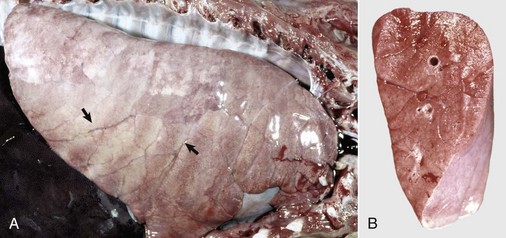
Fig. 9-60 Interstitial pneumonia, lung, feeder pig.
A, The lung is heavy, pale, and rubbery in texture. It also has prominent costal (rib) imprints (arrows), a result of hypercellularity of the interstitium and the failure of the lungs to collapse when the thorax was opened. B, Transverse section. The pulmonary parenchyma has a “meaty” appearance and some edema, but no exudate is present in airways or on the pleural surface. This type of lung change in pigs is highly suggestive of a viral pneumonia. (Courtesy Dr. A. López, Atlantic Veterinary College.)
Chronic Interstitial Pneumonia: When the source of alveolar injury persists, the proliferative and infiltrative lesions of acute interstitial pneumonia can progress into a morphologic stage referred to as chronic interstitial pneumonia whose hallmark is fibrosis of the alveolar walls (with or without intraalveolar fibrosis) and infiltrative and proliferative changes. Infiltrative changes are characterized by the presence of lymphocytes, macrophages, fibroblasts, and myofibroblasts in the alveolar interstitium (Fig. 9-61). Proliferative changes are generally characterized by hyperplasia and persistence of type II pneumonocytes (see Fig. 9-54), and in rare cases, by squamous metaplasia of the alveolar epithelium. It should be emphasized again that, although the lesions in interstitial pneumonia are centered in the alveolar walls and its interstitium, a mixture of desquamated epithelial cells, macrophages, and mononuclear cells are usually present in the lumen of bronchioles and alveoli. Other, concurrent changes that can occur are microscopic granulomas and hyperplasia of smooth muscle in bronchioles and pulmonary arterioles. Ovine progressive pneumonia, hypersensitivity pneumonitis in cattle and dogs, and silicosis in horses are good veterinary examples of chronic interstitial pneumonia. Pneumoconioses (silicosis, asbestosis), paraquat toxicity, pneumotoxic antineoplastic drugs (bleomycin), and extrinsic allergic alveolitis (farmer’s lung) are well-known examples of diseases that lead to chronic interstitial pneumonias in humans. Massive pulmonary migration of ascaris larvae in pigs also causes interstitial pneumonia (Fig. 9-62).
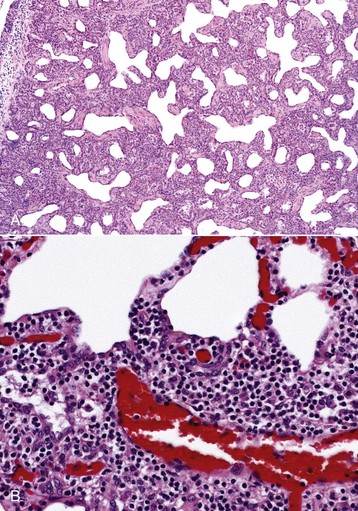
Fig. 9-61 Interstitial pneumonia, lung, aged ewe.
A, The alveolar septa are notably thickened by severe interstitial infiltration of inflammatory cells. H&E stain. B, Higher magnification view of A showing large numbers of lymphocytes and other mononuclear cells infiltrating the alveolar septal interstitium. H&E stain. (A courtesy Western College of Veterinary Medicine. B courtesy of Dr. A. López, Atlantic Veterinary College.)
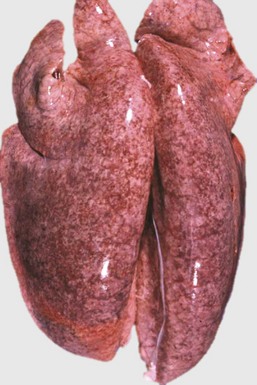
Fig. 9-62 Interstitial pneumonia, edema, and hemorrhages, lungs, pig.
This pig had migrating Ascaris suum larvae. The lungs are heavy and wet and failed to collapse when the thorax was opened, as a result of pulmonary edema. The mottled appearance of lungs is due to the presence of numerous petechiae scattered in the pulmonary parenchyma. Petechiae are likely alveolar hemorrhages caused by migrating larvae. Larvae leave the blood-stream to enter the alveoli by penetrating and rupturing alveolar capillaries and thus damage the air-blood barrier of alveolar septa. (Courtesy Dr. J.M. King, College of Veterinary Medicine, Cornell University.)
There is an insidious and poorly understood group of chronic interstitial diseases, both in humans and animals, that eventually progresses to terminal interstitial fibrosis. These were originally thought to be the result of repeated cycles of alveolar injury, inflammation, and fibroblastic/myoblastic response to an unknown agent. However, aggressive antiinflammatory therapy generally fails to prevent or reduce the severity of fibrosis. Now, it is proposed that a genetic mutation alters the cell-cell communication between epithelial and mesenchymal cell in the lung. This aberrant cellular communication leads to an overexpression of inflammatory and repair molecules (i.e., IL-4, IL-13, TGF-β1, and caveolin) leading to increased apoptosis and interstitial deposition of extracellular matrix (ECM). The chronic interstitial (restrictive) diseases in human medicine include “idiopathic pulmonary fibrosis,” “nonspecific interstitial pneumonia,” “unusual interstitial pneumonia,” and “cryptogenic organizing pneumonia,” also referred to as bronchiolitis obliterans organizing pneumonia (BOOP). Equine multinodular pulmonary fibrosis and feline idiopathic pulmonary fibrosis are examples of this type of progressive interstitial disease in veterinary medicine. It has been reported that in rare cases chronic alveolar remodeling and interstitial fibrosis can progress to lung cancer.
The term bronchointerstitial pneumonia has been introduced into veterinary pathology to describe cases in which pulmonary lesions share some histologic features of both bronchopneumonia and interstitial pneumonia. This combined type of pneumonia is in fact frequently seen in many viral infections in which viruses replicate and cause necrosis in bronchial, bronchiolar, and alveolar cells. Damage to the bronchial and bronchiolar epithelium causes an influx of neutrophils similar to that in bronchopneumonias, and damage to alveolar walls causes proliferation of type II pneumonocytes, similar to that which takes place in the proliferative phase of acute interstitial pneumonias. It is important to emphasize that bronchointerstitial pneumonia is a microscopic not a gross diagnosis. Examples include uncomplicated cases of respiratory syncytial virus infections in cattle and lambs, canine distemper, and influenza in pigs and horses.
Embolic Pneumonia: Embolic pneumonia refers to a particular type of pneumonia in which lung injury is hematogenous, and the inflammatory response is typically centered in pulmonary arterioles and alveolar capillaries. Lungs act as a biologic filter for circulating particulate matter. Sterile thromboemboli, unless extremely large, are rapidly dissolved and removed from the pulmonary vasculature by fibrinolysis, causing little if any ill effects. Experimental studies have confirmed that most types of bacteria when injected intravenously (bacteremia) are phagocytosed by pulmonary intravascular macrophages, or bypass the lungs and are finally trapped by macrophages in the liver, spleen, joints, or other organs. To cause pulmonary infection, circulating bacteria must first attach to the pulmonary endothelium with specific binding proteins or simply attach to intravascular fibrin and then evade phagocytosis by intravascular macrophages or leukocytes. Septic thrombi facilitate entrapment of bacteria in the pulmonary vessels and provide a favorable environment to escape phagocytosis. Once trapped in the pulmonary vasculature, usually in small arterioles or alveolar capillaries, offending bacteria disrupt endothelium and basement membranes, spread from the vessels to the interstitium and then to the surrounding lung, forming finally a new nidus of infection.
Embolic pneumonia is characterized by multifocal lesions randomly distributed in all pulmonary lobes (see Fig. 9-55). Early lesions in embolic pneumonia are characterized grossly by the presence of very small (1 to 10 mm), white foci surrounded by a discrete, red, hemorrhagic halo (Fig. 9-63 and Web Fig. 9-9). Unless emboli arrive in massive numbers, causing fatal pulmonary edema, embolic pneumonia is seldom fatal; therefore these acute lesions are rarely seen at postmortem examination. In most instances, acute lesions if unresolved rapidly progress to pulmonary abscesses. These are randomly distributed in all pulmonary lobes and are not restricted to the cranioventral aspects of the lungs, as is the case of abscesses developing from suppurative bronchopneumonia. The early microscopic lesions in embolic pneumonias are always focal (Fig. 9-64); thus they differ from those of endotoxemia or septicemia, in which endothelial damage and interstitial reactions (interstitial pneumonia) are diffusely distributed in the lungs.
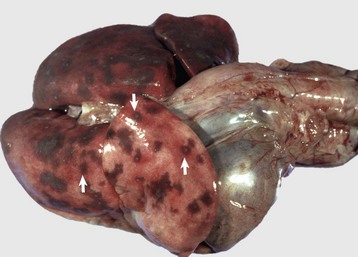
Fig. 9-63 Embolic pneumonia, lungs, 6-week-old puppy.
Large hemorrhagic foci are scattered relatively uniformly throughout all pulmonary lobes (arrows). These hemorrhagic foci are the sites of lodgment of Pseudomonas aeruginosa emboli (septic) that originated from necrotizing enteritis. Note the multifocal distribution of the inflammatory foci, which is typical of embolic pneumonia. Septic emboli were also present in the liver. (Courtesy Atlantic Veterinary College.)
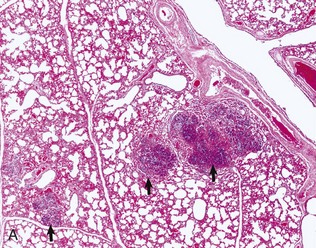
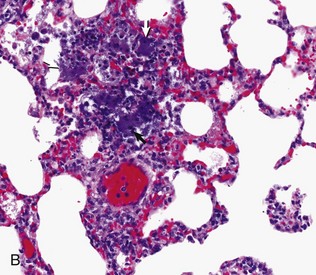
Fig. 9-64 Embolic pneumonia, lung, cow.
A, Foci of necrosis and infiltration of neutrophils (arrows) resulting from septic emboli. Note the multifocal distribution of the lesion, which is typical of embolic pneumonia. Vegetative endocarditis involving the tricuspid valve was the source of septic emboli in this cow. H&E stain. B, Embolic focus in the lung. Note bacterial colonies (arrows) mixed with neutrophils and cellular debris. H&E stain. (Courtesy Dr. A. López, Atlantic Veterinary College.)
When embolic pneumonia or its sequelae (abscesses) is diagnosed at necropsy, an attempt should be made to locate the source of septic emboli. The most common are hepatic abscesses which can have ruptured into the caudal vena cava in cattle, omphalophlebitis in farm animals, chronic bacterial skin or hoof infections, and a contaminated catheter in all species (see Fig. 9-46). Valvular or mural endocarditis in the right heart is a common source of septic emboli and embolic pneumonia in all species. Most frequently, bacterial isolates from septic pulmonary emboli in domestic animals are Arcanobacterium (Actinomyces) pyogenes (cattle), Fusobacterium necrophorum (cattle, pigs, and humans), Erysipelothrix rhusiopathiae (pigs, cattle, dogs, and humans), Streptococcus suis type II (pigs), Staphylococcus aureus (dogs, humans), and Streptococcus equi (horses).
Granulomatous Pneumonia: Granulomatous pneumonia refers to a particular type of pneumonia in which aerogenous or hematogenous injury is caused by organisms or particles that cannot be normally eliminated by phagocytosis and that evoke a local inflammatory reaction with numerous alveolar and interstitial macrophages, lymphocytes, a few neutrophils, and sometimes giant cells. The term granulomatous is used here to describe an anatomic pattern of pneumonia typically characterized by the presence of granulomas.
The pathogenesis of granulomatous pneumonia shares some similarities with that of interstitial and embolic pneumonias. Not surprisingly, some pathologists group granulomatous pneumonias within one of these types of pneumonias (e.g., granulomatous interstitial pneumonia). What makes granulomatous pneumonia a distinctive type is not so much the portal of entry or site of initial injury in the lungs, but the unique type of inflammatory response that results in the formation of granulomas, which can be easily recognized at gross and microscopic examination. The portal of agent entry into lungs can be aerogenous or hematogenous. As a rule, agents causing granulomatous pneumonia are resistant to intracellular killing by phagocytic cells and to the acute inflammatory response and persist in affected tissue for a long time.
The most common causes of granulomatous pneumonia in animals include systemic fungal diseases, such as cryptococcosis (Cryptococcus neoformans), coccidioidomycosis (Coccidioides immitis), histoplasmosis (Histoplasma capsulatum), and blastomycosis (Blastomyces dermatitidis). In most of these fungal diseases, the port of entry is aerogenous and from the lungs the fungi disseminate systemically to other organs, particularly the lymph nodes, liver, and spleen. Granulomatous pneumonia is also caused by some bacterial diseases, such as tuberculosis (Mycobacterium bovis) in all species, and inhaled foreign material (starch). Sporadically, aberrant parasites such as Fasciola hepatica in cattle and aspiration of foreign bodies can also cause granulomatous pneumonia. Feline infectious peritonitis is one of a few viral infections of domestic animals that result in granulomatous pneumonia. Lesions are caused by the deposition of antigen-antibody complexes in the vasculature of many organs, including the lungs, and subsequent vasculitis.
Granulomatous pneumonia is characterized by the presence of variable numbers of caseous or noncaseous granulomas randomly distributed in the lungs (Fig. 9-65; also see Fig. 9-55). On palpation, lungs have a typical nodular character given by well-circumscribed, variably sized nodules that generally have a firm texture, especially if calcification has occurred (see Fig. 9-65). During postmortem examination, granulomas in the lungs occasionally can be mistaken for neoplasms. Microscopically, pulmonary granulomas are composed of a center of necrotic tissue, surrounded by a rim of macrophages (epithelioid cells) and giant cells and an outer delineated layer of connective tissue commonly infiltrated by lymphocytes and plasma cells (Fig. 9-66). Unlike other types of pneumonias, the causative agent in granulomatous pneumonia can, in many cases, be identified microscopically in sections stained by PAS reaction or by Grocott-Gomori’s methenamine silver (G-GMS) stains for fungi or the acid-fast stain for mycobacteria.
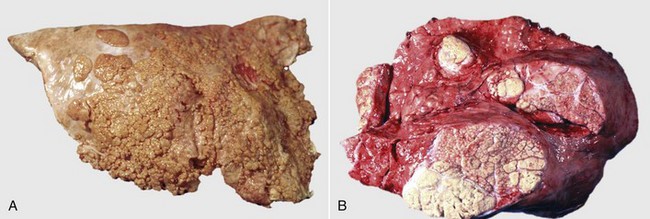
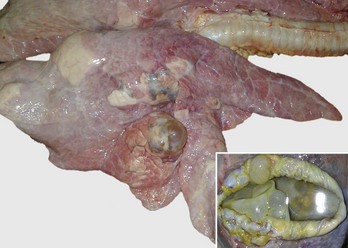
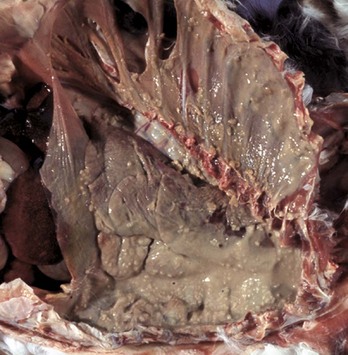
Fig. 9-65 Pulmonary tuberculosis, lung, aged cow.
A, Multifocal, coalescing granulomatous pneumonia involves most of the lung, except for the dorsal portion of the caudal lung lobe. B, Transverse section. Large multifocal to confluent caseating granulomas are present in the pulmonary parenchyma. Note the caseous (“cheesy,” pale yellow-white) appearance of the granulomas, which is typical of bovine tuberculosis. (A courtesy Facultad de Medicina Veterinaria y Zootecnia, UNAM, México. B courtesy Dr. J.M. King, College of Veterinary Medicine, Cornell University.)
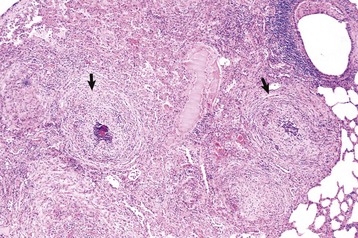
Fig. 9-66 Granulomatous pneumonia, lung, cow.
There are several noncaseous granulomas (arrows), each with a small necrotic center filled with neutrophils, surrounded by histiocytes and mononuclear cells, and with an outer rim of connective tissue. H&E stain. (Courtesy Western College of Veterinary Medicine.)
Species-Specific Pneumonias
Pneumonias of Horses: Viral infections of the respiratory tract, particularly equine viral rhinopneumonitis and equine influenza, are important diseases of horses around the world. The effects of these and other respiratory viruses on the horse can be manifested in three distinct ways. First, as pure viral infections, their severity may range from mild to severe, making them a frequent interfering factor in training and athletic performance. Second, superimposed infections by opportunistic bacteria, such as Streptococcus spp., Escherichia coli, Klebsiella pneumoniae, Rhodococcus equi, and various anaerobes, can cause fibrinous or suppurative bronchopneumonias. Third, it is possible but yet unproved that viral infections may also predispose horses to airway hyperresponsiveness and COPD.
Equine Influenza: Equine influenza is an important and highly contagious flulike respiratory disease of horses characterized by high morbidity and low mortality and explosive outbreaks in susceptible populations of horses. It is an OIE-notifiable disease. Two antigenically unrelated subtypes of equine influenza viruses have been identified (H7N7 [A/equi-1] and H3N8 [A/equi-2]). The course of the disease is generally mild and transient, and its importance is primarily because of its economic impact on horse racing. The types of injury and host response in the conducting system are described in the section on Equine Nasal Diseases. Uncomplicated lesions in the lungs are mild and self-limiting bronchointerstitial pneumonia. In fatal cases, the lungs are hyperinflated with dark red coalescing ares of dark red discoloration. Microscopically, there is a bronchointerstitial pneumonia characterized by necrotizing bronchiolitis that is followed by hyperplastic bronchiolitis, hyperplasia of type II pneumonocytes, hyaline membranes in alveoli, and sporadic multinucleated giant cell. The microscopic changes are ARDS in severe and fatal cases. The influenza virus antigen can be readily demonstrated in ciliated cells and alveolar macrophages. Clinical signs are characterized by fever, cough, abnormal lung sounds (crackles and wheezes), anorexia, and depression. Secondary bacterial infections (Streptococcus equi, Streptococcus zooepidemicus, Streptococcus aureus, and Escherichia coli) commonly complicate equine influenza.
Equine Viral Rhinopneumonitis: Equine viral rhinopneumonitis (EVR), or equine herpesvirus infection, is a respiratory disease of young horses that is particularly important in weanlings between 4 and 8 months of age and to a much lesser extent in young foals and adult horses. The causative agent is a ubiquitous equine herpesvirus (EHV-1 and EHV-4) that in addition to respiratory disease can cause abortion in pregnant mares and neurologic disease (equine herpes myeloencephalopathy) (see the section on Equine Nasal Diseases).
The respiratory form of EVR is a mild and a transient bronchointerstitial pneumonia seen only by pathologists when complications with secondary bacterial infections cause a fatal bronchopneumonia (Streptococcus equi, Streptococcus zooepidemicus, or Staphylococcus aureus). Uncomplicated lesions in EVR are seen only in aborted fetuses or in foals that die within the first few days of life. They consist of focal areas of necrosis (0.5 to 2 mm) in various organs, including liver, adrenal glands, and lungs. In some cases, intranuclear inclusion bodies are microscopically observed in these organs. Recent outbreaks of interstitial pneumonia in donkeys have been attributed to a novel strain of asinine (equine) herpesvirus (AHV-3). Clinically, horses and donkeys affected with the respiratory form of EVR exhibit fever, anorexia, conjunctivitis, cough, and nasal discharge.
Equine Viral Arteritis: Equine viral arteritis (EVA), a pansystemic disease of foals and horses caused by an arterivirus, occurs sporadically throughout the world, sometimes as an outbreak. This virus infects and causes severe injury to macrophages and endothelial cells. Gross lesions are hemorrhagic and edematous in many sites, including lungs, intestine, scrotum, and periorbital tissues and voluminous hydrothorax and hydroperitoneum,. The basic lesion is fibrinoid necrosis and inflammation of the vessel walls (vasculitis), particularly the small muscular arteries (lymphocytic arteritis), which is responsible for the edema and hemorrhage that explain most of the clinical features. Pulmonary lesions are those of interstitial pneumonia with hyperplasia of type II pneumonocytes and vasculitis with abundant edema in the bronchoalveolar spaces and distended pulmonary lymphatic vessels. Viral antigen can be detected by immunoperoxidase techniques in the walls and endothelial cells of affected pulmonary vessels and in alveolar macrophages.
Clinical signs are respiratory distress, fever, abortion, diarrhea, colic, and edema of the limbs and ventral abdomen. Respiratory signs are frequent and consist of serous or mucopurulent rhinitis and conjunctivitis with palpebral edema. Like most viral respiratory infections, EVA can predispose horses to opportunistic secondary bacterial pneumonias.
African Horse Sickness: African horse sickness is a vector-borne, “List A” OIE-notifiable disease of horses, mules, donkeys, and zebras that is caused by an orbivirus (family Reoviridae) and characterized by respiratory distress or cardiovascular failure. It has a high mortality rate—up to 95% in the native population of horses in Africa, the Middle East, India, Pakistan, and most recently Spain and Portugal. Although the virus is transmitted primarily by insects (Culicoides) to horses, other animals, such as dogs, can be infected by eating infected equine flesh. The pathogenesis of African horse sickness remains unclear, but this equine orbivirus has an obvious tropism for pulmonary and cardiac endothelial cells and to a lesser extent mononuclear cells. Based on clinical signs (not pathogenesis), African horse sickness is arbitrarily divided into four different forms: pulmonary, cardiac, mixed, and mild.
The pulmonary form is characterized by severe respiratory distress and rapid death because of massive pulmonary edema, presumably from viral injury to the pulmonary endothelial cells. Grossly, large amounts of froth are present in the airways, lungs fail to collapse, subpleural lymph vessels are distended, and the ventral parts of the lungs are notably edematous (see Fig. 4-44). In the cardiac form, recurrent fever is detected, and heart failure results in subcutaneous and interfascial edema, most notably in the neck and supraorbital region. The mixed form is a combination of the respiratory and cardiac forms. Finally, the mild form, rarely seen in postmortem rooms, is characterized by fever and clinical signs resembling those of equine influenza; it is in most cases transient and followed by a complete recovery. This mild form is most frequently seen in donkeys, mules, and zebras and in horses with some degree of immunity. Detection of viral antigen for diagnostic purposes could be done by immunohistochemistry in paraffin-embedded tissues.
Equine Henipavirus (Hendra Virus): Fatal cases of a novel respiratory disease in horses and humans suddenly appeared around 1994 in Hendra, a suburb of Brisbane, Australia. This outbreak was attributed to a newly recognized zoonotic virus that was tentatively named equine Morbillivirus (Hendra virus) and is currently classified as a member of the Henipavirus (Hendra virus and Nipah virus), new members of the subfamily Paramyxoviridae. Fruit bats (flying foxes) act as natural reservoirs and are involved in the transmission by poorly understood mechanisms. The lungs of affected horses are severely edematous with gelatinous distention of pleura and subpleural lymph vessels. Microscopically, the lungs have diffuse alveolar edema associated with vasculitis, thrombosis, and presence of multinucleated syncytial cells in the endothelium of small pulmonary blood vessels and alveolar capillaries. The lymphatic vessels are notably distended with fluid. The characteristic inclusion bodies seen in other Paramyxovirus infections are not seen in horses; however, the virus can be easily detected by immunohistochemistry in pulmonary endothelial cells and alveolar epithelial cells. Clinical signs are nonspecific and include fever, anorexia, respiratory distress, and nasal discharge.
Rhodococcus Equi: Rhodococcus equi, formerly known as Corynebacterium equi, is an important cause of morbidity and mortality in foals around the world. It is a facultative intracellular Gram-positive bacterium that causes two major forms of disease: the first involves the intestine, causing ulcerative enterocolitis, and the second severe and often fatal bronchopneumonia. Although half of foals with pneumonia have ulcerative enterocolitis, it is rare to find animals with intestinal lesions alone. Occasionally, infection is disseminated to lymph nodes, joints, bones, genital tract, and other organs. Because Rhodococcus equi is present in soil and feces of herbivores (particularly foals), it is not unusual for the disease to become enzootic on farms where the organism has been shed earlier by infected foals. Serologic evidence of infection in horses is widespread, yet clinical disease is sporadic and largely restricted to young foals or to adult horses with severe immunosuppression. Virulence factors encoded by plasmids (virulence-associated protein A [VapA gene]) appear to be responsible for the survival of Rhodococcus equi in macrophages, thus determining the evolution of the disease. This bacterium also has been sporadically incriminated with infections in cattle, goats, pigs, dogs, and cats, and quite often in immunocompromised humans, for example, those infected with the AIDS virus, after organ transplantation, or undergoing chemotherapy.
It is still debatable whether natural infection starts as a bronchopneumonia (aerogenous route) from which Rhodococcus equi reaches the intestine via swallowed sputum or whether infection starts as an enteritis (oral route) with a subsequent bacteremia into the lungs. The results of experimental studies suggest that natural infection likely starts from inhalation of infected dust or aerosols. Once in the lung, Rhodococcus equi is rapidly phagocytosed by alveolar macrophages, but because of defective phagosome-lysosome fusion and premature lysosomal degranulation, bacteria survive and multiply intracellularly, eventually leading to the destruction of the macrophage. Interestingly, Rhodococcus equi appears to be easily killed by neutrophils but not macrophages. Released cytokines and lysosomal enzymes and bacterial toxins are responsible for extensive caseous necrosis of the lungs and the recruitment of large numbers of neutrophils, macrophages, and giant cells containing intracellular Gram-positive organisms in their cytoplasm.
Depending on the stage of infection and the immune status and age of affected horses, pulmonary lesions induced by Rhodococcus equi can vary from suppurative bronchopneumonia to granulomatous pneumonia. In young foals, the infection starts as a suppurative cranioventral bronchopneumonia, which progresses within a few days into small variable-size pulmonary abscesses. These abscesses rapidly transform into pyogranulomatous nodules some of which become confluent and form large masses of caseous exudate (Fig. 9-67). Microscopically the early lesion starts with neutrophilic infiltration, followed by an intense influx of alveolar macrophages into the bronchoalveolar spaces. This type of histiocytic inflammation tends to persist for a long period of time because Rhodococcus equi is a facultative intracellular organism that survives the bactericidal effects of equine alveolar macrophages. In the most chronic cases, the pulmonary lesions culminate with the formation of large necrotic masses with extensive fibrosis of the surrounding pulmonary parenchyma. PCR analysis of tracheal aspirates has successfully been used as an alternative to bacteriologic culture in the diagnosis of Rhodococcus equi infection in live foals.
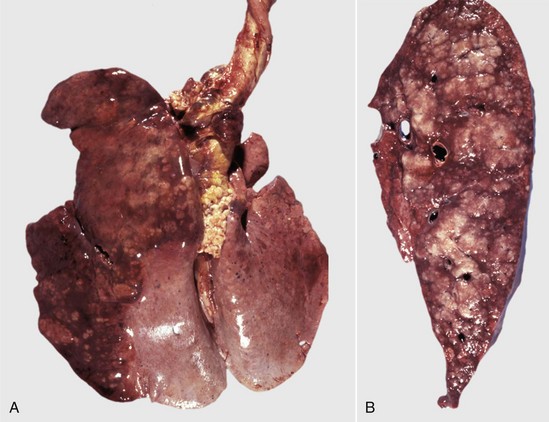
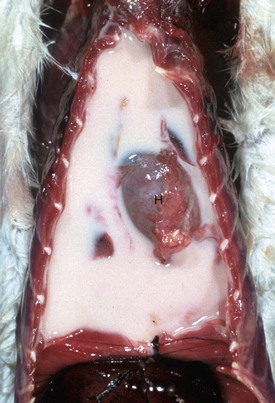
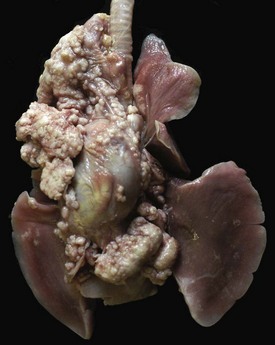
Fig. 9-67 Granulomatous pneumonia (Rhodococcus equi), lungs, foal.
A, Cranioventral consolidation of the lungs with subpleural granulomas. Note that the pneumonic lesions in this foal are unilateral. This was an experimental case in which a foal was intratracheally inoculated with a suspension of Rhodococcus equi. B, Cut surface. Note the large, confluent, caseated granulomas. (Courtesy Drs. J. Yager and J. Prescott, Ontario Veterinary College.)
Clinically, Rhodococcus equi infection can be acute, with rapid death caused by severe bronchopneumonia, or chronic, with depression, cough, weight loss, and respiratory distress. In either form, there may be diarrhea, arthritis, or subcutaneous abscess formation.
Other Pneumonias of Horses: Chlamydophila (Chlamydia) psittaci, an obligatory intracellular zoonotic pathogen, can cause systemic infection in many mammalian and avian species; in horses, it also causes keratoconjunctivitis, rhinitis, pneumonia, abortion, polyarthritis, enteritis, hepatitis, and encephalitis. Serologic studies suggest that infection without apparent disease is common in horses. Horses experimentally infected with Chlamydophila psittaci develop mild and transient bronchointerstitial pneumonia. There are unconfirmed reports suggesting a possible association between this organism and recurrent airway obstruction in horses. Detection of chlamydial organisms in affected tissue is not easy and requires special laboratory techniques such as PCR, immunohistochemistry, and fluorescent antibody tests.
Horses are susceptible to mycobacteriosis (Mycobacterium avium complex, Mycobacterium tuberculosis, and Mycobacterium bovis). The intestinal tract and associated lymph nodes are usually affected, suggesting an oral route of infection with subsequent hematogenous dissemination to the lungs (Fig. 9-68). The tubercles (granulomas) differ from those in ruminants and pigs, being smooth, gray, solid nodules without grossly visible caseous necrosis or calcification; they typically appear more like sarcomas. Microscopically, the tubercles are composed of macrophages, epithelioid cells, and multinucleated giant cells. Fibrosis increases with time, accounting in part for the sarcomatous appearance.
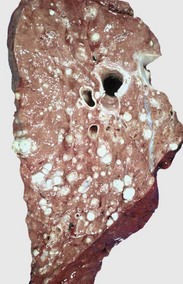
Fig. 9-68 Multifocal granulomatous pneumonia, tuberculosis (Mycobacterium avium-intracellulare), lung, cut surface, aged horse.
Note the large numbers of noncaseating granulomas scattered throughout the pulmonary parenchyma. In horses, granulomas caused by Mycobacteria often resemble sarcomatous nodules. (Courtesy Western College of Veterinary Medicine.)
Adenovirus infections occur commonly in Arabian foals with combined immunodeficiency (CID), a hereditary lack of B and T lymphocytes. In cases of adenoviral infection, large basophilic or amphophilic inclusions are present in the nuclei of tracheal, bronchial, bronchiolar, alveolar, renal, and intestinal epithelial cells. As it occurs in other species, infection with a unique fungal pathogen known as Pneumocystis carinii typically occurs in immunosuppressed or immunoincompetent individuals such as Arabian foals with CID. Diagnosis of Pneumocystis carinii requires microscopic examination of lungs and special stains (see the section on Pneumonias of Pigs).
Interstitial and bronchointerstitial pneumonias of undetermined cause that can progress to severe pulmonary fibrosis have been reported in foals and young horses. The gross and microscopic lesions are reminiscent of those of bovine pulmonary edema and emphysema or ARDS. The lungs are notably congested and edematous and microscopically are characterized by necrosis of the bronchiolar epithelium, alveolar edema, hyperplasia of type II pneumonocytes, and hyaline membranes. The cause of this form of equine interstitial pneumonia is not known, but toxic and particularly viral causes have been proposed.
Equine multinodular pulmonary fibrosis characterized by progressive focal to coalescing fibrotic lesions in the lung has been described in adult horses. The pathogenesis is still under investigation, but equine herpes-5 virus has been proposed as the putative etiology.
Aspiration pneumonia is often a devastating sequela to improper gastric tubing of horses, particularly exogenous lipid pneumonia from mineral oil delivered into the trachea in treatment of colic. Gross and microscopic lesions are described in detail in the section on Aspiration Pneumonias of Cattle.
Parasitic pneumonias of horses: Parascaris equorum is a large nematode (roundworm) of the small intestine of horses; the larval stages migrate through the lungs as ascarid larvae do in pigs. It is still unclear whether migration of Parascaris equorum larvae can cause significant pulmonary lesions under natural conditions. Experimentally, migration of larvae results in coughing, anorexia, weight loss, and small necrotic foci and petechial hemorrhages in the liver, hepatic and tracheobronchial lymph nodes, and lungs. Microscopically, eosinophils are prominent in the interstitium and airway mucosa during the parasitic migration and in focal granulomas caused by dead larvae in the lung.
Dictyocaulus arnfieldi is not a very pathogenic nematode, but it should be considered if there are signs of coughing in horses that are pastured together with donkeys. Donkeys are considered the natural hosts and can tolerate large numbers of parasites without ill effects. Dictyocaulus arnfieldi does not usually become patent in horses, so examination of fecal samples is not useful; BAL is only occasionally diagnostic because eosinophils (but not parasites) are typically found in the lavage fluid. Mature parasites (up to 8 cm in length) cause obstructive bronchitis, edema, and atelectasis, particularly along the dorsocaudal lung. The microscopic lesion is an eosinophilic bronchitis similar to the less acute infestations seen in cattle and sheep with their Dictyocaulus species.
Bovine Respiratory Disease Complex and Acute Undifferentiated Respiratory Disease: Bovine respiratory disease (BRD) complex and acute undifferentiated respiratory disease are general terms often used by clinicians to describe acute and severe bovine respiratory illness of clinically undetermined cause. These terms do not imply any particular type of pneumonia and therefore should not be used in pathology reports. Clinically, the BRD complex includes enzootic pneumonia of calves (multifactorial etiology); pneumonic Mannheimiosis (Mannheimia haemolytica); respiratory histophilosis (Histophilus somni), previously known as respiratory hemophilosis (Haemophilus somnus); Mycoplasma bovis; respiratory viral infections, such as IBR/BoHV-1, PI-3 virus, and BRSV; and noninfectious interstitial pneumonias, such as bovine pulmonary edema and emphysema, reinfection syndrome, and many others.
Bovine Enzootic Pneumonia: Enzootic pneumonia, sometimes simply referred to as calf pneumonia, is a multifactorial disease caused by a variety of etiologic agents that produces an assortment of lung lesions in young, intensively housed calves. Morbidity is often high (up to 90%), but fatalities are uncommon (>5%) unless management is poor or unless new, virulent pathogens are introduced by additions to the herd. Enzootic pneumonia is also called viral pneumonia because it often begins with an acute respiratory infection with PI-3 virus, BRSV, or possibly with one or more of several other viruses (adenovirus, BoHV-1, reovirus, bovine respiratory coronavirus, and rhinovirus). Mycoplasmas, notably Mycoplasma dispar, Mycoplasma bovis, Ureaplasma, and possibly Chlamydophila, may also be primary agents. Following infection with any of these agents, opportunistic bacteria, such as Pasteurella multocida, Arcanobacterium (Actinomyces) pyogenes, Histophilus somni, Mannheimia haemolytica, and Escherichia coli, cause a secondary suppurative bronchopneumonia, the most serious stage of enzootic pneumonia. The pathogenesis of the primary invasion and how it predisposes the host to invasion by the opportunists are poorly understood, but it is likely that there is impairment of pulmonary defense mechanisms. Environmental factors, including air quality (poor ventilation), high relative humidity, and animal crowding, have been strongly incriminated. The immune status of the calf also plays an important role in the development and severity of enzootic pneumonia. Calves with bovine leukocyte adhesion deficiency (BLAD), which prevents the migration of neutrophils from the capillaries, are highly susceptible to bronchopneumonia.
Lesions are variable and depend largely on the agents involved and on the duration of the inflammatory process. In the acute phases, lesions caused by viruses are those of bronchointerstitial pneumonia, which are generally mild and transient, and therefore are seen only sporadically at necropsy. Microscopically, the lesions are necrotizing bronchiolitis, necrosis of type I pneumonocytes with hyperplasia of type II pneumonocytes, and mild interstitial and alveolar edema.
In the case of PI-3 and BRSV infection, intracytoplasmic inclusion bodies and the formation of large multinucleated syncytia, resulting from the fusion of infected bronchiolar epithelial cells, can also be observed in the lungs (Fig. 9-69). Airway hyperreactivity has been described in calves after BRSV infection; however, the significance of this syndrome in relation to enzootic pneumonia of calves is still under investigation.
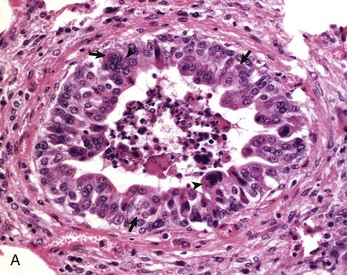
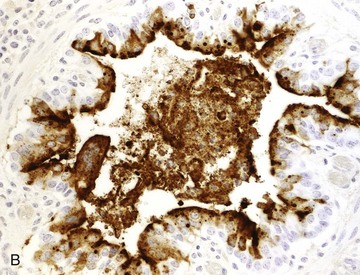
Fig. 9-69 Necrotizing bronchiolitis, bovine respiratory syncytial virus, lung, 5-week-old calf.
This is the reparative stage of necrotizing bronchiolitis and is characterized by epithelial hyperplasia and exfoliation of necrotic cells into the bronchiolar lumen. A, Epithelial cells are swollen, some are multinucleated (arrowheads) and the cytoplasm of some cells contains eosinophilic inclusion bodies surrounded by a clear halo (arrows). Many of these hyperplastic bronchiolar cells eventually undergo apoptosis during the last stage of bronchiolar repair. H&E stain. B, Necrotizing viral bronchiolitis. Note positive staining for bovine respiratory syncytial virus antigen in bronchiolar cells and in exfoliated necrotic material in the bronchiolar lumen. Immunohistochemical stain. (A and B courtesy Dr. A. López, Atlantic Veterinary College.)
The mycoplasmas also can cause bronchiolitis, bronchiolar and alveolar necrosis, and an interstitial reaction, but in contrast to viral-induced pneumonias, mycoplasmal lesions tend to progress to a chronic stage characterized by striking peribronchiolar lymphoid hyperplasia (cuffing pneumonia). When complicated by secondary bacterial infections (e.g., Pasteurella multocida, Arcanobacterium pyogenes), viral or mycoplasmal lesions change from a pure bronchointerstitial to a suppurative bronchopneumonia (Fig. 9-70). In late stages of bronchopneumonia, the lungs contain a creamy-mucoid exudate in the airways and later often have pulmonary abscesses and bronchiectasis (see Fig. 9-51).
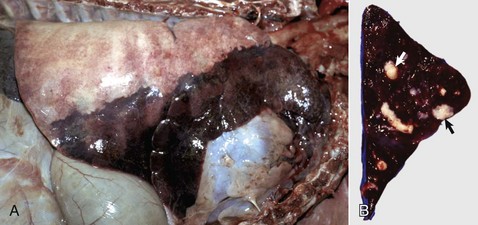
Fig. 9-70 Suppurative bronchopneumonia, right lung, calf.
A, Approximately 40% of the lung parenchyma is consolidated and includes most of the cranial lung lobe and the ventral portions of the middle and caudal lung lobes, a distribution often designated as cranioventral. Note the dark color of the consolidated lung and the normal appearance of the dorsal portion of the caudal lung lobe. B, Transverse section of the cranial lung lobe showing bronchi filled with purulent exudate (arrows). (Courtesy Ontario Veterinary College.)
It should be noted that the same viruses and mycoplasmas involved in the enzootic pneumonia complex can also predispose cattle to other diseases, such as pneumonic Mannheimiosis (Mannheimia haemolytica). Clinically, enzootic pneumonia is usually mild, but fatal cases are occasionally seen even in farms with optimal health management.
Pneumonic Mannheimiosis (Shipping Fever): Shipping fever (transit fever) is a vague clinical term used to denote acute respiratory diseases that occur in cattle several days or weeks after shipment. The disease is characterized by a severe fibrinous bronchopneumonia, reflecting the fact that death generally occurs early or at an acute stage. Because Mannheimia haemolytica (formerly Pasteurella haemolytica) is typically isolated from affected lungs, the names pneumonic Mannheimiosis and pneumonic pasteurellosis have been used synonymously. It is known that pneumonic Mannheimiosis can occur in animals that have not been shipped and that organisms other than Mannheimia haemolytica can cause similar lesions. Therefore the term shipping fever should be relinquished in favor of more specific names such as pneumonic Mannheimiosis or respiratory histophilosis (hemophilosis).
Pneumonic Mannheimiosis (shipping fever) is the most important respiratory disease of cattle in North America, particularly in feedlot animals that have been through the stressful marketing and assembly processes. Mannheimia haemolytica biotype A, serotype 1 is the etiologic agent responsible for the severe pulmonary lesions. A few investigators still consider that Pasteurella multocida and other serotypes of Mannheimia haemolytica are also causes of this disease.
Even after many years of intense investigation, from the gross lesions to the molecular aspects of the disease, the pathogenesis of pneumonic Mannheimiosis remains incompletely understood. Experiments have established that Mannheimia haemolytica A1 alone is usually incapable of causing disease because it is rapidly cleared by pulmonary defense mechanisms. These findings may explain why Mannheimia haemolytica, in spite of being present in the nasal cavity of healthy animals, only sporadically causes disease. For Mannheimia haemolytica to be established as a pulmonary infection, it is first required that stressors impair the defense mechanisms and allow the bacteria to colonize the lung (see section on Impairment of Defense Mechanisms). These stressors include weaning, transport, fatigue, crowding, mixing of cattle from various sources, inclement weather, temporary starvation, and viral infections. Horizontal transmission of viruses and Mannheimia haemolytica occurs during crowding and transportation of cattle.
Viruses that most commonly predispose cattle to pneumonic Mannheimiosis include BoHV-1, PI-3, and BRSV. Once established in the lungs, Mannheimia haemolytica causes lesions by means of different virulence factors, which include endotoxin, lipopolysaccharide, adhesins, and outer membrane proteins, but the most important is probably the production of a leukotoxin (exotoxin), which binds and kills bovine macrophages and neutrophils. The fact that this toxin exclusively affects ruminant leukocytes probably explains why Mannheimia haemolytica is a respiratory pathogen in cattle and sheep but not in other species. During Mannheimia haemolytica infection, alveolar macrophages, neutrophils, and mast cells release maximum amounts of proinflammatory cytokines, particularly TNF-α, IL-1, IL-8, adhesion molecules, histamine, and leukotrienes. By locally releasing enzymes and free radicals, leukocytes further contribute to the injury and necrosis of bronchiolar and alveolar cells.
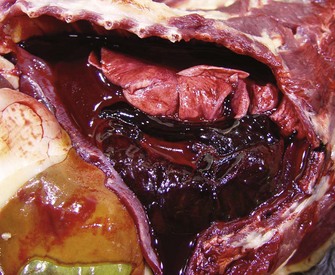
The gross lesions of acute and subacute pneumonic Mannheimiosis are the prototypic fibrinous bronchopneumonia, with prominent fibrinous pleuritis (Fig. 9-71 and Web Fig. 9-10) and pleural effusion. Lesions are always cranioventral and usually ventral to a horizontal line through the tracheal bifurcation. The interlobular septa are distended by yellow, gelatinous edema and fibrin. The “marbling” of lobules is the result of intermixing areas of coagulation necrosis, interlobular interstitial edema, and congestion (Fig. 9-72).
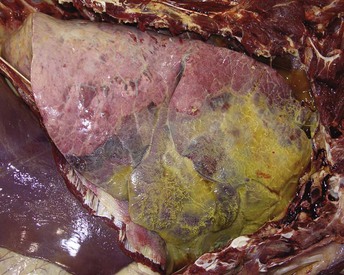
Fig. 9-71 Fibrinous bronchopneumonia (pleuropneumonia), pneumonic Mannheimiosis (Mannheimia haemolytica), right lung, steer.
Note the cranioventral pneumonia involving approximately 85% of the lung parenchyma. The lung is firm and swollen, and the pleura are covered with a thick layer of fibrin. (Courtesy Dr. A. López, Atlantic Veterinary College.)
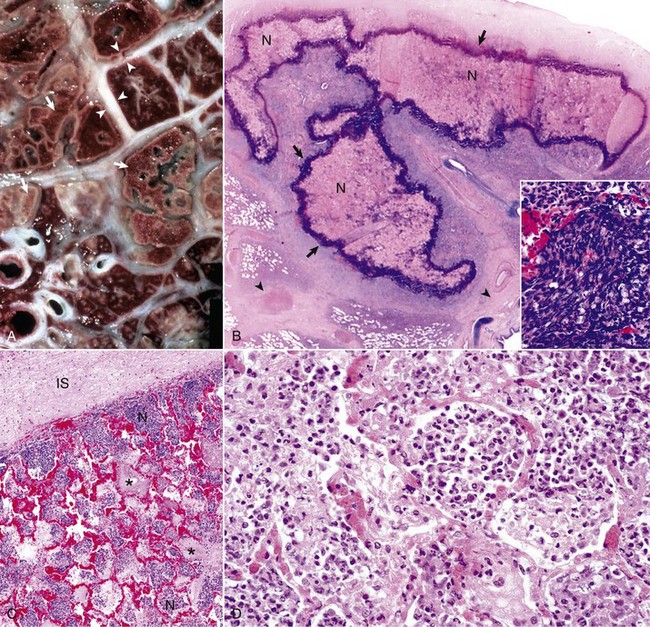
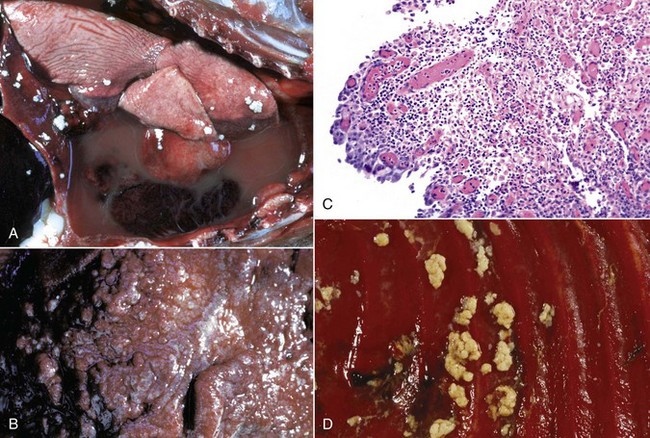
Fig. 9-72 Pneumonic Mannheimiosis (Mannheimia haemolytica), lung, steer.
A, Cut surface. Interlobular septa (arrowheads) are notably distended by edema and fibrin. In the lung parenchyma are irregular areas of coagulative necrosis (arrows) surrounded by a rim of inflammatory cells. B, Note a large irregular area of necrosis (N) of the pulmonary parenchyma. Typically these necrotic areas are surrounded by an outer dense layer of inflammatory cells (arrows). The interlobular septa are distended (arrowheads). Inset (bottom right corner) shows the typical elongated and basophilic appearance of degenerated neutrophils known as oat-shaped cells. H&E stain. C, Note alveoli filled with fibrin (asterisks) and with neutrophils (N). The interlobular septa (IS) is distended with proteinaceous fluid. H&E stain. D, Mannheimia haemolytica produces leukotoxin (cytotoxic for ruminant leukocytes) and lipopolysaccharide. Note the accumulation of cells, chiefly neutrophils, in the alveoli. Also note the active hyperemia of acute inflammation of the alveolar capillaries. H&E stain. (A, B, and C courtesy Dr. A. López, Atlantic Veterinary College. D courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Microscopically, lung lesions are evident 4 hours after experimental infection in which neutrophils fill the bronchial, bronchiolar, and alveolar spaces. Within 24 to 48 hours, the cytotoxic effect of Mannheimia haemolytica is manifested by necrosis of individual alveolar cells and fibrin begins to exude into the alveoli from increased permeability of alveolar capillaries. These changes are exacerbated by endothelial swelling, altered platelet function, increased procoagulant activity, and diminished profibrinolytic activity in the lungs. By 72 hours, alveolar macrophages start to appear in the bronchoalveolar space. At this time, large and irregular areas of coagulative necrosis are typically bordered by a rim of elongated cells often referred to as swirling macrophages or oat-shaped cells, now known to be degenerating neutrophils mixed with a few alveolar macrophages (see Fig. 9-72). In the early stages of the necrosis, there is no evidence of vascular thrombosis, suggesting that necrosis is primarily caused by the cytotoxin of Mannheimia haemolytica and is not the result of an ischemic change. The interlobular septae becomes distended with protein-rich edematous fluid and the lymphatic vessels contain fibrin thrombi. The trachea and bronchi can have considerable amounts of blood and exudate, which are transported by the mucociliary escalator or coughed up from deep within the lungs, but the walls of the trachea and major bronchi may or may not be involved. Because of the necrotizing process, sequelae to pneumonic Mannheimiosis can be serious and can include abscesses, encapsulated sequestra (isolated piece of necrotic lung), chronic pleuritis, fibrous pleural adhesions, and bronchiectasis.
Clinically, pneumonic Mannheimiosis is characterized by a severe toxemia that can kill animals even when considerable parts of the lungs remain functional and structurally normal. Cattle usually become depressed, febrile (104° to 106° F [40° to 41° C]), and anorexic and have a productive cough, encrusted nose, mucopurulent nasal exudate, shallow respiration, or an expiratory grunt.
Hemorrhagic Septicemia: Pneumonic Mannheimiosis should not be confused with hemorrhagic septicemia (septicemic pasteurellosis) of cattle and water buffalo (Bubalus bubalis) caused by inhalation or ingestion of serotypes 6:B and 6:E of Pasteurella multocida. This OIE-notifiable disease does not occur in North America and currently is reported only from some countries in Asia and Africa. In contrast to pneumonic Mannheimiosis, in which lesions are always confined to the lower respiratory tract, the bacteria of hemorrhagic septicemia always disseminates hematogenously to other organs. At necropsy, typically, generalized petechiae are present on the serosal surfaces of the intestine, heart, and lungs and in skeletal muscles. Superficial and visceral lymph nodes are swollen and hemorrhagic. Variable lesions include edematous and hemorrhagic lungs with or without consolidation, hemorrhagic enteritis, blood-tinged fluid in the thorax and abdomen, and subcutaneous edema of the head, neck, and ventral abdomen. Bacteria can be cultured from blood, and animals have high fever and die rapidly (100% case fatality).
Respiratory Histophilosis (Hemophilosis): Respiratory histophilosis is part of the Histophilus somni (Haemophilus somnus) disease complex, which has at least eight different clinicopathologic forms, each one involving different organs. This complex includes septicemia, encephalitis (known as thrombotic meningoencephalitis [TME]), pneumonia (respiratory histophilosis [hemophilosis]), pleuritis, myocarditis, arthritis, ophthalmitis, conjunctivitis, otitis, and abortion. The portals of entry for the different forms of histophilosis have not been properly established.
The respiratory form of bovine histophilosis is the result of the bacterium’s capacity to induce both suppurative and fibrinous bronchopneumonia. The latter is in some cases indistinguishable from that of pneumonic Mannheimiosis. The pathogenesis of respiratory histophilosis is still poorly understood, and the disease cannot be reproduced consistently by administration of Histophilus somni alone. Like Mannheimia haemolytica, it requires predisposing factors such as stress or a preceding viral infection. Histophilus somni is often isolated from the lungs of calves with enzootic pneumonia. The capacity of Histophilus somni to cause septicemia and localized infections in the lungs, brain, eyes, ear, heart, mammary gland, male and female genital organs, or placenta is perhaps attributable to specific virulence factors, such as immunoglobulin-binding proteins (IgBPs) and lipooligosaccharide (LOS). Also, Histophilus somni has the ability to undergo structural and antigenic variation, evade phagocytosis by promoting leukocytic apoptosis, inhibit intracellular killing, reduce transferrin concentrations, and induce endothelial apoptosis in the lungs of affected calves. Mixed pulmonary infections of Histophilus somni, Mannheimia haemolytica, Pasteurella multocida, Arcanobacterium pyogenes, and mycoplasmas are fairly common in calves.
Mycoplasma Bovis Pneumonia: Mycoplasma bovis is the most common Mycoplasma sp. isolated from pneumonic lungs of cattle in Europe and North America. Pulmonary infection is exacerbated by stress or any other adverse factor (e.g., viral infection) that depresses the pulmonary defense mechanisms. Lung lesions are typically those of a chronic necrotizing bronchopneumonia with numerous well-delineated caseated nodules (Fig. 9-73 and Web Fig. 9-11). Microscopically, lesions are quite characteristic and consist of distinct areas of pulmonary necrosis centered around bronchi or bronchioles. The lesion is formed by a core of fine eosinophilic granular debris surrounded by a rim of neutrophils, macrophages, and fibroblasts (see Fig. 9-73). The diagnosis is confirmed by isolation or immunohistochemical labeling of tissue sections for Mycoplasma antigens. Mycoplasma bovis has also been incriminated in arthritis, otitis, mastitis, abortion, and keratoconjunctivitis.
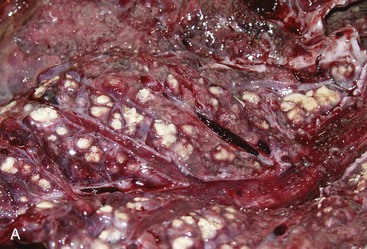
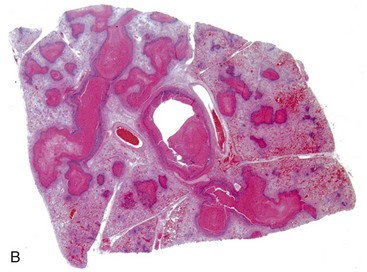
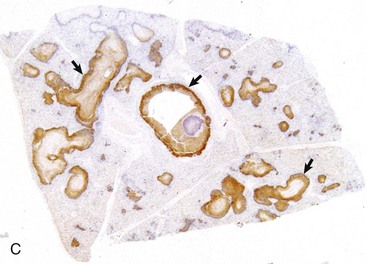
Fig. 9-73 Chronic bronchopneumonia (Mycoplasma bovis), steer.
A, The lung has numerous caseated granulomas scattered throughout the cranioventral lobes. B, Section of lung showing large rounded areas of necrosis filled with hypereosinophilic granular debris. C, Necrotizing bronchopneumonia, immunohistochemistry. Note positive staining for Mycoplasma bovis antigen in the margin of the necrotic lung (arrows). Immunoperoxidase stain. (A courtesy Dr. A. López, Atlantic Veterinary College; B and C courtesy Dr. A. López and Dr. C. Legge, Atlantic Veterinary College.)
Contagious Bovine Pleuropneumonia: Contagious bovine pleuropneumonia is a “List A” OIE-notifiable disease of historic interest in veterinary medicine because it was the object of early national control programs for infectious disease. It was eradicated from North America in 1892 and from Australia in the 1970s, but it is still enzootic in large areas of Africa, Asia, and Eastern Europe. The etiologic agent, Mycoplasma mycoides ssp. mycoides small colony type, was the first Mycoplasma isolated and is one of the most pathogenic of those that infect domestic animals. Natural infection occurs in cattle and Asian buffalo. Portal of entry is aerogenous, and infections occur when a susceptible animal inhales infected droplets. The pathogenic mechanisms are still inadequately understood but are suspected to involve toxin and galactan production, unregulated production of TNF-α, ciliary dysfunction, immunosuppression, and immune-mediated vasculitis. Vasculitis and thrombosis of pulmonary arteries, arterioles, veins, and lymphatics lead to lobular infarction.
The name of the disease is a good indication of the gross lesions. It is a severe, fibrinous bronchopneumonia (pleuropneumonia) similar to that of pneumonic Mannheimiosis (see Figs. 9-71 and 9-72) but having a more pronounced “marbling” of the lobules because of extensive interlobular edema and lymphatic thrombosis. Typically, 60% to 79% of lesions are in the caudal lobes (not cranioventrally), and pulmonary sequestra (necrotic lung encapsulated by connective tissue) are more frequent and larger than pneumonic Mannheimiosis. Unilateral lesions are common in this disease. Microscopically, the appearance again is like that of pneumonic Mannheimiosis, except that vasculitis and thrombosis of pulmonary arteries, arterioles, and capillaries are much more obvious and are clearly the major cause of the infarction and thrombosis of lymphatic vessels in interlobular septa. Mycoplasma mycoides ssp. mycoides small colony type remains viable in the sequestra for many years, and under stress (e.g., starvation), the fibrous capsule may break down, the mycoplasma is released into the airways, and it becomes a source of infection for other animals. Clinical signs are those of severe sepsis, including fever, depression, anorexia followed by severe respiratory signs such as opened-mouth dyspnea and coughing, and crepitation and pleural friction on thoracic auscultation. Vaccination is highly effective in preventing the disease.
Bovine Tuberculosis: Tuberculosis is an ancient, communicable, worldwide, chronic disease of humans and domestic animals. It continues to be a major problem in humans in underdeveloped countries, and it is on the rise in some industrialized nations, largely because of the immunosuppressive effects of AIDS, immigration, and movement of infected animals across borders. The World Health Organization (WHO) estimated that 30 million people, mostly in developing countries, died of tuberculosis between 1990 and 1999. Mycobacterium tuberculosis is transmitted between humans, but where unpasteurized cow’s milk is consumed, Mycobacterium bovis from the milk of cattle with mammary tuberculosis is also an important cause of human tuberculosis.
Bovine tuberculosis is primarily caused by Mycobacterium bovis, but infection with Mycobacterium tuberculosis, the pathogen of human tuberculosis, can occur sporadically. Tuberculosis can be acquired by several routes, but infection of the lungs by inhalation of Mycobacterium bovis is the most common in adult cattle, whereas ingestion of infected milk is more predominant in young animals. Organisms belonging to the Mycobacterium avium-intracellulare complex can also infect cattle, but for infection caused by these organisms, the term mycobacteriosis (not tuberculosis) is currently preferred.
Respiratory infection usually starts when inhaled bacilli reach the alveoli and are phagocytosed by pulmonary alveolar macrophages. If these cells are successful in destroying the bacteria, infection is averted. However, Mycobacterium bovis, being a facultative pathogen of the monocytic-macrophagic system, may multiply intracellularly, kill the macrophage, and initiate infection. From this first nidus of infection, bacilli spread aerogenously via airways within the lungs and eventually via the lymph vessels to tracheobronchial and mediastinal lymph nodes.
The initial focus of infection at the portal of entry (lungs) plus the involvement of regional lymph nodes is termed the primary (Ghon) complex of tuberculosis. If the infection is not contained within this primary complex, bacilli disseminate via the lymph vessels to distant organs and other lymph nodes by the migration of infected macrophages. Hematogenous dissemination occurs sporadically when a granuloma containing mycobacteria erodes the wall of a blood vessel, causes vasculitis, and allows the granuloma to discharge mycobacteria into the alveolar circulation. If dissemination is sudden and massive, mycobacteria are widely disseminated and numerous small foci of infection develop in many tissues and organs and the process is referred to as miliary tuberculosis (like millet seeds). The host becomes hypersensitive to the mycobacterium, which enhances the cell-mediated immune defenses in early or mild infections but can result in host-tissue destruction in the form of caseous necrosis. The evolution and dissemination of the pulmonary infection are closely regulated by cytokines and TNF-α production by alveolar macrophages.
Unlike abscesses that tend to grow rather fast, granulomas evolve slowly at the site of infection. The lesion starts with few macrophages and neutrophils ingesting the offending organism, but because mycobacterium organisms are resistant to phagocytosis, infected macrophages eventually die, releasing viable bacteria, lipids, and cell debris. Cell debris accumulates in the center of the lesion, whereas viable bacteria and bacterial lipids attract additional macrophages and a few lymphocytes at the periphery of the lesion. Some of these newly recruited macrophages are activated by local lymphocytes and become large phagocytic cells with abundant cytoplasm resembling epithelial cells, thus the term epithelioid cells. Multinucleated macrophages also appear at the edges of the lesion, and finally the entire focus of inflammatory process becomes surrounded by fibroblasts and connective tissue. It may take weeks or months for a granuloma to be grossly visible.
Bovine tuberculosis, the prototype for granulomatous pneumonia, is characterized by the presence of a few or many caseated granulomas (see Fig. 9-65). The early gross changes are small foci (tubercles) most frequently seen in the dorsocaudal, subpleural areas. With progression, the lesions enlarge and become confluent with the formation of large areas of caseous necrosis. Calcification of the granulomas is a typical finding in bovine tuberculosis. Single nodules or clusters occur on the pleura and peritoneum, and this presentation has been termed pearl disease. Microscopically the tubercle is composed of mononuclear cells of various types. In young tubercles, which are noncaseous, epithelioid and Langhans’ giant cells are at the center, surrounded by lymphocytes, plasma cells, and macrophages. Later, caseous necrosis develops at the center, secondary to the effects of cell-mediated hypersensitivity and enclosed by fibrosis at the periphery. Acid-fast organisms may be numerous but more often are difficult to find in histologic section or smears.
Clinically, the signs relate to the dysfunction of a particular organ system or to general debilitation, reduced milk production, and emaciation. In the pulmonary form, which is more than 90% of bovine cases, a chronic, moist cough can progress to dyspnea. Enlarged tracheobronchial lymph nodes can contribute to the dyspnea by impinging on airways, and the enlargement of caudal mediastinal nodes can compress the caudal thoracic esophagus and cause bloating.
Interstitial Pneumonias of Cattle: Atypical interstitial pneumonia (AIP) is a vague clinical term well entrenched in veterinary literature, but one that has led to enormous confusion among veterinarians. It was first used to describe acute or chronic forms of bovine pneumonia that did not fit in any of the “classic” forms because of the lack of exudate and lack of productive cough. Microscopically, the criteria for diagnosis of AIP in cattle were based on the absence of obvious exudate and the presence of edema, interstitial emphysema (see the section on Pulmonary Emphysema), hyaline membranes, hyperplasia of type II pneumonocytes, and alveolar fibrosis with interstitial cellular infiltrates. At that time, any pulmonary disease or pulmonary syndrome that had a few of the above lesions was traditionally diagnosed as AIP, and grouping all these different syndromes together was inconsequential because their etiopathogenesis were then unknown.
Field and laboratory investigations have demonstrated that most of the bovine syndromes previously grouped under AIP have rather different causes and pathogeneses (Fig. 9-74). Further, what was “atypical” in the past has become so routine that it is fairly common nowadays to find “typical cases” of AIP. For all these reasons, investigators, largely from Britain, proposed that all these syndromes previously clustered into AIP should be named according to their specific cause or pathogenesis. The most common bovine syndromes characterized by edema, emphysema, hyaline membranes, and hyperplasia of type II pneumonocytes include (1) bovine pulmonary edema and emphysema (fog fever), (2) “extrinsic allergic alveolitis” (hypersensitivity pneumonitis), (3) “reinfection syndromes” (hypersensitivity to Dictyocaulus sp. or BRSV), (4) milk allergy, (5) ingestion of moldy potatoes, (6) Paraquat toxicity, and others.
Fig. 9-74 Schematic diagram of the pathogenesis of toxic and allergic pneumonias (“atypical interstitial pneumonia”) in cattle.
BRSV, Bovine respiratory syncytial virus. (Redrawn with permission from Dr. A. López, Atlantic Veterinary College.)
Acute bovine pulmonary edema and emphysema (fog fever): Acute bovine pulmonary edema and emphysema (ABPEE), known in Britain as fog fever (no association with atmospheric conditions), occurs in cattle usually grazing “fog” pastures (i.e., aftermath or foggage, regrowth after a hay or silage has been cut). Epidemiologically, ABPEE usually occurs in adult beef cattle in the fall when there is a change in pasture, from a short, dry grass to a lush, green grass. It is generally accepted that l-tryptophan present in the pasture is metabolized in the rumen to 3-methylindole, which in turn is absorbed into the bloodstream and carried to the lungs. Mixed function oxidases present in the nonciliated bronchiolar epithelial (Clara) cells metabolize 3-methylindole into a highly pneumotoxic compound that causes extensive and selective necrosis of bronchiolar cells and type I pneumonocytes (see Figs. 9-74 and 9-75) and increases alveolar permeability, leading to edema, thickening of the alveolar interstitium, and alveolar and interstitial emphysema. 3-Methylindole also interferes with the lipid metabolism of type II pneumonocytes.
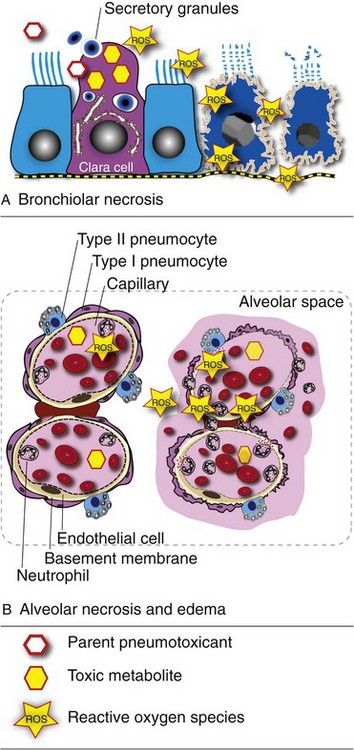
Fig. 9-75 Schematic representation of bronchiolar and alveolar injury caused by pneumotoxicants.
A, Inhaled pneumotoxicants, such as paraquat or 3-methyl-indole, are metabolized into highly reactive compounds (ROS) by bronchiolar Clara cells. ROS reach adjacent bronchiolar cells (blue) by diffusion and cause injury and necrosis (right two cells). Secretory granules contain several proteins such as surfactant-like protein, antiinflammatory protein (CC10) and bronchiolar lining proteins. B, ROS produced by Clara cells are also absorbed into capillaries within the lamina propria and are transferred by the circulatory system to pulmonary capillaries where they also disrupt the air-blood barrier, causing degeneration and necrosis of type I pneumocytes. This process leads to leakage of plasma fluid (alveolar edema [pink color]) and extravasation of erythrocytes (alveolar hemorrhage) and neutrophils (active hyperemia). Ingested pneumotoxicants can also be metabolized by the liver, leading to release of ROS into the circulatory system that then disrupts the air-blood barrier. (Courtesy Dr. A. López, Atlantic Veterinary College.)
The gross lesions are those of a diffuse interstitial pneumonia with severe alveolar and interstitial edema and interlobular emphysema (see Fig. 9-37). The lungs are expanded, pale, and rubbery in texture, and the lesions are most notable in the caudal lobes. Microscopically, the lesions are alveolar and interstitial edema and emphysema, formation of characteristic hyaline membranes within alveoli (see Fig. 9-37), and in those animals that survive for several days, hyperplasia of type II epithelial cells and alveolar interstitial fibrosis.
Clinically, severe respiratory distress develops within 10 days of the abrupt pasture change, and cattle develop expiratory dyspnea, oral breathing, and evidence of emphysema within the lungs and even subcutaneously along the back. Experimentally, reducing ruminal conversion of l-tryptophan to 3-methylindole prevents the development of ABPEE.
A number of other agents cause virtually the same clinical and pathologic syndrome as is seen in ABPEE. The pathogenesis is assumed to be similar, although presumably other toxic factors are specific for each syndrome. One of these pneumotoxic factors is 4-ipomeanol, which is found in moldy sweet potatoes contaminated with the fungus Fusarium solani. Mixed-function oxidases in the lungs activate 4-ipomeanol into a potent pneumotoxicant capable of producing irreversible oxidative injury to type I pneumonocytes and bronchiolar epithelial cells, presumably through lipoperoxidation of cell membranes. Similarly, purple mint (Perilla frutescens), stinkwood (Zieria arborescens), and rapeseed and kale (Brassica species) also cause pulmonary edema, emphysema, and interstitial pneumonia.
Extrinsic allergic alveolitis: Extrinsic allergic alveolitis (hypersensitivity pneumonitis), one of the most common allergic diseases in cattle, is seen mainly in housed, adult dairy cows in the winter. This disease shares many similarities with its human counterpart known as farmer’s lung, which results from a type III hypersensitivity reaction to inhaled organic antigens, most commonly fungal spores, mainly of the thermophilic actinomycete, Saccharopolyspora rectivirgula (Micropolyspora faeni), commonly found in moldy hay. This is followed by an antibody response to inhaled fungal spores and local deposition of antigen-antibody complexes (Arthus reaction) in the lungs (see Fig. 9-74). Because it affects only a few animals of the herd or the sporadic person working in a farm, it is presumed that intrinsic host factors, such as dysregulation of dendritic cells, T lymphocytes, IgG, interleukins, IFN-γ, and surfactant, are involved in the pathogenesis of the disease. Clinically, it can be acute or chronic; the latter has a cyclical pattern of exacerbation during winter months. Weight loss, coughing, and poor exercise tolerance are clinical features.
Grossly, the postmortem lesions vary from subtle, gray, subpleural foci (granulomatous inflammation) to severe lesions, in which the lungs are firm and heavy and have a “meaty appearance” because of type II alveolar epithelial hyperplasia, lymphocytic infiltration, and interstitial fibrosis. Characteristically, discrete noncaseous granulomas formed in response to the deposition of antigen-antibody complexes are scattered throughout the lungs. Chronic cases of extrinsic allergic alveolitis can eventually progress to diffuse fibrosing alveolitis. Full recovery can occur if the disease is recognized and treated early.
Reinfection syndrome: Hypersensitivity to reinfection with larvae of Dictyocaulus viviparus is another allergic syndrome manifested in the lungs that causes signs and lesions indistinguishable from ABPEE, with the exception of eosinophils and possibly larvae in the alveolar exudate. The hypersensitivity reaction in the lung causes diffuse alveolar damage and edema, necrosis of type I pneumonocytes and hyperplasia of type II pneumonocytes. In the later stages of the disease there is formation of small granulomas with interstitial infiltrates of mononuclear cells.
It has been suggested that emphysema with diffuse proliferative alveolitis and formation of hyaline membranes can also occur sporadically in the late stages of BRSV infection in cattle. Presumably, this disease shares many similarities with “atypical” infections occasionally seen in children with respiratory syncytial virus (RSV human strain), in which a hypersensitivity to the virus or virus-induced augmentation of the immune response results in hypersensitivity pneumonitis (see Fig. 9-74).
Other Forms of Bovine Interstitial Pneumonia: Inhalation of manure (“pit”) gases, such as NO2, H2S, and ammonia (NH3), from silos or sewage can be a serious hazard to animals and humans. At toxic concentrations, these gases cause necrosis of bronchiolar cells and type I pneumonocytes, a fulminating pulmonary edema that causes asphyxiation, and rapid death (see Fig. 9-42). Like other oxidant gases, inhalation of NO2 (silo gas) also causes bronchiolitis, edema, and interstitial pneumonia and in survivors, bronchiolitis obliterans (“silo filler’s disease”).
Smoke inhalation resulting from barn or house fires is sporadically seen by veterinarians and pathologists. In addition to skin burns, animals involved in fire accidents suffer extensive thermal injury produced by the heat on the nasal and laryngeal mucosa, and severe chemical irritation caused by inhalation of combustion gases and particles in the lung. Animals that survive or are rescued from fires frequently develop nasal, laryngeal, and tracheal edema, and pulmonary hemorrhage and alveolar edema, which are caused by chemical injury to the blood-air barrier or by ARDS caused by the excessive production of free radicals during the pulmonary inflammatory response. Microscopic examination of the lungs often reveals carbon particles (soot) on mucosal surfaces of the conducting system.
Parasitic Pneumonias of Cattle:
Verminous pneumonia (Dictyocaulus viviparus): Pulmonary lesions in parasitic pneumonias (the word is used here in its restricted sense to mean helminth infestations of the lungs) vary from interstitial pneumonia caused by migrating larvae to chronic bronchitis from intrabronchial adult parasites, to granulomatous pneumonia, which is caused by dead larvae, aberrant parasites, or eggs of parasites. In many cases, an “eosinophilic syndrome” in the lungs is characterized by infiltrates of eosinophils in the pulmonary interstitium and bronchoalveolar spaces and by blood eosinophilia. Atelectasis and emphysema secondary to the obstruction of airways by parasites and mucous secretions are also common findings in parasitic pneumonias. The severity of these lesions relates to the numbers and size of the parasites and the nature of the host reaction, which sometimes includes hypersensitivity reactions (see re-infection syndrome). A common general term for all of these diseases is verminous pneumonia, and the adult nematodes are often visible grossly in the airways (Fig. 9-76).
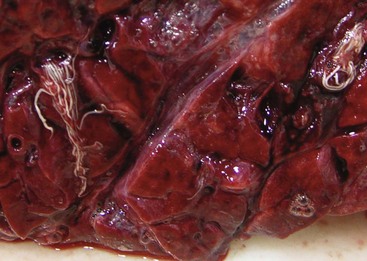
Fig. 9-76 Verminous pneumonia (Dictyocaulus viviparus), bronchus, calf.
The bronchi contains numerous slender lungworms and large amounts of clear foamy fluid, indicative of pulmonary edema. (Courtesy Dr. A. López and Dr. L. Miller, Atlantic Veterinary College.)
Dictyocaulus viviparus is an important pulmonary nematode (lungworm) responsible for a disease in cattle referred to as verminous pneumonia or verminous bronchitis. Adult parasites live in the bronchi of cattle, mainly in the caudal lobes and cause severe bronchial irritation, bronchitis, and pulmonary edema, which in turn is responsible for lobular atelectasis and interstitial emphysema. Atelectasis is confined to the lobules of the lungs ventilated by the obstructed bronchi (dorsocaudal). Interstitial emphysema (interlobular) is caused by forced expiratory movements against a partially obstructed single bronchus. In addition to the inflammation of bronchial mucosa, bronchoaspiration of larvae and eggs also causes an influx of leukocytes into the bronchoalveolar space (alveolitis). Verminous pneumonia is most commonly seen in calves during their first summer grazing pastures that are repeatedly used from year to year, particularly in regions of Europe that have a moist, cool climate. The parasite can overwinter in pastures, even in climates as cold as Canada’s, and older animals may be carriers for a considerable length of time.
At necropsy, lesions appear as dark or gray, depressed, wedge-shaped areas of atelectasis involving few or many lobules usually along the dorsocaudal aspect of the lungs. On a cut surface, edematous foam and mucus mixed with white, slender (up to 80-mm long) nematodes are visible in the bronchi (see Fig. 9-76). In the most severe cases, massive numbers of nematodes fill the bronchial tree. Microscopically the bronchial lumens are filled with parasites admixed with mucus because of goblet cell hyperplasia, and there is also squamous metaplasia of the bronchial and bronchiolar epithelium because of chronic irritation. There is also alveolar edema; hyperplasia of BALT caused by persistent immunologic stimuli; hypertrophy and hyperplasia of bronchiolar smooth muscle because of increased contraction and decreased muscle relaxation; and a few eosinophilic granulomas around the eggs and dead larvae. These granulomas, grossly, are gray, noncaseated nodules (2 to 4 mm in diameter) and may be confused with those seen at the early stages of tuberculosis.
The clinical signs (coughing) vary with the severity of infection, and severe cases can be confused clinically with interstitial pneumonias. Expiratory dyspnea and death can occur with heavy parasitic infestations when there is massive obstruction of airways.
A different form of bovine pneumonia, an acute allergic reaction known as re-infection syndrome, occurs when previously sensitized adult cattle are exposed to large numbers of larvae (Dictyocaulus viviparus). Lesions in this syndrome are those of a hypersensitivity pneumonia as previously described.
Other lung parasites: Ascaris suum is the common intestinal roundworm of pigs; larvae cannot complete their life cycle in calves, but the larvae can migrate through the lungs and cause severe pneumonia and the death of calves within 2 weeks of infection, usually acquired from the soil in which infested pigs were previously kept. Pigs, the natural host, also can be killed if exposed to an overwhelming larval migration. Clinical signs as a result of migration of larvae through the lungs include cough and expiratory dyspnea to the point of oral breathing. The gross lesions are a diffuse interstitial pneumonia with hemorrhagic foci, atelectasis, and interlobular edema and emphysema, similar to what is seen in the lungs of pigs (see Fig. 9-62). Microscopically, there are focal intraalveolar hemorrhages caused by larvae migrating through the alveolar walls. Some larvae admixed with edematous fluid and cellular exudate (including eosinophils) may be visible in bronchioles and alveoli. The alveolar walls are thickened because of edema and a few inflammatory cells.
Hydatid cysts, the intermediate stage of Echinococcus granulosus, can be found in the lungs and liver and other viscera of sheep and to a lesser extent in cattle, pigs, goats, horses, and humans. The adult stage is a tapeworm that parasitizes the intestine of Canidae. Hydatidosis is still an important zoonosis in some countries, and perpetuation of the parasite life cycle results from animals being fed uncooked offal from infected sheep and consumption of uninspected meat. Hydatid cysts are generally 5 to 15 cm in diameter, and numerous cysts can be found in the viscera of affected animals (Fig. 9-77). Each parasitic cyst is filled with clear fluid; numerous daughter cysts attach to the wall, each containing several “brood capsules” with protoscolices inside. Hydatid cysts have little clinical significance in animals but are economically important because of carcass condemnation.
Aspiration Pneumonias of Cattle: The inhalation of regurgitated ruminal contents or iatrogenic deposition of medicines or milk into the trachea can cause severe and often fatal aspiration pneumonia. Bland substances, such as mineral oil, may incite only a mild suppurative or histiocytic bronchopneumonia, whereas some “home remedies” or ruminal contents are highly irritating and cause a fibrinous, necrotizing bronchopneumonia. The right cranial lung lobe tends to be more severely affected because the right cranial bronchus is the most cranial branch and enters the ventrolateral aspect of the trachea. However, the distribution may vary when animals aspirate while in lateral recumbency. In some severe cases, pulmonary necrosis can be complicated by infection with saprophytic organisms present in ruminal contents, causing fatal gangrenous pneumonia. Aspiration pneumonia should always be considered in animals whose swallowing has been compromised, for example those with cleft palate or hypocalcemia (milk fever). On the other hand, neurological diseases such as encephalitis (e.g., rabies) or encephalopathy (e.g., lead poisoning) should be investigated in animals in which the cause of aspiration pneumonia could not be explained otherwise. Depending on the nature of the aspirated material, histopathologic evaluation generally reveals foreign particles such as vegetable cells, milk droplets, and large numbers of bacteria in bronchi, bronchioles, and alveoli. Vegetable cells and milk typically induce an early neutrophilic response followed by a histocytic reaction with “foreign body” multinucleated giant cells. Special stains are used for the microscopic confirmation of aspirated particles in the lung (e.g., PAS for vegetable cells and O-red oil for oil or milk droplets).
Pneumonias of Sheep and Goats: In the past, Pasteurella haemolytica was incriminated in four major ovine diseases known as (1) acute ovine pneumonic pasteurellosis (shipping fever), (2) enzootic pneumonia (nonprogressive chronic pneumonia), (3) fulminating septicemia, and (4) mastitis. Under the new nomenclature, Mannheimia haemolytica is responsible for ovine pneumonia resembling shipping fever in cattle (ovine pneumonic Mannheimiosis), septicemia in young lambs (under 3 months of age), and ovine enzootic pneumonia and sporadic severe gangrenous mastitis in ewes. Bibersteinia (Pasteurella) trehalosi (formerly Pasteurella haemolytica biotype T) is the agent incriminated in septicemia in lambs 5 to 12 months old.
Ovine Pneumonic Mannheimiosis: Ovine pneumonic Mannheimiosis is one of the most common and economically significant diseases in most areas where sheep are raised. It is caused by Mannheimia haemolytica and has pathogenesis and lesions similar to those of pneumonic Mannheimiosis of cattle. Colonization and infection of lungs are facilitated by stressors, such as changes in weather, handling, deworming, or dipping, and viral infections, such as PI-3 virus, RSV, adenovirus, and probably chlamydia and Bordetella parapertussis. Lesions are characterized by a severe fibrinous bronchopneumonia (cranioventral) with pleuritis (Fig. 9-78). Subacute to chronic cases progress to purulent bronchopneumonia, and sequelae include abscesses and fibrous pleural adhesions.
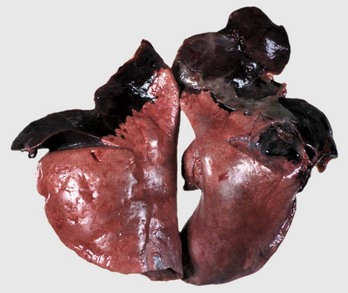
Fig. 9-78 Acute fibrinous bronchopneumonia (pleuropneumonia), pneumonic Mannheimiosis (Mannheimia haemolytica), lungs, lamb.
The cranioventral aspects of the lung are red, swollen, and very firm (consolidated) with some fibrin on the pleural surface. Note that the consolidated lung resembles liver, a change that was previously referred to as “hepatization.” (Courtesy Ontario Veterinary College.)
Chronic Enzootic Pneumonia: In sheep, this entity is a multifactorial disease complex that, in contrast to ovine pneumonic Mannheimiosis, causes only a mild-to-moderate pneumonia, and it is rarely fatal. It generally affects animals younger than 1 year of age. Significant costs associated with chronic enzootic pneumonia include reduction of weight gain, labor costs, veterinary fees, and slaughterhouse waste. The modifier “chronic” is used here to avoid any confusion with pneumonic Mannheimiosis (“acute enzootic pneumonia”). It is also sometimes called atypical pneumonia, chronic nonprogressive pneumonia, proliferative pneumonia, or other names.
Chronic enzootic pneumonia is a clinical epidemiologic term and does not imply a single causal agent but is the result of a combination of infectious, environmental, and managerial factors. The list of infectious agents involved in ovine enzootic pneumonia includes Mannheimia haemolytica, Pasteurella multocida, PI-3, adenovirus, reovirus, RSV, chlamydia, and mycoplasmas (Mycoplasma ovipneumoniae).
In the early stages of the disease, a cranioventral bronchointerstitial pneumonia is characterized by moderate thickening of alveolar walls because of hyperplasia of type II pneumonocytes. In some cases, when lungs are infected with secondary pathogens, such as Pasteurella multocida, pneumonia may progress to fibrinous or suppurative bronchopneumonia. One might expect some specific evidence pointing to the infectious agents (e.g., large intranuclear inclusion bodies in epithelial cells with adenoviral infection), but this is often not the case, either because examination is seldom done at the acute stage when the lesions are still present or because secondary bacterial infections mask the primary lesions. In the late stages, chronic enzootic pneumonia is characterized by hyperplastic bronchitis, atelectasis, alveolar and peribronchiolar fibrosis, and marked peribronchial lymphoid hyperplasia (cuffing pneumonia).
Septicemic Pasteurellosis: Septicemic pasteurellosis, a common ovine disease, is caused by Bibersteinia trehalosi (formerly Pasteurella trehalosi or Mannheimia haemolytica biotype T) in lambs 5 months or older, or by Mannheimia haemolytica (biotype A) in lambs younger than 2 months of age. Both organisms are carried in the tonsils and oropharynx of clinically healthy sheep, and under abnormal circumstances, bacteria can invade adjacent tissues, enter the bloodstream, and cause septicemia, particularly under stress from dietary or environmental changes. Affected animals usually die within few hours of infection, and these animals only rarely have clinical signs such as dullness, recumbency, and dyspnea. Gross lesions include a distinctive necrotizing pharyngitis and tonsillitis, ulcerative esophagitis, severe congestion and edema of the lungs, focal hepatic necrosis, and petechiae in the mucosa of the tongue, esophagus, and intestine and particularly in the lungs and pleura. Microscopically, the hallmark lesion is a disseminated intravascular thrombosis often with bacterial colonies in the capillaries of affected tissues. The alveolar capillaries contain bacteria and microrhombi, and the alveolar lumens have fibrin and red blood cells. Mannheimia haemolytica and Bibersteinia trehalosi are readily isolated from many organs.
Contagious Caprine Pleuropneumonia: Contagious caprine pleuropneumonia in goats is the counterpart of contagious bovine pleuropneumonia in cattle; sheep do not have a corresponding disease. The etiopathogenesis and geographic distribution of contagious caprine pleuropneumonia are yet to be determined. Three mycoplasmas, namely Mycoplasma mycoides ssp. mycoides large colony type, Mycoplasma mycoides ssp. capri, and Mycoplasma capricolum ssp. capripneumoniae, can produce respiratory tract infections; however, only the last is considered to cause typical contagious caprine pleuropneumonia.
Caprine pleuropneumonia (OIE-notifiable disease) is important in Africa, the Middle East, and parts of Asia but is also seen elsewhere. Mycoplasma mycoides ssp. mycoides large colony type and Mycoplasma mycoides ssp. capri are present in North America and have been isolated from respiratory diseases of goats.
The gross lesions caused by Mycoplasma capricolum ssp. capripneumoniae are similar to those of the bovine disease and consist of a severe, often unilateral fibrinous bronchopneumonia and pleuritis; however, distention of the interlobular septa (which are normally not as well developed in sheep as in cattle) and formation of pulmonary sequestra are less obvious than in the bovine disease. Fibrinous polyarthritis, septicemia, meningitis, mastitis, peritonitis, and abortion are other possible manifestations of disease caused by Mycoplasma mycoides ssp. mycoides large colony type and Mycoplasma mycoides ssp. capri. The pathogenicity of other mycoplasmas, such as Mycoplasma ovipneumoniae, Mycoplasma arginini, and Mycoplasma capricolum ssp. capricolum, in sheep and goats is still being defined, and specific description of the lesions would be premature. These organisms probably cause disease only in circumstances similar to those for enzootic pneumonia, where host, infectious, and environmental factors create a complex interaction in the pathogenesis of the disease. Most recently, it has been suggested that IgG antibodies directed against ovine mycoplasmal antigens cross-react with ciliary proteins, causing inflammation and ciliary dysfunction, a condition in lambs referred to as coughing syndrome. Clinically, contagious caprine pleuropneumonia is similar to contagious bovine pleuropneumonia, with high morbidity and mortality, fever, cough, dyspnea, and increasing distress and weakness.
Maedi (Maedi-Visna): Maedi is an important, lifelong, and persistent viral disease of sheep and occurs in most countries, except Australia and New Zealand. Maedi means “shortness of breath” in the Icelandic language, and it is known as Graaff-Reinet disease in South Africa, Zwoegerziekte in The Netherlands, La bouhite in France, and ovine (Montana or Marsh’s) progressive pneumonia (OPP) in the US. More recently, the disease has also been referred to as ovine lentivirus-induced lymphoid interstitial pneumonia or simply, lymphoid interstitial pneumonia (LIP).
Maedi is caused by a nononcogenic small ruminant lentivirus (ovine lentivirus) of the family Retroviridae antigenically related to the lentivirus causing caprine arthritis-encephalitis. Seroepidemiologic studies indicate that infection is widespread in the sheep population, yet the clinical disease seems to be rare.
The pathogenesis is incompletely understood, but it is known that transmission occurs largely through ingestion of infected colostrum and to a lesser extent by close contact between infected and susceptible sheep. Once in the body, the ovine lentivirus remains for long periods of time within monocytes and macrophages, including alveolar and pulmonary intravascular macrophages; clinical signs do not develop until after a long incubation period of 2 years or more.
Pulmonary lesions at the time of death are a severe interstitial pneumonia, and the lungs fail to collapse when the thorax is opened. Notable rib imprints, indicators of uncollapsed lungs, are often present on the pleural surface (Fig. 9-79). The lungs are pale and mottled and typically heavy (2 to 3 times normal weight), and the tracheobronchial lymph nodes are enlarged. Microscopically, the interstitial pneumonia is characterized by BALT hyperplasia and thickening of alveolar walls and peribronchial interstitial tissue by heavy infiltration of lymphocytes, largely T lymphocytes (see Fig. 9-61). Recruitment of mononuclear cells into the pulmonary interstitium is presumably the result of sustainable production of cytokines by retrovirus-infected pulmonary macrophages and lymphocytes. Hyperplasia of type II pneumonocytes is not a prominent feature of maedi likely because in this disease there is no injury to type I pneumonocytes, but there is some alveolar fibrosis and smooth muscle hypertrophy in bronchioles. Secondary bacterial infections often cause concomitant bronchopneumonia. Enlargement of regional lymph nodes (tracheobronchial) is because of severe lymphoid hyperplasia, primarily of B lymphocytes. The virus also can infect many other tissues, causing nonsuppurative encephalitis (visna), lymphocytic arthritis, lymphofollicular mastitis, and vasculitis.
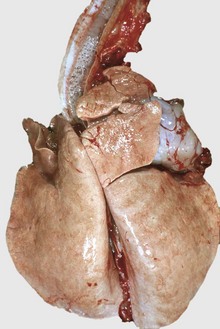
Fig. 9-79 Interstitial pneumonia (unknown etiology), lung, sheep.
The lungs are heavy, rubbery, and show costal (rib) imprints on the visceral pleural surface. The diffuse distribution is typical of interstitial pneumonia. The trachea contains some edema fluid. (Courtesy Western College of Veterinary Medicine.)
Maedi is clinically characterized by dyspnea and an insidious, slowly progressive emaciation despite good appetite. Death is inevitable once clinical signs are present, but may take many months.
Caprine Arthritis-Encephalitis: Caprine arthritis-encephalitis (CAE) is a retroviral disease of goats (small ruminant lentivirus) that has a pathogenesis remarkably similar to that of maedi-visna in sheep. It was first described in the US in the 1970s, but also occurs in Canada, Europe, Australia, and probably elsewhere. This disease has two major clinicopathologic forms: One involves the central nervous system of goat kids and young goats and is characterized by a nonsuppurative leukoencephalomyelitis; the other form involves the joints of adult goats and is characterized by a chronic, nonsuppurative arthritis-synovitis. In addition, infection with CAE virus can cause chronic lymphocytic interstitial pneumonia.
The lentivirus of CAE is closely related to the maedi-visna virus, and, in fact, cross infection with CAE virus in sheep has been achieved experimentally. Similar to maedi, CAE infection presumably occurs during the first weeks of life when the doe transmits the virus to her offspring through infected colostrum or milk. Horizontal transmission between infected and susceptible goats has also been described. After coming into contact with mucosal cells at the portal of entry, the virus is phagocytized by macrophages which migrate to the regional lymph nodes. Infected macrophages are disseminated hematogenously to the central nervous system, joints, lungs, and mammary glands. Like maedi, there is some evidence that the recruitment of lymphocytic cells results from dysregulation of cytokine production by infected macrophages and lymphocytes in affected tissues. It can take several months before serum antibodies can be detected in infected goats.
Grossly, the interstitial pneumonia is diffuse and tends to be most severe in the caudal lobes. The lungs are gray-pink and firm in texture with numerous, 1- to 2-mm, gray-white foci on the cut surface (Fig. 9-80). The tracheobronchial lymph nodes are consistently enlarged. Microscopically, the alveolar walls are thickened by lymphocytes and conspicuous hyperplasia of type II pneumonocytes. One important difference between the pneumonias of CAE and maedi is that in CAE the alveoli is filled with proteinaceous eosinophilic material, which in electron micrographs has structural features of pulmonary surfactant. The pulmonary form of CAE can be mistaken for parasitic pneumonia (Muellerius capillaris) because these two diseases have lymphocytic interstitial pneumonia and can coexist in the same goat.
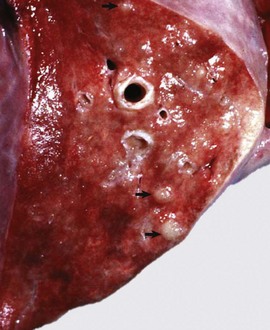
Fig. 9-80 Interstitial pneumonia with alveolar proteinosis, lung, cut surface, sheep.
Note the gray nodules (arrows) and meaty appearance of the lung. These lesions are seen in sheep with the caprine arthritis-encephalitis-pneumonia disease complex. (Courtesy Dr. J.M. King, College of Veterinary Medicine, Cornell University.)
Clinically, goats are active and afebrile but progressively lose weight in spite of normal appetites. The encephalitic or arthritic signs tend to obscure the respiratory signs, which are only evident on exertion. Secondary bacterial bronchopneumonia is common in affected animals.
Tuberculosis: Tuberculosis is uncommon in sheep and goats, but infection with Mycobacterium bovis or with the Mycobacterium avium complex does occur when the disease is prevalent in other species in the locality. The pulmonary form, similar to that seen in cattle, is characterized by a granulomatous pneumonia with multiple, large, caseous, calcified, and well-encapsulated granulomas scattered throughout the lungs. Intralesional acid-fast organisms within macrophages are not as abundant as in bovine tuberculosis.
Parasitic Pneumonias of Sheep and Goats: Dictyocaulus filaria is a serious, worldwide, parasitic disease of the lungs, most commonly of lambs and goat kids, but occurring in adults as well. The life cycle and lesions are similar to those of Dictyocaulus viviparus of cattle. As seen in cattle with Dictyocaulus viviparus, areas of atelectasis secondary to bronchiolar obstruction are present, particularly along the dorsal caudal aspects of the caudal lung lobes. Microscopically, affected lungs are characterized by a catarrhal, eosinophilic bronchitis, with peribronchial lymphoid hyperplasia and smooth muscle hyperplasia of bronchi and bronchioles. Bronchioles and alveoli can contain edematous fluid, eosinophils, and parasitic larvae and eggs. Microscopic granulomas caused by aspirated eggs can be observed in the distal lung. The clinical signs (cough, moderate dyspnea, and loss of condition) and lesions relate mainly to obstruction of the small bronchi by adult worms and filaria. Anemia of undetermined pathogenesis and secondary bacterial pneumonia are common in small ruminants with this parasitic disease.
Muellerius capillaris, also called the nodular lungworm, occurs in sheep and goats in most areas of the world and is the most common lung parasite of sheep in Europe and Northern Africa. It requires slugs or snails as intermediate hosts. The lesions in sheep are typically multifocal, subpleural nodules that tend to be most numerous in the dorsal areas of the caudal lung lobes (Fig. 9-81). These nodules are soft and hemorrhagic in the early stages but later become gray-green and hard or even calcified. Microscopically, a focal, eosinophilic, and granulomatous reaction occurs in the subpleural alveoli where the adults, eggs, and coiled larvae reside (see Fig. 9-81). Clinical signs are usually not apparent.
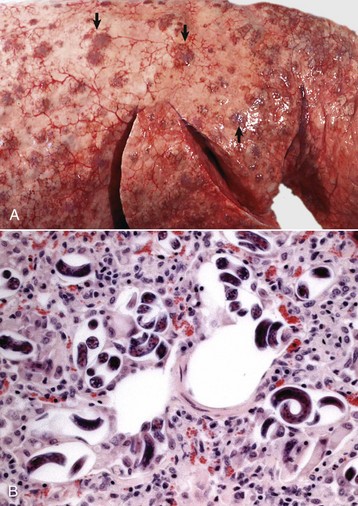
Fig. 9-81 Multifocal granulomatous pneumonia, lungworms (Muellerius spp.), lungs, sheep.
A, Multiple gray nodules (granulomas) (arrows) are scattered throughout the pulmonary parenchyma. On palpation, the lungs have a nodular texture. B, Coiled larvae of Muellerius spp. in the lung. Note also mononuclear cells extending into the surrounding pulmonary interstitium. H&E stain. (A courtesy Dr. J. Edwards, Texas A&M University, Olafson Short Course, Cornell Veterinary Medicine. B courtesy Dr. A. López, Atlantic Veterinary College.)
Goats differ from sheep by having diffuse interstitial rather than focal lesions, and the reaction to the parasites seen microscopically varies from almost no lesions to a severe interstitial pneumonia with heavy infiltrates of mononuclear cells in alveolar walls resembling CAE or mycoplasmal infections. Secondary effects of Muellerius capillaris infection in sheep and goats include decreased weight gain and possibly secondary bacterial infections.
Protostrongylus rufescens is a worldwide parasite of sheep, goats, and wild ruminants. It requires an intermediate snail as a host. Infection is usually subclinical, but Protostrongylus rufescens can be pathogenic for lambs and goat kids and can cause anorexia, diarrhea, weight loss, and mucopurulent nasal discharge. The adult parasite lives in bronchioles as Dictyocaulus spp., but it causes pulmonary nodules similar to those of Muellerius capillaris.
Pneumonias of Pigs: Porcine pneumonias are unequivocally a major component of the problems facing the contemporary swine industry. The incidence, prevalence, and mortality rates of pneumonias in pigs depend on a series of complex, multifactorial interactions. Among the most commonly recognized elements linked to porcine pneumonias are the following:
• Host (age, genetic makeup, immune status)
• Infectious agents (viruses, bacteria, mycoplasmas)
• Environmental determinants (humidity, temperature, ammonia concentrations)
• Management practices (crowding, mixing of animals, air quality, nutrition, stress)
Because of the nature of these multifactorial interactions, it will become obvious in the following paragraphs that more often than not a specific type of pneumonia frequently progresses to or coexists with another one. The term porcine respiratory disease complex (PRDC) has been introduced in clinical practice to describe pigs with signs of respiratory infection involving one or more viruses, bacteria, and mycoplasmas.
Swine Influenza (Swine Flu): It is generally accepted that swine influenza resulted from adaptation of the type A influenza virus, the cause of the human influenza pandemic during World War I. Swine influenza is enzootic worldwide and is known to infect humans who are in close contact with sick pigs. In 2009, an outbreak of swine-human influenza (H1N1), presumably transmitted from pigs to humans, emerged in Mexico and rapidly spread to many countries around the world. This new “pandemic” was attributed to a triple-reassortant of influenza A virus containing gene segments of swine, Eurasian avian, and human strains. Human infection with this novel strain affected mainly children and young adults, as well as individuals of any age with an underlying debilitating condition.
Transmission between influenza-infected and susceptible pigs occurs mainly by aerosols or oral route. The infection of epithelial cells spreads rapidly throughout the nasal, tracheal, and bronchial mucosa, with the more severe outbreaks reflecting more involvement of intrapulmonary airways and secondary infection with Pasteurella multocida, Arcanobacterium pyogenes, or Haemophilus spp. Although uncommon, humans infected with swine influenza (H1N1) can pass the virus back to pigs, therefore it is important that veterinarians or workers with influenza-like illness should stay away from pig farms. Natural transmission of H1N1 and H5N1 from humans to ferrets (Mustela putorius furo) and from humans to cats and dogs has been recently reported.
Pulmonary lesions caused by influenza virus alone are rarely seen in the postmortem room because this disease has very low mortality rate unless complicated with secondary bacterial infections. Grossly a copious catarrhal to mucopurulent inflammation extends from the nasal passages to the bronchioles, the volume of mucus being sufficient to plug small airways and cause a lobular or multilobular atelectasis in the cranioventral regions of the lungs. The appearance can be quite similar grossly, although not microscopically, to that of Mycoplasma pneumoniae. Fatal cases have severe alveolar and interstitial pulmonary edema. Microscopically, the lesions in uncomplicated cases are typical of a virus-induced, necrotizing bronchitis-bronchiolitis, which in severe cases extends into the alveoli as bronchointerstitial pneumonia. It is characterized by thickening and infiltration of the alveolar wall with mononuclear cells and aggregates of macrophages, neutrophils, mucus, and some necrotic cells within the alveolar lumen. If these changes are extensive enough, the lumen of bronchioles can be occluded by exudate, causing lobular atelectasis. Viral antigen can be demonstrated in infected epithelial cells by immunoperoxidase techniques. In the later stages of alveolar inflammation, neutrophils are progressively replaced by intraalveolar macrophages, unless the pneumonia is complicated by secondary bacterial infections. Recent serologic surveys indicate that infection is also prevalent in wild pigs.
Clinically, a sudden onset of painful and often paroxysmal coughing is followed by respiratory distress, nasal discharge, high fever, stiffness, and weakness or even prostration in most or the entire herd, including animals of all age groups. The outbreak subsides virtually without mortality within a week; the clinical appearance is much more alarming than the pathologic changes, unless the pigs have secondary infection with bacteria. Infection can be confirmed using PCR in secretions collected with nasal swabs. The most important effect of most outbreaks of influenza is severe weight loss, but pregnant sows also abort or give birth to weak piglets.
Porcine Reproductive and Respiratory Syndrome: A disease originally named mystery disease of swine was first recognized in the US in 1987. In 1990, it was also seen in Europe, and since then, PRRS has been reported in many Latin American and Asian countries. In 1991, Dutch investigators isolated a virus as the etiologic agent, which is currently classified under the Arterivirus group.
As its name implies, PRRS is characterized by late-term abortions and stillbirths and respiratory problems in young pigs. The respiratory form is generally seen in nursing or young pigs. The pathogenesis has not been completely elucidated, but it is presumed that there is a mucosal portal of entry with virus replication in local macrophages, followed by transient viremia and finally dissemination of infected macrophages to the lungs and other organs, such as the thymus, liver (Kupffer cells), spleen, all lymph nodes, and intestine. The PRRS virus induces apoptosis in many cells including the pulmonary intravascular macrophages, The virus also deregulates the adaptive immune response and interferes with the normal defense mechanisms predisposing pigs to septicemia and bacterial pneumonia. The most common opportunistic organisms are Streptococcus suis, Haemophilus parasuis, Bordetella bronchiseptica, Pasteurella multocida, and Pneumocystis carinii. Dual viral infections with PRRS virus and porcine circovirus-2 (PCV-2) are quite commonly found in pigs.
On postmortem examination, pulmonary lesions vary from very mild changes characterized by failure of the lung to collapse when the thorax is opened and the presence of rib imprints (see Fig. 9-61) to severe changes manifested by consolidation of the lung in cases that have been complicated with bacterial pneumonia. Tracheobronchial and mediastinal lymph nodes are typically enlarged. Microscopically, pulmonary changes are those of interstitial pneumonia characterized by thickening of alveolar walls by infiltrating macrophages and lymphocytes and mild hyperplasia of type II pneumonocytes. Necrotic cells are scattered in the alveolar lumens. Unlike some other viral infections, bronchiolar epithelium does not appear to be affected. Diagnosis of PRRS in tissue collected at necropsy can be confirmed by immunohistochemistry and PCR techniques. Infected pigs may become carriers and transmit the infection through body fluids and semen. Clinically, PRRS is characterized by anorexia, dyspnea, cough, and occasional death. Some piglets develop severe cyanosis of the abdomen and ears, which explains why this syndrome when first described in Europe was named blue ear disease.
Postweaning Multisystemic Wasting Syndrome: Another emerging porcine syndrome, characterized clinically by progressive emaciation in weaned pigs, was originally described in the 1990s in Canada, the US, and Europe. Since then, it has disseminated to many countries, causing economic devastation in pig farms worldwide. Because of the clinical signs and lesions in many organs, this syndrome was named postweaning multisystemic wasting syndrome (PMWS); a PCV-2 has been incriminated as the etiologic agent.
At necropsy, affected pigs are in poor body condition, and the most remarkable changes, not considering other possible secondary infections, are enlargement of the superficial and visceral lymph nodes and a mild interstitial pneumonia characterized by failure of the lungs to collapse when the thorax is opened. Jaundice is occasionally observed. Microscopically, the lymph nodes show necrosis of lymphoid follicles, depletion of lymphocytes, and notable proliferation of follicular macrophages, some of which fuse and form syncytial cells (granulomatous lymphadenitis). In many cases, large basophilic botryoid inclusion bodies resembling grapes are present in the cytoplasm of macrophages, particularly in Peyer’s patches, spleen, and lymph nodes. Similar inclusions are occasionally seen in bronchial and renal epithelial cells. The lungs show thickening of the alveolar walls because of hyperplasia of type II pneumonocytes and interstitial infiltrates of mononuclear cells, some of which may contain intracytoplasmic inclusion bodies. Circovirus antigen can be confirmed in affected tissue by immunohistochemical or PCR techniques.
Secondary infections with Pneumocystis carinii are commonly seen in pigs with PRRS and PMWS. Characteristically, alveoli are filled with a distinctive foamy exudate, which contains the organism not visible in H&E-stained sections but easily demonstrated with Gomori’s methenamine silver stain (Fig. 9-82). In humans, Pneumocystis carinii pneumonia (pneumocystosis) is one of the most common and often fatal complications in AIDS patients. As in AIDS patients, in foals and pigs, abnormal populations of CD4+ and CD8+ T lymphocytes have been incriminated as the underlying mechanism leading to pneumocystosis.
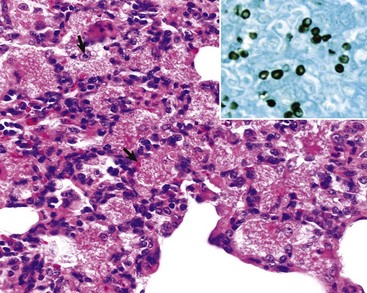
Fig. 9-82 Pneumocystosis (Pneumocystis carinii), lung, pig.
Alveoli are filled with a foamy eosinophilic proteinaceous material in which numerous punctiform organisms (arrows) are present. H&E stain. Inset, Silver-stained oval bodies typical of Pneumocystis carinii. Pneumocystosis is generally a microscopic diagnosis because this condition does not cause remarkable gross lesions. Gomori’s methenamine silver stain. (Courtesy Dr. A. López, Atlantic Veterinary College.)
Porcine Enzootic Pneumonia (Mycoplasmal Pneumonia of Pigs): Porcine enzootic pneumonia, a highly contagious disease of pigs caused by Mycoplasma hyopneumoniae, is grossly characterized by suppurative or catarrhal bronchopneumonia (Fig. 9-83 and Web Fig. 9-12). When its worldwide prevalence and deleterious effect on feed conversion are taken into account, this disease is probably the most economically significant respiratory disease of pigs. Although an infectious disease, it is very much influenced by immune status and management factors, such as crowding (airspace and floor space), ventilation (air exchange rate), concentrations of noxious gases in the air (ammonia, hydrogen sulfide), relative humidity, temperature fluctuations, and mixing of stock from various sources. It has been demonstrated with PCR technique that Mycoplasma hyopneumoniae is present in the air of infected farms.
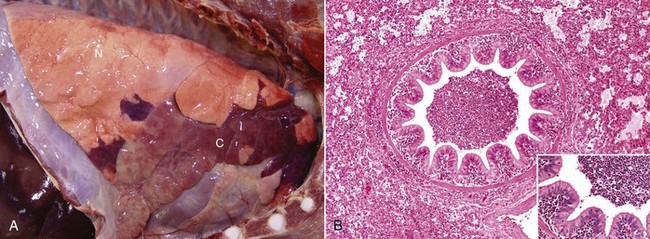
Fig. 9-83 Chronic-active (suppurative) bronchopneumonia (enzootic pneumonia), lung, pig.
A, Cranioventral consolidation of 40% to 50% of pulmonary parenchyma. Consolidated lung (C) is firm, and the outlines of the lobules are accentuated by edema of the interlobular septa. N, Normal lung. B, The bronchiole and alveoli contain numerous neutrophils and macrophages. Some of the neutrophils are migrating from the capillaries of the lamina propria of the bronchiole into its lumen. Alveoli are edematous and contain similar inflammatory cells. Alveolar septa are also widened by inflammation. H&E stain. Inset, Higher magnification of wall of bronchiole in B. H&E stain. (Courtesy Dr. A. López, Atlantic Veterinary College.)
The cause of porcine enzootic pneumonia remained unclear for many years, and so the disease was mistakenly known as “virus pneumonia of pigs” based on the assumption that if the agent was hard to find, it must be a virus. The causative agent, Mycoplasma hyopneumoniae, is a fastidious organism and very difficult to grow; thus the final diagnosis is frequently based on interpretation of lesions alone, or supported by ancillary tests to detect this mycoplasma in affected lungs by immunohistochemical, immunofluorescence, enzyme-linked immunosorbent assay (ELISA), or PCR techniques. The bronchopneumonic lesions of porcine enzootic pneumonia are in most cases mild to moderate, and thus mortality is low unless complicated with secondary pathogens, such as Pasteurella multocida, Arcanobacterium pyogenes, Bordetella bronchiseptica, Haemophilus spp., Mycoplasma hyorhinis, and other mycoplasmas and ureaplasmas. Although the pathogenesis of porcine enzootic pneumonia is not completely elucidated, it is known that Mycoplasma hyopneumoniae first adheres to the cilia of the bronchi by means of a unique adhesive protein, produces ciliostasis, and finally colonizes the respiratory system by firmly attaching to the ciliated epithelial cells of the trachea and the bronchi of the cranioventral regions of the lungs. Once attached to the respiratory epithelium, it provokes an influx of neutrophils into the tracheobronchial mucosa, causes extensive loss of cilia (deciliation), stimulates an intense hyperplasia of lymphocytes in the BALT, and attracts mononuclear cells into the peribronchial, bronchiolar, and alveolar interstitium. Additional virulence factors include the ability of Mycoplasma hyopneumoniae to cause immunosuppression, reduce the phagocytic activity of neutrophils in the lung, and change the chemical composition of mucus. All of these functional alterations can predispose the lung to secondary bacterial infections.
The lesions caused by Mycoplasma hyopneumoniae start as a bronchointerstitial pneumonia microscopically characterized by mononuclear infiltrates in the alveolar walls and a few macrophages and neutrophils in bronchiolar and alveolar lumens. This inflammatory reaction progresses to a suppurative or mucopurulent bronchopneumonia once secondary pathogens, such as Pasteurella multocida, Bordetella bronchiseptica, or Arcanobacterium pyogenes, are involved (commonly seen at necropsy). In most pigs, gross lesions affect only portions of the cranial lobes, but in more severely affected pigs, lesions involve 50% or more of the cranioventral portions of the lungs (see Fig. 9-83). The affected lungs are dark red in the early stages but have a homogeneous pale-gray (“fish flesh”) appearance in the more chronic stages of the disease. On the cut surface, exudate can easily be expressed from airways, and depending on the stage of the lesions and secondary infections, the exudate varies from purulent to mucopurulent to mucoid. Microscopic lesions are characterized by an influx of macrophages and neutrophils into the bronchi, bronchioles, and alveoli, and with time there is also notable BALT hyperplasia. In some cases, accumulation of exudate can be severe enough to cause occlusion of bronchioles and atelectasis of their lobules (see Fig. 9-83). The suppurative bronchopneumonia may be accompanied by a mild fibrinous pleuritis, which is often more severe if other organisms, such as Mycoplasma hyorhinis, Pasteurella multocida, or Actinobacillus pleuropneumoniae, are also involved. Abscesses and fibrous pleural adhesions are sequelae of chronic complicated infections.
Clinically, enzootic pneumonia occurs as a herd problem in two disease forms. A newly acquired infection of a previously clean herd causes disease in all age groups, resulting in acute respiratory distress and low mortality. In a chronically infected herd, the mature animals are immune and clinical signs are usually apparent only in growing pigs at times of particular stress such as at weaning. In such herds, coughing and reduced rate of weight gain are the most notable signs.
Porcine Pasteurellosis: Porcine pasteurellosis is an infectious disease complex with unclear pathogenesis that includes primary infections by Pasteurella multocida alone, or more frequently, after the defense mechanisms are impaired and a secondary bacterium colonizes the lung (porcine pneumonic pasteurellosis). In some rare cases, Pasteurella multocida causes acutely fatal septicemias in pigs. It is important to remember that Pasteurella multocida serotypes A and D are both part of the normal nasal flora and are also causative agents of bronchopneumonia, pleuritis, and atrophic rhinitis in pigs.
Pasteurella multocida is one of the most common secondary pathogens isolated from the lungs of pigs with porcine influenza, PRRS, PCV-2 infection, pseudorabies, classic swine fever (hog cholera), enzootic pneumonia, and porcine pleuropneumonia. Secondary infections with Pasteurella multocida notably change the early and mild bronchointerstitial reaction of enzootic and viral pneumonias into a severe suppurative bronchopneumonia with multiple abscesses and sometimes pleuritis. The other important role of Pasteurella multocida in porcine pneumonias is as a cause of a fulminating, cranioventral, fibrinous bronchopneumonia (pleuropneumonia) after influenza virus infection or stress from inadequate ventilation resulting in high levels of ammonia in the air. The nature of the lesion and the predisposing factors of poor management or coexisting viral infections suggest that fulminating porcine pasteurellosis has a pathogenesis similar to that of pneumonic Mannheimiosis of cattle. Pharyngitis with subcutaneous cervical edema, fibrinohemorrhagic polyarthritis, and focal lymphocytic interstitial nephritis are also present in porcine pneumonic pasteurellosis. Whether this disease is a separate form of pasteurellosis (septicemic) or a change to bronchopneumonia needs to be elucidated. Sequelae of porcine pneumonic pasteurellosis include fibrous pleuritis and pericarditis, pulmonary abscesses, so-called sequestra, and usually death. In contrast to ruminants, Mannheimia haemolytica is not a respiratory pathogen for pigs, but in some instances, it can cause abortion in sows.
Porcine Pleuropneumonia: Porcine pleuropneumonia is a highly contagious, worldwide disease of pigs caused by Actinobacillus pleuropneumoniae (Haemophilus pleuropneumoniae), which is characterized by a severe, often fatal, fibrinous bronchopneumonia with extensive pleuritis (pleuropneumonia). Survivors generally develop notable residual lesions and become carriers of the organisms. Porcine pleuropneumonia is an increasingly important cause of acute and chronic pneumonias, particularly in intensively raised pigs (2 to 5 months old). Transmission of Actinobacillus pleuropneumoniae occurs by the respiratory route, and the disease can be reproduced experimentally by intranasal inoculation of the bacterium. Considered as a primary pathogen, Actinobacillus pleuropneumoniae can sporadically produce septicemia in young pigs and otitis media and otitis interna with vestibular syndrome in weaned pigs. Twelve serotypes of the organism, most of which can cause the disease, have been identified. The pathogenesis is not yet well understood, but specific virulence factors, such as RTX and Apx toxins, capsular factors, fimbriae and adhesins, lipopolysaccharide, hemolysins, cytotoxins, and permeability factors, have been identified. These factors allow Actinobacillus pleuropneumoniae to attach to cells; produce pores in cell membranes; damage capillaries and alveolar walls, resulting in vascular leakage and thrombosis; impair phagocytic function; and elicit failure of clearance mechanisms.
The gross lesions in the acute form consist of a fibrinous bronchopneumonia characterized by severe consolidation and a fibrinous exudate on the pleural surface. Although all lobes can be affected, a common site is the dorsal area of the caudal lobes. In fact, a large area of fibrinous pleuropneumonia involving the caudal lobe of a pig’s lung is considered almost diagnostic for this disease (Fig. 9-84). On the cut surface, consolidated lungs have notably dilated interlobular septa and irregular but well-circumscribed areas of necrosis caused by potent cytotoxins produced by Actinobacillus pleuropneumoniae. Except for the distribution, pulmonary lesions of porcine pleuropneumonia are identical to those of pneumonic Mannheimiosis of cattle. The microscopic lesions are also very similar and include areas of coagulative necrosis surrounded by a thick cluster of “streaming (oat-shaped) leukocytes” and a notable distention of the interlobular septa because of severe edema and lymphatic thrombosis. Bronchioles and alveoli are filled with edematous fluid, fibrin, neutrophils, and few macrophages (see Fig. 9-84). Pigs with the chronic form have multiple pulmonary abscesses and large (2 to 10 cm) pieces of necrotic lung encapsulated by connective tissue (sequestra), changes frequently seen in slaughterhouses.
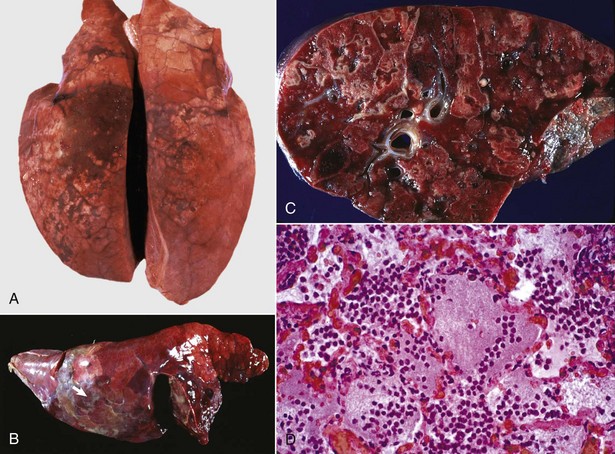
Fig. 9-84 Porcine pleuropneumonia (Actinobacillus pleuropneumoniae), lung, pig.
A, In peracute porcine pleuropneumonia, the pneumonic lesions are locally extensive in the dorsal aspects of the caudal lung lobes. There is lobular congestion, consolidation, and interlobular edema. B, As the disease progresses and becomes acute to subacute, the lesions expand in size and severity. Note the large area of hemorrhagic necrotizing fibrinous bronchopneumonia. Fibrin is abundant (arrow) on the pleural surface and in interlobular septa. C, The cut surface has numerous discrete and coalescing zones of lobular inflammation and necrosis (upper left), which are pale pink to white and often surrounded by a white margin (inflammation). There is extensive congestion (active hyperemia) and hemorrhage throughout the section. D, Alveoli are filled with fibrin, edema fluid, and neutrophils. Capillaries in alveolar septa are congested (active hyperemia), and in many cases, there is necrosis of alveolar septa (not visible at this magnification). (A courtesy Dr. A. López, Atlantic Veterinary College. B courtesy Dr. J. Render, College of Veterinary Medicine and Animal Health Diagnostic Laboratory, Michigan State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. C and D courtesy Dr. A.R. Doster, University of Nebraska; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Clinically, porcine pleuropneumonia can vary from an acute form with unexpected death and blood-stained froth at the nostrils and mouth to a subacute form characterized by coughing and dyspnea accompanied by clinical signs of sepsis such as high fever, hypoxemia, anorexia, and lethargy. A chronic form is characterized by decreased growth rate and persistent cough. Animals that survive often carry the organism in the tonsils, shed the organism, and infect susceptible pigs.
Haemophilus Pneumonia: In addition to Glasser’s disease characterized by polyserositis (pericarditis, pleuritis, peritonitis, polyarthritis, and meningitis), some serotypes of Haemophilus parasuis (originally Haemophilus parasuis suis) can also cause suppurative bronchopneumonia that in some severe cases can be fatal. The causal organism, Haemophilus parasuis, is usually carried in the nasopharynx of normal pigs and requires abnormal circumstances such as those following stress (weaning, cold weather) or viral infections (swine influenza or PCV-2). Specific pathogen-free (SPF) pigs seem to be particularly susceptible to Glasser’s disease (arthritis and serositis) but not to pulmonary infection (bronchopneumonia).
Streptococcal Pneumonia: Streptococcus suis is a common cause of porcine disease worldwide and a serious zoonosis capable of causing death by septic shock or meningitis and residual deafness in butchers, veterinarians, and pig farmers. Typically, Streptococcus suis gains entrance to the susceptible young pig through the oropharyngeal mucosa and is carried in the tonsils, nasal mucosa, and mandibular lymph nodes of healthy animals, particularly in survivors of an outbreak. Infected sows can abort or vertically transmit the infections to their offspring. Some serotypes of Streptococcus suis cause neonatal septicemia, and this can result in suppurative meningitis, otitis, arthritis, polyserositis, myocarditis, valvular endocarditis, and embolic pneumonia (Fig. 9-85). Other serotypes of Streptococcus suis can reach the lung by the aerogenous route and cause a suppurative bronchopneumonia, in combination with Pasteurella multocida, Escherichia coli, or Mycoplasma hyopneumoniae, or in combination with Actinobacillus pleuropneumoniae, which causes a fibrinous bronchopneumonia.
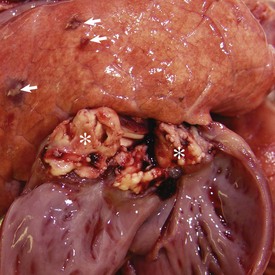
Fig. 9-85 Vegetative endocarditis, heart and multiple embolic lesions, lung, pig.
Note large vegetative (cauliflower-like) mass attached to the tricuspid valve (asterisks). The lung (top half of figure) shows multifocal well-circumscribed nodules (arrows), the result of emboli being released from the tricuspid valve. (Courtesy Dr. A. López, Atlantic Veterinary College.)
Tuberculosis: Tuberculosis is an important disease in domestic and wild pigs that has a much greater prevalence in pigs than in cattle or other domestic mammals in many countries, including those in North America. Porcine tuberculosis is attributed to infection with Mycobacterium bovis and porcine mycobacteriosis to Mycobacterium avium-intracellulare complex. A common scenario in small mixed-farming operations is the diagnosis of avian tuberculosis at the time that pigs are slaughtered, and the source is ingestion of tuberculous chickens or contaminated litter. As would be expected, granulomas are found in the mesenteric, mandibular, and retropharyngeal lymph nodes, to a lesser extent in the intestine, liver, and spleen, and only in rare cases in the lung. The route of infection in pulmonary tuberculosis and mycobacteriosis of pigs is most often hematogenous after oral exposure and intestinal infection. Lung lesions are those of a granulomatous pneumonia. The microscopic lesions are basically those of tubercles, but the degree of encapsulation, caseation, and calcification varies with the type of mycobacterium, age of the lesion, and host immune response.
Other Infectious Pneumonias of Pigs: Porcine respiratory coronavirus (PRCV) is sporadically incriminated in pneumonia in pigs. This viral pneumonia is generally mild, and most pigs fully recover if the pneumonia is not complicated with other infections. Lesions in the lung are those of interstitial pneumonia with necrotizing bronchiolitis. Interestingly, infections with porcine and other respiratory coronaviruses have been used to investigate the pathogenesis of severe acute respiratory syndrome (SARS), an emerging and highly contagious condition recently reported in humans and attributed to a novel human coronavirus (SARS-CoV). The relationship between SARS-CoV and animal coronavirus is still under investigation.
Septicemias in pigs often cause petechial hemorrhages in the lung and pulmonary edema, and these lesions may be present in African swine fever, classic swine fever (hog cholera), pseudorabies, and other diseases. Salmonellae, Escherichia coli, and Listeria monocytogenes can cause severe interstitial pneumonia in very young animals. Salmonella choleraesuis causes a necrotizing fibrinous pneumonia similar to porcine pleuropneumonia, and Salmonella typhisuis causes a chronic suppurative bronchopneumonia.
Foreign body granulomatous pneumonia occurs frequently in pigs after inhalation of vegetable material (starch pneumonia), presumably from dusty (nonpelleted) feed. Lesions are clinically silent but are often mistaken for other pneumonic processes during inspection at slaughterhouses. Microscopically, pulmonary changes are typical of foreign body granulomatous inflammation in which variably sized feed particles are surrounded by macrophages and neutrophils, and often have been phagocytosed by multinucleated giant cells. Feed (vegetable) particles appear as thick-walled polygonal cells that stain positive with PAS because of their rich carbohydrate (starch) content.
Parasitic Pneumonias of Pigs: Metastrongylus apri (elongatus), Metastrongylus salmi, and Metastrongylus pudendotectus (lungworms) of domestic and feral pigs occur throughout most of the world and require earthworms as intermediate hosts for transmission. Lungworms may transmit the virus of swine influenza. The importance of pig lungworms is mainly because infection results in growth retardation of the host. Clinical signs include coughing because of parasitic bronchitis.
The gross lesions, when noticeable, consist of small gray nodules, particularly along the ventral borders of the caudal lobes. The adult worms are grossly visible in bronchi, and microscopically, the parasites cause a catarrhal bronchitis with infiltration of eosinophils and lobular atelectasis (Fig. 9-86).
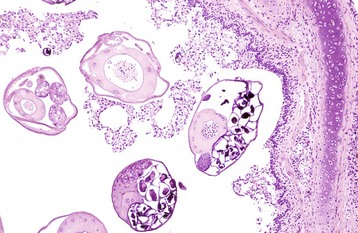
Fig. 9-86 Acute verminous bronchitis (Metastrongylus apri), bronchus, cross section, pig.
Several sections of nematodes (Metastrongylus apri) admixed with mucus, neutrophils, and eosinophils (not visible at this magnification) are present in the lumen of the bronchus. H&E stain. (Courtesy Armed Forces Institute of Pathology and Dr. G. Conboy, Atlantic Veterinary College.)
The larvae of Ascaris suum can cause edema, focal subpleural hemorrhages, and interstitial inflammation (see Fig. 9-62). Along their larval migration tracts, hemorrhages also occur in the liver, and after fibrosis, they become the large, white “milk spots” seen so frequently as incidental findings at necropsy. It has recently been reported that Ascaris suum may cause immunosuppression in severely affected pigs.
Pneumonias of Dogs: In general, inflammatory diseases of the lungs are less of a problem in dogs than in food-producing species and can be subdivided in two major groups, infectious and noninfectious pneumonias. Of the infectious type, two diseases account for most cases: infectious tracheobronchitis (kennel cough), which was discussed previously, and canine distemper. Uremia and paraquat toxicity are perhaps the two most notable noninfectious causes.
Canine Distemper: Canine distemper is an important and ubiquitous infectious disease of dogs, other Canidae, wild Felidae, Mustelidae, and marine mammals around the world. It is caused by a Morbillivirus that is antigenically related to the human measles, rinderpest, “peste de petit ruminants,” and phocine distemper viruses. Distemper virus is transmitted to susceptible puppies through infected body fluids. The virus invades through the upper respiratory tract and conjunctiva, proliferates in regional lymph nodes, becomes viremic, and in dogs with an inadequate antibody response, infects nearly all body tissues (pantropic), particularly the epithelial cells. Distemper virus hampers the immune response and appears to downregulate cytokine production and persist for a long time in some tissues. This virus can target the lungs either directly as a viral pneumonia or by its immunosuppressive effects rendering the lungs susceptible to secondary bacterial and protozoal infections, or as a co-infection with other viruses such as CAV.
Gross lesions in the acute stages include serous to catarrhal to mucopurulent nasopharyngitis and conjunctivitis. The lungs are edematous and have a diffuse interstitial pneumonia (Fig. 9-87) microscopically characterized by necrotizing bronchiolitis, necrosis and exfoliation of pneumonocytes, mild alveolar edema, and several hours later, thickening of the alveolar walls because of interstitial mononuclear cell infiltrates and hyperplasia of type II pneumonocytes. Secondary infections with Bordetella bronchiseptica and mycoplasmas are common and induce life-threatening suppurative bronchopneumonia. The thymus may be small relative to the age of the animal because of viral-induced lympholysis.
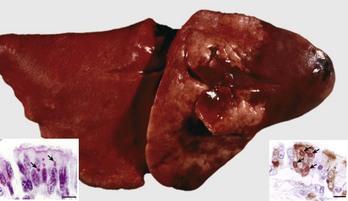
Fig. 9-87 Interstitial pneumonia, canine distemper, lungs, dog.
The lungs are heavy, edematous, and rubbery, with costal (rib) imprints on the pleural surface. Inset, left: Bronchial epithelium contains intracytoplasmic eosinophilic inclusion bodies (arrows). H&E stain. Inset, right: Immunoperoxidase stain revealing canine Morbillivirus antigen (arrows) in the cytoplasm and apical borders of bronchial epithelial cells. Immunoperoxidase stain. Bars = 20 µm. (From Berrocal A, López A: J Vet Diagn Invest 15:292-294, 2003.)
Microscopically, eosinophilic inclusions are present in the epithelial cells of many tissues, in the nuclei or cytoplasm, or in both (see Fig. 9-87). They appear early in the bronchiolar epithelium but are most prominent in the epithelium of the lung, stomach, renal pelvis, and urinary bladder, making these tissues good choices for diagnostic examination. Viral inclusions are rarely seen in the later stages of this disease. The suppurative secondary bronchopneumonias often hinder the detection of viral lesions in the lung, particularly because bronchiolar cells containing inclusion bodies exfoliate and mix with the neutrophils recruited by the bacterial infection. Distemper virus antigens can be readily demonstrated in infected cells by the immunoperoxidase technique (see Fig. 9-87), which can also be used in skin biopsies for the antemortem diagnosis of canine distemper.
Distemper virus also has a tendency to affect developing tooth buds and ameloblasts, causing enamel hypoplasia in dogs that recover from infection. Of all distemper lesions, demyelinating encephalomyelitis, which develops late, is the most devastating (see Chapter 14). Sequelae to distemper include the nervous and pneumonic complications mentioned previously and various systemic infections, such as toxoplasmosis and sarcocystosis, because of depressed immunity. Persistent viral infection occurs in some dogs that survive the disease, and they may become carriers and the source of infection for other susceptible animals.
Clinical signs consist of biphasic fever, diarrhea, vomiting, weight loss, mucopurulent oculonasal discharge, coughing, respiratory distress, and possible loss of vision. Weeks later, hyperkeratosis of foot pads (“hard pad”) and the nose are observed, along with nervous signs, including ataxia, paralysis, convulsions, or residual myoclonus (muscle twitches, tremors, and “tics”).
Canine Adenovirus Type 2 Infection: CAV-2 is a common but transient contagious disease of the respiratory tract of dogs, causing mild fever, oculonasal discharge, coughing, and poor weight gain. The portal of entry is generally by inhalation of infected aerosols followed by viral replication in pneumonocytes. Pulmonary lesions are initially those of a bronchointerstitial pneumonia, with necrosis and exfoliation of bronchiolar and alveolar epithelium and edema, and a few days later, proliferation of type II pneumonocytes, mild infiltration of neutrophils and lymphocytes in alveolar interstitium, and hyperplastic bronchitis and bronchiolitis. Large basophilic intranuclear viral inclusions are typically seen in bronchiolar and alveolar cells (Fig. 9-88). The disease is sometimes associated or may be confused with the infectious tracheobronchitis complex (kennel cough). The infection with CAV is clinically mild unless complicated with a secondary bacterial infection or co-infections with other viruses such as distemper virus. Experimental work suggests CAV-2 reinfection may lead to hyperreactive airways, a nonspecific condition in which the bronchial mucosa becomes highly “responsive” to irritation such as that caused by cold air, gases, or cigarette smoke. However, it is not clear if this outcome is true in natural infections.
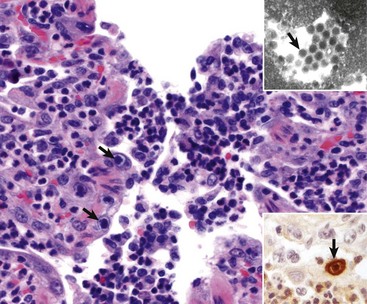
Fig. 9-88 Necrotizing bronchiolitis, canine adenovirus-2 (CAV-2), puppy.
Note necrosis and exfoliation of bronchiolar epithelial cells and the neutrophil infiltrates in the mucosa and bronchiolar lumen. Large basophilic inclusion bodies are present in nuclei of some bronchiolar cells (arrows). H&E stain. Inset right bottom corner, Immunopositive staining for CAV-2 antigen (arrow). Immunoperoxidase stain. Inset top right corner, Paracrystalline arrays of electron dense particles typical of adenovirus (arrow) in a transmission electron photomicrograph. Uranyl acetate and lead citrate stain. (From Rodríguez LE, Ramírez-Romero R, Valdez-Nava Y, et al: Can Vet J 48:632-634, 2007.)
Canine Herpesvirus 1: Canine herpesvirus 1 (CHV-1) can cause fatal generalized disease in newborn puppies, and it is probably part of the variety of factors that result in the “fading puppy syndrome.” Hypothermia has been suggested as a pivotal component in the pathogenesis of fatal infections in puppies. Many dogs are seropositive, suggesting that transient or subclinical infections are more common than realized; the virus remains latent in the trigeminal and other ganglia and can be reactivated after stress, resulting in asymptomatic transmission of CHV-1 virus to offspring via the placenta, resulting in abortion or stillbirths. In puppies, CHV-1 causes ulcerative tracheitis, interstitial pneumonia, and focal necrosis and inflammation in kidneys, liver, and brain. CHV-1 has also been identified as a cause of ulcerative keratoconjunctivitis in older dogs.
Canine Influenza (Canine Flu): Canine influenza is an emerging contagious respiratory infection of dogs that has been recently described in the US. It has a high morbidity (close to 100%), but the mortality, as with most other influenza infections, is relatively low (less than 8%). This disease first diagnosed in greyhounds is caused by a novel influenza-A virus that appears to be a mutation from a previously recognized strain of equine influenza virus, the H3N8 strain. Dog-to-dog transmission does occur and therefore this infection must be distinguished from kennel cough. Pulmonary lesions are generally mild and transient, but infected dogs are susceptible to secondary bacterial bronchopneumonia. The most relevant lesions in dogs dying unexpectedly from canine influenza are pleural and pulmonary hemorrhages. Microscopically, there is necrotizing tracheitis, bronchitis, and bronchiolitis with exudation of neutrophils and macrophages. Vasculitis and thrombosis are occasionally observed in the lungs. Influenza antigen can be demonstrated by immunohistochemistry in airway epithelium and alveolar macrophages. Clinically, dogs with canine influenza are lethargic, inappetent, and hyperthermic and frequently cough and show nasal discharge. These signs resemble those seen in dogs with kennel cough or secondary bacterial pneumonia. In addition, there are confirmed cases of canine influenza caused by the porcine H1N1 presumably transmitted from infected pet owners.
Bacterial Pneumonias of Dogs: Dogs generally have bacterial pneumonias when the pulmonary defense mechanisms have been impaired. Pasteurella multocida, Streptococcus spp., Escherichia coli, Klebsiella pneumoniae, and Bordetella bronchiseptica can be involved in pneumonia secondary to distemper or after aspiration of gastric contents (Fig. 9-89 and Web Fig. 9-13). Streptococcus zooepidemicus also causes acute and fatal hemorrhagic pleuropneumonia with hemorrhagic pleural effusion in dogs. Death is generally a consequence of severe sepsis and septic shock or from β-hemolytic Streptococci bacteremia causing bacteremia and embolisms affecting the lungs, liver, brain, and lymph nodes. The primary source of the infection cannot be determined in most cases. Dental disease in dogs may be a source of systemic and pulmonary infection, which is not a new concept, as it has been recognized in human medicine for many years. The role of mycoplasmas in canine pneumonia is still uncertain because these organisms are frequently isolated from normal nasopharyngeal flora.
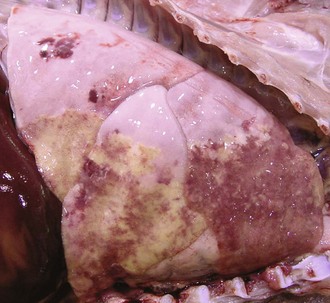
Fig. 9-89 Aspiration pneumonia, bronchopneumonia, right lung, dog.
Subacute to chronic bronchopneumonia. The cranioventral portions of the lung are firm and contain purulent exudate (yellow areas). Aspiration pneumonia starts as an acute necrotizing bronchitis and bronchiolitis caused by aspiration of irritant materials such as gastric acid or a caustic material administered by mouth. The aspirate also contains potentially pathogenic bacteria, and because the mucociliary apparatus is damaged and these bacteria are not removed, they settle into the ventral portions of the lung (from gravity) and provoke a fibrinosuppurative bronchopneumonia. (Courtesy Dr. A. López, Atlantic Veterinary College.)
Tuberculosis is uncommon in dogs because these animals appear to be quite resistant to infection; most cases occur in immunocompromised dogs or in dogs living with infected humans. Dogs are susceptible to the Mycobacterium tuberculosis, Mycobacterium bovis, and Mycobacterium avium-intracellulare complex strains, and therefore canine infection presupposes contact with human or animal tuberculosis. The clinicopathologic manifestation is pulmonary after inhalation or alimentary after oral exposure, but in most cases infection is disseminated to lymph nodes and visceral organs. The gross lesions are multifocal, firm nodules with necrotic centers, most often seen in the lungs, lymph nodes, kidneys, and liver. Diffuse granulomatous pleuritis and pericarditis with copious serofibrinous or sanguineous effusion are common. Microscopically, granulomas are formed by closely packed macrophages, but with very little connective tissue.
Mycotic Pneumonias of Dogs: Mycotic pneumonias are serious diseases seen commonly in animals in some areas. There are two main types: those caused by opportunistic fungi and those caused by a group of fungi associated with systemic “deep” mycoses. All these fungi affect humans and most domestic animals but are probably not transmitted between species.
Opportunistic fungi, such as Aspergillus fumigatus, are important in birds, but in domestic animals, they mainly affect immunosuppressed animals or those animals on prolonged antibiotic therapy. The pulmonary lesion is a multifocal, nodular, pyogranulomatous, or granulomatous pneumonia. Microscopically, there is necrosis and infiltrates of neutrophils, macrophages and lymphocytes, and proliferation of fibroblasts eventually leads to encapsulation of the granuloma. Fungal hyphae are generally visible in the core of the lesion and in the walls of blood vessels.
Systemic (deep) mycoses are caused by Blastomyces dermatitidis, Histoplasma capsulatum, Coccidioides immitis, and Cryptococcus neoformans (Fig. 9-90). Blastomycosis mainly affects dogs and is discussed here, whereas cryptococcosis is discussed in the section on Pneumonias of Cats. In contrast to other fungi, such as Aspergillus spp., organisms of the systemic mycosis group are all primary pathogens of humans and animals and thus do not necessarily require a preceding immunosuppression to cause disease. These fungi have virulence factors that favor hematogenous dissemination and evasion of immune and phagocytic responses. Systemic dissemination is often exacerbated by the administration of immunosuppressant drugs such as corticosteroids. These fungi are usually detected by cytological evaluation of affected tissues.
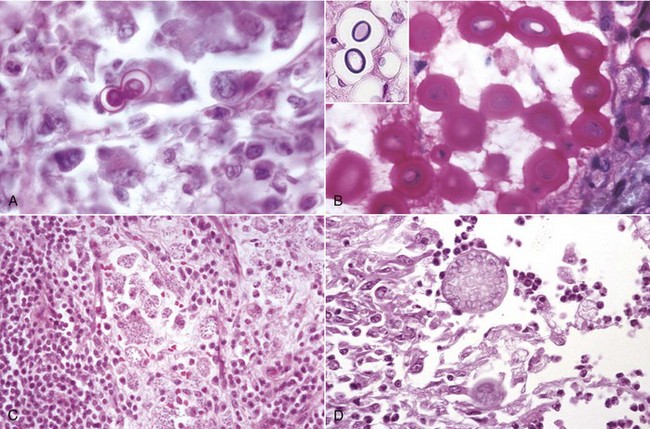
Fig. 9-90 Systemic (deep) mycoses.
A, Blastomyces dermatitidis, 8 to 25 µm in diameter, broad-based budding spherical yeastlike organisms, intracellular or extracellular location. H&E stain. B, Cryptococcus neoformans, spherical, 2 to 10 µm in diameter, usually surrounded by a thick mucus capsule, which can increase the overall diameter up to 30 µm, intracellular or extracellular location. The mucus capsule does not stain with H&E (inset) but is stained by mucicarmine. With routine mountants, the capsule shrinks and distorts, but this effect has been prevented here by using an aqueous mounting medium. Mayer’s mucicarmine stain, aqueous mounting medium. Inset, In H&E–stained sections, the capsule is not visible but appears as a halo around the cell body. C, Histoplasma capsulatum, located intracellularly, is spherical to slightly elongated, 5 to 6 µm in diameter. H&E stain. D, Coccidioides immitis, spherules, 20 to 30 µm in diameter, containing endospores (<5 µm in diameter), intracellular or extracellular location. H&E stain. (A, B, C, and Inset courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. D courtesy College of Veterinary Medicine, University of Illinois.)
Blastomycosis occurs in many countries of the North American continent, Africa, the Middle East, and occasionally in Europe. In the US, it is most prevalent in the Atlantic, St. Lawrence, and Ohio-Mississippi River Valley states, as compared with the Mountain-Pacific region. Blastomyces dermatitidis is a dimorphic fungus (mycelia-yeast) seen mainly in young dogs and occasionally in cats. This fungus is present in the soil, and inhalation of spores is considered the principal route of infection; thus it most frequently affects outdoor and hunting dogs. From the lung, infection is disseminated hematogenously to other organs, mainly bone, skin, brain, and probably eyes.
Pulmonary lesions are characterized by multifocal to coalescing granulomatous pneumonia, generally with firm nodules (pyogranulomas) scattered throughout the lungs (see Fig. 9-66). Microscopically, nodules are granulomas with numerous macrophages (epithelioid cells), some neutrophils, multinucleated giant cells, and thick-walled yeasts (see Fig. 9-91; also see Fig. 9-90, A). Yeasts are 5 to 25 µm in diameter and are much better visualized when they are stained with PAS reaction or Gomori’s methenamine silver stain. Nodules can also be present in other tissues, chiefly lymph nodes, skin, spleen, liver, kidneys, bones, testes, prostate, and eyes. This fungus can be easily identified in properly prepared and stained transtracheal washes or lymph node aspirates.
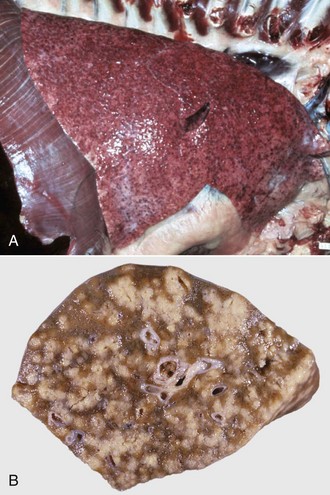
Fig. 9-91 Granulomatous pneumonia, blastomycosis (Blastomyces dermatitidis), right lung, dog.
A, The lung contains large numbers of small granulomas distributed throughout all pulmonary lobes. B, The cut surface of the lung shows multiple discrete and coalescing gray-white granulomas distributed randomly throughout the lung. (Courtesy College of Veterinary Medicine, University of Illinois.)
Clinical signs can reflect involvement of virtually any body tissue; pulmonary effects include cough, decreased exercise tolerance, and terminal respiratory distress.
Coccidioidomycosis (San Joaquin Valley fever), caused by the dimorphic fungus Coccidioides immitis, occurs mainly in animals living in arid regions of the southwestern US, Mexico, and Central and South America. It is a primary respiratory tract (aerogenous) infection commonly seen at slaughterhouses in clinically normal feedlot cattle. In dogs, coccidioidomycosis also has an aerogenous portal of entry and then disseminates systemically to other organs. Clinical signs relate to the location of lesions, so there can be respiratory distress, lameness, generalized lymphadenopathy, or cutaneous lesions, among others.
The lesions caused by Coccidioides immitis consist of focal granulomas or pyogranulomas that can have suppurative or caseated centers. The fungal organisms are readily seen in histologic or cytologic preparation as large (10 to 80 µm in diameter), double-walled, and highly refractile spherules (see Fig. 9-90, D).
Histoplasmosis is a systemic infection that results from inhalation of another dimorphic fungus, Histoplasma capsulatum. Histoplasmosis occurs sporadically in dogs and humans and to a lesser extent, in cats and horses. Bats often eliminate Histoplasma capsulatum in the feces, and droppings from bats and birds, particularly pigeons, heavily promote the growth and survival of this fungus in the soil of enzootic areas.
Pulmonary lesions are grossly characterized by variably sized, firm, poorly-encapsulated granulomas, and, sometimes, more diffuse involvement of the lungs. Microscopically, granulomatous tissue typically has many macrophages filled with small (1 to 3 µm), punctiform, intracytoplasmic, dark oval bodies (yeasts), and best demonstrated with PAS reaction (Fig. 9-90, C) or Gomori’s methenamine silver stain. Similar nodules can be present in other tissues, chiefly lymph nodes, spleen, intestines, and liver.
Parasitic Pneumonias of Dogs: Toxoplasmosis is a worldwide disease caused by the obligate intracellular, protozoal parasite Toxoplasma gondii. Cats and other Felidae are the definitive host where the mature parasite divides sexually in the intestinal mucosa. Humans, dogs, cats, and many wild mammals can become intermediate hosts after accidental ingestion of fertile oocysts shed in cat feces, or fetuses can be infected transplacentally from an infected dam. In most instances, the parasite infects many cells of different tissues and induces an antibody response (seropositive animals) but does not cause clinical disease. Toxoplasmosis is often triggered by immunosuppression, such as that caused by canine distemper virus. Toxoplasmosis is characterized by focal necrosis around the protozoan.
Pulmonary lesions are severe, multifocal necrotizing interstitial pneumonia with notable proliferation of type II pneumonocytes and infiltrates of macrophages and neutrophils. Other lesions in disseminated toxoplasmosis include focal necrotizing hepatitis, myocarditis, splenitis, myositis, encephalitis, and ophthalmitis. The parasites appear microscopically as small (3 to 6 µm) basophilic cysts that can be found free in affected tissues or within the cytoplasm of many epithelial cells and macrophages. Similar findings can be seen sporadically in dogs infected with Sarcocystis canis, and immunohistochemistry would be required to differentiate those protozoal organisms from Toxoplasma gondii.
Pneumocystis carinii has also been reported as a sporadic cause of chronic interstitial pneumonia in dogs with a compromised immune system (see Pneumonias of Horses; also see Fig. 9-82).
Filaroides hirthi, a lungworm of the alveoli and bronchioles of dogs, has long been known as a cause of mild subclinical infection in large colonies of beagle dogs in the US. However, it can on occasion cause severe and even fatal disease in individual pets, presumably as a result of immunosuppression. Clinical signs may include coughing and terminal respiratory distress. Grossly, the lesions are multifocal subpleural nodules, often with a green hue because of eosinophils, scattered throughout the lungs. Microscopically, these nodules are eosinophilic granulomas arising from the alveolar interstitium associated with larvae or dead worms, as little reaction develops to the live adults.
Crenosoma vulpis is a lungworm seen commonly in foxes and sporadically in dogs with access to the intermediate hosts—slugs and snails. The adult lungworms live in small bronchi and bronchioles in the caudal lobes, causing eosinophilic and catarrhal bronchitis manifested grossly as gray areas of inflammation and atelectasis. In some animals, Crenosoma vulpis causes bronchiolar goblet cell metaplasia and mucous obstruction, resulting in lobular atelectasis due to the valve effect of the mucous plug.
Eucoleus aerophilus (Capillaria aerophila) is a nematode parasite typically found in the trachea and bronchi of wild and domestic carnivores. In some cases, this parasite may also involve the nasal passages and sinuses. Although generally asymptomatic, some dogs cough because of the local irritation caused by the parasites on the tracheal or bronchial mucosa.
Paragonimus kellicotti in North America and Paragonimus westermani in Asia are generally asymptomatic fluke infections in fish-eating species; cats and dogs acquire it in North America by eating crayfish. Gross lesions include pleural hemorrhages when the metacercariae migrate into the lungs. Later, multifocal eosinophilic pleuritis, and subpleural cysts up to 7 mm long containing pairs of adult flukes, are found along with eosinophilic granulomas around clusters of eggs. Like many other parasitic pneumonias, lesions and scars are more frequent in the caudal lobes. Pneumothorax can occur if a cyst that communicates with an airway ruptures to the pleural surface.
Angiostrongylus vasorum and Dirofilaria immitis are parasites of the pulmonary arteries and right ventricle and, depending on the stage, can produce different forms of pulmonary lesions. Adult parasites can cause chronic arteritis that leads to pulmonary hypertension, pulmonary arterial thrombosis, interstitial (eosinophilic) granulomatous pneumonia, pulmonary interstitial fibrosis, congestive right-sided cardiac failure, and eventually caudal vena caval syndrome. Other lesions include pleural petechial hemorrhages, and in later stages, diffuse pulmonary hemosiderosis, parasitic granulomas around eggs and larvae, and multifocal pulmonary infarcts. Larvae also cause alveolar injury, thickening of the alveolar walls with eosinophils and lymphocytes (interstitial pneumonia), and multifocal or coalescing granulomas with giant cells.
Aspiration Pneumonia in Dogs: Aspiration pneumonia is an important form of pneumonia in dogs when vomit or regurgitated materials are aspirated into the lungs, or when drugs or radiographic contrast media are accidentally introduced into the airways. As in other animal species, aspiration pneumonia may be unilateral or may more often affect the right cranial lobe (see Fig. 9-89). The severity of lesions depends very much on the chemical and microbiologic composition of the aspirated material. In general, aspiration in monogastric animals, particularly in dogs and cats, is more severe because of the low pH of the gastric contents (chemical pneumonitis). In severe cases, dogs and cats die rapidly from septic shock and ARDS (see Fig. 9-45), which is microscopically characterized by diffuse alveolar damage, protein-rich pulmonary edema, neutrophilic alveolitis, and formation of typical hyaline membranes along the alveolar walls (Fig. 9-92). In animals that survive the acute stages of aspiration, pulmonary lesions progress to bronchopneumonia. Aspiration pneumonia is a common sequela to cleft palate, and in dogs with megaesophagus secondary to either myasthenia gravis or persistent right aortic arch. It is also an important complication of anesthesia or neurologic diseases affecting laryngeal function.
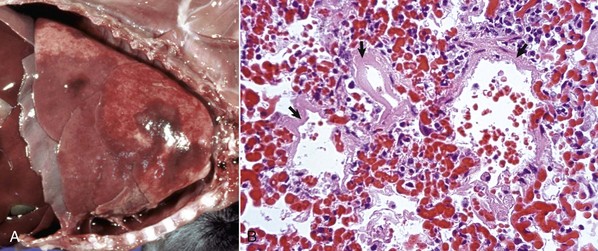
Fig. 9-92 Acute hemorrhagic bronchopneumonia, acute respiratory distress syndrome (ARDS), lungs, 4-week-old puppy.
A, Note that the lungs did not collapse when the thorax was opened (loss of negative pressure) and as a result fill almost the entire thoracic cavity. The cranioventral aspects of the lung are consolidated with hemorrhage. B, Alveolar capillary congestion, thick hyaline membranes along the alveolar septa (arrows), and intraalveolar hemorrhage. These microscopic changes are typical of the diffuse alveolar damage seen in lungs with ARDS. H&E stain. (Courtesy Dr. A. López, Atlantic Veterinary College.)
Toxic Pneumonias in Dogs: Paraquat, a broad-spectrum herbicide widely used in gardening and agriculture, can cause severe and often fatal toxic interstitial pneumonia (pneumonitis) in dogs, cats, humans, and other species. After ingestion or inhalation, this herbicide selectively accumulates in the lung and paraquat metabolites are produced by Clara cells. These metabolites promote local release of free radicals in the lung, which cause extensive injury to Clara cells and to the blood-air barrier, presumably through lipid peroxidation of type I and II pneumonocytes and alveolar endothelial cells (see Fig. 9-75). Paraquat toxicity has been used experimentally as a model of oxidant-induced alveolar injury and pulmonary fibrosis. Soon after poisoning, the lungs are heavy, edematous, and hemorrhagic because of extensive necrosis of epithelial and endothelial cells in the alveolar walls. The lungs of animals that survive acute paraquat toxicosis are pale, fail to collapse when the thorax is opened, and have interstitial emphysema, bullous emphysema, and may have pneumomediastinum. Microscopic findings in the acute and subacute phases include necrosis of type I pneumonocytes, interstitial and alveolar edema, intraalveolar hemorrhages, and proliferation of type II pneumonocytes. In the chronic stages (4 to 8 weeks later), the lesions are typically characterized by severe interstitial and intraalveolar fibrosis.
Uremic pneumonopathy (pneumonitis) is one of the many extrarenal lesions seen in dogs with chronic uremia. Lesions are characterized by a combination of pulmonary edema and calcification of vascular smooth muscle and alveolar basement membrane. In severe cases, alveolar calcification prevents lung collapse when the thorax is opened. In the more advanced cases, the lungs appear diffusely distended, pale red or brown in color, and show a rough pleural surface with rib imprints (see Fig. 9-33). On palpation, the pulmonary parenchyma has a typical “gritty” texture because of mineralization of the alveolar and vascular walls, which is best visualized microscopically by using special stains such as von Kossa (see Fig. 9-33). Because this is not primarily an inflammatory lesion, the term pneumonitis should not be used.
Pneumonias of Cats: Although upper respiratory tract infections are common and important in cats, pneumonias are uncommon except when there is immunosuppression or aspiration of gastric contents. Viral infections such as feline rhinotracheitis and calicivirus may cause lesions in the lungs, but unless there is secondary invasion by bacteria, they do not usually cause a fatal pneumonia (see Specific Diseases of the Nasal Cavity and Sinuses).
Feline Pneumonitis: The term feline pneumonitis is a misnomer because the major lesions caused by Chlamydophila (psittaci) felis are a severe conjunctivitis and rhinitis (see Specific Diseases of the Nasal Cavity and Sinuses). The elucidation of the importance of feline viral rhinotracheitis and feline calicivirus has removed Chlamydophila felis from its previously overstated importance as a lung pathogen.
Bacterial Pneumonias of Cats: Bacteria from the nasal flora such as Pasteurella multocida and Pasteurella-like organisms are occasionally associated with secondary bronchopneumonia in cats (Fig. 9-93). Pasteurella multocida also causes otitis media and meningitis, but its role as a respiratory pathogen is mainly associated with pyothorax. Interestingly, there are reports of Pasteurella multocida pneumonia in older or immunosuppressed humans acquired through contact with domestic cats. Mycoplasmas are often isolated from the lungs of cats with pulmonary lesions but are not definitively established as primary pathogens in feline pneumonias.
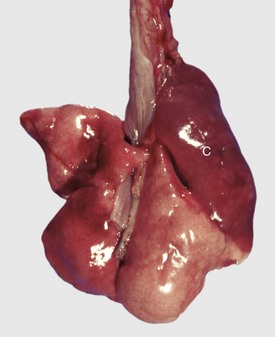
Fig. 9-93 Fibrinopurulent bronchopneumonia, lungs, 5-month-old kitten with history of conjunctivitis, rhinitis, and bacterial pneumonia.
Cranioventral consolidation (C) of the right lung involves approximately 40% of its parenchyma. The consolidated lung is firm, and on the cut surface, some exudate is present in major bronchi. (Courtesy Dr. S. McBurney, Atlantic Veterinary College.)
Cats are susceptible to three types of mycobacterial infections: classic tuberculosis, feline leprosy, and atypical mycobacteriosis. Classic tuberculosis in cats is rare and generally caused by Mycobacterium bovis, but also to a lesser extent by Mycobacterium tuberculosis and Mycobacterium avium. The usual route of infection for feline tuberculosis is oral, through unpasteurized milk or infected meat, so the granulomatous lesions are mainly in the intestine and mesenteric lymph nodes where they may disseminate through infected phagocytes to other organs. The solid and noncaseated appearance of tuberculous nodules is grossly similar to that of neoplasms, so they must be differentiated from pulmonary neoplasms (e.g., lymphosarcoma). Classic tuberculosis with dermal lesions in cats should be differentiated from feline leprosy (localized skin granulomas) caused by Mycobacterium lepraemurium and other nonculturable species of acid-fast bacilli. Atypical mycobacteriosis is caused by contamination of a skin wound with saprophytic and nonsaprophytic mycobacteria such as those of the Mycobacterium avium-intracellulare complex. Advances in PCR techniques have notably reduced the time required for etiologic diagnosis of mycobacteriosis in veterinary diagnostic laboratories.
Mycotic Pneumonias of Cats: Cryptococcosis (pulmonary Cryptococcus neoformans) is the most frequent systemic mycosis in cats, and lesions are akin to those discussed in the section on Mycotic Pneumonias, Pneumonias of Dogs. It occurs worldwide in all species but is diagnosed most frequently in cats, horses, dogs, and humans. Some healthy dogs and cats harbor Cryptococcus neoformans in the nasal cavity and become asymptomatic carriers. Cats that are immunologically compromised, such as by FeLV, FIV, malnutrition, or corticosteroid treatment, are most susceptible to clinical infection. Lesions can occur in nearly any tissue, resulting in a wide variety of clinical signs. However, granulomatous rhinitis, sinusitis, otitis media and interna, pneumonia, ulcerative dermatitis, and meningoencephalitis are most common.
The pulmonary lesion in cryptococcosis is a multifocal granulomatous pneumonia and, like those occurring in other internal organs, they are small, gelatinous, white foci. The gelatinous appearance is because of the broad mucous capsule around the yeast (Fig. 9-90, B). Microscopically, lesions contain great numbers of fungal organisms (4 to 10 µm in diameter without capsule) and only a few macrophages, lymphocytes, and multinucleated giant cells. This thick polysaccharide capsule does not stain well with H&E, and thus there is a large empty space or halo around the yeast.
Endogenous lipid (lipoid) pneumonia: Endogenous lipid pneumonia is an obscure, subclinical pulmonary disease of cats and occasionally of dogs, which is unrelated to aspiration of foreign material. Although the pathogenesis is not understood, it is presumed that lipids from pulmonary surfactant and from degenerated cells accumulate within alveolar macrophages, some of which exit the lung via the mucociliary escalator. The gross lesions are multifocal, white, firm nodules scattered throughout the lungs. Microscopically, the alveoli are filled with foamy, lipid-laden macrophages accompanied by interstitial infiltration of lymphocytes and plasma cells, fibrosis, and alveolar epithelialization.
Lipid (lipoid) pneumonia occurs frequently in the vicinity of cancerous lung lesions in humans, cats, and dogs. The reason for this association remains unknown and frequently unrecognized by pathologists. Recent investigations suggest that lipids are the breakdown products of neoplastic cells.
Exogenous lipid pneumonia: Another form of lipid pneumonia occurs accidentally in cats given mineral oil by their owners in attempts to remove hairballs (aspiration pneumonia).
Aspiration pneumonias: Aspiration pneumonias are common in cats as a result of vomiting, regurgitation, dysphagia, or anesthetic complication or after accidental administration of food, oral medicaments, or contrast media into the trachea (iatrogenic). Pulmonary lesions are similar to those described for dogs, and the type of lung lesion depends on the chemical and bacterial composition of the aspirated material (see the section on Aspiration Pneumonia in Dogs).
Feline idiopathic pulmonary fibrosis: Feline idiopathic pulmonary fibrosis is a rare disease of cats of uncertain etiology characterized by fibrotic nodules in the lung and subpleurally resulting in the pleural surface resembling nodular cirrhosis of the liver (Fig. 9-94). Microscopically, affected alveolar and peribronchiolar interstitium is thickened by excessive fibrosis and abundant deposition of ECM. The alveolar walls are diffusely lined by cuboidal hyperplastic type II pneumonocytes and the alveolar lumen often contains exfoliated cells and necrotic debris. This feline condition has morphologic features similar to “equine multinodular pulmonary fibrosis” and “cryptogenic pulmonary fibrosis” in humans.
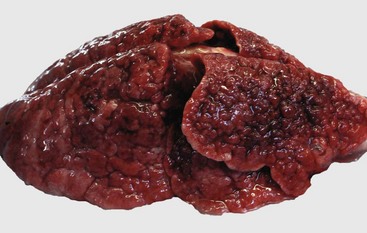
Fig. 9-94 Idiopathic nodular pulmonary fibrosis, lung, cat.
The lung contains large numbers of nodules distributed throughout all pulmonary lobes. These nodules are formed by focal areas of fibrosis with retraction of the pulmonary parenchyma admixed with focal areas of pulmonary hyperinflation. This cat had a history of chronic respiratory problems. (Courtesy Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de México.)
Parasitic Pneumonias of Cats: Aelurostrongylus abstrusus, known as feline lungworm, is a parasite that occurs in cats wherever the necessary slug and snail intermediate hosts are found. It can cause chronic respiratory disease with coughing and weight loss and, sometimes, severe dyspnea and death, particularly if there are secondary bacterial infections. The gross lesions are multifocal, amber, and subpleural granulomatous nodules up to 1 cm in diameter throughout the lungs. On incision, these nodules may contain viscous exudate. Microscopically, the parasites and their eggs and coiled larvae are in the bronchioles and alveoli where they cause catarrhal bronchiolitis, hyperplasia of submucosal glands, and later, granulomatous alveolitis, alveolar fibrosis, and fibromuscular hyperplasia. During routine examination of feline lungs, it is quite common to find fibromuscular hyperplasia in bronchioles and arterioles in otherwise healthy cats. It was alleged in the past that this fibromuscular hyperplasia was a long-term sequela of subclinical infection with Aelurostrongylus abstrusus. However, this view has been challenged, thus the pathogenesis and significance of pulmonary fibromuscular hyperplasia in healthy cats remains uncertain.
Toxoplasma gondii, Paragonimus kellicotti, and Dirofilaria immitis can also affect cats (see the section on Parasitic Pneumonias of Dogs).
Fetal and Perinatal Pneumonias
Fetal Pneumonias: Pneumonia is one of the most frequent lesions found in fetuses submitted for postmortem examination, particularly in foals and food-producing animals. Because of autolysis, lack of inflation, and the lungs being at various stages of development, fetal lesions are often missed or misdiagnosed. In the nonaerated fetal lung, the bronchoalveolar spaces are filled with a viscous, locally produced fluid known as lung fluid or lung liquid. It has been estimated that an ovine fetus produces about 2.5 mL of “lung fluid” per kilogram of body weight per hour. In the fetus, this fluid normally moves along the tracheobronchial tree, reaching the oropharynx, where a fraction is swallowed into the gastrointestinal tract, and a small portion is released into the amniotic fluid. At the time of birth, the lung fluid is rapidly reabsorbed from the lungs by alveolar absorption and lymphatic drainage.
Aspiration of amniotic fluid contaminated with meconium and bacteria from placentitis is the most common route by which microbial pathogens reach the fetal lungs. This form of pneumonia is secondary to fetal hypoxia and acidosis (“fetal distress”), which cause the fetus to relax the anal sphincter, release meconium into the amniotic fluid, and in the terminal stages inspire deeply with open glottis, resulting in the aspiration of contaminated fluid (see Web Fig. 9-14). Gross lesions are only occasionally recognized, but microscopic changes are similar to those of a bronchopneumonia. Microscopically, bronchoalveolar spaces contain variable numbers of neutrophils, macrophages, and pieces of meconium that appear as bright yellow material because of its bile content. In contrast to postnatal bronchopneumonia, lesions in fetuses are not restricted to the cranioventral aspects of the lungs but typically involve all pulmonary lobes.
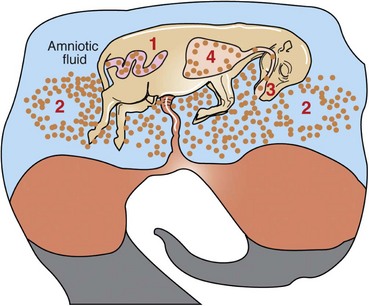
Web Fig. 9-14 Schematic diagram of meconium aspiration resulting from intrauterine hypoxia.
1, Increased peristalsis and relaxation of anal sphincter. 2, Meconium contamination of amniotic fluid. 3, Meconium in the oropharynx. 4, Intrauterine gasping with open glottis causing aspiration of meconium and amniotic fluid into fetal lung. (Redrawn with permission from Dr. J. Martinez-Burnes, Facultad de Medicina Veterinaria y Zootecnia, Universidad Autónoma de Tamaulipas, México.)
In cattle, Brucella abortus and Arcanobacterium (Actinomyces) pyogenes are two of the most common bacteria isolated from the lungs of aborted fetuses. These bacteria are usually present in large numbers in the amniotic fluid of cows with bacterial placentitis. Inflammation of the placenta interferes with oxygen exchange between fetal and maternal tissue, and the resultant fetal hypoxia induces the fetus to “breathe” with an open glottis and aspirate the amniotic fluid. Aspergillus spp. (mycotic abortion) and Ureaplasma diversum cause sporadic cases of placentitis, which results in fetal pneumonia and abortion.
In addition to the respiratory route (aspiration), pathogens, such as bacteria and viruses, can also reach the lungs via fetal blood and cause interstitial pneumonia. Listeriosis (Listeria monocytogenes), salmonellosis (Salmonella spp.), and chlamydiosis (Chlamydophila psittaci) are the best known examples of blood-borne diseases that cause fetal pneumonia in farm animals. Gross lesions in the lungs are generally undetected, but microscopic lesions include focal necrotizing interstitial pneumonia and focal necrosis in the liver, spleen, or brain. Fetal bronchointerstitial pneumonia occurs also in some viral abortions, such as those caused by IBR virus and PI-3 virus in cattle and EVR in horses. Fetal pneumonias in dogs and cats are infrequently described, perhaps because aborted puppies and kittens are rarely submitted for postmortem examination. With advancements in molecular biology techniques, the etiologic diagnosis of abortions and their association with pulmonary fetal lesions is rapidly improving.
Neonatal Pneumonias and Septicemias: These entities are rather common in newborn animals lacking passive immunity from hypogammaglobulinemia because of the lack of either ingestion or absorption of maternal colostrum (failure of passive transfer). In addition to septicemias causing interstitial pneumonia, farm animals with hypogammaglobulinemia can develop bronchopneumonia by inhalation of bacterial pathogens. These include Histophilus somni and Pasteurella multocida in calves, Streptococcus spp. in foals, and Escherichia coli, Listeria monocytogenes, and Streptococcus suis in pigs.
Meconium Aspiration Syndrome: Meconium aspiration syndrome (MAS) is an important but preventable condition in human babies that originates when amniotic fluid contaminated with meconium is aspirated during labor or immediately after birth. The pathogenesis of MAS is basically the same as in those of fetal bronchopneumonia (see Web Fig. 9-14). Fetal hypoxia, a common event during dystocia or prolonged parturition, causes the fetus to relax the anal sphincter and release meconium into the amniotic fluid. Aspiration of meconium can occur directly from aspirating contaminated amniotic fluid before delivery (respiratory movements with an open glottis), or immediately after delivery when the meconium lodged in the nasopharynx is carried into the lung with the first breath of air. This latter form of aspiration is prevented in delivery rooms by routine suction of the nasopharynx in meconium-stained babies. MAS is well known in human babies, but the occurrence and significance in animals remains largely unknown. MAS has been reported in calves, foals, piglets, and puppies. Although pulmonary lesions are generally mild and transient, aspiration of meconium can be life-threatening for newborn babies and animal because it typically occurs in compromised neonates already suffering from intrauterine hypoxia and acidosis. Neonatal acidosis is known to impair colostrum absorption in calves. Common MAS sequels are lobular atelectasis, pulmonary hypertension, and possibly airway hyperreactivity.
In the most severe cases of MAS, focal (patchy) atelectasis can be observed grossly in the lung, indicating failure of the lungs to be fully aerated because of the mechanical obstruction and the chemical effect of meconium on pulmonary surfactant (see Fig. 9-34). Microscopically, meconium and keratin exfoliated from skin of the fetus into the amniotic fluid are present in bronchi, bronchioles, and alveoli and accompanied by mild alveolitis characterized by infiltration of leukocytes followed by alveolar macrophages and occasional giant cells.
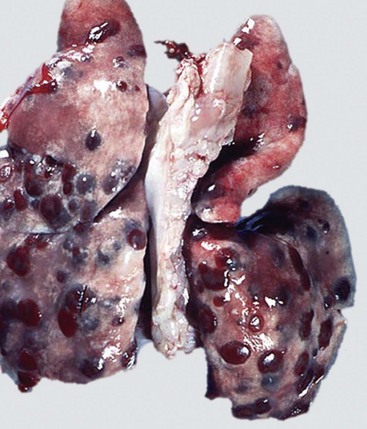
Web Fig. 9-15 Metastatic hemangiosarcoma, lung, dog.
Note the red to dark red bulging masses randomly distributed throughout all lobes of the lung. (Courtesy College of Veterinary Medicine, University of Illinois.)
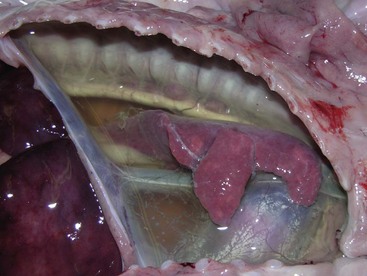
Web Fig. 9-16 Hydrothorax, pleural cavity, neonatal pig.
The pleural cavity contains a large amount of a clear yellow transudate. The fluid in the thoracic cavity has compressed the lungs resulting in compressive atelectasis. The cause of the hydrothorax could not be determined. (Courtesy Dr. V. Hsiao, College of Veterinary Medicine, University of Illinois.)
Neoplasms of the Lungs
General Considerations: Lung cancer in animals is rare, unlike in humans in which the incidence is alarming and continues to be the number one cause of cancer deaths in Canada, the US, and Europe. Interestingly, prostatic and breast cancers, so much feared by men and women, are a distant second. To say that cigarette smoking is responsible for this epidemic of lung cancer is unnecessary. Although dogs have been proposed as valuable “sentinels” for environmental hazards, such as exposure to passive smoking, asbestos, dyes, and insecticides, it is not known if the prevalence of canine lung tumors has increased in geographical areas with high contamination. Alterations in genes (oncogenes) and chromosomes and changes in biologically active molecules have been linked to lung cancer in recent years. As with many other forms of cancer, epidemiologic studies indicate that the incidence of pulmonary neoplasms increases with age, but there are still insufficient data to confirm that particular canine or feline breeds have a higher predisposition to spontaneous lung neoplasms.
A standard nomenclature of pulmonary neoplasms in domestic animals is lacking, and as a consequence, multiplicity of names and synonyms occur in the veterinary literature. Some classifications are based on the primary site, whereas others emphasize more the histomorphologic type. The most common types of benign and malignant pulmonary neoplasms in domestic mammals are listed in Box 9-2.
Clinically, the signs of pulmonary neoplasia vary with the degree of invasiveness, the amount of parenchyma involved, and locations of metastases. Signs may be vague such as cough, lethargy, anorexia, weight loss, and perhaps dyspnea. In addition, paraneoplastic syndromes, such as hypercalcemia, endocrinopathies, and pulmonary hypertrophic osteoarthropathy, have been associated with pulmonary neoplasms.
Primary Neoplasms of the Lungs: Primary neoplasms of the lungs arise from cells normally present in the pulmonary tissue and can be epithelial or mesenchymal, although the latter are rare. Primary benign neoplasms of the lungs, such as pulmonary adenomas, are highly unusual in domestic animals. Most primary neoplasms are malignant and appear as solitary masses of variable size that, with time, can metastasize to other areas of the lungs and to distant organs. It is sometimes difficult on gross and microscopic examination to differentiate primary lung cancer from pulmonary metastasis resulting from malignant neoplasms elsewhere in the body.
It is often difficult to determine the precise topographic origin of a neoplasm within the lungs—for example, whether it originates in the conducting system (bronchogenic carcinoma), transitional system (bronchiolar carcinoma), exchange system (alveolar carcinoma), or bronchial glands (bronchial gland carcinoma). According to the literature, pulmonary carcinomas in animals arise generally from Clara cells or type II pneumonocytes of the bronchioloalveolar region, in contrast to those in humans, which are mostly bronchogenic. Tumors located at the hilus generally arise from major bronchi and tend to be a solitary large mass with occasional small metastasis to the periphery of the lung. In contrast, tumors arising from the bronchioloalveolar region are often multicentric with numerous peripheral metastases in the lung parenchyma. Because of histologic architecture and irrespective of their site of origin, many malignant epithelial neoplasms are frequently classified by the all-encompassing term of pulmonary adenocarcinomas.
Dogs and cats are the species most frequently affected with primary pulmonary neoplasms, largely carcinomas, generally in older animals. The mean age for primary lung tumors is 11 years for dogs and 12 years for cats. Pulmonary carcinomas in other domestic animals are less common, possibly because fewer farm animals are allowed to reach their natural life span. These neoplasms can be invasive or expansive, vary in color (white, tan, or gray) and texture (soft or firm), and often have areas of necrosis and hemorrhage, which result in a “craterous” or “umbilicate” appearance. This umbilicate appearance is frequently seen in rapidly growing carcinomas in which the center of the tumoral mass undergoes necrosis as a result of ischemia. Some lung neoplasms resemble pulmonary consolidation or large granulomas. Cats with moderately differentiated neoplasms had significantly longer survival time (median, 698 days) than cats with poorly differentiated neoplasms (median, 75 days). Dogs with primary lung neoplasms, grades I, II, and III, had survival times of 790, 251, and 5 days, respectively.
Ovine Pulmonary Adenocarcinoma (Ovine Pulmonary Carcinoma): Ovine pulmonary adenocarcinoma, also known as pulmonary adenomatosis and jaagsiekte (from the South African Afrikaans word for “driving sickness”), is a transmissible, retrovirus-induced neoplasia of ovine lungs. It occurs in sheep around the world, with the notable exception of Australia and New Zealand; its incidence is high in Scotland, South Africa, and Peru and unknown but probably low in North America. This pulmonary carcinoma behaves very much like a chronic pneumonia, and the “Jaagsiekte sheep retrovirus” shares many epidemiologic similarities with the ovine lentivirus responsible for Maedi and the retrovirus responsible for enzootic nasal carcinoma in ruminants. Pulmonary adenomatosis has been transmitted to goats experimentally but is not known to be a spontaneous disease in that species.
This disease affects mainly mature sheep but can occasionally affect young stock. Intensive husbandry probably facilitates horizontal transmission by the copious nasal discharge and explains why the disease occurs in Iceland as devastating epizootics with 5% to 80% mortality. Differential diagnosis between maedi and pulmonary adenomatosis can prove difficult because both diseases often coexist in the same flock or in the same animal. Death is inevitable after several months of the initial onset of respiratory signs, and a specific humoral immune response to the Jaagsiekte sheep retrovirus is undetectable in affected sheep.
During the early stages of ovine pulmonary carcinoma, the lungs are enlarged, heavy, and wet and have several firm, gray, variably-sized nodules that tend to be located in the cranioventral lobes (Fig. 9-95, A). In the later stages, the nodules become confluent, and large segments of both lungs are diffusely, but not symmetrically, infiltrated by neoplastic cells. On cross-section, edematous fluid and a copious mucoid secretion are present in the trachea and bronchi (Fig. 9-95, B). Microscopically, the nodules consist of cuboidal or columnar epithelial cells lining airways and alveoli and forming papillary or acinar (glandlike) structures (Fig. 9-95, A inset). Because the cells have been identified ultrastructurally as originating from both type II alveolar epithelial cells and Clara cells, the neoplasm is considered a “bronchioloalveolar” carcinoma. Sequelae often include secondary bronchopneumonia, abscesses, and fibrous pleural adhesions. Metastases occur to tracheobronchial and mediastinal lymph nodes and to a lesser extent to other tissues such as pleura, muscle, liver, and kidneys.
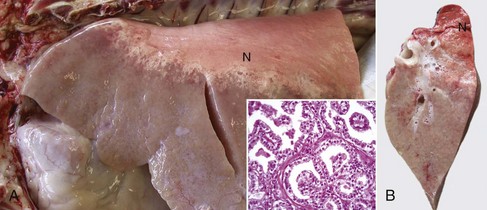
Fig. 9-95 Ovine pulmonary carcinoma (pulmonary adenomatosis, jaagsiekte), lung, 3-year-old sheep.
A, Neoplastic cell infiltration involving the cranial and ventral portions of the lung and mainly sparing the dorsal portions of the caudal lung lobe (N). The affected lung is enlarged and firm. Inset, Papillary proliferation of cuboidal epithelial cells (presumed type II pneumonocytes). H&E stain. B, Transverse section of the cranial lobe. Note the solid appearance of the ventral portion (bottom) of the lung and the frothy fluid (edema) that originates in the alveolar walls. N, Normal lung. (Courtesy Dr. M. Heras, Facultad de Veterinaria, Universidad de Zaragoza, Spain.)
Clinically, pulmonary adenomatosis is characterized by a gradual loss of condition, sometimes coughing, and respiratory distress, especially after exercise (such as herding or “driving”). Appetite and temperature are normal, unless there are secondary bacterial infections. An important differentiating feature from maedi (interstitial pneumonia) can be observed if the animals with pulmonary adenomatosis are raised by their hind limbs; copious, thin, mucoid fluid, produced by neoplastic cells in the lungs, pours from the nostrils of some animals.
Carcinoid (Neuroendocrine) Tumor of the Lungs: Carcinoid tumor of the lungs is a neoplasm presumably arising from neuroendocrine cells that is sporadically seen in dogs as multiple, large, firm pulmonary masses close to the mainstem bronchi. It has also been reported in the nasal cavity of horses. Tumor cells are generally polygonal with finely granular, pale, or slightly eosinophilic cytoplasm. Nuclei are small, and mitotic figures are absent or rare.
Granular Cell Tumor: Granular cell tumor is a rare and locally invasive tumor that has been reported mainly in humans and older horses. The cell origin of this tumor was thought to be the myoblast, but it is currently presumed to be Schwann cells, which are normally present in the bronchovascular bundles of the lung. Microscopically, neoplastic cells are large, polyhedron-shaped with abundant cytoplasm containing numerous acidophilic granules, which are positive for S-100 protein. Although this tumor can caused bronchial obstruction and respiratory signs, in most cases, it is an incidental finding in older horses submitted for postmortem examination.
Lymphomatoid Granulomatosis: Lymphomatoid granulomatosis is a rare but interesting disease of humans, dogs, and cats characterized by nodules or large solid masses in one or more lung lobes. These frequently metastasize to lymph nodes, kidneys, and liver. Microscopically, tumors are formed by large pleomorphic mononuclear (lymphomatoid) cells with a high mitotic rate and frequent formation of binucleated or multinucleated cells. Tumor cells have a distinct tendency to grow around blood vessels and destroy the vascular walls. Lymphomatoid granulomatosis has some resemblance to lymphomas, and phenotypic marking confirms that neoplastic cells are a mixed population of plasma cells, B and T lymphocytes, and histiocytes.
Secondary Neoplasms of the Lungs: Secondary neoplasms of the lungs are all malignant by definition because they are the result of metastasis to the lungs from malignant neoplasms elsewhere. Because the pulmonary capillaries are the first filter met by tumor emboli released into the vena cava or pulmonary arteries, secondary neoplasms in the lung are relatively common in comparison to primary ones. Also, secondary tumors can be epithelial or mesenchymal in origin. Common metastatic tumors of epithelial origin are mammary, thyroid (Fig. 9-96), and uterine carcinomas. Tumors of mesenchymal origin are osteosarcoma (Fig. 9-97, A); hemangiosarcoma (see Fig. 9-97, B and Web Fig. 9-15); malignant melanoma in dogs; malignant lymphoma in cows, pigs, dogs, and cats (Fig. 9-98); and the recently reported vaccination-site fibrosarcoma in cats. Usually, secondary pulmonary neoplasms are multiple, scattered throughout all pulmonary lobes (hematogenous dissemination), of variable size, and according to the growth pattern, can be nodular, diffuse, or radiating.
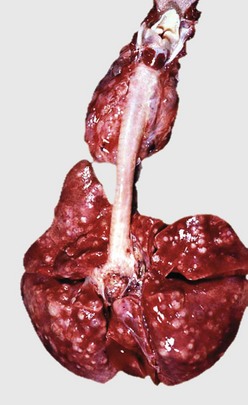
Fig. 9-96 Metastatic thyroid carcinoma, lungs, adult dog.
The lungs contain multiple randomly distributed metastatic nodules, which originated from the enlarged and neoplastic left thyroid gland. (Courtesy Dr. J.M. King, College of Veterinary Medicine, Cornell University.)
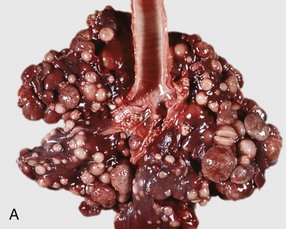
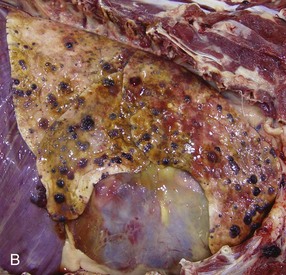
Fig. 9-97 Lung, dog.
A, Metastatic sarcoma (primary site unknown). Large numbers of metastatic nodules are randomly distributed throughout all lung lobes. B, Metastatic hemangiosarcoma. Note the red to dark red masses throughout the lung parenchyma. If these masses were black, metastatic melanoma would be the likely diagnosis. (A courtesy Dr. J.M. King, College of Veterinary Medicine, Cornell University. B courtesy Dr. A. Bourque and Dr. A. López, Atlantic Veterinary College.)
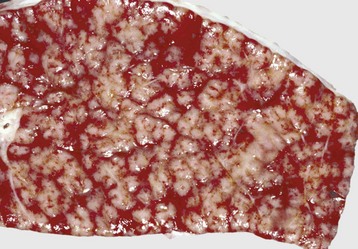
Fig. 9-98 Metastatic lymphoma (lymphosarcoma), lungs, cut surface, cow.
Note the numerous discrete and confluent metastatic nodules with the smooth texture and gray color characteristic of lymphoma. (Courtesy College of Veterinary Medicine, University of Illinois.)
The appearance of metastatic neoplasms differs according to the type of neoplasm. For example, dark red cystic nodules containing blood indicate hemangiosarcoma; dark black solid nodules indicate melanoma; and hard solid nodules (white, yellow, or tan color) with bone spicules indicate osteosarcoma. The gross appearances of metastatic carcinomas are generally similar to the primary neoplasm and sometimes have umbilicated centers. Proper diagnoses of pulmonary neoplasms in live animals require history, clinical signs, radiographs, cytologic analysis of BAL fluid, and when necessary, a lung biopsy. Identification of a specific lineage of neoplastic cells in biopsy or postmortem specimens is often difficult and requires electron microscopy or immunohistochemical techniques. Electron microscopy allows identification of distinctive cellular components such as osmophilic lamellar phospholipid nephritic bodies in alveolar type II epithelial cells or melanosomes in melanomas. Immunohistochemical staining of intermediate filaments is also helpful in identifying tumor cells, for example, a metastatic neurofibrosarcoma can be distinguished from an anaplastic hemangiosarcoma by the presence of S-100 protein and factor VIII–related markers.