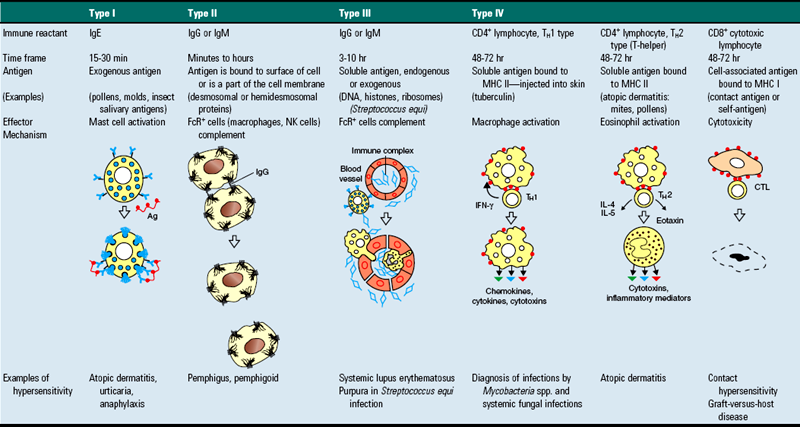
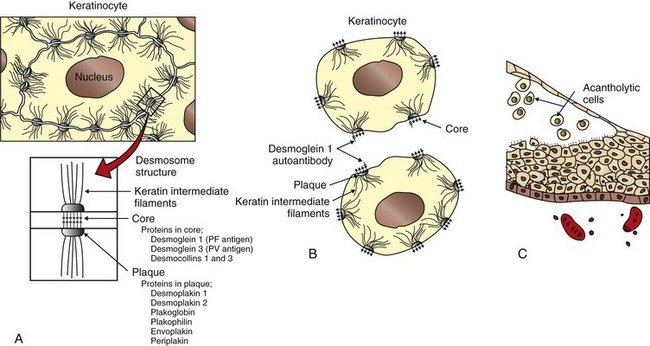
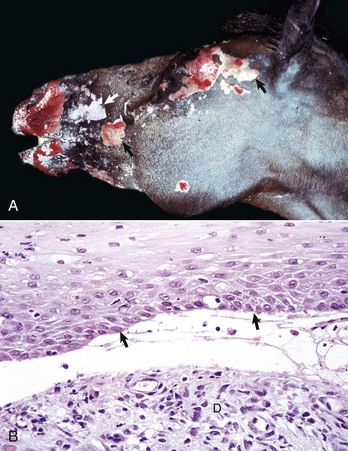
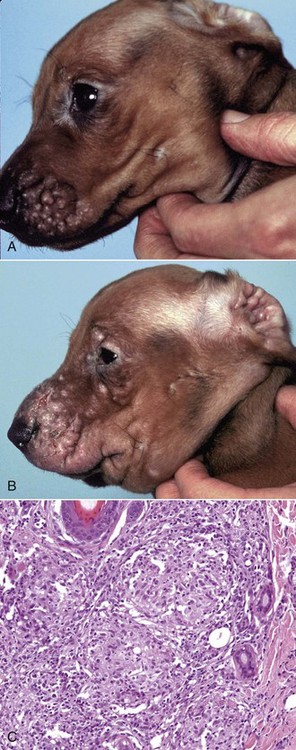
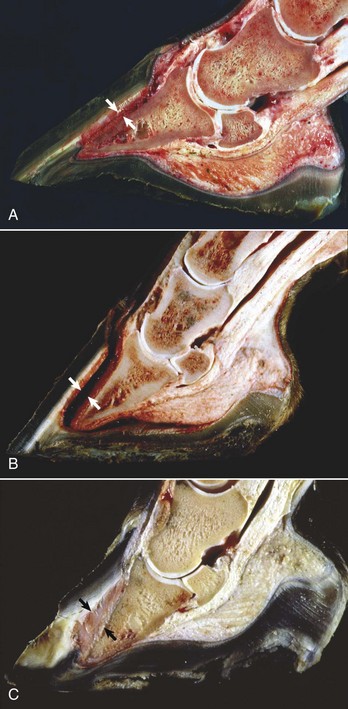
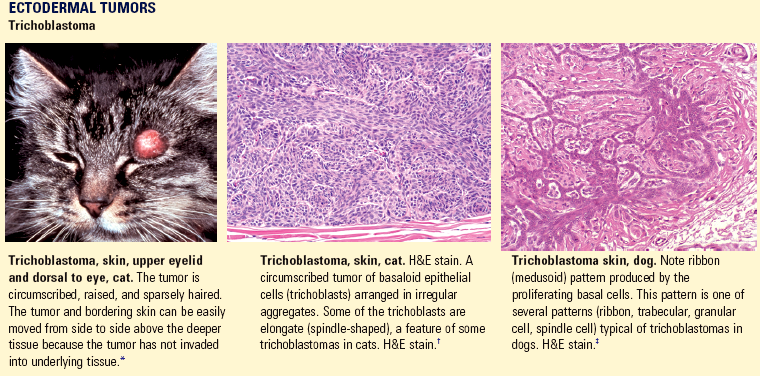
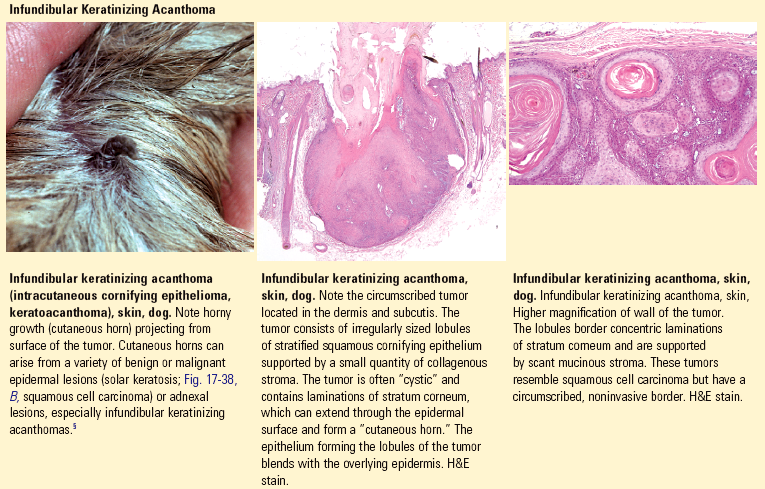
Acantholysis: loss of cohesion between keratinocytes caused by the breakdown of intercellular bridges.
Acanthosis: thickening of the spinous cell layer (stratum spinosum) of the epidermis.
Acral: distal parts of the extremities.
Alopecia: hair loss.
Anagen: phase of hair cycle in which hair synthesis takes place.
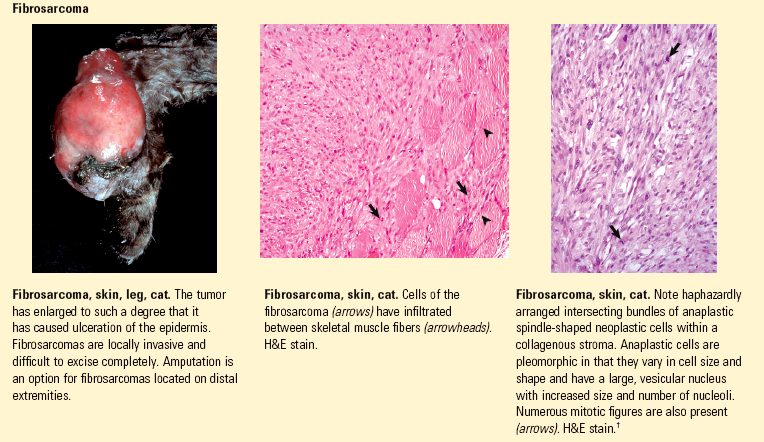
Anaplasia: lack of cellular differentiation and organization, a feature of neoplastic cells.
Angioedema: vascular reaction involving the deep dermis or subcutis and consisting of edema manifested as giant wheals and caused by dilation and increased permeability of capillaries (deeper version of urticaria).
Apoptosis: programmed cell death.
Atrophy: reduction in size of a cell, tissue, organ, or part.
Ballooning degeneration: marked intracellular fluid accumulation in the cells of the epidermis.
Blister (vesicle or bulla): localized collection of fluid usually in or beneath the epidermis.
Bulla: large blister (≥1.0 cm).
Carcinoma in situ: a malignant neoplasm of epithelial origin that has not invaded through the basement membrane.
Catagen: transition phase of the hair cycle between growth and resting phases.
Cellulitis: an acute bacterial infection of the dermis and subcutis that spreads to surrounding soft tissues and is characterized by erythema, warmth, swelling, and pain. The source of the infection is most often a penetrating wound in the area of infection. Cellulitis can also cause fever and enlarged lymph nodes.
Comedo (pl., comedones): plug of follicular stratum corneum and dried sebum in a hair follicle that leads to follicular distention.
Cornification: production of stratum corneum by terminal epidermal differentiation.
Crust: material formed by drying of exudate or secretion on the skin surface.
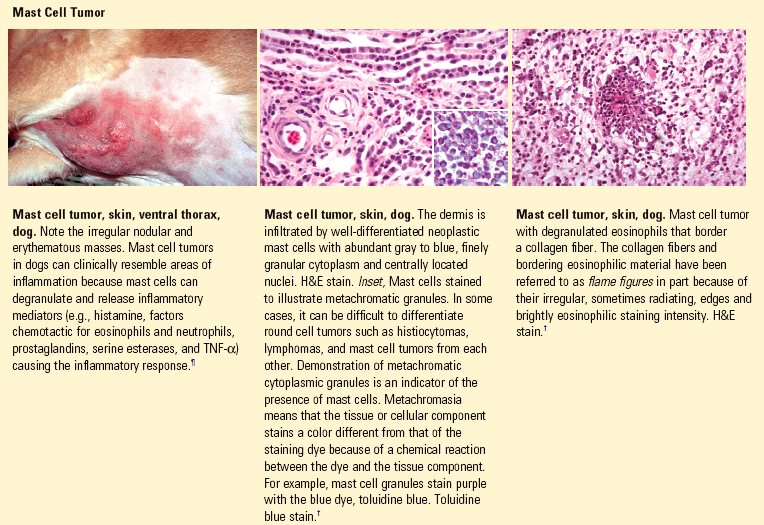
Cytokines: small molecular weight protein molecules (generally <30 kD) that are mediators of inflammation and growth.
Dematiaceous: naturally pigmented black or brown mycelium or conidium.
Dermatitis: inflammation of the skin.
Dermatophytosis: infection of the stratum corneum of the epidermis, hair, or claws with fungi of the genera Microsporum, Epidermophyton, or Trichophyton.
Dermatosis: noninflammatory lesion of the skin.
Dyskeratosis: abnormal, premature, or imperfect keratinization.
Dysplasia: abnormal development; term may be used in association with a congenital or inherited developmental anomaly or in association with an abnormality in maturation of cells within a tissue.
Effluvium: shedding of hair.
Elastosis: degeneration of dermal connective tissue leading to accumulation of elastotic fibers; sometimes seen with solar dermatitis.
Epidermal collarette: peripheral expanding ring of scale.
Epidermitis: inflammation of the epidermis.
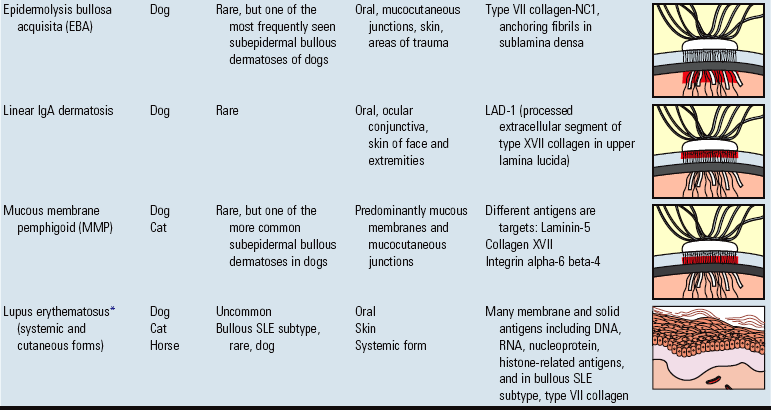
Epidermolysis: separation of the epidermis from the dermis.
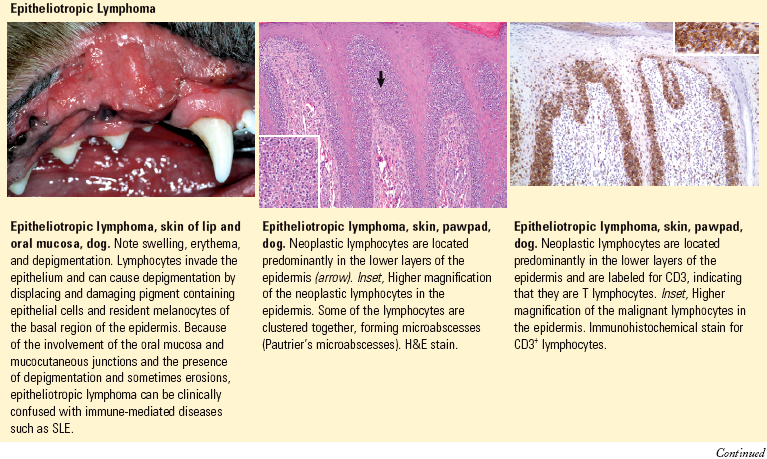
Epidermotropic/Epitheliotropic: having a predilection to enter the epidermis or other epithelial structures as seen with cutaneous T cell lymphoma (mycosis fungoides).
Erosion: loss of all or part of the thickness of the epidermis.
Eruption: rapid development of skin lesion associated with redness.
Erythema: redness of skin caused by congestion of capillaries.
Excoriation: superficial loss of epidermal layers caused by physical trauma (scratching).
Exfoliation: shedding of layers or scales.
Exogen: the stage of the hair cycle where old hairs are shed.
Exudate: fluid, cells, or debris from blood vessels deposited in or on other tissues.
Fissure: cleft or groove.
Folliculitis: inflammation of a hair follicle.
Furuncle: circumscribed, painful nodule (accumulation of pus) in the dermis secondary to follicular rupture.
Furunculosis: rupture of follicles usually caused by inflammation, distention, and/or trauma leading to entry of follicular contents into the dermis.
Genodermatosis: a genetically determined disorder of the skin.
Glabrous: smooth skin, hairless skin.
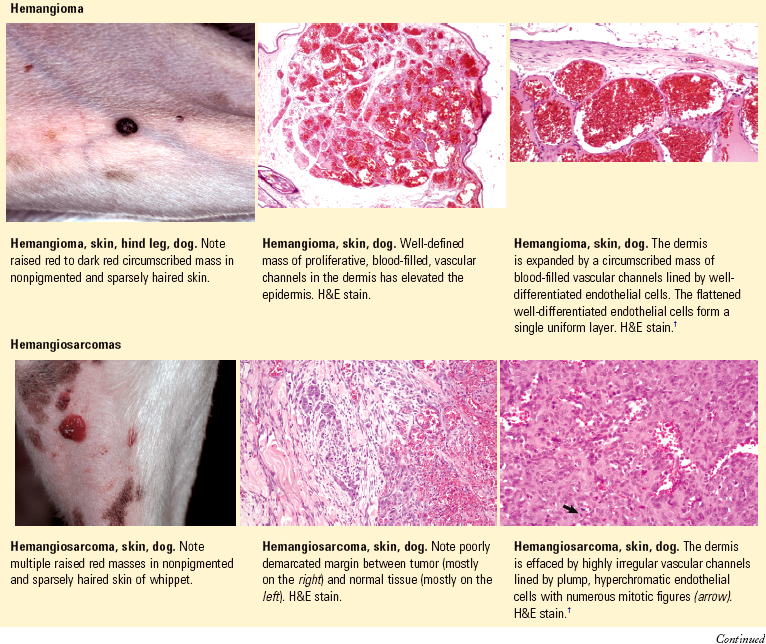
Hamartoma: a localized, tumor-like malformation of mature cells and tissues that includes normal components of the organ in which the hamartoma arises but that is disorganized, present in excess, and sometimes larger than normal. Usually, one tissue element predominates (e.g., follicular hamartoma, vascular hamartoma). A hamartoma is not a true neoplasm because it involves the proliferation of more than one cell type and often includes the development of complex structures such as arteries or follicles.
Hydropic degeneration: intracellular fluid accumulation in cells of the basal epidermis.
Hyperkeratosis: histologic term for thickening of stratum corneum.
Hyperplasia: increase in the number of normal cells.
Hypoplasia: incomplete development.
Hypotrichosis: less hair than normal.
Ichthyosis: congenital skin disorder in which the skin is thickened by scales (hyperkeratosis) that can crack into plates resembling fish scales.
Impetigo: bacterial dermatitis characterized by pustules.
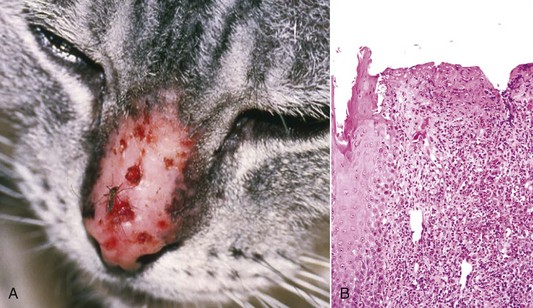
Indolent: slow growing, a term applied to persistent ulcers on the lips of cats, and sometimes incorrectly called “rodent ulcer,” a term from the human literature used to refer to ulcerated basal cell carcinoma.
Indurated: Hardening of the skin as a result of inflammation or fibrosis.
Interface: inflammation arranged in a layer close to and often obscuring the epidermal-dermal junction (interface), and with vacuolated (hydropic degeneration) and sometimes apoptotic basal cells; the inflammation can be mild (cell poor) or extensive (cell rich).
Intertrigo: dermatitis that develops because of friction between apposing skin surfaces (e.g., adjacent folds).
Keratinocytes: the epidermal cells that synthesize keratin and comprise more than 90% of epidermal cells.
Keratosis (pl., keratoses): an uncommon to rare circumscribed papular, plaque-like, or linear focus of proliferative keratinocytes covered by thick stratum corneum; keratoses can be caused by sun exposure (solar or actinic keratoses) or can be idiopathic (lichenoid, linear, cannon [metatarsal bone] keratoses).
Kerion: an intense focal folliculitis usually caused by a dermatophyte infection.
Langerhans’ cells: intraepidermal dendritic antigen-presenting cells.
Lichenification: thickening of skin with accentuation of skin creases caused by marked acanthosis.
Lichenoid: confusing term that generally refers to a dense zone of dermal inflammation parallel to the epidermis usually without basal cell injury.
Lichenoid dermatosis (es): the conventional term for uncommon to rare, often idiopathic, single or grouped papules, plaques, or papillomatous foci covered by scale, and histologically composed of epidermal hyperplasia, lichenoid lymphoplasmacytic dermal inflammation, hyperkeratosis, and parakeratosis. The term dermatitis is probably better than dermatosis as inflammation is present in these lesions.
Macule: flat, circumscribed lesion of altered skin color.
Melanin: the dark granular pigment produced by melanocytes that is responsible for the brown coloration of hair, skin, and other tissues such as the iris and choroid of the eye.
Melanophage: macrophage containing ingested melanin.
Merkel cell: a neuroendocrine cell found in the stratum basale.
Mucin: glycosaminoglycan (GAG), a normal component of the intercellular ground substance of the dermis, consists of protein bound to hyaluronic acid.
Mycelium: a mass of hyphae.
Mycetoma: a slowly progressive infection of the cutaneous and subcutaneous tissue, fascia, and sometimes underlying bone caused by traumatic implantation of actinomycetes (actinomycotic mycetoma) or fungi (eumycotic mycetoma).
Myxedema: nonpitting edema of the skin because of abnormal deposits of mucin in the dermis.
Necrotizing fasciitis: an acute serious life-threatening subtype of cellulitis usually caused by streptococcal bacterial infection and toxin production, and located within the subcutaneous fat and fascial planes. The clinical lesions are painful, hot, and swollen areas with extensive exudation and necrosis. The condition can progress rapidly and result in systemic shock.
Nevus: circumscribed malformation of the skin assumed to be of congenital or inherited origin, and consisting of any component of the skin. The term “hamartoma” is preferred to nevus to avoid confusion with the pigmented nevus (mole) that arises in the skin of humans.
Nodule: a circumscribed, solid elevation of skin (≥1 cm).
Onychodystrophy: abnormal formation of the claw.
Onychomadesis: sloughing of claws.
Panniculitis: inflammation of subcutaneous adipose tissue.
Papule: circumscribed, solid elevation of skin (<1 cm).
Parakeratosis: retention of pyknotic nuclei in epidermal cells of the stratum corneum.
Paronychia: inflammation of skin around the claws.
Pautrier’s microabscess: a localized intraepidermal collection of neoplastic lymphocytes characteristic of epitheliotropic lymphoma (mycosis fungoides).
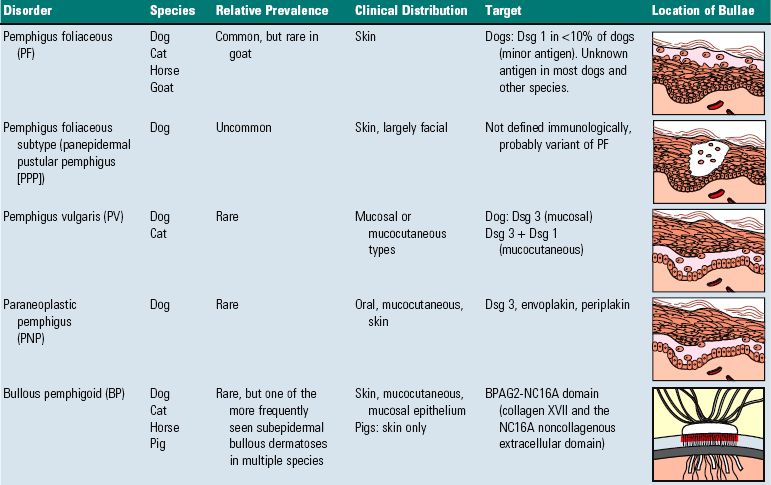
Pemphigus: a group of cutaneous diseases associated with blistering.
Phaeohyphomycosis: mycotic disease caused by pigmented fungi (dematiaceous fungi) of a variety of genera and species that do not form sclerotic bodies or granules.
Pigmentary incontinence: melanin pigment within dermal macrophages or free in the dermis developing via injury to pigment containing basal layer cells.
Plaque: a flat-topped, solid elevation in the skin that occupies a relatively large surface area in comparison with its height (≥1 cm).
Pruritus: itching.
Pustule: small, circumscribed accumulation of pus within the epidermis or within a hair follicle.
Pyoderma: pyogenic (pus-producing) bacterial infection of the skin.
Rodent ulcer: a term used in human medicine to define an ulcerative basal cell carcinoma; sometimes used inappropriately in veterinary medicine to refer to an indolent ulcer affecting the lip of cats.
Scale: a thin, platelike accumulation of stratum corneum on the surface of skin.
Seborrhea: nonspecific term for clinical signs of scaling, crusting, and greasiness. Primary seborrhea is a more specific term applied to inherited cornification disorders.
Sebum: secretion of sebaceous glands.
Spongiosis: intercellular edema, which, by widening of the intercellular space and stretching of the “intercellular bridges,” creates a spongelike appearance to the epidermis.
Telogen: resting phase of the hair cycle.
Ulcer: loss of epidermis and at least the superficial portion of dermis.
Urticaria: usually transient vascular reaction in the upper dermis consisting of edema manifested clinically as wheals (hives); a more superficial version of angioedema.
Vesicle: small blister within the epidermis or at or below the dermal-epidermal interface (<1.0 cm) (see Fig. 17-17).
Vibrissa (pl., vibrissae): long, coarse hair located about the nose (sinus hair, tactile hair).
Vitiligo: acquired disorder characterized by circumscribed areas of depigmentation in the skin.
Wheal: smooth, circumscribed, slightly elevated area on skin caused by dermal edema.
Yeast: unicellular budding fungus.