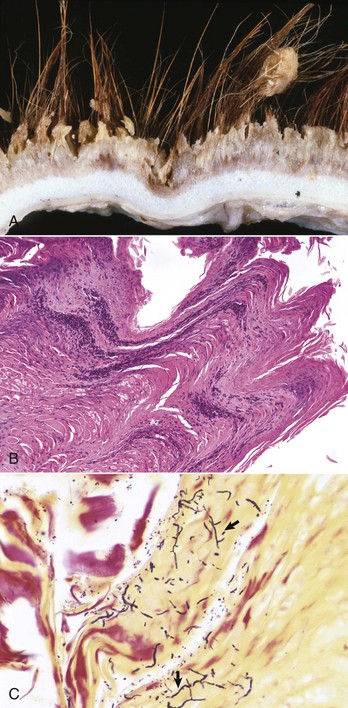
Poxviruses: Poxviruses are DNA epitheliotropic viruses that infect most domestic, wild, and laboratory animals and birds (Table 17-7). Dogs and cats are rarely infected with poxviruses, although infection with contagious ecthyma (parapoxvirus) has been reported in dogs, and cutaneous infection caused by a poxvirus of the orthopoxvirus genus (cowpox) has been reported in cats in Europe. There are rare anecdotal reports of poxvirus infection in the skin of cats in North America, but, with the exception of one case, the poxvirus or viruses involved have not been characterized. In the single case in which the virus was identified, polymerase chain reaction (PCR) and gene sequencing identified raccoonpox, an orthopoxvirus, in the skin lesions. There are major differences between different poxviruses and in the range of species they infect; some are species-specific and others are zoonotic. Many poxviruses of animals, such as the contagious ecthyma parapoxvirus, can cause skin lesions in humans. Poxviruses induce lesions by a variety of mechanisms. Lesions develop secondary to poxviral invasion of epithelium, by ischemic necrosis caused by vascular injury, and by stimulation of host cell DNA, resulting in epidermal hyperplasia. Hyperplasia may be explained by a gene, present in several poxviruses, including molluscum contagiosum, whose product has significant homology with epidermal growth factor. Poxviruses also encode for functions that may counteract host defenses. These include genes related to those encoding the serpins (a superfamily of related proteins important in regulating serine protease enzymes that mediate kinin, complement, fibrinolytic, and coagulation pathways) and genes encoding antiinterferon activities. The severity of poxviral infection varies, depending on whether the infection is localized (cutaneous) or systemic and whether there are secondary infections. The sequence of the cutaneous lesions are macule, papule, vesicle (varies in severity), umbilicated pustule, crust, and scar (see Fig. 17-31). Histologically, pox lesions begin as keratinocyte cytoplasmic swelling and vacuolation, usually first affecting the cells of the outer stratum spinosum. Rupture of the damaged keratinocytes produces multiloculated vesicles, so-called reticular degeneration. The early dermal lesions include edema, vascular dilation, a perivascular mononuclear cell infiltrate, and a variable neutrophilic infiltrate. Neutrophils migrate into the epidermis and aggregate in vesicles to form microabscesses. Large intraepidermal pustules can form and sometimes extend into the superficial dermis. There is usually marked epithelial hyperplasia and sometimes pseudocarcinomatous hyperplasia of the adjacent epithelium. This contributes to the raised border of the umbilicated pustule. Rupture or drying of the pustule produces a crust, often colonized on its surface by bacteria. Poxvirus lesions often contain characteristic intracytoplasmic eosinophilic inclusion bodies. These are single or multiple and of varying size and duration. The inclusions are primarily comprised of proteins. Sheeppox and goatpox are the most pathogenic poxviruses, and infection causes significant mortality, especially in young animals as a result of systemic disease. Sheeppox and goatpox do not occur in the US or Canada.
TABLE 17-7
Viral Infections of the Skin
*Indirect evidence of viral involvement.
Molluscum Contagiosum: Equine molluscum contagiosum is a self-limiting cutaneous infection in the horse caused by a poxvirus from the genera Molluscipoxvirus (molluscum contagiosum virus), a virus closely related to, or possibly the same as, the human molluscum contagiosum virus. The equine lesions are small and often incidental, and may be localized to the penis, prepuce, axillary and inguinal areas, and nose. However, the lesions may become widespread, and hundreds of lesions are present over the neck, shoulders, chest, and legs. The pathogenesis of lesion formation is typical of poxvirus infection. Commencing as multiple, circular, smooth-surfaced, gray-white papules 1 to 2 mm in diameter, the lesions become umbilicated and develop a central pore from which a caseous plug is extruded. The microscopic lesions of molluscum contagiosum consist of well-demarcated foci of epidermal hyperplasia and hypertrophy that form invaginated lobules of the epidermis in the superficial dermis. Keratinocytes containing inclusions exfoliate through a pore that forms in the stratum corneum and enlarges into a central crater. The individual keratinocytes are markedly swollen and contain large intracytoplasmic eosinophilic inclusions, known as molluscum bodies, which can be identified histologically and in cytologic preparations. There is usually no dermal reaction. Rarely, molluscum contagiosum has developed in dogs.
Cowpox: Cowpox virus infections occur rarely in cattle in the United Kingdom and other areas of Europe. There is increasing evidence that small wild rodents (i.e., mice, squirrels, voles) serve as a reservoir for infection and that cattle, cats, and rarely other mammals become infected through contact with the wild rodents. Cutaneous infections in cattle usually develop on the teats and udder of cows and on the muzzle of suckling calves. Lesions follow the typical sequence of cutaneous poxviral infections. In cats, poxvirus infection is uncommon and usually occurs in outdoor cats living in rural areas, presumably because these cats hunt and have contact with rodents harboring the poxvirus. Primary cutaneous lesions typically develop on the face, neck, or forelegs and consist of an ulcerated or crusted macule or plaque. Lesions can develop into deep ulcers that heal with granulation tissue or less commonly develop into abscesses or cellulitis. Rarely, oral or mucocutaneous junctional areas are affected. Additional secondary cutaneous lesions can develop within about 2 weeks after viremic distribution to other cutaneous sites and less commonly to the upper or lower respiratory tract. The microscopic lesions are sharply demarcated, often deep ulcers covered by fibrinonecrotic exudate. Intracytoplasmic inclusion bodies in keratinocytes or follicular or sebaceous glandular epithelial cells help establish the diagnosis.
Bovine Papular Stomatitis: Bovine papular stomatitis virus is distributed worldwide, and although it causes disease more commonly in cattle more than 2 years old, it can occur at any age and in any breed. Lesions occur on the muzzle, nostrils, lips, and mouth, and cows with suckling calves can develop teat and udder lesions. The development and appearance of the lesions are similar to pseudocowpox, with resolution of lesions in days to weeks. A chronic form has been described in which exudative necrotic dermatitis involves the trunk, as well as the mouth, and in which the animals died in 4 to 6 weeks. Transmission to humans induces lesions identical to “milker’s nodule” caused by Pseudocowpox virus infection. The histologic appearance of lesions is typical of other poxviral infections.
Capripoxviral Diseases: Capripoxviruses are the cause of sheeppox and goatpox. These viruses cause significant economic losses in countries where they are endemic, and the geographic distribution of these viruses is expanding. Sheeppox and goatpox are present in Africa, Asia, the Middle East, and most of the Indian subcontinent where, despite attempts at vaccination, capripoxvirus is responsible for cycles of epidemic disease followed by periods of endemic maintenance with low morbidity. The disease is exotic to the Americas, Australia, and New Zealand. Although eradication measures eliminated the disease from Britain in the mid-nineteenth century, they have only recently been successful in Eastern European countries. The diseases they cause lead to constraints on international trade of livestock and related products and can prevent the importation of new breeds of sheep or goats into endemic areas because fatality rates can be very high in nonindigenous breeds. Capripoxviruses are highly contagious and spread by the respiratory tract in times of close contact and mechanically by insect vectors and fomites. Virus is shed in saliva, conjunctival secretions, milk, urine, and feces, as well as in skin lesions and scabs. Vaccination of susceptible animals for sheeppox and goatpox provides lifelong immunity. The viruses share a high percentage of homology at the nucleotide and amino acid levels but are distinguishable phylogenetically using PCR-RFLP techniques. These viruses are also considered potential agents of agroterrorism.
Sheeppox: Sheeppox is caused by the sheeppox virus and is the most serious of the pox diseases of domestic animals. Sheeppox causes extensive economic loss through high mortality, reduced meat, milk or wool yields, commercial inhibitions from quarantine requirements, and the cost of disease prevention programs.
Transmission of infection is by direct contact with diseased sheep or indirect contact via contaminated environment. Sheeppox virus is resistant to desiccation and remains viable for up to 2 months on wool or 6 months in dried crust. There are breed differences in disease susceptibility. Fine-wooled Merino sheep are particularly sensitive, whereas breeds native to endemic areas, such as Algerian sheep, are comparatively resistant. Sheeppox occurs in all ages of sheep with a high morbidity and a mortality as high as 50%; but the disease is most severe in lambs, with mortality reaching 80% to 100%. A high level of background immunity, such as occurs in endemic areas of Kenya, is associated with low mortality, even in the young.
Sheeppox is a systemic disease. Infection is usually by the respiratory route but may occur through skin abrasions. The incubation period varies from 4 to 21 days and is followed by a leukocyte-associated viremia. The virus localizes in many organs, including the skin where the virus concentration is highest 10 to 14 days postinfection. The initial clinical signs are fever, lacrimation, drooling, serous nasal discharge, and hyperesthesia. Skin lesions develop in 1 to 2 days and have a predilection for the sparsely-wooled areas and typically involve eyelids, cheeks, nostrils, vulva, udder, scrotum, prepuce, ventral surface of the tail, and medial thigh. There is usually a concurrent superficial lymphadenopathy.
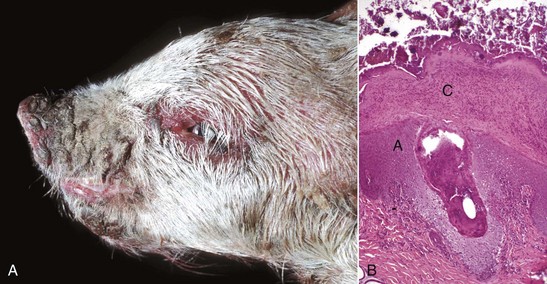
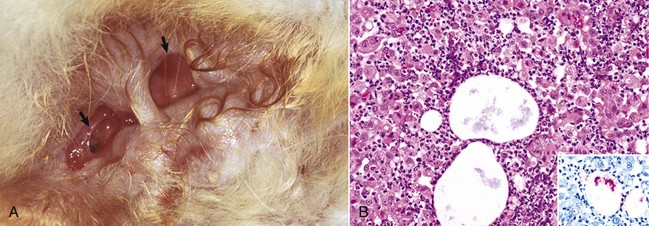
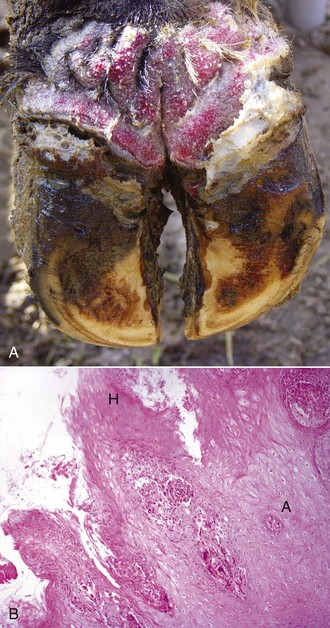
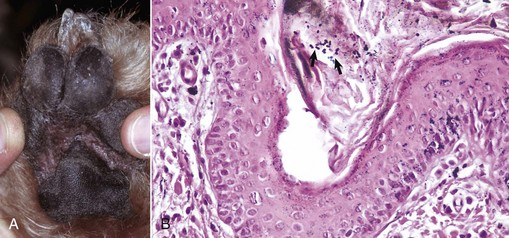
The macroscopic lesions follow the typical pattern for pox infections. Erythematous macules progress to papules, which may be firm. Sheeppox lesions have a variably prominent vesicular stage. The pustule stage is characterized by the formation of a thin crust. In severely affected animals, the lesions coalesce and form areas of edema, hemorrhage, necrosis, and induration, involving all layers of the skin and subcutis (Fig. 17-42). These areas correspond to the development of vasculitis described later with microscopic lesions (Fig. 17-42, B). Highly susceptible animals often develop hemorrhagic mucosal papules early in the course of the disease, and ulcerative lesions in the gastrointestinal and respiratory tracts develop later. Approximately one-third of animals develop multiple pulmonary lesions that comprise foci of pulmonary consolidation. The kidneys have multifocal, circular, and fleshy nodules throughout the renal cortices.
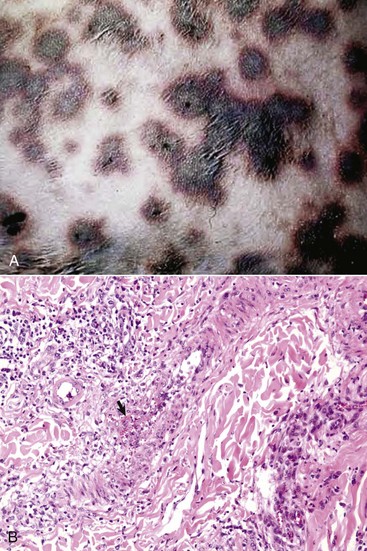
Fig. 17-42 Capripoxvirus, skin, lamb.
A, The clinical lesions are multifocal coalescing macules and plaques that are indurated, hemorrhagic, and necrotic likely a result of vasculitis. B, Note necrosis of vessel wall with fibrin deposition, red blood cells, and neutrophils and lymphocytes in the bordering dermis (vasculitis) (arrow). H&E stain. (A courtesy of Foreign animal diseases, ed 7, 2008, United States Animal Health Association. B courtesy Dr. A.M. Hargis, DermatoDiagnostics. Photographed from slides provided by Division of Animal Medicine, Animal Technology Institute Taiwan. From AFIP WSC October 8, 2008, Conference 5, Case III.)
Healing of the skin lesions is slow, taking up to 6 weeks, and a scar may remain. In the milder form of the disease, seen in endemic areas, the full range of pox lesions does not develop. Instead, epidermal proliferation produces papules covered by scale-crust, which heal with desquamation in a few days. Such lesions often occur on the ventral surface of the tail.
Sheeppox lesions have the typical microscopic poxviral epithelial lesions, including intracytoplasmic inclusion bodies. The lesions affect both surface epithelium and that of the hair follicles. There are also marked dermal lesions reflecting the systemic route of cutaneous involvement and possibly implicating immune-mediated lesions in addition to those caused by direct viral damage. The initial dermal lesions, corresponding to the macroscopic erythematous macule, are marked edema, hyperemia, and neutrophilic exocytosis. During the papular stage, large numbers of mononuclear cells accumulate in the increasingly edematous dermis. These mononuclear cells are called sheeppox cells and are characteristic of the disease. The nuclei of sheeppox cells are vacuolated and have marginated chromatin. The vacuolated cytoplasm contains single, occasionally multiple, eosinophilic intracytoplasmic inclusion bodies. Sheeppox cells are virus-infected monocytes, macrophages, and fibroblasts, but not endothelial cells. Approximately 10 days postinfection and corresponding with the most prominent epithelial lesions and peak of skin infectivity, severe necrotizing vasculitis develops in arterioles and post-capillary venules (Fig. 17-42, B). Virus particles have not been identified in endothelial cells, and the vasculitis may be the result of immune-complex deposition. Ischemic necrosis of the dermis and overlying epidermis follows. The pulmonary lesions are proliferative alveolitis and bronchiolitis with focal areas of caseous necrosis. Alveolar septal cells contain intracytoplasmic inclusion bodies. Additional histologic lesions, characterized by the accumulation of sheeppox cells, may involve heart, kidney, liver, adrenal gland, thyroid gland, and pancreas.
The course and outcome of sheeppox depend not only on the usual host-virus relationship but also on the nature and location of secondary infections. The virus itself may cause death during the febrile, eruptive phase of the disease. Secondary bacterial infection and even septicemia and pneumonia can be the cause of death because animals are susceptible to fly strike and while the skin lesions are in the healing stage.
Goatpox: Goatpox, caused by goatpox virus, occurs in the previously described geographic distribution, and a benign form of goatpox occurs in California and Sweden. The clinical signs of goatpox vary in different geographic areas. The disease is generally milder than sheeppox with a low mortality rate (5%), although generalized eruption with mortality rates approaching 100% may occur, with a course of disease similar to that of sheeppox infections in sheep. The cutaneous lesions have a predilection for the same areas as for sheeppox. In nursing kids, lesions may appear on the buccal mucosa or anterior nares. In animals with higher levels of resistance, the lesions may be confined to the udder, teats, inner aspects of thighs, or ventral surface of the tail.
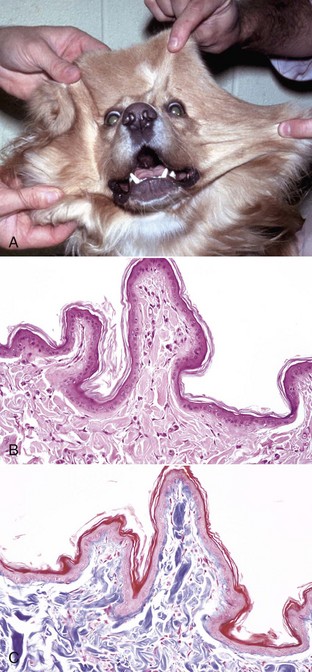
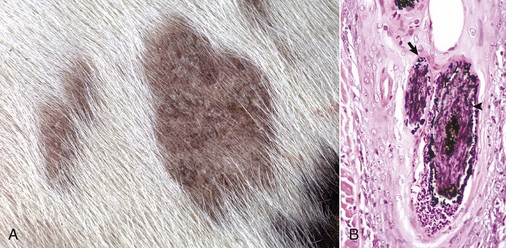
Contagious Ecthyma: Contagious ecthyma (contagious pustular dermatitis, orf, sore mouth) is a common localized infection of young sheep and goats caused by a parapoxvirus with worldwide distribution. Less commonly, humans, cattle, wild ungulates, and dogs are infected. Morbidity in lambs is usually great, and although mortality is usually low, it can approach 15% in lambs. Lesions are initiated by abrasions from pasture grasses or forage, begin at the commissures of the mouth, and spread to the lips (Fig. 17-43), oral mucosa, eyelids, and feet. Lambs can transfer the virus to the teats of ewes, and the lesions can spread to the skin of the udder. Contagious ecthyma is economically important as the result of weight loss in lambs that are reluctant to eat because of the pain associated with oral and perioral lesions. Pathogenesis of lesion formation and gross and microscopic features are consistent with the typical poxvirus lesions (see previous discussion and Fig. 17-31), except that the vesicle stage is very brief, the ulcer and crust stage persists and is clinically prominent, and the epidermis is markedly hyperplastic. Inclusion bodies are only briefly detectable histologically at the vesicular stage.
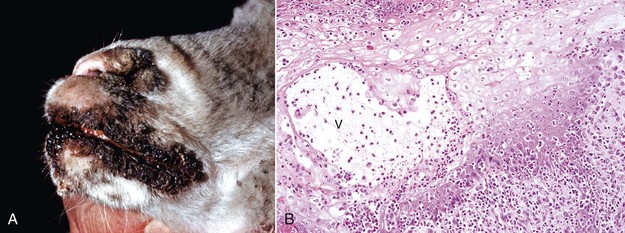
Fig. 17-43 Contagious ecthyma, skin, lamb.
A, Note crusts around nose and lips. These lesions are the late stage of the disease, are formed after rupture of vesicles and pustules, and are responsible for the common name scabby mouth. B, Note the epidermal hyperplasia (acanthosis), ballooning degeneration, vesicle (V), and neutrophils accumulating in the vesicle, which subsequently results in the formation of a pustule. Epidermal hyperplasia, upward movement of the pustule, and rupture of the vesicle or pustule contribute to crust formation as seen in A. H&E stain. (A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. Ann M. Hargis, DermatoDiagnostics.)
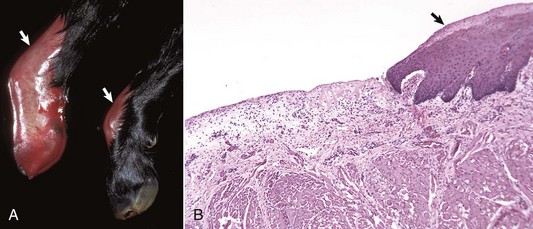
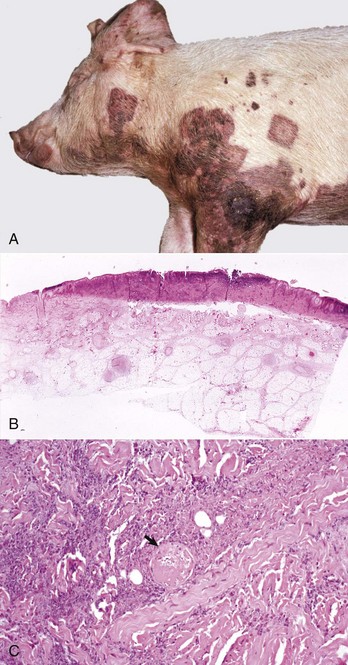
Swinepox: Pox lesions in pigs are caused by the host-specific poxvirus Suipoxvirus (swinepox). Normally, swinepox is transmitted by contact, although transplacental infection has not been ruled out. The sucking louse Haematopinus suis often acts as a mechanical vector and assists infection by causing skin trauma. The virus persists in dried crusts from infected animals. The pathogenesis of lesion formation and morphology of the gross and histologic lesions are consistent with the typical pox infection. The gross lesions typically affect the ventral and lateral abdomen, lateral thorax, and medial foreleg and thigh. Occasionally, lesions on the dorsum predominate. Lesions can be generalized and rarely involve the oral mucosa, pharynx, esophagus, stomach, trachea, and bronchi. The erythematous papules usually transform into umbilicated pustules without a significant vesicular stage (Fig. 17-44). The inflammatory crust eventually sheds to leave a white scar. The disease occurs worldwide and is endemic to areas of intensive swine production. The disease affects young, growing piglets and is mild with very low mortality.
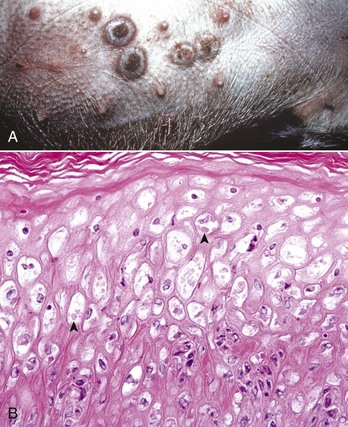
Fig. 17-44 Swinepox, skin, piglet.
A, Note the four umbilicated pustules in the abdominal skin. B, Note keratinocytes with ballooning degeneration and eosinophilic cytoplasmic inclusion bodies (arrowheads). Ballooning degeneration develops before vesicle formation. H&E stain. (A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. A.M. Hargis, DermatoDiagnostics. Photographed from slides provided by Department of Veterinary Pathology, Western College of Veterinary Medicine, University of Saskatchewan. From AFIP WSC January 21, 1998, Conference 15, Case IV.)
Herpesviruses: Herpesviruses are DNA viruses that only occasionally produce cutaneous lesions (see Table 17-7). Cutaneous lesions have rarely been reported in nondermatotropic herpesvirus infections, such as infectious bovine rhinotracheitis (bovine herpesvirus 1) and equine coital exanthema (equine herpesvirus 3), and in cats with FHV-1 infection. Two dermatotropic herpesvirus infections with economic importance are bovine herpesvirus 2 and bovine herpesvirus 4. Herpesviruses can be latent, with inactive virus persisting in tissue such as the trigeminal nerve ganglia. It is speculated that up to 80% of adult cats recovered from FHV-1 infection as kittens or young cats have latent herpesvirus 1 infections. During times of stress, the virus is reactivated and lesions can recur. Herpesviruses infect epithelial cells and replicate in the nucleus, leading to lysis of nuclear contents. As immature viral particles enter the cytoplasm, there is degeneration of cytoplasmic organelles, accumulation of cytoplasmic lipid, and precipitation of protein. Death of keratinocytes leads to spread of virus to neighboring cells, leading to rapid necrosis of focally extensive areas of the epidermis. Gross lesions consist of vesicles that rupture to form ulcers that are then covered by crusts. Microscopic lesions in herpesvirus infections depend on the stage, but early degenerative changes include ballooning and reticular degeneration, the sequelae of degeneration of epidermal cells and acantholysis. Syncytial cells may be seen. Intranuclear inclusions develop, but because of rapidly developing necrosis may not be found except at the margins of ulcers. The appearance of the viral inclusions varies with the specific herpesvirus. Some herpesviruses produce large, hyaline amphophilic inclusions that fill the nucleus (FHV-1), whereas others (bovine herpes mammillitis) produce typical Cowdry type A inclusions, which are also intranuclear but smaller and eosinophilic.
Bovine Herpesvirus 2: Bovine herpesvirus 2, a dermatotropic virus (Allerton virus), can cause generalized disease (pseudo–lumpy skin disease) or as seen in the US, localized infection of the teat called bovine ulcerative mammillitis (bovine herpes mammillitis). Mammillitis is inflammation of the teat or nipple. Localized infection occurs more commonly in lactating dairy cows but can develop in beef cows, pregnant heifers, and suckling calves. Trauma is implicated in the pathogenesis because normal skin is resistant to viral penetration. The pathogenesis of lesion formation was discussed earlier. Bovine ulcerative mammillitis is economically important because of decreased milk production and secondary bacterial mastitis. Lesions develop on the teats and skin of the nearby udder or occasionally the perineum. Suckling calves develop lesions on the muzzle (nose).
Bovine Herpesvirus 4: Bovine herpesvirus 4 (bovine herpes mammary pustular dermatitis) causes a similar but milder disease than the localized form of bovine herpesvirus 2.
Feline Ulcerative Facial Dermatitis and Stomatitis: Feline herpesvirus 1 is an uncommon cause of ulcerative, often persistent, facial dermatitis or stomatitis in cats of various ages and sexes. Glucocorticoid therapy or stresses, such as overcrowding, are thought to play a role in lesion development. Most lesions develop under circumstances suggesting reactivation of latent herpesvirus infection. The pathogenesis is typical of that described for herpesviruses in the previous section. Gross lesions are ulcerative and crusted (see Table 17-3). Histologically, there is extensive necrosis of the epidermal, follicular, and sometimes sebaceous gland epithelium accompanied by prominent mixed dermal inflammation that frequently includes numerous eosinophils. Follicular epithelium can be destroyed, and free keratin in the dermis is associated with eosinophils and foci of eosinophil degranulation bordering collagen fibers and collagen degeneration. Large amphophilic or hyaline intranuclear inclusions are present in the surface and adnexal epithelium. Inclusion bodies are often easily overlooked, variable in number, and sometimes present in small rafts of epithelial cells surrounded by necrotic debris. The lesions are different from those previously reported in domestic cats in that they persist and are limited to the skin of the face or oral mucosa and often have significant eosinophilic inflammation. The inflammation in feline herpesvirus dermatitis overlaps with that of the hypersensitivity reactions, including mosquito bite hypersensitivity, and also with that of eosinophilic ulcers, thus warranting close scrutiny of eosinophilic necrotizing cutaneous lesions for intranuclear inclusion bodies.
Papillomaviruses: Papillomaviruses are typically species- and site-specific pathogens that infect the squamous epithelium and cause benign proliferative masses and less commonly malignant tumors. As more precise methods of papillomavirus identification have been developed, including in situ hybridization, PCR, and DNA sequencing, increasing numbers of papillomaviruses have been identified. Papillomaviruses reproduce exclusively in keratinocyte nuclei. They gain access through defects in the epithelium and enter the basal layer epithelial cells. Once in the cell, there are three possible outcomes: (1) the virus can remain within the basal cell nucleus outside of the chromosomes in a circular DNA episome where the virus replicates synchronously with the host cell causing a latent infection without morphologic changes in keratinocytes; (2) as the basal cells mature, the virus can convert from latent to productive infection with the formation of complete infectious virions and with morphologic changes recognized as viral cytopathology, including epithelial hyperplasia, keratinocytes with clear cytoplasm and pyknotic nuclei, and sometimes with cytoplasmic or intranuclear inclusion bodies; or (3) the virus can become integrated into the genome of the host cell, resulting in malignant transformation and the morphologic changes of neoplasia. Malignant transformation occurs because the viral genes that remain after integration into the host cell are those associated with cellular regulation. These viral genes promote keratinocyte cell growth by inactivating tumor-suppressor proteins such as p53 and pRb. These events lead to uncontrolled cell proliferation, inability to repair DNA damage, and eventual malignant transformation.
All domestic animals are affected by one or more papillomaviruses (see Table 17-7), and some cross-species infections have been detected, particularly with the bovine papillomaviruses. Recently, a papillomavirus in an exophytic papilloma on the dorsal surface of the nose of a cat had 98% similarity to human papillomavirus type 9. The specific type or types of papillomaviruses involved in some infections have yet to be determined. Papillomavirus infections cause diverse clinical and histologic lesions ranging from papillomas, epidermal pigmented plaques, and fibropapillomas, including sarcoids. Papillomavirus DNA has also been identified within in situ and invasive squamous cell carcinomas in animals, but further work is necessary to prove a cause and effect relationship.
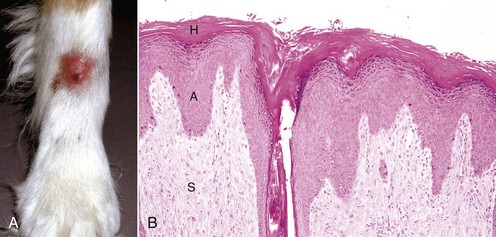
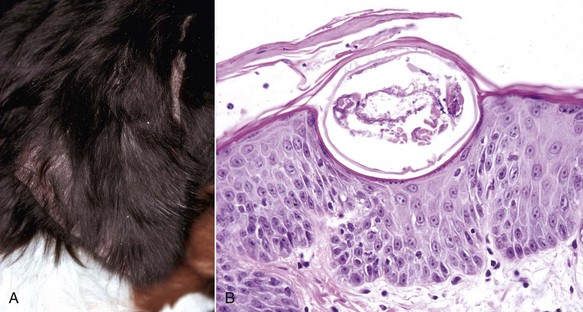
A common type of cutaneous papillomavirus infection (papilloma, wart, cutaneous papillomatosis) consists of clinical lesions that may be exophytic (proliferating to the exterior) (Fig. 17-45) or endophytic (inverted) papilliferous benign masses. Other papillomas may be flat, plaque-like lesions that lack the prominent papilliferous projections. Histologically, stratified squamous epithelium is covered by thickened orthokeratotic or parakeratotic keratin, is acanthotic, and in the exophytic and endophytic papillomas, has elongated epidermal-dermal interdigitations that project outwardly or inwardly, depending on the type. In some papillomas, keratinocytes, especially of the upper stratum spinosum, are swollen, have clear cytoplasm or a perinuclear halo, and a pyknotic nucleus; these keratinocytes are termed koilocytes (meaning hollow or concave). Keratohyalin granules are irregular. Also, pale basophilic intranuclear inclusion bodies, located in degenerating cells in the outer layers of the stratum spinosum and granulosum in which virion production is taking place, occur in some but not all papillomas. Many papillomas spontaneously regress, and in regressing stages, there is reduced epidermal hyperplasia, increased proliferation of fibroblasts, deposition of collagen, and infiltration of T lymphocytes at the epidermal dermal interface and in the epithelium. Some papillomas, such as those on the ears of horses and some affecting the teats of cattle, often do not regress.
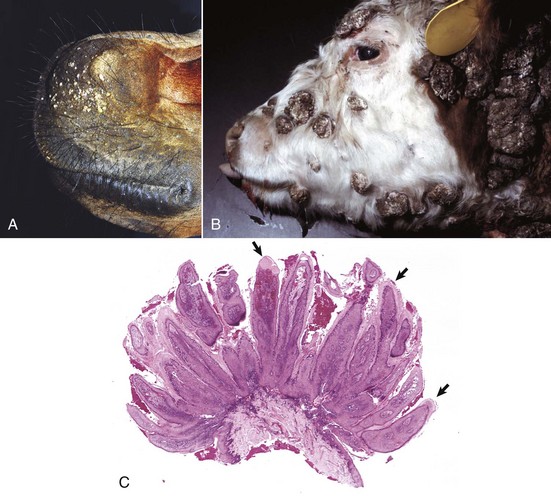
Fig. 17-45 Cutaneous papillomas, skin.
A, Chin, horse. Note multiple, small, verrucous masses arising in the skin. B, Head, cow. Note multiple, irregular, alopecic, verrucous papillomas. C, The papillary projections (arrows), often called fronds, are composed of hyperkeratotic epidermis covering a collagenous core. H&E stain. (A courtesy Dr. David Duclos, Animal Skin and Allergy Clinic. B and C courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
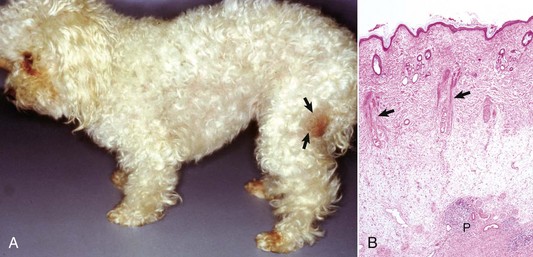
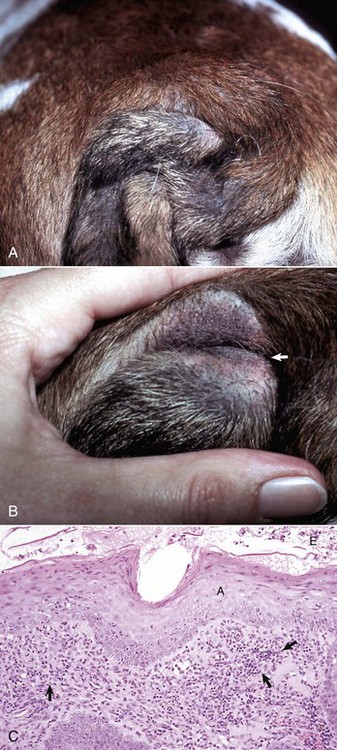
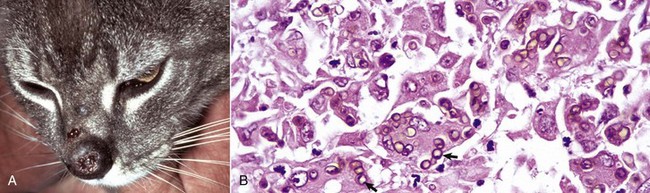
Viral plaques have been described in dogs and cats. Canine pigmented viral plaques are associated with papillomavirus infection in certain breeds of dogs (miniature schnauzers, pugs, and Shar-Peis) or in other breeds of dogs that are immunosuppressed. Novel papillomaviruses have been detected in some of these plaques. Clinical lesions occur most commonly on the ventral abdomen, groin, ventral thorax, or neck and consist of variably irregular, pigmented macules, or plaques. Histologically, the lesions are sharply demarcated, hyperkeratotic foci, or plaques with pigmented, acanthotic epidermis, and large keratohyalin granules. These pigmented epidermal plaques do not regress, are slowly progressive, and occasionally develop into a squamous cell carcinoma. Feline viral plaques are usually multiple, ovoid, slightly raised pigmented or nonpigmented, and slightly scaly and rough plaques less than 8 mm in greatest dimension. Papillomavirus has been detected in the lesions but not specifically identified to type. Histologically, there is an abrupt transition between normal epithelium and the plaque, which consists of epidermis thickened by hyperkeratosis, hypergranulosis, and acanthosis. Keratohyalin granules may be enlarged, and koilocytes (keratinocytes with clear cytoplasm and pyknotic nuclei), and cytoplasmic pseudoinclusions may be present. Malignant transformation commonly occurs; lesions resemble the in situ bowenoid carcinoma (see next paragraph).
Papillomavirus infection also has been implicated in the development of another syndrome in cats and less often in dogs termed multicentric squamous cell carcinoma in situ (Bowen’s-like disease, in situ bowenoid carcinoma). Although not all the factors contributing to lesion formation have been documented, papillomavirus DNA has been identified in these lesions in cats, suggesting a nonproductive infection promotes the epithelial hyperplasia characteristic of this disease. The papillomaviruses identified in some of these lesions have homology with human papillomaviruses. Multicentric squamous cell carcinoma in situ clinically consists of sharply demarcated single, or more often multiple, scaly verrucous or irregular plaque-like lesions 0.5 to 3.0 cm in diameter. Histologically, the epidermis and follicular infundibular epithelium are thickened by proliferation of basal keratinocytes that tend to stream together, providing a “windblown” appearance to the epidermis. Nuclei are often varied in size with hyperchromatic nuclei, large nucleoli, and numerous mitoses, some of which are located above the basal layer. The basement membrane, at the time of histologic examination, is intact. Most lesions remain as “in situ” carcinomas indefinitely, but an occasional lesion has progressed to invasive basal cell carcinoma or invasive squamous cell carcinoma.
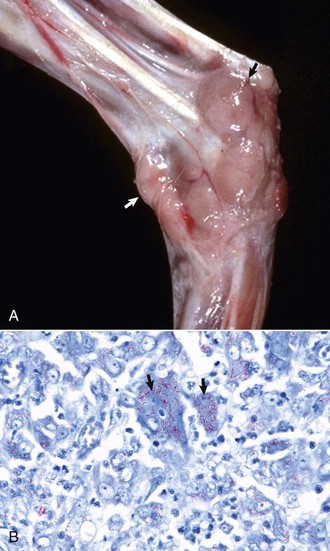
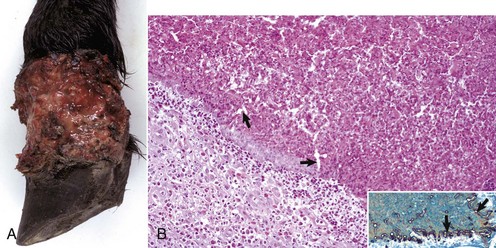
Some types of papillomaviruses, particularly bovine papillomaviruses 1 and 2, can infect fibroblasts and cause fibropapillomas, flat, verrucous, or nodular masses in which the proliferation of dermal fibroblasts is the prominent feature, often surpassing that of the epidermal hyperplasia (Fig. 17-46). Fibropapillomas occur in horses, mules, donkeys, cattle, sheep, and cats. These lesions in horses are called sarcoids, which are thought to represent a nonproductive infection by distinct variants of bovine papillomavirus. Equine sarcoids are locally aggressive, nonmetastatic fibroblastic skin tumors of horses, mules, and donkeys. They are the most common skin tumor of horses, accounting for up to 30% of tumors, and occur in any breed, sex, or age. Young adult horses 3 to 6 years of age are most commonly affected. Sarcoids frequently develop in areas subjected to trauma or at sites of wounds 6 to 8 months after wound healing and develop anywhere but are most common on the head, legs, and ventral trunk. They can be single or multiple. The tumors are classified according to their gross appearance as verrucous, fibroblastic, mixed, or occult. The verrucous type is a small wartlike growth, usually measuring less than 6 cm in diameter, with a dry, rough surface and variable alopecia. The fibroblastic type of sarcoid is more variable in appearance and can range from a well-circumscribed firm nodule with intact surface to a large mass, greater than 25 cm in diameter, with an ulcerated surface prone to hemorrhage, and resembling exuberant granulation tissue. The mixed type is a transitional form in which a verrucous sarcoid becomes a fibroblastic type as a result of trauma or biopsy. The occult form consists of a slow-growing, slightly thickened area of skin with slight surface roughening and alopecia that remains static for a long period.
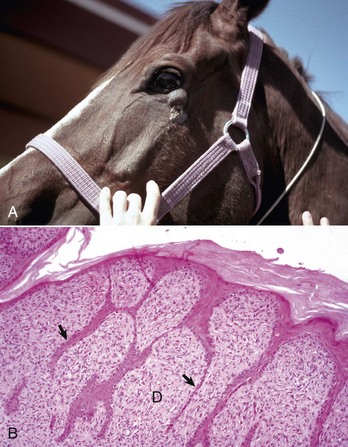
Fig. 17-46 Sarcoid, skin, horse.
A, Equine sarcoid, face. The irregular multinodular mass is present on the ventrolateral periocular skin, especially below the eye. B, Sarcoid. The sarcoid consists of an epidermal and dermal component. The hyperplastic epidermis has thin rete pegs that extend into the dermis (arrows). The dermis (D) is thickened by proliferating fibroblasts and collagen. H&E stain. (A courtesy Dr. Helen Power, Dermatology for Animals. B courtesy Dr. Pamela E. Ginn, College of Veterinary Medicine, University of Florida.)
Histologically, sarcoids are typically biphasic tumors composed of both epidermal and dermal components; however, the epidermal component may be minimal or absent in some tumors, especially those with extensive ulceration. When the epidermis is intact, hyperkeratosis, parakeratosis, and acanthosis with thin rete pegs extending deep into the dermis are common features. The dermal component consists of fibroblasts and collagen in various proportions. The fibroblasts have plump nuclei, and nucleoli may be prominent. The mitotic index is usually low. Fibroblasts at the dermal-epidermal junction are frequently oriented perpendicular to the basement membrane in a “picket fence” pattern, which is a distinctive histologic feature seen in most sarcoids. The cells are arranged in whorls, interlacing bundles, or haphazard arrays of variable density. Tumor margins are typically indistinct and adequacy of excision is frequently difficult to determine. Spontaneous remission can occur after several years in up to 30% of cases. The tumors are characterized by a high rate of recurrence, up to 50%, after surgical excision. Feline fibropapillomas (also called sarcoids) are similar morphologically to the equine lesion, and also likely represent a nonproductive infection with papillomavirus with similarities to bovine, ovine, deer, and European elk papillomaviruses. Affected cats often live in rural areas and have had exposure to cattle. Bovine fibropapillomas are caused by bovine papilloma virus 1 (teats, penis) or bovine papillomavirus 2 (head, neck, shoulder, legs, and teats) and occur in young animals. The lesions generally spontaneously regress within 1 to 12 months.
Papillomaviruses, some as yet to be identified by type, have been found in canine and feline invasive squamous cell carcinoma. However, the presence of the virus in the tumors does not allow differentiation between actual induction of the tumor and mere infection of the tumor. Further work to characterize the types of papillomaviruses and the role the viruses play in epidermal hyperplasia and neoplasia is necessary.
Other Viruses: Cutaneous lesions are seen with foot-and-mouth disease (picornavirus), vesicular stomatitis (rhabdovirus), swine vesicular disease (picornavirus), vesicular exanthema (calicivirus), and malignant catarrhal fever (herpesvirus) (see Table 17-7; see also Chapters 4 and 7). Feline leukemia virus (FeLV) can cause the development of lymphoma and fibrosarcoma. A few cats with FeLV infection have developed dermatitis characterized by epidermal and follicular acanthosis with epidermal giant cells, dyskeratosis, necrosis, and ulceration. In addition, a few FeLV-infected cats have also developed one or more localized areas of marked compact hyperkeratosis of the pawpads that clinically resemble “horns” (cutaneous horns). In immunoperoxidase-stained sections, the hyperplastic epidermis in cases with epidermal giant cells is strongly positive for FeLV. Both FeLV and feline immunodeficiency virus (FIV), because of their immunosuppressive capabilities, can cause cats to be susceptible to chronic skin infections, including abscesses, paronychia, and demodicosis.
Feline calicivirus, usually the cause of upper respiratory infection and oral ulcers with high morbidity but low mortality, rarely produces cutaneous ulcers. More recently, virulent systemic strains of feline calicivirus with mortality rates between 30% and 60% have been described. These strains cause alopecia, cutaneous ulcers, and subcutaneous edema. The pathogenesis involves lysis of epithelial cells of the epidermis, follicles, oral mucosa, bronchioles, alveoli, and exocrine pancreas, in addition to lysis of endothelial cells. The lysis of epithelial and endothelial cells leads to necrosis, edema, fibrin exudation, and thrombosis from vascular injury and release of inflammatory mediators from damaged cells. Clinical lesions include ulcers of the nose, lips, pinnae, feet, and oral mucosa, variable alopecia of limbs and ventrum, and subcutaneous edema, especially of the limbs and face. Systemic signs of fever, anorexia, icterus, red and swollen conjunctival mucosa, and nasal or ocular discharge are present. The most consistent microscopic lesions are epithelial necrosis with subsequent ulceration of the skin and oral and nasal mucosa. Vascular injury consists of edema, microthrombosis, and fibrin exudation. Bronchointerstitial pneumonia and necrosis of the liver, pancreas, spleen, and lymph nodes are present in some cats. In contrast to infection with the more common and less virulent field strains of feline calicivirus, the systemic virulent strains cause more severe disease in adult cats than kittens.