Alimentary System and the Peritoneum, Omentum, Mesentery, and Peritoneal Cavity*
Introduction
The alimentary system is a long and complex tube that varies in its construction and function among animal species. For example, herbivores need a fermentation chamber (either a rumen or an expanded cecum) for the digestion of cellulose, a feature not present in carnivores. Although a large variety of gastrointestinal (GI) disturbances are clinically important in all species of animals, the predominant form of disease varies from species to species. Pet carnivores, partly because of their long lifespan, effective vaccines, and a lifestyle and diet similar to that of humans, develop alimentary neoplasia far more often than herbivores. Meat, milk, and fiber-producing animals (ruminants and pigs) are host to a variety of infectious diseases that are largely resistant to vaccines. These pathogens may have evolved as a result of the herding instinct of these animals, giving the pathogens an opportunity to mutate within a large socially structured host population. Equids are most prone to displacements of alimentary viscera.
In general, the alimentary system, including the salivary glands, pancreas, and liver, functions by adding water, electrolytes, and enzymes to ingested matter and then mixing and grinding it to facilitate its breakdown to water-soluble nutrients for absorption across mucous membranes into the blood circulation and subsequent distribution through the body. Although the alimentary tract is open ended, most ingested substances and secretions produced by the GI system are absorbed.
A large part of the practice of veterinary medicine is devoted to the diagnosis and treatment of alimentary disorders. Many of the newer molecular and imaging methods have been designed specifically to increase the clinician’s ability to make accurate diagnoses of the various conditions of the alimentary system. Additionally, every physical examination includes the opportunity for a fecal analysis that allows the clinician a window into the functioning of the alimentary system as a whole.
The polymerase chain reaction (PCR) is a tool that allows the opportunity to rapidly diagnose an infectious cause of enteritis without having to culture the organism in the traditional manner. Diagnosis of the cause of an infectious disease of the alimentary system can also be made from examination of a biopsy sample by histologic and immunohistochemical staining or by in situ hybridization that allows demonstration of the pathogen within target cells.
Through the use of fiberoptic endoscopes inserted through the mouth or anus or through a small incision in the abdominal wall (laparoscopy), a thorough clinical examination of most of the alimentary system can be made. This knowledge is now a necessity in clinical practice because GI mucosa from the oral cavity, through the esophagus, stomach, duodenum, and the large colon and rectum and the entire serosal surface of the abdominal viscera, can be viewed and sampled directly in the live animal.
Structure and Function
The most important point to keep in mind when examining the alimentary system is that normal mucosal and serosal surfaces should be smooth and shiny (although there may be normal papilla, folds, and ridges). The exception to this rule is the rumen, whose papillae may normally have a roughened, dull, surface appearance. When serosal and mucosal surfaces are not smooth and shiny, animals should be examined thoroughly to determine the reason.
The function of the alimentary system as a whole is to take ingested feedstuffs, grind them and mix them with a variety of secretions from the oral cavity, stomach, pancreas, liver, and intestines (digestion), and then to absorb the constituent nutrients into the bloodstream and lacteals. Undigested ingesta, effete neutrophils, fresh (hematochezia) or digested (melena) blood, and excess secretions are passed from the body into the alimentary lumen and thus become a component of the feces. The quality and quantity of the feces and clinical signs, such as regurgitation and vomiting, are often early indicators of alimentary dysfunction.
Portals of Entry
There are limited numbers of ways that pathogenic agents gain entry into the alimentary system (Box 7-1). The most common, of course, is through ingestion. However, under certain circumstances, pathogens may be coughed up from the lungs into the pharynx and swallowed. Systemic blood-borne infections of viruses (viremia), bacteria (bacteremia), and systemic toxins (septicemia and toxemia) may make their way through the bloodstream and attach to specific receptors on the epithelial lining cells of the alimentary system. Parasites may migrate through various regions of the body to find a home within the mucosa or roam free in the lumen of the alimentary tract.
Defense Mechanisms
Considering the types of materials that are ingested by domestic animals, it is significant that they are not constantly ill. This resistance to disease occurs because the alimentary system is well suited to protect itself against most potentially pathogenic insults (Box 7-2). These protective mechanisms include oral secretions, such as saliva; “normal” resident flora and fauna; the gastric pH; opening of tight junctions between intestinal cells to allow macromolecules, such as immunoglobulins, into the lumen; vomiting; secretions from the liver and pancreas; intestinal proteolytic enzymes, macrophages, and other effector cells, such as neutrophils, within the submucosa, which are exuded into the alimentary lumen; the high rate of epithelial turnover; increased peristalsis resulting in diarrhea; Paneth cells; and the immune system. Paneth cells produce antimicrobial peptides and proteins, including lysozyme and secretory phospholipase A2. They also produce α-defensins (cryptdins).
Oral Cavity
The oral cavity is one of the places that can be examined directly by the clinician and pathologist and where they can use the same criteria for determining abnormality. The same can be said of the rectal mucosa.
Structure and Function
The physiologically normal oral mucosa is smooth, shiny, and pink. It is composed of variably keratinizing, stratified squamous epithelium (mucous membranes). In animals in which the oral mucosa is heavily pigmented (melanosis), assessment of circulatory function (capillary refill time) and color as an indicator of red blood cell concentration (packed cell volume) can be difficult. In these cases, examination of conjunctiva, rectal, and urogenital mucosa can be substituted. The oral cavity is where ingested materials are masticated; mixed with digestive enzymes, such as those in saliva; and passed on through the oropharynx to the esophagus.
Defense Mechanisms
Defense mechanisms of the oral cavity include the stratified epithelial surface that is resistant to trauma and some irritants; taste buds, which reject potentially toxic materials based on taste and tongue feel; an indigenous bacterial flora that occupy attachment sites that would otherwise be available to pathogens; and saliva (Box 7-3). Saliva provides a flushing action so potential pathogens are cleared from the oropharynx and swallowed. Saliva also forms a protective coating of the mucosa and contains antimicrobial lysozyme in the zymogen granules of serous cells and immunoglobulins, especially immunoglobulin A (IgA), in a manner analogous to cryptal enterocytes of the intestine, through the production of a secretory component. Migration through the alimentary tract, including the oral cavity, eliminates neutrophils at the end of their lifespan. In their absence, stomatitis results.
Developmental Anomalies
There are a wide variety of developmental abnormalities in the oral cavity. Some are incompatible with life unless surgically corrected. Only a few of these congenital lesions have a proven hereditary component. Most are idiopathic. Thorough physical examination of neonates must include examination of the oral cavity for these defects.
Palatoschisis, or cleft palate, and cheiloschisis, or cleft lip, are among the most common developmental abnormalities of the oral cavity. Cheiloschisis is sometimes referred to as hare lip because this is a normal feature of the rabbit. It is a failure of fusion of the upper lip along the midline or philtrum. Palatoschisis can be genetic or toxic in origin. It results from a failure of fusion of the lateral palatine processes. It can be caused by steroid administration during pregnancy in primates, including humans. Depending on the size of the defect, which may involve only the soft palate or both the soft and hard palates (Fig. 7-1), the lesion may be surgically correctable. It is a matter of some ethical concern whether to correct such defects without also sterilizing the patient because of the potential for cleft palate to have a genetic cause. Important sequelae to the host from cleft palate are starvation, as the result of the inability of the nursing animal to create a negative pressure in the mouth with a resultant failure to suckle, and aspiration pneumonia, since no effective separation is present between the oral and nasal cavities.
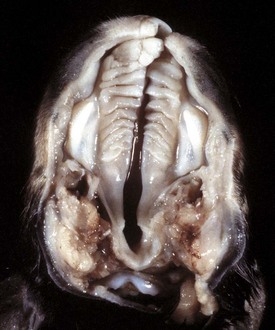
Fig. 7-1 Palatoschisis and cheiloschisis, hard and soft palate, puppy.
The lateral palatine processes have failed to fuse during the first trimester of gestation (palatoschisis). In dogs, palatoschisis has been attributed to genetic abnormalities, excessive intake of vitamin A during gestation, and the administration of cortisone during gestation. The upper lip is also cleft (cheiloschisis). (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Stomatitis and Gingivitis
Stomatitis and gingivitis refer to inflammation of the mucous membranes of the oral cavity and gingiva, respectively. Because the oral cavity is constantly bombarded with ingested substances that are moved around by the tongue, the final result of a variety of insults to the lining of the oral cavity is a loss of mucosa—erosions, ulcerations, and necrosis. Thus, although inflammation is apparent, clues as to the initiating process may be absent. Lesions may be at different stages and are commonly classified as macules, papules, vesicles, erosions, abscesses, granulomas, and ulcers. These lesions can be caused by infectious agents, particularly viruses; chemical injury; trauma; intoxicants; or autoimmune or systemic disease. They often result in anorexia caused by painful mastication. Hypersalivation (ptyalism) is also apparent, whether from overproduction or a failure to swallow. In the cat, gingivitis is the first and most consistent sign of feline immunodeficiency virus (FIV) infection (immune system failure) associated with a reduction in CD4 lymphocytes, thymic atrophy, and lymph node atrophy.
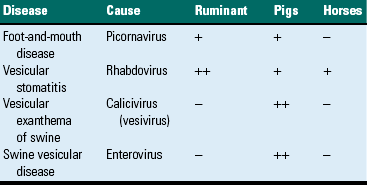
Vesicular Stomatitides
Although vesicular stomatitis correctly refers to oral vesicles and blisters, the term is generally reserved for those lesions caused by epitheliotropic viruses. The vesicular stomatitides are listed in Table 7-1. Their genesis is from virus-induced epithelial cytolysis attended by fluid accumulation and subsequent rupture of the resultant vesicle. Blistering or vesiculation of the oral epithelium is present early in the course of these diseases. All of these diseases are virus-induced, and all have identical appearances at gross and histopathologic examination. None of these conditions is fatal. They produce great economic loss because of poor weight gain in affected animals and sometimes abortions in gravid females. The exact cause of the abortions is unknown, but it is probably related to the stress induced by the painful oral, cutaneous, and pedal (hoof or foot) lesions. Secondary bacterial invaders, both Gram-negative and Gram-positive, of these lesions can result in endotoxemia. Several diseases, such as foot-and-mouth disease and vesicular exanthema, affect the coronary bands of the digits and interdigital clefts, resulting in lameness. Some of these diseases (foot-and-mouth disease, vesicular exanthema, and swine vesicular disease) are exotic to the United States (US) and thus are reportable to state or federal authorities, or both, if the clinician or pathologist suspects the disease. This requirement is due to the great expense involved in eradicating these diseases from the US and their potential use as agents of agroterrorism. Nontariff export/import barriers designed to prevent the introduction of highly contagious agents, such as foot-and-mouth disease, into animal populations of countries with which we trade are often put in place.
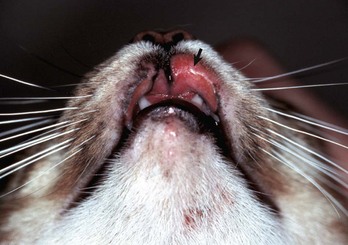
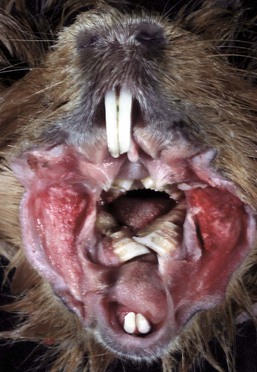
The gross lesions of the vesicular stomatitides are epithelial. Fluid-filled vesicles are present in the oral cavity, lips, rostral palate, and tongue (Fig. 7-2, A). Entry of virus in these cases is most likely oral into areas of temporary loss of mucosa as the result of normal mastication and trauma. The viruses are cytolytic, and the resultant release of virus from cells infects neighboring cells. The lesions enlarge centripetally, forming vesicles. Bullae result from coalescence resulting in erosions and ulcers. These ulcers are typically hyperemic (Fig. 7-2, B). Viremia, often transient, sometimes occurs.
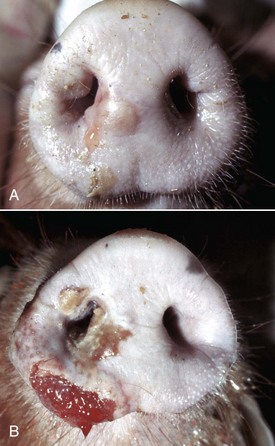
Fig. 7-2 Cutaneous vesicles, vesicular exanthema, snout, pig.
A, Vesicles, both intact (upper vesicle) and ruptured (lower vesicle), are present on the planum rostrale and are caused by the infection of injured mucosal epithelial cells with vesicular exanthema of swine virus, a calicivirus (vesivirus). B, Ruptured vesicles with cutaneous ulceration, vesicular exanthema (later stage of the disease). Note the ruptured vesicles which can cause pain resulting in inappetence. (A from Gelberg H, Lewis RM: Vet Pathol 19:424-443, 1982. B courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Similar vesicular lesions occur in the nasal mucosa, particularly in pigs with vesicular exanthema and in the proximal epithelium of the alimentary system (esophagus and rumen) of cattle with foot-and-mouth disease. Some animals have conjunctivitis and vesicular dermatitis of the teats and vulva. Microscopically, the lesions of these four diseases (foot-and-mouth disease, vesicular stomatitis, vesicular exanthema, and swine vesicular disease) are similar. Virus-induced, intracellular edema progresses to swelling of the cells of the stratum spinosum, cell lysis and intercellular edema, and resultant vesicles. The epithelium overlying the virus-rich vesicular fluid is thin, and even slight friction can rupture vesicles and bullae, creating an ulcer. Healing of the ulcer progresses from the usual fibrin and/or neutrophil-rich acute stages (scab) to the more chronic stages of granulation.
Lesions and signs of the vesicular stomatitides include vesicles, bullae, and detachment of patches of epithelium with resultant raw ulcers, ptyalism, lameness, fever, and anorexia. Besides the lesions that develop from the initially infected cells, the virus spreads centripetally to adjacent susceptible epithelium, causing repeated episodes of this infectious, lytic cycle. The vesicular stomatitides are tentatively diagnosed based on the clinical signs and lesions resulting from oral and nasal ulceration, conjunctivitis, and ulceration of the genitalia and mammary glands. Lameness is secondary to hoof involvement that is focused at the coronary band. Definitive diagnosis is important and performed at federal laboratories equipped to rapidly respond to suspected outbreaks. Federal quarantine of infected herds is an important control mechanism, followed by eradication, slaughter, and carcass disposal.
Specific Vesicular Diseases
Foot-and-mouth disease is an extremely important disease and disease threat of arteriodactyls worldwide but has not appeared in US livestock since 1929, when it was eradicated after an outbreak in California. Virus spreads rapidly and principally by aerosol. The disease is characterized in its early stages by vesicles in the planum nasale, in the oral cavity, and tongue. The picornavirus of foot-and-mouth disease attaches to susceptible cells via integrins on the cell surface. Fluid from ruptured vesicles spreads to areas of abraded skin, for example, skin of a mammary gland. When coronary bands and hooves are affected, coronary band vesiculation may eventually lead to sloughing of the hoof. Although this disease is not fatal, the pain and accompanying inappetence lead to weight loss. If allowed to heal, the hoof will regrow into a ball-like structure. Young animals with foot-and-mouth disease frequently have a viral myocarditis without other signs. Vaccination is short-lived (6 months) and takes time to become effective in individual animals; thus immediate protection is not provided. Persistent infections occur in water buffalo and infected cattle that have been previously vaccinated.
Vesicular stomatitis is common in calves, pigs, and some wildlife species but does not occur in sheep or goats. It is the only vesicular disease to which horses are susceptible. In northern latitudes, it is generally a warm weather disease, suggesting that insects act as vectors. As the name implies, vesicles in the oral cavity characterize the disease. Clinically the disease is often recognized by inappetence in the affected animal, accompanied by ptyalism.
Vesicular exanthema is a specific disease of pigs that is indistinguishable clinically and pathologically from foot-and-mouth disease. This disease is uniquely American and was believed eradicated from pigs in 1956 through enactment of federal laws requiring the cooking of garbage fed to pigs. The evidence indicates that vesicular exanthema of swine serovars are variants of San Miguel sea lion virus. This latter marine calicivirus occurs in coastal sea lion and fur seal populations from California to Alaska (Web Fig. 7-1).

Web Fig. 7-1 Cutaneous vesicles, San Miguel sea lion virus infection, foreflippers, northern fur seal.
On the nonhaired portion of the foreflipper are vesicles both intact (arrow) and ruptured, caused by the infection of injured mucosal epithelial cells with San Miguel sea lion virus, a calicivirus (vesivirus) These vesicles will rupture with trauma, resulting in cutaneous erosion and ulceration. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Swine vesicular disease is indistinguishable from the other vesicular diseases and is exotic to the US. Efforts are underway to develop DNA microarrays to facilitate rapid identification of specific vesicular diseases from a single sample.
Erosive and Ulcerative Stomatitides
Erosions are defined by a loss of part of the thickness of the surface epithelium, whereas ulcers are full-thickness epithelial losses exposing the basement membrane. Thus erosions may progress to ulcers, which in hollow organs may become perforating ulcers. Erosive and ulcerative stomatitis can have a variety of causes. Agents responsible include the viruses of bovine viral diarrhea (BVD) (Fig. 7-3), rinderpest, malignant catarrhal fever (Fig. 7-4), feline calicivirus, and bluetongue, and in equids, nonsteroidal antiinflammatory drugs (NSAIDs). Other causes include uremia (Fig. 7-5); ingested foreign bodies, such as foxtail awns; the feline eosinophilic granuloma complex; and vitamin C deficiency in primates and guinea pigs (Web Fig. 7-2). Often, the oral lesions must be evaluated in the context of the clinical signs, together with histopathologic findings and ancillary testing, to arrive at a definitive diagnosis. Additionally, the vesicular stomatitides can progress to ulceration secondary to abrasion to the point that they cannot be distinguished from the ulcerative stomatitides.
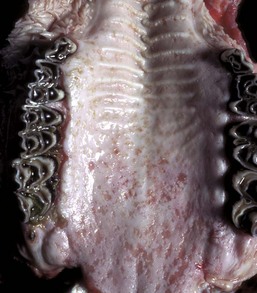
Fig. 7-3 Erosions and ulcers, bovine viral diarrhea virus infection, hard palate, cow.
Erosions and ulcers caused by this pestivirus are particularly evident on the mucosal epithelial surface of the caudal hard palate. These lesions are characteristic of the ulcerative stomatitides, which, unlike the vesicular disease viruses, do not form vesicles. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
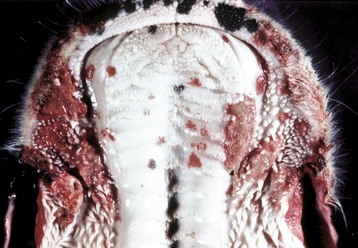
Fig. 7-4 Erosions and ulcers, malignant catarrhal fever, hard palate, dental pad and buccal papillae, cow.
The erosions and ulcers (red areas on mucosal surface) are due to malignant catarrhal fever virus, a herpes virus, but are characteristic of many ulcerative stomatitides. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
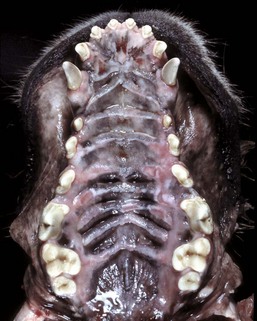
Fig. 7-5 Uremic ulcers, hard palate, dog.
Ulcers present on the transverse palatine ridges and periodontal gingiva are secondary to vascular damage associated with increased concentrations of plasma blood urea nitrogen and creatinine from kidney failure. Affected animals often have an ammoniacal or uremic odor to the breath. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
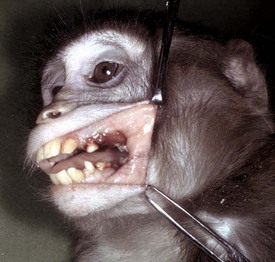
Web Fig. 7-2 Ulcerative gingivitis secondary to scurvy (vitamin C deficiency), gingiva, monkey.
There is a deep ulcer at the commissure of the mouth and smaller ulcers periodontally. Vitamin C deficiency in primates and guinea pigs can result in gingival erosions and ulcers, and even tooth loss. (Courtesy College of Veterinary Medicine, University of Illinois.)
Parapox Stomatitides
The two major diseases in this category, bovine papular stomatitis and contagious ecthyma, are zoonotic. Bovine papular stomatitis is recognized by papules on the nares, muzzle, gingiva, buccal cavity, palate, and tongue (Fig. 7-6). Lesions also occur in the esophagus, rumen, and omasum. Microscopically, acantholysis is responsible for the macule and ballooning degeneration of these cells, which may contain intracytoplasmic eosinophilic parapoxvirus inclusions at a later stage (Fig. 7-7; also see Fig. 1-12). Erosion of the infected cells accompanied by a neutrophilic infiltrate heals readily from the unaffected basal epithelium. The disease is more common in immunosuppressed animals such as those persistently infected with BVD virus. In humans, the disease is called milker’s nodules and is characterized by papules of the hands and arms.
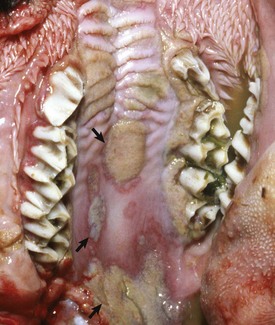
Fig. 7-6 Epithelial plaques, papular stomatitis, hard palate mucosa, calf.
Virus-induced (parapoxvirus) epithelial plaques and papules are present on the mucosal epithelium of the hard palate and adjacent gingiva (arrows). (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
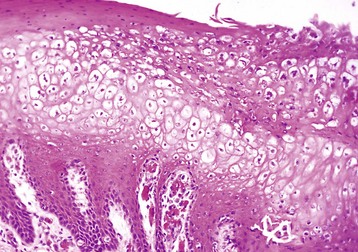
Fig. 7-7 Hydropic change, papular stomatitis, hard palate mucosa, cow.
There is massive cytoplasmic swelling of the epithelial cells of the stratum spinosum. At a later stage, these cells may contain intracytoplasmic eosinophilic parapoxvirus inclusions (not visible here). H&E stain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Contagious ecthyma, sore mouth or infectious pustular dermatitis, is a condition of sheep and goats characterized by progression of the stages typical of pox viruses—macules, papules, vesicles, pustules, scabs, and scars in areas of skin abrasions, including the corners of the mouth (Fig. 7-8; also see Fig. 17-43), mouth, udder, teats, coronary bands, and anus. Occasionally, the mucosa of the esophagus and rumen also can be affected. The virus is quite hardy and can survive for 50 to 60 days in the summer and longer in cold weather. At room temperature, scabs containing virus can be infective after 10 years. Eosinophilic cytoplasmic inclusion bodies are visible at microscopic examination of lesions early in the course of disease. The condition in humans is called orf.
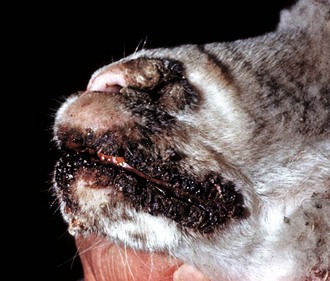
Fig. 7-8 Contagious ecthyma, oral mucous membranes, lamb.
Note crusts around nose and lips. Multiple pustules and coalescing ruptured pustules covered by scabs are present on the skin. The parapoxvirus induces epithelial proliferation (acanthosis), followed by vesicle formation. These vesicles rupture and are quickly covered by scabs. Lesions develop at the sites of trauma, such as occur with a nursing lamb, where damage to the superficial oral epithelium allows entry of the virus into skin. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Necrotizing Stomatitides
Necrotizing stomatitis occurs in cattle, sheep, and pigs. In cattle, it is sometimes referred to as calf diphtheria (Fig. 7-9). Necrotizing stomatitis is the end-stage of all other forms of stomatitis when they are complicated by infection with Fusobacterium necrophorum, a filamentous-to-rodlike-to-coccoid, Gram-negative anaerobe. Bacterial toxins are responsible for the extensive lesions. Necrotizing stomatitis is characterized by yellow-gray, round foci surrounded by a rim of hyperemic tissue in the oral cavity, larynx, pharynx, or tongue. Well-demarcated foci of coagulation necrosis typify the histologic appearance of necrotizing stomatitis. As might be expected in foci of inflammation, there is a circumferential rim of leukocytes and hyperemia. Clinical signs include swollen cheeks, inappetence, pyrexia, and halitosis. Infection may become systemic if severe, resulting in lesions throughout the alimentary system and associated lymphoid tissue.
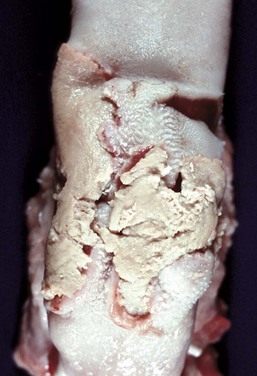
Fig. 7-9 Necrotizing stomatitis, calf diphtheria, tongue, calf.
The dorsal surface of the tongue is ulcerated, and the ulcers are covered by a diphtheric membrane. Calf diphtheria is caused by infection with the bacterium Fusobacterium necrophorum secondary to abrasion and/or trauma to the mucosal epithelium of the oral cavity or larynx. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Noma is a severe form of oral ischemic necrosis with lesional spirochetes and fusiform bacteria. Although rare, it is seen most often in primates, including humans, and dogs. It is characterized by severe necrotizing gingivitis that can extend into adjacent bone causing osteolysis and sometimes death.
Ulcerative gingivitis (trench mouth), caused by anaerobic spirochetes, affects humans, some nonhuman primates, and rarely, puppies. In addition to Fusobacterium spp., Borrelia vincentii may be causative. Debilitated animals and those with intercurrent infections are at increased risk for these secondary invaders, which may be part of normal oral flora. Similar clinically to necrotizing stomatitis, ulcerative gingivitis is characterized by acute inflammation and necrosis, oral ulceration and pain, halitosis, a fragile oral mucosa, and ptyalism. The morphologic diagnosis is an acute, necrotizing gingivitis. Unlike the case in necrotizing stomatitis, the causative agents are readily identified by tissue smears or by culture.
Eosinophilic Stomatitides
Oral granulomas or ulcers (“rodent ulcers”) occur frequently in cats. Similar lesions occur sporadically in a variety of canine breeds. In cats, they are termed oral eosinophilic granulomas. Although the etiology of this condition is unknown, the histologic appearance of lesions suggests an immune-mediated mechanism, possibly a hypersensitivity reaction to an unknown antigen. Antibodies to intercellular material can often be demonstrated in affected cats. In the majority of cases of both dogs and cats, an increase in circulating eosinophils is present.
In cats, lip lesions are commonly visible near the philtrum and may extend through the adjacent haired skin. Oral lesions may occur anywhere in the mouth, including the gingiva, hard and soft palates, oral and nasal pharynx, tongue, and occasionally draining lymphoid tissues, excluding the tonsils, which do not have afferent lymphatic vessels (Fig. 7-10; also see Fig. 17-21, C). In dogs, eosinophilic granulomas typically are raised, fungating masses on the ventral and lateral lingual epithelium and palate. Collagenolysis (because collagen is acellular, it cannot undergo necrosis) is characteristically central in the lesion. The surrounding inflammatory tissue contains mixed inflammatory cells with increased numbers of eosinophils, mast cells, and multinucleated giant cells (see Web Fig. 3-14). Lesions grouped as the eosinophilic granuloma complex of cats include eosinophilic ulcer, linear (collagenolytic) granulomas, and eosinophilic plaques. The latter two lesions are strictly cutaneous and do not affect the oral cavity. No proven etiologic link has been established between these cutaneous conditions (linear granulomas and eosinophilic plaques) and oral eosinophilic granulomas. The cause of the canine lesions is unknown.
Lymphoplasmacytic Stomatitis
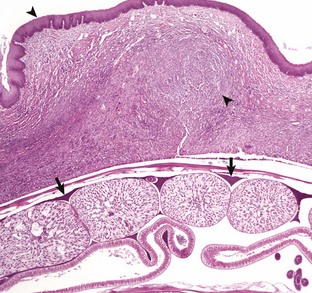
Lymphoplasmacytic stomatitis is an idiopathic condition of the cat named on the basis of the histologic appearance of the lesions (Fig. 7-11). Associations have been hypothesized between this condition and the presence of bacteria or calicivirus associated with feline leukemia virus (FeLV) and/or FIV infection. It is a chronic condition characterized by red, inflamed gums, a fetid breath, and inappetence. The oral mucosa may be hyperplastic and ulcerated. An inefficient immune response may be responsible for the persistence of oral bacteria and the accumulation of lymphocytes and plasma cells.
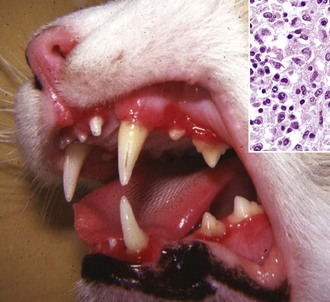
Fig. 7-11 Lymphoplasmacytic stomatitis, gingiva, cat.
This chronic condition of cats is characterized by red, inflamed gums, fetid breath, and inappetence. The oral mucosa can also be hyperplastic and ulcerated. Inset, There is a florid infiltrate of mixed inflammatory cells, including many lymphocytes and plasma cells in the submucosa beneath the epithelium. H&E stain. (Figure courtesy Dr. C. Patrick Ryan, Veterinary Public Health, Los Angeles Department of Health Services; and Noah’s Arkive, College of Veterinary Medicine, University of Georgia. Inset, Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Chronic Ulcerative Paradental Stomatitis
Chronic ulcerative paradental stomatitis, a condition of dogs also known as ulcerative stomatitis and lymphocytic-plasmacytic stomatitis, is caused by apposition of “kissing ulcers” to dental plaque. The condition is painful with resultant inappetence and anorexia. Affected dogs drool and have halitosis. This condition occurs in older dogs of any breed, but Maltese dogs and cavalier King Charles spaniels are particularly susceptible. The lymphocytic-plasmacytic lesions noted on histologic examination are suggestive of an inflammatory rather than infectious etiology possibly caused by mediators released from the plaque. If untreated, bone resorption may occur.
Oral Mucosal Hyperplasia and Neoplasia
Hyperplastic Diseases: Gingival hyperplasia is a simple overgrowth of gum tissue, principally the fibrous submucosa. The hyperplasia can become so severe as to bury incisor teeth (Fig. 7-12). Gingival hyperplasia is most common in brachycephalic dog breeds and is present in 30% of boxer dogs older than 5 years.
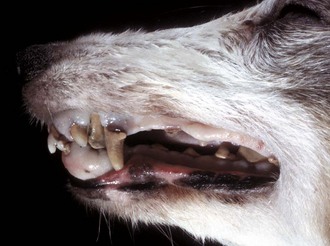
Fig. 7-12 Gingival hyperplasia, gingiva, dog.
Hyperplastic gingiva has enveloped the lower incisor teeth. Dental calculus (tartar, brown) is also present on both upper and lower incisor, canine and molar teeth. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Grossly, gingival hyperplasia can be indistinguishable from an epulis (Fig. 7-13). Epulis is a nonspecific term that designates a growth of the gingiva. The several kinds of epulides can only be distinguished by histopathologic examination. These include fibromatous epulis of periodontal ligament origin—a benign tumor of dental mesenchyme. This distinction is not just an academic exercise because, although all epulides are considered benign, one form, acanthomatous epulis or acanthomatous ameloblastoma, invades bone and can be quite destructive. This growth arises from the epithelial rests of Malassez or epithelial tooth germ. Fortunately, this type of epulis can be managed therapeutically. Whether the epulides represent fibrous and epithelial hyperplasia or benign neoplasms of tooth germ is controversial.
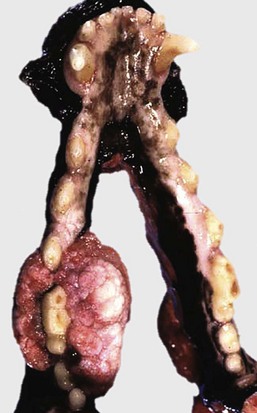
Fig. 7-13 Fibromatous epulis, left mandible, molar teeth, dog.
This growth is an epulis (fibromatous type); however, epulides are often grossly indistinguishable from gingival hyperplasia. Epulis is a term used to designate a growth of the gingiva that is firm, periodontal, and usually solitary, in contrast to gingival hyperplasia. This distinction is not just an academic exercise because, although all epulides are considered benign, one form, acanthomatous ameloblastoma, is locally invasive. It invades bone and can be quite destructive. (Courtesy Dr. J. King, College of Veterinary Medicine, Cornell University.)
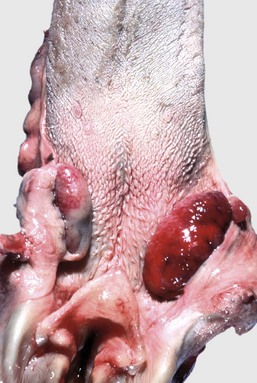
Neoplasia: In the dog, 70% of tumors of the alimentary system are in the oral cavity and oropharynx. These tumors run the gamut of biologic behavior from simple epithelial hyperplasia to malignant neoplasms with metastases to distant sites. Squamous cell carcinomas occur in the oral cavity, particularly in old cats, in which they account for 60% of oral neoplasms. They generally occur on the ventrolateral surface of the tongue and tonsils. Lingual squamous cell carcinomas occur more commonly in felids, and tonsillar squamous cell carcinomas are more common in canids. Although often appearing histologically aggressive, only a small percentage of lingual neoplasms metastasize, most commonly to draining lymph nodes, the mandibular and medial retropharyngeal. Unfortunately, most tonsillar carcinomas metastasize, initially to regional lymph nodes and then to distant sites.
Squamous cell carcinomas vary both in size and in gross appearance—from flat to proliferative (Fig. 7-14). These tumors are often quite aggressive locally, invading subjacent tissues. Some tumors contain more differentiated cells, keratin, often in whorls (keratin pearls) and visible desmosomes (intercellular bridges), whereas others are less well differentiated but with significant mitotic activity. In these latter cases, intracellular immunohistochemical markers for cytokeratin are useful in determining a definitive diagnosis. The amount of fibrous tissue within an individual tumor is variable. Some carcinomas induce a scirrhous response, whereas others have areas of necrosis caused by rapid tumor growth, “collision necrosis,” of the tightly packed proliferating cells and loss of contiguity with the blood supply.
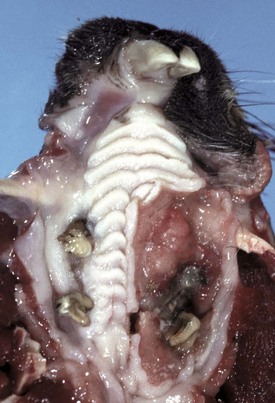
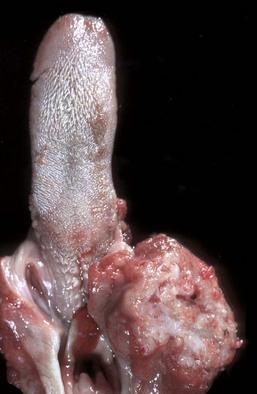
Fig. 7-14 Squamous cell carcinoma, palate, woodchuck.
A mass of proliferating neoplastic squamous epithelial cells has displaced and replaced the mucosa and underlying tissue of the left hard palate and gingiva. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Ninety percent of melanomas of the oral cavities of dogs are malignant. A breed predilection exists for Scottish terriers, Airedales, cocker spaniels, golden retrievers, Bedlington terriers, Duroc pigs, and others. Most melanomas contain copious intracellular pigment and are visibly black. Some melanomas without pigment, termed amelanotic melanomas, present a greater diagnostic challenge to both the clinician and pathologist (Fig. 7-15). Immunohistochemical staining for tryrosinase-related proteins (TRP-1, TRP-2), Melan-A, and melanocytic antigen PNL2 are useful for immunohistochemically identifying amelanotic tumors. Melanomas are composed of melanocytes and are of neural crest origin. Cellular morphology within melanomas varies from spindloid to epithelioid. Thus some neoplasms are histologically difficult to differentiate from squamous cell carcinomas and others from fibrosarcomas.
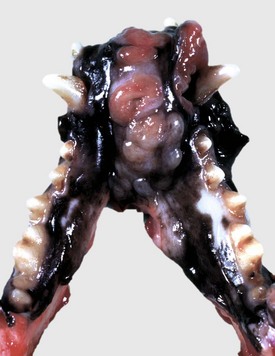
Fig. 7-15 Amelanotic melanoma, mandibular symphysis, dog.
A proliferative, ulcerated, nonpigmented mass is present on the oral mucosa at the mandibular symphysis and protrudes into the oral cavity, likely resulting in malocclusion. Incisor teeth have been lost. Note the absence of pigmentation (melanin) in this tumor. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Canine oral papillomatosis is a papovavirus-induced transmissible condition that usually occurs in animals younger than 1 year. The lesions usually regress spontaneously. Immunity is long lasting. The lesions are papilliform or cauliflower-like and can become quite numerous. They are generally white and friable and occur on the mouth, tongue, palate, larynx, and epiglottis. These oral tumors are usually multiple, white-to-gray, raised, and pedunculated with a keratinized surface and a stromal core. The epithelial cells comprising the lesion can be acanthotic, hyperplastic, and rest on a hyperplastic folded connective tissue stroma. The stratum spinosum is also hyperplastic and ballooned. Cytoplasmic inclusion bodies are sometimes present.
Oral extramedullary plasmacytomas may occur anywhere in the mucous membranes of the oral cavity, esophagus, or intestine. In the oral cavity, they are slow-growing neoplasms and in spite of often-recognized anisokaryosis, mitoses, and multinucleate cells, they rarely invade surrounding tissues and have not been reported to metastasize. Histologic examination is required for accurate diagnosis (see Fig. 13-33).
Fibrosarcomas arise from the collagen-producing cells (fibroblasts) of the oral cavity. Fibrosarcomas are most common in the cat, accounting for 20% of oral neoplasia in that species. They may occur anywhere in the oral cavity. In large breed dogs, histologically benign-appearing fibrosarcomas of the oral cavity invade bone and metastasize.
Teeth*
Teeth provide mechanical advantage for prehension, tearing and/or mastication of food. Among domestic animals, there are differences in the growth pattern and numbers of teeth. Hypsodont teeth, such as in the horse, continue to grow throughout life, and appropriate leveling of the occlusive surfaces (floating) may be a necessary procedure to prevent malocclusion and sharp edges that can lacerate the adjacent buccal mucosa as the horse ages. Brachydont teeth, such as in carnivores, do not continue to grow after they are fully erupted. Most species of mammals have deciduous teeth that are replaced near maturity by permanent teeth. In many species, the approximate age of the animal may be determined by eruption date and examination of wear patterns and shape of the teeth.
Structure and Function
Molar teeth in general are designed for grinding feedstuffs, whereas incisors in ruminants (mandibular only) are for cropping forage. Canine teeth are designed for tearing flesh. Brachydont teeth consist of a crown, which is the portion above the gingiva (the neck that is slightly constricted), and just below the gingiva are the roots, which are embedded in the bony socket (alveolus) of the jaw. Enamel covers the crown, cementum covers the roots, and both cover the dentin. Besides carnivores, the incisor (lower) teeth of ruminants and porcine teeth, except the canines of the boar, are brachydontic.
Hypsodont teeth have an elongated body, but the neck and roots may form later in life. Cementum covers the tooth, and enamel is beneath the cementum. Beneath the enamel is the dentin. The cementum and enamel invaginate into the dentin, forming the infundibula. Enamel crests result from normal wear, with enamel being the hardest of the layers. The cheek teeth of ruminants and tusks of boars and the teeth of horses are hypsodont.
In simple-toothed animals, such as carnivores, the tooth root is not covered by enamel. Receding gum lines therefore expose the dentin, resulting in pain and invasion by bacteria. Domestic animal species seldom get caries, although buildup of plaque can result in gingival infections and tooth loss.
Malocclusions
Abnormal development and positioning of the teeth may affect dental function. Malocclusion refers to a failure of the upper and lower incisors to oppose properly. This feature is “normal” for some dogs, particularly the brachycephalic breeds. In the extreme, malocclusions can lead to difficulty in the prehension and mastication of food. Malocclusions are named according to the position of the mandible. Protrusion of the lower jaw is termed prognathia, whereas a short lower jaw with resultant protrusion of the upper jaw is termed brachygnathia and sometimes hypognathia (Fig. 7-16). Sometimes these terms are incorrectly used, referring to brachygnathia as superior prognathia and prognathia as superior brachygnathia.

Fig. 7-16 Prognathia, head, horse.
The mandible is elongated compared with the maxilla. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Malocclusions result from abnormal jaw conformation or rarely from abnormal tooth eruption patterns. In some animals, such as rodents and rabbits, the teeth continue to grow throughout the animal’s lifetime. If these animals are not provided with sufficient roughage in their diets, the teeth (both incisors and cheek teeth) overgrow and either “lock” the jaw or because of a lack of occlusal grinding surfaces, prevent the animal from receiving proper nutrition (Web Fig. 7-3).
Anomalies of Tooth Development
In simple-toothed animals and rarely in other animals, agenesis of a tooth or teeth occurs and is generally of no clinical significance (see Fig. 17-35). Supernumerary tooth development is less common than tooth agenesis and is similarly of little clinical significance. Some animals, such as elasmobranches (sharks), continue to produce row on row of teeth as the outermost rows are lost. Dental dysgenesis may be primarily due to dysplasia of the enamel-forming organ or secondary to trauma, infection and hyperthermia, toxicosis, or other metabolic irregularities during odontogenesis.
Dentigerous cysts result from dental dysgenesis, and epithelial-lined, cystic structures in tissue, including the bone of the jaw result. Dentigerous cysts develop from abnormal proliferation of the cell rests of Malassez. They appear as variably sized, sometimes fluctuant swellings of the mandible or maxilla. In the maxilla, they sometimes invade the nasal sinuses. Although rare, dentigerous cysts are often painful, and while not usually neoplastic, they can destroy the jaw. Dentigerous cysts are epithelial-lined and may become impacted with keratin. Rudimentary, malformed teeth may be found within these cysts, and painful fistulas may develop, especially in horses. These draining tracts are seen most often rostral and ventral to the ear (ear tooth). Dentigerous cysts occur less frequently in otherwise normal, mature animals.
Segmental enamel hypoplasia occurs before eruption of the permanent teeth of dogs as a result of hyperthermia and viral infection, most often by canine distemper virus infection. Enamel is fully formed when the teeth erupt; therefore virus infection of ameloblasts must occur during enamel formation, which is before the dog is 6 months of age, if enamel hypoplasia is to occur. Canine distemper virus infection causes necrosis and disorganization of the enamel organ. After the virus is cleared, structure and function of the enamel organ return to normal. Thus segmental enamel hypoplasia results from the lack of enamel formation during the period of virus infection (Fig. 7-17). A similar condition in calves is caused by in utero BVD virus infection.
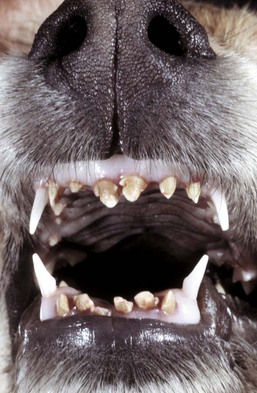
Fig. 7-17 Enamel hypoplasia, permanent incisor teeth, dog.
There is a lack of enamel formation with resultant discrete deep pits and exposure of the dentin (light yellow to beige areas of the teeth), the result of infection with canine distemper virus and necrosis of the ameloblasts during enamel formation. Permanent adult teeth (shown in illustration) are infected with virus before their eruption and while they are still within their sockets (dental alveoli). (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Chemicals, most notably tetracycline antibiotics ingested during the process of mineralization, can cause yellowish, permanent discoloration (see Fig. 1-58). Congenital porphyria, a defect in red blood cell production, may result in incorporation of porphyrins into dentin, resulting in pink discoloration of the teeth (pink tooth) (Web Fig. 7-4). Both tetracycline and porphyrins fluoresce under ultraviolet light, dramatically demonstrating these lesions.
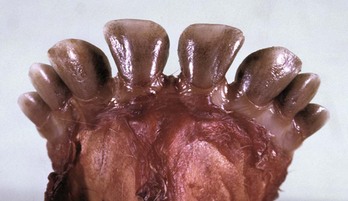
Web Fig. 7-4 Pink tooth, congenital porphyria teeth, adult ox.
The teeth are discolored brown from the accumulation of porphyrins in the dentin. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Fluoride incorporation into the enamel and dentin occurs in fluoride toxicosis, particularly in cattle and sheep. A relationship exists in beef cattle between fluorosis and selenium supplementation, with selenium supplementation being protective in high fluoride areas such as those downwind from aluminum smelters or with high levels of fluoride in ground water. Excessive dietary concentrations of fluorine during odontogenesis (from 6 to 36 months of age) may result in incorporation of the fluoride in the enamel and dentin of the permanent teeth. The result is soft, chalky, discolored enamel, usually yellow, dark brown, or black (Web Fig. 7-5). Occlusal grinding of affected soft teeth against more normal enamel results in rapid dental wear to the extent that severely affected sheep may have almost completely worn down their incisors. One wonders therefore about the cumulative effect of fluoride supplementation in municipal drinking water, vitamins with added fluoride, fluoride-supplemented toothpaste, fluoride treatment of teeth, reconstituted and bottled soft drinks made with fluoridated water, and so forth. It is difficult to calculate the total fluoride load ingested by individuals or what the effects may be of that fluoride supplementation.
Lesions Caused by Attrition and Abnormal Wear
Loss of normal dental structure and function often results from rapid and irregular and/or abnormal wear of occlusal surfaces in many species of domestic animals. In those species with hypsodont teeth, attention to the dentition as the animal ages is often a major factor in overall body conditioning and health (Fig. 7-18). Aggressive treatment of occlusal surface irregularity by filing of high points in the dental arcade (floating) can notably prolong an animal’s life. Rock chewing or other compulsive oral behaviors in dogs may result in accelerated dental wear. Similarly, cribbing in horses and herbivorous animals grazing on sandy soils can cause premature dental wear. In all species, exposure of dentin or the pulp canal may lead to dental infection with serious consequences.
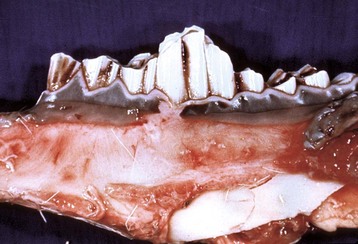
Fig. 7-18 Dental attrition, molar teeth, antelope.
Age-associated dental wear results in improper mastication of feedstuffs and malnutrition. This condition occurs most commonly in horses and is referred to as “step-mouth” or “broken mouth.” (Courtesy College of Veterinary Medicine, University of Tennessee.)
Miscellaneous Dental Lesions
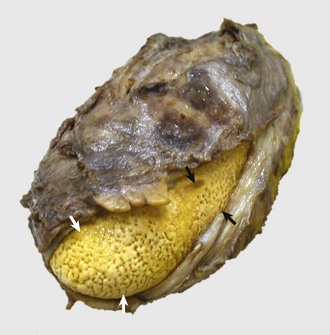
Feline External Resorptive Neck Lesions: Cats suffering from feline external resorptive neck lesions often have pain upon chewing that may be reflected by inappetence and/or abnormal masticatory movements. External neck resorption of the cheek teeth of otherwise dentally normal cats is caused by odontoclastic resorption of cementum, particularly in the neck area or root of the tooth. Osteoclast ingrowths partially or completely line the resorption cavity. The resultant cavity may harbor bacterial plaque, resulting in intense inflammation and further osteoclastic resorption of dental tissue, including dentin and the root canal. The primary cause of this condition is not known.
Infundibular Impaction
Impaction of the infundibulum, also known as infundibular necrosis or infundibular caries, may cause serious dental disease in ruminants and more rarely in horses. Incomplete infundibular cementum formation before the tooth erupts likely predisposes to infundibular impaction. The pathogenic mechanism is comparable to dental caries in simple-toothed animals, which is uncommon in domestic animals. Feed material is ground into the infundibulum, where bacteria metabolize it to form acid, which causes demineralization. Bacterial enzymes digest the organic matrix of enamel and dentin. As a result of this destruction, the pulp cavity becomes exposed and infected, resulting in pulpitis and endodontitis. Dental abscesses and fistulous tracks may develop and rupture into the paranasal sinuses. The inflamed infundibular cavities often continue to become impacted with feed, creating a vicious cycle.
Periodontal Disease
More than 200 species of bacteria and fungi have been associated with dental plaque (a film of an organic matrix, food particles, and bacteria on the tooth surface). This plaque often becomes mineralized (tartar or dental calculus). The mineralized material contributes to atrophy and inflammation of the gingival mucosa and supporting stroma by acting as a nidus for additional plaque accumulation. Bacteria resident in films on the tooth surface produce acids and enzymes that may damage their enamel substrate (cavities) and also destroy the subjacent gingival tissue and periodontal ligament (periodontal disease).
The initial site for destructive inflammation is in the gingival crevice–forming pockets where bacteria lodge. With time, this inflammation spreads distally along the tooth, resulting in gingival-epithelial attachment only on the root of the tooth, deep in the alveolar socket. Progression of inflammation may destroy the connective tissue of the periodontal ligament, resulting in loosening of the tooth. The infection can spread causing alveolar osteomyelitis and pulpitis and can result in apical abscesses and bacteremia. There is significant oral pain, reluctance to masticate, and halitosis. Periodontal disease is common in carnivores and humans. Mildly abrasive diets and brushing of the teeth of pet carnivores, combined with regular dental examination, is preventive as it is in humans.
Dental Neoplasia
Proliferative, cystic, or neoplastic diseases of the dental arcade can originate from cell rests that form from the dental lamina or the enamel organ (the cell rests of Malassez). Dental neoplasms usually arise close to the teeth, either deeply in the jaw or from the oral epithelium. There is a relatively precise method of naming dental neoplasms based on the tissue or cell of origin and the extent of differentiation and odontogenesis present within the neoplastic tissue. The histologic appearance of these neoplasms is complex; pathologists with considerable experience in differentiating these uncommon neoplasms should be consulted when a precise diagnosis is indicated.
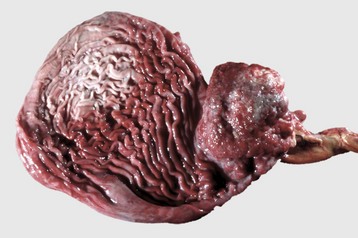
Odontomas are hamartomas originating in the enamel organ and are usually seen in puppies and foals (Fig. 7-19). They usually contain well-recognizable dentin and enamel, as well as ameloblasts, odontoblasts, and dental pulp.
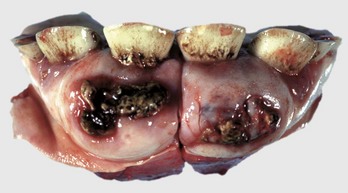
Fig. 7-19 Odontoma, incisor teeth, cow.
This is a hamartoma (a benign tumorlike nodule) of the enamel organ that in this case has expanded bilaterally on the rostral mandibles. There is extensive hemorrhagic ulceration over the tumor. Diagnosis can be confirmed by radiographic and histopathologic examination. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Ameloblastoma is a term applied to epithelial neoplasms of enamel organ origin. Several subtypes, distinguished histologically, are ameloblastic fibroma, ameloblastic odontoma, calcifying epithelial odontogenic tumor, peripheral odontogenic fibroma, and other rare tooth neoplasms. Ameloblastoma appears randomly in the dental arcade, usually in adult dogs. They are often osteolytic and thus are locally invasive. Histologic examination by an expert is often necessary to distinguish ameloblastoma from acanthomatous epulis (acanthomatous ameloblastoma) and squamous cell carcinoma.
Tonsils
The palatine tonsils are pharyngeal lymphoid structures covered by stratified squamous epithelium. Their function is uncertain, although it is likely they serve in lymphocyte production and antibody formation (see Chapter 13). In carnivores, they are found in crypts or recesses at the dorsolateral aspect of the caudal oropharynx. In pigs, they are flat and recognized by tiny pores in the surface epithelium of the caudal soft palate. Equids, ruminants, and pigs have lingual tonsils in addition to palatine tonsils.
Portals of Entry
Tonsils do not possess afferent lymphatic vessels and do not serve as lymph filters. Therefore only primary (or direct) or hematogenous infections occur (tonsillitis) (Fig. 7-20), as well as primary neoplasms of either the lymphoid (lymphoma) (Fig. 7-21) or epithelial (squamous cell carcinoma) (Fig. 7-22) components. In many viremias of mammals, such as pseudorabies of pigs, virus may be isolated from the tonsils.
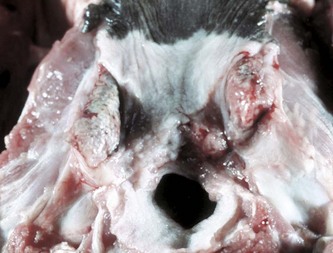
Fig. 7-20 Necrotizing tonsillitis, tonsils, dog.
The palatine tonsils are enlarged and discolored. The right tonsil is covered by a diphtheritic membrane, and the left tonsil is extensively ulcerated. Because there are no afferent lymphatics to the tonsils, infection is either primary (by direct spread) or hematogenous. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Salivary Glands
Salivary glands are found in a variety of locations in the head and neck regions and vary in number and location from species to species. They arise from oral ectoderm. In all species, the major salivary glands include the parotid, mandibular, and sublingual. Carnivores have a zygomatic gland as well. Minor salivary glands include buccal, labial, lingual, palatine, and others similarly named by location.
Structure and Function
Most salivary glands are discrete aggregates of compound tubuloalveolar tissue. Saliva is a mixture of serous and mucoid secretions. Saliva lubricates the mouth and esophagus and moistens ingesta. Saliva also dissolves water-soluble components of food so the taste buds can function. The mucus in saliva binds to masticated food and creates a bolus that is more easily swallowed. Salivary mucus also coats the epithelium of the mouth, preventing mechanical damage to the tissue. Saliva, through its flushing action, reduces bacterial populations. Saliva contains a lysozyme that lyses bacteria. Carbohydrate digestion begins in the oral cavity as a result of the presence of α-amylase, which changes starch into maltose. There are very small quantities of this enzyme in carnivores and cattle. Saliva also is an effective buffer, especially in ruminants, whose forestomachs have no glands. In carnivores, evaporation of saliva is a major mechanism of thermoregulation.
Portal of Entry
Salivary glands are generally affected by blood-borne pathogens, direct penetration by foreign objects, obstruction of the excretory ducts, or bite wounds. In humans, ascending infections from the salivary ducts occur, but there is no evidence that this occurs in domestic animals. The serous portions of the salivary glands are radiosensitive.
Response to Injury
Injury to the salivary gland is accompanied by incomplete regeneration, principally from ductular epithelium. There are often atrophy, fibrosis, and squamous metaplasia of secretory epithelium, sometimes resulting in blockage of ducts.
Inflammatory Diseases
Sialoadenitis, inflammation of a salivary gland, is relatively rare in veterinary medicine. Although diagnosis of systemic diseases is not made by examining the salivary gland, rabies and canine distemper are two very important diseases that cause inflammation of the salivary glands. Saliva is a particularly important medium of spread, by bite wounds, of the rhabdovirus that causes rabies. There are focal necrosis, mononuclear cell inflammation, and sometimes inclusions (Negri bodies) in the nuclei of ganglion cells. In the rat, a coronavirus termed sialodacryoadenitis virus is responsible for inflammation of the salivary gland and some adnexal ocular glands. Salmonella typhisuis has caused suppurative parotid sialoadenitis in pigs.
Gross lesions of sialoadenitis are subtle and include swelling and edema. Sialoadenitis can be accompanied by pain on palpation. Abscesses occasionally occur sometimes secondary to the migration of foreign bodies (grass awns) and are especially noticeable when they occur in the retrobulbar zygomatic gland where they may cause ocular protrusion (proptosis).
Miscellaneous Diseases or Conditions
Changes in the salivary glands are uncommon in domestic animal species. A ranula is a cystic saliva-filled distention of the duct of the sublingual or submaxillary salivary gland that occurs on the floor of the mouth alongside the tongue (Fig. 7-23). It is thus epithelial lined. The cause is generally unknown, although some cases are due to sialoliths. A salivary mucocele, in contrast, is a pseudocyst not lined by epithelium but filled with saliva. The cause of this lesion is also unknown, but it may occur secondary to traumatic rupture of the duct of a sublingual salivary gland with resultant leakage and encapsulation of saliva by reactive connective tissue.
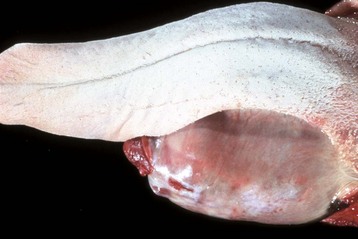
Fig. 7-23 Ranula, mandibular salivary duct, dog.
This is a cystic distention of the left mandibular salivary duct along the ventral-lateral aspect of the tongue. (Courtesy Dr. P. Stromberg, College of Veterinary Medicine, The Ohio State University.)
Sialoliths are rare in domestic animal species. When they do occur, they are considered to be caused by inflammation of the salivary gland with sloughed cells or inflammatory exudate forming a nidus for mineral accretion (Fig. 7-24). Thus they are one cause of ranula formation.
Neoplasia
Salivary gland neoplasms, both benign and malignant, are uncommon but occur in all species (Fig. 7-25). They are composed of glandular or ductular elements or a combination of epithelial and mesenchymal components similar to those in mixed mammary neoplasms. A grossly appearing similar condition, salivary gland infarction, occurs infrequently in cats and rarely in dogs. The cause of the infarction is unknown. The gross appearance of firmness and swelling of an infarcted gland must be distinguished microscopically from neoplasia (Web Fig. 7-6). In salivary gland infarction, there are discrete foci of parenchymal necrosis with peripheral hemorrhage and inflammatory cells. Attempted incomplete regeneration of the gland from ductal epithelium can be mistaken for neoplasia unless one is familiar with the former condition.
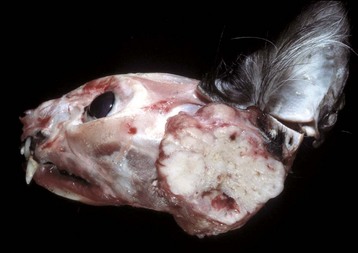
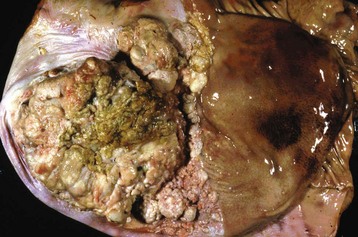
Fig. 7-25 Salivary gland carcinoma, left parotid salivary gland, cat.
A large proliferative carcinoma of the salivary gland has replaced the normal gland. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
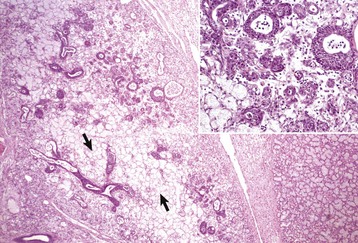
Web Fig. 7-6 Salivary gland infarction, salivary gland, cat.
Note the areas that lack cell definition (necrosis) secondary to infarction (arrows). Normal salivary gland is present in the right third of this illustration. Inset, Abortive regeneration as evidenced by hyperplasia of surviving salivary duct epithelial cells. H&E stain. (Fig. and Inset courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Tongue
The tongue is a muscular organ covered by stratified epithelium and is functionally connected to the esophagus via the epiglottis.
Structure and Function
The tongue is necessary for prehension, mastication, and swallowing of feedstuffs and water. The epithelial covering of the tongue is stratified squamous with various degrees of keratinization dorsally, but ventrally the epithelium is not keratinized and the tongue attaches to the floor of the oral cavity by a frenulum. Keratinized papillae are most prominent in ruminants and cats. There are various types of papillae, some with secondary lamellae. Vallate papillae, for example, are on the dorsal surface of the tongue near its root and are flat structures completely surrounded by a cleft. Some surface macroscopic papillae contain taste buds. The tongue is a highly vascular (functioning in heat loss in many animals, especially carnivores that have no sweat glands) and sensitive organ containing a variety of serous and mucus glands and sensory cells (taste buds). The muscular part of the tongue is striated in randomly arranged bundles. A cordlike structure enclosed in dense collagen extending lengthwise near the ventral central surface of the tongue of carnivores is called the lyssa. Porcine and equine tongues have a similar structure. The lyssa appears to be a structure without a function. Historically, the lyssa was removed as “prevention” for rabies. Lyssa bodies are synonymous with Negri bodies, and rabies used to be called lyssa. Adipose tissue becomes more abundant in the caudal part of the tongue in most species.
Developmental Anomalies
Congenital diseases of the tongue include epithelial defects such as fissures, epitheliogenesis imperfecta, macroglossia and microglossia, bifid tongue, and hair growing from the tongue (choristoma) (Web Fig. 7-7). Lethal glossopharyngeal defect, or bird tongue of dogs, is characterized by a pointed tongue that cannot wrap around a nipple and create the negative pressure required for nursing and without intervention, starvation results. Ventral ankyloglossia, fusion of the tongue to the floor of the oral cavity, has been reported in related Anatolian shepherd dogs. The cause of these congenital lesions is not known, but they sometimes occur in association with other defects. As in the case of other congenital defects, ingestion of unknown teratogenic substances by the dam during gestation is an etiologic possibility, as is mutation of T-box genes.
Systemic Disease: Primary Involvement of the Tongue
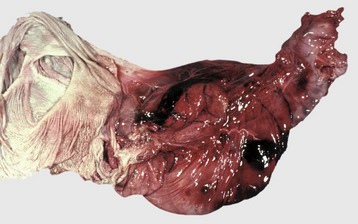
Disease agents that principally target the tongue are relatively rare. The exception to this rule is Actinobacillus lignieresii, a gram-negative bacillus that is a normal inhabitant of the oral cavity. It is an opportunistic invader of damaged lingual tissue, principally in bovids and occasionally in equids and small ruminants. The granulomas resulting from infection contain centrally located actinobacilli rimmed by radiating amorphic, eosinophilic, and clublike structures composed of immunoglobulin molecules from lesion plasmacytes (Fig. 7-26). Mixed mononuclear inflammatory cells, including multinucleated Langhans’ giant cells, often surround these foci (Splendore-Hoeppli phenomenon), and infection may drain and cause similar inflammation in submaxillary and retropharyngeal lymph nodes. The amount of fibrous tissue present depends on the duration of the inflammation and the swelling. Inflammation and fibrosis cause increased firmness of the tongue and linguamegaly, or “wooden tongue” (Fig. 7-27). Horses are rarely affected by Actinobacillus lignieresii infections, but when they are, lesions are cutaneous or lymph node abscesses, mastitis, and occasional glossitis.
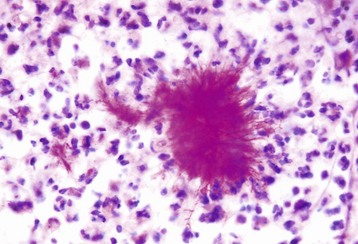
Fig. 7-26 Actinobacillosis (wooden tongue), tongue, cow.
Splendore-Hoeppli reaction (colony of bacteria with surrounding radiating “clubs” of immunoglobulin) is surrounded by suppurative inflammation. H&E stain. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
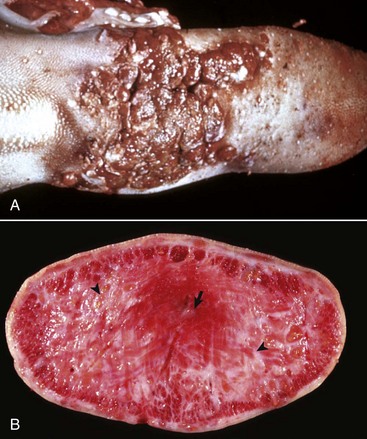
Fig. 7-27 Actinobacillosis, tongue, cow.
A, Dorsal surface. Proliferative and ulcerative chronic-active inflammatory lesions containing neutrophils mixed with mononuclear inflammatory cells (lymphocytes, macrophages, plasma cells) and fibrous tissue are present in the tongue. B, Chronic actinobacillosis (wooden tongue). Chronic inflammation results in loss of muscle of the tongue and its replacement by fibrous tissue during healing. Note the white interwoven bands of fibrous connective tissue (arrowheads) and the focus of granulomatous inflammation (arrow). (A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. R.J. Panciera, School of Veterinary Medicine, Oklahoma State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Systemic Disease: Secondary Involvement of the Tongue
Thrush is a Candida albicans (yeast) infection of intact mucous membranes of the tongue and esophagus (Fig. 7-28). It occurs principally in ungulates but has also been seen in carnivores. Thrush is not a primary disease but often indicates an underlying debility, particularly in young animals. It occurs as a result of antibiotic treatment that kills normal flora, increased serum glucose concentrations as a result of diabetes mellitus, a high-sugar diet, or intravenous glucose therapy. The availability of iron is a limiting factor for the indigenous bacteria, which compete with yeast for mucosal colonization. Immunodeficiency states also contribute to the development of thrush. All of these scenarios provide tissue conditions suitable for the proliferation of yeast forms. Rarely, systemic infection may result. Factors predisposing to systemic infections include multiple antibiotic usage, indwelling catheters, and endotracheal tubes. This infection presents as a gray-green pseudomembrane that is easily scraped off the intact underlying mucosal surface (Fig. 7-29).
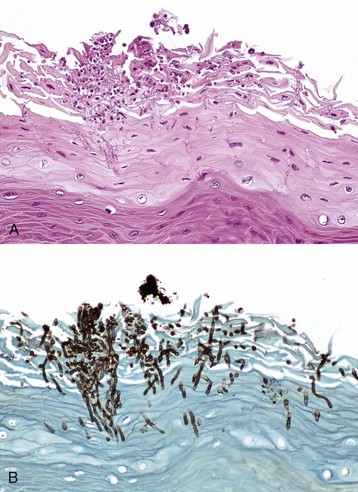
Fig. 7-28 Thrush (oral candidiasis), tongue, foal.
A, Hyphae of Candida albicans are growing in the superficial keratin of the tongue. H&E stain. B, Same specimen as A. Gomori’s methenamine silver stain. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
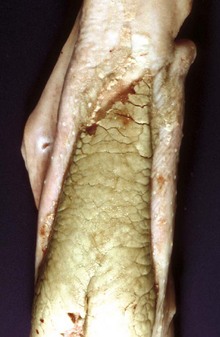
Fig. 7-29 Thrush, tongue, foal.
A pseudomembrane of hyphae of candida is present on the dorsal surface. It has been scraped off the rostral end of the tongue (top) to reveal normal mucosa beneath the fungal mat. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Often, lingual lesions are manifestations of systemic diseases, such as BVD, foot-and-mouth disease, multisystemic amyloidosis, and uremia (Fig. 7-30; also see Fig. 11-27). These diseases are discussed in more detail in this and other chapters of this book.
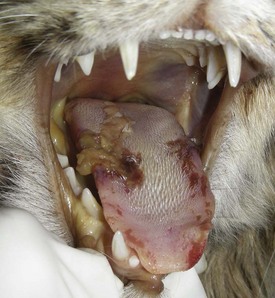
Fig. 7-30 Ulcerative glossitis, uremia (uremic glossitis), tongue, cat.
There is extensive ulceration of the mucosal epithelium of the tongue associated with increased concentrations of serum blood urea nitrogen and creatine from kidney failure. (Courtesy Drs. R.L. Fredrickson and R.A Doty, College of Veterinary Medicine, University of Illinois.)
Hyperplastic and Neoplastic Conditions
Epithelial hyperplasia of the lateral edges of the tongue is common in piglets before nursing, when the fringelike epithelium is rubbed off (Web Fig. 7-8).
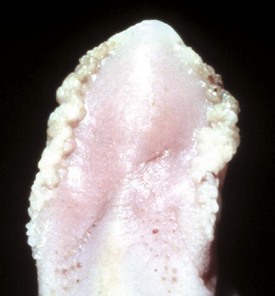
Web Fig. 7-8 Lingual epithelial hyperplasia, tongue, neonatal pig.
The lateral surfaces of the tongue are covered by a hyperplastic epithelial fringe. This fringe is normal at birth and will be lost through mechanical trauma to the fringe during nursing. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Lingual (glossal) neoplasms are rare but when they occur are generally of epithelial origin. Squamous cell carcinomas are most common (Fig. 7-31; also see Fig. 6-10), but papillomas (Fig. 7-32), rhabdomyomas, rhabdomyosarcomas, fibrosarcomas, melanomas, and granular cell tumors have all been reported in domestic animals.
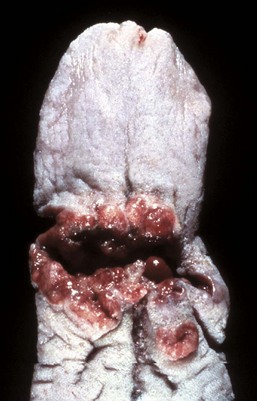
Fig. 7-31 Squamous cell carcinoma, tongue (dorsal surface), dog.
Note the proliferative, ulcerated, and hemorrhagic neoplasm growing transversely across the surface of the tongue. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
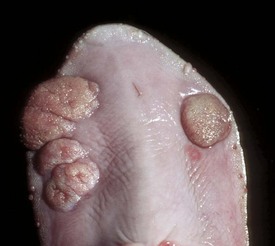
Fig. 7-32 Papillomas, tongue (ventral surface), cow.
Papillomas, often caused by bovine papillomavirus, are present on the ventral surface of the tongue. The virus infects traumatized mucosal epithelial cells and induces epithelial cell proliferation. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Parasites
Parasites of the tongue are uncommon, with the exception of those that reside in muscles such as Sarcocystis spp. in most species and Trichinella spiralis in pigs and occasionally in carnivorous wildlife such as polar bears. Gongylonema spp. can be present in the lingual mucosa of pigs and ruminants and are of no clinical significance.
Esophagus
Under normal circumstances, the esophageal lumen is a potential space. The wall collapses when the esophagus is not transporting ingesta.
Structure and Function
The esophagus extends from the aboral end of the oropharynx, passes through the mediastinum and the diaphragmatic hiatus, and ends at the stomach. The esophagus is lined by nonkeratinizing stratified squamous epithelium in carnivores and is keratinized in pigs, horses, and ruminants. Keratinization is greatest in ruminants, less in horses, and least in pigs. Longitudinal and oblique mucosal folds are present to varying degrees. Transverse, herringbone-like folds are present in the cat.
The tunica muscularis is completely striated in ruminants and dogs. In the horse, the distal third of the esophagus contains smooth muscle. The pig is similar to the horse, except that the middle third of the esophagus contains a mixture of smooth and striated muscle. In cats, opossums, and primates, the distal two-thirds of the esophagus is composed of smooth muscle. The smooth muscle is arranged as an inner circular layer and an outer longitudinal layer.
Mixed mucinous glands are present in the tunica submucosa of pigs and dogs. In pigs, the glands are most abundant in the cranial half of the esophagus, and in dogs, they are present throughout. Glands are present in cats, horses, and ruminants only at the junction of the esophagus and pharynx.
It is important to remember that unlike the rest of the tubular digestive tract, the esophagus is unique in that it lacks a serosa in all but the abdominal portion. This means that there is no serosa to leak serum and fibrin to seal a puncture wound from a perforation of a foreign body or a surgical incision. Likewise, sutures are not likely to seal an incision. Combine this with the strong muscular peristaltic contractions that characterize this organ and it is easy to understand why esophageal surgery is not often performed and is even less often successful. For the same anatomic reasons, perforating foreign bodies of the esophagus do not seal themselves off.
Esophageal innervation is from the vagus nerves. Esophageal smooth muscle contains myenteric ganglia. Striated muscle is innervated by motor end-plates via efferent fibers of the hypoglossal nerve along with contributory neurofibers from cranial nerves 5, 9, and 10, which control voluntary lingual function.
Portals of Entry
Materials from the oral cavity pass via the esophagus to the stomach or rumen, including caustic chemicals. Penetration or obstruction by foreign objects is the most common portal of entry into the mediastinum (Fig. 7-33). Some parasites spend part or all of their life cycles in the esophagus. Iatrogenic puncture of the esophagus is a not uncommon sequela to passage of stomach tubes. Gastric reflux is an additional portal of entry into the esophagus.
Developmental Anomalies
Esophageal motility disorders are termed achalasia. In this condition, the sequential contractility of the esophagus is defective and the lower cricopharyngeal sphincter fails to function properly. Achalasia results in difficulty in swallowing and may be responsible for regurgitation and weight loss.
Cricopharyngeal achalasia is a congenital, possibly neurogenic, disorder of the upper esophageal (cricopharyngeal) sphincter. It occurs in young, small breed dogs, particularly terriers, cocker spaniels, and miniature poodles. Postweaning dysphagia and regurgitation after a meal of solid food is characteristic of this functional disorder. Liquids are generally swallowed without incidence. Gagging or choking behavior of the patient after swallowing is a good indicator in the appropriately aged dog of this condition.
Acquired canine achalasia is extremely uncommon. In this condition, there is often a visible abnormality of the musculature of deglutition (cricopharyngeus). There does not appear to be a characteristic change in the affected musculature. Esophageal myotomy of the appropriate cricopharyngeal sphincter muscle is palliative for these idiopathic conditions.
Megaesophagus
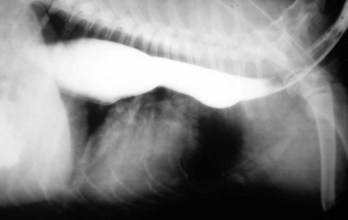
Megaesophagus or esophageal ectasia is dilation of the esophagus because of insufficient, absent, or uncoordinated peristalsis in the mid and cervical esophagus. It has been described in dogs, cats, cows, ferrets, horses, and new world camelids. Causes include innervation or denervation disorders and partial physical obstructions and stenosis, secondary to inflammatory diseases of esophageal musculature or persistence of the right aortic arch. Many cases are idiopathic.
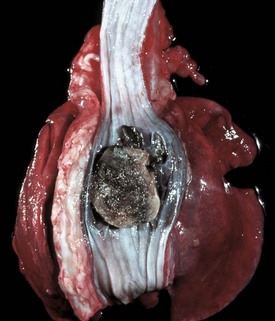
Congenital megaesophagus is usually due to partial blockage of the lumen of the esophagus by a persistent right fourth aortic arch. Because of the persistence of the arch, a vascular ring forms around the esophagus and trachea, preventing full dilation of the esophagus. The ring is formed by the aorta, pulmonary artery, and ductus arteriosus. This form of megaesophagus is unique in that the esophageal obstruction, and thus dilation, occurs cranial to the heart because of the location of the obstructing vascular ring (Fig. 7-34; also see Fig. 10-64). Persistent right aortic arch is likely hereditary in German shepherds, Irish setters, and greyhounds. All other forms of megaesophagus result in dilation cranial to the stomach.
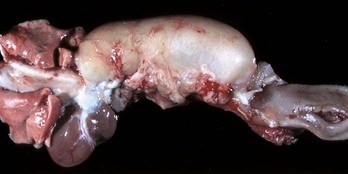
Fig. 7-34 Megaesophagus from a persistent right aortic arch, esophagus, dog.
Dilation of the esophagus cranial to the heart is the result of failure of the right fourth aortic arch to regress during embryonic life (vascular ring abnormality). (Courtesy Dr. C.S. Patton, College of Veterinary Medicine, University of Tennessee.)
Congenital megaesophagus also occurs as an idiopathic denervation of the esophagus, most notably in great Danes, Irish setters, miniature schnauzers, Labrador retrievers, wire hair fox terriers, shar-peis, Newfoundlands, Siamese cats, and in some cases of vagal indigestion, in cattle. Some cases of myasthenia gravis (see later discussion) are congenital and may be of genetic origin.
Acquired megaesophagus (esophageal achalasia) is the result of failure of relaxation of the distal esophageal (cardiac) sphincter of the stomach. The obstruction and thus dilation occurs cranial to the stomach (Fig. 7-35). Although the gross appearance of acquired megaesophagus in animals is similar to that of humans, the cause of the condition in animals does not involve the cardiac sphincter. Causes are idiopathic or secondary to polymyositis (inflammation of the esophageal muscle), myasthenia gravis (a congenital or autoimmune disease directed against acetylcholine receptors of the neuromuscular junction), hypothyroidism (which can result in muscle atrophy and denervation disease), congenital myopathy, lead and thallium poisoning (via effect on innervation), peripheral neuropathies, vagal indigestion, esophagitis, and recurrent gastric dilation. Increased risk in dogs is seen in German shepherds, golden retrievers, and Irish setters.
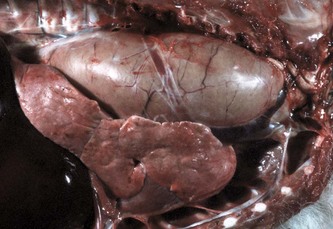
Fig. 7-35 Megaesophagus, thoracic esophagus, dog.
A notably dilated thoracic esophagus cranial to the diaphragm has displaced the right lung caudally and ventrally. This form of megaesophagus is often attributable to an abnormality (mass, foreign body, innervation disorder) affecting the cardiac sphincter. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Megaesophagus is recognized clinically by regurgitation after ingestion of solid food. Thus congenital megaesophagus is often recognized at weaning. Often, animals are thin and may have aspiration pneumonia. Radiographically, the esophagus is dilated anterior to the lesion and retains radiopaque dyes (Web Fig. 7-9). Dilation may vary from diffuse to locally extensive, depending on its cause. Putrid ingesta are sometimes found in the dilated, atonic portions of the esophagus. Although degenerate nerve fibers are occasionally found within vagus nerves, megaesophagus can occur without detectable histologic lesions.
Hiatal Hernia
Protrusion of the abdominal esophagus and cardia of the stomach through the diaphragm into the thoracic cavity is termed a hiatal hernia. This inversion is generally into the esophageal lumen and is self-reducing. Sometimes a gastroesophageal intussusception results.
Eosinophilic Esophagitis
Eosinophilic esophagitis is an emerging disease in humans; in a single dog, clinical signs include regurgitation, dysphagia, and cough, accompanying a diffusely affected, friable, hyperemic, ulcerated esophageal mucosa visible by endoscopy. Inflammation is dominated by granulocytes—half of which are eosinophils. Diagnosis is by elimination of other causes of inflammatory esophagitis. Seventy percent of humans and the single dog described had concurrent allergic skin disease.
Esophageal Parasites
With notable exceptions, parasitic diseases of the esophagus are generally of no clinical importance. The more common parasites of the esophagus are Gongylonema spp., which affect ruminants, pigs, horses, primates, and occasionally rodents. These nematodes reside in the esophageal mucosa and are characteristically thin, red, and serpentine. They can be 10 to 15 cm in length and are easily visible (Fig. 7-36). The intermediate hosts are cockroaches and dung beetles.
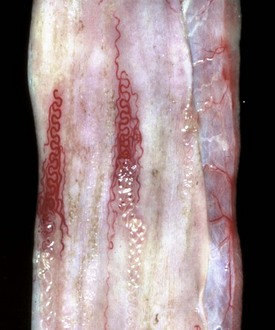
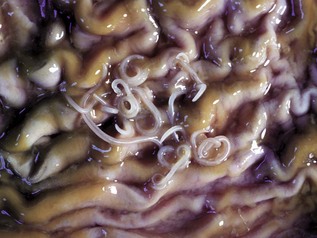
Fig. 7-36 Gongylonemiasis, esophagus, cow.
The serpentine intramucosal nematodes are characteristic of Gongylonema, a nematode of the superfamily Spiruroidea. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Gasterophilus spp. occur in equids. These fly larvae have interesting life cycles because their eggs are laid on the skin in varying locations. The warmth and moisture from licking activates them. The larvae burrow into the oral mucosa, molt, and then migrate down the esophagus. They occur both in the distal esophagus and stomach, where they attach to the mucosa via oral hooks. They eventually detach, leaving craters at the site of attachment, and pass in the feces.
Hypoderma lineatum is the larvae of the warble fly of ruminants. These parasites eventually migrate to the esophageal adventitia and then to the subcutaneous tissue of the back.
Spirocerca lupi of canids is probably the most pathogenic of the esophageal parasites. These nematodes reach the esophageal submucosa after migrating from the stomach. They penetrate through the gastric mucosa to reach the adventitia of arteries and then migrate in the adventitia to the abdominal aorta and aborally to the caudal aorta where they form a granuloma in the adventitia. From here they migrate to the adjacent esophageal submucosa. A passage forms between the esophageal lumen and the granuloma containing the parasite, allowing discharge of ova into the lumen of the alimentary system and eventually into the feces. Clinical sequelae of infestation include dysphagia, aortic aneurysms, hemothorax, and rarely esophageal fibrosarcomas or osteosarcomas (Fig. 7-37). Occasionally, chronic aortic granulomas will extend into the adjacent thoracic vertebral bodies of chronically affected canids to cause spondylosis deformans adjacent to the aortic granulomas. Spirocerca lupi infestations occur in warmer climates. The intermediate hosts are dung beetles, and the paratenic hosts are chickens, reptiles, and rodents.
Miscellaneous Esophageal Lesions and Conditions
Idiopathic muscular hypertrophy of the distal esophagus is a lesion peculiar to horses and pigs that can be quite spectacular at necropsy (Fig. 7-38) but usually is of no clinical significance. The esophageal musculature can be several centimeters thick, and the lesion can extend along the distal quarter of the esophagus. Rarely this condition plays a role in esophageal impaction. Similarly, dilation of the esophageal glands present throughout the esophagus of aged dogs can be a spectacular gross lesion of no clinical consequence. It is therefore important to carefully evaluate these lesions either at necropsy or during fiberoptic viewing in the live animal to determine whether what appear to be erosions and ulcers are instead mucosal elevations caused by glands filled with mucus. Because the lesions are subepithelial, the overlying mucosa is smooth and shiny. Dilated esophageal glands vary in number and location but are generally only a few millimeters in diameter (Fig. 7-39). They are most numerous in the distal esophagus.
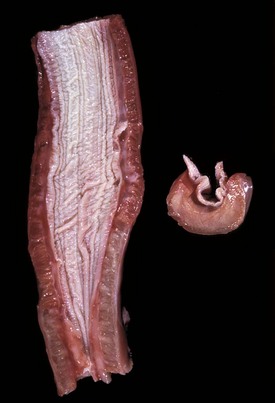
Fig. 7-38 Muscular hypertrophy, distal esophagus, horse.
Longitudinal (left) and transverse (right) sections of the esophagus demonstrate the marked increase in the thickness of the smooth muscle in the tunica muscularis of the distal esophagus. (Courtesy Dr. C.S. Patton, College of Veterinary Medicine, University of Tennessee.)
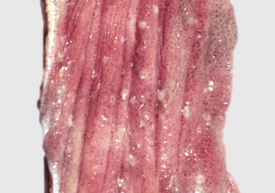
Fig. 7-39 Cystic esophageal glands, distal esophagus, dog.
Multiple white mucosal cysts are present in the esophageal glands of the mucosa and submucosa. These cysts are common and insignificant findings in aged dogs. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Esophageal erosions and ulcers are relatively common and have a variety of causes. One of the more common causes of esophageal erosions and ulcers is reflux of stomach acid. This reflux of gastric acids causes chemical burning of the distal or aboral esophagus and is commonly called acid reflux esophagitis (Fig. 7-40) or clinically, heartburn in humans. Other causes of esophageal ulcers include improper use of stomach tubes, which cause linear scraping on the crests of the longitudinal folds of the esophageal mucosa (Fig. 7-41), foreign bodies such as bones in dogs and infectious diseases, such as BVD (Fig. 7-42), which cause mucosal injury in other locations as well.
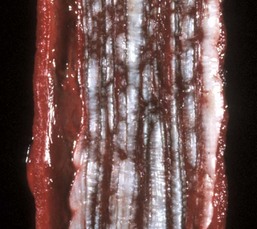
Fig. 7-40 Acid reflux esophagitis, esophagus, horse.
The dark red streaks on the surface of the esophagus are areas of epithelial loss secondary to gastric acid reflux. The white streaks and vertically linear areas on the surface of the esophagus are areas of unaffected and likely hyperplastic mucosal epithelium. As would be expected, erosions are most severe in the esophageal mucosa adjacent to the cardia and extend orad. This distribution is diagnostic of acid reflux esophagitis. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
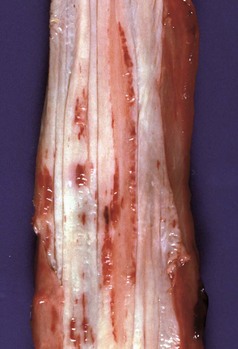
Fig. 7-41 Trauma-induced esophageal ulceration, esophagus, horse.
These red linear ulcers are the result of abrasion from improper stomach tubing, either from an overly large diameter tube, from a too-vigorous insertion, or from a tube with a roughened edge. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
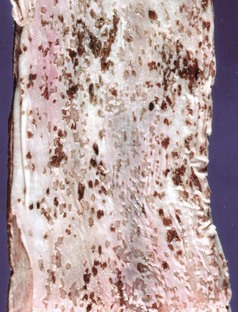
Fig. 7-42 Ulcerative esophagitis, bovine viral diarrhea (BVD), esophagus, cow.
Note the multiple variably sized (millimeter range) and variably shaped esophageal mucosal ulcers caused by the pestivirus of BVD. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Leukoplakia of the esophagus and stomach is characterized by discrete, flat, white mucosal elevations (epithelial plaques) of no clinical significance and of unknown cause. They are sometimes mistaken for thrush lesions or neoplasia. Unlike thrush lesions, they do not scrape off easily, and their regularity, number, and location distinguish them from neoplasms. Histologically, the stratum basale and prickly cell layers are notably thickened, and the surface cells have pyknotic nuclei and some parakeratosis. In humans, about 5% of these lesions become cancerous. They are present in the oral cavity and esophagus of humans and are believed related to chronic irritation most often associated with smoking or chewing tobacco. Alcohol consumption and restorative dental amalgams may also predispose to leukoplakia.
Choke
Choke is a clinical term referring to esophageal obstruction subsequent to stenoses or blockage. Choke most often occurs in anatomic locations in which the esophagus cannot fully expand. These locations are dorsal to the larynx, cranial to the first rib at the thoracic inlet, the base of the heart, and the diaphragmatic hiatus. Choke occurs most frequently as a result of ingestion of large foreign bodies, such as potatoes, apples, bones (Fig. 7-43), or corn cobs (Fig. 7-44), or medicaments, such as large gelatin-filled capsules or tablets (dry boluses). If these bodies are lodged against the epithelium for longer than 2 days, the interaction often results in circumferential pressure necrosis of the esophageal mucosa (Fig. 7-45), which forms strictures during healing. These strictures then can cause reflex regurgitation after ingestion of food with possible starvation or aspiration pneumonia resulting.
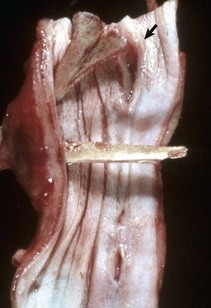
Fig. 7-43 Ulcers and perforation, foreign body, esophagus, dog.
The esophagus has been perforated by an ingested chicken bone. Note that the end of the bone opposite the perforation site has caused a deep ulcer (arrow). There are also several chronic ulcers caudal to the perforation, presumably from abrasion by other bones as they moved down the esophagus. (Courtesy Dr. C.S. Patton, College of Veterinary Medicine, University of Tennessee.)
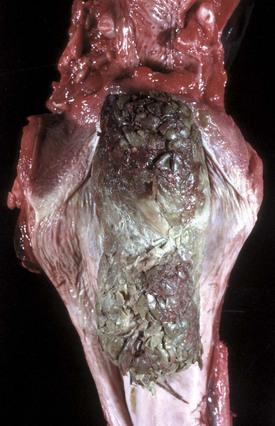
Fig. 7-44 Foreign body (choke), esophagus, cow.
A corn cob has lodged in the esophagus subjacent to the larynx. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
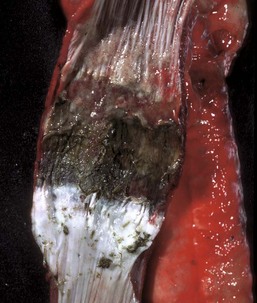
Fig. 7-45 Foreign body (choke), esophagus, horse.
Pressure necrosis of the proximal esophageal mucosa, adjacent to the larynx has occurred secondary to lodgement of a foreign body (compacted chaff). As a general rule, pressure necrosis usually occurs if the foreign body remains in place against the mucosal epithelium for longer than 2 days. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
In older horses, poor dentition causes feed to be incompletely masticated, resulting in impaction in the esophagus. Neoplastic or inflammatory lesions of the esophagus or periesophageal tissues also cause obstruction. Persistence of the right aortic arch has already been discussed as a cause of esophageal stenosis and megaesophagus.
Neoplasia
Neoplasms of the esophagus are rare. Bracken fern (Pteridium aquilinum) consumption sometimes in association with papilloma viruses has been associated with squamous cell carcinomas in bovids. The clinical signs are similar to those of other causes of esophageal blockage and include dysphagia, regurgitation, weight loss, and dilation of the esophagus proximal to the mass. Tumors of the esophagus are occasionally palpable but are most often intraluminal rather than mural. Epithelial tumors include papillomas (Fig. 7-46) and squamous cell carcinomas. The latter have wide metastatic potential. Smooth muscle tumors of the esophagus, whether benign or malignant, are also rare but may result in similar clinical signs (Fig. 7-47). Esophageal fibrosarcomas of dogs often develop in areas with Spirocerca lupi infestation. Esophageal lymphoma occurs sporadically in most species (Fig. 7-48).
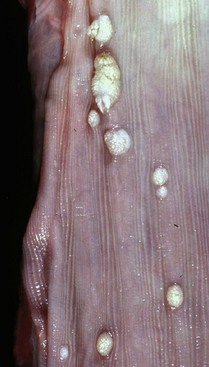
Fig. 7-46 Papillomatosis, bovine papilloma virus, esophagus, bull.
Multiple papillomas, characteristic of this viral-induced disease, occur following trauma to the esophageal mucosa and infection of mucosal epithelial cells. Oral papillomas may be present concurrently. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
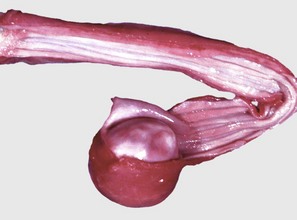
Fig. 7-47 Leiomyoma, esophagus, dog.
A mass consisting of submucosal proliferation of smooth muscle cells bulges into the distal esophageal lumen causing obstruction. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
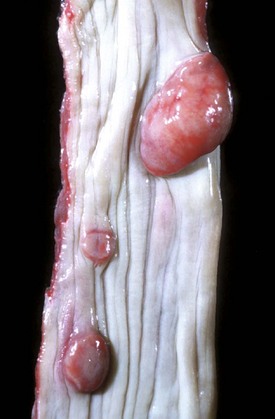
Fig. 7-48 Lymphoma (lymphosarcoma), esophagus, dog.
Masses of submucosal proliferating malignant lymphocytes bulge into the esophageal lumen, causing partial obstruction. Note that the mucosal epithelium is intact (smooth and shiny). (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Rumen, Reticulum, and Omasum
The three compartments of the ruminant forestomach are the reticulum, rumen, and omasum. Folds and compartments subdivide the forestomach. Normal forestomach motility, and thus innervation, is critical in maintaining digestive homeostasis. The forestomach is aglandular. The resident flora and fauna are responsible for digestion and fermentation of cellulose. In general, the rumen is a large fermentation vat where microorganisms break down ingesta by mechanical and chemical action into short-chain fatty acids, which are directly absorbed across the epithelial lining into the blood. These fatty acids supply more than half of the energy from nutrients absorbed by the alimentary tract. The reticulum and omasum act mechanically to further reduce the ingesta to fine particles.
Structure and Function
The rumen has small papillae that vary by diet up to 1.5 cm in length. Their length, shape, and degree of keratinization are affected by diet, and they are longer with high roughage diets and shorter with more concentrates in the ration. These changes are most obvious in the ventral compartment—the ventral ruminal sac. The reticulum has a honeycomb appearance, and the omasum consists of a series of about 100 longitudinal folds similar to the pages of a book. The nonglandular stratified squamous mucosa of the reticulum, rumen, and omasum can be acutely inflamed when their contents have an acid pH and the abnormal milieu permits bacterial and mycotic overgrowth.
The epithelial lining of the forestomach functions as a protective barrier for the forestomach and for the metabolism of ingesta and the absorption of volatile fatty acids. Because the reticulo-omasal orifice is more dorsal than the floor of the compartments, the reticulum can trap foreign bodies, especially dense metallic ones. These can irritate or penetrate the mucosa (“hardware disease”). Problems with motility and imbalances of rumen flora and fauna are the most frequent abnormalities of forestomach function. Often, the changes in flora and fauna are precipitated by a change in ingested substrate, promoting the growth of particular organisms. These changes alter ruminal pH and thus affect the integrity of the mucosal lining of the compartments of the forestomach or cause the production of excessive gas, resulting in ruminal distention.
Parts of compartment one (C1) and C2 and C3 of the camelid forestomach are lined by mucinous glandular epithelium. Concretions of ingesta are sometimes found within the saccules that contain the glands. The saccules are also the sites of water and other solutes. The nonglandular portions of C1 and C2 are lined by nonkeratinized stratified squamous epithelium without papillae. The forestomach of new world camelids contracts at two to three times the rate of other ruminants (and in reverse order), and with each cycle, the saccules empty and refill. This results in high digestive efficiency across the saccules.
Bloat (Ruminal Tympany)
Ruminal tympany or bloat is by definition an overdistention of the rumen and reticulum by gases produced during fermentation. Mortality of affected animals is approximately 50%. A hereditary predisposition to bloat might exist in cattle because cases are on record of bloat in monozygotic twins. Bloat can be divided into primary tympany and secondary tympany.
Primary tympany is also known as legume bloat, dietary bloat, or frothy bloat. It generally occurs up to 3 days after animals begin a new diet. Certain legumes, such as alfalfa, ladino clover, and grain concentrates, promote the formation of stable foam. The nonvolatile acids of legume and ruminal fermentation lower the rumen pH to between 5 and 6, which is optimal for formation of bloat. Foam mixed with rumen contents physically blocks the cardia, preventing eructation and causing the rumen to distend with the gases of fermentation. Clinical signs include a distended left paralumbar fossa, a distended abdomen (see Fig. 1-27), increased respiratory and heart rates, and late in the disease, decreased ruminal movements. When death occurs, it is attributable to distention of the abdomen, which compresses the diaphragm, moving it cranially (orad), with resultant decreased pleural cavity size and respiratory embarrassment. There is also increased intraabdominal and intrathoracic pressure, resulting in decreased venous return to the heart and ultimately generalized congestion cranial to the thoracic inlet.
The lesions of primary tympany are often difficult to detect if there is an interval between death and postmortem examination because the foam can collapse. Conversely, fermentation can occur after death in a nonbloated animal, resulting in the production of abundant gas. The most reliable postmortem indicator of antemortem bloat is the sharp line of demarcation most evident in the mucosa between the pale, bloodless esophagus distal to the thoracic inlet and the congested proximal esophagus cranially (orad) to it. This line may sometimes form even after death before the blood clots. This division is known as a bloat line (Fig. 7-49).
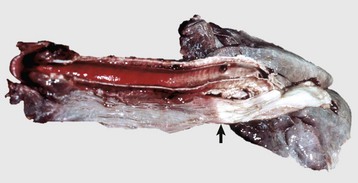
Fig. 7-49 Bloat line, esophagus and trachea at the thoracic inlet, cow.
There is a sharp demarcation between the caudal (blanched) and the cranial (congested) mucosa of the esophagus (arrow). This demarcation is caused by compromised venous return, the result of a grossly distended rumen displacing the diaphragm cranially and causing increased intrathoracic pressure, thus preventing the flow of venous blood into the thorax. In this illustration, a similar demarcation can be seen on the mucosa of the trachea. The subcutaneous tissues of the neck and head are also congested. (Courtesy Department of Veterinary Pathology, Cornell University.)
Secondary tympany is caused by a physical or functional obstruction or stenosis of the esophagus resulting in failure to eructate. Examples of physical causes are esophageal papilloma, lymphoma, esophageal foreign bodies, and enlarged mesenteric or tracheobronchial lymph nodes, usually from lymphoma or tuberculosis. Vagus indigestion or other innervation disorders are examples of functional disorders.
Foreign Bodies
Foreign bodies can collect or lodge in the rumen. These include trichobezoars (hair balls) and phytobezoars (plant balls). Trichobezoars are sometimes a sequela to a habit of bucket-fed calves sucking on the skin of each other to satisfy their nursing instincts. Trichobezoars can form in utero because of hair circulating in the amniotic fluid and being swallowed by the fetus. Phytobezoars result from an excess of indigestible roughage.
Ingestion of nails and wire, common where straw and hay bales are bound by wire, can result in perforation of the wall of the reticulum with resultant reticulitis, peritonitis, or eventually possible pericarditis (hardware disease) (Fig. 7-50). Often, in areas in the US in which ruminants are at high risk of hardware disease because of farming practices, magnets are placed in rumens to prevent the ingested wires and nails from penetrating the reticular mucosa. Occasionally, ruminants ingest plates from storage batteries and suffer lead poisoning.
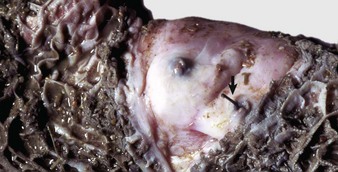
Fig. 7-50 Traumatic reticulitis, reticulum, cow.
Several ingested wires have perforated the wall of the reticulum (arrow) and lodged in the tunica muscularis. Each wire is surrounded by a sinus tract draining to the surface of the reticulum. A chronic ulcer has formed around each area penetrated by the wires. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Inflammatory Diseases
Inflammation of the rumen, rumenitis, is generally considered synonymous with lactic acidosis. Lactic acidosis is synonymous with grain overload, rumen overload, carbohydrate engorgement, and chemical rumenitis. All ruminants are susceptible. The pathophysiology of lactic acidosis usually involves a sudden dietary change to an easily fermentable feed or a change in the feed volume consumed. The latter scenario is most likely to occur during weather changes, especially among feedlot cattle, when a sudden cooling rainstorm will stimulate food intake of cattle that had previously lost appetite because of high environmental temperatures and humidity.
Ruminal microflora is generally rich in cellulolytic gram-negative bacteria necessary for the digestion of hay. A sudden change to a highly fermentable, carbohydrate-rich feed promotes the growth of Gram-positive bacteria, Streptococcus bovis, and Lactobacillus spp. The lactic acid produced by the fermentation of ingested carbohydrates decreases the ruminal pH below 5 (normal = 5.5 to 7.5). This acidic pH eliminates normal ruminal flora and fauna and damages ruminal mucosa. Increased concentrations of dissociated fatty acids lead to ruminal atony. When death occurs, it is due to dehydration secondary to the increased osmotic effect of ruminal solutes (organic acids), causing movement of fluids across the damaged ruminal mucosa into the rumen, acidosis (from absorption of lactate from the rumen), and circulatory collapse. Mortality among animals with lactic acidosis ranges from 25% to 90% and usually occurs within 24 hours.
At necropsy, the ruminal and intestinal contents are watery and acidic. Often, abundant grain is found in the rumen. The mucosa of the ruminal papillae is brown and friable and detaches easily, especially from the ventral ruminal sac. Caution must be exercised in interpreting this latter finding as a lesion because the ruminal mucosa often detaches easily in animals that have been dead for even a few hours at high environmental temperatures. Hydropic change and coagulative necrosis of the ruminal epithelium followed by an influx of neutrophils are common microscopic lesions. Animals surviving lactic acidosis develop stellate scars that are visible because of their color difference from the unaffected surrounding ruminal mucosa. Scars are pale; unaffected mucosa may be light to dark brown to black, depending on the original diet.
New world camelids appear to be more sensitive than ruminants to high carbohydrate diets. While their compartments do not have papillae, widespread ulceration of the squamous mucosa occurs in cases of lactic acidosis. New world camelids retain feed in their stomach longer than ruminants, possibly increasing fermentation and acid production. They rely on emptying of fluid to preserve milieu. High energy feeds may quickly impede motility promoting a drop in pH.
Bacterial rumenitis generally occurs secondary to lactic acidosis or mechanical injury to the ruminal mucosa. Bacteria that colonize the damaged ruminal wall can be transported into the portal circulation and to the liver, resulting in multiple abscesses. Arcanobacterium (Actinomyces) pyogenes is a common cause of bacterial abscesses in the liver. Fusobacterium necrophorum, also transported from the rumen to the liver, results in necrobacillosis, which has distinctive liver lesions.
Mycotic infections of the rumen also occur secondary to the damage to the ruminal mucosa caused by lactic acidosis and mechanical injury. Mycotic rumenitis also results from the administration of antibiotics, usually in calves but also in adult cattle, which reduce the numbers of normal flora and allow fungi to proliferate. In cases of mycotic rumenitis, lesions are generally circular and well delineated and are caused principally by infarction from thrombosis secondary to fungal vasculitis (Fig. 7-51). Offending fungi include Aspergillus, Mucor, Rhizopus, Absidia, and Mortierella spp. These fungi can spread to the placenta hematogenously and cause mycotic placentitis, which leads to abortions.
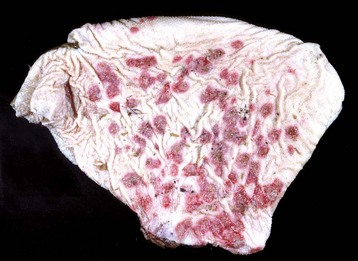
Fig. 7-51 Mycotic rumenitis, rumen, calf.
Note the numerous well-demarcated red foci of necrosis and hemorrhage (infarcts) in the ruminal mucosa that can be caused by angioinvasive fungi such as Aspergillus, Mucor, Rhizopus, Absidia, and Mortierella spp. This type of mycotic infection is usually preceded by a chemical (lactic acid) rumenitis (overeating). (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Ruminal candidiasis occurs as an incidental finding at necropsy. There is usually an underlying debilitating condition, glucose therapy, milk-replacer overload (sour rumen), or an antibiotic-induced kill-off of resident flora and fauna. Ruminal candidiasis is seldom diagnosed in a live animal.
Miscellaneous Diseases or Conditions
Ruminal papillae vary in length, becoming longer with high-roughage diets (Fig. 7-52). Such diets also can cause the papilla to become tongue- or leaf-shaped. Animals consuming diets with less than 10% roughage can develop ruminal parakeratosis. These rumens have hard, brown, often clumped, papillae. This lesion has little to no clinical consequence.

Fig. 7-52 Parakeratosis, reticulo-rumen, calf.
A diet that was almost devoid of roughage has resulted in atrophy and parakeratosis of ruminal papillae. Normal papillae are leaf shaped, but some of these papillae have become finger shaped, cauliflower shaped, or clumped. The parakeratotic epithelium has been stained brown to black by components of the feed because of the lack of abrasion by the ground feed. These lesions are most marked on the ventral floor of the ventral sac of the rumen. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Ruminal papillomas are papilloma virus-induced in some cases, but in certain countries, bracken fern has been implicated as a cofactor in these forestomach neoplasms (Fig. 7-53).
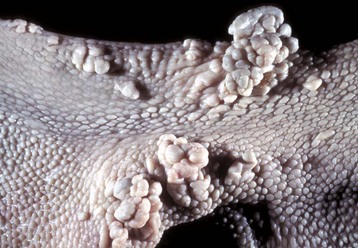
Fig. 7-53 Papillomas, rumen, cow.
Smooth-surfaced, squamous papillomas are present on the dorsal wall. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Vagal indigestion results in a functional outflow problem from the forestomach. Damage to the vagus nerve can occur anywhere along its length and can result in functional pyloric stenosis and omasal dilation. Causes of vagal indigestion include damage to the vagus nerve due to traumatic reticuloperitonitis, liver abscesses with secondary peritonitis, volvulus of the abomasum, and bronchopneumonia. Mechanical obstruction of the forestomach or abomasal outflow can be due to abomasal lymphoma or papillomas or from blockage after ingestion of indigestible or foreign materials. Diet and dwarfism are sometimes associated with vagus indigestion. Many cases are idiopathic. Clinical signs include ruminoreticular distention. The presence of abomasal distention depends on the precise location of the damage to the vagus nerve. Vagal indigestion is divided into the following four types, based on the anatomic location of the functional obstruction.
• Type I is usually caused by inflammatory lesions around the vagal nerve at any location and is a failure of eructation resulting in bloat.
• Type II is a functional or anatomic condition that results in failure of omasal transport into the abomasum. Usual causes are adhesions and abscesses on the medial wall of the reticulum associated with or secondary to traumatic reticuloperitonitis. Abomasal lymphoma and physical obstruction of the omasal canal (e.g., neoplasia or ingested placenta) may also be causative.
• Type III is caused by physical impaction of the abomasum by roughage and thus is dietary in origin. Abomasal displacements and volvulus are also potential causes.
• Type IV is pregnancy related, perhaps as a result of shifting of position of the abomasum secondary to the expanding uterus, causing compression of the abdominal branches of the vagus nerve.
Ruminal Parasitism
Paramphistomiasis is a fluke infestation of the ruminant forestomach in warmer latitudes around the world. These trematodes are in the genera Paramphistomum, Calicophoron, and Cotylophoron. They are similar in size and appearance to ruminal papillae (Fig. 7-54). Although the presence of adult organisms in the forestomach is usually of no clinical significance, heavy infestations of larvae in the proximal small intestine, before migration to the rumen and reticulum, can cause hypoproteinemia, anemia, and death. Larvae burrow deeply into and sometimes through the wall of the small intestine and can be found in the peritoneal cavity. The intermediate host is a snail. Cercariae encyst on aquatic vegetation and are eaten by the ruminant.
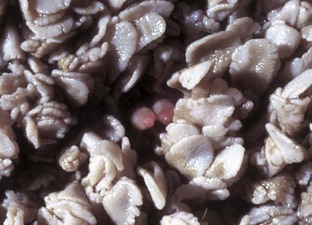
Fig. 7-54 Paramphistomiasis, rumen, cow.
The pink conical structures located in the center of the illustration are paramphistomes (ruminal flukes). They are considered to be innocuous, but massive numbers of immature flukes in the duodenum may cause a severe catarrhal duodenitis. Note the normal leaf-shaped ruminal papillae, indicative of a high-roughage diet. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Stomach and Abomasum
Although some differences exist, the stomachs of the simple-stomached animals and the abomasum of ruminants (third compartment of new world camelids) are very similar in structure and function. A fundus and body make up the cranial portion lined by numerous spiral folds and produce acid and pepsin. The aboral portion, the pyloric part, is lined by epithelium with mucous-secreting glands and G cells that produce gastrin. Stomachs have an indigenous flora. Most of these organisms cannot be cultured by traditional methods. C3 of new world camelids is more tubular than the abomasum, with more peristalsis-like rather than mixing motility. The first two-thirds of C3 is fermentative with a pH around 6.5. At the caudal flexure, the mucosa thickens to 7 to 10 mm and the pH is around 2.0. The final portion surrounding the torus pyloricus has an alkaline pH.
Structure and Function
The gastric mucosa of simple-stomached animals contains numerous folds or rugae that are flattened when the stomach is distended. Foveolas or gastric pits communicate with the lumen of the stomach and transport gastric cell secretions. The glandular stomach functions in the enzymatic and hydrolytic digestion of ingested food substances. The epithelial covering is one cell thick, and the cell types include columnar mucus and bicarbonate-secreting surface epithelial cells, mucous neck cells arranged in tubuloalveolar glands, acid-secreting parietal cells, pepsinogen-secreting chief cells, and neuroendocrine (enterochromaffin, argentaffin) cells that secrete gastrin, enteroglucagon, and somatostatin (Fig. 7-55). The neuroendocrine cells do not communicate with the gastric lumen. The mucous neck cells are the precursor cells for all the other epithelial types in the stomach and are responsible for the replacement of surface epithelial cells as they are lost, either at the end of their normal lifespan or from some type of insult.
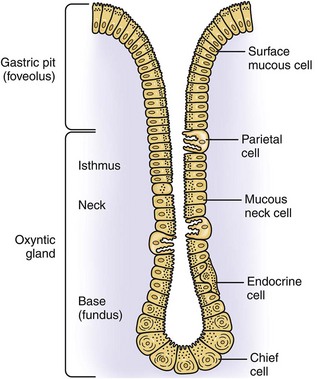
Fig. 7-55 Schematic illustration, microanatomy of the stomach. (Adapted from Ito S, Winchester RJ: J Cell Biol 16:541, 1963.)
Multiple submucosal lymphoid patches are present in monogastric animals. In ruminants, a single lymphoid patch is present at the fold separating the omasum and abomasum.
In some species, such as the horse and rat, the cranial or orad part of the stomach (nonglandular part or pars nonglandularis) is lined by stratified squamous epithelium, whereas the distal portion (pars glandularis) is lined by glandular epithelium. In the horse, the dividing line between the two is called the margo plicatus. The pars nonglandularis in the pig is a small square to rectangular area of stratified squamous epithelium surrounding the esophageal opening.
Defense Mechanisms
The gastric mucosal barrier is significant in preventing autodigestion and bacterial overgrowth. There is, however, a resident flora that is difficult to grow on artificial media. Microorganismal overgrowth is prevented under normal physiologic conditions by abomasal or gastric motility, prostaglandin E2 (PGE2), a protective layer of mucus and bicarbonate, secretory IgA, transforming growth factor-α (TGF-α), epidermal growth factor, an extremely acid luminal pH, and an effective pyloric sphincter that prevents regurgitation into the stomach or abomasum of duodenal, hepatic, and pancreatic secretions. An intact epithelial layer and adequate blood flow also prevent acid-induced damage.
Gastric Dilation and Volvulus
Simple gastric dilation occurs in a variety of animals and in humans (Fig. 7-56). In dogs, particularly in the large, deep-chested breeds, the acute gastric dilation and volvulus syndrome occurs. This lesion is life-threatening and should not be confused with simple gastric dilation that is common in young puppies after overeating. Predisposing factors to acute gastric dilation include a source of distending gas, fluid, or feed; obstruction of the cardia that prevents eructation and emesis; and obstruction of the pylorus that prevents passage of gastric contents into the small intestine. The source of gas is not well understood. Theories include gas production by Clostridium perfringens, spores of which are present in the feed, carbon dioxide (CO2) from physiologic mechanisms of digestion, or simple aerophagia.
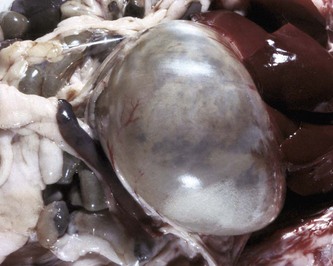
Fig. 7-56 Simple gastric dilation, stomach, rabbit.
The stomach is markedly dilated and filled with gas. Dilation occurs most commonly following aerophagia or overeating, and is relieved by eructation or vomiting. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
The result of repeated episodes of gastric dilation is stretching and relaxation of the gastrohepatic ligament. Recurrent dilation, combined with overfeeding, postprandial exercise, and perhaps a hereditary predisposition, results in gastric rotation. Gastric rotation is recognized by splenic displacement and a twisted esophagus and results in vascular compression and decreased venous drainage and hypoxemia (Figs. 7-57 and 7-58). The stomach generally is rotated clockwise on the ventrodorsal axis when the abdomen is viewed from the ventral surface. Rotation is 180 to 360 degrees. The combination of gastric hypoxemia, acid-base imbalance, obstruction of the pylorus and cardia, and increased intragastric pressure leads to antiperistaltic waves followed by atony, cardiovascular ischemia, arrhythmias, and shock. Decreased portal venous return leads to pancreatic ischemia and release of myocardial depressant factor, cardiac collapse, and death.
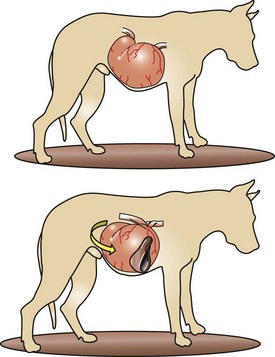
Fig. 7-57 Schematic illustration, gastric dilation and volvulus, stomach, dog.
The stomach is distended with food and gas (top example). It rotates (arrow) on the mesenteric axis (bottom example) clockwise (180, 270, or 360 degrees on a ventrodorsal axis when the abdomen is viewed from the ventral surface), resulting in a gastric volvulus with an obstructed esophagus that prevents eructation and thus further contributes to gastric dilation. The spleen, attached to the stomach by the gastrosplenic ligament, rotates with the stomach and is thus folded back upon itself and located in the right cranial abdomen against the diaphragm (bottom example). The splenic vein is compressed, resulting in a congested spleen, because the arterial blood supply remains patent longer than venous drainage. (Modified from Van Kruiningen HJ, Gregoire K, Meuten DJ: J Am Anim Hosp Assoc 10:294-324, 1974.)
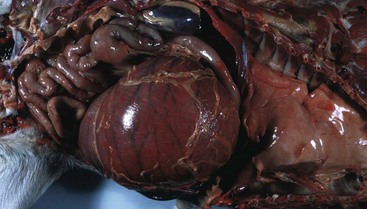
Fig. 7-58 Gastric dilation and volvulus, abdomen, dog.
The stomach is filled with gas and its serosa is congested. The duodenum and engorged spleen have been displaced to the right. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Epidemiologic evidence suggests that dry dog foods that list oils or fats among the first four ingredients increase the risk of the gastric dilation and volvulus syndrome. Gastric dilation and volvulus is sometimes associated with gastric eversion or intussusception into the distal esophagus. This latter condition can also occur independently of gastric dilation and volvulus.
Abomasal Displacement
Normally, the abomasum lies over the xiphoid process at the abdominal ventral midline. Abomasal displacement is usually to the left side, although right-sided displacements also occur (Fig. 7-59). Left-sided displacement of the abomasum is a generally nonfatal entity seen in high-producing dairy cattle during the 6 weeks after parturition. Strenuous activity can predispose nonpregnant cows to displacement. In the postcalving period, abomasal atony can occur as a result of heavy grain feeding (volatile fatty acids decrease motility) and hypocalcemia. Meanwhile, the gravid uterus may have displaced the rumen and abomasum cranially and to the left, rupturing the attachment of the greater omentum to the abomasum. The abomasum then occupies the cranial left quadrant of the abdomen and displaces the rumen medially. This change leads to partial obstruction of abomasal outflow. Metabolic alkalosis contributes to rumen atony and impaired movement of ingesta. The associated hypochloremia is a result of hydrogen chloride (HCl) secretion and is common along with hypokalemia. Abomasal ulcers and peritoneal adhesions can result in cases of chronic displacement.
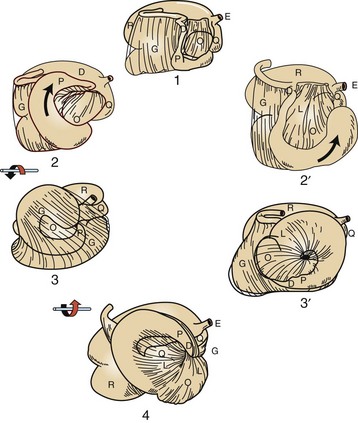
Fig. 7-59 Schematic diagrams illustrating two possible modes of rotation of the omasum, abomasum, and cranial part of the duodenum in volvulus.
1, Normal relations; 2, simple dilation and displacement on the right; 3, 180-degree volvulus around the longitudinal axis of the lesser omentum, counterclockwise as seen from the rear; 2′, 90-degree rotation of the abomasum in a sagittal plane, counterclockwise as seen from the right; 3′, 180-degree rotation of the abomasum and omasum around the transverse axis of the lesser omentum, drawing the duodenum cranially, medial to the omasum; 4, 360-degree counterclockwise volvulus, final stage resulting from either mode of rotation. D, Duodenum; E, esophagus; G, greater omentum; L, lesser omentum; O, omasum; P, pylorus; Q, reticulum; R, rumen. (Modified from Habel RE, Smith DF: J Am Vet Med Assoc 179:447-455, 1981.)
Fifteen percent of abomasal displacements are right-sided. The abomasum can be overdistended, displaced dorsally, and rotated on its mesenteric axis, and 20% of these cases develop abomasal volvulus. Right-sided displacements occur in postparturient dairy cows and in calves.
Clinical features of displaced abomasums, whether right-sided or left-sided, include anorexia, cachexia, dehydration, lack of feces, ketonuria, and a characteristic high-pitched ping subsequent to percussion over the abomasum. Idiopathic abomasal volvulus occurs occasionally in ruminants and calves (Web Fig. 7-10).
Gastric Dilation and Rupture
Gastric dilation occurs in horses as a result of the ingestion of fermentable feeds or grain, a situation analogous to grain overload with lactic acidosis in cattle. Acute gastric dilation and rupture in equids occurs most frequently as a terminal event in intestinal obstruction and displacement. Because gastric dilation and rupture can occur after death, the diagnostic challenge is to determine if the rupture occurred antemortem or postmortem. The only reliable indicator of the time of rupture, in relation to the death of the animal, is the presence of hemorrhage and evidence of inflammation, such as fibrin strands, along the margins of the rupture (usually the greater curvature) because such inflammatory responses occur only in live animals (Figs. 7-60 and 7-61).
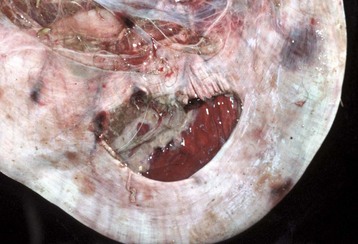
Fig. 7-60 Rupture, abomasum, calf.
Multifocal hemorrhages along the upper margin of the tear and subserosally adjacent to the greater curvature indicate that the rupture occurred antemortem. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
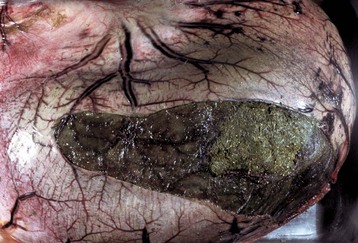
Fig. 7-61 Rupture, stomach, horse.
The hemorrhage visible on the right margin of the rupture indicates that the rupture occurred antemortem. Note also that the rent through the tunica muscularis is longer than that through the mucosa, which still covers the ingesta on the left and right sides. The mucosal and serosal surfaces are congested. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
In Northern Europe, acute gastric dilation occurs in horses on pasture as part of the syndrome called grass sickness or dysautonomia. The esophagus and stomach are often dilated and atonic. Although serologic evidence suggests an association of grass sickness with Clostridium perfringens type A enterotoxin, noninflammatory degeneration of associated autonomic ganglia have also been described. This condition can be experimentally produced in the horse with whole blood from affected animals, suggesting that a soluble toxin may be the cause.
Chronic gastric dilation is also associated with ingestion of poorly digestible substances. Habitual cribbing and aerophagia may also be contributory.
Dysautonomia also occurs in pet carnivores secondary to ganglionic death in cranial nerves, spinal nerves, and autonomic nerves. Associated ganglionic peptides are reduced in an amount consistent with the functional aberrations. Ganglioneuritis creates a similar syndrome to dysautonomia in a variety of species and is infrequently diagnosed. GI signs of dysautonomia include xerostomia, decreased anal tone, vomiting, and regurgitation. See Chapter 14 for a description of the histologic lesions.
Chronic abomasal and/or ruminal dilation may occur in cows with overeating disease, dystocia, exhaustion, poor quality or frozen feedstuffs, abomasal ulcers with or without abomasal lymphoma, and vagal indigestion. A sequela of abomasitis may be a mycotic infection similar to that which occurs in the rumen (Fig. 7-62).
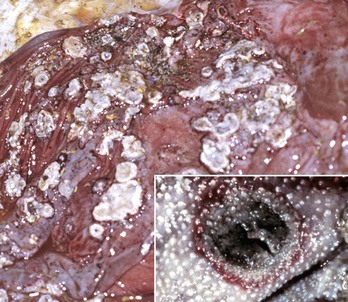
Fig. 7-62 Mycotic abomasitis and omasitis, calf.
The mucosal surface of the abomasum has discrete and coalescing ulcers covered by yellow-white diphtheritic membranes and a red outer margin of active hyperemia and inflammation. These lesions are indicative of infarcts and are likely secondary to vasculitis and thrombosis by angioinvasive fungi such as Aspergillus, Mucor, Rhizopus, Absidia, and Mortierella spp. The diphtheritic membranes are a mixture of necrotic cellular debris from the infarct, inflammatory cells, and hyphae from the inciting fungus. Inset: Mycotic omasitis. The lesion is similar to the one in the abomasum. The diphtheritic membrane has been lost due to omasal peristalsis, but the necrotic center (infarct) and red outer margin of active hyperemia and inflammation are prominent. (Figure courtesy College of Veterinary Medicine, University of Illinois. Inset courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
In monkeys, an increased frequency of acute gastric dilation often occurs during weekends, when there may be changes in feeding behavior secondary to unfamiliar keepers. Studies have implicated Clostridium perfringens overgrowth secondary to an increase in fermentable feed consumption in the pathogenesis of gastric dilation in primates.
Chronic gastric dilation in canids is usually secondary to gastric ulcer, mural gastric lymphomas, uremia affecting gastric structure and function, pyloric stenosis or obstruction, acute gastric dilation, intervertebral disc disease, or vagotomy. Chronic gastric dilation is characterized by reduced feed intake, diminished gastric motility, and increased gastric gas accumulation sometimes resulting in abdominal distention similar to that of bloat.
Abomasal Dilation and Tympany
Abomasal dilation and tympany is a syndrome of young cattle that occurs most commonly in dairy breeds with a history of one or more of the following: only a single milk feeding per day, cold milk or milk replacer, lack of free choice water, inconsistency of feeding time, dosing with high energy oral electrolyte solutions, and sometimes, failure of passive transfer of immunity. The pathophysiology is presumed to be abomasal fermentation of high energy ingesta by gas-producing bacteria. Hyperglycemia and a resultant glycosuria are present. Hemorrhage, edema, necrosis, and sometimes emphysema of the abomasum and other compartments of the forestomach are found at necropsy.
Impaction
Impaction of the monogastric stomach and abomasum has a variety of causes. Intrathoracic lesions, such as pneumonia, pleuritis, lymphadenopathy, and lymphoma of mediastinal lymph nodes, can infiltrate and damage the vagal nerves, resulting in a problem with abomasal/gastric motility and emptying. Roughage, hairballs, and other foreign materials also cause impaction. Gastric trichobezoars and phytobezoars of monogastric animals are similar to those that occur in the rumen (Web Fig. 7-11).
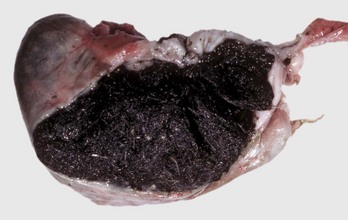
Web Fig. 7-11 Trichobezoar, stomach, rabbit.
The stomach is impacted with ingested hair. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Abomasal emptying defect is principally a condition of Suffolk sheep 2 to 6 years of age. It is characterized by an impacted, dilated abomasum. Clinical signs include anorexia, weight loss, and increased ruminal chloride levels. The latter feature is believed to be secondary to abomasal reflux. Scattered chromatolysis and neuronal necrosis in the celiac and mesenteric ganglia are consistent changes from a neurotoxicosis. The process is likely mediated by excitotoxins, although viruses have not been ruled out. Clusters of affected animals in a single flock are suggestive of an environmental cause. Inflammation is minimal. Abomasal emptying defect may be a form of acquired dysautonomia.
Inflammatory Diseases
Inflammation of the simple stomach or abomasum is designated as gastritis and abomasitis, respectively, and must be differentiated from simple hyperemia and petechiae, which are often nonspecific agonal lesions. Gastritis is often associated clinically with vomiting, dehydration, and metabolic acidosis. Hemorrhage, edema, increased amounts of mucus, abscesses, granulomas, foreign body penetration, parasites, inflammatory cells of various types, erosions, ulcerations, and necrosis characterize the changes in the mucosal surface and subsequent inflammatory reaction.
Clostridium septicum is a cause of hemorrhagic abomasitis with submucosal emphysema of sheep and cattle, a disease known as braxy. Although this disease is most common in the United Kingdom and Europe, it occurs in North America as well. Generally, the disease follows ingestion of frozen feeds contaminated with the causative Clostridium spp. The lesions are produced by the exotoxin of the bacteria, and death therefore is due to an exotoxemia.
Sarcina-like organisms have been reported in association with abomasal bloat in several calves. Sarcinas are anaerobic, gram-positive, nonmotile cocci found in rafts or packets. They are suspected gastric pathogens in a variety of animal species. The lesions are similar to braxy.
In many septicemias of pigs, bacterial emboli lodge in the vessels of the gastric submucosa and cause thrombosis, resulting in hyperemia, hemorrhage, infarction, and ulceration. This occurs in salmonellosis, swine dysentery, Glasser’s disease, and colibacillosis. Certain intoxicants such as vomitoxin produced by Fusarium spp. can cause similar lesions (Fig. 7-63).
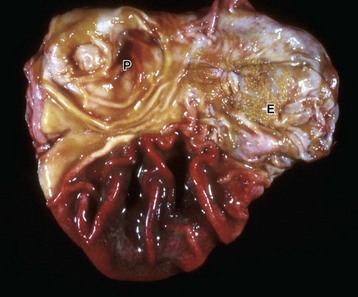
Fig. 7-63 Acute “hemorrhagic gastritis,” stomach, pig.
The fundus of the stomach is hemorrhagic. This type of gastric change is often seen in the pig in acute septicemia, for example, from salmonella, and the severe congestion is attributed to venous infarction from endotoxemia. E, Esophageal os; P, pylorus. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
A deep mycosis that causes a granulomatous gastritis is due to Histoplasma capsulatum (see intestinal diseases of carnivores). Very rarely, Mycobacterium tuberculosis causes granulomatous gastritis in a variety of species. In granulomatous gastritis, epigastric discomfort after eating (postprandial), emesis, progressive cachexia, weakness, vomiting of blood (hematemesis), and pyloric obstruction caused by the space-occupying inflammatory reaction occur. In both histoplasmosis and tuberculosis, regional (gastric, splenic, and hepatic) lymph nodes may be affected. Nodular or diffusely thickened gastric and lymphoid lesions contain predominately macrophages. Mononuclear inflammatory cells, fibroblasts, granulocytes (including eosinophils), and multinucleate giant cells are also present. Often the causative organisms can be demonstrated within the granulomatous inflammation, but special stains, such as acid-fast for mycobacteria and periodic acid–Schiff (PAS) reaction, or Gomori’s methenamine silver stain may be necessary to demonstrate fungi.
Eosinophilic gastritis is uncommon in all species of domestic animals but has been reported in pet carnivores and humans. In general, the etiologic basis for this condition is poorly understood. The three types of gastritis characterized by an influx of eosinophils are as follows:
• A characteristic focal eosinophilic infiltrate is sometimes associated with trapped, intramural nematode larvae, especially Toxocara canis. Larvae of Toxocara canis pass to nursing puppies through the milk, through fecal soiling of bedding, or from dirt or other fomites harboring eggs or larvae. After ingestion, the parasite’s larval sheath, feces, and saliva are antigenic. In dogs and cats, tissue reaction to these larvae in the mucosal and submucosal intestine and gastric epithelial cell is hyperplasia, resulting in a polyp-like proliferation of the antral mucosa. Pyloric obstruction sometimes results.
• In other cases of eosinophilic gastritis, the infiltration of eosinophils is more diffuse and is believed to be a hypersensitivity reaction. The offending antigen is not known. In many of these cases, there is a peripheral eosinophilia, especially when associated with eosinophilic infiltration of the small intestine (eosinophilic gastroenteritis). This form of eosinophilic gastritis may become transmural, with necrosis and scarring.
• The third type, scirrhous eosinophilic gastritis of dogs and cats, for the most part has unknown causes. The fibrosis associated with scirrhous changes in the stomach and lymph nodes result in persistent emesis, weight loss, and malnutrition.
The gross lesions of eosinophilic gastritis are rather nonspecific and consist of diffuse or nodular mural thickenings. Microscopic lesions are characterized by infiltrates of eosinophils in the mucosa and the submucosa and are seen extensively through the muscularis of the stomach. Similar lesions are sometimes present in segments of the small intestine and colon. In the dog, there is sometimes necroproliferative eosinophilic perivasculitis and eosinophilic lymphadenopathy. In the scirrhous form, the eosinophilic infiltrate is followed by transmural fibroplasia and scarring.
Hypertrophic or Hyperplastic Enteritides
Hypertrophic gastritis, characterized by thickened rugae, is the result of hyperplasia of the gastric glands. This effect is believed to be a response to chronic retention of gastric fluid and reflux of intestinal bile. Similar mucosal glandular changes are seen in immune-mediated lymphoplasmacytic gastritis of dogs. Hypertrophic gastritis has also been described in primates, horses, pigs, and rodents. The nematode Nochtia nocti causes this lesion in the stomachs of monkeys. Equine hypertrophic gastritis is a focal lesion or more diffuse lesion associated with the nematodes, Habronema spp. and Trichostrongylus axei, respectively.
Chronic giant hypertrophic gastropathy of dogs affects the basenji, beagle, boxer, and bull terrier breeds, among others. The disease is similar to Ménétrier’s disease in humans. Clinical signs include weight loss, diarrhea, vomiting, and hypoproteinemia. The chronic gastritis results in increased mucosal permeability to serum proteins with subsequent protein-losing gastropathy. Unlike normal gastric mucosal folds, in giant hypertrophic gastropathy, the mucosa does not flatten with distention of the organ (Fig. 7-64). Microscopically, the mucosa is hypertrophic and hyperplastic. The incorporation of folds of submucosa and muscularis mucosa is variable, as is the presence of inflammatory cells, principally lymphocytes and plasma cells. The cause of this condition is unknown.
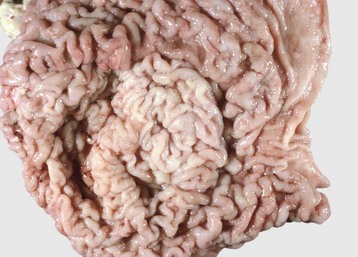
Fig. 7-64 Chronic giant hypertrophic gastropathy, stomach, dog.
A cerebriform mass of redundant mucosa is present in the center of the gastric mucosa. Chronic inflammation in the mass results in increased mucosal permeability to serum proteins and a subsequent protein-losing gastropathy. (Courtesy College of Veterinary Medicine, Cornell University.)
An ulcer is a mucosal defect in which the entire epithelial thickness, down to or through the basement membrane, has been lost. Penetration through the remaining tissue layers to the peritoneal cavity is termed a perforating ulcer. Partial-thickness epithelial loss is termed an erosion. Chronic ulcers differ from acute ulcers by the presence of an indurated rim caused by fibrosis and attempts at epithelial regeneration. The identification of gastric ulcers is not challenging, either at necropsy or by endoscopy. They are sharply bordered cavities, often coated with exudate. Thrombosis of blood vessels is sometimes adjacent to ulcers in ruminants with mycotic vasculitis secondary to ruminal lactic acidosis. Thus it is an infarct.
The pathogenesis of most gastric and duodenal ulcers in humans has been demonstrated to be a result of infection with a helical bacterium, Helicobacter pylori. The same bacterium has been epidemiologically linked to gastric adenocarcinoma. Helicobacter mustelae acts similarly in ferrets. Although similar, gastric Helicobacter-like organisms (GHLOs) are readily demonstrated in dogs and cats, their relationship with ulcer formation or neoplasia is not established. It appears that the stomachs of as many animals without gastritis or ulcers are as heavily colonized by these bacteria as are those animals with ulcers (Fig. 7-65). More than 90% of cats are infected with two Helicobacter spp. Helicobacter felis can be cultured in vitro, but the noncultivatable Helicobacter heilmannii is the more frequent. Pathologic and clinical outcomes appear to depend on a number of bacterial virulence factors as well as on the host response to these agents. Investigations suggest there may be a link between the presence of Helicobacter and other diseases, including coronary and neurologic disease.
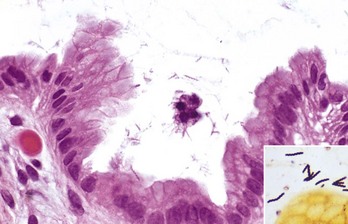
Fig. 7-65 Helicobacter spp. infection, stomach, cat.
Numerous spiral bacteria are present in the superficial mucous layer. There is no inflammation in the adjacent mucosa; however, in some areas the epithelium is hyperplastic. H&E stain. Inset: The helical shape of the helicobacter organisms is demonstrated with a Steiner’s silver stain. (Figure courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University. Inset courtesy Dr. C.S. Patton, College of Veterinary Medicine, University of Tennessee.)
Theories abound as to the causes of most gastric ulcers in animals. None have been proved. There may be a heritable component to ulcer susceptibility. The conditions necessary for ulcer development boil down to an imbalance between acid secretion and mucosal protection. This imbalance occurs as a result of the following:
• Local disturbances or trauma to the mucosal epithelial barrier; this injury can be due to back flush of bile salts from the duodenum or ingestion of lipid solvents such as alcohol.
• Normal or high gastric acidity.
• Local disturbances in blood flow (stress-induced and sympathetic nervous system–mediated arteriovenous shunts) resulting in ischemia.
• Steroids and NSAIDs that depress prostaglandin formation (PGE2, PGI1) or concentration, thus decreasing phospholipid secretions, which are protective
All of these mechanisms allow pepsin and hydrochloric acid into the submucosa.
Severe gastric hyperacidity and gastric ulcers is sometimes associated with the presence of islet cell tumors producing gastrin. Some of these gastrin-producing tumors arise in the duodenum, but the majority originate in the pancreas. These neoplasms release histamine into the bloodstream, which binds to receptors on parietal cells of the stomach, increasing HCl secretion. The gastric ulceration produced associated with these tumors is known as Zollinger-Ellison syndrome.
In dogs, gastric ulceration causes vomiting, inappetence, abdominal pain, and anemia secondary to gastric bleeding (Fig. 7-66). Melena (digested blood in feces) may also be present if the ulceration persists, and significant amounts of blood are lost to the GI system. Gastric ulcers in dogs and cats are generally idiopathic but can occur in those animals with mast cell tumors that stimulate gastric HCl secretion through histamine release and its effect on the surrounding blood vessels or other neoplasia that infiltrate and weaken the gastric wall.
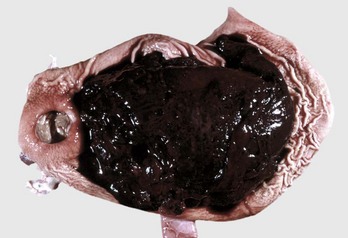
Fig. 7-66 Ulcer, stomach, dog.
The stomach contains a large volume of clotted and unclotted blood from a gastric ulcer (idiopathic) with rounded edges visible in the left side of the photograph. The hemorrhage was so severe that the dog died from exsanguination. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Ulcers are idiopathic in foals. Foals with gastric ulcers may have abdominal pain, bruxism (grinding of the teeth), ptyalism, and gastric reflux and may lie in dorsal recumbency. Gastric ulcers associated with administration of NSAIDs are common in horses and to a lesser extent in other species (Fig. 7-67). Equine gastric ulcer syndrome occurs in 40% to 90% of competitive and performance horses, with the most severe ulcers occurring in those animals that are worked the hardest. More than one-third of horses used less strenuously develop mild ulcers.
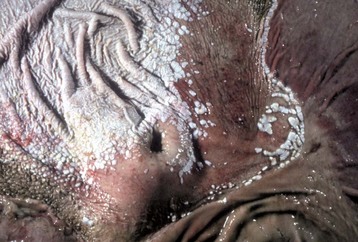
Fig. 7-67 Gastric ulcers, stomach, horse.
Administration of nonsteroidal antiinflammatory drugs (NSAIDs) has caused extensive ulceration of the stratified squamous epithelium of the nonglandular mucosa. The ulceration extends from the cardia (center) to the margo plicatus (right). (Courtesy College of Veterinary Medicine, Cornell University.)
Cattle with abomasal ulcers have partial or complete anorexia, decreased milk production, palpable discomfort on pressure applied to the right xiphoid area, and melena. In any species, the vomiting of coffee grounds–like material (hematemesis) or passage of melena is highly suggestive of gastric ulcer disease. Abomasal ulcers of ruminants vary in significance from subclinical to fatal (Figs. 7-68 and 7-69). In calves, ulcers are associated with dietary changes or mechanical irritation of the abomasum by roughage. Dietary changes involve substitution of roughage for milk or milk replacer, together with the associated stress. In dairy cattle, ulcers are associated with heavy grain feeding (lactic acidosis) at the time of parturition, displacement of the abomasum, BVD, impaction, torsion, and gastric lymphoma. Because cattle have an effective omentum that seals abomasal ulcers, they may live for a long time unless a large perforation occurs, resulting in septic peritonitis.
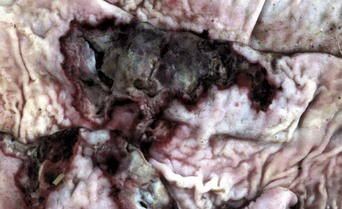
Fig. 7-68 Ulcers, abomasum, cow.
The ulcers consist of a central area of necrosis surrounded by an outer red margin characteristic of active hyperemia and inflammation. The discrete rounded outline of these ulcers suggests that they are infarcts, possibly from vasculitis and thrombosis caused by angioinvasive fungi. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
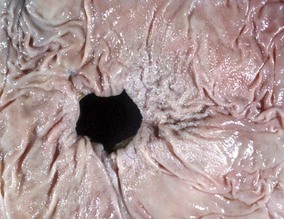
Fig. 7-69 Perforating ulcer, abomasum, cow.
The rounded borders of the ulcer indicate an attempt at repair and therefore chronicity. Death was due to peritonitis. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
In pigs, gastric ulcers are common and occur in penned pigs fed finely ground grain. These ulcers always are limited to the stratified squamous epithelium of the esophageal portion of the gastric mucosa that surrounds the cardia (Fig. 7-70). Death can result from exsanguination into the gastric lumen. Evidence suggests that a high-carbohydrate diet is not sufficient alone to produce erosions and ulcers but rather that the appropriate diet in combination with fermentative commensal bacteria, such as Lactobacillus and Bacillus spp., produces lesions. Lesions progress from parakeratosis to hyperkeratosis through keratolysis to erosive gastritis or perforation of the stomach
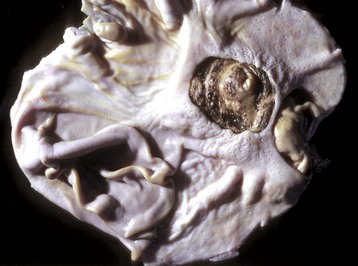
Fig. 7-70 Gastric ulcer (pars esophagea), stomach, pig.
This type of gastric ulcer occurs exclusively in pigs and most commonly in confined growing pigs. The lesion is limited to the stratified squamous epithelium surrounding the cardia (pars esophagea). Ulcers in this location characteristically have a multifactorial cause, including the ingestion of finely ground grain or pelleted feed (possibly deficient in vitamin E), fermentation of sugars in the feed, and stress of confinement rearing. These ulcers frequently bleed and can cause exsanguination. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Miscellaneous Diseases and Conditions
Uremic gastritis occurs most frequently in carnivores as a result of chronic renal disease (Fig. 7-71; also see Fig. 1-54, Fig. 11-28, and Fig. 11-29). In ungulates, it is a rare event and is usually secondary to obstructive kidney disease (postrenal uremia). Uremic gastritis is characterized by mineralization of the glands, vessels, and lamina propria of the gastric mucosa and sometimes results in ulcer formation.
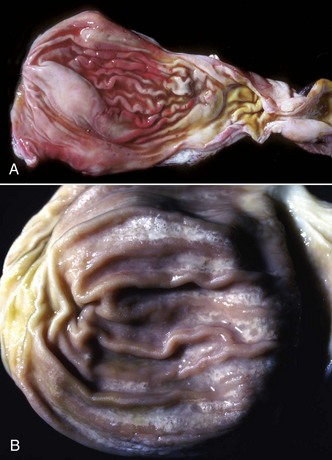
Fig. 7-71 Uremic gastropathy (also called uremic gastritis), stomach, cat.
A, The major lesion is congestion and edema of the gastric mucosa caused by injury to capillaries within the lamina propria associated with elevated concentrations of nitrogen-derived metabolic waste products in the systemic circulation from kidney failure. B, With chronicity, there is mineralization of the gastric mucosa, visible as fine white stippling and lines in the mucosa. (A courtesy Dr. C.S. Patton, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Amyloidosis occasionally is present in the stomach concomitant with systemic amyloid infiltrates. Generalized amyloid A (AA) amyloidosis with gastric deposits of amyloid has been reported in bats, Siamese and Abyssinian cats, goats, rhesus monkeys, sheep, and Siberian tigers.
Pyloric stenosis can be anatomic or physiologic because of an inability of the pyloric sphincter to function properly. This condition may be congenital or acquired. This lesion occurs most often in dogs (particularly brachycephalic breeds), Siamese cats, horses, and humans. Congenital pyloric stenoses may be hereditary, at least in humans. Pyloric stenosis is often first recognized in recently weaned animals by projectile vomiting, retention of gastric contents, gastromegaly, and the presence of strong gastric peristaltic waves (Web Fig. 7-12). Pyloric muscular hypertrophy, variable submucosal edema, vascular ectasia, and degeneration of myenteric ganglion cells (dysautonomia) may be identified histologically in some cases. Functional pyloric stenosis can be a feature of vagus indigestion of ruminants. In general, the many causes of pyloric stenosis are not well understood.
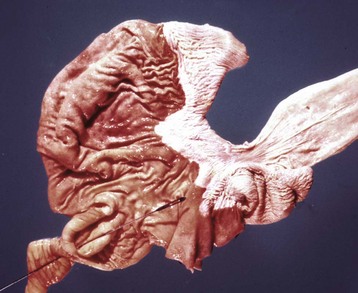
Web Fig. 7-12 Pyloric stenosis, stomach, horse.
The probe passes through the narrowed pyloric canal. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Giant hypertrophic pyloric gastropathy, not to be confused with giant hypertrophic gastropathy of basenjis and other dogs, is an idiopathic condition seen most often in older small breed dogs. To the uninitiated, the gross and microscopic features of this pyloric lesion strongly imitate those of carcinoma (Fig. 7-72). Microscopically, there is notable foveolar and glandular hyperplasia with variable hypertrophy of pyloric smooth muscle, small mucosal erosions, and ulcerations. There is usually a lymphoplasmacytic infiltrate of variable degree in the lamina propria.
Parasites
Horses: Many parasites cause disease of the stomach, especially in ungulates. Modern anthelmintics have made these diseases relatively easy to prevent and control both in individual animals and in herds.
Equine bots, Gasterophilus intestinalis and Gasterophilus nasalis, are commonly seen in animals on inadequate deworming regimens (Fig. 7-73). Both species migrate in the tissues of the oral cavity and often reside in infected spaces adjacent to teeth. Gasterophilus intestinalis colonizes the stratified portion of the stomach. The adult fly lays eggs on the hairs of the distal limbs of the horse. Gasterophilus nasalis lays its eggs around the nose of the horse. The larvae hatch after being moistened and warmed by licking. They are swallowed and live in the glandular stomach and duodenum. Both species attach to the mucosa via their anterior pincers. The larvae pass in the feces, pupate, and develop into flies.
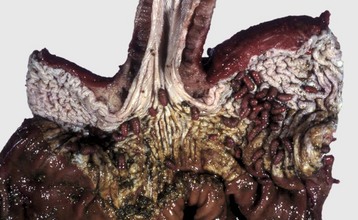
Fig. 7-73 Gasterophiliasis, stomach, horse.
Fly larvae (bots) of Gasterophilus intestinalis are attached to the epithelium of the nonglandular portion of the stomach. Note the muscular hypertrophy of the distal esophagus. Although not shown in this illustration, Gasterophilus nasalis, another similar equine gastric parasite, attaches to the epithelium of the glandular portion of the stomach. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Draschia megastoma is found in “brood pouches” in the glandular mucosa adjacent to the margo plicatus (Fig. 7-74). Infection is sometimes referred to as habronemiasis, based on antiquated taxonomic nomenclature in which these nematodes were classified as Habronema spp. Eggs produced in the cysts are extruded through a pore in the brood pouch to the gastric lumen. The eggs pass out with the feces and are consumed by fly larvae that are the intermediate hosts. Both Draschia and Gasterophilus spp. can cause gastric ulcers. Considering their location and means of survival in the stomach, it is remarkable that they do not cause serious damage more often.
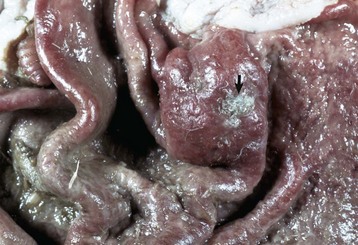
Fig. 7-74 Focal granulomatous gastritis, draschia brood pouch, stomach, horse.
A large parasitic brood pouch is present in the glandular mucosa adjacent to the margo plicatus (right center of illustration). Nematodes have been squeezed from the pouch and are visible on the surface (arrow). Histologically, the mucosa is expanded by focal granulomatous inflammation containing clusters of adult Draschia megastoma. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Ruminants (Cattle, Sheep, and Goats): Haemonchus contortus, known as the barber pole worm, is relatively common in ruminant abomasums. The common name of this parasite is related to the macroscopically visible entwining of the blood-filled intestine and white uterus in the female worm (Fig. 7-75). Hyperinfested pastures containing numerous third-stage larvae are the source of infection. Lambs are particularly at risk. Larvae on grasses are ingested by the host and enter the abomasum, where they may lie dormant within the gastric glands. After development to adults, they exit to the abomasal surface and attach via a buccal tooth. Eggs pass in the feces, completing the life cycle. Haemonchus are blood feeders and can cause severe anemia, hypoproteinemia, and resultant edema. This edema is characteristically present in the intermandibular space, resulting in a physical resemblance to a bottle (“bottle jaw”). As with any process resulting in anemia and hypoproteinemia, there are pale mucous membranes, stunting, and diarrhea. Diagnosis is by fecal egg counts and at necropsy, by semiquantification of abomasal parasite load along with the attendant lesions of anemia and hypoproteinemia. At necropsy, the carcass is pale and has generalized edema and fluid in all body cavities secondary to hypoproteinemia. Abomasal contents are fluid and discolored from free blood. Foci of mucosal hemorrhage are present at sites of worm attachment.
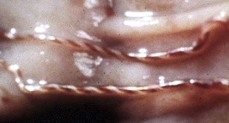
Fig. 7-75 Haemonchus contortus, abomasum, sheep.
The white spiral reproductive tract wrapped around the blood-filled intestine is responsible for the striped appearance, hence the common name “barber’s pole worm.” (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
In temperate climates, ostertagiosis is considered the most important parasitic disease in cattle (Ostertagia ostertagia) and small ruminants (Ostertagia circumcincta). Affected animals are unthrifty. Ostertagia spp. have a direct life cycle similar to that of Haemonchus spp. The nematodes are smaller than those of Haemonchus and are uniformly brown. Third-, fourth-, and fifth-stage larvae reside in the abomasal gastric glands. Ostertagia spp. are often present along with Trichostrongylus spp. in other GI locations. Intercurrent GI parasitism with other trichostrongyles has an additive effect on the clinical signs. Unthriftiness, lack of proper mentation, diarrhea, hypoproteinemia, and ventral edema may result. The multinodular appearance of the abomasum of heavily infested animals resembles Morocco leather (Fig. 7-76). This cobblestone appearance is due to enlargement of the gastric glands because of mucous cell hyperplasia and hyperplasia of lymphoid nodules in the abomasal submucosa elevating the overlying mucosa. Abomasitis produced by Ostertagia spp. is characterized by an infiltration of mononuclear inflammatory cells and eosinophils in the lamina propria. There are also increased numbers of globule leukocytes, a decrease in the number of parietal and chief cells, and hyperplasia of abomasal mucous cells. Differential diagnoses include lymphoma.
Fig. 7-76 Ostertagiosis, abomasum, cow.
The granular Moroccan leather appearance of the abomasal mucosa is characteristic of chronic ostertagiosis and is due to epithelial hyperplasia of the gastric glands, which may also contain ostertagia larvae. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Abomasal coccidiosis has been reported in a sheep. Mucosal lesions are nodular and hemorrhagic with hyperplasia of mucous neck cells, parietal cell atrophy, and lymphoplasmacytic fibrosis of the lamina propria associated with giant schizonts of uncertain taxonomy.
Pigs: Hyostrongylus rubidus of pigs is a gastric parasite that causes a thickening of the mucosa, with mucus accumulation and mucous cell hyperplasia and inflammation of the lamina propria by lymphocytes, plasma cells, and eosinophils. The parasite is threadlike and red. Clinically, hyostrongylosis is associated with the “thin sow syndrome.” Grossly, the gastric mucosa is thickened, catarrhal, and somewhat cobblestone, similar to ostertagiosis of ruminants. Microscopically, there is mucous metaplasia of parasitized and adjacent gastric glands. Submucosal lymphoid follicles develop in chronic infections.
Carnivores (Dogs and Cats): Several genera of nematodes, principally Ollulanus, Gnathostoma, and Cylicospirura, cause gastritis in dogs and cats, but these infections are rare.
Physaloptera spp. are often thought of as gastric parasites of carnivores because they are sometimes found in the stomach on endoscopic examination or at necropsy. They are occasionally responsible for vomiting. They appear similar to ascarids but generally attach by anterior hooks to the proximal duodenal mucosa at the gastric valve (Fig. 7-77). Intermediate hosts are coprophagous beetles.
Neoplasia
Gastric neoplasia, although uncommon, manifests in different ways in domestic animals. Leiomyoma and more rarely leiomyosarcoma arise from the tunica muscularis (Fig. 7-78). Lymphoma can be primary, metastatic, or multicentric in origin (Figs. 7-79 and 7-80; also see Fig. 13-85). In cattle, lymphoma is often caused by the bovine leukemia virus and has a predilection for the abomasum and the right atrium and uterus (Fig. 7-81). Squamous cell carcinoma of the stratified squamous (esophageal) portion of the stomach is relatively common in the horse (Fig. 7-82). Glandular neoplasms, adenomas, and adenocarcinomas occur in all species but are seen most often in dogs and cats. Dogs and rarely cats occasionally develop gastric mast cell tumors.

Fig. 7-78 Leiomyoma, stomach, dog.
This tumor arose from smooth muscle in the tunica muscularis and is covered by intact mucosa. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
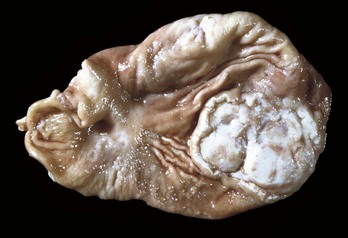
Fig. 7-79 Lymphoma, stomach, cat.
A large expansile mass is present in the submucosa of the stomach (top edge) and is covered by an intact mucosal epithelium. Note the other lymphomatous mass, which is ulcerated (lower right). This latter lesion is somewhat atypical of this disease, because ulceration is uncommon and occurs in late-stage disease when the mass is quite large and protrudes into the gastric lumen. In most cases of gastric lymphoma, the mucosal epithelium is intact and not ulcerated. (Courtesy Dr. C.S. Patton, College of Veterinary Medicine, University of Tennessee.)
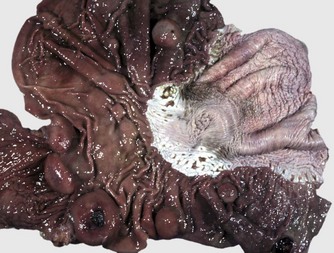
Fig. 7-80 Lymphoma, stomach, horse.
Large smooth-surfaced submucosal nodules, two of which have a central hemorrhagic ulcer, are present in the glandular portion of the stomach. Ulcers in the stratified squamous portion of the stomach are sites of Gastrophilus intestinalis attachment. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Fig. 7-81 Lymphoma, abomasum, cow.
A, Mucosal surface of abomasal folds. Note the folds are thickened and have a pale white-pink color resulting from the infiltration of neoplastic lymphocytes. Overlying mucosae are eroded and ulcerated. B, Transverse section. This cross-section demonstrates a white, space-occupying submucosal mass. The intact mucosa is located at the top of the specimen. (A courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee. B courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
Intestine
The intestines might be thought of as a tube within the body cavity that carries material (ingesta) through the body. The overall anatomic and histologic organization of this digestive tube is illustrated in Fig. 7-83. By the action of enzymes, resident flora, and added secretions from the liver and pancreas, ingesta are broken down, nutrients are absorbed into the body, and waste products are excreted. To perform these functions, the intestine needs a very large surface area, which is accomplished by the following three means:
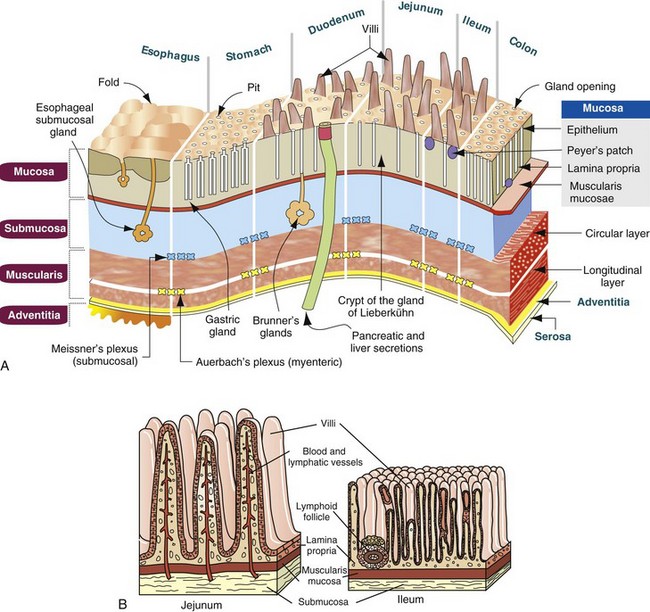
Fig. 7-83 Schematic diagram of the anatomic and histologic organization of the digestive tube.
A, Entire digestive tube. B, Higher magnification of the jejunum and ileum. (A from Kierzenbaum AL: Histology and cell biology: an introduction to pathology, St Louis, 2002, Mosby.)
1. Coiling the intestine in the abdomen.
2. Numerous intestinal folds that contain villi that notably increase the number of cells contacting the ingesta (Fig. 7-84).
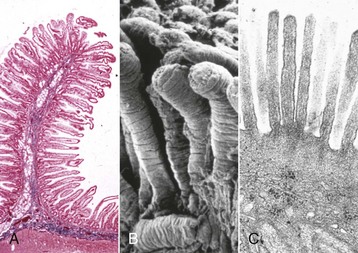
Fig. 7-84 Organization of the intestine.
The digestive and absorptive surfaces of the intestine are markedly increased by the presence of villi and microvilli on the enterocytes. A, Intestinal villus. Villus epithelial cells are present on a basement membrane (not seen) on a core of lamina propria. H&E stain. B, Small intestine, intestinal villi, scanning electron microscopy. Carbon sputter coat. C, Enterocyte microvilli. TEM. Uranyl acetate and lead citrate stain. (From Damjanov I, Linder J: Anderson’s pathology, ed 10, St Louis, 1996, Mosby.)
3. Each enterocyte has a microvillous border, further increasing the surface area available for digestive and absorptive processes.
Herbivores have longer intestines than carnivores or omnivores and need a fermentation vat, either the rumen or cecum, to digest cellulose. Within the smooth muscle layers and villi are the neural network of the enteric nervous system.
Structure and Function
The intestinal mucosa is composed of three layers—a single-cell-thick layer of epithelial cells lining the intestinal lumen, mesenchymal cells of the lamina propria, and the muscularis mucosa. Damage to any of these structures can result in digestive dysfunction and resultant diarrhea.
These cells function as a selectively permeable barrier allowing nutrient, electrolyte, and water absorption, while excluding pathogens, toxins, and other antigens. An understanding of these cell types and their functional roles in digestion and absorption is important in understanding the mechanisms of intestinal disease. Similarly, an understanding of the biology of these cell types is important in predicting clinical outcomes and designing therapeutic strategies for treating intestinal disease.
Epithelial Cells: There are six major types of polarized epithelial cells lining the intestine. These epithelial cells are enterocytes, undifferentiated or crypt epithelial cells, goblet cells, Paneth cells, enterochromaffin (neuroendocrine, argentaffin) cells, and microfold (M) cells (Fig. 7-85).
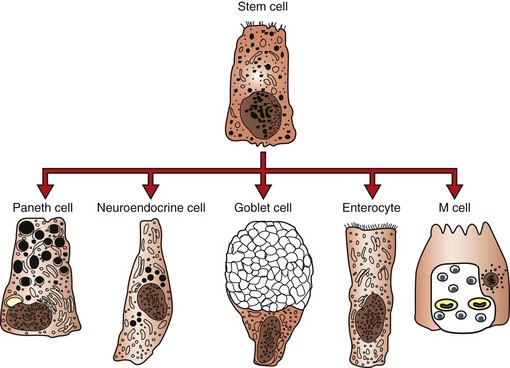
Fig. 7-85 Schematic illustration of the epithelial cell types of the small intestine.
Progenitor cells, located in the intestinal crypts, give rise to all other epithelial cell types lining the crypt and covering the villi. (From Damjanov I, Linder J: Anderson’s pathology, ed 10, St Louis, 1996, Mosby.)
Enterocytes are tall and columnar with luminal microvilli. They contain a surface glycocalyx that houses the digestive and absorptive enzymes. The mature cells do not proliferate, but they provide feedback inhibition of mitosis to the crypt cells by chalones. The cells are attached to each other by tight junctions composed of more than 40 proteins anchored to actin filaments, the most predominant of which are occludin, junctional adhesion molecules, and claudins. Many nutrients are absorbed through the lateral intercellular spaces between cells. Enterocytes move up the crypt and intestinal villus to the extrusion zone at the villus tip, where effete enterocytes are discarded into the fecal mass by an apoptotic mechanism called anoikis. The turnover rate for enterocytes is the most rapid of any fixed-cell population in the body. In neonatal pigs, for example, the turnover rate is 7 to 10 days. In 3-week-old pigs that have achieved a mature or climax flora, that rate accelerates to 2 to 3 days.
Undifferentiated crypt epithelial cells have little or no digestive capability. They are the progenitor cells that replace all of the other epithelial cell types. They have short, sparse microvilli. Crypt cells are the source of secretory component that acts as a receptor for IgA and IgM produced by plasmacytes in the intestinal lamina propria. The migration rate of crypt cells up the villus depends on several factors, one of which is an adaptation to gut microflora. In germ-free or gnotobiotic animals, the enterocyte replacement rate is similar to that of the neonate. Crypt cells are a source of chloride ion secretion into the intestinal lumen.
Goblet cells secrete mucus. They occur in both villous and crypt regions. Their numbers tend to increase aborally throughout the length of the intestine.
Paneth cells are located near the crypt base in some species, notably primates, horses, and rodents. It is not certain if Paneth cells are present in swine. Unlike all the other cells of the intestinal surface, these cells migrate toward the crypts rather than the villus tips. Paneth cells are considered to have both secretory and phagocytic functions. They produce cryptdin and lysins. These substances are toxic to bacteria and probably protect the proliferating crypt cells from infection. Paneth cells also act in a paracrine manner by opening anion channels in enterocytes causing chloride-secretion from crypt enterocytes. It has been suggested that Paneth cells play a role in elimination of heavy metals because they are selectively damaged by methylmercury. Collectively, Paneth cells comprise a cellular mass similar to that of the pancreas.
Enteroendocrine cells are also known as enterochromaffin cells and argentaffin cells because of their affinity for silver stains. They occur primarily in the crypts and produce serotonin, glucoinsulotropic peptide, catecholamines, gastrin, somatostatin, serotonin, cholecystokinin, secretin, bombesin, enteroglucagon, and likely others in response to chemical and mechanical stimuli. They secrete these products into the tissue rather than the gut lumen and thus are truly endocrine. Serotonin for example, activates both the intrinsic and extrinsic primary afferent neurons initiating peristalsis and secretory reflexes that are transmitted to the central nervous system (CNS). Occasionally, enteroendocrine cells form neoplasms called carcinoids.
M cells are so named because they have a microfolded or membranous surface. They form the dome epithelium that covers the gut-associated lymphoid tissue (GALT). They serve important functions in the uptake of protein and peptide antigens from the intestinal lumen and in the transport of these undigested antigens to the GALT where they are taken up by local dendritic cells and macrophages. Similarly, M cells also serve as a portal of entry for some pathogens, including bacteria, such as Salmonella, Yersinia, and Rhodococcus spp., and some viruses, such as BVD virus.
Mesenchymal Cells: Mesenchymal cells reside in the lamina propria. They arise from primitive mesenchyme rather than from ectoderm or endoderm. Their numbers increase with exposure to antigen, although there is a resident population of these cells in normal animals.
The intestinal lymphoid tissue is 25% of the body’s lymphoid mass (Fig. 7-86) and consists of those in the lamina propria and the GALT. This volume is larger than that of the spleen. In spite of the fact that the average person ingests 700 tons of antigens in a lifetime, the gut is adept at not responding to these food antigens. Laminal propria lymphocytes also play a role in intestinal crypt cell differentiation. Data are beginning to accumulate identifying the different effector and regulatory T lymphocyte types in the lamina propria and the functional organization of the GALT (Fig. 7-87).
Fig. 7-86 Normal gut-associated lymphoid tissue (GALT), intestine, pig.
The lymphoid tissue on the antimesenteric surface of the intestine is outlined by arrows and makes up one quarter of the animal’s total lymphoid mass. (Courtesy Dr. H. Gelberg, College of Veterinary Medicine, Oregon State University.)
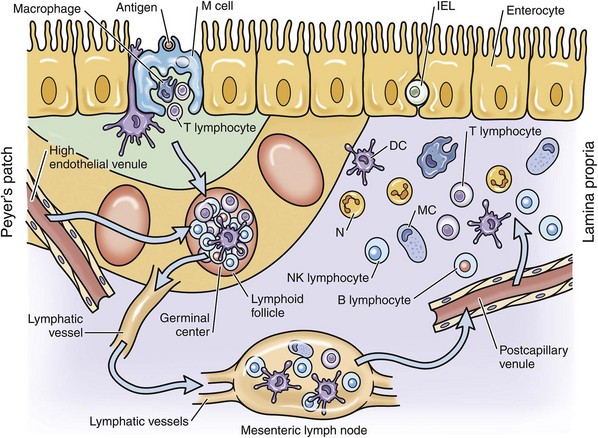
Fig. 7-87 Schematic illustration of GALT.
DC, Dendritic cell; IEL, intraepithelial lymphocyte; M, microfold cell; MC, mast cell; N, neutrophil. (Adapted from Cominelli F, Arseneau KO, Blumberg RS, et al: The mucosal immune system and gastrointestinal inflammation. In Yamata T, editor: Textbook of gastroenterology, ed 5, West Sussex, UK, 2009, Wiley-Blackwell.)
Neutrophils are transient within the lamina propria of the intestine. Neutrophils are short-lived in the blood and tissues; their normal route of removal from the body is to migrate through the wall of the alimentary tract to the lumen and be digested or excreted from the body via feces.
Eosinophils, when present in the intestinal lamina propria and submucosa, indicate a hypersensitivity reaction, often to food antigens or parasites.
Mast cells comprise 2% to 3% of the cells of the lamina propria and under normal conditions help regulate the intestinal epithelial barrier. They help control blood flow and coagulation, smooth muscle contraction, stimulation of the enteric nervous system, and peristalsis. They also help regulate acid, electrolyte, and mucus production by enterocytes. They recognize parasites and microorganisms through antibody-dependent mechanisms and receptor recognition. Mast cells release proinflammatory mediators and attract inflammatory cells through cytokine release that acts in a paracrine manner.
Globule leukocytes are large granular lymphocytes that are interepithelial or within the lamina propria. They are found in all species and occasionally form neoplasms, most notably in the cat. The normal function of these cells is unknown.
Defense Mechanisms
Defense mechanisms of the intestinal tract are diverse. They include indigenous (nonpathogenic) bacterial flora, intestinal and extraintestinal secretions, gastric acidity, intestinal motility, epithelial cell turnover, bile salts, immunologic mechanisms, and although a secondary mechanism, the Kupffer cells of the liver.
Secretions of the oral cavity, saliva, and intestine, called mucins, inhibit the adherence of organisms to the mucosa of the alimentary system. In addition to physically trapping pathogens, intestinal mucus serves to cover glycolipid and glycoprotein receptors on the surface of enterocytes (unstirred layer), thus preventing pathogen attachment and damage by toxins. Mucins are viscous, thus aid in protecting the epithelium from the shear forces of particulates driven against them by peristaltic waves. Because they are extensively glycosylated, mucins can cross-link and trap bacteria, making them more amenable to clearance by passage through the alimentary system.
Normal gastric acidity kills many organisms before they have the chance to reach the small intestine. Very young animals are achlorhydric, thus they may be more susceptible to some organisms such as pathogenic Escherichia coli. Helical bacteria in the stomach are the single greatest cause of gastric ulcers in humans. Although similar organisms occur in the stomachs of domestic animals, particularly carnivores, their role in gastritis of animals is less certain. Normal gastric acidity apparently does not kill all potentially pathogenic bacteria (helical bacteria) in the stomach and proximal small intestine of domestic animals.
Indigenous (nonpathogenic) bacterial flora (microbiota) competitively bind to putative attachment sites on the enterocytes, thus preempting pathogen attachment. These prokaryotic symbionts co-evolved with their hosts and are an integral part of homeostatic mechanisms. Killing of these bacteria through the use of antibiotics sometimes allows pathogens to colonize the intestine and produce disease. Thus gnotobiotic animals are more susceptible to infection. The microbiome enhances the host’s genome in contributing to normal physiology and disease resistance and susceptibility. Bacteria in the intestine outnumber the total somatic and germ cells of the body by approximately a factor of ten. Probiotics are “friendly bacteria” that are sometimes used therapeutically or prophylactically in a variety of products and nutraceuticals. These “friendly bacteria” also compete for substrate with pathogens, alter the microenvironmental pH making the growth of competitive bacteria difficult, and produce short chain fatty acids and inhibitory growth substances (bacteriocins) that are toxic to other bacteria. Colicins are bacteriocins produced by E. coli. Bacterial growth is also inhibited by lactoferrin and peroxidase from the pancreas and lysozyme and defensins from Paneth cells. Transferrin in serum and lactoferrin produced by enterocytes and neutrophils at sites of infection serve to sequester iron that is necessary for bacterial growth.
Intestinal peristalsis is protective in that loss of motility may lead to bacterial overgrowth in the intestine and increased susceptibility of enterocytes to toxins that are not moved out of the gut. Diarrhea can be in part a defense mechanism that rids the body of bacteria and toxins. Conversely, some bacteria secrete toxins that impair intestinal motility thus allowing pathogens a greater opportunity to attach to enterocytes.
Epithelial cells of the intestine have the greatest turnover rate of any fixed cell population in the body. In effect, this means that pathogens with a life cycle that exceeds that of the enterocytes will likely not be successful because their host cell will slough before the pathogen can reproduce. In addition, experimental evidence indicates that enterocyte microvilli form unilaminar vesicles containing digestive enzymes such as catalase and alkaline phosphatase on their surface. These vesicles are shed into the intestinal lumen where they may interact with the pathogen(s), thus preventing contact of pathogens with enterocytes since these vesicles are passed in the feces.
Bile salts inhibit the growth of many organisms. Kupffer cells of the liver act as a secondary line of defense. Because all the blood from the intestine enters the portal vein and percolates through the hepatic sinusoids, the Kupffer cells are perfectly positioned to phagocytose bacteria and endotoxins with which they come in contact. In pigs, goats, and cattle (artiodactyls), these functions are performed by intravascular pulmonary macrophages.
Secretory IgA and IgM constitute very important mechanisms of humoral immunity and function largely to prevent attachment of pathogens to intestinal epithelium. Crypt epithelial cells produce the secretory component of IgA. IgA functions through adhesion to M cells that regulates transepithelial movement of antigens while masking certain other antigens (Fig. 7-88).
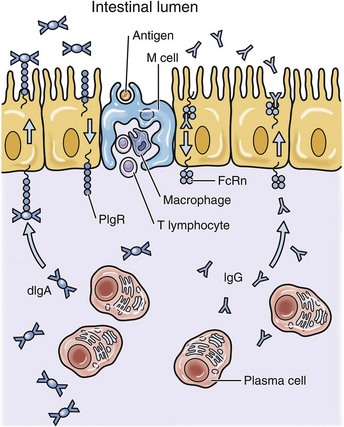
Fig. 7-88 Schematic diagram of immunoglobulin secretion in the intestine.
Dimeric IgA (dIgA), produced by plasma cells via interaction with polymeric IgR (PIgR) is transported across the intestinal epithelium in association with secretory component which is a portion of PIgR secreted into the intestinal lumen. IgG transport is mediated by MHC-I (FcRn). IgG transport is bidirectional, IgA is not. M, Microfold cell. (Adapted from Cominelli F, Arseneau KO, Blumberg RS, et al: The mucosal immune system and gastrointestinal inflammation. In Yamata T, editor: Textbook of gastroenterology, ed 5, West Sussex, UK, 2009, Wiley-Blackwell.)
Although in its infancy, research indicates that genetic polymorphisms of the host, including human leukocyte antigen (HLA), likely play a role in disease susceptibility and resistance.
Diarrhea
Response to Injury: Diarrhea is defined as secretion of abnormally fluid feces accompanied by an increased volume of feces and an increased frequency of defecation. Pathogens causing diarrhea fall into three major categories: those that induce intestinal secretion, such as enterotoxigenic E. coli (ETEC) (noninflammatory or secretory diarrhea); those that induce inflammation, such as Lawsonia; and those that are invasive, such as Salmonella. To simplify this further, there are two mechanistic “types” of diarrhea; noninflammatory and inflammatory. Noninflammatory diarrheas are produced by organisms that disrupt the absorptive or secretory mechanisms of the enterocytes without destroying the cells. Usually, but not always, noninflammatory diarrheas affect the more proximal portions of the bowel (ETEC, rotavirus, or Cryptosporidium parvum). Inflammatory diarrheas are produced by organisms that produce cytotoxins or are invasive and activate cytokines that initiate inflammatory cascades. The inflammatory diarrheas generally affect the ileum, cecum, or colon (Salmonella, Brachyspira, or Lawsonia). Combinations of mechanisms are present in specific diseases and are as follows:
• Malabsorption with or without bacterial fermentation leading to osmotic diarrhea. Generally, this is a problem of the small intestine, but secondary colonic malfunctions can occur because of malabsorption of bile salts and fatty acids that stimulate fluid secretion in the large intestine.
• Chloride (Cl−) hypersecretion by the cystic fibrosis transmembrane regulator (CFTR) of a structurally intact mucosa. CFTR is regulated by kinases, which are dependent on cyclic adenosine monophosphate (cAMP), which acts as second messenger. Prostanoids, bacterial toxins, and protein kinases all increase cAMP, thus increasing Cl− secretion. Calcium ion (Ca2+) also plays a role in opening Cl− channels through increases in acetylcholine interaction with epithelial muscarinic receptors via cholinergic nerves in intestinal plexi. Through a different mechanism, but also involving the CFTR, bicarbonate secretion is also increased. This osmotic activity results in a net efflux of fluid and electrolytes independent of permeability changes, absorptive capacity, or exogenously generated osmotic gradients.
• Exudation caused by increased capillary or epithelial permeability (protein-losing enteropathy) by leaky tight junctions between enterocytes.
• Hypermotility generally is involved in diarrhea but usually not as a primary mechanism in domestic animals. Hypermotility is defined as an increased rate, intensity, or frequency of peristalsis. Theoretically, with decreased mucosal contact time, digestion and absorption of nutrients and water should be less efficient. It is suspected that decreased motility in some diseases allows for increased bacterial proliferation (Fig. 7-89). Conversely, some enterotoxins can stimulate intestinal motility. In some motility disorders of humans, such as achalasia, Hirschsprung’s disease, inflammatory bowel disease (IBD) and others, there is an alteration in the network of interstitial cells of Cajal within the smooth muscle of the bowel wall. Whether this is a cause or effect of bowel motility disorders is not known.
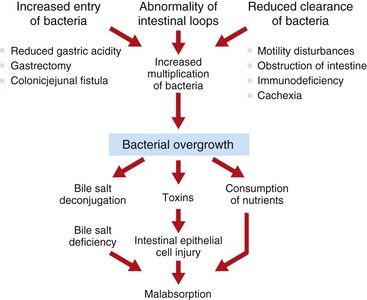
Fig. 7-89 Schematic diagram of the mechanism of how intestinal bacterial overgrowth causes malabsorption and diarrhea. (From Damjanov I, Linder J: Anderson’s pathology, ed 10, St Louis, 1996, Mosby.)
• Toll-like receptors (TLRs) and their associated molecules produced by enterocytes and leukocytes are very important in the regulation of intestinal inflammation and in the host’s response to intestinal pathogens.
• M cells regulate the presentation of antigens to GALT.
• Other factors (prostaglandins, leukotrienes, and platelet-activating factor) act on enteric nerves to induce neurotransmitter-mediated intestinal secretion by crypt cells.
• Cell damage is possibly a consequence of inflammation mediated by T lymphocytes or proteases and oxidants secreted by mast cells. T lymphocytes also may affect epithelial cell maturation, causing villous atrophy and crypt hyperplasia.
• Cell death can result from pathogen invasion into enterocytes, multiplication of the pathogen, and extrusion of the affected enterocytes. These changes lead to notable distortion of villous architecture with a lack of absorptive mature enterocytes accompanied by nutrient malabsorption and osmotic diarrhea.
• Mast cells of the lamina propria are in close association with enteric neurons and the enteric vasculature. They release histamine, prostaglandins, 5HT, and proteolytic enzymes that also play a role in diarrhea production.
As might be expected, the pathogenesis of diarrhea is complicated, involving a complex interplay of cells and factors that are currently being elucidated by a variety of molecular techniques. For example, when a pathogen invades or attaches to an enterocyte, the pathogen can release an enterotoxin. This toxin causes the enterocyte to release cytokines, particularly interleukin-8 (IL-8). These cytokines activate resident macrophages and recruit new macrophages into the lamina propria from the blood. The activated leukocytes release soluble factors (histamine, serotonin, and adenosine) that increase intestinal secretion of chloride ions and water and inhibit absorption (Figs. 7-90 and 7-91). Recruitment of inflammatory cells to areas of injury results in release of a chemical soup of cytokines (Fig. 7-92).
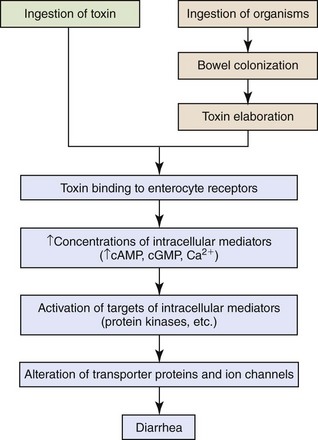
Fig. 7-90 Schematic diagram of the mechanism of action for enterotoxin-mediated bacterial diarrhea. (Adapted from Cominelli F, Arseneau KO, Blumberg RS, et al: The mucosal immune system and gastrointestinal inflammation. In Yamata T, editor: Textbook of gastroenterology, ed 5, West Sussex, UK, 2009, Wiley-Blackwell.)
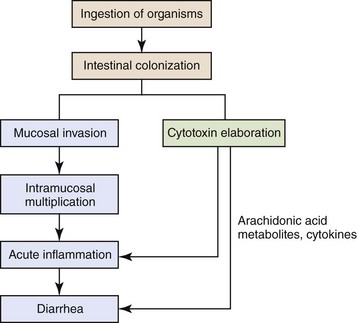
Fig 7-91 Schematic diagram of the mechanism of invasive and cytotoxin-mediated bacterial inflammation. (Adapted from Cominelli F, Arseneau KO, Blumberg RS, et al: The mucosal immune system and gastrointestinal inflammation. In Yamata T, editor: Textbook of gastroenterology, ed 5, West Sussex, UK, 2009, Wiley-Blackwell.)
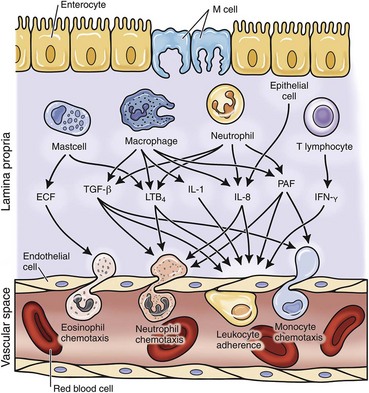
Fig 7-92 Schematic diagram of chemotactic factors during intestinal inflammation.
ECF, Eosinophil chemotactic factor; IFN-γ, interferon-γ; IL, interleukin; LTB4, leukotriene B4; PAF, platelet activating factor; TGF-β, transforming growth factor-β. (Adapted from Cominelli F, Arseneau KO, Blumberg RS, et al: The mucosal immune system and gastrointestinal inflammation. In Yamata T, editor: Textbook of gastroenterology, ed 5, West Sussex, UK, 2009, Wiley-Blackwell.)
Additionally, there are nonintestinal causes of diarrhea that must be considered in addition to diseases of the intestine. Among this group are hyperthyroidism, Addison’s disease, pancreatic insufficiency, pancreatitis, chronic renal failure, and others. These diseases are discussed in their respective chapters of this book.
Consequences: Normal feces are 75% water. Diarrheal feces are greater than 85% water. The consequence of excess fluid loss in the feces through diarrhea is dehydration. Dehydration results in hypovolemia. Hypovolemia results in hemoconcentration that results in inadequate tissue perfusion. Energy therefore is generated in tissue by anaerobic glycolysis. The resultant hypoglycemia leads to ketoacidosis. Acidosis is, by definition, a reduction in blood and tissue pH. Acidosis causes a reduction in pH-dependent enzyme system functions. Acidosis is compounded by fecal bicarbonate loss in diarrhea and the results of inadequate renal excretion of hydrogen ions and inadequate absorption of bicarbonate, which is a late effect of inadequate renal perfusion. The resultant electrolyte imbalance results in an increase in intracellular hydrogen ion concentration and a decrease in intracellular potassium ion concentration. The imbalances decrease neuromuscular control of myocardial contraction, leading to a further decrease in tissue perfusion. A vicious cycle results, culminating in hypovolemic shock.