VERRUCOUS CARCINOMA (SNUFF DIPPER’S CANCER; ACKERMAN’S TUMOR)
Verrucous carcinoma is a low-grade variant of oral squamous cell carcinoma. In 1948, Ackerman described this lesion in detail, although the term verrucous carcinoma had been used in 1944 in a series of cases reported by Burford, Ackerman, and Robinson. Ackerman postulated that some of these lesions might be associated with the use of smokeless tobacco, because 11 of his 31 patients were “tobacco chewers.” However, there was no mention of the type of smokeless tobacco used and no mention of whether any of these patients also had smoked tobacco. In addition to the oral mucosa, verrucous carcinoma has been identified at several extraoral sites, including laryngeal, vulvovaginal, penile, anorectal, sinonasal, and esophageal mucosa, as well as the skin of the breast, axilla, ear canal, and soles of the feet. Tumors at anatomic sites other than the mouth are unrelated to tobacco use. Several investigators have identified human papillomavirus (HPV) subtypes 16 and 18 in a minority of oral verrucous carcinomas, but the significance of this is unclear because this virus often is associated with otherwise normal oral mucosa.
Verrucous carcinoma represents less than 1% to 10% of all oral squamous cell carcinomas, depending on the local popularity of smokeless tobacco use. The only epidemiologic assessment of this tumor in a Western culture reported an average annual incidence rate of one to three oral lesions per 1 million population each year. Among 411,534 cases of head and neck carcinoma recorded in the National Cancer Database from 1985 to 1996, only 0.6% of cases were diagnosed as verrucous carcinoma.
Some verrucous carcinomas arise from the oral mucosa in people who chronically use chewing to-bacco or snuff, typically in the area where the tobacco is habitually placed. Cases also occur in nonusers, but the exact figure is difficult to assess because patients will often deny the tobacco habit. In smokeless tobacco users, conventional squamous cell carcinoma is much more likely to develop than this low-grade variant.
CLINICAL FEATURES: Verrucous carcinoma is found predominantly in men older than 55 years of age (average age, 65 to 70 years). In areas where women are frequent users of dry snuff, however, older females may predominate. The most common sites of oral mucosal involvement include the mandibular vestibule, gingiva, buccal mucosa, tongue, and hard palate. The site of occurrence often corresponds to the site of chronic tobacco placement. In cultural groups who keep the tobacco in the maxillary vestibule or under the tongue, these locations are the most commonly involved sites.
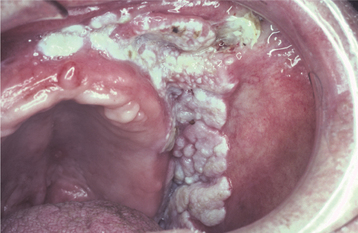
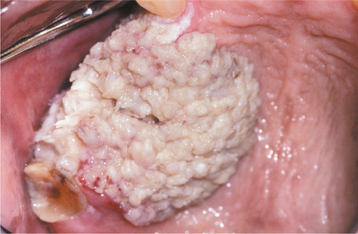
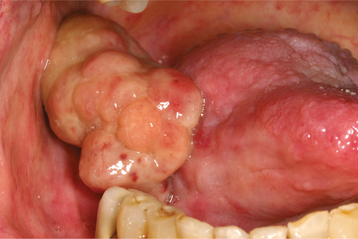
Oral verrucous carcinoma is usually extensive by the time of diagnosis, and it is not unusual for a tumor to be present in the mouth for 2 to 3 years before definitive diagnosis. The lesion appears as a diffuse, well-demarcated, painless, thick plaque with papillary or verruciform surface projections (Figs. 10-122 and 10-123). Lesions are typically white but also may appear erythematous or pink. The color depends on the amount of keratin produced and the degree of host inflammatory response to the tumor. If left untreated, then lesions may destroy underlying structures, such as bone, cartilage, muscle, and salivary glands. Enlarged cervical lymph nodes in patients with verrucous carcinoma usually represent inflammatory reactive changes rather than nodal metastasis.
Leukoplakia or tobacco pouch keratosis may be seen on adjacent mucosal surfaces, and verrucous carcinoma is a lesion that may develop from the high-risk precancer, proliferative verucous leukoplakia (PVL) (see page 391). PVL and verrucous carcinoma may have been reported in the past by the name oral florid papillomatosis.
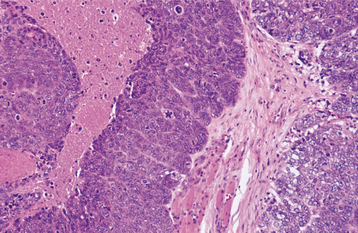
HISTOPATHOLOGIC FEATURES: Verrucous carcinoma has a deceptively benign microscopic appearance; it is characterized by wide and elongated rete ridges that appear to “push” into the underlying connective tissue (Fig. 10-124). Lesions usually show abundant keratin (usually parakeratin) production and a papillary or verruciform surface. Parakeratin typically fills the numerous clefts or crypts (parakeratin plugs) between the surface projections. These projections may be long and pointed or short and blunted. The lesional epithelial cells generally show a normal maturation pattern with no significant degree of cellular atypia. There is frequently an intense infiltrate of chronic inflammatory cells in the subjacent connective tissue.
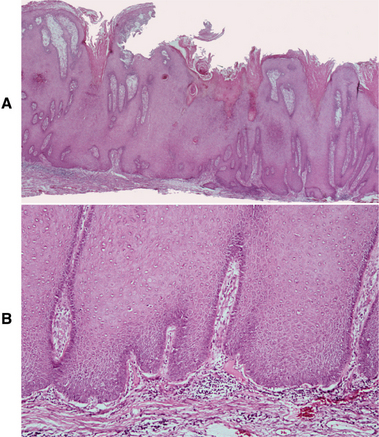
Fig. 10-124 Verrucous carcinoma. A, Low-power photomicrograph showing marked epithelial hyperplasia with a rough, papillary surface and keratin plugging. B, High-power view showing bulbous rete ridges without significant dysplasia.
The histopathologic diagnosis of verrucous carcinoma requires an adequate incisional biopsy. Because the individual cells are not very dysplastic, the pathologist must evaluate the overall histomorphologic configuration of the lesion to arrive at an appropriate diagnosis. Adequate sampling also is important because as many as 20% of these lesions have a routine squamous cell carcinoma developing concurrently within the verrucous carcinoma.
TREATMENT AND PROGNOSIS: Because metastasis is an extremely rare event in verrucous carcinoma, the treatment of choice is surgical excision without radical neck dissection. The surgery generally need not be as extensive as that required for routine squamous cell carcinoma of a similar size. With this treatment, 90% of patients are disease free after 5 years, although some patients will require at least one additional surgical procedure during that time. The treatment failures usually occur in patients with the most extensive involvement or in those unable to tolerate extensive surgery because of unrelated systemic diseases. An additional cause of treatment failure is the initial inability to identify a focal squamous cell carcinoma arising concurrently within the less aggressive lesion. Verrucous carcinomas containing foci of conventional squamous cell carcinoma should be treated as conventional squamous cell carcinomas.
Radiotherapy is an alternative primary treatment modality but provides poorer local control and thus is considered less effective than surgery. In addition, radiotherapy has been unpopular because of published reports of poorly differentiated or anaplastic carcinoma developing within the lesion after treatment. However, more recent analysis suggests that this threat is seriously overexaggerated. Chemotherapy may temporarily reduce the size of verrucous carcinoma and may be an option for inoperable cases, but it is not considered a definitive, stand-alone treatment. In a limited number of cases, tumor regression after radiochemotherapy or photodynamic therapy has been reported, although these treatment alternatives require further study.
SPINDLE CELL CARCINOMA (SARCOMATOID SQUAMOUS CELL CARCINOMA; POLYPOID SQUAMOUS CELL CARCINOMA)
Spindle cell carcinoma is a rare variant of squamous cell carcinoma characterized by dysplastic surface squamous epithelium in conjunction with an invasive spindle cell element. It may be indistinguishable from connective tissue sarcomas or other spindle cell malignancies at the level of the light microscope.
In the past, this biphasic lesion was thought to be a “collision” tumor between a carcinoma and sarcoma, but most authorities now consider the spindle cells to be simply an anaplastic type of carcinoma cell. Electron microscopy and immunohistochemical analysis support the concept that these lesional cells are of epithelial origin, with the ability to produce mesenchymal intermediate filaments. Based on immunohistochemical studies, some investigators have hypothesized that a dysfunctional cadherin-catenin complex important for intercellular adhesion causes the tumor cells to shift from a squamous to a spindled type, with increased infiltrative behavior. More than one third of all mucosal cases develop as recurrences after radiotherapy for a more differentiated squamous cell carcinoma, a phenomenon known as dedifferentiation.
CLINICAL FEATURES: The mean age at diagnosis for spindle cell carcinoma is 57 years (range: 29 to 93 years). There is no sex predilection. The neoplasm occurs predominantly in the upper aerodigestive tract, especially the larynx, oral cavity, and esophagus. Within the mouth, the lower lip, lateral posterior tongue, and alveolar ridges are common sites, but other areas may be involved.
In contrast to other oral cancers, the spindle cell carcinoma typically appears as a pedunculated, polypoid mass, but it may occasionally appear as a sessile, nodular or fungating mass or as an ulcer (Fig. 10-125). Pain and paresthesia are prominent features. The tumor grows rapidly, tends to metastasize early, and is typically diagnosed in a late stage (stages III and IV). Lower lip lesions seem to have a special propensity to travel along nerves through the mental foramen and into the mandibular canal.
HISTOPATHOLOGIC FEATURES: The spindle cell carcinoma is composed predomi-nantly of fascicles of anaplastic spindle-shaped cells (Fig. 10-126). Some spindle cells may appear as obvious epithelial elements, but others strongly resemble atypical mesenchymal cells. On rare occasions, bone, cartilage, or muscle differentiation may be seen. Numerous mitotic figures often are present. The overall picture is similar to that of an anaplastic fibrosarcoma (see page 553), except for the often-inconspicuous squamous element.
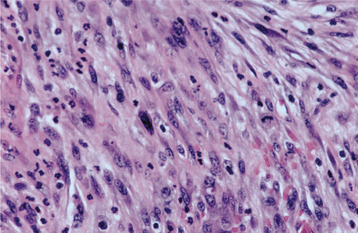
Fig. 10-126 Spindle cell carcinoma. Streaming fascicles of pleomorphic spindle cells that represent anaplastic epithelial cells.
The squamous component usually consists of carcinoma in situ of the overlying surface epithelium but may appear as islands of dysplastic squamous epithelium among the spindle cells. Direct transition be-tween the two cell types may be seen. Metastatic lesions may show only spindle cells, only squamous cells, or a combination of spindle and squamous cells.
Serial sections may be needed to find areas of unequivocal squamous cell carcinoma, and immunohistochemical techniques can be particularly useful in distinguishing this tumor from mesenchymal spindle cell malignancies. The lesional cells of most mesenchymal tumors typically produce vimentin but not cytokeratin. Approximately two thirds of the cases of spindle cell carcinoma react with antibodies directed against cytokeratin, and an equivalent number show vimentin immunoreactivity. Some cases also will be positive for carcinoembryonic antigen (CEA).
TREATMENT AND PROGNOSIS: The treatment of choice for spindle cell carcinoma is radical surgery, with neck dissection when clinically positive nodes are present. Most authors agree that radiotherapy and chemotherapy are ineffective. However, adjuvant radiation therapy may be of benefit in cases where surgical margins are positive for tumor. The 5-year disease-free survival rate is approximately 30% for oral lesions, with most deaths occurring within 1 year of diagnosis. This is somewhat worse than the prognosis for the tumor when it occurs in other anatomic sites, but it is similar to the prognosis for high-grade oral squamous cell carcinoma. Surprisingly, tumor size seems to have little effect on the prognosis, although there is some evidence that the microscopic depth of invasion is a strong prognostic indicator in oral lesions, with superficial tumors demonstrating a better prognosis.
ADENOSQUAMOUS CARCINOMA
Adenosquamous carcinoma is a rare variant of squamous cell carcinoma that is characterized histopathologically by a combination of adenocarcinoma and squamous cell carcinoma. The adenoid (glandular) pattern, which includes mucus production, has been demonstrated clearly in metastatic deposits. Some authorities consider this carcinoma to be merely a high-grade mucoepidermoid carcinoma (see page 487). The cause is unknown.
CLINICAL FEATURES: Cases of adenosquamous carcinoma have been reported from the tongue, oral floor, and other mucosal surfaces, usually in older adults. There is a slight male predilection. The clinical appearance is that of a nodular, broad-based, variably painful mass with or without surface ulceration. Eighty percent of patients have metastatic deposits within the neck nodes at diagnosis.
HISTOPATHOLOGIC FEATURES: Adenosquamous carcinoma appears as an admixture of a surface squamous cell carcinoma and an underlying ductal adenocarcinoma. The glandular component tends to be most prominent in deeper portions of the tumor. Intracytoplasmic mucin is noted by mucicarmine staining in most cases, making differentiation from mucoepidermoid carcinoma difficult but helping to distinguish adenosquamous carcinoma from forms of squamous cell carcinoma that exhibit a pseudoglandular pattern of degeneration. Both squamous and glandular components immunoreact with antibodies directed against high molecular–weight cytokeratin (KL1).
TREATMENT AND PROGNOSIS: Radical surgical excision, with or without radiation therapy, is the treatment of choice for patients with adenosquamous carcinoma. The prognosis is poor; among previously reported cases involving the upper aerodigestive tract, the overall 5-year survival rate is 13%, with 42% of patients dying of disease after a mean period of 2 years after their diagnosis. Cervical lymph node metastasis develops in approximately 65% of patients, and distant metastasis develops in approximately 23% of patients, with the lung being the most common site of dissemination.
BASALOID SQUAMOUS CARCINOMA (BASALOID SQUAMOUS CELL CARCINOMA)
Basaloid squamous carcinoma is a lesion found primarily in the upper aerodigestive tract mucosa and represents the most recently described variant of squamous cell carcinoma. It has a tendency to develop in the hypopharynx, but dozens of oral lesions have been reported.
CLINICAL FEATURES: Basaloid squamous carcinoma of the head and neck occurs predominantly in men, although no significant gender predilection exists among previously reported oral cases. The neoplasm tends to occur in persons 40 to 85 years of age and in abusers of alcohol and smoked tobacco. It most commonly involves the larynx, pyriform sinus, and tongue base, but any region of the upper aerodigestive tract may be affected. The individual lesion clinically appears as a fungating mass or ulcer and may be painful or interfere with swallowing (dysphagia). Almost 80% of patients have cervical metastases at the time of diagnosis of this high-grade, aggressive cancer.
HISTOPATHOLOGIC FEATURES: As its name connotes, basaloid squamous carcinoma has two microscopic components. The first is a superficial, well-differentiated or moderately differentiated squamous cell carcinoma, often with surface ulceration, multifocal origin, and areas of carcinoma in situ. The second, deeper component is an invasive basaloid epithelium arranged in islands, cords, and glandlike lobules. This deeper tumor often shows palisading of peripheral cells, necrosis of central regions, and occasional squamous differentiation (Fig. 10-127). This component appears similar to basal cell carcinoma, adenoid cystic carcinoma, basal cell adenocarcinoma, or neuroendocrine carcinoma. The interface between the two components is typically sharp and distinct, but transition from squamous to basaloid cells may occasionally be seen. Basaloid cells and islands of cells often are surrounded by mucoid stroma (basal lamina material). Microcystic spaces filled with PAS-positive basal lamina material may be interspersed among the tumor islands as well.
TREATMENT AND PROGNOSIS: Basaloid squamous carcinoma is an aggressive malignancy. Affected patients have a mean survival time of only 23 months. Although somewhat controversial, several recent studies have suggested that basaloid squamous cell carcinoma may have a similar outcome compared with conventional squamous cell carcinoma when cases are matched by clinical stage and anatomic location. Therefore, the poor prognosis for basaloid squamous cell carcinoma simply might be caused by a tendency for these patients to be diagnosed with late-stage disease. Surgery followed by radiotherapy is the recommended treatment, usually with adjuvant chemotherapy for the distant metastases.
CARCINOMA OF THE MAXILLARY SINUS
Carcinoma of the maxillary sinus or antrum is an uncommon malignancy of largely unknown cause. It does not appear to be related to sinusitis or nasal polyps. Unlike squamous cell carcinomas in other head and neck sites, squamous cell carcinomas of the paranasal sinuses have been associated only weakly with tobacco use. A strong causal relationship to occupational wood and leather dust exposure has been established for the rarely occurring sinonasal intestinal type of adenocarcinoma. Maxillary sinus carcinomas comprise only 3% of all head and neck carcinomas; however, among paranasal sinus carcinomas, the maxillary sinus is the most common site (accounting for 80% of lesions). Most lesions remain asymptomatic or mimic sinusitis for long periods while the tumor grows to fill the sinus. Therefore, the diagnosis may not be made until the lesion has perforated through the surrounding bone.
The majority of maxillary sinus carcinomas are classified as squamous cell carcinomas. However, additional carcinomas that may arise in this location include sinonasal adenocarcinoma, sinonasal undifferentiated carcinoma (SNUC) (see next section), neuroendocrine (small cell undifferentiated) carcinoma, and salivary gland type of adenocarcinoma.
CLINICAL AND RADIOGRAPHIC FEATURES: Typically, carcinoma of the maxillary sinus is a disease of older adults. There is a slight predilection for males. More than 80% of cases are advanced (stage III or stage IV) at the time of diagnosis. Affected patients generally complain of a chronic unilateral nasal stuffiness or notice an ulceration or mass of the hard palate or alveolar bone (Fig. 10-128). When the second division of the trigeminal nerve is involved, intense pain or paresthesia of the midface or maxilla may occur, perhaps simulating a toothache. Teeth in the area of the tumor may become loosened, and dental radiographs often reveal a “moth-eaten” destruction of the lamina dura and surrounding bone. A panoramic radiograph shows a cloudy sinus with destruction of its bony wall; however, the extent of the tumor is best visualized by computed tomography (CT) or magnetic resonance imaging (MRI).
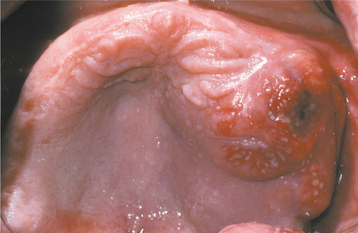
Fig. 10-128 Carcinoma of the maxillary sinus. The tumor has produced a bulge of the posterior maxillary alveolar ridge and is beginning to ulcerate through the surface mucosa.
If the tumor perforates the lateral wall of the sinus, unilateral facial swelling and pain are usually present. If the extension is medial, then nasal obstruction or hemorrhage usually occurs. Extension superiorly results in displacement or protrusion of the eyeball. Approximately 4% of patients have cervical or submandibular lymph node metastasis at the time of diagnosis. Distant metastasis is quite uncommon until late in the progression of disease.
HISTOPATHOLOGIC FEATURES: Although the antrum is lined by respiratory epithelium, the great majority of maxillary sinus carcinomas are squamous cell carcinomas, usually moderately or poorly differentiated.
TREATMENT AND PROGNOSIS: Carcinoma confined within the maxillary sinus usually is treated by hemimaxillectomy; those that have perforated through the surrounding bone are treated by radiotherapy or combined radical surgery and radiotherapy. However, even with radical treatment the prognosis is poor, with a 5-year survival rate of approximately 40%. The presence of metastatic deposits in local lymph nodes reduces the survival rate to less than 8%, as does involvement of the pterygopalatine fossa. With or without cervical node involvement, death usually occurs from local destruction and the inability to control the primary disease.
SINONASAL UNDIFFERENTIATED CARCINOMA
Sinonasal undifferentiated carcinoma (SNUC) is a rare, highly aggressive, and clinicopathologically distinctive neoplasm of the nasal cavity and paranasal sinuses. The tumor was first described in 1986, and since then fewer than 100 cases have been reported. In the earlier literature, tumors of this type were probably reported as anaplastic or undifferentiated carcinomas.
The histogenesis is uncertain; some investigators have theorized that the cell of origin may be related to the schneiderian membrane or olfactory epithelium. The pathogenesis of SNUC is poorly understood. A few cases have been associated with a history of smoking or the presence of Epstein-Barr virus (EBV), although a strong correlation with these factors has not been established. In some instances, patients have developed SNUC secondary to radiation therapy for nasopharyngeal carcinoma or retinoblastoma.
CLINICAL AND RADIOGRAPHIC FEATURES: Although a broad age range (third through ninth decades) has been reported, there is a tendency for older patients to be affected, with a median age at presentation in the sixth decade. Men are affected more commonly than women, with a male-to-female ratio of approximately 2:1 to 3:1.
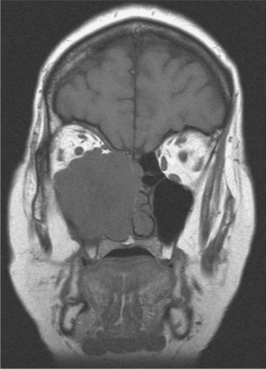
SNUC is well known for rapid development of locally extensive disease. The neoplasm typically appears as a large tumor mass that can involve multiple regions of the sinonasal tract, usually including the nasal cavity, maxillary sinus, and ethmoid sinuses. In addition, extension into contiguous sites—such as the nasopharynx, orbit, and cranial cavity—is common. Inferior penetration into the oral cavity is possible as well. There is usually relatively rapid development of multiple sinonasal symptoms, including nasal obstruction or discharge, epistaxis, swelling, and pain. Orbital involvement may lead to proptosis, periorbital swelling, diplopia, and vision loss. Cranial nerve palsies are a common finding as well.
Radiographic assessment is best performed by CT or MRI, which typically reveals a large, expansile sinonasal mass with bony destruction and invasion of adjacent structures (Fig. 10-129).
HISTOPATHOLOGIC FEATURES: Sinonasal undifferentiated carcinoma is characterized by trabeculae, ribbons, sheets, and nests of polygonal cells with minimal cytoplasm and pleomorphic, hyperchromatic to vesicular nuclei. No squamous or glandular differentiation should be observed. Mitotic figures are numerous. Tumor necrosis, apoptosis, and lymphovascular invasion usually are prominent. The surface epithelium overlying the tumor may exhibit dysplasia or carcinoma in situ. Immunohistochemical staining for cytokeratin or epithelial membrane antigen typically is positive.
TREATMENT AND PROGNOSIS: The standard approach has been aggressive multimodal therapy, including complete surgical resection when feasible followed by adjuvant radiation and/or chemotherapy. The prognosis for this lesion is extremely poor, with an overall 5-year survival rate of less than 20%. However, a few centers recently have reported promising results with induction chemotherapy followed by radiation and surgical resection of any remaining disease. This newer treatment approach has been associated with 2-year survival rates of 64% to 75%. High-dose chemotherapy and bone marrow transplantation may extend the life of the patient. Local recurrence is common and is the major cause of morbidity and mortality. Metastasis is possible, usually to cervical lymph nodes, bone, liver, or brain.
NASOPHARYNGEAL CARCINOMA
Nasopharyngeal carcinoma refers to a group of malignancies that arise from the lining epithelium of the lymphoid tissue–rich nasopharynx; similar tumors are found in the palatine tonsil and base of tongue. These three anatomic sites are collectively called Waldeyer’s tonsillar tissue or Waldeyer’s ring.
Nasopharyngeal carcinoma is rare in most areas of the world. The average annual incidence rate in the United States is less than 1 case per 100,000 persons. In southern Chinese men, however, the rate is a startling 20 to 55 cases per 100,000. Among southern Chinese men who migrate to the United States, the rate is intermediate, which suggests an environmental causative agent. Intermediate rates also are observed among many indigenous people of Southeast Asia (including Thais, Vietnamese, Malays, and Filipinos), Inuits of Alaska and Greenland, and Arabs of North Africa. Infection with Epstein-Barr virus, diets deficient in vitamin C, and consumption of salt fish that contains potentially carcinogenic N-nitrosamines have been implicated as contributory factors. Tobacco also has been implicated as a risk factor; however, the magnitude of risk for carcinoma development for a given level of tobacco exposure is lower in the nasopharynx than in other parts of the upper aerodigestive tract.
CLINICAL FEATURES: Nasopharyngeal carcinoma occurs in all age groups, but most commonly affects those who are 40 to 60 years old. It also occurs three times more commonly in men than in women. The primary lesion, which usually arises from the lateral nasopharyngeal wall, often is small and difficult to detect, even when the area is examined endoscopically. The first sign of disease for 50% to 60% of patients is an enlarged, firm to hard, cervical lymph node, which represents metastatic tumor (Fig. 10-130). Symptoms related to the ear are described by slightly less than half of these patients. If the tumor arises near the eustachian tube, then unilateral serous otitis media, otalgia, or hearing loss from obstruction may be the presenting complaint.
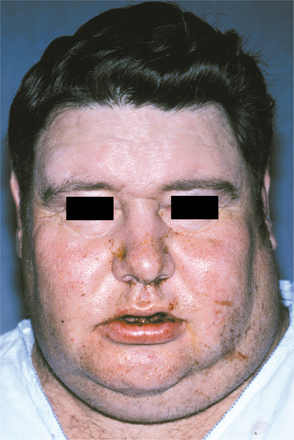
Fig. 10-130 Nasopharyngeal carcinoma. This patient initially appeared with metastatic carcinoma in the left lateral neck. Further evaluation revealed a primary tumor of the nasopharynx. (Courtesy of Dr. D. E. Kenady.)
Epistaxis, nasal obstruction, and pharyngeal pain may be present. The tumor may invade through the foramen lacerum into the brain, producing CNS symptoms, or it may involve cranial nerves in the area, causing specific symptoms related to those nerves. Significantly, 5% to 10% of patients also have distant metastasis at the time of diagnosis.
HISTOPATHOLOGIC FEATURES: The surgeon often has difficulty finding the primary lesion of nasopharyngeal carcinoma clinically, and multiple, systematic biopsy samples of the nasopharyngeal mucosa may be necessary for tumor identification and diagnosis. Microscopic examination of a nasopharyngeal carcinoma typically shows one of three histopathologic patterns:
1. Squamous cell carcinoma (keratinizing squamous cell carcinoma)
2. Differentiated nonkeratinizing carcinoma (nonkeratinizing squamous cell carcinoma)
3. Undifferentiated nonkeratinizing carcinoma (poorly differentiated carcinoma, anaplastic carcinoma, lymphoepithelioma)
More than one histopathologic type may be present in the same biopsy sample, in which case the tumor is classified according to the predominant histologic type.
The histopathologic features of the keratinizing squamous cell carcinoma are identical to those of squamous cell carcinoma of other head and neck regions (see page 419). Evidence of keratinization must be seen at the light microscopic level.
The lesional cells of differentiated nonkeratinizing carcinoma are relatively mature and somewhat squamous in nature, but they produce no keratin. Broad interconnecting bands of oval or round cells are organized in plexiform and papillary patterns.
Undifferentiated nonkeratinizing carcinoma consists of sheets of lesional cells with less distinct margins that show virtually no differentiation in most instances (Fig. 10-131). They have very little cytoplasm and large vesicular nuclei. These tumor cells are often intermixed with the lymphoid cells normally found at this anatomic site. The term lymphoepithelioma has been used to describe this lesion because it was once thought to be a malignancy that originated from both epithelial and lymphoid tissues. This terminology should be discouraged, however, because the lymphoid tissue is not part of the neoplastic process. Such undifferentiated tumors may be difficult to distinguish from lymphoma by light microscopy alone, and immunohistochemical studies often are used to demonstrate cytokeratins within the carcinoma cells. Some of these undifferentiated tumors currently are classified as sinonasal undifferentiated carcinomas (SNUC). Occasional neoplasms show neuroendocrine differentiation.
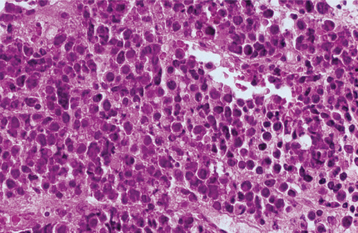
Fig. 10-131 Nasopharyngeal carcinoma. Poorly differentiated tumor exhibiting sheets of rounded tumor cells.
The less-differentiated lesions tend to occur in younger individuals. In fact, virtually all nasopharyngeal carcinomas in people younger than 40 years of age are poorly differentiated. Among southern Chinese patients, the vast majority (95%) of cases are classified as undifferentiated nonkeratinizing carcinomas, where-as in nonendemic areas, 30% to 50% of cases are keratinizing squamous cell carcinomas.
TREATMENT AND PROGNOSIS: Because of the inaccessibility of the nasopharynx and the high frequency of metastasis at diagnosis, nasopharyngeal carcinoma is treated most frequently with radiotherapy to the nasopharynx and neck. Although there has been some controversy regarding the benefit of adding chemotherapy to radiation therapy, emerging evidence favors concurrent chemoradiation over radiation alone for improved survival among patients with locally advanced disease.
The prognosis ranges from good to poor, depending on the stage of the disease. The overall 5-year disease-free survival rate reported in one large series of cases in the United States was 45.5%. For stage I patients, a 100% 5-year survival rate has been demonstrated. Stage II is associated with a 67% 5-year survival rate; stage III, 44%; and stage IV, 34%. Patients with two or more clinical symptoms tend to have a worse prognosis. When treated with radiation therapy, the differentiated and undifferentiated nonkeratinizing types exhibit a higher local control rate but a greater risk of distant metastasis compared with the keratinizing type. In the United States, higher survival rates have been observed among Chinese-American patients compared with other ethnic groups. This trend traditionally has been attributed to the prevalence of the more radiosensitive undifferentiated nonkeratinizing type among Chinese-American patients; however, for reasons unclear, Chinese ethnicity has been shown to be a favorable prognostic factor even independent of histopathologic type. Persons treated for nasopharyngeal carcinoma are also at increased risk of developing a second primary malignancy of the head and neck mucosa.
BASAL CELL CARCINOMA (BASAL CELL EPITHELIOMA; RODENT ULCER)
Basal cell carcinoma, the most common skin cancer (and the most common of all cancers), is a locally invasive, slowly spreading, primary epithelial malignancy that arises from the basal cell layer of the skin and its appendages. About 80% are found on the skin of the head and neck. More than 800,000 new cases of basal cell carcinoma are diagnosed annually in the United States, representing 80% of all skin cancers. The worldwide incidence is increasing by about 10% per year. Incidence generally increases with age, although a recent study suggests a disproportionate increase among young adults (particularly women) as well.
This cancer mainly results from chronic exposure to ultraviolet radiation. Frequent sunburns and tendency for freckling in childhood are associated with an increased risk, whereas occupational sun exposure and sunburns as an adult do not seem to be risk factors. Lesser risk factors include exposure to significant ionizing radiation, ingestion of arsenic, immunosuppressive therapy, and psoralen and ultraviolet A (PUVA) treatment (often used for psoriasis). In addition, several genodermatoses are associated with basal cell carcinoma development, including the nevoid basal cell carcinoma syndrome (see page 688), xeroderma pigmentosum (see page 747), albinism, Rasmussen syndrome, Rombo syndrome, and Bazex-Christol-Dupré syndrome.
Recent molecular genetic studies have shown that dysregulation of the hedgehog signaling pathway is a critical early event in the development of basal cell carcinoma. Inactivating mutations in the patched (PTCH) gene on chromosome 9q22 have been identified in both sporadic cases and patients with the nevoid basal cell carcinoma syndrome. Mutations in other genes participating in this pathway (e.g., smoothened [SMO]) occasionally may be found in sporadic cases as well. These mutations lead to constitutive activation of hedgehog signaling and enhanced cellular proliferation. In addition, mutations in p53 are found in more than 50% of sporadic basal cell carcinomas and may represent a later event in tumor development.
Oral lesions have been reported but are usually considered to be cases of misdiagnosed salivary or odontogenic neoplasms.
CLINICAL FEATURES: Basal cell carcinoma is a disease of adult whites, especially those with fair complexions. Although most patients are older than 40 years of age at the time of diagnosis, some lesions are detected as early as the second decade of life, particularly in patients with red or blonde hair and blue or green eyes. Approximately 80% of lesions occur on the head and neck, with the remainder involving the trunk and limbs.
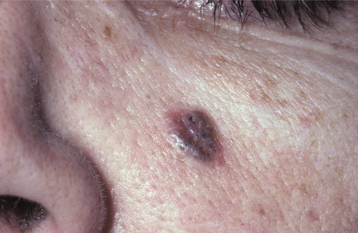
The most common form of this lesion, the nodular (noduloulcerative) basal cell carcinoma, begins as a firm, painless papule that slowly enlarges and gradually develops a central depression and an umbilicated appearance. One or more telangiectatic blood vessels are usually seen coursing over the rolled border surrounding the central depression (Figs. 10-132 and 10-133). When the lesion is pressed, a characteristic pearly opalescent quality is discerned. Expanding ulceration often develops in the central depressed area, and the patient may give a history of intermittent bleeding followed by healing. Untreated lesions continue to enlarge slowly over months and years, with ulceration and destruction of underlying structures, hence their historical name, rodent ulcer. Destruction of underlying bone or cartilage may occur, but metastasis is extremely rare.
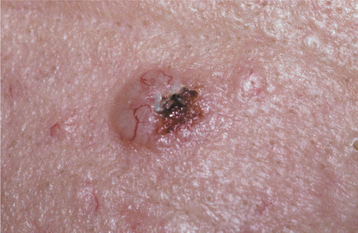
Fig. 10-132 Basal cell carcinoma. Early noduloulcerative basal cell carcinoma of the forehead showing raised, rolled borders and focal ulceration. Fine, telangiectatic blood vessels can be seen on the surface.
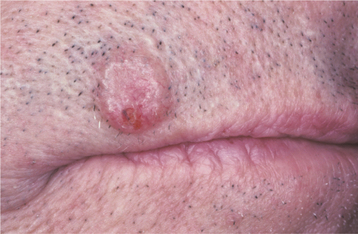
Fig. 10-133 Basal cell carcinoma. Noduloulcerative lesion of the upper lip demonstrating telangiectasia and small ulceration.
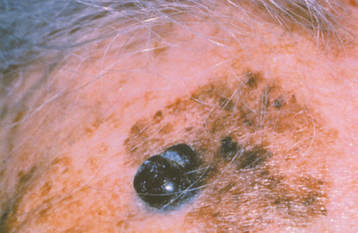
Several other clinicopathologic varieties of this tumor have also been described. Pigmented basal cell carcinoma is seen occasionally and represents a noduloulcerative tumor colonized by benign melanocytes (Fig. 10-134). The melanin production imparts a tan, brown, black, or even bluish color to the lesion, and usually the pigment is not distributed uniformly, as it would be in a melanocytic nevus (see page 382).
Sclerosing (morpheaform) basal cell carcinoma is an insidious lesion that often mimics scar tissue. The overlying skin appears pale and atrophic, and the lesion is firm to palpation with poorly demarcated borders. A slight elevation may be noted at the edges of the tumor. Often a great deal of invasion has occurred before the patient becomes aware of a problem.
The superficial basal cell carcinoma occurs primarily on the skin of the trunk. Often, lesions are multiple and appear as well-demarcated, erythematous, scaly patches that may be mistaken clinically for psoriasis. A fine, elevated, “threadlike” border is seen at the margins.
Some investigators believe that the basal cell carcinoma associated with the nevoid basal cell carcinoma syndrome (see page 688) should be placed in a separate category. These lesions develop in both sun-exposed and protected areas of the skin and may number in the hundreds on a single patient. The tumors associated with this syndrome usually do not produce a significant degree of tissue destruction.
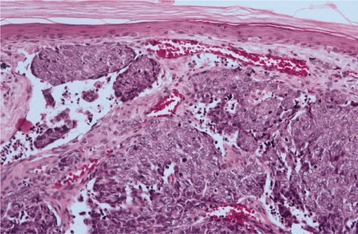
HISTOPATHOLOGIC FEATURES: The basal cell carcinoma displays a considerable diversity of appearances under the microscope: nodulocystic (noduloulcerative), superficial, adenoid, pigmented, infiltrative, morpheaform, and keratotic. The noduloulcerative, pigmented, and syndrome-related basal cell carcinomas are comprised of uniform ovoid, dark-staining basaloid cells with moderate-sized nuclei and relatively little cytoplasm (Fig. 10-135). The cells are arranged into well-demarcated islands and strands, which appear to arise from the basal cell layer of the overlying epidermis and invade into the underlying dermal connective tissue. Epithelial islands typically demonstrate palisading of the peripheral cells; frequently, a clear zone of artifactual retraction is seen between the epithelial islands and the connective tissue. Although most of these neoplasms show no differentiation, some exhibit areas of keratin production, sebaceous differentiation, or interlacing strands of lesional cells that resemble duct formation (“adenoid”). Necrosis of epithelial islands may produce a cystic appearance. Actinic damage in the form of solar elastosis almost always is seen in adjacent stroma.
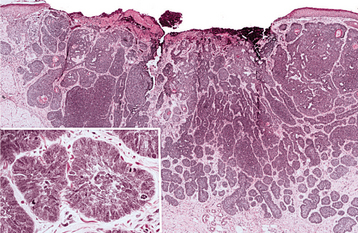
Fig. 10-135 Basal cell carcinoma. Low-power photomicrograph showing ulceration of the epidermal surface associated with an invading tumor of hyperchromatic epithelial cells. Inset demonstrates islands of basophilic epithelium with peripheral palisading.
Pigmented basal cell carcinoma demonstrates dendritic melanocytes within tumor islands, and melanophages may be seen in the surrounding stroma. Sclerosing basal cell carcinoma is characterized by infiltrating thin strands of basaloid tumor cells set in a densely collagenous background. Superficial basal cell carcinoma includes lobules of tumor cells that drop from the epidermis in a multifocal pattern. Occasionally, basal cell carcinoma is seen admixed with an independent primary squamous cell carcinoma of the skin. The resulting “collision” tumor is called basosquamous carcinoma. Some authorities consider the basosquamous carcinoma to be a simple basal cell carcinoma with abundant squamous metaplasia.
TREATMENT AND PROGNOSIS: The treatment of basal cell carcinoma often depends on the size and site of the lesion. Small lesions (<1 cm) are treated by routine surgical excision, laser ablation, or electrodesiccation and curettage, with 3- to 5-mm margins of clinically normal-appearing skin beyond the visible lesion. These methods result in a cure rate of 95% to 98%. Radical surgical excision and radiation therapy are recommended for large or aggressive lesions. For sclerosing type of lesions, recurrent lesions, or lesions situated near embryonic planes of fusion (along which these tumor cells tend to invade), a procedure called Mohs micrographic surgery should be used. This technique essentially uses frozen-section evaluation of specially mapped and marked surgical specimens to determine whether tumor tissue has been left behind. If it has, then the surgeon can return immediately to that particular area and remove more tissue, repeating the process until the patient is free of disease.
Topical treatments have shown promise as an alternative for certain variants of basal cell carcinoma, although long-term follow-up data for these alternative therapies are lacking. Photodynamic therapy is effective for superficial basal cell carcinomas, with good cosmetic outcomes reported. Additional topical treatments include 5% fluorouracil cream for the management of multiple superficial basal cell carcinomas on the trunk and limbs and 5% imiquimod cream for superficial basal cell carcinomas on the trunk, neck, or limbs.
Recurrence of a properly treated basal cell carcinoma is uncommon, and metastasis is exceptionally rare. In patients with uncontrollable disease, death is usually the result of local invasion into vital structures. However, with early detection and the advent of Mohs surgery, such an outcome is unusual today.
Patients with a history of basal cell carcinoma must be evaluated periodically. There is a 30% chance of a second basal cell carcinoma and a 6% chance of a cutaneous squamous cell carcinoma developing within 3 years of treatment of the initial tumor.
MERKEL CELL CARCINOMA (MERKEL CELL TUMOR; NEUROENDOCRINE CARCINOMA OF SKIN; SMALL CELL CARCINOMA OF SKIN; TRABECULAR CARCINOMA OF SKIN)
The Merkel cell carcinoma, first described in 1972, is a rare, aggressive primary malignancy with neuroendocrine features. It occurs primarily on the skin of the head and neck region. As with other skin malignancies, ultraviolet (UV) light exposure is a major risk factor. In addition, an increased frequency has been reported among immunosuppressed individuals, including transplant recipients, patients with chronic lymphocytic leukemia, and patients with HIV infection. Lesional cells contain cytoplasmic granules that resemble the neurosecretory granules found within the epidermal Merkel cells of touch receptor regions. Intraoral and lip vermilion cases have been reported but are rare.
CLINICAL FEATURES: Merkel cell carcinoma typically appears in older people, with more than 76% of cases reported in individuals 65 years or older. The tumor exhibits a predilection for whites and a slight male predominance. It occurs primarily on the sun-exposed areas of fair-skinned individuals, most commonly (75%) on the skin of the face. The vermilion border of the lower lip is also a susceptible site. Merkel cell carcinoma only rarely (4.5%) arises from mucosal sites, including the oral, nasal, pharyngeal, laryngeal, and vaginal mucosa. The tumor usually appears as a slowly enlarging, dome-shaped nodule with a smooth surface and prominent surface vessels (telangiectasias). It is red or violaceous and ranges in size from 0.5 to 5.0 cm. Ulceration rarely is seen. Occasional lesions grow rapidly, and 25% demonstrate local metastasis at diagnosis, belying its innocuous clinical appearance.
HISTOPATHOLOGIC FEATURES: Merkel cell carcinoma consists of infiltrating sheets and anastomosing strands of moderately sized, uniform, undifferentiated basophilic cells in the dermis and subcutaneous fat (Fig. 10-136). Pseudoglandular, trabecular, cribriform (“Swiss cheese”), and sheetlike patterns may be seen. The surface epithelium is usually intact and otherwise unremarkable unless secondarily ulcerated by the tumor. Mitotic figures are abundant, and tumor cells have prominent nuclei, scant cytoplasm, and indistinct cell borders. Intracytoplasmic argyrophilic granules may be demonstrated by the Grimelius stain, and lesional cells typically exhibit a “perinuclear dot” immunoreactivity pattern with antibodies directed against cytokeratin 20 (CK20). Immunopositivity for markers of neuroendocrine differentiation, such as chromogranin A, synaptophysin, and neuron-specific enolase, also may be helpful in establishing the diagnosis. Lack of immunoreactivity for antithyroid transcription factor 1 (anti-TTF-1) may help to exclude the possibility of metastatic small cell carcinoma of the lung, which may have similar histomorphologic features. At times, this entity is difficult to differentiate histopathologically from amelanotic melanoma, metastatic esthesioneuroblastoma, metastatic small cell carcinoma of the lung, malignant lymphoma, and other undifferentiated malignancies. In this situation, a panel of immunohistochemical studies should be used to exclude these other diagnostic possibilities. Careful physical examination of the patient also may provide useful diagnostic information.
TREATMENT AND PROGNOSIS: Merkel cell carcinoma is typically treated by wide local excision. For small primary skin tumors, removal by Mohs micrographic surgery with or without adjuvant radiation has been proven effective and less disfiguring than standard surgery. Lymph node dissection is performed when clinically palpable nodes are found. Sentinel lymph node biopsy may be used to determine whether regional lymph node dissection and/or radiation therapy is indicated in those patients with clinically negative nodes. Some controversy exists regarding adjuvant therapy in the management of Merkel cell carcinoma. Although some authors have not found adjuvant radiation to improve survival, most studies have shown improved survival and a significant decrease in risk of local recurrence and regional metastasis with postoperative radiation therapy. The role of adjuvant chemotherapy remains investigational.
Recurrence develops in 55% of cases, most commonly within the draining lymph nodes. In a recent analysis of U.S. Surveillance, Epidemiology, and End Results (SEER) Program data for a cohort of more than 1000 patients with Merkel cell carcinoma, the 5-year survival rates for patients with localized, regional, and distant disease were 75%, 59%, and 25%, respectively. The overall 5-year disease-specific survival rate is approximately 64%. Female sex, localized disease, limb involvement, and younger age have been found to be positive predictors of survival. In a few rare cases, complete spontaneous regression of the primary tumor has been reported, which suggests that immunologic therapy may be an alternative approach to investigate in the future.
Approximately 25% of patients with Merkel cell carcinoma develop additional malignancies (e.g., squamous cell carcinomas of the skin, hematologic malignancies, or adenocarcinomas of the breast or ovary) before, concurrent with, or after their diagnosis. Thus these patients should be monitored closely.
MELANOMA (MALIGNANT MELANOMA; MELANOCARCINOMA)
Melanoma is a malignant neoplasm of melanocytic origin that arises from a benign melanocytic lesion or de novo from melanocytes within otherwise normal skin or mucosa. Although most melanomas occur on the skin, they may develop at any site where melanocytes are present. Damage from UV radiation is considered a major causative factor, as suggested by the fact that the incidence of melanoma increases for light-complexioned populations as they approach the equator. However, chronic sun exposure does not seem to be as significant as it is for other cutaneous cancers, such as basal and squamous cell carcinoma. Acute sun damage may be of greater causative importance than chronic exposure in melanoma. Lesions of the oral mucosa, of course, are not related to sun exposure.
The risk of melanoma development is two to eight times greater when a relative has a history of the cancer. Additional risk factors include a fair complexion and light hair, a tendency to sunburn easily, a history of painful or blistering sunburns in childhood, an indoor occupation with outdoor recreational habits, a personal history of melanoma, and a personal history of dysplastic or congenital nevus.
Melanoma is the third most common skin cancer, but it accounts for only 5% of the total. Most deaths that are due to skin cancer, however, are caused by melanoma. In the United States for the year 2008, it is estimated that 62,480 people will be diagnosed with cutaneous melanoma and 8420 people will die of the disease. The age-adjusted incidence rate for skin melanoma (23.6 per 100,000 men and 14.9 per 100,000 women) has been increasing dramatically over the past several decades. Based on U.S. incidence rates from 2002 to 2004, it is estimated that 1 in 58 persons will be diagnosed with cutaneous melanoma during their lifetime. Controversy exists regarding this perceived increase in melanoma incidence. Some investigators contend that the increase is due to increased numbers of skin biopsies and improved diagnosis of early-stage disease, whereas others feel that there is a true increase in disease rate. Despite increasing incidence, the mortality rate for cutaneous melanoma has remained relatively constant since the 1990s, apparently because a large proportion of cases are diagnosed at an early stage. According to the National Cancer Database Report on Cutaneous and Noncutaneous Melanoma, 91.2% of all melanomas arise on the skin, whereas ocular, mucosal, and unknown primaries account for 5.2%, 1.3%, and 2.2% of cases, respectively. Almost 25% of cutaneous melanomas arise in the head and neck area, 40% occur on the extremities, and the rest occur on the trunk. More than half of mucosal melanomas occur in the head and neck area (including the oral and sinonasal regions), with the remainder involving the urogenital and rectal mucosa.
Oral mucosal melanoma is rare in the United States, where it occurs in only 1 in every 2 million persons annually and comprises much less than 1% of all melanomas. Several reports suggest it is more frequent in other countries, such as Japan and Uganda; however, other investigators have suggested that the true incidence of mucosal melanomas is not greater in these countries but only appears so because of the comparatively low incidence of cutaneous melanomas in these racial groups. The mucosal melanoma tends to present at a more advanced stage and is much more aggressive than its cutaneous counterpart. At least one in three patients with oral melanoma has a history of a pigmented macule in the region of the tumor for some time before melanoma diagnosis. Melanoma occasionally affects the parotid gland, usually as a metastatic deposit from a scalp, conjunctival, or paranasal tumor.
In recent years, there have been many discoveries regarding recurrent genetic alterations in melanomas, including those involving the Ras-Raf-ERK, mitogen-activating protein (MAP) kinase, and phosphatidylinositol 3-kinase (Pl3K) pathways. In particular, a high proportion (approximately 50% to 70%) of melanomas possess mutations in the gene encoding BRAF, a protein kinase involved in the Ras-Raf-ERK signaling pathway.
CLINICAL FEATURES: Most melanomas are seen in white adults. The average age of affected persons is 50 to 55 years, but cases are rather evenly distributed over the 30- to 80-year age bracket. A few melanomas occur in the second and third decades of life. Four clinicopathologic types of melanoma have been described:
Melanomas tend to exhibit two directional patterns of growth: (1) the radial growth phase and (2) the vertical growth phase. In the early stages of melanoma development, the radial growth phase tends to predominate in lentigo maligna melanoma, superficial spreading melanoma, and acral lentiginous melanoma. In these lesions, the malignant melanocytes have a propensity to spread horizontally through the basal layer of the epidermis. Eventually, however, the malignant cells begin to invade the underlying connective tissue, thus initiating the vertical growth phase. With nodular melanoma, the radial growth phase is very short or nonexistent and the vertical growth phase predominates.
Because many clinical similarities exist between melanoma and its benign counterpart, the melanocytic nevus, an “ABCDE” system of evaluation has been developed to help distinguish a melanoma clinically from a melanocytic nevus (Box 10-4).
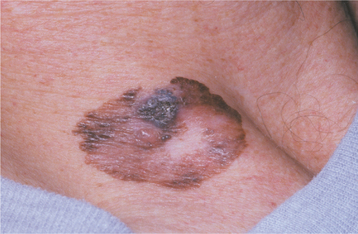
SUPERFICIAL SPREADING MELANOMA: Superficial spreading melanoma is the most common form of melanoma, representing 70% of cutaneous lesions (Fig. 10-137). The most common sites of origin are the interscapular area of males and the back of the legs of females. This form of melanoma appears as a macule with a variety of potential colors (i.e., tan, brown, gray, black, blue, white, pink). Typically, the lesion is smaller than 3 cm in greatest diameter at diagnosis, but it may be several times that size. Many lesions are slightly elevated. Clinically, invasion is indicated by the appearance of surface nodules or induration, and usually occurs within 1 year of discovery of the precursor macule. Satellite macules or nodules of malignant cells may develop around the primary lesion.
NODULAR MELANOMA: Nodular melanoma represents 15% of cutaneous melanomas, and one third of such lesions develop in the head and neck area. Nodular melanoma is thought to begin almost immediately in the vertical growth phase; therefore, it typically appears as a nodular elevation that rapidly invades into the connective tissue. Nodular melanoma is usually a deeply pigmented exophytic lesion, although sometimes the melanoma cells are so poorly differentiated that they no longer can produce melanin, resulting in a nonpigmented amelanotic melanoma.
LENTIGO MALIGNA MELANOMA: Lentigo maligna melanoma, which accounts for 5% to 10% of cutaneous melanomas, develops from a precursor lesion called lentigo maligna (Hutchinson’s freckle). Lentigo maligna occurs almost exclusively on the sun-exposed skin of fair-complexioned older adults, particularly in the midfacial region, and represents a melanoma in situ in a purely radial growth phase.
The lesion appears as a large, slowly expanding macule with irregular borders and a variety of colors, including tan, brown, black, and even white (Fig. 10-138). Patients usually indicate that the lesion has been present and has slowly expanded laterally for years. The average duration of the radial growth phase is 15 years. The appearance of nodularity within a lentigo maligna signals the onset of the invasive or vertical growth phase and the transition to lentigo maligna melanoma.
ACRAL LENTIGINOUS MELANOMA (MUCOSAL LENTIGINOUS MELANOMA): Acral lentiginous melanoma is the most common form of melanoma in blacks, and it is also the most common form of oral melanoma. It typically develops on the palms of the hands, soles of the feet, subungual area, and mucous membranes. It begins as a darkly pigmented, irregularly marginated macule, which later develops a nodular invasive growth phase. Recently, some authorities have separated this lesion into two entities: (1) acral lentiginous melanoma and (2) mucosal lentiginous melanoma.
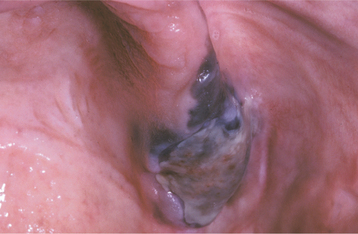
Oral melanoma is often nodular at the time of diagnosis, but early lesions may be flat. Affected persons are usually in their sixth or seventh decade of life. Two thirds of patients are men. Four of every five oral melanomas are found on the hard palate or maxillary alveolus.
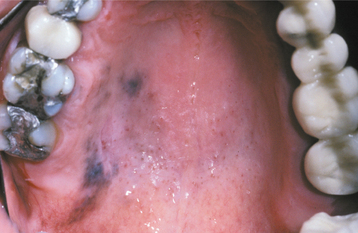
An oral lesion typically begins as a brown to black macule with irregular borders (Figs. 10-139 and 10-140). The macule extends laterally, and a lobulated, exophytic mass develops once the vertical growth is initiated (Fig. 10-141). Ulceration may develop early, but many lesions are dark, lobulated, exophytic masses without ulceration at the time of diagnosis. More than 20% of oral melanomas contain so little pigment that they have an essentially normal mucosal tint. Pain is not a common feature except in ulcerated lesions, and most lesions remain relatively soft to palpation. Underlying or adjacent bone may show radiographic evidence of irregular or “moth-eaten” destruction.
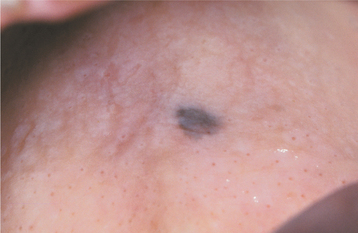
Fig. 10-139 Oral melanoma. This discrete area of pigmentation, measuring approximately 5 mm in diameter, was discovered on the posterior hard palate of a middle-aged woman during a routine oral examination. Biopsy revealed melanoma in situ.
HISTOPATHOLOGIC FEATURES: With cutaneous and oral melanomas, atypical melanocytes are initially seen at the epithelial and connective tissue junction. From here, they have the potential to proliferate throughout the epithelium, laterally along the basal cell layer, and downward into the connective tissue. In the early stages of the neoplasm, atypical melanocytes are seen either scattered singly among the basal epithelial cells or as nests within the basal cell layer. The atypical melanocytes are usually larger than normal melanocytes and have varying degrees of nuclear pleomorphism and hyperchromatism.
With superficial spreading melanoma, pagetoid spread often is seen. Large melanoma cells infiltrate the surface epithelium singly or in nests (Fig. 10-142). The resulting microscopic pattern is called pagetoid because it resembles an intraepithelial adenocarcinoma known as Paget’s disease of skin.
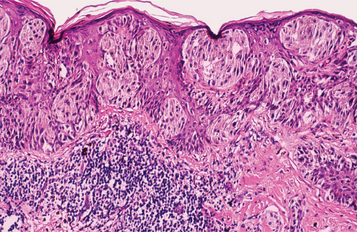
Fig. 10-142 Superficial spreading melanoma. The radial growth phase is characterized by the spread of atypical melanocytes along the basilar portion of the epidermis. Also note the presence of individual melanocytes invading the higher levels of the epithelium.
The spreading of the lesional cells along the basal layer constitutes the radial growth phase of the neoplasm. Such lateral spread of cells within the epithelium, which occurs before invasion into the underlying connective tissue, is characteristically seen in superficial spreading melanoma, lentigo maligna melanoma, and acral lentiginous melanoma. In acral lentiginous melanoma, many of the melanocytes have prominent dendritic processes (Fig. 10-143).
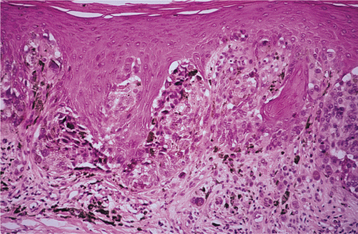
Fig. 10-143 Acral lentiginous melanoma. This palatal melanoma demonstrates numerous atypical melanocytes in the basilar portion of the epithelium with invasion into the superficial lamina propria. This represents the biopsy specimen from Fig. 10-140.
When malignant melanocytes are observed invading the connective tissue, the vertical growth phase has taken place. In nodular melanoma, this vertical growth phase occurs early in the course of the tumor. No radial growth of cells can be observed in the overlying epithelium beyond the edge of the invasive tumor (Fig. 10-144). The invasive melanoma cells usually appear either spindle-shaped or epithelioid and infiltrate the connective tissue as loosely aggregated cords or sheets of pleomorphic cells. Oral lesions tend to show invasion of lymphatic and blood vessels more readily than skin lesions. Several mucosal melanomas have been reported to contain unequivocal bone and cartilage, a feature that may cause diagnostic confusion with pleomorphic adenoma, sarcomatoid carcinoma, osteogenic sarcoma, and mesenchymal chondrosarcoma.
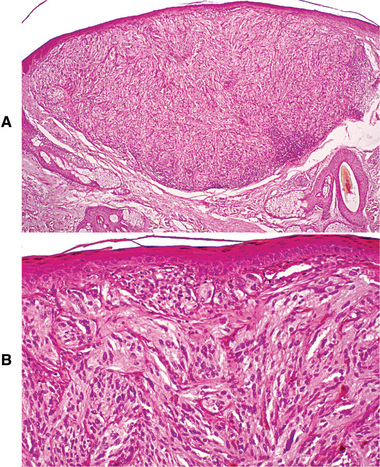
Fig. 10-144 Nodular melanoma. A, Low-power photomicrograph showing a nodular mass of malignant melanocytes invading into the dermis. Note the lack of radial growth in the adjacent overlying epidermis. B, Higher-power photomicrograph showing atypical spindle-shaped melanocytes.
In most instances, the lesional cells of melanoma contain fine melanin granules, but they may demonstrate no melanin production (amelanotic melanoma). A lack of melanin production may cause diagnostic confusion at the light microscopic level because melanoma can mimic a variety of undifferentiated tumors. Immunohistochemical studies showing S-100 protein, HMB-45, Melan-A, and Mart-1 reactivity of the lesional cells are beneficial in distinguishing such melanomas from other malignancies.
TREATMENT AND PROGNOSIS: Microscopic measurement of the depth of invasion is an important component of the histopathologic evaluation of cutaneous melanoma because of its correlation with the prognosis. The Clark system of measurement assigns a “level” to the lesion that depends on the deepest anatomic cutaneous region that has been invaded by tumor cells (Table 10-4). The more recent Breslow classification, however, appears to show a more accurate correlation with the prognosis and is based on the actual measurement of the distance from the top of the granular cell layer to the deepest identifiable point of tumor invasion.
Table 10-4
Clark’s classification in Cutaneous Melanoma
| Clark’s Definition of Level of Tumor Invasion | Clark’s Classification |
| Cells confined to epithelium | Level I |
| Cells penetrating papillary dermis | Level II |
| Cells filling papillary dermis | Level III |
| Cells extending into reticular dermis | Level IV |
| Cells invading subcutaneous fat | Level V |
Clinical staging for cutaneous melanoma is performed using a TNM classification system that takes into account tumor thickness, ulceration, regional nodal metastasis, and distant metastasis (Table 10-5). In this system, tumor thickness as measured by the Breslow classification is an important determinant of tumor (T) classification, and the Clark system is used only to further subtype T1 lesions (thin melanomas 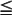 1 mm). Ulceration is an adverse prognostic factor; thus patients with stage I to III disease are upstaged (from A to B) when ulceration is present. Among patients with stage IV disease, elevated serum lactic dehydrogenase (LDH) is associated with an especially poor prognosis.
1 mm). Ulceration is an adverse prognostic factor; thus patients with stage I to III disease are upstaged (from A to B) when ulceration is present. Among patients with stage IV disease, elevated serum lactic dehydrogenase (LDH) is associated with an especially poor prognosis.
Table 10-5
TNM Classification System and Stage Groupings for Cutaneous Melanomaa
| T CLASSIFICATION | TUMOR THICKNESS (BRESLOW’S DEPTH OF INVASION) b | ULCERATION STATUS |
| T1 | ≤1.0 mm | a: Without ulceration or Clark’s level II/III |
| b: With ulceration or Clark’s level IV/V | ||
| T2 | 1.01-2.0 mm | a: Without ulceration |
| b: With ulceration | ||
| T3 | 2.01-4.0 mm | a: Without ulceration |
| b: With ulceration | ||
| T4 | >4.0 mm | a: Without ulceration |
| b: With ulceration | ||
| N CLASSIFICATION | NUMBER OF METASTATIC REGIONAL LYMPH NODES | NODAL METASTATIC MASS |
| N0 | 0 | |
| N1 | One | a: Microscopicc |
| b: Macroscopicd | ||
| N2 | Two to three, or intralymphatic regional metastasis without nodal metastasis | a: Microscopic |
| b: Macroscopic | ||
| c: In-transit metastasis/satellite(s) without metastatic nodes | ||
| N3 | Four or more, or matted nodes, or in-transite metastasis/satellite(s)f with metastatic nodes | |
| M CLASSIFICATION | SITE OF DISTANT METASTASIS | SERUM LACTATE DEHYDROGENASE |
| M0 | No distant metastasis | Normal |
| M1a | Distant skin, subcutaneous, or nodal metastasis | Normal |
| M1b | Lung metastasis | Normal |
| M1c | All other visceral metastases | Normal |
| Any distant metastasis | Elevated | |
| CLINICAL STAGE GROUPING | TNM CLASSIFICATION | FIVE-YEAR SURVIVAL RATEg |
| Stage IA | T1a N0 M0 | 95% |
| Stage IB | T1b N0 M0 | 91% |
| T2a N0 M0 | 89% | |
| Stage IIA | T2b N0 M0 | 77% |
| T3a N0 M0 | 79% | |
| Stage IIB | T3b N0 M0 | 63% |
| T4a N0 M0 | 67% | |
| Stage IIC | T4b N0 M0 | 45% |
| Stage III | Any T N1 M0 | 29%-70% |
| Any T N2 M0 | 24%-63% | |
| Any T N3 M0 | 27% | |
| Stage IV | Any M1 | 10%-19% |
aBased on Greene FL, Page DL, Fleming ID et al, editors: AJCC cancer staging manual, ed 6, New York, 2002, Springer.
bBreslow’s depth is measured from the top of the granular cell layer.
cMicroscopic—clinically occult.
dMacroscopic—clinically apparent.
eIn-transit—intralymphatic metastases occurring within 2 cm of the primary tumor.
fSatellite metastasis—intralymphatic metastases occurring >2 cm from the primary tumor but before the first echelon of regional lymph nodes.
gSurvival data from Balch CM, Buzaid AC, Soong SJ et al: Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma, J Clin Oncol 19:3635-3648, 2001.
Although depth of invasion and presence of ulceration are correlated closely with patient outcome in cutaneous melanomas, such a close association has not been found in mucosal melanomas. In general, there is a marked deterioration in prognosis among patients with oral mucosal melanomas exceeding a depth of 0.5 mm.
Surgical excision is the mainstay of treatment, although the extent of the excision is somewhat controversial. Older literature suggests that surgical margins of 3 to 5 cm around the tumor are necessary to achieve control, regardless of the site of the lesion. More recent studies indicate that a 1-cm margin is adequate for small cutaneous tumors less than 2 mm in thickness. For larger, more deeply invasive tumors, wide surgical excision still is recommended.
Lymph node dissection is usually performed on patients with clinically evident regional metastasis in the absence of distant metastasis. Elective lymph node dissection for patients with intermediate thickness (1 to 4 mm) lesions in the absence of clinically palpable nodes is somewhat controversial. The rationale for this procedure is that these patients have a high probability of occult regional nodal disease and low probability of distant metastasis. However, the reported survival benefit of this treatment strategy is variable, and significant morbidity can be associated with lymph node dissection. To address this problem, sentinel-node biopsy (biopsy of the first lymph node in the lymphatic basin to receive drainage from the tumor) often is used as an alternative to elective lymph node dissection to identify patients with occult nodal metastases who would benefit from total lymphadenectomy.
Although melanomas traditionally are considered to be radioresistant, several clinical studies have demonstrated that radiation may be of some benefit as adjunctive—or less commonly primary—therapy for certain subsets of patients; newer radiotherapy techniques, such as hypofractionation and neutron beam therapy, may play a greater role in the future. In addition, adjunct chemotherapy and immunotherapy are showing promise. With advances in the understanding of the molecular pathogenesis of melanoma, rationally designed targeted therapies (e.g., tyrosine kinase inhibitors, farnesyltransferase inhibitors, Pl3K pathway inhibitors) are being developed as well.
The cutaneous melanoma that is detected early (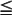 1.0-mm thick with or without ulceration, 1.01- to 2.0-mm thick without ulceration) and removed before metastasis has developed (stages IA and IB) is associated with an 89% to 95% 5-year survival rate. Cutaneous melanoma that is thicker (1.01 to 2.0 mm with ulceration, 2.01 to >4 mm with or without ulceration) but has not yet metastasized at the time of diagnosis (stages IIA to IIC) is associated with a 45% to 77% 5-year survival rate. If regional lymph node metastasis is present at the time of diagnosis (stages IIIA to IIIC), then 5-year survival rates in the range of 24% to 70% can be expected. The prognosis for patients with disseminated disease present at the time of diagnosis (stage IV) is dismal, with a 5-year survival rate of only 7% to 19%. Overall, the 5-year survival rate for cutaneous melanoma is 92%, and the 10-year survival rate is 79%. Current survival is much improved over past decades, primarily as a result of public education. Currently, the clinical features of cutaneous melanoma are so widely known that many lesions are discovered and treated at an early stage.
1.0-mm thick with or without ulceration, 1.01- to 2.0-mm thick without ulceration) and removed before metastasis has developed (stages IA and IB) is associated with an 89% to 95% 5-year survival rate. Cutaneous melanoma that is thicker (1.01 to 2.0 mm with ulceration, 2.01 to >4 mm with or without ulceration) but has not yet metastasized at the time of diagnosis (stages IIA to IIC) is associated with a 45% to 77% 5-year survival rate. If regional lymph node metastasis is present at the time of diagnosis (stages IIIA to IIIC), then 5-year survival rates in the range of 24% to 70% can be expected. The prognosis for patients with disseminated disease present at the time of diagnosis (stage IV) is dismal, with a 5-year survival rate of only 7% to 19%. Overall, the 5-year survival rate for cutaneous melanoma is 92%, and the 10-year survival rate is 79%. Current survival is much improved over past decades, primarily as a result of public education. Currently, the clinical features of cutaneous melanoma are so widely known that many lesions are discovered and treated at an early stage.
Other factors may influence the outcome of the disease besides the depth of invasion. For reasons that are unclear, melanomas affecting certain cutaneous sites seem to carry a worse prognosis than those at other sites with a similar depth of invasion. The areas with a worse prognosis are designated BANS (interscapular area of the Back, posterior upper Arm, posterior and lateral Neck, and Scalp). In addition, the prognosis is better for patients younger than 50 years of age and for women. Follow-up of patients treated for melanoma is important not only to monitor for metastatic disease but also because, in 3% to 5% of these patients, a second primary melanoma will eventually develop.
The prognosis for oral melanoma is extremely poor. Although 5-year survival rates as high as 45% have been reported, in the majority of studies, 5-year survival rates are in the range of only 13% to 22%. The poor prognosis for oral melanoma appears to be related to difficulty in achieving wide resection and a tendency for early hematogenous metastasis. Younger patients have a better survival than older ones, and patients with nonpigmented or amelanotic lesions appear to have a particularly poor prognosis. Patients usually die from distant metastasis rather than from the lack of local control. Radical surgical removal is the treatment of choice; hemimaxillectomy is done for unilateral lesions that invade the overlying maxillary bone.
BIBLIOGRAPHY
Abbey, LM, Page, DG, Sawyer, DR. The clinical and histopathologic features of a series of 464 oral squamous cell papillomas. Oral Surg Oral Med Oral Pathol. 1980;49:419–428.
Bouquot, JE, Wrobleski, GJ. Papillary (pebbled) masses of the oral mucosa, so much more than simple papillomas. Pract Periodontics Aesth Dent. 1996;8:533–543.
Carr, J, Gyorfi, T. Human papillomavirus. Epidemiology, transmission, and pathogenesis. Clin Lab Med. 2000;20:235–255.
Freed, GL, Derkay, CS. Prevention of recurrent respiratory papillomatosis: role of HPV vaccination. Int J Pediatr Otorhinolaryngol. 2006;70:1799–1803.
Kimberlin, DW, Malis, DJ. Juvenile onset recurrent respiratory papillomatosis: possibilities for successful antiviral therapy. Antiviral Res. 2000;45:83–93.
Lee, HJ, Smith, RJ. Recurrent respiratory papillomatosis: pathogenesis to treatment. Curr Opin Otolaryngol Head Neck Surg. 2005;13:354–359.
Stamataki, S, Nikolopoulos, TP, Korres, S, et al. Juvenile recurrent respiratory papillomatosis: still a mystery disease with difficult management. Head Neck. 2007;29:155–162.
Summersgill, KF, Smith, EM, Levy, BT, et al. Human papillomavirus in the oral cavities of children and adolescents. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:62–69.
Syrjänen, S, Puranen, M. Human papillomavirus infections in children: the potential role of maternal transmission. Crit Rev Oral Biol Med. 2000;11:259–274.
Terai, M, Hashimoto, K, Yoda, K, et al. High prevalence of human papillomaviruses in the normal oral cavity of adults. Oral Microbiol Immunol. 1999;14:201–205.
Ward, KA, Napier, SS, Winter, PC, et al. Detection of human papilloma-virus DNA-sequences in oral squamous-cell papillomas by the polymerase chain-reaction. Oral Surg Oral Med Oral Pathol. 1995;80:63–66.
Bacelieri, R, Johnson, SM. Cutaneous warts: an evidence-based approach to therapy. Am Fam Physician. 2005;72:647–652.
Fazel, N, Wilczynski, S, Lowe, L, et al. Clinical, histopathologic, and molecular aspects of cutaneous human papillomavirus infections. Dermatol Clin. 1999;17:521–536.
Gibbs, S, Harvey, I. Topical treatments for cutaneous warts. Cochrane Database Syst Rev. 2006;3:CD001781.
Green, TL, Eversole, LR, Leider, AS. Oral and labial verruca vulgaris: clinical, histologic, and immunohistochemical evaluation. Oral Surg Oral Med Oral Pathol. 1986;62:410–416.
Kuykendall-Ivy, TD, Johnson, SM. Evidence-based review of management of nongenital cutaneous warts. Cutis. 2003;71:213–222.
Lipke, MM. An armamentarium of wart treatments. Clin Med Res. 2006;4:273–293.
Micali, G, Dall’Oglio, F, Nasca, M, et al. Management of cutaneous warts: an evidence-based approach. Am J Clin Dermatol. 2004;5:311–317.
Plunkett, A, Merlin, K, Gill, D, et al. The frequency of common nonmalignant skin conditions in adults in central Victoria, Australia. Int J Dermatol. 1999;38:901–908.
Terai, M, Takagi, M, Matsukura, T, et al. Oral wart associated with human papillomavirus type 2. J Oral Pathol Med. 1999;28:137–140.
Anderson, KM, Perez-Montiel, D, Miles, L, et al. The histologic differentiation of oral condyloma acuminatum from its mimics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:420–428.
Baccaglini, L, Atkinson, JC, Paton, LL, et al. Management of oral lesions in HIV-positive patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(suppl 1):S50.e1–S50.e23.
Brodell, LA, Mercurio, MG, Brodell, RT. The diagnosis and treatment of human papillomavirus-mediated genital lesions. Cutis. 2007;79(4 suppl):5–10.
Brown, DR, Schroeder, JM, Bryan, JT, et al. Detection of multiple papillomavirus types in condylomata acuminata lesions from otherwise healthy and immunosuppressed patients. J Clin Microbiol. 1999;37:3316–3322.
Giraldo, P, Gonçalves, AKS, Pereira, SAS, et al. Human papillomavirus in the oral mucosa of women with genital human papillomavirus lesions. Eur J Obstet Gynecol Reprod Biol. 2006;126:104–106.
Gloster, HM, Roenigk, RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436–441.
Henley, JD, Summerlin, DJ, Tomich, CE. Condyloma acuminatum and condyloma-like lesions of the oral cavity: a study of 11 cases with an intraductal component. Histopathology. 2004;44:216–221.
Kodner, CM, Nasraty, S. Management of genital warts. Am Fam Physician. 2004;70:2335–2342. [2345-2346].
Scheinfeld, N, Lehman, DS. An evidence-based review of medical and surgical treatments of genital warts. Dermatol Online J. 2006;12:5.
Silverman, S, Jr., Migliorati, CA, Lozada-Nur, F, et al. Oral findings in people with or at high risk for AIDS: a study of 375 homosexual males. J Am Dent Assoc. 1986;112:187–192.
Simon, PA. Oral condyloma acuminatum as an indicator of sexual abuse: dentistry’s role. Quintessence Int. 1998;29:455–458.
Suskind, DL, Mirza, N, Rosin, D, et al. Condyloma acuminatum presenting as a base-of-tongue mass. Otolaryngol Head Neck Surg. 1996;114:487–490.
Zunt, SL, Tomich, CE. Oral condyloma acuminatum. J Dermatol Surg Oncol. 1989;15:591–594.
Multifocal Epithelial Hyperplasia
Aykol, A, Anadolu, R, Anadolu, Y, et al. Multifocal papillomavirus epithelial hyperplasia: successful treatment with CO2 laser therapy combined with interferon alpha-2b. Int J Dermatol. 2003;42:733–735.
Benevides dos Santos, PJ, Borborema dos Santos, CM, Mendonça, RR, et al. Human papillomavirus type 13 infecting the conjunctiva. Diagn Microbiol Infect Dis. 2005;53:71–73.
Borborema-Santos, CM, de Castro, MM, Benevides dos Santos, PJ, et al. Oral focal epithelial hyperplasia: report of five cases. Braz Dent J. 2006;17:79–82.
Carlos, R, Sedano, HO. Multifocal papilloma virus epithelial hyperplasia. Oral Surg Oral Med Oral Pathol. 1994;77:631–635.
Cuberos, V, Perez, J, Lopez, CJ, et al. Molecular and serological evidence of the epidemiological association of HPV 13 with focal epithelial hyperplasia: a case-control study. J Clin Virol. 2006;37:21–26.
Garcia-Corona, C, Vega-Memije, E, Mosqueda-Taylor, A, et al. Association of HLA-DR4 (DRB1*0404) with human papillomavirus infection in patients with focal epithelial hyperplasia. Arch Dermatol. 2004;140:1227–1231.
González, LV, Gaviria, AM, Sanclemente, G, et al. Clinical, histopathological and virological findings in patients with focal epithelial hyperplasia from Colombia. Int J Dermatol. 2005;44:274–279.
Harris, AM, van Wyk, CW. Heck’s disease (focal epithelial hyperplasia): a longitudinal study. Community Dent Oral Epidemiol. 1993;21:82–85.
Ledesma-Montes, C, Vega-Memije, E, Garcés-Ortíz, M, et al. Multifocal epithelial hyperplasia. Report of nine cases. Med Oral Patol Oral Cir Bucal. 2005;10:394–401.
Steinhoff, M, Metze, D, Stockfleth, E, et al. Successful topical treatment of focal epithelial hyperplasia (Heck’s disease) with interferon-b. Br J Dermatol. 2001;144:1067–1069.
Tan, AK, Tewfik, TL, Moroz, B, et al. Focal epithelial hyperplasia. Otolaryngol Head Neck Surg. 1995;112:316–320.
Viraben, R, Aquilina, C, Brousset, P, et al. Focal epithelial hyperplasia (Heck disease) associated with AIDS. Dermatology. 1996;193:261–262.
Witkop, CJ, Jr., Niswander, JD. Focal epithelial hyperplasia in Central and South American Indians and Latinos. Oral Surg Oral Med Oral Pathol. 1965;20:213–217.
Batsakis, JG, Suarez, P. Schneiderian papillomas and carcinomas: a review. Adv Anat Pathol. 2001;8:53–64.
Bawa, R, Allen, GC, Ramadan, HH. Cylindrical cell papilloma of the nasal septum. Ear Nose Throat J. 1995;74:179–181.
Busquets, JM, Hwang, PH. Endoscopic resection of sinonasal inverted papilloma: a meta-analysis. Otolaryngol Head Neck Surg. 2006;134:476–482.
Califano, J, Koch, W, Sidransky, D, et al. Inverted sinonasal papilloma: a molecular genetic appraisal of its putative status as a precursor to squamous cell carcinoma. Am J Pathol. 2000;156:333–337.
Eggers, G, Mühling, J, Hassfeld, S. Inverted papilloma of paranasal sinuses. J Craniomaxillofac Surg. 2007;35:21–29.
Holzmann, D, Hegyi, I, Rajan, GP, et al. Management of benign inverted sinonasal papilloma avoiding external approaches. J Laryngol Otol. 2007;121:548–554.
Hyams, VJ. Papillomas of the nasal cavity and paranasal sinuses: a clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol. 1971;80:192–206.
Kaufman, MR, Brandwein, MS, William, L. Sinonasal papillomas: clinicopathologic review of 40 patients with inverted and oncocytic Schneiderian papillomas. Laryngoscope. 2002;112:1372–1377.
Lane, AP, Bolger, WE. Endoscopic management of inverted papilloma. Curr Opin Otolaryngol Head Neck Surg. 2006;14:14–18.
Lawson, W, Kaufman, MR, Biller, HF. Treatment outcomes in the management of inverted papilloma: an analysis of 160 cases. Laryngoscope. 2003;113:1548–1556.
Maitra, A, Baskin, LB, Lee, EL. Malignancies arising in oncocytic Schneiderian papillomas: a report of 2 cases and review of the literature. Arch Pathol Lab Med. 2001;125:1365–1367.
Mansell, NJ, Bates, GJ. The inverted Schneiderian papilloma: a review and literature report of 43 new cases. Rhinology. 2000;38:97–101.
Melroy, CT, Senior, BA. Benign sinonasal neoplasms: a focus on inverting papilloma. Otolaryngol Clin North Am. 2006;39:601–617.
Minovi, A, Kollert, M, Draf, W, et al. Inverted papilloma: feasibility of endonasal surgery and long-term results of 87 cases. Rhinology. 2006;44:205–210.
Mirza, S, Bradley, PJ, Acharya, A, et al. Sinonasal inverted papillomas: recurrence, and synchronous and metachronous malignancy. J Laryngol Otol. 2007;1:1–8.
Pasquini, E, Sciaretta, V, Farneti, G, et al. Inverted papilloma: report of 89 cases. Am J Otolaryngol. 2005;25:178–185.
Sauter, A, Matharu, R, Hörmann, K, et al. Current advances in the basic research and clinical management of sinonasal inverted papilloma (review). Oncol Rep. 2007;17:495–504.
Sulica, RL, Wenig, BM, Debo, RF, et al. Schneiderian papillomas of the pharynx. Ann Otol Rhinol Laryngol. 1999;108:392–397.
Weiner, JS, Sherris, D, Kasperbauer, J, et al. Relationship of human papillomavirus to Schneiderian papillomas. Laryngoscope. 1999;109:21–26.
Yang, YJ, Abraham, JL. Undifferentiated carcinoma arising in oncocytic Schneiderian (cylindrical cell) papilloma. J Oral Maxillofac Surg. 1997;55:289–294.
Yoskovitch, A, Braverman, I, Nachtigal, D, et al. J Sinonasal schneiderian papilloma. Otolaryngology. 1998;27:122–126.
Braue, A, Ross, G, Varigos, G, et al. Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatr Dermatol. 2005;22:287–294.
Brown, J, Janniger, CK, Schwartz, RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93–99.
Fornatora, ML, Reich, RF, Gray, RG, et al. Intraoral molluscum contagiosum: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:318–320.
Smith, KJ, Skelton, H. Molluscum contagiosum: recent advances in pathogenic mechanisms and new therapies. Am J Clin Dermatol. 2002;3:535–545.
Smith, KJ, Yeager, J, Skelton, H. Molluscum contagiosum: its clinical, histopathologic, and immunohistochemical spectrum. Int J Dermatol. 1999;38:664–672.
van der Wouden, JC, Menke, J, Gajadin, S, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2006;2:CD004767.
Watanabe, T, Nakamura, K, Wakugawa, M, et al. Antibodies to molluscum contagiosum virus in the general population and susceptible patients. Arch Dermatol. 2000;136:1518–1522.
Whitaker, SB, Wiegand, SE, Budnick, SD. Intraoral molluscum contagiosum. Oral Surg Oral Med Oral Pathol. 1991;72:334–336.
Allen, CM, Kapoor, N. Verruciform xanthoma in a bone marrow transplant recipient. Oral Surg Oral Med Oral Pathol. 1993;75:591–594.
Connolly, SB, Lewis, EJ, Lindholm, JS, et al. Management of cutaneous verruciform xanthoma. J Am Acad Dermatol. 2000;42:343–347.
Hu, H, Li, Y, Li, S. Verruciform xanthoma of the oral cavity: clinicopathological study relating to pathogenesis. APMIS. 2005;113:629–634.
Huang, JS, Tseng, CC, Jin, YT, et al. Verruciform xanthoma. Case report and literature review. J Periodontol. 1996;67:162–165.
Neville, B. The verruciform xanthoma: a review and report of eight new cases. Am J Dermatopathol. 1986;8:247–253.
Nowparast, B, Howell, FV, Rick, GM. Verruciform xanthoma: a clinicopathologic review and report of fifty-four cases. Oral Surg Oral Med Oral Pathol. 1981;51:619–625.
Oliveira, PT, Jaeger, RG, Cabral, LAG, et al. Verruciform xanthoma of the oral mucosa. Report of four cases and a review of the literature. Oral Oncol. 2001;37:326–331.
Philipsen, HP, Reichart, PA, Takata, T, et al. Verruciform xanthoma—biological profile of 282 oral lesions based on a literature survey with nine new cases from Japan. Oral Oncol. 2003;39:325–336.
Yu, CH, Tsai, TC, Wang, JT, et al. Oral verruciform xanthoma: a clinicopathologic study of 15 cases. J Formos Med Assoc. 2007;106:141–147.
Ellis, DL, Yates, RA. Sign of Leser-Trélat. Clin Dermatol. 1993;11:141–148.
Hafner, C, Hartmann, A, Vogt, T. FGFR3 mutations in epidermal nevi and seborrheic keratoses: lessons from urothelium and skin. J Invest Dermatol. 2007;127:1572–1573.
Izikson, L, Sober, AJ, Mihm, MC, et al. Prevalence of melanoma clinically resembling seborrheic keratosis: analysis of 9204 cases. Arch Dermatol. 2002;138:1562–1566.
Silver, SG, Ho, VCY. Seborrheic keratosis. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s dermatology in general medicine. ed 6. New York: McGraw-Hill; 2003:767–770.
Wade, TR, Ackerman, AB. The many faces of seborrheic keratoses. J Dermatol Surg Oncol. 1979;5:378–382.
Wilborn, WH, Dismukes, DE, Montes, IF. Seborrheic keratoses. J Cutan Pathol. 1978;5:373–375.
Daley, TD. Intraoral sebaceous hyperplasia: diagnostic criteria. Oral Surg Oral Med Oral Pathol. 1993;75:343–347.
Dent, CD, Hunter, WE, Svirsky, JA. Sebaceous gland hyperplasia: case report and literature review. J Oral Maxillofac Surg. 1995;53:936–938.
De Villez, RL, Roberts, LC. Premature sebaceous gland hyperplasia. J Am Acad Dermatol. 1982;6:933–935.
Graham-Brown, RAC, McGibbon, DH, Sarkany, I. A papular plaque-like eruption on the face due to naevoid sebaceous gland hyperplasia. Clin Exp Dermatol. 1983;8:379–382.
Richey, DF. Aminolevulinic acid photodynamic therapy for sebaceous gland hyperplasia. Dermatol Clin. 2007;25:59–65.
Bastiaens, M, Hoefnagel, J, Westendorp, R, et al. Solar lentigines are strongly related to sun exposure in contrast to ephelides. Pigment Cell Res. 2004;17:225–229.
Bastiaens, M, ter Huurne, J, Gruis, N, et al. The melanocortin-1-receptor gene is the major freckle gene. Human Mol Genet. 2001;10:1701–1708.
Bastiaens, MT, Westendorp, RG, Vermeer, BJ, et al. Ephelides are more related to pigmentary constitutional host factors than solar lentigines. Pigment Cell Res. 1999;12:316–322.
Bliss, JM, Ford, D, Swerdlow, AJ, et al. Risk of cutaneous melanoma associated with pigmentation characteristics and freckling: systematic overview of 10 case-control studies. Int J Cancer. 1995;62:367–372.
Andersen, WK, Labadie, RR, Bhawan, J. Histopathology of solar lentigines of the face: a quantitative study. J Am Acad Dermatol. 1997;36:444–447.
Beacham, BE. Solar-induced epidermal tumors in the elderly. Am Fam Physician. 1990;42:153–160.
Fleischer, AB, Jr., Schwartzel, EH, Colby, SI, et al. The combination of 2% 4-hydroxyanisole (Mequinol) and 0.01% tretinoin is effective in improving the appearance of solar lentigines and related hyperpigmented lesions in two double-blind multicenter clinical studies. J Am Acad Dermatol. 2004;42:459–467.
Holzle, E. Pigmented lesions as a sign of photodamage. Br J Dermatol. 1992;127:48–50.
Jarratt, M. Mequinol 2%/tretinoin 0.01% solution: an effective and safe alternative to hydroquinone 3% in the treatment of solar lentigines. Cutis. 2004;74:319–322.
Kang, S, Goldfarb, MT, Weiss, JS, et al. Assessment of adapalene gel for the treatment of actinic keratoses and lentigines: a randomized trial. J Am Acad Dermatol. 2003;49:83–90.
Monestier, S, Gaudy, C, Gouvernet, J, et al. Multiple senile lentigos of the face, a skin ageing pattern resulting from a life excess of intermittent sun exposure in dark-skinned Caucasians: a case-control study. Br J Dermatol. 2006;154:438–444.
Ortonne, JP, Pandya, AG, Lui, H, et al. Treatment of solar lentigines. J Am Acad Dermatol. 2006;54:S262–S271.
Rafal, ES, Griffiths, CE, Ditre, CM, et al. Topical tretinoin (retinoic acid) treatment for liver spots associated with photodamage. N Engl J Med. 1992;326:368–374.
Shimbashi, T, Kamide, R, Hashimoto, T. Long-term follow-up in treatment of solar lentigo and café-au-lait macules with Q-switch ruby laser. Aesthetic Plast Surg. 1997;21:445–448.
Buchner, A, Merrell, PW, Hansen, LS, et al. Melanocytic hyperplasia of the oral mucosa. Oral Surg Oral Med Oral Pathol. 1991;71:58–62.
Coleman, WP, 3rd., Gately, LE, 3rd., Krementz, AB, et al. Nevi, lentigines and melanomas in blacks. Arch Dermatol. 1980;116:548–551.
Gorlin, RJ, Andersen, RC, Blaw, M. Multiple lentigines syndrome. Am J Dis Child. 1969;117:652–662.
Grichnik, JM, Rhodes, AR, Sober, AJ. Lentigo simplex. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s dermatology in general medicine. ed 6. New York: McGraw-Hill; 2003:881–882.
Aloi, F, Solaroli, C, Giovannini, E. Actinic lichen planus simulating melasma. Dermatology. 1997;195:69–70.
Gupta, AK, Gover, MD, Nouri, K, et al. The treatment of melasma: a review of clinical trials. J Am Acad Dermatol. 2006;55:1048–1065.
Kauh, YC, Zachian, TF. Melasma. Adv Exp Med Biol. 1999;455:491–499.
Lim, JT. Treatment of melasma using kojic acid in a gel containing hydroquinone and glycolic acid. Dermatol Surg. 1999;25:282–284.
Rendon, M, Berneburg, M, Arellano, I, et al. Treatment of melasma. J Am Acad Dermatol. 2006;54:S272–S281.
Sanchez, NP, Pathuk, MA, Sato, S, et al. Melasma: a clinical, light microscopic, ultrastructural and immunofluorescence study. J Am Acad Dermatol. 1981;4:698–703.
Victor, FC, Gelber, J, Rao, B. Melasma: a review. J Cutan Med Surg. 2004;8:97–102.
Axéll, T. A prevalence study of oral mucosal lesions in an adult Swedish population. Odontol Revy. 1976;27:1–103.
Bouquot, JE. Common oral lesions found during a mass screening examination. J Am Dent Assoc. 1986;112:50–57.
Bouquot, JE, Nikai, H. Lesions of the oral cavity. In: Gnepp DR, ed. Diagnostic surgical pathology of the head and neck. Philadelphia: WB Saunders; 2001:141–238.
Buchner, A, Hansen, LS. Melanotic macule of the oral mucosa: a clinicopathologic study of 105 cases. Oral Surg Oral Med Oral Pathol. 1979;48:244–249.
Buchner, A, Merrell, PW, Carpenter, WM. Relative frequency of solitary melanocytic lesions of the oral mucosa. J Oral Pathol Med. 2004;33:550–557.
Dohil, MA, Billman, G, Pransky, S, et al. The congenital lingual melanotic macule. Arch Dermatol. 2003;139:767–770.
Kaugars, GE, Heise, AP, Riley, WT, et al. Oral melanotic macules: a review of 353 cases. Oral Surg Oral Med Oral Pathol. 1993;76:59–61.
Weathers, DR, Corio, RL, Crawford, BE, et al. The labial melanotic macule. Oral Surg Oral Med Oral Pathol. 1976;42:196–205.
Yeh, CJ. Simple cryosurgical treatment of the oral melanotic macule. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:12–13.
Andrews, BT, Trask, DK. Oral melanoacanthoma: a case report, a review of the literature, and a new treatment option. Ann Otol Rhinol Laryngol. 2005;114:677–680.
Chandler, K, Chaudhry, Z, Kumar, N, et al. Melanoacanthoma: a rare cause of oral hyperpigmentation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:492–494.
Contreras, E, Carlos, R. Oral melanoacanthosis (melanoachantoma): report of a case and review of the literature. Med Oral Patol Oral Cir Bucal. 2005;10:9–12.
Fatahzadeh, M, Sirois, DA. Multiple intraoral melanoacanthomas: a case report with unusual findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:54–56.
Fornatora, ML, Reich, RF, Haber, S, et al. Oral melanoacanthoma: a report of 10 cases, review of literature, and immunohistochemical analysis of HMB-45 reactivity. Am J Dermatopathol. 2003;25:12–15.
Heine, BT, Drummond, JF, Damm, DD, et al. Bilateral oral melanoacanthoma. Gen Dent. 1996;44:451–452.
Landwehr, DJ, Halkins, LE, Allen, CM. A rapidly growing pigmented plaque. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:332–334.
Tomich, CE, Zunt, SL. Melanoacanthosis (melanoacanthoma) of the oral mucosa. J Dermatol Surg Oncol. 1990;16:231–236.
Allen, CM, Pellegrini, A. Probable congenital melanocytic nevus of the oral mucosa: case report. Pediatr Dermatol. 1995;12:145–148.
Barnhill, RL. The Spitzoid lesion: the importance of atypical variants and risk assessment. Am J Dermatopathol. 2006;28:75–83.
Bett, BJ. Large or multiple congenital melanocytic nevi: occurrence of cutaneous melanoma in 1008 persons. J Am Acad Dermatol. 2005;52:793–797.
Brener, MD, Harrison, BD. Intraoral blue nevus: report of a case. Oral Surg Oral Med Oral Pathol. 1969;28:326–330.
Buchner, A, Hansen, LS. Pigmented nevi of the oral mucosa: a clinicopathologic study of 36 new cases and review of 155 cases from the literature. Part I: a clinicopathologic study of 36 new cases. Oral Surg Oral Med Oral Pathol. 1987;63:566–572.
Buchner, A, Hansen, LS. Pigmented nevi of the oral mucosa: a clinicopathologic study of 36 new cases and review of 155 cases from the literature. Part II: analysis of 191 cases. Oral Surg Oral Med Oral Pathol. 1987;63:676–682.
Buchner, A, Leider, AS, Merrell, PW, et al. Melanocytic nevi of oral mucosa: a clinicopathologic study of 130 cases from northern California. J Oral Pathol Med. 1990;19:197–201.
Casso, EM, Grin-Jorgensen, CM, Grant-Kels, JM. Spitz nevi. J Am Acad Dermatol. 1992;27:901–913.
Chuang, WY, Hao, SP, Yeh, CJ, et al. Blue nevi of the sinonasal mucosa: a report of two cases and review of the literature. Laryngoscope. 2007;117:371–372.
Dorji, T, Cavazza, A, Nappi, O, et al. Spitz nevus of the tongue with pseudoepitheliomatous hyperplasia: report of three cases of a pseudomalignant condition. Am J Surg Pathol. 2002;26:774–777.
Fistarol, SK, Itin, PH. Plaque-type blue nevus of the oral cavity. Dermatology. 2005;211:224–233.
Flaitz, CM, McCandless, G. Palatal blue nevus in a child. Pediatr Dent. 2001;23:354–355.
Frank, SB, Cohen, HJ. The halo nevus. Arch Dermatol. 1964;89:367–373.
Hale, EK, Stein, J, Ben-Porat, L, et al. Association of melanoma and neurocutaneous melanocytosis with large congenital melanocytic naevi—results from the NYU-LCMN registry. Br J Dermatol. 2005;152:512–517.
Laskaris, G, Kittas, C, Triantafyllou, A. Unpigmented intramucosal nevus of palate. An unusual clinical presentation. Int J Oral Maxillofac Surg. 1994;23:39–40.
LeBoit, P. Spitz nevus: a look back and a look ahead. Adv Dermatol. 2000;16:81–109.
Mader, CL, Konzelman, JL. Intraoral blue nevus—a review. Cutis. 1979;24:165–166.
Marghoob, AA, Agero, ALC, Benvenuto-Andrade, C, et al. Large congenital melanocytic nevi, risk of cutaneous melanoma, and prophylactic surgery. J Am Acad Dermatol. 2006;54:868–870.
Meleti, M, Mooi, WJ, Casparie, MK, et al. Melanocytic nevi of the oral mucosa—no evidence of an increased risk for oral malignant melanoma: an analysis of 119 cases. Oral Oncol. 2007;43:976–981.
Mones, JM, Ackerman, AB. “Atypical” Spitz’s nevus, “malignant” Spitz’s nevus, and “metastasizing” Spitz’s nevus: a critique in historical perspective of three concepts and flawed fatally. Am J Dermatopathol. 2004;26:310–333.
Mooi, WJ. Spitz nevus and its histologic simulators. Adv Anat Pathol. 2002;9:209–221.
Nikia, H, Miyauchi, M, Ogawa, I, et al. Spitz nevus of the palate. Report of a case. Oral Surg Oral Med Oral Pathol. 1990;69:603–608.
Pinto, A, Raghavendra, S, Lee, R, et al. Epithelioid blue nevus of the oral mucosa: a rare histologic variant. Oral Surg Oral Med Oral Pathol. 2003;96:429–436.
Sulit, DJ, Guardiano, RA, Krivda, S. Classic and atypical Spitz nevi: review of the literature. Cutis. 2007;79:141–146.
Takata, M, Saida, T. Genetic alterations in melanocytic tumors. J Dermatol Sci. 2006;43:1–10.
Weedon, D, Little, JH. Spindle and epithelioid cell nevi in children and adults: a review of 211 cases of the Spitz nevus. Cancer. 1977;40:217–225.
Zembowicz, A, Mihm, MC. Dermal dendritic melanocytic proliferations: an update. Histopathology. 2004;45:433–451.
Alexander, RE, Wright, JM, Thiebaud, S. Evaluating, documenting and following up oral pathological conditions. A suggested protocol. J Am Dent Assoc. 2001;132:329–335.
Bagán, JV, Jimenez, Y, Sanchis, JM. Proliferative verrucous leukoplakia: high incidence of gingival squamous cell carcinoma. J Oral Pathol Med. 2003;32:379–382.
Bagán, JV, Murillo, J, Poveda, R, et al. Proliferative verrucous leukoplakia: unusual locations of oral squamous cell carcinomas, and field cancerization as shown by the appearance of multiple OSCCs. Oral Oncol. 2004;40:440–443.
Bánóczy, J. Follow-up studies in oral leukoplakia. J Maxillofac Surg. 1977;5:69–75.
Barrett, AW, Kingsmill, VJ, Speight, PM. The frequency of fungal infection in biopsies of oral mucosal lesions. Oral Dis. 1998;4:26–31.
Bernstein, ML. Oral mucosal white lesions associated with excessive use of Listerine mouthwash: report of two cases. Oral Surg Oral Med Oral Pathol. 1978;46:781–785.
Bouquot, JE, Gorlin, RJ. Leukoplakia, lichen planus and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surg Oral Med Oral Pathol. 1986;61:373–381.
Bouquot, J, Kurland, L, Weiland, L. Leukoplakia of the head and neck: characteristics of 568 lesions with 6,720 person-years of follow-up. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:202.
Bouquot, JE, Weiland, LH, Kurland, LT. Leukoplakia and carcinoma in situ synchronously associated with invasive oral/oropharyngeal carcinoma in Rochester, Minnesota, 1935-1984. Oral Surg Oral Med Oral Pathol. 1988;65:199–207.
Bouquot, JE, Whitaker, SB. Oral leukoplakia—rationale for diagnosis and prognosis of its clinical subtypes or “phases,”. Quintessence Int. 1994;25:133–140.
Brennan, M, Migliorati, CA, Lockhart, PB, et al. Management of oral epithelial dysplasia: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(suppl 1):S19.e1–S19.e12.
Cabay, RJ, Morton, TH, Epstein, JB. Proliferative verrucous leukoplakia and its progression to oral carcinoma: a review of the literature. J Oral Pathol Med. 2007;36:255–261.
Chi, AC, Lambert, PR, 3rd., Pan, Y, et al. Is alveolar ridge keratosis a true leukoplakia? A clinicopathologic comparison of 2,153 lesions. J Am Dent Assoc. 2007;138:641–651.
Cowan, CG, Gregg, TA, Napier, SS, et al. Potentially malignant oral lesions in Northern Ireland: a 20-year population-based perspective of malignant transformation. Oral Dis. 2001;7:18–24.
Crissman, JD, Zarbo, RJ. Dysplasia, in situ carcinoma, and progression to invasive squamous cell carcinoma of the upper aerodigestive tract. Am J Surg Pathol. 1989;13(suppl 1):5–16.
Daley, TD, Lovas, JG, Peters, E, et al. Salivary duct involvement in oral epithelial dysplasia and squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:186–192.
Damm, DD, Curran, A, White, DK, et al. Leukoplakia of the maxillary vestibule—an association with Viadent? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:61–66.
Eversole, LR, Eversole, GM, Kopcik, J. Sanguinaria-associated oral leukoplakia: comparison with other benign and dysplastic leukoplakic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:455–464.
Fettig, A, Pogrel, MA, Silverman, S, Jr., et al. Proliferative verrucous leukoplakia of the gingiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:723–730.
Holmstrup, P, Vedtofte, P, Reibel, J, et al. Long-term treatment outcome of oral premalignant lesions. Oral Oncol. 2006;42:461–474.
Holmstrup, P, Vedtofte, P, Reibel, J, et al. Oral premalignant lesions: is a biopsy reliable? J Oral Pathol Med. 2007;36:262–266.
Hsue, SS, Wang, WC, Chen, CH, et al. Malignant transformation in 1458 patients with potentially malignant oral mucosal disorders: a follow-up study based in a Taiwanese hospital. J Oral Pathol Med. 2007;36:25–29.
Kaugars, GE, Burns, JC, Gunsolley, JC. Epithelial dysplasia of the oral cavity and lips. Cancer. 1988;62:2166–2170.
King, GN, Healy, CM, Glover, MT, et al. Increased prevalence of dysplastic and malignant lip lesions in renal-transplant recipients. N Eng J Med. 1995;332(16):1052–1057.
Lee, JJ, Hong, WK, Hittelman, WN, et al. Predicting cancer development in oral leukoplakia: ten years of translational research. Clin Cancer Res. 2000;6:1702–1710.
Lee, JJ, Hung, CH, Cheng, SJ, et al. Carcinoma and dysplasia in oral leukoplakias in Taiwan: prevalence and risk factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;110:472–480.
Lodi, G, Sardella, A, Bez, C, et al. Systematic review of randomized trials for the treatment of oral leukoplakia. J Dent Educ. 2002;66:896–902.
Lodi, G, Sardella, A, Bez, C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006;4:CD001829.
Martin, IC, Kerawala, CJ, Reed, M. The application of toluidine blue as a diagnostic adjunct in the detection of epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:444–446.
Mascarenhas, AK, Allen, CM, Moeschberger, ML. The association between Viadent use and oral leukoplakia. Epidemiology. 2001;12:741–743.
Mascarenhas, AK, Allen, CM, Moeschberger, ML. The association between Viadent use and oral leukoplakia—results of a matched case-control study. J Public Health Dent. 2002;62:158–162.
Mithani, SK, Mydlarz, WK, Grumbine, FL, et al. Molecular genetics of premalignant oral lesions. Oral Dis. 2007;13:126–133.
Nielson, H, Norrild, B, Vedtofte, P, et al. Human papillomavirus in oral premalignant lesions. Eur J Cancer B Oral Oncol. 1996;32B:264–270.
Napier, SS, Cowan, CG, Gregg, TA, et al. Potentially malignant oral lesions in Northern Ireland: size (extent) matters. Oral Dis. 2003;9:129–137.
Pandey, M, Thomas, G, Somanathan, T, et al. Evaluation of surgical excision of non-homogeneous oral leukoplakia in a screening intervention trial, Kerala, India. Oral Oncol. 2001;37:103–109.
Petti, S. Pooled estimate of world leukoplakia prevalence: a systematic review. Oral Oncol. 2003;39:770–780.
Pindborg, JJ, Daftary, DK, Mehta, FS. A follow-up study of sixty-one oral dysplastic precancerous lesions in Indian villagers. Oral Surg Oral Med Oral Pathol. 1977;43:383–390.
Reibel, J. Prognosis of oral pre-malignant lesions: significance of clinical, histopathological, and molecular biological characteristics. Crit Rev Oral Biol Med. 2003;14:47–62.
Roed-Petersen, B. Effect on oral leukoplakia of reducing or ceasing tobacco smoking. Acta Derm Venereol. 1982;62:164–167.
Rosin, MP, Cheng, X, Poh, C, et al. Use of allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin Cancer Res. 2000;6:357–362.
Sankaranarayanan, R, Mathew, B, Varghese, C, et al. Chemoprevention of oral leukoplakia with vitamin A and beta carotene: an assessment. Oral Oncol. 1997;33:231–236.
Scheifele, C, Reichart, PA, Dietrich, T. Low prevalence of oral leukoplakia in a representative sample of the US population. Oral Oncol. 2003;39:619–625.
Schoelch, ML, Sekandari, N, Regezi, JA, et al. Laser management of oral leukoplakias: a follow-up study of 70 patients. Laryngoscope. 1999;109:949–953.
Sciubba, JJ. Oral leukoplakia. Crit Rev Oral Biol Med. 1995;6:147–160.
Shepman, KP, van der Meij, EH, Smeele, LE, et al. Malignant transformation of oral leukoplakia: a follow-up study of a hospital-based population of 166 patients with oral leukoplakia from The Netherlands. Oral Oncol. 1998;34:270–275.
Shiu, MN, Chen, TH, Chang, SH, et al. Risk factors for leukoplakia and malignant transformation to oral carcinoma: a leukoplakia cohort in Taiwan. Br J Cancer. 2000;82:1871–1874.
Silverman, S, Jr., Gorsky, M. Proliferative verrucous leukoplakia: a follow-up study of 54 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:154–157.
Silverman, S, Jr., Gorsky, M, Lozada, F. Oral leukoplakia and malignant transformation: a follow-up study of 257 patients. Cancer. 1984;53:563–568.
Speight, PM, Farthing, PM, Bouquot, JE. The pathology of oral cancer and precancer. Curr Diag Pathol. 1997;3:165–176.
Van der Waal, I, Axéll, T. Oral leukoplakia: a proposal for uniform reporting. Oral Oncol. 2002;38:521–526.
Van der Waal, I, Shepman, KP, van der Meij, EH, et al. Oral leukoplakia: a clinicopathologic review. Oral Oncol. 1998;33:291–301.
Waldron, CA, Shafer, WG. Leukoplakia revisited: a clinicopathologic study of 3256 oral leukoplakias. Cancer. 1975;36:1386–1392.
Zakrzewska, JM, Lopes, V, Speight, P, et al. Proliferative verrucous leukoplakia: a report of ten cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:396–401.
Zhang, L, Rosin, MP. Loss of heterozygosity: a potential tool in management of oral premalignant lesions? J Oral Pathol Med. 2001;30:513–520.
Amagasa, T, Yokoo, E, Sata, K, et al. A study of the clinical characteristics and treatment of oral carcinoma in situ. Oral Surg Oral Med Oral Pathol. 1985;60:50–55.
Bouquot, JE, Gnepp, DR. Epidemiology of carcinoma in situ of the upper aerodigestive tract. Cancer. 1988;61:1685–1690.
Bouquot, JE, Ephros, H. Erythroplakia: the dangerous red mucosa. Pract Periodontics Aesth Dent. 1995;7:59–68.
Bouquot, JE, Kurland, LT, Weiland, LH. Carcinoma in situ of the upper aerodigestive tract: incidence, time trends, and follow-up in Rochester, Minnesota, 1935-1984. Cancer. 1988;61:1691–1698.
Hashibe, M, Mathew, B, Kuruvilla, B, et al. Chewing tobacco, alcohol and the risk of erythroplakia. Cancer Epidemiol Biomarkers Prev. 2000;9:639–645.
Lumerman, H, Freedman, P, Kerpel, S. Oral epithelial dysplasia and the development of invasive squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:321–329.
Mincer, HH, Coleman, SA, Hopkins, KP. Observations on the clinical characteristics of oral lesions showing histologic epithelial dysplasia. Oral Surg Oral Med Oral Pathol. 1972;33:389–399.
Reichart, PA, Philipsen, HP. Oral erythroplakia—a review. Oral Oncol. 2005;41:551–561.
Shafer, WG, Waldron, CA. Erythroplakia of the oral cavity. Cancer. 1975;36:1021–1028.
Axéll, T, Mörnstad, H, Sundström, B. The relation of the clinical picture to the histopathology of snuff dipper’s lesions in a Swedish population. J Oral Pathol. 1976;5:229–236.
Bergström, J, Keilani, H, Lundholm, C, et al. Smokeless tobacco (snuff) use and periodontal bone loss. J Clin Periodontol. 2006;33:549–554.
Bouquot, JE, Glover, ED, Schroeder, KL, Leukoplakia and smokeless tobacco keratosis are two separate precancers. Varma, AD, eds.. Oral oncology, vol 2.. MacMillan: Delhi, India, 1991:67–69.
Centers for Disease Control and Prevention. Tobacco use among adults—United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1145–1148.
Centers for Disease Control and Prevention. Youth risk behavior surveillance—United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1–112.
Ebbert, JO, Carr, AB, Dale, LC. Smokeless tobacco: an emerging addiction. Med Clin North Am. 2004;88:1593–1605.
Ebbert, JO, Rowland, LC, Montori, VM, et al. Treatments for spit tobacco use: a quantitative systematic review. Addiction. 2003;98:569–583.
Fisher, MA, Taylor, GW, Tilashalski, KR. Smokeless tobacco and severe active periodontal disease. NHANES III. J Dent Res. 2005;84:705–710.
Grasser, JA, Childers, E. Prevalence of smokeless tobacco use and clinical oral leukoplakia in a military population. Mil Med. 1997;162:401–404.
Gupta, PC, Hebert, JR, Bhonsle, RB, et al. Influence of dietary factors on oral precancerous lesions in population-based case-controlled study in Kerala, India. Cancer. 1999;85:1885–1893.
Kaugars, GE, Riley, WT, Brandt, RB, et al. The prevalence of oral lesions in smokeless tobacco users and an evaluation of risk factors. Cancer. 1992;70:2579–2585.
Lewin, F, Norell, SE, Johansson, H, et al. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck. A population-based case-referent study in Sweden. Cancer. 1998;82:1367–1375.
Martin, GC, Brown, JP, Eifler, CW, et al. Oral leukoplakia status six weeks after cessation of smokeless tobacco use. J Am Dent Assoc. 1999;130:945–954.
Mumford, EA, Levy, DT, Gitchell, JG, et al. Smokeless tobacco use 1992-2002: trends and measurement in the Current Population Survey-Tobacco Use Supplements. Tob Control. 2006;15:166–171.
Nelson, DE, Mowery, P, Tomar, S, et al. Trends in smokeless tobacco use among adults and adolescents in the United States. Am J Public Health. 2006;96:897–905.
Robertson, PB, Walsh, MM, Greene, JC. Oral effects of smokeless tobacco use by professional baseball players. Adv Dent Res. 1997;11:307–312.
Rodu, B. Smokeless tobacco and oral cancer: a review of the risks and determinants. Crit Rev Oral Biol Med. 2004;15:252–263.
Schildt, E-B, Eriksson, M, Hardell, L, et al. Oral snuff, smoking habits and alcohol consumption in relation to oral cancer in a Swedish case-control study. Int J Cancer. 1998;77:341–346.
Stotts, RC, Schroeder, KL, Burns, DM. Smokeless tobacco and health. Bethesda, Md: National Institutes of Health, 1992. [NIH publication no. 92-3461].
Tomar, SL, Winn, DM, Swango, PA, et al. Oral mucosal smokeless tobacco lesions among adolescents in the United States. J Dent Res. 1997;76:1277–1286.
Walsh, PM, Epstein, JB. The oral effects of smokeless tobacco. J Can Dent Assoc. 2000;66:22–25.
Warnakulasuriya, KA, Ralhan, R. Clinical, pathological, cellular and molecular lesions caused by oral smokeless tobacco—a review. J Oral Pathol Med. 2007;36:63–77.
Avon, SL. Oral mucosal lesions associated with use of quid. J Can Dent Assoc. 2004;70:244–248.
Cox, SC, Walker, DM. Oral submucous fibrosis. A review. Aust Dent J. 1996;41:294–299.
Hague, MF, Meghji, S, Nazir, R, et al. Interferon gamma (IFN-gamma) may reverse oral submucous fibrosis. J Oral Pathol Med. 2001;30:12–21.
Hazarey, VK, Erlewad, DM, Mundhe, KA, et al. Oral submucous fibrosis: study of 1000 cases from central India. J Oral Pathol Med. 2007;36:12–17.
Jacob, BJ, Straif, K, Thomas, G, et al. Betel quid without tobacco as a risk factor for oral precancers. Oral Oncol. 2004;40:697–704.
Kumar, A, Bagewadi, A, Keluskar, V, et al. Efficacy of lycopene in the management of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:207–213.
Lai, DR, Chen, HR, Lin, LM, et al. Clinical evaluation of different treatment methods for oral submucous fibrosis. A 10-year experience with 150 cases. J Oral Pathol Med. 1995;24:402–406.
Maher, R, Lee, AJ, Warnakulasuriya, KA, et al. Role of areca nut in the causation of oral submucous fibrosis: a case-control study in Pakistan. J Oral Pathol Med. 1994;23:65–69.
Merchant, A, Husain, SS, Hosain, M, et al. Paan without tobacco: an independent risk factor for oral cancer. Int J Cancer. 2000;86:128–131.
Murti, PR, Gupta, PC, Bhonsle, RB, et al. Smokeless tobacco use in India: effects on oral mucosa. In: Stotts RC, Schroeder KL, Burns DM, eds. Smokeless tobacco and health. Bethesda, Md: National Institutes of Health, 1992. [NIH publication no. 92-3461].
Nair, U, Bartsch, H, Nair, J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis. 2004;19:251–262.
Norton, SA. Betel: consumption and consequences. J Am Acad Dermatol. 1998;38:81–88.
Rajalallitha, P, Vali, S. Molecular pathogenesis of oral submucous fibrosis—a collagen metabolic disorder. J Oral Pathol Med. 2005;34:321–328.
Rajendran, R, Vidya, R, Saleem, S. Pentoxifylline therapy: a new adjunct in the treatment of oral submucous fibrosis. Indian J Dent Res. 2006;17:190–198.
Ranganathan, K, Devi, MU, Kirankumar, K, et al. Oral submucous fibrosis: a case-control study in Chennai, South India. J Oral Pathol Med. 2004;33:274–277.
Reichart, PA, Philipsen, HP. Betel chewer’s mucosa—a review. J Oral Pathol Med. 1998;27:239–242.
Tilakaratne, WM, Klinikowski, MF, Saku, T, et al. Oral submucous fibrosis: review on aetiology and pathogenesis. Oral Oncol. 2006;42:561–568.
World Health Organization International Agency for Research on Cancer. IARC monographs on the evaluation and carcinogenic risks to humans, betel-quid and areca-nut chewing and some areca-nut-derived nitrosamines, vol 85 . IARC Press: Lyon, France, 2004.
Zheng, X, Reichart, PA. A review of betel quid chewing, oral cancer and precancer in Mainland China. Oral Oncol. 2007;43:424–430.
Ortiz, GM, Pierce, AM, Wilson, DF. Palatal changes associated with reverse smoking in Filipino women. Oral Dis. 1996;2:232–237.
Rossie, KM, Guggenheimer, J. Thermally induced “nicotine” stomatitis: a case report. Oral Surg Oral Med Oral Pathol. 1990;70:597–599.
Saunders, WH. Nicotine stomatitis of the palate. Ann Otol Rhinol Laryngol. 1958;67:618–627.
Schwartz, DL. Stomatitis nicotina of the palate: report of two cases. Oral Surg Oral Med Oral Pathol. 1965;20:306–315.
Alexiades-Armenakas, M. Aminolevulinic acid photodynamic therapy for actinic keratoses/actinic cheilitis/acne: vascular lasers. Dermatol Clin. 2007;25:25–33.
Fu, W, Cockerell, CJ. The actinic (solar) keratosis: a 21st-century perspective. Arch Dermatol. 2003;139:66–70.
Fuchs, A, Marmur, E. The kinetics of skin cancer: progression of actinic keratosis to squamous carcinoma. Dermatol Surg. 2007;33:1099–1101.
Gupta, AK, Cooper, EA, Feldman, SR, et al. A survey of office visits for actinic keratosis as reported by NAMCS, 1990-1999. National Ambulatory Medical Care Survey. Cutis. 2002;70(suppl 2):8–13.
Gupta, AK, Davey, V, Mcphail, H. Evaluation of the effectiveness of imiquimod and 5-fluorouracil for the treatment of actinic keratosis: critical review and meta-analysis of efficacy studies. J Cutan Med Surg. 2005;9:209–214.
Hadley, G, Derry, S, Moore, RA. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol. 2006;126:1251–1255.
Jorizzo, JL. Current and novel treatment options for actinic keratosis. J Cutan Med Surg. 2005;8:13–21.
Lober, BA, Fenske, NA. Optimum treatment strategies for actinic keratosis (intraepidermal squamous cell carcinoma). Am J Clin Dermatol. 2004;5:395–401.
Lober, BA, Lober, CW. Actinic keratosis is squamous cell carcinoma. South Med J. 2000;93:650–655.
Marks, VJ. Actinic keratosis: a premalignant skin lesion. Otolaryngol Clin North Am. 1993;26:23–35.
Memon, AA, Tomenson, JA, Bothwell, J, et al. Prevalence of solar damage and actinic keratosis in a Merseyside population. Br J Dermatol. 2000;142:1154–1159.
Tran, H, Chen, K, Shumack, S. Summary of actinic keratosis studies with imiquimod 5% cream. Br J Dermatol. 2003;149(suppl 66):37–39.
Wade, TR, Ackerman, AB. The many faces of solar keratoses. J Dermatol Surg Oncol. 1978;4:730–734.
Alexiades-Armenakas, M. Aminolevulinic acid photodynamic therapy for actinic keratoses/actinic cheilitis/acne: vascular lasers. Dermatol Clin. 2007;25:25–33.
Dufresne, RG, Jr., Curlin, MU. Actinic cheilitis. A treatment review. Dermatol Surg. 1997;23:15–21.
Huber, MA, Terezhalmy, GT. The patient with actinic cheilitis. Gen Dent. 2006;54:274–282.
Kaugars, GE, Pillion, T, Svirsky, JA, et al. Actinic cheilitis: a review of 152 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:181–186.
Manganaro, AM, Will, MJ, Poulous, E. Actinic cheilitis: a premalignant condition. Gen Dent. 1997;5:492–494.
Markopoulos, A, Albanidou-Farmaki, E, Kayavis, I. Actinic cheilitis: clinical and pathologic characteristics in 65 cases. Oral Dis. 2004;10:212–216.
Menta Simonsen Nico, M, Rivitti, AE, Lourenço, SV. Actinic cheilitis: histologic study of the entire vermilion and comparison with previous biopsy. J Cutan Pathol. 2007;34:309–314.
Orenstein, A, Goldan, O, Weissman, O, et al. A new modality in the treatment of actinic cheilitis using the Er:YAG laser. J Cosmet Laser Ther. 2007;9:23–25.
Smith, KH, Germain, M, Yeager, J, et al. Topical 5% imiquimod for the therapy of actinic cheilitis. J Am Acad Dermatol. 2002;47:497–501.
Cribier, B, Asch, P-H, Grosshans, E. Differentiating squamous cell carcinoma from keratoacanthoma using histopathological criteria. Is it possible? A study of 296 cases. Dermatology. 1999;199:208–212.
Chen, YK, Lin, LM, Lin, CC, et al. Keratoacanthoma of the tongue: a diagnostic problem. Otolaryngol Head Neck Surg. 2003;128:581–582.
Clausen, OPF, Aass, HCD, Beigi, M. Are keratoacanthomas variants of squamous cell carcinoma? A comparison of chromosomal aberrations by comparative genomic hybridization. J Invest Dermatol. 2006;126:2308–2315.
de Visscher, JG, van der Wal, JE, Starink, TM, et al. Giant kerato-acanthoma of the lower lip. Report of a case of spontaneous regression. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:193–196.
Eversole, LR, Leider, AS, Alexander, G. Intraoral and labial keratoacanthoma. Oral Surg Oral Med Oral Pathol. 1982;54:663–667.
Griffiths, RW. Keratoacanthoma observed. Br J Plast Surg. 2004;57:485–501.
Jaber, PW, Cooper, PH, Greer, ICE. Generalized eruptive keratoacanthoma of Grzybowski. J Am Acad Dermatol. 1993;29:299–304.
Janette, A, Pecaro, B, Lonergan, M, et al. Solitary intraoral keratoacanthoma: report of a case. J Oral Maxillofac Surg. 1996;54:1026–1030.
Ponti, G, Ponz de Leon, M. Muir-Torre syndrome. Lancet Oncol. 2005;6:980–987.
Sanchez Yus, E, Simon, P, Requena, L, et al. Solitary keratoacanthoma: a self-healing proliferation that frequently becomes malignant. Am J Dermatopathol. 2000;22:305–310.
Schwartz, RA. Keratoacanthoma. J Am Acad Dermatol. 1994;30:1–19.
Schwartz, RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326–333.
Weedon, D. Keratoacanthoma: a personal perspective. Curr Diagn Pathol. 2003;9:259–265.
American Cancer Society. Cancer facts & figures—2007. Atlanta, Ga: American Cancer Society, 2007;1–56.
Anneroth, G, Hansen, LS, Silverman, S, Jr. Malignancy grading in oral squamous cell carcinoma. 1. Squamous cell carcinoma of the tongue and floor of mouth: histologic grading in the clinical evaluation. J Oral Pathol. 1986;15:162–168.
Baker, SR, Krause, CJ. Carcinoma of the lip. Laryngoscope. 1980;90:19–27.
Ballantyne, AJ, McCarten, AB, Ibanez, ML. The extension of cancer of the head and neck through peripheral nerves. Am J Surg. 1963;106:657–667.
Barasch, A, Safford, M, Eisenberg, E. Oral cancer and oral effects of anticancer therapy. Mt Sinai J Med. 1998;65:370–377.
Black, RJ, Gluckman, JL, Shumrick, DA. Multiple primary tumours of the upper aerodigestive tract. Clin Otolaryngol. 1983;8:277–281.
Blot, WJ, McLaughlin, JK, Winn, DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287.
Bouquot, JE. Common oral lesions found during a mass screening examination. J Am Dent Assoc. 1986;112:50–57.
Bouquot, JE. Epidemiology. In: Gnepp DR, ed. Pathology of the head and neck. New York: Churchill Livingstone; 1988:263–314.
Bouquot, JE, Meckstroth, RL. Oral cancer in a tobacco-chewing U.S. population: incidence and mortality. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:697–706.
Bouquot, JE, Weiland, LH, Kurland, LT. Metastases to and from the upper aerodigestive tract in the population of Rochester, Minnesota, 1935-1984. Head Neck. 1989;11:212–218.
Braakhuis, BJM, Leemans, CR, Brakenhoff, RH. Expanding fields of genetically altered cells in head and neck squamous carcinogenesis. Semin Cancer Biol. 2005;15:113–120.
Brandwein-Gensler, M, Teixeira, MS, Lewis, CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178.
Brugere, J, Guenel, P, Leclerc, A, et al. Differential effects of tobacco and alcohol in cancer of the larynx, pharynx, and mouth. Cancer. 1986;57:391–395.
Bsoul, SA, Huber, MA, Terezhalmy, GT. Squamous cell carcinoma of the oral tissues: a comprehensive review for oral healthcare providers. J Contemp Dent Pract. 2005;4:001–016.
Centers for Disease Control and Prevention. Tobacco use among adults—United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1145–1148.
Chang, KW, Chang, CS, Lai, KS, et al. High prevalence of human papillomavirus infection and possible association with betel quid chewing and smoking in oral epidermoid carcinomas in Taiwan. J Med Virol. 1989;28:57–61.
Choi, SY, Kahyo, H. Effect of cigarette smoking and alcohol consumption in the aetiology of cancer of the oral cavity, pharynx and larynx. Int J Epidemiol. 1991;20:878–885.
Choong, NW, Cohen, EEW. Epidermal growth factor receptor directed therapy in head and neck cancer. Crit Rev Oncol Hematol. 2006;57:25–42.
Cianfriglia, F, Di Gregorio, DA, Manieri, A. Multiple primary tumours in patients with oral squamous cell carcinoma. Oral Oncol. 1999;35:157–163.
Cox, MF, Scully, C, Maitland, N. Viruses in the aetiology of oral carcinoma? Examination of the evidence. Br J Oral Maxillofac Surg. 1991;29:381–387.
Davies, L, Welch, HG. Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg. 2006;135:451–457.
de Visscher, JG, Schaapveld, M, Otter, R, et al. Epidemiology of cancer of the lip in the Netherlands. Oral Oncol. 1998;34:421–426.
Dickenson, AJ, Currie, WJ, Avery, BS. Screening for syphilis in patients with carcinoma of the tongue. Br J Oral Maxillofac Surg. 1995;33:319–320.
Flaitz, CM, Hicks, MJ. Molecular piracy: the viral link to carcinogenesis. Oral Oncol. 1998;34:448–453.
Fukuzawa, K, Noguchi, Y, Yoshikawa, T, et al. High incidence of synchronous cancer of the oral cavity and the upper gastrointestinal tract. Cancer Lett. 1999;144:145–151.
Lip and oral cavity. In: Greene FL, Page DL, Fleming ID, et al, eds. AJCC cancer staging manual. ed 6. New York: Springer-Verlag; 2002:23–32.
Ha, PK, Califano, JA. The molecular biology of mucosal field cancerization of the head and neck. Crit Rev Oral Biol Med. 2003;14:363–369.
Ha, PK, Califano, JA. The role of human papillomavirus in oral carcinogenesis. Crit Rev Oral Biol Med. 2004;15:188–196.
Hart, AK, Karakla, DW, Pitman, KT, et al. Oral and oropharyngeal squamous cell carcinoma in young adults: a report on 13 cases and review of the literature. Otolaryngol Head Neck Surg. 1999;120:828–833.
Hjortdal, O, Naess, A, Berner, A. Squamous cell carcinomas of the lower lip. J Craniomaxillofac Surg. 1995;23:34–37.
Jemal, A, Siegel, R, Ward, E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66.
Kademani, D. Oral cancer. Mayo Clin Proc. 2007;82:878–887.
Kalyankrishna, S, Grandis, JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–2672.
Karja, J, Syrjanen, S, Usenius, T, et al. Oral cancer in children under 15 years of age: a clinicopathological and virological study. Acta Otolaryngol Suppl. 1988;449:145–149.
Kleinman, DV, Crossett, LS, Gloeckler Ries, LA, et al. Cancer of the oral cavity and pharynx: a statistics review monograph, 1973-1987. Bethesda, Md: National Institute of Dental Research, 1992. [NIH Monograph].
Koch, WM, Lango, M, Sewell, D, et al. Head and neck cancer in nonsmokers: a distinct clinical and molecular entity. Laryngoscope. 1999;109:1544–1551.
Kowalski, LP, Bagietto, R, Lara, JR, et al. Prognostic significance of the distribution of neck node metastasis from oral carcinoma. Head Neck. 2000;22:207–214.
Kreimer, AR, Clifford, GM, Boyle, P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475.
Krogh, P, Hald, B, Holmstrup, P. Possible mycological etiology of oral mucosal cancer. Catalytic potential of infecting Candida albicans and other yeasts in production of N-nitrosobenzylmethylamine. Carcinogenesis. 1987;8:1543–1548.
Kurumatani, N, Kirita, T, Zheng, Y, et al. Time trends in the mortality rates for tobacco- and alcohol-related cancers within the oral cavity and pharynx in Japan, 1950-94. J Epidemiol. 1999;9:46–52.
Lindqvist, C, Teppo, L. Epidemiological evaluation of sunlight as a risk factor of lip cancer. Br J Cancer. 1978;37:983–989.
Maserejian, NN, Joshipura, KJ, Rosner, BA, et al. Prospective study of alcohol consumption as risk of oral premalignant lesions in men. Cancer Epidemiol Biomarkers Prev. 2006;15:774–781.
Mashberg, A, Samit, AM. Early diagnosis of asymptomatic oral and oropharyngeal cancer. CA Cancer J Clin. 1995;45:328–351.
Massano, J, Regateiro, FS, Januário, G, et al. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67–76.
Mattson, ME, Winn, DM. Smokeless tobacco: association with increased cancer risk. Bethesda, Md: National Cancer Institute, 1989;13–16. [NCI Monogr No 8].
McDowell, JD. An overview of epidemiology and common risk factors for oral squamous cell carcinoma. Otolaryngol Clin North Am. 2006;39:277–294.
Merchant, A, Husain, SS, Hosain, M, et al. Paan without tobacco: an independent risk factor for oral cancer. Int J Cancer. 2000;86:128–131.
Moreno-Lopez, LA, Esparza-Gomez, GC, Gonzalez-Navarro, A, et al. Risk of oral cancer associated with tobacco smoking, alcohol consumption and oral hygiene: a case-control study in Madrid, Spain. Oral Oncol. 2000;36:170–174.
Morse, DE, Kerr, AR. Disparities in oral and pharyngeal cancer incidence, mortality and survival among black and white Americans. J Am Dent Assoc. 2006;137:203–212.
Morse, DE, Psoter, WJ, Cleveland, D, et al. Smoking and drinking in relation to oral cancer and oral epithelial dysplasia. Cancer Causes Control. 2007;18:919–929.
Nair, S, Pillai, MR. Human papillomavirus and disease mechanisms: relevance to oral and cervical cancers. Oral Dis. 2005;11:350–359.
Neville, BW, Day, TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215.
Niv, A, Sion-Vardi, N, Gatot, A, et al. Identification and typing of human papillomavirus (HPV) in squamous cell carcinoma of the oral cavity and oropharynx. J Laryngol Otol. 2000;114:41–46.
Notani, PN, Jayant, K. Role of diet in upper aerodigestive tract cancers. Nutr Cancer. 1987;10:103–113.
Paleri, V, Rees, G, Arullendran, P, et al. Sentinel node biopsy in squamous cell cancer of the oral cavity and oral pharynx: a diagnostic meta-analysis. Head Neck. 2005;27:739–747.
Partridge, M, Kiguwa, S, Emilion, G, et al. New insights into p53 protein stabilisation in oral squamous cell carcinoma. Oral Oncol. 1999;35:45–55.
Pentenero, M, Gandolfo, S, Carrozzo, M. Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: a review of the literature. Head Neck. 2005;27:1080–1091.
Prince, S, Bailey, BM. Squamous cell carcinoma of the tongue: review. Br J Oral Maxillofac Surg. 1999;37:164–174.
Rodu, B. Smokeless tobacco and oral cancer: a review of the risks and determinants. Crit Rev Oral Biol Med. 2004;15:252–263.
Rodu, B, Cole, P. Oral cavity and pharynx-throat cancer in the United States, 1973-2003. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:653–658.
Rothman, K, Keller, A. The effect of joint exposure to alcohol and tobacco on risk of cancer of the mouth and pharynx. J Chronic Dis. 1972;25:711–716.
Sankaranarayanan, R. Oral cancer in India: an epidemiologic and clinical review. Oral Surg Oral Med Oral Pathol. 1990;69:325–330.
Sciubba, JJ. Oral cancer: the importance of early diagnosis and treatment. Am J Clin Dermatol. 2001;2:239–251.
Shaha, AR, Patel, S, Shasha, D, et al. Head and neck cancer. In: Lenhard RE, Osteen RT, Gansler T, eds. Clinical oncology. Atlanta, Ga: American Cancer Society; 2000:297–331.
Shiboski, CH, Schmidt, BL, Jordan, RC. Racial disparity in stage at diagnosis and survival among adults with oral cancer in the US. Community Dent Oral Epidemiol. 2007;35:233–240.
Silverman, S, Jr., Griffith, M. Smoking characteristics of patients with oral carcinoma and the risk for second oral primary carcinoma. J Am Dent Assoc. 1972;85:637–640.
Specenier, PM, Vermorken, JB. Neoadjuvant chemotherapy in head and neck cancer: should it be revisited? Cancer Lett. 2007;256:166–177.
Speight, PM, Farthing, PM, Bouquot, JE. The pathology of oral cancer and precancer. Curr Diag Pathol. 1997;3:165–176.
Spiro, RH, Guillamondegui, O, Jr., Paulino, AF, et al. Pattern of invasion and margin assessment in patients with oral tongue cancer. Head Neck. 1999;21:408–413.
Head and neck cancer. In: Stewart BW, Kleihues P, eds. World Cancer Report. Lyon, France: IARC Press; 2003:232–236.
Syrjänen, S. HPV infections and tonsillar carcinoma. J Clin Pathol. 2004;57:449–455.
Syrjänen, S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol. 2005;32S:S59–S66.
Strome, SE, To, W, Strawderman, M, et al. Squamous cell carcinoma of the buccal mucosa. Otolaryngol Head Neck Surg. 1999;120:375–379.
Tabor, MP, Brakenhoff, RH, van Houten, VMM, et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7:1523–1532.
Tralongo, V, Rodolico, V, Luciani, A, et al. Prognostic factors in oral squamous cell carcinoma. A review of the literature. Anticancer Res. 1999;19:3503–3510.
van Oijen, MG, Leppers, VD, Straat, FG, et al. The origins of multiple squamous cell carcinomas in the aerodigestive tract. Cancer. 2000;88:884–893.
Velly, AM, Franco, EL, Schlecht, N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34:284–291.
Watts, JM. The importance of the Plummer-Vinson syndrome in the etiology of carcinoma of the upper gastrointestinal tract. Postgrad Med J. 1961;37:523–533.
Winn, DM. Smokeless tobacco and cancer: the epidemiologic evidence. CA Cancer J Clin. 1988;38:236–243.
World Health Organization International Agency for Research on Cancer. IARC monographs on the evaluation and carcinogenic risks to humans, Betel-quid and areca-nut chewing and some areca-nut-derived nitrosamines, vol 85 . IARC Press: Lyon, France, 2004.
Ackerman, LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670–678.
Addante, RR, McKenna, SJ. Verrucous carcinoma. Oral Maxillofac Surg Clin North Am. 2006;18:513–519.
Bouquot, JE. Oral verrucous carcinoma—incidence in two U.S. populations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:318–324.
Burford, WN, Ackerman, LV, Robinson, HBG. Ellis Fischel State Cancer Hospital number: symposium on twenty cases of benign and malignant lesions of the oral cavity, from the Ellis Fischel State Cancer Hospital, Columbia, Missouri. Am J Orthod Oral Surg. 1944;30:353–372.
Jordan, RC. Verrucous carcinoma of the mouth. J Can Dent Assoc. 1995;61:797–801.
Kamath, VV, Varma, RR, Gadewar, DR, et al. Oral verrucous carcinoma: an analysis of 37 cases. J Craniomaxillofac Surg. 1989;17:309–314.
Karthikeya, P, Mahima, VG, Bhavna, G. Sinonasal verrucous carcinoma with oral invasion. Indian J Dent Res. 2006;17:82–86.
Koch, BB, Trask, DK, Hoffman, HT, et al. National survey of head and neck verrucous carcinoma: patterns of presentation, care, and outcome. Cancer. 2001;92:110–120.
McCoy, JM, Waldron, CA. Verrucous carcinoma of the oral cavity: a review of forty-nine cases. Oral Surg Oral Med Oral Pathol. 1981;52:623–629.
Medina, JE, Dichtel, W, Luna, MA. Verrucous-squamous carcinomas of the oral cavity: a clinicopathologic study of 104 cases. Arch Otolaryngol. 1984;110:437–440.
Ogawa, A, Fukua, Y, Nakajima, T, et al. Treatment results of verrucous carcinoma and its biological behavior. Oral Oncol. 2004;40:793–797.
Oliveira, DT, de Moraes, RV, Filho, JFF, et al. Oral verrucous carcinoma: a retrospective study in Sao Paulo Region, Brazil. Clin Oral Invest. 2006;10:205–209.
Schwartz, RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1–24.
Strojan, P, Soba, E, Budihna, M, et al. Radiochemotherapy with vinblastine, methotrexate, and bleomycin in the treatment of verrucous carcinoma of the head and neck. J Surg Oncol. 2005;92:278–283.
Tharp, ME, II., Shidnia, H. Radiotherapy in the treatment of verrucous carcinoma of the head and neck. Laryngoscope. 1995;105:391–396.
Batsakis, JG, Suarez, P. Sarcomatoid carcinomas of the upper aerodigestive tracts. Adv Anat Pathol. 2000;7:282–293.
Benninger, MS, Kraus, D, Sebek, B, et al. Head and neck spindle cell carcinoma: an evaluation of current management. Cleve Clin J Med. 1992;59:479–482.
Ellis, GL, Langloss, JM, Heffner, DK, et al. Spindle-cell carcinoma of the aerodigestive tract: an immunohistochemical analysis of 21 cases. Am J Surg Pathol. 1987;11:335–342.
Gupta, R, Singh, S, Hedau, S, et al. Spindle cell carcinoma of head and neck: an immunohistochemical and molecular approach to its pathogenesis. J Clin Pathol. 2007;60:472–475.
Lewis, JE, Olsen, KD, Sebo, TJ. Spindle cell carcinoma of the larynx: review of 26 cases including DNA content and immunohistochemistry. Hum Pathol. 1997;28:664–673.
Rizzardi, C, Frezzini, C, Maglione, M, et al. A look at the biology of spindle cell squamous carcinoma of the oral cavity: report of a case. J Oral Maxillofac Surg. 2003;61:264–268.
Shibuya, Y, Umeda, M, Yokoo, S, et al. Spindle cell squamous carcinoma of the maxilla: report of a case with immunohistochemical analysis. J Oral Maxillofac Surg. 2000;58:1164–1169.
Slootweg, PJ, Roholl, PJ, Muller, H, et al. Spindle-cell carcinoma of the oral cavity and larynx. Immunohistochemical aspects. J Craniomaxillofac Surg. 1989;17:234–236.
Su, HH, Chu, ST, Hou, YY, et al. Spindle cell carcinoma of the oral cavity and oropharynx: factors affecting outcome. J Chin Med Assoc. 2006;69:478–483.
Abdelsayed, RA, Sangueza, OP, Newhouse, RF, et al. Adenosquamous carcinoma: a case report with immunohistochemical evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:173–177.
Alos, L, Castillo, M, Nadal, A, et al. Adenosquamous carcinoma of the head and neck: criteria for diagnosis in a study of 12 cases. Histopathology. 2004;44:570–579.
Banks, ER, Cooper, PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227–234.
Gerughty, RM, Hennigar, GB, Brown, FM. Adenosquamous carcinoma of the nasal, oral and laryngeal cavities: a clinicopathologic survey of ten cases. Cancer. 1968;22:1140–1155.
Keelawat, S, Liu, CZ, Roehm, PC, et al. Adenosquamous carcinoma of the upper aerodigestive tract: a clinicopathologic study of 12 cases and review of the literature. Am J Otolaryngol. 2002;23:160–168.
Martinez-Madrigal, F, Baden, E, Casiraghi, O, et al. Oral and pharyngeal adenosquamous carcinoma: a report of four cases with immunohistochemical studies. Eur Arch Otorhinolaryngol. 1991;248:255–258.
Scully, C, Porter, SR, Speight, PM, et al. Adenosquamous carcinoma of the mouth: a rare variant of squamous cell carcinoma. Int J Oral Maxillofac Surg. 1999;28:125–128.
Sheahan, P, Fitzgibbon, J, Lee, G, et al. Adenosquamous carcinoma of the tongue in a 22-year-old female: report of a case with immunohistochemistry. Eur Arch Otorhinolaryngol. 2003;260:509–512.
Sheahan, P, Toner, M, Timon, CV, I. Clinicopathologic features of head and neck adenosquamous carcinoma. ORL J Otorhinolaryngol Relat Spec. 2005;67:10–15.
Yoshimura, Y, Mishimma, K, Ohara, S, et al. Clinical characteristics of oral adenosquamous carcinoma: report of a case and an analysis of the reported Japanese cases. Oral Oncol. 2003;39:309–315.
Altavilla, G, Mannara, GM, Rinaldo, A, et al. Basaloid squamous cell carcinoma of oral cavity and oropharynx. ORL J Otorhinolaryngol Relat Spec. 1999;61:169–173.
Banks, ER, Frierson, HF, Jr., Mills, SE, et al. Basaloid squamous cell carcinoma of the head and neck: a clinicopathologic and immunohistochemical study of 40 cases. Am J Surg Pathol. 1992;16:939–946.
Barnes, L, Ferlito, A, Altavilla, G, et al. Basaloid squamous cell carcinoma of the head and neck: clinicopathological features and differential diagnosis. Ann Otol Rhinol Laryngol. 1996;105:75–82.
Coletta, RD, Cotrim, P, Vargas, PA, et al. Basaloid squamous carcinoma of the oral cavity: report of 2 cases and study of AgNOR, PCNA, p53, and MMP expression. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:563–569.
Coletta, RD, Cotrim, P, Almeida, OP, et al. Basaloid squamous carcinoma of oral cavity: a histologic and immunohistochemical study. Oral Oncol. 2002;38:723–729.
De Sampaio Góes, FC, Oliveira, DT, Dorta, RG, et al. Prognoses of oral basaloid squamous cell carcinoma and squamous cell carcinoma: a comparison. Arch Otolaryngol Head Neck Surg. 2004;130:83–86.
Ide, F, Shimoyama, T, Horie, N, et al. Basaloid squamous cell carcinoma of the oral mucosa: a new case and review of 45 cases in the literature. Oral Oncol. 2002;38:120–124.
Ide, F, Shimoyama, T, Suzuki, T, et al. Polypoid carcinoma of the tongue. J Oral Pathol Med. 1996;25:90–92.
Oikawa, K, Tabuchi, K, Nomura, M, et al. Basaloid squamous cell carcinoma of the maxillary sinus: a report of two cases. Auris Nasus Larynx. 2007;34:119–123.
Paulino, AF, Singh, B, Shah, JP, et al. Basaloid squamous cell carcinoma of the head and neck. Laryngoscope. 2000;110:1479–1482.
Sampaio-Góes, FCG, Oliveira, DT, Dorta, RG, et al. Expression of PCNA, p53, Bax, and Bcl-X in oral poorly differentiated and basaloid squamous cell carcinoma: relationships with prognosis. Head Neck. 2005;27:982–989.
Zbären, P, Nuyens, M, Stauffer, E. Basaloid squamous cell carcinoma of the head and neck. Curr Opin Otolaryngol Head Neck Surg. 2004;12:116–121.
Carcinoma of the Maxillary Sinus
Bhattacharyya, N. Factors affecting survival in maxillary sinus cancer. J Oral Maxillofac Surg. 2003;61:1016–1021.
Carrillo, JF, Guemes, A, Ramierz-Ortega, MC, et al. Prognostic factors in maxillary sinus and nasal cavity carcinoma. Eur J Surg Oncol. 2005;31:1206–1212.
Doig, TN, McDonald, SW, McGregor, IA. Possible routes of spread of carcinoma of the maxillary sinus to the oral cavity. Clin Anat. 1998;11:149–156.
Georgiou, AF, Walker, DM, Collins, AP, et al. Primary small cell undifferentiated (neuroendocrine) carcinoma of the maxillary sinus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:572–578.
Giri, SP, Reddy, EK, Gemer, LS, et al. Management of advanced squamous cell carcinomas of the maxillary sinus. Cancer. 1992;69:657–661.
Guntinas-Lichius, O, Kreppel, MP, Stuetzer, H, et al. Single modality and multimodality treatment of nasal and paranasal sinuses cancer: a single institution experience of 229 patients. Eur J Surg Oncol. 2007;33:222–228.
Hoppe, BS, Stegman, LD, Zelefsky, MJ, et al. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting—the MKSCC experience. Int J Radiat Oncol Biol Phys. 2007;67:691–702.
Indudharan, R, Das, PK, Thida, T. Verrucous carcinoma of maxillary antrum. Singapore Med J. 1996;37:559–561.
Itami, J, Uno, T, Aruga, M, et al. Squamous cell carcinoma of the maxillary sinus treated with radiation therapy and conservative surgery. Cancer. 1998;82:104–107.
Paulino, AC, Marks, JE, Bricker, P, et al. Results of treatment of patients with maxillary sinus carcinoma. Cancer. 1998;83:457–465.
Smith, SR, Som, P, Fahmy, A, et al. A clinicopathological study of sinonasal neuroendocrine carcinoma and sinonasal undifferentiated carcinoma. Laryngoscope. 2000;110:1617–1622.
Tiwari, R, Hardillo, JA, Mehta, D, et al. Squamous cell carcinoma of maxillary sinus. Head Neck. 2000;22:164–169.
Waldron, JN, O’Sullivan, B, Gullane, P, et al. Carcinoma of the maxillary antrum: a retrospective analysis of 110 cases. Radiother Oncol. 2000;57:167–173.
Sinonasal Undifferentiated Carcinoma
Ejaz, A, Wenig, BM. Sinonasal undifferentiated carcinoma: clinical and pathologic features and a discussion on classification, cellular differentiation, and differential diagnosis. Adv Anat Pathol. 2005;12:134–143.
Frierson, HF, Jr., Mills, SE, Fechner, RE, et al. Sinonasal undifferentiated carcinoma: an aggressive neoplasm derived from schneiderian epithelium and distinct from olfactory neuroblastoma. Am J Surg Pathol. 1986;10:771–779.
Gorelick, J, Ross, D, Marentette, L, et al. Sinonasal undifferentiated carcinoma: case series and review of literature. Neurosurgery. 2000;47:750–755.
Houston, GD. Sinonasal undifferentiated carcinoma. Report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:185–188.
Houston, GD, Gillies, E. Sinonasal undifferentiated carcinoma: a distinctive clinicopathologic entity. Adv Anat Pathol. 1999;6:317–323.
Jeng, YM, Sung, MT, Fang, CL, et al. Sinonasal undifferentiated carcinoma and nasopharyngeal-type carcinoma: two clinically, biologically, and histopathologically distinct entities. Am J Surg Pathol. 2002;26:371–376.
Jones, AV, Robinson, I, Speight, PM. Sinonasal undifferentiated carcinoma: report of a case and review of literature. Oral Oncol. 2005;41:299–302.
Kim, BS, Vongtama, R, Juillard, G. Sinonasal undifferentiated carcinoma: case series and literature review. Am J Otolaryngol. 2004;25:162–166.
Levine, PA, Frierson, HF, Stewart, FM, et al. Sinonasal undifferentiated carcinoma: a distinctive and highly aggressive neoplasm. Laryngoscope. 1987;97:905–908.
Righi, PD, Francis, F, Aron, BS, et al. Sinonasal undifferentiated carcinoma: a 10-year experience. Am J Otolaryngol. 1996;17:167–171.
Rischin, D, Porceddu, S, Peters, L, et al. Promising results with chemoradiation in patients with sinonasal undifferentiated carcinoma. Head Neck. 2004;26:435–441.
Sharara, N, Muller, S, Olson, J, et al. Sinonasal undifferentiated carcinoma with orbital invasion: report of three cases. Ophthal Plast Reconstr Surg. 2001;17:288–292.
Smith, SR, Som, P, Fahmy, A, et al. A clinicopathological study of sinonasal neuroendocrine and sinonasal undifferentiated carcinoma. Laryngoscope. 2000;110:1617–1622.
Agulnik, M, Siu, LL. State-of-the-art management of nasopharyngeal carcinoma: current and future directions. Br J Cancer. 2005;92:799–806.
August, M, Dodson, TB, Nastri, A, et al. Nasopharyngeal carcinoma: clinical assessment and review of 176 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:205–214.
Chen, CL, Hsu, MM. Second primary epithelial malignancy of nasopharynx and nasal cavity after successful curative radiation therapy of nasopharyngeal carcinoma. Hum Pathol. 2000;31:227–232.
Epstein, JB, Jones, CK. Presenting signs and symptoms of nasopharyngeal carcinoma. Oral Surg Oral Med Oral Pathol. 1993;75:32–36.
Farrow, DC, Vaughan, TL, Berwick, M, et al. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78:675–679.
Ma, BBY, Chan, ATC. Recent perspectives in the role of chemotherapy in the management of advanced nasopharyngeal carcinoma. Cancer. 2005;103:22–31.
O’Meara, WP, Lee, N. Advances in nasopharyngeal carcinoma. Curr Opin Oncol. 2005;17:225–230.
Ou, SHI, Zell, JA, Ziogas, A, et al. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29–35.
Wei, WI, Sham, JST. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–2052.
Wenig, BM. Nasopharyngeal carcinoma. Ann Diagn Pathol. 1999;3:374–385.
Yu, MC, Yuan, JM. Epidemiology of nasopharyngeal carcinoma. Cancer Biol. 2002;12:421–429.
Bath-Hextall, FJ, Perkins, W, Bong, J, et al. Interventions for basal cell carcinoma of the skin. Cochrane Database Syst Rev. 2007;1:CD003412.
Ceilley, RI, Del Rosso, JQ. Current modalities and new advances in the treatment of basal cell carcinoma. Int J Dermatol. 2006;45:489–498.
Christenson, LF, Borrowman, TA, Vachon, CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681–690.
Crowson, AN. Basal cell carcinoma: biology, morphology and clinical implications. Mod Pathol. 2006;19:S127–S147.
Daya-Grosjean, L, Couvé-Privat, S. Sonic hedgehog signaling in basal cell carcinomas. Cancer Lett. 2005;225:181–192.
Del Rosario, RN, Barr, RJ, Jensen, JL, et al. Basal cell carcinoma of the buccal mucosa. Am J Dermatopathol. 2001;23:203–205.
Gutierrez, MM, Mora, RG. Nevoid basal cell carcinoma syndrome: a review and case report of a patient with unilateral basal cell nevus syndrome. J Am Acad Dermatol. 1986;15:1023–1030.
Kato, N, Endo, Y, Tamura, G, et al. Ameloblastoma with basal cell carcinoma-like feature emerging as a nasal polyp. Pathol Int. 1999;49:747–751.
Kuijpers, DIM, Thissen, MRTM, Neumann, MHA. Basal cell carcinoma. Treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247–259.
Martin, RC, 2nd., Edwards, MJ, Cawte, TG, et al. Basosquamous carcinoma: analysis of prognostic factors influencing recurrence. Cancer. 2000;88:1365–1369.
Niederhagen, B, Lindern, J, Berge, S, et al. Staged operations for basal cell carcinoma of the face. Br J Oral Maxillofac Surg. 2000;38:477–479.
Rishiraj, B, Epstein, JB. Basal cell carcinoma: what dentists need to know. J Am Dent Assoc. 1999;130:375–380.
Rubin, AI, Chen, EH, Ratner, D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262–2269.
Telfer, NR, Colver, GB, Bowers, PW. Guidelines for the management of basal cell carcinoma. Br J Dermatol. 1999;141:415–423.
Agelli, M, Clegg, LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–841.
Bickle, K, Glass, LF, Messina, JF, et al. Merkel cell carcinoma: a clinical, histopathologic, and immunohistochemical review. Semin Cutan Med Surg. 2004;23:46–53.
Chan, JK, Suster, S, Wenig, BM, et al. Cytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sites. Am J Surg Pathol. 1997;21:226–234.
Dancey, AL, Rayatt, SS, Soon, C, et al. Merkel cell carcinoma: a report of 34 cases and literature review. J Plast Reconstr Aesthet Surg. 2006;59:1294–1299.
Garneski, KM, Nghiem, P. Merkel cell carcinoma adjuvant therapy: current data support radiation but not chemotherapy. J Am Acad Dermatol. 2007;57:166–169.
Gollard, R, Weber, R, Kosty, MP, et al. Merkel cell carcinoma. Review of 22 cases with surgical, pathologic, and therapeutic considerations. Cancer. 2000;8:1842–1851.
Hendrikx, SMGA, de Wilde, PCM, Kaanders, JHAM, et al. Merkel cell carcinoma in the oral cavity: a case presentation and review of the literature. Oral Oncol. 2005;41:202–206.
Jabbour, J, Cumming, R, Scolyer, RA, et al. Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease—results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol. 2007;14:1943–1952.
Lewis, KG, Weinstock, MA, Weaver, AL, et al. Adjuvant local irradiation for Merkel cell carcinoma. Arch Dermatol. 2006;142:693–700.
Longo, F, Califano, L, Mangone, GM, et al. Neuroendocrine (Merkel cell) carcinoma of the oral mucosa: report of a case with immunohistochemical study and review of the literature. J Oral Pathol Med. 1999;28:88–91.
Mendenhall, WM, Mendenhall, CM, Mendenhall, NP. Merkel cell carcinoma. Laryngoscope. 2004;114:906–910.
Miller, SJ, Alam, M, Andersen, J, et al. Merkel cell carcinoma. J Natl Compr Canc Netw. 2006;4:704–712.
Pectasides, D, Pectasides, M, Economopoulos, T. Merkel cell carcinoma of the skin. Ann Oncol. 2006;17:1489–1495.
Suárez, C, Rodrigo, JP, Ferlito, et al. Merkel cell carcinoma of the head and neck. Oral Oncol. 2004;40:773–779.
Yom, SS, Rosenthal, DI, El-Naggar, AK, et al. Merkel cell carcinoma of the tongue and head and neck oral mucosal sites. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:761–768.
Abassi, NR, Shaw, HM, Rigel, DS, et al. Early diagnosis of cutaneous melanoma. Revisiting the ABCD criteria. JAMA. 2004;292:2771–2776.
Balch, CM, Buzaid, AC, Soong, SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648.
Barnhill, RL, Mihm, MC, Jr. The histopathology of cutaneous malignant melanoma. Semin Diagn Pathol. 1993;10:47–75.
Barrett, AW, Bennett, JH, Speight, PM. A clinicopathological and immunohistochemical analysis of primary oral mucosal melanoma. Oral Oncol. 1995;31:100–105.
Batsakis, JG, Suarez, P. Mucosal melanomas: a review. Adv Anat Pathol. 2000;7:167–180.
Chidzonga, MM, Mahomva, L, Marimo, C, et al. Primary malignant melanoma of the oral mucosa. J Oral Maxillofac Surg. 2007;65:1117–1120.
Curtin, JA, Fridlyand, J, Kageshita, T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147.
Garbe, C, Eigentler, TK. Diagnosis and treatment of cutaneous melanoma: state of the art 2006. Melanoma Res. 2007;17:117–127.
Garzino-Demo, P, Fasolis, M, Maggiore, GM, et al. Oral mucosal melanoma: a series of case reports. J Craniomaxillofac Surg. 2004;32:251–257.
Gorsky, M, Epstein, JB. Melanoma arising from the mucosal surfaces of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:715–719.
Gray-Schopfer, V, Wellbrock, C, Marais, R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857.
Melanoma of the skin. In: Greene FL, Page DL, Fleming ID, et al, eds. AJCC cancer staging manual. ed 6. New York: Springer-Verlag; 2002:209–217.
Hall, HI, Miller, DR, Rogers, JD, et al. Update on the incidence and mortality from melanoma in the United States. J Am Acad Dermatol. 1999;40:35–42.
Hicks, MJ, Flaitz, CM. Oral mucosal melanoma: epidemiology and pathobiology. Oral Oncol. 2000;36:152–169.
Jemal, A, Siegel, R, Ward, E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66.
Kahn, M, Weathers, DR, Hoffman, JG. Transformation of a benign oral pigmentation to primary oral mucosal melanoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:454–459.
Kilpatrick, SE, White, WL, Browne, JD. Desmoplastic malignant melanoma of the oral mucosa. An underrecognized diagnostic pitfall. Cancer. 1996;78:383–389.
Manganaro, AM, Hammond, HL, Dalton, MJ, et al. Oral melanoma: case reports and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80:670–677.
Markovic, SN, Erickson, LA, Rao, RD, et al. Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc. 2007;82:364–380.
Markovic, SN, Erickson, LA, Rao, RD, et al. Malignant melanoma in the 21st century, part 2: staging, prognosis, and treatment. Mayo Clin Proc. 2007;82:490–513.
Miller, AJ, Mihm, MC. Mechanisms of disease. Melanoma. N Engl J Med. 2006;355:51–65.
Morton, DL, Thompson, JF, Cochran, AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med. 2006;355:1307–1317.
Nandapalan, V, Roland, NJ, Helliwell, TR, et al. Mucosal melanoma of the head and neck. Clin Otolaryngol. 1998;23:107–116.
Patrick, RJ, Fenske, NA, Messina, JL. Primary mucosal melanoma. J Am Acad Dermatol. 2007;56:828–834.
Rapini, RP, Golitz, LE, Greer, RO, et al. Primary malignant melanoma of the oral cavity: a review of 177 cases. Cancer. 1985;55:1543–1551.
Ringbörg, U, Afzelius, LE, Lagerlöf, B, et al. Cutaneous malignant melanoma of the head and neck: analysis of treatment results and prognostic factors in 581 patients: a report from the Swedish Melanoma Study Group. Cancer. 1993;71:751–758.
Tanaka, N, Mimura, M, Ogi, K, et al. Primary malignant melanoma of the oral cavity: assessment of outcome from the clinical records of 35 patients. Int J Oral Maxillofac Surg. 2004;33:761–765.
Umeda, M, Komatsubara, H, Shibuya, Y, et al. Premalignant melanocytic dysplasia and malignant melanoma of the oral mucosa. Oral Oncol. 2002;38:714–722.
Wang, BY, Lawson, W, Robinson, RA, et al. Malignant melanomas of the parotid: comparison of survival for patients with metastases from known vs unknown primary tumor sites. Arch Otolaryngol Head Neck Surg. 1999;125:635–639.
Welch, HG, Woloshin, S, Schwartz, LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331:481.
*Lentigines (multiple), electrocardiographic abnormalities, ocular hyper-telorism, pulmonary stenosis, abnormalities of genitalia, retardation of growth, and deafness (sensorineural).