Salivary Gland Pathology
SALIVARY GLAND APLASIA
Salivary gland aplasia is a rare developmental anomaly that can affect one or more of the major salivary glands. The condition may occur alone, although agenesis of the glands is often a component of one of several syndromes, including mandibulofacial dysostosis (Treacher Collins syndrome; see page 45), hemifa-cial microsomia, and lacrimo-auriculo-dento-digital (LADD) syndrome.
CLINICAL AND RADIOGRAPHIC FEATURES: Salivary gland aplasia has been reported more frequently in men than women by a 2:1 ratio. Some individuals are affected by agenesis of all four of the largest glands (both parotids and submandibular glands), but others may be missing only one to three of the four glands. In spite of the absence of the glands, the face still has a normal appearance because the sites are filled in by fat or connective tissue. Intraorally, the orifices of the missing glands are absent.
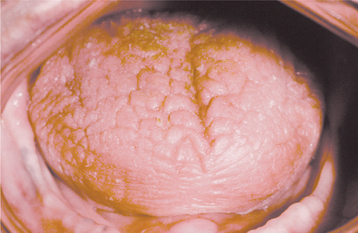
As would be anticipated, the most significant symptom associated with salivary gland aplasia is severe xerostomia with its attendant problems (see page 464). The tongue may appear leathery, and the patient is at greater risk for developing dental caries and erosion (Fig. 11-1). However, some degree of moisture still may be present because of the continued activity of the minor salivary glands. Absence of the major glands can be confirmed by technetium-99m pertechnetate scintiscan, computed tomography (CT), or magnetic resonance imaging (MRI).
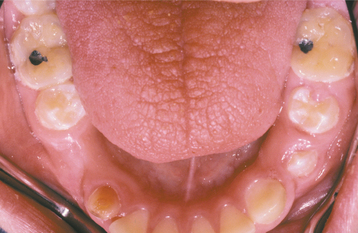
Fig. 11-1 Salivary gland aplasia. Dry, leathery tongue and diffuse enamel erosion in a child with aplasia of the major salivary glands.
LADD syndrome is an autosomal dominant disorder that is caused by mutations in the fibroblast growth factor 10 (FGF10) gene. It is characterized by aplasia or hypoplasia of the lacrimal and salivary glands, cup-shaped ears, and dental and digital anomalies. Dental features may include hypodontia, microdontia, and mild enamel hypoplasia.
TREATMENT AND PROGNOSIS: Patient management is directed toward compensating for the saliva deficiency, and saliva substitutes are often necessary. If any residual functional salivary gland tissue is present, then sialagogue medications such as pilocarpine or cevimeline may be used to increase saliva production. Salivary flow also may be stimulated via the use of sugarless gum or sour candy. Regular preventive dental care is important to avoid xero-stomia-related caries and enamel breakdown.
MUCOCELE (MUCUS EXTRAVASATION PHENOMENON; MUCUS ESCAPE REACTION)
The mucocele is a common lesion of the oral mucosa that results from rupture of a salivary gland duct and spillage of mucin into the surrounding soft tissues. This spillage is often the result of local trauma, although there is no known history of trauma in many cases. Unlike the salivary duct cyst (see page 457), the mucocele is not a true cyst because it lacks an epithelial lining. Some authors, however, have included true salivary duct cysts in their reported series of cases, sometimes under the classification of retention mucocele. Because these two entities exhibit distinctly different clinical and histopathologic features, they are discussed as separate topics in this chapter.
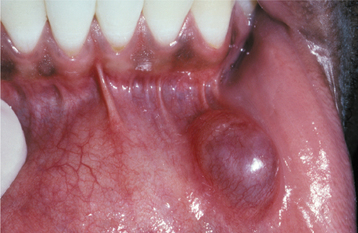
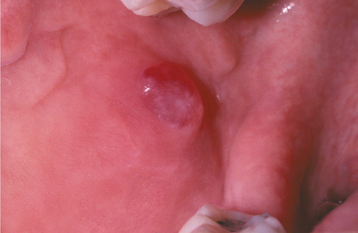
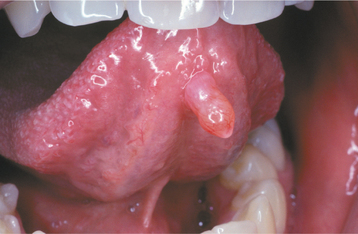
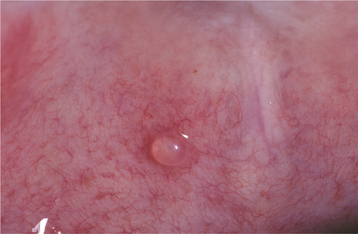
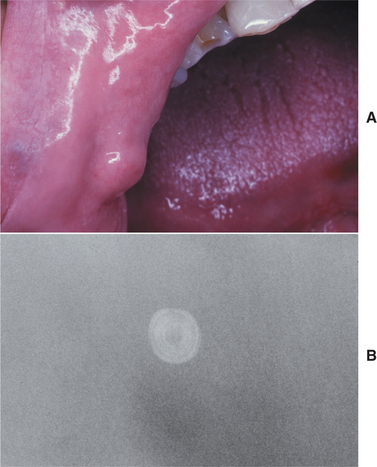
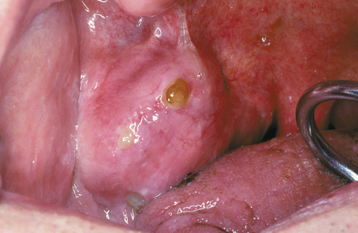
CLINICAL FEATURES: Mucoceles typically appear as dome-shaped mucosal swellings that can range from 1 or 2 mm to several centimeters in size (Figs. 11-2 to 11-4). They are most common in children and young adults, perhaps because younger people are more likely to experience trauma that induces mucin spillage. However, mucoceles have been reported in patients of all ages, including infants and older adults. The spilled mucin below the mucosal surface often imparts a bluish translucent hue to the swelling, although deeper mucoceles may be normal in color. The lesion characteristically is fluctuant, but some mucoceles feel firmer to palpation. The reported duration of the lesion can vary from a few days to several years; most patients report that the lesion has been present for several weeks. Many patients relate a history of a recurrent swelling that periodically may rupture and release its fluid contents.
The lower lip is by far the most common site for the mucocele; a recent large study of 1824 cases found that 81% occurred at this one site (Table 11-1). Lower lip mucoceles usually are found lateral to the midline. Less common sites include the floor of mouth (ranulas: 5.8%), anterior ventral tongue (from the glands of Blandin-Nuhn: 5.7%), buccal mucosa (4.7%), palate (1.4%), and retromolar pad (0.5%). Mucoceles rarely develop on the upper lip. In the large series summarized in Table 11-1, not a single example was identified from the upper lip. This is in contrast to salivary gland tumors, which are not unusual in the upper lip but are distinctly uncommon in the lower lip.
Table 11-1
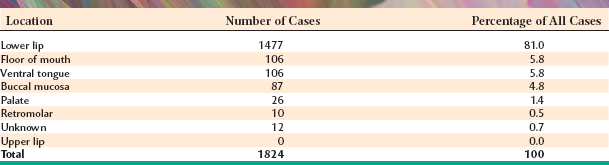
Data from Chi A, Lambert P, Richardson M et al: Oral mucoceles: a clinicopathologic review of 1,824 cases including unusual variants, Abstract No. 19. Paper presented at the annual meeting of the American Academy of Oral and Maxillofacial Pathology, Kansas City, Mo, 2007. American Academy of Oral and Maxillofacial Pathology
As noted, the soft palate and retromolar area are uncommon sites for mucoceles. However, one interesting variant, the superficial mucocele, does develop in these areas and along the posterior buccal mucosa. Superficial mucoceles present as single or multiple tense vesicles that measure 1 to 4 mm in diameter (Fig. 11-5). The lesions often burst, leaving shallow, painful ulcers that heal within a few days. Repeated episodes at the same location are not unusual. Some patients relate the development of the lesions to mealtimes. Superficial mucoceles also have been reported to occur in association with lichenoid disorders, such as lichen planus, lichenoid drug eruptions, and chronic graft-versus-host disease (GVHD). The vesicular appearance is created by the superficial nature of the mucin spillage, which causes a separation of the epithelium from the connective tissue. The pathologist must be aware of this lesion and should not mistake it microscopically for a vesiculobullous disorder, especially mucous membrane (cicatricial) pemphigoid.
HISTOPATHOLOGIC FEATURES: On microscopic examination, the mucocele shows an area of spilled mucin surrounded by a granulation tissue response (Figs. 11-6 and 11-7). The inflammation usually includes numerous foamy histiocytes (macrophages). In some cases a ruptured salivary duct may be identified feeding into the area. The adjacent minor salivary glands often contain a chronic inflammatory cell infiltrate and dilated ducts.
TREATMENT AND PROGNOSIS: Some mucoceles are short-lived lesions that rupture and heal by themselves. Many lesions, however, are chronic in nature, and local surgical excision is necessary. To minimize the risk of recurrence, the surgeon should remove any adjacent minor salivary glands that may be feeding into the lesion when the area is excised. The excised tissue should be submitted for microscopic examination to confirm the diagnosis and rule out the possibility of a salivary gland tumor. The prognosis is excellent, although occasional mucoceles will recur, necessitating reexcision, especially if the feeding glands are not removed.
RANULA: Ranula is a term used for mucoceles that occur in the floor of the mouth. The name is derived from the Latin word rana, which means “frog,” because the swelling may resemble a frog’s translucent underbelly. The term ranula also has been used to describe other similar swellings in the floor of the mouth, including true salivary duct cysts, dermoid cysts, and cystic hygromas. However, the term is best used for mucus escape reactions (mucoceles). The source of mucin spillage is usually from the sublingual gland, but ranulas also may arise from the submandibular duct or, possibly, from minor salivary glands in the floor of the mouth. Larger ranulas usually arise from the body of the sublingual gland, although smaller lesions can develop along the sublingual plica from the superficial ducts of Rivini of this gland.
CLINICAL FEATURES: The ranula usually appears as a blue, dome-shaped, fluctuant swelling in the floor of the mouth (Fig. 11-8), but deeper lesions may be normal in color. Ranulas are seen most frequently in children and young adults. They tend to be larger than mucoceles in other oral locations, often developing into large masses that fill the floor of the mouth and elevate the tongue. The ranula usually is located lateral to the midline, a feature that may help to distinguish it from a midline dermoid cyst (see page 33). Like other mucoceles, ranulas may rupture and release their mucin contents, only to re-form.
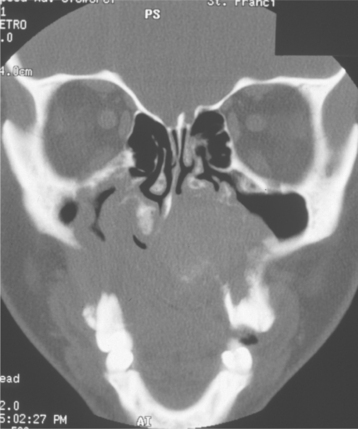
An unusual clinical variant, the plunging or cervical ranula, occurs when the spilled mucin dissects through the mylohyoid muscle and produces swelling within the neck (Fig. 11-9). A concomitant swelling in the floor of the mouth may or may not be present. If no lesion is produced in the mouth, then the clinical diagnosis of ranula may not be suspected. Imaging studies can be helpful in supporting a diagnosis of plunging ranula and in determining the origin of the lesion. CT and MRI images of plunging ranulas that arise from the sublingual gland often exhibit a slight extension of the lesion into the sublingual space (“tail sign”)—an imaging feature not observed in lesions that develop from the submandibular gland.
HISTOPATHOLOGIC FEATURES: The microscopic appearance of a ranula is similar to that of a mucocele in other locations. The spilled mucin elicits a granulation tissue response that typically contains foamy histiocytes.
TREATMENT AND PROGNOSIS: Treatment of the ranula consists of removal of the feeding sublingual gland and/or marsupialization. Marsupialization (exteriorization) entails removal of the roof of the intraoral lesion, which often can be successful for small, superficial ranulas associated with the ducts of Rivini. However, marsupialization is often unsuccessful for larger ranulas developing from the body of the sublingual gland, and most authors emphasize that removal of the offending gland is the most important consideration in preventing a recurrence of the lesion. If the gland is removed, then meticulous dissection of the lining of the lesion may not be necessary for the lesion to resolve, even for the plunging ranula.
SALIVARY DUCT CYST (MUCUS RETENTION CYST; MUCUS DUCT CYST; SIALOCYST)
The salivary duct cyst is an epithelium-lined cavity that arises from salivary gland tissue. Unlike the more common mucocele (see page 454), it is a true developmental cyst that is lined by epithelium that is separate from the adjacent normal salivary ducts. The cause of such cysts is uncertain.
Cystlike dilatation of salivary ducts also may develop secondary to ductal obstruction (e.g., mucus plug), which creates increased intraluminal pressure. Although some authors refer to such lesions as mucus retention cysts, such lesions probably represent salivary ductal ectasia rather than a true cyst.
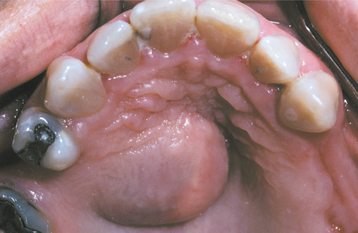
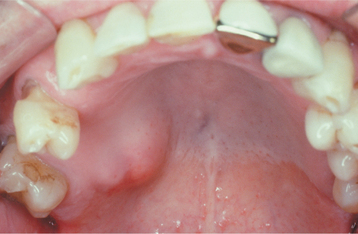
CLINICAL FEATURES: Salivary duct cysts usually occur in adults and can arise within either the major or minor glands. Cysts of the major glands are most common within the parotid gland, presenting as slowly growing, asymptomatic swellings. Intraoral cysts can occur at any minor gland site, but most frequently they develop in the floor of the mouth, buccal mucosa, and lips (Fig. 11-10). They often look like mucoceles and are characterized by a soft, fluctuant swelling that may appear bluish, depending on the depth of the cyst below the surface. Some cysts may feel relatively firm to palpation. Cysts in the floor of the mouth often arise adjacent to the submandibular duct and sometimes have an amber color.
On rare occasions, patients have been observed to develop prominent ectasia of the excretory ducts of many of the minor salivary glands throughout the mouth. Such lesions have been termed “mucus retention cysts,” although they probably represent multi-focal ductal dilatation. The individual lesions often present as painful nodules that demonstrate dilated ductal orifices on the mucosal surface. Mucus or pus may be expressed from these dilated ducts.
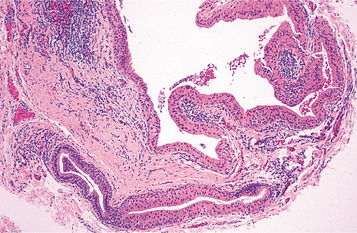
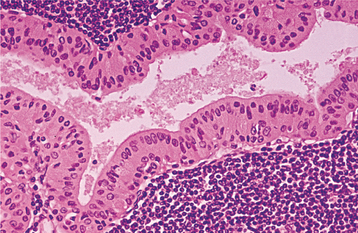
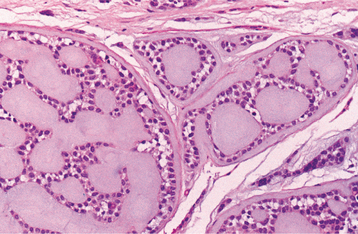
HISTOPATHOLOGIC FEATURES: The lining of the salivary duct cyst is variable and may consist of cuboidal, columnar, or atrophic squamous epithelium surrounding thin or mucoid secretions in the lumen (Fig. 11-11). In contrast, ductal ectasia secondary to salivary obstruction is characterized by oncocytic metaplasia of the epithelial lining. This epithelium often demonstrates papillary folds into the cystic lumen, somewhat reminiscent of a small Warthin tumor (see page 482) but without the prominent lymphoid stroma (Fig. 11-12). If this proliferation is extensive enough, then these lesions sometimes are diagnosed as papillary cystadenoma, although it seems likely that most are not true neoplasms. The individual lesions of patients with multiple “mucus retention cysts” also show prominent oncocytic metaplasia of the epithelial lining.
TREATMENT AND PROGNOSIS: Isolated salivary duct cysts are treated by conservative surgical excision. For cysts in the major glands, partial or total removal of the gland may be necessary. The lesion should not recur.
For rare patients who develop multifocal salivary ductal ectasia (“mucus retention cysts”), local excision may be performed for the more problematic swellings; however, surgical management does not appear feasible or advisable for all of the lesions, which may number as many as 100. In one reported case, systemic erythromycin and chlorhexidine mouth rinses were helpful in relieving pain and reducing drainage of pus. Sialagogue medications also may be helpful in stimulating salivary flow, thereby preventing the accumulation of inspissated mucus within the dilated excretory ducts.
SIALOLITHIASIS (SALIVARY CALCULI; SALIVARY STONES)
Sialoliths are calcified structures that develop within the salivary ductal system. Researchers believe that they arise from deposition of calcium salts around a nidus of debris within the duct lumen. This debris may include inspissated mucus, bacteria, ductal epithelial cells, or foreign bodies. The cause of sialoliths is unclear, but their formation can be promoted by chronic sialadenitis and partial obstruction. Their development is not related to any systemic derangement in calcium and phosphorus metabolism.
CLINICAL AND RADIOGRAPHIC FEATURES: Sialoliths most often develop within the ductal system of the submandibular gland; the formation of stones within the parotid gland system is distinctly less frequent. The long, tortuous, upward path of the submandibular (Wharton’s) duct and the thicker, mucoid secretions of this gland may be responsible for its greater tendency to form salivary calculi. Sialoliths also can form within the minor salivary glands, most often within the glands of the upper lip or buccal mucosa. Salivary stones can occur at almost any age, but they are most common in young and middle-aged adults.
Major gland sialoliths most frequently cause episodic pain or swelling of the affected gland, especially at mealtime. The severity of the symptoms varies, depending on the degree of obstruction and the amount of resultant backpressure produced within the gland. If the stone is located toward the terminal portion of the duct, then a hard mass may be palpated beneath the mucosa (Fig. 11-13).
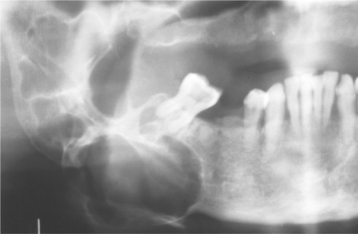
Sialoliths typically appear as radiopaque masses on radiographic examination. However, not all stones are visible on standard radiographs (perhaps because of the degree of calcification of some lesions). They may be discovered anywhere along the length of the duct or within the gland itself (Fig. 11-14). Stones in the terminal portion of the submandibular duct are best demonstrated with an occlusal radiograph. On panoramic or periapical radiographs, the calcification may appear superimposed on the mandible and care must be exercised not to confuse it with an intrabony lesion (Fig. 11-15). Multiple parotid stones radiographically can mimic calcified parotid lymph nodes, such as might occur in tuberculosis. Sialography, ultrasound, and CT scanning may be helpful additional imaging studies for sialoliths.
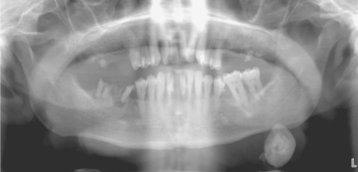
Fig. 11-14 Sialolithiasis. Radiopaque mass located at the left angle of the mandible. (Courtesy of Dr. Roger Bryant.)
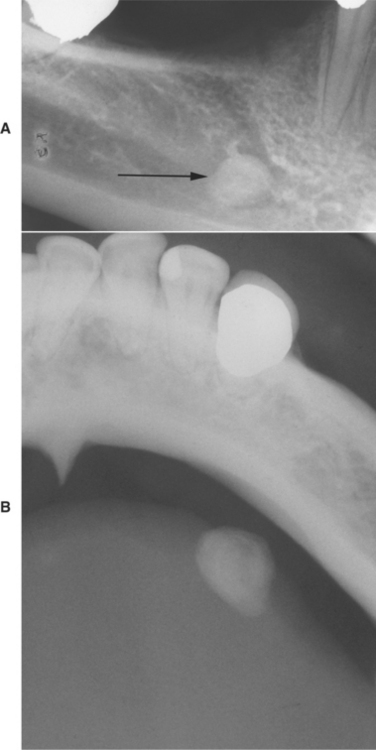
Fig. 11-15 Sialolithiasis. A, Periapical film showing discrete radiopacity (arrow) superimposed on the body of the mandible. Care must be taken not to confuse such lesions with intrabony pathosis. B, Occlusal radiograph of same patient demonstrating radiopaque stone in Wharton’s duct.
Minor gland sialoliths often are asymptomatic but may produce local swelling or tenderness of the affected gland (Fig. 11-16). A small radiopacity often can be demonstrated with a soft tissue radiograph.
HISTOPATHOLOGIC FEATURES: On gross examination, sialoliths appear as hard masses that are round, oval, or cylindrical. They are typically yellow, although they may be a white or yellow-brown color. Submandibular stones tend to be larger than those of the parotid or minor glands. Sialoliths are usually solitary, although occasionally two or more stones may be discovered at surgery.
Microscopically, the calcified mass exhibits concentric laminations that may surround a nidus of amorphous debris (Fig. 11-17). If the associated duct also is removed, then it often demonstrates squamous, oncocytic, or mucous cell metaplasia. Periductal inflammation is also evident. The ductal obstruction frequently is associated with an acute or chronic sialadenitis of the feeding gland.
TREATMENT AND PROGNOSIS: Small sialoliths of the major glands sometimes can be treated conservatively by gentle massage of the gland in an effort to milk the stone toward the duct orifice. Sialagogues (drugs that stimulate salivary flow), moist heat, and increased fluid intake also may promote passage of the stone. Larger sialoliths usually need to be removed surgically. If significant inflammatory damage has occurred within the feeding gland, then the gland may need to be removed. Minor gland sialoliths are best treated by surgical removal, including the associated gland.
Shock wave lithotripsy (extracorporeal or intracorporeal), salivary gland endoscopy, and radiologically guided basket retrieval are newer techniques that have been shown to be effective in the removal of sialoliths from the major glands. These minimally invasive techniques have low morbidity and may preclude the necessity of gland removal.
SIALADENITIS
Inflammation of the salivary glands (sialadenitis) can arise from various infectious and noninfectious causes. The most common viral infection is mumps (see page 263), although a number of other viruses also can involve the salivary glands, including Coxsackie A, ECHO, choriomeningitis, parainfluenza, and cytomegalovirus (CMV) (in neonates). Most bacterial infections arise as a result of ductal obstruction or decreased salivary flow, allowing retrograde spread of bacteria throughout the ductal system. Blockage of the duct can be caused by sialolithiasis (see page 459), congenital strictures, or compression by an adjacent tumor. Decreased flow can result from dehydration, debilitation, or medications that inhibit secretions.
One of the more common causes of sialadenitis is recent surgery (especially abdominal surgery), after which an acute parotitis (surgical mumps) may arise because the patient has been kept without food or fluids (NPO) and has received atropine during the surgical procedure. Other medications that produce xerostomia as a side effect also can predispose patients to such an infection. Most cases of acute bacterial sialadenitis are caused by Staphylococcus aureus, but they also may arise from streptococci or other organisms. Noninfectious causes of salivary inflammation include Sjögren syndrome (see page 466), sarcoidosis (see page 338), radiation therapy (see page 295), and various allergens.
CLINICAL AND RADIOGRAPHIC FEATURES: Acute bacterial sialadenitis is most common in the parotid gland and is bilateral in 10% to 25% of cases. The affected gland is swollen and painful, and the overlying skin may be warm and erythematous (Fig. 11-18). An associated low-grade fever and trismus may be present. A purulent discharge often is observed from the duct orifice when the gland is massaged (Fig. 11-19).
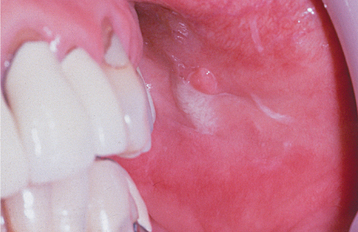
Fig. 11-19 Sialadenitis. A purulent exudate can be seen arising from Stensen’s duct when the parotid gland is massaged.
Recurrent or persistent ductal obstruction (most commonly caused by sialoliths) can lead to a chronic sialadenitis. Periodic swelling and pain occur within the affected gland, usually developing at mealtime when salivary flow is stimulated. In the submandibular gland, persistent enlargement may develop (Küttner tumor), which is difficult to distinguish from a true neoplasm. Sialography often demonstrates sialectasia (ductal dilatation) proximal to the area of obstruction (Fig. 11-20). In chronic parotitis, Stensen’s duct may show a characteristic sialographic pattern known as “sausaging,” which reflects a combination of dilatation plus ductal strictures from scar formation. Chronic sialadenitis also can occur in the minor glands, possibly as a result of blockage of ductal flow or local trauma.
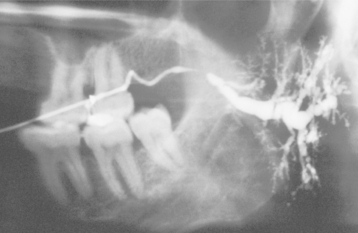
Fig. 11-20 Chronic sialadenitis. Parotid sialogram demonstrating ductal dilatation proximal to an area of obstruction. (Courtesy of Dr. George Blozis.)
Subacute necrotizing sialadenitis is a form of salivary inflammation that occurs most commonly in teenagers and young adults. The lesion usually involves the minor salivary glands of the hard or soft palate, presenting as a painful nodule that is covered by intact, erythematous mucosa. Unlike necrotizing sialometaplasia (see page 471), the lesion does not ulcerate or slough necrotic tissue. An infectious or allergic cause has been hypothesized.
HISTOPATHOLOGIC FEATURES: In patients with acute sialadenitis, accumulation of neutrophils is observed within the ductal system and acini. Chronic sialadenitis is characterized by scattered or patchy infiltration of the salivary parenchyma by lymphocytes and plasma cells. Atrophy of the acini is common, as is ductal dilatation. If associated fibrosis is present, then the term chronic sclerosing sialadenitis is used (Fig. 11-21).
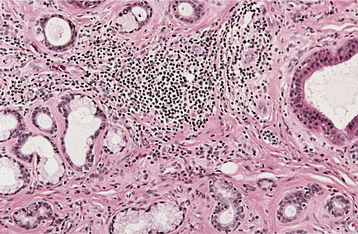
Fig. 11-21 Chronic sclerosing sialadenitis. Chronic inflammatory infiltrate with associated acinar atrophy, ductal dilatation, and fibrosis.
Subacute necrotizing sialadenitis is characterized by a heavy mixed inflammatory infiltrate consisting of neutrophils, lymphocytes, histiocytes, and eosinophils. There is loss of most of the acinar cells, and many of the remaining ones exhibit necrosis. The ducts tend to be atrophic and do not show hyperplasia or squamous metaplasia.
TREATMENT AND PROGNOSIS: The treatment of acute sialadenitis includes appropriate antibiotic therapy and rehydration of the patient to stimulate salivary flow. Surgical drainage may be needed if there is abscess formation. Although this regimen is usually sufficient, a 20% to 50% mortality rate has been reported in debilitated patients because of the spread of the infection and sepsis.
The management of chronic sialadenitis depends on the severity of the condition and ranges from conservative therapy to surgical intervention. Initial management often includes antibiotics, analgesics, sialagogues, and glandular massage. Early cases that develop secondary to ductal blockage may respond to removal of the sialolith or other obstruction. However, if sialectasia is present, then the dilated ducts can lead to stasis of secretions and predispose the gland to further sialolith formation. Sialadenoscopy and ductal irrigation are newer techniques that can be used to dilate ductal strictures and to eliminate sialoliths and mucus plugs. Second-line treatment options for chronic parotitis have included ligation of Stensen’s duct, but this method has a high failure rate. Tympanic neurectomy, which results in decreased secretion by the parotid gland via transection of the parasympathetic secretory fibers at the tympanic plexus, produces improvement in 75% of patients with chronic parotitis. If conservative methods cannot control chronic sialadenitis, then surgical removal of the affected gland may be necessary.
Subacute necrotizing sialadenitis is a self-limiting condition that usually resolves within 2 weeks of diagnosis without treatment.
CHEILITIS GLANDULARIS
Cheilitis glandularis is a rare inflammatory condition of the minor salivary glands. The cause is uncertain, although several etiologic factors have been suggested, including actinic damage, tobacco, syphilis, poor hygiene, and heredity.
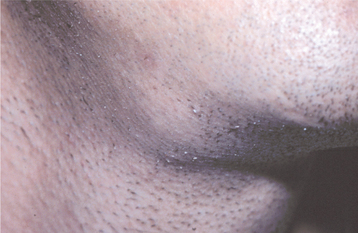
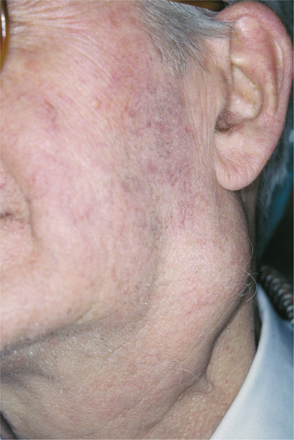
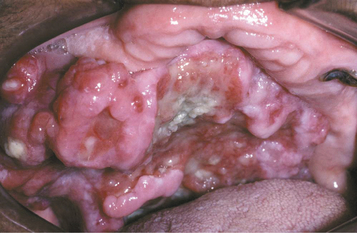
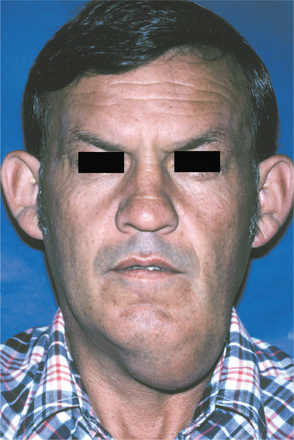
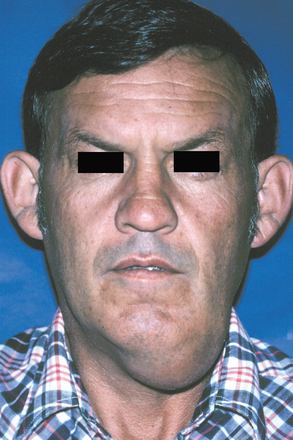
CLINICAL FEATURES: Cheilitis glandularis characteristically occurs on the lower lip, although there are also purported cases involving the upper lip and palate. Affected individuals experience swelling and eversion of the lower lip as a result of hypertrophy and inflammation of the glands (Fig. 11-22). The openings of the minor salivary ducts are inflamed and dilated, and pressure on the glands may produce mucopurulent secretions from the ductal openings. The condition most often has been reported in middle-aged and older men, although cases also have been described in women and children. However, some of the childhood cases may represent other entities, such as exfoliative cheilitis (see page 304).
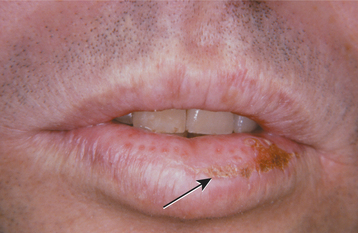
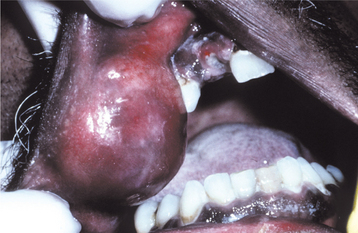
Fig. 11-22 Cheilitis glandularis. Prominent lower lip with inflamed openings of the minor salivary gland ducts. An early squamous cell carcinoma has developed on the patient’s left side just lateral to the midline (arrow). (Courtesy of Dr. George Blozis.)
Historically, cheilitis glandularis has been classified into three types, based on the severity of the disease:
The latter two types represent progressive stages of the disease with bacterial involvement; they are characterized by increasing inflammation, suppuration, ulceration, and swelling of the lip.
HISTOPATHOLOGIC FEATURES: The microscopic findings of cheilitis glandularis are not specific and usually consist of chronic sialadenitis and ductal dilatation. Concomitant dysplastic changes may be observed in the overlying surface epithelium in some cases.
TREATMENT AND PROGNOSIS: The treatment of choice for most cases of persistent cheilitis glandularis associated with actinic damage is vermilionectomy (lip shave), which usually produces a satisfactory cosmetic result. A significant percentage of cases (18% to 35%) have been associated with the development of squamous cell carcinoma of the overlying epithelium of the lip. Because actinic damage has been implicated in many cases of cheilitis glandularis, it is likely that this same solar radiation is responsible for the malignant degeneration.
SIALORRHEA
Sialorrhea, or excessive salivation, is an uncommon condition that has various causes. Minor sialorrhea may result from local irritations, such as aphthous ulcers or ill-fitting dentures. Patients with new dentures often experience excess saliva production until they become accustomed to the prosthesis. Episodic hypersecretion of saliva, or “water brash,” may occur as a protective buffering system to neutralize stomach acid in individuals with gastroesophageal reflux dis-ease. Sialorrhea is a well-known clinical feature of rabies and heavy-metal poisoning (see page 313). It also may occur as a consequence of certain medications, such as antipsychotic agents, especially clozapine, and cholinergic agonists used to treat dementia of the Alzheimer’s type and myasthenia gravis.
Drooling can be a problem for patients who are mentally retarded, who have undergone surgical resection of the mandible, or who have various neurologic disorders such as cerebral palsy, Parkinson’s disease, or amyotrophic lateral sclerosis (ALS). In these instances, the drooling is probably not caused by overproduction of saliva but by poor neuromuscular control.
In addition, there is a second group of patients who report complaints of drooling; however, no obvious clinical evidence of excessive saliva production is observed, and they do not have any of the recognized causes for sialorrhea. Personality analysis has suggested that the complaint of sialorrhea in such otherwise healthy patients does not have an organic basis but may be associated with high levels of neuroticism and a tendency to dissimulate.
CLINICAL FEATURES: The excess saliva production typically produces drooling and choking, which may cause social embarrassment. In children with mental retardation or cerebral palsy, the uncontrolled salivary flow may lead to macerated sores around the mouth, chin, and neck that can become secondarily infected. The constant soiling of clothes and bed linens can be a significant problem for the parents and caretakers of these patients.
An interesting type of supersalivation of unknown cause has been termed idiopathic paroxysmal sialorrhea. Individuals with this condition experience short episodes of excessive salivation lasting from 2 to 5 minutes. These episodes are associated with a prodrome of nausea or epigastric pain.
TREATMENT AND PROGNOSIS: Some causes of sialorrhea are transitory or mild, and no treatment is needed. For individuals with increased salivation associated with gastroesophageal reflux disease, medical management of their reflux problem may be beneficial.
For persistent severe drooling, therapeutic intervention may be indicated. Speech therapy can be used to improve neuromuscular control, but patient cooperation is necessary. Anticholinergic medications can decrease saliva production but may produce unacceptable side effects. Transdermal scopolamine has been tried with some success, but it should not be used in children younger than age 10. Intraglandular injection of botulinum toxin has been shown to be successful in reducing salivary secretions, with a duration of action that varies from 6 weeks to 6 months.
Several surgical techniques have been used successfully to control severe drooling in individuals with poor neuromuscular control:
• Relocation of the submandibular ducts (sometimes along with excision of the sublingual glands)
• Relocation of the parotid ducts
• Submandibular gland excision plus parotid duct ligation
• Ligation of the parotid and submandibular ducts
• Bilateral tympanic neurectomy with sectioning of the chorda tympani
In ductal relocation, the ducts are repositioned posteriorly to the tonsillar fossa, thereby redirecting salivary flow and minimizing drooling. The use of bilateral tympanic neurectomy and sectioning of the chorda tympani destroys parasympathetic innervation to the glands, reducing salivary secretions and possibly inducing xerostomia. However, this procedure also produces a loss of taste to the anterior two thirds of the tongue.
XEROSTOMIA
Xerostomia refers to a subjective sensation of a dry mouth; it is frequently, but not always, associated with salivary gland hypofunction. A number of factors may play a role in the cause of xerostomia, and these are listed in Box 11-1. Xerostomia is a common problem that has been reported in 25% of older adults. In the past, complaints of dry mouth in older patients often were ascribed to the predictable result of aging. However, it is now generally accepted that any reductions in salivary function associated with age are modest and probably are not associated with any significant reduction in salivary function. Instead, xerostomia in older adults is more likely to be the result of other factors, especially medications. More than 500 drugs have been reported to produce xerostomia as a side effect, including 63% of the 200 most frequently prescribed medicines in the United States. A list of the most common and significant drugs associated with xerostomia is provided in Table 11-2. Not only are specific drugs known to produce dry mouth, but the prevalence of xerostomia also increases in relation to the total number of drugs that a person takes, regardless of whether the individual medications are xerogenic or not.
Table 11-2
Medications That May Produce Xerostomia
| Class of Drug | Example |
| Antihistamine agents | Diphenhydramine |
| Chlorpheniramine | |
| Decongestant agents | Pseudoephedrine |
| Antidepressant agents | Amitriptyline |
| Citalopram | |
| Fluoxetine | |
| Paroxetine | |
| Sertraline | |
| Bupropion | |
| Antipsychotic agents | Phenothiazine derivatives |
| Haloperidol | |
| Sedatives and anxiolytic agents | Diazepam |
| Lorazepam | |
| Alprazolam | |
| Antihypertensive agents | Reserpine |
| Methyldopa | |
| Chlorothiazide | |
| Furosemide | |
| Metoprolol | |
| Calcium channel blockers | |
| Anticholinergic agents | Atropine |
| Scopolamine |
CLINICAL FEATURES: Examination of the patient typically demonstrates a reduction in salivary secretions, and the residual saliva appears either foamy or thick and “ropey.” The mucosa appears dry, and the clinician may notice that the examining gloves stick to the mucosal surfaces. The dorsal tongue often is fissured with atrophy of the filiform papillae (see Fig. 11-1). The patient may complain of difficulty with mastication and swallowing, and they may even indicate that food adheres to the oral membranes during eating. The clinical findings, however, do not always correspond to the patient’s symptoms. Some patients who complain of dry mouth may appear to have adequate salivary flow and oral moistness. Conversely, some patients who clinically appear to have a dry mouth have no complaints. The degree of saliva production can be assessed by measuring both resting and stimulated salivary flow.
There is an increased prevalence of oral candidiasis in patients with xerostomia because of the reduction in the cleansing and antimicrobial activity normally provided by saliva. In addition, these patients are more prone to dental decay, especially cervical and root caries. This problem has been associated more often with radiation therapy, and it is sometimes called radiation-induced caries but more appropriately should be called xerostomia-related caries (see page 296).
TREATMENT AND PROGNOSIS: The treatment of xerostomia is difficult and often unsatisfactory. Artificial salivas are available and may help make the patient more comfortable, as may continuous sips of water throughout the day. In addition, sugarless candy can be used in an effort to stimulate salivary flow. One of the better patient-accepted management approaches includes the use of oral hygiene products that contain lactoperoxidase, lysozyme, and lactoferrin (e.g., Biotene toothpaste and mouth rinse, Oralbalance gel). If the dryness is secondary to the patient’s medication, then discontinuation or dose modification in consultation with the patient’s physician may be considered; a substitute drug can also be tried.
Systemic pilocarpine is a parasympathomimetic agonist that can be used as a sialagogue. At doses of 5 to 10 mg, three to four times daily, it can be an effective promoter of salivary secretion. Excess sweating is a common side effect, but more serious problems, such as increased heart rate and blood pressure, are uncommon. The U.S. Food and Drug Administration (FDA) has also approved cevimeline hydrochloride, an acetylcholine derivative, for use as a sialagogue. Both pilocarpine and cevimeline are contraindicated in patients with narrow-angle glaucoma.
Because of the increased potential for dental caries in patients with xerostomia, frequent dental visits are recommended. Office and daily home fluoride applications can be used to help prevent decay, and chlorhexidine mouth rinses minimize plaque buildup.
BENIGN LYMPHOEPITHELIAL LESION (MYOEPITHELIAL SIALADENITIS): In the late 1800s, Johann von Mikulicz-Radecki described the case of a patient with an unusual bilateral painless swelling of the lacrimal glands and all of the salivary glands. Histopathologic examination of the involved glands showed an intense lymphocytic infiltrate, with features that today are recognized microscopically as the benign lymphoepithelial lesion. This clinical presentation became known as Mikulicz disease, and clinicians began using this term to describe a variety of cases of bilateral parotid and lacrimal enlargement. However, many of these cases were not examples of benign lymphoepithelial lesions microscopically; instead, they represented salivary and lacrimal involvement by other disease processes, such as tuberculosis, sarcoidosis, and lymphoma. These cases of parotid and lacrimal enlargement secondary to other diseases were later recognized as being different and termed Mikulicz syndrome, with the term Mikulicz disease reserved for cases associated with benign lymphoepithelial lesions. However, these two terms have become so confusing and ambiguous that they should no longer be used.
Many cases of so-called Mikulicz disease may be examples of what is now more commonly known as Sjögren syndrome (see page 466). Sjögren syndrome is an autoimmune disease that may produce bilateral salivary and lacrimal enlargement, with microscopic features of benign lymphoepithelial lesion. However, not all benign lymphoepithelial lesions are necessarily associated with the clinical disease complex of Sjögren syndrome.
CLINICAL FEATURES: Most benign lymphoepithelial lesions develop as a component of Sjögren syndrome. Those not associated with Sjögren syndrome are usually unilateral, although occasional bilateral examples are seen. On occasion, benign lymphoepithelial lesions occur in association with other salivary gland pathologic conditions, such as sialoliths and benign or malignant epithelial tumors.
The benign lymphoepithelial lesion most often develops in adults, with a mean age of 50. From 60% to 80% of cases occur in women. Eighty-five percent of cases occur in the parotid gland, with infrequent examples also reported in the submandibular gland and minor salivary glands. The lesion usually appears as a firm, diffuse swelling of the affected gland that sometimes is dramatic in size. It may be asymptomatic or associated with mild pain.
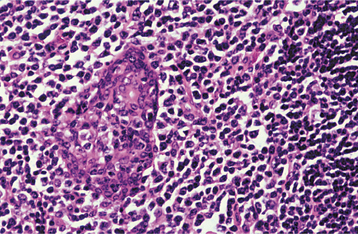
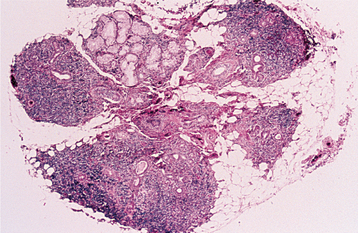
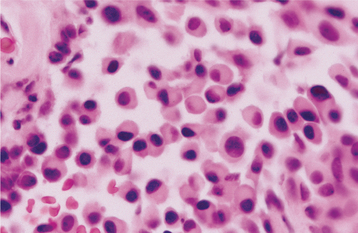
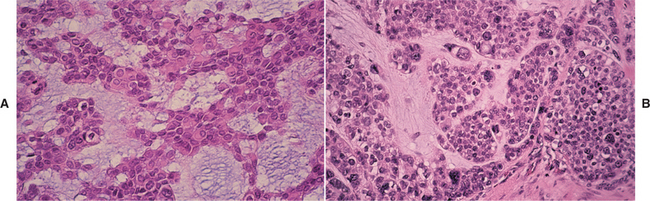
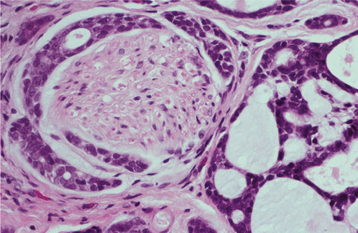
HISTOPATHOLOGIC FEATURES: Microscopic examination of the benign lymphoepithelial lesion shows a heavy lymphocytic infiltrate associated with the destruction of the salivary acini (Fig. 11-23). Germinal centers may or may not be seen. Although the acini are destroyed, the ductal epithelium persists. The ductal cells and surrounding myoepithelial cells become hyperplastic, forming highly characteristic groups of cells, known as epimyoepithelial islands, throughout the lymphoid proliferation. The presence of epimyoepithelial islands once was considered indicative of a benign process, but now it is recognized that these islands also can be found in low-grade salivary lymphomas of the mucosa-associated lymphoid tissue (MALT lymphomas, marginal zone B-cell lymphomas).
TREATMENT AND PROGNOSIS: Although the benign lymphoepithelial lesion fre-quently necessitates surgical removal of the involved gland, the prognosis in most cases is good. However, individuals with benign lymphoepithelial lesions have an increased risk for lymphoma, either within the affected gland or in an extrasalivary site. Although the exact risk is uncertain, one study showed the risk in patients with Sjögren syndrome to be more than 40 times higher than expected in the general population. (The management of patients with Sjögren syndrome is discussed on page 470.)
With the development of modern molecular techniques to assess for gene rearrangements and monoclonality within lymphoid infiltrates, it is now recognized that some lesions originally believed to represent benign lymphoepithelial lesions are actually early-stage MALT lymphomas. Many experts now recognize a spectrum of salivary lymphoid proliferations, which range from benign lymphoepithelial lesions to borderline lesions to frank lymphomas. Because of this, some authors have dropped the term benign from this spectrum and refer to these proliferations only as lymphoepithelial lesions. Fortunately, most MALT lymphomas are low-grade tumors that tend to remain localized with good survival rates. However, occasional tumors transform to higher-grade lymphomas with more aggressive behavior.
In addition, a rare malignant counterpart of this lesion, called a malignant lymphoepithelial lesion or lymphoepithelial carcinoma, represents a poorly differentiated salivary carcinoma with a prominent lymphoid stroma. Most of these lesions have occurred in Inuit and Asian populations and appear to have arisen de novo as carcinomas; however, some cases (especially in non-Inuits) have been reported to develop from a prior benign lymphoepithelial lesion. The Epstein-Barr virus (EBV) has been strongly associated in the cause of malignant lymphoepithelial lesions, especially those cases arising in Inuit and Asian populations. The 5-year survival rate has been reported to range from 70% to 85%.
SJÖGREN SYNDROME
Sjögren syndrome is a chronic, systemic autoimmune disorder that principally involves the salivary and lacrimal glands, resulting in xerostomia (dry mouth) and xerophthalmia (dry eyes). The effects on the eye often are called keratoconjunctivitis sicca (sicca means “dry”), and the clinical presentation of both xerostomia and xerophthalmia is also sometimes called the sicca syndrome. Two forms of the disease are recognized:
1. Primary Sjögren syndrome (sicca syndrome alone; no other autoimmune disorder is present)
2. Secondary Sjögren syndrome (the patient manifests sicca syndrome in addition to another associated autoimmune disease)
The cause of Sjögren syndrome is unknown. Although it is not a hereditary disease per se, there is evidence of a genetic influence. Examples of Sjögren syndrome have been reported in twins or in two or more members of the same family. Relatives of affected patients have an increased frequency of other autoimmune diseases. In addition, certain histocompatibility antigens (HLAs) are found with greater frequency in patients with Sjögren syndrome. HLA-DRw52 is associated with both forms of the disease; HLA-B8 and HLA-DR3 are seen with increased frequency in the primary form of the disease. Researchers have suggested that viruses, such as Epstein-Barr virus (EBV) or human T-cell lymphotropic virus, may play a pathogenetic role in Sjögren syndrome, but evidence for this is still speculative.
CLINICAL AND RADIOGRAPHIC FEATURES: Sjögren syndrome is not a rare condition. Although the exact prevalence depends on the clinical criteria used, current estimates place the population prevalence at 0.5%, with a 9:1 female-to-male ratio. It is seen predominantly in middle-aged adults, but rare examples have been described in children. The revised classification criteria suggested by the American-European Consensus Group are shown in Box 11-2.
When the condition is associated with another connective tissue disease, it is called secondary Sjögren syndrome. It can be associated with almost any other autoimmune disease, but the most common associated disorder is rheumatoid arthritis. About 15% of patients with rheumatoid arthritis have Sjögren syndrome. In addition, secondary Sjögren syndrome may develop in 30% of patients with systemic lupus erythematosus (SLE).
The principal oral symptom is xerostomia, which is caused by decreased salivary secretions; however, the severity of this dryness can vary widely from patient to patient. The saliva may appear frothy, with a lack of the usual pooling saliva in the floor of the mouth. Affected patients may complain of difficulty in swallowing, altered taste, or difficulty in wearing dentures. The tongue often becomes fissured and exhibits atrophy of the papillae (Fig. 11-24). The oral mucosa may be red and tender, usually as a result of secondary candidiasis. Related denture sore mouth and angular cheilitis are common. The lack of salivary cleansing action predisposes the patient to dental decay, especially cervical caries.
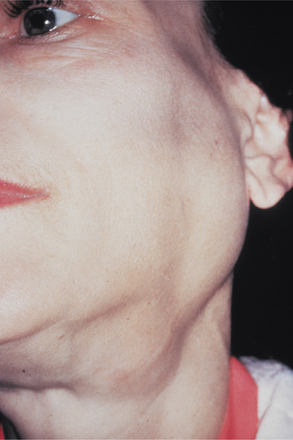
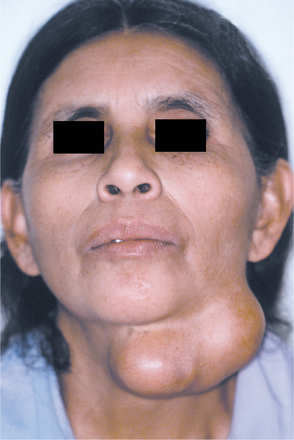
From one third to one half of patients have diffuse, firm enlargement of the major salivary glands during the course of their disease (Fig. 11-25). This swelling is usually bilateral, may be nonpainful or slightly tender, and may be intermittent or persistent in nature. The greater the severity of the disease, the greater the likelihood of this salivary enlargement. In addition, the reduced salivary flow places these individuals at increased risk for retrograde bacterial sialadenitis.
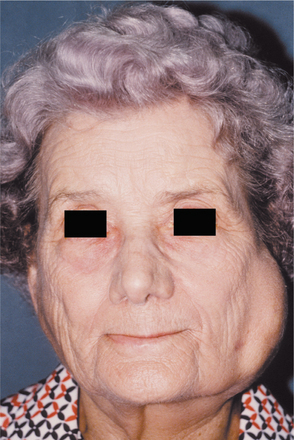
Fig. 11-25 Sjögren syndrome. Benign lymphoepithelial lesion of the parotid gland. (Courtesy of Dr. David Schaffner.)
Although it is not diagnostic, sialographic examination often reveals punctate sialectasia and lack of normal arborization of the ductal system, typically demonstrating a “fruit-laden, branchless tree” pattern (Fig. 11-26). Scintigraphy with radioactive technetium-99m pertechnetate characteristically shows decreased uptake and delayed emptying of the isotope.
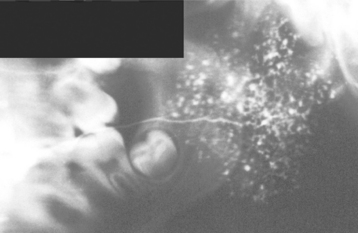
Fig. 11-26 Sjögren syndrome. Parotid sialogram demonstrating atrophy and punctate sialectasia (“fruit-laden, branchless tree”). (Courtesy of Dr. George Blozis.)
The term keratoconjunctivitis sicca describes not only the reduced tear production by the lacrimal glands but also the pathologic effect on the epithelial cells of the ocular surface. As in xerostomia, the severity of xerophthalmia can vary widely from one patient to the next. The lacrimal inflammation causes a decrease of the aqueous layer of the tear film; however, mucin production is normal and may result in a mucoid discharge. Patients often complain of a scratchy, gritty sensation or the perceived presence of a foreign body in the eye. Defects of the ocular surface epithelium develop and can be demonstrated with rose bengal dye. Vision may become blurred, and sometimes there is an aching pain. The ocular manifestations are least severe in the morning on wakening and become more pronounced as the day progresses.
A simple means to confirm the decreased tear secretion is the Schirmer test. A standardized strip of sterile filter paper is placed over the margin of the lower eyelid, between the medial and lateral third of the lid of the unanesthetized eye, so that the tabbed end rests just inside the lower lid. By measuring the length of wetting of the filter paper, tear production can be assessed. Values less than 5 mm (after a 5-minute period) are considered diagnostic of keratoconjunctivitis sicca.
Sjögren syndrome is a systemic disease, and the inflammatory process also can affect various other body tissues. The skin is often dry, as are the nasal and vaginal mucosae. Fatigue is fairly common, and depression sometimes can occur. Other possible associated problems include lymphadenopathy, primary biliary cirrhosis, Raynaud’s phenomenon, interstitial nephritis, interstitial lung fibrosis, vasculitis, and peripheral neuropathies.
LABORATORY VALUES: In patients with Sjögren syndrome, the erythrocyte sedimentation rate is high and serum immunoglobulin (Ig) levels, especially IgG, typically are elevated. A variety of autoantibodies can be produced, and although none of these is specifically diagnostic, their presence can be another helpful clue to the diagnosis. A positive rheumatoid factor (RF) is found in approximately 60% of cases, regardless of whether the patient has rheumatoid arthritis. Antinuclear antibodies (ANAs) are also present in 75% to 85% of patients. Two particular nuclear autoantibodies—anti-SS-A (anti-Ro) and anti-SS-B (anti-La)—may be found, especially in patients with primary Sjögren syndrome. Anti-SS-A antibodies have been detected in approximately 40% of patients, whereas anti-SS-B antibodies have been discovered in about 25% of these individuals. Occasionally, salivary duct autoantibodies also can be demonstrated, usually in secondary Sjögren syndrome. However, because these are infrequent in primary cases, they are believed to occur as a secondary phenomenon (rather than playing a primary role in pathogenesis).
HISTOPATHOLOGIC FEATURES: The basic microscopic finding in Sjögren syndrome is a lymphocytic infiltration of the salivary glands, with destruction of the acinar units. If the major glands are enlarged, then microscopic examination usually shows progression to a lymphoepithelial lesion (see page 466), with characteristic epimyoepithelial islands in a background lymphoid stroma. Lymphocytic infiltration of the minor glands also occurs, although epimyoepithelial islands are rarely seen in this location.
Biopsy of the minor salivary glands of the lower lip has become a useful test in the diagnosis of Sjögren syndrome. A 1.5- to 2.0-cm incision is made on clinically normal lower labial mucosa, parallel to the vermilion border and lateral to the midline, allowing the harvest of five or more accessory glands. These glands then are examined histopathologically for the presence of focal chronic inflammatory aggregates (50 or more lymphocytes and plasma cells). These aggregates should be adjacent to normal-appearing acini and should be found consistently in most of the glands in the specimen. The finding of more than one focus of 50 or more cells within a 4-mm2 area of glandular tissue is considered supportive of the diagnosis of Sjögren syndrome (Fig. 11-27). The greater the number of foci (up to 10 or confluent foci) is, the greater is the correlation with this diagnosis. The focal nature of this chronic inflammation among otherwise normal acini is a highly suggestive pattern; in contrast, the finding of scattered inflammation with ductal dilatation and fibrosis (chronic sclerosing sialadenitis) does not support the diagnosis of Sjögren syndrome.
Although labial salivary gland biopsy has become a widely used test in the diagnosis of Sjögren syndrome, it is not 100% reliable. Some patients diagnosed with Sjögren syndrome will show no significant labial gland inflammation; conversely, examination of labial glands removed incidentally from non-Sjögren patients sometimes will show focal lymphocytic infiltrates. Sjögren syndrome patients who smoke have been shown to have a significantly lower frequency of abnormal lymphocytic foci scores in their labial gland specimens. It also is important that a pathologist experienced in the analysis of these specimens examines the labial gland biopsies. One study showed that slightly more than half of labial gland specimens required a revised diagnosis after being reviewed by a second pathologist.
Other authors have advocated incisional biopsy of the parotid gland through a posterior auricular approach instead of a labial salivary gland biopsy. One study has shown this technique to be more sensitive in demonstrating inflammatory changes that support the diagnosis of Sjögren syndrome; however, other authors think that this technique confers no increased benefit over labial gland biopsy. Parotid biopsy may enable the clinician to evaluate an enlarged gland for the development of lymphoma and rule out the possibility of sialadenosis or sarcoidosis.
TREATMENT AND PROGNOSIS: The treatment of the patient with Sjögren syndrome is mostly supportive. The dry eyes are best managed by periodic use of artificial tears. In addition, attempts can be made to conserve the tear film through the use of sealed glasses to prevent evaporation. Sealing the lacrimal punctum at the inner margin of the eyelids also can be helpful by blocking the normal drainage of any lacrimal secretions into the nose.
Artificial salivas are available for the treatment of xerostomia; sugarless candy or gum can help to keep the mouth moist. Symptoms often can be relieved by the use of oral hygiene products that contain lactoperoxidase, lysozyme, and lactoferrin (e.g., Biotene toothpaste and mouth rinse, Oralbalance gel). Sialagogue medications, such as pilocarpine and cevimeline, can be useful to stimulate salivary flow if enough functional salivary tissue still remains. Medications known to diminish secretions should be avoided, if at all possible. Because of the increased risk of dental caries, daily fluoride applications may be indicated in dentulous patients. Antifungal therapy often is needed to treat secondary candidiasis.
Patients with Sjögren syndrome have an increased risk for lymphoma, up to 40 times higher than the normal population. These tumors may arise initially within the salivary glands or within lymph nodes. With the advent of modern molecular pathology techniques to detect B-cell monoclonality (e.g., in situ hybridization, polymerase chain reaction [PCR]), many salivary gland infiltrates formerly thought to represent benign lymphoepithelial lesions are now being diagnosed as lymphomas. These tumors are predominantly low-grade non-Hodgkin’s B-cell lymphomas of the mucosa-associated lymphoid tissue (i.e., MALT lymphomas), although occasionally, high-grade MALT lymphomas can develop that demonstrate more aggressive behavior. The detection of immunoglobulin gene rearrangements in labial salivary gland biopsies may prove to be a useful marker for predicting the development of lymphoma.
SIALADENOSIS (SIALOSIS)
Sialadenosis is an unusual noninflammatory disorder characterized by salivary gland enlargement, particularly involving the parotid glands. The condition frequently is associated with an underlying systemic problem, which may be endocrine, nutritional, or neurogenic in origin (Box 11-3). The best known of these conditions include diabetes mellitus, general malnutrition, alcoholism, and bulimia.
These conditions are believed to result in dysregulation of the autonomic innervation of the salivary acini, causing an aberrant intracellular secretory cycle. This leads to excessive accumulation of secretory granules, with marked enlargement of the acinar cells.
CLINICAL AND RADIOGRAPHIC FEATURES: Sialadenosis usually appears as a slowly evolving swelling of the parotid glands, which may or may not be painful (Fig. 11-28). The condition is usually bilateral, but it also can be unilateral. In some patients, the submandibular glands may be affected, but involvement of minor salivary glands is distinctly rare. Decreased salivary secretion may occur. Sialography demon-strates a “leafless tree” pattern, which is thought to be caused by compression of the finer ducts by hypertrophic acinar cells.
HISTOPATHOLOGIC FEATURES: Microscopic examination reveals hypertrophy of the acinar cells, sometimes two to three times greater than normal size. The nuclei are displaced to the cell base, and the cytoplasm is engorged with zymogen granules. In cases associated with long-standing diabetes or alcoholism, there may be acinar atrophy and fatty infiltration. Significant inflammation is not observed.
TREATMENT AND PROGNOSIS: The clinical management of sialadenosis is often unsatisfactory because it is closely related to the control of the underlying cause. Mild examples may cause few pro-blems. If the swelling becomes a cosmetic concern, then partial parotidectomy can be performed. Pilocarpine recently has been reported to be beneficial in reducing salivary gland enlargement in bulimic patients.
ADENOMATOID HYPERPLASIA OF THE MINOR SALIVARY GLANDS
CLINICAL FEATURES: Adenomatoid hyperplasia is a rare lesion of the minor salivary glands characterized by localized swelling that mimics a neoplasm. This pseudotumor most often occurs on the hard or soft palate, although it also has been reported in other oral minor salivary gland sites. The pathogenesis of adenomatoid hyperplasia is uncertain, but it has been speculated that local trauma may play a role. It is most common in the fourth to sixth decades of life. Most examples present as sessile, painless masses that may be soft or firm to palpation. They usually are normal in color, although a few lesions are red or bluish.
HISTOPATHOLOGIC FEATURES: Microscopic examination demonstrates lobular aggregates of relatively normal-appearing mucous acini that are greater in number than normally would be found in the area. These glands also sometimes appear to be increased in size. In some instances, the glands are situated close to the mucosal surface. Chronic inflammation occasionally is seen, but it usually is mild and localized.
NECROTIZING SIALOMETAPLASIA
Necrotizing sialometaplasia is an uncommon, locally destructive inflammatory condition of the salivary glands. Although the cause is uncertain, most authors believe it is the result of ischemia of the salivary tissue that leads to local infarction. The importance of this lesion rests in the fact that it mimics a malignant process, both clinically and microscopically.
A number of potential predisposing factors have been suggested, including the following:
Researchers have suggested that these factors may play a role in compromising the blood supply to the involved glands, resulting in ischemic necrosis. However, many cases occur without any known predisposing factors.
CLINICAL FEATURES: Necrotizing sialometaplasia most frequently develops in the palatal salivary glands; more than 75% of all cases occur on the posterior palate. The hard palate is affected more often than the soft palate. About two thirds of palatal cases are unilateral, with the rest being bilateral or midline in location. Necrotizing sialometaplasia also has been reported in other minor salivary gland sites and, occasionally, in the parotid gland. The submandibular and sublingual glands are rarely affected. Although it can occur at almost any age, necrotizing sialometaplasia is most common in adults; the mean age of onset is 46 years. Males are affected nearly twice as often as females.
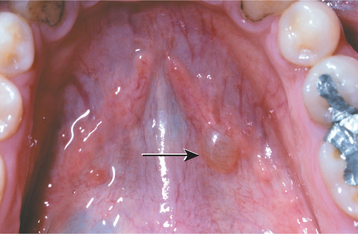
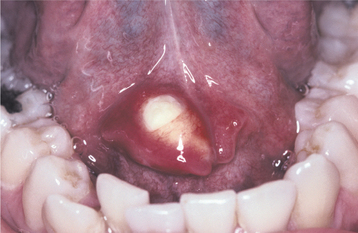
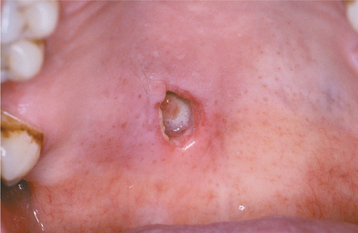
The condition appears initially as a nonulcerated swelling, often associated with pain or paresthesia (Fig. 11-29). Within 2 to 3 weeks, necrotic tissue sloughs out, leaving a craterlike ulcer that can range from less than 1 cm to more than 5 cm in diameter (Fig. 11-30). The patient may report that “a part of my palate fell out.” At this point, the pain often subsides. In rare instances, there can be destruction of the underlying palatal bone.
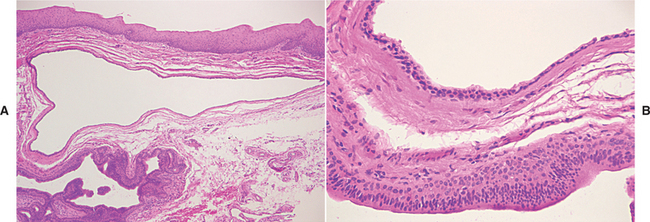
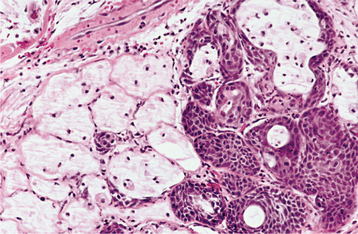
HISTOPATHOLOGIC FEATURES: The microscopic appearance of necrotizing sialometaplasia is characterized by acinar necrosis in early lesions, followed by associated squamous metaplasia of the salivary ducts (Fig. 11-31). Although the mucous acinar cells are necrotic, the overall lobular architecture of the involved glands is still preserved—a helpful histopathologic clue. There may be liberation of mucin, with an associated inflammatory response. The squamous metaplasia of the salivary ducts can be striking and produce a pattern that is easily misdiagnosed as squamous cell carcinoma or mucoepidermoid carcinoma. The frequent association of pseudoepitheliomatous hyperplasia of the overlying epithelium may further compound this mistaken impression. In most cases, however, the squamous proliferation has a bland cytologic appearance.
TREATMENT AND PROGNOSIS: Because of the worrisome clinical presentation of necrotizing sialometaplasia, biopsy usually is indicated to rule out the possibility of malignant disease. Once the diagnosis has been established, no specific treatment is indicated or necessary. The lesion typically resolves on its own accord, with an average healing time of 5 to 6 weeks.
Salivary Gland Tumors
Tumors of the salivary glands constitute an important area in the field of oral and maxillofacial pathology. Although such tumors are uncommon, they are by no means rare. The annual incidence of salivary gland tumors around the world ranges from about 1.0 to 6.5 cases per 100,000 people. Although soft tissue neoplasms (e.g., hemangioma), lymphoma, and metastatic tumors can occur within the salivary glands, the discussion in this chapter is limited to primary epithelial neoplasms.
An often-bewildering array of different salivary tumors has been identified and categorized. In addition, the classification scheme is a dynamic one that changes as clinicians learn more about these lesions. Box 11-4 includes most of the currently recognized tumors. Some of the tumors on this list are not specifically discussed because their rarity places them outside the scope of this text.
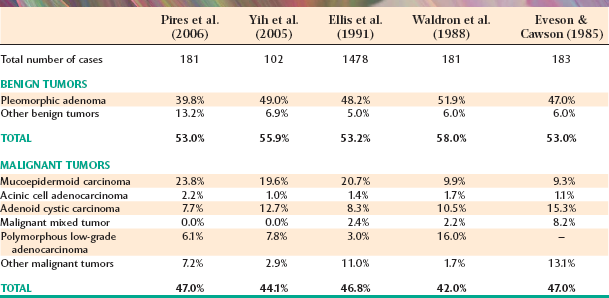
A number of investigators have published their findings on salivary gland neoplasia, but a comparison of these studies is often difficult. Some studies have been limited to only the major glands or have not included all the minor salivary gland sites. In addition, the ever-evolving classification system makes an evaluation of some older studies difficult, especially when researchers attempt to compare them with more recent analyses. (For example, the polymorphous low-grade adenocarcinoma was first identified in 1983, but clinicians now recognize that it is one of the more common malignancies in the minor glands.) Notwithstanding these difficulties, it is still helpful to compare these studies because they provide a good overview of salivary neoplasia in general. An evaluation of various studies shows fairly consistent trends (with minor variations) with regard to salivary gland tumors.
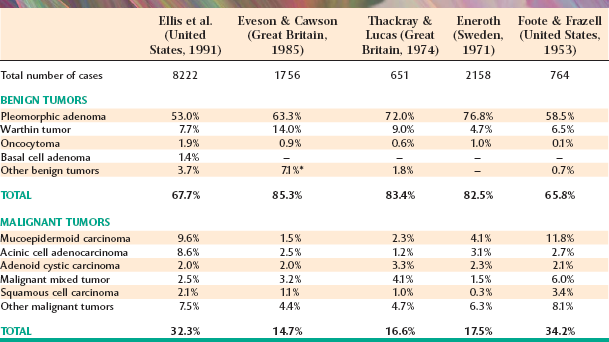
Tables 11-3 and 11-4 summarize four large series of primary epithelial salivary gland tumors, analyzed by sites of occurrence and frequency of malignancy, respectively. Some variations between studies may represent differences in diagnostic criteria, geographic differences, or referral bias in the cases seen. (Some centers may tend to see more malignant tumors on referral from other sources.)
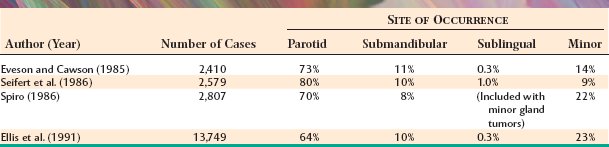
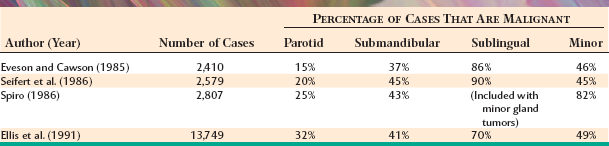
The most common site for salivary gland tumors is the parotid gland, accounting for 64% to 80% of all cases. Fortunately, a relatively low percentage of paro-tid tumors are malignant, ranging from 15% to 32%. Overall, it can be stated that two thirds to three quarters of all salivary tumors occur in the parotid gland, and two thirds to three quarters of these parotid tumors are benign.
Table 11-5 summarizes five large, well-known series of parotid neoplasms. The pleomorphic adenoma is overwhelmingly the most common tumor (53% to 77% of all cases in the parotid gland). Warthin tumors are also fairly common; they account for 6% to 14% of cases. A variety of malignant tumors occur, with the mucoepidermoid carcinoma appearing to be the most frequent overall. However, two studies from Great Britain show a significantly lower prevalence of this tumor, possibly indicative of a geographic difference, especially compared with reports of cases from the United States.
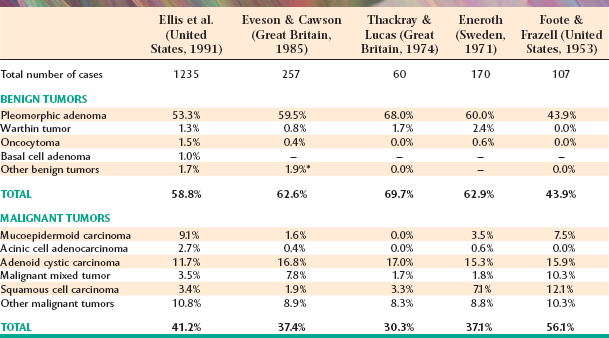
From 8% to 11% of all salivary tumors occur in the submandibular gland, but the frequency of malignancy in this gland is almost double that of the parotid gland, ranging from 37% to 45%. However, as shown in Table 11-6, the pleomorphic adenoma is still the most common tumor and makes up 44% to 68% of all neoplasms. Unlike its occurrence in the parotid gland, the Warthin tumor is unusual in the submandibular gland, making up no more than 1% to 2% of all tumors. Adenoid cystic carcinoma is the most common malignancy, ranging from 12% to 27% of all cases.
Tumors of the sublingual gland are rare, comprising no more than 1% of all salivary neoplasms. However, 70% to 90% of sublingual tumors are malignant.
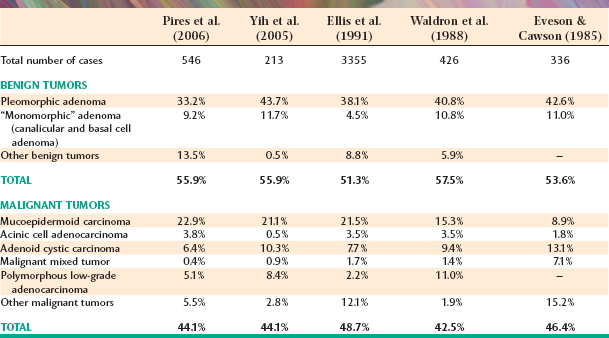
Tumors of the various smaller minor salivary glands make up 9% to 23% of all tumors, which makes this group the second most common site for salivary neoplasia. Table 11-7 summarizes the findings of five large surveys of minor gland tumors. Unfortunately, a relatively high proportion (almost 50%) of these have been malignant in most studies. Excluding rare sublingual tumors, it can be stated that the smaller the gland is, the greater is the likelihood of malignancy for a salivary gland tumor.
As observed in the major glands, the pleomorphic adenoma is the most common minor gland tumor and accounts for about 40% of all cases. Mucoepidermoid carcinoma and adenoid cystic carcinoma generally have been considered the two most common malignancies, although polymorphous low-grade adenocarcinoma also is becoming recognized as one of the more common minor gland tumors.
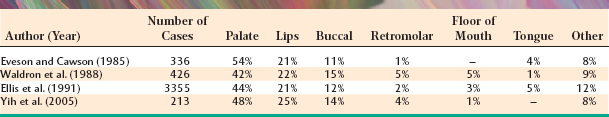
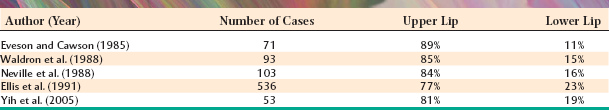
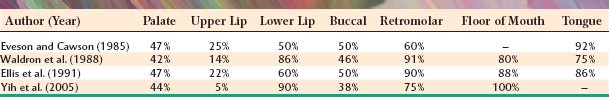
The palate is the most frequent site for minor salivary gland tumors, with 42% to 54% of all cases found there (Table 11-8). Most of these occur on the posterior lateral hard or soft palate, which have the greatest concentration of glands. Table 11-9 shows the relative prevalence of various tumors on the palate. The lips are the second most common location for minor gland tumors (21% to 25% of cases), followed by the buccal mucosa (11% to 15% of cases). Labial tumors are significantly more common in the upper lip, which accounts for 77% to 89% of all lip tumors (Table 11-10). Although mucoceles are commonly found on the lower lip, this is a surprisingly rare site for salivary gland tumors.
Significant differences in the percentage of malignancies and the relative frequency of various tumors can be noted for different minor salivary gland sites. As shown in Table 11-11, 38% to 50% of tumors of the palate and buccal mucosa sites are malignant, similar to the overall prevalence of malignancy in all minor salivary gland sites combined. In the upper lip, however, only 5% to 25% of tumors are malignant because of the high prevalence of the canalicular adenoma, which has a special affinity for this location. In contrast, although lower lip tumors are uncommon, 50% to 90% are malignant (mostly mucoepidermoid carcinomas). Up to 91% of retromolar tumors are malignant, also because of a predominance of mucoepidermoid carcinomas. Unfortunately, most tumors in the floor of the mouth and tongue are also malignant.
PLEOMORPHIC ADENOMA (BENIGN MIXED TUMOR)
The pleomorphic adenoma, or benign mixed tumor, is easily the most common salivary neoplasm. It accounts for 53% to 77% of parotid tumors, 44% to 68% of submandibular tumors, and 33% to 43% of minor gland tumors.
Pleomorphic adenomas are derived from a mixture of ductal and myoepithelial elements. A remarkable microscopic diversity can exist from one tumor to the next, as well as in different areas of the same tumor. The terms pleomorphic adenoma and mixed tumor both represent attempts to describe this tumor’s unusual histopathologic features, but neither term is entirely accurate. Although the basic tumor pattern is highly variable, rarely are the individual cells actually pleomorphic. (However, focal minor atypia is acceptable.) Likewise, although the tumor often has a prominent mesenchyme-appearing “stromal” component, it is not truly a mixed neoplasm that is derived from more than one germ layer.
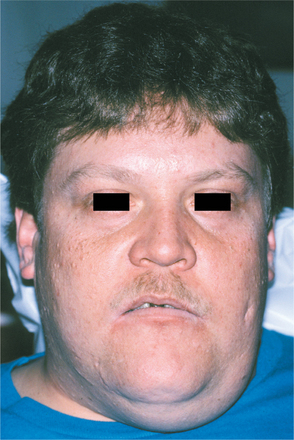
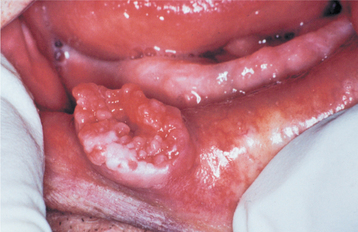
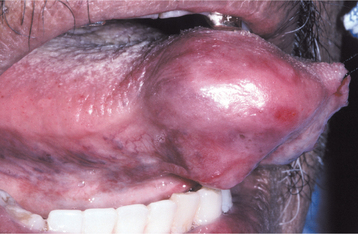
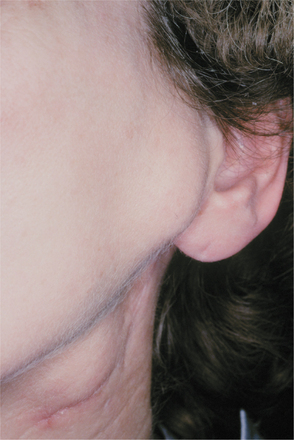
CLINICAL AND RADIOGRAPHIC FEATURES: Regardless of the site of origin, the pleomorphic adenoma typically appears as a painless, slowly growing, firm mass (Figs. 11-32 to 11-34). The patient may be aware of the lesion for many months or years before seeking a diagnosis. The tumor can occur at any age but is most common in young and middle-aged adults between the ages of 30 and 60. Pleomorphic adenoma is also the most common primary salivary gland tumor to develop during childhood. There is a slight female predilection.
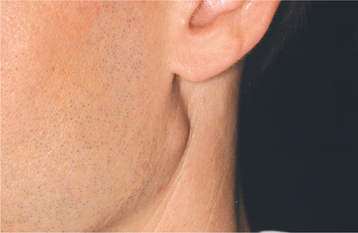
Fig. 11-32 Pleomorphic adenoma. Small, firm nodule located below the left ear in the parotid gland. (Courtesy of Dr. Mike Hansen.)
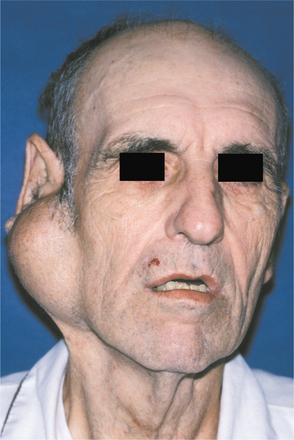
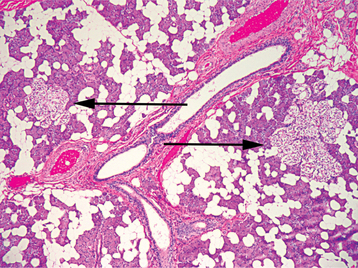
Most pleomorphic adenomas of the parotid gland occur in the superficial lobe and present as a swelling overlying the mandibular ramus in front of the ear. Facial nerve palsy and pain are rare. Initially, the tumor is movable but becomes less mobile as it grows larger. If neglected, then the lesion can grow to grotesque proportions. About 10% of parotid mixed tumors develop within the deep lobe of the gland beneath the facial nerve (Fig. 11-35). Sometimes these lesions grow in a medial direction between the ascending ramus and stylomandibular ligament, resulting in a dumbbell-shaped tumor that appears as a mass of the lateral pharyngeal wall or soft palate. On rare occasions, bilateral pleomorphic adenomas of the parotid glands have been reported, developing in either a synchronous or metachronous fashion.
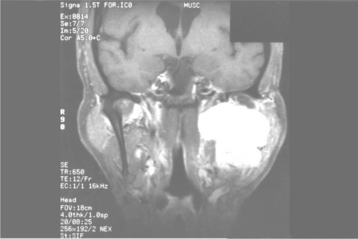
Fig. 11-35 Pleomorphic adenoma. T1-weighted, fat-suppressed, contrast-enhanced coronal magnetic resonance image (MRI) of a tumor of the deep lobe of the parotid gland. (Courtesy of Dr. Joel Curé.)
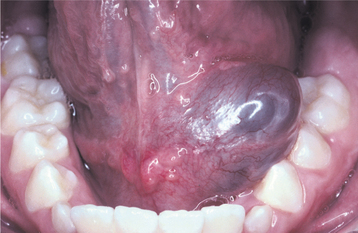
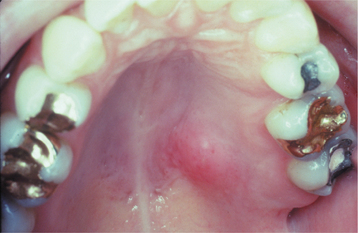
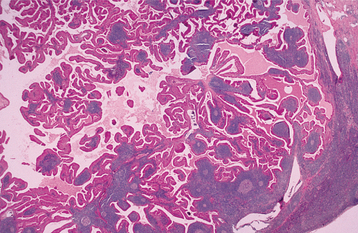
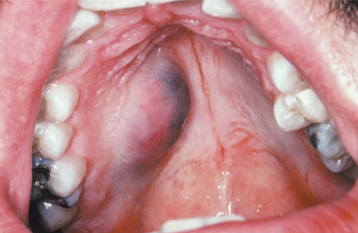
The palate is the most common site for minor gland mixed tumors, accounting for approximately 50% of intraoral examples. This is followed by the upper lip (27%) and buccal mucosa (17%). Palatal tumors almost always are found on the posterior lateral aspect of the palate, presenting as smooth-surfaced, dome-shaped masses (Figs. 11-36 and 11-37). If the tumor is traumatized, then secondary ulceration may occur. Because of the tightly bound nature of the hard palate mucosa, tumors in this location are not movable, although those in the lip or buccal mucosa frequently are mobile.
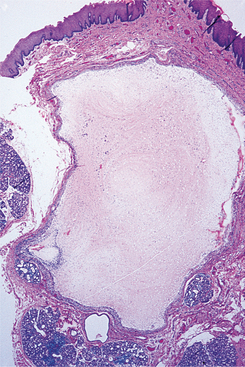
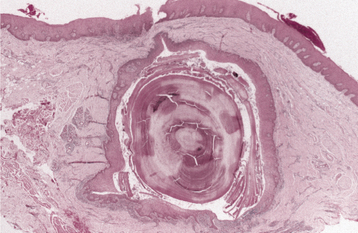
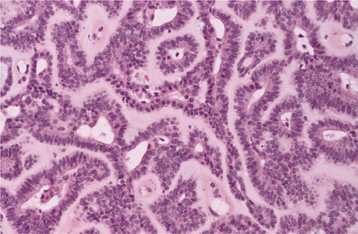
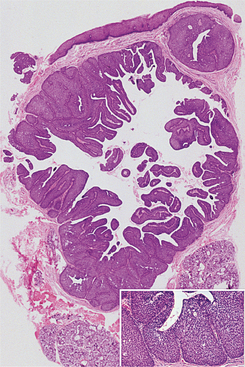
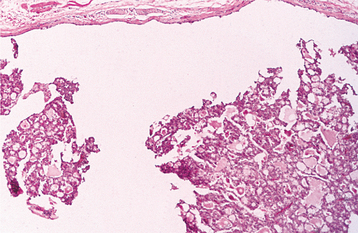
HISTOPATHOLOGIC FEATURES: The pleomorphic adenoma is typically a well-circumscribed, encapsulated tumor (Fig. 11-38). However, the capsule may be incomplete or show infiltration by tumor cells. This lack of complete encapsulation is more common for minor gland tumors, especially along the superficial aspect of palatal tumors beneath the epithelial surface.
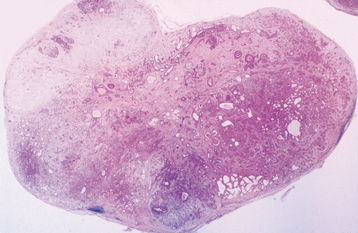
Fig. 11-38 Pleomorphic adenoma. Low-power view showing a well-circumscribed, encapsulated tumor mass. Even at this power, the variable microscopic pattern of the tumor is evident.
The tumor is composed of a mixture of glan-dular epithelium and myoepithelial cells within a mesenchyme-like background. The ratio of the epithelial elements and the mesenchyme-like component is highly variable among different tumors. Some tumors may consist almost entirely of background “stroma.” Others are highly cellular with little background alteration.
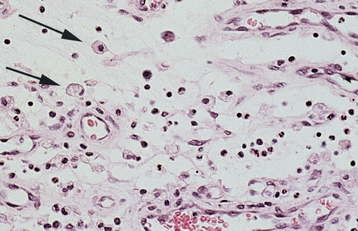
The epithelium often forms ducts and cystic structures or may occur as islands or sheets of cells. Keratinizing squamous cells and mucus-producing cells also can be seen. Myoepithelial cells often make up a large percentage of the tumor cells and have a variable morphology, sometimes appearing angular or spindled. Some myoepithelial cells are rounded and demonstrate an eccentric nucleus and eosinophilic hyalinized cytoplasm, thus resembling plasma cells (Fig. 11-39). These characteristic plasmacytoid myoepithelial cells are more prominent in tumors arising in the minor glands.
The highly characteristic “stromal” changes are believed to be produced by the myoepithelial cells. Extensive accumulation of mucoid material may occur between the tumor cells, resulting in a myxomatous background (Fig. 11-40). Vacuolar degeneration of cells in these areas can produce a chondroid appearance (Fig. 11-41). In many tumors, the stroma exhibits areas of an eosinophilic, hyalinized change (Fig. 11-42). At times, fat or osteoid also is seen.
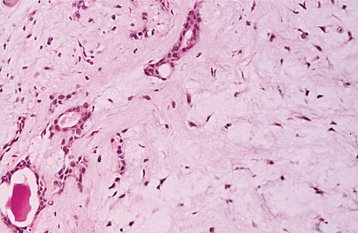
Fig. 11-40 Pleomorphic adenoma. Ductal structures (left) with associated myxomatous background (right).
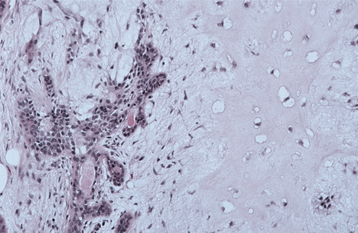
Fig. 11-41 Pleomorphic adenoma. Chondroid material (right) with adjacent ductal epithelium and myoepithelial cells.
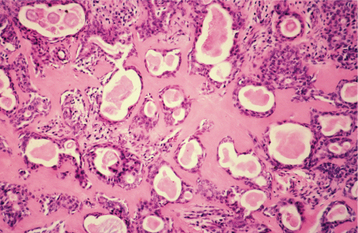
Fig. 11-42 Pleomorphic adenoma. Many of the ducts and myoepithelial cells are surrounded by a hyalinized, eosinophilic background alteration.
Occasionally, salivary tumors are seen that are composed almost entirely of myoepithelial cells with no ductal elements. Such tumors often are called myoepitheliomas, although they probably represent one end of the spectrum of mixed tumors.
TREATMENT AND PROGNOSIS: Pleomorphic adenomas are best treated by surgical excision. For lesions in the superficial lobe of the parotid gland, superficial parotidectomy with identification and preservation of the facial nerve is recommended. Local enucleation should be avoided because the entire tumor may not be removed or the capsule may be violated, resulting in seeding of the tumor bed. For tumors of the deep lobe of the parotid, total parotidectomy is usually necessary, also with preservation of the facial nerve, if possible. Submandibular tumors are best treated by total removal of the gland with the tumor. Tumors of the hard palate usually are excised down to periosteum, including the overlying mucosa. In other oral sites the lesion often enucleates easily through the incision site.
With adequate surgery the prognosis is excellent, with a cure rate of more than 95%. The risk of recurrence appears to be lower for tumors of the minor glands. Conservative enucleation of parotid tumors often results in recurrence, with management of these cases made difficult as a result of multifocal seeding of the primary tumor bed. In such cases, multiple recurrences are not unusual. Tumors with a predominantly myxoid appearance are more susceptible to recur than those with other microscopic patterns.
Malignant degeneration is a potential complication, resulting in a carcinoma ex pleomorphic adenoma (see page 492). The risk of malignant transformation is probably small, but it may occur in as many as 5% of all cases.
ONCOCYTOMA (OXYPHILIC ADENOMA)
The oncocytoma is a benign salivary gland tumor composed of large epithelial cells known as oncocytes. The prefix onco- is derived from the Greek word onkoustai, which means to swell. The swollen granular cytoplasm of oncocytes is due to excessive accumulation of mitochondria. Focal oncocytic metaplasia of salivary ductal and acinar cells is a common finding that is related to patient age; oncocytes are uncommon in persons younger than 50, but they can be found in almost all individuals by age 70. In addition to salivary glands, oncocytes have been identified in a number of other organs, especially the thyroid, parathyroid, and kidney. The oncocytoma is a rare neoplasm, representing approximately 1% of all salivary tumors.
CLINICAL FEATURES: The oncocytoma is predominantly a tumor of older adults, with a peak prevalence in the eighth decade of life. A slight female predilection has been observed but may not be significant. Oncocytomas occur primarily in the major salivary glands, especially the parotid gland, which accounts for about 85% to 90% of all cases. Oncocytomas of the minor salivary glands are exceedingly rare.
The tumor appears as a firm, slowly growing, painless mass that rarely exceeds 4 cm in diameter. Parotid oncocytomas usually are found in the superficial lobe and are clinically indistinguishable from other benign tumors. On occasion, bilateral tumors can occur, although these may represent examples of multinodular oncocytic hyperplasia (oncocytosis).
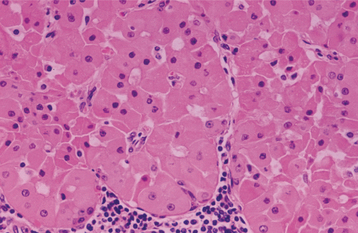
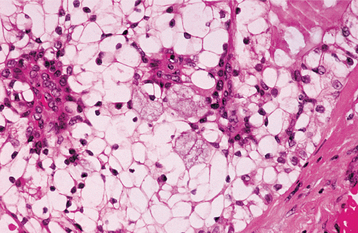
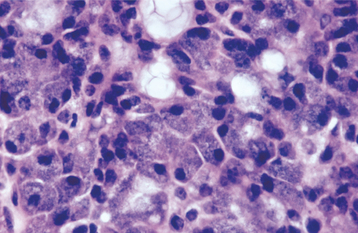
HISTOPATHOLOGIC FEATURES: The oncocytoma is usually a well-circumscribed tumor that is composed of sheets of large polyhedral cells (oncocytes), with abundant granular, eosinophilic cytoplasm (Fig. 11-43). Sometimes these cells form an alveolar or glandular pattern. The cells have centrally located nuclei that can vary from small and hyperchromatic to large and vesicular. Little stroma is present, usually in the form of thin fibrovascular septa. An associated lymphocytic infiltrate may be noted.
The granularity of the cells corresponds to an overabundance of mitochondria, which can be demonstrated by electron microscopy. These granules also can be identified on light microscopic examination with a phosphotungstic acid hematoxylin (PTAH) stain. The cells also contain glycogen, as evidenced by their positive staining with the periodic acid-Schiff (PAS) technique but by negative PAS staining after digestion with diastase.
Oncocytomas may contain variable numbers of cells with a clear cytoplasm. In rare instances, these clear cells may compose most of the lesion and create difficulty in distinguishing the tumor from low-grade salivary clear cell adenocarcinoma or metastatic renal cell carcinoma.
TREATMENT AND PROGNOSIS: Oncocytomas are best treated by surgical excision. In the parotid gland, this usually entails partial parotidectomy (lobectomy) to avoid violation of the tumor capsule. The facial nerve should be preserved whenever possible. For tumors in the submandibular gland, treatment consists of total removal of the gland. Oncocytomas of the oral minor salivary glands should be removed with a small margin of normal surrounding tissue.
The prognosis after removal is good, with a low rate of recurrence. However, oncocytomas of the sinonasal glands can be locally aggressive and have been considered to be low-grade malignancies. Rare examples of histopathologically malignant oncocytomas (oncocytic carcinoma) also have been reported. These carcinomas have a relatively poor prognosis.
ONCOCYTOSIS (NODULAR ONCOCYTIC HYPERPLASIA)
Oncocytic metaplasia is the transformation of ductal and acinar cells to oncocytes. Such cells are uncommon before the age of 50; however, as people get older, occasional oncocytes are common findings in the salivary glands. Focal oncocytic metaplasia also may be a feature of other salivary gland tumors. Oncocytosis refers to both the proliferation and the accumulation of oncocytes within salivary gland tissue. It may mimic a tumor, both clinically and microscopically, but it also is considered to be a metaplastic process rather than a neoplastic one.
CLINICAL FEATURES: Oncocytosis is found primarily in the parotid gland; however, in rare instances, it may involve the submandibular or minor salivary glands. It can be an incidental finding in otherwise normal salivary gland tissue, but it may be extensive enough to produce clinical swelling. Usually the proliferation is multifocal and nodular, but sometimes the entire gland can be replaced by oncocytes (diffuse hyperplastic oncocytosis). As with other oncocytic proliferations, oncocytosis occurs most frequently in older adults.
HISTOPATHOLOGIC FEATURES: Microscopic examination usually reveals focal nodular collections of oncocytes within the salivary gland tissue. These enlarged cells are polyhedral and demonstrate abundant granular, eosinophilic cytoplasm as a result of the proliferation of mitochondria. On occasion, these cells may have a clear cytoplasm from the accumulation of glycogen (Fig. 11-44). The multifocal nature of the proliferation may be confused with that of a metastatic tumor, especially when the oncocytes are clear in appearance.
WARTHIN TUMOR (PAPILLARY CYSTADENOMA LYMPHOMATOSUM)
Warthin tumor is a benign neoplasm that occurs almost exclusively in the parotid gland. Although it is much less common than the pleomorphic adenoma, it represents the second most common benign parotid tumor, accounting for 5% to 14% of all parotid neoplasms. The name adenolymphoma also has been used for this tumor, but this term should be avoided because it overemphasizes the lymphoid component and may give the mistaken impression that the lesion is a type of lymphoma. Analyses of the epithelial and lymphoid components of the Warthin tumor have shown both to be polyclonal; this suggests that this lesion may not represent a true neoplasm but would be better classified as a tumorlike process.
The pathogenesis of these tumors is uncertain. The traditional hypothesis suggests that they arise from heterotopic salivary gland tissue found within parotid lymph nodes. However, researchers have also suggested that these tumors may develop from a proliferation of salivary gland ductal epithelium that is associated with secondary formation of lymphoid tissue. This latter theory is supported by studies that have found cytogenetic abnormalities in the epithelial component. A number of studies have demonstrated a strong association between the development of this tumor and smoking. Smokers have an eightfold greater risk for Warthin tumor than do nonsmokers.
CLINICAL FEATURES: The Warthin tumor usually appears as a slowly growing, painless, nodular mass of the parotid gland (Fig. 11-45). It may be firm or fluctuant to palpation. The tumor most frequently occurs in the tail of the parotid near the angle of the mandible, and it may be noted for many months before the patient seeks a diagnosis. One unique feature is the tendency of Warthin tumor to occur bilaterally, which has been noted in 5% to 17% of reported cases. Most of these bilateral tumors do not occur simultaneously but are metachronous (occurring at different times).
In rare instances, the Warthin tumor has been reported within the submandibular gland or minor salivary glands. However, because the lymphoid component is often less pronounced in these extraparotid sites, the pathologist should exercise caution to avoid overdiagnosis of a lesion better classified as a papillary cystadenoma or a salivary duct cyst with oncocytic ductal metaplasia.
Warthin tumor most often occurs in older adults, with a peak prevalence in the sixth and seventh decades of life. The observed frequency of this tumor is much lower in blacks than in whites. Most studies show a decided male predilection, with some early studies demonstrating a male-to-female ratio up to 10:1. However, more recent investigations show a more balanced sex ratio. Because Warthin tumors have been associated with cigarette smoking, this changing sex ratio may be a reflection of the increased prevalence of smoking in women over the past few decades. This association with smoking also may help explain the frequent bilaterality of the tumor, because any tumorigenic effects of smoking might be manifested in both parotids.
HISTOPATHOLOGIC FEATURES: The Warthin tumor has one of the most distinctive histopathologic patterns of any tumor in the body. Although the term papillary cystadenoma lymphomatosum is cumbersome, it accurately describes the salient microscopic features.
The tumor is composed of a mixture of ductal epithelium and a lymphoid stroma (Figs. 11-46 and 11-47). The epithelium is oncocytic in nature, forming uniform rows of cells surrounding cystic spaces. The cells have abundant, finely granular eosinophilic cytoplasm and are arranged in two layers. The inner luminal layer consists of tall columnar cells with centrally placed, palisaded, and slightly hyperchromatic nuclei. Beneath this is a second layer of cuboidal or polygonal cells with more vesicular nuclei. The lining epithelium demonstrates multiple papillary infoldings that protrude into the cystic spaces. Focal areas of squamous metaplasia or mucous cell prosoplasia may be seen. The epithelium is supported by a lymphoid stroma that frequently shows germinal center formation.
TREATMENT AND PROGNOSIS: Surgical removal is the treatment of choice for patients with Warthin tumor. The procedure usually is easily accomplished because of the superficial location of the tumor. Some surgeons prefer local resection with minimal surrounding tissue; others opt for superficial parotidectomy to avoid violating the tumor capsule and because a tentative diagnosis may not be known preoperatively. A 6% to 12% recurrence rate has been reported. Many authors, however, believe that the tumor is frequently multicentric in nature; therefore, it is difficult to determine whether these are true recurrences or secondary tumor sites. Malignant Warthin tumors (carcinoma ex papillary cystadenoma lymphomatosum) have been reported but are exceedingly rare.
MONOMORPHIC ADENOMA
The term monomorphic adenoma originally was used to describe a group of benign salivary gland tumors demonstrating a more uniform histopathologic pattern than the common pleomorphic adenoma. In some classification schemes, a variety of tumors were included under the broad heading of monomorphic adenoma, including Warthin tumor, oncocytoma, basal cell adenoma, and canalicular adenoma. Other authors have used this term more specifically as a synonym just for the basal cell adenoma or canalicular adenoma. Because of its ambiguous nature, the term monomorphic adenoma probably should be avoided, and each of the tumors mentioned should be referred to by its more specific name.
CANALICULAR ADENOMA
The canalicular adenoma is an uncommon tumor that occurs almost exclusively in the minor salivary glands. Because of its uniform microscopic pattern, the canalicular adenoma also has been called a monomorphic adenoma. However, because this term also has been applied to other tumors, its use probably should be discontinued. Likewise, the term basal cell adenoma sometimes has been used synonymously for this tumor but should be avoided because it refers to a separate tumor with different clinical features (see next topic).
CLINICAL FEATURES: The canalicular adenoma shows a striking predilection for the upper lip, with nearly 75% occurring in this location. It represents the first or second most common tumor (along with pleomorphic adenoma) of the upper lip. The buccal mucosa is the second most common site. Occurrence in other minor salivary glands is uncommon, and canalicular adenomas of the parotid gland are rare.
The tumor nearly always occurs in older adults, with a peak prevalence in the seventh decade of life. There is a definite female predominance, ranging from 1.2 to 1.8 females for each male.
The canalicular adenoma appears as a slowly growing, painless mass that usually ranges from several millimeters to 2 cm (Fig. 11-48). It may be firm or somewhat fluctuant to palpation. The overlying mucosa may be normal in color or bluish and can be mistaken for a mucocele. However, mucoceles of the upper lip are rare. In some instances, the lesion has been noted to be multifocal, with multiple separate tumors discovered in the upper lip or buccal mucosa.
HISTOPATHOLOGIC FEATURES: The microscopic pattern of canalicular adenoma is monomorphic in nature. This pattern is characterized by single-layered cords of columnar or cuboidal epithelial cells with deeply basophilic nuclei (Fig. 11-49). In some areas, adjacent parallel rows of cells may be seen, resulting in a bilayered appearance of the tumor cords. These cells enclose ductal structures, sometimes in the form of long canals. Larger cystic spaces often are created, and the epithelium may demonstrate papillary projections into the cystic lumina. The tumor cells are supported by a loose connective tissue stroma with prominent vascularity. Unlike the appearance in pleomorphic adenomas, stromal alterations, such as chondroid metaplasia, do not occur. A thin, fibrous capsule often surrounds the tumor, although satellite islands are observed in the surrounding salivary gland tissue in approximately 22% to 24% of cases, which explains the tendency for multifocal tumors.
BASAL CELL ADENOMA
The basal cell adenoma is a benign salivary tumor that derives its name from the basaloid appearance of the tumor cells. It is an uncommon neoplasm that represents only 1% to 2% of all salivary tumors. Because of its uniform histopathologic appearance, it often has been classified as one of the monomorphic adenomas. However, as mentioned previously, this term probably should be avoided because of its imprecise and frequently confusing definition. In addition, ultrastructural and immunohistochemical studies have shown that basal cell adenomas are not necessarily composed of only one cell type but sometimes of a combination of salivary ductal epithelium and myoepithelial cells. The basal cell adenoma shows some histopathologic similarity to the canalicular adenoma; in the past, these two terms have been used synonymously. However, histopathologic and clinical differences warrant that they be considered as distinct entities.
CLINICAL FEATURES: Unlike the canalicular adenoma, the basal cell adenoma is primarily a tumor of the parotid gland, with around 75% of all cases occurring there. However, the minor glands represent the second most common site, specifically the glands of the upper lip and buccal mucosa. The tumor can occur at any age but is most common in middle-aged and older adults, with a peak prevalence in the seventh decade of life. The tumor appears to be more common in women, with some studies showing as high as a 2:1 female-to-male ratio.
Clinically, the basal cell adenoma appears as a slowly growing, freely movable mass similar to a pleomorphic adenoma. Most tumors are less than 3 cm in diameter. Parotid tumors usually are located within the superficial lobe of the gland.
One subtype, the membranous basal cell adenoma, deserves separate mention. This form of the tumor appears to be hereditary, often occurring in combination with skin appendage tumors, such as dermal cylindromas and trichoepitheliomas. Multiple bilateral tumors may develop within the parotids. Because these tumors often bear a histopathologic resemblance to the skin tumors, they also have been called dermal analogue tumors.
HISTOPATHOLOGIC FEATURES: The basal cell adenoma is usually encapsulated or well circumscribed. The most common subtype is the solid variant, which consists of multiple islands and cords of epithelial cells that are supported by a small amount of fibrous stroma. The peripheral cells of these islands are palisaded and cuboidal to columnar in shape, similar to the microscopic appearance of basal cell carcinoma. These peripheral cells are frequently hyperchromatic; the central cells of the islands tend to have paler staining nuclei. The central cells occasionally form eddies or keratin pearls.
The trabecular subtype demonstrates narrow cordlike epithelial strands (Fig. 11-50). The tubular subtype is characterized by the formation of small, round, ductlike structures. Frequently, a mixture of histopathologic subtypes is seen.
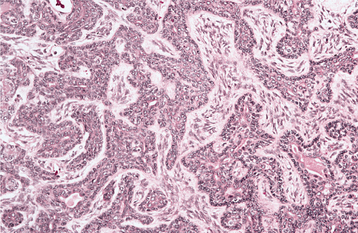
Fig. 11-50 Basal cell adenoma. Parotid tumor showing cords of basaloid cells arranged in a trabecular pattern.
The membranous basal cell adenoma exhibits multiple large lobular islands of tumor that are molded together in a jigsaw puzzle fashion. These islands are surrounded by a thick layer of hyaline material, which represents reduplicated basement membrane. Similar hyaline droplets also are often found among the epithelial cells. The microscopic appearance is similar to that of a dermal cylindroma, one of the skin tumors with which it is often associated.
TREATMENT AND PROGNOSIS: The treatment of basal cell adenoma is similar to that of pleomorphic adenoma and consists of complete surgical removal. Recurrence is rare for most histopathologic subtypes. However, the membranous subtype has a 25% to 37% recurrence rate, possibly related to its multifocal nature.
The malignant counterpart of the basal cell adenoma is the basal cell adenocarcinoma. Most basal cell adenocarcinomas arise de novo, but some examples develop from malignant degeneration of a preexisting basal cell adenoma. Fortunately, these tumors have a relatively good prognosis; although local recurrence is common, the tumor rarely metastasizes or results in death.
DUCTAL PAPILLOMAS (SIALADENOMA PAPILLIFERUM; INTRADUCTAL PAPILLOMA; INVERTED DUCTAL PAPILLOMA)
A number of salivary gland tumors can be characterized microscopically by a papillomatous pattern, the most common being Warthin tumor (papillary cystadenoma lymphomatosum). The sialadenoma papilliferum, intraductal papilloma, and inverted ductal papilloma are three rare salivary tumors that also show unique papillomatous features.
It also should be mentioned that, on occasion, the common squamous papilloma (see page 362) of the oral mucosa will arise at the site where a minor salivary gland duct merges with the surface epithelium. Because of this location, such squamous papillomas also contain scattered mucous cells within the exophytic papillary growth, and these lesions have sometimes been called ductal papillomas. However, it should be emphasized that these lesions are viral (human papillomavirus [HPV]) surface papillomas and not primary salivary gland tumors.
CLINICAL FEATURES: The sialadenoma papilliferum most commonly arises from the minor salivary glands, especially on the palate, although it also has been reported in the parotid gland. It usually is seen in older adults and has a 1.5:1.0 male-to-female ratio. The tumor appears as an exophytic, papillary surface growth that is clinically similar to the common squamous papilloma (Fig. 11-51).
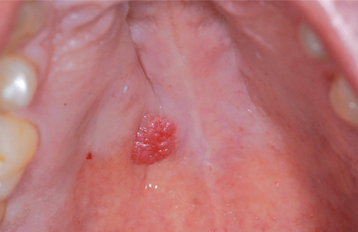
Fig. 11-51 Sialadenoma papilliferum. Exophytic papillary mass on the palate. (Courtesy of Dr. Peter Lyu.)
The intraductal papilloma is an ill-defined lesion that often has been confused with other salivary gland lesions, such as the papillary cystadenoma. It usually occurs in adults and is most common in the minor salivary glands, where it appears as a submucosal swelling.
The inverted ductal papilloma is a rare tumor that has been described only in the minor salivary glands of adults. The lower lip and mandibular vestibule are the most common locations. The lesion usually ap-pears as an asymptomatic nodule, which sometimes may show a pit or indentation in the overlying surface mucosa (Fig. 11-52).
HISTOPATHOLOGIC FEATURES: At low-power magnification, the sialadenoma papilli-ferum is somewhat similar to the squamous papilloma, exhibiting multiple exophytic papillary projections that are covered by stratified squamous epithelium. This epithelium is contiguous with a proliferation of papillomatous ductal epithelium found below the surface and extending downward into the deeper connective tissues (Fig. 11-53). Multiple ductal lumina are formed, which characteristically are lined by a double-rowed layer of cells consisting of a luminal layer of tall columnar cells and a basilar layer of smaller cuboidal cells. These ductal cells often have an oncocytic appearance. An inflammatory infiltrate of plasma cells, lymphocytes, and neutrophils is characteristically present. Because of their microscopic similarity, this tumor has been considered to be an analogue of the cutaneous syringocystadenoma papilliferum.
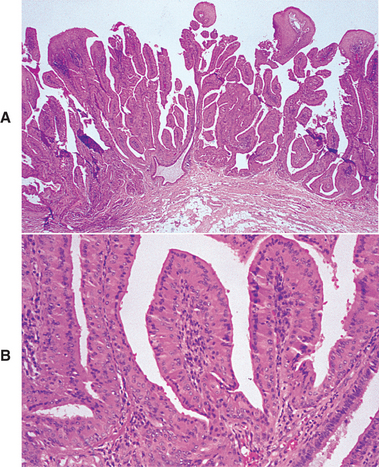
Fig. 11-53 Sialadenoma papilliferum. A, Low-power view showing a papillary surface tumor with associated ductal structures in the superficial lamina propria. B, High-power view of cystic areas lined by papillary, oncocytic epithelium.
The intraductal papilloma exhibits a dilated, unicystic structure that is located below the mucosal surface. It is lined by a single or double row of cuboidal or columnar epithelium, which has multiple arborizing papillary projections into the cystic lumen. In contrast, the inverted ductal papilloma is composed primarily of a proliferation of squamoid epithelium with multiple thick, bulbous papillary projections that fill the ductal lumen (Fig. 11-54). This epithelium may be contiguous with the overlying mucosal epithelium, communicating with the surface through a small porelike opening. Although the tumor is primarily squamous in nature, the luminal lining cells of the papillary projections are often cuboidal or columnar in shape, with scattered mucus-producing cells.
MUCOEPIDERMOID CARCINOMA
The mucoepidermoid carcinoma is one of the most common salivary gland malignancies. Because of its highly variable biologic potential, it was originally called mucoepidermoid tumor. The term recognized one subset that acted in a malignant fashion and a second group that appeared to behave in a benign fashion with favorable prognosis. However, researchers later recognized that even low-grade tumors occasionally could exhibit malignant behavior; therefore, the term mucoepidermoid carcinoma is the preferred designation.
CLINICAL FEATURES: Most studies show that the mucoepidermoid carcinoma is the most common malignant salivary gland neoplasm. In the United States, it makes up 10% of all major gland tumors and 15% to 23% of minor gland tumors. However, British studies have shown a much lower relative frequency, with mucoepidermoid carcinoma accounting for only 1% to 2% of major gland neoplasms and 9% of minor gland tumors. Perhaps a true geographic difference exists in the prevalence of this lesion.
The tumor occurs fairly evenly over a wide age range, extending from the second to seventh decades of life. Rarely is it seen in the first decade of life. However, mucoepidermoid carcinoma is the most common malignant salivary gland tumor in children. Some tumors have been associated with a previous history of radiation therapy to the head and neck region.
The mucoepidermoid carcinoma is most common in the parotid gland and usually appears as an asymptomatic swelling. Most patients are aware of the lesion for 1 year or less, although some report a mass of many years’ duration. Pain or facial nerve palsy may develop, usually in association with high-grade tumors. The minor glands constitute the second most common site, especially the palate (Fig. 11-55). Minor gland tumors also typically appear as asymptomatic swellings, which are sometimes fluctuant and have a blue or red color that can be mistaken clinically for a mucocele. Although the lower lip, floor of mouth, tongue, and retromolar pad areas are uncommon locations for salivary gland neoplasia, the mucoepidermoid carcinoma is the most common salivary tumor in each of these sites (Fig. 11-56). Intraosseous tumors also may develop in the jaws (see page 490).
HISTOPATHOLOGIC FEATURES: As its name implies, the mucoepidermoid carcinoma is composed of a mixture of mucus-producing cells and squamous (epidermoid) cells (Figs. 11-57 to 11-59). The mucous cells vary in shape but contain abundant foamy cytoplasm that stains positively with mucin stains. The epidermoid cells are characterized by squamoid features, often demonstrating a polygonal shape, intercellular bridges, and, rarely, keratinization. In addition, a third type of cell—the intermediate cell—is typically present and is believed to be a progenitor of both the mucous and the epidermoid cells. Intermediate cells vary in appearance from small, basaloid (“maternal”) cells to slightly larger ovoid cells with scant, pale eosinophilic cytoplasm. Some tumors also show variable numbers of clear cells, which sometimes can predominate the microscopic picture (Fig. 11-60). An associated lymphoid infiltrate is not unusual and may be so prominent in some cases that the lesion can be mistaken for a metastatic tumor within a lymph node.
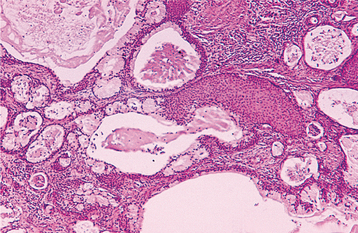
Fig. 11-57 Mucoepidermoid carcinoma. Low-power view of a moderately well-differentiated tumor showing ductal and cystic spaces surrounded by mucous and squamous cells.
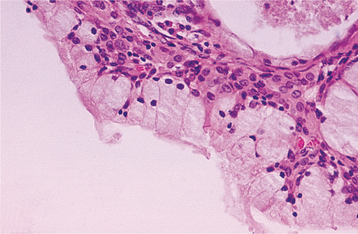
Fig. 11-58 Mucoepidermoid carcinoma. This low-grade tumor shows numerous large mucous cells surrounding a cystic space.
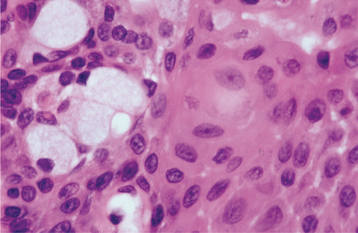
Fig. 11-59 Mucoepidermoid carcinoma. High-power view showing a sheet of squamous cells with focal mucus-producing cells (left).
Traditionally, mucoepidermoid carcinomas have been categorized into one of three histopathologic grades based on the following:
Low-grade tumors show prominent cyst formation, minimal cellular atypia, and a relatively high proportion of mucous cells. High-grade tumors consist of solid islands of squamous and intermediate cells, which can demonstrate considerable pleomorphism and mitotic activity (Fig. 11-61). Mucus-producing cells may be infrequent, and the tumor sometimes can be difficult to distinguish from squamous cell carcinoma.
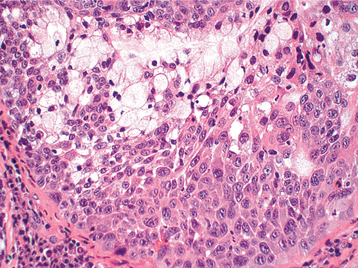
Fig. 11-61 Mucoepidermoid carcinoma. High-power view showing a sheet of pleomorphic squamous epithelial cells intermixed with mucous and intermediate cells.
Intermediate-grade tumors show features that fall between those of the low-grade and high-grade neoplasms. Cyst formation occurs but is less prominent than that observed in low-grade tumors. All three major cell types are present, but the intermediate cells usually predominate. Cellular atypia may or may not be observed.
However, some authors have found that the relative proportion of the three different cell types does not necessarily correlate with prognosis. To overcome this, two expert groups have proposed evaluation schemes based on significant microscopic parameters, to which relative point values have been assigned to determine the grade of the tumor (Table 11-12).
Table 11-12
Mucoepidermoid Carcinoma: Comparison of Two Grading Systems
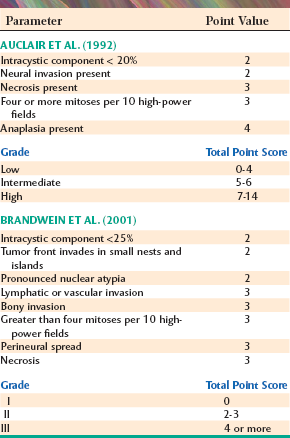
From Auclair PL, Goode RK, Ellis GL: Mucoepidermoid carcinoma of intraoral salivary glands: evaluation and application of grading criteria in 143 cases, Cancer 69:2021-2030, 1992; Brandwein MS, Ivanov K, Wallace DI et al: Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading, Am J Surg Pathol 25:835-845, 2001.
TREATMENT AND PROGNOSIS: The treatment of mucoepidermoid carcinoma is predicated by the location, histopathologic grade, and clinical stage of the tumor. Early-stage tumors of the parotid often can be treated by subtotal parotidectomy with preservation of the facial nerve. Advanced tumors may necessitate total removal of the parotid gland, with sacrifice of the facial nerve. Submandibular gland tumors are treated by total removal of the gland. Mucoepidermoid carcinomas of the minor glands usually are treated by assured surgical excision. For low-grade neoplasms, only a modest margin of surrounding normal tissue may need to be removed, but high-grade or large tumors warrant wider resection, similar to that required for squamous cell carcinomas. If there is underlying bone destruction, then the involved bone must be excised.
Radical neck dissection is indicated for patients with clinical evidence of metastatic disease and also may be considered for patients with larger or high-grade tumors. Postoperative radiation therapy also may be used for more aggressive tumors.
The prognosis depends on the grade and stage of the tumor. Patients with low-grade tumors generally have a good prognosis. For most primary sites, local recurrences or regional metastases are uncommon, and around 90% to 98% of patients are cured. The prognosis for those with intermediate-grade tumors is slightly worse than that for low-grade tumors. The outlook for patients with high-grade tumors is guarded, with only 30% to 54% of patients surviving.
For unknown reasons, submandibular gland tumors are associated with a poorer outlook than those in the parotid gland. Mucoepidermoid carcinomas of the oral minor salivary glands generally have a good prognosis, probably because they are mostly low- to intermediate-grade tumors. However, tumors of the tongue and floor of the mouth are less predictable and may exhibit more aggressive behavior.
INTRAOSSEOUS MUCOEPIDERMOID CARCINOMA (CENTRAL MUCOEPIDERMOID CARCINOMA)
On rare occasions, salivary gland tumors arise centrally within the jaws. The most common and best-recognized intrabony salivary tumor is the intraosseous mucoepidermoid carcinoma. However, other salivary tumors have been reported to develop within the jaws, including adenoid cystic carcinoma, benign and malignant mixed tumors, adenocarcinoma, acinic cell adenocarcinoma, epithelial-myoepithelial carcinoma, and monomorphic adenoma.
Several hypotheses have been proposed to explain the pathogenesis of intraosseous salivary tumors. One theory suggests that they may arise from ectopic salivary gland tissue that was developmentally entrapped within the jaws. However, the discovery of ectopic salivary tissue is uncommon in biopsy specimens from the jaws; therefore, this seems an unlikely source for most intrabony salivary tumors. Some maxillary tumors may arise from glands of the sinus lining, but this is often difficult to prove or disprove. The most likely source for most intraosseous tumors is odontogenic epithelium. Mucus-producing cells are common in odontogenic cyst linings, especially dentigerous cysts (see page 679). In addition, many intraosseous muco-epidermoid carcinomas develop in association with impacted teeth or odontogenic cysts.
CLINICAL AND RADIOGRAPHIC FEATURES: Intraosseous mucoepidermoid carcinomas are most common in middle-aged adults and demonstrate a slight female predilection. They are three times more common in the mandible than in the maxilla and are most often seen in the molar-ramus area. The most frequent presenting symptom is cortical swelling, although some lesions may be discovered as incidental findings on radiographs. Pain, trismus, and paresthesia are reported less frequently.
Radiographs usually reveal either a unilocular or multilocular radiolucency with well-defined borders (Fig. 11-62). However, some examples are characterized by a more irregular and ill-defined area of bone destruction. Some cases are associated with an unerupted tooth and, therefore, clinically may suggest an odontogenic cyst or tumor.
HISTOPATHOLOGIC FEATURES: The microscopic appearance of intraosseous mucoepidermoid carcinoma is similar to that of its soft tissue counterpart. Most tumors are low-grade lesions, although high-grade mucoepidermoid carcinomas also have been reported within the jaws.
TREATMENT AND PROGNOSIS: The primary treatment modality for patients with intraosseous mucoepidermoid carcinoma is surgery; adjunctive radiation therapy also sometimes is used. Radical surgical resection offers a better chance for cure than do more conservative procedures, such as enucleation or curettage. The local recurrence rate with conservative treatment is 40%, in contrast to 13% for more radical treatment. Metastasis has been reported in about 12% of cases. The overall prognosis is fairly good; around 10% of patients die, usually as a result of local recurrence of the tumor.
ACINIC CELL ADENOCARCINOMA
The acinic cell adenocarcinoma is a salivary gland malignancy with cells that show serous acinar differentiation. Because many of these tumors act in a nonaggressive fashion and are associated with a good prognosis, this neoplasm formerly was called acinic cell tumor, a nonspecific designation that did not indicate whether the lesion was benign or malignant. However, because some of these tumors do metastasize or recur and cause death, it is generally agreed today that acinic cell adenocarcinoma should be considered a low-grade malignancy.
CLINICAL FEATURES: Around 85% of all acinic cell adenocarcinomas occur in the parotid gland, a logical finding because this is the largest gland and one that is composed entirely of serous elements (Fig. 11-63). Most surveys have shown that this neoplasm makes up 1% to 3% of all parotid tumors, although one study showed it represented 8.6% of all parotid tumors (see Table 11-4). It is much less common in the submandibular gland, which is the site for only 2.7% to 4% of these tumors. About 9% of all acinic cell adenocarcinomas develop in the oral minor salivary glands, with the buccal mucosa, lips, and palate being the most common sites (Fig. 11-64). Overall, around 2% to 6.5% of all minor salivary gland tumors are acinic cell adenocarcinomas.
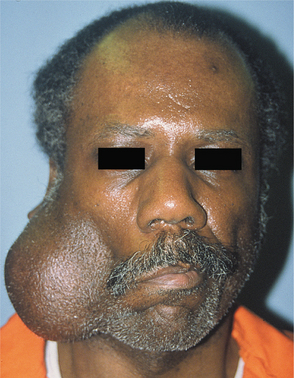
Fig. 11-63 Acinic cell adenocarcinoma. Large, firm mass of the right parotid gland. (Courtesy of Dr. Guillermo Chacon.)
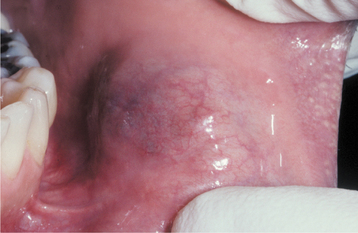
Fig. 11-64 Acinic cell adenocarcinoma. Bluish swelling of the anterior buccal mucosa, which could be mistaken for a mucocele.
The tumor occurs over a broad age range, with a relatively even peak prevalence stretching from the second to the seventh decades of life; the mean age is in the middle 40s. Approximately 60% of cases occur in women. The tumor usually appears as a slowly growing mass, and the lesion often is present for many months or years before a diagnosis is made. The tumor may be otherwise asymptomatic, although associated pain or tenderness sometimes is reported. Facial nerve paralysis is an infrequent but ominous sign for parotid tumors.
HISTOPATHOLOGIC FEATURES: Acinic cell adenocarcinomas are highly variable in their microscopic appearance. The tumor often is well circumscribed and sometimes may even appear encapsulated; however, some tumors exhibit an infiltrative growth pattern. The most characteristic cell is one with features of the serous acinar cell, with abundant granular basophilic cytoplasm and a round, darkly stained eccentric nucleus. These cells are fairly uniform in appearance, and mitotic activity is uncommon. Other cells may resemble intercalated duct cells, and some tumors also have cells with a clear, vacuolated cytoplasm.
Several growth patterns may be seen. The solid variety consists of numerous well-differentiated acinar cells arranged in a pattern that resembles normal parotid gland tissue (Figs. 11-65 and 11-66). In the microcystic variety, multiple small cystic spaces are created that may contain some mucinous or eosinophilic material. In the papillary-cystic variety, larger cystic areas are formed that are lined by epithelium having papillary projections into the cystic spaces (Fig. 11-67). The follicular variety has an appearance similar to that of thyroid tissue. A lymphoid infiltrate, sometimes with germinal center formation, is not unusual.
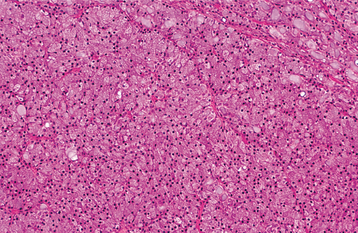
Fig. 11-65 Acinic cell adenocarcinoma. Parotid tumor demonstrating sheet of granular, basophilic serous acinar cells.
TREATMENT AND PROGNOSIS: Acinic cell adenocarcinomas confined to the superficial lobe of the parotid gland are best treated by lobectomy; for those in the deep lobe, total parotidectomy is usually necessary. The facial nerve may need to be sacrificed if it is involved by tumor. Submandibular tumors are managed by total removal of the gland, and minor gland tumors are treated with assured surgical excision. Lymph node dissection is not indicated unless there is clinical evidence of metastatic disease. Adjunctive radiation therapy may be considered for uncontrolled local disease.
The acinic cell adenocarcinoma is associated with one of the better prognoses of any of the malignant salivary gland tumors. Approximately one third of patients have recurrences locally, and metastases develop in 10% to 15% of patients. From 6% to 26% of patients die of their disease. The prognosis for minor gland tumors is better than that for tumors arising in the major glands.
MALIGNANT MIXED TUMORS (CARCINOMA EX PLEOMORPHIC ADENOMA; CARCINOMA EX MIXED TUMOR; CARCINOSARCOMA; METASTASIZING MIXED TUMOR)
Malignant mixed tumors represent malignant counterparts to the benign mixed tumor or pleomorphic adenoma. These uncommon neoplasms constitute 2% to 6% of all salivary tumors and can be divided into three categories:
The most common of these is the carcinoma ex pleomorphic adenoma, which is characterized by malignant transformation of the epithelial component of a previously benign pleomorphic adenoma. The car-cinosarcoma is a rare “mixed” tumor in which both carcinomatous and sarcomatous elements are present. The metastasizing mixed tumor has histopathologic features that are identical to the common pleomorphic adenoma (mixed tumor). In spite of its benign appearance, however, the lesion metastasizes. The metastatic tumor also has a benign microscopic appearance, usually similar to that of the primary lesion.
CARCINOMA EX PLEOMORPHIC ADENOMA: There is fairly convincing evidence that the carcinoma ex pleomorphic adenoma represents a malignant transformation within what was previously a benign neoplasm. First of all, the mean age of patients with this tumor is about 15 years older than that for the benign pleomorphic adenoma. It is most common in middle-aged and older adults, with a peak prevalence in the sixth to eighth decades of life. In addition, patients may report that a mass has been present for many years, sometimes undergoing a recent rapid growth with associated pain or ulceration. However, some tumors may have a short duration. The histopathologic features, which are discussed later, also support malignant transformation of a benign pleomorphic adenoma. It has been noted that the risk for malignant change in a pleomorphic adenoma increases with the duration of the tumor.
More than 80% of cases of carcinoma ex pleomorphic adenoma are seen within the major glands, primarily the parotid gland (Fig. 11-68). Nearly two thirds of minor salivary gland cases occur on the palate (Fig. 11-69). Although pain or recent rapid growth is not unusual, many cases present as a painless mass that is indistinguishable from a benign tumor. Parotid tumors may produce facial nerve palsy.
CARCINOSARCOMA: The carcinosarcoma is an extremely rare tumor. Most cases have been reported in the parotid gland, but the lesion also has been seen in the submandibular gland and minor salivary glands. The clinical signs and symptoms are similar to those of the carcinoma ex pleomorphic adenoma. Some patients have a previous history of a benign pleomorphic adenoma, although other cases appear to arise de novo.
METASTASIZING MIXED TUMOR: The metastasizing mixed tumor is also quite rare. As with other malignant mixed tumors, most cases originate in the parotid gland, but the primary tumor also may occur in the submandibular gland or minor salivary glands. Metastases have been found most frequently in the bones or lung, but they also can occur in other sites, such as regional lymph nodes, skin, or the liver. Most patients have a history of a benign mixed tumor, which may have been excised many years earlier. Many times the primary tumor exhibits multiple recurrences before metastasis occurs.
CARCINOMA EX PLEOMORPHIC ADENOMA: The carcinoma ex pleomorphic adenoma shows a variable microscopic appearance. Areas of typical benign pleomorphic adenoma usually can be found and may constitute most or only a small portion of the lesion. Within the tumor are areas of malignant degeneration of the epithelial component, characterized by cellular pleomorphism and abnormal mitotic activity (Fig. 11-70). This change is most often in the form of a poorly differentiated adenocarcinoma, but other patterns also can develop, including polymorphous low-grade adenocarcinoma, salivary duct carcinoma, mucoepidermoid carcinoma, and adenoid cystic carcinoma. The malignant component often has an aggressive growth pattern, with capsular invasion and infiltration into surrounding tissues. However, in rare instances it is discovered as a small focus within the center of an encapsulated mixed tumor. Because such tumors have a markedly better prognosis than invasive tumors, they have been designated as noninvasive carcinoma ex mixed tumor or carcinoma in situ ex mixed tumor.
CARCINOSARCOMA: The carcinosarcoma is a biphasic tumor, demonstrat-ing both carcinomatous and sarcomatous areas. The epithelial component usually consists of a poorly differentiated adenocarcinoma or an undifferentiated carcinoma. The sarcomatous portion often predominates the tumor and is usually in the form of chondrosar-coma but also may show characteristics of osteosarcoma, fibrosarcoma, liposarcoma, rhabdomyosarcoma, or malignant fibrous histiocytoma. Some lesions have evidence of an origin from a benign mixed tumor.
CARCINOMA EX PLEOMORPHIC ADENOMA: Invasive carcinoma ex pleomorphic adenoma usually is best treated by wide excision, possibly in conjunction with local lymph node dissection and adjunctive radiation therapy. The prognosis is guarded; the overall 5-year survival rate ranges from 25% to 65%, but this rate drops to 10% to 35% at 15 years. The prognosis is related to the histopathologic subtype of the malignant component. One study showed that well-differentiated carcinomas, such as polymorphous low-grade adenocarcinoma, have nearly a 90% 5-year survival rate. In contrast, the outlook is much worse for patients with tumors that are poorly differentiated or that have invaded more than 8 mm beyond the residual capsule or benign residual tumor. However, for cases of in situ (noninvasive) carcinoma ex mixed tumor, the prognosis is similar to that for benign mixed tumor.
ADENOID CYSTIC CARCINOMA
The adenoid cystic carcinoma is one of the more common and best-recognized salivary malignancies. Because of its distinctive histopathologic features, it was originally called a cylindroma, and this term still is used sometimes as a synonym for this neoplasm. However, use of the term cylindroma should be avoided because it does not convey the malignant nature of the tumor, and also because this same term is used for a skin adnexal tumor that has a markedly different clinical presentation and prognosis.
CLINICAL AND RADIOGRAPHIC FEATURES: The adenoid cystic carcinoma can occur in any salivary gland site, but approximately 50% to 60% develop within the minor salivary glands. The palate is the most common site for minor gland tumors (Fig. 11-71). The remaining tumors are found mostly in the parotid and submandibular glands, with a fairly even distribution between these two sites. On an individual basis, however, a striking difference can be seen among the various glands. In the parotid gland, the adenoid cystic carcinoma is relatively rare, constituting only 2% to 3% of all tumors. In the submandibular gland, this tumor accounts for 12% to 17% of all tumors and is the most common malignancy. It is also relatively common among palatal salivary neoplasms; it represents 8% to 15% of all such tumors. The lesion is most common in middle-aged adults and is rare in people younger than age 20. There is a fairly equal sex distribution, although some studies have shown a slight female predilection.
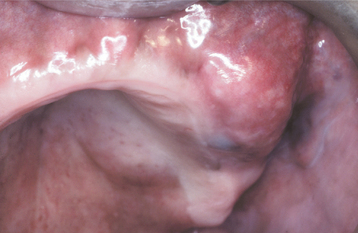
Fig. 11-71 Adenoid cystic carcinoma. Painful mass of the hard palate and maxillary alveolar ridge. (Courtesy of Dr. George Blozis.)
The adenoid cystic carcinoma usually appears as a slowly growing mass. Pain is a common and important finding, occasionally occurring early in the course of the disease before there is a noticeable swelling. Patients often complain of a constant, low-grade, dull ache, which gradually increases in intensity. Facial nerve paralysis may develop with parotid tumors. Palatal tumors can be smooth surfaced or ulcerated. Tumors arising in the palate or maxillary sinus often show radiographic evidence of bone destruction (Fig. 11-72).
HISTOPATHOLOGIC FEATURES: The adenoid cystic carcinoma is composed of a mixture of myoepithelial cells and ductal cells that can have a varied arrangement (Fig. 11-73). Three major patterns are recognized: (1) cribriform, (2) tubular, and (3) solid. Usually a combination of these is seen, and the tumor is classified based on the predominant pattern.
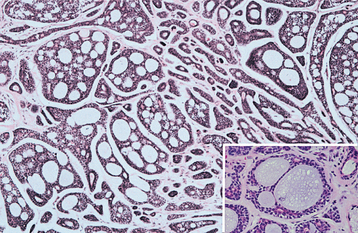
Fig. 11-73 Adenoid cystic carcinoma. Islands of hyperchromatic cells forming cribriform and tubular structures. Inset shows a high-power view of a small cribriform island.
The cribriform pattern is the most classic and best-recognized appearance, characterized by islands of basaloid epithelial cells that contain multiple cylindrical, cystlike spaces resembling Swiss cheese. These spaces often contain a mildly basophilic mucoid material, a hyalinized eosinophilic product, or a combined mucoid-hyalinized appearance. Sometimes the hyalinized material also surrounds these cribriform islands (Fig. 11-74), or small strands of tumor are found embedded within this hyalinized “stroma.” The tumor cells are small and cuboidal, exhibiting deeply basophilic nuclei and little cytoplasm. These cells are fairly uniform in appearance, and mitotic activity is rarely seen. The pathologist should be mindful that other salivary tumors, especially polymorphous low-grade adenocarcinoma, also may exhibit areas with a cribriform pattern.
In the tubular pattern, the tumor cells are similar but occur as multiple small ducts or tubules within a hyalinized stroma. The tubular lumina can be lined by one to several layers of cells, and sometimes both a layer of ductal cells and myoepithelial cells can be discerned.
The solid variant consists of larger islands or sheets of tumor cells that demonstrate little tendency toward duct or cyst formation. Unlike the cribriform and tubular patterns, cellular pleomorphism and mitotic activity, as well as focal necrosis in the center of the tumor islands, may be observed.
A highly characteristic feature of adenoid cystic carcinoma is its tendency to show perineural invasion (Fig. 11-75), which probably corresponds to the common clinical finding of pain in these patients. Sometimes the cells appear to have a swirling arrangement around nerve bundles. However, perineural invasion is not pathognomonic for adenoid cystic carcinoma; it also may be seen in other salivary malignancies, especially polymorphous low-grade adenocarcinomas.
Positive immunostaining reactions for CD43 and c-kit (CD117) in adenoid cystic carcinoma have been reported to be useful diagnostic features that can help to distinguish this tumor from polymorphous low-grade adenocarcinoma, basal cell adenoma, and canalicular adenoma. In addition, the patterns of expression of a variety of other immunohistochemical markers have been suggested to be diagnostically relevant, including stains for vimentin, collagen IV, laminin, integrins, Ki-67, smooth muscle actin, and various cytokeratins.
TREATMENT AND PROGNOSIS: Adenoid cystic carcinoma is a relentless tumor that is prone to local recurrence and eventual distant metas-tasis. Surgical excision is usually the treatment of choice, and adjunct radiation therapy may slightly improve patient survival in some cases. Because metastasis to regional lymph nodes is uncommon, neck dissection typically is not indicated. Because of the poor overall prognosis, regardless of treatment, clinicians should be cautioned against needlessly aggressive and mutilating surgical procedures for large tumors or cases already showing metastases.
Because the tumor is prone to late recurrence and metastasis, the 5-year survival rate has little significance and does not equate to a cure. The 5-year survival rate may be as high as 70%, but this rate continues to decrease over time. The 10-year survival is approximately 50%, and by 20 years, only 25% of patients are still alive. Tumors with a solid histopathologic pattern are associated with a worse outlook than those with a cribriform or tubular arrangement. With respect to site, the prognosis is poorest for tumors arising in the maxillary sinus and submandibular gland. Most studies have shown that microscopic identification of perineural invasion has little effect on the prognosis. Tumor DNA ploidy analysis may help to predict the prognosis of adenoid cystic carcinoma; patients with diploid tumors have been shown to have a significantly better outcome than patients with aneuploid tumors.
Death usually results from local recurrence or distant metastases. Tumors of the palate or maxillary sinus eventually may invade upward to the base of the brain. Metastases occur in approximately 35% of patients, most frequently involving the lungs and bones.
POLYMORPHOUS LOW-GRADE ADENOCARCINOMA (LOBULAR CARCINOMA; TERMINAL DUCT CARCINOMA)
The polymorphous low-grade adenocarcinoma is a more recently recognized type of salivary malignancy that was first described in 1983. Before its identification as a distinct entity, examples of this tumor were categorized as pleomorphic adenoma, an unspecified form of adenocarcinoma, or sometimes as adenoid cystic carcinoma. Once recognized as a specific entity, however, it was realized that this tumor possesses distinct clinicopathologic features and is one of the more common minor salivary gland malignancies.
CLINICAL FEATURES: The polymorphous low-grade adenocarcinoma is almost exclusively a tumor of the minor salivary glands. However, rare examples also have been reported in the major glands, either arising de novo or as the malignant component of a carcinoma ex pleomorphic adenoma. Sixty-five percent occur on the hard or soft palate (Fig. 11-76), with the upper lip and buccal mucosa being the next most common locations. It is most common in older adults, having a peak prevalence in the sixth to eighth decades of life. Two thirds of all cases occur in females.
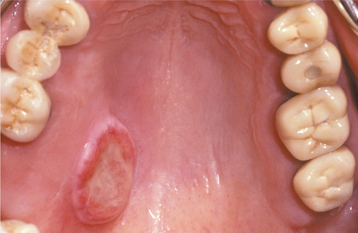
Fig. 11-76 Polymorphous low-grade adenocarcinoma. Ulcerated mass of the posterior lateral hard palate.
The tumor most often appears as a painless mass that may have been present for a long time with slow growth. Occasionally, it is associated with bleeding or discomfort. Tumor can erode or infiltrate the underlying bone.
HISTOPATHOLOGIC FEATURES: The tumor cells of polymorphous low-grade adenocarcinomas have a deceptively uniform appearance. They are round to polygonal in shape, with indistinct cell borders and pale to eosinophilic cytoplasm. The nuclei may be round, ovoid, or spindled; these nuclei usually are pale staining, although they can be more basophilic in some areas. The cells can exhibit different growth patterns, hence, the polymorphous term. The cells may grow in a solid pattern or form cords, ducts, or larger cystic spaces. In some tumors, a cribriform pattern can be produced that mimics adenoid cystic carcinoma (Fig. 11-77). Mitotic figures are uncommon.
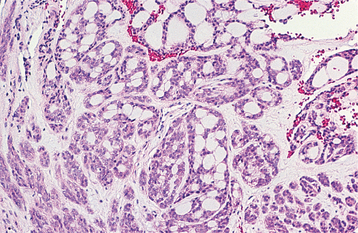
Fig. 11-77 Polymorphous low-grade adenocarcinoma. This medium-power view shows a cribriform arrangement of uniform tumor cells with pale-staining nuclei.
At low power, the tumor sometimes appears well circumscribed. However, the peripheral cells are usually infiltrative, invading the adjacent tissue in a single-file fashion (Fig. 11-78). Extension into underlying bone or skeletal muscle may be observed. The stroma is often mucoid in nature, or it may demonstrate hyalinization. Perineural invasion is common—another feature that may cause the tumor to be mistaken for adenoid cystic carcinoma (Fig. 11-79). However, a distinction between these two tumors is important because of their vastly different prognoses.
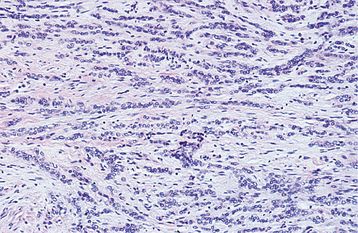
Fig. 11-78 Polymorphous low-grade adenocarcinoma. Pale-staining cells that infiltrate as single-file cords.
Immunohistochemical staining can be helpful in distinguishing polymorphous low-grade adenocarcinoma from other salivary gland tumors that it may mimic. When compared with adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma exhibits significantly weaker expression of CD43 and c-kit (CD117). Likewise, lack of staining for glial fibrillary acidic protein (GFAP) can help to differentiate this tumor from pleomorphic adenoma, which is almost always strongly positive for GFAP.
TREATMENT AND PROGNOSIS: The polymorphous low-grade adenocarcinoma is best treated by wide surgical excision, sometimes including resection of the underlying bone. Metastasis to regional lymph nodes is relatively uncommon, occurring in just under 10% of patients. Therefore, neck dissection seems unwarranted unless there is clinical evidence of cervical metastases. Distant metastasis is rare.
The overall prognosis is relatively good. Recurrent disease has been reported in 9% to 17% of all patients, but this usually can be controlled with reexcision. Death from tumor is rare but may occur secondary to direct extension into vital structures. Microscopic identification of perineural invasion does not appear to affect the prognosis.
SALIVARY ADENOCARCINOMA, NOT OTHERWISE SPECIFIED
In spite of the wide variety of salivary gland malignancies that have been specifically identified and categorized, some tumors still defy the existing classification schemes. These tumors usually are designated as salivary adenocarcinomas, not otherwise specified (NOS).
CLINICAL AND HISTOPATHOLOGIC FEATURES: Because these adenocarcinomas represent such a diverse group of neoplasms, it is difficult to generalize about their clinical and microscopic features. Like most salivary tumors, they appear to be most common in the parotid gland, followed by the minor glands and the submandibular gland (Figs. 11-80 and 11-81). They may present as asymptomatic masses or cause pain or facial nerve paralysis. The microscopic appearance is highly variable but demonstrates features of a glandular malignancy with evidence of cellular pleomorphism, an infiltrative growth pattern, or both. These tumors exhibit a wide spectrum of differentiation, ranging from well-differentiated, low-grade neoplasms to poorly differentiated, high-grade malignancies.
As these tumors are studied more, it should be possible to classify some of them into separate, specific categories and allow more definitive analyses of their clinical and microscopic features.
TREATMENT AND PROGNOSIS: Because of their diversity, it is difficult to predict the prognosis for salivary adenocarcinoma (NOS), but patients with early-stage, well-differentiated tumors appear to have a better outcome. The survival rate is better for tumors of the oral cavity than for those in the major salivary glands. The reported 10-year survival rate for parotid tumors ranges from 26% to 55%; in contrast, one study reported a 10-year survival rate of 76% for intraoral tumors.
BIBLIOGRAPHY
Antoniades, DZ, Markopoulos, AK, Deligianni, E, et al. Bilateral aplasia of parotid glands correlated with accessory parotid tissue. J Laryngol Otol. 2006;120:327–329.
Mandel, L. An unusual pattern of dental damage with salivary gland aplasia. J Am Dent Assoc. 2006;137:984–989.
Matsuda, C, Matsui, Y, Ohno, K, et al. Salivary gland aplasia with cleft lip and palate. A case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:594–599.
Milunsky, JM, Zhao, G, Maher, TA, et al. LADD syndrome is caused by FGF10 mutations. Clin Genet. 2006;69:349–354.
Ramirez, D, Lammer, EJ. Lacrimoauriculodentodigital syndrome with cleft lip/palate and renal manifestations. Cleft Palate Craniofac J. 2004;41:501–506.
Baurmash, HD. Mucoceles and ranulas. J Oral Maxillofac Surg. 2003;61:369–378.
Campana, F, Sibaud, V, Chauvel, A, et al. Recurrent superficial mucoceles associated with lichenoid disorders. J Oral Maxillofac Surg. 2006;64:1830–1833.
Cataldo, E, Mosadomi, A. Mucoceles of the oral mucous membrane. Arch Otolaryngol. 1970;91:360–365.
Chi A, Lambert P, Richardson M et al: Oral mucoceles: a clinicopathologic review of 1,824 cases including unusual variants, Abstract No. 19. Paper presented at the annual meeting of the American Academy of Oral and Maxillofacial Pathology, Kansas City, Mo, 2007.
Eveson, JW. Superficial mucoceles: pitfall in clinical and microscopic diagnosis. Oral Surg Oral Med Oral Pathol. 1988;66:318–322.
Jensen, JL. Superficial mucoceles of the oral mucosa. Am J Dermatopathol. 1990;12:88–92.
Jinbu, Y, Kusama, M, Itoh, H, et al. Mucocele of the glands of Blandin-Nuhn: clinical and histopathologic analysis of 26 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:467–470.
Jinbu, Y, Tsukinoki, K, Kusama, M, et al. Recurrent multiple superficial mucocele on the palate: histopathology and laser vaporization. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:193–197.
Standish, SM, Shafer, WG. The mucous retention phenomenon. J Oral Surg. 1959;17:15–22.
Sugerman, PB, Savage, NW, Young, WG. Mucocele of the anterior lingual salivary glands (glands of Blandin and Nuhn): report of 5 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:478–482.
Anastassov, GE, Haiavy, J, Solodnik, P, et al. Submandibular gland mucocele: diagnosis and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:159–163.
Baurmash, HD. A case against sublingual gland removal as primary treatment of ranulas. J Oral Maxillofac Surg. 2007;65:117–121.
Baurmash, HD. Mucoceles and ranulas. J Oral Maxillofac Surg. 2003;61:369–378.
Davison, MJ, Morton, RP, McIvor, NP. Plunging ranula: clinical observations. Head Neck. 1998;20:63–68.
Galloway, RH, Gross, PD, Thompson, SH, et al. Pathogenesis and treatment of ranula: report of three cases. J Oral Maxillofac Surg. 1989;47:299–302.
Mahadevan, M, Vasan, N. Management of pediatric plunging ranula. Int J Pediatr Otorhinolaryngol. 2006;70:1049–1054.
Kurabayashi, T, Ida, M, Yasumoto, M, et al. MRI of ranulas. Neuroradiology. 2000;42:917–922.
Yoshimura, Y, Obara, S, Kondoh, T, et al. A comparison of three methods used for treatment of ranula. J Oral Maxillofac Surg. 1995;53:280–282.
Zhao, Y-F, Jia, Y, Chen, X-M, et al. Clinical review of 580 ranulas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:281–287.
Eversole, LR. Oral sialocysts. Arch Otolaryngol. 1987;113:51–56.
Takeda, Y, Yamamoto, H. Salivary duct cyst: its frequency in a certain Japanese population group (Tohoku districts), with special reference to adenomatous proliferation of the epithelial lining. J Oral Sci. 2001;43:9–13.
Tal, H, Altini, M, Lemmer, J. Multiple mucous retention cysts of the oral mucosa. Oral Surg Oral Med Oral Pathol. 1984;58:692–695.
Arzoz, E, Santiago, A, Esnal, F, et al. Endoscopic intracorporeal lithotripsy for sialolithiasis. J Oral Maxillofac Surg. 1996;54:847–850.
Baurmash, HD. Submandibular salivary stones: current management modalities. J Oral Maxillofac Surg. 2004;62:369–378.
Capaccio, P, Ottaviani, F, Manzo, R, et al. Extracorporeal lithotripsy for salivary calculi: a long-term clinical experience. Laryngoscope. 2004;114:1069–1073.
Jensen, JL, Howell, FV, Rick, GM, et al. Minor salivary gland calculi: a clinicopathologic study of forty-seven new cases. Oral Surg Oral Med Oral Pathol. 1979;47:44–50.
McGurk, M, Escudier, MP, Brown, JE. Modern management of salivary calculi. Br J Surg. 2005;92:107–112.
Nahlieli, O, Baruchin, AM. Sialoendoscopy: three years’ experience as a diagnostic and treatment modality. J Oral Maxillofac Surg. 1997;55:912–918.
Nahlieli, O, Eliav, E, Hasson, O, et al. Pediatric sialolithiasis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:709–712.
Ottaviani, F, Capaccio, P, Rivolta, R, et al. Salivary gland stones: US evaluation in shock wave lithotripsy. Radiology. 1997;204:437–441.
Williams, MF. Sialolithiasis. Otolaryngol Clin North Am. 1999;32:819–834.
Zenk, J, Bozzato, A, Winter, M, et al. Extracorporeal shock wave lithotripsy of submandibular stones: evaluation after 10 years. Ann Otol Rhinol Laryngol. 2004;113:378–383.
Ziegler, CM, Steveling, H, Seubert, M, et al. Endoscopy: a minimally invasive procedure for diagnosis and treatment of diseases of the salivary glands—six years of practical experience. Br J Oral Maxillofac Surg. 2004;42:1–7.
Fattahi, TT, Lyu, PE, Van Sickels, JE. Management of acute suppurative parotitis. J Oral Maxillofac Surg. 2002;60:446–448.
Fowler, CB, Brannon, RB. Subacute necrotizing sialadenitis: report of 7 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:600–609.
Mandel, L, Surattanont, F. Bilateral parotid swelling: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:221–237.
McQuone, SJ. Acute viral and bacterial infections of the salivary glands. Otolaryngol Clin North Am. 1999;32:793–811.
Motamed, M, Laugharne, D, Bradley, PJ. Management of chronic parotitis: a review. J Laryngol Otol. 2003;117:521–526.
Nahlieli, O, Bar, T, Shacham, R, et al. Management of chronic recurrent parotitis: current therapy. J Oral Maxillofac Surg. 2004;62:1150–1155.
Seifert, G. Aetiological and histological classification of sialadenitis. Pathologica. 1997;89:7–17.
Suresh, L, Aguirre, A. Subacute necrotizing sialadenitis: a clinicopathological study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:385–390.
Vasama, J-P. Tympanic neurectomy and chronic parotitis. Acta Otolaryngol. 2000;120:995–998.
Werning, JT, Waterhouse, JP, Mooney, JW. Subacute necrotizing sialadenitis. Oral Surg Oral Med Oral Pathol. 1990;70:756–759.
Williams, HK, Connor, R, Edmondson, H. Chronic sclerosing sialadenitis of the submandibular and parotid glands: a report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:720–723.
Carrington, PR, Horn, TD. Cheilitis glandularis: a clinical marker for both malignancy and/or severe inflammatory disease of the oral cavity. J Am Acad Dermatol. 2006;54:336–337.
Cohen, DM, Green, JG, Dickmann, SL. Concurrent anomalies: cheilitis glandularis and double lip: report of a case. Oral Surg Oral Med Oral Pathol. 1988;66:397–399.
Doku, HC, Shklar, G, McCarthy, PL. Cheilitis glandularis. Oral Surg Oral Med Oral Pathol. 1965;20:563–571.
Oliver, ID, Pickett, AB. Cheilitis glandularis. Oral Surg Oral Med Oral Pathol. 1980;49:526–529.
Stoopler, ET, Carrasco, L, Stanton, DC, et al. Cheilitis glandularis: an unusual histopathologic presentation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:312–317.
Swerlick, RA, Cooper, PH. Cheilitis glandularis: a re-evaluation. J Am Acad Dermatol. 1984;10:466–472.
Banerjee, KJ, Glasson, C, O’Flaherty, SJ. Parotid and submandibular botulinum toxin A injections for sialorrhoea in children with cerebral palsy. Dev Med Child Neurol. 2006;48:883–887.
Contarino, MF, Pompili, M, Tittoto, P, et al. Botulinum toxin B ultrasound-guided injections for sialorrhea in amyotrophic lateral sclerosis and Parkinson’s disease. Parkinsonism Relat Disord. 2007;13:299–303.
Ethunandan, M, Macpherson, DW. Persistent drooling: treatment by bilateral submandibular duct transposition and simultaneous sublingual gland excision. Ann R Coll Surg Engl. 1998;80:279–282.
Freudenreich, O. Drug-induced sialorrhea. Drugs Today. 2005;41:411–418.
Lamey, P-J, Clifford, TJ, El-Karim, IA, et al. Personality analysis of patients complaining of sialorrhoea. J Oral Pathol Med. 2006;35:307–310.
Lieblich, S. Episodic supersalivation (idiopathic paroxysmal sialorrhea): description of a new clinical syndrome. Oral Surg Oral Med Oral Pathol. 1989;68:159–161.
Mandel, L, Tamari, K. Sialorrhea and gastroesophageal reflux. J Am Dent Assoc. 1995;126:1537–1541.
Meningaud, J-P, Pitak-Arnnop, P, Chikhani, L, et al. Drooling of saliva: a review of the etiology and management options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:48–57.
O’Dwyer, TP, Conlon, BJ. The surgical management of drooling—a 15 year follow-up. Clin Otolaryngol. 1997;22:284–287.
Shirley, WP, Hill, JS, Woolley, AL, et al. Success and complications of four-duct ligation for sialorrhea. Int J Pediatr Otorhinolaryngol. 2003;67:1–6.
Talmi, YP, Finkelstein, Y, Zohar, Y. Reduction of salivary flow with transdermal scopolamine: a four-year experience. Otolaryngol Head Neck Surg. 1990;103:615–618.
Cassolato, SF, Turnbull, RS. Xerostomia: clinical aspects and treatment. Gerodontology. 2003;20:64–77.
Epstein, JB, Emerton, S, Le, ND, et al. A double-blind crossover trial of Oral Balance gel and Biotene toothpaste versus placebo in patients with xerostomia following radiation therapy. Oral Oncol. 1999;35:132–137.
Fox, PC. Salivary enhancement therapies. Caries Res. 2004;38:241–246.
Jensen, SB, Pedersen, AM, Reibel, J, et al. Xerostomia and hypofunction of the salivary glands in cancer therapy. Support Care Cancer. 2003;11:207–225.
Johnson, JT, Ferretti, GA, Nethery, WJ, et al. Oral pilocarpine for post-irradiation xerostomia in patients with head and neck cancer. N Engl J Med. 1993;329:390–395.
Kirstilä, V, Lenander-Lumikari, M, Söderling, E, et al. Effects of oral hygiene products containing lactoperoxidase, lysozyme, and lactoferrin on the composition of whole saliva and on subjective oral symptoms in patients with xerostomia. Acta Odontol Scand. 1996;54:391–397.
Närhi, TO, Meurman, JH, Ainamo, A. Xerostomia and hyposalivation. Causes, consequences and treatment in the elderly. Drugs Aging. 1999;15:103–116.
Nieuw Amerongen, AV, Veerman, ECI. Current therapies for xerostomia and salivary gland hypofunction associated with cancer therapies. Support Care Cancer. 2003;11:226–231.
Porter, SR, Scully, C, Hegarty, AM. An update of the etiology and management of xerostomia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:28–46.
Scully, C. Drug effects on salivary glands: dry mouth. Oral Dis. 2003;9:165–176.
Sreebny, LM, Schwartz, SS. A reference guide to drugs and dry mouth—2nd edition. Gerodontology. 1997;14:33–47.
Warde, P, Kroll, B, O’Sullivan, B, et al. A phase II study of Biotene in the treatment of postradiation xerostomia in patients with head and neck cancer. Support Care Cancer. 2000;8:203–208.
Benign Lymphoepithelial Lesion
Bridges, AJ, England, DM. Benign lymphoepithelial lesion: relationship to Sjögren’s syndrome and evolving malignant lymphoma. Semin Arthritis Rheum. 1989;19:201–208.
Chaudhry, AP, Cutler, LS, Yamane, GM, et al. Light and ultrastructural features of lymphoepithelial lesions of the salivary glands in Mikulicz’s disease. J Pathol. 1986;146:239–250.
DiGiuseppe, JA, Corio, RL, Westra, WH. Lymphoid infiltrates of the salivary glands: pathology, biology, and clinical significance. Curr Opin Oncol. 1996;8:232–237.
Herbst, H, Niedobitek, G. Sporadic EBV-associated lymphoepithelial salivary gland carcinoma with EBV-positive low-grade myoepithelial component. Virchows Arch. 2006;448:648–654.
Penfold, CN. Mikulicz syndrome. J Oral Maxillofac Surg. 1985;43:900–905.
Quintana, PG, Kapadia, SB, Bahler, DW, et al. Salivary gland lymphoid infiltrates associated with lymphoepithelial lesions: a clinicopathologic, immunophenotypic, and genotypic study. Hum Pathol. 1997;28:850–861.
Sato, K, Kawana, M, Sato, Y, et al. Malignant lymphoma in the head and neck associated with benign lymphoepithelial lesion of the parotid gland. Auris Nasus Larynx. 2002;29:209–214.
Saw, D, Lau, WH, Ho, JHC, et al. Malignant lymphoepithelial lesion of the salivary gland. Hum Pathol. 1986;17:914–923.
Wu, DL, Shemen, L, Brady, T, et al. Malignant lymphoepithelial lesion of the parotid gland: a case report and review of the literature. Ear Nose Throat J. 2001;80:803–806.
Al-Hashimi, I. Xerostomia secondary to Sjögren’s syndrome in the elderly. Drugs Aging. 2005;22:887–899.
Biasi, D, Caramaschi, P, Ambrosetti, A, et al. Mucosa-associated lymphoid tissue lymphoma of the salivary glands occurring in patients affected by Sjögren’s syndrome: report of 6 cases. Acta Haematol. 2001;105:83–88.
Daniels, TE. Labial salivary gland biopsy in Sjögren’s syndrome: assessment as a diagnostic criterion in 362 suspected cases. Arthritis Rheum. 1984;27:147–156.
Daniels, TE, Fox, PC. Salivary and oral components of Sjögren’s syndrome. Rheum Dis Clin North Am. 1992;18:571–589.
Delaleu, N, Jonsson, R, Koller, MM. Sjögren’s syndrome. Eur J Oral Sci. 2005;113:101–113.
Fox, RI. Sjögren’s syndrome. Lancet. 2005;366:321–331.
Fox, RI, Törnwall, J, Maruyama, T, et al. Evolving concepts of diagnosis, pathogenesis, and therapy of Sjögren’s syndrome. Curr Opin Rheumatol. 1998;10:446–456.
Friedlaender, MH. Ocular manifestations of Sjögren’s syndrome: keratoconjunctivitis sicca. Rheum Dis Clin North Am. 1992;18:591–608.
García-Carrasco, M, Ramos-Casals, M, Rosas, J, et al. Primary Sjögren syndrome: clinical and immunologic disease patterns in a cohort of 400 patients. Medicine. 2002;81:270–280.
Jonsson, R, Moen, K, Vestrheim, D, et al. Current issues in Sjögren’s syndrome. Oral Dis. 2002;8:130–140.
Jordan, R, Diss, TC, Lench, NJ, et al. Immunoglobulin gene rearrangements in lymphoplasmacytic infiltrates of labial salivary glands in Sjögren’s syndrome. A possible predictor of lymphoma development. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:723–729.
Jordan, RCK, Speight, PM. Lymphoma in Sjögren’s syndrome. From histopathology to molecular pathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:308–320.
Manthorpe, R, Benoni, C, Jacobsson, L, et al. Lower frequency of focal lip sialadenitis (focus score) in smoking patients. Can tobacco diminish the salivary gland involvement as judged by histological examination and anti-SSA/Ro and anti-SSB/La antibodies in Sjögren’s syndrome. Ann Rheum Dis. 2000;59:54–60.
Marx, RE, Hartman, KS, Rethman, KV. A prospective study comparing incisional labial to incisional parotid biopsies in the detection and confirmation of sarcoidosis, Sjögren’s disease, sialosis and lymphoma. J Rheumatol. 1988;15:621–629.
Pedersen, AM, Reibel, J, Nauntofte, B. Primary Sjögren’s syndrome (pSS): subjective symptoms and salivary findings. J Oral Pathol Med. 1999;28:303–311.
Vitali, C, Bombardieri, S, Jonsson, R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558.
Vivino, FB, Gala, I, Hermann, GA. Change in final diagnosis on second evaluation of labial minor salivary gland biopsies. J Rheumatol. 2002;29:938–944.
Chilla, R. Sialadenosis of the salivary glands of the head: studies on the physiology and pathophysiology of parotid secretion. Adv Otorhinolaryngol. 1981;26:1–38.
Coleman, H, Altini, M, Nayler, S, et al. Sialadenosis: a presenting sign in bulimia. Head Neck. 1998;20:758–762.
Mehler, PS, Wallace, JA. Sialadenosis in bulimia: a new treatment. Arch Otolaryngol Head Neck Surg. 1993;119:787–788.
Mignogna, MD, Fedele, S, Lo Russo, L. Anorexia/bulimia-related sialadenosis of palatal minor salivary glands. J Oral Pathol Med. 2004;33:441–442.
Pape, SA, MacLeod, RI, McLean, NR, et al. Sialadenosis of the salivary glands. Br J Plast Surg. 1995;48:419–422.
Satoh, M, Yoshihara, T. Clinical and ultracytochemical investigation of sialadenosis. Acta Otolaryngol Suppl. 2004;553:122–127.
Arafat, A, Brannon, RB, Ellis, GL. Adenomatoid hyperplasia of mucous salivary glands. Oral Surg Oral Med Oral Pathol. 1981;52:51–55.
Barrett, AW, Speight, PM. Adenomatoid hyperplasia of oral minor salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:482–487.
Buchner, A, Merrell, PW, Carpenter, WM, et al. Adenomatoid hyperplasia of minor salivary glands. Oral Surg Oral Med Oral Pathol. 1991;71:583–587.
Giansanti, JS, Baker, GO, Waldron, CA. Intraoral, mucinous, minor salivary gland lesions presenting clinically as tumors. Oral Surg Oral Med Oral Pathol. 1971;32:918–922.
Shimoyama, T, Wakabayashi, M, Kato, T, et al. Adenomatoid hyperplasia of the palate mimicking clinically as a salivary gland tumor. J Oral Sci. 2001;43:135–138.
Abrams, AM, Melrose, RJ, Howell, FV. Necrotizing sialometaplasia: a disease simulating malignancy. Cancer. 1973;32:130–135.
Brannon, RB, Fowler, CB, Hartman, KS. Necrotizing sialometaplasia: a clinicopathologic study of sixty-nine cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1991;72:317–325.
Imbery, TA, Edwards, PA. Necrotizing sialometaplasia: literature review and case reports. J Am Dent Assoc. 1996;127:1087–1092.
Schöning, H, Emshoff, R, Kreczy, A. Necrotizing sialometaplasia in two patients with bulimia and chronic vomiting. Int J Oral Maxillofac Surg. 1998;27:463–465.
Sneige, N, Batsakis, JG. Necrotizing sialometaplasia. Ann Otol Rhinol Laryngol. 1992;101:282–284.
Salivary Gland Tumors: General Considerations
Dardick, I. Color atlas/text of salivary gland tumor pathology. New York: Igaku-Shoin, 1996.
Ellies, M, Schaffranietz, F, Arglebe, C, et al. Tumor of the salivary glands in childhood and adolescence. J Oral Maxillofac Surg. 2006;64:1049–1058.
Ellis, GL, Auclair, PL. Tumors of the salivary glands. Washington DC: Armed Forces Institute of Pathology, 1996.
Ellis, GL, Auclair, PL, Gnepp, DR. Surgical pathology of the salivary glands. Philadelphia: WB Saunders, 1991.
Eneroth, C-M. Salivary gland tumors in the parotid gland, submandibular gland, and the palate region. Cancer. 1971;27:1415–1418.
Eveson, JW, Cawson, RA. Salivary gland tumours: a review of 2410 cases with particular reference to histological types, site, age, and sex distribution. J Pathol. 1985;146:51–58.
Eveson, JW, Cawson, RA. Tumours of the minor (oropharyngeal) salivary glands: a demographic study of 336 cases. J Oral Pathol. 1985;14:500–509.
Foote, FW, Frazell, EL. Tumors of the major salivary glands. Cancer. 1953;6:1065–1113.
Neville, BW, Damm, DD, Weir, JC, et al. Labial salivary gland tumors: an analysis of 103 cases. Cancer. 1988;61:2113–2116.
Pires, FR, Pringle, GA, de Almeida, OP, et al. Intra-oral minor salivary gland tumors: a clinicopathological study of 546 cases. Oral Oncol. 2007;43:463–470.
Seifert, G, Brocheriou, C, Cardesa, A, et al. WHO international histological classification of tumours: tentative histological classification of salivary gland tumours. Pathol Res Pract. 1990;186:555–581.
Seifert, G, Miehlke, A, Haubrich, J, et al. Diseases of the salivary glands. Pathology—diagnosis—treatment—facial nerve surgery. New York: George Thieme Verlag, 1986.
Spiro, RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177–184.
Thackray, AC, Lucas, RB. Tumors of the major salivary glands: atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology, 1974. [series 2, fascicle 10].
Waldron, CA, EI-Mofty, SK, Gnepp, DR. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1988;66:323–333.
Whatley, WS, Thompson, JW, Rao, B. Salivary gland tumors in survivors of childhood cancer. Otolaryngol Head Neck Surg. 2006;134:385–388.
Yih, W-Y, Kratochvil, FJ, Stewart, JCB. Intraoral minor salivary gland neoplasms: review of 213 cases. J Oral Maxillofac Surg. 2005;63:805–810.
Chau, MNY, Radden, BG. A clinical-pathological study of 53 intraoral pleomorphic adenomas. Int J Oral Maxillofac Surg. 1989;18:158–162.
Dardick, I. Myoepithelioma: definitions and diagnostic criteria. Ultrastruct Pathol. 1995;19:335–345.
Friedrich, RE, Li, L, Knop, J, et al. Pleomorphic adenoma of the salivary glands: analysis of 94 patients. Anticancer Res. 2005;25:1703–1705.
Kanazawa, H, Furuya, T, Watanabe, T, et al. Plasmacytoid myoepithelioma of the palate. J Oral Maxillofac Surg. 1999;57:857–860.
Myssiorek, D, Ruah, CB, Hybels, RL. Recurrent pleomorphic adenomas of the parotid gland. Head Neck. 1990;12:332–336.
Noguchi, S, Aihara, T, Yoshino, K, et al. Demonstration of monoclonal origin of human parotid gland pleomorphic adenoma. Cancer. 1996;77:431–435.
Silva, SJ, Costa, GT, Brant Filho, AC, et al. Metachronous bilateral pleomorphic adenoma of the parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:333–338.
Sciubba, JJ, Brannon, R. Myoepithelioma of salivary glands: report of 23 cases. Cancer. 1982;47:562–572.
Stennert, E, Guntinas-Lichius, O, Klussman, JP, et al. Histopathology of pleomorphic adenoma in the parotid gland: a prospective unselected series of 100 cases. Laryngoscope. 2001;111:2195–2200.
Stennert, E, Wittekindt, C, Klussman, JP, et al. Recurrent pleomorphic adenoma of the parotid gland: a prospective histopathological and immunohistochemical study. Laryngoscope. 2004;114:158–163.
Brandwein, MS, Huvos, AG. Oncocytic tumors of major salivary glands: a study of 68 cases with follow-up of 44 patients. Am J Surg Pathol. 1991;15:514–528.
Capone, RB, Ha, PK, Westra, WH, et al. Oncocytic neoplasms of the parotid gland: a 16-year institutional review. Otolaryngol Head Neck Surg. 2002;126:657–662.
Damm, DD, White, DK, Geissler, RH, Jr., et al. Benign solid oncocytoma of intraoral minor salivary glands. Oral Surg Oral Med Oral Pathol. 1989;67:84–86.
Ellis, GL. “Clear cell” oncocytoma of salivary gland. Human Pathol. 1988;19:862–867.
Goode, RK, Corio, RL. Oncocytic adenocarcinoma of salivary glands. Oral Surg Oral Med Oral Pathol. 1988;65:61–66.
Loreti, A, Sturla, M, Gentileschi, S, et al. Diffuse hyperplastic oncocytosis of the parotid gland. Br J Plast Surg. 2002;55:151–152.
Ozolek, JA, Bastacky, SI, Myers, EN, et al. Immunophenotypic comparison of salivary gland oncocytoma and metastatic renal cell carcinoma. Laryngoscope. 2005;115:1097–1100.
Palmer, TJ, Gleeson, MJ, Eveson, JW, et al. Oncocytic adenomas and oncocytic hyperplasia of salivary glands: a clinicopathological study of 26 cases. Histopathology. 1990;16:487–493.
Stomeo, F, Meloni, F, Bozzo, C, et al. Bilateral oncocytoma of the parotid gland. Acta Otolaryngol. 2006;126:324–326.
Thompson, LD, Wenig, BM, Ellis, GL. Oncocytomas of the submandibular gland. A series of 22 cases and a review of the literature. Cancer. 1996;78:2281–2287.
Aguirre, JM, Echebarría, MA, Martínez-Conde, R, et al. Warthin tumor. A new hypothesis concerning its development. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:60–63.
Batsakis, JG. Carcinoma ex papillary cystadenoma lymphomatosum. Malignant Warthin’s tumor. Ann Otol Rhinol Laryngol. 1987;96:234–235.
Dietert, SE. Papillary cystadenoma lymphomatosum (Warthin’s tumor) in patients in a general hospital over a 24-year period. Am J Clin Pathol. 1975;63:866–875.
Fantasia, JE, Miller, AS. Papillary cystadenoma lymphomatosum arising in minor salivary glands. Oral Surg Oral Med Oral Pathol. 1981;52:411–416.
Honda, K, Kashima, K, Daa, T, et al. Clonal analysis of the epithelial component of Warthin’s tumor. Hum Pathol. 2000;31:1377–1380.
Klussmann, JP, Wittekindt, C, Preuss, SF, et al. High risk for bilateral Warthin tumor in heavy smokers—review of 185 cases. Acta Otolaryngol. 2006;126:1213–1217.
Kotwall, CA. Smoking as an etiologic factor in the development of Warthin’s tumor of the parotid gland. Am J Surg. 1992;164:646–647.
Martins, C, Fonseca, I, Roque, L, et al. Cytogenetic characterisation of Warthin’s tumour. Oral Oncol. 1997;33:344–347.
Monk, JS, Jr., Church, JS. Warthin’s tumor: a high incidence and no sex predominance in central Pennsylvania. Arch Otolaryngol Head Neck Surg. 1992;118:477–478.
Takezawa, K, Jackson, C, Gnepp, DR, et al. Molecular characterization of Warthin tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:569–575.
Teymoortash, A, Werner, JA. Tissue that has lost its track: Warthin’s tumour. Virchows Arch. 2005;446:585–588.
van der Wal, JE, Davids, JJ, van der Waal, I. Extraparotid Warthin’s tumours—report of 10 cases. Br J Oral Maxillofac Surg. 1993;31:43–44.
Zappia, JJ, Sullivan, MJ, McClatchey, KD. Unilateral multicentric Warthin’s tumors. J Otolaryngol. 1991;20:93–96.
Daley, TD, Gardner, DG, Smout, MS. Canalicular adenoma: not a basal cell adenoma. Oral Surg Oral Med Oral Pathol. 1984;57:181–188.
Fantasia, JE, Neville, BW. Basal cell adenomas of the minor salivary glands. Oral Surg Oral Med Oral Pathol. 1980;50:433–440.
Gardner, DG, Daley, TD. The use of the terms monomorphic adenoma, basal cell adenoma, and canalicular adenoma as applied to salivary gland tumors. Oral Surg Oral Med Oral Pathol. 1983;56:608–615.
Nelson, JF, Jacoway, JR. Monomorphic adenoma (canalicular type): report of 29 cases. Cancer. 1973;31:1511–1513.
Neville, BW, Damm, DD, Weir, JC, et al. Labial salivary gland tumors: an analysis of 103 cases. Cancer. 1988;61:2113–2116.
Rousseau, A, Mock, D, Dover, DG, et al. Multiple canalicular adenomas: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:346–350.
Suarez, P, Hammond, HL, Luna, MA, et al. Palatal canalicular adenoma: report of 12 cases and review of the literature. Ann Diagn Pathol. 1998;2:224–228.
Yoon, AJ, Beller, DE, Woo, VL, et al. Bilateral canalicular adenomas of the upper lip. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:341–343.
Batsakis, JG, Brannon, RB. Dermal analogue tumours of major salivary glands. J Laryngol. 1981;95:155–164.
Ellis, GL, Wiscovitch, JG. Basal cell adenocarcinomas of the major salivary glands. Oral Surg Oral Med Oral Pathol. 1990;69:461–469.
Fonseca, I, Soares, J. Basal cell adenocarcinoma of minor salivary and seromucous glands of the head and neck region. Semin Diagn Pathol. 1996;13:128–137.
Jayakrishnan, A, Elmalah, I, Hussain, K, et al. Basal cell adenocarcinoma in minor salivary glands. Histopathology. 2003;42:610–614.
Luna, MA, Tortoledo, ME, Allen, M. Salivary dermal analogue tumors arising in lymph nodes. Cancer. 1987;59:1165–1169.
Machado de Sousa, SO, Soares de Araújo, N, Corrêa, L, et al. Immunohistochemical aspects of basal cell adenoma and canalicular adenoma of salivary glands. Oral Oncol. 2001;37:365–368.
Muller, S, Barnes, L. Basal cell adenocarcinoma of the salivary glands: report of seven cases and review of the literature. Cancer. 1996;78:2471–2477.
Parashar, P, Baron, E, Papadimitriou, JC, et al. Basal cell adenocarcinoma of the oral minor salivary glands: review of the literature and presentation of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:77–84.
Pogrel, MA. The intraoral basal cell adenoma. J Craniomaxillofac Surg. 1987;15:372–375.
Zarbo, RJ, Prasad, AR, Regezi, JA, et al. Salivary gland basal cell and canalicular adenomas: immunohistochemical demonstration of myoepithelial cell participation and morphogenetic considerations. Arch Pathol Lab Med. 2000;124:401–405.
Abbey, LM. Solitary intraductal papilloma of the minor salivary glands. Oral Surg Oral Med Oral Pathol. 1975;40:135–140.
Abrams, AM, Finck, FM. Sialadenoma papilliferum: a previously unreported salivary gland tumor. Cancer. 1969;24:1057–1063.
Brannon, RB, Sciubba, JJ, Giulani, M. Ductal papillomas of salivary gland origin: a report of 19 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:68–77.
Cabov, T, Macan, D, Manojlovic, S, et al. Oral inverted ductal papilloma. Br J Oral Maxillofac Surg. 2004;42:75–77.
Fantasia, JE, Nocco, CE, Lally, ET. Ultrastructure of sialadenoma papilliferum. Arch Pathol Lab Med. 1986;110:523–527.
Gomes, APN, Sobral, APV, Loducca, SVL, et al. Sialadenoma papilliferum: immunohistochemical study. Int J Oral Maxillofac Surg. 2004;33:621–624.
Hegarty, DJ, Hopper, C, Speight, PM. Inverted ductal papilloma of minor salivary glands. J Oral Pathol Med. 1994;23:334–336.
Iguchi, H, Yamane, H, Nasako, Y, et al. Intraductal papilloma in the parotid duct. Acta Otolaryngol. 2002;122:314–317.
Maiorano, E, Favia, G, Ricco, R. Sialadenoma papilliferum: an immunohistochemical study of five cases. J Oral Pathol Med. 1996;25:336–342.
Nagao, T, Sugano, I, Matsuzaki, O, et al. Intraductal papillary tumors of the major salivary glands. Case reports of benign and malignant variants. Arch Pathol Lab Med. 2000;124:291–295.
de Sousa, SO, Sesso, A, de Araújo, NS, et al. Inverted ductal papilloma of minor salivary gland origin: morphological aspects and cytokeratin expression. Eur Arch Otorhinolaryngol. 1995;252:370–373.
White, DK, Miller, AS, McDaniel, RK, et al. Inverted ductal papilloma: a distinctive lesion of minor salivary gland. Cancer. 1982;49:519–524.
Auclair, PL, Goode, RK, Ellis, GL. Mucoepidermoid carcinoma of intraoral salivary glands. Cancer. 1992;69:2021–2030.
Batsakis, JG, Luna, MA. Histopathologic grading of salivary gland neoplasms. I. Mucoepidermoid carcinomas. Ann Otol Rhinol Laryngol. 1990;99:835–838.
Brandwein, MS, Ivanov, K, Wallace, DI, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25:835–845.
Evans, HL. Mucoepidermoid carcinoma of salivary glands: a study of 69 cases with special attention to histologic grading. Am J Clin Pathol. 1984;81:696–701.
Goode, RK, Auclair, PL, Ellis, GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82:1217–1224.
Guzzo, M, Andreola, S, Sirizzotti, G, et al. Mucoepidermoid carcinoma of the salivary glands: clinicopathologic review of 108 patients treated at the National Cancer Institute of Milan. Ann Surg Oncol. 2002;9:688–695.
Hicks, J, Flaitz, C. Mucoepidermoid carcinoma of salivary glands in children and adolescents: assessment of proliferation markers. Oral Oncol. 2000;36:454–460.
Luna, MA. Salivary mucoepidermoid carcinoma: revisited. Adv Anat Pathol. 2006;13:293–307.
Spiro, RH, Huvos, AG, Berk, R, et al. Mucoepidermoid carcinoma of salivary gland origin: a clinicopathologic study of 367 cases. Am J Surg. 1978;136:461–468.
Stewart, FW, Foote, FW, Becker, WF. Mucoepidermoid tumors of salivary glands. Ann Surg. 1945;122:820–844.
Védrine, PO, Coffinet, L, Temam, S, et al. Mucoepidermoid carcinoma of salivary glands in the pediatric age group: 18 clinical cases, including 11 second malignant neoplasms. Head Neck. 2006;28:827–833.
Whatley, WS, Thompson, JW, Rao, B. Salivary gland tumors in survivors of childhood cancer. Otolaryngol Head Neck Surg. 2006;134:385–388.
Intraosseous Mucoepidermoid Carcinoma
Bouquot, JE, Gnepp, DR, Dardick, I, et al. Intraosseous salivary tissue: jawbone examples of choristomas, hamartomas, embryonic rests, and inflammatory entrapment—another histogenetic source for intraosseous adenocarcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:205–217.
Brookstone, MS, Huvos, AG. Central salivary gland tumors of the maxilla and mandible: a clinicopathologic study of 11 cases with an analysis of the literature. J Oral Maxillofac Surg. 1992;50:229–236.
Browand, BC, Waldron, CA. Central mucoepidermoid tumors of the jaws. Oral Surg Oral Med Oral Pathol. 1975;40:631–643.
Inagaki, M, Yuasa, K, Nakayama, E, et al. Mucoepidermoid carcinoma in the mandible. Findings of panoramic radiography and computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:613–618.
Martínez-Madrigal, F, Pineda-Daboin, K, Casiraghi, O, et al. Salivary gland tumors of the mandible. Ann Diagn Pathol. 2000;4:347–353.
Pires, FR, Paes de Almeida, O, Lopes, MA, et al. Central mucoepidermoid carcinoma of the mandible: report of four cases with long-term follow-up. Int J Oral Maxillofac Surg. 2003;32:378–382.
Waldron, CA, Koh, ML. Central mucoepidermoid carcinoma of the jaws: report of four cases with analysis of the literature and discussion of the relationship to mucoepidermoid, sialodontogenic, and glandular odontogenic cysts. J Oral Maxillofac Surg. 1990;48:871–877.
Batsakis, JG, Luna, MA, El-Naggar, AK. Histopathologic grading of salivary gland neoplasms. II. Acinic cell carcinomas. Ann Otol Rhinol Laryngol. 1990;99:929–933.
Chen, S-Y, Brannon, RB, Miller, AS, et al. Acinic cell adenocarcinoma of minor salivary glands. Cancer. 1978;42:678–685.
Ellis, GL, Corio, RL. Acinic cell adenocarcinoma: a clinicopathologic analysis of 294 cases. Cancer. 1983;52:542–549.
Hamper, K, Mausch, H-E, Caselitz, J, et al. Acinic cell carcinoma of the salivary glands: the prognostic relevance of DNA cytophotometry in a retrospective study of long duration (1965-1987). Oral Surg Oral Med Oral Pathol. 1990;69:68–75.
Hoffman, HT, Karnell, LH, Robinson, RA, et al. National Cancer Data Base report on cancer of the head and neck: acinic cell carcinoma. Head Neck. 1999;21:297–309.
Lewis, JE, Olsen, KD, Weiland, LH. Acinic cell carcinoma: clinicopathologic review. Cancer. 1991;67:172–179.
Michal, M, Skálová, A, Simpson, RHW, et al. Well-differentiated acinic cell carcinoma of salivary glands associated with lymphoid stroma. Hum Pathol. 1997;28:595–600.
Perzin, KH, LiVolsi, VA. Acinic cell carcinomas arising in salivary glands: a clinicopathologic study. Cancer. 1979;44:1434–1457.
Auclair, PL, Ellis, GL. Atypical features in salivary gland mixed tumors: their relationship to malignant transformation. Mod Pathol. 1996;9:652–657.
Bradley, PJ. “Metastasizing pleomorphic salivary adenoma” should now be considered a low-grade malignancy with a lethal potential. Curr Opin Otolaryngol Head Neck Surg. 2005;13:123–126.
Brandwein, M, Huvos, AG, Dardick, I, et al. Noninvasive and minimally invasive carcinoma ex mixed tumor: a clinicopathologic and ploidy study of 12 patients with major salivary tumors of low (or no?) malignant potential. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:655–664.
Carson, HJ, Tojo, DP, Chow, JM, et al. Carcinosarcoma of salivary glands with unusual stromal components: report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:738–746.
Gnepp, DR. Malignant mixed tumors of the salivary glands: a review. Pathol Annu. 1993;28(pt 1):279–328.
Klijanienko, J, El-Naggar, AK, Servois, V, et al. Clinically aggressive metastasizing pleomorphic adenoma: report of two cases. Head Neck. 1997;19:629–633.
Kwon, MY, Gu, M. True malignant mixed tumor (carcinosarcoma) of parotid gland with unusual mesenchymal component: a case report and review of the literature. Arch Pathol Lab Med. 2001;125:812–815.
Lewis, JE, Olsen, KD, Sebo, TJ. Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol. 2001;32:596–604.
LiVolsi, VA, Perzin, KH. Malignant mixed tumors arising in salivary glands. I. Carcinomas arising in benign mixed tumor: a clinicopathologic study. Cancer. 1977;39:2209–2230.
Nouraei, SAR, Ferguson, MS, Clarke, PM, et al. Metastasizing pleomorphic salivary adenoma. Arch Otolaryngol Head Neck Surg. 2006;132:788–793.
Nouraei, SAR, Hope, KL, Kelly, CG, et al. Carcinoma ex benign pleomorphic adenoma of the parotid gland. Plast Reconstr Surg. 2005;116:1206–1213.
Spiro, RH, Huvos, AG, Strong, EW. Malignant mixed tumor of salivary origin: a clinicopathologic study of 146 cases. Cancer. 1977;39:388–396.
Tortoledo, ME, Luna, MA, Batsakis, JG. Carcinomas ex pleomorphic adenoma and malignant mixed tumors. Arch Otolaryngol. 1984;110:172–176.
Wenig, BM, Hitchcock, CL, Ellis, GL, et al. Metastasizing mixed tumor of salivary glands. A clinicopathologic and flow cytometric analysis. Am J Surg Pathol. 1992;16:845–858.
Araújo, VC, Loducca, SVL, Sousa, SOM, et al. The cribriform features of adenoid cystic carcinoma and polymorphous low-grade adenocarcinoma: cytokeratin and integrin expression. Ann Diagn Pathol. 2001;5:330–334.
Beltran, D, Faquin, WC, Gallagher, G, et al. Selective immunohistochemical comparison of polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma. J Oral Maxillofac Surg. 2006;64:415–423.
Bradley, PJ. Adenoid cystic carcinoma of the head and neck: a review. Curr Opin Otolaryngol Head Neck Surg. 2004;12:127–132.
da Cruz Perez, DE, de Abreu Alves, F, Nobuko Nishimoto, I, et al. Prognostic factors in head and neck adenoid cystic carcinoma. Oral Oncol. 2006;42:139–146.
Darling, MR, Schneider, JW, Phillips, VM. Polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma: a review and comparison of immunohistochemical markers. Oral Oncol. 2002;38:641–645.
Enamorado, I, Lakhani, R, Korkmaz, H, et al. Correlation of histopathological variants, cellular DNA content, and clinical outcome in adenoid cystic carcinoma of the salivary glands. Otolaryngol Head Neck Surg. 2004;131:646–650.
Gurney, TA, Eisele, DW, Weinberg, V, et al. Adenoid cystic carcinoma of the major salivary glands treated with surgery and radiation. Laryngoscope. 2005;115:1278–1282.
Hamper, K, Lazar, F, Dietel, M, et al. Prognostic factors for adenoid cystic carcinoma of the head and neck: a retrospective evaluation of 96 cases. J Oral Pathol Med. 1990;19:101–107.
Jones, AS, Hamilton, JW, Rowley, H, et al. Adenoid cystic carcinoma of the head and neck. Clin Otolaryngol. 1997;22:434–443.
Loducca, SVL, Raitz, R, Araújo, NS, et al. Polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma: distinct architectural composition revealed by collagen IV, laminin and their integrin ligands (a2b1 and a3b1). Histopathology. 2000;37:118–123.
Penner, CR, Folpe, AL, Budnick, SD. C-kit expression distinguishes salivary gland adenoid cystic carcinoma from polymorphous low-grade adenocarcinoma. Mod Pathol. 2002;15:687–691.
Perzin, KH, Gullane, P, Clairmont, AC. Adenoid cystic carcinomas arising in salivary glands: a correlation of histologic features and clinical course. Cancer. 1978;42:265–282.
Spiro, RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg. 1997;174:495–498.
Szanto, PA, Luna, MA, Tortoledo, ME, et al. Histologic grading of adenoid cystic carcinoma of the salivary glands. Cancer. 1984;54:1062–1069.
van der Wal, JE, Snow, GB, van der Waal, I. Intraoral adenoid cystic carcinoma: the presence of perineural spread in relation to site, size, local extension, and metastatic spread in 22 cases. Cancer. 1990;66:2031–2033.
Woo, VL, Bhuiya, T, Kelsch, R. Assessment of CD43 expression in adenoid cystic carcinomas, polymorphous low-grade adenocarcinomas, and monomorphic adenomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:495–500.
Polymorphous Low-Grade Adenocarcinoma
Araújo, VC, Loducca, SVL, Sousa, SOM, et al. The cribriform features of adenoid cystic carcinoma and polymorphous low-grade adenocarcinoma: cytokeratin and integrin expression. Ann Diagn Pathol. 2001;5:330–334.
Araújo, V, Sousa, S, Jaeger, M, et al. Characterization of the cellular component of polymorphous low-grade adenocarcinoma by immunohistochemistry and electron microscopy. Oral Oncol. 1999;35:164–172.
Batsakis, JG, Pinkston, GR, Luna, MA, et al. Adenocarcinomas of the oral cavity: a clinicopathologic study of terminal duct carcinomas. J Laryngol Otol. 1983;97:825–835.
Beltran, D, Faquin, WC, Gallagher, G, et al. Selective immunohistochemical comparison of polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma. J Oral Maxillofac Surg. 2006;64:415–423.
Castle, JT, Thompson, LDR, Frommelt, RA, et al. Polymorphous low grade adenocarcinoma: a clinicopathologic study of 164 cases. Cancer. 1999;86:207–219.
Curran, AE, White, DK, Damm, DD, et al. Polymorphous low-grade adenocarcinoma versus pleomorphic adenoma of minor salivary glands: resolution of a diagnostic dilemma by immunohistochemical analysis with glial fibrillary acidic protein. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:194–199.
Darling, MR, Schneider, JW, Phillips, VM. Polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma: a review and comparison of immunohistochemical markers. Oral Oncol. 2002;38:641–645.
Evans, HL, Luna, MA. Polymorphous low-grade adenocarcinoma: a study of 40 cases with long-term follow up and an evaluation of the importance of papillary areas. Am J Surg Pathol. 2000;24:1319–1328.
Freedman, PD, Lumerman, H. Lobular carcinoma of intraoral minor salivary glands. Oral Surg Oral Med Oral Pathol. 1983;56:157–165.
Nagao, T, Gaffey, TA, Kay, PA, et al. Polymorphous low-grade adenocarcinoma of the major salivary glands: report of three cases in an unusual location. Histopathology. 2004;44:164–171.
Penner, CR, Folpe, AL, Budnick, SD. C-kit expression distinguishes salivary gland adenoid cystic carcinoma from polymorphous low-grade adenocarcinoma. Mod Pathol. 2002;15:687–691.
Vincent, SD, Hammond, HL, Finkelstein, MW. Clinical and therapeutic features of polymorphous low-grade adenocarcinoma. Oral Surg Oral Med Oral Pathol. 1994;77:41–47.
Woo, VL, Bhuiya, T, Kelsch, R. Assessment of CD43 expression in adenoid cystic carcinomas, polymorphous low-grade adenocarcinomas, and monomorphic adenomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:495–500.
Salivary Adenocarcinoma, Not Otherwise Specified
Li, J, Wang, BY, Nelson, M, et al. Salivary adenocarcinoma, not otherwise specified: a collection of orphans. Arch Pathol Lab Med. 2004;128:1385–1394.
Matsuba, HM, Mauney, M, Simpson, JR, et al. Adenocarcinomas of major and minor salivary gland origin: a histopathologic review of treatment failure patterns. Laryngoscope. 1988;98:784–788.
Spiro, RH, Huvos, AG, Strong, EW. Adenocarcinoma of salivary origin: clinicopathologic study of 204 patients. Am J Surg. 1982;144:423–431.
Stene, T, Koppang, HS. Intraoral adenocarcinomas. J Oral Pathol. 1981;10:216–225.
Wahlberg, P, Anderson, H, Biörklund, A, et al. Carcinoma of the parotid and submandibular glands—a study of survival in 2465 patients. Oral Oncol. 2002;38:706–713.