Epithelial Pathology
SQUAMOUS PAPILLOMA
The squamous papilloma is a benign proliferation of stratified squamous epithelium, resulting in a papillary or verruciform mass. Presumably, this lesion is induced by the human papillomavirus (HPV). HPV comprises a large family (more than 100 types) of double-stranded DNA viruses of the papovavirus subgroup A. Research has shown that 81% of normal adults have buccal epithelial cells that contain at least one type of HPV, although case control studies using more rigorous criteria usually have shown distinct differences, with high levels of HPV in oral lesions and low levels in normal controls. The virus is capable of becoming totally integrated with the DNA of the host cell, and at least 24 types are associated with lesions of the head and neck. HPV can be identified by in situ hybridization, immu nohistochemical analysis, and polymerase chain reaction (PCR) techniques, but it is not visible with routine histopathologic staining. Viral subtypes 6 and 11 have been identified in up to 50% of oral papillomas, as compared with less than 5% in normal mucosal cells.
The exact mode of transmission is unknown. Transmission by sexual and nonsexual person-to-person contact, contaminated objects, saliva, or breast milk has been proposed. In contrast to other HPV-induced lesions, the viruses in oral squamous papillomas appear to have an extremely low virulence and infectivity rate. A latency or incubation period of 3 to 12 months has been suggested.
The squamous papilloma occurs in one of every 250 adults and makes up approximately 3% of all oral lesions submitted for biopsy. In addition, researchers have estimated that oral squamous papillomas comprise 7% to 8% of all oral masses or growths in children.
CLINICAL FEATURES: The squamous papilloma occurs with equal frequency in both men and women. Some authors have asserted that it develops predominantly in children, but epidemiologic studies indicate that it can arise at any age and, in fact, is diagnosed most often in persons 30 to 50 years of age. Sites of predilection include the tongue, lips, and soft palate, but any oral surface may be affected. This lesion is the most common of the soft tissue masses arising from the soft palate.
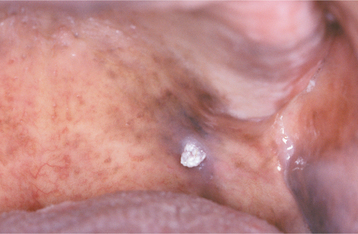
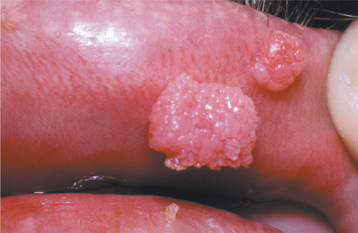
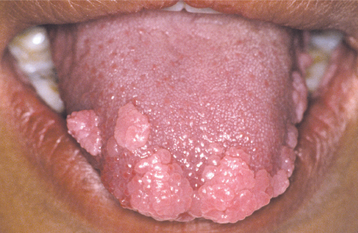
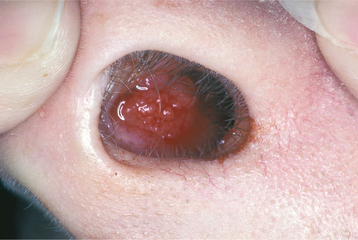
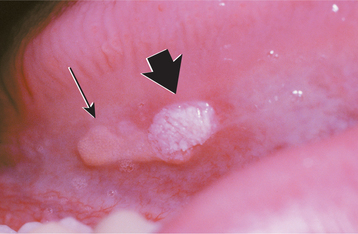
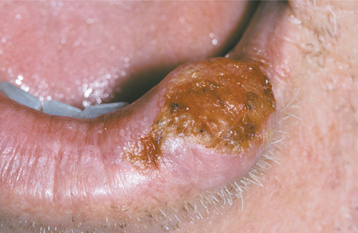
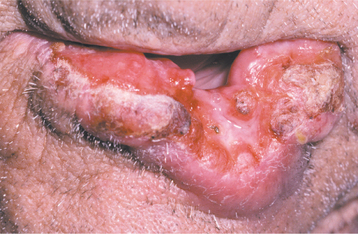
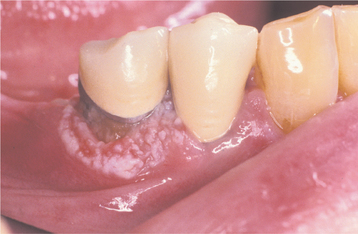
The squamous papilloma is a soft, painless, usually pedunculated, exophytic nodule with numerous fingerlike surface projections that impart a “cauliflower” or wartlike appearance (Fig. 10-1). Projections may be pointed or blunted (Figs. 10-2 and 10-3), and the lesion may be white, slightly red, or normal in color, depending on the amount of surface keratinization. The papilloma is usually solitary and enlarges rapidly to a maximum size of about 0.5 cm, with little or no change thereafter. However, lesions as large as 3.0 cm in greatest diameter have been reported.
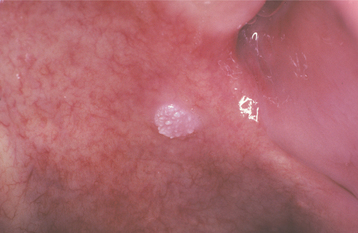
Fig. 10-1 Squamous papilloma. An exophytic lesion of the soft palate with multiple short, white surface projections.
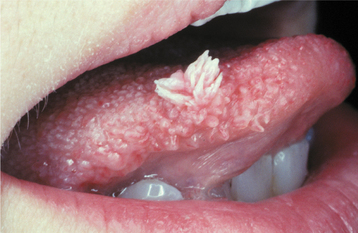
Fig. 10-2 Squamous papilloma. A pedunculated lingual mass with numerous long, pointed, and white surface projections. Note the smaller projections around the base of the lesion.
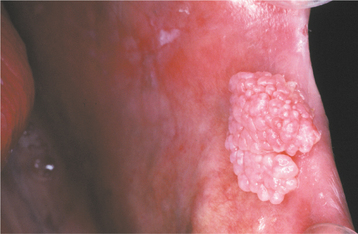
Fig. 10-3 Squamous papilloma. A pedunculated mass of the buccal commissure, exhibiting short or blunted surface projections and minimal white coloration.
It is sometimes difficult to distinguish this lesion clinically from verruca vulgaris (see page 364), condyloma acuminatum (see page 366), verruciform xanthoma (see page 372), or multifocal epithelial hyperplasia (see page 367). In addition, extensive coalescing papillary lesions (papillomatosis) of the oral mucosa may be seen in several skin disorders, including nevus unius lateris, acanthosis nigricans, and focal dermal hypoplasia (Goltz-Gorlin) syndrome. Laryngeal papillomatosis, a rare and potentially devastating disease of the larynx and hypopharynx, has two distinct types: (1) juvenile-onset and (2) adult- onset. Hoarseness is the usual presenting feature, and rapidly proliferating papillomas in the juvenile-onset type may obstruct the airway. The strongest risk factor for juvenile-onset laryngeal papillomatosis is a maternal history of genital warts; transmission of HPV infection via the birth canal, the placenta, or amniotic fluid has been hypothesized.
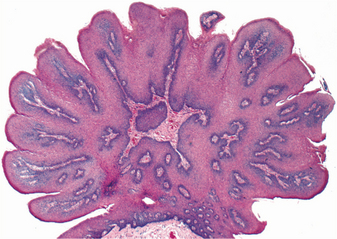
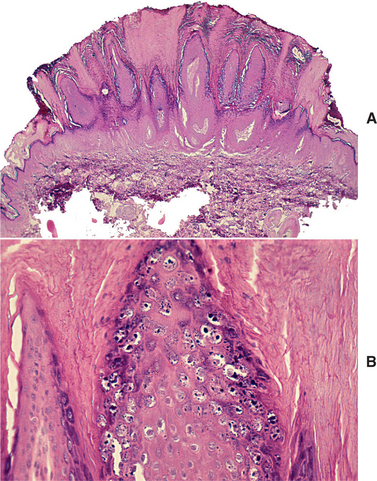
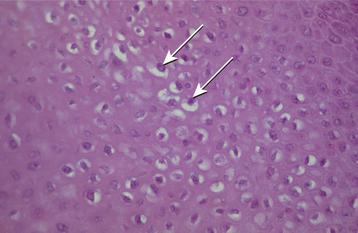
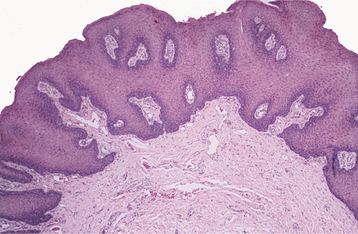
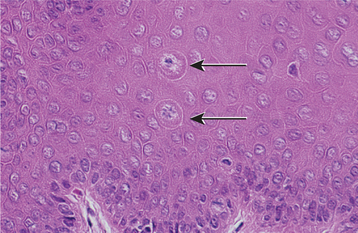
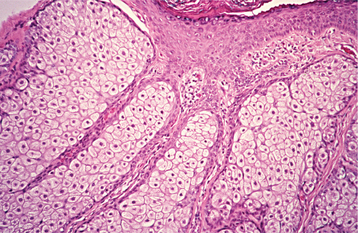
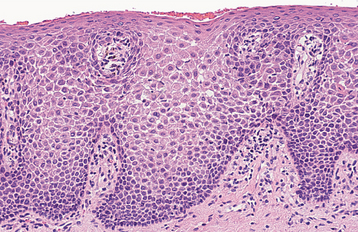
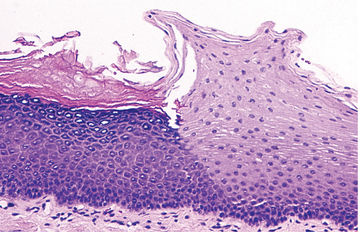
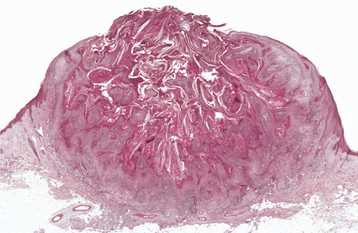
HISTOPATHOLOGIC FEATURES: The papilloma is characterized by a proliferation of keratinized stratified squamous epithelium arrayed in fingerlike projections with fibrovascular connective tissue cores (Fig. 10-4). The connective tissue cores may show inflammatory changes, depending on the amount of trauma sustained by the lesion. The keratin layer is thickened in lesions with a whiter clinical appearance, and the epithelium typically shows a normal maturation pattern. Occasional papillomas demonstrate basilar hyperplasia and mitotic activity, which can be mistaken for mild epithelial dysplasia. Koilocytes, virus-altered epithelial clear cells with small dark (pyknotic) nuclei, are sometimes seen high in the prickle cell layer.
TREATMENT AND PROGNOSIS: Conservative surgical excision, including the base of the lesion, is adequate treatment for the oral squamous papilloma, and recurrence is unlikely. Frequently, lesions have been left untreated for years with no reported transformation into malignancy, continuous enlargement, or dissemination to other parts of the oral cavity.
Although spontaneous remission is possible, juvenile-onset laryngeal papillomatosis tends to be continuously proliferative, sometimes leading to death by asphyxiation. Some investigators have noted especially aggressive behavior among cases associated with HPV type 11 infection. The papillomatosis is treated by repeated surgical debulking procedures to relieve airway obstruction. Adjuvant therapy with agents such as a-interferon may be used for cases exhibiting rapid regrowth or distant spread. Adult-onset lesions are typically less aggressive and tend to be single. Conservative surgical removal may be necessary to eliminate hoarseness from vocal cord involvement. In rare instances, squamous cell carcinoma will develop in long-standing laryngeal papillomatosis, sometimes in a smoker or a patient with a history of irradiation to the larynx.
A vaccine targeted against HPV types 6, 11, 16, and 18 has been introduced recently for the prevention of cervical cancer and genital warts. It is possible that this vaccine may prevent HPV-related lesions of the head and neck as well, such as oral squamous papilloma, laryngeal papillomatosis, and perhaps some cases of oral and oropharyngeal squamous cell carcinoma.
VERRUCA VULGARIS (COMMON WART)
Verruca vulgaris is a benign, virus-induced, focal hyperplasia of stratified squamous epithelium. One or more of the associated human papillomavirus (HPV) types 2, 4, 6, and 40 are found in virtually all examples. Verruca vulgaris is contagious and can spread to other parts of a person’s skin or mucous membranes by way of autoinoculation. It infrequently develops on oral mucosa but is extremely common on the skin.
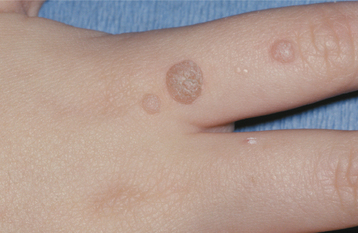
CLINICAL FEATURES: Verruca vulgaris is frequently discovered in children, but occasional lesions may arise even into middle age. The skin of the hands is usually the site of infection (Fig. 10-5). When the oral mucosa is involved, the lesions are usually found on the vermilion border, labial mucosa, or anterior tongue.
Typically, the verruca appears as a painless papule or nodule with papillary projections or a rough pebbly surface (Figs. 10-6 and 10-7). It may be pedunculated or sessile. Cutaneous lesions may be pink, yellow, or white; oral lesions are almost always white. Verruca vulgaris enlarges rapidly to its maximum size (usually <5 mm), and the size remains constant for months or years thereafter unless the lesion is irritated. Multiple or clustered lesions are common. On occasion, extreme accumulation of compact keratin may result in a hard surface projection several millimeters in height, termed a cutaneous horn or keratin horn. Other cutaneous lesions, including seborrheic keratosis (see page 374), actinic keratosis (see page 404), and squamous cell carcinoma, may also create a cutaneous horn.
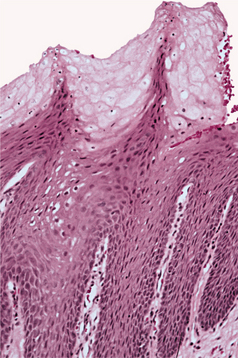
HISTOPATHOLOGIC FEATURES: The verruca vulgaris is characterized by a proliferation of hyperkeratotic stratified squamous epithelium arranged into fingerlike or pointed projections with connective tissue cores (Fig. 10-8). Chronic inflammatory cells often infiltrate the supporting connective tissue. Elongated rete ridges tend to converge toward the center of the lesion, producing a “cupping” effect. A prominent granular cell layer (hypergranulosis) exhibits coarse, clumped keratohyaline granules. Abundant koilocytes are often seen in the superficial spinous layer. Koilocytes are HPV-altered epithelial cells with perinuclear clear spaces and small, dark nuclei (pyknosis). Eosinophilic intranuclear viral inclusions are often noted within the cells of the granular layer.
TREATMENT AND PROGNOSIS: Skin verrucae are treated effectively by topical salicylic acid, topical lactic acid, or liquid nitrogen cryotherapy. Surgical excision is indicated only for cases with an atypical clinical presentation in which the diagnosis is uncertain. Skin lesions that recur or are resistant to standard therapy may be treated by alternative methods, such as intralesional bleomycin, topical or intralesional 5-fluorouracil, or photodynamic therapy.
Oral lesions are usually surgically excised, or they may be destroyed by a laser, cryotherapy, or electrosurgery. Cryotherapy induces a subepithelial blister that lifts the infected epithelium from the underlying connective tissue, allowing it to slough away. All destructive or surgical treatments should extend to include the base of the lesion.
Recurrence is seen in a small proportion of treated cases. Without treatment, verrucae do not transform into malignancy, and two thirds will disappear spontaneously within 2 years, especially in children.
CONDYLOMA ACUMINATUM (VENEREAL WART)
Condyloma acuminatum is a virus-induced proliferation of stratified squamous epithelium of the genitalia, perianal region, mouth, and larynx. One or more of the human papillomavirus (HPV) types 2, 6, 11, 53, and 54 are usually detected in the lesion. However, the high-risk types 16, 18, and 31 also may be present, especially in anogenital lesions. Condyloma is considered to be a sexually transmitted disease (STD), with lesions developing at a site of sexual contact or trauma. This lesion represents 20% of all STDs diagnosed in STD clinics and may be an indicator of sexual abuse when diagnosed in young children. In addition, studies of oral and pharyngeal HPV infection in infants have suggested that vertical transmission from mothers with genital HPV infection may occur perinatally or perhaps in utero; however, reported transmission rates have varied widely (ranging from 4% to >80%). It is not unusual for oral and anogenital condylomata to be present concurrently. The incubation period for a condyloma is 1 to 3 months from the time of sexual contact. Once present, autoinoculation to other mucosal sites is possible.
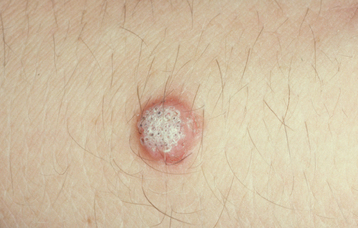
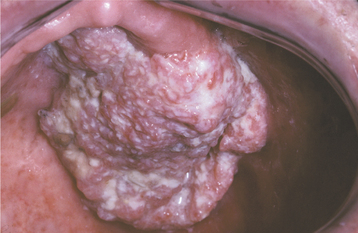
CLINICAL FEATURES: Condylomata are usually diagnosed in teenagers and young adults, but people of all ages are susceptible. Oral lesions most frequently occur on the labial mucosa, soft palate, and lingual frenum. The typical condyloma appears as a sessile, pink, well-demarcated, nontender exophytic mass with short, blunted surface projections (Fig. 10-9). The condyloma tends to be larger than the papilloma and is characteristically clustered with other condylomata. The average lesional size is 1.0 to 1.5 cm, but oral lesions as large as 3 cm have been reported.
HISTOPATHOLOGIC FEATURES: Condyloma acuminatum appears as a benign proliferation of acanthotic stratified squamous epithelium with mildly keratotic papillary surface projections (Fig. 10-10). Thin connective tissue cores support the papillary epithelial projections, which are more blunted and broader than those of squamous papilloma and verruca vulgaris, imparting an appearance of keratin-filled crypts between prominences. In some cases, lesions extending from the surface mucosa to involve underlying salivary ductal epithelium have been reported; such lesions should be distinguished from salivary ductal papillomas (see page 485).
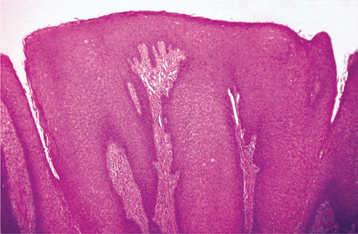
Fig. 10-10 Condyloma acuminatum. Medium-power photomicrograph showing acanthotic stratified squamous epithelium forming a blunted projection.
The covering epithelium is mature and differentiated, but the prickle cells often demonstrate pyknotic, crinkled (or “raisinlike”) nuclei surrounded by clear zones (koilocytes), a microscopic feature of HPV infec tion (Fig. 10-11). Koilocytes may be less prominent in oral lesions compared with genital lesions, in which case distinction from squamous papilloma may be difficult. Ultrastructural examination reveals virions within the cytoplasm or nuclei of koilocytes, and the virus also can be demonstrated by immunohistochemical analysis, in situ hybridization, and PCR techniques.
TREATMENT AND PROGNOSIS: The oral condyloma is usually treated by conservative surgical excision. Laser ablation also has been used, but this treatment has raised some question as to the airborne spread of HPV through the aerosolized microdroplets created by the vaporization of lesional tissue. Nonsurgical, patient-applied topical agents such as imiquimod or podophyllotoxin are becoming the mainstay of treatment for anogenital condylomata, although such treatments are not typically used for oral lesions. Regardless of the method used, a condyloma should be removed because it is contagious and can spread to other oral surfaces and to other persons through direct (usually sexual) contact. In the anogenital area, condylomata infected with HPV-16 or HPV-18 are associated with an increased risk of malignant transformation to squamous cell carcinoma, but this has not been demonstrated in oral lesions.
MULTIFOCAL EPITHELIAL HYPERPLASIA (HECK’S DISEASE; MULTIFOCAL PAPILLOMA VIRUS EPITHELIAL HYPERPLASIA; FOCAL EPITHELIAL HYPERPLASIA)
Multifocal epithelial hyperplasia is a virus-induced, localized proliferation of oral squamous epithelium that was first described in Native Americans and Inuit. Currently, it is known to exist in many populations and ethnic groups and is apparently produced by human papillomavirus (HPV) types 13 and 32. In some populations, as many as 39% of children are affected. The condition often affects multiple members of a given family; this familial tendency may be related to either genetic susceptibility or HPV transmission between family members. An association with the HLA-DR4 (DRB1*0404) allele was found in a recent study of Mexican patients with this condition. Lower socio-economic status, crowded living conditions, and poor hygiene appear to be additional risk factors. Multiple papillary lesions similar to multifocal epithelial hyperplasia arise with increased frequency in patients with acquired immunodeficiency syndrome (AIDS) (see page 276).
CLINICAL FEATURES: Although multifocal epithelial hyperplasia is usually a childhood condition, it occasionally affects young and middle-aged adults. Previous studies have reported either a slight female predilection or no significant gender bias. The most common sites of involvement include the labial, buccal, and lingual mucosa, but gingival, palatal, and tonsillar lesions also have been reported. In addition, involvement of the conjunctiva has been described very rarely.
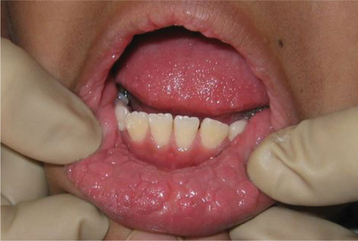
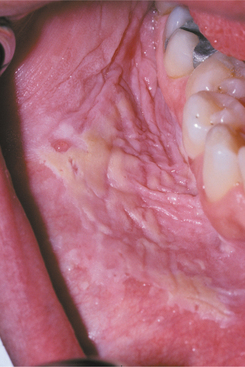
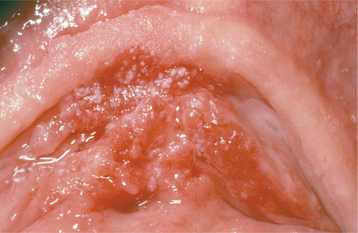
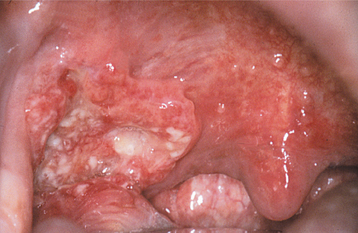
This disease typically appears as multiple soft, nontender, flattened or rounded papules, which are usually clustered and the color of normal mucosa, although they may be scattered, pale, or rarely white (Fig. 10-12). Occasional lesions show a slight papillary surface change (Fig. 10-13). Individual lesions are small (0.3 to 1.0 cm), discrete, and well demarcated, but they frequently cluster so closely together that the entire area takes on a cobblestone or fissured appearance.
HISTOPATHOLOGIC FEATURES: The hallmark of multifocal epithelial hyperplasia is an abrupt and sometimes considerable acanthosis of the oral epithelium (Fig. 10-14). Because the thickened mucosa extends upward, not down into underlying connective tissues, the lesional rete ridges are at the same depth as the adjacent normal rete ridges. The ridges themselves are widened, often confluent, and sometimes club shaped. Some superficial keratinocytes show a koilocytic change similar to that seen in other HPV infections. Others occasionally demonstrate an altered nucleus that resembles a mitotic figure (mitosoid cell) (Fig. 10-15). Viruslike particles have been noted ultrastructurally within both the cytoplasm and the nuclei of cells within the prickle cell layer, and the presence of HPV has been demonstrated with both DNA in situ hybridization and immunohistochemical analysis.
TREATMENT AND PROGNOSIS: Spontaneous regression of multifocal epithelial hyperplasia has been reported after months or years and is inferred from the rarity of the disease in adults. Conservative surgical excision may be performed for diagnostic or aesthetic purposes or for lesions subject to recurrent trauma. Lesions also can be removed by cryotherapy or carbon dioxide (CO2) laser ablation. Use of topical interferon-b and systemic interferon-a has been reported in a few cases, although with variable results. The risk of recurrence after therapy is minimal, and there seems to be no malignant transformation potential.
SINONASAL PAPILLOMAS
Papillomas of the sinonasal tract are benign, localized proliferations of the respiratory mucosa of this region. This mucosa gives rise to three histomorphologically distinct papillomas:
Lesions exhibiting features of both the inverted and the cylindrical cell types may be termed mixed or hybrid papillomas. In addition, a keratinizing squamous papilloma, similar to the oral squamous papilloma (see page 362), rarely may occur in the nasal vestibule.
Collectively, sinonasal papillomas represent 10% to 25% of all tumors of the nasal and paranasal region. Half of the sinonasal papillomas arise from the mucosa of the lateral nasal wall; the remainder predominantly involve the maxillary and ethmoid sinuses and the nasal septum. Multiple lesions may be present.
The cause of sinonasal papillomas remains controversial and unclear. Some authorities say that these lesions represent neoplasms; others consider them to be a reactive hyperplasia secondary to a variety of environmental stimulants, such as allergy, chronic bacterial or viral (HPV type 11) infection, and tobacco smoking. Recent molecular genetic investigations have shown that inverted papillomas arise from a single progenitor cell (i.e., monoclonal), suggesting that these lesions are neoplastic and recurrence may result from growth of residual transformed cells.
FUNGIFORM (SEPTAL; SQUAMOUS; EXOPHYTIC) PAPILLOMA
The fungiform papilloma bears some similarity to the oral squamous papilloma, although it has a somewhat more aggressive biologic behavior and more varied epithelial types. It represents 18% to 50% of all sinonasal papillomas in various investigations. Almost all examples are positive for HPV type 6 or 11.
CLINICAL FEATURES: The fungiform papilloma arises almost exclusively on the nasal septum and is twice as common in men as in women. It occurs primarily in people 20 to 50 years of age. Typically, it exhibits unilateral nasal obstruction or epistaxis and appears as a pink or tan, broad-based nodule with papillary or warty surface projections (Fig. 10-16).
HISTOPATHOLOGIC FEATURES: The fungiform papilloma has a microscopic appearance similar to that of the oral squamous papilloma, although the stratified squamous epithelium covering the fingerlike projections seldom is keratinized. Respiratory epithelium or “transitional” epithelium (intermediate between squamous and respiratory) may be seen in some lesions. Mucous (goblet) cells and intraepithelial microcysts containing mucus often are present. Mitoses are infrequent, and dysplasia is rare. The underlying connective tissue consists of delicate fibrous tissue with a minimal inflammatory component, unless it is irritated.
TREATMENT AND PROGNOSIS: Complete surgical excision is the treatment of choice for the fungiform papilloma. Recurrence is common, developing in approximately one third of all cases; however, this may be caused by incomplete excision. Most authorities consider this lesion to have minimal or no potential for malignant transformation.
INVERTED PAPILLOMA (INVERTED SCHNEIDERIAN PAPILLOMA)
The most common (50% to 78%) sinonasal papilloma, the inverted papilloma, is also the variant with the greatest potential for local destruction and malignant transformation. HPV types 6, 11, 16, and 18 have been identified, with considerable variability in the reported proportion of cases positive for HPV. This variability is likely due to differences in detection methods used; however, more recent studies using quantitative real-time polymerase chain reaction (PCR) suggest that HPV is present in approximately 18 to 30% of lesions.
CLINICAL AND RADIOGRAPHIC FEATURES: The inverted papilloma seldom occurs in patients younger than 20 years of age; the median age is 55 years. A strong male predilection is noted (3:1 male-to-female ratio). This lesion arises predominantly from the lateral nasal cavity wall or a paranasal sinus, usually the antrum. Typically, the inverted papilloma results in unilateral nasal obstruction; additional symptoms may include pain, epistaxis, purulent discharge, or local deformity. The papilloma appears as a soft, pink or tan, polypoid or nodular growth. Multiple lesions may be present.
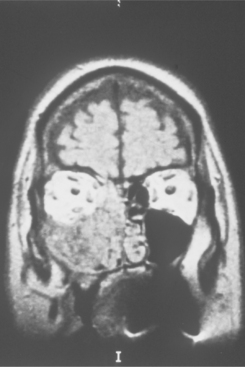
Pressure erosion of the underlying bone is usually present and may be visible radiographically as an irregular radiolucency. Primary sinus lesions may be distinguishable only as a soft tissue radiodensity or mucosal thickening on radiographs; sinus involvement generally represents extension from the nasal cavity. Magnetic resonance imaging (MRI) can help to identify the extent of the lesion (Fig. 10-17).
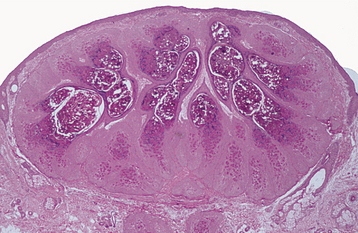
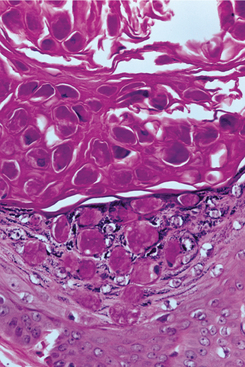
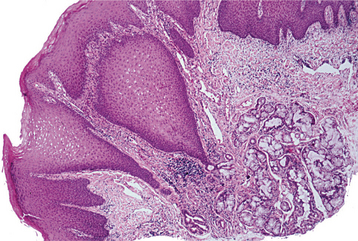
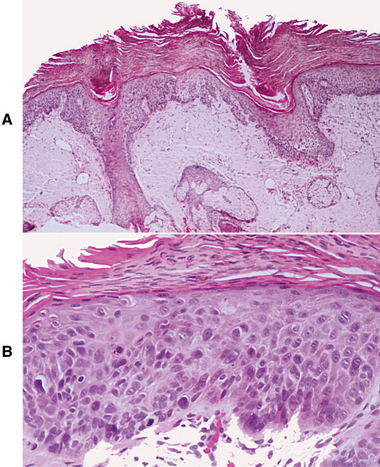
HISTOPATHOLOGIC FEATURES: Microscopically, the inverted papilloma is characterized by squamous epithelial proliferation into the submucosal stroma (Fig. 10-18). The basement membrane remains intact, and the epithelium appears to be “pushing” into underlying connective tissue. Goblet (mucous) cells and mucin-filled microcysts frequently are noted within the epithelium. Keratin production is uncommon, but thin surface keratinization may be seen. Mitoses often are noted within the basilar or parabasilar cells, and varying degrees of dysplasia may be seen. Papillary surface projections are present, and deep clefts may be seen between projections. The stroma consists of dense fibrous or loose myxomatous connective tissue with or without inflammatory cells.
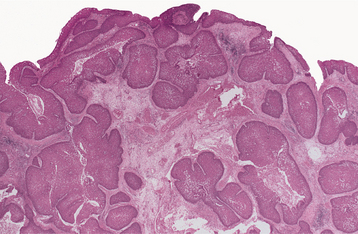
Fig. 10-18 Inverted papilloma. Low-power photomicrograph showing a squamous epithelial proliferation, with multiple “inverting” islands of epithelium extending into the underlying connective tissue.
Destruction of underlying bone frequently is noted. Immunohistochemical expression of CD44, a cell adhesion molecule, is increased in this papilloma, which may help to distinguish it from invasive papillary squamous cell carcinoma, which lacks this feature. Although some authors have suggested that hyperkeratosis, prominent epithelial hyperplasia, and high mitotic index are negative prognostic indicators, no histopathologic parameters have been found to be reliably predictive of recurrence or malignant transformation among inverted papillomas.
TREATMENT AND PROGNOSIS: The inverted papilloma has a significant growth potential and, if neglected, may extend into the nasopharynx, middle ear, orbit, or cranial base. In some studies, recurrence after conservative surgical excision has occurred in nearly 75% of all cases. However, with more aggressive surgical therapy, consisting of medial maxillectomy via a lateral rhinotomy or midfacial degloving approach, recurrence rates of less than 14% have been reported. Although an open surgical approach historically has been regarded as the standard of care, advances in transnasal endoscopic surgery have led to wider acceptance of this method as an alternative, particularly for patients with limited and easily accessible disease. Several investigators, using modern endoscopic techniques and careful patient selection, have reported recurrence rates comparable to those for conventional lateral rhinotomy with medial maxillectomy. Recurrences are usually noted within 2 years of surgery but can happen much later. Hence, long-term follow-up is essential. Continued tobacco smoking is associated with an increased risk of multiple recurrences.
The inverted papilloma also is associated with malignancy, usually squamous cell carcinoma, in 3% to 24% of cases. In such an eventuality, of course, the lesion is treated as a malignancy, typically by performing more radical surgery, with or without adjunctive radiotherapy.
CYLINDRICAL CELL PAPILLOMA (ONCOCYTIC SCHNEIDERIAN PAPILLOMA)
The cylindrical cell papilloma accounts for less than 7% of sinonasal papillomas. This lesion is considered by some authorities to be a variant of the inverted papilloma because of the similarity in clinical and histopathologic features and a similarly low frequency of HPV.
CLINICAL FEATURES: Cylindrical cell papilloma typically occurs in adults 20 to 50 years of age. There is a strong male predominance, with a predilection for the maxillary antrum, lateral nasal cavity wall, and ethmoid sinus. The presenting symptom is usually unilateral nasal obstruction, and it appears as a beefy-red or brown mass with a multinodular surface.
HISTOPATHOLOGIC FEATURES: Microscopically, the cylindrical cell papilloma demonstrates both endophytic and exophytic growth. Surface papillary projections have a fibrovascular connective tissue core and are covered by a multilayered epithelium of tall columnar cells with small, dark nuclei and eosinophilic, occasionally granular, cytoplasm. The lesional epithelial cell is similar to an oncocyte. Cilia may be seen on the surface, and there are numerous intraepithelial microcysts filled with mucin, neutrophils, or both.
MOLLUSCUM CONTAGIOSUM
Molluscum contagiosum is a virus-induced epithelial hyperplasia produced by the molluscum contagiosum virus, a member of the DNA poxvirus group. At least 6% of the population (more in older age groups) has antibodies to this virus, although few ever develop lesions. After an incubation period of 14 to 50 days, infection produces multiple papules of the skin or, rarely, mucous membranes. These remain small for months or years and then spontaneously involute.
During its active phase, the molluscum contagiosum virus is sloughed from a central core in each papule. Routes of transmission include sexual contact (in adults) and such nonsexual contacts (in children and teenagers) as sharing clothing, wrestling, communal bathing, and swimming. Lesions have a predilection for warm portions of the skin and sites of recent injury. Florid cases have been reported in immunocompromised patients, and the prevalence among the human immunodeficiency virus (HIV)-positive patient population is estimated to be 5% to 18% (see page 278). Patients with atopic dermatitis and Darier’s disease also are at risk for developing severe and extensive disease.
CLINICAL FEATURES: Molluscum contagiosum is usually seen in children and young adults. The papules almost always are multiple and occur predominantly on the skin of the neck, face (particularly eyelids), trunk, and genitalia. Infrequently, oral involvement occurs, usually on the lips, buccal mucosa, palate, or gingiva.
Lesions are pink, smooth-surfaced, sessile, nontender, and nonhemorrhagic papules that are 2 to 4 mm in diameter (Fig. 10-19). Many show a small central indentation or keratin-like plug from which a curdlike substance can be expressed. Some are surrounded by a mild inflammatory erythema and may be slightly tender or pruritic. Eczematous eruptions occasionally may develop in the vicinity of molluscum contagiosum lesions, particularly in patients with atopic dermatitis.
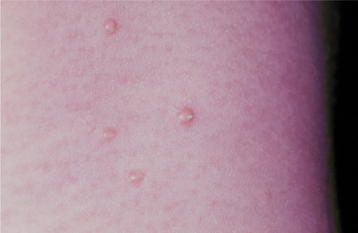
Fig. 10-19 Molluscum contagiosum. Multiple, smooth-surfaced papules, with several demonstrating small keratin-like plugs, are seen on the neck of a child.
In immunocompromised patients, atypical lesions that are unusually large, verrucous, or markedly hyperkeratotic have been described.
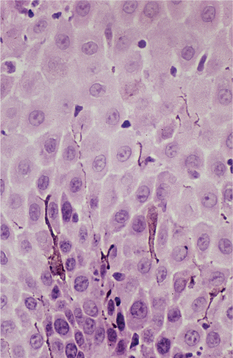
HISTOPATHOLOGIC FEATURES: Molluscum contagiosum appears as a localized lobular proliferation of surface stratified squamous epithelium (Fig. 10-20). The central portion of each lobule is filled with bloated keratinocytes that contain large, intranuclear, basophilic viral inclusions called molluscum bodies (or Henderson-Paterson bodies) (Fig. 10-21). These bodies begin as small eosinophilic structures in cells just above the basal layer. As they approach the surface, these bodies increase so much in size that they frequently become larger than the original size of the invaded cells. A central crater is formed at the surface as stratum corneum cells disintegrate to release their molluscum bodies. These unique features make the diagnosis readily apparent.
TREATMENT AND PROGNOSIS: In most cases of molluscum contagiosum, spontaneous remission occurs within 6 to 9 months. For immunocompetent patients, there is ongoing debate as to whether the disease should be treated or allowed to resolve on its own. Treatment may be performed to decrease the risk of disease transmission, prevent autoinoculation, or provide symptomatic relief.
Few controlled studies of treatment efficacy have been performed, but lesions most commonly are removed by curettage or cryotherapy. Alternative treatment methods include CO2 or pulsed dye laser therapy, electrodessication, trichloroacetic acid, silver nitrate, potassium hydroxide, or the topical blistering agent cantharidin. Topical agents such as tretinoin, podophyllotoxin, and imiquimod are additional alternatives, although generally not as effective or rapid as in-office cryotherapy or curettage
In immunosuppressed patients with recalcitrant lesions, the antiviral agent cidofivir may be effective. Moreover, in patients with AIDS, highly active antiretroviral therapy indirectly counteracts molluscum contagiosum infection by increasing CD4+ T cell counts and improving the immune response.
There is no apparent potential to transform into carcinoma, and the lesions tend not to recur after treatment.
VERRUCIFORM XANTHOMA
Verruciform xanthoma is a hyperplastic condition of the epithelium of the mouth, skin, and genitalia, with a characteristic accumulation of lipid-laden histiocytes beneath the epithelium. First reported in 1971, it remains largely an oral disease; its cause is still unknown. Although verruciform xanthoma is a papillary lesion, human papillomavirus (HPV) has been identified in only a small number of cases, and no definitive role for this virus in the pathogenesis of these lesions has been established. The lesion probably represents an unusual reaction or immune response to localized epithelial trauma or damage. This hypothesis is supported by cases of verruciform xanthoma that have developed in association with disturbed epithelium (e.g., lichen planus, lupus erythematosus, epidermolysis bullosa, epithelial dysplasia, squamous cell carcinoma, pemphigus vulgaris, warty dyskeratoma, graft-versus-host disease [GVHD]). The lesion is histopathologically similar to other dermal xanthomas, but it is not associated with diabetes, hyperlipidemia, or any other metabolic disorder.
CLINICAL FEATURES: Verruciform xanthoma is typically seen in whites, 40 to 70 years of age, with a slight male predilection. Approximately half of the intraoral lesions occur on the gingiva and alveolar mucosa, but any oral site may be involved.
The lesion appears as a well-demarcated, soft, painless, sessile, slightly elevated mass with a white, yellow-white, or red color and a papillary or roughened (verruciform) surface (Figs. 10-22 and 10-23). Rarely, flat-topped nodules are seen without surface projections. Most lesions are smaller than 2 cm in greatest diameter; no oral lesion larger than 4 cm has been reported. Multiple lesions occasionally have been described. Clinically, verruciform xanthoma may be similar to squamous papilloma, condyloma acuminatum, or early carcinoma.
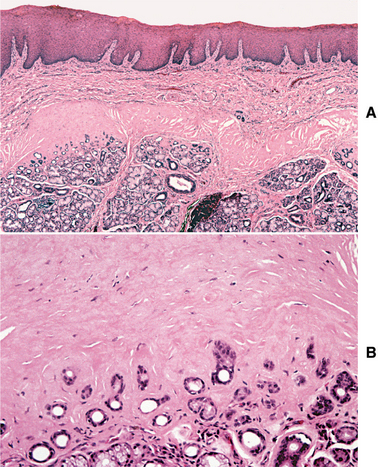
HISTOPATHOLOGIC FEATURES: Verruciform xanthoma demonstrates papillary, acanthotic surface epithelium covered by a thickened layer of parakeratin. On routine hematoxylin and eosin (H&E) staining, the keratin layer often exhibits a distinctive orange coloration (Fig. 10-24). Clefts or crypts between the epithelial projections are filled with parakeratin, and rete ridges are elongated to a uniform depth. The most important diagnostic feature is the accumulation of numerous large macrophages with foamy cytoplasm, which typically are confined to the connective tissue papillae. These foam cells, also known as xanthoma cells, contain lipid and periodic acid-Schiff (PAS)-positive, diastase-resistant granules. With immunohistochemical stains, the xanthoma cells are positive for markers consistent with monocyte-macrophage lineage, including CD68 (KP1) and cathepsin B.
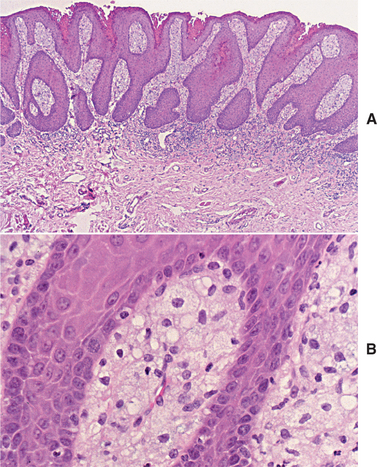
Fig. 10-24 Verruciform xanthoma. A, A slight papillary appearance is produced by hyperparakeratosis, and the rete ridges are elongated to a uniform depth. Note the parakeratin plugging between the papillary projections. B, The connective tissue papillae are composed almost exclusively of xanthoma cells—large macrophages with foamy cytoplasm.
TREATMENT AND PROGNOSIS: The verruciform xanthoma is treated with conservative surgical excision. Recurrence after removal of the lesion is rare, and no malignant transformation has been reported. However, two cases have been reported in which a verruciform xanthoma occurred in association with carcinoma in situ or squamous cell carcinoma. This does not necessarily imply that verruciform xanthoma is a potentially malignant lesion; however, it may indicate that hyperkeratotic or dysplastic oral lesions can undergo degenerative changes to form a verruciform xanthoma.
SEBORRHEIC KERATOSIS
Seborrheic keratosis is an extremely common skin lesion of older people and represents an acquired, benign proliferation of epidermal basal cells. The cause is unknown, although there is a positive correlation with chronic sun exposure, sometimes with a hereditary (autosomal dominant) tendency. In addition, recent genetic studies have suggested that somatic mutations in the FGFR3 (fibroblast growth factor receptor 3) gene are important in the development of these lesions. Seborrheic keratosis does not occur in the mouth.
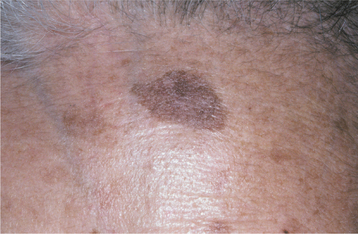
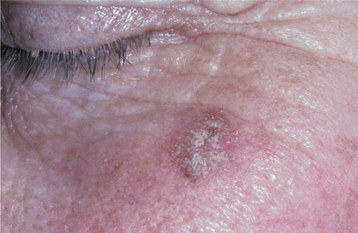
CLINICAL FEATURES: Seborrheic keratoses begin to develop on the skin of the face, trunk, and extremities during the fourth decade of life, and they become more prevalent with each passing decade. Lesions are usually multiple, beginning as small tan to brown macules that are indistinguishable clinically from actinic lentigines (see page 377), and which gradually enlarge and elevate (Figs. 10-25 and 10-26). Individual lesions are sharply demarcated plaques and have surfaces that are finely fissured, pitted, or verrucous, but may be smooth. They tend to appear “stuck onto” the skin and are usually less than 2 cm in diameter.
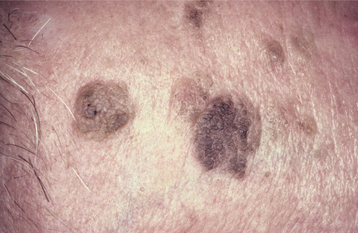
Fig. 10-25 Seborrheic keratosis. Multiple brown plaques on the face of an older man exhibit a fissured surface. They had been slowly enlarging for several years.
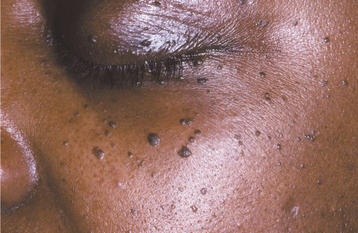
Dermatosis papulosa nigra is a form of seborrheic keratosis that occurs in approximately 30% of blacks and frequently has an autosomal dominant inheritance pattern. This condition typically appears as multiple, small (1 to 2 mm), dark-brown to black papules scattered about the zygomatic and periorbital region (Fig. 10-27).
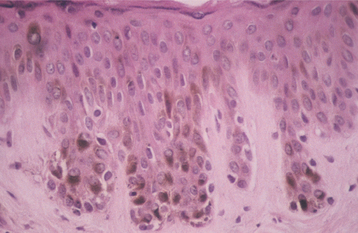
HISTOPATHOLOGIC FEATURES: Seborrheic keratosis consists of an exophytic proliferation of basilar epithelial cells that exhibit varying degrees of surface keratinization, acanthosis, and papillomatosis (Fig. 10-28). Characteristically, the entire epithelial hyperplasia extends upward, above the normal epidermal surface. The lesion usually exhibits deep, keratin-filled invaginations that appear cystic on cross-section; hence, they are called horn cysts or pseudo-horn cysts (Fig. 10-29). Melanin pigmentation often is seen within the basal layer.
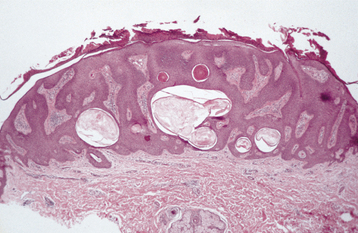
Fig. 10-28 Seborrheic keratosis. The acanthotic form demonstrates considerable acanthosis, surface hyperkeratosis, and numerous pseudocysts. The epidermal proliferation extends upward, above the normal epidermal surface.
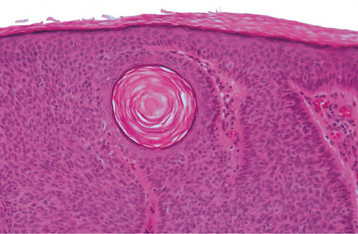
Fig. 10-29 Seborrheic keratosis. Pseudocysts are actually keratin-filled invaginations, as seen toward the left in this high-power photomicrograph. The surrounding epithelial cells are basaloid in appearance.
Several histopathologic patterns may be seen in seborrheic keratoses. The most common is the acanthotic form, which exhibits little papillomatosis and marked acanthosis with minimal surface keratinization. The hyperkeratotic form is characterized by prominent papillomatosis and hyperkeratosis with minimal acanthosis. The adenoid form consists of anastomosing trabeculae of lesional cells with little hyperkeratosis or papillomatosis. The lesions of dermatosis papulosa nigra are predominantly of the adenoid and acanthotic types.
Chronic trauma may alter these histopathologic features, and the lesion known as inverted follicular keratosis of Helwig is thought to represent an irritated seborrheic keratosis. This lesion shows a mild degree of proliferation into the connective tissue and a chronic inflammatory cell infiltrate adjacent to the lesion. Squamous metaplasia of the lesional cells results in whorled epithelial patterns called squamous eddies. Inflamed seborrheic keratosis may show enough nuclear atypia and mitotic activity to cause confusion with squamous cell carcinoma, but enough of the basic attributes of seborrheic keratosis typically remain to allow a proper diagnosis.
TREATMENT AND PROGNOSIS: Except for aesthetic purposes, a seborrheic keratosis seldom is removed. Cryotherapy with liquid nitrogen or simple curettage is the treatment of choice for lesions that are removed. Although the keratosis has no malignant potential, other more significant skin lesions may develop in areas contiguous to it. In rare cases, melanomas may resemble seborrheic keratoses clinically; thus it is important for a dermatologist or other qualified clinician to determine whether it is most appropriate to treat a lesion by cryotherapy or to excise and submit it for histopathologic confirmation. Moreover, the sudden appearance of numerous seborrheic keratoses with pruritus has been associated with internal malignancy, a rare event called the Leser-Trélat sign.
SEBACEOUS HYPERPLASIA
Sebaceous hyperplasia is characterized by a localized proliferation of sebaceous glands of the skin. It has no known cause and is common on the facial skin. In some cases an association with cyclosporine, systemic corticosteroids, hemodialysis, and Muir-Torre syndrome (a rare autosomal dominant disorder characterized by visceral malignancies, sebaceous adenomas and carcinomas, and keratoacanthomas) has been described. The major significance of this entity is its clinical similarity to more serious facial tumors, such as basal cell carcinoma.
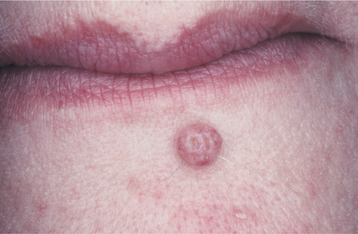
CLINICAL FEATURES: Cutaneous sebaceous hyperplasia usually affects adults older than 40 years of age. It occurs most commonly on the skin of the face, especially the nose, cheeks, and forehead. Less commonly, lesions may involve the genital area, chest, and areola. The condition is characterized by one or more soft, nontender papules with white, yellow, or normal coloration (Fig. 10-30). Lesions are usually umbilicated, with a small central depression, representing the area where the ducts of the involved sebaceous lobules terminate. Most lesions are smaller than 5 mm in greatest diameter and take considerable time to reach even this small size.
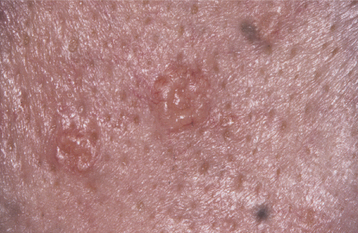
Fig. 10-30 Sebaceous hyperplasia. Multiple soft papules of the midface are umbilicated and small. Sebum can often be expressed from the central depressed area.
Compression of the lesion usually causes sebum, the thick yellow-white product of the sebaceous gland, to be expressed in the central depressed area. This feature helps clinically to distinguish sebaceous hyperplasia from basal cell carcinoma. An oral counterpart, which probably has no relation to the skin lesion, appears as a white to yellow papule or nodular mass with a “cauliflower” appearance, usually of the buccal mucosa.
HISTOPATHOLOGIC FEATURES: Histopathologically, sebaceous hyperplasia is characterized by a collection of enlarged but otherwise nor-mal sebaceous gland lobules grouped around one or more centrally located sebaceous ducts (Fig. 10-31).
TREATMENT AND PROGNOSIS: No treatment is necessary for sebaceous hyperplasia except for aesthetic reasons or unless basal cell carcinoma cannot be eliminated from the clinical differential diagnosis of cutaneous lesions. Excisional biopsy is curative. Cryosurgery, electrodessication, laser therapy, and photodynamic therapy are alternative methods for removal.
EPHELIS (FRECKLE)
An ephelis is a common small hyperpigmented ma-cule of the skin that represents a region of increased melanin production. Ephelides are seen most often on the face, arms, and back of fair-skinned, blue-eyed, red- or light-blond haired persons; they may be associated with a strong genetic predilection (autosomal dominant). Recent studies have demonstrated a strong relationship between certain variants of the MC1R (melanocortin-1-receptor) gene and the development of ephelides. The skin discoloration is produced by a relative excess of melanin deposition in the epidermis, not by a local increase in the number of melanocytes.
CLINICAL FEATURES: Ephelides become noticeable during the first decade of life, and new macules seldom arise after the teenage years. During adult life the macules typically become less prominent. There is no sex predilection; however, persons with blond or red hair are more likely to have ephelides. The lesions become more pronounced after sun exposure and are associated closely with a history of painful sunburns in childhood.
Each individual macule is round or oval, and typically remains less than 3 mm in diameter (Fig. 10-32). It has a uniform light-brown coloration and is sharply demarcated from the surrounding skin. There is great variability in the numbers of ephelides present. Many individuals have less than 10, whereas some have hundreds of macules. The brown color is not as dark as the lentigo simplex (see page 378), and there is never ele vation above the surface of the skin, as may occur in a melanocytic nevus (see page 382).
HISTOPATHOLOGIC FEATURES: The ephelis is composed of stratified squamous epithelium with abundant melanin deposition in the basal cell layer. Despite the increased melanin, the number of melanocytes is normal or may be somewhat reduced. In contrast to lentigo simplex, there is no elongation of rete ridges.
ACTINIC LENTIGO (LENTIGO SOLARIS; SOLAR LENTIGO; AGE SPOT; LIVER SPOT; SENILE LENTIGO)
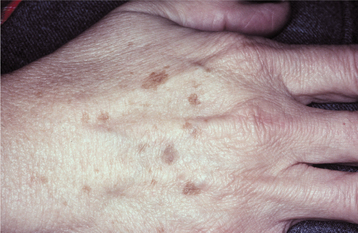
Actinic lentigo is a benign brown macule that results from chronic ultraviolet (UV) light damage to the skin. It is found in more than 90% of whites older than 70 years of age and rarely is seen before age 40. It does not occur within the mouth but is seen frequently on the facial skin. Persons who have facial ephelides (freckles) in childhood are more likely to develop actinic lentigines later in life. In a recent study of actinic lentigines in older whites, patients with multiple facial lesions typically were dark-skinned individuals who repeatedly received intermittent, intense sun exposure during their lifetime and whose facial lesions were preceded by the development of multiple actinic lentigines on the upper back.
CLINICAL FEATURES: Actinic lentigo is common on the dorsa of the hands, on the face, and on the arms of older whites (Figs. 10-33 and 10-34). It is typically multiple, but individual lesions appear as uniformly pigmented brown to tan macules with well-demarcated but irregular borders. Although the lesion may reach more than 1 cm in diameter, most examples are smaller than 5 mm. Adjacent lesions may coalesce, and new ones continuously arise with age. Unlike ephelides, no change in color intensity is seen after exposure to UV light.
HISTOPATHOLOGIC FEATURES: Rete ridges are elongated and club shaped in actinic lentigines, with thinning of the epithelium above the connective tissue papillae (Fig. 10-35). The ridges sometimes seem to coalesce with one another. Within each rete ridge, melanin-laden basilar cells are intermingled with excessive numbers of heavily pigmented melanocytes.
TREATMENT AND PROGNOSIS: No treatment is required for actinic lentigo, except for aesthetic reasons. Lesions may be treated by cryotherapy, although hypopigmentation is a potential side effect. Laser therapy or intense pulsed light also can be effective. In addition, there is a wide range of topical therapies currently available, including hydroquinone, tretinoin, tazarotene, adapalene, and, more recently, a stable fixed combination of mequinol and tretinoin. Generally, sunscreens are recommended as preventive treatment and for maintenance of treatment success. Actinic lentigo does not undergo malignant transfor mation; if removed, then it rarely recurs. New lesions, however, can arise in adjacent or distant skin at any time.
LENTIGO SIMPLEX
Lentigo simplex is one of several forms of benign cutaneous melanocytic hyperplasia of unknown cause. It usually occurs on skin that is not exposed to sunlight, but it may occur on any skin surface and at any age. Its color intensity does not change with variations in sun exposure. Lentigo simplex is darker in color than the common ephelis (see page 376). Ephelides, moreover, are found predominantly on sun-exposed skin, become more pronounced with increased sun exposure, and represent merely an increase in local melanin production rather than an increase in the number of productive melanocytes.
Some investigators believe that lentigo simplex represents the earliest stage of another common skin lesion, the melanocytic nevus (see page 382). Oral lesions have been reported, but they are rare and may be examples of the oral melanotic macule (see page 379).
CLINICAL FEATURES: Lentigo simplex usually occurs in children but may occur at any age. The typical lesion is a sharply demarcated macule smaller than 5 mm in diameter, with a uniformly tan to dark-brown color (Fig. 10-36). It is usually solitary, although some patients may have several lesions scattered on the skin of the trunk and extremities. Lentigo simplex reaches its maximum size in a matter of months and may remain unchanged indefinitely thereafter.
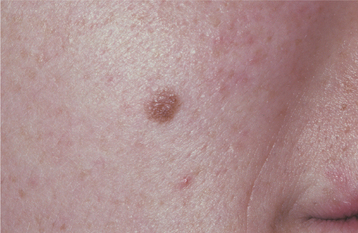
Fig. 10-36 Lentigo simplex. A sharply demarcated lesion of uniform brown coloration is seen on the midface.
Clinically, individual lesions of lentigo simplex are indistinguishable from the nonelevated melanocytic nevus. With multiple lesions, conditions such as lentiginosis profusa, Peutz-Jeghers syndrome (see page 753), and the multiple lentigines or LEOPARD* syndrome must be considered as diagnostic possibilities.
HISTOPATHOLOGIC FEATURES: Lentigo simplex shows an increased number of benign melanocytes within the basal layer of the epidermis, and these often are clustered at the tips of the rete ridges. Abundant melanin is distributed among the melanocytes and basal keratinocytes, as well as within the papillary dermis in association with melanophages (melanin incontinence).
TREATMENT AND PROGNOSIS: Lentigo simplex may fade spontaneously after many years, but most lesions remain constant over time. Treatment is not required, except for aesthetic reasons. Conservative surgical excision is curative, and no malignant transformation potential has been documented for lesions not removed.
MELASMA (MASK OF PREGNANCY)
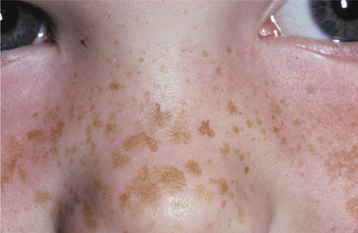
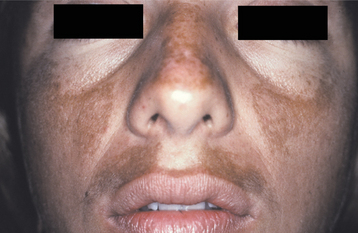
Melasma is an acquired, symmetrical hyperpigmentation of the sun-exposed skin of the face and neck. The cause is unknown, but it is classically associated with pregnancy. Exposure to exogenous estrogen and progesterone in the form of either oral contraceptives or hormone replacement therapy also may cause melasma. Dark-complexioned persons—particularly Asian and Hispanic women—are more likely to develop this condition.
CLINICAL FEATURES: Melasma appears in adult women as bilateral light- to dark-brown cutaneous macules that vary in size from a few millimeters to more than 2 cm in diameter (Fig. 10-37). Lesions develop slowly with sun exposure and occur primarily on the midface, forehead, upper lip, chin, and (rarely) the arms. It is not unusual for the entire face to be involved. The pigmentation may remain faint or darken over time. Rarely, melasma is seen in men.
HISTOPATHOLOGIC FEATURES: Melasma is characterized by increased melanin deposition within an otherwise unremarkable epidermis. Pigment also may be seen within numerous melanophages in the dermis.
TREATMENT AND PROGNOSIS: Melasma is difficult to treat. First-line therapy typically consists of triple-combination topical therapy, such as Tri-Luma cream (a combination of 4% hydroquinone, 0.05% tretinoin, and 0.01% fluocinolone acetonide). Dual-ingredient topical agents (e.g., hydroquinone combined with glycolic acid or kojic acid) or single topical agents (e.g., 4% hydroquinone, 0.1% retinoic acid, or 20% azelaic acid) are alternatives for patients who are sensitive to triple-combination therapy. Options for second-line therapy include glycolic acid chemical peel, laser therapy, and dermabrasion, although variable results have been reported with these alternative therapies. Because sun exposure is an important etiologic factor, sun avoidance and the use of sunscreens containing zinc oxide or titanium dio-xide are crucial for effective clinical management. The lesions may resolve after parturition or after discontinuing oral contraceptives. There is no potential for malignant transformation.
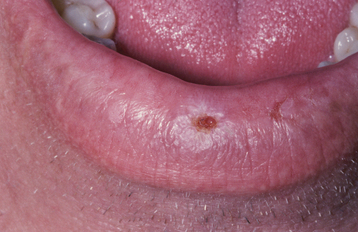
ORAL MELANOTIC MACULE (FOCAL MELANOSIS)
The oral melanotic macule is a flat, brown, mucosal discoloration produced by a focal increase in melanin deposition and possibly a concomitant increase in the number of melanocytes. The cause remains unclear. Unlike the cutaneous ephelis (freckle), the melanotic macule is not dependent on sun exposure. Some authorities have questioned the purported lack of an association with actinic irradiation for the melanotic macule located on the vermilion border and prefer to consider it a distinct entity (labial melanotic macule). In one recent study of more than 773 solitary oral melanocytic lesions submitted to an oral pathology laboratory for histopathologic examination, oral and labial melanotic macules were the most common and comprised 86% of cases; oral and labial melanotic macules were encountered much more frequently than oral melanocytic nevi, melanoacanthomas, and melanomas.
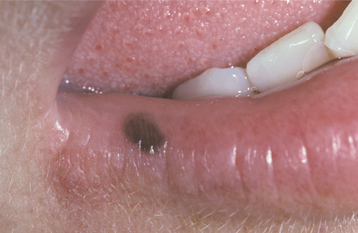
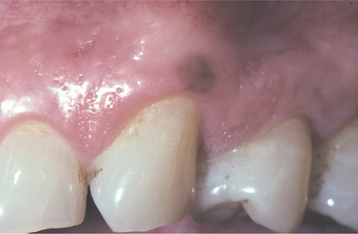
CLINICAL FEATURES: The oral melanotic macule occurs at any age in both men and women; however, biopsy samples demonstrate a 2:1 female predilection. The average age of patients is 43 years at the time of diagnosis. The vermilion zone of the lower lip is the most common site of occurrence (33%), followed by the buccal mucosa, gingiva, and palate. Rare examples have been reported on the tongue in newborns.
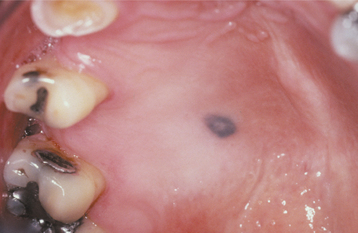
The typical lesion appears as a solitary (17% are multiple), well-demarcated, uniformly tan to dark-brown, asymptomatic, round or oval macule with a diameter of 7 mm or smaller (Figs. 10-38 and 10-39). Occasional lesions may be blue or black. Lesions are not reported to enlarge after diagnosis, which suggests that the maximum dimension is achieved rather rapidly and remains constant thereafter.
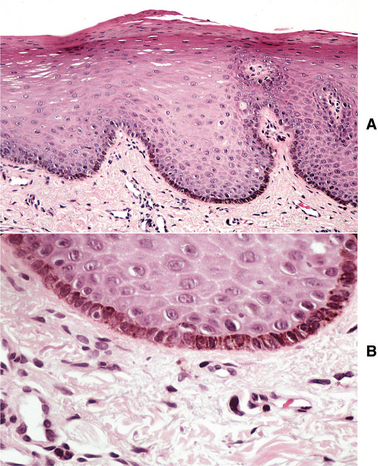
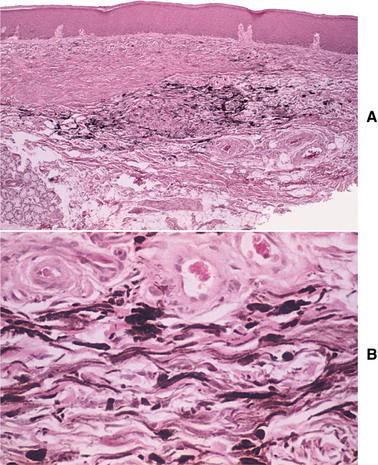
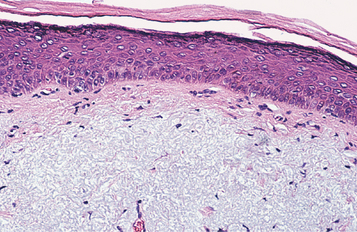
HISTOPATHOLOGIC FEATURES: The oral melanotic macule is characterized by an increase in melanin (and perhaps melanocytes) in the basal and parabasal layers of an otherwise normal stratified squamous epithelium (Fig. 10-40). Melanin also may be seen free or within melanophages in the subepithelial connective tissue (melanin incontinence). The lesion typically does not show elongated rete ridges like actinic lentigo (see page 377).
TREATMENT AND PROGNOSIS: Treatment is usually not required for the melanotic macule, except for aesthetic considerations. When necessary, excisional biopsy is the preferred treatment. Electrocautery, laser ablation, or cryosurgery is effective, but no tissue remains for histopathologic examination after these procedures. The intraoral melanotic macule has no malignant transformation potential, but an early melanoma can have a similar clinical appearance. For this reason, all oral pigmented macules of recent onset, large size, irregular pigmentation, unknown duration, or recent enlargement should be submitted for microscopic examination.
On occasion, flat pigmented lesions are encountered that are clinically and microscopically similar to the melanotic macule; however, these lesions represent a sign of systemic or genetic disease or may be a consequence of the use of certain medications. A list of these conditions is shown in Box 10-1.
ORAL MELANOACANTHOMA (MELANOACANTHOSIS)
Oral melanoacanthoma is a benign, relatively un-common acquired pigmentation of the oral mucosa characterized by dendritic melanocytes dispersed throughout the epithelium. The lesion appears to be a reactive process; in some cases an association with trauma has been reported. Oral melanoacanthoma appears to be unrelated to the melanoacanthoma of skin, which most authorities believe represents a variant of seborrheic keratosis.
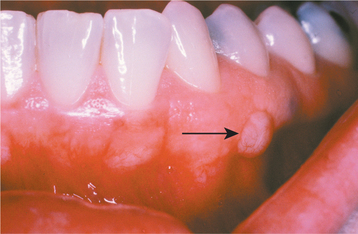
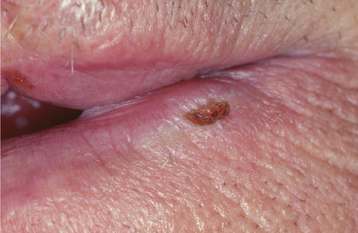
CLINICAL FEATURES: Oral melanoacanthoma is seen almost exclusively in blacks, shows a female predilection, and is most common during the third and fourth decades of life. The buccal mucosa is the most common site of occurrence. The lips, palate, gingiva, and alveolar mucosa also may be involved. Most patients exhibit solitary lesions, although bilateral or multifocal involvement is possible as well. Oral melanoacanthomas typically are asymptomatic; however, pain, burning, and pruritus have been reported in a few unusual cases. The lesion is smooth, flat or slightly raised, and dark-brown to black in color (Fig. 10-41). Lesions often demonstrate a rapid increase in size, and they occasionally reach a diameter of several centimeters within a period of a few weeks.

Fig. 10-41 Oral melanoacanthoma. A, Smooth, darkly pigmented macule of the buccal mucosa in a young adult. B, Appearance of the lesion 2 months later showing dramatic enlargement. C, Resolution of the lesion 3 months after incisional biopsy. Rights were not granted to include this figure in electronic media. Please refer to the printed book. (From Park SK, Neville BW: AAOMP case challenge: rapidly enlarging pigmented lesion of the buccal mucosa, J Contemp Dent Pract 3:69-73, 2002.)
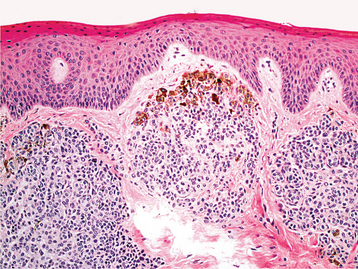
HISTOPATHOLOGIC FEATURES: The oral melanoacanthoma is characterized by numerous benign dendritic melanocytes (cells that are normally confined to the basal cell layer) scattered throughout the lesional epithelium (Figs. 10-42 and 10-43). Basal layer melanocytes are also present in increased numbers. Spongiosis and mild acanthosis are typically noted. In addition, eosinophils and a mild to moderate chronic inflammatory cell infiltrate are usually seen within the underlying connective tissue.
TREATMENT AND PROGNOSIS: Because of the alarming growth rate of oral melanoacanthoma, incisional biopsy is usually indicated to rule out the possibility of melanoma. Once the diagnosis has been established, no further treatment is necessary. In several instances, lesions have undergone spontane-ous resolution after incisional biopsy. Recurrence or development of additional lesions has been reported only rarely. There is no potential for malignant transformation.
ACQUIRED MELANOCYTIC NEVUS (NEVOCELLULAR NEVUS; MOLE)
The generic term nevus refers to malformations of the skin (and mucosa) that are congenital or developmental in nature. Nevi may arise from the surface epithelium or any of a variety of underlying connective tissues. The most commonly recognized nevus is the acquired melanocytic nevus, or common mole—so much so that the simple term nevus is often used synonymously for these pigmented lesions. However, many other developmental nevi also are recognized (Box 10-2).
The acquired melanocytic nevus represents a benign, localized proliferation of cells from the neural crest, often called nevus cells. Although there is little debate as to their neural crest origin and their ability to produce melanin, various authorities are divided on the issue of whether these cells represent melanocytes or are merely “first cousins” of melanocytes. These melanocytic cells migrate to the epidermis during development, and lesions may first appear shortly after birth. The acquired melanocytic nevus is probably the most common of all human “tumors,” and white adults have an average of 10 to 40 cutaneous nevi per person. Intraoral lesions occur but are not common.
CLINICAL FEATURES: Acquired melanocytic nevi begin to develop on the skin during childhood, and most cutaneous lesions are present before 35 years of age. They occur in both men and women, although women usually have a few more than men. Racial differences are seen. Whites have more nevi than Asians or blacks. Most lesions are distributed above the waist, and the head and neck region is a common site of involvement.
Acquired melanocytic nevi evolve through several clinical stages, which tend to correlate with specific histopathologic features. The earliest presentation (known microscopically as a junctional nevus) is that of a sharply demarcated, brown or black macule, typically less than 6 mm in diameter. Although this lesional appearance may persist into adulthood, more often the nevus cells proliferate over a period of years to produce a slightly elevated, soft papule with a relatively smooth surface (compound nevus). The degree of pigmentation becomes less; most lesions appear brown or tan.
As time passes, the nevus gradually loses its pigmentation, the surface may become somewhat papillomatous, and hairs may be seen growing from the center (intradermal nevus) (Figs. 10-44 and 10-45). However, the nevus usually remains less than 6 mm in diameter. Ulceration is not a feature unless, for example, the nevus is situated in an area where a belt or bra strap traumatizes it easily. Throughout the adult years, many acquired melanocytic nevi will involute and disappear; therefore, fewer of these lesions can be detected in older persons.
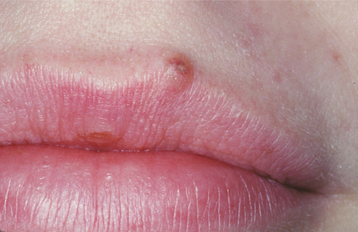
Fig. 10-44 Melanocytic (intradermal) nevus. A well-demarcated, dome-shaped papule is seen at the edge of the vermilion border of the upper lip.
Intraoral melanocytic nevi are distinctly uncommon. Most arise on the palate, mucobuccal fold, or gingiva, although any oral mucosal site may be affected (Fig. 10-46). Intraoral melanocytic nevi have an evolution and appearance similar to skin nevi, although mature lesions typically do not demonstrate a papillary surface change. More than one in five intraoral nevi lack clinical pigmentation (Fig. 10-47). Approximately two thirds of intraoral examples are found in females; the average age at diagnosis is 35 years.
HISTOPATHOLOGIC FEATURES: The acquired melanocytic nevus is characterized by a benign, unencapsulated proliferation of small, ovoid cells (nevus cells). The lesional cells have small, uniform nuclei and a moderate amount of eosinophilic cytoplasm, with indistinct cell boundaries. These cells demonstrate a variable capacity to produce melanin, with the pigment primarily evident in the superficial aspects of the lesion. Nevus cells typically lack the dendritic processes that melanocytes possess. A characteristic microscopic feature is that the superficial nevus cells tend to be organized into small, round aggregates (thèques).
Melanocytic nevi are classified histopathologically according to their stage of development, which is reflected by the relationship of the nevus cells to the surface epithelium and underlying connective tissue. In the early stages, thèques of nevus cells are found only along the basal cell layer of the epithelium, especially at the tips of the rete ridges. Because the lesional cells are found at the junction between the epithelium and the connective tissue, this stage is known as a junctional nevus (Fig. 10-48). As the nevus cells proliferate, groups of cells begin to drop off into the underlying dermis or lamina propria. Because cells are now present along the junctional area and within the underlying connective tissue, the lesion then is called a compound nevus (Fig. 10-49).
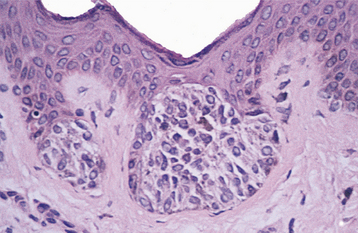
Fig. 10-48 Junctional nevus. Nests of melanocytic nevus cells along the basal layer of the epithelium.
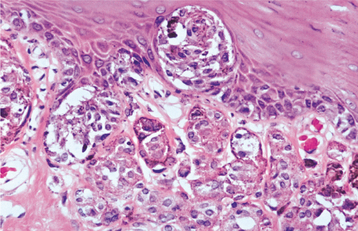
Fig. 10-49 Compound nevus. High-power view showing nests of pigmented nevus cells within the epithelium and the superficial lamina propria.
In the later stages, nests of nevus cells are no longer found within the epithelium but are found only within the underlying connective tissue. Because of the connective tissue location of the lesional cells, on the skin this stage is called an intradermal nevus. The intraoral counterpart is called an intramucosal nevus (Fig. 10-50). Zones of differentiation often are seen throughout the lesion. The superficial cells typically appear larger and epithelioid, with abundant cytoplasm, frequent intracellular melanin, and a tendency to cluster into thèques. Nevus cells of the middle portion of the lesion have less cytoplasm, are seldom pigmented, and appear much like lymphocytes. Deeper nevus cells appear elongated and spindle shaped, much like Schwann cells or fibroblasts. Some authorities classify these variations as type A (epithelioid), type B (lymphocyte-like), and type C (spindle shaped) nevus cells.
Most intraoral melanocytic nevi are classified microscopically as intramucosal nevi. However, this probably reflects the age (average, 35 years) at which most oral nevi undergo biopsy and diagnosis, because these lesions would have earlier evolved through junctional and compound stages.
TREATMENT AND PROGNOSIS: No treatment is indicated for a cutaneous melanocytic nevus unless it is cosmetically unacceptable, is chronically irritated by clothing, or shows clinical evidence of a change in size or color. By midlife, cutaneous melanocytic nevi tend to regress; by age 90, very few remain. If removal is elected, then conservative surgical excision is the treatment of choice; recurrence is unlikely.
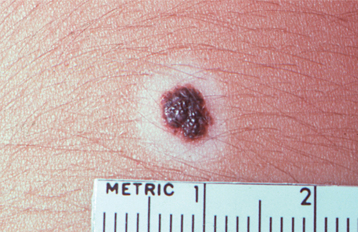
At least some skin melanomas arise from longstanding or irritated nevi of the skin. Overall, the risk of transformation of a particular acquired melanocytic nevus to melanoma is approximately 1 in 1 million. However, because oral melanocytic nevi clinically can mimic an early melanoma, it is generally advised that biopsy be performed for all unexplained pigmented oral lesions, especially because of the extremely poor prognosis for oral melanoma discovered in its later stages.
VARIANTS OF MELANOCYTIC NEVUS
Congenital melanocytic nevus affects approximately 1% of newborns in the United States. This entity is usually divided into two types: (1) small (<20 cm in diameter) and (2) large (>20 cm in diameter). Approximately 15% of congenital nevi are found in the head and neck area, although intraoral involvement is quite rare.
CLINICAL FEATURES: The small congenital melanocytic nevus may be similar in appearance to an acquired melanocytic nevus, but it is frequently larger in diameter (Figs. 10-51 and 10-52). The large congenital lesion classically appears as a brown to black plaque, usually with a rough surface or multiple nodular areas. However, the clinical appearance often changes with time. Early lesions are flat and light tan, becoming elevated, rougher, and darker with age. A common feature is the presence of hypertrichosis (excess hair) within the lesion, which may become more prominent with age (giant hairy nevus). A very large congenital nevus sometimes may be referred to as bathing trunk nevus or garment nevus, because it gives the appearance of the patient wearing an article of clothing.
HISTOPATHOLOGIC FEATURES: The histopathologic appearance of the congenital melanocytic nevus is similar to that of the acquired melanocytic nevus, and some small congenital nevi cannot be distinguished microscopically from the acquired nevus. Both congenital and acquired types are composed of nevus cells, which may have a junctional, compound, or intradermal pattern. The congenital nevus is usually of the compound or intradermal type. In contrast to the acquired melanocytic nevus, the congenital nevus often shows extension of nevus cells into the deeper levels of the dermis, with “infiltration” of cells between collagen bundles. In addition, congenital nevus cells often are seen intermingled with neurovascular bundles in the reticular dermis and surrounding normal adnexal skin structures (e.g., hair follicles, sebaceous glands). Large congenital melanocytic nevi may show extension of nevus cells into the subcutaneous fat.
TREATMENT AND PROGNOSIS: Many congenital melanocytic nevi are excised for aesthetic purposes. In addition, 3% to 15% of large congenital nevi may undergo malignant transformation into melanoma. Therefore, whenever feasible, these lesions should be removed completely by conservative surgical excision. Close follow-up is required for lesions not removed. Patients with multiple large congenital nevi also are at risk for developing neurocutaneous melanosis, a rare congenital syndrome in which patients may develop melanotic neoplasms of the central nervous system (CNS), including meningeal melanosis or melanoma. Unfortunately, no effective therapy is currently available for patients with symptomatic neurocutaneous melanosis.
HALO NEVUS
Halo nevus is a melanocytic nevus with a pale hypopigmented border or “halo” of the surrounding epithelium, apparently as a result of nevus cell destruction by the immune system. The halo develops because the immune cells also attack the melanocytes adjacent to the nevus. The cause of the immune attack is unknown, but regression of the nevus usually results. Interestingly, the development of multiple halo nevi has been seen in patients who have had a recent excision of a melanoma.
CLINICAL FEATURES: The halo nevus is typically an isolated phenomenon associated with a preexisting acquired melanocytic nevus. It is most common on the skin of the trunk during the second decade of life. The lesion typically appears as a central pigmented papule or macule, surrounded by a uniform, 2- to 3-mm zone of hypopigmentation (Fig. 10-53). Sometimes this peripheral zone is much wider.
SPITZ NEVUS (BENIGN JUVENILE MELANOMA; SPINDLE AND EPITHELIOID CELL NEVUS)
Spitz nevus is an uncommon type of melanocytic nevus that shares many histopathologic features with melanoma. It was, in fact, first described as a juvenile melanoma. The distinctly benign biologic behavior of the lesion was first emphasized by Spitz in 1948. The first oral example was not reported until 1990.
CLINICAL FEATURES: The Spitz nevus typically develops on the skin of the extremities or the face during childhood. It appears as a solitary, dome-shaped, pink to reddish-brown papule, usually smaller than 6 mm in greatest diameter. The young age at presentation and the relatively small size of the Spitz nevus are useful features to help distinguish it from melanoma.
HISTOPATHOLOGIC FEATURES: The Spitz nevus has the overall microscopic architecture of a compound nevus, showing a zonal differentiation from the superficial to deep aspects of the lesion and good symmetry. Lesional cells are either spindle shaped or plump (epithelioid), and the two types often are intermixed. The epithelioid cells may be multinucleated and appear somewhat bizarre, often lacking cell cohesiveness. Mitotic figures, all normal in appearance, may be seen in the superficial aspects of the lesion. Ectatic superficial blood vessels, which probably impart much of the reddish color of some lesions, are seen frequently. The nevocellular nature of the lesional cells is demonstrated by immunohistochemical reactivity for S-100 protein and neuron-specific enolase.
BLUE NEVUS (DERMAL MELANOCYTOMA; JADASSOHN-TIèCHE NEVUS)
Blue nevus is an uncommon, benign proliferation of dermal melanocytes, usually deep within subepithelial connective tissue. Two major types of blue nevus are recognized: (1) the common blue nevus and (2) the cellular blue nevus. The common blue nevus is the second most frequent melanocytic nevus encountered in the mouth. The blue color of this melanin-producing lesion can be explained by the Tyndall effect, which relates to the interaction of light with particles in a colloidal suspension. In the case of a blue nevus, the melanin particles are deep to the surface, so that the light reflected back has to pass through the overlying tissue. Colors with long wavelengths (reds and yellows) tend to be more readily absorbed by the tissues; the shorter-wavelength blue light is more likely to be reflected back to the observer’s eyes.
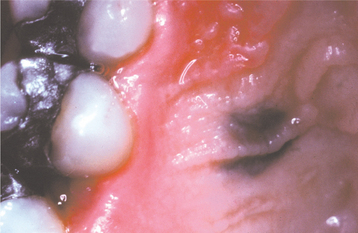
CLINICAL FEATURES: The common blue nevus may affect any cutaneous or mucosal site, but it has a predilection for the dorsa of the hands and feet, the scalp, and the face. Mucosal lesions may involve the oral mucosa, conjunctiva, and, rarely, sinonasal mucosa. Oral lesions are found almost always on the palate. The lesion usually occurs in children and young adults, and a female predilection is seen. It appears as a macular or dome-shaped, blue or blue-black lesion smaller than 1 cm in diameter (Fig. 10-54).
The cellular blue nevus is much less common and usually develops during the second to fourth decades of life, but it may be congenital. More than 50% of cellular blue nevi arise in the sacrococcygeal or buttock region, although they may be seen on other cutaneous or mucosal surfaces. Clinically, this nevus appears as a slow-growing, blue-black papule or nodule that sometimes attains a size of 2 cm or more. Occasional lesions remain macular.
HISTOPATHOLOGIC FEATURES: Histopathologically, the common blue nevus consists of a collection of elongated, slender melanocytes with branching dendritic extensions and numerous melanin globules. These cells are located deep within the dermis or lamina propria (Fig. 10-55) and usually align themselves parallel to the surface epithelium. The cellular blue nevus appears as a well-circumscribed, highly cellular aggregation of plump, melanin-producing spindle cells within the dermis or submucosa. More typical pigmented dendritic spindle cells are seen at the periphery of the lesional tissue. Occasionally, a blue nevus is found in conjunction with an overlying melanocytic nevus, in which case the term combined nevus is used.
TREATMENT AND PROGNOSIS: If clinically indicated, conservative surgical excision is the treatment of choice for the blue nevus of the skin. Recurrence is minimal with this treatment. Malignant transformation to melanoma is rare but has been reported. However, because an oral blue nevus clinically can mimic an early melanoma, it is usually advisable to perform a biopsy of intraoral pigmented lesions, especially because of the extremely poor prognosis for oral melanoma (see page 433).
LEUKOPLAKIA (LEUKOKERATOSIS; ERYTHROLEUKOPLAKIA)
Oral leukoplakia (leuko = white; plakia = patch) is defined by the World Health Organization (WHO) as “a white patch or plaque that cannot be characterized clinically or pathologically as any other disease.” The term is strictly a clinical one and does not imply a specific histopathologic tissue alteration.
The definition of leukoplakia is unusual in that it makes the diagnosis dependent not so much on definable appearances as on the exclusion of other entities that appear as oral white plaques. Such lesions as lichen planus, morsicatio (chronic cheek nibbling), frictional keratosis, tobacco pouch keratosis, nicotine stomatitis, leukoedema, and white sponge nevus must be ruled out before a clinical diagnosis of leukoplakia can be made. As with most oral white lesions, the clinical color results from a thickened surface keratin layer, which appears white when wet, or a thickened spinous layer, which masks the normal vascularity (redness) of the underlying connective tissue.
Although leukoplakia is not associated with a specific histopathologic diagnosis, it is typically considered to be a precancerous or premalignant lesion. When the outcome of a large number of leukoplakic lesions is reviewed, the frequency of transformation into malignancy is greater than the risk associated with normal or unaltered mucosa. Because there is considerable misunderstanding of this concept, Box 10-3 provides definitions that are used throughout the chapter.
INCIDENCE AND PREVALENCE: Although leukoplakia is considered a premalignant lesion, the use of the clinical term in no way suggests that histopathologic features of epithelial dysplasia are present in all lesions. Dysplastic epithelium or frankly invasive carcinoma is, in fact, found in only 5% to 25% of biopsy samples of leukoplakia. The precancerous nature of leukoplakia has been established, not so much on the basis of this association or on the fact that more than one third of oral carcinomas have leukoplakia in close proximity, as on the results derived from clinical investigations that monitored numerous leukoplakic lesions for long periods. The latter studies suggest a malignant transformation potential of 4% (estimated lifetime risk). Specific clinical subtypes or phases, mentioned later, are associated with potential rates as high as 47%. These figures may be artificially low because many lesions are surgically removed at the beginning of follow-up.
Leukoplakia is by far the most common oral precancer, representing 85% of such lesions. Based on pooled, weighted data from previously reported studies, the worldwide prevalence of leukoplakia has been estimated to fall within a range of 1.5% to 4.3%. There is a strong male predilection (70%), except in regional populations in which women use tobacco products more than men. A slight decrease in the proportion of affected males, however, has been noted over the past half century. The disease is diagnosed more frequently now than in the past, probably because of an enhanced awareness on the part of health professionals (rather than because of a real increase in frequency).
CAUSE: The cause of leukoplakia remains unknown, although hypotheses abound.
TOBACCO: The habit of tobacco smoking appears most closely associated with leukoplakia development. More than 80% of patients with leukoplakia are smokers. When large groups of adults are examined, smokers are much more likely to have leukoplakia than nonsmokers. Heavier smokers have greater numbers of lesions and larger lesions than do light smokers, especially after many years of tobacco use. In addition, a large proportion of leukoplakias in persons who stop smoking either disappear or become smaller within the first year of habit cessation.
The smokeless tobacco habit produces a somewhat different result. It often leads to a clinically distinctive white oral plaque called tobacco pouch keratosis (see page 398). This lesion probably is not a true leukoplakia.
ALCOHOL: Alcohol, which seems to have a strong synergistic effect with tobacco relative to oral cancer production, has not been associated with leukoplakia. People who excessively use mouth rinses with an alcohol content greater than 25% may have grayish buccal mucosal plaques, but these are not considered true leukoplakia.
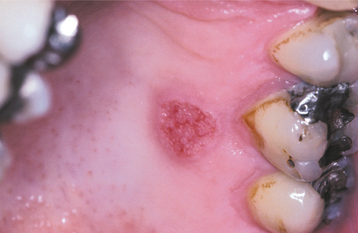
SANGUINARIA: Persons who use toothpaste or mouth rinses containing the herbal extract, sanguinaria, may develop a true leukoplakia. This type of leukoplakia (sanguinaria-associated keratosis) is usually located in the maxillary vestibule or on the alveolar mucosa of the maxilla (Fig. 10-56). More than 80% of individuals with vestibular or maxillary alveolar leukoplakia have a history of using products that contain sanguinaria, compared with 3% of the normal population.
The affected epithelium may demonstrate dysplasia identical to that seen in other leukoplakias, although the potential for the development of cancer is uncertain. The leukoplakic plaque may not disappear even after the patient stops using the product; some lesions have persisted for years afterwards.
ULTRAVIOLET RADIATION: Ultraviolet radiation is accepted as a causative factor for leukoplakia of the lower lip vermilion. This is usually associated with actinic cheilosis (see page 405). Immunocompromised persons, especially transplant patients, are especially prone to the development of leuko-plakia and squamous cell carcinoma of the lower lip vermilion.
MICROORGANISMS: Several microorganisms have been implicated in the cause of leukoplakia. Treponema pallidum, for example, produces glossitis in the late stage of syphilis, with or without the arsenic therapy in popular use before the advent of modern antibiotics. The tongue is stiff and frequently has extensive dorsal leukoplakia.
Tertiary syphilis is rare today, but oral infections by another microorganism, Candida albicans, are not. Candida organisms can colonize the superficial epithelial layers of the oral mucosa, often producing a thick, granular plaque with a mixed white and red coloration (Fig. 10-57). The terms candidal leukoplakia and candidal hyperplasia have been used to describe such a lesion, and biopsy may show dysplastic or hyperplastic histopathologic changes. It is not known whether this yeast produces dysplasia or secondarily infects previously altered epithelium, but some of these lesions disappear or become less extensive, even less severely dysplastic, after antifungal therapy. Tobacco smoking may cause the leukoplakia and also predispose the patient to develop candidiasis.
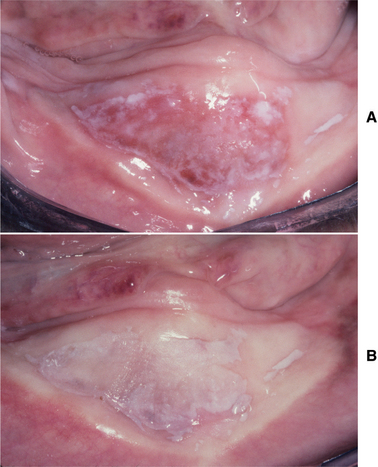
Fig. 10-57 Candidal leukoplakia. A, Well-circumscribed red and white plaque on the anterior floor of mouth, which showed candidal infestation on cytology smears. B, After antifungal therapy, the erythematous component resolved, resulting in a homogeneous white plaque.
Human papillomavirus (HPV), in particular subtypes 16 and 18, has been identified in some oral leukoplakias. These are the same HPV subtypes associated with uterine cervical carcinoma and a subset of oral squamous cell carcinomas. Such viruses, unfortunately, also can be found in normal oral epithelial cells, and so their presence is perhaps no more than coincidental. It may be significant, however, that HPV-16 has been shown to induce dysplasia-like changes in normally differentiating squamous epithelium in an otherwise sterile in vitro environment.
TRAUMA: Several keratotic lesions, which until recently had been viewed as variants of leukoplakia, are now considered not to be precancers. Nicotine stomatitis is a generalized white palatal alteration that seems to be a hyperkeratotic response to the heat generated by tobacco smoking (usually a pipe), rather than a response to the carcinogens within the smoke (see page 403). Its malignant transformation potential is so low as to be about the same as that of normal palatal mucosa.
In addition, chronic mechanical irritation can produce a white lesion with a roughened keratotic surface, termed frictional keratosis. Although the resulting lesion is clinically similar to true leukoplakia, such a lesion is now thought to be no more than a normal hyperplastic response (similar to a callus on the skin). Keratoses of this type are readily reversible after elimination of the trauma, and obviously traumatic lesions such as linea alba (see page 285), morsicatio (see page 286), and toothbrush gingival “abrasion” have not been documented to have transformed into malignancy. In addition, the presence of dentures or broken and missing teeth has not been shown to increase the cancer risk. Alveolar ridge keratoses (Fig. 10-58)—involving the retromolar pad or crest of an edentulous alveolar ridge—represent another form of frictional keratosis caused by masticatory function or denture trauma. Frictional keratosis should be differentiated from the group of oral precancers.
Fig. 10-58 Frictional keratosis. There is a rough, hyperkeratotic change to the posterior mandibular alveolar ridge (“alveolar ridge keratosis”), because this area is now edentulous and becomes traumatized from mastication. Such frictional keratoses should resolve when the source of irritation is eliminated and should not be mistaken for true leukoplakia.
CLINICAL FEATURES: Leukoplakia usually affects persons older than 40 years of age. Prevalence increases rapidly with age, especially for males, and as many as 8% of men older than 70 years of age reportedly are affected (Fig. 10-59). The average age of affected persons (60 years) is similar to the average age for patients with oral cancer; however, in some studies, leukoplakia has been found to occur about 5 years earlier (on average) than oral squamous cell carcinoma.
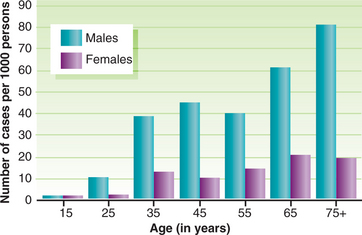
Fig. 10-59 Leukoplakia. Age-specific prevalence (number of new cases per 1000 adults examined at various ages) for oral leukoplakia demonstrates increasing prevalence with increasing age, especially for men.
Approximately 70% of oral leukoplakias are found on the lip vermilion, buccal mucosa, and gingiva. Lesions on the tongue, lip vermilion, and oral floor, however, account for more than 90% of those that show dysplasia or carcinoma. Individual lesions may have a varied clinical appearance and tend to change over time. Early and mild lesions appear as slightly elevated gray or gray-white plaques, which may appear somewhat translucent, fissured, or wrinkled and are typically soft and flat (Fig. 10-60). They usually have sharply demarcated borders but occasionally blend gradually into normal mucosa.
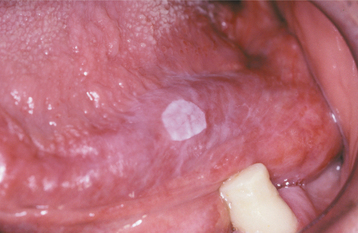
Fig. 10-60 Early or thin leukoplakia. This early lesion of the ventral tongue is smooth, white, and well demarcated from the surrounding normal mucosa.
Mild or thin leukoplakia, which seldom shows dysplasia on biopsy, may disappear or continue unchanged. For tobacco smokers who do not reduce their habit, as many as two thirds of such lesions slowly extend laterally, become thicker, and acquire a distinctly white appearance. The affected mucosa may become leathery to palpation, and fissures may deepen and become more numerous. At this stage or phase, the lesion is often called a homogeneous or thick leukoplakia (Figs. 10-61 and 10-62). Most thick, smooth lesions remain indefinitely at this stage. Some, perhaps as many as one third, regress or disappear; a few become even more severe, develop increased surface irregularities, and are then called granular or nodular leukoplakia (Figs. 10-63 and 10-64). Some lesions demonstrate sharp or blunt projections and have been called verrucous or verruciform leukoplakia (Fig. 10-65).
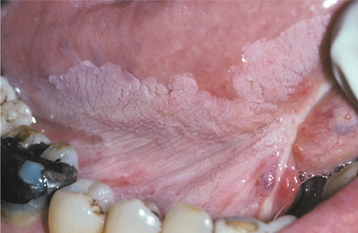
Fig. 10-61 Homogeneous or thick leukoplakia. A diffuse, corrugated white patch on the right ventral surface of the tongue and floor of mouth.
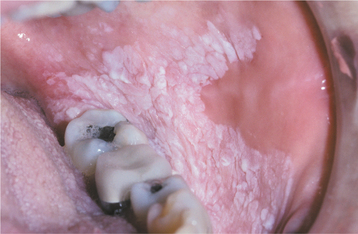
Fig. 10-62 Homogeneous or thick leukoplakia. Extensive buccal mucosa lesion with an uneven whiteness and fissures. Moderate epithelial dysplasia was noted on histopathologic evaluation, and squamous cell carcinoma later developed in this area.
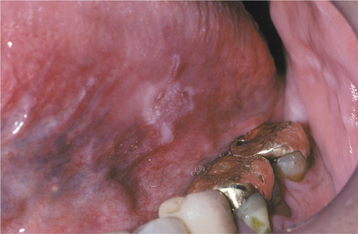
Fig. 10-63 Granular leukoplakia. Focal leukoplakic lesion with a rough, granular surface on the posterior lateral border of the tongue. Biopsy of the lesion revealed an early invasive squamous cell carcinoma.
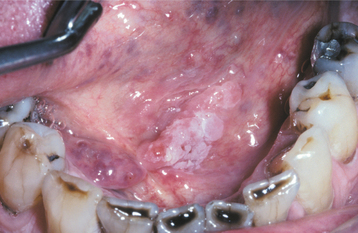
Fig. 10-64 Granular leukoplakia. Irregular white patch in the floor of the mouth of a heavy smoker. Early invasive squamous cell carcinoma was found on biopsy.
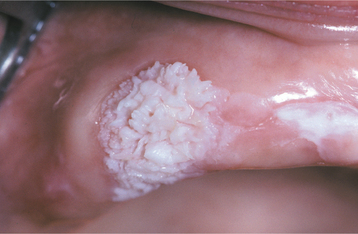
Fig. 10-65 Verruciform leukoplakia. Exophytic papillary lesion of the anterior maxillary alveolar ridge. Biopsy revealed a well-differentiated squamous cell carcinoma.
A special high-risk form of leukoplakia, proliferative verrucous leukoplakia (PVL), is characterized by the development of multiple keratotic plaques with roughened surface projections (Fig. 10-66). The relationship of PVL to cases described as verrucous leukoplakia is uncertain. The multiple PVL plaques tend to slowly spread and involve additional oral mucosal sites. The gingiva frequently is involved, although other sites may be affected as well. Although the lesions typically begin as simple, flat hyperkeratoses that are indistinguishable from ordinary leukoplakic lesions, PVL exhibits persistent growth, eventually becoming exophytic and verrucous in nature. As the lesions progress, they may go through a stage indistinguishable from verrucous carcinoma (see page 422), but they later usually develop dysplastic changes and transform into full-fledged squamous cell carcinoma (usually within 8 years of initial PVL diagnosis). These lesions rarely regress despite therapy. PVL is unusual among the leukoplakia variants in having a strong female predilection (1:4 male-to-female ratio) and minimal association with tobacco use.
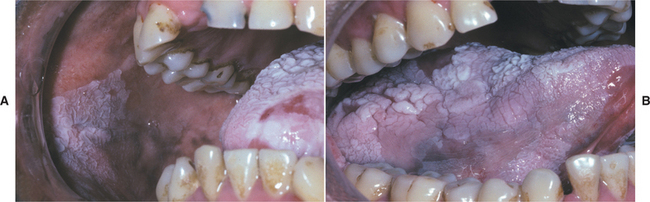
Fig. 10-66 Proliferative verrucous leukoplakia (PVL). A, Large, diffuse, and corrugated white lesions of the buccal mucosa and tongue. B, Same patient showing the extensive thickened and fissured alteration of the tongue.
Leukoplakia may become dysplastic, even invasive, with no change in its clinical appearance. However, some lesions eventually demonstrate scattered patches of redness, called erythroplakia (see page 397). Such areas usually represent sites in which epithelial cells are so immature or atrophic that they can no longer produce keratin. This intermixed red-and-white lesion, called erythroleukoplakia or speckled leukoplakia, represents a pattern of leukoplakia that frequently reveals advanced dysplasia on biopsy (Fig. 10-67).
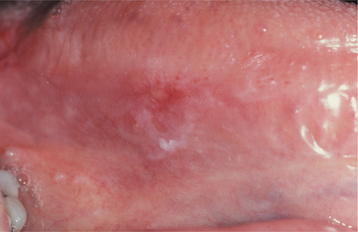
Fig. 10-67 Erythroleukoplakia. Mixed red-and-white lesion of the lateral border of the tongue. Biopsy revealed carcinoma in situ.
Of course, many leukoplakic lesions are a mixture of the previously mentioned phases or subtypes. Because it is important to perform a biopsy of the lesional site with the greatest potential to contain dysplastic cells, Figs. 10-68 and 10-69 provide a clinical and graphic representation of such a lesion. Biopsy sites should be taken from areas with clinical lesional appearances that are most similar to those toward the right in Fig. 10-69.
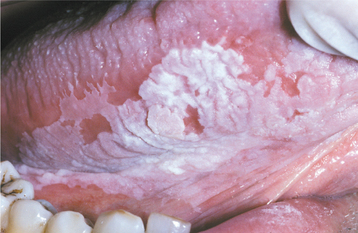
Fig. 10-68 Leukoplakia. Extensive ventral and lateral tongue lesion containing multiple areas representing various possible phases or clinical appearances (compare with Fig. 10-69).

Fig. 10-69 Leukoplakia. Composite representation of the various phases or clinical appearances of oral leukoplakia, with anticipated underlying histopathologic changes. Lesions have increasing malignant transformation potentials as their appearances approach those toward the right. Rights were not granted to include this figure in electronic media. Please refer to the printed book. (From Bouquot JE, Gnepp DR: Laryngeal precancer—a review of the literature, commentary and comparison with oral leukoplakia, Head Neck 13:488-497, 1991.)
In recent years, attempts have been made to develop new techniques to aid in the identification and diagnosis of premalignant and malignant oral lesions. However, at the present time, careful clinical evaluation with directed conventional biopsy remains the best and most accurate means of assessing oral leukoplakic lesions (see Fig. 10-101). In their excellent article, Alexander, Wright, and Thiebaud support this approach when they state, “Noninvasive screening techniques such as cytologic testing (including brush biopsy) and lesion staining with supravital dyes have many pitfalls and should not be considered as substitutes for biopsy when there is concern about malignancy.”
HISTOPATHOLOGIC FEATURES: Microscopically, leukoplakia is characterized by a thickened keratin layer of the surface epithelium (hyperkeratosis), with or without a thickened spinous layer (acanthosis). Some leukoplakias demonstrate surface hyperkeratosis but show atrophy or thinning of the underlying epithelium. Frequently, variable numbers of chronic inflammatory cells are noted within the subjacent connective tissue.
The keratin layer may consist of parakeratin (hyperparakeratosis), orthokeratin (hyperorthokeratosis), or a combination of both (Fig. 10-70). With parakeratin, there is no granular cell layer and the epithelial nuclei are retained in the keratin layer. With orthokeratin, the epithelium demonstrates a granular cell layer and the nuclei are lost in the keratin layer.
Verrucous leukoplakia has papillary or pointed surface projections, varying keratin thickness, and broad, blunted rete ridges. It may be difficult to differentiate it from early verrucous carcinoma.
PVL shows a variable microscopic appearance, depending on the stage of the lesions. Early PVL appears as a benign hyperkeratosis that is indistinguishable from other simple leukoplakic lesions. With time, the condition progresses to a papillary, exophytic proliferation that is similar to localized lesions of verrucous leukoplakia (or what is sometimes termed verrucous hyperplasia). In later stages this papillary proliferation exhibits downgrowth of well-differentiated squamous epithelium with broad, blunt rete ridges. This epithelium demonstrates invasion into the underlying lamina propria; at this stage it is indistinguishable from verrucous carcinoma. In the final stages the invading epithelium becomes less differentiated, transforming into a full-fledged squamous cell carcinoma. Because of the variable clinical and histopathologic appearance of PVL, careful correlation of the clinical and microscopic findings is required for diagnosis.
Most leukoplakic lesions demonstrate no dysplasia on biopsy. Evidence of epithelial dysplasia is found in only 5% to 25% of cases if all oral sites are considered. When present, these dysplastic changes typically begin in the basilar and parabasilar portions of the epithelium. The more dysplastic the epithelium becomes, the more the atypical epithelial changes extend to involve the entire thickness of the epithelium. The histopathologic alterations of dysplastic epithelial cells are similar to those of squamous cell carcinoma and may include the following:
• Large and prominent nucleoli
• Increased nuclear-to-cytoplasmic ratio
• Hyperchromatic (excessively dark-staining) nu-clei
• Pleomorphic (abnormally shaped) nuclei and cells
• Dyskeratosis (premature keratinization of individual cells)
• Increased mitotic activity (excessive numbers of mitoses)
• Abnormal mitotic figures (tripolar or star-shaped mitoses or mitotic figures above the basal layer)
In addition, histomorphologic alterations of dysplastic epithelium are evident at low-power magnification, including the following:
• Bulbous or teardrop-shaped rete ridges
• Loss of polarity (lack of progressive maturation toward the surface)
• Keratin or epithelial pearls (focal, round collections of concentrically layered keratinized cells)
When epithelial dysplasia is present, the pathologist provides a descriptive adjective relating to its “severity” or intensity. Mild epithelial dysplasia refers to alterations limited principally to the basal and parabasal layers (Fig. 10-71). Moderate epithelial dysplasia demonstrates involvement from the basal layer to the midportion of the spinous layer (Fig. 10-72). Severe epithelial dysplasia demonstrates alterations from the basal layer to a level above the midpoint of the epithelium (Fig. 10-73). Sometimes dysplasia will be seen to extend down the duct of a minor salivary gland, especially in lesions of the floor of the mouth (Fig. 10-74).
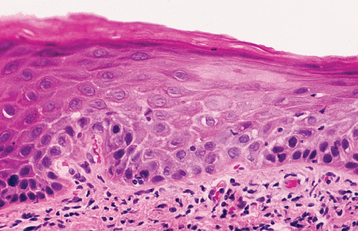
Fig. 10-71 Mild epithelial dysplasia. Hyperchromatic and slightly pleomorphic nuclei are noted in the basal and parabasal cell layers of this stratified squamous epithelium.
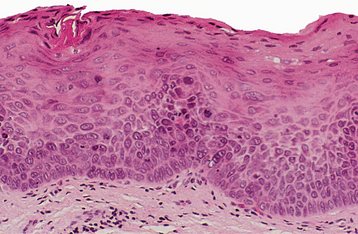
Fig. 10-72 Moderate epithelial dysplasia. Dysplastic changes extend to the midpoint of the epithelium and are characterized by nuclear hyperchromatism, pleomorphism, and cellular crowding.
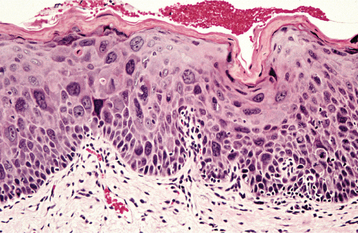
Fig. 10-73 Severe epithelial dysplasia. Epithelium exhibiting marked pleomorphism, hyperchromatism, and scattered mitotic figures. Atypical cells involve most of the epithelial thickness.
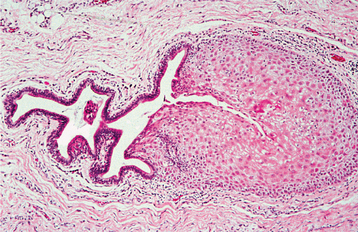
Fig. 10-74 Ductal dysplasia. Salivary gland duct exhibiting squamous metaplasia and dysplasia that originated from an overlying surface epithelial dysplasia.
When the entire thickness of the epithelium is involved, the term carcinoma in situ is used. Carcinoma in situ is defined as dysplastic epithelial cells that extend from the basal layer to the surface of the mucosa (“top-to-bottom” change) (Fig. 10-75). There may or may not be a thin layer of keratin on the surface. The epithelium may be hyperplastic or atrophic. Some authorities consider this entity to be a precancerous lesion; others believe that it represents a genuine malignancy discovered before invasion. Regardless of the concept preferred, the important feature of carcinoma in situ is that no invasion has occurred, despite the fact that the atypical epithelial cells look exactly like those of squamous cell carcinoma (see page 419). Without invasion, the most serious aspect of malignant transformation, metastasis, cannot occur. In this light, it should be mentioned that keratin pearl formation is rare in carcinoma in situ and may indicate the presence of a focus of invasive squamous cell carcinoma in the adjacent tissue.
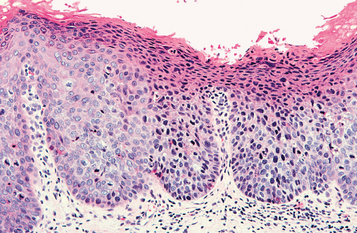
Fig. 10-75 Carcinoma in situ. Dysplastic changes extend throughout the entire thickness of the epithelium.
Sometimes dysplasia will be seen extending down the ducts of the minor salivary glands, especially in lesions in the floor of the mouth. When ductal dysplasia occurs in a precancerous surface dysplasia, the recurrence rate is increased. The depth of ductal dysplasia does not appear to be a significant factor.
TREATMENT AND PROGNOSIS: Because leukoplakia represents a clinical term only, the first step in treatment is to arrive at a definitive histopathologic diagnosis. Therefore, a biopsy is mandatory and will guide the course of treatment. Tissue obtained for biopsy, moreover, should be taken from the clinically most “severe” areas of involvement (with features toward the right side of Fig. 10-69). Multiple biopsies of large or multiple lesions may be required.
Leukoplakia exhibiting moderate epithelial dysplasia or worse warrants complete destruction or removal, if feasible. The management of leukoplakia exhibiting less severe change is guided by the size of the lesion and the response to more conservative measures, such as smoking cessation.
Complete removal can be accomplished with equal effectiveness by surgical excision, electrocautery, cryosurgery, or laser ablation. Long-term follow-up after removal is extremely important because recurrences are frequent and because additional leukoplakias may develop. This is especially true for the verruciform or granular types, 83% of which recur and require additional removal or destruction. Leukoplakia not exhibiting dysplasia often is not excised, but clinical evaluation every 6 months is recommended because of the possibility of progression toward epithelial dysplasia. Additional biopsies are recommended if smoking continues or if the clinical changes increase in severity.
Overall, 4% of oral leukoplakias become squamous cell carcinoma after diagnosis, according to follow-up studies. As previously stated, this figure, and those mentioned later, may be artificially low because so many monitored cases are treated early in an investigation. Not to do so, of course, raises certain ethical questions; hence, more accurate data may never become available. Other confounding features of leukoplakia follow-up investigations include variations in diagnostic definitions and periods of observation. Typically, the latter extend for 5 to 10 years, but several studies have observed patients with lesions for more than 20 years—one study for more than half a century.
With these caveats in mind, follow-up investigations have demonstrated that carcinomatous transformation usually occurs 2 to 4 years after the onset of the white plaque, but it may occur within months or after decades. Transformation does not appear to depend on the age of the affected patient.
Although dysplasia may be present in any leukoplakia, each clinical appearance or phase of leukoplakia has a different malignant transformation potential. Thin leukoplakia seldom becomes malignant without demonstrating a clinical change. Homogeneous, thick leukoplakia undergoes malignant transformation in 1% to 7% of cases. Once the surface becomes granular or verruciform, the malignant transformation potential becomes 4% to 15%. Erythroleukoplakia carries an average transformation potential of 28%, but the rates have varied from 18% to 47% in different investigations.
The increased frequency of transformation of the different phases of leukoplakia is related closely to the degree of dysplasia present. The greater the clinical severity, the greater the chance of significant dysplasia and malignant transformation. Estimates of the malignant potential for histopathologically proven dysplastic lesions are, unfortunately, open to question because so many are excised completely. Thus their true biologic behavior in an unaltered state may not be appreciated fully. With this understanding, however, lesions diagnosed as moderate and severe dysplasia reportedly have malignant transformation potentials of 4% to 11% and 20% to 35%, respectively. Cancers from dysplastic lesions usually develop within 3 years of the dysplasia diagnosis, but they can occur much later. Additionally, one in three dysplasias will recur after complete removal.
In addition to the clinical and histopathologic ap-pearance at diagnosis, several factors may increase the risk for cancer in leukoplakic lesions. These include persistence over several years, occurrence in a female patient, occurrence in a nonsmoker, and occurrence on the oral floor or ventral tongue. Leukoplakia of the latter two locations has shown malignant transformation in 16% to 39% of all cases and 47% of those occurring in females.
There is much interest in the identification of chromosomal, genetic, and molecular alterations that may aid in predicting the risk of malignant transformation for oral leukoplakia. Cytogenetic studies have suggested that loss of heterozygosity (LOH) of chromosome arms 3p and 9p is associated with increased risk of malignant transformation, and additional LOH at 4q, 8p, 11q, 13q, and 17p further increases this risk. Additional alterations such as microsatellite instabil-ity (insertion or deletion of base pairs in repetitive stretches of short DNA sequences), increased telomerase activity (important for cellular longevity), and changes in expression of various molecular markers (e.g., p53 and other markers of apoptosis, p16 and other markers of cell cycle regulation, epidermal growth factor receptor [EGFR], matrix metalloproteinases, vascular endothelial growth factor) have been found to correlate with histopathologic progression in oral premalignant lesions. Despite these interesting observations, many of the previously discussed analy ses require fresh tissue or labor-intensive techniques that are not widely available, which limits their use in routine clinical practice. Thus histopathologic grading of dysplasia remains the standard method for predicting the risk of progression to malignancy.
Some smoking-related leukoplakias with no or minimal dysplasia may disappear or diminish in size within 3 months after the patient stops smoking. Thus habit cessation is recommended. Chemoprevention also may be useful, but it remains primarily experimental. Isotretinoin (13-cis-retinoic acid, a form of vitamin A)—alone or in combination with betacarotene—has been reported to reduce or eliminate some leukoplakic lesions in short-term studies. Toxic reactions to systemic retinoid agents are frequent, however, as is lesion recurrence after the conclusion of therapy. Agents such as bleomycin, lycopene, and cyclooxgenase-2 (COX-2) inhibitors have been investigated as potential chemopreventive agents as well. However, to date there is insufficient evidence from well-designed clinical trials to support the effectiveness of such medical therapies in treating oral dysplasia or preventing the progression of oral dysplasia to squamous cell carcinoma.
ERYTHROPLAKIA (ERYTHROPLASIA; ERYTHROPLASIA OF QUEYRAT)
As with leukoplakia, erythroplakia is defined as a red patch that cannot be clinically or pathologically diagnosed as any other condition. Queyrat originally used the term erythroplasia to describe a precancerous red lesion that develops on the penis. Oral erythroplakia is clinically and histopathologically similar to the genital process. Almost all true erythroplakias demonstrate significant epithelial dysplasia, carcinoma in situ, or invasive squamous cell carcinoma. The causes of erythroplakia are unknown, but they are presumed to be the same as those associated with invasive squamous cell carcinoma of the mouth (see page 409).
The point prevalence rate (number of persons with active lesions at a given point in time) of oral erythroplakia has been estimated as 1 per 2500 adults. The reported prevalence among several large-scale epidemiologic surveys—most of which were conducted in South and Southeast Asia—ranges from 0.02% to 0.83%. The incidence is not known, but the average annual incidence for microscopically proven oral carcinoma in situ, which represents the great majority of erythroplakias, has been estimated to be 1.2 per 100,000 population (2.0 in males and 0.5 in females) in the United States.
Erythroplakia also may occur in conjunction with leukoplakia (see page 388) and has been found concurrently with a large proportion of early invasive oral carcinomas. Although erythroplakia is less common than leukoplakia, it has a much greater potential to be severely dysplastic at the time of biopsy or to develop invasive malignancy at a later time.
CLINICAL FEATURES: Erythroplakia is predominantly a disease of middle-aged to older adults with no significant gender predilection. In the United States, a peak prevalence of 65 to 74 years has been reported. In India, the peak prevalence is in a somewhat younger age range of 45 to 54 years. The floor of mouth, tongue, and soft palate are the most common sites of involvement, and multiple lesions may be present.
The altered mucosa appears as a well-demarcated erythematous macule or plaque with a soft, velvety texture (Figs. 10-76 and 10-77). It is usually asymptomatic and may be associated with an adjacent leukoplakia (erythroleukoplakia) (see Fig. 10-67). Nonspecific mucositis, candidiasis, psoriasis, or vascular lesions may clinically mimic erythroplakia, and biopsy often is required to distinguish between them.
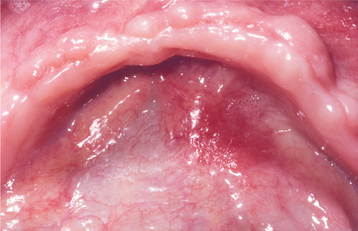
Fig. 10-76 Erythroplakia. An erythematous macular lesion is seen on the right floor of the mouth with no associated leukoplakia. Biopsy showed early invasive squamous cell carcinoma.
Fig. 10-77 Erythroplakia. Well-circumscribed red patch on the posterior lateral hard and soft palate. Rights were not granted to include this figure in electronic media. Please refer to the printed book. (From Neville BW, Chi AC, Jeter M: Diagnostic challenge: a red lesion on the palate, J Am Dent Assoc 137:1537-1538, 2006.)
HISTOPATHOLOGIC FEATURES: According to one large clinicopathologic investigation, 90% of erythroplakic lesions histopathologically represent severe epithelial dysplasia (see page 394), carcinoma in situ (see page 394), or superficially invasive squamous cell carcinoma (see page 419). The epithelium shows a lack of keratin production and often is atrophic, but it may be hyperplastic. This lack of keratinization, especially when combined with epithelial thinness, allows the underlying microvasculature to show through, thereby explaining the red color. The underlying connective tissue often demonstrates chronic inflammation.
TREATMENT AND PROGNOSIS: Red lesions of the oral mucosa, especially those of the oral floor and ventral or lateral tongue, should be viewed with suspicion, and a biopsy should be performed. If a source of irritation can be identified and removed, then biopsy of such a lesion may be delayed for 2 weeks to allow a clinically similar inflammatory lesion time to regress.
As with leukoplakia, the treatment of erythroplakia is guided by the definitive diagnosis obtained by biopsy. Lesions exhibiting moderate dysplasia or worse must be removed completely or destroyed by the methods used for leukoplakia (see page 396). It is best, however, to preserve most of the specimen for microscopic examination because of the possibility that a focal invasive carcinoma might be missed in the initial biopsy material. Recurrence and multifocal oral mucosal involvement are common with erythroplakia; hence, long-term follow-up is suggested for treated patients.
SMOKELESS TOBACCO USE AND SMOKELESS TOBACCO KERATOSIS (SNUFF POUCH; SNUFF DIPPER’S LESION; TOBACCO POUCH KERATOSIS; SPIT TOBACCO KERATOSIS)
The three main types of smokeless tobacco used in the United States include chewing tobacco, moist snuff, and dry snuff. Moist snuff is the most popular, with a 77% increase in sales over the past 15 years, whereas dry snuff and chewing tobacco have been declining in popularity. The increase in popularity of moist snuff may be related in part to the convenience of small, prepackaged pouches that can be used discreetly. Chewing tobacco often is used by men in conjunction with outdoor activities, and dry snuff is used primarily by women in the southern United States. Smokeless tobacco use also has been referred to as spit tobacco use—a term preferred by the U.S. federal government in its attempt to diminish the appeal of the habit.
As part of its Healthy People 2010 objectives, the U.S. Department of Health and Human Services has set a goal to reduce the prevalence of smokeless tobacco use among U.S. adults from 2.3% to 0.4%. At present, the proportion of adult men in the United States who regularly use smokeless tobacco approximates 4.5%. Among male high school students, the proportion is as high as 20% to 27% in some Southeastern and Midwestern states. However, over the past few decades, smokeless tobacco use has declined, particularly among young men 18 to 24 years of age. The habit is started early in life, usually at 8 to 14 years of age, and rarely is initiated after 20 years of age. A national survey detected smokeless tobacco lesions of all types in 1.5% (2.9% in males, 0.1% in females) of U.S. adolescents and teenagers.
In India, smokeless tobacco may be combined in a quid with other products, such as betel leaves, areca nuts, and slaked lime. Oral lesions associated with betel quid use are described separately (see the discussion of oral submucous fibrosis on page 401).
CLINICAL FEATURES: Several health and addiction hazards may be associated with the use of smokeless tobacco because of the ready absorption of nicotine and other molecules through the oral mucosa. A variety of local oral alterations also are found in chronic users. One of the most common local changes is a characteristic painless loss of gingival tissues in the area of tobacco contact (Fig. 10-78). This gingival recession may be accompanied by destruction of the facial surface of the alveolar bone and correlates well with the quantity of daily use and the duration of the smokeless tobacco habit. Although the association between smokeless tobacco and gingival recession is well known, there is some variability across studies regarding the association between smokeless tobacco and periodontal bone loss. Researchers have suggested that this variability may be related to the specific type of smokeless tobacco used or possible confounding by concurrent cigarette smoking.
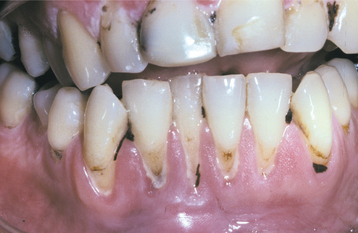
Fig. 10-78 Smokeless tobacco–related gingival recession. Extensive recession of the anterior mandibular facial gingiva.
Dental caries also has been reported to be more prevalent in smokeless tobacco users, perhaps because of the high sugar content of some brands; other reports dispute caries susceptibility. Long-term use may lead to localized or generalized wear of occlusal and incisal surfaces, especially in those using the product in dusty environments. A brown-black extrinsic tobacco stain is typically found on the enamel and cementum surfaces of the teeth adjacent to the tobacco. In addition, halitosis is a frequent finding in chronic users.
A characteristic white plaque, the smokeless tobacco keratosis, also is produced on the mucosa in direct contact with snuff or chewing tobacco. In Western cultures, it affects 15% of chewing tobacco users and 60% of snuff users, if mild examples are included. The development of this lesion is most strongly influenced by habit duration and also by the brand of tobacco used, early onset of smokeless tobacco use, total hours of daily use, amount of tobacco consumed daily, and number of sites routinely used for tobacco placement. Smokeless tobacco keratosis in many Western cultures is usually noted in young adult men and in men older than 65 years of age, because the habit has not been popular among the generation that is now middle-aged. In some populations, the prevalence of smokeless tobacco keratosis (and the smokeless tobacco habit) is most frequent among older women. Individual lesions begin to develop shortly after heavy tobacco use begins, and new lesions seldom arise in persons with a long history of use. The lesion is confined to areas in direct contact with spit tobacco. It is typically a thin, gray or gray-white, almost “translucent,” plaque with a border that blends gradually into the surrounding mucosa (Fig. 10-79). Sometimes mild peripheral erythema is present.
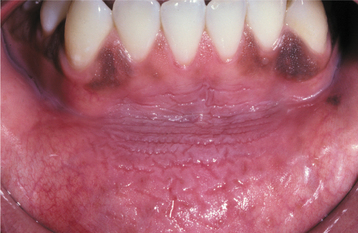
Fig. 10-79 Tobacco pouch keratosis, mild. A soft, fissured, gray-white lesion of the lower labial mucosa located in the area of chronic snuff placement. The gingival melanosis is racial pigmentation and not associated with the keratosis.
The altered mucosa typically has a soft velvety feel to palpation, and stretching of the mucosa often reveals a distinct “pouch” (snuff pouch, tobacco pouch) caused by flaccidity in the chronically stretched tissues in the area of tobacco placement. Because the tobacco is not in the mouth during a clinical examination, the usually stretched mucosa appears fissured or rippled, in a fashion resembling the sand on a beach after an ebbing tide. Similar alterations can occur when other bulky materials are held chronically in the vestibule (e.g., hard candy, sunflower seeds, beef jerky). Induration, ulceration, and pain are not associated with this lesion.
Smokeless tobacco keratosis usually takes 1 to 5 years to develop. Once it occurs, however, the keratosis typically remains unchanged indefinitely unless the daily tobacco contact time is altered. In some cases the white lesion gradually becomes thickened to the point of appearing leathery or nodular (Fig. 10-80).
HISTOPATHOLOGIC FEATURES: The histopathologic appearance of smokeless tobacco keratosis is not specific. The squamous epithelium is hyperkeratinized and acanthotic, with or without intracellular vacuolization or “edema” of glycogen-rich superficial cells. Parakeratin chevrons may be seen as pointed projections above or within superficial epithelial layers (Fig. 10-81). Increased subepithelial vascularity and vessel engorgement often are seen. In some cases an unusual deposition of amorphous eosinophilic material is noted within the subjacent connective tissue and salivary glands (Fig. 10-82). Epithelial dysplasia is uncommon in smokeless tobacco keratosis and, when present, is typically mild. Occasionally, however, significant dysplasia or squamous cell carcinoma may be present.
TREATMENT AND PROGNOSIS: Chronic use of smokeless tobacco in the United States is considered to be carcinogenic, although the risk is less than that associated with cigarette smoking and alcohol abuse. Fortunately, the clinical appearance of smokeless tobacco keratosis is distinct enough and the malignant transformation potential is low enough so that biopsy is needed for only the more severe lesions (i.e., those demonstrating an intense whiteness, a granular or verruciform clinical appearance, ulceration, mass formation, induration, or hemorrhage). Obviously, treatment would then depend on the histopathologic diagnosis. Without microscopic evidence of dysplasia or malignancy, keratoses are not treated. Alternating the tobacco-chewing sites between the left and right sides will eliminate or reduce the keratotic lesion but may result in epithelial alteration or gingival and periodontal difficulties in two sites rather than one.
Squamous cell carcinoma (see page 410) related to smokeless tobacco use typically develops after a long latency period of several decades. In a recent review of case control studies performed in the United States and Western Europe, the reported relative risk of developing oral cancer from chronic smokeless tobacco use ranged from less than 2 to 26, with lower risk associated with chewing tobacco and moist snuff and higher risk associated with dry snuff. Recent studies from Sweden, however, have failed to show an increased risk for users of Swedish moist snuff (also known as snus). Many of the early reports of malignant transformation of snuff-related lesions described lesions among female dry snuff users in the southern United States. Only recently have epidemiologic studies tried to separate the various types of smokeless tobacco with respect to their carcinogenic potential. Squamous cell carcinoma is the most common malignancy resulting from this habit, but an uncommon low-grade oral malignancy, verrucous carcinoma (“snuff dipper’s” cancer), also may be associated with smokeless tobacco use (see page 422).
Significantly, habit cessation leads to a normal mucosal appearance (usually within 2 weeks) in 98% of smokeless tobacco keratosis lesions that are not intensely white (Fig. 10-83). A lesion that remains after 6 weeks without smokeless tobacco contact should be considered to be a true leukoplakia and should be sampled for biopsy and managed accordingly.
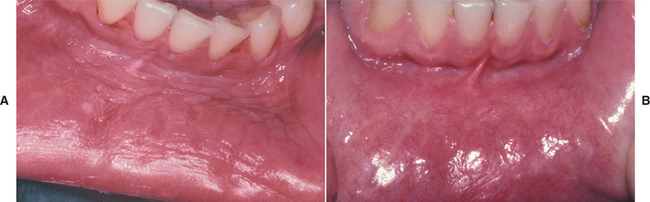
Fig. 10-83 Tobacco pouch keratosis. A, Moderately severe lesion of the lower anterior vestibule and lip in a 15-year-old male subject, which demonstrates a gray-white surface change and fissuring. The patient had been placing snuff in the area for several years. B, Two weeks after cessation of the tobacco habit, the mucosa has returned to an almost normal appearance.
ORAL SUBMUCOUS FIBROSIS
Oral submucous fibrosis is a chronic, progressive, scarring, high-risk precancerous condition of the oral mucosa seen primarily in the Indian subcontinent, Southeast Asia, Taiwan, southern China, and Papua New Guinea. It has been linked to the chronic placement in the mouth of a betel quid or paan and is found in 0.4% of India’s villagers. The quid consists typically of a nut from the areca palm tree and slaked lime, usually with tobacco and sometimes with sweeteners and condiments, wrapped in a betel leaf. The slaked lime acts to release alkaloids (arecoline, arecaidine, guvacine, and guvacoline) from the areca nut, producing a feeling of euphoria and well-being in the user. Villagers habitually chew betel quids from an early age, frequently for 16 to 24 hours daily. Commercial freeze-dried betel quid substitutes (such as pan masala, gutkha, and mawa), conveniently packaged in portable sachets, have become increasingly popular because they have a long shelf life and do not require preparation before use. These products contain a higher concentration of areca nut and appear to cause oral submucous fibrosis more rapidly than conventionally prepared betel quid.
The condition is characterized by a mucosal rigidity of varied intensity caused by a fibroelastic hyperplasia and modification of the superficial connective tissue. The submucosal changes may be a response to the areca nut, whereas the epithelial alterations and carcinogenesis may be the result of tobacco contact. However, several studies suggest that even betel quid without tobacco may be carcinogenic, albeit probably less so than when combined with tobacco. Nutritional deficiency increases the risk and severity of fibrosis, and some persons seem to have a genetic predisposition to it. A few individuals have developed the disease after only a few contacts with areca nut.
The underlying pathogenetic mechanism for oral submucous fibrosis is hypothesized to involve the role of the areca nut in disrupting the homeostatic equilibrium between synthesis and degradation of the extracellular matrix. Cytokines and growth factors produced by activated inflammatory cells may promote fibrosis by inducing proliferation of fibroblasts, upregulating collagen synthesis, and downregulating collagenase production. In addition, considerable amounts of copper have been found in areca nut products; copper may upregulate collagen production by increasing the activity of the enzyme lysyl oxidase involved in collagen synthesis and cross-linking.
CLINICAL FEATURES: Oral submucous fibrosis often is first noted in young adult betel quid users, whose chief complaint is an inability to open the mouth (trismus), often accompanied by mucosal pain while eating spicy foods. An interincisal distance of less than 20 mm is considered severe; in advanced cases, the jaws may actually be inseparable. Females are more susceptible to these changes than males.
Vesicles, petechiae, melanosis, xerostomia, and a generalized oral burning sensation (stomatopyrosis) are usually the first signs and symptoms. The buccal mucosa, retromolar area, and soft palate are the most commonly affected sites. The mucosa in these regions develops a blotchy, marblelike pallor and a progressive stiffness of subepithelial tissues (Fig. 10-84). When the tongue is involved, it becomes rather immobile, is frequently diminished in size, and may be devoid of papillae. Submucosal fibrous bands are palpable on the buccal mucosa, soft palate, and labial mucosa of fully developed cases. Involvement may extend beyond the oral cavity to involve the oropharynx and upper esophagus. Leukoplakia of the surface mucosa often is noted.
Fig. 10-84 Oral submucous fibrosis. Pallor and fibrosis of the soft palate in a betel quid chewer. The uvula has retained its normal color.
Betel quid chewers also may exhibit a brown-red discoloration of the mucosa with an irregular surface that tends to desquamate. This particular change, known as betel chewer’s mucosa, is not believed to be precancerous. In addition, some authors have reported betel quid lichenoid lesions, characterized by white, parallel, wavy striae resembling oral lichen planus (see page 782).
HISTOPATHOLOGIC FEATURES: Oral submucous fibrosis is characterized by the submucosal deposition of dense and hypovascular collagenous connective tissue with variable numbers of chronic inflammatory cells (Fig. 10-85). Epithelial changes include subepithelial vesicles in early lesions and hyperkeratosis with marked epithelial atrophy in older lesions. Epithelial dysplasia is found in 10% to 15% of cases submitted for biopsy, and carcinoma is found in at least 6% of sampled cases.
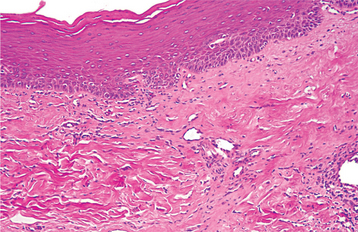
Fig. 10-85 Oral submucous fibrosis. Mucosal biopsy exhibiting hyperparakeratosis, basilar hyperplasia, and fibrosis in the lamina propria.
The lesions of so-called betel chewer’s mucosa are histopathologically similar to morsicatio buccarum (see page 286), except that the ragged keratinaceous surface is covered by encrustations of betel quid ingredients.
TREATMENT AND PROGNOSIS: Unlike tobacco pouch keratosis, oral submucous fibrosis does not regress with habit cessation. Patients with mild cases may be treated with intralesional corticosteroids to reduce the symptoms; surgical splitting or excision of the fibrous bands may improve mouth opening and mobility in the later stages of the disease. One study showed that intralesional injections of inter-feron-g improved maximum mouth opening, reduced mucosal burning, and increased suppleness of the buccal tissues. Additional agents that have been investigated for the treatment of oral submucous fibrosis include lycopene (alone or in combination with intra-lesional steroid injections) and pentoxifylline.
Frequent evaluation for development of oral squamous cell carcinoma is essential because a 17-year malignant transformation rate of 8% has been determined for betel quid users in India. Overall, persons with oral submucous fibrosis are at least 19 times more likely to develop oral cancer than persons without the disease.
NICOTINE STOMATITIS (NICOTINE PALATINUS; SMOKER’S PALATE)
Once a common mucosal change of the hard palate, nicotine stomatitis has become less common as cigar and pipe smoking have lost popularity. Although this lesion is a white keratotic change obviously associated with tobacco smoking, it does not appear to have a premalignant nature, perhaps because it develops in response to heat rather than the chemicals in tobacco smoke. Because pipe smoking generates more heat on the palate than other forms of smoking, nicotine stomatitis has been associated most often with this habit. Similar changes can also be produced by the long-term use of extremely hot beverages.
In some South American and Southeast Asian cultures, hand-rolled cigarettes and cigars are smoked with the lit end held within the mouth. This “reverse smoking” habit produces a pronounced palatal keratosis, or reverse smoker’s palate, which has a significant potential to develop dysplasia or carcinoma.
CLINICAL FEATURES: Nicotine stomatitis most commonly is found in men older than 45 years of age. With long-term exposure to heat, the palatal mucosa becomes diffusely gray or white; numerous slightly elevated papules are noted, usually with punctate red centers (Figs. 10-86 and 10-87). Such papules represent inflamed minor salivary glands and their ductal orifices. The mucosa that covers the papules frequently appears whiter than the surrounding epithelium.
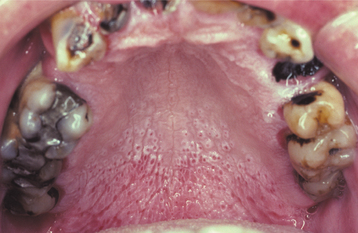
Fig. 10-86 Nicotine stomatitis. This extensive leathery, white change of the hard palate in a pipe smoker is sprinkled throughout with numerous red papules, which represent inflamed salivary duct openings. The gingival mucosa also is keratotic.
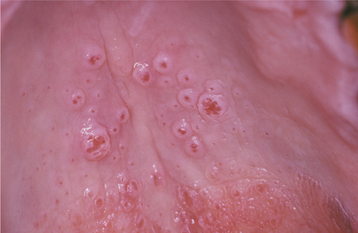
Fig. 10-87 Nicotine stomatitis. Close-up of the inflamed ductal openings of involved salivary glands of the hard palate. Note the white keratotic ring at the lip of many of the inflamed ducts.
The palatal keratin may become so thickened that a fissured or “dried mud” appearance is imparted. The whiteness usually involves marginal gingiva and interdental papillae, and leukoplakia of the buccal mucosa is occasionally seen. A heavy brown or black tobacco stain may be present on the teeth.
HISTOPATHOLOGIC FEATURES: Nicotine stomatitis is characterized by hyperkeratosis and acanthosis of the palatal epithelium and mild, patchy, chronic inflammation of subepithelial connective tissue and mucous glands (Fig. 10-88). Squamous metaplasia of the excretory ducts is usually seen, and an inflammatory exudate may be noted within the duct lumina. In cases with papular elevation, hyperplastic ductal epithelium may be seen near the orifice. The degree of epithelial hyperplasia and hyperkeratosis appears to correlate positively with the duration and the level of heat exposure. Epithelial dysplasia rarely is seen.
TREATMENT AND PROGNOSIS: Nicotine stomatitis is completely reversible, even when it has been present for many decades. The palate returns to normal, usually within 1 to 2 weeks of smoking cessation. Although this is not a precancerous lesion and no treatment is needed, the patient nevertheless should be encouraged to stop smoking (and other high-risk areas should be examined closely). Any white lesion of the palatal mucosa that persists after 1 month of habit cessation should be considered a true leukoplakia and managed accordingly (see page 396).
ACTINIC KERATOSIS (SOLAR KERATOSIS)
Actinic keratosis is a common cutaneous premalignant lesion that is caused by cumulative ultraviolet (UV) radiation to sun-exposed skin, especially in fair-skinned people. A similar phenomenon, actinic cheilosis, is associated with sun damage to the lower lip vermilion (see page 405). UV light exposure can produce mutations in the p53 tumor suppressor gene, an alteration found frequently in this and other precancers and cancers of the head and neck region. Mutations in the telomerase gene represent another early event in lesion development, resulting in delayed apoptosis and immortalization of cells. Although UV light exposure is the major etiologic factor, additional potential risk factors include immunosuppression, arsenic exposure, and certain genetic abnormalities, such as albinism, Rothmund-Thompson syndrome, Cockayne syndrome, xeroderma pigmentosum (see page 747), and Bloom syndrome.
The lesion will develop on the skin of more than 50% of all white adults with significant lifetime sun exposure, and in the U.S. white population the prevalence rate is 15% for older men and 6% for older women. The prevalence increases with advancing age. According to the National Ambulatory Medical Care Survey, more than 47 million physician office visits were conducted in the United States over a 10-year period for the diagnosis of actinic keratosis.
CLINICAL FEATURES: Actinic keratosis seldom is found in persons younger than 40 years of age. The face and neck, the dorsum of the hands, the forearms, and the scalp of bald-headed men are the most common sites of occurrence. Individual lesions are irregular scaly plaques, which vary in color from normal to white, gray, or brown, and may be superimposed on an erythematous background (Fig. 10-89). The keratotic scale peels off with varying degrees of difficulty. Palpation reveals a “sandpaper,” roughened texture, and some lesions can be felt more easily than they can be seen. Typically, a lesion is smaller than 7 mm in diameter but may reach a size of 2 cm, usually with minimal elevation above the surface of the skin. Occasional lesions, however, produce so much keratin that a “horn” may be seen arising from the central area. Other skin lesions, such as verruca vulgaris or seborrheic keratosis, also may produce keratin or cutaneous horns.
HISTOPATHOLOGIC FEATURES: Histopathologically, actinic keratosis is characterized by hyperparakeratosis and acanthosis (Fig. 10-90). Teardrop-shaped rete ridges typically extend down from the epithelium; by definition, some degree of epithelial dysplasia is present. When full-thickness dysplasia is noted, this is termed bowenoid actinic keratosis. Suprabasilar acantholysis may be seen, as may melanosis and a lichenoid inflammatory infiltrate. The dermis exhibits a band of pale basophilic change, which represents sun-damaged collagen and elastic fibers (solar elastosis). In this band of sun-damaged connective tissue, there is a fourfold increase in the amount of elastic fibers, and band thickness is increased with increased exposure to actinic rays. Variable numbers of chronic inflammatory cells are typically present.
TREATMENT AND PROGNOSIS: Because of its precancerous nature, it is usually recommended that actinic keratosis be destroyed by liquid nitrogen cryotherapy, curettage, electrodesiccation, or surgical excision. Alternative treatment methods include topical agents (including 5-fluorouracil, imiquimod, and diclofenac) and photodynamic therapy. Recurrence is rare, but additional lesions frequently arise in adjacent sun-damaged skin. Long-term follow-up, therefore, is recommended.
Although the exact frequency of malignant transformation is unknown, it has been estimated that 10% of actinic keratoses will progress to squamous cell carcinoma, typically over a period of approximately 2 years.
ACTINIC CHEILOSIS (ACTINIC CHEILITIS)
Actinic cheilosis is a common premalignant alteration of the lower lip vermilion that results from long-term or excessive exposure to the ultraviolet component of sunlight. It is a problem confined predominantly to light-complexioned people with a tendency to sunburn easily. Outdoor occupation obviously is associated with this problem, leading to the popular use of terms such as farmer’s lip and sailor’s lip. A person with chronic sunlight exposure and compromised immunity, especially a transplant recipient, has an elevated risk of developing a cancer of the lower lip vermilion.
Actinic cheilosis is similar to actinic keratosis of the skin (see previous topic) in its pathophysiologic and biologic behavior.
CLINICAL FEATURES: Actinic cheilosis seldom occurs in persons younger than 45 years of age. It has a strong male predilection, with a male-to-female ratio as high as 10:1 in some studies.
The lesion develops so slowly that patients often are not aware of a change. The earliest clinical changes include atrophy of the lower lip vermilion border, characterized by a smooth surface and blotchy pale areas. Blurring of the margin between the vermilion zone and the cutaneous portion of the lip is typically seen (Fig. 10-91). As the lesion progresses, rough, scaly areas develop on the drier portions of the vermilion. These areas thicken and may appear as leukoplakic lesions, especially when they extend near the wet line of the lip. The patient may report that the scaly material can be peeled off with some difficulty, only to reform again within a few days.
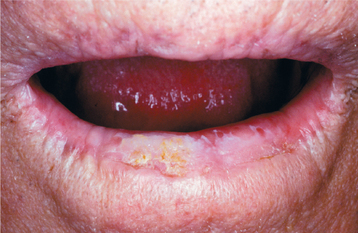
Fig. 10-91 Actinic cheilosis. A blurring of the interface between the vermilion mucosa and the skin of the lip is especially noted in this case.
With further progression, chronic focal ulceration may develop in one or more sites (Fig. 10-92). Such ulcerations may last for months and often suggest progression to early squamous cell carcinoma (Fig. 10-93).
HISTOPATHOLOGIC FEATURES: Actinic cheilosis is usually characterized by an atrophic stratified squamous epithelium, often demonstrating marked keratin production. Varying degrees of epithe lial dysplasia may be encountered. A mild chronic inflammatory cell infiltrate commonly is present subjacent to the dysplastic epithelium. The underlying connective tissue invariably demonstrates a band of amorphous, acellular, basophilic change known as solar (actinic) elastosis, an ultraviolet light–induced alteration of collagen and elastic fibers (Fig. 10-94).
TREATMENT AND PROGNOSIS: Many of the changes associated with actinic cheilosis are probably irreversible, but patients should be encouraged to use lip balms with sunscreens to prevent further damage. Areas of induration, thickening, ulceration, or leukoplakia should be submitted for biopsy to rule out carcinoma. In clinically severe cases without obvious malignant transformation, a lip shave procedure (vermilionectomy) may be performed. The vermilion mucosa is removed, and either a portion of the intraoral labial mucosa is pulled forward or the wound is allowed to heal by secondary intention. The advantage of this technique is that it provides tissue for histopathologic examination should areas of superficially invasive squamous cell carcinoma be present. Alternative treatments include CO2 or erbium:YAG (Er:YAG) laser ablation, electrodesiccation, topical 5-fluorouracil, topical imiquimod, photodynamic therapy, and chemoexfoliation (or “chemical peel”) with trichloroacetic acid. Long-term follow-up is recommended. Of course, if a squamous cell carcinoma is identified, then the involved lip is treated accordingly.
Squamous cell carcinoma, usually well-differentiated, develops over time in 6% to 10% of actinic cheilosis cases reported from medical centers. Such malignant transformation seldom occurs before 60 years of age, with the resulting carcinoma typically enlarging slowly and metastasizing only at a late stage.
KERATOACANTHOMA (“SELF-HEALING” CARCINOMA; PSEUDOCARCINOMA; KERATOCARCINOMA; SQUAMOUS CELL CARCINOMA, KERATOACANTHOMA TYPE)
Keratoacanthoma is a self-limiting, epithelial proliferation with a strong clinical and histopathologic similarity to well-differentiated squamous cell carcinoma. In fact, some authorities consider it to represent an extremely well-differentiated form of squamous cell carcinoma. Cutaneous lesions presumably arise from the infundibulum of hair follicles. Intraoral lesions have been reported, but they are rare; in fact, some authorities do not accept keratoacanthoma as an intraoral disease.
The cause of this lesion is unknown, but sun damage and human papillomavirus (HPV), possibly subtypes 26 or 37, have been proposed. The association with sun damage is suggested by the fact that most solitary lesions are found on sun-exposed skin, predominantly in older adults. Additional potential contributing factors include tar exposure, immunosuppression, and burns or other trauma. Keratoacanthoma-like lesions have been produced in animals by the cutaneous application of carcinogens.
There appears to be a hereditary predisposition for multiple lesions, and the lesions occur with in-creased frequency in patients with Muir-Torre syndrome (sebaceous neoplasms, keratoacanthomas, and gastrointestinal carcinomas).
A number of studies have examined potential genetic alterations or immunohistochemical markers for distinguishing between keratoacanthoma and squamous cell carcinoma. In general, these studies have shown that squamous cell carcinoma tends to have different chromosomal abnormalities and a greater number of genetic aberrations compared with keratoacanthoma, suggesting different pathogenetic mechanisms between these two lesions.
CLINICAL FEATURES: Keratoacanthoma rarely occurs in patients before 45 years of age and shows a male predilection. Almost 95% of solitary lesions are found on sun-exposed skin, and 8% of all cases are found on the outer edge of the vermilion border of the lips, with equal frequency on both the upper and the lower lips.
Keratoacanthoma appears as a firm, nontender, well-demarcated, sessile, dome-shaped nodule with a central plug of keratin (Figs. 10-95 and 10-96), although lesions reported as intraoral keratoacanthoma usually have lacked the central plug. The outer portion of the nodule has a normal texture and color but may be erythematous. The central keratin plug is yellowish, brown, or black and has an irregular, crusted, often verruciform surface.
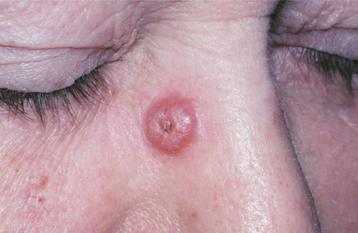
Fig. 10-95 Keratoacanthoma. A nontender, well-demarcated nodule of the skin of the nose in an older woman. The nodule demonstrates a central keratin plug.
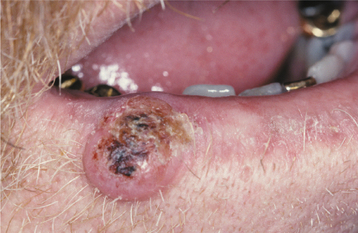
Fig. 10-96 Keratoacanthoma. This lesion, which is located at the outer edge of the vermilion border of the lip, demonstrates a prominent core or plug of keratin.
The evolution of keratoacanthoma can be divided into three phases: (1) growth phase, (2) stationary phase, and (3) involution phase. During the growth phase, rapid enlargement is typical, with the lesion usually attaining a diameter of 1 to 2 cm within 6 weeks. This critical feature helps to distinguish it from the more slowly enlarging squamous cell carcinoma. The lesion stabilizes during the stationary phase, which usually is of a duration similar to that of the growth phase. Most lesions regress spontaneously within 6 to 12 months of onset, frequently leaving a depressed scar in the area (Fig. 10-97). The regression of these lesions is a curious phenomenon, which some investigators have theorized is related to a cytotoxic immune response to the tumor.
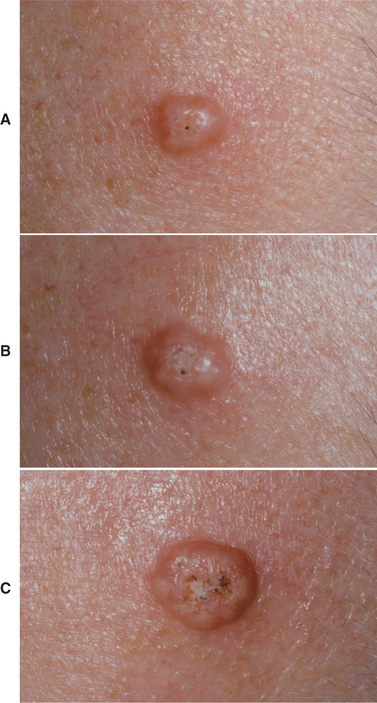
Fig. 10-97 Keratoacanthoma. A, Appearance on initial presentation. Note small, central keratin-filled invagination. B, Same lesion 1 week later showing slight enlargement. C, Same lesion showing further growth 3 weeks after initial presentation. All three photographs were taken at the same magnification. (Courtesy of Dr. John Lovas.)
Occasional patients demonstrate large numbers of keratoacanthomas. One multiple-lesion variant, the Ferguson Smith type, manifests in early life and appears to be hereditary; the lesions are not likely to involute spontaneously. Another variant manifests as hundreds of small papules of the skin and upper digestive tract (eruptive Grzybowski type) and may be associated with internal malignancy.
HISTOPATHOLOGIC FEATURES: Keratoacanthoma of the skin and lip vermilion warrants excisional or large incisional biopsy with inclusion of adjacent, clinically normal epithelium for proper histopathologic interpretation; this is because the overall pattern of the tumor is diagnostically more important than the appearance of individual cells. The cells appear mature, although considerable dyskeratosis (abnormal or premature keratin production) is typically seen in the form of deeply located individually keratinizing lesional cells and keratin pearls similar to those found in well-differentiated squamous cell carcinoma.
The surface epithelium at the lateral edge of the tumor appears normal; at the lip of the central crater, however, a characteristic acute angle (or “buttress”) is formed between the overlying epithelium and the lesion. The crater is filled with keratin, and the epithelium at the base of the crater proliferates downward (Fig. 10-98). This action often elicits a pronounced chronic inflammatory cell response. Downward proliferation does not extend below the level of the sweat glands in skin lesions or into underlying muscle in vermilion lesions. Late-stage lesions show considerably more keratinization of the deeper aspects of the tumor than do early lesions.
TREATMENT AND PROGNOSIS: Despite the propensity of keratoacanthoma to involute of its own accord, surgical excision of large lesions is indicated for optimal aesthetic appearance because significant scarring may otherwise occur. After excision, 4% to 8% of treated patients experience recurrence. Although surgical excision is the preferred treatment, alternative therapies include cryosurgery (reserved for small early lesions), intralesional injection of chemotherapeutic agents (such as 5-fluorouracil, bleomycin, methotrexate, or interferon a-2a), and topical imiquimod. Systemic chemotherapy, often combined with cryotherapy, may be used to treat patients with multiple lesions of the Ferguson Smith or eruptive Grzybowski type.
Aggressive behavior and malignant transformation into carcinoma have been reported in a small proportion of keratoacanthomas—particularly those occurring in the setting of immunosuppression. However, the close histopathologic similarities between this lesion and squamous cell carcinoma sometimes make it difficult to rule out the possibility of misinterpretation of the microscopic section.
SQUAMOUS CELL CARCINOMA
In approximately one of every three Americans now living, a malignancy will develop at some point. It is estimated that in 2007, more than 1,444,000 cancer cases will be diagnosed in the United States, in addition to more than 1 million nonmelanoma skin cancers. Although 66% of affected persons now survive their disease, cancer still causes more than 559,000 deaths each year in the United States and accounts for more than 20% of all deaths. In addition, the current annual death rate from nondermal cancers (186 per 100,000 persons) has increased by 30% since 1930, partially because of a considerable increase in the incidence of lung cancer and partially because people are now less likely to die at an early age of other common disorders, such as cardiovascular disease and infection. From the 1990s through the present, however, this trend has reversed, and the average annual incidence and mortality rates for all cancers combined (excluding nonmelanoma skin cancers) have been declining.
Oral cancer accounts for less than 3% of all cancers in the United States, but it is the eighth most common cancer in males and the fifteenth most common in females. It is the eleventh most common cancer worldwide, with an especially high incidence reported in the Indian subcontinent, Australia, France, Brazil, and southern Africa. Approximately 94% of all oral malignancies are squamous cell carcinoma. In the United States, approximately 22,000 new cases of oral squamous cell carcinoma are diagnosed annually, and slightly more than 5300 individuals die of this disease each year. The average annual incidence and mortality rates, however, vary considerably between different races, genders, and age groups.
As with so many carcinomas, the risk of intraoral cancer increases with increasing age, especially for males. The annual incidence rate (the number of newly diagnosed cases per 100,000 persons each year) for this disease is 5 per 100,000 in the United States, although many texts report an 11 to 15 per 100,000 rate because of the inadvertent inclusion of pharyngeal and vermilion cancers with the intraoral cases. In the United States, white men have a higher risk of intraoral cancer after 65 years of age than does any other group. However, the highest annual incidence rate in middle age is seen in American men of African ancestry (Fig. 10-99). Although the annual incidence rate for intraoral cancer in black males has been decreasing slowly since the middle to late 1980s, marked disparities in survival and mortality rates between black and white males persist. Females, whether white or nonwhite, have a much lower annual incidence rate than males at all age levels. The overall male-to-female ratio is 3:1.
Fig. 10-99 Oral carcinoma. Age-specific incidence rates for intraoral squamous cell carcinoma (number of new cases diagnosed per 100,000 persons each year). Separate rates are provided for white and black men and women in the United States.
Carcinoma of the lip vermilion is somewhat different from intraoral carcinoma. It has a pathophysiology more akin to squamous cell carcinoma of the sun-exposed skin. The average annual incidence rate for white males in the United States is 4 per 100,000, but the rate increases dramatically with age, to almost 30 per 100,000 for men older than 75 years of age. Once the most common oral cancer, the cumulative lifetime risk for developing lip cancer today is only 0.15% for men and 0.07% for women. There was a considerable decrease in the annual incidence rate of this cancer in white males in the United States during the latter half of the twentieth century, because fewer and fewer of them held outdoor occupations. Few women or nonwhite men develop lip carcinoma; there has been little change in the incidence over time for these groups.
Outside the United States, exceptionally wide differences in annual incidence and mortality rates for oral carcinoma are found. These rates vary by as much as twentyfold among different countries. Many of these differences are undoubtedly caused by differing population habits, life expectancies, preventive education, and the quality of medical records in various countries. Despite the difficulties involved in interpreting such data, however, the data have been helpful in identifying potential causative factors.
ETIOLOGY OF ORAL CANCER: The cause of oral squamous cell carcinoma is multifactorial. No single causative agent or factor (carcinogen) has been clearly defined or accepted, but both extrinsic and intrinsic factors may be at work. It is likely that more than a single factor is needed to produce such a malignancy (cocarcinogenesis). Extrinsic factors include such external agents as tobacco smoke, alcohol, syphilis, and (for vermilion cancers only) sunlight. Intrinsic factors include systemic or generalized states, such as general malnutrition or iron-deficiency anemia. Heredity does not appear to play a major causative role in oral carcinoma. Many oral squamous cell carcinomas have been documented to be associated with or preceded by a precancerous lesion, especially leukoplakia (Table 10-1).
Table 10-1
Precancerous Lesions of the Oral, Pharyngeal, and Laryngeal Mucosa (Clinical Terms Only)
| Disease Name | Malignant Transformation Potential |
| Proliferative verrucous leukoplakia (PVL)* |       |
| Nicotine palatinus in reverse smokers† |      |
| Erythroplakia |      |
| Oral submucous fibrosis |      |
| Erythroleukoplakia |     |
| Granular leukoplakia |     |
| Laryngeal keratosis |    |
| Actinic cheilosis |    |
| Smooth, thick leukoplakia |   |
| Smooth, red tongue of Plummer-Vinson syndrome |   |
| Smokeless tobacco keratosis |  |
| Lichen planus (erosive forms)‡ |  ? ? |
| Smooth, thin leukoplakia | +/− |
*PVL: High-risk, high-recurrence form of oral leukoplakia affecting multiple sites.
†Reverse smoking: smoking with the lit end of the cigarette in one’s mouth.
‡Precancer character is controversial.
From Speight PM, Farthing PM, Bouquot JE: The pathology of oral cancer and precancer, Curr Diag Pathol 3:165-176, 1997.
TOBACCO SMOKING: Tobacco smoking reached its greatest popularity in the United States during the 1940s, when at least 65% of white men smoked and other population subgroups were beginning to smoke in large numbers. Today less than 21% of U.S. adults, men and women alike, smoke cigarettes. Unfortunately, the remaining smokers appear to be the heavier users; therefore, the effects on the mouth may be even greater than the typical effects noted in the past.
Much indirect clinical evidence implicates the habit of tobacco smoking in the development of oral squamous cell carcinoma. The proportion of smokers (80%) among patients with oral carcinoma is two to three times greater than the general population. The risk for a second primary carcinoma of the upper aerodigestive tract is two to six times greater for treated patients with oral cancer who continue to smoke than for those who quit after diagnosis.
In addition, case control studies have shown that pipe and cigar smoking carries a greater oral cancer risk than does cigarette smoking, and that the relative risk (smoker’s risk for oral cancer compared with that of a nonsmoker) is dose dependent for cigarette smokers. It is at least five for persons who smoke 40 cigarettes daily, but increases to as much as 17 for persons who smoke 80 or more cigarettes daily. The risk, furthermore, increases the longer the person smokes.
The greatest risk of all probably is found in certain isolated Indian and South American cultures in which the practice of reverse smoking is popular, especially among women. In reverse smoking, the burning end of a handmade cigar or cigarette is held inside the mouth. This habit considerably elevates one’s risk for oral cancer. Where reverse smoking is practiced, as many as 50% of all oral malignancies are found on the hard palate, a site usually spared by this disease.
It should be mentioned that there may be distinct differences between head and neck cancers that develop in smokers compared with those that develop in nonsmokers, although these differences do not appear to affect survival. Tumors in nonsmokers contain a lower frequency of common genetic alterations and have certain clinical differences. For example, affected nonsmokers are more likely to be female, to have oral (especially tongue) rather than pharyngeal or laryngeal disease, to be very young, and to demonstrate mutations of the p53 and other tumor suppressor genes.
SMOKELESS TOBACCO: Smokeless tobacco use in Western cultures may in-crease a chronic user’s risk for oral carcinoma by a factor ranging from less than two to as high as 26. This variation in relative risk reported by different epide-miologic case control studies may be influenced by the type of smokeless tobacco used, with some studies suggesting a lower risk associated with wet snuff and chewing tobacco and a higher risk associated with dry snuff. This apparent increased risk is supported by clinicopathologic investigations that have found an abnormal male-to-female sex ratio for oral carcinoma (>1.0:1.5) in geographic areas where the habit is more popular among women than among men. These geographic areas are typically in the southern United States, where women use dry snuff. In addition, approximately 50% of all oral cancers in smokeless tobacco users occur at the site where the tobacco is habitually placed.
BETEL QUID (PAAN): The betel quid or paan is a compound of natural substances (i.e., areca palm nuts, betel leaf, slaked lime, perhaps tobacco leaf) chewed for their psychostimulating effects. Among betel quid users in Asia, the lifetime risk of developing oral cancer is a remarkable 8%. This habit is also associated with significant development of precancers, such as leukoplakia. More than 600 million persons worldwide chew these quids on a regular basis.
ALCOHOL: Excessive alcohol consumption has been implicated in oral cancer development. It is uncertain whether alcohol alone can initiate carcinogenesis, although it is well established that alcohol in combination with tobacco is a significant risk factor for oral cancer development.
Case control studies have concluded that the risk is dose dependent and time dependent, and the combination of alcohol and tobacco abuse over long periods may increase a person’s risk for oral cancer by a factor of 15 or more (relative risk is 15). In this light, it may be significant that the lowest annual oral cancer incidence rate in the United States is found in Utah, where 75% of the population follows Mormon doctrines that forbid the use of tobacco and alcohol.
Indirect evidence for alcohol’s role in oral cancer production includes the fact that approximately one third of male patients with oral cancer are heavy alcohol users; less than 10% of the general population can be classified as such. Cirrhosis of the liver, likewise, is found in at least 20% of male patients with oral cancer. Nutritional deficiencies associated with heavy alcohol consumption also may contribute to an increased risk of oral cancer development.
PHENOLIC AGENTS: Recent evidence has pointed to an increased oral cancer risk for workers in the wood products industry chronically exposed to certain chemicals, such as phe-noxyacetic acids. Moreover, it has long been known that these workers are at increased risk for nasal and nasopharyngeal carcinoma.
RADIATION: The effects of ultraviolet (UV) radiation on the lips are discussed elsewhere (actinic cheilosis, see page 405), but it is well known that another form of radiation, x-irradiation, decreases immune reactivity and produces abnormalities in chromosomal material. It should not seem surprising, then, that radiotherapy to the head and neck area increases the risk of the later development of a new primary oral malignancy, either a carcinoma or a sarcoma. This effect is dose dependent, but even low-dose radiotherapy for benign entities may increase the local risk to some extent. However, the small amount of radiation from routine diagnostic dental radiographs has not been associated with oral mucosal carcinomas.
IRON DEFICIENCY: Iron deficiency, especially the severe, chronic form known as the Plummer-Vinson or Paterson-Kelly syndrome (see page 828), is associated with an elevated risk for squamous cell carcinoma of the esophagus, oropharynx, and posterior mouth. Malignancies develop at an earlier age than in patients without iron deficiency anemia. People who are deficient in iron tend to have impaired cell-mediated immunity, and iron is essential to the normal functioning of epithelial cells of the upper digestive tract. In deficiency states, these epithelial cells turn over more rapidly and produce an atrophic or immature mucosa. Intertwining fibrous bands of scar tissue also may develop within the esophagus of severely affected patients (esophageal webs). Patients with such esophageal webbing seem to be especially susceptible to malignant transformation.
VITAMIN-A DEFICIENCY: Vitamin-A deficiency produces excessive keratinization of the skin and mucous membranes, and researchers have suggested that the vitamin may play a protective or preventive role in oral precancer and cancer. Some believe that blood levels of retinol and the amount of dietary betacarotene ingested are inversely proportional to the risk of oral squamous cell carcinoma and leukoplakia. Long-term therapy with retinoic acids and betacarotene also has been associated with a regression of at least some leukoplakic lesions and a concomitant reduction in the severity of dysplasia within such lesions.
SYPHILIS: Syphilis (tertiary stage) has long been accepted as having a strong association with the development of dorsal tongue carcinoma. The relative risk ratio approximates four. Conversely, a person with a lingual carcinoma is five times more likely to have a positive serology test for syphilis than someone without such a cancer. The arsenical agents and heavy metals that were used to treat syphilis before the advent of modern antibiotic therapy have carcinogenic properties themselves and may have been responsible for some of the earlier cancer development in this disease. Regardless of the pathophysiologic mechanism at work, however, syphilis-associated oral malignancies are rare today because the infection is typically diagnosed and treated before the onset of the tertiary stage.
CANDIDAL INFECTION: Hyperplastic candidiasis (see page 217) frequently is cited as an oral precancerous condition. Because this lesion appears as a white plaque that cannot be rubbed off, it also has been called candidal leukoplakia. Unfortunately, it is difficult both clinically and histopathologically to distinguish between a true hyperplastic candidiasis and a preexisting leukoplakia with superimposed candidiasis. Experimentally, some strains of Candida albicans have produced hyperkeratotic lesions of the dorsal rat tongue without any other contributing factor. In other studies, certain strains have been shown to produce nitrosamines, chemicals that have been implicated in carcinogenesis. Some candidal strains may have the potential to promote the development of oral cancer; to date, however, the evidence to suggest this role is largely circumstantial.
ONCOGENIC VIRUSES: Oncogenic (tumor producing) viruses may play a major role in a wide variety of cancers, although no virus has definitively been proven to cause oral cancer so far. Viral agents capable of integration into the host’s genetic material may be particularly dangerous and potentially could commandeer the host’s ability to regulate normal growth and proliferation of the infected cell. The oncogenic viruses may immortalize the host cell, thereby facilitating malignant transformation. In the past, retroviruses, adenoviruses, herpes simplex viruses (HSVs), and human papillomaviruses (HPVs) all have been suggested as playing a role in the development of oral carcinoma. It appears, however, that HPV is the only one still implicated, not only in oral cancer but also in carcinoma of the pharyngeal tonsil, larynx, esophagus, uterine cervix, vulva, and penis. HPV subtypes 16, 18, 31, and 33 are the strains most closely associated with dysplasia and squamous cell carcinoma. The underlying mechanisms by which HPV is believed to contribute to oral carcinogenesis primarily involve two virally encoded proteins: (1) E6 (which promotes degradation of the p53 tumor suppressor gene product) and (2) E7 (which promotes degradation of the pRb [retinoblastoma protein] tumor suppressor gene product).
HSV, especially type 2, once was thought to produce a large proportion of cancers of the uterine cervix, and it has been suggested as a causative factor in oral carcinoma. Evidence now suggests that it may be no more than a common companion infection in persons with HPV infections, and that the latter virus plays a much more important carcinogenic role than does HSV. Currently, the evidence gathered to prove a causal relationship between HSV and oral carcinoma is insufficient.
IMMUNOSUPPRESSION: Immunosuppression may play a role in the development of at least some malignancies of the upper aerodigestive tract. Without effective immunologic surveillance and attack, it is thought that newly created malignant cells cannot be recognized and destroyed at an early stage. Persons with AIDS and those who are undergoing immunosuppressive therapy for malignancy or organ transplantation are at increased risk for oral squamous cell carcinoma and other head and neck malignancies, especially when tobacco smoking and alcohol abuse are present.
ONCOGENES AND TUMOR SUPPRESSOR GENES: Oncogenes and tumor suppressor genes are chromosomal components capable of being acted on by a variety of causative agents. Normal genes, or proto-oncogenes, are transformed into activated oncogenes in certain malignancies through the actions of viruses, irradiation, or chemical carcinogens. Once oncogenes are activated, they may stimulate the production of an excessive amount of new genetic material through amplification or overexpression of the involved gene. Oncogenes probably are involved in the initiation and progression of a wide variety of neoplasms, including oral squamous cell carcinoma.
Tumor suppressor genes, on the other hand, allow tumor production indirectly when they become inactivated or mutated. Genetic aberrations commonly identified in oral squamous cell carcinomas include abnormalities of the ras, myc, and EGFR (epidermal growth factor receptor; also known as c-erbB1) oncogenes, and the p53, pRb, p16, and E-cadherin tumor suppressor genes. Most authorities feel that an accumulation of several of these various genetic aberrations is necessary before the affected cell expresses a malignant phenotype.
CLINICAL AND RADIOGRAPHIC FEATURES: Persons with oral squamous cell carcinoma are most often older men who have been aware of an alteration in an oral cancer site for 4 to 8 months before seeking professional help (8 to 24 months among lower socioeconomic groups). There is mini-mal pain during the early growth phase, and this may explain the delay in seeking professional care. If the health care professional does not have a high index of suspicion, then an additional several weeks or months may elapse before a biopsy is performed.
Oral squamous cell carcinoma has a varied clinical presentation, including the following:
• Exophytic (mass forming; fungating, papillary, verruciform)
• Endophytic (invasive, burrowing, ulcerated)
• Leukoplakic (white patch) (Fig. 10-100)

Fig. 10-101 Squamous cell carcinoma. Speckled erythroplakia of the left posterior buccal mucosa. Brush sampling had been reported to be negative for epithelial abnormality, but incisional biopsy revealed invasive squamous cell carcinoma. Rights were not granted to include this figure in electronic media. Please refer to the printed book. (From Chi AC, Ravenel MC: AAOMP case challenge: a “speckled” lesion, J Contemp Dent Pract 6:168-172, 2005.)
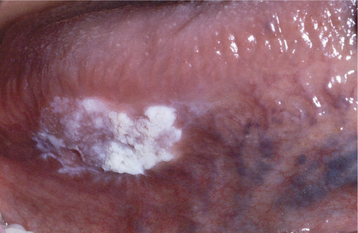
Fig. 10-100 Squamous cell carcinoma. Leukoplakic lesion on the right ventrolateral surface of the tongue.
• Erythroleukoplakic (combined red-and-white patch) (Fig. 10-101)
The leukoplakic and erythroplakic examples are probably early cases that have not yet produced a mass or ulceration, and the clinical features are identical to those described for premalignant leukoplakia and erythroplakia (see pages 388 and page 397). These mucosal surface changes typically are destroyed by the developing exophytic or endophytic carcinoma, but many cases are diagnosed before their complete destruction and show residual precancerous lesions involving adjacent mucosa.
An exophytic lesion typically has a surface that is irregular, fungating, papillary, or verruciform, and its color may vary from normal to red to white, depending on the amount of keratin and vascularity (Figs. 10-102 and 10-103). The surface is often ulcerated, and the tumor feels hard (indurated) on palpation (Fig. 10-104).
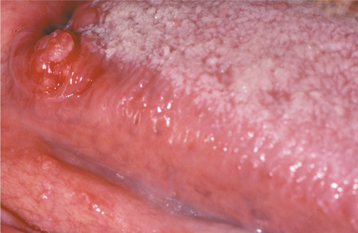
Fig. 10-102 Squamous cell carcinoma. An exophytic lesion of the posterior lateral tongue demonstrates surface nodularity and minimal surface keratin production. It is painless and indurated.
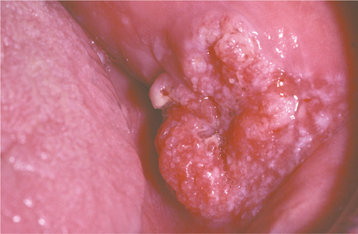
Fig. 10-103 Squamous cell carcinoma. An exophytic buccal lesion shows a roughened and irregular surface with areas of erythema admixed with small areas of white keratosis. Surface ulceration is evident.
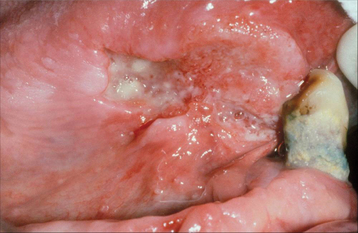
Fig. 10-104 Squamous cell carcinoma. Chronic ulcerated lesion on the right ventral surface of the tongue. The rolled anterior margin felt indurated on palpation.
The endophytic growth pattern has a depressed, irregularly shaped, ulcerated, central area with a surrounding “rolled” border of normal, red or white mucosa (Fig. 10-105). The rolled border results from invasion of the tumor downward and laterally under adjacent epithelium. This appearance is not unique to oral carcinoma because granulomatous lesions, such as deep fungal infections, tuberculosis, tertiary syphi-lis, oral lesions of Wegener’s granulomatosis or Crohn’s disease, and chronic traumatic ulcers, may look similar.
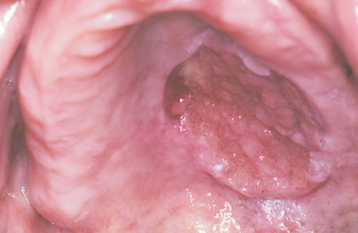
Fig. 10-105 Squamous cell carcinoma. An ulcerated or endophytic lesion of the hard palate demonstrates rolled borders and a necrotic ulcer bed. This cancer was painless, although it had partially destroyed underlying palatal bone.
Destruction of underlying bone, when present, may be painful or completely painless, and it appears on radiographs as a “moth-eaten” radiolucency with ill-defined or ragged margins (an appearance similar to osteomyelitis) (Fig. 10-106). Carcinoma also can extend for many centimeters along a nerve (perineural invasion) without breaking away to form a true metastasis.
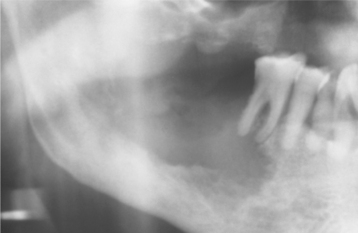
Fig. 10-106 Squamous cell carcinoma. Bone involvement is characterized by an irregular, “moth-eaten” radiolucency with ragged margins—an appearance similar to that of osteomyelitis.
LIP VERMILION CARCINOMA: Carcinoma of the lip vermilion is typically found in light-skinned persons with either long-term exposure to UV radiation from sunlight or a history of acute sun damage (sunburn) early in life. Seventy percent of affected individuals have outdoor occupations. It is usually associated with actinic cheilosis (see page 405) and may arise at the site where the patient holds a cigarette, cigar, or pipe stem. Almost 90% of lesions are located on the lower lip.
The typical vermilion carcinoma is a crusted, oozing, nontender, indurated ulceration that is usually less than 1 cm in greatest diameter when discovered (Figs. 10-107 and 10-108). The tumor is characterized by a slow growth rate, and most patients have been aware of a “problem” in the area for 12 to 16 months before a formal diagnosis is made. Metastasis is a late event; at diagnosis, fewer than 2% of patients have metastatically involved lymph nodes, usually in the submental region. Perineural invasion may result in extension of the tumor into the mandible through the mental foramen. Although this tumor is typically diagnosed and treated at an early stage, patient neglect can result in considerable destruction of normal tissue (Fig. 10-109).
INTRAORAL CARCINOMA: The most common site for intraoral carcinoma is the tongue, usually the posterior lateral and ventral surfaces. The oral floor is affected almost as frequently in men but is involved much less commonly in women. Other sites of involvement (in descending order of frequency) are the soft palate, gingiva, buccal mucosa, labial mucosa, and hard palate.
Carcinoma of the tongue accounts for more than 50% of intraoral cancers in population studies in the United States (Figs. 10-110 and 10-111). Two thirds of lingual carcinomas appear as painless, indurated masses or ulcers of the posterior lateral border; 20% occur on anterior lateral or ventral surfaces, and only 4% occur on the dorsum. The tongue especially is the site of involvement in young patients and, in fact, is the site of the only congenital oral squamous cell carcinoma reported.
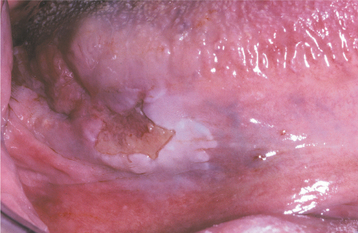
Fig. 10-110 Squamous cell carcinoma. Ulcerated lesion with surrounding leukoplakia on the posterior lateral and ventral tongue.
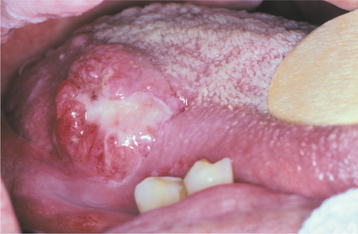
Fig. 10-111 Squamous cell carcinoma. Ulcerated, exophytic mass of the posterior lateral border of the tongue.
Carcinoma of the oral floor represents 35% of all intraoral cancers in epidemiologic surveys and appears to be increasing in frequency among females. It occurs a decade earlier in women than in men but is still usually a disease of older adults. Of all intraoral carcinomas, floor of mouth lesions are the most likely to arise from a preexisting leukoplakia or erythroplakia (Fig. 10-112). It is also the oral cancer site most often associated with the development of a second primary malignancy of another aerodigestive tract location or of a distant organ. The most common site of involvement is the midline near the frenum.
Gingival and alveolar carcinomas are usually painless and most frequently arise from keratinized mucosa in a posterior mandibular site. This tumor has a special propensity to mimic the benign inflammatory and reactive lesions, such as pyogenic granuloma, that are so common to the gingiva. When the cancer develops in an edentulous area, it may give rise to a mass that “wraps around” a denture flange and superficially resembles inflammatory fibrous hyperplasia (epulis fissuratum) (Fig. 10-113). Tumors of the maxillary alveolar ridge may extend onto the hard palate (Fig. 10-114). If the tumor is adjacent to a tooth (Fig. 10-115), then it may mimic periodontal disease or a pyogenic granuloma. Gingival carcinoma often destroys the underlying bone structure, causing tooth mobility. This lesion may not become clinically evident until after tooth extraction, when it proliferates out of the socket to mimic the hyperplastic granulation tissue of epulis granulomatosa. Of all the intraoral carcinomas, this one is least associated with tobacco smoking and has the greatest predilection for females.
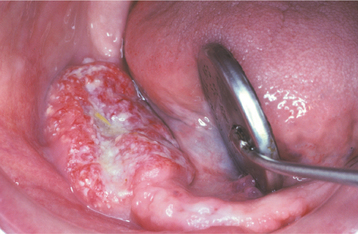
Fig. 10-113 Squamous cell carcinoma. An exophytic lesion with an irregular and pebbled surface has a linear indentation along its facial aspect resulting from pressure from the patient’s lower denture. Underlying alveolar bone was extensively destroyed.
OROPHARYNGEAL CARCINOMA: Carcinoma of the soft palate and oropharyngeal mucosa has the same basic clinical appearance as more anterior carcinomas, except that, in this posterior location, the patient often is unaware of its presence and the diagnosis may be delayed. Tumor size is typically greater than that of more anterior carcinomas, and the proportion of cases with cervical and distant metastasis at diagnosis is higher (Fig. 10-116). Three of every four oropharyngeal carcinomas arise from the tonsillar area or soft palate; most of the others originate on the base of the tongue. The initial symptoms are usually pain or difficulty in swallowing (dysphagia). The pain may be dull or sharp and frequently is referred to the ear.
As a general rule, the more posterior or inferior the oropharyngeal tumor location is, the larger is the lesion and the greater is the chance for lymphatic spread by the time of diagnosis. A soft palate lesion may present as a localized tumor, but 80% of posterior oropharyngeal wall lesions have metastasized or extensively involved surrounding structures by the time of diagnosis.
METASTASIS: The metastatic spread of oral squamous cell carcinoma is largely through the lymphatics to the ipsilateral cervical lymph nodes (Fig. 10-117). A cervical lymph node that contains a metastatic deposit of carcinoma is usually firm to stony hard, nontender, and enlarged (Fig. 10-118). If the malignant cells have perforated the capsule of the node and invaded into surrounding tissues, then the node will feel “fixed,” or not easily movable. Extracapsular spread (extension of metastatic deposits outside of the lymph node capsule) is a microscopic feature associated with poor prognosis, including increased risk of locoregional recurrence, distant metastasis, and lower survival rates.
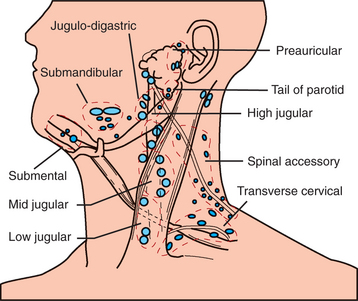
Fig. 10-117 Squamous cell carcinoma, metastatic spread. Diagram demonstrating potential sites for metastatic spread of oral carcinoma to regional lymph nodes.
Fig. 10-118 Squamous cell carcinoma. Metastatic deposits within cervical lymph nodes present as firm, painless enlargements as seen in this patient with metastasis to a superior jugular node from a posterior lateral tongue carcinoma.
Occasionally, contralateral or bilateral metastatic deposits are seen, and at least 2% of patients have distant (“below the clavicles”) metastasis at diagnosis; in some studies this figure is as high as 22%. The most common sites of distant metastasis are the lungs, liver, and bones, but any part of the body may be affected.
Carcinoma of the lower lip and oral floor tends to travel to the submental nodes; tumors from the posterior portions of the mouth travel to the superior jugular and digastric nodes. Lymphatic drainage from the oropharynx leads to the jugulodigastric chain of lymph nodes or to the retropharyngeal nodes, and metastatic deposits from oropharyngeal carcinoma are usually found there.
Metastasis is not an early event for carcinomas of the oral cavity proper. However, because of delay in the diagnosis, approximately 21% of patients have cervical metastases at diagnosis (60% in reports from tertiary care medical centers). In contrast, tumors that arise more posteriorly in the oropharynx are prone to early metastasis. More than 50% of all affected persons in population studies have positive cervical nodes at diagnosis, and one in 10 already have distant metastasis by that time.
STAGING: Tumor size and the extent of metastatic spread of oral squamous cell carcinoma are the best indicators of the patient’s prognosis. Quantifying these clinical parameters is called staging the disease. Table 10-2 summarizes the most popular staging protocol, the tumor-node-metastasis (TNM) system. Individualized TNM classifications are used for most human cancers, with each system pertaining exclusively to a specific anatomic site and a specific tumor type. This staging protocol depends on three basic clinical features:
Table 10-2
Tumor-Node-Metastasis (TNM) Staging System for Oral Carcinoma
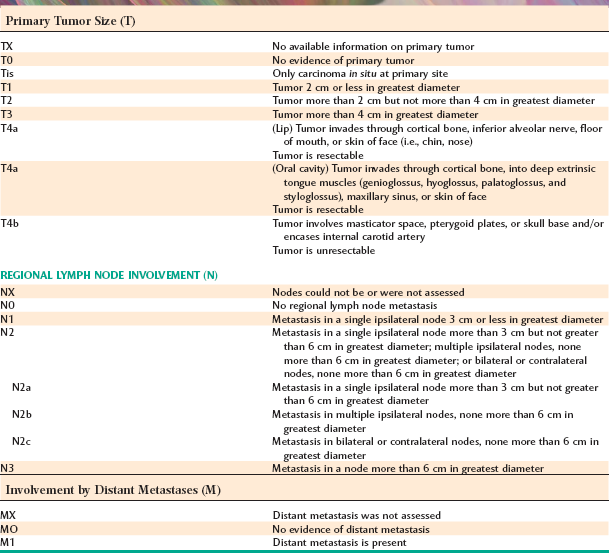
From Tumor-node-metastasis (TNM) staging system for oral carcinoma. In Greene FL, Page DL, Fleming ID et al, editors, AJCC cancer staging manual, ed 4, New York, 2002, Springer.
Once the three parameters are determined, they are tallied together to determine the appropriate stage. The higher the stage classification, the worse the prognosis (Table 10-3). In other words, a stage IV lesion is associated with a much worse prognosis than a stage I lesion. Most head and neck staging protocols do not use histopathologic or immunohistochemical findings beyond those needed for a determination of the diagnosis.
HISTOPATHOLOGIC FEATURES: Squamous cell carcinoma arises from dysplastic sur-face epithelium and is characterized histopathologically by invasive islands and cords of malignant squamous epithelial cells. When the tumor is sampled fortuitously at the earliest moment of invasion, the adjectives superficially invasive or microinvasive often are used. The features of epithelial dysplasia are discussed in more detail in the section pertaining to leukoplakia (see page 394).
Invasion is represented by irregular extension of lesional epithelium through the basement membrane and into subepithelial connective tissue. Individual squamous cells and sheets or islands of cells are seen to be thriving as independent entities within the connective tissues, without attachment to the surface epithelium. Invading cells and cell masses may extend deeply into underlying adipose tissue, muscle, or bone, destroying the original tissue as they progress. Lesional cells may surround and destroy blood vessels and may invade into the lumina of veins or lymphatics. There is often a strong inflammatory or immune cell response to invading epithelium, and focal areas of necrosis may be present. The lesional epithelium is capable of inducing the formation of new small blood vessels (angiogenesis) and, occasionally, dense fibrosis (desmopla-sia or scirrhous change).
Whether the tumor is superficially or deeply invasive, lesional cells generally show abundant eosino-philic cytoplasm with large, often darkly staining (hyperchromatic) nuclei and an increased nuclear-to-cytoplasmic ratio. Varying degrees of cellular and nuclear pleomorphism are seen. The normal product of squamous epithelium is keratin, and keratin pearls (a round focus of concentrically layered keratinized cells) may be produced within lesional epithelium. Single cells also may undergo individual cell keratinization.
Histopathologic evaluation of the degree to which these tumors resemble their parent tissue (squamous epithelium) and produce their normal product (keratin) is called grading. Lesions are graded on a 3-point (grades I to III) or a 4-point (grades I to IV) scale. The less differentiated tumors receive the higher numerals. The histopathologic grade of a tumor is related somewhat to its biologic behavior. In other words, a tumor that is mature enough to closely resemble its tissue of origin seems to grow at a slightly slower pace and to metastasize later in its course. Such a tumor is called low-grade, grade I, or well-differentiated squamous cell carcinoma (Fig. 10-119). In contrast, a tumor with much cellular and nuclear pleomorphism and with little or no keratin production may be so immature that it becomes difficult to identify the tissue of origin. Such a tumor often enlarges rapidly, metastasizes early in its course, and is termed high-grade, grade III/IV, poorly differentiated, or anaplastic (Fig. 10-120). A tumor with a microscopic appearance somewhere between these two extremes is labeled a “moderately differentiated” carcinoma (Fig. 10-121).
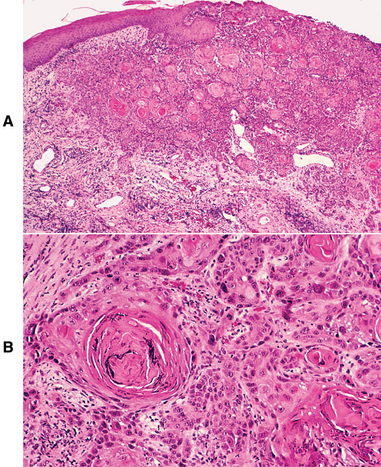
Fig. 10-119 Well-differentiated squamous cell carcinoma. A, Low-power photomicrograph showing islands of malignant squamous epithelium invading into the lamina propria. B, High-power view showing dysplastic epithelial cells with keratin pearl formation.
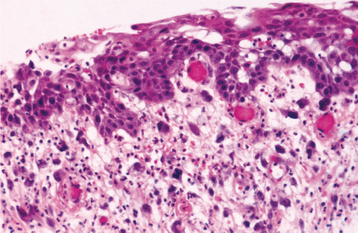
Fig. 10-120 Poorly differentiated squamous cell carcinoma. The numerous pleomorphic cells within the lamina propria represent anaplastic carcinoma.
Fig. 10-121 Moderately differentiated squamous cell carcinoma. Although no keratinization is seen in this medium-power view, these malignant cells are still easily recognizable as being of squamous epithelial origin.
To a certain extent, the grading of squamous cell carcinoma is a subjective process, depending on the area of the tumor sampled and the individual pathologist’s criteria for evaluation. Moreover, clinical staging seems to correlate much better with the prognosis than microscopic grading. Over the past several decades, investigators have proposed various multi-parameter histopathologic assessment systems in an attempt to provide more objective criteria that correlate with prognosis. Variables such as pattern of invasion, tumor thickness, degree of keratinization, nuclear pleomorphism, lymphocytic response, and mitotic rate have been included in such grading systems. However, widespread agreement regarding the use of such methods is lacking.
The diagnosis of squamous cell carcinoma almost always is made with routine light microscopy. Special studies that use monoclonal antibodies directed against cytokeratins may, however, be needed to distinguish high-grade or poorly differentiated squamous cell carcinoma from other malignancies.
TREATMENT AND PROGNOSIS: Carcinoma of the vermilion zone of the lip is usually treated by surgical excision, typically a wedge resection, with excellent results. Only 8% recur, and 5-year survival rates are 95% to 100%. In one study that evaluated all vermilion cancers diagnosed in a population over six decades, not one patient died of his or her disease. Squamous cell carcinomas of the upper lip vermilion appear to have a different biologic behavior than do those of the lower lip. The 5-year survival rate is only 58%, and 25% of lesions recur after treatment. Fortunately, upper lip carcinoma is considerably less common than lower lip carcinoma.
The clinical stage of the disease guides the treatment of intraoral squamous cell carcinoma, which consists of wide (radical) surgical excision, radiation therapy, or a combination of surgery and radiation therapy. The tumor’s location may influence the treatment plan. Oropharyngeal lesions usually receive radiation therapy. Additional indications for radiation therapy include the presence of close or positive resection margins, regional metastasis, high-grade histopathologic features, and perineural or angiolymphatic invasion. A variety of chemotherapeutic agents are used as adjunctive therapy. Chemotherapy typically is administered concurrently with radiation, as induction chemotherapy followed by concurrent chemoradiation, or as palliative therapy. Commonly used agents include platinum-containing compounds (e.g., cisplatin, carboplatin), 5-fluorouracil, and taxanes (e.g., paclitaxel, docetaxel). In addition, clinical trials evaluating monoclonal antibodies or small molecule inhibitors directed against EGFR have shown promise, and molecular-based targeted therapies are anticipated to become important treatment strategies in the future. Although chemotherapeutic agents may reduce the size of a tumor mass temporarily, none has improved survival rates significantly.
For the small intraoral carcinoma, a single treatment modality is usually chosen. Patients with larger lesions or lesions with clinically palpable lymph nodes typically require combined therapy. In addition, patients with early-stage (clinically T1/T2 and N0) but deeply invasive (tumor thickness >3 or 4 mm) tongue carcinomas are at increased risk for subclinical nodal metastasis and thus may receive either postoperative neck irradiation or elective neck dissection. With suspected local lymph node metastasis, either a radical or modified radical neck dissection is performed. Radical neck dissection is essentially an en bloc removal of all fibrofatty tissues of the lateral triangle of the neck, including the superior, middle, and inferior jugular nodes, the supraclavicular group of nodes, and variable portions of the surrounding musculature. The use of sentinel-node biopsy (biopsy of the first lymph node in the lymphatic basin to receive drainage from the tumor) to identify patients with occult neck metastasis has shown promise but remains investigational for patients with oral cancer.
The prognosis for survival from oral cancer depends on tumor stage (see Table 10-3). The 5-year relative survival rate for intraoral carcinoma is 53% to 68% if the tumor is relatively small (<4 cm) and metastasis has not occurred by the time of diagnosis (stage I and II); 41% when the tumor is 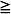 4 cm and either without metastasis or with metastasis in a single ipsilateral regional lymph
4 cm and either without metastasis or with metastasis in a single ipsilateral regional lymph 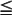 3 cm (stage III); and only 27% when the tumor has invaded adjacent structures, has metastasized to a distant site, or is associated with regional metastasis of multiple nodes, a single ipsilateral node >3 cm and
3 cm (stage III); and only 27% when the tumor has invaded adjacent structures, has metastasized to a distant site, or is associated with regional metastasis of multiple nodes, a single ipsilateral node >3 cm and  6 cm, or a node >6 cm (stage IV). Although some patients die of their disease as many as 10 years after initial treatment, the great majority of deaths occur within the first 5 years.
6 cm, or a node >6 cm (stage IV). Although some patients die of their disease as many as 10 years after initial treatment, the great majority of deaths occur within the first 5 years.
The various molecular markers associated with carcinoma, such as mutation of the p53 tumor suppressor gene, have shown equivocal results as prognostic indicators. The relationship between HPV tumor status and prognosis is unclear. Some studies have suggested that HPV-positive tumors are associated with improved treatment response and prolonged survival; however, other studies have reported no significant difference in survival between patients with HPV-positive and HPV-negative tumors.
The overall 5-year survival rate for intraoral carcinoma in whites in the United States and Europe has increased from 40% in the 1950s to 59% today. During the same period, however, the rate for black Americans has increased only slightly from 36% to 39%. Researchers believe that this disparity in survival results from differences in access to health care services; in addition, some have hypothesized that lifestyle habits (e.g., tobacco and alcohol use, nutrition), cultural differences, and the presence of comorbid conditions may be contributing factors.
Despite advances in treatment and in the understanding of the underlying molecular pathogenesis of oral cancer, survival rates over the last several decades have not improved significantly and have remained in the range of 50% to 59%. Therefore, early diagnosis and prevention are essential for improving patient outcomes. In addition, there is much interest in the development of chemoprevention strategies for preventing the malignant transformation of oral dysplastic lesions or preventing the development of second primary or recurrent tumors in patients with a history of oral squamous cell carcinoma.
MULTIPLE CARCINOMAS: Patients with one carcinoma of the mouth or throat are at increased risk for additional concurrent (synchronous) or later (metachronous) primary surface epithelial malignancies of the upper aerodigestive tract, the esophagus, the stomach, the lungs, and other sites. This risk has been estimated to be as low as 6% and as high as 44% in affected individuals. The highest figures are associated with male patients who continue to smoke and abuse alcohol after therapy. Overall, in 9% to 25% of patients with oral carcinoma, additional mouth or throat malignancies develop.
In patients with more than one upper aerodigestive tract malignancy, approximately one third of the tumors arise simultaneously. Of the rest, the second lesion usually develops within 3 years after the initial cancer. This tendency toward the development of multiple mucosal cancers, sometimes called field cancerization, may reflect diffuse exposure to local carcinogens, a process that increases the malignant transformation potential of all exposed epithelial cells. Molecular analyses of various markers, including loss of heterozygosity (LOH), microsatellite alterations, p53 tumor suppressor gene mutations, and X-chromosome inactivation, have identified genetic alterations shared between tumor tissue and adjacent clinically normal-appearing tissue in one third to one half of cases examined. In addition, investigators have shown that a significant proportion of second primary tumors develop from the same preneoplastic precursor lesion or “field,” with the remaining cases representing tumors that develop independently. Furthermore, researchers have proposed that patches of clonal cells can progress to develop additional mutations and give rise to subclones in a process known as clonal divergence, which would account for the genetic heterogeneity typically seen among these tumors.