Chapter 27 Packaging
Introduction
Pharmaceutical formulations must be suitably contained, protected and labelled from the time of manufacture until the patient uses them. Throughout this period the container must maintain the quality, safety and stability of the medicine and protect the product against physical, climatic, chemical and biological hazards. The British Pharmacopoeia identifies the closure as part of the container.
To promote good patient compliance the container must be user friendly. This is particularly significant for the elderly who have to take more medicines than the general population and have a greater need for improved compliance (see Ch. 46). Thus containers should be easy to open and reclose, most notably for elderly or arthritic patients. However, other factors must also be considered in the selection of the container used to package a pharmaceutical formulation, including the cost and the need for both child-resistant closures and tamper-evident seals.
Repackaging is performed in the community pharmacy for dispensing purposes (see Chs 25 and 36), in hospital pharmacy and in specialized production facilities. Bulk medicines are repackaged into smaller quantities in dispensing containers for distribution to hospital wards, clinics and general practitioners for direct supply to patients. This is mostly carried out with tablets and capsules that are transferred from bulk quantities into smaller amounts that are more suitable for patient use. In the UK this process is performed in the hospital pharmacy where the Medicines and Healthcare products Regulatory Agency (MHRA) allows the repackaging of small batches of up to 25 containers. Larger batches must be packed in licensed manufacturing premises. The facilities used for these repackaging operations are designed to maintain the quality of the medicine and avoid product contamination and mix up.
Medicines originally contained in patient packs are subdivided into small amounts by transferring small quantities of the medicine in strip or blister packs into secondary cardboard containers. The composition of containers and closures used for the repackaging of bulk medicines must be carefully selected and must be of a quality as good as the original container. Both glass and plastic containers are used for repackaging but glass containers are often preferred due to the more inert qualities of glass.
Primary containers used for repackaging must not:
The container used in the repackaging process must protect the product from:
As the medicine has been transferred into a new container, the expiry date of the repackaged medicine must not exceed 12 months unless the stability of the repackaged product justifies a longer shelf life. The details of these repackaging processes must be recorded.
Each container of the repackaged batch is labelled with the:
There are some situations where the repackaging is limited, such as with glyceryl trinitrate tablets, owing to the potential loss of the volatile drug (see Ch. 36). Sterile products cannot easily be repackaged and require effective closure systems to minimize the risk of microbial contamination of the contents within the container. In addition, the pack itself must withstand sterilization procedures. Consequently, care must be applied to the selection of the container and its closure for the packaging of sterile products (see also Chs 38, 39, 40 and 41).
Primary and secondary packaging
Primary packaging materials are in direct contact with the product. This also applies to the closure, which is also part of the primary pack. It is important that this container must not interact with the medicine. It must protect the medicine from damage and from extraneous chemical and microbial contamination. In addition, the primary packaging should support use of the product by the patient. Secondary packages are additional packaging materials that improve the appearance of the product and include outer wrappers or labels that do not make direct contact with the product (Table 27.1). Secondary packages can also supply information about the product and its use. They should provide evidence of tampering with the medicine.
Table 27.1 Types of primary and secondary packaging materials and their use
| Material | Type | Examples of use |
| Glass | Primary | Metric medical bottle, ampoule, vial |
| Plastic | Primary | Ampoule, vial, container, infusion fluid dropper bottle |
| Plastic | Secondary | Wrapper to contain primary pack |
| Board | Secondary | Box to contain primary pack |
| Paper | Secondary | Labels, patient information leaflet |
The following terms are used to describe containers:
An important consideration when selecting the packaging for any product is that its main objective is that the package must contribute to delivering a drug to a specific site of effective activity in the patient.
The selection of packaging for a pharmaceutical product is dependent on the following factors:
See also Chapter 36 in Pharmaceutics: the Science of Dosage Form Design.
Packaging materials
Glass
Historically, glass has been widely used as a drug packaging material. It continues to be the preferred packaging material for many pharmaceutical products.
Glass does have several advantages:
The disadvantages of glass include:The chemical stability of glass for pharmaceutical use is given by the resistance of the glass to the release of soluble minerals into water contacting the glass. This is known as the hydrolytic resistance. Details are given in the British Pharmacopoeia (2007) for three types of glass.
Type I glass
This is also known as neutral glass or borosilicate glass. It possesses a high hydrolytic resistance due to the chemical composition of the glass. It is the most inert type of pharmaceutical glass with the lowest coefficient of thermal expansion. As a result, it is unlikely to crack on exposure to rapid temperature changes. Type I glass is suitable for packing all pharmaceutical preparations. However, it is expensive and this restricts its applications. It is widely used as glass ampoules and vials to package fluids for injection. In addition, it is used to package solutions that could dissolve basic oxides in the glass. This would increase the pH of the formulation and could affect the drug stability and potency.
Type II glass
This is made of soda-lime-silica glass with a high hydrolytic resistance due to surface treatment of the glass. Type II glass is used to package aqueous preparations. In general, it is not used by manufacturers to package parenteral formulations with a pH less than 7. This glass has a lower melting point than Type I glass. It is thus easier to produce and consequently cheaper. It is the glass used to produce containers for eye preparations and other dropper bottles.
Type III glass
This is made of a soda-lime-silica glass. It has a similar composition to Type II glass but contains more leachable oxides. Type III glass offers only moderate resistance to leaching and is commonly used to produce dispensary metric medical bottles. It is also suitable for packaging non-aqueous parenteral products and powders for injection.
Types of glass containers
Bottles
These are commonly used in the dispensary as either amber metric medical bottles or ribbed (fluted) oval bottles. Both types of bottle are available in sizes from 50 mL to 500 mL and are supplied with a screw closure.
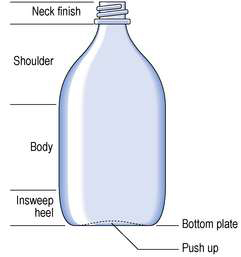
Amber metric medical bottles have a smooth curved side and a flat side (Fig. 27.1). The bottle was designed to permit the curved side of the bottle to fit into the palm of the hand when pouring from the bottle. The flat side was intended to permit the attachment of a label. In practice, however, the label is commonly attached to the curved surface of the bottle. Amber metric medical bottles are used for packaging a wide range of oral medicines.
Ribbed oval bottles have flutes down one side of the container (Fig. 27.2). The characteristic feel of the flutes warns the user that the contents are not to be taken. A label is attached to the plain front of the bottle. Ribbed oval bottles are used to package various products that should not be taken orally; this includes liniments, lotions, inhalations and antiseptic solutions.
Dropper bottles
Eye drop and dropper bottles for ear and nasal use are hexagonal-shaped amber glass containers fluted on three sides. They are fitted with a cap, rubber teat and dropper as the closure. The bottles are used at a capacity of 10 mL or 20 mL. The label is attached to the plain sides of the bottle.
Jars
Powders and semi-solid preparations are generally packed in wide-mouthed cylindrical jars made of clear or amber glass. The capacity of these jars varies from 15 mL to 500 mL. Ointment jars are used for packing extemporaneously prepared ointments and pastes. They are also used to repackage commercial products where microbial contamination by the patient’s fingers is not detrimental to the product.
Containers for parenteral products
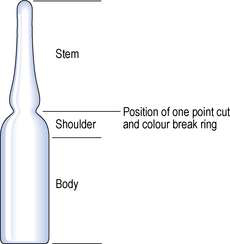
Small-volume parenteral products, such as subcutaneous injections, are typically packaged in various containers made of Type I glass. Glass ampoules (Fig. 27.3) are used to package parenteral solutions intended for single use.
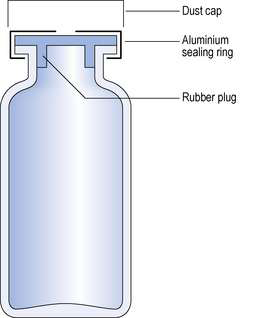
Multiple-dose vials (Fig. 27.4) are used to package parenteral formulations that will be used on more than one occasion. Large-volume parenteral fluids have been packaged in 500 mL glass containers but these have been largely superseded by plastic bags.
Plastics
Plastics have been widely used for several years as containers for the product and as secondary packaging in the form of a carton. In more recent times, plastic has been developed for the packaging of parenteral products including infusion fluids and small-volume injections.
Two classes of plastics are used in the packaging of pharmaceutical products. These are known as thermosets and thermoplastics. The thermosets are used for making screw caps for glass and metal containers. Thermoplastic polymers are used in the manufacture of a wide variety of pharmaceutical packages as detailed in Table 27.2.
Table 27.2 The application of thermoplastic polymers for the packaging of pharmaceutical products
| Polymer | Examples of application |
| High-density polyethylene | Solid dosage form containers |
| Low-density polyethylene | Flexible eye drop bottles |
| Linear low-density polyethylene | Heat-sealable containers |
| Polypropylene | Container closures, intravenous solution bottles |
| Polyvinyl chloride | Laminate for blister packs, intravenous bags |
| Polystyrene | Containers for oils and creams and solid dosage forms |
The advantages of plastics for packaging are that they:
The disadvantages of plastics are that:Plastic pharmaceutical containers are made of at least one polymer together with additives. The additives used will depend on the composition of the polymer and the production methods used.
Additives used in plastic containers include:
Plastic containers
These are used for many types of pack including rigid bottles for tablets and capsules, squeezable bottles for eye drops and nasal sprays, jars, flexible tubes, strip and blister packs. The composition and the physical shape of the containers vary widely to suit the application.
The principal plastic materials used in pharmaceutical packaging
Polyethylene
This is used as high- and low-density polyethylene, both of which are compatible with a wide range of drugs and are extensively used for the packaging of various pharmacy products. Of these two forms of polyethylene, low-density polyethylene (LDPE) is softer, more flexible and more easily stretched than high-density polyethylene (HDPE). Consequently, LDPE is usually the preferred plastic for squeeze bottles. By contrast, HDPE is stronger, stiffer, less clear, less permeable to gases and more resistant to oils, chemicals and solvents. It is commonly pigmented or printed white to block light transmission and improve label clarity. HDPE is widely used in bottles for solid dosage forms.
Polyvinyl chloride (PVC)
This is extensively used as rigid packaging material and as the main component of intravenous bags.
Polypropylene
This is a strong, stiff plastic polymer with good resistance to cracking when flexed. As a result it is particularly suitable for use in closures with hinges which must resist repeated flexing. In addition, polypropylene has been used as tablet containers and intravenous bottles.
Polystyrene
This is a clear, hard, brittle material with low impact resistance. Its use in drug packaging is limited due to its high permeability to water vapour. However, it has been used for tubes and amber-tinted bottles where clarity and stiffness are important and high gas permeability is not a drawback. It is also used for jars for ointments and creams with low water content.
Closures
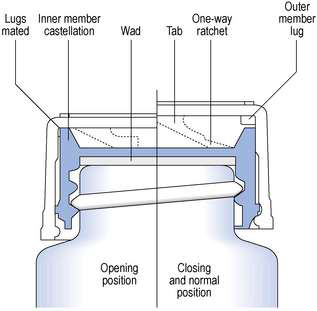
Any closure system should provide an effective seal to retain the container contents and exclude external contaminants. Child-resistant containers (CRCs) commonly consist of a glass or plastic vial or bottle with a specially designed closure. These CRCs are a professional requirement for dispensing of solid and liquid dosage forms in the UK and are ultimately a compromise between child resistance and ease of opening. They are not an absolute barrier to children accessing medicine containers, therefore the containers should be stored in a safe place. Several designs of child-resistant closures are currently used for pharmaceutical packaging, including cap–bottle alignment systems, push down and turn caps and, less commonly, squeeze and turn caps.
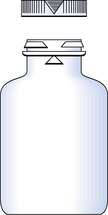
The closures in common use with dispensed medicines are the Snap-safe® alignment closure (Fig. 27.5) and the push down and turn Clic-loc® closure (Fig. 27.6). The Clic-loc® child-resistant closures are based on the assumption that young children are unable to coordinate two separate and dissimilar actions; that is, applying pressure and rotating the closure top. The Clic-loc® closure has a two-piece mechanism with springs between the inner and the outer parts. As a result of this design, the closure produces an audible clicking noise when the cap is turned without first being depressed. The inner cap is composed of polypropylene while the outer overcap is made of HDPE.
Contamination of the screw thread with crystallized sugar arising from syrups can increase the torque necessary to open these Clic-loc® closures. This type of problem can restrict their suitability for use. Owing to opening difficulties experienced by some adults, these closures should not be used on containers supplied to elderly or handicapped patients with poor manual dexterity. They should not be used when a request is made that the product is not dispensed with a child-resistant closure fitted. A Clic-loc® closure must only be dispensed on one occasion as continued use increases the penetration of moisture vapour into the container and decreases the child-resistant properties of the closure.
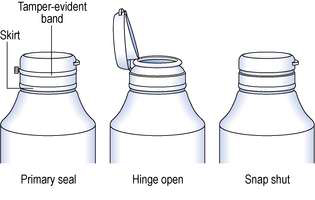
In recent years greater awareness of the vulnerability of products has led to the development of tamper-evident closures. The closures indicate if unlawful access to the container contents has occurred and are currently available in various designs suitable for different containers and closures. Dispensary stock containers are frequently fitted with a Jaycap® type of tamper-evident closure. These closures are made of either white polypropylene or LDPE. With this closure design the tamper-evident closures snap over a security bead on the neck of the container. The closures cannot be opened until the tamper-evident band connecting the cap to the skirt is torn away (Fig. 27.7). Clic-loc® closures are available with this design whereby an external tamper-evident coloured band must be removed before the closure can be turned. Tamper-evident inner seals are positioned within the closure and are attached to the rim of the opening to the container isolating its contents. The seal must be torn or removed from the container to gain access to the packaged product. These seals are commonly made of a combination of paper, plastic and foil.
Collapsible tubes
These are flexible containers for the storage and dispensing of creams and ointments. Tubes made of tin are used to package certain sterile formulations. Typically the formulation is aseptically filled into the pre-sterilized tubes. However, the most common metal tubes in current use are made of aluminium with an internal lacquered surface. With this package the tube remains collapsed as the product is removed. These tubes are frequently sealed at both ends and the nozzle must be punctured to access the product. An alternative seal that can be used with these packages is a heat seal band between the closure and the container. This band must be torn to gain access to the container contents.
Plastic tubes made from a variety of materials are superseding metal tubes. For example, the tube sleeve may be made of LDPE with either a LDPE or HDPE head or the entire tube may be made of polypropylene.
Unit-dose packaging
This term usually means that a single item such as a tablet or capsule or a specific dose is enclosed within its own disposable packaging. The most commonly used methods for unit-dose packaging are blister packs and strip packs.
Blister packs
These are used for packaging unit doses of tablets and capsules and can act as an aid for patient compliance. The medication is placed in a compartment in a base material made of paper, board, plastic or metal foil or a combination of these. The blister is generally composed of a thermoformed plastic sheet such as PVC. The protection given by the plastic blister depends on its composition, design and the method used to form it. Perforations in the base material allow individual sections of the package to be broken off. Blister packages are rigid, unlike strip packs that are flexible.
Strip packaging
With strip packaging, two webs of material sandwich various types of medicine such as tablets, capsules, suppositories or pessaries. Each of these dosage forms is contained within its own compartment. The composition of the two webs can be selected to meet the necessary protective requirements for the medicine. Aluminium foil is commonly used to manufacture strip packs and provides a good barrier against moisture penetration. The foil is used as a laminate in which the other components add strength to the fragile aluminium foil. They also block small holes which can occur in the thinner foil layer.
Paper
Paper is used more than any other material in packaging. Although it has an insignificant role in primary packaging it remains the predominant secondary and tertiary packaging material. In this role it is used as the carton which contains the primary package and, in the form of board, is the corrugated shipping container which contains both.
Patient pack dispensing
A patient pack consists of a course of medication together with a patient information leaflet in a ready to dispense pack. Liquid formulations are supplied in a standard pack. Solid dose forms are supplied as a strip or blister pack. The size of the sealed patient pack is based on a 28, 30 or 56 dose unit appropriate to the medicine. It is supplied in this amount unless a doctor prescribes that a different quantity of medicine is to be dispensed. The patient pack contains an information leaflet as an aid to improving patient compliance and to supply information to patients about their medication. Pharmacists should be prepared to respond to enquiries after the patient has read the leaflet. The patient pack is designed as a balance between the need for child resistance and the need for ease of opening by the elderly. If requested by the patient, the pack contents can be repackaged in a more suitable container.