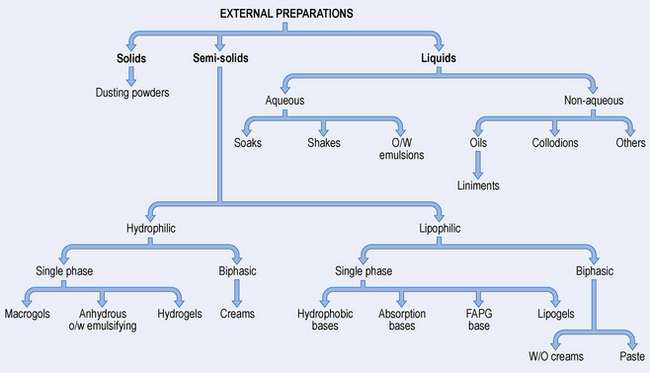
Chapter 33 External preparations
Introduction
Skin is the largest organ in the body and has three distinct regions. The hypodermis is the innermost and is often called subcutaneous fat. The dermis is the bulk of the thickness of the skin and contains blood vessels, nerve fibres, sweat glands and hair follicles. The outermost region is the epidermis, which is made up of several layers. One of these layers is the stratum basale, in which cells divide and as they move towards the surface they change appearance and function. The outermost layer, the stratum corneum, acts as the skin barrier. It is made up of about 20 layers of dead keratinized cells. The hair follicles and sweat ducts pass through the stratum corneum to reach the surface. A simplified diagram showing the main skin structures is given in Figure 33.1.
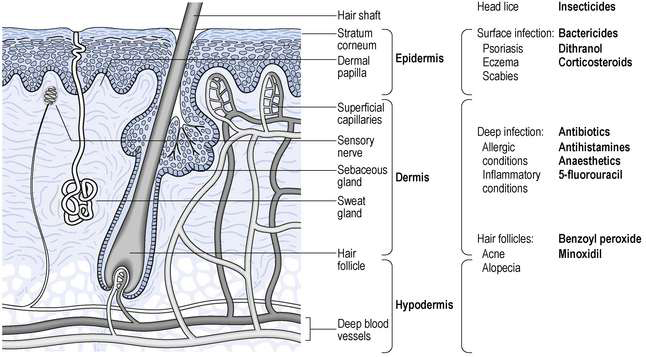
Fig. 33.1 Diagrammatic representation of the skin showing the main structures, location of diseases and the sites of action of drugs.
There are a large number of diseases which may affect different regions of the skin. Any drug used will require to reach the site of the disease in order to act. Unless it is for a surface effect only, the drug must either pass through the stratum corneum or go through hair follicles or sweat ducts. Examples of drugs applied to the skin and their sites of action are shown in Figure 33.1. Once in the skin, a lipid-soluble drug will tend to accumulate in lipid regions, while more water-soluble drugs will tend to enter the blood capillaries and be removed from the skin. There are also many metabolic enzymes in the skin which can deactivate drugs. A more detailed discussion of these factors and the physicochemical principles involved is given in Aulton (2007).
Pharmacokinetics is seldom applied to skin administration and dosage is often imprecise. However, by effective formulation it is possible to achieve adequate and reproducible percutaneous absorption. The main advantage is that close to zero order kinetics can be produced. This also carries an inherent warning because traditionally it has been assumed that absorption from the skin is minimal. As a consequence, the skin is seen as ‘safe’ even if quite toxic materials are applied. This is not true and great care is required, so that gloves should always be warn when extemporaneously preparing external preparations.
There are an increasing number of drugs that are effective against skin diseases, but drugs are not the only way of treating skin conditions. Creating physiological changes in the skin can also be beneficial. The main change is to control the moisture content of the skin. Normal skin has 10–25% moisture in the stratum corneum. This level may be reduced in, for example, eczema, or increased, as in skin maceration between the toes. By using an occlusive product (that is, an oily product), water leaving the body through the skin will be trapped and moisture content will increase. These products are called emollients. An excess of moisture may be removed using an astringent, a hygroscopic material or, to a lesser extent, a dusting powder. Where an oily vehicle is needed, but moisture must not increase, adding solid particles to the vehicle will allow water to escape. Lubrication of sensitive skin is achieved by using finely divided solids, applied either as a powder or, more efficiently, as a suspension. Cooling the skin relieves inflammation and eases discomfort. It is achieved by evaporating a solvent, usually water or a water and alcohol mixture. Volatile solvents sprayed on the skin give intense cooling.
Types of skin preparation
There are a large number of different types of external medicine, ranging from dry powders through semi-solids to liquids. The names are often traditional making classification difficult. Figure 33.2 illustrates the formulation of the main types of preparation used on the skin.
Solids
Dusting powders are applied to the skin for a surface effect such as drying or lubricating, or an antibacterial action. They are made of a fine-particle-size powder which may be a drug alone or together with excipients.
Liquids
Soaks have an active ingredient dissolved in an aqueous solvent and are often used as astringents, for cooling or to leave a film of solid on the skin. Oily vehicles can be used in bath additives to leave an emollient film on the skin surface.
Applications are solutions or emulsions that frequently contain parasiticides (see Example 33.4).
Example 33.4
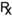 Benzyl Benzoate Application BP
Benzyl Benzoate Application BP
Prepare 100 mL of benzyl benzoate application.
| Master formula | For 100 mL | |
| Benzyl benzoate | 250 g | 25 g |
| Emulsifying wax | 20 g | 2 g |
| Purified water, freshly boiled and cooled | to 1000 mL | to 100 mL |
Liniments are alcoholic or oily solutions or emulsions (see Example 33.3) designed to be rubbed into the skin. The medicament is usually a rubefacient.
Example 33.3
Send 100 mL of turpentine liniment.
| Master formula | To send 100 mL (120 units) | |
| Turpentine oil | 650 mL | 78 mL |
| Racemic camphor | 50 g | 6 g |
| Soft soap | 75 g | 9 g |
| Purified water, freshly boiled and cooled | 225 mL | 27 mL |
Lotions are aqueous solutions, suspensions (see Example 33.1) or emulsions that cool inflamed skin and deposit a protective layer of solid.
Example 33.1
Send 100 mL Compound Sulphur Lotion BPC.
| Master formula | For 100 mL | |
| Precipitated sulphur | 40 g | 4 g |
| Quillaia tincture | 5 mL | 0.5 mL |
| Glycerol | 20 mL | 2 mL |
| Industrial methylated spirit (now IDA) | 60 mL | 6 mL |
| Calcium hydroxide solution | to 1000 mL | to 100 mL |
Paints and tinctures are concentrated aqueous or alcoholic antimicrobial solutions.
Collodions are organic solvents containing a polymer and keratolytic agent for treating corns and calluses.
There are also many other liquid products including shampoos, pomades and foot washes.
Semi-solids
Ointments are usually oily vehicles that may contain a surfactant to allow them to be washed off easily (barrier creams). They are used as emollients, or for drug delivery either to the surface or for deeper penetration.
Creams are traditionally oil-in-water (o/w) emulsions while oily creams are water-in-oil (w/o) emulsions. However, there are also ‘creams’ that are not emulsions. Emulsified creams usually give cooling, are less greasy than ointments and can be used for drug delivery onto or into the skin. They require antimicrobial preservatives.
Pastes are vehicles (aqueous or oily) with a high concentration of added solid. This makes them thick so they do not spread and so localizes drug delivery (e.g. Dithranol in Lassar’s Paste, see Example 33.11). They can also be used for sun blocks.
Example 33.11
Send 100 g of weak dithranol paste.
| Master formula | For 100 g | |
| Dithranol | 1 g | 0.1 g |
| Lassar’s paste | 999 g | 99.9 g |
| Lassar’s paste: | Master formula | For 110 g |
| Zinc oxide | 240 g | 26.4 g |
| Salicylic acid | 20 g | 2.2 g |
| Starch | 240 g | 26.4 g |
| White soft paraffin | 500 g | 55 g |
Gels (jellies) are usually aqueous gels used for lubrication or applying a drug to the skin. Oily gels are also available where occlusion is required.
Ingredients used in skin preparations
Water-miscible vehicles
These include water, alcohol and the macrogols. Alcohol is often added to water to increase the rate of evaporation and produce a more intense cooling effect. Industrial denatured alcohol (IDA) (formerly known as industrial methylated spirit) is normally used for external preparations because it is exempt from excise duty. The macrogols (polyethylene glycols) are available with a range of molecular weights. As chain length increases, so the properties change from liquid, through semi-solid to waxy solid. They have good solvent properties for a wide range of drugs and can be blended to produce intermediate consistencies. They tend to dry the skin, inactivate some antimicrobials, interact with some plastics and can give poor release of drugs.
Oily vehicles
Oils used in external preparations come from one of three sources.
Mineral oils (paraffins) are the most widely used. They are complex mixtures of mainly saturated hydrocarbons which are available in different fractions. Different names are used in different pharmacopoeias (Table 33.1).
Table 33.1 Paraffins used in external preparations: the names used are different in the UK, USA and European pharmacopoeias
| UK | USA | European |
| Light liquid paraffin | Light mineral oil | Paraffinium perliquidum |
| Liquid paraffin | Mineral oil | Paraffinium liquidum |
| Soft paraffin | Petrolatum | Paraffinium molle |
| Hard paraffin | Paraffin | Paraffinium durum |
Light liquid paraffin is not normally used in external medicines. Soft paraffin is the main ingredient in many products, with liquid or hard paraffin being used to thin or thicken them respectively. There are two forms of soft paraffin – yellow and white. The latter has been bleached, residues of which may remain. As a general rule, white is used with white or pale coloured ingredients, while yellow is used for darker ingredients. The paraffins are occlusive and chemically inert, but do not give good skin penetration.
Vegetable oils come from many plant sources such as peanut, castor, olive and coconut. They may be used as a mobile solvent (as in a liniment, see Example 33.2) or as part of an ointment or cream. If they require thickening, a high melting point material such as cetostearyl alcohol can be used. They are occlusive and give good skin penetration, but may go rancid. Sufficient skin penetration may occur to cause severe reactions in patients with nut allergies.
Example 33.2
Prepare 100 mL methyl salicylate liniment.
| Master formula | For 100 mL | |
| Methyl salicylate | 250 mL | 25 mL |
| Arachis oil | to 1000 mL | to 100 mL |
Synthetic oils, such as the silicone oils (Dimeticone BP), are used as water repellents and occlusives because they are very hydrophobic. The semi-synthetic isopropylmyristate is similar to vegetable oil in properties and use.
Emulsifying agents
Liquid and semi-solid emulsions, both o/w and w/o, are used externally and require the addition of emulsifying agents. The latter may also be added to an oil without water as in Emulsifying Ointment BP. The presence of a surfactant usually increases the skin penetration of any drug. A wide range of materials can be used as surfactants, either alone or in combinations. Selection is made in view of the type of emulsion required (o/w or w/o) and the charge on the other ingredients (anionic, cationic or non-ionic).
Emulsifiers – w/o
Wool fat, obtained from sheep wool, is a pale yellow sticky material. It is a complex mixture of fatty acid esters of cholesterol and other sterols and alcohols. While it is similar to human sebum, it has been implicated in sensitization in some people. Where this is a problem there are an increasing number of hypoallergenic commercial products available. Wool alcohol, a solid, is richer in cholesterol and lanesterol and freer from impurities. Both it and wool fat increase the ‘water-holding’ capacity of greasy bases. Hydrous wool fat is 7 parts wool fat and 3 parts water and is a softer material. Different names are used, as shown in Table 33.2. Beeswax is a traditional w/o emulsifier which is occasionally used.
Table 33.2 Materials based on wool fat: different names are used in the UK, USA and European pharmacopoeias
| UK | USA | European |
| Wool fat | Lanolin | Adeps lanae |
| Wool alcohols | Lanolin alcohols | Alcoholes adipis lanae |
| Hydrous wool fat | Hydrous lanolin | Adeps lanae cum aqua |
Emulsifiers – o/w
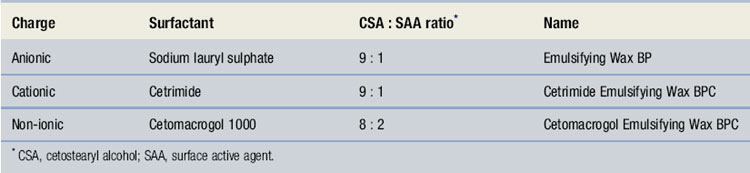
The main group of materials used extemporaneously are the emulsifying waxes. Each one has two ingredients – cetostearyl alcohol and a surface-active agent, as shown in Table 33.3. All three are waxy solids that mix with oily materials. Addition of water produces an o/w emulsion – a cream. Both the non-aqueous blends and the creams are easily washed off the skin. Varying the amount of bodying agent, usually cetostearyl alcohol, can control consistency. The ratio of oil to water will also alter the consistency of a cream.
Other emulsifiers
The gums used in oral emulsions (see Ch. 32) are too sticky for external use, but a number of other emulsifying agents are used.
Calcium soaps are produced by mixing a fatty acid with lime water (calcium hydroxide solution) to form a soap in situ (see Example 32.5). They form w/o emulsions. Soft soap is a sticky green material that can be used to make o/w emulsions (see Example 33.3).
Synthetic surface-active agents are used particularly in commercial products. Low HLB (hydrophilic–lipophilic balance) materials will produce w/o emulsions, while higher HLB surfactants give o/w emulsions.
Suspending agents
These materials can be used for suspending solids in shake lotions, or to produce gels, depending on the concentration used. Those used in oral suspensions (see Ch. 31) are too sticky for use in external liquid suspensions. The main group of materials used for this purpose are the clays, of which there are many forms, including bentonite, attapulgite, montmorrilonite and Veegum® (aluminium magnesium silicate). They leave a lubricant layer of powder on the skin. They are unsuitable for use below pH 3.5 and their consistency may be affected by alcohol and electrolytes (see Example 31.5).
Gelling agents can be used to produce a wide range of consistency from slightly thickened (as in artificial tears), through lubricants and semi-solids for the delivery of drugs to very thick bases used to immobilize the skin. For aqueous gels the materials used include tragacanth, alginates, pectin, gelatin, methylcelluloses, carbomer, polyvinyl alcohol and clays. Oils may be thickened using cetostearyl alcohol, hard paraffin, beeswax (see Example 33.7), wool alcohols and polyvalent soaps such as magnesium stearate. The latter, when heated with an oil, produces a clear ‘lipogel’.
Example 33.7
Send 30 g methyl salicylate ointment.
| Master formula | For 35 g | |
| Methyl salicylate | 500 g | 17.5 g |
| White beeswax | 250 g | 8.75 g |
| Hydrous wool fat | 250 g | 8.75 g |
Other ingredients
Wetting agents are required for hydrophobic solids. Tincture of quillaia is the traditional material (see Example 33.1), but alcohol alone may be effective. Synthetic materials, such as Manoxol OT, can also be used.
Humectants are materials added to reduce the rate of water loss from creams and gels. They are all hygroscopic materials and include glycerol, propylene glycol, PEG 300 and sorbitol syrup, typically used at concentrations of 5–15%.
Solids may be added to semi-solid occlusive bases. They provide channels for the migration of water from the skin surface and so reduce the occlusiveness. Solids used include zinc oxide, talc, starch and Aerosil. Some, such as talc, must be sterilized to kill bacterial spores (see Ch. 35).
Whenever there is a danger of microbial growth, antimicrobial preservation is required.
Dispensing of external preparations
A wide range of dispensing techniques are used in compounding external medicines, some of which have been reviewed in other chapters (see Chs 25, 30, 31, 32 and 35). In the section that follows, only those types of product which require different dispensing techniques are described in detail.
Dusting powders
A simple mixing in a mortar and pestle using ‘doubling-up’ is used (see Ch. 35). Sieving may be necessary to disperse aggregates of cohesive powders. A 180 μm sieve should be used. Powders such as starch, which contains a lot of moisture, may need drying to ensure optimum flow properties. With coloured materials, considerable working with the pestle is required before proceeding to ‘doubling-up’ otherwise a speckled product may result. A liquid may be added by pipette to a small quantity of the powder and be worked in before further mixing. A worked example of a dusting powder is given in Example 35.3.
Liquid preparations
These include solutions, suspensions and emulsions. The same basic dispensing techniques employed in making the corresponding oral systems are used (see Chs 30, 31 and 32). Most liquid preparations are used unsterilized, but if they are intended for application to broken skin, eyes or body cavities, they should be sterilized. They should be packed in ribbed bottles, labelled ‘For external use only’, and carry a ‘Shake the bottle’ label if they are emulsions or suspensions. Worked examples are given of a lotion in Example 31.5 and of an oily lotion in Example 32.5.
Action and uses. This is used as a treatment for acne, scabies and as a mild antiseptic.
Formulation notes. This an example of a shake lotion, an aqueous suspension prepared without a suspending agent, but including a wetting agent for the hydrophobic sulphur.
Method of preparation. Sieve the precipitated sulphur. Weigh out 4 g and place in a glass mortar. Using a 1 mL pipette, add 0.5 mL quillaia tincture and work well into the sulphur using a pestle. Add 6 mL of industrial methylated spirits followed by 2 mL glycerol, working in after each addition (thus achieving maximum wetting before water is added). Add 20–30 mL calcium hydroxide solution to produce a pourable suspension. Transfer to a tared bottle. Rinse the mortar with calcium hydroxide solution, adding it to the bottle, before making up to volume.
Shelf life and storage. There are no special requirements for storage. An expiry date of 4 weeks is suitable.
Action and uses. Methyl salicylate is a rubefacient, used to treat muscular aches and pains.
Formulation notes. The methyl salicylate requires to enter the skin. The vegetable oil, arachis oil, is used as the solvent to assist in this process. Other similar fixed oils can be used.
Method of preparation. Measure 25 mL of methyl salicylate in a 100 mL measure and add arachis oil to make up to volume. Transfer to a dry 100 mL amber ribbed bottle.
Shelf life and storage. This liniment should be kept in a well-closed container in a cool place. An expiry date of 4 weeks is appropriate.
Action and uses. Both turpentine oil and camphor are rubefacients which are rubbed into the skin to relieve muscular aches and pains.
Formulation notes. The BP formula adds up to 1000. However, this is a mixture of weights and volumes so the final volume is not known. It is usual to calculate in the ratio 120 ‘units’ per 100 mL.
This is an emulsion made using an alkali soap. When using soft soap, it is usual to use it at 10% by weight of an oil (as in this example), or 20% by weight of a fat.
Method of preparation. Weigh the camphor and place in a porcelain mortar. Grind it to a small particle size. Choose the softer, greener parts of the soap. Weigh (on a piece of paper) and mix thoroughly with the camphor. Measure the turpentine oil and add small aliquots (5–10 mL at first) to the soap and camphor followed by thorough mixing. When an even, pourable dispersion is obtained, transfer this to a 250 mL stoppered measuring cylinder. Use the remaining oil to rinse the mortar and add to the cylinder. Measure the water and add it, as quickly as possible, to the measure, stopper and shake it vigorously until a creamy white emulsion is formed. Allow it to stand for a few minutes (for air bubbles to separate) before transferring 100 mL to a tared bottle. Avoid plastic containers because turpentine reacts with some plastics.
Shelf life and storage. There are no special storage requirements. An expiry date of 4 weeks is appropriate.
Action and uses. Benzyl benzoate is a liquid insecticide used for treating scabies and lice. It is usually applied with a brush over the whole body. It should not be applied to broken or inflamed skin.
Formulation notes. Benzyl benzoate is water immiscible and is being emulsified using the anionic Emulsifying Wax BP. The application is an o/w emulsion.
Method of preparation. Weigh the emulsifying wax and place it in an evaporating basin on a water bath or hot plate to melt. Add the benzyl benzoate and mix and warm. Warm about 75 mL of the water to the same temperature. Add about half of this to the evaporating basin and mix very gently. Transfer the mixture, again very gently to avoid frothing, to a tared bottle. Add warmed water to volume. Close the bottle and shake vigorously. Care is required to avoid frothing when water is present, because it will be very difficult to make up to the tare mark when froth has formed. Shake frequently during cooling.
Shelf life and storage. The application should be kept in a cool place, but not be allowed to freeze. An expiry date of 4 weeks is appropriate.
Semi-solid preparations
Mixing by fusion
The compounding of many semi-solid preparations includes the blending together of oily materials, some of which are solids at room temperature. The process called ‘mixing by fusion’ achieves this. As the name implies, it involves melting the ingredients together (see Example 33.5). The process is carried out in an evaporating basin on a water bath or hot plate. It should be noted that a high temperature is not required so 60–70°C is usually adequate. Waxy solids should be grated before weighing and should be added first, so that melting can start while other ingredients are being measured. When all the ingredients are melted, remove the basin from the water bath and gently stir until cold. Mixing, which should be gentle to avoid air bubbles, is necessary to avoid lumps forming. This could happen because the higher melting point ingredients in the eutectic system may precipitate out. Any medicament may be added at different stages of preparation depending on its properties. If soluble and stable, it can be added when the base is molten. If it is less stable, or insoluble but easy to disperse, it can be added during cooling. However, if it is unstable or if dispersion is difficult, it should be added when cold using mixing by trituration.
Example 33.5
| Master formula | For 60 g | |
| Wool fat | 50 g | 3 g |
| Hard paraffin | 50 g | 3 g |
| Cetostearyl alcohol | 50 g | 3 g |
| Yellow or white soft paraffin | 850 g | 51 g |
When evaporating basins are being used, recovery of all the product is not possible. Thus, in order to be able to pack the prescribed amount, it is necessary to make an excess of about 10%.
Action and uses. Simple ointment is used as an emollient, or for making other ointments.
Formulation notes. This is a simple blend of solid and semi-solid oily ingredients made by fusion. Yellow or white soft paraffin is chosen according to the colour of the finished product. In this case, since there is nothing else to be added, white soft paraffin should be used; 60 g is made to allow 50 g to be dispensed.
Method of preparation. Grate the hard paraffin and cetostearyl alcohol. Weigh 3 g of each and place in an evaporating basin on a water bath or hot plate. Weigh the wool fat, using a piece of paper to allow full recovery of the material, and add it to the evaporating basin, followed by the soft paraffin (also weighed on paper). Stir gently until fully melted. Remove from the heat and continue to stir gently until cold. Weigh 50 g of base into a tared ointment jar or pack into a collapsible tube (see Example 33.10). If an ointment jar is used, a greaseproof paper disc should be placed on the surface of the ointment to protect the liner of the lid from the greasiness.
Example 33.10
A method for filling a collapsible tube extemporaneously.
Shelf life and storage. Store in a cool place. An expiry date of 4 weeks is appropriate.
Mixing by trituration
Insoluble solids or liquids are incorporated into bases using the technique called ‘mixing by trituration’. Any powders should be passed through a 180 μm sieve before weighing to avoid grittiness in the finished product. Mixing by trituration is carried out on an ointment slab or tile, which may be made of glass or glazed porcelain. A flexible spatula is used to work the materials together. Powders are placed on the tile and incorporated into the base using ‘doubling-up’ as it is worked in. However, it is usually necessary to have two to three times the volume of base to powder, otherwise it will ‘crumble’. Liquids, if present, are usually present in small amounts. To incorporate a liquid, a portion of the base is placed on the slab and a recess made to hold the liquid which is then worked in gently. Larger quantities of liquid should be added a little at a time using the same method. In theory it is possible to recover all material from the slab, but it is normal to allow up to 10% excess for losses. These processes can be carried out in a mortar with a flat base using a pestle with a flat head. However, because recovery of the product is difficult, this is usually reserved for larger-scale batches.
Action and uses. The ointment is used to treat acne and scabies.
Formulation notes. The BP directs that the simple ointment be prepared with white soft paraffin. If simple ointment is available, the trituration can be carried out on a slab and all the product recovered. However, if simple ointment is also being made, 50 g should be adequate to ensure that 45 g are available. Precipitated sulphur, while of smaller particle size than sublimed sulphur, can give a gritty feel unless it is passed through a 180 μm sieve.
Method of preparation. Sieve and then weigh out the precipitated sulphur and place it on the slab. Weigh out the simple ointment (using a piece of paper to prevent it sticking to the balance), and place it on a different part of the slab. Take a portion of the sulphur and a portion of the base of about three times the volume of the sulphur and work them together vigorously until there is no sign of any particles of sulphur. Spreading a thin layer on the slab helps check this. Gradually add the remaining sulphur and base. Collect the ointment together on the slab using the spatula and pack 50 g.
Shelf life and storage. Store in a cool place. An expiry date of 4 weeks is appropriate.
Action and uses. Methyl salicylate is a volatile material used as a rubefacient.
Formulation notes. Methyl salicylate is a liquid. With the high proportion present, the product would be runny without the addition of the beeswax as a thickening agent. The base ingredients require to be blended by fusion.
Method of preparation. Grate and weigh the beeswax. Melt it with the hydrous wool fat (weighed on a piece of paper) in an evaporating basin on a water bath or hot plate. Remove from the heat and stir until almost cold before adding the methyl salicylate (it is volatile). Continue stirring until cold. Pack 30 g in a glass ointment jar (plastic should be avoided with methyl salicylate).
Shelf life and storage. Store in a cool place. An expiry date of 4 weeks is appropriate.
Creams
Creams are emulsified preparations containing water. They are susceptible to microbial growth which may cause spoilage of the cream or disease in the patient. While preservatives are included, they are usually inadequate to cope with a heavy microbial contamination and so the possibility of microbial contamination during preparation should be minimized. Ideally aseptic techniques should be used, but this is not normally possible in extemporaneous dispensing and so thorough cleanliness is employed. As a minimum, all apparatus and final containers should be thoroughly cleaned and rinsed with freshly boiled and cooled purified water, then dried just prior to use. Swabbing of working surfaces, spatulas and other equipment with ethanol will also reduce the possibility of microbial contamination.
The basic method of making an emulsified cream is to warm both the oily phase and aqueous phase separately to a temperature of about 60°C, mix the phases and stir until cold. It is important that the temperatures of the two phases are within a few degrees of each other and it is advisable to use a thermometer to check this. Rapid cooling will cause the separation of high melting point materials, and excessive aeration as a result of vigorous stirring will produce a granular appearance in the product. Medicaments may, if they are stable, be dissolved in the appropriate phase before emulsification, or can be added by trituration when cold.
Action and uses. Aqueous cream is an emollient and can be used as a base for drugs.
Formulation notes. This is an o/w cream made using an anionic emulsifying agent. To reduce the risk of microbial contamination, all equipment should be washed before use. Phenoxyethanol is present as an antimicrobial preservative. It is a liquid, so has to be weighed, or, if its density is obtained, it could be measured by pipette. If the emulsifying ointment has to be made, exactly 16.5 g can be made because the emulsification can be carried out in the same evaporating basin.
Method of preparation. The phenoxyethanol is dissolved in the water warmed to 60°C. Weigh the emulsifying ointment (using a piece of paper to prevent it sticking) and melt it in an evaporating basin on a water bath or hot plate. Ensure that both phases are close to 60°C, then add the aqueous phase to the melted ointment. Remove from the heat and stir continuously until cold, taking care not to incorporate too much air. Weigh 50 g and pack in an ointment jar or collapsible tube.
Shelf life and storage. The preparation should be stored in a cool place, but not allowed to freeze. A shelf life of 2–3 weeks is appropriate because the preparation has not been made in the cleanest conditions.
Actions and uses. Oily cream is used as an emollient in treating dry skin conditions.
Formulation notes. This is a w/o cream prepared using wool alcohols as the emulsifying agent. Phenoxyethanol is present as preservative, but all equipment should be washed before use. Phenoxyethanol is a liquid and so must be weighed, or, if its density is obtained, it can be measured by pipette. Quantities for 55 g produce amounts that cannot be weighed on a dispensing balance, so 60 g is made. If the wool alcohols ointment is also to be made, exactly 30 g is adequate, because it does not have to be removed from the evaporating basin.
Method of preparation. All equipment should be thoroughly cleaned before use. Dissolve the magnesium sulphate and phenoxyethanol in the water and warm to 60°C on a water bath or hot plate. Weigh the wool alcohols ointment, using a piece of paper, and melt it in an evaporating basin at 60°C. Check that the two temperatures are the same. Add the water, little by little, to the ointment, stirring constantly until a smooth creamy mixture is produced, while maintaining the temperature at 60°C. When all the water is added, remove from the heat and stir gently until the cream is at room temperature. Pack 50 g in an ointment jar or collapsible tube.
Shelf life and storage. Store in a cool place but do not allow to freeze. If liquid separates on storage, stirring may reincorporate it. An expiry date of 4 weeks is appropriate.
Dilution of creams
It is sometimes necessary to prepare a dilution of a commercially produced cream, although the practice is undesirable. Choice of diluent is crucial, since the diluent may impair the preservative system in the cream, may affect the bioavailability of the medicament, or be incompatible with other ingredients. The process of dilution also increases the risk of microbial contamination. Thus, dilutions should only be made with the diluent(s) specified in the manufacturer’s data sheet and heat must be avoided. All diluted creams should be freshly prepared and be given a 2-week shelf life.
Pastes
Pastes are dispersions of high concentrations of solid in either an aqueous or oily vehicle. They can be used to treat infections by making use of their high osmotic pressure, or as very thick materials to prevent irritant drugs spreading over the skin surface. Incorporation of the solid is by mixing on an ointment slab.
Action and uses. Dithranol is used to treat psoriasis. There are two strengths of dithranol paste, ‘weak’ is 0.1% and ‘strong’ is 1%, although a range of intermediate strengths are prescribed by dermatologists.
Formulation notes. The Lassar’s paste has to be made first before incorporating the dithranol. Dithranol is prone to oxidation, so contact with metal should be avoided. Gloves should be worn during preparation
Method of preparation. Sieve the zinc oxide and salicylic acid through a 180 μm sieve before weighing. Weigh the soft paraffin (on a piece of paper) and melt in an evaporating basin on a water bath. Take some of the powder and stir into the melted base. Continue until all the powder is added, then stir gently until cold. Weigh out the Lassar’s paste (using paper to avoid sticking). Only when the Lassar’s paste has been completed, weigh out the dithranol. Care is required because it is very irritant to skin. Place it on a slab and incorporate it in a small portion of the paste using a plastic spatula, ensuring that a smooth, even product is produced. Dilute gradually with the remainder of the paste. Pack in a 120 g (4 ounces) brown ointment jar, with a circle of greaseproof paper and a tight-fitting closure, or a collapsible tube. The label should include the words ‘To be spread thinly’ (British National Formulary Label 28).
Shelf life and storage. The product should be kept in a cool place, protected from light. An expiry date of 2 weeks is appropriate because of chemical instability.
Transdermal delivery systems
Transdermal drug delivery systems aim to provide continuous drug release over a period of time which can be from a few hours to 7 days.
The principle of this dosage form is that, by optimization of physicochemical factors, the drug is absorbed through the skin into the systemic circulation. Absorption through the skin is variable so the rate of release of the drug must be controlled to a slower rate than the skin can absorb it. This may be achieved either by using a matrix system or a rate-limiting membrane. These devices are commonly known as ‘patches’. Further details about the pharmaceutics of the patches are given in Aulton (2007). There is also a glyceryl trinitrate ointment (Percutol) which gives systemic activity.
Drugs available as transdermal therapeutic systems include:
Advantages
Although these are benefits, various problems are associated with this type of dosage form. For these reasons, few drugs so far have been formulated in this way.
Disadvantages
Method of use
It is important that patients are informed how to use patches correctly. All patients who purchase or are prescribed patches should be given the following information about their use: