Chapter 30 Solutions
Introduction
Solutions are homogeneous mixtures of two or more components. They contain one or more solutes dissolved in one or more solvents, usually solids dissolved in liquids. The solvent is often aqueous but can be oily, alcoholic or some other solvent.
There are many types of pharmaceutical solutions, based on their composition or medical use. Solutions may be used as oral dosage forms, mouthwashes, gargles, nasal drops and ear drops and externally as lotions, liniments, paints, etc. Solutions may also be used in injections and ophthalmic preparations, which are discussed in Chapters 38 and 39 respectively.
Solutions for oral dosage
Oral solutions are usually formulated so that the patient receives the usual dose of the medicament in a conveniently administered volume, 5 mL or a multiple thereof, given to the patient using a 5 mL medicine spoon. The term ‘teaspoon’ and ‘tablespoon’ should not be used as expressions of a dose for an oral liquid because they are not accurate measures.
Advantages of solutions for oral use over a solid dosage form are that liquids are much easier to swallow than tablets or capsules and the medicament is readily absorbed from the gastrointestinal tract. Ease of taking is especially useful for children, elderly patients or those with chronic conditions such as Parkinson’s disease, who may have difficulty swallowing a solid oral dosage form. An advantage of solutions over suspensions is that the medicament is dispersed homogeneously throughout the preparation, without the need to shake the bottle. This makes the preparation easier for the patient to use and should ensure consistent dosage. Sometimes substances with a low aqueous solubility may be made into solution by the addition of another solvent rather than formulate the medicine as a suspension.
Disadvantages of solutions are that they are bulky and not as convenient to carry around as a solid dosage form. They are also less microbiologically and chemically stable than their solid counterparts. Drugs that have an unpleasant taste may not be suitable for administration as an oral solution. The accuracy of oral dosage is dependent on the patient measuring the dose carefully.
The different forms of oral solutions are:
Containers for dispensed solutions for oral use
Plain, amber medicine bottles should be used, with a reclosable child-resistant closure. Exceptions to this are if the medicine is in an original pack or patient pack, if there are no suitable child-resistant containers for a particular liquid preparation or if the patient requests it, e.g. if they have severe arthritis in their hands. Advice to store away from children should then be given. A 5 mL medicine spoon or an appropriate oral syringe should be supplied to the patient.
Special labels and advice for dispensed oral solutions
An expiry date should appear on the label for extemporaneously prepared solutions. Most ‘official’ mixtures and some oral solutions are freshly or recently prepared (see Ch. 43). ‘Official’ elixirs and linctuses and manufactured products are generally more stable, unless diluted. Diluted products generally have a shorter shelf life than the undiluted preparation. Linctuses should be sipped and swallowed slowly, without the addition of water.
Solutions for other pharmaceutical uses
Topical solutions for external use are considered in Chapter 33. Some topical solutions are designed for use in body cavities, such as the nose, mouth and ear.
Mouthwashes and gargles
Gargles are used to relieve or treat sore throats and mouthwashes are used on the mucous membranes of the oral cavity, rather than the throat, to refresh and mechanically clean the mouth. Both are concentrated solutions, although gargles tend to contain higher concentrations of active ingredients than mouthwashes. Both are usually diluted with warm water before use. They may contain antiseptics, analgesics or weak astringents. The liquid is usually not intended for swallowing. Examples are Phenol Gargle BPC and Compound Sodium Chloride Mouthwash BP (see Example 30.7). Proprietary examples are chlorhexidine (Corsodyl®) mouthwash and povidone-iodine (Betadine®) mouthwash.
Nasal solutions
Most nasal preparations are solutions, administered as nose drops or sprays. They are usually formulated to be isotonic to nasal secretions (equivalent to 0.9% normal saline) and buffered to the normal pH range of nasal fluids (pH 5.5–6.5) to prevent damage to ciliary transport in the nose. The most frequent use of nose drops is as a decongestant for the common cold or to administer local steroids for the treatment of allergic rhinitis. Examples are normal saline nose drops and ephedrine nose drops, 0.5% or 1%. Overuse of topical decongestants can lead to oedema of the nasal mucosa and they should only be used for short periods of time (about 4 days) to avoid rebound congestion, called rhinitis medicamentosa. The nasal route may also be useful for new biologically active peptides and polypeptides which need to avoid the first pass metabolism and destruction by the gastrointestinal fluids. The nasal mucosa rapidly absorbs medicaments applied there to give a systemic effect. There are some products utilizing nasal delivery currently available on the market, e.g. insulin (Exubera®) and desmopressin (e.g. Desmospray®, DDAVP®), used in the treatment of pituitary diabetes insipidus. Accurate dosage is achieved using metered spray devices.
Ear drops
Ear drops are solutions of one or more active ingredient which exert a local effect in the ear, e.g. by softening earwax or treating infection or inflammation. They may also be referred to as otic or aural preparations. Propylene glycol, oils, glycerol (to increase viscosity) and water may be used as vehicles. Examples are aluminium acetate ear drops, almond oil ear drops and Sodium Bicarbonate Ear Drops BP (see Example 30.8).
Containers for nasal and aural preparations
Nose and ear drops that are prepared extemporaneously should be packed in an amber, ribbed hexagonal glass bottle which is fitted with a teat and dropper. Manufactured nasal solutions may be packed in flexible plastic bottles which deliver a fine spray to the nose when squeezed, or in a plain glass bottle with a pump spray or dropper. Manufactured ear drops are usually packed in small glass or plastic containers with a dropper.
Special labels and advice for nasal and aural preparations
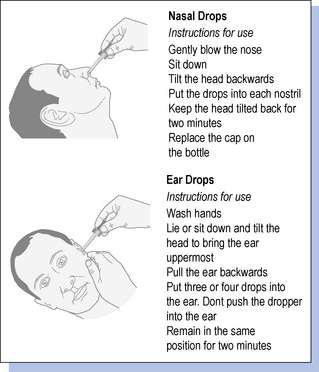
Patients should be advised not to share nasal sprays or nose and ear drops in order to minimize contamination and infection. Manufactured nasal sprays and nose and ear drops will usually contain instructions for administration. Patients should be given advice on how to administer extemporaneously prepared nose and ear drops, accompanied by written information if possible (Fig. 30.1). For nose drops, it may be easier if the patient is lying flat with the head tilted back as far as comfortable, preferably over the edge of a bed. The patient should remain in this position for a few minutes after the drops have been administered to allow the medication to spread in the nose.
For ear drops, it may be easier for someone other than the patient to administer the drops. If desired, the drops can be warmed by holding the bottle in the hands before putting them in, but they must not be overheated. The ear lobe should be held up and back in adults, down and back in children, to allow the medication to run in deeper. They may cause some transient stinging. If the drops are intended to soften earwax, then the ears should be syringed after several days of use.
Extemporaneous preparations should be labelled with the appropriate expiry date following the official monographs. ‘For external use’ is not an appropriate label and so ‘Not to be taken’ is advised.
Enemas
Enemas are oily or aqueous solutions that are administered rectally. They are usually anti-inflammatory, purgative, sedative or given to allow X-ray examination of the lower bowel. Examples are arachis oil enema and magnesium sulphate enema. Retention enemas are administered to give either a local action of the drug, e.g. prednisolone, or for systemic absorption, e.g. diazepam. They are used after defecation. The patient lies on one side during administration and remains there for 30 minutes to allow distribution of the medicament. Microenemas are single-dose, small-volume solutions. Examples are solutions of sodium phosphate, sodium citrate or docusate sodium. They are packaged in plastic containers with a nozzle for insertion into the rectum. Large-volume (0.5–1 litre) enemas should be warmed to body temperature before administration.
Expression of concentration
Strengths of pharmaceutical solutions can be expressed in a number of ways. The two most commonly used are in terms of amount of drug contained in 5 mL of vehicle or percentage strength. Thus, for example, a 100 mg/5 mL solution contains 100 mg of drug in each 5 mL. The volume 5 mL is used because it is the commonest oral dose administered. A percent weight in volume (% w/v) describes the number of grams of a constituent in 100 mL of preparation. For example, a 2% w/v preparation contains 2 g of a constituent in 100 mL of preparation. A percent volume in volume (% v/v) describes the number of millilitres of a constituent in 100 mL of preparation. For example, a 1% v/v preparation contains 1 mL of a constituent in 100 mL of preparation (see also Ch. 26).
Formulation of solutions
Solutions comprise the medicinal agent in a solvent as well as any additional agents. These additional agents are usually included to provide colour, flavour, sweetness or stability to the formulation. Most solutions are now manufactured on a large scale although it may be occasionally required to make up a solution extemporaneously. When compounding a solution, information on solubility and stability of each of the solutes must be taken into account.
Chemical and physical interactions that may take place between constituents must also be taken into account, as these will affect the preparation’s stability or potency. For example, esters of p-hydroxybenzoic acid, which can be used as preservatives in oral solutions, have a tendency to partition into certain flavouring oils. This could reduce the effective concentration of the preservative agent in the aqueous vehicle of the preparation to a level lower than that required for preservative action.
Solubility
The saturation solubility of a chemical in a solvent is the maximum concentration of a solution which may be prepared at a given temperature. For convenience, this is usually simply called solubility. Solubilities for medicinal agents in a given solvent are given in the British Pharmacopoeia (BP) and Martindale and other reference sources. Solubilities are usually stated as the number of parts of solvent (by volume) that will dissolve one part (by weight or volume) of the substance. In other situations, words are used to describe the solubility (see Example 30.2). Using this information it is often possible to calculate whether a solution can be prepared. Most solutions for pharmaceutical use are not saturated with solute.
Vehicles
In pharmacy, the medium which contains the ingredients of a medicine is called the vehicle. In solutions, this is the solvent. The choice of a vehicle depends on the intended use of the preparation and on the nature and physicochemical properties of the active ingredients.
Example 30.2
Diazepam is described as being ‘very slightly soluble’ in water (which means 1 in 1000 to 1 in 10 000), ‘soluble’ in alcohol (which means 1 in 10 to 1 in 30) and ‘freely soluble’ in chloroform (which means 1 in 1 to 1 in 10).
This means that 1 g of diazepam will dissolve in between 10 and 30 mL of alcohol, but would need 1000–10 000 mL of water to dissolve, at a temperature of 20 °C.
Water as a vehicle
Water is the vehicle used for most pharmaceutical preparations. It is widely available, relatively inexpensive, palatable and non-toxic for oral use and non-irritant for external use. It is also a good solvent for many ionizable drugs. Different types of water are available as outlined below:
Other vehicles used in pharmaceutical solutions
Factors affecting solubility
Compounds that are predominantly non-polar tend to be more soluble in non-polar solvents, such as chloroform or a vegetable oil. Polar compounds tend to be more soluble in polar solvents, such as water and ethanol. The pH will also affect solubility, as many drugs are weak acids or bases. The ionized form of a compound will be the most water soluble, therefore a weakly basic drug will be most soluble in an aqueous solution that is acidic. Acid or alkali may therefore be added to manipulate solubility. Most compounds are more soluble at higher temperatures. Particle size reduction will increase the rate of solution.
Increasing the solution of compounds with low solubility
Cosolvency
The addition of cosolvents, such as ethanol, glycerol, propylene glycol or sorbitol, can increase the solubility of weak electrolytes and non-polar molecules in water. They are discussed in more detail in Aulton (2007).
Solubilization
Surfactants may be used as solubilizing agents. Above the critical micelle concentration (CMC), they form micelles which are used to help dissolve poorly soluble compounds. The dissolved compound may be in the centre of the micelle, adsorbed onto the micelle surface, or sit at some intermediate point, depending on the polarity of the compound. Examples of surfactants used in oral solutions are polysorbates, while soaps are used to solubilize phenolic disinfectants for external use.
Preservation of solutions
Most water-containing pharmaceutical solutions will support microbial growth unless this is prevented. Contamination may come from raw materials or be introduced during extemporaneous dispensing.
Preservatives may be added to the formulation to reduce or prevent microbial growth. Chloroform is the most widely used in oral extemporaneous preparations although there are disadvantages to its use, including its high volatility and reported carcinogenicity in animals. Use in the UK is limited to a chloroform content of 0.5% (w/w or w/v). For oral solutions, chloroform at a strength of 0.25% v/v will usually be incorporated as Chloroform Water BP. Alternatively, double strength chloroform water may be included in pharmaceutical formulae as half the total volume of the solution, to effectively give single strength chloroform water in the finished medicine (see Example 30.3). Benzoic acid at a strength of 0.1% w/v is also suitable for oral administration, as are ethanol, sorbic acid, the hydroxybenzoate esters and syrup. Some of the alternative preservatives have pH-dependent activity.
Example 30.3
 Ammonium and Ipecacuanha Mixture BP. Mitte 100 mL.
Ammonium and Ipecacuanha Mixture BP. Mitte 100 mL.
| Master formula | For 100 mL | |
| Ammonium bicarbonate | 200 mg | 2 g |
| Liquorice liquid extract | 0.5 mL | 5 mL |
| Ipecacuanha tincture | 0.3 mL | 3 mL |
| Concentrated camphor water | 0.1 mL | 1 mL |
| Concentrated anise water | 0.05 mL | 0.5 mL |
| Double strength chloroform water | 5 mL | 50 mL |
| Water | to 10 mL | to 100 mL |
Syrups can be preserved by the maintenance of a high concentration of sucrose as part of the formulation. Concentrations of sucrose greater than 65% w/w will usually protect an oral liquid from growth of most microorganisms by its osmotic effects. A problem with their use occurs when other ingredients are added to the syrup, as this decreases the sucrose concentration. This may cause a loss in the preservative action of the sucrose. Accidental dilution by, for example, using a damp bottle, may have a similar effect.
Preservatives used in external solutions include chlorocresol (0.1% w/v), chlorbutanol (0.5% w/v) and the parahydroxybenzoates (parabens).
Additional ingredients
Solutions that are intended for oral use may contain excipients such as flavouring, sweetening and, sometimes, colouring agents. These are added to improve the palatability and appearance of a solution for the patient. Stabilizing and viscosity enhancing agents may also be used.
Flavouring agents
Flavours added to solutions can make a medicine more acceptable to take, especially if the drug has an unpleasant taste. Selection of flavours is a complex process in the pharmaceutical industry. Flavours should be chosen to mask particular taste types, e.g. a fruit flavour helps to disguise an acid taste. The age of the patient should be taken into account when selecting a flavour, as children will tend to enjoy fruit or sweet flavours. Some flavours are associated with particular uses, e.g. peppermint is associated with antacid preparations. The flavour and colour should also complement each other. Extemporaneous medicines tend to use natural flavours added as juices (raspberry), extracts (liquorice), spirits (lemon and orange), syrups (blackcurrant), tinctures (ginger) and aromatic waters (anise and cinnamon). Some synthetic flavours are used in manufactured medicines.
Sweetening agents
Many oral solutions are sweetened with sugars, including glucose and sucrose. Sucrose enhances the viscosity of liquids and also gives a pleasant texture in the mouth. Prolonged use of liquid medicines containing sugar will lead to an increased incidence of dental caries, particularly in children. Attempts should be made to formulate oral solutions without sugar as a sweetening agent, using sorbitol, mannitol, xylitol, saccharin and aspartame as alternatives. Oral liquid preparations that do not contain fructose, glucose or sucrose are labelled ‘sugar free’ in the British National Formulary (BNF). These alternatives should be used where possible.
Colouring agents
Colouring agents are added to pharmaceutical preparations to enhance the appearance of a preparation or to increase the acceptability of a preparation to the patient. Colours are often matched to the flavour of a preparation, e.g. a yellow colour for a banana-flavoured preparation. Colour is also useful to give a consistent appearance where there is natural variation between batches. Colours can give distinctive appearances to some medicines, e.g. the green colour of the Drug Tariff formula of methadone mixture.
Colouring agents should be non-toxic and free of any therapeutic activity themselves. Natural colourants are most likely to meet this criterion and include materials derived from plants and animals, e.g. carotenoids, chlorophylls, saffron, red beetroot extract, caramel and cochineal. As with all natural agents, the disadvantage is that batches may vary in quality. Mineral pigments such as iron oxides are not often used in solutions because of their low solubility in water. Synthetic organic dyes such as the azo compounds are alternatives for colouring pharmaceutical solutions as they give a wide range of bright, stable colours. Colours appear in pharmaceutical formulae less often now, especially in children’s medicines. Some consumers see their use as unnecessary and some colouring agents, e.g. tartrazine, have been implicated in allergic reactions and hyperactivity of children. Additionally, coloured dyes in medicines can lead to confusion when diagnosing diseases, e.g. a red dye appearing in vomit could be wrongly assumed to be blood. In the European Union, colours are selected from a list permitted for medicinal products, with designated ‘E’ numbers between 100 and 180.
Stabilizers
Antioxidants may be used where ingredients are liable to degradation by oxidation, e.g. in oils. Those which are added to oral preparations include ascorbic acid, citric acid, sodium metabisulphite and sodium sulphite. These are odourless, tasteless and non-toxic.
Viscosity-enhancing agents
Syrups may be added to increase the viscosity of an oral liquid. They also improve palatability and ease pourability. Other thickening agents may also be used (see Ch. 31).
Oral syringes
If fractional doses are prescribed for oral liquids, they should not be diluted, but an oral syringe should be supplied with the dispensed oral liquid. The standard 5 mL or 10 mL capacity oral syringe is marked in 0.2 mL divisions to measure fractional doses. An adapter fits into the neck of all common sizes of the medicine bottle. Instructions should be supplied with the oral syringe. Shake the bottle and then remove the lid and insert the adapter firmly into the top of the bottle. Push the tip of the oral syringe into the hole in the adapter and turn the bottle upside down. Pull the syringe plunger to draw liquid to the appropriate volume. It may be desirable to indicate this on the syringe. Turn the bottle right way up and carefully remove the syringe, holding the barrel. Gently put the tip into the child’s mouth to be inside the cheek. Slowly and gently push the plunger in and allow the child to swallow the medicine before removing the syringe. Do not squirt the liquid or direct it towards the throat. After completing the process, remove the adapter and replace the cap on the bottle. The adapter and syringe should be rinsed and left to dry. Patient information leaflets are available to accompany the oral syringe.
Diluents
If a prescriber insists that a manufactured solution is diluted, then a suitable diluent must be selected. Information sources to obtain this information are the Medicines Compendium or the National Pharmaceutical Association (NPA) Diluent Directory. An indication of the expiry date for the diluted preparation is also given in these references. The dilution should be freshly prepared.
A short shelf life for a diluted solution may require patients to return to the pharmacy to collect the balance of their medication. This may happen, for instance, where an oral sodium chloride solution has been prescribed for 1 month. The solution has a 2-week expiry, and must therefore be supplied in two instalments. The patient, or their representative, should be issued with an owing slip, or some similar documentation. This should state the name of the patient, the pharmacy, the item and quantity of medicine owed and the date of issue. A record should also be kept in the pharmacy. Most computer labelling systems have the facility to handle ‘owings’.
Action and uses. Expectorant cough preparation. The benefit of expectorant mixtures is doubtful, but they may be useful as a placebo and they are inexpensive.
Formulation notes. Ammonium bicarbonate, ipecacuanha and camphor water are mild expectorants. Anise water acts as a mild expectorant and a flavouring agent. Liquid liquorice extract is used as a mild expectorant, flavouring and sweetening agent. Chloroform water acts as a sweetener and a preservative. Ammonium bicarbonate is soluble 1 in 5 of water, so will dissolve to give a solution. All other ingredients are liquids.
Method of preparation. Weigh the ammonium bicarbonate on a suitable balance and dissolve in approximately 15 mL water, in a 100 mL conical measure. Add the double strength chloroform water to this solution. Measure the other liquid ingredients and add to the solution. Make up to volume with water in the conical measure. Pack into an amber medicine bottle with a child-resistant closure. Polish the bottle and label, and provide a 5 mL spoon.
Shelf life and storage. Store in a cool, dry place. It is recently prepared, therefore a shelf life of 2–3 weeks is applicable.
Advice and labelling. ‘Shake well before use’. While this is not strictly required, it is good practice to include it.
Action and uses. A cough suppressant in terminal care.
Formulation notes. Oxymel is a solution of acetic acid, water and purified honey, used as a demulcent and sweetening agent in linctuses. Glycerol is also a demulcent and sweetener. Compound tartrazine solution is a colouring agent and syrup is a demulcent vehicle. Diamorphine is soluble 1 in 1.6 of water and 1 in 12 of alcohol, so a solution will be produced.
Method of preparation. Weigh 120 mg diamorphine on an appropriate balance. Transfer to a 200 mL measuring cylinder. Dissolve the diamorphine in the oxymel and glycerol. Add about 50 mL of syrup, then add the compound tartrazine solution. Transfer to a previously tared amber medicine bottle (see Ch. 25). Make up to volume with the syrup in the tared bottle in order to overcome difficulties in draining all the viscous mixture from a measure. Close with a child-resistant closure, polish and label the bottle and give a 5 mL medicine spoon or oral syringe with the medicine (depending on the dosage prescribed).
Shelf life and storage. Store in a cool, dry place. It is recently prepared, therefore a shelf life of 2–3 weeks is applicable.
Advice and labelling. ‘Shake well before use’. The linctus should be sipped and swallowed slowly, undiluted. ‘Warning. May cause drowsiness. Avoid alcoholic drink’ (BNF Label 2). Since this patient is terminally ill, they are unlikely to be driving or operating machinery so this part of the advisory label can be omitted. Alcohol should be avoided as this will increase the sedative effect.
Action and uses. For short-term use in insomnia.
Formulation notes. Chloral hydrate is soluble 1 in 0.3 of water and has an unpleasant taste. Blackcurrant syrup is used as a flavouring agent to mask this.
Method of preparation. Weigh 2 g chloral hydrate on a suitable balance. Transfer it to a 50 mL measuring cylinder and dissolve it in water. Add the blackcurrant syrup. Add some of the syrup (rinsing the measure used for the blackcurrant syrup). Transfer the mixture to a tared, 50 mL amber medicine bottle and make up to volume, to avoid loss of the viscous product in the measures. Polish and label the bottle and give a 5 mL medicine spoon or oral syringe with the medicine.
Shelf life and storage. Store in a cool, dry place. Chloral hydrate is volatile and sensitive to light. It is recently prepared and a shelf life of 2–3 weeks is appropriate.
Advice and labelling. ‘Shake well before use’ and BNF Labels 1 and 27. An appropriate dose for a child up to 1 year is one 5 mL spoonful to be given, well diluted with water, at bedtime. The parent should be advised that this might make the child drowsy.
Example 30.6
 200 mL Potassium Citrate Mixture BP.
200 mL Potassium Citrate Mixture BP.
| Master formula | For 200 mL | |
| Potassium citrate | 3 g | 60 g |
| Citric acid monohydrate | 500 mg | 10 g |
| Syrup | 2.5 mL | 50 mL |
| Quillaia tincture | 0.1 mL | 2 mL |
| Lemon spirit | 0.05 mL | 1 mL |
| Double strength chloroform water | 3 mL | 60 mL |
| Water | to 10 mL | to 200 mL |
Action and uses. Alkalinization of urine to relieve discomfort in mild urinary tract infections or cystitis.
Formulation notes. Citric acid and potassium citrate are the active ingredients; both are soluble 1 in 1 of water. Lemon spirit, which is lemon oil in alcoholic solution, is a flavouring agent. The oil tends to be displaced from solution in an aqueous medium, especially in the presence of a high concentration of salts. The quillaia tincture is a surfactant used to emulsify any displaced lemon oil. Syrup is a sweetening agent.
Example 30.7
 Compound Sodium Chloride Mouthwash BP. Mitte 500 mL.
Compound Sodium Chloride Mouthwash BP. Mitte 500 mL.
| Master formula | For 500 mL | |
| Sodium chloride | 1.5 g | 7.5 g |
| Sodium bicarbonate | 1 g | 5 g |
| Concentrated peppermint emulsion | 2.5 mL | 12.5 mL |
| Double strength chloroform water | 50 mL | 250 mL |
| Water | to 100 mL | to 500 mL |
Method of preparation. The solids should be size reduced, weighed and dissolved in the double strength chloroform water and syrup. The quillaia tincture should be added before the lemon spirit is added with stirring, so that immediate emulsification of the oil will be achieved if required. Make up to volume with water. Pack in an amber medicine bottle with a child-resistant closure. Polish and label the bottle and give a 5 mL medicine spoon with the medicine.
Shelf life and storage. Store in a cool, dry place. It is recently prepared, therefore a shelf life of 2–3 weeks is applicable.
Advice and labelling. ‘Shake well before use’. The medicine should be diluted with plenty of water (BNF Label 27).
Example 30.8
 10 mL Sodium Bicarbonate Ear Drops BP.
10 mL Sodium Bicarbonate Ear Drops BP.
| Master formula | For 10 mL | |
| Sodium bicarbonate | 5 g | 500 mg |
| Glycerol | 30 mL | 3 mL |
| Water | to 100 mL | to 10 mL |
Action and uses. Mechanically cleans and freshens the mouth.
Formulation notes. Concentrated peppermint emulsion is used as a flavouring and the chloroform water is a sweetener and preservative. Sodium chloride is soluble 1 in 3 of water and sodium bicarbonate is soluble 1 in 11 of water.
Method of preparation. The solids are weighed on a suitable balance and dissolved in a 500 mL conical measure in approximately 100 mL of water. Add the double strength chloroform water and the concentrated peppermint emulsion. Make up to volume with water. Pack in an amber ribbed bottle with a child-resistant closure. Polish and label the bottle.
Shelf life and storage. Store in a cool, dry place. It is recently prepared, therefore a shelf life of 2–3 weeks is applicable.
Advice and labelling. ‘Shake well before use’. The patient should be directed to use about 15 mL diluted in an equal volume of warm water, usually morning and night, unless otherwise directed. The solution should be used as a mouthwash and should not be swallowed, although reassure the patient that it is not harmful to swallow small amounts of the mouthwash.
Action and uses. For the softening and removal of earwax (usually prior to syringing with warm water).
Formulation notes. Sodium bicarbonate is soluble 1 in 11 of water. Glycerol is a viscous liquid used to thicken the drops, but presents problems in measuring the volume accurately.
Method of preparation. Weigh 500 mg sodium bicarbonate and dissolve in 6 mL of water, using a 10 mL conical measure. Carefully make up to 7 mL using water. Carefully add glycerol up to the 10 mL mark (this will result in 3 mL of glycerol being added to the solution). Pack in a 10 mL hexagonal, amber, ribbed bottle with a dropper. Polish and label the bottle on the three smooth sides.
Shelf life and storage. Store in a cool, dry place. The drops are recently prepared, therefore a shelf life of 2–3 weeks is applicable.
Advice and labelling. ‘Shake well before use’ and ‘Not to be taken’. The bottle may be warmed in the hands before placing drops in the ears. A patient information leaflet should be used to describe how to use the drops (see Fig. 30.1).