1 Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129.
2 Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290.
3 O’Malley DP. T-cell large granular leukemia and related proliferations. Am J Clin Pathol. 2007;127:850.
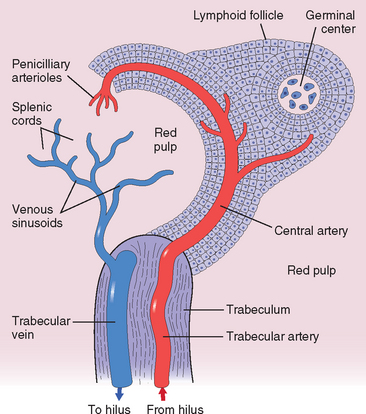
4 Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292.
5 Sagaert X, et al. The pathogenesis of MALT lymphomas: where do we stand? Leukemia. 2007;21:389.
6 Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700.
7 Polo JM, Melnick A. B-cell lymphoma 6 and the molecular pathogenesis of diffuse large B-cell lymphoma. Curr Opin Hematol. 2008;15:381.
8 Dorsett Y, et al. A role for AID in chromosome translocations between c-myc and the IgH variable region. J Exp Med. 2007;204:2225.
9 Ramiro AR, et al. Role of genomic instability and p53 in AID-induced c-myc-Igh translocations. Nature. 2006;440:105.
10 Pasqualucci L, et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341.
11 Harris NL, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting–Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835.
12 Szczepanski T. Why and how to quantify minimal residual disease in acute lymphoblastic leukemia? Leukemia. 2007;21:622.
13 Nabhan C, et al. Minimal residual disease in chronic lymphocytic leukaemia: is it ready for primetime? Br J Haematol. 2007;136:379.
14 Aster JC, et al. Notch signaling in leukemia. Annu Rev Pathol. 2008;3:587.
15 Mullighan CG, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758.
16 Greaves MF, et al. Leukemia in twins: lessons in natural history. Blood. 2003;102:2321.
17 Schultz KR, et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG). Blood. 2007;109:926.
18 Pfeifer H, et al. Kinase domain mutations of BCR-ABL frequently precede imatinib-based therapy and give rise to relapse in patients with de novo Philadelphia-positive acute lymphoblastic leukemia. Blood. 2007;110:727.
19 Calin GA, Croce CM. Genomics of chronic lymphocytic leukemia microRNAs as new players with clinical significance. Semin Oncol. 2006;33:167.
20 Bouley J, et al. New molecular markers in resistant B CLL. Leuk Lymphoma. 2006;47:791.
21 Endo T, et al. BAFF and APRIL support chronic lymphocytic leukemia B-cell survival through activation of the canonical NF-kappaB pathway. Blood. 2007;109:703.
22 Alinari L, et al. Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene. 2007;26:3644.
23 Tsimberidou AM, Keating MJ. Richter’s transformation in chronic lymphocytic leukemia. Semin Oncol. 2006;33:250.
24 Dave SS, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159-2169.
25 Kuppers R. Prognosis in follicular lymphoma—it’s in the microenvironment. N Engl J Med. 2004;351:2152.
26 Staudt LM, Dave S. The biology of human lymphoid malignancies revealed by gene expression profiling. Adv Immunol. 2005;87:163.
27 Abramson JS, Shipp MA. Advances in the biology and therapy of diffuse large B-cell lymphoma: moving toward a molecularly targeted approach. Blood. 2005;106:1164.
28 Parekh S, et al. BCL6 programs lymphoma cells for survival and differentiation through distinct biochemical mechanisms. Blood. 2007;10:2067.
29 Phan RT, et al. BCL6 interacts with the transcription factor Miz-1 to suppress the cyclin-dependent kinase inhibitor p21 and cell cycle arrest in germinal center B cells. Nat Immunol. 2005;6:1054.
30 Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432:635.
31 Hemann MT, et al. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature. 2005;436:807.
32 Dave SS, et al. Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med. 2006;354:2431.
33 Matsui W, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332.
34 Peacock CD, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci U S A. 2007;104:4048.
35 Anderson KC. Targeted therapy of multiple myeloma based upon tumor-microenvironmental interactions. Exp Hematol. 2007;35:155.
36 Terpos E, et al. Significance of macrophage inflammatory protein-1 alpha (MIP-1alpha) in multiple myeloma. Leuk Lymphoma. 2005;46:1699.
37 Hjertner O, et al. Bone disease in multiple myeloma. Med Oncol. 2006;23:431.
38 Bergsagel PL, Kuehl WM. Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol. 2005;23:6333.
39 Terpos E, et al. Clinical implications of chromosomal abnormalities in multiple myeloma. Leuk Lymphoma. 2006;47:803.
40 Zhan F, et al. The molecular classification of multiple myeloma. Blood. 2006;108:2020.
41 Stewart AK, Fonseca R. Prognostic and therapeutic significance of myeloma genetics and gene expression profiling. J Clin Oncol. 2005;23:6339.
42 Rajkumar SV, et al. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23:630.
43 Terpos E, et al. The effect of novel anti-myeloma agents on bone metabolism of patients with multiple myeloma. Leukemia. 2007.
44 Kyle RA, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356:2582.
45 Kyle RA, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362.
46 Kyle RA, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564.
47 Bergsagel PL, Kuehl WM. Chromosome translocations in multiple myeloma. Oncogene. 2001;20:5611.
48 Chiarle R, et al. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer. 2008;8:11.
49 Sun SC, Yamaoka S. Activation of NF-kappaB by HTLV-I and implications for cell transformation. Oncogene. 2005;24:5952.
50 Schmitz R, et al. Pathogenesis of classical and lymphocyte-predominant Hodgkin lymphoma. Annu Rev Pathol Mech Dis. 2009;4:151.
51 Kuppers R, Brauninger A. Reprogramming of the tumour B-cell phenotype in Hodgkin lymphoma. Trends Immunol. 2006;27:203.
52 Jungnickel B, et al. Clonal deleterious mutations in the IkappaBalpha gene in the malignant cells in Hodgkin’s lymphoma. J Exp Med. 2000;191:395.
53 Re D, et al. Molecular pathogenesis of Hodgkin’s lymphoma. J Clin Oncol. 2005;23:6379.
54 Joos S, et al. Classical Hodgkin lymphoma is characterized by recurrent copy number gains of the short arm of chromosome 2. Blood. 2002;99:1381.
55 de Bruijn MF, Speck NA. Core-binding factors in hematopoiesis and immune function. Oncogene. 2004;23:4238.
56 Scaglioni PP, Pandolfi PP. The theory of APL revisited. Curr Top Microbiol Immunol. 2007;313:85.
57 Kelly LM, et al. PML/RARalpha and FLT3-ITD induce an APL-like disease in a mouse model. Proc Natl Acad Sci U S A. 2002;99:8283.
58 Falini B, et al. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood. 2007;109:874.
59 Paschka P, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B Study. J Clin Oncol. 2006;24:3904.
60 Corey SJ, et al. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer. 2007;7:118.
61 Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355:2452.
62 Levine RL, et al. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders. Nat Rev Cancer. 2007;7:673.
63 Goldman JM, Melo JV. Chronic myeloid leukemia—advances in biology and new approaches to treatment. N Engl J Med. 2003;349:1451.
64 Mullighan CG, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453:110.
65 Druker BJ, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408.
66 Melo JV, Barnes DJ. Chronic myeloid leukaemia as a model of disease evolution in human cancer. Nat Rev Cancer. 2007;7:441.
67 Vannucchi AM, et al. Clinical profile of homozygous JAK2 617V > F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007;110:840.
68 Patel RK, et al. Prevalence of the activating JAK2 tyrosine kinase mutation V617F in the Budd-Chiari syndrome. Gastroenterology. 2006;130:2031.
69 Jelinek J, et al. JAK2 mutation 1849G > T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome-negative CML, and megakaryocytic leukemia. Blood. 2005;106:3370.
70 Annels NE, et al. Aberrant chemokine receptor expression and chemokine production by Langerhans cells underlies the pathogenesis of Langerhans cell histiocytosis. J Exp Med. 2003;197:1385.
71 Fleming MD, et al. Coincident expression of the chemokine receptors CCR6 and CCR7 by pathologic Langerhans cells in Langerhans cell histiocytosis. Blood. 2003;101:2473.