Chapter 26 Drugs That Modify Animal Behavior
Neurotransmitters and their Role in Abnormal Behaviors
Little is known about the cellular mechanisms of abnormal behavior in humans or animals. The most likely neurotransmitters (NTs) associated with abnormal behaviors might be identified based on the NTs targeted by drugs used to modify the behaviors. NTs identified for their role or potential role in abnormal behaviors include the biogenic amines serotonin and histamine (H1 subtype), the monoamine dopamine, the catecholamine norepinephrine, acetylcholine, gamma-aminobutyric acid (GABA), and the excitatory amino acids such as glutamate.1-3 Several of these neurotransmitters are discussed in Chapter 27.
Behavior also can be affected by other chemicals, including circulating hormones, opioids, and neurokinins.
The NT most commonly associated with abnormal behaviors is serotonin.4 Accordingly, the more effective drugs for modification of behavior are those (e.g., fluoxetine, clomipramine) that tend to be selective for serotonin. Serotonin is synthesized in the brain from tryptophan. Serotonin receptors differ in anatomic location and behavioral roles, with the majority of 5-hydroxytryptamine (5-HT) receptors being located in the gastrointestinal tract. Receptors in the central nervous system (CNS) are located in the raphe nucleus, the basal ganglia, and the limbic area. At least nine 5-HT receptor subtypes have been identified, four of which appear to be particularly important to behavior or mood.3 The 5–HT1 receptors, located primarily in the brain, are predominantly inhibitory (toward adenylyl cyclase) both presynaptically (autoreceptors; 1A and 1D) and postsynaptically (1A, 1D, 2A, 2C, and 3 and 4). Regulation of serotonin action is complex, involving both presynaptic and postsynaptic mechanisms3 with postsynaptic receptors regulating serotonin release from the presynaptic nerve endings. Serotonin may exert tonic inhibition on the dopaminergic system; drug-mediated increase may result in extrapyramidal effects.4 Badino and coworkers5 compared serotonergic and adrenergic receptor density in dogs neurologically normal except for aggressive behavior (n = 8) and normal, nonaggressive dogs (n = 8). The concentration of high affinity (serotonin) 5-HT receptors was increased in the thalamus, and low affinity 5-HT receptors were increased throughout the regions of the CNS studied (frontal cortex, thalamus, hypothalamus, hippocampus) in aggressive dogs compared with nonaggressive dogs. Riva and coworkers6 demonstrated that plasma levels of serotonin and dopamine were higher in anxious dogs (n = 22) compared with control dogs (n = 13).
KEY POINT 26-1
Drugs that are among the most effective and safest for behavior modification are those that tend to be most selective for serotonin.
In addition to serotonin, dopamine is also increased in dogs with anxiety.6 Dopamine is synthesized in neurons from l-DOPA in presynaptic vesicles. Synthesis begins with oxidation of the dietary amino acid tyrosine, followed by decarboxylation to l-DOPA.3 Those neurons in which dopamine is found lack the enzyme that converts dopamine to norepinephrine. Dopamine is removed by monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT). Dopamine receptors are distributed throughout the brain, although less so than norepinephrine receptors. Dopamine appears to be largely located in the midbrain, hypothalamus, and limbic system (the part of the brain thought to control emotions).3 Dopamine receptors (at least five subtypes) are found in portions of the extrapyramidal system responsible for coordinated movement.1,2 At least four dopamine receptors are affected by mood disorders and stereotypics; increased dopamine appears to stimulate these abnormal behaviors.1,2 Because dopamine generally suppresses acetylcholine activity, blockade of dopamine receptors generally results in an increase in acetylcholine release.4
Norepinephrine is the end product of dopamine oxidation. Its inactivation occurs primarily by active transport or reuptake into presynaptic vesicles. The NT is then deaminated by mitochondrial monoamine oxidases or catechol-o-methyltransferase in the presynaptic nerve terminal. Norepinephrine is located predominantly in the gray matter of the pons and in the medulla; projections in the locus ceruleus into the limbic cortex regulate emotions. Norepinephrine interacts postsynaptically with α1 receptors (G protein–mediated activation of phospholipase C and inositol triphosphate formation) and β receptors (activation of adenylyl cyclase) and presynaptically with α2 receptors (G proteins).The behavioral effects of norepinephrine appear to affect arousal, functional reward systems, and mood. The latter effect may reflect a decrease in depression and an increase in mania.
GABA is a major inhibitory NT, being active at 30% of synapses in the human CNS, and particularly in the cortex and thalamus. Its precursor glutamate is widely distributed throughout the brain. As with norepinephrine, dopamine, and serotonin, GABA has a presynaptic transporter. Two primary receptor types, GABAA and GABAB, appear to cause postsynaptic inhibition by facilitating chloride ion influx into the neuron. Several drugs, including the benzodiazepines and barbiturates (e.g., phenobarbital), interact with the GABAA receptor in an agonistic fashion, causing neuronal inhibition.1 The physiologic and behavioral effects of GABA and its receptors have not yet been totally characterized (see Chapter 27).3
Several abnormal human behaviors have been associated with changes in excitatory NTs, including aggressive, impulsive, and schizophrenic disorders. Among the more important excitatory NTs is glutamate. Glutamate is preformed and stored in synaptic vesicles released by calcium-mediated endocytosis. Barbiturates and progesterone modulate behaviors, in part by inhibiting calcium uptake and thus release of glutamate at the NT.1,2
Acetylcholine, the most widely distributed NT in the brain,3 is preformed from choline and acetyl coenzyme A and stored at the terminal end of the synapse in vesicles. Calcium stimulates its release by exocytosis into the synaptic cleft, where it is rapidly metabolized by acetylcholinesterase. Acetylcholine generally is an excitatory NT, interacting with nicotinic and muscarinic receptors. At least five muscarinic receptors are located in the CNS. Receptors (M1) are also found in the gastrointestinal and cardiovascular systems, accounting for some of the adverse effects associated with several behavior-modifying drugs.1
NTs tend to be formed and degraded locally. Both formation and inhibition offer pharmacologic targets. A number of drugs result in an increase in the presence of NTs in the synaptic cleft by inhibiting either the metabolism (e.g., dopamine) or the reuptake (e.g., serotonin, norepinephrine) of the NT after release (Figure 26-1).
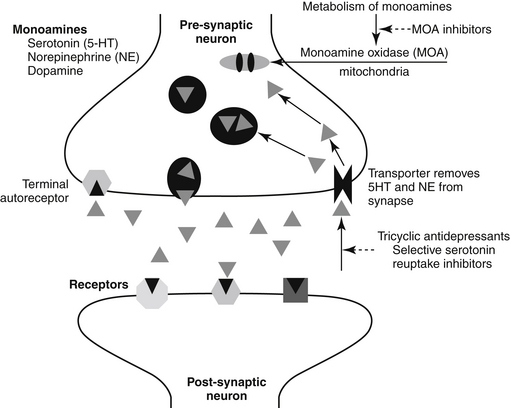
Figure 26-1 Mechanism of action of selected behavior-modifying drugs. Neurotransmitters responsible for behavior are released from the presynaptic neuron into the synaptic cleft and interact with postsynaptic receptors. Following exocytosis, inactivation of the transmitter occurs primarily by reuptake into the presynaptic neuron, the site of action of most behavior-modifying drugs. Activation also may involve metabolic degradation, as in the case of the monoamines (MOA).
Drugs used to Modify Behaviors
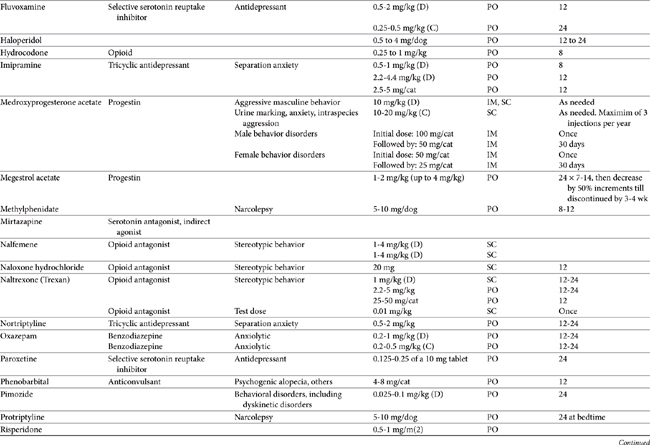
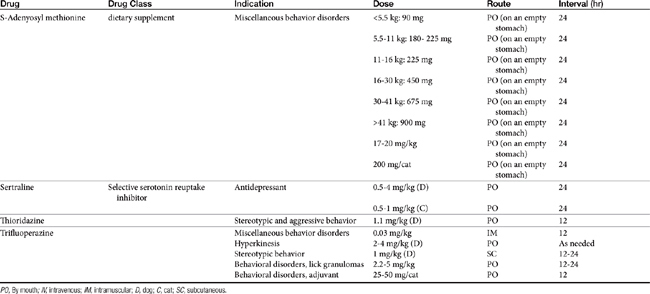
Drugs that modify behavior are classified initially on the basis of their (human) therapeutic use, followed by their chemistry and neurochemical activity.4 Included are the antipsychotic drugs (predominantly antidopaminergic in action), anxioselective drugs such as the azapirones (primarily antiserotonergic in action), drugs used to treat affective or mood disorders (antidepressants, lithium, and selected anticonvulsant drugs), and drugs used to treat anxiety and anxiety-related disorders (anxiolytics or minor tranquilizers, benzodiazepines). Other drugs include antihistamines, beta blockers, progestins, anticonvulsants, and opioid antagonists. Most of the drugs used to modify behaviors are used for treatment of other disorders, and as such may be discussed elsewhere in this volume. Only drugs that have veterinary application are discussed here. Table 26-1 provides dosing information about the use of these drugs.
KEY POINT 26-2
Drugs that modify the central nervous system should not be withdrawn suddenly. Extra precaution is indicated for drugs with short half-lives.
In general, the disposition of behavior-modifying drugs is complex, with characteristics that tend to be different among species. Oral absorption, first-pass metabolism, significant binding to serum proteins, profoundly large volumes of distribution, and metabolism to enantiomers and active metabolites (the latter potentially contributing to more activity than the parent compound) all contribute to these drugs’ lack of predictable behavior when dosing regimens are extrapolated among species. Hepatic metabolism increases the risk of both hepatotoxicity and drug interactions. The former, although rarely reported in humans, appears to be increasing and warrants additional attention as the use of these drugs increases in animals. Many behavior-modifying drugs are inhibitors of cytochrome P450 (CYP). Drug interactions at the level of CYP are well known in human medicine and should be anticipated in animals, particularly if drugs are used in combination. However, differences in CYP distribution and activity preclude predictable extrapolation of reported drug interactions among the species. The potentially toxic nature of these drugs places the animal patient further at risk because many dosing regimens are extrapolated from humans. Exceptions include clomipramine, fluoxetine and selegiline, each of which has been approved for use in dogs. The disposition of several drugs also has been reported in cats.
Antipsychotic Drugs
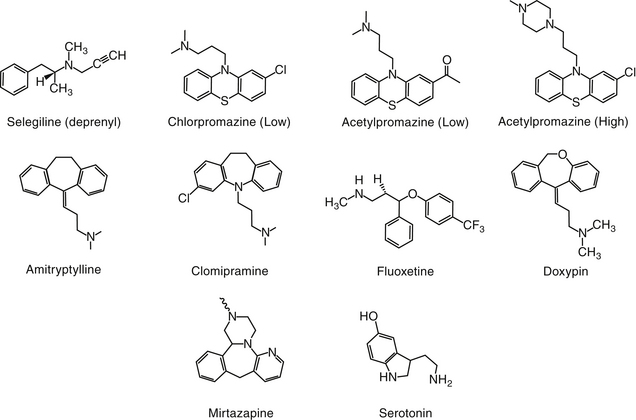
Psychotic disorders in humans involve a severe disturbance of brain function characterized by thought and speech disruption and hallucinations or delusions.3 Although psychotic disorders do not appear to occur in veterinary medicine, drugs developed for their management in humans have proved efficacious for a number of veterinary applications. Antipsychotic drugs (also called neuroleptics or major tranquilizers) are largely structurally dissimilar from one another, but the commonality among them is dopamine, and particularly D2 receptors, as a target. Drugs include the phenothiazines, the thioxanthenes (structurally related to the phenothiazines), heterocyclic dibenzepines, the butyrophenones, and diphenylbutylpiperidines (Figure 26-2).7
Structure–Activity Relationships
Antipsychotic drugs are categorized by structure and by potency. Low-potency drugs (chlorpromazine, acepromazine, promazine) are characterized by greater sedation and cardiac and anticholinergic side effects compared with their high-potency counterparts. High-potency drugs (e.g., haloperidol, fluphenazine, trifluoperazine, prochlorperazine, and thiothixene) are administered at lower doses and are associated with less sedation and fewer anticholinergic and cardiac side effects. They do, however, have a greater incidence of extrapyramidal side effects.
The largest structural class of antipsychotics is the phenothiazines or tricyclic antipsychotics, which should not be confused with the tricyclic antidepressant drugs (discussed later). The tricyclic antipsychotic drugs are represented by phenothiazine, a three-ring structure containing a sulfur and nitro group in the ring connecting two benzene rings. Substitutions on one of the benzene rings yield different drugs (e.g., chlorpromazine, promazine), which differ in efficacy (see Figure 26-2). The pharmacology also is affected by substitutions of nitro groups such that potency (but not efficacy) is reduced by an aliphatic side chain (e.g., chlorpromazine, thorazine, acepromazine, and trifluopromazine).7 The length of the side chain also determines antihistaminergic properties, with two-carbon side chains such as that occurring in promethazine being more antihistaminergic. Drugs with higher potency have a piperazine side chain, as can be found on fluphenazine and rifluoperazone. Esterification with long-chain fatty acids results in long-acting (due to slow hydrolysis and absorption) drugs (e.g., fluphenazine enanthate or decanoate).7 The use of these latter drugs in small animal veterinary medicine has yet to be established. The butyrophenone neuroleptics include haloperidol (the prototype) and droperidol. The latter is very short acting and highly sedative; thus its use is limited to anesthetic regimens.7
Pharmacologic Effects
The pharmacologic effects of antipsychotic drugs generally are similar among humans and animals.7 Phenothiazines are also categorized as tranquilizers. As tranquilizers, the phenothiazines are calming in nature, causing a decrease in spontaneous activity that generally decreases response to external stimuli.1 The predominant antipsychotic action of the phenothiazines is neuroleptic, a term derived from the effect of the drugs on human psychiatric patients and intended to contrast with signs typical of CNS depression.7 The neuroleptic effects are attributed, although not conclusively so, to the antidopaminergic effects at D2 receptors.7
Some of the neuroleptics (e.g., the phenothiazines) have high affinity for and thus also antagonize D1 dopamine receptors, although pharmacologic effects at these receptors appear to be minimal. Phenothiazines also block D3 and D4 (which are D2-like) receptors. Selected “atypical” antipsychotic drugs (e.g., clozapine) have a low affinity for D2 receptors and are not characterized by extrapyramidal effects. They are, however, characterized by α2-adrenergic antagonism. Some of the antipsychotic drugs also have affinity for serotonergic (5-HT2) receptors (e.g., clonazepam). Cholinergic and histaminergic (H1) receptors also are targeted by some of the drugs, resulting in unique pharmacologic effects among the neuroleptics. Variable interactions with different receptor types lead to unpredictable effects on the autonomic system. Among the neuroleptics, chlorpromazine has significant α-adrenergic antagonistic actions. In general, the antimuscarinic actions of neuroleptics are weak.
Neuroleptic effects include suppression of spontaneous movements or complex behaviors but minimal effects on spinal reflexes and unconditioned nociceptive avoidance behaviors. Interest in the environment is minimized (ataraxia), as are manifestations of emotion. Patients are easily aroused; ataxia or incoordination should not be evident at appropriate doses.7 Aggressive or impulsive behavior should gradually diminish. As a result, conditioned avoidance (but not unconditioned escape or avoidance) behavior and exploratory behavior are minimized. Feeding and emesis also are inhibited. At high doses, cataleptic immobility is evident (particularly in cats3), resulting in increased muscle tone (and the ability to place animals in an abnormal posture) and ptosis. Akathisia, an increase in restless activity, is an undesirable side effect that occurs in humans but apparently not in animals. Akathisia occurs as an adaptive response to increased phenothiazines in extrapyramidal tissues.7
The effects of phenothiazines occur throughout the CNS. Cortical effects are responsible for many of the neuroleptic actions. Antagonism of D2 receptors is largely responsible for the various extrapyramidal effects of the drugs. Many of these sites appear to be spared from the adaptive changes of tolerance.7 Neuroleptics have been associated with an increased incidence of seizures.8 Selected drugs lower seizure threshold as well as induce discharges typical of epileptic seizures. Aliphatic, low-potency phenothiazines are particularly characterized by this effect.7 Although the effect is more likely to occur in patients who are epileptic or who are predisposed to seizures, the effect is also a dose-dependent characteristic of some drugs. These drugs are contraindicated in human epileptic patients or patients undergoing withdrawal from central depressants.7 The relevance of this precaution to dogs is not clear. Both Tobias and coworkers9 and McConnell and coworkers10 retrospectively studied the frequency of seizures in epileptic dogs treated with low doses of acepromazine and found no increased risk (see also Chapter 27). Indeed, acepromazine was associated with decreased seizure activity in some of the patients that did not respond to other therapies. However, because the author experienced induction of seizures in a non-epileptic patient (lead poisoning) immediately after intravenous administration of acepromazine, caution is warranted when using phenothiazine derivatives in epileptic patients. Increasing doses slowly and accompanying with anticonvulsant therapy are indicated if the drugs must be used by epileptic patients.
The neuroleptic drugs have a number of effects in the limbic system. Although D2 antagonism occurs in the limbic system, because D3 receptor stimulation may be responsible for many of the behaviors targeted by neuroleptics, attempts are being made to identify D3-selective drugs for treatment of psychoses.7 Neuroleptics stimulate prolactin secretion in human beings. Indeed, the potency of neuroleptic action and ability to cause prolactin secretion are well correlated for most drugs. Pageat and coworkers11 demonstrated that prolactemia correlates with response to selected behavior-modifying drugs. Tolerance is not likely to develop to this side effect. In humans prolactin secretion caused by neuroleptics also is responsible for breast engorgement and galactorrhea. Releases of growth hormone and corticotropin-releasing hormone occur in response to stress; neuroleptics (especially chlorpromazine) also interfere with the release of growth hormone, although apparently not sufficiently for treatment of acromegaly. Impaired release of serotonin may result in weight gain (particularly with low-potency drugs), and impaired glucose tolerance and insulin release may be impaired in “prediabetic” patients (especially with chlorpromazine).7
In the brainstem the neuroleptics have little effect, even in cases of acute overdosing. Life-threatening coma is rare. In contrast, most neuroleptics protect against nausea and emesis at the chemoreceptor trigger zone in the medulla. These effects occur at low doses. Potent piperazines and butyrophenones are also often effective against nausea stimulated by the vestibular system.7
Phenothiazines characterized by lower potency have a predominant sedative effect that is more apparent initially but tends to decline as tolerance develops. The phenothiazines are characterized by anxiolytic effects, but more specific anxiolytic drugs are available. In addition, the risk of either autonomic (e.g., low-potency drugs) or extrapyramidal (e.g., highly potent drugs) effects increases the likelihood of causing anxiety.7
The neuroleptic drugs impart physiologic (especially cardiovascular) effects through peripheral actions. The effects are complex because the neuroleptics interact with a number of receptor types that have cardiovascular effects. Hypotension induced by phenothiazines—low potency in particular—reflects direct effects on the blood vessels, indirect actions in the CNS and autonomic receptors, and a direct negative inotropic effect on the heart. Chlorpromazine also has antiarrhythmic effects on the heart, similar to quinidine.
Disposition
The antipsychotics are characterized by variable bioavailability, high lipophilicity, high protein binding, and accumulation in a number of tissues. Elimination occurs primarily through hepatic metabolism. In humans the elimination half-life is long, ranging from 20 to 40 hours. Biologic effects persist for more than 24 hours, allowing once-daily therapy in humans.7 Metabolites can be detected in urine for several months.
Side Effects and Toxicity
The antipsychotic drugs tend to be very safe; lethal ingestion is rare in human patients. Side effects tend to reflect the pharmacologic actions of the drugs, including effects of the CNS and cardiovascular, endocrine, and autonomic systems.7 In human patients other effects include dry mouth, blurry vision, and constipation. Urinary retention may occur in male patients with prostatitis. At high doses antipsychotics may cause catalepsy, characterized by immobility, increased muscle tone, and abnormal postures.4 Extrapyramidal neurologic side effects reflecting inhibitionof dopamine occur in humans but have not been reported in animals. However, involuntary motor movements associated with extrapyramidal effects may be confused with seizures. The risk of seizures may be increased, but risk factors are not known (discussed previously). Some animals exhibit signs of hyperactivity after treatment with acepromazine.3 In addition, at least one report cites increased agitation and irritability after treatment of aggression with acepromazine.12 Jaundice has occurred in humans after taking chlorpromazine and may resolve despite continued treatment. Blood dyscrasias, including leukopenia, eosinophilia, and leukocytosis, occur but are less common with low-potency phenothiazines. Skin reactions tend to be common in people, again more commonly with low-potency phenothiazines. Small doses of acepromazine (0.1 mg/kg, given subcutaneously 30 minutes before testing) appear to have no effect on intravenous glucose tolerance testing in dogs.13 Acepromazine sedation (0.03 mg/kg given intramuscularly 30 minutes before measurement) markedly increased peak latencies but minimally affected peak amplitude of middle-latency auditory-evoked potential in dogs (n = 12).7,14
Drug Interactions
Chlorpromazine is used in combination anesthetic regimens because of its ability to potentiate central depressants. Effects of analgesics and sedatives also can be enhanced. Interactions with antihypertensive drugs can be unpredictable and are more likely to be adverse with low-potency products.7 Selected phenothiazines can antagonize the positive inotropic effects of digoxin.
Clinical Indications
In general, the use of phenothiazines for treatment of aggressive behavioral abnormalities is inappropriate because both normal and abnormal behaviors are blunted. Acepromazine is particularly problematic. Restraint of aggressive dogs with the drug renders dogs more likely to be reactive to noises and more easily startled.1 In addition, because the degree and duration of tranquilization vary, reactions in dogs are unpredictable. Phenothiazines are not selective as antianxiety drugs but can reduce responsiveness in general, and thus they are useful in some cases of episodic anxiety.3 Thioridazine has been used in one case of aberrant motor behavior.15 Newer atypical antipsychotics (e.g., risperidone) may prove useful for treatment of selected abnormal behaviors (e.g., environmental-specific anxieties, impulsive explosive disorders).4
Antidepressant Drugs
Much of the information regarding the use of these mood-modifying drugs in animals has been extrapolated from human use. These drugs are characterized by clinical pharmacology and mechanisms of action that are likely to differ markedly among animals. Yet limited scientific information is available to guide their use in animals. Among the human mood-modifying drugs that are approved for their use for treatment of behavioral disorders in either dogs or cats are the tricyclic antidepressants (TCAs) (clomipramine), the MAO inhibitors (selegiline), and selective serotonin reuptake inhibitors (SSRIs; e.g., fluoxetine). Affective (behavior) disorders targeted by antidepressants in humans range from depression to manic–depressive disorders. In animals the list of targeted disorders is much greater and may be publically perceived to include any type of behavior deemed unacceptable by pet owners.
For a better understanding of the pharmacologic actions (intended and undesirable) of these drugs, it is necessary to appreciate the extent to which NTs targeted by these drugs are active in the brain. Among the most commonly targeted NTs is the biogenic amine system, with norepinephrine, 5-HT (serotonin), and dopamine serving as primary targets. Acetylcholine and histamine are common, although generally secondary, targets. In addition, α-adrenergic receptors may be stimulated by some of these drugs. The pharmacologic and side effects of these drugs vary with the NT targeted. Drugs that are more specific in their actions tend to be safer. Inability to predict the effect of antidepressant drugs on behavior reflects in part the inability to predict effects at the synapse as well as a lack of knowledge regarding the impact of neurotransmission on behavior. In general, blockade of dopamine transport appears to be stimulatory rather than antidepressant whereas inhibition of either serotonin or epinephrine reuptake appears to produce an antidepressant effect.
Tricyclic Antidepressants
Structure–Activity Relationships
The TCAs are among the most frequently prescribed drugs in human behavior medicine. Their name reflects their chemical structure (see Figure 26-2). The TCAs were identified as a group of potentially useful drugs for the modification of behavior in the 1940s after the generation of a number of drugs with antihistaminergic, sedative, analgesic, and antiparkinsonian effects. Imipramine was selected on the basis of its hypnotic and sedative effects. Imipramine differs from phenothiazine only by the replacement of sulfur with an ethylene bridge, yielding a seven-member ring. This compound proved ineffective in quieting agitated psychotic patients but very effective for selected mood disorders.7
The search for chemically related compounds yielded a number of additional drugs. Clomipramine, amitriptyline, and doxepin are all derivatives of imipramine.7 Each contains a tertiary amine at one of the substitution sites on the seven-member ring. Desipramine, a major metabolite of imipramine, and nortriptyline, the N-demethylated metabolite of amitriptyline, are secondary amine tricyclics. Protriptyline and trimipramine are other TCAs with few veterinary applications.3 Differences in the effects of NT reuptake vary with the different amine structures.7
Mechanism of Action
The mechanism of action of the TCAs (and MAO inhibitors and SSRIs) is blockade of the mechanisms of physiologic inactivation (see Figure 26-1). For the TCAs the mechanism is inhibition of reuptake at presynaptic biogenic amine NT receptors in the brain. As reuptake is inhibited, the concentration of NTs increases, prolonging their actions (CNS stimulation). Differences in NTs affected reflect, in part, the chemical structures.7 Imipramine and its derivatives with a tertiary amine side chain block norepinephrine reuptake but have little effect on dopamine reuptake.
The secondary amine derivatives of imipramine are potent and highly selective inhibitors of norepinephrine reuptake; however, they are characterized by fewer autonomic and anticholinergic effects. Tertiary amines that are metabolized in the patient to secondary amines will have effects on norepinephrine reuptake. Clomipramine has marked effects on serotonin reuptake and minimal effects on other NTs or receptors, although a poorly understood dopaminergic effect has been described. Its active metabolite appears to inhibit norepinephrine uptake as well.16 Doxepin is characterized by greater antihistaminergic actions (thus explaining its frequent recommendation for chronic pruritus). Although amitriptyline is the most commonly prescribed drug for animals, clomipramine has recently been approved for treatment of separation anxiety in dogs.
The effect of clomipramine (3 mg/kg once daily orally for 6 weeks) on the concentration of monoamines (5HIAA [5-hydroxyindoleacetic acid], HVI [homovanillic acid] and MHPG [methoxy4-hydroxyphenylglycol]) in the cerebrospinal fluid was compared with that of placebo using a randomized crossover design in otherwise behaviorally normal dogs (n = 6) with obsessive–compulsive disorders (OCDs).16 The concentration of monoamines did not significantly differ (power of the study now known), leading the authors to conclude that clomipramine did not affect monoamine turnover in the brains of behaviorally normal dogs, further confirming the need for studies in affected dogs.
Pharmacologic Effects
Adaptation to pharmacologic effects
The effects of TCAs at presynaptic and postsynaptic receptors and autoregulation result in complex responses that are not well understood. Although inhibition of reuptake occurs very rapidly, peak effects still take several weeks. This prolonged time to maximal effect reflects in part disposition (e.g., time to steady-state for the parent compound and active metabolites; see later discussion of clinical pharmacology) but also appears to reflect adaptation in the CNS to changes in NT concentrations at the synapse. Administration of a TCA results in an immediate decrease in the synthesis and release of norepinephrine or serotonin (depending on the major target of the TCA) in selected areas of the brain. The effects appear to be mediated presynaptically through autoreceptors (α2 or serotonin, respectively). Turnover, however, gradually normalizes within 1 to 3 weeks. Autoreceptors appear to be downregulated and become desensitized to the presence of the TCA.7 The number and sensitivity of postsynaptic adrenergic receptors do not appear to be affected by continued use of a TCA.
Adaptive responses appear to influence the pharmacologic properties of TCAs at adrenergic receptor sites. The TCAs have a moderate affinity for α1-receptors and only limited affinity for α2-receptors and β-receptors. Changes in serotonin receptors after repeated treatment with TCAs are complex. Although the impact is not clear, the general effect appears to be increased sensitivity to serotonin. This may be an important component to the outcome of prolonged treatment.7 The TCAs also appear to influence the effect of other NTs and their receptors, including GABA (unknown significance), and dopamine (D2; desensitization of autoreceptors resulting in mood elevation). Other factors to consider regarding adaptation to TCA effects include changes in adenosine monophosphate–dependent protein kinases and potential changes at the level of gene expression.7
In addition to tolerance (to sedative and autonomic effects), physical dependence on the TCA can develop. Physical dependence after acute withdrawal is manifested in human patients as malaise, chills, coryza (head cold), and muscle aches.7 Slow discontinuation of the drug is recommended.
Autonomic nervous system
The predominant effect of TCAs on the autonomic nervous system appears to reflect inhibition of norepinephrine transport into adrenergic nerve terminals and antagonism of muscarinic cholinergic and α1-adrenergic responses to the NTs. Blurred vision, dry mouth, constipation, and urinary retention at therapeutic doses (documented in humans) appear to reflect anticholinergic effects.7
Cardiovascular system
Cardiovascular effects of TCAs occur at therapeutic doses and can become life threatening with overdose. Postural hypotension occurs in humans (unlikely in dogs or cats) as a result of α–adrenergic blockade. Mild sinus tachycardia results from inhibition of norepinephrine uptake and muscarinic (M1) blockade.7 Conduction time is prolonged, especially at concentrations above 200 ng/mL. The TCA can also directly suppress the myocardium.7 The myocardial depressant effects are greater in the presence of underlying cardiac disease.
Clinical Pharmacology
The disposition of the TCA favors adverse reactions in that the characteristics of disposition are those that tend to vary greatly among animals, and extrapolation between species is complicated. Unfortunately, the disposition has not been scientifically studied in animals except for clomipramine, and information for other drugs or species is extrapolated from human or canine data. The TCAs are very lipophilic. As such, they are well absorbed after oral administration. They can, however, undergo marked first-pass metabolism. Typical drug concentrations achieved in humans at recommended doses are 100 to 250 ng/mL for amitriptyline, 150 to 500 ng/mL for clomipramine, and 200 to 300 ng/mL for imipramine.7 High doses can cause anticholinergic effects on the gastrointestinal tract, slowing absorption or making it erratic. Absorption in humans can result in peak concentrations as rapidly as 2 hours or as long as 12 hours after administration.7 The drugs are very highly protein bound, but unbound drug is characterized by a very large volume of distribution (10 to 15 L/kg in human patients), contributing to a long elimination half-life of the parent drug or its metabolites. Drug may bind avidly to selected tissues.
Drugs are eliminated by hepatic (oxidative) metabolism with the main reaction being N-demethylation to the active metabolite, desmethylclomipramine (DCMP). This reaction is catalyzed by CYP3A4, CYP2C19, CYP2C9, and CYP1A2. The parent and metabolite are hydroxylated by CYP2D6 and then glucuronidated.17,18 Metabolism is variable among human and canine patients, accounting for plasma concentrations that differ by tenfold to thirtyfold. Species and gender differences in metabolism or pharmacokinetics have been demonstrated for clompramine.17,18 Metabolism of TCA yields active and inactive metabolites. It is not clear what percentage of the antidepressant activity of the TCA is associated with the metabolites. Metabolites generally have an elimination half-life that is twice or more that of the parent compound.7 Thus accumulation of the metabolites can result in a marked proportion of the pharmacologic effect of TCAs.
Clomipramine is approved for use in dogs for treatment of separation anxiety. After multiple oral administration in dogs, both clomipramine and its principal active metabolite, desmethylclomipramine, accumulate in a dose-dependent manner at doses ranging from 1 to 4 mg/kg.19 Food does not appear to alter the rate or the extent of oral absorption,20 with bioavailability of clomipramine being 20% and of the metabolite 140%. The ratio of metabolite to parent in dogs approximates 0.6, varying across time.21 After a single dose of 3 mg/kg in normal dogs (n = 6), clomipramine Cmax was quite variable for both clomipramine (16 to 310 ng/mL at 0.75 to 3.1 hr) and desmethylclomipramine (21 to 134 ng/mL at 1.4 to 8.8 hr).22 After multiple dosing, (28 d) clomipramine Cmax again was markedly variable at 43 to 222 ng/mL (at 3 to 8 hr), compared with 21 to 134 ng/mL at 1.4 to 8.8 hr, for desmethylclomipramine. Hewson and coworkers21 reported trough concentrations of clomipramine ranging from 2 to 17 ng/mL and desmethylclomipramine ranging from 2 to 15 ng/mL following 26 days of dosing at 3 mg/kg; neither compound was detectable in three of six dogs at trough times (24 hr). Protein binding of both the parent compound and its metabolite are greater than 96%. Hepatic clearance of clomipramine in dogs is flow-limited; changes in hepatic blood flow, but not hepatic mass (i.e., CYP activity), should be expected to alter clomipramine elimination. However, rapid clearance is balanced by a very large volume of distribution. Despite this large volume, clomipramine elimination half-life is shorter and markedly variable in dogs ranging from 1.2 to 16 hours after a single oral dose in dogs (n = 6) compared to 20 in humans. After multiple (28 days) dosing, both variability and duration of half-life decreased to 1.5 to 9 hours.21 An average half-life of 5 to 6 hours was reported for dogs in a subsequent study.19 Half-life of the desmethyl clomipramine in dogs also is short (2 to 3.8 hours, compared to 36 hours in humans),19,21 with total concentration of the metabolite being less than that of the parent compound. Steady-state concentrations are reached by 4 days or less, resulting in response within 1 to 2 weeks. Cats metabolize (based on in vitro hepatic microsomal studies) clomipramine more slowly than do dog or rats, with male cats being less efficient metabolizers.17 The pharmacokinetics of clomipramine and its major metabolite desmethylclomipramine have been described in cats (n = 6) using a randomized crossover single-dose oral (0.5 mg/kg) and intravenous (0.25 mg/kg) administration study).23 After oral administration, Cmax (μg/mL) was 0.87 ng/mL at 6.2 hours. After intravenous administration, volume of distribution and clearance were 0.393 L/kg and 0.833 mL ∗ min/kg, respectively, resulting in an elimination half-life of 12.3 hours. Area under the curve (AUC) of clomipramine and desmethylclomipramine after oral administration were 948 and 613 ng ∗ min/mL, respectively. Oral bioavailability of clomipramine and its active metabolite approximated 30%. Variability among cats was marked. On the basis of these data, Lainesse and coworkers18 found no correlation between either clomipramine or desmethylclomipramine concentrations and sleep scores. However, gender differences in metabolism may have contributed to the lack of correlation.
KEY POINT 26-3
For a number of the behavior-modifying drugs, slow elimination and formation of active metabolites complicate the design of dosing regimens.
Of the TCAs, only amitriptyline appears to have been studied after transdermal delivery in cats. Plasma drug concentrations were not detectable in cats (n = 6) receiving a single 5-mg transdermal (pluronic lecithin organo [PLO]) gel dose. This compared with a mean peak concentration of 61 ± 6.2 ng/mL after oral administration of 5 mg.24 The author has demonstrated, using a randomized crossover, dummy-placebo controlled design, that although amitryptylline administered as a transdermal gel achieved detectable concentrations in 5/6 cats receiving 2 mg/kg twice daily for 3 weeks, bioavailability was very poor. After 3 weeks of dosing, serum drug concentrations were 46 to 101 ng/mL after gel administration compared to 1336 to 5230 ng/mL after oral administration at the same dose. The disappearance half-life in the cats receiving the oral drug (based on peak and trough dosing) was markedly variable among cats, ranging from 20 to approximately 85 hrs after 3 weeks. Two of the cats receiving the gel developed marked aural erythema and scaling. Despite the very high concentrations, 4/5 cats tolerated the oral administration well; the one cat with concentrations of 5230 ng/mL was depressed, with resolution occurring once the drug was discontinued. Interestingly, disappearance half-life of amitryptyilline was only 20 hrs in this cat, suggesting that oral bioavailabilitymay have been contributing to the high serum concentrations.
Side effects
Up to 5% of human patients receiving a TCA react adversely. Sedation is common with the TCAs.3 The most common reactions reflect antimuscarinic effect or overdosing. Cardiac toxicity (reflecting a quinidine-like membrane-stabilizing effect) is a less frequently reported but serious effect, but not apparently with clomipramine administration in dogs. The effect may be lethal at 15 mg/kg.4 Tachycardia may also reflect anticholinergic effects and must be differentiated from true cardiotoxicity. Other side effects include dry mouth, gastric distress, constipation, dizziness, tachycardia, or other arrhythmias, blurred vision, and urinary retention (particularly problematic in the presence of prostatic hypertrophy).7 Weakness and fatigue reflect CNS effects. Cardiac toxicity is more likely in patients who start therapy with cardiac disease. In healthy patients the most likely cardiac response is hypotension as a result of α-adrenergic blockade.
An undesirable side effect of antidepressant drugs in humans is referred to as the “switch process.” Patients undergo a transition from depression to hypomanic or manic excitement.7 This effect has not been reported in animals. Confusion and delirium are behavior aberrations that occur commonly in human patients, with the incidence of 10% in all patients, increasing to more than 30% in elderly patients over age 50 years.7 Miscellaneous toxic effects in human patients include leukopenia, jaundice, and skin rashes. Weight gain occurs, particularly with those drugs that are selective for serotonin reuptake. Isolated reports that address the side effects of TCAs in dogs are uncommon. Goldberger and Rapoport25 reported side effects in 5 of 13 dogs receiving clomipramine for lick granuloma. Clinical signs included lethargy, anorexia, diarrhea, and growling.
Acute poisoning with TCAs is common in human patients (accidental or intentional) and appears to be a significant potential problem in animals.26 Symptoms in humans vary and are complex. Excitement and restlessness may be accompanied by myoclonus or tonic–clonic seizures. Coma may rapidly develop, associated with depressed expiration, hypoxia, hypothermia, and hypotension.7 Anticholinergic effects include mydriasis, dry mucosa, absent bowel sounds, urinary retention, and cardiac arrhythmias, including tachycardia. Clinical signs reported after accidental ingestion in animals26 include hyperexcitement and vomiting as early manifestations, followed by ataxia, lethargy, and muscular tremors. Bradycardia and other cardiac arrhythmias occur later. These later signs occurred shortly before death in experimental animal models of TCA toxicosis.26
Treatment for TCA toxicosis is supportive, including respiratory (intubation) and cardiovascular support. Gastric lavage with activated charcoal can be used early. Emetics probably should be avoided because of the risk of aspiration pneumonia in seizuring animals (some antiemetics may further predispose the animal to seizures). Short-acting barbiturates (or similar drugs) without pre-administration of atropine are preferred for anesthetic control during gastric lavage. Cathartics (sorbitol or sodium sulfate–Glauber’s salt) can be of benefit. Magnesium sulfate should not be used because impaired gastrointestinal motility can facilitate absorption of magnesium. Resolution of coma may require several days; the threat of cardiac arrhythmias likewise persists for several days. Pharmacologic interventions for cardiac arrhythmias have not been well established. Alkalinization (sodium bicarbonate sufficient to maintain blood pH above 7.5: 2 to 3 mEq/kg, administered intravenously over 15 to 30 minutes) may prevent death by increasing protein binding and increasing cardiac automaticity (as a result of potassium shifts).26 Traditional cardiac drugs, including antiarrhythmics and digoxin, are contraindicated in human patients. However, phenytoin may provide safe antiarrhythmic effects and in human patients is useful for treatment of seizures.7 Relevance to dogs or cats is not clear: phenytoin has a short half-life in dogs and is associated with adverse events when administered IV (see Chapter 27). Diazepam is indicated for acute management of seizures. β–adrenergic receptor antagonists and lidocaine may be useful.7 The risk of tonic–clonic seizures is increased in human patients, particularly at high doses.
Clomipramine generally is associated with less sedation than with the other TCAs.27 Systemic phospholipidosis (excessive accumulation of phospholipids in tissues) occurs with chronic administration of cationic, amphophilic drugs, including the TCAs, and fluoxetine.
Indications
The TCAs have been recommended among animal behaviorists for most abnormal behaviors manifested in dogs or cats. These include, but are not limited to, behaviors associated with fear and aggression,27,28 stereotypics, OCDs or self-mutilation disorders, and excessive barking. The use of clomipramine is discussed later with specific disorders. However, using an open trial, Seksel and Lindeman29 reported the efficacy and tolerance of clomipramine (1 to 2 mg/kg every 12 hours for the initial dose, increased up to 4 mg/kg twice daily) in a variety of behavioral disorders in dogs (n = 24). Included in the study were OCD (n = 9), separation anxiety (n = 14), and noise phobia (n = 4). Treatment included behavior modification and continued for at least 1 month after resolution of clinical signs. Resolution to marked improvement of clinical signs was reported in 67% of the dogs, whereas 21% reported slight to moderate improvement versus no change in 12.5% (3 of 24) animals. Clomipramine discontinuation (without return of the abnormal behavior) was described as successful in five of the nine animals in which it was attempted.
KEY POINT 26-4
The tricyclic antidepressants have been recommended among animal behaviorists for most abnormal behaviors manifested in dogs or cats. These include, but are not limited to, behaviors associated with fear and aggression, stereotypes, obsessive–compulsive or self-mutilation disorders, and excessive barking.
Contraindications
The TCAs are not recommended for animals with a metabolic disease. Specific contraindications include a history of cardiac or hepatic disease, seizures, glaucoma, hyperthyroidism, and thyroid hormone supplementation.27,28
Drug Interactions
The TCAs can interact with a number of other drugs. Competition for protein-binding sites with other highly protein-bound drugs can result in increased drug concentrations; however, this may be balanced by increased hepatic clearance. Drugs that affect drug-metabolizing enzymes, through either inhibition or induction, also affect the clearance of TCAs. The sequelae of the impact are difficult to predict because active metabolites similarly are affected. In general, however, drugs that inhibit metabolism are likely to result in greater drug accumulation and increased risk of toxicity. TCAs and other antidepressants also can compete with other compounds for metabolism. The drugs themselves may affect metabolism of other drugs. Clomipramine inhibits the metabolism of other drugs; but a more relevant interaction may be the impact of other drugs on the metabolism of clomipramine (e.g., cimetidine).7 The antidepressants potentiate the effects of sedative drugs. The TCAs should not be used in combination with other drugs that modify CNS NTs. Included are MAO inhibitors and amitraz.27,28 In human patients a potentially lethal interaction has been reported when a TCA, particularly one that inhibits serotonin uptake, is combined with an MAO.7 The term serotonin syndrome has been applied to this interaction, which is characterized by restlessness, muscle twitches, hyperreflexia, shivering, and tremors.30 Drugs or herbal products whose combination might result in the serotonin syndrome are indicated in Table 26-2.
Table 26-2 Drugs or Supplements Whose Combined Use Increases the Risk of Serotonin Syndrome
| Drug Class | Interacting Drug Class | Example Drugs |
|---|---|---|
| Monamine oxidase inhibitors | Tricyclic antidepressants | Amitriptyline |
| Imipramine | ||
| Clomipramine | ||
| Miscellaneous | Isocarboxazid | |
| Phenelzine | ||
| Serotonin uptake inhibitors | SSRI | Fluoxetine |
| Fluvoxamine | ||
| Paroxetine | ||
| Sertraline | ||
| Citalopram | ||
| Serotonin and norepinephrine uptake inhibitors | Trazazone | |
| Bupriopion | Venlaflaxine | |
| Opioids | Fentanyl | |
| Mepiridine | ||
| Tramadol | ||
| Dextromethorphan | ||
| Antimicrobials | Antibacterials | Linesolid |
| Antivirals | Ritonavir | |
| Antiemetics | Metoclopramide | |
| Ondansetron | ||
| Granisetron | ||
| Herbals | St. John’s wort | |
| SAMe | ||
| Ginseng | ||
| Substances of abuse | MDMA | |
| Cocaine |
Clinical Use
Most drugs take 2 to 3 weeks for clinical efficacy to be realized. The exception might be amitriptyline, which may cause a response in 3 to 5 days; however, maximum response may take longer, particularly in cats, for which the half-life may be long.27 Dogs can respond to clomipramine in 1 to 2 weeks. In an approval study, clomipramine (Clomicalm) treatment with behavior modification improved separation anxiety in dogs compared with behavior modification alone (according to package insert). King and coworkers31,32 (manufacturer sponsored studies) studied the efficacy of clomipramine for treatment of separation anxiety in dogs. Using a parallel, randomized, double-blinded design, researchers gave dogs with “hyper attachment” behavior (n = 97) either a low dose (0.5 to 1 mg/kg twice daily; n = 25), a standard dose (1 to <2 mg/kg twice daily; n = 28), or no dose of clomipramine (n = 32) along with behavior therapy for 12 weeks. At each of three assessment periods, treatment dogs responded better, and overall, to therapies three times more rapidly than nontreatment dogs in all behaviors (destructive or inappropriate elimination) with the exception of vocalization. Among the treatment dogs was an epileptic; this dog had no seizure episodes during the treatment period. Differences in the frequency of side effects among treatment groups were vomiting and gastritis, which occurred in 13/25 and 8/28 dogs in the low and standard group, respectively, compared with three in the placebo group, and lethargy or sleepiness in four of the standard group compared with two in the other two groups. Interestingly, one healthy dog collapsed with hyperthermia (111°F) and disseminated intravascular coagulopathy. No other underlying illness was reported; the dog survived the episode, but clomipramine was discontinued. In their follow-up questionnaire-based study,32 12 dogs continued to receive clomipramine long term (13 to 16 months or more), with 10 of these dogs improving further. No increases in adverse events were identified. Behavior changes were also studied in dogs for which the drug was either discontinued (n = 48) or had not been administered within 4 months of the questionnaire (n = 16). However, the data are confounded by the use of other behavior-modifying drugs. Behaviors worsened in the first 2 weeks after therapy was discontinued in three of ten dogs receiving low-dose clomipramine; however, no dogs in the standard-dose group exhibited clinical signs potentially consistent with withdrawal. For treatment groups, after 2 weeks behavior worsened in 13% to 15% of dogs compared with 23% of dogs receiving placebo. Six of nine dogs for which clinical signs worsened had not responded totally to therapy during the trial, whereas three of nine had been considered successes. The time to worsening was longest for the standard dose group. On the basis of response to clomipramine, the authors raised the possibility that treatment accompanied by behavioral therapy might allow permanent control.
Clomipramine has been studied for acute management of anxiety in dogs. Frank and coworkers33 prospectively studied transport-induced stress in Beagles treated with clomipramine (2 mg/kg twice daily for 7 days). Compared with placebo, treatment only tended to reduce clinical signs of panting or drooling; however, cortisol concentrations increased less in treated versus untreated controls after three 1-hour trips.
Therapeutic drug monitoring may facilitate the safe and effective use of the drugs. In human patients plasma concentrations that range between 100 and 250 ng/mL are most likely to cause satisfactory antidepressant effects; toxicity can be expected at concentrations above 500 ng/mL, with fatal consequences likely as concentrations approach 1000 ng/mL.7 Variability among human patients (and presumably among animals) supports the use of monitoring to guide therapy. Monitoring to avoid toxicity is, however, complicated by the recognition that serum concentrations by themselves are not reliable predictors of toxic responses. Because of the risk of withdrawal associated with physical dependence, discontinuation of TCAs should occur over a week or longer if therapy has been prolonged.7
Selective Serotonin Reuptake Inhibitors
Structure–Activity Relationships and Mechanism of Action
The SSRIs enhance CNS serotonin by blocking presynaptic neuronal uptake (see Figure 26-1). They may also increase postsynaptic receptor sensitivity.3 Among the drugs currently approved for use in humans are fluoxetine, paroxetine, sertraline, and fluvoxamine. Of these, sertraline appears more potent (see the discussion of monitoring).7 Potency of fluoxetine for 5-HT receptors is similar to that of amitriptyline, but much less than that of amitryptyline for adrenergic, histaminic, muscarinic, opiate, or dopaminergic receptors. Consequently, fluoxetine is considered selective for serotonin. Because of their selectivity for serotonin uptake, the diverse effects that characterize TCAs are generally absent with SSRIs. Fluoxetine is prepared as a 50:50 racemic mixture; enantiomers are equally potent for 5-HT receptors although it is not clear if this is true for all species. However, for the metabolite (also racemic), the S metabolite isomer is fourteenfold more potent than the R isomer for 5-HT receptors.34
The lag time described for maximal efficacy of fluoxetine may reflect, in part, its impact on serotonin reuptake: Initially, increased serotonin in the somatodendritic area leads to 5-HT(1A) downregulation or desensitization. Aznavour and coworkers35 described response of 5-HT(1A) receptors in the brains of cats to acute and chronic administration of fluoxetine. Described as the primary target of SSRI, these receptors are autoreceptors that inhibit the firing and release of serotonin by the neurons they regulate. They are activated in response to increased serotonin concentrations in the synapse associated with SSRI treatment. Their ability to inhibit 5-HT neuronal firing and release, however, is muted by desensitization associated with acute SSRI (or other agonist) therapy. Although initially transient, in 2 to 3 weeks this desensitization allows a return to baseline firing and release and is paramount for successful therapy with SSRI. The acute desensitization probably reflects internalization of the autoreceptors. In cats therapy with 5 mg/kg fluoxetine intravenously resulted in a marked decrease in 5HT(1A) binding in the nucleus raphe dorsalis but not other regions of the brain. However, with chronic administration (5 mg/kg subcutaneously per day for 3 weeks), binding was not altered in any region of the brain, indicating that 5-HT1 autoreceptors are fully desensitized and 5-HT neuronal firing returns to baseline, at least in healthy cats.
Clinical Pharmacology
As with the TCAs, the clinical pharmacology of the SSRIs is complicated. Plasma concentrations thought to be effective for human patients range from 100 to 300 ng/mL for fluoxetine (and its active metabolites). Effective concentrations for paroxetine and sertraline are 30 to 100 and 25 to 50 ng/mL, respectively.7 Fluoxetine disposition is characterized by high protein binding, metabolism to an active compound, long elimination half-life of both parent and metabolite, a delayed onset of action, and drug interactions.7 For both the dog and the cat, because the active metabolite norfluoxetine represents a substantial, if not dominant, portion of the AUC (exposure), response to therapy cannot be evaluated at least until both fluoxetine and its metabolite reach steady state (approximately 10 to 14 days).
Fluoxetine is available as a chewable tablet approved for use in dogs. A number of efficacy studies were implemented during the approval process. Pasloske34 reviewed fluoxetine disposition in dogs and cat in abstract form. In Beagles, after administration of a single 1 mg/kg dose, oral bioavailability averaged 72%, yielding a Cmax for fluoxetine of 49 ng/mL at 1.8 hours, and for norfluoxetine 79 ng/mL. As in humans, the volume of distribution for fluoxetine is large in dogs, averaging 39 L/kg, reflecting accumulation that approximates a 100-fold increase compared with plasma in some tissues. Despite the very large volume of distribution of fluoxetine, elimination half-life averaged between 6 and 10 hours in Beagles. Norfluoxetine is the major metabolite formed from hepatic metabolism, accounting for more than 56% of the total peak area in dogs. The volume of distribution of norfluoxetine is one third of that for fluoxetine, at 10.7 L/kg, yet the elimination half-life of 48 to 57 hours for the metabolite is much longer than that of fluoxetine. The shorter half-life of fluoxetine compared with that of its metabolite reflects, in part, fluoxetine clearance, which, at 6 mL/kg/min, is threefold higher that that for norfluoxetine at 2 mL/kg/min.34 The AUC for the S-isomer of fluoxetine (14 times more potent than R) was threefold higher than that for the R-isomer.
The disposition of fluoxetine also has been reported in cats receiving 1 mg/kg. Oral bioavailability approximates 100%; Cmax is 83 ng/mL (at approximately 2 hours), compared with 25 ng/mL (at 40 hours) for norfluoxetine. The elimination half-life (0.5 mg/kg intravenously) of fluoxetine in cats is 34 to 47 hours, which is much longer than that in dogs. The volume of distribution in cats is smaller (by 50%) than that in dogs (18 L/kg), whereas clearance is similar (6.4 L/kg). The metabolite has a much larger volume of distribution compared with the parent in cats (32.6 L/kg), resulting in a longer elimination half-life (51 to 55 hours) for the metabolite (although clearance is similar to the parent at 7.4 mL/kg/min).
Whereas gradual withdrawal may not be necessary for fluoxetine because of its long half-life (including the metabolite), the same is not true for paroxetine, sertraline, or fluvoxamine. Several weeks of decreasing dosing should follow chronic dosing.
Fluoxetine has been studied after administration of a single dose as a PLO transdermal gel in cats (n = 12) at either 5 or 10 mg/kg, compared with 1 mg/kg orally.36 For oral administration Cmax of 94.8 ± 34 ng/mL was achieved at 8.5 ± 2.9 hours, with AUC being 5.4 ± 2.4 μg∗hr/mL. This compares to a Cmax of 23 ± 20.2 at 51 ± 28 hours for 5 mg/kg transdermal (AUC = 2.9 ± 1.8) and 33 ± 9.1 (Cmax) at 87 ± 34 hours (AUC of 5.4 ± 0.5 μg∗hr/mL) at 10 mg/kg. Although this study demonstrated systemic absorption of fluoxetine that approximated oral administration after transdermal administration (at 10 times the oral dose), single doses may not have been sufficient to generate therapeutic concentrations. Further, the variability in drug concentrations achieved was substantial, suggesting that absorption may not be predictable among animals. Because both the parent compound and metabolite have a long half-life in the cat, accumulation to effective concentrations may increase the potential efficacy after transdermal administration. Monitoring of the parent compound to document drug absorption after transdermal delivery should be considered. Although cats in this aforementioned study tolerated a single 10 mg/kg transdermal dose well, a second study of multiple dosing was terminated because of substantial dermal irritation after several days of therapy.7,36
Drug Interactions
Fluoxetine may inhibit its own metabolism through inhibition (both parent and metabolite) of CYPIID6-mediated demethylation.34 The SSRIs also can inhibit the metabolism of other drugs; the order of potency of inhibition is paroxetine>norfluo-xetine>fluoxetine=sertraline. Because of the risk of drug interactions, SSRIs should not be used in combination with other antidepressants (see earlier discussion of serotonin syndrome and drug interactions of TCAs and MAO inhibitors).7 Fluvoxamine minimally affects drug metabolism.
Side Effects
As a class, the SSRIs are generally considered safer than TCA in humans. Exceptions may occur for clomipramine in dogs. Unlike the TCAs, SSRIs have minimal effects on the cardiovascular system.7 Their safety for patients with underlying cardiac disease has not, however, been established. Sedation is not a common side effect and is least likely with fluoxetine.3 In humans gastrointestinal side effects are the most common, occurring in as many as 25% of patients receiving the drug.3 Their incidence is minimized by starting with a low dose and gradually increasing the dose until efficacy is evident.
Toxicity of fluoxetine has been studied in both dogs and cats.34,37 Steinberg and coworkers37 found no adverse cardiovascular effects of fluoxetine in anesthetized dogs, concluding that it was thus safer than tricyclic antidepressants. Beagles survived up to 100 mg/kg when fluoxetine was administered as a single dose, although emesis, mydriasis, tremors, and anorexia occurred. In contrast, with multiple dosing dogs could not tolerate 10 to 20 mg/kg orally per day for 6 months.34 Intolerance was manifested as tremors, anorexia, slow or incomplete pupillary response, mydriasis, aggressive behavior, nystagmus, emesis, hypoactivity, and ataxia. Aggressive behavior and anorexia also were evident. All of the physical signs of toxicity were reversible within 2 months of drug discontinuation. Tremors, slow or incomplete pupillary response, and occasional anorexia may be present at 1 mg/kg. Histologically, phospholipidosis occurred in the lung, liver, adrenals, lymph nodes, spleen, and peripheral leukocytes at 20 mg/kg and occasionally in the lung and leukocytes at 1 mg/kg. Cats receiving a single dose of 50 mg/kg also developed emesis, mydriasis, tremors, and anorexia. Oral administration to cats of 1 and 3 mg/kg once daily was well tolerated, although sporadic anorexia and vomiting occurred occasionally at 3 mg/kg. At 5 mg/kg daily, toxicity appeared at approximately 60 days and was manifested as body tremors, hypoactivity, convulsions, low food consumption, and dehydration. In contrast to dogs, for which cardiovascular depression did not occur, heart rates decreased in a non–dose-dependent manner in cats. Infiltration of alveolar macrophages, consistent with phospholipidosis, occurred at 3 and 5 mg/kg daily. At 5 mg/kg, centrilobular hepatocellular degeneration and decreased T-lymphocytes were detected.
Side effects also have been reported in animals after clinical use. In 4 of 14 dogs receiving fluoxetine for treatment of lick granuloma,38 side effects included lethargy, anorexia, and hyperactivity. Another study39 reported the same side effects as well as polydipsia, diarrhea, and increased or decreased appetite. At least 50% of animals appeared to develop some type of side effect, although side effects were described as mild. Side effects reported by owners in a study of fluoxetine for treatment of canine dominance-related aggression included fatigue, lethargy, and decreased appetite.40
A very early study41 demonstrated that fluoxetine potentiates morphine hyperthermia in cats. Although SSRIs are considered stimulatory in the sense that serotonin increases and might thus be considered proconvulsant, Jobe and Browning42 argued that they may, in fact, be anticonvulsant and that deficiencies in norepinephrine and serotonin contribute to proconvulsant tendencies.
Citalopram is the most recent SSRI to be approved for use in the United States. However, its approval was delayed after a study in which 50% of dogs receiving 8 mg/kg as part of a preapproval toxicity study (for humans) died as a result of cardiac side effects at 17 to 31 weeks of therapy. Therefore the drug probably should not be used in dogs. Diarrhea is a side effect reported for both paroxetine and sertraline. Paroxetine may be more likely than fluoxetine to cause anticholinergic side effects.4 Initiating therapy with a low dose that is increased after the first week of therapy may prevent this side effect. Paroxetine also is associated with an idiosyncratic, dose-dependent increase in arousal, awakening, and rapid eye movement suppression in dogs.4
Clinical Indications and Use
Clinical use also is addressed with specific indications. Probably no behavior-modifying drug has received more attention in the veterinary and lay literature than fluoxetine.43,44 Despite the plethora of opinions or testimonials regarding the efficacy of this drug for treatment of animal behavioral disorders, few scientific studies existed until its approval in dogs. Efficacy for treatment of lick granulomas is supported by a double-blind crossover study.38 One third of the animals studied did not repeat the abnormal behavior when fluoxetine was discontinued. Fluoxetine also has been studied in an open (nonblinded) study of dogs with a variety of behavioral problems.39 Approximately 65% of dogs with lick granuloma, 100% of animals with separation anxiety, and 85% of animals with tail mutilation disorders responded to fluoxetine. Unfortunately, data were not controlled for other treatments, making interpretation of the success of fluoxetine in this study difficult. The Freedom of Information Act file delineates several clinical trials of field studies that support its use. In a European study, doses of 1 to 4 mg/kg for 2 to 4 weeks were associated with improvement in 80% of dogs (n = 47) with separation anxiety. The most frequent adverse reactions were anorexia, weight loss, constipation, mydriasis and muscle tremors; clinical signs were considered unacceptable at 3 mg/kg. A field trial found improvement compared with control (n = 112) in dogs (n = 117) with separation anxiety treated for 56 days with fluoxetine therapy (1 to 2 mg/kg). All dogs received behavior modification. One dog in the control and three in the treatment groups developed seizures. Seizures developed 10 days after the end of therapy in one dog (this dog eventually died as a result of seizures), 45 days into therapy and 24 days into therapy. Simpson and coworkers45 and Landsberg and coworkers46 reported on the efficacy of fluoxetine for treatment of separation anxiety based on manufacturer-sponsored studies. A multicenter placebo-controlled, parallel double-blinded study was implemented in dogs (n = 208) with separation anxiety using fluoxetine (1-2 mg/kg) as a chewable tablet; treatment occurred for 6 weeks. At each week, dogs in the treatment group had improved compared with those receiving placebo; improvement occurred even in those dogs not receiving behavior modification. Destructive behavior and inappropriate urination were reduced. Seizures occurred in one dog in each group.
Fluoxetine also has been used successfully to treat psychogenic alopecia in a cat,47 dominance aggression in dogs,40 and inappropriate urination in cats.48 As with TCAs, monitoring to guide therapy with SSRI is complicated. Effective concentrations have not been established in animals and must be extrapolated from human studies. The relationship between plasma drug concentrations and therapeutic efficacy has not been well established3 and is complicated by the presence of the active metabolite, which cannot be predicted by the parent compound. Paroxetine and fluvoxamine have no active metabolites (in human patients). One advantage of paroxetine over fluoxetine in veterinary medicine is convenience in dosing with the availability of multiple tablet sizes and scored tablets.
Atypical Antidepressants
Trazodone is a mixed serotonergic agonist–antagonist used to treat sleep disorders and major depression in humans. It is characterized by a wide therapeutic range (typical drug concentrations in humans after administration of the recommended dose are 800 to 1600 ng/mL.7 The likelihood of drug interactions appears to be limited, despite the fact that it is an inhibitor of CYP2D6 metabolism. It has been used in dogs to treat mild thunderstorm phobias either as sole therapy or in combination with a TCA or SSRI. It does not appear to be effective, even at high doses (10 mg/kg), for treatment of severe thunderstorm phobias in dogs. Side effects reported in dogs include vomiting, diarrhea, and sedation. Initiating therapy at a low dose with a gradual increase may minimize this side effect.
Monoamine Oxidase Inhibitors
Structure–Activity Relationships
The recognition that the antitubercular drug isoniazid tended to elevate the mood of patients receiving the drug for treatment of tuberculosis led to further discovery of drugs that inhibit MAO. The first drugs used were structurally related to hydrazine and were associated with marked hepatotoxicity. An attempt was made to synthesize CNS-stimulant compounds unrelated to hydrazine but similar to amphetamine. Ultimately, selegiline was a result of this later effort.7
The MAO inhibitors potentially affect a variety of monoamines by inhibiting mitochondrial MAO and the subsequent degradation of monoamines, most notably dopamine (see Figure 26-1). Most of the clinically relevant drugs are nonselective toward two major enzyme groups7 that are characterized by different substrate specificities. MAO-A prefers serotonin and is inhibited by clorgyline, an MAO-A–selective inhibitor, whereas MAO-B prefers phenylethylamine and is inhibited by selegiline (deprenyl). Selegiline is the only currently used MAO inhibitor characterized by selectivity. The drug targets MAO-B and is relatively selective for dopamine. It is approved for use in dogs for treatment of pituitary-dependent hyperadrenocorticism (purported to be a dopamine deficiency) and cognitive dysfunctions. Binding to the MAO is irreversible, and recovery from effects requires synthesis of new enzyme. In human patients this appears to require 1 to 2 weeks. Metabolism occurs more slowly in geriatric patients.7
Pharmacologic Effects
The potential antiepileptic effects of selegilinle49,50 are briefly addressed in Chapter 27. The behavioral effects of the MAO inhibitors occur on systems affected by sympathomimetic amines and serotonin. Although as a class the MAO inhibitors inhibit a number of enzyme systems other than MAO, generalizations to the class do not necessarily apply to selegiline. Selegiline potentiates dopamine in selected neurons and has been approved to treat Parkinson’s disease in humans and cognitive dysfunctions in animals, conditions assumed to be associated with dopamine deficiency. Selegiline also scavenges oxygen radicals and reduces neuronal damage caused by reactive products of oxidative metabolism of dopamine or other compounds.7 A delay in the therapeutic effect of up to 2 or more weeks characterizes the use of selegiline. Reasons for the delay are not known.7
Clinical Pharmacology
The MAO inhibitors are readily absorbed after oral administration. Maximal inhibition occurs within 5 to 10 days. Despite a long biologic activity, efficacy appears to decrease in human patients if the drugs are administered at an interval longer than 24 hours.7 Selegiline is metabolized to L-amphetamine and L-methamphetamine in dogs and, along with an increase in CNS phenylethylamine, may contribute to clinical and side effects of selegiline.
Side Effects and Drug Interactions
Selective MAO inhibitors appear to be safe. Severe and potentially fatal interactions have, however, been described when MAO inhibitors were combined with other antidepressants. Particularly problematic is the combination of MAO inhibitors with drugs that inhibit the reuptake of serotonin (see earlier discussion of serotonin syndrome with TCAs). Amitraz also is an MAO inhibitor and should not be used concurrently with selegiline. Other drugs with which MAO inhibitors may interact include meperidine and precursors of biogenic amines. Selective MAOs such as selegiline are not necessarily safer than the older or nonselective inhibitors when combined with other drugs. Hypertensive crisis, a serious side effect that occurs when aged cheeses containing tyramine (a bacterial monoamine by-product) are ingested in the presence of nonselective MAO inhibitors, does not occur with selective MAO inhibitors such as selegiline.
Anxiolytics
Pharmacology
The primary anxiolytics used in veterinary medicine are the benzodiazepines (see Chapter 27 for more extensive discussion), including diazepam, its metabolite oxazepam, clorazepate (metabolized in the stomach to N-desmethyldiazepam, a major metabolite of diazepam), lorazepam, alprazolam, and clonazepam. Differences in the drugs largely reflect pharmacokinetic characteristics, although alprazolam is classified as a high potency drug. The assumed mechanism of action of these drugs is gabaminergic through interaction with the GABAA receptor. The anxiolytic effects are separate from the general CNS depressant effects caused by these drugs. Their central effects are somewhat dose dependent. Sedative effects occur at low doses; as a result, excitement is tempered. Antianxiety effects are evident at moderate doses, being beneficial to social interactions. At high doses hypnotic effects become evident. Sedation becomes profound at high doses, ataxia is evident and sleep is facilitated.1 Decreased skeletal muscle activity—particularly of value in animals experiencing seizures—is central in nature and is independent of sedative effects. Cats appear to be more prone than dogs to muscle relaxation.1 Benzodiazepines may distribute differently in cats, with extensive binding of diazepam and its major metabolite, desmethyldiazepam, in the brain.51
The disposition and tolerance to and withdrawal of most of the benzodiazepines are discussed in Chapter 27. The effects of the benzodiazepines reflect in part metabolism to active, inactive, and potentially toxic metabolites. If efficacy reflects formation of an active metabolite (e.g., desmethyldiazepam), accumulation may be necessary before maximum effects are seen. Accumulation will be more likely with twice-daily dosing. The elimination half-life of many benzodiazepines in general is short. Efficacy can be prolonged by metabolism to active metabolites (in humans). Shorter-acting compounds include clorazepate and midazolam. Intermediate half-life drugs such as oxazepam, lorazepam, chlordiazepoxide, and alprazolam have no active metabolites. Longer-acting benzodiazepines include diazepam and clonazepam. Alternatively, clorazepate is available as a sustained-release product that can be administered less frequently.
Tolerance develops to the anticonvulsant and sedative effects of many benzodiazepines (see Chapter 27). Tolerance appears less likely to develop to the anxiolytic effects of these drugs.3 In contrast, withdrawal can accompany rapid discontinuation of the drug. Thus doses should be tapered gradually (e.g., 25% per week) as the drug is discontinued.1,3
The cat has served as a sleep model for studies investigating the effect of therapeutic agents on sleep cycles in humans. Accordingly, a fair amount of dated literature is available regarding the use of benzodiazepines in cats. Although routes (e.g., intraperitoneal, which avoids first-pass metabolism) and doses are not always relevant to therapeutic use in cats, some information might be gleaned from the studies. For example, whereas flurazepam (1.25 to 2 mg/kg intraperitoneally) depresses reticular formation activity for 72 hours or more,52 tolerance develops even after one dose, and dependence (resulting in withdrawal signs) was maximal in 7 days.53 Because of the diverse impact of benzodiazepines on CNS activity (including efficacy, tolerance, and withdrawal) and the variability that characterizes their disposition (e.g., variable first-pass metabolism, variability in the activity and half-life of metabolites), the use of this class of drugs, perhaps more so than others, should be based on scientific studies that establish not only pharmacokinetics but also acute and chronic pharmacodynamic responses.
Side Effects
In addition to changes in behavior, the benzodiazepines have been associated with a number of side effects in human patients. Reaction may be to the parent drug or a metabolite. Long-term use in human patients has been associated with neutropenia and liver disease. Acute fulminating hepatotoxicity has been reported in cats receiving diazepam orally.54 Clinical signs include anorexia, vomiting, lethargy, hypothermia, and jaundice. The adversity appears to be dose dependent (and thus may be idiosyncratic), occurring in most animals within 5 to 11 days after therapy is begun. Mortality rates are high (8 of 11 cats in one report) despite intensive therapy. Histology revealed severe acute to subacute lobular to massive hepatic necroses, suppurative cholangitis, and biliary hyperplasia. Baseline hepatic function data might be collected from cats before therapy is begun and again 3 to 5 days after therapy is begun in order to minimize the damage induced by diazepam administered to cats at risk. Any evidence of illness (or evidence of prolonged elimination) should lead to discontinuation of the drug. Clorazepate used in combination with phenobarbital in dogs for control of seizures has, in the author’s experience, also been associated with liver disease. Tolerance will generally develop toward ataxia and sedation, which may accompany initial therapy. Paradoxical hyperactivity may require transitioning to another class of anxiolytics.4 Drug withdrawal should be gradually tapered for any benzodiazepine administered for more than 1 week, particularly for high-potency drugs such as alprazolam. The duration of withdrawal should be in proportion to the duration of therapy. Care should be taken not to miss doses, particularly with high-potency drugs such as alprazolam; twice-daily administration may be necessary to avoid clinical signs of withdrawal.
The Animal Poison Control Center of the American Society for the Prevention of Cruelty to Animals has reported alprazolam toxicity in dogs. Clinical signs developed within 30 minutes of ingestion and included ataxia/disorientation, depression, hyperactivity, vomiting, weakness, tremors, vocalization, tachycardia, tachypnea, hypothermia, diarrhea, and increased salivation. In addition to supportive treatment, flumazenil was suggested to counter severe CNS depression.55
Clinical Indications
The benzodiazepines are less desirable as behavior-modifying drugs because of their nonspecific nature.1 Thus a notable disadvantage of the long-term use of benzodiazepines is their tendency to interfere with the ability to learn in animals undergoing behavior modification as part of their treatment program.4,56 Animals may forget previous learned behaviors. An exception can be made for chlordiazepoxide, which appears to facilitate operant conditioning in nervous dogs (e.g., Pointer).3 Paradoxical reactions may occur in some animals, including rage, hyperexcitability, and anxiety. In addition, the risk that pet owners may use the animal’s drug should lead to close scrutiny of the animal’s drug needs and use.
KEY POINT 26-7
The short half-life and nonspecific nature of benzodiazepines decrease their efficacy as behavior modifiers.
Benzodiazepines are indicated for the treatment of anxiety. Alprazolam and clonazepam may be associated with fewer side effects and might be preferred1; however, fewer reports exist regarding their use in animals. The benzodiazepines are contraindicated in aggressive patients.1 Simpson and Simpson3 noted that the contraindication may depend on the cause of aggression. If aggression is a manifestation of an underlying fear or anxiety, then the benzodiazepines may reduce aggression. If, however, anxiety or fear is masking aggression, the benzodiazepine may increase aggression. Other indications for benzodiazepines include treatment of inappropriate elimination,1 noise phobias, and selected anxieties such as visits to the veterinarian.1,3 Oxazepam and lorazepam are not metabolized to active metabolites; as such, their use might be preferred in patients with liver disease.
Anxioselective Drugs: Azapirones (Buspirone)
Structure–Activity Relationships
Buspirone also is referred to as a nonspecific anxiolytic. Members of this group were specifically developed for atypical depressions, nonspecific generalized anxiety disorders, and selected OCDs. Buspirone is the first nonsedating antianxiety drug to be marketed.3 Its effects appear to reflect blockade of 5-HT1 receptors at both presynaptic and postsynaptic sites. Presynaptic inhibition increases serotonergic activity when serotonin is low, whereas postsynaptic control reduces serotonin when it is high.3 Buspirone will cause downregulation of 5-HT receptors. In addition, it will act as a dopamine agonist throughout the brain.3 Maximum efficacy may require several weeks.4
Side Effects
In contrast to benzodiazepine anxiolytic drugs, buspirone has no sedative, muscle relaxant, or anticonvulsant actions. It does not impair motor performance.3 Side effects to buspirone manifested in cats include increased aggressiveness (toward other household cats), increased affection toward owners, mild sedation, and agitation.56 Vomiting and tachycardia also have been reported.56 In contrast to the anxiolytic drugs and TCAs, buspirone is associated with a low abuse potential. Withdrawal symptoms after discontinuation of the drug apparently do not occur.1 Because it is not metabolized by CYP enzymes, drug interactions are less likely compared with other behavior-modifying drugs.
Clinical Indications
Buspirone has been used to treat canine aggression, canine and feline stereotypic behaviors, self-mutilation, OCDs, thunderstorm phobias, and feline spraying.1 Buspirone apparently has been particularly useful for treatment of anxiety associated with social situations such as aggression or marking behaviors.1 One week of therapy may be sufficient to evaluate the drug. Buspirone might be used in combination with other drugs that target reuptake of serotonin, particularly if intraneuronal serotonin is depleted.4
Transdermal delivery of buspirone does not appear to be a reasonable method of administration in cats. Drug concentrations were not detectable in circulation in cats (n = 6) receiving a single dose of 2.5 mg transdermally as a PLO gel. This compared with a mean peak concentration of 3.5 + 5.5 ng/mL after oral administration of 2.5 mg.24 Although a therapeutic range has not been established, typical drug concentrations after administration of a recommended dose in humans approximate 75 to 100 ng/mL.7
Miscellaneous (Nonspecific) Drugs Used To Modify Behavior
Progestins
Progestin interaction with GABA receptors is 10 to 50 times more potent than that of barbiturates.1 This may account for the nonspecific calming effects of the drugs observed in veterinary medicine. The advent of newer behavior-modifying drugs (e.g., TCAs, SSRIs) and the incidence of side effects largely limit the use of progestins to animals that have failed other medications and are faced with euthanasia.
Several side effects have been well documented in animals receiving progestins for long periods. Among the more notable side effects, because of their magnitude or life-threatening nature, are gynecomastia, mammary gland neoplasia, diabetes mellitus, aplastic anemia, and pyometra.27,28 Animals should be monitored frequently for evidence of adversities.
Progestins are most wisely reserved for adjuvant short-term therapy until the second drug takes effect (i.e., 4 to 6 weeks), and only the oral form is recommended. The progestins also are an alternative for animals in whom euthanasia is being considered; in such cases, a high dose (4 mg/kg orally every 24 hours) has been recommended in order to stimulate a rapid response.27
Anticonvulsants
A number of anticonvulsant drugs have been used to treat behavioral abnormalities. The most notable used for animals include the barbiturate phenobarbital (and its congener primidone) and phenytoin, a hydantoin derivative. Their use has been somewhat efficacious for treatment of overactive or aggressive behaviors (which actually may have been an expression of psychomotor epilepsy).1 Efficacy is, however, generally dependent on administration of sedative (and, with long-term use, potentially toxic) effects. More notably, their use has largely been replaced by the TCAs or SSRIs. The side effects of these drugs (discussed in Chapter 27) limit their long-term use, although monitoring (as with anticonvulsant therapy) may help prevent toxicity.
Phenytoin has been useful for the treatment of explosive aggression in human patients. Phenobarbital may prove useful for controlling excessive feline vocalization during car travel1 or canine aggression.57 Carbamazepine (an iminodibenzyl derivative of imipramine) also has been used to treat explosive aggression in humans. Valproic acid may be useful for treatment of aggression.57
Opioid Agonists and Antagonists
The drugs are discussed more in depth for pain control (see Chapter 28). The antagonists in particular have proved useful in the treatment of selective OCDs in humans. Efficacy also has been reported to treat selected self-mutilation disorders in dogs (e.g., acral lick dermatitis or lick granuloma).1,3,57 Pure antagonists, including naloxone and naltrexone, the latter an orally bioavailable product, and mixed agonists–antagonists such as pentazocine appear effective. These drugs block mu and kappa receptors. The assumed mechanism of action is blockade of self-reward mediated by endogenous opioid release that may accompany self-destructive behavior. Using a double-blind crossover study, a single dose of naloxone (1 mg/kg subcutaneously) decreased excessive grooming behavior in cats (n = 12) for 2.5 to 24 weeks (median 12 weeks). Likewise, a single dose of haloperidol (2 mg/kg intravenously) decreased grooming behavior, with effects lasting 16 weeks in 6 of 10 cats. The authors postulated that efficacy of naloxone might be limited only to recently developed stereotypic behaviors, whereas haloperidol might be effective for more chronic behaviors.58 Hydrocodone (for destructive behaviors) and naloxone and haloperidone (for OCDs) have shown some efficacy for treatment of behavioral disorders. Dextromethorphan, a non-narcotic opioid, also is an N-meth-D-aspartate receptor antagonist (see Chapter 28) and has shown some efficacy for OCDs. Dodman and coworkers59 prospectively compared the effect of dextromethorphan (2 mg/kg orally twice daily) with placebo for treatment of chewing or excessive grooming in dogs (n = 14) with chronic allergic dermatitis. A randomized double-blinded crossover design was used for each 2-week phase of the study. The percentage of time that abnormal behaviors and pruritus were observed was less in dogs in the treatment group.
Antihistamines
The mildly sedative (e.g., with hydroyzine) or hypnotic (e.g., with diphenhydramine) effects caused by H1 receptor blockade can be of benefit for some behavioral disorders. These drugs are discussed in greater depth as antiinflammatories (see Chapter 29) and antiemetics at the vestibular apparatus (see Chapter 19). Indications as behavior-modifying drugs might include the treatment of chronic pruritus, late-night activity, car travel, and selected transient behaviors accompanied by pacing and vocalization.1
β-Blockers
β-adrenergic blockers (e.g., propranolol, pindolol) have been used in human medicine for the treatment of aggressive outbursts associated with self-mutilation or injury problems, intermittent explosive behaviors, conduct disorders, dementia, and schizophrenia.1 The use of these drugs for similar disorders in animals has not, however, been very successful.1 Nonselective β-blockers also have been used to treat anxiety in human beings. One animal behaviorist reports success with propranolol or pindolol (the latter also affecting serotonin receptors) for the treatment of fear aggression in dogs.57
Stimulants
Stimulants include dextroamphetamine, methylphenidate (Ritalin), and pemoline, a drug whose actions are similar to those of methylphenidate. Stimulants are characterized by paradoxical effects in that they cause excitement in the normal patient but a calming effect in the hyperactive patient. Their indication for human patients is for the treatment of attention deficits. Conditions of hyperactivity are rare in veterinary medicine. Proper diagnosis is imperative to successful therapy with stimulants.
They act to increase sympathomimetic stimulation. Side effects include increased heart and respiratory rate and anorexia. Tremors and hyperthermia may occur. The drugs are contraindicated for patients with cardiovascular disease, glaucoma, and hyperthyroidism. The drugs should not be used in combination with other behavior-modifying drugs.1 Methylphenidate toxicosis was described in a 10-year-old cat treated with a 5-mg tablet. Plasma concentrations of 83 ng/mL (5 to 16 times the therapeutic range recommended in humans) were associated with restlessness, vocalizing, and circling. Treatment was environmental (external stimuli minimized); clinical signs resolved within 25 hours of ingestion.60
Others
Pheromones are increasingly becoming popular adjuvants for the treatment of behavioral and other disorders. Their use has been reviewed.61 Pheromones used to modify behavior include Dog Appeasing Pheromone (DAP). A number of clinical trials have been performed with DAP, most showing some degree of improvement, regardless of the behavior studied. However, study design, including appropriateness of sample size, control, blinding, and randomization procedures, should be carefully critiqued as the validity of the conclusions and their application to clinical practice is considered. Taylor and Mills62 studied the effect of DAP (assumed to be maternal appeasing pheromone) in newly adopted puppies. Based on the number of nights puppies cried, DAP reduced the time compared with placebo, but only in gun dog breeds. Incorporation of DAP into a collar was studied as a means to reduce travel-related problems in dogs (n = 62).63 The study was neither controlled nor blinded. Response was based on assessment of 21 behavioral signs assessed every 3 weeks; dogs were subjected to car rides at least twice weekly for 9 weeks. Significant improvement occurred in nine of the signs, with reduction in fear intensity the most consistent. DAP was also studied for its ability to decrease aggressive behavior associated with veterinary examination (n = 15 dogs), but no significant effect was found regarding aggression. Dogs, however, were more relaxed compared with those in the placebo group.64 Todd and coworkers65studied the effect on barking of DAP administered to shelter dogs treated for 7 consecutive days (n = 37 treated, 13 control). Mean barking amplitude and frequency were reduced. Responses toward strangers (e.g., sniffing frequency) also were reduced. DAP has been compared with clomipramine to treat separation anxiety66 and noise phobias67,68 (see later discussions), and feline facial pheromone (FFP) has been studied for inappropriate urination (see later discussions).69
Herbals
Several herbal products have been recommended in humans for treatment of behavioral problems. Hypercium and St. John’s wort have been studied in controlled trials in humans, with variable activity. Hypercium contains at least 10 active agents, with hypericin and hyperforin identified as inhibitors of amine transport. Sceletium contains mesembrine, which may be clinically active. The mechanism is not clear but appears to be different than that of traditional SSRI s on the basis of neuronal discharge in cats.70 S-Adenyosyl methionine has demonstrated mood elevation in humans, and ginkgo biloba extract has demonstrated some effects with mild dementia.7 The risk of interactions between herbal products and drugs should not be ignored. The combination of herbal ingredients that target serotonin with one another or with drugs that share a similar pharmacologic effect may contribute to manifestations indicative of the serotonin syndrome.
Other Considerations
Drug Combinations
For patients that are refractory to drug therapy, behavioral modification therapy, or environmental changes, combination drug therapy might be considered. Drugs should be selected on the basis of their having mechanisms that will complement one another. However, care must be taken to ensure that combined drugs do not result in adverse drug reactions as a result of drug interactions, particularly at the level of mechanism of action or drug metabolism. Particularly problematic will be the combinations of drugs and herbal agents that affect serotonin. Among the drugs most likely to be safe when combined with other modifying drugs are the benzodiazepines. Examples of combinations that may be reasonable include4 neuroleptic phenothiazines with drugs that target serotonin for treatment of OCDs; buspirone with SSRIs or TCAs, and combined use of beta blockers.
Newer Drugs and Therapies
Treatment of human behavioral disorders continues to be a major focus of research and development. Newer agents are likely to focus on the noradrenergic or serotonergic pathways, with selectivity for either receptor or system to be expected. The plethora of serotonin receptor subtypes should lead to drugs with increasing selectivity (i.e., 5-HT1A, 2A, 2C, or 3). Example drugs selectively targeting neuronal transport of norepinephrine include levoprotiline, and a tomoxetine. Drugs classified as mixed serotonin–norepinephrine transport antagonists such are venlafaxine coupled with serotonergic drugs (thus enhancing serotonergic properties beyond SSRIs or TCAs) has led to studies of duloxetine and milnacipran, the latter an analog of buspirone. Complex atypical antidepressants include the α-2 adrenergic receptors antagonists such as mianserin and mirtazapine. MAO inhibition continues to be a focus of development, although the risk of drug interactions remains high. Other drugs of interest for potential development include drugs that target GABA-A receptors, cerebral peptides (opioids, neurokinin K), and neuroactive steroids.7
Mirtazapine is a piperazino–azepine antidepressant that is structurally similar to serotonin (see Figure 26-2). It is characterized by blockade of 5-HT2 and 5-HT3 receptors and histamine (subtype 1) receptors. Sympathetic (norepinephrine) and serotonergic (5-HT1) actions reflect antagonism of alpha2 autoreceptors (similar to yohimbime) and heteroreceptors (receptors that alter release of mediators other than their ligand). In humans adverse events to mirtazapine tend to be limited in part because of its target selectivity. Its actions at 5-HT1 receptors have been referred to as indirect agonist. Its antiserotonergic effects contribute to its efficacy as an antiemetic, with effects similar to ondansetron. Regarding its behavioral modification, a meta-analysis comparing mirtazapine and amitryptyline treatment for behavioral disorders in humans demonstrated equal efficacy. Mirtazapine has not yet been studied in animals. In humans disposition is complex, warranting scientific studies to support its use in dogs and cats. Oral bioavailability in humans is approximately 50%, largely owing to first-pass metabolism. In addition to plasma protein binding (approximately 85%), 40% of the drug is bound to erythrocytes. Hepatic metabolism is extensive, by way of CYP2D6 and CYP3A4.71 Approximately 50% of the AUC of active drug reflects the demethylmirtazapine metabolite (CYP3A4). However, the potency of the metabolite is only 10% to 20% of the parent compound; as such, the active metabolite contributes to only 5% to 10% of bioactivity. Elimination half-life is both gender and age dependent, ranging from 14 to 32 hours in men and 30 to 40 hours in women. Time to steady state (in humans) is 4 days. The elimination half-life is increased 33% to 40% in the presence of either hepatic or severe (but not mild) renal disease. Not surprisingly, mirtazapine is involved in a number of pharmacokinetic drug interactions, involving selected behavior-modifying drugs, as a result of pharmacokinetic (e.g., cimetidine, risperidone, fluoxetine, and amitryptyline) and pharmacodynamic (behavior-modifying drugs, diazepam) effects. In humans behavior-modifying response does not correlate with plasma drug concentrations; mean concentrations associated with response range from 5.7 to about 111 at the lowest (0.21 mg/kg) and highest (0.64 mg/kg) effective doses, respectively. However, despite its complex disposition, mirtazapine is characterized by a wide therapeutic margin such that increased dosing is not associated with serious toxicity.71Anecdotally, mirtazapine has been used to stimulate appetite in dogs and cats.
Treatment of Specific Behavioral Disorders
Care must be taken to distinguish behavior that is perceived to be abnormal by the pet owner and normal behavior. Pharmacologic management of abnormal behavior should be approached as an adjunct, and specifically as a facilitator, to normalizing behavior rather than as a cure. The treatment of disorders of veterinary behavior has been the topic of a 2008 Veterinary Clinics of North America conference.72 A number of nondrug techniques have been recommended by many animal behaviorists.3,73-77 Abnormal behaviors that require drug therapy should be simultaneously managed with behavior-modification training. For example, decreasing arousal and fear can facilitate learning a new behavior.27,28 The evidence supporting use of behavior-modifying drugs is increasing, but well-designed clinical trials are still limited. Before a study involving pharmacologic control is accepted as guidance for management of behavioral disorders in animals, the study design must be closely scrutinized for evidence of randomization; placebo control; blinding procedures that minimize bias; equal handling of treatment groups; and perhaps most commonly overlooked, a sufficient number of animals to detect a significant difference. Outcome measures most appropriately support the study hypothesis or purpose. The inability of a study to prove a significant treatment effect should not be interpreted as no treatment groups or that the treatment groups are the same.
KEY POINT 26-9
Pharmacologic management of abnormal behavior should be approached as an adjunct, and specifically as a facilitator, to normalizing behavior rather than as a cure.
In addition to the lack of well-designed clinical trials, many of the drugs used to modify behavior can cause serious side effects, and the clinical pharmacology of the drugs increases the likelihood of adversity because of unpredictability of plasma or tissue drug concentrations. Many of the side effects may not be readily observed by the pet owner, further increasing the risk of side effects. Finally, slow response to therapy may lead to unsupervised manipulation of dosing regimens by the pet owner, again predisposing the animal to adverse reactions. Owners should be well counseled regarding the risks and benefits of behavior-modifying drugs, including potential changes in behavior that may be less desirable than the behavior targeted by the drug. Although many drugs recommended for use in dogs and cats are approved for human, but not veterinary, use, behavior-modifying drugs stand out as having potential risks of adversity.26 Obtaining informed owner consent may be prudent before implementing therapy with these drugs. Caution should be taken to prevent substance abuse by pet owners.
Monitoring serum drug concentrations may be of benefit for selected drugs. Monitoring must, however, be performed in conjunction with clinical response, including both efficacy and safety. Antidepressants should be used cautiously or not at all for patients suffering from metabolic illnesses. Adequate time must be allowed before a drug or a dosing regimen is considered to fail. At least two drug elimination half-lives plus the time described to maximum efficacy for the particular drug should elapse. In general, combinations of behavior-modifying drugs should be avoided. One drug should be withdrawn, often slowly, before another is begun. A drug-free time of two drug elimination half-lives is recommended in humans before a new drug is begun. Generally, 10 to 20 days should elapse until a drug-free period has been sufficiently long for a short-acting drug and up to 6 to 8 weeks for longer-acting drugs.1
The following description of drug therapy for selected behaviors is not intended to be a “cookbook” approach to managing abnormal behavior in dogs and cats. Rather, clinicians should familiarize themselves with the assumed behavior and its proper nondrug behavioral modification management. Clinicians should be thoroughly familiar with the drug to be used. Because indications are less clear with these drugs, an emphasis should be placed on side effects, drug interactions, and contraindications. Consultation with a veterinary behaviorist is strongly recommended before implementing any drug therapy.
Cognitive Dysfunction
The use of deprenyl to treat cognitive dysfunction in animals is supported by the efforts of Ruehl.78 Reported in abstract, using a randomized, placebo-controlled design, dogs (n = 199) with cognitive dysfunction received either placebo or 0.2 mg/kg or 1 mg/kg daily of selegiline HCl (Anipryl). Response in the higher-dose group (1 mg/kg) significantly improved compared with the placebo group; response in the lower-dose group was not reported.
Among the postulated causes of cognitive dysfunction is age-related neuropathy. Among the leading causes of neuropathy is the progressive and accumulated changes in the neuron induced by reactive oxygen species generated through normal mitochondrial aerobic respiration. Damage reflects not only direct effects caused by the radicals but also mutations in DNA and formation of aldehydes and secondary toxins.79 Accumulative damage coupled with limited regenerative capacity results in changes associated with age. A direct relationship between age and loss of oxidative scavenging ability supports the free radical theory of aging. As such, attenuation or reversal of the process might be achieved through treatment with compounds that facilitate maintenance of or an increase in radical scavenging ability. For example, Ikdea-Douglas and coworkers79 demonstrated that 90 days of dietary antioxidant supplementation was associated with improved performance on landmark-discrimination tasks in aged (9 to 13 years) Beagles (n = 30). Improvements in behavior could be associated with concentrations of antioxidant supplementation, although the role of inflammation control could not be ruled out. Vitamin E (all-rac- α–tocopherol) was supplemented as a lipid-soluble antioxidant at low (83 ppm) to high (799 ppm) concentrations. Other supplements included vitamin C (an aqueous antioxidant), and L-carnitine and dl-α-lipolic acid as mitochondrial cofactors. Osella and coworkers80 reported the efficacy of a neuroprotective nutraceutical (Senilife) that contains phosphatidylserine, ginkgo biloba extract, d-α-tocopherol, and pyridoxine in dogs (n = 8) with cognitive dysfunction. However, no placebo group was treated. The authors reported that animals were markedly improved, although none underwent complete remission. The use of other nutraceuticals for treatment of age-related diseases has been reviewed by others.81 Finally, efficacy of deprenyl may reflect, in part, its neuroprotectant ability through scavenging of oxygen radicals.
Canine Behaviors
Dominance-Related Aggression
Aggression appears to be related to noradrenergic, dopaminergic, and serotonergic receptors; of these, the serotonergic appear most important. Consequently, drugs that are selective for serotonin receptors may be more effective for aggression. Among the TCAs, clomipramine might be preferred. One group82 found no effect of amitriptyline for treatment of aggressive behaviors using both a prospective and retrospective approach. Prospectively, amitriptyline was compared with placebo using randomized, double-blind, placebo-controlled crossover design in 12 dogs; retrospectively, no treatment effect for amitriptyline was found in 27 dogs. The authors concluded, on the basis of both studies, that amitriptyline did not improve aggressive behaviors. However, their conclusion is likely to be limited by the power of the study. Variability in the underlying causes of aggression may have further limited interpretation. Hewson and coworkers22 also reported no effect for clomipramine on dominance aggression in dogs (n = 29). Their study was well controlled and blinded, but again, the ability to detect a significant difference was not reported.
Fluoxetine, paroxetine, and sertraline are selective for serotonin uptake; among these, fluoxetine has been used in dogs for dominance aggression.83 Fluoxetine (1 mg/kg orally every 24 hours) significantly decreased owner-directed aggression in eight of nine dogs in a single-blind crossover study; although “placebo controlled,” placebo was administered only 1 of the 5 weeks of treatment. Behavioral modification was not included with drug therapy.83
Administration of tryptophan, a serotonin precursor, has been associated with reduced aggression. Other drugs that may reduce aggression include propranolol (episodic aggression in people), carbamazepine, lithium, and phenobarbital.84 Long-term adverse effects outweigh the potential behavior-modifying effect of progestin administration for aggression. Anxiolytic drugs may reduce dominance-related aggression if aggression is a manifestation of fear or anxiety.3,12 Aggression may worsen in some patients treated with benzodiazepine derivatives, however, especially if normal inhibitory mechanisms are suppressed.3 In human patients anticonvulsants have been useful for treatment of “explosive” aggression. Phenytoin and carbamazepine both have been used. In animals carbamazepine is preferred for profound aggression that has not responded to other therapy.1 Monitoring may facilitate successful therapy; concentrations known to be effective for control of seizures in human patients (6 to 10 μg/mL) may be effective for control of aggression in animals.1
Stereotypic Motor Behaviors, Obsessive Disorders, and Self-Mutilating Disorders
Luescher85 has reviewed treatment of compulsive disorders in dogs and cats. Stereotypics are repetitive behaviors without an obvious goal and thus appear pointless and mindless to the observer.57 They are usually derived from normal behaviors and may be a component of displacement behavior (reflecting an inability to perform more than one strongly motivated behavior) or a compulsive disorder. They are generally associated with chronic conflict, confinement, and sensory deprivation. OCDs are poorly defined in veterinary medicine. In humans OCDs are ritualistic and sufficiently invasive either cognitively or physically to interfere with normal function.1,86 They may reflect a chronic state of conflict anxiety, leading to displacement. Abnormal behaviors in animals that might be considered OCDs include stereotypic, ritualistic circling, spinning, pacing, howling, flank sucking, and fly biting; selected ingestive behaviors; polydipsia; and self-mutilation or grooming behaviors, including acral lick granuloma.1,2,86,87 In humans the disorders may reflect aberrant serotonin metabolism and possibly increased dopamine. Therefore treatment has focused on serotonergic metabolism. A similar approach seems to work for dogs with OCDs. A series of cases of obsessive–compulsive behaviors in dogs provides evidence of the potential efficacy of clomipramine but not amitriptyline.1,86 Other reports support the use of clomipramine for treatment of OCDs.25,89 One single-blind crossover study of lick granuloma found clomipramine but not desipramine to reduce licking by 50% in half of patients studied.25 Another clinical report noted the efficacy of clomipramine, but not diazepam, naloxone, or phenobarbital, in a single dog affected with self-trauma.88
Well-designed controlled clinical trials supporting drug therapy for OCDs or stereotypics are increasing. One well-designed study (randomized, placebo-controlled, double-blinded, balanced crossover) in dogs (n = 51) with canine compulsive disorder (including spinning and acral lick dermatitis) were fourfold more likely to improve (although not be cured) after 4 weeks of treatment with clomipramine.22 A retrospective study in dogs (n = 103) and cats (n = 23) with OCDs found first that compliance with behavior modification was high; second, that the combination of behavior modification and medication was associated with a marked decrease (>50%) in intensity and frequency of OCDs in most animals; and third, that clomipramine was significantly more efficacious than amitriptyline.89
Fluoxetine has been reported as useful for treatment of OCDs or self-mutilation, including acral lick granuloma38,39,90 and tail mutilation.39 Wynchank and Berk90 studied the efficacy of fluoxetine (20 mg) using a randomized, double-blinded, placebo-controlled study in dogs (n = 63); dogs were treated for 6 weeks. Both owners and veterinarians reported improvement. Clomipramine also has been studied. In an open clinical trial, clomipramine (2 mg/kg orally once daily) was associated with marked healing of acral lick granuloma in 8 of 10 dogs within 3 weeks. Dogs were studied for 6 months, with response maintained in three dogs at least 3 months after therapy was discontinued.91
The phenothiazine derivative thioridazine was reported as useful in one case of aberrant motor behavior.15
Opioid antagonists also have been reported to be effective for stereotypic behaviors in dogs manifested as self-mutilation acral lick dermatitis. Release of endogenous opioids may serve as a reward system after mutilation. Breaking the reward cycle with antagonists may resolve the behavior.57,87 Both naltrexone and nalmefene, pure opioid antagonists, were found to be useful. In one study self-mutilation activity significantly decreased in seven of eleven dogs and was partially effective in three more.92 On the basis of accompanying pharmacokinetic studies, concentrations of 20 to 50 ng/mL of nalmefene were considered therapeutic. The short half-life of the drug (2 to 3 hours in dogs) may necessitate frequent dosing. Using a double-blind crossover study, a single dose of naloxone (1 mg/kg subcutaneously) decreased excessive grooming behavior in cats (n = 12) for 2.5 to 24 weeks (median 12 weeks). Likewise, a single dose of haloperidol (2 mg/kg intravenously) decreased grooming behavior, with effects lasting 16 weeks in six of ten cats. The authors postulated that efficacy of naloxone might be limited only to recently developed stereotypic behaviors, whereas haloperidol might be effective for more chronic behaviors.58 Dextromethorphan inhibits the uptake of serotonin (see Table 26-2)93 The successful use of dextromethorphan (2 mg/kg every 12 hours orally for 2 weeks) for treatment of self-licking, self-chewing, and self-biting associated with pruritus has been described in a series of dogs.59 Pruritus scores also decreased in the dogs. Animals (n = 14) were studied using a placebo-controlled, randomized, double-blind crossover study. Dextromethorphan was administered as the pure drug substrate, with filler administered in a gelatin capsule. Two dogs were withdrawn because of side effects (lethargy and diarrhea, respectively).
Multiple-Animal Households
Abnormal behaviors sometimes accompanying the addition of a new pet to the household that already has one or more pets (generally dogs) include excessive barking, territorial defense, predatory aggression (exhibited in dogs allowed to roam unsupervised), or intraspecies aggression (aggression toward other dogs either within or outside the household). Drugs that modify anxiety or fearfulness should be considered as adjuvant therapy. Included are the TCAs amitriptyline27 and clomipramine (fear or anxiety), the SSRIs fluoxetine and paroxetine, the azapirone buspirone (antianxiety), and progestins. Of these drugs, fluoxetine and clomipramine may be most preferred. Amitriptyline and buspirone in particular have been cited for a potential increase in aggressive tendencies27 with interdog aggression. Care should be taken to adhere to previously stated concerns regarding progestin therapy.
Excessive Barking
Occasionally, excessive barking reflects an OCD. Most cases are conducive to behavioral modification. Surgical treatment (vocal cordectomy) is a less desirable alternative treatment. The use of behavior-modifying drugs should be reserved for cases in which fear, separation anxiety, or other compulsive component can be identified in association with the behavior.28 Drugs should be administered for a short period (2 to 4 months) and in conjunction with behavior modification. Once the desirable behavior is achieved and maintained for 4 to 6 weeks, medication can be tapered gradually until it is discontinued. Occasional cases may require lifelong medication in conjunction with behavioral modification. Drugs recommended by animal behaviorists include clomipramine, amitriptyline, buspirone, and fluoxetine.28 Thioridazine was reported useful in one case of excessive barking accompanied by tail biting.15
Destructive Behavior
Destructive behavior can be dangerous to the animal (e.g., when foreign or toxic material is ingested) and economically undesirable to the pet owner. Causes of the behavior may vary with age. The underlying cause of the behavior may reflect exploration and play; attention-seeking behavior; or expression of territory, fear, or separation anxiety. Cognitive dysfunction in geriatric animals also may be manifested as destructive behavior.94 Although behavioral modification is an important component of therapy, drug therapy may be urgent for animals in which the behavior is potentially harmful and for animals whose owners are intolerant of the behavior. Treatment of specific causes of destructive behavior is addressed with those disorders.
Tranquilizers such as the phenothiazine derivatives (acepromazine) may seem appropriate for destructive behavior and occasionally might prove useful. However, sedative effects may cause the animal to sleep rather than interact with family members.94 Likewise, use of anxiolytics (e.g., clorazepate, diazepam) can cause sedation. Although rapid acting, the anxiolytics must be administered frequently. In addition, they may interfere with the learning ability of the animal undergoing behavior modification.94 The efficacy of buspirone is generally poor. In addition, it is costly and characterized by a slow onset of action. Because it is safe, however, its use may be warranted in some cases.94 For animals whose destructive behavior reflects separation anxiety, a TCA (amitriptyline, clomipramine) or SSRI (fluoxetine) is indicated. As with buspirone, a long onset of action time and relatively high cost should be anticipated.
Anxieties and Noise Phobias
Sherman and Mills95 reviewed anxieties and noise phobias. A common complaint of owners is that their dogs exhibit disruptive behavior when left alone.74 Behaviors include urination, defecation, barking, howling, chewing, and digging. An Internet survey of owners of dogs with noise phobias indicated a high level of frustration regarding attempts to control the behavior pharmacologically. Of the 69 respondents, 38% attempted some type of therapy, including behavioral therapy, training, prescribed medications, herbal remedies, or some combination of these treatments. Of these, only five reported improvement.96 Riva and coworkers6 demonstrated that plasma serotonin and dopamine were increased in dogs (n = 22) with anxiety disorders compared with control animals; norepinephrine, l-DOPA, and selected other NTs did not differ.
Clomipramine has been approved for use in dogs for treatment of separation anxiety. Its use was reported in 1997 in abstract form97 based on a placebo-controlled clinical trial in dogs (n = 77). In addition to placebo, dogs were administered a low (0.5 to less than 1 mg/kg twice daily; n = 24) or a high (1 to 2 mg/kg) dose of clomipramine every 12 hours. Response was based on changes in four behaviors (vocalization, destruction, defecation, or urination) monitored at 4, 8, and 12 weeks. Significant improvement was detected in the high-dose group at 8 and 12 weeks; side effects were limited to more frequent vomiting, which was nonetheless described as infrequent and mild. In contrast, Podberscek and Serpell98 reported the lack of efficacy of clomipramine for treatment of separation anxiety in dogs (n = 49) following a placebo-controlled, double-blinded clinical trial. However, outcome measures in this study were based on owner-response questionnaires. The power of the study to detect a significant difference was not provided. Gaultier and coworkers66 compared DAP (n = 30) to clomipramine (n = 27) for treatment of separation anxiety. The DAP was administered in paraffin oil by way of a reservoir electrical diffuser, which was placed in a room where the dog spent most of its time. The study design was a multicenter, randomized, positive-controlled, parallel clinical trial. Clomipramine (n = 27) was administered as a positive control at 1 to 2 mg/kg orally twice a day; the study was designed to confirm noninferiority rather than lack of differences between the two treatment groups. A placebo diffuser was placed in the home of positive control dogs. Animals were treated for 28 days. Efficacy outcomes did not differ between the groups; however, the lack of a negative control (because of ethical considerations) and the significant placebo effect that has been demonstrated by other studies98 should lead to cautious interpretation. In contrast to efficacy, undesirable effects were greater in the clomipramine group (gastrointestinal: vomiting, appetite changes).
Melatonin is produced from serotonin in the pineal gland; its use as an adjuvant anticonvulsant is addressed in Chapter 27. Its use as an adjuvant for treatment of thunderstorm phobias is anecdotally promoted, although no scientific studies have been reported. A homeopathic treatment has been studied99 for treatment of noise phobias in dogs (n = 15). Dogs were studied prospectively using a randomized, placebo-controlled (n = 15) design. All treated dogs and 14 of 15 placebo-treated dogs responded, resulting in no significant differences between the groups; this study exemplifies the importance of placebo-controlled studies.
Separation anxiety appears to respond well to fluoxetine; one open study reported a 100% response rate.39 Benzodiazepines also may be effective for treatment of anxiety. Examples include chlordiazepoxide or diazepam.100 An advantage of the latter group is rapid response.
Fear behaviors in dogs are also common.75 Fear is commonly manifested toward loud noises such as thunderstorms and firecrackers, sudden movements, unfamiliar people, or novel environment. Before the advent of specific anxiolytic or antidepressant medications, tranquilizers such as phenothiazines or anticonvulsants such as phenobarbital were recommended for pharmacologic management of fear behaviors in dogs.75 The efficacy of phenothiazine tranquilizers, however, reflects reduction of general responsiveness and is only likely for episodic anxieties.3 Benzodiazepines (including diazepam, clorazepate, and other derivatives) have stood the test of time for treatment of noise phobias.57,75 They have been used to treat thunderstorm or other noise phobias. They must, however, be administered before the inciting event. For thunderstorm phobias oral administration should occur at or before the first atmospheric sign,1 such as changes in atmospheric pressure, wind, or ambient light conditions. Clorazepate (sustained-release form) may be preferred to diazepam, which requires more frequent administration. Alprazolam, characterized by a longer half-life, may also prove more beneficial.1
The combination of benzodiazepines with clomipramine has been anecdotally supported and subsequently studied.101 Using an unblinded, uncontrolled design, dogs (n = 40; 32 completed the study) with storm phobias were treated with clomipramine (2 mg/kg orally every 12 hours for 3 months, then decreased to 1 mg/kg for 2 weeks and 0.5 mg/kg for 2 weeks) combined with alprazolam (0.02 mg/kg orally, as needed 1 hour before anticipated storms and every 4 hours as needed). Dogs also received counter conditioning. Improvement occurred in 94% of patients and was maintained at least 4 months after study completion. Although storm phobia was reported (by caregiver) to resolve in only two dogs, behaviors associated with the phobia (panting, pacing, trembling, remaining near the caregiver, hiding, excessive salivation, destructiveness, excessive vocalization, self-trauma, and inappropriate elimination) decreased significantly during treatment. Improvement was greater during true storms (rain, thunder, and lightning) than during rain only. Response to auditory simulation did not change. The authors concluded that the combination of clomipramine, alprazolam, and behavior modification effectively decreased but did not cure the phobia.101
Dodman and Shuster57 reported that phobias can be palliatively treated but not eradicated with buspirone. Onset of action may, however, take up to 4 weeks.
Nonapproved mediations have been suggested for treatment of noise phobias. Two examples include DAP and melatonin. The pheromone is marketed as spray (VPL) and might prudently be used in combination with other therapies. It has been studied using either retrospective68 or an open noncontrolled clinical trial of 30 dogs.67 Owners rated changes in responses of 14 behavioral signs, with ratings improving in 9 of the 14. The use of melatonin for other behavior disorders and seizures has been variably addressed elsewhere, and noise phobias join other putative, albeit unproven, uses. Melatonin should not be combined with other drugs that increase synaptic serotonin.
Sexual Behaviors
Abnormal sexual behaviors are unusual. Both too “much” and too “little” behavior will benefit from a full reproductive workup, including serum sex steroidal hormone measurements. Behaviors that reflect “too much” generally are those targeted for behavioral modification, which might include drug therapy. Sexual behaviors that might be considered abnormal in uncastrated male dogs include house soiling and possessive or dominance aggression. Care must be taken to distinguish soiling from marking. Castration is the preferred treatment. Abnormal behaviors of castrated dogs include mounting, which may be accompanied by aggression, destructiveness, house soiling, and barking.76,77 Previous discussions regarding these behaviors apply when the behavior is a manifestation of sexual behavior.
Psychogenic Dermatoses
Psychogenic dermatoses generally consist of both a dermatologic and a behavioral component. Discriminating between the two components is difficult but vital to successful therapy. Some psychogenic dermatosis will respond only to behavioral management, others only to dermatologic management, and some will require both behavioral and dermatologic therapy.87 Dermatoses requiring medical management are discussed in greater depth in Chapter 22.
Clinical manifestations of psychogenic dermatoses include pruritus, acral lick dermatitis, OCDs (e.g., trichotillomania), and self-mutilation manifestations (see previous discussion of OCDs). Causes of psychogenic dermatoses are complex and may include boredom; endogenous opioid release (previously discussed); attention-seeking behavior; and, less commonly, separation anxiety.87
A number of drugs have been recommended for treatment of psychogenic dermatoses.87 These include antihistamines such as hydroxyzine or chlorpheniramine; TCAs, in particular clomipramine and doxepin, followed by amitriptyline102; and opioid antagonists (especially for acral lick dermatitis) such as naltrexone. Doxepin stands out among the TCAs for its antihistaminergic effects.
Hyperactivity
Hyperactivity must be distinguished from overactivity. The former is a medical condition. Hyperactivity or hyperkinesia is a very rare behavior in dogs and cats. It has been reported in association with aggression in dogs.57 Low doses of stimulant drugs, such as dextroamphetamine, may be useful. Dextroamphetamine can be used to provocatively diagnose the syndrome (2.5 to 5 mg orally in a medium-size dog). The patient should be calmed, and heart rate and respiratory rate should decrease.57 Dextroamphetamine can be used to manage hyperactive dogs. The addition of β-blockers has proved useful for human patients, but this use has not been documented in animals.57
Narcolepsy
Narcolepsy is an incurable neurologic disease manifested as a disturbance in the normal sleep cycle. It is an inherited (autosomal recessive) disorder in several breeds. Treatment includes methylphenidate or a TCA. Protriptyline is a nonsedative TCA that has been used successfully in human narcoleptic patients. The drug has been used successfully in one dog in which narcolepsy manifested as hyperinsomnia.103
Feline Behaviors
Inappropriate Elimination
Inappropriate elimination (urinary or defecation) was the most commonly identified risk factor for relinquishment of pet cats to an animal shelter in one study.104 Treatment of inappropriate elimination is highly individualized. Inappropriate elimination may reflect a marking behavior (generally identified by the location of urine on vertical surfaces). Abnormal or excessive marking behavior may reflect an increase in territorialism or anxiety.56 Inappropriate elimination also is an abnormal behavior that frequently has a medical rather than a behavioral cause. Care must be taken to distinguish between the two causes so that correct medical care can be provided when indicated.56 For abnormal behaviors, careful history taking should help identify the cause of the abnormal behavior. Care should be taken to discriminate an aversion to the litter box from a desire to eliminate elsewhere; the former might easily be managed by simply moving the litter box.56 For all behavioral causes, environmental and behavioral modification should precede any type of pharmacologic management. Surgical castration is recommended in males. If inappropriate urination reflects a social behavior or anxiety, the use of a behavior-modifying drug may be indicated.56
Buspirone, diazepam, TCA, and progestins have been recommended for treatment of inappropriate elimination in cats. Male and female cats appear equally likely to respond. Buspirone is safe but costly. In an open clinical trial, marking decreased by at least 75% in approximately 55% of cats (n = 62); however, relapse occurred in 50% of the responders.105 Cats that do respond generally do so within 1 week.56 Cats that are the sole cat in a household appear to be less likely to respond to buspirone (compared with diazepam) than are cats from multiple-cat households.106 Cats may, however, become more aggressive (or “assertive”) when treated with buspirone.1 Relapse of inappropriate urination appears less likely when the cat is treated with buspirone (approximately 50%) than with diazepam (approximately 75% to 91% relapse).12,105,107
Diazepam can be effective for treatment of inappropriate elimination in cats (55% to 75% success rates in two studies). As with buspirone, males and females appear to respond equally well, although spayed females are less likely to respond, whereas castrated males are more likely to respond.1,56 However, a potential disadvantage of the benzodiazepines and, most notably, oral diazepam is acute hepatic failure.54 In the report of 12 cases, acute hepatic disease occurred despite use of the drug according to recommended dosing regimens. Drugs within the class of benzodiazepines that are less likely to undergo oxidative metabolism (e.g., oxazepam) may be less likely to induce hepatic failure, although their efficacy for treating abnormal elimination has not been validated.1,56 Cats treated with diazepam are likely to stagger for the first several days of therapy,1 with spontaneous resolution occurring afterward. Chlordiazepoxide and clorazepate also have been useful for suppressing inappropriate elimination in cats,1 although drug concentrations may be less predictable than in diazepam.
Among the TCAs, use of amitriptyline is reported most commonly, although evidence is anecdotal rather than scientific.56 The incidence of adverse reactions may preclude use of amitriptyline. Clomipramine seems reasonable instead of amitriptyline on the basis of a nonrandomized, single-blinded, placebo-controlled crossover study in cats (n = 26) expressing inappropriate elimination behavior. Placebo was administered 5 days before and 3 days after a 7-day treatment period with 5 mg of clomipramine administered orally once daily. Based on the number of urine marks, the inappropriate behavior resolved in 35% of the cats, and a 75 % reduction in the number of marks occurred in 80% of the cats.108 The findings of Landsberg and Horwitz,72 reported in abstract form, were similar. Cats (n = 25) received 0.5 mg/kg daily in an open trial; 84% responded (at least a 75% reduction in marking behaviors), generally within 2 to 3 weeks.
Using a randomized, placebo-controlled multicenter clinical trial, King and coworkers109 reported a response in neutered cats (n = 67) with clomipramine at either a low (0.125 to 0.25 mg/kg), medium (0.25 to 0.5 mg/kg), or high (0.5 to 1 mg/kg) dose when administered once daily for up to 12 weeks. Behavioral and environmental modifications were encouraged. Sedation was reported in 54% of the cats but was not sufficient to cause withdrawal from the study. This study supports the use of a low dose of clomipramine but also supports a gradual increase in dose in nonresponders if necessary.
Fluoxetine also has demonstrated efficacy for control of inappropriate urination. Using a randomized, placebo-controlled, double-blinded design, the effect of fluoxetine (1 mg/kg orally once daily) on urine spraying was studied in neutered cats (n = 17).48 Drug therapy was accompanied by environmental management. Within 1 week of therapy, all but one cat had responded, with the last responding by week 2. Differences between the placebo and treatment group increased as drug therapy continued, supporting a lag time between initiation of therapy and maximal response time noted for fluoxetine in humans. Weekly episodes of spraying reduced from 8.6 ± 2 to 1.7 ± 0.6 compared with 5.5 ± 1.8 episodes in placebo-treated cats. Cats were treated for 8 weeks and monitored for an additional 4 weeks after therapy. Marking behavior returned in six of nine drug-treated cats, with those cats marking most before drug therapy also marking most at the end of the 4-week posttherapy monitoring period. Appetite decreased in four of nine cats receiving therapy; vomiting and lethargy were reported rarely.
FFP has been studied for treatment of urine spraying in cats using a double-blinded placebo-controlled design (n = 22).69 Comparisons were made within each group to baseline but not to one another. Spraying decreased compared with baseline in the treatment but not the placebo group.
The use of progestins (discussed in Chapter 19) to treat inappropriate urination should be reserved for animals that have failed all other alternatives. A single case report cited the successful use of alprazolam (2.5 mg orally every 12 hours) for treatment of soiling. Response occurred within 1 week of therapy, with mild sedation the only reported side effect. The behavior returned after the alprazolam dose was discontinued over the next 6 weeks.110
The use of fluorescein has been recommended to detect the soiling culprit in multicat households. The injectable product is sold as a 10% (10 g/dL; 100 mg/mL) solution, which can be administered orally in the evening (50 mg once daily with food or 30 mg once daily without food) for 4 to 5 days.
Social Behaviors and Aggression
Disorders of aggression are the second most common cause of abnormal behavior in cats.111 Causes associated with aggression include dominance; fear; defensive, territorial, or play aggression toward another cat; or play and fear aggression toward the owner or another person. Intolerance of petting often is manifested as an aggressive behavior. For many types of aggression (an exception being fear aggression), neutering may decrease the undesirable behavior.111 Behavioral modification techniques are the preferred method of treatment. Pharmacologic management is indicated for cats that do not respond to behavioral modification or in conjunction with behavioral modification. Little information is available, however, regarding treatment of aggression in cats. Benzodiazepines have been used with variable results. Chlordiazepoxide or diazepam has been recommended for frustration or social anxiety in cats.12,100,112 Diazepam may, however, increase predatory behavior in cats.3
Psychogenic Dermatoses
Feline psychogenic alopecia manifests as a traumatically induced regional alopecia. Underlying dermatologic causes include flea allergy, food allergy, and allergic inhalant dermatitis. Neurodermatitis also includes evidence of more damage (excoriations, crusting) and is more common in high-strung cats (e.g., Siamese, Burmese, Abyssinian). The lesions reflect overzealous grooming. Recommended treatments include TCAs (clomipramine and amitriptyline) or antihistamines.87 Fluoxetine was successful in a report of a single cat47 and clomipramine in another.113
Sexual Behaviors
Abnormal sexual behaviors in cats that may require management generally occur in toms and include urine spraying and mounting. Spraying by uncastrated cats is more appropriately treated by environmental management or according to previous discussions regarding inappropriate elimination; mounting that has not responded to behavioral modification techniques may respond to amitriptyline or another TCA.76,77
Depression
Among the classic signs of depression in the cat is anorexia; early intervention is important. Benzodiazepines should be used as appetite stimulants as early as possible. For depression, clomipramine hydrochloride or fluoxetine has been recommended (either drug at 0.5 mg/kg orally once daily).114 Clomipramine is preferred if anorexia is intermittent and associated with endogenous or environmental stressors; fluoxetine is preferred for long-term management. Treatment may be required for 4 to 6 months and should be accompanied by behavior and environmental modification.
1. Overall K.L. Pharmacologic treatments for behavior problems. Vet Clin North Am Small Anim Pract. 1997;27(3):637-666.
2. Overall K.L. Pharmacological treatment in behavioral medicine: the importance of neurochemistry, molecular biology and mechanistic hypotheses. Vet J. 2001;162(1):9-23.
3. Simpson B.S., Simpson D.M. Behavioral pharmacotherapy. In: Voith V.L., Borchelt P.L., editors. Readings in companion animal behavior. Trenton, NJ: Veterinary Learning Systems; 1996:100-115.
4. Simpson B.S., Papich M.G. Pharmacologic management in veterinary behavioral medicine. Vet Clin North Am Small Anim Pract. 2003;33(2):365-404.
5. Badino P., Odore R., Osella M.C., et al. Modifications of serotinergic and adrenergic receptor concentrations in the brain of aggressive Canis familiaris. Comp Biochem Physiol A Mol Integr Physiol. 2004;139(3):343-350.
6. Riva J., Bondiolotti G., Michelazzi M., et al. Anxiety-related behavioural disorders and neurotransmitters in dogs. Appl Anim Behav Sci. 2008;114:168-181.
7. Baldessarini Ross J. Drug therapy of depression and anxiety disorders. In Brunton L.L., Lazo J.S., Parker K.L., editors: Goodman & Gilman’s the pharmacological basis of therapeutics, ed 11, New York: McGraw-Hill, 2006.
8. Lipka L.J., Lathers C.M. Psyoactive agents, seizure production, and sudden death in epilepsy. J Clin Pharmacol. 1987;27:169-183.
9. Tobias K.M., Marioni-Henry K., Wagner R. A retrospective study on the use of acepromazine maleate in dogs with seizures. J Am Anim Hosp Assoc. 2006;42:283-289.
10. McConnell J., Kirby R., Rudloff E. Administration of acepromazine maleate to 31 dogs with a history of seizures. J Vet Emerg Crit Care. 2007;17(3):262-267.
11. Pageat P, Lafont C, Falewee C, et al: An evaluation of serum prolactin in anxious dogs and response to treatment with selegiline or fluoxetine, Appl Anim Behav Sci 105(4):342-350
12. Marder A.R. Psychotropic drugs and behavioral therapy. Vet Clin North Am Small Anim Pract. 1991;21:329-342.
13. Ionut V., Kirkman E.L., Bergman R.N. Investigation of the effect of acepromazine on intravenous glucose tolerance test in dogs. Am J Vet Res. 2004;66:1124-1127.
14. Murrell J.C., de Groot H.N.M. Venker-van Haagen AJ: Middle-latency auditory-evoked potential in acepromazine-sedated dogs. J Vet Intern Med. 2004;18:196-200.
15. Jones R.D. Use of thioridazine in the treatment of aberrant motor behavior in a dog. J Am Vet Med Assoc. 1987;191:89-90.
16. Hewson C.J., Luescher U.A., Parent J.M., et al. Effect of clomipramine on monoamine metabolites in the cerebrospinal fluid of behaviorally normal dogs. Can J Vet Res. 2000;64(2):123-129.
17. Lainesse C., Frank D., Beaudry F., et al. Comparative oxidative metabolic profiles of clomipramine in cats, rats and dogs: preliminary results from an in vitro study. J Vet Pharmacol Ther. 2007;30(5):387-393.
18. Lainesse C., Frank D., Beaudry F., et al. Effects of physiological variables on pharmacokinetic parameters of clomipramine in a large population of cats after a single oral administration. J Vet Pharmacol Ther. 2007;30(2):116-126.
19. King J.N., Maurer M.P., Hotz R.P., et al. Pharmacokinetics of clomipramine in dogs following single-dose intravenous and oral administration. Am J Vet Res. 2000;61(1):74-79.
20. King J.N., Maurer M.P., Altmann B.O., et al. Pharmacokineticis of clomipramine in dogs following single-dose and repeated-dose oral administration. Am J Vet Res. 2000;61(1):80-85.
21. Hewson C., Luescher A., Parent J.M., et al. The pharmacokinetics of clomipramine and desmethylclomipramine in dogs: parameter estimates following a single oral dose and 28 consecutive daily oral doses of clomipramine. J Vet Pharmacol Ther. 1998;21(3):214-222.
22. Hewson C.J., Luescher A., Parent J.M., et al. Efficacy of clomipramine in the treatment of canine compulsive disorder. J Am Vet Med Assoc. 1998;213(12):1760-1766.
23. Lainesse C., Frank D., Meucci V., et al. Pharmacokinetics of clomipramine and desmethylclomipramine after single-dose intravenous and oral administrations in cats. J Vet Pharmacol Ther. 2006;29(4):271-278.
24. Mealey K.L., Peck K.E., Bennett B.S. Systemic absorption of amitriptyline and buspirone after oral and transdermal administration to healthy cats. J Vet Intern Med. 2004;18:43-46.
25. Goldberger E., Rapoport J.L. Canine acral lick dermatitis: response to the antiobsessional drug clomipramine. J Am Anim Hosp Assoc. 1991;27:179-182.
26. Johnson L.R. Tricyclic antidepressant toxicosis. Vet Clin North Am Small Anim Pract. 1990;20(2):393-403.
27. Juarbe-Diaz S.V. Social dynamics and behavior problems in multiple dog households. Vet Clin North Am Small Anim Pract. 1997;27(3):497-514.
28. Juarbe-Diaz S.V. Assessment and treatment of excessive barking in the domestic dog. Vet Clin North Am Small Anim Pract. 1997;27(3):515-532.
29. Seksel K., Lindeman M.J. Use of clomipramine in treatment of obsessive–compulsive disorder, separation anxiety and noise phobia in dogs: a preliminary, clinical study. Aust Vet J. 2001;79(40):252-256.
30. Dvir Y., Smallwood P. Serotonin syndrome: a complex but easily avoidable condition. Gen Hosp Psychiatry. 2008;30(3):284-287.
31. King J.N., Simpson B.S., Overall K.L., et al. Treatment of separation anxiety in dogs with clomipramine: results from a prospective, randomised, double-blinded, placebo-controlled, multi-centre clinical trial. Appl Anim Behav Sci. 2000;67:255-275.
32. King J.N., Overall K.L., Appleby D., et al. Results of a follow-up investigation to a clinical trial testing the efficacy of clomipramine in the treatment of separation anxiety in dogs. Appl Anim Behav Sci. 2004;89:233-242.
33. Frank D., Gauthier A., Bergeron R. Placebo-controlled double-blind clomipramine trial for the treatment of anxiety or fear in beagles during ground transport. Can Vet J. 2006;47(11):1102-1108.
34. Pasloske K. New therapeutic horizons: fluoxetine pharmacology and safety in dogs and cats and its role in behavior modification. Proceedings of the American College of Veterinary Medicine. 2003.
35. Aznavour N., Rbah L., Riad M., et al. A PET imaging study of 5-HT(1A) receptors in cat brain after acute and chronic fluoxetine treatment. Neuroimage. 2006;33(3):834-842.
36. Ciribassi J., Luescher A., Pasloske K.S., et al. Comparative bioavailaibility of fluoxetine after transdermal and oral administration to healthy cats. Am J Vet Res. 2003;64:994-999.
37. Steinberg M.I., Smallwood J.K., Holland D.R., et al. Hemodynamic and electrocardiographic effects of fluoxetine and its major metabolite, norfluoxetine, in anesthetized dogs. Toxicol Appl Pharmacol. 1986;82(1):70-79.
38. Rapoport J.L., Ryland D.H., Kriete M. Drug treatment of canine acral lick: an animal model of obsessive–compulsive disorder. Arch Gen Psychiatry. 1992;49:517-521.
39. Melman S.A. Use of Prozac in animals for selected dermatological and behavioral conditions. Vet Forum. 1995;12:19-27.
40. Dodman N.H., Mertens P.A. Fluoxetine (Prozac) for the treatment of dominance-related aggression in dogs [abstract]. Newslett Am Vet Soc Anim Behav. 1995;17:3.
41. French E.D., Vasquez S.A., George R. Potentiation of morphine hyperthermia in cats by pimozide and fluoxetine hydrochloride. Eur J Pharmacol. 1978;48(4):351-356.
42. Jobe P.C., Browning R.A. The serotonergic and noradrenergic effects of antidepressant drugs are anticonvulsant, not proconvulsant. Epilepsy Behav. 2005;7(4):602-619.
43. Kauffman S. Problem pets may now get Prozac. Raleigh News-Observer August. 1994;1:1B-5B.
44. Marder A. The promise of Prozac. Vet Product News 1(May/June). 45, 1995.
45. Simpson B.S., Landsberg G.M., Reisner I.R., et al. Effects of reconcile (fluoxetine) chewable tablets plus behavior management for canine separation anxiety. Vet Ther. 2007;8(1):18-31.
46. Landsberg G.M., Melese P., Sherman B.L., et al. Effectiveness of fluoxetine chewable tablets in the treatment of canine separation anxiety. J Vet Behavior. 2008;3:12-19.
47. Hartmann L. Cats as possible obsessive–compulsive disorder and medications models [letter]. Am J Psychiatry. 1995;152:1236.
48. Pryor P.A., Hart B.L., Cliff K.D., et al. Effects of a selective serotonin reuptake inhibitor on urine spraying behavior in cats. J Am Vet Med Assoc. 2001;219:1557-1561.
49. Gupta M., Kulkarni S.K. Studies on anticonvulsant actions of L-deprenyl. Indian J Exp Biol. 2000;38(4):332-337.
50. Loscher W., Honack D. Anticonvulsant and antiepileptogenic effect of L-deprenyl (selegiline) in the kindling model of epilepsy. J Pharmacol Exp Ther. 1995;274(1):307-314.
51. Placidi G.F., Togoni C., Pacifici G.M., et al. Regional distribution of diazepam and its metabolites in the brain of cats after chronic treatment. Psychopharmacology. 1976;48:133.
52. Novack G.D., Owenburg K.M. Flurazepam and triazolam: dose-response and time-response evaluation on cat sleep. Electroencephalogr Clin Neurophysiol. 1984;57(3):277-288.
53. Rosenberg H.C., Chiu T.H. Time course for development of benzodiazepine tolerance and physical dependence. Neurosci Biobehav Rev. 1985;9(1):123-131.
54. Center S.A., Elson T.H., Rowland P.H., et al. Fulminant hepatic failure associated with oral diazepam in 11 cats. J Am Vet Med Assoc. 1996;190:618-625.
55. Wismer T.A. Accidental ingestion of alprazolam in 415 dogs. Vet Hum Toxicol. 2002;44(1):22-23.
56. Cooper L.L. Feline inappropriate elimination. Vet Clin North Am Small Anim Pract. 1997;27(3):569-600.
57. Dodman N.H., Shuster L. Pharmacologic approaches to managing behavior problems in small animals. Vet Med. 1994;89:960-969.
58. Willemse T., Mudde M., Josephy M., et al. The effect of haloperidol and naloxone on excessive grooming behavior of cats. Eur Neuropsychopharmacol. 1994;4(1):39-45.
59. Dodman N.H., Shuster L., Nesbitt G., et al. The use of dextromethorphan to treat repetitive self-directed scratching, biting, or chewing in dogs with allergic dermatitis. J Vet Pharmacol Ther. 2004;27:99-104.
60. Gustafson B.W. Methylphenidate toxicosis in a cat. J Am Vet Med Assoc. 1996;208(7):1052-1053.
61. Pageat P., Gaultier E. Current research in canine and feline pheromones. Vet Clin North Am Small Anim Pract. 2003;33(2):187-211.
62. Taylor K., Mills D.S. A placebo-controlled study to investigate the effect of dog appeasing pheromone and other environmental and management factors on the reports of disturbance and house soiling during the night in recently adopted puppies. (Canis familiaris). Appl Anim Behav Sci. 105. 4. 2007. 358-368.
63. Gandia Estellés M. Mills DS: Signs of travel-related problems in dogs and their response to treatment with dog-appeasing pheromone. Vet Rec. 2006;159(5):143-148.
64. Mills D.S., Ramos D., Estelles S.G., et al. A triple blind placebo-controlled investigation into the assessment of the effect of dog appeasing pheromone (DAP) on anxiety-related behaviour of problem dogs in the veterinary clinic. Appl Anim Behav Sci. 2006;98:114-126.
65. Tod E., Brander D., Waran N. Efficacy of dog appeasing pheromone in reducing stress and fear related behaviour in shelter dogs. Appl Anim Behav Sci. 2006;93:295-308.
66. Gaultier E., Bonnafous L., Bougrat L., et al. Comparison of the efficacy of a synthetic dog-appeasing pheromone with clomipramine for the treatment of separation-related disorders in dogs. Vet Rec. 2005;156(17):533-538.
67. Sheppard G., Mills D.S. Evaluation of dog-appeasing pheromone as a potential treatment for dogs fearful of fireworks. Vet Rec. 2003;152(14):432-436.
68. Mills D.S., Gandia Estelles M., Coleshaw P.H., et al. Retrospective analysis of the treatment of firework fears in dogs. Vet Rec. 2003;153(18):561-562.
69. Mills D.S., Mills C.B. Evaluation of a novel method for delivering a synthetic analogue of feline facial pheromone to control urine spraying by cats. Vet Rec. 2001;149(7):197-199.
70. Casimir A., Fornal C.A., Metzler C.W., et al. Effects of standardized extracts of St. John’s wort on the single-unit activity of serotonergic dorsal Raphe neurons in awake cats: comparisons with fluoxetine and sertraline. Neuropsychopharmacology. 2001;25(6):858-870.
71. Timmer C.J., Sitsen J.M., Delbressine L.P. Clinical pharmacokinetics of mirtazapine. Clin Pharmacokinet. 2000;38(6):461-474.
72. Landsberg G, Horwitz D, editors: Practical applications and new perspectives in veterinary behavior, Vet Clin North Am 38(5):937-1172, 2008
73. Landsberg G. Products for preventing or controlling undesirable behavior. Vet Med. 1994;89(10):970-983.
74. Voith V., Borchelt P.L. Separation anxiety in dogs. Compend Contin Educ Small Anim Pract. 1985;7:42-53.
75. Voith V.L., Borchelt P.L. Fears and phobias in companion animals. Compend Contin Educ Small Anim Pract. 1985;7:209-218.
76. Houpt K.A. Sexual behavior problems in dogs and cats. Vet Clin North Am Small Anim Pract. 1997;27(3):601-616.
77. Houpt K.A., editor. Progress in companion animal behavior. Philadelphia: Saunders, 1997.
78. Ruehl W.W. Anipryl treatment of canine cognitive dysfunction in a fully blinded, placebo-controlled trial for the Cognitive Study Group. Reno: Presented at the AVSAB Meeting; 1997.
79. Ikdea-Douglas C.J., Zicker S.C., Estrada J., et al. Prior experience, antioxidants and mitochondrial cofactors improve cognitive function in aged beagles. Vet Ther. 2004;5(1):1-16.
80. Osella M.C., Re G., Odore R., et al. Canine cognitive dysfunction syndrome: prevalence, clinical signs and treatment with a neuroprotective nutraceutical. Appl Anim Behav Sci. 2007;105:297-310.
81. Head E., Zicker S.C. Nutraceuticals, aging, and cognitive dysfunction. Vet Clin North Am Small Anim Pract. 2004;34(1):217-228.
82. Virga V., Houpt K.A., Scarlett J.M. Efficacy of amitriptyline as a pharmacological adjunct to behavioral modification in the management of aggressive behaviors in cogs. J Am Anim Hosp Assoc. 2001;37(4):325-330.
83. Dodman N.H., Donnelly R., Shuster L., et al. Use of fluoxetine to treat dominance aggression in dogs. J Am Vet Med Assoc. 1996;209:1585-1587.
84. Reisner L.R. Assessment, management and prognosis of canine dominance-related aggression. Vet Clin North Am Small Anim Pract. 1997;27(3):479-496.
85. Luescher A.U. Diagnosis and management of compulsive disorders in dogs and cats. Vet Clin North Am Small Anim Pract. 2003;33(2):253-267.
86. Overall K.L. Use of clomipramine to treat ritualistic stereotypic motor behavior in three dogs. J Am Vet Med Assoc. 1994;205:1733-1741.
87. Shanley K., Overall K. Psychogenic dermatosis. In: Kirk R.W., Bonagura J.D., editors. Current veterinary therapy XI. Philadelphia: Saunders; 1992:552-558.
88. Thornton L.A. Animal behavior case of the month. J Am Vet Med Assoc. 1995;206:1868-1870.
89. Overall K., Dunham A.E. Clinical features and outcome in dogs and cats with obsessive-compulsive disorder: 126 Cases (1989-2000). J Am Vet Med Assoc. 2002;221(10):1445-1452.
90. Wynchank D., Berk M. Fluoxetine treatment of acral lick dermatitis in dogs: a placebo controlled randomised double blind trial. Eur Neuropsychopharmacol. 1996;6(3):70.
91. Mertens P.A., Dodman N.H. Canine acral lick dermatitis: clinical experiences regarding the pharmacological treatment of stereotypic behavior in dogs. Birmingham, England: Proceedings of the First International Conference on Veterinary Behavioural Medicine; 1997. p 227
92. Dodman N., Shuster L., White S.D., et al. Use of narcotic antagonists to modify stereotypic self-licking, self-chewing and scratching behavior in dogs. J Am Vet Med Assoc. 1988;193:815-819.
93. International Programme on Chemical Safety: Dextromethorphan. Accessed January 7, 2010, at http://www.inchem.org/documents/pims/pharm/pim179.htm.
94. Lindell E.M. Diagnosis and treatment of destructive behavior in dogs. Vet Clin North Am Small Anim Pract. 1997;27(3):533-549.
95. Sherman B.L., Mills D.S. Canine anxieties and phobias: an update on separation anxiety and noise aversions. Vet Clin North Am Small Anim Pract. 2008;38(5):1081-1106.
96. McCobb E.C., Brown E.A., Damiani K., et al. Thunderstorm phobia in dogs: an internet survey of 69 cases. J Am Anim Hosp Assoc. 2001;37(4):319-324.
97. Simpson B. Treatment of separation-related anxiety in dogs with clomipramine. Results from a multicentre, blinded, placebo controlled clinical trial for the clomipramine in canine separation-related anxiety study group. Birmingham, England: Proceedings of the First International Conference on Veterinary Behavioural Medicine; 1997. pp 143-154
98. Podberscek A.L., Serpell J.A. Evaluation of clomipramine as an adjunct to behavioural therapy in the treatment of separation-related problems in dogs. Vet Rec. 1999;145:365-369.
99. Cracknell N.R., Mills D.S. A double-blind placebo-controlled study into the efficacy of a homeopathic remedy for fear of firework noises in the dog. (Canis familiaris), Vet J. 2007;177(1):80-88.
100. Beaver B. Feline behavior: a guide for veterinarians. Philadelphia: Saunders; 1992.
101. Crowell-Davis S.L., Seibert L.M., Sung W., et al. Use of clomipramine, alprazolam, and behavior modification for treatment of storm phobia in dogs. J Am Vet Med Assoc. 2003;222(6):744-748.
102. Miller W.H., Scott D.W., Wellington J.R. Nonsteroidal management of canine pruritus with amitriptyline. Cornell Vet. 1992;82:53-57.
103. Shores A., Redding R.W. Narcoleptic hypersomnia syndrome responsive to protriptyline in a Labrador retriever. J Am Anim Hosp Assoc. 1987;23:455-458.
104. Neville P. Behaviour problems in the cat. In: Fischer J., editor. The behaviour of dogs and cats. Ondon: Stanley Paul & Co; 1993:130-148.
105. Hart B.L., Eckstein R.A., Powell K.L., et al. Effectiveness of buspirone on urine spraying and inappropriate urination in cats. J Am Vet Med Assoc. 1993;203:254-258.
106. Overall K.L. Commentary on “buspirone for use in treating cats,”. Adv Small Anim Med Surg. 1994;7:4.
107. Cooper L., Hart B.L. Comparison of diazepam with progestin for effectiveness in suppression of urine spraying behavior in cats. J Am Vet Med Assoc. 1992;200:797-801.
108. Dehasse J. Feline urine spraying. J Appl Anim Behav Sci. 1997;52:365-371.
109. King J.N., Steffan J., Heath S.E., et al. Determination of the dosage of clomipramine for the treatment of urine spraying in cats. J Am Vet Med Assoc. 2004;225(6):881-887.
110. Seibert L.M. Case report. J Am Vet Med Assoc. 2004;224(10):1594-1596.
111. Crowell-Davis S.L., Barry K., Wolfe R. Social behavior and aggressive problems in cats. Vet Clin North Am Small Anim Pract. 1997;27(3):549-568.
112. Leuscher A. Behavioral disorders. In Ettinger S.J., editor: Textbook of veterinary internal medicine, ed 6, St. Louis: Saunders, 2005.
113. Swanepoel N., Lee E., Stein D.J. Psychogenic alopecia in a cat: response to clomipramine. J South Afr Vet Assoc. 1998;69(1):22.
114. Overall K.L. Treating depression in a cat after it loses a companion, behavior Q & A. Vet Med. 2002;97(7):508-510.