Chapter 22 Dermatologic Therapy
A major advantage of treating diseases of the skin is easy access to the site of disease. Drug delivery can be facilitated by topical therapy, and response can be based on visual examination (clinical signs) rather than solely on supportive diagnostic aids. The advent of topical drug therapy has also, however, led to a plethora of systems designed to deliver drug to the skin; its upper layers; through the skin; and, in some instances, into systemic circulation. The result is an innumerable list of products that vary in active and inactive ingredients. This chapter approaches treatment of skin diseases by first discussing drugs that are intended for topical administration and then drugs intended for systemic therapy. Finally, specific skin diseases are addressed.
KEY POINT 22-1
Successful dermatologic therapy is based on myriad factors. Dosages and frequency of administration should be modified to meet the needs of the individual patient.
Anatomy and Physiology of the Skin as They Relate to Drug Therapy
The skin is the largest organ of the body, accounting for 12% of body weight in the adult dog and 24% in the puppy.1 Although structurally canine and feline skin markedly varies from human skin, some similarity is maintained among the species. Generally, skin is thickest on the head, dorsum, and plantar and palmar surfaces of the feet; thinner on the ventral abdomen, medial aspects of the limbs, and inner pinnae; and thinnest on the scrotum.2 The skin is perforated by several appendages, the number and structure of which varies among the species. In cats and dogs, these include hair follicles, sebaceous and sweat glands, and nails.
Histologically, the skin is composed of the epidermis and dermis. Dermis is essentially composed of connective tissue, including collagen, elastin, and reticular fibers, and amorphous ground substance. It can be roughly separated into a dense, deeper reticular layer that connects the dermis to the hypodermis (composed mostly of fat) and a more superficial, loosely packed papillary layer. The dermis contains an arterial and venous network that provides nutrients to the epidermis and receives topically administered drugs able to penetrate this region, which distribute to the rest of the body.2 Cutaneous blood flow rates can affect percutaneous absorption of drugs. Cutaneous blood flow in the dog and cat is greatest in the skin of the ventral abdomen and pinnea.2 This fact, coupled with skin thickness, leads to these regions serving as the site of drug delivery for many topically applied drugs intended to have systemic effects.
The epidermis is composed of stratified squamous keratinized epithelium that undergoes sequential superficial differentiation. Five layers of the epidermis exist, with the stratum basale being deepest, followed by the stratum spinosum, stratum granulosum, stratum lucidum, and the stratum corneum (the most superficial layer). Among these layers the stratum corneum is the most important to topical drug therapy because it is the primary barrier. The stratum corneum consists of several layers of dead cells, which present a significant lipid barrier to drug penetration. The thickness varies with the area of the body. The cells are aligned to minimize water loss and are surrounded by a plasma membrane that serves as a barrier to movement into or out of the skin.2
The epidermis is anaerobic2 because of the absence of capillaries that directly provide oxygen to the cells.2 Despite the fact that 80% of the total energy requirements of the skin occur by anaerobic glycolysis, the skin is metabolically active. Drugs that are able to pass through the stratum corneum potentially are subjected to drug-metabolizing enzymes similar to those in the liver. The skin has a great capacity to synthesize lipids, which are located in the extracellular (intercellular) material, the primary barrier to drug penetration in this region of the epidermis.2 Lipids are important to intercellular cohesion, permeability (barrier), function, and normal desquamation of mature corneocytes.3 Epidermal lipids include both free and esterified fatty acids, sphingolipids, free and esterified cholesterol, and phospholipids.3 The lipids form a bilipid layer, with the hydrophobic and hydrophilic ends aligning within themselves.2 As the epidermis differentiates, the fatty acid component tends to increase. Alterations in the lipid layer result in the release of arachidonic acid and the subsequent formation of inflammatory mediators. Keratin is the major protein of the skin and is the foundation of the hair.2
Principles of Topical Drug Therapy
Topical drug therapy is indicated as initial therapy until a definitive diagnosis can be made (i.e., while waiting for results from diagnostic tests), as an adjunct to systemic therapy, and as sole treatment for selected specific dermatologic diseases. A definitive diagnosis of the cause of the skin disease should be made if possible before any masking treatments have been initiated. Therapy may be based on the morphology of skin lesions if further diagnostics yield no useful data. The clinician must be able to distinguish between primary and secondary lesions. Primary lesions develop as a direct result of the underlying disease. Secondary lesions may evolve from the primary lesion, trauma (e.g., scratching) induced by patient response to the lesion, or medications. The clinician must also be able to recognize or discriminate between acute versus chronic, deep versus superficial, and benign versus malignant lesions.
Clinicians should be very familiar with one or two drugs from each class (e.g., one keratolytic, two topical antifungals). Lesions should be evaluated frequently to assess therapy and the need to modify treatment because of treatment failure or adverse effects. The clinician must know the adverse effects of each drug and anticipate and look for evidence of these effects. Clients must be well educated regarding topical drug agents and the need for compliance. Several principles can guide effective use of dermatologic agents.
Drug Movement and the Skin
The skin functions as a barrier to prevent loss of water, electrolytes, and macromolecules and to exclude external agents (chemical, physical, and microbiologic) from the internal environment. The stratum corneum is the layer of the epidermis that is primarily responsible for this physical barrier because of the abundance of keratin and the configuration and content of the intercellular lipids. Topically applied drugs can be absorbed by three routes; they are, in order of importance or magnitude, the stratum corneum (between rather than through the cells), hair follicles, and sweat or sebaceous glands that open into the hair follicle. Movement of drug through the stratum corneum occurs by passive diffusion. Only a very small proportion of topically applied drug penetrates the stratum corneum. Despite the alignment of the lipid layer, both lipid-soluble and water-soluble drugs can pass through the stratum corneum, although passage may occur through the appendages. In general, permeability of lipophilic drugs through intact skin is generally greater than that of polar drugs.4 More drug is likely to pass through the skin of heavily haired animals because of the larger number of hair follicles.
Before a drug can move through the stratum corneum, it must first move out of the vehicle. Thus factors that affect percutaneous absorption are not limited to the drug but include factors involving the vehicle. Drug movement through the skin has been mathematically described (Fick’s law)2 to be directly proportional to the partition coefficient between the vehicle and the stratum corneum, the concentration of drug dissolved in the vehicle, the diffusion coefficient, and the surface area of the skin to which the drug is applied. Percutaneous absorption is inversely proportional to the depth of the stratum corneum (and additional layers). The driving force for absorption, as with any drug movement, is concentration of diffusible drug. The higher the concentration of the dissolved drug in the barrier (stratum corneum), the greater the diffusion “gradient.” Drugs with a high degree of lipid solubility achieve higher concentrations in the stratum corneum because it is lipophilic. Large drug molecules are absorbed less readily.
Vehicles
A vehicle is a substance used in a medicinal preparation as the agent for carrying the active ingredient. Occasionally, the vehicle is therapeutic, but usually it is inactive. The vehicle of a topical agent can profoundly affect movement of drug into the skin. Two topical medications may have the same active ingredient at the same concentration but have different vehicles and thus markedly differ in efficacy. A drug must be sufficiently soluble in a vehicle such that it can distribute throughout the vehicle and thus come in contact with the skin in diffusible form. It cannot, however, be so soluble in the vehicle that it does not leave the vehicle and thus penetrate the skin. Vehicle selection is critical to effective topical therapy. A vehicle not only acts as a carrier but also can have a direct impact on the skin and thus on drug movement.
Characteristics of a Vehicle
The physical and chemical characteristics of the vehicle and the drug largely determine drug movement. The partition coefficient describes the relative affinity of a hydrophobic phase and a hydrophilic phase. The greater the partition coefficient, the greater the affinity between the drug and the lipid phase of the skin, which generally results in an increase in percutaneous absorption of the drug. Once the skin is penetrated, however, the drug must be able to leave the lipid phase of the skin if it is to reach systemic circulation. Drugs with very high partition coefficients tend to remain in the lipid layer, causing a reservoir effect. A partition coefficient of 1 is desired for topical medicaments.2
The rate of vehicle penetration through the stratum corneum also influences percutaneous absorption. If vehicle penetration of the stratum corneum is more rapid than penetration of the skin, the concentration of drug in the vehicle on the surface of the skin increases, perhaps to the point of precipitation, slowing absorption. Evaporation of the vehicle will cause the same effect.2 Some vehicles are used to facilitate drug movement into the skin. For example, dimethylsulfoxide (DMSO) is so hygroscopic that it readily moves through the skin, carrying many drugs with it. The vehicle may contain ingredients (e.g., Tween) intended to facilitate percutaneous drug absorption by altering the integrity of the stratum corneum. Disruption of the composition or lipid orientation of the stratum corneum enhances drug penetrability. A vehicle that hydrates the corneum facilitates drug penetration. Occlusion of the skin increases hydration; vehicles can occlude by preventing skin transpiration, the passage of water vapor from the skin. Occlusive bandages also can be used to facilitate drug absorption. Water associated with hydration alters the compact structure of the corneum, decreasing resistance to drug movement. Dehydration of the stratum corneum decreases drug absorption; rehydration might be indicated before drug application.
Other patient factors that influence drug movement include the integrity of the barrier presented by the stratum corneum. Drug absorption dramatically increases if the skin has been traumatized, leading to the disruption of the stratum corneum. Absorption may also be enhanced by rubbing a medicament vigorously onto the skin. Prior removal of debris on the surface of the skin (e.g., dirt, blood, hair) can increase drug absorption, as can increasing the temperature of the skin (with sweats or water). Enhanced blood flow to the area might force drug into the hair follicles and through the stratum corneum. A warmer environmental temperature also might increase drug movement into the skin.
Many vehicles are represented in the commercial products for veterinary use. Compounding of dermatologic products is a relatively common practice in veterinary medicine. Although this can often provide a safe and effective therapeutic agent, it is important to remember that the effect of the vehicle on bioavailability of the active ingredient(s) can be profound. The resulting product may be entirely ineffective because of lack of absorption, or it may cause toxicity as a result of systemic absorption.
Types of Vehicles
Water itself can be therapeutic. Bathing with water vehicles (especially shampoos) contributes to dermatologic therapy by removing debris, including potential allergens, bacteria, and other organisms, from the skin surface and rehydrating and cooling the skin (if cool water is used).5 The addition of other drugs (shampoos, soaks, and dips or rinses) to water, forming an aqueous solution, suspension, or lotion, can create other therapeutic effects. Aqueous medications are often the topical treatment of choice for acute exudative dermatoses.
Shampoos, a type of water vehicle, can be very effective adjuvants for the control of dermatoses.5,6 In general, contact time should be at least 10 minutes. Shampoos generally are applied once to twice weekly. Examples of shampoos with therapeutic intent include hypoallergenic shampoos, which are cleansing and moisturizing; antipruritic shampoos, which often contain colloidal oatmeal along with antihistamines, anesthetics (pramoxine hydrochloride), or cortisone 1%; and insecticidal shampoos containing compounds such as pyrethrins, carbaryl, and permethrin. Application of rinses, sprays, or lotions can enhance the residual effect of shampoos.
Rinses generally are applied after a shampoo and are not necessarily intended to be completely rinsed from the coat. Incomplete rinses may increase the residual effect of the drug but also may leave the coat greasy to the touch and dull (especially on long-haired coats). Rinses include cream rinses (generally rinsed off the animal) and aqueous rinses (generally not rinsed off). Aqueous rinses also can be applied as a soak, which should last at least 10 to 15 minutes. Powders (colloidal oatmeal) intended to be applied as soaks can be placed in cheesecloth or nylon stockings before placement in water. Humectants such as Humilac (Virbac U.S.) may be applied as a spray directly onto the skin or diluted with water and applied as a rinse. Humectants are oil-free products that help draw moisture to the stratum corneum.
Lotions are liquid or semiliquid combinations of active ingredient with a water, alcohol, glycerin, or propylene glycol base. Often the liquid base evaporates, and a thin film of powder remains on the skin. For this reason lotions may have a drying effect on the skin. Examples include calamine lotion and the antifungal Resizole (Virbac U.S.). Lotions also are available with a variety of antipruritic and parasitic medications.
Both aerosol and pump sprays are available for many veterinary products. Those with alcohol bases may be drying to the target area. Clipping animals may be necessary to facilitate penetration of the hair coat. Foams consist of a mixture of finely divided gas bubbles interspersed in a liquid. These preparations provide an effective way of spreading a small amount of liquid over a large surface area. A potential drawback of sprays is the noise of the application, which may frighten the patient. Both sprays and lotions may be easier to use than creams and ointments, especially for dogs with long hair coats.
Creams and ointments are mixtures of grease or oil and water that are blended together into an emulsion. In general, ointments are greasy to the touch and form an occlusive layer over the skin, reducing water loss. Creams are smooth to the touch and, once applied to the skin, are rapidly absorbed or evaporate (i.e., there is no occlusive layer left on the surface of the skin). In general, ointments are contraindicated in exudative areas. Examples include triple antibiotic ointments and creams and hydrocortisone ointments and creams. Hydrocarbon bases are emollient, being composed of vegetable oils and animal fats. Examples include oleic acid, paraffin, petrolatum, and wax. They generally are hydrophobic and occlusive, causing the stratum corneum to hydrate. They are greasy, however, and cannot be washed off. Anhydrous absorption bases contain little to no water but readily accept large amounts of water while maintaining a thick consistency. Examples include hydrophilic petrolatum and anhydrous lanolin.
Emulsions are oil and water combinations. Water–oil emulsion bases are water-washable bases that are easily removed from the skin surface. The oil phase generally is petrolatum with an alcohol; the aqueous phase may be water, propylene glycol, polyethylene glycol, or glycerin. Oil-water emulsion bases are composed of an aqueous phase that is greater than the oil component. These tend to be water washable, nongreasy, and nonocclusive. Finally, water-soluble–based ointments have no hydrophobic lipid base. They are completely water soluble, do not hydrolyze, and do not support the growth of microorganism contaminants in the product. If the preparation is in a gelled medium, the product is a gel (e.g., a combination of propylene glycol, propylene gallate, methylcellulose, polyethylene glycol, and others). DMSO is commonly prepared as a gel.2 Gels are clear, colorless, and water miscible. Gels are becoming more popular because they can be rubbed into the skin to completely disappear and do not leave a sticky feeling. Examples of gels in veterinary medicine are KeraSolv, OxyDex and Pyoben.
DMSO has the ability to allow some substances ordinarily unable to penetrate the skin to be carried through it. DMSO is a waste product of wood processing that has been used in a large number of topical medicaments. In addition to its hydrophilic actions, DMSO is characterized by bacteriostatic, antiinflammatory, fibrinolytic, and vasodilatory actions. Topical analgesia may reflect a thermal effect, which occurs with direct application.2 At concentrations greater than 70%, however, DMSO can cause skin irritation. In concentrations greater than 50%, DMSO has been shown to enhance the percutaneous absorption of a large number of drugs, including glucocorticoids, antibiotics, hormones, and antiinflammatory agents. Absorption increases as DMSO concentration reaches 100%.2 DMSO increases percutaneous absorption of fluocinolone (a potent glucocorticoid) by a factor of five and other compounds by as much as a factor of 25. DMSO is approved for use only in the horse (for traumatic musculoskeletal injuries) and in the dog (in Synotic, a commercial steroid ear preparation). Any other use for DMSO is considered extralabel use. Toxic effects that should be considered when DMSO is used include teratogenicity (contraindicated in pregnant animals); potential for inducing degranulation of mast cells in underlying skin; and, in cats, hemolysis with hemoglobinuria and methemoglobinuria. DMSO has been shown to induce lenticular changes in animals and humans. Rubber gloves should be worn when DMSO is handled.
Adsorbents act to bind potentially noxious agents, keeping them from damaging the skin. Protectants provide an occlusive layer that physically protects the skin from the external environment. Together these two classes of vehicles are represented by dusting powders and mechanical protectives (kaolin, lanolin, mineral oil, petrolatum, zinc stearate). Dusting powders generally are inert, composed of starch, calcium carbonate, talc, titanium dioxide, zinc oxide, and boric acid. Smooth-surfaced powders prevent friction, protecting abraded and raw skin. Rough or porous powder surfaces absorb water, tending to occlude the skin surface when wetted. Rough powders should be avoided on moist or exudative lesions because of the risk of secondary bacterial or fungal infections. Care should be taken to make sure that powders, and in particular talc, are not used within a body cavity because of the potential for a massive granulomatous response.
Demulcents are high-molecular-weight water-soluble compounds that reduce irritation. Like protectants, they can coat the surface of damaged skin, protecting the stratum corneum and its underlying structures, and they inherently reduce irritation. Examples include mucilages, gums, dextrins, starches, methylcelluloses, and polyvinyl alcohol.2 Among those most commonly used in veterinary medicine are glycerin, propylene glycol, and polyethylene glycols. Glycerin, when used in high concentrations on the skin, can dehydrate and irritate it by increasing transepidermal water loss. Propylene glycol is miscible with water. Like glycerin, it is hygroscopic, is not occlusive, and also is bacteriostatic and fungistatic. As such, it might be considered the ideal vehicle. It spreads easily on the skin surface, has a low evaporation rate, is not greasy, and may hydrate rather than dehydrate the skin.2 A mixture of one part propylene glycol to one part water has been used to treat canine sebaceous adenitis. Topical hypersensitivity occurs occasionally. Several polyethylene glycols are available. They differ markedly in molecular weight, with the number directly correlating with size and viscosity. Polyethylene glycols that are 900 or above tend to be semihard to waxy solids at room temperature; lower-molecular-weight products are liquid. These compounds are not easily hydrolyzed but are very water soluble and nontoxic.2
Astringents cause precipitation of proteins and prevent exudation. Because of their inability to penetrate the skin, their action is predominantly on the surface. Many astringents are also antiseptic. Astringents can arrest hemorrhage by coagulating plasma proteins (ferric chloride, silver nitrate). Burow’s solution is available commercially as Domeboro (aluminum acetate powder or tablets) for use as an astringent in exudative dermatoses. Commercially available otic products containing Burow’s solution (Bur-Otic, Virbac U.S.)) can be used to treat dogs that spend a lot of time in the water or after bathing to reduce the risk of secondary infection. Magnesium sulfate (Epsom salt) is not an astringent but acts to dehydrate or “draw” water from the tissues.
Emollients are fatty or oleaginous substances that soften, protect, and soothe the skin. They are often used to make the cream or ointment vehicle in many dermatologic preparations. Examples include mineral oil, petrolatum, glycerin, and vegetable and animal oils. In veterinary medicine emollients are commonly used as cream rinses after baths. Most of these chemicals have a characteristic medicinal odor that appeals to owners. Active ingredients can be added (pramoxine hydrochloride) to enhance therapeutic effect.
Classes of Topical Drugs
Many topical products are commercially available, and many have multiple effects. Some of these agents are also discussed in other chapters (e.g., those on parasitology, antibacterials, and antifungals).
Antiseborrheics
Antiseborrheic drugs include keratolytics and keratoplastics. The appropriate antiseborrheic depends on the patient’s condition (seborrhea sicca versus seborrhea oleosa).
Sulfur is keratolytic (keratolytics hydrate and soften the stratum corneum, promoting its mechanical removal) and keratoplastic (keratoplastics normalize cornification). It has a mild follicular flushing action but is not a good degreaser. It also has antibacterial and antipruritic effects. Its keratolytic effects may reflect inflammation that ultimately causes sloughing of the stratum corneum. Keratoplastic effects probably reflect cytostatic effects.2 Many commercially available products containing sulfur are available. Shampoo products containing sulfur may have additional active ingredients for enhanced therapy. Lime sulfur (LymDip) is used for its antifungal, antipruritic, and antiparasitic effects.
Salicylic acid is keratoplastic, bacteriostatic, and mildly antipruritic. It is frequently used in combination with sulfur products, including most of the sulfur products listed previously. When salicylic acid is combined with sulfur, a synergistic effect results. In stronger concentrations (6%) it acts as a keratolytic.
Coal tar is keratolytic and keratoplastic and has good degreasing action. It is also frequently used in combination with sulfur and salicylic acid. Commercial shampoos are frequently used in veterinary medicine and include Lytar, Allerseb-T, and Mycodex Tar & Sulfur. Straight tar lotions should not be used in veterinary medicine. Coal tar is toxic to cats. Coal tar preparations are potentially irritating, photosensitizing, carcinogenic, and staining. In general, these products are reserved for severe seborrheic disorders.
Benzoyl peroxide (2% to 5%) is keratolytic, bactericidal, degreasing, and follicular flushing. It also is a strong oxidizer, free radical generator (and therefore antibacterial), and antimicrobial.2 Benzoyl peroxide is metabolized by viable epidermal cells in the skin to benzoic acid. In high concentrations it can irritate the skin. It may be too drying for some patients with seborrhea sicca. These products are not well tolerated by cats and should not be used on them. Commercial products available for veterinary patients include Oxydex, Pyoben, Derma Ben SS, and Benzoyl-Plus shampoos. A gel form of 5% is available for veterinary use, primarily for treatment of chin acne.7 Other uses include fold pyodermas and local superficial or deep pyodermas. Benzoyl peroxide will bleach clothing.
Resorcinol is a keratolytic agent that also has bactericidal and fungicidal effects. It is a protein precipitant that promotes keratin hydration, acting as a keratolytic.2 It often is combined with another keratolytic (e.g., sulfur, salicylic acid).2
Selenium sulfide is antiseborrheic, keratolytic, and keratoplastic by virtue of its antimitotic effects. Cell proliferation and sebum formation are slowed. It tends to be irritating, however, and can stain hair. Mucous membrane irritation may result if accidental contact occurs. A product for human beings is Selsun Blue. Fatty acids (e.g., undecylenic acid [Desenex]) are also keratolytic.
Retinoids
Retinoids are natural or synthetic derivatives of retinol (vitamin A) that exhibit vitamin A activity (Figure 22-1).8 Dermatologic effects of vitamin A include epithelial differentiation. Vitamin A deficiency causes metaplasia of glandular epithelia; excessive vitamin A causes keratinizing epithelia to differentiate into a secretory epithelia.9 The antikeratinizing effects are the target of drug therapy.9 Retinoids tend to “normalize” the skin. Although natural retinoids have proved to be too toxic for clinical use, the synthetic products are characterized by specific effectiveness with decreased toxicity. They tend to vary in bioavailability, in metabolism to active versus inactive metabolites, and in tissue distribution patterns. First-generation compounds include retinol and its derivatives tretinoin and isotretinoin. The second-generation products are synthetic and include etretinate and acitretin, approved for treatment of human acne and psoriasis, respectively. The third-generation compounds, arytenoids, are in development.8
The effects of the retinoids include cellular proliferation and differentiation, immunomodulation, inflammation, and production of sebum. Their actions are mediated by retinoic acid receptors, members of the thyroid/steroid receptors.8 Retinoids may influence genomic expression of cells by altering RNA synthesis, typical of other steroids. Tretinoin increases dermal thickness and granular layer thickness, decreases melanocytic activity, and increases the secretion of a polysulfated glycosaminoglycan intercellular matrix. In humans wrinkling is reduced. It is formulated as a 0.01% to 0.1% topical preparation. Therapy begins with lower concentrations and gradually increases. Adverse effects include erythema, peeling, burning, and stinging, which tend to decrease with time and are less likely to occur when the drug is prepared as an emollient.
Isotretinoin normalizes keratinization of the follicular epithelium and reduces sebum synthesis and, in human beings, Proprionobacterium acnes. It is administered orally, however, with cumulative doses being important to efficacy. Toxicity is manifested in the skin and mucous membranes; is dose dependent; and, in humans, may facilitate the growth of Staphylococcus aureus. Dermatologic manifestations include epistaxis, dry eyes, blepharoconjunctivitis, erythematous eruptions, and dry mucous membranes.8 Systemic manifestations can be minimized with short-term therapy and include increased liver enzymes, myalgia, and arthralgia. Teratogenicity occurs with all retinoids; the drugs generally are contraindicated in pregnancy. Isotretinoin was studied in dogs.10 Four of 29 developed conjunctivitis, which resolved once therapy was discontinued. Cats may have a higher incidence of side effects, including periocular erythema, epiphora, and blepharospasm. The potential veterinary applications of isotretinoin include selected abnormalities of sebum production such as primary idiopathic seborrhea and comedo syndromes.
Etretinate is a synthetic aromatic retinoid that is effective for the treatment of inflammatory psoriasis. The retinoids are highly potent aromatic analogs of retinoic acid and represent the third generation. Etretinate is extremely lipophilic and is stored in adipose tissue. Accumulation is sufficient to allow detection of the drug in humans 2 to 3 years after its use is discontinued. It normalizes keratin expression in epidermal cells, suppresses chemotaxis, decreases stratum corneum cohesiveness, and may impair cytokine function.8 It is less likely than isotretinoin to cause conjunctivitis, but hair loss, cutaneous exfoliation, bruising, and liver dysfunction are more common. Collection of a baseline minimum database is recommended for humans before its use. Its teratogenicity precludes use by women of childbearing age; owners of animals using the drug should be warned of its contraindications.8 The drug may no longer be available because of its adverse effects. Acitretin has now replaced etretinate in the U.S. market. A dose of 0.5 to 1 mg/kg orally per day was suggested.11
The use of retinoids in clinical veterinary medicine has not been well established. Animals are not afflicted by skin diseases typical of those for which retinoids are indicated in human patients (e.g., psoriasis, acne). Use is limited by lack of known effects and indications, cost, and the risk of side effects. Dogs, however, appear to be more tolerant of the retinoids than human beings.10,12 Side effects that have been reported in dogs include inappetence, vomiting, diarrhea, thirst, pruritis, conjunctivitis, cheilitis, stiffness, and hyperactivity.12 Keratoconjunctivitis has been reported in dogs.10,12 Tear composition is changed, leading to more rapid evaporation. Schirmer’s tear test should be monitored monthly for the first 6 months of therapy. Clinical pathology changes are rare, but monitoring before and 30 days after the start of therapy is recommended for dogs receiving synthetic retinoid therapy.12 In cats the most common side effect is anorexia. Teratogenicity is a likely problem, particularly with etretinate, when used for intact females.
The most common use of synthetic retinoids in dogs has been for treatment of keratinization disorders of dogs, particularly primary seborrhea of cocker spaniels. Etretinate has been evaluated in spaniels with idiopathic seborrheic dermatitis (approximately 10 mg or 0.75 to 1 mg/kg orally per day). Animals generally respond well with a decrease in scaling, a softening and thinning of seborrheic plaques, decreased pruritis, and reduction in odor. Response occurs within 2 months, and improvement continues for at least 2 more months.12 The more severe the syndrome, the slower the time to response; discontinuation of therapy is likely to result in recrudescence of clinical signs within 3 to 12 weeks.9 The drug was minimally effective in the treatment of ceruminous otitis associated with seborrhea.12 Maintenance therapy ranges from 10 mg every other day to 10 mg daily, alternating 30 days on and 30 days off. Isotretinoin (1 and 3 mg/kg per day) appears to be much less effective.9 Neither isotretinoin nor etretinate has proved to be effective in West Highland White Terriers, and etretinate was ineffective in Basset Hounds. Both etretinate and isotretinoin have proved effective in the treatment of Schnauzer comedo syndrome9,12 and canine ichthyosis. Newer applications of the synthetic retinoids include hair follicle dysplasia (etretinate), which should respond in approximately 30 days, and selected dermatologic cancers. These include solar-induced squamous cell carcinoma (etretinate 2 mg/kg divided or once daily for 6 months), mycosis fungoides (etretinate or isotretinoin 3 to 4 mg/kg divided or once daily), and selected benign cutaneous neoplasms (multiple sebaceous adenomas, epidermal cysts, inverted papillomas, and infundibular keratinizing acanthomas).12
Isotretinoin for the treatment of disorders of the sebaceous glands has been variably successful; success may be breed dependent. It was proved effective in sebaceous adenitis of standard poodles in one study but ineffective in another.9 A higher dose (2 to 3 mg/kg) has been recommended. Hair growth in poodles that do respond is abnormal, however, in that kinks are lost. Vizslas appear to respond to isotretinoin very well.9 In contrast, neither isotretinoin nor etretinate appears to be effective in sebaceous adenitis of Akitas.
Retinoids appear to be safe for cats but do not appear to be effective for solar-induced squamous cell carcinoma. Either isotretinoin or etretinate (2 to 2.5 mg/kg) can, however, be beneficial for preneoplastic actinic disease. Cats tolerate retinoids well, although anorexia is more common in cats than in dogs.12 Reducing treatment to every other day or every other week may limit this side effect. Topical tretinoin (0.025% cream) may be efficacious for treatment of feline acne. The product must be used very sparingly, however, so as not to incur severe tissue irritation.12
Although there are no reports of either acute or chronic toxicity in animals receiving retinoids, animals nonetheless should be closely monitored. Monitoring should begin with a physical examination before implementation of therapy, including measurement of tear production. These tests should be repeated at 4- to 6-month intervals. A complete blood count and serum chemistries, including triglycerides, should be monitored at baseline, at 1 and 2 months of therapy, and then every 4 to 6 months during therapy. Care should be taken to counsel clients regarding the cost, importance of compliance, and risk of accidental human ingestion.
Ceruminolytics
Ceruminolytics are topical products that emulsify, soften, and break up waxy debris and exudate. Generally, they are detergents or surfactants used for cleaning or flushing the ear. Examples include dioctyl sodium sulfosuccinate, which is water soluble (and perhaps less messy); squalene, which is an oil-based product; propylene glycol; glycerin; and oil. Carbamide peroxide differs from most other ceruminolytics in that it is a humectant, releasing urea and oxygen to cause its foaming action. Ceruminolytics and drying products are often combined with alpha hydroxy acid such as lactic, salicylic, benzoic, and malic acids. These acids have the added advantage of decreasing local pH and are mildly antibacterial and antifungal, along with their keratolytic effects. These products need to be placed in the ear 3 to 15 minutes before flushing with water or saline.
Antipruritics
Antipruritics (topical)5 are used to provide temporary relief of itching, but their efficacy is debatable. In general, antipruritics relieve itching by four mechanisms. (1) The itching sensation can be substituted with another sensation (such as heat or cold). Examples of agents with this mechanism of action include menthol, camphor, warm soaks or baths, and ice packs. (2) The skin can be protected from external factors such as scratching, biting, irritants, and changes in humidity or temperature. This can be accomplished with bandages or impermeable protective agents. (3) Peripheral sensory nerves can be anesthetized by local anesthetics (benzocaine, lidocaine). These drugs may, however, cause allergic sensitization. A new product in this category for small animals is pramoxine (Dermacool). (4) Biochemical agents used topically to treat pruritus include glucocorticoids and antihistamines. Despite the fact that the skin contains large numbers of mast cells, topical antihistamines do not seem to be efficacious. Systemic antihistamines, on the other hand, may be useful.
Topical glucocorticoids may not be as potent as their oral or injectable counterparts.13 Like systemic glucocorticoids, however, the active ingredients vary in potency and risk of side effects. For topical glucocorticoids ointments provide greater efficacy than creams. Topical glucocorticoids can be absorbed through the skin and cause systemic effects. This is more likely to be a problem with the potent fluorinated agents (betamethasone, dexamethasone, triamcinolone, flumethasone, and flucinolone) and when combined with DMSO (Synotic). Gloves should be worn to apply these drugs. There are many forms of glucocorticoids available for topical use, including use on extensions of the skin such as the external ear canal and anal sacs. Once absorbed through the skin, topical corticosteroids are handled by the body in the same capacity as systemically administered glucocorticoids. The extent of percutaneous absorption of topical glucocorticoids depends on factors such as the vehicle, the ester form of the steroid (greater lipid solubility enhances percutaneous absorption), duration of exposure, surface area, and integrity of the epidermal barrier. A new product containing 0.015% triamcinolone (Genesis spray, Virbac U.S.) has been formulated to be applied topically to the entire skin surface for its antipruritic effect.11 This product may also be used for spot treatment of pruritic regions. Ointment bases are occlusive and are therefore more likely to increase percutaneous absorption of the same glucocorticoid in a cream base. Highly potent preparations in any form should not be used on abraded skin.
Irritants
A number of products are used to inflame or irritate the skin to various degrees. Examples include those that cause hyperemia (rubefacients), inflammation (irritants), and cutaneous blisters (vesicants). Caustics are corrosive agents that destroy tissue after one or more applications. Examples include camphor, coal tar, creosote, menthol, methyl salicylate, iodine, mercuric iodide, alcohols, and pine tar. Among these, only coal tar is used to any degree in veterinary medicine. It is a by-product of bituminous coal distillation and, as an irritant, decreases epidermal synthesis of DNA.2 Escharotics also are corrosives that precipitate proteins, causing the formation of a scab and eventually a scar. Examples include glacial acetic acid, aluminum chloride, gentian violet, phenol, salicylic acid, and silver nitrate. The uses in veterinary medicine are few.2 Irritant products have been used empirically for many centuries. Their proposed mode of action is masking of moderate to severe pain by milder pain caused by the application. Another desired effect of irritants is to induce a healing action on chronic wounds. The idea is to heal chronic inflammation by converting it to acute inflammation. Chemicals used include phenol, formalin, mercuric iodide, and camphor. Menthol-containing products are sometimes used to treat acral lick dermatitis (“lick granuloma”) in dogs but may be painful upon initial application. Capsaicin has been used topically on human beings for relieving arthritis pain. It also has been used to treat acral lick dermatitis in dogs.
Antimicrobials
Alcohols, iodine, chlorhexidine, iodophors, and hexachlorophene can be effective in the treatment of infectious skin diseases (see Chapters 10, 11, and 13).
Benzoyl peroxide, discussed with the antiseborrheics, is a potent broad-spectrum antibacterial agent. It is an excellent adjunctive treatment for pyoderma. In a clinical trial of four antibacterial shampoos (containing 3% benzoyl peroxide, 0.5% chlorhexidine, 1% available iodine in a povidone complex, and 0.5% triclosan combined with 2% salicylic acid and 2% sulfur), although each was effective prophylactically, the product containing benzoyl peroxide was most effective.14 Use of the veterinary products (as opposed to the human proprietary products) is strongly recommended. Benzoyl peroxide is very irritating to cats and should be avoided.
Many antibiotics are available in topical form as ointments. Examples include neomycin, bacitracin, polymyxin B, gramicidin, and nitrofurazone. Often these drugs are available in combination with each other or with steroids.
Mupirocin is a compound produced by Pseudomonas fluorescens that is effective against superficial (topical) infections caused by Staphylococcus species. It is less active against gram-negative organisms and in humans is not active against normal skin flora. It inhibits protein synthesis by binding to bacterial tRNA synthetase. Prepared as an ointment, it often is used for prophylaxis of superficial infections resulting from wounds and injuries.8 Veterinary products containing mupirocin recently have become available (Muricin ointment 2%, Dechra).
Treatment of otitis externa often involves a topical antibiotic–steroid combination such as Tresaderm or Panalog. Any of the agents contained in these products, neomycin in particular, can cause allergic sensitization or irritation. Cats appear to be more sensitive to topically applied otics than dogs.
In general, topical therapy of dermatophytoses in dogs and cats is not highly effective because of the thick hair coat and the location of the organisms deep in the hair follicle. The drugs are often unable to reach the site of the infection in adequate concentrations. Gentle clipping of the affected area(s) may aid in topical application, but whole body clipping is generally not recommended. Amphotericin B (Fungizone), available as 3% cream, lotion, or ointment, can be used for Candida infections. Chlorhexidine is a mild antifungal as well as antibacterial and is available as a rinse or shampoo (1% to 4% recommended). By itself it is not very effective for the treatment of dermatophytosis. New formulations combined with miconazole (Malaseb rinse, shampoo and spray DVM) or ketoconazole (Ketochlor, Virbac US)) shampoo can be used for Malassezia dermatitis or adjunct therapy for dermatophytosis. Clotrimazole 1% (Lotrimin, Veltrim) is effective against dermatophytes, Candida, and Malassezia. It may cause mild irritation. Miconazole is available as a 2% cream or 1% lotion (Conofite and Resizole 2%) and shampoo (Dermazole) and is effective against dermatophytes, Malassezia, and Candida. Nystatin (Panalog ointment or cream, nystatin cream) is effective against some yeast and some dermatophytes but not Malassezia species. Sulfur is effective against dermatophytes and therefore may be used for localized or generalized dermatophytosis (LymDyp, DVM). Cats may become ill if they groom after treatment so it is recommended that they wear an Elizabethan collar until the product is dry. Lime sulfur dip is also antiparasitic and antipruritc and very cost effective compared with other topical treatments. Lime sulfur dip may stain fabric and can tarnish jewelry. It is a very efficacious product, but because of the strong odor, owner compliance may be weak. Thiabendazole is effective for dermatophytes and some yeast, including Malassezia. Products including thiabendazole include Tresaderm, a combination product with neomycin and dexamethasone, which is best used for local lesions. In general, the use of a topical agent containing corticosteroid is not recommended when treating dermatophytosis.
Antiparasitics
Drug delivery systems
Antiparasitics are available as sprays, powders, shampoos, foams, spot-ons, tablets, and dips. The use of parasiticides is discussed in Chapter 15. Spot-on products are commonly used as broad-spectrum antiparasitic agents. Their ease of use and efficacy make them a preferred product for many circumstances. Powders are the safest formulation but must be frequently applied. They often are messy and must be applied deep into the coat to be effective. Sprays may have little residual effect depending on the active ingredient and concentration of the product, and the noise made during application often frightens the animal. Efficacy can be enhanced by ensuring adequate penetration of the hair. The hair should be brushed away from the skin so that the spray can reach the skin. The face can be treated by spraying into a glove and then rubbing the face. A water-based spray may cause less drooling than an alcohol-based spray.
Shampoos have little residual effect and must stay on the skin at least 10 minutes to kill fleas and ticks. The active ingredients (pyrethrin, pyrethroids, carbaryl) in these preparations are not intended to be absorbed systemically. Any factor that would increase the absorption of these drugs (see earlier discussion) may result in system toxicity. Because cats are especially susceptible to the toxicities of certain parasiticides, only those products specifically intended for use on cats should be used. Flea shampoos should not be used more than once weekly to avoid drying of the skin. A humectant rinse may help compensate for the drying effects. Shampoos remove flea eggs, flea feces, and other debris, facilitating other topical therapy and making the animal look and feel better. High-concentration pyrethrins may be effective as repellents for fleas and ticks. They are rapidly destroyed by ultraviolet light. Toxicity can follow ingestion (grooming) or percutaneous absorption. Toxicity is manifested as salivation, tremors, and seizures. Treatment is symptomatic but should include bathing.
A number of spot-on products act as adulticides and some are effective against the juvenile stages as well. They are applied to the infrascapular area from where they diffuse over the body. Some products are intended to be systemically absorbed for their antiparasitic effects. They can cause contact allergy and irritation. Examples include imidacloprid (Advantage, Bayer), fipronil (Frontline, Merial) selemectin (Revolution, Pfizer), metaflumazone (ProMeris, Fort Dodge), and dinotefuran (Vectra 3D, Summit).
In most cases flea collars have limited efficacy. Collars containing carbaryl, pyrethrin, or organophosphates are readily available in stores. Besides having limited efficacy, collars occasionally cause irritation reactions. Collars containing methoprene (Ovitrol) act to “sterilize” the fleas and are helpful in flea eradication. To keep clients from being disappointed with the results, clinicians should inform them that these products do not kill adult fleas. Collars containing amitraz (Preventic) are effective against ticks but not fleas. The feeding ticks will detach and die. Recent studies demonstrate that the tick will be killed before being able to transmit borreliosis, but other tick-borne diseases were not evaluated. This collar will not affect nonfeeding ticks. Ingestion of the collar is associated with acute toxicity. Yohimbine can be an effective antidote. Even though the active ingredient is amitraz, it has been shown to have many beneficial effects for the treatment of demodicosis.
Active ingredients
Pyrethrins are extracts from the chrysanthemum flower. Their mechanism of action involves disrupting neurologic function by prolonging Na+ in nerve membranes. They rapidly kill fleas, flies, lice, cheyletiella, otodectes, and mosquitoes but have little residual activity. Although among the safest products for use in cats, only those products approved for cats should be used. Pyrethrin-containing products are available in many formulations. Both pyrethrins and permethrins have repellent properties, but their residual effects are not well documented.
Pyrethroids are synthetic analogs of pyrethrins with the same mechanism of action but greater ultraviolet stability and thus longer action. Microencapsulation of pyrethroids provides further residual activity. They have a slower knockdown than pyrethrins, and thus they are often combined with them. Toxicity and treatment thereof is the same as for pyrethrins. Permethrins are available as 0.05% to 25% flea sprays but also up to 65% as spot flea and tick products. A relatively new product (K9 Advantix) combines imidacloprid with permethrin for increased efficacy against ticks. Toxicities have been seen with this product when it was inadvertently applied to a cat.
Chlorinated hydrocarbons should not be used (if there happens to be any still on the market). There are two types of cholinesterase inhibitors available: carbamates and organophosphates.
Carbamates such as carbaryl are available in sprays, dips, collars, and premise-control sprays, and they are safe for dogs and cats. Toxicity of carbamates as well as organophosphates reflects overstimulation of the parasympathetic system and should be treated with atropine and 2-pyridine aldoxime methylchloride.
Organophosphates are the most toxic insecticides used in veterinary medicine. With one exception, these agents should not be used around cats. Care should be taken to avoid cumulative exposure if animals are exposed to this class of insecticide in lawn and garden preparations. Examples of commonly used organophosphates are chlorpyrifos (Dursban, Duratrol), used for flea sprays and dips; diazinon, used for environmental flea and tick control; malathion, used on both cats and dogs and often combined with other insecticides (noncholinesterase inhibitors); phosmet (Paramite Dip), useful for flea control and sometimes used for scabies; cythioate (Proban), a systemic insecticide; and fenthion (Pro-Spot), topically applied for a systemic effect. Organophosphates are disappearing from the market because of concerns of safety of animals and human beings.
Fipronil (Frontline) is a new synthetic molecule in the phenylpyrazole family. It acts at gamma-aminobutyric acid (GABA) receptors and inhibits GABA-regulated chloride flux into the nerve cell. It is a flea adulticide and has efficacy against ticks. It may also be effective in preventing scabies mite infestation. Preliminary studies indicate good residual activity even after bathing. This product is available as an on-animal spray or as a spot-on product. Frontline Plus contains fipronil and methoprene for its ovicidal properties.
Imidacloprid (Advantage and K9 Advantix) is a spot-on application product that kills adult fleas. It works by preventing postsynaptic binding of acetylcholine, leading to respiratory paralysis of the flea. This product may be removed by frequent bathing but is labeled as waterproof. It must be applied every 30 days to be effective. Advantage may be applied as frequently as every 7 days if needed to treat severe infestations of fleas. The addition of permethrin to the K9 Advantix provides efficacy against ticks and mosquitoes and repellent properties against mosquitoes and other parasites. Again, this product is to be used only on dogs.
A combination product containing imidacloprid and moxidectin (Advantage Multi) is available in a spot-on formulation for dogs and cats. The age restriction is 7 weeks and 9 weeks, respectively. This product is used to treat for fleas, heartworm prevention, intestinal worms, and ear mites. It is not approved for use in the United States for the treatment of sarcoptes (scabies) or demodicosis but may prove to be efficacious.
Selamectin (Revolution, Pfizer) is available as a spot-on formulation. It is approved for the treatment of fleas, heartworm prevention, tick (Dermacentor sp.) infestations, sarcoptes (scabies), and otodectes (ear mites) for dogs. The age restriction is 6 weeks. The formulation for cats has an age restriction of 8 weeks. It is approved for the treatment of fleas, heartworm prevention, otodectes, roundworms, and hookworms. Anecdotal reports indicate efficacy for feline scabies mites, Notoedres, though not approved for this use.
Nitenpyram (Capstar, Novartis) is an oral medication for the treatment of flea infestations in dogs and cats. The age restriction is 4 weeks, and the animal’s body weight must be greater than 2 pounds. This product may be given daily but is often used in conjunction with other flea medications on an as-needed basis. Rapid and complete kill of adult fleas is the main reason for using this product. Anectotal reports indicate efficacy when inserted rectally (i.e., during a surgical procedure when fleas are discovered). It has also been used to treat subcutaneous maggot infestations of dogs and cats. Neither of these uses are licensed or approved by the Food and Drug Administration.
Amitraz (Mitaban) is a monoamine oxidase inhibitor. It is the only licensed product for treatment of generalized demodicosis. Mitaban is also efficacious against scabies and ticks, but this is considered an off-label use. This product rapidly oxidizes on exposure to light and air, and the breakdown product is more toxic than the parent compound. Mitaban should be mixed fresh each time, and the entire contents should be used to avoid toxicity. Side effects include sedation and lethargy (sometimes for 24 hours), pruritus, bradycardia, hypothermia, hypotension, and hyperglycemia. Hyperexcitability is an uncommon side effect. Amitraz is not appropriate for epileptic dogs and animals receiving behavior-modifying drugs. Yohimbine works as a reversing agent. The large animal form of amitraz (Taktic) should not be used on dogs. ProMeris for dogs (Fort Dodge) is a spot-on formulation containing amitraz and metaflumazone. It has recently been approved to treat localized and generalized demodicosis in dogs greater than 8 weeks of age. The recommended application rate is every 14 days until remission. Potential side effects include those associated with amitraz application. Anecdotal skin reactions have been noted.
Ivermectin (Ivomec 1%) is a GABA agonist that leads to parasite paralysis. In mammals GABA in the central nervous system is protected by the blood–brain barrier. Ivermectin is a large molecule that cannot pass the blood–brain barrier except in certain breeds. It is efficacious against scabies, lice, otodectes (ear mites), and cheyletiella. It is not approved for use in small animals for treatment of parasites other than as a heartworm preventive. Ivomec is rapidly absorbed orally or subcutaneously. The administration into the ear canal is not recommended except for the 0.01% ivermectin product Acarex. It should not be used in Collies, Border Collies, Shetland Sheepdogs, Australian Shepherds, Old English Sheepdogs, or any dog that looks like a Collie. Also, it is not recommended for any animal younger than 12 weeks of age. Daily ivermectin has been used to treat demodicosis. Enzodiazepines are contraindicated for concurrent use. Treatment of ivermectin toxicity is symptomatic and supportive. There is no good antagonist available.
Milbemycin (Interceptor) has a similar action to ivermectin. Its use in dermatology is confined to daily oral administration for the treatment of refractory demodicosis. Therapy may take 6 to 9 months, and relapses are common. Cost is a limiting factor. This drug is not approved for use for demodex treatment. Although not contraindicated in Collies and Collielike dogs, caution is advised for side effects. A new otic product containing milbemycin (Milbemite) is a very effective treatment for ear mites in dogs and cats.
Spinosad is the active ingredient in Comfortis (Eli Lilly). This is an oral tablet that is given monthly to control fleas on dogs only. The age restriction is 14 weeks. Safety in pregnant animals is not evaluated. Side effects have been noted when this medication has been used in conjunction with off-label usage of ivermectin; this is not recommended. This product has no efficacy against ticks. Vomiting was the most common adverse reaction noted. Absorption of the medication is adequate after 1 hour.
A combination product containing dinotefuran, permethrin, and pyriproxyfen (Vectra 3D, Summit VetPharm) has been approved for use on dogs older than 7 weeks of age. This spot-on product is effective against fleas and ticks and will repel and kill mosquitoes. It is recommended to apply this product monthly. The feline formulation does not contain permethrin. The age restriction is 8 weeks for kittens.
A combination product containing metaflumizone and amitraz (ProMeris, Fort Dodge) is a spot-on product approved for dogs older than 8 weeks of age. It is effective for fleas and ticks and has recently been approved for the treatment of localized and generalized demodicosis. ProMeris for cats (8 weeks or older) does not contain amitraz and is for the treatment of fleas only.
Flea insect growth regulators (IGRs) are endogenous chemicals in insects that control the early stages of their metabolism, morphogenesis, and reproduction. Synthetic compounds mimic the effects of the natural chemicals. Because IGRs maintain high levels of these chemicals during maturation and development of larvae, insects are prevented from developing. Natural levels decrease over time and allow normal maturation. IGRs have no effects on mammals and are very safe. They are combined with pyrethrins or pyrethroidsor fipronil to increase the spectrum of activity to include adults. Several products are available: Methoprene (Ovitrol spray) is a juvenile hormone analog available for on-animal and environmental use. It is degraded by ultraviolet light and hormone esterase. Pyriproxifen (Nylar) is a juvenile hormone mimetic for fleas. Preliminary studies indicate very long residual activity even when the animal is bathed and excellent environmental stability. This ingredient is available mixed with 2.5% permethrin (KnockOut spray) and is a very effective treatment for canine flea allergy. The high concentration of pemethrin precludes its use on cats. Pyriproxifen is also included in Vectra 3D (Summit Labs).
Lufenuron (Program) is a benzoylphenylurea that inhibits synthesis and deposition of chitin within the ova and larval exoskeleton of developing fleas. It is strongly lipophilic and stored in adipose tissue with slow release into the blood vasculature, providing long residual activity from a single dose. It does not affect adult fleas. Lufenuron is taken up by the feeding flea and incorporated into the developing egg. It is excreted in the flea feces. If the flea larvae consume flea feces containing lufenuron, they will be unable to mature into the pupal stage. Because of the slow absorption from the gastrointestinal tract, this product is given with food. Cats do not absorb this product as well as dogs, and therefore a higher dosage on a per-pound basis is needed for the same efficacy and duration of effect. This product is very safe and carries no contraindications.
Miscellaneous
Pennyroyal oil is a volatile oil extracted from plants in the mint family. Because of its limited efficacy and evidence of hepatotoxicity, this product is not recommended. D‑limonene is from oils of citrus fruits. Toxicities have been noted in the cat, especially depression, ataxia, and toxic epidermal necrolysis. This product is not recommended. Tea tree oil contains various monoterpenes. Toxicities with this oil have been reported, and there is no scientific evidence to support claims of its efficacy as an antiparasitic agent.
Systemic Dermatologic Therapy
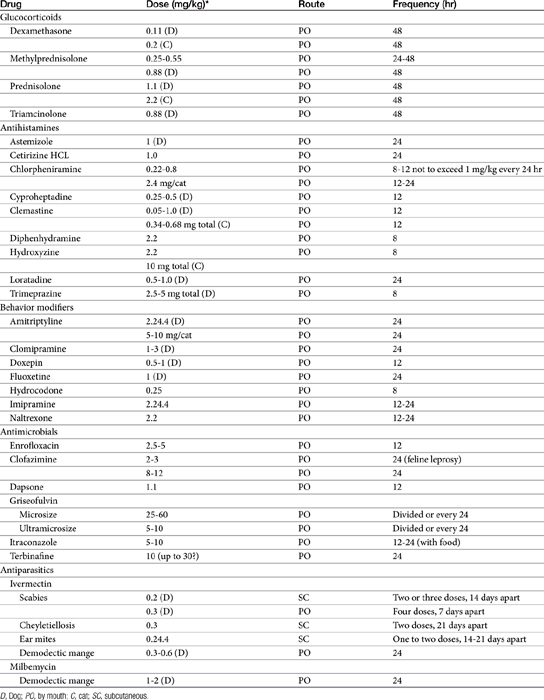
Systemic dermatologic therapy is indicated for diffuse, serious, or chronic conditions (Table 22-1).
Antimicrobials
Antimicrobials indicated for the treatment of dermatologic disorders are discussed in Chapters 10 and 11. A number of antimicrobials are effective for the treatment of bacterial skin diseases (pyoderma). Care should be taken when using sulfonamides, often selected as first-choice therapy, because of their ability to suppress the synthesis of thyroid hormones (see Chapter 32). A study in 20 dogs receiving sulfamethoxazole (with trimethoprim) at 30 mg/kg every 12 hours for 6 weeks found marked suppression of thyronine concentrations and response to thyroid-stimulating hormone. Suppression did not occur with 15 mg/kg of sulfadiazine (trimethoprim) once daily for 4 weeks.15 Thyroid function returned to normal within 3 weeks of discontinuing therapy. Suppression may result from inhibition of thyroid peroxidase by the amino group in the sulfonamide. The effect of sulfonamides on feline thyroid function has not been reported.
Antiinflammatory and Antipruritic Agents
Glucocorticoids
Glucocorticoids are discussed in Chapter 30. Glucocorticoids continue to be overused and abused for treatment of dermatologic diseases.13,16 Scott13 noted that more than 50% of his referral cases are complicated by the excessive use of the drugs. Yet glucocorticoids remain an important and legitimate component of both acute and chronic treatment of a variety of skin diseases associated with pruritus or inflammation resulting from allergic diseases (e.g., atopy, flea bite, other insect- and arachnid-mediated hypersensitivity, food hypersensitivity, contact dermatitis), pyotraumatic dermatitis (“hot spots”), and acral lick dermatitis.13 Note that glucocorticoids for acral lick dermatitis may largely be replaced with behavior-modifying drugs for this syndrome. Glucocorticoids also remain the cornerstone of therapy for many of the autoimmune diseases affecting the skin, including the eosinophilic granuloma complex, pemphigus complex, systemic lupus erythematosus, and discoid lupus erythematosus. Optimal therapy of each of these diseases varies, insofar as some may respond to glucocorticoids alone and some may require a combination of glucocorticoids and alternative immunosuppressive drugs such as azathioprine, chlorambucil, cyclosporine, or cyclophosphamide. Routes of administration vary with lesion and intent and include oral, topical, intralesional, and systemic (intravenous, subcutaneous, intramuscular). The use of topical glucocorticoids was previously discussed; note that side effects of glucocorticoids will not necessarily be prevented by limiting therapy to topical application. In general, oral administration is preferred for its convenience and ability to regulate dosage safely (including rapid withdrawal relative to other routes). Although some animals may appear to respond better to injectable rather than oral drugs, differences in response may reflect an insufficient oral dose. This is particularly likely to occur if the oral drug is one that is less potent than the injectable drug.13
The choice of glucocorticoid should be based on desired potency (e.g., dexamethasone is more potent than prednisolone and may be preferred for acute needs) balanced with avoidance of side effects (prednisolone is characterized by a smaller tendency to affect the hypothalamic–pituitary–adrenal axis negatively; see Chapter 30).16 Personal preference among clinicians ultimately also will determine the selection of specific drugs: Whereas prednisolone may be efficacious for some situations, it may not be for others. In addition, an animal may develop intolerable side effects with one glucocorticoid but not another. The development of steroid tachyphylaxis may lead to deselection of a steroid that previously was efficacious.13 Remission in the case of acute exacerbation of clinical signs might respond to a pulse-dose approach using the original antiinflammatory dose.16
Dermatologic conditions requiring glucocorticoid therapy range from mild to serious. Dermatoses associated with life-threatening conditions generally are limited to diseases that also are accompanied by diseases of multiple organs (e.g., immune-mediated disease). In such cases glucocorticoid therapy should be aggressive, with doses sufficiently high to control disease. Regardless of the indication of glucocorticoids, alternate-day therapy should be a goal of maintenance.16 Not all conditions will, however, be sufficiently controlled with alternate-day therapy.13 Because high doses of glucocorticoids are often required to adequately treat immune-mediated diseases, adverse effects are likely to occur. Concurrent administration of additional immunosuppressive drugs (azathioprine, chlorambucil, cyclosporine, or cyclophosphamide) may allow the glucocorticoid dose to be decreased. Dose reduction for patients with autoimmune diseases should be conducted gradually and should occur for at least 2 weeks (longer if time to clinical remission was prolonged), and the actual dose should be decreased by no more than half. Relapse may occur if the dosage is decreased too rapidly. Clinical reassessment should continue until a minimally effective dose is established for maintenance therapy.
Chronic inflammatory disorders (e.g., atopy or flea allergy dermatitis) should be treated less aggressively. A minimum effective dose should be determined by trial and error and reevaluated such that the dose is reduced when possible. Agents that are amenable to alternate-day administration include the first-choice drugs prednisone and prednisolone and the second-choice drug methylprednisolone.16 The ideal alternate-day dose for these drugs is 0.22 to 0.55 mg/kg.16 The durations of action of hydrocortisone and cortisone may be too short for effective alternate-day therapy. Although triamcinolone’s duration of antiinflammatory action is longer than that of prednisolone and methylprednisolone, suppression of the hypothalamic–pituitary–adrenal axis is more likely but less typical of the long-acting agents such as dexamethasone and betamethasone.
All patients receiving long-term glucocorticoids should be monitored, with physical examinations occurring at least twice yearly. Urinalysis is recommended with a urine culture because of the risk of subclinical urinary tract infection.16 In cases of relapse, the animal should be reevaluated for complicating diseases or conditions such as pyoderma, dermatophyte infection, and demodicosis.13,16 Glucocorticoids should be discontinued whenever possible; however, discontinuation may not be possible for immune-mediated disorders.13,16 Concurrent treatment with nonglucocorticoid antipruritics such as antihistamines or fatty acid supplements, either systemically or topically, should be attempted to reduce the glucocorticoid dose16 Other agents being studied include misoprostol and cyclosporine.
Cyclosporine
Cyclosporine may be used to control pruritus in dogs and cats with atopic dermatitis.17,18 It appears to be quite effective at 5 mg/kg orally once daily, and doses range from 2.5 to 6 mg/kg daily. Initially it should be given with food to decrease the likelihood of vomiting, but it is best given on an empty stomach for improved absorption. Food decreased the bioavailability by 22%.19 The water-soluble forms should be selected (Atopica, Neoral). Long-term use of cyclosporine for the treatment of canine atopic dermatitis has been reviewed.20 Laboratory abnormalities were detected in 25% of the dogs; 0.039% developed oral growths, and 0.058 % developed hirsutism. Many dogs were tapered to 2 to 3 times per week to control clinical signs. Interestingly, 24% of the dogs did not require ongoing therapy when cyclosporine was discontinued. In another report approximately 40% of dogs treated with cyclosporine for 4 months did not relapse during a 2-month follow-up.19 There appears to be good evidence to support the beneficial response to cyclosporine therapy in atopic dogs.21 A poor response to therapy, not due to side effects of cyclosporine, may indicate secondary skin infections, ectoparasitism, or non–atopy-related causes of the pruritus. Cyclosporine has also been used for treating canine perianal fistulae, erythema multiforme, sterile granuloma, pemphigus complex disease, and occasionally sebaceous adenitis.22 Tacrolimus ointment (Protopic 0.1%) applied topically once daily for 4 weeks led to clinical improvement of localized lesions associated with atopic dermatitis in the dog.23 Improvement was not noted in dogs with generalized lesions. Tacrolimus ointment may also be used for the treatment of autoimmune diseases with localized severity such as discoid lupus erythematosus confined to the planum nasale. This potent immunosuppressive agent is still in its infancy with respect to the uses in veterinary dermatology.
Dapsone
Dapsone is a sulfone product that has been used dermatologically for its antiinflammatory effects.8 Prevention of myeloperoxidase respiratory burst impairs white blood cell activity, and blocking of integrin-mediated adherence impairs neutrophil migration. Antibody adherence to neutrophils also is blocked. Dapsone is approved for use in humans for a number of immune-mediated diseases. Dapsone is metabolized to a toxic compound (dapsone hydroxylamine) that depletes glutathione in cells with a glucose-6-phosphate dehydrogenase deficiency in people; the importance of this effect has not been documented in animals. The metabolite, however, causes rapid hemolysis. Cimetidine can be used to minimize toxicity by competing for drug-metabolizing enzymes.8
Antihistamines
Despite structural differences, all classes of H1 antihistamines have similar antiinflammatory actions and side effects. The primary mechanism of action of these drugs reflects competitive inhibition of histamine at the receptor. Newer H1 antagonists also block histamine release and have proved effective for treatment of atopy in humans (see the discussion of antihistamines in Chapter 31). However, differences in response among drugs, species, or disorders might also reflect impaired histamine release from mast cells or altered T-cell function. Side effects of these drugs also vary with the product and include gastrointestinal upset and neurologic manifestations, including drowsiness (the most common side effect) and hyperexcitability. Contraindications include central nervous system disorders (including epilepsy), glaucoma, and smooth muscle motility disorders such as might occur in the gastrointestinal or urinary tract. These products are generally used safely in human pregnancy, although safety has not been established in the pregnant or nursing cat and dog. Products with antihistamine activity only generally have fewer contraindications; the particular product should be reviewed before use and those with other pharmacologic effects avoided when appropriate.
Antihistaminergics can be beneficial in some cases of pruritus in dogs and cats. Because of varying effects among the drugs, each antihistaminergic should be tried for at least 1 week before an alternative medication is sought. Among the drugs tested in clinical trials, clemastine appears to be the most effective in stopping itching associated with pruritus in dogs and cats and is the antihistamine of choice.24 Cost, however, can be prohibitive. A recent study found that after oral administration of clemastine, bioavailability was only 1% to 6%. It may be necessary to modify dose regimens for greater systemic effect.25 Chlorpheniramine should be considered next for both dogs and cats.7 The bitter taste of chlorpheniramine might be avoided by use of time-release capsules, which need to be administered only once daily. Diphenhydramine and hydroxyzine may be less efficacious; in addition, hyperexcitability may limit use in cats. A minimally effective dose may reduce the incidence of side effects. Astemizole is another recently approved human product. Although it appears safe (1 mg/kg), its efficacy has not been established in animals. In human medicine a combination of H1 (the traditional antihistaminergic selection) and H2 blockers has been recommended. The immunomodulating effects of H2 blockers may benefit the dermatologic patient.8 Newer H1-blocking drugs (loratadine) do not cause anticholinergic effects and are not sedating. Caution should be taken when combining these products with H2 receptors, however, because of an increased risk of cardiac arrhythmias, probably due to inhibition of drug-metabolizing enzymes.8 Antihistamines may act synergistically with misoprostol in controlling pruritus. There is fair evidence for its efficacy in controlling pruritus in dogs with atopy21; studies currently are under way.
Omega-3 (Omega-6) Polyunsaturated Fatty Acids
Body fats are stored as either adipose tissue or structural fat. Adipose tissue is rich in triglycerides, which are composed of a glycerol backbone and three fatty acids. Structural fats are represented by phospholipids, also composed of a glycerol backbone; two fatty acids (at the 1 and 2 positions); and a phosphate group (at the 3 position). Fatty acids can be released from glycerol by phospholipase. Saturated fatty acids have no double bonds. Unsaturated fatty acids (UFAs) include monounsaturated fatty acids, which have one double bond, and polyunsaturated fatty acids (PUFAs), which have two or more double bonds. The shorthand identification system of PUFA reflects the number of carbon atoms, the number (n) of double bonds, and the position of the first double bond from the terminal or omega methyl group end of the molecule.3 For example, the formula for linoleic acid, an essential fatty acid for mammals, is 18:2n‑6; it contains 18 carbons and two double bonds, with the first double bond located between the sixth and seventh carbon. Alpha-linolenic acid (ALA) (18:3n-3) also contains 18 carbons but has three double bonds, with the first located between the third and fourth carbon (Figure 22-2).
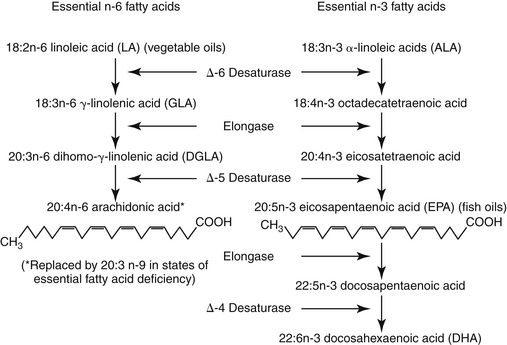
Figure 22-2 The three families of fatty acids are the plant-derived (α-linolenic acid) and fish oil-derived (eicosapentaenoic acid) n-3 family, the plant-derived n-6 family, and the de novo (nonessential) fatty acid family. Fatty acid biosynthesis includes desaturation, wherein a double bond is added, and elongation, wherein two carbon atoms are added. The same enzymes are used for fatty acid biosynthesis; the fatty acids in different families are not interconvertible.
Essential fatty acids (EFAs) are those fatty acids required for normal physiologic function that cannot be synthesized by the animal and thus must be obtained in the diet.3 Among the functions of EFAs are serving as a structural component of cell membranes, primarily as arachidonic acid and in specialized tissues (retina and brain) as eicosapentaenoic acid (EPA) and docosahexaenoic acid.3 The PUFA component is important in determining fluidity of the membrane, rendering it more stable, and maintaining cellular permeability. The epidermal water barrier of the skin depends on linoleic acid (LA) lipids located in the intercellular lamellar granules at the level of the stratum granulosum–corneum interface. EFAs are also the source of eicosanoids, from which are derived prostaglandins, leukotrienes, platelet-activating factor, and related compounds. Prostaglandins are notable for their protective effects in many body systems; both classes of eicosanoids also are potent inflammagens (see Chapter 16).
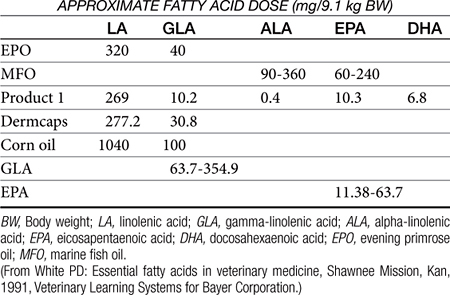
Two families of UFAs are essential for mammals (see Figure 22-2). Fatty acids of the n-3 (omega-3) series include ALA; EPA (20:5n-3) is a metabolic product of ALA found in fish oil. Fatty acids of the n-6 (omega-6) family include LA, the precursor to arachidonic acid (AA), a fundamental component of cell membranes and thus the most important of the EFAs in mammals.3 Gamma-linolenic acid (GLA) is a product of LA found in certain plant oils. It is elongated to dihomo-gamma-linolenic acid, which is then converted to AA. Of the omega-3 series, ALA is elongated to EPA and then docosahexaenoic acid (DHA) (see Figure 22-2). Because of the absence of microsomal desaturase enzymes necessary to make double bonds, mammals are unable to synthesize LA and ALA.3 Most mammals are, however, able to synthesize AA from dietary sources of LA, EPA, and DHA from dietary sources of ALA. Thus, although LA and ALA are EFAs, their products and end products are conditionally essential because their synthesis requires the precursor. However, cats cannot synthesize AA.3 In addition, enzymes necessary for conversion of LA to AA apparently are not present; thus AA must be consumed in their diet. Dietary supplements contain PUFAs rich in LA and GLA (plant sources) and EPA (fish oil), although the quantity or ratio of plant and animal oils varies with the source (Table 22-2).
After ingestion from the diet, and metabolism and restructuring in the body, the end products of n-3 and n-6 PUFAs are EPA and AA, respectively. Both of these products are inserted as components of phospholipids into cell membranes. When the membrane is damaged, both EPA and AA are released into the cell, where they are converted by lipoxygenase and cyclooxygenase to various eicosanoid (leukotriene and prostaglandin) end products (see Chapter 16). The activities of these end products vary with the fatty acid: Those formed from EPA are much less inflammogenic than those formed from AA. The composition of PUFA in cell membranes can be nutritionally modified by replacing AA with either EPA or GLA.3 These nutritional modifications can result in changes in inflammagen mediators. Inclusion of GLA (an n-6 EFA) in the diet specifically should reduce the formation of two inflammatory eicosanoids found in skin: leukotriene B4 (LTB4) and prostaglandin E2 (PGE2) because GLA may be elongated to DGLA, apparently increasing the concentrations of PGE1, an antiinflammatory prostaglandin.3 In addition, DGLA has a higher affinity for lipoxygenases than AA.
Inclusion of EPA and DHA (n-3 EFA) causes replacement of AA in the cell membrane; increasing the proportion of EPA or ALA also can decrease inflammatory responses by the cell. Because EPA has a high affinity for but is a poor substrate for cyclooxygenase, the generation of inflammatory prostaglandins is inhibited.3 In addition, LTB5, a leukotriene that is less chemotactic than LTB4, is preferentially formed. Immunologic reactivity also may be modulated with fatty acid supplementation. Mobility of cell surface receptors and movement of materials between the inside and outside might be affected. The cell-mediated response can be reduced, especially at high doses.26 Combinations of EFA of the n-3 (EPA) and n-6 (GLA) series appear to enhance control of inflammation, and the two should be given in combination for maximum effects.3 Several combinations are available in commercial preparations (see Table 22-2). The most appropriate combination of n-3 versus n-6 fatty acids has not, however, been documented, but a ratio of 5 to 10:1 has been suggested on the basis of a study that found a decrease in LTB4 and an increase in LTB5 in the skin of dogs whose diets were supplemented with these ratios.3
Current therapeutic indications for EFA include pruritus caused by atopy and other disorders and keratinization disorders. EFAs may have steroid-sparing effects, but these products may take 12 weeks or longer for full therapeutic effect.27 Newer inflammatory conditions being studied include lupus erythematosus, rheumatoid arthritis, hypothyroidism, and cancer cachexia and neoplasia.3
Noninflammatory prostaglandins also are affected by replacement of AA with EPA. The normal role of prostaglandins (reviewed in Chapter 16) can be modified with fatty acid supplementation, and this can account for some of their side effects. Side effects reported in humans include nose bleeds and hemoptysis; gastrointestinal distress; and decreased serum vitamin E, which could increase inflammation owing to increased oxygen radical. Side effects reported in dogs include lethargy, pruritus, vomiting, diarrhea, and urticaria. Use of fatty acids may increase the incidence of pancreatitis in dogs prone to that disease; fatty acid supplementation should begin with a low dose that is gradually increased over several weeks.
Behavior Modifiers
Tricyclic and other antidepressant drugs are used to treat stereotypic behaviors, including those resulting in skin lesions (see Chapter 25). The most common behaviors are related to grooming, such as acral lick dermatitis (lick granuloma) and hair chewing. Pruritus has also become a common indication for antidepressant therapy. Most behavior modifiers (psychotropic drugs) have the ability to alter multiple central nervous system neurotransmitters. Most notably affected are the biogenic amines, including serotonin and dopamine (and, to a lesser degree, epinephrine). Acetylcholine and histamine may also be affected. Because these neurotransmitters are affected centrally, they can affect many physiologic behaviors, including control of the endocrine system, motor control, appetite, and so forth. Because physiology varies markedly among animals, adverse reactions to the drugs should be anticipated on account of differences in response (i.e., pharmacodynamics) and differences in drug disposition. The behavior-modifying drugs should be used cautiously, and their use is best based on previous studies or experiences noted in the veterinary literature. Antidepressants interact with cholinergic, histaminergic, and α-adrenergic receptors and thus are associated with a variety of adverse effects. Adverse reactions have been noted in cats (central nervous system signs, gastrointestinal upset). Amitriptyline, imipramine, and clomipramine are tricyclic antidepressants that have been used in dogs and cats. Doxepin is a tricyclic antidepressant with potent antihistaminergic (H1) effects that has also been used to treat pruritus. Fluoxetine is a nontricyclic antidepressant that specifically binds serotonergic receptors.
The behavioral aspects of the self-mutilation syndrome of acral lick dermatitis have been treated with tricyclic antidepressants, fluoxetine (Prozac), and narcotic antagonists. Narcotic antagonists are reportedly effective for humans and animals for the control of certain behavioral disorders. The mechanism of action of these drugs is unknown. Increased release of endogenous opioids has, however, been detected in self-mutilative behaviors in experimental animals. The use of chemical antagonists may be a reasonable alternative to the plethora of treatments recommended by various authors for this syndrome. Other postulated benefits of the opioid antagonists include eradication of endorphin-mediated “self-reward” and analgesia. Like naloxone, naltrexone appears to be a pure opioid antagonist. Naltrexone is, however, characterized by a higher oral efficacy and longer duration of action. The success of treatment with naltrexone varies; if there has been no response after 10 days, the dose is doubled. Treatment for 1 to 2 months will cause remission in up to 60% of animals with lick granulomas, although relapses are likely in some when the drug is discontinued. Response has also been reported with hydrocodone bitartrate (Hycodan).
Antiparasitic Drugs
A limited number of agents are administered systemically (orally or by injection) for control of dermatologic parasites. Milbemycin and lufenuron are two examples. These drugs are discussed in greater depth in Chapter 15.
Macrolide Antibiotics
Ivermectin
Ivermectin is used to treat a number of parasitic dermatoses in animals. The drug stimulates the release of the inhibitory neurotransmitter GABA at peripheral neuronal synapses, resulting in paralysis and death of the parasite (see Chapter 15). Selective activity in animals reflects a difference in the peripheral role (nematodes and arthropods) versus central role (mammals) of this neurotransmitter. In most mammals ivermectin does not penetrate the blood–brain barrier in sufficient concentrations to cause side effects. The drug is administered orally and parenterally; up to 3 weeks may elapse before the drug is totally eliminated in feces. The primary indication for ivermectin in small animals is mite infestation: feline and canine scabies, cheyletiellosis, and ear mites. The drug has also been used for mite infestations of guinea pigs and birds. Although clinical response may occur in several weeks, animals may remain skin-scrape positive for several to many months. Adverse reactions are idiosyncratic and have been reported primarily in herding breeds (Collies, Border Collies, Shetland Sheepdogs, Old English Sheepdogs, Australian Shepherds). Adverse reactions are associated with a mutation in the MDR1 gene. Testing is available at Washington State University for this mutation to help identify dogs at risk for life-threatening reactions. Reactions have ranged from mydriasis to tremors, hypersalivation, ataxia, coma, and death. The 1% bovine product may be diluted with propylene glycol to allow more accurate dosing in small animals. Ivermectin can be used (off-label) for the treatment of generalized canine demodicosis. Treatment is usually started at 0.1 mg/kg daily and gradually increased. The commonly used dosage ranges from 0.2 mg/kg daily to 0.6 mg/kg daily, but some clinicians recommend dosages as high as 0.8 mg/kg/day. Careful patient monitoring for side effects and response to therapy is warranted.
Milbemycin
Like ivermectin, milbemycin at 1.5 to 2 mg/kg daily is effective for the treatment of demodectic mange. Cost may be a limiting factor. Response to treatment is lengthy, and relapses may occur. The dosage may be modified if an inadequate response is seen after 1 month of therapy. Also like ivermectin, milbemycin can cause toxicity, particularly in Collie-type dogs, although this drug is slightly safer than ivermectin. Moxidectin also may be efficacious; Collies also appear to be more sensitive to its adverse effects. Moxidectin is not approved for the treatment of canine demodicosis in the United States.
Immunomodulators
Immunomodulators (biologic response modifiers) potentiate some facet of the immune response (see Chapter 19). Many compounds described as immunomodulators have been used to treat a variety of dermatologic conditions. Immunosuppressants used to treat immune-mediated dermatologic disorders include glucocorticoids, cytotoxic (antineoplastic) drugs, and selected hormones. Immunostimulants tend to be less effective (see Chapter 19).
Staphylococcus aureus phage lysate (Staphage Lysate) is a vaccine containing parts of S. aureus, a bacteriophage, and culture medium ingredients. It is indicated for treatment of canine staphylococcal pyoderma. Vaccination is intended to result in stimulation of antibacterial antibodies. There may also be a hyposensitizing effect. Concomitant antimicrobial therapy should be used for the initial 4 to 6 weeks. Staphage Lysate is administered subcutaneously once a week initially, and then the dosage interval is lengthened. The vaccine is relatively expensive. Possible side effects include allergic reactions and local redness and swelling at the injection site. This vaccine should be reserved for patients with recurrent staphylococcal pyoderma that responds to antimicrobial therapy but recurs when therapy is discontinued. It is extremely important that other causes of pyoderma (e.g., allergies, ectoparasites, endocrinopathies) are identified and treated.
Propionibacterium acnes killed suspension (ImmunoRegulin) is available as an adjunct to antimicrobial therapy in the treatment of canine pyoderma. It has been shown to induce cell-mediated immunity. This bacterium is the species associated with human acne. Treatment with ImmunoRegulin involves intravenous injections twice weekly initially and then once weekly. Potential adverse effects include anaphylactic reactions. Disadvantages include expense and the need for intravenous injections (client cannot administer at home). It is the author’s opinion that use of this agent should be reserved for patients in which all other forms of therapy have failed.
Hyposensitization involves parenteral administration of allergens (antigens) in allergic patients. Although the mechanism of action of this therapy is unknown, the most appealing theory is that repeated exposure to the allergen may result in reduced cellular sensitivity (tolerance). Hyposensitization is one of best therapies for long-term management of the dog and cat. Patient and client selection are critical for success because therapy generally lasts as long as the patient is alive. Hyposensitization solutions may be based on intradermal allergy testing or serum allergy testing. Many protocols are available insofar as patient response may be quite variable.
Transdermal Delivery of Drugs
Most drugs in human and veterinary medicine that are applied to the skin are intended to provide their pharmacologic effect where they are applied. Systemic absorption is intended to be minimal so that systemic effects will not be encountered. There are, however, a small number of drug products that are designed to be applied to the skin and absorbed transdermally, whereupon they attain sufficiently high plasma concentrations to produce systemic effects. The same factors mentioned in the discussion of the principles of topical drug therapy affect drug absorption from cutaneous sites.
Transdermal drug delivery offers several advantages. It is easier to administer drugs transdermally, and drug delivery can be sustained, thus ensuring continued therapeutic effects. Drug input might be more precisely controlled over oral therapy because there are fewer factors complicating transdermal drug absorption, such as first-pass hepatic metabolism.
Not all drugs may be administered transdermally. The drug should not be irritating to the skin and should be transdermally bioavailable. Effective transdermal drug delivery formulations are very difficult to design, which is probably why so few products are available. Some products used in veterinary medicine, although not intended for transdermal absorption, can result in systemic absorption in the person treating the animal. Such drugs should be handled with nonpermeable gloves (e.g., DMSO, all anticancer drugs, nitroglycerin ointment, all antiparasitics [dips and shampoos], and altrenogest [Regu-Mate]). Gloves might also be used when administering chloramphenicol, although percutaneous absorption in association with oral medication of small animals has not been documented.
Several products are used in veterinary medicine with the intent of achieving systemic therapeutic effects. Nitroglycerin ointment relaxes vascular smooth muscle primarily on the venous side and is used for dogs and cats to treat cardiogenic edema. The ointment is applied to glabrous areas (i.e., axillary, inguinal, or inside the ears). Onset of action is approximately 1 hour. Selemectin (Revolution, Fort Dodge) and other systemic insecticides (discussed previously) are applied topically. Scopolamine, nitroglycerin, and clonidine transdermal patches are available for use in human patients to treat motion sickness, angina, and hypertension, respectively. Fentanyl, a narcotic analgesic available in transdermal patches, has proved to be an effective and safe alternative to injectable opioid delivery for control of pain in dogs and cats (see Chapter 28).
Treatment of Specific Dermatologic Disorders
Selected skin diseases are discussed in other chapters. Otitis externa, pyoderma, and other bacterial infections of the skin are discussed in Chapter 8. Fungal disorders are additionally discussed in Chapter 9, external parasites are additionally discussed in Chapter 13, immune-mediated diseases of the skin are discussed in Chapter 31, behavioral disorders of the skin are discussed in Chapter 26, and endocrinopathies affecting the skin are addressed in Chapter 21.
Chronic Pruritus
Pathophysiology
Pruritus is a cutaneous sensation that leads the animal to resolve the feeling by scratching, rubbing, licking, or chewing. The cause of the sensation is complex, as can be its resolution. Ideally, the underlying cause of the sensation is identified and treated, ultimately leading to resolution of the pruritus. Discussion of or even provision of a comprehensive list of the causes of pruritus is beyond the scope of this chapter.
Like pain, the sensation of pruritus consists of a peripheral signal, originating in the skin, and central perception, ending at the sensory cortex. A number of chemicals are responsible for signal generation and transmission in the skin, although the precise types that predominate in the dog and cat are not known. The chemicals largely are the same as those generating the inflammatory response and include both preformed chemicals (histamine, serotonin) and those synthesized in situ (prostaglandins, leukotrienes, proteases). The signal generated at the level of the skin can be modified by emotional, biochemical, or central factors, leading to behavior that appears to be disproportionate to the inciting cause (i.e., skin trauma induced in response to a single flea bite).28 Drugs oriented toward control of pruritus can target either peripheral or central transmission. For chronic pruritus drugs with systemic effects generally are more effective.
Drug Therapy
Topical Therapy
A number of nonglucocorticoid topical preparations may control pruritus.5,13 Care should be taken to select products that are not irritating or drying. Shampoos generally should be applied once to twice weekly. Cool water may be effective in some animals; antipruritic efficacy can be enhanced by the addition of moisturizing agents, colloids, antiinflammatories, or anesthetics. Preparations that can be applied in “spots” include Dermacool (hamamelis extract and menthol.13 ResiSoothe (Virbac U.S.) and Relief cream rinse (DVM) are lotions that are also effective for spot treatment.
Other medications available in sprays that might be helpful for control of pruritus include local anesthetics such as lidocaine (Dermacool) or promaxine (with colloidal oatmeal: Relief Spray), 2% benzyl alcohol with 0.05% benzalkonium chloride, and hamamelis distillate (PTD). In addition to anthistaminergic medications, sprays also may contain glucocorticoids for treatment of localized pruritus such as pyotraumatic pruritus and allergic dermatitis.5 More potent sprays intended for acute management contain 0.1% triamcinolone, 0.025% fluocinolone, or 0.1% betamethasone; “milder” glucocorticoid sprays for long-term maintenance contain 0.5% to 2.5% hydrocortisone. Sprays can be applied two to three times per day.5 Sprays should be applied every 12 hours until inflammation and pruritus are controlled and then tapered to an as-needed basis. Systemic side effects may be seen with extended use. Localized adverse effects such as the development of thin skin or alopecia may also occur.
Glucocorticoid ointments and creams may be difficult to use because of lack of penetration through the hair coat into the skin. A hydrocortisone lotion that is not greasy or staining may be useful for treatment of large areas with some residual activity.5 ResiCort (1% hydrocortisone) lotion (Virbac U.S.) is a formulation that is easier to apply to the skin surface than ointments and creams.
Total body application of moisturizing and hypoallergenic shampoos, or colloidal oatmeal soaks or shampoos with or without antihistamines, local anesthetics (Relief Shampoo DVM), or hydrocortisone (Cortisoothe, Virbac U.S.) are indicated for patients with generalized pruritus.6,13 Colloidal shampoos containing pyrethrins, carbaryl, or pyrethrins and permethrin will facilitate control of pruritus associated with fleas.5 Application of a rinse, spray, or lotion after a shampoo will provide some residual effect.5 Cream rinses containing colloidal oatmeal with (pramoxine) or without a local anesthetic are available for control of pruritus. Incomplete rinsing of a cream rinse may facilitate residual activity in dogs with short hair coats (coats of long hair become greasy). Antipruritic cream rinses might also be applied locally for control of localized pyoderma (e.g., Relief lotion).5 Several aqueous rinses also are available for control of pruritus. These include 100% colloidal oatmeal (Aveeno), oilated oatmeal with 43% colloid (Aveeno Oilated Bath) (for very dry skin and hair coats, also applied as a soak), and moisturizing bath oils or humectants (Humilac spray, Virbac U.S.). Aqueous rinses generally also can be applied as sprays, although efficacy is greater as a total body rinse.5
Topical shampoo therapy is an important component of treatment or prevention of superficial and deep pyodermas. Antibacterial shampoos containing benzoyl peroxide, chlorhexidine, or ethyl lactate are indicated as adjuvant therapy for pruritus associated with pyoderma (see Chapter 8). Those most effective contain benzoyl peroxide or chlorhexidine.6 Following the shampoo with a chlorhexidine rinse (dilution of a 2% solution to 0.5%) further enhances antibacterial activity. Maximum ChlorhexiDerm HC shampoo (DVM), a new product containing 4% chlorhexidine and 1% hydrocortisone, has been developed to treat secondary pyoderma associated with an underlying pruritic disorder. Very few controlled and blinded studies exist to guide the selection and use of drugs for treatment of chronic pruritus in dogs or cats. In general, those that have provided support for selected drugs define success or response as a reduction in pruritus by 50%.There is good evidence, however, that glucocorticoids and cyclosporine are beneficial for the treatment of canine atopy.21
Antibiotics
Antibiotics may be indicated to control secondary infection (generally Staphylococcus) that can contribute to pruritus. The use of antibiotics for treatment of dermatologic disorders is discussed in Chapter 8. For some animals antibiotic therapy may be the sole therapy needed to control pruritus. Some antibiotics (e.g., erythromycin, tetracyclines) directly provide some relief from pruritus (rather than by controlling infection).29 Tetracyclines in general are not, however, effective for treating pyoderma associated with staphylococcal infections. Erythromycin can be effective against Staphylococcus species, but it can cause gastrointestinal upset.
Glucocorticoids
Glucocorticoids remain the most effective drugs for control of chronic pruritus associated with allergies.13,28 The principles of glucocorticoid therapy (see Chapter 30) must be closely observed when a glucocorticoid is used to control pruritus. Injectable products should be avoided when possible. Miller and coworkers28 suggest that injections may be acceptable for animals in which a single injection provides pruritic relief for 3 to 4 months or more. The temptation to increase the frequency of administration of an injectable product to less than 3-month intervals should be resisted. Prednisone and prednisolone (0.55 to 1.1 mg/kg per day [dog]; 2.2 mg/kg per day [cat]) remain the glucocorticoids most commonly used for long-term treatment. The dose can be divided and given twice or once daily. The dose is titrated to a minimum effective dose defined by the point at which the animal is “tolerably itchy”—that is, the itch does not disrupt the animal’s (or owner’s) life or cause secondary trauma to the skin.28 The lack of pruritus suggests that the dose is too high. Many animals cannot tolerate even an alternate-day regimen of glucocorticoids; other animals remain pruritic at doses associated with marked side effects. Long-term side effects will be seen with continued use. It has been suggested that alternative therapies should be sought for animals that require glucorticoid therapy for more than 3 months out of the year.
Some clinicians find glucocorticoids devoid of mineralocorticoid to be less frequently associated with polyuria and polydipsia28; however, the rationale behind this observation is not clear because this side effect is related to anti–antidiuretic hormone effects rather than to increased mineralocorticoid effects. Nevertheless, an alternative glucocorticoid might be tried; however, triamcinolone and dexamethasone have increasingly longer biologic effect times, and the risk of hypothalamic–pituitary–adrenal suppression increases with continued use. Methylprednisolone may be a viable alternative. If undesirable side effects occur with glucocorticoid use, combination therapy might be tried (e.g., antihistamines, bathing, fatty acid therapy) in an attempt to decrease the dose of glucocorticoids. In some animals, however, only continued use of glucocorticoids in the face of side effects will control pruritus sufficiently. In such patients the risk of therapy must be weighed carefully against the disadvantages of continued pruritus. Glucocorticoid-sparing drugs should be attempted before selecting glucocorticoids. If proved effective as sole drug therapy, then combined use should be considered to reduce the dose and thus likelihood of side effects. These include antihistamines, misoprostol, and omega series fatty acids.
Antihistamines
Antihistamines traditionally have been considered ineffective as a sole therapy for controlling pruritus in dogs or cats. However, with the advent of newer drugs and modification of the goal of therapy (e.g., reduction in the glucocorticoid dose), the following approaches may enhance the use of antihistamine therapy for control of pruritus. The clinician should wait at least 1 week before evaluating efficacy. If one drug does not work, an alternative should be tried. The clinician also should aim to reduce the itching sensation rather than eradicate it. The antihistamine also can be combined with other drugs that might decrease itching (e.g., fatty acids). Drugs most likely to be useful include diphenhydramine (Benadryl), hydroxyzine, chlorpheniramine, and clemastine. Cyproheptadine, also an antiserotinergic, and the tricyclic antidepressants amitriptyline and doxepine (both with antihistaminergic effects) also may be useful. It may be necessary to increase the dosage of doxepin to as high as 5 mg/kg for it to be effective in some dogs.
Few studies have properly evaluated the use of antihistamines for the treatment of pruritus in dogs. One study30 provided support for a reduction in pruritus in allergic dogs treated with one of three antihistamines. In general, however, response occurred in at most 25% of animals treated. Thus response rates tend to be low. Response to chlorpheniramine was best, followed by diphenhydramine or hydroxyzine. Follow-up studies28,31 found clemastine to be much more effective than astemizole or trimeprazine. Thus, of the antihistamines studied in clinical patients, clemastine appears to be the most effective. Because of low bioavailability when clemastine is given orally, a higher dose (1 mg/kg twice daily) may be more clinically effective.32 Misoprostol may enhance the efficacy of antihistamines used to treat pruritus.
Cats appear to respond to antihistamines better than dogs. Chlorpheniramine can be used to treat cats without causing the excitement that often occurs with diphenhydramine (the latter effect probably reflects overdosing). One study found response to chlorpheniramine to be excellent in 73% of cats when dosed at 2 mg every 12 hours within 2 weeks. Other reports consistently have been favorable with doses of 2 to 4 mg/cat twice daily. Occasionally, a 24-hour dosing interval may be effective. The 8‑mg time-release capsule (sprinkling one fourth to one half of the contents on food) may prove easier to administer than the bitter-tasting tablets. Alternatively, the 4-mg tablet can be broken in half and dipped in tuna fish.7 It is generally recommended that the 4-mg tablet be administered unbroken, however. An over-the-counter solution is also available. Side effects reported in cats include vomiting, diarrhea, and hyperexcitability. Hydroxyzine and amitriptyline can cause adverse reactions in cats. Clemastine (0.34 to 0.68 mg every 12 hours orally) may be effective.
A combination of both H1 and H2 receptor antagonists has been recommended but has not proved useful in animals thus far.28
Fatty Acid Supplements
Studies that focus on the effects of dietary supplementation of EFA on canine atopy vary. Interpretation among clinical trials is complicated by the quantity and source of the PUFAs (GLA, EPA, and DHA), the lack of tissue fatty acid analysis, the duration of therapy (which probably was not sufficiently long), and differences in outcome measures and adjuvant therapy. In addition, many of the studies were not controlled or blinded.
White3 provides a summary of the results of selected clinical trials implemented by various dermatologists. A study evaluating LA, linolenic acid, and EPA in dogs with atopy, flea allergy, and idiopathic pruritus (nonseasonal) found the response rate to the PUFA to be low (20% or fewer being controlled well; another 36% responding by a 50% reduction in pruritus). An open comparison of evening primrose oil, marine fish oil, and EFA products administered for 2 weeks found response in 25% of animals, but no product appeared more effective than the others. Responders worsened when supplementation was removed. Comparison of evening primrose oil alone or combined with marine fish oil found significant improvement with the sole product and increased (but not significant) efficacy with the combined product. A double-blind placebo-controlled crossover study with olive oil (placebo) or evening primrose administered for 9 weeks found clinical improvement with evening primrose oil. Two recent blinded studies have reported the benefits of EFA (EPA 180 mg and DHA 120 mg per 4.55 mg/kg) supplementation when administered for 6 weeks. Eleven of 16 dogs responded to fish oil, 3 of 16 responded better to corn oil, and 2 of 16 dogs responded to neither oil. A parallel study of 28 dogs supplemented with high doses of GLA (350 mg), EPA (250 mg), or linolenic acid (250 mg) and EPA (50 mg) found a benefit with all three supplements, suggesting that higher doses may be important to efficacy.
Cats seem to respond better than dogs, with up to 40% efficacy reported by some authors.28 Again, however, results of clinical trials tend to be conflicting. Pruritic cats with miliary dermatitis and other nonlesional causes of pruritus responded to 2 and 6 weeks of therapy of a linolenic acid–EPA supplement. In contrast, 12 weeks of therapy with evening primrose oil (3.7 mg linolenic acid/kg) or olive oil did not improve feline skin disease in another study. A 6-week course of evening primrose alone or when combined with marine fish oil resulted in improvement in feline pruritus and dermatitis; in contrast, marine fish oil by itself caused worsening of the skin disease. A 12-week course of therapy with evening primrose and sunflower oil also improved the skin and coat of cats with miliary dermatitis.3 White3 suggests that atopy should be most responsive to EFAs that are low in LA and high in linolenic acid and/or EPA/DHA. Addition of vitamins to the EFA supplement may facilitate efficacy of the EFA product. The appropriate ratio (n‑6:n-3) is not clear, nor is the duration of therapy. A ratio of 5 to 10:1 has been suggested on the basis of a study that found a decrease in LTB4 and an increase in LTB5 in the skin of dogs supplemented with diets containing these ratios.3 Response has been reported in as few as 7 to 14 days or as many as 9 to 12 weeks.
The most appropriate use of EFA for control of pruritus may be in combination with antihistamines or glucocorticoids.3 Success with fatty acid supplements also is most likely if an alternative product is tried if and when the first fails, adequate time is allowed to elapse before clinical assessment is complete, the goal is reduction rather than eradication of pruritus, or the goal is reduction of the dose of other drugs associated with adverse effects (e.g., glucocorticoids).
Behavior-Modifying Drugs
The use of behavior-modifying drugs is increasingly popular; the proper use and side effects associated with these drugs are discussed in Chapter 25. Among the behavior-modifying drugs, those that are characterized by antihistaminergic effects might be preferred (e.g., doxepin or amitriptyline).
Behavior-modifying drugs have been used to treat feline pruritus, particularly those associated with psychogenic alopecia.7 Psychogenic alopecia also has responded to naloxone (1 mg/kg subcutaneously, one dose), although time to recurrence of the abnormal behavior ranged from as short as 1 week to as long as 6 months.7 The dopamine antagonist haloperidol (2 mg/kg intravenously) did not appear very useful long term when compared with a control for treatment of psychogenic alopecia.7 Cats did, however, respond within 48 hours. Buspirone does not appear to be effective for control of psychogenic alopecia.7 Fluoxetine has been useful for chronic, idiopathic licking in cats.
Combination Therapy
Combinations of drugs that alter the sensation or response to the sensation through different mechanisms of action should be considered. The combinations that appear to be most effective include fatty acid supplements (Dermcaps) with glucocorticoids (leading to a 25% to 50% reduction in the glucocorticoid dose)28; antihistamines (trimeprazine) and glucocorticoids (an increase from 50% improvement with glucocorticoids alone to 76% improvement with the combination, with a 50% to 75% reduction in the glucocorticoid dose)33; and fatty acid supplements with antihistamines (chlorpheniramine and Dermcaps in combination caused a 35% response in animals that responded to neither drug alone).28 Combination of misoprostol with antihistamines is being investigated. Assessment at weekly intervals should be accompanied by a change in therapy if response has not been sufficient.
Nonsteroidal Antiinflammatory Drugs
Nonsteroidal antiinflammatory drugs should be avoided in pruritic skin diseases. Inhibition of prostaglandin formation may result in greater synthesis of leukotriene and related compounds. Newer lipoxygenase-specific inhibitors (approved for use in the treatment of human bronchial asthma) may be beneficial, but these drugs have not been studied with the intent of controlling pruritus.
Future Therapies
Future therapies may focus on biologic response modifiers.34 Products with potential efficacy for human patients with atopy include interferon-γ, thymopentin (an active pentapeptide of a thymic hormone that promotes differentiation of mature T lymphocytes), and interleukin-2.
Acral Lick Dermatitis
Acral lick dermatitis (lick granuloma) is a self-traumatizing syndrome that afflicts dogs, occurring almost exclusively in larger breeds. The cause is not known, but it has patterns similar to an obsessive–compulsive disorder. It is often considered psychogenic in origin; suggested behavioral causes include boredom, loneliness, and confinement.35 Underlying bony pathology or allergy may also be the cause of the lesions. Chronic lesions are frequently infected with bacteria or fungal organisms, which can also contribute to the pruritic stimulation. Local irritation has, however, been suggested as a provoking cause. Not surprisingly, drugs used to treat obsessive–compulsive disorders in humans have proved effective. The proper use of these drugs is discussed in Chapter 26. In one clinical trial with 43 dogs, the drugs most effective were those that increase serotonin at the neurotransmitter. This is not surprising because the serotonin system has been implicated in a variety of abnormal behaviors.35 Dose ranges of the drugs (orally per day) proven successful in clinical trials36 include clomipramine (2.4 to 3.6 mg/kg),36 fluoxetine (0.55 to 1.36 mg/kg), and sertraline (2.7 to 4.3 mg/kg). Of these, clomipramine followed by fluoxetine appeared to be most effective, causing response (50% or more reduction in behavior) in close to 50% of animals studied. Clinical response was not evident until the second week of therapy and continued to decline through the 5 weeks of one study.35 Adverse effects occurred in approximately 25% of dogs in each treatment group, but they tended to be mild and subsided with time. Discontinuation of the behavior-modifying drug may cause recrudescence of clinical signs. Drugs to which there was little response included desipramine and fenfluramine. In most cases, secondary infection is present and must be treated. Glucocorticoid therapy is usually indicated in the early stage of treatment to decrease the inflammation. Antiinflammatory dosages rather than immunosuppressive dosages are recommended. Oral prednisone or prednisolone are the favored products. Additional topical therapy with Synotic applied twice daily may be selected instead of oral glucocorticoid therapy. Appropriate antibiotic therapy based on culture and susceptibility is indicated.
Both naloxone and naltrexone, both pure narcotic antagonists, have been used to resolve abnormal behavior in dogs with lick granulomas. Increased release of endogenous opioids has been detected in self-mutilating behaviors in experimental animals. Opioid antagonists may eradicate endorphin-mediated self-reward and analgesia. Like naloxone, naltrexone is a pure opioid antagonist but is characterized by a higher oral efficacy and longer duration of action. An uncontrolled study of the use of naltrexone in dogs with acral lick dermatitis reported a 64% success rate.37 A dose of 2.2 mg/kg orally once daily (1 mg/kg subcutaneously) was increased to 2.2 mg/kg orally twice daily when there was no response after 10 days. Dogs were treated for 1 month; lesions returned when the drug was discontinued, and time of recurrence varied from 1 week to 3 years after the drug was discontinued. One animal that failed to respond to naltrexone responded to nalmefene, another narcotic antagonist. Chronic cases or those with a poor response to therapy should be biopsied to rule out neoplasia and cultured for infectious agents.
Canine Seborrhea
Canine idiopathic seborrhea is an inherited disorder of keratinization characterized by pruritus, epidermal thickening, and formation of dry or greasy crusts and seborrheic plaques.3,38 The pathophysiology is not well understood, but hyperproliferation of the epidermis, hair follicle infundibulum, and sebaceous glands have been reported. Renewal time for the epidermis is reduced from 22 to 8 days.38 Increased cutaneous concentrations of AA have been measured in affected dogs. Treatment controls but generally does not cure the condition. The production of increased scale can be caused by myriad diseases and problems. The search for the cause of the scale is more important than symptomatic therapy. Primary idiopathic seborrhea as a diagnosis is fairly uncommon.
Therapy for seborrhea oleosa should include shampoos that are keratolytic, keratoplastic, and degreasing. Ingredients that serve this purpose contain sulfur; salicylic acid; benzoyl peroxide; or, less commonly, coal tar. Among these, benzoyl peroxide is probably the preferred ingredient, although a sulfur salicylic shampoo might be tried first. Benzoyl peroxide activity is enhanced with the addition of sulfur (SulfOxydex, DVM). In general, products for human beings should not be used on dogs. Products also can be alternated.
Animals should be bathed twice a week until scaling, greasiness, and odor are controlled; the frequency of bathing is then decreased to the minimum necessary to control clinical signs. Contact with the shampoo (completely lathered) must occur for at least 10 minutes. For animals with an extremely thickened accumulation, bathing with a mild cleanser such as D-Basic (DVM) before a medicated shampoo is used may prove beneficial and will be sparing of the more expensive products. PUFAs have recently been used for treatment of seborrhea. Supplementation should begin as topical therapy is started. Corn, sunflower, or safflower oil (1.5 mL/kg every 24 hours orally) or commercial EFA products can be used.38 It is possible that the skin of affected dogs cannot obtain or metabolize LA, suggesting that topical administration of EFA (LA) may be more appropriate than oral administration.3
Generalized Demodicosis
Therapy for generalized demodicosis should be accompanied by proper nutrition, minimization of stress, and control of accompanying pyoderma. Self-cure can be expected in 50% of dogs younger than 12 months of age, although no factors appear to be predictive of self-cure.39 The most efficacious treatment for generalized demodicosis appears to vary in the literature. No single drug will cure all cases; up to 10% cannot be cured.39 Amitraz (Mitaban)19.9%, and amitraz in ProMeris (Fort Dodge) are the only drugs approved by the Food and Drug Administration for treatment of demodicosis, although an Environmental Protection Agency–registered solution is available (Taktic). Amitraz (Mitaban)is labeled for treatment at 2-week intervals (diluted to 250 ppm or 10.6 mL in 7.6 L). Original reports regarding its efficacy using the approved protocol apparently were inappropriately optimistic (90% predicted cure rates). Long-term cure rates according to the approved treatment regimen range from as little as 0% to 50% (0.025% or 250 ppm dip).40 Follow-up studies at greater concentrations have more favorable results.
Concentrations of 500 ppm (double strength) may be used for refractory cases. Weekly dips of 250 to 500 ppm are curative in 75% to 80% of cases, respectively.40 Long hair coats should be clipped and animals bathed first. Treatment should continue until two dips have occurred after two negative skin scrapings, regardless of how many dips must occur. Weekly application may be implemented if biweekly therapy is not successful. Oral vitamin E therapy at 200 IU/dog five times daily may enhance efficacy of amitraz, although this has not been proved.40 ProMeris (Fort Dodge) is to be applied every 14 days until a remission is reached. Adverse side effects similar to the those of of Mitaban have been reported. Immunomodulators such as Staphage Lysate, ImmunoRegulin, and levamisole appear to be ineffective.40
Should amitraz fail, a macrolide antibiotic should be considered. Milbemycin at 1 and 2 mg/kg daily appears to cure 50% to 90% of dogs, respectively. Duration of therapy in one study ranged from 90 to 300 days; treatment should occur for 60 days past multiple negative skin scrapings. Side effects should be limited as long as 2.5 mg/kg is not exceeded.39 Ivermectin at 0.3 to 0.6 mg/kg per day orally (but not 0.2 to 0.4 mg/kg per week subcutaneously) also may be efficacious, with treatments being necessary for as long as 210 days. Because the drug is very bitter, administration in vanilla ice cream or apple sauce is recommended.40 This dosing regimen is not safe for Collies and related breeds. Testing for reaction to ivermectin involves administration of 0.125 mg/kg per day for 7 days. Alternatively, hospitalization might occur for the initial period during which a starting dose of 0.1 mg/kg is gradually increased to the maintenance dose. Signs of pending toxicity include mydriasis and excessive salivation. Neurologic signs are also possible. Testing for the MDR1 gene mutation is available through Washington State University. This test will help identify those dogs that are sensitive to ivermectin toxicity.
Should relapse occur within 3 months, inadequate treatment is the most likely cause,39 and the therapeutic protocol can be repeated but for a longer duration. Later relapses probably reflect recurrence; repetition of the initial treatment protocol is not likely to be effective,39 and an alternative protocol should be selected. It is essential to resolve any complicating skin infections and to search for an underlying cause of refractory demodicosis. Also, underlying allergic disorders must be addressed.
Dermatophytosis
Treatment of dermatophytes is also addressed in Chapter 9. Establishment of the efficacy of selected treatment regimens is handicapped by the often self-resolving nature of the infection. Moriello41 emphasizes that, as with pyoderma, topical therapy alone is not sufficient for effective treatment of dermatophytosis. Indeed, several disadvantages are associated with topical therapy. Hairs protect spores; in addition, many commonly used topical antifungal solutions and shampoos are not sporicidal. Among the more efficacious are lime sulfur (1:16) and enilconazole (bottle dilution, but not licensed for use in the United States).41 Unfortunately, both of these topical products are associated with side effects: Enilconazole has caused death in some cats, and lime sulfur can be very irritating. Grooming after topical treatment increases the risk of toxicity in cats if they are allowed to groom while the product is wet. Pet owners are exposed to topical agents, increasing their risk. Occasionally, topical agents worsen infection, perhaps because of damage to the skin (mechanical scrubbing) during shampooing. The efficacy of topical ointments, creams, and gels is questionable. Among the topical ointments and creams, bifonazole is more effective than miconazole, but its efficacy also is questionable, particularly if infection involves haired skin. In addition, topical agents tend to be messy, are groomed by the animal, and encourage “spot” therapy. Nonetheless, topical therapy is an important adjuvant for treatment of dermatophytosis. Topical therapy will limit environmental contamination and contagion if spores on the hair coat are killed. The preferred topical product41 is lime sulfur (4 to 8 oz/gallon); higher concentrations are potentially irritating and cause exfoliation. Gauze sponges should be used to pat, not rub, the dip onto the skin. An Elizabethan collar should reduce the risk of ingestion.
Five drugs are used to systemically treat dermatophytosis: griseofulvin, ketoconazole, itraconazole, and fluconazole and terbinafine (see Chapter 9). Even in infections that will be self-resolving (self-cure generally occurs in 60 to 100 days), systemic antifungal therapy will hasten the time to recovery and thus is beneficial, limiting environmental contamination and exposure to human beings. Griseofulvin remains the drug of choice because it is licensed and approved for use in dogs and cats. Either the microsize dose (25 to 50 mg/kg divided or once every 24 hours) or the ultramicrosize dose (5 to 10 mg/kg orally once daily) can be administered. Because many animals are intolerant to ketoconazole, itraconazole is the second drug of choice should an animal not tolerate griseofulvin therapy or fail to respond to it. Ketoconazole is significantly less expensive than itraconazole and is a reasonable choice for dogs. Although improvement should be evident within 2 to 3 weeks of therapy, several weeks (generally 30 to 70 days) must lapse before a cure is achieved. A comparison study in kittens infected with Microsporum canis found that time to resolution with griseofulvin (50 mg/kg orally divided or once every 24 hours) was 70 days versus 56 days with itraconazole (10 mg/kg orally once daily); the control group remained positive at 100 days. Therapy should be monitored by fungal cultures biweekly after 4 to 6 weeks of therapy and treatment continued until two or three consecutive weekly cultures are negative. Note that false-positive fungal cultures can occur after clinical recovery because of environmental contamination. Cats with questionable positive results should be isolated from the environment for 3 to 5 days and subsequently recultured. Griseofulvin should not be used in pregnant animals, those younger than 12 weeks of age, or those that test positive for feline immunodeficiency virus. This drug should be discontinued immediately if the cat becomes anorexic or lethargic or if gastrointestinal signs are seen. Terbinafine, a systemic antifungal approved for use in human beings for treatment of dermatophytosis, has not been studied for efficacy or safety in animals. However, an unpublished pharmacokinetic study with cats (in Europe) indicates that a dose of 10 mg/kg once daily is appropriate; 10 to 30 mg/kg orally once daily has been suggested in Internet resources. Fluconazole may also be effective when other treatments fail or are associated with side effects. The recent change to a generic drug has reduced the cost dramatically. Terbinafine has been shown to be effective for treating Malassezia and may be an alternative antidermatophyte treatment.
Cleansing of the environment is obviously critical in a cattery or kennel situation but can also be important in a single-animal household. Undiluted bleach and 1% formalin are the most effective products for resolving environmental contamination.41 This regimen is likely to kill 100% of spores; residual activity is likely to be greatest with undiluted bleach, followed by 1% formalin, enilconazole (not available in the United States), and common household bleach diluted 1:10. Common household bleach is not as efficacious in killing spores as the other two products, but it is the least expensive and most readily available. Aqueous chlorhexidine is ineffective.
Canine Perianal Fistulae (Anal Furunculosis)
Very little has been written on the topic of canine perianal fistulae. Nonsurgical management is the new standard of therapy.42 Cyclosporine orally at 5 mg/kg twice daily until a remission is reached is the treatment of choice. The available water-soluble forms should be selected (Neoral, Atopica).43 Vomiting and diarrhea are common side effects. Additional side effects can include gingival hyperplasia and secondary infections. Tacrolimus ointment (protopic 0.1%) is a topical analog of cyclosporine. It has been shown anecdotally to be effective for the treatment of perianal fistulae when used as a sole treatment. In the author’s experience, this product alone is not beneficial. Cyclosporine orally with or without tacrolimus ointment appears to be the current treatment of choice. Treatment may take several weeks to induce a remission before tapering off the dosage to every other day. Relapses are common. Secondary infections are common and should be treated on the basis of culture and susceptibility testing.
1. Pavletic M.M. Anatomy and circulation of the canine skin. Microsurgery. 1991;12:103-112.
2. Riviere J.E., Spoo H.W. Dermatopharmacology: drugs acting locally on the skin. In: Adams H.R., editor. Veterinary pharmacology and therapeutics. Ames, Iowa: Iowa State University Press; 1995:1050-1089.
3. White P.D. Essential fatty acids in veterinary medicine, Shawnee Mission, Kansas. Bayer Corporation and Veterinary Learning Systems, Bayer Corporation, Agriculture Division, Animal Health; 1995.
4. Millis P.C., Magnusson B.M., Cross S.E. The effect of solute lipophilicity on penetration through feline skin. J Vet Pharmacol Ther. 2003;26:311-314.
5. Kwochka K.W. Topical antipruritic therapy. In Dermatology: 19th Annual Waltham/OSU for the treatment of small animal disease. Waltham USA: Vernon, Calif; 1995. pp 41–45
6. Rosenkrantz W.S. Shampoo therapy. In: Bonagura J.D., Twedt D.C., editors. Kirk’s current veterinary therapy XIV. St Louis: Saunders, 2009.
7. Messinger L.M. Therapy for feline dermatoses. Vet Clin North Am Small Anim Pract. 1995;25:981-1005.
8. Guzzo C.A., Lazarus G.S., Werth V.P. Dermatologic pharmacology. In: Hardman J.G., Limberd L.E., editors. Goodman and Gilman’s the pharacologic basis of therapeutics. ed 9. New York: McGraw-Hill; 1995:1593-1616.
9. Power H.T., Ihrke P.J. Synthetic retinoids in veterinary dermatology. Vet Clin North Am Small Anim Pract. 1990;20:1525-1540.
10. Kwochka K.W. Retinoids in dermatology. In: Kirk R.W., editor. Current veterinary therapy X: small animal practice. Philadelphia: Saunders; 1989:553-560.
11. Kwochka K.W. Treatment of scaling disorders in dogs. Proceedings Atlantic Coast Veterinary Conference. 2003.
12. Power H.T., Ihrke P.J. The use of synthetic retinoids in veterinary medicine. In: Bonagura J., editor. Current veterinary therapy XII: small animal practice. Philadelphia: Saunders; 1995:585-590.
13. Scott D.W. Rational use of glucocorticoids in dermatology. In: Bonagura J., editor. Current veterinary therapy XII: small animal practice. Philadelphia: Saunders; 1995:573-581.
14. Kwochka K.W., Kowalski J.J. Prophylactic efficacy of four antibacterial shampoos against Staphylococcus intermedius in dogs. Am J Vet Res. 1991;52:115-118.
15. Hall J.A., Campbell K.L. The effect of potentiated sulfonamides on canine thyroid function. In: Bonagura J., editor. Current veterinary therapy XII: small animal practice. Philadelphia: Saunders; 1995:595-597.
16. Kunkle G. Steroid therapy for the skin. In: In Dermatology: 19th Annual Waltham/OSU for the treatment of small animal disease. Waltham USA: Vernon, Calif; 1995:37-40.
17. Guaguère E., Steffan J., Olivry T., Cyclosporine A. a new drug in the field of canine dermatology. Vet Derm. 2004;15:61-74.
18. Olivry T., Steffan J., Fisch R.D., et al. Randomized controlled trial of the efficacy of cyclosporine in the treatment of atopic dermatitis in dogs. J Am Vet Med Assoc. 2002;221(3):370-377.
19. Steffan J., Strehlau G., Maurer M., et al. Cyclosporin A pharmacokinetics and efficacy in the treatment of atopic dermatitis in dogs. J Vet Pharmacol Ther. 2004;27(4):231-238.
20. Radowicz S.N., Power H.T. Long-term use of cyclosporine in the treatment of canine atopic dermatitis. Vet Derm. 2005;16:81-86.
21. Olivry T., Mueller R.S. Evidence-based veterinary dermatology: a systematic review of the pharmacotherapy of canine atopic dermatitis. Vet Derm. 2003;14:121-146.
22. Steffan J., Horn J., Gruet P., et al. Remission of the clinical signs of atopic dermatitis in dogs after cessation of treatment with cyclosporine A or methylprednisolone. Vet Record. 2004;154:681-684.
23. Marsella R., Nicklin C.F., Saglio S., et al. Investigation on the clinical efficacy and safety of 0.1% tacrolimus ointment (Protopic) in canine atopic dermatitis: a randomized, double-blinded, placebo-controlled, cross-over study. Vet Derm. 2004;15:294-303.
24. Logas D. Systemic nonsteroidal therapy for pruritus: the North American experience. In: In Dermatology: 19th Annual Waltham/OSU for the treatment of small animal disease. Waltham USA: Vernon, Calif; 1995:32-36.
25. Hansson H., et al. Clinical pharmacology of clemastine in healthy dogs. Vet Dermatol. 2004;15:152-158.
26. Campbell K.L. Fatty acid supplementation and skin disease. Vet Clin North Am Small Anim Pract. 1990;20:1475-1486.
27. Saevik B.K., Bergvall K., Holm B.R., et al. A randomized controlled study to evaluate the steroid sparing effect of essential fatty acid supplements in the treatment of canine atopic dermatitis. Vet Derm. 2004;15:137-145.
28. Miller W.H., Scott D.W., Wellington J.R. A clinical trial on the efficacy of clemastine in the management of allergic pruritus in dogs. Can Vet J. 1993;34:2-27.
29. White S.D., Rosychuk R.A.W., Reinke S.I., et al. Use of tetracycline and niacinamide for treatment of autoimmune skin diseases in 31 dogs. J Am Vet Med Assoc. 1992;200:1497-1500.
30. Scott D.W., Buerger R.G. Nonsteroidal antiinflammatory agents in the management of canine pruritus. J Am Anim Hosp Assoc. 1988;24:424-428.
31. Paradis M., Lemay S., Scott D.W. The efficacy of clemastine, a fatty acid containing product (Dermcaps) and the combination of both products in the management of canine pruritus. Vet Dermatol. 1991;2:17-20.
32. Hansson H., Bergvall K., Bondesson U. Clinical pharmacology of clemastine in healthy dogs. Vet Derm. 2004;15:152-158.
33. Paradis M., et al. Further investigations on the use of nonsteroidal and steroidal anti-inflammatory agents in the management of canine pruritus. J Am Anim Hosp Assoc. 1991;27:44.
34. Cooper K.D. New therapeutic approaches in atopic dermatitis. Clin Rev Allergy. 1993;11:543-559.
35. Rapoport J.L., Ryland D.H., Kriete M. Drug treatment of canine acral lick. An animal model of obsessive–compulsive disorder. Arch Gen Psychiatry. 1992;49:517-521.
36. Goldberger E., Rapoport J. Canine acral lick dermatitis: response to the antiobsessional drug clomipramine. J Am Anim Hosp Assoc. 1991;22:179-182.
37. Dodman N., Shuster L., White S., et al. Use of narcotic antagonists to modify stereotypic self-lick, self-chewing, and scratching behavior in dogs. J Am Vet Med Assoc. 1988;193:815-819.
38. Kwochka K.W. Treatment of seborrhea in the American cocker spaniel. In: Kirk R.W., editor. Current veterinary therapy XI: small animal practice. Philadelphia: Saunders; 1992:523-527.
39. Miller W.H. Treatment of generalized demodicosis in dogs. In: Bonagura J., editor. Current veterinary therapy XII: small animal practice. Philadelphia: Saunders; 1995:625-628.
40. Scott D.W. Update on the treatment of generalized demodicosis in dogs. In Dermatology: 19th Annual Waltham/OSU for the treatment of small animal disease. Waltham USA: Vernon, Calif; 1995. pp 77–81
41. Moriello K.A. Treatment of dermatophytosis in dogs and cats: an update. In: In Dermatology: 19th Annual Waltham/OSU for the treatment of small animal disease. Waltham USA: Vernon, Calif; 1995:46-54.
42. Patricilli A.J., Hardie R.J., McAnulty J.F. Cyclosporine and ketoconazole for the treatment of perianal fistulas in dogs. J Am Vet Med Assoc. 2002;220(7):1009-1016.
43. Patterson A.P., Campbell K.L. Managing anal furunculosis in dogs. Compend Contin Educ Vet. 2005;27(5):339-355.