25 The Head and Ventral Neck of the Ruminant
The account contained in this and the following chapters (Chapter 26, Chapter 27, Chapter 28, Chapter 29, Chapter 30, Chapter 31) is predominantly of bovine anatomy. Sheep and goats differ from one another and, more obviously, from cattle in many features of their anatomy, but it seems unnecessary to include any but the most significant and clinically relevant distinctions.
CONFORMATION AND EXTERNAL FEATURES
CONFORMATION AND EXTERNAL FEATURES IN CATTLE
The features of the bovine head that first attract notice are the angular, pyramidal form, the bare muzzle, and the horns (when these are present). The form owes much to the late development of the frontal sinuses that invade the bones of the cranial vault, transforming the domed contours of the calf’s head into the broad, flattened forehead and upright nuchal surface of the adult (Figures 25–1, 25–2, and 25–3). The proportions are also much altered after birth by the greater growth of the facial part than of the neurocranium.

Figure 25–1 Lateral view of bovine skull.
1, Incisive bone; 2, mental foramen; 3, maxilla; 3′, facial tuberosity; 3″, infraorbital foramen; 4, nasal bone; 4′, nasoincisive notch; 5, frontal bone; 5′, horn surrounding cornual process of frontal bone; 5″, temporal line; 6, orbit; 7, zygomatic bone; 7′, zygomatic arch; 8, temporal fossa; 9, temporal bone; 9′, temporomandibular joint; 10, occipital condyle; 11, paracondylar process.
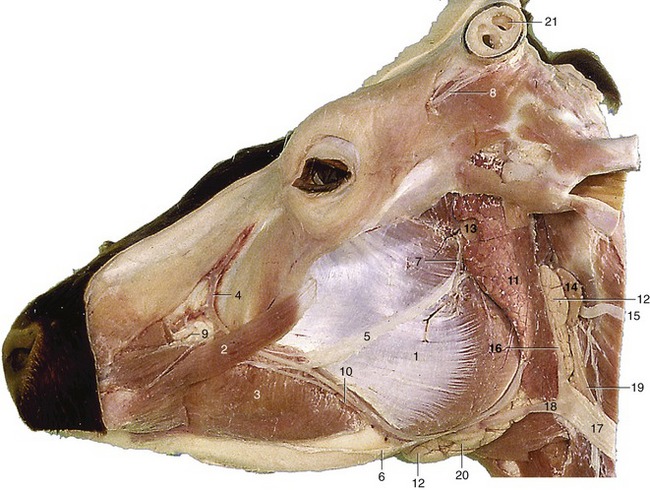
Figure 25–2 Superficial dissection of the head.
1, Masseter; 2, zygomaticus; 3, buccinator; 4, facial vein, 5, 6, dorsal and ventral buccal branches of facial nerve; 7, auriculotemporal nerve; 8, cornual nerve; 9, infraorbital nerve; 10, parotid duct and facial artery and vein; 11, parotid gland; 12, mandibular gland; 13, parotid lymph node; 14, lateral retropharyngeal lymph node; 15, spinal accessory nerve; 16, maxillary vein; 17, external jugular vein; 18, linguofacial vein; 19, common carotid artery; 20, mandibular lymph node; 21, cornual diverticulum of frontal sinus.

Figure 25–3 Paramedian section of the head of a 2-week-old calf. Note the rounded vault.
1, Frontal sinus; 2, ethmoidal conchae; 3, vomer; 4, pharyngeal septum; 5, palatine sinus; 6, hard palate; 7, soft palate; 8, nasopharynx; 9, medial retropharyngeal lymph node; 10, mandibular gland; 11, nuchal ligament; 12, cerebellomedullary cistern; 13, cerebellum; 14, cerebrum; 15, larynx.
The modified skin around the nostrils extends to the margin of the upper lip, forming the slightly cobbled naked nasolabial plate. This is kept moist by the watery secretion of a thick layer of eccrine glands massed below the skin.
The naked integument continues through the large oval nostril into the nasal vestibule where it blends with the mucosa. The opening of the nasolacrimal duct is placed just caudal to the mucocutaneous junction. It is concealed on the ventromedial side of the fold that prolongs the ventral concha rostrally but may be uncovered for cannulation by bending the wing of the nostril outward.
The lips are thick, relatively immobile, and insensitive; they take little part in prehension of food. The upper one is the larger and overlaps the lower lip to the front and sides when at rest.
The size and conformation of the horns depend on breed, age, and sex. The horns are based on much smaller cornual processes that grow from the frontal bones at the caudolateral angles of the forehead. The cornual process has a ridged and porous surface and is covered by a papillated dermis that also serves as periosteum. The specialized dermis blends with that of the surrounding skin at the base of the projection. The major part of the horn wall or sheath grows from the epithelium that covers the dermis over the horn process; the softer outermost layer (epiceras) is produced by an irregular epithelial strip at the base that is transitional to the ordinary epidermis. The horn sheath represents a modification of the cornified stratum of the epithelium and consists chiefly of tubules formed over the dermal papillae; the tubules run lengthwise and are welded together by irregular, intertubular horn produced by the interpapillary regions of the epithelium. Since the whole epithelial surface is productive and the older horn is thrust apically by that of more recent origin, it follows that the horn sheath increases in thickness toward the tip (Figure 25–4). Although horn growth is continuous, the rate of production varies according to the stresses to which the animal is subjected, and it is usual to find the horns marked by alternating rings of greater and lesser thickness. The latter represent periods when production was less active and the horn that was produced was softer and more prone to wear. In cows these periods commonly correspond to calvings. Since the first calf is generally born when the cow is about 2 years of age and subsequent calves are born at yearly intervals thereafter, the number of rings is commonly one fewer than the animal’s age in years (Figure 25–5).
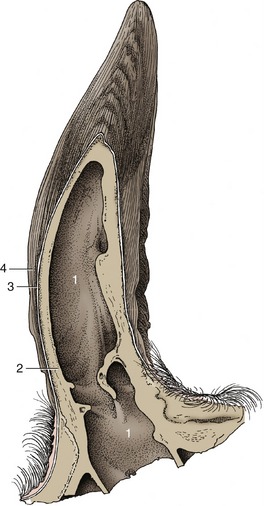
Figure 25–4 Longitudinal section of a bovine horn.
1, Cornual diverticulum of frontal sinus; 2, cornual process; 3, periosteum, dermis, and epidermis; 4, horn tubules.
The sensitive dermis of the horn is supplied mainly by the cornual nerve (Figure 25–6/1), a branch of the zygomaticotemporal division of the maxillary nerve. The cornual nerve arises within the orbit and then passes backward through the temporal fossa, where it is sheltered by the prominent ridge of the temporal line. The nerve later divides into two or more branches that wind around this ridge and approach the horn separately under cover of the thin frontalis muscle. The cornual nerve is often blocked for dehorning operations and is then sought where it crosses the ridge, roughly midway between the postorbital bar and the horn (Figure 25–6/1). The anesthetic technique is not always successful; among the explanations advanced to account for its failure are variation in the relationship of the nerve to the bony ridge, precocious division into divergent branches, and the existence of unusually substantial contributions from the supraorbital or infratrochlear nerves. Since the nerve to the frontal sinus may extend to the diverticulum within the horn, even infiltration around the horn base does not ensure complete loss of sensitivity.

Figure 25–6 Bovine skull with cornual nerve (1) and auriculopalpebral nerve (3). The cornual nerve follows the temporal line (2) to the base of the horn. The auriculopalpebral nerve is palpable where it crosses the zygomatic arch.
The cornual nerve is accompanied by a considerable artery and vein that branch from the superficial temporal vessels within the temporal fossa. The artery ramifies before it reaches the horn. Its smaller branches run in the grooves and canals of the cornual process and retract when severed so that they cannot be easily grasped with hemostats; because of this, dehorning is accompanied by spurting arterial hemorrhage unless the cut is made close to the skull where the arteries are still embedded in soft tissue.
The horns are barely indicated in the newborn calf, and their development can be prevented by cauterization of the germinal epithelium at an early age (2 to 4 weeks). The epidermis, which spreads to heal the wound, lacks the specialized inductive capacity of the original covering. An extension from the frontal sinus invades the cornual process when the calf is about 6 months old.
CONFORMATION AND EXTERNAL FEATURES IN SHEEP AND GOATS
The shape and appearance of the head show many specific, breed, sex, and age differences, but although they determine the “character” of the animal, they are for the most part of no great clinical interest. It is, however, important to note that the dorsal profile of the skull, unlike that of adult cattle, is domed over the cranial cavity and slopes caudally toward the nuchal plane; this feature is commonly masked by the location and size of the horns (Figure 25–7).
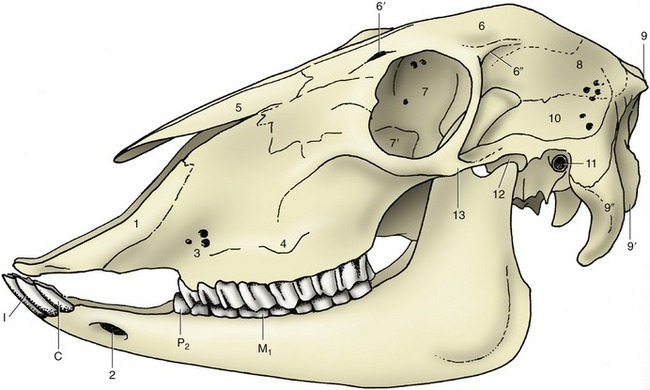
Figure 25–7 Lateral view of the skull of a sheep.
1, Incisive bone; 2, mental foramen; 3, infraorbital foramina; 4, facial tuberosity; 5, nasal bone; 6, frontal bone; 6′, supraorbital foramen and groove; 6″, temporal line; 7, orbit; 7′, lacrimal bulla; 8, parietal bone; 9, external occipital protuberance; 9′, occipital condyle; 9″, paracondylar process; 10, temporal fossa; 11, external acoustic meatus; 12, temporomandibular joint; 13, zygomatic arch.
The goat’s head has a fairly long coat of hair, but that of sheep is shorter, and in some breeds wool extends considerably onto the face. The nasal plate resembles that of the dog but has a more limited extent, particularly in goats. It is confined to a narrow strip to each side of the deep median philtrum, with lateral prolongations along the upper edges of the long, slitlike nostrils.
The horns arise close behind the orbits in a parietal position (see Figure 26–2). Each is based on a separate ossification center that makes a secondary fusion to a projection of the skull quite close to its contralateral fellow. In both sheep and goats the frontal sinus later excavates the horn core at the base but does not reach so far toward the tip as in cattle. Polled breeds are common, but when horns occur they are generally present in both sexes, although those of males are more strongly formed. In a few rare breeds, two (in rams occasionally three) pairs may exist. The multiple-horn (polycerate) condition is frequently associated with defects of cranial sutural closure and also of the eyelids.
The horns of goats generally have an oval section and grow caudally over the skull. Those of sheep are triangular in section and pursue a helical course that carries them first caudally, then successively ventrally, rostrally, and dorsally in a form of increasing complexity. This growth sometimes carries the inner surface of the horn close to the skin of the face, which may suffer from pressure necrosis if contact is made. Shepherds of flocks of the vulnerable breeds are on watch for this occurrence and often remove a surface slice from the horn in treatment or in prevention. The operation can be performed without anesthetic if only “horn” is sawn away; on occasion sensitive dermis and bone must also be removed.
The horns of the sheep and goat are placed so close to the orbit that the supplying structures ascend directly behind the zygomatic process, where the nerve may be blocked. The horn of the goat receives a subsidiary supply through branches of the infratrochlear nerve; these can be reached by a second injection at the dorsomedial margin of the orbit.
Certain glands of the skin of the heads of sheep and goats are mentioned in Chapter 10.
SUPERFICIAL STRUCTURES
Other organs that are visible or palpable in life may be identified with the assistance of Figure 25–2. Relatively little of the skull lies directly below the skin, but large areas have thin coverings of fascia and cutaneous muscle that offer little obstacle to palpation. In addition to the broad forehead and dorsum of the nose, the temporal line, zygomatic arch, facial tuberosity, nasoincisive notch, and ventral border of the mandible are all easily palpated. The supraorbital, infraorbital, and mental foramina can also be identified (Figures 25–1, 25–2, and 25–8).
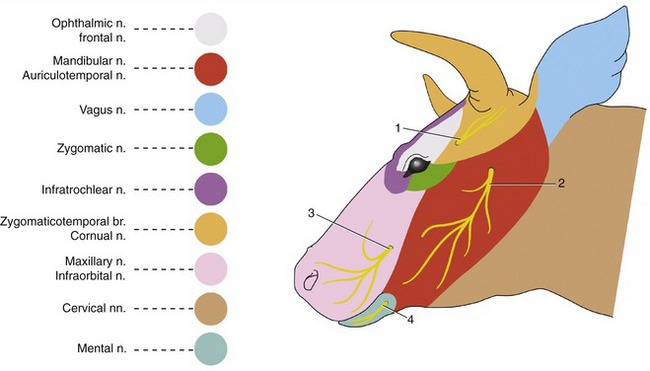
Figure 25–8 Skin innervation of the head.
1, Cornual n.; 2, auriculotemporal n.; 3, infraorbital n.; 4, mental n.
Few specific features of the mimetic musculature are important. It is supplied by the facial nerve (VII), which divides into its principal terminal branches under cover of the parotid gland. The auriculopalpebral nerve supplies muscles of the external ear and eyelids. It reaches these by crossing the zygomatic arch directly in front of the temporomandibular joint, where its superficial position makes it vulnerable (Figure 25–6/3). Damage to the nerve may be evidenced by drooping of the ear and sagging of the eyelids, particularly the lower one. Paralysis of the orbicularis makes it impossible to close the eye. It is therefore clear that it may be advantageous to block the nerve to eliminate the blink reflex when examining the eye. It is most easily palpated where it passes over the zygomatic arch.
The dorsal buccal branch continues the parent trunk, crossing the masseter muscle in an exposed position that carries considerable risk of injury. The effects of such injury include loss of innervation to the muscles of the nose and upper lip and to the buccinator. The first loss leads to slight distortion of the face, which is drawn toward the unaffected side; the second allows food to collect in a wad within the oral vestibule. The ventral buccal branch takes a more protected course caudomedial to the ramus of the mandible and reaches the face in company with the facial artery and vein. It has a limited distribution, and the visible effects of injury are minimal (Figure 25–2/5,6).
The distribution of the cutaneous nerves is shown in Figure 25–8. Specific “blocks” of certain of these nerves are occasionally attempted. The large infraorbital nerve can be palpated where it leaves the infraorbital foramen, about 3 cm dorsal to the first cheek tooth. The mental nerve is found where it leaves the mental foramen of the mandible, about 3 to 4 cm caudal to the lateral incisor tooth.
The facial artery and vein are the most important superficial vessels. They cross the ventral margin of the mandible in front of the masseter muscle and are distributed to the lips, cheeks, muzzle, and periocular structures. The pulse may be examined where the artery lies on the side of the bone; it is less easily located in the notch of the ventral border.
The position of the frontal vein should also be noted because this fair-sized vessel is at some risk in trepanation of the caudal frontal sinus. The vein takes a caudorostral course in a palpable groove over the frontal bone to enter the supraorbital foramen; it then traverses a canal in the lateral part of the sinus. The foramen is located about 2 cm medial to the temporal line and about 2 cm caudal to the lateral angle of the eye (see Figure 25–12/4). A system of veins on the external surface of the pinna becomes engorged and prominent when a tourniquet is applied around the base of the ear. The central member of the set is sometimes used as an alternative to the jugular vein for the placement of an indwelling catheter. Neither site is free from problems.
The ventral end of the mandibular gland forms a conspicuous swelling in the intermandibular space. When palpated, this gland is often mistaken for the adjacent mandibular lymph node (Figure 25–2/20); its larger size, softer consistency, and more medial and more rostral extent make confusion unnecessary. The lymph node can be separately identified on the medial aspect of the sternomandibularis tendon. Normally the parotid lymph node is also palpable rostroventral to the temporomandibular joint.
In the last part of its course along the rostral margin of the masseter, the parotid duct accompanies the facial vessels and ventral buccal nerve. The duct penetrates the cheek opposite the fifth upper cheek tooth.
THE NASAL CAVITY AND PARANASAL SINUSES
The nasal cavity is much smaller than would be supposed from the exterior because its walls are widened and hollowed by air sinuses, while much of the internal space is occupied by the conchae. Caudally, the nasal septum fails to reach the floor, which results in the formation of a single median channel that continues the paired nasal passages into the nasopharynx (Figures 25–9 and 25–10).

Figure 25–9 Paramedian section of the head.
1, Dorsal nasal concha; 2, ventral nasal concha; 3, middle nasal concha; 4, ethmoidal conchae; 5, vomer; 6, choana; 7, nasopharynx; 8, rostral frontal sinus; 8′, caudal frontal sinus; 9, palatine sinus; 10, soft palate; 11, apex of tongue; 12, torus linguae; 13, basihyoid; 14, thyroid cartilage; 15, epiglottis; 16, arytenoid cartilage; 17, cricoid cartilage; 18, medial retropharyngeal lymph node; 19, venous plexus surrounding hypophysis; 20, cerebrum; 21, cerebellum; 22, entrance to tonsillar sinus.

Figure 25–10 Transverse section of a bovine head at the level of the last premolars.
1, Nasal septum; 2, dorsal nasal concha; 3, ventral nasal concha; 4, thick nasal mucosa containing venous plexus; 5, nasolacrimal duct; 6, infraorbital canal with infraorbital nerve; 7, dorsal nasal meatus; 8, middle nasal meatus; 9, ventral nasal meatus; 10, common nasal meatus; 11, maxillary sinus; 12, palatine sinus; 13, hard palate.
Each nasal passage is divided by the major conchae into dorsal, middle, and ventral meatuses that branch from the common meatus located against the nasal septum. The deeper part of the cavity is further subdivided by the numerous ethmoidal conchae; the largest of these projects rostrally and is known as the middle concha. The dorsal meatus leads to the ethmoidal meatuses; the middle meatus communicates with certain sinuses; and the ventral meatus is the principal respiratory pathway. The nasal route is occasionally chosen for the passage of a sound when the instrument is directed to follow the largest space, formed at the junction of the ventral and common meatuses (Figure 25–10/9).
The wall of each nasal passage is clothed by a thick, generously vascularized mucous membrane that ventrally encloses the vomeronasal organ.
The paranasal sinus system is very poorly developed in the young calf, and several years must elapse before it attains full size. Even in the mature animal, the maxillary compartment continues to adjust to extrusion of the cheek teeth (Figures 25–3/1,5 and 25–11).
The complete set of sinuses is very complicated. It comprises frontal compartments within the bones of the cranial roof and side walls; a palatomaxillary complex within the caudal part of the hard palate and the face, both before and below the orbit; a lacrimal sinus within the medial orbital wall; sphenoidal sinuses that extend past the orbit into the rostral part of the cranial floor; and conchal sinuses within the nasal conchae. Any of these may be infected or otherwise become an object of clinical interest, but in practice attention is concentrated on the maxillary and caudal frontal sinuses. The surface projections over which these spaces may be percussed are illustrated in Figures 25–11 and 25–12.
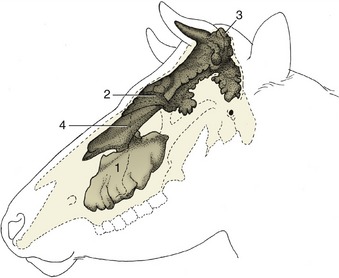
Figure 25–11 Topography of the paranasal sinuses, which are filled with casting material.
1, Maxillary sinus; 2, rostral frontal sinuses; 3, caudal frontal sinus; 4, dorsal conchal sinus.
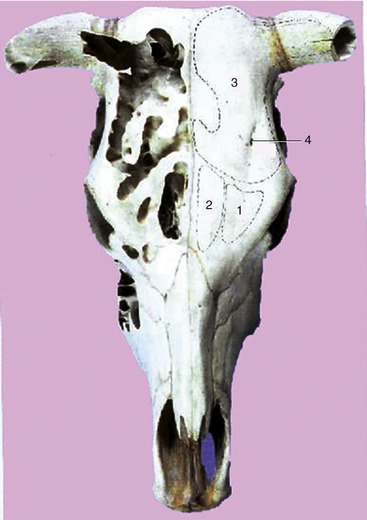
Figure 25–12 Dorsal projection of the frontal sinuses.
1, Lateral rostral frontal sinus; 2, medial rostral frontal sinus; 3, caudal frontal sinus with cornual diverticulum; 4, supraorbital foramen.
The maxillary sinus occupies much of the upper jaw above the alveoli of the cheek teeth. It communicates with the nasal cavity via a large nasomaxillary opening, but natural drainage of pus or other fluid is hindered by the location of this opening high in the medial wall. The maxillary sinus is continuous with the palatine sinus over the plate of bone that carries the infraorbital nerve in its free margin (Figure 25–10/6). It also extends caudally (as the lacrimal sinus in front of the orbit) and within the fragile lacrimal bulla that intrudes into the ventral part of the orbit.
The frontal sinus comprises several compartments that communicate separately with ethmoidal meatuses. The two or, occasionally, three small rostral compartments are of little clinical interest. The caudal compartment, by far the largest and most important, spreads mainly within the frontal bone. It covers the dorsal part of the brain case and also extends into the lateral and nuchal walls and into the horn core. It is separated from its fellow and from the smaller ipsilateral compartments by partitions of rather variable position (Figure 25–12). The openings in these partitions, visible in dry skulls, are closed by mucosa in the fresh state. The major cavity, which continues to increase throughout life, is further subdivided by irregular and perforate septa. Inflammation of its mucosa is a common sequel to surgical dehorning.
The protection that the frontal sinus affords the cranial cavity makes it impossible to predict the extent of the latter by simple inspection of the head. The cranial cavity is in fact surprisingly small, rather globular, and so tilted that its rostral extremity is placed above as well as behind the nasal cavity (Figure 25–9). It is protected above, behind, and to the sides by the pneumatized bones of the cranial vault. The topography is relevant to the usual humane slaughter technique. The target spot is defined by the intersection of the diagonals joining the lateral angles of the eyes to the nearest parts of the opposite horn bases (or equivalent points in polled breeds). The bolt or bullet then has to pass through the shallowest part of the frontal sinus en route to the brain.
The maxillary sinus is shallower and simpler in the sheep and goat. It does not communicate with the lacrimal sinus, which may open into the nasal cavity separately or via the lateral frontal sinus. The frontal sinus comprises separate medial and lateral compartments in both these species. They lie medial to the orbit (and extend slightly beyond this, both rostrally and caudally) and are of irregular form. The lateral compartment corresponds to the caudal sinus of cattle and provides the extension into the horn core.
The most common clinical involvement of the sinuses of sheep is that caused by invasion of the frontal sinus by larvae of oestrid flies. Treatment involves surgical puncture, and the preferred sites are rostral to the horn or medial to the middle of the orbital rim, where there is no risk of injury to the frontal vein.
THE MOUTH
Since cattle do not ingest large mouthfuls, the small size of the oral opening is no disadvantage to the animal; however, it is a considerable hindrance to clinical inspection of the mouth parts and pharynx. The vestibule between the cheeks and the margin of the jaws is surprisingly roomy; the inner surface of the lips and cheeks bears large, backward-pointing papillae that are most prominent toward the corners of the mouth (Figure 25–13/3).
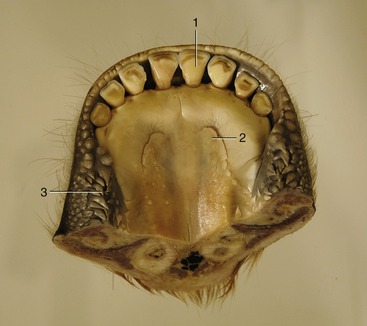
Figure 25–13 Floor of the bovine mouth.
1, Central incisor; 2, sublingual caruncle; 3, buccal papillae.
The mouth cavity proper is long and narrow and is largely occupied by the tongue. The hard palate is most constricted directly in front of the cheek teeth. It is sculpted to display a dozen or more transverse ridges that progressively decrease in prominence and at last fade out toward the back of the mouth; their crests carry numerous papillae (Figure 25–14). The region occupied in other species by the upper incisor teeth here carries the paired dental pads; these are crescentic elevations that are pliant when compressed, though cornified on the surface (Figure 25–14/2). Cattle do not graze by edge-to-edge biting but, after drawing a tuft into the mouth with the assistance of the tongue, sever it by pressing the incisor blades against these pads; the risk of injury to the pads is reduced by their tough covering and pliant consistency and by the procumbent arrangement and rather loose implantation of the incisors (Figures 25–15 and 25–16). The incisive papilla behind the pads is flanked by the small openings of the incisive ducts.

Figure 25–14 The roof of the bovine oral cavity.
1, Incisive papilla; 2, dental pad; 3, buccal papillae; 4, palatine ridges; 5, palatine raphe; 6, first upper cheek tooth (P2).
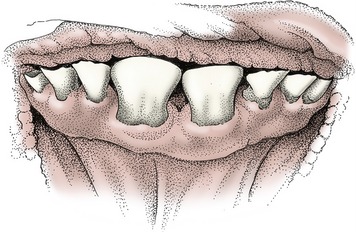
Figure 25–15 Front view of the incisors of a 2-year-old cow. The central incisors are permanent, the others deciduous.

Figure 25–16 Front view of the incisors of a  - to 5-year-old cow. The fourth incisors have reached the height of their neighbors and are coming into wear.
- to 5-year-old cow. The fourth incisors have reached the height of their neighbors and are coming into wear.
The lips of small ruminants are much more mobile than those of cattle. They are the principal organs of prehension and enable these species to crop a pasture closely.
In cattle it is the pointed tongue that is the principal organ of prehension. Its caudal part is raised to form a large torus that is marked off in front by a transverse lingual fossa in which food tends to collect; it is a potential portal for infection because the epithelium, quite delicate within the fossa, is easily pricked by sharp particles (Figure 25–17/5). The papillae that give the surface of the tongue a characteristic roughness are concentrated over the dorsum and toward the apex. Harsh, caudally directed, filiform papillae are freely spread over the apex, while flat and conical lenticular papillae are present on the torus (Figure 25–17/4′,4″); all of these have a purely mechanical function. As usual, it is the fungiform papillae scattered on the apex and the numerous vallate papillae (Figure 25–17/3) present toward the root that carry the sensory receptors concerned with taste. An accumulation of lymphoid tissue toward the root constitutes the diffuse lingual tonsil.
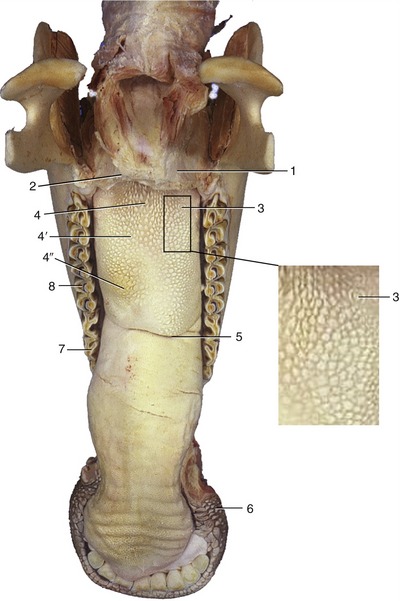
Figure 25–17 Bovine tongue and lower jaw.
1, Soft palate, cut; 2, palatoglossal arch; 3, vallate papillae; 4, filiform papillae; 4′, lenticular; 4′, conical; 5, lingual fossa; 6, buccal papillae; 7, first lower cheek tooth (P2); 8, M1.
The oral floor below the apex of the tongue presents a fleshy sublingual caruncle to each side; the ducts of the mandibular and monostomatic sublingual glands open beside this (see Figure 25–13).
The orientation of the projections on the cheeks, palate, and tongue encourages the backward movement of material within the mouth; this, combined with the general insensitivity of the mouth parts and the copious salivary secretion, may explain the frequency with which cattle swallow foreign bodies concealed within their forage.
THE DENTITION AND MASTICATORY APPARATUS
The most unusual features of the dentition are the absence of incisor and canine teeth in the upper jaw and the assimilation of the canines to the incisors in the lower one. Since both upper and lower first premolar teeth fail to develop, the dental formulae read
for the temporary set, and
for the permanent set. It is customary to refer to the canine tooth as the fourth or corner incisor.
The eight incisor teeth toward the front of the lower jaw are arranged in a continuous crescent that is opposed to the dental pads when the mouth is closed. Each tooth presents a wide spatulate crown abruptly joined to a narrow, peglike root; the crown is asymmetrical, and in young animals it overlaps the lingual aspect of its medial neighbor (see Figure 25–15). The convex labial and concave lingual surfaces initially meet at a ridge, but this becomes increasingly broadened and the dentine increasingly exposed with continuing use (see Figures 25–19, D-E and 25–16). The crowns are sometimes wholly eroded in old animals, and then only narrow but widely spaced roots remain in the margin of the jaw. Often the incisors are shed before this state is reached.
The wide gap or diastema that separates the front from the cheek teeth makes it easy to grasp the tongue to force the animal to permit examination of its mouth. The six cheek teeth in each jaw increase in size from front to back and are so arranged that most occlude with two opponents. The upper tooth rows are more widely separated than those of the lower jaw; consequently, only narrow strips of opposing teeth are in contact when the mouth is closed in central occlusion (see Figure 25–10). The tables slope transversely; the buccal edge is raised on the maxillary teeth, and the lingual edge is raised on those in the mandible. The masticatory surfaces of unworn teeth bear a series of crescentic enamel cusps arranged in two rows parallel to the axis of the jaw: the premolars have one pair of these cusps, and the molars have two. Once wear has exposed the dentine, the alternation of softer and more resistant tissues creates an uneven surface that is a very efficient shredding mechanism when the lower teeth are swung inward across their upper counterparts (Figure 25–18). Attrition of the crowns is compensated by their continuing growth for a time; when growth eventually ceases, the roots are formed, and the height of the exposed part is then maintained only by gradual extrusion of the embedded portion. Eventually the crowns completely erode in animals that survive to very advanced age.

Figure 25–18 Left half of upper and right half of lower jaw of cow. Note the different shapes of the upper and lower cheek teeth and the large diastema (1).
Most temporary teeth closely resemble their replacements, but the temporary premolars, which initially bear the full burden of mastication, are larger and more complicated than those that succeed them. The eruption dates of the teeth are given in Table 25–1.
Table 25–1 Eruption Dates of the Teeth of Cattle
| Temporary Tooth (wk) | Permanent Tooth (mo) | |
|---|---|---|
| Incisor 1 | Birth–2 | 18–24 |
| Incisor 2 | Birth–2 | 24–30 |
| Incisor 3 | Birth–2 | 36–42 |
| Incisor 4 | Birth–2 | 42–48 |
| Premolar 2 | Birth–1 | 24–30 |
| Premolar 3 | Birth–1 | 18–30 |
| Premolar 4 | Birth–1 | 20–36 |
| Molar 1 | 6 | |
| Molar 2 | 12–18 | |
| Molar 3 | 24–30 |
Estimation of age is based on these dates and on the state of wear of the incisors. Neither factor is very reliable. The dates of eruption are influenced by breed and reflect differences in the general rate of maturation. The rate of wear provides a somewhat more useful criterion, though it obviously depends on the nature of the fodder. Wear converts the cutting edge into a surface that gradually broadens. The lingual edge of this surface is originally jagged (because of the ridging of the distal part of the lingual surface of the crown) but becomes smooth when the tooth is worn down; the change in character occurs at 6 years on the first incisor and at 7, 8, and 9 years on the second, third, and fourth incisors, respectively. The teeth are then said to be “level.” Exposure of the root coincides with this alteration in the crown (Figure 25–19, E). The changes at later ages are too unreliable to be of value.
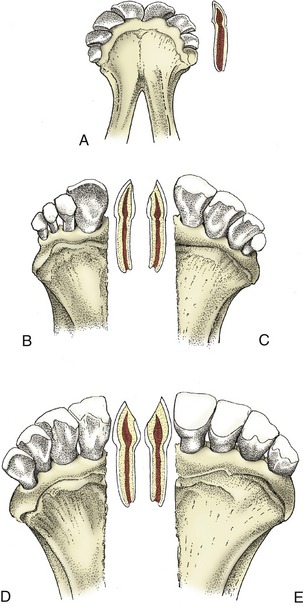
Figure 25–19 Changes in the bovine incisors with increasing age. A, Deciduous incisors in the newborn calf. In the longitudinal section of I1 the enamel still surrounds the crown. B, Two years: I1 has been replaced. The other incisors are deciduous. The distal border of I1 is slightly worn, and the dentine is exposed. C, Three and a half years: I1, I2, and I3 are permanent; I4 is deciduous. The occlusal surface of I2, wider than that of I3, is shown in the longitudinal section. D, Five years. E, Eight years. Note the size of the occlusal surface in the longitudinal section. The lingual edge of the occlusal surface of I1 and I2 is smooth; these two teeth are said to be “level.”
The dentition of the small ruminants broadly resembles that of cattle. The teeth of sheep are often exposed to very rough wear, and tooth loss (“broken mouth”) is a frequent reason for culling older animals. The dates of tooth eruption and replacement in sheep and goats are given in Table 25–2.
Table 25–2 Eruption Dates of the Teeth of Sheep and Goats
| Temporary Tooth (wk) | Permanent Tooth (mo) | |
|---|---|---|
| Incisor 1 | Before birth–1 (at birth) | 12–18 |
| Incisor 2 | Before birth–1 (at birth) | 18–24 |
| Incisor 3 | Before birth–1 (at birth) | 30–36 |
| Incisor 4 | Birth–1 wk (1–3) | 36–48 |
| Premolar 2 | Birth–4 wk (3) | 18–24 |
| Premolar 3 | Birth–4 wk (3) | 18–24 |
| Premolar 4 | Birth–4 wk (3) | 18–24 |
| Molar 1 | 3 (3–4) | |
| Molar 2 | 9 (8–10) | |
| Molar 3 | 18 (18–24) |
From Habermehl KH: Altersbestimmung bei Haus- und Labortieren, ed 2, Berlin, 1975, Blackwell Wissenschafts-Verlag.
Because of the unequal width of the upper and lower dental arcades, mastication is unilateral, and although both sides are used in alternation, most animals tend to favor one. The usual action comprises three phases. In the first, the jaw is dropped and carried laterally; in the second, it is raised while displaced farther to the side; and in the third, which is performed much more swiftly and vigorously, it is carried upward and medially so that the tooth crescents of the lower row engage between those of the upper row as the jaw is returned to its resting position.
The pterygoids of the active side and the masseter of the passive side are the most important muscles in the work stroke.
THE SALIVARY GLANDS
Cattle produce an enormous volume of saliva—perhaps as much as 100 L a day—which contributes to the fermentation medium within the forechambers of the stomach, where it helps to buffer the fatty acids that are produced. Interference with the normal flow to the stomach results in serious depletion of the electrolytes that are normally reabsorbed and recycled.
Although the parotid gland is almost continuously active, it is smaller than might be expected. It lies ventral to the ear along the caudal border of the masseter, where it partly covers the parotid lymph node. A spurt in its growth is coordinated with the initiation of ruminant digestion by the calf. The duct was encountered in the description of the face (Figure 25–2/10).
The mandibular gland is considerably larger. It produces a mixed secretion but only when the animal is actually feeding or remasticating; the flow is most copious when the fodder is dry. The gland extends in an arc on the inner aspect of the lower jaw. Its palpable ventral end projects below the mandible and often almost meets its fellow in the midline; its dorsal end is within the atlantal fossa. The duct runs below the oral mucosa to open by the sublingual caruncle (Figure 25–13/2).
The sublingual gland has the usual two divisions. The polystomatic part lies in the mouth floor, lateral to the tongue, and drains through many small openings beside the frenulum. It is overlapped by the more compact rostral part, whose single duct opens close by or together with that of the mandibular gland.
Many minor salivary glands are scattered below the labial, buccal, palatine, and lingual mucosae; those in the cheeks are particularly well developed. In the aggregate, these lesser glands must contribute a considerable volume of secretion.
THE PHARYNX
The pharynx is divided in the customary fashion.
The nasopharynx extends the nasal cavity caudally. In the ruminants it is incompletely divided by a median membranous fold (pharyngeal septum) that prolongs the nasal septum to the dorsal pharyngeal wall (Figure 25–9/7). The caudal end of this septum is thickened by a mass of lymphoid tissue, the pharyngeal tonsil. Other lymphoid aggregations are found around the slitlike entrances to the auditory tubes on the lateral pharyngeal walls (see Figure 3–25).
The oropharynx is narrow, and this significantly restricts the size of the morsels that can be swallowed. It contains within each lateral wall the palatine tonsil, which projects away from the lumen around a deep, branching tonsillar sinus. The entrance to this sinus (Figure 25–9/22), not the tonsil itself, is visible on the surface.
The laryngopharynx tapers caudally before joining the esophagus, and its lumen is normally held closed by the investing muscles; the muscle principally involved, the cricopharyngeus (Figure 25–20/7), is sometimes described as the cranial sphincter of the esophagus. The piriform recesses to each side of the entrance to the larynx allow a continuous dribble of saliva to reach the esophagus without need for active swallowing.
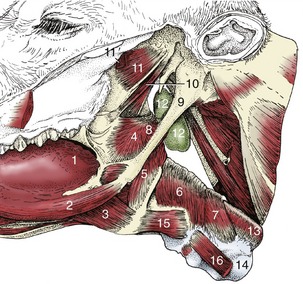
Figure 25–20 Connections of the pharynx and larynx with the base of the skull and the tongue.
1, Root of tongue; 2, styloglossus; 3, hyoglossus; 4, rostral pharyngeal constrictor; 5, middle pharyngeal constrictor; 6, 7, caudal pharyngeal constrictors (thyropharyngeus and cricopharyngeus); 8, stylopharyngeus caudalis; 9, stylohyoid; 10, tensor and levator veli palatini; 11, pterygoideus lateralis; 11′, remnants of pterygoideus medialis; 12, medial retropharyngeal lymph node; 13, esophagus; 14, trachea; 15, thyrohyoideus; 16, sternothyroideus.
The pharynx may be examined by palpation, externally or through the mouth, and its interior may also be inspected with the use of an oral speculum. Swelling of lymphoid tissue in the pharyngeal wall may intrude on the food and air pathways. The pharynx may also be compressed when the adjacent medial retropharyngeal lymph nodes are inflamed (Figure 25–20/12).
The pharynx receives and transmits the regurgitated cud to the mouth. It also receives the gas that is eructated from the stomach in large amounts; some of this gas is lost to the exterior, but a significant portion is directed to the lungs when the communication with the nasopharynx is shut off. The significance of this phenomenon is not fully understood; in animals on certain rations, absorption of eructated gas may lead to tainting of the milk and to pathology of the lung.
THE LARYNX
The larynx is largely situated between the mandibular rami but extends into the upper part of the neck, where it may be felt. The appreciation of its palpable features requires the correct identification of three midline skeletal structures: the basihyoid and the thyroid and cricoid cartilages. Those familiar with the surface anatomy of the horse may experience an initial uncertainty when first examining cattle. The different spacing of the ventral prominences is due to the shape of the bovine thyroid cartilage, which is complete ventrally and most salient toward its caudoventral point.
The bovine larynx shows few other peculiarities of note. The entrance, which may be inspected with the assistance of a laryngoscope, is bounded by the low, curled margin of the epiglottis and the prominent corniculate extensions of the arytenoid cartilages (Figure 25–9/15,16). Intubation is made difficult by a slight caudal deflection of the entrance (see Figure 25–9).
The vestibule possesses neither median nor lateral ventricles, and its side walls shelve smoothly to the glottis. The size of the glottic cleft varies with the phase of respiration, but the changes are not pronounced during quiet breathing. It is narrower than might be supposed, and this limits the caliber of the endotracheal tube that may be passed. The relationship to the medial retropharyngeal lymph nodes is important; when much enlarged, these may seriously compress the larynx as well as the pharynx (Figure 25–9/18).
THE EYE
The orbital rim projects above the surrounding surfaces. The orbital cavity is capacious, although reduced ventrorostrally by the fragile, thin-walled swelling of the lacrimal bulla into which the maxillary sinus extends. The orbital axes diverge upward, outward, and forward and together subtend an angle of approximately 120°. It is therefore clear that, as is usual in ungulates, the field of monocular vision is large, the binocular one small.
The eyelids are supported by dense fibrous plates or “tarsi.” The skin adheres tightly over the orbicularis muscle; however, elsewhere its attachment is looser, and the lid becomes furrowed when the eye is open. The lashes are long and are more densely spread on the upper lid. The muscles of the lids include the frontalis, which extends from the forehead into the upper lid, and the malaris, which radiates from the lower lid onto the face. These are supplied by the facial nerve, mainly through the auriculopalpebral nerve. The levator, supplied as always by the oculomotor nerve, remains active in facial paralysis, which mitigates the effects of this injury.
The conjunctiva contains considerable scattered lymphoid accumulations in its palpebral part. The usual glands are present within the eyelids. The largest, the tarsal (meibomian) glands, occupy the deeper layers of the tarsi; they may be visible through the conjunctiva of the everted lid.
The medial corner of the palpebral opening forms a bay containing the fleshy lacrimal caruncle. The third eyelid covers a variable part of the bulb. The supporting cartilage sinks medial to the eyeball, where it is associated with superficial and deep accessory lacrimal glands. Only a small part of the third eyelid is normally visible. A larger part is brought into view when the eyeball is withdrawn or pressed into its socket; this displaces the retrobulbar fat, which in turn pushes the cartilage and therefore the fold outward.
The lobulated, bipartite lacrimal gland lies dorsolaterally on the eyeball. It drains by numerous ducts of varying caliber into the upper conjunctival fornix. The tears collect by the lacrimal caruncle before entering the slitlike puncta lacrimalia that lead to the lacrimal sac. The sac lies within a depression of the cranial part of the orbital wall. It tapers to the nasolacrimal duct, which first traverses the maxillary sinus and then runs on the lateral nasal wall to discharge within the nasal vestibule.
The extrinsic muscles, which exhibit no especially notable features, are shown schematically in Figure 9–19.
The eyeball is small in relation to the orbit. The sclera is thin and locally obtains a bluish tinge from the dark underlying choroid. Some pigmentation is common, especially toward the junction with the cornea, and tends to increase with age. The cornea is ovoid, and its pointed end is lateral. It is rather thick, especially toward its margin.
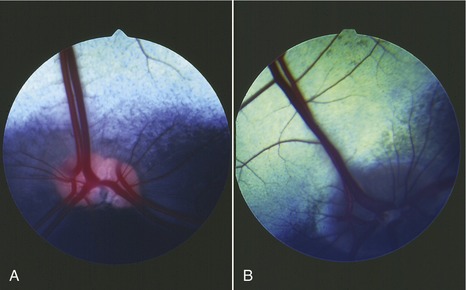
The bovine pupil is widened from side to side when constricted but becomes circular on dilation. Its upper and lower margins are broken by irregular projections, the iridic granules, which are smaller than in the horse; they are more prominent along the upper margin. The ciliary muscles are poorly developed, and the capacity for accommodation is limited accordingly. The vascular and choroidocapillary layers of the choroid are separated in the caudal part of the bulb by the brilliantly colored reflective tapetum (Figure 25–21). The tapetum is triangular, and its base is directly above the optic disc. Its peripheral parts are most colorful and display an array of metallic blues and greens, while the area close to the optic disc is reddish, especially in the calf. Ophthalmoscopic examination of the tapetum reveals scattered dark flecks, where capillaries enter, and larger vessels, which appear as red lines. Four pairs of arteries and veins radiate in cruciate fashion from the optic disc, which is lateroventral to the posterior pole of the eye. The dorsal vein is especially large and is entwined by a spiraling artery. A clear spot in the center of the disc indicates the vestige of the hyaloid artery; as would be expected, the remnant is more obvious in the newborn calf. The macula of the retina consists of two rather ill-defined parts: a rounded area placed dorsolateral to the optic disc is concerned with binocular vision, and a horizontal strip below the tapetum is concerned with monocular vision. Their extents are suggested by their relatively poor vascularization.
Evisceration of the orbit is sometimes performed under local anesthesia. The anesthetic technique, though simple, is exacting because it requires the deposit of anesthetic solution deep in the orbit, precisely by the single foramen (orbitorotundum) through which emerge the nerves that supply the structures within the periorbita. The nerves are thus blocked where bundled together before dispersing to their scattered destinations. Movement of the eyelids may be prohibited in the usual way, that is, by blockage of the palpebral branch of the facial nerve where it crosses the zygomatic arch (Figure 25–6/3).
THE VENTRAL PART OF THE NECK
Dorsal cervical structures are described with the vertebral column (Chapter 26). The skin of the ventral aspect is freely movable and redundant in amount; it becomes folded and creased when the head is lowered to the ground. In addition, the caudal part of the neck carries the large dewlap that continues onto the brisket (breast) between the forelimbs (Figure 25–22). There is scant evidence for the belief that this increase in surface area is important in heat dissipation as is sometimes claimed, for the Zebu in particular. Zebu cattle do possess, here and elsewhere, more numerous, larger, and more saclike sweat glands than are found in cattle of European origin.
The groove over the course of the external jugular vein is generally obvious, at least in cows. It is bounded dorsally by the brachiocephalicus (cleidomastoideus) extending from the arm to the skull and ventrally by the part (sternomandibularis) of the sternocephalicus that runs between the manubrium of the sternum and the angle of the jaw. Except in the most caudal part of the neck, a second part of the sternocephalicus (sternomastoideus) forms the floor of the groove and provides a substantial separation between the vein and the common carotid artery (Figure 25–23/7). The external jugular vein is easily raised for injection and blood sampling because only the caudal part is covered by the cutaneous muscle, and even this is rather weak. The vein is formed caudal to the parotid gland by the confluence of maxillary and linguofacial radicles (see Figure 25–2). It is the principal drainage of the head and neck but is assisted by the internal jugular vein, the vertebral vein, and the internal vertebral plexus. Variation in the prominence of the vein may reflect conditions within the thorax. Gentle undulation in time with respiration is due to a change in intrathoracic pressure. Pulsation in time with the heartbeat in healthy cattle indicates the recurrence of atrial systole; in other animals it points to atrioventricular valvular incompetence. The normal jugular pulse does not persist after compression of the cranial part of the vein, but the pathological pulse does.
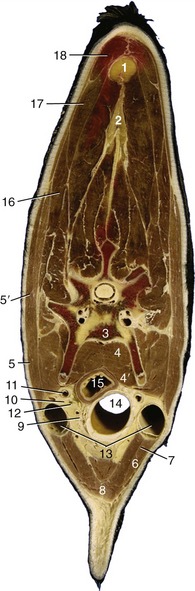
Figure 25–23 Transverse section through the middle of the bovine neck.
1, 2, Nuchal ligament (funiculus and lamina nuchae); 3, vertebra; 4, longus colli; 4′, longus capitis; 5, 5′, brachiocephalicus; 5, cleidooccipitalis; 5′, cleidomastoideus; 6, 7, sternocephalicus; 6, sternomandibularis; 7, sternomastoideus; 8, combined sternohyoideus and sternothyroideus; 9, thymus and internal jugular vein; 10, recurrent laryngeal nerve; 11, common carotid artery; 12, vagosympathetic trunk; 13, external jugular vein; 14, trachea; 15, esophagus; 16, omotransversarius; 17, trapezius; 18, rhomboideus.
The superficial muscles enclose the space that contains the cervical viscera and the vessels and nerves that make their way between the thorax and the head (see Figure 25–23). All of these organs are invested by tough fascia and are joined by looser tissue.
The trachea may be identified on deep palpation and is most easily appreciated toward the upper end of the neck, between the diverging sternocephalic muscles; even here it is not directly subcutaneous because the thin straplike sternothyrohyoid muscles follow its whole length. The trachea (Figure 25–23/14) is small in section and slightly deeper than it is wide; its form makes it susceptible to narrowing by local pressure. The symmetry of its relations is disturbed by the devious course of the esophagus. Its structure is mainly remarkable for the concentration of lymphoid tissue in the dorsal retromucosal space (external to the tracheal muscle but within the cartilage rings).
Although the esophagus cannot be identified by palpation, its position is made evident by the swift movement along its track when the animal swallows. In its cervical course the esophagus gradually slips to the left of the trachea only to creep back to a more dorsal position as the thorax is approached. However, its position varies with posture; its course is considerably straightened when the neck is extended. The relations in the middle of the neck are shown in Figure 25–23.
The ruminant esophagus is very distensible, but its wide appearance in the cadaver gives a misleading impression of the usual condition in life. The mucosa is remarkably insensitive, which is one reason why cattle rarely appear to be distressed by the passage of a stomach tube or probang. Although transport is normally rapid in both directions, chunks of food quite commonly become lodged in the esophagus. The predilection sites are at the origin from the pharynx, at the thoracic inlet, and level with the tracheal bifurcation.
The thyroid gland is almost completely divided into two lobes, each shaped like an inverted pyramid and placed laterally over the cricoid cartilage. They are tenuously joined by an isthmus that crosses the second tracheal ring ventrally. They are finely granular and brick-red in the adult but paler in the calf (see Figure 6–4, C).
The parathyroid glands are small (ca. 8 to 10 mm) and, because they are irregular in shape and inconstant in position, frequently difficult to find. They may be embedded in other structures—usually the thyroid, thymus, or mandibular gland. The external parathyroid most often lies cranial to the thyroid but caudal to the carotid bifurcation; the internal one is perhaps most often embedded in the thyroid or located between this and the trachea. They have been confused with lymph nodes, which they resemble superficially.
The thymus is large and lobulated and extends from the larynx to the pericardium in young animals (Figure 25–24/1,2). Its cervical part is connected to the thoracic thymus by a narrow isthmus ventral to the trachea. The cervical part comprises two horns that taper over the lateral aspects of the trachea, possibly reaching the larynx; the cranial tip may be, or appear to be, detached and fragmented and more closely associated with the medial retropharyngeal lymph node and the mandibular and parathyroid glands. The thymus grows rapidly during the first 6 or 9 months of postnatal life, although it attains its greatest relative size much earlier. Indeed, involution may begin as early as the 8th week after birth. The tempo of regression varies, and the thymus, particularly its thoracic part, may still be quite large in animals several years old. Ultimately the isthmus and neck part disappear almost completely. The thymus of young calves is bright pink or even red, but the organ lightens with age; its consistency also firms as the active tissue is progressively replaced by fatty fibrous tissue.
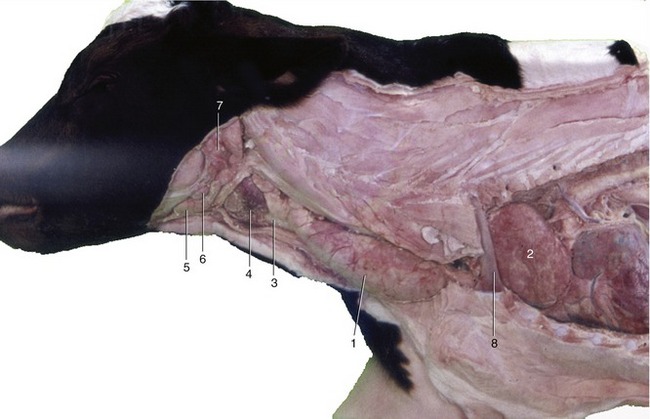
Figure 25–24 The thymus in the newborn calf.
1, Cervical part of thymus; 2, thoracic part of thymus; 3, trachea; 4, thyroid gland; 5, mandibular gland; 6, mandibular lymph node; 7, parotid gland; 8, first rib.
The common carotid artery runs dorsolateral to the trachea within a fascial sheath shared with the vagosympathetic trunk. The internal jugular vein and the recurrent laryngeal nerve are closely related to the sheath on the right side; the esophagus intervenes on the left. The artery ends over the lateral pharyngeal wall, where it detaches a small occipital artery; the parent trunk is continued (without alteration of course) as the external carotid artery. In the fetus an internal carotid artery arises with the occipital artery, but the part proximal to the rete mirabile (see Figure 7–35) begins to close even before birth; complete obliteration is usually achieved a few months after birth, although a residual lumen sometimes persists for a year or two (Figure 25–25/4). The common carotid artery detaches no branches of individual consequence before its termination. Pulsation in the common carotid may sometimes be detected when the artery is pressed against the transverse processes of the vertebrae.

Figure 25–25 Branching of the left common carotid artery.
1, Common carotid a.; 2, occipital a.; 3, ascending palatine a.; 4, remnant of internal carotid a.; 5, medial meningeal a.; 6, external carotid a.; 7, linguofacial trunk; 8, lingual a.; 9, facial a.; 10, deep lingual a.; 11, sublingual a.; 12, submental a.; 13, inferior labial aa.; 14, superior labial a.; 15, infraorbital foramen; 16, caudal auricular a.; 17, masseteric branch; 18, superficial temporal a.; 19, transverse facial a.; 20, cornual a.; 21, maxillary a.; 22, inferior alveolar a.; 23, mental a.; 24, rostral and caudal branches to rete mirabile; 25, malar a.; 26, angular a. of the eye; 27, caudal lateral nasal a.; 28, dorsal nasal a.; 29, infraorbital a.; 30, sphenopalatine a.; 31, major and minor palatine aa.
At this point brief mention may be made of the blood supply to the brain, less because of any clinical significance than on account of its relevance to the controversial Jewish and Muslim slaughter techniques, in which the animals are killed by a deep ritual slash of the neck without preliminary stunning.
The brain is supplied by a combination of vessels that feed very intricate arterial plexuses within the cranial cavity, external to the dura mater and submerged within the cavernous and associated venous sinuses. These plexuses, the retia mirabilia, are formed by many closely wound, anastomosing arteries. The retia are entered on their peripheral aspect from several sources (see Figure 7–35); on the distal or cerebral side the network narrows to one emissary trunk that pierces the dural membrane to form the cerebral arterial circle with its fellow. The circle lies on the ventral aspect of the brain and gives off branches according to the conventional pattern. The basilar artery, which runs caudally over the medulla and continues down the spinal cord, is a contributor to the circle in cattle but leads blood from it in sheep. Although difficult to explain on hemodynamic grounds, all parts of the bovine brain are supplied by a mixture of carotid and vertebral blood, whereas in sheep the vertebral blood is restricted to the caudal part of the brainstem. These differences are germane to the ritual slaughter technique because the vertebral arteries are spared when the common carotid trunks are severed. The suggestion that abrupt reduction of the pressure within the cerebral arteries produces almost immediate loss of consciousness has been questioned.
The vagosympathetic trunk exhibits no particular features of note. The vagus and sympathetic components loosen their association and part company before entering the thorax. Their further courses and connections are described elsewhere. The recurrent laryngeal nerves resemble those of other species.
THE LYMPHATIC STRUCTURES OF THE HEAD AND NECK
The most important lymph nodes of the head were mentioned in their topographical contexts; other smaller nodes that are usually found medial to the ramus of the mandible are of slight practical concern.
The parotid node (Figure 25–2/13) receives lymph from the skin covering most of the head, especially the more dorsal areas. It also collects from the upper jaw, temporomandibular joint, masticatory muscles, nasal cavity, hard palate, orbit, and the region about the external ear. The efferent vessels pass to the lateral retropharyngeal node.
The territory of the mandibular node (Figure 25–2/20) overlaps those of the parotid and medial retropharyngeal nodes. The chief afferent vessels come from the skin and underlying structures of the ventral part of the head and from the rostral part of the mouth, including the apex of the tongue. The efferent vessels pass to the lateral retropharyngeal node.
The large medial retropharyngeal node lies embedded in fat between the pharynx and the muscles below the cranial base (Figures 25–9/18 and 25–20/12). It collects lymph from most of the deeper structures of the head, including the nasal and oral cavities, pharynx, larynx, cranium, and jaw muscles, and from the ventral part of the upper end of the neck. The efferent vessels once again drain into the lateral retropharyngeal node, which is the collecting center for the entire head (Figure 25–26/4). This lateral node, which is placed below the atlantal wing (Figure 25–2/14), also acts as a primary center for additional lymph vessels draining deeper structures of the head. It channels its outflow into a single large vessel, the tracheal duct, that runs down the neck within the fascia covering the lateral aspect of the trachea. The duct ends by joining the thoracic duct or by opening into one or another vein at the thoracic inlet; most usually the left tracheal duct opens into the thoracic duct while the right one drains directly into a major tributary of the cranial vena cava (Figure 25–26/9).
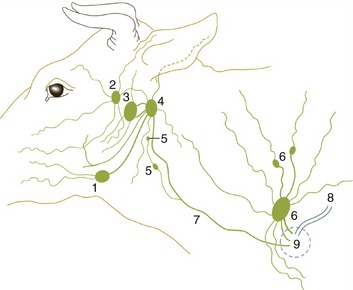
Figure 25–26 The lymph drainage of the head and neck.
1, Mandibular lymph node; 2, parotid lymph node; 3, medial retropharyngeal lymph node; 4, lateral retropharyngeal lymph node; 5, deep cervical lymph nodes; 6, superficial cervical lymph nodes; 7, tracheal duct; 8, thoracic duct; 9, area within which lymphatic vessels enter veins.
A series of small deep cervical lymph nodes is spread along the course of each tracheal duct. These are supposedly divided into cranial, middle, and caudal clusters and receive lymph from the structures within the cervical visceral space. They transmit this lymph to the tracheal duct, sometimes directly and sometimes after serial passage through several nodes within the group. Usually one or more of the most caudal of these nodes receive the efferent vessels of the axillary lymph center of the forelimb, as well as smaller trunks coming directly from the brisket.
A single, much larger node lies in the lower part of the neck in front of the scapula. This is the superficial cervical (prescapular) node (Figure 25–26/6), which rests on the deep muscles over the cervical vertebrae; it is easily palpated, though covered by the omotransversarius. It collects from the skin and underlying muscles over a very wide area extending from the middle of the neck to the caudal part of the thorax, including the proximal part of the forelimb. The flow through the node is compartmentalized; particular portions of the node are related to different parts of the drainage field. The large efferent vessels open variously into the major lymph and venous trunks in the vicinity.