8 The Nervous System
INTRODUCTORY CONCEPTS
Every living organism must be able to react appropriately to changes in its environment if it is to survive; by surviving, it increases the chance of survival of the species. The regulation of these reactions is the responsibility of the nervous system, incomparably the most complicated of the body systems.
A purely descriptive account, of the brain in particular, has a very limited value or appeal; an account that attempts an adequate explanation of function encounters certain problems. Many of the structures and pathways of which the central nervous system is composed are neither discrete nor identifiable by the usual methods of anatomy; the majority of the “functional units” that it is convenient to recognize have multifarious and complex connections with other such units. There are parts to which it is impossible to pin specific functional labels, either because their significance is unknown or because of a multiplicity of associations.
The compromise adopted in this chapter is the presentation of an initial formal description followed by short and rather elementary digressions on the functional significance of a few selected units. These digressions have as their prime purpose the attachment of some “meaning” to the structures previously described. We do so knowing that more complete functional analyses will be provided by concurrent or later courses of physiology or neurology.
THE STRUCTURAL ELEMENTS
An appropriate environmental change provides a stimulus that is recognized by a receptor organ; the reaction or response that may be provoked in answer to the stimulus is performed by an effector organ (Figure 8–1). In multicellular organisms the receptor and effector organs are separate and are connected by a chain of neurons, highly specialized cells in which the general cytoplasmic properties of excitability and conductivity are developed to extreme degrees. Whatever the stimulus, the receptor neuron translates it into an electrical potential, and the message is transmitted in this coded form. The impulse travels the length of the neuron before transmission to the next cell in the chain; this may be another neuron or interneuron, but ultimately, at the end of the chain, the motor neuron will end on an effector muscle or gland cell. Neurons thus provide the basic units from which the nervous system is constructed.
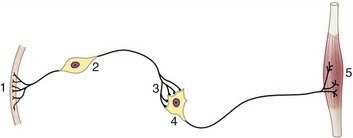
Figure 8–1 A simplified receptor–effector system. 1, Skin receptor; 2, afferent neuron; 3, synapse; 4, efferent neuron; 5, striated muscle (effector organ).
The typical neuron is an elongated cell that consists of a cell body containing the nucleus (therefore known as the perikaryon) and various processes (Figure 8–2). The processes, which vary considerably in number, length, and form, are of two varieties obviously and usefully distinguished by the direction in which they transmit impulses. One variety, the dendrite, is usually multiple and transmits impulses toward the perikaryon; the other, the axon, is always single at its origin (although it may divide at some distance from the perikaryon) and conveys impulses away. The nerve cell is thus clearly polarized. The arrangement of the processes permits a simple morphological classification of neurons. Most are multipolar and possess a number (often a very large number) of branching dendrites that join the perikaryon at scattered points (see Figure 8–2). In a second type, bipolar, the dendrites join in a common trunk before reaching the perikaryon at a site remote from the origin of the axon. In the third type, unipolar, the dendrite tree and axon first combine in a single extension of the perikaryon that later branches; such neurons are also described as pseudounipolar because they initially develop as bipolar cells. Dendrites and axons are superficially alike, and both are commonly described as nerve fibers. As a general rule to which there are many exceptions, dendrites are relatively short and axons relatively long.
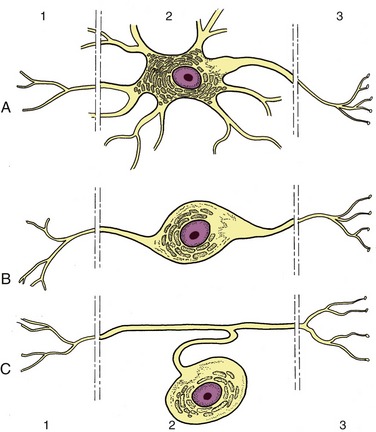
Figure 8–2 Multipolar (A), bipolar (B), and pseudounipolar (C) neurons. 1, Receptor side (dendrites); 2, cell body (perikaryon); 3, effector side (axon).
The different varieties of a neuron have specific distributions that are related to their particular functions. Clearly, a much-branched dendritic tree enables a neuron to receive impulses from many sources. Conversely, a much-branched axon makes connection with and stimulates many cells. The first arrangement allows a convergence of impulses from various origins; the second provides for a divergence or diffusion of a message.
Interneuronal connections are known as synapses, a term usually broadened to also include neuromuscular connections. An axon may establish synaptic connections with the bodies, dendrites, or axons of other neurons, which are varieties of synapses distinguished as axosomatic, axodendritic, and axoaxonic. Most neurons establish many synapses: some have many thousands of synaptic sites, though not in relation to so many other cells. Synapses have a variable but always complicated morphology, but only an elementary description is required here. The participating cells are neither continuous nor in direct contact but are always separated by a very narrow gap. A nerve impulse (action potential) arriving at the presynaptic part of an axon does not jump from cell to cell; instead, it prompts the release of a specific chemical transmitter substance that diffuses across the gap. When this substance arrives at the postsynaptic plasma membrane (of the following cell), it produces one of two effects: it either depolarizes the membrane, initiating a fresh impulse, which is then propagated the length of the postsynaptic cell, or it hyperpolarizes the membrane, producing a blocking or inhibitory effect. The existence of both excitatory and inhibitory synapses, sometimes on the same cell, provides a means for a great diversity of response. Many transmitter substances are known; the most common include acetylcholine, glutamate (excitatory), GABA (inhibitory) noradrenaline, serotonin, and many neuropeptides.
Neurons are supported by other specialized cells. The supporting tissue of the brain and spinal cord is known as neuroglia and comprises several cell types that we shall not distinguish. Neuroglial cells not only support the neurons but also assist in their nutrition and neurotransmission; additionally, neuroglial cells provide nerve fibers within the brain and spinal cord with cytoplasmic products that insulate them from their surroundings and prevent leakage of the impulses they convey. The insulating material, myelin, incidentally imparts a white color to nerve fibers seen en masse. Nerve fibers within peripheral trunks (outside the brain and spinal cord) receive similar insulation—of very variable thickness—from another type of supporting cell, the Schwann cell (neurolemmocytus; Figure 8–3). Peripheral nerve trunks are further protected, supported, and subdivided by connective tissue sheaths and septa, but the brain and cord, although included within a series of connective tissue investments (meninges), are not penetrated by connective tissue in this way.
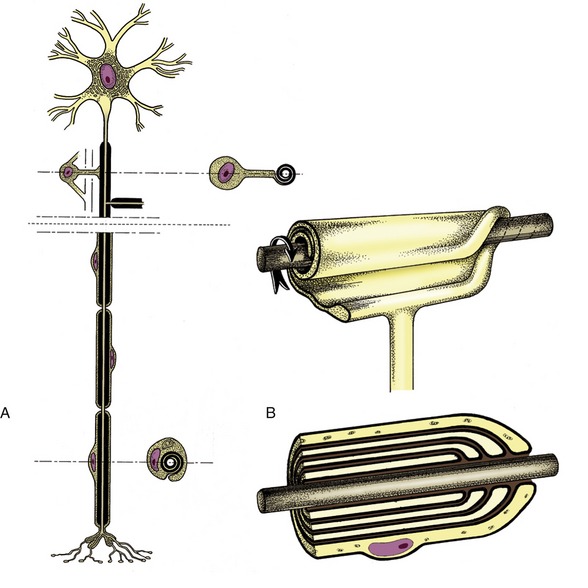
Figure 8–3 A, Neuron with its axon enwrapped within a cytoplasmic sheath supplied by a series of Schwann cells. B, The cell membrane of the Schwann cell is rolled around the axon. The investment may consist of several plasmalemma layers forming a thick myelin sheath.
Groups of perikarya are distinguished by their darker color, especially when set off by the whiteness of adjacent fiber bundles; this permits the ready distinction of the “gray” (in fact beige in the fresh specimen) and white substance of the brain and cord. Isolated neuronal aggregations within the brain are generally known as nuclei; many are too small to be distinguished by the naked eye.
Fiber bundles of common origin, destination, and function tend to be aggregated within the brain and cord into fasciculi or tracts, although the limits of these are not normally evident and can be made so only by experimental means. Most such tracts are named by the combination of their origin, employed as prefix, with their destination, employed as suffix; the significance of such names as the spinocerebellar and cerebellospinal tracts is thus revealed directly.
Neuronal aggregations on peripheral nerves may form visible swellings; they may also be distinguished by their color and texture, which are darker and firmer than those of the related nerve trunks. They are universally known as ganglia.
STIMULUS-RESPONSE APPARATUS
Having established these fundamental points, we may now return to consider the stimulus-response apparatus. In the simplest form found in mammals, this apparatus comprises five elements arranged in series: a receptor region adapted to respond to a stimulus of a particular modality* (sound, touch, and so forth); an afferent neuron that conveys an impulse centrally, toward the brain or cord; a synapse; the remainder of the efferent neuron that conveys an impulse from the center to the periphery; and an effector, which may be a muscle, gland, or neurosecretory cell (see Figure 8–1). This sequence constitutes a primary, elementary, or monosynaptic reflex arc.
The monosynaptic reflex arc is actually a most uncommon arrangement, although it does provide the basis of one familiar example, the patellar or knee-jerk reflex (Figure 8–4). This is a stretch (myotactic) reflex that, as many readers will know, can be elicited by an appropriate tap on the patellar ligament, which is the functional continuation of the quadriceps femoris muscle. The tap stretches the muscle and thus stimulates muscle spindles and other receptors within its belly and tendon; an impulse travels along afferent fibers within the femoral nerve to reach the spinal cord, where it is projected on efferent (lower motor) neurons. The axons of these neurons return within the femoral nerve, and the impulse is then projected on the constituent fibers of the muscle, stimulating their contraction to effect the abrupt extension of the joint.

Figure 8–4 The monosynaptic patellar reflex. The stretch stimulus on the tendon (1) travels via the afferent neuron (2) to the spinal cord. The impulse is then transmitted to the efferent neuron (3), which stimulates the quadriceps muscle (4).
In most reflexes one or more additional neurons are interposed in the chain between the afferent and efferent neurons (Figure 8–5). These are conveniently known as interneurons, although several synonyms exist. The system may still be described as simple if only an unbranched neuronal chain is involved. However, most reflexes involve more complicated circuitry in which additional neurons are stimulated (or inhibited). Collateral branching enables the exercise of a more refined control and possibly the intrusion of the activity on consciousness.
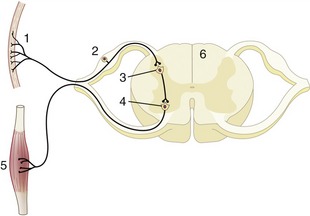
Figure 8–5 Schematic representation of a reflex chain in which an interneuron is interposed. 1, Skin receptor; 2, afferent neuron; 3, synapse at interneuron; 4, synapse at efferent neuron; 5, muscle; 6, spinal cord.
A good example of an integrated response is given by the limb of a standing animal subjected to a prick or other noxious stimulus. The limb is withdrawn by the coordinated action of the flexor muscles of several joints; these movements are facilitated by the relaxation of the previously active and antagonistic extensor muscles. The branching pathways involved in securing this response extend through several segments of the cord to reach and excite, or inhibit, the efferent neurons that supply the various muscles. At the same time, the animal has to adjust to the removal of one of its supporting props by redistributing its weight over the other limbs; the pathways necessary for this wider adjustment extend through considerable stretches of the cord, some of which cross to the contralateral side (Figure 8–6).
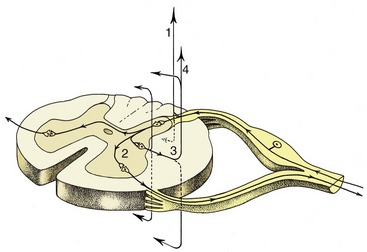
Figure 8–6 The course of fibers within the spinal cord. Some afferent fibers in the dorsal funiculus travel directly toward the brain (1); others end on interneurons in the dorsal horn. From here impulses can be transmitted directly to efferent neurons (2) or to other interneurons that transmit impulses caudally or cranially within the spinal cord (3), some extending to the brain (4).
Coordination of the changes so that balance may be maintained involves higher centers within the brain, to which the message must ascend, in addition to integration within the cord. The process is unlikely to go unnoticed; the cortex is involved, and the animal assesses the situation and considers whether a more general response, such as flight or retaliation against the aggressor, would be appropriate. This considered response is a far cry from the simple, monosynaptic, and monosegmental response of the knee jerk and involves integrative apparatuses of various degrees of complexity, spread through the cord and brain, and drawing on those higher centers that are concerned with memory and judgment.
THE SUBDIVISIONS OF THE NERVOUS SYSTEM
Although the nervous system forms a single, integrated whole in reality, it is convenient, indeed necessary for many purposes, to divide it into parts. The most fundamental division can be made on topographical grounds, distinguishing the central nervous system (brain and spinal cord, or neuraxis) from the peripheral nervous system (the cranial, spinal, and autonomic nerve trunks with their associated ganglia). The division facilitates description but at the cost of making an artificial distinction that even assigns different parts of the same neurons to the two divisions: for example, the perikarya and axons of the efferent neurons of the patellar reflex arc.
An alternative division that would purport to have more regard to function is based on the direction in which impulses travel and on the nature of the information these impulses convey. It distinguishes afferent from efferent systems. The former conduct impulses toward the spinal cord and particular brain parts; the latter convey impulses away from these structures. Afferent pathways within the peripheral nerves are frequently termed sensory; the impulses travel from the periphery toward the brain or spinal cord. Within the cord they are more often described as ascending; the impulses travel from “lower” (more caudal) toward “higher” (more cranial) parts. Efferent pathways usually conduct impulses from “higher” to “lower” levels within the brain and cord and from these to the periphery; the alternative names to describe these systems are descending and motor. The equivalence of certain of these terms does not withstand close scrutiny, particularly when applied to integrative systems within the spinal cord; many descending fiber bundles are not motor, and many ascending bundles are not sensory.
The nature of the information that is conveyed, as well as the nature of activities that are directed, permits the further distinction of somatic and visceral nervous systems. The somatic system is concerned with those functions, like locomotion, that determine the relationship of the organism to the outside world. The visceral system is concerned with functions that relate to the internal environment: the regulation of the vascular system and heart rate, the control of glandular activity and digestive processes, and so forth. As a general but not invariable rule, there is a greater awareness and greater voluntary control of somatic than visceral functions; of course they work in close collaboration.
A more elaborate classification is possible. Afferent systems are initially divisible into somatic and visceral divisions, and these in turn are divisible into general and special subdivisions.
Somatic afferent pathways originate in receptors within the skin and deeper somatic tissues of the body wall and limbs. The pathways that arise from skin receptors are concerned with the exteroceptive sensations, such as touch, temperature, and pain, that respond to stimuli delivered from outside the organism. Receptors within the deeper tissues include the additional proprioceptive category concerned with such “deep” sensations as those that inform on the present angulation of the joints and tension within muscles and tendons and on changes in these conditions. Somatic afferent fibers are carried by all spinal nerves and by the fifth cranial (trigeminal) nerve (see Table 8–2, p. 286).
Special somatic afferent pathways have a more restricted origin within certain special sense organs: the retina of the eye and the cochlear and vestibular components of the inner ear, which are concerned with vision, hearing, and balance, respectively. The fibers concerned with vision and hearing are exteroceptive, those concerned with balance proprioceptive. Special somatic afferent fibers are thus found only within two cranial nerves, the optic and vestibulocochlear nerves.
Visceral afferent pathways originate in the (enteroceptive) receptors of vessels and glands and the viscera of the head and trunk that mostly respond to stretch and chemical stimuli. The fibers of this division are found in the cranial nerves III, V, VII, IX, and X, certain sympathetic and parasympathetic nerves, and all spinal nerves.
Special visceral afferent pathways arise from the special sense organs of smell and taste. Fibers conveying olfactory information are confined to the olfactory nerve; those conveying gustatory (taste) information are confined to a small group of cranial nerves.
Efferent systems are divided more simply.
Somatic efferent pathways lead to striated muscles of somitic and branchiomeric* origin.
Visceral efferent pathways lead to the smooth muscle of the viscera and vessels, to heart muscle, and to glands. Most of these organs receive a double innervation through the sympathetic and parasympathetic divisions of the autonomic nervous system (p. 327), which are often described as antagonistic, although “balancing” might better suggest their cooperative role. Visceral efferent fibers of the sympathetic division leave the central nervous system via the spinal nerves in the thoracolumbar regions of the cord; those of the parasympathetic division are limited to a small group of cranial nerves and to the sacral contingent of spinal nerves. However, many visceral efferent fibers later join other nerves so that they finally obtain a very widespread peripheral distribution.
SOMATOTOPY
The fibers and cell bodies within many tracts and relaying nuclei and within areas of the cerebral and cerebellar cortices on which these may project preserve very orderly point-to-point arrangements that reflect the topography of the parts of the body from which afferent impulses arise or to which efferent impulses are delivered. These do not always, or even usually, reproduce the true proportions but represent the parts of the body in relation to the densities of their innervation. The representations take the form of grotesque caricatures, sometimes known as homunculi—although animalcula would better fit veterinary anatomy—in which very sensitive parts, such as the lips and muzzle of the horse, or those capable of very refined and accurate movements, such as the fingers of a human or the prehensile tail of a monkey, are of exaggerated size. The concept of somatotopy is of great importance in the consideration of the significance of pathological lesions, in the conduct of neurosurgery, and in experimental stimulation.
GENERAL MORPHOLOGY AND EMBRYOLOGY OF THE CENTRAL NERVOUS SYSTEM
INTRODUCTORY SURVEY
The brain† and spinal cord‡ are continuous without any clear demarcation. The brain is a very irregular organ whose shape conforms very approximately to the cranial cavity in which it is lodged, whereas the slender elongated cord has a more regular and uniform appearance.
The size of the brain bears no linear relationship to that of the animal from which it came but is relatively smaller in large species and is certainly proportionately greater in more advanced mammals. It is the relative weights that signify. The ratio of brain weight to body weight is of the order of 1 : 50, 1 : 200, and 1 : 800 in human, dog, and horse, respectively. As a rule domestication leads to reduction in weight; the process cannot be reversed by putting domesticated animals back into the wild. A more clear significance attaches to the relative development of particular parts of the brain; there is a relative preponderance of “newer” parts (in the phylogenetic sense), particularly in the cerebrum in mammals generally and in “higher” mammals specifically, when comparison is made with lower forms. The great size and complexity of the human cerebral hemispheres provide the extreme example of this evolutionary trend (Figure 8–7).
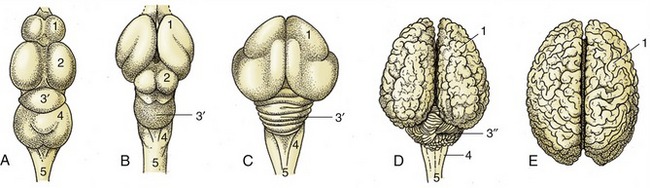
Figure 8–7 Vertebrate brains illustrating the phylogenetic development. The increase in volume and complexity of the telencephalon and cerebellum is most striking. A, fish (carp); B, reptile (python); C, bird (duck); D, mammal (cattle); E, mammal (human). 1, Telencephalon; 2, mesencephalon; 3′, 3″, metencephalon; 3′, archicerebellum; 3″, neocerebellum; 4, myelencephalon; 5, spinal cord.
More detailed descriptions of the parts of the central nervous system are given shortly, but a first appreciation of these will be facilitated by an initial survey of the brain as a whole followed by an account of its development. Repeated references should be made to the figures so that the structures named can be located and identified.
When viewed from the dorsal direction the dominant features of the brain are the cerebral hemispheres and cerebellum; only a small part of the medulla oblongata is visible in continuity with the spinal cord (see Figure 8–20). The semiovoid cerebral hemispheres are divided from each other by a deep longitudinal fissure and from the cerebellum by a transverse fissure; when the brain is in situ, both fissures are occupied by folds of the tough dural membrane that lines the cranial cavity. Each hemisphere is molded to display ridges (gyri) and grooves (sulci) in patterns that differ significantly among the various species. The cerebellum has an even more pronounced surface marking.
The ventral aspect of the brain is flatter overall and reveals the subdivisions of the brain more clearly. The caudal part is provided by the medulla oblongata, which expands when followed forward until it terminates behind a prominent transverse ridge, the pons, which can be traced over the lateral aspect to join the cerebellum (see Figure 8–19). The midbrain in front of this, hidden in dorsal view, appears as two divergent columns, the crura cerebri (see Figure 8–19/12), which continue rostrally to disappear into the depths of the hemispheres. They are separated by the interpeduncular fossa (see Figure 8–19/13). The forebrain lies in front of this; its most prominent ventral median features are the hypothalamus (to which the hypophysis [pituitary gland] is attached by a stalk) and the crossing or chiasm formed by the optic nerves. The larger part of the forebrain is provided by the paired cerebral hemispheres, which have as their most prominent ventral features the rounded piriform lobes (see Figure 8–19/3), flanking the crura cerebri, and the olfactory tracts (see Figure 8–19/2), which originate in the olfactory bulbs that project at the rostral extremity. The superficial origins of the cranial nerves, all except the trochlear (IV) pair, are also visible on the ventral surface.
The cerebral hemispheres and cerebellum develop dorsal to the other parts, and when they are removed, all that remains is referred to as the brainstem (see Figure 8–23). This is a direct, though highly modified, continuation of the spinal cord.
DEVELOPMENT
Because the anatomy of the brain is most easily understood by reference to its development, it may be useful to give a general account of this before proceeding further; additional details will be mentioned later.
The nervous system makes a very early appearance, becoming evident at the embryonic disk stage as an elongated thickening (neural plate) of the ectoderm that overlies the notochord and paraxial mesoderm. The lateral parts of the neural plate are soon raised above the surrounding surface by growth of the underlying mesoderm and form bilateral neural folds that slope toward an axial crease, the neural groove. As the process continues, the edges of the folds become increasingly prominent and then bend inward toward each other; eventually they meet and fuse, which converts the neural groove into a neural tube (Figure 8–8). The tube, which is the primordium of the brain and spinal cord, then sinks below the surface, which is simultaneously closed above it by the fusion of the nonneural ectoderm to each side. At the same time, cells within the margins of the folds break away to form continuous cords, the neural crests, that run almost the whole length of the tube at its dorsolateral aspects. The neural crests contribute to peripheral ganglia, both somatic (dorsal root) and visceral, to the enteric nervous system, to the medullary parts of the adrenal glands, to glia, to skin melanocytes, and to a variety of craniofacial connective tissues. The sympathetic ganglia develop in the mid trunk of the embryo while neural crest cells from more cranial and more caudal regions migrate into the gut to form the enteric nervous system.

Figure 8–8 Three stages in the closure of the neural plate. 1, Neural plate; 2, notochord; 3, paraxial mesoderm; 4, endoderm; 5, neural tube; 6, somite.
Closure of the neural tube is initially limited to the presumptive occipital region but soon spreads rostrally and caudally until only two small openings (neuropores; Figure 8–9/3,5) remain to provide communication at the surface of the embryo between the lumen of the tube and the amniotic cavity. These openings do not persist long: the rostral neuropore closes first, and the caudal one remains open for another day or two while the tube continues to lengthen at its caudal extremity by extension and subsequent infolding of the neural plate. The abnormal persistence of these openings produces relatively common defects of the brain and spinal cord in which nerve tissue may be exposed on the surface of the body. Failure at the rostral extremity leads to malformation of the forebrain and midbrain with accompanying anomalies of the skull; it is known as anencephaly and, although the term implies complete failure of brain development, it can show considerable variation in severity. Most forms are incompatible with life after birth. Failure at the caudal extremity is more common and is known as spina bifida. It is associated with defective closure of the vertebral arches. Children and young animals with this malformation may live after birth, though with severe functional disturbance; affected animals are not usually permitted to survive.
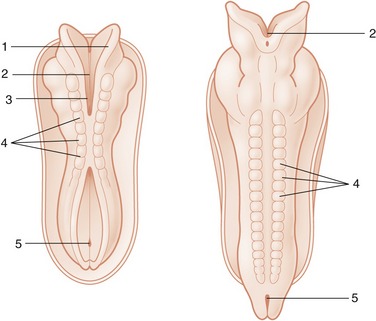
Figure 8–9 Dorsal views of developing embryos. Two stages in the formation and fusion of the neural folds are illustrated. 1, Neural fold; 2, neural groove; 3, rostral neuropore; 4, somites; 5, caudal neuropore.
The part of the neural tube that forms the brain is wider from the outset and shows localized expansions even before the tube is completely closed. These define three primary brain vesicles: prosencephalon (forebrain), mesencephalon (midbrain), and rhombencephalon (hindbrain). The remaining, more uniform part of the tube becomes the spinal cord. The differentiation of the wall is initially similar along the length of the tube but becomes modified later in the part that becomes the brain, increasingly so toward its rostral extremity. It is convenient to consider first the differentiation of the spinal cord.
A transverse section of the tube at its formation reveals three concentric layers in its structure (Figure 8–10). These are unequally developed around the circumference, which is divisible into thick lateral parts connected by thinner roof and floor plates. The innermost layer bounding the lumen is provided by a sheet of neuroepithelial cells that persists as the ependyma lining the central canal and ventricular system of the adult cord and brain. These cells proliferate rapidly, and although some daughter cells remain as a surface lining, most migrate outward into the middle (mantle) layer of the lateral wall. These immigrant cells are neuroblasts, precursors of neurons and glia. The mantle layer itself becomes the gray substance to which the bodies of the neurons are confined. The processes from the cells within the mantle layer extend outward and form the outer (marginal) layer consisting of dendrites and axons. The marginal layer becomes the white substance of the cord in which fibers descend or ascend for various distances.
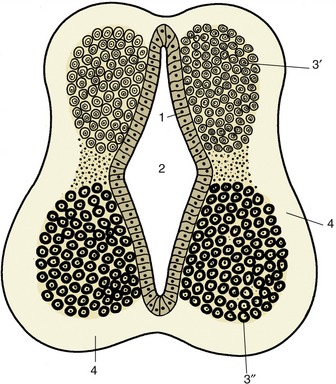
Figure 8–10 Differentiation of the neural tube. 1, Neuroepithelial (ependymal) layer; 2, central canal; 3′, 3″, mantle layer; 3′, dorsal column (alar lamina); 3″, ventral column (basal lamina); 4, marginal layer.
The cells of the mantle layer now become arranged in dorsal and ventral columns that bulge into the lumen of the tube, where they are separated by a longitudinal limiting groove (Figure 8–11/4). The dorsal bulge (alar plate) provides the dorsal horn or column of the gray substance of the cord; its constituent neurons are those of the afferent systems. The ventral bulge (basal plate) becomes the ventral horn or column, which is the location of the efferent neurons; both horns also contain many interneurons. Neurons with somatic functions segregate from those with visceral functions, and four groups of neurons are then arranged in dorsoventral sequence: somatic afferent, visceral afferent, visceral efferent, and somatic efferent (Figure 8–12). The roof and floor plates provide commissures through which nerve fibers pass from one side of the cord to the other.

Figure 8–11 Further differentiation of the neural tube (spinal cord). 1, Neuroepithelial layer, 2, central canal; 3, dorsal column of mantle layer; 4, longitudinal limiting groove; 5, ventral column of mantle layer; 6, marginal layer.

Figure 8–12 Organization of the gray substance of the spinal cord (A) and medulla oblongata (B). 1, Somatic afferent column; 2, visceral afferent column; 3, visceral efferent column; 4, somatic efferent column (lower motor neurons); 5, dorsal root; 6, ventral root; 7, central canal or fourth ventricle; 8, sulcus limitans; 9, basal lamina; 10, alar lamina.
Further growth of the alar and basal plates causes the lateral parts of the tube wall to expand outward in all directions, submerging the roof and floor plates and creating the dorsal sulcus and the ventral fissure that divide the adult cord into its right and left halves. A serial segmentation is created by the appearance of the roots of the spinal nerves. The dorsal roots are provided by neurons within the dorsal root ganglia, local condensations of neural crest cells. The axon processes of these cells extend medially to reach and penetrate the marginal layer, where they divide. Branches of these axons diffuse over several segments before entering the mantle layer to terminate on dorsal column cells; some of greater length extend to reach higher levels within the central nervous system (see Figure 8–6). The ventral roots are formed by axons of efferent neurons within the ventral column, which grow through the marginal layer to emerge on the surface of the cord, where they converge. The appearance of the roots divides the white substance into the dorsal, lateral, and ventral funiculi (Figure 8–13/7,8,9).
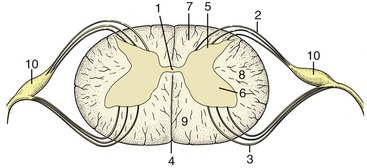
Figure 8–13 Transverse section of spinal cord showing the subdivision of the white substance by the dorsal and ventral roots of the spinal nerves. 1, Central canal; 2, fibers of dorsal root; 3, fibers of ventral root; 4, ventral median fissure; 5, dorsal horn; 6, ventral horn; 7, dorsal funiculus; 8, lateral funiculus; 9, ventral funiculus; 10, dorsal root ganglion.
Although the histogenesis of the nervous system will not be described, two points must be made. In most parts of the brain the full complement of neurons is established shortly after, if not before, birth. However, contrary to former beliefs, in some regions there is a significant, more protracted postnatal recruitment in areas such as the cerebellum and hippocampus that continues into later life. Adult life is marked by a slight depletion of their number. Different authors provide very different estimates of neuronal loss in the human brain, in which the phenomenon is of the most obvious interest. The second point relates to the process of myelinization of the fibers within the central nervous system. Different tracts within the brain and cord acquire adequate insulation (essential to their function) at different stages of development. There are important species differences in this process.
The three primary brain vesicles are evident before closure of the neural tube. At this time the prosencephalon has already extended the evaginations that become the optic cups. The brain grows more rapidly than the tissues that enclose it, and the constraint that this exercises enforces a remodeling of its form. Flexures appear at three locations. The most caudal flexure is more marked in ourselves than in quadrupeds. It bends the brain ventrally at its junction with the cord. A second flexure at midbrain level is almost simultaneous and is sufficiently pronounced to bring the ventral surfaces of the forebrains and hindbrains close together; this relationship is later reversed by the third flexure, which folds the hindbrain dorsally on itself (Figure 8–14). The plan of the chief parts is completed by the appearance of paired lateral evaginations from the alar plates of the prosencephalon, directly behind the rostral limit of this part. These outgrowths, the future cerebral hemispheres, constitute the telencephalon; the unpaired median portion of the prosencephalon, hereafter known as the diencephalon, differentiates as the thalamus and related structures. The telencephalic vesicles expand in all directions but chiefly in a curve that extends dorsally and caudally to overlap the diencephalon, to which they make secondary fusions at the apposed surfaces (see Figure 8–33).
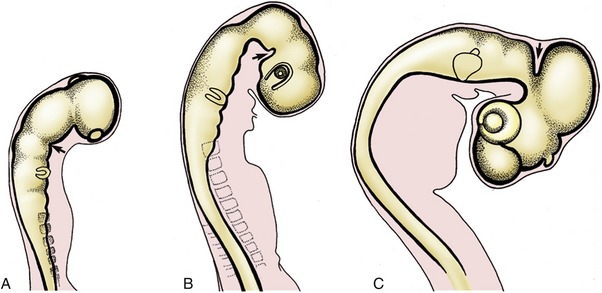
Figure 8–14 Formation of the caudal ventral (A), rostral ventral (B), and dorsal (C) flexures (arrows).
The development of the cerebellum is initially by bilateral formations in the alar plates of the metencephalon; these later extend to a median fusion.
The origin of the major components and cavities of the brain may be conveniently summarized in tabular form (Table 8–1).
Table 8–1 Derivatives of the Neural Tube
The neural tube receives an early direct envelopment provided by mesodermal cells; the forebrain region is supplemented somewhat from cells that migrate from the neural crests. They form two sheets (pia mater and arachnoid). An outer covering (dura mater) is provided by condensation from the surrounding mesoderm; it is separated from the arachnoid by a narrow space.
DESCRIPTIVE ANATOMY OF THE CENTRAL NERVOUS SYSTEM
THE SPINAL CORD
The spinal cord (medulla spinalis) is an elongated structure that is more or less cylindrical but with some dorsoventral flattening and certain regional variations in form and dimensions. The most important of these are the thickenings (intumescentiae; Figure 8–15) of the parts that give origin to the nerves supplying the forelimbs and hindlimbs and the final caudal tapering (conus medullaris). The cord is divided into segments corresponding to the somites by the serial origins of the roots of the paired spinal nerves; the formation of these nerves has been described (p. 29). The relation of the segments to the vertebrae are considered in later chapters (see Figure 8–15).
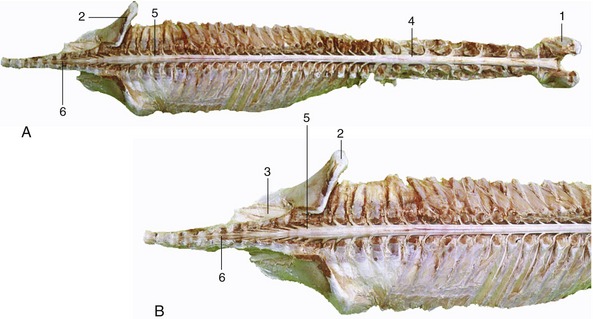
Figure 8–15 A, Dorsal view of the spinal cord and the vertebral pedicles of the horse. The spinal cord is shorter than the vertebral canal (ascensus medullae spinalis). B, Enlargement of the caudal part. 1, Atlas; 2, ilium; 3, sacrum; 4, cervical intumescence; 5, lumbar intumescence; 6, cauda equina.
A simple transverse section shows a central mass of gray substance perforated in the midline by a small central canal, which is the residue of the lumen of the embryonic neural tube (see Figure 8–13). The gray substance, which has a crude resemblance to a butterfly or an H, is commonly described as exhibiting dorsal and ventral horns or columns; the former is a rather misleading term as the horns extend the length of the cord (Figure 8–16). The grayness is of course produced by the restriction of the perikarya to this part. The dorsal horn corresponds to the alar plate. It contains somatic afferent neurons dorsomedially and visceral afferent neurons dorsolaterally (see Figure 8–17). The ventral horn corresponds to the basal plate; it is composed of somatic efferent neurons, which are located ventrally, and visceral efferent neurons, which form an additional lateral horn confined to the thoracolumbar and sacral regions of the cord.
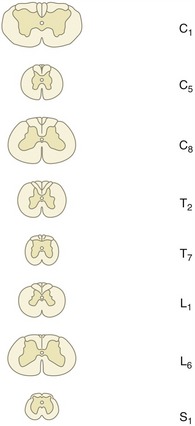
Figure 8–16 Transverse sections of the canine spinal cord (the levels are indicated). Note the changes in diameter of the cord and in the relative proportions of gray and white substance.
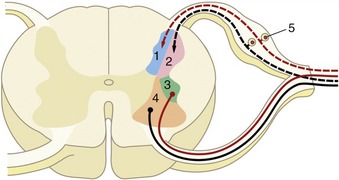
Figure 8–17 Schematized subdivision of the gray substances in the spinal cord. 1, Somatic afferent neurons; 2, visceral afferent neurons (1 and 2 form the dorsal horn); 3, visceral efferent neurons; 4, somatic efferent neurons (3 and 4 form the ventral horn); 5, dorsal root ganglion.
The neurons within each horn are more specifically grouped according to their functional and topical associations, but this is not grossly discernible.
The white substance that envelops the gray is divided into three funiculi on each side (Figure 8–18/I,II,III). The dorsal funiculus is contained between a shallow dorsal sulcus, extended deeply by a median glial septum, and the line of origin of the dorsal roots of the spinal nerves (see Figure 8–13). The lateral funiculus is contained between the lines of the dorsal and ventral roots, and the ventral funiculus is contained between the line of the ventral roots and a ventral fissure that penetrates far into the white substance, although it leaves a considerable commissure connecting the right and left halves. This ventral fissure is occupied by a mass of pia that appears as a glistening streak on the surface of the cord.
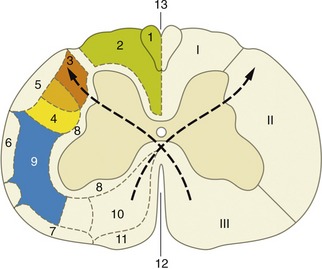
Figure 8–18 Hypothetical transverse section of the canine spinal cord showing the location of some principal tracts. The curved arrows indicate the crossing of the pyramidal tracts. (The drawing has been simplified for the sake of clarity.) I, Dorsal funiculus; II, lateral funiculus; III, ventral funiculus. 1, Fasciculus gracilis; 2, fasciculus cuneatus; 3, lateral corticospinal tract; 4, rubrospinal tract; 5, dorsal spinocerebellar tract; 6, ventral spinocerebellar tract; 7, spinoolivary and olivospinal tracts; 8, propriospinal system (fasciculi proprii); 9, spinothalamic tract; 10, ventral corticospinal tract; 11, vestibulospinal tract; 12, ventral median fissure; 13, dorsal median sulcus.
The funiculi are composed of ascending and descending nerve fibers, of which many are grouped within bundles (fasciculi or tracts) of common origin, destination, and function (see Figure 8–18). Certain of these are mentioned later.
THE HINDBRAIN
The hindbrain (rhombencephalon) comprises the medulla oblongata, pons, and cerebellum. These parts differentiate from the caudal brain vesicle shortly after closure of the neural tube. Attenuation of the roofplate weakens the structure and causes the vesicle to flatten as the pontine flexure develops. The flattening splays the side walls outward so that the luminal surfaces come to face dorsomedially; the alar plates are now lying lateral to the basal plates (see Figure 8–24). The part caudal to the flexure (myelencephalon) becomes the medulla oblongata of adult anatomy. The rostral part develops to become metencephalon, externally marked by the pons and cerebellum. The parts of the roofplate caudal and rostral to the cerebellum remain thin and constitute the medullary vela that complete the enclosure of the lumen, now known as the fourth ventricle (see Figure 8–24).
The Medulla Oblongata and Pons
The medulla oblongata and pons form successive portions of the brainstem. The pons corresponds in extent to the large transverse bar that encloses the ventral and lateral aspects and continues into the cerebellum as the middle cerebellar peduncles (see Figure 8–23/9). Despite the clear external distinction, continuity of the internal organization makes the division of pons from medulla a rather artificial concept.
Although the medulla oblongata continues the spinal cord directly, it widens toward its rostral end as the result of the developmental flattening. Its ventral surface is marked by a median fissure continuous with that of the cord and flanked by longitudinal ridges, the pyramids (Figure 8–19/17). Many of the constituent fibers of the pyramids decussate at the transition of spinal cord and medulla, forming interlacing bundles within the fissure. A lesser transverse ridge, the trapezoid body, crosses the ventral surface of the medulla oblongata directly caudal to the larger pontine bar. The other noteworthy features on this surface are the superficial origins of many of the cranial nerves. The trigeminal nerve (V) appears at the lateral aspect of the transverse pontine bar; the abducent nerve (VI) emerges caudal to this and more medially, through the trapezoid body lateral to the pyramid; the facial (VII) and vestibulocochlear (VIII) nerves appear to continue the trapezoid body laterally; the glossopharyngeal (IX), vagus (X), and accessory (XI) nerves arise from the lateral aspect of the medulla oblongata in close succession; and the hypoglossal nerve (XII) takes a more ventral origin in line with that of the abducent nerve and the ventral roots of the spinal nerves (Figure 8-19 and Figure 8-21).
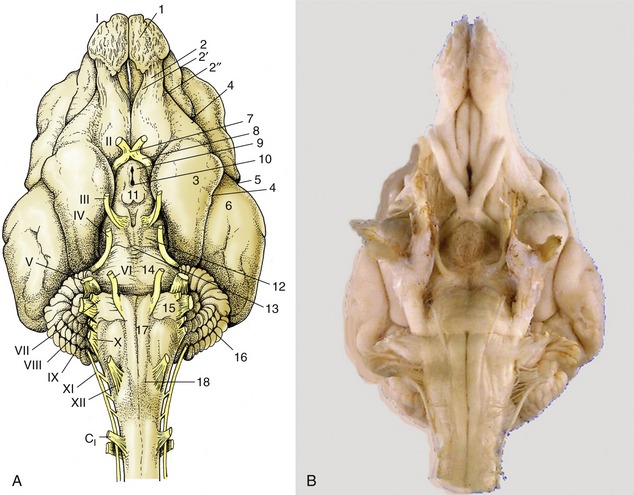
Figure 8–19 A, Ventral view of the canine brain. 1, Olfactory bulb; 2, olfactory tract; 2′, medial olfactory tract; 2″, lateral olfactory tract; 3, piriform lobe; 4, rhinal sulcus; 5, sylvian sulcus; 6, ectosylvian gyrus; 7, optic chiasm; 8, optic tract; 9, tuber cinereum; 10, infundibulum (the hypophysis has been detached and the third ventricle is opened); 11, mamillary body; 12, crus cerebri; 13, interpeduncular fossa; 14, pons; 15, trapezoid body; 16, cerebellar hemisphere; 17, pyramidal tract; 18, crossing of pyramidal tracts. I–XII designate the appropriate cranial nerves. B, The real specimen of the dog.
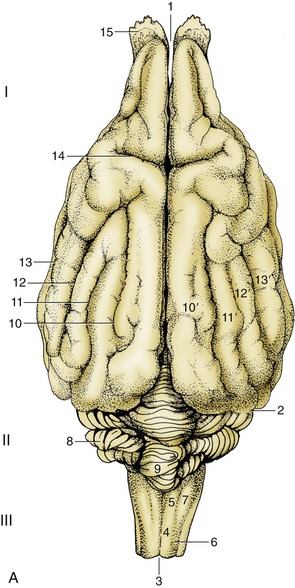
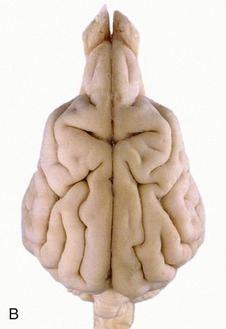
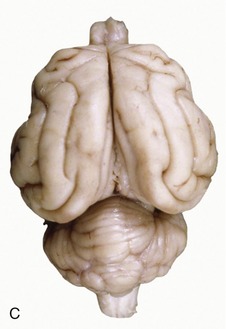
Figure 8–20 A, Dorsal view of the canine brain. I, Cerebral hemispheres; II, cerebellum; III, medulla oblongata. 1, Longitudinal fissure; 2, transverse fissure; 3, dorsal median sulcus; 4, tractus gracilis; 5, nucleus gracilis; 6, tractus cuneatus; 7, nucleus cuneatus; 8, cerebellar hemisphere; 9, cerebellar vermis; 10, marginal sulcus; 10′, marginal gyrus; 11, ectomarginal sulcus; 11′, ectomarginal gyrus; 12, suprasylvian sulcus; 12′, suprasylvian gyrus; 13, ectosylvian sulcus; 13′, ectosylvian gyrus; 14, cruciate sulcus; 15, olfactory bulb. B, The real specimen of the dog. C, The real specimen of the cat.
Figure 8–21 A, Lateral view of the canine brain. 1, Olfactory bulb; 2, olfactory tract; 3, piriform lobe; 4, rhinal sulcus; 5, sylvian sulcus; 5′, sylvian gyrus; 6, ectosylvian sulcus; 6′, ectosylvian gyrus; 7, suprasylvian sulcus; 7′, suprasylvian gyrus; 8, ectomarginal sulcus; 8′, ectomarginal gyrus; 9, coronal sulcus; 9′, coronal gyrus; 10, cruciate sulcus; 11, cerebellar vermis; 12, cerebellar hemisphere; 13, paraflocculus; 14, pons. B, Lateral view of the canine brain. 1, Olfactory bulb; 2, ectosylvian gyrus; 3, optic nerve; 4, cerebellar hemisphere. C, Lateral view of the feline brain.
It may be helpful to study a median section (Figure 8–22) of the brain before examining the dorsal aspect of the medulla oblongata and pons. This section shows that the fourth ventricle is brought close to the upper surface of the brainstem by a dorsal inclination of the central canal within the short caudal part of the medulla. The ventricle is covered by a tented roof formed by the cerebellum and the rostral and caudal medullary vela (Figure 8–22/15,15′), which extend from the cerebellum to the closed caudal part of the medulla oblongata and to the midbrain, respectively. Exposure of the dorsal surface of the medulla and pons requires the removal of the cerebellum by transection of its peduncles, which is an operation that almost inevitably destroys the fragile vela (Figure 8–23).
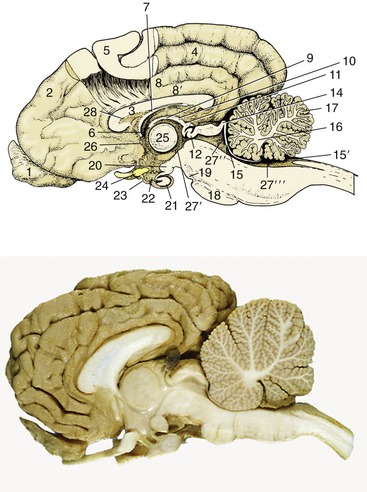
Figure 8–22 Median section of the canine brain. Part of the medial wall of the hemisphere has been removed. 1, Olfactory bulb; 2, hemisphere; 3, corpus callosum; 4, splenial sulcus; 5, cerebral cortex; 6, interventricular foramen; 7, fornix; 8, cingulate gyrus; 8′, supracallosal gyrus; 9, thalamus; 10, epithalamus; 11, epiphysis; 12, posterior commissure; 13, 14, commissures of rostral and caudal colliculi; 15, rostral medullary velum; 15′, caudal medullary velum; 16, corpus medullare; 17, cerebellar cortex; 18, pons; 19, crus cerebri; 20, mamillary body; 21, hypophysis; 22, infundibulum; 23, tuber cinereum; 24, optic chiasm; 25, interthalamic adhesion; 26, anterior commissure; 27′, third ventricle; 27″, mesencephalic aqueduct; 27′″, fourth ventricle; 28, septum telencephali (pellucidum).

Figure 8–23 A, Dorsal view of the canine brainstem with the cerebellum removed and the fourth ventricle opened. 1, Cut fibers of internal capsule; 2, dorsal part of thalamus; 3, epiphysis; 4, lateral geniculate body; 5, medial geniculate body; 6, rostral colliculus; 7, caudal colliculus; 8, decussating fibers of trochlear nerves in the rostral velum; 9, middle cerebellar peduncle; 10, caudal cerebellar peduncle; 11, rostral cerebellar peduncle; 12, dorsal cochlear nucleus; 13, cuneate tubercle; 14, fasciculus cuneatus; 15, fasciculus gracilis; 16, superficial arcuate fibers; 17; median sulcus; 18, medial eminence; 19, sulcus limitans; 20, optic tract; 21, margin of roof of third ventricle. B, Dorsal view of equine brainstem.
The fourth ventricle is diamond-shaped and is aptly named the rhomboidal fossa; it has its widest part at the pontinomedullary junction. The margins of the fossa are provided by the three pairs of cerebellar peduncles. The floor is rather irregular and is marked by a median sulcus and paired lateral (limiting) sulci. The most rostral part of the rostral velum, a part that commonly survives removal of the cerebellum, shows the superficial origins of the trochlear nerves (IV), the only nerves to emerge from the dorsal aspect of the brain.
In the lateral floor of the fourth ventricle and close to the midline the locus coeruleus shines through. Its blue color is due to the presence of neuromelanin granules formed by the polymerization of norepinephrine.
The dorsal surface of the medulla oblongata flanking the caudal part of the fourth ventricle presents inconspicuous eminences, the gracile and cuneate nuclei (Figure 8–20/5,7), at the termination of the like-named fasciculi within the dorsal funiculus of the spinal cord.
The principal features of the internal anatomy of the medulla oblongata and pons are as follows: the nuclei of the cranial nerves, the olivary and pontine nuclei, and the reticular formation and certain ascending and descending fiber tracts that connect the spinal cord with higher levels within the brain. The various categories of structure are described seriatim but without excessive attention to establishing their topographical relationships.
The Nuclei of the Cranial Nerves
The nuclei of the cranial nerves represent the continuation of the four functional components, somatic afferent, visceral afferent, visceral efferent, and somatic efferent, that compose the gray matter of the spinal cord (see Figure 8–12), supplemented by two additional components, special somatic afferent and special visceral afferent, that appear in the medulla oblongata in connection with the innervation of structures of the head that have no counterparts in the trunk or limbs (Figure 8–24). The first four components are massed together within the gray matter of the cord but separate into parallel columns within the medulla (see Figure 8–12). In part, this is the consequence of the flattening and the widening of the medulla and dorsal shift in the position of its lumen.
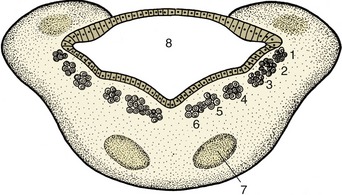
Figure 8–24 Schematic transverse section of the metencephalon. The special somatic afferent nuclei are not shown. 1, Somatic afferent column; 2, visceral afferent column; 3, special visceral afferent column; 4, visceral efferent column; 5, 6, somatic efferent column; 7, nuclei of pons; 8, fourth ventricle.
These components now exhibit a lateromedial rather than dorsoventral sequence with a lateral somatic afferent column and a medial somatic efferent column. Certain of the columns also fragment into discrete parts (nuclei), while at some levels the relationships are further adjusted to allow the intrusion of the additional components. The consequences of all this are that those cranial nerves that contain more than one functional component arise from more than one nucleus and that certain nuclei give rise to similar components of more than one nerve. The general arrangement of the six components is illustrated in Figure 8–25 in a schematic fashion sufficient for most needs.
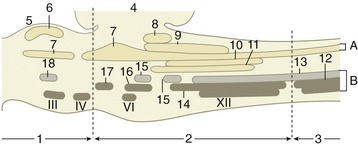
Figure 8–25 Schematic representation of the brainstem showing the nuclei in an adult mammal. Roman numerals are used for nuclei of some cranial nerves. A, afferent nuclei; B, efferent nuclei. 1, Mesencephalon; 2, rhombencephalon; 3, spinal cord; 4, cerebellum; 5, tectum mesencephali; 6, rostral colliculus (SSA); 7, trigeminal nuclei (SA); 8, cochlear nuclei (SSA); 9, vestibular nuclei (SSA); 10, solitary nucleus of VII, IX, X (VA); 11, gustatory nuclei of VII, IX (SVA); 12, motor nucleus of XI (SE); 13, motor nucleus of X (VE); 14, nucleus ambiguus of IX, X (SE); 15, salivatory nuclei of VII, IX (VE); 16, motor nucleus of VII (SE); 17, motor nucleus of V (SE); 18, parasympathetic nucleus of III (VE). SSA, special somatic afferent; SA, somatic afferent; VA, visceral afferent; SVA, special visceral afferent; SE, somatic efferent; VE, visceral efferent.
The somatic efferent column serves muscles that have originated from somites and branchiomeres of the head. Its medial part is fragmented into a long hypoglossal nucleus and a smaller abducent nucleus within the floor of the fourth ventricle (and trochlear and oculomotor nuclei within the tegmentum of the midbrain). The fibers from the oculomotor, abducent, and hypoglossal nuclei take the expected courses to emerge on the ventral aspect of the brain, close to the midline and in line with each other and the ventral roots of the spinal nerves (Figure 8–19). Those that compose the trochlear nerve emerge from the dorsal aspect of the brain after decussation within the rostral medullary velum (Figure 8–23/IV); it is an aberrant course for which there is no satisfactory explanation.
The lateral (branchiomeric) portion of the somatic efferent column (see Figure 8–25) supplies the striated masticatory, mimetic, laryngeal, and pharyngeal muscles through the trigeminal, facial, glossopharyngeal, vagus, and accessory nerves. This portion is divided into the motor nuclei of the trigeminal and facial nerves (Figure 8–25/16,17) and the nucleus ambiguus (Figure 8–25/14) shared by the glossopharyngeal and vagus nerves. The fibers emerge from the ventrolateral surface of the brainstem but do not always take the most direct internal course to do so.
The visceral efferent column supplies the autonomic (parasympathetic) motor component of certain cranial nerves. It is the lateral of the efferent columns (Figure 8–24/4) and is divided into the parasympathetic nucleus of the vagus (Figure 8–25/13), the caudal salivatory nucleus of the glossopharyngeal, and the rostral salivatory nucleus of the facial nerve (Figure 8–25/15) (and the parasympathetic nucleus of the oculomotor nerve [Figure 8–25/18] in the midbrain). The distribution of the vagal fibers of this category is to the cervical, thoracic, and abdominal (but not pelvic) viscera, and the distribution of those within the glossopharyngeal and facial nerves is to glands of the head (and the distribution of those within the oculomotor nerve is to intrinsic muscles of the eyeball).
The visceral afferent column (Figure 8–24/2,3) is in fact double and is shared by visceral and special visceral afferent neurons. It forms a single very long nucleus (of the solitary tract [Figure 8–25/10]) that is subdivided in relation to the associated facial, glossopharyngeal, and vagus nerves. Many neurons are concerned with visceral sensation in the caudal part of the mouth and the cervical, thoracic, and abdominal viscera; the special component, which is concerned with taste, is spread between all three named nerves.
The somatic afferent column (Figure 8–24/1) extends from the cervical part of the spinal cord through the medulla and pons into the mesencephalon. It is broken into several nuclei. One, the mesencephalic nucleus of the trigeminal nerve (Figure 8–25/7), is concerned with proprioception; it presents a unique feature, the inclusion of the primary afferent neuron cell bodies within the central nervous system (the one exception to an otherwise inviolable rule that the cell bodies of primary afferent neurons are located within peripheral ganglia). The two exteroceptive nuclei (Figure 8–25/7) are the nucleus of the descending (spinal) tract of the trigeminal nerve, which extends from the level of the nerve’s entrance into the cervical part of the spinal cord, and the principal sensory nucleus of the trigeminal nerve within the pons.
The special somatic afferent column is associated with the optic and vestibulocochlear nerves and therefore with the special somatic senses of vision (II), balance (vestibular division of VIII), and hearing (cochlear division of VIII) (Figure 8–25/6,8,9). The afferent pathways of these important senses are considered elsewhere; our present purpose is to locate the relevant nuclei within the brainstem. The four closely related vestibular nuclei are spread through part of the medulla oblongata and pons, medial to the caudal cerebellar peduncle. The two (dorsal and ventral) cochlear nuclei are located within the most rostral part of the medulla oblongata close to the entry of the eighth nerve.
The fiber composition of the nerves are summarized conveniently within Table 8–2.
Other Internal Features
The olivary nuclear complex occupies a position in the caudal part of the medulla oblongata, dorsolateral to the pyramidal tract, where it sometimes raises a gentle surface swelling (Figure 8–26/10). It is composed of several parts and varies considerably in form among species, generally taking the form of a nuclear lamina folded onto itself to form a bag. It is an important feature of the motor feedback regulatory mechanism (pp. 302–303). Several other nuclei within the pons (Figure 8–27) are also concerned with motor control (p. 301).
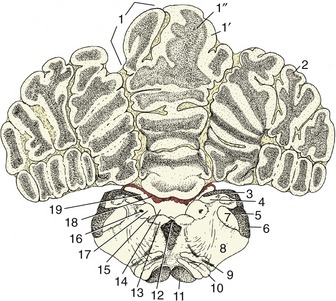
Figure 8–26 Transverse section of the canine brain at the level of the hypoglossal nerve (XII). 1, Cerebellar vermis; 1′, cortex; 1″, medulla; 2, cerebellar hemisphere; 3, fasciculi gracilis and cuneatus; 4, gracile and cuneate nuclei; 5, caudal cerebellar peduncle; 6, spinal tract of the trigeminal nerve; 7, nucleus of the spinal tract of the trigeminal nerve; 8, reticular formation; 9, root of hypoglossal nerve; 10, caudal olivary nucleus; 11, pyramidal tract; 12, medial longitudinal tract; 13, motor nucleus of XII; 14, sulcus limitans; 15, motor nucleus of X; 16, solitary tract (special visceral afferents of VII, IX, and X); 17, solitary nucleus; 18, choroid plexus; 19, fourth ventricle.
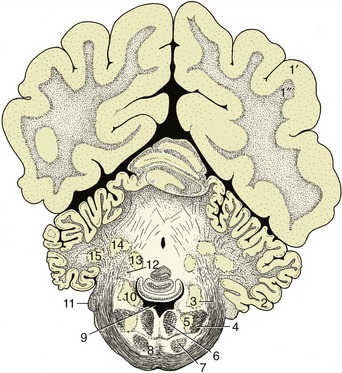
Figure 8–27 Transverse section of the canine brain at the level of the middle cerebellar peduncle. 1′, 1″, cerebral hemisphere; 1′, neocortex; 1″, fibers; 2, paraflocculus lateralis; 3, middle cerebellar peduncle; 4, spinal tract of the trigeminal nerve; 5, nucleus of the spinal tract of the trigeminal nerve; 6, medial longitudinal fasciculus; 7, pyramidal tract; 8, pontine nuclei; 9, fourth ventricle; 10, nuclei of the vestibulocochlear nerve (VIII); 11, root of VIII; 12, rostral cerebellar peduncle; 13, fastigial nucleus; 14, nucleus interpositus; 15, lateral cerebellar nucleus.
The reticular formation is a diffuse system of nuclei and fiber tracts (Figures 8–26/8 and 8–28/13) that extends from the spinal cord to the forebrain and occupies a large part of the core of the medulla oblongata and pons. It is discussed on p. 298.
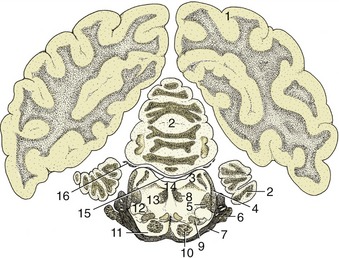
Figure 8–28 Transverse section of the canine brain at the level of the trigeminal nerve. 1, Cerebral hemisphere; 2, cerebellum; 3, rostral cerebellar peduncle; 4, lateral lemniscus; 5, rubrospinal tract; 6, root of V; 7, middle cerebellar peduncle; 8, medial longitudinal fasciculus; 9, medial lemniscus; 10, pyramidal tract; 11, pontine nuclei; 12, nucleus of lateral lemniscus; 13, reticular formation; 14, fourth ventricle; 15, rostral medullary velum; 16, root of IV.
The principal fiber tracts that pass through this part of the brainstem also receive attention later. The large descending tract that produces the pyramid externally (Figure 8–26/11) and the ascending tract known as the medial lemniscus (Figure 8–28/9) are prominent in transverse sections. The medial lemniscus is formed of fibers that issue from the gracile and cuneate nuclei, run ventrally (as the deep [internal] arcuate fibers), and cross the midline in the ventral part of the caudal medulla before turning rostrally as a large medial lemniscal bundle. This area also includes fibers of the trigeminothalamic and cervicothalamic tracts, which emanate from the principal sensory nucleus of the trigeminal nerve and the lateral cervical nucleus, respectively. Other conspicuous fiber aggregations compose the three cerebellar peduncles whose composition, origin, and destination are given later.
The Cerebellum
The cerebellum is a roughly globular, much-fissured mass that is located above the pons and medulla oblongata and is connected to the brainstem by three peduncles on each side (Figure 8–23/9,10,11). It is separated from the cerebral hemispheres by the transverse fissure occupied by the membranous tentorium cerebelli (p. 308) when the brain is in situ.
The cerebellum consists of large lateral hemispheres and a narrow median ridge named the vermis from its fancied resemblance to an earthworm. A division of greater functional and phylogenetic significance is created by a series of transverse fissures. The deepest divide a small caudal flocculonodular lobe from the larger mass, which is itself divided into caudal and rostral lobes (Figure 8–21). Smaller fissures divide the lobes into lobules and these into yet smaller units known as folia. The caudal lobe is particularly well developed in higher forms and especially so in primates. The lobules are individually named, but neither their names nor their exact forms are important.
The arrangement of the gray and white substance sharply contrasts that found in the spinal cord and medulla oblongata. In the cerebellum the bulk of the gray substance is arranged as an external cortex that encloses the white substance or “medulla” (Figure 8–22). The medulla arises from the peduncles and radiates through the various lobes, lobules, and folia, forming a branching structure with some resemblance to a tree. Because of this appearance and because of an ancient belief that it is the seat of the soul, it is sometimes known as the arbor vitae—the tree of life. Some additional gray substance forms a series of nuclei embedded within the medulla; the most important of these are the fastigial nuclei (Figure 8–27/13) close to the midline, the lateral cerebellar (dentate) nucleus (Figure 8–27/15) laterally, and the nuclei interpositi (Figure 8–27/14).
The cerebellum is attached to the brainstem by the three cerebellar peduncles on each side and by the caudal and rostral medullary vela (see Figure 8–23). The caudal peduncle (Figure 8–23/10) connects with the medulla oblongata and is largely composed of afferent fibers, of which some run from origins within the spinal cord and others run from the vestibular nuclei, the olivary nucleus, and the reticular formation. The middle peduncle (brachium pontis; Figure 8–23/9) is also composed of afferent fibers; these arise from pontine nuclei. The rostral peduncle (brachium conjunctivum; Figure 8–23/11) is attached to the midbrain; it is largely composed of efferent fibers dispatched toward the red nucleus, reticular formation, and thalamus but also includes a considerable afferent component that continues the ventral spinocerebellar tract. The three peduncles are closely compressed together at their attachments to the cerebellum.
The functions of the cerebellum are concerned with the control of balance and the coordination of postural and locomotor activities. Balance is located in the flocculonodular node. The caudal lobe is concerned with the feedback regulation of motor function, and to this end it receives a direct input from pontine and olivary nuclei and an indirect input from the other parts of the cerebellum. The rostral lobe receives an input of proprioceptive information. There is a somatotopic representation of the body in the cerebellar cortex.
THE MIDBRAIN
The midbrain (mesencephalon) is a short, rather constricted portion that better preserves the basic organization of the neural tube than do other parts of the brainstem.
The midbrain is exposed on the ventral surface of the intact brain, to which it contributes the crura cerebri, the interpeduncular fossa, and the superficial origin of the oculomotor nerves (III). It is concealed dorsally by the overhanging cerebral hemispheres and cerebellum. Its lumen, the aqueduct, is a simple passage joining the much larger cavities of the third and fourth ventricles. The mesencephalon has a stratified structure, comprising tectum, tegmentum, ventral tegmentum and cerebral peduncle in dorsoventral sequence (Figure 8–29). Formally, all parts except the tectum are included within the cerebral peduncles, but in practice the latter term is frequently equated with the crus cerebri, the part ventral to the tegmentum.

Figure 8–29 Schematic transverse section of the mesencephalon. 1, Tectum; 2, tegmentum; 3, crus cerebri; 4, mesencephalic aqueduct; 5, oculomotor nucleus (III); 6, red nucleus; 7, substantia nigra. 8, locus coeruleus.
The tectum lies dorsal to the aqueduct. Its major features are four rounded surface swellings (see Figure 8–23). The paired caudal swellings, the caudal colliculi, are widely spaced and are joined by a substantial commissure. They are integration centers on auditory pathways (p. 300). There is a connection with the ipsilateral medial geniculate body (a swelling of the thalamus) via a distinct ridge (brachium). The rostral colliculi are placed closer together and are joined to the lateral geniculate bodies by similar but less obtrusive brachia. The rostral colliculi are staging posts on the visual pathways and are involved in somatic reflexes resulting from visual input, such as the response to being startled by a flash of intense light. They are also spatial integration centers.
The tegmentum comprises the core of the midbrain and is directly continuous with the corresponding stratum of the metencephalon. Much of it is formed by the reticular formation. The principal mesencephalic nuclei are the mesencephalic nuclei of the trigeminal nerves (V), the trochlear nuclei (IV), the principal and parasympathetic oculomotor nuclei (III), the red nuclei (named for their pronounced vascularity), and the periaqueductal gray, a core of gray substance about the aqueduct. The substantia nigra is a prominent lamina that can be identified in transverse sections by its darker color, which is due to the gradual accumulation of pigment within the constituent neurons. Like the red nucleus, it is associated with the basal nuclei (p. 291) in the control of voluntary movement.
The crura cerebri are visible on the ventral surface of the brain. They comprise fiber tracts that are in passage between the telencephalon and caudal brainstem. On emerging from the telencephalon, they converge, although they are separated by the interpeduncular fossa (Figure 8–19). The oculomotor nerves (III) emerge in this region, directly rostral to the pons.
THE FOREBRAIN
The forebrain comprises the median diencephalon and the paired cerebral hemispheres (telencephalon). The hemispheres overlap the dorsolateral aspects of the diencephalon to which they have become fused by the growth of fiber tracts across the gaps.
The Diencephalon
The diencephalon (there is no convenient alternative name) forms the most rostral part of the brainstem. Only its most ventral part, the hypothalamus, is visible on the external surface of the intact brain (Figure 8–19), but it is more extensively revealed in median section (see Figure 8–22). The diencephalon comprises three parts: epithalamus, thalamus (including subthalamus), and hypothalamus, which develop in relation to the roof, walls, and floor of the third ventricle, respectively.
The epithalamus, the most dorsal part, comprises the pineal gland (epiphysis cerebri), habenular striae, habenulae, and habenular commissure (Figure 8–30). The pineal gland (Figure 8–30/6) is a small, median body projecting dorsally from the brainstem behind an evagination of the roof of the third ventricle that is composed only of pia and ependyma. Although the pineal gland has long been suspected to play some part in sexual development and behavior, its functions are only now becoming clear; it is believed to be particularly concerned in the seasonal regulation of ovarian activity in response to changing day length. The pineal gland produces melatonin, the pineal antigonadotropin that is also important in circadian and seasonal rhythms (p. 218). The habenular stria is a fiber bundle that among others connects the septal area with the habenular nuclei (Figure 8–30/5′). It is an important pathway in the limbic system. The habenulae are nuclear complexes of enigmatic function that develop within the most dorsal parts of the ventricular walls. They receive fibers (habenular stria) from the hippocampus and other parts of the telencephalon and send fibers to mesencephalic nuclei. The left and right habenular nuclei are interconnected via the habenular commissure.

Figure 8–30 Dorsal view of the canine brain. Part of the left hemisphere has been removed, which opens the lateral ventricle. On the right, the hippocampus and basal nuclei have also been removed, which exposes the thalamus and the internal capsule. 1, Septal nuclei; 2, dorsal surface of thalamus; 3, fornix (cut); 4, internal capsule; 5, dorsal part of third ventricle; 5′, habenular nuclei (in roof of third ventricle); 6, epiphysis; 7, rostral colliculus; 8, caudal colliculus; 9, cerebellum; 10, cut lateral wall of hemisphere; 11, lumen of lateral ventricle; 12, hippocampus; 12′, cut-edge of denticulate gyrus; 13, tail of caudate nucleus; 14, head of caudate nucleus.
The thalamus is the largest component of the diencephalon. It develops within the lateral walls of the third ventricle, but in many species, including domestic ones, it later bulges into the ventricle to form a bridge with its fellow. This, the intermediate mass or interthalamic adhesion, reduces the ventricle to an encircling annular space (Figure 8–31/3). The relations of the thalamus are difficult to envisage because of its deep position and lack of separation from neighboring structures. It extends to the lamina terminalis grisea rostrally and to the midbrain caudally. Its dorsal surface faces toward the fornix and floor of the lateral ventricle, its ventral surface rests on the hypothalamus, and its lateral face is covered by the internal capsule of fibers ascending to and descending from the cerebral cortex (see Figure 8–30).
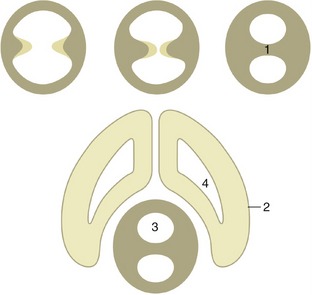
Figure 8–31 The formation of the interthalamic adhesion by median fusion of outgrowths of the lateral walls of the diencephalon. 1, Interthalamic adhesion; 2, telencephalon; 3, third ventricle; 4, lateral ventricle.
The thalamus is composed of a very large number of nuclei named according to their topographical relationships to each other. These nuclei have various specific functions and collectively form one of the most important relay and integration centers of the brainstem. The ventral group receives most afferent systems (excluding the pathways concerned with olfaction) and also provides relays on feedback control systems of motor pathways (Figure 8–33).
The subthalamus contains the subthalamic and endopeduncular nuclei and the zona incerta. The subthalamic nucleus acts as a relay station on the extrapyramidal motor pathway, whereas the other nuclei serve as links between the limbic system and the somatic and visceral motor systems.
The metathalamus, the caudolateral part of the thalamus, comprises the medial and lateral geniculate bodies (Figure 8–32/3,5), whose presence and position were noted in the description of the midbrain. The lateral geniculate body, although not conspicuous in itself, is joined by the optic tract, which sweeps caudodorsally toward it, over the surface of the thalamus. The medial geniculate body lies ventromedial to the lateral one and receives acoustic fibers via the caudal colliculus (p. 300). The nuclei within these swellings relay visual and acoustic information to the cerebral cortex.
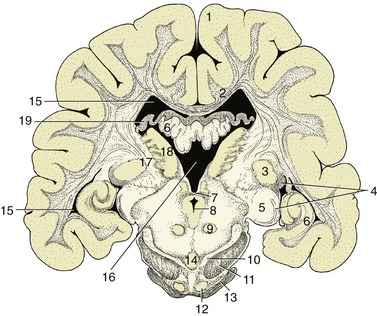
Figure 8–32 Transverse section of the canine brain at the boundary between the mesencephalon and diencephalon. 1, Cerebral hemisphere; 2, corpus callosum; 3, lateral geniculate nucleus; 4, optic tract; 5, medial geniculate nucleus; 6, hippocampus; 7, caudal commissure; 8, mesencephalic aqueduct; 9, red nucleus; 10, substantia nigra; 11, crus cerebri; 12, rostral extension of pontine nuclei; 13, middle cerebellar peduncle; 14, interpeduncular nucleus; 15, lateral ventricle; 16, third ventricle; 17, internal capsule; 18, thalamic nuclei; 19, fornix.
The hypothalamus forms the lower parts of the lateral walls of the third ventricle. It appears on the external surface of the brain between the preoptic region (rostral to the optic chiasm) and the cerebral peduncles and interpeduncular fossa (see Figure 8–19). Its salient surface features are the region known as the tuber cinereum, which extends the stalk or infundibulum that suspends the hypophysis below the brain, and the rounded mamillary body (see Figure 8–22) that receives information from the hippocampal complex and sends information to the thalamus (mammillothalamic tract of Vicq d’Azyr). As such it is an important structure for memory. Internally it contains a number of nuclei associated with the visceral nervous system and hormonal regulation.
The gonadotropin-releasing hormone (GnRH)–producing neurons have a curious history. They originate from outside the brain in the olfactory placode and migrate along the route taken by the developing olfactory, vomeronasal, and terminal nerves to enter the forebrain. Pheromone stimuli can directly influence the GnRH cells (see p. 352).
The hypophysis is a dark, solid body. It is located within a recess of the floor of the cranial cavity and is usually left behind when the brain is removed because the infundibulum, hollowed by a recess of the third ventricle, is easily torn across. The hypophysis is also held in place by a fold of dura mater (p. 308). The functions of the hypophysis are described elsewhere (p. 217).
The Telencephalon (Cerebrum)
The telencephalon consists of the paired hemispheres and the lamina terminalis grisea, the thin plate forming the rostral wall of the third ventricle with the organon vasculosum laminae terminalis griseae (Figure 8–66/7). Because the hemispheres develop as outgrowths of the diencephalon, their walls and lumina (lateral ventricles) remain in direct continuity with the corresponding features of that part. The adult hemispheres are semiovoid structures that form the largest part of the brain; their growth causes them to extend caudally over the brainstem to reach to within a short distance of the cerebellum. This growth brings them close together, and their flattened medial surfaces face toward each other across the narrow longitudinal fissure into which the falx cerebri fits when the brain is in situ. The remainder of the outer wall is divided between convex dorsolateral and flattish ventral (basal) surfaces (see Figures 8–20, 8–32, and 8–33).
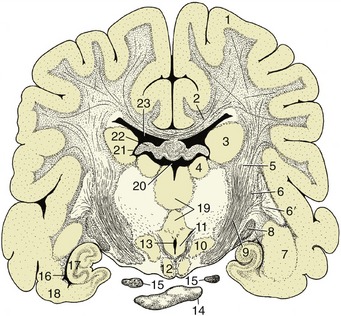
Figure 8–33 Transverse section of the canine brain at the transition between crus cerebri and internal capsule. 1, Cerebral hemisphere; 2, corpus callosum; 3, caudate nucleus; 4, thalamic nuclei; 5, internal capsule; 6, 6′, lentiform nucleus; 6, globus pallidus; 6′, putamen; 7, amygdala; 8, optic tract; 9, crus cerebri; 10, hypothalamic nuclei; 11, mammillothalamic tract; 12, mamillary body; 13, ventral part of third ventricle; 14, hypophysis; 15, oculomotor nerve; 16, ventral part of lateral ventricle; 17, hippocampus; 18, piriform lobe; 19, interthalamic adhesion; 20, dorsal part of third ventricle; 21, interventricular foramen; 22, fornix; 23, lateral ventricle.
The walls of the hemispheres thicken unequally. Much of the medial wall of each hemisphere remains particularly thin, and in fetal life a part rolls inward, invaginating the pia mater and blood vessels covered by the ependymal lining into the ventricle, where it develops into the choroid plexus (p. 310) associated with this cavity. This structure produces the cerebrospinal fluid. The ventrolateral (striatal) part of the wall becomes much thickened when a number of large nuclei, the basal nuclei, develop within it. The alternation of these nuclei with the fiber aggregations in which they are embedded lends this region a striated appearance when exposed by section (see Figure 8–33); it is therefore appropriately known as the corpus striatum. The remainder of the wall is initially known as the pallium, but when it acquires an external covering of gray substance, again by migration from the ependyma, it is more frequently termed the cortex, although this term strictly designates only the outer gray substance.
Three regions of the pallium (or cortex) are distinguished on the basis of evolutionary history, structure, and function. The paleopallium initially served a purely olfactory function; it has retained this association in the highly developed mammals. The archipallium was also initially concerned with olfaction, but unlike the paleopallium, it has largely lost this association. The youngest part, the neopallium, made a very modest initial appearance in vertebrate history but has undergone a spectacular enlargement in mammals, in which it is both the largest and the functionally dominant part of the mammalian telencephalon. These parts are now described separately, but in a different order for convenience. First, it may be helpful to dispose of the concept of a rhinencephalon (“smell-brain”) of primary olfactory function. Although it is true that the telencephalon of lower vertebrates developed specifically in relation to this sense, many parts have since discarded their original function and acquired new roles. The term rhinencephalon therefore no longer describes the functions of these parts at all adequately, and because it is now used in many conflicting ways, there is little in favor of its retention.
The Paleopallium
The paleopallium is confined to the basal part of the brain; it is separated from the neopallium by the rhinal sulcus (Figure 8–34/4) on the lateral surface and, although less clearly, from the archipallium medially. Its rostral extremity is provided by an appendage, the olfactory bulb (Figure 8–34/1), that fits into a recess of the ethmoid bone. The surface apposed to the bone is made shaggy by the entrance of the numerous filaments that together form the olfactory nerve (I); these arise from receptors within the nasal mucosa and pass through the many perforations in the cribriform plate of the ethmoid bone. In the bulb the olfactory stimuli are conveyed to second-stage neurons. The bulb is continued caudally by the common olfactory tract (Figure 8–19/2), which soon divides into medial and lateral divisions separated by a triangular area. The medial tract runs toward the medial aspect of the hemisphere (precommissural area), where the information is conveyed to third-stage neurons. Some of the continuing fibers terminate within certain cortical gyri; others pass through the narrow anterior commissure in the rostral wall of the third ventricle to reach the corresponding region of the opposite hemisphere. The lateral tract continues caudally to join the large piriform lobe (Figure 8–19/3), the most salient feature of the basal surface of the hemisphere; not all the fibers in this tract reach the piriform lobe, as some are precociously detached en route, mainly to the amygdaloid body.
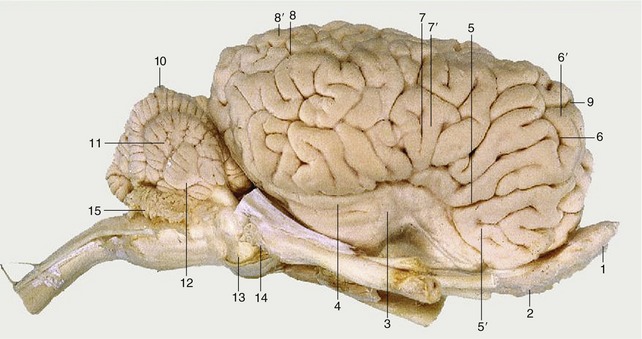
Figure 8–34 Lateral view of the equine brain. 1, Olfactory bulb; 2, olfactory tract; 3, piriform lobe; 4, rhinal sulcus; 5, sylvian sulcus; 5′, sylvian gyrus; 6, ectosylvian sulcus; 6′, ectosylvian gyrus; 7, suprasylvian sulcus; 7′, suprasylvian gyrus; 8, ectomarginal sulcus; 8′, ectomarginal gyrus; 9, cruciate sulcus; 10, cerebellar vermis; 11, cerebellar hemisphere; 12, paraflocculus; 13, pons; 14, crus cerebri; 15, caudal medullary velum.
The Basal Nuclei
The large nuclei known by this title lie dorsal to the paleopallium, where a number of them combine with the white substance to form the corpus striatum. The complex may have had its original importance in relation to olfaction but has now acquired additional functions in relation to other sensory input and to the regulation of motor function.
The nuclei composing the striatal complex are listed variously but most commonly as follows: the caudate nucleus, lentiform nucleus, amygdala, and claustrum. The caudate nucleus (Figure 8–33/3) has the general form of a comma with a large head bulging into the floor of the main part of the lateral ventricle, a body following the caudal bend of the cavity, and a tail related to the roof of its ventral extension (Figure 8–30/13,14). The lentiform nucleus is more lateral and is divided by a fiber intersection into two parts: the medial globus pallidus and the lateral putamen (Figure 8–33/6,6′). The lentiform nucleus is separated from the caudate nucleus by the rostral limb of the fiber mass known as the internal capsule (Figure 8–33/5) and is separated from the thalamus by the caudal limb of the same formation. The nucleus accumbens, the reward center, is located in the ventral striatum.
The other basal nuclei are the smaller amygdala (Figure 8–33/7), located near the tail of the caudate nucleus, and the claustrum, which is interposed between the lentiform nucleus and neopallium. It is separated from these by other fiber laminae; the one on its lateral face is known as the external capsule.
The Neopallium
The neopallium constitutes the major part of the telencephalon: all that is visible in dorsal view and the bulk of that visible in lateral and medial views. References to the cortex, or even to the cerebrum without further qualification, usually have the neopallium specifically in mind. It is divided from the paleopallium by the rhinal sulcus on the lateral side of the hemisphere (Figure 8–21/4) and from the archipallium by the splenial sulcus medially (Figure 8–22/4). In some mammals, generally those that are of smaller size, its outer surface is smooth; however, in larger mammals, including domestic species, it displays a complicated arrangement of alternating ridges (gyri) and grooves (sulci) (see Figure 8–20). Though it is tempting to regard the more intricate modeling as evidence of greater intelligence and increased capacity for complex responses, the underlying cause appears to be physical. The ridges, which are mainly longitudinal, are produced by restraints imposed on the expanding telencephalic vesicle by the rigid corpus striatum and corpus callosum, while additional folding is necessary to maintain the relationship between volume (which increases by the cube) and cortical area (which increases by the square) in large brains.
The pattern of the gyri is reasonably constant within one species but differs among species. The features of greatest consistency include the cruciate sulcus, running transversely on the rostrodorsal aspect, a few sulci and gyri that follow the dorsomedial border, and the sylvian sulcus on the lateral side. Although other features provide useful landmarks for the investigator seeking to establish the functional significance of particular cortical areas, the names of most are of little consequence to the student. A simpler, rather arbitrary division of more general utility distinguishes four regions or lobes named for their proximity to overlying bones; this division recognizes frontal, parietal, and occipital lobes in rostrocaudal sequence and a temporal lobe lying lateral to the last two. Only the frontal lobe is clearly demarcated because it is bounded caudally by the cruciate sulcus (see Figures 8–20/14 and 8–35).
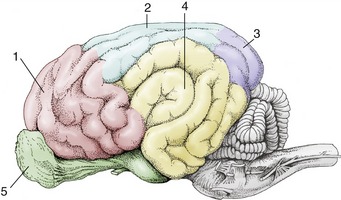
Figure 8–35 Cortical lobes of the canine brain. Lateral view. 1, Frontal lobe; 2, parietal lobe; 3, occipital lobe; 4, temporal lobe; 5, olfactory lobe.
The structure of the neopallium is more elaborate than that of other cortical areas and is remarkably uniform. It exhibits six superimposed strata that are densely populated by neurons and are separated by cell-free divisions. The neurons are broadly of two types: some more or less spherical (granular) neurons are provided with processes of very limited extent, and other (pyramidal) neurons have processes that range more distantly within the underlying white substance. The pyramidal neurons can be classified by their connections. Association fibers connect parts of the neopallium of the same hemisphere after passage directly below the cortex. Commissural fibers connect the two hemispheres, generally linking equivalent contralateral parts. They run over the roof of the lateral ventricle and mainly cross within the corpus callosum, the major telencephalic commissure that is shaped to form a rostral genu, middle trunk, and caudal splenium (Figure 8–22/3). Descending projection fibers from the cortex connect with lower parts of the central nervous system; most converge on the internal capsule squeezed between the basal nuclei and thalamus (Figures 8–36/7 and 8–37/1). In their courses to, from, and within the capsule the projection fibers are ordered according to their functional associations and somatotopic relationships.
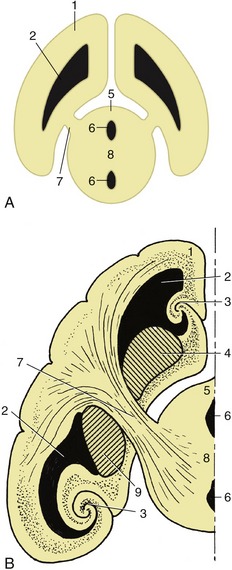
Figure 8–36 A, The connection between the cerebral hemisphere and diencephalon via the internal capsule (7). B, The lateral ventricle, basal nuclei, and hippocampus form concentric arches over the internal capsule. 1, Cerebral hemisphere; 2, lateral ventricle; 3, hippocampus; 4, caudate nucleus; 5, diencephalon; 6, third ventricle; 7, internal capsule; 8, interthalamic adhesion; 9, globus pallidus and putamen.
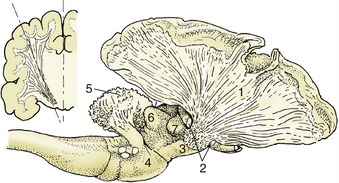
Figure 8–37 The internal capsule in the canine brain. A part of the cerebral cortex and the cortex of the cerebellum have been removed. The resected part of the telencephalon is indicated in the inset. 1, Fibers of the internal capsule; 2, optic tract, partly removed; 3, crus cerebri; 4, pons; 5, corpus medullare of cerebellum; 6, caudal colliculus; 7, medial geniculate body.
The Archipallium
This part of the cortex was once concerned with the correlation of olfactory with other sensory information but has acquired new functions in modern mammals. It is included in the limbic system, which comprises the cingulate, supracallosal and geniculate gyri, the hippocampal formation, and the dentate gyrus.
The archipallium is no longer a conspicuous feature of the telencephalon. The relatively reduced importance of the olfactory sense and the enormous development of the neopallium have caused the archipallium to be displaced to the medial wall of the hemisphere; it is further reduced in prominence by a large part being rolled inward to lie on the floor of the lateral ventricle. The archipallium is topographically divided by the corpus callosum into a dorsal part that remains on the surface of the hemisphere (forming the cingulate and supracallosal gyri between the splenial sulcus and the corpus callosum; Figure 8–22/8,8′) and a ventral part composed of the inflected portion usually known as the hippocampus (Figure 8–38/2). The archipallium is curved in conformity with the shape assumed by the expanding telencephalic vesicle and fits around the dorsal, caudal, and ventral aspects of the thalamus. This arrangement is difficult to envisage, and it is helpful to remember that the archipallium is interposed between the olfactory bulb and the hypothalamus. The pathway is thus bent into a hairpin loop by the expansion of the hemisphere (Figure 8–39); the proximal limb extends, with a ventral concavity, caudally toward the apex of the loop, where a spiral twist sets the distal limb on a parallel returning course.
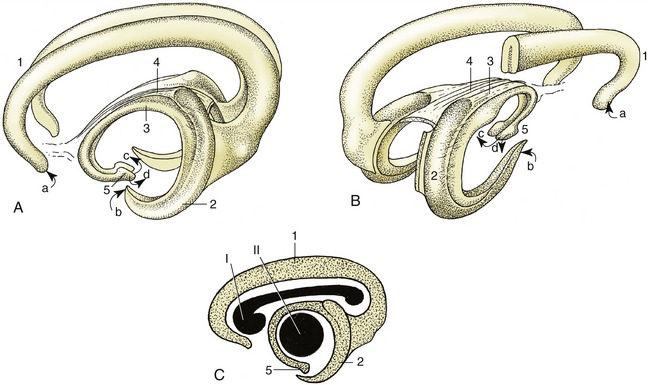
Figure 8–38 Three-dimensional representation of the archipallium. A, Left lateral view. B, Right caudolateral view. C, The positions of the corpus callosum (I) and the thalamus (II) are shown in lateral projection. 1, Supracallosal and cingulate gyri; 2, hippocampus; 3, fornix; 4, commissure of fornix; 5, hypothalamus with mamillary body. a, Input from the medial olfactory tract; b, input from the piriform lobe; c, output to the mammillothalamic tract; d, output to the brainstem.
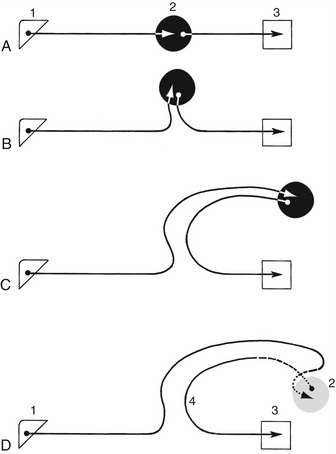
Figure 8–39 Diagram illustrating conjectured course of fibers running to and from the hippocampus. Because of differential growth of various parts of the brain, the hippocampus extends first dorsally (B), then caudally (C), and finally laterally (D). 1, Olfactory bulb; 2, hippocampus; 3, hypothalamus; 4, fornix.
The proximal limb is provided by the surface gyri; beneath that run the longitudinal association fibers (cingulum) from the septal area. The fibers of this multisynaptic pathway enter the caudal extremity of the hippocampus and form a covering to it. The fibers leaving the hippocampus run rostrally over its surface, gradually consolidating into a thick bundle, the fornix. The fornix lies directly below the corpus callosum at its commencement but deviates ventrally as it passes forward; it curves around the rostral extremity of the thalamus to enter the hypothalamus, where it terminates within the mamillary body (Figures 8–38 and 8–40). The right and left hippocampi are joined by the commissure of the fornix. There are thus three telencephalic commissures: the neopallial corpus callosum, the paleopallial anterior commissure, and the archipallial fornical commissure (also known as the commissure of the hippocampus).
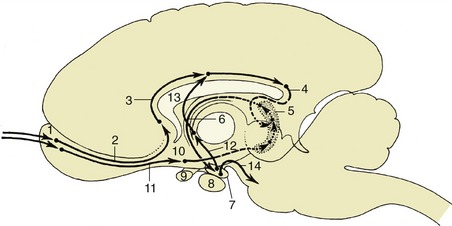
Figure 8–40 A simplified conjectured diagram of the relay scheme of the limbic system. The fiber tracts indicated by dotted lines are bent laterally out of the plane of the drawing. 1, Olfactory bulb; 2, medial olfactory tract; 3, cingulum (in gyri supracallosus and cinguli); 4, gyrus dentatus; 5, hippocampus; 6, fornix; 7, mamillary body; 8, hypophysis; 9, optic chiasm; 10, piriform lobe; 11, lateral olfactory tract; 12, mammillothalamic tract; 13, projection fibers entering the cingulum; 14, projection fibers to reticular formation.
When the fornix parts company with the corpus callosum, it remains connected to it by a thin septum that increases in depth toward its rostral end. This septum telencephali (pellucidum) forms part of the medial wall of the lateral ventricle (Figure 8–22/28). It is a bilateral structure that is separated from its neighbor by a narrow, completely enclosed cleft and in its ventrorostral part contains septal nuclei in which fibers from the medial olfactory tract terminate.
THE FUNCTIONAL MORPHOLOGY OF THE CENTRAL NERVOUS SYSTEM
Despite the pretensions of the heading, this section will deal only with certain rather fundamental topics involving, for the most part, relatively discrete structured pathways.
SOMATIC AFFERENT PATHWAYS
The designation somatic afferent is applied to those pathways of fiber tracts and intercalated nuclei that convey information from the wide array of receptors of various types that are scattered throughout the skin and the deeper somatic tissues. It excludes the special somatic afferent pathways from the eye and inner ear and, obviously, the pathways from visceral receptors.
The somatic afferent system is concerned with a variety of sensory modalities: touch, pressure, vibratory sensation, thermal sensation, pain, and the kinesthetic sensations relating to joint angulation and muscle tension. The primary neurons concerned with all these senses are located within the dorsal root ganglia of the spinal nerves (and corresponding ganglion of the trigeminal nerve where structures of the head are concerned), and their axons enter the central nervous system by way of the dorsal roots of the spinal nerves (and afferent root of the trigeminal nerve). The axons branch on entering the central nervous system. Some branches end on interneurons within the gray substance of the segment of entry or of an adjacent segment; these neurons in turn project on ventral horn cells of the same or neighboring segment, so completing the short neuron chain that provides the anatomical basis for local reflex responses. (The interneuron is omitted from the simplest reflex arc of all—that of the tendon jerk; Figure 8–4.) The ventral horn neuron whose axon ends directly on the effector is called the lower motor neuron.
Other branches of the primary axons connect directly, or through interneurons, with higher centers, thus providing pathways that initiate more complex integrated responses. The ascending pathways concerned with most sensory modalities (including a fraction of those concerned with registering joint position) ultimately reach the somatosensory area of the cerebral cortex, providing the mechanism for conscious perception. None of these ascending pathways is entirely isolated from other parts of the brain; all are variously connected to other centers by collateral branches at different levels.
The Lemniscal System
There are two large ascending pathways that enter consciousness. One, termed here the lemniscal system though other names are used, is followed by impulses that provide for a high degree of spatial discrimination of touch, for accurate assessment of the intensity of pressure, for repetitive vibratory sensation, and for a part of joint proprioception. The initial link in this pathway is provided by the chief branches of the axons of the primary sensory neurons that enter the cord (Figure 8–6). These pass at once to the dorsal funiculus of the cord, where they adopt a very orderly arrangement (Figure 8–18); those that enter through sacral nerves occupy the most medial positions, while those that enter at more cranial levels assume progressively more lateral positions. A glial septum that appears within the dorsal funiculus at midthoracic level divides it into two parts: the medial division, which constitutes the gracile fasciculus, contains fibers from the hindlimb and caudal trunk; the lateral division, the cuneate fasciculus, contains fibers from the forelimb, the cranial part of the trunk, and neck. Both tracts end within like-named nuclei of the dorsal part of the medulla oblongata, where they raise slight surface elevations, the gracile and cuneate tubercles (Figure 8–23/13,14). The axons of the second-stage neurons leave the ventral aspects of the gracile and medial cuneate nuclei and at once decussate to the opposite side and turn rostrally as the large fiber tract known as the medial lemniscus. The medial lemniscus runs forward within the ventral part of the medulla, dorsal to the pyramid and close to the median plane, to reach a specific part of the caudoventral nuclear complex of the thalamus (MCV) (Figure 8–41). After synapses within the thalamus, axons of third-stage neurons project through the thalamic radiation to the somatosensory area of the cerebral cortex (neopallium), chiefly to a region directly caudal to the cruciate sulcus. In its course through the brainstem the medial lemniscus is joined by equivalent fibers from the lateral cervical nucleus, the nucleus of the descending tract of the trigeminal nerve, and the rostral (principal) sensory nucleus of the trigeminal nerve after a decussation within the metencephalon (Figures 8–41 and 8–42).
Figure 8–41 The lemniscal (black) and extralemniscal (white) projections from the trunk and head to the telencephalon. d, Decussation; LCV, lateral part; MCV, medial part of the caudoventral thalamic nucleus.
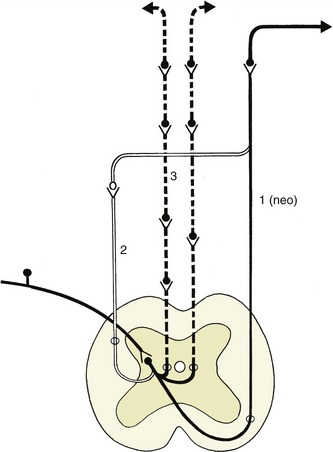
Figure 8–42 A simplified scheme of the extralemniscal projections ascending from the spinal cord to the telencephalon. The uninterrupted black and white lines represent the projections within the lateral system; the interrupted black lines represent the bilateral and multisynaptic projections within the medial system. The (paleo)spinothalamic tract is not represented in this scheme. 1, Spinothalamic tract; 2, spinocervicothalamic tract; 3, spinoreticulothalamic tract.
The somatotopic organization of this pathway is preserved throughout its length, including the thalamic nucleus and the cortex. The cortical representation is of contralateral parts of the body and reflects the generosity of their sensory innervation, not their absolute sizes. There is also some segregation by modality.
The Extralemniscal System
The extralemniscal system conveys a second group of somatic afferent modalities, characterized by slower propagation and less precise localization of the originating stimuli. The information conveyed relates to the cruder varieties of touch and pressure, to temperature, and to pain. The primary axons of this system end on neurons of the dorsal horns within a segment or two of entry. The information is processed via several interneurons before leaving the dorsal horn (Figure 8–6). The axons of the second-stage neuron then pass into the white substance of the cord and ascend to higher brain centers. The projection of pain signals from the spinal cord to the brain occurs via multiple ascending systems, which can be divided into medial and lateral groups by their projections.
The tracts of the medial group tend to project into the core of the neuraxis to the level of the limbic system. The group comprises the spinothalamic tract (see Figure 8–42) that projects into the medial and intralaminar thalamic nuclei, the spinoreticular tract composed of fiber bundles located bilaterally within the ventral and ventrolateral zones of the spinal white substance and ending in the reticular formation of the brainstem as far rostrally as the diencephalon, and a loosely organized group of propriospinal pathways that originates and ends in the spinal gray substance and forms a multisynaptic ascending fiber system. The medial group, in contrast to the lateral, shows little variation among vertebrates.
The lateral group also comprises tracts projecting onto the medial MCV and thence to the neocortex: the spinothalamic tract, the spinocervicothalamic system, and the second-order dorsal column pathway (Figures 8–42 and 8–43).
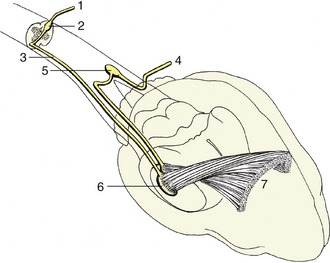
Figure 8–43 Three-dimensional representation of the extralemniscal projection in the dog. 1, Spinal nerve; 2, dorsal horn of spinal cord; 3, spinothalamic tract; 4, trigeminal nerve; 5, nucleus of the spinal tract of the trigeminal nerve; 6, medial part of the caudoventral thalamic nucleus; 7, somatosensory cortex.
The (neo)spinothalamic tract constitutes the classical pain tract of primates, including humans. It is entirely crossed and ascends on the ventrolateral aspect of the ventral horn toward the MCV.
The spinocervicothalamic system is well developed in subprimate mammals, particularly carnivores. Second-order axons ascend ipsilaterally as the spinocervical tract, which occupies the dorsolateral quadrant of the white substance and ends in the lateral cervical nucleus, located at the junction of the spinal cord and brainstem. The axons that emanate from this nucleus cross the midline and follow the medial lemniscus to end in the MCV, where they overlap the projection site of the (neo)spinothalamic tract.
The third system has been found in cats. It is composed of second-order axons that, surprisingly, ascend through the dorsal columns; in addition to being nonnociceptive, these are mainly composed of primary afferents. The postsynaptic, pain-conveying axons end in ipsilateral dorsal column nuclei. The third-order axons that cross the midline also run to the MCV. Second-order trigeminal axons arise from the caudal part of the descending trigeminal nucleus. Axons either join the lateral system and ascend to the MCV or join the medial system into the thalamic reticular formation. The third-stage axons project on an area of the somatosensory cortex rostral to the area allocated to the lemniscal system.
Models have been proposed to explain the respective roles of the lateral and medial pain-signaling systems in the generation of pain sensation and behavior. It has been proposed that the lateral and medial systems contribute differentially to the psychological dimensions of pain experience: one suggestion is that the lateral system conveys information regarding the sensory-discriminative dimensions of pain, whereas the medial is mainly involved in the motivational-affective dimension via the reticular formation, medial thalamus, and limbic system. Another model suggests that the lateral system is tuned preferentially to the sudden onset of noxious stimuli and thus may be related to the threat modality of pain. In contrast, the medial system is tuned to persistent components of pain and is thus better suited to mediate signals relating to existing tissue damage.
Other Ascending Pathways
Ascending pathways transmit information—from muscle and tendon receptors—of which there is no conscious appreciation. The pathways commence in the usual way with primary axons that terminate on dorsal horn cells within the initial and adjacent segments. The axons of the second-stage neurons associate in dorsal and ventral spinocerebellar tracts (Figure 8–18/5,6), which follow separate routes to their projections on the cerebellar cortex. The dorsal tract takes a direct ipsilateral pathway that enters the cerebellum through the caudal peduncle; the information it conveys is obtained from stimulation of muscle spindles. In contrast, the ventral spinocerebellar tract is mainly concerned with transmitting information provided by tendon receptors. The fibers of this tract decussate within the cord close to their origins; they then ascend to midbrain level before they turn back to enter the cerebellum through the rostral peduncle. A second decussation within the cerebellar medulla restores the fibers to the side of the origin of the stimulus before they terminate within the cerebellar cortex. These two tracts are concerned only with information from the trunk and hindlimb; the equivalent representation of the forelimb follows a different pathway that will not be described.
A further diffuse ascending pathway is provided within the reticular formation, the subject of the following section. It provides a means for integrating information conveyed by the pathways previously described with information from other afferent systems, somatic and visceral, general and special.
The Reticular Formation
The reticular formation extends from the spinal cord throughout the brainstem as a diffuse arrangement of neurons interspersed with fiber tracts. In the evolutionary sense it is an old system.
Despite the impression of diffusion and lack of organization it initially creates, closer analysis permits the recognition of numerous nuclear aggregations of varying size and architectonic character; some are sufficiently distinctive for their homologues to be recognizable in different species.
The reticular formation is connected to all projection systems within the central nervous system, whether afferent or efferent, and has reciprocal connections with the major integration centers within the brain. Thus, among its many ascending, descending, and transverse connections, there are such tracts as reticulocerebellar and cerebelloreticular, reticulothalamocortical and corticoreticular tracts. The inescapable inference is that the reticular formation plays an important role in modulating the activities of these integration centers.
The reticular formation occupies a large part of the brainstem; it is spread within the core, and when it reaches the thalamus, it contributes some of the nuclear groups of this complex structure. It also extends into the cervical part of the spinal cord.
The formation may be divided into parts distinguished by their morphology. The medial part, the periventricular gray, is arranged mainly in relation to the ventricular system of the brain. It has proved impossible to analyze in detail but appears to provide multisynaptic pathways composed of an indeterminable number of neurons with short and much-branched processes.
The second component exhibits a more obvious organization with more readily identifiable nuclei and tracts. It is restricted to the brainstem, extending from the floor of the medulla oblongata through the midbrain to the “reticular” nuclei of the thalamus. The reticular nuclei of the thalamus receive an input from lower parts of the formation and project diffusely on the entire neopallium. The spinoreticulothalamic tract, an important component of the system, may provide an alternative or complementary route to the spinothalamic system. The spinoreticulothalamic tract commences with the projection of primary afferent neurons on neurons within the dorsal horn. It contains axons that project for long distances and conduct more rapidly than those found in the spinothalamic tract.
One extensive ascending pathway that ultimately projects beyond the thalamus to the cortex is known as the ascending reticular activating system. It receives an input through collateral branches from all sensory systems, whether exteroceptive or enteroceptive (Figure 8–44). Its activation arouses the animal, making it more conscious of its circumstances and surroundings; diminution of activity induces lethargy or sleep. The reticular activating system has been regarded as the seat of consciousness, but most neurologists would assert that “there is no room or place where consciousness dwells.”
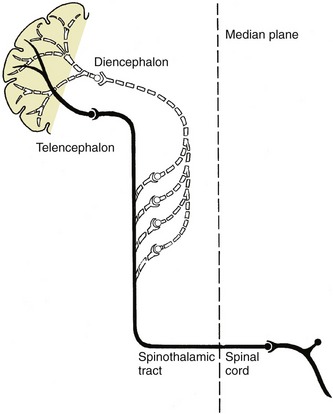
Figure 8–44 A multisynaptic ascending tract (white dotted line) to the telencephalon via the reticular formation. The collateral tract in this example represents the extralemniscal projection (black).
The reticular system also plays an essential role in motor control by means of a descending pathway that extends from the telencephalon to ultimate destinations on lower motor neurons of the brainstem and cord (p. 301).
SPECIAL SOMATIC AFFERENT PATHWAYS
The Visual Pathways
Visual information is conveyed from the retina by the optic nerve. After entering the cranial cavity by the optic foramen, the nerve converges to meet its fellow in the optic chiasm on the ventral surface of the brain. Here, there is a partial decussation of fibers, and the proportion crossing has been correlated with the degree of binocular vision enjoyed by the species. In birds all fibers cross and vision was considered to be monocular; however, some recent information indicates that some birds have an even larger field of binocular vision than humans. In ungulates, the binocular field of vision is much restricted, and a very large percentage (85% to 90%) of fibers cross. A smaller proportion (75%) cross in carnivores, and about 50% cross in primates, in which binocular vision is best developed.
This reassortment brings fibers from both retinae into each optic tract, which arches over the lateral surface of the thalamus (Figure 8–23/20). The larger proportion terminates within the lateral geniculate nucleus, which raises a swelling on the upper end of the tract, or within the pulvinar nucleus medial to it. The primary optic pathway ends here. The fibers of the second-stage neurons project, via the optic radiation within the internal capsule, on the visual cortex, which is located within the occipital lobe of the cerebrum and is the seat of conscious visual perception (Figure 8–45/6).

Figure 8–45 A simplified schema of the visual and pupillary reflex pathways. Thick lines, special somatic visual fibers; thin lines, sympathetic fibers; broken lines, parasympathetic fibers. 1, Retina; 1′, dilated and constricted pupils; 2, optic nerve; 3, optic chiasm; 4, optic tract; 5, lateral geniculate nucleus; 6, optic radiation; 7, rostral colliculus and pretectal nuclei; 8, oculomotor nucleus (parasympathetic part); 9, ciliary ganglion; 10, lateral visceral efferent column; 11, cranial cervical ganglion.
A smaller number of the fibers project on various mesencephalic nuclei; some do so after preliminary relay in the lateral geniculate nucleus. The foremost of these mesencephalic visual integration centers and nuclei are the rostral colliculi. From the mesencephalic nuclei there are relays through various neuronal chains by which the various visual and optic reflexes—concerned with direction of gaze, accommodation, and pupillary diameter—are effected. Fibers from the rostral colliculi also end on lower motor neurons in the cervical spinal cord and constitute the tectospinal tract, part of the so-called extrapyramidal system.
Vestibular Pathways
The vestibular fibers enter the brainstem within the common vestibulocochlear trunk that penetrates the trapezoid body. They then terminate on, or detach collateral branches to, neurons of the vestibular nuclei (Figure 8–46/2). Those that continue unbroken reach the cerebellum by way of the caudal peduncle. The secondary fibers from the vestibular nuclei are divided between those that also pass to the cerebellum and the remainder, which run to the spinal cord via the vestibulospinal tract and medial longitudinal fasciculus. Within the cord they project via a series of interneurons on lower motor neurons of the ventral column. Other fibers proceed to the nuclei of the cranial nerves supplying the external ocular muscles; they follow the medial longitudinal fasciculus (Figure 8–46/4) and the reticular formation. These tracts are part of the extrapyramidal system.
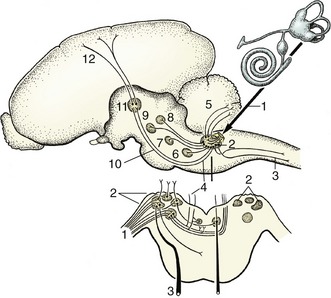
Figure 8–46 A simplified scheme of the vestibular pathways. 1, Vestibular fibers in vestibulocochlear nerve; 2, vestibular nuclei; 3, vestibulospinal tract; 4, medial longitudinal fasciculus; 5, vestibulocerebellar tract; 6, abducent nucleus; 7, trochlear nucleus; 8, oculomotor nucleus; 9, red nucleus; 10, vestibulothalamic tract (in lateral lemniscus); 11, thalamic nuclei; 12, thalamocortical projection fibers.
The fibers that lead to conscious perception of vestibular stimuli proceed via the lateral lemniscus and thalamic nuclei to a particular region of the cerebral cortex of the temporal lobe.
Auditory Pathways
The fibers of the cochlear component of the vestibulocochlear nerve relay within the dorsal and ventral cochlear nuclei located on the surface of the brainstem (Figure 8–47/1,2). The second-stage fibers from the ventral nucleus then proceed to a further synapse within an ipsilateral or contralateral nucleus of the trapezoid body (Figure 8–47/3). The pathway is then continued by fibers of third-stage neurons carried within the lateral lemniscus. A proportion of these synapse within the nucleus of this tract, a second contingent proceeds to the caudal colliculus (Figure 8–47/6), and a third, concerned with the conscious perception of sound, synapses in the medial geniculate nucleus before going to the auditory cortex, which is located within the temporal lobe.
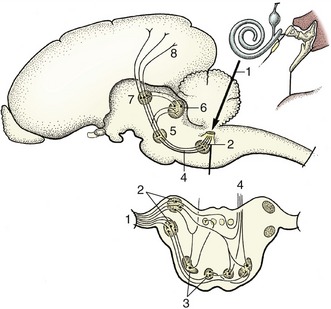
Figure 8–47 A simplified scheme of the auditory pathways. 1, Cochlear fibers in the vestibulocochlear nerve; 2, cochlear nuclei (dorsal and ventral); 3, nuclei in trapezoid body; 4, lateral lemniscus; 5, nucleus in lateral lemniscus; 6, caudal colliculus; 7, medial geniculate nucleus; 8, projection fibers for conscious perception.
The fibers that emerge from the dorsal cochlear nuclei join the ipsilateral or contralateral lateral lemniscus and thereafter follow the same courses as those that proceed from the ventral cochlear nuclei.
SOMATIC MOTOR PATHWAYS
Somatic motor activity is regulated at two levels within the central nervous system by separate groups of nerve cells conveniently designated the lower and upper motor neurons.
The lower motor neurons are located within the ventral column of the gray substance of the spinal cord (Figure 8–12/4) and within the somatic motor nuclei of those cranial nerves that contain somatic efferent components. Their axons are conveyed within the spinal and relevant cranial nerves to the skeletal muscles, where each terminates on a group of muscle fibers (Figure 8–48); the size of the group varies with the precision of performance required of the particular muscle (p. 25). Lower motor neurons provide the efferent limbs of simple reflexes but are in most other circumstances directed by upper motor neurons.
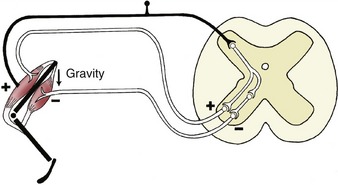
Figure 8–48 A myotactic reflex arc. Gravity (arrow) stretches the extensor muscle, stimulating its contraction via the reflex arc. The flexor muscle is inhibited by a collateral fiber and an inhibiting interneuron.
The upper motor neurons are involved in more complicated reflexes and also initiate voluntary movements. They are mainly located within the motor area of the neopallium but also in other regions of the brain, including the reticular formation and red nucleus. The cortical areas allocated to neurons controlling the muscles of different parts of the body vary in extent with the importance and complexity of the movements of these parts in the habitual activities of the species; thus the hand occupies a relatively much larger area of the human cortex than that allocated to the whole limb in ungulates. Upper motor neurons do not project directly on muscle fibers but exert their control by excitation or inhibition of lower motor neurons.
The connections of the upper with lower motor neurons follow various pathways that vary considerably among species in their relative development and details of organization. The primary distinction is made between so-called pyramidal and extrapyramidal systems, although the two are coordinated and work in close collaboration. The pyramidal system is mostly concerned with the exercise of finely adjusted movements, while the extrapyramidal system is employed in the control of coarser movements, particularly in stereotyped locomotor patterns. It follows that the pyramidal system must be better developed in primates than in domestic species, which is a distinction that explains the different consequences of lesions to the pyramidal pathway. Severe damage to the pyramidal pathway produces a complete and permanent paralysis of the contralateral voluntary musculature in ourselves, while the effects in domestic species are mainly confined to disturbance of contralateral postural reactions from which partial recovery occurs after a few days. Both pyramidal and extrapyramidal systems are provided with elaborate feedback mechanisms that allow for the continuous monitoring and adjustment of motor activity.
The Pyramidal System
The pyramidal system takes origin from neurons within various regions of the neopallium, particularly the primary motor area. The axons of these neurons converge toward their exit from the telencephalon and form an important fraction of the internal capsule; in their passage they preserve the orderly point-to-point arrangement of the cortical representation. They then continue over the lateral aspect of the thalamus to enter the crus cerebri on the ventral surface of the brain (Figure 8–33/9); after traversing the ventral portion of the pons, they reappear on the surface as the pyramids of the medulla oblongata (Figure 8–19/17). Three fiber groups may be distinguished within the system: corticospinal fibers continue through the medulla oblongata into the spinal cord; corticobulbar fibers peel off at appropriate levels of the brainstem to reach various nuclei of contralateral cranial nerves; and corticopontine fibers pass to various nuclei in the pons (Figure 8–49/a,b,c).
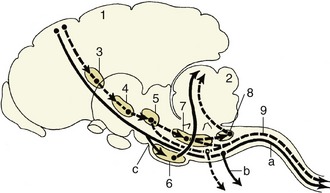
Figure 8–49 Relay diagram of the pyramidal (continuous line) and the extrapyramidal (interrupted line) systems. 1, Motor cortex; 2, cerebellum; 3, basal nuclei; 4, substantia nigra (mesencephalon); 5, red nucleus (mesencephalon); 6, pontine nuclei (metencephalon); 7, reticular formation; 8, olivary nucleus; 9, rubrospinal tract.
a, Corticospinal fibers; b, corticobulbar fibers; c, corticopontine fibers.
Certain of the corticospinal fibers decussate within the medulla oblongata, while the others continue directly into the cord and decussate only when close to their terminations. The fibers with a medullary decussation form a lateral corticospinal tract within the lateral funiculus; those that continue uncrossed constitute a ventral corticospinal tract within the ventral funiculus (Figure 8–18/3,10). The fibers of both tracts finally project on ventral column cells of the side contralateral to their origin. In domestic species, as in the generality of mammals, a short interneuron is always interposed; this interneuron is omitted from certain connections of the primate system.
There are other differences among species. In primates and carnivores, pyramidal fibers reach all levels of the cord; in the dog about 50% terminate in cervical segments, 20% in thoracic segments, and 30% in lumbosacrocaudal segments. In contrast, the pyramidal system of ungulates appears to have terminated by the level of origin of the brachial plexus (Figure 8–50), although there are hints of a diffuse continuation to lower levels within the dorsal funiculus—the route, incidentally, that is favored by the entire system in rodents. The proportion of fibers that decussate within the medulla oblongata also varies: about 50% do so in ungulates, 75% in primates, while all, or almost all, do so in the dog and cat.

Figure 8–50 Comparison of the pyramidal (P) and extrapyramidal (E) systems of human, horse, and dog. The multisynaptic composition of the extrapyramidal system is indicated by the interruptions in this column; the width of the columns is an indication of their importance.
The corticopontine fibers end within nuclei of the ventral pons; the axons of the neurons of the second order then decussate and pass within the transverse lamina of the pons to enter the cerebellum through its middle peduncle. Further successive synapses occur within the cerebellar cortex and then within the nuclei of the cerebellum, whence the return to the cerebral cortex is completed by relay through ventral thalamic nuclei (Figure 8–51). This arrangement constitutes the pyramidal feedback system.
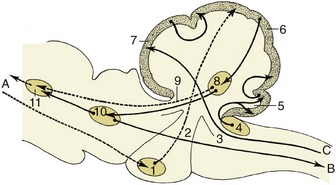
Figure 8–51 Some important fiber connections of the cerebellum. The connections with the neocortex are represented by broken lines. A, Tracts to and from the neocortex; B, tracts to the motor column of the spinal cord (extrapyramidal); C, proprioceptive tracts. 1, Pontine nuclei; 2, middle cerebellar peduncle; 3, caudal cerebellar peduncle; 4, cochlear nuclei; 5, flocculonodular lobe of the cerebellum; 6, neocerebellum; 7, rostral cerebellar lobe; 8, cerebellar nuclei; 9, rostral cerebellar peduncle; 10, red nucleus; 11, thalamic nuclei.
The Extrapyramidal System
The extrapyramidal motor system includes all brain areas involved in regulating motor functions that are not included within the pyramidal system. It is more complicated and involves various multisynaptic pathways that relay within a series of nuclei dispersed through the brain from the telencephalon to the medulla oblongata. Some of these nuclei are large, grossly visible structures; others are small or diffuse, constituting a descending reticular system within the reticular formation of the brainstem. Tracts originating in the tectum and in the lateral vestibular nucleus are dealt with under visual and vestibular pathways (p. 299).
The extrapyramidal system also takes origin from various parts of the cortex, including the primary motor area. The relay stations include the caudate nucleus among the basal nuclei, small subthalamic nuclei, the substantia nigra and red nucleus of the mesencephalon, the reticular formation, and the olive in the medulla oblongata (see Figure 8–49). Only the reticular formation and the red nucleus contain neurons that project directly (via interneurons) on the lower motor neurons of the brainstem and spinal cord; the other nuclei and also some neurons within the red nucleus project only on cells within nuclei lower in the series.
The fibers from the red nucleus decussate at once before descending through the ventrolateral part of the medulla oblongata to constitute a discrete (rubrospinal) tract bordering on the lateral corticospinal tract within the lateral funiculus of the cord (Figure 8–18/4). This tract reaches to the most caudal part of the cord, projecting en route on ventral column cells (lower motor neurons) via short interneurons. This is an important tract in carnivores and is the best developed of all motor pathways in ungulates (see Figure 8–50). It serves as a modulator of pattern generators that are located in the spinal cord itself.
The reticulospinal system is divided between well-defined dorsal and ventral tracts located within the lateral funiculus and a third (pontine reticulospinal) tract within the ventral funiculus.
The activities of the various nuclei and connecting tracts of the extrapyramidal system are closely coordinated and so finely balanced that damage to any part may seriously impair the ability to maintain posture or to execute intended movements. Different parts of the system play different roles: some are facilitatory, others inhibitory, and yet others facilitatory through removal of other inhibitory influences.
The numerous feedback circuits associated with the extrapyramidal system maintain the necessary balance between these facilitatory and inhibitory influences. The various circuits are, however, all subordinated to the overall control of the cerebellum to which all nuclei of the system project via relays within the olivary nuclear complex (see Figure 8–49). This complex projects, by way of the caudal cerebellar peduncle, to the contralateral cerebellar cortex before returning from the cerebellum to the various nuclei. The most important return limb runs from the cerebellum to the thalamic nuclei and thence to the motor cortex and basal nuclei; other pathways take shorter courses to project on the red nucleus and reticular formation.
Cerebellar Function
Although the cerebellum does not itself initiate movement, it ensures that movements are executed as intended by controlling both pyramidal and extrapyramidal systems. To this end, it receives a continuous stream of information that flows from the pyramidal and extrapyramidal feedbacks to the caudal lobe, from the vestibular apparatus via the vestibular nuclei to the flocculonodular lobe, and from proprioceptors that feed into the rostral lobe.
The directions that are based on the integration of these various inputs within the cerebellar cortex are relayed through the cerebellar nuclei before issuing through the various peduncles to the contralateral red and thalamic nuclei, the reticular formation, and the vestibular nuclei (for the coordination of vestibular reflexes).
THE VISCERAL NERVOUS SYSTEM
The visceral nervous system governs the visceral functions. It has many particular responsibilities, which in summation may be defined as the maintenance of the internal environment within the permissible limits. The common concentration on the peripheral (sympathetic and parasympathetic) motor pathways distracts attention from the central controlling structures and the afferent pathways that supply the information necessary for appropriate responses.
THE HYPOTHALAMUS
An important integration center is the hypothalamus, of which the rostral part is concealed and the caudal part—exemplified by the tuber cinereum and mamillary bodies—is exposed on the surface of the brain (Figures 8–19 and 8–22). The hypothalamus includes many areas of specialized function and responsibility. A brief summation of its functions must include the control of biological rhythms, appetite, water balance, body temperature, cardiovascular performance, sexual behavior and activity, sleep, muscle tension (orexin), and emotion. Deficiencies in the orexin/hypocretin system may cause severe cataplexy and narcolepsy (also in the dog). Several hypothalamic cell groups implicated in reproductive function are sexually dimorphic. Because almost every body function has visceral implications, the hypothalamus must receive (and coordinate) information from most other parts of the nervous system, including those of ostensibly somatic function. Information on the somatic activities is projected via the basal nuclei and relays on the extrapyramidal motor pathways via the thalamic nuclei to which the somatic afferent pathways lead. Information concerning visceral function is received from mesencephalic nuclei and the reticular formation. The nucleus of the solitary tract is the principal visceral sensory nucleus that receives topographically organized input from major organ systems by way of the glossopharyngeus (IX) and vagus (X) nerves. As such it is the region of initial processing of visceral, cardiovascular and respiratory, and gustatory information. A further very important contribution comes from the telencephalon (from the prefrontal cortex) and especially from the hippocampus, via the fornix. This enables emotional inputs to be related to and coordinated with the rest. Hypothalamic input from peripheral organ systems is also possible by way of blood-borne signals.
The hypothalamus regulates activity through both nervous and humoral mechanisms, sometimes in combination. The nervous pathways extend to the brainstem and spinal cord by direct routes or by multisynaptic pathways within the reticular formation, in which final integration takes place. Other projections provide a feedback to the forebrain routed through rostral thalamic nuclei.
The humoral pathway operates through neurosecretory cells whose products may enter the bloodstream directly for general distribution or whose products may be conveyed specifically to the hypophysis by means of a system of portal vessels (see Figure 6–3).
THE HYPOPHYSIS
The hypophysis (pituitary gland; Figure 8–52) suspended below the hypothalamus by the infundibulum, consists of two parts. One, the neurohypophysis (posterior lobe), is an outgrowth of the brain itself; the other, the adenohypophysis, is developed from oral ectoderm (p. 217) and comprises anterior and intermediate lobes. Interspecific differences in the topographical interrelationship of the lobes are not of present concern (see Figure 6–2).
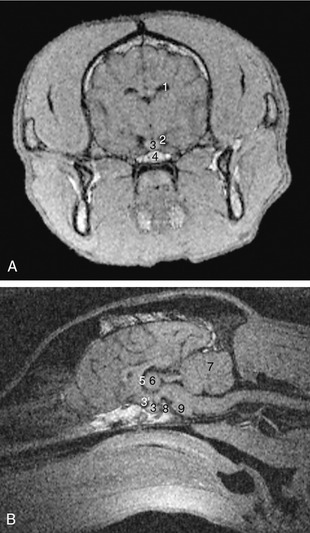
Figure 8–52 Transverse image at the level of the pituitary fossa (A) and median image (B) of 1-mm-thick T1-weighted gradient-echo magnetic resonance slices of the canine head. 1, Lateral ventricle; 2, basal cistern; 3, pituitary gland; 3′, infundibulum; 4, fat in sphenoid bone; 5, third ventricle; 6, interthalamic adhesion; 7, cerebellum; 8, dorsum sellae; 9, pons.
The three lobes produce or store several hormones (p. 217). The posterior lobe hormones (vasopressin and oxytocin) are produced by neurosecretory cells within the supraoptic and paraventricular nuclei of the hypothalamus and are conveyed along the axons for direct release into the neurohypophysial capillary bed (see Figure 6–3).
VISCERAL AFFERENT PATHWAYS
There are both “general” and special visceral afferent pathways, and the latter is concerned with taste and smell. The receptors of the general visceral afferent pathway are found within viscera and blood vessels; most are mechanoreceptors responsive to pressure, stretch, and, less commonly, flow, while a minority are chemoreceptors responsive to such stimuli as the carbon dioxide content of the blood. The fibers that convey impulses from these receptors travel within any conveniently located nerve trunk, utilizing those of mainly somatic composition as well as those whose other components are visceral efferent. The bodies of the primary neurons are located within the dorsal root ganglia of all spinal nerves (and the equivalent ganglia of certain cranial nerves); the axons project on interneurons and projection neurons within the visceral afferent column of the spinal cord and brainstem (Figure 8–12/2).
Short chains of interneurons provide for simple visceral reflexes that have their last two relays within the visceral efferent column and the peripheral autonomic ganglia. The projection neurons form ascending pathways that follow somatic systems, both lemniscal and extralemniscal, to end (like these) within nuclei of the ventrocaudal thalamus. A final projection to the cortex may give rise to conscious perception, although most visceral activity goes unnoticed. (The sense of fullness arising from digestive organs or the bladder is among the visceral activities of which awareness is most common.) Pronounced contraction and serious overdistention of visceral organs may be perceived as pain. Pain of visceral origin may be “referred” to the surface of the body, presumably as a consequence of the convergence of the cutaneous somatic and visceral afferent pathways on the same neurons at some point along their course.
The special visceral afferent pathway concerned with taste follows a similar route to that taken by the general visceral sensory modalities. The course from the taste buds within the facial, glossopharyngeal, and vagus nerves terminates in the nucleus of the solitary tract.
The more complicated olfactory pathways are described elsewhere (see Figure 8–40).
VISCERAL EFFERENT PATHWAYS
Unlike the afferent component, the efferent component of the visceral nervous system is arranged in two divisions, sympathetic and parasympathetic, distinguished by morphology, pharmacology, and physiology. The final conducting pathway of both divisions, unlike that of the somatic system, includes two motor neurons in succession: the first has its perikaryon within the central nervous system, and the second is stationed within a peripheral ganglion (Figure 8–53). The two are most frequently distinguished as preganglionic and postganglionic neurons and together are equivalent to the lower motor neuron of the somatic system.
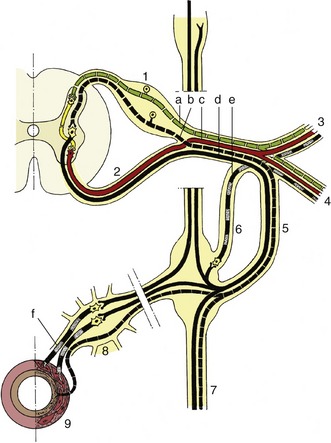
Figure 8–53 Comparison of the organization of the visceral (black) and the somatic (red) nervous system at the thoracolumbar level of the spinal cord. Afferent fibers are indicated by interrupted lines, efferent fibers by solid lines. The postganglionic sympathetic fibers are indicated by alternating black and stippled lines. 1, Dorsal root ganglion; 2, ventral root; 3, dorsal branch of spinal nerve; 4, ventral branch of spinal nerve; 5, 6, white (preganglionic) and gray (postganglionic) communicating branches, often fused; 7, sympathetic trunk with ganglia; 8, prevertebral ganglion; 9, gut. a, Somatic afferent fibers; b, visceral afferent fibers; c, somatic efferent fibers; d, visceral efferent fibers (preganglionic sympathetic); e, postganglionic sympathetic (to peripheral structures); f, postganglionic sympathetic (to abdominal organs).
The preganglionic neurons of the sympathetic division are located within the lateral (visceral efferent) column of the spinal cord between the first thoracic and middle lumbar segments (with some interspecific variation) (see Figure 8–74). The postganglionic neurons are found in paravertebral ganglia of the sympathetic chain or subvertebral ganglia on the aorta; both groups are relatively close to the cord.
The parasympathetic preganglionic neurons are restricted to the nuclei of origin of the oculomotor, facial, glossopharyngeal, and vagus nerves within the brainstem and the lateral columns of certain sacral segments of the cord (see Figure 8–73). The postganglionic neurons are stationed within small ganglia in close proximity to or actually incorporated within the walls of the organs they supply.
The transmitter substance at the last sympathetic relay is norepinephrine and that of the parasympathetic division is acetylcholine; both are collocated with a host of neuropeptides. The two divisions therefore react differently to autonomic stimulant and depressant drugs.
The two systems have broadly similar distributions and are frequently described as antagonist: one inhibits while the other stimulates a particular activity. This rule is less absolute than was once supposed, and their roles are better regarded as collaborative. The more diffuse anatomy of the peripheral sympathetic nerves (which are described later) and the use of norepinephrine as a transmitter indicate the more general effects produced by sympathetic activity, in contrast to those of parasympathetic activity, which are often local, effecting single specific functions.
The central control is exerted by neurons within the hypothalamus; those that influence the sympathetic division are generally caudal to those controlling the parasympathetic division. The pathways from both sets follow various routes, of which some are direct and others are via multisynaptic chains within the reticular formation.
THE LIMBIC SYSTEM
The limbic system has a complex organization and is composed of the limbic cortex and many subcortical nuclei. The cortical part forms a ring at the medial surface of the cerebral hemisphere, including, among other structures, the cingulate and supracallosal gyri, the piriform lobe, and the hippocampus. The subcortical part is composed of the hypothalamus, septal area, amygdala, habenular nuclei, and dorsal part of the mesencephalic tegmentum. There are numerous associations between these structures and other regions of the brain. The limbic system is often considered to be primarily a “visceral brain” because its major functions are expressed by visceral motor activity.
Olfactory impulses passing by way of the piriform lobes may influence many structures of the system. Of all the sensory inputs, olfaction exhibits the most profound effects on visceral motor activities that are associated with emotional behavior such as eating, rage, sexual activity, fear, and drinking. The system also receives optic, auditory, extroceptive, and introceptive stimuli.
The efferent pathways from the cortical regions involve nearly all the subcortical nuclei of the system. A major portion of the influences of the limbic cortex is mediated through the efferent systems of the amygdaloid nuclei. Electrical stimulation of the amygdala produces a wide variety of visceral and somatic reactions and many behavioral reactions such as aggression and anxiety. The types of behavior most influenced by the limbic system are those essential for the preservation of the individual or the species.
The hippocampus probably plays the predominant part in the limbic system’s control of emotional expression and behavior through regulation of autonomic, endocrine, and somatic functions. It is also concerned with memory functions, such as the processing of recently acquired memory and its more permanent consolidation. In its activities the limbic system is closely associated with the reticular formation of the brainstem.
THE TOPOGRAPHY, ENVIRONMENT, AND VASCULARIZATION OF THE BRAIN AND SPINAL CORD
TOPOGRAPHY
The brain and spinal cord are contained within a continuous space provided by the cranial cavity of the skull and the canal formed by successive bony rings and connecting ligaments and disks of the vertebral column.
The cranial cavity lies directly behind the nasal cavities. Smaller than is commonly supposed, its form and extent are not easily predicted from external inspection because the paranasal sinuses, horns, muscular ridges and other projections of the skull, and the temporal muscles all contribute significantly to the conformation of this part of the head. The closest agreement between the external contours and the cavity within the cranium is found in the newborn of all species; among adults this agreement is best retained in cats and in dogs of brachycephalic breeds. Fortunately, the exact location of the brain is rarely of practical significance except in the humane slaughter techniques mentioned in later chapters. It is probably sufficient to state meanwhile that the caudal limit of the cavity extends to the caudal wall of the skull—thickened by the frontal sinus in cattle—while the rostral limit shows considerable variation; it ends level with the caudal margin of the zygomatic processes of the frontal bones in dogs and cats and with the rostral level of these processes in horses and cattle, but it extends to the middle of the orbit in pigs and small ruminants.
The interior of the cranial cavity shows a fairly close correspondence with the contours of the brain, although significant intracranial space is required for the meninges and intermeningeal spaces that surround the brain and for the capacious intracranial venous sinuses. While the roof (calvaria) of the cavity remains largely undivided, the base is divided into three fossae; these need not be described in detail as the main features are depicted in Figure 8–54. The rostral fossa is formed by the sphenoid and ethmoid bones and extends to the level of the optic canals, the passages of exit of the optic nerves. It contains the olfactory bulbs, within recesses of the cribriform plate (Figure 8–54/1), and the rostral parts of the cerebral hemispheres. The middle fossa extends from the optic canals to the sharp petrosal crests (Figure 8–54/9) that project inward from the petrous temporal bones of the lateral walls. The floor is formed by the sphenoid bone, which carries the median hypophysial fossa (sella turcica) into which the hypophysis fits; it also presents various foramina of exit—the orbital fissure and the round and oval foramina—that were encountered in the previous description of the skull (p. 61). This, the widest part of the cranial cavity, contains the temporal and parietal lobes of the cerebral hemispheres. The caudal fossa extends from the caudal limit of the hypophysial fossa to the foramen magnum in the caudal wall. Its principal features are the contributions to its lateral walls made by the petrous parts of the temporal bones (each perforated by an internal acoustic meatus) and the jugular and hypoglossal foramina in the floor. The caudal fossa lodges the midbrain, pons, and medulla ventrally and the cerebellum dorsally.
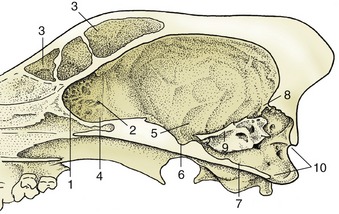
Figure 8–54 Sagittal section of the cranium of the dog. 1, Cribriform plate; 2, ethmoid foramen; 3, frontal sinus; 4, rostral fossa; 5, middle fossa; 6, hypophysial fossa; 7, caudal fossa; 8, tentorium cerebelli osseum; 9, petrosal crest; 10, foramen magnum.
The caudal, dorsal, and lateral walls of the entire cranial cavity blend together. Their most prominent internal feature is the tentorium cerebelli osseum (Figure 8–54/8), a large projection at the junction of the dorsal and caudal walls forming the middle portion of the tentorium cerebelli within the transverse fissure of the brain. It presents passages through which emerge emissary branches of the dorsal intracranial venous sinuses.
The vertebral canal is widest within the atlas and tapers rapidly within the sacrum; in between, it is most expanded where it contains the cervical and lumbar swellings of the spinal cord from which arise the nerves that form the limb plexuses (Figure 8–15). The topography of the spinal cord is of considerable importance in veterinary practice because injections into the canal are frequently made, particularly of local anesthetic solution, with the intention of blocking specific spinal nerves; in addition, there is sometimes a need to locate central nervous lesions to specific vertebral levels, which is a procedure made possible by association of specific sensory and motor deficits with particular spinal segments.
Even with the inclusion of its meningeal wrappings, the spinal cord is considerably smaller than the vertebral canal (see Figure 8–55). It is also considerably shorter. This is due to the unequal later growth of the spinal cord and vertebral column, which is an inequality that begins well before birth and continues after it. The relative shift in position (ascensus medullae) carries the segments of the cord cranially from their original positions within vertebrae of the same numerical designations. The shift of the more caudal segments is most pronounced and explains the peculiar arrangement of the associated spinal nerves. These take progressively longer courses within the canal to reach their fixed foramina of exit, forming a leash (known as the cauda equina because of a superficial resemblance to a horse’s tail) to each side of the conus medullaris (see Figure 12–9/9). The level at which the cord ends varies among species (and, in early life, with age); it is within L5 or L6 in the pig, L6 in ruminants, L6 or L7 in the dog, S2 in the horse, and rather variably between L6 and S3 in the cat (Figure 8–56).
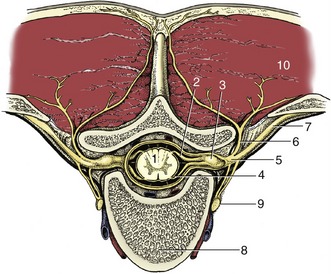
Figure 8–55 Transection of the vertebral column to show the formation of a spinal nerve. 1, Spinal cord; 2, dorsal root; 3, spinal ganglion; 4, ventral root; 5, spinal nerve; 6, dorsal branch of spinal nerve; 7, ventral branch of spinal nerve; 8, body of vertebra; 9, sympathetic trunk; 10, epaxial muscles.
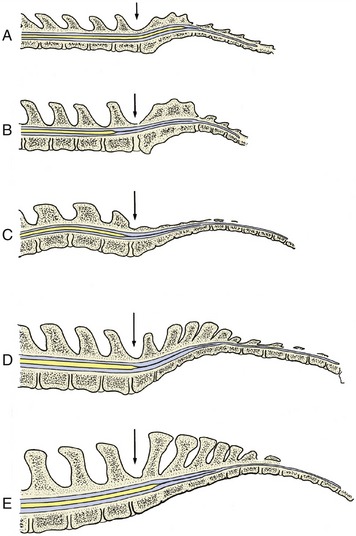
Figure 8–56 Median section of the vertebral canal and spinal cord of cat (A), dog (B), pig (C), cattle (D), and horse (E). The lumbosacral interarcuate space is indicated by an arrow. Notice the difference in caudal extent of the spinal cord in the different species. The thin extension of the spinal cord is the filum terminale that ends on the caudal vertebrae (not shown).
THE MENINGES AND FLUID ENVIRONMENT
The brain and spinal cord are surrounded by three continuous membranes or meninges that exhibit certain topographical differences of importance in their cranial and vertebral parts.
The tough outermost membrane, the dura mater, is fused with the inner periosteum of the skull bones; it splits from this within the margin of the foramen magnum to form a free tube separated from the wall of the vertebral canal by a wide though varying epidural space. The epidural space is occupied by fat, more fluid in life than in the postmortem specimen, and by the internal vertebral venous plexus; the fat and vessels together cushion the spinal cord and allow it to adjust to the movements of the neck and back (see Figure 8–55). The dural tube is attached at its caudal end, where the several meninges finally combine in a fibrous strand (filum terminale) that fuses with the upper surface of the caudal vertebrae.
The fusion of the cranial dura with the periosteum obliterates the epidural space within the skull, and the cranial venous sinuses thus come to be enclosed within the thickness of the combined membrane. In addition to lining the cavity, the cranial dura forms certain folds that project inward and limit shuddering movements of the brain; these are a considerable hindrance to the removal of the intact brain at autopsy. One, the falx cerebri, extends from the dorsal and rostral cranial walls between the two cerebral hemispheres; caudally it joins a second, transverse fold, the membranous tentorium cerebelli, which separates the cerebellum from the cerebrum (Figure 8–57/7). The tentorium is ossified in its median part. A third specialization of the dura roofs the hypophysial fossa in which the hypophysis is seated, forming a diaphragm around the infundibular stalk.
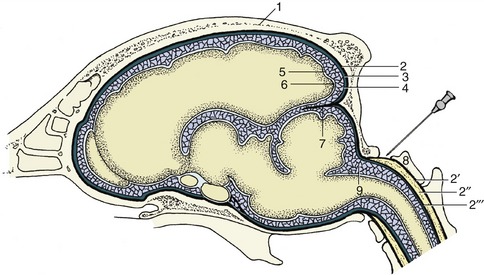
Figure 8–57 Schematic representation of the meninges of the brain. The needle points to the atlantooccipital space and the cerebellomedullary cistern. 1, Calvaria; 2, dura mater (also connected to the bone as periosteum); 2′, periosteum of vertebral canal; 2″, epidural space (with fat); 2‴, dura mater of spinal cord; 3, subdural space; 4, arachnoid; 5, arachnoid space; 6, pia mater; 7, membranous tentorium cerebelli; 8, atlas; 9, cerebellomedullary cistern.
A capillary space divides the dura from the subarachnoid, the first of the two more delicate inner membranes. This subdural space normally contains only a minute amount of a clear lymphlike fluid but may be enlarged by effusion of blood after an injury. The spinal part of the subdural space is crossed by a bilateral series of triangular (denticulate) ligaments that alternates with the origins of the spinal nerves; they attach the inner meninges to the dural tube and thus indirectly sling the cord (Figure 8–58/4). The outer part of the arachnoid forms a continuous membrane molded against the dural envelope. Its inner surface is joined to the pia mater by numerous trabeculae and filaments, imaginatively compared to a spider’s web (the origin of the name subarachnoid). The pia mater is directly attached to the brain and cord and follows every change in their contours. The arachnoid space, which contains the clear, watery cerebrospinal fluid, is much wider than the subdural space but less uniform, particularly in its cranial part (see Figure 8–57).

Figure 8–58 Dorsal view of the opened vertebral canal. The dura mater has been dissected and is reflected. 1, Dura mater; 2, dorsal rootlets of a spinal nerve; 3, spinal cord (covered by pia mater); 4, denticulate ligament.
The widest parts (“cisterns”) of the cranial subarachnoid space are located between the more salient parts of the ventral surface of the brain and in the angle between the cerebellum and the dorsal aspect of the medulla. The dorsal widening, the cerebellomedullary cistern, is especially large and may be reached in the living animal if one passes a needle between the atlas and the skull (see Figure 8–57). Cisternal puncture is employed in both clinical and experimental work for obtaining samples of cerebrospinal fluid. The spinal subarachnoid space is more uniform but widens around the conus medullaris, which is a fortunate circumstance as access to the vertebral canal is easiest through the lumbosacral interarcuate space (Figure 8–59).

Figure 8–59 Schematic median section of the vertebral canal and its contents. The needle points to the lumbosacral interarcuate space. 1, Lumbar vertebra; 2, sacrum; 3, caudal vertebra; 4, conus medullaris; 5, filum terminale; 6, epidural space; 7, dura mater; 8, arachnoid space with cerebrospinal fluid.
The pia mater is firmly attached to the outer surface of the brain and cord, and many branches from arteries within the pia penetrate the brain and cord substance. These vessels are initially enclosed by pial sleeves but these soon merge with the vascular walls. A thickening of the pia fills the ventral fissure of the spinal cord, where it appears as a glittering silver line.
All three meninges form cuffs around the roots of origin of the cranial and spinal nerves.
The cerebrospinal fluid within the arachnoid space forms a water jacket that buoys up and protects the soft brain and cord. It is largely a product of the ependymal lining of the ventricular system within the brain, and the overwhelmingly larger part is produced where this covers the choroid plexuses, vascular tufts that invaginate the ventricles (Figure 8–60/6,9). An additional contribution to the fluid is made by the pial vessels.
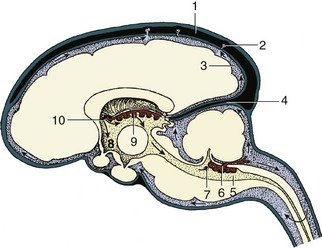
Figure 8–60 The production and circulation of cerebrospinal fluid (sagittal section). The blood vessels are in black, the subarachnoid spaces are heavily shaded, the ventricles are lightly shaded, and the nervous tissue is white. The direction of the flow of the cerebrospinal fluid is indicated by arrows. The cerebrospinal fluid is secreted by the choroid plexus (6, 9) of the lateral, third, and fourth ventricles. It escapes into the subarachnoid space via the aperture of the fourth ventricle (7). The cerebrospinal fluid is transferred to the systemic circulation (1) at the arachnoid villi (2). 1, Dorsal sagittal sinus; 2, arachnoid villus; 3, subarachnoid space; 4, membranous tentorium cerebelli; 5, fourth ventricle; 6, choroid plexus of fourth ventricle; 7, aperture of fourth ventricle; 8, third ventricle; 9, choroid plexus of third ventricle; 10, interventricular foramen, connecting the lateral and third ventricles
The ventricles are local modifications of the lumen of the neural tube; they have complicated shapes, but as these are illustrated (Figure 8–61) and as the details have little veterinary significance, they need not be described. It is more important to understand their relationship to the choroid plexuses. The plexuses of the lateral and third ventricles, which merge within the interventricular foramen, develop within a fold of the pia that becomes entrapped between the expanding telencephalic vesicles and the roof of the diencephalon (Figure 8–62). The plexuses of the fourth ventricle develop separately within the pia over the caudal medullary velum. In the course of development these plexuses thrust themselves into the lumen of the fourth ventricle; parts later reemerge into the arachnoid space by herniating through paired lateral openings in the roof (Figure 8–63).
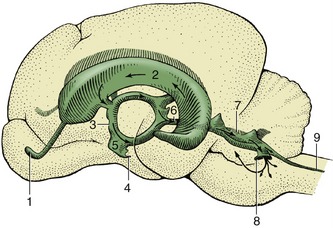
Figure 8–61 Lateral view of a cast of the ventricles of the brain of the dog. 1, Cavity of olfactory bulb; 2, lateral ventricle; 3, third ventricle; 4, infundibular recess; 5, optic recess; 6, mesencephalic aqueduct; 7, fourth ventricle; 8, lateral recess; 9, central canal.
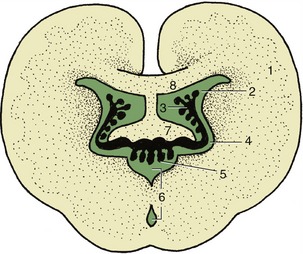
Figure 8–62 Schematic section of the brain illustrating the interrelations of the third and lateral ventricles and their choroid plexuses. 1, Cerebral hemisphere; 2, lateral ventricle; 3, choroid plexus of lateral ventricle; 4, interventricular foramen; 5, choroid plexus of third ventricle; 6, third ventricle; 7, fornix; 8, corpus callosum.
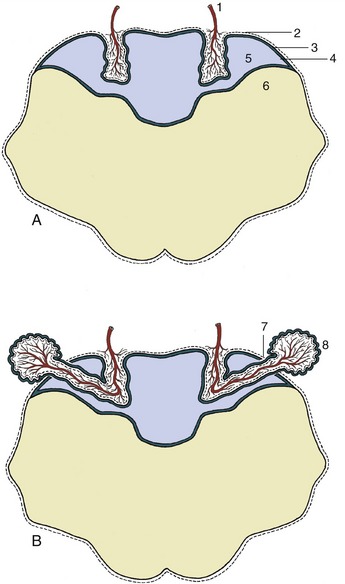
Figure 8–63 The formation of the choroid plexus in the roof of the fourth ventricle (A) and its later extension into the subarachnoid space (B). 1, Blood vessel invagination; 2, pia mater; 3, caudal medullary velum; 4, ependyma; 5, fourth ventricle; 6, myelencephalon; 7, aperture of fourth ventricle; 8, choroid plexus extending into subarachnoid space.
The clear colorless cerebrospinal fluid is to a certain extent also formed from the blood plasma by ultrafiltration through the “blood–cerebrospinal fluid barrier” (blood–brain barrier) composed of vascular endothelial cells. The fluid has a higher concentration of potassium and calcium ions and a lower concentration of sodium, magnesium, and chloride ions than the plasma; it is also rather deficient in glucose and, most importantly, contains little protein because the barrier is impermeable to larger molecules, which of course include those of many antibiotic and other drugs.
In addition to its mechanical role, the cerebrospinal fluid protects the brain through its chemical buffering capacity, which provides a rather stable milieu. It also transports nutrients, flushes away waste products, and serves as a medium for the diffusion of neuroendocrine and neurotransmitter substances.
The fluid is produced continuously, at a rate of some 30 mL per hour in the dog, and first circulates through the ventricular system, moved onward by the filtration pressure and ciliary activity of the ependymal lining. It then escapes from the interior of the brain through the lateral apertures of the fourth ventricle (Figure 8–60/7; in some species there is a third median opening unrelated to the plexus). The fluid bathes the brain and cord before returning to the blood, mostly through the arachnoid granulations (villi) (Figure 8–64/10), projections of the arachnoid and subarachnoid space that pierce the dura to enter the dorsal sagittal venous sinus of the brain; these formations become increasingly prominent with age. (Obliteration of the villi results in hydrocephalus because drainage of the fluid is hampered while its production continues and is not influenced by a feedback mechanism.) A smaller part of the fluid percolates along the meningeal cuffs that surround the cranial and spinal nerves at their origins and is eventually absorbed by perineural lymphatics; these connections are believed to provide potential routes for the retrograde (i.e., toward the meninges and nervous tissue) spread of infection.
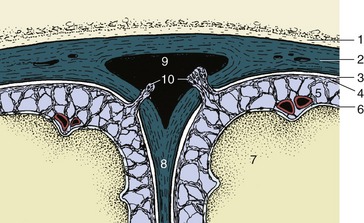
Figure 8–64 Transverse section of the dorsal sagittal sinus and adjacent meninges. Cerebrospinal fluid is transferred from the subarachnoid space to the sinus via the arachnoid granulations (villi). 1, Roof of cranial cavity; 2, fused dura mater and periosteum; 3, subdural space; 4, arachnoid; 5, subarachnoid space; 6, pia mater; 7, cerebral hemisphere; 8, falx cerebri; 9, dorsal sagittal sinus; 10, arachnoid granulations (villi).
THE ARTERIAL BLOOD SUPPLY
The blood supply to the brain comes mainly from the circulus arteriosus cerebri (formerly known as the circle of Willis), which lies ventral to the hypothalamus where it forms a ring around—but at some distance from—the infundibular stalk. The appearance of the circle and the pattern of its major branches are remarkably constant among mammals, although the sources from which the circle is supplied and the directions in which blood flows in certain vessels vary. For this reason, the initial account is based on the arrangements in the dog, an animal in which the arrangement is not only relatively simple but also of the most common pattern.
The arterial circle of the dog is supplied from three sources: paired internal carotid arteries laterally and the basilar artery caudally (Figure 8–65). The internal carotid artery (Figure 8–65/5) is a terminal branch of the common carotid from which it springs opposite the pharynx. It then runs toward the base of the skull. In many species the artery makes immediate entry to the cranial cavity through a carotid foramen in the cranial floor, but in the dog it must first traverse a tunnel (carotid canal) in the bone medial to the tympanic bulla. The artery is released at the rostral end of the tunnel and describes a loop that first carries it ventrally, then dorsally, before it finally gains the cranial cavity. It then penetrates the outer meninges, which involves passage through the cavernous venous sinus enclosed within a splitting of the dura, before dividing into divergent branches. The rostral branch unites with its fellow to complete the rostral half of the circle, the half from which the large rostral and middle cerebral arteries arise. The caudal branch anastomoses with a branch of the basilar artery (which reaches the circle along the midventral surface of the brainstem) to complete the circle (Figure 8–65/11). The caudal cerebral and rostral cerebellar arteries leave the caudal half of the circle; the fifth major artery to the brain, the caudal cerebellar, leaves the basilar artery directly.
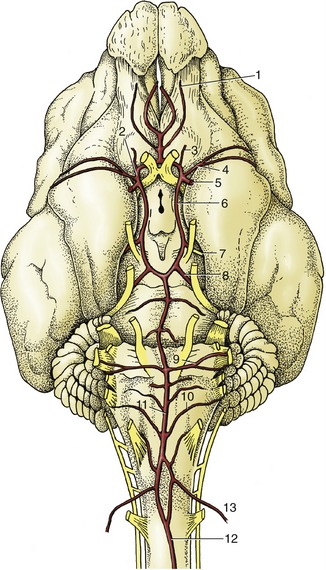
Figure 8–65 Arteries on the ventral surface of the canine brain. 1, Internal ethmoidal a.; 2, rostral cerebral a.; 3, internal ophthalmic a., 4, middle cerebral a.; 5, internal carotid a.; 6, caudal communicating a.; 7, caudal cerebral a.; 8, rostral cerebellar a.; 9, labyrinthine a.; 10, caudal cerebellar a; 11, basilar a.; 12, ventral spinal a.; 13, vertebral a.
The blood within the basilar artery has a composite origin. The artery appears to be the direct continuation of the small ventral spinal artery but is greatly reinforced by anastomosis with the vertebral artery (Figure 8–65/13), which passes into the vertebral canal through the atlas. The vertebral artery itself receives anastomotic branches (dog and horse) from the occipital artery (another branch of the common carotid) before entering the canal, and it would thus appear that this vessel (occipital artery) also contributes to the supply of the brain. However, the vertebral artery is the main if not sole supply to the occipital lobes of the cerebral hemispheres and other caudal parts of the brain.
The arrangement is more complicated in many other species. In these the internal carotid connects with other arteries of the head, especially the maxillary, before discharging into the arterial circle. The anastomosis may be small initially, but in many species it later enlarges and detaches many tortuous branches, which together substitute for the original single channel. This arrangement, which may present a rather tangled appearance, is known as a rete mirabile and has a rather enigmatic significance; the arrangement enhances the efficiency of the blood-cooling mechanism that is discussed shortly. In some species the lumen of the part of the internal carotid artery proximal to the rete becomes obliterated, sometimes only a considerable time after birth; when this happens, the emissary artery from the rete delivers blood that is wholly of external carotid origin (see Figure 7–35). This arrangement is found in both sheep and cattle, although these species differ in other features of the arterial supply to the brain (p. 661).
The brain, particularly its gray substance, has very high metabolic requirements, and the arterial supply is commensurate with this, amounting to 15% or 20% of the cardiac output. Despite this, the vessels that actually penetrate the brain are uniformly small, which is a feature that may be related to the need to avoid large, pulsating trunks within the delicate brain tissue. Moreover, in sharp contrast to the wide anastomoses between the feeding vessels, any intracerebral anastomoses are narrow and mostly connect functional end-arteries. This fact, coupled with the very limited regeneration capacity of brain tissue, explains why the most serious consequences may attend occlusion or rupture of a small vessel that may be the sole effective supply to some vital nucleus or tract. Notorious examples are provided by the small arteries within the human corpus striatum, where an infarct is so often the cause of a stroke.
The permeability of the blood capillaries of the nervous tissue is reduced, resulting in the blood–brain barrier. The main structural components of this barrier are provided by the continuity between the endothelial cells of these capillaries and pericytes, astrocytes, and the basement membrane surrounding these capillaries (see p. 217; Figure 8–66).
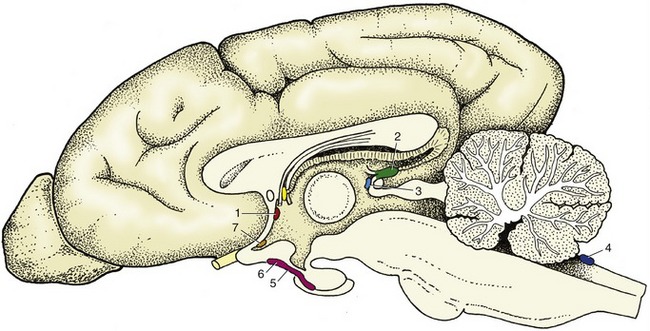
Figure 8–66 Schematic median section of the canine brain with an indication of the locations of the circumventricular organs. 1, Subfornical organ; 2, pineal body; 3, subcommissural organ; 4, area postrema; 5, posterior and intermediate lobes of pituitary; 6, median eminence; 7, vascular organ of lamina terminalis.
The spinal cord is supplied by three arteries that run its length. The largest, the ventral spinal artery, follows the surface of the ventral fissure of the cord; paired dorsolateral spinal arteries run close to the furrow from which the dorsal roots of the spinal nerves arise. All three are periodically reinforced by branches from regional arteries: vertebral in the neck and intercostal, lumbar, and sacral in the trunk. These enter at the intervertebral foramina, often in the form of narrow vessels that accompany the roots of the spinal nerves; they form plexuses on the surface of the cord with which the major longitudinal arteries connect. This theoretically regular pattern is subject to much variation, both specific and individual, in which many expected reinforcing arteries are lacking, the plexus is unevenly developed, and stretches of the longitudinal trunks are attenuated.
Branches of the ventral spinal artery supply the “core” of the cord, the gray substance, and the adjacent layer of white substance by an approach through the ventral fissure (see Figure 19–5). The greater part of the white substance is supplied by radial twigs from the dorsolateral arteries and surface plexus. Internal anastomoses between the two sets of vessels, although common, are of questionable efficiency.
THE VENOUS DRAINAGE
A complicated system of venous sinuses within the cranial cavity and vertebral canal is connected at intervals to the exposed regional veins. The cranial sinuses enclosed within the dura mater are divided into dorsal and ventral systems between which there is only limited communication (Figure 8–67). The dorsal system collects blood from the dorsal parts of the brain and the diploë of the bones of the cranial vault. It includes a dorsal sagittal sinus within the falx cerebri. The dorsal sagittal sinus receives numerous tributary veins directly from the cerebral hemispheres, and it is joined toward its caudal end by the straight sinus, which runs within the ventral part of the falx and collects blood from a major vein draining deeper parts of the brain. The dorsal sinus splits (in a variable manner) into bilateral transverse sinuses within the tentorium cerebelli; each later divides—one branch leaving the skull through a foramen, the other connecting with the ventral system.
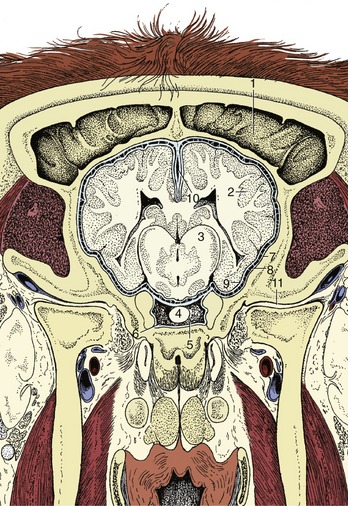
Figure 8–67 Position of the brain in relation to the roof of the bovine skull. Some features of the meninges are also shown. 1, Frontal sinus; 2, cerebral cortex; 3, diencephalon; 4, hypophysis; 5, sella turcica; 6, cavernous sinus; 7, dura mater; 8, arachnoid; 9, pia mater; 10, falx cerebri with dorsal sagittal sinus; 11, temporomandibular joint.
The ventral or basilar system drains the ventral part of the brain (and other cranial contents and walls) and also receives a major inflow from a vein that enters the cranial cavity from the orbit after draining much of the face, including the nasal cavity. The rostral part of the longitudinal trunk of the ventral system, the cavernous sinus (Figure 8–67/6), is connected with its fellow both before and behind the hypophysis. It divides caudally into the basilar sinus, which continues through the foramen magnum as the main component of the internal vertebral plexus, and a branch that receives a connection from the dorsal system before emerging through a ventral foramen to contribute to the maxillary vein.
The flow of blood into the cranial cavity from the face is noteworthy for two reasons. First, it provides a potential pathway for the spread of infection from the face to the cranial contents. Secondly, it provides for cooling of the arterial supply to the hypothalamus, the part of the brain responsive to and concerned with the regulation of body temperature. The cooling is due to the passage of the internal carotid artery (or rete substitute) through the cavernous sinus, where bathing by venous blood at a somewhat lower temperature (because it is drained from the nose and superficial structures of the head) promotes heat exchange. (An additional mechanism for protecting the brain from damaging hyperthermia is provided by the course of the common carotid artery, which is extensively related to the trachea at no great depth below the skin. These relationships promote heat loss, especially because any physical exertion that tends to raise body temperature also increases the flow of air within the upper respiratory tract.)
The vertebral venous plexus is probably more important clinically. It runs the whole length of the vertebral column and drains blood from the vertebrae, the adjacent musculature, and the structures within the vertebral canal. It gives rise to segmental veins that leave the canal through the intervertebral foramina to join the principal venous channels of the neck and trunk: the vertebral, cranial caval, azygous, and caudal caval veins (see Figure 7–43/18). The major part of the plexus consists of paired channels within the epidural space ventral to the cord. They are composed of crescentic segments that extend between successive intervertebral foramina (see Figure 26–5). The enlarged midpart of each segment swings toward and is generally joined to its neighbor over the middle of the vertebra, which produces a ladderlike pattern of vessels. The connections with segmental veins through the intervertebral foramina form a plexus around the emerging spinal nerves, protecting them from injury.
The veins composing the plexus are thin walled and, being without valves, may pass blood in either direction. They are capacious and adjust in size to compensate for variations in venous return to the heart induced by the intrathoracic pressure changes that accompany respiration. Since the system provides alternative channels to the major systemic veins, it may mitigate the effects of jugular obstruction (when the neck is compressed) or caudal caval obstruction (when pressure within the abdomen is raised). The intermittency of flow caused by these several factors facilitates the spread of septic or neoplastic disease to the vertebral column when the lungs would be the expected destination; blood diverted into the vertebral plexus when the flow through other channels is impeded may be temporarily held stagnant, which allows tumor seeds or microorganisms to settle within the tributaries that issue from the bones.
A further point of clinical importance lies in the risk of hemorrhage when epidural or subarachnoid puncture is performed. The risk is greatest at the atlantooccipital space, where tributaries of the plexus most often encircle the dural tube.
THE CRANIAL NERVES
The names and sequence of the cranial nerves should now be familiar. Although these nerves lack the relative uniformity of makeup and distribution pattern that is found with the spinal nerves, it is possible to arrange them in three groupings: those exclusively concerned with special senses (the olfactory, optic, and vestibulocochlear nerves); those that supply head muscles of somitic origin (the oculomotor, trochlear, abducent, and hypoglossal nerves); and those primarily concerned with structures of pharyngeal arch origin (the trigeminal, facial, glossopharyngeal, vagus, and accessory nerves). However, it is probably more convenient to deal with them in numerical, that is, in rostrocaudal, sequence.
THE OLFACTORY NERVE (I)
The fibers that compose the olfactory nerve arise as the central processes of the olfactory cells of the nasal mucosa. They are collected into a number of filaments that separately traverse the cribriform plate to join the adjacent surface of the olfactory bulb (Figure 8–19/1). The further course of the olfactory pathways has been described (p. 291).
The short course and deep location protect these nerves against casual injury, and though they may be involved in infectious or neoplastic disease, interference with the sense of smell is more often due to blockage of the air passages leading to the olfactory mucosa. The filaments are surrounded by meningeal sheaths enclosing extensions of the subarachnoid space, which provide potential routes for the spread of infection from the nose to the cranial cavity.
The vomeronasal organ is also part of the olfactory system (see p. 352)
THE OPTIC NERVE (II)
The optic nerve mediates the visual sense and is in fact a brain tract connecting the retina with the diencephalon (from which it originated). The intracranial part of the nerve extends from the optic chiasm (Figure 8–19/7), where varying proportions of the fibers decussate (p. 299), to the optic foramen at the apex of the orbital cone; the intraorbital course is described elsewhere (p. 345) (see Figure 9–17/9). The optic nerve is also enclosed within extensions of the meninges, and the dura blends with the sclera where the nerve joins the eyeball. Section of the nerve obviously results in blindness of that eye.
THE OCULOMOTOR NERVE (III)
The oculomotor nerve consists of somatic efferent fibers from the principal (motor) nucleus and visceral efferent fibers from the parasympathetic nucleus (of Edinger–Westphal), both of which are within the tegmentum of the midbrain (see Figure 8–25). Fibers of the two categories emerge together at the superficial origin from the ventral aspect of the midbrain, close to the midline and in series with other cranial nerves of predominantly somatic efferent composition and with the ventral roots of spinal nerves (Figure 8–19). In its intracranial course the oculomotor is related to the trochlear, abducent, and ophthalmic nerves and to the cavernous sinus, and it passes through the orbital fissure in their company. It divides within the orbit to supply the dorsal, medial, and ventral recti, the ventral oblique, and the levator muscle of the upper eyelid (some authors also include part of the retractor bulbi). The preganglionic parasympathetic fibers synapse within the small ciliary ganglion placed on one of the branches (Figure 8–45/9 and 8–70/1,6). From here, postganglionic fibers pass within the short ciliary nerves to supply the intraocular ciliary and constrictor pupillae muscles. Isolated injury or involvement in disease is not common; the effects can be deduced from consideration of the actions of the muscles it supplies (p. 345).
THE TROCHLEAR NERVE (IV)
The trochlear nerve, which is small, is motor to the dorsal oblique muscle. The nucleus of origin within the tegmentum of the midbrain gives rise to a fiber bundle that decussates internally before emerging from the rostral medullary velum (Figure 8–23/8). The nerve then follows the edge of the tentorium cerebelli to the floor of the cranial cavity. In some species it makes a separate entrance to the orbit, but usually it passes through the orbital fissure. The effects of section are those of paralysis of the dorsal oblique (p. 341).
THE TRIGEMINAL NERVE (V)
The trigeminal nerve, the largest of the cranial nerves, is sensory to the skin and deeper tissues of the face and motor to the muscles of first pharyngeal (mandibular) arch origin. The proprioceptive fibers, which include many from muscles that receive their motor innervation from other cranial nerves, pass to the rostral sensory mesencephalic nucleus; the other afferent fibers pass to the pontine and spinal nuclei. The efferent fibers originate in the motor nucleus (Figure 8–25/7,17). The peripheral nerve is formed by the fusion of sensory and motor roots that attach to the ventrolateral aspect of the pons. The larger sensory root carries the massive trigeminal ganglion and, just beyond this, divides into the trio of primary branches that give the trunk its name. The mandibular branch unites with the motor root to constitute the mixed mandibular nerve; the ophthalmic and maxillary divisions remain purely sensory at this level, although peripheral connections with other cranial nerves introduce somatic and visceral efferent fibers into certain branches. The mandibular nerve emerges through the oval foramen in the floor of the cranial cavity. The ophthalmic and maxillary nerves run rostrally to emerge through the orbital fissure and round foramen, respectively (in ruminants the two openings are combined).
The three primary divisions are initially each restricted to a different process of the embryonic face, which explains the crisply defined adult territories (cf. the dermatomes of the trunk). The ophthalmic nerve supplies the frontonasal process, the primordium of the forehead and nose regions; the maxillary nerve supplies the maxillary process, the primordium of the upper jaw and associated parts; and the mandibular nerve supplies the mandibular process, the primordium of the lower jaw and associated parts, which include the masticatory and other first pharyngeal arch muscles (Figure 8–68).

Figure 8–68 Distribution pattern of the trigeminal nerve of the dog. 1, Ophthalmic n.; 2, frontal n.; 3, lacrimal n.; 4, nasociliary n.; 4′, infratrochlear n.; 4″, long ciliary n.; 5, maxillary n; 6, infraorbital n.; 7, zygomatic n.; 8, pterygopalatine n.; 9, lesser palatine n.; 10, greater palatine n.; 11, caudal nasal n.; 12, mandibular n.; 13, masticatory n.; 14, deep temporal n.; 15, buccal n.; 16, pterygoid n.; 17, auriculotemporal n.; 18, lingual n.; 18′, sublingual n.; 19, inferior alveolar n.; 19′, mylohyoid n.; 19″, mental n.
The ophthalmic nerve (Figure 8–68/1), for which the convenient notation is V-1, divides into three divergent branches soon after entering the orbit. The lacrimal nerve (Figure 8–68/3) passes to the lateral part of the orbital perimeter and, after detaching branches to the lacrimal gland and other deeper structures, emerges to supply the skin about the lateral angle of the eye. The more considerable territory of the frontal nerve (Figure 8–68/2) includes much of the upper eyelid, the forehead, and, through branches that penetrate the bone, the mucosa of the frontal sinus.
The nasociliary nerve (Figure 8–68/4) runs toward the medial wall of the orbit. One branch, the infratrochlear nerve (Figure 8–68/4′), emerges on the face after supplying structures at the medial angle; it supplies another portion of the mucosa of the frontal sinus and in small ruminants detaches the principal nerve to the horn. Other branches of the nasociliary nerve include long ciliary and ethmoidal nerves. The long ciliary nerves (Figure 8–68/4″) penetrate the posterior aspect of the eyeball to supply sensitive tissues, including the cornea; the ethmoidal nerve first re-enters the cranial cavity through the ethmoidal foramen and subsequently passes to the nasal cavity via the cribriform plate before dividing into medial and lateral branches to the mucosa.
The maxillary nerve (V-2) runs across the wall of the pterygopalatine fossa ventral to the orbit (Figure 8–68/5). It bears, or lies close to, the pterygopalatine ganglion, but the relationship is purely topographical. It then enters the infraorbital canal at the maxillary foramen, where it becomes known as the infraorbital nerve (Figure 8–68/6) in anticipation of its reappearance on the face at the infraorbital foramen.
Collateral branches detached within the pterygopalatine fossa include the zygomatic nerve (Figure 8–68/7), which supplies the lower eyelid and adjacent skin and is the origin of the principal nerve of the horn in cattle.
The second branch, the pterygopalatine nerve (Figure 8–68/8), detaches the lesser palatine nerve (Figure 8–68/9) to the soft palate; the greater palatine nerve (Figure 8–68/10), which reaches the hard palate after traversing the palatine canal and supplies both the palatine mucosa and the floor of the nasal vestibule; and the caudal nasal nerve (Figure 8–68/11), which passes through the pterygopalatine foramen to supply mucosa of the ventral part of the nasal cavity, maxillary sinus, and palate.
Within the infraorbital canal the infraorbital nerve (Figure 8–68/6) detaches short twigs to the alveoli of the cheek teeth and nasal mucosa and longer rostral alveolar branches that continue within the bone, beyond the infraorbital foramen, to the alveoli of the canine and incisor teeth. After emerging at the infraorbital foramen the infraorbital nerve supplies various labial and nasal branches to the structures of the muzzle, including some branches that run back over the nose to the edge of the infratrochlear territory. Although covered by muscle at its emergence, the infraorbital nerve may usually be palpated, stimulated by pressure, or blocked by injection of local anesthetic solution.
On leaving the cranium, the mandibular nerve (V-3) detaches in close succession several nerves that pass to the masseter, temporalis, medial and lateral pterygoid, tensor veli palatini, and tensor tympani muscles (Figure 8–68/12). There are minor variations in their pattern, and those to the masseter and temporalis are often initially conjoined in a short masticatory nerve (Figure 8–68/13). The masseteric nerve passes to that muscle between the coronoid and condylar processes of the mandible. The deep temporal nerves (Figure 8–68/14) run dorsomedially to the temporalis. The otic ganglion lies close to the origin of the pterygoid nerves (Figure 8–68/16).
The next branch, the buccal nerve (Figure 8–68/15), is sensory to the tissues of the cheek, which it reaches after first passing between the pterygoideus and temporalis and then between the maxillary tuber and mandible. Its origin is followed by that of the auriculotemporal nerve (Figure 8–68/17), which bends around the caudal border of the mandible to enter the face a little ventral to the temporomandibular joint. It is sensory to the skin of the temporal region and over much of the external ear, including the lining of the canal leading to the eardrum. The continuation onto the face, the transverse facial branch, supplies a strip of skin extending to the corner of the mouth.
The mandibular nerve continues between the medial and lateral pterygoid muscles before dividing into its end-branches, the lingual and inferior alveolar nerves.
The lingual nerve (Figure 8–68/18) detaches twigs to the oropharyngeal mucosa before dividing into a deep branch that enters the tongue and a superficial branch, the sublingual nerve (Figure 8–68/18′), that runs medial to the mylohyoideus below the mucosa of the oral floor that it supplies. The branch to the tongue is joined by the chorda tympani, a branch of the facial, which introduces visceral efferent fibers to salivary glands that synapse in the adjacent mandibular ganglion and gustatory fibers (special visceral afferent) from the taste buds of the rostral two thirds of the tongue. Other sensory fibers supply general sensation in the same area of the lingual mucosa.
The inferior alveolar nerve (Figure 8–68/19) detaches the mylohyoid nerve (Figure 8–68/19′) for the mylohyoideus and rostral belly of the digastricus before entering the mandibular canal at the mandibular foramen. The inferior alveolar nerve supplies the lower cheek teeth before a large part reappears at the mental foramen as the mental nerve (Figure 8–68/19″), which supplies tissues of the lower lip and chin. In some species several mental branches exit through as many foramina. Although also covered by muscle, the mental nerve(s) can be palpated, compressed, and blocked at emergence.
Injuries to, or disease of, the branches of the trigeminal nerve produce sensory deficiencies in their territories and sometimes manifest themselves by chronic facial irritation; some branches are frequently blocked for minor surgery of the head. Destructive lesions of the mandibular nerve produce paralysis of the muscles that raise the jaw; when the lesion is unilateral, the resulting atrophy may be more obvious than any motor disability. A temporary idiopathic bilateral paralysis of the trigeminal musculature, characterized by a dropped jaw, has been reported in dogs.
THE ABDUCENT NERVE (VI)
The fibers of the abducent nerve originate within the caudal brainstem and make the expected appearance (for general somatic efferent fibers) close to the midline (Figure 8–19). The intracranial course leads to the orbital fissure (or the foramen orbitorotundum); within the orbit the nerve branches to go to the lateral rectus and the retractor, although the exact innervation of the latter muscle is still controversial. Injury produces inability to deviate the eyeball laterally (p. 341).
THE FACIAL NERVE (VII)
The facial nerve is sometimes known as the intermediofacial nerve, a term that indicates its composite nature. The intermediate component is a visceral nerve with sensory (including gustatory) and motor (parasympathetic) functions; the facial component is the nerve of the second pharyngeal arch whose main distribution is to the mimetic musculature.
The facial and vestibulocochlear nerves arise close together at the lateral extremity of the trapezoid body (Figure 8–21/VII,VIII) and run within common meningeal investments to the internal acoustic meatus of the petrous temporal bone. They part here, and the facial nerve enters a passage (facial canal) within the bone that leads, via a sharp caudal convexity (“genu”), to the stylomastoid foramen, where the nerve appears at the surface of the skull. The facial nerve carries the appropriately named geniculate ganglion at the summit of the bend. With the exception of the small branch to the stapedius muscle, the branches detached within the bone represent the intermediate component and those detached after leaving the bone represent the facial component (Figure 8–69/1).
Figure 8–69 Distribution pattern of the facial nerve of the dog. 1, Facial n.; 2, auriculopalpebral n.; 3, dorsal buccal branch; 4, ventral buccal branch; 5, cervical branch.
The greater petrosal nerve is detached at the level of the ganglion and emerges through an independent foramen. It is initially parasympathetic but is shortly joined by sympathetic fibers to form a composite autonomic nerve; this, the nerve of the pterygoid canal, runs through that fine passage to reach the pterygopalatine ganglion within the pterygopalatine fossa (Figure 8–70/7,11). The nerve of the pterygoid canal is discussed more fully later (p. 327). The stapedial nerve, which arises next, is motor to the stapedius muscle of the middle ear. The next branch, the chorda tympani (Figure 8–70/13), crosses the tympanic cavity to emerge at the petrotympanic fissure, after which it converges on and becomes incorporated within the lingual branch of the mandibular nerve (p. 353).
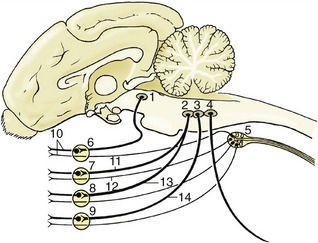
Figure 8–70 Schematic representation of the autonomic innervation of structures of the head. 1, Parasympathetic oculomotor nucleus (III); 2, parasympathetic facial nucleus (VII); 3, parasympathetic glossopharyngeal nucleus; 4, parasympathetic vagus nucleus; 5, cranial cervical ganglion; 6, ciliary ganglion; 7, pterygopalatine ganglion; 8, mandibular ganglion; 9, otic ganglion; 10, short ciliary nerves; 11, greater petrosal nerve; 12, deep petrosal nerve; 13, chorda tympani; 14, tympanic plexus, short petrosal nerve.
The first branches of the free portion of the facial nerve are the internal and caudal auricular nerves, which supply muscles of the external ear and other branches to some hyoid muscles, including the caudal belly of the digastricus. The main trunk enters the face by turning around the mandible, where it is first contained between the masseter and the parotid gland. It divides at about this level (although there are species differences) into three terminal branches.
In some species the auriculopalpebral nerve (Figure 8–69/2) is detached before the main trunk reaches the face, and it is then less vulnerable to injury from superficial trauma to the side of the head. It crosses the zygomatic arch, heading for the space between the upper eyelid and external ear, before dividing into branches that supply the muscles of the eyelids (excluding levator palpebrae superioris) and the auricular muscles in front of the external ear.
The dorsal buccal branch (Figure 8–69/3), which may take the form of a leash of divergent branches, crosses the masseter en route to the muzzle.
In some species the ventral buccal branch (Figure 8–69/4) may take a similar path at a slightly more ventral level, but in others it takes a divergent course, first running within the intermandibular space before entering the face with the parotid duct and facial vessels, where they cross the mandible in front of the masseter. Together, the buccal branches supply the muscles of the cheek, lips, and nostrils. Their peripheral branches join with those of the trigeminal nerve at various levels, and many of the smaller trunks combine motor (facial) and sensory (trigeminal) fibers.
The effects of injury or disease clearly depend on the site of the lesion. Lesions that are situated more centrally, which tend to have more sinister origins, affect the whole facial field and lead to loss of secretory activity by the lacrimal and salivary (except the parotid) glands in addition to muscular paralysis. Lesions involving the main trunk near its exit from the bone paralyze the entire mimetic musculature, while more peripheral lesions may spare some groups, depending on their site and specific and individual variations in the branching pattern. Those confined to the auriculopalpebral nerve produce drooping of the external ear and narrowing of the palpebral fissure with inability to close the eye. Damage to the buccal branches may paralyze the muscles of the lips and cheeks, allowing a quid of food to collect in the oral vestibule. It may also lead to deformation of the muzzle, which is drawn out of symmetry by the unopposed activity of the muscles on the sound side. The alteration in appearance is not always very striking, and the uninjured side, to which the muzzle is drawn, may sometimes appear to have the more distorted aspect. The distortion tends to be more pronounced in the horse and sheep than in other domestic species. (It is important to be aware that in unilateral facial spasm, seen occasionally in the dog, the nose may be drawn toward the affected side.)
The auriculopalpebral nerve is sometimes blocked to facilitate examination of the eye.
THE VESTIBULOCOCHLEAR NERVE (VIII)
The vestibulocochlear nerve divides at the internal acoustic meatus into its vestibular and cochlear parts, which make their separate ways through the petrous temporal bone to the vestibular and cochlear components of the membranous labyrinth of the inner ear. They are discussed further with the special sense organs of balance and hearing (p. 351).
THE GLOSSOPHARYNGEAL NERVE (IX)
The glossopharyngeal nerve combines fibers concerned with the innervation of structures of third pharyngeal arch origin with important visceral efferent (parasympathetic) and afferent components. It is motor to part of the palatopharyngeal musculature and to certain salivary glands and sensory to mucosa of the root of the tongue, palate, and pharynx. In addition, there is an important branch to the carotid sinus and body.
The glossopharyngeal nerve arises from the ventrolateral aspect of the medulla oblongata, from the most rostral rootlets of the linear series that also gives origin to the vagus and the medullary part of the accessory nerve (Figures 8–19 and 8–21). It runs with these nerves to the jugular foramen and at about this level bears two small and rather indistinct ganglia. The first branch, the tympanic nerve, enters the tympanic cavity, where it participates with branches of the facial and internal carotid (sympathetic) nerves in forming a plexus from which a nerve leads to the otic ganglion for the supply of the parotid gland (Figure 8–70/3,14).
The main trunk cleaves for a spell to the vagus and accessory nerves and at this level detaches the carotid sinus branch, which proceeds to the carotid sinus, where it terminates in baroreceptors within the sinus wall and chemoreceptors of the carotid body. The glossopharyngeal nerve then turns rostroventrally, parallel to the stylohyoid, before dividing into pharyngeal and lingual branches. The pharyngeal branches include one to the stylopharyngeus caudalis; the others become diffused within the pharyngeal plexus to which the vagus also contributes. Although most fibers are sensory to the mucosa, the possibility of a further contribution to the pharyngeal musculature seems likely.
The larger lingual branch enters the tongue parallel to the lingual artery, the lingual branch of the mandibular nerve, and the hypoglossal nerve. It is sensory to the mucosa of the root of the tongue (including the taste buds in this area) and motor to the levator palatini muscle and the glands of the soft palate. Damage to the nerve, which is most common in horses as the result of inflammation of the guttural pouch, may lead to difficulties in swallowing. Because the vagus may also be affected, it is difficult to know the extent to which the paresis of palate and pharynx is due to glossopharyngeal involvement. Experimental studies suggest that the role of the glossopharyngeal nerve is more important than many authors have claimed.
THE VAGUS NERVE (X)
The vagus nerve is the nerve of the fourth and subsequent pharyngeal arches. It also contains the parasympathetic fibers that innervate the cervical, thoracic, and abdominal viscera. The second component gives it by far the most widespread distribution of any cranial nerve (Figure 8–73/5).
The vagus forms part of the bundle of nerves that passes through the jugular foramen. It bears two small ganglia on the stretch that lies within and immediately external to the foramen and beyond this runs in close association with the glossopharyngeal and accessory nerves. After the glossopharyngeal nerve turns rostrally, the vagus continues with the accessory and later acquires a relationship with the cranial cervical ganglion. It then continues down the neck in close contact with the sympathetic trunk, with which it is bound within a common fascial sheath, on the dorsal margin of the common carotid artery and related to the trachea. The left vagosympathetic trunk has an additional contact with the esophagus (Figure 8–75/5). The vagus and sympathetic nerves part at the entrance to the chest, after which the vagus continues more or less horizontally through the mediastinum until it divides over the pericardium into dorsal and ventral branches. These combine with the corresponding contralateral branches to form the dorsal and ventral vagal trunks that enter the abdomen along the corresponding borders of the esophagus. Within the abdomen the two nerves branch freely, participating with the sympathetic fibers in forming the plexuses from which the abdominal viscera are supplied (p. 327).
The first significant detachment after the nerve leaves the skull is an auricular branch that takes part in the innervation of the skin of the external ear. This is followed by pharyngeal branches that combine with those of the glossopharyngeal, cranial laryngeal, and sympathetic nerves in forming the pharyngeal plexus. An extension of the plexus supplies the cervical esophagus. The cranial laryngeal nerve goes to the larynx, where it divides into an external branch for the cricothyroid muscle and an internal branch for the laryngeal mucosa from the aditus to the glottis. This branch makes connections with the recurrent laryngeal nerve. The depressor nerve to the heart is formed partly of fibers from the cranial laryngeal nerve and partly of fibers from the main vagal nerve; it is difficult to follow because in most animals it rejoins the main trunk for its further progress through the neck and thorax to the heart.
The thoracic portion of the vagus detaches cardiac branches that form a mediastinal plexus with sympathetic fibers with the same destination. A large caudal (recurrent) laryngeal nerve is also detached within the thorax. That of the right side changes direction by winding around a branch of the subclavian artery, while the left one winds around the aorta. The recurrent laryngeal nerve reascends the neck ventral to the common carotid artery in a course that leads it back to the larynx, where it supplies the bulk of the intrinsic laryngeal musculature (all but the cricothyroideus) and the mucosa caudal to the glottis. Small twigs detached en route pass to the cardiac plexus and to the trachea and esophagus. The distribution of the main trunk is completed by pulmonary branches that combine in a common plexus with sympathetic nerves.
Damage to the vagus nerve and its branches may be manifested in a variety of ways, including difficulties in swallowing and altered functioning of the heart and the other viscera. Degeneration of the recurrent laryngeal nerve is especially common in horses, producing the condition known as roaring (p. 526); it also occurs in dogs.
THE ACCESSORY NERVE (XI)
The accessory nerve is curiously formed of two roots. The spinal root is provided by filaments that emerge midway between the dorsal and ventral roots of the first five (or so) spinal nerves (Figures 8–19 and 8–21). These combine in a trunk that runs within the spinal subarachnoid space to enter the skull through the foramen magnum; it then approaches the cranial root, which is formed by the most caudal rootlets of the glossopharyngeal–vagus series. There is only brief contact between the two roots, and although some fibers may be exchanged, the cranial root then amalgamates with the vagus to which it probably furnishes the fibers that reach the laryngeal musculature via the recurrent laryngeal nerve. It is the spinal root that forms the accessory nerve of descriptive anatomy. This passes through the jugular foramen to divide within the atlantal fossa into dorsal and ventral branches.
The dorsal branch runs caudally over the splenius and serratus ventralis before it supplies the covering brachiocephalicus, omotransversarius, and trapezius. The ventral branch supplies only one muscle, the sternocephalicus, which it enters close to its cranial attachment.
There is no convincing explanation for the curious detour made by the spinal fibers of this nerve.
THE HYPOGLOSSAL NERVE (XII)
The hypoglossal nerve is motor to the intrinsic and extrinsic muscles of the tongue, which originate in the myotomes of occipital somites. After leaving the ventral aspect of the medulla oblongata (Figure 8–19), the nerve passes through the hypoglossal canal before crossing the nerves of the vagus group to continue toward the tongue, which it enters ventral to the glossopharyngeal nerve. It ramifies within the tongue substance to reach the various muscles.
A destructive lesion of this nerve paralyzes the ipsilateral muscles, allowing a deviation of the tongue toward the normal side. A marked atrophy eventually develops.
THE SPINAL NERVES
A general account of the formation and distribution of the spinal nerves has been given (see p. 29). That account described the formation of each nerve by the union of dorsal and ventral roots and its later division into dorsal and ventral primary rami, which diverge on passing through the intervertebral foramen (Figure 8–55). The rather consistent pattern of distribution of the dorsal rami may be represented by a single description; important regional features of the ventral rami require separate attention.
THE DORSAL RAMI
As a rule, the dorsal rami are considerably smaller than the ventral and have simpler distributions. Each divides into a medial branch that supplies the local part of the epaxial musculature of the neck, trunk, or tail and a lateral branch that is distributed to the dorsal part of the skin segment (dermatome) served by the particular spinal nerve. These areas extend from the dorsal midline for a variable distance over the side of the animal. The territories of the first few cervical nerves extend onto the poll region in addition to supplying skin over the neck, those of the nerves to each side of the cervicothoracic junction supply skin over the upper part of the shoulder, and those of the middle and caudal thoracic and lumbar regions serve increasingly larger areas of the skin of the chest wall and flank; however, those of the sacral nerves are again restricted. Inconspicuous connections between neighboring nerves form a continuous plexus through which exchange of fibers blurs the boundaries between the areas supplied by individual nerves; indeed, it is probable that every part of the skin receives sensory fibers from two, if not three, spinal nerves.
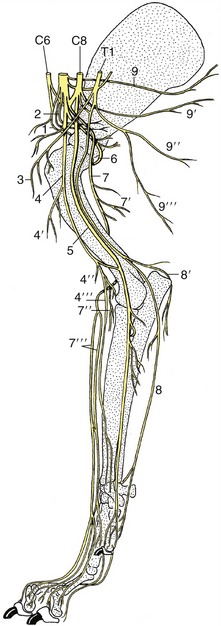
Figure 8–71 The nerves of the right forelimb of the dog; medial view. 1, Suprascapular n.; 2, subscapular nn.; 3, cranial pectoral nn.; 4, musculocutaneous n.; 4′, proximal muscular branch; 4″, distal muscular branch; 4′″, medial cutaneous antebrachial n.; 5, median n.; 6, axillary n.; 7, radial n.; 7′, muscular branches to triceps; 7″, muscular branches to extensors; 7′″, cranial cutaneous antebrachial n.; 8, ulnar n.; 8′, caudal cutaneous antebrachial n.; 9, long thoracic n.; 9′, thoracodorsal n.; 9″, lateral thoracic n.; 9′″, caudal pectoral n.
THE VENTRAL RAMI
The larger ventral rami supply the hypaxial muscles, including those of the limbs (excepting the thoracic girdle muscles supplied by the eleventh cranial nerve and the rhomboideus supplied in some species by dorsal rami) and the remaining skin of the neck, trunk, and limbs. Except in the thoracic region, where a more precise segmental distribution is retained, the ventral rami are also joined with their neighbors by connecting branches. These connections are greatly exaggerated at the levels of origin of the nerves to the forelimb and hindlimb, where they constitute the brachial and lumbosacral plexuses, respectively.
The Cervical Ventral Rami
The cutaneous distribution of the first two cervical ventral rami extends to the external ear and the masseteric and throat regions. The more caudal members of the series contribute to the phrenic nerve and brachial plexus while retaining local responsibilities.
In domestic species the phrenic nerve is generally formed by the fifth, sixth, and seventh cervical nerves. The contributions run ventrally over the scalenus muscle to join in a trunk (see Figure 1–38) that winds below the muscle to enter the mediastinum between the two first ribs. The phrenic nerve runs caudally within the mediastinum, crossing the lateral face of the pericardium, to reach the diaphragm; the right nerve utilizes the plica venae cavae in the last part of its course (see Figure 13–15/7). The phrenic nerves ramify within the diaphragm to which they are the sole motor innervation; their sensory fibers are supplemented by others channeled through intercostal nerves. It is worth emphasizing that the phrenic nerves are typical muscle nerves; it must not be inferred from the normally involuntary nature of breathing that they are autonomic. Experiments (in several species) have shown that bilateral section of the phrenic nerves has little effect, although respiratory embarrassment may become evident when the animal is severely stressed.
The Brachial Plexus
The brachial plexus supplies almost all structures of the forelimb—except for the trapezius, omotransversarius, brachiocephalicus, and rhomboideus—and the skin over the upper shoulder region.
The plexus is usually formed by contributions from the last three cervical and first two thoracic nerves; the fifth cervical nerve sometimes participates, and the contribution of the second thoracic nerve is then reduced or lacking. The plexus reaches the axilla by passing between the parts of the scalenus and quickly splits into peripheral branches that diverge toward their separate destinations (see Figure 8–71). Several branches have very restricted local distributions, and bare mention of their names and destinations is all that is required; they comprise the long thoracic nerve (Figure 8–71/9) to the serratus ventralis, the thoracodorsal nerve (Figure 8–71/9′) to the latissimus dorsi, cranial and caudal pectoral nerves (Figure 8–71/3,9′″) to the pectoral muscles (including the subclavius), the subscapular nerve (Figure 8–71/2) to the subscapularis, and the lateral thoracic nerve (Figure 8–71/9″) to the cutaneous trunci and to skin over the ventral part of the thorax and abdomen. The other branches require fuller description. Though some exhibit interspecies differences, these are rarely of importance except in the manus, and even these will be largely disregarded in the meantime.
The suprascapular nerve (Figure 8–71/1) leaves the cranial part of the brachial plexus (C6–C7). It passes between the supraspinatus and subscapularis to reach the cranial margin of the neck of the scapula, around which it winds to the lateral aspect of the bone, where it is expended within the supraspinatus and infraspinatus muscles. Like other nerves directly related to bone, it is vulnerable to injury; in this case it is usually stretched against the scapula when the limb is overabducted or violently retracted. The resulting paralysis of the lateral shoulder muscles does not affect the standing posture but may result in an obvious lateral movement of the shoulder joint (“shoulder slip”) during the stride. The condition occurs most frequently in horses, in which it is also known as “sweeny”; it manifests itself after a time by obvious wasting of the muscles beside the scapular spine.
The musculocutaneous nerve (Figure 8–71/4) is also of cervical origin (C7–C8). After a short course within the axilla the nerve branches off the proximal muscular branch (Figure 8–71/4′), which supplies the coracobrachialis and biceps in the upper part of the arm. In the dog the continuation beyond the proximal muscular branch remains separate from the median nerve until in the distal third of the arm a communicating branch passes distocaudally to the median nerve. The continuing nerve passes under the terminal part of the biceps brachii, where it divides into the distal muscular branch (Figure 8–71/4″), which supplies the brachialis, and the medial cutaneous nerve of the forearm (Figure 8–71/4′″), which crosses the flexor aspect of the elbow before ramifying in skin.
In ungulates generally, the musculocutaneous nerve loops around the axillary artery to join the median nerve in which its identity is for a time submerged; the musculocutaneous fibers again separate from the median nerve in the upper and lower parts of the arm, where they form the proximal and distal muscular branches of the musculocutaneous nerve. In the horse alone the cutaneous branch extends beyond the carpus to the fetlock.
Section of the main musculocutaneous trunk is an unlikely injury; it would paralyze the main flexors of the elbow, although compensation would probably be found from activity of the carpal and digital extensors.
The axillary nerve (C8) (Figure 8–71/6) passes behind the shoulder joint to reach the lateral aspect of the limb. En route it supplies the teres major, teres minor, capsularis, and deltoideus—the true flexors of the shoulder joint. It also supplies twigs to the distal part of the brachiocephalicus, which, it will be recalled, is of deltoid origin. A cutaneous branch supplies skin over the cranial aspect of the arm and forearm.
The three remaining branches of the plexus have the most complicated courses and the most extensive distributions. The radial nerve (Figure 8–71/7) arises from the last two cervical and first thoracic nerves (C7–T1). It first runs distally within the arm, caudal to the brachial artery, before diving between the long and medial heads of the triceps to follow the spiral groove of the humerus, which leads it to the craniolateral aspect of the limb. While buried by the triceps, it supplies branches to the various heads of this muscle (Figure 8–71/7′) and to tensor fasciae antebrachii and anconeus. In the lower part of the arm the radial nerve supplies a further set of muscular branches (Figure 8–71/7″) to all carpal and digital extensor muscles, including the anomalous ulnaris lateralis. A cutaneous branch (Figure 8–71/7′″), often replicated, descends over the craniolateral aspect of the forearm and carpus to reach the dorsal surface of the digits, except in the horse, in which it fades about the level of the carpus because part of the more distal duty is assumed by the musculocutaneous nerve.
Damage to the radial nerve can have three obvious consequences: paralysis of the elbow extensors, paralysis of the carpal and digital extensors, and anesthesia of the skin territory. The combination of all three disabilities points to injury proximal to the middle of the arm, the combination of the second and third points to injury in the distal part of the arm, and a purely sensory deficit suggests injury beyond the origin of the distal motor branches. Injury in the arm is quite common because in places only a thin layer of muscle separates the nerve from the humerus, and it may be involved in fracture or tumor of this bone. Extensive damage to the radial nerve proximal to the origin of the tricipital branches is serious because it prevents fixation of the elbow, prohibiting the limb from bearing weight; the foot is dragged with its dorsal surface on the ground. More distal lesions are less serious because the elbow can be fixed and most animals learn to compensate for paralysis of the forearm muscles by flicking the limb forward and planting the foot before the impetus is lost.
The median nerve (Figure 8–71/5) comes mainly from the last cervical and first thoracic nerves (C8–T1). It runs down the medial surface of the arm caudal to the main artery and enters the forearm over the medial collateral ligament of the elbow joint. It then inclines caudally, passes under the flexor carpi radialis, and maintains this protected situation until it reaches the carpus. It divides in the distal part of the forearm, or within the carpal canal, into two or more divisions that descend through the carpal canal to supply most structures of the palmar part of the foot. The median nerve supplies most of the flexor muscles of the carpus and digit in a pattern that overlaps (but does not quite coincide) with the distribution of the ulnar. Because of this, damage confined to the median nerve does not usually manifest itself through any abnormality of posture or gait.
The ulnar nerve (Figure 8–71/8) leaves the caudal part of the plexus (C8–T2). It runs down the arm beside and possibly (as in the dog) for a stretch united to the median nerve before deviating in the direction of the olecranon to cross the caudal aspect of the elbow joint. Within the arm it detaches the caudal cutaneous antebrachial nerve. The main trunk is severely depleted by detachment of the branches to the carpal and digital flexor muscles in the upper part of the forearm, and the narrow continuation runs down the caudal aspect of the forearm. It finally divides a short distance above the accessory carpal bone. The dorsal branch emerges between the tendons of the ulnar carpal flexor and ulnaris lateralis and descends over the lateral face of the accessory bone to supply the skin on the lateral aspect of the forefoot. The palmar branch continues through the carpal canal and later supplies the interosseous and other small muscles of the foot. It also supplies sensory branches to skin and deeper structures. The distribution within the foot is in close collaboration with the median nerve, partly through combined trunks. The innervation of the forefoot, a topic of considerable practical importance in horses, is later considered separately.
Damage confined to this nerve is unlikely to impair locomotion; the sensory deficits show considerable interspecies variation.
The Thoracic Ventral Rami
These show a more strictly segmental distribution than is found in other regions. The first two contribute to the brachial plexus, but generally the thoracic ventral rami provide the intercostal nerves that run ventrally within the intercostal spaces, either directly below the pleura or between the two intercostal muscle layers; the relation varies according to location and species. Apart from supplying the intercostal muscles, the intercostal nerves detach lateral cutaneous branches that supply a band of skin over the lateral aspect and ventral cutaneous branches that supply the ventral aspect of the chest wall; the more caudal members of the series are also concerned in the supply of the abdominal floor. There are a few minor connections with nerves of the brachial plexus. In the sow, bitch, and cat the lateral cutaneous branches detach twigs to thoracic mammary glands.
The last thoracic ventral branch (costoabdominal nerve) is slightly different in its course and distribution because it runs behind the last rib. It collaborates with lumbar ventral branches in the supply of the flank.
The Lumbar Ventral Rami
The lumbar and sacral ventral rami form a continuous plexus, best developed where the last three or four lumbar and first two sacral nerves form the lumbosacral plexus that supplies the hindlimb. The more cranial lumbar ventral rami have a considerable importance in cattle because they are frequently blocked for abdominal surgery. They are given individual names; in species (including cattle) in which there are six lumbar nerves, the first ventral ramus is known as the iliohypogastric, the second is known as the ilioinguinal, and the third and fourth combine to form the genitofemoral nerve. In species with seven lumbar nerves the first two ventral rami are distinguished as the cranial and caudal iliohypogastric; the third supplies the ilioinguinal and also makes a contribution to the genitofemoral nerve. The genitofemoral nerve divides into a femoral branch that supplies the skin over the medial aspect of the thigh and a genital branch that supplies the spermatic fasciae, the scrotum, and the prepuce.
The caudoventral inclination of the ventral rami that becomes increasingly apparent with the caudal intercostal nerves is further accentuated with the lumbar rami; the locations where the nerves can most easily be reached by injection of local anesthetic solution and the positions of their dermatomes are both considerably more caudal than would naturally be supposed (see Figure 28–2). The nerves pass through the transversus close to the tip of the transverse processes and then run deep to the internal oblique toward the abdominal floor (see Figure 1–37). In addition to supplying the flank and rectus muscles, they detach lateral and ventral cutaneous branches; the former appear subcutaneously at increasingly dorsal levels as the series is followed caudally.
The Lumbosacral Plexus
The lumbosacral plexus that gives origin to the nerves of the hindlimb (with the minor exceptions of those to certain proximal skin areas) is an enhancement of the continuous plexus. It usually begins with the ventral ramus of the fourth lumbar nerve and ends with that of the second sacral (L4–S2); it thus has an additional root in species possessing seven lumbar nerves (Figure 8–72).
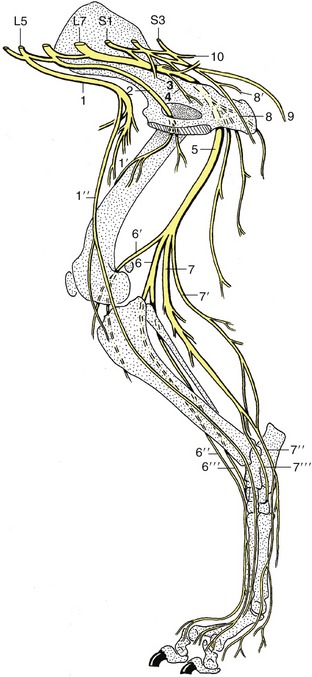
Figure 8–72 The lumbar and sacral nerves of the dog; medial view. 1, Femoral n.; 1′, branches to quadriceps; 1″, saphenous n.; 2, obturator n.; 3, pelvic n.; 4, branch to obturator internus, gemelli, and quadratus femoris; 5, sciatic n.; 6, peroneal n.; 6′, lateral cutaneous sural n.; 6″, superficial peroneal n.; 6′″, deep peroneal n.; 7, tibial n.; 7′, caudal cutaneous sural n.; 7″, medial plantar n.; 7′″, lateral plantar n.; 8, pudendal n.; 8′, deep perineal n.; 9, caudal cutaneous femoral n.; 10, caudal rectal n.
The femoral nerve (Figure 8–72/1) arises from the cranial part (L4–L6) of the plexus and pursues a course through the psoas muscles to reach the gap between the dorsocaudal corner of the flank and the iliopsoas muscle. It is accompanied by the external iliac artery and vein, and on entering the thigh it runs in a protected position between the sartorius and pectineus. It soon detaches the saphenous nerve, and after a very short further course it dives between the rectus femoris and vastus medialis to be expended within the quadriceps mass (Figure 8–72/1′). Severe damage to this nerve, though relatively infrequent, has serious consequences because paralysis of the quadriceps precludes fixation of the stifle joint, which renders the whole limb incapable of supporting weight. No compensation for this defect is possible.
The saphenous nerve (Figure 8–72/1″) gives a branch to the sartorius before continuing to supply skin over the medial aspect of the limb from the stifle to the metatarsus.
The obturator nerve (Figure 8–72/2) has broadly the same origin (L4–L6) as the femoral nerve. It follows the medial aspect of the shaft of the ilium to reach the obturator foramen through which it passes to the adductor muscles of the thigh; the group comprises gracilis, pectineus, adductor, and obturator externus—and obturator internus in ruminants and the pig.* The relationship to bone is potentially dangerous, exposing the nerve to the risk of laceration in fractures and to the risk of compression during calving and foaling (the risk is less in species in which the young are small relative to the pelvic cavity). The effects of injury vary with its extent but are greater in heavier animals and are exaggerated by a requirement to walk on smooth ground, when the limb tends to slip sideways.
The remaining branches of the plexus arise from a common lumbosacral trunk that is largely formed by the last lumbar and first two sacral nerves, along with a smaller contribution from the penultimate lumbar nerve. The trunk leaves the pelvis through the greater sciatic foramen and almost at once detaches three branches.
The short cranial gluteal nerve supplies the tensor fasciae latae, the middle and deep, and in some species part of the superficial gluteal muscles, a group that—contrary to the usual expectation—includes both flexor and extensor muscles of the hip.
The caudal gluteal nerve supplies the superficial gluteal muscle and the vertebral heads of origin of the hamstring muscles (biceps femoris, semitendinosus, and semimembranosus), parts supposed to represent assimilation of elements of the superficial gluteal. It thus supplies extensor muscles of the hip.
The caudal cutaneous femoral nerve (8–72/9) supplies skin over the caudal aspect of the thigh.
The sciatic nerve (Figure 8–72/5) continues the lumbosacral trunk distally, passing between the middle and deep gluteal muscles before turning into the thigh caudal to the hip joint, where it is protected by the greater trochanter of the femur. It then runs between the biceps femoris laterally and the semitendinosus medially before dividing into its terminal branches, the common peroneal and tibial nerves, at a level that varies among species. In the proximal part of its course it detaches twigs to the unimportant internal obturator (except in ruminants and pigs), gemelli, and quadratus femoris (Figure 8–72/4); other muscular branches that may appear to arise directly from the sciatic nerve are usually referred to its common peroneal and tibial divisions.
The common peroneal nerve (Figure 8–72/6), the lesser of the terminal branches, arises from the lumbar roots of the lumbosacral trunk. It runs first with the tibial nerve but separates from this to pass over the lateral head of the gastrocnemius to enter the leg. It detaches a branch, the lateral sural nerve (Figure 8–72/6′), to the skin over the lateral aspect of the leg before dividing into superficial and deep branches when close to the head of the fibula. The superficial peroneal nerve (Figure 8–72/6″) supplies skin over the dorsal aspect of the leg and entire foot, except in the horse, in which it fades about the level of the fetlock joint. The deep peroneal nerve (Figure 8–72/6′″) supplies the dorsolateral muscles of the leg (flexors of the hock and extensors of the digits) and is also sensory to the structures of the foot. Because the sensory innervation of pedal structures varies considerably, the details are deferred until the accounts of individual species.
Paralysis of the common peroneal nerve produces overextension of the hock and flexion of the digits, which may be rested on their dorsal surfaces. The foot may be passively placed to support weight, and in time compensation may be possible (cf. radial paralysis, p. 322). There is also a considerable sensory deficit.
The tibial nerve (Figure 8–72/7) arises from the sacral roots of the lumbosacral trunk. It detaches important proximal muscular branches to the pelvic heads of the hamstring muscles before freeing itself from the parent trunk to enter the leg by passing between the two heads of the gastrocnemius. About this level it first detaches a caudal sural nerve (Figure 8–72/7′) to the skin of this aspect of the leg and later detaches distal muscular branches to the gastrocnemius, soleus, popliteus, and caudal crural muscles. The nerve continues as an almost exclusively sensory trunk (although it will supply short digital muscles) within the fascial plate between the common calcanean tendon and the caudal crural muscles; it ends by dividing into medial and lateral plantar nerves when level with the point of the hock. The plantar nerves (Figure 8–72/7″,7′″) continue into the plantar aspect of the foot to supply sensation to plantar structures chiefly but with some dorsal penetration that varies among species.
Section or severe damage to the tibial nerve is manifested by overflexion of the hock and overextension of the digits. Similar damage to the parent trunk combines the effects of common peroneal and tibial nerve injuries, rendering the limb largely incapable, although fixation of the stifle joint by the unaffected quadriceps may allow it to support some weight.
The Sacral and Caudal Ventral Rami
The sacral ventral rami caudal to and overlapping the roots of the lumbosacral plexus give rise to other important individual nerves. The pelvic nerves (Figure 8–72/3) composed of the parasympathetic outflow are considered in the following section.
The pudendal nerve (Figure 8–72/8) arises variously (S1–S3 in the dog, S2–S4 in ruminants, S[2]3–S4 in the horse). It is sensory to the rectum, internal and external reproductive organs, and perineal skin and motor to much of the striated perineal musculature. It has both physiological and applied importance, but because it is variable, it must for the present suffice to say that its course takes it obliquely through the pelvis toward the ventral part of the outlet (see Figure 29–5/7). It provides deep and superficial perineal nerves in addition to various cutaneous branches and finally continues as the dorsal nerve of the penis (or clitoris). The superficial perineal branch supplies the skin of the anus, vulva, and ventral perineal region, the strict perineal location.
The deep perineal nerve supplies the ventral part of the striated musculature of the perineum, particularly that of the reproductive organs. The main trunk also supplies branches to the skin of the prepuce and scrotum in the male and of the caudal part of the udder in the ungulates.
The caudal rectal nerves (Figure 8–72/10) arise from the most caudal sacral nerves, sometimes overlapping the origin of the pudendal nerve. They supply sensory fibers to the rectum, anus, and perianal skin, and motor fibers to the dorsal perineal striated musculature, including the levator ani. The division of territory between these nerves and the pudendal is rather variable.
The ventral rami of the caudal nerves supply the ventral or depressor muscles of the tail.
THE PERIPHERAL AUTONOMIC NERVOUS SYSTEM
Although the appropriate regulation of “visceral” activities clearly presumes the existence of receptors in the viscera and vessels, the autonomic nervous system was originally defined as wholly efferent. This offers a certain convenience because visceral afferent pathways are in general indistinguishable in structure and arrangement from their somatic counterparts. The visceral efferent pathways, on the other hand, are clearly distinguished, particularly by the location of the last neuron in the chain within a peripheral ganglion and by the restriction of the neurons that drive these ganglion cells to specific nuclei of the brainstem and particular regions of the cord (Figures 8–73 and 8–74). The peripheral efferent pathway thus consists of a preganglionic (myelinated, and therefore white) fiber and a postganglionic (little myelinated, and therefore gray) fiber. Moreover, certain anatomical, physiological, and pharmacological features distinguish two contrasting—sympathetic and parasympathetic—efferent systems, whereas no similar distinction is possible for the visceral afferent fibers presumed to be included in all cranial and spinal nerves (if only because of the ubiquitous distribution of blood vessels). It has been shown (p. 304) that cerebrospinal (somatic) and autonomic (visceral) mechanisms cannot be entirely separated because the cerebral cortex directs both types.
Figure 8–73 Origin and distribution of the parasympathetic nervous system. Ventral view, schematic. 1, Parasympathetic oculomotor nucleus; 2, rostral and middle parasympathetic nuclei of the medulla oblongata; 3, dorsal vagal nucleus; 4, sacral outflow; 5, vagus nerve; 6, recurrent laryngeal nerve; 7, parasympathetic fibers to heart and lungs; 8, ventral vagal trunk; 9, dorsal vagal trunk; 10, parasympathetic fibers to the abdominal organs; 11, pelvic nerves.
Figure 8–74 Origin and distribution of the sympathetic nervous system. Ventral view, schematic. The parasympathetic nuclei in brain and spinal cord are indicated in gray. 1, Sympathetic outflow from T1 to L3; 2, communicating branches; 3, 4, sympathetic trunk; 5, cranial cervical ganglion; 6, cervicothoracic ganglion; 6′, middle cervical ganglion; 6″, ansa subclavia; 7, vertebral n.; 8, greater splanchnic n.; 9, lesser splanchnic nn.; 10, celiac ganglion; 11, cranial mesenteric ganglion; 12, caudal mesenteric ganglion; 13, hypogastric n.
Some contrasting physiological actions of the two systems are summarized later (p. 331), but it may be said now that they partly rest on the use of norepinephrine as the mediating substance at the last synapse of the sympathetic pathway, while acetylcholine is used at the corresponding parasympathetic synapse. Epinephrine is produced by the adrenal medulla, and when generally diffused by the bloodstream, it evokes a mass sympathetic response. Acetylcholine is liberated and destroyed locally. The activities of the parasympathetic system therefore tend to be more specific and discrete than those of the sympathetic system. The narrower localization of parasympathetic responses is further assisted by the location of parasympathetic ganglia close by or even within the target organ, whereas the sympathetic ganglia are closer to the central nervous system and the postganglionic fibers radiate more widely.
THE PARASYMPATHETIC SYSTEM
The preganglionic cells of the parasympathetic system are restricted to a number of discrete nuclei within the brainstem and to the lateral column of a short stretch of the spinal cord, generally the second, third, and possibly fourth sacral segments (see Figure 8–73). The aptly designated craniosacral outflow is confined to the oculomotor, facial, glossopharyngeal, vagus, and pelvic nerves.
The cranial parasympathetic pathways have rather limited anatomical independence. Varying parts of their courses are incorporated within nerves of predominantly somatic composition. Exclusively parasympathetic bundles are found only close to the target organs. The chief, grossly visible features of the cranial parasympathetic outflow have been described with the relevant nerves, and it now remains to draw these threads together.
The most rostral parasympathetic nucleus, the parasympathetic oculomotor nucleus, lies within the midbrain in association with the motor nucleus of the third cranial nerve. The parasympathetic preganglionic fibers emerge from the main trunk within the orbit to constitute the oculomotor (short) root of the ciliary ganglion. Beyond the ganglion, the postganglionic fibers proceed as the short ciliary nerves, which also incorporate sympathetic and sensory fibers; these nerves penetrate the sclera to form the ciliary plexus from which the parasympathetic fibers extend to the ciliary and pupillary sphincter muscles (Figure 8–70/6,10).
The parasympathetic component of the facial nerve originates in the rostral parasympathetic (salivatory) nucleus of the medulla oblongata (Figure 8–70/2). The preganglionic fibers are incorporated within the main facial trunk, run through the somatic geniculate ganglion without interruption, and later leave in the chorda tympani and the greater petrosal nerve (Figure 8–70/11,13). The chorda tympani introduces its complement to the lingual nerve from which the parasympathetic fibers later emerge to synapse within the mandibular ganglion; the postganglionic fibers supply the mandibular and sublingual salivary glands.
The greater petrosal nerve is joined by the deep petrosal (sympathetic) nerve (Figure 8–70/12) to constitute the nerve of the pterygoid canal, which leads to the pterygopalatine ganglion (Figure 8–70/7). The postganglionic fibers join the lacrimal nerve (after passage through the zygomatic nerve) en route to the lacrimal gland and various other branches of the maxillary nerve en route to glands within the nasal and palatine mucosae.
The parasympathetic component of the glossopharyngeal nerve originates from the middle parasympathetic nucleus in the medulla oblongata (Figure 8–70/3). The preganglionic fibers pass through the somatic ganglion of this nerve before joining the tympanic plexus; from this they proceed to the otic ganglion (Figure 8–70/9). The postganglionic fibers are carried via the pterygoid nerve and a communicating branch of the auriculotemporal nerve to the parotid gland.
The parasympathetic component supplies the bulk of the vagus nerve; indeed it is the whole complement distal to the origin of the recurrent laryngeal nerve (Figure 8–73/5,6). The preganglionic fibers proceed to numerous small ganglia scattered along the nerve plexuses that supply and are often located within the tissues of the target organs. The plexuses include the cardiac and pulmonary plexuses within the chest (Figure 8–73/7) and the gastric, hepatic, mesenteric, gonadal, and renal plexuses formed by the confluence of branches of the vagal trunks with sympathetic nerves within the abdomen (Figure 8–73/10). Broadly, the dorsal vagal trunk supplies hepatic and gastric plexuses, and the larger ventral vagal trunk supplies celiac, mesenteric, renal, and gonadal plexuses.
The fibers of the sacral parasympathetic outflow are initially incorporated in certain sacral ventral rami from which they emerge to constitute the pelvic nerves (Figure 8–73/11). These form a retroperitoneal plexus that is joined by sympathetic fibers delivered by the hypogastric nerves that descend from the caudal mesenteric ganglion. Numerous minute ganglia are found scattered in the plexus, whereas other (terminal) ganglia are embedded within the walls of predominantly pelvic viscera: the descending colon, rectum, bladder, uterus, and vagina (in the female); accessory reproductive glands (in the male); and the genital erectile tissue. The parasympathetic pathways have their peripheral synapses exclusively in the terminal ganglia, whereas some sympathetic peripheral synapses are divided among the plexus and terminal ganglia.
THE SYMPATHETIC SYSTEM
The preganglionic fibers of the sympathetic system take their origin from the lateral column of the thoracolumbar part of the spinal cord (Figure 8–74/1) and pass into the ventral roots of the thoracic and first several lumbar nerves. They continue into the spinal nerves and then issue from the ventral rami, constituting the white communicating branches, which join the ganglia of the sympathetic trunks (Figure 8–53/5,7). The bilateral trunks run the length of the neck and back, and each have a basically segmental arrangement, although strict correspondence of the ganglia with spinal nerves is evident only in the thoracic and cranial lumbar regions.
The cervical part of the trunk begins at the large, spindle-shaped, cranial cervical ganglion placed close to the base of the skull (Figure 8–74/5). The trunk is associated with the vagus within the carotid sheath and forms the vagosympathetic trunk that proceeds down the neck. The two components part company at the entrance to the chest, where the sympathetic trunk often bears a middle cervical ganglion by the first rib (Figure 8–75/7′). The trunk then continues subpleurally, over the line of the costovertebral articulations, before passing dorsal to the diaphragm to gain admission to the abdomen. Its thoracic part shows a regular arrangement of ganglia, although the first one or two are fused with a caudal cervical element to form the large cervicothoracic ganglion deep to the head of the first rib (Figure 8–75/7). The lumbar part of the trunk, which lies between the psoas musculature and vertebral bodies, at first also carries a regular complement of ganglia, but the arrangement later becomes more erratic in that some caudal lumbar ganglia split into two or, less commonly, fuse with their neighbors. The sacral part is even less regular and may fuse, temporarily or finally, with its fellow before extending into the tail, where it rapidly fades (Figure 8–74/3).

Figure 8–75 Distribution of sympathetic (black) and parasympathetic (dotted yellow) nervous systems, semischematic. 1, Parasympathetic oculomotor nucleus; 2, salivatory nuclei (rostral and middle parasympathetic nuclei); 3, dorsal vagal nucleus; 4, cranial cervical ganglion; 5, vagosympathetic trunk; 6, vertebral nerve; 7, cervicothoracic ganglion; 7′, middle cervical ganglion; 8, ansa subclavia; 9, sympathetic outflow from spinal cord; 10, sympathetic trunk with paravertebral ganglia; 11, celiac ganglion; 12, cranial mesenteric ganglion; 13, caudal mesenteric ganglion; 14, vagus nerve with distribution to thoracic and abdominal organs; 15, sacral outflow of parasympathetic nervous system.
Because the sympathetic outflow is restricted, it follows that only the thoracic and cranial lumbar ganglia are joined by white communicating branches. However, all spinal and many cranial nerves are joined by bundles (gray communicating branches) of postganglionic fibers destined for vessels, skin glands, and so forth. It should be stressed that the body wall and limbs are innervated only by these postganglionic sympathetic fibers. Those to most cervical nerves join within a single trunk, the vertebral nerve, which runs from the cervicothoracic ganglion through the foramina of successive cervical transverse processes (Figure 8–74/7). The postganglionic sympathetic fibers to the first two cervical nerves and to cranial nerves extend from the cranial cervical ganglion; many form the internal carotid nerve that follows the like-named artery.
Several alternative fates are open to the preganglionic fibers that enter the sympathetic chain, each to project on many ganglion cells. Some fibers synapse immediately within the local ganglion, others run cranially or caudally within the trunk to synapse within ganglia that are more cranial or caudal in the series, and yet others pass uninterruptedly through the trunk to proceed to a second set of (prevertebral) ganglia placed about the origin of the visceral branches of the abdominal aorta (Figures 8–74/10,11 and 8–75/11,12). This last group constitutes the splanchnic nerves, which are rather variable in arrangement; usually one greater splanchnic nerve is formed by preganglionic fibers that leave the trunk from about the sixth to the penultimate thoracic ganglia with lesser thoracic and lumbar splanchnic nerves arising at more caudal levels (Figure 8–74/8,9).
The viscera and vessels of the head receive their sympathetic innervation via the cranial cervical ganglion. The postganglionic fibers that emerge from this ganglion radiate in a number of directions that carry them into the territories of the cranial and first two cervical nerves. Though many pass through parasympathetic ganglia, they of course do so without interruption. The details are of rather limited clinical importance (although relevant to experimental work), and only a few points are presented here (see Figure 8–70).
One large group of fibers follows the internal carotid artery into the cranial cavity and there provides twigs to the intracranial vessels and fiber bundles that join various nerves, especially the trigeminal and those to the extraocular muscles. Another group of fibers passes through the ciliary ganglion to the eyeball for ultimate distribution to the dilator pupillae. At a more proximal level, the internal carotid nerve gives off the deep petrosal nerve, which combines with the greater petrosal nerve (Figure 8–70/11) in its passage through the pterygoid canal to the pterygopalatine ganglion (Figure 8–70/7). These fibers are ultimately dispersed with the various nerves that supply structures within the orbit, nasal cavity, sinuses, and palate.
Other branches concur with parasympathetic fibers in forming a plexus within the tympanic cavity from which the parotid gland is supplied after passage beyond the otic ganglion. Yet other bundles of fibers entwine the external carotid artery and its branches.
The thoracic organs—heart, trachea, and lungs—are supplied by postganglionic fibers that form cardiac and pulmonary plexuses within the mediastinum after leaving the thoracic portion of the sympathetic trunk. These plexuses combine with the corresponding parasympathetic component (see Figure 8–75).
The abdominal and pelvic organs receive their sympathetic innervation through the various splanchnic nerves that lead to the celiac, cranial mesenteric, renal, aorticorenal, gonadal, and caudal mesenteric ganglia placed on the ventral face of the aorta by the origins of the visceral arteries. The preganglionic fibers synapse in these ganglia and in the postganglionic fibers that emerge from intricate plexuses (combining vagal contributions) that enmesh, and run parallel to, the visceral arteries from which they obtain their names (Figure 8–76).
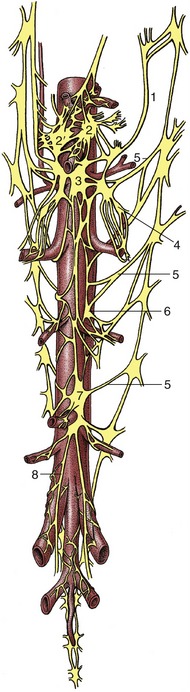
Figure 8–76 Ganglia and plexuses of the abdominal cavity. Ventral view. 1, Greater splanchnic n.; 2, left celiac ganglion; 2′, right celiac ganglion; 3, cranial mesenteric ganglion; 4, renal ganglion; 5, lumbar splanchnic nn.; 6, gonadal ganglion; 7, caudal mesenteric ganglion; 8, right hypogastric n.
The pelvic organs are supplied with postganglionic fibers that leave the caudal mesenteric ganglion within the paired hypogastric nerves (Figure 8–76/8). These enter the pelvic cavity below the peritoneum to form a common pelvic plexus with the parasympathetic pelvic nerves (Figure 8–75). As already mentioned, the sympathetic contribution to the pelvic plexus includes preganglionic fibers that have deferred their synapses to peripheral locations within the pelvis.
SUMMARY OF AUTONOMIC INNERVATION
Certain effects of the autonomic nervous system are tabulated (Table 8–3) by way of illustration, but for more controversial points, such as the innervation of the bladder and urethra, or those requiring more detailed description, the reader is referred to modern works of physiology.


