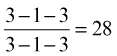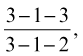11 The Head and Ventral Neck of the Dog and Cat
This chapter is the first of a series that covers the regional anatomy of the two companion animals, the dog and cat. Although the dog and cat are included in different suborders of Carnivora (Canoidea and Feloidea, respectively), the general anatomies are sufficiently alike for it to be possible to consider them together. Although cats rival and in many countries now surpass dogs in popularity, it is both conventional and convenient (because of the greater wealth of literature) to base the initial accounts on the dog and follow these with mention of the clinically significant differences in the cat. Dogs of course differ considerably among themselves, and where no specific breed features are mentioned, it may be assumed that the description refers to animals of moderate size and generalized conformation, such as are represented by the Beagle.
The reader is also reminded that the systemic chapters are largely based on the anatomy of the dog, which supplies the bulk of their illustrations. To facilitate review, page and figure references to this material will be found under many subheadings in the chapters that now follow.
CONFORMATION AND EXTERNAL FEATURES
Conformation varies much more considerably in dogs than in other domesticated species. The preferences of fanciers have produced a variety of breeds that are strikingly different from each other and from their common wolf ancestor. The current popularity of purebred cats has increased awareness of the variation between breeds, even if the differences are much less considerable than among dogs. In both species this variation is nowhere better expressed than in the head.
The appearance of the dog’s head is largely determined by the shape of the skull, the position and size of the eyes, and the form and carriage of the ears. The ears may be held erect, hang from the side, or have an intermediate carriage that is erect at the base and pendulous toward the tip. Certain differences are permanent attributes of a breed, but others are no more than temporary expressions of mood.
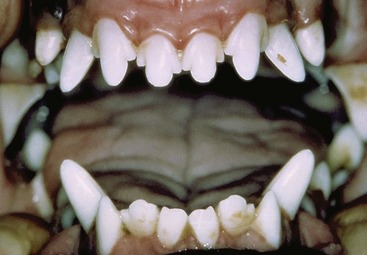
The skull of the adult dog is characterized by a well-developed facial part, large orbits and temporal fossae, incomplete postorbital bars, prominent tympanic bullae, and the absence of supraorbital foramina. It is widest behind the eyes, where the zygomatic arches are widely spread. Breed differences in the skull largely relate to the relative length of the facial part. Dolichocephalic, brachycephalic, and mesaticephalic or mesocephalic (long, short, and intermediate head length, respectively) breeds are recognized (Figure 11–1). In dolichocephalic breeds like the Greyhound, the head is long and narrow. The dorsal surfaces of nose and cranium form two nearly parallel planes that are divided at the level of the eyes by a break (nasofrontal angle or stop) where the cranium descends to the level of the nose. The long facial part is often accompanied by an underbite jaw (brachygnathism). The external sagittal crest is well developed for attachment of the temporal muscles, and the zygomatic arches project less than in the other groups. In brachycephalic breeds, like the English Bulldog and the Pekingese, the facial part is short and the cranium wide and globular. The stop is pronounced, and the dorsal surface of the cranium is convex and has a much reduced external sagittal crest. In some breeds the fontanelles remain open throughout life. Numerous skin folds mark the face, and the eyes are widely spaced. Brachycephalic breeds are most often prognathic; the term indicating that the lower jaw protrudes in front of the upper jaw (Figure 11–1, C). Most breeds belong to the mesaticephalic type, in which the length of the skull is more harmoniously proportional to its width.
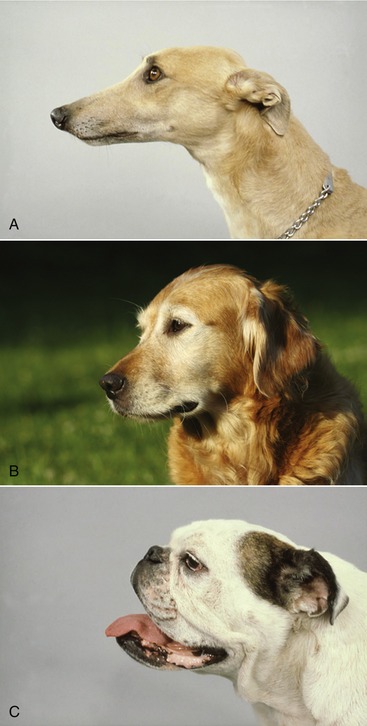
Figure 11–1 Representatives of dolichocephalic (A), mesaticephalic (B), and brachycephalic (C) breeds.
The face of the dog is more expressive of emotion than that of other species, and everyone is familiar with the signs that indicate aggressive intent (Figure 11–2), submission, or pain, even if unable to particularize them. Age is also clearly revealed in dogs of pigmented coat by a “graying” that begins at the upper lip and later spreads, reaching the area about the eyes by about the eighth year or a little later (Figure 11–3).
Redundancy of facial skin is a feature of several breeds such as the Bulldog, Shar Pei (Figure 11–4), and Bloodhound. In extreme form it may result in frontal folds that obscure the vision, and because the upper eyelid is turned inward (entropion), it may irritate the cornea through contact with hairy skin.
In cats, in contrast to the breeds of dogs recently mentioned, the bare sufficiency of the skin of the scalp creates problems when it is necessary to close large wounds. The cat’s head also exhibits features distinctive of breed or type. In most cats the face is relatively short, but in certain Oriental breeds, especially the Siamese, it is proportionally longer and the whole head is more wedge-shaped with a less pronounced stop. In contrast, Persian cats have very short “pushed-in” faces; when exaggerated, this trait may be associated with blockage of tear ducts, leading to persistent weeping. The eyes and orbits are relatively large and face more directly forward than those of dogs, providing a wider field of binocular vision (see Figure 9–1). The ears are wide at the base and are carried erect, except in the Scottish Fold in which the distal part of the pinna flops. The contrast between the rather short, rounded ears of most European breeds and the larger pointed ears of the Oriental has little practical importance but contributes much to breed “character.” Tactile hairs (whiskers) are prominent (see Figure 10–11).
SUPERFICIAL STRUCTURES
Much of the surface of the skull can be palpated because it is either directly subcutaneous or covered only by a thin layer of muscle. Palpable features of the face include the infraorbital and mental foramina and the ridge over the long root of the upper canine tooth. In the cat the infraorbital foramen is small and not easily found on palpation; it lies very close to the orbit.
MASTICATORY MUSCLES
The masticatory muscles are massive; the temporalis and masseter remove the lateral plate of the frontal and parietal bones and the ramus of the mandible from direct reach. The boundary between these muscles is provided by the zygomatic arch, a relatively vulnerable region of the skull prone to traumatic separation at the oblique suture between the zygomatic and temporal bones (see Figure 2–34).
SKULL
The brain case is surmounted by the sagittal crest and by the nuchal crest, which connects the caudal end of the sagittal crest with the base of the ear, providing the dorsal boundary of the triangular caudal (nuchal) surface of the skull. Both crests are palpable, although little of the nuchal surface can be appreciated. In the puppy’s skull the cranial exceeds the facial part in size, being relatively much wider than it is in the adult (see Figure 1–18); the sagittal crest has yet to form, and the nuchal crest, although visible on the skull, is not palpable. The fontanelle, characteristic of the neonatal skull, may persist into adult life in certain toy breeds in which it remains a palpable feature. The ventral border of the mandible and the prominent angular process at its caudal end are easily palpated. The halves of the mandible meet in a cartilaginous joint that persists throughout life.
SALIVARY GLANDS AND LYMPH NODES
The parotid and mandibular glands and the mandibular lymph nodes can be palpated caudal to the mandible. The mandibular gland is embraced by the maxillary and linguofacial veins, which join to form the external jugular vein. The parotid duct (Figure 11–6, A/8-B/8) crosses the masseter, midway between two branches of the facial nerve; it can sometimes be palpated before it passes deep to the communicating nerves and the facial vessels to open into the cheek cavity. Accessory lobes of the parotid gland may accompany the duct. The end of this duct is occasionally transplanted into the conjunctival sac when the flow of tears is insufficient to keep the conjunctiva moist.
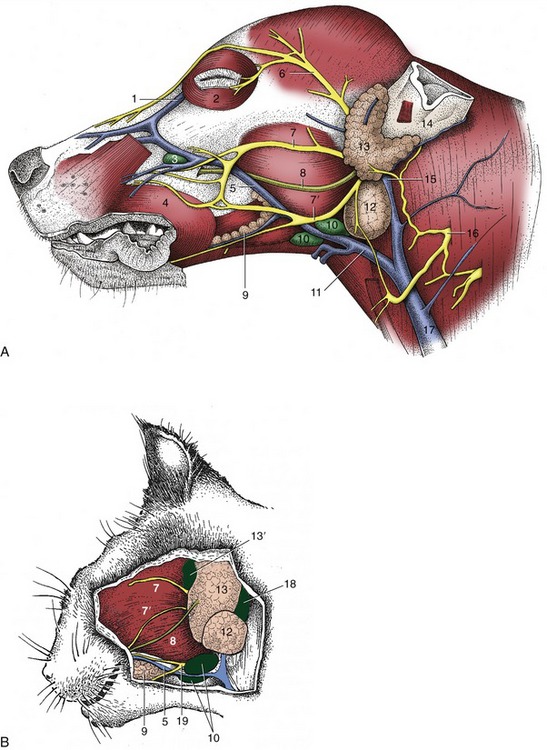
Figure 11–6 Superficial dissection of the canine (A) and feline (B) heads. 1, Angularis oculi vein; 2, orbicularis oculi; 3, facial lymph node; 4, orbicularis oris; 5, facial vein; 6, auriculopalpebral nerve; 7,7′, dorsal and ventral buccal branches of facial nerve; 8, parotid duct; 9, buccal salivary glands; 10, mandibular lymph nodes; 11, linguofacial vein; 12, mandibular gland; 13, parotid gland; 13′, parotid lymph node; 14, base of ear; 15, maxillary vein; 16, second cervical nerve; 17, external jugular vein; 18, retropharyngeal lymph node; 19, facial nerve, ventral branch.
SUPERFICIAL VESSELS
The linguofacial vein is short (Figure 11–6, A/11-B/11). In the dog, the left and right lingual vein unite and form the superficially situated hyoid arch; in the cat, this arch is formed by the left and right linguofacial veins.The facial vein, when followed rostrally, first passes over the mandibular lymph nodes and then along the ventral border of the masseter before crossing the face obliquely. It arises from the fusion of prominent dorsal nasal and angularis oculi veins rostral to the eye. These run the risk of injury when surgical access is made to the nasal cavity and frontal sinuses. The angularis oculi vein, which emerges from the orbit, is also vulnerable during enucleation (removal) of the eye. The facial artery and accompanying vein serve the lips, cheek, and muzzle. The side of the nose is supplied by an artery that emerges from the infraorbital foramen.
SUPERFICIAL NERVES
The distribution of the cutaneous nerves follows the general pattern (see Figures 8–68 and 8–69). The dorsal branch (Figure 11–6, A/7-B/7) of the facial nerve runs across the dorsal half of the masseter; the ventral branch takes the more protected course along the ventral edge. They are joined together by communicating branches at the rostral border of the muscle. The auriculopalpebral branch of the facial nerve (Figure 11–6/6) passes across the zygomatic arch, where it can be blocked to eliminate blinking (m. orbicularis oculi) during examination of the eye.
THE NASAL PLATE, NASAL CAVITY, AND PARANASAL SINUSES
THE EXTERNAL NOSE
The moist bare skin around the nostrils, the nasal plate, is divided by a median philtrum that continues ventrally to groove the upper lip (see Figure 4–1). The nasal plate is covered with a thick keratinized epidermis. In cats its surface is made up of fine tubercles, but in dogs it is made of irregularly formed plaques and sulci that create a pattern that is believed to be individual and therefore available as a means of identification (nose printing). The nasal plate of dogs is kept moist by an overflow of the secretion of glands of the nasal cavity, principally the lateral nasal glands (pp. 148 and 381). There are no local glands in the plate.
A curved alar cartilage supports the roof and the wing of the nose. The floor is strengthened by a small accessory nasal cartilage. The wing, the thickened dorsolateral portion of the nostril, is the most mobile part. The nostrils of dogs are comma-shaped: the tail curves laterally beneath the wing. It is suggested that this separation of the wing from the floor of the nostril allows directional scenting (Figure 4–1). The alar fold is an extension of the ventral nasal concha that terminates within the nasal vestibule at a bulbous enlargement fused with the wing of the nostril.
Congenital malformation of the nasal plane is a common finding in brachycephalic dogs and Persian cats. In this condition the cartilage supporting the nostrils is too weak; the resulting collapse of the wings narrows the nostrils, especially during inspiration. This condition can be relieved by surgery, in which parts of the alar folds are removed. The tissue is highly vascular and bleeds profusely when cut.
THE NASAL CAVITY
The nasal cavity extends from the nostrils to the level of the eyes. Its rostral part, the nasal vestibule, is roughly tubular; caudal to the level of the infraorbital foramen, it widens and gains in height (Figure 11–7). The nasal vestibule is occupied by the alar fold.
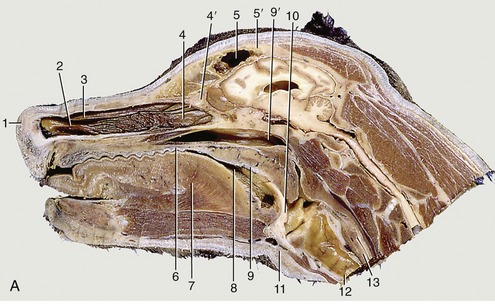
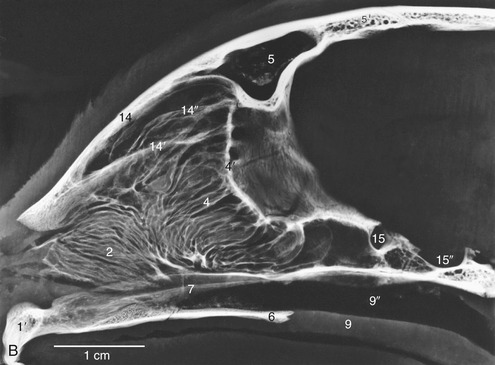
Figure 11–7 Paramedian section of the canine head (A) and tomogram of the feline nasal cavity (B). 1, Right nostril; 2, ventral nasal concha; 3, dorsal nasal concha; 4, ethmoidal conchae; 4′, cribriform plate; 5, frontal sinus; 5′, frontal bone; 6, hard palate; 7, tongue; 8, oropharynx; 9, soft palate; 9′, nasopharynx; 10, epiglottis; 11, basihyoid; 12, trachea; 13, esophagus; 14, nasal bone; 14′, horizontal crest of nasal bone; 14″, dorsal part of nasal cavity invaded by ethmoidal conchae; 15, optic canal; 15′, hypophysial fossa.
The nasal cavity is divided into two halves by the nasal septum. In dogs, only the caudal and dorsal parts of the septum ossify; the rostral extremity projecting beyond the skull remains cartilaginous and accounts for the passive mobility of the tip of the nose. The middle section of the septum is membranous. A cat’s nose is not actively mobile, and its cartilages resemble shortened canine nasal cartilages.
In dogs, the cavity is more tightly filled with nasal and ethmoidal conchae than in other species, and the intervening meatuses are narrow. The rostral half lodges the dorsal and ventral conchae. The dorsal one (Figure 11–7/3) is a simple plate where it arises from the nasal bone; it widens caudally where it attaches to the ethmoid. The ventral concha, which is thick but short, arises from the maxilla and breaks up to form many scrolls that greatly enlarge the area that is covered by a richly vascularized mucosa (Figure 11–7/2). It extends from the level of the first to the third premolar teeth and is attached to the conchal crest on the medial surface of the maxilla. This crest creates a linear shadow that is a very distinctive radiographic feature (Figure 11–7, B/14′). The ventral concha is continued rostrally by the alar fold. The caudal half of the nasal cavity is almost filled by ethmoidal conchae covered with olfactory mucosa. These conchae also invade the lower part of the frontal sinus. The olfactory mucosa in the German Shepherd reportedly covers an area of 150 cm2 and possesses more than 20 million receptors. The appearance of the olfactory membrane differs little from the remainder of the mucous membrane, although it may be slightly thicker and grayer. Collectively, the ethmoidal conchae are larger than the nasal conchae, which is an indication of the dog’s keen sense of smell (see Figure 11–10/11).
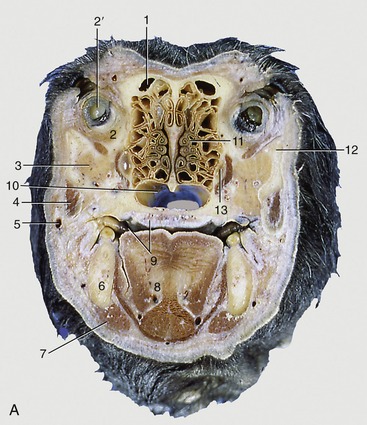
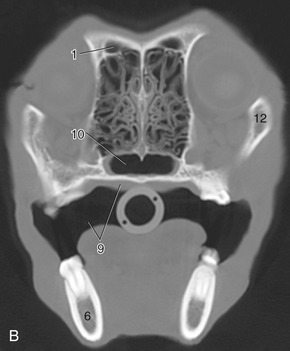
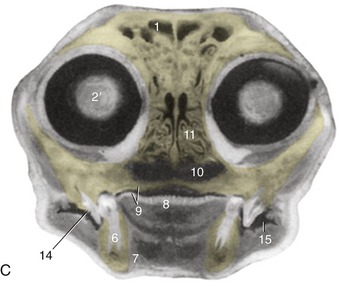
Figure 11–10 Transverse section of the canine (A) and feline (C) heads through the rostral part of the orbit, rostral surface. B, CT scan (bone window) of canine head at the level of A. 1, Frontal sinus; 2, orbital structures; 2′, eye; 3, zygomatic gland; 4, masseter; 5, facial vein; 6, mandible; 7, digastricus; 8, tongue; 9, oral cavity and hard palate; 10, choana; 11, ethmoidal conchae; 12, zygomatic arch; 13, maxillary recess; 14, sectorial teeth, P4 engaging M1; 15, oral vestibule.
The nasal cavity of cats resembles the one of brachycephalic dogs. However, the ventral nasal concha is smaller, compensated for by enlargement and development of the middle concha and its lamellae. The middle concha reaches to the level of the entrance of the maxillary recess that it covers.
In both species, the nasolacrimal duct (Figure 11–8) opens where the floor of the vestibule meets the alar fold and is visible when the nostril is spread. As often as not, there is a second, more caudal opening level with the canine tooth. The duct is described more extensively later. The duct of the lateral nasal gland opens at the rostral end of the dorsal nasal concha, but as it is only about 0.5 mm in diameter, it can be difficult to identify, even at dissection. The gland lies in the lateral nasal wall close to the entrance of the maxillary recess. Its secretion may have a social significance that accounts for the nose-to-nose sniffing common when dogs meet. In cats, the lateral nasal gland and its duct are not visible macroscopically; the secretion is mucous instead of serous.
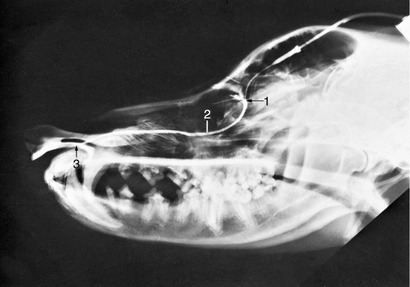
Figure 11–8 Contrast medium outlining the canine nasolacrimal duct in a radiograph. 1, Position of ventral punctum; 2, nasolacrimal duct; 3, opening of duct at the nostril.
A few much smaller nasal glands found on the rostral part of the septum open at the caudal limit of the vestibule and contribute marginally to the wetness of the nose.
The watery secretions of the lacrimal, lateral nasal, and scattered minor nasal glands moisten the nasal plate. As is well-known, a moist nose is generally regarded as a sign of health in dogs.
The nasal cavity has an extremely good blood supply from both the external and the internal carotid arteries; anastomoses occur between the internal carotid artery and the maxillary arteries (the main branch of the external carotid artery) of both sides. The maxillary artery is the major supply to the nasal cavity. Ligation of the external carotid artery in dogs (in cases of persistent nose bleeding) gives rise to collateral connections between corresponding vessels of both sides.
THE PARANASAL SINUSES
The sinus system of the dog is poorly developed. The largest sinus is the frontal one, which occupies much of the frontal bone including its zygomatic process, which is separated from its fellow by a median septum. It may extend to the level of the temporomandibular joints in larger animals (especially if long-headed) (Figure 11–11). Each sinus is composed of three cavities (lateral, medial, and rostral), which communicate separately with the nasal cavity via nasofrontal openings (ethmoidal meatuses). The lateral compartment is the largest and may be subdivided by incomplete septa; ethmoturbinates are present in its rostral part. The medial and rostral compartments are also filled with ethmoturbinates, which hampers their identification on radiographs. The ethmoturbinates are covered with olfactory mucosa, in contrast to the sinus walls that are lined with nonolfactory mucoperiosteum. The sinuses are smaller and may even be absent in brachycephalic breeds. Absence is not associated with clinical signs and is usually only found when radiographs are made for another reason.
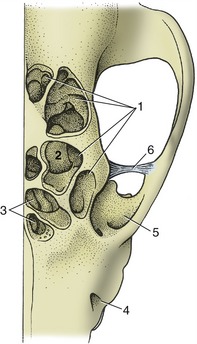
Figure 11–11 The canine frontal sinuses, dorsal view. 1, Lateral frontal sinus; 2, ethmoidal concha invading the sinus; 3, medial and rostral frontal sinuses; 4, infraorbital foramen; 5, orbit; 6, orbital ligament.
The sinus system of the cat comprises frontal, sphenoidal, and maxillary compartments, among which the frontal is the most important (Figure 11–7, B, and Figure 11–12/1). It occupies the same general position as the corresponding sinus in the dog, but it is undivided and extends rather far ventrally within the medial wall of the orbit. The communication with the nasal cavity is in its rostral part and may provide ineffective drainage in the bacterial sinusitis that commonly complicates viral infections of the upper respiratory tract. Surgical drainage may then be required. In mature cats, the sinus can be surgically approached just lateral to the midline, on the line connecting the rostral margins of the supraorbital processes. In 3 to 4 month-old kittens the approach is made midway between the line connecting the rostral margins of the supraorbital processes and that connecting the medial angles of the eyes.
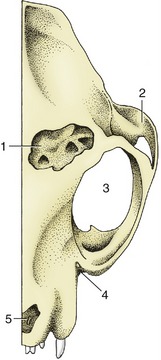
Figure 11–12 The feline frontal sinus, dorsal view. 1, Frontal sinus, opened; 2, zygomatic arch; 3, orbit; 4, position of infraorbital foramen; 5, nasal aperture.
In both dogs and cats, the maxillary sinus (Figure 11–10/13) communicates so freely with the nasal cavity that the term nasal recess is preferred. It is not a true sinus because it is not formed between two plates of maxillary bone but is bounded by the maxilla laterally and the ethmoid medially. The recess occupies the face immediately rostral to the orbit, above the roots of the last three cheek teeth, and communicates with the middle meatus by a wide nasomaxillary opening flanked by the nasal conchae. The recess houses on its lateral wall the broad, flat, lateral nasal gland, which appears as a thickening of the mucosa. Root abscesses of the sectorial tooth P4 may break into the recess and later onto the surface of the skull. Surgical drainage is most conveniently achieved by the extraction of the sectorial tooth to open a passage to the mouth; the presence of the infraorbital canal makes the direct lateral approach unwise.
In cats, a small sphenoidal sinus is present; the similar cavity found in dogs is filled with ethmoturbinates.
THE MOUTH
The wide gape of carnivores is made possible by the caudal situation of the angles of the mouth and the correspondingly short cheeks. The interior of the mouth, including the oropharynx, is therefore easily examined. The edge of the lower lip carries blunt papillae. The upper lip is pendulous and presses on the lower one, which is everted near the commissure in certain breeds with ample head skin, such as the Spaniel (Figure 11–6, A, and Figure 11–13). The resulting folds predispose to infection. The general looseness of the lips creates a large vestibule—an advantage when administering liquid medicines, which then escape behind the cheek teeth into the central cavity.
The ducts of the parotid (Figures 11–6, A/13-B/13 and 11–14/3) and zygomatic (Figures 11–10/3 and 11–27/8) salivary glands open into the vestibule: the former by a single orifice in a small papilla opposite the upper fourth premolar P4, and the latter by a row of four or five orifices on a mucosal ridge a little farther caudally. The ducts of the mandibular and compact (monostomatic) sublingual glands open to the floor of the mouth at the sublingual caruncle. They run below the mucous membrane that connects the side of the tongue with the gums; when a duct is damaged, saliva may escape to form a large mucosal swelling (ranula) lateral to the tongue. The larger salivary ducts are occasionally cannulated to remove obstructions or to inject a contrast medium for radiographic examination (sialography, Figure 11–15).
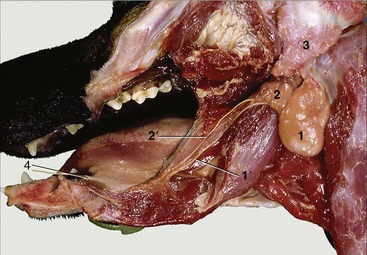
Figure 11–14 Salivary glands. 1, Mandibular gland; 1′, mandibular duct; 2, sublingual gland, monostomatic part; 2′, its duct; 3, parotid gland; 4, sublingual caruncle.
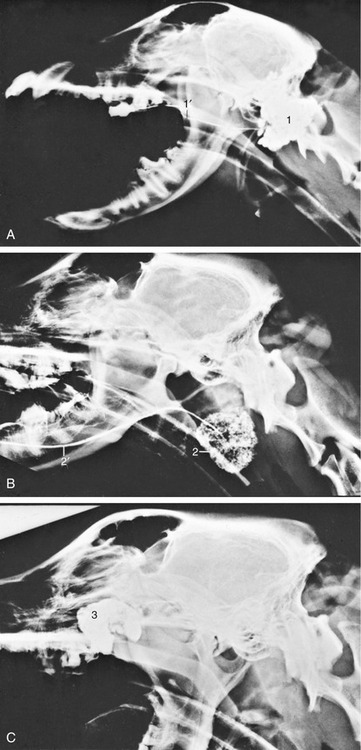
Figure 11–15 Contrast medium outlining the canine parotid (A), mandibular (B), and zygomatic (C) glands. 1, Parotid gland; 1′, duct; 2, mandibular gland; 2′, duct; 3, zygomatic gland.
The oral cavity proper, like the nasal cavity above, widens from front to back before contracting at the level of the palatoglossal arches, beyond which it is continued by the oropharynx.
The hard palate presents transverse ridges and a prominent incisive papilla (see Figure 3–5). The slit to each side of the incisive papilla opens into an incisive duct that extends caudodorsally for 1 or 2 cm through the palatine fissure to open onto the floor of the nasal cavity. Before doing so, the duct communicates with the cavity of the vomeronasal organ. The Flehmen reaction associated with the perception of pheromones is exhibited in both dogs and cats but is less clearly demonstrated than in animals such as in horses (Figure 11–16).
The oral mucosa, generally pink, may be pigmented locally. The wide and flat apex of the tongue is depressed centrally (like a spoon) when liquids are lapped. A short median rod (lyssa) of connective, muscular, and cartilaginous tissue is embedded close to the ventral surface of the tongue. Its significance is not known, although a fanciful connection with rabies was postulated in former times.
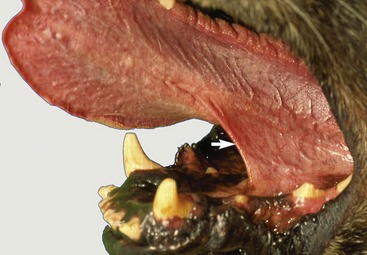
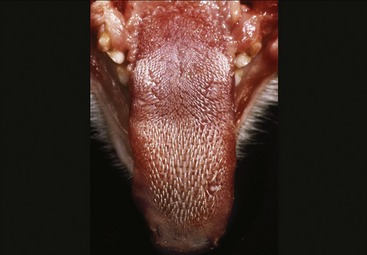
The dorsal surface of the tongue is roughened by papillae. Filiform papillae predominate but are replaced by stouter conical papillae toward the root; both have protective and mechanical functions. Other papillae are concerned with the perception of taste; round fungiform papillae are dotted among the filiform papillae; foliate papillae, represented by a few shallow grooves, are present on the lateral border, near the palatoglossal arch; and four to six vallate papillae form a rostrally open V on the root (Figure 11–17). The tongue of the newborn is fringed with lacelike (marginal) papillae that persist for the first 2 weeks and are thought to assist in fitting the tongue to the dam’s teat.
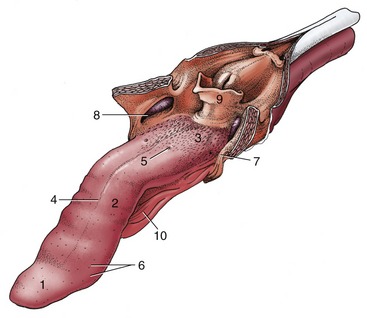
Figure 11–17 The tongue of the dog. The soft palate and the esophagus are sectioned in the median plane. 1, Apex; 2, body; 3, root, forming floor of oropharynx; 4, median groove; 5, vallate papilla; 6, fungiform papillae; 7, palatoglossal arch; 8, palatine tonsil in tonsillar fossa; 9, epiglottis; 10, frenulum.
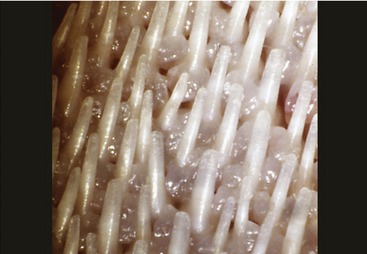
The oral cavity of the cat is short and wide and is easily examined in cooperative subjects (Figure 11–18). The abrasive nature of the cat’s tongue is due to the strong keratinization of the epithelium of the large conical papillae that replace the delicate filiform papillae of most species. On the dorsum of the tongue these papillae are caudally directed and hooked; this assists in grooming but makes it more difficult to eject threadlike objects that have been taken into the mouth (Figure 11–19 and Figure 11–20). Hairs removed from the coat during grooming therefore accumulate in the stomach (hairballs); they mingle with the ingesta and may be expelled with the feces or ejected through the mouth.
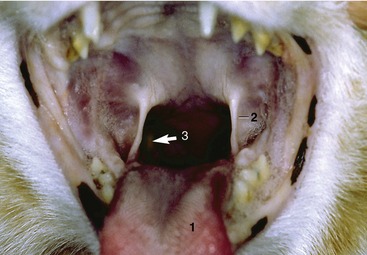
Figure. 11–18 Oropharynx (cat). 1, Tongue; 2, palatoglossal arch; 3, position of right palatine tonsil (arrow).
In addition to diffuse labial salivary glands, the lips of cats contain large sebaceous and apocrine glands. The secretion of these circumoral glands is used in grooming and may be frequently rubbed off on objects, apparently as a scent marker substance (see Figure 10–11).
Congenital clefts of the primary (harelip) or secondary palate has been reported in both dogs and cats, especially Siamese. In dogs, the incidence of cleft palate is higher in brachycephalic breeds, although other breeds (Labrador, Cocker Spaniel) may be affected. The primary palate forms the lips and premaxilla, and the secondary palate forms the hard and soft palates. The incomplete closure of these structures is attributed to inherited and environmental factors. Clefts may be inherited as either recessive or irregular dominant traits. Toxic agents and intrauterine viral infections can produce animals with clefts if the insult occurs at a very specific time in fetal development (25th to 28th day in dogs).
Fractures of the mandible and separation at the symphysis are fairly common in both species, often as the result of traffic accidents. Concurrent involvement of the maxilla, nasal structures, teeth, and soft tissues of the face is more frequent in cats that have fallen from heights.
THE DENTITION
Much of the general description of the teeth was based on the dentition of the dog, in which the most remarkable features are the prominence of the canine teeth and the marked regional specialization of the others (see Figure 3–16). The upper dental arch, despite having fewer teeth, is slightly longer than the lower one; the upper teeth therefore bite on the buccal side of the lower ones in a shearing action. This feature precludes lateral movement of the lower jaw, making grinding impossible. There is little occlusal contact between upper and lower teeth except caudally, where some crushing of food is possible. The first few premolars do not touch at all, which creates the so-called carrying space. Dogs and cats bolt rather than chew their food.
The formula for temporary dentition in dogs is
and for the permanent set is
The Triadan system is also available for reference to specific teeth. In this system, each tooth is assigned a three-digit number. The first digit (in the hundreds place) indicates the quadrant of the mouth: 1(00) indicates the right upper, 2(00) the left upper, 3(00) the left lower, and 4(00) the right lower quadrant. The other two digits indicate the place of the tooth in the dental arcade, 01 being the most mesial. Thus 102 specifies the upper right second incisor, 409 the lower right first molar.
The incisor teeth are rather loosely embedded in the incisive bones and mandible. On eruption the upper incisor crowns present a central cusp flanked by two smaller ones; the mesial cusp is lacking on the lower incisors (Figure 11–21). These features are lost as wear reduces the incisors to simple prismatic pegs. The wear gives some indication of a dog’s age but is not very reliable because of differences in skull size, frequency of malocclusion, and individual variation in the diet and habits (Figure 11–22). All incisors have a single root. They are mainly for nibbling, both in grooming and when detaching small morsels.
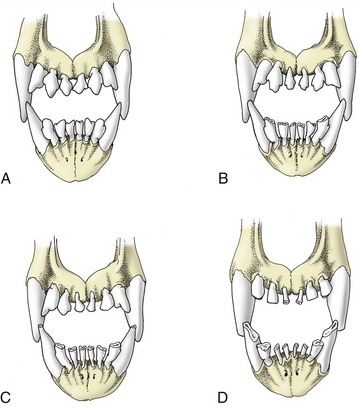
Figure 11–22 Changes in the canine incisors with increasing age. A, Six months. B, About 21/2 years. C, About 6 years. D, About 10 years.
The root of the canine is especially massive—larger indeed than the crown—and curves caudally to lie dorsal (or ventral) to the first premolar (Figure 11–23). These teeth are occasionally removed in aggressive dogs. Simple extraction is made impossible by the size and firm implantation of the root; the attempt to draw one free risks fracture of the jaw. It is necessary to resect the bone over the lateral surface of the root before it can be elevated from its socket. Abscesses of the upper canine teeth may fistulate into the nasal cavity.
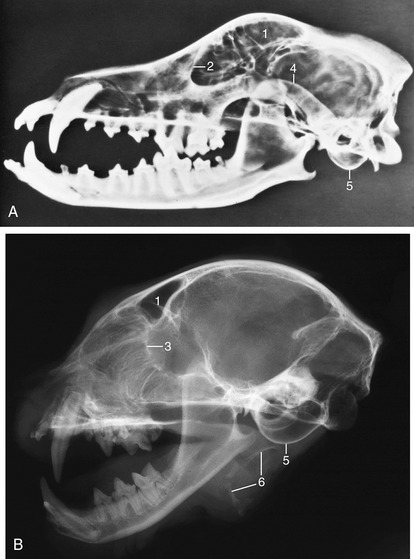
Figure 11–23 A, Radiograph of half of a canine skull showing the permanent teeth and their roots. B, Radiograph of half of a feline head. 1, Frontal sinus; 2, orbital rim; 3, cribriform plate; 4, zygomatic arch; 5, tympanic bulla; 6, hyoid apparatus.
In adult dogs, there are four premolar teeth; the first one may have either one or two roots, whereas the others have two. The one exception is the upper fourth premolar or sectorial tooth, which has three roots (Figure 11–24). The four premolars increase in size and complexity from the first to last in both jaws. The laterally compressed crowns are triangular in profile, presenting small mesial and distal cusps to each side of the principal one. The last upper premolar, P4, is massive and has a small medial part, with its own root, which encroaches on the hard palate. The molars decrease in size from first to last. The two upper molars, though still tuberculate, have flatter crowns than the premolars and are orientated transversely rather than rostrocaudally (see Figure 11–24). They have three diverging roots. The first of the lower molars, M1, the sectorial tooth, is the largest in the lower series. It is flattened from side to side and has two thick divergent roots that occupy most of the width of the jaw. Extraction must be performed carefully to avoid fracture of the mandible. M2 and M3 are much smaller; they engage the last upper molar and, like it, have flat tuberculate crowns. They also have two roots.
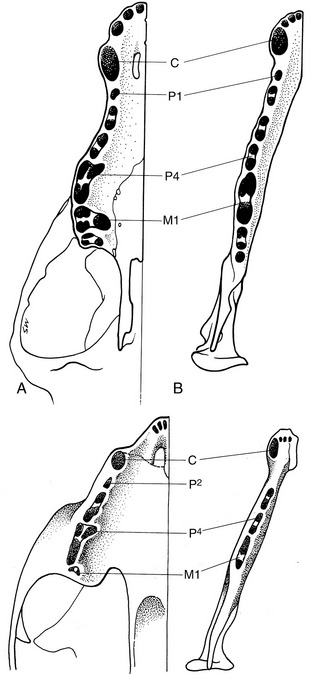
Figure 11–24 The tooth sockets in canine (top) and feline (bottom) upper (A) and lower (B) jaws to show the number and disposition of the roots.
It is important to know the pattern of the sockets to ensure that no part is left behind after extraction of a tooth (see Figure 11–24). Multiple roots always diverge, and it is frequently necessary to split a tooth before it can be extracted to avoid causing excessive trauma.
Brachygnathic breeds often have less than the full complement of teeth: upper and lower P1 and M3 are those most often missing. The cheek teeth of these breeds may be more obliquely placed than normal to fit in the foreshortened jaws.
At birth, a puppy is toothless. The first teeth appear within a few weeks, and the deciduous set is complete and functional by the end of the second month. The first replacement tooth erupts after a further month, or little more, and the permanent set is complete by the sixth or seventh month, which is a remarkably early age (Table 11–1). Permanent teeth erupt earlier in larger breeds of dogs. The temporary teeth in general resemble those of the definitive set but are smaller and sharper. They have long slender roots. A temporary canine is sometimes retained after the replacement tooth has erupted because the latter appears beside its predecessor and produces asymmetrical and sometimes insufficient resorption pressure. In such cases the temporary canine is found caudal to its replacement in the upper jaw and lateral to it in the lower jaw. Retained teeth should be removed to allow their replacements to attain their normal positions. The three temporary premolars are properly designated p2, p3, and p4; the tooth known as the first premolar erupts several weeks later than these and is part of the permanent dentition (Table 11–1).
Table 11–1 Eruption Dates of the Dog’s Teeth
| Eruption of Temporary Tooth (wk) | Eruption of Permanent Tooth (mo)* | |
|---|---|---|
| Incisor 1 | 4–6 | 3–5 |
| Incisor 2 | 4–6 | 3–5 |
| Incisor 3 | 4–6 | 4–5 |
| Canine | 3–5 | 5–7 |
| Premolar 1 | 4–5 | |
| Premolar 2 | 5–6 | 5–6 |
| Premolar 3 | 5–6 | 5–6 |
| Premolar 4 | 5–6 | 4–5 |
| Molar 1 | 5–6 | |
| Molar 2 | 5–6 | |
| Molar 3 | 6–7 |
* Permanent teeth erupt slightly earlier in large breeds. Modified from Schummer A, Nickel R, Sack WO: The viscera of the domestic mammals, ed 2, New York, 1979, Springer-Verlag; and Evans HE: Miller’s anatomy of the dog, ed 3, Philadelphia, 1993, Saunders.
The upper teeth are innervated by the infraorbital nerve, and the rostral members of the series can be desensitized by blocking the nerve within the infraorbital foramen. The lower teeth are supplied by the inferior alveolar nerve, which can be blocked at a site a centimeter or so caudal to the last tooth, before it enters the mandible. The rostral members of this series can also be desensitized by blocking the nerve within the mental foramen.
The cat has sharp and pointed teeth. The formula for the temporary dentition reads
and for the permanent dentition reads
The reduction of the number of cheek teeth is due to the absence of P1 and M2 and of P1, P2, M2, and M3 (see Figure 3–17). The molar loss deprives the cat of flat-crowned crushing teeth, leaving an exclusively shearing bite (Figure 11–25). P4, the upper sectorial, is the only tooth to have three roots, which are implanted only a few millimeters from the ventral wall of the orbit. Its lower counterpart is M1. It is not uncommon to find that one or more of the smaller incisor teeth have been shed by the time cats settle into middle age, without obvious cause.
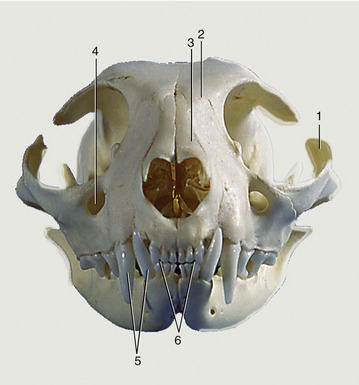
Figure 11–25 Feline skull, rostral view. 1, Zygomatic arch; 2, frontal bone; 3, nasal bones; 4, infraorbital foramen; 5, upper and lower canine teeth; 6, upper and lower incisors, in incisive bones and mandible, respectively.
In kittens, eruption of deciduous teeth typically begins during the third postnatal week. The permanent teeth are all in place by about 6 months of age. However, there is so much individual and breed variation that the eruption and replacement dates, given in Table 11–2, are unreliable guides to age.
Table 11–2 Eruption Dates of the Cat’s Teeth
| Eruption of Temporary Tooth (wk) | Eruption of Permanent Tooth (mo) | |
|---|---|---|
| Incisor 1 | 3–4 |
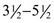 |
| Incisor 2 | 3–4 |
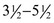 |
| Incisor 3 | 3–4 |
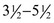 |
| Canine | 3–4 |
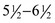 |
| Premolar 2 | 5–6 | 4–5 |
| Premolar 3 | 5–6 | 4–5 |
| Premolar 4 | 5–6 | 4–5 |
| Molar 1 | 5–6 |
From Schummer A, Nickel R, Sack WO: The viscera of the domestic mammals, ed 2, New York, 1979, Springer-Verlag.
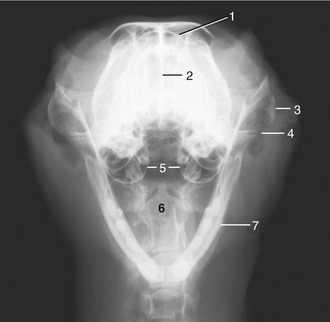
Figure 11–26 Rostrocaudal open-mouth radiograph of a feline head. 1, Frontal sinus; 2, nasal septum; 3, zygomatic arch; 4, temporomandibular joint; 5, tympanic bullae; 6, axis with dens; 7, mandible.
Plaque deposition and consequent periodontal disease are common in both companion species. In cats, this is often accompanied by resorptive lesions at the necks of the teeth.
THE TEMPOROMANDIBULAR JOINT
The articular surfaces of the temporomandibular joint are nearly congruent. The transverse cylinder provided by the mandible fits within a trough on the undersurface of the zygomatic process of the temporal bone (Figures 11–23, 11–28, and 11–29). The trough is enlarged caudally by a prominent retroarticular process that securely cups the cylinder and prevents its luxation in a caudal direction. In keeping with the congruence of the joint, the articular disk is thin. The joint capsule is strengthened by a lateral ligament.
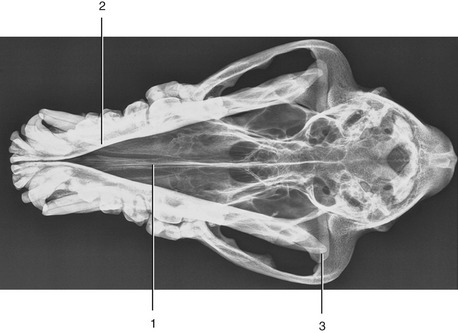
Figure 11–28 Ventrodorsal radiograph of the canine head. Note the position and size of the brain case. 1, Nasal septum; 2, mandible; 3, temporomandibular joint.
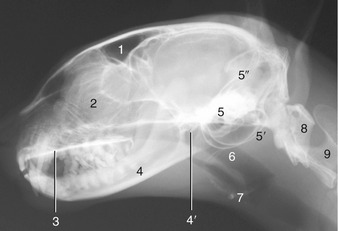
Figure 11–29 Radiograph of the feline head. 1, Frontal sinus; 2, cribriform plate and ethmoidal conchae; 3, hard palate; 4, mandible; 4′, temporomandibular joint; 5, petrous temporal bone; 5′, tympanic bullae; 5″, tentorium cerebelli; 6, nasopharynx; 7, basihyoid; 8, atlas; 9, axis.
Movement of the mandible is almost exclusively of a hinge nature; only slight protrusion is possible when the mouth is fully open. Lateral movement may be produced by trauma and occasionally is so severe that the coronoid process engages the zygomatic arch, locking the jaws in the depressed position.
The joint lies under cover of the caudal part of the masseter, where the dorsal buccal branch of the facial nerve crosses the border of the muscle. It is rostral to the parotid gland.
The masticatory muscles have been sufficiently described (p. 113).
THE SALIVARY GLANDS
PAROTID GLAND
The parotid gland (see Figure 11–6) is roughly triangular, relatively thin, and molded around the proximal portion of the auricular cartilage, against which it can be rolled on palpation. It occupies a depression between the masseter, the wing of the atlas, and the auricular cartilage. Ventral to the cartilage, it is related medially to the facial nerve and maxillary vein and more rostrally to the parotid lymph node and temporomandibular joint. The parotid duct leaves the cranial aspect of the gland and continues over the lateral aspect of the masseter muscle between the buccal branches of the facial nerve. The duct opens into the vestibule at a small parotid papilla opposite the caudal part of the upper fourth premolar tooth, approximately 5 mm from the margin of the gum. The duct makes a right-angle bend just before opening at the papilla; cannulation is made easier by grasping the mucosa just caudal to the opening and pulling it rostrally to straighten the bend.
ZYGOMATIC GLAND
The ventral buccal glands comprise a few small, solitary units located in the submucosa, rostral to the masseter muscle, medial to the ventral part of the buccinator and lateral to the mandible.
The dorsal buccal glands are consolidated in a mass generally known as the zygomatic gland (see Figures 11–10, A, 11–27/28, and 11–36/2). This is a large mixed gland located in the ventral part of the orbit, covered by the zygomatic arch, and related medially to the maxillary artery and nerve and medial pterygoid muscle and dorsally to the periorbita. Its swelling, when diseased, may cause protrusion of the eyeball (exophthalmus) or bulging of the oral mucosa near the last upper cheek tooth where the duct opens into the vestibule. Facial trauma may cause leakage of saliva, and the resulting zygomatic mucocele may produce exophthalmus.
The main duct of the zygomatic gland (Figure 11–15, C) opens on a small papilla lateral to the caudal part of the upper first molar tooth. A small ridge connects the main zygomatic and parotid gland duct openings. Usually there are one to four small accessory ducts opening caudal to the main one. These openings are usually obvious and easily cannulated.
MANDIBULAR GLAND
The large ovoid mandibular gland is contained within a strong fibrous capsule that gives it form. This, with its firm attachment, makes it easily palpable, in contrast to the adjacent mandibular lymph nodes that “float” under exploring fingers. The gland has these relations: rostrally, the mandibular lymph nodes, sublingual gland, and masseter and digastric muscles; medially, the digastricus, external carotid artery, and medial retropharyngeal lymph node; and caudally, the muscles of the neck. Its capsule continues rostrally onto the compact part of the sublingual gland to which it is firmly fused (see Figure 11–14). The course of the mandibular duct is described with the sublingual gland.
SUBLINGUAL GLAND
The narrow compact sublingual gland continues forward from the mandibular gland. It follows the mandibular duct between the digastricus ventrally and the medial pterygoid dorsally and soon gains a position lateral to the root of the tongue, before ending variously at the level of the cheek teeth. Its duct accompanies that of the mandibular gland to the sublingual caruncle; together they raise the sublingual fold, near the body of the mandible. A variable number of lobules of the polystomatic portion of the sublingual gland are present in the sublingual fold, located rostral to the lingual branch of the trigeminal nerve; they open on the floor of the mouth next to the tongue through several ducts. The lingual nerve crosses the lateral surfaces of the mandibular and sublingual ducts just caudal to the level of the orbits.
The slitlike openings of the mandibular and sublingual ducts are recognizable on the lateroventral surface of the lingual caruncles, at the end of the frenulum of the tongue (see Figure 11–14/4). The mandibular duct (Figure 11–15, B) is the larger and more rostral of the two and is easily cannulated. The sublingual duct is more difficult to cannulate. In 20% to 40% of dogs the sublingual duct joins the mandibular duct along its course.
The most common clinical condition of these glands in both dogs and cats is the salivary mucocele, which is the accumulation of mucoid saliva leaked from a damaged gland or duct. The sublingual gland is most frequently affected. Extravasated saliva most commonly collects in the subcutaneous tissues of the intermandibular, sublingual tissues (ranula) or cranial cervical area. A less common site is the wall of the pharynx. Treatment requires the removal of the mandibular–sublingual gland complex. Removal of both these glands does not affect the animal adversely, even if bilateral, but care must be taken to avoid the lingual nerve.
The cat’s salivary glands are shown in Figure 11–6, B, and Figure 11–27.
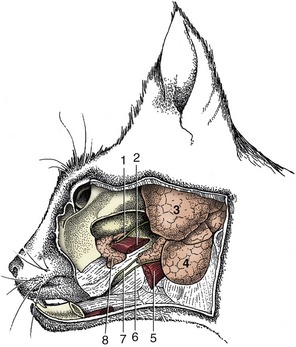
Figure 11–27 Deep dissection of the feline head to expose the zygomatic salivary gland (8). 1, Parotid duct, cut; 2, medial pterygoid muscle; 3, parotid gland; 4, mandibular gland; 5, digastricus muscle; 6, mandibular duct; 7, sublingual duct emerging from the rostral end of the monostomatic sublingual salivary gland; 8, zygomatic salivary gland.
THE PHARYNX
The auditory tubes open high on the lateral walls of the nasopharynx, immediately rostral to small mucosal cushions, measuring about 10 mm long in dogs and 4 mm in cats. Nasopharyngeal polyps, common in cats, originate in the middle ear as focal hypertrophies of the mucosa, develop stalks, and extend through the auditory tube to reach the nasopharynx. There is a flat pharyngeal tonsil in the roof of the nasopharynx. Digital pressure in this area may stimulate respiration.
The oropharynx is dorsoventrally flattened; it extends from the palatoglossal arches, which are not easily detected unless made to stand out by pulling the tongue forward. During normal breathing the soft palate lies on the tongue with it free edge rostral to the epiglottis (Figures 11–29 and 11–33). In many brachycephalic dogs the soft palate is disproportionally long and rests over the entrance to the larynx, causing respiratory difficulties. The overlong soft palate can be shortened using the palatine vessels laterally and the palatines muscle toward the midline, as landmarks. Additional guidance is provided by the wrinkling of the palatine mucosa where it does not lie over muscle. For different reasons, the epihyoid provides a useful landmark where it crosses the lateral wall of the oropharynx. Contact with the oropharyngeal wall during examination of the mouth normally causes dogs to retch; the absence of this (gag) reflex suggests damage to the glossopharyngeal and vagal nerves.
Oral breathing is possible with the palate in the normal position (Figure 11–30), and the panting dog is a familiar sight. Cats may also breathe through the mouth but more discretely, sitting quietly and letting the air slip in and out through lips slightly parted toward the commissure. Occasionally the mouth is opened more widely, allowing a brief glimpse of the tongue.
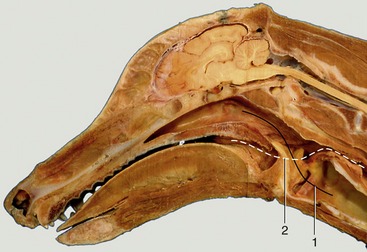
Figure 11–30 Median section of head and neck. 1, Route from nasopharynx to trachea (solid line); 2, route of food from mouth to esophagus (broken line).
The fusiform palatine tonsils occupy fossae in the lateral walls of the oropharynx caudal to the palatoglossal arch and ventral to the soft palate and are covered medially by semilunar folds, which arise from the ventrolateral part of the soft palate (Figures 11–17/8 and 11–31). In cats the palatine tonsil is very small and is covered by a mucosal fold.
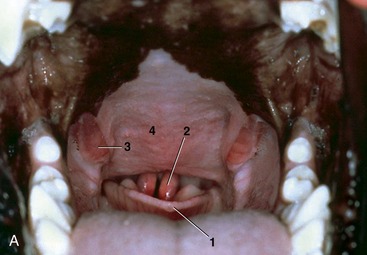
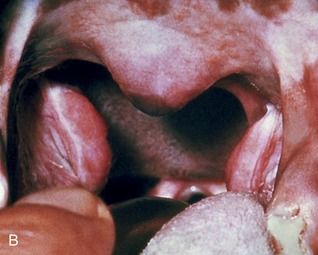
Figure 11–31 A, Oropharynx. 1, Epiglottis; 2, cuneiform processes of arytenoid cartilages; 3, palatine tonsils; 4, soft palate. B, Palatine tonsils; the caudal part of the soft palate is missing.
The tonsils are relatively large in young dogs and often protrude from the fossae; similar protrusion in the adult usually indicates pathological swelling. In the performance of tonsillectomy the reddish lymphoid tissue that lines the fossa dorsal to the tonsil must also be removed; it is exposed when the main part is retracted from the fossa. The tonsil is related laterally to the lingual nerve and the mandibular and sublingual ducts, all of which are at some risk in this operation. The tonsil is supplied by tonsillar and hyoid branches of the lingual artery, which courses ventrolateral to the tonsil. Sensory innervation to the tonsil is from the glossopharyngeal nerve. The efferent lymph vessels drain to the medial retropharyngeal and mandibular lymph nodes. There are of course no afferents.
On each side the caudal border of the soft palate is continued to the dorsolateral wall of the palatopharyngeal arch. The palatopharyngeal muscle and the mucosa that covers it form this arch.
DEGLUTITION
During the act of swallowing, the regurgitation of food into the nasopharynx and its aspiration into the larynx are both prevented by the coordinated activity of the pharyngeal muscles. These muscles arch over the roof of the pharynx to meet their contralateral fellows at a median raphe, and their contractions occur in sequence but overlap, ensuring that in cooperation they effect the movement of food toward and into the esophagus. The more rostral contrictor muscles also draw the pharynx forward and upward for the better reception of the food bolus as it is passed from the mouth. An essential feature of the process is the sphincterlike closure of the intrapharyngeal ostium that involves elevation of the soft palate, in part effected by the small muscles (tensor and levator) that pull the palate taut between the pterygoid bones. The timely relaxation of the cricopharyngeus allows food to escape into the esophagus. During the process, the larynx is raised while its entrance is partially blocked and the glottis closed.
Inappropriate closure of the intrapharyngeal ostium provokes sneezing.
THE LARYNX
The larynx is located caudal to the intermandibular space and ventral to the first two or three cervical vertebrae. Its cranial parts can be examined through the mouth in the sedated dog when the soft palate is raised with a spatula (Figure 11–31, A). Palpation through the skin reveals, in caudorostral succession, the cricoid cartilage (especially its arch), the rounded ventral surface of the thyroid cartilage, and the prominent thyrohyoids that connect the rostral horns of the thyroid cartilage with the basihyoid. The remaining bones of the hyoid apparatus, other than the stylohyoid, are also palpable (Figures 2–34, 11–9, and 11–32, A-B).
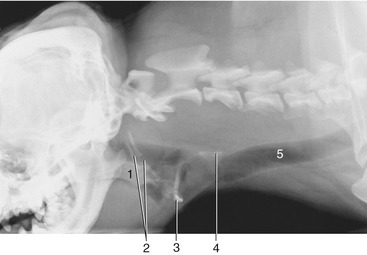
Figure 11–9 Radiograph of the cramped pharyngeal region of the brachiocephalic dog. (The space available is rather cramped.) 1, Soft palate; 2, hyoid apparatus; 3, basihyoid; 4, cricoid cartilage; 5, trachea.
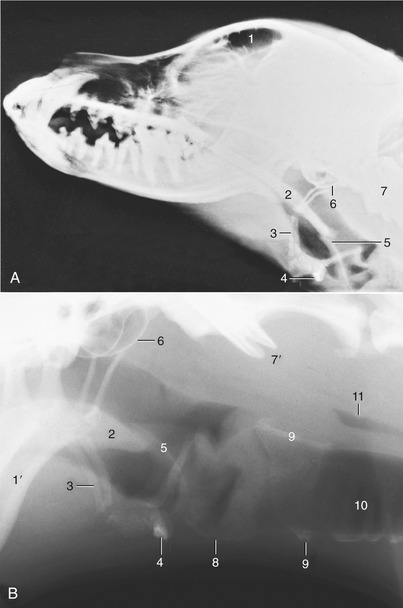
Figure 11–32 A, Radiograph of the canine head to show the relation of the hyoid apparatus to the skull and atlas. B, Enlargement of the laryngeal region of another dog. 1, Frontal sinus, 1′, mandible; 2, soft palate; 3, hyoid apparatus (epihyoid); 4, basihyoid; 5, epiglottis; 6, tympanic bulla; 7, atlas; 7′, wings of atlas; 8, thyroid cartilage; 9, cricoid cartilage; 10, trachea; 11, air in esophagus.
The epiglottis resembles a pointed spade that is connected to the body of the hyoid bone and the cranioventral part of the thyroid cartilage. The aryepiglottic folds link the sides of the epiglottis to the dorsal parts of the arytenoid cartilages and their corniculate processes (Figure 11–33). The channel lateral to the aryepiglottic folds is called the piriform recess, through which fluids leave the laryngopharynx for the esophagus during swallowing.
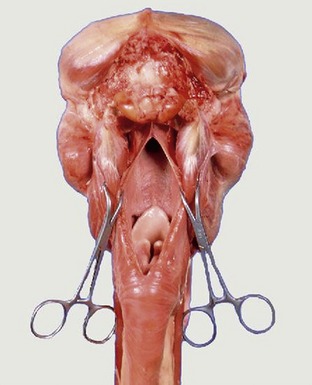
Figure 11–33 The nasopharyngeal cavity exposed by median incision of the roof. Note the postvelar position of the tip of the epiglottis.
The laryngeal vestibule extends caudally from the entrance to the vocal folds. The vestibular folds are short but wide plicae of mucosa that run from the expanded ventral margins of the arytenoid cartilage to the dorsal surface of the thyroid cartilage. The vocal folds visible through the entrance are formed by the vocal ligaments, straps of elastic fibers continuous caudally with the vocalis muscles. The vocal folds are separated from the more rostral vestibular folds by the large laryngeal ventricles, lateral evaginations of the mucosa that extend to the thyroid cartilage. The opening to the ventricles is about 1.5 mm wide and extends the length of the vocal fold that bounds it. Each ventricle has two parts. One part extends cranially lateral to the vestibule and a separate one caudally lateral to the vocal cord. The secretion of glands within the saccule prevents dessication of the vestibular and vocal folds. Solitary lymph nodules are present in the wall of the ventricles. The saccules may provide room for the vocal folds to vibrate during barking, which is a theory supported by the reduction, even absence, of ventricles in the Basenji, a breed of dog that never barks.
The parts of the larynx surrounding the entrance project into the pharynx, and except when the dog swallows or breathes through the mouth, the free border of the soft palate is lodged below the epiglottis, which aligns the laryngeal lumen with that of the nasopharynx (see Figure 11–33).
The larynx is covered ventrally by the subcutaneous sternohyoid muscles (see Figure 11–45). It is related laterally to the medial retropharyngeal lymph node, the common carotid artery and vagosympathetic trunk, linguofacial vein, and mandibular lymph nodes. It is related dorsally to the caudal part of the laryngopharynx leading to the esophagus.
The sensory nerve supply to the laryngeal mucosa is from the cranial laryngeal nerve, entering the laryngeal cavity through the rostral thyroid notch. The recurrent laryngeal nerves that supply the remainder of the intrinsic laryngeal musculature, except for the cricothyroids (supplied by a branch of the cranial laryngeal nerve), leave the parent vagal trunks within the chest. The right one arises level with the middle cervical ganglion and winds dorsally around the subclavian artery to proceed cranially in the angle between the longus colli muscle and the trachea. The left one leaves the vagus level of the aortic arch, which it loops around, distal to the ligamentum arteriosum. It ascends the neck ventromedial to the esophagus. Both nerves supply the trachea and esophagus before termination at the larynx.
Laryngeal paralysis as a genetic disorder occurs in certain breeds, notably the Bouvier and Leonberger, but it has also been encountered as an occasional disorder of older dogs of other large breeds.
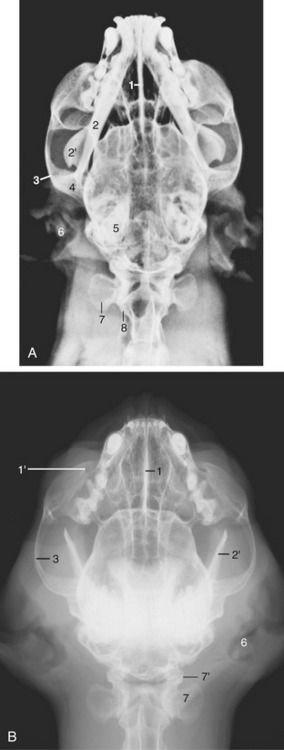
Figure 11–34 Radiographs of the feline head. A, Ventrodorsal view. B, Ventrodorsal view with mouth fully opened. 1, Nasal septum; 1′, infraorbital foramen; 2, mandible; 2′, coronoid process; 3, zygomatic arch; 4, temporomandibular joint; 5, petrous temporal bone; 6, external ear; 7, wing of atlas; 7′, atlantooccipital joint; 8, axis.
The cranial laryngeal arteries provide the principal blood supply. They originate from the external carotid arteries and, with the cranial laryngeal nerves, pass through the rostral thyroid notches. Satellite veins drain into the external maxillary veins. Lymphatics drain into the medial retropharyngeal lymph nodes.
The cat’s larynx is depicted in radiographs (see Figure 11–29) and in a median section (see Figure 11–35). The arytenoid cartilages have a simpler shape than those in the dog. The aryepiglottic folds bypass the arytenoid cartilages and connect the sides of the epiglottis directly to the cricoid cartilage. The vocal cords are thick and round; in contrast, the vestibular folds are thin and sharp-edged. There is no genuine ventricle, but small pouches of the vestibular mucosa extend lateral to the fold. Solitary lymph nodules are present on the laryngeal surface of the epiglottis, while aggregated nodules (paraepiglottic tonsils) thicken the aryepiglottic folds.
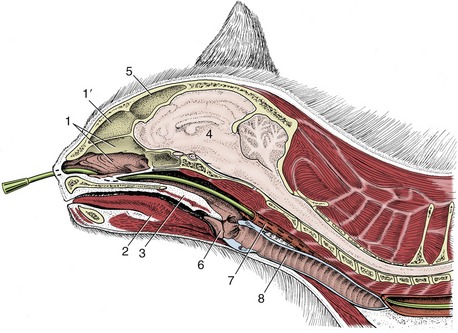
Figure 11–35 Paramedian section of the feline head and neck. A nasogastric tube is in place. 1, Nasal cavity; 1′, dorsal part of nasal cavity; 2, tongue; 3, soft palate; 4, brain; 5, frontal sinus; 6, epiglottis; 7, esophagus; 8, trachea.
Electromyographic studies show that purring in cats is produced by fast twitching of muscles in the larynx and diaphragm. The laryngeal muscles rapidly narrow and widen the glottis, which causes respiratory air to vibrate and make the sound.
Differences between the upper airways of brachycephalic and mesaticephalic breeds have been mentioned from time to time. In certain breeds, the specific proportionalities in the upper airways have an adverse influence on respiratory function resulting in the brachycephalic obstruction syndrome. In this syndrome, the nostrils can be stenotic, the pharynx short and narrow with thickened redundant mucosa, the root of the tongue massive, and the soft palate overlong. The progressive dyspnea is caused by increasing body weight, relatively insufficient growth of the laryngeal structures, increasing mass of the pharyngeal mucosa, and insufficient opening of the glottis. In addition, there is progressive collapse of the laryngeal structures and eversion of the laryngeal ventricles because of the increased traction caused by the increased velocity of exhaled air passing the relatively small laryngeal opening.
THE EYE AND ORBIT
The margins of the orbit are easily palpable. They are formed by the frontal, lacrimal, and zygomatic bones, with the gap in the dorsolateral segment closed by the orbital ligament (Figure 11–11/6). Only the medial third of the orbital wall is osseous; the remainder is provided by the periorbita. The orbital axis takes a dorsal, lateral, and anterior direction from the apex of the cone. In brachycephalic dogs, particularly those with wide skulls, the axes point more laterally, restricting binocular vision.
The openings into the orbit comprise the optic canal, orbital fissure, duplicated ethmoidal foramina, and the fossa of the lacrimal sac. The optic canal transmits the optic nerve and internal ophthalmic artery; the orbital fissure transmits the oculomotor, trochlear, abducent, and ophthalmic nerves; the ethmoidal foramina transmit divisions of the like-named nerve and artery; and the fossa contains the slight enlargement at the origin of the nasolacrimal duct.
The osseous wall of the orbit is related dorsomedially to the frontal sinus and rostromedially to the maxillary recess; infection in either of these cavities can easily spread to orbital structures. The periorbita is related as follows: medioventrally to the medial pterygoid muscle; ventrally to a pad of fat caudal to the orbital margin, the zygomatic gland, and the large deep facial vein; laterally to the zygomatic arch; and caudodorsally to the orbital ligament and temporalis muscle. The dorsolateral aspect of the orbit is accessible to surgery without resection of bone.
The important maxillary artery and nerve and their branches to the face and palate course ventral to the orbit between the medial pterygoid and the zygomatic gland (Figure 11–36). The maxillary artery gives off the external ophthalmic artery, which pierces the periorbita near its apex to supply structures within the cone. The temporalis, which surrounds the coronoid process of the mandible, impinges on the periorbita when the mouth is opened. This may cause pain when the orbital contents are diseased as, for example, by a retrobulbar abscess. The proximity to the oral cavity permits drainage of such abscesses into the mouth, behind the last cheek tooth.
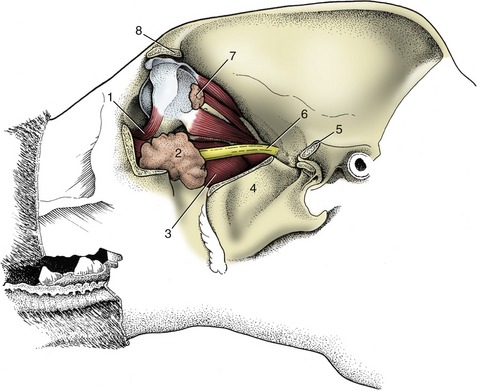
Figure 11–36 Dissection of the canine orbit and pterygopalatine fossa, lateral view. 1, Ventral oblique muscle; 2, zygomatic gland; 3, medial pterygoid muscle; 4, coronoid process of mandible, cut; 5, caudal stump of zygomatic arch; 6, maxillary nerve; 7, lacrimal gland; 8, zygomatic process of frontal bone.
The dimensions of the orbital rim of large and small dogs differ less than might be expected; because the diameter of the eyeball varies even less, the surgical working “space” is generally narrower in larger dogs. However, the position of the eyeball within the orbit differs markedly. In dolichocephalic dogs the eyeball is deeply placed and the palpebral fissure is small. The eyes of brachycephalic dogs protrude and are more susceptible to injury to the cornea.
The lacrimal gland (Figure 11–36/7) is flat, lobulated, and about 12 to 15 mm in width. It lies between the eyeball and the orbital ligament, dorsal to the lateral angle of the eye. The gland must be identified and removed in enucleation (removal) of the eye. The thin edge of the third eyelid is visible in the medial angle of the eye in the “resting” state. More is seen when the upper and lower lids are retracted with the fingers, while full protrusion is obtained by gentle pressure on the eyeball through the upper lid (see Figure 9–21/6). Although the superficial gland that surrounds the cartilage of the third lid is not normally visible, it appears when the eyelid is retracted because the increased retrobulbar pressure pushes it to the fore. Active protrusion of the third eyelid, effected by a specific muscular arrangement, is common in cats and may have an emotional or physical origin. Abnormal retrobulbar pressure may cause the gland of the third eyelid to be everted into the medial angle of the eye, where it appears as a round swelling below a covering of conjunctiva. Subepithelial lymph nodules present on the bulbar surface of the third eyelid may become inflamed.
In cross section the eyelids display the external skin, the orbicular muscle of the eye, the tarsal plate, the meibomian glands, and the palpebral conjunctiva. The openings of the tarsal glands (20 to 40 in each lid) can be seen at the lid margins. When the lids are everted, these glands appear as white cords extending 5 to 7 mm from the lid margin under the conjunctiva. Occasionally aberrant hairs protrude from the openings of the tarsal glands and may irritate the cornea. The eyelashes in dogs are found on the outer surface of the upper lid margin; there are none on the lower lid. Both lids of cats are without lashes.
The orbicular muscle of the eye, rostral to the tarsal plate, is anchored to the orbit by fascia medially and by the retractor muscle of the lateral angle laterally. These attachments preserve the elliptical shape of the palpebral fissure.
The puncta lacrimalia are 2 to 4 mm from the medial angle of the eye and are usually located at the junction of pigmented and nonpigmented epithelia. Although they may be difficult to find or the lower one may in fact be absent or displaced to the bulbar surface of the lid, it is possible to cannulate them. The puncta are the openings to the upper and lower canaliculi, which join to form the lacrimal sac, from which the nasolacrimal duct takes origin (see Figure 9–21). The duct continues rostrally in the medial wall of the maxilla, deep to the nasal mucosa. An accessory, or more rarely the sole, opening of the nasolacrimal duct may enter the nose at the level of the canine tooth in a significant proportion of dogs. The duct makes an abrupt 90° turn about 2 mm before opening onto the floor of the nasal cavity (see Figure 11–8).
The feline lacrimal system is similar; however, an opening with the oral cavity has been recorded, located on a small papilla just behind the upper incisor teeth.
One or both puncta may be absent in several dog breeds, as well as Persian cats. If both are absent, a slight depression in the conjunctiva may indicate where the opening would normally have been located.
The eyeball is nearly spherical and relatively large. The cornea is slightly oval, its larger diameter being mediolateral in keeping with the shape of the globe itself. It is slightly thicker at the pole than at the periphery. The canine iris is brown, golden yellow, or bluish, and whether dilated or contracted the pupil remains round. It is said to be smaller in older dogs under standard light conditions. Remnants of the papillary membrane may be seen on its upper margin in puppies up to the age of 5 weeks.
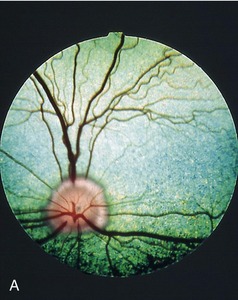
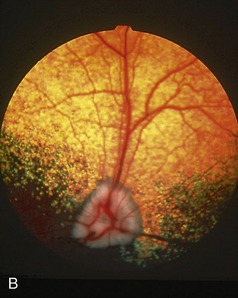
The fundus is illustrated in Figure 11–37, A-B. The triangular tapetum lucidum, which nearly fills the dorsal half, includes the optic disc in large dogs. The retinal vessels radiate from the disc; prominent venules form a partial circle from which tributaries usually spread dorsally, medioventrally, and lateroventrally. Thinner arterioles extend in all directions, many accompanying the venules.
In the cat there is little surgical working space between the eye and the orbital margin. The third eyelid is large, and in certain circumstances it may be drawn completely over the cornea. As in the dog, it responds to retraction of the eyeball. The cornea is relatively large and permits a wide visual field. The color of the iris ranges from blue through green to golden. In certain breeds iris color is strictly prescribed to meet show standards. Kittens are usually born with blue eyes that later change color.
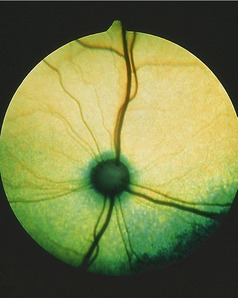
The pupils of domestic cats are round when dilated but are vertical slits when constricted (those of some wild felids remain round at all times) (Figure 11–38, A-B). The vertical form is due to the dorsoventral orientation of muscle fibers that extend to the periphery of the iris and decussate at the extremities of the pupil. The fundus is dominated by a large tapetum lucidum that surrounds the optic disc. The tapetum is yellowish- or bluish-green and because of its brilliance is thought to be more effective in reflecting light than that of the dog, which may be a convenience in nocturnal wandering (Figure 11–39).
THE EAR
(See p. 346.)
EXTERNAL EAR
The external ear consists of the external auditory canal and its cartilaginous extension, the auricle (pinna). The auricle, sometimes known as the ear leather to dog fanciers, is shaped like a lopsided funnel, with a small cutaneous pouch on the caudal border a short distance above the ear opening (Figure 11–40 and Figure 11–41). There is a wide diversity in the shape, size, and posture (erect or folded) of dog ears. It is not clear if this diversity influences the hearing ability. Most cats have erect auricles, but an exception is the Scottish Fold cat, in which the most distal portion of the auricle bends rostroventrally, beginning at 3 to 4 weeks of age.
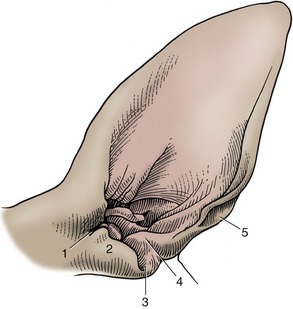
Figure 11–41 Left canine ear, shaved. 1, Pretragic notch; 2, tragus; 3, intertragic notch; 4, antitragus; 5, cutaneous pouch.
The basis of the auricle is a plate of fibroelastic cartilage that is covered by subcutaneous tissue and skin. The skin on the inner (concave) surface adheres more firmly to the cartilage than that on the outer part.
The features of the auricular cartilage provide important surgical landmarks known as the helix, antihelix, tragus, antitragus, and scapha (see Figure 11–41).
The tragus forms the lateral rim of the ear canal opening; it is separated from the more caudal antitragus by the intertragic notch. Both consist of rolled up articular cartilage that supports the external ear opening. The antitragus forms the caudal part of the ear opening and ascends toward the end of the lateral side.
The proximal part of the auricular cartilage is rolled to form a partial tube called the concha, which serves as the enlarged entry of the auditory canal. This first part of the canal connects to the short annular cartilage, which terminates in a short osseous external canal. The ear canal is first directed ventrally (auricular cartilage) before turning medially to form the horizontal canal (portion of the auricular and annular cartilages), which is surrounded and supported by the temporal bone. This course hampers passage of the straight otoscope for examination of the proximal part of the canal and the eardrum. The canal must be straightened by pulling the ear first caudally, then ventrally as the otoscope is advanced (Figure 11–42). The canal is about 7 cm long.
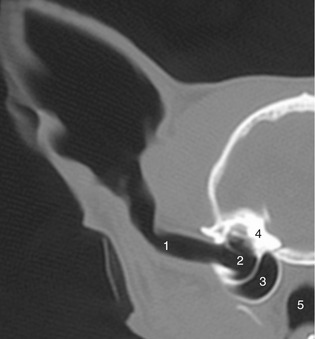
Figure 11–42 Transverse CT scan (bone window) of half of a feline head showing ear canal and middle ear. 1, Ear canal; 2, tympanic cavity; 3, tympanic bulla; 4, petrous temporal bone; 5, nasopharynx.
The horizontal ear canal ends at the eardrum. The tympanic membrane consists of an outer epithelial layer, which is a continuation of the skin of the external auditory canal, an inner mucosal layer, and a fibrous layer in between. The tympanic membrane is thin, slightly oval, semitransparent, and concave owing to traction on its medial side by the tensor tympani muscle. The appearance of the tympanic membrane (eardrum) through an otoscope is shown in Figure 11–43, A-B. The tympanic membrane consists of a small upper portion, the pars flaccida, and a large lower portion, the pars tensa (thin, tough, and glistening). The outline of the manubrium of the malleus is clearly visible.
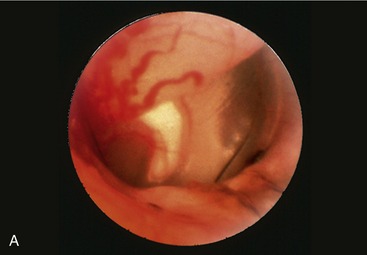
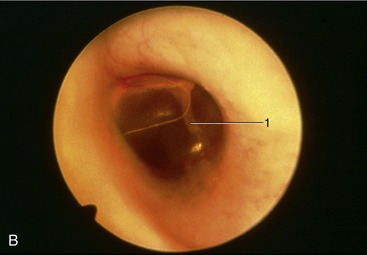
Figure 11–43 A, Otoscopic view of eardrum showing handle of malleus. B, Otoscopic view of the ear drum (cat). 1, Malleus.
The auricular skin continues as the lining of the auditory canal. This skin is thin, and its lateral part possesses both ceruminous and sebaceous glands. It generally contains only a few hairs, but in some breeds (Poodles) hair is abundant. The skin of the bony part of the ear canal is much thinner than that of the cartilaginous portion and is continuous with the epithelial layer of the tympanic membrane. There are no glands or hair follicles here where, because of its thinness, it is more sensitive to trauma.
The base of the auricle and the ear canal are related laterally and ventrally to the parotid gland. The facial nerve crosses the ventral surface of the canal deep to the gland before breaking into the auriculopalpebral nerve and the two buccal branches. The former passes dorsally in front of the ear with the superficial temporal vessels. This stretch of the facial nerve also detaches a caudal auricular nerve and a branch to the middle ear. The sensory innervation is provided by the trigeminal, glossopharyngeal, vagus, and second cervical nerves. The innervation of the muscles of the external ear is by the facial nerve.
The veins of the area join the maxillary vein, which descends toward the mandibular gland from its formation by substantial caudal and cranial auricular and superficial temporal veins that may pass through the parotid gland (Figure 11–44).
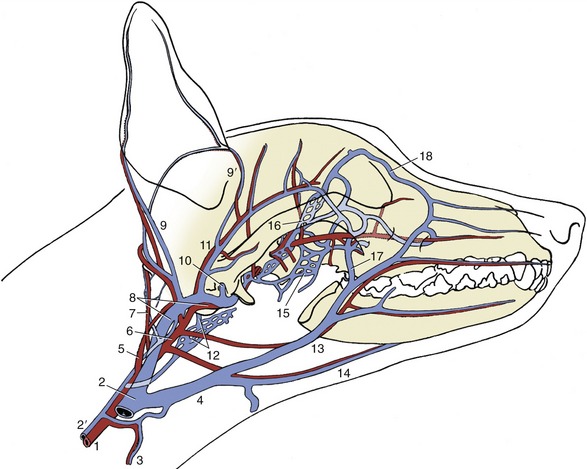
Figure 11–44 The major arteries (red) and veins (blue) of the canine head. The ramus of the mandible has been removed. 1, Common carotid; 2, external jugular; 2′, internal jugular; 3, cranial thyroid; 4, linguofacial; 5, internal carotid; 6, external carotid; 7, occipital; 8, maxillary; 9, 9′, caudal and rostral auricular; 10, dorsal emissary; 11, superficial temporal; 12, ventral emissary and pharyngeal plexus; 13, facial; 14, lingual; 15, pterygoid plexus; 16, ophthalmic plexus; 17, deep facial; 18, angularis oculi.
The arteries lie more deeply. The external carotid having detached the caudal auricular artery to the convex surface of the auricle ends rostroventral to the ear canal by dividing into maxillary and superficial temporaral arteries. The latter, with the like-named vein, lies deep to the parotid gland close to the rostral surface of the ear canal.
The caudal auricular artery branches in the convex outer surface of the auricle; it sends finer branches to the skin over the concave surface through small holes in the cartilage. Vigorous and repeated head shaking or scratching, in most instances elicited by parasites or infection of the ear canal, may injure the vessels and cause hematomas by rupture of the penetrating small branches. Because the hematoma is lined by cartilage on both sides, splitting of the auricular cartilage also takes place. Once begun, the bleeding between the cartilages continues until the internal pressure equals the pressure in the feeder arteries.
MIDDLE AND INNER EAR
The middle and inner ears show few special features of importance. The auditory tubes are narrow and open on the dorsolateral wall of the nasopharynx, level with the landmark provided by the hamulus of the pterygoid bone, which is palpable through the mouth caudomedial to the last cheek tooth in the dog. The tympanic bullae are large, hemispherical, and, except for a serrated septum in their rostral halves, undivided (see Figure 11–23).
In the cat an incomplete bony septum bullae subdivides the middle ear into a small dorsolateral and a large ventromedial compartment. The two compartments communicate with each other through an opening at the caudodorsal margin of the septum near the cochlear window.
In both species middle ear infections (otitis media) may be drained into the nasopharynx through the bulla, which can be palpated through the oropharynx and soft palate, caudal to the hamulus. The inflated tympanic bulla of the cat is also easily found on palpation through the skin, between the wing of the atlas and the zygomatic arch.
The bulla can be approached surgically from the ventral side, with the use of the medial border of the rostral digastric muscle, the mylohyoid muscle, and the stylohyoid and the tympanohyoid cartilages of the hyoid apparatus as landmarks; care should be taken to avoid damage to the nerves of the pharyngeal plexus (Figure 11–44/12) and the vascular supply of the mandibular lymph node.
Several nerves pass through the middle ear, but only two are of clinical significance. The facial nerve travels in the facial canal of the petrous temporal bone; in its course it detaches a branch, the chorda tympani, that enters the cavity of the middle ear.
Postganglionic fibers of the cranial cervical ganglion, located just behind the tympanic bulla, participate in a plexus within the middle ear. The resulting dysfunction is Horner syndrome, a complication of otitis media. The signs are miosis and retraction of the globe, which causes protrusion of the third eyelid, and narrowing of the palpebral fissure. The syndrome usually disappears spontaneously in about 3 months.
THE VENTRAL PART OF THE NECK
It is convenient to describe with the head the part of the neck that lies ventral to the vertebrae. The dorsal part of the neck will be dealt with in the next chapter. The skin on the ventral surface of the neck is loose and in some breeds forms longitudinal folds. Subcutaneous fat tends to be concentrated caudally, especially in the depression dorsolateral to the manubrium.
The external jugular vein sinks into this depression after following a course along the lateral surface of the sternocephalic muscle (see Figure 2–42). It does not lie in a distinct jugular groove as in the larger species. Although it is the principal vein draining the head, it is assisted by small vessels associated with the vertebrae (vertebral vein, internal vertebral plexus) and accompanying the common carotid artery (internal jugular vein) (see Figure 11–46, A-B); these mainly drain deeper structures. The external jugular vein is formed by tributaries embracing the mandibular gland; these are easily raised by pressure on the jugular and provide an additional means for the positive distinction of the gland from the mandibular lymph nodes (see Figure 11–6). The large diameter of the jugular vein makes it a convenient alternative to the cephalic when considerable amounts of bloods have to be collected. It is especially useful in the cat, in which the limb veins are naturally small.
Parts of the hyoid and larynx can be palpated immediately caudal to the angle of the mandible. The transverse basihyoid, the most rostral component, is flanked by the ceratohyoid bones, which project forward, and the thyrohyoids, which pass obliquely caudally. Two further prominences, easily identifiable in the midline, are the thyroid prominence and the cricoid cartilage.
THE CONTENTS OF THE VISCERAL SPACE
The visceral space of the neck is enclosed by four superficial and two deep muscles. The sternohyoid muscle ventral to the trachea extends from the manubrium to the basihyoid; it is loosely connected with its fellow in the midline. The sternothyroideus, also thin and straplike, lies lateral to the trachea, ending on the lateral surface of the thyroid cartilage. These are the only structures that intervene between the larynx and trachea and the skin in the cranial half of the neck (Figure 11–45). They are covered by the sternocephalicus in the caudal half. This muscle consists of two parts, the sternomastoideus and the sternooccipitalis, which diverge toward the head (Figure 11–46). The dorsal sternooccipitalis ends on the back of the skull.
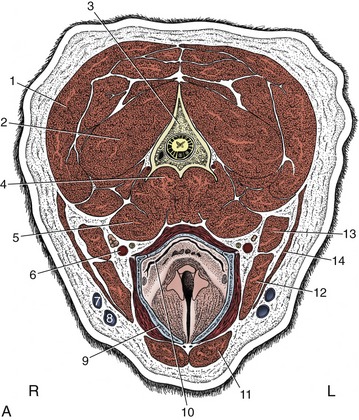
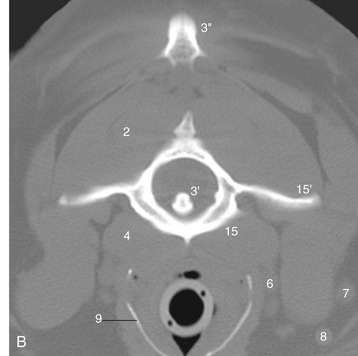
Figure 11–45 A, Transverse section of the canine neck at the level of the axis. B, Corresponding CT scan (bone window), slightly more cranial than A. 1, Splenius; 2, obliquus capitis caudalis; 3, axis; 3′, dens of axis; 3″, cranial tip of spine of axis; 4, longus colli; 5, longus capitis; 6, common carotid artery, vagosympathetic trunk, and medial retropharyngeal lymph node; 7, maxillary vein; 8, linguofacial vein; 9, thyroid cartilage (calcified); 10, laryngopharynx, leading into esophagus; 11, sternohyoideus; 12, sternomastoideus; 13, cleidomastoideus; 14, sternooccipitalis; 15, atlas; 15′, wing of atlas.
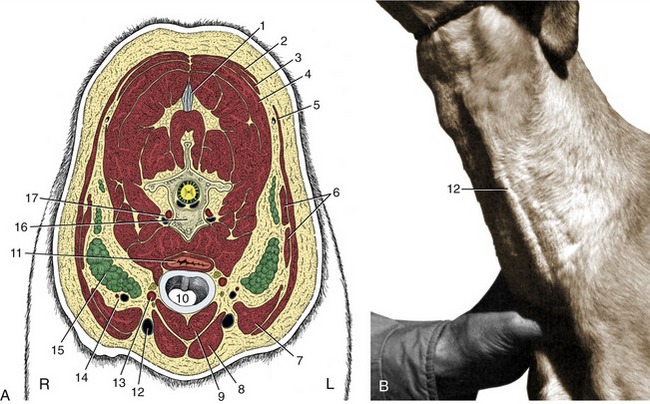
Figure 11–46 A, Transverse section of the canine neck at the level of the fifth cervical vertebra. B, Left external jugular vein raised by thumb pressure at the base of the neck. 1, Nuchal ligament; 2, trapezius; 3, rhomboideus; 4, splenius; 5, cleidocervicalis; 6, omotransversarius; 7, cleidomastoideus; 8, sternocephalicus; 9, sternothyrohyoideus; 10, trachea; 11, esophagus; 12, external jugular vein; 13, common carotid artery, vagosympathetic trunk, and recurrent laryngeal nerve; 14, superficial cervical vessels; 15, superficial cervical lymph nodes; 16, fifth cervical vertebra; 17, vertebral vessels.
The brachiocephalicus also has two parts in the neck, the cleidomastoideus and the cleidocervicalis. The former passes deep to the sternooccipitalis to a common insertion with the sternomastoideus on the mastoid process of the temporal bone. The latter sweeps over the lateral surface of the neck to meet its fellow in the dorsal midline (see Figure 2–55/2).
The sternocephalicus and brachiocephalicus are fused except caudally, where there separation allows the external jugular vein to become more superficial (see Figure 11–46).
The deep muscles comprise the longus capitis, ventrolateral to the cervical vertebrae, and the longus colli more medially (Figure 11–45/4,5). The fascia that covers these muscles ventrally detaches a superficial leaf that encloses the many structures in the visceral space: the esophagus, trachea, thyroid and parathyroid glands, common carotid arteries, vagosympathetic trunks, internal jugular veins, recurrent nerves and tracheal lymph nodes (see Figure 11–46, A). There is no cervical component of the thymus.
The esophagus continues from the laryngopharynx. It first lies dorsal to the trachea, but it deviates to the left in the middle of the neck and maintains this position through the thoracic inlet. The esophagus and trachea are thus both in contact with the longus colli in the caudal half of the neck. The esophagus may be felt with the fingertips as a pliable tube sinistrodorsal to the trachea. The habit of dogs of bolting their food often leads to obstructions. Large pieces of meat, gristle, or bone—and not infrequently stones—tend to lodge at the thoracic inlet, where the esophagus is unable to expand fully.
The trachea continues from the larynx and, because of its firmness, is easily palpated. Unlike the esophagus, it can be grasped so that the flat dorsal surface between the ends of the tracheal rings can be appreciated. In normal dogs it may be possible to demonstrate modest changes in tracheal diameter in synchrony with the phases of respiration. The cervical trachea, especially its caudal part, narrows slightly during inspiration only to recover during expiration. The changes in the thoracic trachea are reciprocal. This physiological variation is not to be confused with the more severe narrowing of the tracheal lumen, possibly amounting to collapse, that sometimes develops with congenital or acquired degeneration of the supporting cartilages. In this pathological condition the cervicothoracic transitional portion of the trachea is most often affected.
There is radiographic evidence that brachycephalic breeds have relatively narrow tracheas, whereas Dachshunds and Basset Hounds have wide ones. Estimates of the normality of the tracheal diameter may be made by comparing it with the height of the thoracic inlet; in some breeds the ratio may be as high as 0.5, whereas in severely affected Bulldogs it may be as low as 0.05.
The trachea is loosely enclosed in a sleeve of fascia. A deeper leaf forms part of the prevertebral fascia that separates the trachea from the longus colli muscle. It also contributes to the carotid sheath, which encloses the vagus and sympathetic nerves, carotid artery, internal jugular vein, and sometimes the tracheal lymph trunk. The carotid sheath is found dorsolateral to the trachea; the recurrent laryngeal nerve follows a similar but independent course.
Each tracheal ring is thickest ventrally and thins along the curves to end dorsally as flexible, potentially overlapping blades. Only the first ring is completely closed in dogs and is partially covered by the cricoid cartilage. The dorsal part of the trachea is composed of connective tissue and muscle. In carnivores, this smooth muscle inserts on the external surface of the cartilages some distance from their tips.
The thyroid gland consists of two elongated rather flattened lobes placed against and loosely attached to the lateral aspects of the first few tracheal cartilages under cover of the sternothyroid muscle (see Figure 6–4, A). Their caudal poles are sometimes connected across the ventral surface of the trachea by a vestigial isthmus. They are embedded in the deep cervical fascia. The sternocephalicus and sternohyoideus muscles pass immediately lateral to the convex surface of each gland, and the sternothyroideus covers each thyroid ventrally. The recurrent laryngeal nerve passes dorsally. In medium-sized dogs the lobes are about 5 cm long (spanning the first five to eight tracheal rings) and 1.5 cm wide. In immature dogs and in those of brachycephalic breeds, they are larger. In cats each thyroid gland lobe is about 2 cm long and 0.3 cm wide. Frequently, accessory thyroid tissue occurs along the trachea at the thoracic inlet, within the mediastinum, and along the thoracic portion of the descending aorta. During development islets of the rapidly proliferating cells of the thyroid primordium separate from the main mass and become incorporated in the developing structures of the branchial arch region and thorax.
The major blood supply to each lobe is provided by a cranial thyroid artery (branching from the common carotid artery), a vessel with a larger distribution than its name suggests. Its thyroid branches include one that follows the dorsal margin caudally to an anastomosis with the much smaller and inconsistent caudal thyroid artery (branching from the brachiocephalic artery); one that follows the ventral margin; and others that pass directly to the cranial pole (and to the external parotid gland). Twigs from all these result in the thyroid being supplied at scattered points around most of its periphery. Blood leaving the gland enters the nearby internal jugular vein, while some is conveyed to the large veins at the thoracic inlet by an unpaired (caudal thyroid) vein lying on the ventral surface of the trachea.
Each lobe is closely associated with two parathyroid glands (discounting the possible existence of accessory parathyroid tissue) in a relationship of obvious relevance to the performance of thyroid surgery. The external (III) parathyroid gland is generally found close to or against the cranial pole of the thyroid to which it is loosely joined; in cats more often than in dogs, this glands descends unusually far from its site of origin (p. 220) and comes to rest near the caudal pole. The internal (IV) parathyroid is located within the connective tissue capsule of the thyroid and may be difficult to discover, especially when completely submerged within thyroid glandular tissue, as sometimes happens. Recognition is assisted by its pale color, which contrasts with the brownish-red thyroid tissue; it can be identified on or within the thyroid gland by ultrasonography. The sizes of the parathyroid glands are rather variable, but on average they are about 3 mm in diameter in dogs. Partial or complete thyroidectomy may be performed in the treatment of thyroid hyperplasia or neoplasia, the former condition now recognized as occurring with great frequency in cats. Certain surgical procedures (intracapsular thyroidectomy) make the concomitant removal of a considerable fraction of the total parathyroid tissue more or less inevitable; this loss is generally tolerated, provided the integrity of the blood supply to the remaining part is preserved. Caution is obviously most necessary when surgery is bilateral.
The common carotid artery runs dorsolateral to the trachea (though the left one is commonly displaced to the side of the esophagus in the caudal half of the neck). It arises from the brachiocephalic trunk about 1 cm apart (sometimes a bicarotid trunk is formed) within the chest and crosses the lateral surface of the trachea (esophagus on the left) obliquely to gain a dorsolateral position in the neck. The cranial thyroid artery, which arises level with the larynx, is the only cervical branch of consequence. The common artery ends at the level of the atlantooccipital joint by dividing into internal and external carotid arteries. The former enters the skull through the carotid foramen after pursuing a rather unusual course (p. 311).
The internal carotid artery (much smaller than the external one) leaves the medial side of the parent vessel and almost at once displays the bulbous enlargement, the carotid sinus (see Figure 7–32). It makes its way between deep structures of the head, crossing the lateral surface of the pharynx, without detaching any branches and enters rostral to the tympanic bulla in the skull to supply the brain. The internal carotid artery is regressed in the adult cat; the main blood supply to the brain is then coming from branches of the maxillary artery.
The external carotid artery forms a sigmoid flexure as it winds its way under the hypoglossal nerve, submandibular salivary gland, and the digastric muscle. Its many branches include the occipital, cranial laryngeal, ascending pharyngeal, lingual, facial, caudal auricular, parotid, superficial temporal, and maxillary arteries (see Figure 7–39). The occipital sometimes arises independently from the common carotid.
The internal jugular vein is formed by the confluence of the vertebral vein, the sigmoid sinus, and, occasionally, the vein of the hypoglossal canal. The internal jugular is first associated with the internal carotid artery in the sheath of the common carotid. This vein usually terminates in the caudal part of the external jugular vein, which is the main channel for venous return from the head. It begins by the union of the linguofacial and maxillary veins. In the adult it contains a few ineffective, irregularly spaced valves.
THE LYMPHATIC STRUCTURES OF THE HEAD AND NECK
Except for the unimportant facial node, the lymph nodes of the head are concentrated caudal to the mandible; those of the neck are found at shoulder level and, inconstantly, scattered along the trachea (Figure 11–47).
Figure 11–47 Lymphatic structures of the canine head and neck. The inset shows the approximate areas of drainage of the principal nodes. 1, Parotid lymph node; 2, mandibular lymph nodes; 3, 3′, medial and lateral retropharyngeal lymph nodes; 4, 4′, 4″, cranial, middle, and caudal deep cervical lymph nodes; 5, superficial cervical lymph nodes; 6, tracheal lymph trunk; 7, thyroid gland; 8, external jugular vein.
PAROTID LYMPH NODE
The parotid lymph node lies on the caudal border of the masseter cranial to the base of the ear, under the rostrodorsal border of the parotid gland. It drains superficial structures such as, broadly, those dorsal to the palate and the ear, including the eyelids and associated glands and the temporomandibular joint. Its efferents drain to the medial retropharyngeal lymph node. It is not always palpable.
MANDIBULAR LYMPH NODE
Two or three mandibular lymph nodes are grouped around the facial vein near the angle of the mandible. They drain superficial structures of the face and also the intermandibular space. Overlap with the region drained by the parotid and the mandibular nodes is present. Their efferents drain to the medial retropharyngeal lymph node. They are always palpable (Figure 11–6/10).
MEDIAL RETROPHARYNGEAL LYMPH NODE
The large medial retropharyngeal lymph node lies medial to the mandibular gland and sternomastoideus, between the wing of the atlas and the larynx. Coursing along its medial surface is the terminal portion of the common carotid artery, as well as the hypoglossal, vagus, and sympathetic nerves and the internal jugular vein. It drains deep structures of the head, including the tongue, palatine tonsil, salivary glands, and the deep parts of the external ear, and also receives lymph from the other nodes in the head. It also receives afferents from the larynx and esophagus in the upper part of the neck. Its efferents form the tracheal lymph trunk. It cannot be palpated (Figure 11–47/3).
LATERAL RETROPHARYNGEAL LYMPH NODE
The lateral retropharyngeal lymph node is placed at the caudal border of the parotid and mandibular glands, when present. It drains deep structures dorsal to it and may be palpated.
TRACHEAL TRUNK
The tracheal trunk arises from the caudal pole of the ipsilateral medial retropharyngeal lymph node and runs in or adjacent to the lateral wall of the carotid sheath. The left trunk usually terminates in the thoracic duct, and the right one terminates in the angle formed by the mergence of the right external jugular and the right axillary veins to form the brachiocephalic vein.
DEEP CERVICAL LYMPH NODES
Small, deep cervical lymph nodes are occasionally found in the vicinity of the thyroid gland and the cervical portion of the trachea. They receive afferents from the larynx, thyroid glands, trachea, esophagus, and the cervical vertebrae. They send their efferents from the cranial caudally to others in the chain and thence the thoracic duct, tracheal trunk, or cranial mediastinal lymph node. The cranial node of the group is located between the caudal end of the medial retropharyngeal lymph node and the thyroid gland, either dorsomedially to the gland along the carotid sheath or on the pharynx cranial to the thyroid. The middle node is positioned along the carotid sheath or ventral to the trachea, in the middle third of the neck.
The caudal node lies on the ventral surface of the caudal third of the cervical trachea.