CHAPTER 14 Diagnostic Tests for the Nasal Cavity and Paranasal Sinuses
NASAL IMAGING
Nasal imaging is a key component of the diagnostic assessment of animals with signs of intranasal disease, allowing assessment of bone and soft tissue structures that are not visible by physical examination or rhinoscopy. Nasal radiography is the type of imaging most readily available and is described in some detail. However, computed tomography (CT) provides images that are superior to radiographs in the majority of cases. The role of magnetic resonance imaging (MRI) in the evaluation of canine and feline nasal disease has not been well established, but it likely provides more accurate images of soft tissue than does CT. MRI is not used routinely on account of its limited availability and relatively high expense.
Because nasal imaging rarely provides a definitive diagnosis, it is usually followed by rhinoscopy and nasal biopsy. All of these procedures require general anesthesia. Imaging should be performed before, rather than after, these procedures for two reasons: (1) The results of nasal imaging help the clinician direct biopsy instruments to the most abnormal regions, and (2) rhinoscopy and biopsy cause hemorrhage, which obscures soft tissue detail.
RADIOGRAPHY
Nasal radiographs are useful for identifying the extent and severity of disease, localizing sites for biopsy within the nasal cavity, and prioritizing the differential diagnoses. The dog or cat must be anesthetized to prevent motion and facilitate positioning. Radiographic abnormalities are often subtle. At least four views should be taken: lateral, ventrodorsal, intraoral, and frontal sinus or skyline. Radiographs of the tympanic bullae are obtained in cats because of the frequent occurrence of otitis media in cats with nasal disease (Detweiler et al., 2006). Determination of involvement of the middle ear is particularly important in cats with suspected nasopharyngeal polyps. Lateral-oblique views or dental films are also indicated in dogs and cats with possible tooth root abscess. The intraoral view is particularly helpful for detecting subtle asymmetry between the left and right nasal cavities.
The intraoral view is taken with the animal in sternal recumbency. The corner of a nonscreen film is placed above the tongue as far into the oral cavity as possible, and the radiographic beam is positioned directly above the nasal cavity (Figs. 14-1 and 14-2). The frontal sinus view is obtained with the animal in dorsal recumbency. Adhesive tape can be used to support the body and draw the forelimbs caudally, out of the field. The head is positioned perpendicular to the spine and the table by drawing the muzzle toward the sternum with adhesive tape. Endotracheal tube and anesthetic tubes are displaced lateral to the head to remove them from the field. A radiographic beam is positioned directly above the nasal cavity and frontal sinuses (Figs. 14-3 and 14-4). The frontal sinus view identifies disease involving the frontal sinuses, which in diseases such as aspergillosis or neoplasia may be the only area of disease involvement. The tympanic bullae are best seen with an open-mouth projection in which the beam is aimed at the base of the skull (Figs. 14-5 and 14-6). The bullae are also evaluated individually by lateral-oblique films, offsetting each bulla from the surrounding skull.
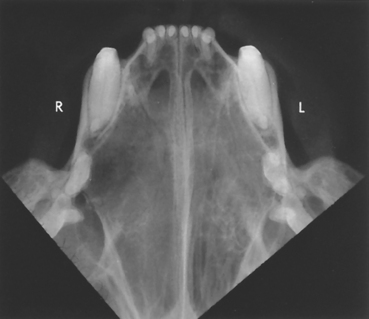
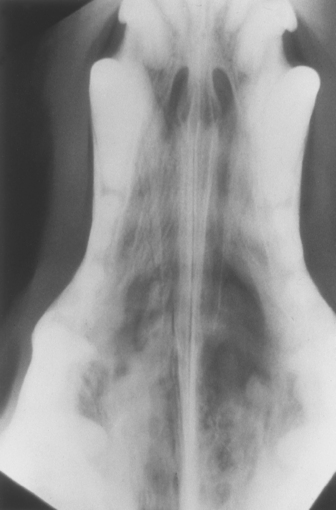
FIG 14-2 Intraoral radiograph of a cat with carcinoma. Normal fine turbinate pattern is visible on the left side (L) of nasal cavity and provides basis for comparison with the right side (R). Turbinate pattern is less apparent on right side, and an area of turbinate lysis can be seen adjacent to the first premolar.
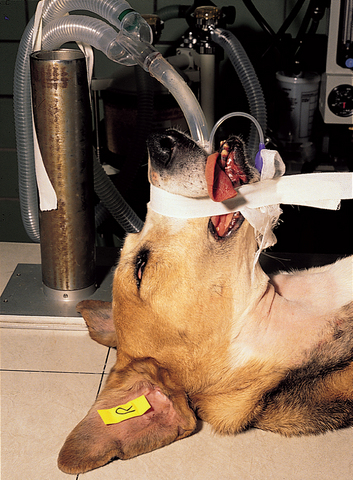
FIG 14-3 Positioning of a dog for frontal sinus radiographs. The endotracheal and anesthetic tubes are displaced laterally in this instance by taping them to an upright metal cylinder.
FIG 14-4 Frontal sinus view of a dog with a nasal tumor. The left frontal sinus (L) has increased soft tissue density compared with the air-filled sinus on the right side (R).

FIG 14-5 Positioning of a cat for open-mouth projection of the tympanic bullae. Beam (arrow) is aimed through the mouth toward the base of the skull. Adhesive tape (t) is holding head and mandible in position.
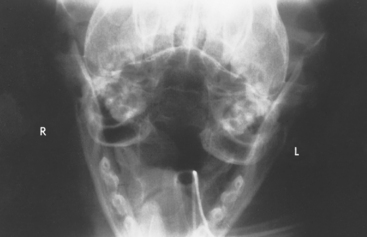
FIG 14-6 Radiograph obtained from a cat with nasopharyngeal polyp using the open-mouth projection demonstrated in Fig. 14-5. The left bulla has thickening of bone and increased fluid density, indicating bulla osteitis and probable extension of the polyp.
Nasal radiographs are evaluated for increased fluid density, loss of turbinates, lysis of facial bones, radiolucency at the tips of the tooth roots, and the presence of radiodense foreign bodies (Box 14-1). Increased fluid density can be caused by mucus, exudate, blood, or soft tissue masses such as polyps, tumors, or granulomas. Soft tissue masses may appear localized, but the surrounding fluid often obscures their borders. A thin rim of lysis surrounding a focal density may represent a foreign body. Fluid density within the frontal sinuses may represent normal mucus accumulation caused by obstruction of drainage into the nasal cavity, extension of disease into the frontal sinuses from the nasal cavity, or primary disease involving the frontal sinuses.
 BOX 14-1 Radiographic Signs of Common Nasal Diseases*
BOX 14-1 Radiographic Signs of Common Nasal Diseases*
Nasopharyngeal Polyp
Soft tissue opacity above soft palate
Soft tissue opacity within nasal cavity, usually unilateral
Bulla osteitis: soft tissue opacity within bulla, thickening of bone
Nasal Aspergillosis
Well-defined lucent areas within the nasal cavity
Increased radiolucency rostrally
Increased soft tissue opacity possibly also present
No destruction of vomer or facial bones, although signs often bilateral
Vomer bone sometimes roughened
Fluid density within the frontal sinus; frontal bones sometimes thickened or moth-eaten
Foreign Bodies
Mineral and metallic dense foreign bodies readily identified
Plant foreign bodies: focal, ill-defined, increased soft tissue opacity
* Note that these descriptions represent typical cases and are not specific findings.
Loss of the normal fine turbinate pattern in combination with increased fluid density within the nasal cavity can occur with chronic inflammatory conditions of any etiology. Early neoplastic changes can also be associated with an increase in soft tissue density and destruction of the turbinates (see Figs. 14-2 and 14-4). More aggressive neoplastic changes may include marked lysis or deformation of the vomer and/or facial bones. Multiple, well-defined lytic zones within the nasal cavity and increased radiolucency in the rostral portion of the nasal cavity suggest aspergillosis (Fig. 14-7). The vomer bone may be roughened but is rarely destroyed. Previous traumatic fracture of the nasal bones and secondary osteomyelitis can also be detected radiographically.
COMPUTED TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING
CT provides excellent visualization of the nasal turbinates, nasal septum, hard palate, and cribriform plate (Fig. 14-8). In cats CT is also useful for determining middle ear involvement with nasopharyngeal polyps or other nasal disease. CT is more accurate than conventional radiography in assessing the extent of neoplastic disease insofar as it allows more accurate localization of mass lesions for subsequent biopsy than nasal radiography, and it is instrumental for radiotherapy treatment planning. Determination of the integrity of the cribriform plate is important in treatment planning for nasal aspergillosis. CT may also identify the presence of lesions in animals with undiagnosed nasal disease when other techniques have failed. Typical lesions are as described in Box 14-1. MRI may be more accurate than CT in the assessment of soft tissues, such as nasal neoplasia.
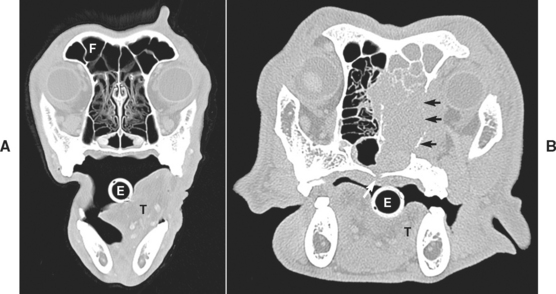
FIG 14-8 CT scans of nasal cavity of two different dogs at the level of the eyes. A, Normal nasal turbinates and intact nasal septum are present. B, Neoplastic mass is present within the right cavity; it is eroding through the hard palate (white arrow), the frontal bone into the retrobulbar space (small black arrows), and the nasal septum. The tumor also extends into the right frontal sinus. F, Frontal sinus; E, endotracheal tube; T, tongue.
RHINOSCOPY
Rhinoscopy allows visual assessment of the nasal cavity through the use of a rigid or flexible endoscope or an otoscopic cone. Rhinoscopy is used to visualize and remove foreign bodies; to grossly assess the nasal mucosa for the presence of inflammation, turbinate erosion, mass lesions, fungal plaques, and parasites; and to aid in the collection of nasal specimens for histopathologic examination and culture. Complete rhinoscopy always includes a thorough examination of the oral cavity and caudal nasopharynx, in addition to visualization of the nasal cavity through the external nares.
The extent of visualization depends on the quality of the equipment and the outside diameter of the rhinoscope. A narrow (2- to 3-mm diameter), rigid fiberoptic endoscope provides good visualization through the external nares in most patients. Endoscopes without biopsy or suction channels are preferable because of their small outside diameter. Some of these systems are relatively inexpensive, including one model that can be attached to a standard otoscope handle for the light source (Fig. 14-9). Scopes designed for arthroscopy, cystoscopy, and sexing of birds also work well. In medium to large dogs, a flexible pediatric bronchoscope (e.g., 4-mm outer diameter) can be used. Flexible endoscopes are now available in smaller sizes, similar to small rigid scopes, although they are relatively more expensive and fragile. If an endoscope is not available, the rostral region of the nasal cavity can be examined with an otoscope. Human pediatric otoscopic cones (2- to 3-mm diameter) can be purchased for examining cats and small dogs.
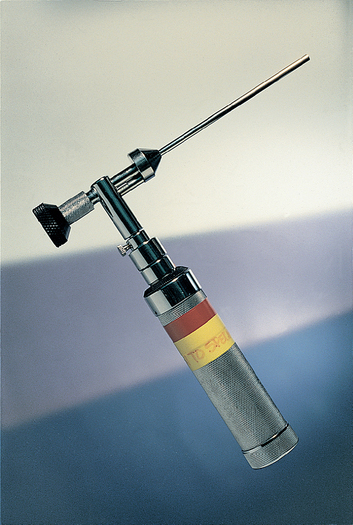
FIG 14-9 Rigid endoscope (diameter, 3.5 mm; length, 4 inches) suitable for rhinoscopy that uses a standard otoscope handle as a light source. (MDS, Inc., Brandon, Fla.)
General anesthesia is required for rhinoscopy. Rhinoscopy is usually performed immediately after nasal imaging unless a foreign body is strongly suspected. The oral cavity and caudal nasopharynx should be assessed first. During the oral examination the hard and soft palates are visually examined and palpated for deformation, erosions, or defects, and the gingival sulci are probed for fistulae.
The caudal nasopharynx is evaluated for the presence of nasopharyngeal polyps, neoplasia, and foreign bodies. Foreign bodies, particularly grass or plant material, are commonly found in this location in cats and occasionally in dogs. The caudal nasopharynx is best visualized with a flexible endoscope that is passed into the oral cavity and retroflexed around the soft palate (Figs. 14-10 through 14-12). Alternatively, the caudal nasopharynx can be evaluated with the aid of a dental mirror, penlight, and spay hook, which is attached to the caudal edge of the soft palate and pulled forward to improve visualization of the area. It may be possible to visualize nasal mites of infected dogs by observing the caudal nasopharynx while flushing anesthetic gases (e.g., halothane and oxygen) through the nares.
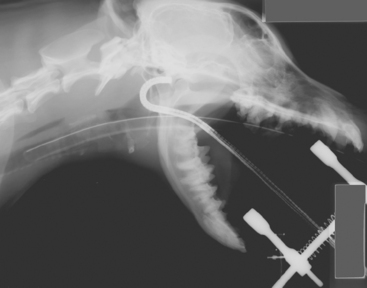
FIG 14-10 The caudal nasopharynx is best examined with a flexible endoscope that is passed into the oral cavity and retroflexed 180 degrees around the edge of the soft palate, as shown in this radiograph.
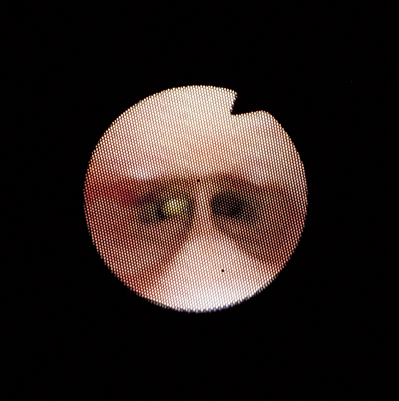
FIG 14-11 View of the internal nares obtained by passing a flexible bronchoscope around the edge of the soft palate in a dog with sneezing. A small white object is seen within the left nasal cavity adjacent to the septum. Note that the septum is narrow and the right internal naris is oval in shape and not obstructed. On removal, the object was found to be a popcorn kernel. The dog had an abnormally short soft palate, and the kernel presumably entered the caudal nasal cavity from the oropharynx.
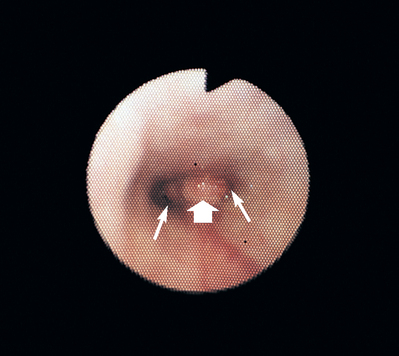
FIG 14-12 View of the internal nares (thin arrows) obtained by passing a flexible bronchoscope around the edge of the soft palate in a dog with nasal discharge. A soft tissue mass (broad arrow) is blocking the normally thin septum and is partially obstructing the airway lumens. Compare this view with the appearance of the normal septum and right internal naris in Fig. 14-11
Rhinoscopy must be performed patiently, gently, and thoroughly to maximize the likelihood of identifying gross abnormalities and minimize the risk of hemorrhage. The more normal side of the nasal cavity is examined first. The tip of the scope is passed through the naris with the tip pointed medially. Each nasal meatus is evaluated, beginning ventrally and working dorsally to ensure visualization should hemorrhage develop during the procedure. Each nasal meatus should be examined as far caudally as the scope can be passed without trauma.
Although the rhinoscope can be used to evaluate the large chambers of the nose, many of the small recesses cannot be examined, even with the smallest endoscopes. Thus disease or a foreign body may be missed if only these small recesses are involved. Swollen and inflamed nasal mucosa, hemorrhage caused by the procedure, and the accumulation of exudate and mucus can also interfere with visualization of the nasal cavity. Foreign bodies and masses are frequently coated and effectively hidden by seemingly insignificant amounts of mucus, exudate, or blood. The tenacious material must be removed using a rubber catheter with the tip cut off attached to a suction unit. If necessary, saline flushes can also be used, although resulting fluid bubbles may further interfere with visualization. Some clinicians prefer to maintain continuous saline infusion of the nasal cavity using a standard intravenous administration set attached to a catheter or, if available, the biopsy channel of the rhinoscope. The entire examination is done “under water.”
No catheter should ever be passed blindly into the nasal cavity beyond the level of the medial canthus of the eye to avoid entering the cranial vault through the cribriform plate. The clinician must be sure the endotracheal tube cuff is fully inflated and the back of the pharynx is packed with gauze to prevent aspiration of blood, mucus, or saline flush into the lungs. The clinician must be careful not to overinflate the endotracheal tube cuff, which could result in a tracheal tear.
The nasal mucosa is normally smooth and pink, with a small amount of serous to mucoid fluid present along the mucosal surface. Potential abnormalities visualized with the rhinoscope include inflammation of the nasal mucosa; mass lesions; erosion of the turbinates (Fig. 14-13, A); mats of fungal hyphae (Fig. 14-13, B); foreign bodies; and, rarely, nasal mites or Capillaria worms (Fig. 14-14). Differential diagnoses for gross rhinoscopic abnormalities are provided in Box 14-2.
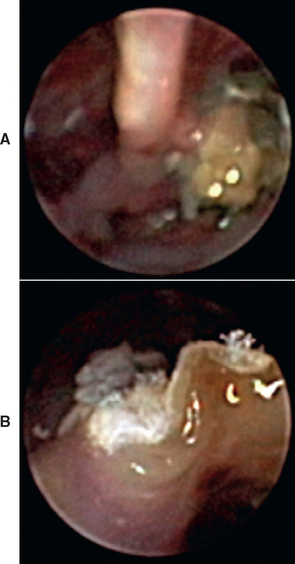
FIG 14-13 A, Rhinoscopic view through the external naris of a dog with aspergillosis showing erosion of turbinates and a green-brown granulomatous mass. B, A closer view of the fungal mat shows white, filamentous structures (hyphae).
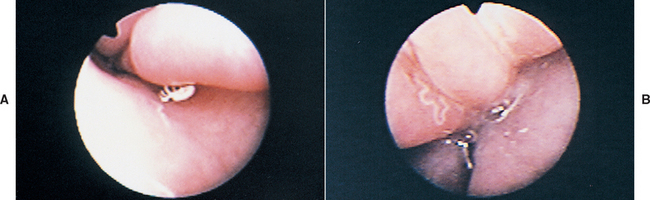
FIG 14-14 Rhinoscopic view through the external naris. A, A single nasal mite is seen in this dog with Pneumonyssoides caninum. B, A thin white worm is seen in this dog with Capillaria (Eucoleus) boehmi.
 BOX 14-2 Differential Diagnoses for Gross Rhinoscopic Abnormalities in Dogs and Cats
BOX 14-2 Differential Diagnoses for Gross Rhinoscopic Abnormalities in Dogs and Cats
Inflammation (Mucosal Swelling, Hyperemia, Increased Mucus, Exudate)
Nonspecific finding; consider all differential diagnoses for mucopurulent nasal discharge (infectious, inflammatory, neoplastic)
The location of any abnormality should be noted, including the meatus involved (common, ventral, middle, dorsal), the medial-to-lateral orientation within the meatus, and the distance caudal from the naris. Exact localization is critical for directing instruments for the retrieval of foreign bodies or nasal biopsy should visual guidance become impeded by hemorrhage or size of the cavity.
NASAL BIOPSY: INDICATIONS AND TECHNIQUES
Visualization of a foreign body or nasal parasites during rhinoscopy establishes a diagnosis. For many dogs and cats, however, the diagnosis must be based on cytologic, histologic, and microbiologic evaluation of nasal biopsy specimens. Nasal biopsy specimens should be obtained immediately after nasal imaging and rhinoscopy while the animal is still anesthetized. These earlier procedures can help localize the lesion, maximizing the likelihood of obtaining material representative of the primary disease process.
Nasal biopsy techniques include nasal swab, nasal flush, pinch biopsy, and turbinectomy. Fine-needle aspirates can be obtained from mass lesions as described in Chapter 75. Pinch biopsy is the preferred nonsurgical method of specimen collection. It is more likely to provide pieces of nasal tissue that extend beneath the superficial inflammation, which is common to many nasal disorders, than nasal swabs or flushes. In addition, the pieces of tissue obtained with this more aggressive method can be evaluated histologically, whereas the material obtained with the less traumatic techniques may be suitable only for cytologic analysis. Histopathologic examination is preferred over cytologic examination in most cases because the marked inflammation that accompanies many nasal diseases makes it difficult to cytologically differentiate primary from secondary inflammation and reactive from neoplastic epithelial cells. Carcinomas can also appear cytologically as lymphoma and vice versa.
Regardless of the technique used (except for nasal swab), the cuff of the endotracheal tube should be inflated (avoiding overinflation) and the caudal pharynx packed with gauze sponges to prevent the aspiration of fluid. Intravenous crystalloid fluids (10 to 20ml/kg/h plus replacement of estimated blood loss) are recommended during the procedure to counter the hypotensive effects of prolonged anesthesia and blood loss from hemorrhage after biopsy. Blood-clotting capabilities should be assessed before the more aggressive biopsy techniques are performed if there is any history of hemorrhagic exudate or epistaxis or any other indication of coagulopathy.
NASAL SWAB
The least traumatic techniques are the nasal swab and nasal flush. Unlike the other collection techniques, nasal swabs can be collected from an awake animal. Nasal swabs are useful for identifying cryptococcal organisms cytologically and should be collected early in the evaluation of cats with chronic rhinitis. Other findings are generally nonspecific. Exudate immediately within the external nares or draining from the nares is collected using a cotton-tipped swab. Relatively small swabs are available (e.g., Dacron swabs; Puritan Medical Products Co. LLC) that can facilitate specimen collection from cats with minimal discharge. The swab is then rolled on a microscope slide. Routine cytologic stains are generally used, although India ink can be applied to demonstrate cryptococcal organisms (see Chapter 98).
NASAL FLUSH
Nasal flush is a minimally invasive technique. A soft catheter is positioned in the caudal region of the nasal cavity via the oral cavity and internal nares, with the tip of the catheter pointing rostrally. With the animal in sternal recumbency and the nose pointed toward the floor, approximately 100ml of sterile saline solution is forcibly injected in pulses by syringe. The fluid exiting the external nares is collected in a bowl and can be examined cytologically. Occasionally nasal mites can be identified in nasal flushings. Magnification or placement of dark paper behind the specimen for contrast may be needed to visualize the mites. A portion of fluid can also be filtered through a gauze sponge. Large particles trapped in the sponge can be retrieved and submitted for histopathologic analysis. These specimens are often insufficient for providing a definitive diagnosis.
PINCH BIOPSY
Pinch biopsy is the author’s preferred method of nasal biopsy. In the pinch biopsy technique, alligator cup biopsy forceps (minimum size, 2 × 3 mm) are used to obtain pieces of nasal mucosa for histologic evaluation (Fig. 14-15). Full-thickness tissue specimens can be obtained, and guided specimen collection is more easily performed with this technique than with previously described methods. The biopsy forceps are passed adjacent to a rigid endoscope and directed to any gross lesions. If a flexible scope is used, biopsy instruments can be passed through the biopsy channel of the endoscope. The resulting specimens are extremely small and may not be of sufficient quality for diagnostic purposes. Larger alligator forceps are preferred. If lesions are not present grossly but are present radiographically or by CT, the biopsy instrument can be guided using the relationship of the lesion to the upper teeth.
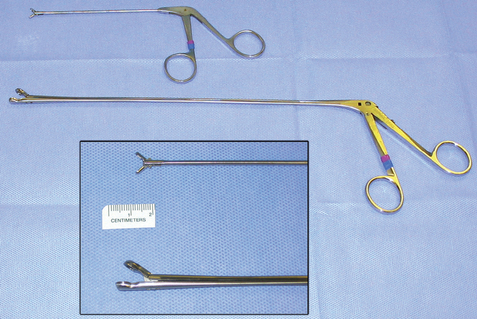
FIG 14-15 Cup biopsy forceps are available in different sizes. To obtain sufficient tissue, a minimum size of 2 × 3 mm is recommended. The larger forceps are particularly useful for obtaining biopsies from nasal masses in dogs.
After the first piece is taken, bleeding will prevent further visual guidance; therefore the forceps are passed blindly to the position identified during rhinoscopic examination (e.g., meatus involved and depth from external naris). If a mass is present, the forceps are passed in a closed position until just before the mass is reached. The forceps are then opened and passed a short distance farther until resistance is felt. Larger forceps, such as a mare uterine biopsy instrument, are useful for collecting large volumes of tissue from medium to large size dogs with nasal masses. No forceps should ever be passed into the nasal cavity deeper than the level of the medial canthus of the eye without visual guidance to keep from penetrating the cribriform plate.
A minimum of six tissue specimens (using a 2 × 3 mm forcep or larger) should be obtained from any lesion. If no localizable lesion is identified radiographically or rhinoscopically, multiple biopsies (usually 6 to 10) are obtained randomly from both sides of the nasal cavity.
TURBINECTOMY
Turbinectomy provides the best tissue specimens for histologic examination and allows the clinician to remove abnormal or poorly vascularized tissues, debulk fungal granulomas, and place drains for subsequent topical nasal therapy. Turbinectomy is performed through a rhinotomy incision and is a more invasive technique than those previ ously described. Turbinectomy is a reasonably difficult surgical procedure that should be considered only when other less invasive techniques have failed to establish the diagnosis. Potential operative and postoperative complications include pain, excessive hemorrhage, inadvertent entry into the cranial vault, and recurrent nasal infections. Cats may be anorectic postoperatively. Placement of an esophagostomy or gastrostomy tube (see Chapter 30) should be considered if necessary to provide a means for meeting nutritional requirements during the recovery period. (See Suggested Readings in Chapter 13 for information on the surgical procedure.)
Complications
The major complication associated with nasal biopsy is hemorrhage. The severity of hemorrhage depends on the method used to obtain the biopsy, but even with aggressive techniques the hemorrhage is rarely life threatening. When any technique is used, the floor of the nasal cavity is avoided to prevent damage to major blood vessels. For minor hemorrhage, the rate of administration of intravenous fluids should be increased and manipulations within the nasal cavity should be stopped until the bleeding subsides. Cold saline solution with or without diluted epinephrine (1 : 100,000) can be gently infused into the nasal cavity. Persistent severe hemorrhage can be controlled by packing the nasal cavity with umbilical tape. The tape must be packed through the nasopharynx as well as through the external nares or the blood will only be redirected. Similarly, placing swabs or gauze in the external nares serves only to redirect blood caudally. In the rare event of uncontrolled hemorrhage, the carotid artery on the involved side can be ligated without subsequent adverse effects. Rhinotomy should not be attempted. In the vast majority of animals, only time or cold saline infusions are required to control hemorrhage. The fear of severe hemorrhage should not prevent the collection of good-quality tissue specimens.
Trauma to the brain is prevented by never passing any object into the nasal cavity beyond the level of the medial canthus of the eye without visual guidance. The distance from the external nares to the medial canthus is noted by holding the instrument or catheter against the face, with the tip at the medial canthus. The level of the nares is marked on the instrument or catheter with a piece of tape or marking pen. The object should never be inserted beyond that mark.
Aspiration of blood, saline solution, or exudate into the lungs must be avoided. A cuffed endotracheal tube should be in place during the procedure, and the caudal pharynx should be packed with gauze after visual assessment of the oral cavity and nasopharynx. The cuff should be sufficiently inflated to prevent audible leakage of air during gentle compression of the reservoir bag of the anesthesia machine. Overinflation of the cuff may lead to tracheal trauma or tear. The nose is pointed toward the floor over the end of the examination table, allowing blood and fluid to drip out from the external nares after rhinoscopy and biopsy. Finally, the caudal pharynx is examined during gauze removal and before extubation for visualization of continued accumulation of fluid. Gauze sponges are counted during placement and then recounted during removal so that none is inadvertently left behind.
NASAL CULTURES: SAMPLE COLLECTION AND INTERPRETATION
Microbiologic cultures of nasal specimens are recommended but can be difficult to interpret. Aerobic and anaerobic bacterial cultures, mycoplasmal cultures, and fungal cultures can be performed on material obtained by swab, nasal flush, or tissue biopsy. According to Harvey (1984), the normal nasal flora can include Escherichia coli, Staphylococcus, Streptococcus, Pseudomonas, Pasteurella, and Aspergillus organisms and a variety of other aerobic and anaerobic bacteria and fungi. Thus bacterial or fungal growth from nasal specimens does not necessarily confirm the presence of infection.
Cultures should be performed on specimens collected within the caudal nasal cavity of anesthetized patients. Bacterial growth from superficial specimens, such as nasal discharge or swabs inserted into the external nares of unanesthetized patients, is unlikely to be clinically significant. It is difficult for a culture swab to be passed into the caudal nasal cavity without its being contaminated with superficial (insignificant) organisms. Guarded specimen swabs can prevent contamination but are relatively expensive. Alternatively, mucosal biopsies from the caudal nasal cavity can be obtained for culture using sterilized biopsy forceps; the results may be more indicative of true infection than those from swabs because, in theory, the organisms have invaded the tissues. Superficial contamination may still occur.
Regardless of the method used, the growth of many colonies of one or two types of bacteria more likely reflects infection than the growth of many different organisms. The microbiology laboratory should be asked to report all growth. Otherwise, the laboratory may report only one or two organisms that are more often pathogenic and provide misleading information about the relative purity of the culture. The presence of septic inflammation based on histologic examination of nasal specimens and a positive response to antibiotic therapy support a diagnosis of bacterial infection contributing to clinical signs. Although bacterial rhinitis is rarely a primary disease entity, improvement in nasal discharge may be seen if the bacterial component of the problem is treated; however, the improvement is generally transient unless the underlying disease process can be corrected. Some animals in which a primary disease process is never identified or cannot be corrected (e.g., cats with chronic rhinosinusitis) respond well to long-term antibiotic therapy. Sensitivity data from bacterial cultures considered to represent significant infection may help in antibiotic selection. (See Chapter 15 for further therapeutic recommendations.)
The role of Mycoplasma spp. in respiratory tract infections of dogs and cats is still being elucidated. Cultures for Mycoplasma spp. and treatment with appropriate antibiotics are a consideration for cats with chronic rhinosinusitis.
A diagnosis of nasal aspergillosis or penicilliosis requires the presence of several supportive signs, and fungal cultures are indicated whenever fungal disease is one of the differential diagnoses. The growth of Aspergillus or Penicillium organisms is considered along with other clinical data, such as radiographic and rhinoscopic findings, and serologic titers. Fungal growth supports a diagnosis of mycotic rhinitis only when other data also support the diagnosis. The fact that fungal infection occasionally occurs secondary to nasal tumors should not be overlooked during initial evaluation and monitoring of therapeutic response. The sensitivity of fungal culture can be greatly enhanced by collecting a swab or biopsy for culture directly from a fungal plaque or granuloma with rhinoscopic guidance.
Codner EC, et al. Comparison of computed tomography with radiography as a noninvasive diagnostic technique for chronic nasal disease in dogs. J Am Vet Med Assoc. 1993;202:1106.
Detweiler DA, et al. Computed tomographic evidence of bulla effusion in cats with sinonasal disease: 2001-2004. J Vet Intern Med. 2006;20:1080.
Harvey CE. Therapeutic strategies involving antimicrobial treatment of the upper respiratory tract in small animals. J Am Vet Med Assoc. 1984;185:1159.
Lefebvre J. Computed tomography as an aid in the diagnosis of chronic nasal disease in dogs. J Small Anim Pract. 2005;46:280.
McCarthy TC. Rhinoscopy: the diagnostic approach to chronic nasal disease. In: McCarthy TR, editor. Veterinary endoscopy for the small animal practitioner. St Louis: Saunders; 2005:137.
Padrid PA, et al. Endoscopy of the upper respiratory tract of the dog and cat. In: Tams TR, editor. Small animal endoscopy. ed 2. St Louis: Mosby; 1999:357.
Schoenborn WC, et al. Retrospective assessment of computed tomographic imaging of feline sinonasal disease in 62 cats. Vet Rad Ultrasound. 2003;44:198.
Willard MD, et al. Endoscopic examination of the choane in dogs and cats: 118 cases (1988–1998). J Am Vet Med Assoc. 1999;215:1301.