CHAPTER 15 Disorders of the Nasal Cavity
FELINE UPPER RESPIRATORY INFECTION
Etiology
Upper respiratory infections (URIs) are common in cats. Feline herpesvirus (FHV), also known as feline rhinotracheitis virus, and feline calicivirus (FCV), cause nearly 90% of these infections. Bordetella bronchiseptica and Chlamydophila felis (previously known as Chlamydia psittaci) are less commonly involved. Other viruses and Mycoplasmas may play a primary or secondary role, whereas other bacteria are considered secondary pathogens.
Cats become infected through contact with actively infected cats, carrier cats, and fomites. Cats that are young, stressed, or immunosuppressed are most likely to develop clinical signs. Infected cats often become carriers of FHV or FCV after resolution of the clinical signs. The duration of the carrier state is not known but may last from weeks to years. Bordetella can be isolated from asymptomatic cats, although the effectiveness of transmission of disease from such cats is not known.
Clinical Features
Clinical manifestations of feline URI can be acute, chronic and intermittent, or chronic and persistent. Acute disease is the most common. The clinical signs of acute URI include fever, sneezing, serous or mucopurulent nasal discharge, conjunctivitis and ocular discharge, hypersalivation, anorexia, and dehydration. FHV can also cause corneal ulceration, abortion, and neonatal death, whereas FCV can cause oral ulcerations, mild interstitial pneumonia, or polyarthritis. Rare, short-lived outbreaks of highly virulent strains of calicivirus have been associated with severe upper respiratory disease, signs of systemic vasculitis (facial and limb edema progressing to focal necrosis) and high rates of mortality. Bordetella can cause cough and, in young kittens, pneumonia. Signs of Chlamydophila infection are usually limited to conjunctivitis.
Some cats that recover from the acute disease have periodic recurrence of acute signs, usually in association with stressful or immunosuppressive events. Other cats may have chronic, persistent signs, most notably a serous to mucopurulent nasal discharge with or without sneezing. Chronic nasal discharge can presumably result from persistence of an active viral infection or from irreversible damage to turbinates and mucosa by FHV; the latter predisposes the cat to an exaggerated response to irritants and secondary bacterial rhinitis. Unfortunately, correlation between tests to confirm exposure to or the presence of viruses and clinical signs is poor (Johnson et al., 2005). Because the role of viral infection in cats with chronic rhinosinusitis is not well understood, cats with chronic signs of nasal disease are discussed in the section on feline chronic rhinosinusitis (p. 232).
Diagnosis
Acute URI is usually diagnosed on the basis of history and physical examination findings. Specific tests that are available to identify FHV, FCV, Bordetella, and Chlamydophila organisms include fluorescent antibody testing, virus isolation procedures or bacterial cultures, polymerase chain reaction (PCR), and serum antibody titers. Fluorescent antibody tests for FHV and FCV are performed on smears prepared from conjunctival scrapings, pharyngeal swabs, or tonsillar swabs or on impression smears from tonsillar biopsy specimens. Virus isolation tests and PCR can be performed on pharyngeal, conjunctival, or nasal swabs (using sterile swabs made of cotton) or on tissue specimens such as tonsillar biopsy specimens or mucosal scraping. Tissue specimens are preferred for virus isolation and PCR. Specimens are placed in appropriate transport media. Routine cytologic preparations of conjunctival smears can be examined for intracytoplasmic inclusion bodies suggestive of Chlamydophila infections, but these findings are nonspecific. Although routine bacterial cultures of the oropharynx can be used to identify Bordetella, the organism can be found in healthy and infected cats. Demonstration of rising antibody titers against a specific agent over 2 to 3 weeks suggests active infection. Regardless of the method used, close coordination with the pathology laboratory on specimen collection and handling is recommended for optimal results.
Tests to identify specific agents are particularly useful in cattery outbreaks in which the clinician is asked to recommend specific preventive measures. Multiple cats, both with and without clinical signs, should be tested when performing cattery surveys. Specific diagnostic tests are less useful for testing individual cats because their results do not alter therapy; false-negative results may occur if signs are the result of permanent nasal damage or if the specimen does not contain the agent, and, positive results may merely reflect a carrier cat that has a concurrent disease process causing the clinical signs. The exception to this generalization is individual cats with suspected Chlamydophila infection, in which case specific effective therapy can be recommended.
Treatment
In most cats URI is a self-limiting disease, and treatment of cats with acute signs includes appropriate supportive care. Hydration and nutritional needs should be provided when necessary. Dried mucus and exudate should be cleaned from the face and nares. The cat can be placed in a steamy bathroom or a small room with a vaporizer for 15 to 20 minutes two or three times daily to help clear excess secretions. Severe nasal congestion is treated with pediatric topical decongestants such as 0.25% phenylephrine or 0.025% oxymetazoline. A drop is gently placed in each nostril daily for a maximum of 3 days. If longer therapy is necessary, the decongestant is withheld for 3 days before beginning another 3-day course to prevent possible rebound congestion after withdrawal of the drug (based on problems with rebound congestion that occurs in people). Another option for prolonged decongestant therapy is to alternate daily the naris treated.
Antibiotic therapy to treat secondary infection is indicated in cats with severe clinical signs. The initial antibiotic of choice is ampicillin (22 mg/kg q8h) or amoxicillin (22 mg/kg q8h to q12h), because they are often effective, are associated with few adverse reactions, and can be administered to kittens. If Bordetella, Chlamydophila, or Mycoplasma spp. is suspected, doxycycline (5 to 10 mg/kg q12h, followed by a bolus of water) or chloramphenicol (10 to 15 mg/kg q12h) should be used. Azithromycin (5 to 10 mg/kg q24h for 3 days, then q72h) can be prescribed for cats that are difficult to medicate.
Cats with FHV infection may benefit from treatment with lysine. It has been postulated that excessive concentrations of lysine may antagonize arginine, a promoter of herpesvirus replication. Lysine (500mg/cat q12h), obtained from health food stores, is added to food. Administration of feline recombinant omega interferon or human recombinant α-2b interferon may also be of some benefit in FHV-infected cats (Siebeck et al., 2006).
Chlamydophila infection should be suspected in cats with conjunctivitis as the primary problem and in cats from catteries in which the disease is endemic. Oral antibiotics are administered for 3 weeks. In addition, chloramphenicol or tetracycline ophthalmic ointment should be applied at least three times daily and continued for a minimum of 14 days after the resolution of signs.
Corneal ulcers resulting from FHV are treated with topical antiviral drugs, such as trifluridine, idoxuridine, or adenine arabinoside. One drop should be applied to each affected eye five to six times daily for no longer than 2 to 3 weeks. Routine ulcer management is also indicated. Tetracycline or chloramphenicol ophthalmic ointment is administered two to four times daily. Topical atropine is used for mydriasis as needed to control pain. Treatment is continued for 1 to 2 weeks after epithelialization has occurred.
Topical and systemic corticosteroids are contraindicated in cats with acute URI or ocular manifestations of FHV infection. They can prolong clinical signs and increase viral shedding.
Treatment of cats with chronic signs is discussed on p. 233.
Prevention in the Individual Pet Cat
Prevention of URI in all cats is based on avoiding exposure to the infectious agents (e.g., FHV, FCV, Bordetella and Chlamydophila organisms) and strengthening immunity against infection. Most household cats are relatively resistant to prolonged problems associated with URIs, and routine health care with regular vaccination using a subcutaneous product is adequate. Vaccination decreases severity of clinical signs resulting from URIs but does not prevent infection. Owners should be discouraged from allowing their cats to roam freely outdoors.
Subcutaneous modified-live virus vaccines for FHV and FCV are used for most cats and are available in combination with panleukopenia vaccine. These vaccines are convenient to administer, do not result in clinical signs when used correctly, and provide adequate protection for cats that are not heavily exposed to these viruses. These vaccines are not effective in kittens while maternal immunity persists. Kittens are usually vaccinated beginning at 6 to 10 weeks of age and again in 3 to 4 weeks. At least two vaccines must be given initially, with the final vaccine administered after the kitten is 16 weeks old. A booster vaccination is recommended 1 year after the final vaccine in the initial series. Subsequent booster vaccinations are recommended every 3 years, unless the cat has increased risk of exposure to infection. A study by Lappin et al. (2002) indicates that detection of FHV and FCV antibodies in the serum of cats is predictive of susceptibility to disease and therefore may be useful in determining need for revaccination. Queens should be vaccinated before breeding.
Subcutaneous modified-live vaccines for FHV and FCV are safe but can cause disease if introduced into the cat by the normal oronasal route of infection. The vaccine should not be aerosolized in front of the cat. Vaccine inadvertently left on the skin after injection should be washed off immediately before the cat licks the area.
Modified-live vaccines should not be used in pregnant queens. Killed products are available for FHV and FCV that can be used in pregnant queens. Killed vaccines have also been recommended for cats with feline leukemia virus (FeLV) or feline immunodeficiency virus (FIV) infection.
Modified-live vaccines for FHV and FCV are also available for intranasal administration. Signs of acute URI occasionally occur after vaccination. Attention should be paid to ensure that panleukopenia is included in the intranasal product or that a panleukopenia vaccine is administered subcutaneously.
Vaccines against Bordetella or Chlamydophila are recommended for use only in catteries or shelters where these infections are endemic. Infections with Bordetella or Chlamydophila are less common than FHV and FCV infection, and disease resulting from Bordetella infections occurs primarily in cats housed in crowded conditions. Furthermore, these diseases can be effectively treated with antibiotics.
BACTERIAL RHINITIS
Acute bacterial rhinitis caused by Bordetella bronchiseptica occurs occasionally in cats (see the section on feline upper respiratory infection) and rarely in dogs (see the section on canine infectious tracheobronchitis in Chapter 21). It is possible that Mycoplasma can act as primary nasal pathogens. In the vast majority of cases, bacterial rhinitis is a secondary complication and not a primary disease process. Bacterial rhinitis occurs secondarily to almost all diseases of the nasal cavity. The bacteria that inhabit the nasal cavity in health are quick to overgrow when disease disrupts normal mucosal defenses. Antibiotic therapy often leads to clinical improvement, but the response is usually temporary. Therefore management of dogs and cats with suspected bacterial rhinitis should include a thorough diagnostic evaluation for an underlying disease process, particularly when signs are chronic.
Diagnosis
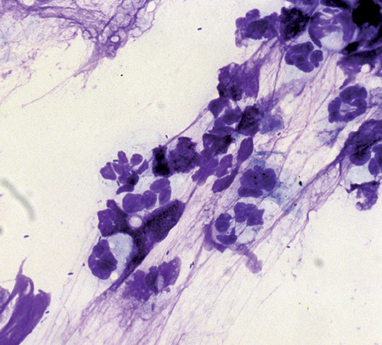
Most dogs and cats with bacterial rhinitis have mucopurulent nasal discharge. No clinical signs are pathognomonic for bacterial rhinitis, and it is difficult to make a definitive diagnosis because of the diverse flora in the normal nasal cavity (see Chapter 14). Microscopic evidence of neutrophilic inflammation and bacteria is a nonspecific finding in the majority of animals with nasal signs (Fig. 15-1). Bacterial cultures of swabs or nasal mucosal biopsies collected deep within the nasal cavity can be performed. The growth of many colonies of only one or two organisms may represent significant infection. Growth of many different organisms or small numbers of colonies probably represents normal flora. The microbiology laboratory should be requested to report all growth. Specimens for Mycoplasmal cultures should be placed in appropriate transport media for culture using specific isolation methods. Beneficial response to antibiotic therapy is often used to support a diagnosis of bacterial involvement.
Treatment
The bacterial component of nasal disease is treated with antibiotic therapy. If growth obtained by bacterial culture is believed to be significant, sensitivity information can be used in selecting antibiotics. Anaerobic organisms may be involved. Broad-spectrum antibiotics that may be effective include amoxicillin (22mg/kg q8-12h), trimethoprimsulfadiazine (15mg/kg q12h), chloramphenicol (50mg/kg q8h for dogs; 10 to 15mg/kg q12h for cats), or clindamycin (5.5 to 11mg/kg q12h). Doxycycline (5 to 10mg/kg q12h, followed by a bolus of water) or chloramphenicol is often effective against Bordetella and Mycoplasma organisms.
For acute infection or in cases in which the primary etiology (e.g., foreign body, diseased tooth root) has been eliminated, antibiotics are administered for 7 to 10 days. Chronic infections require prolonged treatment. Antibiotics are administered initially for 1 week. If a beneficial response is seen, the drug is continued for a minimum of 4 to 6 weeks. If signs recur after discontinuation of drug after 4 to 6 weeks, the same antibiotic is reinstituted for even longer periods.
If no response is seen after the initial week of treatment, the drug should be discontinued. Another antibiotic can be tried, although further evaluation for another, as yet unidentified, primary disorder should be pursued. Further diagnostic evaluation is particularly warranted in dogs because, compared with cats, they less frequently have idiopathic disease. Frequent stopping and starting of different antibiotics every 7 to 14 days is not recommended and may predispose the animal to the growth of resistant gram-negative infections.
NASAL MYCOSES
CRYPTOCOCCOSIS
Cryptococcus neoformans is a fungal agent that infects cats and, less commonly, dogs. It most likely enters the body through the respiratory tract and, in some animals, may disseminate to other organs. In cats clinical signs usually reflect infection of the nasal cavity, central nervous system (CNS), eyes, or skin and subcutaneous tissues. In dogs signs of CNS involvement are most common. The lungs are commonly infected in both species, but clinical signs of lung involvement (e.g., cough, dyspnea) are rare. Clinical features, diagnosis, and treatment of cryptococcosis are discussed in Chapter 98.
ASPERGILLOSIS
Aspergillus fumigatus is a normal inhabitant of the nasal cavity in many animals. In some dogs and, rarely, cats, it becomes a pathogen. The mold form of the organism can develop into visible fungal plaques that invade the nasal mucosa (“fungal mats”) and fungal granulomas. An animal that develops aspergillosis may have another nasal condition, such as neoplasia, foreign body, prior trauma, or immune deficiency that predisposes the animal to secondary fungal infection. Excessive exposure to Aspergillus organisms may explain the frequent occurrence of disease in otherwise healthy animals. Another type of fungus, Penicillium, can cause signs similar to those of aspergillosis.
Clinical Features
Aspergillosis can cause chronic nasal disease in dogs of any age or breed but is most common in young male dogs. Nasal infection is rare in cats. The discharge can be mucoid, mucopurulent with or without hemorrhage, or purely hemorrhagic. The discharge can be unilateral or bilateral. Sneezing may be reported. Features that are highly suggestive of aspergillosis are sensitivity to palpation of the face or depigmentation and ulceration of the external nares (see Fig. 13-1). Lung involvement is not expected.
Systemic aspergillosis in dogs is generally caused by Aspergillus terreus and other Aspergillus spp. rather than A. fumigatus. It is an unusual, generally fatal disease that occurs primarily in German Shepherd Dogs. Nasal signs are not reported.
Diagnosis
No single test result is diagnostic for infection with aspergillosis. The diagnosis is based on the cumulative findings of a comprehensive evaluation of a dog with appropriate clinical signs. In addition, aspergillosis can be an opportunistic infection, and underlying nasal disease must always be considered.
Radiographic signs of aspergillosis include well-defined lucent areas within the nasal cavity and increased radiolucency rostrally (see Fig. 14-7). Typically no destruction of the vomer or facial bones occurs, although the bones may appear roughened. However, destruction of these bones or the cribriform plate may occur in dogs with advanced disease. Increased fluid opacity may be present. Fluid opacity within the frontal sinus can represent a site of infection or mucus accumulation from obstructed drainage. In some patients the frontal sinus is the only site of infection.
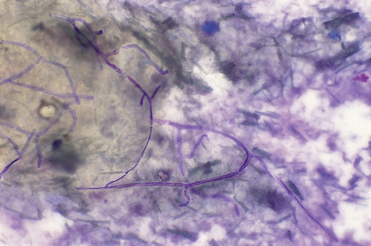
Rhinoscopic abnormalities include erosion of nasal turbinates and fungal plaques, which appear as white-to-green plaques of mold on the nasal mucosa (see Fig. 14-13). Failure to visualize these lesions does not rule out aspergillosis. Confirmation that presumed plaques are indeed fungal hyphae can be achieved by cytology (Fig. 15-2) and culture of material collected by biopsy or swab under visual guidance. During rhinoscopy, plaques are mechanically debulked by scraping or vigorous flushing to increase the efficacy of topical treatment.
Aspergillus organisms can generally be seen histologically in biopsy specimens of affected nasal mucosa after routine staining techniques, although special staining can be performed to identify subtle involvement. Neutrophilic, lymphoplasmacytic, or mixed inflammation is usually also present. Multiple biopsy specimens should be obtained because the mucosa is affected multifocally rather than diffusely. Invasion of fungal organisms into the nasal mucosa is indicative of infection.
Results of fungal cultures are difficult to interpret, unless the specimen is obtained from a visualized plaque. The organism can be found in the nasal cavity of normal animals, and false-negative culture results can also occur. A positive culture, in conjunction with other appropriate clinical and diagnostic findings, supports the diagnosis.
Positive serum antibody titers also support a diagnosis of infection. Although titers are indirect evidence of infection, animals with Aspergillus organisms as a normal nasal inhabitant do not usually develop measurable antibodies against the organism. Pomerantz et al. (2007) found that serum antibodies had a sensitivity of 67%, a specificity of 98%, a positive predictive value of 98%, and a negative predictive value of 84% for the diagnosis of nasal aspergillosis. Their study included 21 dogs with aspergillosis, 25 dogs with nonfungal rhinitis, and 12 dogs with nasal neoplasia.
Treatment
The current treatments of choice for nasal aspergillosis are topical clotrimazole, with a success rate of 80% to 90% with one or more treatments, and oral itraconazole, with a success rate of 60% to 70%. Oral therapy is simpler to administer than topical therapy but is somewhat less successful, requires prolonged treatment, and is relatively expensive. Itraconazole is administered orally at a dose of 5mg/kg every 12 hours and must be continued for 60 to 90 days or longer. (See Chapter 98 for a complete discussion of this drug.)
Successful topical treatment of aspergillosis was originally documented with enilconazole administered through tubes placed surgically into both frontal sinuses and both sides of the nasal cavity. The drug was administered through the tubes twice daily for 7 to 10 days. Subsequently, it was discovered that the over-the-counter drug clotrimazole was equally efficacious when infused through surgically placed tubes over a 1-hour period. During the 1-hour infusion, the dogs were kept under anesthesia and the caudal nasopharynx and external nares were packed to allow filling of the nasal cavity. It has since been demonstrated that good distribution of the drug can be achieved using a noninvasive technique (discussed in the next paragraphs). Success with clotrimazole using this technique has been similar to that documented with infusion through surgically placed tubes. Debridement of visible fungal plaques during rhinoscopy and before topical therapy appears to increase the rate of success.
The animal is anesthetized and oxygenated through a cuffed endotracheal tube. The dog is positioned in dorsal recumbency with the nose pulled down parallel with the table (Figs. 15-3 and 15-4). For a large-breed dog, a 24Fr Foley catheter with a 5-ml balloon is passed through the oral cavity, around the soft palate, and into the caudal nasopharynx such that the bulb is at the junction of the hard and soft palates. The bulb is inflated with approximately 10ml of air to ensure a snug fit. A laparotomy sponge is inserted within the oropharynx, caudal to the balloon and ventral to the soft palate to help hold the balloon in position and further obstruct the nasal pharynx. Additional laparotomy sponges are packed carefully into the back of the mouth around the tracheal tube to prevent any drug that might leak past the nasopharyngeal packing from reaching the lower airways.
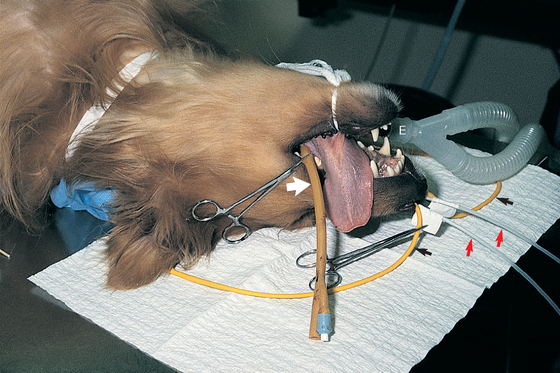
FIG 15-3 Dog with nasal mycotic infection prepared for 1-hour soak with clotrimazole. A cuffed endotracheal tube is in place (E). A 24Fr Foley catheter (broad arrow) is in the caudal nasopharynx. A 12Fr Foley catheter (narrow arrows) is obstructing each nostril. A 10Fr polypropylene catheter (red arrowheads) is placed midway into each dorsal meatus for infusion of the drug. Laparotomy sponges are used to further pack the caudal nasopharynx, around the tracheal tube and the caudal oral cavity.
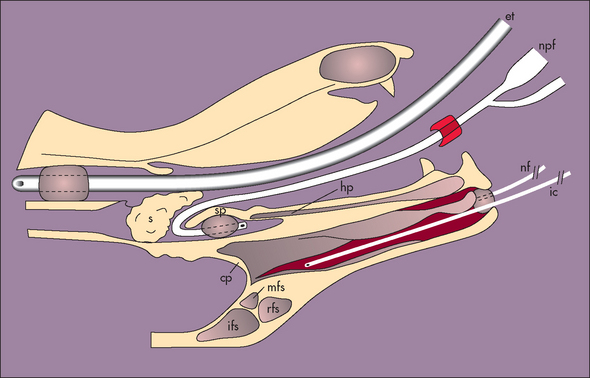
FIG 15-4 Schematic diagram of a cross section of the head of a dog prepared for 1-hour soak with clotrimazole. et, Endotracheal tube; npf, Foley catheter placed in caudal nasopharynx; s, pharyngeal sponges; ic, polypropylene infusion catheter; nf, rostral Foley catheter obstructing nostril; hp, hard palate; sp, soft palate; cp, cribriform plate; rfs, rostral frontal sinus; mfs, medial frontal sinus; lfs, lateral frontal sinus.
(Reprinted with permission from Mathews KG et al: Computed tomographic assessment of noninvasive intranasal infusions in dogs with fungal rhinitis, Vet Surg 25:309, 1996.)
A 10 Fr polypropylene urinary catheter is passed into the dorsal meatus of each nasal cavity to a distance approximately midway between the external naris and the medial canthus of the eye. The correct distance is marked on the catheters with tape to prevent accidentally inserting the catheters too far during the procedure. A 12Fr Foley catheter with a 5-ml balloon is passed adjacent to the polypropylene catheter into each nasal cavity. The cuff is inflated and pulled snugly against the inside of the naris. A small suture is placed across each naris lateral to the catheter to prevent balloon migration. A gauze sponge is placed between the endotracheal tube and the incisive ducts behind the upper incisors to minimize leakage.
A solution of 1% clotrimazole is administered through the polypropylene catheters. Approximately 30ml is used for each side in a typical retriever-size dog. Each Foley catheter is checked for filling during the initial infusion and is then clamped when clotrimazole begins to drip from the catheter. The solution is viscous, but excessive pressure is not required for infusion. Additional clotrimazole is administered during the next hour at a rate that results in approximately 1 drop every few seconds from each external naris. In dogs of the size described, a total of approximately 100 to 120ml will be used.
After the initial 15 minutes, the head is tilted slightly to one side and then the other for 15 minutes each and then back into dorsal recumbency for 15 minutes. After this hour of contact time, the dog is rolled into sternal recumbency with the head hanging over the end of the table and the nose pointing toward the floor. The catheters are removed from the external nares, and the clotrimazole and resulting mucus are allowed to drain. Drainage will usually subside in 10 to 15 minutes. A flexible suction tip may be used to expedite this process. The laparotomy pads are then carefully removed from the nasopharynx and oral cavity and counted to ensure that all are retrieved. The catheter in the nasopharynx is removed. Any drug within the oral cavity is swabbed or suctioned.
Two potential complications of clotrimazole treatment are aspiration pneumonia and meningoencephalitis. Meningoencephalitis is generally fatal and results when clotrima zole and its carrier, polyethylene glycol (PEG), make contact with the brain through a compromised cribriform plate. It is difficult to determine the integrity of the cribriform plate before treatment without the aid of computed tomography (CT) or magnetic resonance imaging (MRI), although marked radiographic changes in the caudal nasal cavity should increase concern. Fortunately, complications are not common.
Clinical signs generally resolve in 1 to 2 weeks. A second 1-hour soak is performed if signs persist after 2 weeks. One cause of treatment failure is the inability of clotrimazole to reach all sites of infection. As previously mentioned, removal of fungal plaques with rhinoscopic guidance is thought to improve response to therapy. One or both frontal sinuses are often infected, and it may be necessary to trephine the affected sinus, debulk any fungal granulomas, and directly administer clotrimazole into the sinus. In rare cases, infection extends beyond the nasal cavity (e.g., into the retrobulbar space). Itraconazole treatment is indicated in these patients.
Some clinicians have had success using the combination of itraconazole and another oral antifungal agent, terbinafine, for the treatment of aspergillosis. Published studies are not available (see Chapter 98).
Some dogs have a persistant nasal discharge after treatment for aspergillosis in the absence of identifiable active fungal infection. These dogs may have secondary bacterial rhinitis or sensitivity to inhaled irritants because of the damaged nasal anatomy and mucosa and are managed as described in the section on canine chronic/lymphoplasmacytic rhinitis in this chapter.
NASAL PARASITES
NASAL MITES
Pneumonyssoides caninum is a small white mite approximately 1 mm in size (see Fig. 14-14, A). Most infestations are clinically silent, but some dogs may have moderate-to-severe clinical signs.
Clinical Features and Diagnosis
A common clinical feature of nasal mites is sneezing, which is often violent. Head shaking, pawing at the nose, reverse sneezing, chronic nasal discharge, and epistaxis can also occur. These signs are similar to those caused by nasal foreign bodies. The diagnosis is made by visualizing the mites during rhinoscopy or by retrograde nasal flushing, as described in Chapter 14. The mites can be easily overlooked in the retrieved saline solution; they should be specifically searched for with slight magnification or by placing dark material behind the specimen for contrast. Further, the mites are often located in the frontal sinuses and caudal nasal cavity. Marks et al. (1994) report the greatest success in identifying mites by flushing the nasal cavities with halothane in oxygen. The anesthetic mixture causes the mites to migrate to the caudal nasopharynx, where the mites are visualized using an endoscope.
Treatment
Milbemycin oxime (0.5 to 1mg/kg, orally, every 7 to 10 days for three treatments) has been used successfully for treating nasal mites. Ivermectin has also been used for treatment (0.2mg/kg, administered subcutaneously and repeated in 3 weeks), but it is not safe for certain breeds. Any dogs in direct contact with the affected animal should also be treated.
NASAL CAPILLARIASIS
Nasal capillariasis is caused by a nematode, Capillaria (Eucoleus) boehmi, originally identified as a worm of the frontal sinuses in foxes. The adult worm is small, thin, and white and lives on the mucosa of the nasal cavity and frontal sinuses of dogs (see Fig. 14-14, B). The adults shed eggs that are swallowed and pass in the feces. Clinical signs include sneezing and mucopurulent nasal discharge, with or without hemorrhage. The diagnosis is made by identifying double operculated Capillaria (Eucoleus) eggs on routine fecal flotation (similar to the eggs of Capillaria (Eucoleus) aerophila; see Fig. 20-12, C) or visualizing adult worms during rhinoscopy. Treatments include ivermectin (0.2mg/kg, orally, once) or fenbendazole (25 to 50mg/kg q12h for 10 to 14 days). Success of treatment should be confirmed with repeated fecal examinations, in addition to resolution of clinical signs. Repeated treatments may be necessary, and reinfection is possible if exposure to contaminated soil continues.
NASOPHARYNGEAL POLYPS
Nasopharyngeal polyps are benign growths that occur most often in kittens and young adult cats, although they are occasionally found in older animals. Their origin is unknown, but they are often attached to the base of the eustachian tube. They can extend into the external ear canal, middle ear, pharynx, and nasal cavity. Grossly, they are pink, polypoid growths, often arising from a stalk (Fig. 15-5). Because of their gross appearance, they are sometimes mistaken for neoplasia.
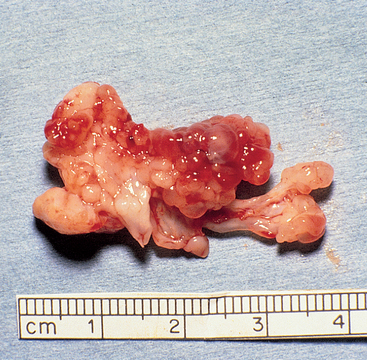
FIG 15-5 A nasopharyngeal polyp was visualized during rhinoscopy through the exterior naris of a cat with chronic nasal discharge. The polyp was excised by traction and has an obvious stalk.
Clinical Features
Respiratory signs caused by nasopharyngeal polyps include stertorous breathing, upper airway obstruction, and serous-to-mucopurulent nasal discharge. Signs of otitis externa or otitis media/interna, such as head tilt, nystagmus, or Horner’s syndrome, can also occur.
Diagnosis
Identification of a soft tissue opacity above the soft palate radiographically and gross visualization of a mass in the nasopharynx, nasal cavity, or external ear canal support a tentative diagnosis of nasopharyngeal polyp. Complete evaluation of cats with polyps also includes a deep otoscopic examination and radiographs or CT scans of the osseous bullae to determine the extent of involvement. The majority of cats with polyps have otitis media, detectable radiographically as thickened bone or increased soft tissue opacity of the bulla (see Fig. 14-6). The definitive diagnosis is made by histopathologic analysis of tissue biopsy; the specimen is usually obtained during surgical excision. Nasopharyngeal polyps are composed of inflammatory tissue, fibrous connective tissue, and epithelium.
Treatment
Treatment of nasopharyngeal polyps consists of surgical excision. Surgery is usually performed through the oral cavity by traction. In addition, bullae osteotomy should be considered in cats with radiographic or CT evidence of involvement of the osseous bullae. Rarely, rhinotomy is required for complete removal.
An early study by Kapatkin et al. (1990) reported that 5 of 31 cats had regrowth of an excised polyp. Of the five cats with regrowth, four had not had bulla osteotomies. These findings support the importance of addressing involvement of the osseous bulla in cats with polyps. However, a subsequent study by Anderson et al. (2000) reported successful treatment with traction alone, particularly when followed by a course of prednisolone in some cats. Prednisolone was administered at 1 to 2mg/kg every 24 hours for 2 weeks, then at half the original dose for 1 week, then every other day for 7 to 10 more days. A course of antibiotics (e.g., amoxicillin) was also administered.
Prognosis
The prognosis is excellent, but treatment of recurrent dis-ease may be necessary. Regrowth of a polyp can occur at the original site if abnormal tissue remains, with signs of recurrence typically appearing within 1 year. Bulla osteotomies should be considered in cats with recurrence and signs of otitis media if not performed with initial treatment.
NASAL TUMORS
The majority of nasal tumors in the dog and cat are malignant. Adenocarcinoma, squamous cell carcinoma, and undifferentiated carcinoma are common nasal tumors in dogs. Lymphoma and adenocarcinoma are common in cats. Fibrosarcomas and other sarcomas also occur in both species. Benign tumors include adenomas, fibromas, papillomas, and transmissible venereal tumors (the latter only in dogs).
Clinical Features
Nasal tumors usually occur in older animals but cannot be excluded from the differential diagnosis of young dogs and cats. No breed predisposition has been consistently identified. Collies and Irish Setters were overrepresented in a report of malignant nasal tumors in dogs by Evans et al. (1989).
The clinical features of nasal tumors (usually chronic) reflect the locally invasive nature of these tumors. Nasal discharge is the most common complaint. The discharge can be serous, mucoid, mucopurulent, or hemorrhagic. One or both nostrils can be involved. With bilateral involvement, the discharge is often worse from one nostril compared with the other. For many animals the discharge is initially unilateral and progresses to bilateral. Sneezing may be reported. Obstruction of the nasal cavity by the tumor may cause decreased or absent air flow through one of the nares.
Deformation of the facial bones, hard palate, or maxillary dental arcade may be visible (see Fig. 13-4). Tumor growth extending into the cranial vault can result in neurologic signs. Growth into the orbit may cause exophthalmos or inability to retropulse the eye. Animals only rarely experience neurologic signs (e.g., seizures, behavior changes, abnormal mental status) or ocular abnormalities as the primary complaints (i.e., no signs of nasal discharge). Weight loss and anorexia may accompany the respiratory signs but are often absent.
Diagnosis
A diagnosis of neoplasia is based on clinical features and supported by typical abnormalities detected by imaging of the nasal cavity and frontal sinuses or rhinoscopy. A definitive diagnosis requires histopathologic examination of a biopsy specimen, although fine needle aspirates of nasal masses may provide conclusive results. Imaging (radiography, CT, or MRI) and rhinoscopic abnormalities can reflect soft tissue mass lesions; turbinate, vomer bone, or facial bone destruction (see Figs. 14-2, 14-4, and 14-8, B); or diffuse infiltration of the mucosa with neoplastic and inflammatory cells.
Biopsy specimens, including tissue from deep within the lesion, should be obtained in all patients for histologic confirmation. Nasal neoplasms frequently cause a marked inflammatory response of the nasal mucosa and, in some patients, secondary bacterial or fungal infection. A cytologic diagnosis of neoplasia must be accepted cautiously, taking into consideration concurrent inflammation and potentially marked hyperplastic and metaplastic change. Furthermore, in some cases the cytologic characteristics of lymphoma and carcinoma will mimic each other, which may lead to an erroneous classification.
Not all cases of neoplasia will be diagnosed on initial evaluation of the dog or cat. Imaging, rhinoscopy, and biopsy may need to be repeated in 1 to 3 months in animals with persistent signs in which a definitive diagnosis has not been made. CT and MRI are more sensitive techniques for imaging nasal tumors than routine radiography, and one of these should be performed when available (see Fig. 14-8, B). Surgical exploration is occasionally necessary to obtain a definitive diagnosis.
Once a definitive diagnosis is made, determining the extent of disease can help in assessing the feasibility of surgical or radiation therapy versus chemotherapy. Some information can be obtained from high-quality nasal radiographs, but CT and MRI are more sensitive methods for evaluating the extent of abnormal tissue. Aspirates of mandibular lymph nodes should be examined cytologically for evidence of local spread. Thoracic radiographs are evaluated, although pulmonary metastases are uncommon at the time of initial diagnosis. Cytologic evaluation of bone marrow aspirates and abdominal radiographs or ultrasound are indicated for patients with lymphoma. Cats with lymphoma are also tested for FeLV and FIV.
Treatment
Treatment of benign tumors should include surgical excision. Malignant nasal tumors can be treated with radiation therapy (with or without surgery) and/or chemotherapy. Palliative treatment can also be tried. The treatments of choice for cats with nasal lymphoma are chemotherapy using standard lymphoma protocols (see Chapter 80), radiation therapy, or both. Radiation therapy avoids the systemic adverse effects of chemotherapeutic drugs but may be insufficient if the tumor involves other organs.
Radiation therapy is the treatment of choice for most other malignant nasal tumors. Surgical debulking before radiation is recommended if orthovoltage radiation will be used. Surgery is not beneficial before megavoltage radiation (cobalt or linear accelerator), but improved success of treatment has been recently reported with surgical debulking performed after megavoltage radiotherapy (Adams et al., 2005).
Treatment of malignant nasal tumors with surgery alone does not result in prolonged survival times; it may indeed shorten survival times. It is doubtful that all abnormal tissue can be excised in the majority of cases.
Chemotherapy may be attempted when radiation therapy has failed or is not a viable option. Carcinomas may be responsive to cisplatin, carboplatin, or multiagent chemotherapy. (See Chapter 77 for a discussion of general principles for the selection of chemotherapy.)
Treatment with piroxicam, a nonsteroidal antiinflammatory drug, can be considered for dogs with carcinoma for which radiation therapy is not elected. Partial remissions or improvement in clinical signs have been reported for some dogs with transitional cell carcinoma of the urinary bladder, oral squamous cell carcinoma, and several other carcinomas. Potential side effects include gastrointestinal ulceration (which can be severe) and kidney damage. For dogs with other types of tumors and cats, improvement of clinical signs may be seen with antiinflammatory doses of glucocorticoids. Prednisolone is prescribed for cats, and either prednisone or prednisolone for dogs (0.5 to 1mg/kg/day; tapered to lowest effective dose). Neither drug should be given in conjunction with piroxicam.
Prognosis
The prognosis for dogs and cats with untreated malignant nasal tumors is poor. Survival after diagnosis is usually only a few months. Euthanasia is often requested because of persistent epistaxis or discharge, labored respirations, anorexia and weight loss, or neurologic signs. Epistaxis is a poor prognostic indicator. In a study of 132 dogs with untreated nasal carcinoma by Rassnick et al. (2006), the median survival time of dogs with epistaxis was 88 days (95% confidence interval (CI), 65-106 days) and of dogs without epistaxis was 224 days (95% CI, 54-467 days). The overall median survival time was 95 days (range 7-1114 days).
Radiation therapy can prolong survival and improve quality of life in some animals. The therapy is well tolerated by most animals, and in those that achieve remission the quality of life is usually excellent. Studies of dogs treated with megavoltage radiation, with or without prior surgical treatment, by Theon et al. (1993) and Henry et al. (1998) found median survival times of approximately 13 months. Survival rates for 1 and 2 years were 55% to 60% and 25% to 45%, respectively. For dogs receiving megavoltage radiation followed by surgical debulking, median survival time was 47.7 months, with survival rates for 2 and 3 years of 69% and 58%, respectively (Adams et al., 2005). The dogs in the study by Adams et al. (2005) that did not receive postradiotherapy surgery had a median survival of 19.7 months and lower 2- and 3-year survival rates (44% and 24%, respectively).
A study by Evans et al. (1989) of dogs receiving orthovoltage radiation therapy after surgical debulking reported a median survival time of 16.5 months, a 1-year survival rate of 54% and a 2-year survival rate of 43%. Northrup et al. (2001) report a median survival time of approximately 7 months, a 1-year survival rate of 37%, and a 2-year survival rate of only 17% in dogs treated with surgery and orthovoltage radiation.
Less information is available concerning prognosis in cats. According to Straw et al. (1986), six cats with malignant neoplasms (three with lymphoma) that received radiation therapy had a mean survival time of 19 months. A study by Theon et al. (1994) of 16 cats with nonlymphoid neoplasia receiving radiation therapy showed a 1-year survival rate of 44% and a 2-year survival rate of 17%. Of eight cats with nasal lymphoma treated with cyclophosphamide, vincristine, and prednisolone (COP), six (75%) achieved complete remission (Teske et al., 2002). Median survival time was 358 days, and the estimated 1-year survival rate was 75%. According to preliminary data from Arteaga et al. (2007), cats with nasal lymphoma treated with radiation and chemotherapy had a median survival time of 511 days.
ALLERGIC RHINITIS
Etiology
Allergic rhinitis has not been well characterized in dogs or cats. However, dermatologists provide anecdotal reports of atopic dogs rubbing the face (possibly indicating nasal pruritus) and experiencing serous nasal discharge, in addition to dermatologic signs. Allergic rhinitis is generally considered to be a hypersensitivity response within the nasal cavity and sinuses to airborne antigens. It is possible that food allergens play a role in some patients. Other antigens are capable of inducing a hypersensitivity response as well, and thus the differential diagnoses must include parasites, other infectious diseases, and neoplasia.
Clinical Features
Dogs or cats with allergic rhinitis experience sneezing and/or serous or mucopurulent nasal discharge. Signs may be acute or chronic. Careful questioning of the owner may reveal a relationship between signs and potential allergens. For instance, signs may be worse during certain seasons; in the presence of cigarette smoke; or after the introduction of a new brand of kitty litter, new perfumes, cleaning agents, furniture, or fabric in the house. Note that worsening of signs may simply be a result of exposure to irritants rather than an actual allergic response. Debilitation of the animal is not expected.
Diagnosis
Identifying a historical relationship between signs and a particular allergen and then achieving resolution of signs after removal of the suspected agent from the animal’s environment support the diagnosis of allergic rhinitis. When this approach is not possible or successful, a thorough diagnostic evaluation of the nasal cavity is indicated (see Chapters 13 and 14). Nasal radiographs reveal increased soft tissue opacity with minimal or no turbinate destruction. Classically, nasal biopsy reveals eosinophilic inflammation. It is possible that with chronic disease, a mixed inflammatory response occurs, obscuring the diagnosis. There should be no indication in any of the diagnostic tests of an aggressive disease process, parasites or other active infection, or neoplasia.
Treatment
Removing the offending allergen from the animal’s environment or diet is the ideal treatment of allergic rhinitis. When this is not possible, a beneficial response may be achieved with antihistamines. Chlorpheniramine can be administered orally at a dose of 4 to 8mg/dog every 8 to 12 hours or 2mg/cat every 8 to 12 hours. The second-generation antihistamine cetirizine (Zyrtec, Pfizer) may be more successful in cats. A pharmacokinetic study of this drug in healthy cats found a dosage of 1mg/kg, administered orally every 24 hours, to maintain plasma concentrations similar to those reported in people (Papich et al., 2006). Glucocorticoids may be used if antihistamines are unsuccessful. Prednisone is initiated at a dose of 0.25mg/kg every 12 hours until signs resolve. The dose is then tapered to the lowest effective amount. If treatment is effective, signs will generally resolve within a few days. Drugs are continued only as long as needed to control signs.
IDIOPATHIC RHINITIS
Idiopathic rhinitis is a more common diagnosis in cats compared with dogs. The diagnosis cannot be made without a thorough diagnostic evaluation to rule out specific diseases (see Chapters 13 and 14).
FELINE CHRONIC RHINOSINUSITIS
Etiology
Feline chronic rhinosinusitis has long been presumed to be a result of viral infection with FHV or FCV (see the section on feline upper respiratory infection, p. 223). Persistent viral infection has been implicated, but studies have failed to show an association between tests indicating exposure to or infection with these viruses and clinical signs. It is possible that infection with these viruses results in damaged mucosa that is more susceptible to bacterial infection or that mounts an excessive inflammatory response to irritants or normal nasal flora. Preliminary studies have failed to show an association with feline chronic rhinosinusitis and Bartonella infection, based on serum antibody titers or PCR of nasal tissue (Berryessa et al., 2007). In the absence of a known etiology, this disease will be denoted by the term idiopathic feline chronic rhinosinusitis.
Clinical Features and Diagnosis
Chronic mucoid or mucopurulent nasal discharge is the most common clinical sign of idiopathic feline chronic rhinosinusitis. The discharge is typically bilateral. Fresh blood may be seen in the discharge of some cats but is not usually a primary complaint. Sneezing may occur. Given that this is an idiopathic disease, the lack of specific findings is important. Cats should have no funduscopic lesions, no lymphadenopathy, no facial or palate deformities, and healthy teeth and gums. Anorexia and weight loss are rarely reported. Thorough diagnostic testing is indicated, as described in Chapters 13 and 14. Results of such testing do not support the diagnosis of a specific disease. Usual nonspecific findings include turbinate erosion, mucosal inflammation, and increased mucus accumulation as assessed by nasal imaging and rhinoscopy; neutrophilic or mixed inflammation with bacteria on cytology of nasal discharge; and neutrophilic and/or lymphoplasmacytic inflammation on nasal biopsy. Nonspecific abnormalities attributable to chronic inflammation, such as epithelial hyperplasia and fibrosis, may also be seen. Secondary bacterial rhinitis or Mycoplasma infection may be identified.
Treatment
Cats with idiopathic chronic rhinosinusitis often require management for years. Fortunately, most of these cats are healthy in all other respects. Treatment strategies include facilitating drainage of discharge; decreasing irritants in the environment; controlling secondary bacterial infections; treating possible Mycoplasmal or FHV infection; reducing inflammation; and, as a last resort, performing a turbinectomy and frontal sinus ablation (Box 15-1).
 BOX 15-1 Management Considerations for Cats with Idiopathic Chronic Rhinosinusitis
BOX 15-1 Management Considerations for Cats with Idiopathic Chronic Rhinosinusitis
Keeping secretions moist, performing intermittent nasal flushes, and judiciously using topical decongestants facilitate drainage. Keeping the cat in a room with a vaporizer, for instance, during the night, can provide symptomatic relief by keeping secretions moist. Alternatively, drops of sterile saline can be placed into the nares. Some cats experience a marked improvement in clinical signs for weeks after flushing of the nasal cavity with copious amounts of saline or dilute betadine solution. General anesthesia is required, and the lower airways must be protected with an endotracheal tube, gauze sponges, and positioning of the head to facilitate drainage from the external nares. Topical decongestants, as described for feline upper respiratory infection (see page 224), may provide symptomatic relief during episodes of severe congestion.
Irritants in the environment can further exacerbate mucosal inflammation. Irritants such as smoke (from tobacco or fireplace) and perfumed products should be avoided. Motivated clients can take steps to improve the air quality in their homes, such as by cleaning the carpet, furniture, drapery, and furnace; regularly replacing air filters; and using an air cleaner. The American Lung Association has a useful Web site with nonproprietary recommendations for improving indoor air quality (www.lungusa.org).
Chronic antibiotic therapy may be required to manage secondary bacterial infections. Broad-spectrum antibiotics such as amoxicillin (22mg/kg q8-12h) or trimethoprimsulfadiazine (15mg/kg q12h) are often successful. Chloramphenicol (10 to 15mg/kg q12h) and doxycycline (5 to 10mg/kg q12h, followed by a bolus of water) have activity against some bacteria and Chlamydophila and Mycoplasma organisms and can be effective in some cats when other drugs have failed. This author reserves fluoroquinolones for cats with documented resistant gram-negative infections. If a beneficial response to antibiotic therapy is seen within 1 week of its initiation, the antibiotic should be continued for at least 4 to 6 weeks. If a beneficial response is not seen, the antibiotic is discontinued. Note that the frequent stopping and starting of different antibiotics every 7 to 14 days is not recommended and may predispose the cat to resistant gram-negative infections. Cats that respond well during the prolonged course of antibiotics but that relapse shortly after discontinuation of the drug despite 4 to 6 weeks of relief are candidates for continuous long-term antibiotic therapy. Treatment with the previously used antibiotic often can be successfully reinstituted. Amoxicillin administered twice daily is often sufficient.
Treatment with lysine may be effective in cats with active herpesvirus infections. It has been postulated that excessive concentrations of lysine may antagonize arginine, a promoter of herpesvirus replication. Because the specific organism(s) involved is rarely known, trial therapy is initiated. Lysine (500mg/cat q12h), which can obtained from health food stores, is added to food. A minimum of 4 weeks is necessary to assess success of treatment.
Anecdotal success in occasional cats has been reported with treatment with the second-generation antihistamine cetirizine (Zyrtec, Pfizer) as described for allergic rhinitis (see p. 232). No efficacy studies are available.
Cats with severe signs that persist despite the previously described methods of supportive care may benefit from glucocorticoids to reduce inflammation. However, certain risks are involved. Glucocorticoids may further predispose the cat to secondary infections, increase viral shedding, and mask signs of a more serious disease. Glucocorticoids should be prescribed only after a complete diagnostic evaluation has been performed to rule out other diseases. Prednisolone is administered at a dose of 0.5mg/kg every 12 hours. If a beneficial response is seen within 1 week, the dose is gradually decreased to the lowest effective dose. A dose as low as 0.25mg/kg every 2 to 3 days may be sufficient to control clinical signs. If a clinical response is not seen within 1 week, the drug should be discontinued.
Cats with severe or deteriorating signs that persist despite conscientious care are candidates for turbinectomy and frontal sinus ablation, assuming a complete diagnostic evaluation to eliminate other causes of chronic nasal discharge has been performed (Chapters 13 and 14). Turbinectomy and frontal sinus ablation are difficult surgical procedures. Major blood vessels and the cranial vault must be avoided, and tissue remnants must not be left behind. Anorexia can be a postoperative problem; placement of an esophagostomy or gastrostomy tube (see p. 30) provides an excellent means for meeting nutritional requirements if necessary after surgery. Complete elimination of respiratory signs is unlikely, but signs may be more easily managed. The reader is referred to surgical texts by Fossum or Slatter for a description of the surgical techniques (see Suggested Readings).
CANINE CHRONIC/LYMPHOPLASMACYTIC RHINITIS
Etiology
Idiopathic chronic rhinitis in dogs is sometimes characterized by the inflammatory infiltrates seen in nasal mucosal biopsies; thus the disease lymphoplasmacytic rhinitis has been described. It was originally reported to be a steroid-responsive disorder (Burgener et al., 1987), but a subsequent report by Windsor et al. (2004) and clinical experience suggest that corticosteroids are not always effective in the treatment of lymphoplasmacytic rhinitis. It is not uncommon for neutrophilic inflammation to be found, predominantly or along with lymphoplasmacytic infiltrates. For these reasons, the less specific term idiopathic canine chronic rhinitis will be used.
Many specific causes of nasal disease result in a concurrent inflammatory response because of the disease itself or as a response to the secondary effects of infection or enhanced response to irritants; this makes a thorough diagnostic evaluation of these cases imperative. Windsor et al. (2006) performed multiple PCR assays on paraffin-embedded nasal tissue from dogs with idiopathic chronic rhinitis and failed to find evidence for a role of bacteria (based on DNA load), canine adenovirus-2, parainfluenza virus, Chlamydophila spp. or Bartonella spp. in affected dogs. High amounts of fungal DNA were found in affected dogs, suggesting a possible contribution to clinical signs. Alternatively, the result may simply reflect decreased clearance of fungal organisms from the diseased nasal cavity.
Although not supported in the previously quoted study, a potential role for Bartonella infection has been suggested on the basis of a study that found an association between seropositivity for Bartonella spp. and nasal discharge or epistaxis (Henn et al., 2005) and a report of three dogs with epistaxis and evidence of infection with Bartonella spp. (Breitschwerdt et al., 2005). A study in our laboratory (Hawkins et al., 2008) failed to find an obvious association between bartonellosis and idiopathic rhinitis, in agreement with findings by Windsor et al. (2006).
Clinical Features and Diagnosis
The clinical features and diagnosis of idiopathic canine chronic rhinitis are similar to those described for idiopathic feline chronic rhinosinusitis. Chronic mucoid or mucopurulent nasal discharge is the most common clinical sign and is typically bilateral. Fresh blood may be seen in the discharge of some dogs, but it is not usually a primary complaint. Given that it is an idiopathic disease, the lack of specific findings is important. Dogs should have no funduscopic lesions, no lymphadenopathy, no facial or palate deformities, and healthy teeth and gums. Anorexia and weight loss are rarely reported. Thorough diagnostic testing is indicated, as described in Chapters 13 and 14. Results of such testing do not support the diagnosis of a specific disease. Usual nonspecific findings include turbinate erosion, mucosal inflammation, and increased mucus accumulation as assessed by nasal imaging and rhinoscopy; neutrophilic or mixed inflammation with bacteria on cytology of nasal discharge; and lymphoplasmacytic and/or neutrophilic inflammation on nasal biopsy. Nonspecific abnormalities attributable to chronic inflammation, such as epithelial hyperplasia and fibrosis, can also be seen. Secondary bacterial rhinitis or Mycoplasma infection may be identified.
Treatment
Treatment of idiopathic canine chronic rhinitis is also similar to that described for idiopathic feline rhinosinusitis. Dogs are treated for secondary bacterial rhinitis (as described on p. 233), and efforts are made to decrease irritants in the environment (see p. 233). As with cats, some dogs will benefit from efforts to facilitate the draining of nasal discharge by humidification of air or instillation of sterile saline into the nasal cavity.
Burgener et al. (1987) reported successful treatment of dogs with lymphoplasmacytic rhinitis using immunosuppressive doses of prednisone (1mg/kg q12h). A positive response is expected within 2 weeks, at which time the dose of prednisone is decreased gradually to the lowest effective amount. If no response to initial therapy occurs, other immunosuppressive drugs such as azathioprine can be added to the treatment regimen (see Chapter 103). Unfortunately, immunosuppressive treatment is not always effective. If clinical signs worsen during treatment with corticosteroids, the clinician should discontinue therapy and carefully reevaluate the dog for other diseases.
Other treatments that may be effective in some dogs include antihistamines or itraconazole. According to preliminary data from Kuehn (2006), administration of itraconazole (5mg/kg q12h) resulted in dramatic improvement in clinical signs in some dogs with idiopathic chronic rhinitis. Treatment was required for a minimum of 3 to 6 months. The rationale for this treatment may be supported by the findings of increased fungal load in affected dogs by Windsor et al. (2006).
Dogs with severe or nonresponsive signs are candidates for rhinotomy and turbinectomy, as described for cats on p. 234.
Adams WM, et al. Outcome of accelerated radiotherapy alone or accelerated radiotherapy followed by exenteration of the nasal cavity in dogs with intranasal neoplasia: 53 cases (1990-2002). J Am Vet Med Assoc. 2005;227:936.
Anderson DM, et al. Management of inflammatory polyps in 37 cats. Vet Record. 2000;147:684.
Arteaga T, et al. A retrospective analysis of nasal lymphoma in 71 cast (1999-2006). Abst. J Vet Intern Med. 2007;21:573.
Berryessa NA, et al. The role of Bartonella spp. in feline chronic rhinosinusitis. Abst. J Vet Intern Med. 2007;21:608.
Binns SH, et al. Prevalence and risk factors for feline Bordetella bronchiseptica infection. Vet Rec. 1999;144:575.
Bredal W, et al. Use of milbemycin oxime in the treatment of dogs with nasal mite (Pneumonyssoides caninum) infection. J Small Anim Pract. 1998;39:126.
Breitschwerdt, et al. Bartonella species as a potential cause of epistaxis in dogs. J Clin Micro. 2005;43:2529.
Burgener DC, et al. Lymphoplasmacytic rhinitis in five dogs. J Am Anim Hosp Assoc. 1987;23:565.
Coutts AJ, et al. Studies on natural transmission of Bordetella bronchiseptica in cats. Vet Microbiol. 1996;48:19.
Davidson AP, et al. Treatment of nasal aspergillosis with topical clotrimazole. In: Bonagura JD, et al, editors. Current veterinary therapy XII. Philadelphia: WB Saunders; 1995:899.
Evans SM, et al. Prognostic factors and survival after radiotherapy for intranasal neoplasms in dogs: 70 cases (1974–1985). J Am Vet Med Assoc. 1989;194:1460.
Evinger JV, et al. Ivermectin for treatment of nasal capillariasis in a dog. J Am Vet Med Assoc. 1985;186:174.
Fossum TW. Small animal surgery, ed 3. St Louis: Mosby, 2007.
Gunnarsson LK, et al. Clinical efficacy of milbemycin oxime in the treatment of nasal mite infection in dogs. J Am Anim Hosp Assoc. 1999;35:81.
Hawkins EC, et al. Failure to identify an association between serologic or molecular evidence of Bartonella spp infection and idiopathic rhinitis in dogs (Accepted). J Am Vet Med Assoc. 2008.
Henn JB, et al. Seroprevalence of antibodies against Bartonella species and evaluation of risk factors and clinical signs associated with seropositivity in dogs. Am J Vet Res. 2005;66:688.
Henry CJ, et al. Survival in dogs with nasal adenocarcinoma: 64 cases (1981–1995). J Vet Intern Med. 1998;12:436.
Johnson LR, et al. Assessment of infectious organisms associated with chronic rhinosinusitis in cats. J Am Vet Med Assoc. 2005;227:579.
Kapatkin AS, et al. Results of surgery and long-term follow-up in 31 cats with nasopharyngeal polyps. J Am Anim Hosp Assoc. 1990;26:387.
Kuehn NF: Prospective long term pilot study using oral itraconazole therapy for the treatment of chronic idiopathic (lymphoplasmacytic) rhinitis in dogs. Abstr, British Small Animal Veterinary Association Annual Congress, Prague, Czech Republic, 2006.
Lappin MR, et al. Use of serologic tests to predict resistance to feline herpesvirus 1, feline calicivirus, and feline parvovirus infection in cats. J Am Vet Med Assoc. 2002;220:38.
Maggs DJ, et al. Effects of L-lysine and L-arginine on in vitro replication of feline herpesvirus type-1. Am J Vet Res. 2000;61:1474.
Marks SL, et al. Pneumonyssoides caninum: the canine nasal mite. Compend Contin Educ Pract Vet. 1994;16:577.
Mathews KG, et al. Computed tomographic assessment of noninvasive intranasal infusions in dogs with fungal rhinitis. Vet Surg. 1996;25:309.
Northrup NC, et al. Retrospective study of orthovoltage radiation therapy for nasal tumors in 42 dogs. J Vet Intern Med. 2001;15:183.
Papich MG, et al. Cetirizine (Zyrtec) pharmacokinetics in healthy cats. Abstr. J Vet Intern Med. 2006;20:754.
Pomerantz JS, et al. Comparison of serologic evaluation via agar gel immunodiffusion and fungal culture of tissue for diagnosis of nasal aspergillosis in dogs. J Am Vet Med Assoc. 2007;230:1319.
Rassnick KM, et al. Evaluation of factors associated with survival in dogs with untreated nasal carcinomas: 139 cases (1993-2003). J Am Vet Med Assoc. 2006;229:401.
Schmidt BR, et al. Evaluation of piroxicam for the treatment of oral squamous cell carcinoma in dogs. J Am Vet Med Assoc. 2001;218:1783.
Seibeck N, et al. Effects of human recombinant alpha-2b interferon and feline recombinant omega interferon on in vitro replication of feline herpesvirus. Am J Vet Res. 2006;67:1406.
Sharp N. Nasal aspergillosis. In: Kirk RW, editor. Current veterinary therapy X. Philadelphia: WB Saunders; 1989:1106.
Slatter D. Textbook of small animal surgery, ed 3. St Louis: Saunders, 2003.
Speakman AJ, et al. Antimicrobial susceptibility of Bordetella bronchiseptica isolates from cats and comparison of agar dilution and E-test methods. Vet Microbiol. 1997;54:53.
Straw RC, et al. Use of radiotherapy for the treatment of intranasal tumors in cats: six cases (1980–1985). J Am Vet Med Assoc. 1986;189:927.
Tasker S, et al. Aetiology and diagnosis of persistent nasal disease in the dog: a retrospective study of 42 cases. J Small Anim Pract. 1999;40:473.
Teske E, et al. Chemotherapy with cyclophosphamide, vincristine and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol. J Vet Intern Med. 2002;16:179.
The 2006 American Association of Feline Practitioners Feline Vaccine Advisory Panel Report. J Am Vet Med Assoc. 2006;229:1405.
Theon AP, et al. Megavoltage irradiation of neoplasms of the nasal and paranasal cavities in 77 dogs. J Am Vet Med Assoc. 1993;202:1469.
Theon AP, et al. Irradiation of nonlymphoproliferative neoplasms of the nasal cavity and paranasal sinuses in 16 cats. J Am Vet Med Assoc. 1994;204:78.
Windsor RC, et al. Idiopathic lymphoplasmacytic rhinitis in dogs: 37 cases (1997-2002). J Am Vet Med Assoc. 2004;224:1952.