CHAPTER 36 Diagnostic Tests for the Hepatobiliary System
DIAGNOSTIC APPROACH
Because the liver is physiologically and anatomically diverse, no single test adequately identifies liver disease or its underlying cause. For this reason, a battery of tests must be used to assess the hepatobiliary system. Many of these tests just show liver involvement in a disease process and do not evaluate liver function. A reasonable package of screening tests recommended for an animal suspected of having hepatobiliary disease includes a complete blood count (CBC), serum biochemical profile, urinalysis, fecal analysis, and survey abdominal radiographs or ultrasonography. Results of these tests may suggest evidence of hepatobiliary disease that can be confirmed by other, more specific tests. It is important at this stage to rule out secondary hepatopathy and rule in primary liver disease because with hepatopathies secondary to other diseases, time and resources should be devoted as soon as possible to identifying and treating the underlying cause rather than investigating the liver. The need for other laboratory tests (e.g., serum bile acid [SBA], abdominocentesis, coagulation profile) is determined by each animal’s history and physical examination findings.
Of the recommended screening tests for hepatobiliary disease, the serum biochemistry profile offers specific information regarding the distribution and activity or status (e.g., hyperbilirubinemia, enzyme activities) of a hepatobiliary disorder and an estimate of the degree of functional impairment (e.g., inadequate protein synthesis, altered toxin excretion). Determining hepatic functional capacity adds a meaningful dimension to the diagnostic evaluation and permits construction of a reasonable list of differential diagnoses and tentative assignment of prognosis. It is important to remember that some hepatobiliary diseases are characterized by subtle changes in enzyme activity in association with severe functional disturbance, and some have high enzyme activities and normal functional indices. Because of the large reserve capacity of the liver, detection of global hepatic functional impairment by conventional means is not possible until there is at least 55% loss of hepatic mass. Diseases that cause acute hepatocyte loss show evidence of functional impairment more quickly than diseases with chronic hepatocyte loss, wherein the remaining hepatocytes have time to compensate. In dogs with chronic hepatitis, signs of functional impairment may not be evident until 75% of hepatic mass has been lost. The recommended serum biochemistry profile for liver disease includes, in addition to liver enzymes, albumin, urea nitrogen, bilirubin, cholesterol, and glucose concentrations, which are used to assess the ability of the liver to synthesize proteins, detoxify protein degradation products, excrete organic anions and other substances, and help maintain euglycemia, respectively. Development of automated methods for laboratory analysis has made measurement of many substances in the blood easy; these laboratory analytic methods are available at competitive prices through commercial laboratories or as point-of-care test kits or systems. For this reason, there is no excuse for excluding a multiple component serum biochemistry profile from the initial diagnostic plan for a cat or dog suspected of having hepatobiliary disease.
A sensitive, although relatively nonspecific, test of hepatobiliary function is determination of fasting and postprandial SBA concentrations. Serum bile acid concentrations are measured if there are persistent liver-specific serum biochemical abnormalities or a liver problem is suspected (e.g., microhepatia, ammonium biurate crystalluria) but results of routine diagnostic tests are inconclusive. Serum bile acids are not a helpful test of liver function in a jaundiced animal because they are also elevated in cholestasis because of decreased excretion, independent of liver function. Bile acids are not available on usual practice analyzers, but a point-of-care snap test for SBA estimation has recently become available in the United States (IDEXX Laboratories, Westbrook, ME).
Results of laboratory evaluation reflect one point in time in a spectrum of dynamic changes. If the test results are equivocal and the clinical signs are vague, sequential evaluation may be necessary to allow time for the disease to be fully expressed.
By using a combination of history, physical examination findings, and results of screening and hepatobiliary-specific laboratory tests, the clinician should be able to describe the disorder as primary or secondary (reactive) hepatopathy, active or quiescent; characterize the pattern of hepatobiliary disease as primarily hepatocellular, primarily biliary, or mixed hepatobiliary; and estimate the degree of hepatobiliary dysfunction. From this same information, an animal may be described clinically as having hepatic disease, with evidence of hepatic abnormalities such as high liver enzyme activities and hepatomegaly, or hepatic failure, in which there is a state of multiple function loss. Some primary hepatic diseases may progress to failure; most secondary hepatic diseases do not (Tables 37-1 and 38-1). Use of the term failure often inappropriately connotes a poor prognosis. If the underlying cause can be removed full recovery is possible. Most important, before an accurate prognosis can be given, a complete evaluation must be conducted, including, for most primary hepatobiliary diseases in both dogs and cats, a liver biopsy.
DIAGNOSTIC TESTS
TESTS TO ASSESS STATUS OF THE HEPATOBILIARY SYSTEM
Serum Enzyme Activities
Liver-specific serum enzyme activities are included routinely in screening serum biochemistry panels and are regarded as markers of hepatocellular and biliary injury and reactivity. Because marked hepatic disease can be present in patients with normal serum enzyme activity, finding normal values should not preclude further investigation, especially if there are clinical signs or other laboratory test results that suggest hepatobiliary disease. Increased serum activity of enzymes normally located in hepatocyte cytosol in high concentration reflects structural or functional cell membrane injury that would allow these enzymes to escape or leak into the blood. The two enzymes found to be of most diagnostic use in cats and dogs are alanine transaminase (ALT; glutamic-pyruvic transaminase [GPT]) and aspartate transaminase (AST; glutamic-oxaloacetic transaminase [GOT]). Because ALT is found principally in hepatocytes and AST (also located within hepatocyte mitochondria) has a wider tissue distribution (e.g., in muscle), ALT is the enzyme selected to most accurately reflect hepatocellular injury. Less is known about the behavior of AST in various hepatobiliary diseases in companion animals, although some studies have indicated that AST is a more reliable indicator of liver injury in cats. Several studies have demonstrated mild to moderately high serum ALT activity (without histologic or biochemical evidence of liver injury), in addition to expected high serum activities of muscle-specific creatine kinase and AST, in dogs with skeletal muscle necrosis.
In general, the magnitude of serum ALT and AST activity elevation approximates the extent, but not the reversibility, of hepatocellular injury. Rather than clinical relevance being assigned to absolute values for ALT or AST activity (e.g., serum ALT activity of 200 IU/L is worse than 100 IU/L), the values should be assessed in terms of number of fold elevations from normal. Twofold to threefold elevations in serum ALT activity are associated with mild hepatocellular lesions, fivefold to tenfold elevations are seen with moderately severe lesions, and greater than tenfold increases suggest marked hepatocellular injury. ALT (and to a lesser extent AST) activity is also often increased by glucocorticoids in dogs, although to a lesser extent than ALP.
Serum enzyme activities that reflect new synthesis and release of enzyme from the biliary tract in response to certain stimuli are alkaline phosphatase (AP) and γ-glutamyltransferase (GGT). Bile retention (i.e., cholestasis) is the strongest stimulus for accelerated production of these enzymes. Unlike ALT and AST, AP and GGT are in low concentration in the cytoplasm of hepatocytes and biliary epithelium and are membrane-associated, so the fact that they simply leak out of damaged cells does not account for increased serum activity. Measurable AP activity is also detectable in nonhepatobiliary tissues of cats and dogs (including osteoblasts, intestinal mucosa, renal cortex, and placenta), but serum activity in healthy adult cats and dogs arises only from the liver, with some contribution by the bone isoenzyme in young, rapidly growing dogs and in kittens less than 15 weeks old. The renal form is mainly measurable in the urine, and the gut form has a very short half-life so is not usually measurable (although the steroid-induced isoenzyme of AP in dogs is believed to be an altered gut isoenzyme with a prolonged half-life). The half-life of feline AP is shorter than that of canine AP; thus serum activity is relatively lower in cats than in dogs with a similar degree of cholestasis, and, conversely, even mild elevations of AP in cats are clinically very significant. Markedly high serum AP activity of bone origin (mean total serum AP values more than fivefold higher than those in nonaffected individuals, with only the bone isoenzyme detected) was identified in certain healthy juvenile (7 months old) members of a family of Siberian Huskies (Lawler et al., 1996). This change is believed to be benign and familial and should be considered when results of serum AP activity are interpreted in this breed. A young, growing dog of any breed can have a mild increase in serum AP. Increased serum AP activity of unknown origin has also been described in adult Scottish Terriers and is believed to be benign and possibly familial (Gallagher et al., 2006).
Certain drugs, the most common of which are anticonvulsants (specifically phenytoin, phenobarbital, and primidone) and corticosteroids, can elicit striking increases (up to hundredfold) in serum AP activity (and to a lesser extent GGT and also ALT activity) in dogs but not in cats. There usually is no other clinicopathologic or microscopic evidence of cholestasis (i.e., hyperbilirubinemia). Anticonvulsant drugs stimulate production of AP identical to the normal liver isoenzyme; GGT activity does not change. Pharmacologic levels of corticosteroids administered orally, by injection, or topically reliably provoke a unique AP isoenzyme that is separable from the others by electrophoretic and immunoassay techniques. This characteristic is useful when interpreting high total serum AP activity in a dog with subtle clinical signs suggestive of iatrogenic or naturally occurring hypercortisolism. The corticosteroid AP isoenzyme is a component of routine canine serum biochemistry profiles at several veterinary colleges and commercial laboratories. However, measurement of AP isoenzymes has been shown to be of limited usefulness either in dogs treated with phenobarbital (Gaskill et al., 2004) or in dogs with hyperadrenocorticism (Jensen et al., 1992). In the latter, it has a high sensitivity but very low specificity, so finding a low steroid-induced isoenzyme rules out hypercortisolism, but a high concentration of steroid-induced isoenzyme may be found in many disease other than hypercortisolism. Serum GGT activity rises similarly in response to corticosteroid influence but less spectacularly. Serum AP and GGT activities tend to be parallel in cholestatic hepatopathies of cats and dogs, although they are much less dramatic in cats. Simultaneous measurement of serum AP and GGT may aid in differentiating seemingly benign drug-induced effects from nonicteric cholestatic hepatic disease in dogs. Assessing serum AP and GGT activities together may also offer clues to the type of hepatic disorder in cats. Both enzymes are in low concentration in feline liver tissue compared with that in the canine liver and have short half-lives, so relatively smaller increases in serum activity, especially of GGT, are important signs of the presence of hepatic disease in cats. In cats a pattern of high serum AP activity with less strikingly abnormal GGT activity is most consistent with hepatic lipidosis (see Chapter 37), although extrahepatic bile duct obstruction (EBDO) must also be considered.
TESTS TO ASSESS FUNCTION OF THE HEPATOBILIARY SYSTEM
Serum Albumin Concentration
The liver is virtually the only source of albumin production in the body; thus hypoalbuminemia could be a manifestation of hepatic inability to synthesize this protein. Causes other than lack of hepatic synthesis (i.e., massive glomerular or gastrointestinal loss or bleeding) must be considered before ascribing hypoalbuminemia to hepatic insufficiency. Renal protein loss can be detected presumptively by routine urinalysis. Consistent identification of positive protein dipstick reactions, especially in dilute urine with inactive sediment, justifies further evaluation by at least measurement of random urine protein : creatinine ratio (normal ratio is <0.5 in cats and dogs). If proteinuria is ruled out, diseases that cause gastrointestinal protein loss should be considered; however, these diseases usually result in equivalent loss of globulins and thus panhypoproteinemia, although this is not invariably the case in inflammatory gastrointestinal disease wherein concurrent increase in gamma-globulins masks the gut loss. Conversely, although panhyproteinemia is reportedly not typical of hypoproteinemia of hepatic origin, globulin concentrations can be low in liver disease, particularly portosystemic shunts, because all plasma globulins except gamma globulins are made in the liver. In fact, globulin concentrations frequently are normal to increased in dogs and cats with chronic inflammatory hepatic disease. Because the plasma half-life of albumin is long in cats and dogs (8 to 10 days) and there must be loss of approximately 80% of functioning hepatocytes before hypoalbuminemia is expressed, the finding of hypoalbuminemia usually indicates severe chronic hepatic insufficiency. The exception to this is the hypoalbuminemia associated with a “negative acute phase” response in acute or acute-on-chronic inflammatory liver disease. Serum albumin can decrease when there is an increase in hepatic production of acute phase proteins in animals without hepatic insufficiency. Serum protein electrophoresis can help differentiate this condition from a true lack of hepatic function: Sevelius et al. (1995) showed that a low albumin concentration combined with a low concentration of acute phase proteins in electrophoresis indicated severe hepatic dysfunction with a poor prognosis, whereas hypoalbuminemia combined with normal or elevated acute phase proteins indicated a good prognosis. Hypoalbuminemia of any cause is unusual in cats, except in those with nephrotic syndrome. When interpreting serum protein concentrations, the clinician should remember that total protein values for young cats and dogs are lower than those for adults and that puppy serum albumin concentration is similar to that in adults, whereas kitten serum albumin concentration is lower than that in adult cats.
Serum Urea Nitrogen Concentration
Formation of urea as a means of detoxifying ammonia derived from intestinal sources takes place only in the liver. Despite this apparent advantage as a specific measure of hepatic function, serum urea concentration is commonly affected by several nonhepatic factors and the capacity of the liver to detoxify urea is so great that it is not noticeably reduced until severe, extensive end-stage liver disease ensues. Prolonged restricted protein intake because of complete anorexia or intentional reduction in protein intake for therapeutic purposes (e.g., chronic kidney disease; urate, cystine, or struvite urolithiasis) is the most common cause of low blood urea nitrogen (BUN) content. Prior fluid therapy and/or marked polydipsia/poluria of nonrenal causes will also result in a decrease in BUN. As always, reference ranges should be considered for each species when interpreting BUN values. For example, a BUN concentration of 12 mg/dl is well within normal limits for dogs but is subnormal for cats. If low BUN values are noted in a cat or dog with normal water intake and a good appetite for a diet with the appropriate protein content for the species (on a dry matter basis: 22% for dogs, 35% to 40% for cats), then the possibility of hepatic inability to convert ammonia to urea should be investigated.
Serum Bilirubin Concentration
Because of the large reserve capacity of the mononuclear-phagocytic system and liver to process bilirubin (e.g., 70% hepatectomy will not cause jaundice), hyperbilirubinemia can occur only from greatly increased production or decreased excretion of bile pigment. Specific inborn errors of bilirubin uptake, conjugation, and excretion have not been documented in cats or dogs. Increased production of bilirubin from red blood cell destruction arises from intravascular or extravascular hemolysis and rarely from resorption of a large hematoma; hyperbilirubinemia also occurs in association with rhabdomyolysis in Greyhounds and other dog breeds. Under these circumstances in dogs, serum bilirubin concentrations are usually lower than 10 mg/dl. Values usually do not increase above 10 mg/dl unless there is a concurrent flaw in bilirubin excretion. This has been borne out clinically in studies of dogs with immune-mediated hemolytic anemia in which high liver enzyme activities are observed, even before treatment with corticosteroids, and moderately delayed bilirubin excretion has been documented. It has been proposed that cholestasis results from liver injury associated with hypoxia and in some cases due to early disseminated intravascular coagulation (DIC). Because increased production and decreased excretion of bilirubin occur in dogs with severe hemolysis, serum bilirubin concentrations therefore can be as high as 35 mg/dl. Icterus in cats with pure hemolytic disease is an inconsistent finding and mild if present; specific bilirubin concentrations associated with experimentally induced or naturally occurring hemolytic diseases in cats are not available.
Because nearly all diseases associated with hyperbilirubinemia in cats and dogs are characterized by a mixture of conjugated and unconjugated bilirubinemia, quantifying the two fractions by use of van den Bergh’s test achieves little in discriminating primary hepatic or biliary disease from nonhepatobiliary disease in a clinical setting. This lack of benefit in using van den Bergh’s test may relate to the time between onset of illness and examination, which is usually at least several days. Under conditions of acute massive hemolysis, the total serum bilirubin concentration may consist primarily of the unconjugated form initially. As hemolysis continues, the liver is able to take up and conjugate bilirubin, accounting for a combination of unconjugated and conjugated bilirubin.
Because red blood cell membrane changes are often a component of many primary hepatobiliary disorders, accelerated red blood cell destruction often contributes to hyperbilirubinemia. In such cases, there is strong clinicopathologic evidence of cholestasis (high serum AP and GGT activities with moderate to high ALT activity), and if there is anemia, it is mild and poorly regenerative. Hyperbilirubinemia is attributed primarily to hemolysis when there is moderate to marked anemia with strong evidence of regeneration (except in the first 1 to 3 days, when the response is less regenerative) and minimal changes in serum markers of cholestasis.
Serum Cholesterol Concentration
Total cholesterol concentration is included in serum chemistry profiles by many commercial laboratories but affords useful information for only a limited number of hepatobiliary diseases. High total cholesterol values are observed in cats and dogs with severe intrahepatic cholestasis involving bile ducts or EBDO because of impaired excretion of free cholesterol into the bile and subsequent regurgitation into the blood. Low total serum cholesterol concentrations have been noted in dogs with chronic severe hepatocellular disease and frequently in cats and dogs with congenital portosystemic shunts (PSS). It has been speculated that hypocholesterolemia is a sign of markedly altered intestinal absorption of (and increased use of) cholesterol for bile acid synthesis when the enterohepatic recirculation of bile acids is disturbed, as occurs with PSS. In other hepatobiliary diseases of cats and dogs, the total cholesterol values vary considerably within the reference range. Normal values in 4-week-old kittens are higher than those for adults; 8-week-old puppy reference ranges are the same as those for adults.
Serum Glucose Concentration
Hypoglycemia is an unusual event associated with hepatobiliary disease in dogs and especially in cats. Lost capacity to maintain normal serum glucose concentrations occurs in animals with acquired chronic progressive hepatobiliary disease when 20% functional hepatic mass or less is remaining. This inability to maintain normal serum glucose concentrations is presumably caused by the loss of hepatocytes with functioning gluconeogenic and glycolytic enzyme systems and impaired hepatic degradation of insulin. Hypoglycemia is often a near-terminal event in dogs with chronic progressive hepatobiliary disease. In striking contrast is the frequent observation of hypoglycemia in dogs with congenital PSS, particularly small-breed dogs. In PSS hypoglycemia may be due to an increase in circulating insulin concentration caused by reduced first pass metabolism in the liver, as observed in humans, but this has never been investigated in dogs. Hypoglycemia is also common as a paraneoplastic syndrome in dogs with large hepatocellular carcinomas and can be associated with production in insulin-like growth factor by the tumour (Zini et al., 2007). In either case, if hypoglycemia is identified and confirmed by repeating the test using sodium fluoride tubes if necessary, and if nonhepatic causes (functional hypoglycemia, sepsis, insulinoma, or other neoplasm producing an insulin-like substance, Addison’s disease; see Chapter 53) are excluded, a primary hepatic tumor (e.g., hepatocellular carcinoma), a PSS, or severe generalized hepatopathy is suspected.
Serum Electrolyte Concentrations
Serum electrolyte determinations facilitate supportive care of cats and dogs with hepatobiliary disease but give no particular hints as to the character of the disorder. The most common abnormality is hypokalemia, which is attributed to a combination of excessive renal and gastrointestinal losses, reduced intake, and secondary hyperaldosteronism in dogs and cats with severe chronic hepatobiliary disease. Metabolic alkalosis, presumptive evidence of which might be abnormally high serum total carbon dioxide content confirmed by blood gas analysis, is usually caused by overzealous diuretic therapy in dogs with chronic hepatic failure and ascites. Hypokalemia and metabolic alkalosis potentiate each other and may also worsen signs of hepatic encephalopathy (HE) by promoting persistence of readily membrane-diffusible ammonia (NH3).
Serum Bile Acid Concentrations
Recent validation of rapid, technically simple methods for SBA analysis in cats and dogs has provided a sensitive, variably specific test of hepatocellular function and the integrity of the enterohepatic portal circulation. “Primary” bile acids (i.e., cholic, chenodeoxycholic) are synthesized only in the liver, where they are conjugated with various amino acids (primarily taurine) before secretion into the bile. Bile is stored in the gallbladder, where it is concentrated until, under the influence of cholecystokinin, it is released into the duodenum. After facilitating fat absorption in the small intestine, the primary bile acids are efficiently absorbed into the portal vein and returned to the liver for reuptake and resecretion into the bile. A small percentage of primary bile acids that escapes resorption is converted by intestinal bacteria to “secondary” bile acids (i.e., deoxycholic, lithocholic), some of which are also resorbed into the portal circulation. Absorption of bile acids by the intestine is extremely efficient, but hepatic extraction from portal venous blood is not. This accounts for small concentrations of cholic, chenodeoxycholic, and deoxycholic acids that are released into the peripheral blood of healthy cats and dogs in the fasting state (total <5 μmol/L by enzymatic method and 5 to 10 μmol/L by radioimmunoassay [RIA]). During a meal a large load of bile acids is delivered to the intestine and portal circulation for recycling; postprandial values in normal dogs and cats may increase up to threefold to fourfold over fasting values (15 μmol/L with the enzymatic method for cats and dogs; 25 μmol/L with the RIA method for dogs). Normal values for juvenile animals are similar to adult reference ranges. Abnormally high fasting and/or postprandial SBA concentrations reflect disturbance in hepatic secretion into the bile or at any point along the path of portal venous return to the liver and hepatocellular uptake. Low SBA concentrations may be attributable to small intestinal (ileal) malabsorption of bile acids but might be difficult to interpret because both fasting and postprandial SBA concentrations may not be measurable in healthy animals.
The standard way to assess SBA concentrations is outlined in Box 36-1. Collective experience indicates that the likelihood of precipitating an episode of HE during this part of the test is extremely low, even in predisposed animals. After the serum is harvested, the samples may be refrigerated for several days or frozen almost indefinitely before assay. The stability of the blood sample is one of the major advantages over the much more labile serum ammonia test.
 BOX 36-1 Summary of Techniques for Bile Acid Stimulation Test and Postprandial Ammonia Challenge Test
BOX 36-1 Summary of Techniques for Bile Acid Stimulation Test and Postprandial Ammonia Challenge Test
Studies of SBAs have confirmed their value in detecting clinically relevant hepatobiliary disease requiring definitive diagnostic testing in cats and dogs, especially in anicteric animals with equivocal clinical signs and unexplained high liver enzyme activity. There continues to be controversy as to whether a fasting or postprandial value alone is sufficient or whether fasting and postprandial measurements are required. If only one sample can be obtained (and the animal will eat or can tolerate being force-fed a small meal), the postprandial value is most useful to determine the presence or absence, but not the type, of clinically relevant hepatobiliary disease in most cats and dogs. Current recommendations state that for animals suspected of having acquired hepatobiliary disease, biopsy is needed when postprandial SBA concentration using the enzymatic method exceeds 20 μmol/L in cats and 25 μmol/L in dogs, although other authors (particularly in the United Kingdom) suggest that SBA between 20 and 40 μmol/L in dogs represents a grey area (Hall et al., 2005). Elevations in this region have been seen with secondary hepatopathies (particularly hyperadrenocorticism) and with small intestinal bacterial overgrowth because of reduced hepatic clearance of deconjugated bile acids. Therefore the authors would recommend a liver biopsy with a higher cut-off for postprandial bile acids of 40 μmol/L. No pattern of preprandial and postprandial values is pathognomonic for any particular hepatic disorder, although it is safe to make certain generalizations. Magnitude of elevation above 20 μmol/L in cats and 25 μmol/L in dogs roughly correlates with the severity, but not the reversibility, of the hepatobiliary disorder, although with PSS, the magnitude of elevation does not correlate with the degree of shunting or severity of clinical signs. The change between the fasting value and the postprandial value likely corresponds to portosystemic shunting, either microscopic (intrahepatic) or macroscopic. There is so much overlap in fasting and postprandial SBA patterns among primary hepatobiliary diseases that no particular statement can be made regarding the specific causative hepatobiliary disease. Occasionally, fasting SBA levels are higher than postprandial levels, which signifies nothing more than occasional, normal, spontaneous gallbladder contraction in fasting. In general, secondary hepatic diseases cause more modest hepatobiliary dysfunction (SBA values <100 μmol/L).
For the diagnosis of congenital PSS, fasting and postprandial SBA determinations are recommended to enhance detection ability because it is relatively common for fasting values to be well within normal limits and for postprandial values to be as high as tenfold to twentyfold higher than normal postprandial values.
Now that simplified methods for SBA measurement have been developed (i.e., enzymatic, RIA) and are accessible, determination of total SBA has become a convenient, practical test of hepatobiliary function in cats and dogs. Some reference laboratories use an adapted enzymatic method, a commercial enzymatic kit (Enzabile; Nyegaard and Co., Olso, Norway), or a commercial RIA (Conjugated Bile Acids Solid Phase Radioimmunoassay Kit 125I; Becton Dickinson, Orangeburg, N.Y.). Each yields comparable diagnostic results, although the sample size needed for the RIA assay is quite small (50 μl) compared with the enzymatic method (400 to 500 μl). Because the measurement of fasting and postprandial SBA concentrations assesses the same functions as the ammonium chloride (NH4Cl) tolerance test without potentially dangerous consequences, it is the preferred test. As with any specially requested test, the laboratory chosen should use methods verified for clinical use in the target species and be able to provide reference ranges.
A benchtop “snap” test for bile acids has recently become available from IDEXX Laboratories (see www.idexx.com/animalhealth/analyzers/vetlabnotes/2005snapreader.jsp). The disadvantage of the snap test is that it has a low cut-off, which means that it does not differentiate secondary from primary hepatobiliary disease.
Several factors may affect SBA values and therefore their interpretation. One aspect of the SBA challenge test that has not been standardized is the feeding step. The ideal quantity and composition of the test meal have not been determined. Size of the test meal and therefore consumption of the entire meal or only part of the meal may affect gastric emptying. Delayed gastric emptying could cause the peak SBA concentration to occur more than 2 hours later. Hastened or delayed intestinal transit time or the presence of intestinal disease (especially of the ileum) may also impede and blunt peak absorption of the test meal. It is likely that fat content of the test meal is important because fat is the primary stimulus for the small intestinal mucosa to secrete cholecystokinin, which causes gallbladder contraction. Expulsion of bile during periodic physiologic gallbladder contraction between meals may complicate interpretation of the fasted sample result. Lipemia of the sample can seriously affect the validity of the test, particularly on heparinized blood. For this reason, it is far preferable to use serum, both for the external samples and for the snap test.
Several questions remain to be answered regarding the clinical use of SBA measurement in cats and dogs. Investigation of individual SBA profiles in cats and dogs with various hepatobiliary diseases has provided interesting information but no clear and specific profile for any one disease. Can sequential SBA values be used to more precisely monitor a cat’s or dog’s progress? Until this and other questions are answered, use of SBA analysis is limited to measuring total serum values as a sensitive and relatively specific screening test for the presence or absence of clinically significant hepatobiliary disease. Additional diagnostic testing must always follow to identify the specific cause.
Urinary Bile Acid Concentrations
Determination of bile acids accumulating in urine over time can be used to assess hepatobiliary function. Urine bile acids are believed to reflect average serum bile acid concentrations during the interval of urine formation. Expression of urine bile acid concentrations as a ratio with the urine creatinine concentration eliminates the influence of urine concentration and flow. Random urine sampling for bile acid determination does not require attending to the timing of an enterohepatic challenge or obtaining a sample after withholding food. In recent studies urinary bile acid concentrations were increased in dogs and cats with hepatobiliary disease and portosystemic vascular anomalies, compared with dogs and cats with nonhepatic disorders (except for hepatic neoplasia in dogs; Balkman et al., 2003; Trainor et al., 2003). The urine nonsulfated bile acid : creatinine ratio and the urine sulfated plus nonsulfated bile acid : creatinine ratio positively correlated with serum bile acid test results and had similar overall diagnostic performance and substantially higher (dogs) or similar (cats) specificity, compared with the serum bile acid test, and are recommended. The urine sulfated bile acid : creatinine ratio had lower sensitivity in dogs and cats, compared with the serum bile acid test.
Plasma Ammonia Concentration
One test that is not included in a standard screening battery of tests but is available through reference or human hospital laboratories is plasma ammonia concentration. Fasting plasma ammonia can be measured in any cat or dog with historic or physical examination findings suggestive of HE. Signs of HE (see Box 35-2), whether they have a congenital or acquired basis, appear the same. Quantifying plasma ammonia concentration not only can confirm HE, although normal fasting values in animals with hepatobiliary disease are relatively common, but can also provide baseline data and help in evaluating response to treatment. However, SBA values (particularly postprandial) provide very similar information. Some investigators have suggested that arterial ammonia concentrations may more accurately represent blood ammonia status in dogs with hepatobiliary disease than venous measurements because skeletal muscle can metabolize ammonia. High plasma ammonia concentration usually indicates reduced hepatic mass available to process ammonia and/or the presence of portosystemic shunting, which disrupts presentation of ammonia to the liver for detoxification. However, ammonia is very labile in the blood sample and, for example, can be falsely elevated if the blood sample is taken in an environment contaminated with urine. Sample handling has to be undertaken with caution, and some benchtop analyzers are inaccurate, particularly in the moderately elevated range. For these reasons, SBAs are often a preferred test. The exception to this would be an animal with suspected hepatic encephalopathy and concurrent cholestasis. As outlined in the preceding paragraphs, bile acid concentrations will be high in cholestasis (because they are excreted in the bile) independent of any reduction in liver function or shunting. Measuring blood ammonia in these circumstances will give useful additional information about potential shunting and HE.
In a recent study the 12-hour fasting plasma ammonia concentration had higher sensitivity and specificity than the 12-hour fasting bile acid concentration for detecting portosystemic shunting in a general population of dogs and in dogs with liver disease (Gerritzen-Bruning et al., 2006). However, a bile acid stimulation test (fasting and 2-hour postprandial bile acid) has a much higher sensitivity for detecting PSS than a single fasting bile acid, and a single postprandial bile acid concentration is likely as sensitive as a fasting ammonia concentration, although the authors did not test this.
Although reference ranges vary among laboratories, fasting plasma ammonia values for normal dogs are typically 100 mg/dl or less and 90 mg/dl or less for normal cats. At least 6 hours of fasting should precede sample collection. Samples must be collected into iced ammonia-free heparinized tubes and spun immediately in a refrigerated centrifuge. Plasma must be removed within 30 minutes so that values will not be spuriously elevated by hemolysis because red blood cells contain two to three times the ammonia concentration of plasma. To obtain accurate values, feline plasma can be frozen at −20° C and assayed within 48 hours; canine plasma must be assayed within 30 minutes.
If signs are compatible with HE at the time of sample collection, a single fasting sample will suffice. If there are no signs of HE and results of other tests are equivocal, a postprandial challenge test may be performed (see Box 36-1). The older ammonium chloride challenge tests (either oral or rectal) are contraindicated because of the significant potential for either test to trigger a severe encephalopathic crisis in the patient. The postprandial ammonia test is a safer test and has a 91% sensitivity for portosystemic shunting but only a 31% sensitivity for diffuse hepatocellular disease (Walker et al., 2001).
Plasma Protein C Activity
Plasma protein C activity was recently evaluated as a marker of hepatobiliary disease in dogs. Protein C is an anticoagulant protein that is synthesized in the liver and circulates as a plasma zymogen. Low protein C activity has been associated with thrombotic disorders in humans and animals. Low protein C activity has also been documented in dogs with acquired and congenital hepatobiliary disorders, and dogs with PSS appear to develop the lowest protein C activity. In a recent study by Toulza et al. (2006), protein C acivity was significantly lower in dogs with congenital or acquired portal systemic shunts, compared with dogs without PSS. Plasma protein C activity improved or normalized after surgery for the shunt. These findings suggest that plasma protein C activity reflects the adequacy of hepatoportal perfusion in dogs and that protein C activity may prove useful as a means to monitor improvement of hepatic-portal perfusion after ligation of portosystemic vascular anomalies. Plasma protein C activity may also help differentiate dogs with intrahepatic portal vein hypoplasia from those with portal systemic vascular anomaly (plasma protein C activity ≥70% versus <70%, respectively).
URINALYSIS
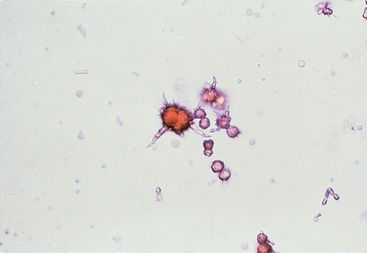
Common findings in urinalysis consistent with hepatobiliary disease include excessive bilirubinuria in a nonanemic dog (≥2+ in urine of specific gravity ≤1.025), presence of bilirubin in the urine of cats, and ammonium biurate crystalluria in properly processed urine specimens (Fig. 36-1). In dogs excessive bilirubinuria may precede the onset of hyperbilirubinemia and jaundice. Small numbers of bilirubin crystals may be found in concentrated urine specimens from normal dogs and ammonium biurate crystals are also occasionally found in normal animals and also in Dalmatian dogs with a defect in urate metabolism (see Chapter 46) and therefore are not pathognomonic for PSS. Hyperammonemia combined with excess uric acidemia from diminished hepatic conversion to allantoin exceeds the renal threshold and favors precipitation of crystals, especially in alkaline urine. Their presence in the urine may fluctuate, but alkalinizing the urine specimen with a few drops of sodium or potassium hydroxide may increase the likelihood of identifying ammonium biurate crystals during sediment examination.
Measurement of urinary urobilinogen by dipstick analysis has traditionally been used to assess the patency of the extrahepatic biliary system. So many factors influence detection of urobilinogen in the urine (e.g., intestinal flora and transit time, renal function, urine pH and specific gravity, exposure of the urine specimen to light) that the test is now considered to be of minimal value in diagnosing EBDO. If urine samples are obtained serially and processed properly, repeated absence of urobilinogen suggests, but is not diagnostic of, complete EBDO.
Consistently dilute urine (specific gravity as low as 1.005) may be a feature of congenital and acquired PSS and severe hepatocellular diseases because of the associated polydipsia/polyuria and hypercortisolism, as discussed in Chapter 35. Urine specific gravity must also be interpreted in light of concurrent drug therapy, such as administration of diuretics, corticosteroids, or anticonvulsants.
FECAL EVALUATION
Fecal specimen analysis rarely provides useful information in the evaluation of a dog or cat with suspected hepatobiliary disease, except for a change in appearance associated with two specific conditions. Absence of fecal pigment (acholic feces; see Fig. 35-6) and steatorrhea are consequences of chronic complete EBDO, and dark, orange-colored feces reflect increased bilirubin production and excretion after marked hemolysis or rhabdomyolysis. It should also be noted that gastrointestinal ulceration is a serious and important complication of portal hypertension, particularly in dogs (see Chapter 39), so the clinician should always be alert to the development of melena in a dog with chronic liver disease.
ABDOMINOCENTESIS/FLUID ANALYSIS
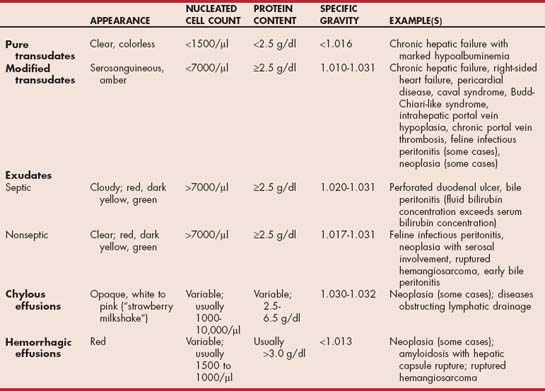
If abdominal fluid is detected during physical examination, abdominal radiography, or ultrasonography, a sample must always be obtained for analysis. For moderate to large volume effusion, simple needle paracentesis is sufficient to obtain 5 to 10 ml of fluid for gross inspection; determination of protein content; cytologic evaluation; and, in selected cases, special biochemical analysis. Larger volumes are removed using an over-the-needle-style catheter with extension tubing or a needle with attached tubing (E-Z infusion set) if clinical signs secondary to fluid accumulation are present (e.g., dyspnea) or if removal of abdominal fluid is part of the treatment (e.g., bile peritonitis). Removal of a significant volume of abdominal fluid for clinical reasons should be avoided unless it is absolutely necessary because doing so often causes a precipitous decrease in serum protein concentrations in animals with liver disease owing to the inability of the liver to replace proteins removed in the fluid. It is preferable in cases other than peritonitis to remove fluid gradually, using diuretics. In cases in which large volume fluid removal is necessary (e.g., for dyspnea), concurrent administration of fresh frozen plasma or a colloid solution is essential. In dogs with chronic hepatic failure and sustained intrahepatic portal hypertension, abdominal fluid is usually a modified transudate with moderate nucleated cell count and protein content (Table 36-1). A pure transudate with low cell count (<2500 cells/μl) and protein concentration (<2.5 g/dl), and a clear, minimally colored appearance is found when the dog is hypoproteinemic. Abdominal fluid in dogs with intrahepatic postsinusoidal venous obstruction (e.g., venoocclusive disease) or posthepatic venous obstruction (e.g., any cause of right-sided heart failure) can be any color but is typically red- or yellow-tinged and is classified as a modified transudate. Feline infectious peritonitis fluid and neoplastic effusions are also commonly classified as modified transudates or nonseptic exudates. Bile peritonitis also results in an exudate, which is initially sterile but can become septic with time. With neoplasia, effusions can occasionally be chylous or even hemorrhagic, and the latter can also be seen in amyloidosis as a result of rupture of the liver capsule. Reactive mesothelial cells can be mistaken for neoplastic cells, emphasizing the need for experience in evaluating cytologic specimens. Exudates have high cell counts (>20,000 cells/μl) and protein content (>2.5 g/dl) and, on the basis of whether the inflammatory cells look toxic or contain ingested bacteria, are further classified as septic or nonseptic. Fluid analysis provides additional clues to the origin of hepatobiliary disease and must not be overlooked. A guide to interpreting fluid analysis results is given in Table 36-1.
COMPLETE BLOOD COUNT
There are few changes in blood cells that suggest hepatobiliary disease. Most are changes in erythrocytes associated with fragmentation or changes in cell size or membrane composition (Fig. 36-2). Microcytosis (mean cell volume [MCV] <60 fl in canine breeds other than the Japanese Akita or Shiba Inu) with normochromia or slight hypochromia (mean cell hemoglobin concentration: 32 to 34 g/dl) is a rather common finding in dogs with congenital PSS (≥60%); it is less common in cats with congenital PSS (≤30%). Most affected animals are not anemic. The cause of microcytosis, which has also been observed with less frequency in dogs with chronic hepatic failure and acquired PSS, is chelation of iron within the liver rather than absolute iron deficiency; therefore iron supplementation does not help. However, the change in the size of red blood cells is reversible upon restoration of portal blood flow. If anemia is also present, microcytosis must be distinguished from anemia of inflammatory disease, which can occasionally cause small red blood cells and relative iron deficiency, or from iron deficiency anemia associated with chronic gastrointestinal blood loss (see Chapter 83).
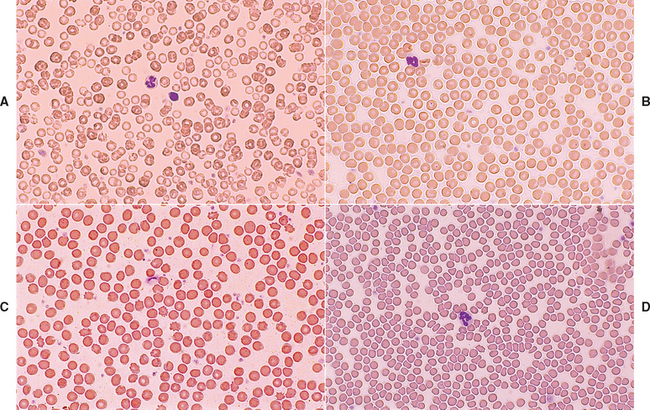
FIG 36-2 Erythrocyte morphologic changes often associated with hepatobiliary disease in cats and dogs (Wright-Giemsa stain). A, Microcytic red blood cells (mean corpuscular volume [MCV] = 45 fl) from dog with congenital portosystemic shunt; compare the microcytic red blood cells with the size of a nearby normal small lymphocyte 6 to 9 μm in diameter. B, Normal canine red blood cells (MCV = 70 fl) for comparison. C, Acanthocytes from dog with severe chronic hepatic passive congestion. D, Poikilocytes from cat with cholangitis.
Strongly regenerative anemia, with macrocytosis, high reticulocyte count, and normal to slightly increased serum protein concentration in a jaundiced dog, especially if spherocytes are also identified, indicates hemolytic anemia and increased bilirubin formation as the cause of jaundice. Cats and dogs with hemolytic anemia typically also have high serum liver enzyme activities and bile acid concentrations, pointing to hepatic consequences developing secondary to the effects of marked hemolysis, such as hypoxia and thromboembolism.
Certain red blood cell morphologic changes are consistent with serious hepatobiliary disease and are related to alterations in lipoprotein metabolism and irregularities in red blood cell membrane structure. Acanthocytes, leptocytes, and codocytes (target cells) are good examples (see Fig. 36-2). Poikilocytosis of unknown pathogenesis is a consistent finding in cats with congenital PSS and occasionally with other hepatobiliary diseases; cats with chronic hepatobiliary disease frequently have Heinz bodies in their red blood cells. Fragmented red blood cells or schistocytes constitute an expected finding in animals with DIC; hemangiosarcoma is considered when an inappropriate number of nucleated red blood cells is also detected. Mild to moderate nonregenerative anemia is common in cats with many different illnesses, including those of the hepatobiliary tract.
Few changes in the leukon are expected in cats or dogs with hepatobiliary disease, except when an infectious agent is present as the initiating event (histoplasmosis, bacterial cholangitis, or leptospirosis in dogs); where there is concurrent pancreatitis, which is particularly common in cats (see Chapter 40); or when infection has complicated a primary hepatobiliary disorder (e.g., gram-negative sepsis in a dog with cirrhosis, septic bile peritonitis). Neutrophilic leukocytosis is likely in such cases, whereas pancytopenia is typical of disseminated histoplasmosis and severe toxoplasmosis in cats and of early infectious canine hepatitis.
COAGULATION TESTS
Clinically relevant coagulopathies are unusual in cats and dogs with hepatobiliary disease except for those with acute hepatic failure (including acute hepatic lipidosis in cats or hepatic lymphoma in both species), complete EBDO, or active DIC. It is more common to have subtle prolongation of activated partial thromboplastin time (APTT; 1.5 times normal), abnormal fibrin degradation products (10 to 40 or higher), and variable fibrinogen concentration (<100 to 200 mg/dl) in cats and dogs with severe parenchymal hepatic disease. Elevated D-dimers are common in patients with liver disease and do not always indicate DIC in these cases. It has been proposed that nonspecific elevation can occur in liver disease as a result of reduced clearance by the liver. Platelet numbers may be normal or low; mild thrombocytopenia (130,000 to 150,000 cells/μl) is usually associated with splenic sequestration or chronic DIC. More severe thrombocytopenia (≤100,000 cells/μl) is expected in acute DIC or decompensated chronic DIC. Some animals with severe hepatic disease and relatively unremarkable routine coagulation test results have high serum activity of proteins induced by vitamin K antagonism (PIVKA) that could impart bleeding tendencies. Primary or metastatic cancer of the liver could also cause coagulopathy unrelated to loss of hepatocellular ability to make or degrade coagulation proteins.
A summary of laboratory tests for cats and dogs with hepatobiliary disease and interpretation of their results is given in Table 36-2.
 TABLE 36-2 Summary of First- and Second-Line Clinicopathologic Tests Useful in the Diagnosis of Hepatobiliary Disease
TABLE 36-2 Summary of First- and Second-Line Clinicopathologic Tests Useful in the Diagnosis of Hepatobiliary Disease
| SCREENING TEST | PRINCIPLE EXAMINED | COMMENTS |
|---|---|---|
| Serum ALT, AST activities | Integrity of liver cell membranes; escape from cells | Degree of increase roughly correlates with number of hepatocytes involved but not severity of disease |
| Serum AP, GGT activities | Reactivity of biliary epithelium to various stimuli; increased synthesis and release | Increase associated with intrahepatic or extrahepatic cholestasis or drug effect (dogs only): corticosteroids, anticonvulsants (AP only, not GGT) |
| Serum albumin concentration | Protein synthesis | Rule out other causes of low concentration (glomerular or intestinal loss); low value indicates ≥80% overall hepatic function loss or negative acute phase response |
| Serum urea concentration | Protein degradation and detoxification | With low values, rule out prolonged anorexia; dietary protein restriction; severe PU/PD; urea cycle enzyme deficiency (rare); congenital PSS; severe, acquired chronic hepatobiliary disease |
| Serum bilirubin concentration | Uptake and excretion of bilirubin | Rule out marked hemolysis first; if PCV is normal, intrahepatic or extrahepatic cholestasis is present |
| Serum cholesterol concentration | Biliary excretion, intestinal absorption, integrity of the enterohepatic circulation | High values compatible with severe cholestasis of any kind; low values suggest congenital PSS; anticonvulsant drug-induced change; severe, acquired chronic hepatobiliary disease; or severe intestinal malassimilation |
| Serum glucose concentration | Hepatocellular gluconeogenic or glycolytic ability; insulin and other hormone metabolism | Low values indicate severe hepatocellular dysfunction, PSS, or presence of a primary liver tumor |
| Plasma ammonia concentration | Integrity of the enterohepatic circulation, hepatic function and mass | High fasting or postprandial values suggest congenital or acquired PSS or acute hepatocellular inability to detoxify ammonia to urea (massive necrosis) |
| Serum bile acid concentrations | Integrity of the enterohepatic circulation, hepatic function and mass | High fasting or postprandial values compatible with hepatocellular dysfunction, congenital PSS, or loss of hepatic mass. Elevated in cholestasis independent of hepatocellular dysfunction or shunting so rule this out first |
| Coagulation profile | Hepatocellular function, adequacy of vitamin K absorption and stores | Abnormal values may indicate marked hepatocellular dysfunction, acute or chronic DIC, complete EBDO |
ALT, Alanine aminotransferase; AST, aspartate aminotransferase; AP, alkaline phosphatase; GGT, γ-glutamyltransferase; PU/PD, polyuria/polydipsia; PSS, portosystemic shunting; PCV, packed cell volume; DIC, disseminated intravascular coagulation; EBDO, extrahepatic bile duct obstruction.
DIAGNOSTIC IMAGING
SURVEY RADIOGRAPHY
Radiographic evaluation of the abdomen is used to complement physical examination findings and to confirm suspicions regarding the character and location of the hepatobiliary disease suggested by results of clinicopathologic examination. Survey radiographs provide subjective information regarding the size and shape of the liver (see Table 35-1). Optimally, the animal should have an empty gastrointestinal tract at the time the radiographs are obtained. In the normal dog and cat in right lateral recumbency, the gastric axis is parallel to the ribs at the tenth intercostal space, and the caudoventral border of the liver (the left lateral liver lobe) appears sharp; the image is made possible by the con trasting fat-filled falciform ligament (Fig. 36-3). In dog breeds with narrow, deep chests, the entire liver shadow may be contained within the caudal rib cage. In dogs with wide, shallow thoracic conformation, the liver may extend slightly beyond the costal arch. In the ventrodorsal view the borders of the liver are defined by the cranial duodenum and the gastric fundus; in this view the gastric shadow is perpendicular to the spine. This view is less useful for assessing liver size unless it is markedly and asymmetrically enlarged. Immature animals have a relatively larger liver than do adults. The gallbladder and extrahepatic biliary tree are not visible separately radiographically in healthy animals.
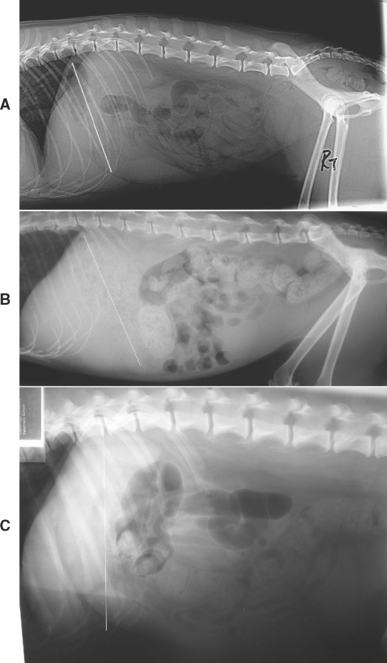
FIG 36-3 Lateral abdominal radiographs demonstrating gastric axis (white line) as an indication of liver size. A, Lateral abdominal radiograph of a normal cat with normal liver size. B, Lateral abdominal radiograph of a cat with diffuse hepatic amyloidosis demonstrating hepatomegally and caudal displacement of the gastric axis. C, Lateral abdominal radiograph of a middle-aged English Springer Spaniel with cirrhosis demonstrating microhepatica and cranial displacement of the gastric axis.
(Radiographs courtesy the diagnostic imaging department, The Queen’s Veterinary School Hospital, University of Cambridge.)
Survey radiography is of minimal to no benefit if there is moderate to marked abdominal effusion because the similar radiographic opacities of the liver and fluid preclude distinction of liver size and shape except by indirect assessment (e.g., malposition of a gas-filled stomach and duodenum; Fig 36-4). However, because abdominal fluid increases ultrasonographic contrast, this is the imaging modality of choice in animals with ascites. Poor abdominal detail in emaciated or very young animals lacking abdominal fat stores also makes detection of subtle hepatic changes difficult.
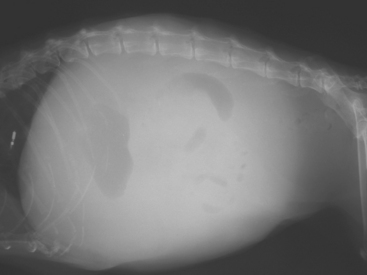
FIG 36-4 Lateral abdominal radiograph of an 8-year-old Bearded Collie with chronic hepatitis, portal hypertension, and ascites demonstrating the loss of abdominal detail associated with free abdominal fluid, which renders radiography unhelpful.
(Radiograph courtesy the diagnostic imaging department, The Queen’s Veterinary School Hospital, University of Cambridge.)
In cats and dogs with generalized hepatomegaly, the liver extends beyond the costal arch; it causes displacement of the gastric axis and pylorus caudally and dorsally in the lateral projection and shifting of the gastric shadow caudally and to the left in the ventrodorsal view (see Fig 36-3). In addition, the edges of the liver in the lateral view may appear rounded (see Fig. 36-3). Occasionally, the spleen and liver cannot be differentiated when they are in direct contact, as seen in the right lateral view. A ventrodorsal view would help to determine the size, shape, and position of each organ. Increased intrathoracic volume associated with deep inspiration, severe pleural effusion, or overinflation of the lungs may result in caudal displacement of the liver, giving the erroneous impression of hepatomegaly using other radiographic criteria.
Because the liver may be contained entirely within the rib cage in normal cats and dogs, microhepatia is more difficult to recognize than hepatomegaly. Changes in the angle of the gastric fundus in the right lateral projection (see Fig. 36-3) could indicate a small hepatic shadow if the angle is more upright or perpendicular to the spine and especially if the stomach seems rather close to the diaphragm. The liver may also seem small in animals with traumatic diaphragmatic hernia and herniation of liver lobes into the thorax or in those with congenital peritoneopericardial hernia.
Focal hepatic enlargement is indicated by displacement of organs adjacent to the affected lobe. The most common radiographically detectable focal hepatic enlargement is that of the right lateral lobe, an example of which is shown in Fig. 36-5. In this case the body and pyloric regions of the stomach are shifted dorsally (lateral view) and to the patient’s left (ventrodorsal view); the gastric fundus remains in normal position. Shifting of the stomach to the left is normal in cats and should not be mistaken for right hepatomegaly. If the left lateral lobe or lobes are enlarged, the gastric fundus moves to the left and caudally; the lesser curvature of the stomach may appear indented. Primary or metastatic neoplasia, hyperplastic or regenerative nodules, and cysts most commonly account for focal hepatic enlargement or for irregular liver margins without enlargement. If the gallbladder is massively enlarged because of EBDO, it may mimic a right cranial abdominal mass or an enlarged, rounded liver lobe. Changes in hepatic radiographic opacity are rare and are usually associated with hepatic or biliary tract infection caused by gas-forming bacteria (patchy and/or linear areas of decreased opacity) or mineralization (focal or diffuse spots of mineralization or mineralized biliary calculi).
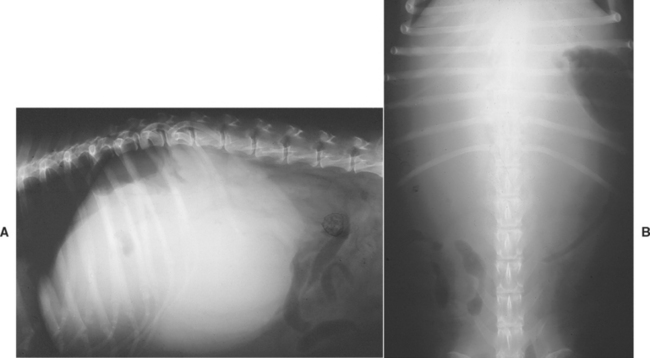
FIG 36-5 Lateral (A) and ventrodorsal (B) abdominal radiographs of a 9-year-old spayed female mixed-breed dog with a hepatocellular carcinoma enlarging the right lateral liver lobe. The dog was also severely hypoglycemic.
With the advent of ultrasonography, contrast radiographic procedures are seldom needed to confirm the presence of hepatic masses, cholelithiasis, EBDO, congenital PSS, and other structural diseases. The contrast study that is still necessary to localize some types of congenital PSS and is achievable in private practice is portal venography. Acceptable approaches for this technique are splenoportography, operative mesenteric portography, and operative splenoportography. The two operative procedures require general anesthesia and a small abdominal incision; however, little sophisticated equipment is needed, and the procedures are associated with few complications. A 22-gauge catheter is placed in the splenic vein or a mesenteric vein (Fig. 36-6), and the resting portal venous pressure is measured with a water manometer (N = 6 to 13 cm H2O). Portal pressure is measured as soon as possible in the procedure because prolonged anesthesia may complicate its interpretation. An injection of iodine-based contrast medium at a dose of 0.5 to 1 ml/kg is then quickly made. Lateral and possibly ventrodorsal and oblique radiographs are made at the end of the injection. Contrast medium given to a normal cat or dog should flow into the portal vein, enter the liver, and branch multiple times, opacifying the extrahepatic and intrahepatic portal vasculature. Diversion of the contrast medium into the systemic circulation indicates PSS (Fig. 36-7). Measurement of portal pressure and a liver biopsy can be performed during the operative techniques; they are required to distinguish acquired PSS from congenital PSS, which is essential to rendering an accurate prognosis and developing the correct treatment plan. As a general rule, cases of congenital PSS are usually single whereas acquired PSS are multiple, so the mesenteric portography may suggest a diagnosis. It may be necessary to repeat the contrast study after congenital PSS ligation if there is concern about the adequacy of the intrahepatic portal vasculature. In addition, it has been shown that the degree of intrahepatic portal vessel opacification on post-ligation portography is predictive for outcome (Lee et al., 2006).
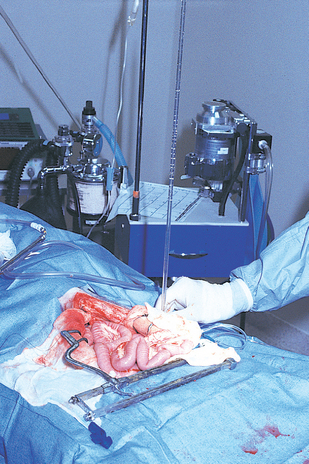
FIG 36-6 A 22-gauge intravenous catheter attached to an extension set, three-way stopcock, and water manometer has been placed in a mesenteric vein in preparation for intraoperative measurement of resting portal pressure. The catheter may also be secured in place and used for operative portal venography.
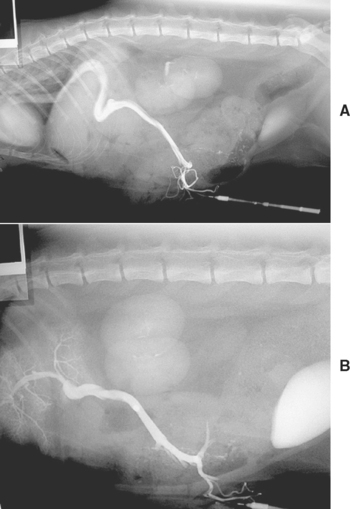
FIG 36-7 Operative mesenteric portal venography in a young domestic shorthaired cat before (A) and after (B) surgical correction (note improvement in hepatic portal blood flow in B with arborization of the contrast material within the small portal vessels in the liver).
(Radiographs courtesy the diagnostic imaging department, The Queen’s Veterinary School Hospital, University of Cambridge.)
ULTRASONOGRAPHY
Abdominal ultrasonagraphy (US) is the preferred diagnostic modality for evaluating the hepatobiliary system in dogs and cats. Operating on the principle that a pulse of sound (echo) can be reflected when it passes through the interface between two different materials, US can detect differences between homogeneous liquids of low echogenicity, such as blood and bile, and more heterogeneous echogenic structures made up of several soft tissues. Whereas abdominal effusion obscures abdominal detail on survey radiography, it enhances the ability of US to detect abnormalities (Fig. 36-8). However, bone and gas-filled organs reflect the sound beam completely (acoustic shadowing) so that structures beneath cannot be imaged by US. The procedure does not require anesthesia, but the patient must be still, and good contact between the transducer and abdominal skin must be ensured by clipping the hair coat and applying acoustic coupling gel. Animals are usually positioned in dorsal or lateral recumbency. The hepatic parenchyma, gallbladder, large hepatic and portal veins, and adjacent caudal vena cava are all visible in the liver of the normal cat and dog. Unlike plain radiography, which requires two views to complete the study, US makes many slices through several planes to create a three-dimensional reconstruction of the target structures.
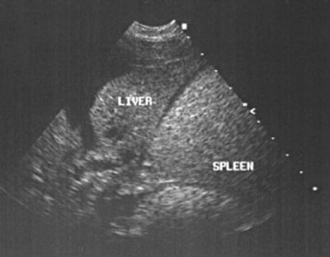
FIG 36-8 Abdominal ultrasound is enhanced by the presence of ascites. Ultrasound of the abdomen of a dog with chronic hepatitis and ascites.
(Image courtesy the diagnostic imaging department, The Queen’s Veterinary School Hospital, University of Cambridge.)
Performing US and interpreting the recorded images are a blend of technical skill and experience. It is also important to remember that US is very sensitive to the presence of lesions but does not diagnose what the lesions are (i.e., it cannot yield a histological diagnosis). With a few exceptions, which predominantly involve lesions of the biliary tract and vessels, the ultrasonographic appearance of a variety of both benign and malignant hepatic lesions can appear very similar and histology of a liver biopsy is usually required for diagnosis. An animal should never be euthanized on the basis of an ultrasonographically identified “tumor” without histological confirmation because benign nodular hyperplasia or focal inflammatory lesions can look the same. Table 36-3 outlines the typical appearances of different hepatic lesions on ultrasonography.
 TABLE 36-3 Ultrasonographic Findings in Dogs and Cats with Hepatobiliary Diseases
TABLE 36-3 Ultrasonographic Findings in Dogs and Cats with Hepatobiliary Diseases
| FINDING | POSSIBLE INTERPRETATIONS |
|---|---|
| Parenchyma | |
| Anechogenicity | |
| Focal | |
| Hypoechogenkity | |
| Focal | |
| Diffuse | |
| Hyperechogenicity | |
| Focal | |
| Diffuse | |
| Tubular Structures—Biliary Tract | |
| Dilated intrahepatic and extrahepatic bile ducts | |
| Distended gallbladder | Normal (prolonged fasting) |
| Distended gallbladder and cystic duct | Cystic duct obstruction |
| Distended gallbladder and common bile duct | Extrahepatic bile duct obstruction; persistent or recently relieved |
| Focal areas of gravity-dependent hyperechogenicity biliary tract or gallbladder that cause acoustic shadowingwithin | Cholelithiasis |
| Focal areas of hyperechogenicity within gallbladder that settle to dependent portion of gallbladder when animal’s position changes | “Sludged” or inspissated bile from severe cholestasis, prolonged anorexia, and dehydration |
| Stellate or “kiwi fruit” appearance to gallbladder | Gallbladder mucocele |
| Intraluminal echoic masses in gallbladder | |
| Apparent thickened gallbladder wall | |
| Tubular Structures—Blood Vessels | |
| Dilated hepatic veins and portal veins | |
| Prominent hepatic arteries | Reduced portal blood flow |
| Distended portal vein with reduced velocity and flow | Portal hypertension of any cause (by Doppler) |
| Inapparent hepatic vessels | |
| Inapparent portal veins | |
| Aberrant vessel that communicates with systemic circulation | Intrahepatic or extrahepatic congenital portosystemic shunt |
| Connection between a portal vein and an artery within one or more liver lobes | Arterioportal venous fistula |
| Many tortuous veins clustered around left kidney and along colon | Acquired portosystemic shunts associated with portal hypertension |
Neoplasia may appear as hyperechoic or hypoechoic and focal or diffuse. Hepatic lymphoma often appears diffusely hypoechoic but can also appear hyperechoic. Some tumors, such as metastatic hemangiosarcomas, have a classically nodular hypoechoic appearance (Fig. 36-9). Contrast-enhanced ultrasonography has recently been used to improve visualization of small hepatic metastases in dogs (O’Brien 2007). Typically, hepatic lipidosis in cats causes an increase in echogenicity and so does diffuse fibrosis such as cirrhosis in dogs. However, a cirrhotic liver may appear normal ultrasonographically.
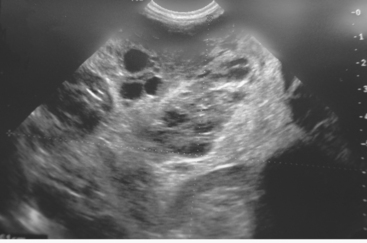
FIG 36-9 Ultrasonographic appearance of a hepatic hemangiosarcoma in a dog. Note the multiple hypoechoic nodules.
(Image courtesy the diagnostic imaging department, The Queen’s Veterinary School Hospital, University of Cambridge.)
Dilated anechoic (black) vascular channels and echoic bile ducts can be identified; biliary tract imaging is particularly useful in cats with suspected biliary tract disease (Fig. 36-10) or dogs and cats with suspected EBDO. The bile duct can be followed ultrasonographically along its course toward the small intestine, and lesions in the pancreas or duodenum obstructing it can be identified.
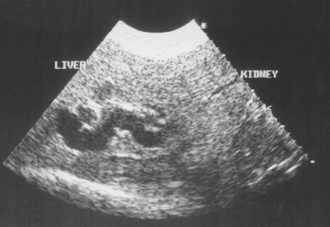
FIG 36-10 Ultrasonographic appearance of dilated biliary tract in a cat with chronic cholangitis.
(Image courtesy the diagnostic imaging department, The Queen’s Veterinary School Hospital, University of Cambridge.)
A dilated gallbladder may indicate prolonged anorexia, unless dilated bile ducts, particularly the common bile duct, are also seen, which supports EBDO or chronic cholangitis/cholangiohepatitis in cats (see Fig. 36-10). Intrahepatic or extrahepatic anomalous vessels may also be identified in animals with clinicopathologic evidence of severe chronic hepatobiliary disease or congenital PSS (Fig. 36-11). Congenital PSSs are typically single vessels, whereas acquired PSSs are usually multiple. Use of Doppler color-flow imaging confirms the location of the suspicious vessel(s) and the direction of blood flow within it. Doppler imaging can also provide supportive evidence of intrahepatic portal hypertension by allowing the assessment of the speed and direction of portal flow. Portal blood flow toward the liver (hepatopetal) is normal; away from the liver (hepatofugal) is abnormal. Whether the lesion is determined to be focal or diffuse, US can also be used as a guide to obtain diagnostic specimens for cytologic or histopathologic evaluation. US has developed into a valuable and critically important adjunct to diagnosis of hepatobiliary disease of cats and dogs by allowing characterization of structural changes not possible by any other modality and by providing a way to obtain needle liver biopsy specimens and bile duct samples in a visualized manner without the need for general anesthesia.
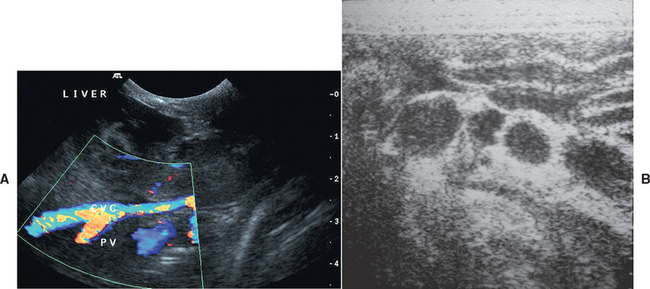
FIG 36-11 A, Doppler ultrasonographic findings of a congenital extrahepatic portocaval shunt in a young English Springer spaniel. B, Ultrasonographic appearance of multiple extrahepatic acquired portosystemic shunts in a 6-year-old German Shepherd Dog with noncirrhotic portal hypertension. CVC, Caudal vena cava; PV, portal vein.
(Images courtesy the diagnostic imaging department, The Queen’s Veterinary School Hospital, University of Cambridge.)
SCINTIGRAPHY
Other imaging modalities, such as scintigraphy (nuclear imaging), magnetic resonance imaging, contrast-enhanced harmonic ultrasound, and computed tomography, are available primarily through teaching or larger referral institutions. Of these imaging modalities, scintigraphy has been evaluated most thoroughly for diagnosis of hepatobiliary disease in cats and dogs. The isotope selected most often for clinical use is technetium-99m (99mTc), which is incorporated into the radiopharmaceutical specific for the planned study. For example, 99mTc bound to sulfur colloid, which is phagocytized by monocyte-macrophage cells of the liver and spleen, is given to assess liver mass. Images are made by collection of emissions from decaying isotope using a gamma camera focused over the animal’s liver region and recorded on radiographic film. The isotope has a short (6-hour) half-life; thus, although the animal must be relatively isolated for 24 to 48 hours and urinary and fecal waste stored until radioactivity has fallen to background levels, there is minimal radiation hazard to the animal or involved personnel. To distinguish medical from surgical causes of jaundice, 99mTc is combined with disofenin (Hepatolite). After an intravenous injection of radiopharmaceutical, scintigraphic images are made sequentially over 3 hours to determine whether the isotope has been taken up by the liver, excreted into the biliary tract, and expelled into the intestine. In cats and dogs with EBDO, no evidence of radiopharmaceutical is detected in the gallbladder or intestine.
Another application of scintigraphy is used in the diagnosis of PSS in cats and dogs. Following placement of pertechnetate labeled with 99mTc into the descending colon, the vascular path taken by the isotope after absorption is plotted. Time/activity curves determine whether the isotope arrived in the liver first, which is normal, or in the heart and lungs, which is compatible with any kind of portal venous bypass of the liver (Fig. 36-12). This approach has the advantage of specifically evaluating the portal blood supply rather than the hepatic mass, which may or may not be reduced in animals with congenital PSS or primary hepatobiliary disease and acquired PSS. The test results do not provide anatomic detail but only evidence of the presence or absence of congenital or acquired portosystemic shunting. Transcolonic portal scintigraphy is most helpful in confirming the presence of a congenital PSS in a cat or dog with atypical clinicopathologic test results, equivocal abdominal ultrasound findings (e.g., normal-size liver, no identifiable vessel arising from the portal vein), and no evidence of portal hypertension (e.g., ascites).
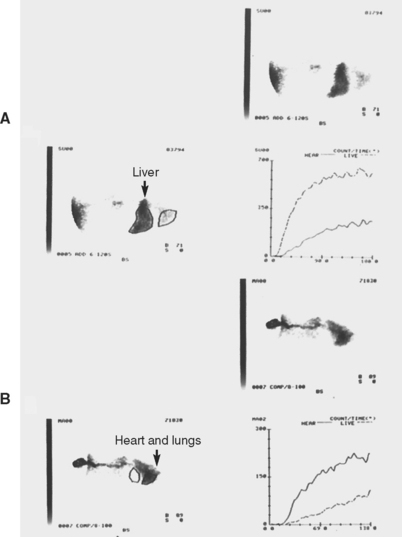
FIG 36-12 Transcolonic scintigraphy demonstrating the portal vascular path to the liver. A, Normal dog with isotope following a direct path to the liver and a small (5%) shunt fraction. B, Abnormal arrival of isotope in the heart and lungs of 1-year-old male Miniature Schnauzer with congenital portosystemic shunt and large (84%) shunt fraction. In each scan image the dog’s head is to the right.
(Courtesy Dr. Lisa J. Forrest, North Carolina State University, College of Veterinary Medicine.)
LIVER BIOPSY
General Considerations
For many primary hepatobiliary diseases of cats and dogs, a hepatic biopsy is needed to establish a final diagnosis and prognosis. In some cases bile culture is also imperative. Biopsy is indicated to (1) explain abnormal results of hepatic status and/or function tests, especially if they persist for longer than 1 month; (2) explain hepatomegaly of unknown cause; (3) determine hepatic involvement in systemic illness (although biopsy is not always necessary for this); (4) stage neoplastic disease; (5) objectively assess response to therapy; or (6) evaluate progress of previously diagnosed, not specifically treatable disease. Percutaneous hepatic biopsy is not performed if there is a good chance that the disease can be corrected surgically, such as in some cases of EBDO or congenital PSS; instead, a specimen is obtained at the time of surgery to complete the diagnostic evaluation. Several approaches are available; choice is dictated by patient and operator considerations (Box 36-2). In addition, in most cases of hepatic disease the accuracy of histological diagno sis is better with larger (i.e., surgical or laparoscopic) rather than smaller (i.e., needle) biopsies.
 BOX 36-2 Patient and Operator Considerations for Hepatic Biopsy
BOX 36-2 Patient and Operator Considerations for Hepatic Biopsy
All cats and dogs undergoing hepatic biopsy are fasted for at least 12 hours, regardless of the approach selected. In general, percutaneous needle core biopsy or aspiration (for cytologic analysis) of a single cavitary or solid lesion highly likely to be nonlymphoid cancer should be avoided unless the owner is unwilling to permit surgery for complete resection. Fine-needle aspiration of the liver for cytologic analysis is rarely advisable because of low diagnostic yield and often misleading results. The exceptions to this are for quick diagnosis of hepatic lipidosis in cats and possibly for suspected hepatic lymphoma, although even then the diagnosis may need to be confirmed histologically (Fig. 36-13). However, an overall correlation of only 30% in dog and 51% in cats was found in one study comparing the cytologic diagnosis with the histopathologic diagnosis of a variety of liver diseases (Wang et al., 2004).

FIG 36-13 A 4-year-old spayed female domestic short-haired cat with suspected hepatic lipidosis positioned in right lateral recumbency for blind fine-needle aspirate for cytology. With care taken to avoid the spleen, the needle is directed craniomedially into the liver.
In an especially small and/or firm fibrotic liver, it is difficult to obtain a biopsy specimen by percutaneous needle methods; small, fragmented specimens that are challenging to interpret are often the result (Fig. 36-14). There is less than a 40% correlation between 18-gauge needle biopsy and wedge biopsy for certain hepatobiliary diseases (i.e., chronic hepatitis/cirrhosis, cholangitis, portovascular anomaly, fibrosis). If a needle technique is selected, the largest available instrument is used (preferably 14 gauge; minimum 16 gauge) and multiple samples are taken to ensure samples adequate for examination.
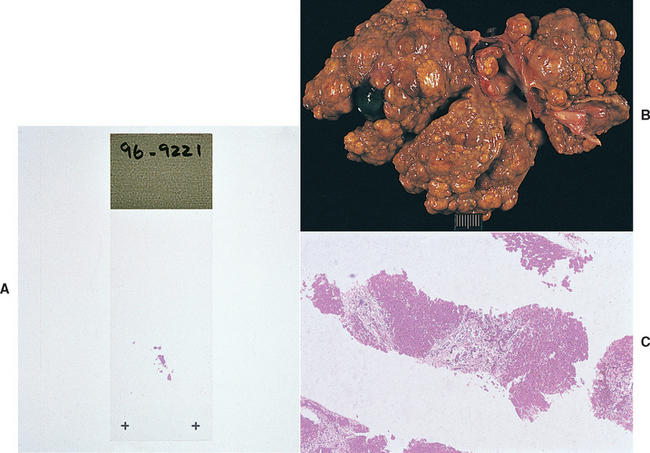
FIG 36-14 A, Liver specimen obtained percutaneously (with ultrasound guidance) from a dog with hepatic fibrosis and nodular regeneration (B). The specimen was difficult to obtain because the liver was firm and rubbery in texture. C, The resultant sample was difficult to interpret histologically.
The animal’s coagulation status is determined before a liver biopsy is performed, regardless of the approach. Ideally, a complete coagulation profile (one-stage prothrombin time [OSPT], APTT, fibrin degradation products, fibrinogen content, platelet count) is obtained; a platelet count and an activated clotting time or whole blood clotting time in a glass tube, as a screening test of the intrinsic coagulation cascade, are also acceptable. Bleeding after ultrasound-guided biopsy is more likely if the platelet count is less than 80,000 cells/μl or if the OSPT (dogs) or APTT (cats) is prolonged (Bigge et al., 2001). If possible, von Willebrand’s factor is measured in susceptible breeds in advance of biopsy because results of standard coagulation tests are usually normal in affected dogs. A buccal mucosa bleeding time test provides indirect assessment of platelet function (see Chapter 87). In dogs with von Willebrand’s disease, desmopressin acetate (DDAVP) is given (1 μg/kg intranasal preparation subcutaneously) before surgery to enhance shift of von Willebrand’s factor activity from endothelial cells to the plasma.
Mild abnormalities in coagulation test results do not preclude liver biopsy. In fact, results of routine coagulation tests may not correlate with liver bleeding times, as was found in one study of human patients. Liver biopsy should be delayed if there is clinical evidence of bleeding or marked abnormalities in results of coagulation tests. Because animals with complete EBDO may be vitamin K deficient (manifested by prolongation of both OSPT and APTT), treatment with vitamin K1 (0.5 to 1.0 mg/kg [maximum of 10 mg] subcutaneously q12h for 3 treatments) is indicated for 1 or 2 days before surgery. Vitamin K supplementation can also improve coagulation times in animals with other liver disease, particularly cats. Repeating the OSPT and APTT within 24 hours after administration of vitamin K1 should demonstrate normal or near-normal values. If not, the dose can be adjusted and the procedure delayed. Although it may not seem rational to give vitamin K1 to animals with severe parenchymal hepatic disease before surgery, it has been of benefit to some animals and, if given properly, can do no harm. These animals may have high serum activity of proteins induced by vitamin K antagonism (PIVKA) that could impart bleeding tendencies. If there has been minimal improvement in coagulation test results after vitamin K1 has been administered, fresh frozen plasma is administered before biopsy. If bleeding is excessive during or after biopsy and cannot be controlled locally with direct pressure or application of clot-promoting substances, fresh whole blood or plasma is given (see Chapter 83 for transfusion guidelines).
Techniques
Information gathered before biopsy must support the fact that the likelihood of acquiring a diagnostic sample without complications is high. A specimen procured from any area of the liver is considered representative of the disease. Because only a small stab incision large enough to accommodate the biopsy needle is needed (a No. 11 blade is the perfect choice for this purpose), healing in hypoalbuminemic animals is not compromised. If the operator is confident with the biopsy procedure, there is little time involved and only heavy sedation is required. If the results are nondiagnostic, a larger specimen is obtained for histopathologic examination, usually by laparoscopy or laparotomy.
Biopsy can be performed blindly if the cat or dog has generalized hepatomegaly and the operator is confident of the path of the needle. The most common needle biopsy instruments are the Tru-Cut (Cardinal Health) and Jamshidi Menghini (Cardinal Health; Kormed Inc.) needles. The Jamshidi Menghini biopsy needles can be operated with one hand, and aspiration is used to sever and contain the specimen within the barrel of a 6- or 12-ml syringe. The Tru-Cut needle requires two hands to operate and relies on the tissue falling into the specimen trough and then being severed by the sharp outer cannula (Fig. 36-15). One-handed operatable semi-automatic (e.g., Tenmo Evolution biopsy needle, Cardinal Health; VET-core biopsy needle, Global Veterinary Products Inc) and automatic (e.g., Pro-Mag Ultra Automatic biopsy instrument, Manan Medical Products; Bard Biopty biopsy instrument and Bard Biopty-Cut biopsy needle, Bard Inc) versions of this instrument are also available. These biopsy needles are intended for single use. Either the automatic biopsy instrument or the semi-automatic biopsy needle device can be used to obtain liver biopsies in dogs, but only the semi-automatic biopsy needle device should be used in cats. A recent study identified a high risk of fatal complications (i.e., unexpected fatal shock reaction) when an automatic biopsy instrument was used to obtain liver biopsies in cats (Proot and Rothuizen, 2006).
FIG 36-15 A, Tru-Cut biopsy needle with the specimen trough exposed (left) and then covered by the sharp outer cannula (right). B, Liver tissue filling the specimen trough (between arrows).
Biopsy can be done of any palpably enlarged lobe as long as care is taken to angle the needle to avoid puncturing the gallbladder. Most often, the animal is placed in right lateral recumbency for this purpose and biopsy of the left lateral lobe is done. Elevating the head and thorax slightly may assist in “presenting” the liver to the operator. Two or three complete core specimens are obtained; if indicated, one core specimen is placed in a sterile container for culture and sensitivity testing. Gently rolling a specimen on a slide for cytologic assessment is a good way to attempt to identify the disease process quickly and inexpensively. Each of the remaining core specimens is placed on a piece of stiff paper (e.g., filter paper) in correct orientation (Fig. 36-16) before immersion in fixative for histologic examination and/or special testing.
FIG 36-16 Needle biopsy specimen affixed to a stiff piece of paper to preserve orientation of the sample during formalin fixation for histopathologic examination.
After biopsy, a small bandage is applied to keep the site clean during recovery, and the animal is placed in a position to allow body weight to compress the region of the biopsy sites in the liver (e.g., left lateral recumbency). Consideration should be given to postoperative analgesia; puncture of the liver capsule can be painful. The animal should be monitored carefully for any evidence of hemorrhage for several hours after the procedure. As long as the biopsy procedure proceeded smoothly and without unpleasant surprises (animal awake and struggling), only basic monitoring of mucous membrane color and the skin puncture site is needed. Naturally, if excessive hemorrhage or damage to other organs occurs with this blind technique, detection and treatment may be delayed.
Visualized percutaneous needle biopsy, with the aid of either US (Fig. 36-17) or modified laparoscopic equipment (Fig. 36-18), allows selection of the site(s) and direct or indirect inspection after the biopsy. When the procedure is properly performed, serious complications are few. In an animal in which diffuse or multifocal hepatobiliary disease is suspected, multiple biopsy specimens are obtained safely. General anesthesia is usually required for use of a modified laparoscope. Aspiration of the gallbladder for cytologic analysis and culture can be accomplished with US guidance or by laparoscopy. Bile leakage may occur, even if a small-gauge needle is used, so attempts are made to completely evacuate the gallbladder, and the needle should be placed in the gallbladder through the liver parenchyma to help prevent leakage. Some surgeons prefer to obtain bile during laparotomy when a purse-string suture can be applied to the aspiration site to prevent seepage. Large-volume abdominal effusion hinders direct inspection of the liver and associated structures and must be removed before laparoscopic biopsy is attempted.
FIG 36-17 Biopsy gun instrument with accompanying biopsy needle used for obtaining liver specimens with ultrasonographic guidance.
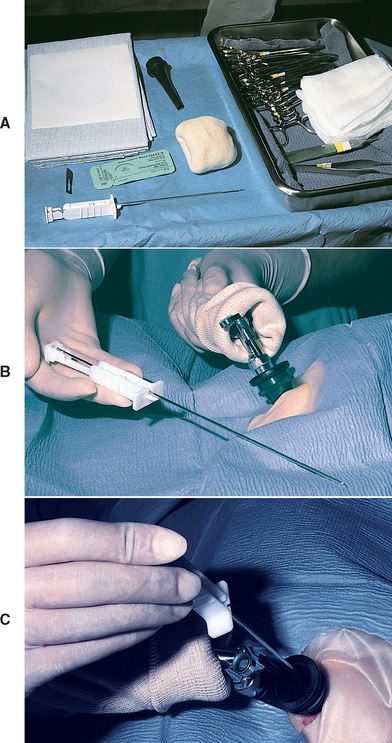
FIG 36-18 Modified laparoscopic approach for liver biopsy. A, Readily available materials needed for the procedure. B, A Tru-Cut biopsy needle is used for obtaining liver specimens. C, The liver is first inspected, and then the needle is passed through a sterile otoscope cone into the liver for tissue sampling. See Bunch et al. (1985) in Suggested Readings for further details on this procedure.
An operative approach (laparoscopy [Fig. 36-19], laparotomy) is preferred for liver biopsy if the liver is small, US equipment is not available, or the operator is not experienced with the aforementioned percutaneous needle methods. Laparotomy is perfectly acceptable for dogs and cats that are good anesthetic risks and allows thorough examination of the liver, biliary tract, and portal vein as well as other abdominal structures, such as lymph nodes. Bile can be acquired easily and safely. The procedure is more prolonged, and healing complications may arise in severely hypoalbuminemic animals, notably those with intractable ascites, but larger samples for histopathologic examination and special staining techniques are obtainable (Fig. 36-20) and hemorrhage can be arrested directly. Use of nonabsorbable suture material and small cranial or flank incisions may lessen incision complications. Obviously, this is the approach of choice for surgically correctable diseases; a liver biopsy specimen is obtained concurrently.
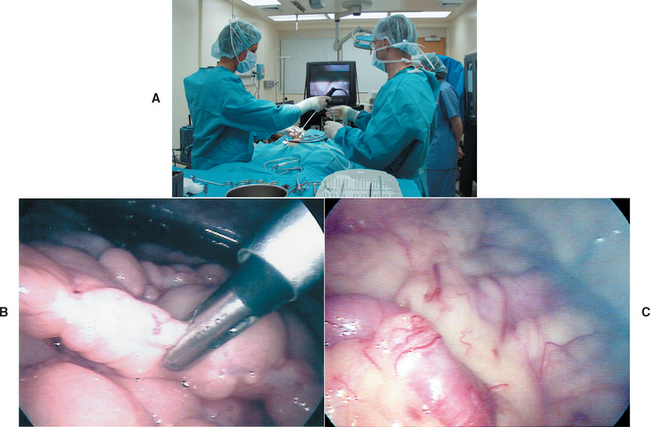
FIG 36-19 A, Laparoscopic liver biopsy. B, Tip of the biopsy instrument that is passed through one of the preplaced cannulas. C, Intraabdominal view of a dog with chronic hepatic disease and portal hypertension. Note the prominent, tortuous omental veins.

FIG 36-20 Comparison of liver specimens obtained by different methods and mounted on glass slides. These samples are adequate for histopathologic examination: percutaneous needle sample (left); samples obtained intraoperatively by use of an 8-mm skin biopsy punch (middle) or removal of a wedge specimen (right).
As for the percutaneous biopsy techniques, liver and/or bile specimens for microbiologic culture are aseptically processed first. Impression smears for cytologic analysis are then made by gently touching the specimen to a slide before placing it in fixative. Excess blood is removed by blotting the sample on gauze before slides are made. Abnormal populations of cells (e.g., mast cells, lymphoblasts) are readily detectable using rapid stain systems such as Diff Quik (Harleco, Gibbstown, N.J.). For routine processing and histopathologic examination, liver tissue specimens are submerged in buffered 10% formalin at a ratio of at least 10 parts formalin to 1 part tissue. Samples for copper histochemical staining or tissue quantification are harvested and fixed or preserved according to the specifications of the pathology laboratory selected to do the assays. Other special stains for infectious agents or fibrous tissue, amyloid, glycogen, and other metabolic products are available, and their use is discussed with the attending pathologist before the tissue specimen is obtained. A portion of the specimen can be frozen for molecular studies (e.g., PCR for organisms or tumor clonality).
Balkman CE, et al. Evaluation of urine sulfated and nonsulfated bile acids as a diagnostic test for liver disease in dogs. J Am Vet Med Assoc. 2003;222:1368.
Bexfield NJ, et al. Diagnosis of canine liver disease. In Practice. 2006;28:444.
Bigge LA, et al. Correlation between coagulation profile findings and bleeding complications after ultrasound-guided biopsies: 434 cases (1993–1996). J Am Anim Hosp Assoc. 2001;37:228.
Bunch SE, et al. A modified laparoscopic approach for liver biopsy in dogs. J Am Vet Med Assoc. 1985;187:1032.
Clifford CA, et al. Magnetic resonance imaging of focal splenic and hepatic lesions in the dog. J Vet Intern Med. 2004;18:330.
Cole T, et al. Diagnostic comparison of needle biopsy and wedge biopsy specimens of the liver in dogs and cats. J Am Vet Med Assoc. 2002;220:1483.
Gallagher AE, et al. Hyperphosphatasemia in Scottish Terriers: 7 cases. J Vet Intern Med. 2006;20:418.
Gaskill CL, et al. Serum alkaline phosphatase isoenzyme profiles in phenobarbital-treated epileptic dogs. Vet Clin Pathol. 2004;33:215.
Gerritzen-Bruning MJ, et al. Diagnostic value of fasting plasma ammonia and bile acid concentrations in the identification of portosystemic shunting in dogs. J Vet Intern Med. 2006;20:13.
Hall EJ, et al. Laboratory evaluation of hepatic disease. In Villiers E, Blackwood L, editors: BSAVA manual of canine and feline clinical pathology, ed 2, Gloucestershire, United Kingdom: British Small Animal Veterinary Association, 2005.
Head LL, Daniel GB. Correlation between hepatobiliary scintigraphy and surgery or postmortem examination findings in dogs and cats with extrahepatic biliary obstruction, partial obstruction, and patency of the biliary system: 18 cases (1995-2004). J Am Vet Med Assoc. 2005;227:1618.
Jensen AL, et al. Preliminary experience with the diagnostic value of the canine corticosteroid-induced alkaline phosphatase isoenzyme in hypercorticism and diabetes mellitus. Zentralbl Veterinarmed. 1992;39:342.
Koblik PD, et al. Transcolonic sodium pertechnetate Tc 99m scintigraphy for diagnosis of macrovascular portosystemic shunts in dogs, cats, and pot-bellied pigs: 176 cases (1988–1992). J Am Vet Med Assoc. 1995;207:729.
Lawler DF, et al. Benign familial hyperphosphatasemia in Siberian Huskies. Am J Vet Res. 1996;57:612.
Lee KC, et al. Association of portovenographic findings with outcome in dogs receiving surgical treatment for single congenital portosystemic shunts: 45 cases (2000–2004). J Am Vet Med Assoc. 2006;229:1122.
Liptak JM. Hepatobiliary tumors. In Withrow SJ, Vail DM, editors: Withrow and MacEwen’s small animal clinical oncology, ed 4, St Louis: Saunders Elsevier, 2007.
Müller PB, et al. Effects of long-term phenobarbital treatment on the liver in dogs. J Vet Intern Med. 2000;14:165.
O’Brien RT. Improved detection of metastatic hepatic hemangiosarcome nodules with contrast ultrasound in three dogs. Vet Radiol Ultrasound. 2007;48:146.
Proot SJ, Rothuizen J. High complication rate of an automatic Tru-Cut biopsy gun device for liver biopsy in cats. J Vet Intern Med. 2006;20:1327.
Sevelius, et al. Serum protein electrophoresis as a prognostic marker of chronic liver disease in dogs. Vet Rec. 1995;137:663.
Toulza O, et al. Evaluation of plasma protein C activity for detection of hepatobiliary disease and portosystemic shunting in dogs. J Am Vet Med Assoc. 2006;229:1761.
Trainor D, et al. Urine sulfated and nonsulfated bile acids as a diagnostic test for liver disease in cats. J Vet Intern Med. 2003;17:145.
Walker MC, et al. Postprandial venous ammonia concentrations in the diagnosis of hepatobiliary disease in dogs. J Vet Intern Med. 2001;15:463.
Wang KY, Panciera DL, Al-Rukibat RK, Radi ZA. Accuracy of ultrasound-guided fine-needle aspiration of the liver and cytologic findings in dogs and cats: 97 cases (1990-2000). J Am Vet Med Assoc. 2004;224:75.
Zini E, et al. Paraneoplastic hypoglycemia due to an insulin-like growth factor type-II secreting hepatocellular carcinoma in a dog. J Vet Intern Med. 2007;21:193.