CHAPTER 33 Disorders of the Intestinal Tract
ABBREVIATIONS USED IN THE CHAPTER
ARE: Antibiotic-responsive enteropathy (previously known as small intestinal bacterial overgrowth—IBO)
EGE: Eosinophilic gastroenteritis
EHEC: Enterohemorrhagic Escherichia coli
EPI: Exocrine pancreatic insufficiency
FIV: Feline immunodeficiency virus
GDV: Gastric dilation and volvulus
GUE: Gastric ulceration/erosion
HES: Hypereosinophilic syndrome
IBD: Inflammatory bowel disease
IL: Intestinal lymphangiectasia
LPC: Lymphocytic-plasmacytic colitis
LPE: Lymphoplasmacytic enteritis
ACUTE DIARRHEA
ACUTE ENTERITIS
Etiology
Acute enteritis can be caused by infectious agents, poor diet, abrupt dietary changes, inappropriate foods, additives (e.g., chemicals), and/or parasites. Except for parvovirus, parasites, and obvious dietary indiscretions, the cause is rarely diagnosed because most affected animals spontaneously improve, although supportive therapy may be needed.
Clinical Features
Diarrhea of unknown cause occurs commonly, especially in puppies and kittens. Signs consist of diarrhea with or without vomiting, dehydration, fever, anorexia, depression, crying, and/or abdominal pain. Very young animals may become hypothermic, hypoglycemic, and stuporous.
Diagnosis
History and physical and fecal examinations are used to identify possible causes. Fecal flotation (preferably a centrifugal flotation using zinc sulfate flotation solution) and direct fecal examinations are always indicated because parasites may worsen the problem, even when they are not the main cause. The need for other diagnostic procedures depends on the severity of the illness and on whether the risk of contagion exists. Clinically mild enteritis is usually treated symptomatically, with few diagnostic tests being performed. If the animal is febrile, has hemorrhagic stools, is part of an outbreak of enteritis, or is particularly ill, then additional tests (e.g., complete blood count [CBC] to identify neutropenia, fecal enzyme-linked immunosorbent assay (ELISA) for canine parvovirus, serologic analysis for feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV), blood glucose to identify hypoglycemia, and serum electrolytes to detect hypokalemia) are indicated. Abdominal radiographs and/or ultrasonography should be evaluated if abdominal pain, masses, obstruction, or foreign body are suspected.
Treatment
Symptomatic therapy usually suffices. The cause is usually unknown or is a virus for which there is no specific therapy. The goal of symptomatic therapy is reestablishment of fluid, electrolyte, and acid-base homeostasis. Animals with severe dehydration (i.e., ≥8% to 10% as determined by sunken eyes; fast, weak pulse; and marked depression; or a history of significant fluid loss coupled with inadequate fluid intake) should receive intravenous fluids, whereas fluids administered orally or subcutaneously usually suffice for patients that are less severely dehydrated. Potassium supplementation is usually indicated, but bicarbonate is rarely needed. Oral rehydration is sometimes useful in allowing home management of animals, especially when litters of young animals are affected. (See the discussion on fluid, electrolyte, and acid-base therapy in Chapter 30 for details.)
Antidiarrheals are seldom necessary except when excessive fecal losses make maintenance of fluid and electrolyte balance difficult, but they are often requested by clients. Opiates are usually the most effective antidiarrheals. Bismuth subsalicylate (see Table 30-6) is useful in stopping diarrhea in dogs with mild to moderate enteritis. However, absorption of the salicylate may cause nephrotoxicity in some animals (especially when combined with other potentially nephrotoxic drugs), and many dogs dislike the taste. Cats rarely need these medications. (See the discussion on drugs that prolong intestinal transit time in Chapter 30.) If antidiarrheals are needed for more than 2 to 5 days, the animal should be carefully reassessed.
Severe intestinal inflammation often causes vomiting that is difficult to control. Central-acting antiemetics (e.g., dolasteron, ondansetron, maropitant, or prochlorperazine; see Table 30-3) are more likely to be effective than peripheral-acting drugs. The animal should be well hydrated before receiving phenothiazine derivatives, which dilate blood vessels and can produce hypotension.
Although food is typically withheld from animals with severe enteritis to “rest” the intestinal tract, such starvation may be detrimental. Administering even small amounts of food to the intestines helps them recover sooner and prevent breakdown of the mucosal barrier to bacteria. Denying any oral intake is occasionally necessary in animals in which eating causes severe vomiting or explosive diarrhea with substantial fluid loss. However, if feeding does not make the pet’s vomiting and diarrhea much worse, feeding small amounts of food is probably more beneficial than withholding food. Frequent, small feedings of easily digested, nonirritative foods (e.g., cottage cheese, boiled chicken, potato) is the most common approach. If food must be withheld, it should be reoffered as soon as possible. Some animals with severe enteritis may need parenteral nutrition to establish a positive nitrogen balance.
If the animal is febrile or neutropenic or has systemic inflammatory response syndrome (SIRS) (e.g., septic shock), broad-spectrum systemic antibiotics (e.g., β-lactam antibiotic plus an aminoglycoside) are indicated (see the discussion of drugs used in gastrointestinal disorders, pp. 409–410). The clinician should observe for hypoglycemia, especially in young animals. Adding dextrose (2.5% to 5%) to the intravenous fluids or administering an intravenous bolus of 50% dextrose (2 to 5 ml/kg) may be necessary to counter hypoglycemia.
If the cause of the diarrhea is unknown, the clinician should assume it to be infectious and disinfect the premises accordingly. Bleach diluted in water (i.e., 1 : 32) destroys parvovirus and many other infectious agents causing diarrhea. Animals must not be injured by inappropriate contact with such disinfectants. Personnel coming in contact with the animals, cages, and litter should wear protective clothing (e.g., boots, gloves, gowns) that can be discarded or disinfected when leaving the area.
After the enteropathy appears to be clinically resolved, the animal is gradually returned to its normal diet over a 5- to 10-day period. If this change is associated with more diarrhea, then the switch is postponed for another 5 days.
Prognosis
The prognosis depends on the animal’s condition and can be influenced by its age and other gastrointestinal (GI) problems. Very young or emaciated animals and those with SIRS or substantial intestinal parasite burdens have a more guarded prognosis. Intussusception may occur secondary to acute enteritis, thus worsening the prognosis.
ENTEROTOXEMIA
Etiology
The cause is assumed to be bacterial, although causative organisms are almost never isolated.
Clinical Features
An acute onset of severe, often mucoid-bloody diarrhea that may be associated with vomiting is typical. In severe cases mucus casts of the intestines are expelled, making it appear as if the intestinal mucosa is being lost. In contrast to animals with acute enteritis, these patients usually feel quite ill and may exhibit symptoms of shock early in the course of the disease. CBCs typically reveal a neutrophilic leukocytosis, often with a left shift and sometimes with white blood cell (WBC) toxicity.
Diagnosis
Exclusion of other causes by history and physical examination coupled with severe WBC changes (e.g., toxicity, left shift) on the CBC allow for presumptive diagnosis. The pet should be checked for intestinal parasites, which may be contributing to the problem. Fecal cultures are rarely useful diagnostically.
Treatment
These patients typically need aggressive intravenous (IV) fluid therapy plus broad-spectrum antibiotic therapy (e.g., ticarcillin plus clavulinic acid). The serum albumin concentration must be monitored and colloids given if needed. Disseminated intravascular coagulation (DIC) may require plasma and/or heparin therapy.
DIETARY-INDUCED DIARRHEA
Etiology
Dietary causes of diarrhea are common, especially in young animals. Poor-quality ingredients (e.g., rancid fat), bacterial enterotoxins or mycotoxins, allergy or intolerance to ingredients, or inability of the animal to digest normal foods are common causes. The latter mechanism revolves around intestinal brush border enzymes that are produced in response to the presence of substrates (e.g., disaccharidases). If the diet is suddenly changed, some animals (especially puppies and kittens) are unable to digest or absorb certain nutrients until the intestinal brush border adapts to the new diet. Other animals may never be able to produce the necessary enzymes (e.g., lactase) to digest certain nutrients (e.g., lactose).
Clinical Features
Diet-induced diarrhea occurs in both dogs and cats. The diarrhea tends to reflect small intestinal dysfunction (i.e., there is usually no fecal blood or mucus) unless there is colonic involvement. The diarrhea usually starts shortly after the new diet is initiated (e.g., 1 to 3 days) and is mild to moderate in severity. Affected animals infrequently have other signs unless parasites or complicating factors are present.
Diagnosis
History and physical and fecal examinations are used to eliminate other common causes. If diarrhea occurs shortly after a suspected or known dietary change (e.g., after the pet is brought home), a tentative diagnosis of diet-induced disease is reasonable. However, the pet may also be showing the first clinical signs of a recently acquired infection. The animal should always be checked for intestinal parasites because they may contribute to the problem even when they are not the principal cause.
Treatment
A bland diet (e.g., boiled potato plus boiled skinless chicken) fed in multiple, small feedings (see p. 397) usually causes resolution of the diarrhea in 1 to 3 days. Once the diarrhea resolves, the diet can be gradually changed back to the pet’s regular diet.
INFECTIOUS DIARRHEA
CANINE PARVOVIRAL ENTERITIS
Etiology
There are two types of parvoviruses that infect dogs. Canine parvovirus-1 (CPV-1), also known as “minute virus of canines,” is a relatively nonpathogenic virus that sometimes is associated with gastroenteritis, pneumonitis, and/or myocarditis in puppies 1 to 3 weeks old. Canine parvovirus-2 (CPV-2) is responsible for classic parvoviral enteritis. CPV-2 usually causes signs 5 to 12 days after the dog is infected via the fecal-oral route, and it preferentially invades and destroys rapidly dividing cells (i.e., bone marrow progenitors, intestinal crypt epithelium).
Clinical Features
The virus has mutated since it was first recognized, and the most recently recognized mutations, CPV-2b, may be more pathogenic in some dogs. CPV-2b and the even more recently identified CPV-2c can also infect cats. The clinical signs depend on the virulence of the virus, the size of the inoculum, the host’s defenses, the age of the pup, and the presence of other enteric pathogens (e.g, parasites). Doberman Pinschers, Rottweilers, Pit Bulls, Labrador Retrievers, and German Shepherd dogs may be more susceptible than other breeds. Viral destruction of intestinal crypts may produce villus collapse, diarrhea, vomiting, intestinal bleeding, and subsequent bacterial invasion; however, some animals have mild or even subclinical disease. Many dogs are initially presented because of depression, anorexia, and/or vomiting (which can resemble foreign object ingestion) without diarrhea. Diarrhea is often absent for the first 24 to 48 hours of illness and may not be bloody if and when it does occur. Intestinal protein loss may occur secondary to inflammation, causing hypoalbuminemia. Vomiting is usually prominent and may be severe enough to cause esophagitis. Damage to bone marrow progenitors may produce transient or prolonged neutropenia, making the animal susceptible to serious bacterial infection, especially if a damaged intestinal tract allows bacteria access to the body. Fever and/or septic shock (i.e., systemic inflammatory response syndrome) are common in severely ill dogs but are often absent in less severely affected animals. Puppies that are infected in utero or before 8 weeks of age may develop myocarditis.
Diagnosis
Diagnosis is often tentatively made on the basis of history and physical examination findings. Neutropenia is suggestive but is neither sensitive nor specific for canine parvovirus enteritis; salmonellosis or any overwhelming infection can cause similar changes in the CBC. Regardless of whether diarrhea occurs, infected dogs shed large numbers of viral particles in the feces (i.e., >109 particles/g). Therefore ELISA for CPV-2 in the feces is the best diagnostic test. Vaccination with a modified live parvoviral vaccine may cause a weak positive result for 5 to 15 days after vaccination. However, the ELISA results may be negative if the assay is performed early in the clinical course of the disease, and the clinician should not hesitate to repeat this test in dogs that seem likely to have parvoviral enteritis but that initially have negative findings. Shedding decreases rapidly and may be undetectable 10 to 14 days after infection. The real advan-tage to testing is that either a presumptive diagnosis of parvoviral enteritis is confirmed or other diseases that can mimic parvovirus but require different therapy (e.g., salmonellosis, intussusception) must be considered. Electron microscopic evaluation of feces detects the presence of the virus; however, CPV-1 (which is usually nonpathogenic except perhaps in neonates) is morphologically indistinguishable from CPV-2. If the dog dies, there are typical histologic lesions (i.e., crypt necrosis), and fluorescent antibody and in situ hydridization techniques can establish a definitive diagnosis.
Treatment
Treatment of canine parvoviral enteritis is fundamentally the same as for any severe, acute, infectious enteritis (see p. 441). Fluid and electrolyte therapy is crucial and is typically combined with antibiotics (Box 33-1). Most dogs will live if they can be supported long enough. However, very young puppies, dogs in severe septic shock, and certain breeds seem to have more problems and may have a more guarded prognosis. Mistakes include inadequate fluid therapy (common), overzealous fluid administration (especially in dogs with severe hypoproteinemia), failure to administer glucose to hypoglycemic patients, failure to supplement adequate potassium, unrecognized sepsis, and unsuspected concurrent GI disease (e.g., parasites, intussusception).
 BOX 33-1 General Guidelines for Treatment of Canine Parvoviral Enteritis*
BOX 33-1 General Guidelines for Treatment of Canine Parvoviral Enteritis*
Fluids†‡
Administer balanced electrolyte solution with 30-40 mEq potassium chloride/L.
Calculate maintanence requirements (i.e., 66 ml/kg/day with dogs <5 kg needing up to 80 ml/kg/day).
Estimate deficit (better to slightly overestimate rather than underestimate the deficit).
Dogs with very mild cases may receive subcutaneous fluids (intravenous fluids still preferred), but watch for sudden worsening of the disease.
Dogs with moderate to severe cases should receive fluids via intravenous or intramedullary route.
Add 2.5%-5% dextrose to the intravenous fluids if hypoglycemia or systemic inflammatory response syndrome is present or is a risk.
Administer plasma or hetastarch if dog has serum albumin ≤2.0 g/dl.
Plasma: 6-10 ml/kg over 4 hours; repeat until the desired serum albumin concentration is attained
Antibiotics†
Administer to febrile or severely neutropenic dogs.
Prophylactic antibiotics for nonfebrile neutropenic patients (e.g., cefazolin).
Broad-spectrum antibiotics for febrile, neutropenic patients (e.g., ticarcillin/clavulinic acid plus amikacin).
Antiemetics
Serotonin receptor antagonists
Maropitant (minimal clinical experience at the time of this writing)
Metoclopramide (constant rate infusion is more effective than intermittent bolusing)
Anthelmintics
Pyrantel (should be given after feeding)
Ivermectin (this drug is absorbed in the oral mucous membranes; do not give to breeds that are likely to have adverse effects, such as Collies, Old English Sheepdogs, etc.)
Dogs With Secondary Esophagitis
If regurgitation occurs in addition to vomiting, administer:
Special Nutritional Therapy
Try to feed dog small amounts as soon as feeding does not cause major exacerbation in vomiting.
“Microenteral” nutrition (slow drip of enteral diet administered via nasoesophageal tube) if dog refuses to eat and administration does not make vomiting worse
Administer parenteral nutrition if prolonged anorexia occurs
Peripheral parenteral nutrition is more convenient than total parenteral nutrition
Monitor Physical Status
Physical examination (1-3 times per day depending on severity of signs)
Body weight (1-2 times per day to assess changes in hydration status)
Potassium (every 1-2 days depending on severity of vomiting/diarrhea)
Serum protein (every 1-2 days depending on severity of signs)
Glucose (every 4-12 hours in dogs that have systemic inflammatory response syndrome or were initially hypoglycemic)
Packed cell volume (every 1-2 days)
White blood cell count: either actual count or estimated from a slide (every 1-2 days in febrile animals)
Controversial Therapies
Recominant feline IFN-ω: One report suggests that this therapy was useful.
Tamiflu (anecdotally beneficial if used early in the course of the disease)
Flunixin Meglumine: Sometimes used for patients with systemic inflammatory response syndrome, but perforation and bleeding are significant risks.
* The same guidelines generally apply to dogs with other causes of acute enteritis/gastritis.
† Usually the first considerations when an animal is presented.
‡ A history of decreased intake plus increased loss such as vomiting and/or diarrhea confirms dehydration, regardless of whether dog appears to be dehydrated.
If the serum albumin concentration is less than 2.0 g/dl, it is advantageous to administer plasma. Colloids such as hetastarch may be substituted for plasma, but they do not contain antibodies that might be beneficial. Antibiotic therapy is needed if evidence of infection (i.e., fever, septic shock) exists or there is risk of infection (i.e., severe neutropenia). If the animal is neutropenic but afebrile, the admin istration of a first-generation cephalosporin is reasonable. If the animal is in septic shock (i.e., systemic inflammatory response syndrome), then an antibiotic combination with a broad aerobic and anerobic spectrum is recommended (e.g., ticarcillin or ampicillin plus amikacin or enrofloxacin). Aminoglycosides should not be administered until the patient is rehydrated and renal perfusion is re-established. Caution should be used when administering enrofloxacin to young, large-breed dogs lest cartilage damage occur. Severe vomiting complicates therapy and may require administration of dolasetron, ondansetron, or maropitant (see Table 30-3). If esophagitis occurs, H2-receptor antagonists may be useful (see Table 30-4). Human granulocyte colony–stimulating factor (G-CSF) (5 μg/kg q24h) to increase neutrophil numbers and tamiflu (oseltamivir phosphate) (2 mg/kg q12-24h) to combat the virus have been advocated; however, there is no evidence that either substantively benefits the patient. Flunixin meglamine has been suggested for patients in septic shock, but care must be taken lest iatrogenic ulceration/perforation occurs. Recombinant feline IFN-ω (2.5 × 106 units per kg) has been suggested to improve the chance of survival.
If possible, feeding small amounts of liquid diet via a nasoesophageal (NE) tube seems to help the intestines to heal more rapidly. A bland diet may be fed once vomiting has ceased for 18 to 24 hours. Parenteral nutrition can be life saving for patients that are persistently unable to hold down oral food. It can be equally critical for patients unable to accept any enteral nutrition. Partial parenteral nutrition is easier and less expensive than total parenteral nutrition. The dog should be kept away from other susceptible animals for 2 to 4 weeks after discharge, and the owner should be conscientious about the disposal of feces. Vaccination of other dogs in the household should be considered.
When trying to prevent the spread of parvoviral enteritis, the clinician must remember that (1) parvovirus persists for long periods of time (i.e., months) in the environment, making it difficult to prevent exposure; (2) asymptomatic dogs may shed virulent CPV-2; (3) maternal immunity sufficient to inactivate vaccine virus may be present in some puppies; and (4) dilute bleach (1 : 32) is one of the few readily available disinfectants that kills the virus, but it can take 10 minutes to achieve effectiveness.
Vaccination of pups should generally commence at 6 to 8 weeks of age. The antigen density and immunogenicity of the vaccine as well as the amount of antibody transferred from the bitch determine when the pup can be successfully immunized. Inactivated vaccines generally are not as successful as attenuated vaccines, and giving a series of these vaccinations seems best. Attenuated vaccines are generally more successful in producing a long-lasting immunity. When the immune status of the pup is unknown, administering an attenuated vaccine at 6, 9, and 12 weeks of age is usually successful. If vaccination before 5 to 6 weeks of age is deemed necessary, an inactivated vaccine is safer. Regardless of the vaccine used, it appears that there is typically a 2- to 3-week window during which the pup is susceptible to parvovirus infection and yet cannot be successfully immunized. Annual revaccination is generally recommended for parvovirus, although it is possible that vaccination every 3 years may be sufficient after the initial series as a puppy. Adults that were previously not vaccinated usually receive two doses 2 to 4 weeks apart. There is no strong evidence that parvoviral vaccination should be given separately from modified-live canine distemper vaccinations. However, modified-live vaccinations should not be administered to patients younger than 5 weeks of age or those suspected of incubating or being affected with distemper.
If parvoviral enteritis develops in one dog in a multiple-dog household, it is reasonable to administer booster vaccinations to the other dogs, preferably using an inactivated vaccine in case they are incubating the infection at the time of immunization. If the client is bringing a puppy into a house with a dog that has recently had parvoviral enteritis, the puppy should be kept elsewhere until it has received its immunizations.
Prognosis
Dogs treated in a timely fashion with proper therapy typically live, especially if they survive the first 4 days of clinical signs. The possible sequela of intussusception may cause persistent diarrhea in pups recovering from the viral infection. Dogs that have recovered from CPV-2 enteritis develop long-lived immunity that may be lifelong. Whether immunization against CPV-1 will be needed is unknown.
FELINE PARVOVIRAL ENTERITIS
Etiology
Feline parvoviral enteritis (feline distemper, feline panleukopenia) is caused by feline panleukopenia virus (FPV), which is distinct from CVP-2b. However, CPV-2a, CPV-2b, and CPV-2c can infect cats and cause disease.
Clinical Features
Many infected cats never show clinical signs of disease. Signs in affected cats are usually similar to those described for dogs with parvoviral enteritis. Kittens affected in utero may develop cerebellar hypoplasia.
Diagnosis
Diagnosis is similar to that described for canine parvovirus. The ELISA test for fecal CPV is also a good test for feline parvovirus. However, it is important to note that the test may be positive for only 1 to 2 days after infection, and by the time the cat is clinically ill, this test may not be able to detect viral shedding in the feces.
Treatment
Cats with parvoviral infection are treated much in the same way as described for dogs with the disease. A major difference between dogs and cats centers on immunization: Parvoviral vaccine seems to engender a better protective response in cats than in dogs. However, kittens younger than 4 weeks of age should not be vaccinated with modified live virus vaccines lest cerebellar hypoplasia occur. Also, the vaccine cannot be administered orally, but intranasal adminstration is effective.
CANINE CORONAVIRAL ENTERITIS
Etiology
Canine coronaviral enteritis occurs when coronavirus invades and destroys mature cells on the intestinal villi. Because intestinal crypts remain intact, villi regenerate more quickly in dogs with coronaviral enteritis than in dogs with parvoviral enteritis; bone marrow cells are not affected.
Clinical Features
Coronaviral enteritis is typically less severe than classic parvoviral enteritis and rarely causes hemorrhagic diarrhea, septicemia, or death. Dogs of any age may be infected. Signs usually last less than 1 to 11/2 weeks, and small or very young dogs may die as a result of dehydration or electrolyte abnormalities if they are not properly treated. Dual infection with parvovirus may produce a high incidence of morbidity and mortality.
Diagnosis
Because canine coronaviral enteritis is usually much less severe than many other enteritides, it is seldom definitively diagnosed. Most dogs are treated symptomatically for acute enteritis until they improve. Electron microscopic examination of feces obtained early in the course of the disease can be diagnostic. However, the virus is fragile and easily disrupted by inappropriate handling of the feces. A history of contagion and elimination of other causes are reasons to suspect canine coronaviral enteritis.
Treatment
Fluid therapy, motility modifiers (see Chapter 30), and time should resolve most cases of coronaviral enteritis. Symptomatic therapy (see p. 441) is usually successful except, perhaps, for very young animals. A vaccination is available but of uncertain value except, perhaps, in animals at high risk of infection (e.g., those in infected kennels or dog shows).
FELINE CORONAVIRAL ENTERITIS
Infections in adults are often asymptomatic, whereas kittens may have mild, transient diarrhea and fever. Deaths are rare, and the prognosis for recovery is excellent. This disease is important because (1) affected animals seroconvert and may become positive on feline infectious peritonitis serologic analysis and (2) mutation by the feline coronavirus may be the cause of feline infectious peritonitis.
FELINE LEUKEMIA VIRUS–ASSOCIATED PANLEUKOPENIA (MYELOBLASTOPENIA)
Etiology
FeLV-associated panleukopenia (myeloblastopenia) may actually be caused by co-infection with FeLV and FPV. The intestinal lesion histologically resembles that produced by feline parvovirus. The bone marrow and lymph nodes are not consistently affected as they are in cats with parvoviral enteritis.
Clinical Features
Chronic weight loss, vomiting, and diarrhea are common. The diarrhea often has characteristics of large bowel disease. Anemia is common.
Diagnosis
Finding FeLV infection in a cat with chronic diarrhea is suggestive. Cats are typically neutropenic. Histologic lesions of FPV in a cat with FeLV should be definitive.
FELINE IMMUNODEFICIENCY VIRUS–ASSOCIATED DIARRHEA
Etiology
FIV may be associated with severe, purulent colitis. The pathogenesis is unclear and may involve multiple mechanisms.
Clinical Features
Severe large bowel disease is common and can occasionally result in colonic rupture. These animals generally appear ill, whereas most cats with chronic large bowel disease caused by inflammatory bowel disease (IBD) or dietary intolerance seemingly feel fine.
Diagnosis
Detection of antibodies to FIV plus severe, purulent colitis allows presumptive diagnosis.
SALMON POISONING/ELOKOMIN FLUKE FEVER
Etiology
Salmon poisoning is caused by Neorickettsia helminthoeca. Dogs are infected when they eat fish (primarily salmon) infected with a fluke (Nanophyetus salmincola) that carries the rickettsia. The rickettsia spreads to the intestines and most lymph nodes, causing inflammation. This disease is principally found in the Pacific northwestern United States because the snail intermediate host (Oxytrema silicula) for N. salmincola lives there. The Elokomin fluke fever agent may be a strain of N. helminthoeca.
Clinical Features
Dogs, not cats, are affected. The severity of signs varies and typically consists of initial fever that eventually falls and becomes subnormal. Fever is followed by anorexia and weight loss, which may also involve vomiting and/or diarrhea. The diarrhea is typically small bowel but may become bloody.
Diagnosis
Presumptive diagnosis is usually based on the animal’s habitat plus a history of recent consumption of raw fish or exposure to streams or lakes. Finding Nanophyetus spp. ova (operculated trematode ova) in the stool is very suggestive, and finding rickettsia in fine-needle aspirates of enlarged lymph nodes is confirmatory.
Treatment
Treatment consists of symptomatic control of dehydration, vomiting, and diarrhea and elimination of the rickettsia and fluke. Tetracycline, oxytetracycline, doxycycline, or chloramphenicol (see Chapter 93) eliminates the rickettsia. The fluke is killed with praziquantel (see Table 30-7).
BACTERIAL DISEASES: COMMON THEMES
The following bacterial diseases all have certain aspects in common. First, all of these bacteria may be found in feces from clinically normal dogs and cats. Simply growing the bacteria or finding toxin produced by the bacteria in the patient’s feces are insufficient by themselves to definitively diagnose intestinal disease as being caused by this particular organism. Diagnosis can be made only by finding clinical disease consistent with a particular organism, evidence of the organism or its toxin, eliminating other causes of the clinical signs, and seeing the expected response to appropriate therapy. If the clinician undertakes culture, it is crucial to call the laboratory ahead of time, tell staff members what is being sought through culture, and follow their instructions regarding submission of the sample.
The problems with making a diagnosis using the previously mentioned criteria are obvious, and caution is warranted before making definitive statements regarding cause and effect. In many cases, the best chance of making a definitive diagnosis involves following the guidelines described and using molecular techniques on isolates to demonstrate toxin production.
CAMPYLOBACTERIOSIS
Etiology
There are several species of Campylobacter. Campylobacter jejuni is the species routinely associated with GI disease, although Campylobacter upsaliensis has been implicated. These organisms prefer high temperatures (i.e., 39° to 41° C); hence poultry is probably a reservoir. These organisms are found in the intestinal tract of healthy dogs and cats.
Clinical Features
Symptomatic campylobacteriosis is principally diagnosed in animals younger than 6 months old living in crowded conditions (e.g., kennels, humane shelters) or as a nosocomial infection. Mucoid diarrhea (with or without blood), anorexia, and/or fever are the primary signs. Campylobacteriosis tends to be self-limiting in dogs, cats, and people; however, it occasionally causes chronic diarrhea.
Diagnosis
Occasionally, classic Campylobacter forms may be found during cytologic examination of a fecal smear (i.e., “commas,” “seagull wings”). This cytology is thought to be specific but of uncertain sensitivity. Polymerase chain reaction (PCR) analysis of feces is available.
Treatment
If campylobacteriosis is suspected, erythromycin (11 to 15 mg/kg administered orally q8h) or neomycin (20 mg/kg administered orally q12h) is usually effective. β-lactam antibiotics (i.e., penicillins, first-generation cephalosporins) are often ineffective. The length of treatment necessary for cure has not been firmly established. The animal should be treated for at least 1 to 3 days beyond resolution of clinical signs; however, antibiotic therapy may not eradicate the bacteria, and reinfection is likely in kennel conditions. Chronic infections may require prolonged therapy (e.g., weeks).
This bacterium is potentially transmissible to people, and there are cases in which there is convincing evidence of transmission from pets to people. Infected dogs and cats should be isolated, and individuals working with the animal or its environment or wastes should wear protective clothing and wash with disinfectants.
SALMONELLOSIS
Etiology
There are numerous Salmonella serotypes that may cause disease; Salmonella typhimurium is one of the serovars that is more commonly associated with disease. The bacteria may originate from animals shedding the organism (e.g., infected dogs and cats) or from contaminated foods (especially poultry and eggs).
Clinical Features
Salmonella spp. may produce acute or chronic diarrhea, septicemia, and/or sudden death, especially in very young or geriatric animals. Salmonellosis in young animals can produce a syndrome that closely mimics parvoviral enteritis (including severe neutropenia), which is one reason that ELISA testing for parvovirus is useful. The fact that salmonellosis occasionally develops during or after canine parvoviral enteritis makes the situation more confusing.
Diagnosis
Culture of Salmonella spp. from normally sterile areas (e.g, blood) confirms that it is causing disease. Identification by PCR can be a sensitive method of diagnosis.
Treatment
Treatment depends on the clinical signs. Animals with diarrhea as the sole sign may need only supportive fluid therapy (including plasma in hypoalbuminemic patients). Nonsteroidal drugs (to lessen intestinal secretion) and lactulose have been used in such patients. Antibiotics are of dubious value and might promote a carrier state. Septicemic (i.e., febrile) animals should receive supportive therapy and parenteral antibiotics as determined by susceptibility testing, but quinolones, potentiated sulfa drugs, amoxicillin, and chloramphenicol are often good initial choices (see the discussion of drugs used in gastrointestinal disorders, pp. 409–410). Aggressive plasma therapy might be beneficial in such patients.
Infected animals are public health risks (especially for infants and older adults) and should be isolated from other animals, at least until they are asymptomatic. Even when signs disappear, reculturing of feces is reasonable to ensure that shedding has stopped. Individuals in contact with the animal, its environment, and its waste should wear protective clothing and wash with disinfectants such as phenolic compounds and bleach (1 : 32 dilution).
CLOSTRIDIAL DISEASES
Etiology
Clostridium perfringens and Clostridium difficile can be found in clinically normal dogs but appear to cause diarrhea in some. For C. perfringens to produce disease, the bacteria must possess the ability to produce toxin, and environmental conditions must be such that toxin is produced.
Clinical Features
C. perfringens apparently may produce an acute, bloody, self-limiting nosocomial diarrhea; an acute, potentially fatal hemorrhagic diarrhea; or a chronic large bowel or small bowel (or both) diarrhea (with or without blood or mucus). This clostridial disease is primarily recognized in dogs. Disease associated with C. difficile is poorly characterized in small animals but may include large bowel diarrhea, especially after antibiotic therapy.
Diagnosis
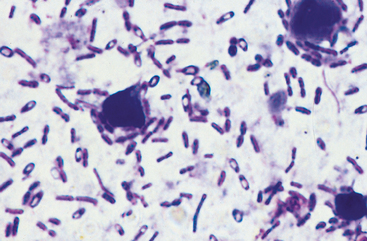
In particular, finding spore-forming bacteria on fecal smears (Fig. 33-1) is not diagnostic. Commercially available toxin assays for C. difficile toxin have not been validated for the dog or cat, and results do not necessarily correlate with the patient’s clinical condition. Determining that the patient has large bowel diarrhea without weight loss or hypoalbuminemia, elimination of other causes, and resolution of signs when treated appropriately (see next paragraph) is typically the basis for presumptive diagnosis.
Treatment
If C. perfringens disease is suspected, the animal may be treated with tylosin or amoxicillin, and response is expected shortly. Some animals are cured after a 1- to 3-week course of therapy. However, antibiotic treatment does not necessarily eliminate the bacteria, and some dogs need indefinite therapy. Tylosin (20 to 80 mg/kg/day, divided, q12h) or amoxicillin (22 mg/kg q12h) seems to be effective and yet has minimal adverse effects in these animals. Some animals can eventually be maintained with once daily or every-other-day antibiotic therapy. Some dogs with chronic diarrhea seemingly caused by C. perfringens respond well to fiber-supplemented diets. Metronidazole is not as consistently effective as tylosin or amoxicillin. The prognosis is good, and there is no obvious public health risk, although there is anecdotal evidence of transmission between people and dogs.
If disease caused by C. difficile is suspected, supportive fluid and electrolyte therapy may be necessary depending on the severity of signs. Metronidazole should be effective in killing this bacterium, but one must be sure to use a sufficiently high dose to achieve adequate metronidazole concentrations in the feces. Vancomycin is often used to treat people with this disease but has not generally been necessary in dogs or cats.
MISCELLANEOUS BACTERIA
Etiology
Yersinia enterocolitica, Aeromonas hydrophila, and Plesiomonas shigelloides may cause acute or chronic enterocolitis in dogs and/or cats as well as in people. However, these bacteria (especially the latter two) are uncommonly diagnosed in the United States. Y. enterocolitica is primarily found in cold environments and in pigs, which may serve as a reservoir. It is also a cause of food poisoning because of its ability to grow in cold temperatures. Enterohemorrhagic Escherichia coli (EHEC) may seemingly be associated with canine and feline diarrhea, although it does not appear to be especially common.
Clinical Features
Small bowel diarrhea may be caused by any of these bacteria. Yersiniosis usually affects the colon and produces chronic large bowel diarrhea. Affected people report substantial abdominal pain.
Diagnosis
Animals with persistent colitis, especially those that are in contact with pigs, may reasonably be cultured for Y. enterocolitica.
Treatment
Therapy is supportive. The affected animal should be isolated from other animals. People in contact with the animal and/or its environment and wastes should wear protective clothing and clean themselves with disinfectants. Although antibiotics intuitively seem indicated, their use has not shortened clinical disease caused by EHEC. Nonetheless, appropriate antibiotics as determined by culture and sensitivity are used (e.g., Y. enterocolitica is often sensitive to tetracyclines). The preferred length of antibiotic therapy has not been established, but treatment should probably be continued for 1 to 3 days beyond clinical remission.
HISTOPLASMOSIS
Etiology
Caused by Histoplasma capsulatum, histoplasmosis is a mycotic infection that may affect the GI, respiratory, and/or reticuloendothelial systems, as well as the bones and eyes. Principally found in animals from the Mississippi and Ohio River valleys, it occurs in other areas as well.
Clinical Features
Alimentary tract involvement is primarily found in dogs; diarrhea (with or without blood or mucus) and weight loss are common signs. The lungs, liver, spleen, lymph nodes, bone marrow, bones, and/or eyes may also be affected. Symptomatic alimentary involvement is much less common in cats, in which respiratory dysfunction (e.g., dyspnea, cough), fever, and/or weight loss are more common.
In GI histoplasmosis, the colon is usually the most severely affected segment. Diffuse, severe, granulomatous, ulcerative mucosal disease can produce bloody stool, intestinal protein loss, intermittent fever, and/or weight loss. Small intestinal involvement occasionally occurs. The disease may smolder for long periods of time, causing mild to moderate, nonprogressive signs. Occasionally, histoplasmosis causes focal colonic granulomas or is present in grossly normalappearing colonic mucosa.
Diagnosis
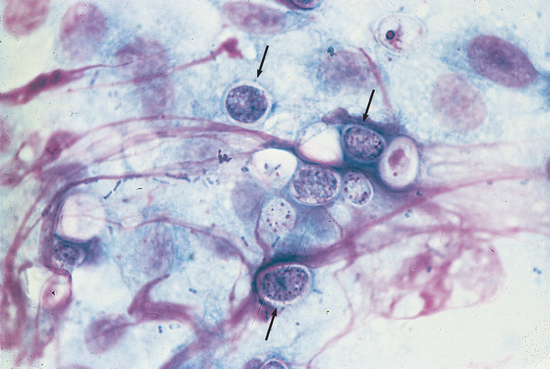
Diagnosis requires finding the yeast (Fig. 33-2), although a recent test for antigen present in urine is being evaluated. Dogs from endemic areas with chronic large bowel diarrhea are especially suspect. Protein-losing enteropathy is common in dogs with severe histoplasmosis, and hypoalbuminemia in dogs with large bowel disease is suggestive of the disease, regardless of the location.
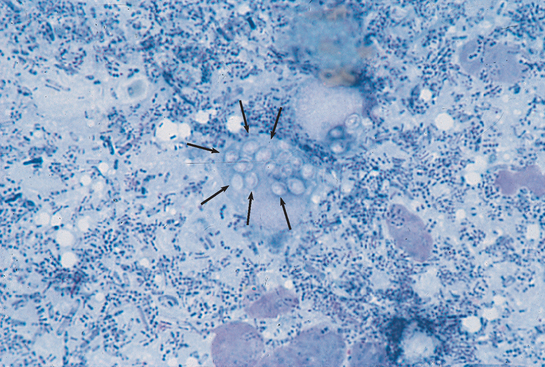
FIG 33-2 Cytologic preparation of a colonic mucosal scraping demonstrating Histoplasma capsulatum. Note the macrophage with numerous yeasts in the cytoplasm (arrows). (Wright-Giemsa stain; magnification ×400.)
(From Allen D, editor: Small animal medicine, Philadelphia, 1991, JB Lippincott.)
Rectal examination sometimes reveals thickened rectal folds, which can easily be scraped with a dull curette or syringe cap to obtain material for cytologic preparations. Evaluation of colonic biopsy specimens is usually diagnostic, but special stains may be necessary. Mesenteric lymph node samples or repeated colonic biopsy is rarely required. Fundic examination occasionally reveals active chorioretinitis. Abdominal radiographs might reveal hepatosplenomegaly, and thoracic radiographs sometimes demonstrate pulmonary involvement (e.g., miliary interstitial involvement and/or hilar lymphadenopathy). Cytologic evaluation of hepatic or splenic aspirates may be diagnostic. The CBC rarely reveals yeasts in circulating WBCs. Thrombocytopenia may occur. Cytologic examination of bone marrow or of buffy coat smears may reveal the organism. Serologic tests and fecal culture for the yeast are unreliable.
Treatment
It is crucial to look for histoplasmosis before beginning empiric corticosteroid therapy for suspected canine colonic IBD. Corticosteroid therapy lessens host defenses and may allow a previously treatable case to rapidly progress and kill the animal. Itraconazole by itself or preceded by amphotericin B is often effective (see Chapter 98). Treatment should be continued long enough (i.e., at least 4 to 6 months) to lessen chances for relapse.
PROTOTHECOSIS
Etiology
Prototheca zopfii is an alga that invades tissue. It appears to be acquired from the environment, and some type of deficiency in the host’s immune system might be needed for the organism to produce disease.
Clinical Features
Affecting dogs and occasionally cats, protothecosis principally involves the skin, colon, and eyes but may disseminate throughout the body. Collies may be overrepresented. Colonic involvement causes bloody stools and other signs of colitis, much like histoplasmosis. Protothecosis is much less common than histoplasmosis, and the GI form primarily affects dogs.
ALIMENTARY TRACT PARASITES
WHIPWORMS
Etiology
Trichuris vulpis is principally found in the eastern United States. Animals acquire the infection by ingesting ova; the adults burrow into the colonic and cecal mucosa and may cause inflammation, bleeding, and intestinal protein loss.
Clinical Features
Dogs and rarely cats acquire whipworms, which produce a wide spectrum of mild to severe colonic disease that can include hematochezia and protein-losing enteropathy. Severe trichuriasis may cause severe hyponatremia and hyperkalemia, mimicking hypoadrenocorticism. Marked hyponatremia might be responsible for CNS signs (e.g., seizures). Whipworms generally do not affect cats as severely as dogs.
Diagnosis
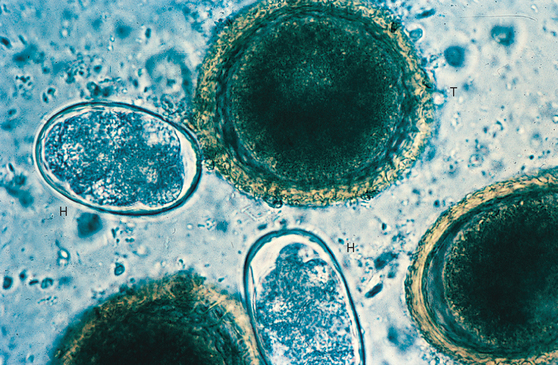
T. vulpis should always be sought in dogs with bloody stools or other colonic disease. Diagnosis is made through finding ova (Fig. 33-4) in the feces or seeing the adults at endoscopic evaluation. However, these ova are relatively dense and float only in properly prepared flotation solutions. Furthermore, ova are shed intermittently and sometimes can be found only if multiple fecal examinations are performed.
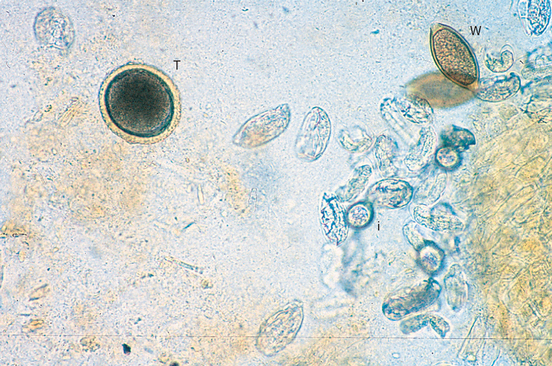
FIG 33-4 Photomicrograph of a fecal flotation analysis from a dog, demonstrating characteristic ova from whipworms (W), Toxocara canis (T), and Isospora spp. (I). The remaining ova are those of an unusual tapeworm, Spirometra sp. (Magnification ×250.)
(Courtesy Dr. Tom Craig, Texas A & M University.)
Treatment
Because of the potential difficulty in diagnosing T. vulpis, it is reasonable to empirically treat dogs with chronic large bowel disease with fenbendazole or other appropriate drugs (see Table 30-7) before proceeding to endoscopy. If a dog is treated for whipworms, it should be treated again in 3 months to kill worms that were not in the intestinal lumen at the time of the first treatment. The ova persist in the environment for long periods.
ROUNDWORMS
Etiology
Roundworms are common in dogs (Toxocara canis and Toxascaris leonina) and cats (Toxocara cati and Toxascaris leonina). Dogs and cats can obtain roundworms from ingesting the ova (either directly or via paratenic hosts). T. canis is often obtained transplacentally from the mother; T. cati may use transmammary passage, and T. leonina can use intermediate hosts. Tissue migration of immature forms can cause hepatic fibrosis and significant pulmonary lesions. Adult roundworms live in the small intestinal lumen and migrate against the flow of ingesta. They can cause inflammatory infiltrates (e.g., eosinophils) in the wall of the intestine.
Clinical Features
Roundworms may cause or contribute to diarrhea, stunted growth, a poor haircoat, and poor weight gain, especially in young animals. Runts with “potbellies” suggest severe roundworm infection. Sometimes, roundworms gain access to the stomach, in which case they may be vomited. If parasites are numerous, they may obstruct the intestines or bile duct.
Diagnosis
Diagnosis is easy because ova are produced in large numbers and are readily found by fecal flotation (Fig. 33-5; see also Fig. 33-4). Occasionally, neonates develop clinical signs of roundworm infestation but ova cannot be found in the feces. Transplacental migration results in large worm burdens, causing signs in these animals before the parasites mature and produce ova.
Treatment
Various anthelmintics are effective (see Table 30-7), but pyrantel is especially safe for young dogs and cats, particularly those with diarrhea. Affected animals should be retreated at 2- to 3-week intervals to kill roundworms that were initially in tissues but migrated into the intestinal lumen since the last treatment.
High-dose fenbendazole therapy (i.e., 50 mg/kg/day from day 40 of gestation until 2 weeks postpartum) has been sug gested to reduce the somatic roundworm burden in bitches and lessen transplacental transmission to puppies. Newborn puppies can be treated with fenbendazole (100 mg/kg for 3 days), which kills more than 90% of prenatal larvae. This treatment can be repeated 2 to 3 weeks later. Preweaning puppies should be treated at 2, 4, 6, and 8 weeks of age to lessen contamination of the environment because T. canis and T. cati pose a human health risk (i.e., visceral and ocular larval migrans). Preweaning kittens should be treated at 6, 8, and 10 weeks of age.
HOOKWORMS
Etiology
Ancylostoma spp. and Uncinaria spp. are more common in dogs than in cats. Infestation is usually via ingestion of the ova or through transcolostral transmission; freshly hatched larvae may also penetrate the skin. The adults live in the small intestinal lumen, where they attach to the mucosa. Plugs of intestinal mucosa and/or blood is ingested, depending on the worm species. In severe infestations hookworms may be found in the colon.
Clinical Features
Dogs are more severely affected than cats. Young animals may have life-threatening blood loss or iron-deficiency anemia, melena, frank fecal blood, diarrhea, and/or failure to thrive. Older dogs rarely have disease solely caused by hookworms unless they harbor a massive infestation, but these worms may still contribute to disease caused by other intestinal problems.
Diagnosis
Finding ova in the feces is diagnostic (see Fig. 33-5) and easy because hookworms are prolific egg producers. However, 5- to 10-day-old puppies may be exsanguinated by transcolostrally obtained hookworms before ova appear in the feces. Such prepatent infections rarely occur in older animals that have received a sudden, massive exposure. Diagnosis is suggested by signalment and clinical signs in these animals. Iron deficiency anemia in a puppy or kitten free of fleas is highly suggestive of hookworm infestation.
Treatment
Various anthelmintics are effective (see Table 30-7). Treatment should be repeated in approximately 3 weeks to kill parasites entering the intestinal lumen from the tissues. In anemic puppies and kittens, blood transfusions may be life saving.
Application of moxidectin to pregnant bitches on day 55 of pregnancy reduces transcolostral transmission to puppies. Hookworms are a potential human health hazard (i.e., cutaneous larval migrans). Use of heartworm preventives containing pyrantel or milbemycin helps to minimize hookworm infestations.
TAPEWORMS
Etiology
Several tapeworms infect dogs and cats, the most common being Dipylidium caninum. Tapeworms usually have an indirect life cycle; the dog or cat is infected when it eats an infected intermediate host. Fleas and lice are intermediate hosts for D. caninum, whereas wild animals (e.g., rabbits) are intermediate hosts for some Taenia spp.
Clinical Features
Aesthetically offensive, tapeworms are rarely pathogenic in small animals, although Mesocestoides spp. can reproduce in the host and cause disease (e.g., abdominal effusion). The most common sign in infested dogs and cats is anal irritation associated with shed segments “crawling” on the area. Typically, the owner sees motile tapeworm segments on the feces and requests treatment. Occasionally, a segment enters an anal sac and causes inflammation. Very rarely, large numbers of tapeworms cause intestinal obstruction.
Diagnosis
Taenia spp. and especially D. caninum eggs are typically confined in segments not detected by routine fecal flotations. Echinococcus spp. and some Taenia spp. ova may be found in the feces. Tapeworms are usually diagnosed when the owner reports tapeworm segments (e.g., “rice grains”) on feces or the perineal area.
Treatment
Praziquantel and episprantel are effective against all species of tapeworms (see Table 30-7). Prevention of tapeworms involves controlling the intermediate hosts (i.e., fleas and lice for D. caninum). Echinococcus spp. are a human health hazard.
STRONGYLOIDIASIS
Etiology
Strongyloides stercoralis principally affects puppies, especially those in crowded conditions. These parasites produce motile larvae that penetrate unbroken skin or mucosa; thus the animal may be infested from its own feces even before the larvae are evacuated from the colon. In this manner, animals can quickly acquire large parasitic burdens. Most animals are infested after being exposed to fresh feces containing the motile larvae. Humane shelters and pet stores are likely sources for infestation.
Clinical Features
Infested animals usually have mucoid or hemorrhagic diarrhea and are systemically ill (e.g., lethargy). Respiratory signs (i.e., verminous pneumonia) occur if parasites penetrate the lungs.
Diagnosis
S. stercoralis is diagnosed by finding the larvae in fresh feces, either by direct fecal examination or by Baermann sedimentation. Strongyloides larvae must be differentiated from Oslerus spp. larvae. The feces must be fresh because old feces may contain hatched hookworm larvae, which resemble those of Strongyloides spp.
Treatment
Fenbendazole (when used for 5 days instead of 3; see Table 30-7), thiabendazole, and ivermectin are effective anthelmintics. This disease is a human health hazard because larvae penetrate unbroken skin. Immunosuppressed people are at risk for severe disease after being infected.
COCCIDIOSIS
Etiology
Isospora spp. are principally found in young cats and dogs. The pet is usually infested by ingesting infective oocysts from the environment. The coccidia invade and destroy villous epithelial cells.
Clinical Features
Coccidia may be clinically insignificant (especially in an asymptomatic, older animal), or they may be responsible for mild to severe diarrhea, sometimes with blood. Rarely, a kitten or puppy may lose enough blood to require a blood transfusion.
Diagnosis
Coccidiosis is diagnosed by finding oocysts on fecal flotation examination (see Fig. 33-4); however, repeated fecal examinations may be needed, and small numbers of oocysts do not ensure that the infestation is insignificant. These oocysts should not be confused with giardial cysts. If a necropsy is performed, multiple areas of the intestine should be sampled because the infection may be localized to one area. Occasionally, Eimeria oocysts will be seen in the feces of dogs that eat deer or rabbit excrement.
Treatment
If coccidia are believed to be causing a problem, sulfadimethoxine or trimethoprim-sulfa should be administered for 10 to 20 days (see Table 30-7). The sulfa drug does not eradicate the coccidia but inhibits it so that body defense mechanisms can reestablish control. Amprolium (25 mg/kg administered orally q24h for 3 to 5 days) can be used in puppies but is not approved for use in dogs; it is potentially toxic in cats. Toltrazuril (15 mg/kg q24h for 3 days) has been found to decrease oocyst shedding, at least temporarily.
CRYPTOSPORIDIA
Etiology
Cryptosporidium parvum may infect animals that ingest the sporulated oocysts. These oocysts originate from infested animals but may be carried in water. Thin-walled oocysts are produced, which can rupture in the intestine and produce autoinfection. The organism infests the brush border of small intestinal epithelial cells and causes diarrhea.
Clinical Features
Diarrhea is the most common clinical sign in dogs and cats, although many infested cats are asymptomatic. Dogs with diarrhea are usually under 6 months of age, but a similar age predilection has not been recognized for cats.
Diagnosis
Diagnosis requires finding the oocysts or a positive ELISA. C. parvum is the smallest of the coccidians and is easy to miss on fecal examination. Examination should be performed at ×1000 magnification. Use of acid-fast stains on fecal smears and fluorescent antibody techniques improves sensitivity. It is best to submit the feces to a laboratory experienced in diagnosing cryptosporidiosis. The laboratory must be warned that the feces may contain C. parvum, which is potentially infective for people. The ELISA is more sensitive than fecal examination.
Treatment/Prognosis
There are no known reliable treatments. Immunocompetent people and cattle often spontaneously eliminate the infestation, but whether small animals do so is unknown. Most young dogs with diarrhea associated with cryptosporidiosis die or are euthanized. Many cats have asymptomatic infestations, and those with diarrhea have an unknown prognosis.
GIARDIASIS
Etiology
Giardiasis is caused by a protozoan, Giardia sp. Animals are infected when they ingest cysts shed from infected animals, often via water. Organisms are principally found in the small intestine, where they interfere with digestion through uncertain mechanisms. In people Giardia organisms may occasionally ascend into the bile duct and cause hepatic problems.
Clinical Features
Signs vary from mild to severe diarrhea, which may be persistent, intermittent, or self-limiting. Typically the diarrhea is “cow patty”–like, without blood or mucus; however, there is substantial variation. Some animals experience weight loss; others do not. Diarrhea caused by Giardia can mimic large bowel diarrhea in some patients. In cats there may be an association between shedding giardial oocysts and shedding either cryptosporidial or coccidian oocysts.
Diagnosis
Giardiasis is diagnosed by finding motile trophozoites (Fig. 33-6) in fresh feces or duodenal washes, by finding cysts with fecal flotation techniques, or by finding giardial proteins in feces using an ELISA. Zinc sulfate solutions seem to be the best medium for demonstrating cysts (especially when centrifugal flotation is performed) because other solutions may distort them. At least three fecal examinations should be performed over the course of 7 to 10 days before discounting giardiasis. Some fecal ELISA techniques (e.g., SNAP Giardia Test, Idexx Laboratories) appear to have excellent sensitivity and are easier than centrifugal fecal flotation examinations. Washes of the duodenal lumen (performed endoscopically or surgically by instilling and then retrieving 5 to 10 ml of physiologic saline solution from the duodenal lumen) or cytologic evaluation of the duodenal mucosa occasionally reveal Giardia organisms when other techniques do not.
Treatment
Because of the occasional difficulty in finding Giardia organisms (especially in animals that have had various symptomatic antidiarrheal medications), response to treatment is often the retrospective basis of diagnosis (see Table 30-7). This approach has limitations. Quinacrine is effective but no longer available. Metronidazole has few adverse effects and seems reasonably effective (approximately 85% cured after 7 days of therapy). However, clinical response to metronidazole therapy may occur in animals without giardiasis. Furazolidone (5 days of therapy) is probably as effective as metronidazole and is available as a suspension, making it easier to treat infected kittens. Albendazole (3 days of therapy in dogs, 5 days of therapy in cats) and fenbendazole (5 days of therapy in dogs or cats) are also effective, and recent data suggest that ronidazole may also be effective (see the section on tritrichomoniasis). However, none of these drugs is 100% effective, meaning that failure to respond to drug therapy does not rule out giardiasis.
There are several reasons why it can be difficult to eliminate Giardia spp. First, Giardia organisms seemingly may become resistant to some drugs. Second, immunodeficiency or concurrent host disease may make it difficult to eliminate the organism. Third, reinfection is easy because giardial cysts are rather resistant to environmental influences and relatively few are needed to reinfect a dog or person. Bathing the patient and cleansing the environment can be very important to successful treatment in many patients. Quaternary ammonium compounds and pine tars are effective disinfectants for the premises. Fourth, sometimes other protozoal agents (e.g, Tritrichomonas) are mistaken for Giardia. Vaccination is not generally successful as a treatment modality for patients that do not respond to the aforementioned drugs.
TRICHOMONIASIS
Etiology
Trichomoniasis in cats appears to be caused by Tritrichomonas foetus/suis. Animals are probably infected by the fecal-oral route.
Clinical Features
Trichomoniasis typically is associated with large bowel diarrhea, which rarely contains blood or mucus. Exotic cat breeds (e.g., Somalis, Ocicats, Bengals) are the breeds primarily affected with clinical signs. Affected cats are typically otherwise normal, although there may be anal irritation and defecation in inappropriate places. The diarrhea typically resolves spontaneously, although it may persist for months.
Diagnosis
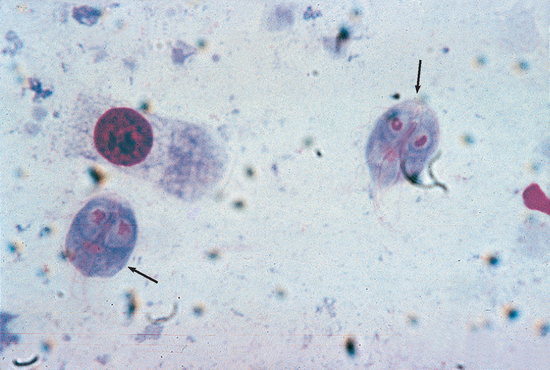
Diagnosis requires identifying the motile trophozoite, but live Tritrichomonas trophozoites can be mistaken for Giardia trophozoites (Fig. 33-7). Timely examination of fresh feces diluted with warm saline solution is the easiest technique, but it is insensitive. Fecal culture using the pouch technique developed for bovine venereal trichomoniasis is more sensitive.
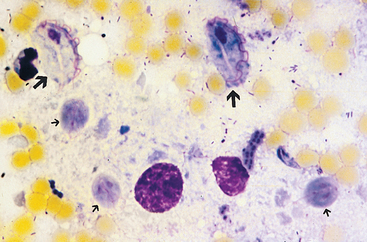
FIG 33-7 Comparison of Giardia trophozoites (small arrows) and Tritrichomonas trophozoites (large arrows) in a smear that has been stained to enhance internal structures. Note that the Tritrichomonas trophozoites are larger and have one large undulating membrane. (Magnification ×1000.)
(Courtesy Dr. Tom Craig, Texas A & M University.)
Treatment/Prognosis
Ronidazole (30 to 50 mg/kg q12h for 14 days) is the only drug currently known to safely eliminate Tritrichomonas, but neurologic signs have been reported with its use. If trichomoniasis is diagnosed, it is still important to look for other causes of diarrhea (e.g., C. perfringens, diet, Cryptosporidium spp.) because treatment for one of these other causes may cause resolution of the diarrhea. Most affected cats will eventually resolve the clinical signs of trichomoniasis, although diarrhea may recur if the patient undergoes stressful events (e.g., elective surgery).
HETEROBILHARZIA
Etiology
Heterobilharzia americana infects dogs and establishes itself in the liver. Ova laid in the veins end up in the intestinal wall, where they elicit a granulomatous inflammation. The organism is primarily found in Gulf coast states and the southern Atlantic coast states.
Clinical Features
Large bowel disease is the primary sign, although the ova can be found in large and small bowel. Diarrhea, hematochezia, and weight loss are typical findings. Protein-losing enteropathy may occur, and the granulomatous reaction is associated with hypercalcemia in some dogs. Hepatic disease may be mild or severe.
MALDIGESTIVE DISEASE
EXOCRINE PANCREATIC INSUFFICIENCY
Etiology
Canine exocrine pancreatic insufficiency (EPI) is caused by pancreatic acinar cell atrophy or destruction associated with pancreatitis.
Clinical Features
EPI is principally found in dogs and rarely in cats. Chronic small intestinal diarrhea, a ravenous appetite, and weight loss are classic findings. Steatorrhea (i.e., slate-gray stools) is sometimes seen, and animals occasionally have weight loss without diarrhea. The diarrhea is classified as a small bowel problem (because of the weight loss and the nature of the diarrhea). Physical examination and routine clinical pathologic findings are not diagnostic. The most sensitive and specific test for canine EPI is measurement of serum trypsin-like immunoreactivity (TLI; i.e., low activity in affected dogs). Finding undetectable levels of canine pancreatic lipase immunoreactivity (cPLI) might be suggestive of EPI but is not as specific as decreased TLI. Treatment involves the administration of pancreatic enzymes with the food and manipulation of dietary fat content. The reader is referred to Chapter 40 for more information on EPI.
MALABSORPTIVE DISEASES
ANTIBIOTIC-RESPONSIVE ENTEROPATHY
Etiology
Antibiotic-responsive enteropathy (ARE) is a syndrome in which the duodenum or jejunum (or both) has high numbers of bacteria (i.e., usually >105 colony forming units/ml) and the host seemingly has an abnormal response to these bacteria. The abnormal host response is important, as seen by the fact that dogs with comparable numbers of bacteria in their small intestine (i.e., ≥108/ml of fasting fluid) do not have clinical disease. The bacteria may be present because of (1) an anatomic defect allowing retention of food (e.g., a partial stricture or an area of hypomotility), (2) other diseases (e.g., intestinal mucosal disease), (3) impaired host defenses (i.e., hypochlorhydria, IgA deficiency), or (4) no identifiable reason. Bacteria causing ARE are usually present in mixed culture, and they probably gain access to the alimentary tract by being swallowed (i.e., originating from the oral cavity or in the food). Any species of bacteria may be present, but Escherichia coli, enterococci, and anaerobes such as Clostridium spp. seem to be especially common. Presumably, enterocytes are damaged by deconjugation of bile acids, fatty acid hydroxylation, generation of alcohols, and potentially other mechanisms.
Clinical Features
ARE can be found in any dog. Clinical signs are principally diarrhea or weight loss (or both), although vomiting may also occur.
Diagnosis
Currently available diagnostic tests for ARE have questionable sensitivity and specificity. Quantitative duodenal fluid cultures are difficult to obtain in most private practices and are difficult to interpret. The major value of small bowel cultures may be in patients in which the diagnosis of ARE is not in doubt but the patient is no longer responding to commonly used antibiotics, and the question is which antibiotic(s) might be effective. Serum cobalamin and folate concentrations have questionable sensitivity and specificity for this disorder. Duodenal mucosal cytology and histopathology are routinely nondiagnostic for ARE. Because of these problems in diagnosing ARE, many clinicians treat and observe for response.
Treatment
Because of the difficulty in diagnosing ARE, therapy is reasonable when this disorder is suspected. Therapy consists of antibiotics and the removal of potential causes (e.g., blind or stagnant loops of intestine). Because mixed bacterial populations are expected, broad-spectrum antibiotics effective against aerobic and anaerobic bacteria are recommended. Tylosin (10 to 40 mg/kg q12h) is often effective. A combination of metronidazole (15 mg/kg q24h) and enrofloxacin (7 mg/kg q24h) also seems effective in many patients. Recent work suggests that simultaneously feeding a high-quality, highly digestible or hypoallergenic diet makes the antibiotic therapy more effective.
Occasionally, a pure culture of a specific bacteria will be found in the duodenum, such that a specific antibiotic is required. However, such cases appear to be rare. When treating dogs with suspected ARE, the clinician should wait 2 to 3 weeks before deciding that the therapy was not effective. Because there may be an underlying cause that cannot be corrected, some animals need long-term to indefinite antibiotic therapy. This may be especially true in dogs that have had repeated episodes of illness since they were a few months old. It seems as though these patients may have some genetic predisposition to ARE, probably because of a defect in host defense mechanisms. The clinician should warn the owner that the goal is typically control, not cure. Patients that have nearly constant diarrhea when not being treated may need antibiotics and dietary therapy indefinitely. Patients who have episodes every 2 to 4 months might best be treated when they relapse as opposed to having them on antibiotics constantly
DIETARY-RESPONSIVE DISEASE
Etiology
Dietary-responsive disease is an all-inclusive term that includes dietary allergy (a hyperimmune response to a dietary antigen) and dietary intolerance (a nonimmune-mediated response to a dietary substance). From a clinical standpoint, there is minimal value in distinguishing between the two unless there are concurrent cutaneous signs of allergic disease.
Clinical Features
Affected patients may have vomiting and/or diarrhea (large and/or small bowel) as well as allergic skin disease.
Diagnosis
Diagnosis consists of showing response to feeding an elimination diet that is appropriate for the patient (see the discussion of dietary management in Chapter 30). There is typically minimal value in distinguishing between allergy and intolerance. Tests for IgE antibodies in the patient’s blood to specific antigens are not as valuable as seeing the response to an elimination diet. The diet must be carefully chosen; it must consist of nonallergenic substances or foods to which the patient has not previously been exposed. Most animals respond to an appropriate diet within 3 weeks, although some take longer.
SMALL INTESTINAL INFLAMMATORY BOWEL DISEASE
Clinical Features
IBD involves idiopathic intestinal inflammation. IBD can affect any portion of the canine or feline intestine. Although the cause of IBD is unknown, it is speculated to involve an exaggerated or inappropriate response by the immune system to bacterial and/or dietary antigens as at least part of the mechanism. The clinical and histologic features of IBD can closely resemble those of alimentary lymphoma (see p. 467). Lymphocytic-plasmacytic enteritis (LPE) is the most commonly diagnosed form of canine and feline IBD. Chronic small intestinal diarrhea is common, but some patients have weight loss with normal stools. If the duodenum is severely affected, vomiting may be the major sign, and diarrhea can be either mild or absent. Protein-losing enteropathy can occur with the more severe forms.
Eosinophilic gastroenterocolitis (EGE) is usually an allergic reaction to dietary substances (e.g., beef, milk) and as such is not IBD. However, the clinical signs do not always respond to dietary change and may represent true IBD in some dogs. It is less common than LPE. Some cats have eosinophilic enteritis as part of a hypereosinophilic syndrome (HES). The cause of feline HES is unknown, but immune-mediated and neoplastic mechanisms may be responsible. Less severely affected cats without HES seem to have a condition similar to canine EGE.
Diagnosis
Because IBD is idiopathic intestinal inflammation, it is a diagnosis of exclusion; it is not just a histologic diagnosis. No physical examination, historic, clinical pathology, imaging, or histologic findings are diagnostic of IBD. Diagnosis requires elimination of known causes of diarrhea plus histology showing mucosal inflammatory infiltrates, architectural changes (e.g., villus atrophy, crypt changes), and/or epithelial changes. Mucosal cytologic evaluation is unreliable for diagnosing lymphocytic inflammation because lymphocytes and plasma cells are normally present in intestinal mucosa. Histologic diagnosis of mucosal inflammation is unfortunately subjective, and biopsy samples are frequently overinterpreted. “Mild” LPE often refers to essentially normal tissue. Even descriptions of “moderate” or “severe” LPE may be dubious because of substantial inconsistency among pathologists. It can be extremely difficult to distinguish a well-differentiated lymphocytic lymphoma from severe LPE, even with full-thickness samples. Some animals with intense dietary reactions have biopsy findings that resemble lymphoma. If the biopsy specimens are of marginal quality (either from the standpoint of size or artifacts present), it is easy to mistakenly diagnose LPE instead of lymphoma if the latter is causing a secondary tissue reaction. Recent data document that biopsy of more than one site (e.g., duodenum and ileum, as opposed to just duodenum) is sometimes critical in finding inflammatory (and neoplastic) changes. Diagnosis of feline LPE is similar to that of canine LPE, but it is important to note that cats with IBD may have mild to moderate mesenteric lymphadenopathy, and such lymphadenopathy is not diagnostic of intestinal lymphoma.
Diagnosis of EGE is similar to diagnosis of LPE. Dogs with EGE may have eosinophilia and/or concurrent eosinophilic respiratory or cutaneous dietary allergies with pruritus. German Shepherd dogs seem to be overrepresented. Diagnosis of feline EGE centers on finding intestinal eosinophilic infiltrates; however, splenic, hepatic, lymph node, and bone marrow infiltrates and peripheral eosinophilia are common.
Treatment
Canine LPE treatment begins with elimination diets and antibiotics in case what appears to be IBD is actually dietary intolerance or ARE. Other therapy depends on the severity of the LPE. Somewhat more severe disease warrants metronidazole with or without high-dose corticosteroid therapy (e.g., prednisolone, 2.2 mg/kg/day or budesonide in steroid-intolerant patients). More severe disease, especially if associated with hypoalbuminemia, usually requires immunosuppressives (e.g., azathioprine or cyclosporine). Cyclosporine seems to be reasonably effective and works faster than azathioprine administered every other day; however, it is also more expensive. Elemental diets, although expensive, can be invaluable in severely emaciated or severely hypoproteinemic patients with severe inflammation as a way to feed the patient and the intestinal mucosa without causing more mucosal irritation. Failure of a dog to respond to “appropriate” therapy can be the result of inadequate therapy, owner noncompliance, or misdiagnosis (i.e., diagnosing LPE when the problem is lymphoma).
Feline LPE treatment is somewhat similar to that for canine LPE. Highly digestible elimination diets may be cura tive if what was thought to be IBD is actually food intolerance, and therapeutic diets should always be used if the cat will eat them. High doses of corticosteroids are typically administered early in cats because of their beneficial effects and the cat’s relative resistance to iatrogenic hyperadrenocorticism. Prednisolone is preferred to prednisone in the cat, and methylprednisolone is typically more effective than prednisolone. Budesonide is primarily indicated in cats that cannot tolerate the systemic effects of steroids (e.g., those with diabetes mellitus). Low-dose metronidazole (10 to 15 mg/kg administered orally q12h), either alone or in combination with corticosteroids and diet, may also be effective. Azathioprine is not used in cats; instead, chlorambucil is used for cats with biopsy-proven, severe LPE that does not respond to other therapy (see Chapter 79) or for cats with well-differentiated lymphoma. Enteral or parenteral nutritional supplementation may be useful in emaciated cats (see Chapter 30). Parenteral administration of cobalamin to cats with severely decreased serum concentrations may aid or be necessary for remission of diarrhea. If the cat responds to this therapy, the elimination diet should be continued while the medications are gradually tapered one at a time.
Canine EGE treatment should focus on a strict hypoallergenic diet (e.g., fish and potato, turkey and potato). Partially hydrolyzed diets may also be helpful, but they are not a panacea for all GI dietary allergies/intolerances. It is important to determine what the dog was fed previously when selecting the dietary therapy. If signs do not resolve with dietary therapy, the addition of corticosteroid therapy is usually curative. Animals usually respond better to elimination diets than to corticosteroids. Sometimes, an animal initially responds to dietary management but relapses while still eating this diet because it becomes allergic to one of the ingredients. This situation necessitates administration of another elimination diet. In some animals that are very prone to developing such intolerances, switching back and forth from one elimination diet to another at 2-week intervals helps to prevent this relapse from happening. (See Chapter 30 for more information on these therapies.)
Feline EGE associated with hypereosinophilic syndrome usually requires high-dose corticosteroid therapy (i.e., prednisolone, 4.4 to 6.6 mg/kg/day); response is often poor. Cats with eosinophilic enteritis not caused by HES often respond favorably to elimination diets plus corticosteroid therapy.
If the dog or cat responds clinically, then the therapy should be continued without change for another 2 to 4 weeks to ensure that the clinical improvement is the result of the therapy and not an unrelated transient improvement. Once the clinician is convinced that the prescribed therapy is responsible for the improvement seen, the animal should be slowly weaned from the drugs, starting with those that have the greatest potential for adverse effects. If antiinflammatory or immunosuppressive therapy was initially required, the clinician should attempt to maintain the pet on every-other-day corticosteroid and azathioprine therapy. If that regimen is successful, then the lowest effective dose of each should be slowly determined. Only one change should be made at a time, and the dose should not be decreased more frequently than once every 2 to 3 weeks, if possible. If a homemade diet was used initially, the clinician should seek to transition the patient to a complete, balanced commercial elimination diet. Dietary and antibiotic therapy are usually the last to be altered. There is no obvious benefit to rebiopsying patients that are clinically improving.
Prognosis
The prognosis for dogs and cats with LPE is often good, if therapy is begun before the patient is emaciated. Severe hypoalbuminemia and a very poor body condition are thought to be suggestive that the patient may have more difficulty responding. A markedly low serum cobalamin concentration in the dog might be a poor prognostic sign, but that is uncertain. Many animals will need to be on a special diet for the rest of their lives. Many with moderate to severe disease will need prolonged medical therapy, which should be tapered cautiously. Iatrogenic Cushing’s syndrome should be avoided. Severely affected animals may initially benefit from enteral or parenteral nutritional therapy. Although the relationship is unclear, LPE has been suggested to be a potentially prelymphomatous lesion (see p. 460 for immunoproliferative enteropathy in Basenjis); however, this is uncertain. If a dog or cat with a prior diagnosis of LPE is later diagnosed as having lymphoma, it may be just as likely that either the initial diagnosis of IBD was wrong (i.e., the patient had lymphoma) or that the lymphoma developed independently of the IBD.
LARGE INTESTINAL INFLAMMATORY BOWEL DISEASE
Clinical Features
In the author’s practice, Clostridium colitis, parasites, dietary intolerance, and fiber-responsive diarrhea are responsible for most cases referred and previously diagnosed as having “intractable” large bowel “IBD.” Canine lymphocyticplasmacytic colitis (LPC) typically causes large bowel diarrhea (i.e., soft stools with or without blood or mucus; no appreciable weight loss). In general, affected dogs are fundamentally healthy except for soft stools. In cats hematochezia is the most common clinical sign, and diarrhea is the second most common sign. Feline LPC may occur by itself or concurrently with LPE, whereas canine large bowel IBD seems to be infrequently associated with small bowel IBD.
Diagnosis
Diagnosis (i.e., excluding other causes and finding mucosal histologic changes) is similar to that for small bowel IBD. In particular, Tritrichomonas can cause substantial mononuclear infiltrates into feline colonic mucosa.
Treatment
Steroids, metronidazole, sulfasalazine (Azulfidine), mesalamine, or olsalazine may be used in dogs with moderate to severe LPC. Corticosteroids and/or metronidazole may be effective by themselves and/or allow lower doses of sulfasalazine to be successful. Hypoallergenic and fiber-enriched diets are often very helpful. It is critical to eliminate colonic fungal infections before begining immunosuppressive therapy.
High-fiber and hypoallergenic diets are also often beneficial in cats; in fact, most “intractable” feline LPC cases seen in the author’s practice are ultimately determined to be related to diet. Most cats with LPC respond well to prednisolone and/or metronidazole, and sulfasalazine is rarely needed.
GRANULOMATOUS ENTERITIS/GASTRITIS
Canine granulomatous enteritis/gastritis is uncommon, and it can be diagnosed only histopathologically. The clinician should search diligently for an etiology (e.g., fungal). Clinical signs are similar to those of other forms of IBD. Although compared to Crohn’s disease in people, the two are dissimilar. If the disease is localized, surgical resection should be considered if the clinician is sure that there is not a systemic cause (e.g., fungal). If it is diffuse, corticosteroids, metronidazole, antibiotics, azathioprine, and dietary therapy should be considered. Too few cases have been described and treated to allow generalizations. The prognosis is poor.
Feline granulomatous enteritis is a rare type of IBD that causes weight loss, protein-losing enteropathy, and perhaps diarrhea; it also requires histopathologic confirmation. Affected cats seem to respond to high-dose corticosteroid therapy, but attempts to reduce the dose of glucocorticoids may cause recurrence of clinical signs. The prognosis is guarded.
IMMUNOPROLIFERATIVE ENTEROPATHY IN BASENJIS
Etiology
Immunoproliferative enteropathy in Basenjis is an intense lymphocytic-plasmacytic small intestinal infiltrate often associated with villous clubbing, mild lacteal dilation, gastric rugal hypertrophy, lymphocytic gastritis, and/or gastric mucosal atrophy. It probably has a genetic basis or predisposition, and intestinal bacteria may play an important role.
Clinical Features
The disease tends to be a severe form of LPE that waxes and wanes, particularly as the animal is stressed (e.g., traveling, disease). Weight loss, small intestinal diarrhea, vomiting, and/or anorexia are commonly seen. Most affected Basenjis start showing clinical signs by 3 to 4 years of age.
Diagnosis
Marked hypoalbuminemia and hyperglobulinemia are common, especially in advanced cases. The early stages of the disease resemble many other intestinal disorders. In advanced cases the clinical signs are so suggestive that a presumptive diagnosis is often made without biopsy. However, because other diseases (e.g., lymphoma, histoplasmosis) may mimic immunoproliferative enteropathy, alimentary tract biopsy is needed before aggressive immunosuppressive therapy is begun.
Treatment
Therapy may include highly digestible, elimination, or elemental diets; antibiotics for ARE (see p. 457); high-dose corticosteroids; metronidazole; and azathioprine. Response to therapy is variable, and affected dogs that respond are at risk for relapse, especially if stressed.
Although a genetic basis is suspected, not enough is known to be able to confidently recommend a breeding program. Performing biopsy of the intestines of asymptomatic dogs to identify animals in which the disease will develop is dubious because clinically normal Basenjis may have lesions similar to those of dogs with diarrhea and weight loss, although the changes tend to be milder.
ENTEROPATHY IN CHINESE SHAR-PEIS
Etiology
Chinese Shar-Peis have a poorly characterized enteropathy that may be unique to them or may be a severe form of IBD. Chinese Shar-Peis have immune system abnormalities that may predispose them to exaggerated inflammatory reactions.
Clinical Features
Diarrhea and/or weight loss (i.e., small intestinal dysfunction) are the main clinical signs.
Diagnosis
Small intestinal biopsy is necessary for diagnosis. Eosinophilic and lymphocytic-plasmacytic intestinal infiltrates are typically found. Serum cobalamin concentrations are often quite low.
PROTEIN-LOSING ENTEROPATHY
CAUSES OF PROTEIN-LOSING ENTEROPATHY
Any intestinal disease that produces sufficient inflammation, infiltration, congestion, or bleeding can produce a protein- losing enteropathy (PLE; or gastropathy if it affects the stomach; see Box 28-10). IBD and alimentary tract lymphoma have been suggested as particularly common causes in adult dogs, whereas hookworms and chronic intussusception are common causes in very young dogs. When IBD is responsible, it is usually a severe form of LPE, although EGE or granulomatous disease may be responsible. Immunoproliferative enteritis of Basenjis, GI ulceration/erosion, and bleeding tumors may also produce PLE. Lymphangiectasia appears to be more common (in dogs) than was once thought; the problem is that it can be difficult to diagnose. Cats infrequently have PLE, but when it occurs, it is usually caused by LPE or lymphoma. Therapy should be directed at managing the underlying cause.
INTESTINAL LYMPHANGIECTASIA
Etiology
Intestinal lymphangiectasia (IL) is a disorder of the intestinal lymphatic system of dogs. Lymphatic obstruction causes dilation and rupture of intestinal lacteals with subsequent leakage of lymphatic contents (i.e., protein, lymphocytes, and chylomicrons) into the intestinal submucosa, lamina propria, and lumen. Although these proteins may be digested and resorbed, excessive loss exceeds the intestine’s ability to resorb them, thus resulting in hypoalbuminemia. Leakage of lymphatic fat into the intestinal wall may cause granuloma formation, which exacerbates lymphatic obstruction. Not reported in cats, the condition has many potential causes in dogs (e.g., lymphatic obstruction, pericarditis, infiltrative mesenteric lymph node disease, infiltrative intestinal mucosal disease, congenital malformations). Most cases of symptomatic IL are idiopathic.
Clinical Features
Yorkshire Terriers, Soft Coated Wheaten Terriers, and Lundehunds appear to be at higher risk than other breeds. Soft Coated Wheaten Terriers also have an unusually high incidence of protein-losing nephropathy. The first sign of disease caused by IL may be transudative ascites. Diarrhea is inconsistent and may occur early or late in the course of the disease, if at all. Intestinal lipogranulomas (i.e., white nodules in the intestinal serosa or mesentery) are sometimes found at surgery. They are probably secondary to IL (i.e., fat leaking out of dilated lymphatic vessels), but they might worsen existing IL by further obstructing lymphatics.
Diagnosis
Clinical pathologic evaluation is not diagnostic, but hypoalbuminemia and hypocholesterolemia are expected. Although panhypoproteinemia is classically attributed to PLE, animals that were initially hyperglobulinemic may lose most of their serum proteins and still have normal serum globulin concentrations. Lymphopenia is common but inconsistent. Diagnosis requires intestinal histopathology. Feeding the animal fat the night before the biopsy seems to make lesions more obvious, and classic mucosal lesions may be seen endoscopically (Fig. 33-8). Endoscopic biopsies are often diagnostic if done appropriately, but surgical biopsies are sometimes required. If full-thickness surgical biopsies are performed, serosal patch grafting and nonabsorbable suture material may decrease the risk of dehiscence. IL may be localized to one area of the intestines (e.g., ileum).
Treatment
The underlying cause of IL is rarely determined, necessitating reliance on symptomatic therapy. An ultra–low-fat diet essentially devoid of long-chain fatty acids helps to prevent further intestinal lacteal engorgement and subsequent protein loss. Prednisolone (1.1 to 2.2 mg/kg/day) or azathioprine (2.2 mg/kg q48h) or cyclosporine (3-5 mg/kg q24h to q12h) sometimes lessens inflammation around the lipogranulomas and improves lymphatic flow.
Monitoring serum albumin concentration may be the best way of assessing response to therapy. If the animal improves with dietary therapy, it should probably be fed that diet indefinitely. Azathioprine or cyclosporine therapy might help solidify response to dietary therapy and maintain remission.
PROTEIN-LOSING ENTEROPATHY IN SOFT-COATED WHEATEN TERRIERS
Etiology
Soft Coated Wheaten Terriers (SCWTs) have a predisposition to PLE and protein-losing nephropathy. The cause is uncertain, although food hypersensitivity has been reported to be present in some affected dogs.
Clinical Features
Individual dogs may have PLE or protein-losing nephropathy (or both). Typical clinical signs may include vomiting, diarrhea, weight loss, and ascites. Affected dogs are often middle aged when diagnosed.
FUNCTIONAL INTESTINAL DISEASE
IRRITABLE BOWEL SYNDROME
Etiology
Irritable bowel syndrome (IBS) in people is characterized by diarrhea, constipation, and/or cramping (usually of the large intestines) in which an organic lesion cannot be identified. It is an idiopathic large bowel disease in which all known causes of diarrhea have been eliminated and a “functional” disorder is presumed. IBS in dogs is different and primarily involves an idiopathic, chronic large bowel diarrhea in which parasitic, dietary, bacterial, and inflammatory causes have been eliminated. There are probably various causes of this syndrome in dogs.
Clinical Features
Chronic large bowel diarrhea is the principal sign. Fecal mucus is common, blood in the feces is infrequent, and weight loss is very rare. Some dogs with IBS are small breeds that are heavily imprinted on a single family member. Clinical signs may develop following separation of the dog from the favored person. Other dogs with IBS are nervous and high-strung (e.g., police or guard dogs, especially German Shepherd Dogs). Some dogs have no apparent initiating cause.
Diagnosis
Diagnosis consists of eliminating known causes by physical examination, clinical pathologic data, fecal analysis, colonoscopy/biopsy, and appropriately performed therapeutic trials.
Treatment
Treatment with fiber-supplemented diets (i.e., ≥7% to 9% fiber on a dry matter basis) is often helpful (see p. 398). Many animals must receive fiber chronically to prevent relapse. Anticholinergics occasionally are useful (e.g., propantheline, 0.25 mg/kg; or dicyclomine, 0.15 mg/kg up to q8h, as needed).
INTESTINAL OBSTRUCTION
SIMPLE INTESTINAL OBSTRUCTION
Etiology
Simple intestinal obstruction (i.e., the intestinal lumen is obstructed but without peritoneal leakage, severe venous occlusion, or bowel devitalization) is usually caused by foreign objects. Infiltrative disease and intussusception may also be responsible.
Clinical Features
Simple intestinal obstructions usually cause vomiting with or without anorexia, depression, or diarrhea. Abdominal pain is uncommon. The more orad the obstruction is, the more frequent and severe the vomiting tends to be. If the intestine becomes devitalized and septic peritonitis results, the obstruction becomes complicated and the animal may be presented in a moribund state or in septic shock (systemic inflammatory response syndrome, or SIRS).
Diagnosis
Abdominal palpation, plain abdominal radiographs, or ultrasonographic imaging can be diagnostic if they reveal a foreign object, mass, or obvious obstructive ileus (see Fig. 29-5, A). Masses or dilated intestinal loops may be found with either technique. Abdominal ultrasonography tends to be the most sensitive technique (unless the intestines are filled with gas) and can reveal dilated or thickened intestinal loops that are not obvious on radiographs (e.g., poor serosal contrast caused by abdominal fluid or lack of abdominal fat) or palpation. If it is difficult to distinguish obstruction from physiologic ileus, abdominal contrast radiographs may be considered. Many intestinal foreign bodies cause hypochloremic, hypokalemic metabolic alkalosis, a metabolic change that is supposedly suggestive of gastric outflow obstruction.
Finding a foreign object is usually sufficient to establish a diagnosis. If an abdominal mass or an obvious obstructive ileus is found, a presumptive diagnosis of obstruction is made, and ultrasonography or exploratory surgery should be planned. Aspirate cytologic evaluation of masses may be used to diagnose some diseases (e.g., lymphoma) before surgery.
Treatment
Once intestinal obstruction is diagnosed, the clinician should perform routine preanesthetic laboratory tests (serum electrolyte and acid-base abnormalities are common in vomiting animals), stabilize the animal, and promptly proceed to surgery. Vomiting of gastric origin classically produces a hypokalemic, hypochloremic metabolic alkalosis and paradoxical aciduria, whereas vomiting caused by intestinal obstruction may produce metabolic acidosis and varying degrees of hypokalemia. However, these changes cannot be predicted even when the cause of the vomiting is known, making serum electrolyte and acid-base determinations important in therapy planning.
INCARCERATED INTESTINAL OBSTRUCTION
Etiology
Incarcerated intestinal obstruction involves a loop of intestine trapped or “strangulated” as it passes through a hernia (e.g., abdominal wall, mesenteric) or similar rent. The entrapped intestinal loop quickly dilates, accumulating fluid in which bacteria flourish and release endotoxins. SIRS occurs rapidly. This is a true surgical emergency, and animals deteriorate quickly if the entrapped loop is not removed.
Clinical Features
Dogs and cats with incarcerated intestinal obstruction typically have acute vomiting, abdominal pain, and progressive depression. Palpation of the entrapped loop often causes severe pain and occasionally vomiting. On physical examination, “muddy” mucous membranes and tachycardia may be noted, suggesting endotoxic shock.
Diagnosis
A presumptive diagnosis is made by finding a distended, painful intestinal loop, especially if the loop is contained within a hernia. Radiographically, a markedly dilated segment of intestine is detected (Fig. 33-9) that is sometimes obviously outside the peritoneal cavity. Otherwise, an obviously strangulated loop of intestine will be found at exploratory surgery.
MESENTERIC TORSION/VOLVULUS
Etiology
In mesenteric torsion/volvulus, the intestines twist about the root of the mesentery, causing severe vascular compromise. Much of the intestine is typically devitalized by the time surgery is performed.
Clinical Features
This uncommon cause of intestinal obstruction principally occurs in large dogs (especially German Shepherd Dogs). Mesenteric torsion is denoted by an acute onset of severe nausea, retching, vomiting, abdominal pain, and depression. Bloody diarrhea may or may not occur. Abdominal distention is not as evident as it is in animals with gastric dilation/volvulus (GDV).
Diagnosis
Abdominal radiographs are often diagnostic and typically show widespread, uniform ileus (see Fig. 29-6).
LINEAR FOREIGN OBJECTS
Etiology
Numerous objects can assume a linear configuration in the alimentary tract (e.g., string, thread, nylon stockings, cloth). The foreign object lodges or fixes at one point (e.g., the base of the tongue, pylorus), and the rest trails off into the intestines. The small intestine seeks to propel the object aborally via peristaltic waves and in this manner gathers around it and becomes pleated. As the intestines continue trying to propel it aborally, the linear object cuts or “saws” into the intestines, often perforating them at multiple sites on the antimesenteric border. Fatal peritonitis can result.
Clinical Features
Linear foreign objects appear to be more frequent in cats than in dogs. Vomiting food, bile, and/or phlegm is common, but some animals show only anorexia or depression. A few (especially dogs with chronic linear foreign bodies) can be relatively asymptomatic for days to weeks while the foreign body continues to embed itself in the intestines.
Diagnosis
The history may be suggestive of a linear foreign body (e.g., the cat was playing with cloth or string). Bunched, painful intestines are occasionally detected by abdominal palpation. The object is sometimes seen lodged at the base of the tongue; however, failure to find a foreign object at the base of the tongue does not eliminate linear foreign body as a diagnosis. Even when such objects lodge under the tongue, they can be very difficult to find despite a careful, thorough oral examination; some become embedded in the frenulum. If necessary, chemical restraint (e.g., ketamine, 2 mg/kg administered intravenously) should be used to allow adequate oral examination.
Foreign objects lodged at the pylorus and trailing off into the duodenum must be diagnosed by abdominal palpation, imaging, or endoscopy. The objects themselves are infrequently seen radiographically and only rarely produce dilated intestinal loops suggesting anatomic ileus; the proximity to the stomach and the pleating of the intestines around the object usually prevents the intestines from dilating. Plain radiographs may reveal small gas bubbles in the intestines, especially in the region of the duodenum, and obvious intestinal pleating may occasionally be seen (Fig. 33-10). If contrast radiographs are performed, they typically reveal a pleated or bunched intestinal pattern, which is diagnostic of linear foreign body. Finally, these objects are sometimes seen endoscopically lodged at the pylorus.
FIG 33-10 A, Plain abdominal radiograph of a cat with a linear foreign body lodged at the pylorus. Note the small gas bubbles in the mass of intestines (arrows). B, Plain abdominal radiograph of a cat with a linear foreign body. Note the obviously pleated small bowel (arrows). C, Contrast radiograph of a cat with a linear foreign body. Note the pleated, bunched pattern of intestines (arrows).
(A from Allen D, editor: Small animal medicine, Philadelphia, 1991, JB Lippincott.)
Treatment
Abdominal surgery is often needed to remove linear foreign objects. However, if the animal is otherwise healthy, if the linear foreign object has been present for only 1 or 2 days, and if it is fixed under the tongue, the object may be cut loose to see if it will now pass through the intestines without further problem. Surgery is indicated if the animal does not feel better 12 to 24 hours after the object is cut free from its point of fixation.
If there is doubt as to the length of time that the object has been present, or if it is fixed at the pylorus, surgery is usually a safer therapeutic approach. Endoscopic removal occasionally succeeds, but the clinician must be careful because it is easy to rupture devitalized intestine and cause peritonitis. If the clinician can pass the tip of the endoscope to near the aborad end of the object and pull it out by grabbing the aborad end, surgery is sometimes unnecessary.
Prognosis
The prognosis is usually good if severe septic peritonitis is absent and massive intestinal resection is unnecessary. If a linear foreign object has been present a long time, it may embed itself in the intestinal mucosa, making intestinal resection necessary. When massive intestinal resection is necessary, short bowel syndrome can result; this condition has a guarded to poor prognosis.
INTUSSUSCEPTION
Etiology
Intussusception is a telescoping of one intestinal segment (the intussusceptum) into an adjacent segment (the intussuscipiens). It may occur anywhere in the alimentary tract, but ileocolic intussusceptions (i.e., the ileum entering the colon) seem more common. Ileocolic intussusceptions seem to be associated with active enteritis (especially in young animals), which ostensibly disrupts normal motility and promotes the smaller ileum to intussuscept into the larger diameter colon. However, ileocolic intussusception may occur in animals with acute renal failure, leptospirosis, prior intestinal surgery, and other problems.
Clinical Features
Acute ileocolic intussusception causes obstruction of the intestinal lumen and congestion of the intussusceptum’s mucosa. Scant bloody diarrhea, vomiting, abdominal pain, and a palpable abdominal mass are common. Chronic ileocolic intussusceptions typically produce less vomiting, abdominal pain, and hematochezia. These animals often have intractable diarrhea and hypoalbuminemia because of protein loss from the congested mucosa. PLE in a young dog without hookworms or a puppy that seems to be having an unexpectedly long recovery from parvoviral enteritis should prompt suspicion of chronic intussusception. Acute jejunojejunal intussusceptions usually do not cause hematochezia. Mucosal congestion can be more severe than that in ileocolic intussusception; intestinal devitalization eventually occurs, and bacteria and their toxins gain access to the peritoneal cavity.
Diagnosis
Palpation of an elongated, obviously thickened intestinal loop establishes a presumptive diagnosis; however, some infiltrative diseases produce similar findings. Ileocolic intussusceptions that are short and do not extend far into the descending colon may be especially difficult to palpate because they are high up and under the rib cage. Occasional intussusceptions “slide” in and out of the colon and can be missed during abdominal palpation. If the intussusception protrudes as far as the rectum, it may resemble a rectal prolapse. Therefore if tissue is protruding from the rectum, the clinician should perform a careful rectal palpation to ascertain that a fornix exists (i.e., it is a rectal prolapse) as opposed to an intussusception (in which a fornix cannot be found).
Plain abdominal radiographs infrequently allow the diagnosis of ileocolic intussusceptions because they usually cause minimal intestinal gas accumulation. A properly performed barium contrast enema may reveal a characteristic colonic filling defect caused by the intussuscepted ileum (Fig. 33-11). Abdominal ultrasonography is quick and reasonably sensitive and specific for detecting intussusceptions (see Fig. 29-8, B). Colonoscopy can be definitive if the intussuscepted intestine is seen extending into the colon (Fig. 33-12). Jejunojejunal intussusceptions may be easier to palpate because of their location. Furthermore, plain abdominal radiographs may be more likely to demonstrate obstructive ileus (i.e., gas-distended bowel loops) because the obstruction is not so far aborad.
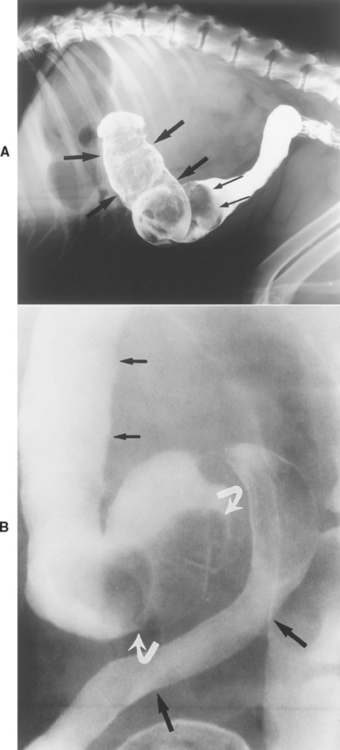
FIG 33-11 A, Lateral radiograph taken during a barium enema of a dog. Contrast medium outlines the end of a large ileocolic intussusception (thin arrows). Note that barium does not fill up the normally positioned colonic lumen because of a long filling defect (large arrows). B, Spot radiograph taken during a barium enema of a dog. The colon is descending on the left (short arrows), and the ileum (long arrows) is entering the colon. There is an area in which barium is displaced, representing an intussuscepted cecum (curved arrows).
(A courtesy Dr. Alice Wolf, Texas A & M University.)
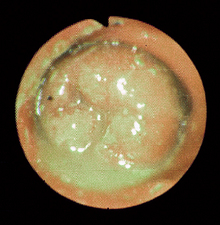
FIG 33-12 Endoscopic view of the ascending colon of a dog with an ileocolic intussusception. Note the large, “hot dog”-like mass in the colonic lumen, which is the intussusception.
A reason for the intussusception (e.g., parasites, mass, enteritis) should always be sought. Fecal examination for parasites and evaluation of full-thickness intestinal biopsy specimens obtained at the time of surgical correction of the intussusception should be performed. In particular, the tip of the intussuscepted bowel (i.e., the intussusceptum) should be examined for a mass lesion (e.g., tumor), which could have served as a focus and allowed the intussusception to occur. Additional diagnostic tests may be warranted depending on the history, physical examination findings, and results of clinical pathologic evaluation.
MISCELLANEOUS INTESTINAL DISEASES
SHORT BOWEL SYNDROME
Etiology
Short bowel syndrome occurs when extensive resection of intestines results in the need for special nutritional therapy until the intestines are able to adapt. This is typically an iatrogenic disorder caused by resection of more than 75% to 90% of the small intestine. The remaining intestine is unable to adequately digest and absorb nutrients. Large numbers of bacteria may reach the upper small intestines, especially if the ileocolic valve is removed. However, not all animals with substantial small intestinal resections develop this syndrome. Dogs and cats seem better able than people to tolerate loss of a large percentage of small intestine.
Clinical Features
Affected animals usually have severe weight loss and intractable diarrhea (typically without mucus or blood), which often occurs shortly after eating. Undigested food particles are often seen in the feces.
Diagnosis
A history of substantial resection in conjunction with the clinical signs is sufficient for diagnosis. It is wise to deter mine how much small intestine is left by performing contrast radiographs; estimates made at surgery can be surprisingly inaccurate.
Treatment
The best treatment is prevention. One should avoid massive resections if at all possible, even if it means doing a “second look” surgery 24 to 48 hours later. If massive resection occurs and the animal cannot maintain its body weight with oral feedings alone, total parenteral nutrition is needed until intestinal adaptation has occurred and treatments have become effective in controlling clinical signs. It is important to continue to feed the animal orally to stimulate intestinal mucosal hypertrophy. The diet should be highly digestible (e.g., low-fat cottage cheese, potato) and should be fed in small amounts, at least three to four times per day. Opiate antidiarrheals (e.g., loperamide), and H2-receptor antagonists may be useful in lessening diarrhea and decreasing gastric hypersecretion. Antibiotics might be needed to control the large bacterial populations now present in the small intestine (pp. 409–410).
Prognosis
If intestinal adaptation occurs, the animal may eventually be fed a near-normal diet. However, some animals will never be able to resume regular diets, and others die despite all efforts. Animals that are initially malnourished seem to have a worse prognosis than those that are well nourished. Some dogs and cats do better than one would intuitively expect them to do, despite the loss of approximately 85% of the small intestines.
NEOPLASMS OF THE SMALL INTESTINE
ALIMENTARY LYMPHOMA
Etiology
Lymphoma is a neoplastic proliferation of lymphocytes (see Chapter 80) that could also be placed in the section on malabsorptive diseases. It may be caused by FeLV in cats, but the etiology in dogs is unknown. LPE has been sugges-ted to be prelymphomatous in some animals, but the frequency of malignant transformation of LPE to lymphoma is unknown. Lymphoma often affects the intestines, although extraintestinal forms (e.g., lymph nodes, liver, spleen) are more common in dogs (see Chapter 80). Alimentary lymphoma appears to be more common in cats than in dogs.
Clinical Features
Chronic, progressive weight loss, anorexia, small intestinal diarrhea, and/or vomiting may occur. Alimentary lym-phoma may cause nodules, masses, diffuse intestinal thickening resulting from infiltrative disease (see Fig. 29-9), dilated sections of intestine that are not obstructed, and/or focal constrictions. It may also be present in grossly normal-appearing intestine; PLE may also occur. Mesenteric lymphadenopathy (i.e., enlargement) is typical but not invariable, and it is important to note that IBD can cause mild to moderate mesenteric lymphadenopathy. Extraintestinal abnormalities (e.g., peripheral lymphadenopathy) are inconsistently found in dogs and cats with alimentary lymphoma.
Diagnosis
Diagnosis requires demonstration of neoplastic lymphocytes, which may be obtained by fine-needle aspiration, imprint, or squash cytologic preparations. However, histopathologic evaluation of intestinal biopsy specimens is the most reliable diagnostic method. It is important to biopsy the ileum because many patients (especially cats) do not have lymphoma in the duodenum. If endoscopic biopsy samples are obtained, a poor sample or one that is not sufficiently deep may cause the erroneous diagnosis of LPE instead of lymphoma. Finding lymphocytes in the submucosa is not specific for lymphoma: Lymphocytes can be found in the submucosa of cats with IBD. However, cats with IBD generally do not have the dramatic numbers that can be found in some cases with lymphoma. Occasionally, neoplastic lymphocytes are found only in the serosal layer and full-thickness surgical biopsy specimens are necessary, but this scenario is extremely uncommon. Animals with extremely well-differentiated lymphocytic lymphoma may be impossible to distinguish from those with LPE using routine histopathology, even with multiple full-thickness biopsy samples. This seems to be a particularly important problem in cats. In such cases, diagnosis often depends on finding lymphocytes in organs where they should not be found (e.g., liver) or in performing immunohistochemical studies to determine if the lymphoid population is monoclonal. Paraneoplastic hypercalcemia occasionally occurs but is neither sensitive nor specific for lymphoma.
Treatment
Chemotherapy is of questionable value in dogs; many patients become quite ill if given aggressive chemotherapy. Cats with well-differentiated small cell lymphoma treated with prednisolone and chlorambucil may do as well as cats with IBD that receive the same therapy. Treatment protocols are outlined in Chapter 80.
INTESTINAL ADENOCARCINOMA
Intestinal adenocarcinoma is more common in dogs than in cats. It typically causes diffuse intestinal thickening or focal circumferential mass lesions. Primary clinical signs are weight loss and vomiting caused by intestinal obstruction. Diagnosis requires demonstrating neoplastic epithelial cells. Endoscopy, surgery, and ultrasound-guided fine-needle aspiration may be diagnostic. Scirrhous carcinomas have very dense fibrous connective tissue that often cannot be adequately biopsied with fine-needle aspiration or a flexible endoscope; therefore surgery is sometimes required to obtain diagnostic biopsies. The prognosis is good if com-plete surgical excision is possible, but metastases to regional lymph nodes are common by the time of diagnosis. Postoperative adjuvant chemotherapy does not appear to be beneficial.
INTESTINAL LEIOMYOMA/ LEIOMYOSARCOMA
Intestinal leiomyomas and leiomyosarcomas are connective tissue tumors that usually form a distinct mass and are primarily found in the small intestine and stomach of older dogs. Primary clinical signs are intestinal hemorrhage, iron deficiency anemia, and obstruction. They can also cause hypoglycemia as a paraneoplastic effect. Diagnosis requires demonstration of neoplastic cells. Evaluation of ultrasound-guided fine-needle aspirate may be diagnostic, but these tumors do not exfoliate as readily as many carcinomas or lymphomas, and biopsy is often necessary. Surgical excision may be curative if there are no metastases. Metastases make the prognosis poor, although some animals are palliated by chemotherapy.
INFLAMMATION OF THE LARGE INTESTINE
ACUTE COLITIS/PROCTITIS
Etiology
Acute colitis has many causes (e.g., bacteria, diet, parasites). The underlying cause is seldom diagnosed because this problem tends to be self-limiting. Acute proctitis probably has similar causes but may also be secondary to passage of a rough foreign object that traumatizes the rectal mucosa.
Clinical Features
Animals with acute colitis, which is more common in dogs than in cats, often feel good despite large bowel diarrhea (i.e., hematochezia, fecal mucus, tenesmus). Vomiting occurs infrequently. The major clinical signs of acute proctitis are constipation, tenesmus, hematochezia, dyschezia, and/or depression.
Diagnosis
Rectal examination is important; animals with acute colitis may have rectal discomfort and/or hematochezia. Eliminating obvious causes (e.g., diet, parasites) and resolving the problem with symptomatic therapy allow the clinician to make a presumptive diagnosis. Colonoscopy and biopsy are definitive but seldom performed or needed unless the initial presentation is unduly severe. Rectal examination of animals with acute proctitis may reveal roughened, thick, and/or obviously ulcerated mucosa. Proctoscopy and rectal mucosal biopsy are definitive but seldom required.
Treatment
Symptomatic therapy is typically sufficient because acute proctitis and colitis are usually idiopathic. Withholding food for 24 to 36 hours lessens the severity of clinical signs. The animal should then be fed small amounts of a bland diet (e.g., cottage cheese and rice) with or without fiber. After resolution of the clinical signs, the animal may be gradually returned to its original diet. Areas of anal excoriation should be cleansed, and an antibiotic-corticosteroid ointment should be applied. Most animals recover within 1 to 3 days. For proctitis, stool softeners and broad-spectrum antimicrobial therapy effective against anaerobic bacteria may also be used.
INTUSSUSCEPTION/PROLAPSE OF THE LARGE INTESTINE
CECOCOLIC INTUSSUSCEPTION
Etiology
Cecocolic intussusception, in which the cecum intussuscepts into the colon, is rare. The cause is unknown, although some suggest that whipworm-induced typhlitis may be responsible.
Clinical Features
Primarily occurring in dogs, intussuscepted cecums can bleed to the point where some dogs become anemic. Hematochezia is the major sign. It does not lead to intestinal obstruction and infrequently causes diarrhea.
Diagnosis
Cecocolic intussusception is rarely palpated during physical examination. Flexible endoscopy, ultrasonography, and contrast enema (see Fig. 33-11, B) usually reveal the intussusception.
RECTAL PROLAPSE
Etiology
Rectal prolapse usually occurs secondary to enteritis or colitis in young animals. They begin to strain because of rectal irritation, and eventually some or all of the rectal mucosa prolapses. Mucosal exposure increases irritation and perpetuates straining, which promotes prolapse. Hence a positive feedback cycle is initiated. Manx cats appear to be predisposed to rectal prolapse.
Clinical Features
Dogs and cats (especially juveniles) are affected. The presence of colonic or rectal mucosa extending from the anus is obvious during the physical examination.
Diagnosis
The diagnosis is based on physical examination. Rectal examination is needed to differentiate rectal prolapse from an intussusception protruding from the rectum (see p. 465).
Treatment
Treatment consists of resolving the original cause of straining if possible, repositioning the rectal mucosa, and preventing additional straining/prolapse. A well-lubricated finger is used to reposition the mucosa. If it readily prolapses after being replaced, a purse-string suture in the anus is used for 1 to 3 days to hold it in position. The subsequent rectal opening must be large enough so that the animal can defecate. Occasionally, an epidural anesthetic is needed to prevent repeated prolapse. If the everted mucosa is so irritated that straining continues, retention enemas with kaolin or barium may provide relief. If a massive prolapse is present or the rectal mucosa is irreversibly damaged, resection may be necessary.
NEOPLASMS OF THE LARGE INTESTINE
ADENOCARCINOMA
Etiology
The cause of adenocarcinoma is unknown. Contrary to adenocarcinoma in people, relatively few cases of colonic adenocarcinoma in dogs have been found to arise from polyps. These tumors can extend into the lumen or be infiltrative and produce a circumferential narrowing.
Clinical Features
Principally found in dogs, colonic and rectal adenocarcinomas are more common in older animals. Hematochezia is common. Infiltrative tumors are likely to cause tenesmus and/or constipation secondary to obstruction.
Diagnosis
Finding carcinoma cells is necessary for a diagnosis. Histopathologic evaluation is often preferable to cytologic analysis because epithelial dysplasia may be present in benign lesions, causing a false-positive cytologic diagnosis of carcinoma. Relatively deep biopsies obtained with rigid biopsy forceps are usually required to diagnose submucosal carcinomas and distinguish benign polyps from carcinomas because invasion of the submucosa is an important feature of rectal adenocarcinomas. Because most colonic neoplasms arise in or near the rectum, digital examination is the best screening test. Colonoscopy is required for masses farther orad. Imaging is used to detect sublumbar lymph node or pulmonary involvement (i.e., metastases).
RECTAL POLYPS
Clinical Features
Principally found in dogs, hematochezia (which may be considerable) and tenesmus are the primary clinical signs. Obstruction is rare.
Diagnosis
Usually detected during rectal examination, some adenomatous polyps resemble sessile adenocarcinomas because they are so large that the narrow, stalklike attachment cannot be readily discerned. Occasionally, multiple small polyps may be palpated throughout one segment of the colon, usually within a few centimeters of the rectum (Fig. 33-13). Histopathology is required for diagnosis and to distinguish polyps from malignancies.
Treatment
Complete excision via surgery or endoscopy is curative. If possible, a thorough endoscopic or imaging evaluation of the colon should be done before surgery to ensure that additional polyps are not present. If they are incompletely excised, polyps return and must be excised again. Multiple polyps within a defined area may necessitate segmental colonic mucosal resection.
MISCELLANEOUS LARGE INTESTINAL DISEASES
PYTHIOSIS
Clinical Features
Pythiosis of the large bowel usually occurs at or near the rectum. However, it can involve any area of the intestinal tract. Rectal lesions often cause partial obstruction. Fistulae may develop, resembling perianal fistulae. The dog may be presented for constipation and/or hematochezia. Animals with advanced disease often lose weight. In rare cases there will be infarction of mucosa or vessels with subsequent ischemia. Cats are rarely affected.
Diagnosis
Because the lesion is submucosal and very fibrotic, rigid biopsy forceps are typically necessary to obtain deep, diagnostic samples that include substantial amounts of submucosa (i.e., where the organism is found; Fig. 33-14). Special stains (e.g., Warthin-Starry) are needed to find the organism. Sometimes, the organism cannot be found, but a suggestive pyogranulomatous, eosinophilic inflammation is present. Serologic tests for antigen and antibodies are available (see Chapter 29).
PERINEAL/PERIANAL DISEASES
PERINEAL HERNIA
Etiology
Perineal hernia occurs when the pelvic diaphragm (i.e., coccygeus and levator ani muscles) weakens and allows the rectal canal to deviate laterally.
Clinical Features
This condition is principally found in older intact male dogs (especially Boston Terriers, Boxers, Cardigan Welsh Corgis, and Pekingeses); cats are rarely affected. Most animals present because of dyschezia, constipation, or perineal swelling; however, urinary bladder herniation into this defect may cause severe, potentially fatal postrenal uremia with depression and vomiting.
Diagnosis
Digital rectal examination should detect rectal deviation, lack of muscular support, and/or a rectal diverticulum. The clinician should check for retroflexion of the urinary bladder into the hernia. If such herniation is suspected, it can be confirmed by ultrasonography, radiographs, catheterizing the bladder, or aspirating the swelling (after imaging) to see if urine is present.
Treatment
Animals with postrenal uremia constitute an emergency; the bladder should be emptied and repositioned, and intravenous fluids should be administered. The preferred treatment is surgical reconstruction of the muscular support; however, surgery may fail, and clients should be prepared for the fact that their pet may require additional reconstructive procedures.
PERIANAL FISTULAE
Etiology
The cause of perianal fistulae is unknown. Impacted anal crypts and/or anal sacs have been hypothesized to become infected and rupture into deep tissues. An immune-mediated mechanism is likely to be involved, as seen by the clinical response to immunosuppressive drugs.
Clinical Features
Perianal fistulae occur in dogs and are more common in breeds with a sloping conformation and/or a broad base to the tail head (e.g., German Shepherd Dogs). There are typically one or more painful draining tracts around the anus. Animals are usually presented because of constipation (caused by the pain), odor, rectal pain, and/or rectal discharge.
Diagnosis
Diagnosis is made by physical and rectal examination. Care should be taken when examining the patient because the rectal area can be very painful. Draining tracts are sometimes absent, but granulomas and abscesses can be palpated via the rectum. Rectal pythiosis rarely mimics perianal fistulae.
Treatment
Most affected dogs are cured with immunosuppressive therapy (e.g., cyclosporine, 3 to 5 mg/ kg q12h or azathioprine, 50 mg/m2 q48h, or topical 0.1% tacrolimus q24h to q12h) with or without antibacterial drugs (e.g., metronidazole, erythromycin). Administering oral ketoconazole (5 mg/kg q12h) may allow a lower dose of cyclosporine to be effective, thus decreasing the client’s cost. If cyclosporine is used, the clinician should monitor therapeutic blood levels of the drug to ensure that adequate blood levels are present. Hypoallergenic diets may also be beneficial. Rarely, animals will not respond to medical therapy and will require surgery. Surgery may cause fecal incontinence. Postoperative care is important and consists of keeping the area clean. Fecal softeners are sometimes useful.
ANAL SACCULITIS
Clinical Features
Anal sacculitis is relatively common in dogs and occasionally occurs in cats. Small dogs (e.g., Poodles, Chihuahuas) probably have a higher incidence of this disorder than other breeds. Mild cases cause irritation (i.e., scooting, licking, or biting the area). Anal sacs occasionally bleed onto the feces. Severe cases may be associated with obvious pain, swelling, and/or draining tracts. Dyschezia or constipation may develop because the animal refuses to defecate. Fever may occur in dogs and cats with severe anal sacculitis.
Diagnosis
Physical and rectal examination is usually diagnostic. The anal sacs are often painful; the sac contents may appear purulent, bloody, or normal but increased in volume. In severe cases it may be impossible to express the affected sac. If the sac ruptures, the fistulous tract is usually in a 4 o’clock or 7 o’clock position in relation to the anus. Occasionally, there is an obvious abscess.
Treatment
Mild cases require only that the anal sac be expressed and an aqueous antibiotic-corticosteroid preparation be infused. Infusion with saline solution may aid in expressing impacted sacs. If clients express the anal sacs at home, they can often prevent impaction and reduce the likelihood of severe complications.
Abscesses should be lanced, drained, flushed, and treated with a hot pack; systemic antibiotics should also be administered. Hot packs also help soft spots form in early abscesses. If the problem recurs, is severe, or is nonresponsive to medical therapy, affected sacs can be resected.
PERIANAL NEOPLASMS
ANAL SAC (APOCRINE GLAND) ADENOCARCINOMA
Etiology
Anal sac adenocarcinomas are derived from the apocrine glands and are usually found in older female dogs.
Clinical Features
An anal sac or pararectal mass can often be palpated, but some are not obvious. Paraneoplastic hypercalcemia causing anorexia, weight loss, vomiting, and polyuria-polydipsia is common. Occasionally, constipation occurs as a result of the hypercalcemia or perineal mass. Metastatic sublumbar lymphadenopathy occurs early in the course of the disease, but metastases to other organs are rare.
Diagnosis
Cytologic and/or histopathologic evaluation is necessary to establish a diagnosis. Hypercalcemia in an older female dog should lead to careful examination of both anal sacs and pararectal structures. Abdominal ultrasonography may reveal sublumbar lymphadenopathy.
Treatment
Hypercalcemia, if present, must be treated (see Chapter 55). The tumor should be removed, but these tumors have often metastasized to regional lymph nodes by the time of diagnosis. Palliative chemotherapy (see Chapter 77) may be transiently beneficial in some dogs.
PERIANAL GLAND TUMORS
Etiology
Perianal gland tumors arise from modified sebaceous glands. Perianal gland adenomas have testosterone receptors.
Clinical Features
Perianal gland adenomas are often sharply demarcated, raised, and red and may be pruritic. Commonly found around the anus and base of the tail, they may be solitary or multiple and can occur over the entire back half of the dog. Male hormones appear to stimulate their growth, and they are often found in older intact male dogs (especially Cocker Spaniels, Beagles, and German Shepherd Dogs). Pruritus may lead to licking and ulceration of the tumor. Perianal gland adenocarcinomas are rare; they are usually large, infiltrative, ulcerated masses with a high metastatic potential.
Diagnosis
Cytologic and/or histopathologic evaluation is needed for diagnosis, but neither reliably distinguishes malignant from benign masses. Finding metastases (e.g., regional lymph nodes, lungs) is the most certain method of diagnosing malignancy.
Treatment
Surgical excision is preferred for benign or solitary tumors that have not metastasized. Neutering is recommended for dogs with adenomas. Radiation is recommended for multicentric and some malignant tumors. Chemotherapy (vincristine, adriamycin, cyclophosphamide [VAC] protocol) is helpful in dogs with adenocarcinomas (see Chapter 77).
CONSTIPATION
Constipation may be caused by any perineal or perianal disease that causes pain (e.g., perianal fistulae, perineal hernia, anal sacculitis), obstruction, or colonic weakness. It may also be caused by other disorders (see Box 28-15).
PELVIC CANAL OBSTRUCTION CAUSED BY MALALIGNED HEALING OF OLD PELVIC FRACTURES
Etiology
Prior trauma (e.g., automobile-associated injuries) is a common cause of pelvic canal obstruction in cats because they frequently sustain pelvic trauma that heals if they are allowed to rest. Cats appear clinically normal once the fractures heal, but the diminution of the pelvic canal can produce megacolon and/or dystocia.
Diagnosis
Digital rectal examination should be diagnostic. Radiographs will further define the extent of the problem.
Treatment
Constipation caused by minimal pelvic narrowing may be controlled with stool softeners, but orthopedic surgery may be needed. The prognosis depends somewhat on how severely the colon has been distended. Unless the colon is massively stretched out of shape, it can often resume function if it is kept empty and allowed to regain its normal diameter. Prokinetic drugs such as cisapride (0.25 mg/kg administered orally q8-12h) may stimulate peristalsis; however, prokinetic drugs must not be used if there is residual obstruction.
BENIGN RECTAL STRICTURE
Diagnosis
Digital rectal examination detects a stricture, although this sign can be missed if a large dog is palpated carelessly or if the stricture is beyond reach. Proctoscopy and evaluation of a deep biopsy specimen (i.e., including submucosa) of the stricture are needed to confirm that the lesion is benign and fibrous as opposed to neoplastic or fungal.
Treatment
In some animals, simple dilation via balloon or retractor will tear the stricture and allow normal defecation; other animals require surgery. Owners should be warned that strictures may re-form during healing, and surgery can cause incontinence in rare cases. Corticosteroids (prednisolone, 1.1 mg/kg/day) might impede stricture re-formation.
DIETARY INDISCRETION LEADING TO CONSTIPATION
Etiology
Dogs often eat inappropriate foods or other materials (e.g., paper, popcorn, hair, bones). Excessive dietary fiber supplements can cause constipation if the animal becomes dehydrated.
Diagnosis
Dietary causes are common in dogs that eat trash. Dietary indiscretion is best diagnosed by examining fecal matter retrieved from the colon.
Treatment
Controlling the pet’s eating habits, adding appropriate amounts of fiber to the diet, and feeding a moist diet (especially in cats) help prevent constipation. Repeated retention and cleansing (not hypertonic) enemas may be needed. Manual disruption of hard feces should be avoided, but if it is necessary, the animal should be anesthetized to help prevent colonic trauma during the procedure, and sponge forceps or curved hemostats should be used to mechanically break apart the feces. It often helps to insert a rigid colonoscope up to the fecal mass and then insert a tube with a vigorous stream of running water at body temperature issuing from the tip. This will soften the fecal mass and wash away debris that breaks off.
IDIOPATHIC MEGACOLON
Etiology
The cause is unknown but may involve behavior (i.e., refusal to defecate) or altered colonic neurotransmitters.
Clinical Features
Idiopathic megacolon is principally a feline disease, although dogs are occasionally affected. Affected animals may be depressed and anorectic and are often presented because of infrequent defecation.
Diagnosis
Diagnosis requires palpating a massively dilated colon (not one just filled to normal capacity) plus elimination of dietary, behavioral, metabolic, and anatomic causes. Abdominal radiographs should be evaluated if proper abdominal palpation cannot be performed.
Treatment
Impacted feces must be removed. Multiple warm water retention and cleansing enemas over 2 to 4 days usually work. Future fecal impaction is prevented by adding fiber to a moist diet (e.g., Metamucil, pumpkin pie filling), making sure clean litter is always available, and using osmotic laxatives (e.g., lactulose) and/or prokinetic drugs (e.g., cisapride). Lubricants are not helpful, because they do not change fecal consistency. If this conservative therapy fails or is refused by the client, subtotal colectomy is indicated in cats (not dogs). Cats typically have soft stools for a few weeks postoperatively, some for the rest of their lives.
Abdelmagid OY, et al. Evaluation of the efficacy and duration of immunity of a canine combination vaccine against virulent parvovirus, infectious canine hepatitis virus, and distemper virus experimental challenges. Vet Therap. 2004;5:173.
Allenspach K, et al. Pharmacokinetics and clinical efficacy of cyclosporine treatment of dogs with steroid-refractory inflammatory bowel disease. J Vet Intern Med. 2006;20:239.
Baez JL, et al. Radiographic, ultrasonographic, and endoscopic findings in cats with inflammatory bowel disease of the stomach and small intestine: 33 cases (1990–1997). J Am Vet Med Assoc. 1999;215:349.
Batchelor DJ, et al. Breed associations for canine exocrine pancreatic insufficiency. J Vet Intern Med. 2007;21:207.
Bender JB, et al. Epidemiologic features of Campylobacter infection among cats in the upper midwestern United States. J Am Vet Med Assoc. 2005;226:544.
Boag AK, et al. Acid-base and electrolyte abnormalities in dogs with gastrointestinal foreign bodies. J Vet Intern Med. 2005;19:816.
Bowman DD, et al. Efficacy of moxidectin 6 month injectable and milbemycin oxime/lufenurono tablets against naturally acquired Trichuris vulpis infections in dogs. Vet Therap. 2002;3:286.
Brissot H, et al. Use of laparotomy in a staged approach for resolution of bilateral or complicated perineal hernia in 41 dogs. Vet Surg. 2004;33:412.
Carmichael L. An annotated historical account of canine parvovirus. J Vet Med B. 2005;52:303.
Coyne MJ. Seroconversion of puppies to canine parvovirus and canine distemper virus: a comparison of two combination vaccines. J Am Anim Hosp Assoc. 2000;36:137.
Craven M, et al. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995-2002). J Small Anim Pract. 2004;45:336.
Doherty D, et al. Intestinal intussusception in five postparturient queens. Vet Rec. 2000;146:614.
Dryden M, et al. Accurate diagnosis of Giardia spp. and proper fecal examination procedures. Vet Therap. 2006;7:4.
Eleraky NZ, et al. Virucidal efficacy of four new disinfectants. J Am Anim Hosp Assoc. 2002;38:231.
Epe C, et al. Investigations into the prevention of neonatal Ancyclostoma caninum infections in puppies by application of moxidectin to the bitch. J Vet Med. 1999;46:361.
Evans SE, et al. Comparison of endoscopic and full-thickness biopsy specimens for diagnosis of inflammatory bowel disease and alimentary tract lymphoma in cats. J Am Vet Med Assoc. 2006;229:1447.
Evermann JF, et al. Canine coronavirus-associated puppy mortality without evidence of concurrent canine parvovirus infection. J Vet Diagn Invest. 2005;17:610.
Foster DM, et al. Outcome of cats with diarrhea and Tritrichomonas foetus infection. J Am Vet Med Assoc. 2004;225:888.
Fradkin JM, et al. Elevated parathyroid hormone-related protein and hypercalcemia in two dogs with schistosomiasis. J Am Anim Hosp Assoc. 2001;37:349.
Garci-Sancho M, et al. Evaluation of clinical, macroscopic, and histopathologic response to treatment in nonhypoproteinemic dogs with lymphocytic-plasmacytic enteritis. J Vet Intern Med. 2007;21:11.
German AJ, et al. Comparison of direct and indirect tests for small intestinal bacterial overgrowth and antibiotic-responsive diarrhea in dogs. J Vet Intern Med. 2003;17:33.
German AJ, et al. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med. 2003;17:8.
Gookin JL, et al. Experimental infection of cats with Tritrichomonas foetus. Am J Vet Res. 2001;62:1690.
Gookin J, et al. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. J Vet Intern Med. 2006;20:536.
Gorman S, et al. Extensive small bowel resection in dogs and cats: 20 cases (1998-2004). Am J Vet Res. 2006;228:403.
Graham JP, et al. Ultrasonographic features of canine gastrointestinal pythiosis. Vet Radiol Ultrasound. 2000;41:273.
Guilford WG, et al. Food sensitivity in cats with chronic idiopathic gastrointestinal problems. J Vet Intern Med. 2001;15:7.
Hill SL, et al. Prevalence of enteric zoonotic organisms in cats. J Am Vet Med Assoc. 2000;216:687.
Holt PE. Evaluation of transanal endoscopic treatment of benign canine rectal neoplasia. J Small Anim Pract. 2007;48:17-25.
Jergens AE, et al. Diseases of the large intestine. In Ettinger SJ, et al, editors: Textbook of veterinary internal medicine, ed 5, Philadelphia: WB Saunders, 2000.
Jergens AE. Clinical assessment of disease activity for canine inflammatory bowel disease. J Am Anim Hosp Assoc. 2004;40:437.
Johnson KL. Small intestinal bacterial overgrowth. Vet Clin N Am. 1999;29:523.
Johnston SP, et al. Evaluation of three commercial assays for detection of Giardia and Cryptosporidium organisms in fecal specimens. J Clin Microbiol. 2003;41:623.
Kimmel SE, et al. Hypomagnesemia and hypocalcemia associated with protein-losing enteropathy in Yorkshire terriers: five cases (1992–1998). J Am Vet Med Assoc. 2000;217:703.
Kull PA, et al. Clinical, clinicopathologic, radiographic, and ultrasonographic characteristics of intestinal lymphangiectasia in dogs: 17 cases (1996–1998). J Am Vet Med Assoc. 2001;219:197.
Kupanoff P, et al. Colorectal plasmacytomas: a retrospective study of nine dogs. J Am Anim Hosp Assoc. 2006;42:37.
Leib MS. Treatment of chronic idiopathic large-bowel diarrhea in dogs with a highly digestible diet and soluble fiber: a retrospective review of 37 cases. J Vet Intern Med. 2000;14:27.
Littman MP, et al. Familial protein-losing enteropathy and protein-losing nephropathy in Soft Coated Wheaten Terriers: 222 cases (1983–1997). J Vet Intern Med. 2000;14:68.
Mantione N, et al. Characterization of the use of antiemetic agents in dogs with parvoviral enteritis treated at a veterinary teaching hospital: 77 cases (1997-2000). J Am Vet Med Assoc. 2005;227:1787.
Marks SL, et al. Dietary trial using commercial hypoallergenic diet containing hydrolyzed protein for dogs with inflammatory bowel disease. Vet Therap. 2002;3:109.
Marks SL, et al. Bacterial-associated diarrhea in the dog: a critical appraisal. Vet Clin N Am. 2003;33:1029.
Marks SL, et al. Editorial: small intestinal bacterial overgrowth in dogs—less common than you think? J Vet Intern Med. 2003;17:5.
McCaw DL, et al. Canine viral enteritis. In Greene CE, editor: Infectious diseases of the dog and cat, ed 3, St Louis: Elsevier, 2006.
Misseghers BS, et al. Clinical observations of the treatment of canine perianal fistulas with topical tacrolimus in 10 dogs. Can Vet J. 2000;41:623.
Miura T, et al. Endoscopic findings on alimentary lymphoma in 7 dogs. J Vet Med Science. 2004;66:577.
Mochizuki M, et al. Feline coronavirus participation in diarrhea of cats. J Vet Med Sci. 1999;61:1071.
Mohr AJ, et al. Effect of early enteral nutrition on intestinal permeability, intestinal protein loss, and outcome in dogs with severe parvoviral enteritis. J Vet Int Med. 2003;17:791.
Morely P, et al. Evaluation of the association between feeding raw meat and Salmonella enterica infections at a Greyhound breeding facility. J Amer Vet Med Assoc. 2006;228:1524.
Nakamura K, et al. Patholgenic potential of canine paravovirus types 2a and 2c in domestic cats. Clin Diag Lab Imm. 2001;8:663.
O’Neill T, et al. Efficacy of combined cyclosporine A and ketconazole treatment of anal furunculosis. J Small Anim Pract. 2004;45:238.
Patterson EV, et al. Effect of vaccination on parvovirus antigen testing in kittens. J Am Vet Med Assoc. 2007;230:359.
Payne PA, et al. Efficacy of a combination febantel-praziquantel-pyrantel product, with or without vaccination with a commerical Giardia vaccine, for treatment of dogs with naturally occurring giardiasis. J Am Vet Med Assoc. 2002;220:330.
Peterson PB, et al. Protein-losing enteropathies. Vet Clin N Am. 2003;33:1061.
Pratelli A, et al. Fatal canine parvovirus type-1 infection in pups from Italy. J Vet Diagn Invest. 1999;11:365.
Ragaini L, et al. Inflammatory bowel disease mimicking alimentary lymphosarcoma in a cat. Vet Res Comm. 2003;27(Suppl 1):791.
Rewerts JM, et al. CVT update: diagnosis and treatment of parvovirus. In Bonagura JD, editor: Current veterinary therapy XIII, ed 13, Philadelphia: WB Saunders, 2000.
Richter KP. Feline gastrointestinal lymphoma. Vet Clin N Am. 2003;33:1083.
Stein JE, et al. Efficacy of Giardia vaccination in the treatment of giardiasis in cats. J Am Vet Med Assoc. 2003;222:1548.
Steiner JM, et al. Serum lipase activities and pancreatic lipase immunoreactivity concentrations in dogs with exocrine pancreatic insufficiency. Am J Vet Res. 2006;67:84.
Tauni MA, et al. Outbreak of Salmonella typhimurium in cats and humans associated with infections in wild birds. J Small Anim Pract. 2000;41:339.
Vasilopulos RJ, et al. Prevalence and factors associated with fecal shedding of Giardia spp. in domestic cats. J Am Anim Hosp Assoc. 2006;42:424.
Villeneuve V, et al. Efficacy of oxfendazole for the treatment of giardiasis in dogs: experiments in dog breeding kennels. Parasitology. 2000;7:221.
Weese JS, et al. Outbreak of Clostridium difficile-associated disease in a small animal veterinary teaching hospital. J Vet Intern Med. 2003;17:813.
Westermarck E, et al. Exocrine pancreatic insufficiency in dogs. Vet Clin N Am. 2003;33:1165.
Westermarck E, et al. Tylosin-responsive chronic diarrhea in dogs. J Vet Intern Med. 2005;19:177.
Westermarck E, et al. Effect of diet and tylosin on chronic diarrhea in Beagles. J Vet Intern Med. 2005;19:822.
Wiberg ME, et al. Exocrine pancreatic atrophy in German shepherd dogs and rough-coated Collies: an end result of lymphocytic pancreatitis. Vet Pathol. 1999;36:530.
Willard MD, et al. Intestinal crypt lesions associated with protein-losing enteropathy in the dog. J Vet Intern Med. 2000;14:298.
Williams LE, et al. Carcinoma of the apocrine glands of the anal sac in dogs: 113 cases (1985–1995). J Am Vet Med Assoc. 2003;223:825.