CHAPTER 97 Polysystemic Viral Diseases
There are multiple viral infections of dogs and cats. Several, including canine distemper virus, some feline coronaviruses, feline leukemia virus, and feline immunodeficiency virus, can cause systemic signs of disease. See other chapters for discussions of viral diseases specific to one organ system.
CANINE DISTEMPER VIRUS
Etiology and Epidemiology
Canine distemper virus (CDV) induces disease predominantly in terrestrial carnivores, but many other species, including seals, ferrets, skunks, badgers, porpoises, and exotic Felidae, have been infected by either CDV or related morbilliviruses. The virus replicates in lymphoid, nervous, and epithelial tissues and is shed in respiratory exudates, feces, saliva, urine, and conjunctival exudates for up to 60 to 90 days after natural infection. After inhalation, the virus is engulfed by macrophages and within 24 hours is carried by lymphatics to tonsillar, pharyngeal, and bronchial lymph nodes, where replication occurs. Central nervous system (CNS) and epithelial tissues are infected approximately 8 to 9 days after initial infection.
The degree of clinical illness and the tissues involved vary depending on the strain of the virus and the immune status of the host (Greene et al., 2006). Nonimmune dogs of any age are susceptible, but the disease is most common in puppies between 3 and 6 months of age. An estimated 25% to 75% of susceptible dogs are subclinically infected after exposure. Massive replication of the virus in the epithelial cells of the respiratory tract, gastrointestinal system, and genitourinary system occurs in dogs with poor immune responses by postinfection days 9 to 14; these dogs usually die from polysystemic disease. In dogs with moderate immune responses by postinfection days 9 to 14, the virus replicates in epithelial tissues and may result in clinical signs of disease. Dogs with good cell-mediated responses and virus-neutralizing antibody titers by postinfection day 14 clear the virus from most tissues and may not be clinically affected. Most infected dogs develop CNS infection, but clinical signs of CNS disease occur only in dogs with low or no antibody response. Acute demyelination results from restrictive infection of oligodendrogliocytes and subsequent necrosis; chronic demyelination is caused by immune-mediated mechanisms, including antimyelin antibodies and CDV immune complex formation and removal.
Clinical Features
Many clinically affected dogs are unvaccinated, failed to receive colostrum from an immune bitch, were inappropriately vaccinated, or are immunosuppressed and also have a history of exposure to infected animals. Owners generally present affected dogs for evaluation of depression, malaise, oculonasal discharge, cough, vomiting, diarrhea, or CNS signs. Dogs with poor immune responses generally have the most severe signs and progress rapidly to life-threatening disease. Some partially immune dogs have only mild respiratory disease, presumptively diagnosed as kennel cough syndrome. Tonsillar enlargement, fever, and mucopurulent ocular discharge are common physical examination findings. Increased bronchial sounds, crackles, and wheezes are usually auscultated in dogs with bronchopneumonia.
Hyperesthesia, seizures, cerebellar or vestibular disease, paresis, and chorea myoclonus are common CNS signs that generally develop within 21 days of recovery from systemic disease (Table 97-1). CNS disease is generally progressive and carries a poor prognosis. Some dogs with signs of CNS disease never had systemic signs of disease recognized. Old dog encephalitis is a chronic, progressive panencephalitis in older dogs (older than 6 years) thought to be attributable to CDV infection in which microglial proliferation and neuronal degeneration in the cerebral cortex result in depression, circling, head pressing, and visual deficits (see Chapter 69 for more information on CNS distemper).
 TABLE 97-1 Clinical Manifestations of CDV Infection
TABLE 97-1 Clinical Manifestations of CDV Infection
| In utero infection | |
| Gastrointestinal tract disease | |
| Respiratory tract disease | |
| Ocular disease | |
| Neurologic Disease | |
| Spinal cord disease | Paresis and ataxia |
| Central vestibular disease | Head tilt, nystagmus, other cranial nerve and conscious proprioception deficits |
| Cerebellar disease | Ataxia, head bobbing, hypermetria |
| Cerebral disease | |
| Chorea myoclonus | Rhythmic jerking of single muscles or muscle groups |
| Miscellaneous |
CDV, Canine distemper virus; CNS, central nervous system.
Ocular abnormalities associated with CDV infection include anterior uveitis, optic neuritis with resultant blindness and dilated pupils, and retinochoroiditis. The combination of retinochoroiditis and encephalitis is detected in approximately 40% of affected dogs. Keratoconjunctivitis sicca and hyperreflective retinal scars called medallion lesions occur in some dogs with chronic infection (Fig. 97-1).
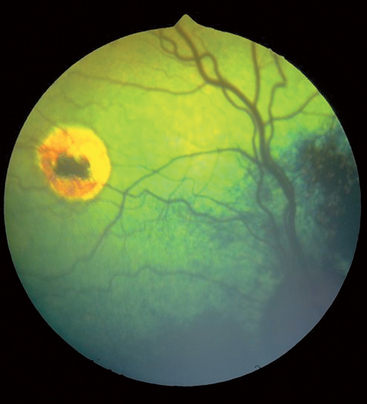
FIG 97-1 Medallion lesions resulting from canine distemper virus infection.
(Courtesy Dr. Cynthia Powell, Colorado State University, Fort Collins.)
Other less-common syndromes have been attributed to CDV infection. Dogs infected before the development of permanent dentition usually have enamel hypoplasia. Hyperkeratosis of the nose and footpads and pustular dermatitis are the most common dermatologic abnormalities. Puppies infected transplacentally can be stillborn, aborted, or born with CNS disease.
Diagnosis
The combination of clinical findings and routine clinicopathologic and radiographic evaluation usually leads to a presumptive diagnosis of CDV infection. Lymphopenia and mild thrombocytopenia are consistent hematologic abnormalities. Interstitial and alveolar pulmonary infiltrates are common radiographic findings in dogs with respiratory disease. Although some dogs with CNS infection have normal cerebrospinal fluid (CSF) analyses, most have mononuclear cell pleocytosis and increased protein concentrations. The ratio of serum/CSF immunoglobulin G (IgG) and albumin is commonly high in dogs with encephalitis, but this only documents inflammation of the CNS, not CDV infection.
Measurement of serum or CSF antibody titers can aid in the diagnosis of CDV infection. Documentation of a fourfold increase in the serum IgG titer over a 2- to 3-week period or detection of IgM antibodies in serum is consistent with recent infection or recent vaccination but does not prove clinical disease. CSF antibody titers to CDV are increased in some dogs with encephalitis. False-positive results can occur in CSF samples contaminated with blood. If CSF antibody titers are greater than those in serum, the antibody in the CSF had to be produced locally and is consistent with CNS CDV infection. If increased CSF protein concentrations, mononuclear pleocytosis, and antibodies against CDV are detected in a CSF sample not contaminated with peripheral blood, a presumptive diagnosis of CDV encephalitis can be made.
Definitive diagnosis of CDV infection requires demonstration of viral inclusions by cytologic examination, direct fluorescent antibody staining of cytologic or histopathologic specimens, histopathologic evaluation, virus isolation, or reverse transcriptase polymerase chain reaction (RT-PCR) documentation of CDV RNA in peripheral blood, CSF, or conjunctival scrapings. Viral inclusions are rarely found in erythrocytes, leukocytes, and leukocyte precursors of infected dogs. Inclusions are generally present for only 2 to 9 days after infection and therefore often are not present when clinical signs occur. Inclusions may be easier to find in smears made from buffy coats or bone marrow aspirates than in those made from peripheral blood. Viral particles can be detected by immunofluorescence in cells from the tonsils, respiratory tree, urinary tract, conjunctival scrapings, and CSF for 5 to 21 days after infection. Recent administration of modified-live CDV-containing vaccines can lead to positive results in direct fluorescent antibody assays and RT-PCR assays. False-positive results have been detected occasionally in direct fluorescent antibody assays performed on conjunctival cells from specific pathogen-free puppies, so results of these tests should be interpreted cautiously (Lappin et al., unpublished data, 2007).
Treatment
Therapy for CDV infection is nonspecific and supportive. Secondary bacterial infections of the gastrointestinal tract and respiratory system are common and, if indicated, should be treated with appropriate antibiotics (see Chapter 93). Anticonvulsants are administered as needed to control seizures (see Chapter 67), but chorea myoclonus has no known effective treatment. Glucocorticoid administration may be beneficial in some dogs with CNS disease from chronic CDV infection, but it is contraindicated in acutely infected dogs. The prognosis for dogs with CNS distemper is poor.
Prevention and Zoonotic Aspects
The CDV survives in exudates only for approximately 1 hour at body temperature and 3 hours at room temperature and is susceptible to most routine hospital disinfectants. Dogs with gastrointestinal or respiratory signs of disease should be housed in isolation to avoid aerosolization to susceptible populations. Care should be taken to avoid transmission by contaminated fomites (see Chapter 94). All puppies should receive at least three CPV-2, CAV-2, and CDV-containing vaccines, every 3 to 4 weeks, between 6 and 16 weeks of age, with the last booster administered at 14 to 16 weeks of age (see Chapter 94). Modified-live CDV vaccines and the recombinant CDV (rCDV) vaccine are considered adequate by the AAHA Task Force (Paul et al., 2006). Maternal antibodies can block CDV vaccines; therefore in high-risk puppies a modified-live measles virus vaccine has been used between 4 and 12 weeks of age to induce heterologous antibodies that will protect puppies against CDV as maternal antibodies wane. The need for this product is now in question because the rCDV vaccine immunizes puppies in the face of maternal immunity (see Chapter 94). Vaccination against CDV is not as effective if the body temperature is 39.9° C or higher or if other systemic diseases are detected. Vaccines should be boosted at 1 year of age. Recent data suggest that after the 1-year booster, repeat boosters are not needed again for a minimum of 3 years (see Chapter 94).
Disease from CDV infection has occurred in some vaccinated dogs and rarely is attributed to modified-live virus vaccination. Clinical disease in vaccinated dogs develops if the host was immunocompromised, infected with the virus before vaccination, had vaccine-suppressive levels of maternal antibodies, or was incompletely vaccinated. Alternately, the vaccine may have been inactivated by improper handling or may not have protected against all field strains of CDV. Distemper virus encephalitis develops after modified-live vaccination of some dogs coinfected with canine parvovirus; administration of modified-live CDV vaccines should be delayed in dogs with clinical signs of disease consistent with parvovirus infection. Mild, transient thrombocytopenia can be induced by modified CDV vaccination but has not been associated with spontaneous bleeding unless the patient has an underlying subclinical coagulopathy. No proven public health risks are associated with CDV.
FELINE CORONAVIRUS
Etiology and Epidemiology
Coronaviruses causing disease in cats include feline infectious peritonitis (FIP) virus and feline enteric coronavirus (FECV). Enteric infection generally results in mild gastrointestinal signs; systemic infection can induce a clinical syndrome with diverse manifestations commonly referred to as FIP. Multiple field strains of FECV and FIP virus have varying degrees of virulence. Mutations or recombinant strains of endemic FECV capable of inducing FIP are believed to develop in the gastrointestinal tract of some infected cats (Vennema et al., 1998).
Enteric coronaviruses are commonly shed in feces and rarely in saliva (Addie et al., 2001) and are highly contagious. Although the prevalence of transplacental transmission is unknown, one epidemiologic study suggested that it is unlikely (Addie et al., 1993). By RT-PCR testing, coronaviruses can be detected in feces as early as 3 days after infection. In studies of FECV-infected, closed cat colonies, almost every cat becomes infected. In one study of 155 pet cats with naturally occurring FECV infection, viral RNA was shed continuously (n = 18) or intermittently (n = 44) in the feces of some cats (Addie et al., 2001). Others were initially shedding viral RNA and then ceased shedding (n = 56), and some were resistant to infection (n = 4). The cats that stopped viral shedding were susceptible to reinfection. Viral RNA was detected in the ileum, colon, and rectum of cats with persistent shedding.
Coronaviruses with the ability to infect monocytes can cause viremia and disseminate throughout the body, potentially resulting in FIP. Between 1986 and 1995, one of every 200 feline accessions at veterinary teaching hospitals in North America was given a clinical diagnosis of FIP (Rohrbach et al., 2001). Most cases of FIP develop in multiple-cat households or catteries. The effusive form of disease develops in cats with poor cell-mediated immune responses; the noneffusive form develops in cats with partial cell-mediated immunity. The effusive form of disease is an immune complex vasculitis characterized by leakage of protein-rich fluid into the pleural space, the peritoneal cavity, the pericardial space, and the subcapsular space of the kidneys. In the noneffusive form pyogranulomatous or granulomatous lesions develop in multiple tissues, particularly the eyes, brain, kidneys, omentum, and liver. Some affected cats have characteristics of both forms of FIP.
Clinical disease associated with FIP virus may be influenced by a number of factors, including the virulence of the strain, the dose of the virus, the route of infection, the immune status of the host, genetically determined host factors, the presence of other concurrent infections, and whether the cat had been previously exposed to a coronavirus. Some breeds appear to be predisposed to the development of FIP (Pesteanu-Somogyi et al., 2006). Feline leukemia virus infection and respiratory tract infection increase the risk for FIP, suggesting that the immune status of the host is important in determining the development of clinical disease. Cats concurrently infected with FIV shed 10 to 100 times more FECV in stool than FIV-naive cats. Experimentally infected, seropositive kittens develop accelerated FIP compared with seronegative kittens when exposed to FIP virus. This antibody-dependent enhancement of virus infectivity occurs because macrophages are more effectively infected by virus complexed with antibody than by virus alone. This phenomenon appears to be rare in naturally infected cats.
Clinical Features
Enteric replication of coronaviruses commonly results in fever, vomiting, and mucoid diarrhea. With FECV infection clinical signs are self-limiting and generally respond to supportive care within days. Fulminant FIP can occur in cats of any age but is generally recognized in cats younger than 5 years; most cases are younger than 1 year. Intact males are overrepresented in some studies. In cattery outbreaks, usually only one or two kittens in a litter are clinically affected. This may relate to poor transmissibility of strains capable of inducing FIP. Anorexia, weight loss, and general malaise are common presenting complaints (Box 97-1). Icterus, ocular inflammation, abdominal distension, dyspnea, or CNS abnormalities are occasionally noted by the owner.
 BOX 97-1 Clinical Findings Suggestive of FIP in Cats
BOX 97-1 Clinical Findings Suggestive of FIP in Cats
FIP, Feline infectious peritonitis; FeLV, feline leukemia virus; CSF, cerebrospinal fluid; RT-PCR, reverse transcriptase polymerase chain reaction.
Signalment and History
Cats <5 years or>10 years of age
Purchase from a cattery or multiple-cat household
Previous history of a mild, self-limiting gastrointestinal or respiratory disease
Serologic evidence of infection by FeLV
Nonspecific signs of anorexia, weight loss, or depression
Seizures, nystagmus, or ataxia
Acute, fulminant course in cats with effusive disease
Chronic, intermittent course in cats with noneffusive disease
Physical Examination
Pale mucous membranes with or without petechiae
Dyspnea with a restrictive breathing pattern
Abdominal distension with a fluid wave with or without scrotal swelling
Abdominal mass from focal intestinal granuloma or lymphadenopathy
Icterus with or without hepatomegaly
Chorioretinitis or iridocyclitis
Multifocal neurologic abnormalities
Clinicopathologic Abnormalities
Neutrophilic leukocytosis with or without a left shift
Hyperglobulinemia characterized as a polyclonal gammopathy; rare monoclonal gammopathies
Nonseptic, pyogranulomatous exudate in pleural space, peritoneal cavity, or pericardial space
Increased protein concentrations and neutrophilic pleocytosis in CSF
Positive coronavirus antibody titer in the majority (especially noneffusive)
Pyogranulomatous or granulomatous inflammation in perivascular location on histologic examination of tissues
Positive results of immunofluorescence or RT-PCR performed on pleural or peritoneal exudate
Fever and weight loss are common with both the effusive and noneffusive forms of the disease. Pale mucous membranes or petechiae are noted in some cats. FIP is one of the most common causes of icterus in cats younger than 2 years; liver size can be normal or enlarged, and the margins are usually irregular. Abdominal distension is common, a fluid wave can often be balloted, and occasionally masses (pyogranulomas or lymphadenopathy) can be palpated in the omentum, mesentery, or intestines. A solitary ileocecocolic or colonic mass, resulting in obstruction leading to vomiting and diarrhea, occurs in some cats. Kidneys can be small (chronic disease) or large (acute disease or subcapsular effusion); renal margins are usually irregular. Pleural effusion can result in dyspnea and a restrictive breathing pattern (shallow and rapid) as well as muffled heart and lung sounds. Male cats sometimes have scrotal enlargement from fluid accumulation.
Anterior uveitis and chorioretinitis occur most frequently with the noneffusive form of the disease and can be its only manifestation. Pyogranulomatous disease can develop anywhere in the CNS, leading to a variety of neurologic signs that include seizures, posterior paresis, and nystagmus.
Feline coronaviruses have been suggested as a cause of failure to conceive, abortion, stillbirth, and congenital defects as well as the fading kitten syndrome (kitten mortality complex). However, one epidemiologic study failed to link feline coronavirus with reproductive failure or neonatal kitten death (Addie et al., 1993).
Diagnosis
Multiple hematologic, serum biochemical, urinalysis, diagnostic imaging, and CSF abnormalities develop in cats with FIP. Several authors have assessed the predictive values of individual and combinations of tests (Hartmann et al., 2003; Sparkes et al., 1994). Other than histopathology, the positive predictive values of tests used to aid in the diagnosis of FIP are less than 100%. A presumptive diagnosis of FIP is usually based on the combination of clinical and clinicopathologic findings.
Normocytic, normochromic, nonregenerative anemia; neutrophilic leukocytosis; and lymphopenia are common. Disseminated intravascular coagulation resulting in thrombocytopenia occurs in some cats. Hyperproteinemia with or without hypoalbuminemia can occur. Polyclonal gammopathies from increases in α2-globulin and γ-globulin concentrations are most commonly detected; monoclonal gammopathies are rare. Most of these findings are consistent with chronic inflammation and do not prove FIP.
Hyperbilirubinemia with variable increases in alanine aminotransferase and alkaline phosphatase activities occurs in some cats with hepatic disease. Prerenal azotemia, renal azotemia, and proteinuria are the most common renal abnormalities. Radiographs can reveal pleural, pericardial, or peritoneal effusions; hepatomegaly; or renomegaly. Mesenteric lymphadenopathy may result in mass lesions in some cats. Ultrasonography can be used to confirm the presence of abdominal fluid in cats with minimal fluid volumes and to evaluate the pancreas, liver, lymph nodes, and kidneys. Magnetic resonance imaging showed periventricular contrast enhancement, ventricular dilation, and hydrocephalus in one group of cats with neurologic FIP (Foley et al., 1998). Protein concentrations and nucleated cell counts (neutrophils predominate in most cases) are commonly increased in CSF from cats with CNS involvement. Although high coronavirus antibody titers are com-mon in the CSF of cats with neurologic FIP, the antibodies appear to be derived from blood and, as the authors of one study concluded, were of equivocal value (Boettcher et al., 2007).
Effusions from cats with FIP are sterile, colorless to straw colored, may contain fibrin strands, and may clot when exposed to air. The protein concentration on fluid analysis commonly ranges from 3.5 g/dL to 12 g/dL and is generally higher than that associated with other diseases. Mixed inflammatory cell populations of lymphocytes, macrophages, and neutrophils occur most commonly; neutrophils predominate in most cases, but in some cats macrophages are the primary cell type seen. In some cats the coronavirus antibody titers are greater in the effusion than in serum. Measurement of protein concentrations in effusions and calculation of the albumin/globulin ratio can aid in the diagnosis of effusive FIP. In one study an albumin/globulin ratio of 0.5 had a positive predictive value of 89%, and an albumin/globulin ratio of 1.0 had a negative predictive value of 91% (Hartmann et al., 2003). Coronavirus antigens are commonly detected by direct immunofluorescence in the effusions of cats with FIP but not in the effusions of cats with other diseases. In addition, viral RNA can be detected by RT-PCR in effusions and is unlikely to be in effusions from other causes.
Detection of serum antibodies is of limited benefit in the diagnosis of FIP. Infection of cats by any coronavirus can cause cross-reacting antibodies; therefore a positive antibody titer does not diagnose FIP, protect against disease, or predict when a cat may develop clinical FIP. Because coronavirus antibody tests are not standardized, results from different laboratories commonly do not correlate. Cats with FIP are occasionally serologically negative because of rapidly progressive disease, with a delayed rise in titer, disappearance of antibody in terminal stages of the disease, or immune complex formation. Maternal antibodies decline to undetectable concentrations by 4 to 6 weeks of age; kittens infected in the postnatal period become seropositive at 8 to 14 weeks of age. Thus serologic testing of kittens can be used to prevent the spread of coronaviruses (see below).
Because virus isolation is not practical clinically, RT-PCR is used most frequently to detect coronaviruses in feces. However, positive test results do not differentiate FIP virus from FECV. RNA of both FIP virus and FECV can be amplified from the blood of cats, so positive test results do not always correlate with the development of FIP. Amplification of the mRNA of the M gene by RT-PCR has had mixed results in two studies performed to date (Simons et al., 2005; Can-S Ahna K et al., 2007). In the latter study, 13 of 26 apparently normal cats were positive for FECV mRNA in blood, suggesting that the positive predictive value of this assay for the diagnosis of FIP was low.
Definitive diagnosis of FIP is based on detection of characteristic histopathologic findings, virus isolation, demonstration of the virus in effusions or tissue by use of immunocytochemical or immunohistochemical staining, or demonstration of viral RNA in effusions or tissues by RT-PCR.
Treatment
Because an antemortem diagnosis of FIP is difficult to make, assessment of studies reporting successful treatment is virtually impossible. A small percentage of cats have spontaneous remission, adding to the confusion concerning therapeutic response. Supportive care, including correction of electrolyte and fluid balance abnormalities, should be provided to cats with FIP as needed.
Optimal treatment of cats with FIP would ideally combine virus elimination with suppression of B-lymphocyte function and stimulation of T-lymphocyte function. In vitro inhibition of FIP virus replication has been demonstrated with a number of drugs, including ribavirin, human interferon-α, feline fibroblastic interferon-β, adenine arabinoside, and amphotericin B. However, to date no uniformly successful antiviral treatment has been developed, and the drugs typically have potentially serious adverse effects.
Because disease from FIP is secondary to immune-mediated reactions against the virus, modulation of the inflammatory reaction is the principal form of palliative therapy. Low-dose prednisolone (1 to 2 mg/kg PO q24h) may lessen clinical manifestations of noneffusive FIP. However, the use of immune-suppressive drugs is controversial because cats with FIP have impaired immune responses (Knotek, 2000). The use of prednisolone and feline interferon has recently been promoted for the treatment of both effusive and noneffusive FIP (Isida et al., 2004). In that study four cats with effusive disease believed to be from FIP virus had prolonged remission. However, the results should be viewed cautiously because the cases were atypical (older cats), the diagnosis of FIP was not confirmed, no control group was used and, if a treatment response occurred, whether it was from the prednisolone or interferon-γ was impossible to determine because both drugs were administered to all cats. Procurement of feline interferon is currently difficult in the United States; whether a positive effect could be achieved by use of human interferons is unknown.
Antibiotics do not have primary antiviral effects but may be indicated for the treatment of secondary bacterial infection. Other supportive care treatments, such as anabolic steroids (stanozolol, 1 mg PO q12h), aspirin (10 mg/kg PO q48-72h), and ascorbic acid (125 mg PO q12h) have also been recommended for the treatment of FIP. Most cats with systemic clinical signs of FIP die or require euthanasia within days to months of diagnosis. The effusive form of disease carries a grave prognosis. Depending on the organ system involved and the severity of polysystemic clinical signs, cats with noneffusive disease have variable survival times. Cats with only ocular FIP may respond to antiinflammatory treatment or enucleation of the affected eye(s) and have a better prognosis than cats with systemic FIP.
Prevention and Zoonotic Aspects
Prevention of coronavirus infections is best accomplished by avoiding exposure to the virus. Although viral particles of FIP can survive in dried secretions for up to 7 weeks, routine disinfectants inactivate the virus. Epidemiologic studies suggest the following:
These findings have lead to recommendations that kittens born in a breeding situation with coronavirus-seropositive cats should be housed only with the queen and littermates until sold, should be tested for coronavirus antibodies at 14 to 16 weeks of age, and should be sold only if seronegative. Maintaining a coronavirus-seronegative household and not allowing cats to have contact with other cats would be optimal. Cats can eliminate coronavirus infections; a previously infected cat should be shown to be negative for viral RNA in feces for 5 months and should be seronegative to be considered coronavirus naïve (Addie et al., 2001).
An intranasally administered, mutant strain of coronavirus that induces mucosal immune response but minimal systemic immune response is available (Primucell FIP, Pfizer Animal Health, Exton, Pa.). This strain does not induce FIP; the majority of cats with adverse effects have exhibited only mild signs associated with placement of liquid in the nares, and the vaccine does not appear to potentiate antibody-dependent enhancement of virus infectivity when administered to previously seropositive cats (see Chapter 94). The vaccine appears to be effective in at least some cats, but whether the vaccine protects against all field strains, mutations, or recombinants is unknown. The vaccine is not likely to be effective in cats that have previously been infected by a coronavirus. The only indication for the vaccine is for seronegative cats with risk of exposure to coronaviruses, and the American Association of Feline Practitioners considers the vaccine generally not recommended (see Chapter 94). Zoonotic transfer of FIP virus or FECV to human beings has not been documented.
FELINE IMMUNODEFICIENCY VIRUS
Etiology and Epidemiology
Feline immunodeficiency virus (FIV) is an exogenous, single-strand RNA virus in the family Retroviridae, subfamily Lentivirinae. The virus is morphologically similar to the human immunodeficiency virus (HIV) but it is antigenically distinct. Like FeLV, FIV produces reverse transcriptase to catalyze the insertion of viral RNA into the host genome. Multiple subtypes of the virus exist, and some isolates have differing biologic behavior. For example, immune deficiency is induced much more quickly by some isolates, and clinical diseases, such as uveitis, are induced by some but not all isolates.
Aggressive biting behavior is thought to be the primary route of transmission of FIV; older, male, outdoor cats with clinical signs of disease are most commonly infected. The prevalence of FIV antibodies in North America was 2.5% in a recent study (Levy et al., 2006). FIV is present in semen and can be transmitted by artificial insemination. Transplacental and perinatal transmission occurs from infected queens to kittens. Arthropod transmission appears to be unlikely. Transmission by routes other than biting is less common because high levels of viremia are of short duration. FIV infection of cats has worldwide distribution, and prevalence rates vary greatly by region and the lifestyle of the cats tested. FIV replicates in several cell types, including T-lymphocytes (CD4+ and CD8+, B-lymphocytes, macrophages, and astrocytes. The primary phase of infection occurs as the virus disseminates throughout the body, initially leading to low-grade fever, neutropenia, and generalized reactive lymphadenopathy. A subclinical, latent period of variable length then develops; the length of this period is related in part to the strain of virus and the age of the cat when infected. The median age of healthy, naturally infected cats and clinically ill naturally infected cats is approximately 3 years and 10 years, respectively, suggesting a latent period of years for most strains of FIV. Chronic experimental and naturally occurring infection results in a slow decline in circulating CD4+ lymphocyte numbers, response to mitogens, and decreased production of cytokines associated with cell-mediated immunity, such as interleukin (IL)-2 and IL-10; neutrophil function and natural killer cell function are also affected. Humoral immune responses are often intact, and a polyclonal gammopathy develops from nonspecific B-lymphocyte activation. Within months to years, an immune deficiency stage similar to acquired immunodeficiency syndrome (AIDS) in human beings develops. Coinfection with FeLV potentiates the primary and immune deficiency phases of FIV. However, coinfection with Mycoplasma haemofelis, Toxoplasma gondii, feline herpesvirus, and feline calicivirus, as well as immunization, failed to potentiate FIV-associated immunodeficiency.
Clinical Features
Clinical signs of infection with FIV can arise from direct viral effects or secondary infections that ensue after the development of immunodeficiency (Table 97-2). Most of the clinical syndromes diagnosed in FIV-seropositive cats also occur in FIV-naïve cats, which makes proving disease causation difficult during the subclinical stage of infection. A positive FIV antibody test does not prove immunodeficiency or disease from FIV and does not necessarily indicate a poor prognosis. The only way to determine accurately whether an FIV-seropositive cat with a concurrent infectious disease has a poor prognosis is to treat the concurrent infection.
 TABLE 97-2 Clinical Syndromes Associated with FIV Infection and Possible Opportunistic Agents
TABLE 97-2 Clinical Syndromes Associated with FIV Infection and Possible Opportunistic Agents
| CLINICAL SYNDROME | PRIMARY VIRAL EFFECT | OPPORTUNISTIC AGENTS |
|---|---|---|
| Dermatologic/otitis externa | None | Bacterial; atypical Mycobacterium; Otodectes cynotis; Demodex cati; Notoedres cati; dermatophytosis; Cryptococcus neoformans; cowpox |
| Gastrointestinal | Yes; small-bowel diarrhea | Cryptosporidium spp.; Cystoisospora spp.; Giardia spp.; Salmonella spp.; Campylobacter jejuni; others |
| Glomerulonephritis | Yes | Bacterial; FeLV, FIP, SLE |
| Hematologic | Yes; nonregenerative anemia; neutropenia; thrombocytopenia | M. haemofelis; FeLV; Bartonella henselae? |
| Neoplasia | Yes; myeloproliferative disorders and lymphoma | FeLV |
| Neurologic | Yes; behavioral abnormalities | T. gondii; C. neoformans; FIP; FeLV, B. henselae? |
| Ocular | Yes; pars planitis, anterior uveitis | T. gondii; FIP; C. neoformans, FHV-1, B. henselae |
| Pneumonia/pneumonitis | None | Bacterial; T. gondii; C. neoformans |
| Pyothorax | None | Bacterial |
| Renal failure | Yes | Bacterial; FIP; FeLV |
| Stomatitis | None | Calicivirus; overgrowth of bacteria flora; candidiasis, B henselae? |
| Upper respiratory tract | None | FHV-1; calicivirus; overgrowth of bacterial flora; Cryptococcus neoformans |
| Urinary tract infection | None | Bacterial |
FIV, Feline immunodeficiency virus; FeLV, feline leukemia virus; FIP, feline infectious peritonitis; SLE, systemic lupus erythematosus; FHV-1, feline herpesvirus type 1.
Primary (acute) FIV infection is characterized by fever and generalized lymphadenopathy. Owners commonly present FIV-infected cats in the immunodeficiency stage for evaluation of nonspecific signs such as anorexia, weight loss, and depression or for evaluation of abnormalities associated with specific organ systems. When a clinical syndrome is diagnosed in a cat seropositive for FIV, the workup should include diagnostic tests for other potential causes (see Table 97-2).
Clinical syndromes reportedly from primary viral effects include chronic small-bowel diarrhea, nonregenerative anemia, thrombocytopenia, neutropenia, lymphadenopathy, pars planitis (inflammation in the anterior vitreous humor), anterior uveitis, glomerulonephritis, renal insufficiency, and hyperglobulinemia. Behavioral abnormalities, with dementia, hiding, rage, inappropriate elimination, and roaming, are the most common neurologic manifestations of FIV infection. Seizures, nystagmus, ataxia, and peripheral nerve abnormalities may occasionally be attributable to primary viral effects. Lymphoid malignancies, myeloproliferative diseases, and several carcinomas and sarcomas have been detected in FIV-infected, FeLV-naïve cats, suggesting a potential association between FIV and malignancy; FIV-infected cats are at higher risk for the development of lymphoma. Although FIV is not oncogenic, it predisposes to neoplasia because of its immunosuppressive effects.
Diagnosis
Neutropenia, thrombocytopenia, and nonregenerative anemia are the most common hematologic abnormalities associated with FIV infection. Monocytosis and lymphocytosis occur in some cats and may be caused by the virus or chronic infection with opportunistic pathogens. Cytologic examination of bone marrow aspirates may reveal maturation arrest (i.e., myelodysplasia), lymphoma, or leukemia. A progressive decline in CD4+ lymphocytes, a plateau or progressive increase in CD8+ lymphocytes, and an inversion of the CD4+/CD8+ ratio occurs in experimentally infected cats over time. A multitude of serum biochemical abnormalities is possible depending on what FIV-associated syndrome is occurring. Renal azotemia and polyclonal gammopathy are the changes most likely to be attributable to direct viral effects. No pathognomonic imaging abnormalities are associated with FIV infection.
Antibodies against FIV are detected in serum in clinical practice most frequently by enzyme-linked immunosorbent assay (ELISA). Comparisons between different tests have shown the results of most assays are comparable (Hartmann et al., 2007). Clinical signs can occur before seroconversion in some cats and some infected cats never seroconvert; thus false-negative reactions can occur. Results of virus isolation or PCR on blood are positive in some antibody-negative cats. False-positive reactions are common with ELISA; therefore positive ELISA results in healthy or low-risk cats should be confirmed by Western blot immunoassay or RT-PCR. Kittens can have detectable, colostrum-derived antibodies for several months. Kittens younger than 6 months that are FIV seropositive should be tested every 60 days until the result is negative. If antibodies persist at 6 months of age, the kitten is likely infected. Virus isolation or PCR on blood can also be performed to confirm infection. The biggest problem with FIV RT-PCR assays to date is lack of standardization among laboratories and the potential for both false-positive and false-negative results (Crawford et al., 2005). A vaccine against FIV has been licensed in the United States (see Chapter 94). This vaccine induces antibodies that cannot be distinguished from those induced by naturally occurring disease with currently available tests (see below).
Detection of antibodies against FIV in the serum of cats that have not been vaccinated against FIV documents exposure and correlates well with persistent infection but does not correlate with disease induced by the virus. Because many clinical syndromes associated with FIV can be caused by opportunistic infections, further diagnostic procedures may determine treatable etiologies (see Table 97-2). For example, some FIV-seropositive cats with uveitis are coinfected by T. gondii and often respond to the administration of anti-Toxoplasma drugs (see Chapter 99).
Treatment
Because FIV-seropositive cats are not necessarily immunosuppressed or diseased from FIV, the cat should be evaluated and treated for other potential causes of the clinical syndrome. Some FIV-seropositive cats are immunodeficient; if infectious diseases are identified, bacteriocidal drugs administered at the upper end of the dosage should be chosen. Long-term antibiotic therapy or multiple treatment periods may be required. The only way to determine if an FIVseropositive cat with a concurrent infection has a poor prognosis is to treat the concurrent infection.
A variety of antiviral drugs and immune stimulation therapies have been administered to cats with FIV or FeLV infection (Table 97-3). Administration of interferons has shown promise in some studies. Oral administration of 10 IU/kg of human interferon-α led to improved clinical signs and prolonged survival compared with a placebo-treated control group in one study (Pedretti et al., 2006). In another study feline recombinant interferon was administered at 106 U/kg/day SQ for 5 days in three series (starting on days 0, 14, and 60) and was shown to improve clinical signs early in the study and prolong survival in treated cats (de Mari et al., 2004). Administration of antiviral agents such as the reverse transcriptase inhibitor azidothymidine (AZT) has had mixed success in the treatment of FIV. Use of AZT at a dosage of 5 mg/kg PO or SQ q12h improved overall quality of life and stomatitis in FIV-infected cats and is believed to aid in the treatment of neurologic signs (Hartmann et al., 1995a, 1995b). Cats treated with AZT should be monitored for the development of anemia. Administration of bovine lactoferrin by mouth was beneficial in the treatment of intractable stomatitis in FIV-seropositive cats (Sato et al., 1996). Removal of all premolar and molar teeth has also been effective for treatment of intractable stomatitis in some FIV-seropositive cats (see Chapter 31). Immunomodulators have not been shown to have reproducible clinical effect, but owners sometimes report positive responses. Human recombinant erythropoietin administration increased red blood cell and white blood cell counts, did not increase viral load, and had no measurable adverse clinical effects in FIV-infected cats compared with placebo (Arai et al., 2000). In contrast, although administration of human recombinant granulocyte-monocyte colony-stimulating factor (GM-CSF) to FIV-infected cats increased white blood cell counts in some treated cats, it also induced fever, anti–GM-CSF antibodies, and increased viral load. GM-CSF therefore appears to be contraindicated for the treatment of FIV in cats.
 TABLE 97-3 Drug Treatment Regimens for Viremic, Clinically Ill Cats with FIV or FeLV Infections
TABLE 97-3 Drug Treatment Regimens for Viremic, Clinically Ill Cats with FIV or FeLV Infections
| THERAPEUTIC AGENT* | ADMINISTRATION |
|---|---|
| Acemannan | 2 mg/kg intraperitoneal once weekly for 6 weeks |
| AZT | 5 mg/kg PO or SQ q12h; monitor for the development of anemia |
| Bovine lactoferrin | 175 mg PO in milk or VAL syrup, q12-24h for treatment of stomatitis |
| Erythropoietin | 100 U/kg SQ three times weekly and then titrate to effect |
| Interferon-a* | 10 IU/kg PO q24h as long as effective |
| Interferon-feline | 1 million U, SQ, q24h for 5 days in three series starting on days 0, 14, and 60 |
| Staphylococcus A | 10 μg/kg intraperitoneal twice weekly for 10 weeks and then monthly |
| Propionibacterium acnes | 0.5 mL IV once or twice weekly to effect |
Limited information from controlled studies is available for any of these protocols.
AZT, Azidothymidine; FIV, feline immunodeficiency virus; FeLV, feline leukemia virus.
* Several human interferon-α products are available in the United States.
Modified from Hartmann K et al: Treatment of feline leukemia virus infection with 3′-azido-2,3-dideoxythymidine and human alpha-interferon, J Vet Intern Med 16:345, 2002.
Prevention and Zoonotic Aspects
Housing cats indoors to avoid fighting and testing new cats before introduction to an FIV-seronegative, multiple-cat household will prevent most cases of FIV. Transmission by fomites is unusual because the virus is not easily transmitted by casual contact, is susceptible to most routine disinfectants, and dies when out of the host after minutes to hours, especially when dried. Cleaning litter boxes and dishes shared by cats with scalding water and detergent inactivates the virus. Cats with potential exposure from fighting should be retested 60 days after the potential exposure. Cats that are FIV infected should be housed indoors at all times to avoid exposing FIV-naïve cats in the environment to the virus and to lessen the affected animal’s chance of acquiring opportunistic infections. Kittens queened by FIV-infected cats should not be allowed to nurse to avoid transmission by ingestion of milk. Kittens queened by FIV-infected cats should be shown to be serologically negative at 6 months of age to document failure of lactogenic or transplacental transmission before being sold. A killed vaccine containing immunogens from two FIV isolates was recently licensed for use in the United States (Fel-O-Vax FIV, Fort Dodge Animal Health, Overland Park, Kan). However, the efficacy and safety of the vaccine has not been assessed under field conditions in large numbers of cats, so the American Association of Feline Practitioners considers the vaccine noncore (see Chapter 94). In addition, the vaccine induces antibodies that cannot be distinguished from those induced by natural exposure by currently available tests.
HIV and FIV are morphologically similar but antigenically distinct. Antibodies against FIV have not been documented in the serum of human beings, even after accidental exposure to virus-containing material (Butera et al., 2000). Cats with FIV infection resulting in immunodeficiency may be more likely to spread other zoonotic agents into the human environment; clinically ill, FIV-seropositive cats should therefore undergo a thorough diagnostic evaluation (see Chapter 100).
FELINE LEUKEMIA VIRUS
Etiology and Epidemiology
Feline leukemia virus (FeLV) is a single-strand RNA virus in the family Retroviridae, subfamily Oncovirinae. The virus produces reverse transcriptase, which catalyzes the reaction, resulting in the formation of a DNA copy (provirus) of FeLV viral RNA in the cytoplasm of infected cells; the provirus is inserted into the host cell genome. On subsequent host cell divisions the provirus serves as a template for new virus particles formed in the cytoplasm and is released across the cell membrane by budding. FeLV is composed of several core and envelope proteins. Envelope protein p15e induces immunosuppression. Core protein p27 is present in the cytoplasm of infected cells, peripheral blood, saliva, and tears of infected cats; detection of p27 is the basis of most FeLV tests. The envelope glycoprotein 70 (gp70) contains the subgroup antigens A, B, or C, which are associated with the infectivity, virulence, and disease caused by individual strains of the virus. Neutralizing antibodies are produced by some cats after exposure to gp70. Antibodies against feline oncornavirus-associated cell membrane antigen (FOCMA) are formed by some cats but are generally not used clinically.
The principal route of infection by FeLV is prolonged contact with infected cat saliva and nasal secretions; grooming or sharing common water or food sources effectively results in infection. Because the organism does not survive in the environment, feces, or urine, fomite and aerosol transmission is unlikely. Transplacental, lactational, and venereal transmission is less important than casual contact. FeLV infection has worldwide distribution; the seroprevalence of infection varies geographically and by the population of cats tested. Infection is most common in outdoor male cats between ages 1 and 6 years. In a recent study (Levy et al., 2006) the prevalence of FeLV antigenemia in cats in North America was 2.3%. FeLV can be detected in feces of infected fleas for 2 weeks (Vobis et al., 2006). However, the prevalence rates for FeLV vary little across regions of the United States with high and low prevalence rates of fleas, so this is an unlikely route of infection.
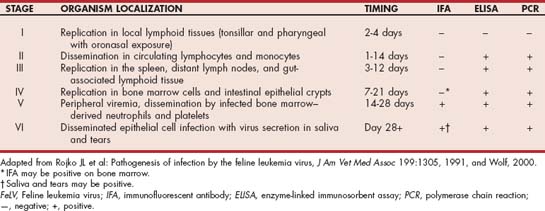
The virus replicates first in the oropharynx, followed by dissemination through the body to the bone marrow (Table 97-4). If persistent bone marrow infection occurs, infected white blood cells and platelets leave the bone marrow with ultimate infection of epithelial structures, including salivary and lacrimal glands. Whether infection occurs after natural exposure to FeLV is determined by the virus subtype or strain, the virus dose, the age of the cat when exposed, and the cat’s immune responses. Using realtime PCR and antigen ELISA results, four classes of FeLV infection were defined (Torres et al. 2005; Levy et al. 2008). Some FeLV-exposed cats can eliminate the infection (abortive) whereas others progress to clinical illness and persistent viremia (progressive). Other FeLV-exposed cats will develop regressive infection characterized by antigen-negative results and lower transiently positive realtime PCR results. Latent FeLV infections are transiently antigen positive but have persistently positive realtime PCR results. Latent and regressive infections can be potentially activated by the administration of glucocorticoids or other immunosuppressive drugs.
The pathogenesis of various syndromes induced by FeLV is complex but includes induction of lymphoma from activation of oncogenes by the virus or insertion of a provirus into the genome of lymphoid precursors; subgroup C induction of aplastic anemia from increased secretion of tumor necrosis factor-α; immunodeficiency attributable to T-lymphocyte depletion (both CD4+ and CD8+ lymphocytes) or dysfunction; neutropenia; neutrophil function disorders; malignant transformation; and viral induction of bone marrow growth-promoting substances leading to myeloproliferative diseases.
Clinical Features
Owners generally present FeLV-infected cats for evaluation of nonspecific signs such as anorexia, weight loss, and depression or abnormalities associated with specific organ systems. Of the FeLV-infected cats evaluated at necropsy, 23% had evidence of neoplasia (96% lymphoma/leukemia); the remainder died from nonneoplastic diseases (Reinacher, 1989). Specific clinical syndromes can result from specific effects of the virus or from opportunistic infections caused by immunosuppression. A positive FeLV test result does not prove disease induced by FeLV. When a clinical syndrome is diagnosed in a FeLV-seropositive cat, the workup should include diagnostic tests for other potential causes. The opportunistic agents discussed for FIV also are common in FeLV-infected cats (see Table 97-2).
Bacterial or calicivirus-induced stomatitis occurs in some FeLV-infected cats as a result of immunosuppression. FeLV infection can result in vomiting or diarrhea from a form of enteritis clinically and histopathologically resembling panleukopenia, from alimentary lymphoma, or from secondary infections attributable to immunosuppression. Icterus in FeLV-infected cats can be prehepatic from immunemediated destruction of red blood cells induced by FeLV or secondary infection by Mycoplasma haemofelis or “Candidatus Mycoplasma haemominutum”; hepatic from hepatic lymphoma, hepatic lipidosis, or focal liver necrosis; or posthepatic from alimentary lymphoma. Some FeLV-infected cats with icterus may be concurrently infected by FIP virus or T. gondii.
Clinical signs of rhinitis or pneumonia occur in some FeLV-infected cats as a result of secondary infections. Dyspnea or dysphagia from mediastinal lymphoma occurs in some cats. These cats are generally younger than 3 years and may have decreased cranial chest compliance on palpation as well as muffled heart and lung sounds if pleural effusion is present.
Mediastinal, multicentric, and alimentary lymphomas are the most common neoplasms associated with FeLV; lymphoid hyperplasia also occurs. Alimentary lymphoma most commonly involves the small intestine, mesenteric lymph nodes, kidneys, and liver of older cats; most cats with alimentary lymphoma are FeLV negative. Renal lymphoma can involve one or both kidneys, which are usually enlarged and irregularly marginated on physical examination. Fibrosarcomas occasionally develop in young cats coinfected with FeLV and feline sarcoma virus. Lymphocytic, myelogenous, erythroid, and megakaryocytic leukemia all are reported with FeLV infection; erythroleukemia and myelomonocytic leukemia are the most common. The history and physical examination findings are nonspecific.
Renal failure occurs in some FeLV-infected cats from renal lymphoma or glomerulonephritis. Affected cats are presented for evaluation of polyuria, polydipsia, weight loss, and inappetence during the last stages of disease. Urinary incontinence from sphincter incompetence or detrusor hyperactivity occurs in some cats; small-bladder nocturnal incontinence is reported most frequently.
Some FeLV-infected cats are presented for miosis, blepharospasm, or cloudy eyes from ocular lymphoma. Aqueous flare, mass lesions, keratic precipitates, lens luxations, and glaucoma are often found on ocular examination. FeLV does not likely induce uveitis without lymphoma. Neurologic abnormalities associated with FeLV infection include anisocoria, ataxia, weakness, tetraparesis, paraparesis, behavioral changes, and urinary incontinence. Nervous system disease is likely to develop as a result of polyneuropathy or lymphoma. Intraocular and nervous system disease in FeLV-infected cats can occur from infection with other agents, including FIP virus, Cryptococcus neoformans, or T. gondii.
Abortion, stillbirth, or infertility occurs in some FeLV-infected queens. Kittens infected in utero that survive to birth generally develop accelerated FeLV syndromes or die as part of the kitten mortality complex.
Some FeLV-seropositive cats present for lameness or weakness from neutrophilic polyarthritis attributed to immune complex deposition. Multiple cartilaginous exostoses occur in some cats and may be FeLV related.
Diagnosis
A variety of nonspecific hematologic, biochemical, urinalysis, and radiographic abnormalities occur in FeLV-infected cats. Nonregenerative anemia alone or in combination with decreases in lymphocyte, neutrophil, and platelet counts is common. The presence of increased numbers of circulating nucleated red blood cells or macrocytosis in association with severe nonregenerative anemia occurs frequently; examination of bone marrow often documents a maturation arrest in the erythroid line (erythrodysplasia). Immune-mediated destruction of erythrocytes can be induced by FeLV and occurs in cats coinfected with hemoplasmas; regenerative anemia, microagglutination or macroagglutination of erythrocytes, and a positive result on the direct Coombs test are common in these cats. Neutropenia and thrombocytopenia occur from bone marrow suppression or immune-mediated destruction. FeLV-infected cats with the panleukopenia-like syndrome have gastrointestinal tract signs and neutropenia and are difficult to differentiate from cats with panleukopenia virus infection or salmonellosis. Cats with FeLV-induced panleukopenia-like syndrome usually have anemia and thrombocytopenia, abnormalities rarely associated with panleukopenia virus infection. Azotemia, hyperbilirubinemia, bilirubinuria, and increased activity of liver enzymes are common biochemical abnormalities. Proteinuria occurs in some FeLV-infected cats with glomerulonephritis. Cats with lymphoma have mass lesions radiographically depending on the organ system affected. Mediastinal lymphoma can result in pleural effusion; alimentary lymphoma can cause obstructive intestinal patterns.
Lymphoma can be diagnosed by cytologic or histopathologic evaluation of affected tissues (see Chapters 75 and 80). Because lymphoma can be diagnosed cytologically and treated with chemotherapy, cats with mediastinal masses, lymphadenopathy, renomegaly, hepatomegaly, splenomegaly, or intestinal masses should be evaluated cytologically before surgical intervention. Malignant lymphocytes are also occasionally identified in peripheral blood smears, effusions, and CSF.
Most cats with suspected FeLV infection are screened for FeLV antigens in neutrophils and platelets by immunofluorescent antibody (IFA) testing or in whole blood, plasma, serum, saliva, or tears by ELISA. Serum is the most accurate fluid to assess in ELISA tests. IFA results are not positive until the bone marrow has been infected (see Table 97-4). The results of IFA testing are accurate more than 95% of the time. False-negative reactions may occur when leukopenia or thrombocytopenia prevents evaluation of an adequate number of cells. False-positive reactions can occur if the blood smears submitted for evaluation are too thick. A positive IFA result indicates that the cat is viremic and contagious; approximately 90% of cats with positive IFA results are viremic for life. The rare combination of IFA-positive and ELISA-negative results suggests technique-related artifact. Negative ELISA results correlate well with negative IFA results and an inability to isolate FeLV. Comparisons of different antigen tests have shown the results of most assays to be comparable (Hartmann et al., 2007).
The virus can be detected in serum by ELISA before infection of bone marrow and can therefore be positive in some cats during early progressive stages of infection or during early latent infection even though IFA results are negative. Other possibilities for discordant results (ELISA positive, IFA negative) are false-positive ELISA results or false-negative IFA results. Cats with positive ELISA results and negative IFA results are probably not contagious at that time but should be isolated until retested 4 to 6 weeks later because progression to persistent viremia and epithelial cell infection may be occurring.
ELISA-positive cats that revert to negative have developed latent infections or regressive infection. Virus isolation, IFA performed on bone marrow cells, immunohistochemical staining of tissues for FeLV antigen, and PCR can be used to confirm latent or regressive infection in some cats. Cats with latent or regressive infection are not likely contagious to other cats, but infected queens may pass the virus to kittens during gestation or parturition or by milk. Cats with regressive or latent infection can be immunodeficient and may become viremic (IFA and ELISA positive) after receiving corticosteroids or after extreme stress.
A delay of 1 to 2 weeks generally occurs after the onset of viremia before ELISA tear and saliva test results become positive; therefore these test results can be negative even when results with serum are positive. Antibody titers to FeLV envelope antigens (neutralizing antibody) and against virus-transformed tumor cells (FOCMA antibody) are available in some research laboratories, but the diagnostic and prognostic significance of results from these tests is unknown. Realtime PCR assays are more sensitive than conventional PCR for FeLV infections, but validated and standardized assays are not currently available in the United States (Torres et al. 2005).
Treatment
Several antiviral agents have been proposed for the treatment of FeLV; the reverse transcriptase inhibitor AZT has been studied the most (see Table 97-3). Unfortunately, administration of AZT to persistently viremic cats does not appear to clear viremia in most, and it had minimal benefits for clinically ill cats in a recent study (Hartmann et al., 2002). Interferons have an effect against FeLV in vivo and in vitro (Collado et al., 2007; de Mari et al., 2004). Immunotherapy with drugs such as Staphylococcus protein A, Propionibacterium acnes, or acemannan (see Table 97-3) improves clinical signs of disease in some cats, but controlled studies are lacking.
Chemotherapy should be administered to cats with FeLV-associated neoplasia (see Chapters 77 and 80). Opportunistic agents should be managed as indicated; the upper dose range and duration of antibiotic therapy are generally required. Supportive therapies such as hematinic agents, vitamin B12, folic acid, anabolic steroids, and erythropoietin generally have been unsuccessful in the management of nonregenerative anemia. Blood transfusion is required in many cases. Cats with autoagglutinating hemolytic anemia require immunosuppressive therapy, but this may activate virus replication. The prognosis for persistently viremic cats is guarded; the majority die within 2 to 3 years.
Prevention and Zoonotic Aspects
Avoiding contact with FeLV by housing cats indoors is the best form of prevention. Potential fomites such as water bowls and litter pans should not be shared by seropositive and seronegative cats. Testing and removal of seropositive cats can result in virus-free catteries and multiple-cat households.
Because of variations in challenge study methods and the difficulty of assessing the preventable fraction of a disease with a relatively low infection rate, long subclinical phase, and multiple field strains, the efficacy of individual vaccines continues to be in question (see Chapter 94). Vaccination of cats not previously exposed to FeLV should be considered in cats at high risk (i.e., contact with other cats), but owners should be warned of the potential efficacy of less than 100%. Cats with persistent FeLV viremia do not benefit from vaccination. Vaccination is related to the development of fibrosarcoma in some cats (see Chapter 94). Cats developing these tumors may be genetically predisposed (Banerji et al., 2007).
FeLV-infected cats should be housed indoors to avoid infecting other cats and avoid exposure to opportunistic agents. Flea control should be maintained to avoid exposure to hemoplasmas, and Bartonella spp. FeLV-infected cats should not be allowed to hunt or be fed undercooked meats to avoid infection by T. gondii, Cryptosporidium parvum, Giardia spp., and other infectious agents carried by transport hosts.
Antigens of FeLV have never been documented in the serum of human beings, suggesting that the zoonotic risk is minimal. However, FeLV-infected cats may be more likely than FeLV-naïve cats to pass other zoonotic agents, such as C. parvum and Salmonella spp., into the human environment.
Amude AM, et al. Clinicopathological findings in dogs with distemper encephalomyelitis presented without characteristic signs of the disease. Res Vet Sci. 2007;82:416.
Elia G, et al. Detection of canine distemper virus in dogs by real-time RT-PCR. J Virol Methods. 2006;136:171.
Greene CE, et al. Canine distemper. In: Greene CE, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Saunders; 2006:25.
Krakowka S, et al. Canine parvoviruses potentiate canine distemper encephalitis attributable to modified live-virus vaccine. J Am Vet Med Assoc. 1982;180:137.
Kubo T, et al. Distribution of inclusion bodies in tissues from 100 dogs infected with canine distemper virus. J Vet Med Sci. 2007;69:527.
Larson LJ, et al. Effect of vaccination with recombinant canine distemper virus vaccine immediately before exposure under shelter-like conditions. Vet Ther. 2006;7:113.
McCandlish IAP, et al. Distemper encephalitis in pups after vaccination of the dam. Vet Rec. 1992;130:27.
Paul MA, et al. 2006 AAHA canine vaccine guidelines. http://www.aahanet.org/About_aaha/About_Guidelines_Canine06.html. accessed
Saito TB, et al. Detection of canine distemper virus by reverse transcriptase-polymerase chain reaction in the urine of dogs with clinical signs of distemper encephalitis. Res Vet Sci. 2006;80:116.
Feline Infectious Peritonitis Virus
Addie DD, et al. Control of feline coronavirus infection in kittens. Vet Rec. 1990;126:164.
Addie DD, et al. A study of naturally occurring feline coronavirus infections in kittens. Vet Rec. 1992;130:133.
Addie DD, et al. Feline coronavirus is not a major cause of neonatal kitten mortality. Fel Pract. 1993;21:13.
Addie DD, et al. Use of a reverse-transcriptase polymerase chain reaction for monitoring the shedding of feline coronavirus by healthy cats. Vet Rec. 2001;148:649.
Benetka V, et al. Prevalence of feline coronavirus types I and II in cats with histopathologically verified feline infectious peritonitis. Vet Microbiol. 2004;99:31.
Boettcher IC, et al. Use of anti-coronavirus antibody testing of cerebrospinal fluid for diagnosis of feline infectious peritonitis involving the central nervous system in cats. J Am Vet Med Assoc. 2007;230:199.
Can-S Ahna K, et al. The detection of feline coronaviruses in blood samples from cats by mRNA RT-PCR. J Feline Med Surg. 2007;9:369.
Foley JE, et al. The inheritance of susceptibility to feline infectious peritonitis in purebred catteries. Fel Pract. 1996;24:14.
Foley JE, et al. Risk factors for feline infectious peritonitis among cats in multiple-cat environments with endemic feline enteric coronavirus. J Am Vet Med Assoc. 1997;210:1313.
Foley JE, et al. Diagnostic features of clinical neurologic feline infectious peritonitis. J Vet Intern Med. 1998;12:415.
Gunn-Moore DA, et al. Detection of feline coronaviruses by culture and reverse transcriptase-polymerase chain reaction of blood samples from healthy cats and cats with clinical feline infectious peritonitis. Vet Microbiol. 1998;62:193.
Harvey CJ, et al. An uncommon intestinal manifestation of feline infectious peritonitis: 26 cases (1986–1993). J Am Vet Med Assoc. 1996;209:1117.
Hartmann K, et al. Comparison of different tests to diagnose feline infectious peritonitis. J Vet Intern Med. 2003;17:781.
Ishida T, et al. Use of recombinant feline interferon and glucocorticoid in the treatment of feline infectious peritonitis. J Feline Med Surg. 2004;6:107.
Paltrinieri S, et al. Some aspects of humoral and cellular immunity in naturally occurring feline infectious peritonitis. Vet Immunol Immunopathol. 1998;65:205.
Paltrinieri S, et al. In vivo diagnosis of feline infectious peritonitis by comparison of protein content, cytology, and direct immunofluorescence test on peritoneal and pleural effusions. J Vet Diagn Invest. 1999;11:358.
Paltrinieri S, et al. Laboratory profiles in cats with different pathological and immunohistochemical findings due to feline infectious peritonitis (FIP). J Fel Med Surg. 2001;3:149.
Pesteanu-Somogyi, et al. Prevalence of feline infectious peritonitis in specific cat breeds. J Feline Med Surg. 2006;8:1.
Poland AM, et al. Two related strains of feline infectious peritonitis virus isolated from immunocompromised cats infected with a feline enteric coronavirus. J Clin Microbiol. 1996;34:3180.
Rohrbach BW, et al. Epidemiology of feline infectious peritonitis among cats examined at veterinary medical teaching hospitals. J Am Vet Med Assoc. 2001;218:1111.
Rottier PJ, et al. Acquisition of macrophage tropism during the pathogenesis of feline infectious peritonitis is determined by mutations in the feline coronavirus spike protein. J Virol. 2005;79:14122.
Saverio P, et al. Critical assessment of the diagnostic value of feline alpha1-acid glycoprotein for feline infectious peritonitis using the likelihood ratios approach. J Vet Diagn Invest. 2007;19:266.
Shelly SM, et al. Protein electrophoresis in effusions from cats as a diagnostic test for feline infectious peritonitis. J Am Anim Hosp Assoc. 1998;24:495.
Simons FA, et al. A mRNA PCR for the diagnosis of feline infectious peritonitis. J Virol Methods. 2005;124:111.
Sparkes AH, et al. Feline infectious peritonitis: a review of clinicopathological changes in 65 cases and a critical assessment of their diagnostic value. Vet Rec. 1991;129:209.
Sparkes AH, et al. An appraisal of the value of laboratory tests in the diagnosis of feline infectious peritonitis. J Am Anim Hosp Assoc. 1994;30:345.
Vennema H, et al. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology. 1998;243:150.
Arai M, et al. The use of human hematopoietic growth factors (rhGM-CSF and rhEPO) as a supportive therapy for FIV-infected cats. Vet Immunol Immunopathol. 2000;77:71.
Butera ST, et al. Survey of veterinary conference attendees for evidence of zoonotic infection by feline retroviruses. J Am Vet Med Assoc. 2000;217:1475.
Crawford PC, et al. Accuracy of polymerase chain reaction assays for diagnosis of feline immunodeficiency virus infection in cats. J Am Vet Med Assoc. 2005;226:1503.
de Mari K, et al. Therapeutic effects of recombinant feline interferon-omega on feline leukemia virus (FeLV)-infected and FeLV/feline immunodeficiency virus (FIV)-coinfected symptomatic cats. J Vet Intern Med. 2004;18:477.
English R, et al. Preliminary report of the ocular manifestations of feline immunodeficiency virus infections. J Am Vet Med Assoc. 1990;196:1116.
Hartmann K, et al. AZT in the treatment of feline immunodeficiency virus infection I. Fel Pract. 1995;23:16.
Hartmann K, et al. AZT in the treatment of feline immunodeficiency virus infection II. Fel Pract. 1995;23:16.
Jordan HL, et al. Shedding of feline immunodeficiency virus in semen of domestic cats during acute infection. Am J Vet Res. 1999;60:211.
Kohmoto M, et al. Eight-year observation and comparative study of specific pathogen-free cats experimentally infected with feline immunodeficiency virus (FIV) subtypes A and B: terminal acquired immunodeficiency syndrome in a cat infected with FIV petaluma strain. J Vet Med Sci. 1998;60:315.
Lappin MR, et al. Primary and secondary Toxoplasma gondii infection in normal and feline immunodeficiency virus-infected cats. J Parasitol. 1996;82:733.
Levy JK, et al. Effect of vaccination against feline immunodeficiency virus on results of serologic testing in cats. J Am Vet Med Assoc. 2004;225:1558.
Levy JK, et al. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in North America and risk factors for seropositivity. J Am Vet Med Assoc. 2006;228:371.
Levy J, et al. 2008 American Association of Feline Practitioners’ feline retrovirus management guidelines. J Fel Med Surg. 2008;10:300.
MacDonald K, et al. Effects of passive transfer of immunity on results of diagnostic tests for antibodies against feline immunodeficiency virus in kittens born to vaccinated queens. J Am Vet Med Assoc. 2004;225:1554.
O’Neil LL, et al. Frequent perinatal transmission of feline immunodeficiency virus by chronically infected cats. J Virol. 1996;70:2894.
Papasouliotis K, et al. Assessment of intestinal function in cats with chronic diarrhea after infection with feline immunodeficiency virus. Am J Vet Res. 1998;59:569.
Pedersen NC, et al. Isolation of a T-lymphotrophic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790.
Pedersen NC, et al. Feline leukemia virus infection as a potentiating cofactor for the primary and secondary stages of experimentally induced feline immunodeficiency virus infection. J Virol. 1990;64:598.
Pedretti E, et al. Low-dose interferon-alpha treatment for feline immunodeficiency virus infection. Vet Immunol Immunopathol. 2006;109:245.
Poli A, et al. Renal involvement in feline immunodeficiency virus infection: a clinicopathological study. Nephron. 1993;64:282.
Sato R, et al. Oral administration of bovine lactoferrin for treatment of intractable stomatitis in feline immunodeficiency virus (FIV)-positive and FIV-negative cats. Am J Vet Res. 1996;57:1443.
Steigerwald ES, et al. Effects of feline immunodeficiency virus on cognition and behavioral function in cats. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:411.
Tasker S, et al. Effect of chronic FIV infection, and efficacy of marbofloxacin treatment, on Mycoplasma haemofelis infection. Vet Microbiol. 2006;117:169.
Tasker S, et al. Effect of chronic feline immunodeficiency infection, and efficacy of marbofloxacin treatment, on “Candidatus Mycoplasma haemominutum” infection. Microbes Infect. 2006;8:653.
Addie DD, et al. Long-term impact on a closed household of pet cats of natural infection with feline coronavirus, feline leukaemia virus and feline immunodeficiency virus. Vet Rec. 2000;146:419.
Arjona A, et al. Evaluation of a novel nested PCR for the routine diagnosis of feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV). J Feline Med Surg. 2007;9:14.
Banerji N, et al. Association of germ-line polymorphisms in the feline p53 gene with genetic predisposition to vaccine-associated feline sarcoma. J Hered. 2007. Jul 19; [Epub ahead of print]
Collado VM, et al. Effect of type I interferons on the expression of feline leukaemia virus. Vet Microbiol. 2007;123:180.
Gomes-Keller MA, et al. Detection of feline leukemia virus RNA in saliva from naturally infected cats and correlation of PCR results with those of current diagnostic methods. J Clin Microbiol. 2006;44:916.
Hartmann K, et al. Treatment of feline leukemia virus infection with 3′-azido-2,3-dideoxythymidine and human alpha-interferon. J Vet Intern Med. 2002;16:345.
Hartmann K, et al. Quality of different in-clinic test systems for feline immunodeficiency virus and feline leukaemia virus infection. J Feline Med Surg. 2007. Jun 30 [Epub ahead of print]
Hayes KA, et al. Incidence of localized feline leukemia virus infection in cats. Am J Vet Res. 1992;53:604.
Herring ES, et al. Detection of feline leukaemia virus in blood and bone marrow of cats with varying suspicion of latent infection. J Fel Med Surg. 2001;3:133.
Hofmann-Lehmann R, et al. Vaccination against the feline leukaemia virus: outcome and response categories and long-term follow-up. Vaccine. 2007;25:5531.
Hoover EA, et al. Feline leukemia virus infection and diseases. J Am Vet Med Assoc. 1991;199:1287.
Kass PH, et al. Epidemiologic evidence for a causal relationship between vaccination and fibrosarcoma tumorigenesis in cats. J Am Vet Med Assoc. 1993;203:396.
Lafrado LJ, et al. Immunodeficiency in latent feline leukemia virus infections. Vet Immunol Immunopathol. 1989;21:39.
McCaw DL, et al. Immunomodulation therapy for feline leukemia virus infection. J Am Anim Hosp Assoc. 2001;37:356.
Pinches MD, et al. An update on FIV and FeLV test performance using a Bayesian statistical approach. Vet Clin Pathol. 2007;36:141.
Pinches MD, et al. Diagnosis of feline leukaemia virus infection by semi-quantitative real-time polymerase chain reaction. J Feline Med Surg. 2007;9:8.
Reinacher M. Feline leukemia virus-associated enteritis: a condition with features of feline panleukopenia. Vet Pathol. 1987;24:1.
Reinacher M. Diseases associated with spontaneous feline leukemia virus (FeLV) infection in cats. Vet Immunol Immunopathol. 1989;21:85.
Rojko JL, et al. Pathogenesis of infection by the feline leukemia virus. J Am Vet Med Assoc. 1991;199:1305.
Sellon R, et al. Therapeutic effects of diethylcarbamazine and 3′-azido-3′-deoxythymidine on feline leukemia virus lymphoma formation. Vet Immunol Immunopathol. 1995;46:181.
Torres AN, et al. Re-examination of feline leukemia virus: host relationships using realtime PCR. Virology. 2005;332:272.
Vobis M, et al. Experimental quantification of the feline leukaemia virus in the cat flea (Ctenocephalides felis) and its faeces. Parasitol Res. 2005;1:S102.