CHAPTER 82 Selected Neoplasms in Dogs and Cats
HEMANGIOSARCOMA IN DOGS
Hemangiosarcomas (HSAs, hemangioendotheliomas, angiosarcomas) are malignant neoplasms that originate from the vascular endothelium. They occur predominantly in older dogs (8 to 10 years of age) and in males; German Shepherd Dogs and Golden Retrievers are at high risk for this neoplasm.
The spleen, right atrium, and subcutis are common sites of involvement at the time of presentation. Approximately 50% of the tumors originate in the spleen, 25% in the right atrium, 13% in subcutaneous tissue, 5% in the liver, 5% in the liver-spleen–right atrium, and 1% to 2% simultaneously in other organs (i.e., kidney, urinary bladder, bone, tongue, prostate). The latter are referred to as multiple tumor, undeterminable primary. In Greyhounds most of the HSAs evaluated have been intramuscular.
In general, the biologic behavior of this neoplasm is highly aggressive, with most anatomic forms of the tumor infiltrating and metastasizing early in the disease. The exception are primary dermal and conjunctival or third eyelid HSAs, which have a low metastatic potential.
Clinical and Clinicopathologic Features
The nature of owners’ complaints and the clinical signs at presentation are usually related to the site of origin of the primary tumor; to the presence or absence of metastatic lesions; and to the development of spontaneous tumor rupture, coagulopathies, or cardiac arrhythmias. More than half of the dogs with HSA are evaluated because of acute collapse after spontaneous rupture of the primary tumor or a metastatic lesion. Some episodes of collapse may stem from ventricular arrhythmias, which are relatively common in dogs with splenic or cardiac HSA. In addition, dogs with splenic HSA often are seen because of abdominal distention secondary to tumor growth or hemoabdomen.
Dogs with cardiac HSA usually are presented for evaluation of right-sided congestive heart failure (caused by cardiac tamponade or obstruction of the posterior vena cava by a neoplasm) or cardiac arrhythmias (see the chapters on cardiovascular system disorders for additional information). Dogs with cutaneous or subcutaneous neoplasms are usually evaluated because of a lump. Greyhounds with intramuscular HSA typically present with a swollen and bruised rear limb; the tumor is frequently in the biceps femoris or quadriceps.
Two common problems in dogs with HSA, regardless of the primary location or stage, are anemia and spontaneous bleeding. The anemia is usually the result of intracavitary bleeding or microangiopathic hemolysis (MAHA), whereas the spontaneous bleeding is usually caused by disseminated intravascular coagulation (DIC) or thrombocytopenia secondary to MAHA (see later discussion). HSA is so highly associated with clinical DIC (see Chapter 87) that at our hospital dogs with DIC of acute onset but without an obvious primary cause are evaluated for HSA first.
Hemangiosarcomas are usually associated with a wide variety of hematologic and hemostatic abnormalities. Hematologic abnormalities in dogs with HSA have been well characterized and include anemia; thrombocytopenia; the presence of nucleated red blood cells (RBCs), RBC fragments (schistocytes), and acanthocytes in the blood smear; and leukocytosis with neutrophilia, a left shift, and monocytosis. In addition, hemostatic abnormalities are also common in dogs with HSAs. However, these hematologic abnormalities are location dependent; for example, in our clinic anemia, thrombocytopenia, schistocytosis, and acanthocytosis were significantly more common in dogs with splenic, right atrial, or visceral HSA than in dogs with subcutaneous or dermal HSA (Alvarez et al., 2006).
Most dogs with HSA (83%) evaluated at our clinic were anemic; more than one half had RBC fragmentation and acanthocytosis (Hammer et al., 1991b). The pretreatment coagulograms of these dogs were normal in only four dogs (17%). Most dogs (75%) had thrombocytopenia, with a mean platelet count of 137,000/μl. Approximately one half of the coagulograms met three or more criteria for diagnosis of DIC, whereas fewer than 12% of them were compatible with microangiopathic thrombocytopenia. Approximately 25% of these dogs died as a result of their hemostatic abnormalities.
Diagnosis
Hemangiosarcomas can be diagnosed cytologically on the basis of the appearance of fine-needle aspirates (FNA) or impression smears. The neoplastic cells are similar to those in other sarcomas in that they are spindle-shaped or polybedral; however, they are quite large; have large nuclei with a lacy chromatin pattern and one or more nucleoli; and a bluish gray, usually vacuolated cytoplasm (Fig. 82-1). Nucleated RBCs are frequently present cytologically in HSAs. Although HSA cells are relatively easy to identify in tissue aspirates or impression smears, they are extremely difficult to identify in HSA-associated effusions. The probability of establishing a cytologic diagnosis of HSA after evaluating effusions is less than 25%. A further problem with effusions is that a specimen may contain reactive mesothelial cells that may resemble neoplastic cells, leading to a false-positive diagnosis of HSA.
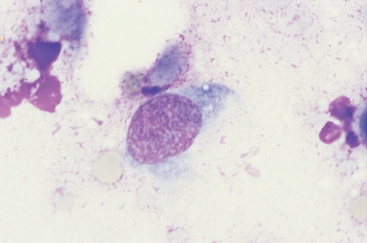
FIG 82-1 Cytologic features of canine hemangiosarcoma. Note the spindle-shaped cells, with a dark, vacuolated cytoplasm, and the fine nuclear chromatin pattern with prominent nucleolus. (×1000.)
In general, a presumptive clinical or cytologic diagnosis of HSA should be confirmed histopathologically. Because of the large size of some splenic HSAs, however, multiple samples (from different morphologic areas) should be submitted in appropriate fixative. Histochemically, HSA cells are positive for von Willebrand factor antigen in approximately 90% of the cases; CD31 is a relatively new marker of endothelial origin positive in most HSAs.
Metastatic sites can be detected radiographically, ultrasonographically, or on computed tomography (CT). Our routine staging system for dogs with HSA includes a complete blood count (CBC), serum biochemistry profile, hemostasis screen, urinalysis, thoracic radiographs, abdominal ultrasonography, and echocardiography. The latter is used to identify cardiac masses and determine the baseline fractional shortening before instituting doxorubicin-containing chemotherapy (see the section on treatment and prognosis).
Thoracic radiographs in dogs with metastatic HSA are typically characterized by the presence of interstitial or alveolar infiltrates, as opposed to the common “cannonball” metastatic lesions seen with other tumors. The radiographic pattern may be due to true metastases or to DIC and intrapulmonary bleeding, or adult respiratory distress syndrome (ARDS).
Ultrasonography constitutes a reliable way to evaluate dogs with suspected or confirmed HSA for intraabdominal disease. Neoplastic lesions appear as nodules with variable echogenicity, ranging from anechoic to hyperechoic (Fig. 82-2). Hepatic metastatic lesions can often be identified using this imaging technique. However, the clinician should bear in mind that what appear to be metastatic nodules in the liver of a dog with a splenic mass may represent regenerative hyperplasia rather than true metastatic lesions. Contrast ultrasonography appears to enhance the operator’s ability to detect hepatic metastatic nodules from HSA.
Treatment and Prognosis
Historically, the mainstay of treatment for dogs with HSA has been surgery, although the results have been poor. Survival times vary with the location and stage of the tumor, but in general (with the exception of dermal and conjunctival or third eyelid HSAs), they are quite short (approximately 20 to 60 days, with a 1-year survival rate of <10%). Results of treatment combining surgery and postoperative adjuvant chemotherapy with doxorubicin; doxorubicin and cyclophosphamide (AC protocol); and vincristine, doxorubicin, and cyclophosphamide (VAC protocol) are better than with surgery alone. Median survival times range from 140 to 202 days.
The median survival times of dogs with HSA treated with the VAC protocol (see box on cancer chemotherapy protocols in Chapter 81) are approximately 190 days, with a 30% 1-year survival rate. Interestingly, in a recent study conducted in our clinic, the presence of metastasis was not a negative prognostic factor in dogs with HSA receiving VAC chemotherapy (Alvarez et al., 2007). Dogs with metastatic HSA had a 70% response rate (Fig. 82-3). Adverse effects associated with this protocol include myelosuppression, gastroenteritis, alopecia and hyperpigmentation, and cardiotoxicity. There was no apparent difference in the survival times between dogs with bulky disease (i.e., no surgical cytoreduction) and those that had undergone surgery. Similar results were reported for dogs treated either with doxorubicin and cyclophosphamide or with doxorubicin alone; however, in my experience, the prognosis for dogs with HSA is better if a three-drug combination, instead of a two-drug combination or monochemotherapy, is used. In our clinic we have rarely been able to administer more than 3 or 4 doses of single agent doxorubicin in dogs with HSA because they have already relapsed. The coagulopathies in HSA patients should be managed simultaneously, as discussed in Chapter 85.
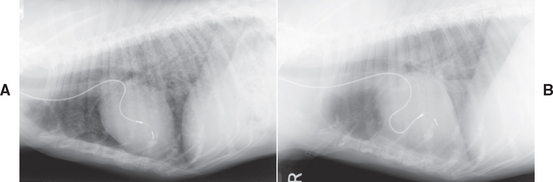
FIG 82-3 Thoracic radiographs of a 10-year-old, spayed female German Shepherd Dog with pulmonary metastases from a primary splenic hemangiosarcoma before (A) and 9 weeks after initiating VAC chemotherapy (B). Notice the complete disappearance of the pulmonary nodules. The radiopaque line is the lead of a permanent pacemaker.
Biologic response modifiers and antiangiogenic factors have also been used in dogs with HSA in combination with doxorubicin-containing chemotherapy. Dogs receiving liposome-encapsulated MTP (muramyl tripeptide—the active immunomodulatory molecule in bacille Calmette-Guérin [BCG]) and AC chemotherapy after splenectomy for HSA had significantly longer survival times (277 days) than those receiving chemotherapy and placebo (144 days; Vail et al., 1995). However, liposomal MTP is not readily available to the practicing veterinarian. Recently, minocycline, an antiangiogenic antibiotic, used at a dosage of 5 mg/kg by mouth every 24 hours, was added to the AC protocol in dogs with HSA (Sorenmo et al., 2000); the median survival time was 170 days, similar to the median survival times obtained when using chemotherapy alone.
In summary, HSAs are usually diagnosed on the basis of historical, physical examination, and clinicopathologic findings, in conjunction with ultrasonographic and radiographic changes. A morphologic diagnosis can usually be made on the basis of cytologic or histopathologic findings. Although surgery is the preferred treatment, survival times in such animals are extremely short (except in dogs with dermal or conjunctival/third eyelid HSA). Postoperative adjuvant chemotherapy using doxorubicin-containing protocols prolongs survival in dogs with this malignancy.
OSTEOSARCOMA IN DOGS AND CATS
Etiology and Epidemiology
Primary bone neoplasms are relatively common in dogs but rare in cats. Most primary bone tumors in dogs are malignant in that they usually cause death as a result of local infiltration (e.g., pathologic fractures or extreme pain leading to euthanasia) or metastasis (e.g., pulmonary metastases in osteosarcoma [OSA]). In cats most primary bone neoplasms, although histologically malignant, are cured by wide surgical excision (i.e., amputation). Neoplasms that metastasize to the bone are rare in dogs; some that occasionally metastasize to bones in dogs are transitional cell carcinoma of the urinary tract, osteosarcoma of the appendicular skeleton, hemangiosarcoma, mammary adenocarcinoma, and prostatic adenocarcinoma. Bone metastases are exceedingly rare in cats.
Osteosarcomas are the most common primary bone neoplasm in dogs. They can affect either the appendicular or axial skeletons, and they occur primarily in large- and giant-breed dogs and in Greyhounds; they are common in middle-age to older dogs. There is a distinct genetic predisposition to OSA in dogs; for example, in former racing Greyhounds OSA is the most common cause of death (i.e.; 25%), whereas OSAs are extremely rare in show Greyhounds in the U.S. The biologic behavior of OSA is characterized by aggressive local infiltration of the surrounding tissues and rapid hematogenous dissemination (usually to the lungs). Although historically it was believed that OSAs of the axial skeleton had a low metastatic potential, it now appears that their metastatic rate is similar to that of the appendicular OSAs.
Clinical Features
Appendicular OSAs occur predominantly in the metaphyses of the distal radius, distal femur, and proximal humerus, although other metaphyses can also be affected. As just mentioned, they typically affect Greyhounds and male dogs of large (and giant) breeds, and owners seek veterinary care because of lameness or swelling of the affected limb. Physical examination usually reveals a painful swelling in the affected area, with or without soft tissue involvement. The pain and swelling can be acute in onset, leading to the presumptive diagnosis of a nonneoplastic orthopedic problem and thus considerably delaying diagnosis and definitive therapy for the neoplasm. Pathologic fractures are common in Greyhounds with OSA but rare in other breeds.
Diagnosis
Radiographically, OSAs exhibit a mixed lytic-proliferative pattern in the metaphyseal region of the affected bone (Fig. 82-4). Adjacent periosteal bone formation leads to the development of the so-called Codman’s triangle, which is composed of the cortex in the affected area and the periosteal proliferation. OSAs typically do not cross the articular space, but occasionally they can infiltrate adjacent bone (e.g., ulnar lysis resulting from an adjacent radial OSA). Because other primary bone neoplasms and some osteomyelitis lesions can mimic the radiographic features of OSAs, cytology or biopsy specimens of every lytic or lytic-proliferative bone lesion should be obtained before the owners decide on a specific treatment. An exception to this rule is an owner who has already decided that amputation is the initial treatment of choice for that lesion (i.e., the limb is amputated and the lesion is submitted for histopathologic evaluation).
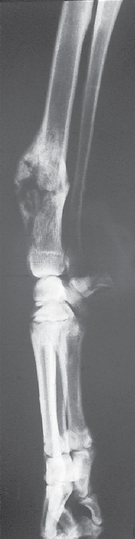
FIG 82-4 Radiographic appearance of a typical osteosarcoma of the radius in a dog. Note the lytic and proliferative changes characteristic of this neoplasm.
(Courtesy RM Gamblin.)
Once a presumptive radiographic diagnosis has been established and if the owners are contemplating treatment, thoracic and/or bone (i.e., skeletal survey) radiographs should be obtained to determine the extent of the disease. We usually obtain three radiographic views of the thorax and do not perform a skeletal radiographic survey (or radionuclide bone scan). Fewer than 10% of dogs with OSA initially have radiographically detectable lung lesions; the presence of metastases is a strong negative prognostic factor.
The radiographic diagnosis can be confirmed before surgery (i.e., limb amputation or limb salvage) on the basis of the findings yielded either by FNA or by aspiration of the affected area using a bone marrow aspiration needle. In most cases a blind percutaneous FNA can be performed with only manual restraint; if the operator cannot penetrate through the cortex, ultrasonographic guidance almost always allows visualization of a “window” through which the needle is inserted. OSA cells are usually round or oval; have distinct cytoplasmic borders; have a bright blue, granular cytoplasm; and have eccentric nuclei with or without nucleoli (Fig. 82-5); multinucelatedgiant cells are common, and there is frequenly pink amorphous material (osteoid) in the background or in the cytoplasm of the osteoblasts. If the round cells cannot be convincingly identified as osteoblast, most diagnostic laboratories can perform an alkaline phosphatase (ALP) cytochemical stain in unstained slides; osteoblasts are typically ALP-positive. A preamputation diagnosis can also be made after histopathologic evaluation of core biopsy specimens from the affected areas. To obtain a bone biopsy, a 13- or 11-gauge Jamshidi bone marrow biopsy needle (Monoject) is used with the animal under general anesthesia, and a minimum of two (and preferably three) cores of tissue are obtained from both the center of the lesion and the area between affected and unaffected bone. The diagnostic yield of this procedure is quite high (approximately 70% to 75%). In our clinic we obtain cytologic diagnoses in the vast majority of patients with OSA; we rarely need to perform a biopsy in order to confirm a diagnosis.
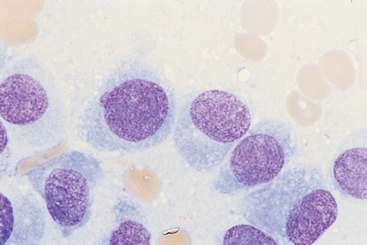
FIG 82-5 Characteristic cytologic features of osteosarcoma in a fine-needle aspirate of a lytic/proliferative lesion in the proximal scapula of a 12-year-old Wirehaired Terrier. Note the round to oval eccentric nuclei with a fine chromatin pattern and prominent nucleoli. (×1000.)
As long as the owners understand the biologic behavior of the neoplasm (i.e., the high likelihood of their dog dying of metastatic lung disease within 4 to 6 months of amputation if no chemotherapy is used) and as long as the clinical and radiographic features of the lesion are highly suggestive of OSA, the limb can be amputated in the absence of a histopathologic diagnosis. The amputated leg (or representative samples) and the regional lymph nodes should always be submitted for histopathologic evaluation. The presence of pulmonary or lymph node metastases is a negative prognostic factor for survival in dogs with OSA.
Treatment and Prognosis
The treatment of choice for dogs with OSA is amputation with adjuvant single-agent or combination chemotherapy. The median survival time in dogs with appendicular OSA treated with amputation alone is approximately 4 months, whereas in dogs treated with amputation and cisplatin, amputation and carboplatin, amputation and doxorubicin, or amputation and combination chemotherapy it is approximately 1 year. The dosages for chemotherapy in dogs with OSA are given in the box on cancer chemotherapy protocols at the end of this chapter and Box 82-1. At our hospital we use either doxorubicin or carboplatin immediately after amputation for a total of five and four treatments, respectively. With the advent of generic carboplatin, the cost is now acceptable to most owners.
 BOX 82-1 Chemotherapy Protocols and Palliative Treatment for Dogs with Osteosarcoma
BOX 82-1 Chemotherapy Protocols and Palliative Treatment for Dogs with Osteosarcoma
IV,Intravenous; CRI, continuous rate infusion; PO, by mouth.
* Other nonsteroidal antiinflamatories are also effective.
An alternative therapeutic approach for dogs with distal radial or ulnar OSAs consists of sparing the limb in affected dogs. Instead of amputation, the affected bone is resected and an allograft from a cadaver (or a prosthetic device) is used to replace the neoplastic bone; novel biomaterials are also currently being investigated for this purpose. The dogs are also treated with intravenous (IV) chemotherapy and, in general, have almost normal limb function. Survival times in dogs treated with limb-sparing procedures are comparable to those in dogs that undergo amputation plus chemotherapy, with the added benefit to the owners of having a four-legged pet. The main complication is the development of osteomyelitis in the allograft; if that occurs, the limb frequently needs to be amputated. However, in patients with infected allografts that eventually undergo amputation, the survival times are significantly longer than in dogs that did not experience complications (Lascelles et al., 2005).
If owners are reluctant to allow the veterinarian to amputate the limb, local radiotherapy plus chemotherapy may be beneficial. We usually avoid using doxorubicin as the chemotheraputic agent to prevent radiosensitization and severe cutaneous reactions to irradiation. In addition to radiation therapy, we use bisphosphonates (pamidronate 1-2 mg/kg, IV constant rate infusion, q2-4 weeks) and analgesics (see Box 82-1) for pain control and palliative care.
Chemotherapy may modify the biologic behavior of the tumor, resulting in a higher prevalence of bone metastases and a lower prevalence of pulmonary metastases. Moreover, the doubling time (i.e., growth rate) of metastatic lesions appears to be longer than that in dogs that have not received chemotherapy, and there appear to be fewer metastatic nodules in treated than in untreated dogs. Therefore surgical removal of the metastatic nodules (i.e., metastasectomy) followed by additional chemotherapy may be recommended for a dog that has been treated with chemotherapy after amputation of the limb and in which one to three pulmonary metastatic lesions are detected (O’Brien et al., 1993).
As discussed in previous paragraphs, the treatment of choice for OSAs in cats is limb amputation alone. Extremely long survival times (in excess of 2 years) are common in such cats. As discussed in Chapter 69, cisplatin is extremely toxic in cats and should therefore not be used in this species. If necessary, carboplatin or doxorubicin can be used instead.
MAST CELL TUMORS IN DOGS AND CATS
Mast cell tumors (MCTs) are among the most common skin tumors in dogs and are relatively common in cats. They originate from mast cells, which are intimately involved in the local control of vascular tone and which contain a large array of intracytoplasmic bioactive molecules, including heparin, histamine, leukotrienes, and several cytokines. Given their unpredictable biologic behavior, the term mast cell tumor is preferred to mastocytoma or mast cell sarcoma. Because of differences in the clinical and pathologic features of canine and feline MCTs, they are discussed separately.
MAST CELL TUMORS IN DOGS
Etiology and Epidemiology
MCTs constitute approximately 20% to 25% of the skin and subcutaneous tumors seen by practicing veterinarians. Brachiocephalic breeds (Boxer, Boston Terrier, Bull Mastiff, English Bulldog) are at high risk for MCTs. These tumors are also more common in middle-age to older dogs (mean age, approximately 8.5 years) than in younger dogs, but there is no gender-related predilection. MCTs have been found in sites of chronic inflammation or injury, such as burn scars.
Clinical and Pathologic Features
MCTs occur either as dermoepidermal masses (i.e., a superficial mass that moves with the skin) or subcutaneous masses (i.e., the overlying skin moves freely over the tumor). Grossly, MCTs can mimic any primary or secondary skin lesion, including a macula, papula, nodule, tumor, and crust. Approximately 10% to 15% of all MCTs in dogs are clini-cally indistinguishable from the common subcutaneous lipomas. As a rule, an MCT cannot be definitively diagnosed until the lesion has been evaluated cytologically or histopathologically.
Most MCTs are solitary, although multifocal MCTs can occur in dogs. Regional lymphadenopathy caused by metastatic disease is also common in dogs with invasive MCTs. Occasionally, splenomegaly or hepatomegaly is present in dogs with systemic dissemination.
Given the fact that mast cells produce a variety of bioactive (mainly vasoactive) substances, dogs with MCTs may be evaluated because of diffuse swelling (i.e., edema and inflammation around a primary tumor or its metastatic lesion), erythema, or bruising of the affected area. These episodes may be acute, and they may occur during or shortly after exercise or exposure to cold weather. Percutaneous FNA of an unexplained subcutaneous swelling in dogs should always be performed as part of the workup.
A “typical” MCT is a dermoepidermal, dome-shaped, alopecic, and erythematous lesion. However, as discussed in previous paragraphs, MCTs rarely have a typical appearance. A clinical feature that may aid in the diagnosis of an MCT is Darier’s sign, which is the erythema and wheal that form after the tumor is slightly traumatized (i.e., scraped or compressed).
Most dogs with MCTs have a normal CBC, although eosinophilia (sometimes marked), basophilia, mastocythemia, neutrophilia, thrombocytosis, or anemia (or a combination of these) may be present. Serum biochemistry abnormalities are uncommon.
From a histopathologic standpoint, MCTs are traditionally classified into three categories: well differentiated (grade 1), moderately differentiated (grade 2), and poorly differentiated (grade 3). Several studies have shown that dogs with grade 1 tumors treated with surgery or radiotherapy have longer survival times than identically treated dogs with grade 3 tumors, mainly because well-differentiated neoplasms have a lower metastatic potential (i.e., most tumors in dogs with systemic mast cell disease are grade 3). Special stains may be required to identify the typical intracytoplasmic granules in poorly differentiated neoplasms. The mitotic index is of prognostic relevance in dogs with MCTs, so it should be provided by the pathologist (Romansik et al., 2007). In addition to the grading of the tumor, the pathologist should provide the clinician with information regarding the completeness of the excision. A dog with an incompletely excised MCT is rarely cured by the initial surgical procedure and requires either a second surgery or irradiation of the affected area.
From a molecular standpoint, a variable percentage of canine MCTs have c-kit mutations; c-kit is the stem cell growth factor receptor, and its mutation results in immortalized clones that do not undergo apoptosis (Jones et al., 2004).
Biologic Behavior
The biologic behavior of canine MCTs can be summed up in one word: unpredictable. Even though several criteria may help in establishing the biologic behavior of these neoplasms, they rarely apply to an individual dog (i.e., they may be meaningful from the statistical viewpoint).
In general, well-differentiated (grade 1), solitary cutaneous MCTs have a low metastatic potential and low potential for systemic dissemination. However, the clinician may encounter a dog with several dozen cutaneous MCTs, which on histopathologic evaluation are well differentiated.
Grade 2 and 3 tumors have a higher metastatic potential and a higher potential for systemic dissemination than grade 1 MCTs. Metastases to the regional lymph nodes commonly occur (particularly in dogs with grade 3 tumors), although occasionally a tumor “skips” the draining lymph node and metastasizes to the second or third regional node (e.g., a digital MCT in the rear limb metastasizing to the iliac or sublumbar node). Because nodal metastases can be present in normal-size lymph nodes, every lymph node in the region of an MCT should be aspirated regardless of whether it is enlarged or not. Pulmonary metastases are extremely rare. Although not evident from published clinical data, it appears that MCTs in certain anatomic locations are more aggressive than tumors in other areas. For example, distal limb (e.g., toe), perineal, inguinal, and extracutaneous (e.g., oropharyngeal, intranasal) MCTs appear to have a higher metastatic potential than similarly graded tumors in other regions (e.g., trunk, neck).
Another biologic characteristic of canine MCTs is that they may become systemic, behaving like a hematopoietic malignancy (i.e., a lymphoma or leukemia). These dogs usually have a history of a cutaneous MCT that was excised. Most dogs with systemic mast cell disease (SMCD) are evaluated because of lethargy, anorexia, vomiting, and weight loss in association with splenomegaly, hepatomegaly, pallor, and (occasionally) detectable cutaneous masses. The CBC in affected dogs commonly reveals cytopenias, with or without circulating mast cells.
MCTs can release bioactive substances that may cause edema, erythema, or bruising of the affected area. Gastrointestinal tract ulceration may also occur as a result of hyperhistaminemia (approximately 80% of dogs euthanized because of advanced MCTs have gastroduodenal ulceration). Therefore any dog with an MCT should undergo occult fecal blood testing. Profuse intraoperative and postoperative bleeding and delayed wound healing occur in some dogs as a consequence of the bioactive substances released from mast cells.
Diagnosis
The evaluation of a dog with a suspected MCT should include FNA of the affected area. MCTs are extremely easy to diagnose cytologically. They consist of a monomorphic population of round cells with prominent intracytoplasmic purple granules; eosinophils are frequently present in the smear (see Fig. 75-6). In approximately one third of MCTs, the granules do not stain with Diff-Quik; hence if agranular round cells are found in a dermal or subcutaneous mass resembling an MCT, the clinician should stain the slide with Giemsa or Wright’s stain to reveal the characteristic purple granules. A cytologic diagnosis of MCT allows the clinician to discuss treatment options with the owner and to plan therapeutic strategies (see the section on treatment and prognosis).
Although clinical pathologists frequently state the degree of differentiation of the cells in a cytologic specimen of an MCT, that scheme does not necessarily correlate with the histopathologic grading system. In other words, a cytologic diagnosis of a well-differentiated MCT does not necessarily imply that it will be a grade 1 tumor when evaluated histopathologically (i.e., cytologic grading may not have the same prognostic implications as histopathologic grading).
The clinical evaluation of a dog with a cytologically confirmed MCT should include careful palpation of the affected area and its draining lymph nodes; abdominal palpation, radiography, or ultrasonography to search for hepatosplenomegaly; a CBC, serum biochemistry profile, and urinalysis; and thoracic radiography if the neoplasm is in the anterior one half of the body (i.e., to detect intrathoracic lymphadenopathy). If lymphadenopathy, hepatomegaly, or splenomegaly is present, FNA of the enlarged lymph node or organ should be performed to detect mast cells (i.e., local neoplasm versus metastatic tumor versus SMCD).
The use of a buffy coat smear to search for circulating mast cells is controversial. It was thought that the presence of mast cells in a buffy coat smear indicated systemic dissemination and therefore a poor prognosis. However, dogs with a solitary, potentially curable MCT occasionally have low numbers of circulating mast cells that disappear from circulation shortly after the primary tumor is excised or irradiated. Moreover, a recent study revealed that circulating mast cells are more common in dogs with diseases other than MCTs; over 95% of the CBCs with circulating mast cells were from dogs with inflammatory disorders, regenerative anemia, tumors other than MCTs, and trauma (McManus, 1999). Also, dogs with MCT had significantly lower circulating mast cell counts (71 per buffy coat smear) than those with other diseases (276 per buffy coat smear). Cytologic evaluation of a bone marrow aspirate may therefore be more beneficial for staging purposes. Dogs with more than five mast cells per 500 nucleated cells are believed to have SMCD; however, bone marrow mast cells have also been documented to disappear after excision or irradiation of the primary tumor. Therefore the appropriate staging procedures in dogs with MCTs remains controversial. At our clinic we do not use buffy coat smears or bone marrow aspirates routinely in dogs with MCT and a normal CBC; if cytopenias or leukoerythroblastic reactions are present, we perform a bone marrow aspirate.
As discussed previously, all dogs with MCTs should be tested for occult blood in the stool even if melena is not evident. There are several kits for this purpose. The presence of blood in the stool is suggestive of upper gastrointestinal tract bleeding. If this is found on repeat testing, the dog should be treated with H2 antihistamines (i.e., famotidine, ranitidine) with or without a coating agent (i.e., sucralfate; see Chapter 30). Once this clinical information is obtained, the tumor should be staged to determine the extent of disease (Table 82-1).
 TABLE 82-1 Clinical Staging Scheme for Dogs with Mast Cell Tumors
TABLE 82-1 Clinical Staging Scheme for Dogs with Mast Cell Tumors
| STAGE | DESCRIPTION |
|---|---|
| I | |
| II | |
| III | |
| IV |
Treatment and Prognosis
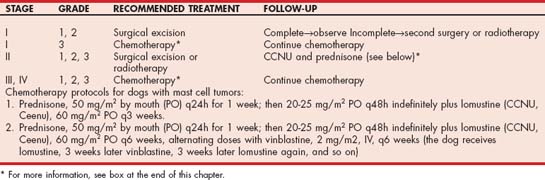
As discussed previously, it is imperative to know whether the mass the clinician is preparing to excise is an MCT because this information is useful when discussing treatment options with the client and when planning the treatment strategy. Dogs with MCT can be treated with surgery, radiotherapy, chemotherapy, or a combination of these. However, the first two treatment options are potentially curative, whereas chemotherapy is usually only palliative. Treatment guidelines are provided in Table 82-2.
A solitary MCT in an area in which complete surgical excision is feasible should be removed by aggressive en bloc resection (i.e., 2- to 3-cm margins around and underneath the tumor). If a complete excision is obtained (according to the pathologist evaluating the specimen), the tumor is grade 1 or 2, and no metastatic lesions are present; there is usually no need for further treatment (i.e., the dog is most likely cured). If the excision appears incomplete, the clinician can take one of three courses of action: (1) perform a second surgery in an attempt to excise the remaining tumor (the excised area should be submitted for histopathologic evaluation to assess the completeness of excision); (2) irradiate the surgical site (35 to 40 Gy delivered in 10 to 12 fractions); or (3) administer a short course (3-6 months) of lomustine chemotherapy (discussed later). The three options appear to be equally effective, resulting in approximately an 80% probability of long-term survival.
A solitary MCT in an area in which surgical excision is difficult or impossible, or at a site where the cosmetic or functional results are unacceptable (e.g., prepuce, eyelid), can be successfully treated with radiotherapy. Approximately two thirds of dogs with a grade 1 or 2 localized MCT treated with radiotherapy alone are cured. Irradiation is also recommended for the management of tumors in high-risk areas. Intralesional injections of corticosteroids (triamcinolone [Vetalog], 1 mg intralesionally per centimeter of tumor diameter q2-3 weeks) can also successfully shrink the tumor (although it is usually only palliative). Intralesional injections of deionized water have also been reported to be beneficial in managing local MCTs, although that has not been my experience. An alternative approach is to use neodajuvant chemotherapy (ie; chemotherapy before and after surgery). In these dogs a combination of lomustine and prednisone is used, with or without vinblastine, in order to decrease the tumor size; then surgery is performed, followed by chemotherapy (discussed later).
Once metastatic or disseminated MCTs (or SMCD) develop, a cure is rarely obtained. Treatment in these dogs consists of chemotherapy and supportive therapy and is aimed at palliating the neoplasm and its complications. Results of prospective studies of chemotherapy in dogs with MCTs have not been very encouraging; two chemotherapy protocols have been widely used (see box on cancer chemotherapy protocols at the end of this chapter): (1) prednisone and (2) the CVP protocol (cyclophosphamide, prednisone, vinblastine). Over the past several years, lomustine (CCNU) has been used with a high degree of success in dogs with nonresectable, metastatic, or systemic MCTs. The probability of response is high >50%), and remissions in excess of 18 months in dogs with metastatic grade 2 and 3 MCTs have been documented. Lomustine can be combined with prednisone, vinblastine, or both (see Table 82-2).
Traditionally, I used lomustine, with or without prednisone (see Table 82-2), and famotidine and/or sucralfate in dogs with metastatic or nonresectable MCTs. Although lomustine is potentially myelosuppressive, clinically relevant cytopenias are rare; however, hepatotoxicity is common (see Chapter 78), so chemistry profiles should be evaluated periodically. The addition of vinblastine allows administration of lomustine every 6 weeks instead of every 3 weeks; this may decrease the prevalence of hepatotoxicity.
Small molecule tyrosine kinase inhibitors have demonstrated efficacy against some canine MCTs with c-kit mutations and will likely be available in the near future (Pryer et al., 2003; London et al., 2003)
MAST CELL TUMORS IN CATS
Etiology and Epidemiology
Although MCTs are relatively common in cats, they rarely result in the considerable clinical problems seen in dogs with this neoplasm. Most cats with MCTs are middle-age or older (median, 10 years old), there is apparently no gender-related predilection, and Siamese cats may be at high risk. Feline leukemia virus and feline immunodeficiency virus do not play a role in the development of this tumor.
As opposed to the dog, in which most of the MCTs are cutaneous or subcutaneous, cats exhibit two main forms of feline MCTs: visceral and cutaneous. There is controversy as to whether cutaneous forms are more common than visceral forms and whether both forms can co-exist in the same cat. At our clinic the cutaneous form is considerably more common than the visceral form, and it is extremely rare for the cutaneous and visceral forms to coexist.
Clinical and Pathologic Features
Visceral MCTs are characterized by either hemolymphatic or intestinal involvement. Cats with hemolymphatic disease are classified as having SMCD (or mast cell leukemia) because the bone marrow, spleen, liver, and blood are commonly involved. Most cats initially have nonspecific signs such as anorexia and vomiting; abdominal distention caused by massive splenomegaly is a consistent feature. As in dogs, the hematologic abnormalities in cats with SMCD are extremely variable and include cytopenias, mastocythemia, basophilia, eosinophilia, or a combination of these; however, a high percentage of cats may have normal CBCs. Cats with the intestinal form of SMCD usually are evaluated because of gastrointestinal signs such as anorexia, vomiting, or diarrhea. Abdominal masses are palpated in approximately one half of these cats. Most tumors involve the small intestine, where they can be solitary or multiple. Metastatic disease affecting the mesenteric lymph nodes, liver, spleen, and lungs is commonly found at the time of presentation. Multiple intestinal masses in cats are most commonly associated with lymphoma and with MCT, although both neoplasms can co-exist. Gastrointestinal tract ulceration has also been documented in affected cats.
Cats with cutaneous MCTs usually initially have solitary or multiple, small (2 to 15 mm), white to pink dermoepidermal masses primarily in the head and neck regions, although solitary dermoepidermal or subcutaneous masses also occur in other locations. It has been reported that, on the basis of the clinical, epidemiologic, and histologic features, MCTs in cats can be classified as either mast cell–type MCTs (common) or histiocytic-type MCTs (rare). Cats with mast cell–type MCTs are usually older than 4 years of age and have solitary dermal masses; there is no apparent breed predilection. Cats with histiocytic-type MCTs are primarily Siamese cats younger than 4 years of age. Typically, such cats have multiple (miliary) subcutaneous masses that exhibit a benign biologic behavior. Some of these neoplasms appear to regress spontaneously. We have never seen the histiocytic type of disease in cats treated at our clinic, even in Siamese cats with multiple dermoepidermal nodules. The subcutaneous MCTs commonly seen in dogs are extremely rare in cats. Unlike the situation in dogs, the histopathologic grade does not appear to correlate well with the biologic behavior of MCTs in cats (Molander-McCrary et al., 1998).
Diagnosis and Treatment
The diagnostic approach to cats with MCT is similar to that in dogs. As in dogs, some mast cells in cats are poorly granulated and the granules may not be easily identified during a routine cytologic or histopathologic evaluation.
The treatment for cats with MCTs is controversial. As a general rule, surgery is indicated for cats with a solitary cutaneous mass, for cats with two to five skin masses, and for cats with intestinal or splenic involvement. As discussed previously, cutaneous MCTs in cats are less aggressive than in dogs, and in most affected cats removal of a solitary dermoepidermal MCT using a biopsy punch is curative; the same applies to cats with fewer than five dermoepidermal MCTs. The combination of splenectomy, prednisone, and chloramibucid (Leukeran) treatment is recommended for cats with SMCD, in which survival times in excess of approximately 1 year are common; splenectomy alone does not result in prolonged survival. Surgical excision and prednisone treatment are recommended for cats with intestinal MCT. Prednisone alone (4 to 8 mg/kg by mouth q24-48h) may also be beneficial for cats with systemic or metastatic MCTs. Cats with multiple skin MCTs are best treated with prednisone, in the dosage just given. Although radiotherapy is as effective in cats as in dogs, it is rarely necessary in cats with this neoplasm. When an additional chemotherapeutic agent is needed in cats with MCTs, I usually use chlorambucil (Leukeran, 20 mg/m2 by mouth q2 weeks); this drug seems to be quite effective and well tolerated. In my limited experience, lomustine (CCNU) is not very effective in cats with MCTs.
INJECTION SITE SARCOMAS IN CATS
An association between injections/vaccination and the development of sarcomas has been recently recognized in cats, and epidemiologic studies have confirmed the association. In this syndrome fibriosarcomas (FSAs) or occasionally other types of sarcomas develop in the subcutis or muscle in the interscapular region or the thigh, common sites of injection/vaccination. It is estimated that a sarcoma develops in one to two of 10,000 cats that receive an injection. Although the exact pathogenesis is still unclear, both the adjuvants and the local immune response against the antigens (i.e.; inflammation) have been implicated as causative agents.
A rapidly growing soft tissue mass develops in the region weeks to months after vaccination or injection in cats with injection site sarcomas (ISSs). A vaccine- or injection-associated inflammatory reaction may precede the development of this neoplasm. Therefore an ISS should be suspected in any cat with a superficial or deep mass in the interscapular or thigh regions, and every effort should be made to establish a diagnosis immediately. Although FNA findings may provide a definitive answer, more often a surgical biopsy is necessary because sarcomas do not consistently exfoliate cells (see Chapter 75).
Although most FSAs in dogs and cats have a low metastatic potential, ISSs are quite aggressive and should be treated accordingly. Although studies are currently in progress, on the basis of the results of studies reported in the literature and on the findings in cats seen at our clinic, the rate of metastasis of ISSs is high (probably as high as 50% to 70%). Pulmonary metastatic lesions can be detected at presentation in a high proportion of cats; we have also seen ocular metastases as the main presenting feature in a few cats with ISSs.
The treatment of choice for cats with ISS is aggressive surgical excision (see Chapter 76). In keeping with the maxim “cut it once, but cut it all,” an en bloc resection (to include any biopsy tracts) should be performed immediately after the diagnosis is established, provided there is no metastatic disease. In a recent study cats treated with aggressive surgery had significantly longer disease-free survival times than cats treated with conservative surgery (274 versus 66 days); also, cats with tumors in the limbs had significantly longer disease-free survival times than cats with tumors in the trunk (325 versus 66 days; Hershey et al., 2000). Complete surgical excision of a relatively small ISS (i.e., <2 cm in diameter) is usually associated with long-term remissions. Although the role of postoperative adjuvant chemotherapy has not been thoroughly evaluated, cats with large or incompletely excised tumors may benefit from treatment with mitoxantrone and cyclophosphamide, doxorubicin and cyclophosphamide, or carboplatin. We have seen objective complete or partial responses in cats with nonresectable or metastatic ISS treated with doxorubicin/ cyclophosphamide combinations or with carboplatin alone; some of these cats have been in remission for longer than 1 year. If metastatic disease is already present, chemotherapy is not usually effective.
Alvarez FJ et al: Hematological changes in dogs with hemangiosarcoma, Proc 26th Annual Vet Cancer Society Conf, Huntington Beach, Calif, 2006.
Alvarez FJ et al: Treatment of dogs with stage III hemangiosarcoma using the VAC protocol, Proc 27th Annual Vet Cancer Society Conf, Fort Lauderdale, Fla, 2007.
Bertazzolo W, et al. Canine angiosarcoma: cytologic, histologic, and immunohistochemical correlations. Vet Clin Pathol. 2005;34:28.
Brown NO, et al. Canine hemangiosarcoma: retrospective analysis of 104 cases. J Am Vet Med Assoc. 1985;186:56.
Clifford CA, et al. Treatment of canine hemangiosarcoma: 2000 and beyond. J Vet Intern Med. 2000;14:479.
Hammer AS, et al. Efficacy and toxicity of VAC chemotherapy (vincristine, doxorubicin, and cyclophosphamide) in dogs with hemangiosarcoma. J Vet Intern Med. 1991;5:16.
Hammer AS, et al. Hemostatic abnormalities in dogs with hemangiosarcoma. J Vet Intern Med. 1991;5:11.
Lamerato-Kozicki AR, et al. Canine hemangiosarcoma originates from hematopoietic precursors with potential for endothelial differentiation. Exp Hematol. 2006;34:870.
Lana S, et al. Continuous low-dose oral chemotherapy for adjuvant therapy of splenic hemangiosarcoma in dogs. J Vet Intern Med. 2007;21:764.
Liptak JM, et al. Retroperitoneal sarcomas in dogs: 14 cases (1992-2002). J Am Vet Med Assoc. 2004;224:1471.
O’Brien RT. Improved detection of metastatic hepatic hemangiosarcoma nodules with contrast ultrasound in three dogs. Vet Radiol Ultrasound. 2007;48:146.
Ogilvie GK, et al. Surgery and doxorubicin in dogs with hemangiosarcoma. J Vet Intern Med. 1996;10:379.
Pirie CG, et al. Canine conjunctival hemangioma and hemangiosarcoma: a retrospective evaluation of 108 cases (1989-2004). Vet Ophthalmol. 2006;9:215.
Prymak C, et al. Epidemiologic, clinical, pathologic, and prognostic characteristics of splenic hemangiosarcoma and splenic hematomas in dogs: 217 cases (1985). J Am Vet Med Assoc. 1988;193:706.
Sorenmo KU, et al. Chemotherapy of canine hemangiosarcoma with doxorubicin and cyclophosphamide. J Vet Intern Med. 1993;7:370.
Sorenmo KU, et al. Canine hemangiosarcoma treated with standard chemotherapy and minocycline. J Vet Intern Med. 2000;14:395.
Sorenmo KU, et al. Efficacy and toxicity of a dose-intensified doxorubicin protocol in canine hemangiosarcoma. J Vet Intern Med. 2004;18:209.
Vail DM, et al. Liposome-encapsulated muramyl tripeptide phosphatidylethanolamine adjuvant immunotherapy for splenic hemangiosarcoma in the dog: a randomized multiinstitutional clinical trial. Clin Cancer Res. 1995;1:1165.
Weisse C, et al. Survival times in dogs with right atrial hemangiosarcoma treated by means of surgical resection with or without adjuvant chemotherapy: 23 cases (1986-2000). J Am Vet Med Assoc. 2005;226:575.
Boston SE, et al. Evaluation of survival time in dogs with stage III osteosarcoma that undergo treatment: 90 cases (1985-2004). J Am Vet Med Assoc. 2006;228:1905.
Chun R, et al. Toxicity and efficacy of cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma. J Am Anim Hosp Assoc. 2005;41:382.
Fan TM, et al. Single-agent pamidronate for palliative therapy of canine appendicular osteosarcoma bone pain. J Vet Intern Med. 2007;21:431.
Hillers KR, et al. Incidence and prognostic importance of lymph node metastases in dogs with appendicular osteosarcoma: 228 cases (1986-2003). J Am Vet Med Assoc. 2005;226:1364.
LaRue SM, et al. Limb-sparing treatment for osteosarcoma in dogs. J Am Vet Med Assoc. 1989;195:1734.
Lascelles BD, et al. Improved survival associated with postoperative wound infection in dogs treated with limb-salvage surgery for osteosarcoma. Ann Surg Oncol. 2005;12:1073.
Moore AS, et al. Doxorubicin and BAY 12-9566 for the treatment of osteosarcoma in dogs: a randomized, double-blind, placebo-controlled study. J Vet Intern Med. 2007;21:783.
Mueller F, et al. Palliative radiotherapy with electrons of appendicular osteosarcoma in 54 dogs. In Vivo. 2005;19:713.
O’Brien MG, et al. Resection of pulmonary metastases in canine osteosarcoma: 36 cases. Vet Surg. 1993;22:105.
Rosenberger JA, Pablo NV, Crawford PC. Prevalence of and intrinsic risk factors for appendicular osteosarcoma in dogs: 179 cases (1996-2005). J Am Vet Med Assoc. 2007;231:1076.
Antognoni MT, et al. Characteristic clinical, haematological and histopathological findings in feline mastocytoma. Vet Res Commun. 2003;27((Suppl) 1):727.
Jones CL, et al. Detection of c-kit mutations in canine mast cell tumors using fluorescent polyacrylamide gel electrophoresis. J Vet Diagn Invest. 2004;16:95.
Lepri E, et al. Diagnostic and prognostic features of feline cutaneous mast cell tumours: a retrospective analysis of 40 cases. Vet Res Commun. 2003;27((Suppl) 1):707.
London CA, et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin Cancer Res. 2003;9:2755.
Macy DW, et al. Mast cell tumor. In: Withrow SJ, et al, editors. Clinical veterinary oncology. Philadelphia: JB Lippincott, 1989.
McManus PM. Frequency and severity of mastocythemia in dogs with and without mast cell tumors: 120 cases (1995–1997). J Am Vet Med Assoc. 1999;215:355.
Molander-McCrary H, et al. Cutaneous mast cell tumors in cats: 32 cases (1991–1994). J Am Anim Hosp Assoc. 1998;34:281.
O’Keefe DA. Canine mast cell tumors. Vet Clin N Am. 1990;20:1105.
Pryer NK, et al. Proof of target for SU11654: inhibition of KIT phosphorylation in canine mast cell tumors. Clin Cancer Res. 2003;9:5729.
Rassnick KM, et al. Treatment of canine mast cell tumors with CCNU (lomustine). J Vet Intern Med. 1999;13:601.
Romansik EM, et al. Mitotic index is predictive for survival for canine cutaneous mast cell tumors. Vet Pathol. 2007;44:335.
Séguin B, et al. Clinical outcome of dogs with grade-II mast cell tumors treated with surgery alone: 55 cases (1996–1999). J Am Vet Med Assoc. 2001;218:1120.
Séguin B, et al. Recurrence rate, clinical outcome, and cellular proliferation indices as prognostic indicators after incomplete surgical excision of cutaneous grade II mast cell tumors: 28 dogs (1994-2002). J Vet Intern Med. 2006;20:933.
Thamm DH, Mauldin EA, Vail DM. Prednisone and vinblastine chemotherapy for canine mast cell tumor—41 cases (1992–1997). J Vet Intern Med. 1999;13:491.
Turrel JM, et al. Prognostic factors for radiation treatment of mast cell tumors in 85 dogs. J Am Vet Med Assoc. 1988;193:936.
Barber L, et al. Combined doxorubicin and cyclophosphamide chemotherapy for nonresectable feline fibrosarcoma. J Am Anim Hosp Assoc. 2000;36:416.
Couto CG, et al. Review of treatment options for vaccine-associated feline sarcoma. In Vaccine-Associated Feline Sarcoma Symposium. J Am Vet Med Assoc. 1998;213:1426.
Dean R, et al. Study of feline injection site sarcomas. Vet Rec. 2006;159:641.
Esplin DG, et al. Postvaccination sarcomas in cats. J Am Vet Med Assoc. 1992;202:1245.
Hershey AE, et al. Prognosis for presumed feline vaccine-associated sarcoma after excision: 61 cases (1986–1996). J Am Vet Med Assoc. 2000;216:58.
Kass PH, et al. Epidemiologic evidence for a causal relation between vaccination and fibrosarcoma tumorigenesis in cats. J Am Vet Med Assoc. 1993;203:396.
Kirpensteijn J. Feline injection site-associated sarcoma: Is it a reason to critically evaluate our vaccination policies? Vet Microbiol. 2006;117:59.
Lester S, et al. Vaccine-site associated sarcomas in cats: clinical experience and a laboratory review (1982–1993). J Am Anim Hosp Assoc. 1996;32:91.
Macy DW, et al. Vaccine-associated sarcomas in cats. Feline Pract. 1995;23:24.
 Cancer Chemotherapy Protocols Commonly Used at the l Ohio State University Veterinary Teaching Hospital
Cancer Chemotherapy Protocols Commonly Used at the l Ohio State University Veterinary Teaching Hospital
|
I. Lymphoma
As in COP protocol but with the addition of l-Asparaginase at a dosage of 10,000-20,000 IU/m2 IM q4-6 wk
DOGS
VI. Multiple myeloma
|
The duration of chemotherapy using this protocol varies.
PO, Orally; IV, intravenously; SC, subcutaneously; IM, intramuscularly; CRI, constant rate infusion.
* The daily dose should be divided into two to four daily administrations.
 TABLE 82-2
TABLE 82-2