CHAPTER 49 Disorders of the Hypothalamus and Pituitary Gland
POLYURIA AND POLYDIPSIA
Water consumption and urine production are controlled by complex interactions among plasma osmolality and volume, the thirst center, the kidney, the pituitary gland, and the hypothalamus. Dysfunction in any of these areas results in the clinical signs of polyuria (PU) and polydipsia (PD). In dogs normal water intake is usually less than 60 ml/kg of body weight/24 h, with an upper normal limit of 100 ml/kg. Similar values are used for cats, although most cats drink considerably less than these amounts. Normal urine output varies between 20 and 45 ml/kg/24 h. PD and PU in the dog and cat have been defined as water consumption that exceeds 100 ml/kg/24 h and urine production greater than 50 ml/kg/24 h, respectively. It is possible, however, for thirst and urine production to be abnormal within the limits of these normal values in individual dogs and cats.
A variety of metabolic disturbances can cause PU/PD (see Box 41-3). Primary polyuric disorders can be classified on the basis of the underlying pathophysiology into primary pituitary and nephrogenic diabetes insipidus, secondary nephrogenic diabetes insipidus, osmotic diuresis-induced polyuria, and interference with the hypothalamic-pituitary secretion of arginine vasopressin (AVP). The most common form of diabetes insipidus is acquired secondary nephrogenic diabetes insipidus. This form includes a variety of renal and metabolic disorders in which the renal tubules lose the ability to respond adequately to AVP. Most of these acquired forms are potentially reversible after elimination of the underlying illness.
Secondary nephrogenic diabetes insipidus results from interference with the normal interaction of AVP and renal tubular AVP receptors, problems with the generation of intracellular cAMP, problems with renal tubular cell function, or loss of the renal medullary interstitial concentration gradient. Primary polydipsic disorders occur in dogs and usually have a psychogenic or behavioral basis for the compulsive water consumption (see the discussion of psychogenic PD, p. 702). A complete discussion of the diagnostic approach to PU/PD is presented on p. 704. An index of suspicion for most of the endocrinopathies that cause PU/PD can be raised after a review of the history, physical examination findings, and results of a complete blood count (CBC), serum biochemistry panel, and urinalysis. Specific tests may be necessary to confirm the diagnosis (Table 49-1). See the appropriate chapters in this section for a more complete discussion of the diagnosis and treatment of each of these endocrinopathies.
 TABLE 49-1 Endocrine Disorders Causing Polyuria and Polydipsia in the Dog and Cat
TABLE 49-1 Endocrine Disorders Causing Polyuria and Polydipsia in the Dog and Cat
| DISORDER | TESTS TO ESTABLISH THE DIAGNOSIS |
|---|---|
| Diabetes mellitus | Fasting blood glucose, urinalysis |
| Hyperadrenocorticism | Urine C/C ratio, low-dose dexamethasone suppression test |
| Hypoadrenocorticism | Blood electrolytes, ACTH stimulation test |
| Primary hyperparathyroidism | Blood calcium/phosphorus, cervical ultrasound, serum PTH concentration |
| Hyperthyroidism | Serum T4 and free T4 concentration |
| Diabetes insipidus Pituitary Nephrogenic | Modified water deprivation test, response to dDAVP therapy |
| Acromegaly | Baseline GH or IGF-I concentration, CT or MR scan |
| Primary Hyperaldosteronism | Blood electrolytes, plasma aldosterone concentration |
C/C, Cortisol/creatinine; ACTH, Adrenocorticotropic hormone; PTH, parathyroid hormone; GH, growth hormone; IGF-I, Insulin-like growth factor-I; CT, computed tomographic; MR, magnetic resonance.
Occasionally, the physical examination findings and initial blood and urine tests are normal in dogs and cats with PU and PD. Differential diagnoses in these dogs and cats include diabetes insipidus, psychogenic PD, hyperadrenocorticism, mild renal insufficiency without azotemia, and mild hepatic insufficiency, most notably with portosystemic shunts. Hyperadrenocorticism, renal insufficiency, and hepatic insufficiency should be ruled out before performing diagnostic tests for diabetes insipidus or psychogenic PD. Diagnostic tests to consider include evaluating the range of urine specific gravities obtained from several urine samples (discussed in more detail below), tests for hyperadrenocorticism (e.g., urine cortisol : creatinine ratio, low-dose dexamethasone suppression test), liver function tests (e.g., measurement of preprandial and postprandial bile acid levels), determination of the urine protein : creatinine (P/C) ratio, and abdominal ultrasonography. Ideally, all realistic causes of secondary acquired nephrogenic diabetes insipidus should be ruled out before performing tests (especially the modified water deprivation test) for primary pituitary and nephrogenic diabetes insipidus and psychogenic PD.
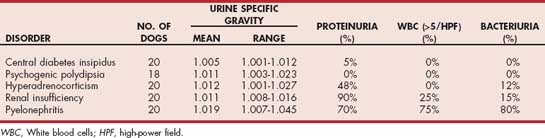
Critical evaluation of urine specific gravity measured from several urine samples obtained by the client at different times of the day for 2 to 3 days may provide clues to the underlying disorder (Table 49-2). Urine samples should be stored in the refrigerator until they can be brought to the veterinary hospital for determination of urine specific gravity. Urine specific gravity varies widely among healthy dogs and can range from 1.006 to greater than 1.040 within a 24-hour period. Wide fluctuations in urine specific gravity have not been reported in healthy cats. If the urine specific gravity is consistently in the isosthenuric range (1.008 to 1.015), renal insufficiency should be considered the primary differential diagnosis, especially if the blood urea nitrogen and serum creatinine concentration are high normal or increased (i.e., 25 mg/dl or more and 1.6 mg/dl or more, respectively). Isosthenuria is relatively common in dogs with hyperadrenocorticism, psychogenic water consumption, hepatic insufficiency, pyelonephritis, and partial diabetes insipidus with concurrent water restriction, but urine specific gravities above (e.g., hyperadrenocorticism, pyelonephritis, hepatic insufficiency, psychogenic water consumption) or below (e.g., hyperadrenocorticism, hepatic insufficiency, partial diabetes insipidus) the isosthenuric range also occur with these disorders. If urine specific gravities less than 1.005 (i.e., hyposthenuric) are identified, renal insufficiency and pyelonephritis are ruled out and diabetes insipidus, psychogenic water consumption, hyperadrenocorticism, and hepatic insufficiency should be considered. Primary pituitary and nephrogenic diabetes insipidus are ruled out if the urine specific gravity exceeds 1.020. Urine specific gravities that range from less than 1.005 to greater than 1.030 are suggestive of psychogenic PD.
DIABETES INSIPIDUS
Etiology
AVP plays a key role in the control of renal water resorption, urine production and concentration, and water balance. AVP is produced in the supraoptic and paraventricular nuclei of the hypothalamus, is stored in and secreted from the posterior pituitary gland in response to an increase in plasma osmolality or decrease in extracellular fluid volume, and interacts with distal tubular and collecting duct cells of the kidney to promote water resorption and the formation of concentrated urine. The defective synthesis or secretion of AVP or an inability of the renal tubules to respond to AVP causes diabetes insipidus.
CENTRAL DIABETES INSIPIDUS
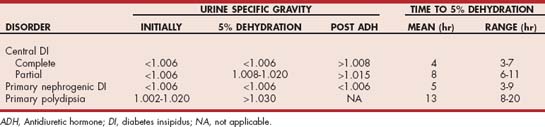
Central diabetes insipidus (CDI) is a polyuric syndrome that results from insufficient secretion of AVP to concentrate urine for water conservation. This deficiency may be absolute or partial. An absolute deficiency of AVP, referred to as complete CDI, causes persistent hyposthenuria and severe diuresis. The urine specific gravity in dogs and cats with complete CDI remains hyposthenuric (i.e., 1.005 or less), even with severe dehydration. A partial deficiency of AVP, referred to as partial CDI, also causes persistent hyposthenuria and a marked diuresis as long as the dog or cat has unlimited access to water. During periods of water restriction the urine specific gravity can increase into the isosthenuric range (i.e., 1.008 to 1.015), but typically the urine cannot be concentrated to more than 1.015 to 1.020 even when the animal is severely dehydrated. In any dog or cat with partial CDI the maximum urine-concentrating ability during dehydration is inversely related to the severity of the deficiency in AVP secretion—that is, the more severe the AVP deficiency, the less concentrated the urine specific gravity during dehydration.
CDI may result from any condition that damages the neurohypophyseal system (Box 49-1). Idiopathic CDI is the most common form, appearing at any age, in any breed, and affecting animals of either sex. Necropsies performed in dogs and cats with idiopathic CDI fail to identify an underlying reason for the AVP deficiency. Although CDI is well documented in kittens and puppies, a hereditary form of CDI has not yet been documented. The most common identifiable causes of CDI in dogs and cats are head trauma (accidental or neurosurgical), neoplasia, and hypothalamic-pituitary malformations (e.g., cystic structures). Head trauma may cause a transient (typically lasting 1 to 3 weeks) or permanent CDI, depending on the viability of the cells in the supraoptic and paraventricular nuclei.
 BOX 49-1 Recognized Causes of Diabetes Insipidus in Dogs and Cats
BOX 49-1 Recognized Causes of Diabetes Insipidus in Dogs and Cats
| CENTRAL DIABETES INSIPIDUS | NEPHROGENIC DIABETES INSIPIDUS |
|---|---|
| Idiopathic | Primary idiopathic |
| Traumatic | Primary familial (Huskies) |
| Secondary acquired (see Box 41-4) |
Primary intracranial tumors that are associated with diabetes insipidus in dogs and cats include craniopharyngioma, pituitary chromophobe adenoma, and pituitary chromophobe adenocarcinoma. Metastatic mammary carcinoma, lymphoma, malignant melanoma, and pancreatic carcinoma have been reported to cause CDI in dogs through their presence in the pituitary gland or hypothalamus. Metastatic neoplasia has not yet been reported to be a cause of CDI in cats.
NEPHROGENIC DIABETES INSIPIDUS
Nephrogenic diabetes insipidus (NDI) is a polyuric disorder that results from impaired responsiveness of the nephron to AVP. Plasma AVP concentrations are normal or increased in animals with this disorder. NDI is classified as either primary (familial) or secondary (acquired). Primary NDI is a rare congenital disorder in dogs and cats, with only a few reports in the literature. The etiology of primary NDI in dogs and cats is unknown, although decreased binding affinity of AVP receptors was identified in a family of Siberian Huskies. Affected puppies showed antidiuretic responses to high doses of synthetic vasopressin (desmopressin [dDAVP]).
SIGNALMENT
There is no apparent breed-, sex-, or age-related predilection for CDI. In one study the age at the time of the diagnosis of CDI in dogs ranged from 7 weeks to 14 years, with a median of 5 years. Similarly, most cats with CDI are domestic short- and long-haired cats, although the disorder has also been documented in Persians and Abyssinians. The age at the time of diagnosis of CDI in cats ranged from 8 weeks to 6 years, with a mean of 1.5 years. Primary NDI has been identified only in puppies, kittens, and young adult dogs and cats younger than 18 months of age. PU and PD have been present since the clients acquired these pets.
CLINICAL SIGNS
PU and PD are the hallmark signs of diabetes insipidus and are typically the only signs seen in dogs and cats with congenital and idiopathic CDI and in those with primary NDI. Clients may believe that affected animals are incontinent because of the frequency of urination and loss of normal housebroken behavior. Owners of cats with diabetes insipidus often complain that they need to change the kitty litter more frequently than expected. Additional clinical signs may be found in dogs and cats with secondary causes of diabetes insipidus. The most worrisome are neurologic signs, which may indicate the presence of an expanding hypothalamic or pituitary tumor in the adult dog or cat that has not had head trauma.
PHYSICAL EXAMINATION
The physical examination findings are usually unremarkable in animals with CDI, although some dogs and cats are thin, presumably because the pet’s strong desire for water overrides its normal appetite. As long as access to water is not restricted, the animal’s hydration status, mucous membrane color, and capillary refill time remain normal. The presence of neurologic abnormalities is variable in dogs and cats with either trauma-induced CDI or neoplastic destruction of the hypothalamus or pituitary gland. When present, neurologic signs may include stupor, disorientation, ataxia, circling, pacing, and convulsions. Severe hypernatremia may also cause neurologic signs in the traumatized dog or cat with undiagnosed CDI given inadequate fluid therapy (see Chapter 55). Hyposthenuria in the presence of persistent hypernatremia should raise suspicion for diabetes insipidus.
Diagnosis
The diagnostic workup for PU and PD should initially rule out causes of acquired secondary NDI (see Chapter 41). Recommended initial diagnostic studies include a CBC; biochemistry panel; urinalysis with bacterial culture; abdominal ultrasonography; and a urine cortisol : creatinine ratio, low-dose dexamethasone suppression test, or both. Results of these screening tests are normal in dogs and cats with CDI, primary NDI, and psychogenic water consumption, although a low-normal serum urea nitrogen concentration (5 to 10 mg/dl) may be found. Random urine specific gravity is usually less than 1.006 and is often as low as 1.001 if the dog or cat has unlimited access to water. The urine osmolality is less than 300 mOsm/kg. A urine specific gravity in the isosthenuric range (i.e., 1.008 to 1.015) does not rule out diabetes insipidus (Fig. 49-1), especially if the urine has been obtained after water is knowingly or inadvertently withheld (e.g., a long car ride and wait in the veterinary office). The urine of dogs and cats with partial diabetes insipidus can be concentrated into the isosthenuric range if they are dehydrated. Erythrocytosis (packed cell volume of 50% to 60%), hyperproteinemia, hypernatremia, and azotemia may be found in animals if their access to water has been restricted.
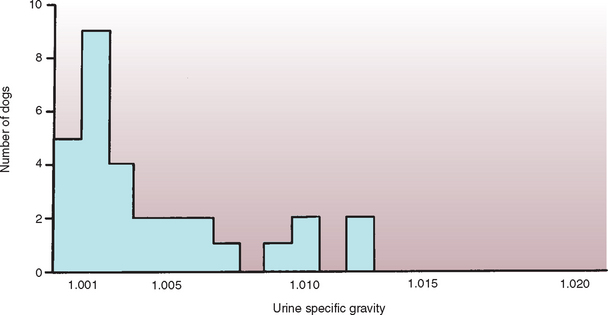
FIG 49-1 Urine specific gravity measured in 30 dogs with central diabetes insipidus at the time of initial presentation to the veterinarian.
(From Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
Diagnostic tests to confirm and differentiate among CDI, primary NDI, and psychogenic water consumption include the modified water deprivation test, random plasma osmolality determination, and the response to AVP supplementation. The results of these tests can be interpreted only after the causes for acquired secondary NDI have been ruled out.
MODIFIED WATER DEPRIVATION TEST
The technique, interpretation, contraindications, and complications of the modified water deprivation test are described in Chapter 42. The test consists of two phases. In phase I the AVP secretory capabilities and renal distal and collecting tubule responsiveness to AVP are evaluated by assessing the effects of dehydration (i.e., water restriction until the animal loses 3% to 5% of its body weight) on urine specific gravity. The normal dog and cat, as well as those with psychogenic water consumption, should be able to concentrate urine to greater than 1.030 (1.035 in the cat) if dehydrated. Dogs and cats with partial and complete CDI and primary NDI have an impaired ability to concentrate urine in the face of dehydration (Table 49-3 and Fig. 49-2). The time required to attain 3% to 5% dehydration can sometimes be helpful in establishing the diagnosis. It often takes less than 6 hours for dogs and cats with complete CDI to attain 3% to 5% dehydration, whereas it often takes more than 8 to 10 hours for dogs and cats with partial CDI, and especially those with psychogenic water consumption, to attain 3% to 5% dehydration.
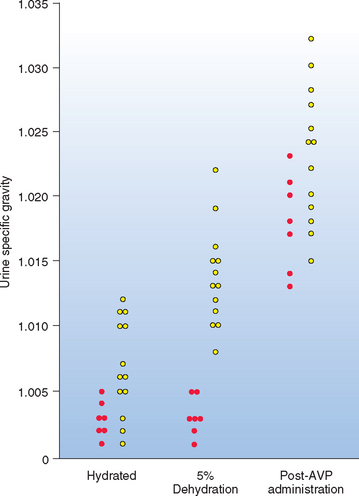
FIG 49-2 Urine specific gravity in seven dogs with complete central diabetes insipidus (red circle) and 13 dogs with partial central diabetes insipidus (yellow circle) at the beginning (hydrated), end of phase I (5% hydrated), and end of phase II (after arginine vasopressin administration) of the modified water deprivation test.
(From Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
Phase II of the water deprivation test is indicated for dogs and cats that do not concentrate urine to greater than 1.030 during phase I of the test. Phase II determines the effect, if any, that exogenous AVP has on the renal tubular ability to concentrate urine in the face of dehydration (see Fig. 49-2). This phase differentiates impaired AVP secretion from impaired renal tubular responsiveness to AVP (see Table 49-3).
RESPONSE TO DESMOPRESSIN (dDAVP)
An alternative approach to establishing the diagnosis is to evaluate the animal’s response to trial therapy with dDAVP (desmopressin acetate, Aventis Pharmaceuticals). One 0.1-mg or one-half of a 0.2-mg (dog) and one-half of a 0.1-mg (cat) dDAVP tablet is administered orally every 8 hours, or 1 to 4 drops of dDAVP nasal spray is administered from an eye dropper into the conjunctival sac every 12 hours for 5 to 7 days. The effect of dDAVP should not be critically evaluated until after 5 to 7 days of therapy because renal medullary solute washout may prevent a dog or cat with CDI from forming concentrated urine in response to only one or two administrations. Clients should notice a decrease in PU and PD by the end of the treatment period if the PU and PD are caused by CDI. Urine specific gravity should be measured on several urine samples collected by the client on the last couple of days of trial therapy. An increase in urine specific gravity by 50% or more, compared with pretreatment specific gravities, supports the diagnosis of CDI, especially if the urine specific gravity exceeds 1.030. There should be only minimal improvement in dogs and cats with primary NDI, although a response may be observed with very high doses of dDAVP. Dogs and cats with psychogenic water consumption may exhibit a mild decline in urine output and water intake because the chronically low serum osmolality tends to depress AVP production.
This approach to diagnosis requires that all other causes of PU and PD, except CDI, primary NDI, and psychogenic PD, be previously ruled out. Tests for hyperadrenocorticism should always be evaluated before trial therapy with dDAVP is considered. Hyperadrenocorticism mimics partial CDI, in part because of the suppression of vasopressin secretion with hyperadrenocorticism. Dogs with hyperadrenocorticism typically have a positive, albeit moderate, response to dDAVP treatment, which can result in a misdiagnosis of partial CDI as the cause of PU and PD. Unlike partial CDI, the beneficial response to dDAVP wanes over the ensuing weeks in dogs with hyperadrenocorticism.
Although less time-consuming than the water deprivation test, the expense is often comparable, in part because of the cost of the dDAVP. In addition, the modified water deprivation test may still have to be performed if ambiguous results are obtained using this simpler approach.
RANDOM PLASMA OSMOLALITY
Measurement of random plasma osmolality may help identify primary or psychogenic PD. Plasma osmolality in normal dogs and cats is approximately 280 to 310 mOsm/kg. Diabetes insipidus is a primary polyuric disorder, with compensatory PD to prevent severe hyperosmolality. Random plasma osmolality should be greater than 300 mOsm/kg. Psychogenic PD is a primary polydipsic disorder, with compensatory PU to prevent hyposmolality and water intoxication. Random plasma osmolality should be less than 280 mOsm/kg. Unfortunately, there is considerable overlap in random plasma osmolality in animals with these disorders (Fig. 49-3). A random plasma osmolality of less than 280 mOsm/kg obtained while the dog or cat has free access to water suggests the presence of psychogenic PD, whereas a plasma osmolality greater than 280 mOsm/kg is consistent with CDI, NDI, or psychogenic PD.
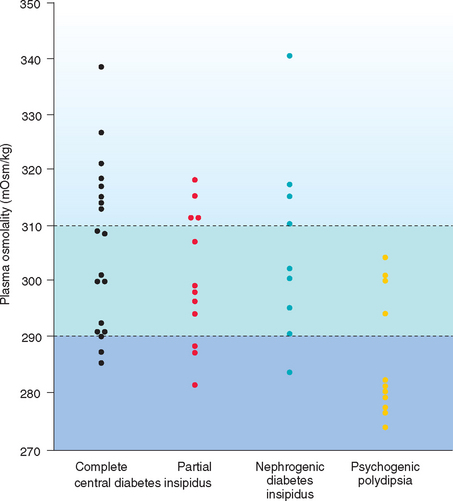
FIG 49-3 Random plasma osmolality in 19 dogs with complete central diabetes insipidus, 12 dogs with partial central diabetes insipidus, 9 dogs with primary nephrogenic diabetes insipidus, and 11 dogs with primary (psychogenic) polydipsia. Note the overlap in values between groups of dogs. Dashed lines, Upper and lower limits for normal plasma osmolality.
(From Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
ADDITIONAL DIAGNOSTIC TESTS
Neoplasia in the region of the pituitary and hypothalamus should be considered in the older dog or cat in which CDI develops. A complete neurologic evaluation, including computed tomographic (CT) or magnetic resonance (MR) scan may be warranted before idiopathic CDI is arbitrarily diagnosed, especially if the client is willing to consider radiotherapy or chemotherapy should a tumor be identified. Similarly, a more complete evaluation of the kidney (e.g., creatinine clearance studies, intravenous pyelogram, CT or MR scan, renal biopsy) may be warranted in the older dog or cat tentatively considered to have primary NDI.
Treatment
Therapeutic options for dogs and cats with diabetes insipidus are listed in Box 49-2. The synthetic analog of vasopressin, dDAVP, is the standard therapy for CDI. dDAVP has almost three times the antidiuretic action of AVP, with minimal-to-no vasopressor or oxytocic activity. The intranasal dDAVP preparation (dDAVP nasal drops, 2.5- and 5.0-ml bottles containing 100 μg dDAVP/ml) is used most commonly for treating CDI in dogs and cats. Administration of medication to animals via the intranasal route is possible but not recommended. The dDAVP nasal preparation may be transferred to a sterile eye dropper bottle and drops placed into the conjunctival sac of the dog or cat. Although the solution is acidic, ocular irritation rarely occurs. One drop of dDAVP contains 1.5 to 4 μg of dDAVP, and a dosage of one to four drops administered once or twice daily controls signs of CDI in most animals.
 BOX 49-2 Therapies Available for Polydipsic/Polyuric Dogs and Cats with Central Diabetes Insipidus, Nephrogenic Diabetes Insipidus, or Primary (Psychogenic) Polydipsia
BOX 49-2 Therapies Available for Polydipsic/Polyuric Dogs and Cats with Central Diabetes Insipidus, Nephrogenic Diabetes Insipidus, or Primary (Psychogenic) Polydipsia
ME, Metabolizable energy
Oral dDAVP (dDAVP tablets, 0.1 and 0.2 mg) can be used to treat CDI, although the clinical response is variable. The bioavailability of oral dDAVP is approximately 5% to 15% of the intranasal dose in humans. Similar information is not available for dogs and cats. The initial oral dDAVP dose is 0.1 mg (dogs) and 0.05 mg (cats) given three times a day. The dose is gradually increased to effect if unacceptable PU and PD persist 1 week after therapy is initiated. Decreasing the frequency of administration to twice a day, decreasing the dose of dDAVP, or both can be tried once clinical response has been documented. To date, most dogs have required 0.1 to 0.2 mg of dDAVP two to three times a day, and most cats have required 0.025 to 0.05 mg of dDAVP two to three times a day to control PU and PD. Treatment should be switched to the intranasal dDAVP preparation if there is minimal to no response to 0.2 mg (dog) or 0.05 mg (cat) of oral dDAVP administered three times a day.
The maximal effect of dDAVP, regardless of the route of administration, occurs from 2 to 8 hours after administration, and the duration of action varies from 8 to 24 hours. Larger doses of dDAVP appear both to increase its antidiuretic effects and to prolong its duration of action; however, expense becomes a limiting factor. The medication may be administered exclusively in the evening as insurance against nocturia.
Chlorpropamide, thiazide diuretics, and oral sodium chloride restriction have a limited efficacy in the treatment of NDI. dDAVP may control the clinical signs if administered in massive amounts (i.e., five to ten times the amount used for the treatment of CDI), but the cost of the drug obviously detracts from the attractiveness of this therapeutic approach. Fortunately, therapy for CDI or NDI is not mandatory as long as the dog or cat has unlimited access to water and is housed in an environment that cannot be damaged by severe PU. A constant water supply is of paramount importance because relatively short periods of water restric tion can have catastrophic results (i.e., the development of hypernatremic, hypertonic dehydration and neurologic signs).
Prognosis
Dogs and cats with idiopathic or congenital CDI become relatively asymptomatic in response to appropriate therapy, and with proper care these animals have an excellent life expectancy. PU and PD frequently resolve in dogs and cats with trauma-induced CDI, often within 2 weeks of the traumatic incident. The prognosis in dogs and cats with hypothalamic and pituitary tumors is guarded to grave. Neurologic signs typically develop within 6 months after the diagnosis of CDI, and clinical response to radiotherapy and chemotherapy is variable and unpredictable.
The prognosis for animals with primary NDI is guarded to poor because of limited therapeutic options and the generally poor response to therapy. The prognosis for animals with secondary NDI depends on the prognosis of the primary problem.
PRIMARY (PSYCHOGENIC) POLYDIPSIA
Primary PD is defined as a marked increase in water intake that cannot be explained as a compensatory mechanism for excessive fluid loss. In humans primary PD results from a defect in the thirst center or may be associated with mental illness. Primary dysfunction of the thirst center resulting in compulsive water consumption has not been reported in the dog or cat, although an abnormal vasopressin response to hypertonic saline infusion has been reported in dogs with suspected primary PD. A psychogenic or behavioral basis for compulsive water consumption does occur in the dog but has not been reported in the cat. Psychogenic PD may be induced by concurrent disease (e.g., hepatic insufficiency, hyperthyroidism) or may represent a learned behavior following a change in the pet’s environment. PU is compensatory to prevent overhydration.
Dogs (and presumably cats) with primary or psychogenic PD have an intact hypothalamic-pituitary-renal axis for controlling fluid balance and variable severity of renal medullary solute washout. Because AVP production and renal tubular response to AVP are normal, these dogs can concentrate urine in excess of 1.030. Depending on the severity of renal medullary solute washout, a period of 24 hours or longer of water deprivation may be necessary to attain concentrated urine. Psychogenic PD is diagnosed by exclusion of other causes of PU and PD and by demonstrating that the dog or cat can concentrate urine to a specific gravity in excess of 1.030 during water deprivation.
Treatment is aimed at gradually limiting water intake to amounts in the high-normal range. The client should determine the dog’s approximate water intake in a 24-hour period when free-choice water is allowed, and this volume of water is then reduced by 10% per week until water volumes of 60 to 80 ml/kg/24 h are reached. The total 24-hour volume of water should be divided into several aliquots, with the last aliquot given at bedtime. Oral salt (1 g/30 kg q12h) and/or oral sodium bicarbonate (0.6 g/30 kg q12h) may also be administered for 3 to 5 days to help reestablish the renal medullary concentration gradient. Changes in the dog’s environment or daily routine should be considered, such as initiating a daily exercise routine; bringing a second pet into the home; providing some distraction, such as a radio playing when the clients are not home; or moving the dog to an area with an increased amount of contact with humans.
ENDOCRINE ALOPECIA
Symmetric alopecia without historical or clinical evidence of inflammation usually results from hair cycle arrest induced by hormonal diseases—hence the term endocrine alopecia (Fig. 49-4). Hair follicles are atrophic, hairs are easily epilated, the skin is often thin and hypotonic, and hyperpigmentation is common. Other dermatologic lesions, such as scales, crusts, and papules, are absent. Seborrhea and pyoderma may develop, depending on the underlying cause.
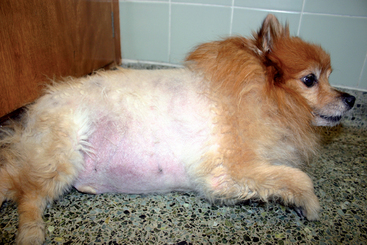
FIG 49-4 Endocrine alopecia, thin skin, and severe obesity in a 7-year-old male castrated Pomeranian with iatrogenic hyperadrenocorticism caused by chronic administration of prednisone for a seizure disorder. Note the symmetric truncal alopecia with sparing of the head and distal extremities.
Causes of endocrine alopecia are listed in Table 49-4. In dogs the most common causes are hypothyroidism and glucocorticoid excess (iatrogenic or spontaneous). Feline endocrine alopecia is perhaps the most common endocrine alopecia in cats. The diagnostic evaluation for endocrine alopecia begins with a complete history, physical examination, and routine blood and urine tests, (i.e., CBC, serum biochemistry panel, and urinalysis). Results of these tests will often provide evidence for hypothyroidism and hyperadrenocorticism, and appropriate diagnostic tests can then be performed to confirm these diagnoses (see Chapters 51 and 53, respectively).
 TABLE 49-4 Disorders Causing Endocrine Alopecia
TABLE 49-4 Disorders Causing Endocrine Alopecia
| DISORDER | COMMON CLINICOPATHOLOGIC ABNORMALITIES | DIAGNOSTIC TESTS |
|---|---|---|
| Hypothyroidism | Lipemia, hypercholesterolemia, mild nonregenerative anemia | Serum T4, free T4, TSH concentrations |
| Hyperadrenocorticism | Stress leukogram, increased ALP, hypercholesterolemia, hyposthenuria, proteinuria, urinary tract infection | Urine cortisol/creatinine ratio, low-dose dexamethasone suppression test, abdominal US |
| Hyperestrogenism | ||
| Functional Sertoli cell tumor in male dog | None (bone marrow depression uncommon) | Physical findings, abdominal US, cytologic or histopathologic findings, plasma estrogen concentration |
| Hyperestrogenism in intact female dog | None (bone marrow depression uncommon) | Vaginal cytology, abdominal US, plasma estrogen concentration, response to ovariohysterectomy |
| Hyperprogesteronism | None | Physical findings, abdominal US, serum progesterone concentration |
| Increased adrenocortical steroid hormone intermediates (adrenal hyperplasia-like syndrome, Alopecia-X) | None | Measure adrenocortical steroid hormone intermediates before and after ACTH administration |
| Growth hormone deficiency pituitary dwarfism | None | Signalment, physical findings, growth hormone response test |
| Growth hormone-responsive dermatosis—adult dog | None | Growth hormone response test, response to growth hormone replacement therapy |
| Castration-responsive dermatosis Hypoestrogenism (?) | None | Response to castration |
| Estrogen-responsive dermatosis of spayed female dogs | None | Response to estrogen therapy |
| Hypoandrogenism (?) | ||
| Testosterone-responsive dermatosis—male dog | None | Response to testosterone therapy |
| Feline endocrine alopecia | None | Response to progestin therapy |
| Telogen defluxion (effluvium) | None | History of recent pregnancy or diestrus |
| Diabetes mellitus | Hyperglycemia, glycosuria | Blood and urine glucose measurement |
T4, Tetraiodothyronine; TSH, thyroid-stimulating hormone; ALP, alkaline phosphatase; US, ultrasonography; ACTH, adrenocorticotropic hormone.
Once hypothyroidism and hyperadrenocorticism have been ruled out, the next diagnostic step is to rule out an excess of one of the sex hormones or one of the adrenocortical steroid hormone intermediates. Dermatologic manifestations are similar for most sex hormone–induced dermatoses and include endocrine alopecia that initially begins in the perineal, genital, and ventral abdominal regions and spreads cranially; dull, dry, easily epilated hair; failure of the haircoat to regrow after clipping; and variable presence of seborrhea and hyperpigmentation. Additional clinical signs of hyperestrogenism may include gynecomastia, a pendulous prepuce, the attraction of other male dogs, squatting to urinate, and unilateral testicular atrophy (contralateral to the testicular tumor) in the male dog and vulvar enlargement and persistent proestrus or estrus in the bitch. Results of a CBC may reveal aplastic anemia. Histologic assessment of a skin biopsy specimen can be used to identify nonspecific endocrine-related alterations and support the diagnosis of endocrine alopecia (Table 49-5). There are no pathognomonic histologic changes for sex hormone–induced dermatoses. The identification of an increased plasma estrogen (i.e., estradiol) concentration would support the presence of a functional Sertoli cell tumor in the dog and hyperestrogenism in the bitch (assuming that the bitch is not in proestrus or early estrus). Abdominal ultrasound may identify ovarian cysts or neoplasia in the bitch with hyperestrogenism, and abdominal and testicular ultrasound may identify testicular neoplasia in the male dog. Hyperestrogenism and endocrine alopecia will resolve after surgical removal of the ovarian cyst, ovarian tumor, or testicular tumor.
 TABLE 49-5 Dermatohistopathologic Alterations Associated with Endocrinopathy-Induced Alopecia
TABLE 49-5 Dermatohistopathologic Alterations Associated with Endocrinopathy-Induced Alopecia
| ABNORMALITY | SPECIFIC ENDOCRINE DISORDER |
|---|---|
| Nonspecific Abnormalities Supporting an Endocrinopathy | |
| Orthokeratotic hyperkeratosis | — |
| Follicular keratosis | — |
| Follicular dilatation | — |
| Follicular atrophy | — |
| Predominance of telogen hair follicles | — |
| Sebaceous gland atrophy | — |
| Epidermal atrophy | — |
| Epidermal melanosis | — |
| Thin dermis | — |
| Dermal collagen atrophy | — |
| Abnormalities Suggestive of Specific Endocrine Disorder | |
| Decreased amount and size of dermal elastin fibers | Hyposomatotropism |
| Excessive trichilemmal keratinization (flame follicles) | Growth hormone–and castration-responsive dermatosis |
| Vacuolated and/or hypertrophied arrector pilae muscles | Hypothyroidism |
| Increased dermal mucin content | Hypothyroidism |
| Thick dermis | Hypothyroidism |
| Comedones | Hyperadrenocorticism |
| Calcinosis cutis | Hyperadrenocorticism |
| Absence of arrector pilae muscles | Hyperadrenocorticism |
An abnormal increase in serum progesterone may result from adrenocortical neoplasia, functional ovarian luteal cysts in the bitch, and as a component of an imbalance in adrenocortical steroid hormone intermediates. Functional luteal cysts may cause prolonged anestrus or failure to cycle in the bitch. Clinical features of progesterone-secreting adrenocortical tumors mimic hyperadrenocorticism (see Chapter 53). Documenting increased serum progesterone concentration establishes the diagnosis, especially in a male or female spayed animal. Serum progesterone is normally increased in an intact female dog or cat in diestrus. A history of recent cycling behavior and examination of the ovaries and adrenal glands with abdominal ultrasound will help differentiate diestrus, functional luteal cysts, and adrenal neoplasia.
An increase in one or more of the adrenocortical steroid hormone intermediates often occurs in association with pituitary-dependent and adrenocortical tumor–dependent hyperadrenocorticism (Fig. 49-5). The predominant clinical signs in these dogs result from an excess of cortisol. An imbalance of adrenocortical steroid hormone intermediates such as 17-hydroxyprogesterone, progesterone, and androstenedione has been proposed as an explanation for hair cycle arrest, endocrine alopecia, and hyperpigmentation in dogs that do not have hyperadrenocorticism. A partial deficiency of 21-hydroxylase enzyme may account for the clinical and hormonal findings. Clinical signs for this syndrome (referred to as adrenal hyperplasia-like syndrome or Alopecia-X) are characterized by hair cycle arrest; bilaterally symmetric, nonpruritic alopecia; and hyperpigmentation of the skin and have been identified in many breeds, most notably in the American Eskimo, Pomeranian, Chow Chow, Keeshond, Malamute, Poodle, Samoyed, and Siberian Husky (Frank et al., 2003). Males are overrepresented. Routine blood and urine test results are typically normal. Skin biopsies from affected dogs show the typical changes of endo crine alopecia (see Table 49-5) and may also show features of follicular dysplasia. Diagnosis requires evaluation of adrenocortical steroid hormone intermediates and sex hormones before and after adrenocorticotropic hormone (ACTH) administration (see Chapter 53). The most common abnormality is an increase in serum 17-hydroxyprogesterone concentration. Currently, the only laboratory with established normal values for intermediate and sex steroids is the Endocrinology Laboratory at the University of Tennessee, College of Veterinary Medicine, Knoxville, TN 37901-1071. Treatment has included trilostane and mitotane.

FIG 49-5 A 7-year-old Poodle mix with hyperadrenocorticism and an increase in adrenocortical steroid hormone intermediates. Clinical signs included polyuria, polydipsia, and thinning of the haircoat on the trunk and tail. Tests of the pituitary-adrenocortical axis were inconclusive, and serum 17-hydroxyprogesterone concentrations were increased.
The differential diagnoses become more nebulous and the ability to establish a definitive cause of the alopecia more difficult once hypothyroidism, hyperadrenocorticism, and increased sex hormone and/or adrenocortical steroid hormone intermediates have been ruled out. Clinical manifestations of growth hormone (GH)–responsive dermatosis are similar to those described for increased adrenocortical steroid hormone intermediates (Fig. 49-6). Commonly affected breeds include Chow Chows, Pomeranians, Toy and Miniature Poodles, Keeshonds, American Water Spaniels, and Samoyeds; males are overrepresented; routine blood and urine test results are normal; and the endocrine alopecia responds to GH treatment. Unfortunately, there is no commercially available assay for measuring GH in dogs, and an effective GH product for treatment is not available for dogs.
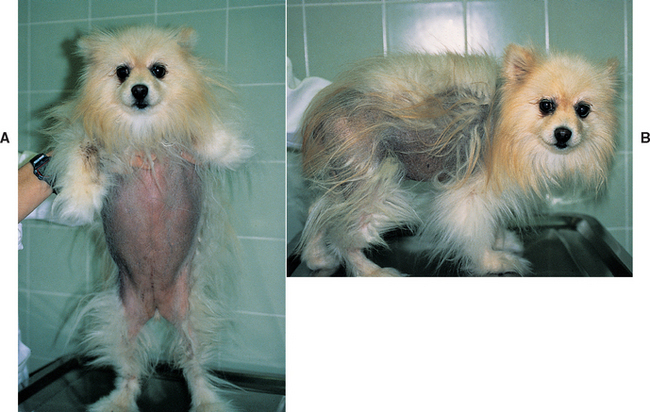
FIG 49-6 A and B, Endocrine alopecia in a 6-year-old Pomeranian with suspected adult-onset, GH-responsive dermatosis. Note the symmetric truncal alopecia with lesser involvement of the extremities and sparing of the head.
Endocrine alopecia may result from a deficiency of one of the sex hormones, most notably estrogens or androgens, or may be responsive to treatment with one of the sex hormones (see Table 49-4). Dermatologic manifestations are similar for most sex hormone–induced and sex hormone–responsive dermatoses and mimic the syndrome induced by alterations in sex hormone and adrenocortical steroid hormone intermediates (adrenal hyperplasia-like syndrome, Alopecia-X) and GH-responsive dermatosis, creating a difficult diagnostic challenge for the veterinarian, especially when the alopecia occurs in a breed such as the Chow Chow or Pomeranian. Diagnosis of sex hormone–deficiency or sex hormone–responsive dermatosis is based on response to treatment (Table 49-6). Castration of intact male dogs or sex hormone replacement therapy (e.g., diethylstilbestrol, methyltestosterone) in previously castrated or spayed dogs can be considered in dogs with endocrine alopecia of undetermined cause. Because of potentially serious adverse reactions to sex hormone replacement therapy, the more common causes of endocrine alopecia should always be ruled out before initiating treatment. The haircoat should improve within 3 months of the start of therapy. If there is no improvement within this time, another diagnosis should be considered.
 TABLE 49-6 Treatment for Sex Hormone-Induced or Sex Hormone-Responsive Endocrine Alopecia
TABLE 49-6 Treatment for Sex Hormone-Induced or Sex Hormone-Responsive Endocrine Alopecia
| DISORDER | PRIMARY TREATMENT | POTENTIAL ADVERSE REACTIONS TO THERAPY |
|---|---|---|
| Sertoli cell neoplasia | Castration | None |
| Castration-responsive dermatosis | Castration | None |
| Hyperestrogenism in the intact female dog | Ovariohysterectomy | None |
| Estrogen-responsive dermatosis of spayed female dogs | Diethylstilbestrol, 0.1-1.0 mg PO q24h 3 weeks per month; once responds, 0.1-1 mg q4-7 days | Aplastic anemia |
| Feline endocrine alopecia | Megestrol acetate, 2.5-5 mg/cat q48h until hair regrows; then 2.5-5 mg/cat q7-14 days | Adrenocortical suppression, benign mammary hypertrophy, mammary neoplasia, pyometra (female cats); infertility (male cats), diabetes mellitus |
| Testosterone-responsive dermatosis | Methyltestosterone, 1 mg/kg (maximum 30 mg) PO q48h until hair regrows, then q4-7 days | Aggression, hepatopathy |
| Telogen defluxion (effluvium) | None | None |
| Adrenal hyperplasia-like syndrome, Alopecia-X | Mitotane, trilostane Melatonin (see Chapter 53) | Hypoadrenocorticism |
PO, By mouth.
Response to melatonin treatment (3 to 6 mg q12-24h for 6 weeks) is perhaps the most innocuous nonspecific treatment option if diagnostic options have been exhausted and a definitive diagnosis for the endocrine alopecia has not been established. The mechanism of action of melatonin for promoting hair growth is not clear. Proposed mechanisms of action include inhibition of gonadotropin-releasing hormone (GnRH) secretion, thereby decreasing follicle-stimulating hormone (FSH), luteinizing hormone (LH), and sex hormone concentrations; stimulation of prolactin secretion; stimulation of GH or insulin-like growth factor-I (IGF-I) secretion; and a direct effect on hair follicles.
Many clients elect not to treat their dog once hypothyroidism, hyperadrenocorticism, ovarian cysts, and neoplasia of the adrenal gland, ovary, and testis have been ruled out. For these dogs the long-term prognosis is good, even without treatment. Dogs remain healthy aside from the alopecia and hyperpigmentation.
FELINE ACROMEGALY
Etiology
Chronic excessive secretion of GH in adult cats results in acromegaly, a disease characterized by overgrowth of connective tissue, bone, and viscera. In cats acromegaly is caused by a functional adenoma of the somatotropic cells of the pituitary pars distalis that secretes excess GH (Fig. 49-7). In most cats the pituitary tumor is a macroadenoma that extends dorsally above the sella turcica. Progestogen-induced acromegaly has not been documented in the cat. Progestogens, including megestrol acetate, do not appear to stimulate GH or IGF-I secretion in the cat. In contrast, acromegaly in the dog is seen most commonly after prolonged exposure to progestogens, either exogenously administered (e.g., medroxyprogesterone acetate) or late in life after years of endogenous progesterone secretion during the diestrual phase of the estrous cycle in the intact bitch.
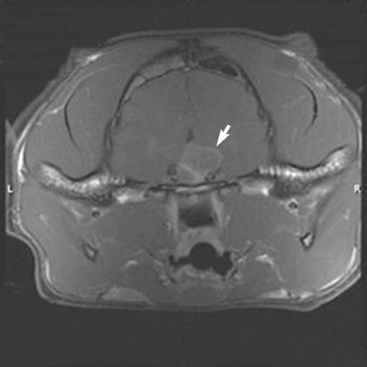
FIG 49-7 Magnetic resonance image of the pituitary region of a 6-year-old male, castrated domestic short-haired cat with insulin-resistant diabetes mellitus and acromegaly (see Fig. 49-8, A). A mass is evident in the hypothalamic-pituitary region (arrow).
Chronic excess secretion of GH has catabolic and anabolic effects. The anabolic effects are caused by increased concentrations of IGF-I. The growth-promoting effects of IGF-I result in proliferation of bone, cartilage, and soft tissues and in organomegaly, most notably of the kidney and heart. These anabolic effects are responsible for producing the classic clinical manifestations of acromegaly (Box 49-3). The catabolic effects of GH are a direct result of GH-induced insulin resistance that ultimately results in carbohydrate intolerance, hyperglycemia, and the development of diabetes mellitus that quickly becomes resistant to insulin treatment. Most but not all cats with acromegaly have diabetes mellitus at the time acromegaly is diagnosed, and most eventually develop severe resistance to exogenously administered insulin.
 BOX 49-3 Clinical Signs Associated with Acromegaly in Dogs and Cats
BOX 49-3 Clinical Signs Associated with Acromegaly in Dogs and Cats
IGF-I, Insulin-like growth factor-I; GH, growth hormone.
* Common findings.
Clinical Features
Acromegaly typically occurs in male, mixed-breed cats that are 8 years of age or older. Clinical signs result from the catabolic, diabetogenic effects of GH, the anabolic actions of chronic IGF-I secretion by the liver, and growth of the pituitary macroadenoma (see Box 49-3). The earliest clinical signs are usually PU, PD, and polyphagia resulting from concurrent diabetes mellitus. Polyphagia can become quite intense. Weight loss varies and depends in part on whether the anabolic effects of IGF-I or the catabolic effects of uncontrolled diabetes predominate. Most cats initially lose weight and then experience a period of stabilization followed by a slow, progressive gain in body weight as the anabolic effects of IGF-I begin to dominate the clinical picture. Severe insulin resistance eventually develops. Insulin dosages in cats with acromegaly frequently exceed 2 to 3 U/kg of body weight twice a day, with no apparent decline in the blood glucose concentration.
Clinical signs related to the anabolic actions of excess GH secretion (see Box 49-3) may be evident at the time diabetes mellitus is diagnosed. More commonly, however, they become apparent several months after diabetes has been diagnosed, often in conjunction with the realization that hyperglycemia is difficult to control with exogenous insulin therapy. Because of the insidious onset and slowly progressive nature of the anabolic clinical signs, clients are often not aware of the subtle changes in the appearance of their cat until the clinical signs are quite obvious. Anabolic changes in acromegalic cats include an increase in body size, enlargement of the abdomen and head, development of prognathia inferior, and weight gain (Fig. 49-8). Weight gain in a cat with poorly regulated diabetes mellitus is an important diagnostic clue to acromegaly. With time, organomegaly, especially of the heart, kidney, liver, and adrenal gland, develop. Diffuse thickening of soft tissues in the pharyngeal region can lead to extrathoracic upper airway obstruction and respiratory distress.

FIG 49-8 A, A 6-year-old male, castrated domestic short-haired cat with insulin-resistant diabetes mellitus and acromegaly. Note the broad face and mildly protruding mandible (prognathia inferior). B and C, An 8-year-old male, castrated domestic short-haired cat with insulin-resistant diabetes mellitus and acromegaly. Note the broad head, mildly protruding mandible, and prognathia inferior with displacement of the lower canine teeth.
(From Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
Neurologic signs may develop as a result of pituitary tumor growth and the resultant invasion and compression of the hypothalamus and thalamus. Signs include stupor, somnolence, adipsia, anorexia, temperature deregulation, circling, seizures, and changes in behavior. Blindness is not common because the optic chiasm is located anterior to the pituitary gland. Papilledema may be evident during an ophthalmic examination. Peripheral neuropathy causing weakness, ataxia, and a plantigrade stance may develop as a result of poorly controlled diabetes mellitus. Other endocrine and metabolic abnormalities resulting from the compressive effects of the tumor on the pituitary are uncommon.
Clinical Pathology
Concurrent, poorly controlled diabetes mellitus is responsible for causing most of the abnormalities identified on a serum biochemistry panel and urinalysis, including hyperglycemia, glycosuria, hypercholesterolemia, and a mild increase in alanine transaminase and alkaline phosphatase activities. Ketonuria is an infrequent finding. Mild erythrocytosis, persistent mild hyperphosphatemia without concurrent azotemia, and persistent hyperproteinemia (total serum protein concentration of 8.2 to 9.7 mg/dl) with a normal pattern of distribution on protein electrophoretic studies may also be found. Renal failure is a potential sequela of acromegaly and, if present, will be associated with azotemia, isosthenuria, and proteinuria.
Diagnosis
Clinical suspicion for acromegaly is based on the identification of conformational alterations (e.g., increased body size, large head, prognathia inferior, organomegaly) associated with acromegaly and a stable or progressive increase in body weight in a cat with insulin-resistant diabetes mellitus. Measurement of serum IGF-I concentration provides further evidence for the diagnosis of acromegaly. Measurement of serum IGF-I is commercially available (e.g., Diagnostic Endocrinology Laboratory, College of Veterinary Medicine, Michigan State University, East Lansing, MI 48909-7576). Concentrations are usually increased in acromegalic cats, but values may be in the reference range in the early stages of the disease (Fig. 49-9). Repeat measurements performed 4 to 6 months later will usually demonstrate an increase in serum IGF-I if acromegaly is present. The increase in serum IGF-I typically coincides with development and growth of the pituitary somatotropic adenoma. Increased serum IGF-I concentrations have been identified in a small number of poorly controlled diabetic cats in which the poor control was not caused by acromegaly. Interpretation of serum IGF-I test results should always take into consideration the status of control of the diabetic state, the presence and severity of insulin resistance, and the index of suspicion for acromegaly based on review of the history, physical examination, and results of routine blood and urine tests and diagnostic imaging. Identifying an increased serum IGF-I concentration in a poorly controlled diabetic cat with insulin resistance and clinical features suggestive of acromegaly supports the diagnosis and provides justification for CT or MR imaging of the pituitary gland. Documenting a pituitary mass by CT or MR scanning (see Fig. 49-7) adds further evidence for the diagnosis and is indicated whenever the client is considering radiation treatment. It is usually necessary to administer a positive contrast agent to visualize a pituitary mass using CT or MR imaging.
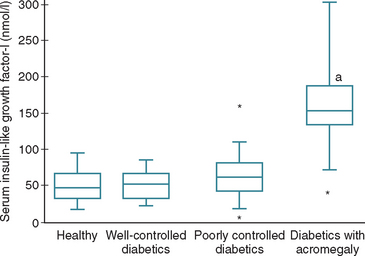
FIG 49-9 Box plots of serum concentrations of insulin-like growth factor-I (IGF-I) in 38 healthy cats, 15 well-controlled diabetic cats, 40 poorly controlled diabetic cats, and 19 poorly controlled diabetic cats with acromegaly. For each box plot, T-bars represent the main body of data, which in most instances is equal to the range. Each box represents the interquartile range (twenty-fifth to seventy-fifth percentile). The horizontal bar in each box is the median. Asterisks represent outlying data points. (a) P < 0.0001, compared with healthy cats and well-controlled and poorly controlled diabetic cats.
(From Berg RIM et al: Serum insulin-like growth factor-I concentration in cats with diabetes mellitus and acromegaly, J Vet Intern Med 21:892, 2007.)
A definitive diagnosis of acromegaly requires documentation of an increased baseline serum GH concentration. Baseline serum GH concentration in cats with acromegaly typically exceeds 10 ng/ml (normal concentration is less than 5 ng/ml). Unfortunately, a commercial GH assay is not available for cats.
ACROMEGALY VERSUS HYPERADRENOCORTICISM
Hyperadrenocorticism and acromegaly are uncommon disorders that occur in older cats, have a strong association with diabetes mellitus, can cause severe insulin resistance, and are often caused by a functional pituitary macrotumor. Clinical signs related to poorly controlled diabetes mellitus are common in cats with hyperadrenocorticism and acromegaly. Additional clinical signs differ dramatically between these two disorders. Hyperadrenocorticism is a debilitating disease that results in progressive weight loss leading to cachexia and dermal and epidermal atrophy leading to extremely fragile, thin, easily torn and ulcerated skin (i.e., feline fragile skin syndrome). In contrast, conformational changes caused by the anabolic actions of chronic IGF-I secretion dominate the clinical picture in acromegaly, most notably an increase in body size, prognathia inferior, and weight gain despite poorly regulated diabetes mellitus. Feline fragile skin syndrome does not occur with acromegaly. With both disorders most of the abnormalities identified on routine blood and urine tests are caused by concurrent poorly controlled diabetes mellitus. Abdominal ultrasound may also reveal mild bilateral adrenomegaly with both disorders. Ultimately, the differentiation between the two diseases is based on results of tests of the pituitaryadrenocortical axis (see Chapter 53) and serum GH and/or IGF-I concentrations.
Treatment
Radiotherapy is currently considered the most viable treatment option for acromegaly in cats. Cobalt teletherapy involves the administration of a total dose of 45 to 48 Gy in daily fractions five days per week for 3 to 4 weeks. The clinical response to cobalt teletherapy is unpredictable and ranges from no response to a dramatic response, characterized by shrinkage of the tumor; elimination of hypersomatotropism; resolution of insulin resistance; and, in some cats, reversion to a subclinical diabetic state (see Fig. 49-7). Typically, tumor size and plasma GH and serum IGF-I concentrations decrease and insulin responsiveness improves after cobalt teletherapy, although this improvement may take 6 months or longer to occur after radiation treatment. In most treated cats that respond to radiation therapy, diabetes mellitus and/or insulin resistance recurs 6 months or longer after treatment, although growth of the pituitary mass is often not evident on CT or MR imaging.
Microsurgical transsphenoidal hypophysectomy has been shown to be effective for the treatment of feline pituitary-dependent hyperadrenocorticism, but use of this specialized surgical technique for the treatment of acromegaly has not been reported. Successful use of transsphenoidal cryotherapy of a pituitary tumor has been described in a cat with acromegaly. An effective medical treatment for acromegaly in cats has not been identified.
Prognosis
The short- and long-term prognosis for cats with tumor-induced acromegaly is guarded to good and poor, respectively. The survival time has ranged from 4 to 60 months (typically 1.5 to 3 years) from the time the diagnosis of acromegaly is established. The GH-secreting pituitary tumor usually grows slowly, and neurologic signs associated with an expanding tumor are uncommon until late in the disorder. Diabetes mellitus is difficult to control, even with the administration of large doses of insulin (20 U or more/injection) given twice daily. Administration of large doses of insulin is not recommended. The severity of insulin resistance fluctuates unpredictably in cats with acromegaly, and severe, life-threatening hypoglycemia may suddenly develop after months of insulin resistance and blood glucose concentrations in excess of 400 mg/dl. To prevent severe hypoglycemia, insulin doses should not exceed 12 to 15 units per injection. Most cats with acromegaly eventually die or are euthanized because of the development of severe congestive heart failure, renal failure, respiratory distress, the neurologic signs of an expanding pituitary tumor, or coma caused by severe hypoglycemia.
PITUITARY DWARFISM
Etiology
Pituitary dwarfism results from a congenital deficiency of GH. Studies in German Shepherd Dog dwarfs suggest that congenital GH deficiency is caused by primary failure of differentiation of the craniopharyngeal ectoderm into normal tropic hormone–secreting pituitary cells. Pituitary cysts are commonly identified with diagnostic imaging of the pituitary region using CT or MR imaging and may enlarge as the pituitary dwarf ages. However, current belief is that pituitary cysts develop secondary to primary failure of anterior pituitary formation in most pituitary dwarfs. Pituitary dwarfism is encountered most often as a simple, autosomal recessive inherited abnormality in the German Shepherd Dog. A similar mode of inheritance has been reported in Carnelian Bear dogs. Inherited pituitary dwarfism may be due to isolated GH deficiency or may be part of a combined pituitary hormone deficiency. Concurrent deficiency in thyroid-stimulating hormone (TSH) and prolactin are most commonly identified in affected German Shepherd Dogs; ACTH secretion is preserved. Kooistra et al. (2000) hypothesize that the disorder is caused by a mutation in a developmental transcription factor that precludes effective expansion of a pituitary stem cell after differentiation of the corticotropic cells that produce ACTH. Pituitary dwarfism resulting from a mutant GH or an insensitivity to GH owing to a lack of or defect in GH receptors (e.g., Laron-type dwarfism in human beings) has not been documented in dogs or cats.
SIGNALMENT
Pituitary dwarfism occurs primarily in German Shepherd Dogs, although pituitary dwarfism in other dog breeds, including the Weimaraner, Spitz, Miniature Pinscher, Carnelian Bear dog, and Labrador Retriever, and in cats has also been observed. There does not appear to be a sex-related predilection.
CLINICAL SIGNS
The most common clinical manifestations of pituitary dwarfism are lack of growth (i.e., short stature), endocrine alopecia, and hyperpigmentation of the skin (Box 49-4). Affected animals are usually normal in size during the first 2 to 4 months of life but after that grow more slowly than their litter mates. By 5 to 6 months of age, affected dogs and cats are obviously runts of the litter and do not attain full adult dimensions. Dwarfs with an isolated GH deficiency typically maintain a normal body contour and body proportions as they age (i.e., proportionate dwarfism), whereas dwarfs with combined deficiencies (most notably TSH) may acquire a square or chunky contour typically associated with congenital hypothyroidism (i.e., disproportionate dwarfism; Fig. 49-10).
 BOX 49-4 Clinical Signs Associated with Pituitary Dwarfism
BOX 49-4 Clinical Signs Associated with Pituitary Dwarfism
* Common findings.

FIG 49-10 A, A 9-month-old male domestic short-haired cat with pituitary dwarfism. The size of the pituitary dwarf cat was similar to that of an 8-week-old kitten. Note the normal body contour and juvenile appearance. B and C, A 7-month-old female German Shepherd Dog with pituitary dwarfism. Note the normal body contour, puppy haircoat, and juvenile appearance. D, A 2-year-old female spayed Labrador Retriever with pituitary dwarfism sitting next to an age-matched normal Labrador Retriever to illustrate the small stature and juvenile appearance of the pituitary dwarf. All of the pituitary dwarfs presented with the primary owner complaint of failure of their pet to grow.
The most notable dermatologic sign is retention of the lanugo or secondary hairs, with concurrent lack of the primary or guard hairs. As a result, the haircoat in a dwarf is initially soft and wooly. The lanugo hairs are easily epilated, and a bilateral symmetric alopecia gradually develops. Initially, hair loss is confined to areas of wear, such as the neck (collar) and posterolateral aspects of the thighs (from sitting). Eventually, the entire trunk, neck, and proximal limbs become alopecic, with primary hairs remaining only on the face and distal extremities. The skin is initially normal but becomes hyperpigmented, thin, wrinkled, and scaly. Comedones, papules, and secondary pyoderma frequently develop in the adult dwarf. Secondary bacterial infections are common long-term complications.
Hypogonadism may also develop, although normal reproductive function has been observed in some animals with pituitary dwarfism. In the male animal cryptorchidism, testicular atrophy, azoospermia, and a flaccid penile sheath are typical; in the female persistent anestrus is common with impaired secretion of pituitary gonadotropins.
Clinical Pathology
Results of a CBC, serum biochemical panel, and urinalysis are usually normal in animals with uncomplicated pituitary dwarfism and isolated GH deficiency. Concurrent deficiency of TSH may result in clinicopathologic abnormalities affiliated with hypothyroidism, such as hypercholesterolemia and anemia (see Chapter 51). Deficiency of GH, IGF-I, and TSH may also affect kidney development and function, resulting in azotemia.
Diagnosis
The signalment, history, and physical examination usually provide sufficient evidence for pituitary dwarfism to be included among the tentative diagnoses of short stature. Strong presumptive evidence can be obtained by ruling out other potential causes of small size (Box 49-5) after a thorough evaluation of the history and physical examination findings, results of routine laboratory studies (i.e., CBC, fecal examinations, serum biochemical panel, urinalysis), and radiographic studies (Fig. 49-11). Serum IGF-I concentrations are decreased in pituitary dwarfs. Because baseline plasma GH concentrations may be low in healthy dogs and cats, a definitive diagnosis of hyposomatotropism requires evaluation of plasma GH concentrations during a stimulation test (Table 49-7). GH-releasing hormone (GHRH, 1 μg/kg body weight), clonidine (10 μg/kg), or xylazine (100 μg/kg) can be used. Blood for plasma GH measurements should be obtained immediately before and 20 and 30 minutes after intravenous administration of the secretagogue. In pituitary dwarfs there is no increase in plasma GH concentration after the administration of a GH secretagogue. A partial GH deficiency should be suspected whenever subnormal results are obtained.
 BOX 49-5 Some Potential Causes of Small Stature in Dogs and Cats
BOX 49-5 Some Potential Causes of Small Stature in Dogs and Cats
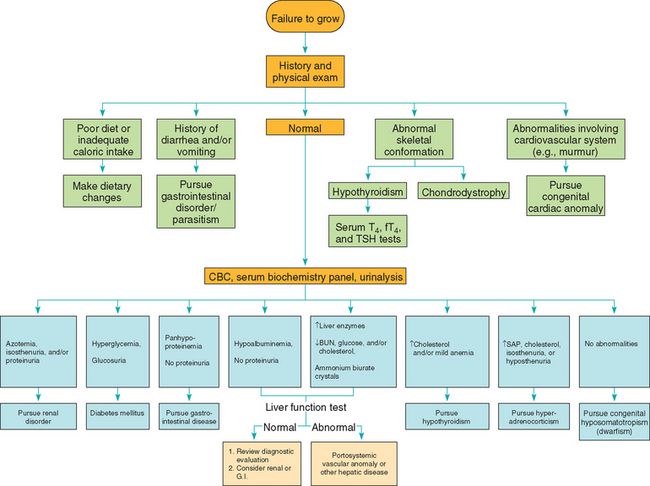
FIG 49-11 Diagnostic approach to the puppy or kitten that fails to grow.
(From Feldman EC, Nelson RW: Canine and feline endocrinology and reproduction, ed 3, St Louis, 2004, WB Saunders.)
 TABLE 49-7 Growth Hormone–Stimulation Testing Protocols
TABLE 49-7 Growth Hormone–Stimulation Testing Protocols
| TEST | DESCRIPTION AND RESULTS |
|---|---|
| Xylazine stimulation test* | |
| Protocol | 100 μg/kg IV; plasma samples obtained before and 20 and 30 minutes after administration of xylazine |
| Normal results | Twofold to fourfold increase in plasma GH 20 to 30 minutes after xylazine administration; poststimulation plasma GH > 10 ng/ml |
| Adverse reactions | Sedation (common), bradycardia, hypotension, collapse, shock, seizures |
| Clonidine-stimulation test | |
| Protocol | 10 μg/kg, IV; plasma samples obtained before and 20 and 30 minutes after administration of clonidine |
| Normal results | Twofold to fourfold increase in plasma GH 20 to 30 minutes after clonidine administration; poststimulation plasma GH > 10 ng/ml |
| Adverse reactions | Sedation (common), bradycardia, hypotension, collapse, aggressive behavior |
| GHRH-stimulation test | |
| Protocol | 1 μg/kg human GHRH, IV; plasma samples before and 20 and 30 minutes after GHRH |
| Normal results | 2 to 4 fold increase in plasma GH 20 to 30 minutes after GHRH administration; post- stimulation plasma GH > 10 ng/ml |
| Adverse reactions | None reported |
IV, Intravenous; GH, growth hormone; GHRH, growth hormone–releasing hormone.
Treatment
The therapy for pituitary dwarfism relies on the administration of GH. Unfortunately, an effective GH product is not available for use in dogs. Canine GH is not available for therapeutic use, GH antibody formation and legal restrictions preclude the use of biosynthetic human GH, and the concentration of biosynthetic bovine GH in commercial products for use in cattle precludes its use in dogs. The amino acid sequence of porcine GH is identical to canine GH, but porcine GH is difficult to find. If available, the recommended subcutaneous dose is 0.1 to 0.3 IU/kg three times per week for 4 to 6 weeks. Because of the synergistic influence of GH and thyroid hormone on growth processes, subnormal concentrations of thyroid hormone may diminish the effectiveness of GH therapy. Dogs and cats with suspected concurrent TSH deficiency should be treated with daily thyroid hormone supplementation, as discussed in Chapter 51.
Hypersensitivity reactions (including angioedema), carbohydrate intolerance, and overt diabetes mellitus are the primary adverse reactions associated with GH injections. Frequent monitoring of urine for glycosuria and blood for hyperglycemia should be done, and GH therapy should be stopped if either develops. Regrowth of hair, thickening of the skin, and changes in serum IGF-I and glucose concentrations are used to monitor therapy. A beneficial response in the skin and haircoat usually occurs within 6 to 8 weeks of the start of GH and thyroid hormone supplementation. The hair that grows back is lanugo or secondary hairs; the growth of primary or guard hairs is variable and may occur sporadically over the body. An increase in height is dependent on the status of the growth plates at the time treatment is initiated. A significant increase in height may occur if the growth plates are open, and minimal to no change in height will occur if the growth plates have closed or are about to close at the time treatment is initiated.
An increase in body size and regrowth of a complete haircoat has been reported in pituitary dwarfs treated with medroxyprogesterone acetate at doses of 2.5 to 5.0 mg/kg body weight, initially at 3-week intervals and subsequently at 6-week intervals. Progestogens induce the expression of the GH gene in the mammary gland of dogs, resulting in GH secretion from foci of hyperplastic ductular epithelial cells and increased plasma concentrations of GH and IGF-I. Adverse reactions with progestogen treatment include recurrent pruritic pyoderma, abnormal skeletal development, mammary tumors, diabetes mellitus, acromegaly, and cystic endometrial hyperplasia. Female dogs should be ovariohysterectomized before progestogen treatment. Serum IGF-I and glucose concentrations should be monitored.
Feldman EC, Nelson RW. Canine and feline endocrinology and reproduction, ed 3. St Louis: WB Saunders, 2004.
Aroch I, et al. Central diabetes insipidus in five cats: clinical presentation, diagnosis and oral desmopressin therapy. J Fel Med Surg. 2005;7:333.
Harb MF, et al. Central diabetes insipidus in dogs: 20 cases (1986–1995). J Am Vet Med Assoc. 1996;209:1884.
Nichols R. Clinical use of the vasopressin analogue dDAVP for the diagnosis and treatment of diabetes insipidus. In: Bonagura JD, editor. Kirk’s current veterinary therapy XIII. Philadelphia: WB Saunders, 2000.
van Vonderen IK, et al. Intra- and interindividual variation in urine osmolality and urine specific gravity in healthy pet dogs of various ages. J Vet Intern Med. 1997;11:30.
van Vonderen IK, et al. Disturbed vasopressin release in 4 dogs with so-called primary polydipsia. J Vet Intern Med. 1999;13:419.
van Vonderen IK, et al. Vasopressin response to osmotic stimulation in 18 young dogs with polyuria and polydipsia. J Vet Intern Med. 2004;18:800.
Ashley PF, et al. Effect of oral melatonin administration on sex hormone, prolactin, and thyroid hormone concentrations in adult dogs. J Am Vet Med Assoc. 1999;215:1111.
Frank LA. Growth hormone-responsive alopecia in dogs. J Am Vet Med Assoc. 2005;226:1494.
Frank LA, et al. Steroid hormone concentration profiles in healthy intact and neutered dogs before and after cosyntropin administration. Domest Animl Endocrinol. 2003;24:43.
Frank LA, et al. Retrospective evaluation of sex hormones and steroid hormone intermediates in dogs with alopecia. Vet Derm. 2003;4:91.
Paradis M. Melatonin therapy for canine alopecia. In: Bonagura JD, editor. Kirk’s current veterinary therapy XIII. Philadelphia: WB Saunders, 2000.
Schmeitzel LP, et al. Congenital adrenal hyperplasia-like syndrome. In: Bonagura JD, editor. Kirk’s current veterinary therapy XII. Philadelphia: WB Saunders, 1995.
Scott DW, et al, editors. Muller and Kirk’s small animal dermatology, ed 6, Philadelphia: WB Saunders, 2001.
Berg RIM, et al. Serum insulin-like growth factor-I concentration in cats with diabetes mellitus and acromegaly. J Vet Intern Med. 2007;21:892.
Goossens MMC, et al. Cobalt 60 irradiation of pituitary gland tumors in three cats with acromegaly. J Am Vet Med Assoc. 1998;213:374.
Reusch CE, et al. Measurements of growth hormone and insulin-like growth factor 1 in cats with diabetes mellitus. Vet Rec. 2006;158:195.
Starkey SR, et al. Investigation of serum IGF-I levels amongst diabetic and nondiabetic cats. J Feline Med Surg. 2004;6:149.