CHAPTER 67 Seizures
GENERAL CONSIDERATIONS
A seizure is the clinical manifestation of excessive or hypersynchronous abnormal electrical activity in the cerebral cortex. The clinical features of seizures can be separated into four components: the prodrome, aura, ictal period, and postictal period. The prodrome is the period of time before the seizure begins, when the owner may report unusual behavior such as hiding, attention seeking, whining, or agitation. The prodrome may be barely noticeable in some animals and distinct enough to enable owners to accurately predict seizure onset in others. The aura is the initial manifestation of the seizure, when animals exhibit stereotypical sensory or motor activity (pacing, licking, swallowing), autonomic patterns (salivation, vomiting, urination) or abnormal behavior (barking, attention seeking) for seconds to minutes before seizure onset. The ictal period is the seizure itself, when the animal exhibits a variety of signs that may include loss or derangement of consciousness, altered muscle tone, jaw chomping, salivation, and involuntary urination and defecation. This phase usually lasts only seconds to minutes. The postictal period immediately follows the seizure and can last from a few seconds to several hours, during which time the animal may exhibit abnormal behavior, disorientation, altered thirst or appetite, somnolence, or blindness as well as defined sensory and motor neurological deficits. Epilepsy is a chronic neurologic condition characterized by recurrent seizures.
Dogs and cats are occasionally affected by nonepileptic paroxysmal disorders, during which they may experience altered behavior, collapse, abnormal movements, transient neurologic symptoms, or paralysis. Distinguishing these disorders from seizures is important for diagnosis and treatment. Cardiac arrhythmias causing syncope; weakness caused by hypoglycemia, hypocortisolemia, or electrolyte disturbances; acute vestibular “attacks”; narcoleptic or cataplexic events; and weakness caused by myasthenia gravis are all examples of such paroxysmal events. Descriptions of the event and the animal’s activity and demeanor immediately preceding and following the event will help distinguish these events from seizures (Box 67-1). One helpful distinguishing feature is that only seizures should have an associated postictal period.
SEIZURE DESCRIPTIONS
Most seizures in dogs and cats are tonic-clonic, generalized motor seizures in which the animal experiences a period of extremely increased extensor muscle tone (tonus), falls into lateral recumbency, and then has periods of tonus alternating with periods of relaxation (clonus), resulting in rhythmic contractions of muscles manifested as paddling or jerking of the limbs and chewing movements. Animals are usually unconscious during these seizures, although their eyes may remain open.
Less common than generalized, symmetric tonic-clonic seizures in dogs and cats are focal partial motor seizures. These seizures arise in part of one cerebral hemisphere, resulting in asymmetric signs that may include turning of the head away from the side of the lesion and focal twitching or tonic-clonic contractions of the contralateral facial or limb muscles. Some focal seizures are primarily manifested as altered consciousness and bizarre behaviors (psychomotor seizures), which may include aggression, howling, “fly biting,” pacing, circling, restlessness, and staggering. It can sometimes be very difficult to distinguish psychomotor seizures from compulsive stereotypic behavior. Focal seizures may progress to generalized motor seizures in some animals. Although it is often stated that partial motor seizures are usually associated with structural brain disease, many dogs with idiopathic epilepsy experience focal seizures with secondary generalization.
SEIZURE CLASSIFICATION AND LOCALIZATION
Seizure disorders are classified according to their cause as being idiopathic, intracranial, or extracranial in origin (Box 67-2). Idiopathic epilepsy is diagnosed in approximately 25% to 30% of dogs having seizures but is uncommon in cats. Animals with idiopathic epilepsy have no identifiable extracranial or intracranial cause for their seizures and no concurrent neurologic abnormalities, and their seizures are presumed to be genetically based. Approximately 35% of dogs with seizures and most cats with seizures have an identifiable structural intracranial lesion (e.g., anomaly, inflammation, neoplasia, trauma) that is causing seizures, and these animals are said to have symptomatic epilepsy. A very small number of patients have seizures believed to be secondary to a scar or residual brain damage following a previous insult, but this structural lesion is difficult to demonstrate; such animals are classified as having probable symptomatic epilepsy. Extracranial causes such as the ingestion of toxins or metabolic or endocrine derangements also result in seizures.
Seizure activity always indicates a functional or structural abnormality of the forebrain, particularly of the frontal or temporal lobes of the cerebrum. Metabolic and toxic disorders cause seizures through functional alterations of the balance between inhibitory and excitatory neurotrans-mitters. Defined, localizing neurologic deficits are unlikely to be detected interictally (between seizures) in patients with extracranial causes of seizures. Animals with an intracranial lesion causing symptomatic epilepsy may exhibit myriad signs leading to forebrain neurolocalization, including behavior change, circling toward the side of the lesion, contralateral hemiparesis and postural reaction deficits, and contralateral vision loss and facial hypalgesia. Some animals with small lesions will, however, be normal interictally, with no other defined neurologic deficits.
Idiopathic epilepsy is a condition wherein the seizure threshold is decreased. This can be caused by intrinsic neurotransmitter imbalances, genetic mutations affecting ion channels, or other functional abnormalities. Epileptic foci contain cells with an intrinsic pattern of high spontaneous firing, leading to seizure activity. Idiopathic epilepsy has been shown to be inherited in a few dog breeds, and a familial basis for the condition is suspected in others. Affected animals are normal interictally, and extensive diagnostic evaluation, including histologic examination of the brain, is normal.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis for a patient with seizures includes idiopathic epilepsy, intracranial disease (symptomatic epilepsy), probable symptomatic epilepsy, and extracranial disease.
IDIOPATHIC EPILEPSY
Idiopathic epilepsy is the most common cause of seizures in the dog and is characterized by repeated episodes of seizures with no demonstrable cause. Affected dogs are normal between seizures. Idiopathic epilepsy is uncommon in cats; most cats with seizures have an identifiable intracranial cause, such as neoplasia or encephalitis.
Idiopathic epilepsy is inherited in German Shepherd Dogs, Belgian Tervurens, Keeshonds, Beagles, and Dachshunds. On the basis of pedigree analysis, genetic factors are also strongly suspected in Labrador Retrievers, Golden Retrievers, and Collies. Epilepsy is also commonly seen in Saint Bernards, Cocker Spaniels, Irish Setters, Boxers, Siberian Huskies, English Springer Spaniels, Alaskan Malamutes, Border Collies, Shetland Sheepdogs, Miniature Poodles, and Wire Fox Terriers. It is seen sporadically in almost all breeds, mixed-breed dogs, and cats.
The initial onset of seizures usually occurs between 6 months and 3 years of age, although seizures are not observed until 5 years of age in some dogs. In most breeds it seems that the younger the age at the onset of a seizure disorder, the more difficult the disorder will be to control. A difficult-to-control seizure disorder develops at a very young age in some purebred dogs (e.g., 8- to 12-week-old Cocker Spaniels), but such animals may then outgrow the problem by 4 to 6 months of age. This form of epilepsy is termed juvenile epilepsy.
The seizures in dogs and cats with idiopathic epilepsy are usually generalized, tonic-clonic, and associated with a loss of consciousness lasting from 1 to 2 minutes. Some dogs, especially Labrador Retrievers and Miniature Poodles, may instead experience a mild, generalized type of seizure in which they remain alert but anxious while they exhibit a crouched stance, uncontrollable trembling, muscular rigidity, or disequilibrium. Many of these dogs experience a postictal phase and develop more classical generalized tonic-clonic seizures later in life, confirming that these events are seizures. A similar syndrome identified in Chinooks (a Northern breed) may be a paroxysmal movement disorder (dyskinesia) rather than a seizure disorder.
Simple or complex focal seizures with or without secondary generalization may also occur in animals with idiopathic epilepsy. Seizures typically recur at regular intervals, with weeks or months intervening between the seizures. As the animal ages, the frequency and severity of seizures may increase, especially in large-breed dogs. In some dogs, particularly those of large breeds, seizures can eventually occur in clusters, in which multiple seizures occur during a 24-hour period. Clusters of seizures are not usually seen in association with the first seizure in dogs with idiopathic epilepsy, except in Border Collies, Dalmatians, and German Shepherd Dogs. If more than two seizures occur during the first week of a seizure disorder, a progressive intracranial or extracranial cause should be suspected.
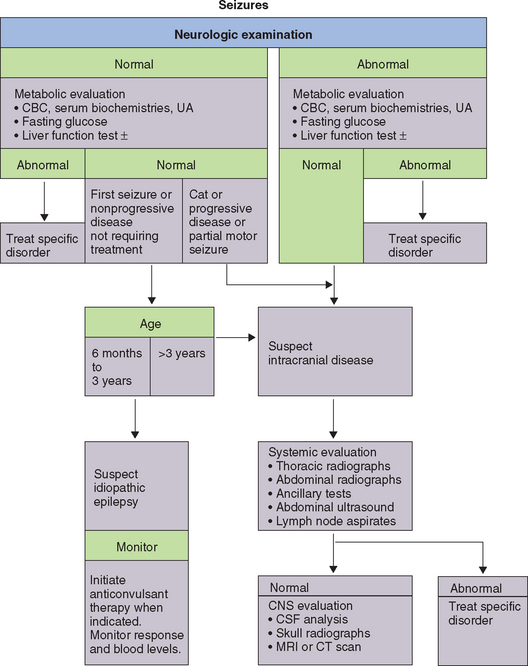
Idiopathic epilepsy is the most likely diagnosis in a young adult, neurologically normal animal with a long history (>1 year) of a nonprogressive intermittent seizure disorder and a lengthy interictal period (>4 weeks). Findings from a complete physical, neurologic, and ophthalmologic evaluation and results of routine clinicopathologic tests are normal. Intracranial evaluation, when performed, is normal (Fig. 67-1).
INTRACRANIAL DISEASE
Symptomatic epilepsy is a direct result of intracranial disease localized in the forebrain. Congenital and infectious inflammatory conditions are most often seen in young animals, whereas neoplasia is the most common cause in dogs and cats older than 6 years of age. Most of the intracranial disorders discussed in Chapter 65 and the inflammatory disorders discussed in Chapter 69 can cause symptomatic epilepsy (see Box 67-2). Focal or multifocal neurological deficits identified interictally may suggest structural forebrain pathology, but not all patients with symptomatic epilepsy will have an abnormal neurologic examination. Diagnosis requires careful physical, neurological, and ophthalmologic examination; evaluation for concurrent systemic manifestations of infectious and neoplastic disorders; and often intracranial evaluation, including cerebrospinal fluid (CSF) analysis, and advanced diagnostic imaging (computed tomography [CT] or magnetic resonance imaging [MRI]).
PROBABLE SYMPTOMATIC EPILEPSY
Scar tissue–related acquired epilepsy can occur after an inflammatory, traumatic, toxic, metabolic, or vascular insult. If a history of significant trauma or infection can be ascertained, the event usually precedes the onset of the seizure disorder by 6 months to 3 years. Findings from physical and neurologic examinations, clinicopathologic tests, and CSF analysis are normal. It is not usually possible to detect a structural abnormality using MRI, and even necropsy will not reliably demonstrate a lesion. The treatment is the same as for idiopathic epilepsy (i.e., anticonvulsant therapy), but the prognosis for seizure control in some large-breed dogs may be better for those with scar tissue–related acquired epilepsy than for those with idiopathic epilepsy.
EXTRACRANIAL DISEASE
Hypoglycemia, hepatic encephalopathy, hypocalcemia, and primary hyperlipoproteinemia may cause seizures in dogs and cats. Other metabolic alterations, including hyperviscosity syndromes (e.g., multiple myeloma, polycythemia), severe electrolyte disturbances (e.g., hypernatremia), hyperosmolality (e.g., untreated diabetes mellitus), heatstroke, and prolonged severe uremia, also occasionally cause seizures (see Box 67-2). In many of these disorders intermittent nonneurologic clinical signs and physical examination findings point toward an extracranial cause of the seizures. Most metabolic encephalopathies also intermittently or permanently alter consciousness, manifesting as confusion, delirium, or depression at least intermittently. Results of a complete blood count (CBC), serum biochemistry panel, and urinalysis often help establish the diagnosis. Hepatic encephalopathy resulting from portosystemic shunting can occasionally cause seizures in the absence of other clinical or clinicopathologic abnormalities, especially in cats, so evaluation of liver function is an important component of the initial evaluation for metabolic causes of seizures. More detailed information on the diagnosis and management of these metabolic disorders is contained elsewhere in this text. Common intoxications causing seizures are described in Box 67-3, and treatment of intoxications is outlined in Box 67-4.
 BOX 67-3 Intoxications Resulting in Acute Neurologic Dysfunction
BOX 67-3 Intoxications Resulting in Acute Neurologic Dysfunction
Strychnine
Metaldehyde
Chlorinated Hydrocarbons
Organophosphates and Carbamates
Lead
Ethylene Glycol
 BOX 67-4 Emergency Treatment of Intoxications
BOX 67-4 Emergency Treatment of Intoxications
Prevent Further Absorption of Intoxicant
Induce emesis
Gastrointestinal adsorbents
DIAGNOSTIC EVALUATION
A complete and accurate history must be obtained in every animal presenting for a seizure. The owner’s description is critical to determine whether the observed paroxysmal event was actually a seizure and to characterize any seizures as generalized, focal, or psychomotor. The relationship of seizures to daily activity (e.g., exercise, sleep, eating, excitement), seizure duration, and a description of any observed postictal abnormalities should be recorded. Owners should be asked whether they have noticed any changes in the animal’s behavior, gait, vision, or sleep patterns in the weeks or months preceding the seizure, characteristics that might indicate a structural forebrain lesion. Recent systemic signs such as cough, vomiting, diarrhea, polyuria, polydipsia, and weight loss or weight gain should also be recorded. Vaccination status, diet, potential exposure to infectious causes of encephalitis, access to drugs or toxins, and history of serious head injury should also be determined. When seizures have occurred intermittently over a prolonged period of time (weeks to months), the seizure pattern and frequency should be assessed and the owner should be asked to record frequency and severity of all future seizures on a calendar to allow objective evaluation of disease progression or response to therapy. When idiopathic epilepsy is considered likely, owners should be encouraged to contact the breeder to ascertain whether litter mates or other related dogs are affected.
Physical, ophthalmologic, and neurologic examinations should be obtained in every animal presented for seizures. In the immediate postictal period transient symmetric neurologic abnormalities such as blindness, altered consciousness, and postural reaction deficits are common, so these should not be overinterpreted. Neurologic abnormalities that persist beyond the postictal period suggest an intracranial cause for seizures requiring further evaluation. Lymph node and abdominal palpation as well as mammary gland and prostate examination should be performed to evaluate for primary neoplasia that could have spread to the brain. Some animals with toxic or metabolic causes of seizures will also have specific abnormal findings on physical examination, which aid in diagnosis. Results of these examinations will be normal in dogs and cats with idiopathic epilepsy as well as in many patients with intracranial and extracranial causes of seizures.
Every animal evaluated for seizures should undergo routine screening laboratory tests, including a CBC, serum biochemistry panel, and urinalysis. Blood glucose should also be measured during observed neurologic signs or after a 12-hour fast. Liver function should be evaluated in dogs and cats that are less than 1 year of age at the time of their first seizure and in all animals with initial laboratory results suggesting hepatic dysfunction (see Chapter 36).
The animal’s signalment and history as well as the onset and progression of the seizure disorder allow ranking of likely differential diagnoses. Congenital structural disorders such as hydrocephalus and lissencephaly are the most likely causes of a seizure disorder in a very young animal. Infectious causes of encephalitis usually cause rapidly progressive neurologic dysfunction rather than seizures alone. In aging animals cerebral neoplasia, vascular accidents, and acquired metabolic disturbances are more likely causes of seizures. Animals with idiopathic epilepsy typically have their first observed seizure between 6 months and 3 years of age; thus it is not a likely diagnosis in a dog or cat with seizures that began late in life.
When the systemic, neurologic, and screening laboratory tests are all normal, recommendations for further testing are based on history and signalment. Dogs between 1 and 3 years of age when their first seizure is observed, presenting with a single generalized seizure or a history of a few generalized seizures weeks or months apart, most likely have idiopathic epilepsy; further evaluation may not be required. Typically, the frequency and severity of the seizures are monitored, and, when necessary, treatment is initiated with anticonvulsant therapy. Idiopathic epilepsy is uncommon in cats; therefore, even when all routine screening tests are normal, cats should be tested for feline leukemia virus and antibody against feline immunodeficiency virus, and intracranial evaluation should be recommended.
Further testing, including intracranial evaluation, should be recommended in all dogs with interictal neurologic abnormalities, in dogs older than 5 years of age when their first seizure is observed, and in dogs with focal seizures or multiple seizures that take place within a 1-month period. When neurologic or systemic signs are present that could be caused by infections diseases endemic to the region, noninvasive and relatively inexpensive serologic testing may be beneficial. Thoracic and abdominal radiographs and abdominal ultrasound should be performed to look for systemic manifestations of infectious causes of symptomatic epilepsy and for primary or metastatic neoplasia. If these tests are negative, advanced imaging of the brain with MRI or CT is performed, as well as CSF collection and analysis.
ANTICONVULSANT THERAPY
Management of dogs and cats with seizures can be attempted using anticonvulsant therapy. Because this requires a large financial, emotional, and time commitment by owners, they should be involved in the decision to initiate treatment. Not every animal with seizures requires anticonvulsant therapy, but there is compelling evidence that dogs treated early in the course of their seizure disorder may have better long-term control of their seizures compared with dogs that are allowed to have many seizures before treatment is initiated. Anticonvulsant therapy should be initiated in all dogs and cats with the following: (1) seizures caused by an intracranial lesion, (2) one or more episodes of cluster seizures or status epilepticus, (3) seizures that occur more often than once every 12 to 16 weeks, or (4) seizures that are becoming more frequent (Box 67-5).
Complete control of seizures in dogs and cats with idiopathic epilepsy is rarely possible, but a decrease in the frequency and severity of seizures is a realistic goal that can be accomplished in 70% to 80% of animals. Owners should keep a log detailing the frequency and severity of seizures so that the effects of the medication can be monitored. Adverse effects of the medication and plans for monitoring blood concentrations and dose adjustments should be discussed. Emergency situations, such as status epilepticus, should be described to owners and specific recommendations for treatment and veterinary assistance provided. A minimum database, including a CBC, serum biochemistry profile, and urinalysis, should always be obtained immediately before the start of anticonvulsant therapy, and if one was not recently performed, a liver function test is also recommended. Whenever possible, animals should be initially treated with a single anticonvulsant drug (monotherapy) to decrease the prevalence of adverse effects, optimize owner compliance, and decrease overall costs of drugs and monitoring. Clinical response and therapeutic drug concentrations should be monitored to determine the proper dose of anticonvulsant drug for the individual animal. If the initial drug adminis tered is ineffective in spite of optimal serum drug concentrations, then another antiepileptic drug should be added or substituted (Box 67-6).
 BOX 67-6 Guidelines for Anticonvulsant Therapy in Dogs
BOX 67-6 Guidelines for Anticonvulsant Therapy in Dogs
PB, Phenobarbital; PO, by mouth.
ANTICONVULSANT DRUGS
PHENOBARBITAL
Phenobarbital (PB) has been considered the drug of choice for the initial and ongoing treatment of seizures in dogs and cats for decades. PB is a relatively safe, effective, and inexpensive anticonvulsant drug. It has a high bioavailability and is rapidly absorbed, with peak plasma concentration 4 to 8 hours after oral administration. An appropriate starting dose is 2.5 mg/kg given orally twice a day.
After 2 weeks of therapy the animal should be examined and its morning prepill (trough) blood PB concentration determined. The trough serum PB concentration should be in the therapeutic range of 25 to 35 μg/ml (107 to 150 μmol/L) in dogs and 10 to 30 μg/ml (45 to 129 μmol/L) in cats. If the serum concentration is too low, the dose of PB should be increased by approximately 25% (see Box 67-6) and the trough serum concentration determined again 2 weeks later. If the serum concentration is still inadequate, the dose of PB should be increased in 25% increments every 2 weeks while the blood concentration is monitored. Once the measured blood concentration of PB is adequate, the dog or cat should be observed through two or three cycles of seizures, and if control is determined to be acceptable, therapy is maintained at that dosage. Long-term dosing of PB can be complicated by the drug’s induction of hepatic microsomal enzyme activity, increasing its own elimination and necessitating dosage increases. Blood PB concentrations should be reevaluated routinely every 6 months, 2 weeks after any change in dosage, and whenever two or more seizures occur between scheduled PB evaluations. Serum separator tubes should not be used to collect serum for this purpose because their use will underestimate the concentration of PB.
PB is well tolerated in most dogs at therapeutic serum concentrations. Sedation, depression, and ataxia may be pronounced for the first 7 to 10 days of therapy, but these adverse effects resolve with time (10 to 21 days) as the animal acquires a tolerance for the sedative effects of the drug. Transient (7 days) hyperexcitability can occur as an idiosyncratic effect in up to 40% of dogs and cats. The most common persistent adverse effects of PB include polyuria, polydipsia, and polyphagia. Owners should be advised to refrain from overfeeding animals receiving this anticonvulsant, even though their pet seems ravenous. Many animals acquire a dependence on the drug, and sudden withdrawal of the drug can precipitate seizures; therefore it is important for owners to administer the drug consistently once treatment is started.
Immune-mediated neutropenia or thrombocytopenia has been recognized in a few dogs within the first 6 months of starting PB, but these blood dyscrasias resolve when the PB is discontinued. PB administration may also be a risk factor for the development of superficial necrolytic dermatitis in dogs. The most life-threatening potential complication of PB therapy is drug-induced hepatotoxicity. PB is a potent inducer of hepatic enzymes, and mild to moderate elevations in serum alkaline phosphatase (ALP) and alanine transaminase (ALT) activities are seen in virtually all dogs receiving the anticonvulsant. Significant hepatotoxicity is uncommon but is most likely to occur when peak serum PB concentrations are at the high end of the therapeutic range (35 μg/ml; >150 μmol/L). Clinical features of significant hepatotoxicity include anorexia, sedation, ascites, and occasionally icterus. Laboratory testing typically reveals a large increase in ALT, decreased serum albumin, and abnormal bile acids. When hepatotoxicity is discovered, the patient should be rapidly switched to an alternative anticonvulsant and supportive measures initiated for liver failure. All animals receiving chronic PB therapy should be evaluated every 6 months to assess the effectiveness of the drug regimen, the serum concentration of PB, liver enzyme activities, and liver function.
PB increases the biotransformation of drugs metabolized by the liver, decreasing the systemic effects of many drugs administered concurrently. PB also increases the rate of thyroid hormone elimination, decreasing measured serum total and free T4 and increasing serum thyroid-stimulating hormone concentrations, but this is rarely associated with clinical signs of hypothyroidism (see Chapter 51). Drugs that inhibit microsomal enzymes (e.g., chloramphenicol, tetracycline, cimetidine, ranitidine, enilconazole) may dramatically inhibit the hepatic metabolism of PB, resulting in increased serum concentrations of PB and potentially causing toxicity.
Seizures are controlled in 70% to 80% of dogs and most cats treated with PB monotherapy if serum PB concentrations are maintained within the target range. If seizures continue to occur at an unacceptable frequency or severity despite adequate serum concentrations, therapy with additional drugs must be considered.
POTASSIUM BROMIDE
Control of refractory seizures can be improved through the addition of potassium bromide (KBr) to already established PB therapy in animals with poorly controlled seizures despite adequate serum concentrations of PB, decreasing seizure numbers by 50% or more in approximately 70% to 80% of dogs (see Box 67-6). KBr is also effective as a single agent and is considered by many to be the initial drug of choice in dogs with hepatic dysfunction and dogs that do not tolerate PB. KBr monotherapy is also commonly administered to large dogs with idiopathic epilepsy and a low frequency of seizures. The drug should not be administered to cats because of a high prevalence of drug-associated severe progressive bronchitis in that species. Bromide is excreted unchanged by the kidney. It is not metabolized by the liver and does not cause hepatotoxicity. Potassium bromide is typically administered as the inorganic salt dissolved in double distilled water to achieve a concentration of 200 to 250 mg/ml. Administration of the salt in gelatin capsules is also possible, but the concentrated drug in this form often causes gastric irritation and vomiting. Dietary chloride should remain constant in dogs treated with KBr because high chloride intake (e.g., chips, rawhide bones) results in increased renal excretion of KBr and decreased serum concentrations. An appropriate starting dose of KBr is 20 mg/kg orally twice daily for monotherapy and 15 mg/kg orally twice daily when used as an add-on drug to PB. KBr serum concentrations should be measured 1 month after initiating therapy, 8 to 12 weeks later when a steady state is achieved, and then annually. The goal is to achieve a serum concentration of 2.5 to 3.0 mg/ml (25 to 30 mmol/L) of KBr when used as monotherapy and 1.0 to 2.0 mg/ml (10 to 20 mmol/L) when used together with PB. Serum PB concentrations should also be maintained in the midtherapeutic range in animals receiving KBr and PB.
When maintenance doses of KBr are administered, there is a long lag period between the initiation of treatment and achieving steady-state serum concentrations. KBr is therefore not recommended as monotherapy in dogs with frequent seizures in which rapid control is required. If KBr must be administered as the only anticonvulsant therapy in a dog with a severe or progressive seizure disorder or in a dog that must be switched from PB to KBr because of toxicity, it is possible to achieve therapeutic serum concentrations of KBr rapidly using a loading-dose protocol. Oral loading can be accomplished by administering 30 mg/kg of KBr orally four times a day for 5 days with food, followed by the administration of maintenance doses.
Adverse effects of KBr include polyuria, polydipsia, and polyphagia, but these may be less dramatic than the changes induced by PB therapy. Transient sedation, incoordination, anorexia, and constipation can also occur. Reversible limb stiffness, lameness, and muscle weakness will occur if serum bromide levels are excessive. Vomiting is a very common problem caused by gastric irritation from the hyperosmolality of the drug; this toxicity can be diminished by further splitting the daily dose (into four equal doses administered approximately every 6 hours) and by feeding a small amount of food with each dose. Pancreatitis occurs rarely. Dramatic sedation can occur in dogs being concurrently treated with PB; this is usually temporary but can be decreased by lowering the dose of PB administered by 25% or by administering intravenous saline to increase the renal excretion of KBr, keeping in mind that dramatically lowering the serum concentration of either drug may cause increased seizure activity. Biochemical abnormalities are not common in dogs treated with KBr monotherapy, but because some laboratory assays cannot distinguish bromide from chloride, there may be an artifactual increase in measured chloride.
DIAZEPAM
Diazepam (Valium; Roche) is of limited use as a primary anticonvulsant in dogs because of its expense, its very short half-life, physical dependence, and the rapid development of tolerance to its anticonvulsant effects. Oral diazepam has been shown to be of some benefit for the long-term management of seizures in cats because tolerance to its anticonvulsant effect does not seem to occur in that species. Diazepam can be administered orally (0.3 to 0.8 mg/kg q8h) to achieve trough blood concentrations of 200 to 500 ng/ml. The drug is eliminated by hepatic metabolism, and the only common adverse effect is sedation, although idiosyncratic severe, life-threatening hepatotoxicity has been documented in a few cats receiving daily diazepam for 5 to 11 days. This potentially fatal reaction warrants close owner observation of appetite and attitude and periodic monitoring of liver enzymes in all cats treated with diazepam. PB is a better choice for chronic anticonvulsant therapy in cats.
Diazepam also has a place in the emergency management of seizures and in the at-home treatment of dogs with idiopathic epilepsy experiencing cluster seizures. In dogs with a recognizable preictal phase or an aura preceding the seizure, an injectable preparation of diazepam (5 mg/ml) can be administered rectally (2 mg/kg) by the owner at the onset of these premonitory signs. Alternatively, this dose can be administered just after each observed seizure, with a maximum of three doses in 24 hours (each dose separated by at least 10 minutes). At-home rectal administration of diazepam decreases the occurrence of cluster seizures and the development of status epilepticus as well as dramatically decreasing the need for owners to seek expensive emergency treatment for their epileptic dogs. Diazepam dispensed for at-home rectal administration should be stored in a glass vial because plastic will adsorb the drug, decreasing its effectiveness. For administration the drug can be drawn into a syringe and injected through a 1-inch plastic teat cannula or rubber catheter directly into the rectum.
CLORAZEPATE
Clorazepate (Traxene; Abbott Laboratories) is a benzodiazepine with a slightly more prolonged action than that of diazepam. This drug is effective as a sole anticonvulsant or when administered as an add-on drug. Chronic administration can result in tolerance to its antiseizure effects, potentially making all benzodiazepines ineffective for emergency use. The only recognized adverse effects are sedation, ataxia, and polyphagia, although acute hepatic necrosis might be a concern in cats because of shared metabolites with diazepam. There is also a potential for severe withdrawal seizure activity with this drug. The starting dose is 1 to 2 mg/kg, administered orally q12h, with desired therapeutic concentration of 300 to 500 ng/ml. Clorazepate administration to dogs being chronically treated with PB will increase serum PB concentrations, requiring monitoring and dosage adjustments.
FELBAMATE
Felbamate (Felbatol; Wallace) is an effective anticonvulsant in dogs when used alone or as an add-on drug in dogs refractory to anticonvulsant therapy with PB and KBr. Following urinary excretion of 70% of the orally administered dose, Felbamate is metabolized by hepatic microsomal P450 enzymes. The recommended starting dose is 15 mg/kg q8h. Felbamate appears to have a wide margin of safety, and the daily dose can be increased in 15 mg/kg increments until the seizures are adequately controlled, with reports of dosages as high as 70 mg/kg q8h without toxicity. Felbamate is an unusual anticonvulsant in that it does not cause sedation. Because approximately 30% of dogs treated with felbamate as an add-on drug with PB develop hepatotoxicity, monitoring of biochemistry panels and liver function tests is recommended. Aplastic anemia has been reported in humans receiving this drug but has not been documented in dogs. Serial monitoring of CBC and serum biochemistry panel is recommended at 1 month and every 3 months during treatment. Trough serum concentrations between 25 and 100 mg/L are reported to be therapeutic.
GABAPENTIN
Gabapentin (Neurontin; Parke-Davis) is a structural analog of GABA, with a poorly understood mechanism of action. The drug is rapidly absorbed and renally excreted with some hepatic metabolism. The elimination half-life in dogs is very short (3 to 4 hours), requiring dosing every 6 to 8 hours. Moreover, the drug has a very high therapeutic index and very little potential for drug-drug interaction. Starting doses of 10 to 20 mg/kg q8h have been recommended. The dose should be increased gradually as needed (up to 80 mg/kg q6h) to avoid excessive sedation, which is the only reported adverse effect. Serum concentrations are rarely monitored, but the suspected therapeutic range for dogs is 4 to 16 mg/L. Preliminary clinical evaluation of gabapentin as an add-on drug in dogs with refractory epilepsy has recorded decreased seizure frequency in 50% of cases.
ZONISAMIDE
Zonisamide (Zonegran; Elan) is a sulfonamide-based anticonvulsant that suppresses epileptic foci and blocks the propagation of epileptic discharges. This drug is well absorbed and hepatically metabolized, with a relatively long half-life (15 hours) in dogs not concurrently receiving PB or other drugs that induce microsomal enzymes. Zonisamide is effective as a sole agent or as an add-on drug. Mild adverse effects reported include sedation, ataxia, vomiting, and inappetence. The initial starting dose is 5 mg/kg twice daily in dogs not receiving PB and 10 mg/kg twice daily in dogs receiving concurrent PB. A serum concentration of 10 to 40 μg/ml is reported to be therapeutic.
LEVITIRACETAM
Levitiracetam (Keppra) is a new anticonvulsant that is well tolerated and effective in human patients. The drug is well absorbed and rapidly metabolized, with a half-life of 3 to 4 hours in dogs. Most of the drug is excreted unchanged in the urine, and the remainder is metabolized by hydrolysis in multiple organs, with no significant hepatic metabolism. Limited information is available on its use in dogs and cats, but it reportedly decreases seizure frequency by over 50% in epileptic dogs when used as an add-on drug and has also been effective in cats with refractory seizures. A starting dose of 20 mg/kg q8h is recommended, with some reports of administration of much higher doses without toxicity. Adverse effects include minimal sedation and salivation and vomiting in a few dogs.
ALTERNATIVE THERAPIES
Approximately 20% to 25% of dogs treated for epilepsy using standard anticonvulsant therapy are never well controlled, despite attempts at therapeutic drug monitoring and appropriate dose adjustments. It is important to evaluate poorly controlled animals for underlying metabolic or intracranial disease that could be specifically treated. Alternative treatments should also be considered in these animals, including hypoallergenic diets, acupuncture, surgical division of the corpus callosum, and vagus nerve stimulation.
EMERGENCY THERAPY FOR DOGS AND CATS IN STATUS EPILEPTICUS
Status epilepticus is a series of seizures or continuous seizure activity lasting for 5 minutes or longer without periods of intervening consciousness. Status epilepticus increases arterial blood pressure, body temperature, heart rate, cerebral blood flow, and cerebral oxygen consumption. It also decreases blood pH (because of lactic acidosis) and may decrease effective ventilation. As seizures continue, metabolic deterioration, increased intracranial pressure, acidosis, hyperthermia, and cardiac dysrhythmias are common, leading to progressive cerebral ischemia and neuronal death. Permanent neurologic damage and even death can result.
Status epilepticus is always a medical emergency. The most common reasons for a known idiopathic epileptic patient to present in status include poor chronic seizure control of cluster seizures and abrupt withdrawal of anticonvulsant medications (missed doses). Nonepileptics may present in status as a result of various metabolic (e.g., hypoglycemia, hypocalcemia, hepatic encephalopathy, hyperosmolality, renal failure, intoxications) and intracranial (e.g., neoplasia, trauma, infarct, malformation, heat stroke, granulomatous meningoencephalitis, infectious meningoencephalitis) disorders. History and physical examination findings help determine the cause of status epilepticus in an individual patient. Diagnostic testing for metabolic causes of seizures (especially hypoglycemia, hypocalcemia, electrolyte disturbances) should always be performed and specific treatment initiated when warranted. When intoxication is suspected, treatment should be directed at reducing further absorption of the toxin, increasing toxin excretion, and controlling the neurologic manifestation of seizures (see Box 67-4).
The goals of treatment are to stabilize the animal, stop the seizure activity, protect the brain from further damage, and allow recovery from the systemic effects of prolonged seizure activity. Oxygen is administered, as well as fluid therapy and supportive care, to minimize systemic effects. Diazepam is administered (intravenously or rectally) to stop the seizures; this is followed by phenobarbital to prevent seizure recur rence. More aggressive treatment is required if seizures continue, usually involving a propofol or pentobarbital infusion to stop seizure activity. Mannitol and furosemide are also recommended (as for head trauma, Box 65-2) to decrease the brain edema secondary to prolonged seizure activity. Details regarding the treatment of status epilepticus are outlined in Box 67-7.
 BOX 67-7 Status Epilepticus Treatment in Dogs and Cats
BOX 67-7 Status Epilepticus Treatment in Dogs and Cats
IV, Intravenous; CRI, constant rate infusion.
Barnes HL, et al. Clinical signs, underlying cause and outcome in cats with seizures: 17 cases (1997-2002). J Am Vet Med Assoc. 2004;225:1723.
Bergman RL, Coates JR. Seizures in young dogs and cats: management. Compend Vet. 2005;July:539.
Boothe DM. Anticonvulsant therapy in small animals. Vet Clin N Am Small Anim Pract. 1998;28(2):411.
Dewey CW, et al. Alternative anticonvulsant drugs for dogs with seizure disorders. Vet Med. September, 2004:786.
Heynold Y, et al. Clinical, epidemiological and treatment results of idiopathic epilepsy in 54 Labrador Retrievers: a long term study. J Small Anim Pract. 1997;38:7.
Podell M. Seizures. In: Platt SR, Olby NJ, editors. BSAVA manual of canine and feline neurology. Gloucester: BSAVA, 2004.
Thomas WB. Idiopathic epilepsy in dogs. Vet Clin N Am Small Anim Pract. 2000;30(1):183.